Abstract
Background and Objectives
The aim of this study was to compare 2 large clinicopathologic cohorts of participants aged 90+ and to determine whether the association between neuropathologic burden and dementia in these older groups differs substantially from those seen in younger-old adults.
Methods
Autopsied participants from The 90+ Study and Adult Changes in Thought (ACT) Study community-based cohort studies were evaluated for dementia-associated neuropathologic changes. Associations between neuropathologic variables and dementia were assessed using logistic or linear regression, and the weighted population attributable fraction (PAF) per type of neuropathologic change was estimated.
Results
The 90+ Study participants (n = 414) were older (mean age at death = 97.7 years) and had higher amyloid/tau burden than ACT <90 (n = 418) (mean age at death = 83.5 years) and ACT 90+ (n = 401) (mean age at death = 94.2 years) participants. The ACT 90+ cohort had significantly higher rates of limbic-predominant age-related TDP-43 encephalopathy (LATE-NC), microvascular brain injury (μVBI), and total neuropathologic burden. Independent associations between individual neuropathologic lesions and odds of dementia were similar between all 3 groups, with the exception of μVBI, which was associated with increased dementia risk in the ACT <90 group only (odds ratio 1.5, 95% CI 1.2–1.8, p < 0.001). Weighted PAF scores indicated that eliminating μVBI, although more prevalent in ACT 90+ participants, would have little effect on dementia. Conversely, eliminating μVBI in ACT <90 could theoretically reduce dementia at a similar rate to that of AD neuropathologic change (weighted PAF = 6.1%, 95% CI 3.8–8.4, p = 0.001). Furthermore, reducing LATE-NC in The 90+ Study could potentially reduce dementia to a greater degree (weighted PAF = 5.1%, 95% CI 3.0–7.3, p = 0.001) than either ACT cohort (weighted PAFs = 1.69, 95% CI 0.4–2.7).
Discussion
Our results suggest that specific neuropathologic features may differ in their effect on dementia among nonagenarians and centenarians from cohorts with different selection criteria and study design. Furthermore, microvascular lesions seem to have a more significant effect on dementia in younger compared with older participants. The results from this study demonstrate that different populations may require distinct dementia interventions, underscoring the need for disease-specific biomarkers.
Introduction
Globally, persons aged 65 years and older are the fastest growing age group. In the United States, the population aged 90 years and older is expected to increase four-fold by 2050, notably faster than the younger-old age groups.1 The corresponding rise in dementia and other chronic health conditions associated with an aging population presage a mounting public health burden.2 Despite substantial developments in our understanding of dementia risk factors and corresponding potential intervention foci, there is rising concern that dementia etiology may differ for these older groups.3-6 Alzheimer disease (AD) neuropathologic change (ADNC) has been shown to level off or even decrease past age 90 across multiple cohorts.7-9 Certain modifiable risk factors that are currently identified as potential targets for dementia intervention, including hypertension, hyperlipidemia, and obesity, may lessen in effect or even be protective to some extent in the oldest age groups.10-12 The negative effect of APOE ε4, the strongest known genetic risk factor for AD, is lower past age 90 as well.5,13 These findings expose a concerning gap in our understanding of dementia risk in the nonagenarians and centenarians and highlight the importance of identifying the different potential neuropathologic underpinnings of dementia in this age group.
By surviving into the later decades, nonagenarians and centenarians represent a segment of the population that is largely either resistant to the lifetime accumulation of potential health conditions and environmental hazards or resilient to the compounding risk to mortality and morbidity posed by such conditions.14 Estimates are that approximately one-third of people aged 90 years or older with ADNC severe enough to meet pathologic criteria for high likelihood of AD do not develop cognitive impairments that substantially interfere with daily function, suggesting that other factors may be important in progression to dementia in this age group.15-17 Thus, applying interventions that solely target ADNC in this age group has the potential to lead to overtreatment among those whose cognition is not substantially affected by moderate/severe ADNC and may miss an estimated one-half of dementia cases considered not attributable to ADNC.18
We previously described the importance of multiple underlying neuropathologic changes, including amyloid deposition, neurofibrillary tangles (NFTs), limbic-predominant age-related TDP-43 encephalopathy (LATE-NC), microvascular brain injury (μVBI), Lewy body disease (LBD), and hippocampal sclerosis (HS), in the development of dementia among participants from The 90+ Study, a longitudinal, community-based study of aging and dementia in people aged older than 90 years, using contemporary consensus guidelines for neuropathologic change. LATE-NC, gaining increasing attention for its role in cognitive decline both in combination with ADNC or alone,19 was identified as a prominent factor in dementia in The 90+ Study, where it seemingly compounded the effects of ADNC in increasing dementia risk and underscored the importance of developing methods for in vivo identification of and intervention for LATE-NC.20 Because others have also shown that LATE-NC may present differently in the older age groups,21 it is important to determine whether these results can be generalized to other community-based cohorts of participants aged older than 90 years.
The aim of this study was to compare findings from 2 large clinicopathologic cohorts of participants aged 90 years and older following identical contemporary consensus neuropathologic guidelines and to determine whether the association between neuropathologic burden and dementia in these older groups differs substantially from those seen in younger-old adults.22,23 Our objectives were to (1) compare the occurrence of and associations between neuropathologic abnormalities, dementia, and age among the 3 groups, (2) determine the association between dementia and individual neuropathologic change types and total neuropathologic burden, and (3) establish whether the estimated fraction of dementia attributed to the types of neuropathologic change differs among the groups.
Methods
Participants
Participants were those who consented to postmortem brain donation for research and were enrolled in either of 2 ongoing, longitudinal, community-based cohort studies of aging and dementia: The 90+ Study, which enrolls participants aged 90 years or older, or the Adult Changes in Thought (ACT) Study, which enrolls participants aged 65 years or older.
