Abstract
Cancer remains a formidable adversary, challenging medical advancements with its dismal prognosis, low cure rates and high mortality rates. Within this intricate landscape, long non-coding RNAs (lncRNAs) emerge as pivotal players, orchestrating proliferation and migration of cancer cells. Harnessing the potential of lncRNAs as therapeutic targets and prognostic markers holds immense promise. The present comprehensive review delved into the molecular mechanisms underlying the involvement of lncRNAs in the onset and progression of the top five types of cancer. By meticulously examining lncRNAs across diverse types of cancer, it also uncovered their distinctive roles, highlighting their exclusive oncogenic effects or tumor suppressor properties. Notably, certain lncRNAs demonstrate diverse functions across different cancers, confounding the conventional understanding of their roles. Furthermore, the present study identified lncRNAs exhibiting aberrant expression patterns in numerous types of cancer, presenting them as potential indicators for cancer screening and diagnosis. Conversely, a subset of lncRNAs manifests tissue-specific expression, hinting at their specialized nature and untapped significance in diagnosing and treating specific types of cancer. The present comprehensive review not only shed light on the intricate network of lncRNAs but also paved the way for further research and clinical applications. The unraveled molecular mechanisms offer a promising avenue for targeted therapeutics and personalized medicine, combating cancer proliferation, invasion and metastasis.
Key words: lncRNAs, oncogenic, cancer-suppressing, proliferation, invasion metastasis
1. Introduction
Cancer stands as one of the most perilous diseases, with its incidence and fatality rates consistently occupying the top positions among non-communicable diseases (1). In 2020, the world witnessed ~19.3 million fresh cases of cancer and nearly 10.0 million cancer-related fatalities (2). There have been significant advancements in cancer treatment, leading to substantial successes in recent decades. Nevertheless, the prognosis remains grim for the majority of cancer patients, particularly those in advanced stages with metastasis, as their survival rates continue to be low (3). Hence, there is an urgent need to gain deeper insights into the molecular mechanisms that control the development and progression of tumors and to devise more efficient clinical tactics for treating cancer.
Traditionally, long non-coding RNA (lncRNA) was thought of as mere background noise in genomic transcription (4). However, recent research has uncovered that lncRNA indeed serves a crucial role in governing gene expression. This includes functions such as gene suppression, modifying chromatin structure, influencing epigenetic gene activation, regulating transcription, post-transcriptional modifications and even affecting translation processes (5-8). Consequently, lncRNA is actively involved in various biological processes such as cell proliferation, cell cycle regulation, immune regulation and programmed cell death (apoptosis) (9-11).
Cancer, among other human diseases, is closely associated with lncRNAs (12). Irregular levels of lncRNA expression can play a pivotal role in the development of various types of cancer, serving as a significant regulatory factor in their onset with a predictive potential (8). lncRNAs can perform diverse biological functions in different types of cancers, such as regulating gene expression, metabolism, immunity, influencing cell proliferation, controlling apoptosis and affecting tumor metastasis (13-16). These versatile molecules can act as oncogenes, promoting cancer growth, or tumor suppressors, inhibiting tumor progression, or both depending on the specific context and type of cancer. The present study provided a comprehensive discussion of how lncRNAs contribute to both cancer-promoting and cancer-inhibiting processes in the top five types of cancer with the highest incidence rates. Additionally, it offered a summary of the oncogenic and suppressive roles of lncRNAs across all types of cancer.
2. Overview of the top five types of cancer
Breast cancer (BC), the leading cause of mortality in women, surpassed lung cancer (LC) in prevalence in 2020, resulting in over 685,000 fatalities (2). Nearly 680 million women worldwide were estimated to have suffered from BC in 2018 (2). LC, the second most common tumor globally (2), claims ~1.6 million lives annually (17). It is primarily categorized into small-cell LC (SCLC) and non-small cell LC (NSCLC) (18), with NSCLC accounting for 80% of cases (19). Early diagnosis of LC is challenging due to its asymptomatic nature (20). Gastric cancer (GC), the fifth most common cancer and the fourth most fatal, maintains high mortality rates worldwide (2). Diagnosis and treatment involve techniques such as endoscopic biopsies, CT scans and laparoscopic staging (21). Despite a gradual decline in the incidence of GC over the past few decades, the 5-year overall survival rate for GC patients remains low, partly because GC patients display no symptoms in the early stages (21). Prostate cancer (PCa) is a common malignant tumor in elderly men and ranks as the second most common cancer and the fifth leading cause of mortality in men worldwide (2). Metastatic diseases, particularly bone metastasis, contribute to the mortality rate. Treatment resistance to androgen deprivation therapy and chemotherapy drugs poses a significant challenge (22,23). Colorectal cancer (CRC), the most prevalent gastrointestinal tract cancer, ranks third in terms of incidence and second in mortality (2). Sporadic CRC develops slowly through the adenoma-cancer sequence and is associated with long-term colorectal inflammation. Early detection remains challenging, despite the use of endoscopy and biopsy (24).
The significance of lncRNAs in the progression and development of BC, LC, GC, PCa, CRC and other types of cancer has been demonstrated in various studies (25-28). These lncRNAs have been found to play essential roles in cancer-related mechanisms, functioning as either oncogenes or tumor suppressors (Fig. 1). Consequently, there is considerable potential for lncRNAs to serve as diagnostic indicators and therapeutic targets. Further exploration of lncRNAs may result in advancements in early detection, personalized treatment strategies and overall management of various types of cancer.
Figure 1.
A central schematic illustrating the lncRNAs associated with various types of cancer. lncRNAs can act as oncogenes, promoting cancer growth, or tumor suppressors, inhibiting tumor progression, depending on the specific context and cancer type. lncRNA, long non-coding RNA.
3. lncRNAs as oncogenes in the top five types of cancer
BC
Several lncRNAs including, lncRNA-CDC6 (25), SNHG1 (29) and MALATA1 (30) have been implicated in the proliferation and metastasis of cancer cells during the progression and development of BC (Table I). When Kong et al (25) sequenced BC sample tissues from the TCGA database, they discovered that the expression of lncRNA-CDC6 was significantly upregulated and the expression level was positively linked with the BC stage. The proliferation and metastasis of MB-231 and MCF-7 cells were inhibited by the suppression of lncRNA-CDC6, which was connected to the arrest of BC cells in the G1 phase (25). At the same time, overexpression of lncRNA-CDC6 was demonstrated to promote the proliferation and metastasis of BC cells (25). Further experiments by the authors revealed that lncRNA-CDC6 may bind to micro(miR)-215, which would then promote the proliferation and metastasis of MCDA-MB-231 and MCF-7 cells (25) (Fig. 2). A study has also shown an association between low expression of the miRNA-215, which is related to BC and a poor clinical outcome in BC patients (31).
Table I.
The oncogenic roles of lncRNAs in the top five types of cancer.
| First author, year | Type of cancer | lncRNA | Species | Expression | Target Spot | Effect | (Refs.) |
|---|---|---|---|---|---|---|---|
| Kong et al, 2019 | Breast cancer | CDC6 | Human MCF-10A, MCF-10AT, MCF-10CA1A, MCF-10CA1H cell lines | ↑ | miR-215/CDC6 | Proliferation and metastasis of BC cells | (25) |
| Zong et al, 2021 | SNHG1 | Human BC cell line MCF-7 cells and mouse macrophage cell line RAW264.7 cells | ↑ | STAT6 protein | Polarization of M2 macrophages in BC | (29) | |
| Zhang et al, 2021 | Tissue samples from BC patients, BC cell lines MCF-7 and MDA-MB-231 and normal epithelial cell line MCF-10A, mice | ↑ | miR-381/EZH2 | DDP resistance in BC cells | (97) | ||
| Yue et al, 2021 | MALAT1 | Human BC cell lines (MCF-7, SK-BR3, MDA-MB-468, MDA-MB-231, T-47d and MDA-MB-453) and normal mammary epithelial cell lines (MCF-10A) | ↑ | miR-570-3p | Proliferation and migration of BC cells | (30) | |
| Li et al, 2019 | LOXL1-AS1 | Human BC cell line (MCF-7, MDA-MB-231 and BT549 SKBR-3) and Human breast epithelial normal cells (HBL-100) | ↑ | miR-143-3p | Proliferation, migration and invasion | (35) | |
| Du et al, 2020 | DLX6-AS1 | Human breast fibroblast CCD-1095Sk and human triple negative BC cell lines HCC1599, MDA-MB-231, HCC1806 and HS578 T | ↑ | miR-199b-5p/Paxillin | Proliferation, EMT and DDP resistance in BC cells | (98) | |
| Liu et al, 2020 | CYTOR | Human BC cell line MCF7 | ↑ | miR-125a-5p/SRF | Tamoxifen resistance in BC cells | (99) | |
| Shi et al, 2020 | DILA1 | Human MCF-7, T47D BC cell lines and 293T cell lines | ↑ | Thr286/Cyclin D1 | Tamoxifen resistance in BC cells and proliferation | (100) | |
| Yu et al, 2021 | OIP5-AS1 | Human BC cell lines SKBR3 and BT474 | ↑ | miR-381-3p/HMGB3 | Trastuzumab resistance in BC cells | (101) | |
| Jen et al, 2017 | Lung cancer | MALAT1 | Human lung adenocarcinoma cell line A549 and CL1-0 and normal bronchial epithelial cell line BEAS-2B | ↑ | 4-Oct | Proliferation migration | (39) |
| Hua et al, 2019 | LINC01123 | NSCLC tissue and its adjacent normal lung tissue | ↑ | miR-199a-5p | Proliferation, glycolysis | (42) | |
| Wang et al, 2019 | LINC00336 | Lung cancer cell line, clinical samples of patients with lung adenocarcinoma | ↑ | ELAV1 | Inhibition of ferroptosis and proliferation | (45) | |
| Chen et al, 2020 | LINC00173.v1 | Endothelial cells and lung squamous cell carcinoma cells | ↑ | miR-511-5p/VEGFA | Proliferation and migration of vascular endothelial cells and tumorigenesis of SQC cells | (102) | |
| Zeng et al, 2020 | Linc00173 | Human SCLC cell lines NCI-H69, NCI-H446 and the drug resistant H69AR purchased from the American Type Culture Collection (ATCC, USA), cisplatin-resistant NCIH446DDP cell line. | ↑ | miR-218/Etk axis | DDP resistance in SCLC cell | (103) | |
| Hua et al, 2021 | XIST | Human normal lung epithelial cell lines BEAS-2B and three lung cancer cell lines, A549, H460 and SKMES-1 | ↑ | miR-101-3p | Glycolysis and swelling | (104) | |
| Xu et al, 2020 | Human A549 and H1299 cells | ↑ | SMAD2 | DDP resistance | (105) | ||
| Zhang et al, 2020 | Colorectal cancer | H19 | Human CRC cells HCT116, SW480 and DLD1/female BALB/c nude mice | ↑ | H19/hnRNPA2B1/EMT axis | EMT and CRC metastasis | (48) |
| Ding et al, 2018 | Human HT29, HCT116, SW480 and SW620 cells and normal colorectal mucosa cell line (NCM460) | ↑ | H19/miR-29b-3p/PGRN/Wnt signal | EMT | (51) | ||
| Zhang et al, 2018 | NEAT1 | Human HCT116 and SW1116 cells/male BALB/c nude mice | ↑ | NEAT1/DDX5/Wnt/β-catenin axis | CRC cell proliferation, migration and invasion | (52) | |
| Zhuang et al, 2020 | Colon cancer cell lines (SW620 HT-29, HCT 116, LoVo and SW480) and normal colon epithelial cells (NCM460) | ↑ | NEAT1/miR-185-5p/IGF2 axis | migration and invasion | (106) | ||
| Wang et al, 2020 | Patient samples, SW480 and HCT116 cells, normal colorectal epithelial cells (NCM460) and male BALB/c-nude mice. | ↑ | lnc-NEAT1/miR-150-5p/CPSF4 axis | 5-FU resistance, inhibition of CRC cell apoptosis and promoting CRC cell invasion. | (107) | ||
| Zhu et al, 2020 | Human CRC tissue, human HCT116 and SW480 cells | ↑ | NEAT1/ALDH1/c-Myc | 5-Fu resistance | (108) | ||
| Bian et al, 2016 | UCA1 | Patients with CRC, cell lines (HEK-293T, HCT8, HCT116, HT29, LoVo and SW480) | ↑ | UCA1/miR-204-5p/CREB1 | Proliferation, inhibition of apoptosis of CRC cells and 5-FU resistance of CRC | (109) | |
