Abstract
The Carter Center has estimated that the addiction crisis in the United States (US), if continues to worsen at the same rate, may cost the country approximately 16 trillion dollars by 2030. In recent years, the well-being of youth has been compromised by not only the coronavirus disease 2019 pandemic but also the alarming global opioid crisis, particularly in the US. Each year, deadly opioid drugs claim hundreds of thousands of lives, contributing to an ever-rising death toll. In addition, maternal usage of opioids and other drugs during pregnancy could compromise the neurodevelopment of children. A high rate of DNA polymorphic antecedents compounds the occurrence of epigenetic insults involving methylation of specific essential genes related to normal brain function. These genetic antecedent insults affect healthy DNA and mRNA transcription, leading to a loss of proteins required for normal brain development and function in youth. Myelination in the frontal cortex, a process known to extend until the late 20s, delays the development of proficient executive function and decision-making abilities. Understanding this delay in brain development, along with the presence of potential high-risk antecedent polymorphic variants or alleles and generational epigenetics, provides a clear rationale for embracing the Brain Research Commission’s suggestion to mimic fitness programs with an adaptable brain health check (BHC). Implementing the BHC within the educational systems in the US and other countries could serve as an effective initiative for proactive therapies aimed at reducing juvenile mental health problems and eventually criminal activities, addiction, and other behaviors associated with reward deficiency syndrome.
Keywords: Brain health check, Cognition, Dopaminergic dysregulation, Executive function, Reward deficiency syndrome, Genetics, Epigenetics
1. Introduction
The purpose of the brain health check (BHC) is to integrate objective assessments across cognition, neurological imaging, psychiatry, and genomics to identify youths who are at risk for juvenile mental health problems, criminal activities, addiction, and other behaviors associated with reward deficiency syndrome (RDS). Identifying vulnerable youths through these assessments can provide insights into proper interventions, such as genome-matched amino acid therapies that can treat reward/dopamine dysregulation and prevent the inheritance of epigenetic insults associated with addiction to future generations. Amidst the increasing drug abuse crisis in the United States (US) and the potential for long-term enormous societal costs, a brain research consortium developed this approach. The group is comprised experienced teachers, educators, drug abuse counselors, psychiatrists, clinicians, scientists, neuroscientists, geneticists, and addiction medicine physicians, who encourage the adoption of the standardized BHC in K1–K12 education. In addition, they endorse basic and clinical scientific research into brain health prophylaxis for developing brains.
2. Understanding reward dysregulation and potential therapeutic approaches
As defined in the Sage Encyclopedia of Psychiatric Disorders (2017), there is emerging evidence of an over-representation of the antecedent to RDS, encompassing both substance- and non-substance-related addictive behaviors, within the general US population.
It is well established that dopamine resistance in individuals with food and drug addiction is caused by dysfunctional genetic neurotransmitter polymorphisms, such as the A1 allele of the DRD2 gene, and epigenetic insults. A burgeoning line of evidence shows that a natural, non-addictive, and safe putative D2 agonist may aid in the treatment of and recovery from these RDS behaviors in patients addicted to substances. The impact of the patented KB220 nutrigenomic technology, known as “Synaptamine Complex,” acts as an activator of the mesolimbic system, as observed through quantitative electroencephalography (qEEG) imaging. A published pilot study demonstrated that the intravenous administration of KB220 was observed to normalize the aberrant electrophysiological parameters of the reward circuitry site.1 The study also revealed that the qEEG graphs of an alcoholic and a heroin abuser with existing abnormalities (widespread theta and alpha activity, respectively) during protracted abstinence were significantly normalized after the administration of a single intravenous dose of KB220® Synaptamine Complex Formulation.1 Both patients were genotyped for several neurotransmitter reward genes to determine if they carried any putative dopaminergic risk alleles that may predispose them to alcohol or heroin dependence, respectively. The genes examined included the dopamine transporter (DAT1, locus symbol SLC6A3), dopamine D4 receptor exon 3 VNTR (DRD4), DRD2 TaqIA (rs1800497), COMT val158 met SNP (rs4680), monoamine oxidase A upstream VNTR (MAOA-uVNTR), and serotonin transporter-linked polymorphic region (5HTTLPR, locus symbol SLC6A4). It should be emphasized that these findings stem from case studies, and it is improbable for individuals to carry all putative risk alleles. Based on the previous research and our qEEG studies, we cautiously suggest that long-term activation of dopaminergic receptors may increase their proliferation, leading to enhanced “dopamine sensitivity” and a heightened sense of happiness, particularly in carriers of the DRD2 A1 allele.2
The intravenous administration of the Synaptamine Complex Variant KB220 in >600 alcoholic patients resulted in a significant reduction in RDS behaviors; this effect was further supported by an expanded study involving oral KB220Z3 and functional magnetic resonance imaging conducted on abstinent heroin addicts.4 For a deeper understanding, future studies, including functional positron emission tomography scanning, are required to determine the acute and chronic effects of oral KB220Z on the number of D2 receptors and its interaction with the nucleus accumbens (NAc). In addition, further confirmation of these findings through large, population-based, and case-controlled experiments could ultimately lead to significant improvements in the treatment and recovery of patients with RDS and dopamine deficiency resulting from disruptions in the transduction of multiple neurotransmitter signals within the Brain Reward Cascade (BRC).5
Moreover, recent neuroimaging studies have highlighted the potent effects of KB220Z, underscoring the importance of Pro-dopamine regulation along the BRC (Figure 1).
Figure 1.
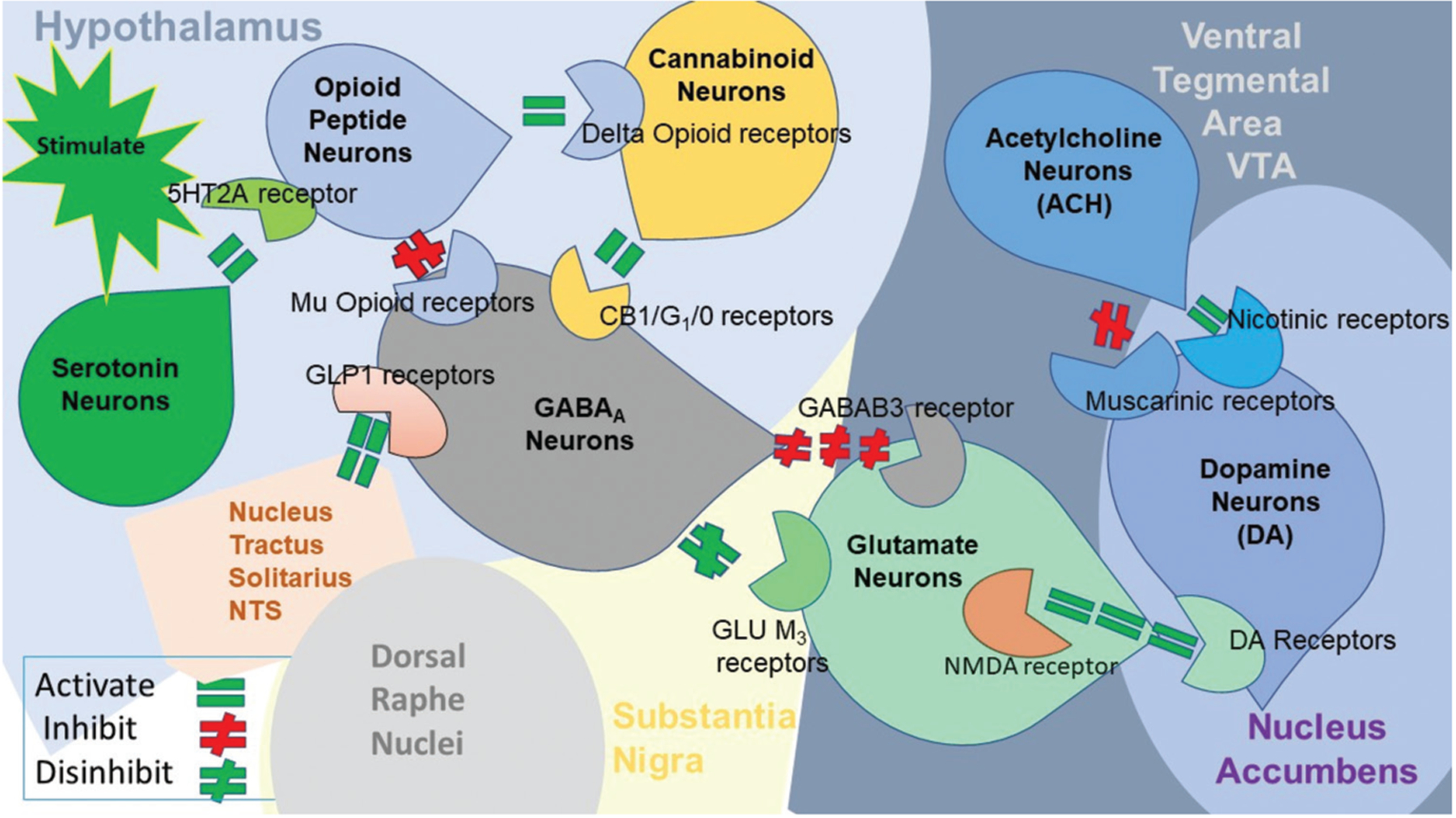
Interaction of at least eight major neurotransmitter-pathways involved in the brain reward cascade. In the hypothalamus, environmental stimulation triggers the release of serotonin, which, through receptors such as 5HT-2a, activates (green equal sign) the subsequent release of opioid peptides from opioid peptide neurons, also located in the hypothalamus. These opioid peptides, in turn, exert two distinct effects, possibly through two different opioid receptors. One effect inhibits (red hash sign) the mu-opioid receptor (possibly through enkephalin) and projects to GABAA neurons in the substantia nigra. The other effect stimulates (green equal sign) cannabinoid neurons (e.g., anandamide and 2-arachidonoylglycerol) through beta-endorphin-linked delta receptors, which further inhibit GABAA neurons in the substantia nigra. In addition, cannabinoids, primarily 2-arachidonoylglycerol, when activated, can indirectly disinhibit (red hash sign) GABAA neurons through the activation of G1/0 coupled to CB1 receptors in the substantia nigra. Not depicted in the figure, the dorsal raphe nuclei feature glutamate neurons that can indirectly disinhibit GABAA neurons in the substantia nigra through activation of GLU M3 receptors (red hash sign). When stimulated, GABAA neurons powerfully (red hash signs) inhibit VTA glutaminergic drive through GABAA neurons. It is also possible that stimulation of ACH neurons at the NAc can stimulate both muscarinic (red hash) and nicotinic (green hash) receptors. Glutamate neurons in the VTA project dopamine neurons through NMDA receptors (green equal sign) to preferentially release dopamine at the NAc, resulting in a sense of euphoria, or “wanting” response. Figure 1 also depicts that GLP1 from the nucleus tractus solitarius stimulates GABAA in the Substantia Nigra. As a result, dopamine release is low (endorphin deficiency), followed by feelings of unhappiness. On the other hand, overall (healthy) happiness depends on the optimal balance of dopamine, regulated by the dopamine homeostatic tonic set point.6
It is also possible that ACH neurons at the NAc ACH can stimulate both muscarinic (red hash) and nicotinic (green hash) receptors. Finally, glutamate neurons in the VTA will project to dopamine neurons through NMDA receptors (green equal sign) to preferentially release dopamine at the NAc (shown as a bullseye), indicating euphoria or a “wanting” response. The result is that when dopamine release is low, there can be a state of unhappiness characterized by endorphin deficiency. At the same time, general (usual) happiness depends on the dopamine homeostatic tonic set point.6 In addition to the coronavirus disease 2019 pandemic, there is a global addiction crisis. While being highest in the US, the devastation and deaths from drug overdose are global issues requiring “out of the box” thinking.7 Even in the face of harm reduction, relying on opioids to treat issues caused by other potent opioids seems counterintuitive and perpetuates unwanted addictions.8 Several investigative groups have been cognizant that addressing the root cause is one of the approaches to reducing harm.9,10 Another approach is using a narcotic antagonist (like naltrexone) to induce “psychological extinction” through blocking D2 receptors.11 The latter approach appears more acceptable; however, compliance remains a deterring issue.12 The approved drug acamprosate, an NMDA receptor antagonist and a positive allosteric modulator of GABAA receptors, also disrupts dopaminergic signaling.13 The growing acceptance of the RDS concept, introduced by Blum in 1995, facilitates the common mechanism hypothesis for substance and non-substance addiction. Understanding the in-common neuromodulating features of neurotransmission and its disruption through chronic exposure to substance and non-substance addictions requires the utilization of an approach that involves “dopamine homeostasis.”14
3. Review of evidence
The “out of the box” approach involves coupling genetic risk polymorphic testing with a safe and well-researched complex, KB220Z. The KB220Z is customized to match the presence of resultant alleles and provide a precision nutraceutical with known prodopamine regulatory pharmacological properties.2,15 High-tier publications strongly support a shared neuromechanism underlying both substance and non-substance addiction, such as alcohol, opioids, gambling, and food.
In the 1970s, Blum’s laboratory developed an amino-acid-based enkephalinase inhibitory pro-dopamine regulator with the KB220 nutraceutical complex as its cornerstone ingredient, now validated by over 45 clinical studies published in peer-reviewed journals.16,17 The basis of this complex is its ability to mimic the BRC,17 an established model of reward processing. The most striking feature is the activation of BOLD by the KB220Z across the BRC,18 including the NAc, anterior cingulate gyrus, anterior thalamic nuclei, hippocampus, prelimbic, and infralimbic parts of the prefrontal cortex (PFC). Evidence of genetic vulnerability as an antecedent to unwanted RDS behaviors may be a determining factor, which could be identified early in life. Based on previously published literature, the role of reward gene polymorphisms puts individuals at an increased risk for various forms of RDS behaviors, including anhedonia.19,20 This insight spurred the development of the patented genetic addiction risk severity (GARS) test, aimed at identifying genetic risk for these behaviors. Specifically, published studies have illustrated the coupling of GARS with KB220Z formulations of semi-customized precision pro-dopamine regulators tailored to one’s GARS profiles.21 The biological approach of this system enhances the effectiveness of RDS treatment.22
Balancing the BRC or achieving “dopamine homeostasis” is generally preferred and considered a commendable objective, as opposed to interventions that involve blocking natural dopamine or administering potent opioids to overcome opioid addiction.21 In the face of the current addiction pandemic, we urge addiction neuroscientists and clinicians to embrace this innovative technology and establish a “standard of care” for treating and preventing addiction and all related RDS neuro-sequala.23 While further research is required, it is crucial to establish a set of acceptable guidelines that include an understanding of the RDS concept. Understanding neurogenetics by utilizing a “systems biology” approach such as precision behavioral management, as outlined herein, seems prudent and represents a step forward in restoring well-being to the billions afflicted globally.24–27 In terms of a system biology approach, Rosen et al. outlined the theory behind complex trait analysis and systems genetics. They describe web-accessible resources, including GeneNetwork, that facilitate rapid exploratory analysis and hypothesis testing. Moreover, GeneNetwork is a tightly bioinformatic integrated tool and data set, allowing investigation into complex networks of gene variants, molecules, and cellular processes that modulate complex traits such as behavior and disease susceptibility. This technique will enable scientists to analyze gene expression across various specific brain regions and tissues, explore genetic covariance among traits, and map loci that modulate these traits. Rosen et al. further suggested that these tools enable investigators to assess the complex interactions of gene networks, employing a systems approach.28
4. Neurogenetic and epigenetic correlates of adolescent predisposition to and risk for addictive behaviors as a function of PFC dysregulation
Within the medical community, especially among addiction professionals, there is growing concern about how preteens, adolescents, and young adults turn to substance abuse to cope with stress and anger. The turbulence of the underdeveloped central nervous system (CNS), especially the PFC, underscores the need for continued neuroimaging studies in both human and animal models, as well as encourages preventive measures and regulatory actions taken by governmental bodies.