The 90+ Study, started in 2003, enrolled surviving participants of the Leisure World Cohort Study (LWCS), a mailed health survey which began in 1981 and was sent to all residents in a retirement community in Southern California. Participants were invited to enroll in The 90+ Study if they were aged 90 years or older. Additional participants who were not part of the original LWCS and lived in the same region were also invited to participate. Participants were followed at 6-month intervals with neurologic and physical examinations, cognitive testing, functional assessments, and study partner interviews.24 Participants unable to come to the clinic were administered telephone visits, including cognitive assessments. For this study, we included all research brain autopsies completed as of April 30, 2022.
The ACT Study, started in 1994, enrolls participants who are randomly selected from dementia-free members of Kaiser Permanente Washington. Participants are followed at biennial intervals with assessments including demographics, medical history, and cognition.25 For this study, we included brain autopsies for participants who donated their brains to the University of Washington BioRepository and Integrated Neuropathology Laboratory, completed as of March 5, 2020. We excluded ACT participants with a final biennial visit more than 2 years before death without a dementia diagnosis due to the potential for cognitive progression during the interval from visit to death. ACT participants were divided into 2 cohorts based on age: ACT <90 and ACT 90+.
Standard Protocol Approvals, Registrations, and Patient Consents
All participants or their designated surrogates provided written consent to participate in the study. Procedures were reviewed and approved by the institutional review boards at the University of California, Irvine, University of Washington, and Kaiser Permanente Washington.
Neuropathologic Indices
Neuropathologic index scores were assigned as follows: (1) neuritic plaque (NP) density score: 0 = none; 1 = Consortium to Establish a Registry for Alzheimer's Disease (CERAD) NP score sparse; 2 = CERAD score moderate; 3 = CERAD score frequent (multisite interrater agreement: κ = 0.77, 95% CI 0.58–0.8826)22,23,27; (2) NFT distribution score: 0 = none, 1 = Braak stage I or II, 2 = Braak stage III or IV, 3 = Braak stage V or VI (multisite interrater agreement: κ = 0.70, 95% CI 0.45–0.8326)22,23,28; (3) LATE-NC: 0 = no TDP-43 inclusions; 1 = amygdala only; 2 = plus hippocampus; 3 = plus middle frontal gyrus (Stanford/ACT interrater agreement: κ = 0.71, 95% CI 0.59–0.84)29; (4) μVBI, defined according to the number of lesions observed in a defined set of standard screening sections: 0 = no microinfarcts; 1 = low (1 microinfarct); 2 = moderate (2 microinfarcts), and 3 = severe (≥3 microinfarcts) (interrater reliability not formally assessed)22,23; (5) LBD: 0 = none; 1 = brainstem-predominant; 2 = limbic (transitional); 3 = neocortical (diffuse) (multisite interrater agreement: Krippendorff α = 0.5930)23,31; and (6) HS, classified as present/absent.22,23 Interrater reliability was less for HS (Stanford/ACT interrater agreement: κ = 0.34, 95% CI 0.16–0.52) likely because there are as yet no consensus criteria. The total neuropathologic burden score was calculated by adding the individual neuropathologic scores. For analyses requiring binary variables, NP density and NFT scores were binarized following previous recommendations (e.g., none/sparse = 0; moderate/frequent = 1; Braak stages 0, I, II = 0; Braak stages III, IV, V, or VI = 1),22 while the non-ADNC neuropathologic lesion scores (LBD, μVBI, LATE-NC, and HS) were defined as present vs absent.
Dementia Diagnosis
Dementia diagnosis for both studies was made according to Diagnostic and Statistical Manual of Mental Disorders, 4th Edition diagnostic criteria; however, the procedures for diagnosis differed. For The 90+ Study, dementia diagnosis of any severity was not an exclusion for study entry for the LWCS. Non-LWCS participants were enrolled if they had no or mild dementia. Every participant was assigned a final cognitive diagnosis at the time of death during a multidisciplinary consensus conference using all available clinical information (including any prior evaluations and interim clinical data) and blinded to neuropathologic diagnoses.24 For the ACT Study, dementia was an exclusion for study entry. Participants whose scores fell below a threshold score on cognitive screening or whose study partners/clinicians expressed concern about cognitive decline were referred for a full diagnostic evaluation. Dementia diagnosis for the screened group was determined at a consensus conference diagnosis.25
Statistical Analyses
Three groups were examined: The 90+ Study, ACT participants aged 90 years or older at death (ACT 90+), and ACT participants aged younger than 90 years at death (ACT <90). Group differences were evaluated using ordinal logistic regression (proportional odds) for the ordinal neuropathologic lesion scores, binary logistic regression for the presence/absence of dementia, and linear regression for the total neuropathologic burden score, with group being the categorical independent variable and ACT <90 being the reference group. Proportional odds assumptions were tested using the Brant and Wolf-Gould tests. Post hoc pairwise comparisons of the log odds or means of the dependent variables between ACT 90+ and The 90+ Study groups were conducted. Associations between neuropathologic variables and age, sex, and education level were measured separately for each group using ordered logistic regression, with the neuropathologic variable being the dependent variable and age, sex, or education level being the independent variables. Model outcomes were inspected for influential observations using Pregibon delta beta statistic and plot. To evaluate the odds of dementia associated with individual neuropathologic variables, logistic regression models, with dementia status (present/absent) being the dependent variable and ordinal neuropathologic lesion being the independent variable, were conducted separately for each neuropathologic variable within each group, controlling for age at death, sex, education, and enrollment cohort. Owing to differences in collecting education data, a binary variable (college degree or higher vs no college degree) was used. Next, all 3 groups were entered into a single logistic regression model for each neuropathologic variable, with dementia status, group, group by dementia interaction, age at death, sex, and education being the predictors. Linear combinations of estimators were calculated to determine the difference in differences between groups at each level. APOE genotype was available for a subsample; thus separate sensitivity analyses were conducted secondarily, entering APOE genotype (ε2/-, ε3/3, ε4/-; participants with ε2/4 genotype were omitted). Additional sensitivity analyses were conducted within the older groups that included only those participants diagnosed with dementia at or after age 90. To determine the population attributable fraction (PAF), or the theoretical proportional reduction in dementia that is estimated if a risk factor was eliminated in the population, we entered all binary neuropathologic change variables and covariates as independent variables in a logistic regression in which dementia is the dependent variable. The logs of 2 scenario means (the baseline and the “fantasy” scenario in which 1 neuropathologic variable is set to 0 while keeping all other predictor variables the same) are estimated, as well as the log of the ratio of the “fantasy” scenario to the baseline scenario, known as the population unattributable fraction. This is then subtracted from 1 to derive the PAF.32 We further adjusted for nonindependence of the neuropathologic variables, using previously described methods,33,34 and present the resulting weighted PAFs. Bonferroni adjustment was used to control the family-wise type I error set a priori at 0.05 for analyses involving multiple testing. All analyses were performed using Stata/SE 17.0.
