Abstract
Purpose
The Bone And MicroBiOme Onset (BAMBOO) study is an ongoing prospective observational cohort study conducted in Tianjin, China, aiming to determine age-appropriate trajectories for microbiome maturation and bone development and to identify the influence of dietary factors in the process.
Participants
The recruitment started in September 2021 and was completed in February 2023. A total of 1380 subjects were recruited, 690 at birth (group 1) and 690 at 6 months of age (group 2). Groups 1 and 2 will be followed up for 12 months and 36 months, respectively.
Findings to date
The age of the mothers was 31.1±3.7 (mean±SD), and the birth weight of infants was 3.3±0.5 kg with an incidence of caesarean section 50.4%. Food diary information of the first 100 subjects showed that 64 food items were introduced by 6 months. A pilot microbiome analysis revealed that at the species level, bacterial communities were composed of mostly Bacteroides dorei, Bacteroides vulgatus and Escherichia coli, which were consistent with that of previous reports. Feasibility assessments of breast milk vitamin D and human milk oligosaccharides were validated through certified reference measurements. The early data assessment showed a high reliability of the data generated from this study.
Future plans
Data collection will be completed in August 2025. Four stage-statistical analyses will be performed as the cohort reaches certain age thresholds before the final report. Analysis of BAMBOO data will be used to develop age-appropriate trajectories for microbiome maturation and bone development for children aged 0–3 years and investigate the contribution of dietary factors in the process.
Trial registration number
ChiCTR2100049972
Keywords: Microbiome, bone health, early life nutrition, early life growth
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is a large prospective longitudinal cohort study covering the first 3 years of life, measuring multiple aspects of health at multiple time points.
The study investigates the interplay of dietary factors, microbiome maturation and bone development, as well as growth and health status.
As an observational study, a limitation is that only associations between early life nutrition, microbiome maturation and bone development can be investigated; thus, the causal relationships could not be determined.
The study is being conducted in a major metropolitan area of northern China; the infant feeding and dietary habits could differ in other regions across China.
Introduction
Early childhood is a critical period for growth and development benefitting long-term health. The role of the gut microbiome in health has been investigated in many studies. Differences in gut microbiome composition and function have been associated with various chronic diseases such as metabolic conditions, neurological disorders and cardiovascular illnesses.1 2 Microbiome maturation in infants parallels human neurodevelopmental processes and growth. It plays a role in gut-brain signalling3 and is associated with cognitive development.4 An essential aspect of microbiome function is its impact on the immune system. The establishment of host-microbial symbiosis relies on the mutualistic co-development of the host microbiota and immune system.5 Microbiome diversity has been associated with respiratory tract infections,6 and its composition may influence mucosal IgA response to the vaccine in infants.7
A critical window for microbiome maturation is from birth to 4 years of age.5 Stewart and colleagues reported three distinct phases of microbiome composition evolution in early life: a developmental phase (months 3–14), a transitional phase (months 15–30) and a stable phase (months 31–46).8 Considering that after these phases the microbiome composition remains fairly stable across life stages, it is important to define the optimal age-appropriate microbiome acquisition, selection and maturation from birth up to toddlerhood in relation to health benefits. Moreover, further understanding of the influence of weaning and nutrition during early childhood on an age-appropriate microbiome maturation is needed. While multiple factors influence the establishment and development of infant gut microbiomes, such as the delivery mode and daycare attendance,9 10 there is also growing evidence of the influence of dietary factors on microbiome maturation, both breastfeeding (BF) and complementary foods. BF has a critical role, especially during the first 6 months of age.8 9 11 After 6 months of age when weaning foods are gradually being introduced to the diet, microbiota composition and environment change rapidly.12 An intervention with microbiota-directed complementary foods (ie, the combination of chickpea flour, peanut flour, soy flour and raw banana) modified the microbiota and increased plasma biomarkers and mediators of growth, bone formation, neurodevelopment and immune function in a randomised, double-blind controlled feeding study in malnourished infants in Bangladesh.13 Understanding the impact and mechanisms underlying these maturation changes requires information on two aspects: establishment of normal age-appropriate microbiome maturation trajectory and investigation of the relationship between specific dietary factors and microbiomes.5 Although some studies have been carried out in Bangladesh,14 USA and Europe,8 12 15 no longitudinal data have been reported for Chinese infants.
