Abstract
The self-renewal capacity of the stem cell pool determines tissue function and health. Cadherin-22 (Cdh22), a member of the cadherin superfamily, has two splicing patterns in rats, and the short type that lacks a catenin binding domain is closely related to spermatogonial stem cell self-renewal. Previously, we reported that CDH22 was highly expressed in mouse ovary germ cells, especially in female germ line stem cells (FGSCs). However, its underlying function in FGSCs is still not clear. Here, we found that Cdh22 encodes only one type of protein product in mice and demonstrated that CDH22 was required for FGSC self-renewal. In addition, JAK2 and β-catenin were found to interact with CDH22 and be involved in CDH22 signaling in mouse FGSCs. Moreover, extrinsic CDH22 was identified as a potential molecule that participates in FGSC adhesion and is pivotal for FGSC maintenance and self-renewal. These results reveal that CDH22 functions as an essential molecule in FGSC maintenance and self-renewal via different mechanisms, including interaction with the JAK-STAT signaling pathway and β-catenin.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-017-2689-4) contains supplementary material, which is available to authorized users.
Keywords: Apoptosis, Alternative splicing, Cell proliferation, Co-culture, Molecular interaction, Niche
Introduction
Ovarian aging is a chronological process manifested by an increasing incidence of health complications commonly associated with post-menopausal life, including obesity, loss of bone strength and neurological defects. Maintenance of ovarian functions in aging females is closely correlated with germ cell health in the ovary. The central dogma in the field of female reproductive biology is that the postnatal mammalian ovary cannot supply an infinite and replenishable pool of germ cells [1–4]. All germ cells in the ovary after birth are arrested in meiosis I and form follicle structures enclosed by somatic cells [5–8]. However, in the past decade, several groups have challenged this prevailing dogma with progressive achievements, such as count re-establishment of the primordial follicle pool in mice [9, 10]; isolation of FGSCs from many species [11–14], including humans [15]; and identification of the physiological activity of FGSCs in mouse ovaries [16]. Therefore, for the first time, there is evidence that FGSCs in the ovary exist as a counterpart germ line stem cell population, such as the spermatogonial stem cell (SSC) population in testes, and are able to produce gametes; many ovarian diseases are probably closely correlated to FGSCs. Thus, FGSCs have been a model of choice to study the consequences of age-related ovarian disease in vitro. However, the evidence of FGSCs in postnatal mammalian ovaries has caused some debate [17–20], primarily because the mechanisms of self-renewal and differentiation of FGSCs are poorly understood. On the other hand, increasing progress in FGSC research has been reported [21–23]. Accordingly, the isolation technique used to obtain FGSCs from the cortex of the mouse ovary has been used to isolate FGSCs from humans [14, 15, 23–33], which unequivocally supports the challenge to the reproductive biology dogma that postnatal mammals lack germ line stem cells. Subsequently, FGSCs cultured in vitro were able to develop meiotic markers and form oocyte-like cells [34, 35] and could also replenish the follicle pool and develop to live pups after transplantation into an infertile ovary and fertilization [24, 26, 29, 33]. These findings add strong evidence that adult mammalian ovaries are capable of primordial follicle pool renewal. However, to definitively understand the biological functions of FGSCs in the postnatal mammalian ovary, it is still indispensable to reveal the exact molecular mechanism of FGSC self-renewal.
Cadherins are widely expressed in germ cells and stem cells, and are involved in adhesion and signal transduction [36–38]. As a member of this superfamily, Cdh22 was first discovered in the rat pituitary gland and brain and was identified with alternative splicing isoforms: the long type and short type [39]. The cytosolic portion of long-type CDH22 includes the catenin-binding domain, whereas this domain is deficient in the short type, which is unique among cadherin members. Subsequently, short-type CDH22 has been shown to promote SSC survival in rats through the JAK–STAT, PI3K–Akt and TGF-beta signaling pathways [40–42]. Thus, the two alternative isoforms of Cdh22 may be involved in separate processes during embryonic development, and short-type CDH22 may play a pivotal role in SSC self-renewal. Moreover, we previously detected CDH22 expression in all stages of germ cells in mouse ovaries, including FGSCs [25]. However, its functions and potential mechanisms, i.e., how CDH22 mediates cell adhesion, remain largely unknown.