As autopsy consent was not a requirement of either study, inverse probability weights were incorporated in all models to address potential selection bias due to factors associated with selection into the autopsy group. Weights were derived from separate logistic regression models (eTable 1, links.lww.com/WNL/D315) for each group (The 90+ Study, ACT <90, and ACT >90) that estimated the probability of selection into the autopsy sample from the entire deceased sample as a function of factors that may influence consent to autopsy (age, sex, education, marital status, study cohort, and dementia diagnosis). Bias-corrected bootstrap standard errors and CIs were calculated for each model to account for uncertainty in the selection weights. Receiver operating characteristic analyses were used to examine the predictive ability of the weights (eFigure 1). Demographic data for the entire deceased sample vs those included in the autopsy sample are included in the supplement (eTables 2–4).
Data Availability
Data for the analyses and results reported in this article were acquired from The 90+ Study and The ACT Study. Data not published within the article will be shared on reasonable request from a qualified investigator.
Results
In the ACT <90 group, 16 participants without dementia were excluded because of interval from final visit to death >2 years (n = 418). In the ACT 90+ group, 15 participants without dementia were excluded because of interval from final visit to death >2 years (n = 401). In The 90+ Study, 4 participants missing final cognitive diagnosis were excluded (n = 414). The 90+ Study participants were older at death; higher proportions were female, White and had a college degree or higher; and average brain weight was lower than both the younger and older ACT participants. Although the proportion of The 90+ Study sample with dementia was lower than that of the ACT 90+ autopsy sample, once selection bias was accounted for in the analyses, there was no significant difference between ACT 90+ and The 90+ Study. Among those with dementia, The 90+ Study participants were also older at dementia onset and had shorter disease duration than both the ACT <90 and ACT 90+ participants (Table 1).
Table 1.
The Adult Changes in Thought Study and the 90+ Study Sample Characteristics
| ACT Study <90 (n = 418) | ACT Study 90+ (n = 401) | The 90+ Study (n = 414) | Overall p valuea Pairwise |
|
| Age at death, y | <0.0001 ACT <90 < ACT 90+ < 90+ |
|||
| Mean (SD) | 83.5 (4.6) | 94.2 (3.2) | 97.7 (3.6) | |
| Range | 68.0–89.0 | 90.0–106.0 | 90.1–110.6 | |
| Sex, female, n (%) | 221 (52.9) | 246 (61.4) | 285 (68.8) | <0.0001 ACT <90 < ACT 90+ < 90+ |
| Race, n (%) | 0.026 ACT <90 | ACT 90+ < White participants than 90+ |
|||
| American Indian/Alaska Native | — | — | — | |
| Asian | 8 (1.9) | 4 (1.0) | 3 (0.7) | |
| Black/African American | 4 (1.0) | 6 (1.5) | — | |
| Other, including mixed | — | 1 (0.3) | — | |
| White | 390 (93.3) | 380 (94.8) | 409 (98.8) | |
| Missing/unknown | 16 (3.8) | 10 (2.5) | 2 (0.5) | |
| Hispanic/Latino, yes, n (%) | 4 (1.0) | 1 (0.3) | 2 (0.5) | 0.463 |
| Education, % with college degree or higher | 185 (44.3) | 162 (40.4) | 210 (50.7) | 0.011 ACT 90+ < 90+ |
| Brain weight, g | n = 402 | n = 388 | n = 383 | <0.0001 ACT <90 > ACT 90+ > 90+ |
| Mean (SD) | 1,240.2 (139.5) | 1,178.1 (128.8) | 1,130.3 (126.8) | |
| Range | 650.0–1,635.0 | 820.0–1,760.0 | 728.0–1,670.0 | |
| Postmortem interval, h | n = 391 | n = 391 | n = 399 | <0.0001 ACT <90 > ACT 90+ > 90+ |
| Mean (SD) | 18.7 (22.6) | 14.2 (18.8) | 8.5 (11.5) | |
| Range | 1.2–178.8 | 1.7–144 | 1.0–98.2 | |
| APOE genotype, n (%) | n = 400 | n = 391 | n = 380 | 0.002 ACT <90 more likely have an ε4 allele than older groups |
| ε2/ε2 or ε2/ε3 | 44 (11.0) | 32 (8.2) | 47 (12.4) | |
| ε3/ε3 | 222 (55.5) | 272 (69.6) | 255 (67.4) | |
| ε3/ε4 or ε4/ε4 | 123 (30.8) | 81 (20.7) | 67 (17.6) | |
| ε2/ε4 | 11 (2.8) | 6 (1.5) | 11 (2.9) | |
| Cognitive status, dementia, n (%) | 157 (37.6) | 229 (57.1) | 182 (44.0) | <0.0001 ACT <90 < ACT 90+ | 90+ |
| Age at dementia diagnosis, y | <0.0001 ACT <90 < ACT 90+ < 90+ |
|||
| Mean (SD) | 80.1 (4.4) | 88.6 (4.4) | 93.7 (4.9) | |
| Range | 68.0–88.0 | 77.0–102.0 | 77.0–108.0 | |
| Dementia duration, y | <0.0001 ACT <90 < ACT 90+ ACT 90+ > 90+ |
|||
| Mean (SD) | 4.8 (2.7) | 5.6 (3.3) | 4.1 (3.4) | |
| Range | 0.9–13.2 | 0.8–20.0 | 0b–15.6 |
Abbreviations: ACT = Adult Changes in Thought; CERAD = Consortium to Establish a Registry for Alzheimer's Disease; LATE-NC = limbic-predominant age-related TDP-43 encephalopathy.
