Abstract
We propose a two-mode (pursuit/maintenance) model of metabolism defined by usable resource availability. Pursuit, consisting of anabolism and catabolism, dominates when usable resources are plentiful and leads to the generation of metabolic waste. In turn, maintenance of a system is activated by elevated metabolic waste during resource depletion. Interaction with the environment results in pendulum-like swings between these metabolic states in thriveless attempts to maintain the least deleterious organismal state - ephemeral homeostasis. Imperfectness of biological processes during these attempts supports the accumulation of the deleteriome, driving organismal aging. We discuss how metabolic adjustment by the environment and resource stabilization may modulate healthspan and lifespan
Keywords: Aging, Metabolism, Homeostasis, Maintenance, Pursuit
Introduction
Metabolism is the term used to describe the sum of chemical reactions within biological systems to maintain them responsive to environmental cues over time, i.e., to be alive [1]. Traditionally, metabolism is divided into catabolism and anabolism. Catabolism encompasses the processes of breaking down organic matter to obtain energy, and anabolism supports the synthesis of organic matter utilizing energy-carrier molecules [2, 3]. Catabolism and anabolism are usually viewed as opposing processes despite many similarities between them. Most importantly, catabolism and anabolism require usable resources, such as ATP, amino acids, proteins, glucose, polysaccharides. When food is ingested, catabolic reactions break macromolecules into smaller components, which can be further used as building blocks or as a source of energy. Anabolism can further exploit energy and building blocks to construct necessary cellular compartments, proteins (including those participating in catabolic processes), nucleic acids, receptors and organelles. Therefore, anabolism and catabolism represent organismal co-dependent resource generation programs, which maintain the necessary ratio between energy carriers (ATP, NADH, NADPH), building blocks (glucose, amino acids, fatty acids) and complex cellular structures (organelles, cell compartments, proteins, nucleic acids) within the system, ensuring its optimal functionality.
Lack of usable resources supports the metabolic state of organismal maintenance
Contrary to anabolism and catabolism, maintenance (see Glossary) of an organism happens when usable resources are scarce and, therefore, when waste products are abundant as a result of metabolizing usable resources. All forms of metabolic waste, such as adenosine, lactate, CO2, urea, and ammonia, exhibit concentration-dependent toxicity, and their elimination from the organism by degradation, conversion or excretion is critical to stay healthy and alive. Metabolic waste is typically represented by small molecules that could engage in numerous unwanted reactions due to their relatively simple structure and small size, but also more complex molecules [4]. Unwanted reactions increase entropy inside the cell, leading to an accelerated accumulation of damage and enhanced system deterioration [4, 5]. Continued presence of waste in an organism, or even its presence before it is completely removed, can inflict damage and further activate the maintenance state. Maintenance includes but is not limited to repair of misfolded proteins, degradation or extrusion of molecular waste, DNA repair, DNA and RNA proofreading mechanisms, chaperones, and autophagy, which support cellular well-being and reduce the burden of damage.
One of the best examples of the induction of metabolic waste-dependent maintenance is sleep. Prolonged organismal metabolic activity during the awake period results in the depletion of glycogen, ATP, ADP, and eventually AMP levels, but adenosine, an important CNS inhibitory neurotransmitter, gradually accumulates in the basal forebrain, inducing the sleep state [6–9]. Importantly, at low concentrations, adenosine may be protective, but higher adenosine concentrations present for a longer time could lead to multiple forms of cellular, tissue, and system damage [10–14]. Further, adenosine receptor activation by adenosine or adenosine agonists increases the permeability of the blood-brain barrier, thereby promoting macromolecule entry, which increases the chances of brain damage [15]. Natural accumulation of adenosine in the brain, as well as injection of adenosine or an adenosine agonist directly into an animal brain, promotes sleep [9, 16, 17]. Sleep is a low metabolic activity state with a unique restorative ability to clear metabolic waste, such as misfolded proteins and adenosine, through the glymphatic system [18, 19]. At the same time, long sleep may lead to energy depletion - when metabolism reaches its lowest point during sleep so that it becomes dangerous and exhausting to sleep further, the organism wakes up and shifts into the active state [20, 21].
Another example is one of the highly conserved signaling systems in eukaryotic species, the AMPK pathway, which is activated by the lack of usable resources and presence of metabolic waste [22] (Box 1).
Box 1.
AMPK regulation by usable resource depletion
Since AMPK has its highest binding sensitivity to AMP, with ADP being about 10-fold lower, this heterotrimer reflects the cellular ATP:AMP ratio. In particular, the AMPK heterotrimer senses high AMP concentrations within the cell, with 3 AMP molecules needed to bind the γ subunit of AMPK to trigger downstream cascade activation, such as activation of pathways linked to ATP production [23]. Interestingly, both salicylate, which is a severely toxic compound at levels greater than 100 mg/dL, and A-769662, which is toxic for cells due to inhibition of proteasomal function likely via an AMPK-dependent mechanism, can directly activate AMPK, consistent with a possible role of AMP as a deleterious metabolic waste [24–28]. This is further supported by the fact that mitochondrial poisons, such as hydrogen peroxide, berberine, metformin, and resveratrol, as well as various types of stress, such as oxidative, nutritional, hypoxic, exercise and heat stresses, increase the AMP to ATP ratio, thereby indirectly activating AMPK [24, 29, 30].
