Abstract
In nature, bacteria are constantly adapting to various stressful conditions. Timely activation of stress response programs is crucial for bacteria to smoothly survive under stressful conditions. Stress response, demanding the de novo synthesis of many defense proteins, is generally activated at the transcriptional level by specific regulators. However, the effect of the global protein translational status on stress response has been largely overlooked. The translational capacity is limited by the number of translating ribosomes and the translational elongation rate. Recent work has shown that certain environmental stressors (e.g. oxidative stress) could severely compromise the stress response progress of bacteria by causing either slow-down or even complete stalling of the translational elongation process. The maintenance of ribosome elongation rate, being crucial for timely synthesis of stress defense proteins, becomes the physiological bottleneck that limits the survival of bacteria in some stressful conditions. Here, we briefly summarize some recent progress on the translational status of bacteria under two distinct stress conditions, nutrient deprivation and oxidative stress. We further discuss several important open questions on the translational regulation of bacteria during stress. The ribosome translation should be investigated in parallel with traditional transcriptional regulation in order to gain a better understanding on bacterial stress defense.
Keywords: Stress response, Translational elongation rate, Oxidative stress, Nutrient deprivation, Ribosome profiling
An overview of stress response
The natural environment of bacteria is constantly changing. For E. coli cells living inside the human gut, they may encounter various stressful conditions such as nutrient deprivation, hyper/hypo-osmolarity, low pH and oxidants. To survive under those harsh environments, bacterial cells have evolved various adaptive stress responses [1]. Exploring the mechanism of stress response is of perennial interest to microbiologists. Stress response is generally depicted as a collection of gene regulatory responses from lots of signaling cascades [1, 2]. Several sigma factors are responsible to activate the stress response at the transcriptional level. The central regulator of the general stress response, RpoS (σS), is responsible for activating the expression of over 100 genes during nutrient-deprived stationary phase and other stressful conditions [2–4]. The σE and σH factors mediate the bacterial transcriptional response to envelope stress and heat shock, respectively [5, 6]. In addition, OxyR and SoxSR proteins mediate the transcriptional response provoked by H2O2 and superoxide anion, respectively [7–11]. Although those stress responses are mainly regulated at the transcriptional level, it is clear that the post-transcriptional event, the ribosome translation process, is crucial for the successful occurrence of stress response [12]. Early work has shown that treatment of E. coli cells with the translation-targeted drug, chloramphenicol, causes severe loss of cell viability during carbon starvation [13]. Therefore, it is important to investigate the effect of specific stress on the ribosome translation process of bacteria.
Bacterial protein translation capacity during stress
Protein synthesis lies at the core of bacterial growth as protein accounts for 60–80% of cellular biomass and its synthesis consumes two-thirds of the cellular energy budget [14–16]. The ribosome is the “worker” to make proteins inside cells. The overall protein translation capacity of bacteria is determined by the number of actively translating ribosomes (number of “workers”, depending on translational initiation rate) and the translational elongation rate (ER, the speed of “workers”) [17–20]. Since stress-related genes are preferentially transcribed during stress, their highly abundant mRNA should naturally attract high amount of ribosomes to translate related proteins. In this case, the number of ribosomes engaged in translating stress-related proteins is guaranteed. Then it comes to the issue of ribosome speed. When growing at rich medium, the ribosome of E. coli cell translates protein at a high elongation rate (ER), 16–17 amino acids per second (aa/s) [18, 19]. At such a high ribosome speed, synthesis of a protein containing 1000 amino acid residues (e.g. LacZ protein) only requires ~ 1 min. However, ER of E. coli substantially decreases at certain stressful conditions [18–24]. Therefore, it is conceivable that slow-down of ribosome speed may negatively affect the timely synthesis of stress defense protein, which further limits the survival of bacteria at some adverse conditions. Taking nutrient starvation and oxidative stress as the examples, below we discuss the impact of translational elongation status on stress response in E. coli cells.
