Abstract
Pancreatic ductal adenocarcinoma is prone to distant metastasis and is expected to become the second leading cause of cancer-related death. In an extremely nutrient-deficient and hypoxic environment resulting from uncontrolled growth, vascular disturbances and desmoplastic reactions, pancreatic cancer cells utilize “metabolic reprogramming” to satisfy their energy demand and support malignant behaviors such as metastasis. Notably, pancreatic cancer cells show extensive enhancement of glycolysis, including glycolytic enzyme overexpression and increased lactate production, and this is caused by mitochondrial dysfunction, cancer driver genes, specific transcription factors, a hypoxic tumor microenvironment and stromal cells, such as cancer-associated fibroblasts and tumor-associated macrophages. The metabolic switch from oxidative phosphorylation to glycolysis in pancreatic cancer cells regulates the invasion–metastasis cascade by promoting epithelial–mesenchymal transition, tumor angiogenesis and the metastatic colonization of distant organs. In addition to aerobic glycolysis, oxidative phosphorylation also plays a critical role in pancreatic cancer metastasis in ways that remain unclear. In this review, we expound on the intracellular and extracellular causes of the enhancement of glycolysis in pancreatic cancer and the strong association between glycolysis and cancer metastasis, which we expect will yield new therapeutic approaches targeting cancer metabolism.
Keywords: Warburg effect, Mitochondrial respiration, Tumor microenvironment, Epithelial–mesenchymal transition, Metastatic niche, Hybrid metabolic phenotype
Introduction
Pancreatic ductal adenocarcinoma (PDAC) accounts for more than 85% of all malignant pancreatic exocrine tumors [1]. PDAC accounts for approximately 3% of all new cancer cases, and it is the fourth leading cause of cancer-related death in both males and females in the U.S. [2]. Over the past several decades, the survival rate for PDAC has shown poor improvement, in contrast to the steady improvement in the survival rate for most types of cancer [2]. Moreover, PDAC is expected to be the second most common cause of cancer-related death by 2030 in the U.S. [3]. The lack of clinically informative early diagnostic symptoms and biomarkers leads to less than 20% of patients being diagnosed at a stage amenable to resection [4]. Most patients with PDAC are diagnosed with distant metastasis. Although patients with resectable tumors have longer survival than patients with unresectable tumors, distant metastases still occur among a great majority [5].
Malignant cells proliferate indefinitely and are prone to distant metastasis, which requires both sufficient energy and biosynthetic precursors to fuel cell division, invasion and migration. However, hypovascularization in PDAC reduces the delivery of nutrients for biosynthesis into cancer cells and leads to an energy crisis. Nevertheless, tumor cells have a robust ability to survive in harsh environments by changing their energy metabolism, which is known as “metabolic reprogramming”; the most common example of this is enhanced glycolysis, which is characterized by increase in glucose uptake and the production of lactate and was initially described as the “Warburg Effect” [6]. This metabolic reprogramming is of great significance for the abnormal survival and growth of cancer cells, as it provides energy, macromolecular precursors and reducing equivalents [7]. Otto Warburg discovered the enhancement of glycolysis under normoxic conditions and hypothesized that respiratory injury led to aerobic glycolysis, which may be the origin of cancer. However, the enhancement of glycolysis can be induced by hypoxia or hypoxia-induced factor 1 (HIF-1), even in the presence of fully functioning mitochondria, and is present in different heterogeneous cancer cells depending on oxidation phosphorylation or glycolysis and in cancer-associated fibroblasts (CAFs) that also launch metabolic reprogramming to support cancer cell growth and metastatic dissemination [8, 9]. In fact, the coexistence of increased glycolysis and oxidative phosphorylation (OXPHOS) is observed in some cancer cells, and these metabolic phenotypes can be induced to mutually switch in response to drug or microenvironmental stimulation [10, 11].
Notably, to ensure its survival, PDAC is characterized by a high-glycolysis rate due to hypovascularization and the desmoplastic reaction, which create a nutrient-poor and highly hypoxic microenvironment [12, 13]. Glycolysis in PDAC supports the vigorous growth of tumor cells by generating large amounts of substrates and promoting invasion and migration via the interaction of glycolytic enzymes and actin, which most likely increases the supply of ATP more easily. Furthermore, important enzymes and intermediates of glycolysis can regulate PDAC metastasis through participating in signaling transduction or epigenetic regulation related to epithelial–mesenchymal transition (EMT), angiogenesis and colonization [14–17]. This review aims to provide an overview of the metabolic plasticity of PDAC, the causes of enhanced glycolysis, and the mechanisms underlying the regulation by glycolysis of the invasion–metastasis cascade and to discuss the potential use of therapeutic strategies to target cancer metabolism. In addition, we also discuss the unclear role of OXPHOS in pancreatic cancer metastasis.
Promotion of pancreatic cancer by the high-glycolysis phenotype
Mitochondrial dysfunction promotes glycolysis
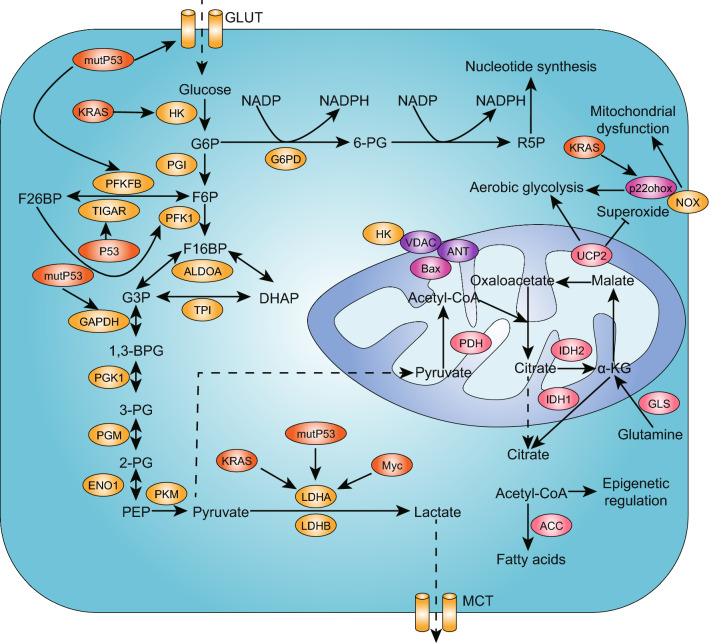
Decades ago, Otto Warburg theorized that cancer originates when a nonneoplastic cell adopts anaerobic metabolism as a means of survival after injury to its respiratory system [18, 19]. Warburg believed that irreversible oxidative phosphorylation (OXPHOS) injury or insufficiency led to aerobic glycolysis. Many studies have discovered abnormalities in mitochondrial DNA (mtDNA), proteomics, lipidomics and structures that compromise the ability of OXPHOS to provide enough energy, and they also revealed that glucose transporters and glycolytic enzymes were apparently upregulated (Fig. 1).
Fig. 1.
Warburg effect in PDAC. The “Warburg effect” exists in the majority of tumors with enhanced glycolysis and lactate production under aerobic conditions, which was initially considered to be the result of mitochondrial dysfunction. PDAC cells present a high-glycolysis phenotype in which glucose uptake is increased and the glycolysis rate is accelerated, which is regulated by Kras, mutp53 and c-Myc, as a result of respiratory injury and the overexpression of glycolytic enzymes, which are marked in the yellow ellipses in this figure. The enhancement of glycolysis shunts more glucose into the pentose phosphate pathway and results in the accumulation of lactate in the microenvironment
Mitochondrial-to-nucleus retrograde signaling regulates tumor properties and can be triggered by altered mitochondrial functioning resulting from mtDNA mutations, mtDNA copy number alterations, mitochondrial respiratory chain complex defects and reactive oxygen species (ROS) production [20]. A reduction in mtDNA copy numbers causes a membrane potential (ΔΨm) disruption that simulates Ca2+/calcineurin signaling, activating a number of oncogenic factors and kinases such as IGF-1R, NF-kB, PI3 K and AKT that are associated with the upregulation of glycolytic enzymes [21]. Due to changes in mitochondria-generated ROS levels, mtDNA mutations could be induced and affect tumor cell metastasis [22]. Notably, an early study that sequenced the complete mtDNA genome in pancreatic cancer cells discovered a large increase in the intracellular mass of mtDNA mutations compared to that in normal cells [23]. Furthermore, 24 somatic mtDNA mutations and 18 somatic nuclear DNA mutations encoding mitochondrial and metabolic genes were identified, and the changes in the metabolic phenotypes of patient-derived pancreatic cancer cell lines (PDLCs) were correlated with mitochondrial dysfunction, including decreased oxygen consumption and an increased glycolysis rate [24]. In recent years, the role of mitochondrial uncoupling protein 2 (UCP2) in suppressing OXPHOS has been discovered, and this promotes aerobic glycolysis and facilitates the malignant progression of PDAC [25]. Moreover, it was found that NADPH oxidase (NOX), a specific enzyme regulating ROS production, was important for maintaining high glycolytic activity in PDAC cells with mitochondrial respiratory dysfunction. The suppression of NOX expression in PANC-1 cells decreased glucose uptake and lactate generation, which inhibited tumor growth in vivo [26].
Induction of PDAC by driver genes
Soon after the publication of Warburg’s discovery, other cancer researchers criticized his hypothesis, because Warburg’s theory did not address the role of tumor-associated mutations and the phenomenon of metastasis. It was hoped that the debate regarding respiratory impairment could be settled to move on to the field of molecular genetic changes in cancer cells in the later part of the twentieth century. However, by the twenty-first century, this issue emerged as a focal point once again. Robert A. Weinberg highlighted the essential role of genome instability in the hallmarks of cancer and acknowledged that metabolic reprogramming was an emerging hallmark associated with activated oncogenes [27]. Although aerobic glycolysis apparently impacts cancer cell survival, it is controlled by proteins involved in cellular programs involved in other core hallmarks of cancer. The Warburg effect (enhanced glycolysis) may constitute a certain phenotype driven by genomic alterations. Studies have shown that Kras and Tp53 are the most important driver genes, with mutation rates of 98% and 50%, respectively, that play vital roles in PDAC development by controlling the regulatory processes or pathways involved in the G1/S phase cell cycle transition, transforming growth factor β (TGF-β) signaling, DNA damage control and Kras signaling; however, they have also been revealed to regulate the “Warburg Effect” in recent years [28]. Kras mutation drives the uncontrolled proliferation of PDAC cells through activating downstream signaling pathways such as the MAPK and PI3 K–AKT–mTOR pathways, promotes local invasion and distant metastasis and plays a key role in regulating glucose utilization and anabolic metabolism [29, 30]. Oncogenic Kras promotes glycolysis through the stimulation of glucose uptake and regulates the glucose transporter (GLUT-1) and several rate-limiting glycolytic enzymes, including hexokinase 1 (HK1), HK2, phosphofructokinase 1 (PFK1) and lactate dehydrogenase A (LDHA), without affecting the glycolytic metabolites involved in the three carboxylic acid (TCA) cycles.
