Abstract
Introduction
The evidence for spinal cord stimulation (SCS) has been criticized for the absence of blinded, parallel randomized controlled trials (RCTs) and limited evaluations of the long-term effects of SCS in RCTs. The aim of this study was to determine whether evoked compound action potential (ECAP)-controlled, closed-loop SCS (CL-SCS) is associated with better outcomes when compared with fixed-output, open-loop SCS (OL-SCS) 36 months following implant.
Methods
The EVOKE study was a multicenter, participant-blinded, investigator-blinded, and outcome assessor-blinded, randomized, controlled, parallel-arm clinical trial that compared ECAP-controlled CL-SCS with fixed-output OL-SCS. Participants with chronic, intractable back and leg pain refractory to conservative therapy were enrolled between January 2017 and February 2018, with follow-up through 36 months. The primary outcome was a reduction of at least 50% in overall back and leg pain. Holistic treatment response, a composite outcome including pain intensity, physical and emotional functioning, sleep, and health-related quality of life, and objective neural activation was also assessed.
Results
At 36 months, more CL-SCS than OL-SCS participants reported ≥50% reduction (CL-SCS=77.6%, OL-SCS=49.3%; difference: 28.4%, 95% CI 12.8% to 43.9%, p<0.001) and ≥80% reduction (CL-SCS=49.3%, OL-SCS=31.3%; difference: 17.9, 95% CI 1.6% to 34.2%, p=0.032) in overall back and leg pain intensity. Clinically meaningful improvements from baseline were observed at 36 months in both CL-SCS and OL-SCS groups in all other patient-reported outcomes with greater levels of improvement with CL-SCS. A greater proportion of patients with CL-SCS were holistic treatment responders at 36-month follow-up (44.8% vs 28.4%), with a greater cumulative responder score for CL-SCS patients. Greater neural activation and accuracy were observed with CL-SCS. There were no differences between CL-SCS and OL-SCS groups in adverse events. No explants due to loss of efficacy were observed in the CL-SCS group.
Conclusion
This long-term evaluation with objective measurement of SCS therapy demonstrated that ECAP-controlled CL-SCS resulted in sustained, durable pain relief and superior holistic treatment response through 36 months. Greater neural activation and increased accuracy of therapy delivery were observed with ECAP-controlled CL-SCS than OL-SCS.
Trial registration number
Keywords: CHRONIC PAIN, Neuromodulation, Spinal Cord Stimulation
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is an absence of blinded, parallel randomized controlled trials (RCTs) of spinal cord stimulation (SCS) and limited evaluations of the long-term effects of SCS in RCTs that has led to criticisms of the evidence base for this therapy.
WHAT THIS STUDY ADDS
This study represents the only multicenter, participant-blinded, investigator-blinded, and outcome assessor-blinded parallel-arm RCT of SCS.
We evaluated whether a physiological closed-loop SCS (CL-SCS) system that measures the neural response through evoked compound action potentials and continuously adjusts the stimulation output to maintain a target neural response, can generate superior, durable, long-term treatment effects for chronic pain when compared with open-loop SCS.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Patients who received CL-SCS maintained consistently greater neural activation and accuracy and demonstrated superior and sustained long-term improvements in pain relief and patient-reported outcomes, including greater holistic treatment response.
The objective physiological response to SCS can be controlled to provide consistent neural activation and thus reproducible long-term clinical outcomes.
Introduction
Spinal cord stimulation (SCS) is an established therapy for the management of chronic refractory pain syndromes.1 2 For nearly 50 years since SCS was first described,3 the evidence base for SCS was limited to fixed-output, open-loop sensation-based stimulation.4 5 For activation of spinal cord cells and/or fibers that contribute to the inhibition of pain transmission, fixed-output, open-loop SCS (OL-SCS) relies on the patient’s report of paresthesia or is assumed by the anatomical position of the SCS leads in paresthesia-free stimulation.4 Following lead placement, the patient’s subjective response to pain is typically evaluated during a screening trial prior to implantation of the permanent SCS device.6
The benefits of OL-SCS have often not been observed in long-term analyses.7 8 Systematic reviews of randomized controlled trials (RCTs) of SCS suggested that the pain reduction with SCS was not clinically meaningful compared with sham or placebo stimulation.7 9 Placebo and/or sham-controlled evidence, however, is limited to crossover trials with phases of short duration and several methodological weaknesses.10 Furthermore, one of the main criticisms of the SCS evidence is the inherent high risk of bias of open-label studies, the absence of double-blind, parallel RCTs and limited evaluations of the long-term effects of SCS in RCTs.
