Abstract
Background and Objectives
Amyloid-related imaging abnormalities (ARIA) were the most common adverse events reported in the phase 3 ENGAGE and EMERGE trials of aducanumab, an anti-amyloid monoclonal antibody. APOE ε4 carrier status has been shown to increase risk of ARIA in prior trials of aducanumab and other anti-amyloid therapies; however, the remainder of the human genome has not been evaluated for ARIA risk factors. Therefore, we sought to determine in a hypothesis-free manner whether genetic variants beyond APOE influence risk of ARIA in aducanumab-treated patients.
Methods
We performed genome-wide association studies (GWAS) of ARIA in participants in the ENGAGE and EMERGE trials. Participants had mild cognitive impairment due to Alzheimer disease or mild Alzheimer disease dementia and were amyloid-positive on PET scans. All participants underwent regular MRI monitoring to detect and diagnose ARIA.
Results
Of the 3,285 participants in the intent-to-treat population, this analysis included 1,691 with genotyping array data who received at least one dose of aducanumab with at least one post-baseline MRI. All participants in the study cohort were of European ancestry; 51% were female. The mean age was 70.3 years. 31% had ARIA-E, 19% had ARIA-H microhemorrhage, and 14% had ARIA-H superficial siderosis. We identified one genome-wide significant (p < 5.0 × 10−8) association at the chromosome 19 locus encompassing APOE. The APOE association with ARIA was stronger in ε4/ε4 homozygotes (OR = 4.28, 4.58, 7.84; p < 2.9 × 10−14 for ARIA-E, ARIA-H microhemorrhage, and ARIA-H superficial siderosis, respectively) than in ε3/ε4 heterozygotes (OR = 1.74, 1.46, 3.14; p ≤ 0.03). We found greater odds of radiographically severe ARIA (OR = 7.04–24.64, p ≤ 2.72 × 10−5) than radiographically mild ARIA (OR = 3.19–5.00, p ≤ 1.37 × 10−5) among ε4/ε4 homozygotes. APOE ε4 was also significantly associated with both symptomatic (ε4/ε4 OR = 3.64–9.52; p < 0.004) and asymptomatic (ε4/ε4 OR = 4.20–7.94, p < 1.7 × 10−11) cases, although among ARIA cases, APOE did not appear to modulate symptomatic status. No other genome-wide significant associations were found.
Discussion
We identified a strong, genome-wide significant association between APOE and risk of ARIA. Future, larger studies may be better powered to detect associations beyond APOE. These findings indicate that APOE is the strongest genetic risk factor of ARIA incidence, with implications for patient management and risk-benefit treatment decisions.
Trial Registration Information
Both trials (ENGAGE [221AD301]: NCT02477800 and EMERGE [221AD302]: NCT02484547) were registered in June 2015 at clinicaltrials.gov and enrolled patients from August 2015 to July 2018.
Introduction
Amyloid-related imaging abnormalities (ARIA) refer to a range of findings detected on brain MRI that are associated with the use of monoclonal antibodies targeting amyloid beta (Aβ) in patients with Alzheimer disease.1 ARIA can occur with edema or sulcal effusion (ARIA-E) or hemorrhage (ARIA-H) characterized by hemosiderin deposition, which occurs with microhemorrhage or with superficial siderosis.1 Most ARIA cases are asymptomatic2 (e.g., for ARIA-E, 74% of 10 mg/kg aducanumab,3 78% of donanemab,4 78% of lecanemab,5 >80% of gantenerumab,6 85% of bapineuzumab APOE ε4 carriers7 were asymptomatic). Clinical manifestations of symptomatic cases range from headaches (47% in phase 3 trials of aducanumab3) to, in rare instances, seizures (<1%), the majority of which are transient (83% of ARIA-E resolved radiographically within 16 weeks3).
The biological underpinnings of ARIA remain poorly understood, but several nonexclusive mechanistic hypotheses implicating cerebral amyloid angiopathy (CAA) have been developed to explain the occurrence of ARIA after treatment with anti-Aβ antibodies. Direct clearance of CAA, followed by disruption of the vascular integrity and vascular leakage, is often cited as the primary cause of ARIA.8 Mobilization of amyloid cleared from the parenchymal plaques into an already dysfunctional perivascular drainage system, because of the presence of CAA, has also been suggested to potentially play a role. In addition, the current therapeutic antibodies, having full effector function and the ability to engage microglial cells, might also trigger and/or exacerbate CAA-related perivascular inflammation.9,10
ARIA was the most common adverse event in the phase 3 clinical trials of aducanumab, which has been approved by the US Food and Drug Administration for the treatment of Alzheimer disease under the accelerated approval pathway.3,11 ARIA has been observed in other anti-amyloid antibody therapies including bapineuzumab,7 crenezumab,12 donanemab,4 ponezumab,13 solanezumab,14 gantenerumab,6 and lecanemab.5 In clinical trials of these therapies, ARIA-E incidence ranged from 0.7% in ponezumab to 35.2% in aducanumab while ARIA-H incidence ranged from 4.9% in crenezumab and solanezumab to 19.1% in aducanumab.15
A widely reported risk factor of ARIA is carriage of the APOE ε4 allele, which is also found to be the strongest risk factor of late-onset Alzheimer disease.16 In addition, the APOE ε4 genotype has been shown to influence ARIA risk in a dose-dependent manner based on carriage of one (heterozygote) or 2 (homozygote) copies of the ε4 allele.5,6,17,18 It is not known, however, whether APOE ε4 is the primary genetic driver of ARIA risk or whether other genetic factors, including AD risk variants such as those found in TREM2,16 influence risk of aducanumab-related ARIA. The ENGAGE and EMERGE trials represent a unique opportunity to investigate genetic factors beyond APOE because they are the largest phase 3 pool of safety data of a monoclonal antibody to date. We therefore conducted a genome-wide association study (GWAS) to determine, in a hypothesis-free manner, the genetic associations with ARIA among aducanumab-treated patients in the ENGAGE and EMERGE trials.
