Abstract
Quantification of trace element concentrations in human and animal tissues has acquired great importance in the last few years, considering the pivotal role of these elements in several physiological and pathological processes. Variations in their concentrations appear to have a role in the development and advancement of diseases in both humans and animals, for example, cancer. The purpose of this study was to investigate the concentration of rare earth elements and metals in healthy and neoplastic Formalin-Fixed Paraffin-Embedded (FFPE) mammary gland tissue of dogs. All samples were processed to have a quantitative determination of inorganic elements including metals of known toxicological interest such as Pb, Cd, Tl, As, Hg, the trace elements Mn, Fe, Co, Cu, Zn, Se, and other elements including Cr, V, Mo, Ni, Sb, W, Sn. Moreover, rare earth elements (REEs) (Sc, Y, Lu, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb) were also investigated. Cu and Mo concentrations in mammary cancerous tissue were greater than those in normal mammary glands (p < 0.05). In non-neoplastic tissue increased concentrations of Cd, Co, Ni, Tl, and V were also reported (p < 0.05). The mammary tissue of healthy individuals had greater concentrations of REEs than the neoplastic mammary glands (p < 0.05). The results of our study confirmed differences in mammary inorganic element concentrations between healthy and neoplastic groups, highlighting the potential relevance of these fluctuations in toxicologic pathology.
Keywords: Inorganic elements, Domestic animals, Toxicologic pathology, Sentinel, cancer, Rare earth
Highlights
-
•
Variations of trace element concentrations in animal tissues are still less studied.
-
•
An imbalance of inorganic elements in observed in neoplastic tissue.
-
•
Concentrations of metals were different in healthy and neoplastic tissue of dogs.
-
•
Mammary concentrations of rare earth elements were higher in healthy dogs.
1. Introduction
The deployment of animals as harbingers for human health hazards is a practice with historical roots, dating back to the late 19th century when canaries were employed by miners to gauge the quality of the air within coal mines [1]. There is now a growing push to clarify the toxicological relationships between humans and animals, especially in the context of the One Health paradigm, which acknowledges the interdependence of environmental, animal, and human health [2]. As demonstrated by several studies on exposure to environmental carcinogens such as asbestos and herbicides, pet dogs can be considered a valuable sentinel for environmental and human health [3,4]. Pet tissues such as genital organs, adipose tissue, blood, urine, and fur have been shown to contain a variety of endocrine-disrupting chemicals (EDCs), including pesticides, dioxins, and dioxin-like compounds, phthalates, and bisphenols [5]. There is still a lack of academic literature about the harmful effects of EDCs in canine and feline populations, despite the growth of studies on EDC exposure and its impacts on human health. According to Backer et al. (2001) [1], the negative health effects linked to toxicant exposure in companion animals are similar to those seen in humans, indicating that disease manifestation is caused by similar pathogenic processes [1]. Pets come with several beneficial characteristics that make it easier to investigate the intricate clinical and molecular aspects of human illnesses. Spontaneous tumors in dogs are a strong model for comparative oncology research because they share a wide range of epidemiological, biological, and clinical similarities with human malignancies [6]. Due to similarities in hormonal etiology, age of beginning, and disease course, clinical and molecular data highlight canine mammary cancers is a comparable model for researching human breast cancer [7].
In the field of toxicologic pathology, there is growing interest in the possibility that exposure to environmental contaminants—particularly toxic metals—may have a role in the development of breast tumors. Even though the term “toxic metal” is often used, chemistry does not always use it precisely. Considering the fundamental idea of toxicology as stated by Paracelsus (1493–1541), it is recognized that any chemical can become poisonous in high enough dosage. Significant differences exist in the toxicity spectrum of metals between various metals and species [8]. Even though these elements are naturally found in the crust of the earth, environmental pollution—a byproduct of human activity—significantly increases the exposure of humans and animals to these elements [9].
Some metals, including those used in the manufacturing of aluminum, inorganic arsenic, cadmium, chromium VI, and nickel compounds, have been categorized as human carcinogens (Group I) by the International Agency for Research on Cancer (IARC) [10]. Based on enough evidence from animal research, but inadequate data from human studies, the IARC classified inorganic lead and its compounds as potential carcinogens (Group 2A) [11]. According to reports, metals can build up in breast tissue and disrupt DNA integrity, resulting in gene mutations, reducing antioxidant defense, and changing epigenetics [12]. Furthermore, because certain metals can activate estrogen receptors (ER), which encourage cell proliferation, they can function as metalloestrogens [13].
Despite metals, emerging contaminants have also become attractive in the field of toxicologic pathology, as they are considered new risk factors for the development of human and animal diseases.
Because of their possible effects on the environment and human health, rare earth elements (REEs) are among them and are acknowledged as pollutants of developing concern. As a result, research on REEs has increased globally [14]. In spite of their name, REEs are not uncommon in the natural world, and like other newly discovered pollutants, they are not well-tracked in programs related to human and environmental health [15]. Anthropogenic sources of REE pollution are linked to their usage as contrast agents in medical imaging, such as magnetic resonance imaging (MRI), and their manufacturing of electronic gadgets, sophisticated weaponry, super-capacitors, and conductors [16].
Considering the pivotal role of domestic dogs as a sentinel for assessing pollution mediated by “old” and “new” contaminants and the development of exposure-related diseases, the purpose of this investigation was to determine the levels of metals and rare earth elements in healthy and neoplastic Formalin-Fixed Paraffin-Embedded (FFPE) mammary gland tissue of dogs.