Healthy skeleton development is an important aspect of child growth, particularly around 2–4 years where fast growth occurs with some consequence on fracture risk elevated in child peaks at age 3 years.16 Bones are dynamic and highly specialised connective tissues. Major functions of bones include provision of mechanical support for muscular activity and physical protection to the tissues and internal organs and to act as a repository for systemic mineral homeostasis.17 Bone mass is related to fracture risk. Studies have shown that, for every SD decrease in size-adjusted bone mass, there is an 89% increase in fracture risk in childhood.18 At the early 20s, a 10–15% higher peak bone mass has been estimated to decrease the risk of fracture by 25–50% later in life.19 Therefore, one strategy to reduce later-life fracture risk is to build peak bone mass. There are two windows of opportunity to maximise peak bone mass, infancy and adolescence.20 The bone mass is assessed by both geometry measures (length and width, which are relatively easier to measure in clinical studies) and mineral deposition. Bone mineralisation provides rigidity and resistance to the skeleton while maintaining a certain degree of elasticity.17 Chronological bone physiology highlights the importance of bone mineralisation in infants and toddlers to achieve developmental milestones, such as climbing, walking and running. However, the knowledge on infant bone mineral deposition is limited due to the difficulties in measurement. Moreover, in this rapid-growing phase, the balance between rigidity and elasticity at different ages also requires further investigation.21 Breastmilk was found to have a positive influence on peak bone microarchitecture22 and might be protective for long-term bone health.23 Non-digestible human milk oligosaccharides (HMOs), which have a significant influence on infant microbiome,24 were found to improve bone mass and structure in different mouse models, suggesting a potential HMO-microbiome-bone interaction.25–27 A prospective randomised clinical trial showed that calcium supplementation increased the acquisition of bone mass in children, adolescents and early adulths.28 Notwithstanding such studies, age-appropriate bone development, and the influence of feeding types and complementary food introduction on bone development, in infants and toddlers is still less investigated.
To fill the knowledge gaps on both optimal age-appropriate microbiome maturation and bone development, the interaction of these two and the influence of dietary factors, the Tianjin Women and Children’s Health Centre (TJWCH), Beijing Genomic Institute (BGI) and Nestlé Research (NR) jointly designed and initiated theBone And MicroBiOme Onset (BAMBOO) study. The results of this unique Chinese project will help researchers establish age-appropriate trajectories for microbiome maturation and bone development from birth to pre-school-aged toddlers. The insights gathered may support local nutrition–healthcare programmes for infants and toddlers to benefit long-term health status. The focus of this article is to report the study protocol used in this longitudinal cohort study.
Cohort description
Aim of BAMBOO
BAMBOO is a prospective cohort observational study aiming to characterise normal age-appropriate microbiome maturation and bone development and to assess how early life nutrition influences these developmental processes.
Setting of BAMBOO
The BAMBOO study is conducted in Tianjin, the fourth largest municipality in China, covering an area of 12 000 square kilometres with a resident population of 13.8 million. It is comprised of 16 districts, including six central urban districts, 4 surrounding urban districts and six suburb districts. All children aged 0–6 years in Tianjin are registered in the Maternal and Child Healthcare system administered by TJWCH. Routine healthcare records from pregnancy until children’s 6 years are kept in the system. Participants in this cohort are mainly from the six central urban districts (Hedong, Nankai, Hebei, Hexi and Hongqiao) and four surrounding urban districts (Beichen, Jinnan, Xiqing and Dongli).
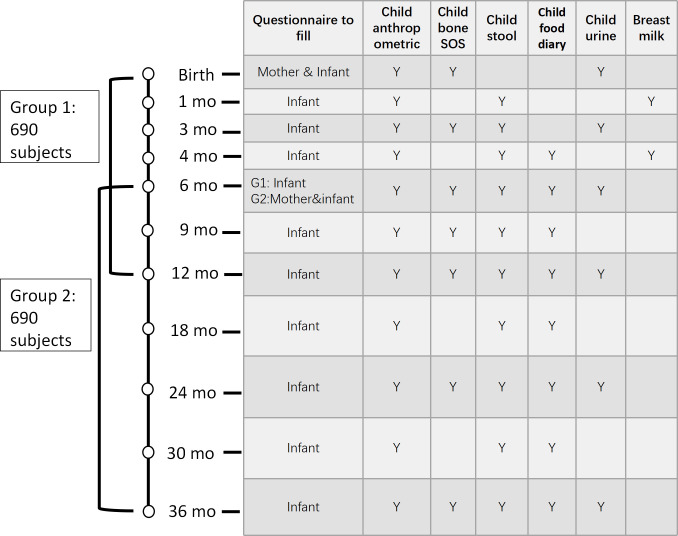
The cohort is composed of children aged between 0 and 3 years. In an accelerated cohort design, two parallel groups covering different age ranges are recruited with an overlap age from 6 to 12 months. Group 1 subjects will be followed from birth to 12 months of age with infant/toddler development information and samples collected at birth and then at ages 1, 3, 4, 6, 9 and 12 months. Group 2 subjects will be followed from age 6 to 36 months with infant/toddler development information, and samples were collected at ages 6, 9, 12, 18, 24, 30 and 36 months. Detailed visit time points and sample collection schedule are shown in figure 1.
Figure 1.
Data and sample collection time points.
This international scientific collaboration was approved by China Human Genetic Resource Admission
Committee (HGRAC) in August 2021. The study is conducted according to the principles of the Declaration of Helsinki and in accordance with the guidelines and regulations of HGRAC. Ethics approval of the research was obtained from the Tianjin Women’s and Children’s Health Centre Medical Ethical Committee and the Institutional Review Board of BGI-Shenzhen (BGI-IRB 21056). For this study, informed consent will be obtained from the parents or legal guardians of the participants. Informed consent will also be obtained from all the mothers involved in the study. The study is registered in the clinical trial registration, and the trial number is ChiCTR2100049972.