ER is maintained during nutrient deprivation
Bacteria seldom encounter conditions that support exponential growth in their natural niche. Even in laboratory conditions, the nutrients contained in the medium are rapidly used up by the exponentially growing bacteria, after which the cells enter into stationary phase. Therefore, nutrient deprivation (e.g. starved for carbon, ammonia or amino acid) is a frequent adverse condition faced by bacteria in their feast-or-famine life cycles [3, 4, 25, 26]. The RpoS regulon lies at the core of bacterial response to nutrient deprivation [2, 3, 6]. The RpoS-mediated general stress response includes the activation of a diverse set of proteins functioning in stress management, membrane stabilization, DNA repair, and central metabolism as well as cell morphology control [2, 3]. The RpoS regulon is activated during the onset of stationary phase (within ~ 1 h) to maintain long-term viability for E. coli cells [25]. Inhibiting the protein synthesis by chloramphenicol at initial stage of stationary phase cause rapid loss of cell viability [13]. Instead, the viability loss is much milder if protein translation is inhibited after cells have been in stationary phase for several hours [27]. Therefore, there is no doubt that the timely synthesis of stress responsive proteins of the RpoS regulon is crucial for bacterial survival during nutrient deprivation. So, what is the status of ribosome speed in this case?
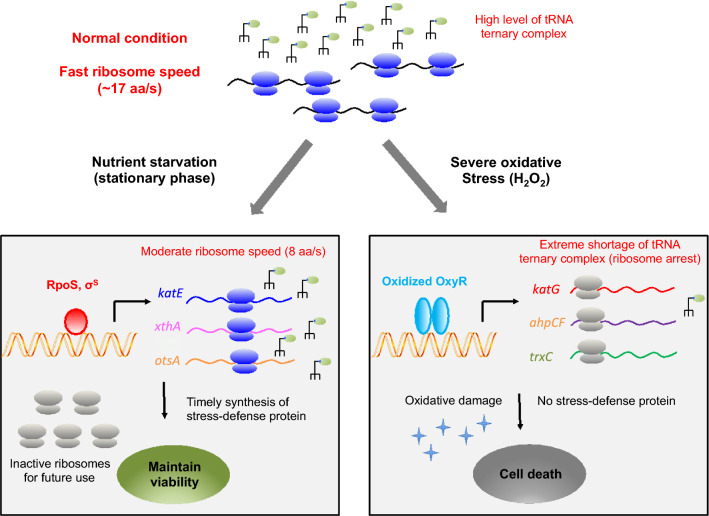
During exponential growth, the ER of E. coli cells is as high as 17 aa/s in rich medium (e.g. 20 min per doubling) and becomes slower at poor nutrient conditions. However, ER remains at a moderate value (9 aa/s) at extremely slow growth condition (1 day per doubling) [19, 20]. A moderate ER is still maintained (8 aa/s) even when growth is completely arrested during stationary phase or nutrient deprivation conditions including carbon deprivation, ammonium deprivation and amino acid starvation [19, 21, 22, 24]. The ER depends on the concentration of ternary complex (TC) under different nutrient conditions [18, 19]. The level of TC decreases, but is still significant under poor nutrient conditions and nutrient starvation conditions, enabling the maintenance of ER at those conditions [19, 28]. Overall, the maintenance of ER guarantees the timely synthesis of stress responsive proteins in RpoS regulon during nutrient deprivation, enabling long-term survival at later stages of the stationary phase (Fig. 1).
Fig. 1.