2. Material and methods
2.1. Sample collection and histological analysis
40 post-surgery breasts for suspected breast cancer (tumor group) and 42 breasts (control group) without gross lesions collected during routine postmortem examinations, were used in the study. Informed agreement was given by owners, veterinarians, and local authorities for the necropsy of the sampled dogs. Mammary tissues were preserved in 10% buffered formalin, and embedded in paraffin. Hematoxylin-eosin (HE) staining was performed after obtaining sections of 3 μm sections. All dogs included in the study lived in the Abruzzo region (Italy) (Fig. S1) and they were fed with mixed diet (both commercial and house-made diet).
At the histological evaluation for the tumor group, we selected only the malignant mammary neoplasia, and the group was divided into 3 subgroups: simple carcinoma (SC); non-simple carcinoma (NSC), and ductal-associated carcinoma (DAC) [17].
The grading according to Peña et al. (2013) [18] was also assessed for each tumor. When different macroscopical lesions were sampled from the same animal for histological analysis, we selected the most common pattern for the microscopical categorization of the tumor. All information regarding age, breed, and tumor location was collected and showed in Tables S1 and S2.
2.2. Chemicals
The nitric acid (HNO3) at a concentration ≥ 69%, used for all dilutions and sample digestion,
was grade trace analysis and oxygen peroxide at 30% was grade suprapure, both supplied by Merck (Darmstadt, Germany).
The multielement stock standard solution, at 100 mgL−1 in HNO3 2÷5% (v/v) with HF traces, of lead (Pb), cadmium (Cd), thallium (Tl), arsenic (As), copper (Cu), iron (Fe), manganese (Mn), zinc (Zn), cobalt (Co), selenium (Se), chromium (Cr), vanadium (V), molybdenum (Mo), nickel (Ni), antimony (Sb) was purchased from Panreac Química (Barcellona, Spain). The single element stock standard solutions at 1000 mgL−1 of mercury (Hg) in HNO3 12% (v/v), Gallium (Ga) in 5% HNO3 (v/v), Indium (In) and Rhenium (Re) in 2% HNO3 (v/v) were provided from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The multielement of tungsten (W) and tin (Sn) stock standard solution at 1000 mgL−1 in 5% HNO3 (v/v) was bought from CPAchem Ltd. (Bogomilovo, Bulgaria).
The stock solution of rare earth elements (lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), lutetium (Lu), scandium (Sc) and yttrium (Y), at 100 mg L−1 in 3.5% HNO3 (v/v) was purchased from Merck KGaA, (Darmstadt, Germany).
All standards have a degree of purity suitable for trace metal analysis. Certified reference materials used as quality control samples were DOLT5 Dogfish Liver and DORM4 Fish Protein supplied from National Research Council Canada (Ottawa, Ontario, Canada), and BCR 668 (mussel's tissue) from Merck KGaA, (Darmstadt, Germany). Another reference material not certified, Fapas 01120 Canned Meat (Fera Science Limited, London, United Kingdom), was used to check moisture determination.
All working solutions, standard, blanks, and samples, were prepared with Type I (resistivity 18.2 MΩ.cm) water, obtained from ELGA LabWater PURELAB Option-Q water purification system (High Wycombe, United Kingdom).
Argon and helium gases of 99.9995% purity were supplied by Sapio (Monza, Italy).
2.3. Sample preparation
All samples were processed in order to have a quantitative determination of inorganic elements including metals of known toxicological interest such as Pb, Cd, Tl, As, Hg, trace elements Cu, Fe, Mn, Zn, Co, Se, other elements Cr, V, Mo, Ni, Sb, W, Sn and REEs (Ce, La, Nd, Sc, Pr, Y, Sm, Gd, Dy, Eu, Yb, Er, Lu, Tb, Ho, Tm).
FFPE was excised from the blocks with a disposable surgical scalpel and left to soak in 25 ÷ 30 mL of toluene (CHROMASOLV™, Sigma-Aldrich) for at least 12 h after initial heating on a plate at 50 ÷ 55 °C for 30 min. After dewaxing, the samples deprived of the solvent were dried on a plate at 50 ÷ 55 °C until constant weight [19]. Subsequently, the tissues under investigation were subjected to the determination of metals and REEs which included an initial digestion step according to the official method EN 13805:2014 and the following instrumental analysis by inductively coupled plasma-quadrupole mass spectrometry (Q-ICP-MS).
First, all the equipment and glassware that will be used in the analyses have been washed with 1÷2% (v/v) HNO3 and then rinsed with Type I water.
The samples obtained from the dewaxing process with toluene had a variable weight between 50 and 200 mg, compatible with the availability of the tissue taken from the animal.
Samples were weighed into PTFE/TFM (tetrafluoroethylene, modified) vessels, and 5 mL HNO3 and 1 mL H2O2 were added. Mineralization was performed in a Multiwave 3000 microwave digestion system (Anton Paar, Graz, Austria) according to the program shown in Table S3.
The influence of the methodological process on element fluctuations in FPPE tissue was also assessed. Levels of investigated elements in formalin and paraffin were under LOQ values. Concentrations of inorganic elements in the paraffin block where the tissue was included and in the residual paraffin after the deparaffinization of the tissue were also evaluated (Tables S5, S6). The resultant clear solutions were then quantitatively transferred into volumetric flasks and precisely diluted with Type I water to a volume of 15 mL after chilling. The same process was used to produce reference materials and blank solutions.
2.4. Analytical determination
All samples, blanks, and standards were diluted or prepared at a final HNO3 concentration in the range of 2 ÷ 4%. The measurement of all the elements under investigation was performed using, as internal standards, 71Ga, 115In, and 187Re, to offset any erratic oscillations in the signal, respectively for isotopes at low, medium, and high mass.
