Abstract
PURPOSE
Pembrolizumab is standard therapy for patients with metastatic urothelial cancer (mUC) who progress after first-line platinum-based chemotherapy; however, only approximately 21% of patients respond. Sacituzumab govitecan (SG) is a trophoblast cell surface antigen-2–directed antibody-drug conjugate with US Food and Drug Administration–accelerated approval to treat patients with locally advanced or mUC who previously received platinum-based chemotherapy and a checkpoint inhibitor (CPI). Here, we report the primary analysis of TROPHY-U-01 cohort 3.
METHODS
TROPHY-U-01 (ClinicalTrials.gov identifier: NCT03547973) is a multicohort, open-label phase II study. Patients were CPI-naïve and had mUC progression after platinum-based chemotherapy in the metastatic setting or ≤12 months in the (neo)adjuvant setting. Patients received 10 mg/kg of SG once on days 1 and 8 and 200 mg of pembrolizumab once on day 1 of 21-day cycles. The primary end point was objective response rate (ORR) per central review. Secondary end points included clinical benefit rate (CBR), duration of response (DOR) and progression-free survival (PFS) per central review, and safety.
RESULTS
Cohort 3 included 41 patients (median age 67 years; 83% male; 78% visceral metastases [29% liver]). With a median follow-up of 14.8 months, the ORR was 41% (95% CI, 26.3 to 57.9; 20% complete response rate), CBR was 46% (95% CI, 30.7 to 62.6), median DOR was 11.1 months (95% CI, 4.8 to not estimable [NE]), and median PFS was 5.3 months (95% CI, 3.4 to 10.2). The median overall survival was 12.7 months (range, 10.7-NE). Grade ≥3 treatment-related adverse events occurred in 61% of patients; most common were neutropenia (37%), leukopenia (20%), and diarrhea (20%).
CONCLUSION
SG plus pembrolizumab demonstrated a high response rate with an overall manageable toxicity profile in patients with mUC who progressed after platinum-based chemotherapy. No new safety signals were detected. These data support further evaluation of SG plus CPI in mUC.
INTRODUCTION
Patients with metastatic urothelial cancer (mUC) have poor prognosis with an estimated 5-year overall survival (OS) rate of <5%.1 Recommended first-line therapy is platinum-based chemotherapy followed by avelumab switch maintenance for patients with a response or stable disease (SD), resulting in a median OS of approximately 24 months from the start of avelumab therapy.2-5 Patients with progression on first-line platinum-based chemotherapy have poorer prognosis, are not eligible for switch maintenance avelumab, and therefore require effective second-line treatment.6
CONTEXT
Key Objective
Approximately 21% of patients with metastatic urothelial cancer (mUC) who progress on or after platinum-based chemotherapy respond to second-line pembrolizumab monotherapy. The TROPHY-U-01 cohort 3 study evaluated sacituzumab govitecan (SG), a trophoblast cell surface antigen-2–directed antibody-drug conjugate (ADC), in combination with pembrolizumab as a second-line treatment for patients with mUC who progressed after prior platinum-based chemotherapy.
Knowledge Generated
Of 41 patients who received SG plus pembrolizumab, central review confirmed an objective response rate (ORR) of 41%, meeting the primary study end point and demonstrating higher ORR compared with historical pembrolizumab monotherapy data in this setting, with a promising survival signal. The combination was generally well tolerated with no new safety signals observed. These data support further evaluation of this combination in mUC while biomarker evaluation is being pursued.
Relevance (M.A. Carducci)
-
SG and pembrolizomab combination therapy for metastatic urothelial carcinoma is a novel regimen moving forward in a number of clinical trials, along with other ADCs and immune checkpoint inhibitor combinations for urothelial cancer. This study provides a summary of early clinical activity and the rationale for future study.*
*Relevance section written by JCO Associate Editor Michael A. Carducci, MD, FACP, FASCO.
Three checkpoint inhibitors (CPIs) are US Food and Drug Administration (FDA)–approved for postplatinum second-line therapy, including the preferred option pembrolizumab, which has been shown in a phase III randomized clinical trial (Keynote-045) to extend median OS to 10.3 months compared with 7.4 months with single-agent chemotherapy.3,6-9 Other CPI options in this setting include the alternative preferred regimens nivolumab and avelumab.3 In addition, patients with confirmed activating FGFR2 or FGFR3 alterations who progress after platinum-based chemotherapy can receive erdafitinib, which has a confirmed response rate of 40% (3% complete response [CR] rate).10-12 Despite these advances, less than one fifth of patients with urothelial cancer are eligible for erdafitinib treatment,11 and only 18%-21% of patients respond to approved second-line CPIs13; thus, new treatment strategies are urgently needed in this setting.
Two antibody-drug conjugates (ADCs) were recently added to the mUC armamentarium for patients who have progressed after platinum-containing chemotherapy and a CPI: enfortumab vedotin (EV) and sacituzumab govitecan (SG).14,15 EV is a nectin-4–targeting ADC that demonstrated a significant improvement in OS and progression-free survival (PFS) compared with chemotherapy (OS, 12.9 v 9 months; hazard ratio [HR], 0.7; 5.6 v 3.7 months; HR, 0.6; respectively) in a phase III study in patients with locally advanced or mUC who had previously received a CPI and platinum-containing chemotherapy.16 EV is also FDA-approved for cisplatin-ineligible patients in combination with pembrolizumab and as a monotherapy for cisplatin-ineligible patients who previously received one or more lines of therapy.14
SG is an ADC composed of a trophoblast cell surface antigen-2 (Trop-2) antibody coupled to SN-38, the active metabolite of the topoisomerase 1 inhibitor irinotecan, via a hydrolyzable linker, with a high drug-to-antibody ratio (7.6:1).17 Trop-2 is a transmembrane glycoprotein involved in various cellular functions including proliferation, cell-to-cell adhesion, and mobility. Its expression is elevated in several epithelial cancers, including UC, and has been correlated with bladder cancer severity.18 SG is FDA-approved for patients with metastatic triple-negative breast cancer who received two or more prior chemotherapy regimens (one or more in the metastatic setting) and for patients with metastatic hormone receptor–positive, human epidermal growth factor receptor 2 (HER2)–negative breast cancer who received endocrine therapy and two or more additional systemic therapies in the metastatic setting. SG also received accelerated FDA approval for patients with locally advanced or mUC who have previously received platinum-containing chemotherapy and a CPI on the basis of the results of the pivotal TROPHY-U-01 cohort 1 study.15
TROPHY-U-01 is a multicohort phase II open-label study of SG in patients with locally advanced unresectable or mUC.19 The registrational cohort 1 assessed SG monotherapy in 113 patients with progression after platinum-based chemotherapy and CPI, demonstrating an objective response rate (ORR) of 27% and a manageable toxicity profile.19 These results led to FDA-accelerated approval in this patient population and provided support for further assessment of SG monotherapy and SG-based combinations in mUC.
Various preclinical and clinical studies have demonstrated that Trop-2–directed cytotoxicity (likely via internalization and the bystander effect) is the main mechanism of action of SG.17,20-23 Historically, pembrolizumab has been successfully combined with several other cytotoxic chemotherapies with combination approvals by the FDA in non–small-cell lung cancer, head and neck squamous cell cancer, esophageal cancer, and cervical cancer indications.8 In addition, the combination of irinotecan and a CPI has previously demonstrated a synergistic antitumor effect in a syngeneic mouse tumor model possibly due to irinotecan augmenting CPI-mediated T-cell activation, via increased expression of major histocompatibility complex class I and PD-L1 on tumor cells and depletion of regulatory T cells, providing further rationale for combining SG and CPI.24 Moreover, both pembrolizumab and SG monotherapy have demonstrated antitumor efficacy with manageable, limited-overlapping safety profiles in patients with platinum-relapsed/refractory mUC.7,19 As such, it was hypothesized that the combination of these two therapies would be a safe and effective treatment strategy in this setting.7,19 To test this hypothesis, we conducted a phase II study of SG plus pembrolizumab in patients with advanced or mUC following progression after platinum-based chemotherapy, TROPHY-U-01 cohort 3. Here, we report the safety and efficacy outcomes from the primary analysis of the phase II TROPHY-U-01 cohort 3 study.
METHODS
Study Design and Patient Population
TROPHY-U-01 (ClinicalTrials.gov identifier: NCT03547973) is an open-label phase II study assessing the safety and antitumor activity of SG (alone or in combinations) in patients with advanced or mUC across various lines of therapy. In cohort 3, eligible patients (18 years or older) had histologically confirmed locally advanced or mUC with progression after platinum-containing chemotherapy in the advanced/metastatic setting or progression within 12 months after platinum-based (neo)adjuvant therapy and had not received prior CPI therapy. Eligible patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) score of ≤1 and creatinine clearance ≥30 mL/min as calculated by the Cockcroft-Gault formula and could not have active autoimmune disease that required systemic treatment within 2 years of enrollment or have received immunosuppressive therapy within 3 years of receiving study drug. There was no Trop-2 expression requirement for enrollment.
