Abstract
Background:
Post-operative delirium after lung transplantation is common. Its associations with health-related quality of life (HRQL), depression, and mortality remains unknown.
Methods:
In 236 lung transplant recipients, HRQL and depressive symptoms were assessed as part of a structured survey battery before and after transplantation. Surveys included the Geriatric Depressive Scale (GDS) and Short Form 12 (SF12). Delirium was assessed throughout the post-operative intensive care unit (ICU) stay with Confusion Assessment Method for ICU. Delirium and mortality data were extracted from electronic medical records. We examined associations between delirium and changes in depressive symptoms and HRQL using linear mixed effects models and association between delirium and mortality with Cox-proportional hazard models.
Results:
Post-operative delirium occurred in 34 participants (14%). Delirium was associated with attenuated improvements in SF12-PCS (difference ₋4.0; 95%CI: −7.4, −0.7) but not SF12-MCS (difference 2.2; 95%CI: −0.7,5.7) or GDS (difference ₋0.4; 95%CI: −1.5,0.7). Thirty-two participants died during the study period. Delirium was associated with increased adjusted hazard risk of mortality (HR 17.9, 95%CI: 4.4,72.5).
Conclusions:
Delirium after lung transplantation identifies a group at increased risk for poorer HRQL and death within the first post-operative year. Further studies should investigate potential causal links between delirium, and poorer HRQL and mortality risk after lung transplantation.
Keywords: Lung transplantation, lung transplant, delirium, critical care medicine, delirium outcomes
Introduction
Post-operative delirium is common after lung transplantation, occurring in 30–40% of lung transplant recipients.1–4 Delirium among critically ill patients is associated with more complications, longer duration of mechanical ventilation, longer length of stay (LOS) in the intensive care unit (ICU), and increased mortality.5 In addition, among survivors of critical illness, delirium is associated with greater risk of developing neuropsychiatric disorders and cognitive impairment.5–7
Preliminary studies evaluating delirium after lung transplantation have reported comparable associations to those among general critically ill populations, with increased resource utilization, including prolonged need for mechanical ventilation, longer ICU and overall hospital stays.2–4,8 Although delirium has been associated with subsequent neurocognitive impairments in this population, the association between delirium and impairments in patient-centered outcomes such as depression or health-related quality of life (HRQL) after lung transplantation remains unknown.9 Further, the association between delirium following lung transplantation and other important clinical outcomes, such as mortality, has been mixed.1–3
Post-operative delirium is an increasingly important perioperative outcome among lung transplant recipients, as recent organ allocation trends prioritize older and sicker patients, both of which independently confer greater delirium risk.10 In the USA, nearly one-third of patients undergoing lung transplantation are aged 65 years or older.11 Further, the proportion of lung transplant recipients who were hospitalized in the ICU at the time of donor offer has tripled in the last decade, from nearly 4% in 2003 to 12% in 2017.11 Therefore, it is not surprising that post-operative complication rates and resource utilization have increased as well. After 2005, with the advent of the Lung Allocation Score (LAS), lung transplant recipients in the United States had a 140% increase in odds of post-transplant extracorporeal membrane oxygenation, 50% increased odds of tracheostomy, and 60% increased odds of prolonged hospital admission.12 Identifying and targeting modifiable risk factors for complications and death are, therefore, urgently needed to improve outcomes among an older, sicker, and complex recipient population. In the present study, we examined the association of ICU delirium after lung transplantation with depression, HRQL, and mortality.
Methods
Study design, participants, and setting
We performed a secondary analysis of the “Breathe Again” cohort study.13 Breathe Again was a prospective cohort study of adults undergoing first-time lung transplantation at the University of California, San Francisco (UCSF) from January 2010 to April 2017.13 All candidates for lung transplantation at our center undergo standardized psychosocial assessments by licensed clinical social workers who use standardized screening instruments in accordance with consensus recommendations.14 We enrolled 96% of all English-speaking lung transplant candidates who underwent transplant during that time period. For this analysis, we included only those Breathe Again participants who underwent lung transplantation. We also included 29 additional UCSF lung transplant recipients who consented for medical record review as part of a separate study, but did not consent to survey completion, and were transplanted during the same time period. The Institutional Review Board at UCSF approved both studies; all participants provided written informed consent for participation.
Exposure variable
Delirium was determined by the Confusion Assessment Method for the ICUs (CAM-ICU). The CAM-ICU has excellent interrater reliability and sensitivity of 80–100% and specificity of 96–100%.15–17 At UCSF, the CAM-ICU is performed by the bedside registered nurse, while patients are in the ICU and documented in the electronic medical record once per 12-hour shift, or more frequently if indicated. We abstracted CAM-ICU assessments from the medical record and coded as CAM-ICU positive, negative, or unable to assess (UTA). Common reasons for UTA scorings included patients who were deeply sedated, those who were deemed too medically unstable to undergo testing, and those who could not speak English. In the event of multiple assessments (positive, negative, and/or UTA) during a single shift, participants were deemed delirious if they ever screened CAM-ICU positive at any point. Causes for delirium were evaluated through review of provider notes in the electronic medical record. Delirium was not assessed after transfer out of the ICU.
Outcomes measures
Measures of depressive symptoms and HRQL were captured as part of a structured psychometric survey battery administered before and at 3, 6, 12, 18, 24, 30, and 36 months after transplantation. While on the waitlist for transplant, surveys were repeated approximately every three months. Survey responses closest to the date of transplant were used as baseline. Depressive symptoms were evaluated using the Geriatric Depression Scale (GDS, range 0–15). We reversed the coding such that lower scores indicate fewer depressive symptoms; 0.5 standard deviation was used as a distribution-based minimally clinically important difference [MCID]: 1.7).18, 19 To evaluate generic HRQL, we utilized the Medical Outcomes Survey Short Form-12 version 2 (SF12) Physical and Mental Component Summary Scores (SF12-PCS and -MCS, respectively). Scores range 0–100, with higher scores indicating better HRQL, with a change of 5 points was considered to meet the MCID.18, 20, 21
Dates of death were abstracted from electronic medical records and verified with site-specific United Network for Organ Sharing reports.