Based on linear regression for continuous variables and logistic regression for categorical variables, with group being the categorical independent variable (reference group = ACT <90). All models were weighted to the full deceased sample for each group using a bootstrapping procedure to account for error in estimating the weights.
A value of 0 indicates that the participant was diagnosed with dementia during the same year as their death.
Group Differences in Neuropathologic Burden
Figure 1, A–F shows group differences in neuropathologic burden. ACT <90 participants (reference group) had lower proportions of NFTs, NPs, and HS than both older groups and lower LBD than The 90+ Study. The proportion of μVBI in the ACT <90 participants was greater than in The 90+ Study. Post hoc pairwise comparisons of the 2 older groups demonstrated that The 90+ Study group had higher NP and LBD scores than ACT 90+. Although the overall NFT score did not differ significantly between the ACT 90+ and The 90+ Study participants, The 90+ Study had a higher proportion of moderate/high NFTs (odds ratio [OR] 3.13, 95% CI 1.80–5.43, p = 0.0001). Conversely, ACT 90+ participants had higher LATE-NC and μVBI. The total neuropathologic burden score was higher in the 2 older groups compared with ACT <90 (ACT 90+: B = 1.73, 95% CI 0.81–1.65, p < 0.0001, 90+: B = 1.23, 95% CI 1.31–2.16, p < 0.0001); post hoc comparisons yielded a higher total burden score in ACT 90+ compared with The 90+ Study (p < 0.014). Detailed neuropathologic data and ORs are provided in eTable 5 (links.lww.com/WNL/D315).
Figure 1. Comparison of Group Differences in Neuropathologic Change Scores NFT (A), NP (B), LATE-NC (C), μVBI (D), LBD (E), and HS (F) Between ACT <90, ACT 90+, and the 90+ Study.
The y-axis represents the predicted probabilities of neuropathologic change for each group based on unadjusted ordinal logistic regression models and is plotted as the predicted combination of moderate-severe NPs/NFTs, and all nonzero outcomes for the other lesions. All models were weighted to the full deceased sample for each group using a bootstrapping procedure to account for error in estimating the weights. ***p < 0.0001, **p < 0.001 level, *p < 0.01. All reported significant values are significant after correcting for multiple comparisons. ACT = Adult Changes in Thought; HS = hippocampal sclerosis; LATE-NC = limbic-predominant age-related TDP-43 encephalopathy; LBD = Lewy body disease; μVBI = microvascular brain injury; NFT = neurofibrillary tangle; NP = neuritic plaque.
Associations Between Demographic Factors, Dementia, and Neuropathologic Burden
Older age at death was associated with higher dementia odds in ACT <90, but not in ACT 90+ nor The 90+ Study (Figure 2A). Older age at death was also associated with higher proportions of ACT <90 participants with higher levels of NFTs and NPs (Figure 2B), LATE-NC (Figure 2C), and μVBI (Figure 2D), but not with higher LBD (Figure 2E) or HS (Figure 2F). Conversely, age at death was not significantly associated with any neuropathologic variables after accounting for multiple comparisons in The 90+ Study and only with HS in the ACT 90+ cohort. Delta beta analyses yielded no evidence for unduly influential observations. Associations between sex and dementia or any neuropathologic lesion scores did not reach statistical significance after accounting for multiple comparisons. The education level (college degree vs no college degree) was significantly associated with a lower rate of dementia in The 90+ Study, but not in the ACT groups after accounting for multiple comparisons. Neuropathologic lesion scores were not associated with the level of education for any groups after accounting for multiple comparisons. The presence of an APOE ε4 was significantly associated with increased dementia odds in the ACT <90 group (OR 2.27, 95% CI 1.38–3.73, p = 0.001) but not in either of the 90+ groups. Among participants with dementia, disease duration was significantly associated with NFT and LATE in the ACT <90 group and NFT in The 90+ Study, but associations with other neuropathologic lesion scores failed to meet statistical significance after accounting for multiple comparisons. eTable 6 (links.lww.com/WNL/D315) provides weighted ORs and bias-corrected 95% CIs for all associations.
Figure 2. Percentage of Participants (Unadjusted) With Dementia (A), Moderate to High NPs or Moderate to Frequent NFTs (B), Any LATE-NC (C), Any μVBI (D), Any LBD (E), and Any HS (F) in the Adult Changes in Thought Study and the 90+ Study by Age at Death.
ACT = Adult Changes in Thought; HS = hippocampal sclerosis; LATE-NC = limbic-predominant age-related TDP-43 encephalopathy; LBD = Lewy body disease; mVBI = microvascular brain injury; NFT = neurofibrillary tangle; NP = neuritic plaque.