Recruitment
Children who meet the following requirements are invited to participate in the study: (1) full-term gestational birth (≥37 and ≤42 weeks), (2) singleton and (3) signed informed consent by the infant’s parents (or his/her legally authorised representative [LAR]) and agree to fulfil the requirements of the study protocol.
The exclusion criteria are as follows: (1) birth after a complicated pregnancy, such as pre-eclampsia, gestational diabetes or bowel disease, determined by medical interview/medical record; (2) infant’s parents/LAR not willing and/or unable to comply with scheduled visits and the requirements of the study protocol; and (3) currently participating or having participated in another clinical trial within 4 weeks prior to the start of this cohort.
For group 1, infants begin their journey in the study within 10 days after birth. Participant recruitment is ongoing at seven local delivery hospitals. Investigators introduce the study to the parents and ask for their consent, with screening based on the inclusion/exclusion criteria at 3–5 days after giving birth. For group 2, infants join the cohort at 6 months of age. Investigators introduce the study to the parents based on the inclusion/exclusion criteria and ask for their consent when the child is around 5.5 months.
The sample size was calculated based on bone development outcomes. For the bone trajectory analysis, Limanovitz et al found that a sample size of 60 infants per group would be required to demonstrate a significant difference of 70 m/s in SoS between breastfed and formula-fed infants with an SD of 133 m/s, assuming an overall type I error of 5% and 80% statistical power.29 Based on a previous study in Tianjin, 10% of women performed exclusively formula feeding, 45% performed exclusively BF and 45% performed mixed feeding. Therefore, given that at least 60 infants are required in each feeding group, a total of 600 completed infants are needed. Considering a drop-out rate of 13%, the total number of infants to be enrolled is 690 per group. For the microbiome trajectory development, based on literature in which a small number of subjects with frequent time points of faecal sampling were used to derive a trajectory,14 we believe we will have a sufficient reference subset of infants selected based on our criteria, starting from 690 subjects per group. A total of 1380 healthy normal developing Chinese infants and toddlers are planned to be recruited in this study. Recruitment of participants started in September 2021 and completed in February 2023.
Data collection
Data collection includes questionnaires, medical history (MH), concomitant medication, adverse events/serious adverse events (AE/SAE) record, food diary, biological sample collection and anthropometric measurements (length/height, weight and bone measurements).
-
Mother and infant questionnaires. A mother’s questionnaire is filled at enrolment (group 1 at around birth; group 2 when infants aged 6 months), including basic information (mother’s height, weight, education level and history of gestation), tobacco and alcohol use, work and physical activities after 28 weeks of gestation, vitamin, mineral and probiotic supplements, health and medication during pregnancy and the last B-ultrasound examination result during pregnancy (only for group 1).
The infant’s questionnaires are gathered at each visit from main caregivers in a face-to-face interview or phone interview if the person cannot attend the visit. Information on baby feeding practice, sleep, vitamin, mineral and probiotic supplements, health status, growth and development and antibiotic use is collected from the infant’s questionnaires. Infant Gastrointestinal Symptom Questionnaire (up to 12 months of age) and Toddler Gut Comfort Questionnaire (18 m to 3 years of age) are also administered.30 MH, AEs/SAEs and concomitant medications and non-pharmacological treatments are recorded from the beginning till the final visit.
Definition of feeding types. In this study, exclusive BF is defined as a subject reported as exclusively breastfed at 1 and 3 months. For exclusive infant formula feeding (IF), a subject is either exclusively infant formula fed at 1 and 3 months or with a mixed feeding and an IF ratio higher than 80% at 1 and 3 months. Feeding practice is classified as mixed feeding if the subject has an estimated IF ratio of lower than 80% of total intake at 1 and 3 months. We applied the principle of flexible feeding-type description,29 IF ratio=total daily infant formula intake in ml/780 mL.
Food diary. Starting from 4 months of age, a food diary is filled by the main caregiver at home, assisted by photos and tools, for 3 consecutive days, starting 4 days prior to each visit. The content is reviewed during the visit by a dietitian. A nutrition evaluation report is shared with the parents by the dietitian at age s6 months and 1 year. Each food diary is checked by the principal investigator to ensure quality. Nutrient intakes are calculated using the Chinese Food Composition database,31 complemented by other sources such as pack labels and literature review in case of lack of information in the current database.
