Abstract
Previously we described safe and efficient three-component human immunodeficiency virus type 1 (HIV-1)-based gene transfer systems for delivery of genes into nondividing cells (H. Mochizuki, J. P. Schwartz, K. Tanaka, R. O. Brady, and J. Reiser, J. Virol. 72:8873–8883, 1998). To apply such vectors in anti-HIV gene therapy strategies and to express multiple proteins in single target cells, we have engineered HIV-1 vectors for the concurrent expression of multiple transgenes. Single-gene vectors, bicistronic vectors, and multigene vectors expressing up to three exogenous genes under the control of two or three different transcriptional units, placed within the viral gag-pol coding region and/or the viral nef and env genes, were designed. The genes encoding the enhanced version of green fluorescent protein (EGFP), mouse heat-stable antigen (HSA), and bacterial neomycin phosphotransferase were used as models whose expression was detected by fluorescence-activated cell sorting, fluorescence microscopy, and G418 selection. Coexpression of these reporter genes in contact-inhibited primary human skin fibroblasts (HSFs) persisted for at least 6 weeks in culture. Coexpression of the HSA and EGFP reporter genes was also achieved following cotransduction of target cells using two separate lentivirus vectors encoding HSA and EGFP, respectively. For the regulated expression of transgenes, tetracycline (Tet)-regulatable lentivirus vectors encoding the reverse Tet transactivator (rtTA) and EGFP controlled by a Tet-responsive element (TRE) were constructed. A binary HIV-1-based vector system consisting of a lentivirus encoding rtTA and a second lentivirus harboring a TRE driving the EGFP reporter gene was also designed. Doxycycline-modulated expression of the EGFP transgene was confirmed in transduced primary HSFs. These versatile vectors can potentially be used in a wide range of gene therapy applications.
Gene transfer vectors based on retroviruses including oncogenic retroviruses and lentiviruses provide effective means for the delivery, integration, and expression of exogenous genes in mammalian cells in vitro and in vivo (48, 65). In contrast to oncogenic retroviruses which are dependent on mitotic cells (40, 61), lentiviruses including human immunodeficiency virus type 1 (HIV-1) are independent of cell division to complete their replicative cycle (37). Therefore, they provide attractive gene delivery vehicles in the context of nondividing cells. Lentivirus vectors including HIV-1-based vectors, HIV-2-based vectors, simian immunodeficiency-based vectors, feline immunodeficiency virus-based vectors, and equine infectious anemia virus-based vectors are being increasingly used for gene delivery in vitro (2, 11, 16, 29, 32, 44, 46, 47, 49, 55, 58, 60, 62, 67, 68). They are also promising for long-term gene expression in vivo in cells of the central nervous system (4, 5, 34, 46, 47, 69), hematopoietic system (42), retina (43), muscle and liver (30, 51), lung (23, 28), pancreatic islets (19), cochlea (24), and corneal tissue (66).
As safe and high-titer lentivirus vectors are being developed, efficient delivery of reporter genes into target cells is becoming customary and the application of these vectors to deliver therapeutic genes is emerging. In a therapeutic setting involving animal disease models, the expression of more than one gene may be needed because the therapeutic protein may consist of multiple subunits. Multigene vectors may also be desirable in anti-HIV gene therapy strategies involving ribozymes, antisense RNA, transdominant proteins, and intracellular antibodies (14). Moreover, the expression of a reporter gene in addition to the functional transgene from a multigene vector could aid in the experimental assessment of gene transfer efficiency and in the optimization of the gene transfer process.
In common with all replication-competent retroviruses, the HIV-1 genome contains the gag, pol, and env coding regions that encode the core proteins, the virion-associated enzymes, and the envelope (Env) glycoprotein, respectively, flanked by the long terminal repeats (LTRs). HIV-1 also possesses regulatory functions encoded by the tat and rev genes, as well as accessory genes that include vif, vpr, vpu, and nef, many of which are not required for virus replication in vitro (20). HIV-1 generates a complex pattern of multiply spliced RNAs to encode the Tat and Rev regulatory proteins and the Vpr and Nef accessory proteins. Several transcripts can express each of the regulatory and accessory proteins, and most of these transcripts have the potential to encode two or more proteins with different efficiencies (56). These results highlight the remarkable transcriptional potential of the HIV-1 genome and underscore the flexibility of HIV-1 in the coexpression of multiple genes.
Utilizing the transcriptional flexibility of HIV-1, we constructed gene transfer vectors that simultaneously express several genes of interest from the viral LTR and from heterologous internal promoters. In one class of vectors, heterologous genes are expressed from the viral 5′ LTR in a Tat-dependent fashion and from heterologous internal promoters and a second set of vectors uses heterologous promoters exclusively. We also report on the design of vectors that allow modulation of transgene expression in response to doxycycline (DOX).
MATERIALS AND METHODS
Plasmid constructs.
The following plasmids were obtained through the AIDS Research and Reference Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, Bethesda, Md.: pHIVgpt from Kathleen Page and Dan Littman (50), pNL4-3 from Malcom Martin (1), and pNL4-3.HSA.R−E− from Nathaniel Landau (25). All nucleotides are numbered in accordance with Korber et al. (33). The two-gene HIV-EGFP-HSAΔE vector is based on the original HIV-EGFPΔE vector (44). The sequences between the BamHI (position 8464) and XhoI (position 8886) sites were replaced with the BamHI/XhoI fragment from pNL4-3.HSA.R−E− carrying the HSA reporter gene within the nef coding region (25). The HIV-EGFP-HSAΔE tat(−) vector contains two consecutive termination codons after amino acid 10 within the 5′ tat exon. It is based on the pTat(−)GV/4GSTm construct (27) that was kindly provided by K.-T. Jeang (NIAID). The HIV-EGFP-HSAΔE rev(−) vector encodes a truncated version of Rev. It was created by filling up the unique BamHI site present within rev exon 2 using T4 DNA polymerase, leading to a 4-bp insertion. The HIV-EGFP-HSAΔE tat(−) and HIV-EGFP-HSAΔE rev(−) vectors were combined to yield HIV-EGFP-HSAΔE tat(−)/rev(−). The three-gene HIV-EGFP-neo-HSAΔE vector was derived from the original HIV-neoΔE construct (44). An expression cassette consisting of the human cytomegalovirus (CMV) immediate-early (IE) promoter linked to the EGFP coding region was derived from pEGFP-C1 (Clontech) and inserted between the NsiI (position 1247) and EcoRI (position 5743) sites, and the sequences between the BamHI and XhoI sites were replaced with sequences carrying the HSA coding region as described above. The bicistronic HIV-HSA-IRES-EGFPΔE vector was constructed as follows. The gag, pol, vif, and vpr sequences between the SpeI (nucleotide 1506) and EcoRI (nucleotide 5742) sites were deleted from the original HIV-HSA construct harboring HSA sequences driven by the CMV IE promoter (58). A 1.34-kb fragment carrying the encephalomyocarditis virus (ECMV) internal ribosome entry site (IRES) sequence (45) and EGFP gene sequences was derived from pIRES-EGFP (Clontech). The fragment was inserted downstream from the HSA coding region at position 7611. All NL vectors are based on the NL4-3 molecular clone (1) with the sequences between the NsiI (position 1246) and BglII (position 7611) sites deleted. A 168-bp simian virus 40 (SV40) origin of replication fragment and a 133-bp fragment harboring HIV-1 polypurine tract sequences (10) were placed between these two sites (57). Various expression cassettes were inserted between the BamHI (nucleotide 8464) and XhoI (nucleotide 8886) sites. NL-EGFP carries an expression cassette consisting of the CMV IE promoter linked to the EGFP coding region. NL-HSA carries a similar expression cassette encoding the mouse HSA cDNA. The CEF hybrid promoter was derived from pCE-490 (SnaBI-BamHI fragment) (63). To construct the NL-HSA-IRES (ECMV)-EGFP and NL-HSA-IRES (ECMV)-EGFP/CEF bicistronic vectors, a fragment carrying the HSA and EGFP genes linked by an ECMV IRES sequence was used as described above. The NL-HSA-IRES (Gtx)-EGFP vector contains an IRES [(Gtx133-141)10(SI)9β; 208-bp SpeI/NcoI fragment] derived from the 5′ untranslated region of the mRNA encoding the Gtx homeodomain protein (9). The tetracycline-regulatable NL-rtTA/TRE-EGFP vector carries a 1,860-bp fragment encoding the rtTA linked to the CMV IE promoter (derived from pRevTet-On; Clontech) and a 1,240-bp fragment carrying the Tet-responsive element (TRE) and EGFP sequences (derived from pBI-EGFP; Clontech). The NL-TRE-EGFP vector contains a 1,240-bp fragment carrying the TRE and EGFP sequences derived from pBI-EGFP, and the NL-rtTA vector contains a 1,860-bp fragment encoding the rtTA linked to the CMV IE promoter derived from pRevTet-On.