Associations Between Dementia and Individual Neuropathologic Lesion Scores
In separate logistic regression analyses, adjusted for age at death, level of education, sex, and enrollment cohort, dementia odds were higher with more NFTs and NPs across all groups after accounting for multiple comparisons. LATE-NC was also associated with higher dementia risk across all groups, although this result was not statistically significant after accounting for multiple comparisons in the ACT <90 cohort. Similarly, LBD and HS were associated with higher dementia risk across all groups to varying degrees: after accounting for multiple comparisons, the association between dementia and LBD was statistically significant in ACT <90 and The 90+ Study, and the association between dementia and HS was statistically significant only in the ACT 90+ cohort. Finally, μVBI was associated with higher dementia risk in the ACT <90 group only (Figure 3; eTable 7, links.lww.com/WNL/D315). When all groups were entered into a single model for each ordinal neuropathologic variable, there were significant groups by dementia diagnosis interactions for μVBI such that the dementia/μVBI associations were stronger in the younger group as compared with the 2 older groups (ACT <90–90+: OR 2.23, 95% CI 1.19–4.21, p = 0.0127; ACT <90–90+: OR 2.28, 95% CI 1.16–4.47, p = 0.017), but not when comparing the 2 older groups with one another. Dementia/LATE-NC associations were stronger in The 90+ Study as compared with the ACT <90 group (ACT <90–90+: OR 0.50, 95% CI 0.26–0.95, p = 0.035), but not for any other combinations.
Figure 3. Odds of Dementia for Each Neuropathologic Lesion Across Groups.

Adjusted ORs are based on separate logistic regression models, controlling for age at death, level of education, sex, and enrollment cohort. All models were weighted to the full deceased sample for each group using a bootstrapping procedure to account for error in estimating the weights. Bias-corrected ORs and 95% CIs are reported. ***p < 0.0001, **p < 0.001, *p < 0.01 (not significant after Bonferroni correction). ACT = Adult Changes in Thought; HS = hippocampal sclerosis; LATE-NC = limbic-predominant age-related TDP-43 encephalopathy; LBD = Lewy body disease; μVBI = microvascular brain injury; NFT = neurofibrillary tangle; NP = neuritic plaque; OR = odds ratio.
In sensitivity analyses, including APOE as a covariate did not change these results substantially (eTable 8, links.lww.com/WNL/D315). Including only those participants who were diagnosed with dementia at or after age 90, only NFTs and NPs were significantly associated with dementia in the ACT 90+ group (total n = 254), although there were no substantial differences in the results for The 90+ Study (total n = 382) (eTable 9).
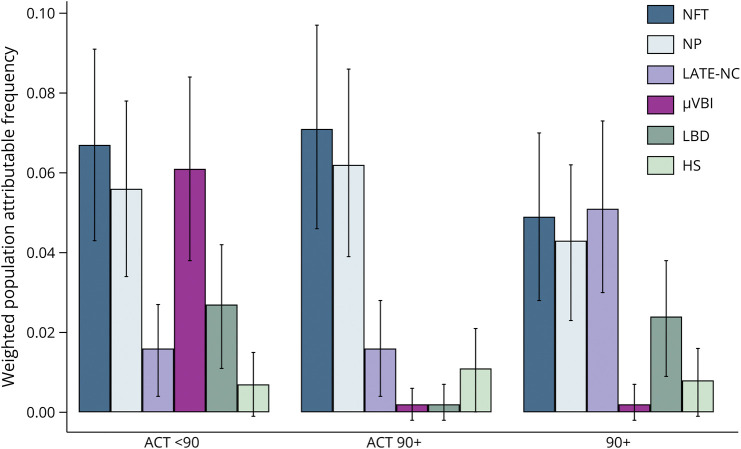
To evaluate the PAF of each neuropathologic lesion, binary variables for all neuropathologic abnormalities examined were first entered into a single logistic regression (eTable 10, links.lww.com/WNL/D315). Next, the weighted PAF was determined for each neuropathologic lesion in each group (Figure 4). The most impressive difference in PAFs was for microinfarcts, which was a very strong predictor in the ACT <90 sample but had a null contribution to dementia risk in both of the 90+ samples. LATE-NC was a stronger predictor in the 90+ study sample than the ACT <90 or the ACT 90+ sample. LBD was a significant predictor in the ACT <90 and The 90+ Study sample, but not in the ACT 90+ sample.
Figure 4. Population Attributable Fraction.
Bars represent the percent of dementia that would be theoretically reduced if the individual lesion were reduced to 0, weighted to account for interdependence between variables. Error bars represent 95% CIs. ACT = Adult Changes in Thought; HS = hippocampal sclerosis; LATE-NC = limbic-predominant age-related TDP-43 encephalopathy; LBD = Lewy body disease; μVBI = microvascular brain injury; NFT = neurofibrillary tangle; NP = neuritic plaque; OR = odds ratio.
Discussion
This study examines neuropathologic profiles of participants aged 90 years and older from 2 community-based cohorts and compares these with a cohort of participants aged younger than 90 years. Participants in The 90+ Study had higher common dementia-associated features (older age, lower brain weight, and higher proportion of female participants) and higher rate of moderate to high NFT, NP, and LBD burden, while the ACT 90+ cohort had significantly higher rates of LATE-NC and μVBI, and higher total neuropathologic burden. Our results suggest that there may be differences in dementia risk associated with specific neuropathologic features between younger and older participants and between the 90+ cohorts with different selection criteria, for example, a stronger association and higher PAFs for μVBI in younger participants, and for LATE-NC in The 90+ Study participants.