The samples were introduced into the inductively coupled plasma quadrupole mass spectrometer IcapRQ (Thermo Scientific, USA). The ICP-MS parameters settings are listed in Table S4.
Promethium (Pm), which is not found in the natural environment, has not been examined in this work. Except for As, Se, and transition elements (Sc, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn), all elements were determined using standard mode. These elements were analyzed using the collision cell mode (KED) which exploits the ability of helium (non-reactive gas) to remove, by collision, the most of all polyatomic interferences typical of medium-low masses. The matrix-matching calibration method was used to quantify all monitored elements. This method allows you to compensate for the matrix effect and consists in using a sample solution with a composition similar to those being analyzed (in our work a mixture of cattle, pigs and poultry meat), with a low content of analytes under examination, to reproduce in the plasma, the same conditions as the real samples [2]. The calibration range was built with concentrations ranging from 0.20 to 50 μgL−1 for 51V, 52Cr, 55Mn, 59Co, 60Ni, 75As, 78Se, 95Mo, 111Cd, 117Sn, 121Sb, 182W, 199Hg, 205Tl, 206Pb, from 5.0 to 120 μgL−1 for 57Fe, 65Cu, 67Zn and from 0.010 to 2.5 μgL−1 for 45Sc, 89Y, 139La, 140Ce, 141Pr, 146Nd, 147Sm, 153Eu, 157Gd, 159Tb, 163Dy, 165Ho, 166Er, 169Tm, 172Yb and 175Lu.
In each analytical session, to verify the accuracy of the analysis the certified reference materials (DOLT5, DORM4 and BCR668) as quality control samples for all the elements investigated were also analyzed.
3. Statistical analysis
For general comparisons between k groups, a Kruskal-Wallis test was used, and where significant, Dunn's post-hoc testing with Bonferroni correction were employed. Two sets of independent samples were compared using non-parametric Mann-Whitney tests. R Statistical Software (version 4.3.0; R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analysis.
4. Results
4.1. Histology
21 out of 42 breast samples without macroscopical lesions were included in the control group. For the definition of this group, we selected the mammary tissue without pathological alteration referable to inflammation, hyperplasia, and/or benign neoplasia; furthermore, all breasts in the lactation stage were excluded. All the animals included in the control group were intact females dead of non-neoplastic diseases. The ranging age of the control group was between 1 and 16 years, however, in our preliminary analysis, no change were observed in the overall content of inorganic elements between dogs with an age included between 1 and 7 years and dogs older than 7 years (Figs. S2, S3).
20 out of 40 breast samples suspected of breast cancer were included in the tumor group. All the animals in the tumor group were intact females with age ranging from 7 to 17 years.
Based on histological diagnoses, tumors were divided as follows: 12/20 were SC, 7/20 were diagnosed as DAC and 1/20 was a non-simple carcinoma. About grading, we found 5/20 mammary tumors of grading 1, 8/20 of grading 2, and 7/20 of grading 3. Detailed information about dog breed, age, histotype, grading, and sampled mammary location are in Tables S1 and S2.
4.2. Metals concentrations
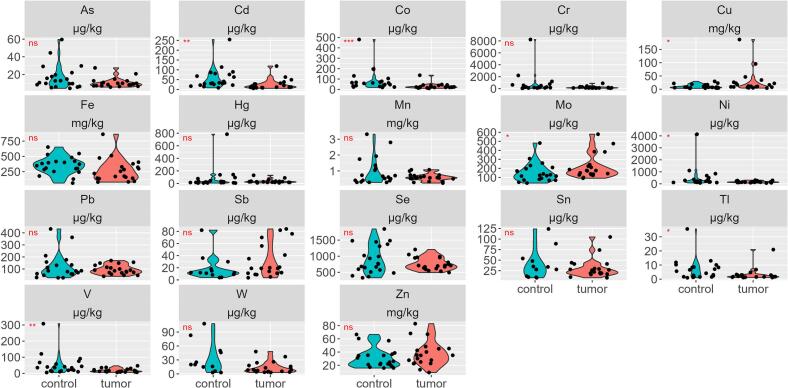
At first, we evaluated the concentration of 18 metals in breast tissues from tumor and control groups. Our analysis revealed a significant rise in Cu and Mo concentrations while a reduction in the concentration of Cd, Co, Ni, Tl, and V in tumor group vs control group (p < 0.05), as shown in Fig. 1 and Table 1.
Fig. 1.
Violin plot showing metal concentrations in tumor and control groups; n.s. not significant; *p < 0.05; **p < 0.01; ***p < 0.001.
Table 1.
Median concentrations of metals in tumor and control groups.