In this study, we analyzed the expression of CDH22 in mice and observed that Cdh22 in mice encodes only one type of functional protein product, suggesting that Cdh22 in mice probably possesses distinct functions and mechanisms. Then, we interfered with CDH22 expression in an FGSC line and revealed that CDH22 is involved in FGSC self-renewal by interacting with the JAK–STAT and β-catenin signaling pathways. The effect of extrinsic CDH22 on FGSC self-renewal examined via a co-culture system also suggested that CDH22 is an extrinsic adhesion molecule that regulates the fates of FGSCs. This study provides a new mechanism of FGSC self-renewal mediated by the transmembrane molecule CDH22.
Materials and methods
Animals
CD-1 mice were supplied by the Comparative Medicine Centre of Yangzhou University. All the procedures for animal experiments were approved by the ethical committee at Nanjing Agricultural University.
Isolation and culture of FGSCs
FGSCs were isolated from 5 dpp mouse ovaries and sorted using MACS following a previously reported protocol [25]. For FGSC sorting, antibody against Fragilis (Thermo, PA5-34598) and goat IgG-coated magnetic beads (BD, Cat.551518) were used. FGSCs were cultured on mitosis-inactivated STO or L929 feeder layers. To make the feeder layers, STO or L929 cells were incubated with medium containing 10 µg/ml mitomycin C (Roche, cat. 101074090001) at 37 °C with 5% CO2 for 2 h and plated on gelatin-coated plates.
RNA extraction and RT-PCR
Ovaries or FGSCs were treated with TRNzol (Tiangen, DP405) for RNA extraction, and RNA was reverse-transcribed to cDNA using a GoScript™ Reverse Transcription System (Progema, A5001). The primer information is listed in Table S1.
BrdU incorporation, immunofluorescence and immunohistochemistry
Immunofluorescence in FGSCs was performed as described previously [14]. For BrdU incorporation assay, FGSCs sorted by Fragilis-beads MACS were maintained in vitro for 3–4 passages, and most of oocytes were removed during change medium. To prevent loss of FGSCs after HCl treatment, FGSCs were transferred to poly-lysine-coated dishes, and were incubated with BrdU (50 µg/ml)-containing medium for 2 h. Subsequently, FGSCs were treated with 2 M HCl to expose DNA, and then, the HCl was neutralized with 0.1 M boric acid (pH 8.5) before serum blocking and incubation with primary antibodies. For immunohistochemistry, mouse ovaries were fixed in 4% neutral paraformaldehyde and dehydrated and embedded in paraffin. Histological sections were dewaxed and rehydrated in an ethanol series, followed by microwave antigen retrieval in 0.01 M citrate (pH 6.0) and methanol/H2O2 treatment. After blocking with 5% goat serum, the slides were sequentially incubated with primary and biotin-labeled secondary antibodies. Streptavidin-HRP (Jackson Lab, 1:500) and a DAB kit (Vector, sk4100) were used for visualization (see antibodies information in Table S2).
Transfection
siRNA was synthesized by Genapharma company, and Cyclin D1 overexpression plasmid was purchased from GeneCopoeia (EX-Mm14092-M02). The Cdh22 open reading frame (ORF) sequence (Table S4) was cloned into a pCDNA3-Flag plasmid for overexpression. Cells were transfected with plasmids or siRNA using lipofectamine 3000 (Life Technologies).
siRNA sequences:
Cdh22-Mus-961 GGACAUCAACGACAGUGAATT UUCACUGUCGUUGAUGUCCTT.
mouse Ccnd1 CCCACGAUUUCAUCGAACATT UGUUCGAUGAAAUCGUGGGTT.
mouse β-catenin GCGCUUGGCUGAACCAUCATT UGAUGGUUCAGCCAAGCGCTT.
