Abstract
Background
Coronary arteriovenous fistulas present an abnormal connection between the coronary arteries and an adjacent systemic or pulmonary vessel. They are rare, representing 0.002% of the general population. The majority is congenital but may additionally occur related to trauma or interventional cardiac procedures.
Case summary
We present the case of a 48-year-old male with a history of untreated bacterial endocarditis developing a right coronary/superior vena cava fistula. We detail the imaging findings of this rare phenomenon to arrive at this diagnosis. We describe his clinical course and the interventions considered, including surgical extraction. Unfortunately, this patient left against medical advice before completing recommended treatment.
Discussion
We present the first documentation of a right coronary/superior vena cava fistula secondary to chronic untreated bacterial endocarditis. Clinicians should be aware of this rare complication.
Keywords: Fistula, Coronary vessel anomaly, Cardiac magnetic resonance, Drug abuse, Endocarditis, Case report
Learning points.
Coronary artery fistulae are rare abnormalities, the majority of which is congenital. However, clinicians should be aware that they may additionally present as a secondary complication of endocarditis.
Clinicians should consider using electrocardiogram-gated venous-phase computed tomography angiographies to obtain better resolution for coronary artery fistulas as they allow for more precise visualization of its communication.
Introduction
Coronary artery fistulas vary widely in their appearance and presentation. They are rare, affecting 0.002% of the general population.1 While most are congenital, acquired fistulas may occur and be organized into disease-related, traumatic, or iatrogenic aetiologies.1,2 Sites of insertion in these acquired cases vary, with published instances of communication between atria, ventricles, pulmonary arteries, or pericardium.2
Here, we describe a coronary fistula between the right coronary artery (RCA) and superior vena cava (SVC) secondary to chronic untreated endocarditis. Current literature reflects several cases of congenital fistulas complicated by endocarditis but few endocarditis-associated acquired fistulas.3–5 Our patient was undergoing surgical or percutaneous evaluation for definitive treatment but left against medical advice (AMA). Treatment is recommended for patients at risk for haemodynamic compromise. Clinicians should be aware of this rare complication of endocarditis.
Summary figure
| Date | Events |
|---|---|
| May 2021 (Hospitalization #1) |
Presented with persistent MSSA bacteraemia in the setting of worsening cavitary lesions secondary to septic pulmonary emboli. Completed two weeks of intravenous nafcillin therapy before leaving against medical advice. |
| May 2021 (Hospitalization #1) |
Transoesophageal echocardiogram (TEE) showed a 3.5 × 1.5 cm vegetation with mild pulmonic regurgitation. Patient left hospital before further evaluation and no outpatient follow-up. |
| June 2021 (Hospitalization #2) |
Presented with methamphetamine overdose. |
| June 2021 (Hospitalization #2) |
TEE suggests an uncharacterized coronary artery/atrial fistula, but the patient left the hospital before further evaluation. |
| February 2023 (Hospitalization #3) |
Presented with a fistula between the right coronary artery (RCA) and SVC secondary to chronic untreated, infective endocarditis confirmed via EKG-gated CTA. Treated with intravenous antibiotics and considered for surgical repair. The patient left the hospital prior to further management. |
Case presentation
A 48-year-old Caucasian male with a past medical history of methamphetamine use and chronic alcohol substance use disorder initially presented in May 2021 after being found altered in his home. An initial physical exam showed bradypnoea and normal cardiac auscultation. Work-up demonstrated blood cultures positive for methicillin-sensitive Staphylococcus aureus (MSSA) and several septic pulmonary emboli. A transthoracic echocardiogram showed no valvular vegetations, while a transoesophageal echocardiogram (TEE) showed a 3.5 × 1.5 cm vegetation on the end of the peripherally inserted central catheter (PICC) line that was subsequently removed. Other findings on TEE were mild pulmonic regurgitation with no other valvular anomalies. The patient received two weeks of intravenous nafcillin before leaving AMA without completing the recommended six-week antibiotic course. The patient was readmitted in mid-June 2021 for a methamphetamine overdose with decreased responsiveness and a normal cardiopulmonary exam. On this admission, TEE suggested an uncharacterized coronary artery/atrial fistula but could not be adequately evaluated as the patient left AMA again.