| Tumor group |
Control group |
p < 0.05 | |||||
|---|---|---|---|---|---|---|---|
| Median Concentrations | 5th percentile | 95th percentile | Median Concentrations | 5th percentile | 95th percentile | ||
| As (μg/kg) | 9.53 | 6.33 | 21.11 | 12.83 | 5.06 | 44.46 | |
| Cd (μg/kg) | 17.14 | 8.23 | 65.73 | 33.26 | 14.40 | 113.77 | * |
| Co (μg/kg) | 23.32 | 13.25 | 51.5 | 58.24 | 22.79 | 185.75 | * |
| Cr (μg/kg) | 165.65 | 87.50 | 447.57 | 243.31 | 88.36 | 2207.96 | |
| Hg (μg/kg) | 26.87 | 9.0 | 90.01 | 22.61 | 6.57 | 140.49 | |
| Mo (μg/kg) | 174.10 | 98.27 | 479.34 | 123.61 | 43.17 | 307.69 | * |
| Ni (μg/kg) | 143.96 | 82.11 | 287.23 | 211.62 | 104.23 | 1107.1 | * |
| Sb (μg/kg) | 19.0 | 4.83 | 82.1 | 12 | 4.06 | 52.1 | |
| Se (μg/kg) | 708.9 | 529.37 | 1060.28 | 761.05 | 365.74 | 1483.18 | |
| Sn (μg/kg) | 24.0 | 9.81 | 77.55 | 31.0 | 8.92 | 101.25 | |
| Tl (μg/kg) | 1.86 | 1.14 | 7.47 | 5.65 | 1.32 | 13.0 | * |
| V (μg/kg) | 13.04 | 6.80 | 37.41 | 35.14 | 9.97 | 120.67 | * |
| W (μg/kg) | 8.6 | 3.57 | 37.55 | 20.0 | 3.0 | 94.25 | |
| Cu (mg/kg) | 11.20 | 5.03 | 100.0 | 7.72 | 3.86 | 22.89 | * |
| Fe (mg/kg) | 238.18 | 82.37 | 530.51 | 359.82 | 125.17 | 551.59 | |
| Mn (mg/kg) | 0.57 | 0.27 | 1.07 | 0.63 | 0.28 | 2.82 | |
| Zn (mg/kg) | 35.06 | 13.09 | 68.86 | 25.39 | 15.92 | 60.74 | |
4.3. Rare earth elements concentrations
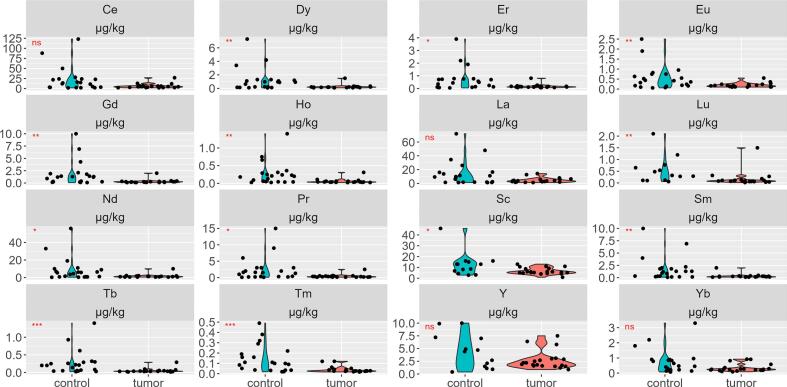
At first, we compared the concentration of 16 REEs in tumor and control groups. Fig. 2 and Table 2 showed that tumor group has lower concentrations of 12 (Dy, Er, Eu, Gd, Ho, Lu, Nd, Pr, Sc, Sm, Tb, and Tm) out of 16 REE vs control group (p < 0.05).
Fig. 2.
Violin plot showing REEs concentrations in tumor and control groups; n.s. not significant; *p < 0.05; **p < 0.01; ***p < 0.001.
Table 2.
Median REEs concentrations in tumor and control groups.
| Tumor group |
Control group |
p < 0.05 | |||||
|---|---|---|---|---|---|---|---|
|
Median concentrations (μg/kg) |
5th percentile | 95th percentile |
Median concentrations (μg/kg) |
5th percentile | 95th percentile | ||
| Ce | 4.75 | 2.19 | 13.7 | 14.0 | 2.2 | 88.0 | |
| Dy | 0.19 | 0.09 | 0.39 | 0.92 | 0.10 | 4.2 | * |
| Er | 0.13 | 0.07 | 0.29 | 0.49 | 0.08 | 2.2 | * |
| Eu | 0.17 | 0.10 | 0.37 | 0.42 | 0.07 | 1.9 | * |
| Gd | 0.26 | 0.11 | 0.53 | 1.2 | 0.13 | 6.9 | * |
| Ho | 0.04 | 0.02 | 0.12 | 0.2 | 0.03 | 0.75 | * |
| La | 3.7 | 1.53 | 12.74 | 9.3 | 1.27 | 47.92 | |
| Lu | 0.08 | 0.03 | 0.38 | 0.32 | 0.08 | 1.56 | * |
| Nd | 1.3 | 0.52 | 2.59 | 5.4 | 0.44 | 33.0 | * |
| Pr | 0.31 | 0.13 | 0.71 | 1.5 | 0.1 | 9.0 | * |
| Sc | 5.65 | 3.85 | 11.1 | 13 | 3.02 | 28.0 | * |
| Sm | 0.24 | 0.12 | 0.55 | 1.2 | 0.16 | 6.9 | * |
| Tb | 0.04 | 0.02 | 0.10 | 0.2 | 0.03 | 0.93 | * |
| Tm | 0.03 | 0.02 | 0.12 | 0.10 | 0.02 | 0.38 | * |
| Y | 2.05 | 1.18 | 6.45 | 4.4 | 0.71 | 9.94 | |
| Yb | 0.25 | 0.11 | 0.91 | 0.47 | 0.09 | 2.2 | |
5. Discussion
According to Sarafanov et al. (2008) [19], FFPE tissues are a potentially significant source of material but aren't often utilized to measure exposure to environmental pollutants [19]. FFPE tissues may provide a useful and feasible substitute if the metal or chemical concentrations in the tissue are not changed. These might lead to new avenues for studies using these clinical biospecimens [20]. Pb concentrations were not considered in this investigation since the background levels of paraffin blocks and the levels evaluated in FFPE mammary tissue were quite comparable (Table S6).
The type of tissue analyzed, extended exposure to formalin, contamination of paraffin, the use of solvents or acids during the paraffin embedding process, deparaffinization, and/or digestion, are some of the factors that may cause variations in the metal concentrations measured in FFPE tissues [19,20].