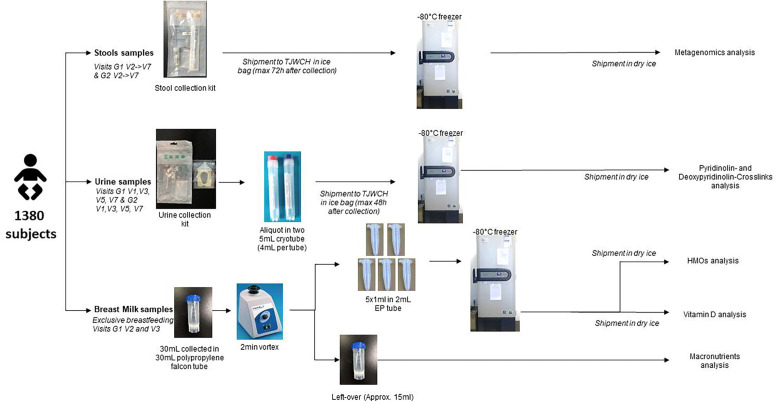
Biological sample collection. All biological samples (child faecal and urine samples and human milk samples) are temporarily stored at −80° freezer at TJWCH and regularly shipped on dry ice to different labs for analysis. The sample flow chart is shown in figure 2. Detailed methods for sample handling and analysis are described in Supplemental material.
Height and weight measurements. Children’s length/height and weight are measured by trained investigators using calibrated electronic scale and measuring bed (Suhong RCS-20), with the nearest accuracy of 0.1 cm and 5 g, respectively.
Bone length and mass index measurement. The bone mass index measurement is conducted using a non-invasive and radiation-free ultrasound sonometer (Sunlight Omnisense Mini) measuring bone transmission time and speed of sound (SoS) at the tibia site, together with measurements of both tibia and radius length. In the study, two ultrasound devices are used to measure the bone transmission time and SoS at the radius and tibia . To calibrate the two devices, a phantom is scanned to detect deviation of the ultrasound source daily. To maximise the reproducibility, the research staff who had past experience with similar devices were trained and tested in children with the reproducibility of two devices CV of <6%.
Figure 2.
Biological sample collection and storage
Data management and statistical analysis
Data are entered from the source document into an electronic Case Report Form (a web database named ClinFlash) within 15 days after the subject’s visit. Data quality review meetings are held quarterly, and the statistical results on sample enrolment and descriptive statistics on key measurements are included into a data quality report and submitted to the research committee for review. Protocol deviations are predefined and divided into major and minor categories, which trigger corresponding corrective and preventive actions in time. All protocol deviations in listing format will be reported to the TJWCH Ethics Committee.
Data analyses are planned to be conducted in R (R Core Team, 2014), and figures will be produced using the package ggplot2 (Wickham, 2009). Microbiome data will be further explored using a microbiome toolbox (version 1.0).32 Data will be initially checked for distribution normality (using qq-plot and residuals vs fitted values plot). Feeding groups, complementary food and nutrient intakes will be summarised. Dietary patterns will be identified using principal component analysis and/or cluster analysis. Microbiome trajectories were derived using a machine learning model to estimate the microbiome maturation index. Microbiota-for-age z-scores will be calculated, and associations between normal/abnormal microbiota-for-age z-scores and various health and dietary factors will be investigated using x² test of independence. Bone (tibia and radius length and SoS) trajectories over time will be summarised. Associations between different measures will also be investigated using appropriate methods, such as linear mixed-effect model adjusting for confounding factors. The model will include the computed propensity scores as weights.
Patient and public involvement
Patients and/or the public were not involved in the development of study design and dissemination of this research.
Findings to date
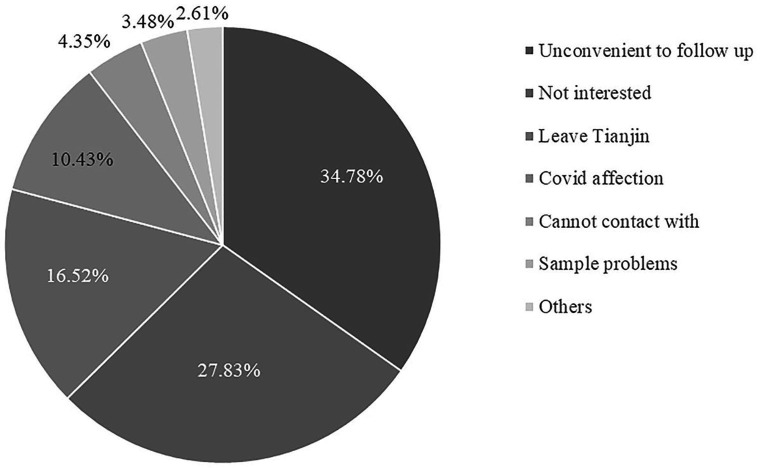
The BAMBOO study was started in September 2021, and the recruitment was completed by the end of February 2023. A total of 1380 mother-child pairs were recruited in this study (690 in group 1 and 690 in group 2). Table 1 shows the basic characteristics of the participants, including mother’s age, delivery weeks, birth mode, infants’ gender, nation, birth weight or birth length. In total, 239 subjects dropped out from the study. Figure 3 shows the reasons for the drop out, where the top three reasons are ‘inconvenient to follow-up,’ ‘not interested in the study’ and ‘leaving Tianjin’. We compared the basic characteristics between participants still in the group and those who dropped out and found no difference, except that mothers who dropped out were more likely younger than the mothers remaining in the study (table 1). The population characteristics of this study are comparable to those in another large cohort study, the Tianjin Birth Cohort (TJBC), conducted in the same city with a large number of mother and infant dyads.33 In both studies, the average age of mothers was around 30–31 years, children’s average body length and weight at birth were around 50 cm and 3.3 kg, respectively. We observed a slightly lower proportion of girls enrolled in this study (45%) as compared with TJBC (48%), but the difference is not statistically significant (detailed data of TJBC have not yet been published).