Treatment (phase II)
The recommended phase II dose of SG in combination with the standard pembrolizumab dose of 200 mg was determined to be 10 mg/kg by a 10-patient safety lead-in phase. SG (10 mg/kg) was administered intravenously once on days 1 and 8, and pembrolizumab (200 mg) was administered intravenously once on day 1 (after SG administration) of a 21-day treatment cycle until progression, unacceptable toxicity, or withdrawal of consent. Growth factors, blood product transfusions, and other supportive/palliative care were allowed as clinically indicated. Premedication with a 2-drug antiemetic for nausea followed by a 3-drug antiemetic (5-HT3 inhibitor, an NK1-receptor antagonist, and dexamethasone [10 mg oral or IV]) for persistent nausea and vomiting and premedication for infusion-related reactions were recommended. The scheduled infusions could be delayed for up to 3 weeks for treatment-related toxicities with a maximum duration of 5 weeks between dosing.
Assessments
Efficacy assessments for all treated patients were performed using computed tomography (CT) or magnetic resonance imaging (MRI) scans obtained at baseline and every 6 weeks up to cycle 12 and then every 9 weeks. Confirmatory CT/MRI scans were obtained 4-6 weeks after first evidence of response. Responses were evaluated by central review via RECIST v1.1 criteria. Safety evaluations included adverse event (AE) assessments that were graded on the basis of investigator assessment according to the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0.
End Points
The primary end point of this study was ORR (defined as a best overall response of CR or partial response [PR]) per central review using RECIST 1.1 criteria. Secondary end points included duration of response (DOR; defined as the time from first documented response, PR or CR, to progressive disease [PD] or death; patients who neither progressed nor died were censored on the date of their last radiographic tumor assessment), clinical benefit rate (CBR; defined as CR plus PR plus SD ≥6 months), and PFS (defined as the time from first dose to objective tumor progression or death, whichever came first; patients without progression or death were censored at the time of last response assessment) on the basis of central review and OS (defined as time from first dose to death from any cause; patients not known to have died were censored on the date they were last known to be alive) and safety and tolerability of SG in combination with pembrolizumab.
Study Oversight
The Protocol (online only) was approved by institutional review boards or independent ethics committees at participating institutions. This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines and other applicable local regulatory requirements and laws. All patients provided written informed consent.
Statistical Analysis
Target enrollment was 41 patients on the basis of a Simon two-stage design for 90% power at one-sided α of .05 to demonstrate a 21% improvement in ORR with a null hypothesis of historical ORR ≤20%7 and an alternate hypothesis of ORR ≥41%. If 13 or more responses were observed among the total of 41 patients, the null hypothesis would be rejected.
Efficacy assessments were performed on all treated patients, defined as all patients who received one or more doses of SG. ORRs were assessed for prespecified subgroups. The Clopper-Pearson method was used to calculate 95% CI estimates for ORR. The Kaplan-Meier method was used to analyze time to event end points with medians and corresponding 95% CIs. Frequency and percentages were used to characterize and present AEs. Median follow-up was defined as the time from first dose to death or end of study (whichever occurs first) for patients discontinued from the study or the database cutoff date for ongoing patients. The Kaplan-Meier method was used to analyze follow-up using a reverse approach, where censoring is treated as an event and deaths are censored. All statistical analyses were performed using SAS (SAS Institute, version 9.2 or later, Cary, NC).
RESULTS
Study Participants
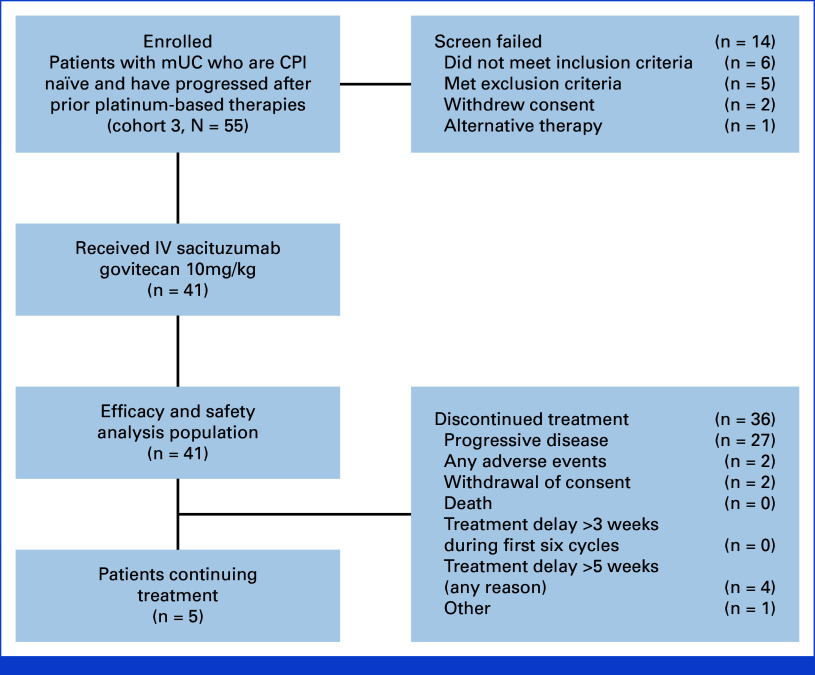
Between March 16, 2020, and August 13, 2021, 55 patients were enrolled, and 41 patients were treated in cohort 3 (Fig 1) with a median follow-up of 14.8 months (95% CI, 12.6 to 16.8) in all treated patients. The median age was 67 years (range, 46-86), and most patients were male (83%), had ECOG PS scores of 1 (61%), and had at least one adverse Bellmunt risk factor (76%; Table 1). Visceral metastases were present in 32 patients (78%) including 12 patients with liver metastases (29%). All 41 patients received prior platinum-based chemotherapy (71% cisplatin, 29% carboplatin) with a median of one prior anticancer regimen (range, 1-2).
FIG 1.
Flow diagram. CPI, checkpoint inhibitor; IV, intravenous; mUC, metastatic urothelial cancer.
TABLE 1.
Patient Demographics and Baseline Characteristics
| Characteristic | Cohort 3 (N = 41) |
|---|---|
| Age, years, median (range) | 67 (46-86) |
| ≥65, No. (%) | 26 (63) |
| Male, No. (%) | 34 (83) |
| Race, No. (%) | |
| White | 22 (54) |
| Other | 1 (2) |
| Not reported | 18 (44) |
| ECOG PS score, No. (%) | |
| 0 | 16 (39) |
| 1 | 25 (61) |
| Type of disease, No. (%) | |
| Metastatic urothelial cancer | 32 (78) |
| Locally advanced unresectable | 9 (22) |
| Visceral metastases, No. (%) | 32 (78) |
| Lung/pleura | 22 (54) |
| Liver | 12 (29) |
| Other | 8 (20) |
| Time since initial diagnosis, months, median (range) | 13.5 (2.3-98.1) |
| Prior anticancer regimens, No. (%) | |
| 1 | 32 (78) |
| 2 | 9 (22) |
| Duration of last anticancer regimen, months, median (range) | 2.7 (0-13) |
| Prior platinum-based chemotherapy, No. (%) | |
| Cisplatin | 29 (71) |
| Carboplatin | 12 (29) |
| Setting of last prior systemic therapy, No. (%) | |
| (Neo)adjuvant | 17 (41) |
| Metastatic | 24 (59) |
| Time since the end of last prior systemic therapy, months, median (range) | |
| (Neo)adjuvant | 7.2 (2.5-12.8) |
| Metastatic | 2.0 (0.3-60.6) |
| Best response to prior systemic platinum therapy with metastatic intent, No. | 24 |
| CR, No. (%) | 1 (4) |
| PR, No. (%) | 2 (8) |
| SD, No. (%) | 13 (54) |
| PD, No. (%) | 6 (25) |
| Not reported/NA, No. (%) | 2 (8) |
| Bellmunt risk factors | |
| 0, No. (%) | 10 (24) |
| 1, No. (%) | 20 (49) |
| 2, No. (%) | 11 (27) |
| 3, No. | 0 |
| UGT1A1 status, No. | 35 |
| Wild type *1/*1, No. (%) | 13 (37) |
| Heterozygous *1/*28, No. (%) | 15 (43) |
| Homozygous *28/*28, No. (%) | 7 (20) |
| Missing, No. | 6 |
Abbreviations: CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; NA, not available; PD, progressive disease; PR, partial response; SD, stable disease.
Seventeen patients (41%) received prior (neo)adjuvant systemic therapy, with a median time since the end of last systemic therapy to screening start date of 7.2 months (range, 2.5-12.8; Table 1). Twenty-four patients (59%) received prior systemic therapy in the metastatic setting, with a median time since the end of last prior systemic therapy to screening start date of 2.0 months (range, 0.3-60.6; Table 1). Most patients were primarily refractory to prior platinum-based chemotherapy in the metastatic setting, with 19 of 24 patients (79%) having no response (SD or PD) as their best response to prior platinum (Table 1).