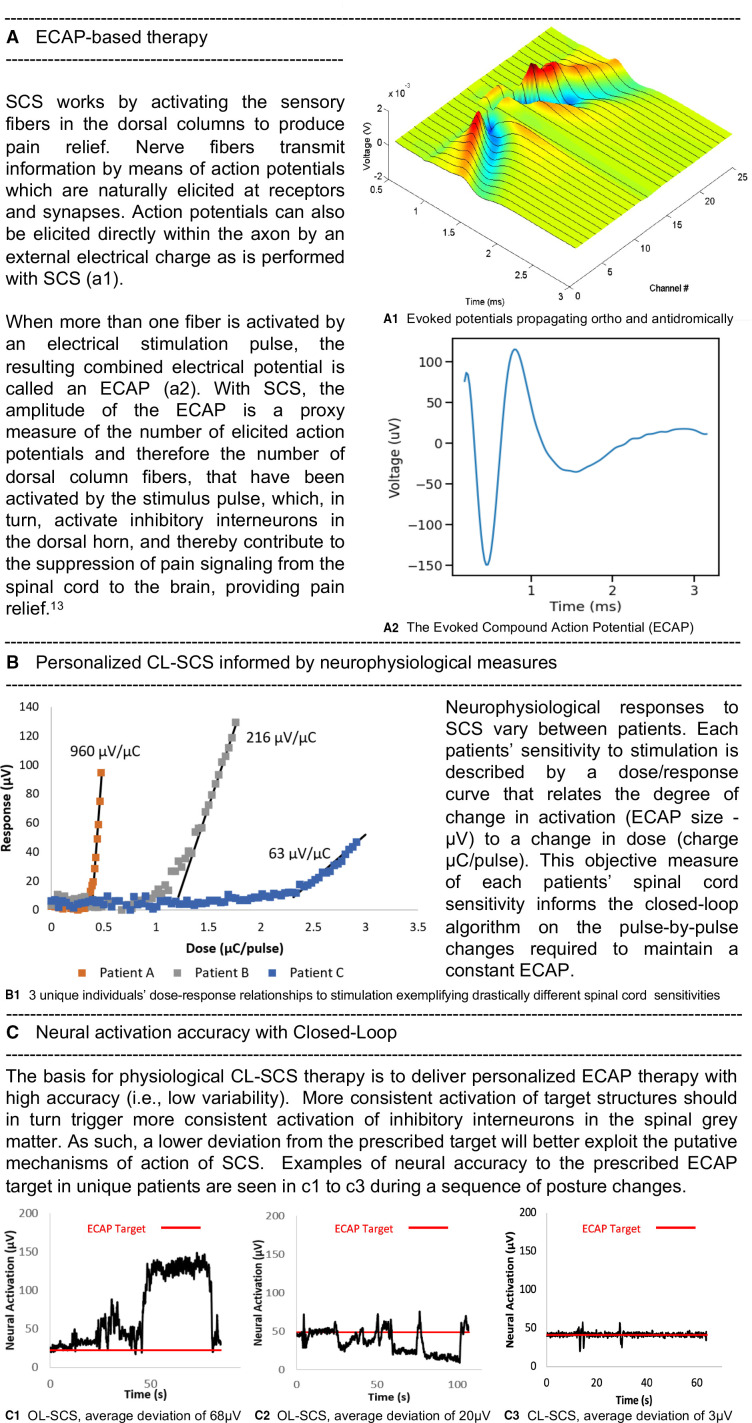
A novel SCS system uses evoked compound action potentials (ECAPs) in a closed-loop SCS (CL-SCS) system. ECAPs provide an objective physiologic biomarker for therapeutic activation of the spinal cord to guide programming and optimize the neural activation and accuracy of the stimulation. ECAP-controlled CL-SCS automatically adjusts the output with each electrical pulse utilizing real-time measured ECAPs to respond to the dynamic environment between the electrodes and spinal cord and subsequently deliver controlled energy to maintain neural activation accuracy using an individualized CL-SCS ECAP amplitude target (figure 1).
Figure 1.
ECAP-controlled closed-loop SCS fundamentals. AP, action potential; CL, closed-loop; ECAP, evoked compound action potential; OL, open-loop; SCS, spinal cord stimulation.
The EVOKE study is the only published evaluation of SCS in a parallel-arm RCT that blinded patients, investigators, and staff including outcome assessors. Results up to 24-month follow-up showed the superiority of ECAP-controlled CL-SCS over OL-SCS in the treatment of chronic, intractable back and leg pain.11 12 In this report, we present the 36-month follow-up results of the EVOKE study.
Methods
Study design
This pivotal, multicenter, participant, investigator, and outcome assessor-blinded, parallel-arm RCT was conducted at 13 investigation sites throughout the USA under an Investigational Device Exemption to gain US Food and Drug Administration (FDA) approval (registered on ClinicalTrials.gov, October 5, 2016; NCT02924129). An approved investigational device exemption permits a device that otherwise would be required to comply with a performance standard or to have premarket approval to be shipped lawfully for the purpose of conducting investigations of that device.13 14 This study was conducted under US FDA regulatory requirements, Good Clinical Practice, and the ethical principles of the Declaration of Helsinki.15
Participants
Candidates with chronic, intractable back and leg pain refractory to conservative therapy with a minimum Visual Analog Scale (VAS) score of 60 mm or higher (where 100 mm indicates the worst imaginable pain), who provided written informed consent, were screened for enrollment. The study was conducted from January 27, 2017 (first patient enrolled) to September 9, 2022 (last patient complete). The full eligibility criteria are presented in the protocol.14 An independent medical monitor confirmed the consistent interpretation of the eligibility criteria before patient enrollment.11 14
Randomization and concealment
Patients were randomized 1:1 to ECAP-controlled CL-SCS (investigational group) or fixed-output OL-SCS (control group). Treatment allocation was concealed from the patients, investigator, and site staff including outcome assessors for the full study duration. Randomization and masking procedures were described previously (also presented in online supplemental eAppendix 1).11 14
rapm-2023-104751supp001.pdf (220.5KB, pdf)
Procedures
Randomized patients underwent a temporary SCS trial lasting on average 6 days (range 2–11). Patients with 50% or more overall back and leg pain VAS score reduction were eligible for permanent implantation. During the temporary trial and permanent implant procedures, two percutaneous leads were implanted in the dorsal epidural space as per standard practice (online supplemental eAppendix 1). The same neuromodulation system (Evoke System; Saluda Medical, Artarmon, Australia) was the investigational and control device as it offered both ECAP-controlled, CL-SCS and fixed output, OL-SCS and the ability to measure the neural activation in both groups. The only difference between groups was having the feedback loop on in the CL-SCS group and off in the OL-SCS group. The closed-loop control system is a proportional-integral-derivative controller, which minimizes the difference between the measured ECAP amplitude and the ECAP amplitude target by automatically varying the stimulation current amplitude in real time in a frequency dependent manner. The system maintains a consistent neural response where the average error between the prescribed ECAP amplitude target and the measured ECAP amplitude is zero.16 ECAP-guided programming was performed for both treatment arms as previously described (online supplemental eAppendix 1).11 14
In addition to baseline prerandomization assessment, outcome follow-up assessments were conducted at 1, 3, 6, 9, and 12 months postrandomization and biannually thereafter for up to 3 years following permanent implant. Patients were permitted to crossover after their 24-month follow-up. Crossover was self-selected with all patients allowed to crossover independently of level of pain relief. Patients could choose to return to the original therapy arm or remain in the crossover arm at 1 and 3 months post-crossover. Irrespective of crossover, treatment allocation remained concealed until the final follow-up assessment at 36 months.