The effect of ribosome speed on stress defense response of Escherichia coli upon nutrient starvation and severe oxidative stress. At normal growth conditions, the abundance of cellular tRNA ternary complex is high, supporting a fast ribosome translational elongation rate (17 aa/s). Upon entry into stationary phase due to nutrient starvation provoked by carbon, phosphate, ammonia and amino acid limitation, E. coli still maintains a moderate translational elongation (8 aa/s). In this case, those RpoS-induced stress defense proteins such as KatE (catalase E), XthA (exonuclease III), OtsAB (trehalose synthesis proteins), could be timely synthesized to maintain long-term cell survival under starvation conditions. At the same time, cells store a large pool of inactive ribosomes for future use. The situation of H2O2-mediated oxidative stress is quite different. High H2O2 causes dramatic slow-down or complete stalling of ribosome elongation through triggering substantial tRNA degradation. In that case, although stress defense program of OxyR regulon can be induced at the transcriptional level, it is severely inhibited at the translational level. Therefore, failure in timely synthesizing oxidative defensive proteins causes cell death upon severe oxidative stress
The dramatic reduction of overall protein synthesis rate upon nutrient deprivation is mainly attributed to two factors: decreased ribosome content and ribosome inactivation [29, 30]. The drastic accumulation of guanosine tetra- and penta-phosphate, (p)ppGpp, mediated by RelA or SpoT (known as stringent response), strongly inhibits the rRNA synthesis during nutrient deprivation [29, 31, 32]. In such case, cells only manage to maintain a basal level of cellular ribosome pool. The decrease in ribosome content also occurs during slow exponential growth since (p)ppGpp level also increases under poor nutrient conditions [17, 33, 34]. Ribosome inactivation is another important contributor to the decrease in protein synthesis rate. The 70S ribosomes were dimerized into the inactive 100S forms during stationary phase provoked by nutrient starvation [30]. This process, known as ribosome hibernation, is mediated by three ribosome hibernation factors including ribosome modulation factor (RMF), hibernation promoting factor (HPF) and ribosome-associated inhibitor A (RaiA) [30]. The inactive 100S ribosome form can account for up to 60% of the total ribosome pool during stationary phase [35]. Marked ribosome inactivation also occurs during slow exponential growth [19, 20]. However, the underlying mechanism may differ from stationary phase since no accumulation of 100S ribosome was observed. Instead, polysome profiling has shown that the fraction of 70S ribosome monomer increases dramatically during slow growth, suggesting the inhibition of translation initiation [19, 20]. A plausible origin could be the inhibition of initiation factor 2 (IF2) by (p)ppGpp [36]. The phenomenon of ribosome inactivation may provide bacterial cells with important physiological and ecological benefits. During adverse conditions, bacterial cells maintain a pool of inactive ribosomes so that they can rapidly resume growth when the environmental condition becomes favorable [37]. The inactive 100S ribosome dissociates into active 70S monomer within 1–2 min of transferring starved-cells to fresh growth medium so that cells immediately use them for protein synthesis [35, 38].
Overall, as depicted in Fig. 1, the E. coli cells maintain a moderate translational elongation rate during nutrient deprivation so that they can timely synthesize related stress defense proteins for long-term survival. At the same time, they store a basal pool of inactive ribosomes for future use so that they can quickly resume growth during nutrient upshift.
ER is severely compromised by oxidative stress
Reactive oxygen species (ROS) mediated oxidative stress is a universal stressor for both prokaryotic cells and eukaryotic cells. It is also a common stressful condition faced by bacterial cells in their natural niches. For example, pathogens could encounter high levels of ROS produced by oxidative burst of activated immune cells during infections [39]. The OxyR regulon is the major stress response program activated by E. coli cells during hydrogen peroxide (H2O2) shock [10, 11]. H2O2 treatment could cause immediate arrest of cell growth through inactivating metabolic enzymes [11]. At low doses of H2O2, the OxyR regulon of E. coli and Salmonella typhimurium is strongly induced within 10–20 min [7, 40], synthesizing various kinds of oxidative protective proteins such as KatG (catalase G), AhpCF (peroxiredoxin/alkyl hydroperoxide reductase), TrxC (thioredoxin 2), GrxA (glutaredoxin A), to remove intracellular H2O2 and maintain redox homeostasis [10, 11]. Cells can then resume normal growth after the excess H2O2 has been removed and protein damages are repaired [23]. However, high levels of H2O2 are lethal to cells by causing irreversible damages to proteins and nucleic acids. For example, many proteins have their cysteine and methionine residues (thiol-containing proteins) be oxidized upon H2O2 shock, leading to substantial protein