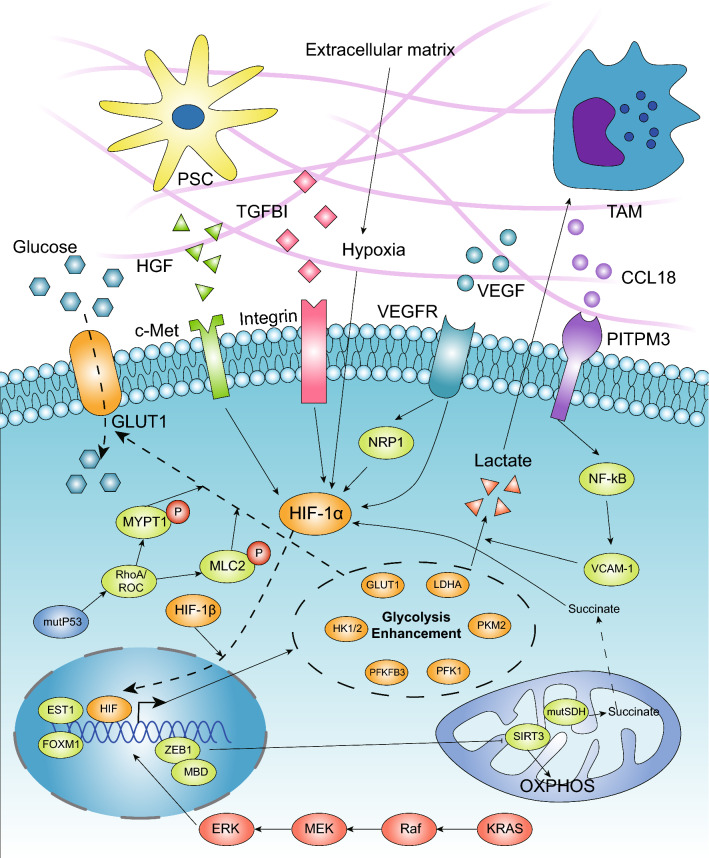
Moreover, hypoxia and HIF-1α also activate glycolytic enzyme expression and coordinate with mutant Kras to maintain cytosolic ATP generation, although the knockdown of HIF-1α has minimal impact on metabolic enzyme expression [30, 31]. In addition, oncogenic Kras activates the hexosamine biosynthetic pathway (HBP) to enhance the generation of the precursor moieties required for protein glycosylation and promote ribose biogenesis by enhancing the activity of the nonoxidative arm of the pentose phosphate pathway (PPP) [30]. Furthermore, KrasG12D mediates this metabolic reprogramming via downstream MAPK signaling pathways and the transcriptional control of c-Myc (Fig. 2). As an oncogenic transcription factor, c-Myc regulates cell growth, differentiation and malignant transformation. It has been demonstrated that c-Myc exerts a crucial effect on the glycolytic phenotype of PDAC and impacts its development [32]. Oncogenic Kras in pancreatic cancer inhibits the tumor suppressor FBW7, which negatively regulates glucose uptake by targeting the c-Myc/TXNIP axis [33]. The overexpression of the kinase IKKε in pancreatic cancer is associated with poor prognosis, and it can promote aerobic glycolysis in PDAC cells by promoting c-Myc accumulation in the nucleus by targeting related genes [34].
Fig. 2.
Driver genes and TME promote the enhancement of glycolysis in PDAC. The high-glycolysis phenotype is considered to be an emerging hallmark associated with activated oncogenes. Mutant Kras can upregulate the expression of glycolytic enzymes via the Raf/MEK/ERK pathway, which is dependent on Myc and functions transcriptionally. GOF mutp53 also promotes glycolysis mainly by regulating GLUT1 translocation to the cytoplasmic membrane. In the pancreatic microenvironment, hypoxia is considered the main regulator that transcriptionally upregulates the expression of multiple glycolytic enzymes. SDH mutations leading to succinate accumulation can promote glycolysis through stabilizing HIF-1α. Additionally, stroma cells, such as TAMs and pancreatic stellate cells, secrete cytokines that interact with membrane receptors in cancer cells, and soluble factors in the TME, such as VEGF and TGFBI, promote glycolysis mainly via the HIF-1α and NF-kB pathways. ZEB1, known as an EMT regulator, can also promote glycolysis via its interaction with MBD1, which transcriptionally suppresses the expression of SIRT3
The tumor suppressor gene Tp53 plays a central role in pancreatic tumor prevention. In addition to the loss of tumor suppression, the gain-of-function version of p53 (GOF mutp53) generated through missense mutation can stimulate cancer cell growth by modulating a set of genes via its interaction with a number of transcription factors [35]. In particular, GOF mutp53 was found to stimulate the Warburg effect by promoting GLUT1 translocation to the plasma membrane that was mediated by the RhoA/ROCK/GLUT1 signaling pathways (Fig. 2) [36]. Through stabilizing the cytosolic localization of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) via regulation by Sirtuin1 (SIRT1) and avoiding its nuclear transport, GOF mutp53 also promotes glycolysis, which enhances the sensitivity of cancer cells to metabolic drugs [35]. For instance, tumors harboring mutant versions of Tp53 exhibit increased apoptosis and reduced proliferation rates when exposed to FX11, a small-molecule inhibitor of LDHA [37]. In cancer cells without Tp53 mutations, when glucose uptake reduces the level of p53, p53 is phosphorylated by AMPK to upregulate Tp53-induced glycolysis via Tp53-induced glycolysis and apoptosis regulator (TIGAR), SCO2 cytochrome c oxidase, guanidinoacetate N-methyltransferase (GAMT), and glutaminase 2 (GLS-2) and to downregulate the expression of phosphoglycerate mutase (PGM) to provide more ATP and reduce the level of ROS [38]. Thus, p53 can block ROS-induced apoptosis in cancer cells under nutrient stress; however, when Tp53 mutations occur, cancer cells exhibit enhanced glycolysis that supports rapid proliferation.
Tp53-induced glycolysis and apoptosis regulator (TIGAR) suppresses glycolysis and can be upregulated by p53, which directly binds to its promoter at the BS2 site [39]. Although TIGAR expression has been found to be upregulated in most human PDAC tissues, it was decreased in Tp53-mutated tumors in a patient-derived tumor xenograft (PDTX) mouse model [37, 40]. In addition, p53 was demonstrated to decrease 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 (PFKFB4) expression, which plays an important role in glycolysis by binding to its promoter and mediating transcriptional repression via histone deacetylases in multiple cancer cell lines. Cancer cells deficient in p53 are highly dependent on the function of PFKFB4 [41]. In addition, p53 can transcriptionally repress paraoxonase 2 (PON2), and mutant p53 in PDAC cells increases PON2 expression, which activates GLUT1-mediated glucose transport and is necessary for anoikis resistance [42].
Regulation of glycolytic enzyme expression and activity
In fact, due to the lack of sufficient evidence of mitochondrial metabolism alteration, enhanced glycolysis is now believed to be the main alteration in metabolism of tumors and a hallmark of PDAC with multiple overexpressed glycolytic enzymes (Table 1) [43]. Within pancreatic cancer cells, there are multiple levels of regulation controlling the expression and activity of glycolytic enzymes to impact the glycolysis rate that involve genome stability, transcriptional regulation and posttranslational modification.
Table 1.
Glycolytic enzymes are overexpressed in various types of cancer
| Glycolytic enzyme | Organs with overexpression | Promote metastasis | References |
|---|---|---|---|
| Hexokinase 2 (HK2) | Colon, brain, tongue, liver, pancreas | Colon, brain, tongue, liver, pancreas | [135, 136] |
| Lactate dehydrogenase A (LDHA) | Lung, ovary, pancreas | Lung, ovary, pancreas | [32, 50, 137–139] |
| Phosphoglucose isomerase/Autocrine motility factor (PGI/AMF) | Breast, pancreas | Skin, brain, breast, fibroblast, pancreas | [15, 140–147] |
| 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) | Lung, bone marrow, colon, nasopharynx, stomach, breast, brain, pancreas | Nasopharynx, stomach, breast, pancreas | [56, 57, 60, 82, 83, 148–150] |
| 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB4) | Stomach, prostate, liver, bladder, breast, pancreas | Prostate, breast | [57, 58, 60, 151–153] |
| TP53-induced glycolysis and apoptosis regulator (TIGAR) [attenuates glycolysis] | Colon, brain, breast, bone marrow, nasopharynx, lung, pancreas | Nasopharynx, lung | [40, 154–160] |
Multiple glycolytic enzymes are highly expressed in malignancies in many organs and metastatic subclones, including PDAC. In addition to the glycolytic enzymes listed above, ENO1, PKM2 and ALDOA are overexpressed in PDAC and also promote metastasis
The alteration of mitochondrial function and the abnormal accumulation of metabolites promote the enhancement of glycolysis through affecting the nuclear genome by activating HIF-dependent pathways and histone modification [20]. The accumulation of succinate due to the mutation of succinate dehydrogenase (SDH) can promote glycolysis via the stabilization of HIF-1α, which promotes the expression of multiple glycolytic enzymes [44]. Intramitochondrial acetyl-CoA and oxaloacetate combine to form citrate, which is transported out of the mitochondria and converted to acetyl-CoA by ATP citrate lyase (ACL). In addition to being utilized for fatty acid synthesis, acetyl-CoA is also an important source of acetyl for histone acetylation, which results in global transcriptional upregulation and S phase progression. The downstream transcriptional targets of ACL-mediated histone acetylation include GLUT4, HK2, PFK1 and LDHA. The regulation of these metabolic enzymes controls aerobic glycolysis in cancer cells [38, 45]. Thus, there must be unknown mechanisms that regulate glycolytic enzymes through epigenetic changes to enhance glycolysis in cancers.
In addition to c-Myc, there are many transcription factors, such as EST1 and FOXM1, that have been known to regulate PDAC invasion and metastasis and can promote glycolysis in pancreatic cancer by binding to the promoters of glycolytic enzyme genes, including HK1, PFKFB3, LDHA and GLUTs [46, 47]. After their expression, the posttranslational modification of glycolytic enzymes can change their enzymatic activity to regulate glycolysis. For example, PFKFB3 can be modified by phosphorylation under energy crisis conditions and as a result of di-methylation and acetylation to enhance cancer cell glycolysis [48, 49]. Li et al. elucidated the mechanism by which cisplatin induced the acetylation of PFKFB3 at lysine 472 (K472) to impair its nuclear localization signal (NLS), thereby causing its accumulation in the cytoplasm; this facilitated the phosphorylation of PFKFB3 by AMPK, leading to PFKFB3 activation and enhanced glycolysis [49]. In addition, LDHA is overexpressed in PDAC cells because of the degradation of lysine 5 (K5)-acetylated LDHA. Acetylation at K5 decreased the LDHA levels to inhibit glycolysis and the migration of PDAC cells [50].