Cells.
Human embryonic kidney 293T cells (15) were kindly provided by Warren Pear (Rockefeller University). Human osteosarcoma (HOS) cells (CRL-1543), HT1080 cells (CCL-121), and primary human skin fibroblasts (HSFs; CRL 2072; passages 8 and 9) were obtained from the American Type Culture Collection. The cells were grown in Dulbecco's modified Eagle's medium (DMEM; Life Technologies Inc.) containing 10% heat-inactivated fetal bovine serum (FBS). The human H9 and A3.01 T-cell lines were obtained from Robert Gallo (38) and Thomas Folks (18), respectively, through the AIDS Research and Reference Program, Division of AIDS, NIAID, National Institutes of Health. The cells were grown in RPMI 1640 medium supplemented with 2 mM l-glutamine, gentamicin at 50 μg/ml, and 10% FBS. Contact-inhibited primary HSFs were cultivated in DMEM–10% FBS for up to 1 month prior to transduction.
Virus production and transduction of cells.
Vector particles pseudotyped with the vesicular stomatitis virus G glycoprotein (VSV-G) were produced using a three-plasmid expression system by transient transfection of human 293T cells with a defective packaging construct (44), a plasmid with the VSV-G coding region driven by the HIV LTR (58) and a HIV-1-based vector construct. Five micrograms of each of the three plasmid DNAs was cotransfected into subconfluent 293T cells using the calcium phosphate precipitation method (54). Cells were seeded into six-well plates 24 to 30 h prior to transfection. Chloroquine (25 μM final concentration) was added to the cells immediately before transfection, and the medium was replaced with 2 ml (per well) of fresh DMEM supplemented with 10% FBS 12 to 14 h later. The virus was harvested 60 to 65 h later, filtered through a Millipore Millex-HA 0.45μ filter unit, aliquoted, and frozen at −80°C. p24 assays were performed using a commercial kit (Cellular Products Inc.). The generation of replication-competent virus was tested by serially passaging transduced H9 cells over a period of 4 weeks followed by measurement of p24 levels (44). Vector titers were derived from quantitative fluorescence-activated cell sorter (FACS) analysis using HOS cells. To calculate titers, the number of target cells was multiplied by the percentage of EGFP- or HSA-positive cells divided by the volume of the input virus.
FACS analysis of transduced cells.
Transductions were typically performed in six-well plates in medium containing 8 μg of Polybrene per ml in a total volume of 0.5 ml. At various times, the virus was removed, 2 ml of fresh medium was added, and the cells were incubated at 37°C. G418-resistant cells were selected in medium supplemented with G418 (Life Technologies Inc.) (0.35 to 0.5 mg of active drug per ml). The medium was changed every 3 to 4 days. Cells expressing HSA and EGFP were detached from the plate using trypsin-EDTA (Life Technologies Inc.), collected into DMEM–10% FBS, and subsequently washed with Hanks balanced salt solution (Life Technologies Inc.) containing 2% FBS (Hanks-FBS). The cells were stained with a phycoerythrin (PE)-labeled anti-HSA monoclonal antibody (Pharmingen) for 30 min on ice in Hanks-FBS. The cells were washed with Hanks-FBS, fixed in 2% formaldehyde for 5 min, resuspended in Hanks-FBS, and then subjected to FACS analysis.
Fluorescence microscopy.
Cells expressing EGFP were fixed with 4% formaldehyde in Hanks-FBS for 15 min at room temperature. Cells were then washed three times with Hanks-FBS and then analyzed by fluorescence microscopy. Cells coexpressing EGFP and HSA were blocked with 10% goat serum for 20 min at room temperature. PE-labeled anti-HSA monoclonal antibody was added, and the cells were incubated for 20 min at room temperature. Cells were then washed three times with Hanks-FBS and analyzed by fluorescence microscopy.
Southern blot analysis.
Genomic DNA from transduced HOS cells was isolated using a QIAamp DNA Mini Kit (Qiagen) and digested with ScaI or AflII. The DNA fragments were separated on a 0.6% agarose gel and then processed for Southern blot analysis using Zeta-Probe GT membranes (Bio-Rad). Blots were probed using a 32P-labeled EGFP gene fragment.
RESULTS
Design of multigene HIV-1-based vector systems.
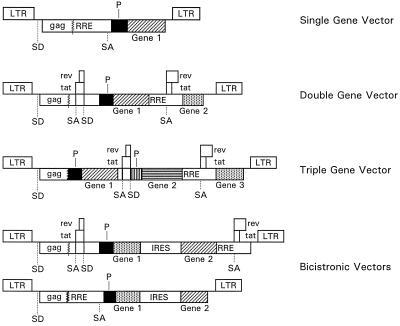
We previously described two different classes of HIV-1-based gene transfer vectors encoding single reporter genes such as EGFP, HSA, and ShlacZ and the application of such vectors to deliver reporter genes into nondividing cells (44). These vectors also contained cis-acting sequences required for packaging, reverse transcription, and integration, including the 5′ and 3′ LTRs, and Env-derived sequences encompassing the Rev-responsive element (RRE). One class of vectors was defective for all HIV-1 genes but encoded functional Tat and Rev with the transgene placed within the env coding region 5′ to the RRE. Vectors lacking Tat and Rev with the expression cassette located 3′ to the RRE were also constructed in accordance with the design of Parolin et al. (52) and Naldini et al. (47). We have now modified these vectors for the concurrent expression of multiple transgenes. Single-gene vectors, bicistronic vectors, or multigene vectors able to express up to three exogenous genes under the control of two or three different transcriptional units placed within the viral gag-pol coding region and/or the viral nef and env genes were designed (Fig. 1). The genes encoding EGFP, HSA, a cell surface marker, and bacterial neomycin phosphotransferase (Neo) were used as models whose expression was monitored by FACS, fluorescence microscopy, and G418 selection. The additional components of the gene transfer system include a packaging (helper) plasmid and an envelope (Env) plasmid encoding VSV-G driven by the HIV-1 LTR (44, 58). Pseudotyped vectors were produced in human embryonic kidney 293T cells using a three-component transient packaging system (44).
FIG. 1.
HIV-1-based gene transfer vectors. Boxes interrupted by jagged lines contain partial deletions. Abbreviations: P, heterologous transcription promoter; SD, splice donor site; SA, splice acceptor site.
Effects of internal promoters on transgene expression levels.
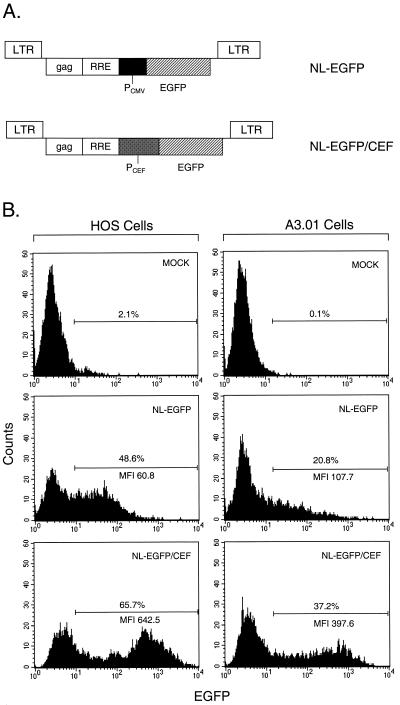
To identify heterologous promoters that work efficiently in the context of HIV-1-based lentivirus vectors, we compared the efficiency of the human CMV IE promoter, which is widely used for transgene expression off such vectors, with that of the CEF hybrid promoter. The CEF hybrid promoter consists of the CMV IE enhancer fused to sequence elements derived from the human translation elongation factor 1α promoter (63). These promoters were tested in the context of a single-gene HIV-1 reporter vector encoding EGFP (Fig. 2A). To analyze the expression of the EGFP reporter gene, HOS cells and human A3.01 T cells were used. HOS cells were chosen primarily because they have been shown in the past to be readily transduced by pseudotyped vectors (36, 44, 58). A3.01 cells were tested because we wanted to investigate the efficiency of the CEF promoter relative to that of the CMV IE promoter in the context of a T-cell line. Cells were transduced in parallel with the NL-EGFP and NL-EGFP/CEF vector stocks at multiplicities of infection (MOIs) of 0.20 and 0.22, respectively, for HOS cells and at MOIs of 8.4 and 9.4, respectively, for A3.01 cells, collected 3 days later, and processed for FACS analysis. The mean fluorescence intensity (MFI) of the transduced cell population was used as a measure of EGFP reporter gene expression. The results presented in Fig. 2B show that the MFI of the EGFP-positive cell population varied, depending on the promoter used. The MFI of HOS cells transduced with the NL-EGFP/CEF vector was about 10-fold higher than the MFI of cells transduced with the NL-EGFP vector. The MFI of A3.01 cells after transduction with NL-EGFP/CEF was about fourfold higher than the MFI of cells transduced with the NL-EGFP vector. This indicates that the hybrid CEF promoter in the context of a single-gene lentivirus vector is more efficient than the CMV IE promoter in HOS and A3.01 T cells.