The PFC is known to undergo significant developmental changes before individuals reach their 20s, impacting decision-making ability within this population. Furthermore, early genetic testing for addiction risk alleles will provide valuable information that could potentially be utilized by parents and caregivers before any psychoactive drug use begins. Beyond genomic testing, a more straightforward approach could be the widespread adoption of a standard BHC, such as school fitness programs.
Family history, parenting styles, and relationship attachments, modified by various reward genes, including the well-known bonding substances oxytocin/vasopressin, may affect dopaminergic function. In addition, well-characterized neuroimaging studies indicate region-specific differential responses to drugs, food, and non-substance-addictive behaviors via either “surfeit” or “deficit.”29,30 Therefore, a “reward deficiency solution system” that combines early genetic risk assessment, medical monitoring, including a BHC, and nutrigenomic dopamine agonist modalities to combat reward deficiency risk may help address the global crisis that is hindering youth from leading normal, productive, and happier lives.31
Unlike fully developed adults, preteens transitioning into adolescence may lack adequate decision-making capacity due to incomplete brain development and myelination. The PFC area, known as the “braking/inhibitory system,” supports executive function and decision-making but can be hijacked by subcortical structures in the midbrain. Impairments in the midbrain region, which regulates social and emotional responses, may lead to deficits in neurotransmitter function.
We must be cognizant of the impact of stress on the brain’s developmental process and how substance abuse, such as alcohol, cocaine, and opioids, alters the integrity of white and gray matter volume.32 Furthermore, it is well known that myelination in the PFC begins when people are in their early 20s.33–40 Myelination regulates brain speed and can be compromised by stress and drug exposure, especially during prenatal and other developmental phases.37–39 During the turbulent years before adulthood, youth may encounter stressful situations, resulting in frustration that could trigger epigenetic changes that exacerbate genetic antecedent risk for drug abuse.40,41 The D2 dopamine receptor (DRD2) is the most extensively investigated gene in diverse neuropsychiatric disorders. Numerous international studies have been performed since the first association of the TaqI A DRD2 minor (A1) allele with severe alcoholism in 1990. As of October 10, 2022, there are 5351 articles listed in PUBMED, with 120 meta-analyses yielding mixed results. In our opinion, negative reports on the association of various DRD2 gene polymorphisms are due to poorly screened controls, resulting in the non-elimination of many hidden RDS behaviors. Moreover, pleiotropic effects of DRD2 variants have been observed in neurophysiologic, neuropsychologic, stress response, social stress defeat, maternal deprivation, and gambling disorders, whereby epigenetic DNA methylation and histone post-translational negative methylation have been identified in many citations.14,42–56 Methylation of DRD2 has been observed in many facets of addiction, including increased striatal response to reward cues in alcoholics,54 decreased functional connectivity of the executive control network,43 and withdrawal.44,46 Blum and Noble characterized the DRD2 Taq A1 allele as a generalized reward gene rather than one specific to alcoholism. This underscores the need for the field to find ways to either use effector moieties to edit the neuroepigenetic insults or possibly harness the idea of potentially removing negative mRNA-reduced expression by inducing “dopamine homeostasis.”
It is important to consider oxytocin as a crucial element in inducing dopamine balance within the brain. Evidence suggests an important interaction between oxytocin/vasopressin and dopamine function, as demonstrated by Modestino et al.57 This important interaction should not be ignored, especially in instances of antisocial behavior in youth, including those with conditions such as autism spectrum disorder.58
5. Opting for immediate satisfaction relative to delayed higher reward value in you
According to Volkow and Baler,41 it is imperative and critical for survival to learn how to balance behaviors that provide a reward NOW versus behaviors that provide an advantage LATER. Specifically, Volkow’s group proposed a model in which dopamine can favor NOW processes through phasic signaling in reward circuits or LATER processes through tonic signaling in control circuits. At the same time, through modulation of the orbitofrontal cortex, which processes salience attribution, dopamine enables shifting from NOW to LATER. In addition, modulation of the insula, which processes interoceptive information, influences the probability of selecting actions NOW versus LATER based on an individuals physiological state. Disruptions along these circuits contribute to diverse pathologies, including obesity, excessive reward-seeking behaviors, and various types of addiction.59
It is noteworthy that adolescents with a family history of substance use disorder (SUD) are at a greater risk for SUD. Rodriguez-Moreno et al.60 suggested that this may be partly attributed to the inheritance of behavioral impulsivity. They employed a delay discounting task to compare impulsivity in decision-making and its associated brain functioning among adolescents with and without a family history of substance abuse. During the task, subjects had to choose between “smaller, sooner” or “larger, later” rewards. The group with a family history of substance abuse displayed greater impatience by responding to “smaller, sooner” rewards more frequently compared to those without a family history of abuse. Behavioral impulsivity is ascribed to the differential developmental trajectories of two brain systems in young individuals. To provide clarity for those unfamiliar, it is known that children can be described with regard to how closely they are functioning to age-expected development in the three early childhood outcomes measured for federal reporting purposes. This is evaluated by collecting a variety of formative assessment data and using it to rate the child’s functioning on a 1 – 7 Likert scale, with 6 and 7 being the age-expected functioning level. In fact, the aim is to link performance with age expectation by comparing the functioning of children with disabilities to those developing according to age expectation. Specifically, Steinberg61 reported on the dominating role of the socioemotional brain systems in driving reward-seeking behavior in the face of an underdeveloped self-regulatory system. Casey’s group62,63 suggested that adolescent developmental changes are hierarchical in subcortical and cortical regions and their interconnections. For clarity, a hierarchy (from Greek: ἱεραρχία, hierarkhia, “rule of a high priest,” from hierarkhes, “president of sacred rites”) is an arrangement of items (objects, names, values, categories, etc.) represented as being “above,” “below,” or “at the same level as” one another.
Most importantly, it is plausible that in adolescence, over-activation of the brain’s reward system and under-activation of the cognitive control brain mechanisms can lead to unwanted substance-seeking behavior driven by impulsivity and sensation-seeking tendencies.64 Others suggested that choosing Now versus Later involves developmental changes that load onto poor decisions due in part to an undeveloped reward and cognitive control system, unlike their adult counterpart.65–69
6. Cognitive impairment in youth
In terms of cognitive impairment, especially concerning deficient executive cognitive functioning (ECF) in children, Aytaclar et al.70 reported that early adolescents at high risk for addictive behavior due to fathers with SUD demonstrated significantly poorer performance on ECF compared to lower risk adolescences. High-risk individuals in early adolescence displayed an earlier initiation of cannabis use and a greater prevalence of lifetime cannabis and tobacco use. Importantly, the level of ECF activity was predictive of the severity of drug involvement, including conduct problems and the number of drugs ever tried.
Several contributing factors are associated with cognitive impairment in youth, including but not limited to excessive opioid/alcohol intake in mothers during pregnancy,71,72 substance abuse, food addiction, and neuropsychiatric illnesses such as attention deficit hyperactivity disorder (ADHD) and attention deficit disorder.73 Bihlar Muld et al.74 highlighted that the clinical characteristics of patients with both ADHD and SUD differed from those with only SUD or ADHD and other psychiatric conditions, indicating the disabling nature of ADHD when combined with SUD. Specifically, the combination of severe substance abuse and ADHD resulted in poor general cognitive ability, including antisocial behavior. In addition, disruptions in the nascent synaptic networks and glia induced by opioids can impact brain connectivity and cognition after the opioid supply is abruptly stopped after birth.75 Neuroimaging has revealed abnormalities in brain structure, including cortical development, white matter microstructure, and functional connectivity, in newborns with fetal alcohol syndrome. These impairments in brain development modify developmental trajectories, leading to deficits in cognition, executive function, memory, behavior, and social adaptation.72 These catastrophic deficits in brain development pose risks for impending RDS behaviors, including SUD.
Undoubtedly, the prevalence of sugar in food and beverages has led to excessive consumption across all age groups, especially children and adolescents. It is staggering to note that over 60 countries consume sugar more than 4 times (>100 g/person/day), exceeding the World Health Organization’s (WHO) recommendations (25 g/person/day). Utilizing a validated mouse model, Beecher et al.73 reported that prolonged sugar overconsumption induces an abnormal response to novelty and changes both episodic and spatial memory. Their findings revealed that hippocampal-dependent learning and memory deficits accompany altered hippocampal neurogenesis. Specifically, there was an overall reduction in the proliferation and differentiation of neurons, especially within the dentate gyrus of newborns.
While the global obesity epidemic has been widely publicized in the media, understanding the evolution of sugar addiction could shed light on this dilemma. Avena’s group76 highlighted that the dopaminergic system in the mesolimbic region of the human brain is involved in hedonic rewards as a function of eating highly addictive, palatable foods like sugar. Particularly interesting is the role of acetylcholine in counteracting the dopaminergic surge as a plausible mechanistic action to help curb uncontrollable sugar cravings.
7. Proposing BHC as a novel program in the US’s educational system
In 2021, over 100,000 individuals died prematurely from an opioid overdose. Neuropsychiatric and cognitive impairments are underreported comorbidities of reward dysregulation due to genetic antecedents and epigenetic insults. Recent genome-wide association studies involving millions of subjects revealed frequent comorbidity with SUD in a sizeable meta-analysis of depression.77 Significant associations were identified between the expression of NEGR1 in the hypothalamus and DRD2 in the NAc, among other genetic factors. However, despite the rise in SUD and neuropsychiatric illness, especially in youth, routine standard objective assessments of brain function remain absent.
The importance of exercise programs in the global educational system was emphasized in 2020 by the release of updated global guidelines by the WHO on physical activity and sedentary behavior for children, adolescents, adults, older adults, sub-populations such as pregnant and postpartum women, and those living with chronic conditions or disabilities. According to Chaput et al.,78 increased and higher intensities of physical activity, as well as a diversity of physical activity (i.e., aerobic, muscle, and bone strengthening activities), are associated with improved health outcomes (primarily intermediate outcomes), as supported by various systematic reviews. Similarly, Thanos’s group79 reported that exercised rats had 18% and 21% lower dopamine D1R-like binding levels than sedentary rats within the olfactory tubercle and NAc shell, respectively. In addition, there was greater dopamine D2R-like binding in the NAc core (24%) and shell (25%) of exercised rats compared with sedentary rats. These observations support the hypothesis that aerobic exercise results in changes in the mesolimbic pathway that could mediate exercise-induced attenuation of drug-seeking behavior. The role of exercise, especially in the educational system, may have potential benefits for assisting school-age children with a positive family history of SUD, for example, through formal fitness programs.80
We propose that integrating existing education-based fitness programs with a standard BHC could synergistically not only improve the health of individuals but could also facilitate early identification of cognitive impairments. For early identification of cognitive abilities, DNA analysis through genetic testing, such as the GARS test, could provide important information, reflecting students’ brain neurotransmitter function at a genetic level.19,21,78,80,81
The rationale for encouraging a standard objective BHC is to acquire an extensive dataset to treat clinical syndromes in psychiatric patients and high-risk populations. While we advocate for implementing a generalized BHC across all K1–K12 students, its importance is especially pronounced for high-risk children attending “recovery high school (RHS).” Spearheaded by one of us (AJF) and others is the needed development of RHSs that provide a supportive educational and therapeutic environment for students following SUD treatment. According to Weimer et al.,82 most students served by RHSs have concurrent mental health disorders and are at risk for school failure, dropout, and substance use relapse. Fairly recently, RHS student high school graduation rates were 21 – 25 percentage points higher compared to students not attending RHS.82 This finding was statistically significant, albeit with limitations related to non-randomized design, selection bias in the study conditions, and uncertainty in calculating school costs. In another study by Tanner-Smith et al.,83 students attending RHS exhibited less frequent delinquent behavior while intoxicated and fewer days of substance use after discharge from SUD treatment than students attending non-RHS. Therefore, we propose RHS students as suitable candidates to test out the utilization of the BHC.
The proposed BHC comprises a set of reliable, accurate, and cost-effective objective assessments involving the following domains: (i) episodic and general memory; (ii) processing speed; (iii) attention; (iv) neuropsychiatry; and (iv) neurological imaging. After a review of over 36 years of computerized and written assessments primarily from PUBMED of memory, attention, psychiatric, and neurological imaging, the following recommendations have been selected for inclusion in the BHC: (i) MemTrax (episodic memory and processing speed); (ii) CNS vital signs (general and remote memory); (iii) test of variables of attention (attention); (iv) millon clinical multiaxial inventory III (neuropsychiatric); and (v) quantitative electroencephalogram/P300/evoked potential (neurological imaging). Continued research aims to simplify the BHC by including qEEG/P300/evoked potentials and genetically guided precision induction of “dopamine homeostasis.”84 This approach allows the assessment and treatment of reward deficiency and helps prevent dopamine dysregulation from being epigenetically transmitted to future generations.
During adolescence, developmental changes in the neural circuitry of reward processing, motivation, cognitive control, and stress may contribute to vulnerability to increased engagement in substance use and nonsubstance addictive behaviors.85 It has been suggested that the adolescent’s liability for addictions involves changes in the function and structure of the midbrain dopaminergic system, genetic antecedents, and epigenetic insults such as stress-induced neuroplasticity, contributing to imbalances between cognitive control and reward response.
Potenzas’ group85 suggests that leveraging genetics, epigenetics, and intermediate phenotypes/endophenotypes may help identify children and adolescents at risk. Once identified, it is crucial for these individuals to participate in a guidance program, essentially brain health coaching (BHCo). The advent of molecular neurobiological tools to uncover neurotransmitter cascade surfeits or deficits and possibilities for restoring dopamine balance across these brain regions, including the PFC, can improve screening of cognitive abilities, which would enhance prevention and intervention approaches. However, implementing changes in educational programs requires top-down public policy strategies. A detailed description of our proposed BHC can be found in Braverman et al.86
8. Epigenetics of reward processing in adolescence
It is widely acknowledged that the adolescent brain matures through a prolonged reorganization of gray matter, white matter, and associated neurochemical systems. Interestingly, this period of enhanced cognitive ability in adolescents coincides with a reduction in cortical gray matter thickness, resulting from epigenetic experience-dependent loss of synapses and a concomitant strengthening of the remaining connections.87–89 In addition, during adolescence, gray matter volume and density decrease in the brain, specifically in the parietal cortex, PFC, and basal ganglia, all of which are critical for executive function, motivated behaviors, and sensory processing.34,90,91 Furthermore, Paus89 demonstrated that there were corresponding increases in white matter, potentially reflecting augmented myelination and axonal diameter, leading to enhanced efficiency of impulse transduction. Notably, Gogtay et al.92 observed that phylogenetically older brain regions mature earlier than the newer ones. This delayed, uneven maturation of subcortical, emotional, and reward-focused systems, including cortical executive and impulse control systems, could underlie many RDS behaviors, including SUD.93–95
The prevalence of mental health disorders, including addictive behaviors, in children and adolescents has increased at least two- to three-fold from the 1990s to the present day.87 According to Monaco,95 one plausible mechanistic reason for this increase may be the transmission of altered brain circuits epigenetically across generations through non-DNA-based mechanisms (intergenerational and transgenerational effects). These epigenetic insults to the developing brain may be due to a family history of SUD, obesity, or a poor diet (e.g., processed, palatable foods). These insults may cause intergenerational and transgenerational effects for at least up to 2 years, influencing set points in neuropathways integrating sensory-motor, reward, and feeding behaviors.
