Abstract
Mixed anion halide-chalcogenide materials have recently attracted attention for a variety of applications, owing to their desirable optoelectronic properties. We report the synthesis of a previously unreported mixed-metal chalcohalide material, CuBiSeCl2 (Pnma), accessed through a simple, low-temperature solid-state route. The physical structure is characterized through single-crystal X-ray diffraction and reveals significant Cu displacement within the CuSe2Cl4 octahedra. The electronic structure of CuBiSeCl2 is investigated computationally, which indicates highly anisotropic charge carrier effective masses, and by experimental verification using X-ray photoelectron spectroscopy, which reveals a valence band dominated by Cu orbitals. The band gap is measured to be 1.33(2) eV, a suitable value for solar absorption applications. The electronic and thermal properties, including resistivity, Seebeck coefficient, thermal conductivity, and heat capacity, are also measured, and it is found that CuBiSeCl2 exhibits a low room temperature thermal conductivity of 0.27(4) W K–1 m–1, realized through modifications to the phonon landscape through increased bonding anisotropy.
1. Introduction
Employing a multiple anion approach to material design provides a route to achieving potentially improved properties beyond single anion compositions.1 In comparison with single-anion materials, mixed-anion compounds are less well characterized. The myriad opportunities afforded through the exploration of novel multianionic phase spaces have recently stimulated more research into this area. Mixed anion chemistry drives material design in two ways: by increasing the number of degrees of freedom available in a system and by modifying the bonding within a material. This can lead to increased structural anisotropy, changes in dimensionality, and the evolution of novel structure types.2,3 Structural changes arising from such bonding variations facilitate the emergence of new physical phenomena4−6 and also provide opportunities for optoelectronic property tuning through modifications to the electronic structure.7,8 A multiple anion approach has already been successfully applied to engineer desirable properties in a variety of energy materials, including battery cathode materials,9,10 solid-state electrolytes,11 and thermoelectrics.12−14
Halide-chalcogenide, or “chalcohalide”, materials are an emerging class of mixed anion materials that contain one or more different metal cations, at least one chalcogenide anion, and at least one halide anion. The multifunctionality of these materials has made them a suitable platform for property tuning to suit a variety of applications, including photocatalysis15 and X-ray detection.16 In particular, they have gained attention as potential solar absorbers, with the aim of using a split anion approach17 to combine the desirable optoelectronic properties of lead halide perovskites (LHPs) with the enhanced stability of chalcogenide-based materials.18,19 The opportunities for wider compositional and structural exploration through employing the mixed anion chemistry of chalcohalide materials make them a valuable starting point for this task.20 Several mixed-metal chalcohalides are suitable for photovoltaic (PV) applications, including Sn2SbS2I321,22 and CuBiSCl2. The latter was recently applied in a single-junction cell by Ming and co-workers and demonstrated exceptional environmental stability, a suitable band gap of 1.44 eV, and a PCE of 1%.23
Here, we report the synthesis, crystal structure, and thermal, electronic, and optical properties of CuBiSeCl2, a previously unreported mixed-metal chalcohalide material. CuBiSeCl2 is a p-type semiconductor with a low thermal conductivity of 0.27(4) W K–1 m–1 and a band gap of 1.33(2) eV. The material was also determined to be stable in ambient air for over a month.
2. Experimental Section
2.1. Reagents
CuCl (99.999%), Bi2Se3 (99.995%), and BiCl3 (99.995%) were purchased from Sigma-Aldrich and were used without further purification. Reagents were stored and handled in an Ar-filled glovebox with <0.1 ppm of O2 and <0.1 ppm of H2O.
2.2. Synthesis of CuBiSeCl2
Powder samples of CuBiSeCl2 on a 1 g scale were synthesized by grinding stoichiometric amounts of reagents in an agate mortar, pressing the reagent mix into an 8 mm diameter pellet, and loading into a flame-dried quartz ampule of dimensions 1 cm × 20 cm. The ampule was evacuated to 1 × 10–3 mbar and sealed under a vacuum. The sample was placed horizontally into a high-temperature oven. The sample was then heated using a ramp rate of 5 °C/min, fired at 430 °C for 10 h, and cooled to 200 °C using a ramp rate of 0.1 °C/min before shutting off the oven and allowing it to cool to room temperature. Single crystals of CuBiSeCl2 were grown using the same reaction conditions used for powder synthesis, but a constant cooling rate of 1 °C/h was used between 430 °C and room temperature.
2.3. Powder X-ray Diffraction
Preliminary phase identification was carried out using a Rigaku SmartLab diffractometer with Mo Kα radiation (λ = 0.7107 Å) in the Debye–Scherrer geometry. Samples were sealed inside 0.2 mm diameter borosilicate capillaries in an Ar-filled glovebox. 16 h scans were conducted on a Bruker D8 Advance with monochromatic Cu Kα1 radiation (λ = 1.54056 Å) in the Debye–Scherrer geometry to obtain high quality data for structural refinement. A LaB6 internal standard was ground into the CuBiSeCl2 mixture to extract accurate lattice parameters during refinement. 12 hour scans were conducted on an X’Pert Panalytical diffractometer with monochromatic Co Kα1 radiation (λ = 1.788965 Å) in the Bragg–Brentano geometry to quantify the extent of preferred orientation in pressed pellets of CuBiSeCl2. Rietveld refinements against CuBiSeCl2 powder x-ray diffraction (PXRD) data were conducted using TOPAS Academic V5.24 The background, lattice parameters, atomic positions, and atomic displacement parameters were refined against the data; a Chebyshev polynomial function was used to model the background; and the peak shapes were modeled using Stephens orthorhombic functions.25 Structural refinement parameters are available in the Supporting Information (S17).
2.4. Single Crystal X-ray Diffraction
A black single crystal with a platelet shape was selected under a polarizing microscope and then studied by single crystal x-ray diffraction (SCXRD) on beamline I19, Diamond Light Source, Didcot, U.K. using silicon double crystal monochromatic synchrotron radiation (λ = 0.6889 Å, Pilatus 2 M detector).26 The synchrotron data were collected with the sample at 100 K. Cell refinement and data reduction were performed using Xia27 and Dials programs.28 The structure was solved and refined using SHELX-2013,29 implemented through Olex2.30 The final residual factors converged to R1 = 0.0119 and wR2 = 0.0114 for reflections with I > 2σ (I). Single crystal solution structure refinement parameters, isotropic and anisotropic thermal displacement parameters, and main bond distances are available in the Supporting Information (S13–S16).
2.5. Compositional Analysis
Scanning electron microscopy (SEM) was performed on a Tescan S8000. Pelletized powder and single crystal samples were attached to an adhesive carbon tape stuck on an aluminum SEM stub. To reduce the charging effects, the samples were coated with a thin layer of carbon. Energy dispersive X-ray (EDX) spectroscopy and wavelength dispersive X-ray (WDX) spectroscopy were performed on the same instrument using X-MaxN and Wave detectors from Oxford Instruments. WDX calibrations for the different elements were obtained by measuring the WDX spectra of appropriate standards. Standard purity was confirmed by using X-ray diffraction and electron microscopy. Quantification was performed by using Aztec software.
2.6. Transmission Electron Microscopy Imaging
Transmission electron microscopy (TEM) images were collected on CuBiSeCl2 particles by using a JEOL JEM 2100+ transmission electron microscope, and compositions were confirmed by using EDX. Samples were loaded onto a Au TEM grid, which was then mounted to a JEOL common specimen holder (EM-21010) before being introduced into the microscope.
2.7. Spark Plasma Sintering
Dense pellets (∼88% theoretical density) were obtained by spark plasma sintering (SPS) of the phase-pure CuBiSeCl2 powder at 300 MPa and 270 °C for 5 min in a 10–3 mbar vacuum using a commercial Thermal Technology LLC DCS10 furnace. Powder samples (∼0.45 g) were pressed in a 10 mm diameter, graphite-foil-lined tungsten carbide die set (with 6% Co binder). Heating and pressure ramp rates were set to 20 °C/min and 100 MPa/min, respectively. The temperature was monitored through a borehole on the side of the die set using a thermocouple. After pressing, the pellets were lightly polished with SiC paper to remove the graphite foil from the pellet surface.
2.8. UV–Vis Spectrometry
Diffuse reflectance of CuBiSeCl2 powder was measured using an Agilent Cary 5000 instrument between 200 and 2500 nm with a step size of 1 nm. Calibration to 100 and 0% reflectance was performed prior to measurement using a PTFE standard and a light trap, respectively. The band gap was determined from a Tauc plot using a method described by Makuła et al.31
2.9. X-ray Photoelectron Spectroscopy
Core-level and valence band X-ray photoelectron spectroscopy (XPS) measurements were collected at HarwellXPS, Didcot, U.K. XPS analysis was performed using a Kratos Axis SUPRA XPS fitted with a monochromatic Al Kα X-ray source (hν = 1486.6 eV), a spherical sector analyzer, and 3 multichannel resistive plates, 128 channel delay line detectors. All data were recorded at 150 W and a spot size of 700 × 300 μm2. Survey scans were recorded at a pass energy of 160 eV, and high-resolution scans were recorded at a pass energy of 20 eV. Electronic charge neutralization was achieved using a magnetic immersion lens. All sample data were recorded at a pressure below 10–8 Torr and temperature of 294 K. Data were analyzed using CasaXPS v2.3.19PR1.0. Peaks were fit with a Shirley background prior to component analysis. Mixed Gaussian–Lorentzian lineshapes [GL(50)] were used to fit components. All binding energies were measured with respect to the Fermi edge of a Ag foil reference sample.
The ionization potential (IP) of CuBiSeCl2 was determined by collecting the secondary electron cutoff (SEC) region using a monochromatic Al Kα SPECS X-ray source with a PSP Vacuum Technology hemispherical electron energy analyzer with a mean radius of 120 mm. Pass energies of 2 eV for the SEC, 10 eV for core levels, and 50 eV for survey scans were used to measure the emitted photoelectrons. Measurements were performed in an ultrahigh-vacuum chamber with a base pressure of 2 × 10–10 mbar. SEC data were recorded at 16 W to prevent overloading the analyzer. A 10 V bias was applied to the sample to remove any effects from the material work function. The Cu 2p region was also measured to use as a reference. The spectrometer resolution was determined to be 0.40 eV by fitting a Fermi–Dirac function convolved with a Gaussian function to the Fermi edge of the Ag foil.