FIG. 2.
Influence of internal promoters on expression of EGFP transgene in HOS cells and in A3.01 T cells. (A) NL-EGFP and NL-EGFP/CEF single-gene vector constructs harboring CMV IE and CEF promoters, respectively. PCMV, human CMV IE promoter; PCEF, hybrid promoter consisting of the enhancer region of the CMV IE promoter fused to translation elongation factor 1α promoter elements. (B) FACS analysis of transduced HOS cells and A3.01 cells. HOS cells were transduced with NL-EGFP and NL-EGFP/CEF vector stocks at MOIs of 0.20 and 0.22, respectively, in DMEM–10% FBS containing Polybrene at 8 μg/ml. A3.01 cells were transduced with the NL-EGFP and NL-EGFP/CEF vector stocks at MOIs of 8.4 and 9.4, respectively, in RPMI 1640 medium–10% FBS containing Polybrene at 8 μg/ml. Cells were incubated with unconcentrated viral supernatants for 18.5 h at 37°C. Forty-eight hours later, the cells were subjected to single-color FACS analysis.
Transgene coexpression following cotransduction of target cells.
We next investigated the possibility of coexpressing two separate transgenes following cotransduction of target cells with a mixture of two separate lentivirus reporter vectors encoding EGFP (NL-EGFP) and HSA (NL-HSA), respectively. HOS cells were transduced separately with the NL-EGFP or NL-HSA reporter vector (Fig. 3, upper right and lower left panels) or using a mixture of the two vectors (Fig. 3, lower right panel) and subjected to double-color FACS analysis 3 days later following staining of the cells with anti-HSA antibodies. The results presented in Fig. 3 show that a substantial fraction of the cells were doubly positive upon exposure to both vectors, indicating efficient cotransduction. It is also evident from Table 1 that the percentage of doubly positive cells correlated, in an MOI-dependent manner, with the product of the individual transduction efficiencies observed with cells transduced separately with the two different vector stocks.
FIG. 3.
Coexpression of EGFP and HSA transgenes following cotransduction of HOS cells with NL-EGFP and NL-HSA vectors. NL-EGFP virus (upper right panel) and NL-HSA virus (lower left panel) were used at MOIs of 0.55 and 0.52, respectively. Cotransduction was carried out using a mixture of NL-EGFP and NL-HSA vector stocks (lower right panel) at the MOIs indicated above. The cells were incubated with virus supernatant for 18 h at 37°C. Forty-eight hours later, cells were detached using trypsin-EDTA and then incubated with a PE-labeled anti-HSA monoclonal antibody (5-μg/ml final concentration) in a total volume of 0.3 ml for 30 min on ice, washed twice with Hanks–2% FBS and then subjected to double-color FACS analysis.
TABLE 1.
Efficiency of cotransduction of HOS cells as a function of MOIa
| Virus(es) | MOI(s) | % of cells positive for:
|
||
|---|---|---|---|---|
| HSAb | EGFPc | HSA + EGFP | ||
| Expt 1 | ||||
| NL-HSA | 0.18 | 19.6 | 0.1 | 0.1 |
| NL-EGFP/CEF | 1.90 | NDd | 98.4 | ND |
| NL-HSA, NL-EGFP/CEF | 0.18, 1.90 | 21.9 | 98.5 | 20.8 |
| Expt 2 | ||||
| NL-HSA | 0.68 | 66.9 | 0 | 0 |
| NL-EGFP/CEF | 0.58 | 0 | 55.7 | 0 |
| NL-HSA, NL-EGFP/CEF | 0.68, 0.58 | 78.0 | 62.1 | 58.2 |
HOS cells were transduced and subjected to FACS analysis as described in the legend to Fig. 3.
Refers to the percentage of all HSA-positive cells (including singly and doubly positive cells).
Refers to the percentage of all EGFP-positive cells.
ND, not determined.
Multigene vectors involving two separate transcriptional units.
With a view toward designing vectors that are useful in anti-HIV gene therapy strategies, HIV-1-based vectors with the potential to coexpress multiple transgenes as separate transcriptional units were designed next. To construct a two-gene vector expressing two separate genes from two independent promoters, the original HIV-EGFPΔE vector (44) containing the EGFP reporter gene linked to the CMV IE promoter was engineered to express the HSA cell surface marker. To generate the two-gene HIV-EGFP-HSAΔE vector (Fig. 4A), the nef coding region was replaced with the mouse HSA cDNA. In this construct, a functional tat coding region was retained, allowing expression of gene sequences placed within the nef coding region from a multiply spliced mRNA through activation of the viral LTR. Coexpression of the EGFP and HSA genes in HOS cells was investigated using quantitative FACS analysis. The FACS data presented in Fig. 4B show that both genes were coexpressed at high levels in transduced HOS cells. More than 61% of the cells were both EGFP and HSA positive (Fig. 4B, middle), while less than 1% of the mock-infected cells were positive for both markers (Fig. 4B, top). Figure 4B (bottom) shows expression results obtained with the HIV-EGFP-HSAΔE tat(−) vector, whose tat coding region had been inactivated by point mutations carrying two consecutive stop codons after amino acid 10, leading to a truncated version of Tat (27). Sixty-five percent of the cells were EGFP positive, but less than 2% of the cells were doubly positive, indicating that HSA gene expression was fully dependent on Tat activity. The MFI of the EGFP-positive HOS cell population transduced with the Tat-containing vector was 19.7. HOS cells transduced with the vector lacking Tat had an MFI of 6.0. Thus, CMV IE promoter-driven EGFP gene expression was reduced about threefold in the absence of Tat.
FIG. 4.
Analysis of HOS cells and contact-inhibited primary HSFs following transduction with Tat-dependent double-gene vectors. (A) Vector construct. An EGFP expression cassette consisting of EGFP sequences and the CMV IE promoter was inserted within the viral env coding region. HSA sequences were inserted at the 5′ end of nef. (B) FACS analysis of transduced cells. HOS cells in six-well plates were incubated with HIV-EGFP-HSAΔE and HIV-EGFP-HSAΔE tat(−) vector stocks for 5.5 h at MOIs of 0.65 and 0.67, respectively. Three days after transduction, the cells were detached and then incubated with a PE-labeled anti-HSA monoclonal antibody (5-μg/ml final concentration) in a total volume of 0.3 ml for 30 min on ice. The cells were washed twice and then subjected to double-color FACS analysis. (C) Analysis of transduced HSFs by fluorescence microscopy. Contact-inhibited HSFs on coverslips were incubated with HIV-EGFP-HSAΔE and HIV-EGFP-HSAΔE tat(−) vector stocks for 5.5 h at MOIs of 5.0 and 2.2, respectively. Twenty-eight days later, the cells were stained with a PE-labeled anti-HSA monoclonal antibody (0.4-μg/ml final concentration) and processed for fluorescence microscopy. Top: HSFs transduced with a vector containing functional Tat. Bottom: HSFs transduced with a vector lacking functional Tat. HSA-positive cells are red, and EGFP-positive cells are green.