| Chen et al, 2020 | CCAT2 | Patient sample CRC | ↑ | CCAT2/BOP1 axis | 5-FU and OxPt resistance | (110) | |
| Ding et al, 2017 | CRNDE | Human CRC cell lines (DLD1, HCT116, SW480 and LOVO cells) and the human colonic epithelial cells HCoEpiC | ↑ | CRNDE/EZH2/DUSP5/CDKN1A | Proliferation and CRC metastasis | (111) | |
| Li et al, 2017 | HOTAIR | Human CRC cell lines (HT29, SW480 and FHC cells) | ↑ | Axis of HOTAIR/miR-218/NF-κB or HOTAIR/ | 5-FU resistance | (112) | |
| Shang et al, 2019 | Prostate cancer | LINPCAT1 | Surgical specimen of human prostate tissue and nude mice | ↑ | PCAT1\FKBP51 | Cancer cell proliferation | (28) |
| He et al, 2014 | LINPCGEM1 | Mice, human non-cancerous RWPE-1 cells, HEK293T cells and LNCaP cells | ↑ | miR-145 | Cancer cell proliferation and colony formation | (55) | |
| Lang et al, 2021 | N6-m6PCAT6 | Human prostate tissue, nude mice and mice | ↑ | PCAT6/IGF2BP2/IGF1R axis | Cancer cell invasion, migration, proliferation and tumor growth | (59) | |
| Wen et al, 2020 | N6- m6LncNEAT1 | Human prostate cancer specimens, patient samples, mice, nude mice | ↑ | CYCLINL1/CDK19/NEAT-1 | Carcinogenesis in metastatic bone prostate cancer | (61) | |
| Hu et al, 2021 | NORAD | Human PCa cell lines (22Rv1, DU145 and PC-3) and mice | ↑ | NORAD/miR- 541-3p/PKM2 | Proliferation and migration of prostate cancer cells | (65) | |
| Mo et al, 2021 | NEAT1 | Human prostate epithelial cell line RWPE-1, human low metastatic MDA-PCa-2PCA cell line, human PCA bone metastasis related cell line, mouse | ↑ | SFPQ/PTBP2 | Participate in the osteogenic differentiation of human bone marrow mesenchymal stem cells | (66) | |
| Zhang et al, 2020 | DSCAM-AS1 | PCa cell line 22Rv1, nude mice | ↑ | FOXA1and ERα | Pedigree-specific carcinogenesis regulated by FOXA1 | (57) | |
| Zhang et al, 2021 | PCBP1-AS1 | CRPC patients, LNCaP and C4-2 cells and mice | ↑ | AR\AR-V7 | Proliferation, migration and cancer growth | (113) | |
| Xie et al, 2021 | Gastric cancer | lncRNA SNHG3 | Human GES-1 Gastric Epithelial Cells, MGC-803, AGS, BGC-823 and HGC-27 Cell Lines and BALB/c Nude Mice GC Cell Lines | ↑ | miR-139-5p/MYB axis | Upregulation of MYB drives GC proliferation, migration and invasion | (67) |
| Ma et al, 2021 | Linc01503 | Human MKN-74, NCI-N87 and SGC-7901GC cell lines | ↑ | EGR1 protein | GC cell proliferation and metastasis | (72) | |
| Xie et al, 2020 | lncRNA ANCR | Patient sample human GC cell and TAMs, nude mouse | ↑ | FOXO1 protein | GC cell metastasis and invasion | (73) | |
| Jiang et al, 2022 | HNF1A-AS1 | Human GC cell lines HGC-27, MKN-45, AGS, NCI-N87, human embryonic kidney (HEK) 293T cells, immortalized gastric mucosal epithelial cell line GES-1 and BALB/c mouse GC cell lines | ↑ | miR-30b-5p/EIF5A2 axis | EMT, enhanced 5-FU resistance of GC cells | (114) | |
| Xin et al, 2021 | lncRNA CRNDE | Human GC cell line SGC7901 and mouse GC cell line MFC | ↑ | PI3K/AKT pathway | PI3K/Akt pathway, reduced the sensitivity of GC cells to DDP | (115) | |
| Zhu et al, 2022 | LINC00942 | Human GC cell line, SGC7901, BGC823, human 293T cell line, SGC7901, BGC823 (SGC-R and BGC-R) and nude mice GC cell line | ↑ | MSI2 protein | Enhance the stability of c-Myc mRNA expression, promote GC chemoresistance | (116) | |
| Fu et al, 2021 | ASB16-AS1 | Human GC cell lines (AGS, MKN-45, HGC-27 and MKN-7) | ↑ | miR-3918, miR-4676-3p | NF-κB pathway, enhance the stemness and cisplatin resistance of GC cells | (117) |
lncRNA, long non-coding RNA; BC, breast cancer; miR, microRNA; DDP, cisplatin; EMT, epithelial-mesenchymal transition; LC, lung cancer; NSCLC, non-small cell LC; SCLC, small cell LC; CRC, colorectal cancer; 5-FU, fluorouracil; OxPt, oxaliplatin; PCa, prostate cancer GC, gastric cancer; TAMs, tumor-associated macrophages.
Figure 2.
The mechanism of lncRNAs in BC. lncRNAs, such as MALAT1, lncRNA-SNHG1, lncRNA-CDC6 and LOXL1-AS1 could enhance the proliferation and metastasis of BC cells, whereas lncRNAs, including lncRNA XIST and lncRNA-TSLNC8 could inhibit the development of BC via the downregulation of miR-155 and miR-214-3p, respectively. lncRNA, long non-coding RNA; BC, breast cancer; miR, microRNA.
Up to 50% of tumor-associated macrophages (TAMs) exist in BC. TAMs can differentiate into M1 and M2 cells (32). Studies have found that M2 cells are the main cells in BC tissues and are implicated in BC progression (33,34). The STAT signaling pathway is an important pathway to drive M2 macrophage polarization (29). According to Zong et al (29) the expression level of small nucleolar RNA host gene 1 (SNHG1) was related to the polarization of M2 macrophages. By enhancing STAT6 phosphorylation in the STAT pathway, lncRNA-SNHG1 may facilitate M2 macrophage polarization, ultimately promoting metastasis in BC cells (29) (Fig. 2).
The lncRNA Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) plays a crucial role in regulating tumor progression across various cancers. In BC, MALAT1 interacts with miR-570-3p, resulting in the promotion of BC cell proliferation and metastasis (30). This interaction underscores the significant effect of MALAT1 on BC progression (30). According to Yue et al (30), the expression level of MALAT1 in BC tissues and cell lines (MCF-7, SK-BR-3, MDA-MB-468, MDA-MB-231, T-47d and MDA-MB-453) were higher compared with that in normal tissues. Bioinformatics analysis and luciferase reporter gene experiments revealed that MALAT1 binds to miR-570-3p, reducing its expression. This interaction promotes BC proliferation and metastasis (30) (Fig. 2). However, the specific targets of miR-570-3p downstream of this regulation remain poorly understood, warranting further investigation.
The lncRNA LOXL1-AS1 is highly expressed in BC cell lines (MCF-7, MDAMB-231, BT549 and SKBR-3) compared with normal a breast tissue cell line (HBL100) (35). Silencing LOXL1-AS1 expression leads to the inhibition of BC cell proliferation, migration and invasion (35). miR-143-3p, a known tumor suppressor in various types of cancer such as triple-negative breast cancer (36), plays a role here. Decreasing the expression of miR-143-3p counteracts the inhibitory effect of LOXL1-AS1 silencing on BC cell proliferation (35). Low LOXL1-AS1 expression promotes BC tumor suppression by upregulating the expression of directly bound miR-143-3p (35) (Fig. 2). This suggests a crucial regulatory pathway involving LOXL1-AS1 and miR-143-3p in BC that warrants further investigation (35).
LC
In recent years, it has been found that a number of lncRNAs are involved in the proliferation and metastasis of LC (Table I). MALAT1 is a lncRNA initially noted by Ji et al (26) in 2003 through subtractive hybridization during their investigation of human NSCLC metastasis. This particular lncRNA has since gained significant attention due to its association with LC and has become one of the most extensively researched lncRNAs in the field (37). Octamer-binding transcription factor 4 (Oct4), a product of the Pou5f1 gene and part of the POU-domain transcription factor family, plays a crucial role in preserving the pluripotent state of both embryonic stem cells and cancer stem cells (38). The expression of Oct4 in cancer cells can indicate a dedifferentiated or stem cell-like state, which may contribute to the aggressiveness and metastatic potential of certain tumors, including LC. Jen et al (39) performed unbiased ChIP-seq sequencing in A549 and CL1-0 cells and the overexpression of Oct4 showed that this transcription factor could activate the transcription of MALAT1 by directly binding to the MALAT1 enhancer region (Fig. 3). In LC, MALAT1 does not alter alternative splicing but actively modulates gene expression including a set of metastasis-associated genes (40). According to Gutschner et al (40) suppressing MALAT1 in human lung tumor cells by genomically integrating RNA destabilizing elements using zinc finger nucleases led to a reduction in cell migration ability and a decreased formation of tumor nodules in a mouse xenograft model.
Figure 3.
The mechanism of lncRNAs in LC. lncRNAs, such as MALAT1, LINC00336 and LINC01123 could act as oncogenes and promote the proliferation and invasion of LC cells, whereas lncRNAs, including HAND2-AS1, LINC00472, WT1-AS and BRAT54 could serve as suppressors during the development of LC. lncRNA, long non-coding RNA; LC, lung cancer; miR, microRNA.
A study found that LC cells maintain growth by increasing glucose uptake and lactic acid release, a unique metabolic phenotype of LC cells (41). Hua et al (42) identified 364 differentially expressed genes between NSCLC tissues with high uptake of 18F-FDG and adjacent normal lung tissues by RNA sequencing, among which LINC01123 was the most significant lncRNA. Functional analysis showed that LINC01123 enhanced the proliferation and aerobic glycolysis of NSCLC cells (42). RNA immunoprecipitation and luciferase analysis confirmed that LINC01123 promoted the transcription of c-myc by competitively binding to miR-199a-5p and isolating it from c-myc mRNA, thus promoting the glycolysis and proliferation of LC cells (42) (Fig. 3).
Ferroptosis is a recently discovered cell death mode different from apoptosis (43). A study has shown that LC cells fight against ferroptosis by regulating lipid metabolism through lymphoid-specific helicase (LSH) (44). Wang et al (45) found that lncRNA LINC00336 was abnormally upregulated by sequencing the RNA of LC cells and examining clinical samples of numerous patients with lung adenocarcinoma. LSH can enhance the expression of the RNA binding protein known as Elav-like RNA binding protein 1 (ELAVL1) by suppressing the activity of p53 in relation to iron-mediated cell death (45). At the same time, ELAVL1 can promote its expression by stabilizing the transcription level of LINC00336 (45). Overexpressing LINC00336 as a competitive endogenous RNA adsorbed miR 6852 and reduced its activity, thus upregulating the expression of cystathionine-β-synthase (CBS), the target gene of miR6852 (45) (Fig. 3). CBS serves as an indicator of the sulfur pathway in ferroptosis and an increase in its expression suggests the inhibition of ferroptosis in LC cells (45-47).
CRC
A number of lncRNAs have been implicated in the proliferation, metastasis, invasion and epithelial-mesenchymal transition (EMT) of CRC cells (Table I) (27,48). H19 is an early-discovered lncRNA initially identified as a carcinoembryonic transcript. It is located on chromosome 11p15.5 and is exclusively expressed from the maternal allele (27). Zhang et al (48) found that the overexpression of H19 was related to distant metastasis of CRC and poor prognosis of patients by database and reverse transcription-quantitative (RT-q) PCR sequencing analysis. Heterogeneous nuclear ribonucleoprotein (hnRNPA2B1) is a protein reportedly associated with CRC metastasis and EMT (49). RNA pull-down analysis found that hnRNPA2B1 could combine with H19 in CRC cancer cells HCT116 (48). Immunoprecipitation reaction further found that H19 can specifically bind to hnRNPA2B1 protein and translocate H19 from the nucleus to the cytoplasm, stabilize Raf-1 mRNA, activate the Raf-ERK signal pathway and promote the proliferation, metastasis and EMT of CRC cancer cells (48). Granular precursor protein (PGRN) is a protein that promotes the occurrence and proliferation of epithelial ovarian cancer cells (50). Ding et al (51) found that H19 can promote the expression of PGRN by downregulating miR-29b-3p which ultimately promote the occurrence and increase of EMT through the Wnt signal pathway in CRC (Fig. 4).
Figure 4.
The mechanism of lncRNAs in colorectal cancer. lncRNAs, including NEAT1 and H19 could act as oncogenes and enhance the proliferation, metastasis, invasion and EMT of CRC cells. By contrast, SATB2-AS1, NBR2 and GAS5 can serve as suppressors via the modulation of SATB2, miRNA-21 and YAP, respectively, subsequently inhibiting the proliferation, metastasis, invasion and EMT of CRC cells. lncRNA, long non-coding RNA; EMT, epithelial-mesenchymal transition; CRC, colorectal cancer; miR, microRNA.
Additionally, a study by Zhang et al (52) found that abnormal expression of lncRNA NEAT1 was related to CRC development. They discovered that NEAT1 activated the Wnt/β-catenin signal pathway, stabilizing the expression of the DEAD box p68 RNA helicase (DDX5) through targeted binding, acetylation and thiolation of DDX5 (52) (Fig. 4).