Pancreatic cancer microenvironment
The interaction of stromal, immune, and malignant cells creates a tumor microenvironment (TME) that imposes physical pressure, oxidative stress, nutrient deprivation, competition, hypoxia, and immune surveillance on cancer cells [51]. Notably, PDAC creates a strong desmoplastic reaction and low vascular density, which reduces the deliverability of nutrients and oxygen and results in enhanced glycolysis and lactate deposition [12]. Hypoxia can induce the transcription of various genes involved in anaerobic metabolism, angiogenesis and metastasis, which is considered the main inducer of the glycolytic switch in cancer. A lack of oxygen leads to reduced ATP levels, which reduces the inhibition of glycolytic enzymes [52]. In addition, hypoxia activates HIF-1α, which regulates the expression of many glycolytic enzymes via its binding to hypoxia-response elements, and HIF-2α also responds to hypoxic conditions to promote glycolysis (Fig. 2) [53]. In PDAC cells, hypoxia promotes the expression of pyruvate dehydrogenase kinase (PDK1), LDHA, pyruvate kinase muscle isozyme (PKM2), glucose-6-phosphate isomerase/autocrine motility factor (PGI/AMF) and HK2, which is mediated by HIF-1α [54, 55]. As important enzymes involved in the glycolysis process, PFKFB3 and PFKFB4 are highly expressed in various types of cancer, including pancreatic cancer, when induced by hypoxia or HIF-1α [56–60]. However, the deletion of HIF-1α does not impact the expression of glycolytic enzymes [54]. In tumor tissues from PDAC patients, TIGAR expression was shown to be increased, but hypoxic conditions could not induce its overexpression [40]. Moreover, TIGAR can translocate to the outer mitochondrial membrane, to bind to and activate HK2 during hypoxia or activate HIF-1α, thus regulating glycolysis and cell apoptosis [61].
PDAC stromal cells comprise pancreatic stellate cells, various leukocytes and endothelial cells, such as cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs), in the TME that can be activated to release growth factors and cytokines to impact cancer cell metabolism (Fig. 2). TAMs (M2) highly express CCL18 to interact with PITPNM3 in PDAC cells and activate the NF-kB signaling pathway, which induces the overexpression of VCAM-1 and promotes aerobic glycolysis to increase the secretion of lactate and promote the conversion of M0 macrophages to M2 macrophages; this creates a feedback loop facilitating tumor survival [62]. Pancreatic stellate cells in tumors secrete hepatocyte growth factor (HGF), which activates its cognate receptor c-MET to regulate YAP translocation and HIF-1α stabilization, enhance the expression of HK2 and promote glycolytic metabolism in PDAC cells [63].
The TME comprises malignant cells, stromal cells and extracellular components such as VEGF, transforming growth factor beta-induced (TGFBI) and extracellular matrix (ECM). In addition to promoting angiogenesis, vascular epidermal growth factor (VEGF) stimulation was reported to lead to metabolic transition from mitochondrial OXPHOS to glycolysis in PDAC cells via HIF-1α upregulation [64]. Transforming growth factor beta-induced (TGFBI) is an ECM-interacting protein associated with an invasive phenotype in cancer and is highly expressed in PDAC lesions versus nonneoplastic pancreatic lesions. Secreted TGFBI could bind to integrin and activate the focal adhesion kinase (FAK) signaling pathway, which promotes the stabilization of HIF-1α and results in increased glycolysis [65]. In addition, the presence of bioavailable copper in pancreatic TME has been associated with tumor growth and metabolism. When using copper chelation, cancer cell proliferation was suppressed with the attenuation of OXPHOS and reduced ATP levels, despite the enhancement of glycolysis. Therefore, glycolysis activation in PDAC may reflect in part low copper bioavailability in the TME [66].
It is noteworthy that a subset of aerobic PDAC cells can use the lactate derived from stroma to fuel the TCA cycle for cell proliferation, resulting in less dependence on glucose metabolism. This process was described as “the reverse Warburg effect”, because a glycolytic phenotype in stromal cells such as CAFs supports adjacent cancer cell survival with increased OXPHOS activity and invasive ability [67–69]. Hence, stromal cells that make up the majority of pancreatic tumors can facilitate cancer cell growth and progression by promoting a glycolytic phenotype and supporting mitochondrial metabolism in oxidative PDAC cells. Therefore, targeting the stroma of PDAC seems to be a promising anti-cancer strategy for use in the future.
Glycolysis regulates the PDAC metastasis cascade
The metastasis process in malignant tumor cells consists of several steps, known as the invasion–metastasis cascade, that include invasion, intravasation, extravasation and colonization in distant organs. Regarding the regulation of the metastatic behaviors of PDAC in the cascade involving EMT, angiogenesis and distant colonization, recent studies have predominantly focused on the fields of genome instability, noncoding RNA, exosomes and TME [70–74]. However, an increasing number of studies have reported the association between the Warburg effect and glycolysis and malignant behaviors in PDAC in recent years. The Warburg effect can benefit pancreatic cancer cells by providing energy, macromolecular precursors and reducing equivalents, which promote cancer cell growth and inhibit apoptosis [7, 30]. Notably, overexpressed glycolytic enzymes and enhanced glucose anabolism promote metastasis in PDAC cells (Table 1) [14, 15]. The overexpression of glycolytic enzymes leads to the deposition of lactate in the TME, which can be transported out of PDAC cells through MCTs and connexin-43 to not only serve as an energetic fuel for oxidative PDAC cells but also promote invasion and metastasis as a signaling molecule [69, 75, 76]. Here, we summarize the mechanisms by which glycolysis regulates the metastasis of PDAC cells in three aspects: EMT, angiogenesis and distant colonization. Robert A. Weinberg considered the deposition of cancer cells in the lymphatic system as a surrogate marker revealing the extent of dissemination [77]. Therefore, the discussion of metastasis mainly focuses on the vascular route through which cancer cells reach and survive in the secondary site.
Regulation of EMT
EMT confers to epithelial cells the neoplastic properties of dissemination and the ability to degrade components of the ECM, which is orchestrated by a series EMT-inducing transcription factors (EMT-TFs) [77]. In contrast to Snail and Twist1, which had no effect on PDAC metastasis, Zeb1 was found to have a crucial impact on the formation of lesions and tumor metastasis in a KPC mouse model [78, 79]. EMT programs seem to be triggered by signals, including TGF-β, Wnts and certain interleukins, from nearby tumor-associated stroma. However, the influence of somatic mutations acquired during primary tumor formation and that of the metabolic reprogramming in cancer cells on the activation and expression of EMT programs remains unclear.
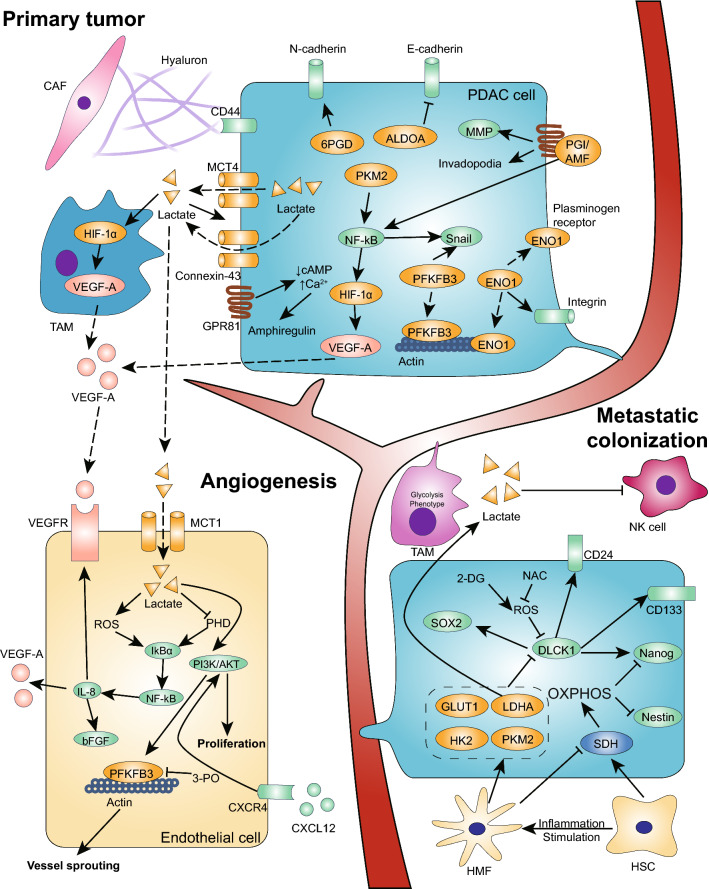
Hypoxic PDAC cells with a high level of glycolysis exhibit a “mesenchymal phenotype” [80]. Glycolytic enzymes activate and maintain EMT programs mainly through regulating the expression of EMT-TFs. The mechanism by which PGI/AMF promotes cancer metastasis is mediated by its binding to gp78, a 78-kDa seven-transmembrane glycoprotein that activates the invasion–metastasis cascade through various intracellular effectors. The overexpression of PGI/AMF leads to the downregulation of E-cadherin expression associated with the upregulation of Snail expression, contributing to the aggressive phenotype of human pancreatic cancer (Fig. 3) [15]. In addition, PGI/AMF can promote the relocalization of RhoA and Rac1, small GTPase proteins regulating the cytoskeleton that are associated with the formation of filopodia and lamellipodia. Moreover, PGI/AMF increases the expression of the integrins a2b3 and a5b1 to regulate cell adhesion, stimulates matrix metalloproteinase (MMP2) activity and promotes MMP3 expression via the MAPK signaling pathway to promote the invasion of malignant tumor cells [81].
Fig. 3.