The ability of the newly designed two-gene lentivirus vector system to mediate gene transfer into nondividing cells was analyzed by transduction of contact-inhibited HSFs. Primary HSFs were growth arrested by being allowed to reach contact inhibition upon cultivation in medium containing 10% FBS for 23 days. Such HSFs have been shown to be highly enriched for cells in the G0 and/or G1 stages of the cell cycle (58, 64). HSFs previously transduced with the HIV-EGFP-HSAΔE vector were both EGFP and HSA positive as analyzed by fluorescence microscopy (Fig. 4C) 28 days after transduction. Cells transduced with the HIV-EGFP-HSAΔE tat(−) vector were HSA negative by immunofluorescence assay but produced abundant EGFP fluorescence (Fig. 4C). This confirms the results of the FACS analysis and also indicates that both genes were coexpressed in resting HSFs for at least 28 days.
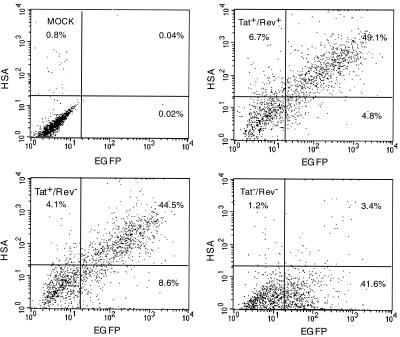
The contribution of Rev with regard to expression of the EGFP and HSA reporter genes was tested using the HIV-EGFP-HSAΔE rev(−) vector harboring a frameshift mutation within the second rev exon. An identical mutation has previously been shown to generate an inactive Rev protein, leading to a change in the relative proportion of the various viral transcripts (17). The HIV-EGFP-HSAΔE rev(−) vector produced titers on HOS cells similar to the ones observed with vectors encoding wild-type Rev (X.-Y. Zhang, unpublished results). HOS cells transduced with this vector showed distributions of doubly positive cells and MFI values similar to those obtained with a vector encoding a wild-type Rev protein (Fig. 5, upper right and lower left). A vector encoding inactive Tat and Rev was also designed and tested in HOS cells. The HIV-EGFP-HSAΔE tat(−)/rev(−) vector produced similar titers on HOS cells (X.-Y. Zhang, unpublished results) and revealed a reduced number of doubly positive cells, but the number of EGFP-positive cells was similar to the number of EGFP-positive cells observed after transduction with the HIV-EGFP-HSAΔE rev(−) vector (Fig. 5, lower left and lower right). The MFI of the EGFP-positive cells was reduced twofold. Taken together, these results indicate that the presence of Rev in the context of the unmodified HIV-EGFP-HSAΔE vector did not negatively (or positively) affect expression of the HSA and EGFP reporter genes and that functional Tat was essential for efficient HSA gene expression.
FIG. 5.
FACS analysis of HOS cells following transduction with a double-gene vector lacking functional Rev. Cells were incubated with HIV-EGFP-HSAΔE, HIV-EGFP-HSAΔE rev(−), and HIV-EGFP-HSAΔE tat(−)/rev(−) vector stocks for 14 h at MOIs of 1.1, 1.3, and 1.0, respectively. Forty-six hours later, the cells were detached and then incubated with a PE-labeled anti-HSA monoclonal antibody (2.5-μg/ml final concentration) in a total volume of 0.2 ml for 30 min on ice. The cells were washed twice and then subjected to double-color FACS analysis. Upper left, mock-transduced cells (using a HIV-neoΔE vector stock); upper right, cells transduced with the HIV-EGFP-HSAΔE vector; lower left, cells transduced with the HIV-EGFP-HSAΔE rev(−) vector stock; lower right, cells transduced with the HIV-EGFP-HSAΔE tat(−)/rev(−) vector stock.
Multigene vectors involving three separate transcriptional units.
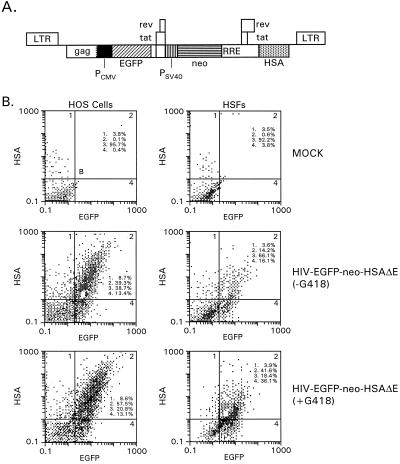
To investigate the potential to express three independent transcriptional units in the context of a Tat-containing lentivirus vector, a construct coexpressing three different transgenes under the control of three separate promoters was designed (Fig. 6A). In this vector, the CMV IE promoter and EGFP gene were placed within the viral gag-pol coding region. The env gene was deleted to accommodate the bacterial neo gene driven by the SV40 early promoter, and the HSA gene was placed within the nef coding region. Pseudotyped vectors were prepared and used to transduce HOS cells, as well as contact-inhibited primary HSFs. Transduced HOS cells were split and subjected to G418 selection 3 days after transduction. Parallel cultures of transduced HOS cells lacked G418. The cells were split once more 10 days later, and the fraction of EGFP- and HSA-positive cells was determined by quantitative FACS analysis 6 days after the second split. Transduced HSFs were split 3 days after infection and grown in the presence or absence of G418 for 39 days. Up to 75% of the G418-selected HOS cells and approximately 78% of the G418-selected HSFs expressed EGFP and/or HSA above background levels. Approximately 57% of the selected HOS cells and 42% of the selected HSFs coexpressed EGFP and HSA (Fig. 6B, bottom). Cells not previously selected with G418 were also analyzed. Sixty-one percent of the infected HOS cells and about one-third of the infected HSFs were EGFP and/or HSA positive; 39.3 and 14.2% of them were doubly positive (Fig. 6B, middle). This shows that G418 selection markedly enriched the fraction of positive cells.
FIG. 6.
Analysis of HOS cells and contact-inhibited primary HSFs following transduction with a triple-gene vector. (A) Vector construct. An EGFP expression cassette consisting of EGFP sequences and the CMV IE promoter was inserted within the viral gag-pol coding region. A second expression cassette consisting of neo sequences driven by the SV40 early promoter was placed within the env coding region. HSA sequences were inserted at the 5′ end of nef. (B) FACS analysis of transduced HOS cells and HSFs. Top, mock-infected cells; middle, cells grown in the absence of G418; bottom, cells grown in the presence of G418. HOS cells were incubated for 7.5 h with unconcentrated virus at an MOI of 1.3. Contact-inhibited primary HSFs were kept in culture for 27 days prior to transduction. Cells were incubated with unconcentrated virus for 7.5 h using an MOI of 1.1. Three days later, the cells were removed from the wells with trypsin-EDTA and diluted into medium with or without G418 (0.3- to 0.4-mg/ml final concentration). HOS cells were diluted at a ratio of 1:2, and HSFs were diluted at a ratio of 1:4. HOS cells were split one more time at a ratio of 1:10 10 days later and processed for FACS analysis after 6 more days. HSFs were processed for FACS analysis 39 days after the first transfer. The cells were stained and processed for FACS analysis as described in the legend to Fig. 4.
A Southern blot analysis with genomic DNA from transduced HOS cells, cut once in each LTR using ScaII or AflII, resulted in a single band that comigrated with purified vector DNA treated in the same way (X.-Y. Zhang, unpublished data). This indicates that the proviral structures were not rearranged.
Expression from bicistronic vectors.
Bicistronic vectors rely on a single promoter driving two or more separate protein coding regions linked by IRES sequences. Cassettes carrying HSA and EGFP genes linked by IRES sequences in one transcriptional unit were designed and introduced into two different HIV-1-based vector backbones. A vector (HIV-HSA-IRES-EGFPΔE) containing the ECMV IRES with functional tat and rev coding regions and the bicistronic expression cassette placed 5′ to the RRE was constructed first (Fig. 7A). The capacity of this vector to coexpress two genes was assessed by FACS analysis by monitoring the expression of the EGFP and HSA reporter genes in transduced HOS cells and in human HT1080 cells. Over 60% of the infected HOS cells were HSA positive, and 15% of them were both EGFP and HSA positive. Close to 80% of the transduced HT1080 cells expressed the upstream HSA gene, and up to 58% were doubly positive (Fig. 7B). These results show that HIV-HSA-IRES-EGFPΔE-driven coexpression of the two genes did occur in both cell lines.
FIG. 7.
FACS analysis of HOS and HT1080 cells following transduction with a bicistronic HIV-1 vector. (A) Vector construct containing EGFP and HSA reporter genes linked by the ECMV IRES. (B) FACS analysis of transduced HOS and HT1080 cells. HOS cells were incubated with virus at an MOI of 0.86 (HSA units) for 7 h at 37°C. HT1080 cells were incubated with the virus for 5.5 h at an MOI of 0.61 (HSA units). The cells were processed for FACS analysis 3 days later as described in the legend to Fig. 4.