Patients received a median of eight cycles (range, 1-32) and 15 doses (range, 2-63) of SG, with a median treatment duration of 5.1 months (range, 0-23). The median relative dose intensity of SG was 92% (range, 57-102), with 44% of patients requiring at least one dose reduction for any reason. Patients received a median of eight cycles (range, 1-31) of pembrolizumab, with a median treatment duration of 4.9 months (range, 0-23). At data cutoff, most patients (88%) had permanently discontinued treatment, primarily due to PD (66%; Fig 1).
Efficacy
The primary end point was met with an observed ORR per central review of 41% (17 of 41 patients; 95% CI, 26.3 to 57.9), with eight patients achieving a CR (20%) and nine patients achieving a PR (22%) as best response in all treated patients (Table 2). Nine patients (22%) had SD (two with SD ≥6 months at data cutoff), and 10 patients (24%) had PD as best response (Table 2). The CBR was 46% (95% CI, 30.7 to 62.6). ORRs were largely similar (with a few numerical differences) across prespecified subgroups, including patients with visceral and liver metastases (41% and 50% ORRs, respectively) at baseline and by the number of Bellmunt risk factors (Appendix Fig A1). The ORR was 58.8% (95% CI, 32.9 to 81.6) in patients who had progression within 12 months from (neo)adjuvant therapy (n = 17) and 29.2% (95% CI, 12.6 to 51.1) in patients who had progression after platinum-based chemotherapy given as first-line therapy in the metastatic setting (n = 24).
TABLE 2.
Summary of Treatment Efficacy
| Variable | Cohort (N = 41) |
|---|---|
| Best response, No. (%) | |
| CR | 8 (20) |
| PR | 9 (22) |
| SD | 9 (22) |
| SD ≥6 months | 2 (5) |
| PD | 10 (24) |
| Not evaluable | 3 (7) |
| Not assesseda | 2 (5) |
| ORR | |
| Patients, No. | 17 |
| Patients, % (95% CI) | 41 (26.3 to 57.9) |
| CBRb | |
| Patients, No. | 19 |
| Patients, % (95% CI) | 46 (30.7 to 62.6) |
| Time to onset of objective response, months | |
| Median | 1.4 |
| Range | 1.2-5.5 |
| Median DOR, months | |
| Median | 11.1 |
| 95% CI | 4.76 to NA |
Abbreviations: CBR, clinical benefit rate; CR, complete response; DOR, duration of response; NA, not available; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
These patients had no postbaseline radiologic tumor assessments.
CBR defined as CR + PR + SD ≥6 months.
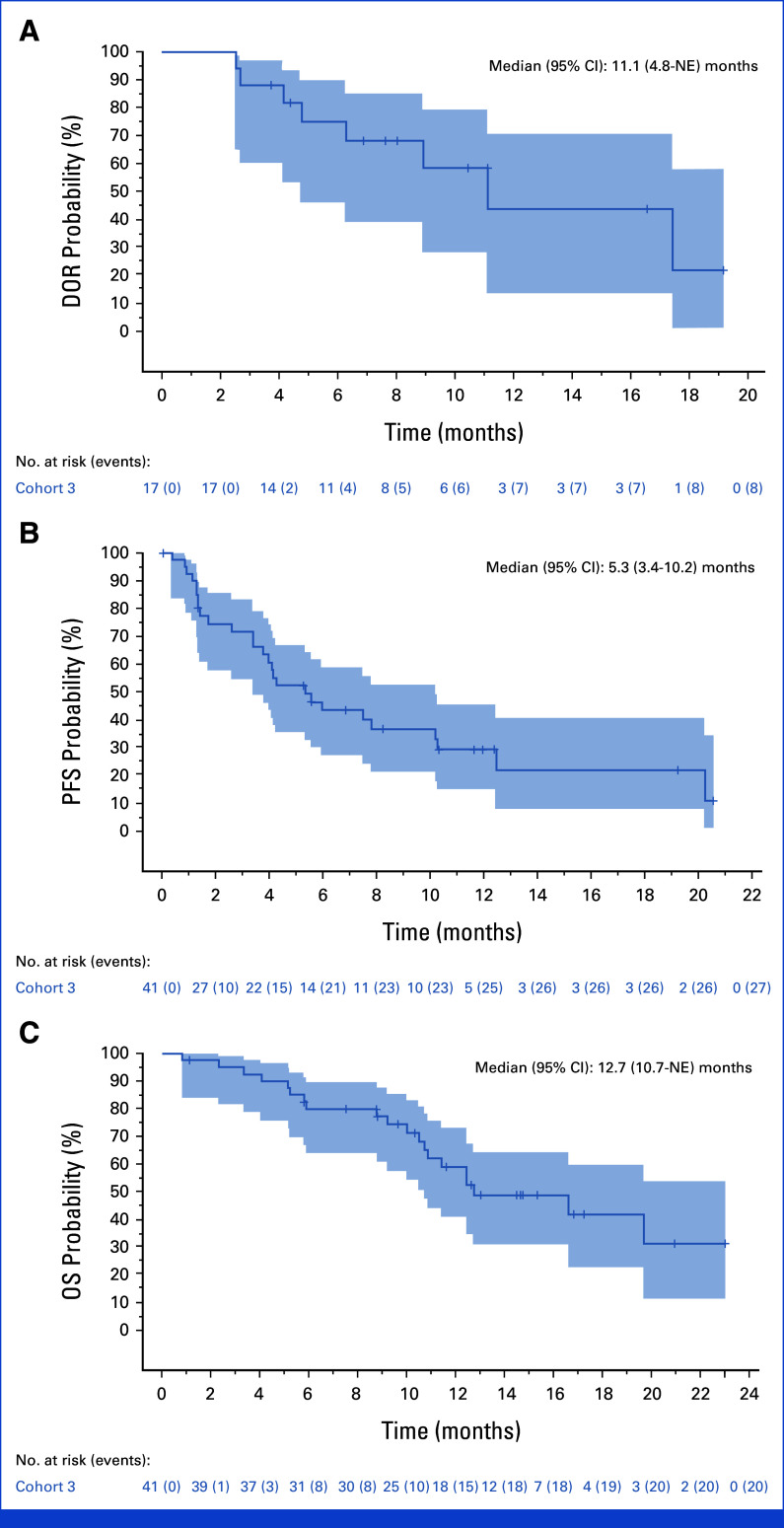
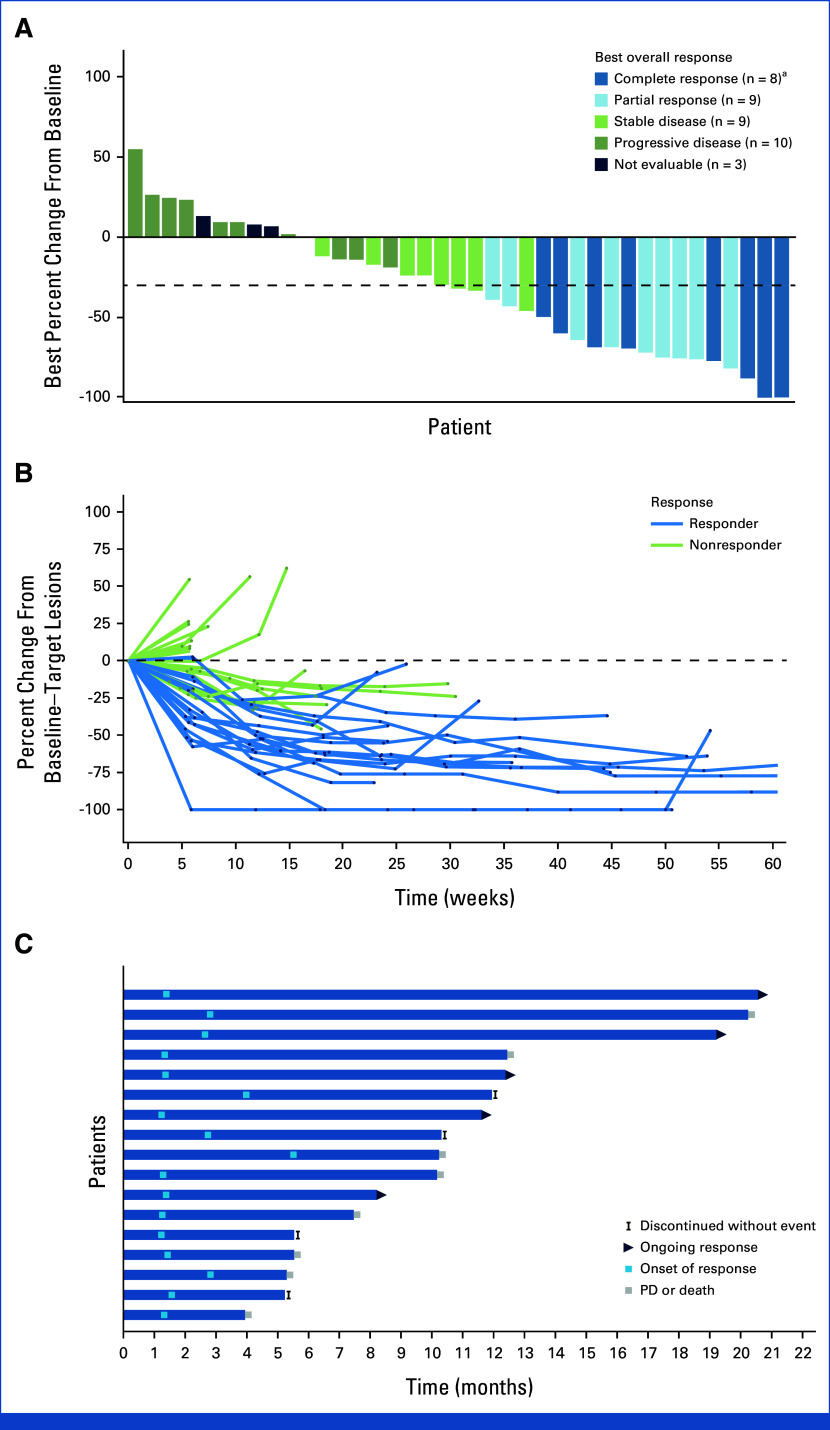
For all patients, the median DOR was 11.1 months (95% CI, 4.8 to not estimable [NE]; n = 17; Fig 2A), and the median time to onset of response was 1.4 months (95% CI, 1.3 to 1.5; 11 patients responded by first assessment). Target lesions were reduced in 72% of patients (28 of 39 evaluable patients) with at least one postbaseline target lesion measurement per central review (Fig 3A). For most responders and a proportion of nonresponders, the best reduction in the size of target lesions from baseline was durable per central review (Fig 3B). The onset and DOR (CR or PR) by central review is summarized in the swimmer plot, with five patients having ongoing response at the time of data cutoff (Fig 3C). The median PFS was 5.3 months (95% CI, 3.4 to 10.2; Fig 2B), and the median OS was 12.7 months (95% CI, 10.7 to NE; Fig 2C). As of July 26, 2022, 20 patients had died, primarily due to disease progression (19 of 20).