2.10. Properties Measurement
SPS prepared pellets were cut into semicircles with 1.13 mm thickness and 4.9 mm radius by using a low-speed, diamond-blade saw for Seebeck coefficient, electronic resistivity, and thermal conductivity measurements. Copper electrodes were attached to the pellet using Ag epoxy and left to dry overnight. The offcuts from the pellets were used for powder diffraction and compositional analysis. The thermal conductivity, electronic conductivity, and Seebeck coefficient were measured simultaneously between 2 and 300 K using two probe geometry on a dense pellet of CuBiSeCl2 using the Thermal Transport Option (TTO) on a Quantum Design physical properties measurement system (PPMS). Heat capacity measurements were performed on a small fragment of dense pellet, with a mass of 0.0125 g, of CuBiSeCl2 using the Heat Capacity Option (HCO) on the PPMS. The sample was mounted using N grease. An addenda measurement was performed on the sample holder and grease prior to mounting and measuring the pellet fragment. Both the addenda and pellet fragment measurements were performed between 2 and 300 K.
2.11. Environmental Stability
The stability of CuBiSeCl2 in ambient air and water was determined as part of this study. The water stability of CuBiSeCl2 was determined by mixing CuBiSeCl2 powder with distilled water for 10 min and pipetting several drops of the suspension onto a glass slide. The slide was then left under ambient conditions for 24 h to allow the water to evaporate. PXRD data were measured before and after water exposure. The air stability was determined by sprinkling CuBiSeCl2 powder onto a glass slide, leaving the slide under ambient conditions for several weeks, and measuring PXRD data at regular time intervals.
2.12. Computational Details
Electronic structure calculations were performed using the periodic density functional theory (DFT) program, Vienna Ab initio simulation package (VASP).32 Core electrons were treated using the projector-augmented wave approach33 with Bi 5d electrons included as valence electrons. The hybrid functional, HSE06,34 was used with the inclusion of spin–orbit coupling (SOC) effects35 and a plane-wave cut off energy of 400 eV. The structure of CuBiSeCl2 was relaxed until forces fell below 0.01 eV Å–1 with a Γ-point centered 10 × 5 × 3 k-point grid. The high-symmetry path for calculation of the band structure was obtained using the automatic flow library.36
3. Results and Discussion
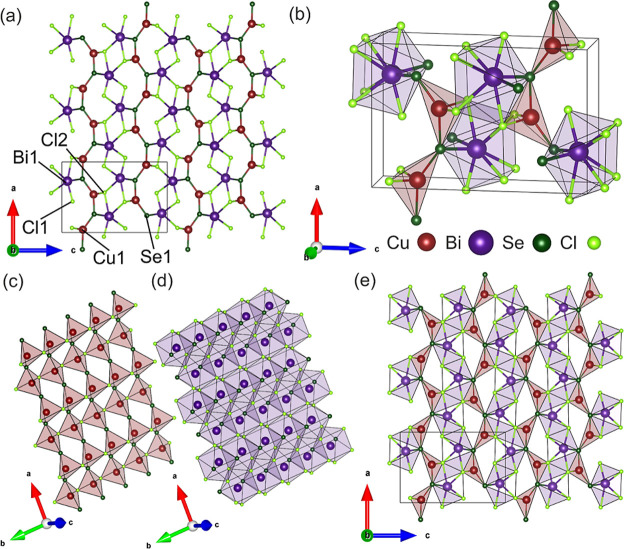
Figure 1a represents the crystal structure of CuBiSeCl2 solved from SCXRD data, viewed along the b axis. CuBiSeCl2 adopts the orthorhombic symmetry of Pnma, with lattice parameters a = 8.78415(6) Å; b = 3.99803(3) Å; and c = 13.13998(9) Å. In this structure, the oxidation states of the species are Cu+, Bi3+, Se2–, and Cl–, confirmed with the analysis of core-level binding energies from XPS (S1–S8). The unit cell of CuBiSeCl2 is depicted in Figure 1b and highlights the local bonding environments present in the overall structure. Cu+ exhibits a coordination number of four, with two Cu–Se bonds and two Cu–Cl bonds forming CuSe2Cl2 tetrahedra. Bi3+ is eight coordinate, forming two Bi–Se bonds and six Bi–Cl bonds to create square antiprismatic BiSe2Cl6 polyhedra. These copper- and bismuth-centered polyhedra are arranged such that two distinct layers are formed within the structure (Figure 1c,d). Layers of CuSe2Cl2 tetrahedra, which are connected over common Cl–Cl edges along the b direction and share a Se vertex in the a direction (Figure 1c), alternate with layers of Bi polyhedra, which face-share along the b direction and share two Cl–Cl edges in the a direction (Figure 1d). These layers are stacked vertically along the c direction such that a single CuSe2Cl2 tetrahedron is interconnected via edge sharing of two Cl–Se edges with two neighboring BiSe2Cl6 polyhedra, forming the overall CuBiSeCl2 structure depicted in Figure 1e.
Figure 1.
(a) Ball and stick model of the structure of CuBiSeCl2 (Pnma) as solved from SCXRD data viewed along the b axis and including the unit cell with labeled atomic sites. (b) Unit cell of CuBiSeCl2 depicting bonding environments of CuSe2Cl2 tetrahedra and BiSe2Cl6 polyhedra. (c) Layers of CuSe2Cl2 tetrahedra and (d) BiSe2Cl6 polyhedra, which are stacked alternately along the c direction in CuBiSeCl2. (e) Polyhedral structure of CuBiSeCl2 viewed along the b axis.
Cu+ and Bi3+ are displaced from the center of their respective environments, visible in Figure 1e. Though it is tetrahedrally coordinated, Cu+ occupies an octahedral environment created by the Se2– and Cl– anions. Within this environment, Cu+ is displaced in the direction of the Cl–Cl octahedral edge to achieve sufficient valence. Bi is then displaced in the direction of the now under-bonded Cl– at the octahedral corner. The origin of these displacements can be understood through comparisons with known compounds that exhibit similar structures: CuBiSCl2 (Cmcm) and MnBiSe2Br (Pnma).
CuBiSCl2 adopts the higher symmetry Cmcm space group40 and exists as a “post-perovskite” structure, first observed in a high-pressure polymorph of MgSiO3.41 The structure of CuBiSCl2 is made up of alternating layers of CuS2Cl4 octahedra and BiS2Cl6 polyhedra and shows the same packing of polyhedra as the selenide compound, but the polyhedra are much less distorted in the sulfide analogue. Figure 2 presents a structural comparison of CuBiSeCl2 and CuBiSCl2. Here, octahedral Cu environments are included in the CuBiSeCl2 unit cell and local environments to allow for better comparison between the two materials, though the long Cu–Cl “bonds” [3.4039(6) Å] are shown with dashed lines to indicate they are not physically reasonable. The following discussion will also consider the octahedral CuSe2Cl4 environment, again for ease of comparison.
Figure 2.
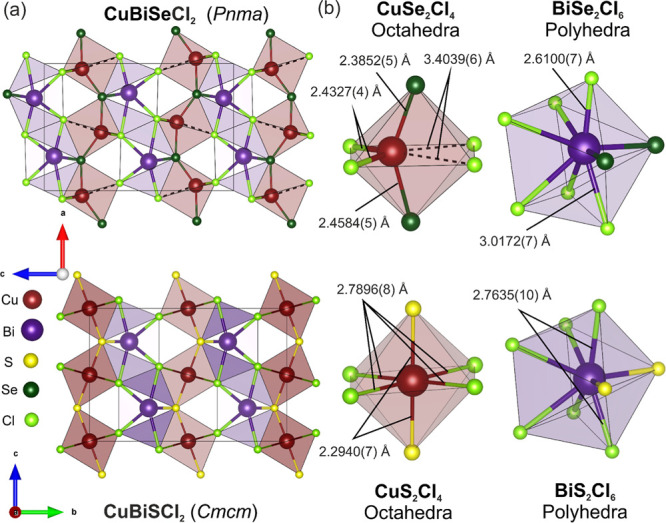
(a) Comparison of the unit cell of CuBiSeCl2 (Pnma) viewed along the b axis (top) with the unit cell of the higher symmetry CuBiSCl2 (Cmcm) viewed along the a axis (bottom). To highlight the structural differences between the two materials, Cu is shown in an octahedral environment in CuBiSeCl2, with the longer Cu–Cl bond represented by a dashed line to indicate that this bond is not physically observed. Furthermore, an additional row of CuSe2Cl4 octahedra are shown to allow for easier comparison of the CuBiSCl2 and CuBiSeCl2 unit cells. (b) Local bonding environments of CuChCl4 octahedra and BiCh2Cl6 polyhedra (Ch = S, Se) in CuBiSCl2 and CuBiSeCl2 are shown with specific Cu–Cl and Cu–Ch bonds labeled to highlight the variation in bond length with increasing anion size. Fully labeled CuChCl4 octahedra and BiCh2Cl6 polyhedra can be found in the Supporting Information (S9).
The comparison between the structures of CuBiSeCl2 and CuBiSCl237 indicates that increasing the chalcogen anion size is the primary driving force for the transition to lower symmetry observed for CuBiSeCl2. A group–subgroup relation scheme detailing the symmetry lowering that occurs between CuBiSCl2 and CuBiSeCl2 is included in the Supporting Information (S10). Figure 2a depicts the unit cells of both structures and Figure 2b shows labeled local bonding environments of CuBiSeCl2 and CuBiSCl2 to highlight the variation in bond lengths between the two materials.
Figure 2b highlights the anisotropic bond lengths present in the CuSe2Cl4 octahedral environment, with the contracted Cu–Cl bonds in the direction of Cu+ displacement [2.4327(4) Å] almost 1 Å shorter than the longer Cu–Cl “bonds” opposite [3.4039(6) Å]. The Cu–Se bond lengths also show some anisotropy, though these differences are less pronounced than in the Cu–Cl bond lengths. As the Cu+ is displaced toward the Cl– at the octahedral vertex, it is also displaced in the a direction toward one of the Se2– species. Figure 2b depicts the shortening of the Cu–Se bond in the direction of displacement [2.3856(9) Å] and the lengthening of the other bond [2.4573(9) Å]. The Cu+ displacement only happens in the a–c plane, and there is no displacement along the b direction.
Bi3+ exhibits the same coordination of six chloride ions and two chalcogen ions in both materials; however, the Cu+ displacements in CuBiSeCl2 also affect Bi3+, which is displaced from the center of the polyhedra, introducing differences in the Bi–Cl and Bi–Se bond lengths. As shown in Figure 2b, the Bi1–Cl1 bond [2.6100(6) Å] contracts as Bi shifts toward the under-bonded Cl of the CuSe2Cl4 octahedra, which induces lengthening of the Bi1–Cl2 bond [3.0172(7) Å] opposite. Again, Bi3+ displacement happens only in the a–c plane.
