Abstract
Objective:
Identification of robust biomarkers that predict individualized response to antipsychotic treatment at the early stage of psychotic disorders remains a challenge in precision psychiatry. The aim of this study was to investigate whether any functional connectome-based neural traits could serve as such a biomarker.
Methods:
In a discovery sample, 49 patients with first-episode psychosis received multi-paradigm fMRI scans at baseline and were clinically followed up for 12 weeks under antipsychotic monotherapies. Treatment response was evaluated at the individual level based on the psychosis score of the Brief Psychiatric Rating Scale. Cross-paradigm connectivity and connectome-based predictive modeling were employed to train a predictive model that uses baseline connectomic measures to predict individualized change rates of psychosis scores, with model performance evaluated as the Pearson correlations between the predicted change rates and the observed change rates, based on cross-validation. The model generalizability was further examined in an independent validation sample of 24 patients in a similar design.
Results:
The results revealed a paradigm-independent connectomic trait that significantly predicted individualized treatment outcome in both the discovery sample (predicted-versus-observed r=0.41) and the validation sample (predicted-versus-observed r=0.47, mean squared error=0.019). Features that positively predicted psychosis change rates primarily involved connections related to the cerebellar-cortical circuitry, and features that negatively predicted psychosis change rates were chiefly connections within the cortical cognitive systems.
Conclusions:
This study discovers and validates a connectome-based functional signature as a promising early predictor for individualized response to antipsychotic treatment in first-episode psychosis, thus highlighting the potential clinical value of this biomarker in precision psychiatry.
Treatment response to antipsychotic medication in patients with first-episode psychosis is highly heterogeneous and hard to predict, making the exploration of prognostic predictors critically important for precision psychiatry. Such exploration is particularly challenging during early treatment, when no prior treatment history is available. Since early response to antipsychotics is highly relevant to patients’ long-term clinical and functional outcome (1–3), the identification of predictors at an early stage of illness would help physicians make optimal individualized treatment plans and benefit long-term quality of life for patients.
Antipsychotic medications are essentially dopamine receptor antagonists, which may exert their effects via changes in brain circuitry. Therefore, neurobiological measures evaluating the function of brain circuitry would theoretically be potential candidates for such predictors. In line with this hypothesis, previous studies have shown that baseline brain activity and connectivity during certain functional MRI (fMRI) paradigms may relate to patients’ response status after antipsychotic treatment. Specifically, using resting-state fMRI, our own work has revealed that individualized calculators of striatal-cortical connectivity (4) and cerebellar-cortical connectivity (5) may be potential predictors for treatment outcome in first-episode psychosis. Similar findings have been reported in studies from independent laboratories with different treatment profiles (6–8), suggesting relative robustness of these results. In addition, functional connectivity measures regarding the default mode network areas (9, 10), frontoparietal regions (10), and sensorimotor cortices (11, 12) were also found to be significantly correlated with symptom changes after medication, suggesting their potential clinical value as prognostic biomarkers. Beyond resting-state measures, task-based fMRI has also been demonstrated to be a useful tool for investigating potential predictors in patients. For instance, a recent study used a cognitive task to evaluate the function of performance monitoring in psychotic patients receiving treatment (13). The investigators found that task-related activity in the frontoparietal regions at baseline significantly predicted the degree of symptom reduction after 1-year follow-up, which provides initial evidence for task-based imaging predictors. Nevertheless, most studies to date have used a hypothesis-driven approach that focuses on selected regions of interest with a specific fMRI paradigm. Since dopamine receptors are widely distributed across the whole brain and dopamine functions are widely involved in a variety of human behaviors (14–16), it is unlikely that neural predictors are located in highly circumscribed brain areas that can be detected during only one functional state. However, whether any trait-like functional biomarkers at the whole-brain connectome level would predict treatment outcome with antipsychotics is unknown.
We previously proposed “cross-paradigm connectivity” (CPC) (17) as a valid approach to evaluate “trait” organizations of the individual functional connectomes, which essentially extracts shared components across functional connectivity matrices constructed from different imaging paradigms (for a review, see reference 18). Compared with connectome measures constructed from a single paradigm (or “state”), the CPC matrices quantify individualized connectomic traits independent of brain functional state and show significantly higher test-retest reliability and individual predictability (17). Implementing this approach in psychosis studies, we have successfully identified state-independent biomarkers for the prediction of illness onset among individuals at clinical high risk (19) and state-independent neural mechanisms underlying schizophrenia polygenic risk (20), implying its feasibility and utility in search of predictive biomarkers. Moreover, these past findings also suggest that psychotic disorders are associated with trait-like abnormalities at the connectome level, which encourages the exploration of such signatures for treatment prediction.
Here, by combining CPC and machine learning, we investigated whether any trait-like functional connectomic measures prior to medication would predict individualized treatment response after antipsychotic monotherapies in patients with first-episode psychosis. To this end, two independent clinical samples were collected, where patients underwent multi-paradigm fMRI scans at baseline and were clinically followed up for 12 weeks. In the discovery sample, we trained a predictive model using connectome-based predictive modeling (CPM) (21) and examined the prediction performance with cross-validation. The generalizability of the derived predictive model was further tested in a validation sample. We expected to see a common connectomic trait predictive of treatment outcome across different brain functional states, and the prediction performance of such a trait would show good generalizability to an independent data set.