FIG 2.
Kaplan-Meier analysis of (A) DOR, (B) PFS, and (C) OS. DOR, duration of response; NE, not estimable; OS, overall survival; PFS, progression-free survival.
FIG 3.
Tumor responses per central review. (A) Waterfall plot showing best percentage change from baseline in the sum of the diameters of the target lesions in 39 patients (excludes two patients who were missing percentage change from baseline). The dashed line indicates threshold for partial response, according to RECIST v1.1. Target lesions were reduced in 72% of patients (28 of 38) with at least one postbaseline target lesion measurement. (B) Spider plot of tumor response by week. (C) Swimmer plot of response and duration. aPatients who achieved a complete response without a 100% reduction relative to the assessment at baseline had a lymph node as the target lesion. PD, progressive disease.
Safety
All 41 patients experienced at least one treatment-emergent AE and at least one treatment-related AE (TRAE). The most common any-grade TRAEs that occurred in ≥15% of patients included diarrhea (71%), nausea (56%), neutropenia (51%), anemia (49%), asthenia (41%), alopecia (39%), fatigue (32%), vomiting (29%), decreased appetite (29%), leukopenia (27%), pruritus (24%), stomatitis (17%), and hypomagnesemia (17%; Table 3). TRAEs led to SG interruptions in 46% of patients, SG dose reductions in 39% of patients, and SG discontinuations in 15% of patients.
TABLE 3.
TRAEs of Any Grade Reported by ≥15% of Patients or Grade ≥3 TRAEs Reported by ≥5% of Patients
| Category | Event | All Grades, No. (%) | Grade 3, No. (%) | Grade 4, No. (%) |
|---|---|---|---|---|
| GI disorders | Diarrhea | 29 (71) | 8 (20) | 0 |
| Nausea | 23 (56) | 3 (7) | 0 | |
| Vomiting | 12 (29) | 0 | 0 | |
| Stomatitis | 7 (17) | 0 | 0 | |
| General disorders and administration site conditions | Fatigue | 13 (32) | 3 (7) | 0 |
| Asthenia | 17 (41) | 3 (7) | 0 | |
| Skin and subcutaneous tissue disorders | Alopecia | 16 (39) | 0 | 0 |
| Pruritus | 10 (24) | 0 | 0 | |
| Blood and lymphatic system disorders | Anemia | 20 (49) | 7 (17) | 0 |
| Neutropenia | 21 (51) | 8 (20) | 7 (17) | |
| Febrile neutropenia | 4 (10) | 4 (10) | 0 | |
| Leukopenia | 11 (27) | 4 (10) | 4 (10) | |
| Metabolism and nutrition disorders | Decreased appetite | 12 (29) | 2 (5) | 0 |
| Hypomagnesemia | 7 (17) | 0 | 0 | |
| Respiratory, thoracic, and mediastinal disorders | Pneumonitis | 2 (5) | 0 | 2 (5) |
Abbreviation: TRAEs, treatment-related adverse events.
Grade ≥3 TRAEs occurred in 61% of patients; most common grade ≥3 TRAEs that occurred in ≥5% of patients included neutropenia (37%), diarrhea (20%), leukopenia (20%), anemia (17%), febrile neutropenia (10%; 0 of 4 received prophylactic granulocyte colony-stimulating factor [G-CSF]) nausea (7%), fatigue (7%), asthenia (7%), decreased appetite (5%), and pneumonitis (5%; Table 3). G-CSF was used by nine patients (22%) for prophylactic use and eight patients (20%) for treatment of an AE. The most common pembrolizumab-related AEs of any grade were diarrhea (24%), asthenia (22%), pruritus (20%), and fatigue (17%; Appendix Table A1, online only). No patients received topical steroids, and five patients (12%) received systemic steroids (oral [four] or IV [one]) because of pembrolizumab-related AEs including diarrhea (three patients), pruritus (one patient), and pneumonitis (one patient). Grade ≥3 serious TRAEs that occurred in more than one patient included pneumonitis (n = 2), febrile neutropenia (n = 3; UGT1A1 status: two patients homozygous *28|*28; one missing), and diarrhea (n = 4; UGT1A1 status: two patients homozygous *28|*28; two patients homozygous *1|*1). No treatment-related deaths occurred.
DISCUSSION
Despite the availability of CPIs for patients in the platinum-relapsed/refractory second-line mUC setting, outcomes remain poor, with only a fifth of patients responding to CPIs resulting in a median OS of <11 months for these patients.7,25 In this study, SG in combination with pembrolizumab showed encouraging antitumor efficacy with a 41% ORR and a CR of 20% in a platinum-relapsed/refractory patient population, meeting the primary end point. With 1 year of follow-up, responses lasted a median of 11.1 months, translating to a median OS of 12.7 months in a primarily platinum-refractory patient population; however, interpreting PFS and OS results in single-arm trials should be pursued with caution. Clinical benefit was observed across all prespecified subgroups, including patients with liver metastases and one or more Bellmunt risk factors, although patient numbers were small in many subgroups, warranting further evaluation in larger trials.
Despite inherent differences in the trial design and baseline patient and disease characteristics, these results compare favorably with historical data in the postplatinum setting, including KEYNOTE-045, CheckMate 275, and JAVELIN 100 Bladder Solid Tumor studies. In KEYNOTE-045, pembrolizumab was associated with significantly longer OS compared with chemotherapy (10.3 v 7.4 months) and a higher ORR compared with chemotherapy (21% v 11%; CR rates, 7% v 3%) in patients with disease progression on or after platinum-based chemotherapy, suggesting a likely additive efficacy effect with the combination of SG and pembrolizumab.7 Nivolumab demonstrated an ORR of 19.6% in CheckMate 275 in the postplatinum setting.26 Finally, in the JAVELIN 100 Bladder Solid Tumor study, avelumab demonstrated an ORR of 17% in patients with mUC who progressed after platinum-based chemotherapy.27 Notably, most patients in the TROPHY cohort 3 study had a short duration of time between the end of prior platinum-based chemotherapy in the metastatic setting and the beginning of SG plus pembrolizumab (median of 2 months). A duration of <3 months from the start of platinum-based chemotherapy to the beginning of second-line CPI therapy has been shown to be a negative prognostic factor associated with poorer outcomes after second-line CPI therapy.28 Therefore, this patient population should be prioritized for clinical trials. Moreover, the site of metastasis can be relevant to the therapeutic decision making in platinum-refractory mUC and the possible differences among clinical trials.29
With limited treatment options for patients with platinum-relapsed/refractory mUC, the addition of two ADCs (EV and SG) to the postplatinum and post-CPI setting and an FGFR inhibitor (erdafitinib) to the postplatinum setting represent a shift in the treatment paradigm away from single-agent chemotherapy.14,15,30 In addition, two HER2-targeting ADCs (disitamab vedotin and trastuzumab deruxtecan) are also under clinical investigation in the postplatinum therapy setting.30,31 In the TROPHY-U-01 cohort 1 study, SG monotherapy demonstrated a high ORR (27%) with a median DOR of 7.2 months and a median OS of 10.9 months in a heavily pretreated patient population.19 TROPiCS-04 (ClinicalTrials.gov identifier: NCT04527991), a confirmatory phase III study of SG versus taxane or vinflunine in mUC, is ongoing.19 In the phase III EV-301 study, EV was associated with a significant improvement in OS compared with chemotherapy (12.9 v 9.0; HR, 0.7) in patients with locally advanced or mUC who previously received platinum-containing therapy and a CPI.16
In addition, EV plus pembrolizumab demonstrated a high ORR (73%) with a median DOR of 25.6 months as a first-line treatment in 45 cisplatin-ineligible patients in the EV-103 cohort A and a 64.5% ORR in the cohort K leading to accelerated approval by the FDA in this patient population.14,32,33 Confirmatory phase III studies, EV-302 (ClinicalTrials.gov identifier: NCT04223856) in this setting, EV-303 (ClinicalTrials.gov identifier: NCT03924895), and EV-304 (ClinicalTrials.gov identifier: NCT04700124) in the perioperative setting, as well as other studies assessing novel ADC and CPI combinations, are ongoing (ClinicalTrials.gov identifier: NCT04879329). Although the ORRs appear to be higher with the combination of EV and pembrolizumab compared with the combination SG and pembrolizumab, it is important to note that the patients in the EV-103 trial were treatment-naïve and thus a much different patient population from the platinum-refractory/relapsed, higher-risk population in TROPHY-U-01, cohort 3.32,33 Additional differences in patient characteristics and study designs as well as possible selection and confounding factors cannot be ruled out and thus a cross-trial comparison here is inherently difficult to make.