Methods
Cohort Description
A full description of the ENGAGE and EMERGE trials (N = 3,285) has been published previously.3,11 Briefly, ENGAGE and EMERGE were 2 phase 3 clinical trials which evaluated safety and efficacy of aducanumab, in participants with mild cognitive impairment due to Alzheimer disease and early Alzheimer disease dementia. Trial participants were aged 50–85 years, with confirmed Aβ pathology by amyloid-positive PET scans. The main inclusion and exclusion criteria are presented in eTable 1 (links.lww.com/WNL/D269).19,20 Participants were recruited from 348 sites in 20 countries.11 Participants were randomized 1:1:1 to low-dose (3 mg/kg for APOE ε4 carriers, 6 mg/kg for APOE ε4 noncarriers), high-dose (before protocol version 4: 6 mg/kg for APOE ε4 carriers, 10 mg/kg for APOE ε4 noncarriers; after protocol version 4: 10 mg/kg regardless of APOE ε4 status), and placebo groups over 76 weeks, with a long-term extension (LTE) period for up to an additional 5 years.
The analyses presented here included all the participants in the ENGAGE and EMERGE studies who received at least one infusion of aducanumab (at any dose, either during the placebo-controlled period or switched to aducanumab during the LTE period) and who consented for genetic testing and had at least one post-baseline MRI assessment.
Standard Protocol Approvals, Registrations, and Patient Consents
The original trials were conducted in accordance with the Declaration of Helsinki and the International Conference for Harmonisation and Good Clinical Practice guidelines and were approved by ethics committees or institutional review boards at each site. Study participants provided written informed consent.
ARIA
A detailed description of ARIA assessment has been published previously.3 Briefly, ARIA was detected using MRI and evaluated by a centralized reader with expertise in the diagnosis of ARIA. MRIs were performed for all participants at baseline and at weeks 14, 22, 30, 42, 54, 66, and 78 of the placebo-controlled period; regular monitoring continued during the LTE period. If ARIA was detected, additional MRI scans were conducted every 4 weeks until ARIA was resolved or stabilized. ARIA was categorized as ARIA with edema or effusion (ARIA-E) or as ARIA with hemorrhage (ARIA-H), characterized either by microhemorrhage or superficial siderosis. ARIA-H macrohemorrhage was also identified, but was not included in this genetic analysis due to very small sample size (N = 4 in the genetic cohort).
All forms of ARIA were categorized by radiographic severity. ARIA-E was considered mild (single edematous region <5 cm), moderate (a single region 5–10 cm or multiple regions summing up < 10 cm), or severe (any region >10 cm). Similarly, ARIA-H microhemorrhage cases were classified by the number of new (incident) microhemorrhages, including mild (N = 1–4), moderate (N = 5–9), or severe (N ≥ 10) microhemorrhages. ARIA-H superficial siderosis was classified by the number of new (incident) areas of superficial siderosis: mild (N = 1), moderate (N = 2), and severe (N > 2 incident areas). In addition, ARIA cases were classified as symptomatic or asymptomatic based on participant self-report.
Genotyping Data
Two thousand eight hundred and eighty two participants provided consent for genetic research related to aducanumab. Of these, 2,408 had sufficient DNA concentrations for genotyping. All samples were genotyped on the Illumina Infinium Global Screening Array-24v3.0 at the Broad Institute (Cambridge, MA).
Standard quality control (QC) metrics were applied to samples and genetic variants. Variants were excluded if they had high levels of missingness (>0.98; N = 28,024), low minor allele frequency (MAF <0.02; N = 187,930), or were out of Hardy-Weinberg equilibrium (HWE >10−50; N = 18,934). Samples were excluded if they had high levels of missingness (>2%; N = 2), sex inconsistencies (N = 37), or excess heterozygosity (>6 SD from the mean; N = 14). Samples were also evaluated for relatedness and unexpected duplicates. All duplicates (pihat >0.9; N = 16) and one member of each related pair (pihat >0.4; N = 3) were excluded.
We conducted principal component analysis to determine genetic ancestry and generate principal components (PCs) to control for as covariates in the GWAS. We did not analyze participants of non-European ancestries (N = 271) because the small number of samples of other ancestries prevented stratified analysis or multiancestry approaches. We used SmartPCA in EIGENSTRAT to identify outliers (N = 63) and then further excluded PC1 >0.01 to remove a distinct cluster primarily comprising self-reported Asian race (N = 194) and finally excluded self-reported race other than “White” or “Not Reported” (N = 14). To determine the number of PCs to include as covariates in our genetic models, we used Tracy-Widom (TW) statistics.16 PCs were included if TW p was < 0.05 that accounted for ≥1% of variance. This resulted in a list of 12 PCs; however, we chose to include the top 10 PCs as covariates to limit the loss of power incurred with an increasing number of variables in a model. We excluded samples with discordant APOE genotypes between targeted genotyping results generated during trial screening and APOE genotypes generated by exome sequencing (N = 11).
Post-QC genotypes were imputed to the 1,000-genome reference panel using the Michigan Imputation server.21 We removed imputed variants with low imputation scores (R2 < 0.3), low-frequency variants (MAF <0.01), and variants out of HWE (>10−50), leaving a total of 9.5 million SNPs in 2,054 samples for analysis.
Exome Sequencing Data
Samples underwent exome sequencing at the Broad Institute (Cambridge, MA). First, libraries were constructed, which included library preparation, hybrid capture, sequencing with 150bp paired-end reads, and sample identification QC check. Libraries were then sequenced using paired-end sequencing on an Illumina platform. The Laboratory Picard bioinformatics pipeline was implemented, with a goal of 85% of targets with >20× coverage. The Broad Institute provided jointly called variant call files (VCFs) generated using GATK best practices.22 This process converted raw CRAM files to FASTQ files, followed by mapping to a reference sequence, producing BAM files. Variants were then called using HaploypteCaller to produce VCFs. Individual VCFs were merged and jointly called.
