Abstract
Smoking continues to pose a global threat to morbidity and mortality in populations. The detrimental impact of smoking on health and disease includes bone destruction and immune disruption in various diseases. Osteoimmunology, which explores the communication between bone metabolism and immune homeostasis, aims to reveal the interaction between the osteoimmune systems in disease development. Smoking impairs the differentiation of mesenchymal stem cells and osteoblasts in bone formation while promoting osteoclast differentiation in bone resorption. Furthermore, smoking stimulates the Th17 response to increase inflammatory and osteoclastogenic cytokines that promote the receptor activator of NF-κB ligand (RANKL) signaling in osteoclasts, thus exacerbating bone destruction in periodontitis and rheumatoid arthritis. The pro-inflammatory role of smoking is also evident in delayed bone fracture healing and osteoarthritis development. The osteoimmunological therapies are promising in treating periodontitis and rheumatoid arthritis, but further research is still required to block the smoking-induced aggravation in these diseases.
Translational potential
This review summarizes the adverse effect of smoking on mesenchymal stem cells, osteoblasts, and osteoclasts and elucidates the smoking-induced exacerbation of periodontitis, rheumatoid arthritis, bone fracture healing, and osteoarthritis from an osteoimmune perspective. We also propose the therapeutic potential of osteoimmunological therapies for bone destruction aggravated by smoking.
Keywords: Arthritis, Bone, Fractures, Mesenchymal stem cells, Osteoarthritis, Osteoclasts, Osteoblasts, Periodontitis, Rheumatoid, Smoking, Th17 cells
Graphical abstract
1. Introduction
Tobacco use remains a global concern with substantial prevalence rates. It is reported that the latest global prevalence of cigarette smoking is 11.3 % of boys and 6.1 % of girls aged 13–15 years, whereas the median prevalence is 22.5 % among people 15 years of age and older [1,2]. Smoking contributes to the annual 7.69 million deaths and 200 million disability-adjusted life years estimated to maintain growth in the following decades [3]. Cigarette smoking, whether active or passive, increases the risk of overall mortality and numerous disease prevalence, especially in lung cancer, stroke, and heart attack [2,4]. Furthermore, smoking is associated with the development of periodontitis, rheumatoid arthritis, bone fractures, etc. [5,6].
Osteoimmunology is an interdisciplinary field that synergizes the skeletal and immune systems and primarily elucidates the interplay between these systems [7]. Notably, bone and immune cells significantly overlap in crucial molecules, including cytokines, chemokines, receptors, and transcription factors [8]. In bone modeling and remodeling, osteoblasts and osteoclasts are essential in maintaining the dynamic balance of bone metabolism, while osteocytes coordinate the functions of osteoblasts and osteoclasts with environmental cues. The immune cells in osteoimmunology mainly include T cells, especially osteolytic T helper 17 (Th17) cells and bone protective T regulatory (Treg) cells [9], B cells, monocytes/macrophages, and regenerative mesenchymal stem cells (MSCs). Recent research has confirmed that innate and adaptive immune responses impact bone metabolism and that bone cells regulate the immune system [10].
Osteoimmune mechanisms underlying periodontitis [7,11], rheumatoid arthritis [7,8], and bone fracture healing [12] have provided novel insights into the therapeutic strategies for skeletal- and immune-related diseases. Although the negative role of smoking on health and disease has been widely accepted [2,[4], [5], [6]], the specific mechanism underlying smoking and bone metabolism remains unknown. Therefore, this review summarizes the adverse effect of smoking on MSCs, osteoblasts, and osteoclasts and elucidates the smoking-induced exacerbation of periodontitis, rheumatoid arthritis, bone fracture healing, and osteoarthritis from an osteoimmune perspective.
2. RANKL/RANK/OPG signaling in osteoimmunology
2.1. RANKL/RANK/OPG in osteoclastogenesis
RANKL, a tumor necrosis factor (TNF) family cytokine encoded by the Tnfsf11 gene, and its receptor (RANK) encoded by the Tnfrsf11a gene, which is highly expressed in dendritic cells (DCs), were initially found to augment the viability of DCs, thus stimulating the proliferation of T cells and enhancing the survival of DCs and RANK+ T cells [13,14]. Osteocytes express more RANKL than osteoblasts and bone marrow MSCs and mediate osteoclastogenesis by combining with RANK in osteoclast precursors [15,16]. Notably, OPG encoded by the Tnfrsf11b gene suppresses osteoclast differentiation and modulates the function of T cells and DCs by competitively binding to RNAKL with a higher affinity [17]. RANK contains three putative TRAF-binding domains, which interact with TRAF2, TRAF5, and TRAF6 [18], and subsequent signaling from TRAF6 is essential for functional osteoclast formation [19]. With the connection of TAB2, TRAF6 integrates with TAK1 to activate the downstream pathways [20], namely the MAPK and NF-κB pathways. The activated MAPK mainly induces the activation of JNK1 to promote the phosphorylation of c-Jun [21]. Activated IKK induces the NF-κB pathway to generate c-Fos [22]. The Jun and Fos family proteins, mainly c-Jun and c-Fos, comprise the AP-1, which is critical for osteoclast differentiation [8].
2.2. Costimulatory signals and master transcriptional factor for RANK
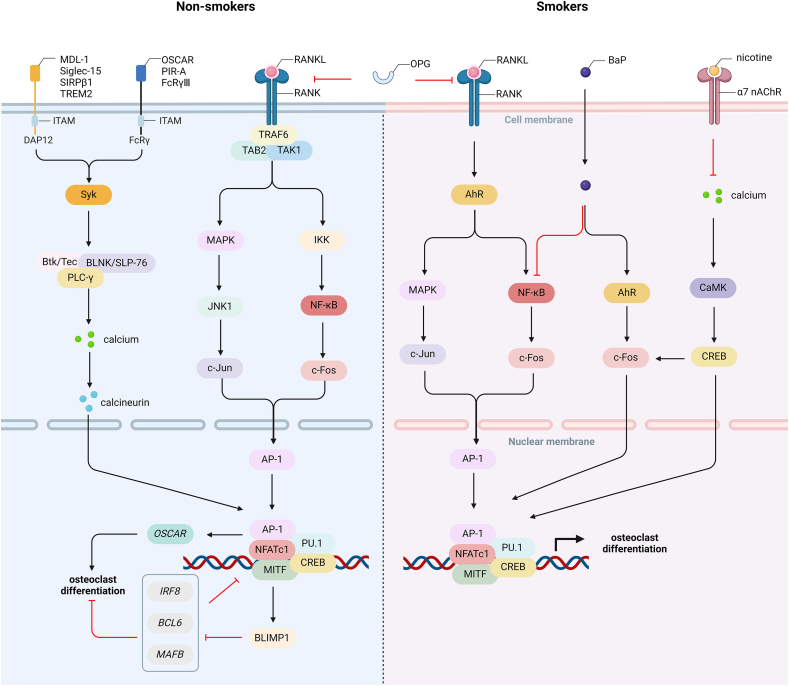
Costimulatory signals are induced by ITAM-containing FcRγ and DAP12 [23]. It is reported that OSCAR, PIR-A, and FcRγⅢ are associated with FcRγ, while MDL-1 receptor, lectin Siglec-15, SIRPβ1, TREM2 are associated with DAP12 [[24], [25], [26]]. The phosphorylation of ITAM recruits and activates Syk to generate Btk(Tec)/BLNK(SLP-76)-containing complex and activate PLC-γ, subsequently triggering calcium oscillation [27,28]. The increased intracellular calcium iron induced by the PLC-γ/IP3/IP3R (inositol-1,4,5-trisphosphate receptor) pathway is sufficient for the calcineurin-mediated activation of NFATc1, the master transcription factor for RANK [29]. Induced dependently by c-Fos, NF-κB, and calcineurin, the increased NFATc1, with the synergistic regulation of AP-1, CREB, PU.1, and MITF, upregulates the expression of osteoclast-specific genes, such as OSCAR gene, and induces BLIMP1 to downregulate the anti-osteoclastogenic genes, such as IRF8, BCL6, and MAFB, leading to the formation of mature osteoclasts [7,30] (Fig. 1).
Figure 1.
RANKL/RANK/OPG signaling in non-smokers and smokers a. RANKL upregulates AhR expression to mediate the phosphorylation of MAPK and NF-κB, thus increasing c-Fos expression. b. BaP regulates the interaction between the AhR and NF-κB pathways to mediate osteoclast differentiation. c. Long-term nicotine treatment inhibits the calcium oscillation to downregulate c-Fos and NFATc1 expression. Therefore, smoking regulates osteoclast formation and bone destruction by RANKL/RANK signaling. AhR, aryl hydrocarbon receptor; AP-1, activator protein-1; BaP, benzo [a]pyrene; BLIMP1, B lymphocyte-induced maturation protein 1; CaMK, Ca2+/calmodulin-dependent kinase; CREB, cAMP response element-binding protein; DAP12, DNAX-activating protein of 12kD; FcRγ, Fc receptor common gamma subunit; IKK, IκB kinase; ITAM, immunoreceptor tyrosine-based activation motif; JNK1, Jun kinase 1; MAPK, mitogen-activated protein kinase; MDL-1, myeloid DAP12-associating lectin-1; MITF, melanocyte-induced transcriptional factor; NFATc1, nuclear factor of activated T-cells 1; OPG, osteoprotegerin; OSCAR, osteoclast-associated receptor; PIR-A, paired immunoglobin-like receptor-A; PLC-γ, phospholipase C-γ; RANKL, receptor activator of NF-κB ligand; SIRPβ1, signal-regulatory protein β1; Syk, spleen tyrosine kinase; TAB2, TAK1-binding protein 2; TAK1, TGF-β-activated kinase 1; TGF-β, transforming growth factor-β; TNF, tumor necrosis factor; TRAF, TNF receptor-associated factor; TREM2, triggering receptor expressed on myeloid cells 2; α7 nAChR, α7 nicotinic acetylcholine receptor.
3. Effect of smoking on bone metabolism
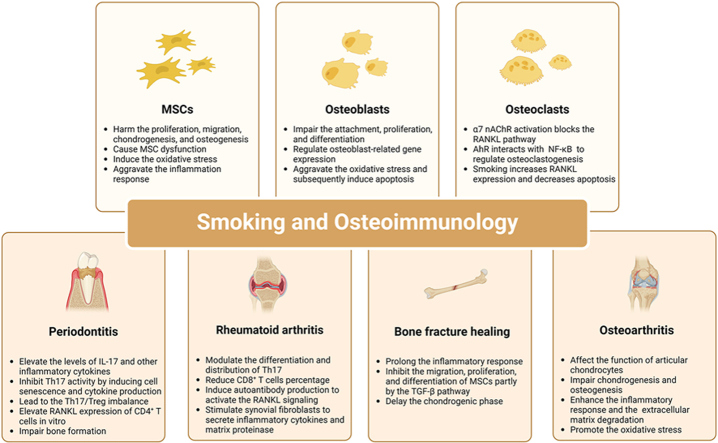
Smoking and its independent components have a harmful effect on bone metabolism as it impairs the osteogenic differentiation of MSCs and osteoblasts through various mechanisms. Additionally, smoking triggers the activation of the RANKL signaling pathway, which promotes osteoclast formation, ultimately leading to bone destruction. Furthermore, smoking negatively impacts the immune function of MSCs and osteoblasts, and smoking-induced regulators interact with the RANKL signaling pathway to modulate osteoclast differentiation.
3.1. Smoking alters the differentiation of mesenchymal stem cells
MSCs are known as milestones in tissue regeneration because MSCs are responsible for the generation of adipocytes, osteoblasts, and chondrocytes. Cigarette smoke harms the proliferation, migration, chondrogenesis, and osteogenesis of MSCs [31]. Cigarette smoke extract (CSE) induced a significant reduction in osteogenic markers, including alkaline phosphatase (ALP) activity, collagen type I (CloⅠ), and Runt-related transcription factor 2 (Runx2), as well as matrix mineralization and calcium deposition, in bone marrow-derived MSCs [32,33]. Besides, CSE exposure not only significantly reduced ALP activity, osteocalcin, and bone morphogenetic protein-2 (BMP-2) but also decreased the total and cortical bone fraction, trabecular thickness, and connectivity density in the femurs from CSE-injected mice [34]. Notably, the production of RANKL significantly increased in CSE-treated MSCs, accompanied by a significant decrease in OPG production [34]. An acute low concentration of CSE slowed the osteoblast differentiation and deteriorated the cartilaginous mechanical function with a decreased extracellular matrix [35]. These results suggest that cigarette smoke directly impairs the osteogenic differentiation of MSCs.
Independent components of cigarette smoke, especially nicotine, also impair MSCs. Firstly, nicotine at 1 mmol/L to 2 mmol/L decreased osteocalcin expression, and nicotine at 2 mmol/L significantly reduced calcium deposition, ALP activity, ALP mRNA expression, bone sialoprotein (BSP), CloIα1, and Runx2 in human alveolar bone marrow-derived MSCs [36]. However, nicotine at 0.1 μmol/L and 1 μmol/L significantly upregulated CloII during the chondrogenesis of bone marrow-derived MSCs, indicating that low-dose nicotine facilitates MSC chondrogenesis [37]. Secondly, benzo [a]pyrene (BaP), a major toxic component of cigarette smoke, can also significantly reduce the expression of Runx2, osteocalcin, and osteopontin in BMP-2-induced osteogenic differentiation [38]. The negative role of BaP is dependent on the aryl hydrocarbon receptor (AhR) signaling, which subsequently decreases the expression of BMP receptor 2 to impair the BMP/Smad pathway [38]. Thirdly, a low dose of carbon black (3 ng/mL and 30 ng/mL) significantly decreased the ALP activity and Runx2 expression at mRNA and protein levels in bone marrow-derived MSCs, partly resulting from impaired mitochondrial biogenesis [39]. Therefore, cigarette smoke, as well as its independent components, is proven to cause MSC dysfunction.