PCa
Some lncRNAs have been shown to promote the proliferation, invasion and migration of PCa cells (Table I). A study by Shang et al (28) revealed a high expression of LINPCAT1 in tissue samples of castrated refractory PCa (CRPC) patients. Subsequent animal experiments in nude mice with LINPCAT1 knockout showed that LINPCAT1 activated AKT and NF-κB pathways by promoting phosphorylation levels of AKT and NF-κB (p65) (28). FK506 binding protein 51 (FKBP51) is a molecular chaperone that interacts with the nuclear factor IκB kinase α subunit (IKKα) and participates in NF-κB activation (53). In addition, FKBP51 acts as a scaffold protein for AKT interaction with a domain-rich leucine repeat protein phosphatase (PHLPP) (54). The enhanced PHLPP activity combined with FKBP51 can play a negative role in AKT signal transduction by binding to AKT (54). Low expression of FKBP51 can reduce the activity of PHLPP and phosphorylate AKT (S437) to activate its pathway (28). Highly expressed LINPCAT1 can competitively bind to FKBP51 and replace the position of PHLPP, ultimately inhibiting the negative regulation of PHLPP on AKT signaling (28). Moreover, the binding of PCAT1 to FKBP51 further stabilizes the FKBP51/IKKα complex, resulting in increased NF-κB signaling and ultimately promoting the growth of CRPC (28) (Fig. 5). LINPCAT1 may be a viable target for treating CRPC, as evidenced by the preclinical mouse model of decreasing CRPC development after targeting PCAT1 (28).
Figure 5.
Mechanism of lncRNAs in PCa. Increase expression of lncRNAs, such as DSCAM-AS1, LINPCAT1, LINPCGEM1, PCAT6 and NEAT1 could promote the proliferation, invasion and migration of PCa cells, whereas overexpression of MEG3 could inhibit the progression of PCa via the downregulation of EN2. lncRNA, long non-coding RNA; PCa, prostate cancer; miR, microRNA.
Research based on the interplay between lncRNA LINPCGEM1 and miR-145 (55) has shown that the mutual suppression of LINPCGEM1 and miR-145 has a role in regulating the proliferation of LNCaP cells. Elevated levels of miR-145 have the potential to hinder the in vitro proliferation and invasion of PCa cells by directly binding to a specific sequence within the 3' untranslated region (UTR) of LINPCGEM1, leading to a reduction in LINPCGEM1 expression (55). Thus, high expression of LINPCGEM1 can downregulate the expression of miR-145 to promote the growth and proliferation of PCa cells (55) (Fig. 5).
The positive feedback loop formed by lncRNAs and other protein expression levels can promote PCa progression. Forkhead box (FOX) A1 is a key transcription factor in the occurrence and development of PCa, driving the malignant transformation of normal prostate epithelial cells (56). Zhang et al (57) found that FOXA1 expression was upregulated through public CHIP-seq experiments and LINDSCAM-AS1 regulated by FOXA1 was explicitly expressed in PCa. DSCAM-AS1 can bind to Y box binding protein 1 (YBX1), a DNA- and RNA-binding protein, and enhance its recruitment in the FOXA1 and estrogen receptor (ER) α initiation region (57). This may result in the formation of PCa and increase the transcription of FOXA1 and ER (57). FOXA1 and ER overexpression can increase the upregulation of LINDSCAM-AS1, generating a positive feedback loop (57) (Fig. 5). Finally, xenograft experiments also revealed that the knockout of DSCAM-AS1 can inhibit the formation of PCa (57).
Bone metastasis is one of the leading causes of death in PCa patients (58) and lncRNA can promote bone metastasis in PCa. Lang et al (59) discovered that N6-methyladenosine (m6A) modified PCa-related transcript 6 (lncRNAPCAT6), which was considerably enhanced in PCa tissues, by sequencing PCa tissue samples from the TCGA database. Functional analysis experiments verified that PCAT6 could promote PCa growth and bone metastasis by regulating the cell cycle (59). Type I insulin-like growth factor receptor (IGF1R) is expressed in prostate cells and promotes bone metastasis of PCa (60). PCAT6 experiences an increase in expression following m6A modification mediated by methyltransferase-like 3 (METTL3). This modification process leads to the regulation of IGF1R mRNA stability through the formation of PCAT6/IGF2BP2/IGF1R complex. As a result, it enhances the stability of IGF1R mRNA, elevates the levels of IGF1R and ultimately stimulates the invasion and migration of PCa cells (Fig. 5). These effects are brought about through the activation of the IGF/IGF1R signaling pathway, contributing to the promotion of PCa bone metastasis (60).
Bone-specific expression protein (CYCLINL1) was found to be expressed in cultured primary prostate cells and patient-derived xenografts of patients with bone metastasis (61). In the cancer genome map data set, NEAT1-1 expression was higher in PCa than in normal tissues (61). The m6A-modified nuclear-rich transcript 1 (lncNEAT1) (61) acts as a binding bridge between CYCLINL1 and prostate-specific expression protein (CDK19), activating CYCLINL1 to form the CYCLINL1/CDK19 complex that is recruited to the RUNX2 promoter, a key protein for bone and prostate metastasis (62-64). The formed LINNEAT1/CYCLINL1/CDK19 complex enhances PolII Ser2 phosphorylation and finally promotes bone metastasis of PCa (61) (Fig. 5). Related lncRNA may also promote PCa cell bone metastasis by binding mRNA to release extracellular vesicles (65) and affect the microenvironment of bone metastasis (66).
GC
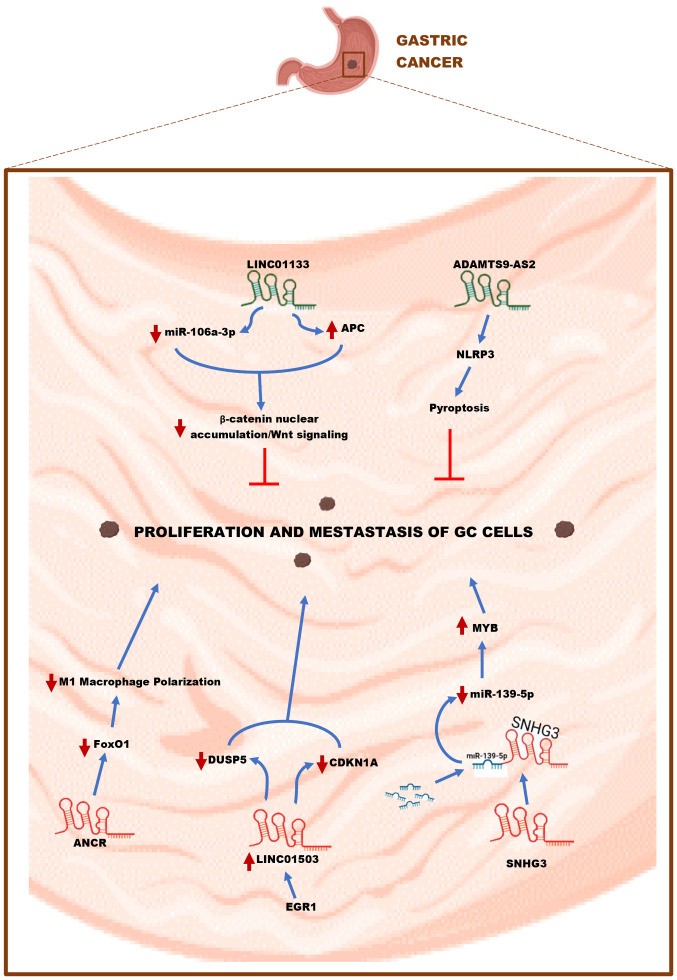
In GC, numerous lncRNAs have been associated with the growth and advancement of cancer cells (Table I). Xie et al (67) found that the expression of lncRNA SNHG3 was upregulated in BGC-823 and AGS cell lines transfected with pcDNA3.1-SNHG3 plasmid by gene sequencing. SNHG3 overexpression can increase the proliferation ability of GC cells and enhance the migration and invasion activity by reducing the G0/G1 phase and increasing the relative frequency of S phase and G2 phase cells (67). miR-139-5p has been shown to inhibit the progression of bladder cancer (68), GC (69) and CRC (70). MYB, a target gene of miR-139-5p, is connected with GC cell proliferation and MYB expression is inversely correlated with miR-139-5p expression (67). High SNHG3 expression can reduce miR-139-5p to promote MYB expression, which in turn drives GC cell proliferation, migration and invasion (67) (Fig. 6).
Figure 6.
Mechanism of lncRNAs in GC. lncRNAs, including ANCR, LINC01503 and SNHG3 could act as oncogenes and enhance the proliferation and metastasis of GC cells. In contrast, LINC01133 and ADAMTS9-AS2 could serve as suppressors by inhibiting the proliferation and metastasis of GC cells. GC gastric cancer; miR, microRNA.
LINC01503 is a new lncRNA located on human chromosome 19, which was first discovered as an oncogene in invasive squamous cell carcinoma (SCC) (71). Ma et al (72) studied whether overexpression of LINC01503 is related to GC progression. The public data analysis results of GEO and TCGA datasets showed that LINC01503 was overexpressed in GC patient tissue samples and gene sequencing showed that the expression level of this gene was upregulated in GC cells (72). Downregulation of LINC01503 expression increases the number of GC cells in G0/G1, which is connected to cell cycle arrest in G0/G1 (72). Early growth response protein 1 (EGR1) is an upstream target effector of LINC01503 (72). The binding of EGR1 to LINC01503 can activate the transcription of LINC01503 (72). The upregulation of LINC01503 mediated by EGR1 promotes the methylation of histone inhibitory chromatin marker (H3K27) and the demethylation of histone 3 tetra-amino acid in GC cells (72). It also inhibits the transcription of dual specific phosphatase 5 and cyclin 1 dependent kinase inhibitor 1A, subsequently downregulating their expressions in an epigenetic manner, promoting the transition of GC cells to the G0/G1 phase and ultimately enhancing the proliferation and metastasis of GC cells (72) (Fig. 6). Xie et al (73) found that lncRNA anti-differentiation RNA (ANCR) is highly expressed in GC tissues. It has been reported that recombinant Forkhead box O1 protein (FOXO1) can promote macrophages to produce inflammatory factors and polarization to M1 macrophages, thereby playing an anti-tumor role (74-76). Overexpression of the lncRNA ANCR can decrease the level of FOXO1 protein, inhibit the polarization of macrophages to M1, and reduce the production of inflammatory factors by M1 macrophages, thereby indirectly promoting the metastasis and invasion of GC cells (73) (Fig. 6). The molecular mechanisms underlying the involvement of numerous lncRNAs in the growth of GC cells are still in progress.
4. lncRNAs as tumor suppressors in the top five types of cancer
BC
lncRNAs are also capable of suppressing the proliferation, migration and invasion of BC cells (Table II). lncRNA XIST is a transcript of chromosome Xq13.2 located in the X inactivation center, affecting the reactivation of X-linked genes (77). XIST is underexpressed in BC tissue samples and cells (78). RT-qPCR results of MCF-7 and MDA-MB-231 cells transfected with pc-XIST showed that overexpression of XIST could inhibit cell proliferation, migration and invasion (78). miR-155 is a potential BC oncogene (79). The overexpression of XIST can directly bind to miR-155 and inhibit its expression, subsequently upregulating the miR-155 downstream target CDX1 and inhibiting the proliferation, migration and invasion of BC cells (78) (Fig. 2).
Table II.