Two years later (without any interval history), in February 2023, he complained of fever, chills, weakness, and pleuritic pain. He was pyrexial, tachycardic, and tachypnoeic. The patient admitted to using methamphetamines intravenously. The general exam showed alertness and cachexia, with no evidence of cyanosis, oedema, or trauma. Initial laboratory data showed a white blood cell count of 16.31 K/mm3 (normal value 3.7–10.5 K/mm3). C-reactive protein was elevated at 4.1 mg/dL (normal value < 0.5 mg/dL), and erythrocyte sedimentation rate returned elevated at 71 mm/h. (normal value 0–15 mm/h). Later in his course, his peripheral blood cultures returned positive for MSSA in 2/2 culture bottles. Computed tomography (CT) of the chest revealed multiple septic emboli, right middle lobe pulmonary embolus, and an enlarged right pulmonary artery secondary to pulmonary artery hypertension (Figure 1). There was also evidence of an unclear abnormal connection emerging from the right atrium (Figure 2).
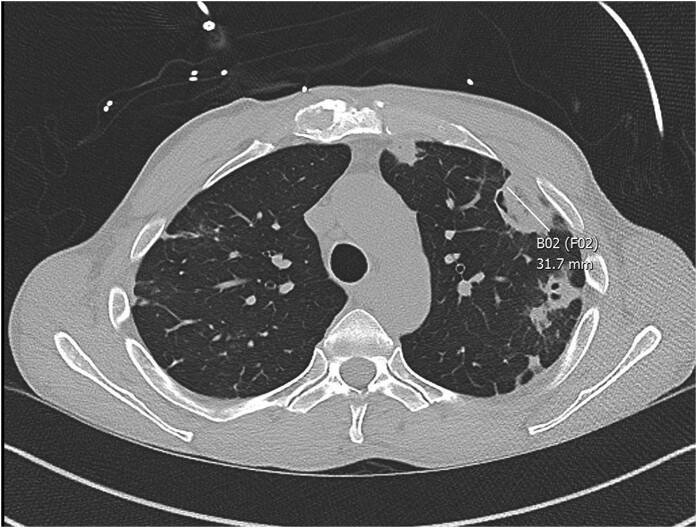
Figure 1.
CT chest, view 1. This image shows evidence of septic emboli (marked B02), measuring 31.7 mm.
Figure 2.
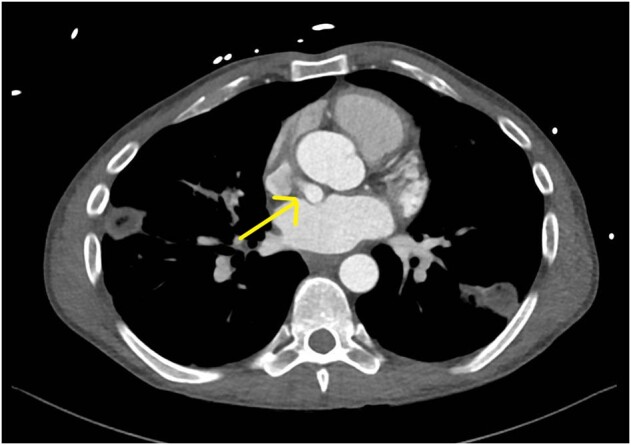
CT chest, view 2. There is evidence of an abnormal connection (arrow) that appears to be emerging from the right atrium.