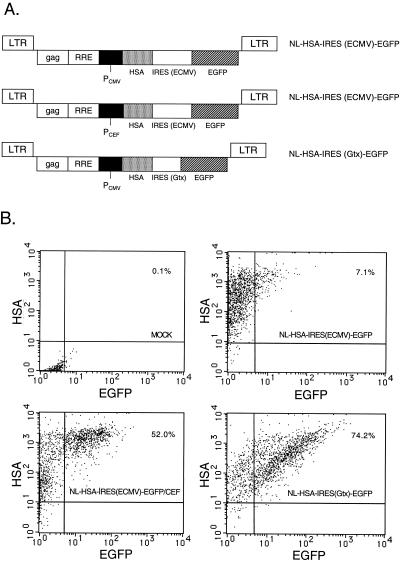
Bicistronic vectors lacking Tat and Rev with the expression cassette located 3′ to the RRE were designed next (Fig. 8A). The ECMV IRES was used along with the homeobox-derived Gtx IRES to yield NL-HSA-IRES (ECMV)-EGFP and NL-HSA-IRES (Gtx)-EGFP, respectively. Up to 99% of the HOS cells transduced with the NL-HSA-IRES (ECMV)-EGFP vector containing the CMV IE promoter were HSA positive. However, only 7.1% of the cells were doubly positive (Fig. 8B, upper right). The NL-HSA-IRES (ECMV)-EGFP/CEF vector construct harboring the CEF promoter in place of the CMV IE promoter produced 52% doubly positive cells (Fig. 8B, lower left panel). Thus, the CEF promoter increased the proportion of doubly positive HOS cells. The Gtx IRES in the context of the NL-HSA-IRES (Gtx)-EGFP vector containing the CMV IE promoter produced 74.2% doubly positive cells (Fig. 8B, lower right). Taken together, these results show that the expression of the downstream EGFP cistron in HOS cells was strongly affected by the promoter used and by the IRES sequence.
FIG. 8.
Effects of internal promoter and IRES on efficiency of expression of downstream cistron in a minimal bicistronic HIV-1 vector. (A) Vector constructs containing the CMV IE or CEF promoter and an ECMV or Gtx IRES element. (B) FACS analysis of transduced HOS cells. Upper left, mock-transduced cells; upper right, cells transduced with the NL-HSA-IRES (ECMV)-EGFP vector (MOI, 3.6); lower left, cells transduced with the NL-HSA-IRES (ECMV)-EGFP/CEF vector (MOI, 3.9); lower right, cells transduced with the NL-HSA-IRES (Gtx)-EGFP vector (MOI, 3.7). Cells were incubated for 24 h at 37°C with the various virus stocks at the indicated MOIs (HSA units) and stained and processed for FACS analysis 2 days later as described in the legend to Figure 4.
Regulated expression of transgenes.
We next investigated the capacity of a heterologous transactivator to modulate the expression of a transgene in the context of HIV-1-based lentivirus vectors. The gene encoding the Tet-controlled rtTA and a minimal promoter (TRE) driving the EGFP reporter gene were placed into a HIV-1 vector 3′ to the viral RRE to yield NL-rtTA/TRE-EGFP (Fig. 9A). A binary Tet-controlled lentivirus vector system was also designed. The rtTA gene and the TRE promoter driving the EGFP reporter gene were placed into two separate HIV-1 vectors 3′ to the viral RRE to yield NL-rtTA and NL-TRE-EGFP, respectively (Fig. 9A). Primary HSFs were transduced sequentially with VSV-G-pseudotyped NL-TRE-EGFP, followed by pseudotyped NL-rtTA. Such cells in the presence of DOX were highly fluorescent (Fig. 9B). Transduced cells in the absence of DOX were also fluorescent, but their relative fluorescence intensities were some two to four times lower than the ones seen in the presence DOX (Fig. 9C, groups 4 and 5), supporting the notion that EGFP gene expression in the presence of DOX was more efficient. The relative fluorescence intensities of primary HSFs transduced with the NL-rtTA/TRE-EGFP vector were some 25-fold lower than the ones obtained with the binary vector system (Fig. 9C, groups 3 and 5). There was a 1.7-fold difference in the fluorescence intensities of NL-rtTA/TRE-EGFP-transduced cells grown in the presence or absence of DOX (Fig. 9C, groups 2 and 3). Additional cells, including the human HT1080, HOS, and 293 Tet-On cell lines and the Chinese hamster ovary CHO-AA8-Luc Tet-Off cell line, were tested to see how general the DOX-dependent regulation of EGFP gene expression would be. Cells were transduced with the various vector constructs in the presence or absence of DOX and subjected to quantitative FACS analysis. The results presented in Table 2 show that the MFI values of transduced cells grown in the presence or absence of DOX varied between 1.5- and 6.5-fold, depending on the vector and cell line investigated. It is also evident from these results that cells transduced with the NL-TRE-EGFP vector alone produced substantial levels of EGFP fluorescence, indicating that there was significant EGFP gene expression from this vector in the absence of rtTA. 293 Tet-On cells transduced with the NL-TRE-EGFP vector alone showed an increased MFI value in the presence of DOX, while the MFI value of CHO-AA8-Luc Tet-Off cells in the presence of DOX was decreased. This was expected, since 293 Tet-On cells produce the rtTA protein, which upregulates EGFP gene expression in the presence of DOX, while CHO-AA8-Luc Tet-Off cells produce the tTA transactivator, which downregulates EGFP gene expression in the presence of DOX. It is also noteworthy that the MFI levels of cells transduced with the binary vector system in the presence of DOX were lower than the MFI values observed with cells transduced with the NL-EGFP vector containing the constitutive CMV IE promoter.
FIG. 9.
Tetracycline-modulated HIV-1 vectors. (A) Vector constructs. Top, NL-rtTA/TRE-EGFP vector carrying rtTA sequences driven by the CMV IE promoter and EGFP reporter gene sequences driven by a TRE. Bottom, binary vector system consisting of the NL-rtTA vector encoding rtTA and a second vector carrying the EGFP reporter gene driven by the TRE promoter. (B) Fluorescence microscopy of transduced HSFs. HSFs were transduced sequentially with the NL-TRE-EGFP and NL-rtTA vectors. The cells were grown in the absence or presence of DOX (1 μg/ml) for 6 days. (C) Quantitation of EGFP gene expression in HSFs using confocal fluorescence microscopy. Groups: 1, mock-transduced HSFs; 2 and 3, HSFs transduced with the NL-rtTA/TRE-EGFP vector grown in the absence (group 2) or presence (group 3) of DOX; 4 and 5, HSFs transduced with the binary vector system grown in the absence (group 4) or presence (group 5) of DOX. Cells were incubated with the vector stocks for 22 h in the presence or absence of DOX at MOIs of 2.7 (single-vector system) and 4.0 (binary system). The fluorescence of intracellular EGFP was quantitated by confocal laser scanning fluorescence microscopy (Leica TCS4D). The fluorescence intensity of individual cells was measured using Leica quantitation software. For each group, the relative intensity of three or more typical cells was measured and the mean fluorescence per unit area of the cell was calculated.
TABLE 2.
DOX-regulated EGFP reporter gene expression in different cell linesa
| Cell type | Virus(es) | MFI
|
|
|---|---|---|---|
| −DOX | +DOXb | ||
| Expt 1,c HT1080 | NL-TRE-EGFP, NL-rtTA | 9.6 | 46.1 |
| NL-TRE-EGFP | 9.1 | 7.5 | |
| NL-EGFP | 160.3 | NDd | |
| Expt 2e | |||
| HT1080 | NL-rtTA/TRE-EGFP | 10.7 | 69.9 |
| NL-TRE-EGFP, NL-rtTA | 27.9 | 121.8 | |
| NL-EGFP | 455.3 | ND | |
| HOS | NL-TRE-EGFP, NL-rtTA | 111.5 | 166.2 |
| NL-EGFP | 455.3 | ND | |
| 293 Tet-On | NL-TRE-EGFP | 3.8 | 14.5 |
| NL-EGFP | 138.7 | ND | |
| CHO Tet-Off | NL-TRE-EGFP | 43.7 | 17.1 |
| NL-EGFP | 227.4 | ND | |
Cells were subjected to FACS analysis.
DOX concentration used: 1 μg/ml.