METHODS
Study Subjects
This study included two independent clinical samples with a similar study design, both recruited from the Zucker Hillside Hospital. The discovery sample comprised 49 patients with first-episode psychosis (30 males; mean age, 24.1 years), and the validation sample consisted of 24 patients with first-episode psychosis (20 males; mean age, 21.9 years); the age range in both samples was 15–40 years. For both samples, patients were diagnosed as having a psychosis spectrum disorder based on the Structured Clinical Interview for DSM-IV (i.e., schizophrenia, schizophreniform disorder, schizoaffective disorder, brief psychotic disorder, psychotic disorder not otherwise specified, bipolar disorder with psychosis features, and major depressive disorder with psychotic features). All patients were in an early phase of illness, as defined by having taken antipsychotic medications for a cumulative lifetime period of less than 2 weeks. Written informed consent was provided by all participants, following protocols approved by the Institutional Review Board of Northwell Health. The demographic and clinical characteristics of the samples are detailed in Table 1 and in the online supplement.
TABLE 1.
Demographic and clinical characteristics of the studied samplesa
| Discovery Sample (N=49) | Validation Sample (N = 24) | |||
|---|---|---|---|---|
|
| ||||
| Characteristic | Mean | SD | Mean | SD |
|
| ||||
| Age (years) | 24.1 | 6.3 | 21.9 | 3.9 |
| Duration of untreated psychosis (months) | 23.3b | 34.1 | 23.2 | 28.7 |
| BPRS-A psychosis score | ||||
| Baseline | 20.1 | 3.5 | 18.3 | 4.5 |
| Week 12 | 8.8c | 5.0 | 7.9d | 4.3 |
| Mean modal antipsychotic dosagee (chlorpromazine equivalents, mg/day) | 283.8 | 184.2 | 278.4 | 85.6 |
|
| ||||
| N | % | N | % | |
|
| ||||
| Sex | ||||
| Male | 30 | 61 | 20 | 83 |
| Female | 19 | 39 | 4 | 17 |
| Diagnosis | ||||
| Schizophrenia | 24 | 49 | 19 | 79 |
| Schizophreniform disorder | 9 | 19 | 4 | 17 |
| Schizoaffective disorder | 2 | 4 | ||
| Brief psychotic disorder | 3 | 6 | ||
| Psychotic disorder NOS | 5 | 10 | 1 | 4 |
| Bipolar disorder with psychosis | 3 | 6 | ||
| Depression with psychosis | 3 | 6 | ||
| Medication | ||||
| Risperidone | 33 | 67 | 12 | 50 |
| Aripiprazole | 16 | 33 | 12 | 50 |
BPRS-A=Brief Psychiatric Rating Scale–Anchored version; NOS=not otherwise specified.
Data available from 28 patients in the sample.
Data available from 36 patients in the sample.
Data available from 18 patients in the sample.
Dosage calculated based on the international consensus study (48).
Patients in both samples underwent baseline clinical assessment (evaluated with the Brief Psychiatric Rating Scale–Anchored version [BPRS-A] [22]) and baseline scans with four fMRI paradigms (of which two paradigms were the same for both samples). The patients were then randomized to receive a single antipsychotic treatment with either risperidone or aripiprazole for 12 weeks. Follow-up visits were performed weekly during the first 4 weeks and biweekly for the next 8 weeks, and the BPRS-A was readministered during each visit. Thirty-six of the 49 participants in the discovery sample and 18 of the 24 patients in the validation sample completed 12-week follow-ups, with the rest having at least one follow-up assessment. As psychosis symptoms are the major target of antipsychotic drugs, our prediction was focused on this symptom domain. Here, psychosis symptoms were calculated as a “psychosis score” that sums five psychosis items in the BPRS-A scale: conceptual disorganization (item 4), grandiosity (item 8), suspiciousness (item 11), hallucinatory behavior (item 12), and unusual thought content (item 15).
Evaluation of Treatment Response
The treatment response for each subject was evaluated as the individualized change rates of psychosis scores over the follow-up period. We treated response as a continuous variable without arbitrarily classifying patients into responder or nonresponder groups, in order to better tailor individualized treatment for precision psychiatry. Intuitively, patients with a larger negative change rate in psychosis scores have a larger degree of symptom reduction over time and thus better treatment response. Similar to the approach in our prior work (5), a linear mixed model was employed in which psychosis scores were included as dependent variable and time points as fixed independent variable, with random slopes and intercepts estimated for each individual. Here, the subject-specific slope coefficients were indicative of individualized mean change rates in psychotic symptoms through the follow-up period and were used for the CPM analysis.
Cross-Paradigm Connectivity
Details on imaging acquisition and preprocessing are provided in the online supplement. The overall processing pipeline is presented in Figure 1. We used the Seitzman atlas (23) to parcellate the whole brain into 300 nodes, which generated 300×300 connectivity matrix for each subject during each fMRI paradigm. These connectivity matrices were further submitted to the CPC analysis, which followed the procedures used in our prior work (17, 19, 20, 24). Here, the four paradigm-specific matrices for each individual were vectorized, mean centered, and then decomposed into a set of principal components (PCs) using singular value decomposition. As shown in our prior work, the first PC scores generated from the analysis captured the vast majority of shared variance across all paradigms in a subject and therefore were reflective of individualized “trait” organizations of the functional connectome. These first PC scores were termed “CPC matrices” and were used for the CPM analysis.
FIGURE 1. Flowchart of the data processing pipelinea.