Given the critical role that trace elements play in several physiological processes, there has been a significant increase in interest in evaluating the quantities of these elements in human tissues in recent years. Certain metals are really necessary for biological processes; they are often found in proteins, enzymes, and cellular membranes [21]. On the other hand, at greater expression levels, they appear to be involved in several pathological processes, including metastasis, invasion, and tumor formation [22]. In this work, we assessed metals and REE levels in dog tumors and healthy FFPE breast tissues. Cu and Mo concentrations in mammary cancerous tissue were greater than those in normal mammary glands (p < 0.05). In non-neoplastic tissue increased concentrations of Cd, Co, Ni, Tl, and V were also reported (p < 0.05). Increased amounts of toxic metals including Fe, Cu, Zn, Pb, Cr, and Ni were reported in human breast malignant tumors [23]. However, literature data on canine mammary concentrations of metals are very limited. According to our results, increased Cu concentrations were found in canine neoplastic mammary tissue by Skibniewska and colleagues [24]. One of the most important trace metals is copper, a crucial element for the development of tumor microenvironments, angiogenesis, and metastasis [25]. According to Finney et al. (2009) [26], copper ions activate many proangiogenic factors, including vascular endothelial growth factor, basic fibroblast growth factor, tumor necrosis factor-α, and interleukine, via stimulating the proliferation and migration of endothelial cells [26]. This element facilitates the invasion or metastatic phenotype of breast cancer cells by activating the copper-binding protein through the epithelial-mesenchymal transition (EMT) [27]. Cu contributes to the biological harm that superoxide causes by inhibiting hydrogen peroxidase activity, which leads to damage to proteins, lipids, and DNA [28]. Cu can activate ERα and induce estrogen-regulated pathways because of its high affinity for the ERa [28].
In the present study, Zn concentrations in cancerous tissue were increased more than to in mammary health tissue, however the increase was not significant. This data is in accordance with those reported in humans, which showed that there is a significant increase in Zn levels in breast cancer tissue when compared to normal tissue as summarised by Rusch et al. (2021) [29]. Metalloproteinases and ROS damage protection, respectively, are the most significant pathways and factors in zinc deficiency and the pathophysiology of breast cancer. The blood's zinc content decreases as zinc enters a cell, and zinc compartmentalization—which is facilitated by matrix metalloproteinases—increases tumor invasion, metastasis, and angiogenesis [30]. The structure of CuZnSOD's active site is also maintained by Zn [31].
Because Mo is a co-factor of numerous enzymes, including xanthine oxidase-dehydrogenase (XO), aldehyde oxidase (AO), sulfite oxidase (SUOX), and mitochondrial amidoxime reducing component (mARC), it is regarded as an essential element for both plants and animals [32]. In the present study, the mammary concentrations of Mo were raised in dogs with tumors compared to healthy subjects. The connection between molecular concentrations and breast cancer is currently poorly understood in the literature and requires more research. Experimental investigations have demonstrated that Mo trioxide exhibits weak carcinogenicity in mice following short-term exposure at elevated doses, whereas no carcinogenic effects were observed at lower doses [33]. Furthermore, epidemiological research has revealed an association between Mo exposure and a decreased incidence of breast cancer risk overall [34].
Ni and Cd have been classified as metalloestrogens, capable of binding to ERs and emulating the physiological actions of estrogen, leading to the hypothesis that they may contribute to the pathogenesis of breast cancer [35]. Nonetheless, epidemiological investigations into the breast tissue concentrations of Cd have yielded inconsistent findings. Jablonska et al. (2017) [36] observed that elevated levels of this metal were associated with smaller or lower-grade tumors. On the contrary, Strumylaite et al. (2014) [37] reported significantly higher concentrations of Cd in malignant breast tissue in comparison to non-tumorous breast tissue, a finding further corroborated by Mansouri et al. (2022) [38]. Conversely, a recent meta-analysis indicated that the concentrations of Cd and Ni in hair and tissue samples did not significantly differ between individuals with and without breast cancer [39]. A potential explanation for these incongruencies is related to the inadequate quantity of studies on Cd and Ni in breast tissue.
As for Cd and Ni, the number of studies aimed to evaluate the mammary concentrations of Co and Tl is still low. It has been suggested that Co could cause breast cancers by mimicking hypoxia in angiogenesis and apoptosis and interfering with signaling pathways, including ERα activation [40]. The potential of Tl to alter glutathione activity and induce mitochondrial dysfunction is one potential mechanism of Tl general toxicity [41], however, these processes have not been ever correlated with cancer development and progression.
Mammary concentrations of V were higher in healthy animals compared to those with mammary tumors. This data is in agreement with the anti-neoplastic activity of V proven by research conducted in vitro and in vivo. In particular, Apoptosis was induced in MCF7 cells in a dose-dependent manner upon exposure to vanadium [42]. Moreover, the chemopreventive properties of vanadium were also confirmed in various experimental studies on chemical-induced mammary cancer in rat models [43].
To our knowledge, this study represents the inaugural effort to investigate the mammary concentrations of REEs in domestic animals. In a recent study, the REE levels in the liver were investigated in domestic and wild carnivores of Italy, and domestic dogs exhibited lower concentrations of REEs in comparison to wolves [2]. REE concentrations increased in the mammary tissue of healthy subjects compared to the neoplastic mammary glands. Although REEs have been widely used in the last decades, there is still fragmentary information on their toxicological effects. Adverse effects associated with exposure to REEs encompass growth inhibition, cytogenetic impacts, and toxicity specific to certain organs.
