Abstract
Cirrhosis consists of two main stages: compensated and decompensated, the latter defined by the development/presence of ascites, variceal hemorrhage and hepatic encephalopathy. Survival is entirely different depending on the stage. Treatment with non-selective beta-blockers prevents decompensation in patients with clinically significant portal hypertension, changing the previous paradigm based of the presence of varices. In patients with acute variceal hemorrhage at high risk of failure with standard treatment (defined as those with a Child-Pugh score of 10–13 or those with Child-Pugh score of 8–9 with active bleeding at endoscopy), pre-emptive transjugular intra-hepatic porto-systemic shunt (TIPS) improves mortality and has become the standard of care in many centers. In patients with bleeding from gastrofundal varices, retrograde transvenous obliteration (in those with a gastrorenal shunt) and/or variceal cyanoacrylate injection have emerged as alternatives to TIPS. In patients with ascites, emerging evidence suggests that TIPS might be used earlier, before strict criteria for refractory ascites are met.. Long term albumin use is under assessment for improving prognosis of patients with uncomplicated ascites and confirmatory studies are ongoing. Hepatorenal syndrome is the least common cause of acute kidney injury in cirrhosis, and first line treatment is the combination of terlipressin and albumin. Hepatic encephalopathy has a profound impact on the quality of life of patients with cirrhosis. Lactulose and rifaximin are first and second-line treatment for hepatic encephalopathy. Newer therapies such as L-ornithine L-aspartate and albumin require further assessment.
Keywords: cirrhosis, portal hypertension, variceal hemorrhage, ascites, hepatic encephalopathy
Cirrhosis consists of two main stages: compensated (asymptomatic) and decompensated, the latter defined by the development/presence of ascites, variceal hemorrhage and hepatic encephalopathy 1. Survival is entirely different depending on stage. While the median survival in the compensated stage exceeds 15 years, the median survival in the decompensated stage is around 1.5 years (ranging from 2 to 4 years) 2. Therefore, compensated and decompensated cirrhosis should be considered separate entities.
The main pathophysiological mechanism leading to cirrhosis decompensation is the severity of portal hypertension. This was demonstrated in a sub-analysis of a prospective cohort study of patients with compensated cirrhosis with no or small varices, 29% of whom developed decompensation on follow-up 3. In these patients, the main determinant of decompensation was a portal pressure (as determined by the hepatic venous pressure gradient) ≥ 10 mmHg, the pressure gradient that now defines “clinically-significant portal hypertension” (CSPH).
Until recently, the approach and management of patients with compensated cirrhosis had been mostly focused on preventing variceal hemorrhage in those with high-risk varices on endoscopy. However, ascites, not variceal hemorrhage, is the most common decompensating event 2, 3 even in a recent series of patients with cirrhosis due to nonalcoholic steatohepatitis 4. Ascites is also the decompensating event associated with the highest mortality 5. Therefore, a loftier goal in the patient with compensated cirrhosis would be the ability to prevent not only variceal hemorrhage, but also ascites. It follows that measures to decrease portal pressure, particularly in those with CSPH, would result in prevention of cirrhosis decompensation.
Non-selective beta-blockers (NSBB) such as propranolol and nadolol decrease portal pressure via both β1 (decrease in cardiac output) and β2 (splanchnic vasoconstriction) adrenergic blockade. Carvedilol, in addition to blocking β1 and β2 receptors, also has α1 adrenergic blockade activity that may act by decreasing intrahepatic resistance, thereby resulting in a greater decrease in portal pressure compared to propranolol 6.
In a landmark randomized double-blind, placebo-controlled trial (PREDESCI) that included patients with cirrhosis mostly due to chronic hepatitis C, and with clinically-significant portal hypertension (CSPH), NSBB were associated with a significant, 49% lower risk of decompensation compared to placebo 7. Ascites was the decompensating event that was significantly lower in the NSBB arm (58% reduction in the risk of ascites). Additionally, the risk of developing high risk varices (the study excluded patients with large varices at entry) was 40% lower in the NSBB group. The specific NSBB used in those randomized to this arm was propranolol (67%) or carvedilol (33%) based on pre-randomization response to IV propranolol. Post-hoc analysis showed that carvedilol outperformed propranolol both in terms of reducing portal pressure and in preventing decompensation. These results further support the recent Baveno consensus statement that carvedilol should be the preferred NSBB to be used in cirrhosis. 8
In the PREDESCI trial, patients with compensated cirrhosis at a higher risk of decompensation were selected based on the presence of CSPH. Determining the presence of CSPH requires an invasive and nuanced procedure (catheterization of the hepatic vein) that, while invaluable in clinical research, is impractical and not recommended in daily clinical practice. This is particularly the case now that there are non-invasive methods that can accurately predict the presence of CSPH. Two multicenter studies have now demonstrated that liver stiffness measurements (LSM) (by transient elastography) and platelet count can predict the probability of having CSPH 9, 10The risk of having CSPH can be calculated using a nomogram included in these studies and that have been translated into two easier to use cutoffs that will predict a >60% probability of having CPSH: a LSM >25 kPa or a LSM 20–25 kPa plus a platelet count <150/mm3. Although the positive predictive value of these thresholds were described as being lower in obese patients with NASH cirrhosis, a recent large study in NASH cirrhosis shows that they are applicable to all patients with NASH cirrhosis 11.. Therefore, these cutoffs have been recommended by the Baveno consensus that state that, although the concept of CSPH is HVPG-driven, noninvasive tests are sufficiently accurate to identify CSPH in clinical practice.