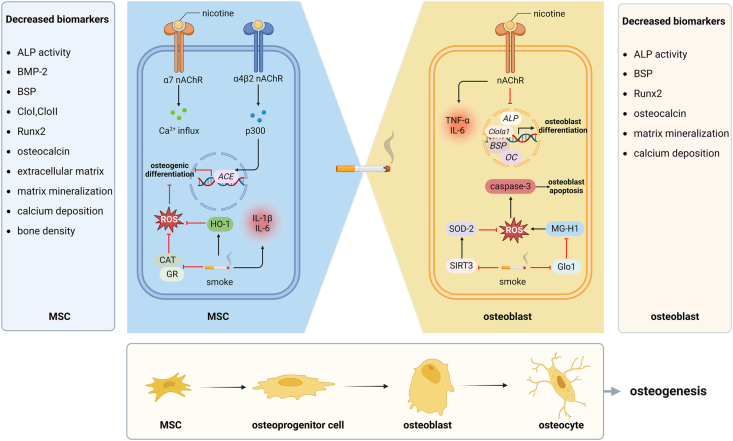
Mechanisms underlying smoking-induced MSC dysfunction may vary. Firstly, reactive oxygen species (ROS) levels increased in CSE-exposed MSCs [32]. Nicotine and cotinine aggravated ROS accumulation induced by cigarette smoke exposure by repressing the activity of catalase and glutathione reductase [40]. This negative effect can be improved by activating nuclear factor erythroid 2-related factor 2 (Nrf2) signaling and eliminating excessive ROS by N-acetylcysteine or l-ascorbate cotreatment [40]. Besides, nicotine increased the expression of heme oxygenase-1 (HO-1) to prevent its further detrimental effect on osteogenesis in MSCs [41,42]. These results suggest that smoking impairs MSCs partly through inducing oxidative stress. Secondly, CSE enhanced the gene expression of pro-inflammatory cytokines, especially the anti-osteogenic IL-1β and IL-6 in MSCs [34]. Nicotine at 100 μmol/L increased TNF-α, IL-1β, and IL-12 in progenitor stem cells from dental pulp, follicle, and gingival tissues [41]. These results show that smoking aggravates the inflammation response in MSCs. Thirdly, human MSCs express α7 nicotinic acetylcholine receptor (nAChR), and nicotine can stimulate α7 nAChR to induce calcium influx into human MSCs [43]. Besides, nicotine can also activate α4β2 nAChR to induce histone acetylase p300 into the nucleus of the bone marrow MSCs and subsequently promote the expression of angiotensin-converting enzyme, which explains the prenatal nicotine-induced osteopenia [44] (Fig. 2).
Figure 2.
Smoking impairs the differentiation of mesenchymal stem cells and osteoblasts a. Nicotine stimulates α7 nAChR to induce calcium influx and activates α4β2 nAChR to induce the nuclear translocation of p300 and subsequently promote ACE expression. b. CSE represses CAT and GR activity to aggravate ROS accumulation. c. CSE enhances pro-inflammatory and anti-osteogenic IL-1β and IL-6 in MSCs, and nicotine increases IL-6 and TNF-α in mouse and human osteoblasts, respectively. d. High-dose nicotine significantly downregulates the gene expression of ALP, CloⅠα1, BSP, and OC in rat primary osteoblasts. e. Nicotine inhibits the SOD-2 activity by reducing SIRT3 and drives MG-H1 aggravation by inhibiting Glo1 activity, contributing to osteoblast apoptosis, possibly through the ROS/caspase-3 pathway. ACE, angiotensin-converting enzyme; ALP, alkaline phosphatase; BMP-2, bone morphogenetic protein-2; BSP, bone sialoprotein; CAT, catalase; CloⅠα1, collagen type I α1; CSE, cigarette smoke extract; Glo1, glyoxalase 1; GR, glutathione reductase; HO-1, heme oxygenase-1; MG, methylglyoxal; MG-H1, hydroimidazoline; MSC, mesenchymal stem cell; OC, osteocalcin; ROS, reactive oxygen species; Runx2, Runt-related transcription factor 2; SIRT3, sirtuin3; SOD-2, superoxide dismutase-2.
Notably, MSCs also have a critical role in regulating the immune system. It has been thoroughly reviewed that the immunoregulatory function of MSCs depends on the inflammatory environment where MSCs are located [45]. In response to high levels of TNF-α and interferon-γ (IFN-γ) in a pro-inflammation environment, MSCs suppress the antigen presentation of DCs and macrophages, inhibit the cytotoxicity of natural killer cells, natural killer T cells, and CD8+ T cells, repress the expression of pro-inflammatory Th1 and Th17 cells, and elevate the anti-inflammatory Treg production, eventually leading to a reduction in TNF-α, IFN-γ, IL-1β, IL-18, IL-17, IL-22, and IL-23, and augmentation in TGF-β, IL-10, and IL-35 [31,45]. Lung-resident MSCs preincubated with 2.5 % and 5 % CSE significantly failed to suppress the CD8+ T cell proliferation, but this effect was reversible [46]. Besides, CSE was sufficient to induce the pro-inflammatory phenotype of MSCs with higher expressions of TNF-α, IFN-γ, IL-6, and IL-17 and lower TGF-β and IL-10 than the control group [47]. These results claim that smoking negatively affects the immunoregulatory function of MSCs, and future studies should attach more importance to this respect, especially the subsequent effect on the bone system.
3.2. Smoking alters the development of osteoblasts
Cigarette smoke and its independent components impair the attachment, proliferation, and differentiation of osteoblasts. Firstly, osteoblast attachment to the titanium implant material was impeded by whole e-cigarette smoke and nicotine alone, which decreased adhesion proteins (e.g., F-actin) and tissue mineralization [48]. Besides, fewer pseudopodia and less spread of MC3T3-E1 cells corresponded with the increased CSE concentrations [49]. Secondly, osteoblast proliferation was downregulated by CSE exposure in a dose-dependent manner [49]. A low dose of nicotine (0.3 μg/mL and 3 μg/mL) for 24 h increased the number of human osteoblast-like cells (MG-63), while a high dose of nicotine (30 μg/mL and 300 μg/mL) to the counterpart [50]. The nAChR antagonist abrogated the positive role of 1 μmol/L nicotine for days 3 and 6 in the proliferation of MC3T3-E1 cells [51]. However, BaP enhanced the proliferation of rat osteoblasts and MG-63 cells, possibly through the estrogen receptor, because estrogen receptor antagonist or estrogen receptor-negative cells inhibited BaP-induced proliferation [52]. Thirdly, osteoblast differentiation is negatively affected by cigarette smoking and its components, indicated by the gradual decrease in Runx2 expression with the increase of CSE concentration at days 7 and 14 [49]. Besides, 4 % cigarette smoke significantly reduced the ALP activity of MC3T3 cells on days 7 and 14 and the calcium deposition on days 28 [53,54]. Nicotine (1 μmol/L to 100 μmol/L) significantly reduced the ALP activity on day 7 and inhibited matrix maturation and mineralization on day 21 in MC3T3-E1 cells [51]. However, over a range of 1 nmol/L to 10 mmol/L, nicotine increased the ALP activity in a dose-dependent manner in UMR106-01 osteoblast-like cells [55]. The nicotine-induced osteoblast proliferation may explain this [55], and the sensibility of different cells to nicotine should also be considered. Even at low concentrations, carbon black significantly reduced the ALP activity and the expression of Runx2, BMP-2, and osteocalcin in MC3T3-E1 and MG-63 cells [56]. Taken together, smoking has a negative role in the development of osteoblasts.
Nicotine regulates gene expression related to bone metabolism in osteoblasts. Nicotine at 100 μmol/L altered the expression of 842 genes in MG-63 cells, which was related to cellular apoptosis, proliferation, and differentiation [57]. Besides, nicotine at 100 μmol/L upregulated the expression of genes that support osteoblast proliferation or suppress cellular apoptosis [57]. ALP, BSP, and Runx2 overexpression were observed in human osteoblasts with sub-toxic nicotine concentrations (0.1 μmol/L to 10 μmol/L) [58]. However, nicotine at 1 mmol/L significantly downregulated gene and protein expression of ALP, CloⅠα1, BSP, and OC in primary osteoblasts from Sprague–Dawley rats [59]. RT-PCR analysis indicated that the mRNA expression of CloⅠ, ALP, and OC experienced an increase with higher nicotine concentrations but dramatically decreased above 1 mmol/L nicotine in MG63 cells when cotreated for 24 h [60]. However, when cotreated for 72 h, the mRNA expression of these genes decreased with higher nicotine concentrations [60]. Therefore, nicotine has a biphasic role in regulating osteoblast-related gene expression as low dose and short-term nicotine upregulates the osteoblast differentiation, while high dose and long-term nicotine to the counterpart.
Smoking aggravates the oxidative stress in osteoblasts and subsequently induces apoptosis. On the one hand, mitochondrial oxidative stress caused by nicotine reduced sirtuin3 (SIRT3) to inhibit the activity of superoxide dismutase-2 (SOD-2), a mitochondrial anti-oxidative enzyme, eventually damaging mitochondrial DNA in osteoblasts [61]. Besides, nicotine drove hydroimidazoline aggravation, major advanced glycation end products deprived of methylglyoxal (MG), by inhibiting glyoxalase 1, eventually causing ROS-induced osteoblast apoptosis [62]. On the other hand, caspase-3 increased in cigarette smoke or nicotine-exposed osteoblasts [48]. The caspase-3 level was significantly higher in bone tissue samples from smokers and former smokers than those from non-smokers [63]. These results suggest that smoking and nicotine induce osteoblast apoptosis, possibly through the ROS/caspase-3 pathway (Fig. 2).
Antioxidants and AhR antagonists can ameliorate smoking-induced oxidative damage. Firstly, the Keap1 inhibitor alleviated nicotine-induced oxidative damage and osteoblast apoptosis by activating the anti-oxidative Nrf2 signaling [64]. Vitamin E, especially combined with vitamin C, significantly improved the viability, proliferation, and migration of MG-63 osteoblast-like cells and reduced nicotine-induced cellular apoptosis [65,66]. Quercetin and green tea extract promoted the expression of HO-1 and SOD-1 to scavenge cigarette smoke-induced ROS in human primary osteoblasts [67,68]. Secondly, AhR was responsible for initiating the adverse effect of cigarette smoke, and AhR antagonists, like resveratrol, α-Naphthoflavone, and 3,3′-Diindolylmethane, restored the inhibited cell adhesion and differentiation of MG63 cells [69]. Therefore, the adverse influence of smoking on osteoblasts can be improved by alleviating oxidative stress and inhibiting AhR signaling.
Smoking and nicotine negatively affect the osteoimmune function of osteoblasts. Nicotine induced a significant increase of IL-6 and TNF-α in mouse and human osteoblasts, respectively [70], partly explaining the elevated IL-1β, IL-6, and TNF-α in bone tissues from smokers [63]. Nicotine and lipopolysaccharide increased prostaglandin E2 production, which integrated with the Ep4 receptor on human osteoblastic Saos-2 cells to induce the macrophage colony-stimulating factor (M-CSF) in an autocrine or paracrine manner [71,72]. These increased molecules support the osteoclast formation and the initiation of Th17 response in osteoimmunology. Besides, AhR activation reduced the expression of CXCL12 and its receptor CXCR4 [69], the former of which contributed to a loss of B lymphoid progenitors in bone marrow [73]. Therefore, smoking influences the osteoimmune function of osteoblasts, favoring pro-inflammation response and bone destruction.
3.3. Smoking changes the differentiation of osteoclasts
Osteoclast differentiation is regulated by cigarette smoke and nicotine in a dose-dependent manner. Nicotine treatment (10 μmol/L to 1 mmol/L) decreased the number of osteoclasts with large nuclei (at least 10 nuclei), as well as the expression of cathepsin K and matrix metallopeptidase-9 (MMP-9), which was associated with the increased α7 nAChR expression on RAW264.7 cells [74]. Nicotine at 2.5 μg/mL and tobacco smoke mixture decreased the total number of tartrate-resistant acid phosphatase (TRAP)-positive cells in RANKL-stimulated rat primary pre-osteoclasts [75]. However, after RANKL stimulation in RAW264.7 cells, the number of TRAP-positive multinucleate osteoclasts was significantly higher in the smoking group with a similar serum nicotine level in smokers [53]. Nicotine at 1 μmol/L did not affect osteoclast differentiation in peripheral blood mononuclear cells (PBMCs) [76]. Taken together, nicotine at a low concentration supports osteoclast differentiation, while a high nicotine concentration inhibits osteoclastogenesis [77].
The RANKL/RANK signaling pathway is indispensable in smoking-induced osteoclast differentiation. Without the stimulation of M-CSF and RANKL, nicotine only initiated the early stages of osteoclast differentiation instead of promoting osteoclast maturation and bone resorption [78]. Firstly, the α7 nAChR is central in linking nicotine with osteoclastogenesis. Long-term treatment of nicotine, as well as other nAChR ligands, inhibited the calcium oscillation to downregulate the expression of c-Fos and NFATc1 in mouse macrophages, thus ultimately reducing the number of TRAP-positive mononuclear cells in a dose-dependent manner [77]. Nicotine activated α7 nAChR on macrophages to inhibit TNF-α expression, which favored OPG and inhibited RANKL in osteoblasts [79]. It can be supported by an elevated serum level of OPG/RANKL in mice lacking the α7 nAChR, which coincided with decreased osteoclast formation and improved bone loss [79]. These results suggest that α7 nAChR activation blocks the RANKL pathway directly or indirectly. Secondly, AhR also interacts with the RANK system. RANKL upregulated AhR expression earlier than the expression of osteoclast-specific genes, such as NFATc1 and cathepsin K, and AhR regulated the phosphorylation of MAPK and NF-κB to increase the expression of c-Fos and subsequently osteoclastogenesis [80]. When activating AhR by BaP administration, osteoclast differentiation and mitochondrial biogenesis were promoted in a RANKL/c-Fos-dependent manner, whereas these outcomes were reversed in AhR-deleted mice [80]. However, BaP inhibits RANKL-induced NF-κB activation and nuclear translocation in RAW264.7 cells and enhances the interaction between AhR and NF-κB response without RANKL [81]. These results show that AhR interacts with the NF-κB pathway to regulate osteoclastogenesis. Thirdly, cigarette smoke exposure elevated the expression of RANKL at days 3 and 14, while the production of RANK and OPG remained unchanged in male Wistar rats [82]. In the co-culture of osteoblasts and osteoclasts, CSE-induced osteoclast activation increased the RANKL/OPG ratio [83]. A daily administration of 84 μg nicotine for 3 weeks significantly increased the number of osteoclasts in mice bones, while nicotine withdrawal reduced the TRAP level and alleviated bone loss [84]. The CSE increased osteoclast formation from bone marrow cells and decreased osteoclast apoptosis by suppressing the mitochondrial ROS/cytochrome C/caspase-3 pathway in vitro [85]. Therefore, smoking regulates RANKL/RANK signaling to induce osteoclast formation, thus aggravating bone destruction (Fig. 1).