Outcomes
Pain relief was assessed by determining the percent change from baseline in VAS score and the proportion of patients with ≥50% and ≥80% reduction in overall back and leg pain. Pain medication17 and selected validated patient-reported outcome measures including the Oswestry Disability Index (ODI), Profile of Mood States (POMS), Pittsburgh Sleep Quality Index (PSQI), and generic health-related quality of life (EQ-5D-5L) were collected in accord with the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials recommendations.4 18
Treatment response was assessed by attaining minimal clinically important differences (MCIDs) for VAS, ODI, POMS, PSQI, and EQ-5D. The breadth of treatment response refers to the number of domains in which at least one MCID was achieved while the depth of treatment response refers to the number of MCIDs obtained in each domain. Holistic treatment response19 was determined for each patient based on attaining at least one MCID improvement in all domains that were impaired at baseline when compared with normative US values. In addition, the total amount of MCIDs achieved were calculated for each domain and pooled for all domains to derive a cumulative responder score (online supplemental eAppendix 1).
Objective measurements associated with SCS, including program parameters, the degree of neural activation, the accuracy of neural activation, and system utilization were collected on the device (online supplemental eAppendix 1). Out-of-clinic neural activation was defined as the most frequent spinal cord activation level (mode ECAP (µV)) for the week leading up to the scheduled visit. An in-clinic metric of device performance was calculated using root mean square error to determine the deviation of the observed ECAP response from the target ECAP response, programmed in a sitting position, during various posture changes, representing neural activation accuracy. System utilization was defined as the proportion of time the system was on for the week prior to the scheduled visit.
All adverse events were reported by the investigators throughout the study and reviewed and adjudicated by a blinded, independent clinical events committee.
Statistical analysis
The sample size calculation, primary analysis at 3 months, and additional analysis at 12 and 24 months following permanent implant have been described previously.11 12 The 36-month analysis of the primary outcome of pain and secondary outcomes included all randomized patients and followed the intention-to-treat principle (ie, analyzed by group according to original random allocation) with missing data imputed using last value carried forward in accord with our 24-month follow-up analyses.12 This was performed as a conservative measure to minimize the potential bias of an enriched population (ie, where only patients benefiting from treatment remained in the study, and those not benefiting withdrew early).20 For one patient, in which the patient reported ≥50% reduction in VAS pain, but the reason for exit was the patient ‘felt no significant difference in pain’, baseline value carried forward was used and was considered a treatment failure. A secondary analysis was performed for groups as randomized with patients who crossed over and received the alternative therapy considered as treatment failures (online supplemental eAppendix 1).21 22 Descriptive statistics are provided as mean (SD), median (IQR), or number of observations (percentage), as appropriate. Differences in categorical variables between treatment groups were evaluated using χ2 or Fisher’s exact test and continuous measures with two-sample t-tests. For all tests, p values less than 0.05 (two-tailed tests) were considered significant and are reported together with 95% CIs where appropriate. Statistical analyses were conducted using SAS statistical software V.9.4 (SAS Institute).
Results
Summary of participation and crossover
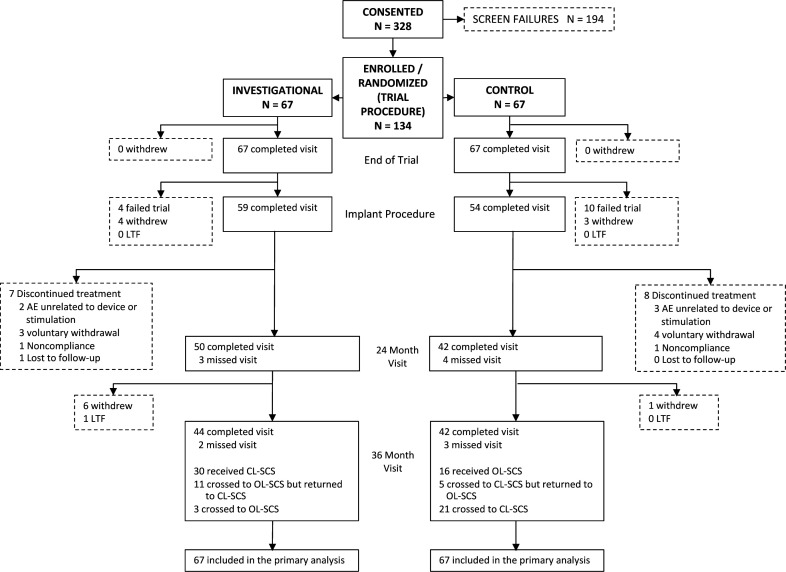
Of 328 screened patients, 134 were enrolled, with 67 randomized to each treatment group (figure 2). Of these, 113 patients underwent implantation (59 in the CL-SCS and 54 in the OL-SCS group). Baseline demographics, diagnoses and other characteristics were well-balanced between the groups.11 During self-selected blinded crossover at 24 months, OL-SCS patients were significantly more likely to crossover (χ2 (1, N=90)=7.3, p=0.007). The most common reason to crossover from CL-SCS to OL-SCS was curiosity (ie, an opportunity to experience the alternative therapy) (81.3%), and to crossover from OL-SCS to CL-SCS was hope for improved pain relief (61.5%). Patients could select to return to the therapy they were initially randomized to, at 1 months or 3 months after the crossover decision or select to continue with the post-crossover therapy. At the end of crossover, 80% (32/40) of patients who participated in the crossover phase selected to continue with CL-SCS. Patients were more likely to return to or stay in CL-SCS rather than return to or stay in OL-SCS (χ2 (1, N=40)=14.1, p<0.001). Forty-four CL-SCS patients and 42 OL-SCS patients completed the 36-month follow-up. Of those that experienced CL-SCS, either randomized or crossed over to CL-SCS, 89% (62/70) completed the study in CL-SCS. Patients, investigators, and outcome assessors remained blinded for the full study duration.
Figure 2.
Consolidated Standards of Reporting Trials (CONSORT) diagram. AE, adverse event; CL-SCS, closed-loop SCS; LTF, lost to follow-up; OL-SCS, open-loop spinal cord stimulation.