Cells were incubated with the virus for a total of 25.5 h in the presence of DOX. Incubations with NL-TRE-EGFP and NL-rtTA were done sequentially (8.5 h with NL-TRE-EGFP and 17 h with NL-rtTA). Equal volumes of the various virus supernatants were used. Cells were subjected to FACS analysis 72 h later.
ND, not determined.
Cells were incubated with the virus for a total of 22 h in the presence of DOX. Incubations with TRE-EGFP and NL-rtTA were done sequentially (6 h with NL-TRE-EGFP and 16 h with NL-rtTA). Cells were subjected to FACS analysis 74 h later.
DISCUSSION
Many of the initial gene transfer studies involving lentivirus vectors were conducted using single reporter genes, such as lacZ or EGFP. However, since these vectors are being used increasingly to deliver therapeutic genes into cells in vitro and in vivo, it would be advantageous to maintain the versatility of reporter gene expression in addition to the coexpression of the gene(s) of interest. This approach will be particularly relevant in in vivo applications where the effects of a therapeutic protein are to be assessed. Transduced cells can be visualized by fluorescence microscopy first following in vivo gene transfer and, once identified, analyzed with regard to the effect(s) of the therapeutic gene. Alternatively, cells transduced in vitro could be enriched by using a FACS. Multigene delivery strategies will also be needed to express complex proteins, and they may be useful in anti-HIV gene therapy strategies involving transdominant proteins, intracellular antibodies, antisense RNA, and ribozymes (14, 22). Finally, it might be desirable, under certain conditions, to express a transgene in a regulated fashion.
As shown in this report, coexpression of different transgenes was achieved by cotransduction of target cells using two different lentivirus vectors expressing different reporter genes. One attractive feature of this cotransduction approach relates to the fact that constraints due to the packaging limit of the vector that may arise as a consequence of incorporating large multigenic expression cassettes can be bypassed. Coexpression of different transgenes was also achieved using multigene reporter vectors. Multigene vectors harboring two or three independent transcriptional units placed within the viral gag-pol coding region and/or the viral nef and env genes were constructed. Transcription of the different units was mediated by heterologous internal promoters and by the viral LTR. LTR-driven reporter gene expression strongly depended on the presence of a functional tat coding region. Experiments carried out with the HIV-EGFP-HSAΔE two-gene vector showed that in the absence of functional Tat, expression in HOS cells and primary HSFs of the HSA reporter gene placed within the nef coding region was reduced to background levels. Although LTR-driven transcription in the absence of Tat was generally low, HIV-1-based vectors were found to display significant LTR activity in cell lines expressing heterologous transactivators such as the adenovirus early protein E1A (41, 68). Thus, these vectors may provide valuable tools with which to investigate mechanisms that lead to the up-regulation (or down-regulation) of LTR-derived transcripts in transduced cells in vitro and in vivo. Moreover, it is conceivable that the U3 region of the vector LTRs can be replaced with heterologous enhancers that make the LTR constitutive and Tat independent (8). CMV IE promoter-driven EGFP gene expression in HOS cells in the context of a two-gene HIV-1 vector was reduced some two- to threefold in the absence of Tat. Recent studies by Zufferey et al. (68) also revealed weak promoter interference in a promoter- and cell type-specific manner.
It was interesting that the HIV-EGFP-HSAΔE rev(−) vector harboring an inactivated Rev coding region was not affected in its capacity to express the EGFP and HSA reporter genes in HOS cells, relative to vectors encoding functional Rev, as judged from the number of doubly positive cells and the MFI of such cells. An HXB2 molecular clone harboring an identical Rev mutation resulted in an altered pattern of HIV-1 transcripts with increased levels of doubly spliced transcripts but decreased levels of singly spliced Env transcripts and of genomic transcripts in transfected monkey COS-1 cells and in infected human T-cell lines and was biologically inactive (17). At a similar MOI, the HIV-EGFP-HSAΔE tat(−)/rev(−) vector revealed a 14-fold reduction in the number of doubly positive HOS cells, most likely due to reduced expression of the HSA reporter gene. The Tat-dependent two-gene vectors will be especially useful for quick establishment of stable cell lines expressing the HSA reporter gene in an LTR-dependent fashion. Such cell lines will be helpful in the screening of compounds that act on Tat or through other mechanisms affecting LTR-driven gene expression. As EGFP gene expression in such vectors appears to be relatively independent of Tat, EGFP would serve as a reference.
The three-gene vector presented here harbors a third independent transcriptional unit within the gag-pol coding region. Since a large fraction of the G418-resistant primary HSFs and HOS cells were EGFP and HSA positive, we conclude that all three genes were coexpressed. However, a significant portion of the G418-resistant cells were EGFP positive but lacked HSA fluorescence (Fig. 6B, bottom), supporting the notion that the three transcriptional units are independent. A Southern blot analysis carried out with DNA prepared from transduced HOS cells revealed intact proviral structures. Thus, selective deletion of proviral sequences does not appear to account for the lack of coexpression observed. Uncoupled CMV IE promoter-driven EGFP gene expression and LTR-driven HSA gene expression was also seen in the context of two-gene vectors (Fig. 4B, middle). This may be due to the fact that HSA is translated from transcripts which are initiated at the viral 5′ LTR. Such transcripts have been shown to be spliced in a complex way in a cell type- and time-dependent fashion (56). This may potentially affect HSA production in transduced cells. The transcripts encoding EGFP and Neo are initiated at heterologous internal promoters and expressed independently of the HSA transgene and of each other. Thus, it is perhaps not surprising that the relative distribution of the various classes of RNA may be variable from cell to cell. A similar phenomenon was encountered before with double-gene vectors based on oncogenic retroviruses and with splicing vectors, in particular in that sequences installed upstream affected the formation of the subgenomic RNA from which the downstream gene was expressed (12, 21). Also, it was found that the inhibition of viral RNA splicing was caused by some inserts but not others (6).
Bicistronic vectors are different, because both genes are contained within the same transcriptional unit and coexpression at the RNA level is ensured. Using the bicistronic vectors described in this report, it was observed that the relative proportion of doubly positive cells varied in an IRES-, vector-, promoter-, and cell-dependent manner. While vectors containing the ECMV IRES and functional Tat and Rev produced a substantial number of doubly positive cells, the minimal vector containing the ECMV IRES but lacking Tat and Rev revealed a reduced number of EGFP-positive HOS cells. Similar observations were made when the lacZ coding region was used as the downstream gene (J. Reiser, unpublished data). However, use of the efficient CEF promoter in the context of such vectors greatly increased the percentage of doubly positive HOS cells. This may indicate that CEF promoter-driven production of the bicistronic transcript was enhanced, leading to increased production of the corresponding protein products. Interestingly, however, the MFI of the HSA signal was unaltered in cells transduced with bicistronic vectors containing the CEF promoter relative to vectors containing the CMV IE promoter. This suggests that other mechanisms, including RNA splicing or other RNA processing steps, contributed to the increased numbers of doubly positive cells. This view is contradicted, however, by Northern blot experiments which revealed one major transcript hybridizing with EGFP- and HSA-specific DNA probes (Z. Lai, unpublished results). Use of the Gtx IRES in place of the ECMV IRES allowed very efficient expression of the downstream reporter gene in HOS cells even from vectors containing the CMV IE promoter. Therefore, the Gtx IRES appears to be superior to the ECMV IRES as far as the above lentivirus vectors and HOS cells are concerned. Richardson et al. (59) and Marcello and Giaretta (39) have described Tat- and Rev-dependent bicistronic HIV-1-based vectors containing the ECMV IRES similar to the ones described here. These vectors expressed puromycin resistance or thymidine kinase, respectively. Both downstream genes were expressed at sufficiently high levels to exert a biological effect (i.e., puromycin resistance, sensitivity to ganciclovir and aciclovir).
Recent studies have provided evidence that HIV-1-based vectors are copackaged and subsequently mobilized to untransduced cells by resident HIV-1 genomes present in HIV-1-infected cells (3, 7, 13). This opens up the possibility of transferring HIV-1 vectors harboring anti-HIV therapeutic genes into HIV-infected target cells (14). An et al. (3) have shown that Tat-inducible vectors with the tat and rev genes ablated were very efficient in this respect. The Tat-dependent HIV-EGFP-HSAΔE tat(−) and HIV-EGFP-HSAΔE tat(−)/rev(−) vectors described above are analogous to the vectors described by An et al. (3), except that they carry an extra reporter gene that is expressed in a Tat-independent fashion. This may facilitate the analysis of vector mobilization by wild-type HIV-1 in vitro and in vivo and thus adds flexibility to the system.