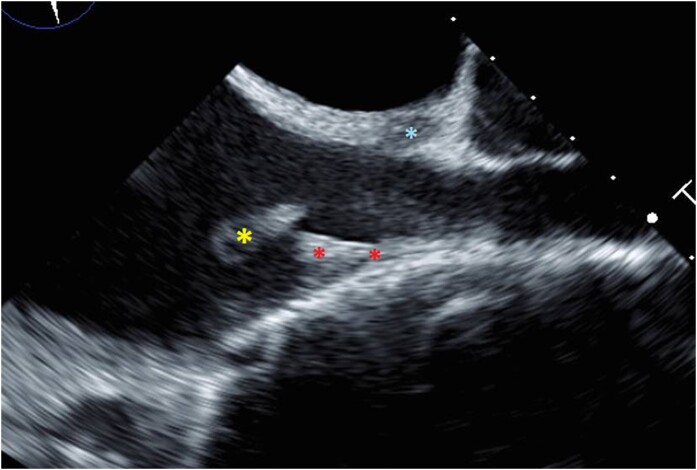
This patient was admitted to internal medicine due to sepsis. Transthoracic echocardiogram showed normal left ventricular systolic and diastolic functions with preserved left ventricular ejection fraction. The estimated pulmonary artery systolic pressure was 44 mmHg, indicating possible pulmonary hypertension. Right atrial and right ventricular size was estimated to be grossly normal. A subsequent TEE showed a mobile echoic density in the right atrium (1.2 × 1 cm) attached via a stalk at the junction of the SVC and right atrium that was consistent with vegetation (Figure 3).
Figure 3.
Vegetation at RA/SVC junction. This still image from the patient’s TEE shows the mobile echoic density (yellow asterisk) at the RA/SVC junction at the edge of the PICC line (red asterisk) suggestive of a vegetation. There is evidence of an abnormal connection (blue asterisk) into the SVC area.
The patient proceeded with a cardiac magnetic resonance imaging (cMRI) for further evaluation. This showed a filling defect in the cavo-atrial junction measuring 1.7 cm without significant perfusion, suggesting a thrombus. A tubular vascular enhancing structure was noted between the left atrium anteriorly and medial wall of the inferior SVC/cavo-atrial junction measuring 0.9 cm, concerning for a fistula (Figure 4, Supplementary material online, Figure S1). An electrocardiogram (EKG)-gated computed tomography angiography (CTA) chest with venous phase acquisition showed communication of the RCA with the SVC (Figure 5, Supplementary material online, Figure S2). The suspicion of a fistula first arose on a TEE two years prior, and the imaging series confirmed and helped to characterize the origin and insertion of the fistula.
Figure 4.
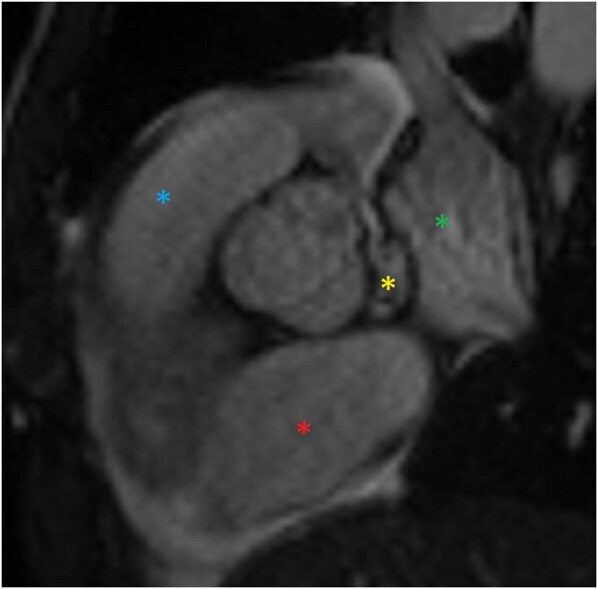
RCA/SVC coronary fistula via cardiac MRI, sagittal. A view showing the left atrium (green asterisk), the right atrium (red asterisk), the right ventricular outflow tract (blue asterisk), and an abnormal connection (yellow asterisk) in between these structures.
Figure 5.