A chief aim of this study was to compare 2 cohorts of participants aged 90 years and older using contemporary consensus neuropathologic guidelines. The differences noted in specific neuropathologic changes between the cohorts may be a result of cohort differences that potentially influence the number and degree of non–ADNC-related neuropathologic changes. Although both cohorts are community-based by design, there are important distinctions between them. First, The 90+ Study enrolls participants who survived and were able to actively participate in study procedures at age 90 years or older. Conversely, the ACT study enrolls participants at initially younger ages (96% of this sample were enrolled at age younger than 90 years); although it captures those who survived to age 90 years and older, more were likely to have developed significant dementia symptoms or other serious illness before that age. Indeed, disease duration among those with dementia was significantly longer in ACT 90+ participants. Thus, the 90+ Study may represent a group that is especially resilient to a lifetime of biological, environmental, and genetic stressors.14,35,36 Second, both the ACT and 90+ Study cohorts have a large majority of White participants (not unusual given their surrounding communities); however, the ACT sample encompasses a wider range of educational, racial, and socioeconomic backgrounds and thus may be more representative of the general population.
We found the rate of μVBI to be substantially higher in ACT 90+ participants than The 90+ Study, contributing to an overall higher total neuropathologic burden score. This finding may be closely related to the differences in study design described above (e.g., participants with vascular risk factors may be less likely to enroll in a study for the first time at age 90 years or older). It is of interest that despite the substantial difference in amount of vascular pathology identified, the presence of μVBI seemed to exert little influence on a clinical diagnosis of dementia for either older group. Another interesting difference between the groups is that although the ACT 90+ cohort has higher LATE-NC than The 90+ Study, eliminating LATE-NC alone may not substantially reduce dementia burden in the ACT cohort. Conversely, LATE-NC may play a much more prominent role in effect on dementia risk in The 90+ Study, supporting what we and others have previously reported.20,21 Additional work to determine the reasons that underlie these differences will be vital to better understanding the role of LATE-NC in dementia in those aged older than 90 years.
A second focus of this study was to identify how nonagenarians and centenarians may differ from young-old age groups in neuropathologic contributions to dementia. It is important that our weighted PAF results support both the previously described importance of comorbid pathologies,37,38 while also highlighting that there is still a large proportion of dementia unaccounted for by these combined pathologic changes, particularly in the older groups. Specifically, the contribution of vascular neuropathologic processes to the development of dementia is well established, but prior reports suggest that this may diminish in people aged older than 90 years.11 Indeed, we found that the proportion of people with μVBI was significantly higher in the older ACT group compared with the younger ACT cohort, yet its presence seems to be more influential on cognitive impairment in the younger group. Theoretically, eliminating μVBI in the ACT <90 sample could contribute to a significant reduction in the population burden of dementia but would likely have little effect on dementia in nonagenarians and centenarians. Although in part this likely represents a survival effect (those most affected by vascular disease do not survive into the oldest age groups), it is also consistent with prior reports that risk factors such as hypertension and hyperlipidemia may have less negative effect in nonagenarians and centenarians.10-12,39,40
There are important limitations in this study. First, because we are comparing 2 separate cohorts, we included only the most common pathologies with consensus neuropathologic guidelines for each (except for HS, which does not have consensus guidelines); all have been previously used to compare neuropathologic change across large autopsy cohorts.38,41 Although the methods for identifying neuropathologic change were based on consensus criteria, differences across pathologists may exist. It is important that prior assessment of multisite interrater agreement shows high concordance across sites for ADNC and LBD, and interrater agreement for TDP-43 assessed for this study was substantial. The microinfarct protocol used here has not been formally evaluated for interrater reliability. In the context of evaluating a condensed sampling protocol, interrater kappa values for microinfarcts were low (approximately 0.30),42 likely due to limited sampling issues (e.g., μVBI occurs less frequently than other lesions). With this as a lower bound, we also note that the protocol used here has been used independently in multiple cohorts and yielded very similar OR for dementia. Our between group comparisons (90+ vs ACT) for μVBI may thus be affected by site differences in neuropathologic protocol. However, comparisons within the ACT group (old vs younger) are of greatest interest here. Finally, HS interrater agreement is low (likely due to lack of consensus criteria), and thus, intersite comparisons must be made carefully.
Second, both studies used the same criteria for dementia; however, the ACT Study required a participant to prompt a full dementia evaluation based on screening criteria applied every 2 years, while The 90+ Study evaluated all available evidence collected at 6-month intervals to arrive at a final cognitive diagnosis at the time of death, based on data that potentially varied between participants (e.g., telephone vs in-person visits). These methods could affect dementia in that (1) participants scoring above the screening criteria in ACT could still have dementia,43 (2) the interval likelihood of progression over time meant that some who had dementia at the time of death were missed, and (3) ACT participants with no dementia who were not seen within 2 years of death were excluded because of unknown cognitive status and the possibility for interval cognitive progression. Examining selection bias in the 2 cohorts yielded an interesting distinction: while ACT participants who died but did not participate in the autopsy study had a lower rate of dementia, those in The 90+ study who died but did not participate in the autopsy study had a slightly higher rate of dementia. This may lead to marked difficulty comparing dementia between cohorts unless efforts are made such as the inverse probability-weighting approach adopted here. A further limitation is that despite the ACT cohort being more representative of the general population, it is still largely White and had a higher percentage of participants attaining a college degree or higher than the general population of the United States aged 65 years or older.44 Finally, only a very small proportion (4%) of ACT participants were enrolled at age 90 years or older. As a result, we are not able to determine whether it is people who survive into their 90s or rather those who survive into the 10th decade and are willing/able to enroll into an involved cohort study who are especially resilient.
Delaying the onset of dementia symptoms and disabilities in a rapidly aging population is a community health imperative. Resistance to the pathophysiologic processes that produce increased neuropathologic burden associated with AD (NFTs and beta amyloid accumulation) in nonagenarians and centenarians is rare; thus, increasing resilience to these processes is of utmost importance. The results from this study demonstrate that different populations may require distinct interventions, underscoring the need for disease-specific biomarkers. Furthermore, the reduction of specific risk factors according to age, as well as prevention of additional neuropathologic change, is likely a key factor in reducing the population burden of dementia.