More detail about the origin of Cu+ displacements in CuBiSeCl2 can be understood using bond valence sum (BVS) analysis (S12–S14). The location of Cu+ on the 4a site at the center of the octahedron in CuBiSeCl2 would yield a BVS of 0.716, indicating that Cu+ is underbonded in such an environment. Displacement from the center to the 4c position toward the Cl–Cl edge achieves higher valence for Cu+ of 0.913. For comparison, in the reported structure for CuBiSCl2, Cu+ occupies a site at the center of the octahedron with a BVS of 0.865. The volume of the CuSe2Cl4 environment increases from 23.8 Å3 in CuBiSCl2 to 33.1 Å3 in CuBiSeCl2, which is the reason the Cu+ displacement is observed in the latter. This is supported by calculation of BVS maps (Figures S13 and S14) that show a cuboidal region with a side length of ∼0.7 Å about the center of the octahedron where Cu+ would achieve a valence of 1. Calculation of the BVS parameters for octahedrally coordinated Cu+ positioned on the 4c site in the experimentally observed Pnma CuBiSeCl2 material confirms the tetrahedral coordination of Cu+ by showing that the long Cu–Cl “bond” [3.4039(6) Å] contributes minimally to the overall valence of Cu+.
We also note that in the structure of CuBiSCl2, a large Uiso of 0.068 Å2 is observed for Cu, which may infer some unresolved structural displacement around this position. This is further supported by BVS maps calculated for CuBiSCl2, which depict a cuboidal region of similar size to that observed for CuBiSeCl2.
Considering the coordination chemistry of Cu+ can also provide insights into the structural displacements present in CuBiSeCl2. Typically, Cu+ forms 3 and 4-fold coordinate environments. In compounds with similar chemistries to CuBiSeCl2 like Cu3BiS3 and CuInxGa1–xSe2, Cu+ preferentially occupies a tetrahedral coordination. In addition to this, Xiao et al. report that, as of yet, there are no reported compounds whereby Cu+ is 6-fold coordinated with halides.38
The crystal structure of CuBiSeCl2 is most similar to that of MnBiSe2Br, which also crystallizes in the space group Pnma.39 A structural comparison between the unit cells of CuBiSeCl2 and MnBiSe2Br can be found in the Supporting Information (S11). Again, though Cu+ is tetrahedrally coordinated in CuBiSeCl2, we will consider the CuSe2Cl4 octahedra for ease of comparison with the MnSe4Br2 octahedra in MnBiSe2Br.
The structure of MnBiSe2Br consists of layers of MnSe4Br2 octahedra and BiSe5Br3 polyhedra and, like the Cu displacement in CuBiSeCl2, MnBiSe2Br exhibits a deviation in the position of Mn from the center of the MnSe4Br2 octahedra and toward the Br–Br octahedral edge. Bi occupies split sites in MnBiSe2Br, with the Bi1 site exhibiting an occupancy of 0.97 and the Bi2 site possessing an occupancy of 0.03. In CuBiSeCl2, the Cu is displaced much more significantly from the center of the octahedra compared to the displacement of Mn observed in MnBiSe2Br. The differences in the cationic displacement magnitude observed in the two structures are partly due to the stoichiometric variation in the local environments, which results in different coordination and bonding.
In the MnSe4Br2 octahedra, the two Br– ions occupy equatorial cis positions around the central Mn2+ ion with the Se2– ions occupying the remaining four sites (S9). In contrast, in the CuSe2Cl4 octahedra the four Cl– ions occupy all equatorial positions and the two Se2– ions are positioned trans on the axial positions. One factor that influences the magnitude of the cationic displacement in CuBiSeCl2 relative to MnBiSe2Br is the size of the ionic radii in the octahedral environment. In the MnSe4Br2 environment, all of the anionic species exhibit similar ionic radii (Br 1.96 Å, Se 1.98 Å),40 which causes minimal displacement of Mn2+. The difference in anion size in the CuSe2Cl4 octahedra is more significant (Cl 1.81 Å, Se 1.98 Å) and results in a more anisotropic displacement of Cu+. The variation in structural displacements achieved in these materials by using different coordination environments in three similar structures suggests that using a mixed anion approach provides a route to establishing control over specific structural features.
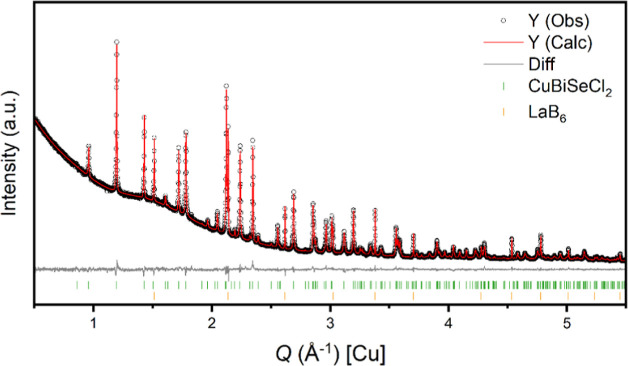
Rietveld analysis of CuBiSeCl2 PXRD data, shown in Figure 3, was used to confirm the structural model determined from SCXRD data and demonstrate the phase purity of the bulk samples. TEM images (S21) show the particles to be needle-like structures of approximately 1.5 μm × 0.25 μm. Compositional analysis was performed on both single crystal and powder samples of CuBiSeCl2 to corroborate the stoichiometry determined from the single crystal model, as shown in S22–S26. SEM–EDX elemental mapping was performed on a single crystal of CuBiSeCl2 to confirm the homogeneous distribution of elements as well as the absence of oxygen in the structure. Single crystal EDX elemental mapping in Figure S22 indicates that Cu, Bi, Se, and Cl are all uniformly distributed in the sample and the presence of oxygen is minimal. To further confirm the material stoichiometry and account for the significant overlap of Bi3+ and Cl– peaks in the EDX spectra, SEM–EDX and WDX were also measured on CuBiSeCl2 powder, and these data are depicted in S23 and S24. SEM–WDX was used to quantify the Cu/Bi and Bi/Cl ratios, while SEM–EDX measured the Bi/Se ratio. The overall composition of CuBiSeCl2 was determined to be Cu1.10(3)Bi1.0(4)Se1.15(5)Cl2.17(3) consistent with the stoichiometry established from the structural solution.
Figure 3.
Rietveld analysis of the PXRD data measured for CuBiSeCl2 with LaB6 used as an internal standard.
The band structure and effective masses were calculated for CuBiSeCl2 to provide insights into the optical band gap and mobility of charge carriers within the material as well as elucidate possible applications. The band structure of CuBiSeCl2 is shown in Figure 4a.
Figure 4.
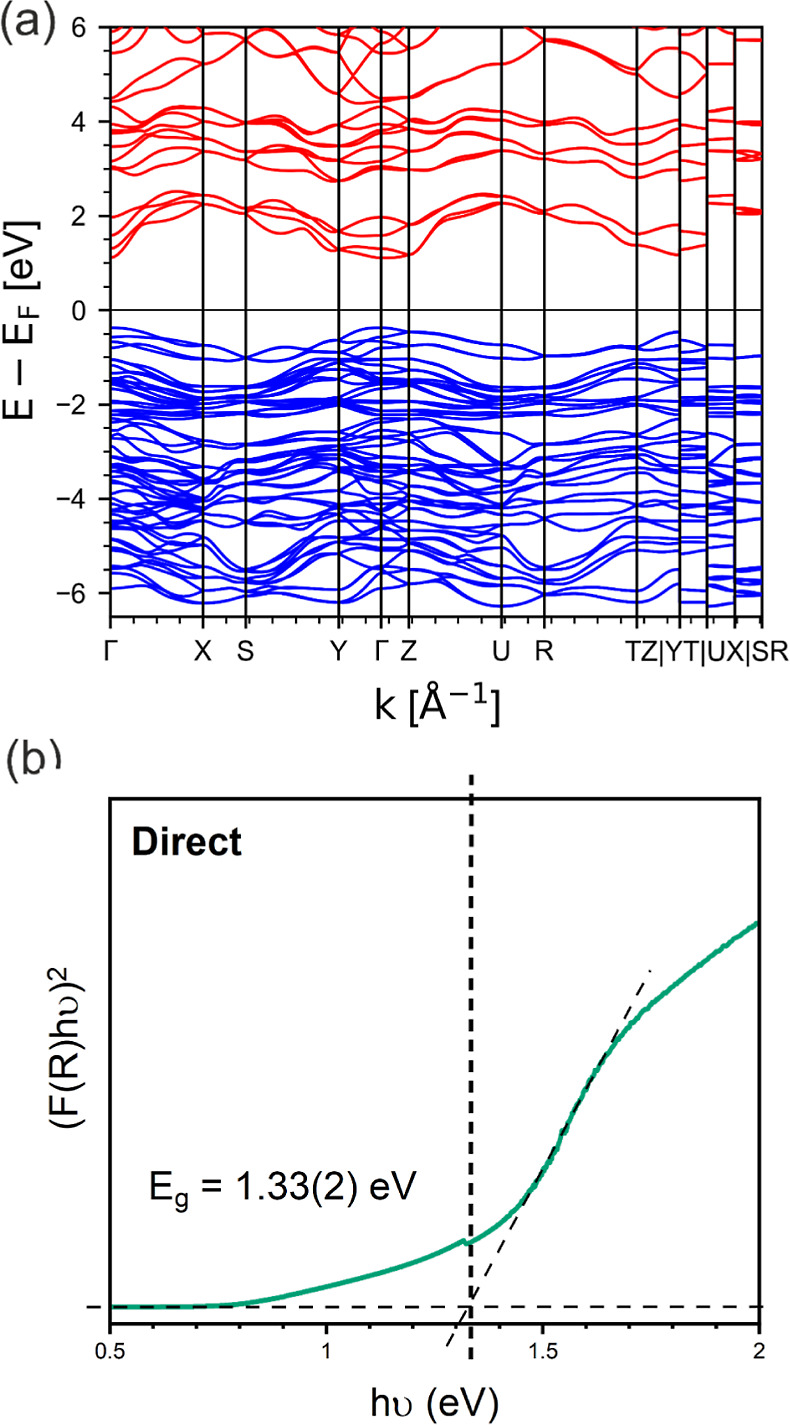
(a) Band structure of CuBiSeCl2 calculated using the HSE06 functional with SOC contributions. Valence band electronic states are shown in blue and the conduction band electronic states in red. (b) Tauc plot derived from UV–vis spectrometry measurements. The direct band gap for CuBiSeCl2 is extrapolated using the Tauc plot and determined to be 1.33(2) eV.