After transfer of jointly called files to a secure server, we performed sample-level and variant-level QC. The GATK pipeline generates a quality score for each variant, referred to as Variant Quality Score Recalibration (VQSR). This variable is generated through a machine learning method which incorporates multiple-variant QC metrics to determine whether a variant is likely to pass or fail quality control.22 We first filtered out variants which failed VQSR (N = 334,149). After manual review of additional variant QC metrics, we excluded variants with Fisher strand bias >40, strand odds ratio >3, mapping quality rank sum < −5, mapping quality rank sum >5, read position rank sum < −4, quality by depth <2, mapping quality <40, and depth across samples >2× the mean (147,000; N = 259,516). Individual analyses also excluded variants which were out of Hardy-Weinberg equilibrium (HWE >10−50). We also excluded samples with a low call rate (<0.80; N = 1), samples with sex inconsistencies (N = 40), and unexpected duplicates (N = 16). Because genotyping data (which includes variants across the genome) can more accurately evaluate relatedness and genetic ancestry than exome sequencing data (which includes variants only in the coding region), we excluded one sample from each pair identified as related using the genotyping data (N = 3) and non-European ancestry samples identified using the genotyping data (N = 290) and APOE inconsistencies (N = 11). Post-QC exome sequencing data resulted in >560,000 variants and 2,053 samples for analysis.
Statistical Analysis
To maximize sample size, analyses were based on the aducanumab-treated period, consisting of both the placebo-controlled period of each study (for participants who received aducanumab in the placebo-controlled period) and the LTE period (for all participants who received at least one dose). Analyses included only participants on aducanumab treatment, with genetic data which passed QC, and at least one post-baseline MRI. For the logistic models analyzing association with ARIA risk, all participants with available data were included in the risk set as they contribute information to the model. The logistic model results, therefore, can be interpreted as the increased odds of experiencing ARIA-E compared with not experiencing ARIA-E, regardless of other events (with a corresponding interpretation for ARIA-H).
For both genome-wide and exome-wide analyses, we ran 2 models. Model 1 controlled for age at study baseline, sex, study (ENGAGE or EMERGE), last dose level before the ARIA event or censor, and genetic ancestry measured by PCs 1–10. To identify genetic variants independent of APOE, Model 2 adjusted for all covariates used in Model 1, with the addition of the 2 SNPs defining APOE genotype, rs429358-C and rs7412-T. We ran analyses for any ARIA; any ARIA-H; and separately for ARIA-E, ARIA-H microhemorrhage, and ARIA-H superficial siderosis. In addition, we stratified by symptomatic status and radiographic severity. ARIA-H can occur with or without ARIA-E; therefore, we performed secondary analyses among isolated ARIA-H cases. All analyses were run in PLINK2.
APOE genotypes were calculated using the SNPs rs429358-C and rs7412-T, and logistic regression was run evaluating the association between each form of ARIA and the APOE genotype, using the so-called neutral APOE genotype ε3/ε3 category as the reference group, using the same covariates as in the GWAS Model 1. For analyses stratifying ARIA outcomes by radiographic severity or symptomatic status, we excluded the ε2/ε3 and ε2ε4 genotypes because the small number of participants in those groups led to potential model convergence issues. Logistic regression evaluating association between APOE genotype and ARIA as well as plots were generated with R (version 4.1.1). Kaplan-Meier plots were produced using SAS software version 9.4 (SAS Institute). We additionally ran gene-based analyses in an attempt to increase power from the single-variant exome-wide analysis. We used the R package SKAT to run SKAT-O tests, after annotating variants with MAF < 0.01 using Variant Effect Predictor.23
To determine whether genetic variants associated with Alzheimer disease across the genome contributed to risk of ARIA, we ran a polygenic risk score (PRS) analysis. The PRS was constructed using GWAS summary statistics from Schwartzentruber et al.,24 applying a clumping and thresholding approach, including variants with p < 0.1 and excluding the APOE region (chr19:44.4–46.5 Mb).25 The PRS was then inverse rank normal transformed. We performed linear regression for the association between each type of ARIA and the Alzheimer disease PRS, controlling for number of APOE ε2 alleles, number of APOE ε4 alleles, age, sex, study, last dose level before the ARIA event or censor, and the first 10 PCs.
Data Availability
While the data described in this article are not publicly available, the authors and Biogen are supportive of data sharing. Biogen has established processes to share protocols, clinical study reports, study-level data, and deidentified patient-level data. These data and materials will be made available to qualified scientific researchers in support of the objective(s) in their approved, methodologically sound research proposal. Proposals should be submitted through Vivli (vivli.org/). To gain access, data requestors will need to sign a data sharing agreement. For general inquiries, please contact datasharing@biogen.com. Biogen's data sharing policies and processes are detailed on the website biogentrialtransparency.com/.
Results
Of the participants who underwent genome-wide genotyping and passed QC metrics (N = 2,054), 1,691 were also treated with aducanumab and had at least one post-baseline MRI. Of these, 529 had ARIA-E, 324 had ARIA-H microhemorrhage, 229 had ARIA-H superficial siderosis, and 1,047 experienced none of these events (Table 1). Incidences of all types of ARIA were similar in this genetic cohort compared with the full cohort, as were demographic characteristics, with the exception of race and region.3 Incidences of all ARIA types were similar between ENGAGE and EMERGE trials. Sex was evenly split across ARIA-E and ARIA-H microhemorrhage; however, of those who experienced ARIA-H superficial siderosis, 40% were female. The mean age was similar. A greater proportion of participants homozygous for APOE ε4 experienced ARIA, as was seen in the full cohort.
Table 1.