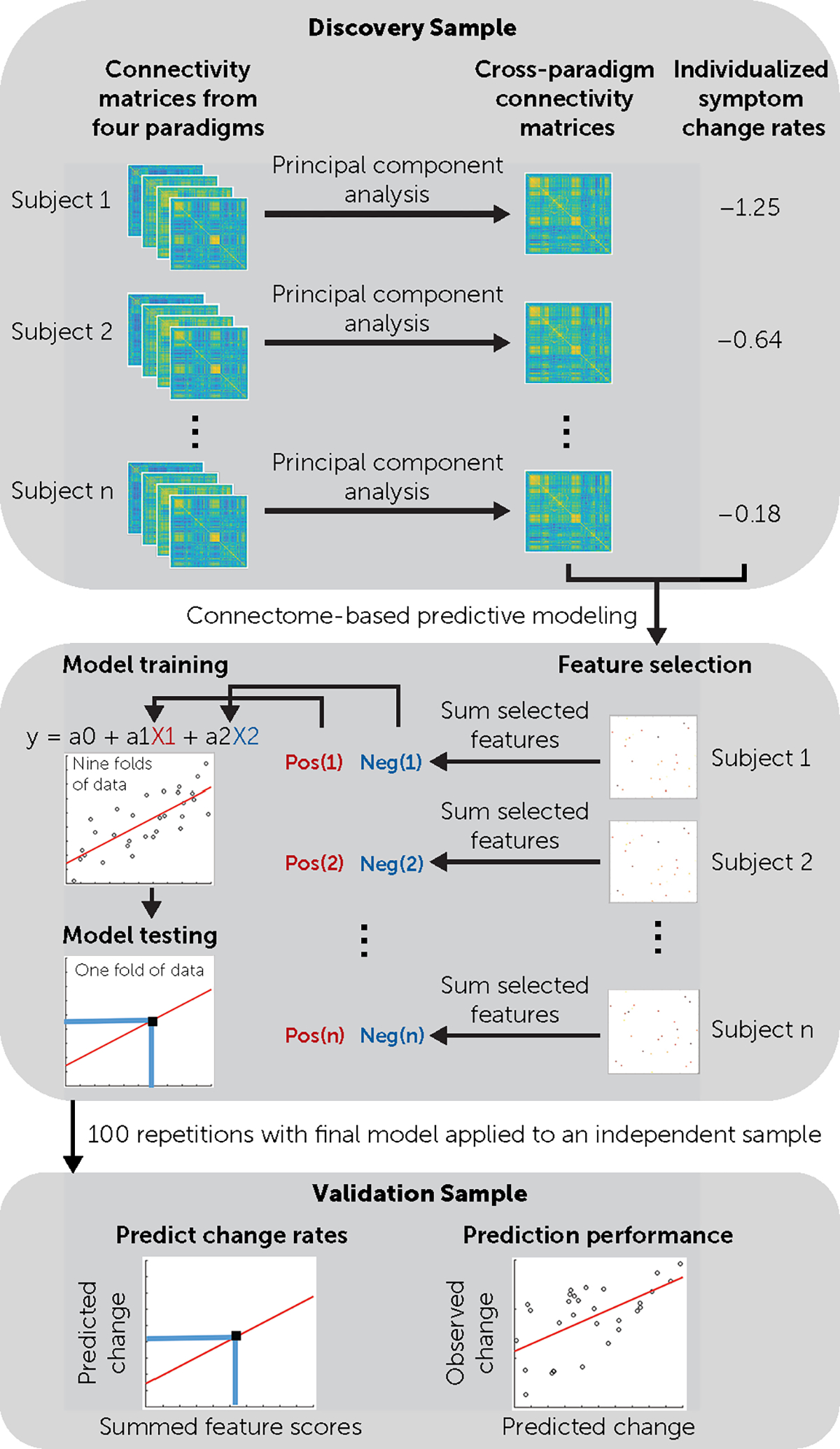
aBy combining cross-paradigm connectivity and connectome-based predictive modeling, the predictive model was trained and tested in the discovery sample. The generalizability of the model was further examined in the validation sample.
Connectome-Based Predictive Modeling
To examine whether the individualized CPC matrices would predict individualized change rates of psychotic symptoms, the CPM was used in the discovery sample to train a supervised machine learning model. On an iterative basis, nine folds of the discovery sample were used as the training set and one fold as the testing set. Within the training set, the value of each connection (N = 300×299/2 = 44,850) in the CPC matrices was first adjusted for age, sex, and framewise displacement and associated by Pearson correlation with the slope coefficients across patients. Connections with significant correlations with slope coefficients at p<0.0005 were subsequently selected as features. These selected features were further summed for each individual to generate a single subject score, separately for positive features (i.e., features positively correlated with slope coefficients) and negative features (i.e., features negatively correlated with slope coefficients). These single subject scores were then entered as predictors into a linear regression model where slope coefficients were included as dependent variable. The fitted model parameters were subsequently applied to the testing set to estimate the predicted slope coefficients. After cross-validation, the prediction performance was calculated as the Pearson correlation coefficient between the predicted and observed slopes across all subjects in the discovery sample. To reduce noise and increase the accuracy of the predictive model, we repeated the entire CPM analysis 100 times, and the performance for the discovery sample was reported as the median of the 100 repetitions. Significance was subsequently determined by 5,000 permutations, with subjects randomly shuffled during each permutation. The p value was calculated as the proportion of correlation coefficients in the permutation distribution that were larger than the observed one.