There is a need for lentivirus vectors that can be regulated by cell-external signals. The results presented in this report show that the Tet-controlled rtTA is capable of regulating the expression of an EGFP reporter gene from a Tet-responsive promoter in the context of HIV-1 vectors in primary HSFs and in human and hamster cell lines. However, there was substantial EGFP transgene expression in the absence of DOX and even in the absence of the rtTA. This is most likely due to the fact that there is substantial promoter activity off the viral LTR in such vectors. This was observed with NL-EGFP vectors lacking an internal promoter altogether (J. Reiser, unpublished data). As shown in this report, the rtTA can either be part of the same vector construct or it can be cotransduced into the same target cell via a separate vector carrying rtTA sequences as part of a binary vector system. The binary vector system was found to be more potent in terms of EGFP gene expression, indicating that there may be interferences between the various expression cassettes present in the NL-rtTA/TRE-EGFP vector, leading to reduced EGFP gene expression. While this report was being revised for publication, Kafri et al. (31) described a HIV-1-based lentivirus vector containing the entire Tet-regulated system, including the tTA transactivator. This vector is similar to the NL-rtTA/TRE-EGFP vector described above. Although substantial GFP expression was observed under repressed conditions in the presence of DOX, a more-than-500-fold increase in GFP levels was seen 2 weeks later, following DOX withdrawal. In agreement with our results, the magnitude of the increase was much less pronounced 3 to 5 days after DOX withdrawal. Thus, longer induction times favorably affected the ratio of induced GFP expression to basal GFP expression. Retrovirus vectors based on Moloney murine leukemia virus allowing reversible induction of transgene expression in response to Tet were developed in the past (26, 53). These vectors contained both components of the Tet system. More recently, Kringstein et al. (35) have described a binary tetracycline-inducible retrovirus vector system. A self-inactivating retrovirus backbone was used for the reporter virus to eliminate transcriptional interference from the viral LTR. Tetracycline-sensitive transactivators were provided from a second retrovirus after sequential transduction of cells harboring reporter virus constructs. Improved vector design strategies will be needed in the future in order to generate Tet-controlled lentivirus vectors with much-reduced basal activity.
ACKNOWLEDGMENTS
We thank Sumio Sugano and Tatsuyuki Takada for providing the CEF promoter plasmid. The Gtx IRES was kindly provided by Vincent P. Mauro. We thank Hideki Mochizuki and Zachary Miller for support during the early phase of this work. We are most grateful to Kazuyo Takeda for help with the quantitative fluorescence microscopy.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akkina R K, Walton R M, Chen M L, Li Q-X, Planelles V, Chen I S Y. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J Virol. 1996;70:2581–2585. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An D S, Morizono K, Li Q-X, Mao S H, Lu S, Chen I S Y. An inducible human immunodeficiency virus type 1 (HIV-1) vector which effectively suppresses HIV-1 replication. J Virol. 1999;73:7671–7677. doi: 10.1128/jvi.73.9.7671-7677.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blömer U, Kafri T, Randolph-Moore L, Verma I M, Gage F H. Bcl-xL protects adult septal cholinergic neurons from axotomized cell death. Proc Natl Acad Sci USA. 1998;95:2603–2608. doi: 10.1073/pnas.95.5.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blömer U, Naldini L, Kafri T, Trono D, Verma I M, Gage F H. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown A M, Wildin R S, Prendergast T J, Varmus H E. A retrovirus vector expressing the putative mammary oncogene int-1 causes partial transformation of a mammary epithelial cell line. Cell. 1986;46:1001–1009. doi: 10.1016/0092-8674(86)90699-9. [DOI] [PubMed] [Google Scholar]
- 7.Bukovsky A A, Song J-P, Naldini L. Interaction of human immunodeficiency virus-derived vectors with wild-type virus in transduced cells. J Virol. 1999;73:7087–7092. doi: 10.1128/jvi.73.8.7087-7092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang L-J, McNulty E, Martin M. Human immunodeficiency viruses containing heterologous enhancer/promoters are replication competent and exhibit different lymphocyte tropisms. J Virol. 1993;67:743–752. doi: 10.1128/jvi.67.2.743-752.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chappell S A, Edelman G M, Mauro V P. A 9-nt segment of a cellular mRNA can function as an internal ribosome entry site (IRES) and when present in linked multiple copies greatly enhances IRES activity. Proc Natl Acad Sci USA. 2000;97:1536–1541. doi: 10.1073/pnas.97.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charneau P, Mirambeau G, Roux P, Paulous S, Buc H, Clavel F. HIV-1 reverse transcription. A termination step at the center of the genome. J Mol Biol. 1994;241:651–662. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- 11.Corbeau P, Kraus G, Wong-Staal F. Transduction of human macrophages using a stable HIV-1/HIV-2-derived gene delivery system. Gene Ther. 1998;5:99–104. doi: 10.1038/sj.gt.3300563. [DOI] [PubMed] [Google Scholar]
- 12.Dougherty J P, Temin H M. High mutation rate of a spleen necrosis virus-based retrovirus vector. Mol Cell Biol. 1986;6:4387–4395. doi: 10.1128/mcb.6.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dropulic B, Hermankova M, Pitha P M. A conditionally replicating HIV-1 vector interferes with wild-type HIV-1 replication and spread. Proc Natl Acad Sci USA. 1996;93:11103–11108. doi: 10.1073/pnas.93.20.11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dropulic B, Jeang K T. Gene therapy for human immunodeficiency virus infection: genetic antiviral strategies and targets for intervention. Hum Gene Ther. 1994;5:927–939. doi: 10.1089/hum.1994.5.8-927. [DOI] [PubMed] [Google Scholar]
- 15.DuBridge R B, Tang P, Hsia H C, Leong P-M, Miller J H, Calos M P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dull T, Zufferey R, Kelly M, Mandel R J, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinberg M B, Jarrett R F, Aldovini A, Gallo R C, Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986;46:807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- 18.Folks T, Benn S, Rabson A, Theodore T, Hoggan M D, Martin M, Lightfoote M, Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci USA. 1985;82:4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallichan W S, Kafri T, Krahl T, Verma I M, Sarvetnick N. Lentivirus-mediated transduction of islet grafts with interleukin 4 results in sustained gene expression and protection from insulitis. Hum Gene Ther. 1998;9:2717–2726. doi: 10.1089/hum.1998.9.18-2717. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs J S, Regier D A, Desrosiers R C. Construction and in vitro properties of HIV-1 mutants with deletions in “nonessential” genes. AIDS Res Hum Retrovir. 1994;10:343–350. doi: 10.1089/aid.1994.10.343. [DOI] [PubMed] [Google Scholar]
- 21.Gilboa E, Eglitis M A, Kantoff P W, Anderson W F. Transfer and expression of cloned genes using retroviral vectors. BioTechniques. 1996;4:504–512. [Google Scholar]
- 22.Giordano V, Jin D Y, Rekosh D, Jeang K T. Intravirion targeting of a functional anti-human immunodeficiency virus ribozyme directed to pol. Virology. 2000;267:174–184. doi: 10.1006/viro.1999.0112. [DOI] [PubMed] [Google Scholar]
- 23.Goldman M J, Lee P S, Yang J S, Wilson J M. Lentiviral vectors for gene therapy of cystic fibrosis. Hum Gene Ther. 1997;8:2261–2268. doi: 10.1089/hum.1997.8.18-2261. [DOI] [PubMed] [Google Scholar]
- 24.Han J J, Mhatre A N, Wareing M, Pettis R, Gao W Q, Zufferey R N, Trono D, Lalwani A K. Transgene expression in the guinea pig cochlea mediated by a lentivirus-derived gene transfer vector. Hum Gene Ther. 1999;10:1867–1873. doi: 10.1089/10430349950017545. [DOI] [PubMed] [Google Scholar]