AMPK activation is known for its pro-longevity potential. Experiments using Caenorhabditis elegans demonstrate that overexpression of the C. elegans AMPK α subunit AAK-2 extends lifespan by 13% and mimics dietary restriction in well-fed worms, whereas the AMP:ATP ratio can be used to predict animal life expectancy [31]. Mutation of aak-2 results in a 12% shorter lifespan, indicating a dose-dependent pro-longevity action of this gene. Another study showed a 37.5% lifespan extension of C. elegans overexpressing aak-2 by targeting CREB-regulated transcriptional coactivators [32]. Experiments in flies also show that overexpression of the AMPK subunit extends, while suppressing AMPK reduces lifespan [24]. Experiments with transgenic mice having a liver-specific inducible AMPK system showed that AMPK activation dramatically improves the outcome of diet-induced obesity by reprogramming lipid metabolism, decreasing inflammation and fibrosis [33]. Interaction of AMPK with “anti-aging” small molecules, such as metformin, resveratrol, and rapamycin, genetic and biochemical links of AMPK to other known longevity aging pathways, such as mTOR and sirtuins, information about the role of AMPK in processes that affect aging, such as autophagy, mitochondrial biogenesis, lipid metabolism, stem cells and rejuvenation, and finally the role of AMPK in various pathologies, such as cancer and neurodegeneration, all support the notion that AMPK is regulated by the lack of usable resources and possibly by the abundance of metabolic waste [34].
Another example of a usable resource-responsive conserved signaling system in eukaryotes is mTOR. It is one of key regulators of cell growth and metabolism, and its activity is mainly regulated by the presence of nutrients and growth factors [22].
In particular, mTORC1, a mechanistic target of rapamycin complex 1, is activated most efficiently in the presence of leucine, arginine, and glutamine. Active mTORC1 promotes rapid cell growth, ribosome biogenesis, transcription, protein translation, and cellular nutrient import, as well as inhibits organismal maintenance mechanisms, such as autophagy and several stress response mechanisms [22, 35]. Inhibition of mTORC1 can be achieved by AMPK activation, genetic manipulations or the use of rapamycin, leading to lifespan extension in yeast, nematodes, water flea, flies, and mice, together indicating that metabolism is shifted towards better maintenance [36–41]. DNA damage can also suppress mTOR, and further mTOR inhibition causes upregulation of OGG1, a DNA repair enzyme, and fails to extend lifespan in DNA repair-deficient mice, pointing to the ability of organisms to repair DNA better in the absence of mTOR activity [42–44].
The examples of sleep, AMPK, and TOR reveal the existence of two types of repair feedback loops in response to usable resource depletion and metabolic waste accumulation. The data suggest that a lack of resources or the presence of deleterious metabolic waste can both activate and stop inhibiting maintenance. Further examples of usable resource-mediated metabolic signaling systems are described in Box 2.
Box 2.
Systems activated by resource depletion or metabolic waste
Inhibition of insulin-like receptor activity, or, in other words, deficiency of insulin-like molecules in nematodes, flies, and mice, results in lifespan extension [45–47].
Hypoxia (deficiency of molecular oxygen) or hypercapnia (increased CO2 levels) inhibit hydroxylation of HIFα, which extends nematode lifespan in a concentration-dependent manner [48].
Deficiency of high-energy coenzyme NADH and an increase of its oxidized form NAD+ results in the activation of sirtuins, important modulators of health and lifespan [49]. Sirtuin 6 is a key protector of the genome from double-strand breaks due to its DNA repair-mediated lifespan extension capability [50–52].
Based on such usable resources/deleterious metabolic waste-mediated activities, we propose to categorize metabolism into pursuit and maintenance states. The pursuit state can be further classified into catabolism and anabolism, and maintenance can be further divided into damage clearance and damage pre-emption (Figure 1). The notion of dividing metabolism into maintenance and pursuit is supported by a quantitative model, describing growth as a process of metabolic effort allocation between the production of new biomass and maintenance of the existing tissue [53]. It should be noted that catabolism, anabolism, damage clearance and damage pre-emption could further be dissected into specific, highly accurate metabolic signatures, corresponding to a variety of environmental input spectrum shades that would require the organism to adapt to.
Figure 1.

Metabolism exists in two major states: pursuit and maintenance. The shift between these states is mediated by the ratio between usable resources and deleterious metabolic waste. The pursuit state consists of catabolism and anabolism, which maintain the necessary ratios of simple (ATP, glucose, amino acids, etc.) and complex (cell compartments, proteins, nucleic acids, etc.) usable organismal resources. The maintenance state consists of damage clearance and damage pre-emption, which together ensure the preservation of healthy cellular status. It should be noted that maintenance mechanisms are non-spontaneous, require energy and maintenance agents, which are produced during the pursuit state of metabolism.
According to the principles described above, a shift between pursuit and maintenance states would happen in a concentration threshold-dependent manner [54]. Briefly, for AMPK to stay active and promote repair during the maintenance state, the probability of interaction with AMP compared to ATP should be above a particular threshold value, representing a probability of a certain number of AMPK receptors being simultaneously activated in a system.
The purpose of metabolism is to increase and maintain organismal fitness, which is challenging as biological systems constantly experience myriad interactions with the aggressive environment that generate and inflict damage to an organism, thereby forcing it to react. Since the state of constant maintenance would result in the death of an organism from the lack of energy and other resources, and the pursuit of resources without repair would result in an overaccelerated accumulation of damage, the best way to interact with an environment appears to be having the ability to effectively maintain homeostasis.
Relationship between metabolic homeostasis and aging
Maintaining homeostasis is a process of regulating biological system variables in a way that the internal environment will remain relatively stable in response to changes in the external environment [55, 56]. For example, mammals’ blood parameters, such as pH, concentration of dissolved gases and macromolecule levels, are kept within a reasonably constant range. One of key milestones in the current understanding of metabolism was the introduction of the concept of homeodynamics as an alternative to homeostasis [57, 58]. It was proposed that homeostasis fails to account for development, reproduction, and aging, and therefore the term homeodynamics attempted to describe the ability of organisms to survive in a constantly changing environment [59]. Further, the term homeodynamic space was introduced to describe the ability of living systems to react to stress, repair and remove damaged parts of the system, and undergo remodeling and adaptation. A progressive shrinkage of homeodynamic space due to the accumulation of damage was proposed to define the aging process [60].