Table 1.
Characteristics of enrolled participants (February 2023)
| Characteristics | Group 1 | Group 2 | Total | Active | Drop out | Statistics | P value | |
| Number of subjects | 690 | 690 | 1380 | 1141 | 239 | |||
| Mother’s age | 30.83±3.80 | 31.44±3.59 | 31.14±3.71 | 31.28±3.73 | 30.44±3.54 | t=3.205 | 0.001 | |
| Delivery weeks | 39.05±0.937 | 39.19±1.072 | 39.12±1.01 | 39.14±1.01 | 39.03±1.02 | t=1.605 | 0.109 | |
| Birth mode | Natural childbirth | 314 (45.5%) | 381 (55.2%) | 695 (49.6%) | 586 (51.4%) | 109 (45.6%) | χ2=2.615 | 0.106 |
| Caesarean section | 376 (54.5%) | 309 (44.8%) | 685 (50.4%) | 555 (48.6%) | 130 (54.4%) | |||
| Infant’s gender | Male | 396 (57.4%) | 369 (53.5%) | 765 (55.4%) | 631 (55.3%) | 134 (56.1%) | χ2=0.047 | 0.829 |
| Female | 294 (42.6%) | 321 (46.5%) | 615 (44.6%) | 510 (44.7%) | 105 (43.9%) | |||
| Nation | Han | 619 (89.7%) | 639 (92.6%) | 1258 (91.2%) | 1041 (91.2%) | 217 (90.8%) | χ2=0.048 | 0.827 |
| Other | 71 (10.3%) | 51 (7.4%) | 122 (8.8%) | 100 (8.8%) | 22 (9.2%) | |||
| Birth weight (kg) | 3.33±0.372 | 3.33±0.389 | 3.33±0.380 | 3.34±0.375 | 3.29±0.401 | t=1.733 | 0.083 | |
| Birth length (cm) | 49.63±1.36 | 49.97±1.29 | 49.80±1.33 | 49.84±1.32 | 49.65±1.39 | t=1.925 | 0.054 |
Figure 3.
Breakdown of drop-out reasons.
A review of dietary intake data was conducted using food diary information from the first 100 subjects at 6 months of age in group 2 since most children have been exposed to the complementary foods at this age. In total, 64 food items were reported (table 2). Nutrient intake estimation is ongoing based on the food intake using the Chinese Food Composition database,31 complemented by labels on the pack.
Table 2.
Foods recorded in the food diary*
| Food name | Count | Percent, % | Food name | Count | Percent, % |
| Breastmilk | 1552 | 54.0 | Orange | 8 | 0.3 |
| Formula | 510 | 17.8 | Tangerine | 6 | 0.2 |
| Ground rice | 179 | 6.2 | Avocado | 1 | 0.1 |
| Ground rice with vegetable puree | 1 | 0.0 | Grape | 2 | 0.1 |
| Ground rice with zucchini puree | 2 | 0.1 | Pomegranate | 1 | 0.0 |
| Ground rice with potato puree | 1 | 0.0 | Red date | 2 | 0.1 |
| Formula with ground rice | 1 | 0.0 | Fruit puree | 6 | 0.2 |
| Rice cracker | 8 | 0.3 | Banana | 7 | 0.2 |
| Rice water | 2 | 0.1 | Yolk | 14 | 0.5 |
| Rice porridge | 1 | 0.0 | Liver | 3 | 0.1 |
| Noodle | 5 | 0.2 | Shrimp | 3 | 0.1 |
| Steamed buns | 3 | 0.1 | Dried meat | 1 | 0.0 |
| Millet puree | 1 | 0.0 | Cookie | 1 | 0.1 |
| Millet porridge | 3 | 0.1 | Puff | 1 | 0.0 |
| Millet sweet potato porridge | 1 | 0.0 | Mousse | 1 | 0.0 |
| Mixed porridge | 1 | 0.0 | Oil | 23 | 0.8 |
| Purple rice porridge | 2 | 0.1 | Linseed oil | 2 | 0.1 |
| Sweet potato | 4 | 0.1 | Sesame butter | 3 | 0.1 |
| Potato | 3 | 0.1 | Soy sauce | 3 | 0.1 |
| Purple sweet potato | 1 | 0.0 | Water | 34 | 1.2 |
| Yum | 7 | 0.2 | Vitamin AD | 188 | 6.5 |
| Spanish | 4 | 0.1 | Vitamin D3 | 61 | 2.1 |
| Bok choy | 1 | 0.0 | Vitamin supplement | 1 | 0.0 |
| Broccoli | 5 | 0.2 | Mineral supplement | 7 | 0.2 |
| Carrot | 14 | 0.5 | Calcium | 47 | 1.6 |
| Cucumber | 5 | 0.2 | Zinc | 2 | 0.1 |
| Pumpkin/squash | 21 | 0.7 | Probiotics | 37 | 1.3 |
| Apple | 29 | 1.0 | Fish oil | 3 | 0.1 |
| Pitaya | 1 | 0.0 | DHA | 25 | 0.9 |
| Blueberry | 2 | 0.1 | B12 | 2 | 0.1 |
| Pear, blueberry | 1 | 0.0 | Albumen powder | 2 | 0.1 |
| Pear | 2 | 0.1 | Iron protein succinylate oral liquid | 2 | 0.1 |
*Data was based on the first 100 subjects at 6 months in group 2.