Validation of Prediction Performance
The generalizability of the predictive model was tested in the validation sample. The final features were determined as the connections that were consistently selected from at least 90% of the 100 CPM repetitions. These connections were further condensed into single subject scores and were used to fit the aforementioned model on the entire discovery sample. The derived model parameters were subsequently applied to the validation sample without modification, thereby generating predicted slope coefficients for each subject in the validation sample using the same features and parameters from the discovery sample. Similarly, the prediction performance for the validation sample was computed as the Pearson correlation coefficient between the predicted and observed slopes, and significance was estimated by 5,000 permutations, with subjects randomly shuffled during each permutation.
RESULTS
Treatment Response in Both Samples
At the group level, the mixed models revealed significant reductions of psychosis scores after treatment (discovery sample: slope = −0.69, p<0.001; validation sample: slope = −0.68, p<0.001) (Figure 2), suggesting that on average the psychosis scores decreased by 0.69 and 0.68 per week in the two patient samples.
FIGURE 2. Trajectories of psychosis scores across treatmenta.
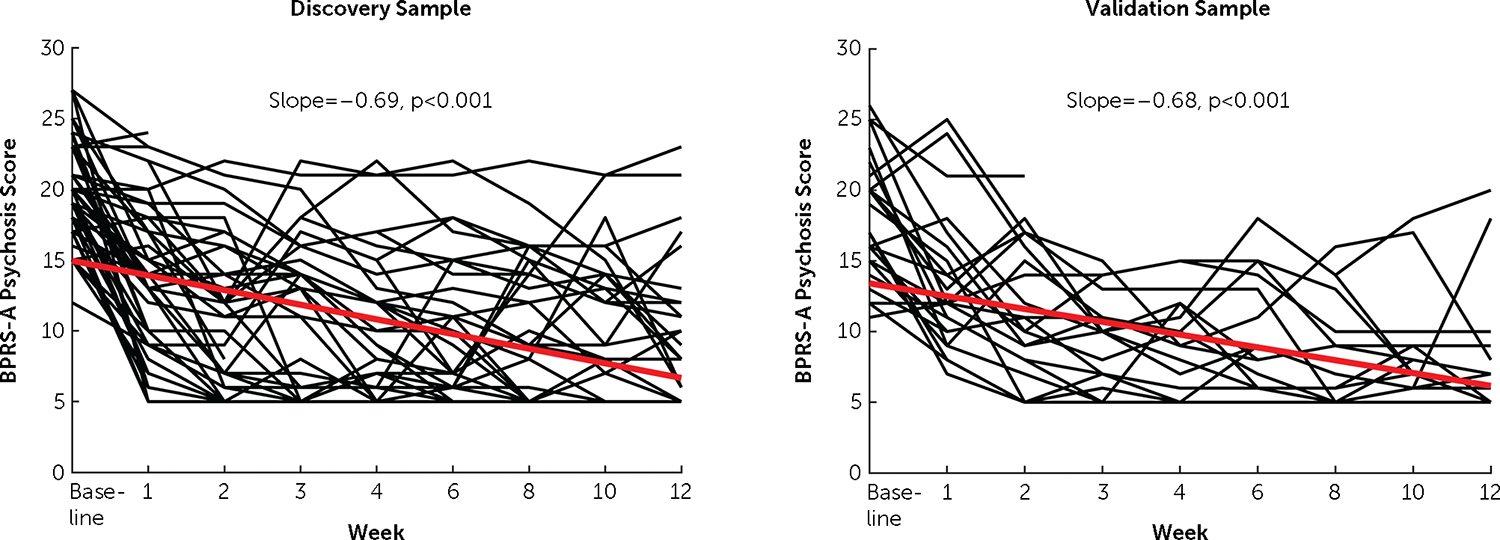
aThe slopes for both samples were highly significant. The black lines and red lines indicate individual trajectories and group trajectories, respectively.
Prediction of Treatment Response
In the discovery sample, the prediction performance for 100 repetitions of CPM analysis ranged between 0.26 and 0.51 (see Figure S1 in the online supplement), with a median predicted-versus-observed performance r of 0.41 (mean squared error = 0.007), which was significant with permutations (p = 0.01) (Figure 3B). Specifically, a total of 14 connections in the CPC matrices were consistently selected from at least 90% of CPM repetitions and thus were considered as final features for the prediction model (five positive features and nine negative features) (Figure 3A; see also Table S1 in the online supplement). Node-wise, the positive features predominantly consisted of connections between the cerebellum and the cerebral cortex. Lower connectivity in these connections at baseline predicted larger negative slope coefficients and thus better treatment response. By contrast, the negative features largely comprised connections between different cerebral cortical areas, where higher connectivity at baseline predicted better treatment response. System-wise, the majority of the positive features mapped to connections between the visual cortex and the higher-order cognitive systems (default mode and frontoparietal networks), while the negative features were chiefly connections within the higher-order cognitive systems (default mode, frontoparietal, cingulo-opercular, salience, and ventral attention networks).
FIGURE 3. The connectome-based features and their prediction performance in the studied samplesa.