Although logical and well-conceived, the homeodynamic model overlooks the discreteness principle, wherein every dynamic process consists of fixed states [54, 61]. Instead, we propose that homeostasis is a fixed point of a threshold between the coherent, i.e., synchronized in space and time, pursuit and maintenance metabolic states, and is achievable for relatively short discrete periods of time when an organism transforms from one state into another. Most of time, an organism exists in one of the metabolic states and attempts to reach homeostasis. However, due to imperfectness of chemical interactions in biological systems and additional imperfectness within cells caused by the propensity of metabolic waste molecules to engage in unwanted reactions, attempts to reach homeostasis generate and accumulate molecular damage and other deleterious consequences of metabolism cumulatively described as the deleteriome [62]. Eventually, metabolism leads to chronic accumulation of damage and progressive decline in fitness (Figure 2).
Figure 2. Integration of the concept of homeostasis and the deleteriome model of aging.

Swings of metabolism in an attempt to achieve homeostasis cause accumulation of deleterious consequences for an organism in the form of the deleteriome. Accumulation of the deleteriome, in turn, causes organismal aging.
Conventional thinking about metabolism is that the accumulation of damage during aging causes homeodynamic impairment, suggesting a causal role of the environment and the resultant role of metabolism [63], whereas we stress that the organismal unceasing attempts to reach homeostasis generate damage and are among key causal contributors to organismal aging. This view further implies that the accumulation of damage per metabolic activity caused by system perturbation accelerates in older organisms because an old organism with a larger deleteriome and, therefore, severely damaged systems, will generate more damage while trying to reach homeostasis than a young, lightly damaged one. At some point in life, the level of damage becomes so high that the organism needs to compensate for the pace of damage accumulation by decreasing its metabolic rate [64].
The role of sensors in metabolism
In order to successfully maintain homeostasis, biological systems should have the capability to effectively sense internal conditions. We further extend our model wherein living organisms shift between two global sensory states, comfort and discomfort (Box 3), which represent sensing pursuit and maintenance states (Figure 3).
Box 3.
Comfort and discomfort global sensory states
A feeling of comfort is mediated by satiety, comfortable temperature, optimal oxygen levels, and being well-rested. Comfort represents sufficient energy and resources, but excessive comfort from overconsumption of resources is predicted to result in severe damage accumulation, aging acceleration, and consequent development of age-related diseases, such as cancer, diabetes, and heart disease. Interestingly, the notion that the unrestrained pursuit type of metabolism, mediated by excessive resources and represented by overactive anabolism or catabolism, leads to cancer development, is well-supported by data [65]. Moreover, alterations of cancer metabolism include activation of oncogenes and suppression of tumor-suppressor genes. This happens because the metabolism state swung beyond the comfort/pursuit threshold into a disease section and no longer has a maintenance state of homeostasis active (follow arrows in Figure 3 from the discomfort and maintenance point to disease).
A feeling of discomfort may be caused by uncomfortable temperature, hunger, thirst, hypoxia, tiredness, and other types of stress. Mild stress, or hormesis, may have a positive effect on health and longevity [66–73] as the model suggests that when stress generates damage, which is severe enough to be sensed by an organism, it will shift organismal state into maintenance. Further, in the maintenance state, previously ïnvisiblë damage that was too mild to generate the shift alone is eliminated too. For example, short-term water deficiency indirectly temporarily increases metabolic waste concentration, stimulates maintenance activation, and extends the lifespan of C. elegans [74].
Therefore, discomfort caused by stress activates maintenance mechanisms, but excessive stress severely damages the system directly and may lead to exhaustion. It is supported by recent data showing that severe stress (discomfort) rapidly increases biological age in mice and humans, which is followed by age reversal after stress relief [75].
Figure 3. The Sensory Pendulum Model of Metabolism integrated with the Deleteriome Model of Aging.
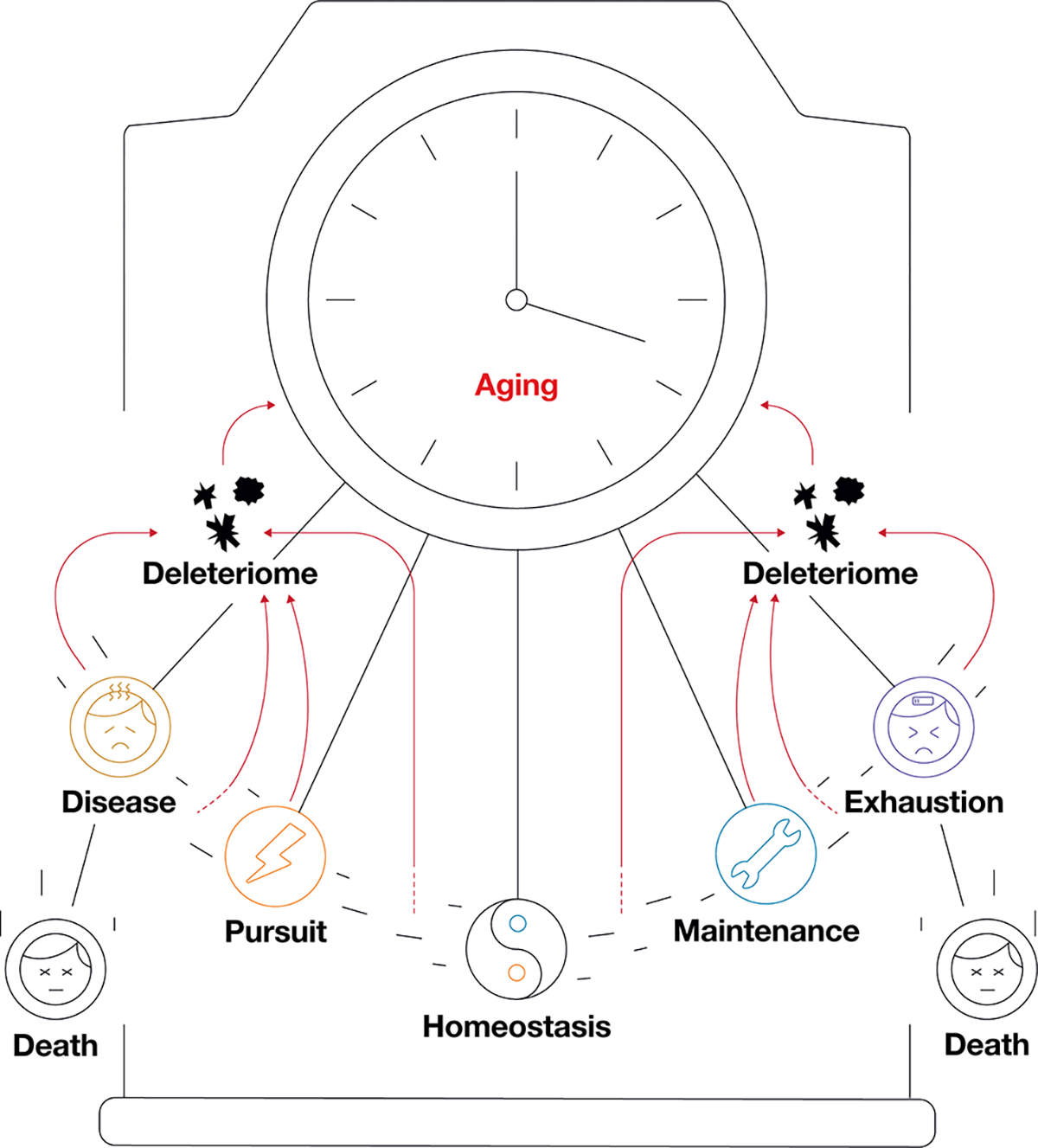
A greater distance of the pendulum from the point of homeostasis results in an increased deleteriome accumulation. In a biological system, unrestrained pursuit results in disease development, whereas excessive maintenance leads to resource exhaustion. Further progression of disease or exhaustion results in the death of an organism.