4. Effect of smoking on bone by the immune system
The osteoimmunological mechanisms underlying periodontitis, rheumatoid arthritis, and bone fracture healing have brought new insights into the pathology and treatment of these diseases. Smoking regulates the quantity and function of osteoimmune cells, particularly Th17 and Treg cells, leading to increased inflammatory and osteoclastogenic cytokines, further aggravating bone destruction during disease progression. Therapeutic interventions targeting the osteoimmune system show promise in alleviating the detrimental effects of smoking on periodontitis and rheumatoid arthritis.
4.1. Smoking and periodontitis
Periodontitis is a common osteoimmune disease featuring inflamed gingival and bone destruction caused by oral microbial dysbiosis and local inflammation, eventually leading to systematic bacterial dissemination and multiple-organ inflammatory lesions when treated inappropriately [86]. Porphyromonas gingivalis triggers periodontitis and microbial dysbiosis in the gingiva, which initiates local inflammation, activates host immune response, and subsequently aggravates alveolar bone destruction [87,88]. Smoking plays a negative role in the occurrence and progression of periodontitis and poses a global threat to periodontitis [89,90].
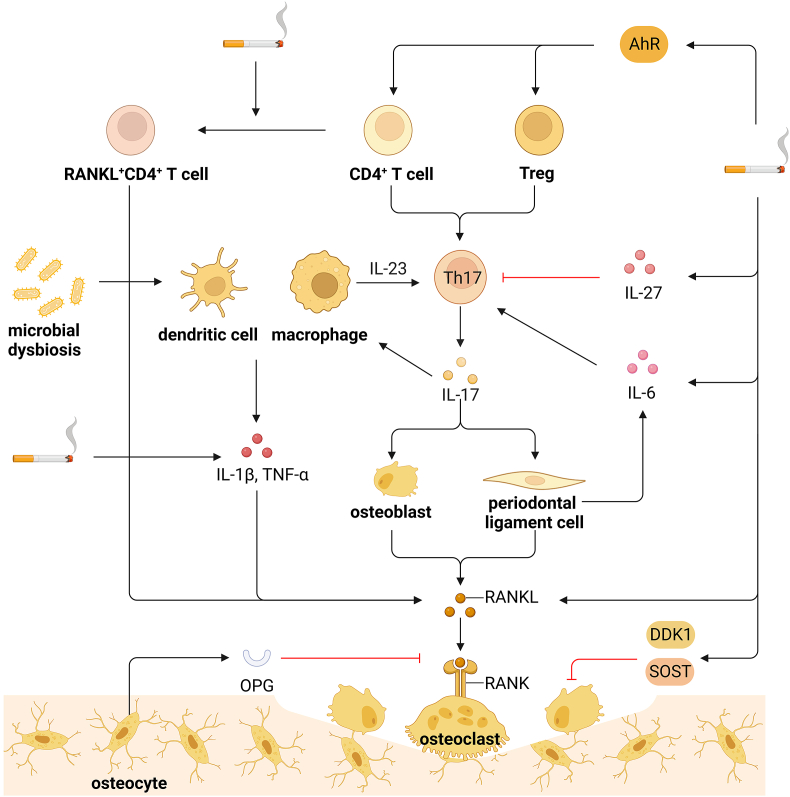
Th17 is critical in the osteoimmune pathogenesis of periodontitis, as Th17 secretes IL-17 and IL-23 to enhance T cell activation and bone destruction [7,91]. The generation of Th17 is triggered by bacterial invasion, and Th17 activates mucosal immunity and induces bone destruction to protect against local infection in a cytokine-dependent manner [92]. Although the RANKL on Th17 is insufficient for osteoclast differentiation, the elevated level of IL-17 can induce local inflammation and RANKL expression in osteoblasts and periodontal ligament cells to accelerate osteoclast differentiation [91,92]. In patients with primary chronic apical periodontitis, the quantitative evaluation showed a significant increase of IL-1β and IL-17 in periapical lesions extracted from smokers [93]. It can be supported by the increased IL-8, IL-17, and IFN-γ in gingival crevicular fluid from smokers [94]. Besides, the IL-17 A and IL-23 levels in the whole saliva were higher among cigarette smokers than non-smokers with periodontitis [95]. In addition, smoking exacerbated local and systematic inflammatory infiltrate supported by an increased concentration of IL-1β, IL-6, and TNF-α in the mandibles and serum of periodontitis patients [96,97]. These results suggest that smoking elevates IL-17 and other inflammatory cytokines to accelerate osteoclast differentiation and exacerbate bone destruction in periodontitis.
The proportion of Th17, as well as Th17-like cells, in CD4+ T cells and the Th17/Th1 ratio were increased in the blood of smokers [98,99]. CSE promoted the primary CD4+ lymphocytes to differentiate into Th17 cells [100]. Cigarette smoke and morphine promoted the Tregs conversion to Th17-like phenotype, which lost immune inhibitory capability while obtaining pro-inflammatory expression [101]. Cigarette smoke-induced Th17 differentiation was not observed in AhR antagonist-treated or AhR-deficient mice that were thus protected from arthritis aggravation [102,103]. In transgenic mice expressing human shared epitope, AhR antagonist treatment caused severe arthritis aggravation, overabundant osteoclasts, and IL-17-expressing cells in arthritic joints, which interacted with the NF-κB pathway [104]. Therefore, it is plausible to claim that smoking promotes Th17 differentiation partly through the AhR pathway and interacts with the NF-κB pathway to induce bone destruction in autoimmune arthritis and periodontitis. The mucosa-homing CCR6+ Th17 cells are more sensitive to CSE-induced senescence than peripheral Tregs [105]. Notably, the IL-27 in serum and IL-27 receptor in naive CD4+ T cells were elevated by cigarette smoke, and IL-27 enhanced IFN-γ-producing CD4+ T cells and inhibited IL-17-expressing subsets [106]. These results confirm that smoking can also negatively affect Th17 activity by inducing cell senescence and cytokine production, indicating that smoking-induced Th17 overabundance is the comprehensive effect of multiple mechanisms.
The upregulated Th17 in the peripheral blood of patients with periodontitis coincided with the downregulated Treg, and the Th17/Treg imbalance is involved in periodontitis [107,108]. Although the impact of smoking on disturbing Th17/Treg balance is widely accepted [109], the effect of smoking on Treg is controversial. Clinical statistics show that the percentage of Treg and forkhead helix transcription factor 3 (Foxp3) mRNA expression in the peripheral blood is higher in smokers than in non-smokers [[110], [111], [112]]. In C57BL/6 mice, decreased levels of TGF-β and IL-10 and an increased level of IL-17 occurred after cigarette smoke exposure for 3 months and 6 months, respectively, indicating that smoking induces Treg expression in an early stage [113]. However, passive smoking significantly reduced Treg-associated Foxp3 and TGF-β levels and increased Th17-associated IL-17 A and IL-23 levels in PBMCs [114]. CSE suppressed the differentiation of naive CD4+ T cells into Treg when stimulated with α-CD3 and α-CD28 monoclonal antibodies [115]. Notably, Treg secretes IL-4, IL-10, and TGF-β to inhibit osteoclast formation [116,117]. Therefore, smoking has a complicated role in Treg differentiation and reprogramming, especially in vivo experiments [118], and more research on smoking and Treg function in periodontitis is warranted.
The increased IL-17-expressing Th17 cell frequencies in smokers were also accompanied by higher percentages of RANKL+CD4+ T cells and RANKL+ IL-17+CD4+ T cells and CSE elevated RANKL expression of CD4+ T cells in vitro [119]. Notably, cigarette smoke significantly increased TNF-α and soluble RANKL and decreased OPG in the plasma of periodontitis patients [120]. In ligature-induced periodontitis model mice, nicotine and cigarette smoke condensate enhanced RANKL expression in the submandibular lymph nodes and the number of TRAP-positive osteoclasts in the ligated tissues, leading to alveolar bone loss [121]. Therefore, it is conceivable that smoking increases the RANKL expression in RANKL-expressing Th17 and other T cells, contributing to bone destruction in the progression of periodontitis. In addition, smoking upregulated sclerostin and dickkopf-1, two antagonists of Wnt/β-catenin signaling, to block the osteoblast differentiation at gingival protein and mRNA levels in periodontitis patients [122]. Nicotine reduced the ALP activity and calcium deposition in BMP-2-induced differentiation in human periodontal ligament-derived stem cells [123]. These results identify the detrimental role of smoking in osteoblast differentiation, leading to impaired bone formation in periodontitis (Fig. 3).
Figure 3.
Smoking and periodontitis a. Microbial dysbiosis stimulates dendritic cells and macrophages to trigger Th17 response and local inflammation. b. Th17 cells elevate the IL-17 level and subsequently induce RANKL expression in osteoblasts and periodontal ligament cells, the latter of which produces IL-6 to accumulate Th17 cells. c. Smoking elevates IL-17 and other inflammatory cytokines, including IL-1β, IL-6, and TNF-α, to activate RANKL signaling. d. Smoking promotes the primary CD4+ T cells to differentiate into Th17 cells and the Tregs conversion to Th17-like phenotype, which depends on the AhR pathway. e. Smoking increases the RANKL expression in RANKL-expressing Th17 and other RANKL+CD4+ T cells. f. Cigarette smoke elevates IL-27 to inhibit IL-17-expressing subsets. g. Smoking upregulates DKK1 and SOST to inhibit Wnt/β-catenin signaling and block the osteoblast differentiation. Overall, smoking enhances pro-inflammatory Th17 expression to aggravate bone destruction in periodontitis. DKK1, dickkopf-1; SOST, sclerostin.
Resveratrol is effective in ameliorating cigarette smoke-induced bone destruction aggravation in periodontic rats, as resveratrol reduced the Th17/Th2 ratio, increased the expression of IL-4, which suppresses osteoclast differentiation, and significantly decreased the RANKL expression in gingival tissues compared with placebo rats [116,124]. The local delivery of neutralizing IL-17 A antibodies in the periodontium of periodontic mice prevented alveolar bone loss and osteoclastic activity by decreasing IL-6 and IL-17 A [125]. Given the similar effect of IL-6 and IL-23 in activating IL-17-expressing Th17, both tocilizumab (an IL-6R inhibitor) and ustekinumab (an IL-12 and IL-23 antibody) significantly alleviated the periodontal condition of patients in clinical trials [126,127]. In IL-10-deficient mice, the expression of IL-17 and RANKL in the gingiva was elevated, with observed alveolar bone loss that was reduced by neutralizing IL-17 antibody injection in the palatal gingiva [128]. These results suggest that IL-17-targeted osteoimmunological therapies effectively reduce smoking-induced exacerbation of bone loss in periodontitis. In summary, smoking disturbs Th17/Treg balance by enhancing pro-inflammatory Th17 expression in a cytokine-dependent manner to aggravate alveolar bone destruction in periodontitis, and local IL-17 antibody treatment collaborated with antibiotics is promising in reversing periodontic deterioration brought by smoking (Table 1).
Table 1.
Th17-targeted osteoimmunological therapies in periodontitis.
| Treatment | Effects | Subject | Ref. |
|---|---|---|---|
| All-trans retinoic acid | downregulated IL-17 A; upregulated IL-10 and TGF-β1; decreased retinoid-related orphan receptor γτ-positive CD4+ cells; increased CD4+ Foxp3+ cells | Female C57BL/6 mice | [129] |
| Halofuginone | decreased IL-1β, IL-6, and TNF-α; inhibited immune cell infiltration and Th17 cell differentiation | C57BL/6 mice | [130] |
| Resveratrol | increased IL-4; decreased Th17/Th2 levels; downregulated mRNA expression of RANKL | Male Wistar rats | [124] |
| Tamibarotene | downregulated mRNA expression of IL-1β, IL-6, IL-17 A, MCP-1, and RANKL; upregulated IL-10 and TGF-β1 in gingival tissues | Female BALB/c mice | [131] |
| IL-1 receptor antagonist | decreased TNF-α, IL-1β, and IL-6 in vivo and in vitro; inhibited the NF-κB pathway; inhibited gingivitis and alveolar bone loss | RAW 264.7 cells male Sprague–Dawley rats | [132] |
| IL-17 A antibody | decreased IL-6; inhibited osteoclastic activity and alveolar bone loss | Male BALB/c mice | [125] |
| IL-21, anti-Tim1 and CD40L | increased gingival IL-10 mRNA and protein expressions; decreased gingival RANKL and periodontal bone loss | C57BL/6 J mice | [133] |
| Tocilizumab (IL-6R inhibitor) | decreased gingival index, bleeding on probing, probing depth, clinical attachment level, and serum IL-6 and MMP-3 | Patients with RA and chronic periodontitis | [126] |
| Ustekinumab (IL-12 and IL-23 antibody) | decreased bleeding on probing and oral inflammation; decreased IL-17 and IL-23; improved sacral wound | A patient with LAD1 | [127] |
Foxp3, forkhead helix transcription factor 3; IL-6R, interleukin 6 receptor; LAD1, leukocyte adhesion deficiency type 1; MCP-1, monocyte chemotactic protein-1; MMP-3, matrix metallopeptidase-3; RA, rheumatoid arthritis; Tim1, T-cell immunoglobulin and mucin domain 1.
4.2. Smoking and rheumatoid arthritis
Rheumatoid arthritis (RA) is a typical autoimmune disease characterized by chronic inflammation and cartilage and bone erosion in the inflamed joints, which has a similar osteoimmunological pathology to periodontitis, especially the Th17/Treg imbalance [134]. Both genetic and environmental factors, especially human leukocyte antigen (HLA) shared epitope (HLA-SE) alleles and smoking, can trigger the development of RA [135]. Smoking is involved in the development and severity of RA, while chronic inflammation, autoantibody formation, and epigenetic alterations are implicated in the pathology of RA in smokers [136].
Cigarette smoke exposure aggravated collagen-induced arthritis in transgenic mice carrying RA-susceptible HLA genes DQ8, which coincided with the elevated expression of Th17 genes [137]. IL-1β promoted differentiation, drove Th17 distribution, and enhanced IL-17 expression to exacerbate arthritis and systematic inflammation, which was reduced in IL-1R-depleted CD4+ cells [138]. In IL-17Ra-deficient mice, the IL-17 signaling was suppressed, as well as the smoking-induced arthritis aggravation [102]. The pro-inflammatory role of smoking is dependent on the AhR pathway in Th17, and the induced Th17 specifically expressed miRNA132 to increase osteoclasts in the inflamed joints [104,139]. Therefore, smoking modulates the differentiation and distribution of Th17 to aggravate inflammation and bone erosion in RA.