Lentivirus packaging and infection
The lentivirus packaging system for shRNA infection (pMD2.G, pCMVR8.91, and pLVTHM-shRNA) was a gift from Prof. Zijie Sun (School of Medicine, Stanford University). The validated Cdh22 shRNA oligos (Table S3) were synthesized by Shanghai Generay Company. The lentivirus package system for overexpression was ViraPower™ Lentiviral Packaging system (Life Technologies). 293T cells were transfected with plasmids using Lipofectamine 3000 (Life Technologies), and lentivirus in the supernatant was harvested 72 h later and concentrated with a GML-PCTM kit (Genomeditech Shanghai, GM-040801-15). For infection, FGSCs were seeded onto a 24-well culture plate containing a mitotically inactivated STO feeder layer. Then, 0.5 × 105 cells were infected with lentivirus using 6 µg/ml polybrene the next day according to the standard procedure. The cells were incubated with lentivirus-containing medium for 8–12 h and were then cultured in normal FGSC culture medium.
Western blotting and co-immunoprecipitation (Co-IP)
For western blotting, the routine protocol was used. For detection of phosphorylation levels, skimmed milk was replaced with bovine serum albumin (Amresco) for blocking and antibody dilution. See antibody information in Table S2.
Immunoprecipitation assays—FGSCs (transfected with a FLAG tag expression vector, if necessary) or ovary tissue were resuspended in lysis buffer supplemented with protease inhibitors (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100). The lysates were pre-cleared with protein A+G agarose beads (Beyotime Biotechnology, P2012), and the supernatant was incubated overnight with an anti-Jak2 or anti-β-catenin antibody on a rotating platform at 4 °C and then incubated with protein A + G agarose beads. The beads were collected, washed, and resuspended in equal volumes of 5× SDS loading buffer. The immunoprecipitation fractions were separated using 8% SDS-PAGE. A western blotting assay was performed as described.
Statistical analysis
For cell counting, sections or immunofluorescence visual fields were selected randomly. For FGSC counting in bright field, the cells with a size ranging from 10 to 15 µm were counted. The data were analyzed with Excel and are presented as the mean ± SD (standard deviation). Statistical significance was determined with a t test.
Results
Mouse FGSCs highly express CDH22
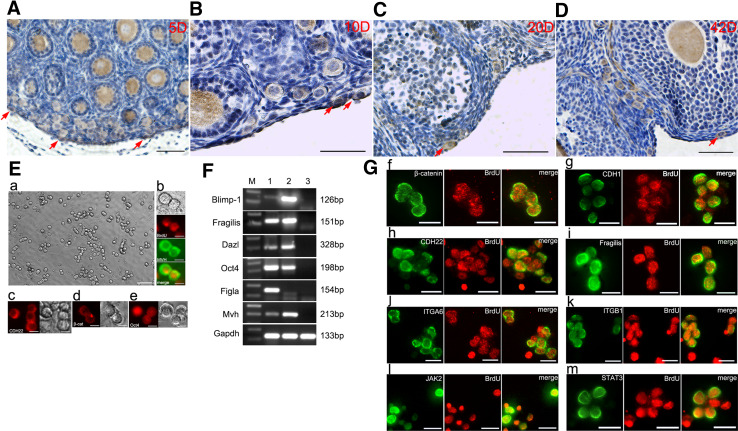
To investigate the CDH22 expression pattern in FGSCs, sections of 5-, 10-, 20-, and 42-day mouse ovaries were analyzed using IHC. Briefly, CDH22 staining was observed in the germ cells at all stages, including in FGSCs and in oocytes in the primordial follicle, primary follicle, secondary follicle, and preantral follicles (Fig. 1A–D), suggesting that CDH22 is a germ line marker. However, we also frequently observed regions of the preantral follicles that contained a weak CDH22 signal (Fig. 1A–D), which demonstrated that CDH22 expression was not restricted to germ cells but was also present in neighboring somatic cells. Based