Feelings of comfort and discomfort are determined by sensors that assess the availability of usable resources or sense the signals from metabolic waste. Depending on the ratio between those types of compounds, sensors initiate shifts between metabolic states to maintain homeostasis. Examples of such sensors are glucokinase in the beta cells of the pancreas (glucose sensors), osmoreceptors of the hypothalamus (fluid balance sensors), oxygen level sensors in carotid and aortic bodies, AMPK (AMP sensor), mTORC1 (amino acid sensor), IGF-1 (insulin-like growth factor sensor), sirtuins (NAD+ sensors), PARP1 (DNA damage sensor) and many others.
A shift of the balance between usable resources and deleterious metabolic waste results in active metabolic processes -- attempts of a system to return to homeostasis resembling a pendulum. Due to the imperfectness of these processes, deleterious changes are generated and accumulated [62]. The equilibrium position of the pendulum indicates homeostasis, and the pursuit of homeostasis from both directions results in damage accumulation and aging. An excessive movement in both directions away from homeostasis is increasingly deleterious for the system. The pendulum position of a particular intraorganismal system can be detected by measuring the corresponding biomarkers. For example, simultaneously measuring glucose and insulin levels in a young, healthy organism could help determine the pendulum architecture for the blood glucose system of that age. The homeostatic equilibrium would be represented by the point when insulin stops increasing and glucose stops decreasing, right before both trends reverse.
Application and predictions using the Sensory Pendulum Model
Homeostasis could be achieved and maintained for a longer time when interaction with the environment causes minimal or no net metabolic response and, therefore, relatively negligible accumulation of the deleteriome. Hydra is an example of an organism with excellent fitness and longevity under close-to-perfect conditions - it does not seem to age or lose reproductive ability in a lab setting and dies very infrequently and not in an age-dependent manner [76]. However, finding the best environmental conditions for more complex organisms is challenging and requires a systematic approach. The data on C. elegans indicate that lower temperatures could extend lifespan under certain conditions at least in part due to alterations of metabolism, such as activation of autophagy, TRPA-1 mediated DAF-16/FOXO signaling, and increased antioxidant levels, rather than exclusively due to slow-down of the metabolic rate as previously thought [77–80]. In addition, experiments using mice and hamsters demonstrate a critical causal role of body temperature, but not of a metabolic rate in modulating aging [81, 82]. Body temperature in warm-blooded animals depends on the environmental temperature in the sense that metabolism of a whole system needs to be remodeled in accordance with the need to spend a certain amount of resources to warm or cool an organism to stabilize the body temperature. Multiple studies show a striking difference between metabolism of mice grown in standard, or thermoneutral conditions, suggesting a possibility of developing interventions for increased healthspan and longevity by modulating the temperature [83]. Mice at a thermoneutral temperature exhibit lower blood pressure, heart rate, food intake, oxygen consumption, greater survival following infection, healthier response to inflammation, improved efficiency of chemotherapeutics, and reduced tumor growth due to the better immune response. Naked mole rats (NMRs) are exceptionally long-lived rodents, which show increased resistance to cancer, cardiovascular disease progression and hypoxia, display no signs of reproductive aging, and do not exhibit an age-related increase in mortality [84–86]. One of the NMRś important characteristics is their inability to maintain a stable body temperature in a cold environment via thermogenic metabolism [87, 88]. This feature has evolutionary roots, as NMRs live strictly in the subterranean niche and are mainly exposed to temperature fluctuations within their thermoneutral zone, which removes the necessity to invest metabolic efforts into body temperature maintenance [87, 89, 90]. When NMRs are placed into a colder environment, their primary response to maintain homeothermy is behavioral, such as an increase in activity, huddling in the nest, stimulation of muscle activity, relocation with the burrow, etc. [87, 89, 90]. Interestingly, hypoxia at colder temperatures decreases thermogenesis attempts of NMRs, presumably due to the need to put metabolic efforts into oxygen conservation [86]. According to the Sensory Pendulum Model, NMRs live close to the point of homeostasis for the part of metabolism responsible for body temperature maintenance. This eliminates a vast source of damage to the system and may stabilize other systems by putting available metabolic efforts into them. However, the contribution of the temperature to the exceptional longevity and stress resistance of NMR is currently unknown.