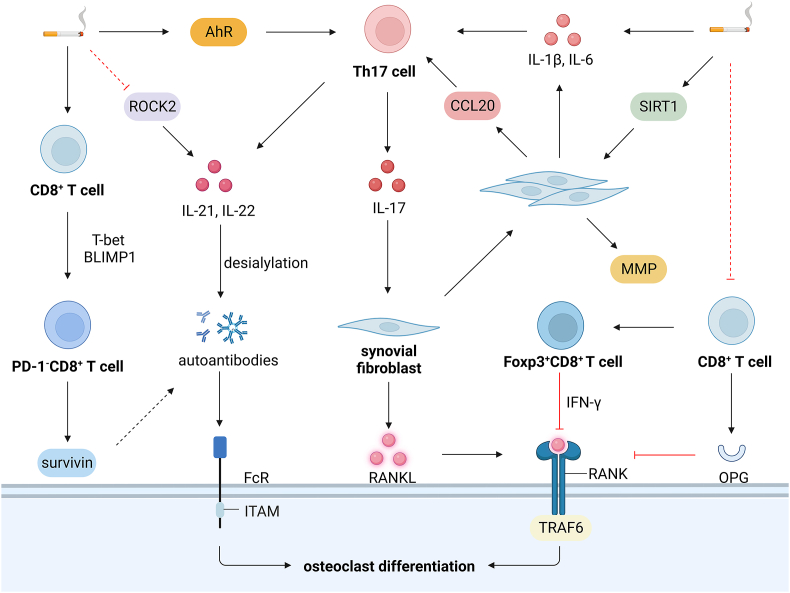
Smoking supported the PD-1 deficient phenotype of CD8+ T cells and increased survivin in the serum in RA patients [140]. Nicotine exposure stimulated the nicotinic receptors on CD8+ cells to induce the inhibitory transcriptional factors T-bet and BLIMP1, contributing to PD-1−CD8+ T cells and the release of survivin [140]. Notably, a persistently high level of survivin is associated with anti-citrullinated peptide antibodies (ACPA) and rheumatoid factor (RF) antibodies, as well as the disease severity and joint damage in RA patients [141]. The decreased serum soluble PD-1 ligand was found in RA smokers and was associated with aggravated systemic inflammation and ACPA positivity in RA [142]. These results implicate that CD8+ T cells are activated by the depletion of the PD-1 signaling in RA, and the concomitant survivin is involved in RA pathology. However, the percentage of CD8+ T cells was significantly lower in smokers than non-smokers, and CD8+ T cells inhibited osteoclast formation by directly integrating with the substantial OPG on CD8+ T cells [143,144]. In addition, osteoclasts acted as the APCs to induce the Foxp3+CD8+ T cells, which produced IFN-γ and degraded TRAF6 to inhibit osteoclast differentiation [145,146]. A low concentration of RANKL caused the Foxp3+CD8+ T cells to suppress bone resorption by Notch signaling, while a high-dose RANKL to the counterparts [145]. Therefore, smoking reduces the percentage of CD8+ T cells to aggravate bone erosion in RA, but the role of smoking on Foxp3+CD8+ T cells is unclear.
Smoking duration increases the risk of developing ACPA-positive RA, while smoking cessation diminishes the harmful effects [147]. Smoking history was associated with the seropositivity of ACPA IgA in RA patients, regardless of the presence of ACPA IgG, which was related to HLA-SE [148]. Besides, cigarette smoke conferred even more risks of a high level of RF antibodies despite HLA-SE and interacted with HLA-SE alleles on ACPA level, while prompt smoking cessation decreased the risks of excessive ACPA and RF [149]. Cigarette smoke exposure induced the production of citrullinated antigens and ACPA in the serum of collagen-induced arthritis mice [150]. The osteoclastogenesis depends on the activation of FcγR signaling, and the autoantibody IgG2 immune complex initiates this process by binding to FcγRI and FcγRIV under inflammatory conditions [151]. Besides, the desialylated IgG immune complex substantially affected osteoclast differentiation, determining bone structure in RA patients [152]. Smoking activates Th17 cells to generate IL-21 and IL-22 to promote the accumulation of desialylated IgG to strengthen osteoclastogenesis [153]. In addition, cigarette smoke increased the production of IL-22, possibly by inhibiting serine–threonine kinase ROCK2, which augmented the production of IL-17 and IL-21 b y interferon regulatory factor 4 (IRF4), a crucial transcriptional factor in Th17 differentiation [154]. Therefore, smoking induces autoantibody production to activate the RANKL signaling, thus aggravating bone erosion in RA.
Synovial fibroblasts play a crucial role in the local inflammation and bone erosion in RA, which express CCL20 to recruit CCR6+ Th17 cells [155], secrete IL-6 to stimulate Th17 differentiation, produce MMP to degrade cartilage and bone [156], and express the majority of RANKL to induce osteoclast formation and bone erosion in RA [157]. GNF351, an AhR antagonist, effectively inhibited the expression of IL-1β and IL-6 by separating AhR from the related promotors in human synovial fibroblasts [158]. CSE also enhanced the capability of RA synovial fibroblasts to express pro-inflammatory IL-8 and matrix-destructive MMP1, specifically by silencing SIRT 6 [159]. In synovial tissues of RA smokers, SIRT1 was upregulated, which enhanced the proliferation and leukocyte adhesion to RA synovial fibroblasts, but CSE-treated RA synovial fibroblasts displayed a decreased SIRT1 [160]. BaP facilitated the invasive properties of RA synovial fibroblasts, which depended on the Slug expression [161]. Therefore, smoking unlocks the potential of synovial fibroblasts to secrete inflammatory cytokines and matrix proteinase to aggravate bone erosion in RA (Fig. 4).
Figure 4.
Smoking and rheumatoid arthritis a. Nicotine stimulates CD8+ cells and induces T-bet and BLIMP1 to increase PD-1−CD8+ T cells and release survivin associated with autoantibody production. b. Smoking decreases the percentage of CD8+ T cells, and CD8+ T cells inhibit osteoclast formation by integrating with OPG on CD8+ T cells. c. Foxp3+CD8+ T cells produce IFN-γ and degrade TRAF6 to inhibit osteoclast differentiation, but the role of smoking on Foxp3+CD8+ T cells is unclear. d. Smoking activates Th17 cells in an AhR-dependent way to generate IL-21 and IL-22 and promote the desialylation of the IgG immune complex, substantially initiating osteoclast differentiation by binding to FcγRI and FcγRIV. e. Smoking increases IL-22 production, possibly by inhibiting ROCK2/IRF4 pathway. f. SIRT1 is upregulated in synovial tissues of RA smokers to enhance the proliferation and leukocyte adhesion to synovial fibroblasts. Foxp3, forkhead helix transcription factor 3; IFN-γ, interferon-γ; IRF4, interferon regulatory factor 4; MMP, MMP-3, matrix metallopeptidase; PD-1, programmed cell death protein 1; ROCK2, rho-associated coiled-coil containing protein kinase 2.
Recently, the most often utilized therapies in treating RA are TNFα and IL-6 monoclonal antibodies and JAK inhibitors, which are directly targeted at the inflammatory cytokines and the subsequent signaling pathway. Although inhibition of TNFα and IL-17 A displayed acceptable tolerability and consistent efficacy in treating RA, clinical evidence still denied the incrementally beneficial role of IL-17 A inhibition [162,163]. A low dose of IL-2 restored the Th17/Treg balance by increasing CD4+ Treg cells with efficacy and safety [164,165]. Besides, peresolimab, which stimulates PD-1 signaling, was effective in treating RA patients in a phase Ⅱ clinical trial [166]. Inhibiting IL-1 by anakinra, a recombinant human IL-1 receptor antagonist, reached the therapeutic targets of both RA and type 2 diabetes [167]. The addition of denosumab, a RANKL monoclonal antibody, inhibited bone erosion and improved bone architecture compared with a placebo, but the efficacy of denosumab was not significant [168,169]. Considering the correlation between fibroblast signatures, such as synovial B cell molecular signature, in patients and response to rituximab and tocilizumab, novel machine learning algorithms have been developed to optimize the use of drugs according to diverse molecular signatures [170]. These clinical trial outcomes demonstrate the promising therapeutic approaches targeted at the osteoimmune system, but the interactions between various therapies are rather complicated, and personalized interventions should be encouraged.
4.3. Smoking and bone fracture healing
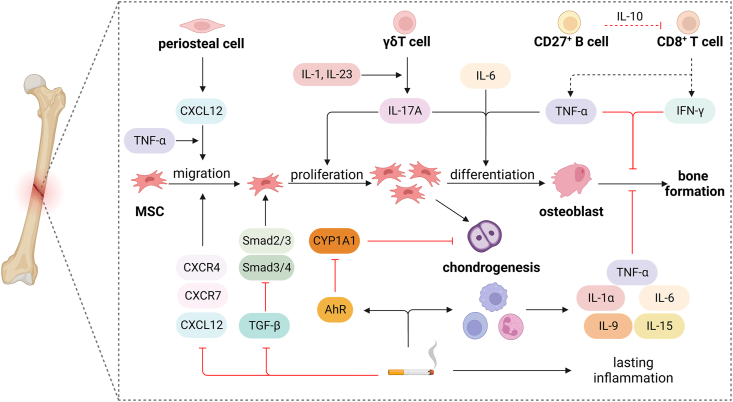
Bone fracture healing is a multistage process of bone regeneration comprising several events, including hematoma formation, inflammation response, callus generation, bone formation and remodeling, eventually restoring the shape, structure, and mechanical strength of the injured bone [8]. Upon hematoma formation, the macrophages and inflammatory cells produce TNF-α and IL-1 to remove necrotic tissue and initiate the differentiation of MSCs [171]. Notably, TNF-α promotes the integration of CXCL12 expressed by periosteal cells to CXCR4 on MSCs and enhances the MSC differentiation process, which is also enhanced by IL-17 A produced by γδT cells and IL-6 [12]. Besides, the increase of TNF-α and IL-1 in the later phase of bone repair indicates the vital role of inflammatory cytokines in bone formation and remodeling, and the levels of OPG, RANKL, and M-CSF are elevated asynchronously during fracture healing [171]. However, CD8+ T cells inhibit the healing process by secreting TNF-α and IFN-γ, which can be suppressed by IL-10 produced by CD27+ B cells [12]. These results suggest the vital role of the osteoimmune system in bone fracture healing.
Limited studies focus on the impact of smoking on the inflammatory response in bone fracture healing. A significant increase of TNF-α was detected 4 h and 48 h after hematoma formation in smoking groups, which was accompanied by significantly decreased levels of osteogenic markers, including Runx2, BMP-4, ALP activity, indicating that inflammation is boosted in hematoma of smokers and smoking impairs bone formation [172]. In bone fracture-induced hematoma, cigarette smoke significantly enhanced the number of macrophages, neutrophils, and lymphocytes, as well as the production of IL-1, IL-2, IL-6, IL-9, IL-15, and TNF-α, among which IL-1α, IL-6, IL-9, and IL-15 remained high concentrations 24 h after hematoma formation [173]. These results support the pro-inflammatory role of smoking in bone fracture healing.
Smoking harmed tibial shaft fracture healing as cigarette smoke increased the risk of nonunion, delayed union, and prolonged fracture healing time [174]. Low concentrations of CSE inhibited the osteogenic differentiation of MSCs, indicated by significantly reduced ALP activity and matrix mineralization [33]. Low-dose nicotine downregulated CXCR4, CXCR7, and CXCL12 to inhibit the migration of bone marrow stem cells to the fracture site [175]. CSE decreased TGF-β and downstream active Smad2/3 and Smad3/4 complex to impair the function of MSCs [176]. Besides, 82 activated the AhR pathway to inhibit TGF-β1/Smad4 and TGF-β1/ERK/AKT signaling pathways in bone marrow MSCs, partially explaining the BaP-induced tibial fracture delay [177]. Therefore, smoking inhibits the migration, proliferation, and differentiation of MSCs partly by the TGF-β pathway to delay bone fracture healing.
Smoking delays the chondrogenic phase of bone fracture healing. In mice with surgical tibial fracture, cigarette smoke exposure induced a smaller fracture callus on day 7 after injury and a larger fracture callus on day 28, and chondrogenesis was more active on day 14 in smoking groups [178]. The cartilage size and percentage of cartilage in total callus also decreased in early chondrogenesis [173]. Notably, chondrocytes expressed AhR during differentiation, and the strong inhibitory role of CSE on chondrogenesis depended on the AhR/CYP1A1 signaling pathway [179]. In addition, exposing mice to CSE for 10 days was sufficient and effective in inhibiting osteoblast differentiation and enhancing osteoclast activity, and the NF-κB signaling was activated to initiate bone remodeling in osteoclasts [180]. Eventually, smoking disrupts the balance of bone formation and absorption and leads to decreased maturity, density, and mechanical strength in bone fracture healing [181] (Fig. 5).
Figure 5.
Smoking and bone fracture healing a. Low-dose nicotine downregulates CXCR4, CXCR7, and CXCL12 to inhibit the migration of bone marrow stem cells to the fracture site. b. CSE decreases TGF-β and downstream active Smad2/3 and Smad3/4 complex to impair the function of MSCs. c. Smoking strongly inhibits chondrogenesis through the AhR/CYP1A1 signaling pathway. d. Cigarette smoke significantly enhances the number of macrophages, neutrophils, and lymphocytes to maintain high concentrations of TNF-α, IL-1α, IL-6, IL-9, and IL-15 after hematoma formation.
4.4. Smoking and osteoarthritis
Osteoarthritis is the most common joint disease, mainly affecting the whole knee, hand, and hip joint [182]. The mechanisms underlying osteoarthritis include cartilage degradation, subchondral bone damage, synovial and systemic inflammation, mechanical and metabolic alterations [183,184]. Smoking is positively associated with osteoarthritis (OR 1.31, 95 % CI 1.24–1.38, n = 84,898 cases) [185]. In the United States general population, the current and former smoking rate is significantly higher in participants with osteoarthritis than those without [186]. However, in the Korean general older adult population, an inverse association is reported between smoking and the prevalence of knee osteoarthritis [187]. These results suggest that there is a complex association between smoking and osteoarthritis, which may differ in different populations and osteoarthritic phenotypes.