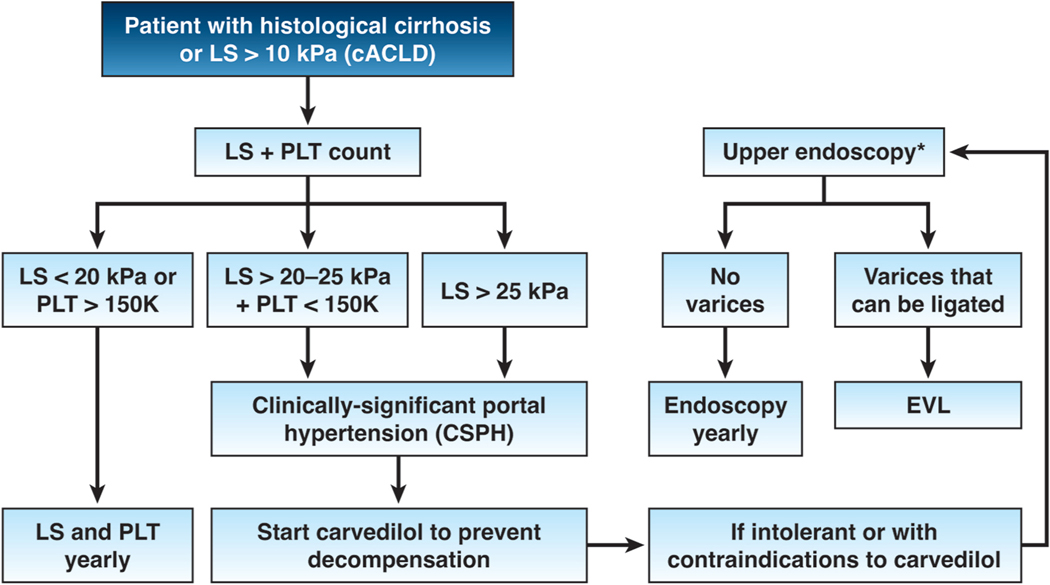
Therefore, the greatest paradigm change in the management of compensated cirrhosis is to forego screening endoscopy and instead, to screen for the presence of CSPH by using noninvasive tools and to start carvedilol (dose 3.25 to 25 mg/day) in those with CSPH. In patients with compensated cirrhosis with contraindications/intolerance to NSBB, one would revert to the old paradigm by which the patient would have a screening endoscopy and high-risk varices would undergo ligation, which would only prevent variceal hemorrhage but not ascites (Figure 1).
Figure 1.
The presence of clinically-significant portal hypertension (CSPH, determined noninvasively) establishes the indication for carvedilol with the goal of preventing cirrhosis decompensation. cACLD= compensated advanced chronic liver disease (noninvasive surrogate of compensated cirrhosis); LS = liver stiffness; PLT= platelet count; EVL= endoscopic variceal ligation. *Patients with LS <20 kPa and PLT >150,000/mm3 can circumvent endoscopy because the risk of having high-risk varices is minimal
1). Variceal Hemorrhage
a). Prevention of a first variceal hemorrhage in patients with decompensated cirrhosis: when to start.
While in patients with compensated cirrhosis the presence of varices has become less relevant for therapeutic decisions, in patients with decompensated cirrhosis an upper endoscopy is still indicated in all cases. Small varices in decompensated cirrhosis carry the same risk of bleeding as large varices in compensated cirrhosis. Therefore, Baveno VII consensus 8 recommends that in patients with ascites, NSBBs may be used to prevent first variceal hemorrhage in varices of any size. Considering the greater portal pressure-reducing effect 12 and the easier titration, carvedilol is preferred in cirrhosis 8.
b). Advances in the treatment of acute esophageal variceal hemorrhage and the concept of pre-emptive TIPS
Mortality from acute esophageal variceal hemorrhage has markedly decreased from 40% at 6 weeks in series published in the 1980s 13 to 15–20% in the 1990s 14, but this has not further improved in the 2000s 15. The advances in hemostatic treatment with drugs, endoscopy and TIPS resulted in most deaths no longer being due to ongoing bleeding, but to infections and worsening liver and kidney failure 14, which made it difficult to further reduce mortality. A breakthrough arose with the concept of pre-emptive TIPS 16. The idea was that most patients experiencing early rebleeding were also those at higher risk of dying, i.e. those with worse liver failure that would not tolerate a second hit. Hence, the proposal was to place TIPS pre-emptively in patients that were at high risk of rebleeding (Child-Pugh B with active bleeding or Child-Pugh C) 17, even if bleeding was initially controlled with pharmacological and endoscopic treatment.
Four trials have been conducted assessing pre-emptive TIPS (pTIPS) as compared with standard therapy (pharmacological and endoscopic therapy, using TIPS only as rescue therapy). Meta-analysis of the four trials 18 shows a large effect on mortality (RR: 0.33, or a 67% reduction in the relative risk of death), but with a wide confidence interval (0.08–1.36), reflecting the small number of patients (n=302) included in those trials.
Another recent individual patient meta-analysis, using data from additional observational studies, suggested a minimal threshold of Child-Pugh B of 8 points (with active bleeding at endoscopy) for pTIPS to improve survival 19. On this basis, recent North American consensus on the use of TIPS 20 and Baveno 8 recommend pTIPS in patients with Child Pugh class C<14 points or Child class B >7 with active bleeding at initial endoscopy. This seems reasonable until the results of a large UK randomized trial (n=294) of pTIPS (https://fundingawards.nihr.ac.uk/award/NIHR130883) become available.
2). Prevention of recurrent bleeding (secondary prophylaxis)
In patients not undergoing pTIPS during the index admission, the standard first line therapy is the combination of NSBBs and endoscopic variceal ligation 8 with TIPS reserved for treatment failures. An exception may be patients with portal vein thrombosis. In these patients, a recent RCT 21 showed that TIPS was superior to NSBBs + variceal ligation in preventing rebleeding and was associated with increased portal vein recanalization, without differences in survival or encephalopathy. This suggests that TIPS might be the preferred first-line option in patients with portal vein thrombosis.
a). The controversy regarding the therapeutic window for beta-blockers
The potential of NSBBs to induce acute kidney injury (AKI) in patients with ascites was recognized early after the first proposal of propranolol for the treatment of varices 22. However, it was not until 2010 that a prospective observational study raised for the first time the possibility of harm with NSBBs in patients with refractory ascites 23, ushering the concept of the “therapeutic window” 24. However, subsequent meta-analysis of observational studies showed no association between NSBBs and mortality in patients with refractory ascites 25. Furthermore, a recent RCT in patients with ACLF challenged the concept of a therapeutic window, showing a decreased 28-day mortality in patients treated with carvedilol as compared with placebo 26. Arterial pressure has shown to be an excellent biomarker to determine when NSBBs might not offer benefit. A recent observational study 27 showed that transplant-free survival was not improved if NSBB were associated with MAP<65 mmHg or systolic blood pressure <90 mmHg.