unfolding and inactivation [9]. It was recently found that E. coli K-12 cells could smoothly resume growth at 5 mM H2O2, but fail to tolerate a slightly high H2O2 level of 6 mM [23], posing the issue of what’s the intrinsic factor that limits the oxidative stress resistance of the bacterial cells? Systematic studies have shown that H2O2 severely inhibits the translational elongation process of E. coli cells through causing substantial degradation of cellular tRNA pools [23]. The ER of E. coli cells drops to as slow as ~ 1 aa/s during the initial stage of 5 mM H2O2 shock, for which the accumulation of OxyR-controlled proteins is significantly delayed compared with low doses of H2O2 shock [23]. Therefore, the cells undergo a lag phase before resuming normal growth. At the threshold level of H2O2 (6 mM), although the OxyR-mediated stress response was dramatically induced at the transcriptional level, it was completely abolished at the translational level due to complete stalling of ribosome movement (ER = 0 aa/s) [23]. In this case, cells fail to accumulate oxidative protective proteins and ultimately fail to tolerate the oxidative damage from high doses of H2O2 (Fig. 1). Instead, if the cells have been pre-adapted to a low dose of H2O2 to pre-accumulate enough stress defense proteins, they could tolerate a much higher lethal H2O2 [7, 23]. The tRNA-limited translational elongation process imposes a strong physiological burden on bacterial cells during counteraction of oxidative stress.
Outlook
Although the in vivo protein translational status of bacteria has been extensively studied in recent years, it is clear that we are still lacking a detailed picture of translational regulation of bacterial cells in various stressful conditions. Some important future directions are highlighted below:
Apply ribosome profiling to investigate details of translational elongation status and its regulation in bacteria during stress
Owing to the emergency of the revolutionary ribosome-profiling approach [41], we have seen tremendous progress on the protein translational status of cells in vivo during the last decade. Ribosome profiling is a high-resolution deep-sequencing-based tool that allows us to monitor every process of translation in vivo. Through mapping the ribosome occupancy on mRNA, it provides a snapshot of the instantaneous protein synthesis rate of each gene as well as the information of ribosome pause in vivo [42, 43]. In addition, the combination of ribosome profiling and mRNA-seq allows quantitative measurement of translational efficiency of individual genes [42, 44, 45]. The application of ribosome profiling has shed new light into the translational regulation of bacterial cells in vivo. For example, it is traditionally proposed that rare codon limits the translational elongation rate in vivo [46–48]. However, ribosome profiling has shown that ribosome pause mainly occurs at internal SD-like sequences instead of rare codon regions [49]. In another study, ribosome profiling was used to measure the protein synthesis rate of individual genes in bacterial operons such as the operon encoding the eight different subunits of F0F1-ATP synthase [44]. Although the mRNA levels of the eight subunits are very similar with each other, ribosome profiling shows that the individual ORFs that encode the subunits of the F0F1-ATP synthase operon are translated at a ratio of 1:1:1:1:2:3:3:10, which precisely reflects the stoichiometry of these components in the ATP synthase [44]. This observation reflects the importance of regulation of translational efficiency in bacterial cells.
Given the central role of protein synthesis in biomass growth, it is important to apply ribosome profiling to obtain a detailed picture of the in vivo translational status of bacteria during various kinds of stress. Ribosome profiling studies on yeast cells or animal cells have shown that three stressful conditions, including oxidative stress, heat shock, protein misfolding, all cause translation arrest at ~ 50 codons downstream of the translational start site, suggesting a common mode of translational regulation in vivo in eukaryotic cells under those stressful conditions [50–52]. However, the details of the ribosome slow-down in bacterial cells during stress remain largely unclear. For example, it is unclear whether ribosome just stalls in specific regions of mRNA or it is a uniform slow-down of elongation rate at each codon under oxidative stress. Similar issue applies to the case of nutrient starvation. It is expected that ribosome stalls at specific codons due to lack of related charged tRNA under amino acids starvation. However, the case is unclear under carbon starvation where translational elongation rate also drops by half compared with normal growth conditions [21, 24]. Another important issue is the variation of translational efficiency among different genes [45]. The combination of ribosome profiling and mRNA-seq could obtain the translational efficiency of each individual gene under stress. This information will shed light on strategies of translational regulation in bacterial cells during stress response and its relation with cell survival.