However, numerous studies have revealed that REEs, like many other chemical compounds, exhibit concentration-related hormetic trends, indicating that low concentrations may have protective or stimulatory effects while higher concentrations have negative effects [44]. The biological characteristics of lanthanides stem mainly from their calcium-like nature, which has led to their possible use in medicine as anticancer agents [45]. The anticancer activity of REEs is mainly related to their link with other molecules in the form of REE-containing biomaterials. For instance, Gd (III) DTPA is frequently utilized in MRI imaging for tumor detection. Additionally, initial clinical observations have indicated that Ce (III) iodide possesses efficacy in treating solid tumors [46]. Uptake of REEs was documented in one out of 113 cases of female breast cancer, attributed to occupational exposure. Specifically, Eu, Dy, and Pr were identified within the neoplastic cells of a singular case of triple-negative breast cancer in a woman previously employed in the ceramic industry [47]. Nonetheless, the detection of REEs in only one among 113 cases evaluated suggests a negligible role in the pathogenesis of breast cancer, while concurrently unveiling potential avenues for the therapeutic utilization of these elements in the treatment of triple-negative breast cancer.
6. Conclusions
In this research, the levels of metals and rare earth elements were assessed in both healthy and neoplastic canine mammary tissues. Each tumor was properly characterized as well as the control tissue was confirmed as “healthy” by histology. Moreover, the determination of REEs concentrations in mammary glands will help to obtain new data for the toxicological risk assessment of these elements. The results of the present study highlighted the importance of domestic dogs as valuable sentinel in toxicological investigations and the relevance of including comparative toxicologic pathology studies in the One Health paradigm. Most often, this concept is traditionally applied to zoonotic infectious and parasitic diseases and is not focused on environmental chemical- or poison-related illnesses in animals and humans.
This research also emphatizing how utilizing the One Health approach may improve the evaluation of chemical concentrations in healthy and neoplastic tissues to correlate chemicals exposure to the adverse outcome pathways of disease. However, additional research involving larger sample sizes and a broader range of tissue types is essential to elucidate the association between exposure to metals and REEs and the risk of mammary cancer.
Funding
This work was supported by Regione Abruzzo, Piano Regionale di Prevenzione -Registro tumori - RAPRP2125H.
CRediT authorship contribution statement
Sabrina V.P. Defourny: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Giulia Caioni: Visualization, Validation, Software, Formal analysis. Mirella Bellocci: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Valeria Melai: Writing – review & editing, Writing – original draft, Supervision, Methodology, Formal analysis, Data curation, Conceptualization. Giampiero Scortichini: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition. Romolo Salini: Writing – original draft, Visualization, Validation, Software, Formal analysis. Michele Martino: Visualization, Validation, Formal analysis. Giovanni Di Teodoro: Visualization, Validation, Methodology, Formal analysis. Antonio Cocco: Visualization, Validation, Formal analysis. Maria Chiara Cantelmi: Visualization, Validation, Formal analysis. Carmine Merola: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Antonio Petrini: Visualization, Validation, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Carmine Merola reports financial support was provided by Abruzzo Region. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2024.100749.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- 1.Backer L.C., Grindem C.B., Corbett W.T., Cullins L., Hunter J.L. Pet dogs as sentinels for environmental contamination. Sci. Total Environ. 2001;274(1–3):161–169. doi: 10.1016/s0048-9697(01)00740-9. [DOI] [PubMed] [Google Scholar]
- 2.Bellocci M., Defourny S.V., Melai V., Scortichini G., Salini R., Di Bernardo G., Lomellini L., Coccaro A., Merola C., Petrini A. Comparative analysis of rare earth elements concentrations in domestic dogs and Apennine wolves of Central Italy: influence of biological, nutritional, and lifestyle factors. Sci. Total Environ. 2024;170358 doi: 10.1016/j.scitotenv.2024.170358. [DOI] [PubMed] [Google Scholar]
- 3.Glickman L.T. In: In Situ Evaluation of Biological Hazards of Environmental Pollutants. Sandhu S.S., Lower W.R., De Serres F.J., Suk W.A., Tice R.R., editors. Springer US; Boston, MA: 1990. Analytical epidemiology in pet populations for environmental risk assessment; pp. 133–143. [Google Scholar]
- 4.Knapp D.W., Peer W.A., Conteh A., Diggs A.R., Cooper B.R., Glickman N.W., Bonney P.L., Stewart J.C., Glickman L.T., Murphy A.S. Detection of herbicides in the urine of pet dogs following home lawn chemical application. Sci. Total Environ. 2013;456–457:34–41. doi: 10.1016/j.scitotenv.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Pocar P., Grieco V., Aidos L., Borromeo V. Endocrine-disrupting chemicals and their effects in pet dogs and cats: an overview. Animals. 2023;13(3):378. doi: 10.3390/ani13030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinho S.S., Carvalho S., Cabral J., Reis C.A., Gärtner F. Canine tumors: a spontaneous animal model of human carcinogenesis. Transl. Res. 2012;159(3):165–172. doi: 10.1016/j.trsl.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Queiroga F.L., Raposo T., Carvalho M.I., Prada J., Pires I. Canine mammary tumours as a model to study human breast cancer: most recent findings. Vivo. 2011;25(3):455–465. [PubMed] [Google Scholar]