In cohort 3, SG in combination with pembrolizumab had a tolerable safety profile with no new safety signals detected with the combination compared with SG or pembrolizumab monotherapy. The most common grade ≥3 AEs associated with treatment were neutropenia, leukopenia, and diarrhea, which is consistent with SG monotherapy in mUC.19 The AEs were predictable and relatively manageable, resulting in a low study drug discontinuation rate (15%). Education and proactive management is recommended for neutropenia, diarrhea, and other common AEs using established guidelines.
Limitations inherent to this study design include its single-arm, nonrandomized, open-label design and moderate sample size that could lead to potential selection and confounding biases. As such, PFS and OS should be interpreted with caution. In addition, because of the single-arm design, the individual contributions of SG or pembrolizumab alone cannot be ascertained. Moreover, a large proportion of patients (44%) did not report race, and molecular biomarkers and patient-reported outcomes were not analyzed.
Additional TROPHY-U-01 cohorts are being assessed, including SG monotherapy post-CPI in platinum-ineligible patients with mUC (cohort 2 is closed to accrual and data readouts are expected soon); SG plus cisplatin in patients with mUC who are platinum-naïve followed by avelumab plus SG as maintenance (cohort 4); SG maintenance after first-line cisplatin-based chemotherapy (cohort 5); and continuous SG versus continuous SG plus zimberelimab (anti–PD-1 monoclonal antibody)34 versus carboplatin plus gemcitabine and avelumab maintenance in first-line cisplatin-ineligible mUC (cohort 6). TROPiCS-04, the phase III confirmatory study of SG monotherapy in mUC that has progressed after platinum-based chemotherapy and CPI, is ongoing, as noted above.
In conclusion, SG in combination with pembrolizumab in the phase II TROPHY-U-01 cohort 3 study demonstrated encouraging antitumor activity with a manageable toxicity profile in platinum-refractory/relapsed CPI-naïve patients with mUC who progressed on/after platinum-based chemotherapy and support further evaluation of SG in combination with pembrolizumab in mUC.
ACKNOWLEDGMENT
We thank the patients, their caregivers, and families for their participation and commitment to clinical research, as well as the clinical study investigators and their team members, without whom this work would not have been possible. Medical writing and editorial assistance were provided by Ashly Pavlovsky, PhD, from Team 9 Science and funded by Gilead Sciences, Inc. The members of TROPHY-U-01 cohort 3 investigators are listed in Appendix 1.
APPENDIX 1. LIST OF TROPHY-U-01 COHORT 3 INVESTIGATORS
The United States: Petros Grivas, Chandler H. Park, Manojkumar Bupathi, Daniel P. Petrylak, Neeraj Agarwal, Sumati Gupta, Chethan Ramamurthy, Nancy B. Davis, Alejandro Recio-Boiles, Cora N. Sternberg, and Scott T. Tagawa. France: Damien Pouessel, Philippe Barthelemy, Aude Fléchon, and Yohann Loriot.
APPENDIX 2
TABLE A1.
Pembrolizumab-Related Adverse Events of Any Grade Reported by ≥15% of Patients or Grade ≥3 Pembrolizumab-Related Adverse Events Reported by ≥5% of Patients
| Category | Event | All Grades, No. (%) | Grade ≥3, No. (%) |
|---|---|---|---|
| GI disorders | Diarrhea | 10 (24) | 2 (5) |
| Nausea | 6 (15) | 2 (5) | |
| General disorders and administration site conditions | Fatigue | 7 (17) | 2 (5) |
| Asthenia | 9 (22) | 1 (2) | |
| Skin and subcutaneous tissue disorders | Pruritus | 8 (20) | 0 |
| Respiratory, thoracic, and mediastinal disorders | Pneumonitis | 2 (5) | 2 (5) |
FIG A1.
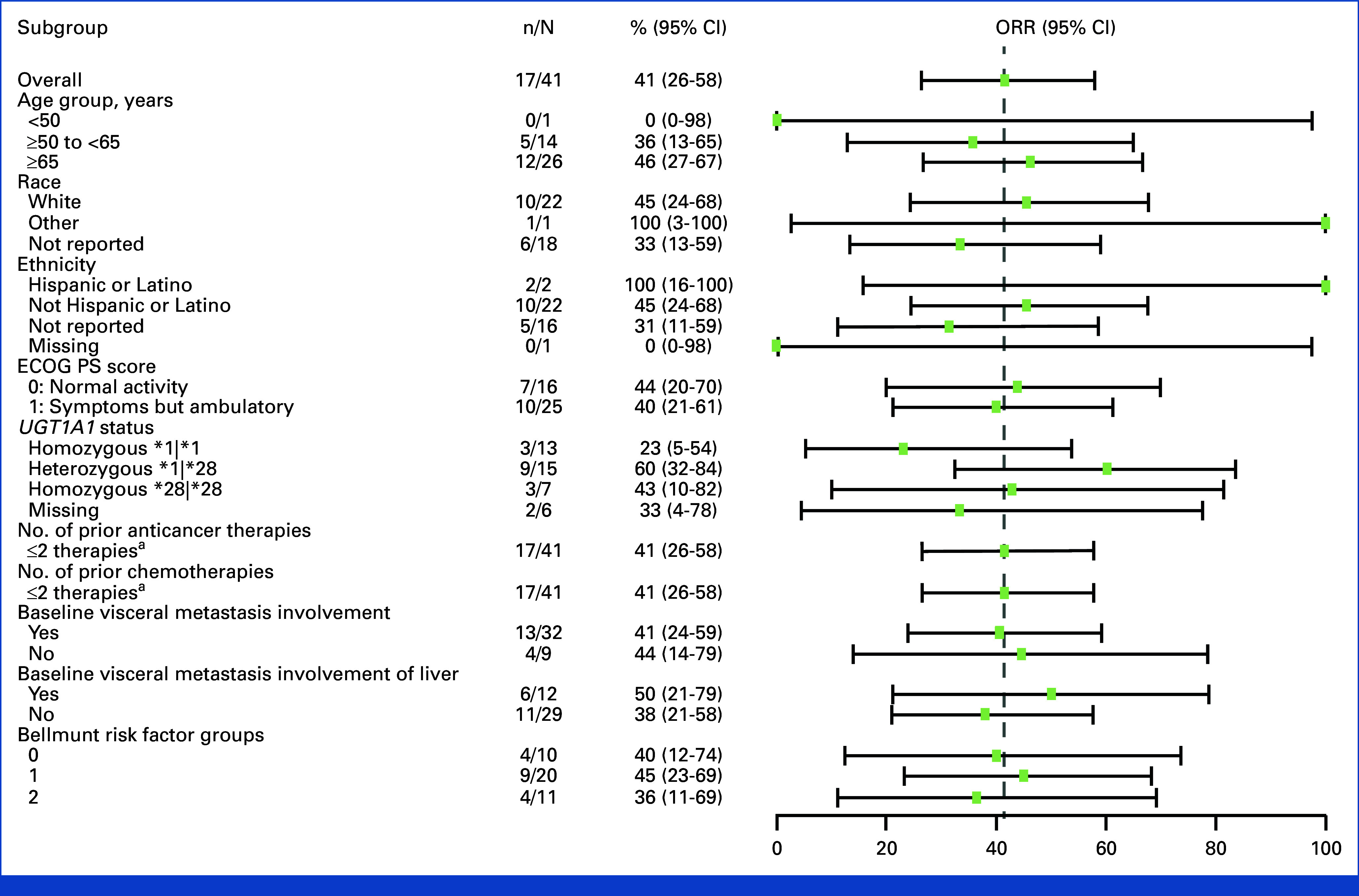
ORR in key prespecified subgroups per central review: forest plot showing ORR in different subgroups. Horizontal line represents CI. aAll patients had two or fewer therapies and two or fewer chemotherapies. ECOG PS, Eastern Cooperative Oncology Group performance status; NA, not available; ORR, objective response rate.