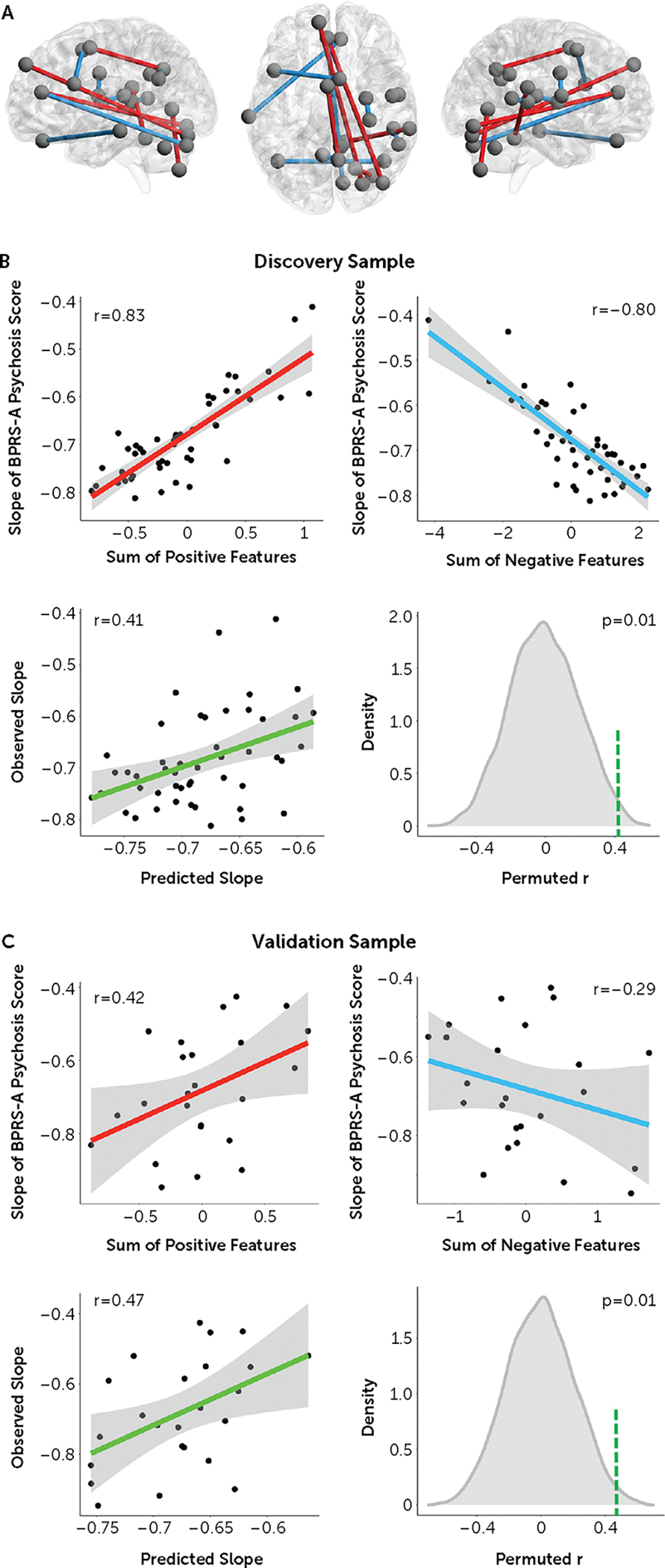
aPanel A shows the final features (five positive and nine negative) selected from the 100 repetitions of cross-validated connectome-based predictive modeling in the discovery sample. The positive and negative features are marked in red and blue, respectively. In panel B, the upper graphs show associations between the selected features and symptom changes in the discovery sample, and the lower graphs show median prediction performance (correlations between the predicted and observed slopes) in the discovery sample, which was significant with permutations. In panel C, the upper graphs show associations between the selected features and symptom changes in the validation sample, and the lower graphs show prediction performance in the validation sample, which remained significant with permutations. The green dashed lines in the histograms in panels B and C indicate the position of the observed slopes.
Specificity of the Identified Features
To test the specificity of the identified features, we deliberately removed these 14 connections from the CPC matrices and reran the entire CPM analysis. We found that the prediction performance significantly dropped after feature removal (median predicted-versus-observed r = 0.28, with no single r above 0.41 from 100 repetitions; see Figure S1 in the online supplement). This indicates high specificity of the observed features in prediction of treatment response.
Validation of Results in an Independent Sample
When the same model was applied, the same 14 features significantly predicted individualized slope coefficients in the independent data set (predicted-versus-observed r = 0.47, mean squared error = 0.019, p = 0.01) (Figure 3C). This indicates good generalizability of our predictive model to clinical studies with a similar design. Additional results to support the validity and robustness of the predictive model are presented in the online supplement.
DISCUSSION
By combining cross-paradigm connectivity and connectome-based predictive modeling in a clinical sample, we identified a functional connectome-based neural signature for the prediction of individualized treatment outcome in patients with first-episode psychosis. This signature consisted of positive predictors predominantly related to the cerebellar-cortical circuitry and negative predictors chiefly within the cognitive systems in the cerebral cortex. We further demonstrated good generalizability of these predictors in an independent data set. Together, these findings highlight a promising trait-like functional biomarker that has potential to be leveraged as a prognostic predictor of treatment outcome in precision psychiatry.
While an increasing number of studies in the literature have aimed to investigate functional imaging predictors for treatment outcome in psychosis (4–12, 25, 26), they have so far focused on a single fMRI paradigm. Therefore, findings reported from past studies may reflect, at least in part, a state-related measure, making it difficult to distinguish whether the discovered biomarkers are related to an individualized trait that is predictive of treatment or a state-dependent change evoked by a specific paradigm that covaries with medication (18). Our study overcame this limitation by using a completely data-driven approach combining multiple fMRI paradigms to identify neural traits most predictive of treatment response across the whole brain connectome regardless of the brain’s functional state. Our findings thus may point to a rudimentary neural mechanism tuning individualized responses to antipsychotic therapies in patients. One notable advantage of such an approach is that the findings would by nature facilitate clinical translation because they are not affected by experiment- or state-related factors and are generalizable between fMRI paradigms and data sets. As a proof of concept, our results indeed showed good generalizability across samples with different scan protocols, imaging paradigms, and preprocessing pipelines, suggesting that the detected predictors are robust and may show relatively high clinical value.