Nicotine affects the function of articular chondrocytes and the process of chondrogenesis and osteogenesis in a dose-dependent manner, with lower doses usually causing positive effects and higher doses to the counterpart [188]. Prenatal nicotine exposure impairs articular cartilage development and MSC-related osteochondral repair, ultimately leading to osteoarthritis in offspring [188]. Nicotine induces an inflammatory response to enhance the extracellular matrix degradation, which can be interestingly inhibited by estrogen [189]. Cigarette smoke deteriorates osteoarthritis progression and systemic inflammation by increasing the MMP-13 expression and activating the NF-κB pathway [190]. BaP exposure aggravates the mandibular subchondral bone loss in the osteoarthritic mouse, indicated by increased osteoclastic bone resorption [191]. Besides, smoking promotes systemic oxidative stress in young adults and increases pain and cartilage loss in the development of osteoarthritis [192]. CSE increases oxidative stress to induce chondrocyte death and disrupt cartilage homeostasis, as 10 % CSE produces sufficient free radicals to lead to cell death [193]. These results support the detrimental role of smoking in osteoarthritis development.
5. Conclusion
The risk of smoking on health and disease is still a global concern. Smoking is believed to aggravate bone destruction in various skeletal diseases, but the understanding of the underlying mechanisms and therapeutic approaches is limited. Osteoimmunology is a field that focuses on the interplay between bone metabolism and immune homeostasis to understand the relationship between osteoimmune interaction and disease development. Smoking impairs the osteogenic differentiation of MSCs and osteoblasts and promotes osteoclast formation by activating the RANKL signaling pathway, ultimately leading to bone destruction. Additionally, smoking regulates the quantity and function of osteoimmune cells, particularly Th17 and Treg cells, leading to increased inflammatory and osteoclastogenic cytokines, further aggravating bone destruction. Therapeutic interventions targeting the osteoimmune system show promise in alleviating the detrimental effects of smoking on periodontitis and rheumatoid arthritis. However, further research is still required to assess the effectiveness of therapeutic strategies targeting osteoimmune cytokines.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2019YFA0111900); National Natural Science Foundation of China (No.82072506, 92268115, 82372500); Hunan Provincial Science Fund for Distinguished Young Scholars (No. 2024JJ2089), Science and Technology Innovation Program of Hunan Province (No. 2021RC3025); Wu Jieping Medical Foundation (320.6750.2020-03-14), National Clinical Research Center for Geriatric Disorders (Xiangya Hospital, No. 2021KF02); National High Level Hospital Clinical Research Funding (No. 2022-NHLHCRF-LX-02-0104) & Elite Medical Professionals Project of China-Japan Friendship Hospital (ZRJY2021-QM21).
References
- 1.Ma C., Xi B., Li Z., Wu H., Zhao M., Liang Y., et al. Prevalence and trends in tobacco use among adolescents aged 13-15 years in 143 countries, 1999-2018: findings from the Global Youth Tobacco Surveys. Lancet Child Adolesc Health. 2021;4:245–275. doi: 10.1016/S2352-4642(20)30390-4. the number of cases is 54. [DOI] [PubMed] [Google Scholar]
- 2.Ahluwalia I.B., Smith T., Arrazola R.A., Palipudi K.M., Garcia de Quevedo I., Prasad V.M., et al. Current tobacco smoking, quit attempts, and knowledge about smoking risks among persons aged >/=15 Years - global adult tobacco survey, 28 countries, 2008-2016. MMWR Morb Mortal Wkly Rep. 2018;67(38):1072–1076. doi: 10.15585/mmwr.mm6738a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborators G.B.D.T. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397(10292):2337–2360. doi: 10.1016/S0140-6736(21)01169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan K.H., Wright N., Xiao D., Guo Y., Chen Y., Du H., et al. Tobacco smoking and risks of more than 470 diseases in China: a prospective cohort study. Lancet Public Health. 2022;7(12):e1014–e1026. doi: 10.1016/S2468-2667(22)00227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apatzidou D.A. The role of cigarette smoking in periodontal disease and treatment outcomes of dental implant therapy. Periodontol. 2000 2022;90(1):45–61. doi: 10.1111/prd.12449. [DOI] [PubMed] [Google Scholar]
- 6.Mahdi H., Fisher B.A., Kallberg H., Plant D., Malmstrom V., Ronnelid J., et al. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat Genet. 2009;41(12):1319–1324. doi: 10.1038/ng.480. [DOI] [PubMed] [Google Scholar]
- 7.Tsukasaki M., Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat Rev Immunol. 2019;19(10):626–642. doi: 10.1038/s41577-019-0178-8. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto K., Nakashima T., Shinohara M., Negishi-Koga T., Komatsu N., Terashima A., et al. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev. 2017;97(4):1295–1349. doi: 10.1152/physrev.00036.2016. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Osuna L., Sierra-Cristancho A., Rojas C., Cafferata E.A., Melgar-Rodriguez S., Cardenas A.M., et al. Premature senescence of T-cells favors bone loss during osteolytic diseases. A new concern in the osteoimmunology arena. Aging Dis. 2021;12(5):1150–1161. doi: 10.14336/AD.2021.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh M.C., Takegahara N., Kim H., Choi Y. Updating osteoimmunology: regulation of bone cells by innate and adaptive immunity. Nat Rev Rheumatol. 2018;14(3):146–156. doi: 10.1038/nrrheum.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alghamdi B., Jeon H.H., Ni J., Qiu D., Liu A., Hong J.J., et al. Osteoimmunology in periodontitis and orthodontic tooth movement. Curr Osteoporos Rep. 2023;21(2):128–146. doi: 10.1007/s11914-023-00774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono T., Takayanagi H. Osteoimmunology in bone fracture healing. Curr Osteoporos Rep. 2017;15(4):367–375. doi: 10.1007/s11914-017-0381-0. [DOI] [PubMed] [Google Scholar]
- 13.Josien R., Wong B.R., Li H.L., Steinman R.M., Choi Y. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J Immunol. 1999;162(5):2562–2568. [PubMed] [Google Scholar]
- 14.Anderson D.M., Maraskovsky E., Billingsley W.L., Dougall W.C., Tometsko M.E., Roux E.R., et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390(6656):175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 15.Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J.Q., et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 16.Xiong J., Onal M., Jilka R.L., Weinstein R.S., Manolagas S.C., O'Brien C.A. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17(10):1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95(7):3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darnay B.G., Haridas V., Ni J., Moore P.A., Aggarwal B.B. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J Biol Chem. 1998;273(32):20551–20555. doi: 10.1074/jbc.273.32.20551. [DOI] [PubMed] [Google Scholar]
- 19.Kim N., Kadono Y., Takami M., Lee J., Lee S.H., Okada F., et al. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202(5):589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizukami J., Takaesu G., Akatsuka H., Sakurai H., Ninomiya-Tsuji J., Matsumoto K., et al. Receptor activator of NF-kappaB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB2, and TRAF6. Mol Cell Biol. 2002;22(4):992–1000. doi: 10.1128/MCB.22.4.992-1000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David J.P., Sabapathy K., Hoffmann O., Idarraga M.H., Wagner E.F. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J Cell Sci. 2002;115(Pt 22):4317–4325. doi: 10.1242/jcs.00082. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita T., Yao Z., Li F., Zhang Q., Badell I.R., Schwarz E.M., et al. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem. 2007;282(25):18245–18253. doi: 10.1074/jbc.M610701200. [DOI] [PubMed] [Google Scholar]
- 23.Koga T., Inui M., Inoue K., Kim S., Suematsu A., Kobayashi E., et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428(6984):758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 24.Kim N., Takami M., Rho J., Josien R., Choi Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med. 2002;195(2):201–209. doi: 10.1084/jem.20011681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joyce-Shaikh B., Bigler M.E., Chao C.C., Murphy E.E., Blumenschein W.M., Adamopoulos I.E., et al. Myeloid DAP12-associating lectin (MDL)-1 regulates synovial inflammation and bone erosion associated with autoimmune arthritis. J Exp Med. 2010;207(3):579–589. doi: 10.1084/jem.20090516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishida-Kitagawa N., Tanaka K., Bao X., Kimura T., Miura T., Kitaoka Y., et al. Siglec-15 protein regulates formation of functional osteoclasts in concert with DNAX-activating protein of 12 kDa (DAP12) J Biol Chem. 2012;287(21):17493–17502. doi: 10.1074/jbc.M111.324194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mocsai A., Humphrey M.B., Van Ziffle J.A., Hu Y., Burghardt A., Spusta S.C., et al. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci U S A. 2004;101(16):6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinohara M., Koga T., Okamoto K., Sakaguchi S., Arai K., Yasuda H., et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132(5):794–806. doi: 10.1016/j.cell.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 29.Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H., et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 30.Kim K., Kim J.H., Lee J., Jin H.M., Lee S.H., Fisher D.E., et al. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J Biol Chem. 2005;280(42):35209–35216. doi: 10.1074/jbc.M505815200. [DOI] [PubMed] [Google Scholar]
- 31.Harrell C.R., Djonov V., Volarevic V. The effects of cigarette smoking and nicotine on the therapeutic potential of mesenchymal stem cells. Histol Histopathol. 2022;37(2):93–100. doi: 10.14670/HH-18-400. [DOI] [PubMed] [Google Scholar]
- 32.Shaito A., Saliba J., Husari A., El-Harakeh M., Chhouri H., Hashem Y., et al. Electronic cigarette smoke impairs normal mesenchymal stem cell differentiation. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-14634-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aspera-Werz R.H., Ehnert S., Muller M., Zhu S., Chen T., Weng W., et al. Assessment of tobacco heating system 2.4 on osteogenic differentiation of mesenchymal stem cells and primary human osteoblasts compared to conventional cigarettes. World J Stem Cell. 2020;12(8):841–856. doi: 10.4252/wjsc.v12.i8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cyprus G.N., Overlin J.W., Hotchkiss K.M., Kandalam S., Olivares-Navarrete R. Cigarette smoke increases pro-inflammatory markers and inhibits osteogenic differentiation in experimental exposure model. Acta Biomater. 2018;76:308–318. doi: 10.1016/j.actbio.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Wahl E.A., Schenck T.L., Machens H.G., Egana J.T. Acute stimulation of mesenchymal stem cells with cigarette smoke extract affects their migration, differentiation, and paracrine potential. Sci Rep. 2016;6 doi: 10.1038/srep22957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim B.S., Kim S.J., Kim H.J., Lee S.J., Park Y.J., Lee J., et al. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sci. 2012;90(3–4):109–115. doi: 10.1016/j.lfs.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Ying X., Zhang W., Cheng S., Nie P., Cheng X., Shen Y., et al. Nicotine-induced chondrogenic differentiation of human bone marrow stromal cells in vitro. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2329–2336. doi: 10.1007/s00167-012-1890-0. [DOI] [PubMed] [Google Scholar]
- 38.An L., Shi Q., Fan M., Huang G., Zhu M., Zhang M., et al. Benzo[a]pyrene injures BMP2-induced osteogenic differentiation of mesenchymal stem cells through AhR reducing BMPRII. Ecotoxicol Environ Saf. 2020;203 doi: 10.1016/j.ecoenv.2020.110930. [DOI] [PubMed] [Google Scholar]
- 39.Shen Y., Wu L., Qin D., Xia Y., Zhou Z., Zhang X., et al. Carbon black suppresses the osteogenesis of mesenchymal stem cells: the role of mitochondria. Part Fibre Toxicol. 2018;15(1):16. doi: 10.1186/s12989-018-0253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aspera-Werz R.H., Ehnert S., Heid D., Zhu S., Chen T., Braun B., et al. Nicotine and cotinine inhibit catalase and glutathione reductase activity contributing to the impaired osteogenesis of SCP-1 cells exposed to cigarette smoke. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/3172480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bin Homran F.M., Alaskari A.A., Devaraj A., Udeabor S.E., Al-Hakami A., Joseph B., et al. Chronic metabolic and induced stress impacts mesenchymal stromal cell differentiation and modulation of dental origin in-vitro. Saudi J Biol Sci. 2022;29(4):2230–2237. doi: 10.1016/j.sjbs.2021.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim D.H., Liu J., Bhat S., Benedict G., Lecka-Czernik B., Peterson S.J., et al. Peroxisome proliferator-activated receptor delta agonist attenuates nicotine suppression effect on human mesenchymal stem cell-derived osteogenesis and involves increased expression of heme oxygenase-1. J Bone Miner Metabol. 2013;31(1):44–52. doi: 10.1007/s00774-012-0382-0. [DOI] [PubMed] [Google Scholar]
- 43.Yang X., Qi Y., Avercenc-Leger L., Vincourt J.B., Hupont S., Huselstein C., et al. Effect of nicotine on the proliferation and chondrogenic differentiation of the human Wharton's jelly mesenchymal stem cells. Bio Med Mater Eng. 2017;28(s1):S217–S228. doi: 10.3233/BME-171644. [DOI] [PubMed] [Google Scholar]