-
2.
The status of transcription-translation coordination of bacteria during stress
It is generally accepted that bacterial cells tightly maintain the coordination of transcription and translation process in vivo, as reflected by the same elongation speed of RNAP and ribosome in rich medium [22, 53]. Loss of transcription-translation coordination upon nonsense mutation or ribosome stalling could cause Rho-factor mediated premature transcriptional termination, affecting the integrity of gene expression in long operon [54–57]. It has been found that transcriptional-translation coordination in bacteria is still largely maintained under nutrient starvation conditions, in which (p)ppGpp assists in matching the speed of ribosome with RNAP [22, 58, 59]. However, it remains unclear regarding the status of transcription-translation coordination in bacterial cells during various kinds of other stress conditions. During oxidative stress, it has been shown that the expression of OxyR-regulon genes of E. coli is smoothly induced at the transcriptional level although the ribosome translation is slown down or stalled, suggesting the occurrence of transcription-translation dissociation [23]. In the future, it is important to deeply investigate the transcription–translation coordination during stress and elucidate how bacterial cells manage to integrate the control of transcription and translation during stress response.
-
3.
Does slow-down of ribosome also limit the stress response on eukaryotic cells?
Many stress conditions (e.g. oxidative stress, nutrient deprivation, heat shock, osmotic shock) are universal for both bacterial cells and eukaryotic cells. For example, oxidative stress causes transient growth arrest of both bacterial cells and eukaryotic cells, during which both types of cells could initiate stress response to fight against ROS [60]. In addition, previous studies have shown that substantial tRNA degradation is also a common phenomenon observed in eukaryotic cells during many kinds of stress including oxidative stress, heat shock and hypothermia [61]. Therefore, it is interesting to investigate the relation between ribosome translation and stress response in eukaryotic cells. For example, does slow-down of translation elongation also occur in eukaryotic cells during stress? Does ribosome slow-down also limit stress response and cell fitness of eukaryotic cells? Moreover, how does the translational efficiency of different genes vary during stress? Exploring those issues will help to elucidate the translational regulation strategy of eukaryotic cells in response to stress.
Concluding remarks
In summary, the maintenance of ribosome speed is crucial for bacteria to cope with stress. The ribosome speed is moderately maintained at nutrient deprivation, enabling the long-term survival of bacteria. However, ribosome speed is severely compromised under oxidative stress, strongly affecting the growth fitness of bacteria during stress. Therefore, translational status should be investigated in parallel with transcriptional analysis for a more comprehensive understanding of the stress response of bacteria. In the future, it is important to investigate the molecular details of ribosome slow-down in vivo and the strategies of translational regulation of bacteria during stress. Moreover, it is conceivable to explore whether similar notion could be applied to eukaryotic cells, such as yeasts and animal cells during stress.
Acknowledgements
This work was supported by the National Natural Science Fund of China (No. 31700089, No. 31970027, No. 31700039 and No. 31870028) and by self-determined research funds of CCNU from the colleges’ basic research and operation of MOE (CCNU18QN028, CCNU18KFY01, CCNU19TS028 and CCNU18ZDPY05).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Manlu Zhu, Email: zhumanlu@mail.ccnu.edu.cn.
Xiongfeng Dai, Email: daixiongfeng@mail.ccnu.edu.cn.