The direct band gap calculated from this band structure is found to be 1.5 eV, suggesting that CuBiSeCl2 may have potential as a solar absorber. Interestingly, the calculated direct and indirect bandgaps are very close in energy such that the band gap in CuBiSeCl2 can be considered to be quasi-direct. This occurs since the conduction band in the Γ → Z direction is extremely flat close to the Γ point and dips slightly as it goes away from the Γ point such that the computed conduction band minimum (CBM) is actually displaced slightly away from the Γ point itself. This leads to an indirect gap that is only very slightly smaller than the direct gap (0.1 meV). This effect is also observed in the band structure of CuBiSCl2, whereby, when SOC is accounted for, the CBM shifts from Y toward Γ, but this energy difference is very small (10 meV); hence, the band gap in this material is considered to be quasi-direct.23
To provide insight into the effect of the physical structure on the electronic transport properties in CuBiSeCl2, the band structures of the reported sulfide analogue CuBiSCl2 (Cmcm) and the hypothetical, higher-symmetry CuBiSeCl2 (Cmcm) material were also calculated (S28). The associated charge carrier effective masses are reported for the experimentally observed CuBiSeCl2 (Pnma), the hypothetical higher symmetry CuBiSeCl2 structure (Cmcm) and CuBiSCl2 (Cmcm) in Table 1. The effective masses for CuBiSeCl2 are reported for the Γ → X, Γ → Y, and Γ → Z directions. For the Cmcm structures, the effective masses are reported for the Y → X1, Y → Γ, and Y → T reciprocal space paths.
Table 1. Electron (me*) and Hole (mh*) Effective Masses (Relative to the Mass of an Electron, m0) Calculated as Part of This Study from the Band Structures of CuBiSCl2 (Cmcm), CuBiSeCl2 (Cmcm), and CuBiSeCl2 (Pnma) in the kx, ky, and kz Directions.
| CuBiSeCl2 (Pnma)a | ky (Γ → Y) | kz (Γ → Z) | kx (Γ → X) |
|---|---|---|---|
| me* (m0) | 1.0 | 12 | 0.2 |
| mh* (m0) | 8 | 1.6 | 4 |
| CuBiSeCl2 (Cmcm) | kx (Y → X1) | ky (Y → Γ) | kz (Y → T) |
|---|---|---|---|
| me* (m0) | 3 | 4.2 | 0.7 |
| mh* (m0) | 2.5 | 0.7 | 1.3 |
| CuBiSCl2 (Cmcm) | |||
| me* (m0) | 0.25 | 3 | 0.65 |
| mh* (m0) | 2.9 | 0.75 | 1.1 |
Indicates that the effective masses for the Pnma structure of CuBiSeCl2 have been reordered relative to the Cmcm structures such that the directions are related by kx → kz, kz → ky, and ky → kx to account for symmetry lowering effects between Cmcm and Pnma and allow for ease of comparison between different symmetries.
Though the band structure of CuBiSeCl2 exhibits visibly low dispersion, the calculated effective masses within CuBiSeCl2 are highly anisotropic, with particularly heavy electron effective masses in the kz direction but much lighter electrons in the kx direction.
The very light electron effective masses in the kx direction result from the highly connected face-sharing Bi3+ polyhedra and corner-sharing Cu+ octahedra, creating “channels” for the charge carriers. These channels are visible in Figure 1a,d. In a similar way, the moderate effective masses in the ky direction result from the edge-sharing Cu+ octahedra and face-sharing Bi3+ polyhedra, providing pathways for conductivity. Electrons are more mobile than holes in both the kx and ky directions.
The heavy electrons in the kz direction may be a result of the displacement of Cu+ away from the octahedral center, with the reduced orbital overlap between Cu+ and Cl– weakening the pathway for charge carriers and therefore reducing the conductivity through the network. Therefore, these anisotropic effective masses are likely to result from the anisotropic bonding in CuBiSeCl2 that arises due to the inclusion of mixed anions in the structure.
The calculated effective masses for all compounds are anisotropic, suggesting that this is characteristic of the structure of these materials. CuBiSCl2 (Cmcm) exhibits light effective masses along kx (0.25 m0) and kz (0.65 m0), with electrons being more mobile than holes in both directions. Substituting S for Se into the same Cmcm structure has the effect of increasing the electron effective masses observed along kx (3 m0), with holes now being more mobile than electrons in this direction. The electron effective mass in the kz direction also increases slightly (0.7 m0). Increases in the calculated effective masses observed going from the sulfide to the selenide Cmcm structures probably arise due to longer bond distances, which reduce the orbital overlap between atomic species.
Interestingly, when comparing with the calculated effective masses for the experimentally observed Pnma structure of CuBiSeCl2, it is found that me in the kz direction of CuBiSCl2 decreases to 0.2 m0 in the corresponding kx direction in Pnma CuBiSeCl2. Likewise, me along kx also decreases from 3 to 1 m0 in the corresponding ky direction between the Cmcm and Pnma structures of CuBiSeCl2, respectively.
The reduction in electron effective masses in the kx and kz directions between hypothetical CuBiSeCl2 (Cmcm) and experimentally observed CuBiSeCl2 (Pnma) corresponds to the Cu+ displacement, which occurs in the a–c plane. In particular, the electron effective mass in the c direction is substantially reduced as a result of the significant Cu+ displacement toward the octahedral edge in this direction, which affords increased orbital overlap within the structure. Consequently, the Cu+ displacement also has the effect of increasing me in the ky direction in CuBiSeCl2 (Pnma) to 12 m0, which is a sharp increase compared to the analogous direction in CuBiSCl2 (Cmcm) (3 m0).
Though CuBiSeCl2 possesses heavy charge carriers in the kz direction, this should not be a barrier to its implementation as a thin film for optical absorbing applications, provided it is grown in the correct orientation. For example, Sb2Se3 is a well-established solar cell absorber that also exhibits highly anisotropic effective masses.41 These anisotropic properties are overcome by orienting crystal growth perpendicular to the substrate, allowing for efficient transport along one axis.42 Saparov et al. also report a method to deposit preferentially oriented thin films,43 and depositing a axis-aligned CuBiSeCl2 could reduce the effects of the heavy charge carriers along the c axis on the material conductivity.
CuBiSeCl2 may be particularly suited to the deposition of orientated thin films as a result of the effects of the preferred orientation. Analysis of PXRD data measured on CuBiSeCl2 powder and pellets in reflection geometry reveals that CuBiSeCl2 particles preferentially orient in the [h00] and [00l] directions (S24) such that the [0k0] direction is oriented parallel to the surface normal direction, supported by refining the March–Dollase parameters using the Rietveld method.
Diffuse reflectance UV–vis spectrometry was measured on CuBiSeCl2 powder to ascertain the optical band gap. The band gap was determined from a Tauc plot, as shown in Figure 4b, and is found to be 1.33(2) eV, in good agreement with the value derived from the band structure calculations (1.5 eV). Complete agreement between the experiment and calculations is not expected since the DFT calculates the band structure at 0 K, whereas UV–vis diffuse reflectance was measured at 300 K. However, as the temperature is increased, the calculated band gap will decrease, indicating even better agreement between calculation and experiment. CuBiSeCl2 is measured to have a lower band gap than CuBiSCl2, with the value increasing from 1.33(2) to 1.44 eV between the two compounds, respectively. A reduction in band gap with increasing Se content is observed in materials with related chemistries, including CuSb(SxSe1–x)2,44 BiCuSxSe1–xO,45 and BaCu2Sn(SexS1–x)4.46
A long sub-bandgap absorption (Urbach) edge is present in the Tauc plot of CuBiSeCl2, indicative of either structural (static) or thermal (dynamic) disorder present in the material.47 More specifically, the size of this Urbach edge is directly influenced by lattice vibrations in the material,48 and in LHPs, it has been shown that increased electron–phonon scattering enlarges the Urbach edge.49 Modeling of heat capacity data from CuBiSeCl2 (see Section 3.2) confirms the presence of several localized phonon modes, suggesting that a complex landscape of lattice vibrations in the material influence band edge disorder, resulting in the extended Urbach edge.
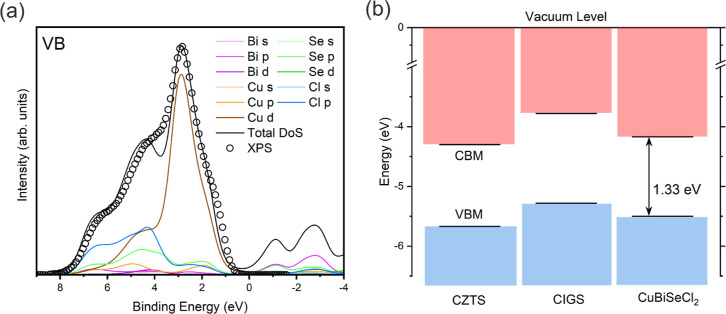
To understand the electronic structure of CuBiSeCl2 in more detail, experimental measurement of the valence band using XPS was combined with partial density of states (pDoS) calculations. Figure 5a shows valence band XPS compared to pDoS that has been corrected to account for realistic photoemission processes, including lifetime broadening and spectrometer resolution. The broadening processes used are discussed in greater detail in the Supporting Information (S29 and S30).
Figure 5.
(a) CuBiSeCl2 valence band DoS, calculated using the HSE06 functional with additional SOC contributions and broadened to account for realistic photoemission processes, plotted with valence band XPS data experimentally measured on CuBiSeCl2 powder. (b) Band alignments of CuBiSeCl2 in comparison with optical absorbers with similar chemistries, CZTS and CIGS. The conduction band and valence band states are colored red and blue, respectively.
The corrected pDoS indicates that Cu 3d states dominate the top of the valence band, while Cl 3p states occupy higher binding energies much deeper into the valence band. Se 4p, Cu 3p, and, to a lesser extent, Bi 5p/5d states cover a broad range of binding energies between 0 and 6 eV in the valence band. There is some hybridization between the Cu 3d, Se 4p, and Cl 3p states at higher binding energy in the valence band, likely due to the overlap of Cu, Se, and Cl orbitals in the tetrahedral CuSe2Cl2 bonding environment. Similarly, hybridization between Bi 5p and Se 4p/Cl 3p states at the bottom of the conduction band may arise from the Bi–Se/Bi–Cl bonding in the Bi polyhedra.
A sharp peak arising from Cu orbital contributions is also observed in the pDoS of similar chalcogenide and chalcohalide materials, including Cu3BiS3 (P212121)50 and Bi2CuSe3I (C2/m),51 in which Cu occupies different bonding environments. This suggests that Cu has a commanding effect on both chalcogen and halogen orbitals across different structures with similar chemistries, heavily influencing the top of the valence band. Comparisons between the pDoS of CuBiSCl2 and CuBiSeCl2 reveal that the valence band consists of a hybridization of Cu 3d, Ch 3p (where Ch = S, Se), and Cl 4p states in both materials, though the number of Se 3p states in the CuBiSeCl2 valence band is lower than the number of S 3p states in the CuBiSCl2 valence band. This is attributed to the reduced orbital overlap in CuBiSeCl2 as a result of longer bond distances.