Demographics of ENGAGE and EMERGE Participants With ARIA and Genetic Data
| ARIA-E (N = 529) | ARIA-H microhemorrhage (N = 324) | ARIA-H superficial siderosis (N = 229) | ARIA-H (any) (N = 454) | ARIA (any) (N = 644) | Nonea (N = 1,047) | Full genetic cohort (N = 1,691) | |
| Study | |||||||
| ENGAGE No. (%) | 264 (50) | 153 (47) | 122 (53) | 222 (49) | 313 (49) | 491 (47) | 804 (48) |
| EMERGE No. (%) | 265 (50) | 171 (53) | 107 (47) | 232 (51) | 331 (51) | 556 (53) | 887 (52) |
| Sex, % female | 267 (50) | 167 (52) | 91 (40) | 212 (47) | 322 (50) | 535 (51) | 857 (51) |
| Age, mean (SD) | 70.1 (7.4) | 70.9 (7.2) | 70.4 (7.0) | 70.7 (7.3) | 70.5 (7.4) | 70.1 (7.4) | 70.3 (7.4) |
| APOE genotype, n (%) | |||||||
| ε2/ε2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ε2/ε3 | 9 (2) | 3 (1) | 4 (2) | 7 (2) | 12 (2) | 24 (2) | 36 (2) |
| ε2/ε4 | 13 (2) | 6 (2) | 4 (2) | 8 (2) | 15 (2) | 19 (2) | 34 (2) |
| ε3/ε3 | 86 (16) | 54 (17) | 22 (10) | 67 (15) | 112 (17) | 354 (34) | 466 (28) |
| ε3/ε4 | 258 (49) | 144 (44) | 113 (49) | 219 (48) | 315 (49) | 527 (50) | 842 (50) |
| ε4/ε4 | 163 (31) | 117 (36) | 86 (38) | 1,553 (34) | 190 (30) | 123 (12) | 313 (19) |
| Dose at ARIA or censor, n (%) | |||||||
| 1 | 2 (<1) | 2 (1) | 1 (<1) | — | — | — | — |
| 3 | 300 (57) | 188 (58) | 122 (53) | — | — | — | — |
| 6 | 106 (20) | 51 (16) | 39 (17) | — | — | — | — |
| 10 | 121 (23) | 83 (26) | 67 (29) | — | — | — | — |
| Radiographic severity, n (%) | — | — | — | ||||
| Mild | 155 (29) | 237 (73) | 113 (49) | ||||
| Moderate | 304 (57) | 41 (13) | 62 (27) | ||||
| Severe | 70 (13) | 46 (14) | 54 (24) | ||||
| Symptomatic ARIA | 133 (25) | 39 (12) | 33 (14) | — | — | — | — |
All participants were of European ancestry and from geographic regions Europe/Canada/Australia and the United States.
None = no ARIA-E, ARIA-H microhemorrhage, or ARIA-H superficial siderosis.
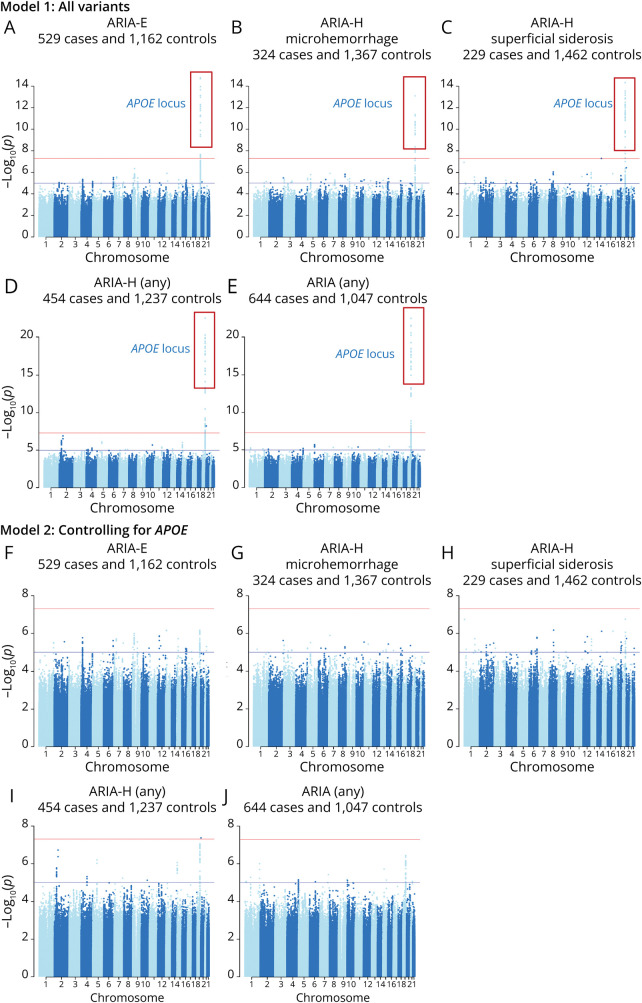
In the GWAS, the most significant association was a locus in the APOE region, which was strongly and genome-wide significantly associated with risk of ARIA overall and with all 3 types of ARIA analyzed as well as any ARIA-H (Figure, Table 2). There was no evidence of genomic inflation (lambda <1.03, eFigure 1, links.lww.com/WNL/D269). When conditioning on the APOE genotype, no additional variants were genome-wide significant, indicating a single independent hit in this locus. The association with ARIA occurred in an APOE ε4 dose-dependent manner: participants homozygous for APOE ε4 exhibited 4.28 greater odds of experiencing ARIA-E, 4.58 greater odds of experiencing ARIA-H microhemorrhage, and 7.84 greater odds of experiencing ARIA-H superficial siderosis when compared with ε3/ε3 homozygotes (Table 2). There was a less pronounced effect of carrying one copy of the ε4 allele (ε2/ε4 or ε3/ε4) in ARIA-E (OR = 1.74–2.60), ARIA-H microhemorrhage (OR = 1.46–1.63), and ARIA-H superficial siderosis (OR = 2.64–3.14). We did not find a protective effect of the APOE ε2 allele in a small, underpowered group of ε2/ε3 carriers (N = 36; p = 0.12–0.69). Time to first ARIA analysis showed similar results to the main analysis (eFigure 2). Analysis of targeted APOE genotyping data from the full cohort showed similar results (eTable 2).
Figure. Manhattan Plots for GWAS for ARIA-E, ARIA-H Microhemorrhage, ARIA-H Superficial Siderosis, and Any ARIA.
All ARIA outcomes show a strong peak in the APOE locus (Model 1) and no additional genome-wide significant loci (as indicated by horizontal red line). Model 2, controlling for APOE-tagging SNPs, rs429358_C and rs7412_T, resulted in no genome-wide significant hits in any ARIA analysis, indicating a single independent peak within this locus. ARIA = amyloid-related imaging abnormality.