In line with this, Hurd’s group linked parental THC exposure in rats to reduced proenkephalin mRNA expression in the NAc during early development, along with elevated expression during adulthood. Perinatal THC exposure also resulted in shorter latency to the first active lever press, greater responses to low heroin doses, and more heroin-seeking during mild stress and after extinction.96 Studies by Yuan et al.,97 and others98 reveal that persistent alterations in neuronal signaling and cognitive ability result from chronic nicotine exposure, likely due to altered dopamine function in the brain. Dopamine D2 receptor activation of fast-spiking interneurons in the PFC does not occur until late adolescence, along with the recruitment and maturation of local GABAergic activity.99,100 In addition, Tseng and O’Donnell99 point out that D1-NMDA receptor interactions in cortical pyramidal neurons that are necessary for mature cognitive and attentional processing continue to develop during this period. Flores-Barrera et al.101 discovered that ventral hippocampal input to the medial PFC is strengthened during late adolescence due to the D1 receptor-mediated emergence of NMDA receptor GluN2B subunit function. Unfortunately, in the mesolimbic system, particularly in the NAc, D1 and D2 receptor responses are immature, leading to reduced synaptic interaction between NAc and the PFC.102 Furthermore, the stimulation of the D2 receptor has an age-specific influence on AMPA-evoked cell excitability, and interactions between D2 and AMPA receptors elicit the activation of GABA interneurons, primarily in adults but not adolescents.103 In summary, these observations suggest a functional switch in reward processing during adolescent development mediated by dopamine regulation of GABA interneurons. It is well known that enhanced GABA transmission following chronic alcohol intake significantly reduces dopamine release at the NAc.104 In addition, stimulation of GABAB receptors inhibits dopaminergic VTA neurons.105 However, Pandey’s group demonstrated that the inhibition of VTA neuronal firing by bath‐applied GABA is primarily mediated by GABAA receptors.106
The risk of all addictive drug and non-drug behaviors, especially in the unmyelinated PFC of adolescents, is both critical and complex. Many animal and human studies have highlighted the epigenetic impact on the developing brain in adolescents compared to adults. Some studies reveal an underlying hyperdopaminergia, which predisposes young individuals to risky behaviors by inducing high quanta presynaptic dopamine release at reward site neurons. In addition, altered reward gene expression in adolescents caused by epigenetically transferred social defeat, such as bullying, can persist into adulthood. However, there is also evidence that overstimulating epigenetic events can elicit adolescent hypodopaminergia. This complexity (Figure 2) suggests that neuroscience cannot definitively claim that all adolescents carry a hyperdopaminergic trait. To help dissect these seemingly opposing views, Blum’s laboratory reported a high risk for any addictive behavior (hypodopaminergia), especially drug-seeking (95%) and alcohol-seeking (64%) based on GARS testing of 24 Caucasians, ages 12–19 (derived from families with RDS). These results, although from a small cohort, should encourage further extensive studies in this area.
Figure 2.
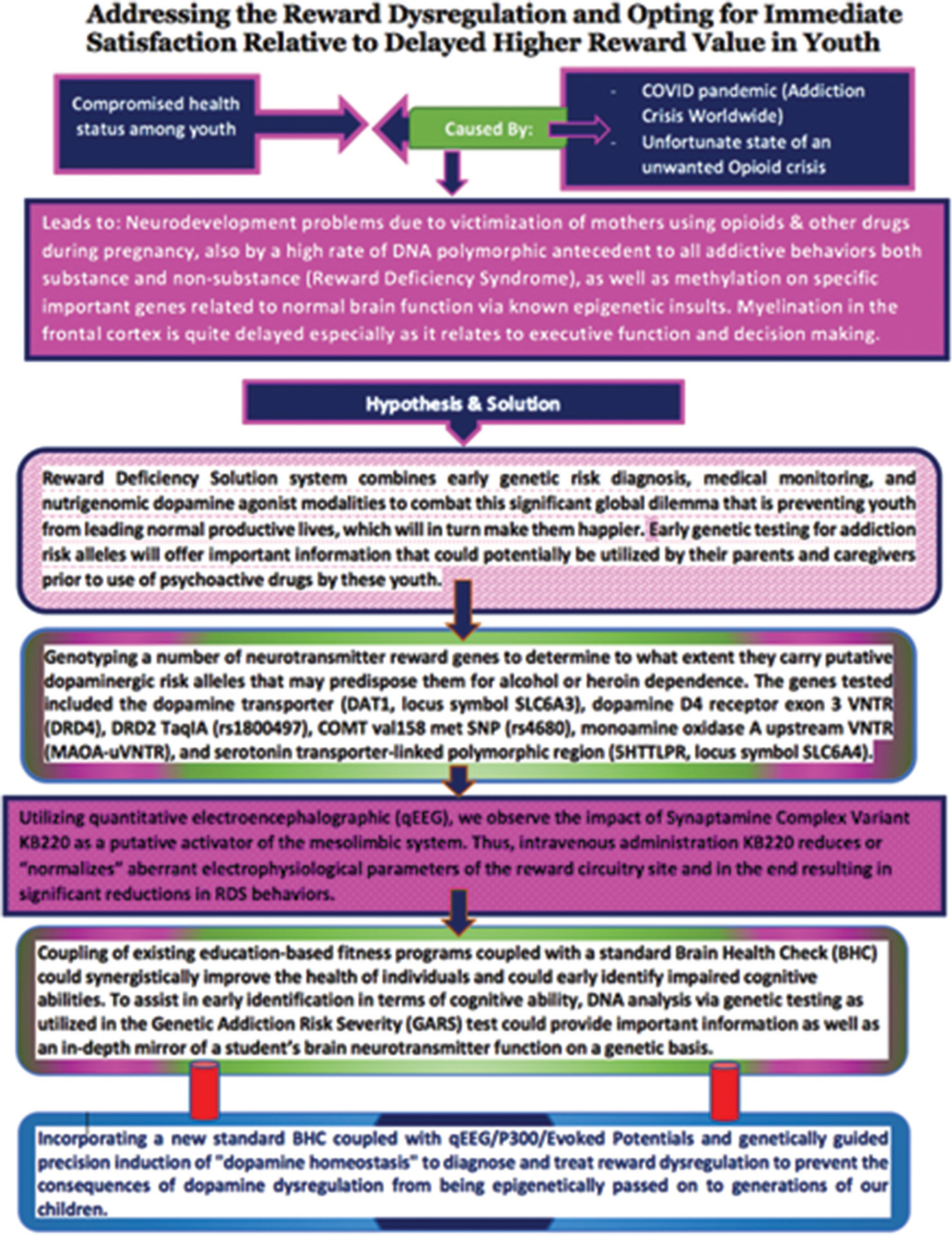
A conceptual schematic that summarizes reward dysregulation in youth and how the reward deficiency solution and brain health check can be used to diagnose and treat reward dysregulation.
Mental disorders are widespread globally, influencing every community and age group, and contribute substantially to the overall disease burden, with major economic and social consequences as well as effects on human health and rights. Alarmingly, the largest inequities exist across nations, with 80% of people affected by mental disorders living in low- and middle-income countries, which benefit from scarcely 10% of global mental health resources. Unfortunately, poor rural areas in the US experience a significantly higher rate of mental disorders, including RDS behaviors such as SUD. Furthermore, due to low income and high juvenile delinquency in rural communities, possibly linked to cognitive inabilities such as poor decision-making, the recommendation of a standard BHC seems prudent. While globally accepted diagnostic categories and classifications, such as the Research Domain Criteria project, WHO International Statistical Classification of Diseases-11, or DSM-5, can help overcome global mental health challenges, our concern is that anomalous brain activity is not being adequately considered within the context of “systems biology,” neglecting educational, economic, and behavioral consequences that require appropriate and effective interventions. The best approach to achieving positive clinical outcomes is to initiate novel strategic alternative modalities targeting the etiology rather than just the symptoms.107,108
9. Positive thinking in adolescence
Positive emotions and cognition have been widely recognized for their beneficial effects on overall mental health and well-being, particularly when people focus on positive thought processes. In positive psychology, the goal is typically to engender character traits such as optimism and hope, which reduce anxiety and depression while fostering strong social interactions.109 There are many interventions aimed at developing and adjusting emotional and social skills in school, such as social and emotional learning programs110 or positive youth development interventions.111 However, research concerning positive thinking in adolescents has been relatively limited.112 Data indicate that negative emotions, such as anxiety or depression, are associated with the dysregulation of the amygdala – PFC circuitry.113 Positive emotional words are associated with increased activation in the ventral medial PFC.114 Other studies have uncovered important connections between positive emotions and brain processes relevant to prosocial behaviors. For example, a study of the positive emotion of professional pride revealed a relationship to empathy, reward, and emotion regulation, as well as the theory-of-mind network.115
From a neurotransmitter perspective, positive emotions are associated with increases in dopamine function within the reward network.116–119 Altered activity in serotonin modulates negative emotional responses.120 Oxytocin, which supports affiliative behaviors, may also play a role in responses to positive versus negative emotional processes.121 Thus, fostering positive emotions and implementing interventions that support them lead to substantial changes in the brain, involving various areas associated with reward, positive self-image, prosocial behaviors, and empathy. Working toward instilling positive emotions in adolescents is likely to yield short- and long-term benefits regarding their overall mental health and well-being.
10. Conclusion
Importantly, initial engagement in rehabilitation and detoxification bears similarities to experiencing a first stroke or heart attack in that the brain has already been impacted by pathological events that led to the manifestations of SUD and the need for treatment. The tools to prevent the progression of SUD are available and must be implemented urgently because deaths attributed to SUD have continued to increase unabated. Therefore, a reexamination of approaches to brain health and addiction and novel perspectives needs to be implemented by the medical community.
The clinical evidence accumulated during the past three decades underscores the necessity for establishing a BHC focused on precision neuropsychiatric testing, including episodic memory and processing speed (MemTrax),122–124 general memory (CNSVS),125–130 attention (T.O.V.A),131–137 neuropsychiatric (MCMI-III),138–140 and neurological imaging (qEEG/P300/EP),131,137,141–154 for patients at risk of or presenting with problematic drug misuse. Since addiction is related to learning mechanisms, refocusing on learning and memory may change the perspective on the beneficial use of these brain mechanisms. For example, the online program MemTrax (www.memtrax.com) can help individuals monitor their memory as frequently as needed and observe how it is being impacted by substance abuse. Such feedback can lead to behavioral improvements and serve as a valuable tool for those providing therapeutic interventions, such as BHCo.
One of the basic neurochemical mechanisms in the brain, the midbrain dopamine system, participates in pacing critical cognition functions, including reward and the facilitation of addictive behaviors.155–158 A study conducted by Rouhani and Niv,155 through fitting reinforcement learning models to behavior, demonstrated that both signed (cue predicting the reward) and unsigned (unexpected, surprising reward) prediction errors (RPEs) contribute to learning by modulating the learning rate. They further characterized the effects of these RPE signals on memory, demonstrating that both signed and unsigned RPEs augment memory, aligning with midbrain dopamine and locus-coeruleus modulation of hippocampal plasticity. Further research by this group supports the complex nature of reward and learning involving dopaminergic mechanisms.156 Finally, Katzman and Hartley’s work indicates that both children and adults tend to remember past events more when the value of choice is beneficial compared to non-beneficial.157 These proposed BHC/BHCo can be used as a standardized approach for school-aged children, akin to fitness programs. Our suggestion introduces a set of objective brain assessments parallel to those used in cardiology for diagnosing and following the clinical course of cardiac diseases. The coaching approach, including close evaluation and management guidance (including GARS testing and subsequent KB220z variant matching), could easily be adapted for implementation throughout the US and global educational systems. We understand that this initiative would require a substantial and bold approach to the care of the general US population. This commission believes that the BHC/BHCo would synergize with current fitness programs, particularly in addressing co-occurring RDS behaviors. Thanos et al.158 underscore the role of exercise in preventing the initiation of cocaine use in adolescence, suggesting that the implementation of exercise programs might be an important preventive measure and significantly improve students’ mental health. Based on the reviewed research, this appeal promises to stop/prevent the increased prevalence of SUD through early detection utilizing robust brain screening159–209 as recently proposed by the Society of Brain Mapping and Therapeutics,84 as well as psychological and pharmacological treatment approaches espoused herein.210–257
Notably, each year, over a million adolescents globally succumb to preventable or treatable causes. Psychosocial factors are the strongest factors associated with drug abuse, bullying, attempted suicide, and sleep deprivation resulting from bullying.258–316 The Carter Center has estimated that the addiction crisis, if continues to worsen at the same rate, may cost the US approximately 16 trillion dollars by 2030. Furthermore, the neurodevelopment of children could be compromised by maternal usage of opioids and other drugs during pregnancy. A high rate of DNA polymorphic antecedents compounds the epigenetic insults involving the methylation of specific essential genes related to normal brain function. Myelination in the frontal cortex, a process known to extend until the late 20s, delays proficient executive function and decision-making abilities. Understanding this delay in brain development, along with the presence of potential high-risk antecedent polymorphic variants or alleles and generational epigenetics, provides a clear rationale to mimic fitness programs with an adaptable BHC. Implementing the BHC within the educational systems in the US and other countries might be a good starting point for proactive therapies aimed at reducing juvenile mental health problems and, eventually, criminal activities, addiction, and other behaviors associated with RDS.
Footnotes
Conflict of interest
KENNETH BLUM is the holder of both USA and foreign patents related to kb220 and gars. Other authors declare no conflicts of interest.