Overall back and leg pain intensity reduction
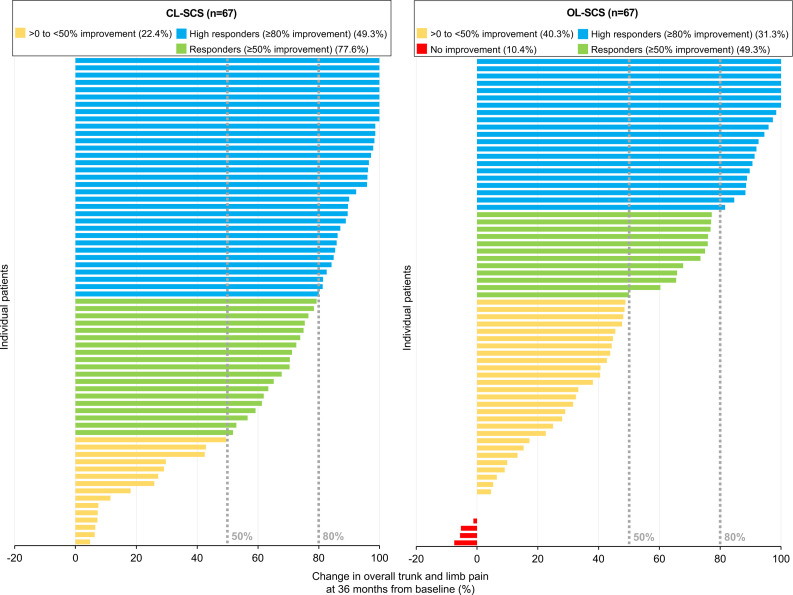
At 36 months, the reduction in overall back and leg pain intensity was significantly greater for CL-SCS (mean (SD) score, 25.4 (25.6); point decrease, 56.6; per cent decrease, 69.6%) than OL-SCS patients (mean (SD) score, 38.3 (29.8); point decrease, 43.9; per cent decrease, 53.9%) with a mean between groups difference of −12.9 (95% CI −22.4 to −3.4), p=0.008; point decrease difference, 12.7 (95% CI 3.5 to 21.9), p=0.007; per cent decrease difference, 15.7% (95% CI 4.5% to 26.9%), p=0.006). A greater proportion of CL-SCS patients achieved ≥50% reduction (CL-SCS=77.6%, OL-SCS=49.3%; difference: 28.4%, 95% CI 12.8% to 43.9%, p<0.001) and ≥80% reduction (CL-SCS=49.3%, OL-SCS=31.3%; difference: 17.9, 95% CI 1.6% to 34.2%, p=0.032) in overall back and leg pain intensity when compared with OL-SCS patients (figure 3).
Figure 3.
Individual patient percent change from baseline in overall back and leg pain at 36 months. CL-SCS, closed-loop spinal cord stimulation; OL-SCS, open-loop spinal cord stimulation.
Other patient-reported outcome measures
Statistically significant and clinically meaningful improvements from baseline were observed at 36 months in both treatment groups in all other patient-reported outcomes including ODI, POMS, EQ-5D-5L, and PSQI (online supplemental eAppendix 1) with improvement greater with CL-SCS compared with OL-SCS. Eighty-one per cent of CL-SCS patients compared with 66.0% of OL-SCS patients indicated their health status was ‘very much improved’ or ‘much improved’ following SCS implant.
Holistic treatment assessment (depth and breadth)
A greater proportion of CL-SCS patients (44.8%) compared with OL-SCS patients (28.4%) were categorized as responders for each of the impaired domains (table 1) and were holistic treatment responders at 36 months (risk difference: 16.4%, 95% CI 0.3% to 32.5%, p=0.072), thus obtaining a greater breadth of response with CL-SCS.
Table 1.
Proportion of responders for each impaired domain
| Closed-loop SCS | Open-loop SCS | |
| Pain intensity responders (VAS overall ≥30%) | 55/67 (82.1%) | 49/67 (73.1%) |
| Risk difference (%) and 95% CI | 9.0 (−5.1to 23.0), p=0.211 | |
| Physical function responders (ODI Score ≥10) | 47/67 (70.1%) | 42/67 (62.7%) |
| Risk difference (%) and 95% CI | 7.5 (−8.5 to 23.4), p=0.465 | |
| HRQoL responders (EQ-5D-5L Index Score ≥0.074) | 46/67 (68.7%) | 41/67 (61.2%) |
| Risk difference (%) and 95% CI | 7.5 (−8.6 to 23.6), p=0.469 | |
| Sleep responders (PSQI Global Score≥3) | 39/66 (59.1%) | 27/64 (42.2%) |
| Risk difference (%) and 95% CI | 16.9 (−0.0 to 33.8), p=0.079 | |
| Emotional function responders (POMS TMD Score≥10) | 31/44 (70.5%) | 18/38 (47.4%) |
| Risk difference (%) and 95% CI | 23.1 (2.3 to 43.9), p=0.043 | |
| Multimodal treatment responders (MCID in at least two impaired domains out of VAS≥30%, ODI≥10, EQ-5D≥0.074, PSQI≥3, POMS≥10) | ||
| Responders in ≥1 impaired domain | 63/67 (94.0%) | 57/67 (85.1%) |
| Responders in ≥2 impaired domains | 52/67 (77.6%) | 46/67 (68.7%) |
| Responders in ≥3 impaired domains | 47/67 (70.1%) | 41/67 (61.2%) |
| Responders in ≥4 impaired domains | 35/66 (53.0%) | 26/66 (39.4%) |
| Responders in 5 impaired domains | 21/44 (47.7%) | 7/36 (19.4%) |
| Holistic treatment responder | 30/67 (44.8%) | 19/67 (28.4%) |
| Risk difference (%) and 95% CI | 16.4 (0.3 to 32.5), p=0.072 | |
HRQoL, health-related quality of life; MCID, minimal clinically important difference; ODI, Oswestry Disability Index; POMS, Profile of Mood States; PSQI, Pittsburgh Sleep Quality Index; SCS, spinal cord stimulation; TMD, total mood disorder; VAS, Visual Analog Scale.