Although it may be impossible to achieve a perfect environment, a close-to-ideal environment seems feasible. At least, finding an optimal temperature, oxygen, food, and water availability is possible by subjecting animals to a range of these conditions followed by the measurements of damage accumulation. Combining the data from all optimal conditions may offer information about the limits of maximum lifespan extension for a specific organism.
Importantly, biological systems require a systematic approach, and identifying an optimal atmospheric oxygen:carbon dioxide ratio is suggested to be the first step in finding an ideal environment. Oxygen metabolism is one of the fastest types of resource pursuit; therefore, biological systems would try to maintain oxygen homeostasis by doing respective maintenance most frequently. Presumably, such frequent use will result in a faster destruction of the system, leading to oxygen imbalance, but it should be noted that it is not the oxidative damage itself, but the redox regulatory metabolism wearout due to the imperfect frequent use and the consequent inability to maintain redox homeostasis that are causal to the accumulation of damage.
After finding the least deleterious atmospheric oxygen:carbon dioxide ratio, the most optimal temperature in the ideal gas environment may be the next target. Eventually, finding all the environmental conditions that result in fewer metabolic swings and, consequently, more frequent passes through homeostasis points could potentially significantly adjust health and lifespan across different species, including humans.
Environmental conditions include the availability of usable resources and the presence of deleterious metabolic waste in different ratios. According to the model, the optimal resource level is a balance between comfort and discomfort in global sensory states so that there is a minimal net signal to the given group of sensors. Identification and maintenance of the close-to-perfect internal biomarker concentration leading to the reduction of pendulum swing amplitude could provide valuable insights into the influence of particular biomarkers on longevity. Interestingly, the idea that the immune system consists of various immune cell types, each representing a biomarker that needs to be in a certain homeostatic ratio, was described earlier [91].
Pendulums tend to synchronize in a coherent manner as described previously [58], so maintaining optimal levels of some biomarkers could help optimize the levels of the other ones. Therefore, identifying and synchronizing multiple biomarkers’ homeostasis ranges could be an attractive strategy to improve health and longevity across species. The importance of organismal biological age consideration during analysis of biomarker homeostasis is discussed in Box 4.
Box 4.
The pendulum architecture changes during aging
Importantly, the pendulum architecture is predicted to be dissimilar for organisms at different ages. At an advanced age, the pendulum is less resistant and consecutively overreactive to the environment, causing increased vulnerability to get into the disease or exhaustion state, which could result in sudden death. Additionally, a pendulum rope loses elasticity, and an organism is not as readily returned to the point of homeostasis as it was able to do it when younger. Therefore, older organisms remain in the non-homeostatic state longer, crossing the homeostatic point rarer than before, altogether suffering from enhanced damage generation and accumulation.
On the other hand, an organism during development actively seeks resources to build itself. Scarcity of resources or stress and consequently enhanced maintenance during development might negatively affect organismal fitness, although lifespan could be extended [40, 92]. Therefore, the model predicts that relative to the organism in adulthood, the homeostasis for the metabolism of developing animals will be shifted toward the pursuit state while in older animals toward the maintenance state (Figure I).
The Sensory Pendulum Model predicts that weakening of a signal from the sensor could have a pro-longevity effect on the system since a homeostatic disbalance would take longer to be sensed. As a result, there would be a weaker metabolic response during stress, resulting in an attenuated accumulation of damage. Therefore, an organism could live longer, although not necessarily healthier life, due to the possibility of missing severe external damage from the environment. At the same time, coupling signal attenuation with organismal life in a protected environment could rescue the animal from this type of damage. Importantly, the complete removal of a sensor could have a detrimental effect on health, especially during development, due to an impaired reaction to critical environmental stimuli.
Lastly, identifying deleterious metabolic waste molecules and tracking their interaction could reveal previously unknown mechanisms of organismal maintenance and repair. Importantly, some of the metabolic waste could be recycled in anabolic pathways but could still have additional distinct signaling leading to maintenance pathway activation, which can potentially be utilized in order to design new health and longevity interventions.
Concluding remarks
An accurate understanding of metabolism is critical for further deconvolution of how biological systems function and for the consequent effective design of longevity interventions. Here, we introduced the Sensory Pendulum Model of Metabolism, discussed evidence in support of the model, and integrated it with the Deleteriome Model of Aging. Finally, we discussed practical applications of the model that can be used to adjust healthspan and lifespan across species and outlined outstanding questions that remain to be answered (see the Outstanding questions section).
Outstanding questions.
Can lowering metabolic burden through “neutralizing” environment extend organismal lifespan?
How precisely can we measure the point of homeostasis for organisms?
Will mammals at thermoneutral temperatures live significantly longer?
What is the contribution of temperature to the extreme longevity of naked mole rats?
What are the least deleterious temperature and atmospheric oxygen concentration for mice and humans?
Can we track metabolic waste interactions within the organism to discover novel damage-sensing systems that could potentially be targeted to slow aging?
Figure I. Age-related changes in homeostasis.

During development, organisms require more usable resources to build itself, and therefore the point of homeostasis is shifted toward the pursuit type of metabolism. In contrast, older organisms require fewer resources, but more repair, therefore the least deleterious environment would be on the maintenance side of the pendulum.
Highlights.
Metabolism can be categorized by resource abundance or depletion.
Resource abundance drives the pursuit type of metabolism and consists of anabolism and catabolism.
Resource depletion, accompanied by metabolic waste accumulation as a result of pursuit, activates the maintenance type of metabolism and consists of damage pre-emption and clearance mechanisms.
The organismal sensory system mediates pendulum-like swings between pursuit and maintenance states in an attempt to sustain the least deleterious state - homeostasis.
Swings represent imperfect metabolic reactions and generate deleterious metabolic consequences.
Adjusting environmental signals close to neutral may lower the magnitude of metabolic swings, extend the periods near the homeostatic point and extend lifespan.
Analyzing pathways activated by metabolic waste could help identifying new interventions to slow aging.