Excellent agreement is observed between the XPS data and pDoS, suggesting that the DFT calculations provide accurate insights into the electronic character of the material. To enable the development of thin films and prototype devices based on CuBiSeCl2, it is essential to understand the band alignments of the material to allow the selection of suitable device structures and n-type window layers. Figure 5b depicts the band alignments for CuBiSeCl2 alongside well-established semiconductors with similar chemistries, Cu2ZnSnS4 (CZTS)52 and CuInxGa1–xSe2 (CIGS).53 By collecting the SEC region using very low-power XPS, it was possible to determine the IP and the valence band minimum (full description in the Supporting Information S32–S34). The CBM could then be located using the band gap, determined from diffuse reflectance UV–vis Spectroscopy, and found to be 1.33(2) eV, in good agreement with computational predictions. CdS has already been demonstrated as a successful window layer for CZTS and CIGS54,55 and, given the comparable band alignments, could also find application as a suitable window layer for devices based on CuBiSeCl2.
The environmental stability of CuBiSeCl2 was characterized to understand the powder degradation over time with exposure to air and moisture. CuBiSeCl2 powder was found to be stable in ambient air for over 1 month (S35). Analysis of PXRD data measured at regular time intervals shows that there is no change in the data taken after 10 min and 3 weeks of air exposure. However, after 9 weeks in ambient conditions, broad peaks consistent with the formation of BiOCl become visible. After mixing with water, depositing onto a glass slide, and drying for 24 h, it was observed that the black powder underwent a slight color change to pink around the very edges. A comparison of PXRD data from before and after water exposure reveals the appearance of small impurity peaks, consistent with the formation of BiOCl (S36).
3.1. Electronic Properties
The electronic resistivity and Seebeck coefficient were measured on a dense pellet (∼88%) of CuBiSeCl2, parallel to the pressing direction, between 2 and 300 K (Figure 6) to understand the electronic transport within the material. The pellet was highly textured, as described above, with a preferential orientation in both the a and c directions (S24). Below 250 K, the resistance of CuBiSeCl2 became too high for any meaningful resistivity or Seebeck coefficient data to be collected; hence, these properties are only shown at approximately 250 K.
Figure 6.
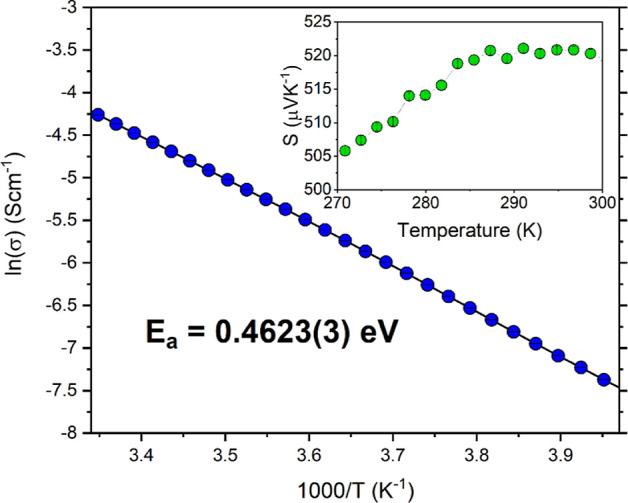
Arrhenius plot calculated from measuring the resistivity of CuBiSeCl2 parallel to the pressing direction between 250 and 300 K, with Seebeck coefficient measurement between 270 and 300 K inset. The activation energy is determined to be 0.4623(3) eV.
At 300 K, CuBiSeCl2 is measured to have a large, positive Seebeck coefficient of 519.0(6) μVK–1, confirming that the material is a p-type semiconductor.
The electronic resistivity of CuBiSeCl2 exhibits behavior typical of a semiconductor, and a large resistivity of ∼6376(3) Ωm is measured at 300 K, consistent with the prediction of heavy charge carriers. Using the Arrhenius equation and by plotting ln(σ), where σ is the electronic conductivity, as a function of 1000/T, the activation energy (Ea) could be extracted from the gradient and was found to be 0.4623(3) eV.
For an intrinsic semiconductor, it is expected that Ea is half the band gap, which in this case would be equal to 0.65 eV. We attribute the lower observed Ea to the p-type conductivity of CuBiSeCl2, which results in an additional extrinsic charge carrier density. It is likely that the measured Ea corresponds to the ionization energy of an acceptor level positioned above the valence band, an effect that has been observed in other p-type semiconductors. For example, p-type CuAlO2 possesses a direct band gap of 3.47 eV and a 0.7 eV activation energy.56 Similarly, Ga2O3 exhibits an ultrawide 4.8 eV band gap, which is almost four times the Ea (1.24 eV).57 In both cases, the discrepancy between the band gap and activation energy is associated with the presence of an acceptor level above the valence band.
3.2. Thermal Properties
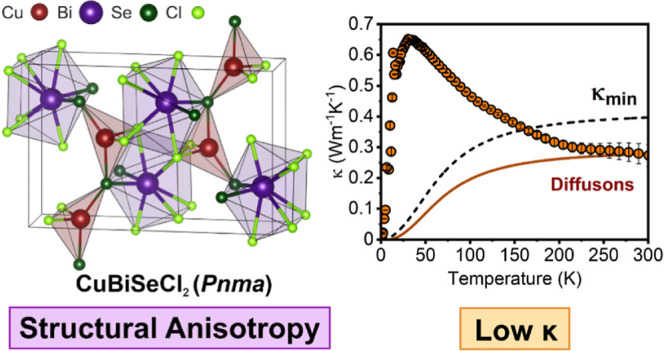
Along with the resistivity and Seebeck coefficient, the thermal conductivity was simultaneously measured on the same dense pellet of CuBiSeCl2, parallel to the pressing direction, between 2 and 300 K (Figure 7a). The behavior of the thermal conductivity of CuBiSeCl2 between 2 and 300 K is consistent with that of a crystalline material in which heat is transported by propagating phonons. At low temperatures, the thermal conductivity increases rapidly and peaks at 0.646(1) W K–1 m–1 at ∼35 K before decreasing to 0.27(4) W K–1 m–1 at 225 K, where it plateaus and is almost temperature-independent up to 300 K. Observation of the peak at ∼35 K in κ confirms the phonon-crystal characteristics of CuBiSeCl2.
Figure 7.
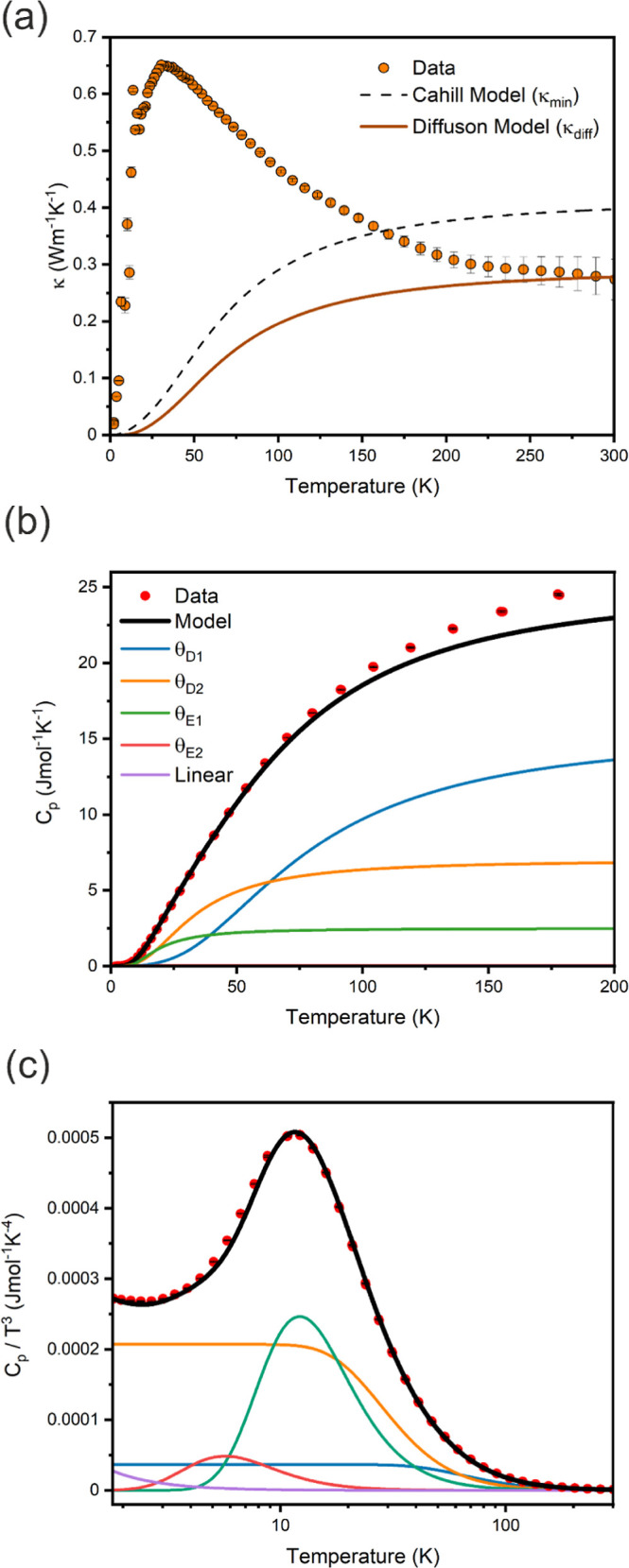
(a) Thermal conductivity of CuBiSeCl2 measured parallel to the pressing direction shown with Cahill (κmin) and diffuson-based (κdiff) models; fitted heat capacity data of CuBiSeCl2 shown as (b) Cp and (c) Cp/T3 with Debye, Einstein, and linear components shown.
The heat capacity of CuBiSeCl2 was measured on a fragment of the same dense pellet to provide insights into the behavior of phonons within the material and the origin of the particularly low thermal conductivity (Figure 7b,c). The data were modeled using a combination of linear, Debye, and Einstein terms, the parameters of which are summarized in Table 2.
Table 2. Heat Capacity Parameters Determined from Modeling Heat Capacity Measurements for CuBiSeCl2.
| compound | θD1 (K) | θD2 (K) | θE1 (K) | θE2 (K) | γ (J mol–1 K–2) |
|---|---|---|---|---|---|
| CuBiSeCl2 | 320 | 138 | 60 | 28 | 0.00009 |
The observation of an excess of specific heat at low temperatures, which often arises from highly localized vibrations,58 is accurately modeled using 2 Einstein temperatures, in addition to a linear term. This indicates the presence of several highly localized phonon modes within the structure.59 The Debye temperatures obtained from the heat capacity modeling enable the associated speeds of sound in CuBiSeCl2 to be calculated, which can provide information on the low thermal conductivity in the material. Further detail on the modeling used in this study may be found in Supplementary Information S37.