Petros Grivas
Consulting or Advisory Role: Merck, Bristol Myers Squibb, AstraZeneca, EMD Serono, Seagen, Pfizer, Janssen, Roche, Dyania Health, 4D Pharma, Astellas Pharma, Guardant Health, Urogen Pharma, Gilead Sciences, Silverback Therapeutics, BostonGene, Fresenius Kabi, Lucence, PureTech, G1 Therapeutics, AADi, CG Oncology, Strata Oncology, ImmunityBio, Asieris Pharmaceuticals
Research Funding: Pfizer (Inst), Clovis Oncology (Inst), Bavarian Nordic (Inst), Bristol Myers Squibb (Inst), Debiopharm Group (Inst), Merck (Inst), QED Therapeutics (Inst), GlaxoSmithKline (Inst), Mirati Therapeutics (Inst), EMD Serono (Inst), G1 Therapeutics (Inst), Gilead Sciences (Inst), Acrivon Therapeutics (Inst), ALX Oncology (Inst)
Damien Pouessel
Honoraria: Ipsen, Bristol Myers Squibb, AstraZeneca, Astellas Pharma, MSD Oncology, Pfizer/Astellas
Consulting or Advisory Role: Astellas Pharma, Pfizer, MSD Oncology, Bristol Myers Squibb/Medarex, Merck
Research Funding: Merck Sharp & Dohme (Inst), Roche (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Seagen (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Pfizer
Chandler H. Park
Consulting or Advisory Role: Bristol Myers Squibb/Celgene, Exelixis, Eisai, Gilead Sciences, Seagen, Merck
Speakers' Bureau: Eisai, Seagen, Gilead Sciences, Pfizer, AstraZeneca, Merck
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb/Celgene (Inst), Eisai (Inst), Exelixis (Inst), Gilead Sciences (Inst), Merck (Inst), SeaGen (Inst), Roche
Philippe Barthelemy
Honoraria: BMS, MSD, Astellas Pharma, Janssen-Cilag, Pfizer, Merck KGaA, Novartis, Seagen, Ipsen, Gilead Sciences, Bayer
Consulting or Advisory Role: Ipsen, BMS, MSD Oncology, Pfizer, Janssen-Cilag, AstraZeneca, Amgen, Merck KGaA, Eisai, Gilead Sciences, Bayer, AAA/Endocyte/Novartis
Research Funding: Ipsen (Inst)
Travel, Accommodations, Expenses: BMS, Pfizer, Janssen-Cilag, MSD, Ipsen, Merck/Pfizer
Manojkumar Bupathi
Honoraria: Bristol Myers Squibb, Exelixis, AstraZeneca, Pfizer, Astellas Pharma, Myovant Sciences, Bayer, Agendia, Janssen
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Exelixis
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Pfizer, Exelixis, Astellas Pharma, Janssen Oncology
Daniel P. Petrylak
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Lilly, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Incyte, Janssen, Pharmacyclics, Seagen, Urogen Pharma, Advanced Accelerator Applications, Ipsen, Bicycle Therapeutics, Mirati Therapeutics, Monopteros Therapeutics, Regeneron, Gilead Sciences
Research Funding: Progenics (Inst), Sanofi (Inst), Endocyte (Inst), Genentech (Inst), Merck (Inst), Astellas Medivation (Inst), Novartis (Inst), AstraZeneca (Inst), Bayer (Inst), Lilly (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Pfizer (Inst), Roche (Inst), Seagen (Inst), Clovis Oncology (Inst), Bristol Myers Squibb (Inst), Advanced Accelerator Applications (Inst), Agensys (Inst), BioXCel Therapeutics (Inst), Eisai (Inst), Mirati Therapeutics (Inst), Replimune (Inst), Medivation (Inst), Gilead Sciences (Inst)
Expert Testimony: Celgene, Sanofi
Neeraj Agarwal
Research Funding: Bayer (Inst), Bristol Myers Squibb (Inst), Takeda (Inst), Pfizer (Inst), Exelixis (Inst), Amgen (Inst), AstraZeneca (Inst), Calithera Biosciences (Inst), Celldex (Inst), Eisai (Inst), Genentech (Inst), Immunomedics (Inst), Janssen (Inst), Merck (Inst), Lilly (Inst), Nektar (Inst), ORIC Pharmaceuticals (Inst), CRISPR Therapeutics (Inst), Arvinas (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Pfizer, Exelixis
Sumati Gupta
Stock and Other Ownership Interests: Salarius Pharmaceuticals
Research Funding: Mirati Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), Viralytics (Inst), Hoosier Cancer Research Network (Inst), Rexahn Pharmaceuticals (Inst), Five Prime Therapeutics (Inst), Incyte (Inst), MedImmune (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Clovis Oncology (Inst), LSK BioPharma (Inst), QED Therapeutics (Inst), Daiichi Sankyo/Lilly (Inst), Immunocore (Inst), Seagen (Inst), Astra Zeneca (Inst), Acrotech Biopharma (Inst)
Travel, Accommodations, Expenses: QED Therapeutics
Uncompensated Relationships: Astellas Pharma
Aude Fléchon
Honoraria: MSD Oncology, AstraZeneca, BMS, Janssen-Cilag, Astellas Pharma, Pfizer, Sanofi/Aventis, Roche/Genentech, Bayer, Ipsen, AAA HealthCare, Novartis, Gilead Sciences
Travel, Accommodations, Expenses: Astellas Pharma, Sanofi/Aventis, Janssen-Cilag, Bayer, Pfizer, Ipsen, BMS, AstraZeneca, MSD Oncology, Roche/Genentech, AAA HealthCare
Chethan Ramamurthy
Employment: Merck
Stock and Other Ownership Interests: Merck
Honoraria: Gilead Sciences
Consulting or Advisory Role: SeaGen, Exelixis
Research Funding: Dispersol (Inst), Novartis (Inst), SeaGen (Inst), Gilead Sciences (Inst), Mirati Therapeutics (Inst), Nuvation Bio (Inst)
Nancy B. Davis
Employment: Merck, Sharp & Dohme, Inc
Consulting or Advisory Role: Janssen Biotech
Research Funding: AstraZeneca (Inst), Roche (Inst), Pfizer (Inst), Merck (Inst), Incyte (Inst), Mirati Therapeutics (Inst), Seattle Genetics/Astellas (Inst), Calithera Biosciences (Inst), Immunomedics (Inst), Bristol Myers Squibb (Inst), Exelixis (Inst), Gilead Sciences (Inst)
Alejandro Recio-Boiles
Honoraria: Janssen
Cora N. Sternberg
Consulting or Advisory Role: Bayer, MSD, Pfizer, Roche, Incyte, AstraZeneca, Merck, Medscape, UroToday, Astellas Pharma, Genzyme, Immunomedics, Foundation Medicine, Bristol Myers Squibb/Medarex, IMPAC Medical Systems, Amgen, Gilead Sciences, Janssen Oncology
Astha Bhatia
Employment: Zentalis, Bayer
Stock and Other Ownership Interests: Zentalis
Cabilia Pichardo
Employment: Novocure
Stock and Other Ownership Interests: Novocure
Consulting or Advisory Role: None
Expert Testimony: None
Mitch Sierecki
Employment: Gilead Sciences
Leadership: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences, Bayer HealthCare Pharmacuticals
Travel, Accommodations, Expenses: Gilead Sciences
Julia Tonelli
Employment: Gilead Sciences
Stock and Other Ownership Interests: Myovant Sciences, Gilead Sciences
Huafeng Zhou
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Scott T. Tagawa
Stock and Other Ownership Interests: Convergent Therapeutics
Consulting or Advisory Role: Medivation, Astellas Pharma, Dendreon, Janssen, Genentech, Endocyte, Immunomedics, Karyopharm Therapeutics, AbbVie, Tolmar, QED Therapeutics, Amgen, Sanofi, Pfizer, Clovis Oncology, Novartis, Genomic Health, POINT Biopharma, Blue Earth Diagnostics, Seagen, AIkido Pharma, 4D Pharma, Clarity Pharmaceuticals, Gilead Sciences, Telix Pharmaceuticals, Bayer, Myovant Sciences, Convergent Therapeutics, Hookipa Pharma, Merck, Daiichi Sankyo, Regeneron, TransThera Biosciences, Bicycle Therapeutics
Research Funding: Lilly (Inst), Sanofi (Inst), Janssen (Inst), Astellas Pharma (Inst), Progenics (Inst), Millennium (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Dendreon (Inst), Rexahn Pharmaceuticals (Inst), Bayer (Inst), Genentech (Inst), Newlink Genetics (Inst), Inovio Pharmaceuticals (Inst), AstraZeneca (Inst), Immunomedics (Inst), Novartis (Inst), AVEO (Inst), Boehringer Ingelheim (Inst), Merck (Inst), Stem CentRx (Inst), Karyopharm Therapeutics (Inst), AbbVie (Inst), Medivation (Inst), Endocyte (Inst), Exelixis (Inst), Clovis Oncology (Inst), POINT Biopharma (Inst), Ambrx (Inst), Clarity Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Patent Royalty from Immunomedics/Gilead
Travel, Accommodations, Expenses: Sanofi, Immunomedics, Amgen
Uncompensated Relationships: ATLAB Pharma, Phosplatin Therapeutics, Ambrx
Yohann Loriot
Consulting or Advisory Role: Janssen, Janssen (Inst), Astellas Pharma, Roche, AstraZeneca, MSD Oncology, MSD Oncology (Inst), Seagen, Bristol Myers Squibb, Immunomedics, Taiho Pharmaceutical, Loxo/Lilly, Pfizer/EMD Serono
Research Funding: Janssen Oncology (Inst), MSD Oncology (Inst), AstraZeneca (Inst), Exelixis (Inst), Incyte (Inst), Pfizer (Inst), Nektar (Inst), Sanofi (Inst), Seagen (Inst), Astellas Pharma (Inst), Gilead Sciences (Inst), Merck KGaA (Inst), Taiho Pharmaceutical (Inst), Basilea (Inst), BMS (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology, Roche, MSD Oncology, AstraZeneca, Seagen
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, February 16-18, 2023.