- 8.Duffus J.H. “Heavy metals”—a meaningless term?: (IUPAC technical report) Pure Appl. Chem. 2002;74 doi: 10.1515/iupac.74.0076. [DOI] [Google Scholar]
- 9.Fatema K., Shoily S.S., Ahsan T., Haidar Z., Sumit A.F., Sajib A.A. Effects of arsenic and heavy metals on metabolic pathways in cells of human origin: similarities and differences. Toxicol. Rep. 2021;8:1109–1120. doi: 10.1016/j.toxrep.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mérida-Ortega Á., Rothenberg S.J., Cebrián M.E., López-Carrillo L. Breast cancer and urinary metal mixtures in Mexican women. Environ. Res. 2022;210:112905. doi: 10.1016/j.envres.2022.112905. [DOI] [PubMed] [Google Scholar]
- 11.IARC . 2006. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume. [Google Scholar]
- 12.Romaniuk А., Lyndin M., Sikora V., Lyndina Y., Romaniuk S., Sikora K. Heavy metals effect on breast cancer progression. J. Occup. Med. Toxicol. 2017;12(1):32. doi: 10.1186/s12995-017-0178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pamphlett R., Satgunaseelan L., Jew S.K., Doble P.A., Bishop D.P. Elemental bioimaging shows mercury and other toxic metals in normal breast tissue and in breast cancers. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0228226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Pereira W.V.S., Ramos S.J., LCA Melo, de Braz A.M.S., Dias Y.N., de Almeida G.V., Fernandes A.R. Levels and environmental risks of rare earth elements in a gold mining area in the Amazon. Environ. Res. 2022;211:113090. doi: 10.1016/j.envres.2022.113090. [DOI] [PubMed] [Google Scholar]
- 15.Gwenzi W., Mangori L., Danha C., Chaukura N., Dunjana N., Sanganyado E. Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants. Sci. Total Environ. 2018;636:299–313. doi: 10.1016/j.scitotenv.2018.04.235. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Chen Z., Chen Z., Zhang Y. A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China. Chemosphere. 2013;93(6):1240–1246. doi: 10.1016/j.chemosphere.2013.06.085. [DOI] [PubMed] [Google Scholar]
- 17.Zappulli V., Peña L., Rasotto R., Goldschmidt M., Gama A., Scruggs J., Kiupel M. vol. 2. Davis-Thompson DVM Foundation; Gurnee, IL: 2019. Surgical Pathology of Tumors of Domestic Animals; p. 280. [Google Scholar]
- 18.Peña L., De Andrés P.J., Clemente M., Cuesta P., Pérez-Alenza M.D. Prognostic value of histological grading in noninflammatory canine mammary carcinomas in a prospective study with two-year follow-up: relationship with clinical and histological characteristics. Vet. Pathol. 2013;50(1):94–105. doi: 10.1177/0300985812447830. [DOI] [PubMed] [Google Scholar]
- 19.Sarafanov A.G., Todorov T.I., Kajdacsy-Balla A., Gray M.A., Macias V., Centeno J.A. Analysis of iron, zinc, selenium and cadmium in paraffin-embedded prostate tissue specimens using inductively coupled plasma mass-spectrometry. J. Trace Elem. Med. Biol. 2008;22(4):305–314. doi: 10.1016/j.jtemb.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Coyte R.M., Darrah T.H., Barrett E., O’Connor T.G., Olesik J.W., Salafia C.M., Shah R., Love T., Miller R.K. Comparison of trace element concentrations in paired formalin-fixed paraffin-embedded and frozen human placentae. Placenta. 2023;131:98–103. doi: 10.1016/j.placenta.2022.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva M.P., Soave D.F., Ribeiro-Silva A., Poletti M.E. Trace elements as tumor biomarkers and prognostic factors in breast cancer: a study through energy dispersive x-ray fluorescence. BMC. Res. Notes. 2012;5(1):194. doi: 10.1186/1756-0500-5-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan M.P., Cooke M.M., McCarthy G.M. Microcalcifications associated with breast cancer: an epiphenomenon or biologically significant feature of selected tumors? J. Mammary Gland Biol. Neoplasia. 2005;10(2):181–187. doi: 10.1007/s10911-005-5400-6. [DOI] [PubMed] [Google Scholar]
- 23.Koual M., Tomkiewicz C., Cano-Sancho G., Antignac J.-P., Bats A.-S., Coumoul X. Environmental chemicals, breast cancer progression and drug resistance. Environ. Health. 2020;19(1):117. doi: 10.1186/s12940-020-00670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skibniewska E.M., Skibniewski M., Kosla T. Dependence between Cu concentration in the liver, kidneys and skeletal muscles of canine females. Cent.Eur.J.Biol. 2012;7(5):817–824. doi: 10.2478/s11535-012-0083-7. [DOI] [Google Scholar]
- 25.Lowndes S.A., Harris A.L. The role of copper in tumour angiogenesis. J. Mammary Gland Biol. Neoplasia. 2005;10(4):299–310. doi: 10.1007/s10911-006-9003-7. [DOI] [PubMed] [Google Scholar]
- 26.Finney L., Vogt S., Fukai T., Glesne D. Copper and angiogenesis: unravelling a relationship key to cancer progression. Clin. Exp. Pharmacol. Physiol. 2009;36(1):88–94. doi: 10.1111/j.1440-1681.2008.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D., Dai L., Wang L., Tan C., Cai J., Wang H., Zhao X. Copper complexes suppress stemness, reverse epithelial-to-mesenchymal transition progression, and induce apoptosis on triple negative breast cancer. Appl. Organomet. Chem. 2023;37(4) doi: 10.1002/aoc.7055. [DOI] [Google Scholar]