Confounding and precision variables
We abstracted additional variables from the electronic medical record based on their known or plausible association with delirium and our outcomes of interest. They included age at transplant, sex, and diagnostic indication for transplant including chronic obstructive pulmonary disease (COPD)/emphysema, pulmonary hypertension (PH), cystic fibrosis (CF) and other suppurative lung diseases, and pulmonary fibrosis (PF).10 We collected the Lung Allocation Score (LAS) at the time of transplantation. We also collected if participants experienced primary graft dysfunction (PGD), with severe PGD defined as grade III at 48 and/or 72 hours after transplant.10, 22
Analysis approach
In order to characterize the association between delirium and early post-transplant outcomes, we a priori limited our primary analyses to the first post-transplant year. This time period was selected because of its well-known importance for delirium-related outcomes and its central relevance as a marker of post-transplant success. Where available, follow-up data up to 18-months were incorporated in order to provide more a more comprehensive characterization of temporal changes in our outcomes of interest.
In order to examine the association between delirium and depressive symptoms and HRQL, we employed mixed effect models (MEM) that account for possible non-linear changes over time, which we have previously documented.13 When using time as a categorical variable in the mixed effects model, it is assumed that the difference between the two groups is constant over the course of the study. To account for survivorship, HRQL was jointly modeled with death, which allowed us to estimate changes in HRQL and depression among the participants who were still living despite observable missing data (subject who is alive but did not complete survey) and unobservable missing data (subject who has died). We included study visit as a categorical variable and a subject-specific random effect to account for correlation among serial measures within the same individual.
To test the association between delirium and mortality, we first plotted the unadjusted association between delirium and mortality using Kaplan Meier methods. We next fit Cox proportional hazards models. Nonproportionality was tested using martingale residuals.
Not all subjects completed all study visits. The number of missing surveys was small. Mixed models can handle some missing data, but only data missing at random. We did not impute these missing data because the maximum likelihood methods used to fit MEM provide valid estimates for missing values.23
For both the joint MEM and Cox analyses, we fit two adjusted models. In our primary model, we adjusted for age, sex, native disease diagnosis, ventilator at transplant, and LAS at the time of transplant. We also gave careful consideration to the potential influence of PGD, recognizing that the development of delirium may occur in the setting of critical illness that is often accompanied by acute inflammatory and immunological changes, such as those caused by severe PGD. Thus, in a second a priori model, we included PGD as an additional covariate to test whether the association of delirium with depressive symptoms and HRQL was independent of PGD. Finally, to test for any residual confounding from PGD, we performed a sensitivity analysis in which we excluded the 48 participants with severe PGD from our Cox models. While exploratory, sensitivity analyses employing such stratification techniques have been advocated as an effective method to test for potential confounding factors. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
During the study period, 236 participants underwent lung transplantation and were included in this study (Figure 1). Of the participants, 141 (60%) were male, 143 (69%) white, and mean age at transplant of 57 years (+/− 11.5) (Table 1). Most participants underwent lung transplantation for pulmonary fibrosis (74%).
FIGURE 1:
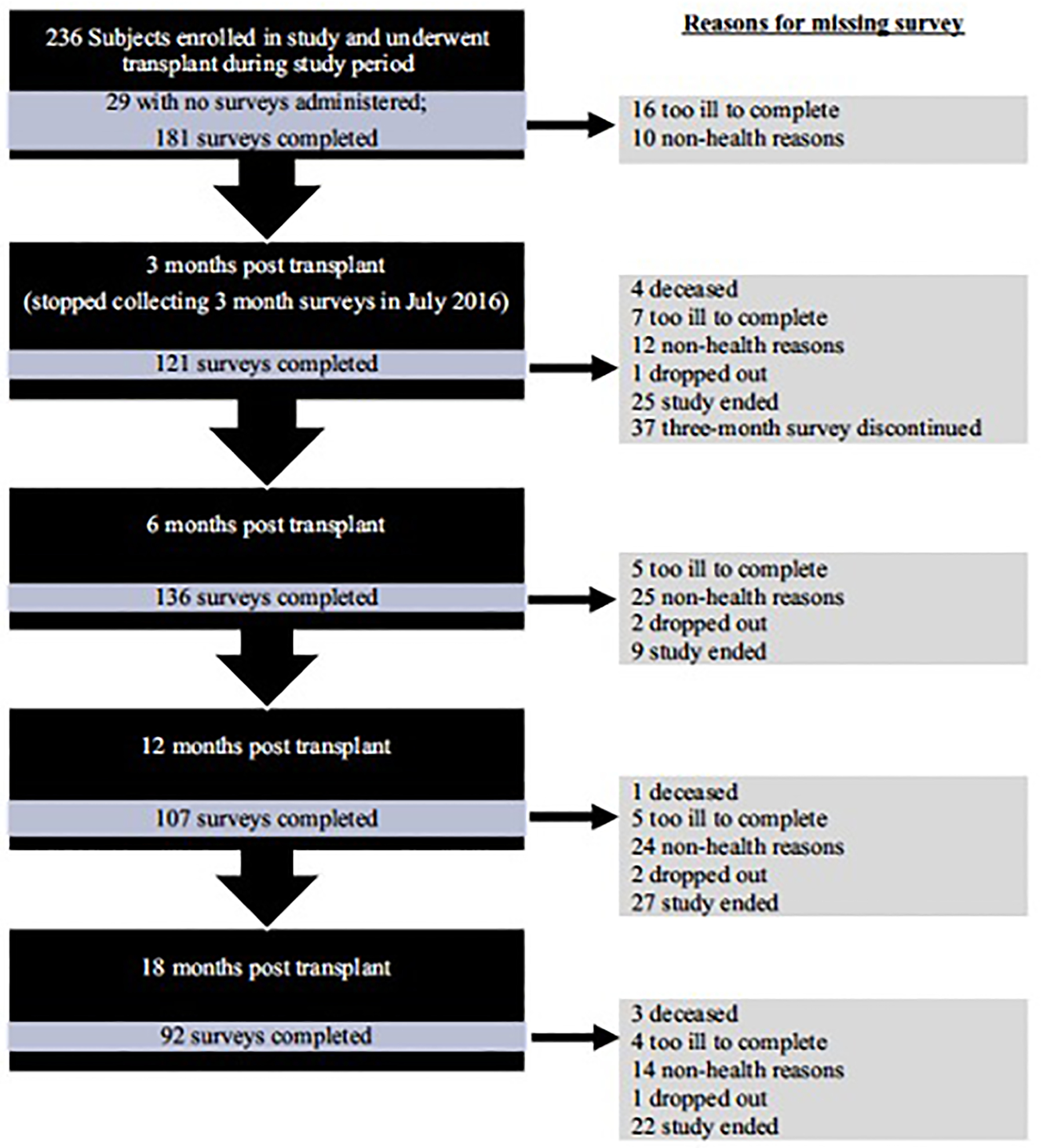
Flowchart Of Subjects Throughout The Study
TABLE 1:
Characteristics Of Study Population
| Variable | All n=236 |
Delirium n=34 |
No Delirium n=202 |
|---|---|---|---|