Using these values, the minimum thermal conductivity under the assumptions of the Cahill model (κmin) for CuBiSeCl2 was calculated (shown by the dashed line in Figure 7a).60 Unusually, the calculated minimum thermal conductivity (0.40 W K–1 m–1) is greater than the measured data (0.27 (4) W K–1 m–1) at 300 K. However, a diffuson model (κdiff) of the thermal conductivity, which includes the experimentally determined θD1, θD2, θE1, θE2, and vs, accurately captures the high temperature behavior of the measured data (shown in Figure 7a in red).61 This indicates that the thermal conductivity of the material must likely be understood through a two-channel model, where propagons dominate at low temperatures (resulting in the peak at 35 K), while diffusons dominate at higher temperatures (resulting in the plateau in thermal conductivity above 225 K).62 This effect has previously been observed in Ag9GaSe6 and was attributed to small interband spacings between phonon modes.63
The origin of the low thermal conductivity in CuBiSeCl2 can be understood by considering the structure and bonding present in the material. Achieving bonding anisotropy and structural anharmonicity are key design criteria for novel materials with ultralow thermal conductivities,64,65 as reducing or disrupting the bonding between species reduces the speed of sound and, therefore, the transport of heat through a material. The need for multiple Debye terms to accurately model the observed heat capacity can arise from the contributions of species with distinct bonds or masses within the structure. The higher-frequency represented by θD1 (320 K) is associated with the more ionic character of Cu–Se/Cl bonds and the lower mass of Cu, while the lower frequency contributions of θD2 (138 K) arise from the increased mass and more covalent bonding of Bi and can be understood when considering the local CuSe2Cl2 and BiSe2Cl6 environments within CuBiSeCl2. Certainly, the room temperature phonon mean free paths calculated from these Debye temperatures are of the order of 2–3 Å (S38 and S39), comparable to the interatomic distances within CuBiSeCl2, which indicates strong suppression of phonon propagation throughout the structure. In addition, 2 Einstein temperatures of θE1 = 60 K and θE2 = 28 K are required to model the excess specific heat evident from the experimentally measured heat capacity, the contributions of which represent those of localized oscillators with singular vibrational frequencies and thus nonpropagating modes. Again, the heavier mass of Bi is associated with a lower frequency θE2 term. The higher-frequency Einstein temperature of θE1 can be attributed to the local environment of Cu. As described above, Cu displaces from the center of an octahedral environment, observed in CuBiSCl2, to yield a tetrahedral CuSe2Cl2 environment in CuBiSeCl2. We note that the isotropic displacement parameter Uiso for the Cu site [0.01027(6) Å2] in the single crystal structure of CuBiSeCl2 solved at 100 K is approximately double that of any other species in the structure (Table S16). This difference in Uiso values for each site is significantly increased at room temperature from refinement of the model against PXRD data (Table S20), where the Uiso for Cu is 0.044(2) Å2, and the next largest is that of the Cl2 site [Uiso = 0.014(3) Å2]. Large Uiso values can be indicative of weak restoring forces and consequently weak bonding and anharmonicity.66,67 This indicates that Cu is loosely bound in the structure of CuBiSeCl2, despite the displacement to achieve higher bond valence. It is well established that loosely bound atoms can disrupt the propagating vibrational modes within the surrounding structural framework and are commonplace in materials such as clathrates and skutterudites;68−71 hence, Cu acts as a localized oscillator attributed to θE1 and significantly enhances phonon scattering within the structure of CuBiSeCl2. The combination of multiple anions, and therefore bonding environments in CuBiSeCl2, infers a complex phonon landscape with localized vibrations that result in low speeds of sound and a low κ that is comparable to many other inorganic materials that exhibit low thermal conductivity.
For example, a similar effect is observed in the structurally complex Cu4Bi4Ch9 (Ch = S, Se) materials, whereby substitution of S for Se increases the volume of the coordination environment and reduces the localization of the Cu atoms.72 This increases the lattice anharmonicity in the selenide material, increasing the frequency of phonon scattering events, which manifests itself as a reduction in room temperature κ between Cu4Bi4S9 (0.44 K–1 m–1) and Cu4Bi4Se9 (0.29 W K–1 m–1). The similarity in κ between CuBiSeCl2 and Cu4Bi4Se9 points to the fact that increased Cu delocalization and the associated phonon scattering are contributing factors to the low thermal conductivity of CuBiSeCl2.
The thermal conductivity of CuBiSeCl2 is also comparable with that observed in other mixed anion materials, including 2D layered materials such as Bi4O4SeCl2. Gibson et al. employed the effect of bond anisotropy at the interfaces between layers of BiOCl and Bi2O2Se to achieve a material with ultralow thermal conductivity (0.1 W K–1 m–1) by minimizing the contribution of longitudinal and transverse phonons to heat transport.64 Bi4O4Cu1.7Se2.7Cl0.3, a system based on the intergrowth of Bi2O2Se and BiCuSeO layers, also realizes a low thermal conductivity [0.4(1) W K–1 m–1] through additional structural complexity.2
CuBiSeCl2 is an example of how anionic substitution can introduce structural anharmonicity, modifying the phonon landscape to drive the reduction of heat transport through the material.
4. Conclusions
We have reported the synthesis, crystal structure, and properties of CuBiSeCl2, a previously unreported p-type chalcohalide semiconductor with low thermal conductivity and a suitable band gap for solar-absorbing applications. Cationic displacements that arise from heterogeneous bonding induced by the different anions result in anisotropic charge carrier transport, with light effective masses observed in the a direction. As such, for the development of this material as a thin film for application as a potential solar absorber, the a axis-oriented deposition is suggested to utilize the superior effective masses along this direction. The low thermal conductivity observed in CuBiSeCl2 demonstrates how the combination of multiple anions can lead to separate structural motifs that significantly reduce κ, as evidenced through experimental heat capacity and structural refinement through loosely bound Cu species and bonding anharmonicity. As such, CuBiSeCl2 is an example of the opportunities for property modification through the application of mixed anion chemistry, as shown by the variations in the structural, electronic, and physical properties of CuBiSeCl2 relative to known materials with similar structures.
Acknowledgments
The authors thank EPSRC for funding the work (EP/V026887 and EP/X038777). The X-ray Photoelectron Spectroscopy (XPS) data collection was performed at the EPSRC National Facility for XPS (“HarwellXPS”), operated by Cardiff University and UCL, under contract no. PR16195. We thank Diamond Light Source for access to beamline I-19 (proposal CY30461). This work used the ARCHER2 UK National Supercomputing Service (https://www.archer2.ac.uk).
Data Availability Statement
The data underlying this study are openly available in University of Liverpool Data Repository at https://doi.org/10.17638/datacat.liverpool.ac.uk/2550.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.chemmater.4c00188.
The authors declare no competing financial interest.
Special Issue
Published as part of Chemistry of Materialsvirtual special issue “C. N. R. Rao at 90”.
Supplementary Material
References
- Kageyama H.; Hayashi K.; Maeda K.; Attfield J. P.; Hiroi Z.; Rondinelli J. M.; Poeppelmeier K. R. Expanding frontiers in materials chemistry and physics with multiple anions. Nat. Commun. 2018, 9, 772. 10.1038/s41467-018-02838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. D.; Dyer M. S.; Whitehead G. F. S.; Alaria J.; Pitcher M. J.; Edwards H. J.; Claridge J. B.; Zanella M.; Dawson K.; Manning T. D.; Dhanak V. R.; Rosseinsky M. J. Bi4O4Cu1.7Se2.7Cl0.3: Intergrowth of BiOCuSe and Bi2O2Se Stabilized by the Addition of a Third Anion. J. Am. Chem. Soc. 2017, 139 (44), 15568–15571. 10.1021/jacs.7b06168. [DOI] [PubMed] [Google Scholar]
- Yan M.; Tang R.-L.; Zhou W.; Liu W.; Guo S.-P. Pb3SBrI3: the first Pb-based chalcohalide with multiple halogens features a unique two-dimensional structure composed of diverse Pb-centered polyhedra. Dalton Trans. 2022, 51 (34), 12921–12927. 10.1039/D2DT02038F. [DOI] [PubMed] [Google Scholar]