SUPPORT
Supported by Gilead Sciences, Inc.
CLINICAL TRIAL INFORMATION
NCT03547973 (TROPHY U-01)
DATA SHARING STATEMENT
Gilead Sciences shares anonymized individual patient data on request or as required by law or regulation with qualified external researchers on the basis of submitted curriculum vitae and reflecting no conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science's discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.
AUTHOR CONTRIBUTIONS
Conception and design: Mitch Sierecki
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: Mitch Sierecki, Julia Tonelli, Huafeng Zhou
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Sacituzumab Govitecan in Combination With Pembrolizumab for Patients With Metastatic Urothelial Cancer That Progressed After Platinum-Based Chemotherapy: TROPHY-U-01 Cohort 3
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Petros Grivas
Consulting or Advisory Role: Merck, Bristol Myers Squibb, AstraZeneca, EMD Serono, Seagen, Pfizer, Janssen, Roche, Dyania Health, 4D Pharma, Astellas Pharma, Guardant Health, Urogen Pharma, Gilead Sciences, Silverback Therapeutics, BostonGene, Fresenius Kabi, Lucence, PureTech, G1 Therapeutics, AADi, CG Oncology, Strata Oncology, ImmunityBio, Asieris Pharmaceuticals
Research Funding: Pfizer (Inst), Clovis Oncology (Inst), Bavarian Nordic (Inst), Bristol Myers Squibb (Inst), Debiopharm Group (Inst), Merck (Inst), QED Therapeutics (Inst), GlaxoSmithKline (Inst), Mirati Therapeutics (Inst), EMD Serono (Inst), G1 Therapeutics (Inst), Gilead Sciences (Inst), Acrivon Therapeutics (Inst), ALX Oncology (Inst)
Damien Pouessel
Honoraria: Ipsen, Bristol Myers Squibb, AstraZeneca, Astellas Pharma, MSD Oncology, Pfizer/Astellas
Consulting or Advisory Role: Astellas Pharma, Pfizer, MSD Oncology, Bristol Myers Squibb/Medarex, Merck
Research Funding: Merck Sharp & Dohme (Inst), Roche (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Seagen (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Pfizer
Chandler H. Park
Consulting or Advisory Role: Bristol Myers Squibb/Celgene, Exelixis, Eisai, Gilead Sciences, Seagen, Merck
Speakers' Bureau: Eisai, Seagen, Gilead Sciences, Pfizer, AstraZeneca, Merck
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb/Celgene (Inst), Eisai (Inst), Exelixis (Inst), Gilead Sciences (Inst), Merck (Inst), SeaGen (Inst), Roche
Philippe Barthelemy
Honoraria: BMS, MSD, Astellas Pharma, Janssen-Cilag, Pfizer, Merck KGaA, Novartis, Seagen, Ipsen, Gilead Sciences, Bayer
Consulting or Advisory Role: Ipsen, BMS, MSD Oncology, Pfizer, Janssen-Cilag, AstraZeneca, Amgen, Merck KGaA, Eisai, Gilead Sciences, Bayer, AAA/Endocyte/Novartis
Research Funding: Ipsen (Inst)
Travel, Accommodations, Expenses: BMS, Pfizer, Janssen-Cilag, MSD, Ipsen, Merck/Pfizer
Manojkumar Bupathi
Honoraria: Bristol Myers Squibb, Exelixis, AstraZeneca, Pfizer, Astellas Pharma, Myovant Sciences, Bayer, Agendia, Janssen
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Exelixis
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Pfizer, Exelixis, Astellas Pharma, Janssen Oncology
Daniel P. Petrylak
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Lilly, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Incyte, Janssen, Pharmacyclics, Seagen, Urogen Pharma, Advanced Accelerator Applications, Ipsen, Bicycle Therapeutics, Mirati Therapeutics, Monopteros Therapeutics, Regeneron, Gilead Sciences
Research Funding: Progenics (Inst), Sanofi (Inst), Endocyte (Inst), Genentech (Inst), Merck (Inst), Astellas Medivation (Inst), Novartis (Inst), AstraZeneca (Inst), Bayer (Inst), Lilly (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Pfizer (Inst), Roche (Inst), Seagen (Inst), Clovis Oncology (Inst), Bristol Myers Squibb (Inst), Advanced Accelerator Applications (Inst), Agensys (Inst), BioXCel Therapeutics (Inst), Eisai (Inst), Mirati Therapeutics (Inst), Replimune (Inst), Medivation (Inst), Gilead Sciences (Inst)
Expert Testimony: Celgene, Sanofi
Neeraj Agarwal
Research Funding: Bayer (Inst), Bristol Myers Squibb (Inst), Takeda (Inst), Pfizer (Inst), Exelixis (Inst), Amgen (Inst), AstraZeneca (Inst), Calithera Biosciences (Inst), Celldex (Inst), Eisai (Inst), Genentech (Inst), Immunomedics (Inst), Janssen (Inst), Merck (Inst), Lilly (Inst), Nektar (Inst), ORIC Pharmaceuticals (Inst), CRISPR Therapeutics (Inst), Arvinas (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Pfizer, Exelixis
Sumati Gupta
Stock and Other Ownership Interests: Salarius Pharmaceuticals
Research Funding: Mirati Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), Viralytics (Inst), Hoosier Cancer Research Network (Inst), Rexahn Pharmaceuticals (Inst), Five Prime Therapeutics (Inst), Incyte (Inst), MedImmune (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Clovis Oncology (Inst), LSK BioPharma (Inst), QED Therapeutics (Inst), Daiichi Sankyo/Lilly (Inst), Immunocore (Inst), Seagen (Inst), Astra Zeneca (Inst), Acrotech Biopharma (Inst)
Travel, Accommodations, Expenses: QED Therapeutics
Uncompensated Relationships: Astellas Pharma
Aude Fléchon
Honoraria: MSD Oncology, AstraZeneca, BMS, Janssen-Cilag, Astellas Pharma, Pfizer, Sanofi/Aventis, Roche/Genentech, Bayer, Ipsen, AAA HealthCare, Novartis, Gilead Sciences
Travel, Accommodations, Expenses: Astellas Pharma, Sanofi/Aventis, Janssen-Cilag, Bayer, Pfizer, Ipsen, BMS, AstraZeneca, MSD Oncology, Roche/Genentech, AAA HealthCare
Chethan Ramamurthy
Employment: Merck
Stock and Other Ownership Interests: Merck
Honoraria: Gilead Sciences
Consulting or Advisory Role: SeaGen, Exelixis
Research Funding: Dispersol (Inst), Novartis (Inst), SeaGen (Inst), Gilead Sciences (Inst), Mirati Therapeutics (Inst), Nuvation Bio (Inst)
Nancy B. Davis
Employment: Merck, Sharp & Dohme, Inc
Consulting or Advisory Role: Janssen Biotech
Research Funding: AstraZeneca (Inst), Roche (Inst), Pfizer (Inst), Merck (Inst), Incyte (Inst), Mirati Therapeutics (Inst), Seattle Genetics/Astellas (Inst), Calithera Biosciences (Inst), Immunomedics (Inst), Bristol Myers Squibb (Inst), Exelixis (Inst), Gilead Sciences (Inst)
Alejandro Recio-Boiles
Honoraria: Janssen
Cora N. Sternberg
Consulting or Advisory Role: Bayer, MSD, Pfizer, Roche, Incyte, AstraZeneca, Merck, Medscape, UroToday, Astellas Pharma, Genzyme, Immunomedics, Foundation Medicine, Bristol Myers Squibb/Medarex, IMPAC Medical Systems, Amgen, Gilead Sciences, Janssen Oncology
Astha Bhatia
Employment: Zentalis, Bayer
Stock and Other Ownership Interests: Zentalis
Cabilia Pichardo
Employment: Novocure
Stock and Other Ownership Interests: Novocure
Consulting or Advisory Role: None
Expert Testimony: None
Mitch Sierecki
Employment: Gilead Sciences
Leadership: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences, Bayer HealthCare Pharmacuticals
Travel, Accommodations, Expenses: Gilead Sciences
Julia Tonelli
Employment: Gilead Sciences
Stock and Other Ownership Interests: Myovant Sciences, Gilead Sciences
Huafeng Zhou
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Scott T. Tagawa
Stock and Other Ownership Interests: Convergent Therapeutics
Consulting or Advisory Role: Medivation, Astellas Pharma, Dendreon, Janssen, Genentech, Endocyte, Immunomedics, Karyopharm Therapeutics, AbbVie, Tolmar, QED Therapeutics, Amgen, Sanofi, Pfizer, Clovis Oncology, Novartis, Genomic Health, POINT Biopharma, Blue Earth Diagnostics, Seagen, AIkido Pharma, 4D Pharma, Clarity Pharmaceuticals, Gilead Sciences, Telix Pharmaceuticals, Bayer, Myovant Sciences, Convergent Therapeutics, Hookipa Pharma, Merck, Daiichi Sankyo, Regeneron, TransThera Biosciences, Bicycle Therapeutics
Research Funding: Lilly (Inst), Sanofi (Inst), Janssen (Inst), Astellas Pharma (Inst), Progenics (Inst), Millennium (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Dendreon (Inst), Rexahn Pharmaceuticals (Inst), Bayer (Inst), Genentech (Inst), Newlink Genetics (Inst), Inovio Pharmaceuticals (Inst), AstraZeneca (Inst), Immunomedics (Inst), Novartis (Inst), AVEO (Inst), Boehringer Ingelheim (Inst), Merck (Inst), Stem CentRx (Inst), Karyopharm Therapeutics (Inst), AbbVie (Inst), Medivation (Inst), Endocyte (Inst), Exelixis (Inst), Clovis Oncology (Inst), POINT Biopharma (Inst), Ambrx (Inst), Clarity Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Patent Royalty from Immunomedics/Gilead