References
- 1.Miller M, Chen ALC, Stokes SD, et al. Early intervention of intravenous KB220IV--neuroadaptagen amino-acid therapy (NAAT) improves behavioral outcomes in a residential addiction treatment program: A pilot study. J Psychoactive Drugs. 2012;44(5):398–409. doi: 10.1080/02791072.2012.737727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller DK, Bowirrat A, Manka M, et al. Acute intravenous synaptamine complex variant KB220™ “normalizes” neurological dysregulation in patients during protracted abstinence from alcohol and opiates as observed using quantitative electroencephalographic and genetic analysis for reward polymorphisms: Part 1, pilot study with 2 case reports. Postgrad Med. 2010;122(6):188–213. doi: 10.3810/pgm.2010.11.2236 [DOI] [PubMed] [Google Scholar]
- 3.Blum K, Chen TJH, Downs BW, et al. Synaptamine (SG8839) an amino-acid enkephalinase inhibition nutraceutical improves recovery of alcoholics, a subtype of reward deficiency syndrome (RDS). Trends Appl Sci Res. 2007;2(2):132–138. [Google Scholar]
- 4.Blum K, Liu Y, Wang W, et al. rsfMRI effects of KB220Z™ on neural pathways in reward circuitry of abstinent genotyped heroin addicts. Postgrad Med. 2015;127(2):232–241. doi: 10.1080/00325481.2015.994879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blum K, Chen AL, Chen TJ, et al. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): A commentary. Theor Biol Med Model. 2008;5:24. doi: 10.1186/1742-4682-5-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold MS, Baron D, Bowirrat A, Blum K. Neurological correlates of brain reward circuitry linked to opioid use disorder (OUD): Do homo sapiens acquire or have a reward deficiency syndrome? J Neurol Sci. 2020;418:117137. doi: 10.1016/j.jns.2020.117137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martins SS, Sampson L, Cerdá M, Galea S. Worldwide prevalence and trends in unintentional drug overdose: A systematic review of the literature. Am J Public Health. 2015;105(11):e29–e49. doi: 10.2105/AJPH.2015.302843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorpe HHA, Hamidullah S, Jenkins BW, Khokhar JY. Adolescent neurodevelopment and substance use: Receptor expression and behavioral consequences. Pharmacol Ther. 2020;206:107431. doi: 10.1016/j.pharmthera.2019.107431 [DOI] [PubMed] [Google Scholar]
- 9.Strulik H Opioid epidemics. Econ Hum Biol. 2020;37:100835. doi: 10.1016/j.ehb.2019.100835 [DOI] [PubMed] [Google Scholar]
- 10.Horseman C, Meyer A. Neurobiology of addiction. Clin Obstet Gynecol. 2019;62(1):118–127. doi: 10.1097/GRF.0000000000000416 [DOI] [PubMed] [Google Scholar]
- 11.Blum K, Modestino EJ, Badgaiyan RD, et al. Analysis of evidence for the combination of pro-dopamine regulator (KB220PAM) and naltrexone to prevent opioid use disorder relapse. EC Psychol Psychiatr. 2018;7(8):564–579. [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2018;85:90–96. doi: 10.1016/j.jsat.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daoust M, Legrand E, Gewiss M, et al. Acamprosate modulates synaptosomal GABA transmission in chronically alcoholised rats. Pharmacol Biochem Behav. 1992;41(4):669–674. doi: 10.1016/0091-3057(92)90210-7 [DOI] [PubMed] [Google Scholar]
- 14.Blum K, Baron D, Lott L, et al. In search of reward deficiency syndrome (RDS)-free controls: The “Holy Grail” in genetic addiction risk testing. Curr Psychopharmacol. 2020;9(1):7–21. [PMC free article] [PubMed] [Google Scholar]
- 15.Baron D, Blum K, Chen A, Gold M, Badgaiyan RD. Conceptualizing addiction from an osteopathic perspective: Dopamine homeostasis. J Am Osteopath Assoc. 2018;118(2):115–118. doi: 10.7556/jaoa.2018.026 [DOI] [PubMed] [Google Scholar]
- 16.Blum K, Modestino EJ, Gondré-Lewis M, et al. “Dopamine homeostasis” requires balanced polypharmacy: Issue with destructive, powerful dopamine agents to combat America’s drug epidemic. J Syst Integr Neurosci. 2017;3(6). doi: 10.15761/JSIN.1000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenneth B, Edward MJ, Marjorie GLC, et al. Pro-dopamine regulator (KB220) a fifty year sojourn to combat reward deficiency syndrome (RDS): Evidence based Bibliography (Annotated). CPQ Neurol Psychol. 2018;1:2. [PMC free article] [PubMed] [Google Scholar]
- 18.Febo M, Blum K, Badgaiyan RD, et al. Enhanced functional connectivity and volume between cognitive and reward centers of naïve rodent brain produced by pro-dopaminergic agent KB220Z. PLoS One. 2017;12(4):e0174774. doi: 10.1371/journal.pone.0174774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum K, Chen ALC, Thanos PK, et al. Genetic addiction risk score (GARS)™, a predictor of vulnerability to opioid dependence. Front Biosci (Elite Ed). 2018;10(1):175–196. doi: 10.2741/e816 [DOI] [PubMed] [Google Scholar]
- 20.Gold MS, Blum K, Febo M, et al. Molecular role of dopamine in anhedonia linked to reward deficiency syndrome (RDS) and anti- reward systems. Front Biosci (Schol Ed). 2018;10(2):309–325. doi: 10.2741/s518 [DOI] [PubMed] [Google Scholar]
- 21.Blum K, Modestino EJ, Gondre-Lewis M, et al. The benefits of genetic addiction risk score (GARS™) testing in substance use disorder (SUD). Int J Genom Data Min. 2018;2018(1):115. doi: 10.29014/IJGD-115.000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blum K, Thanos PK, Wang GJ, et al. The food and drug addiction epidemic: Targeting dopamine homeostasis. Curr Pharm Des. 2018;23(39):6050–6061. doi: 10.2174/1381612823666170823101713 [DOI] [PubMed] [Google Scholar]
- 23.Blum K, Badgaiyan RD, Braverman ER, et al. Hypothesizing that, A pro-dopamine regulator (KB220Z) should optimize, but not hyper-activate the activity of trace amine-associated receptor 1 (TAAR-1) and induce anti-craving of psychostimulants in the long-term. J Reward Defic Syndr Addict Sci. 2016;2(1):14–21. doi: 10.17756/jrdsas.2016-023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blum K, Modestino EJ, Neary J, et al. Promoting precision addiction management (PAM) to combat the global opioid crisis. Biomed J Sci Tech Res. 2018;2(2):1–4. doi: 10.26717/BJSTR.2018.02.000738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blum K, Gondré-Lewis MC, Baron D, et al. Introducing precision addiction management of reward deficiency syndrome, the construct that underpins all addictive behaviors. Front Psychiatry. 2018;9:548. doi: 10.3389/fpsyt.2018.00548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volkow ND. Toward individualized treatment for substance abuse. Addict Sci Clin Pract. 2010;5(2):2. [PMC free article] [PubMed] [Google Scholar]
- 27.Blum K, Baron D, McLaughlin T, Gold MS. Molecular neurological correlates of endorphinergic/dopaminergic mechanisms in reward circuitry linked to endorphinergic deficiency syndrome (EDS). J Neurol Sci. 2020;411:116733. doi: 10.1016/j.jns.2020.116733 [DOI] [PubMed] [Google Scholar]
- 28.Rosen GD, Chesler EJ, Manly KF, Williams RW. An informatics approach to systems neurogenetics. Methods Mol Biol. 2007;401:287–303. doi: 10.1007/978-1-59745-520-6_16 [DOI] [PubMed] [Google Scholar]
- 29.Blum K, Febo M, Smith DE, et al. Neurogenetic and epigenetic correlates of adolescent predisposition to and risk for addictive behaviors as a function of prefrontal cortex dysregulation. J Child Adolesc Psychopharmacol. 2015;25(4):286–292. doi: 10.1089/cap.2014.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blum K, Bowirrat A, Gondre Lewis MC, et al. Exploration of epigenetic state hyperdopaminergia (Surfeit) and genetic trait hypodopaminergia (Deficit) during adolescent brain development. Curr Psychopharmacol. 2021;10:181–196. doi: 10.2174/2211556010666210215155509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blum K, Febo M, McLaughlin T, Cronjé FJ, Han D, Gold SM. Hatching the behavioral addiction egg: Reward deficiency solution system (RDSS)™ as a function of dopaminergic neurogenetics and brain functional connectivity linking all addictions under a common rubric. J Behav Addict. 2014;3(3):149–156. doi: 10.1556/JBA.3.2014.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackey S, Stewart JL, Connolly CG, Tapert SF, Paulus MP. A voxel-based morphometry study of young occasional users of amphetamine-type stimulants and cocaine. Drug Alcohol Depend. 2014;135:104–111. doi: 10.1016/j.drugalcdep.2013.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012 [DOI] [PubMed] [Google Scholar]
- 34.Giedd JN, Rumsey JM, Castellanos FX, et al. A quantitative MRI study of the corpus callosum in children and adolescents. Brain Res Dev Brain Res. 1996;91(2):274–280. doi: 10.1016/0165-3806(95)00193-x [DOI] [PubMed] [Google Scholar]
- 35.Spear L Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24(2):115–123. [PMC free article] [PubMed] [Google Scholar]
- 36.Yurgelun-Todd DA, Killgore WD, Young AD. Sex differences in cerebral tissue volume and cognitive performance during adolescence. Psychol Rep. 2002;91(3 Pt 1):743–757. doi: 10.2466/pr0.2002.91.3.743 [DOI] [PubMed] [Google Scholar]
- 37.Melo P, Moreno VZ, Vázquez SP, Pinazo-Durán MD, Tavares MA. Myelination changes in the rat optic nerve after prenatal exposure to methamphetamine. Brain Res. 2006;1106(1):21–29. doi: 10.1016/j.brainres.2006.05.020 [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Yang B, Yan C, et al. Effects of duration and timing of prenatal stress on hippocampal myelination and synaptophysin expression. Brain Res. 2013;1527:57–66. doi: 10.1016/j.brainres.2013.06.025 [DOI] [PubMed] [Google Scholar]
- 39.De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescentonset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29(9):1590–1600. doi: 10.1097/01.alc.0000179368.87886.76 [DOI] [PubMed] [Google Scholar]
- 40.Kofink D, Boks MPM, Timmers HT, Kas MJ. Epigenetic dynamics in psychiatric disorders: Environmental programming of neurodevelopmental processes. Neurosci Biobehav Rev. 2013;37(5):831–845. doi: 10.1016/j.neubiorev.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 41.Volkow ND, Baler RD. NOW vs LATER brain circuits: Implications for obesity and addiction. Trends Neurosci. 2015;38(6):345–352. doi: 10.1016/j.tins.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 42.Dalterio S, Blum K, DeLallo L, Sweeney C, Briggs A, Bartke A. Perinatal exposure to delta 9-THC in mice: Altered enkephalin and norepinephrine sensitivity in vas deferens. Subst Alcohol Actions Misuse. 1980;1(5–6):467–471. [PubMed] [Google Scholar]
- 43.Hagerty SL, YorkWilliams SL, Bidwell LC, et al. DRD2 methylation is associated with executive control network connectivity and severity of alcohol problems among a sample of polysubstance users. Addict Biol. 2020;25(1):e12684. doi: 10.1111/adb.12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hillemacher T, Rhein M, Burkert A, et al. DNA-methylation of the dopamin receptor 2 gene is altered during alcohol withdrawal. Eur Neuropsychopharmacol. 2019;29(11):1250–1257. doi: 10.1016/j.euroneuro.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 45.Groleau P, Joober R, Israel M, Zeramdini N, DeGuzman R, Steiger H. Methylation of the dopamine D2 receptor (DRD2) gene promoter in women with a bulimia-spectrum disorder: Associations with borderline personality disorder and exposure to childhood abuse. J Psychiatr Res. 2014;48(1):121–127. doi: 10.1016/j.jpsychires.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 46.Hillemacher T, Frieling H, Buchholz V, et al. Alterations in DNA-methylation of the dopamine-receptor 2 gene are associated with abstinence and health care utilization in individuals with a lifetime history of pathologic gambling. Prog Neuropsychopharmacol Biol Psychiatry. 2015;63:30–34. doi: 10.1016/j.pnpbp.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 47.Staes N, White CM, Guevara EE, et al. Chimpanzee Extraversion scores vary with epigenetic modification of dopamine receptor gene D2 (DRD2) and early rearing conditions. Epigenetics. 2022;17(12):1701–1714. doi: 10.1080/15592294.2022.2058224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frieling H, Römer KD, Scholz S, et al. Epigenetic dysregulation of dopaminergic genes in eating disorders. Int J Eat Disord. 2010;43(7):577–583. doi: 10.1002/eat.20745 [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Fan Y, Zhou J, et al. Methylation quantitative trait locus rs5326 is associated with susceptibility and effective dosage of methadone maintenance treatment for heroin use disorder. Psychopharmacology (Berl). 2021;238(12):3511–3518. doi: 10.1007/s00213-021-05968-8 [DOI] [PubMed] [Google Scholar]
- 50.Nohesara S, Ghadirivasfi M, Barati M, et al. Methamphetamine-induced psychosis is associated with DNA hypomethylation and increased expression of AKT1 and key dopaminergic genes. Am J Med Genet B Neuropsychiatr Genet. 2016;171(8):1180–1189. doi: 10.1002/ajmg.b.32506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feltmann K, Borroto-Escuela DO, Rüegg J, et al. Effects of long-term alcohol drinking on the dopamine D2 receptor: Gene expression and heteroreceptor complexes in the striatum in rats. Alcohol Clin Exp Res. 2018;42(2):338–351. doi: 10.1111/acer.13568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hill SY, Sharma VK. DRD2 methylation and regional grey matter volumes in young adult offspring from families at ultra-high risk for alcohol dependence. Psychiatry Res Neuroimaging. 2019;286:31–38. doi: 10.1016/j.pscychresns.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klaus K, Vaht M, Pennington K, Harro J. Interactive effects of DRD2 rs6277 polymorphism, environment and sex on impulsivity in a population-representative study. Behav Brain Res. 2021;403:113131. doi: 10.1016/j.bbr.2021.113131 [DOI] [PubMed] [Google Scholar]
- 54.Bidwell LC, Karoly HC, Thayer RE, et al. DRD2 promoter methylation and measures of alcohol reward: Functional activation of reward circuits and clinical severity. Addict Biol. 2019;24(3):539–548. doi: 10.1111/adb.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandey SC, Kyzar EJ, Zhang H. Epigenetic basis of the dark side of alcohol addiction. Neuropharmacology. 2017;122:74–84. doi: 10.1016/j.neuropharm.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Q, Xu Y, Mao Y, et al. Genetic and epigenetic analysis revealing variants in the NCAM1-TTC12-ANKK1-DRD2 cluster associated significantly with nicotine dependence in Chinese Han smokers. Nicotine Tob Res. 2020;22(8):1301–1309. doi: 10.1093/ntr/ntz240 [DOI] [PubMed] [Google Scholar]
- 57.Modestino EJ, Blum K, Oscar-Berman M, et al. Reward deficiency syndrome: Attentional/arousal subtypes, limitations of current diagnostic nosology, and future research. J Reward Defic Syndr. 2015;1(1):6–9. doi: 10.17756/jrds.2015-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamasue H, Domes G. Oxytocin and autism spectrum disorders. Curr Top Behav Neurosci. 2018;35:449–465. doi: 10.1007/7854_2017_24 [DOI] [PubMed] [Google Scholar]
- 59.Lindgren E, Gray K, Miller G, et al. Food addiction: A common neurobiological mechanism with drug abuse. Front Biosci (Landmark Ed). 2018;23(5):811–836. doi: 10.2741/4618 [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez-Moreno DV, Cycowicz YM, Figner B, et al. Delay discounting and neurocognitive correlates among inner city adolescents with and without family history of substance use disorder. Dev Cogn Neurosci. 2021;48:100942. doi: 10.1016/j.dcn.2021.100942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinberg L A social neuroscience perspective on adolescent risk-taking. Dev Rev. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1189–1285. doi: 10.1016/j.jaac.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage. 2011;54(2):1344–1354. doi: 10.1016/j.neuroimage.2010.08.067 [DOI] [PubMed] [Google Scholar]