It is noteworthy that treatment prediction in this study was conducted at the individual level, which is different from the majority of studies in the literature dichotomizing patients into responders and nonresponders. Clearly, the degree of individualized response to antipsychotic drugs is a continuous variable, and binarized classification of treatment response is not only arbitrary but also statistically inefficient (27, 28). In addition, such dichotomization completely ignores individual variabilities within each assigned group, as response heterogeneities are still evident among patients, even when they are uniformly assigned to a “responder” or “nonresponder” group. Our research therefore has the advantage of predicting the degree of symptom reduction for each individual. According to the mean squared error in the validation sample, it can be estimated that the mean deviation between the predicted and observed psychosis scores at week 12 is rather small (~1.6) at the individual level, suggesting its potential to assist clinical judgment for individual patients.
The predictors identified here are broadly consistent with the psychosis literature, encompassing nodes in several key sensory processing systems (sensorimotor, auditory, visual) and higher-order cognitive systems (default mode, cingulo-opercular, frontoparietal, salience) that have repeatedly been reported in previous studies (4–12). Notably, two recent review articles suggested that connectivity in the default mode network and sensory networks were the most consistent predictors of treatment response (29, 30). This conclusion generally aligns with the present results, as nodes in these systems were among those most frequently observed (see Table S1 in the online supplement). Features negatively predictive of response were predominantly connections within the cognitive systems, in particular the default mode network, where patients with higher connectivity had better treatment response. Tentatively, since dopamine plays a key role in maintaining the normal function of cognitive processing, which gates the information input and output in order to balance goal-directed signals and background noise (31, 32), stronger connectivity may reflect a result of dopamine dysregulation in the cortical cognitive systems. In this case, stronger connectivity may imply higher dopamine sensitivity (33), rendering those individuals more responsive to antipsychotic treatment. Alternatively, it may also reflect a better cognitive capacity in the brain that facilitates the function of dopamine antagonists in rectification of a psychosis state (34). It should be noted that the identified nodes may not be sufficient to represent these predefined networks, and therefore more comprehensive research is needed to further illustrate the function of these networks in treatment response. In addition, these nodes may also reflect a spatial shift of the network organization, as previously identified in patients (35), and therefore individual variation in the topology of these networks needs to be further studied.
Intriguingly, the positive predictors are predominantly connections between the cerebellum and the cerebral cortex, where lower connectivity at baseline predicts better response to treatment. This is remarkably consistent with our previous findings that increased connectivity in the cerebellar-cortical circuitry is a trait abnormality for psychotic disorders (19, 24). Such change can be robustly detected across different brain functional states and disease stages (5, 19, 24, 36) and is predictive of illness onset in individuals at clinical high risk (19). Moreover, higher connectivity in the cerebellar-cortical circuitry also significantly predicts worse clinical outcome after 2 years of continuous antipsychotic medication (5). These lines of evidence converge to show that cerebellar-cortical hyperconnectivity is a highly robust pathological finding in psychosis, which has potential to be clinically utilized as a predictor of illness development and prognosis. While speculative, such abnormality has been suggested as a downstream effect of N-methyl-d-aspartate (NMDA) receptor hypofunction (37, 38), which leads to the overactivity of pyramidal glutamatergic neurons and in turn disrupts the error processing signals conveyed between the cerebral cortex and the cerebellum (39), thereby generating a series of aberrant thoughts and behaviors collectively theorized as “cognitive dysmetria” (40, 41). Since the function of dopamine neurons is downstream modulated by glutamate signaling (42–44), it is plausible that such a trait would also affect antipsychotic treatment outcome in patients.
Beyond the glutamate hypothesis, it has increasingly been recognized that the bidirectional interactions between the cerebellum and the midbrain is a key and indispensable regulator of the brain’s dopaminergic system. In particular, it has been shown that the direct projection from the cerebellum to the midbrain powerfully modulates the firing rate of midbrain dopaminergic neurons and serves as a required mechanism for activating the reward circuitry and further contributing to social and cognitive behaviors (45, 46). In addition, enriched dopamine D2 receptors on the cerebellar Purkinje cells directly receive dopaminergic input from the midbrain, whose dysfunction has been shown to be sufficient to alter social functioning (46, 47). As a result, cerebellar connectivity may be a pivotal factor in regulating dopamine function and in turn the antipsychotic effect on the brain. It remains to be determined, however, whether the functional modulation of cerebellar-cortical circuitry would boost individualized capacity for antipsychotic response.
We note that this study has some limitations. First, our study design did not include a placebo group, and therefore it is difficult to determine whether the detected predictors would relate to the placebo effect. This limitation may be common to psychosis medication studies in the literature, given that the inclusion of a placebo group is ethically difficult. Second, while we observed and validated the findings in two independent clinical samples, both samples were relatively small. Therefore, the generalizability of the results still merits further investigation in larger cohorts. For the same reason, we did not train predictive models for each drug (risperidone and aripiprazole) separately, and the reported findings may reflect a mixed effect of both drugs. However, in an exploratory analysis shown in the online supplement, we found that the predictive model applied to both drugs, suggesting potentially similar effects underlying the studied drugs. Third, our treatment response measure was primarily based on positive symptoms, which is the major target of antipsychotic drugs. Outcomes of other symptom domains, such as negative symptoms and social functioning, are also clinically important and should be investigated in the future. Fourth, while collecting multiple task–based fMRI data is demanding in clinical populations, further research is certainly warranted to optimize data acquisition strategy for better clinical translation. One possible solution, based on the state-independent nature of our findings, is to implement a similar approach in paradigms with much less cognitive demand, such as naturalistic stimuli. Finally, because of small sample sizes, we did not calculate slopes separately for the training and testing samples in the discovery data set, and therefore the estimated slopes may not be independent due to the partial pooling effect in the linear mixed model. However, since our findings were validated in an independent data set in which the slopes were calculated separately, the prediction performance is unlikely to be driven simply by data leakage.