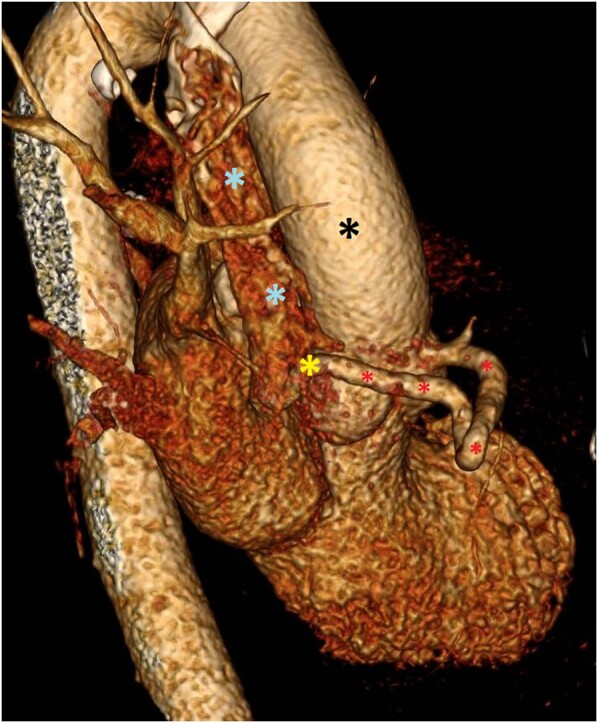
EKG-gated CTA chest, side view. This image depicts a 3D reconstruction from EKG-gated CTA. The aorta (black asterisk) and SVC (blue asterisk) are visible, with an abnormal RCA–SVC connection (yellow asterisk) via a fistula (red asterisk).
It is difficult to rule out a congenital fistula entirely, especially as this patient is presented with no haemodynamic percussions. However, this patient’s clinical course suggests against a pre-existing congenital anomaly. Over 85% of congenital fistula patients present with signs of congestive heart failure with a continuous heart murmur. Moreover, the most common fistula is between the left anterior descending (LAD) to the pulmonary artery.6 As his prior CT scan did not suggest any evidence of an abnormal connection, with repeated imaging later showing an fistula, this suggests that his fistulization developed in the interim. Thus, this patient was subsequently diagnosed with a RCA to SVC fistula.
The patient was treated with intravenous cefazolin at 2 g every 8 h. Cardiothoracic surgery was consulted for percutaneous extraction of the right atrial density with AngioVac (a percutaneous thrombus aspiration system). Per the European Society of Cardiology endocarditis guidelines, surgery is a class IB recommendation for complications of locally advanced infections such as fistulas.7 Discussions were directed towards surgical intervention. However, the patient declined any surgical intervention and left AMA.
The patient was contacted several months after discharge. He declined to follow up with any specialists. Notably, there is limited literature on the prognosis of similar shunts, but the prognosis appears related to the haemodynamic stability of the patient.1 This patient reports that he continues to remain normotensive, suggesting that he is likely to remain stable in the short-term.
Discussion
Coronary artery fistulas are a rare pathology and may be difficult to diagnose. Confirmation of coronary artery fistulas by imaging is traditionally done with an echocardiogram, coronary CT angiogram, or cardiac catheterization. According to Lim et al., non-invasive coronary CTAs detect coronary artery fistulas at a higher rate than invasive cardiac catheterization and provide high-quality spatial resolution.8 Prior literature suggests that cMRI presents higher quality images with better tissue characterization for intracardiac masses, such as abscesses and vegetations, however cMRI and CT are comparable in detecting paravalvular involvement in endocarditis.9
In this case, we utilized EKG-gated venous-phase CTA to obtain the best resolution for this specific fistula given its location. On review, his pathology was likely present on prior CT imaging but could not be adequately evaluated without EKG gating. Treatment choices for these cases include embolization via cardiac catheterization or surgical repair. Embolization is still the preferred treatment option over open surgical repair for haemodynamically unstable fistulas. Indications for repair are haemodynamically significant left to right shunt, signs of congestive heart failure, and myocardial ischaemia.10,11 Though cardiac catheterization can supply real-time information and provide both diagnostic and therapeutic modalities, CTA remains superior at detecting coronary artery fistulas and for the follow-up after successful coronary embolization.11 In the setting of uncontrolled infection, surgery is recommended, while rare cases with contraindications to surgery are suggested to be monitored with close follow-up and imaging.7
This fistula presented incidentally during the work-up, without any overt initial signs. Of note, the presence of the infected PICC line, its proximity to the location of the acquired fistula, and lack of a complete antibiotic course are thought to have contributed to its development. The patient left before further haemodynamic investigation, which limited our evaluation of long-term outcomes.