Data assessment was conducted using early available samples, which is a small proportion of the cohort data. The data that support the findings of this study have been deposited into China National GeneBank Sequence Archive,34 35 with accession number CNP0003576. A total of 20 stool samples were sequenced and subjected to subsequent metagenomic analysis. All DNA concentrations of the 20 samples are greater than 3 ng/µL, the minimum amount required for library construction. Adaptor contaminated, low-quality and host reads were removed from raw sequencing read sets. An average of 33.6 GB (9.52 to 48.36 GB) data of high-quality clean reads per sample was generated, equivalent to 96.1% of raw reads on average. Comparison of the compositional features of the gut microbiota alongside the age spectrum revealed several characteristic patterns. At the species level, bacterial communities were composed mostly of Bacteroides dorei, Bacteroides vulgatus and Escherichia coli (online supplemental figure S1). Microbial abundances were shown in the Supplemental materials. Progressive changes in microbial diversity and abundance of the infant gut microbiome are likely to reshape the metabolic functions of the hosts over time. The results of the pilot assessment were consistent with the discoveries reported previously.8 36 The urine creatine assay has been tested and validated previously in infants and toddlers with similar age to the BAMBOO cohort, with results in the correct range.37 38 A cross-validation of breast milk vitamin D and HMOs measurement has been conducted with internal data previously published39 40 and also validated through certified reference values (online supplemental tables S1, S2 and S3). In summary, the early data assessment shows that high reliability of the data generated from this study.
bmjopen-2023-075417supp001.pdf (150.3KB, pdf)
The next step will focus on completing recruitment and minimising the drop out, especially in group 1. Additionally, an interim statistical analysis will be performed to provide an initial overview of different aspects of the study, including microbiome maturation, bone development and dietary intake of children at different ages. Finally, four stage-statistical analyses before the final data analysis will be performed as the complete cohort reaches certain age thresholds.
Strengths and limitations
There are several limitations in this study. First, as an observational study, only associations between early life nutrition, microbiome maturation and bone development can be investigated, but their causal relationships could not be determined. However, these findings will provide insights that may help develop scientific hypotheses and identify potentially relevant timing and interventions to support optimal microbiome or bone development and contribute new evidence to inform nutrition policy setting. Second, as the study is conducted in a major metropolitan area of northern China, we could anticipate differences in feeding and dietary habits from other regions across China. Future studies could focus on such regional differences.
The strength of the study is its large sample size with longitudinal follow-up, measuring multiple aspects of health consistently at multiple time points. To our knowledge, this is the first study with such comprehensive coverage in infant and toddlers in China, making it possible to investigate the interplay of dietary factors, microbiome maturation and bone development, as well as growth and health status.
bmjopen-2023-075417supp002.pdf (62.5KB, pdf)
Supplementary Material
Acknowledgments
We thank Dr Norbert Sprenger for his contribution in questionnaire design and guidance in microbiome data analysis and Dr Giles Major for his critical review of this manuscript. We thank Qiaoji Li, Dr Alice Pannérec, Dr Marie Boutant Lys and Dr Marie Bachelet for setting up the collaboration and their professional management in planning and executing the project. We thank all the investigators from Tianjin Women and Children’s Health Center for conducting the field investigation and sample collection and research staff from BGI-Shenzhen for preparing sample collecting kits and performing laboratory testing. This work was supported by China National GeneBank. We thank all participants in this study.
Footnotes
Contributors: All authors contributed to the study design, interpretation of the data and writing the manuscript. Data and sample collection were performance by JW, CJ, SW, LF, YZ, YG and GL. Sample measurement and analyses were performed by XL, GZ, XZ, FR, LG, JC and YG. Data management and statistical protocol were set up by MC, ND, MNH, NB, SKD and DW. The first draft of the manuscript was written by JW, SCM, MNH, NB, SKD and DW. All authors read and approved the manuscript, and acted as the guarantor of the over all content.
Funding: The study is funded by Société des Produits Nestlé SA and BGI-Research.