| Age, years, mean ± SD | 57 ± 12 | 57 (11) | 57 (12) |
| Age ≥ 65 years, n (%) | 69 (29) | 8 (24) | 61 (30) |
| Sex: male, n (%) | 141 (60) | 18 (53) | 123 (61) |
| Race: white, n (%) | 143 (69) | 19 (73) | 124 (69) |
| LAS diagnostic category, n (%) | |||
| A (eg, Obstructive lung disease) | 31 (13) | 5 (15) | 26 (13) |
| B (eg, Pulmonary hypertension) | 13 (6) | 1 (3) | 12 (6) |
| C (eg, Suppurative lung disease) | 17 (7) | 0 (0) | 17 (8) |
| D (eg, Pulmonary fibrosis) | 175 (74) | 28 (82) | 147 (73) |
| BMI (kg/m2), mean | 25.8 ± 4.0 | 26.4 (3.7) | 25.7 (4.0) |
| FEV1% predicted, mean | 46.8 ± 20.1 | 51.8 (23.2) | 46.1 (19.7) |
| 6 MWD (m), median [IQR] | 278 [153, 370] | 215 [122, 341] | 284 [154, 377] |
| Pre-transplant HRQL instruments | |||
| SF12-PCS, mean | 24 ± 9 | 24 ± 8 | 24 ± 9 |
| SF12-MCS, mean | 49.03 (10.89) | 50.60 (11.55) | 48.84 (10.83) |
| GDS, mean | 9 ± 4 | 10 ± 4 | 9 ± 3 |
| LAS at transplant, mean | 58.2 ± 21.0 | 61.8 ± 23.5 | 57.6 ± 20.5 |
| Inpatient at transplant, n (%) | 76 (35) | 13 (41) | 63 (34) |
| Ventilator at transplant, n (%) | 21 (10) | 5 (16) | 16 (9) |
| Bilateral transplant, n (%) | 210 (90) | 32 (94) | 178 (89) |
| Ischemic time (minutes), median [IQR] | 320 [283, 367] | 310 [283, 386] | 321 [283, 366] |
| Cardiopulmonary bypass time (minutes), median [IQR] | 168 [140, 209] | 185 [160, 226] | 167 [134, 209] |
| Severe PGD (grade = 3), n (%) | 53 (22) | 18 (53) | 35 (17) |
| Length of ICU stay, median [IQR] | 6 [4,10] | 21 [12–41] | 5 [4–8] |
Abbreviations: 6MWD, Six Minute Walk Distance (meters); BMI, Body Mass Index; FEV1, Forced Expiratory Volume in the first second; GDS, Geriatric Depression Scale, range 0–15 and reverse-coded for analysis; HRQL, Health-Related Quality of Life; LAS, Lung Allocation Score; PGD, Primary Graft Dysfunction. Severe PGD was defined as grade III, pulmonary edema on chest X-ray and PaO2/FiO2 ratio < 200 at 48 and/or 72 hours after transplant.18 ICU, Intensive Care Unit; SF12-MCS, Short Form 12—Mental Component Score, range 0–100; SF12-PCS, Short Form 12—Physical Component Score, range 0–100.
Delirium occurred in 34 participants (14%). Participants had a median of 2 days of delirium (IQR 1, 4) and a maximum 12 days of delirium. In clinical notes, the majority of delirium was deemed ICU delirium without further specification (Supplemental Table 1). Although all participants experienced substantial improvements in depressive symptoms and HRQL, HRQL improved less among those who developed delirium (Supplemental Table 2). Delirium was associated with lower SF12-PCS (difference between participants with delirium and those without, −4.0; 95%CI: −7.4, −0.7) (Table 2, Figure 2a). Although the improvements in SF12-MCS and GDS were lower in participants with delirium, the differences were not statistically different nor clinically meaningful (Table 2, Figure 2b, and Figure 2c).
TABLE 2:
Differences In Depressive Symptoms And HRQL In Participants With And Without Delirium Over 12 Months
| Measurement | Difference between recipients who had delirium and those who did not | 95% CI | p-value |
|---|---|---|---|
| Adjusted for recipient characteristics | |||
| GDS | −0.4 | (−1.5, 0.7) | .5 |
| SF12-PCS | −4.0 | (−7.4, −0.7) | .02 |
| SF12-MCS | 2.2 | (−0.9, 5.3) | .17 |
| Adjusted for recipient characteristics and severe PGD | |||
| GDS | −0.2 | (−1.3, 1.0) | .8 |
| SF12-PCS | −4.1 | (−7.6, −0.6) | .02 |
| SF12-MCS | 2.5 | (−0.7, 5.7) | .12 |
Lower scores reflect worse depressive symptoms and poorer HRQL. The association between delirium and depressive symptoms or HRQL was quantified by linear mixed effects models jointly modeled with death. Recipient characteristics include age at transplant, sex, diagnosis, and Lung Allocation Score. PGD, Primary Graft Dysfunction. Severe PGD was defined as grade III, pulmonary edema on chest X-ray and PaO2/FiO2 ratio < 200 at 48 and/or 72 hours after transplant.18 MCID, Minimally clinically important difference.17
Abbreviations: GDS, Geriatric Depression Scale, range 0–15 and reverse-coded for analysis; MCID 1.7; MCID 5; MCID 5; SF12-MCS, Short Form 12—Mental Component Score, range 0–100; SF12-PCS, Short Form 12—Physical Component Score, range 0–100.