Suppressive roles of lncRNAs in the top five types of cancer.
| First author, year | Cancer | lncRNA | Species | Expression | Target Spot | Effect | (Refs.) |
|---|---|---|---|---|---|---|---|
| Qin et al, 2019 | Breast cancer | TSLNC8 | BC cell lines (MDA-MB-231, HCC1559, BT-549, UACC-812 and MDA-MB-453) and Human BC tissues | ↓ | miR-214-3p | Inhibits G1/S phase progression | (80) |
| Zheng et al, 2018 | XIST | Human BC cells (MCF-7, ZR-75-1, HCC1937, MDA-MB-231, MDA-MB-468, MDA-MB-453) and normal breast epithelial cell line MCF-10 A | ↓ | miR-155/CDX | Inhibits proliferation, migration and invasion | (78) | |
| Yang et al, 2021 | Lung cancer | LINBRCAT54 | Plasma samples from NSCLC patients | ↓ | RPS9 | Inhibits proliferation, invasion and promote apoptosis | (81) |
| Wan et al, 2021 | WT1-AS | Tissue samples of NSCLC patients | ↓ | UCA1 | Reduces proliferation and migration | (82) | |
| Deng et al, 2020 | LINC00472 | Lung adenocarcinoma A549 and PC-9 cell lines, Human normal lung epithelial cells (BEAS-2B) | ↓ | YBX1 | Inhibits cancer cell migration and invasion | (83) | |
| Ni et al, 2019 | Colorectal cancer | GAS5 | Human DLD1, LOVO, SW480, SW620, LS174T, HCT116, RKO and HT29 cells | ↓ | The lncRNA GAS5- YAP-YTHDF3 axis | Inhibits proliferation and invasion | (85) |
| Xu et al, 2019 | SATB2-AS1 | Human CRC cell lines HCT-116, HT-29, SW-620, HCT-8, SW-480 and DLD-1 | ↓ | SATB2-AS1/WDR5/GADD45A/SATB2 axis | Inhibits metastasis | (87) | |
| Bai et al, 2020 | NBR2 | Human CRC tissue, human CRC cell line RKO, HT115 | ↓ | NBR2/miRNA-21 axis | Reduces migration and invasion | (88) | |
| Zhou et al, 2018 | HAND2-AS1 | patient sample, CRC cell line, mouse | ↓ | HAND2-AS1/miR-1275/KLF14 axis | Reduces 5-FU resistance, cell proliferation, migration and invasion | (118) | |
| Zhou et al, 2020 | Prostate cancer | LncMEG3 | Human prostate cells, nude mice | ↓ | EZH2 | Reduces cancer cell vitality, migration and invasion | (91) |
| Ghildiyal et al, 2022 | NXTAR | Prostate, mouse, normal human prostate cell line | ↓ | AR\AR-V7 | Inhibits proliferation of cancer cells | (119) | |
| Ren et al, 2020 | Gastric cancer | lncRNA ADAMTS9- AS2 | GES-1, DDP-sensitive GC (CS-GC) cell lines (SGC7901, MKN74, NUGC-4, HGC-27 and BGC-823) and DDP-resistant GC (CR-GC) cell lines (SGC7901/DDP and BGC-823/DDP) | ↓ | miR-223-3p/NLRP3 axis | Reduces pyroptosis and sensitivity of cancer cells to cisplatin | (95) |
| Yang et al, 2018 | LINC01133 | Human GC cell lines (SUN-216, BGC-823, AGS, BGC-803, NUGC4, MKN74, MKN45, SGC-7901 and HGC-27), nonmalignant gastric mucosal epithelial cell line GES-1 and human embryonic kidney (HEK) 293FT cell lines | ↓ | miR-106a-3p/APC axis | Inhibits Wnt/β-Catenin pathway, EMT and GC cell metastasis | (96) |
lncRNA, long non-coding RNA; BC, breast cancer; miR, microRNA; LC, lung cancer; NSCLC, non-small cell LC; DDP; cisplatin; EMT, epithelial-mesenchymal transition; CRC, colorectal cancer; 5-FU, fluorouracil; GC, gastric cancer.
In addition, the expression of lncRNA TSLNC8 is downregulated in breast cancer tissues and cells (80). Upregulated TSLNC8 can block the proliferation of BC cells, which is related to its inhibition of cell G1/S phase progression (80). TSLNC8 interacts with miR-214 and overexpression of TSLNC8 inhibits downstream miR-214-3p expression, upregulating Forkhead box P2 (FOXP2) expression and ultimately inhibiting BC cell proliferation (80) (Fig. 2).
LC
A number of studies have shown that lncRNAs, such as LINBRAT54 (81), WT1-AS (82) and LINC00472 (83) can also inhibit the growth of LC cells and tissues (Table II). After a comprehensive microarray investigation of lncRNAs in preoperative and postoperative plasma samples of NSCLC patients, Yang et al (81) identified LINBRAT54 as a tumor suppressor that can reduce NSCLC proliferation in vitro and in vivo. A study also revealed that BRAT54 can directly bind to ribosomal protein S9 (RPS9) to activate the Janus tyrosine kinase/activator (JAK/STAT) and calcium signaling pathways to inhibit proliferation and invasion of NSCLC cells, ultimately promoting apoptosis of these cells (81) (Fig. 3). Gao et al (84) detected the expression of lncRNA in lung tissues and serum of NSCLC patients and healthy controls by RT-qPCR. They discovered that the expression level of lncRNA HAND2-AS1 in tumor tissues was lower compared with healthy tissues and that its downregulation influenced LC proliferation. HAND2-AS1 can inhibit the proliferation and promote the apoptosis of NSCLC cells by inactivating the PI3K/Akt pathway, thus inhibiting the growth of tumors (84) (Fig. 3).
Wan et al (82) took the lung tissues of 66 patients with NSCLC for RNA sequencing and found that Wilms tumor 1 antisense RNA (WT1-AS) was downregulated in NSCLC, which was related to the low survival rate of NSCLC patients but was not associated with clinical stage. Overexpression of WT1-AS inhibited the proliferation and migration of NSCLC cells (82). Urinary tract cancer-related 1 (UCA1) is mostly upregulated in LC (82). Correlation analysis found that the expression level of UCA1 negatively correlated with the expression level of WT1-AS in NSCLC and non-cancerous tissues (82). Its overexpression increased the proliferation, EMT, migration and invasion of NSCLC cells and was inhibited by WT1-AS (82). Gene sequencing results of H1993 and H1581 cells transfected with UCA1 and WT1-AS1 showed that the overexpression of UCA1 had little effect on the expression of WT1-AS1; however, the decrease in proliferation and migration of NSCLC cells mediated by overexpression of WT1-AS1 might be achieved by reducing the expression of UCA1 (82) (Fig. 3). Deng et al screened and identified lncRNA LINC00472 by using published expression profile data from lung adenocarcinoma and integrating bioinformatics analysis and proved that this lncRNA could bind with YBX1 to regulate cell hardness and inhibit the migration and invasion of lung adenocarcinoma, thus playing a role as a tumor suppressor (83) (Fig. 3).
CRC
lncRNAs including lncRNA GAS5, SATB2-AS1 and NBR2 have been shown to limit the growth of CRC cells (Table II). Biosphere interference and RNA fluorescence in situ hybridization study demonstrate that lncRNA GAS5 can bind to the WW domain of Yes-related protein (YAP) (85). YAP is the main participant of the Hippo YAP signaling pathway, which can regulate cell proliferation and apoptosis (85).
lncRNA GAS5 interacts with YAP to cause its translocation from the nucleus to the cytoplasm, which in turn results in the phosphorylation and degradation of YAP. This inhibits YAP signal transduction, ultimately preventing the growth of CRC cells (85) (Fig. 4). Special AT-rich binding protein (SATB2) is a nuclear gene protein. As an important transcription factor, it plays a vital role in biological development, gene expression regulation and chromatin remodeling (86). SATB2-AS1 is an antisense RNA located on chromosome 2q33 of SATB2 (87). According to Xu et al (87), silencing lncRNA SATB2-AS1 can significantly reduce the RNA and protein levels of SATB2. By recruiting WD repeat domain 5 (WDR5), a growth arrest and DNA damage indigenous protein, SATB2-AS1 can increase the expression of SATB2 and ultimately prevent CRC cells from metastasizing (87) (Fig. 4). In addition, lncRNA NBR2 has been shown to inhibit CRC metastasis and invasion by downregulating the level of miRNA-21 (88) (Fig. 4). Future treatment targets for CRC are expected to be these lncRNAs and their associated miRNAs (89,90).
PCa
Some lncRNAs have also been shown to slow the course of PCa (91) (Table II). Using RT-qPCR sequencing, Zhou et al (91) discovered that the expression of lncRNA MEG3 was downregulated in PCa cells, but western blotting revealed that the expression of EN railed-2 (EN2) was elevated in PCa cells. EN2 is a well-known carcinogenic gene (92,93). PCa cells transfected with MEG3 demonstrate that overexpression of MEG3 inhibited cancer cell growth, whereas overexpression of EN2 reverses this effect (91). MEG3 in the nucleus can bind to EZH2, causing EN2 to undergo H3K27 trimethylation, thereby inhibiting the proliferation of PCa (91) (Fig. 5).
GC
lncRNA can also prevent GC progression (Table II). Pyroptosis is a type of programmed cell death that is caused by the activation of the NOD-like receptor hot protein domain-associated protein 3 inflammasome (NLRP3) (94). Ren et al (95) discovered that overexpression of the lncRNA ADAMTS9-AS2 activated NLRP3, induced pyroptosis in cisplatin (DDP)-treated cells, increased the cytotoxicity of high-dose DDP on GC cells and ultimately prevented GC cell growth (Fig. 6). By contrast, using pyroptosis inhibitors Z-VAD-FMK and NSA to treat GC cells treated with high-dose DDP can abolish the inhibitory effect of overexpressed lncRNA ADAMTS9-AS2 on GC cell growth and increase GC cell development (95).
In a study by Yang et al (96) the colon adenomatous polyposis gene (APC) was used as the main study object. It was found that overexpression of LINC01133 lowered miR-106a-3p expression in GC samples and eight elevated mRNAs were seen in cells inhibited by AGS-miR-106a-3p. The overexpression of LINC01133 elevated the production of APC mRNA, indicating that the control of miR-106a-3p and APC was primarily responsible for the suppression of GC metastasis by LINC01133 overexpression (96). To determine whether the Wnt signaling pathway is involved in the regulation of GC metastasis by LINC01133/miR-106a-3p, the authors conducted a comprehensive bioinformatics analysis and Gene Ontology bioprocess enrichment analysis and found that 93 mRNAs were potential targets of miR-106a-3p (including the APC gene) (96). These genes are mainly enriched in the Wnt signaling and cell migration pathways and the low expression of LINC01133 affects the Wnt signaling pathway (96). The enhanced transcriptional activity of the gene plasmid TOP/FOP weakens the activity of the Wnt signaling pathway and enhances the EMT and metastasis of GC cells (96). β-catenin is involved in GC cell adhesion through the catenin-cadherin complex and the Wnt signaling pathway (96). It can also reduce the dependence of the LINC01133/miR-106a-3p/APC axis on the Wnt pathway and promote GC cell migration (96). In a nutshell, LINC01133 overexpression can downregulate miR-106a-3p and promote APC gene expression, inhibit β-catenin nuclear accumulation and Wnt signaling pathway in GC cells and ultimately prevent GC cell metastasis (96) (Fig. 6). To verify the reliability and correctness of the aforesaid research, they must be supplemented with several clinical experiments.
5. lncRNAs acting as both oncogenes and tumor suppressors during cancer development
A number of lncRNAs have been shown to display a dual role as both oncogenes and tumor suppressors in different types of cancer. This duality of such lncRNAs makes them essential characters in the complex story of cancer development. Based on literature, the present study identified 10 lncRNAs demonstrating this dual role in cancer research, including GAS5, HAND2-AS1, lncRNA ADAMTS9-AS2, PCBP1-AS1, ASB16-AS1, CRNDE, LINC00173.v1, LINC01133, lncRNA ANCR and MALAT1, which has been condensed in Table III. Due to their contrasting roles in various cancers, as diagnostic markers, they must closely match the type of cancer and propose that they are not suitable as therapeutic targets for cancer.
Table III.
lncRNAs playing a dual role in cancer development.
| lncRNA | Effect | Type of cancer |
|---|---|---|
| GAS5 | Oncogene | Cholangiocarcinoma (120) |
| Suppressor | Colorectal cancer (85), gastric cancer (121), cervical cancer (122), liver cancer (123), ovarian cancer (124) | |
| HAND2-AS1 | Oncogene | Liver cancer (125) |
| Suppressor | Breast cancer (126), colorectal cancer (118,127), gastric adenocarcinoma (128), cervical cancer (129), bladder cancer (130), lung cancer (84) | |
| lncRNA ADAMTS9-AS2 | Oncogene | Squamous cell carcinoma of tongue (131) |
| Suppressor | Gastric cancer (95) | |
| PCBP1-AS1 | Oncogene | Liver cancer (132), cervical cancer (133), prostate cancer (113) |
| Suppressor | Vulvar squamous cell carcinoma (134) | |
| ASB16-AS1 | Oncogene | Glioma (135), gastric cancer (117) |
| Suppressor | Clear cell renal cell carcinoma (136) | |
| CRNDE | Oncogene | Colorectal cancer (110,137), pancreatic cancer (138), cervical cancer (139), ovarian cancer (140), Wilms' tumor (141), hepatocellular carcinoma (142,143), papillary thyroid cancer (144), non-small cell lung cancer (145), prostate cancer (146), osteosarcoma (147), gastric cancer (115,148), breast cancer (149), acute myeloid leukemia (150), medulloblastoma (151), intrahepatic cholangiocarcinoma (152), glioma (153,154), myeloma (155), melanoma (156), gallbladder carcinoma (157) |
| Suppressor | Chronic lymphocytic leukemia (158) | |
| LINC00173.v1 | Oncogene | Lung cancer (102), colorectal cancer (159) |
| Suppressor | Cervical cancer (160) | |
| LINC01133 | Oncogene | Lung cancer (161), cervical cancer (162), epithelial ovarian cancer (163), pancreatic cancer (164) |
| Suppressor | Breast cancer (165), colorectal cancer (166), gastric cancer (96,167) | |
| lncRNA ANCR | Oncogene | Gastric cancer (73), colorectal cancer (168) |
| Suppressor | Breast cancer (169), lung cancer (170) | |
| MALAT1 | Oncogene | Colorectal cancer (171,172), lung cancer (39,173), prostate cancer (174,175), gastric cancer (176,177), breast cancer (30) |
| Suppressor | Breast cancer (178,179), colon cancers (178) |
lncRNA, long non-coding RNA.