Table 2.
| APOE genotype | ARIA-E | ARIA-H microhemorrhage | ARIA-H superficial siderosis | |||||||||
| (N = 529 vs 1,162 controls) | (N = 324 vs 1,367 controls) | (N = 229 vs 1,462 controls) | ||||||||||
| OR | 95% CI | RRc | p Value | OR | 95% CI | RRc | p Value | OR | 95% CI | RRc | p Value | |
| ε2/ε3 | 1.47 | 0.66–3.28 | 1.37 | 0.34 | 0.78 | 0.23–2.65 | 0.81 | 0.69 | 2.47 | 0.79–7.7 | 2.15 | 0.12 |
| ε2/ε4 | 2.60 | 1.23–5.50 | 2.07 | 0.01 | 1.63 | 0.64–4.17 | 1.47 | 0.31 | 2.64 | 0.84–8.29 | 2.27 | 0.10 |
| ε3/ε4 | 1.74 | 1.30–2.31 | 1.55 | 0.0002 | 1.46 | 1.03–2.06 | 1.35 | 0.03 | 3.14 | 1.94–5.09 | 2.59 | 3.2E-06 |
| ε4/ε4 | 4.28 | 3.04–6.02 | 2.81 | 6.2E-17 | 4.58 | 3.09–6.78 | 2.85 | 2.9E-14 | 7.84 | 4.67–13.19 | 4.66 | 7.8E-15 |
Values are compared with the ε3/ε3 reference group.
Models controlled for age at study baseline, sex, study (ENGAGE or EMERGE), last dose level before the ARIA event or censor, and genetic ancestry measured by PCs 1–10.
RR was calculated from OR using the equation RR = OR/(1−p0 + (p0 × OR)), where p0 is the baseline risk defined as ε3/ε3 incidence (16% for ARIA-E, 17% for ARIA-H microhemorrhage, 10% for ARIA-H with superficial siderosis).
To further investigate the effect of APOE on risk of ARIA, we stratified by radiographic severity and symptomatic/asymptomatic status. APOE ε4/ε4 genotypes were significantly associated with mild, moderate, and severe radiographic ARIA (Table 3). The effect was stronger among ε4/ε4 homozygotes than ε3/ε4 heterozygotes and showed a larger effect in severe (ε4/ε4 OR = 7.04–24.64, p ≤ 2.38 × 10−6) vs mild (ε4/ε4 OR = 3.19–5.00, p ≤ 1.37 × 10−5) cases.
Table 3.
| ARIA-E | ||||||||||||
| Mild (N = 146 vs 1,114 controls) | Moderate (N = 295 vs 1,114 controls) | Severe (N = 66 vs 1,114 controls) | ||||||||||
| APOE genotype | OR | 95% CI | RR | p Value | OR | 95% CI | RR | p Value | OR | 95% CI | RR | p Value |
| ε3/ε4 | 1.74 | 1.11–2.71 | 1.55 | 0.02 | 1.70 | 1.18–2.46 | 1.53 | 0.005 | 2.09 | 0.88–4.98 | 1.78 | 0.09 |
| ε4/ε4 | 3.37 | 1.95–5.82 | 2.44 | 1.37E-05 | 4.19 | 2.75–6.39 | 2.77 | 2.89E-11 | 7.04 | 2.83–17.53 | 3.58 | 2.72E-05 |
| ARIA-H microhemorrhage | ||||||||||||
| Mild (N = 230 vs 1,306 controls) | Moderate (N = 39 vs 1,306 controls) | Severe (N = 46 vs 1,306 controls) | ||||||||||
| APOE genotype | OR | 95% CI | RR | p Value | OR | 95% CI | RR | p Value | OR | 95% CI | RR | p Value |
| ε3/ε4 | 1.33 | 0.91–1.93 | 1.26 | 0.14 | 2.23 | 0.72–6.9 | 1.84 | 0.16 | 2.60 | 0.72–9.37 | 2.04 | 0.14 |
| ε4/ε4 | 3.19 | 2.06–4.93 | 2.32 | 1.83E-07 | 8.27 | 2.49–27.52 | 3.70 | 0.001 | 21.40 | 5.99–76.42 | 4.79 | 2.38E-06 |
| ARIA-H with superficial siderosis | ||||||||||||
| Mild (N = 108 vs 1,400 controls) | Moderate (N = 60 vs 1,400 controls) | Severe (N = 53 vs 1,400 controls) | ||||||||||
| APOE genotype | OR | 95% CI | RR | p Value | OR | 95% CI | RR | p Value | OR | 95% CI | RR | p Value |
| ε3/ε4 | 33.30 | 1.77–6.15 | 2.68 | 0.0002 | 2.11 | 0.89–5.01 | 1.90 | 0.09 | 6.48 | 1.5–28.04 | 4.18 | 0.01 |
| ε4/ε4 | 5.00 | 2.48–10.08 | 3.57 | 6.76E-06 | 8.17 | 3.34–19.96 | 4.76 | 4.05E-06 | 24.64 | 5.64–107.68 | 7.32 | 2.06E-05 |
Values are compared with the ε3/ε3 reference group.
Models controlled for age at study baseline, sex, study (ENGAGE or EMERGE), last dose level before the ARIA event or censor, and genetic ancestry measured by PCs 1–10. ε2/ε3 and ε2/ε4 genotypes were excluded from these analyses because small sample sizes in these groups led to potential model convergence issues.
APOE was also associated with both symptomatic (ε4/ε4 OR = 3.64–9.52; p < 0.004) and asymptomatic (ε4/ε4 OR = 4.20–7.94, p < 1.7 × 10−11) ARIA (Table 4). These associations showed a stronger effect in APOE ε4/ε4 homozygotes vs ε3/ε4 heterozygotes. However, in an analysis among only those who experienced ARIA, no association was seen for APOE when comparing symptomatic vs asymptomatic cases (p > 0.05; Table 4).
Table 4.