FIGURE 2:
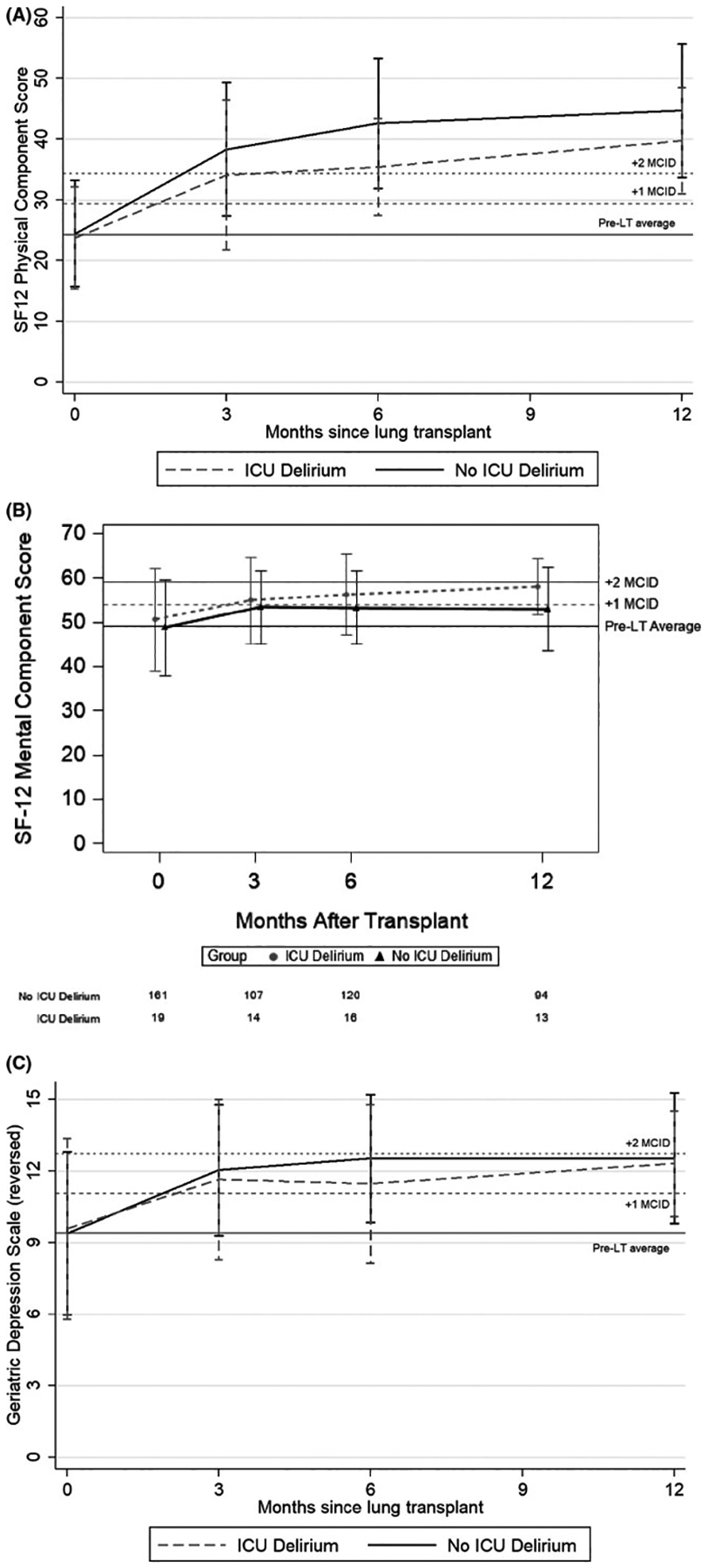
(A) Unadjusted plot of average health-related quality of life (HRQL), by short-form 12 physical component score (SF-12PCS) From before to 12 months after lung transplantation. Solid line represents subjects who did not experience ICU delirium after lung transplantation. Dashed line represents subjects who experienced delirium after lung transplantation. Whiskers represent 1 standard deviation above and below the mean HRQL value for each time point. On the y-axis, the first horizontal black line denotes pre-transplantation mean scores, the dotted horizontal lines above denote a change in score equal to 1 time and 2 times the minimally clinically important difference, respectively. MCID, Minimally clinically important difference.
(B) Unadjusted plot of average health-related quality of life (HRQL), by short-form 12 mental component score (SF12-MCS) from before to 12 months after lung transplantation. Solid line represents subjects who did not experience ICU delirium after lung transplantation. Dashed line represents subjects who experienced delirium after lung transplantation. Whiskers represent 1 standard deviation above and below the mean HRQL value for each time point. On the y-axis, the first horizontal black line denotes pre-transplantation mean scores, the dotted horizontal lines above denote a change in score equal to 1 time and 2 times the minimally clinically important difference, respectively. MCID, Minimally clinically important difference.
(C) Unadjusted plot of depressive symptoms, by geriatric depression scale (GDS) from before to 12 months after lung transplantation. Black line represents subjects who did not experience ICU delirium after lung transplantation. Dashed line represents subjects who experienced delirium after lung transplantation. Whiskers represent 1 standard deviation above and below the mean GDS value for each time point. On the y-axis, the first horizontal black line denotes pre-transplantation mean scores, the dotted horizontal lines above denote a change in score equal to 1 time and 2 times the minimally clinically important difference, respectively. MCID, Minimally clinically important difference.