Acknowledgements
We thank Albina Tskhay (McGill University) and Dr. Andrei Tarkhov (Brigham and Women’s Hospital, Harvard Medical School) for their comments and suggestions. We are grateful to Nikita Gavrilenko for help with figure design.
Funding
Supported by NIA and Impetus grants.
Glossary
- Comfort
organismal sensory state under conditions of usable resource abundance and metabolic waste scarcity
- Deleteriome
a set of deleterious age-related changes within a biological system
- Discomfort
global organismal sensory state under conditions of usable resource scarcity and metabolic waste abundance
- Maintenance
global organismal metabolic state of active damage pre-emption and clearance and basal pursuit
- Pursuit
global organismal metabolic state of active catabolism and anabolism, and basal maintenance
- Sensor
an organismal structure that can adjust metabolism in response to environmental changes
Footnotes
Conflict of Interest
The authors declare they have no conflicts of interest.
References
- [1].Metabolism Kornberg H.. Encyclopedia Britannica., https://www.britannica.com/science/metabolism (2021, accessed 17 March 2022). [Google Scholar]
- [2].Britannica TE of E Catabolism. Encyclopedia Britannica., https://www.britannica.com/science/catabolism (2019, accessed 17 March 2022). [Google Scholar]
- [3].Britannica TE of E Anabolism. Encyclopedia Britannica., https://www.britannica.com/science/anabolism (2019, accessed 17 March 2022). [Google Scholar]
- [4].Gladyshev VN. The Origin of Aging: Imperfectness-Driven Non-Random Damage Defines the Aging Process and Control of Lifespan. Trends Genet 2013; 29: 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hayflick L Entropy Explains Aging, Genetic Determinism Explains Longevity, and Undefined Terminology Explains Misunderstanding Both. PLOS Genet 2007; 3: e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nat 2005 4377063 2005; 437: 1257–1263. [DOI] [PubMed] [Google Scholar]
- [7].Kong J, Shepel PN, Holden CP, et al. Brain Glycogen Decreases with Increased Periods of Wakefulness: Implications for Homeostatic Drive to Sleep. J Neurosci 2002; 22: 5581–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Porkka-Heiskanen T, Strecker RE, Thakkar M, et al. Adenosine: A Mediator of the Sleep-Inducing Effects of Prolonged Wakefulness. Science (80- ) 1997; 276: 1265–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Strecker RE, Morairty S, Thakkar MM, et al. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res 2000; 115: 183–204. [DOI] [PubMed] [Google Scholar]
- [10].Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 2007 147 2007; 14: 1315–1323. [DOI] [PubMed] [Google Scholar]
- [11].Borea PA, Gessi S, Merighi S, et al. Pathological overproduction: the bad side of adenosine. Br J Pharmacol 2017; 174: 1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang Y, Xia Y. Adenosine signaling in normal and sickle erythrocytes and beyond. Microbes Infect 2012; 14: 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang Y, Dai Y, Wen J, et al. Detrimental effects of adenosine signaling in sickle cell disease. Nat Med 2010 171 2010; 17: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dai Y, Zhang W, Wen J, et al. A2B Adenosine Receptor–Mediated Induction of IL-6 Promotes CKD. J Am Soc Nephrol 2011; 22: 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bynoe MS, Viret C, Yan A, et al. Adenosine receptor signaling: a key to opening the blood–brain door. Fluids Barriers CNS 2015; 12: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Scammell TE, Gerashchenko DY, Mochizuki T, et al. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience 2001; 107: 653–663. [DOI] [PubMed] [Google Scholar]
- [17].Basheer R, Strecker RE, Thakkar MM, et al. Adenosine and sleep–wake regulation. Prog Neurobiol 2004; 73: 379–396. [DOI] [PubMed] [Google Scholar]
- [18].Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med; 4. Epub ahead of print 2012. DOI: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science (80- ) 2013; 342: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brebbia DR, Altshuler KZ. Oxygen Consumption Rate and Electroencephalographic Stage of Sleep. Science (80- ) 1965; 150: 1621–1623. [DOI] [PubMed] [Google Scholar]
- [21].Goldberg GR, Prentice AM, Davies HL, et al. Overnight and basal metabolic rates in men and women. Eur J Clin Nutr 1988; 42: 137–144. [PubMed] [Google Scholar]
- [22].González A, Hall MN, Lin SC, et al. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab 2020; 31: 472–492. [DOI] [PubMed] [Google Scholar]
- [23].Ross FA, Jensen TE, Hardie DG. Differential regulation by AMP and ADP of AMPK complexes containing different γ subunit isoforms. Biochem J 2016; 473: 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stenesen D, Suh JM, Seo J, et al. Adenosine Nucleotide Biosynthesis and AMPK Regulate Adult Life Span and Mediate the Longevity Benefit of Caloric Restriction in Flies. Cell Metab 2013; 17: 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Runde TJ, Nappe TM. Salicylates Toxicity. StatPearls, https://www.ncbi.nlm.nih.gov/books/NBK499879/ (2022, accessed 26 July 2022). [PubMed] [Google Scholar]
- [26].Ducommun S, Ford RJ, Bultot L, et al. Enhanced activation of cellular AMPK by dual-small molecule treatment: AICAR and A769662. Am J Physiol - Endocrinol Metab 2014; 306: E688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moreno D, Knecht E, Viollet B, et al. A769662, a novel activator of AMP-activated protein kinase, inhibits non-proteolytic components of the 26S proteasome by an AMPK-independent mechanism. FEBS Lett 2008; 582: 2650–2654. [DOI] [PubMed] [Google Scholar]
- [28].Viana R, Aguado C, Esteban I, et al. Role of AMP-activated protein kinase in autophagy and proteasome function. Biochem Biophys Res Commun 2008; 369: 964–968. [DOI] [PubMed] [Google Scholar]