6. Conclusion and prospects
Over the last decade, research into the role of lncRNAs in cancer diagnosis and treatment has steadily increased, becoming an emerging focus in cancer diagnosis and treatment. The development and metastasis of tumors are linked to the aberrant expression of lncRNAs. Overall, the oncogenic and suppressive roles of lncRNAs in various cancers were reviewed as displayed in Fig. 1. Based on the two aforementioned roles of lncRNAs in cancers, distinct strategies for treating different types of cancer must be employed based on the lncRNAs involved. The discovery of several lncRNAs, their widespread expression patterns in various types of cancer, their tumor specificity and their stability in circulating body fluids (plasma and urine) provides a new basis for developing cancer diagnostics and treatments. Although the structure and function of lncRNAs need to be further explored, these molecules seem very promising to develop new diagnoses and targeted treatment strategies, which offers new paradigms for cancer research and may become a major treatment strategy for cancer in the near future.
Acknowledgments
Not applicable.
Funding Statement
The present study was supported by The National Natural Science Foundation of China (grant no. 81700055 to RT), the Outstanding Talent Research Funding of Xuzhou Medical University (grant no. D2016021 to RT), the Natural Science Foundation of Jiangsu Province (grant no. BK20160229 to RT) and Jining Medical University (grant no. 600791001 to JY).
Availability of data and materials
Not applicable.
Authors' contributions
RT and JY were responsible for conceptualization, resources and original draft preparation. HS, JA-A and QZ were responsible for original draft preparation, data collection, analysis, reviewing and editing. DY, KL, AS and JZ were responsible for reviewing and editing. All authors read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the global burden of disease study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Jiang MC, Ni JJ, Cui WY, Wang BY, Zhuo W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res. 2019;9:1354–1366. [PMC free article] [PubMed] [Google Scholar]
- 4.Ivanov KI, Samuilova OV, Zamyatnin AA., Jr The emerging roles of long noncoding RNAs in lymphatic vascular development and disease. CellCell Mol Life Sci. 2023;80:197. doi: 10.1007/s00018-023-04842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Liu M, Li M, Zhang S, Hiju H, Sun J, Mao Z, Zheng M, Feng B. Epigenetic modulations of noncoding RNA: A novel dimension of cancer biology. Mol Cancer. 2020;19:64. doi: 10.1186/s12943-020-01159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattick JS, Amaral PP, Carninci P, Carpenter S, Chang HY, Chen LL, Chen R, Dean C, Dinger ME, Fitzgerald KA, et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol. 2023;24:430–447. doi: 10.1038/s41580-022-00566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mo Y, Adu-Amankwaah J, Qin W, Gao T, Hou X, Fan M, Liao X, Jia L, Zhao J, Yuan J, Tan R. Unlocking the predictive potential of long non-coding RNAs: A machine learning approach for precise cancer patient prognosis. Ann Med. 2023;55:2279748. doi: 10.1080/07853890.2023.2279748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet. 2010;42:1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandes JCR, Acuña SM, Aoki JI, Floeter-Winter LM, Muxel SM. Long non-coding RNAs in the regulation of gene expression: Physiology and disease. Noncoding RNA. 2019;5:17. doi: 10.3390/ncrna5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z, Jiang S, Shang J, Jiang Y, Dai Y, Xu B, Yu Y, Liang Z, Yang Y. LncRNA: Shedding light on mechanisms and opportunities in fibrosis and aging. Ageing Res Rev. 2019;52:17–31. doi: 10.1016/j.arr.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan YT, Lin JF, Li T, Li JJ, Xu RH, Ju HQ. LncRNA-mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Commun (Lond) 2021;41:109–120. doi: 10.1002/cac2.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Zhou Y, Zhang B, Sheng Z, Sun N, Yuan B, Wu X. Identification of lncRNA, miRNA and mRNA expression profiles and ceRNA Networks in small cell lung cancer. BMC Genomics. 2023;24:217. doi: 10.1186/s12864-023-09306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, Kryczek I, Nam J, Li X, Li S, Li J, Wei S, Grove S, Vatan L, Zhou J, et al. LIMIT is an immunogenic lncRNA in cancer immunity and immunotherapy. Nat Cell Biol. 2021;23:526–537. doi: 10.1038/s41556-021-00672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Wang Z, Zhu L, Sun Y, Castellano L, Stebbing J, Yu Z, Peng L. A pyroptosis-related lncRNA signature in bladder cancer. Cancer Med. 2023;12:6348–6364. doi: 10.1002/cam4.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 18.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, et al., editors. SEER cancer statistics review, 1975-2016. National Cancer Institute; Bethesda, MD: 2019. [Google Scholar]
- 19.Tannenbaum SL, Koru-Sengul T, Zhao W, Miao F, Byrne MM. Survival disparities in non-small cell lung cancer by race, ethnicity, and socioeconomic status. Cancer J. 2014;20:237–245. doi: 10.1097/PPO.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 20.Birring SS, Peake MD. Symptoms and the early diagnosis of lung cancer. Thorax. 2005;60:268–269. doi: 10.1136/thx.2004.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 22.Nevedomskaya E, Baumgart SJ, Haendler B. Recent advances in prostate cancer treatment and drug discovery. Int J Mol Sci. 2018;19:1359. doi: 10.3390/ijms19051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciccarese C, Nobili E, Grilli D, Casolari L, Rihawi K, Gelsomino F, Tortora G, Massari F. The safety and efficacy of enzalutamide in the treatment of advanced prostate cancer. Expert Rev Anticancer Ther. 2016;16:681–696. doi: 10.1080/14737140.2016.1192468. [DOI] [PubMed] [Google Scholar]
- 24.Kastenberg D, Bertiger G, Brogadir S. Bowel preparation quality scales for colonoscopy. World J Gastroenterol. 2018;24:2833–2843. doi: 10.3748/wjg.v24.i26.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong X, Duan Y, Sang Y, Li Y, Zhang H, Liang Y, Liu Y, Zhang N, Yang Q. LncRNA-CDC6 promotes breast cancer progression and function as ceRNA to target CDC6 by sponging microRNA-215. J Cell Physiol. 2019;234:9105–9117. doi: 10.1002/jcp.27587. [DOI] [PubMed] [Google Scholar]
- 26.Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 27.Ghafouri-Fard S, Esmaeili M, Taheri M. H19 lncRNA: Roles in tumorigenesis. Biomed Pharmacother. 2020;123:109774. doi: 10.1016/j.biopha.2019.109774. [DOI] [PubMed] [Google Scholar]
- 28.Shang Z, Yu J, Sun L, Tian J, Zhu S, Zhang B, Dong Q, Jiang N, Flores-Morales A, Chang C, Niu Y. LncRNA PCAT1 activates AKT and NF-κB signaling in castration-resistant prostate cancer by regulating the PHLPP/FKBP51/IKKα complex. Nucleic Acids Res. 2019;47:4211–4225. doi: 10.1093/nar/gkz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zong S, Dai W, Guo X, Wang K. LncRNA-SNHG1 promotes macrophage M2-like polarization and contributes to breast cancer growth and metastasis. Aging (Albany NY) 2021;13:23169–23181. doi: 10.18632/aging.203609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue X, Wu WY, Dong M, Guo M. LncRNA MALAT1 promotes breast cancer progression and doxorubicin resistance via regulating miR-570-3p. Biomed J. 2021;44(6 Suppl 2):S296–S304. doi: 10.1016/j.bj.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou SW, Su BB, Zhou Y, Feng YQ, Guo Y, Wang YX, Qi P, Xu S. Aberrant miR-215 expression is associated with clinical outcome in breast cancer patients. Med Oncol. 2014;31:259. doi: 10.1007/s12032-014-0259-2. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37:14–16. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- 33.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM. TH2-polarized CD4(+) T cells and macrophages limit efficacy of radiotherapy. Cancer Immunol Res. 2015;3:518–525. doi: 10.1158/2326-6066.CIR-14-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li GH, Yu JH, Yang B, Gong FC, Zhang KW. LncRNA LOXL1-AS1 inhibited cell proliferation, migration and invasion as well as induced apoptosis in breast cancer via regulating miR-143-3p. Eur Rev Med Pharmacol Sci. 2019;23:10400–10409. doi: 10.26355/eurrev_201912_19679. [DOI] [PubMed] [Google Scholar]
- 36.Li D, Hu J, Song H, Xu H, Wu C, Zhao B, Xie D, Wu T, Zhao J, Fang L. miR-143-3p targeting LIM domain kinase 1 suppresses the progression of triple-negative breast cancer cells. Am J Transl Res. 2017;9:2276–2285. [PMC free article] [PubMed] [Google Scholar]
- 37.Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: A new paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q, Han Z, Zhu Y, Chen J, Li W. The role and specific mechanism of OCT4 in cancer stem cells: A review. Int J Stem Cells. 2020;13:312–325. doi: 10.15283/ijsc20097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jen J, Tang YA, Lu YH, Lin CC, Lai WW, Wang YC. Oct4 transcriptionally regulates the expression of long non-coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. Mol Cancer. 2017;16:104. doi: 10.1186/s12943-017-0674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gutschner T, Hämmerle M, Eissmann M, Hsu J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–1189. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, et al. Lactate metabolism in human lung tumors. Cell. 2017;171:358–371.e9. doi: 10.1016/j.cell.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hua Q, Jin M, Mi B, Xu F, Li T, Zhao L, Liu J, Huang G. LINC01123, a c-Myc-activated long non-coding RNA, promotes proliferation and aerobic glycolysis of non-small cell lung cancer through miR-199a-5p/c-Myc axis. J Hematol Oncol. 2019;12:91. doi: 10.1186/s13045-019-0773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang Y, Mao C, Yang R, Yan B, Shi Y, Liu X, Lai W, Liu Y, Wang X, Xiao D, et al. EGLN1/c-Myc induced lymphoid-specific helicase inhibits ferroptosis through lipid metabolic gene expression changes. Theranostics. 2017;7:3293–3305. doi: 10.7150/thno.19988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang M, Mao C, Ouyang L, Liu Y, Lai W, Liu N, Shi Y, Chen L, Xiao D, Yu F, et al. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2019;26:2329–2343. doi: 10.1038/s41418-019-0304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayano M, Yang WS, Corn CK, Pagano NC, Stockwell BR. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23:270–278. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Huang W, Yuan Y, Li J, Wu J, Yu J, He Y, Wei Z, Zhang C. Long non-coding RNA H19 promotes colorectal cancer metastasis via binding to hnRNPA2B1. J Exp Clin Cancer Res. 2020;39:141. doi: 10.1186/s13046-020-01619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y, Yang X, Chen Z, Tian L, Jiang G, Chen F, Li J, An P, Lu L, Luo N, et al. m6A-induced lncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1. Mol Cancer. 2019;18:87. doi: 10.1186/s12943-019-1014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong T, Yang D, Li R, Zhang L, Zhao H, Shen Y, Zhang X, Kong B, Wang L. PGRN promotes migration and invasion of epithelial ovarian cancer cells through an epithelial mesenchymal transition program and the activation of cancer associated fibroblasts. Exp Mol Pathol. 2016;100:17–25. doi: 10.1016/j.yexmp.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Ding D, Li C, Zhao T, Li D, Yang L, Zhang B. LncRNA H19/miR-29b-3p/PGRN axis promoted epithelial-mesenchymal transition of colorectal cancer cells by acting on Wnt signaling. Mol Cells. 2018;41:423–435. doi: 10.14348/molcells.2018.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang M, Weng W, Zhang Q, Wu Y, Ni S, Tan C, Xu M, Sun H, Liu C, Wei P, Du X. The lncRNA NEAT1 activates Wnt/β-catenin signaling and promotes colorectal cancer progression via interacting with DDX5. J Hematol Oncol. 