Thirty-two participants (14%) died during the study period (Supplemental Table 3). Ten participants of the 32 (31%) developed post-operative ICU delirium. Delirium was associated with an 11-fold unadjusted increased risk of subsequent death through the first post-operative year (HR 11.2; 95%CI: 3.3–38.4) (Table 3, Figure 3). After controlling for age, sex, diagnosis, ventilator at transplant, and LAS, delirium was associated with a nearly 18-fold increased risk of mortality (HR 17.9, 95%CI: 4.4–72.5). Further, each additional day with delirium was associated with a roughly 50% increased risk of mortality (HR 1.4; 95%CI: 1.2–1.7) that was not substantially different after further adjusting for recipient characteristics (HR 1.5; 95%CI: 1.2–1.7) that was not substantially different after further adjusting for recipient characteristics (HR 1.7; 95%CI 1.4–2.1) or additionally for severe PGD (1.5; 95%CI: 1.2–1.9) (Table 3).
TABLE 3:
Association Between Delirium And One-Year Mortality
| Death within the first year after lung transplant by delirium status | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| Unadjusted | 11.2 | (3.3, 38.4) | <.01 |
| Adjusted for recipient characteristics | 17.9 | (4.4, 72.5) | <.01 |
| Adjusted for recipient characteristics and severe PGD | 13.4 | (3.0, 58.7) | .01 |
| Death within the first year after lung transplant by number of days with delirium | Hazard ratio | 95% CI | p-value |
| Unadjusted | 1.4 | (1.2, 1.7) | <.01 |
| Adjusted for recipient characteristics | 1.7 | (1.4, 2.1) | <.01 |
| Adjusted for recipient characteristics and severe PGD | 1.5 | (1.2, 1.9) | <.01 |
Effect estimates are hazard ratios (95% confidence intervals) for mortality within the first post-operative year associated with ICU delirium. Recipient characteristics include age at transplant, sex, diagnosis, use of ventilator at transplant, and Lung Allocation Score.
Abbreviations: PGD, Primary graft dysfunction. PGD, Primary Graft Dysfunction. Severe PGD was defined as grade III, pulmonary edema on chest X-ray and PaO2/FiO2 ratio < 200 at 48 and/or 72 hours after transplant.18
FIGURE 3:
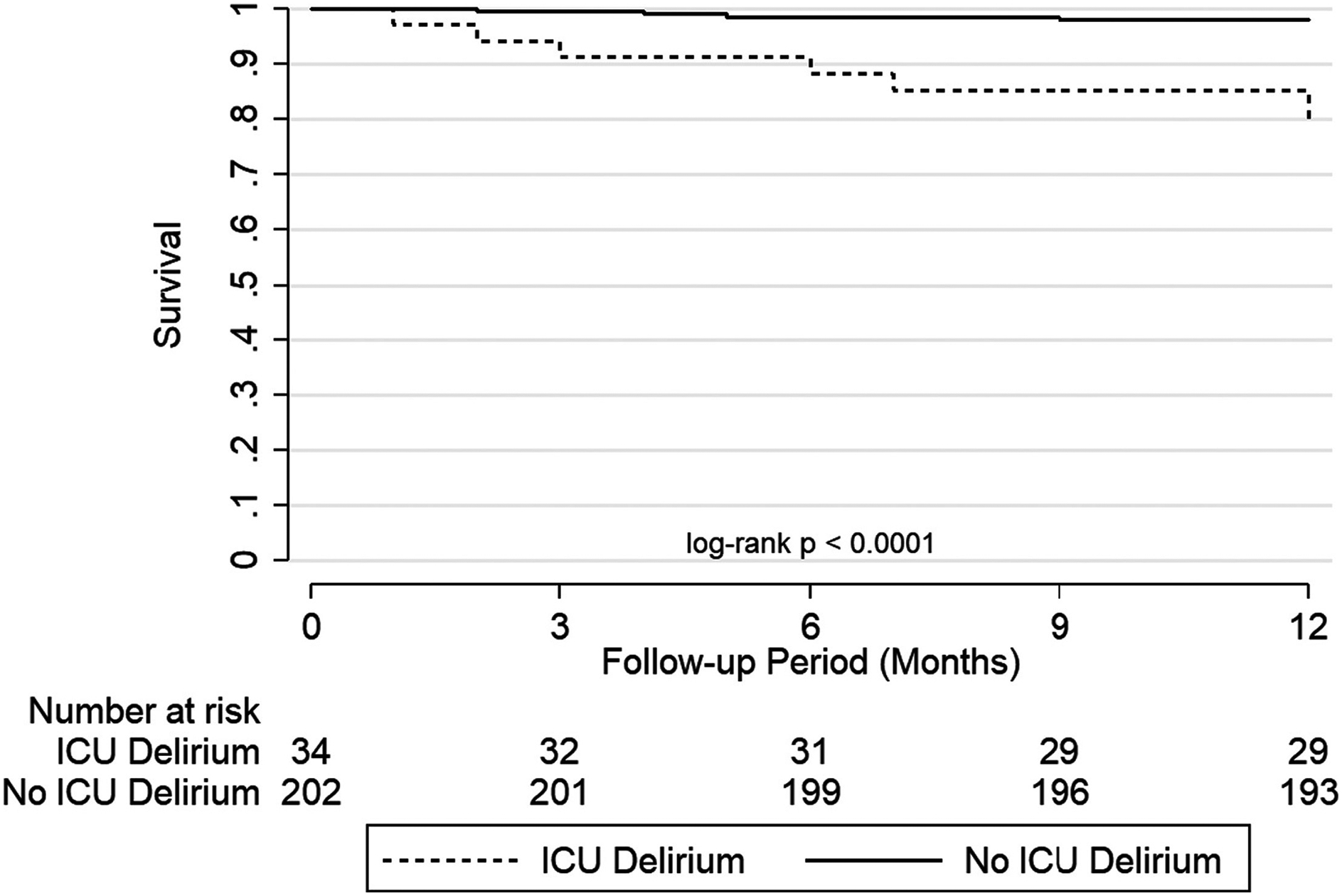
Unadjusted Survival Curve By Delirium Status After Lung Transplant
Sensitivity analyses were conducted to explore the possible influence of PGD to the observed associations between delirium and subsequent outcomes. Delirium remained associated with a 10-fold adjusted increased risk of death when the 53 participants with severe PGD were excluded (HR 10.4; 95%CI: 1.5–74.1) (Supplemental Table 4). Further adjusting for recipient characteristics did not substantively change the effect estimate (HR 19.2; 95%CI: 1.5–244.8).