- 66.Christakou A, Brammer M, Giampietro V, Rubia K. Right ventromedial and dorsolateral prefrontal cortices mediate adaptive decisions under ambiguity by integrating choice utility and outcome evaluation. J Neurosci. 2009;29(35):11020–11028. doi: 10.1523/JNEUROSCI.1279-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ripke S, Hübner T, Mennigen E, et al. Reward processing and intertemporal decision making in adults and adolescents: The role of impulsivity and decision consistency. Brain Res. 2012;1478:36–47. doi: 10.1016/j.brainres.2012.08.034 [DOI] [PubMed] [Google Scholar]
- 68.de Water E, Cillessen AH, Scheres A. Distinct age-related differences in temporal discounting and risk taking in adolescents and young adults. Child Dev. 2014;85(5):1881–1897. doi: 10.1111/cdev.12245 [DOI] [PubMed] [Google Scholar]
- 69.Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting [published correction appears in Child Dev. 2010;81(3):1024]. Child Dev. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x [DOI] [PubMed] [Google Scholar]
- 70.Aytaclar S, Tarter RE, Kirisci L, Lu S. Association between hyperactivity and executive cognitive functioning in childhood and substance use in early adolescence. J Am Acad Child Adolesc Psychiatry. 1999;38(2):172–178. doi: 10.1097/00004583-199902000-00016 [DOI] [PubMed] [Google Scholar]
- 71.Brancato A, Castelli V, Lavanco G, Marino RAM, Cannizzaro C. In utero Δ9-tetrahydrocannabinol exposure confers vulnerability towards cognitive impairments and alcohol drinking in the adolescent offspring: Is there a role for neuropeptide Y? J Psychopharmacol. 2020;34(6):663–679. doi: 10.1177/0269881120916135 [DOI] [PubMed] [Google Scholar]
- 72.Wozniak JR, Riley EP, Charness ME. Clinical presentation, diagnosis, and management of fetal alcohol spectrum disorder. Lancet Neurol. 2019;18(8):760–770. doi: 10.1016/S1474-4422(19)30150-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beecher K, Alvarez Cooper I, Wang J, et al. Long-term overconsumption of sugar starting at adolescence produces persistent hyperactivity and neurocognitive deficits in adulthood. Front Neurosci. 2021;15:670430. doi: 10.3389/fnins.2021.670430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bihlar Muld B, Jokinen J, Bölte S, Hirvikoski T. Attention deficit/hyperactivity disorders with co-existing substance use disorder is characterized by early antisocial behaviour and poor cognitive skills. BMC Psychiatry. 2013;13:336. doi: 10.1186/1471-244X-13-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boggess T, Risher WC. Clinical and basic research investigations into the long-term effects of prenatal opioid exposure on brain development. J Neurosci Res. 2022;100(1):396–409. doi: 10.1002/jnr.24642 [DOI] [PubMed] [Google Scholar]
- 76.Wiss DA, Avena N, Rada P. Sugar addiction: From evolution to revolution. Front Psychiatry. 2018;9:545. doi: 10.3389/fpsyt.2018.00545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levey DF, Stein MB, Wendt FR, et al. Bi-ancestral depression GWAS in the million veteran program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat Neurosci. 2021;24(7):954–963. doi: 10.1038/s41593-021-00860-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaput JP, Willumsen J, Bull F, et al. 2020 WHO guidelines on physical activity and sedentary behaviour for children and adolescents aged 5–17 years: Summary of the evidence. Int J Behav Nutr Phys Act. 2020;17(1):141. doi: 10.1186/s12966-020-01037-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robison LS, Swenson S, Hamilton J, Thanos PK. Exercise reduces dopamine D1R and increases D2R in rats: Implications for addiction. Med Sci Sports Exerc. 2018;50(8):1596–1602. doi: 10.1249/MSS.0000000000001627 [DOI] [PubMed] [Google Scholar]
- 80.Swenson S, Blum K, McLaughlin T, Gold MS, Thanos PK. The therapeutic potential of exercise for neuropsychiatric diseases: A review. J Neurol Sci. 2020;412:116763. doi: 10.1016/j.jns.2020.116763 [DOI] [PubMed] [Google Scholar]
- 81.Blum K, Gondré-Lewis MC, Modestino EJ, et al. Understanding the scientific basis of post-traumatic stress disorder (PTSD): Precision behavioral management overrides stigmatization. Mol Neurobiol. 2019;56(11):7836–7850. doi: 10.1007/s12035-019-1600-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weimer DL, Moberg P, French F, Tanner-Smith EE, Finch AJ. Net benefits of recovery high schools: Higher cost but increased sobriety and educational attainment. J Ment Health Policy Econ. 2019;22(3):109–120. [PMC free article] [PubMed] [Google Scholar]
- 83.Tanner-Smith EE, Nichols LM, Loan CM, Finch AJ, Moberg DP. Recovery high school attendance effects on student delinquency and substance use: The moderating role of social problem solving styles. Prev Sci. 2020;21(8):1104–1113. doi: 10.1007/s11121-020-01161-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nami M, Thatcher R, Kashou N, et al. A proposed brain-, spine-, and mental- health screening methodology (NEUROSCREEN) for healthcare systems: Position of the society for brain mapping and therapeutics. J Alzheimers Dis. 2022;86(1):21–42. doi: 10.3233/JAD-215240 [DOI] [PubMed] [Google Scholar]
- 85.Hammond CJ, Mayes LC, Potenza MN. Neurobiology of adolescent substance use and addictive behaviors: Treatment implications. Adolesc Med State Art Rev. 2014;25(1):15–32. [PMC free article] [PubMed] [Google Scholar]
- 86.Braverman ER, Dennen CA, Gold MS, et al. Proposing a “brain health checkup (BHC)” as a global potential “standard of care” to overcome reward dysregulation in primary care medicine: Coupling genetic risk testing and induction of “dopamine homeostasis”. Int J Environ Res Public Health. 2022;19(9):5480. doi: 10.3390/ijerph19095480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29(38):11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gogtay N, Thompson PM. Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010;72(1):6–15. doi: 10.1016/j.bandc.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paus T Growth of white matter in the adolescent brain: Myelin or axon? Brain Cogn. 2010;72(1):26–35. doi: 10.1016/j.bandc.2009.06.00 [DOI] [PubMed] [Google Scholar]
- 90.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- 91.Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9(6 Pt 1):587–597. doi: 10.1006/nimg.1999.0436 [DOI] [PubMed] [Google Scholar]
- 92.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith SS. α4βδ GABAA receptors and tonic inhibitory current during adolescence: Effects on mood and synaptic plasticity. Front Neural Circuits. 2013;7:135. doi: 10.3389/fncir.2013.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blum K, McLaughlin T, Bowirrat A, et al. Reward deficiency syndrome (RDS) surprisingly is evolutionary and found everywhere: Is it “blowin’ in the wind”? J Pers Med. 2022;12(2):321. doi: 10.3390/jpm12020321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Monaco AP. An epigenetic, transgenerational model of increased mental health disorders in children, adolescents and young adults. Eur J Hum Genet. 2021;29(3):387–395. doi: 10.1038/s41431-020-00726-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spano MS, Ellgren M, Wang X, Hurd YL. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol Psychiatry. 2007;61(4):554–563. doi: 10.1016/j.biopsych.2006.03.073 [DOI] [PubMed] [Google Scholar]
- 97.Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. J Physiol. 2015;593(16):3397–3412. doi: 10.1113/JP270492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Slotkin TA, Seidler FJ. Nicotine exposure in adolescence alters the response of serotonin systems to nicotine administered subsequently in adulthood. Dev Neurosci. 2009;31(1–2):58–70. doi: 10.1159/000207494 [DOI] [PubMed] [Google Scholar]
- 99.Tseng KY, O’Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61(10):843–850. doi: 10.1002/syn.20432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Donnell P Adolescent maturation of cortical dopamine. Neurotox Res. 2010;18(3–4):306–312. doi: 10.1007/s12640-010-9157-3 [DOI] [PubMed] [Google Scholar]
- 101.Flores-Barrera E, Thomases DR, Heng LJ, Cass DK, Caballero A, Tseng KY. Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic protein kinase A and D1 dopamine receptor signaling. Biol Psychiatry. 2014;75(6):508–516. doi: 10.1016/j.biopsych.2013.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Benoit-Marand M, O’Donnell P. D2 dopamine modulation of corticoaccumbens synaptic responses changes during adolescence. Eur J Neurosci. 2008;27(6):1364–1372. doi: 10.1111/j.1460-9568.2008.06107.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huppé-Gourgues F, O’Donnell P. D1-NMDA receptor interactions in the rat nucleus accumbens change during adolescence. Synapse. 2012;66(7):584–591. doi: 10.1002/syn.21544 [DOI] [PubMed] [Google Scholar]
- 104.You C, Vandegrift BJ, Zhang H, Lasek AW, Pandey SC, Brodie MS. Histone deacetylase inhibitor suberanilohydroxamic acid treatment reverses hyposensitivity to γ-aminobutyric acid in the ventral tegmental area during ethanol withdrawal. Alcohol Clin Exp Res. 2018;42(11):2160–2171. doi: 10.1111/acer.13870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mueller AL, Brodie MS. Intracellular recording from putative dopamine-containing neurons in the ventral tegmental area of Tsai in a brain slice preparation. J Neurosci Methods. 1989;28(1–2):15–22. doi: 10.1016/0165-0270(89)90005-8 [DOI] [PubMed] [Google Scholar]
- 106.Arora DS, Nimitvilai S, Teppen TL, et al. Hyposensitivity to gamma-aminobutyric acid in the ventral tegmental area during alcohol withdrawal: Reversal by histone deacetylase inhibitors. Neuropsychopharmacology. 2013;38(9):1674–1684. doi: 10.1038/npp.2013.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jacob KS, Patel V. Classification of mental disorders: A global mental health perspective. Lancet. 2014;383(9926):1433–1435. doi: 10.1016/S0140-6736(13)62382-X [DOI] [PubMed] [Google Scholar]
- 108.Casey BJ, Craddock N, Cuthbert BN, Hyman SE, Lee FS, Ressler KJ. DSM-5 and RDoC: Progress in psychiatry research? Nat Rev Neurosci. 2013;14(11):810–814. doi: 10.1038/nrn3621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seligman MEP. The Optimistic Child: A Proven Program to Safeguard Children against Depression and Build Lifelong Resilience. Boston: Houghton Mifflin Harcourt; 2007. [Google Scholar]
- 110.Domitrovich CE, Durlak JA, Staley KC, Weissberg RP. Social-emotional competence: An essential factor for promoting positive adjustment and reducing risk in school children. Child Dev. 2017;88(2):408–416. doi: 10.1111/cdev.12739 [DOI] [PubMed] [Google Scholar]
- 111.Ciocanel O, Power K, Eriksen A, Gillings K. Effectiveness of positive youth development interventions: A meta-analysis of randomized controlled trials. J Youth Adolesc. 2017;46(3):483–504. doi: 10.1007/s10964-016-0555-6 [DOI] [PubMed] [Google Scholar]
- 112.Benoit V, Gabola P. Effects of positive psychology interventions on the well-being of young children: A systematic literature review. Int J Environ Res Public Health. 2021;18(22):12065. doi: 10.3390/ijerph182212065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Young KS, Sandman CF, Craske MG. Positive and negative emotion regulation in adolescence: Links to anxiety and depression. Brain Sci. 2019;9(4):76. doi: 10.3390/brainsci9040076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Straube T, Sauer A, Miltner WH. Brain activation during direct and indirect processing of positive and negative words. Behav Brain Res. 2011;222(1):66–72. doi: 10.1016/j.bbr.2011.03.037 [DOI] [PubMed] [Google Scholar]
- 115.Hong YJ, Park S, Kyeong S, Kim JJ. Neural basis of professional pride in the reaction to uniform wear. Front Hum Neurosci. 2019;13:253. doi: 10.3389/fnhum.2019.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86(3):646–664. doi: 10.1016/j.neuron.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Volkow ND, Tomasi D, Wang GJ, et al. Positive emotionality is associated with baseline metabolism in orbitofrontal cortex and in regions of the default network. Mol Psychiatry. 2011;16(8):818–825. doi: 10.1038/mp.2011.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blum K, Merritt JH, Wallace JE, Owen R, Hahn JW, Geller I. Effects of catecholamine synthesis inhibition on ethanol narcosis in mice. Curr Ther Res Clin Exp. 1972;14(6):324–329. [PubMed] [Google Scholar]
- 119.Dackis CA, Gold MS. New concepts in cocaine addiction: The dopamine depletion hypothesis. Neurosci Biobehav Rev. 1985;9(3):469–477. doi: 10.1016/0149-7634(85)90022-3 [DOI] [PubMed] [Google Scholar]
- 120.Selvaraj S, Mouchlianitis E, Faulkner P, et al. Presynaptic serotoninergic regulation of emotional processing: A multimodal brain imaging study. Biol Psychiatry. 2015;78(8):563–571. doi: 10.1016/j.biopsych.2014.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen X, Gautam P, Haroon E, Rilling JK. Within vs. Between-subject effects of intranasal oxytocin on the neural response to cooperative and non-cooperative social interactions. Psychoneuroendocrinology. 2017;78:22–30. doi: 10.1016/j.psyneuen.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ashford JW, Tarpin-Bernard F, Ashford CB, Ashford MT. A computerized continuous-recognition task for measurement of episodic memory. J Alzheimers Dis. 2019;69(2):385–399. doi: 10.3233/JAD-190167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu X, Chen X, Zhou X, et al. Validity of the memtrax memory test compared to the Montreal cognitive assessment in the detection of mild cognitive impairment and dementia due to Alzheimer’s disease in a Chinese cohort. J Alzheimers Dis. 2021;80(3):1257–1267. doi: 10.3233/JAD-200936 [DOI] [PubMed] [Google Scholar]
- 124.van der Hoek MD, Nieuwenhuizen A, Keijer J, Ashford JW. The MemTrax test compared to the Montreal cognitive assessment estimation of mild cognitive impairment. J Alzheimers Dis. 2019;67(3):1045–1054. doi: 10.3233/JAD-181003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21(7):623–643. doi: 10.1016/j.acn.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 126.Campman C, van Ranst D, Meijer JW, Sitskoorn M. Computerized screening for cognitive impairment in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3075–3083. doi: 10.2147/COPD.S142871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Olofsen E, Noppers I, Niesters M, et al. Estimation of the contribution of norketamine to ketamine-induced acute pain relief and neurocognitive impairment in healthy volunteers. Anesthesiology. 2012;117(2):353–364. doi: 10.1097/ALN.0b013e31825b6c91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meador KJ, Seliger J, Boyd A, et al. Comparative neuropsychological effects of carbamazepine and eslicarbazepine acetate. Epilepsy Behav. 2019;94:151–157. doi: 10.1016/j.yebeh.2019.02.034 [DOI] [PubMed] [Google Scholar]
- 129.Littleton AC, Register-Mihalik JK, Guskiewicz KM. Test-retest reliability of a computerized concussion test: CNS vital signs. Sports Health. 2015;7(5):443–447. doi: 10.1177/1941738115586997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brooks BL, Plourde V, Fay-McClymont TB, MacAllister WS, Sherman EMS. Factor structure of the CNS vital signs computerized cognitive battery in youth with neurological diagnoses. Child Neuropsychol. 2019;25(7):980–991. doi: 10.1080/09297049.2019.1569609 [DOI] [PubMed] [Google Scholar]
- 131.Braverman ER, Chen TJ, Schoolfield J, et al. Delayed P300 latency correlates with abnormal test of variables of attention (TOVA) in adults and predicts early cognitive decline in a clinical setting. Adv Ther. 2006;23(4):582–600. doi: 10.1007/BF02850047 [DOI] [PubMed] [Google Scholar]