2018;11:113. doi: 10.1186/s13045-018-0656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romano S, D'Angelillo A, Staibano S, Ilardi G, Romano MF. FK506-binding protein 51 is a possible novel tumoral marker. Cell Death Dis. 2010;1:e55. doi: 10.1038/cddis.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z, Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He JH, Zhang JZ, Han ZP, Wang L, Lv YB, Li YG. Reciprocal regulation of PCGEM1 and miR-145 promote proliferation of LNCaP prostate cancer cells. J Exp Clin Cancer Res. 2014;33:72. doi: 10.1186/s13046-014-0072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pomerantz MM, Li F, Takeda DY, Lenci R, Chonkar A, Chabot M, Cejas P, Vazquez F, Cook J, Shivdasani RA, et al. The androgen receptor cistrome is extensively reprogrammed in human prostate tumorigenesis. Nat Genet. 2015;47:1346–1351. doi: 10.1038/ng.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Huang YX, Wang DL, Yang B, Yan HY, Lin LH, Li Y, Chen J, Xie LM, Huang YS, et al. LncRNA DSCAM-AS1 interacts with YBX1 to promote cancer progression by forming a positive feedback loop that activates FOXA1 transcription network. Theranostics. 2020;10:10823–10837. doi: 10.7150/thno.47830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lang C, Dai Y, Wu Z, Yang Q, He S, Zhang X, Guo W, Lai Y, Du H, Wang H, et al. SMAD3/SP1 complex-mediated constitutive active loop between lncRNA PCAT7 and TGF-β signaling promotes prostate cancer bone metastasis. Mol Oncol. 2020;14:808–828. doi: 10.1002/1878-0261.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lang C, Yin C, Lin K, Li Y, Yang Q, Wu Z, Du H, Ren D, Dai Y, Peng X. m6A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2-mediated IGF1R mRNA stabilization. Clin Transl Med. 2021;11:e426. doi: 10.1002/ctm2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burfeind P, Chernicky CL, Rininsland F, Ilan J, Ilan J. Antisense RNA to the type I insulin-like growth factor receptor suppresses tumor growth and prevents invasion by rat prostate cancer cells in vivo. Proc Natl Acad Sci USA. 1996;93:7263–7268. doi: 10.1073/pnas.93.14.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen S, Wei Y, Zen C, Xiong W, Niu Y, Zhao Y. Long non-coding RNA NEAT1 promotes bone metastasis of prostate cancer through N6-methyladenosine. Mol Cancer. 2020;19:171. doi: 10.1186/s12943-020-01293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baniwal SK, Khalid O, Gabet Y, Shah RR, Purcell DJ, Mav D, Kohn-Gabet AE, Shi Y, Coetzee GA, Frenkel B. Runx2 transcriptome of prostate cancer cells: Insights into invasiveness and bone metastasis. Mol Cancer. 2010;9:258. doi: 10.1186/1476-4598-9-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akech J, Wixted JJ, Bedard K, van der Deen M, Hussain S, Guise TA, van Wijnen AJ, Stein JL, Languino LR, Altieri DC, et al. Runx2 association with progression of prostate cancer in patients: Mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene. 2010;29:811–821. doi: 10.1038/onc.2009.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao JC, Yu J, Runkle C, Wu L, Hu M, Wu D, Liu JS, Wang Q, Qin ZS, Yu J. Cooperation between Polycomb and androgen receptor during oncogenic transformation. Genome Res. 2012;22:322–331. doi: 10.1101/gr.131508.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu CY, Chen J, Qin XH, You P, Ma J, Zhang J, Zhang H, Xu JD. Long non-coding RNA NORAD promotes the prostate cancer cell extracellular vesicle release via microRNA-541-3p-regulated PKM2 to induce bone metastasis of prostate cancer. J Exp Clin Cancer Res. 2021;40:98. doi: 10.1186/s13046-021-01891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mo C, Huang B, Zhuang J, Jiang S, Guo S, Mao X. LncRNA nuclear-enriched abundant transcript 1 shuttled by prostate cancer cells-secreted exosomes initiates osteoblastic phenotypes in the bone metastatic microenvironment via miR-205-5p/runt-related transcription factor 2/splicing factor proline- and glutamine-rich/polypyrimidine tract-binding protein 2 axis. Clin Transl Med. 2021;11:e493. doi: 10.1002/ctm2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie Y, Rong L, He M, Jiang Y, Li H, Mai L, Song F. LncRNA SNHG3 promotes gastric cancer cell proliferation and metastasis by regulating the miR-139-5p/MYB axis. Aging (Albany NY) 2021;13:25138–25152. doi: 10.18632/aging.203732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jia Y, Ding X, Zhou L, Zhang L, Yang X. Mesenchymal stem cells-derived exosomal microRNA-139-5p restrains tumorigenesis in bladder cancer by targeting PRC1. Oncogene. 2021;40:246–261. doi: 10.1038/s41388-020-01486-7. [DOI] [PubMed] [Google Scholar]
- 69.Hou J, Zhuo H, Chen X, Cheng J, Zheng W, Zhong M, Cai J. MiR-139-5p negatively regulates PMP22 to repress cell proliferation by targeting the NF-κB signaling pathway in gastric cancer. Int J Biol Sci. 2020;16:1218–1229. doi: 10.7150/ijbs.40338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu X, Luo C, Bu F, Lin K, Zhu Z. Long non-coding RNA RP11-59H7.3 promotes cell proliferation and invasion metastasis in colorectal cancer by miR-139-5p/NOTCH1 axis. Aging (Albany NY) 2020;12:11653–11666. doi: 10.18632/aging.103331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie JJ, Jiang YY, Jiang Y, Li CQ, Lim MC, An O, Mayakonda A, Ding LW, Long L, Sun C, et al. Super-enhancer-driven long non-coding RNA LINC01503, regulated by TP63, is over-expressed and oncogenic in squamous cell carcinoma. Gastroenterology. 2018;154:2137–2151.e1. doi: 10.1053/j.gastro.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 72.Ma Z, Gao X, Shuai Y, Wu X, Yan Y, Xing X, Ji J. EGR1-mediated linc01503 promotes cell cycle progression and tumorigenesis in gastric cancer. Cell Prolif. 2021;54:e12922. doi: 10.1111/cpr.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie C, Guo Y, Lou S. LncRNA ANCR promotes invasion and migration of gastric cancer by regulating FoxO1 expression to inhibit macrophage M1 polarization. Dig Dis Sci. 2020;65:2863–2872. doi: 10.1007/s10620-019-06019-1. [DOI] [PubMed] [Google Scholar]
- 74.Chung S, Ranjan R, Lee YG, Park GY, Karpurapu M, Deng J, Xiao L, Kim JY, Unterman TG, Christman JW. Distinct role of FoxO1 in M-CSF- and GM-CSF-differentiated macrophages contributes LPS-mediated IL-10: Implication in hyperglycemia. J Leukoc Biol. 2015;97:327–339. doi: 10.1189/jlb.3A0514-251R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moritoki Y, Zhang W, Tsuneyama K, Yoshida K, Wakabayashi K, Yang GX, Bowlus C, Ridgway WM, Ueno Y, Ansari AA, et al. B cells suppress the inflammatory response in a mouse model of primary biliary cirrhosis. Gastroenterology. 2009;136:1037–1047. doi: 10.1053/j.gastro.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 76.Yang JB, Zhao ZB, Liu QZ, Hu TD, Long J, Yan K, Lian ZX. FoxO1 is a regulator of MHC-II expression and anti-tumor effect of tumor-associated macrophages. Oncogene. 2018;37:1192–1204. doi: 10.1038/s41388-017-0048-4. [DOI] [PubMed] [Google Scholar]
- 77.Engreitz JM, Pandya-Jones A, McDonel P, Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander ES, et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science. 2013;341:1237973. doi: 10.1126/science.1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng R, Lin S, Guan L, Yuan H, Liu K, Liu C, Ye W, Liao Y, Jia J, Zhang R. Long non-coding RNA XIST inhibited breast cancer cell growth, migration, and invasion via miR-155/CDX1 axis. Biochem Biophys Res Commun. 2018;498:1002–1008. doi: 10.1016/j.bbrc.2018.03.104. [DOI] [PubMed] [Google Scholar]
- 79.Petrović N, Kolaković A, Stanković A, Lukić S, Řami A, Ivković M, Mandušić V. MiR-155 expression level changes might be associated with initial phases of breast cancer pathogenesis and lymph-node metastasis. Cancer Biomark. 2016;16:385–394. doi: 10.3233/CBM-160577. [DOI] [PubMed] [Google Scholar]
- 80.Qin CX, Yang XQ, Jin GC, Zhan ZY. LncRNA TSLNC8 inhibits proliferation of breast cancer cell through the miR-214-3p/FOXP2 axis. Eur Rev Med Pharmacol Sci. 2019;23:8440–8448. doi: 10.26355/eurrev_201910_19156. [DOI] [PubMed] [Google Scholar]
- 81.Yang W, Qian Y, Gao K, Zheng W, Wu G, He Q, Chen Q, Song Y, Wang L, Wang Y, et al. LncRNA BRCAT54 inhibits the tumorigenesis of non-small cell lung cancer by binding to RPS9 to transcriptionally regulate JAK-STAT and calcium pathway genes. Carcinogenesis. 2021;42:80–92. doi: 10.1093/carcin/bgaa051. [DOI] [PubMed] [Google Scholar]
- 82.Wan Y, Yao D, Fang F, Wang Y, Wu G, Qian Y. LncRNA WT1-AS downregulates lncRNA UCA1 to suppress non-small cell lung cancer and predicts poor survival. BMC Cancer. 2021;21:104. doi: 10.1186/s12885-020-07767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deng X, Xiong W, Jiang X, Zhang S, Li Z, Zhou Y, Xiang B, Zhou M, Li X, Li G, et al. LncRNA LINC00472 regulates cell stiffness and inhibits the migration and invasion of lung adenocarcinoma by binding to YBX1. Cell Death Dis. 2020;11:945. doi: 10.1038/s41419-020-03147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao T, Dai X, Jiang Y, He X, Yuan S, Zhao P. LncRNA HAND2-AS1 inhibits proliferation and promotes apoptosis of non-small cell lung cancer cells by inactivating PI3K/Akt pathway. Biosci Rep. 2020;40:BSR20201870. doi: 10.1042/BSR20201870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, Liu J, Che L, Li J. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m6A reader YTHDF3. Mol Cancer. 2019;18:143. doi: 10.1186/s12943-019-1079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naik R, Galande S. SATB family chromatin organizers as master regulators of tumor progression. Oncogene. 2019;38:1989–2004. doi: 10.1038/s41388-018-0541-4. [DOI] [PubMed] [Google Scholar]
- 87.Xu M, Xu X, Pan B, Chen X, Lin K, Zeng K, Liu X, Xu T, Sun L, Qin J, et al. LncRNA SATB2-AS1 inhibits tumor metastasis and affects the tumor immune cell microenvironment in colorectal cancer by regulating SATB2. Mol Cancer. 2019;18:135. doi: 10.1186/s12943-019-1063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bai J, Xu J, Zhao J, Zhang R. LncRNA NBR2 suppresses migration and invasion of colorectal cancer cells by downregulating miRNA-21. Hum Cell. 2020;33:98–103. doi: 10.1007/s13577-019-00265-1. [DOI] [PubMed] [Google Scholar]
- 89.Osei GY, Adu-Amankwaah J, Koomson S, Beletaa S, Ahmad MK, Asiamah EA, Smith-Togobo C, Abdul Razak SR. Revolutionizing colorectal cancer treatment: Unleashing the potential of miRNAs in targeting cancer stem cells. Future Oncol. 2023;19:2369–2382. doi: 10.2217/fon-2023-0426. [DOI] [PubMed] [Google Scholar]
- 90.Osei GY, Adu-Amankwaah J, Koomson S, Beletaa S, Asiamah EA, Smith-Togobo C, Razak SRA. MicroRNAs and colorectal cancer: Clinical potential and regulatory networks. Mol Biol Rep. 2023;50:9575–9585. doi: 10.1007/s11033-023-08810-w. [DOI] [PubMed] [Google Scholar]
- 91.Zhou Y, Yang H, Xia W, Cui L, Xu R, Lu H, Xue D, Tian Z, Ding T, Cao Y, et al. LncRNA MEG3 inhibits the progression of prostate cancer by facilitating H3K27 trimethylation of EN2 through binding to EZH2. J Biochem. 2020;167:295–301. doi: 10.1093/jb/mvz097. [DOI] [PubMed] [Google Scholar]