References
- 1.Ron EZ. Bacterial stress response. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The pro-karyotes. New York: Springer; 2006. pp. 1012–1027. [Google Scholar]
- 2.Battesti A, Majdalani N, Gottesman S. The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol. 2011;65:189–213. doi: 10.1146/annurev-micro-090110-102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henggearonis R. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell. 1993;72(2):165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- 4.Hengge-Aronis R. The Role of rpoS in early stationary-phase gene regulation in Escherichia coli K12. In: Kjelleberg S, editor. Starvation in bacteria. Boston: Springer; 1993. pp. 171–200. [Google Scholar]
- 5.Guisbert E, Yura T, Rhodius VA, Gross CA. Convergence of molecular, modeling, and systems approaches for an understanding of the Escherichia coli heat shock response. Microbiol Mol Biol Rev. 2008;72(3):545–554. doi: 10.1128/MMBR.00007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornforth DM, Foster KR. Competition sensing: the social side of bacterial stress responses. Nat Rev Microbiol. 2013;11(4):285–293. doi: 10.1038/nrmicro2977. [DOI] [PubMed] [Google Scholar]
- 7.Christman MF, Morgan RW, Jacobson FS, Ames BN. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg JT, Monach P, Chou JH, Josephy PD, Demple B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci USA. 1990;87(16):6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezraty B, Gennaris A, Barras F, Collet JF. Oxidative stress, protein damage and repair in bacteria. Nat Rev Microbiol. 2017;15(7):385. doi: 10.1038/nrmicro.2017.26. [DOI] [PubMed] [Google Scholar]
- 10.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11(7):443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imlay JA. Transcription factors that defend bacteria against reactive oxygen species. Annu Rev Microbiol. 2015;69(1):93–108. doi: 10.1146/annurev-micro-091014-104322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moll I, Engelberg-Kulka H. Selective translation during stress in Escherichia coli. Trends Biochem Sci. 2012;37(11):493–498. doi: 10.1016/j.tibs.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeve CA, Amy PS, Matin A. Role of protein synthesis in the survival of carbon-starved Escherichia coli K-12. J Bacteriol. 1984;160(3):1041–1046. doi: 10.1128/jb.160.3.1041-1046.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klumpp S, Scott M, Pedersen S, Hwa T. Molecular crowding limits translation and cell growth. Proc Natl Acad Sci USA. 2013;110(42):16754–16759. doi: 10.1073/pnas.1310377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basan M, et al. Inflating bacterial cells by increased protein synthesis. Mol Syst Biol. 2015;11(10):836. doi: 10.15252/msb.20156178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu M, Dai X. On the intrinsic constraint of bacterial growth rate: M. tuberculosis’s view of the protein translation capacity. Crit Rev Microbiol. 2018;44(4):455–464. doi: 10.1080/1040841X.2018.1425672. [DOI] [PubMed] [Google Scholar]
- 17.Bremer H, Dennis PP. Modulation of chemical composition and other parameters of the cell at different exponential growth rates. In: Neidhardt FC, editor. Escherichia coli and Salmonella. 2. Washington: American Society for Microbiology; 1996. pp. 1553–1569. [Google Scholar]
- 18.Dai X, et al. Slowdown of translational elongation in Escherichia coli under hyperosmotic stress. mBio. 2018;9(1):02375-18. doi: 10.1128/mBio.02375-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai X, et al. Reduction of translating ribosomes enables Escherichia coli to maintain elongation rates during slow growth. Nat Microbiol. 2016;2:16231. doi: 10.1038/nmicrobiol.2016.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li SH, et al. Escherichia coli translation strategies differ across carbon, nitrogen and phosphorus limitation conditions. Nat Microbiol. 2018;3(8):939–947. doi: 10.1038/s41564-018-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iyer S, Le D, Park BR, Kim M. Distinct mechanisms coordinate transcription and translation under carbon and nitrogen starvation in Escherichia coli. Nat Microbiol. 2018;3(6):741. doi: 10.1038/s41564-018-0161-3. [DOI] [PubMed] [Google Scholar]