b). Treating gastric varices
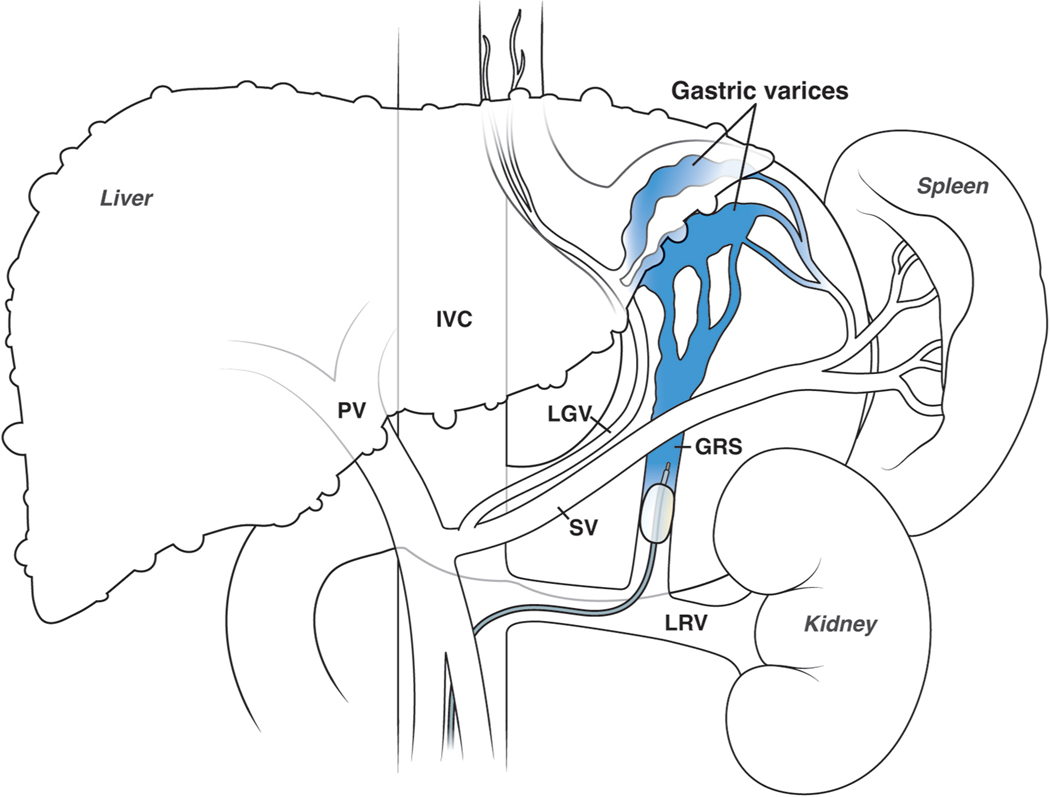
Hemorrhage from gastric varices is much less frequent than hemorrhage from esophageal varices, and there are few RCTs to support treatment decisions. The management of gastroesophageal varices type 1 (GOV1, those extending into the lesser curvature) is comparable to the management of esophageal varices 8. Gastrofundal varices which consist of varices in the fundus with (GOV-2) or without (IGV-1)extension into the esophagus,) have specific vascular anatomy and distinct management (Figure 2). First-line endoscopic treatment is cyanoacrylate injection 28. TIPS is effective, although usually requires variceal embolization, and can be used as first-line especially in centers with limited experience with cyanoacrylate.
Figure 2.
RTO procedure (performed with balloon-occlusion or BRTO). A gastro-renal shunt (GRS), which usually connects the splenic vein (SV) and the left renal vein (LRV) creating the gastrofundal varices (GV) nidus in the fundus of the stomach, is a requisite for performing the procedure. The efferent of the GRS is catheterized and occluded with a balloon. Subsequent retrograde injection of a sclerosant obliterates the gastric varices.
Retrograde transvenous obliteration (RTO) was for many years a routine treatment in Japan and Korea, but a rarity in Western countries. In the last decade it has become widely used in many centers in North America and Europe 28. RTO requires the presence of a gastrorenal shunt (Figure 2), that is cannulated retrogradely allowing the obliteration of the varices. The original technique (balloon-occluded RTO or BRTO) required maintaining an intravenous catheter with an inflated balloon for a prolonged time (6– 36 hours), which created significant logistical issues. The technique has evolved with the development of plug assisted RTO (PARTO) and coil assisted RTO (CARTO) 29 that achieve complete variceal obliteration in less than an hour and have contributed to a wider applicability. By redirecting flow from the gastrorenal shunt to the liver, RTO may improve liver function and hepatic encephalopathy and, thus, can be performed in patients that are poor candidates for TIPS. However, it can result in an increase in portal pressure, with the development of new ascites, hydrothorax and esophageal varices. A recent RCT compared RTO with repeated cyanoacrylate injection in the prevention of gastric variceal rebleeding in 64 patients, showing a higher efficacy of RTO, withoutdifferences in survival, complications or in the rate of worsening esophageal varices 30.
3). Ascites and Acute Kidney Injury
Ascites is the most frequent complication of cirrhosis, with an annual incidence of 5–10% in compensated cirrhosis 1, 31 While the prognostic significance of ascites detected only by ultrasound (mild or grade 1) is still undetermined, clinically-evident ascites (moderate or grade 2 and large or grade 3) is associated with a survival of only 30% at 5 years 1.
Ascites is a risk factor for spontaneous bacterial peritonitis (SBP) and other infections, hyponatremia and acute kidney injury (AKI), including the hepatorenal syndrome (HRS-AKI). It also predisposes to malnutrition, thus contributing to sarcopenia, frailty, and disability. These complications explain not only its higher mortality but also its association with frequent hospitalizations, poor quality of life and a heavy socio-economic burden.
AASLD and EASL guidelines provide consensus recommendations on diagnosis and treatment of ascites 32, 33. Herein we discuss some issues that are still not fully clarified.
From a therapeutic perspective, ascites can be classified as responsive, recurrent or refractory (Table 1) 32, 33. However, while responsive and refractory ascites represent the relatively homogeneous ends of the spectrum with a clear clinical phenotype and prognosis, the current definition of recurrent ascites does not fully reflect the heterogeneity of patients in this category. Further characterization of these patients will allow for a more precise allocation of patients to different treatments.
Table 1.
Classification of ascites according to response to treatment
| Responsive | Ascites that can be fully mobilized or limited to grade 1 with diuretic therapy associated or not to moderate dietary sodium restriction |
| Recurrent | Ascites that recurs on at least 3 occasions within a 12-month period despite dietary sodium restriction and adequate diuretic dosage |
| Refractory | Ascites that cannot be mobilized or the early recurrence of which (i.e., after large-volume paracentesis) cannot be satisfactorily prevented by medical therapy |
a). First line treatments
At present, no treatment is recommended for grade 1 ascites, as there is no evidence that any treatment will improve patient outcomes. In fact, these patients are strictly still compensated and could be treated with NSBB (see above). In patients with grade 2 and 3 ascites, a moderate low-sodium diet and diuretics are recommended with the goal of eliminating ascites or at least decreasing to grade 1. Rather than pursuing strict sodium restriction that will aggravate the already reduced caloric/nutrient intake of these patients 34, a major goal is to avoid excessive salt intake with food, even though this may be insufficient to obtain a negative sodium balance. Regular nutritional counselling is essential in patients with ascites and should be started even with grade 1 ascites.