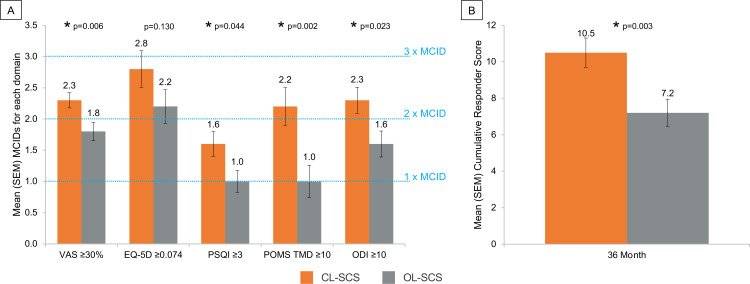
Although improvement was observed in all impaired domains in both groups, the depth of the treatment response was significantly greater for CL-SCS compared with OL-SCS (figure 4A). CL-SCS patients obtained 0.5–1.3 additional MCIDs in each domain (overall back and leg pain MD 0.5, 95% CI 0.2 to 0.9, p=0.006; ODI MD 0.7, 95% CI 0.1 to 1.3, p=0.023; PSQI MD 0.5, 95% CI 0.0 to 1.1, p=0.044; POMS MD 1.3, 95% CI 0.5 to 2.1, p=0.002; and more than 3 additional MCIDs across all impaired baseline domains (cumulative responder score MD 3.3, 95% CI 1.1 to 5.5, p=0.003) (figure 4B).
Figure 4.
(A) Minimal clinically important improvements observed for each impaired domain at 36-month follow-up. (B) Cumulative responder score at 36-month follow-up. *Statistical significant at p<0.05 level. CL-SCS, closed-loop SCS; MCID, minimal clinically important difference; ODI, Oswestry Disability Index; OL-SCS, open-loop SCS; POMS, Profile of Mood States; PSQI, Pittsburgh Sleep Quality Index; SCS, spinal cord stimulation; TMD, total mood disorder; VAS, Visual Analog Scale.
Programming
There were no differences between treatment groups in prescribed stimulation parameters with average frequency of approximately 40 Hz (mean (range) 36.1 (10.0–60.0) CL-SCS, 36.4 (10.0–60.0) OL-SCS, p=0.997), pulse duration of approximately 300 μs (305.4 (140.0–600.0) CL-SCS, 331.5 (180.0–800.0) OL-SCS, p=0.080); and stimulation amplitude of approximately 6 mA (6.6 (1.5–22.3) CL-SCS, 6.0 (1.3–17.7) OL-SCS, p=0.198) in both groups. The neural responses (ECAP amplitude (μV)) measured from the dose-response curves were comparable between groups for perception threshold (median (IQR) 5.0 (3.0–12.0) CL-SCS, 5.0 (2.0–8.0) OL-SCS, p=0.281), and comfort activation level (28.0 (15.0–60.0) CL-SCS, 22.0 (10.0–47.0) OL-SCS, p=0.149). The maximum tolerable activation level was significantly lower in OL-SCS as compared with CL-SCS (92.0 (59.0–167.0) CL-SCS, 76.5 (35.0–140.0) OL-SCS, p=0.030). Measured sensitivity (slope of the dose-response; μV/μC per pulse) was not significantly different between groups (median (IQR)):53.9 (22.0–85.5) CL-SCS, 39.8 (22.8–61.8) OL-SCS; p=0.073).
Neural activation and system utilization
System utilization was similar between treatment groups with patients having their device switched on greater than 75% of the time (CL-SCS=77.6% (IQR 0.6%–96.1%), OL-SCS=75.5% (IQR 7.7%–97.4%), p=0.263). However, neural activation was statistically greater for CL-SCS compared with OL-SCS (online supplemental eAppendix 1). The most frequent neural activation (ECAP) was two times greater in CL-SCS (19.8 µV (IQR 7.0–46.5)) than in OL-SCS patients (9.8 µV (IQR 1.0–23.0)), p=0.049. Neural activation accuracy (the deviation of the observed ECAP response from the target ECAP response) was three times more accurate in CL-SCS (4.1 µV (IQR 2.7–6.2)) compared with OL-SCS (12.4 µV (IQR 3.6–25.8)), p<0.001. There were no significant differences in the estimated median number of days to fully deplete the battery between therapy administered as CL-SCS (6.2 days (IQR 4.1–9.0)) compared with OL-SCS (6.3 days (IQR 4.8–7.7)), p=0.85 at 36 months.
Adverse events
The type, nature, and severity of adverse events were similar between CL-SCS and OL-SCS groups. All patients received the same device and underwent the same procedure; the only difference between groups was the stimulation mode (open-loop or closed-loop stimulation). There were no differences between groups in stimulation therapy-related adverse events. Over the course of 36 months, there were 18 explants (6 in year 1, 7 in year 2, and 5 in year 3) out of 113 patients implanted (CL-SCS: 10 (16.9%); OL-SCS: 8 (14.8%)). The most common reason for device explant was the need for MRI (5/18 (27.8%) explants) (online supplemental eAppendix 1). There were three explants due to loss of efficacy (CL-SCS: 0 (0%); OL-SCS: 3 (5.6%)) and three explants due to procedure-related infections (CL-SCS: 2 (3.4%); OL-SCS: 1 (1.8%)). One patient in each arm requested a device explant as they were pain free. The remaining five explants were for different reasons (online supplemental eTable 5) and only one of these was device related.