Discussion
In this single-center prospective cohort study, we found that delirium following lung transplant surgery was associated with poorer HRQL and increased risk of death through the first post-operative year. Specifically, post-operative delirium was associated with an attenuation in SF12-PCS generic HRQL improvements by nearly 5 points, a difference that approaches the minimally clinically important difference. Further, delirium was associated with more than a 10-fold increased risk of death, independent of age, disease indication, sex, and PGD.
Our findings support the growing body of literature identifying delirium as an understudied and deleterious event that adversely impacts lung transplantation recipients in numerous ways. Sher et al and Anderson et al identified that patients who experienced delirium after lung transplantation had worse survival through the first post-operative year. Their findings, however, were not significant after adjusting for key clinical variables including age, sex, diagnosis group, LAS, and PGD.1,3 Smith and colleagues identified an association between early neurologic sequelae that included delirium and mortality after transplantation, independent of native disease and LAS that persisted at two years after transplant.2 Our study adds to this literature by demonstrating an association between delirium and poorer HRQL and increased mortality, even after adjusting for recipient characteristics.
This study identified that delirium is associated with less improvements in generic-physical HRQL among survivors of lung transplant recipients. In our previous work, a substantial subset of lung transplant recipients (8–46% depending on the HRQL measure applied) do not appear to derive significant HRQL benefits from lung transplantation.13 However, we were previously unable to identify any demographic or clinical factors that could discriminate who would have impaired HRQL following lung transplantation. Delirium may, therefore, represent a novel peri-operative factor that impacts the magnitude of HRQL improvement after transplant.
Our finding that delirium was not associated with depressive symptoms was surprising, given the association between delirium and depression among survivors of critical illness.5,6,8 There are several potential reasons for this difference. One possible explanation may be that lung transplant recipients have generally had a prolonged pulmonary illness prior to their transplant, which significantly limited their functional status. Successful transplants would be expected to lead to overall improved functional status, which may also be protective against mental illness post-transplant. Alternatively, it is well recognized that depression is prevalent after lung transplant, with some studies identifying that up to 20% of patients will have depression after lung transplant.24 The association of delirium with mental illness may be masked given the overall prevalence of depression.
Our finding of an independent association between ICU delirium and one-year mortality, independent of PGD, is particularly notable. In our study, participants who experienced post-operative delirium had more than 10-fold greater risk of mortality. We caution against over interpreting this point estimate given our wide confidence intervals. Nevertheless, the lower bounds of the 95% confidence interval that shows a 3.1-fold higher risk of death, independent of PGD, highlights the importance of delirium as a key perioperative risk factor for poor outcomes after transplant. Further, the risk associated with time spent delirious after transplant appears to be additive. For each additional day of delirium in the ICU, patients had 43% greater odds of death.
The mechanism for association between delirium and mortality is unknown. While speculative, possible explanations include decreased intraoperative cerebral oxygenation and systemic inflammation.25 Intra- and perioperative insults independently increase risk of post-operative cognitive impairments with resultant risk of medication and behavioral non-adherence.26 Importantly, delirium can be prevented and treated. Interventions that prevent delirium can be cost effective by reducing iatrogenic complications of hospitalizations and discharges to locations other than home.27–29 Patients that undergo delirium interventions report improved subjective health and HRQL compared to those who did not.30
While overall first post-operative year mortality has decreased, recent work has identified concerning trends towards more complications during this time.1–3, 11, 12, 31, 32 The factors driving these trends have not been elucidated but delirium may be a contributor. Lung transplant candidates are increasingly older and are more frequently inpatient or in the ICU at time of transplant.10–12 These characteristics can increase risk for delirium. In the USA, contemporary lung transplant recipients suffer from more complications, longer hospital stays, are more frequently discharged to places other than home, and have a higher risk of death after the first post-operative year.2, 12, 31, 32 Similar outcomes are observed among generally critically-ill patients who experience delirium compared to those who do not. Applying approaches to delirium, similar to those that are used with patients who are critically ill, to lung transplantation might help to reverse these concerning developments. Most patient care models to prevent or manage delirium have centered on education for both providers and families, and integration of a variety of strategies, including better pain control and sleep, improved mobilization, optimized nutrition, pharmacist review of medication lists for deliriogenic medications, increased sedation interruptions and spontaneous breathing trials, and improved monitoring for delirium.33–45 More efforts must be focused on delirium as an important intervention to influence HRQL and survival, two of the primary aims of lung transplantation.
Our study has several limitations. First, the incidence of ICU delirium in our sample population was approximately 14%, which is markedly lower than that reported at other centers.1–4 The lower incidence at our center may reflect practice variation in candidate selection for transplant and/or post-operative management. However, our center has a high proportion of transplantation for inpatients and our cohort had a high acuity, reflected by the mean lung allocation scores at transplant. Finally, methodological variations in the frequency and modality of delirium assessment across studies might have impacted the incidence of delirium at our center compared to others. We utilized the CAM-ICU to define delirium, which is an imperfect tool. Despite its limitations, it is one of the two screening tools recommended for delirium in the 2018 Society of Critical Care Medicine practice guidelines. A recent systematic review and meta-analysis reviewed the diagnostic properties of the CAM-ICU and identified a summary sensitivity of 0.85 and summary specificity of 0.95.16 A systematic review by Gelinas et al, 2018 reports excellent reliability, validity, feasibility, and implementation of the CAM-ICU, ultimately concluding that the CAM-ICU is one of the two the most valid and reliable delirium assessment tools for critically ill adults.17 It is possible that screening of our cohort with psychiatrists utilizing more extensive research-based delirium batteries might have identified additional subjects with delirium. While it is possible that some subjects deemed not delirious had “sub-clinical” delirium, if true, it would suggest that sub-clinical delirium is not associated with mortality and that universal screening with psychologists or psychiatrists or advanced imaging may not be cost-effective. Also, to the higher level of medical acuity in our sample, we were unable to obtain delirium assessments for some sicker recipients perhaps reflected in UTA score rates that are higher than prior literature reports.46 It is, therefore, possible that delirious patients who were too sick for assessment may have biased our estimated incidence, although the observed association between delirium and clinical outcomes would likely have been strengthened if these individuals were included. We also did not have access to detailed information on multimodal treatment of delirium. While some of these treatments might have mediated the observed relationship between delirium and mortality, if true, it would suggest that despite standardized multimodal treatment, delirium is still associated with mortality after lung transplant and would argue additional work is needed to mitigate this risk. Although the associations between delirium and mortality were robust, they were based on a relatively small number of delirious patients and a small number of deaths during the first year of follow-up. It will, therefore, be important for future studies to replicate the observed associations using larger patient cohorts with careful control of potential confounders.