Travel, Accommodations, Expenses: Sanofi, Immunomedics, Amgen
Uncompensated Relationships: ATLAB Pharma, Phosplatin Therapeutics, Ambrx
Yohann Loriot
Consulting or Advisory Role: Janssen, Janssen (Inst), Astellas Pharma, Roche, AstraZeneca, MSD Oncology, MSD Oncology (Inst), Seagen, Bristol Myers Squibb, Immunomedics, Taiho Pharmaceutical, Loxo/Lilly, Pfizer/EMD Serono
Research Funding: Janssen Oncology (Inst), MSD Oncology (Inst), AstraZeneca (Inst), Exelixis (Inst), Incyte (Inst), Pfizer (Inst), Nektar (Inst), Sanofi (Inst), Seagen (Inst), Astellas Pharma (Inst), Gilead Sciences (Inst), Merck KGaA (Inst), Taiho Pharmaceutical (Inst), Basilea (Inst), BMS (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology, Roche, MSD Oncology, AstraZeneca, Seagen
No other potential conflicts of interest were reported.
REFERENCES
- 1. Saginala K, Barsouk A, Aluru JS, et al. Epidemiology of bladder cancer. Med Sci. 2020;8:15. doi: 10.3390/medsci8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powles T, Bellmunt J, Comperat E, et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:244–258. doi: 10.1016/j.annonc.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 3. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Bladder Cancer V.3.2022. https://NCCN.org.
- 4. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 5. Powles T, Park SH, Voog E, et al. Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (UC): Long-term follow-up results from the JAVELIN Bladder 100 trial. J Clin Oncol. 2022;40 doi: 10.1200/JCO.22.01792. suppl 6; abstr 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. EMD Serono and Pfizer Inc: BAVENCIO (avelumab) injection, for intravenous use [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761049s009lbl.pdf.
- 7. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Merck, Inc: KEYTRUDA (pembrolizumab) injection for intravenous use [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf.
- 9. Bristol-Myers Squibb Company: OPDIVO (nivolumab) injection for intravenous use [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125554s058lbl.pdf.
- 10. Janssen Pharmaceutical Companies: BALVERSA (erdafitinib) tablets, for oral use [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212018s000lbl.pdf.
- 11. Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381:338–348. doi: 10.1056/NEJMoa1817323. [DOI] [PubMed] [Google Scholar]
- 12. Siefker-Radtke AO, Necchi A, Park SH, et al. Efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma: Long-term follow-up of a phase 2 study. Lancet Oncol. 2022;23:248–258. doi: 10.1016/S1470-2045(21)00660-4. [DOI] [PubMed] [Google Scholar]
- 13. Rhea LP, Mendez-Marti S, Kim D, et al. Role of immunotherapy in bladder cancer. Cancer Treat Res Commun. 2021;26:100296. doi: 10.1016/j.ctarc.2020.100296. [DOI] [PubMed] [Google Scholar]
- 14. Astellas Pharma US, Inc and Seagen Inc: PADCEV (enfortumab vedotin-ejfv) for injection, for intravenous use [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761137s000lbl.pdf.
- 15. Gilead, Inc: TRODELVY (sacituzumab govitecan-hziy) for injection, for intravenous use [prescribing information]. https://www.gilead.com/-/media/files/pdfs/medicines/oncology/trodelvy/trodelvy_pi.pdf.
- 16. Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384:1125–1135. doi: 10.1056/NEJMoa2035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldenberg DM, Cardillo TM, Govindan SV, et al. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC) Oncotarget. 2015;6:22496–22512. doi: 10.18632/oncotarget.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Avellini C, Licini C, Lazzarini R, et al. The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget. 2017;8:58642–58653. doi: 10.18632/oncotarget.17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: A phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39:2474–2485. doi: 10.1200/JCO.20.03489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cardillo TM, Govindan SV, Sharkey RM, et al. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: Preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res. 2011;17:3157–3169. doi: 10.1158/1078-0432.CCR-10-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cardillo TM, Govindan SV, Sharkey RM, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2/SN-38 antibody-drug conjugate: Characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem. 2015;26:919–931. doi: 10.1021/acs.bioconjchem.5b00223. [DOI] [PubMed] [Google Scholar]
- 22. Starodub AN, Ocean AJ, Shah MA, et al. First-in-human trial of a novel anti-Trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res. 2015;21:3870–3878. doi: 10.1158/1078-0432.CCR-14-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ocean AJ, Starodub AN, Bardia A, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: Safety and pharmacokinetics. Cancer. 2017;123:3843–3854. doi: 10.1002/cncr.30789. [DOI] [PubMed] [Google Scholar]
- 24. Iwai T, Sugimoto M, Wakita D, et al. Topoisomerase I inhibitor, irinotecan, depletes regulatory T cells and up-regulates MHC class I and PD-L1 expression, resulting in a supra-additive antitumor effect when combined with anti-PD-L1 antibodies. Oncotarget. 2018;9:31411–31421. doi: 10.18632/oncotarget.25830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19:51–64. doi: 10.1016/S1470-2045(17)30900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 27. Apolo AB, Ellerton JA, Infante JR, et al. Avelumab as second-line therapy for metastatic, platinum-treated urothelial carcinoma in the phase Ib JAVELIN Solid Tumor study: 2-year updated efficacy and safety analysis. J Immunother Cancer. 2020;8:e001246. doi: 10.1136/jitc-2020-001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Talukder R, Makrakis D, Lin GI, et al. Association of the time to immune checkpoint inhibitor (ICI) initiation and outcomes with second line ICI in patients with advanced urothelial carcinoma. Clin Genitourin Cancer. 2022;20:558–567. doi: 10.1016/j.clgc.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Makrakis D, Talukder R, Lin GI, et al. Association between sites of metastasis and outcomes with immune checkpoint inhibitors in advanced urothelial carcinoma. Clin Genitourin Cancer. 2022;20:e440–e452. doi: 10.1016/j.clgc.2022.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sheng X, He Z, Shi Y, et al. RC48-ADC for metastatic urothelial carcinoma with HER2-positive: Combined analysis of RC48-C005 and RC48-C009 trials. J Clin Oncol. 2022;40 suppl 16; abstr 4520. [Google Scholar]
- 31. Galsky MD, Del Conte G, Foti S, et al. Primary analysis from DS8201-A-U105: A phase 1b, two-part, open-label study of trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing urothelial carcinoma (UC) J Clin Oncol. 2022;40 suppl 6; abstr 438. [Google Scholar]
- 32. Hoimes CJ, Flaig TW, Milowsky MI, et al. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J Clin Oncol. 2023;41:22–31. doi: 10.1200/JCO.22.01643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosenberg JE, Milowsky M, Ramamurthy C, et al. LBA73 Study EV-103 Cohort K: Antitumor activity of enfortumab vedotin (EV) monotherapy or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (la/mUC) Ann Oncol. 2022;33:S1441. [Google Scholar]
- 34. Lin N, Zhang M, Bai H, et al. Efficacy and safety of GLS-010 (zimberelimab) in patients with relapsed or refractory classical Hodgkin lymphoma: A multicenter, single-arm, phase II study. Eur J Cancer. 2022;164:117–126. doi: 10.1016/j.ejca.2021.07.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Gilead Sciences shares anonymized individual patient data on request or as required by law or regulation with qualified external researchers on the basis of submitted curriculum vitae and reflecting no conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science's discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.