Enhancement of glycolysis promotes PDAC metastasis. In the invasion–metastasis cascade in PDAC cells, glycolytic enzymes and lactate facilitate the process in three main ways. (1) Glycolysis promotes the EMT program by upregulating EMT-TFs, downregulating E-cadherin, and increasing MMP secretion and cytoskeleton remodeling. PFKFB3, ALDOA, ENO1, PKM2 and PGI/AMF can function in cytoplasmic, nuclear and extracellular forms. (2) Glycolysis promotes angiogenesis by increasing VEGF and amphiregulin secretion via a self-induced pathway and the regulation of stromal cells, which is dependent on the accumulation of lactate in the TME. Additionally, glycolytic enzymes such as PKM2 can be secreted into the blood stream to interact with receptors in the EC membrane to promote EC proliferation and migration. In addition, enhanced glycolysis in ECs also promotes vessel sprouting to facilitate angiogenesis. (3) Glycolysis promotes PDAC cell metastatic colonization. Glycolytic TAMs promote the extravasation of CTCs. In a distant microenvironment, the enhancement of glycolysis in metastatic PDAC cells maintains stemness through low ROS levels. In the hepatic microenvironment, many HSCs promote the elevated expression of SDH and enhance OXPHOS activity, reducing the self-renewal ability of pancreatic cancer metastatic cells via the reduced expression of Nanog and Nestin. In contrast, HMFs promote the downregulation of SDH and activate glycolysis, which promotes cancer cell proliferation and the formation of visible metastases. In addition, the secretion of lactate from PDAC cells reduces NK cell cytotoxicity, which promotes metastatic colonization
TGF-β1 was demonstrated to increase glycolysis by inducing PFKFB3 expression and mediating the invasion of PANC-1 cells via PFKFB3, which could regulate the expression of Snail [82]. In squamous cell carcinoma cells, PFKFB3 could be detected in invadopodia and lamellipodia, although most PFKFB3 was localized in the nuclei of tumor cells [83]. Thus, cytoplasmic PFKFB3 may promote cancer cell mobility through interacting with actin, stimulating protrusion formation and supplying ATP for migration. Upon TGF-β treatment, the expression of aldolase A (ALDOA) was found to increase the most significantly among the glycolysis genes. Furthermore, the high expression of ALDOA and low expression of E-cadherin were found in highly metastatic pancreatic cancer tissue samples, and the overexpression of ALDOA indicated a poorer prognosis for pancreatic cancer patients. In PANC-1 cells, the silencing of ALDOA decreased proliferation and metastasis, increased E-cadherin levels, and decreased the levels of N-cadherin and vimentin [84]. Conversely, lactate metabolism can promote TGF-β2 expression, which induces glioma cell migration by enhancing the production and activation of MMP-2 and b1-integrin [85]. Therefore, TGF-β promotes cancer cell glycolysis, which in turn upregulates its expression, forming a feedback loop. Intriguingly, during pancreatic cancer cell EMT progression, TGFβ-1 treatment was found to induce mitochondrial dysfunction simultaneously [86].
Alpha-enolase (ENO1), a well-known glycolytic enzyme, also acts as a plasminogen receptor on the cell surface, promoting metastatic cancer invasion via interacting cytoskeletal proteins such as F-actin and tubulin. After the knockdown of ENO1 in pancreatic cancer cells, their adhesion to fibronectin and collagen I/IV decreases, and their adhesion to vitronectin increases. Importantly, shENO1 strongly represses the expression of the integrin alpha v/beta 3 complex, which is involved in binding these extracellular matrices to induce cancer cell invasion (Fig. 3) [87]. In addition, cancer cells secrete lactate into the microenvironment, which can induce the secretion of hyaluronan by cancer-associated fibroblasts [88]. Hyaluronan deposition is usually higher in malignant tumors and promotes tumor progression via the interaction of hyaluronan/RHAMM/CD44 [89], which affects adhesion functions, rearranges the cytoskeleton, increases the activity of ECM-degrading enzymes and promotes angiogenesis toward the metastatic lesion. Graviola, also known as the tropical tree Annona muricata, can decrease the expression of GLUT1, HK2 and LDHA by inhibiting the NF-kB/HIF-1α signaling pathway, which is correlated with the attenuation of PDAC cell migration characterized by the disruption of microtubule dynamics and the reduction of MMP9 [90]. This indicates a potential therapeutic approach to inhibit the metastasis of PDAC cells via the targeting of the regulatory system of glycolysis.
The activity of the metastatic pathway can be further increased by epigenetic alterations. For example, in distant metastases in PDAC, Oliver G observed increased glucose uptake, elevated lactate secretion and enhanced PPP activity. The enhanced PPP activity induced broad enrichment of H3 K27ac over that of CDH2/N-cadherin in distant metastatic subclones compared to normal cells or regional PDAC cells. Treatment with 6AN, an inhibitor of 6-phosphogluconate dehydrogenase (6-PGD) with no effects on glutamine metabolism, could reverse this reprogrammed histone modification [14].
In summary, glycolytic enzymes play vital roles in pancreatic cancer EMT and metastasis. However, few studies have reported the impact of EMT regulators, such as ZEB1, on glycolysis, which was found to promote aerobic glycolysis in pancreatic cancer cells by transcriptionally suppressing the expression of SIRT3 via its interaction with methyl-CpG binding domain protein 1 (MBD1) [91]. Thus, the association between EMT and metabolism in PDAC requires more research.
Promotion of angiogenesis
Angiogenesis is new blood vessel formation derived from preexisting endothelial cells [92]. The early steps in the metastatic cascade include invasion and migration into tissues and the circulatory system. Therefore, an “angiogenic switch” is always activated and remains on during tumor progression, causing quiescent vasculature to sprout new vessels and facilitating the expansion of neoplastic growth and cancer cell metastasis. A variety of angiogenic factors contribute to new vessel formation, such as members of the well-known VEGF-A and fibroblast growth factor (FGF) family [27]. Hypoxia can promote angiogenesis by directly inducing VEGF-A overexpression or enhancing glycolysis in cancer cells. PKM2 depletion in pancreatic cancer cells results in impaired tumor growth and angiogenesis in vivo. The hypoxic environment could induce the translocation of PKM2 to the nucleus, and PKM2 regulated HIF-1α-induced VEGF secretion in an NF-kB/p65-dependent manner (Fig. 3) [16]. Additionally, PKM2 can be secreted by cancer cells to promote endothelial cell (EC) proliferation, migration and adhesion to the ECM via the PI3 K/AKT and Wnt/β-catenin signaling pathways in prostate and colon cancer [93].
High levels of glycolysis and mitochondrial dysfunction in cancer cells result in the accumulation of lactate, succinate and a reduction in β-hydroxybutyrate, and these molecules are reported to interact with specific cell-surface G-protein-coupled receptors to promote tumorigenesis and progression [94]. G-protein-coupled receptors 81 (GPR81) are upregulated in most cancers, including PDAC, and are associated with tumor growth and metastasis [75, 95]. Lactate released in the microenvironment can activate GPR81 to promote the proliferation and overexpression of monocarboxylate transporter 1 (MCT1), MCT4 and CD147. GPR81 activation also enhances angiogenesis via the increased secretion of the pro-angiogenic factor amphiregulin, which is involved in the PI3 K/Akt pathway, coupled to decreased cAMP (Fig. 3). In addition, lactate secreted by glycolytic cancer cells induces TAMs to express VEGF dependent on HIF-1α and promotes the conversion of M0 macrophages to M2 macrophages, which in turn enhances aerobic glycolysis in PDAC cells [62, 96]. Additionally, endothelial cells can take up lactate through MCT1, which independently triggers the NF-kB/IL-8 (CXCL8) pathway to promote human colorectal and breast cancer angiogenesis by increasing the expression of VEGF-A, VEGFR-2 and bFGF; this, in turn, is mediated by elevated ROS and the suppression of prolyl hydroxylase 2 (PHD2) [97].
The glycolytic phenotype of endothelial cells can promote tumor angiogenesis. The knockdown of PFKFB3 could suppress cultured endothelial cell proliferation and migration, which can be rescued by the addition of lactate [98]. It has been reported that PFKFB3 can be activated by the CXCL12/CXCR4-induced phosphorylation of PI3 K/Akt to promote microvascular sprouting [99]. The targeting of PFKFB3 to suppress cancer metastasis has already been used in clinical trials [100, 101]. De Bock et al. elucidated the mechanism by which PFKFB3 promoted vessel sprouting in HUVECs. PFKFB3 gene deletion in ECs caused vascular defects in vivo, and the knockdown of PFKFB3 impaired the formation of lamellipodia. Interestingly, PFKFB3 silencing or overexpression did not alter gene expression in tip or stalk cells. PFKFB3 was enriched along with F-actin in membrane ruffles at the leading front of lamellipodia and was confirmed to interact with actin. In addition, PFKFB3 knockdown decreased ATP consumption and reduced protein synthesis, which led to the suppression of hypermetabolism induced by vessel sprouting [102]. Thus, in the hypoxic TME in PDAC, glycolytic cancer cells and stromal cells, including TAMs and endothelial cells, synergistically promote angiogenesis that helps cancer cells hematogenously spread.
Metastatic colonization
In the bloodstream, circulating tumor cells (CTCs) will be challenged by shear forces, the innate immune system and oxidative stress. However, interactions with platelets and neutrophils can protect CTCs from killing by immune cells and promote their capability for extravasation by enhancing the cancer cell EMT program [103–106]. Oxidative stress limits distant metastasis, and treatment with the antioxidant N-acetyl-cysteine (NAC) can promote the survival of CTCs and increase the metastatic burden in vivo [107]. Therefore, the enhancement of glycolysis can help CTCs to metastasize via increase in antioxidant capacity. To colonize distant organs, CTCs must overcome many obstacles to infiltrate distant organs, avoid immune defenses, settle in supportive niches and eventually overtake host tissues [108]. Although it has been shown that glycolysis promotes the distant metastasis of PDAC cells in vivo, the mechanism underlying metastatic colonization is only just starting to be understood, and studies on metabolic regulation have been rare [109].
To induce metastatic colonization, CTCs first need infiltrate the distant tissues, which partly depend on their mesenchymal cell properties that can be enhanced by glycolysis. Additionally, macrophages can contact CTCs to pull them across capillary walls in the lungs. Pancreatic cancer cell-conditioned macrophages were found to promote angiogenesis and augment CTC extravasation. The inhibition of TAM glycolysis with competitive inhibitors of hexokinase II (HK2) and 2-deoxyglucose (2DG) blocked pro-metastatic functioning [110]. Thus, the glycolytic phenotype of CTCs and the regulation of the intravascular microenvironment by CTCs could synergistically promote PDAC cell migration across the vessel wall.
Metastatic cells depend on supportive niches, including premetastatic niches, perivascular niches, ad hoc niches and native stem cell niches, to survive in distant tissues. Metabolic reprogramming has been shown to be involved in premetastatic niche-promoted tumor metastasis, such as that observed in colorectal tumor-initiating cells, and increased lysine catabolism was found to facilitate survival in the liver [108]. When cancer cells infiltrate distant organs, most of them will die due to immune surveillance in the new challenging microenvironment, for example, NK cells, which are enriched in liver, suppress metastatic cell survival [108]. However, tumor-derived lactate plays an immunosuppressive role that facilitates metastatic cell survival, and LDHA-deficient PanO2 cell-injected mice show significantly higher cytotoxic activity in their NK cells, suppressing the dissemination of cancer cells [111]. Additionally, bone marrow neutrophils with immunosuppressive activity exhibit elevated glycolysis and more spontaneous chemokine-induced migration to distant tissue, which aids in early cancer cell dissemination [112]. The liver is one of the most frequently infiltrated organs by distant metastatic PDAC cells [113]. A hepatic microenvironment with hepatic stellate cells (HSCs) has a negative impact on metastatic cancer cells that present the elevated expression of succinate dehydrogenase (SDH) and high OXPHOS activity, leading to a dormant stage with reduced CSC activity and proliferation capability. However, the presence of liver inflammation during the transdifferentiation of HSCs to hepatic myofibroblasts (HMFs) promotes the downregulation of SDH and increased glycolysis, which reactivates the dormant metastatic cancer cells and fosters their proliferation and self-renewal capabilities, thus facilitating the development of visible metastases [114].