Diuretic therapy is based on aldosterone antagonists alone or, more frequently, in combination with loop diuretics. However, with the epidemiological changes in the etiology of cirrhosis, there is an increase in concomitant renal injury, even subtle, due to hypertensive and/or diabetic nephropathy that will require lowering the dose of aldosterone antagonists and increasing the dose of loop diuretics with as yet uncertain effects. Recommendations on the use of diuretics are reviewed in recent AASLD and EASL guidelines 32, 33.
b). Second line treatments
There is more controversy regarding the management of patients who stop responding to diuretics or develop diuretic-related side-effects. Currently, two major options, still under evaluation, have emerged: TIPS, and long-term treatment with intravenous albumin (LTA).
Limited evidence indicates that TIPS placement at a stage earlier than refractory ascites is more effective and safer. First, a meta-analysis of individual data from RCTs all performed using bare stents, showed a more favorable impact on survival in the two trials that also included patients with recurrent ascites, compared to those enrolling only patients with refractory ascites 35. Second, the only RCT assessing the new covered TIPS stents, included patients (median MELD 12) who had had no more than 6 large-volume paracenteses (LVP) in the last 3 months, showing a significant improvement in 1-year transplant free survival 36, even though some of the patients included could have met criteria for refractory ascites.
At present, TIPS should be considered in patients with recurrent ascites irrespective of the presence or absence of varices or history of variceal hemorrhage 8.
On the other hand, a large open-label Italian RCT (ANSWER), in patients with uncomplicated persistent grade 2/3 ascites despite moderate diuretic doses (median MELD 12), showed that LTA, in addition to standard medical treatment, significantly increased the number of patients remaining free of LVP and prolonged the interval between LVPs. Importantly, there was a decrease in the rate of complications of ascites, hospitalizations and death 37. However, several factors limit LTA outside Italy, mainly the cost and logistics/adherence to weekly intravenous infusion. Also, a placebo-controlled RCT performed in somewhat sicker patients with ascites (median MELD 17) showed no benefits of LTA (biweekly) plus midodrine in the need for LVP, complications of ascites or death 38. Ongoing/future trials will help better define the patient population that will benefit from TIPS vs. LTA.
c). Treatment for refractory ascites
In patients with refractory ascites, TIPS should always be considered. Unfortunately, only in some of these patients can TIPS be performed safely and its use is limited by contraindications such as renal failure, cardiac diseases, and recurrent hepatic encephalopathy. With the aim of reducing side-effects, the use of small diameter PTFE-covered stents have provided promising results and a staged approach can be used with reassessment for need to further dilate the stent according to clinical response 20.
Standard of care is also based on periodic LVP followed by albumin supplementation to prevent circulatory dysfunction 39. The administration of vasoconstrictor drugs aiming to expand effective volemia, including midodrine or clonidine, has been investigated in small studies which have not produced sufficient data to recommend their use 40, 41.
Finally, in patients with no other treatment options and need for frequent LVP, palliative care with implantation of a peritoneal catheter or, at least in some centers, the automated alpha-pump (not available in the USA) could be considered 42, 43.
d). General approach to the management of ascites
Treatment of cirrhosis etiology, when available, should be implemented as this represents the most effective approach and may lead to cirrhosis recompensation 8, 44. Also, liver transplant evaluation should be undertaken with development of ascites. Finally, implementation of a “liver home” providing coordinated care among all caregivers should be encouraged and a remote monitoring and intervention strategy using digital systems should be further explored 45.
e). Acute kidney injury (AKI)
AKI is defined by an increase in serum creatinine of ≥0.3 mg/dL within 48 hours or ≥50% increase in serum creatinine that is known or presumed to have occurred within the preceding 7 days and is graded in three stages 46 Notably, in patients with cirrhosis, serum creatinine overestimates glomerular filtration rate because of a combination of decreased creatinine production and muscle wasting.
AKI is present in 25–50% of hospitalized patients with cirrhosis and is associated with a poor prognosis. The main causes of AKI are, in order of frequency, hypovolemia, acute tubular necrosis, and hepatorenal syndrome (HRS-AKI) 32.
The AASLD and EASL guidelines provide consensus recommendations on classification, differential diagnosis and management of AKI 32, 33. Herein we discuss some specific aspects related to HRS-AKI, the type of AKI unique to patients with cirrhosis and ascites.
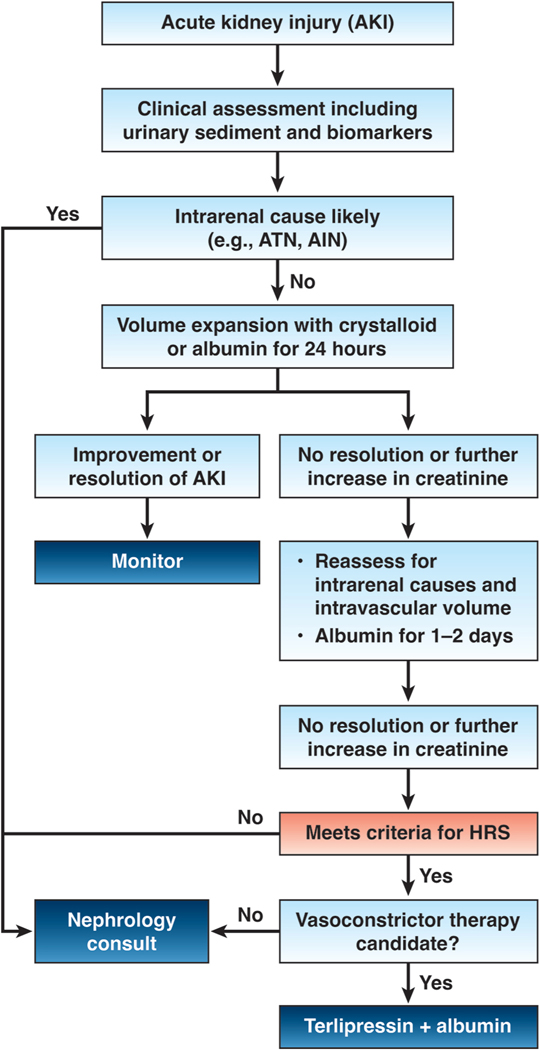
HRS-AKI is the least common type of AKI, representing about 15–30% of all AKI cases 18. Diagnosis of HRS-AKI is challenging and is usually a diagnosis of exclusion (Figure 3) 32, 33, 46. Patients with cirrhosis, ascites and AKI not responding to withdrawal of nephrotoxic drugs, reduction or withdrawal of diuretics, treatment of infections, and fluid replacement in presence of severe volume depletion, should receive plasma expansion with albumin (1 g/kg up to a maximum of 100 grams) for 2 consecutive days. HRS-AKI is a likely diagnosis in patients who do not respond and should be started on vasoconstrictors (Figure 3). Terlipressin is the vasoconstrictor of choice as it has shown to be superior to the combination of midodrine and octreotide 47, achieving the reversal of AKI-HRS in about 30–50% of cases 47, 48. Norepinephrine represents a valid alternative 49, but its administration usually requires admission to an intensive care unit (ICU) and a recent report has shown that it is less effective than terlipressin at least in patients with ACLF 50.