| ARIA-E | ||||||||||||
| APOE genotype | Symptomatic (N = 125 vs 1,114 controls) | Asymptomatic (N = 382 vs 1,114 controls) | Symptomatic vs asymptomatic (N = 125 vs 382) | |||||||||
| OR | 95% CI | RR | p Value | OR | 95% CI | RR | p Value | OR | 95% CI | RR | p Value | |
| ε3/ε4 | 1.79 | 1.04–3.06 | 1.59 | 0.03 | 1.70 | 1.24–2.35 | 1.53 | 0.001 | 1.10 | 0.6–2.03 | 1.09 | 0.75 |
| ε4/ε4 | 3.64 | 1.97–6.73 | 2.56 | 3.8E-05 | 4.56 | 3.13–6.63 | 2.90 | 2.2E-15 | 0.79 | 0.41–1.54 | 0.82 | 0.49 |
| ARIA-H microhemorrhage | ||||||||||||
| APOE genotype | Symptomatic (N = 38, vs 1,306 controls) | Asymptomatic (N = 277 vs 1,306 controls) | Symptomatic vs asymptomatic (N = 38 vs 277) | |||||||||
| OR | 95% CI | RR | p Value | OR | 95% CI | RR | p Value | OR | 95% CI | RR | p Value | |
| ε3/ε4 | 1.03 | 0.37–2.88 | 1.02 | 0.96 | 1.50 | 1.04–2.16 | 1.38 | 0.03 | 0.58 | 0.19–1.74 | 0.62 | 0.33 |
| ε4/ε4 | 7.41 | 2.73–20.08 | 3.55 | 8.3E-05 | 4.20 | 2.76–6.37 | 2.72 | 1.7E-11 | 1.44 | 0.5–4.11 | 1.34 | 0.50 |
| ARIA-H with superficial siderosis | ||||||||||||
| APOE genotype | Symptomatic (N = 32 vs 1,400 controls) | Asymptomatic (N = 189 vs 1,400 controls) | Symptomatic vs asymptomatic (N = 32 vs 189) | |||||||||
| OR | 95% CI | RR | p Value | OR | 95% CI | RR | p Value | OR | 95% CI | RR | p Value | |
| ε3/ε4 | 4.81 | 1.08–21.35 | 3.48 | 0.04 | 3.01 | 1.81–5.00 | 2.51 | 2.1E-05 | 2.16 | 0.43–11.00 | 1.94 | 0.35 |
| ε4/ε4 | 9.52 | 2.03–44.61 | 5.14 | 0.004 | 7.94 | 4.59–13.73 | 4.69 | 1.2E-13 | 1.27 | 0.24–6.77 | 1.24 | 0.78 |
Values are compared with the ε3/ε3 reference group.
Models controlled for age at study baseline, sex, study (ENGAGE or EMERGE), last dose level before the ARIA event or censor, and genetic ancestry measured by PCs 1–10. Ε2/ε3 and ε2/ε4 genotypes were excluded from these analyses because small sample sizes in these groups led to potential model convergence issues.
There was no significant association between APOE and isolated ARIA-H either microhemorrhage or superficial siderosis, although sample sizes were small, and hence, analyses were underpowered (Table 5). Odds ratios trended in a similar direction toward ARIA-H combined with ARIA-E.
Table 5.
| ARIA-H microhemorrhage | ARIA-H superficial siderosis | |||||||||||
| APOE genotype | Isolated | Combined with ARIA-E | Isolated | Combined with ARIA-E | ||||||||
| (N = 84 vs 1,537 controls) | (N = 231 vs 1,390 controls) | (N = 29 vs 1,592 controls) | (N = 192 vs 1,429 controls) | |||||||||
| OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | |
| ε3/ε4 | 1.05 | 0.6–1.84 | 0.85 | 1.66 | 1.09–2.54 | 0.02 | 1.88 | 0.66–5.35 | 0.24 | 3.45 | 2.02–5.92 | 6.42E-06 |
| ε4/ε4 | 1.69 | 0.86–3.32 | 0.13 | 5.55 | 3.51–8.77 | 2.16E-13 | 2.66 | 0.8–8.81 | 0.11 | 8.90 | 5.03–15.77 | 6.75E-14 |
Values are compared with the ε3/ε3 reference group.
Models controlled for age at study baseline, sex, study (ENGAGE or EMERGE), last dose level before the ARIA event or censor, and genetic ancestry measured by PCs 1–10. Ε2/ε3 and ε2/ε4 genotypes were excluded from these analyses because small sample sizes in these groups led to potential model convergence issues.
Isolated ARIA-H is defined as ARIA-H in patients who experienced no ARIA-E during the aducanumab-treated period; all other ARIA-H is considered to be combined with ARIA-E (regardless of whether the ARIA-E and ARIA-H events were contemporaneous).
After controlling for the APOE locus, we found no additional genome-wide significant associations independent of the APOE region (Figure). Similarly, the exome-wide association study found no significant, high-confidence associations beyond the APOE locus (eFigure 3, links.lww.com/WNL/D269). Gene-based tests did not identify additional significant signals associated with the ARIA traits (eTable 3). Note that no APOE SNPs met the allele frequency threshold of MAF <0.01, thus APOE was not tested in the gene-based analysis.
The PRS analysis revealed a significant association between increased polygenic risk of Alzheimer disease and all 3 types of ARIA (ARIA-E: beta = 0.14, p = 0.04; ARIA-H microhemorrhage: beta = 0.20, p = 0.01; ARIA-H with superficial siderosis beta = 0.20, p = 0.03; eTable 4, links.lww.com/WNL/D269).
Discussion
In this genome-wide assessment of genetic risk factors of ARIA, we identified a robust association between genetic variants at the APOE locus and risk of developing aducanumab-related ARIA-E and ARIA-H, with no additional associations observed beyond this region. The strong association observed at APOE in this analysis is consistent with observations from other amyloid-lowering therapies, which also found an elevated incidence of ARIA among APOE ε4 carriers5,6,17,18 and in a recent systematic review of ARIA.26 A phase 2 study of bapineuzumab found a hazard ratio of 3.8 for APOE ε4 heterozygotes and 7.15 for APOE ε4 homozygotes for ARIA-E.17 A phase 3 study of solanezumab did not show a significantly increased incidence of ARIA-E among ε4 heterozygotes.18 Similarly, a phase 3 study of gantenerumab showed that both ARIA-E and ARIA-H occurred more frequently among APOE ε4 heterozygotes vs noncarriers and among APOE ε4 homozygotes vs APOE ε4 heterozygotes.6 In a phase 3 trial of lecanemab, ARIA-E was more frequent among homozygotes (32.6%) than heterozygotes (10.9%) or noncarriers (5.4%) in the treatment arm. A similar pattern was seen for ARIA-H (homozygotes: 39%, heterozygotes 14%, noncarrier: 11.9%).5 In addition, in the phase 3 trials of aducanumab, more APOE ε4 carriers experienced aducanumab-related ARIA than noncarriers (35.9% of APOE ε4 heterozygous and 66% of APOE ε4 homozygotes experienced ARIA).3,11 Similarly, in a phase 3 study of donanemab, among treated participants ARIA-E occurred in 15.7% of APOE ε4 noncarriers, 22.8% of APOE ε4 heterozygotes, and 40.6% of APOE ε4 homozygotes.27
In addition, this study highlights the importance of reporting heterozygosity by genotype when evaluating its association with ARIA, given the substantially larger effects observed among ε4 homozygotes vs heterozygotes in contrast to earlier publications addressing ARIA in aducanumab, which reported APOE primarily by the presence or absence of ε4 alleles,3,11 masking differences between the groups. Notably, from a patient care perspective, the same laboratory genotyping test is used to determine the number of APOE ε4 alleles a patient carries as that used to determine whether the patient is an APOE ε4 carrier or not. Thus, this information is readily available for individuals who have APOE carrier status tested and does not require more sophisticated or further testing for number of APOE ε4 alleles.