Competing interests: XL, GZ, XZ, FR, LG, JC and YG are employed by BGI Research. MC, ND, SCM, MNH, NB, SKD and DW are employed by Nestle Research. The authors declared that they have no competing interests.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Microbiota data that support the findings of this study have been deposited intoChina National GeneBank Sequence Archive and made publicly available upon publication of the results in scientific articles. Ethical considerations related to personal data, informed consent and human research act restrict human data sharing in public repositories. Fully anonymised data may be shared upon request to the corresponding author accompanied by a proposed research plan for the evaluation and approval by the BAMBOO scientific council, followed by relevant ethics committee approval.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
For this study the informed consent was obtained from parents or legal guardians of the infants. Informed consents were also obtained from all the mothers involved in the study. This study involves human participants and was approved by ethics approval of research was obtained from Tianjin Women’s and Children’s Health Center Medical Ethical Committee and the Institutional Review Board of BGI-Shenzhen (BGI-IRB 21056). Participants gave informed consent to participate in the study before taking part. The study is registered in the clinical trial registration, and the trial number is ChiCTR2100049972.
References
- 1. Vijay A, Valdes AM. Role of the gut microbiome in chronic diseases: a narrative review. Eur J Clin Nutr 2022;76:489–501. 10.1038/s41430-021-00991-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2. Parekh PJ, Balart LA, Johnson DA. The influence of the gut microbiome on obesity, metabolic syndrome and gastrointestinal disease. Clin Transl Gastroenterol 2015;6:e91. 10.1038/ctg.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cong X, Xu W, Romisher R, et al. Gut microbiome and infant health: brain-gut-microbiota axis and host genetic factors. Yale J Biol Med 2016;89:299–308. [PMC free article] [PubMed] [Google Scholar]
- 4. Carlson AL, Xia K, Azcarate-Peril MA, et al. Infant gut microbiome associated with cognitive development. Biol Psychiatry 2018;83:148–59. 10.1016/j.biopsych.2017.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dogra SK, Kwong Chung C, Wang D, et al. Nurturing the early life gut microbiome and immune maturation for long term health. Microorganisms 2021;9:2110. 10.3390/microorganisms9102110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woodall CA, McGeoch LJ, Hay AD, et al. Respiratory tract infections and gut microbiome modifications: a systematic review. PLoS One 2022;17:e0262057. 10.1371/journal.pone.0262057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao T, Li J, Fu Y, et al. Influence of gut microbiota on mucosal IgA antibody response to the polio vaccine. NPJ Vaccines 2020;5:47. 10.1038/s41541-020-0194-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stewart CJ, Ajami NJ, O’Brien JL, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018;562:583–8. 10.1038/s41586-018-0617-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ho NT, Li F, Lee-Sarwar KA, et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat Commun 2018;9:4169. 10.1038/s41467-018-06473-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amir A, Erez-Granat O, Braun T, et al. Gut Microbiome development in early childhood is affected by day care attendance. NPJ Biofilms Microbiomes 2022;8:2. 10.1038/s41522-021-00265-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baumann-Dudenhoeffer AM, D’Souza AW, Tarr PI, et al. Infant diet and maternal gestational weight gain predict early metabolic maturation of gut microbiomes. Nat Med 2018;24:1822–9. 10.1038/s41591-018-0216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bäckhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015;17:690–703. 10.1016/j.chom.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 13. Gehrig JL, Venkatesh S, Chang H-W, et al. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science 2019;365:eaau4732. 10.1126/science.aau4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Subramanian S, Huq S, Yatsunenko T, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 2014;510:417–21. 10.1038/nature13421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Depner M, Taft DH, Kirjavainen PV, et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat Med 2020;26:1766–75. 10.1038/s41591-020-1095-x [DOI] [PubMed] [Google Scholar]
- 16. Jones IE, Williams SM, Goulding A. Associations of birth weight and length, childhood size, and smoking with bone fractures during growth: evidence from a birth cohort study. Am J Epidemiol 2004;159:343–50. 10.1093/aje/kwh052 [DOI] [PubMed] [Google Scholar]
- 17. Ma NS, Gordon CM. Pediatric osteoporosis: where are we now. J Pediatr 2012;161:983–90. 10.1016/j.jpeds.2012.07.057 [DOI] [PubMed] [Google Scholar]