Figure 3.
Management algorithm in a patient with cirrhosis and ascites presenting with AKI.
Treatment with terlipressin is combined with intravenous albumin and is not infrequently associated with potentially severe side-effects, mainly related to tissue ischemia and pulmonary edema 48. The risk of ischemic side-effects can be reduced by administering terlipressin in intravenous continuous infusion instead of boluses every 4–6 hours 51. However, in US centers this may require transfer to a more advanced level of care. Treatment should be avoided in patients with hypoxemia and in those with ongoing coronary, peripheral or mesenteric ischemia. Careful assessment of volume status and oxygenation should be performed at bedside (e.g. point-of-care ultrasound) throughout treatment.
Although the absence of structural kidney damage is a diagnostic criterion for HRS-AKI, it could develop in patients with pre-existing subtle organic nephropathy particularly with a higher prevalence of metabolic and alcoholic cirrhosis. Furthermore, the kidneys of patients with HRS-AKI may have a certain degree of histological damage 52. This will make even more challenging the diagnosis and treatment of HRS-AKI and will likely lead to an even worse response.
Because treatment of HRS-AKI has no effect on mortality, patients should be evaluated for liver transplantation. In patients not responding to vasoconstrictors and albumin, candidacy for renal replacement therapy and/or transplantation (liver or kidney/liver) should be discussed by a multidisciplinary team.
4). Hepatic Encephalopathy
Hepatic encephalopathy (HE) is defined as brain dysfunction caused by hepatic insufficiency and or portosystemic shunting and is one of the most common and incapacitating complications of advanced liver disease 53, 54. It ranges in severity from covert HE (reliably detectable only by neuropsychometric testing and affecting 20–80% of patients with cirrhosis) to overt HE (defined as ≥West Haven grade II and affecting up to 40% of patients with cirrhosis) 53, 54. The AASLD and EASL guidelines provide consensus recommendations on multiple facets of diagnosis and treatment of HE 53, 54. Here we highlight current concepts of pathophysiology-linked-to-clinical diagnosis and treatment of overt HE in patients with cirrhosis (Type C HE) to guide further reading.
a). Pathophysiology
Though not fully understood, the pathophysiology of HE is known to be multifactorial and is elegantly updated in a recent review 55. Hyperammonemia and gut dysbiosis are key mechanisms in the pathogenesis of HE, and are linked to the mainstays of treatment, as well as novel therapies for HE.
b). Hyperammonemia
Hyperammonemia in stable outpatients with cirrhosis has been shown to predict not only the risk and frequency of HE, but also the risk of hospitalization for liver-related complications and mortality 56, 57. In addition, ammonia scavengers combined with standard of care have been shown to reduce the risk of recurrent HE 58. These findings support hyperammonemia as a key protagonist in HE. However, the only bed-side value for measured blood ammonia in cirrhosis with altered mental status is when it is normal, as this should direct a search for alternate reasons for altered mentation 54.
c). Gut Microbiome
The gut microbiome interacts with the host, food and medications, and significantly impacts cognitive function in cirrhosis and HE 53–55. Dysbiosis in cirrhosis includes increased pathogenic and decreased autochthonous taxa, and impaired microbial functionality. Although poorly understood, the gut microbiome can predict clinical outcomes such as hospitalization, organ failure and death in cirrhosis 59. Key HE therapies are thought to act through modulation of the gut microbiome function and associated dysbiosis and endotoxemia. Although rifaximin does not significantly reduce bacterial burden per se, it mitigates dysbiosis by driving a shift from pathogenic to beneficial metabolite linkages 60. Probiotics also mitigate dysbiosis and endotoxemia, and have had some success in the prevention of recurrent HE 61, 62 Fecal microbial transplantation (FMT) can restore bacterial diversity and function, and reduce HE recurrence in cirrhosis, and is an exciting future direction in HE therapeutics 63.
d). Hepatic Encephalopathy in Acute-on-Chronic Liver Failure
Hepatic encephalopathy is a leading cause of hospitalization and readmission in patients with decompensated cirrhosis 64. However, HE in the context of acute-or-chronic liver failure (ACLF), is characterized by distinct pathophysiology (exaggerated inflammatory responses with infection, sepsis, neuroinflammation, vasoconstriction, hyponatremia and oxidative stress) and worse prognosis (higher mortality) 65, 66. Though more studies are needed, there is mounting evidence that these distinct features of HE in ACLF may warrant specific therapies targeting hyperammonemia and inflammation 55.
e). Medical management
AASLD and EASL guidelines cover the treatment and secondary prevention of HE in detail, with lactulose as first-line and rifaximin as second line treatments, as well as nutritional measures to mitigate sarcopenia and addressing large portosystemic shunts 53, 54. It is important to recognize racial and ethnic disparities in access to rifaximin for HE in the US, highlighting the need for legislative efforts to improve access to care 67.
Standard and other therapies for HE warrant mention and have been succinctly summarized in recent reviews (Table 2) 55, 68. The targets of other therapies include (i) gut absorption of ammonia (osmotic laxatives), (ii) gut dysbiosis and production of ammonia (FMT, probiotics, engineered bacteria, carbon microspheres) (iii) nitrogen scavenging and ammonia clearance through ureagenesis (hepatic) and glutamine (hepatic and muscle) synthesis (L-Ornithine L-Aspartate (LOLA), ornithine phenylacetate, sodium/glycerol phenylbutyrate), (iv) toxin binding (albumin, albumin dialysis), and (v) direct vigilance modulators such as golexanolone, a GABA-A receptor antagonist 55, 68. Selected clinically available therapies are briefly discussed.