In addition, there are glycolytic enzymes that can be secreted by cancer cells into circulation, such as LDHA and PKM2, which can function as extracellular activators to promote metastasis [93]. We hypothesize that glycolytic enzymes in the circulation can be transported to distant organs to interact with ECs, facilitating their proliferation and supporting metastatic cancer cell survival.
Multiple metabolic signaling pathways, such as the PI3 K-AKT, MAPK, HIF and NF-kB pathways, support metastatic cell growth and survival, and these pathways are mostly driven by cancer-associated genes that also regulate glycolysis and are considered to increase stem cell capacity via the upregulation of CD133, CD24, Nanog and Sox2 [17]. A proteomic analysis of pancreatic stem cells revealed that PANC-1 CSCs were characterized by the upregulation of glycolysis [115]. However, in a subset of pancreatic CSCs, the enhancement of glycolysis mediated by MYC reduced their stemness, while high OXPHOS activity preserved full CSC functionality; however, the glycolytic capacity of CSCs facilitate their survival during the energy crisis induced by metformin [11]. Additionally, cancer cells can obtain support through physical contact with stromal cells. Pancreatic stellate cells secrete hepatocyte growth factor (HGF) which promotes HK2 expression in PDAC cells through HGF/c-MET/YAP/HIF-1α signaling, enhances stem cell potential via the overexpression of Nanog, Oct-4, and Sox-2 and promotes stemness [63].
OXPHOS activity in PDAC metastasis
Although we consider the enhancement of glycolysis to be the dominant metabolic alteration in malignant tumors, OXPHOS activity also plays critical roles in some cancer cells due to metabolic heterogeneity [116, 117]. However, the role of OXPHOS in the regulation of PDAC metastasis remains unclear and complicated. Due to the dysregulation of mitochondrial function in the glycolysis phenotype, we can indirectly conclude that OXPHOS may be negatively associated with PDAC metastasis. A study investigating mitochondrial function during EMT in pancreatic cancer cells found that TGFβ-1 treatment resulted in a high mtDNA copy number, increased intrinsic ROS stress and decreased the ΔΨm, suggesting that mitochondrial dysfunction may play a role in PDAC progression [86]. In liver metastases, dormant cancer cells could reacquire CSC potential along with the enhancement of glycolysis when HSCs transdifferentiated into HMFs, which was induced by the hepatic inflammatory microenvironment [114].
However, many studies have also demonstrated the role of OXPHOS in promoting PDAC metastasis. Coculture of CAFs and pancreatic cancer cells could enhance the migration and invasion abilities of the latter, which could be eliminated by MCT1-specific inhibitors [69]. Myoferlin, a ferlin family member protein overexpressed in PDAC, was reported to promote pancreatic cancer cell metastasis by enhancing OXPHOS activity [118]. Moreover, it was found that high OXPHOS activity could maintain CSC functionality; in contrast, enhanced glycolysis repressed stemness via increased MYC expression, which can promote glycolytic gene expression and suppress PGC-1α [11]. In another study, a subpopulation of dormant tumor cells surviving Kras ablation was found to rely on mitochondrial respiration, show high sensitivity to OXPHOS inhibitors and exhibit a decreased dependence on glycolysis [116]. For other types of cancer, such as breast cancer, cervical carcinoma and melanoma, many studies have demonstrated the association between increased OXPHOS and metastatic properties [20, 119]. Valerie et al. systematically reported the effect of PGC-1α on the promotion of breast cancer metastasis via mediating mitochondrial biogenesis and OXPHOS activity [119].
In summary, we believe that pancreatic CSCs obtain energy mainly through OXPHOS, whereas non-CSCs rely more on aerobic glycolysis. However, many factors can impact the fate of pancreatic cancer cells. The nuclear genetic background, including Kras mutations and MYC expression, restrict metabolic plasticity [11, 116]. Additionally, the number of mtDNA mutations, lipid utilization and the microenvironment collectively influence the metabolic phenotype of PDAC to maintain high OXPHOS activity, facilitating PDAC growth and metastasis [10, 69].
Therapeutic strategies for targeting glycolysis
The glycolytic phenotype has been widely demonstrated to promote tumor growth and metastasis, suggesting that it is a potential therapeutic target, and the limitation of metabolic processes or glycolytic enzymes must be examined and confirmed to be safe. Until now, only the glycolytic inhibitor PFK158 targeting PFKFB3 has been tested in a clinical trial, which confirmed it to be safe and provide a clinical benefit in late-stage PDAC patients [120, 121]. In addition, combining PFK158 with immunotherapeutic agents may result in additional clinical benefits due to the immunosuppressive role of PFKFB3 in cancer cells. HK2 has potential value as a target of anti-cancer therapy, such as treatment with 3-bromopyruvate (3BP), a HK2 inhibitor that was reported to suppress pancreatic cancer growth and progression in animals compared with gemcitabine [122]. Additionally, systematic HK2 deletion in adult mice reduced the tumor burden of lung cancer without adverse physiological consequences [123]. Thus, clinical trials of HK2 inhibitors in patients with PDAC should be considered. The targeting of the Warburg effect with LDHA inhibitors proved to be effective, particularly under hypoxic conditions, and could reduce the expression of MMP and CD133+ to suppress pancreatic cancer metastasis and exhibited synergistic cytotoxic activity with gemcitabine [124]. In addition, pancreatic tumors with Tp53 mutations exhibit a therapeutic response when treated with FX11, a small-molecule inhibitor of LDHA [37, 125]. In addition, ECs exhibited a high level of glycolysis during vessel sprouting, and targeting ECs has recently emerged as a novel approach for cancer therapy. For instance, treatment with the PFKFB3 inhibitor 3PO at a low dose can tighten the vascular barrier and induce vessel maturation to reduce cancer cell metastasis in orthotopic pancreatic mouse models [101].
Targeting glycolytic enzymes seems to be successful for cancer therapy and has been shown to work in animal models. However, such inhibitors have not been used in the clinic, even in clinical trials, suggesting that more potential enzymes need to be identified, especially those that function against cells with the mutation of specific genes such as Tp53 and Kras.
Future perspectives
Disturbances in carbohydrate metabolism in pancreatic cancer have been studied for more than 70 years and have received the most attention in investigations of the crosstalk between metabolic regulation and signaling. We have summarized the causes of the switch to a high-glycolysis phenotype in pancreatic cancer and its role in cancer metastasis. However, cancer cells have many energy resources involving glucose, lipid and glutamine metabolism that can also promote the progression of PDAC [126–131]. In contrast to the dependence of cancer cells on glutamate dehydrogenase, which converts glutamine-derived glutamate into α-ketoglutarate, PDAC cells rely on the transaminases GOT2 and GOT1 for metabolism to promote the conversion of aspartate to OAA, malate and then pyruvate, which supports PDAC cell growth by maintaining the redox balance. Therefore, there must be a great deal of complex crosstalk among the metabolic processes of different energy sources that cooperatively regulate the malignant behaviors of PDAC.
In addition to aerobic glycolysis, OXPHOS also plays a critical role in cancer growth, metastasis and therapy resistance. It is worth noting that cancer cells with higher OXPHOS activity, which supports CSC survival and metastasis, showed no decrease in glycolytic activity and even exhibited a dependence on glycolysis that was promoted by therapy resistance or an inflammatory state, indicating the important role played by the hybrid metabolic phenotype [11, 114, 119]. Jia et al. demonstrated that metastatic TNBC cells could acquire a stable hybrid metabolic phenotype and that repressing either of the metabolic phenotypes would activate the fine-tuning genes (HIF-1 and AMPK) of the other. Only combining glycolysis inhibition and FAO inhibition could lead to a major reduction in both the proliferation and clonogenic potential of breast cancer cells [10]. Additionally, the LDHA inhibitor GEN140 could not suppress the growth of pancreatic cancer cells that utilized OXPHOS rather than glycolysis unless the compounds targeting OXPHOS, such as phenformin, were combined [132]. Overall, due to the enriched metabolic plasticity of cancer cells, future therapeutic strategies might consider targeting the hybrid glycolysis/OXPHOS phenotype and eliminating the capability of cancer cells for metabolic phenotype transition to improve cancer treatment outcomes.
The high-glycolysis phenotype of PDAC favors cancer cell metastasis by promoting EMT, angiogenesis and metastatic colonization. In addition to their cytosolic and nuclear roles in regulating metastasis, glycolytic enzymes can regulate metastasis through multiple pathways as extracellular nonenzymatic regulators [15, 16, 61, 93]. The secretory mechanisms of glycolytic enzymes may be associated with exosomes or microvesicles, and this requires further investigation. Additionally, the metabolic reprogramming of endothelial cells also contributes to cancer growth and metastasis significantly. Glycolysis in ECs benefits metastasis mainly due to the rapid increase in the energy supply, the massive increase in lactate production during pro-angiogenic signaling, and the binding of glycolytic enzymes to actin in invadopodium to directly supply ATP and decrease ROS-mediated apoptosis [133]. The investigation of metabolic factors in tumor ECs is expected to be a fruitful direction for the elucidation of tumor metabolism.
Finally, it is worth noting that metabolic factors can regulate PDAC metastasis by modifying the epigenetic status of cells, which is suggested by studies in which no metastasis-specific driver mutations were identified [14, 134]. Hence, reversible epigenetic modifications induced by enzymatic catalysis can be easily targeted for cancer therapy.