- 22.Vogel U, Sorensen M, Pedersen S, Jensen KF, Kilstrup M. Decreasing transcription elongation rate in Escherichia coli exposed to amino acid starvation. Mol Microbiol. 1992;6(15):2191–2200. doi: 10.1111/j.1365-2958.1992.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhu M, Dai X. Maintenance of translational elongation rate underlies the survival of Escherichia coli during oxidative stress. Nucleic Acids Res gkz467. 2019;47(14):7592–7604. doi: 10.1093/nar/gkz467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu M, Dai X, Wang YP. Real time determination of bacterial in vivo ribosome translation elongation speed based on LacZα complementation system. Nucleic Acids Res. 2016;44(20):e155. doi: 10.1093/nar/gkw698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolter R, Siegele DA, Tormo A. The stationary phase of bacteiral life cycles. Annu Rev Microbiol. 1993;47(1):855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 26.Nyström T. Stationary-phase physiology. Annu Rev Microbiol. 2004;58(1):161–181. doi: 10.1146/annurev.micro.58.030603.123818. [DOI] [PubMed] [Google Scholar]
- 27.Nystrom T, Flardh K, Kjelleberg S. Responses to multiple-nutrient starvation in marine Vibrio sp. strain CCUG 15956. J Bacteriol. 1990;172(12):7085–7097. doi: 10.1128/jb.172.12.7085-7097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svenningsen SL, Kongstad M, Stenum TS, Muñoz-Gómez AJ, Sørensen MA. Transfer RNA is highly unstable during early amino acid starvation in Escherichia coli. Nucleic Acids Res. 2017;45(2):793. doi: 10.1093/nar/gkw1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 30.Prossliner T, Skovbo Winther K, Sorensen MA, Gerdes K. Ribosome Hibernation. Annu Rev Genet. 2018;52:321–348. doi: 10.1146/annurev-genet-120215-035130. [DOI] [PubMed] [Google Scholar]
- 31.Baracchini E, Bremer H. Stringent and growth control of rRNA synthesis in Escherichia coli are both mediated by ppGpp. J Biol Chem. 1988;263(6):2597–2602. [PubMed] [Google Scholar]
- 32.Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol. 2015;13(5):298–309. doi: 10.1038/nrmicro3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu M, Dai X. Growth suppression by altered (p)ppGpp level results from non-optimal resource allocation in Escherichia coli. Nucleic Acids Res gkz 211. 2019;47(9):4684–4693. doi: 10.1093/nar/gkz211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu M, Pan Y, Dai X. (p)ppGpp: the magic governor of bacterial growth economy. Curr Genet. 2019;65(5):1121–1125. doi: 10.1007/s00294-019-00973-z. [DOI] [PubMed] [Google Scholar]
- 35.Wada A. Growth phase coupled modulation of Escherichia coli ribosomes. Genes Cells. 1998;3(4):203–208. doi: 10.1046/j.1365-2443.1998.00187.x. [DOI] [PubMed] [Google Scholar]
- 36.Milon P, et al. The nucleotide-binding site of bacterial translation initiation factor 2 (IF2) as a metabolic sensor. Proc Natl Acad Sci USA. 2006;103(38):13962–13967. doi: 10.1073/pnas.0606384103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori M, Schink S, Erickson DW, Gerland U, Hwa T. Quantifying the benefit of a proteome reserve in fluctuating environments. Nat Commun. 2017;8(1):1225. doi: 10.1038/s41467-017-01242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aiso T, Yoshida H, Wada A, Ohki R. Modulation of mRNA stability participates in stationary-phase-specific expression of ribosome modulation factor. J Bacteriol. 2005;187(6):1951–1958. doi: 10.1128/JB.187.6.1951-1958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burton NA, et al. Disparate impact of oxidative host defenses determines the fate of Salmonella during systemic infection in mice. Cell Host Microbe. 2014;15(1):72–83. doi: 10.1016/j.chom.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Zheng M, et al. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol. 2001;183(15):4562–4570. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324(5924):218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brar GA, Weissman JS. Ribosome profiling reveals the what, when, where and how of protein synthesis. Nat Rev Mol Cell Biol. 2015;16(11):651–664. doi: 10.1038/nrm4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ingolia NT. Ribosome profiling: new views of translation, from single codons to genome scale. Nat Rev Genet. 2014;15(3):205–213. doi: 10.1038/nrg3645. [DOI] [PubMed] [Google Scholar]