- Chen X.; Jing Q.; Ok K. M. Pb18O8Cl15I5: A Polar Lead Mixed Oxyhalide with Unprecedented Architecture and Excellent Infrared Nonlinear Optical Properties. Angew. Chem., Int. Ed. 2020, 59 (46), 20323–20327. 10.1002/anie.202009541. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y.; Sathish C. I.; Hong K.-P.; Oka K.; Azuma M.; Guo Y.; Matsushita Y.; Yamaura K.; Takayama-Muromachi E. Crystal Structural, Magnetic, and Transport Properties of Layered Cobalt Oxyfluorides, Sr2CoO3+xF1–x (0 ≤ x ≤ 0.15). Inorg. Chem. 2012, 51 (8), 4802–4809. 10.1021/ic300116h. [DOI] [PubMed] [Google Scholar]
- Kamihara Y.; Hiramatsu H.; Hirano M.; Kawamura R.; Yanagi H.; Kamiya T.; Hosono H. Iron-Based Layered Superconductor: LaOFeP. J. Am. Chem. Soc. 2006, 128 (31), 10012–10013. 10.1021/ja063355c. [DOI] [PubMed] [Google Scholar]
- Wu H.; Erbing A.; Johansson M. B.; Wang J.; Kamal C.; Odelius M.; Johansson E. M. J. Mixed-Halide Double Perovskite Cs2AgBiX6 (X = Br, I) with Tunable Optical Properties via Anion Exchange. ChemSusChem 2021, 14 (20), 4507–4515. 10.1002/cssc.202101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges J. M.; Xia Y.; Malliakas C. D.; Slade T. J.; Wolverton C.; Kanatzidis M. G. Mixed-Valent Copper Chalcogenides: Tuning Structures and Electronic Properties Using Multiple Anions. Chem. Mater. 2020, 32 (23), 10146–10154. 10.1021/acs.chemmater.0c03620. [DOI] [Google Scholar]
- Kim S.-W.; Pereira N.; Chernova N. A.; Omenya F.; Gao P.; Whittingham M. S.; Amatucci G. G.; Su D.; Wang F. Structure Stabilization by Mixed Anions in Oxyfluoride Cathodes for High-Energy Lithium Batteries. ACS Nano 2015, 9 (10), 10076–10084. 10.1021/acsnano.5b03643. [DOI] [PubMed] [Google Scholar]
- Yao W.; Sougrati M.-T.; Hoang K.; Hui J.; Lightfoot P.; Armstrong A. R. Na2Fe(C2O4)F2: A New Iron-Based Polyoxyanion Cathode for Li/Na Ion Batteries. Chem. Mater. 2017, 29 (5), 2167–2172. 10.1021/acs.chemmater.6b04859. [DOI] [Google Scholar]
- Tang W. S.; Yoshida K.; Soloninin A. V.; Skoryunov R. V.; Babanova O. A.; Skripov A. V.; Dimitrievska M.; Stavila V.; Orimo S.-I.; Udovic T. J. Stabilizing Superionic-Conducting Structures via Mixed-Anion Solid Solutions of Monocarba-closo-borate Salts. ACS Energy Lett. 2016, 1 (4), 659–664. 10.1021/acsenergylett.6b00310. [DOI] [Google Scholar]
- Sato N.; Kuroda N.; Nakamura S.; Katsura Y.; Kanazawa I.; Kimura K.; Mori T. Bonding heterogeneity in mixed-anion compounds realizes ultralow lattice thermal conductivity. J. Mater. Chem. A 2021, 9 (39), 22660–22669. 10.1039/D1TA04958E. [DOI] [Google Scholar]
- Li F.; Li J.-F.; Zhao L.-D.; Xiang K.; Liu Y.; Zhang B.-P.; Lin Y.-H.; Nan C.-W.; Zhu H.-M. Polycrystalline BiCuSeO oxide as a potential thermoelectric material. Energy Environ. Sci. 2012, 5 (5), 7188. 10.1039/c2ee21274a. [DOI] [Google Scholar]
- Pei Y.-L.; He J.; Li J.-F.; Li F.; Liu Q.; Pan W.; Barreteau C.; Berardan D.; Dragoe N.; Zhao L.-D. High thermoelectric performance of oxyselenides: intrinsically low thermal conductivity of Ca-doped BiCuSeO. NPG Asia Mater. 2013, 5 (5), e47 10.1038/am.2013.15. [DOI] [Google Scholar]
- Ran M.-Y.; Zhou S.-H.; Wei W.; Song B.-J.; Shi Y.-F.; Wu X.-T.; Lin H.; Zhu Q.-L. Quaternary Chalcohalides CdSnSX2 (X = Cl or Br) with Neutral Layers: Syntheses, Structures, and Photocatalytic Properties. Inorg. Chem. 2021, 60 (5), 3431–3438. 10.1021/acs.inorgchem.1c00010. [DOI] [PubMed] [Google Scholar]
- Johnsen S.; Liu Z.; Peters J. A.; Song J.-H.; Nguyen S.; Malliakas C. D.; Jin H.; Freeman A. J.; Wessels B. W.; Kanatzidis M. G. Thallium Chalcohalides for X-ray and γ-ray Detection. J. Am. Chem. Soc. 2011, 133 (26), 10030–10033. 10.1021/ja202540t. [DOI] [PubMed] [Google Scholar]
- Sun Y.-Y.; Shi J.; Lian J.; Gao W.; Agiorgousis M. L.; Zhang P.; Zhang S. Discovering lead-free perovskite solar materials with a split-anion approach. Nanoscale 2016, 8 (12), 6284–6289. 10.1039/C5NR04310G. [DOI] [PubMed] [Google Scholar]
- Perera S.; Hui H.; Zhao C.; Xue H.; Sun F.; Deng C.; Gross N.; Milleville C.; Xu X.; Watson D. F.; Weinstein B.; Sun Y. Y.; Zhang S.; Zeng H. Chalcogenide perovskites - an emerging class of ionic semiconductors. Nano Energy 2016, 22, 129–135. 10.1016/j.nanoen.2016.02.020. [DOI] [Google Scholar]
- Gupta T.; Ghoshal D.; Yoshimura A.; Basu S.; Chow P. K.; Lakhnot A. S.; Pandey J.; Warrender J. M.; Efstathiadis H.; Soni A.; Osei-Agyemang E.; Balasubramanian G.; Zhang S.; Shi S. F.; Lu T. M.; Meunier V.; Koratkar N. An Environmentally Stable and Lead-Free Chalcogenide Perovskite. Adv. Funct. Mater. 2020, 30 (23), 2001387. 10.1002/adfm.202001387. [DOI] [Google Scholar]
- Palazon F. Metal Chalcohalides: Next Generation Photovoltaic Materials?. Sol. RRL 2022, 6 (2), 2100829. 10.1002/solr.202100829. [DOI] [Google Scholar]
- Nie R.; Lee K. S.; Hu M.; Paik M. J.; Seok S. I. Heteroleptic Tin-Antimony Sulfoiodide for Stable and Lead-free Solar Cells. Matter 2020, 3 (5), 1701–1713. 10.1016/j.matt.2020.08.020. [DOI] [Google Scholar]
- Nicolson A.; Breternitz J.; Kavanagh S. R.; Tomm Y.; Morita K.; Squires A. G.; Tovar M.; Walsh A.; Schorr S.; Scanlon D. O. Interplay of Static and Dynamic Disorder in the Mixed-Metal Chalcohalide Sn2SbS2I3. J. Am. Chem. Soc. 2023, 145 (23), 12509–12517. 10.1021/jacs.2c13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming C.; Chen Z.; Zhang F.; Gong S.; Wu X.; Jiang J.; Ye T.; Xu Q.; Yang K.; Wang L.; Cao X.; Yang S.; Zhang S.; Zhang Y.; Shi J.; Sun Y. Y. Mixed Chalcogenide-Halides for Stable, Lead-Free and Defect-Tolerant Photovoltaics: Computational Screening and Experimental Validation of CuBiSCl2 with Ideal Band Gap. Adv. Funct. Mater. 2022, 32, 32. 10.1002/adfm.202112682. [DOI] [Google Scholar]
- Coelho A. A. Whole-profile structure solution from powder diffraction data using simulated annealing. J. Appl. Crystallogr. 2000, 33 (3), 899–908. 10.1107/S002188980000248X. [DOI] [Google Scholar]
- Stephens P. W. Phenomenological model of anisotropic peak broadening in powder diffraction. J. Appl. Crystallogr. 1999, 32 (2), 281–289. 10.1107/S0021889898006001. [DOI] [Google Scholar]
- Nowell H.; Barnett S. A.; Christensen K. E.; Teat S. J.; Allan D. R. I19, the small-molecule single-crystal diffraction beamline at Diamond Light Source. J. Synchrotron Radiat. 2012, 19 (3), 435–441. 10.1107/S0909049512008801. [DOI] [PubMed] [Google Scholar]
- Winter G. xia2: an expert system for macromolecular crystallography data reduction. J. Appl. Crystallogr. 2010, 43 (1), 186–190. 10.1107/S0021889809045701. [DOI] [Google Scholar]
- Winter G.; Waterman D. G.; Parkhurst J. M.; Brewster A. S.; Gildea R. J.; Gerstel M.; Fuentes-Montero L.; Vollmar M.; Michels-Clark T.; Young I. D.; Sauter N. K.; Evans G. DIALS: implementation and evaluation of a new integration package. Acta Crystallogr., Sect. D: Struct. Biol. 2018, 74 (2), 85–97. 10.1107/S2059798317017235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. A short history of SHELX. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64 (1), 112–122. 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- Dolomanov O. V.; Bourhis L. J.; Gildea R. J.; Howard J. A. K.; Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42 (2), 339–341. 10.1107/S0021889808042726. [DOI] [Google Scholar]
- Makuła P.; Pacia M.; Macyk W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9 (23), 6814–6817. 10.1021/acs.jpclett.8b02892. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B: Condens. Matter Mater. Phys. 1996, 54 (16), 11169–11186. 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B: Condens. Matter Mater. Phys. 1999, 59 (3), 1758–1775. 10.1103/PhysRevB.59.1758. [DOI] [Google Scholar]
- Krukau A. V.; Vydrov O. A.; Izmaylov A. F.; Scuseria G. E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125 (22), 224106. 10.1063/1.2404663. [DOI] [PubMed] [Google Scholar]
- Steiner S.; Khmelevskyi S.; Marsmann M.; Kresse G. Calculation of the magnetic anisotropy with projected-augmented-wave methodology and the case study of disordered Fe1-xCox. Phys. Rev. B 2016, 93 (22), 224425. 10.1103/physrevb.93.224425. [DOI] [Google Scholar]
- Setyawan W.; Curtarolo S. High-throughput electronic band structure calculations: Challenges and tools. Comput. Mater. Sci. 2010, 49 (2), 299–312. 10.1016/j.commatsci.2010.05.010. [DOI] [Google Scholar]
- Ruck M.; Poudeu Poudeu P. F.; Söhnel T. Synthese, Kristallstruktur und elektronische Bandstruktur der isotypen Sulfidchloride CuBiSCl2 und AgBiSCl2. Z. Anorg. Allg. Chem. 2004, 630, 63–67. 10.1002/zaac.200300248. [DOI] [Google Scholar]
- Xiao Z.; Du K. Z.; Meng W.; Mitzi D. B.; Yan Y. Chemical Origin of the Stability Difference between Copper (I)- and Silver (I)-Based Halide Double Perovskites. Angew. Chem., Int. Ed. 2017, 56 (40), 12107–12111. 10.1002/anie.201705113. [DOI] [PubMed] [Google Scholar]
- Doussier C.; Moëlo Y.; Léone P. Synthesis and crystal structures of four new bromo-chalcogenides: MnSbS2Br, MnBiSe2Br and two allotropic forms of MnSbSe2Br. Crystal chemistry of the Mn PnQ2 X family (Pn = Sb, Bi; Q = S, Se; X = Cl, Br, I). Solid State Sci. 2006, 8, 652–659. 10.1016/j.solidstatesciences.2006.01.009. [DOI] [Google Scholar]