- 92.Morgan R. Engrailed: Complexity and economy of a multi-functional transcription factor. FEBS Lett. 2006;580:2531–2533. doi: 10.1016/j.febslet.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 93.Zhou YJ, Yang HQ, Xia W, Cui L, Xu RF, Lu H, Xue Z, Zhang B, Tian ZN, Cao YJ, et al. Down-regulation of miR-605 promotes the proliferation and invasion of prostate cancer cells by up-regulating EN2. Life Sci. 2017;190:7–14. doi: 10.1016/j.lfs.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 94.Yang Y, Liu PY, Bao W, Chen SJ, Wu FS, Zhu PY. Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC Cancer. 2020;20:28. doi: 10.1186/s12885-019-6491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ren N, Jiang T, Wang C, Xie S, Xing Y, Piao D, Zhang T, Zhu Y. LncRNA ADAMTS9-AS2 inhibits gastric cancer (GC) development and sensitizes chemoresistant GC cells to cisplatin by regulating miR-223-3p/NLRP3 axis. Aging (Albany NY) 2020;12:11025–11041. doi: 10.18632/aging.103314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang XZ, Cheng TT, He QJ, Lei ZY, Chi J, Tang Z, Liao QX, Zhang H, Zeng LS, Cui SZ. LINC01133 as ceRNA inhibits gastric cancer progression by sponging miR-106a-3p to regulate APC expression and the Wnt/β-catenin pathway. Mol Cancer. 2018;17:126. doi: 10.1186/s12943-018-0874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang M, Yang L, Hou L, Tang X. LncRNA SNHG1 promotes tumor progression and cisplatin resistance through epigenetically silencing miR-381 in breast cancer. Bioengineered. 2021;12:9239–9250. doi: 10.1080/21655979.2021.1996305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Du C, Wang Y, Zhang Y, Zhang J, Zhang L, Li J. LncRNA DLX6-AS1 contributes to epithelial-mesenchymal transition and cisplatin resistance in triple-negative breast cancer via modulating Mir-199b-5p/paxillin axis. Cell Transplant. 2020;29:963689720929983. doi: 10.1177/0963689720929983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Liu Y, Li M, Yu H, Piao H. lncRNA CYTOR promotes tamoxifen resistance in breast cancer cells via sponging miR-125a-5p. Int J Mol Med. 2020;45:497–509. doi: 10.3892/ijmm.2019.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shi Q, Li Y, Li S, Jin L, Lai H, Wu Y, Cai Z, Zhu M, Li Q, Li Y, et al. LncRNA DILA1 inhibits cyclin D1 degradation and contributes to tamoxifen resistance in breast cancer. Nat Commun. 2020;11:5513. doi: 10.1038/s41467-020-19349-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu Q, Li Y, Peng S, Li J, Qin X. Exosomal-mediated transfer of OIP5-AS1 enhanced cell chemoresistance to trastuzumab in breast cancer via up-regulating HMGB3 by sponging miR-381-3p. Open Med (Wars) 2021;16:512–525. doi: 10.1515/med-2021-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen J, Liu A, Wang Z, Wang B, Chai X, Lu W, Cao T, Li R, Wu M, Lu Z, et al. LINC00173.v1 promotes angiogenesis and progression of lung squamous cell carcinoma by sponging miR-511-5p to regulate VEGFA expression. Mol Cancer. 2020;19:98. doi: 10.1186/s12943-020-01217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zeng F, Wang Q, Wang S, Liang S, Huang W, Guo Y, Peng J, Li M, Zhu W, Guo L. Linc00173 promotes chemoresistance and progression of small cell lung cancer by sponging miR-218 to regulate Etk expression. Oncogene. 2020;39:293–307. doi: 10.1038/s41388-019-0984-2. [DOI] [PubMed] [Google Scholar]
- 104.Hua G, Zeng ZL, Shi YT, Chen W, He LF, Zhao GF. LncRNA XIST contributes to cisplatin resistance of lung cancer cells by promoting cellular glycolysis through sponging miR-101-3p. Pharmacology. 2021;106:498–508. doi: 10.1159/000512621. [DOI] [PubMed] [Google Scholar]
- 105.Xu X, Zhou X, Chen Z, Gao C, Zhao L, Cui Y. Silencing of lncRNA XIST inhibits non-small cell lung cancer growth and promotes chemosensitivity to cisplatin. Aging (Albany NY) 2020;12:4711–4726. doi: 10.18632/aging.102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhuang ST, Cai YJ, Liu HP, Qin Y, Wen JF. LncRNA NEAT1/miR-185-5p/IGF2 axis regulates the invasion and migration of colon cancer. Mol Genet Genomic Med. 2020;8:e1125. doi: 10.1002/mgg3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang X, Jiang G, Ren W, Wang B, Yang C, Li M. LncRNA NEAT1 regulates 5-Fu sensitivity, apoptosis and invasion in colorectal cancer through the MiR-150-5p/CPSF4 axis. Onco Targets Ther. 2020;13:6373–6383. doi: 10.2147/OTT.S239432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu Y, Hu H, Yuan Z, Zhang Q, Xiong H, Hu Z, Wu H, Huang R, Wang G, Tang Q. LncRNA NEAT1 remodels chromatin to promote the 5-Fu resistance by maintaining colorectal cancer stemness. Cell Death Dis. 2020;11:962. doi: 10.1038/s41419-020-03164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bian Z, Jin L, Zhang J, Yin Y, Quan C, Hu Y, Feng Y, Liu H, Fei B, Mao Y, et al. LncRNA-UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci Rep. 2016;6:23892. doi: 10.1038/srep23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen B, Dragomir MP, Fabris L, Bayraktar R, Knutsen E, Liu X, Tang C, Li Y, Shimura T, Ivkovic TC, et al. The long noncoding RNA CCAT2 induces chromosomal instability through BOP1-AURKB signaling. Gastroenterology. 2020;159:2146–2162.e33. doi: 10.1053/j.gastro.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ding J, Li J, Wang H, Tian Y, Xie M, He X, Ji H, Ma Z, Hui B, Wang K, Ji G. Long noncoding RNA CRNDE promotes colorectal cancer cell proliferation via epigenetically silencing DUSP5/CDKN1A expression. Cell Death Dis. 2017;8:e2997. doi: 10.1038/cddis.2017.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li P, Zhang X, Wang L, Du L, Yang Y, Liu T, Li C, Wang C. lncRNA HOTAIR contributes to 5FU resistance through suppressing miR-218 and activating NF-κB/TS signaling in colorectal cancer. Mol Ther Nucleic Acids. 2017;8:356–369. doi: 10.1016/j.omtn.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang B, Zhang M, Shen C, Liu G, Zhang F, Hou J, Yao W. LncRNA PCBP1-AS1-mediated AR/AR-V7 deubiquitination enhances prostate cancer enzalutamide resistance. Cell Death Dis. 2021;12:856. doi: 10.1038/s41419-021-04144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang L, Zhang Y, Su P, Ma Z, Ye X, Kang W, Liu Y, Yu J. Long non-coding RNA HNF1A-AS1 induces 5-FU resistance of gastric cancer through miR-30b-5p/EIF5A2 pathway. Transl Oncol. 2022;18:101351. doi: 10.1016/j.tranon.2022.101351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xin L, Zhou LQ, Liu C, Zeng F, Yuan YW, Zhou Q, Li SH, Wu Y, Wang JL, Wu DZ, Lu H. Transfer of LncRNA CRNDE in TAM-derived exosomes is linked with cisplatin resistance in gastric cancer. EMBO Rep. 2021;22:e52124. doi: 10.15252/embr.202052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu Y, Zhou B, Hu X, Ying S, Zhou Q, Xu W, Feng L, Hou T, Wang X, Zhu L, Jin H. LncRNA LINC00942 promotes chemoresistance in gastric cancer by suppressing MSI2 degradation to enhance c-Myc mRNA stability. Clin Transl Med. 2022;12:e703. doi: 10.1002/ctm2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fu T, Ji K, Jin L, Zhang J, Wu X, Ji X, Fan B, Jia Z, Wang A, Liu J, et al. ASB16-AS1 up-regulated and phosphorylated TRIM37 to activate NF-κB pathway and promote proliferation, stemness, and cisplatin resistance of gastric cancer. Gastric Cancer. 2021;24:45–59. doi: 10.1007/s10120-020-01096-y. [DOI] [PubMed] [Google Scholar]
- 118.Zhou J, Lin J, Zhang H, Zhu F, Xie R. LncRNA HAND2-AS1 sponging miR-1275 suppresses colorectal cancer progression by upregulating KLF14. Biochem Biophys Res Commun. 2018;503:1848–1853. doi: 10.1016/j.bbrc.2018.07.125. [DOI] [PubMed] [Google Scholar]
- 119.Ghildiyal R, Sawant M, Renganathan A, Mahajan K, Kim EH, Luo J, Dang HX, Maher CA, Feng FY, Mahajan NP. Loss of long noncoding RNA NXTAR in prostate cancer augments androgen receptor expression and enzalutamide resistance. Cancer Res. 2022;82:155–168. doi: 10.1158/0008-5472.CAN-20-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Q, Fu L, Han L, Li S, Zhang Y, Wang J. Long noncoding RNA GAS5 accelerates cholangiocarcinoma progression by regulating hsa-miR-1297. Cancer Manag Res. 2021;13:2745–2753. doi: 10.2147/CMAR.S297868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun M, Jin FY, Xia R, Kong R, Li JH, Xu TP, Liu YW, Zhang EB, Liu XH, De W. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14:319. doi: 10.1186/1471-2407-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cao S, Liu W, Li F, Zhao W, Qin C. Decreased expression of lncRNA GAS5 predicts a poor prognosis in cervical cancer. Int J Clin Exp Pathol. 2014;7:6776–6783. [PMC free article] [PubMed] [Google Scholar]
- 123.Chang L, Li C, Lan T, Wu L, Yuan Y, Liu Q, Liu Z. Decreased expression of long non-coding RNA GAS5 indicates a poor prognosis and promotes cell proliferation and invasion in hepatocellular carcinoma by regulating vimentin. Mol Med Rep. 2016;13:1541–1550. doi: 10.3892/mmr.2015.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dong Q, Long X, Cheng J, Wang W, Tian Q, Di W. LncRNA GAS5 suppresses ovarian cancer progression by targeting the miR-96-5p/PTEN axis. Ann Transl Med. 2021;9:1770. doi: 10.21037/atm-21-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y, Zhu P, Luo J, Wang J, Liu Z, Wu W, Du Y, Ye B, Wang D, He L, et al. LncRNA HAND2-AS1 promotes liver cancer stem cell self-renewal via BMP signaling. EMBO J. 2019;38:e101110. doi: 10.15252/embj.2018101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dong G, Wang X, Jia Y, Jia Y, Zhao W, Zhang J, Tong Z. HAND2-AS1 works as a ceRNA of miR-3118 to suppress proliferation and migration in breast cancer by upregulating PHLPP2. Biomed Res Int. 2020;2020:8124570. doi: 10.1155/2020/8124570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jiang Z, Li L, Hou Z, Liu W, Wang H, Zhou T, Li Y, Chen S. LncRNA HAND2-AS1 inhibits 5-fluorouracil resistance by modulating miR-20a/PDCD4 axis in colorectal cancer. Cell Signal. 2020;66:109483. doi: 10.1016/j.cellsig.2019.109483. [DOI] [PubMed] [Google Scholar]
- 128.Xu Z, Lv H, Wang Y, Hu C, Chen S, Du Y, Shi C, Cheng X. HAND2-AS1 inhibits gastric adenocarcinoma cells proliferation and aerobic glycolysis via miRNAs sponge. Cancer Manag Res. 2020;12:3053–3068. doi: 10.2147/CMAR.S222878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gong J, Fan H, Deng J, Zhang Q. LncRNA HAND2-AS1 represses cervical cancer progression by interaction with transcription factor E2F4 at the promoter of C16orf74. J Cell Mol Med. 2020;24:6015–6027. doi: 10.1111/jcmm.15117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shan L, Liu W, Zhan Y. LncRNA HAND2-AS1 exerts anti-oncogenic effects on bladder cancer via restoration of RARB as a sponge of microRNA-146. Cancer Cell Int. 2021;21:361. doi: 10.1186/s12935-021-02063-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 131.Li Y, Wan Q, Wang W, Mai L, Sha L, Mashrah M, Lin Z, Pan C. LncRNA ADAMTS9-AS2 promotes tongue squamous cell carcinoma proliferation, migration and EMT via the miR-600/EZH2 axis. Biomed Pharmacother. 2019;112:108719. doi: 10.1016/j.biopha.2019.108719. [DOI] [PubMed] [Google Scholar]
- 132.Luo T, Gao Y, Zhangyuan G, Xu X, Xue C, Jin L, Zhang W, Zhu C, Sun B, Qin X. lncRNA PCBP1-AS1 aggravates the progression of hepatocellular carcinoma via regulating PCBP1/PRL-3/AKT pathway. Cancer Manag Res. 2020;12:5395–5408. doi: 10.2147/CMAR.S249657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li L, Peng Q, Gong M, Ling L, Xu Y, Liu Q. Using lncRNA sequencing to reveal a putative lncRNA-mRNA correlation network and the potential role of PCBP1-AS1 in the pathogenesis of cervical cancer. Front Oncol. 2021;11:634732. doi: 10.3389/fonc.2021.634732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang H, Ouyang L, Gao S. Effects of antisense lncRNA PCBP1-AS1 on biological behaviors of vulvar squamous carcinoma cells by regulating TRAF5 and NF-κB expression. Transl Cancer Res. 2019;8:1578–1590. doi: 10.21037/tcr.2019.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang D, Zhou H, Liu J, Mao J. Long noncoding RNA ASB16-AS1 promotes proliferation, migration, and invasion in glioma cells. Biomed Res Int. 2019;2019:5437531. doi: 10.1155/2019/5437531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li M, Yin B, Chen M, Peng J, Mu X, Deng Z, Xiao J, Li W, Fan J. Downregulation of the lncRNA ASB16-AS1 decreases LARP1 expression and promotes clear cell renal cell carcinoma progression via miR-185-5p/miR-214-3p. Front Oncol. 2021;10:617105. doi: 10.3389/fonc.2020.617105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Han P, Li JW, Zhang BM, Lv JC, Li YM, Gu XY, Yu ZW, Jia YH, Bai XF, Li L, et al. The lncRNA CRNDE promotes colorectal cancer cell proliferation and chemoresistance via miR-181a-5p-mediated regulation of Wnt/β-catenin signaling. Mol Cancer. 2017;16:9. doi: 10.1186/s12943-017-0583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhu HY, Gao YJ, Wang Y, Liang C, Zhang ZX, Chen Y. LncRNA CRNDE promotes the progression and angiogenesis of pancreatic cancer via miR-451a/CDKN2D axis. Transl Oncol. 2021;14:101088. doi: 10.1016/j.tranon.2021.101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bai X, Wang W, Zhao P, Wen J, Guo X, Shen T, Shen J, Yang X. LncRNA CRNDE acts as an oncogene in cervical cancer through sponging miR-183 to regulate CCNB1 expression. Carcinogenesis. 2020;41:111–121. doi: 10.1093/carcin/bgz166. [DOI] [PubMed] [Google Scholar]