Abbreviations
- PDAC
Pancreatic ductal adenocarcinoma
- EMT
Epithelial–mesenchymal transition
- CAFs
Cancer-associated fibroblasts
- OXPHOS
Oxidative phosphorylation
- NOX
NADPH oxidase
- mtDNA
Mitochondrial DNA
- ΔΨm
Membrane potential
- PFK
Phosphofructokinase
- ALDOA
Aldolase A
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- PGM
Phosphoglycerate mutase
- ENO
Enolase
- PKM
Pyruvate kinase muscle isozyme
- LDHA
Lactate dehydrogenase A
- TME
Tumor microenvironment
- TGF
Transforming growth factor
- IGF1R
Insulin-like growth factor 1 receptor
- MAPK
Mitogen-activated protein kinase
- PI3K/AKT
Phosphoinositide 3-kinase/protein kinase B
- mTOR
Mammalian target of rapamycin
- NF-kB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PDLCs
Patient-derived pancreatic cancer cell lines
- PGC-1α
Peroxisome proliferator-activated receptor γ coactivator-1α
- SDH
Succinate dehydrogenase
- HK
Hexokinase
- TCA
Tricarboxylic acid cycle
- HIF
Hypoxia-induced factor
- PPP
Pentose phosphate pathway
- GOF mutp53
Gain-of-function of mutant p53
- GLUT
Glucose transporter
- AMPK
AMP-activated protein kinase
- TIGAR
Tp53-induced glycolysis and apoptosis regulator
- GAMT
Guanidinoacetate N-methyltransferase
- GLS2
Glutaminase-2
- ROS
Reactive oxygen species
- PFKFB
6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase
- ACL
ATP citrate lyase
- PDK
Pyruvate dehydrogenase kinase
- PGI/AMF
Glucose-6-phosphate isomerase/Autocrine motility factor
- VEGF
Vascular epidermal growth factor
- TGFBI
Transforming growth factor beta-induced
- FAK
Focal adhesion kinase
- ECM
Extracellular matrix
- EMT-TFs
EMT-TF
- MMP
Matrix metalloproteinase
- 6-PGD
6-Phosphogluconate dehydrogenase
- FGF
Fibroblast growth factor
- GPR81
G-protein-coupled receptors 81
- MCT
Monocarboxylate transporters
- TAMs
Tumor-associated macrophages
- CXCL
CXC chemokine ligand
- CXCR
CXC chemokine receptor
- ECs
Endothelial cells
- CTCs
Circulating tumor cells
- CSCs
Cancer stem cells
- HSCs
Hepatic stellate cells
- HMFs
Hepatic myofibroblasts
- GOT
Glutamic-oxaloacetic transaminase
Author contributions
Study concept and design: All authors. Drafting of the manuscript: JY and BR. Critical revision of the manuscript for important intellectual content: GY, HW and GC. Obtained the funding: LY, TZ and YZ. All authors read and approved the final manuscript.
Funding
This study was supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2018PT32014), the CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2 M-1-001), and the National Nature Science Foundation of China (81672960; 81672443).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jinshou Yang and Bo Ren contributed equally.
Contributor Information
Lei You, Email: florayo@163.com.
Taiping Zhang, Email: tpingzhang@yahoo.com.
Yupei Zhao, Email: zhao8028@263.net.
References
- 1.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L., Smith B. D., Aizenberg R., Rosenzweig A. B., Fleshman J. M., Matrisian L. M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Research. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Poruk KE, Firpo MA, Adler DG, Mulvihill SJ. Screening for pancreatic cancer: why, how, and who? Ann Surg. 2013;257:17–26. doi: 10.1097/SLA.0b013e31825ffbfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovannetti E, et al. Never let it go: Stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin Cancer Biol. 2017;44:43–59. doi: 10.1016/j.semcancer.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 7.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 9.Robertson-Tessi M., Gillies R. J., Gatenby R. A., Anderson A. R. A. Impact of Metabolic Heterogeneity on Tumor Growth, Invasion, and Treatment Outcomes. Cancer Research. 2015;75(8):1567–1579. doi: 10.1158/0008-5472.CAN-14-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia D, et al. Elucidating cancer metabolic plasticity by coupling gene regulation with metabolic pathways. Proc Natl Acad Sci USA. 2019;116:3909–3918. doi: 10.1073/pnas.1816391116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sancho P, et al. MYC/PGC-1alpha balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metab. 2015;22:590–605. doi: 10.1016/j.cmet.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, et al. MiR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase-1. Nat Commun. 2019;10:809. doi: 10.1038/s41467-019-08759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sousa CM, Kimmelman AC. The complex landscape of pancreatic cancer metabolism. Carcinogenesis. 2014;35:1441–1450. doi: 10.1093/carcin/bgu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald OG, et al. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat Genet. 2017;49:367–376. doi: 10.1038/ng.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsutsumi S, Yanagawa T, Shimura T, Kuwano H, Raz A. Autocrine motility factor signaling enhances pancreatic cancer metastasis. Clin Cancer Res. 2004;10:7775–7784. doi: 10.1158/1078-0432.CCR-04-1015. [DOI] [PubMed] [Google Scholar]
- 16.Azoitei N, et al. PKM2 promotes tumor angiogenesis by regulating HIF-1alpha through NF-kappaB activation. Mol Cancer. 2016;15:3. doi: 10.1186/s12943-015-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao H, et al. Up-regulation of glycolysis promotes the stemness and EMT phenotypes in gemcitabine-resistant pancreatic cancer cells. J Cell Mol Med. 2017;21:2055–2067. doi: 10.1111/jcmm.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 19.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 20.Jia D, Park JH, Jung KH, Levine H, Kaipparettu BA. Elucidating the metabolic plasticity of cancer: mitochondrial reprogramming and hybrid metabolic states. Cells. 2018;7:21. doi: 10.3390/cells7030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guha M, Avadhani NG. Mitochondrial retrograde signaling at the crossroads of tumor bioenergetics, genetics and epigenetics. Mitochondrion. 2013;13:577–591. doi: 10.1016/j.mito.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa K, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 23.Jones JB, et al. Detection of mitochondrial DNA mutations in pancreatic cancer offers a “Mass”-ive advantage over detection of nuclear DNA mutations. Cancer Res. 2001;61:1299. [PubMed] [Google Scholar]
- 24.Hardie RA, et al. Mitochondrial mutations and metabolic adaptation in pancreatic cancer. Cancer Metab. 2017;5:2. doi: 10.1186/s40170-017-0164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donadelli M, Dando I, Dalla Pozza E, Palmieri M. Mitochondrial uncoupling protein 2 and pancreatic cancer: a new potential target therapy. World J Gastroenterol. 2015;21:3232–3238. doi: 10.3748/wjg.v21.i11.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu W, et al. Novel role of NOX in supporting aerobic glycolysis in cancer cells with mitochondrial dysfunction and as a potential target for cancer therapy. PLoS Biol. 2012;10:e1001326. doi: 10.1371/journal.pbio.1001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg Robert A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Jones S, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun J, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–1559. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ying H, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halbrook CJ, Lyssiotis CA. Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell. 2017;31:5–19. doi: 10.1016/j.ccell.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 32.He TL, et al. The c-Myc-LDHA axis positively regulates aerobic glycolysis and promotes tumor progression in pancreatic cancer. Med Oncol. 2015;32:187. doi: 10.1007/s12032-015-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji S, et al. FBW7 (F-box and WD Repeat Domain-Containing 7) negatively regulates glucose metabolism by targeting the c-Myc/TXNIP (Thioredoxin-Binding Protein) axis in pancreatic cancer. Clin Cancer Res. 2016;22:3950–3960. doi: 10.1158/1078-0432.CCR-15-2380. [DOI] [PubMed] [Google Scholar]
- 34.Zubair H, et al. Glucose metabolism reprogrammed by overexpression of IKKepsilon promotes pancreatic tumor growth. Cancer Res. 2016;76:7254–7264. doi: 10.1158/0008-5472.CAN-16-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butera G, et al. Mutant p53 prevents GAPDH nuclear translocation in pancreatic cancer cells favoring glycolysis and 2-deoxyglucose sensitivity. BBA-Mol Cell Res. 2018;1865:1914–1923. doi: 10.1016/j.bbamcr.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, et al. Tumour-associated mutant p53 drives the Warburg effect. Nat Commun. 2013;4:2935. doi: 10.1038/ncomms3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajeshkumar NV, et al. Therapeutic targeting of the warburg effect in pancreatic cancer relies on an absence of p53 function. Cancer Res. 2015;75:3355–3364. doi: 10.1158/0008-5472.CAN-15-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchakjian MR, Kornbluth S. The engine driving the ship: metabolic steering of cell proliferation and death. Nat Rev Mol Cell Biol. 2010;11:715–727. doi: 10.1038/nrm2972. [DOI] [PubMed] [Google Scholar]
- 39.Bensaad K, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 40.Kotiah S. D., Caro J. Elevation of PFKFB3 and TIGAR levels in pancreatic cancer. Journal of Clinical Oncology. 2010;28(15_suppl):e14679–e14679. [Google Scholar]
- 41.Ros S, et al. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 is essential for p53-null cancer cells. Oncogene. 2017;36:3287. doi: 10.1038/onc.2016.477. [DOI] [PubMed] [Google Scholar]
- 42.Nagarajan A, et al. Paraoxonase 2 facilitates pancreatic cancer growth and metastasis by stimulating GLUT1-Mediated glucose transport. Mol Cell. 2017;67:685–701.e6. doi: 10.1016/j.molcel.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–1020. doi: 10.1016/j.ygeno.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 44.King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25:4675–4682. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- 45.Wellen K. E., Hatzivassiliou G., Sachdeva U. M., Bui T. V., Cross J. R., Thompson C. B. ATP-Citrate Lyase Links Cellular Metabolism to Histone Acetylation. Science. 2009;324(5930):1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui J, et al. FOXM1 promotes the warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin Cancer Res. 2014;20:2595–2606. doi: 10.1158/1078-0432.CCR-13-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hua S, et al. miR-139-5p inhibits aerobic glycolysis, cell proliferation, migration, and invasion in hepatocellular carcinoma via a reciprocal regulatory interaction with ETS1. Oncogene. 2018;37:1624–1636. doi: 10.1038/s41388-017-0057-3. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto T, et al. Reduced methylation of PFKFB3 in cancer cells shunts glucose towards the pentose phosphate pathway. Nat Commun. 2014;5:3480. doi: 10.1038/ncomms4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li FL, et al. Acetylation accumulates PFKFB3 in cytoplasm to promote glycolysis and protects cells from cisplatin-induced apoptosis. Nat Commun. 2018;9:508. doi: 10.1038/s41467-018-02950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao D, et al. Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell. 2013;23:464–476. doi: 10.1016/j.ccr.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neesse A, et al. Stromal biology and therapy in pancreatic cancer: ready for clinical translation? Gut. 2019;68:159–171. doi: 10.1136/gutjnl-2018-316451. [DOI] [PubMed] [Google Scholar]