Discussion
The results of this study demonstrated that ECAP-controlled CL-SCS provided superior outcomes compared with OL-SCS and sustained durability through 36-month follow-up. In addition to superior pain reduction, greater improvements were observed in all other patient-reported outcomes alongside a greater breadth and depth of response to ECAP-controlled CL-SCS for each of the impaired domains at baseline. The CL-SCS group obtained more than three additional MCIDs across all impaired baseline domains when compared with the OL-SCS group. Consideration of a holistic treatment response provides a more comprehensive characterization of the chronic pain experience and treatment response than a simple evaluation of reduction in pain intensity.23 24
ECAP-guided programming in both CL-SCS and OL-SCS provides an enhancement to other available OL-SCS systems. The comparative evidence for follow-ups of RCTs of SCS at a 36-month time point or later is limited to two studies.25 26 Kemler et al reported a 5-year follow-up for their RCT of OL-SCS plus physiotherapy versus physiotherapy alone for complex regional pain syndrome.26 The group of patients that received a permanent implant in addition to physiotherapy reported a reduction in mean VAS score (−2.5±2.2 cm) compared with patients that received physiotherapy alone (−1.0±2.9 cm; p=0.06).26 No significant differences were observed for secondary outcomes including EQ-5D.
In their study of patients undergoing a screening trial followed by OL-SCS implant versus going directly to OL-SCS implant, Eldabe et al reported clinically important reductions in pain intensity and EQ-5D at 36 months for both groups.25 A ≥50% reduction in pain was observed for 33% (21/66) of the patients at 36 months. In the Eldabe et al study, different types of OL-SCS were used; the mean change in pain intensity measured on a Numerical Rating Scale from baseline was −2.80 for paresthetic stimulation, −1.87 for high-frequency stimulation and −2.04 for burst stimulation.25 In the current study, we observed a mean change in pain intensity of −56.6 for CL-SCS on a 0–100 VAS.
Significant differences in therapy delivered were observed with ECAP-controlled CL-SCS resulting in significantly greater neural activation and increased accuracy of spinal cord activation. ECAP-controlled CL-SCS therapy maintains a consistent level of neural activation at the spinal cord target in real time. OL-SCS, as with commercially available OL systems, is not able to maintain neural activation at the ECAP target, which is the likely reason for the poorer treatment response compared with CL-SCS.
Prior literature listed loss of efficacy or inadequate pain relief as the most common reasons for device explant.27–30 However, at 36 months follow-up, there were no explants in the CL-SCS group due to a lack or loss of efficacy. The safety profile in the current study including the all-cause explant rate at 36 months of 15.9% (18/113) was consistent with previous SCS reports.27–31 The most common reason for device explant was the need for MRI. However, current models of the device are now MR-Conditional thus avoiding this complication. For reasons other than need for MRI (5/18) and being pain free (2/18), the explant rate at 36 months was 9.7% (11/113).
Strengths and limitations
To our knowledge, the EVOKE study is the first published patient, investigator, and outcome assessor-blinded evaluation of SCS using a parallel-arm RCT design. The EVOKE study 36-month report is the longest follow-up evidence from an investigational device exemption trial of SCS. All patients included in the analysis were blinded to allocation of their therapy.11 Although the 36-month analysis was not prespecified, the analysis is consistent with previously published trial statistical analysis.12 Both the CL-SCS and OL-SCS groups in this RCT received the same device and ECAP-guided programming. Using ECAP recordings to maximize activation while setting stimulation parameters may infer benefits that are not available to other OL-SCS paradigms. Thus, the greater than expected improvement in patient-reported outcomes for the OL-SCS group may be attributable to ECAP-guided programming.
Conclusions
At 36-month follow-up, ECAP-controlled CL-SCS resulted in superior and durable improvements in patient reported outcomes of pain, sleep, disability, emotional function, and health-related quality of life and the composite holistic treatment response. Greater neural activation and increased accuracy of spinal cord activation were also observed with CL-SCS. This evaluation demonstrated the long-term benefits of objective measurement, accurate therapy delivery, and enhanced neural activation achieved with CL-SCS therapy.
Footnotes
@doctdeer, @CAGPain, @jonhagedornmd
The Evoke Study Group: Dan Brounstein, Rui V. Duarte, Gerrit E. Gmel, Robert GormanIan Gould, Erin Hanson, Dean M. Karantonis, Abeer Khurram, Angela Leitner, Dave Mugan, Milan Obradovic, Zhonghua Ouyang, John Parker, Peter Single, Nicole Soliday.
Contributors: All authors made substantial contributions to the study design, data analysis, and data interpretation, actively participated in drafting and critically revising the manuscript, provided final approval of the submitted version, and agree to be held accountable for the accuracy and integrity of the finished publication. NM is a guarantor who accepts full responsibility for the finished work and the conduct of the study as well as having access to the data and controlled the decision to publish.
Funding: This study was funded by Saluda Medical.