Table 2.
A summary of therapeutic targets and related therapies in the treatment of hepatic encephalopathy in cirrhosis.
| Therapeutic target | Therapies |
|---|---|
| Laxatives to decrease gut absorption of ammonia | Non-absorbable disaccharides (lactulose) Osmotic laxatives (polyethylene glycol) |
| Gut dysbiosis and gut production of ammonia | Antimicrobials- rifaximin (other antibiotics not favored, eg neomycin, metronidazole or vancomycin) Solid soluble dispersion rifaximin (in clinical trials) Lactulose (putative intraluminal pH effect) Fecal microbiota transplant (clinical protocols) Probiotics (limited success) Engineered bacteria (early-stage clinical trials) Carbon microspheres (early-stage clinical trials) |
| Nutritional measures | Adequate nutrition and protein intake to mitigate sarcopenia/malnutrition Branched-chain amino acids Avoid and treat hypokalemia and hyponatremia Zinc repletion (primes the urea cycle) |
| Closure of portosystemic shunts | Interventional radiology embolization of portosystemic shunts, ideally MELD<12 |
| Enhance nitrogen scavenging | Glycerol and sodium phenylbutyrate (used in urea cycle disorders) Sodium benzoate (used in urea cycle disorders) L-Ornithine L-Aspartate (urea cycle substrate and activator of glutamine synthetase in peripheral organs) Ornithine phenylacetate |
| Ammonia lowering and prevent ammonia-induced neurotoxity | Acetyl L-carnitine (reduce blood/brain ammonia, enhance cellular/mitochondrial energy production) |
| Toxin binding | Albumin infusion Albumin dialysis |
| Direct vigilance modulators | Golexanolone (early-stage clinical trials) Caffeine (limited data) |
f). L-Ornithine L-Aspartate
L-Ornithine L-Aspartate (LOLA), a urea cycle substrate and an activator of glutamine synthetase, has been studied in both oral and intravenous form in the treatment and prevention of HE 69. Interestingly, LOLA is now available as an over-the-counter oral supplement in the US. Therefore, it is worth understanding its potential therapeutic role in HE although there remains uncertainty regarding its benefit 68.
g). Albumin
Recent evidence shows that weekly albumin infusions in outpatients with HE is associated with improved neurocognitive testing and HRQOL, and less severe HE with improved survival in those with uncomplicated ascites 70, 71. At present however, there are no guideline recommendations for albumin use in the prevention of recurrent HE in cirrhosis 53, 54.
h). Primary prevention of HE
There are scant data on the primary prevention of HE, but it has been recently re-examined in a large cohort of patients undergoing TIPS. Contrary to prior findings 72, initiation of rifaximin treatment 2 weeks before TIPS insertion reduced the risk of HE events within 6 months by approximately 50% 73. Current EASL guidelines support the consideration of this strategy for prevention of HE post TIPS, although the role of treatment and risk of HE beyond 6 months is not known 54. Outside of TIPS, a soluble solid dispersion of rifaximin has been studied for the prevention of cirrhosis-related hospitalizations or mortality and recurrent HE 74, and is currently under investigation for the prevention of HE in cirrhosis (ClinicalTrials.gov Identifier: NCT05071716).
Conflicts of interest:
Juan G. Abraldes received a research grant (paid to the University of Alberta) from Cook
Paolo Caraceni received research grants from Grifols SA and Octapharma SA, and speaker or advisory board fees from Grifols SA, CSL Behring SA, Takeda SA, and SOBI SA;
Marwan Ghabril received research support (paid to institution) from Salix/Bausch, and served on the Data Safety Boards for Zydus, PTC Therapeutics, ACTC, and Cymabay.
Guadalupe Garcia-Tsao: no COI
Abbreviations used in this paper:
- ACLF
acute-on-chronic liver failure
- AKI
acute kidney injury
- CSPH
clinically significant portal hypertension
- HE
hepatic encephalopathy
- HRS
hepatorenal syndrome
- LOLA
L-ornithine L-aspartate
- LSM
liver stiffness measurement
- LTA
long-term treatment with intravenous albumin
- LVP
large-volume paracenteses
- MELD
Model for End-stage Liver Disease
- NASH
nonalcoholic steatohepatitis
- NSBB
nonselective b-blocker
- pTIPS
pre-emptive transjugular intrahepatic portosystemic shunt
- RTO
retrograde transvenous obliteration
- TIPS
transjugular intrahepatic portosystemic shunt
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.D’Amico G, Garcia-Tsao G and Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis. A systematic review of 118 studies. J Hepatol. 2006; 44: 217–31. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico G, Pasta L, Morabito A, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014; 39: 1180–93. [DOI] [PubMed] [Google Scholar]
- 3.Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007; 133: 481–8. [DOI] [PubMed] [Google Scholar]
- 4.Allen AM, Therneau TM, Ahmed OT, et al. Clinical course of non-alcoholic fatty liver disease and the implications for clinical trial design. J Hepatol. 2022; 77: 1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno S, Saibeni S, Bagnardi V, et al. Mortality risk according to different clinical characteristics of first episode of liver decompensation in cirrhotic patients: a nationwide, prospective, 3-year follow-up study in Italy. Am J Gastroenterol. 2013; 108: 1112–22. [DOI] [PubMed] [Google Scholar]
- 6.Zacharias AP, Jeyaraj R, Hobolth L, Bendtsen F, Gluud LL and Morgan MY. Carvedilol versus traditional, non-selective beta-blockers for adults with cirrhosis and gastroesophageal varices. Cochrane Database Syst Rev. 2018; 10: CD011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villanueva C, Albillos A, Genesca J, et al. Beta blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019; 393: 1597–608. [DOI] [PubMed] [Google Scholar]
- 8.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T and Ripoll C. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022; 76: 959–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraldes JG, Bureau C, Stefanescu H, et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: The “Anticipate” study. Hepatology. 2016; 64: 2173–84. [DOI] [PubMed] [Google Scholar]