- 52.Yeung SJ, Pan J, Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis—the seventh hallmark of cancer. Cell Mol Life Sci. 2008;65:3981–3999. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Q, et al. Hypoxia-inducible factor-2alpha promotes tumor progression and has crosstalk with Wnt/beta-catenin signaling in pancreatic cancer. Mol Cancer. 2017;16:119. doi: 10.1186/s12943-017-0689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He G, Jiang Y, Zhang B, Wu G. The effect of HIF-1alpha on glucose metabolism, growth and apoptosis of pancreatic cancerous cells. Asia Pac J Clin Nutr. 2014;23:174–180. doi: 10.6133/apjcn.2014.23.1.14. [DOI] [PubMed] [Google Scholar]
- 55.Yoon DY, et al. Identification of genes differentially induced by hypoxia in pancreatic cancer cells. Biochem Biophys Res Commun. 2001;288:882–886. doi: 10.1006/bbrc.2001.5867. [DOI] [PubMed] [Google Scholar]
- 56.Clem BF, et al. Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther. 2013;12:1461–1470. doi: 10.1158/1535-7163.MCT-13-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bobarykina AY, et al. Hypoxic regulation of PFKFB-3 and PFKFB-4 gene expression in gastric and pancreatic cancer cell lines and expression of PFKFB genes in gastric cancers. Acta Biochim Pol. 2006;53:789–799. [PubMed] [Google Scholar]
- 58.Zhang H, et al. HIF-1alpha activates hypoxia-induced PFKFB4 expression in human bladder cancer cells. Biochem Biophys Res Commun. 2016;476:146–152. doi: 10.1016/j.bbrc.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 59.Goidts V, et al. RNAi screening in glioma stem-like cells identifies PFKFB4 as a key molecule important for cancer cell survival. Oncogene. 2012;31:3235–3243. doi: 10.1038/onc.2011.490. [DOI] [PubMed] [Google Scholar]
- 60.Minchenko OH, Tsuchihara K, Minchenko DO, Bikfalvi A, Esumi H. Mechanisms of regulation of PFKFB expression in pancreatic and gastric cancer cells. World J Gastroenterol. 2014;20:13705–13717. doi: 10.3748/wjg.v20.i38.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheung EC, Ludwig RL, Vousden KH. Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc Natl Acad Sci USA. 2012;109:20491–20496. doi: 10.1073/pnas.1206530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye H, et al. Tumor-associated macrophages promote progression and the Warburg effect via CCL18/NF-kB/VCAM-1 pathway in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9:453. doi: 10.1038/s41419-018-0486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan B, et al. Paracrine HGF/c-MET enhances the stem cell-like potential and glycolysis of pancreatic cancer cells via activation of YAP/HIF-1alpha. Exp Cell Res. 2018;371:63–71. doi: 10.1016/j.yexcr.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 64.Shi S, et al. VEGF promotes glycolysis in pancreatic cancer via HIF1alpha up-regulation. Curr Mol Med. 2016;16:394–403. doi: 10.2174/1566524016666160316153623. [DOI] [PubMed] [Google Scholar]
- 65.Costanza B, et al. Transforming growth factor beta-induced, an extracellular matrix interacting protein, enhances glycolysis and promotes pancreatic cancer cell migration. Int J Cancer. 2019;145:1570–1584. doi: 10.1002/ijc.32247. [DOI] [PubMed] [Google Scholar]
- 66.Ishida S, Andreux P, Poitry-Yamate C, Auwerx J, Hanahan D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc Natl Acad Sci USA. 2013;110:19507–19512. doi: 10.1073/pnas.1318431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilde L, et al. Metabolic coupling and the Reverse Warburg Effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44:198–203. doi: 10.1053/j.seminoncol.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen S, et al. MiR-21-mediated metabolic alteration of cancer-associated fibroblasts and its effect on pancreatic cancer cell behavior. Int J Biol Sci. 2018;14:100–110. doi: 10.7150/ijbs.22555. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Shan T, et al. Cancer-associated fibroblasts enhance pancreatic cancer cell invasion by remodeling the metabolic conversion mechanism. Oncol Rep. 2017;37:1971–1979. doi: 10.3892/or.2017.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weissmueller S, et al. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor beta signaling. Cell. 2014;157:382–394. doi: 10.1016/j.cell.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roe JS, et al. Enhancer reprogramming promotes pancreatic cancer metastasis. Cell. 2017;170:875–888.e20. doi: 10.1016/j.cell.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deng SJ, et al. Hypoxia-induced LncRNA-BX111 promotes metastasis and progression of pancreatic cancer through regulating ZEB1 transcription. Oncogene. 2018;37:5811–5828. doi: 10.1038/s41388-018-0382-1. [DOI] [PubMed] [Google Scholar]
- 73.Li Z, et al. Tumor-derived exosomal lnc-Sox2ot promotes EMT and stemness by acting as a ceRNA in pancreatic ductal adenocarcinoma. Oncogene. 2018;37:3822–3838. doi: 10.1038/s41388-018-0237-9. [DOI] [PubMed] [Google Scholar]
- 74.Gao H, et al. Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell. 2016;166:47–62. doi: 10.1016/j.cell.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roland CL, et al. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. 2014;74:5301–5310. doi: 10.1158/0008-5472.CAN-14-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dovmark TH, Saccomano M, Hulikova A, Alves F, Swietach P. Connexin-43 channels are a pathway for discharging lactate from glycolytic pancreatic ductal adenocarcinoma cells. Oncogene. 2017;36:4538. doi: 10.1038/onc.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng X, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krebs AM, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19:518–529. doi: 10.1038/ncb3513. [DOI] [PubMed] [Google Scholar]
- 80.Daemen A, et al. Metabolite profiling stratifies pancreatic ductal adenocarcinomas into subtypes with distinct sensitivities to metabolic inhibitors. Proc Natl Acad Sci USA. 2015;112:E4410–E4417. doi: 10.1073/pnas.1501605112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Payen VL, Porporato PE, Baselet B, Sonveaux P. Metabolic changes associated with tumor metastasis, part 1: tumor pH, glycolysis and the pentose phosphate pathway. Cell Mol Life Sci. 2016;73:1333–1348. doi: 10.1007/s00018-015-2098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yalcin A, et al. 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase-3 is required for transforming growth factor beta1-enhanced invasion of Panc1 cells in vitro. Biochem Biophys Res Commun. 2017;484:687–693. doi: 10.1016/j.bbrc.2017.01.178. [DOI] [PubMed] [Google Scholar]
- 83.Li H-M, et al. Blockage of glycolysis by targeting PFKFB3 suppresses tumor growth and metastasis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36:7. doi: 10.1186/s13046-016-0481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ji S, et al. ALDOA functions as an oncogene in the highly metastatic pancreatic cancer. Cancer Lett. 2016;374:127–135. doi: 10.1016/j.canlet.2016.01.054. [DOI] [PubMed] [Google Scholar]
- 85.Baumann F, et al. Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro Oncol. 2009;11:368–380. doi: 10.1215/15228517-2008-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo Q. Changes in mitochondrial function during EMT induced by TGFbeta-1 in pancreatic cancer. Oncol Lett. 2017;13:1575–1580. doi: 10.3892/ol.2017.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Principe M, et al. Alpha-enolase (ENO1) controls alpha v/beta 3 integrin expression and regulates pancreatic cancer adhesion, invasion, and metastasis. J Hematol Oncol. 2017;10:16. doi: 10.1186/s13045-016-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71:6921–6925. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 89.Nikitovic D, Kouvidi K, Karamanos NK, Tzanakakis GN. The roles of hyaluronan/RHAMM/CD44 and their respective interactions along the insidious pathways of fibrosarcoma progression. Biomed Res Int. 2013;2013:929531. doi: 10.1155/2013/929531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Torres MP, et al. Graviola: a novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett. 2012;323:29–40. doi: 10.1016/j.canlet.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu W-Y, et al. Zinc finger E-box-binding homeobox 1 mediates aerobic glycolysis via suppression of sirtuin 3 in pancreatic cancer. World J Gastroenterol. 2018;24:4893–4905. doi: 10.3748/wjg.v24.i43.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Funasaka T, Raz A. The role of autocrine motility factor in tumor and tumor microenvironment. Cancer Metastasis Rev. 2007;26:725–735. doi: 10.1007/s10555-007-9086-7. [DOI] [PubMed] [Google Scholar]
- 93.Amin S, Yang P, Li Z. Pyruvate kinase M2: a multifarious enzyme in non-canonical localization to promote cancer progression. BBA-Rev Cancer. 2019;1871:331–341. doi: 10.1016/j.bbcan.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 94.Ristic B, Bhutia YD, Ganapathy V. Cell-surface G-protein-coupled receptors for tumor-associated metabolites: a direct link to mitochondrial dysfunction in cancer. Biochim Biophys Acta Rev Cancer. 2017;1868:246–257. doi: 10.1016/j.bbcan.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wagner W, Ciszewski WM, Kania KD. l- and d-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell Commun Signal. 2015;13:36. doi: 10.1186/s12964-015-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Colegio OR, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 98.Xu Y, et al. Endothelial PFKFB3 plays a critical role in angiogenesis. Arterioscler Thromb Vasc Biol. 2014;34:1231–1239. doi: 10.1161/ATVBAHA.113.303041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ziegler ME, Hatch MM, Wu N, Muawad SA, Hughes CC. mTORC2 mediates CXCL12-induced angiogenesis. Angiogenesis. 2016;19:359–371. doi: 10.1007/s10456-016-9509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Redman RA, Pohlmann PR, Kurman MR, Tapolsky G, Chesney JA. A phase I, dose-escalation, multi-center study of PFK-158 in patients with advanced solid malignancies explores a first-in-man inhibitor of glycolysis. J Clin Oncol. 2015;33:TPS2606. doi: 10.1200/jco.2015.33.15_suppl.tps2606. [DOI] [Google Scholar]
- 101.Conradi LC, et al. Tumor vessel disintegration by maximum tolerable PFKFB3 blockade. Angiogenesis. 2017;20:599–613. doi: 10.1007/s10456-017-9573-6. [DOI] [PubMed] [Google Scholar]
- 102.De Bock K, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 103.Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2:1091. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Labelle M, Begum S, Hynes Richard O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coffelt SB, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spiegel A, et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6:630. doi: 10.1158/2159-8290.CD-15-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peiris-Pages M, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Metastasis and oxidative stress: are antioxidants a metabolic driver of progression? Cell Metab. 2015;22:956–958. doi: 10.1016/j.cmet.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 108.Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng TY, et al. Pyruvate kinase M2 promotes pancreatic ductal adenocarcinoma invasion and metastasis through phosphorylation and stabilization of PAK2 protein. Oncogene. 2018;37:1730–1742. doi: 10.1038/s41388-017-0086-y. [DOI] [PubMed] [Google Scholar]