- 132.Braverman ER, Chen AL, Chen TJ, et al. Test of variables of attention (TOVA) as a predictor of early attention complaints, an antecedent to dementia. Neuropsychiatr Dis Treat. 2010;6:681–690. doi: 10.2147/NDT.S12243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bodkyn CN, Holroyd CB. Neural mechanisms of affective instability and cognitive control in substance use. Int J Psychophysiol. 2019;146:1–19. doi: 10.1016/j.ijpsycho.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 134.Gruber R, Grizenko N, Schwartz G, Bellingham J, Guzman R, Joober R. Performance on the continuous performance test in children with ADHD is associated with sleep efficiency. Sleep. 2007;30(8):1003–1009. doi: 10.1093/sleep/30.8.1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rotem A, Danieli Y, Ben-Sheetrit J, et al. Apparent lack of practice effects in the test of variables of attention (TOVA) in adult ADHD. Atten Defic Hyperact Disord. 2019;11(1):73–81. doi: 10.1007/s12402-018-0278-5 [DOI] [PubMed] [Google Scholar]
- 136.Wojcik CM, Beier M, Costello K, et al. Computerized neuropsychological assessment devices in multiple sclerosis: A systematic review. Mult Scler. 2019;25(14):1848–1869. doi: 10.1177/1352458519879094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lubar JF, Swartwood MO, Swartwood JN, O’Donnell PH. Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in T.O.V.A. scores, behavioral ratings, and WISC-R performance. Biofeedback Self Regul. 1995;20(1):83–99. doi: 10.1007/BF01712768 [DOI] [PubMed] [Google Scholar]
- 138.Ball SA, Nich C, Rounsaville BJ, Eagan D, Carroll KM. Millon clinical multiaxial inventory-III subtypes of opioid dependence: Validity and matching to behavioral therapies. J Consult Clin Psychol. 2004;72(4):698–711. doi: 10.1037/0022-006X.72.4.698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Strack S, Millon T. Contributions to the dimensional assessment of personality disorders using Millon’s model and the Millon Clinical Multiaxial Inventory (MCMI9-III). J Pers Assess. 2007;89(1):56–69. doi: 10.1080/00223890701357217 [DOI] [PubMed] [Google Scholar]
- 140.Choca JP, Grossman SD. Evolution of the millon clinical multiaxial inventory. J Pers Assess. 2015;97(6):541–549. doi: 10.1080/00223891.2015.1055753 [DOI] [PubMed] [Google Scholar]
- 141.Blum K, Chen TJ, Morse S, et al. Overcoming qEEG abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and polydrug abusers utilizing putative dopamine D2 agonist therapy: Part 2. Postgrad Med. 2010;122(6):214–226. doi: 10.3810/pgm.2010.11.2237 [DOI] [PubMed] [Google Scholar]
- 142.Sokhadze TM, Cannon RL, Trudeau DL. EEG biofeedback as a treatment for substance use disorders: Review, rating of efficacy, and recommendations for further research. Appl Psychophysiol Biofeedback. 2008;33(1):1–28. doi: 10.1007/s10484-007-9047-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van Dinteren R, Arns M, Jongsma ML, Kessels RP. P300 development across the lifespan: A systematic review and meta-analysis. PLoS One. 2014;9(2):e87347. doi: 10.1371/journal.pone.0087347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Braverman ER, Han D, Oscar-Berman M, et al. Menopause analytical hormonal correlate outcome study (MAHCOS) and the association to brain electrophysiology (P300) in a clinical setting. PLoS One. 2014;9(9):e105048. doi: 10.1371/journal.pone.0105048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Braverman ER, Blum K. P300 (latency) event-related potential: An accurate predictor of memory impairment. Clin Electroencephalogr. 2003;34(3):124–139. doi: 10.1177/155005940303400306 [DOI] [PubMed] [Google Scholar]
- 146.Braverman ER, Chen TJ, Chen AL, et al. Preliminary investigation of plasma levels of sex hormones and human growth factor(s), and P300 latency as correlates to cognitive decline as a function of gender. BMC Res Notes. 2009;2:126. doi: 10.1186/1756-0500-2-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang GY, Kydd R, Russell BR. Resting EEG and ERPs findings in methadone-substituted opiate users: A review. Acta Neurol Belg. 2015;115(4):539–546. doi: 10.1007/s13760-015-0476-2 [DOI] [PubMed] [Google Scholar]
- 148.Porcaro C, Balsters JH, Mantini D, Robertson IH, Wenderoth N. P3b amplitude as a signature of cognitive decline in the older population: An EEG study enhanced by Functional Source Separation. Neuroimage. 2019;184:535–546. doi: 10.1016/j.neuroimage.2018.09.057 [DOI] [PubMed] [Google Scholar]
- 149.Campanella S, Pogarell O, Boutros N. Event-related potentials in substance use disorders: A narrative review based on articles from 1984 to 2012. Clin EEG Neurosci. 2014;45(2):67–76. doi: 10.1177/1550059413495533 [DOI] [PubMed] [Google Scholar]
- 150.Kalechstein AD, De la Garza R 2nd, Newton TF, Green MF, Cook IA, Leuchter AF. Quantitative EEG abnormalities are associated with memory impairment in recently abstinent methamphetamine-dependent individuals. J Neuropsychiatry Clin Neurosci. 2009;21(3):254–258. doi: 10.1176/jnp.2009.21.3.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bjørk MH, Sand T, Bråthen G, et al. Quantitative EEG findings in patients with acute, brief depression combined with other fluctuating psychiatric symptoms: A controlled study from an acute psychiatric department. BMC Psychiatry. 2008;8:89. doi: 10.1186/1471-244X-8-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McVoy M, Lytle S, Fulchiero E, Aebi ME, Adeleye O, Sajatovic M. A systematic review of quantitative EEG as a possible biomarker in child psychiatric disorders. Psychiatry Res. 2019;279:331–344. doi: 10.1016/j.psychres.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 153.Haghighi M, Ludyga S, Rahimi B, et al. In patients suffering from major depressive disorders, quantitative EEG showed favorable changes in left and right prefrontal cortex. Psychiatry Res. 2017;251:137–141. doi: 10.1016/j.psychres.2017.02.012 [DOI] [PubMed] [Google Scholar]
- 154.Gerez M, Tello A. Clinical significance of focal topographic changes in the electroencephalogram (EEG) and evoked potentials (EP) of psychiatric patients. Brain Topogr. 1992;5(1):3–10. doi: 10.1007/BF01129964 [DOI] [PubMed] [Google Scholar]
- 155.Rouhani N, Niv Y. Signed and unsigned reward prediction errors dynamically enhance learning and memory. Elife. 2021;10:e61077. doi: 10.7554/eLife.61077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rouhani N, Norman KA, Niv Y. Dissociable effects of surprising rewards on learning and memory. J Exp Psychol Learn Mem Cogn. 2018;44(9):1430–1443. doi: 10.1037/xlm0000518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Katzman PL, Hartley CA. The value of choice facilitates subsequent memory across development. Cognition. 2020;199:104239. doi: 10.1016/j.cognition.2020.104239 [DOI] [PubMed] [Google Scholar]
- 158.Thanos PK, Tucci A, Stamos J, et al. Chronic forced exercise during adolescence decreases cocaine conditioned place preference in Lewis rats. Behav Brain Res. 2010;215(1):77–82. doi: 10.1016/j.bbr.2010.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dong GH, Wang Z, Dong H, et al. More stringent criteria are needed for diagnosing internet gaming disorder: Evidence from regional brain features and whole-brain functional connectivity multivariate pattern analyses. J Behav Addict. 2020;9(3):642–653. doi: 10.1556/2006.2020.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ismail S, Odland ML, Malik A, et al. The relationship between psychosocial circumstances and injuries in adolescents: An analysis of 87,269 individuals from 26 countries using the Global School-based Student Health Survey. PLoS Med. 2021;18(9):e1003722. doi: 10.1371/journal.pmed.1003722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kalindjian N, Hirot F, Stona AC, Huas C, Godart N. Early detection of eating disorders: A scoping review [published correction appears in Eat Weight Disord. 2021:]. Eat Weight Disord. 2022;27(1):21–68. doi: 10.1007/s40519-021-01164-x [DOI] [PubMed] [Google Scholar]
- 162.Murthy P, Mahadevan J, Chand PK. Treatment of substance use disorders with co-occurring severe mental health disorders. Curr Opin Psychiatry. 2019;32(4):293–299. doi: 10.1097/YCO.0000000000000510 [DOI] [PubMed] [Google Scholar]
- 163.Dervaux A Early detection and treatment of Alcohol dependence. Presse Med. 2018;47(6):532–534. [DOI] [PubMed] [Google Scholar]
- 164.Crunelle CL, van den Brink W, Moggi F, et al. International consensus statement on screening, diagnosis and treatment of substance use disorder patients with comorbid attention deficit/hyperactivity disorder. Eur Addict Res. 2018;24(1):43–51. doi: 10.1159/000487767168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bertulies-Esposito B, Sicotte R, Iyer SN, et al. Early detection and intervention for psychosis: Why and how? Sante Ment Que. 2021;46(2):45–83. [PubMed] [Google Scholar]
- 166.Gross M Hepatic cirrhosis: Early diagnosis and prevention of complications. MMW Fortschr Med. 2019;161(7):48–52. doi: 10.1007/s15006-019-0408-9 [DOI] [PubMed] [Google Scholar]
- 167.Falissard B Early detection of child and adolescent mental disorders: Some elements of a necessary debate. Eur Child Adolesc Psychiatry. 2016;25(10):1041–1043. doi: 10.1007/s00787-016-0906-6 [DOI] [PubMed] [Google Scholar]
- 168.Chan SY, Nickerson LD, Pathak R, Öngür D, Hall MH. Impact of substance use disorder on between-network brain connectivity in early psychosis. Schizophr Bull Open. 2022;3(1):sgac014. doi: 10.1093/schizbullopen/sgac014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Perugi G, Pallucchini A, Rizzato S, et al. Pharmacotherapeutic strategies for the treatment of attention-deficit hyperactivity (ADHD) disorder with comorbid substance-use disorder (SUD). Expert Opin Pharmacother. 2019;20(3):343–355. doi: 10.1080/14656566.2018.1551878 [DOI] [PubMed] [Google Scholar]
- 170.Fortier CB, Whitworth JW, Fonda JR, et al. Early adolescent binge drinking increases risk of psychopathology in post-9/11 veterans and mild traumatic brain injury exacerbates symptom severity. Alcohol Alcohol. 2021;56(1):116–124. doi: 10.1093/alcalc/agaa075 [DOI] [PubMed] [Google Scholar]
- 171.Blanco C, Volkow ND. Management of opioid use disorder in the USA: Present status and future directions. Lancet. 2019;393(10182):1760–1772. doi: 10.1016/S0140-6736(18)33078-2 [DOI] [PubMed] [Google Scholar]
- 172.Beckmann D, Lowman KL, Nargiso J, McKowen J, Watt L, Yule AM. Substance-induced psychosis in youth. Child Adolesc Psychiatr Clin N Am. 2020;29(1):131–143. doi: 10.1016/j.chc.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Aly SM, Omran A, Gaulier JM, Allorge D. Substance abuse among children. Arch Pediatr. 2020;27(8):480–484. doi: 10.1016/j.arcped.2020.09.006 [DOI] [PubMed] [Google Scholar]
- 174.Lalli M, Brouillette K, Kapczinski F, de Azevedo Cardoso T. Substance use as a risk factor for bipolar disorder: A systematic review. J Psychiatr Res. 2021;144:285–295. doi: 10.1016/j.jpsychires.2021.10.012 [DOI] [PubMed] [Google Scholar]
- 175.Kong LZ, Chandimali N, Han YH, et al. Pathogenesis, early diagnosis, and therapeutic management of alcoholic liver disease. Int J Mol Sci. 2019;20(11):2712. doi: 10.3390/ijms20112712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mondi CF, Giovanelli A, Ou SR, Reynolds AJ. Psychiatric and substance use disorders in a predominately low-income, black sample in early midlife. J Psychiatr Res. 2022;148:332–339. doi: 10.1016/j.jpsychires.2022.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Duffy A, Horrocks J, Milin R, Doucette S, Persson G, Grof P. Adolescent substance use disorder during the early stages of bipolar disorder: A prospective high-risk study. J Affect Disord. 2012;142(1–3):57–64. doi: 10.1016/j.jad.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 178.Benningfield MM, Riggs P, Stephan SH. The role of schools in substance use prevention and intervention. Child Adolesc Psychiatr Clin N Am. 2015;24(2):291–303. doi: 10.1016/j.chc.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 179.Hawk KF, Glick RL, Jey AR, et al. Emergency medicine research priorities for early intervention for substance use disorders. West J Emerg Med. 2019;20(2):386–392. doi: 10.5811/westjem.2019.1.39261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.González RA, Vélez-Pastrana MC, Blankers M, et al. Onset and severity of early disruptive behavioral disorders in treatment-seeking substance use disorder patients with and without attention-deficit/hyperactivity disorder. Eur Addict Res. 2020;26(4–5):211–222. doi: 10.1159/000508653 [DOI] [PubMed] [Google Scholar]
- 181.Kim Y, Kim K, Chartier KG, Wike TL, McDonald SE. Adverse childhood experience patterns, major depressive disorder, and substance use disorder in older adults. Aging Ment Health. 2021;25(3):484–491. doi: 10.1080/13607863.2019.1693974 [DOI] [PubMed] [Google Scholar]
- 182.Fuller-Thomson E, Lewis DA, Agbeyaka S. Attention-deficit/hyperactivity disorder and alcohol and other substance use disorders in young adulthood: Findings from a Canadian nationally representative survey. Alcohol Alcohol. 2022;57(3):385–395. doi: 10.1093/alcalc/agab048 [DOI] [PubMed] [Google Scholar]
- 183.Winters DE, Brandon-Friedman R, Yepes G, Hinckley JD. Systematic review and meta-analysis of socio-cognitive and socio-affective processes association with adolescent substance use. Drug Alcohol Depend. 2021;219:108479. doi: 10.1016/j.drugalcdep.2020.108479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Speed TJ, Hanks L, Turner G, et al. A comparison of cognitive behavioral therapy for insomnia to standard of care in an outpatient substance use disorder clinic embedded within a therapeutic community: A RE-AIM framework evaluation. Trials. 2022;23(1):965. doi: 10.1186/s13063-022-06885-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Davis JP, Janssen T, Dworkin ER, Dumas TM, Goldbach J, Monterosso J. Influences of victimization and comorbid conditions on substance use disorder outcomes in justice-involved youth: A discrete time survival mixture analysis. Dev Psychopathol. 2020;32(3):1045–1058. doi: 10.1017/S0954579419000750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Veseth M, Svendsen TS, Nesvaag S, Moltu C, Davidson L, Bjornestad J. “And then the rest happened”- A qualitative exploration of the role that meaningful activities play in recovery processes for people with a diagnosis of substance use disorder. Subst Abus. 2022;43(1):260–266. doi: 10.1080/08897077.2021.1941506 [DOI] [PubMed] [Google Scholar]
- 187.Coetzee C, Schellekens AFA, Truter I, Meyer A. Effect of past pharmacotherapy for attention-deficit/hyperactivity disorder on substance use disorder. Eur Addict Res. 2023;29(1):9–18. doi: 10.1159/000526386 [DOI] [PubMed] [Google Scholar]
- 188.Messinger JC, Suzuki J. Recognizing and reducing the impact of trauma of hospitalization: Considerations for persons who use drugs. J Addict Med. 2022;16(1):7–9. doi: 10.1097/ADM.0000000000000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Long Y, Pan N, Ji S, et al. Distinct brain structural abnormalities in attention-deficit/hyperactivity disorder and substance use disorders: A comparative meta-analysis. Transl Psychiatry. 2022;12(1):368. doi: 10.1038/s41398-022-02130-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Darbinian N, Darbinyan A, Sinard J, et al. Molecular markers in maternal blood exosomes allow early detection of fetal alcohol spectrum disorders. Int J Mol Sci. 2022;24(1):135. doi: 10.3390/ijms24010135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Metz VE, Palzes VA, Kline-Simon AH, et al. Substance use disorders among primary care patients screening positive for unhealthy alcohol use. Fam Pract. 2022;39(2):226–233. doi: 10.1093/fampra/cmab171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Klein RJ, Gyorda JA, Jacobson NC. Anxiety, depression, and substance experimentation in childhood. PLoS One. 2022;17(5):e0265239. doi: 10.1371/journal.pone.0265239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schnabl B, Arteel GE, Stickel F, et al. Liver specific, systemic and genetic contributors to alcohol-related liver disease progression. Leberspezifische, systemische und genetische Faktoren, die zum Fortschreiten der alkoholbedingten Lebererkrankung beitragen. Z Gastroenterol. 2022;60(1):36–44. doi: 10.1055/a-1714-9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.West ML, Sharif S. Cannabis and psychosis. Child Adolesc Psychiatr Clin N Am. 2023;32(1):69–83. doi: 10.1016/j.chc.2022.07.004 [DOI] [PubMed] [Google Scholar]