- [29].Sanli T, Steinberg GR, Singh G, et al. AMP-activated protein kinase (AMPK) beyond metabolism. 10.4161/cbt26726 2013; 15: 156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Auciello FR, Ross FA, Ikematsu N, et al. Oxidative stress activates AMPK in cultured cells primarily by increasing cellular AMP and/or ADP. FEBS Lett 2014; 588: 3361–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Apfeld J, O’Connor G, McDonagh T, et al. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev 2004; 18: 3004–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mair W, Morantte I, Rodrigues APC, et al. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nat 2011 4707334 2011; 470: 404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Garcia D, Hellberg K, Chaix A, et al. Genetic Liver-Specific AMPK Activation Protects against Diet-Induced Obesity and NAFLD. Cell Rep 2019; 26: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Burkewitz K, Zhang Y, Mair WB. AMPK at the Nexus of Energetics and Aging. Cell Metab 2014; 20: 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wullschleger S, Loewith R, Hall MN. TOR Signaling in Growth and Metabolism. Cell 2006; 124: 471–484. [DOI] [PubMed] [Google Scholar]
- [36].Fabrizio P, Pozza F, Pletcher SD, et al. Regulation of longevity and stress resistance by Sch9 in yeast. Science (80- ) 2001; 292: 288–290. [DOI] [PubMed] [Google Scholar]
- [37].Robida-Stubbs S, Glover-Cutter K, Lamming DW, et al. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab 2012; 15: 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bjedov I, Toivonen JM, Kerr F, et al. Mechanisms of Life Span Extension by Rapamycin in the Fruit Fly Drosophila melanogaster. Cell Metab 2010; 11: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nat 2009 4607253 2009; 460: 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shindyapina A V, Cho Y, Kaya A, et al. Rapamycin treatment during development extends lifespan and healthspan. bioRxiv 2022; 2022.02.18.481092. [Google Scholar]
- [41].Marín-Aguilar F, Pavillard LE, Giampieri F, et al. Adenosine Monophosphate (AMP)-Activated Protein Kinase: A New Target for Nutraceutical Compounds. Int J Mol Sci; 18. Epub ahead of print 1 February 2017. DOI: 10.3390/IJMS18020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Birkisdóttir MB, Jaarsma D, Brandt RMC, et al. Unlike dietary restriction, rapamycin fails to extend lifespan and reduce transcription stress in progeroid DNA repair-deficient mice. Aging Cell 2021; 20: e13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Habib SL, Kasinath BS, Arya RR, et al. Novel mechanism of reducing tumourigenesis: Upregulation of the DNA repair enzyme OGG1 by rapamycin-mediated AMPK activation and mTOR inhibition. Eur J Cancer 2010; 46: 2806–2820. [DOI] [PubMed] [Google Scholar]
- [44].Ma Y, Vassetzky Y, Dokudovskaya S. mTORC1 pathway in DNA damage response. Biochim Biophys Acta - Mol Cell Res 2018; 1865: 1293–1311. [DOI] [PubMed] [Google Scholar]
- [45].Tatar M, Kopelman A, Epstein D, et al. A Mutant Drosophila Insulin Receptor Homolog That Extends Life-Span and Impairs Neuroendocrine Function. Science (80- ) 2001; 292: 107–110. [DOI] [PubMed] [Google Scholar]
- [46].Kimura KD, Tissenbaum HA, Liu Y, et al. daf-2, an Insulin Receptor-Like Gene That Regulates Longevity and Diapause in Caenorhabditis elegans. Science (80- ) 1997; 277: 942–946. [DOI] [PubMed] [Google Scholar]
- [47].Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nat 2003 4216919 2002; 421: 182–187. [DOI] [PubMed] [Google Scholar]
- [48].Zhang Y, Shao Z, Zhai Z, et al. The HIF-1 Hypoxia-Inducible Factor Modulates Lifespan in C. elegans. PLoS One 2009; 4: e6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Haigis MC, Sinclair DA. Mammalian Sirtuins: Biological Insights and Disease Relevance. 10.1146/annurev.pathol4110807092250 2010; 5: 253–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mao Z, Hine C, Tian X, et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science (80- ) 2011; 332: 1443–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kanfi Y, Naiman S, Amir G, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nat 2012 4837388 2012; 483: 218–221. [DOI] [PubMed] [Google Scholar]
- [52].Taylor JR, Wood JG, Mizerak E, et al. Sirt6 regulates lifespan in Drosophila melanogaster. Proc Natl Acad Sci U S A; 119. Epub ahead of print 1 February 2022. DOI: 10.1073/PNAS.2111176119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].West GB, Brown JH, Enquist BJ. A general model for ontogenetic growth. Nat 2001 4136856 2001; 413: 628–631. [DOI] [PubMed] [Google Scholar]
- [54].Moldakozhayev A, Tskhay A, Gladyshev VN. Applying deductive reasoning and the principles of particle physics to aging research. Aging (Albany NY) 2021; 13: 22611–22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cannon WB. Organization for physiological homeostasis. 10.1152/physrev192993399 1929; 9: 399–431. [DOI] [Google Scholar]
- [56].Britannica TE of E homeostasis | Definition, Examples, & Facts | Britannica. Encyclopedia Britannica, https://www.britannica.com/science/homeostasis (2022, accessed 18 March 2022). [Google Scholar]
- [57].Yates FE. Order and complexity in dynamical systems: Homeodynamics as a generalized mechanics for biology. Math Comput Model 1994; 19: 49–74. [Google Scholar]
- [58].Lloyd D, Aon MA, Cortassa S. Why Homeodynamics, Not Homeostasis? Sci World J 2001; 1: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rattan SIS. Aging Is Not a Disease: Implications for Intervention. Aging Dis 2014; 5: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rattan SIS. Biogerontology: from here to where? The Lord Cohen Medal Lecture-2011. Biogerontology 2011 131 2011; 13: 83–91. [DOI] [PubMed] [Google Scholar]
- [61].Rovelli C, Smolin L. Discreteness of area and volume in quantum gravity. Nucl Phys B 1995; 442: 593–619. [Google Scholar]
- [62].Gladyshev VN. Aging: progressive decline in fitness due to the rising deleteriome adjusted by genetic, environmental, and stochastic processes. Aging Cell 2016; 15: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Rattan SIS. Increased molecular damage and heterogeneity as the basis of aging. Biol Chem 2008; 389: 267–272. [DOI] [PubMed] [Google Scholar]