Our evaluation of genetic risk factors of ARIA across the full genome did not detect genome-wide significant associations beyond the APOE locus. However, we observed a significant association between polygenic risk of Alzheimer disease and ARIA, indicating that additional genetic variants beyond APOE likely contribute toward ARIA risk. Although this study represents the largest published GWAS of ARIA to date, it is still underpowered to detect more modest associations. For example, we were well-powered (∼80% power) to detect a relative risk of 1.6 for ARIA-E, 1.8 for ARIA-H microhemorrhage, and 2.0 for ARIA-H superficial siderosis for common variants with allele frequencies of 0.3 (eTable 5, links.lww.com/WNL/D269). This allowed us to detect the strong association with the APOE locus. We were underpowered to detect associations of more rare variants or smaller effect sizes of the range often observed in large GWAS. Larger studies in the future may detect additional associations, shedding light on the biological mechanisms contributing toward ARIA. For example, studies with 1,000–2,000 cases would be well-powered to detect moderate effect sizes for common variants (eTable 5). This may be achieved through meta-analysis of multiple amyloid-targeting clinical trials. For potential clinical risk prediction, our results indicate that no other loci in the genome are associated with ARIA at a comparable magnitude with APOE; thus, APOE remains the strongest genetic candidate for prospective clinical utility.
Potential limitations of this study include the following considerations. Although treatment randomization was stratified dichotomously by APOE ε4 carrier status (i.e., carrier or noncarrier), the number of copies of ε4 was not a stratification factor, leaving potential for imbalance across planned dose groups. For some types of events (severe ARIA-H superficial siderosis in particular), the estimated odds ratios may be sensitive to the small number of events in the ε3/ε3 reference group. The genetic population included only those with European ancestry in the United States, Europe, Canada, and Australia; the results may not generalize to other regions or ancestral groups.
ARIA can occur both with and without symptoms and with varying degrees of radiographic severity.15 The clinical relevance and long-term outcomes associated with ARIA is yet to be determined. Our study indicated that APOE is associated with both symptomatic and asymptomatic ARIA but that APOE is not predictive of symptomatic vs asymptomatic ARIA. Nonetheless, we found significant associations between APOE ε4 genotype and all levels of radiographic severity in ARIA, with larger effect sizes for the radiographically severe ARIA than for radiographically mild ARIA.
ARIA represents the most common adverse event for aducanumab, and APOE genotype provides a useful indication of ARIA risk. Given the large number of patients with Alzheimer disease who carry APOE ε4 alleles (46%–52% heterozygotes, 15%–19% homozygotes in the UK Biobank and BioFINDER cohorts (internal analysis) and ENGAGE and EMERGE), a large proportion of patients with AD may be affected by ARIA. Indeed, the recently published Appropriate Use Guidelines call for incorporating APOE genotyping into clinical care.28
This work highlights the value genetics can add to providing additional information to guide clinical treatment decisions. Although not yet standard in clinical settings, targeted genotyping of APOE is relatively inexpensive, highly accurate, and consistent over time, making it an attractive biomarker for patient risk stratification.
Acknowledgment
The authors thank the trial participants and study investigators in the ENGAGE and EMERGE trials of aducanumab for facilitating this study. They also thank Kumar Kandadi Muralidharan, Patrick Burkett, Christina Grassi, Heiko Runz, and Sally John for their input on results interpretation; Chia-Yen Chen, Ben Sun, and Danai Chasioti for their expertise and consultation on PRS methods; and Eric Marshall, David Sexton, and Mehool Patel for their pharmacogenomics database support.
Glossary
- ARIA
amyloid-related imaging abnormality
- ARIA-E
ARIA with edema
- ARIA-H
ARIA with hemorrhage
- CAA
cerebral amyloid angiopathy
- GWAS
genome-wide association studies
- HWE
Hardy-Weinberg equilibrium
- LTE
long-term extension
- MAF
minor allele frequency
- PCs
principal components
- PRS
polygenic risk score
- QC
quality control
- VCF
variant call file
- VQSR
Variant Quality Score Recalibration
Appendix. Authors
| Name | Location | Contribution |
| Stephanie J. Loomis, PhD, MPH | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Ryan Miller, MD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Carmen Castrillo-Viguera, MD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Kimberly Umans, PhD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Wenting Cheng, PhD, MA | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| John O'Gorman, PhD, MS | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Richard Hughes, MD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Samantha Budd Haeberlein, PhD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Christopher D. Whelan, MSc, PhD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Footnotes
Editorial, page e208096
Study Funding
This study was sponsored by Biogen.
Disclosure
S.J. Loomis is an employee of Biogen, and may hold stocks/stock options in Biogen; R. Miller is a former employee of Biogen, and may hold stocks/stock options in Biogen; C. Castrillo-Viguera is a former employee of Biogen, and may hold stocks/stock options in Biogen; K. Umans is a former employee of Biogen, and may hold stocks/stock options in Biogen; W. Cheng is an employee of Biogen, and may hold stocks/stock options in Biogen; J. O'Gorman is an employee of Biogen, and may hold stocks/stock options in Biogen; R. Hughes is an employee of Biogen, and may hold stocks/stock options in Biogen; S. Budd Haeberlein is a former employee of Biogen, and may hold stocks/stock options in Biogen; C.D. Whelan is a former employee of Biogen, and may hold stocks/stock options in Biogen. Go to Neurology.org/N for full disclosures.