- 44.Xiao H., Wen Y., Pan Z., Shangguan Y., Magdalou J., Wang H., et al. Nicotine exposure during pregnancy programs osteopenia in male offspring rats via alpha4beta2-nAChR-p300-ACE pathway. Faseb J. 2019;33(11):12972–12982. doi: 10.1096/fj.201901145RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gazdic M., Volarevic V., Arsenijevic N., Stojkovic M. Mesenchymal stem cells: a friend or foe in immune-mediated diseases. Stem Cell Rev Rep. 2015;11(2):280–287. doi: 10.1007/s12015-014-9583-3. [DOI] [PubMed] [Google Scholar]
- 46.Cruz T., Lopez-Giraldo A., Noell G., Guirao A., Casas-Recasens S., Garcia T., et al. Smoking impairs the immunomodulatory capacity of lung-resident mesenchymal stem cells in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2019;61(5):575–583. doi: 10.1165/rcmb.2018-0351OC. [DOI] [PubMed] [Google Scholar]
- 47.Pavlovic D., Miloradovic D., Stojanovic M.D., Harrell C.R., Polosa R., Rust S., et al. Cigarette smoke attenuates mesenchymal stem cell-based suppression of immune cell-driven acute liver failure. Toxicol Lett. 2023;385:12–20. doi: 10.1016/j.toxlet.2023.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Rouabhia M., Alanazi H., Park H.J., Goncalves R.B. Cigarette smoke and E-cigarette vapor dysregulate osteoblast interaction with titanium dental implant surface. J Oral Implantol. 2019;45(1):2–11. doi: 10.1563/aaid-joi-D-18-00009. [DOI] [PubMed] [Google Scholar]
- 49.Yang J., Shao S.Y., Chen W.Q., Chen C., Zhang S.M., Qiu J. Cigarette smoke extract exposure: effects on the interactions between titanium surface and osteoblasts. BioMed Res Int. 2019;2019 doi: 10.1155/2019/8759568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papaioannou K.A., Markopoulou C.E., Gioni V., Mamalis A.A., Vayouraki H.N., Kletsas D., et al. Attachment and proliferation of human osteoblast-like cells on guided bone regeneration (GBR) membranes in the absence or presence of nicotine: an in vitro study. Int J Oral Maxillofac Implants. 2011;26(3):509–519. [PubMed] [Google Scholar]
- 51.Sato T., Abe T., Nakamoto N., Tomaru Y., Koshikiya N., Nojima J., et al. Nicotine induces cell proliferation in association with cyclin D1 up-regulation and inhibits cell differentiation in association with p53 regulation in a murine pre-osteoblastic cell line. Biochem Biophys Res Commun. 2008;377(1):126–130. doi: 10.1016/j.bbrc.2008.09.114. [DOI] [PubMed] [Google Scholar]
- 52.Tsai K.S., Yang R.S., Liu S.H. Benzo[a]pyrene regulates osteoblast proliferation through an estrogen receptor-related cyclooxygenase-2 pathway. Chem Res Toxicol. 2004;17(5):679–684. doi: 10.1021/tx0499517. [DOI] [PubMed] [Google Scholar]
- 53.Ko C.H., Chan R.L., Siu W.S., Shum W.T., Leung P.C., Zhang L., et al. Deteriorating effect on bone metabolism and microstructure by passive cigarette smoking through dual actions on osteoblast and osteoclast. Calcif Tissue Int. 2015;96(5):389–400. doi: 10.1007/s00223-015-9966-8. [DOI] [PubMed] [Google Scholar]
- 54.Nishino K., Tamai K., Orita K., Hashimoto Y., Nakamura H. Heated tobacco products impair cell viability, osteoblastic differentiation, and bone fracture-healing. J Bone Joint Surg Am. 2021;103(21):2024–2031. doi: 10.2106/JBJS.20.02227. [DOI] [PubMed] [Google Scholar]
- 55.Fang M.A., Frost P.J., Iida-Klein A., Hahn T.J. Effects of nicotine on cellular function in UMR 106-01 osteoblast-like cells. Bone. 1991;12(4):283–286. doi: 10.1016/8756-3282(91)90077-v. [DOI] [PubMed] [Google Scholar]
- 56.Ren Q., Wu Y., Ma J., Shan Q., Liu S., Liu Y. Carbon black-induced detrimental effect on osteoblasts at low concentrations: remarkably compromised differentiation without significant cytotoxicity. Ecotoxicol Environ Saf. 2019;178:211–220. doi: 10.1016/j.ecoenv.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 57.Rothem D.E., Rothem L., Dahan A., Eliakim R., Soudry M. Nicotinic modulation of gene expression in osteoblast cells, MG-63. Bone. 2011;48(4):903–909. doi: 10.1016/j.bone.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Marinucci L., Bodo M., Balloni S., Locci P., Baroni T. Sub-toxic nicotine concentrations affect extracellular matrix and growth factor signaling gene expressions in human osteoblasts. J Cell Physiol. 2014;229(12):2038–2048. doi: 10.1002/jcp.24661. [DOI] [PubMed] [Google Scholar]
- 59.Liang D., Wang K.J., Tang Z.Q., Liu R.H., Zeng F., Cheng M.Y., et al. Effects of nicotine on the metabolism and gene expression profile of Sprague-Dawley rat primary osteoblasts. Mol Med Rep. 2018;17(6):8269–8281. doi: 10.3892/mmr.2018.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rothem D.E., Rothem L., Soudry M., Dahan A., Eliakim R. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. J Bone Miner Metabol. 2009;27(5):555–561. doi: 10.1007/s00774-009-0075-5. [DOI] [PubMed] [Google Scholar]
- 61.Li Y., Yu C., Shen G., Li G., Shen J., Xu Y., et al. Sirt3-MnSOD axis represses nicotine-induced mitochondrial oxidative stress and mtDNA damage in osteoblasts. Acta Biochim Biophys Sin. 2015;47(4):306–312. doi: 10.1093/abbs/gmv013. [DOI] [PubMed] [Google Scholar]
- 62.Marinucci L., Balloni S., Fettucciari K., Bodo M., Talesa V.N., Antognelli C. Nicotine induces apoptosis in human osteoblasts via a novel mechanism driven by H(2)O(2) and entailing Glyoxalase 1-dependent MG-H1 accumulation leading to TG2-mediated NF-kB desensitization: implication for smokers-related osteoporosis. Free Radic Biol Med. 2018;117:6–17. doi: 10.1016/j.freeradbiomed.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 63.Kohler J.B., da Silva A.F., Farias W.A., Sampaio B.F.C., Neves M.A.S., Lima L.G., et al. Smoking induces increased apoptosis in osteoblasts: changes in bone matrix organic components. Sci Rep. 2023;13(1):6938. doi: 10.1038/s41598-023-33965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng Y.H., Yang J.J., Tang P.J., Zhu Y., Chen Z., She C., et al. A novel Keap1 inhibitor iKeap1 activates Nrf2 signaling and ameliorates hydrogen peroxide-induced oxidative injury and apoptosis in osteoblasts. Cell Death Dis. 2021;12(7):679. doi: 10.1038/s41419-021-03962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torshabi M., Rezaei Esfahrood Z., Jamshidi M., Mansuri Torshizi A., Sotoudeh S. Efficacy of vitamins E and C for reversing the cytotoxic effects of nicotine and cotinine. Eur J Oral Sci. 2017;125(6):426–437. doi: 10.1111/eos.12375. [DOI] [PubMed] [Google Scholar]
- 66.Torshabi M., Esfahrood Z.R., Gholamin P., Karami E. Effects of nicotine in the presence and absence of vitamin E on morphology, viability and osteogenic gene expression in MG-63 osteoblast-like cells. J Basic Clin Physiol Pharmacol. 2016;27(6):595–602. doi: 10.1515/jbcpp-2015-0143. [DOI] [PubMed] [Google Scholar]
- 67.Braun K.F., Ehnert S., Freude T., Egana J.T., Schenck T.L., Buchholz A., et al. Quercetin protects primary human osteoblasts exposed to cigarette smoke through activation of the antioxidative enzymes HO-1 and SOD-1. Sci World J. 2011;11:2348–2357. doi: 10.1100/2011/471426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holzer N., Braun K.F., Ehnert S., Egana J.T., Schenck T.L., Buchholz A., et al. Green tea protects human osteoblasts from cigarette smoke-induced injury: possible clinical implication. Langenbeck's Arch Surg. 2012;397(3):467–474. doi: 10.1007/s00423-011-0882-8. [DOI] [PubMed] [Google Scholar]
- 69.Yun C., Katchko K.M., Schallmo M.S., Jeong S., Yun J., Chen C.H., et al. Aryl hydrocarbon receptor antagonists mitigate the effects of dioxin on critical cellular functions in differentiating human osteoblast-like cells. Int J Mol Sci. 2018;19(1) doi: 10.3390/ijms19010225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamer A.R., El-Ghorab N., Marzec N., Margarone J.E., Dziak R. Nicotine induced proliferation and cytokine release in osteoblastic cells. Int J Mol Med. 2006;17(1):121–127. [PubMed] [Google Scholar]
- 71.Tanaka H., Tanabe N., Shoji M., Suzuki N., Katono T., Sato S., et al. Nicotine and lipopolysaccharide stimulate the formation of osteoclast-like cells by increasing macrophage colony-stimulating factor and prostaglandin E2 production by osteoblasts. Life Sci. 2006;78(15):1733–1740. doi: 10.1016/j.lfs.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 72.Shoji M., Tanabe N., Mitsui N., Suzuki N., Takeichi O., Katono T., et al. Lipopolysaccharide enhances the production of nicotine-induced prostaglandin E2 by an increase in cyclooxygenase-2 expression in osteoblasts. Acta Biochim Biophys Sin. 2007;39(3):163–172. doi: 10.1111/j.1745-7270.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 73.Greenbaum A., Hsu Y.M., Day R.B., Schuettpelz L.G., Christopher M.J., Borgerding J.N., et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanaka H., Tanabe N., Kawato T., Nakai K., Kariya T., Matsumoto S., et al. Nicotine affects bone resorption and suppresses the expression of cathepsin K, MMP-9 and vacuolar-type H(+)-ATPase d2 and actin organization in osteoclasts. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0059402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagaie M., Nishiura A., Honda Y., Fujiwara S., Matsumoto N. A comprehensive mixture of tobacco smoke components retards orthodontic tooth movement via the inhibition of osteoclastogenesis in a rat model. Int J Mol Sci. 2014;15(10):18610–18622. doi: 10.3390/ijms151018610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ternes S., Trinkaus K., Bergen I., Knaack S., Gelinsky M., Kilian O., et al. Impact of acetylcholine and nicotine on human osteoclastogenesis in vitro. Int Immunopharm. 2015;29(1):215–221. doi: 10.1016/j.intimp.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 77.Mandl P., Hayer S., Karonitsch T., Scholze P., Gyori D., Sykoutri D., et al. Nicotinic acetylcholine receptors modulate osteoclastogenesis. Arthritis Res Ther. 2016;18:63. doi: 10.1186/s13075-016-0961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costa-Rodrigues J., Rocha I., Fernandes M.H. Complex osteoclastogenic inductive effects of nicotine over hydroxyapatite. J Cell Physiol. 2018;233(2):1029–1040. doi: 10.1002/jcp.25956. [DOI] [PubMed] [Google Scholar]
- 79.Mito K., Sato Y., Kobayashi T., Miyamoto K., Nitta E., Iwama A., et al. The nicotinic acetylcholine receptor alpha7 subunit is an essential negative regulator of bone mass. Sci Rep. 2017;7 doi: 10.1038/srep45597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Izawa T., Arakaki R., Mori H., Tsunematsu T., Kudo Y., Tanaka E., et al. The nuclear receptor AhR controls bone homeostasis by regulating osteoclast differentiation via the RANK/c-Fos signaling Axis. J Immunol. 2016;197(12):4639–4650. doi: 10.4049/jimmunol.1600822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Voronov I., Li K., Tenenbaum H.C., Manolson M.F. Benzo[a]pyrene inhibits osteoclastogenesis by affecting RANKL-induced activation of NF-kappaB. Biochem Pharmacol. 2008;75(10):2034–2044. doi: 10.1016/j.bcp.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 82.Arnez M.F.M., Monteiro P.M., Paula-Silva F.W.G., Dessotti G.B., Menezes L.M., Kuchler E.C., et al. Impact of cigarette smoke on osteogenic and osteoclast signaling in middle palatal suture. Braz Dent J. 2022;33(2):99–108. doi: 10.1590/0103-6440202203966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu S., Haussling V., Aspera-Werz R.H., Chen T., Braun B., Weng W., et al. Bisphosphonates reduce smoking-induced osteoporotic-like alterations by regulating RANKL/OPG in an osteoblast and osteoclast Co-culture model. Int J Mol Sci. 2020;22(1) doi: 10.3390/ijms22010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kiyota Y., Muramatsu H., Sato Y., Kobayashi T., Miyamoto K., Iwamoto T., et al. Smoking cessation increases levels of osteocalcin and uncarboxylated osteocalcin in human sera. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-73789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qin Y., Liu Y., Jiang Y., Mei S., Liu Y., Feng J., et al. Cigarette smoke exposure inhibits osteoclast apoptosis via the mtROS pathway. J Dent Res. 2021;100(12):1378–1386. doi: 10.1177/00220345211009471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kharlamova N., Jiang X., Sherina N., Potempa B., Israelsson L., Quirke A.M., et al. Antibodies to porphyromonas gingivalis indicate interaction between oral infection, smoking, and risk genes in rheumatoid arthritis etiology. Arthritis Rheumatol. 2016;68(3):604–613. doi: 10.1002/art.39491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan W., Wang Q., Chen Q. The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci. 2019;11(3):30. doi: 10.1038/s41368-019-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leite F.R.M., Nascimento G.G., Scheutz F., Lopez R. Effect of smoking on periodontitis: a systematic review and meta-regression. Am J Prev Med. 2018;54(6):831–841. doi: 10.1016/j.amepre.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 90.Schwendicke F., Dorfer C.E., Meier T. Global smoking-attributable burden of periodontal disease in 186 countries in the year 2015. J Clin Periodontol. 2018;45(1):2–14. doi: 10.1111/jcpe.12823. [DOI] [PubMed] [Google Scholar]
- 91.Sato K., Suematsu A., Okamoto K., Yamaguchi A., Morishita Y., Kadono Y., et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsukasaki M., Komatsu N., Nagashima K., Nitta T., Pluemsakunthai W., Shukunami C., et al. Host defense against oral microbiota by bone-damaging T cells. Nat Commun. 2018;9(1):701. doi: 10.1038/s41467-018-03147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thuller K., Armada L., Valente M.I., Pires F.R., Vilaca C.M.M., Gomes C.C. Immunoexpression of interleukin 17, 6, and 1 beta in primary chronic apical periodontitis in smokers and nonsmokers. J Endod. 2021;47(5):755–761. doi: 10.1016/j.joen.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 94.Peruzzo D.C., Gimenes J.H., Taiete T., Casarin R.C., Feres M., Sallum E.A., et al. Impact of smoking on experimental gingivitis. A clinical, microbiological and immunological prospective study. J Periodontal Res. 2016;51(6):800–811. doi: 10.1111/jre.12363. [DOI] [PubMed] [Google Scholar]