Previous studies have identified several baseline characteristics that are associated with delirium after lung transplantation, including pre-transplant benzodiazepine use or neurocognitive impairment.1–4 We found no significant differences in baseline characteristics between participants who developed ICU delirium and those who did not, but did not investigate some of these factors. However, we suspect many of the factors that contribute to increased risk of post-transplant delirium also contribute to pre-transplant delirium and it may be impossible to determine exactly when any of these result in clinically significant delirium. Regardless, additional investigation of characteristics prior to transplant may be useful in candidate selection and in identifying patients at higher risk of delirium.4
Elevated neuroinflammation and early immunological changes are increasingly suspected as contributors to postoperative delirium. This influence is likely multifactorial. It is plausible that proinflammatory factors, such as infection, could influence risk of and recovery from delirium. Not analyzing anti-inflammatory medications or pro-inflammatory factors is another limitation of the study, but it is likely that both patient-level risk factors and perioperative management factors are playing some role that future studies should examine.
While the CAM-ICU is validated in patients who are intubated, over 80% of our cohort had CAM scores of “unable to assess” (UTA) at some point during their ICU admission. Global rates of inappropriate UTA scores may be higher than what is reported in the literature.29 It is possible our findings might be different had the UTA incidence been lower in a controlled research setting in which CAM-ICU scores were performed by investigators.
Finally, our modeling approach for HRQL addresses survivorship bias and is most relevant for patient-specific decision-making and counseling. However, it limits our ability to provide estimates of the effect of lung transplantation on population-level HRQL. It is likely, though, that those who died had poorer HRQL prior to death than survivors. Given the association of a higher risk of death, it is likely that the association between post-operative delirium and HRQL would be larger had we used a modeling approach that assigned poorer HRQL scores to those that had died.
Despite these limitations, our study had several strengths. We studied a relatively large cohort of lung transplantation recipients that was rigorously characterized and followed prospectively for patient-reported clinical outcomes following transplant. Our prospective, repeated assessments of HRQL and depression over several years is a particular strength. We had little missing data and no loss to follow-up. Finally, the size of our cohort allowed us to control for multiple relevant covariates.
In sum, our findings suggest that the occurrence of ICU delirium after lung transplantation is associated with attenuated improvement in HRQL and increased risk of mortality. These findings further highlight the importance of delirium in the immediate post-operative period following lung transplantation. Future research is needed to determine the mechanisms for our observed associations and to investigate best practices to reduce the incidence of delirium.
Supplementary Material
Funding sources:
VA ORD CSR&D career development award (IK2CX001034)
JPS NHLBI K23HL111115 R01HL134851
References
- 1.Anderson BJ, Chesley CF, Theodore M, et al. Incidence, risk factors, and clinical implications of post-operative delirium in lung transplant recipients. J Heart Lung Transplant. 2018;37(6):755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith PJ, Stonerock GL, Ingle KK, et al. Neurological Sequelae and Clinical Outcomes After Lung Transplantation. Transplant Direct. 2018;4(4):e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sher Y, Mooney J, Dhillon G, Lee R, Maldonado JR. Delirium after lung transplantation: Association with recipient characteristics, hospital resource utilization, and mortality. Clin Transplant. 2017;31(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith PJ, Rivelli SK, Waters AM, et al. Delirium affects length of hospital stay after lung transplantation. J Crit Care. 2015;30(1):126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salluh JI, Wang H, Schneider EB, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015;350:h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolters AE, van Dijk D, Pasma W, et al. Long-term outcome of delirium during intensive care unit stay in survivors of critical illness: a prospective cohort study. Crit Care. 2014;18(3):R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith PJ, Rivelli S, Waters A, et al. Neurocognitive changes after lung transplantation. Ann Am Thorac Soc. 2014;11(10):1520–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant. 2016;35(4):433–439. [DOI] [PubMed] [Google Scholar]
- 11.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Lung. Am J Transplant. 2019;19 Suppl 2:404–484. [DOI] [PubMed] [Google Scholar]
- 12.Maxwell BG, Mooney JJ, Lee PH, et al. Increased resource use in lung transplant admissions in the lung allocation score era. Am J Respir Crit Care Med. 2015;191(3):302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer JP, Katz PP, Soong A, et al. Effect of Lung Transplantation on Health-Related Quality of Life in the Era of the Lung Allocation Score: A U.S. Prospective Cohort Study. Am J Transplant. 2017;17(5):1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dew MA, DiMartini AF, Dobbels F, et al. The 2018 ISHLT/APM/AST/ICCAC/STSW recommendations for the psychosocial evaluation of adult cardiothoracic transplant candidates and candidates for long-term mechanical circulatory support. Psychosomatics. 2018; 59(5): 415–440. [DOI] [PubMed] [Google Scholar]
- 15.Guenther U, Popp J, Koecher L, et al. Validity and reliability of the CAM-ICU Flowsheet to diagnose delirium in surgical ICU patients. J Crit Care. 2010;25(1):144–151. [DOI] [PubMed] [Google Scholar]