Acknowledgment
The authors sincerely thank their research participants and their families for their participation in this study. They also thank the testers and examiners of The 90+ Study and the Adult Changes in Thought Study.
Glossary
- ACT
Adult Changes in Thought
- AD
Alzheimer disease
- ADNC
AD neuropathologic change
- CERAD
Consortium to Establish a Registry for Alzheimer's Disease
- HS
hippocampal sclerosis
- LATE-NC
limbic-predominant age-related TDP-43 encephalopathy
- LBD
Lewy body disease
- LWCS
Leisure World Cohort Study
- μVBI
microvascular brain injury
- NFT
neurofibrillary tangle
- NP
neuritic plaque
- OR
odds ratio
- PAF
population attributable fraction
Appendix. Authors
| Name | Location | Contribution |
| Brenna Cholerton, PhD | Department of Pathology, Stanford University School of Medicine, CA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Caitlin S. Latimer, MD, PhD | Department of Laboratory Medicine and Pathology, University of Washington, Seattle | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Paul K. Crane, MD, MPH | Department of Medicine, University of Washington, Seattle | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Maria M. Corrada, ScD | Department of Neurology, and Department of Epidemiology, University of California, Irvine | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Laura E. Gibbons, PhD | Department of General Internal Medicine, University of Washington, Seattle | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Eric B. Larson, MD, MPH | Department of General Internal Medicine, University of Washington; Kaiser Permanente Washington Health Research Institute, Seattle | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Claudia H. Kawas, MD | Department of Neurology, and Department of Neurobiology & Behavior, University of California, Irvine | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| C. Dirk Keene, MD, PhD | Department of Laboratory Medicine and Pathology, University of Washington, Seattle | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Thomas J. Montine, MD, PhD | Department of Pathology, Stanford University School of Medicine, CA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
This work was supported by funding from the NIH (UF1 AG057707, UF1 AG053983, R01 AG021055, U01 AG 006781, U19 AG 066567, P30 AG066509, P50 AG016573, P30AG066519, and K08 AG065426 [to C.S.L.]) and the Nancy and Buster Alvord Endowment (to C.D.K.). APOE genotyping by the National Centralized Repository for Alzheimer's Disease and Related Dementias (NCRAD), which receives government support under a cooperative agreement grant (U24 AG021886) awarded by the National Institute on Aging (NIA), was used in this study.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.He W, Muenchrath MN. 90+ in the United States: 2006-2008 [online]. Accessed September 9, 2023. www2.census.gov/library/publications/2011/acs/acs-17.pdf. [Google Scholar]
- 2.GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):E105-E125. doi: 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos SJB, van Boxtel MPJ, Schiepers OJG, et al. Modifiable risk factors for prevention of dementia in midlife, late life and the oldest-old: validation of the LIBRA index. J Alzheimers Dis. 2017;58(2):537-547. doi: 10.3233/JAD-161208 [DOI] [PubMed] [Google Scholar]
- 4.Paganini-Hill A, Kawas CH, Corrada MM. Lifestyle factors and dementia in the oldest-old: the 90+ study. Alzheimer Dis Assoc Disord. 2016;30(1):21-26. doi: 10.1097/WAD.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall A, Pekkala T, Polvikoski T, et al. Prediction models for dementia and neuropathology in the oldest old: the Vantaa 85+ cohort study. Alzheimers Res Ther. 2019;11(1):11. doi: 10.1186/s13195-018-0450-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawas CH, Legdeur N, Corrada MM. What have we learned from cognition in the oldest-old. Curr Opin Neurol. 2021;34(2):258-265. doi: 10.1097/WCO.0000000000000910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Gunten A, Ebbing K, Imhof A, Giannakopoulos P, Kovari E. Brain aging in the oldest-old. Curr Gerontol Geriatr Res. 2010;2010:358531. doi: 10.1155/2010/358531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imhof A, Kovari E, von Gunten A, et al. Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm? J Neurol Sci. 2007;257(1-2):72-79. doi: 10.1016/j.jns.2007.01.025 [DOI] [PubMed] [Google Scholar]
- 9.Savva GM, Wharton SB, Ince PG, et al. Age, neuropathology, and dementia. N Engl J Med. 2009;360(22):2302-2309. doi: 10.1056/NEJMoa0806142 [DOI] [PubMed] [Google Scholar]
- 10.Corrada MM, Hayden KM, Paganini-Hill A, et al. Age of onset of hypertension and risk of dementia in the oldest-old: the 90+ Study. Alzheimers Dement. 2017;13(2):103-110. doi: 10.1016/j.jalz.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legdeur N, van der Lee SJ, de Wilde M, et al. The association of vascular disorders with incident dementia in different age groups. Alzheimers Res Ther. 2019;11(1):47. doi: 10.1186/s13195-019-0496-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmeidler J, Mastrogiacomo CN, Beeri MS, Rosendorff C, Silverman JM. Distinct age-related associations for body mass index and cognition in cognitively healthy very old veterans. Int Psychogeriatr. 2019;31(6):895-899. doi: 10.1017/S1041610218001412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corrada MM, Paganini-Hill A, Berlau DJ, Kawas CH. Apolipoprotein E genotype, dementia, and mortality in the oldest old: the 90+ Study. Alzheimers Dement. 2013;9(1):12-18. doi: 10.1016/j.jalz.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montine TJ, Cholerton BA, Corrada MM, et al. Concepts for brain aging: resistance, resilience, reserve, and compensation. Alzheimers Res Ther. 2019;11(1):22. doi: 10.1186/s13195-019-0479-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azarpazhooh MR, Avan A, Cipriano LE, et al. A third of community-dwelling elderly with intermediate and high level of Alzheimer's neuropathologic changes are not demented: a meta-analysis. Ageing Res Rev. 2020;58:101002. doi: 10.1016/j.arr.2019.101002 [DOI] [PubMed] [Google Scholar]