- [64].Pontzer H, Yamada Y, Sagayama H, et al. Daily energy expenditure through the human life course. Science (80- ); 373. Epub ahead of print 13 August 2021. DOI: 10.1126/SCIENCE.ABE5017/SUPPL_FILE/SCIENCE.ABE5017_SM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, et al. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol 2016 141 2016; 14: 11–31. [DOI] [PubMed] [Google Scholar]
- [66].Rattan SIS. Aging, anti-aging, and hormesis. Mech Ageing Dev 2004; 125: 285–289. [DOI] [PubMed] [Google Scholar]
- [67].Cypser JR, Tedesco P, Johnson TE. Hormesis and aging in Caenorhabditis elegans. Exp Gerontol 2006; 41: 935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Masoro EJ. Role of hormesis in life extension by caloric restriction. Dose Response 2006; 5: 163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Rattan SIS. Hormesis in aging. Ageing Res Rev 2008; 7: 63–78. [DOI] [PubMed] [Google Scholar]
- [70].Kumsta C, Chang JT, Schmalz J, et al. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat Commun 2017 81 2017; 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sun T, Wu H, Cong M, et al. Meta-analytic evidence for the anti-aging effect of hormesis on Caenorhabditiselegans. Aging (Albany NY) 2020; 12: 2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Proshkina E, Lashmanova E, Dobrovolskaya E, et al. Geroprotective and Radioprotective Activity of Quercetin, (−)-Epicatechin, and Ibuprofen in Drosophila melanogaster. Front Pharmacol 2016; 7: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Dues DJ, Andrews EK, Senchuk MM, et al. Resistance to Stress Can Be Experimentally Dissociated From Longevity. Journals Gerontol Ser A 2019; 74: 1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dues DJ, Andrews EK, Schaar CE, et al. Aging causes decreased resistance to multiple stresses and a failure to activate specific stress response pathways. Aging (Albany NY) 2016; 8: 777–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Poganik JR, Zhang B, Baht GS, et al. Biological age is increased by stress and restored upon recovery. bioRxiv 2022; 2022.05.04.490686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Schaible R, Scheuerlein A, Dańko MJ, et al. Constant mortality and fertility over age in Hydra. Proc Natl Acad Sci U S A 2015; 112: 15701–15706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Xiao R, Zhang B, Dong Y, et al. A Genetic Program Promotes C. elegans Longevity at Cold Temperatures via a Thermosensitive TRP Channel. Cell 2013; 152: 806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhang B, Xiao R, Ronan EA, et al. Environmental temperature differentially modulates C. elegans longevity through a thermosensitive TRP channel. Cell Rep 2015; 11: 1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Henderson D, Huebner C, Markowitz M, et al. Do developmental temperatures affect redox level and lifespan in C. elegans through upregulation of peroxiredoxin? Redox Biol 2018; 14: 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen YL, Tao J, Zhao PJ, et al. Adiponectin receptor PAQR-2 signaling senses low temperature to promote C. elegans longevity by regulating autophagy. Nat Commun 2019 101 2019; 10: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Conti B, Sanchez-Alavez M, Winsky-Sommerer R, et al. Transgenic mice with a reduced core body temperature have an increased life span. Science (80- ) 2006; 314: 825–828. [DOI] [PubMed] [Google Scholar]
- [82].Zhao Z, Cao J, Niu C, et al. Body temperature is a more important modulator of lifespan than metabolic rate in two small mammals. Nat Metab 2022 2022; 1–7. [DOI] [PubMed] [Google Scholar]
- [83].Ganeshan K, Chawla A. Warming the mouse to model human diseases. Nat Rev Endocrinol 2017; 13: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ruby JG, Smith M, Buffenstein R. Naked mole-rat mortality rates defy gompertzian laws by not increasing with age. Elife; 7. Epub ahead of print 24 January 2018. DOI: 10.7554/ELIFE.31157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kerepesi C, Meer MV., Ablaeva J, et al. Epigenetic aging of the demographically non-aging naked mole-rat. Nat Commun 2022 131 2022; 13: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Cheng H, Sebaa R, Malholtra N, et al. Naked mole-rat brown fat thermogenesis is diminished during hypoxia through a rapid decrease in UCP1. Nat Commun 2021 121 2021; 12: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Woodley R, Buffenstein R. Thermogenic changes with chronic cold exposure in the naked mole-rat (Heterocephalus glaber). Comp Biochem Physiol Part A Mol Integr Physiol 2002; 133: 827–834. [DOI] [PubMed] [Google Scholar]
- [88].Oiwa Y, Oka K, Yasui H, et al. Characterization of brown adipose tissue thermogenesis in the naked mole-rat (Heterocephalus glaber), a poikilothermic mammal. bioRxiv 2020; 2020.04.28.062737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Holtze S, Braude S, Lemma A, et al. The microenvironment of naked mole-rat burrows in East Africa. Afr J Ecol 2018; 56: 279–289. [Google Scholar]
- [90].Haupt M, Bennett NC, Oosthuizen MK. Locomotor Activity and Body Temperature Patterns over a Temperature Gradient in the Highveld Mole-Rat (Cryptomys hottentotus pretoriae). PLoS One; 12. Epub ahead of print 1 January 2017. DOI: 10.1371/JOURNAL.PONE.0169644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Fulop T, Larbi A, Dupuis G, et al. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or Foes? Front Immunol 2018; 8: 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sun L, Sadighi Akha AA, Miller RA, et al. Life-Span Extension in Mice by Preweaning Food Restriction and by Methionine Restriction in Middle Age. Journals Gerontol Ser A Biol Sci Med Sci 2009; 64A: 711. [DOI] [PMC free article] [PubMed] [Google Scholar]