In summary, using multi-paradigm fMRI data from two clinical samples, we discovered and validated a trait-like connectomic signature as individualized predictors of antipsychotic response in first-episode psychosis. The identified predictors highlight the potential roles of the cerebellar-cortical circuitry and cortical cognitive systems in prediction of psychosis outcome. Additional studies are encouraged to further test the translational value of this neural signature in clinical settings.
Supplementary Material
Acknowledgments
This study was supported by NIMH grants P50MH080173 and R01MH108654 to Dr. Malhotra and grant R01MH060004 to Dr. Robinson.
Dr. Cao acknowledges funding support from the Feinstein Institutes for Medical Research and Zucker Hillside Hospital.
Footnotes
Dr. Gallego has served as a speaker for Tecnoquimicas. Dr. Rubio has served as a consultant for Janssen, Karuna, and TEVA, has received research funding from Alkermes, and has received royalties from UpToDate. Dr. Birnbaum has served as a consultant for Northshore Therapeutics and HearMe. Dr. Robinson has served as a consultant for Acadia, Advocates for Human Potential, Amalyx, APA, C4 Innovations, Costello Medical Consulting, Health Analytics, Innovative Science Solutions, Janssen, Lundbeck, Neurocrine, Neuronix, Otsuka, Teva, and US WorldMeds and has received grant support from Otsuka. Dr. Malhotra has served as a consultant for Acadia Pharma, Clarivate, Genomind, Health Advances, InformedDNA, Iqvia, and Janssen Pharma. The other authors report no financial relationships with commercial interests.
REFERENCES
- 1.Fusar-Poli P, McGorry PD, Kane JM: Improving outcomes of first-episode psychosis: an overview. World Psychiatry 2017; 16:251–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emsley R, Chiliza B, Schoeman R: Predictors of long-term outcome in schizophrenia. Curr Opin Psychiatry 2008; 21:173–177 [DOI] [PubMed] [Google Scholar]
- 3.Peralta V, García de Jalón E, Moreno-Izco L, et al. : Long-term outcomes of first-admission psychosis: a naturalistic 21-year follow-up study of symptomatic, functional, and personal recovery and their baseline predictors. Schizophr Bull 2022; 48:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarpal DK, Argyelan M, Robinson DG, et al. : Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am J Psychiatry 2016; 173:69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao H, Wei X, Hu N, et al. : Cerebello-thalamo-cortical hyperconnectivity classifies patients and predicts long-term treatment outcome in first-episode schizophrenia. Schizophr Bull 2022; 48:505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li A, Zalesky A, Yue W, et al. : A neuroimaging biomarker for striatal dysfunction in schizophrenia. Nat Med 2020; 26:558–565 [DOI] [PubMed] [Google Scholar]
- 7.Li H, Guo W, Liu F, et al. : Enhanced baseline activity in the left ventromedial putamen predicts individual treatment response in drug-naive, first-episode schizophrenia: results from two independent study samples. EBioMedicine 2019; 46:248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNabb CB, Tait RJ, McIlwain ME, et al. : Functional network dysconnectivity as a biomarker of treatment resistance in schizophrenia. Schizophr Res 2018; 195:160–167 [DOI] [PubMed] [Google Scholar]
- 9.Li H, Ou Y, Liu F, et al. : Reduced connectivity in anterior cingulate cortex as an early predictor for treatment response in drug-naive, first-episode schizophrenia: a global-brain functional connectivity analysis. Schizophr Res 2020; 215:337–343 [DOI] [PubMed] [Google Scholar]
- 10.Doucet GE, Moser DA, Luber MJ, et al. : Baseline brain structural and functional predictors of clinical outcome in the early course of schizophrenia. Mol Psychiatry 2020; 25:863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Xu L, Hu Y, et al. : Functional connectivity between sensory-motor subnetworks reflects the duration of untreated psychosis and predicts treatment outcome of first-episode drug-naïve schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging 2019; 4:697–705 [DOI] [PubMed] [Google Scholar]
- 12.Cao B, Cho RY, Chen D, et al. : Treatment response prediction and individualized identification of first-episode drug-naïve schizophrenia using brain functional connectivity. Mol Psychiatry 2020; 25:906–913 [DOI] [PubMed] [Google Scholar]
- 13.Smucny J, Lesh TA, Carter CS: Baseline frontoparietal task-related BOLD activity as a predictor of improvement in clinical symptoms at 1-year follow-up in recent-onset psychosis. Am J Psychiatry 2019; 176:839–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Björklund A, Dunnett SB: Dopamine neuron systems in the brain: an update. Trends Neurosci 2007; 30:194–202 [DOI] [PubMed] [Google Scholar]
- 15.Iversen SD, Iversen LL: Dopamine: 50 years in perspective. Trends Neurosci 2007; 30:188–193 [DOI] [PubMed] [Google Scholar]
- 16.Montague PR, Hyman SE, Cohen JD: Computational roles for dopamine in behavioural control. Nature 2004; 431:760–767 [DOI] [PubMed] [Google Scholar]
- 17.Cao H, Chen OY, McEwen SC, et al. : Cross-paradigm connectivity: reliability, stability, and utility. Brain Imaging Behav 2021; 15:614–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao H: Towards the understanding of state-independent neural traits underlying psychiatric disorders. Neurosci Biobehav Rev 2022; 133:104515. [DOI] [PubMed] [Google Scholar]