- 95.Javed F., Al-Zawawi A.S., Allemailem K.S., Almatroudi A., Mehmood A., Divakar D.D., et al. Periodontal conditions and whole salivary IL-17a and -23 levels among young adult cannabis sativa (Marijuana)-Smokers, heavy cigarette-smokers and non-smokers. Int J Environ Res Publ Health. 2020;17(20) doi: 10.3390/ijerph17207435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vasques A.M.V., da Silva A.C.R., Bueno C.R.E., Cury M.T.S., Ervolino E., Cintra L.T.A., et al. Inflammatory profile of apical periodontitis exacerbated by cigarette smoke inhalation: histological and immunohistochemical analysis in rats. Int Endod J. 2023;56(4):465–474. doi: 10.1111/iej.13883. [DOI] [PubMed] [Google Scholar]
- 97.Miranda T.S., Heluy S.L., Cruz D.F., da Silva H.D.P., Feres M., Figueiredo L.C., et al. The ratios of pro-inflammatory to anti-inflammatory cytokines in the serum of chronic periodontitis patients with and without type 2 diabetes and/or smoking habit. Clin Oral Invest. 2019;23(2):641–650. doi: 10.1007/s00784-018-2471-5. [DOI] [PubMed] [Google Scholar]
- 98.Xu W., Li R., Sun Y. Increased IFN-gamma-producing Th17/Th1 cells and their association with lung function and current smoking status in patients with chronic obstructive pulmonary disease. BMC Pulm Med. 2019;19(1):137. doi: 10.1186/s12890-019-0899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ammitzboll C., von Essen M.R., Bornsen L., Petersen E.R., McWilliam O., Ratzer R., et al. GPR15(+) T cells are Th17 like, increased in smokers and associated with multiple sclerosis. J Autoimmun. 2019;97:114–121. doi: 10.1016/j.jaut.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 100.He S., Sun S., Lu J., Chen L., Mei X., Li L., et al. The effects of the miR-21/SMAD7/TGF-beta pathway on Th17 cell differentiation in COPD. Sci Rep. 2021;11(1):6338. doi: 10.1038/s41598-021-85637-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shao Y., Saaoud F., Cornwell W., Xu K., Kirchhoff A., Lu Y., et al. Cigarette smoke and morphine promote treg plasticity to Th17 via enhancing trained immunity. Cells. 2022;11(18) doi: 10.3390/cells11182810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Talbot J., Peres R.S., Pinto L.G., Oliveira R.D.R., Lima K.A., Donate P.B., et al. Smoking-induced aggravation of experimental arthritis is dependent of aryl hydrocarbon receptor activation in Th17 cells. Arthritis Res Ther. 2018;20(1):119. doi: 10.1186/s13075-018-1609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jaligama S., Patel V.S., Wang P., Sallam A., Harding J., Kelley M., et al. Radical containing combustion derived particulate matter enhance pulmonary Th17 inflammation via the aryl hydrocarbon receptor. Part Fibre Toxicol. 2018;15(1):20. doi: 10.1186/s12989-018-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fu J., Nogueira S.V., Drongelen V.V., Coit P., Ling S., Rosloniec E.F., et al. Shared epitope-aryl hydrocarbon receptor crosstalk underlies the mechanism of gene-environment interaction in autoimmune arthritis. Proc Natl Acad Sci U S A. 2018;115(18):4755–4760. doi: 10.1073/pnas.1722124115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baskara I., Kerbrat S., Dagouassat M., Nguyen H.Q., Guillot-Delost M., Surenaud M., et al. Cigarette smoking induces human CCR6(+)Th17 lymphocytes senescence and VEGF-A secretion. Sci Rep. 2020;10(1):6488. doi: 10.1038/s41598-020-63613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qiu S.L., Duan M.C., Liang Y., Tang H.J., Liu G.N., Zhang L.M., et al. Cigarette smoke induction of interleukin-27/WSX-1 regulates the differentiation of Th1 and Th17 cells in a smoking mouse model of emphysema. Front Immunol. 2016;7:553. doi: 10.3389/fimmu.2016.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng Y., Dong C., Yang J., Jin Y., Zheng W., Zhou Q., et al. Exosomal microRNA-155-5p from PDLSCs regulated Th17/Treg balance by targeting sirtuin-1 in chronic periodontitis. J Cell Physiol. 2019;234(11):20662–20674. doi: 10.1002/jcp.28671. [DOI] [PubMed] [Google Scholar]
- 108.Deng J., Lu C., Zhao Q., Chen K., Ma S., Li Z. The Th17/Treg cell balance: crosstalk among the immune system, bone and microbes in periodontitis. J Periodontal Res. 2022;57(2):246–255. doi: 10.1111/jre.12958. [DOI] [PubMed] [Google Scholar]
- 109.Melnik B.C., John S.M., Chen W., Plewig G. T helper 17 cell/regulatory T-cell imbalance in hidradenitis suppurativa/acne inversa: the link to hair follicle dissection, obesity, smoking and autoimmune comorbidities. Br J Dermatol. 2018;179(2):260–272. doi: 10.1111/bjd.16561. [DOI] [PubMed] [Google Scholar]
- 110.Li H., Liu Q., Jiang Y., Zhang Y., Zhang Y., Xiao W. Disruption of th17/treg balance in the sputum of patients with chronic obstructive pulmonary disease. Am J Med Sci. 2015;349(5):392–397. doi: 10.1097/MAJ.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 111.Zhang J.C., Chen G., Chen L., Meng Z.J., Xiong X.Z., Liu H.J., et al. TGF-beta/BAMBI pathway dysfunction contributes to peripheral Th17/Treg imbalance in chronic obstructive pulmonary disease. Sci Rep. 2016;6 doi: 10.1038/srep31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hou J., Sun Y., Hao Y., Zhuo J., Liu X., Bai P., et al. Imbalance between subpopulations of regulatory T cells in COPD. Thorax. 2013;68(12):1131–1139. doi: 10.1136/thoraxjnl-2012-201956. [DOI] [PubMed] [Google Scholar]
- 113.Silva L.E.F., Lourenco J.D., Silva K.R., Santana F.P.R., Kohler J.B., Moreira A.R., et al. Th17/Treg imbalance in COPD development: suppressors of cytokine signaling and signal transducers and activators of transcription proteins. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-72305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jing W., Wang W., Liu Q. Passive smoking induces pediatric asthma by affecting the balance of Treg/Th17 cells. Pediatr Res. 2019;85(4):469–476. doi: 10.1038/s41390-019-0276-0. [DOI] [PubMed] [Google Scholar]
- 115.Liu H.J., Chen G., Chen L., Zhou M., Xiong X.Z., Meng Z.J., et al. Cytokine-induced alterations of BAMBI mediate the reciprocal regulation of human Th17/Treg cells in response to cigarette smoke extract. Int J Mol Med. 2018;42(6):3404–3414. doi: 10.3892/ijmm.2018.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim Y.G., Lee C.K., Nah S.S., Mun S.H., Yoo B., Moon H.B. Human CD4+CD25+ regulatory T cells inhibit the differentiation of osteoclasts from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2007;357(4):1046–1052. doi: 10.1016/j.bbrc.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 117.Kelchtermans H., Geboes L., Mitera T., Huskens D., Leclercq G., Matthys P. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis. 2009;68(5):744–750. doi: 10.1136/ard.2007.086066. [DOI] [PubMed] [Google Scholar]
- 118.Shao Y., Cornwell W., Xu K., Kirchhoff A., Saasoud F., Lu Y., et al. Chronic exposure to the combination of cigarette smoke and morphine decreases CD4(+) regulatory T cell numbers by reprogramming the treg cell transcriptome. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.887681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen Y., Bai P., Liu L., Han J., Zeng H., Sun Y. Increased RANKL expression in peripheral T cells is associated with decreased bone mineral density in patients with COPD. Int J Mol Med. 2016;38(2):585–593. doi: 10.3892/ijmm.2016.2629. [DOI] [PubMed] [Google Scholar]
- 120.Nile C.J., Sherrabeh S., Ramage G., Lappin D.F. Comparison of circulating tumour necrosis factor superfamily cytokines in periodontitis patients undergoing supportive therapy: a case-controlled cross-sectional study comparing smokers and non-smokers in health and disease. J Clin Periodontol. 2013;40(9):875–882. doi: 10.1111/jcpe.12134. [DOI] [PubMed] [Google Scholar]
- 121.Kubota M., Yanagita M., Mori K., Hasegawa S., Yamashita M., Yamada S., et al. The effects of cigarette smoke condensate and nicotine on periodontal tissue in a periodontitis model mouse. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miranda T.S., Napimoga M.H., Feres M., Marins L.M., da Cruz D.F., da Silva HDP., et al. Antagonists of Wnt/beta-catenin signalling in the periodontitis associated with type 2 diabetes and smoking. J Clin Periodontol. 2018;45(3):293–302. doi: 10.1111/jcpe.12854. [DOI] [PubMed] [Google Scholar]
- 123.Ng T.K., Huang L., Cao D., Yip Y.W., Tsang W.M., Yam G.H., et al. Cigarette smoking hinders human periodontal ligament-derived stem cell proliferation, migration and differentiation potentials. Sci Rep. 2015;5:7828. doi: 10.1038/srep07828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ribeiro F.V., Pino D.S., Franck F.C., Benatti B.B., Tenenbaum H., Davies J.E., et al. Resveratrol inhibits periodontitis-related bone loss in rats subjected to cigarette smoke inhalation. J Periodontol. 2017;88(8):788–798. doi: 10.1902/jop.2017.170025. [DOI] [PubMed] [Google Scholar]
- 125.Pacheco C.M.F., Maltos K.L.M., Shehabeldin M.S., Thomas L.L., Zhuang Z., Yoshizawa S., et al. Local sustained delivery of anti-IL-17a antibodies limits inflammatory bone loss in murine experimental periodontitis. J Immunol. 2021;206(10):2386–2392. doi: 10.4049/jimmunol.2001432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kobayashi T., Okada M., Ito S., Kobayashi D., Ishida K., Kojima A., et al. Assessment of interleukin-6 receptor inhibition therapy on periodontal condition in patients with rheumatoid arthritis and chronic periodontitis. J Periodontol. 2014;85(1):57–67. doi: 10.1902/jop.2013.120696. [DOI] [PubMed] [Google Scholar]
- 127.Moutsopoulos N.M., Zerbe C.S., Wild T., Dutzan N., Brenchley L., DiPasquale G., et al. Interleukin-12 and interleukin-23 blockade in leukocyte adhesion deficiency type 1. N Engl J Med. 2017;376(12):1141–1146. doi: 10.1056/NEJMoa1612197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun L., Girnary M., Wang L., Jiao Y., Zeng E., Mercer K., et al. IL-10 dampens an IL-17-mediated periodontitis-associated inflammatory network. J Immunol. 2020;204(8):2177–2191. doi: 10.4049/jimmunol.1900532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang L., Wang J., Jin Y., Gao H., Lin X. Oral administration of all-trans retinoic acid suppresses experimental periodontitis by modulating the Th17/Treg imbalance. J Periodontol. 2014;85(5):740–750. doi: 10.1902/jop.2013.130132. [DOI] [PubMed] [Google Scholar]
- 130.Wang J., Wang B., Lv X., Wang Y. Halofuginone functions as a therapeutic drug for chronic periodontitis in a mouse model. Int J Immunopathol Pharmacol. 2020;34 doi: 10.1177/2058738420974893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jin Y., Wang L., Liu D., Lin X. Tamibarotene modulates the local immune response in experimental periodontitis. Int Immunopharm. 2014;23(2):537–545. doi: 10.1016/j.intimp.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 132.Ren B., Lu J., Li M., Zou X., Liu Y., Wang C., et al. Anti-inflammatory effect of IL-1ra-loaded dextran/PLGA microspheres on Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages in vitro and in vivo in a rat model of periodontitis. Biomed Pharmacother. 2021;134 doi: 10.1016/j.biopha.2020.111171. [DOI] [PubMed] [Google Scholar]
- 133.Hu Y., Yu P., Yu X., Hu X., Kawai T., Han X. IL-21/anti-Tim1/CD40 ligand promotes B10 activity in vitro and alleviates bone loss in experimental periodontitis in vivo. Biochim Biophys Acta, Mol Basis Dis. 2017;1863(9):2149–2157. doi: 10.1016/j.bbadis.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 135.Finckh A., Gilbert B., Hodkinson B., Bae S.C., Thomas R., Deane K.D., et al. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol. 2022;18(10):591–602. doi: 10.1038/s41584-022-00827-y. [DOI] [PubMed] [Google Scholar]
- 136.Nielsen S.F., Bojesen S.E., Schnohr P., Nordestgaard B.G. Elevated rheumatoid factor and long term risk of rheumatoid arthritis: a prospective cohort study. BMJ. 2012;345 doi: 10.1136/bmj.e5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vassallo R., Luckey D., Behrens M., Madden B., Luthra H., David C., et al. Cellular and humoral immunity in arthritis are profoundly influenced by the interaction between cigarette smoke effects and host HLA-DR and DQ genes. Clin Immunol. 2014;152(1–2):25–35. doi: 10.1016/j.clim.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chevalier N., Tan J.K., Mason L.J., Robert R., McKenzie C.I., Lim F., et al. Avenues to autoimmune arthritis triggered by diverse remote inflammatory challenges. J Autoimmun. 2016;73:120–129. doi: 10.1016/j.jaut.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 139.Donate P.B., Alves de Lima K., Peres R.S., Almeida F., Fukada S.Y., Silva T.A., et al. Cigarette smoke induces miR-132 in Th17 cells that enhance osteoclastogenesis in inflammatory arthritis. Proc Natl Acad Sci U S A. 2021;118(1) doi: 10.1073/pnas.2017120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wasen C., Turkkila M., Bossios A., Erlandsson M., Andersson K.M., Ekerljung L., et al. Smoking activates cytotoxic CD8(+) T cells and causes survivin release in rheumatoid arthritis. J Autoimmun. 2017;78:101–110. doi: 10.1016/j.jaut.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 141.Svensson B., Hafstrom I., Erlandsson M.C., Forslind K., Bokarewa M.I. Smoking in combination with antibodies to cyclic citrullinated peptides is associated with persistently high levels of survivin in early rheumatoid arthritis: a prospective cohort study. Arthritis Res Ther. 2014;16(1) doi: 10.1186/ar4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wasen C., Erlandsson M.C., Bossios A., Ekerljung L., Malmhall C., Toyra Silfversward S., et al. Smoking is associated with low levels of soluble PD-L1 in rheumatoid arthritis. Front Immunol. 2018;9:1677. doi: 10.3389/fimmu.2018.01677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Andreoli C., Bassi A., Gregg E.O., Nunziata A., Puntoni R., Corsini E. Effects of cigarette smoking on circulating leukocytes and plasma cytokines in monozygotic twins. Clin Chem Lab Med. 2015;53(1):57–64. doi: 10.1515/cclm-2013-0290. [DOI] [PubMed] [Google Scholar]