We describe a rare case of a coronary fistula between the RCA and SVC secondary to chronic untreated, infective endocarditis in an intravenous drug user. This patient’s rare pathology was discovered incidentally on imaging. Clinicians should be vigilant of this pathology, as it is generally overlooked as a complication of endocarditis.
Lead author biography

Michaela Kiel is a second year medical student at the University of Iowa Carver College of Medicine in Iowa City, IA. She obtained her Bachelor of Science in Biology from Dordt University in Sioux Center, Iowa. Upon completion of her bachelor degree, she spent a couple years researching transcriptional regulation of neural crest cells in craniofacial and cardiac development. Her current interests include congenital and acquired cardiac anomalies. She will be graduating from medical school in May 2025.
Supplementary Material
Contributor Information
Michaela D Kiel, University of Iowa Carver College of Medicine, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 52241, USA.
Sruti Prathivadhi-Bhayankaram, Department of Internal Medicine, University of Iowa Carver College of Medicine, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 52241, USA.
Arun K Singhal, Department of Cardiothoracic Surgery, University of Iowa Carver College of Medicine, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 52241, USA.
Mahi L Ashwath, Department of Internal Medicine, University of Iowa Carver College of Medicine, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 52241, USA; Heart and Vascular Center, University of Iowa Carver College of Medicine, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 52241, USA.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Consent: The authors confirm that written consent for submission and publication of this case report, including image(s) and associated text, has been obtained from the patient in line with COPE guidance.
Funding: The authors did not use any funding for the creation of this manuscript.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
References
- 1. Challoumas D, Pericleous A, Dimitrakaki IA, Danelatos C, Dimitrakakis G. Coronary arteriovenous fistulae: a review. Int J Angiol 2014;23:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gowda RM, Vasavada BC, Khan IA. Coronary artery fistulas: clinical and therapeutic considerations. Int J Cardiol 2006;107:7–10. [DOI] [PubMed] [Google Scholar]
- 3. Lee D, Jung M-H, Youn H-J, Choi Y, Byeon JH, Jung HO. Giant right coronary artery with coronary artery fistula complicated by infective endocarditis: multimodality imaging approach. Korean Circ J 2017;47:288–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jariwala U, Hasan RK, Thorn EM, Zakaria S. An unusual case of infective endocarditis involving a right coronary artery to superior vena cava fistula. Catheter Cardiovasc Interv 2015;85:620–624. [DOI] [PubMed] [Google Scholar]
- 5. Pagni S, Austin E, Abraham J. Right coronary artery to superior vena cava fistula presenting with ‘steal’ phenomenon. Interact Cardiovasc Thorac Surg 2005;3:573–574. [DOI] [PubMed] [Google Scholar]
- 6. Albeyoglu S, Aldag M, Ciloglu U, Sargin M, Oz TK, Kutlu H, et al. . Coronary arteriovenous fistulas in adult patients: surgical management and outcomes. Brazilian J Cardiovasc Surg 2017;32:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delgado V, Ajmone Marsan N, de Waha S, Bonaros N, Brida M, Burri H, et al. . 2023 ESC Guidelines for the management of endocarditis: developed by the task force on the management of endocarditis of the European Society of Cardiology (ESC) endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Nuclear Medicine (EANM). Eur Heart J 2023;44:3948–4042.37622656 [Google Scholar]
- 8. Dehaene A, Jacquier A, Falque C, Gorincour G, Gaubert JY. Imaging of acquired coronary diseases: from children to adults. Diagn Interv Imaging 2016;97:571–580. [DOI] [PubMed] [Google Scholar]
- 9. Horgan SJ, Mediratta A, Gillam LD. Cardiovascular imaging in infective endocarditis. Circ Cardiovasc Imaging 2020;13:e008956. [DOI] [PubMed] [Google Scholar]
- 10. Armsby LR, Keane JF, Sherwood MC, Forbess JM, Perry SB, Lock JE. Management of coronary artery fistulae. Patient selection and results of transcatheter closure. J Am Coll Cardiol 2002;39:1026–1032. [DOI] [PubMed] [Google Scholar]
- 11. Sunkara A, Chebrolu LH, Chang SM, Barker C. Coronary artery fistula. Methodist Debakey Cardiovasc J 2017;13:78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.