Competing interests: NM reports personal fees from Saluda Medical for acting as independent medical monitor for the EVOKE study during the conduct of the study; he reports receiving grants from Neuros and Mesoblast, as well as consulting as a medical monitor for Nevro, Vivex, Mainstay, and Vertos outside the submitted work. RL is an uncompensated consultant for Nalu, Saluda Medical, and Mainstay Medical and has stock options from Nalu and Saluda Medical obtained before 2019, not exercisable through the duration of his term as International Neuromodulation Society President and editor-in-chief of the journal Neuromodulation: Technology at the Neural Interface. TD reports personal fees from Saluda Medical during the conduct of the study; consultancy for Axonics, Abbott, Nalu, Vertos, SpineThera, Mainstay, Cornerloc, Ethos, SPR Therapeutics, Medtronic, Boston Scientific, PainTeq, Tissue Tech, Spinal Simplicity, and Avanos outside the submitted work. He is a minor equity holder for Saluda Medical, Nalu, SpineThera, Stimgenics, Vertiflex, Vertos, and Bioness and an advisory board member for Abbott, Vertos, Nalu, SPR Therapeutics, and Tissue Tech. LK reports receiving grants from Nevro, Neuros, Avanos, Medtronic, Neuralace, and Xalud Therapeutics and financial support from Nevro, Avanos, and Saluda Medical outside the submitted work. SL reports receiving grants and personal fees from Saluda Medical during the conduct of the study; he reports grants from Avanos, Boston Scientific, Nalu Medical, SPR Therapeutics, Averitas Pharma, Biotronik, SGX Therapeutics, and PainTeq, as well as consultancy for Abbott, Avanos, Boston Scientific, Nevro, SPR Therapeutics, Averitas Pharma, Biotronik, Nalu Medical, and PainTeq, outside the submitted work, as well as holding stock options for Nalu Medical. KA reports consultancy for Medtronic, Nevro, Boston Scientific, Nalu, Presidio, Biotronik, Mesoblast, Vivex Laboratories outside the submitted work. JP reports research and consulting fees from Saluda Medical during the conduct of the study; consultancy for Abbott, Medtronic, Saluda Medical, Flowonix, SpineThera, Vertos, Vertiflex, SPR Therapeutics, Tersera, Aurora, Spark, Ethos, Biotronik, Mainstay, WISE, Boston Scientific, and Thermaquil outside the submitted work; has received grant and research support from: Abbott, Flowonix, Aurora, Painteq, Ethos, Muse, Boston Scientific, SPR Therapeutics, Mainstay, Vertos, AIS, and Thermaquil outside the submitted work; and is a shareholder of Vertos, SPR Therapeutics, Painteq, Aurora, Spark, Celeri Health, Neural Integrative Solutions, Pacific Research Institute, Thermaquil, and Anesthetic Gas Reclamation. CH reports grants from Saluda Medical during the conduct of the study; consultancy fees from Genecentrix outside the submitted work. SC reports grants from Cleveland Clinic during the conduct of the study; and grants from Vertos, Mainstay, and Vivex outside the submitted work. SMF reports consulting fees from Abbott, Medtronic, Saluda, VertiFlex, Vertos, Surgentec, CornerLoc, Mainstay and Relievant outside the submitted work, has received grant for research funding from Mainstay, Relievant, Medtronic, Abbott, VertiFlex, Saluda, Nalu, CornerLoc, Aurora, Biotronik, and Stimgenics outside the submitted work, and has an equity position in SynerFuse, Aurora Spine, Thermaquil. SPR Therapeutics, Saluda, CornerLoc, PainTEQ, Stimgenics, Anesthetic Gas Reclamation, Neural Integrative Solutions, SpineThera, and Celeri Health. CG reports clinical trial funding from Saluda Medical during the conduct of the study; reports personal fees and other from SPR, and personal fees from Nevro, Nalu, Biotronik, and Boston Scientific outside the submitted work. PSS has received consultancy fees from Medtronic, Saluda Medical, Nalu, and Biotronic outside the submitted work, and has stock options from Saluda Medical and Nalu. JS reports personal fees from Nevro during the conduct of the study and personal fees from Saluda Medical and Boston Scientific outside the submitted work. TM reports research fees from Saluda Medical during the conduct of the study and personal fees from Nevro outside the submitted work. JC reports personal fees from Saluda Medical during the conduct of the study; personal fees from Abbott, Boston Scientific, Nevro, Medtronic, Mainstay, SPR Therapeutics, CornerLoc, PillNurse, Biotronik, and Vivex outside the submitted work; and stock from Mainstay, CornerLoc, and PillNurse. EP has received research support from Mainstay, Medtronic, Neuros Medical, Nevro Corp, ReNeuron, SPR, and Saluda Medical outside the submitted work, as well as personal fees from Abbott Neuromodulation, Biotronik, Medtronic Neuromodulation, Nalu, Neuros Medical, Nevro, Presidio Medical, Saluda Medical, and Vertos outside the submitted work. She holds stock options from SynerFuse and neuro42. JMH reports consulting fees from Abbott, Boston Scientific, Nevro, and Saluda Medical outside the submitted work. RR reports grants from SPR, Nalu and Nevro outside the submitted work,l fees from Presidio, and grants and personal fees from Boston Scientific and Saluda Medical outside the submitted work. JWK is an advisory board member for Boston Scientific, Medtronic, Abbott, and Saluda Medical. GB reports consulting fees from Medtronic, Boston Scientific, and Saluda Medical outside the submitted work, and has a consulting agreement and is on the advisory board for Nevro Corp, Nalu Medical Inc, Abbott, and Boston Scientific. RST reports consulting fees from Medtronic, Nevro and Saluda Medical outside the submitted work. LP reports personal fees from Saluda Medical; is a member of the data monitoring board of Saluda Medical during the conduct of the study; and reports personal consulting fees from Medtronic and Nalu outside the submitted work. Members of the EVOKE study group report being employees of Saluda Medical. No other disclosures were reported.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: the EVOKE Study Group, Dan Brounstein, Rui V Duarte, Gerrit E Gmel, Robert GormanIan Gould, Erin Hanson, Dean M Karantonis, Abeer Khurram, Angela Leitner, Dave Mugan, Milan Obradovic, Zhonghua Ouyang, John Parker, Peter Single, and Nicole Soliday
Data availability statement
Data are available on reasonable request. Saluda Medical is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), provided the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided after review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit https://www.saludamedical.com/us/contact-us/.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Western Institutional Review Board (1168219, 1168118, 1168713, 1174388, 1171961, 1172489, 1169008, 1173993, 1178269, 1180823), Forsyth Medical Center Institutional Review Board (16-518), St. Luke’s University Health Network IRB (SLUHN 2016-92), Cleveland Clinic Foundation Institutional Review Board (16-1465). Participants gave informed consent to participate in the study before taking part.
References
- 1. Medical Advisory Secretariat . Spinal cord stimulation for neuropathic pain: an evidence-based analysis. Ont Health Technol Assess Ser 2005;5:1–78. [PMC free article] [PubMed] [Google Scholar]
- 2. National Institute for Health and Care Excellence (NICE) . Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin. Technology appraisal guidance [TA159]. 2008. Available: https://www.nice.org.uk/guidance/ta159 [Accessed 31 Oct 2022].