- 10.Pons M, Augustin S, Scheiner B, et al. Noninvasive Diagnosis of Portal Hypertension in Patients With Compensated Advanced Chronic Liver Disease. Am J Gastroenterol. 2020. [DOI] [PubMed]
- 11.Rabiee A, Deng Y, Ciarleglio M, et al. Noninvasive predictors of clinically significant portal hypertension in NASH cirrhosis: Validation of ANTICIPATE models and development of a lab-based model. Hepatol Commun. 2022. [DOI] [PMC free article] [PubMed]
- 12.Sinagra E, Perricone G, D’Amico M, Tine F and D’Amico G. Systematic review with meta-analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Aliment Pharmacol Ther. 2014; 39: 557–68. [DOI] [PubMed] [Google Scholar]
- 13.Graham DY and Smith JL. The course of patients after variceal hemorrhage. Gastroenterology. 1981; 80: 800–9. [PubMed] [Google Scholar]
- 14.D’Amico G and de Franchis R. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003; 38: 599–612. [DOI] [PubMed] [Google Scholar]
- 15.Reverter E, Tandon P, Augustin S, et al. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014; 146: 412–9. [DOI] [PubMed] [Google Scholar]
- 16.Monescillo A, Martinez-Lagares F, Ruiz-del-Arbol L, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004; 40: 793–801. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Pagan JC, Di PM, Caca K, et al. Use of early-TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. J Hepatol. 2013; 58: 45–50. [DOI] [PubMed] [Google Scholar]
- 18.Hussain I, Wong YJ, Lohan R, Lin S and Kumar R. Does preemptive transjugular intrahepatic portosystemic shunt improve survival after acute variceal bleeding? Systematic review, meta-analysis, and trial sequential analysis of randomized trials. J Gastroenterol Hepatol. 2022; 37: 455–63. [DOI] [PubMed] [Google Scholar]
- 19.Nicoară-Farcău O, Han G, Rudler M, et al. Effects of Early Placement of Transjugular Portosystemic Shunts in Patients With High-Risk Acute Variceal Bleeding: a Meta-analysis of Individual Patient Data. Gastroenterology. 2021; 160: 193–205 e10. [DOI] [PubMed] [Google Scholar]
- 20.Boike JR, Thornburg BG, Asrani SK, et al. North American Practice-Based Recommendations for Transjugular Intrahepatic Portosystemic Shunts in Portal Hypertension. Clin Gastroenterol Hepatol. 2022; 20: 1636–62 e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv Y, Qi X, He C, et al. Covered TIPS versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: a randomised controlled trial. Gut. 2018; 67: 2156–68. [DOI] [PubMed] [Google Scholar]
- 22.Conn HO. Propranolol in the treatment of portal hypertension: a caution. Hepatology. 1982; 2: 641–4. [DOI] [PubMed] [Google Scholar]
- 23.Serste T, Melot C, Francoz C, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010; 52: 1017–22. [DOI] [PubMed] [Google Scholar]
- 24.Krag A, Wiest R, Albillos A and Gluud LL. The window hypothesis: haemodynamic and non-haemodynamic effects of beta-blockers improve survival of patients with cirrhosis during a window in the disease. Gut. 2012; 61: 967–9. [DOI] [PubMed] [Google Scholar]
- 25.Chirapongsathorn S, Valentin N, Alahdab F, et al. Nonselective beta-Blockers and Survival in Patients With Cirrhosis and Ascites: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016; 14: 1096–104. [DOI] [PubMed] [Google Scholar]
- 26.Kumar M, Kainth S, Choudhury A, et al. Treatment with carvedilol improves survival of patients with acute-on-chronic liver failure: a randomized controlled trial. Hepatol Int. 2019; 13: 800–13. [DOI] [PubMed] [Google Scholar]
- 27.Tergast TL, Kimmann M, Laser H, et al. Systemic arterial blood pressure determines the therapeutic window of non-selective beta blockers in decompensated cirrhosis. Aliment Pharmacol Ther. 2019; 50: 696–706. [DOI] [PubMed] [Google Scholar]
- 28.Henry Z, Patel K, Patton H and Saad W. AGA Clinical Practice Update on Management of Bleeding Gastric Varices: Expert Review. Clin Gastroenterol Hepatol. 2021; 19: 1098–107 e1. [DOI] [PubMed] [Google Scholar]
- 29.Lee EW, Shahrouki P, Alanis L, Ding P and Kee ST. Management Options for Gastric Variceal Hemorrhage. JAMA Surg. 2019; 154: 540–8. [DOI] [PubMed] [Google Scholar]
- 30.Luo X, Xiang T, Wu J, et al. Endoscopic Cyanoacrylate Injection Versus Balloon-Occluded Retrograde Transvenous Obliteration for Prevention of Gastric Variceal Bleeding: A Randomized Controlled Trial. Hepatology. 2021; 74: 2074–84. [DOI] [PubMed] [Google Scholar]
- 31.Villanueva C, Albillos A, Genescà J, et al. Bacterial infections adversely influence the risk of decompensation and survival in compensated cirrhosis. J Hepatol. 2021; 75: 589–99. [DOI] [PubMed] [Google Scholar]
- 32.Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021; 74: 1014–48. [DOI] [PubMed] [Google Scholar]
- 33.EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018; 69: 406–60. [DOI] [PubMed] [Google Scholar]
- 34.Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015; 35: 1508–15. [DOI] [PubMed] [Google Scholar]
- 35.Bai M, Qi XS, Yang ZP, Yang M, Fan DM and Han GH. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. 2014; 20: 2704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bureau C, Thabut D, Oberti F, et al. Transjugular Intrahepatic Portosystemic Shunts With Covered Stents Increase Transplant-Free Survival of Patients With Cirrhosis and Recurrent Ascites. Gastroenterology. 2017; 152: 157–63. [DOI] [PubMed] [Google Scholar]
- 37.Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018; 391: 2417–29. [DOI] [PubMed] [Google Scholar]
- 38.Sola E, Sole C, Simon-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018; 69: 1250–9. [DOI] [PubMed] [Google Scholar]
- 39.Bernardi M, Caraceni P, Navickis RJ and Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012; 55: 1172–81. [DOI] [PubMed] [Google Scholar]
- 40.Singh V, Dhungana SP, Singh B, et al. Midodrine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot study. J Hepatol. 2012; 56: 348–54. [DOI] [PubMed] [Google Scholar]
- 41.Singh V, Singh A, Singh B, et al. Midodrine and clonidine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot study. Am J Gastroenterol. 2013; 108: 560–7. [DOI] [PubMed] [Google Scholar]