References
- 1.Sperling RA, Jack CR Jr., Black SE, et al. . Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7(4):367-385. doi: 10.1016/j.jalz.2011.05.2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barakos J, Purcell D, Suhy J, et al. . Detection and management of amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with anti-amyloid beta therapy. J Prev Alzheimers Dis. 2022;9(2):211-220. doi: 10.14283/jpad.2022.21 [DOI] [PubMed] [Google Scholar]
- 3.Salloway S, Chalkias S, Barkhof F, et al. . Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 2022;79(1):13-21. doi: 10.1001/jamaneurol.2021.4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mintun MA, Lo AC, Duggan Evans C, et al. . Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384(18):1691-1704. doi: 10.1056/NEJMoa2100708 [DOI] [PubMed] [Google Scholar]
- 5.van Dyck CH, Swanson CJ, Aisen P, et al. . Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9-21. doi: 10.1056/NEJMoa2212948 [DOI] [PubMed] [Google Scholar]
- 6.Ostrowitzki S, Lasser RA, Dorflinger E, et al. . A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease. Alzheimers Res Ther. 2017;9(1):95. doi: 10.1186/s13195-017-0318-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salloway S, Sperling R, Fox NC, et al. . Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370(4):322-333. doi: 10.1056/nejmoa1304839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zago W, Schroeter S, Guido T, et al. . Vascular alterations in PDAPP mice after anti-Aβ immunotherapy: implications for amyloid-related imaging abnormalities. Alzheimers Dement. 2013;9(5 Suppl):S105-S115. doi: 10.1016/j.jalz.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 9.Kozberg MG, Yi I, Freeze WM, et al. . Blood-brain barrier leakage and perivascular inflammation in cerebral amyloid angiopathy. Brain Commun. 2022;4(5):fcac245. doi: 10.1093/braincomms/fcac245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease—one peptide, two pathways. Nat Rev Neurol. 2020;16(1):30-42. doi: 10.1038/s41582-019-0281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budd Haeberlein S, Aisen PS, Barkhof F, et al. . Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9(2):197-210. doi: 10.14283/jpad.2022.30 [DOI] [PubMed] [Google Scholar]
- 12.Guthrie H, Honig LS, Lin H, et al. . Safety, tolerability, and pharmacokinetics of crenezumab in patients with mild-to-moderate Alzheimer's disease treated with escalating doses for up to 133 weeks. J Alzheimers Dis. 2020;76(3):967-979. doi: 10.3233/JAD-200134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landen JW, Cohen S, Billing CB Jr., et al. . Multiple-dose ponezumab for mild-to-moderate Alzheimer's disease: safety and efficacy. Alzheimers Dement (N Y). 2017;3:339-347. doi: 10.1016/j.trci.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doody RS, Thomas RG, Farlow M, et al. . Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370(4):311-321. doi: 10.1056/NEJMoa1312889 [DOI] [PubMed] [Google Scholar]
- 15.Cogswell PM, Barakos JA, Barkhof F, et al. . Amyloid-related imaging abnormalities with emerging Alzheimer disease therapeutics: detection and reporting recommendations for clinical practice. AJNR Am J Neuroradiol 2022;43(9):E19–E35. doi: 10.3174/ajnr.A7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellenguez C, Küçükali F, Jansen IE, et al. . New insights into the genetic etiology of Alzheimer's disease and related dementias. Nat Genet. 2022;54(4):412-436. doi: 10.1038/s41588-022-01024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperling R, Salloway S, Brooks DJ, et al. . Amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11(3):241-249. doi: 10.1016/S1474-4422(12)70015-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson C, Siemers E, Hake A, et al. . Amyloid-related imaging abnormalities from trials of solanezumab for Alzheimer's disease. Alzheimers Dement (Amst). 2016;2:75-85. doi: 10.1016/j.dadm.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). February 29, 2000. Identifier NCT02477800, 221AD301 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer's Disease (ENGAGE). Accessed June 1, 2023. https://clinicaltrials.gov/study/NCT02477800?term=ENGAGE&intr=Aducanumab&rank=1
- 20.ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). February 29, 2000. Identifier NCT02484547, 221AD302 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer's Disease (EMERGE). Accessed June 1, 2023. https://clinicaltrials.gov/study/NCT02484547?term=EMERGE&intr=Aducanumab&rank=1
- 21.Das S, Forer L, Schönherr S, et al. . Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284-1287. doi: 10.1038/ng.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Auwera GA & O'Connor BD. (2020). Genomics in the Cloud: Using Docker, GATK, and WDL in Terra (1st Edition). O'Reilly Media.
- 23.McLaren W, Gil L, Hunt SE, et al. . The Ensembl Variant Effect Predictor. Genome Biol. 2016;17(1):122. doi: 10.1186/s13059-016-0974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartzentruber J, Cooper S, Liu JZ, et al. . Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer's disease risk genes. Nat Genet. 2021;53(3):392-402. doi: 10.1038/s41588-020-00776-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonenko G, Baker E, Stevenson-Hoare J, et al. . Identifying individuals with high risk of Alzheimer's disease using polygenic risk scores. Nat Commun. 2021;12(1):4506. doi: 10.1038/s41467-021-24082-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filippi M, Cecchetti G, Spinelli EG, Vezzulli P, Falini A, Agosta F. Amyloid-related imaging abnormalities and beta-amyloid-targeting antibodies: a systematic review. JAMA Neurol. 2022;79(3):291-304. doi: 10.1001/jamaneurol.2021.5205 [DOI] [PubMed] [Google Scholar]
- 27.Sims JR, Zimmer JA, Evans CD, et al. . Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi: 10.1001/jama.2023.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cummings J, Rabinovici GD, Atri A, et al. . Aducanumab: appropriate use recommendations update. J Prev Alzheimers Dis. 2022;9(2):221-230. doi: 10.14283/jpad.2022.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
While the data described in this article are not publicly available, the authors and Biogen are supportive of data sharing. Biogen has established processes to share protocols, clinical study reports, study-level data, and deidentified patient-level data. These data and materials will be made available to qualified scientific researchers in support of the objective(s) in their approved, methodologically sound research proposal. Proposals should be submitted through Vivli (vivli.org/). To gain access, data requestors will need to sign a data sharing agreement. For general inquiries, please contact datasharing@biogen.com. Biogen's data sharing policies and processes are detailed on the website biogentrialtransparency.com/.