- 28.Feng Y., Zeng J.-W., Ma Q., Zhang S., Tang J., Feng J.-F. Serum copper and zinc levels in breast cancer: a meta-analysis. J. Trace Elem. Med. Biol. 2020;62:126629. doi: 10.1016/j.jtemb.2020.126629. [DOI] [PubMed] [Google Scholar]
- 29.Rusch P., Hirner A.V., Schmitz O., Kimmig R., Hoffmann O., Diel M. Zinc distribution within breast cancer tissue of different intrinsic subtypes. Arch. Gynecol. Obstet. 2021;303(1):195–205. doi: 10.1007/s00404-020-05789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Do Holanda A.O.N., ARS De Oliveira, KJC Cruz, Severo J.S., JBS Morais, Da Silva B.B., Do Marreiro D.N. Zinc and metalloproteinases 2 and 9: What is their relation with breast cancer? Rev. Assoc. Med Bras (1992) 2017;63(1):78–84. doi: 10.1590/1806-9282.63.01.78. [DOI] [PubMed] [Google Scholar]
- 31.Rao A.K., Ziegler Y.S., McLeod I.X., Yates J.R., Nardulli A.M. Effects of Cu/Zn superoxide dismutase on estrogen responsiveness and oxidative stress in human breast cancer cells. Mol. Endocrinol. 2008;22(5):1113–1124. doi: 10.1210/me.2007-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odularu A.T., Ajibade P.A., Mbese J.Z. Impact of molybdenum compounds as anticancer agents. Bioinorg. Chem. Appl. 2019;2019 doi: 10.1155/2019/6416198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoner G.D., Shimkin M.B., Troxell M.C., Thompson T.L., Terry L.S. Test for carcinogenicity of metallic compounds by the pulmonary tumor response in strain A mice. Cancer Res. 1976;36(5):1744–1747. [PubMed] [Google Scholar]
- 34.Niehoff N.M., O’Brien K.M., Keil A.P., Levine K.E., Liyanapatirana C., Haines L.G., Waidyanatha S., Weinberg C.R., White A.J. Metals and breast cancer risk: a prospective study using toenail biomarkers. Am. J. Epidemiol. 2021;190(11):2360–2373. doi: 10.1093/aje/kwab204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aquino N.B., Sevigny M.B., Sabangan J., Louie M.C. The role of cadmium and nickel in estrogen receptor signaling and breast cancer: metalloestrogens or not? J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2012;30(3):189–224. doi: 10.1080/10590501.2012.705159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jablonska E., Socha K., Reszka E., Wieczorek E., Skokowski J., Kalinowski L., Fendler W., Seroczynska B., Wozniak M., Borawska M.H., Wasowicz W. Cadmium, arsenic, selenium and iron- implications for tumor progression in breast cancer. Environ. Toxicol. Pharmacol. 2017;53:151–157. doi: 10.1016/j.etap.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Strumylaite L., Kregzdyte R., Bogusevicius A., Poskiene L., Baranauskiene D., Pranys D. Association between cadmium and breast cancer risk according to estrogen receptor and human epidermal growth factor receptor 2: epidemiological evidence. Breast Cancer Res. Treat. 2014;145(1):225–232. doi: 10.1007/s10549-014-2918-6. [DOI] [PubMed] [Google Scholar]
- 38.Mansouri B., Ramezani Z., Yousefinejad V., Nakhaee S., Azadi N., Khaledi P., Nikkhoo B., Hassanzadeh K., Rahimi A. Association between trace elements in cancerous and non-cancerous tissues with the risk of breast cancers in western Iran. Environ. Sci. Pollut. Res. 2022;29(8):11675–11684. doi: 10.1007/s11356-021-16549-9. [DOI] [PubMed] [Google Scholar]
- 39.Liu L., Chen J., Liu C., Luo Y., Chen J., Fu Y., Xu Y., Wu H., Li X., Wang H. Relationships between biological heavy metals and breast cancer: a systematic review and meta-analysis. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.838762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho J., Kim D., Lee S., Lee Y. Cobalt chloride-induced estrogen receptor alpha down-regulation involves hypoxia-inducible factor-1alpha in MCF-7 human breast cancer cells. Mol. Endocrinol. 2005;19(5):1191–1199. doi: 10.1210/me.2004-0162. [DOI] [PubMed] [Google Scholar]
- 41.Genchi G., Carocci A., Lauria G., Sinicropi M.S., Catalano A. Thallium use, toxicity, and detoxification therapy: an overview. Appl. Sci. 2021;11(18):8322. doi: 10.3390/app11188322. [DOI] [Google Scholar]
- 42.Ray R.S., Rana B., Swami B., Venu V., Chatterjee M. Vanadium mediated apoptosis and cell cycle arrest in MCF7 cell line. Chem. Biol. Interact. 2006;163(3):239–247. doi: 10.1016/j.cbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Bishayee A., Oinam S., Basu M., Chatterjee M. Vanadium chemoprevention of 7,12-dimethylbenz(a)anthracene-induced rat mammary carcinogenesis: probable involvement of representative hepatic phase I and II xenobiotic metabolizing enzymes. Breast Cancer Res. Treat. 2000;63(2):133–145. doi: 10.1023/A:1006476003685. [DOI] [PubMed] [Google Scholar]
- 44.Pagano G., Guida M., Tommasi F., Oral R. Health effects and toxicity mechanisms of rare earth elements-knowledge gaps and research prospects. Ecotoxicol. Environ. Saf. 2015;115:40–48. doi: 10.1016/j.ecoenv.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 45.Pałasz A., Czekaj P. Toxicological and cytophysiological aspects of lanthanides action. Acta Biochim. Pol. 2000;47(4):1107–1114. [PubMed] [Google Scholar]
- 46.Fricker S.P. The therapeutic application of lanthanides. Chem. Soc. Rev. 2006;35(6):524–533. doi: 10.1039/B509608C. [DOI] [PubMed] [Google Scholar]
- 47.Roncati L., Gatti A.M., Barbolini G., Piscioli F., Pusiol T., Maiorana A. In vivo uptake of rare earth metals by triple-negative breast cancer cells. Pathol. Oncol. Res. 2018;24(1):161–165. doi: 10.1007/s12253-017-0209-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.