- 3. Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg 1967;46:489–91. [PubMed] [Google Scholar]
- 4. Katz N, Dworkin RH, North R, et al. Research design considerations for randomized controlled trials of spinal cord stimulation for pain: initiative on methods, measurement, and pain assessment in clinical trials/Institute of neuromodulation/International neuromodulation society recommendations. Pain 2021;162:1935–56. 10.1097/j.pain.0000000000002204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linderoth B, Foreman RD. Conventional and novel spinal stimulation algorithms: hypothetical mechanisms of action and comments on outcomes. Neuromodulation 2017;20:525–33. 10.1111/ner.12624 [DOI] [PubMed] [Google Scholar]
- 6. Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the neuromodulation appropriateness consensus committee. Neuromodulation 2014;17:515–50; 10.1111/ner.12208 [DOI] [PubMed] [Google Scholar]
- 7. O’Connell NE, Ferraro MC, Gibson W, et al. Implanted spinal neuromodulation interventions for chronic pain in adults. Cochrane Database Syst Rev 2021;12:CD013756. 10.1002/14651858.CD013756.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brill S, Defrin R, Aryeh IG, et al. Short- and long-term effects of conventional spinal cord stimulation on chronic pain and health perceptions: a longitudinal controlled trial. Eur J Pain 2022;26:1849–62. 10.1002/ejp.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duarte RV, Nevitt S, McNicol E, et al. Systematic review and meta-analysis of placebo/sham controlled randomised trials of spinal cord stimulation for neuropathic pain. Pain 2020;161:24–35. 10.1097/j.pain.0000000000001689 [DOI] [PubMed] [Google Scholar]
- 10. Duarte RV, McNicol E, Colloca L, et al. Randomized Placebo-/Sham-controlled trials of spinal cord stimulation: a systematic review and methodological appraisal. Neuromodulation 2020;23:10–8. 10.1111/ner.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol 2020;19:123–34. 10.1016/S1474-4422(19)30414-4 [DOI] [PubMed] [Google Scholar]
- 12. Mekhail N, Levy RM, Deer TR, et al. Durability of clinical and quality-of-life outcomes of closed-loop spinal cord stimulation for chronic back and leg pain: a secondary analysis of the evoke randomized clinical trial. JAMA Neurol 2022;79:251–60. 10.1001/jamaneurol.2021.4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Food and Drug Administration . Code of Federal regulations title 21. investigational device exemptions (Part 812). 2023. Available: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRsearch.cfm?CFRPart=812 [Accessed Jul 2023].
- 14. Levy R, Deer TR, Poree L, et al. Double-blind study protocol using human spinal cord recording comparing safety, efficacy, and neurophysiological responses between patients being treated with evoked compound action potential-controlled closed-loop spinal cord stimulation or open-loop spinal cord stimulation (the evoke study). Neuromodulation 2019;22:317–26. 10.1111/ner.12932 [DOI] [PubMed] [Google Scholar]
- 15. World Medical Association . World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16. Hellerstein JL, Diao Y, Parekh S, et al. Feedback Control of Computing Systems. Wiley: IEE Press, 2004. [Google Scholar]
- 17. Dougherty MC, Woodroffe RW, Wilson S, et al. Predictors of reduced opioid use with spinal cord stimulation in patients with chronic opioid use. Neuromodulation 2020;23:126–32. 10.1111/ner.13054 [DOI] [PubMed] [Google Scholar]
- 18. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19. 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 19. Levy RM, Mekhail N, Abd-Elsayed A, et al. Holistic treatment response: an international expert panel definition and criteria for a new paradigm in the assessment of clinical outcomes of spinal cord stimulation. Neuromodulation 2023;26:1015–22. 10.1016/j.neurom.2022.11.011 [DOI] [PubMed] [Google Scholar]
- 20. National Research Council Panel on Handling Missing Data in Clinical T . The Prevention and Treatment of Missing Data in Clinical Trials. Washington (DC): National Academies Press (US), 2010. [PubMed] [Google Scholar]
- 21. North RB, Kidd DH, Farrokhi F, et al. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery 2005;56:98–106; 10.1227/01.neu.0000144839.65524.e0 [DOI] [PubMed] [Google Scholar]
- 22. Kumar K, Taylor RS, Jacques L, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery 2008;63:762–70. 10.1227/01.NEU.0000325731.46702.D9 [DOI] [PubMed] [Google Scholar]
- 23. Turk DC, Dworkin RH, Revicki D, et al. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain 2008;137:276–85. 10.1016/j.pain.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 24. Patel KV, Allen R, Burke L, et al. Evaluation of composite responder outcomes of pain intensity and physical function in neuropathic pain clinical trials: an ACTTION individual patient data analysis. Pain 2018;159:2245–54. 10.1097/j.pain.0000000000001324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eldabe S, Nevitt S, Griffiths S, et al. Does a screening trial for spinal cord stimulation in patients with chronic pain of neuropathic origin have clinical utility (TRIAL-STIM)? 36-month results from a randomized controlled trial. Neurosurgery 2023;92:75–82. 10.1227/neu.0000000000002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kemler MA, de Vet HCW, Barendse GAM, et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg 2008;108:292–8. 10.3171/JNS/2008/108/2/0292 [DOI] [PubMed] [Google Scholar]
- 27. Van Buyten J-P, Wille F, Smet I, et al. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation 2017;20:642–9. 10.1111/ner.12642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hagedorn JM, Lam CM, D’Souza RS, et al. Explantation of 10 kHz spinal cord stimulation devices: a retrospective review of 744 patients followed for at least 12 months. Neuromodulation 2021;24:499–506. 10.1111/ner.13359 [DOI] [PubMed] [Google Scholar]
- 29. Pope JE, Deer TR, Falowski S, et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation 2017;20:543–52. 10.1111/ner.12634 [DOI] [PubMed] [Google Scholar]
- 30. Dupré DA, Tomycz N, Whiting D, et al. Spinal cord stimulator explantation: motives for removal of surgically placed paddle systems. Pain Pract 2018;18:500–4. 10.1111/papr.12639 [DOI] [PubMed] [Google Scholar]
- 31. Wang VC, Bounkousohn V, Fields K, et al. Explantation rates of high frequency spinal cord stimulation in two outpatient clinics. Neuromodulation 2021;24:507–11. 10.1111/ner.13280 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rapm-2023-104751supp001.pdf (220.5KB, pdf)
Data Availability Statement
Data are available on reasonable request. Saluda Medical is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), provided the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided after review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit https://www.saludamedical.com/us/contact-us/.