- 18. Clark EM, Ness AR, Bishop NJ, et al. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res 2006;21:1489–95. 10.1359/jbmr.060601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weaver CM, Gordon CM, Janz KF, et al. The National osteoporosis foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 2016;27:1281–386. 10.1007/s00198-015-3440-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu J, Shin Y, Yen M-S, et al. Peak bone mass and patterns of change in total bone mineral density and bone mineral contents from childhood into young adulthood. J Clin Densitom 2016;19:180–91. 10.1016/j.jocd.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ambrose CG, Soto Martinez M, Bi X, et al. Mechanical properties of infant bone. Bone 2018;113:151–60. 10.1016/j.bone.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 22. Yang Y, Wu F, Dwyer T, et al. Associations of breastfeeding, maternal smoking, and birth weight with bone density and microarchitecture in young adulthood: a 25-year birth-cohort study. J Bone Miner Res 2020;35:1652–9. 10.1002/jbmr.4044 [DOI] [PubMed] [Google Scholar]
- 23. Carter SA, Parsons CM, Robinson SM, et al. Infant milk feeding and bone health in later life: findings from the Hertfordshire cohort study. Osteoporos Int 2020;31:709–14. 10.1007/s00198-020-05296-1 [DOI] [PubMed] [Google Scholar]
- 24. Berger B, Porta N, Foata F, et al. Linking human milk oligosaccharides, infant fecal community types, and later risk to require antibiotics. mBio 2020;11:e03196-19. 10.1128/mBio.03196-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charbonneau MR, O’Donnell D, Blanton LV, et al. Sialylated milk oligosaccharides promote microbiotadependent growth in models of infant undernutrition. Cell 2016;164:859–71. 10.1016/j.cell.2016.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blanton LV, Barratt MJ, Charbonneau MR, et al. Childhood undernutrition, the gut microbiota, and microbiotadirected therapeutics. Science 2016;352:1533. 10.1126/science.aad9359 [DOI] [PubMed] [Google Scholar]
- 27. Cowardin CA, Ahern PP, Kung VL, et al. Mechanisms by which sialylated milk oligosaccharides impact bone biology in a gnotobiotic mouse model of infant undernutrition. Proc Natl Acad Sci U S A 2019;116:11988–96. 10.1073/pnas.1821770116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dibba B, Prentice A, Ceesay M, et al. Effect of calcium supplementation on bone mineral accretion in Gambian children accustomed to a low-calcium diet. Am J Clin Nutr 2000;71:544–9. 10.1093/ajcn/71.2.544 [DOI] [PubMed] [Google Scholar]
- 29. Litmanovitz I, Davidson K, Eliakim A, et al. High beta-palmitate formula and bone strength in term infants: a randomized, double-blind, controlled trial. Calcif Tissue Int 2013;92:35–41. 10.1007/s00223-012-9664-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riley AW, Trabulsi J, Yao M, et al. Validation of a parent report questionnaire: the infant gastrointestinal symptom questionnaire. Clin Pediatr (Phila) 2015;54:1167–74. 10.1177/0009922815574075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. China Nutrition Society . China Food Composition Table. Beijing: Peking University Medical Press, 2019. [Google Scholar]
- 32. Banjac J, Sprenger N, Dogra SK. Microbiome toolbox: methodological approaches to derive and visualize microbiome trajectories. Bioinformatics 2023;39:2022. 10.1093/bioinformatics/btac781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang S, Zhang G, Wang J, et al. Study design and baseline profiles of participants in the Tianjin birth cohort (TJBC) in China. J Epidemiol 2022;32:44–52. 10.2188/jea.JE20200238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo X, Chen F, Gao F, et al. CNSA: a data repository for archiving omics data. Database (Oxford) 2020;2020:baaa055. 10.1093/database/baaa055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen FZ, You LJ, Yang F, et al. CNGBdb: China National Genebank database. Yi Chuan 2020;42:799–809. 10.16288/j.yczz.20-080 [DOI] [PubMed] [Google Scholar]
- 36. Niu J, Xu L, Qian Y, et al. Evolution of the gut Microbiome in early childhood: a cross-sectional study of Chinese children. Front Microbiol 2020;11:439. 10.3389/fmicb.2020.00439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang W, Du C, Lin L, et al. Anthropometry-based 24-H urinary creatinine excretion reference for Chinese children. PLoS ONE 2018;13:e0197672. 10.1371/journal.pone.0197672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kwak BO, Lee ST, Chung S, et al. Microalbuminuria in normal Korean children. Yonsei Med J 2011;52:476–81. 10.3349/ymj.2011.52.3.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oberson JM, Bénet S, Redeuil K, et al. Quantitative analysis of vitamin D and its main metabolites in human milk by supercritical fluid chromatography coupled to tandem mass spectrometry. Anal Bioanal Chem 2020;412:365–75. 10.1007/s00216-019-02248-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Austin S, De Castro CA, Bénet T, et al. Temporal change of the content of 10 oligosaccharides in the milk of Chinese urban mothers. Nutrients 2016;8:346. 10.3390/nu8060346 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-075417supp001.pdf (150.3KB, pdf)
bmjopen-2023-075417supp002.pdf (62.5KB, pdf)
Data Availability Statement
Microbiota data that support the findings of this study have been deposited intoChina National GeneBank Sequence Archive and made publicly available upon publication of the results in scientific articles. Ethical considerations related to personal data, informed consent and human research act restrict human data sharing in public repositories. Fully anonymised data may be shared upon request to the corresponding author accompanied by a proposed research plan for the evaluation and approval by the BAMBOO scientific council, followed by relevant ethics committee approval.