- 16.Burke BT, Latimer C, Keene CD, et al. Theoretical impact of the AT(N) framework on dementia using a community autopsy sample. Alzheimers Dement. 2021;17(12):1879-1891. doi: 10.1002/alz.12348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neltner JH, Abner EL, Jicha GA, et al. Brain pathologies in extreme old age. Neurobiol Aging. 2016;37:1-11. doi: 10.1016/j.neurobiolaging.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brookmeyer R, Kawas CH, Abdallah N, Paganini-Hill A, Kim RC, Corrada MM. Impact of interventions to reduce Alzheimer's disease pathology on the prevalence of dementia in the oldest-old. Alzheimers Dement. 2016;12(3):225-232. doi: 10.1016/j.jalz.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson PT, Brayne C, Flanagan ME, et al. Frequency of LATE neuropathologic change across the spectrum of Alzheimer's disease neuropathology: combined data from 13 community-based or population-based autopsy cohorts. Acta Neuropathol. 2022;144(1):27-44. doi: 10.1007/s00401-022-02444-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montine TJ, Corrada MM, Kawas C, et al. Association of cognition and dementia with neuropathologic changes of Alzheimer's disease and other conditions in the oldest-old. Neurology. 2022;99(10):e1067-e1078. doi: 10.1212/WNL.0000000000200832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SJ, Guo Y, Ervin JF, Lusk JB, Luo S. Neuropathological associations of limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) differ between the oldest-old and younger-old. Acta Neuropathol. 2022;144(1):45-57. doi: 10.1007/s00401-022-02432-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8(1):1-13. doi: 10.1016/j.jalz.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. doi: 10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corrada MM, Berlau DJ, Kawas CH. A population-based clinicopathological study in the oldest-old: the 90+ study. Curr Alzheimer Res. 2012;9(6):709-717. doi: 10.2174/156720512801322537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737-1746. doi: 10.1001/archneur.59.11.1737 [DOI] [PubMed] [Google Scholar]
- 26.Montine TJ, Monsell SE, Beach TG, et al. Multisite assessment of NIA-AA guidelines for the neuropathologic evaluation of Alzheimer's disease. Alzheimers Dement. 2016;12(2):164-169. doi: 10.1016/j.jalz.2015.07.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479-486. doi: 10.1212/wnl.41.4.479 [DOI] [PubMed] [Google Scholar]
- 28.Nagy Z, Yilmazer-Hanke DM, Braak H, Braak E, Schultz C, Hanke J. Assessment of the pathological stages of Alzheimer's disease in thin paraffin sections: a comparative study. Dement Geriatr Cogn Disord. 1998;9(3):140-144. doi: 10.1159/000017038 [DOI] [PubMed] [Google Scholar]
- 29.Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503-1527. doi: 10.1093/brain/awz099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Attems J, Toledo JB, Walker L, et al. Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: a multi-centre study. Acta Neuropathol. 2021;141(2):159-172. doi: 10.1007/s00401-020-02255-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. doi: 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newson R. Attributable and unattributable risks and fractions and other scenario comparisons. Stata J. 2013;13(4):672-698. doi: 10.1177/1536867X1301300402 [DOI] [Google Scholar]
- 33.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794. doi: 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- 34.Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. Lancet Glob Health. 2019;7(5):e596-e603. doi: 10.1016/S2214-109X(19)30074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverman JM, Schmeidler J. The protected survivor model: using resistant successful cognitive aging to identify protection in the very old. Med Hypotheses. 2018;1109-1114. doi: 10.1016/j.mehy.2017.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snitz BE, Chang Y, Tudorascu DL, et al. Predicting resistance to amyloid-beta deposition and cognitive resilience in the oldest-old. Neurology. 2020;95(8):e984-e994. doi: 10.1212/WNL.0000000000010239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen MT, Mattek N, Woltjer R, et al. Pathologies underlying longitudinal cognitive decline in the oldest old. Alzheimer Dis Assoc Disord. 2018;32(4):265-269. doi: 10.1097/WAD.0000000000000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White LR, Edland SD, Hemmy LS, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology. 2016;86(11):1000-1008. doi: 10.1212/WNL.0000000000002480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karlsson IK, Zhan Y, Wang Y, et al. Adiposity and the risk of dementia: mediating effects from inflammation and lipid levels. Eur J Epidemiol. 2022;37(12):1261-1271. doi: 10.1007/s10654-022-00918-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White LR, Corrada MM, Kawas CH, et al. Neuropathologic changes of Alzheimer's disease and related dementias: relevance to future prevention. J Alzheimers Dis. 2023;95(1):307-316. doi: 10.3233/JAD-230331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flanagan ME, Marshall DA, Shofer JB, et al. Performance of a condensed protocol that reduces effort and cost of NIA-AA guidelines for neuropathologic assessment of Alzheimer disease. J Neuropathol Exp Neurol. 2017;76(1):39-43. doi: 10.1093/jnen/nlw104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trittschuh EH, Crane PK, Larson EB, et al. Effects of varying diagnostic criteria on prevalence of mild cognitive impairment in a community based sample. J Alzheimers Dis. 2011;25(1):163-173. doi: 10.3233/JAD-2011-101821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan CL, Bauman K. Educational Attainment in the United States: 2015 [online]. Accessed June 15, 2023. files.eric.ed.gov/fulltext/ED572028.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for the analyses and results reported in this article were acquired from The 90+ Study and The ACT Study. Data not published within the article will be shared on reasonable request from a qualified investigator.