- 140.Wang Q, Wang LX, Zhang CY, Bai N, Feng C, Zhang ZM, Wang L, Gao ZZ. LncRNA CRNDE promotes cell proliferation, migration and invasion of ovarian cancer via miR-423-5p/FSCN1 axis. Mol Cell Biochem. 2022;477:1477–1488. doi: 10.1007/s11010-022-04382-8. [DOI] [PubMed] [Google Scholar]
- 141.Cui WW, Sun YL, Chen C, Feng RR, Xu W, Meng JJ, Zhang K. LncRNA CRNDE promotes the development of Wilms' tumor by regulating microRNA-424. Eur Rev Med Pharmacol Sci. 2020;24:1088–1097. doi: 10.26355/eurrev_202002_20159. [DOI] [PubMed] [Google Scholar]
- 142.Tang D, Zhao L, Peng C, Ran K, Mu R, Ao Y. LncRNA CRNDE promotes hepatocellular carcinoma progression by upregulating SIX1 through modulating miR-337-3p. J Cell Biochem. 2019;120:16128–16142. doi: 10.1002/jcb.28894. [DOI] [PubMed] [Google Scholar]
- 143.Zhu Y, Li B, Xu G, Han C, Xing G. Knockdown of long noncoding RNA colorectal neoplasia differentially expressed inhibits hepatocellular carcinoma progression by mediating the expression of nuclear autoantigenic sperm protein. Oncol Rep. 2021;46:252. doi: 10.3892/or.2021.8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun H, He L, Ma L, Lu T, Wei J, Xie K, Wang X. LncRNA CRNDE promotes cell proliferation, invasion and migration by competitively binding miR-384 in papillary thyroid cancer. Oncotarget. 2017;8:110552–110565. doi: 10.18632/oncotarget.22819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fan YF, Yu ZP, Cui XY. lncRNA colorectal neoplasia differentially expressed (CRNDE) promotes proliferation and inhibits apoptosis in non-small cell lung cancer cells by regulating the miR-641/CDK6 axis. Med Sci Monit. 2019;25:2745–2755. doi: 10.12659/MSM.913420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fu D, Zang L, Li Z, Fan C, Jiang H, Men T. Long non-coding RNA CRNDE regulates the growth and migration of prostate cancer cells by targeting microRNA-146a-5p. Bioengineered. 2021;12:2469–2479. doi: 10.1080/21655979.2021.1935402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ding Q, Mo F, Cai X, Zhang W, Wang J, Yang S, Liu X. LncRNA CRNDE is activated by SP1 and promotes osteosarcoma proliferation, invasion, and epithelial-mesenchymal transition via Wnt/β-catenin signaling pathway. J Cell Biochem. 2020;121:3358–3371. doi: 10.1002/jcb.29607. [DOI] [PubMed] [Google Scholar]
- 148.Hu CE, Du PZ, Zhang HD, Huang GJ. Long noncoding RNA CRNDE promotes proliferation of gastric cancer cells by targeting miR-145. Cell Physiol Biochem. 2017;42:13–21. doi: 10.1159/000477107. [DOI] [PubMed] [Google Scholar]
- 149.Huan J, Xing L, Lin Q, Xui H, Qin X. Long noncoding RNA CRNDE activates Wnt/β-catenin signaling pathway through acting as a molecular sponge of microRNA-136 in human breast cancer. Am J Transl Res. 2017;9:1977–1989. [PMC free article] [PubMed] [Google Scholar]
- 150.Wang Y, Zhou Q, Ma JJ. High expression of lnc-CRNDE presents as a biomarker for acute myeloid leukemia and promotes the malignant progression in acute myeloid leukemia cell line U937. Eur Rev Med Pharmacol Sci. 2018;22:763–770. doi: 10.26355/eurrev_201802_14310. [DOI] [PubMed] [Google Scholar]
- 151.Song H, Han LM, Gao Q, Sun Y. Long non-coding RNA CRNDE promotes tumor growth in medulloblastoma. Eur Rev Med Pharmacol Sci. 2016;20:2588–2597. [PubMed] [Google Scholar]
- 152.Xia XL, Xue D, Xiang TH, Xu HY, Song DK, Cheng PG, Wang JQ. Overexpression of long non-coding RNA CRNDE facilitates epithelial-mesenchymal transition and correlates with poor prognosis in intrahepatic cholangiocarcinoma. Oncol Lett. 2018;15:4105–4112. doi: 10.3892/ol.2018.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Y, Wang Y, Li J, Zhang Y, Yin H, Han B. CRNDE, a long-noncoding RNA, promotes glioma cell growth and invasion through mTOR signaling. Cancer Lett. 2015;367:122–128. doi: 10.1016/j.canlet.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 154.Zhao Z, Liu M, Long W, Yuan J, Li H, Zhang C, Tang G, Jiang W, Yuan X, Wu M, Liu Q. Knockdown lncRNA CRNDE enhances temozolomide chemosensitivity by regulating autophagy in glioblastoma. Cancer Cell Int. 2021;21:456. doi: 10.1186/s12935-021-02153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.David A, Zocchi S, Talbot A, Choisy C, Ohnona A, Lion J, Cuccuini W, Soulier J, Arnulf B, Bories JC, et al. The long non-coding RNA CRNDE regulates growth of multiple myeloma cells via an effect on IL6 signalling. Leukemia. 2021;35:1710–1721. doi: 10.1038/s41375-020-01034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu L, Zhang Y, Zhao Z, Chen Z, Wang Z, Xu S, Zhang X, Liu T, Yu S. The long non-coding RNA CRNDE competed endogenously with miR-205 to promote proliferation and metastasis of melanoma cells by targeting CCL18. Cell Cycle. 2018;17:2296–2308. doi: 10.1080/15384101.2018.1526602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shen S, Liu H, Wang Y, Wang J, Ni X, Ai Z, Pan H, Liu H, Shao Y. Long non-coding RNA CRNDE promotes gallbladder carcinoma carcinogenesis and as a scaffold of DMBT1 and C-IAP1 complexes to activating PI3K-AKT pathway. Oncotarget. 2016;7:72833–72844. doi: 10.18632/oncotarget.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ni J, Hong J, Li Q, Zeng Q, Xia R. Long non-coding RNA CRNDE suppressing cell proliferation is regulated by DNA methylation in chronic lymphocytic leukemia. Leuk Res. 2021;105:106564. doi: 10.1016/j.leukres.2021.106564. [DOI] [PubMed] [Google Scholar]
- 159.Yu Y, Lu X, Yang C, Yin F. Long noncoding RNA LINC00173 contributes to the growth, invasiveness and chemo-resistance of colorectal cancer through regulating miR-765/PLP2 axis. Cancer Manag Res. 2020;12:3363–3369. doi: 10.2147/CMAR.S251029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang J, Zhou M, Zhao X, Wang G, Li J. Long noncoding RNA LINC00173 is downregulated in cervical cancer and inhibits cell proliferation and invasion by modulating the miR-182-5p/FBXW7 axis. Pathol Res Pract. 2020;216:152994. doi: 10.1016/j.prp.2020.152994. [DOI] [PubMed] [Google Scholar]
- 161.Zhang J, Zhu N, Chen X. A novel long noncoding RNA LINC01133 is upregulated in lung squamous cell cancer and predicts survival. Tumour Biol. 2015;36:7465–7471. doi: 10.1007/s13277-015-3460-9. [DOI] [PubMed] [Google Scholar]
- 162.Feng Y, Qu L, Wang X, Liu C. LINC01133 promotes the progression of cervical cancer by sponging miR-4784 to up-regulate AHDC1. Cancer Biol Ther. 2019;20:1453–1461. doi: 10.1080/15384047.2019.1647058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu S, Xi X. LINC01133 contribute to epithelial ovarian cancer metastasis by regulating miR-495-3p/TPD52 axis. Biochem Biophys Res Commun. 2020;533:1088–1094. doi: 10.1016/j.bbrc.2020.09.074. [DOI] [PubMed] [Google Scholar]
- 164.Zhang J, Gao S, Zhang Y, Yi H, Xu M, Xu J, Liu H, Ding Z, He H, Wang H, et al. MiR-216a-5p inhibits tumorigenesis in pancreatic cancer by targeting TPT1/mTORC1 and is mediated by LINC01133. Int J Biol Sci. 2020;16:2612–2627. doi: 10.7150/ijbs.46822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Song Z, Zhang X, Lin Y, Wei Y, Liang S, Dong C. LINC01133 inhibits breast cancer invasion and metastasis by negatively regulating SOX4 expression through EZH2. J Cell Mol Med. 2019;23:7554–7565. doi: 10.1111/jcmm.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kong J, Sun W, Li C, Wan L, Wang S, Wu Y, Xu E, Zhang H, Lai M. Long non-coding RNA LINC01133 inhibits epithelial-mesenchymal transition and metastasis in colorectal cancer by interacting with SRSF6. Cancer Lett. 2016;380:476–484. doi: 10.1016/j.canlet.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 167.Foroughi K, Amini M, Atashi A, Mahmoodzadeh H, Hamann U, Manoochehri M. Tissue-specific down-regulation of the long non-coding RNAs PCAT18 and LINC01133 in gastric cancer development. Int J Mol Sci. 2018;19:3881. doi: 10.3390/ijms19123881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang ZY, Yang F, Zhang YL, Liu B, Wang M, Hong X, Yu Y, Zhou YH, Zeng H. LncRNA-ANCR down-regulation suppresses invasion and migration of colorectal cancer cells by regulating EZH2 expression. Cancer Biomark. 2017;18:95–104. doi: 10.3233/CBM-161715. [DOI] [PubMed] [Google Scholar]
- 169.Li Z, Hou P, Fan D, Dong M, Ma M, Li H, Yao R, Li Y, Wang G, Geng P, et al. The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 2017;24:59–71. doi: 10.1038/cdd.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang S, Lan F, Xia Y. lncRA ANCR inhibits non-small cell lung cancer cell migration and invasion by inactivating TGF-β pathway. Med Sci Monit. 2018;24:6002–6009. doi: 10.12659/MSM.911492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Si Y, Yang Z, Ge Q, Yu L, Yao M, Sun X, Ren Z, Ding C. Long non-coding RNA Malat1 activated autophagy, hence promoting cell proliferation and inhibiting apoptosis by sponging miR-101 in colorectal cancer. Cell Mol Biol Lett. 2019;24:50. doi: 10.1186/s11658-019-0175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zheng X, Ren J, Peng B, Ye J, Wu X, Zhao W, Li Y, Chen R, Gong X, Bai C, et al. MALAT1 overexpression promotes the growth of colon cancer by repressing β-catenin degradation. Cell Signal. 2020;73:109676. doi: 10.1016/j.cellsig.2020.109676. [DOI] [PubMed] [Google Scholar]
- 173.Chen W, Tan X, Yang Q, Fang Z, Xu Y. MALAT1 enhances gemcitabine resistance in non-small cell lung cancer cells by directly affecting miR-27a-5p/PBOV1 axis. Cell Signal. 2022;94:110326. doi: 10.1016/j.cellsig.2022.110326. [DOI] [PubMed] [Google Scholar]
- 174.Zhang D, Fang C, Li H, Lu C, Huang J, Pan J, Yang Z, Liang E, Liu Z, Zhou X, et al. Long ncRNA MALAT1 promotes cell proliferation, migration, and invasion in prostate cancer via sponging miR-145. Transl Androl Urol. 2021;10:2307–2319. doi: 10.21037/tau-20-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mao Y, Li W, Weng Y, Hua B, Gu X, Lu C, Xu B, Xu H, Wang Z. METTL3-mediated m6A modification of lncRNA MALAT1 facilitates prostate cancer growth by activation of PI3K/AKT signaling. Cell Transplant. 2022;31:9636897221122997. doi: 10.1177/09636897221122997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Deng QJ, Xie LQ, Li H. Overexpressed MALAT1 promotes invasion and metastasis of gastric cancer cells via increasing EGFL7 expression. Life Sci. 2016;157:38–44. doi: 10.1016/j.lfs.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 177.Lu Z, Luo T, Pang T, Du Z, Yin X, Cui H, Fang G, Xue X. MALAT1 promotes gastric adenocarcinoma through the MALAT1/miR-181a-5p/AKT3 axis. Open Biol. 2019;9:190095. doi: 10.1098/rsob.190095. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 178.Kwok ZH, Roche V, Chew XH, Fadieieva A, Tay Y. A non-canonical tumor suppressive role for the long non-coding RNA MALAT1 in colon and breast cancers. Int J Cancer. 2018;143:668–678. doi: 10.1002/ijc.31386. [DOI] [PubMed] [Google Scholar]
- 179.Kim J, Piao HL, Kim BJ, Yao F, Han Z, Wang Y, Xiao Z, Siverly AN, Lawhon SE, Ton BN, et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat Genet. 2018;50:1705–1715. doi: 10.1038/s41588-018-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.