- 42.Macken L, Corrigan M, Prentice W, et al. Palliative long-term abdominal drains for the management of refractory ascites due to cirrhosis: a consensus document. Frontline Gastroenterol. 2022; 13: e116–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lepida A, Marot A, Trépo E, Degré D, Moreno C and Deltenre P. Systematic review with meta-analysis: automated low-flow ascites pump therapy for refractory ascites. Aliment Pharmacol Ther. 2019; 50: 978–87. [DOI] [PubMed] [Google Scholar]
- 44.Caraceni P, Abraldes JG, Ginès P, Newsome PN and Sarin SK. The search for disease-modifying agents in decompensated cirrhosis: From drug repurposing to drug discovery. J Hepatol. 2021; 75 Suppl 1: S118–S34. [DOI] [PubMed] [Google Scholar]
- 45.Wu T, Simonetto DA, Halamka JD and Shah VH. The digital transformation of hepatology: The patient is logged in. Hepatology. 2022; 75: 724–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015; 62: 968–74. [DOI] [PubMed] [Google Scholar]
- 47.Cavallin M, Kamath PS, Merli M, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology. 2015. [DOI] [PubMed]
- 48.Wong F, Pappas SC, Curry MP, et al. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N Engl J Med. 2021; 384: 818–28. [DOI] [PubMed] [Google Scholar]
- 49.Singh V, Ghosh S, Singh B, et al. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol. 2012; 56: 1293–8. [DOI] [PubMed] [Google Scholar]
- 50.Arora V, Maiwall R, Rajan V, et al. Terlipressin Is Superior to Noradrenaline in the Management of Acute Kidney Injury in Acute on Chronic Liver Failure. Hepatology. 2020; 71: 600–10. [DOI] [PubMed] [Google Scholar]
- 51.Cavallin M, Piano S, Romano A, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatology. 2016; 63: 983–92. [DOI] [PubMed] [Google Scholar]
- 52.Trawale JM, Paradis V, Rautou PE, et al. The spectrum of renal lesions in patients with cirrhosis: a clinicopathological study. Liver Int. 2010; 30: 725–32. [DOI] [PubMed] [Google Scholar]
- 53.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014; 60: 715–35. [DOI] [PubMed] [Google Scholar]
- 54.European Association for the Study of the Liver. Electronic address eee and European Association for the Study of the L. EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. J Hepatol. 2022; 77: 807–24. [DOI] [PubMed] [Google Scholar]
- 55.Rose CF, Amodio P, Bajaj JS, et al. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J Hepatol. 2020; 73: 1526–47. [DOI] [PubMed] [Google Scholar]
- 56.Vierling JM, Mokhtarani M, Brown RS Jr., et al. Fasting Blood Ammonia Predicts Risk and Frequency of Hepatic Encephalopathy Episodes in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2016; 14: 903–6 e1. [DOI] [PubMed] [Google Scholar]
- 57.Tranah TH, Ballester MP, Carbonell-Asins JA, et al. Plasma ammonia levels predict hospitalisation with liver-related complications and mortality in clinically stable outpatients with cirrhosis. J Hepatol. 2022. [DOI] [PubMed]
- 58.Rockey DC, Vierling JM, Mantry P, et al. Randomized, double-blind, controlled study of glycerol phenylbutyrate in hepatic encephalopathy. Hepatology. 2014; 59: 1073–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trebicka J, Macnaughtan J, Schnabl B, Shawcross DL and Bajaj JS. The microbiota in cirrhosis and its role in hepatic decompensation. J Hepatol. 2021; 75 Suppl 1: S67–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bajaj JS, Heuman DM, Sanyal AJ, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013; 8: e60042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bajaj JS, Heuman DM, Hylemon PB, et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther. 2014; 39: 1113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dhiman RK, Rana B, Agrawal S, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014; 147: 1327–37 e3. [DOI] [PubMed] [Google Scholar]
- 63.Bajaj JS, Salzman NH, Acharya C, et al. Fecal Microbial Transplant Capsules Are Safe in Hepatic Encephalopathy: A Phase 1, Randomized, Placebo-Controlled Trial. Hepatology. 2019; 70: 1690–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirode G, Vittinghoff E and Wong RJ. Increasing Burden of Hepatic Encephalopathy Among Hospitalized Adults: An Analysis of the 2010–2014 National Inpatient Sample. Dig Dis Sci. 2019; 64: 1448–57. [DOI] [PubMed] [Google Scholar]
- 65.Romero-Gomez M, Montagnese S and Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015; 62: 437–47. [DOI] [PubMed] [Google Scholar]
- 66.Cordoba J, Ventura-Cots M, Simón-Talero M, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). Journal of Hepatology. 2014; 60: 275–81. [DOI] [PubMed] [Google Scholar]
- 67.Tapper EB, Essien UR, Zhao Z, Ufere NN and Parikh ND. Racial and ethnic disparities in rifaximin use and subspecialty referrals for patients with hepatic encephalopathy in the United States. J Hepatol. 2022; 77: 377–82. [DOI] [PubMed] [Google Scholar]
- 68.Mangini C and Montagnese S. New Therapies of Liver Diseases: Hepatic Encephalopathy. J Clin Med. 2021; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butterworth RF and McPhail MJW. L-Ornithine L-Aspartate (LOLA) for Hepatic Encephalopathy in Cirrhosis: Results of Randomized Controlled Trials and Meta-Analyses. Drugs. 2019; 79: 31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fagan A, Gavis EA, Gallagher ML, et al. A double-blind randomized placebo-controlled trial of albumin in outpatients with hepatic encephalopathy: HEAL study. Journal of Hepatology. 2022. [DOI] [PubMed]
- 71.Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018; 391: 2417–29. [DOI] [PubMed] [Google Scholar]
- 72.Riggio O, Masini A, Efrati C, et al. Pharmacological prophylaxis of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: a randomized controlled study. J Hepatol. 2005; 42: 674–9. [DOI] [PubMed] [Google Scholar]
- 73.Bureau C, Thabut D, Jezequel C, et al. The Use of Rifaximin in the Prevention of Overt Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt : A Randomized Controlled Trial. Ann Intern Med. 2021; 174: 633–40. [DOI] [PubMed] [Google Scholar]
- 74.Bajaj JS, Hassanein TI, Pyrsopoulos NT, et al. Dosing of Rifaximin Soluble Solid Dispersion Tablets in Adults With Cirrhosis: 2 Randomized, Placebo-controlled Trials. Clin Gastroenterol Hepatol. 2022. [DOI] [PubMed]