- 195.Gainer DM, Wong C, Embree JA, Sardesh N, Amin A, Lester N. Effects of telehealth on dropout and retention in care among treatment-seeking individuals with substance use disorder: A retrospective cohort study. Subst Use Misuse. 2023;58(4):481–490. doi: 10.1080/10826084.2023.2167496 [DOI] [PubMed] [Google Scholar]
- 196.Thakral N, Deutsch-Link S, Singal AK. Therapeutic pipeline in alcohol-associated liver disease. Semin Liver Dis. 2023;43(1):60–76. doi: 10.1055/s-0042-1759614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sambo D, Goldman D. Genetic influences on fetal alcohol spectrum disorder. Genes (Basel). 2023;14(1):195. doi: 10.3390/genes14010195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gómez-Medina C, Melo L, Martí-Aguado D, Bataller R. Subclinical versus advanced forms of alcohol-related liver disease: Need for early detection. Clin Mol Hepatol. 2023;29(1):1–15. doi: 10.3350/cmh.2022.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kumar P, Sharma A, Kumar D, Sharma L. Use of spectroscopic methods and their clinical applications in drug abuse: A review. Crit Rev Anal Chem. 2023;53(2):360–373. doi: 10.1080/10408347.2021.1958196 [DOI] [PubMed] [Google Scholar]
- 200.Tsai AP, Youngstrom EA, Gadow KD, et al. Diagnostic accuracy of the Child and adolescent symptom inventory (CASI-4R) substance use subscale in detecting substance use disorders in youth. Psychol Assess. 2023;35(2):178–187. doi: 10.1037/pas0001182 [DOI] [PubMed] [Google Scholar]
- 201.Patrick ME, Pang YC, Jang BJ, Arterberry BJ, Terry-McElrath YM. Alcohol use disorder symptoms reported during midlife: Results from the monitoring the future study among US adults at modal ages 50, 55, and 60. Subst Use Misuse. 2023;58(3):380–388. doi: 10.1080/10826084.2022.2161826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ishii A, Sato K, Kusakabe K, Kato N, Wada T. Identification and quantitative analysis of 2-fluoromethamphetamine and its metabolites in human urine [published correction appears in J Anal Toxicol. 2022]. J Anal Toxicol. 2023;47(1):59–65. doi: 10.1093/jat/bkac026 [DOI] [PubMed] [Google Scholar]
- 203.Greenwald MK, Moses TEH, Lundahl LH, Roehrs TA. Anhedonia modulates benzodiazepine and opioid demand among persons in treatment for opioid use disorder. Front Psychiatry. 2023;14:1103739. doi: 10.3389/fpsyt.2023.1103739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hintze TD, Miller JL, Neely SB, Lim SY, Gupta N, Johnson PN. Characterization of early versus late opioid iatrogenic withdrawal syndrome in critically ill children transitioning from fentanyl -infusions to methadone. J Opioid Manag. 2023;19(1):43–56. doi: 10.5055/jom.2023.0758 [DOI] [PubMed] [Google Scholar]
- 205.Dobson MS, Blackhall LJ. Letter to the Editor: Universal screening for substance use disorder in a palliative care clinic: Results and longitudinal outcomes for patients with cancer. J Palliat Med. 2023;26(2):160–161. doi: 10.1089/jpm.2022.0502 [DOI] [PubMed] [Google Scholar]
- 206.Sharma K, Ghosh A, Krishnan NC, et al. Digital screening and brief intervention for illicit drug misuse in college students: A mixed methods, pilot, cluster, randomized trial from India. Asian J Psychiatr. 2023;81:103432. doi: 10.1016/j.ajp.2022.103432 [DOI] [PubMed] [Google Scholar]
- 207.Henderson EMA, Tappin D, Young D, Favretto D, Mactier H. Assessing maternal alcohol consumption in pregnancy: Does phosphatidylethanol measured from day 5 newborn blood spot cards have any value? An observational, population-based study. Arch Dis Child. 2023;108(1):36–41. doi: 10.1136/archdischild-2022-324394 [DOI] [PubMed] [Google Scholar]
- 208.Syrjanen R, Schumann J, Fitzgerald J, et al. The emerging drugs network of Australia - Victoria clinical registry: A state-wide illicit substance surveillance and alert network. Emerg Med Australas. 2023;35(1):82–88. doi: 10.1111/1742-6723.14059 [DOI] [PubMed] [Google Scholar]
- 209.Pautrat M, Renard C, Riffault V, et al. Cross-analyzing addiction specialist and patient opinions and experiences about addictive disorder screening in primary care to identify interaction-related obstacles: A qualitative study. Subst Abuse Treat Prev Policy. 2023;18(1):12. doi: 10.1186/s13011-023-00522-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Blum K, Han D, Bowirrat A, et al. Genetic addiction risk and psychological profiling analyses for “preaddiction” severity index. J Pers Med. 2022;12(11):1772. doi: 10.3390/jpm12111772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci. 2015;16(5):305–312. doi: 10.1038/nrn3939 [DOI] [PubMed] [Google Scholar]
- 212.Merians AN, Spiller T, Harpaz-Rotem I, Krystal JH, Pietrzak RH. Post-traumatic stress disorder. Med Clin North Am. 2023;107(1):85–99. doi: 10.1016/j.mcna.2022.04.003 [DOI] [PubMed] [Google Scholar]
- 213.Zhou J, Friedel M, Rosmarin DH, Pirutinsky S. Internet addiction and the treatment of depression? A prospective naturalistic outcome study. Cyberpsychol Behav Soc Netw. 2023;26(2):121–126. doi: 10.1089/cyber.2022.0184 [DOI] [PubMed] [Google Scholar]
- 214.Ray LA, Nieto SJ, Grodin EN. Translational models of addiction phenotypes to advance addiction pharmacotherapy. Ann N Y Acad Sci. 2023;1519(1):118–128. doi: 10.1111/nyas.14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ghosh A, Sharma N, Noble D, et al. Predictors of treatment completion in an inpatient substance use treatment service in India. J Addict Med. 2023;17(2):e101–e109. doi: 10.1097/ADM.0000000000001076 [DOI] [PubMed] [Google Scholar]
- 216.Osińska M, Towpik I, Sanchak Y, Franek E, Śliwczyński A, Walicka M. Cost of surgical treatment of obesity and its impact on healthcare expense-nationwide data from a polish registry. Int J Environ Res Public Health. 2023;20(2):1118. doi: 10.3390/ijerph20021118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lyes M, Yang KH, Castellanos J, Furnish T. Microdosing psilocybin for chronic pain: A case series. Pain. 2023;164(4):698–702. doi: 10.1097/j.pain.0000000000002778 [DOI] [PubMed] [Google Scholar]
- 218.Cunningham CO, Starrels JL. Guideline promoting buprenorphine for treatment of chronic pain: Transformative yet underdeveloped. Ann Intern Med. 2023;176(3):419–420. doi: 10.7326/M23-0229 [DOI] [PubMed] [Google Scholar]
- 219.Bernal-Sobrino JL, Arias-Horcajadas F, Varela-Rodríguez C, et al. A 3-year retrospective study of the impact of integrating an addiction liaison team into an outpatient alcoholism treatment programme. Alcohol Alcohol. 2023;58(5):515–522. doi: 10.1093/alcalc/agad004 [DOI] [PubMed] [Google Scholar]
- 220.Sussman S, Galimov A, Ayala N, Sinclair DL. Web-based evidence on the treatment of behavioral addictions in United States model treatment centers. Eval Health Prof. 2023;46(1):23–29. doi: 10.1177/01632787221130543 [DOI] [PubMed] [Google Scholar]
- 221.Natal S, Young CC, Kaur K, et al. Applications of isradipine in human addiction studies: A systematic literature review. Exp Clin Psychopharmacol. 2023;31(2):507–522. doi: 10.1037/pha0000633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rubenis AJ, Nation JA, Katz EC, Arunogiri S. Increasing attendance in addiction treatment with limited resources: A narrative review. J Addict Med. 2023;17(1):13–20. doi: 10.1097/ADM.0000000000001033 [DOI] [PubMed] [Google Scholar]
- 223.Husain JM, Cromartie D, Fitzelle-Jones E, Brochier A, Borba CPC, Montalvo C. A qualitative analysis of barriers to opioid agonist treatment for racial/ethnic minoritized populations. J Subst Abuse Treat. 2023;144:108918. doi: 10.1016/j.jsat.2022.108918 [DOI] [PubMed] [Google Scholar]
- 224.Salehi M, Abbaspour Z, Molana A, Shahini N. Impulsivity, inhibition, and internet addiction in medical students of North of Iran. Front Psychiatry. 2023;13:1002625. doi: 10.3389/fpsyt.2022.1002625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Barnett KB, Amason J, Wagner D, Ray HE. Characteristics of substance-addicted mothers that predict graduation from the family treatment court. J Nurs Scholarsh. 2023;55(3):637–645. doi: 10.1111/jnu.12890 [DOI] [PubMed] [Google Scholar]
- 226.Basenach L, Renneberg B, Salbach H, Dreier M, Wölfling K. Systematic reviews and meta-analyses of treatment interventions for Internet use disorders: Critical analysis of the methodical quality according to the PRISMA guidelines. J Behav Addict. 2023;12(1):9–25. doi: 10.1556/2006.2022.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.van der Meer PB, Fuentes JJ, Kaptein AA, et al. Therapeutic effect of psilocybin in addiction: A systematic review. Front Psychiatry. 2023;14:1134454. doi: 10.3389/fpsyt.2023.1134454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cowie ME, Hodgins DC. Contingency management in Canadian addiction treatment: Provider attitudes and use. J Stud Alcohol Drugs. 2023;84(1):89–96. doi: 10.15288/jsad.22-00036 [DOI] [PubMed] [Google Scholar]
- 229.Mide M, Arvidson E, Gordh AS. Clinical differences of mild, moderate, and severe gambling disorder in a sample of treatment seeking pathological gamblers in Sweden. J Gambl Stud. 2023;39(3):1129–1153. doi: 10.1007/s10899-022-10183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Péter L, Paksi B, Magi A, et al. Severity of behavioral addiction symptoms among young adults using non-prescribed sedatives/hypnotics. Addict Behav Rep. 2023;17:100485. doi: 10.1016/j.abrep.2023.100485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tauscher JS, DePue MK, Swank J, Salloum RG. Determinants of preference for telehealth versus in-person treatment for substance use disorders: A discrete choice experiment. J Subst Use Addict Treat. 2023;146:208938. doi: 10.1016/j.josat.2022.208938 [DOI] [PubMed] [Google Scholar]
- 232.Lo C, Mane M, Kim JH, et al. Treating addiction with deep brain stimulation: Ethical and legal considerations. Int J Drug Policy. 2023;113:103964. doi: 10.1016/j.drugpo.2023.103964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Davidson M, Rashidi N, Hossain MK, Raza A, Nurgali K, Apostolopoulos V. Tryptophan and substance abuse: Mechanisms and impact. Int J Mol Sci. 2023;24(3):2737. doi: 10.3390/ijms24032737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wolfschlag M, Håkansson A. Drug-induced gambling disorder: Epidemiology, neurobiology, and management. Pharmaceut Med. 2023;37(1):37–52. doi: 10.1007/s40290-022-00453-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Brandt L, Hu MC, Liu Y, et al. Risk of experiencing an overdose event for patients undergoing treatment with medication for opioid use disorder. Am J Psychiatry. 2023;180(5):386–394. doi: 10.1176/appi.ajp.20220312 [DOI] [PubMed] [Google Scholar]
- 236.Zhang X, Yang H, Zhang K, et al. Effects of exercise or tai chi on Internet addiction in college students and the potential role of gut microbiota: A randomized controlled trial. J Affect Disord. 2023;327:404–415. doi: 10.1016/j.jad.2023.02.002 [DOI] [PubMed] [Google Scholar]
- 237.Potenza MN, Baldacchino AM. Virtually there and back again: An overview of the 2021 and 2022 scientific annual conferences of the international society of Addiction medicine. Drug Alcohol Depend. 2023;245:109805. doi: 10.1016/j.drugalcdep.2023.109805 [DOI] [PubMed] [Google Scholar]
- 238.Ellis JD, Rabinowitz JA, Ware OD, Wells J, Dunn KE, Huhn AS. Patterns of polysubstance use and clinical comorbidity among persons seeking substance use treatment: An observational study. J Subst Use Addict Treat. 2023;146:208932. doi: 10.1016/j.josat.2022.208932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lake S, Buxton J, Walsh Z, et al. Methadone dose, Cannabis use, and treatment retention: Findings from a community-based sample of people who use unregulated drugs. J Addict Med. 2023;17(1):e18–e26. doi: 10.1097/ADM.0000000000001032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hickey TR, Henry JT, Edens EL, Gordon AJ, Acampora G. Perioperative management of extended-release buprenorphine. J Addict Med. 2023;17(1):e67–e71. doi: 10.1097/ADM.0000000000001024 [DOI] [PubMed] [Google Scholar]
- 241.Aslan M, Sala M, Gueorguieva R, Garrison KA. A network analysis of cigarette craving. Nicotine Tob Res. 2023;25(6):1155–1163. doi: 10.1093/ntr/ntad021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bonnet U, Kanti AK, Scherbaum N, Specka M. The role of gabapentinoids in the substance use pattern of adult Germans seeking inpatient detoxification treatment - A pilot study. J Psychoactive Drugs. 2023;55(1):102–111. doi: 10.1080/02791072.2022.2050858 [DOI] [PubMed] [Google Scholar]
- 243.Novet B, Simon O, Bonjour S, et al. Addiction: What’s new in 2022. Rev Med Suisse. 2023;19(N 809–10):12–15. doi: 10.53738/REVMED.2023.19.809-10.12 [DOI] [PubMed] [Google Scholar]
- 244.Yue Y, Zou L, Li H, et al. Therapeutic effect of implanted and non-invasive vagus nerve stimulation on heroin-induced anxiety. Biochem Biophys Res Commun. 2023;652:46–54. doi: 10.1016/j.bbrc.2023.02.041 [DOI] [PubMed] [Google Scholar]
- 245.Wunsch C, Wightman R, Pratty C, et al. Thirty-day treatment continuation after audio-only buprenorphine telehealth initiation. J Addict Med. 2023;17(2):206–209. doi: 10.1097/ADM.0000000000001077 [DOI] [PubMed] [Google Scholar]
- 246.Basheer M, Hassan Z, Gam LH. Upregulation of brain’s calcium binding proteins in mitragynine dependence: A potential cellular mechanism to addiction. Int J Med Sci. 2023;20(1):102–113. doi: 10.7150/ijms.78861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bach P, de Timary P, Gründer G, Cumming P. molecular imaging studies of alcohol use disorder. Curr Top Behav Neurosci. 2023;1:1–31. doi: 10.1007/7854_2022_414 [DOI] [PubMed] [Google Scholar]
- 248.Bagley SM, Schoenberger SF, dellaBitta V, et al. Ambivalence and stigma beliefs about medication treatment among young adults with opioid use disorder: A qualitative exploration of young adults’ perspectives. J Adolesc Health. 2023;72(1):105–110. doi: 10.1016/j.jadohealth.2022.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Singh N, Varshney U. Adaptive interventions for opioid prescription management and consumption monitoring. J Am Med Inform Assoc. 2023;30(3):511–528. doi: 10.1093/jamia/ocac253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Santino F, Gentilucci L. Design of κ-opioid receptor agonists for the development of potential treatments of pain with reduced side effects. Molecules. 2023;28(1):346. doi: 10.3390/molecules28010346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hendricks MA, El Ibrahimi S, Ritter GA, et al. Association of household opioid availability with opioid overdose. JAMA Netw Open. 2023;6(3):e233385. doi: 10.1001/jamanetworkopen.2023.3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Laks J, Walley AY, Bagley SM, et al. Developing a women’s health track within addiction medicine fellowship: Reflections and inspirations. Addict Sci Clin Pract. 2023;18(1):3. doi: 10.1186/s13722-022-00357-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mestre-Bach G, Potenza MN. Potential biological markers and treatment implications for binge eating disorder and behavioral addictions. Nutrients. 2023;15(4):827. doi: 10.3390/nu15040827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dydyk AM, Sizemore DC, Trachsel LA, Conermann T, Porter BR. Vermont controlled substance abuse, diversion, storage, disposal, monitoring, and legal issues. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 255.Sussman S, Sinclair DL. Treating behavioural addictions that lack diagnostic criteria. Nature. 2023;613(7942):27. doi: 10.1038/d41586-022-04567-7 [DOI] [PubMed] [Google Scholar]