- Shannon R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr., Sect. A: Found. Adv. 1976, 32 (5), 751–767. 10.1107/S0567739476001551. [DOI] [Google Scholar]
- Wang X.; Li Z.; Kavanagh S. R.; Ganose A. M.; Walsh A. Lone pair driven anisotropy in antimony chalcogenide semiconductors. Phys. Chem. Chem. Phys. 2022, 24 (12), 7195–7202. 10.1039/D1CP05373F. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Wang L.; Chen S.; Qin S.; Liu X.; Chen J.; Xue D.-J.; Luo M.; Cao Y.; Cheng Y.; Sargent E. H.; Tang J. Thin-film Sb2Se3 photovoltaics with oriented one-dimensional ribbons and benign grain boundaries. Nat. Photonics 2015, 9 (6), 409–415. 10.1038/nphoton.2015.78. [DOI] [Google Scholar]
- Saparov B.; Hong F.; Sun J.-P.; Duan H.-S.; Meng W.; Cameron S.; Hill I. G.; Yan Y.; Mitzi D. B. Thin-Film Preparation and Characterization of Cs3Sb2I9: A Lead-Free Layered Perovskite Semiconductor. Chem. Mater. 2015, 27 (16), 5622–5632. 10.1021/acs.chemmater.5b01989. [DOI] [Google Scholar]
- Takei K.; Maeda T.; Wada T. Crystallographic and optical properties of CuSbS2 and CuSb(S1-xSex)2 solid solution. Thin Solid Films 2015, 582, 263–268. 10.1016/j.tsf.2014.11.029. [DOI] [Google Scholar]
- Berardan D.; Li J.; Amzallag E.; Mitra S.; Sui J.; Cai W.; Dragoe N. Structure and Transport Properties of the BiCuSeO-BiCuSO Solid Solution. Materials 2015, 8 (3), 1043–1058. 10.3390/ma8031043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J.; Yu Y.; Yan Y. Earth-abundant trigonal BaCu2Sn(SexS1–x)4 (x = 0–0.55) thin films with tunable band gaps for solar water splitting. J. Mater. Chem. A 2016, 4 (48), 18885–18891. 10.1039/C6TA06702F. [DOI] [Google Scholar]
- Ugur E.; Ledinský M.; Allen T. G.; Holovský J.; Vlk A.; De Wolf S. Life on the Urbach Edge. J. Phys. Chem. Lett. 2022, 13 (33), 7702–7711. 10.1021/acs.jpclett.2c01812. [DOI] [PubMed] [Google Scholar]
- Ledinsky M.; Schönfeldová T.; Holovský J.; Aydin E.; Hájková Z.; Landová L.; Neyková N.; Fejfar A.; De Wolf S. Temperature Dependence of the Urbach Energy in Lead Iodide Perovskites. J. Phys. Chem. Lett. 2019, 10 (6), 1368–1373. 10.1021/acs.jpclett.9b00138. [DOI] [PubMed] [Google Scholar]
- Galvani B.; Suchet D.; Delamarre A.; Bescond M.; Michelini F. V.; Lannoo M.; Guillemoles J.-F.; Cavassilas N. Impact of Electron–Phonon Scattering on Optical Properties of CH3NH3PbI3 Hybrid Perovskite Material. ACS Omega 2019, 4 (25), 21487–21493. 10.1021/acsomega.9b03178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittles T. J.; Veal T. D.; Savory C. N.; Yates P. J.; Murgatroyd P. A. E.; Gibbon J. T.; Birkett M.; Potter R. J.; Major J. D.; Durose K.; Scanlon D. O.; Dhanak V. R. Band Alignments, Band Gap, Core Levels, and Valence Band States in Cu3BiS3 for Photovoltaics. ACS Appl. Mater. Interfaces 2019, 11 (30), 27033–27047. 10.1021/acsami.9b04268. [DOI] [PubMed] [Google Scholar]
- Liang I. C.; Bilc D. I.; Manoli M.; Chang W. Y.; Lin W. F.; Kyratsi T.; Hsu K. F. Syntheses, crystal Structures and electronic Structures of new metal chalcoiodides Bi2CuSe3I and Bi6Cu3S10I. J. Solid State Chem. 2016, 234, 1–8. 10.1016/j.jssc.2015.09.030. [DOI] [Google Scholar]
- Burton L. A.; Walsh A. Band alignment in SnS thin-film solar cells: Possible origin of the low conversion efficiency. Appl. Phys. Lett. 2013, 102 (13), 132111. 10.1063/1.4801313. [DOI] [Google Scholar]
- Hinuma Y.; Oba F.; Kumagai Y.; Tanaka I. Ionization potentials of (112) and (112) facet surfaces of CuInSe2 and CuGaSe2. Phys. Rev. B: Condens. Matter Mater. Phys. 2012, 86 (24), 245433. 10.1103/physrevb.86.245433. [DOI] [Google Scholar]
- Yan C.; Huang J.; Sun K.; Johnston S.; Zhang Y.; Sun H.; Pu A.; He M.; Liu F.; Eder K.; Yang L.; Cairney J. M.; Ekins-Daukes N. J.; Hameiri Z.; Stride J. A.; Chen S.; Green M. A.; Hao X. Cu2ZnSnS4 solar cells with over 10% power conversion efficiency enabled by heterojunction heat treatment. Nat. Energy 2018, 3 (9), 764–772. 10.1038/s41560-018-0206-0. [DOI] [Google Scholar]
- Cho K. S.; Jang J.; Park J.-H.; Lee D.-K.; Song S.; Kim K.; Eo Y.-J.; Yun J. H.; Gwak J.; Chung C.-H. Optimal CdS Buffer Thickness to Form High-Quality CdS/Cu(In,Ga)Se2 Junctions in Solar Cells without Plasma Damage and Shunt Paths. ACS Omega 2020, 5 (37), 23983–23988. 10.1021/acsomega.0c03268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate J.; Ju H. L.; Moon J. C.; Zakutayev A.; Richard A. P.; Russell J.; McIntyre D. H. Origin of p-type conduction in single-crystal CuAlO2. Phys. Rev. B: Condens. Matter Mater. Phys. 2009, 80 (16), 165206. 10.1103/physrevb.80.165206. [DOI] [Google Scholar]
- Chikoidze E.; Sartel C.; Mohamed H.; Madaci I.; Tchelidze T.; Modreanu M.; Vales-Castro P.; Rubio C.; Arnold C.; Sallet V.; Dumont Y.; Perez-Tomas A. Enhancing the intrinsic p-type conductivity of the ultra-wide bandgap Ga2O3 semiconductor. J. Mater. Chem. C 2019, 7 (33), 10231–10239. 10.1039/C9TC02910A. [DOI] [Google Scholar]
- Takabatake T.; Suekuni K.; Nakayama T.; Kaneshita E. Phonon-glass electron-crystal thermoelectric clathrates: Experiments and theory. Rev. Mod. Phys. 2014, 86 (2), 669–716. 10.1103/RevModPhys.86.669. [DOI] [Google Scholar]
- Acharyya P.; Ghosh T.; Pal K.; Kundu K.; Singh Rana K.; Pandey J.; Soni A.; Waghmare U. V.; Biswas K. Intrinsically Ultralow Thermal Conductivity in Ruddlesden–Popper 2D Perovskite Cs2PbI2Cl2: Localized Anharmonic Vibrations and Dynamic Octahedral Distortions. J. Am. Chem. Soc. 2020, 142 (36), 15595–15603. 10.1021/jacs.0c08044. [DOI] [PubMed] [Google Scholar]
- Cahill D. G.; Watson S. K.; Pohl R. O. Lower limit to the thermal conductivity of disordered crystals. Phys. Rev. B: Condens. Matter Mater. Phys. 1992, 46 (10), 6131–6140. 10.1103/PhysRevB.46.6131. [DOI] [PubMed] [Google Scholar]
- Agne M. T.; Hanus R.; Snyder G. J. Minimum thermal conductivity in the context of diffuson-mediated thermal transport. Energy Environ. Sci. 2018, 11 (3), 609–616. 10.1039/C7EE03256K. [DOI] [Google Scholar]
- Allen P. B.; Feldman J. L.; Fabian J.; Wooten F. Diffusons, locons and propagons: Character of atomic vibrations in amorphous Si. Philos. Mag. B 1999, 79 (11–12), 1715–1731. 10.1080/13642819908223054. [DOI] [Google Scholar]
- Bernges T.; Peterlechner M.; Wilde G.; Agne M. T.; Zeier W. G. Analytical model for two-channel phonon transport engineering. Mater. Today Phys. 2023, 35, 101107. 10.1016/j.mtphys.2023.101107. [DOI] [Google Scholar]
- Gibson Q. D.; Zhao T.; Daniels L. M.; Walker H. C.; Daou R.; Hébert S.; Zanella M.; Dyer M. S.; Claridge J. B.; Slater B.; Gaultois M. W.; Corà F.; Alaria J.; Rosseinsky M. J. Low thermal conductivity in a modular inorganic material with bonding anisotropy and mismatch. Science 2021, 373 (6558), 1017–1022. 10.1126/science.abh1619. [DOI] [PubMed] [Google Scholar]
- Zeier W. G.; Zevalkink A.; Gibbs Z. M.; Hautier G.; Kanatzidis M. G.; Snyder G. J. Thinking Like a Chemist: Intuition in Thermoelectric Materials. Angew. Chem., Int. Ed. 2016, 55 (24), 6826–6841. 10.1002/anie.201508381. [DOI] [PubMed] [Google Scholar]
- Bhui A.; Dutta M.; Mukherjee M.; Rana K. S.; Singh A. K.; Soni A.; Biswas K. Ultralow Thermal Conductivity in Earth-Abundant Cu1.6Bi4.8S8: Anharmonic Rattling of Interstitial Cu. Chem. Mater. 2021, 33 (8), 2993–3001. 10.1021/acs.chemmater.1c00659. [DOI] [Google Scholar]
- Long S. O.; Powell A. V.; Hull S.; Orlandi F.; Tang C. C.; Supka A. R.; Fornari M.; Vaqueiro P. Jahn–Teller Driven Electronic Instability in Thermoelectric Tetrahedrite. Adv. Funct. Mater. 2020, 30 (12), 1909409. 10.1002/adfm.201909409. [DOI] [Google Scholar]
- Christensen M.; Abrahamsen A. B.; Christensen N. B.; Juranyi F.; Andersen N. H.; Lefmann K.; Andreasson J.; Bahl C. R. H.; Iversen B. B. Avoided crossing of rattler modes in thermoelectric materials. Nat. Mater. 2008, 7 (10), 811–815. 10.1038/nmat2273. [DOI] [PubMed] [Google Scholar]
- Koza M. M.; Johnson M. R.; Viennois R.; Mutka H.; Girard L.; Ravot D. Breakdown of phonon glass paradigm in La- and Ce-filled Fe4Sb12 skutterudites. Nat. Mater. 2008, 7 (10), 805–810. 10.1038/nmat2260. [DOI] [PubMed] [Google Scholar]
- Nolas G. S.; Cohn J. L.; Slack G. A.; Schujman S. B. Semiconducting Ge clathrates: Promising candidates for thermoelectric applications. Appl. Phys. Lett. 1998, 73 (2), 178–180. 10.1063/1.121747. [DOI] [Google Scholar]
- Nolas G. S.; Cohn J. L.; Slack G. A. Effect of partial void filling on the lattice thermal conductivity of skutterudites. Phys. Rev. B: Condens. Matter Mater. Phys. 1998, 58 (1), 164–170. 10.1103/PhysRevB.58.164. [DOI] [Google Scholar]
- Jiang Y.; Jia F.; Chen L.; Wu L.-M. Cu4Bi4Se9: A Thermoelectric Symphony of Rattling, Anharmonic Lone-pair, and Structural Complexity. ACS Appl. Mater. Interfaces 2019, 11 (40), 36616–36625. 10.1021/acsami.9b11115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are openly available in University of Liverpool Data Repository at https://doi.org/10.17638/datacat.liverpool.ac.uk/2550.