- 19.Cao H, Chén OY, Chung Y, et al. : Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun 2018; 9:3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao H, Zhou H, Cannon TD: Functional connectome-wide associations of schizophrenia polygenic risk. Mol Psychiatry 2021; 26:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen X, Finn ES, Scheinost D, et al. : Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc 2017; 12:506–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woerner MG, Mannuzza S, Kane JM: Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull 1988; 24:112–117 [PubMed] [Google Scholar]
- 23.Seitzman BA, Gratton C, Marek S, et al. : A set of functionally-defined brain regions with improved representation of the subcortex and cerebellum. Neuroimage 2020; 206:116290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao H, Ingvar M, Hultman CM, et al. : Evidence for cerebello-thalamo-cortical hyperconnectivity as a heritable trait for schizophrenia. Transl Psychiatry 2019; 9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraguljac NV, White DM, Hadley N, et al. : Aberrant hippocampal connectivity in unmedicated patients with schizophrenia and effects of antipsychotic medication: a longitudinal resting state functional MRI study. Schizophr Bull 2016; 42:1046–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadley JA, Nenert R, Kraguljac NV, et al. : Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology 2014; 39:1020–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Royston P, Altman DG, Sauerbrei W: Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 2006; 25:127–141 [DOI] [PubMed] [Google Scholar]
- 28.Altman DG, Royston P: The cost of dichotomising continuous variables. BMJ 2006; 332:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta UM, Ibrahim FA, Sharma MS, et al. : Resting-state functional connectivity predictors of treatment response in schizophrenia: a systematic review and meta-analysis. Schizophr Res 2021; 237:153–165 [DOI] [PubMed] [Google Scholar]
- 30.Chan NK, Kim J, Shah P, et al. : Resting-state functional connectivity in treatment response and resistance in schizophrenia: a systematic review. Schizophr Res 2019; 211:10–20 [DOI] [PubMed] [Google Scholar]
- 31.Braver TS, Cohen JD: Dopamine, cognitive control, and schizophrenia: the gating model. Prog Brain Res 1999; 121:327–349 [DOI] [PubMed] [Google Scholar]
- 32.Ott T, Nieder A: Dopamine and cognitive control in prefrontal cortex. Trends Cogn Sci 2019; 23:213–234 [DOI] [PubMed] [Google Scholar]
- 33.Cole DM, Beckmann CF, Oei NY, et al. : Differential and distributed effects of dopamine neuromodulations on resting-state network connectivity. Neuroimage 2013; 78:59–67 [DOI] [PubMed] [Google Scholar]
- 34.Garety PA, Kuipers E, Fowler D, et al. : A cognitive model of the positive symptoms of psychosis. Psychol Med 2001; 31:189–195 [DOI] [PubMed] [Google Scholar]
- 35.Nawaz U, Lee I, Beermann A, et al. : Individual variation in functional brain network topography is linked to schizophrenia symptomatology. Schizophr Bull 2021; 47:180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao H, Wei X, Zhang W, et al. : Cerebellar functional dysconnectivity in drug-naïve patients with first-episode schizophrenia. Schizophr Bull 2023; 49:417–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen SM, Tsien RW, Goff DC, et al. : The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res 2015; 167:98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Burgos G, Lewis DA: NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull 2012; 38:950–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao H: Prefrontal-cerebellar dynamics during post-success and post-error cognitive controls in major psychiatric disorders. Psychol Med 2022:1–8 [DOI] [PubMed] [Google Scholar]
- 40.Andreasen NC, Paradiso S, O’Leary DS: “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 1998; 24:203–218 [DOI] [PubMed] [Google Scholar]
- 41.Cao H, Cannon TD: Cerebellar dysfunction and schizophrenia: from “cognitive dysmetria” to a potential therapeutic target. Am J Psychiatry 2019; 176:498–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone JM, Morrison PD, Pilowsky LS: Glutamate and dopamine dysregulation in schizophrenia: a synthesis and selective review. J Psychopharmacol 2007; 21:440–452 [DOI] [PubMed] [Google Scholar]
- 43.Duncan GE, Sheitman BB, Lieberman JA: An integrated view of pathophysiological models of schizophrenia. Brain Res Brain Res Rev 1999; 29:250–264 [DOI] [PubMed] [Google Scholar]
- 44.Goff DC, Coyle JT: The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 2001; 158:1367–1377 [DOI] [PubMed] [Google Scholar]
- 45.Carta I, Chen CH, Schott AL, et al. : Cerebellar modulation of the reward circuitry and social behavior. Science 2019; 363:eaav0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flace P, Livrea P, Basile GA, et al. : The cerebellar dopaminergic system. Front Syst Neurosci 2021; 15:650614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cutando L, Puighermanal E, Castell L, et al. : Cerebellar dopamine D2 receptors regulate social behaviors. Nat Neurosci 2022; 25:900–911 [DOI] [PubMed] [Google Scholar]
- 48.Gardner DM, Murphy AL, O’Donnell H, et al. : International consensus study of antipsychotic dosing. Am J Psychiatry 2010; 167:686–693 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