- 256.Elbau IG, Lynch CJ, Downar J, et al. Functional connectivity mapping for rTMS target selection in depression. Am J Psychiatry. 2023;180(3):230–240. doi: 10.1176/appi.ajp.20220306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mergelsberg ELP, de Ruijter D, Crone MR, Smit ES, Hoving C. Active ingredients of interventions improving smoking cessation support by dutch primary care providers: A systematic review. Eval Health Prof. 2023;46(1):3–22. doi: 10.1177/01632787221099941 [DOI] [PubMed] [Google Scholar]
- 258.Zhang W, Pu J, He R, et al. Demographic characteristics, family environment and psychosocial factors affecting internet addiction in Chinese adolescents. J Affect Disord. 2022;315:130–138. doi: 10.1016/j.jad.2022.07.053 [DOI] [PubMed] [Google Scholar]
- 259.Guinchat V, Baeza-Velasco C, Bulbena A, Castori M. Editorial: Neurodevelopmental, neuropsychiatric and psychosocial correlates of joint hypermobility and related disorders. Front Psychiatry. 2022;13:1109515. doi: 10.3389/fpsyt.2022.1109515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hughes JW, Serber ER, Kuhn T. Psychosocial management in cardiac rehabilitation: Current practices, recommendations, and opportunities. Prog Cardiovasc Dis. 2022;73:76–83. doi: 10.1016/j.pcad.2021.12.006 [DOI] [PubMed] [Google Scholar]
- 261.Lakhdir MPA, Hameed AN, Hasnani FB, et al. Demographic and psychosocial factors associated with internet addiction among the Pakistani population during COVID-19: A web-based survey. Inquiry. 2022;59:469580221138671. doi: 10.1177/00469580221138671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Solé B, Varo C, Torrent C, et al. Sex differences in neurocognitive and psychosocial functioning in bipolar disorder. J Affect Disord. 2022;296:208–215. doi: 10.1016/j.jad.2021.09.066 [DOI] [PubMed] [Google Scholar]
- 263.Martin-Fernandez KW, Creel DB, Schuh LM. Psychosocial and behavioral correlates of weight loss 12 to 15 years after bariatric surgery. J Behav Med. 2022;45(2):252–259. doi: 10.1007/s10865-021-00263-5 [DOI] [PubMed] [Google Scholar]
- 264.Romm KF, Wang Y, Duan Z, et al. Psychosocial predictors of longitudinal changes in tobacco and cannabis use among young adults. Addict Behav. 2022;129:107264. doi: 10.1016/j.addbeh.2022.107264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wu YQ, Liu F, Chan KQ, et al. Childhood psychological maltreatment and internet gaming addiction in Chinese adolescents: Mediation roles of maladaptive emotion regulation strategies and psychosocial problems. Child Abuse Negl. 2022;129:105669. doi: 10.1016/j.chiabu.2022.105669 [DOI] [PubMed] [Google Scholar]
- 266.Daldegan-Bueno D, Révész D, Morais PR, Barbosa PCR, Maia LO. Psychosocial and drug use assessment of regular vs. non-regular Ayahuasca users in a Brazilian sample: A web-based survey. Subst Use Misuse. 2022;57(7):1072–1081. doi: 10.1080/10826084.2022.2063896 [DOI] [PubMed] [Google Scholar]
- 267.Farooqui AM, Arya A, Singh A, Dalal PK. Psychiatric comorbidity, psychosocial problems, and functioning of people who inject opioids: An observational study. Addict Health. 2022;14(3):218–223. doi: 10.34172/ahj.2022.1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Arslan G, Coşkun M. Social exclusion, self-forgiveness, mindfulness, and internet addiction in college students: A moderated mediation approach. Int J Ment Health Addict. 2022;20(4):2165–2179. doi: 10.1007/s11469-021-00506-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Blakey SM, Dillon KH, Wagner HR, et al. Psychosocial well-being among veterans with posttraumatic stress disorder and substance use disorder. Psychol Trauma. 2022;14(3):421–430. doi: 10.1037/tra0001018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mottershead R The social prescribing of psychosocial interventions in the treatment of addictions and substance use disorders with military veterans: A reclamation of identity and belonging. F1000Res. 2022;11:944. doi: 10.12688/f1000research.124768.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Mehmood Qadri H, Waheed A, Munawar A, et al. Physiological, psychosocial and substance abuse effects of pornography addiction: A narrative review. Cureus. 2023;15(1):e33703. doi: 10.7759/cureus.33703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gallassi AD, Nakano EY, de Miranda KG, Dos Santos JE, da Silva Rodrigues D, de Oliveira FM. The increased alcohol and marijuana use associated with the quality of life and psychosocial aspects: A study during the Covid-19 pandemic in a Brazilian University community. Int J Ment Health Addict. 2022:1–21. doi: 10.1007/s11469-022-00937-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Offer S, Alexander E, Barbara K, Hemmingsson E, Flint SW, Lawrence BJ. The association between childhood trauma and overweight and obesity in young adults: The mediating role of food addiction. Eat Weight Disord. 2022;27(8):3257–3266. doi: 10.1007/s40519-022-01454-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Remondi C, Compare A, Tasca GA, et al. The effects of attachment, temperament, and self-esteem on technology addiction: A mediation model among young adults. Cyberpsychol Behav Soc Netw. 2022;25(4):258–263. doi: 10.1089/cyber.2021.0237 [DOI] [PubMed] [Google Scholar]
- 275.Feng H, Gao K, Chen D, et al. Machine learning analysis of cocaine addiction informed by DAT, SERT, and NET-based interactome networks. J Chem Theory Comput. 2022;18(4):2703–2719. doi: 10.1021/acs.jctc.2c00002 [DOI] [PubMed] [Google Scholar]
- 276.Lee CM, Calhoun BH, Abdallah DA, et al. Simultaneous alcohol and Marijuana use among young adults: A scoping review of prevalence, patterns, psychosocial correlates, and consequences. Alcohol Res. 2022;42(1):8. doi: 10.35946/arcr.v42.1.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lu H, Xie C, Lian P, Yu C, Xie Y. Psychosocial factors predict the level of aggression of people with drug addiction: A machine learning approach. Psychol Health Med. 2022;27(5):1168–1175. doi: 10.1080/13548506.2021.1910321 [DOI] [PubMed] [Google Scholar]
- 278.André F, Munck I, Håkansson A, Claesdotter-Knutsson E. Game addiction scale for adolescents-psychometric analyses of gaming behavior, gender differences and ADHD. Front Psychiatry. 2022;13:791254. doi: 10.3389/fpsyt.2022.791254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sharma MK, Anand N, Amudhan S, Vashisht A. Online gaming and tilting: Psychosocial exploration for promotion of emotional regulation. Int J Soc Psychiatry. 2022;68(3):699–701. doi: 10.1177/00207640211028602 [DOI] [PubMed] [Google Scholar]
- 280.Putchavayala CK, Singh D, Sashidharan RK. A perspective of yoga on smartphone addiction: A narrative review. J Family Med Prim Care. 2022;11(6):2284–2291. doi: 10.4103/jfmpc.jfmpc_1765_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Unwin J, Delon C, Giæver H, et al. Low carbohydrate and psychoeducational programs show promise for the treatment of ultra-processed food addiction. Front Psychiatry. 2022;13:1005523. doi: 10.3389/fpsyt.2022.1005523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Andrade ALM, Di Girolamo Martins G, Scatena A, et al. The effect of psychosocial interventions for reducing co-occurring symptoms of depression and anxiety in individuals with problematic internet use: A systematic review and meta-analysis. Int J Ment Health Addict. 2022;21:4141–62. doi: 10.1007/s11469-022-00846-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kacar D, Ayaz-Alkaya S. The effect of traditional children’s games on internet addiction, social skills and stress level. Arch Psychiatr Nurs. 2022;40:50–55. doi: 10.1016/j.apnu.2022.04.007 [DOI] [PubMed] [Google Scholar]
- 284.Yang W, Singla R, Maheshwari O, Fontaine CJ, Gil-Mohapel J. Alcohol use disorder: Neurobiology and therapeutics. Biomedicines. 2022;10(5):1192. doi: 10.3390/biomedicines10051192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Müller KW, Scherer L. Excessive use patterns and internet use disorders: Effects on psychosocial and cognitive development in adolescence. Prax Kinderpsychol Kinderpsychiatr. 2022;71:345–361. doi: 10.13109/prkk.2022.71.4.345 [DOI] [PubMed] [Google Scholar]
- 286.Bhatia U, Velleman R, Velleman G, Garber A, Catalano A, Nadkarni A. Supporting addiction affected families effectively: A feasibility randomised controlled trial of a psychosocial intervention delivered by lay counsellors in Goa, India. Glob Ment Health (Camb). 2022;9:448–459. doi: 10.1017/gmh.2022.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kolla NJ, Smaragdi A, Gainham G, et al. Psychosocial intervention for youth with high externalizing behaviors and aggression is associated with improvement in impulsivity and brain gray matter volume changes. Front Psychiatry. 2022;12:788240. doi: 10.3389/fpsyt.2021.788240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bozkurt M Neuroscientific basis of treatment for substance use disorders. Noro Psikiyatr Ars. 2022;59(Suppl 1):S75–S80. doi: 10.29399/npa.28172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Shyu C, Chavez S, Boileau I, Le Foll B. Quantifying GABA in addiction: A review of proton magnetic resonance spectroscopy studies. Brain Sci. 2022;12(7):918. doi: 10.3390/brainsci12070918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Yue H, Pena E. Addiction psychotherapeutic care. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 291.Dresp-Langley B, Hutt A. Digital addiction and sleep. Int J Environ Res Public Health. 2022;19(11):6910. doi: 10.3390/ijerph19116910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.López-Muciño LA, García-García F, Cueto-Escobedo J, Acosta-Hernández M, Venebra-Muñoz A, Rodríguez-Alba JC. Sleep loss and addiction. Neurosci Biobehav Rev. 2022;141:104832. doi: 10.1016/j.neubiorev.2022.104832 [DOI] [PubMed] [Google Scholar]
- 293.Chang FC, Chiu CH, Chen PH, et al. Smartphone addiction and victimization predicts sleep problems and depression among children. J Pediatr Nurs. 2022;64:e24–e31. doi: 10.1016/j.pedn.2022.01.009 [DOI] [PubMed] [Google Scholar]
- 294.Bian WJ, Atrooz F, Patel S, Rababa’h AM. Editorial: Sleep deprivation, circadian misalignment and addiction vulnerability in adolescents. Front Neurosci. 2022;16:940039. doi: 10.3389/fnins.2022.940039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Day E, Daly C. Clinical management of the alcohol withdrawal syndrome. Addiction. 2022;117(3):804–814. doi: 10.1111/add.15647 [DOI] [PubMed] [Google Scholar]
- 296.Kim E, Lee K. Relationship between smartphone addiction and sleep satisfaction: A cross-sectional study on Korean adolescents. Healthcare (Basel). 2022;10(7):1326. doi: 10.3390/healthcare10071326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Boness CL, Hasler BP, Sheehan H, Pedersen SL. Associations between specific sleep and circadian characteristics and alcohol use disorder criteria and problems. Addict Behav. 2022;132:107348. doi: 10.1016/j.addbeh.2022.107348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zaman M, Babar MS, Babar M, et al. Prevalence of gaming addiction and its impact on sleep quality: A cross-sectional study from Pakistan. Ann Med Surg (Lond). 2022;78:103641. doi: 10.1016/j.amsu.2022.103641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Acikgoz A, Acikgoz B, Acikgoz O. The effect of internet addiction and smartphone addiction on sleep quality among Turkish adolescents. PeerJ. 2022;10:e12876. doi: 10.7717/peerj.12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Doke M, McLaughlin JP, Baniasadi H, Samikkannu T. Sleep disorder and cocaine abuse impact purine and pyrimidine nucleotide metabolic signatures. Metabolites. 2022;12(9):869. doi: 10.3390/metabo12090869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Berro LF, Overton JS, Rowlett JK. Methamphetamine-induced sleep impairments and subsequent slow-wave and rapid eye movement sleep rebound in male Rhesus monkeys. Front Neurosci. 2022;16:866971. doi: 10.3389/fnins.2022.866971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.He J, Wang R, Liu J, Yip P. Effects of adverse childhood experiences on sleep quality in people with drug addiction and non-addicts and the role of resilience between them. Psychol Rep. 2023;126(5):2158–2171. doi: 10.1177/00332941221076776 [DOI] [PubMed] [Google Scholar]
- 303.Lu JX, Zhai YJ, Chen J, et al. Network analysis of internet addiction and sleep disturbance symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 2023;125:110737. doi: 10.1016/j.pnpbp.2023.110737 [DOI] [PubMed] [Google Scholar]
- 304.Kavaliotis E, Boardman JM, Clark JW, Ogeil RP, Verdejo-García A, Drummond SPA. The relationship between sleep and appetitive conditioning: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2023;144:105001. doi: 10.1016/j.neubiorev.2022.105001 [DOI] [PubMed] [Google Scholar]
- 305.Yang H, Yang K, Zhang L, et al. Acupuncture ameliorates mobile phone addiction with sleep disorders and restores salivary metabolites rhythm. Front Psychiatry. 2023;14:1106100. doi: 10.3389/fpsyt.2023.1106100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Mlouki I, Naimi A, Sioud I, Bouanene I, El Mhamdi S. Adverse childhood experiences and sleep disorders among Tunisian adolescents: The mediating role of internet addiction. Child Abuse Negl. 2023;136:106028. doi: 10.1016/j.chiabu.2023.106028 [DOI] [PubMed] [Google Scholar]
- 307.Kao PC. The interrelationship of loneliness, smartphone addiction, sleep quality, and students’ attention in english as a foreign language class. Int J Environ Res Public Health. 2023;20(4):3460. doi: 10.3390/ijerph20043460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ouellet J, Spinney S, Assaf R, et al. Sleep as a mediator between Cannabis use and psychosis vulnerability: A longitudinal cohort study. Schizophr Bull Open. 2022;4(1):sgac072. doi: 10.1093/schizbullopen/sgac072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Xie G, Wu Q, Guo X, Zhang J, Yin D. Psychological resilience buffers the association between cell phone addiction and sleep quality among college students in Jiangsu Province, China. Front Psychiatry. 2023;14:1105840. doi: 10.3389/fpsyt.2023.1105840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Karim MR, Haque MJ, Akhter S, Ahmed HU. Facebook addiction and its related factors among medical students; a cross- sectional study in Bangladesh. PLoS Glob Public Health. 2023;3(2):e0001597. doi: 10.1371/journal.pgph.0001597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Galanter M, White WL, Khalsa J, Hansen H. A scoping review of spirituality in relation to substance use disorders: Psychological, biological, and cultural issues. J Addict Dis. 2023:1–9. doi: 10.1080/10550887.2023.2174785 [DOI] [PubMed] [Google Scholar]
- 312.Hassan AN, Agabani Z, Ahmed F, Shapiro B, Le Foll B. The impact of religiosity/spirituality on slowing the progression of substance use: Based on the national epidemiological survey of alcohol and related conditions (NESARC-III). Int J Soc Psychiatry. 2023;69(6):1399–1408. doi: 10.1177/00207640231162819 [DOI] [PubMed] [Google Scholar]
- 313.Galanter M, White WL, Hunter B. Narcotics Anonymous members in recovery from methamphetamine use disorder. Am J Addict. 2023;32(1):54–59. doi: 10.1111/ajad.13362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Johnson F Jr., RedCloud A, Mootz J, et al. Community member perspectives on adapting the cascade of care for opioid use disorder for a tribal nation in the United States. Addiction. 2023;118(8):1540–1548. doi: 10.1111/add.16184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Rosmarin DH, Pirutinsky S, Kaufman CC, Harris SK, Sneider JT, Silveri MM. Religious affiliation protects against alcohol/substance use initiation: A prospective study among healthy adolescents. J Adolesc. 2023;95(2):372–381. doi: 10.1002/jad.12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Sharif-Nia H, Froelicher ES, Hejazi S, Moshtagh M, Goudarzian AH, Ebrahimi F. Cross-cultural evaluation of the psychometric properties of the spiritual well-being scale: A systematic review. J Relig Health. 2023;62(3):2226–2251. doi: 10.1007/s10943-023-01778-8 [DOI] [PubMed] [Google Scholar]