- 25.He J, Choe S, Walker R, Di Marzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofmann A, Nolan G P, Blau H M. Rapid retroviral delivery of tetracycline-inducible genes in a single autoregulatory cassette. Proc Natl Acad Sci USA. 1996;93:5185–5190. doi: 10.1073/pnas.93.11.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang L M, Joshi A, Willey R, Orenstein J, Jeang K T. Human immunodeficiency viruses regulated by alternative trans-activators: genetic evidence for a novel non-transcriptional function of Tat in virion infectivity. EMBO J. 1994;13:2886–2896. doi: 10.1002/j.1460-2075.1994.tb06583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson L G, Olsen J C, Naldini L, Boucher R C. Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Ther. 2000;7:568–574. doi: 10.1038/sj.gt.3301138. [DOI] [PubMed] [Google Scholar]
- 29.Johnston J C, Gasmi M, Lim L E, Elder J H, Yee J K, Jolly D J, Campbell K P, Davidson B L, Sauter S L. Minimum requirements for efficient transduction of dividing and nondividing cells by feline immunodeficiency virus vectors. J Virol. 1999;73:4991–5000. doi: 10.1128/jvi.73.6.4991-5000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kafri T, Blömer U, Peterson D A, Gage F H, Verma I M. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 31.Kafri T, van Praag H, Gage F H, Verma I M. Lentiviral vectors: regulated gene expression. Mol Ther. 2000;1:516–521. doi: 10.1006/mthe.2000.0083. [DOI] [PubMed] [Google Scholar]
- 32.Kim V N, Mitrophanous K, Kingsman S M, Kingsman A J. Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1. J Virol. 1998;72:811–816. doi: 10.1128/jvi.72.1.811-816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korber B, Kuiken C, Foley B, Hahn B, McCutchan F, Mellors J, Sodroski J. Human retroviruses and AIDS 1998. A compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N.Mex: Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; 1998. [Google Scholar]
- 34.Kordower J H, Bloch J, Ma S Y, Chu Y, Palfi S, Roitberg B Z, Emborg M, Hantraye P, Deglon N, Aebischer P. Lentiviral gene transfer to the nonhuman primate brain. Exp Neurol. 1999;160:1–16. doi: 10.1006/exnr.1999.7178. [DOI] [PubMed] [Google Scholar]
- 35.Kringstein A M, Rossi F M V, Hofmann A, Blau H M. Graded transcriptional response to different concentrations of a single transactivator. Proc Natl Acad Sci USA. 1998;95:13670–13675. doi: 10.1073/pnas.95.23.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landau N R, Page K A, Littman D R. Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J Virol. 1991;65:162–169. doi: 10.1128/jvi.65.1.162-169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis P F, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann D L, O'Brien S J, Gilbert D A, Reid Y, Popovic M, Read-Connole E, Gallo R C, Gazdar A F. Origin of the HIV-susceptible human CD4+ cell line H9. AIDS Res Hum Retrovir. 1989;5:253–255. doi: 10.1089/aid.1989.5.253. [DOI] [PubMed] [Google Scholar]
- 39.Marcello A, Giaretta I. Inducible expression of herpes simplex virus thymidine kinase from a bicistronic HIV1 vector. Res Virol. 1998;149:419–431. doi: 10.1016/s0923-2516(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 40.Miller D G, Adam M A, Miller A D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyoshi H, Blömer U, Takahashi M, Gage F H, Verma I M. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyoshi H, Smith K A, Mosier D E, Verma I M, Torbett B E. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science. 1999;283:682–686. doi: 10.1126/science.283.5402.682. [DOI] [PubMed] [Google Scholar]
- 43.Miyoshi H, Takahashi M, Gage F H, Verma I M. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mochizuki H, Schwartz J P, Tanaka K, Brady R O, Reiser J. High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. J Virol. 1998;72:8873–8883. doi: 10.1128/jvi.72.11.8873-8883.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan R A, Couture L, Elroy-Stein O, Ragheb J, Moss B, Anderson W F. Retroviral vectors containing putative internal ribosome entry sites: development of a polycistronic gene transfer system and applications to human gene therapy. Nucleic Acids Res. 1992;20:1293–1299. doi: 10.1093/nar/20.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naldini L, Blömer U, Gage F H, Trono D, Verma I M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 48.Naldini L, Verma I M. Lentiviral vectors. In: Friedmann T, editor. The development of human gene therapy. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1999. pp. 47–60. [Google Scholar]
- 49.Olsen J C. Gene transfer vectors derived from equine infectious anemia virus. Gene Ther. 1998;5:1481–1487. doi: 10.1038/sj.gt.3300768. [DOI] [PubMed] [Google Scholar]
- 50.Page K A, Landau N R, Littman D R. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J Virol. 1990;64:5270–5276. doi: 10.1128/jvi.64.11.5270-5276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park F, Ohashi K, Chiu W, Naldini L, Kay M A. Efficient lentiviral transduction of liver requires cell cycling in vivo. Nat Genet. 2000;24:49–52. doi: 10.1038/71673. [DOI] [PubMed] [Google Scholar]
- 52.Parolin C, Taddeo B, Palú G, Sodroski J. Use of cis- and trans-acting viral regulatory sequences to improve expression of human immunodeficiency virus vectors in human lymphocytes. Virology. 1996;222:415–422. doi: 10.1006/viro.1996.0438. [DOI] [PubMed] [Google Scholar]
- 53.Paulus W, Baur I, Boyce F M, Breakefield X O, Reeves S A. Self-contained, tetracycline-regulated retroviral vector system for gene delivery to mammalian cells. J Virol. 1996;70:62–67. doi: 10.1128/jvi.70.1.62-67.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poeschla E M, Wong-Staal F, Looney D J. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- 56.Purcell D F J, Martin M A. Alternative splicing of human immunodeficiency virus type 1 mRNA modulates viral protein expression, replication, and infectivity. J Virol. 1993;67:6365–6378. doi: 10.1128/jvi.67.11.6365-6378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reiser J. Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther. 2000;7:910–913. doi: 10.1038/sj.gt.3301188. [DOI] [PubMed] [Google Scholar]
- 58.Reiser J, Harmison G, Kluepfel-Stahl S, Brady R O, Karlsson S, Schubert M. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci USA. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richardson J H, Hofmann W, Sodroski J G, Marasco W A. Intrabody-mediated knockout of the high-affinity IL-2 receptor in primary human T cells using a bicistronic lentivirus vector. Gene Ther. 1998;5:635–644. doi: 10.1038/sj.gt.3300644. [DOI] [PubMed] [Google Scholar]
- 60.Schnell T, Foley P, Wirth M, Münch J, Überla K. Development of a self-inactivating, minimal lentivirus vector based on simian immunodeficiency virus. Hum Gene Ther. 2000;11:439–447. doi: 10.1089/10430340050015905. [DOI] [PubMed] [Google Scholar]
- 61.Springett G M, Moen R C, Anderson S, Blaese R M, Anderson W F. Infection efficiency of T lymphocytes with amphotropic retroviral vectors is cell cycle dependent. J Virol. 1989;63:3865–3869. doi: 10.1128/jvi.63.9.3865-3869.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutton R E, Wu H T M, Rigg R, Böhnlein E, Brown P O. Human immunodeficiency virus type 1 vectors efficiently transduce human hematopoietic stem cells. J Virol. 1998;72:5781–5788. doi: 10.1128/jvi.72.7.5781-5788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takada T, Iida K, Awaji T, Itoh K, Takahashi R, Shibui A, Yoshida K, Sugano S, Tsujimoto G. Selective production of transgenic mice using green fluorescent protein as a marker. Nat Biotechnol. 1997;15:458–461. doi: 10.1038/nbt0597-458. [DOI] [PubMed] [Google Scholar]
- 64.Tobey R A, Valdez J G, Crissman H A. Synchronization of human diploid fibroblasts at multiple stages of the cell cycle. Exp Cell Res. 1988;179:400–416. doi: 10.1016/0014-4827(88)90279-0. [DOI] [PubMed] [Google Scholar]
- 65.Verma I M, Somia N. Gene therapy—promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 66.Wang X, Appukuttan B, Ott S, Patel R, Irvine J, Song J, Park J H, Smith R, Stout J T. Efficient and sustained transgene expression in human corneal cells mediated by a lentiviral vector. Gene Ther. 2000;7:196–200. doi: 10.1038/sj.gt.3301075. [DOI] [PubMed] [Google Scholar]
- 67.White S M, Renda M, Nam N-Y, Klimatcheva E, Zhu Y, Fisk J, Halterman M, Rimel B J, Federoff H, Pandya S, Rosenblatt J D, Planelles V. Lentivirus vectors using human and simian immunodeficiency virus elements. J Virol. 1999;73:2832–2840. doi: 10.1128/jvi.73.4.2832-2840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zufferey R, Dull T, Mandel R J, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zufferey R, Nagy D, Mandel R J, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]