- 16.Ho MH, Montgomery A, Traynor V, et al. Diagnostic performance of delirium assessment tools in critically ill patients: a systematic review and meta-analysis. Worldviews Evid Based Nurs. 2020; 17(4): 301–310. [DOI] [PubMed] [Google Scholar]
- 17.Gelinas C, Berube M, Chevrier A, et al. Delirium assessment tools for use in critically ill adults: a psychometric analysis and systematic review. Crit Care Nurse. 2018; 38(1): 38–49. [DOI] [PubMed] [Google Scholar]
- 18.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. [DOI] [PubMed] [Google Scholar]
- 19.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. [DOI] [PubMed] [Google Scholar]
- 20.Busija L, Pausenberger E, Haines TP, Haymes S, Buchbinder R, Osborne RH. Adult measures of general health and health-related quality of life: medical outcomes study short form 36-item (SF-36) and short form 12-item (SF-12) health surveys, nottingham health profile (NHP), sickness impact profile (SIP), medical outcomes study short form 6D (SF-6D), health utilities index mark 3 (HUI3), quality of well-being scale (QWB), and assessment of quality of life (AQoL). Arthritis Care Res (Hoboken). 2011; 63(Suppl 11): S383–S412. [DOI] [PubMed] [Google Scholar]
- 21.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 22.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1097–1103. [DOI] [PubMed] [Google Scholar]
- 23.Laird NM. Missing data in longitudinal studies. Stat Med. 1988;7(1–2):305–315. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberger EM, DiMartini AF, DeVito Dabbs AJ, et al. Psychiatric Predictors of Long-term Transplant-Related Outcomes in Lung Transplant Recipients. Transplantation. 2016;100(1):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65(3):229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott RA, Goeman D, Beanland C, Koch S. Ability of older people with dementia or cognitive impairment to manage medicine regimens: a narrative review. Curr Clin Pharmacol. 2015;10(3):213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizzo JA, Bogardus ST Jr., Leo-Summers L, Williams CS, Acampora D, Inouye SK. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value? Med Care. 2001;39(7):740–752. [DOI] [PubMed] [Google Scholar]
- 28.Rubin FH, Neal K, Fenlon K, Hassan S, Inouye SK. Sustainability and scalability of the hospital elder life program at a community hospital. Journal of the American Geriatrics Society. 2011;59(2):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitkala KH, Laurila JV, Strandberg TE, Kautiainen H, Sintonen H, Tilvis RS. Multicomponent geriatric intervention for elderly inpatients with delirium: effects on costs and health-related quality of life. J Gerontol A Biol Sci Med Sci. 2008;63(1):56–61. [DOI] [PubMed] [Google Scholar]
- 31.Arnaoutakis GJ, Allen JG, Merlo CA, et al. Impact of the lung allocation score on resource utilization after lung transplantation in the United States. J Heart Lung Transplant. 2011;30(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller CA, Gonwa TA, White LJ, et al. Utilization and Cost Analysis of Lung Transplantation and Survival After 10 Years of Adapting the Lung Allocation Score. Transplantation. 2019;103(3):638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marra A, Pandharipande PP, Patel MB. Intensive care unit delirium and intensive care unit-related posttraumatic stress disorder. Surg Clin North Am. 2017; 97(6): 1215–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundstrom M, Edlund A, Lundstrom G, Gustafson Y. Reorganization of nursing and medical care to reduce the incidence of postoperative delirium and improve rehabilitation outcome in elderly patients treated for femoral neck fractures. Scand J Caring Sci. 1999; 13(3): 193–200. [PubMed] [Google Scholar]
- 35.Lundstrom M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007; 19(3): 178–186. [DOI] [PubMed] [Google Scholar]
- 36.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. [DOI] [PubMed] [Google Scholar]
- 37.Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999; 340(9): 669–676.2010; 91(4): 536–542. [DOI] [PubMed] [Google Scholar]
- 38.Balas MC, Rice M, Chaperon C, Smith H, Disbot M, Fuchs B. Management of delirium in critically ill older adults. Crit Care Nurse. 2012; 32(4): 15–26. [DOI] [PubMed] [Google Scholar]
- 39.Bjorkelund KB, Hommel A, Thorngren KG, Gustafson L, Larsson S, Lundberg D. Reducing delirium in elderly patients with hip fracture: a multi-factorial intervention study. Acta Anaesthesiol Scand. 2010; 54(6): 678–688. [DOI] [PubMed] [Google Scholar]
- 40.Rice KL, Bennett MJ, Berger L, et al. A pilot randomized controlled trial of the feasibility of a multicomponent delirium prevention intervention versus usual care in acute stroke. J Cardiovasc Nurs. 2017; 32(1): E1–E10. [DOI] [PubMed] [Google Scholar]
- 41.Dale CR, Kannas DA, Fan VS, et al. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am Thorac Soc. 2014; 11(3): 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balas MC, Weinhouse GL, Denehy L, et al. Interpreting and implementing the 2018 pain, agitation/sedation, delirium, immobility, and sleep disruption clinical practice guideline. Crit Care Med. 2018; 46(9): 1464–1470. [DOI] [PubMed] [Google Scholar]
- 43.Balas MC, Burke WJ, Gannon D, et al. Implementing the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle into everyday care: opportunities, challenges, and lessons learned for implementing the ICU pain, agitation, and delirium guidelines. Crit Care Med. 2013; 41: S116–S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bryczkowski SB, Lopreiato MC, Yonclas PP, Sacca JJ, Mosenthal AC. Delirium prevention program in the surgical intensive care unit improved the outcomes of older adults. J Surg Res. 2014; 190(1): 280–288. [DOI] [PubMed] [Google Scholar]
- 45.Hughes CG, Boncyk CS, Culley DJ, et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative delirium prevention. Anesth Analg. 2020; 130(6): 1572–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terry KJ, Anger KE, Szumita PM. Prospective evaluation of inappropriate unable-to-assess CAM-ICU documentations of critically ill adult patients. J Intensive Care. 2015;3:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