- 44.Li GW, Burkhardt D, Gross C, Weissman JS. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell. 2014;157(3):624–635. doi: 10.1016/j.cell.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li GW. How do bacteria tune translation efficiency? Curr Opin Microbiol. 2015;24:66–71. doi: 10.1016/j.mib.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedersen S. Escherichia coli ribosomes translate in vivo with variable rate. EMBO J. 1984;3(12):2895–2898. doi: 10.1002/j.1460-2075.1984.tb02227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sorensen MA, Kurland CG, Pedersen S. Codon usage determines translation rate in Escherichia coli. J Mol Biol. 1989;207(2):365–377. doi: 10.1016/0022-2836(89)90260-x. [DOI] [PubMed] [Google Scholar]
- 48.Sorensen MA, Pedersen S. Absolute in vivo translation rates of individual codons in Escherichia coli. The two glutamic acid codons GAA and GAG are translated with a threefold difference in rate. J Mol Biol. 1991;222(2):265–280. doi: 10.1016/0022-2836(91)90211-n. [DOI] [PubMed] [Google Scholar]
- 49.Li GW, Oh E, Weissman JS. The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature. 2012;484(7395):538–541. doi: 10.1038/nature10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerashchenko MV, Lobanov AV, Gladyshev VN. Genome-wide ribosome profiling reveals complex translational regulation in response to oxidative stress. Proc Natl Acad Sci USA. 2012;109(43):17394–17399. doi: 10.1073/pnas.1120799109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu B, Han Y, Qian SB. Cotranslational response to proteotoxic stress by elongation pausing of ribosomes. Mol Cell. 2013;49(3):453–463. doi: 10.1016/j.molcel.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shalgi R, et al. Widespread regulation of translation by elongation pausing in heat shock. Mol Cell. 2013;49(3):439–452. doi: 10.1016/j.molcel.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Proshkin S, Rahmouni AR, Mironov A, Nudler E. Cooperation between translating ribosomes and RNA polymerase in transcription elongation. Science. 2010;328(5977):504–508. doi: 10.1126/science.1184939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newton WA, Beckwith JR, Zipser D, Brenner S. Nonsense mutants and polarity in the lac operon of Escherichia coli. J Mol Biol. 1965;14(1):290–296. doi: 10.1016/s0022-2836(65)80250-9. [DOI] [PubMed] [Google Scholar]
- 55.Adhya S, Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- 56.Elgamal S, Artsimovitch I, Ibba M. Maintenance of transcription–translation coupling by elongation factor P. mBio. 2016;7(5):01373-16. doi: 10.1128/mBio.01373-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu M, Mori M, Hwa T, Dai X. Disruption of transcription-translation coordination in Escherichia coli leads to premature transcriptional termination. Nat Microbiol. 2019 doi: 10.1038/s41564-019-0543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iyer S, Le D, Park BR, Kim M. Distinct mechanisms coordinate transcription and translation under carbon and nitrogen starvation in Escherichia coli. Nat Microbiol. 2018;3(6):741. doi: 10.1038/s41564-018-0161-3. [DOI] [PubMed] [Google Scholar]
- 59.Vogel U, Jensen KF. Effects of guanosine 3′,5′-bisdiphosphate (ppGpp) on rate of transcription elongation in isoleucine-starved Escherichia coli. J Biol Chem. 1994;269(23):16236–16241. [PubMed] [Google Scholar]
- 60.Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2010;50(4–5):279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 61.Nawrot B, Sochacka E, Düchler M. tRNA structural and functional changes induced by oxidative stress. Cell Mol Life Sci. 2011;68(24):4023–4032. doi: 10.1007/s00018-011-0773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]