- 144.Choi Y., Woo K.M., Ko S.H., Lee Y.J., Park S.J., Kim H.M., et al. Osteoclastogenesis is enhanced by activated B cells but suppressed by activated CD8(+) T cells. Eur J Immunol. 2001;31(7):2179–2188. doi: 10.1002/1521-4141(200107)31:7<2179::aid-immu2179>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 145.Buchwald Z.S., Yang C., Nellore S., Shashkova E.V., Davis J.L., Cline A., et al. A bone anabolic effect of RANKL in a murine model of osteoporosis mediated through FoxP3+ CD8 T cells. J Bone Miner Res. 2015;30(8):1508–1522. doi: 10.1002/jbmr.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shashkova E.V., Trivedi J., Cline-Smith A.B., Ferris C., Buchwald Z.S., Gibbs J., et al. Osteoclast-primed Foxp3+ CD8 T cells induce T-bet, eomesodermin, and IFN-gamma to regulate bone resorption. J Immunol. 2016;197(3):726–735. doi: 10.4049/jimmunol.1600253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hedstrom A.K., Stawiarz L., Klareskog L., Alfredsson L. Smoking and susceptibility to rheumatoid arthritis in a Swedish population-based case-control study. Eur J Epidemiol. 2018;33(4):415–423. doi: 10.1007/s10654-018-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Svard A., Skogh T., Alfredsson L., Ilar A., Klareskog L., Bengtsson C., et al. Associations with smoking and shared epitope differ between IgA- and IgG-class antibodies to cyclic citrullinated peptides in early rheumatoid arthritis. Arthritis Rheumatol. 2015;67(8):2032–2037. doi: 10.1002/art.39170. [DOI] [PubMed] [Google Scholar]
- 149.Ishikawa Y., Ikari K., Hashimoto M., Ohmura K., Tanaka M., Ito H., et al. Shared epitope defines distinct associations of cigarette smoking with levels of anticitrullinated protein antibody and rheumatoid factor. Ann Rheum Dis. 2019;78(11):1480–1487. doi: 10.1136/annrheumdis-2019-215463. [DOI] [PubMed] [Google Scholar]
- 150.Kang J., Jeong S.H., Lee K., Park N., Jung H., Lee K., et al. Exacerbation of symptomatic arthritis by cigarette smoke in experimental arthritis. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Negishi-Koga T., Gober H.J., Sumiya E., Komatsu N., Okamoto K., Sawa S., et al. Immune complexes regulate bone metabolism through FcRgamma signalling. Nat Commun. 2015;6:6637. doi: 10.1038/ncomms7637. [DOI] [PubMed] [Google Scholar]
- 152.Harre U., Lang S.C., Pfeifle R., Rombouts Y., Fruhbeisser S., Amara K., et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat Commun. 2015;6:6651. doi: 10.1038/ncomms7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pfeifle R., Rothe T., Ipseiz N., Scherer H.U., Culemann S., Harre U., et al. Regulation of autoantibody activity by the IL-23-T(H)17 axis determines the onset of autoimmune disease. Nat Immunol. 2017;18(1):104–113. doi: 10.1038/ni.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weng C.H., Gupta S., Geraghty P., Foronjy R., Pernis A.B. Cigarette smoke inhibits ROCK2 activation in T cells and modulates IL-22 production. Mol Immunol. 2016;71:115–122. doi: 10.1016/j.molimm.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hirota K., Yoshitomi H., Hashimoto M., Maeda S., Teradaira S., Sugimoto N., et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Qin Y., Cai M.L., Jin H.Z., Huang W., Zhu C., Bozec A., et al. Age-associated B cells contribute to the pathogenesis of rheumatoid arthritis by inducing activation of fibroblast-like synoviocytes via TNF-alpha-mediated ERK1/2 and JAK-STAT1 pathways. Ann Rheum Dis. 2022;81(11):1504–1514. doi: 10.1136/ard-2022-222605. [DOI] [PubMed] [Google Scholar]
- 157.Danks L., Komatsu N., Guerrini M.M., Sawa S., Armaka M., Kollias G., et al. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheum Dis. 2016;75(6):1187–1195. doi: 10.1136/annrheumdis-2014-207137. [DOI] [PubMed] [Google Scholar]
- 158.Lahoti T.S., John K., Hughes J.M., Kusnadi A., Murray I.A., Krishnegowda G., et al. Aryl hydrocarbon receptor antagonism mitigates cytokine-mediated inflammatory signalling in primary human fibroblast-like synoviocytes. Ann Rheum Dis. 2013;72(10):1708–1716. doi: 10.1136/annrheumdis-2012-202639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Engler A., Niederer F., Klein K., Gay R.E., Kyburz D., Camici G.G., et al. SIRT6 regulates the cigarette smoke-induced signalling in rheumatoid arthritis synovial fibroblasts. J Mol Med (Berl) 2014;92(7):757–767. doi: 10.1007/s00109-014-1139-0. [DOI] [PubMed] [Google Scholar]
- 160.Engler A., Tange C., Frank-Bertoncelj M., Gay R.E., Gay S., Ospelt C. Regulation and function of SIRT1 in rheumatoid arthritis synovial fibroblasts. J Mol Med (Berl) 2016;94(2):173–182. doi: 10.1007/s00109-015-1332-9. [DOI] [PubMed] [Google Scholar]
- 161.Lee J., Jeong H., Park E.J., Hwang J.W., Bae E.K., Ahn J.K., et al. A role for benzo[a]pyrene and Slug in invasive properties of fibroblast-like synoviocytes in rheumatoid arthritis: a potential molecular link between smoking and radiographic progression. Joint Bone Spine. 2013;80(6):621–625. doi: 10.1016/j.jbspin.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 162.Genovese M.C., Weinblatt M.E., Mease P.J., Aelion J.A., Peloso P.M., Chen K., et al. Dual inhibition of tumour necrosis factor and interleukin-17A with ABT-122: open-label long-term extension studies in rheumatoid arthritis or psoriatic arthritis. Rheumatology. 2018;57(11):1972–1981. doi: 10.1093/rheumatology/key173. [DOI] [PubMed] [Google Scholar]
- 163.Khatri A., Klunder B., Peloso P.M., Othman A.A. Exposure-response analyses demonstrate no evidence of interleukin 17A contribution to efficacy of ABT-122 in rheumatoid or psoriatic arthritis. Rheumatology. 2019;58(2):352–360. doi: 10.1093/rheumatology/key312. [DOI] [PubMed] [Google Scholar]
- 164.Wang J., Zhang S.X., Chang J.S., Cheng T., Jiang X.J., Su Q.Y., et al. Low-dose IL-2 improved clinical symptoms by restoring reduced regulatory T cells in patients with refractory rheumatoid arthritis: a randomized controlled trial. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.947341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang X., Miao M., Zhang R., Liu X., Zhao X., Shao M., et al. Efficacy and safety of low-dose interleukin-2 in combination with methotrexate in patients with active rheumatoid arthritis: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Targeted Ther. 2022;7(1):67. doi: 10.1038/s41392-022-00887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tuttle J., Drescher E., Simon-Campos J.A., Emery P., Greenwald M., Kivitz A., et al. A phase 2 trial of peresolimab for adults with rheumatoid arthritis. N Engl J Med. 2023;388(20):1853–1862. doi: 10.1056/NEJMoa2209856. [DOI] [PubMed] [Google Scholar]
- 167.Ruscitti P., Masedu F., Alvaro S., Airo P., Battafarano N., Cantarini L., et al. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): a multicentre, open-label, randomised controlled trial. PLoS Med. 2019;16(9) doi: 10.1371/journal.pmed.1002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Iwamoto N., Chiba K., Sato S., Shiraishi K., Watanabe K., Oki N., et al. Inhibition of bone erosion, determined by high-resolution peripheral quantitative computed tomography (HR-pQCT), in rheumatoid arthritis patients receiving a conventional synthetic disease-modifying anti-rheumatic drug (csDMARD) plus denosumab vs csDMARD therapy alone: an open-label, randomized, parallel-group study. Arthritis Res Ther. 2022;24(1):264. doi: 10.1186/s13075-022-02957-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.So H., Cheng I.T., Lau S.L., Chow E., Lam T., Hung V.W., et al. Effects of RANKL inhibition on promoting healing of bone erosion in rheumatoid arthritis using HR-pQCT: a 2-year, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2021;80(8):981–988. doi: 10.1136/annrheumdis-2021-219846. [DOI] [PubMed] [Google Scholar]
- 170.Rivellese F., Surace A.E.A., Goldmann K., Sciacca E., Cubuk C., Giorli G., et al. Rituximab versus tocilizumab in rheumatoid arthritis: synovial biopsy-based biomarker analysis of the phase 4 R4RA randomized trial. Nat Med. 2022;28(6):1256–1268. doi: 10.1038/s41591-022-01789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kon T., Cho T.J., Aizawa T., Yamazaki M., Nooh N., Graves D., et al. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16(6):1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 172.Rinderknecht H., Nussler A.K., Steinestel K., Histing T., Ehnert S. Smoking impairs hematoma formation and dysregulates angiogenesis as the first steps of fracture healing. Bioengineering. 2022;9(5) doi: 10.3390/bioengineering9050186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hao Z., Li J., Li B., Alder K.D., Cahill S.V., Munger A.M., et al. Smoking alters inflammation and skeletal stem and progenitor cell activity during fracture healing in different murine strains. J Bone Miner Res. 2021;36(1):186–198. doi: 10.1002/jbmr.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mahajan A., Kumar N., Gupta B. Delayed tibial shaft fracture healing associated with smoking: a systematic review and meta-analysis of observational studies conducted worldwide. Int J Environ Res Publ Health. 2021;18(19) doi: 10.3390/ijerph181910228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang J., Wan Q., Yu X., Cheng G., Ni Y., Li Z. Low-dose nicotine reduces the homing ability of murine BMSCs during fracture healing. Am J Transl Res. 2018;10(9):2796–2809. [PMC free article] [PubMed] [Google Scholar]
- 176.Aspera-Werz R.H., Chen T., Ehnert S., Zhu S., Frohlich T., Nussler A.K. Cigarette smoke induces the risk of metabolic bone diseases: transforming growth factor beta signaling impairment via dysfunctional primary cilia affects migration, proliferation, and differentiation of human mesenchymal stem cells. Int J Mol Sci. 2019;20(12) doi: 10.3390/ijms20122915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhou Y., Jiang R., An L., Wang H., Cheng S., Qiong S., et al. Benzo[a]pyrene impedes self-renewal and differentiation of mesenchymal stem cells and influences fracture healing. Sci Total Environ. 2017;587-588:305–315. doi: 10.1016/j.scitotenv.2017.02.152. [DOI] [PubMed] [Google Scholar]
- 178.El-Zawawy H.B., Gill C.S., Wright R.W., Sandell L.J. Smoking delays chondrogenesis in a mouse model of closed tibial fracture healing. J Orthop Res. 2006;24(12):2150–2158. doi: 10.1002/jor.20263. [DOI] [PubMed] [Google Scholar]
- 179.Kung M.H., Yukata K., O'Keefe R.J., Zuscik M.J. Aryl hydrocarbon receptor-mediated impairment of chondrogenesis and fracture healing by cigarette smoke and benzo(a)pyrene. J Cell Physiol. 2012;227(3):1062–1070. doi: 10.1002/jcp.22819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu Y., Di Y.P., Chang M., Huang X., Chen Q., Hong N., et al. Cigarette smoke-associated inflammation impairs bone remodeling through NFkappaB activation. J Transl Med. 2021;19(1):163. doi: 10.1186/s12967-021-02836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Santiago H.A., Zamarioli A., Sousa Neto M.D., Volpon J.B. Exposure to secondhand smoke impairs fracture healing in rats. Clin Orthop Relat Res. 2017;475(3):894–902. doi: 10.1007/s11999-016-5184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 183.Glyn-Jones S., Palmer A.J., Agricola R., Price A.J., Vincent T.L., Weinans H., et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 184.Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., et al. Osteoarthritis. Nat Rev Dis Prim. 2016;2 doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 185.Larsson S.C., Burgess S. Appraising the causal role of smoking in multiple diseases: a systematic review and meta-analysis of Mendelian randomization studies. EBioMedicine. 2022;82 doi: 10.1016/j.ebiom.2022.104154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhu S., Ji L., He Z., Zhang W., Tong Y., Luo J., et al. Association of smoking and osteoarthritis in US (NHANES 1999-2018) Sci Rep. 2023;13(1):3911. doi: 10.1038/s41598-023-30644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kwon H.M., Yang I.H., Park K.K., Cho B.W., Byun J., Lee W.S. Cigarette smoking and knee osteoarthritis in the elderly: data from the Korean national health and nutritional examination survey. Exp Gerontol. 2020;133 doi: 10.1016/j.exger.2020.110873. [DOI] [PubMed] [Google Scholar]
- 188.Cai X., Gao L., Cucchiarini M., Madry H. Association of nicotine with osteochondrogenesis and osteoarthritis development: the state of the art of preclinical research. J Clin Med. 2019;8(10) doi: 10.3390/jcm8101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Peng X., Qiao Z., Wang Y., Li H., Xie Y., Xin M., et al. Estrogen reverses nicotine-induced inflammation in chondrocytes via reducing the degradation of ECM. Int J Rheum Dis. 2019;22(4):666–676. doi: 10.1111/1756-185X.13476. [DOI] [PubMed] [Google Scholar]
- 190.Rose B.J., Weyand J.A., Liu B., Smith J.F., Perez B.R., Clark J.C., et al. Exposure to second-hand cigarette smoke exacerbates the progression of osteoarthritis in a surgical induced murine model. Histol Histopathol. 2021;36(3):347–353. doi: 10.14670/HH-18-311. [DOI] [PubMed] [Google Scholar]
- 191.Yoshikawa Y., Izawa T., Hamada Y., Takenaga H., Wang Z., Ishimaru N., et al. Roles for B[a]P and FICZ in subchondral bone metabolism and experimental temporomandibular joint osteoarthritis via the AhR/Cyp1a1 signaling axis. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-94470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fernandez-Torres J., Aztatzi-Aguilar O.G., Zamudio-Cuevas Y., Sierra-Vargas M.P., Martinez-Nava G.A., Montano-Armendariz N., et al. Effect of smoking on the redox status of knee osteoarthritis: a preliminary study. Exp Biol Med. 2023;248(20):1754–1767. doi: 10.1177/15353702231199072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen T., Ehnert S., Tendulkar G., Zhu S., Arnscheidt C., Aspera-Werz R.H., et al. Primary human chondrocytes affected by cigarette smoke-therapeutic challenges. Int J Mol Sci. 2020;21(5) doi: 10.3390/ijms21051901. [DOI] [PMC free article] [PubMed] [Google Scholar]