- 110.Penny HL, et al. Warburg metabolism in tumor-conditioned macrophages promotes metastasis in human pancreatic ductal adenocarcinoma. Oncoimmunology. 2016;5:1191731. doi: 10.1080/2162402X.2016.1191731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191:1486. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 112.Patel S, et al. Unique pattern of neutrophil migration and function during tumor progression. Nat Immunol. 2018;19:1236–1247. doi: 10.1038/s41590-018-0229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133:413–422. doi: 10.5858/133.3.413. [DOI] [PubMed] [Google Scholar]
- 114.Fabian A, et al. Metastasis of pancreatic cancer: An uninflamed liver micromilieu controls cell growth and cancer stem cell properties by oxidative phosphorylation in pancreatic ductal epithelial cells. Cancer Lett. 2019;453:95–106. doi: 10.1016/j.canlet.2019.03.039. [DOI] [PubMed] [Google Scholar]
- 115.Brandi J, et al. Proteomic analysis of pancreatic cancer stem cells: functional role of fatty acid synthesis and mevalonate pathways. J Proteomics. 2017;150:310–322. doi: 10.1016/j.jprot.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 116.Viale A, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou C, et al. Oncogenic HSP60 regulates mitochondrial oxidative phosphorylation to support Erk1/2 activation during pancreatic cancer cell growth. Cell Death Dis. 2018;9:161. doi: 10.1038/s41419-017-0196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rademaker G, et al. Myoferlin contributes to the metastatic phenotype of pancreatic cancer cells by enhancing their migratory capacity through the control of oxidative phosphorylation. Cancers. 2019;11:853. doi: 10.3390/cancers11060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.LeBleu VS, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Redman RA, Pohlmann PR, Kurman MR, Tapolsky G, Chesney J (2015) A phase I, dose-escalation, multicenter study of ACT-PFK-158, 2HCl in patients with advanced solid malignancies explores a first-in-human inhibitor of glycolysis. J Clin Oncol 33. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L71836038. Accessed 17 Aug 2019
- 121.Telang S et al (2016) PFK-158 is a first-in-human inhibitor of PFKFB3 that selectively suppresses glucose metabolism of cancer cells and inhibits the immunosuppressive Th17 cells and MDSCs in advanced cancer patients. Cancer Res 76. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L617902344. Accessed 17 Aug 2019
- 122.Chapiro J, et al. Systemic delivery of microencapsulated 3-bromopyruvate for the therapy of pancreatic cancer. Clin Cancer Res. 2014;20:6406–6417. doi: 10.1158/1078-0432.CCR-14-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Patra KC, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maftouh M, et al. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer. 2014;110:172–182. doi: 10.1038/bjc.2013.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Le A et al (2010) Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. P Natl Acad Sci USA 107:2037. http://www.pnas.org/content/107/5/2037.abstract. Accessed 17 Aug 2019 [DOI] [PMC free article] [PubMed]
- 126.Hui S, et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deng Shi-Jiang, Chen Heng-Yu, Zeng Zhu, Deng Shichang, Zhu Shuai, Ye Zeng, He Chi, Liu Ming-Liang, Huang Kang, Zhong Jian-Xin, Xu Feng-Yu, Li Qiang, Liu Yang, Wang Chunyou, Zhao Gang. Nutrient Stress–Dysregulated Antisense lncRNA GLS-AS Impairs GLS-Mediated Metabolism and Represses Pancreatic Cancer Progression. Cancer Research. 2018;79(7):1398–1412. doi: 10.1158/0008-5472.CAN-18-0419. [DOI] [PubMed] [Google Scholar]
- 128.Son J, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li W, et al. HIF-2α regulates non-canonical glutamine metabolism via activation of PI3 K/mTORC2 pathway in human pancreatic ductal adenocarcinoma. J Cell Mol Med. 2017;21:2896–2908. doi: 10.1111/jcmm.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Swierczynski J, Hebanowska A, Sledzinski T. Role of abnormal lipid metabolism in development, progression, diagnosis and therapy of pancreatic cancer. World J Gastroenterol. 2014;20:2279–2303. doi: 10.3748/wjg.v20.i9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guillaumond F, et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. P Natl Acad Sci USA. 2015;112:2473–2478. doi: 10.1073/pnas.1421601112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boudreau A, et al. Metabolic plasticity underpins innate and acquired resistance to LDHA inhibition. Nat Chem Biol. 2016;12:779–786. doi: 10.1038/nchembio.2143. [DOI] [PubMed] [Google Scholar]
- 133.Draoui N, de Zeeuw P, Carmeliet P. Angiogenesis revisited from a metabolic perspective: role and therapeutic implications of endothelial cell metabolism. Open Biol. 2017;7:170219. doi: 10.1098/rsob.170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Makohon-Moore AP, et al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat Genet. 2017;49:358–366. doi: 10.1038/ng.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Qin Y, Cheng C, Lu H, Wang Y. miR-4458 suppresses glycolysis and lactate production by directly targeting hexokinase2 in colon cancer cells. Biochem Biophys Res Commun. 2016;469:37–43. doi: 10.1016/j.bbrc.2015.11.066. [DOI] [PubMed] [Google Scholar]
- 136.Lin YH, et al. Taurine up-regulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology. 2018;67:188–203. doi: 10.1002/hep.29462. [DOI] [PubMed] [Google Scholar]
- 137.Rong Y, et al. Lactate dehydrogenase A is overexpressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumour Biol. 2013;34:1523–1530. doi: 10.1007/s13277-013-0679-1. [DOI] [PubMed] [Google Scholar]
- 138.Yang B, et al. Overexpression of lncRNA IGFBP4-1 reprograms energy metabolism to promote lung cancer progression. Mol Cancer. 2017;16:154. doi: 10.1186/s12943-017-0722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bhattacharya R, Ray Chaudhuri S, Roy SS. FGF9-induced ovarian cancer cell invasion involves VEGF-A/VEGFR2 augmentation by virtue of ETS1 upregulation and metabolic reprogramming. J Cell Biochem. 2018;119:8174–8189. doi: 10.1002/jcb.26820. [DOI] [PubMed] [Google Scholar]
- 140.Liotta L. A., Mandler R., Murano G., Katz D. A., Gordon R. K., Chiang P. K., Schiffmann E. Tumor cell autocrine motility factor. Proceedings of the National Academy of Sciences. 1986;83(10):3302–3306. doi: 10.1073/pnas.83.10.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Timar J, et al. Autocrine motility factor signals integrin-mediated metastatic melanoma cell adhesion and invasion. Cancer Res. 1996;56:1902–1908. [PubMed] [Google Scholar]
- 142.Li Y, Wei Z, Dong B, Lian Z, Xu Y. Silencing of phosphoglucose isomerase/autocrine motility factor decreases U87 human glioblastoma cell migration. Int J Mol Med. 2016;37:998–1004. doi: 10.3892/ijmm.2016.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gallardo-Perez JC, Adan-Ladron de Guevara A, Marin-Hernandez A, Moreno-Sanchez R, Rodriguez-Enriquez S. HPI/AMF inhibition halts the development of the aggressive phenotype of breast cancer stem cells. BBA-Mol Cell Res. 2017;1864:1679–1690. doi: 10.1016/j.bbamcr.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 144.Funasaka T, Yanagawa T, Hogan V, Raz A. Regulation of phosphoglucose isomerase/autocrine motility factor expression by hypoxia. Faseb J. 2005;19:1422–1430. doi: 10.1096/fj.05-3699com. [DOI] [PubMed] [Google Scholar]
- 145.Mishra Suresh, Raz Avrahram, Murphy Liam J. Insulin-Like Growth Factor Binding Protein-3 Interacts with Autocrine Motility Factor/Phosphoglucose Isomerase (AMF/PGI) and Inhibits the AMF/PGI Function. Cancer Research. 2004;64(7):2516–2522. doi: 10.1158/0008-5472.can-03-2877. [DOI] [PubMed] [Google Scholar]
- 146.Ahmad A, et al. Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res. 2011;71:3400–3409. doi: 10.1158/0008-5472.CAN-10-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Haga A, Funasaka T, Deyashiki Y, Raz A. Autocrine motility factor stimulates the invasiveness of malignant cells as well as up-regulation of matrix metalloproteinase-3 expression via a MAPK pathway. FEBS Lett. 2008;582:1877–1882. doi: 10.1016/j.febslet.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gu M, et al. PFKFB3 promotes proliferation, migration and angiogenesis in nasopharyngeal carcinoma. J Cancer. 2017;8:3887–3896. doi: 10.7150/jca.19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Han J, Meng Q, Xi Q, Wang H, Wu G. PFKFB3 was overexpressed in gastric cancer patients and promoted the proliferation and migration of gastric cancer cells. Cancer Biomark. 2017;18:249–256. doi: 10.3233/CBM-160143. [DOI] [PubMed] [Google Scholar]
- 150.Peng F, et al. PFKFB3 is involved in breast cancer proliferation, migration, invasion and angiogenesis. Int J Oncol. 2018;52:945–954. doi: 10.3892/ijo.2018.4257. [DOI] [PubMed] [Google Scholar]
- 151.Ros S, et al. Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discov. 2012;2:328–343. doi: 10.1158/2159-8290.CD-11-0234. [DOI] [PubMed] [Google Scholar]
- 152.Yun SJ, et al. PFKFB4 as a prognostic marker in non-muscle-invasive bladder cancer. Urol Oncol. 2012;30:893–899. doi: 10.1016/j.urolonc.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 153.Dasgupta S, et al. Metabolic enzyme PFKFB4 activates transcriptional coactivator SRC-3 to drive breast cancer. Nature. 2018;556:249–254. doi: 10.1038/s41586-018-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cheung Eric C, et al. TIGAR is required for efficient intestinal regeneration and tumorigenesis. Dev Cell. 2013;25:463–477. doi: 10.1016/j.devcel.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wanka C, Steinbach JP, Rieger J. Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by up-regulating respiration and improving cellular redox homeostasis. J Biol Chem. 2012;287:33436–33446. doi: 10.1074/jbc.M112.384578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Won KY, et al. Regulatory role of p53 in cancer metabolism via SCO2 and TIGAR in human breast cancer. Hum Pathol. 2012;43:221–228. doi: 10.1016/j.humpath.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 157.Qian S, et al. TIGAR cooperated with glycolysis to inhibit the apoptosis of leukemia cells and associated with poor prognosis in patients with cytogenetically normal acute myeloid leukemia. J Hematol Oncol. 2016;9:128. doi: 10.1186/s13045-016-0360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu J, et al. High expression of synthesis of cytochrome c oxidase 2 and TP53-induced glycolysis and apoptosis regulator can predict poor prognosis in human lung adenocarcinoma. Hum Pathol. 2018;77:54–62. doi: 10.1016/j.humpath.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 159.Kim SH, Choi SI, Won KY, Lim SJ. Distinctive interrelation of p53 with SCO2, COX, and TIGAR in human gastric cancer. Pathol Res Pract. 2016;212:904–910. doi: 10.1016/j.prp.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 160.Shen M, et al. Met is involved in TIGAR-regulated metastasis of non-small-cell lung cancer. Mol Cancer. 2018;17:88. doi: 10.1186/s12943-018-0839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]