on these observations, we hypothesize that CDH22 is a functional molecule in the ovary microenvironment. Subsequently, we detected the expression of CDH22 in FGSCs cultured in vitro. FGSCs isolated from neonatal mouse ovaries were enriched using MACS, maintained in vitro (Fig. 1E-a), and identified by BrdU/MVH (BrdU is an analog of thymine; MVH is a ubiquitous germ cell marker) dual immunostaining (Fig. 1E-b). Immunofluorescence further demonstrated that FGSCs still highly expressed CDH22 (Fig. 1E-c), β-catenin (Fig. 1E-d) and Oct4 (Fig. 1E-e). Moreover, we evaluated the expression of germ line markers (the early germ cell markers Blimp-1 and Fragilis, the germ cell markers Dazl and Mvh, the undifferentiated cell marker Oct4, and the oocyte marker Filga) at the mRNA level (Fig. 1F) and the expression of a series of known stem cell and germ cell markers combined with BrdU dual immunofluorescence in FGSCs (Fig. 1G), including β-catenin (Fig. 1G-f), E-cadherin (Fig. 1G-g), CDH22 (Fig. 1G-h), Fragilis (Fig. 1G-i), ITGA6 (Fig. 1G-j), ITGB1 (Fig. 1G-k), JAK2 (Fig. 1G-l) and STAT3 (Fig. 1G-m). The results indicate that FGSCs are stably maintained in vitro, and CDH22 is highly expressed in FGSCs.
Fig. 1.
Expression of CDH22 in developmental ovaries and in FGSCs. Examination of CDH22 expression (brown) in 5-day (A), 10-day (B), 20-day (C) and 42-day (D) ovaries using IHC. Red arrows indicate FGSCs in the cortex. Bar = 50 µm. The morphology of FGSCs after ten passages maintained on STO feeders (E-a). FGSCs were identified with BrdU/MVH dual immunostaining (E-b). Immunofluorescence was used to detect the expression of CDH22 (E-c), β-catenin (E-d) and Oct4 (E-e) in FGSCs in vitro. The expression of germ line markers in FGSCs was evaluated with RT-PCR; 1, 2, and 3 represent cDNA from the ovary, FGSCs and feeders, respectively (F), and the expression of a series of known stem cell and germ cell markers was examined in FGSCs, combined with BrdU immunofluorescence (G, red), including β-catenin (G-f), E-cadherin (G-g), CDH22 (G-h), Fragilis (G-i), ITGA6 (G-j), ITGB1 (G-k), JAK2 (G-l) and STAT3 (G-m) (green). Bar = 20 µm
Mouse Cdh22 encodes only one type of protein product in FGSCs
Before studying the role and mechanism of CDH22 in FGSCs, we determined how many protein products are encoded by Cdh22 in mice. Bioinformatics tools predicted that only one type of protein was encoded by Cdh22 in mice (http://www.ensembl.org/Mus_musculus/Transcript/Summary?db=core;g=ENSMUSG00000053166;r=2:165111507-165234853;t=ENSMUST00000065438), (http://genome-asia.ucsc.edu/cgi-bin/hgTracks?db=mm10&lastVirtModeType=default&lastVirtModeExtraState=&virtModeType=default&virtMode=0&nonVirtPosition=&position=chr2%3A165091889%2D165338350&hgsid=471636369_r9e7daJAWPDAgLDOdtq9aAPR9Ded), which is consistent with a previous report [43]. This is distinctly different from that in rats and led us to ask whether CDH22 in mice functions via a different mechanism.
Disturbance of Cdh22 expression in FGSCs suppresses their proliferation and induces apoptosis
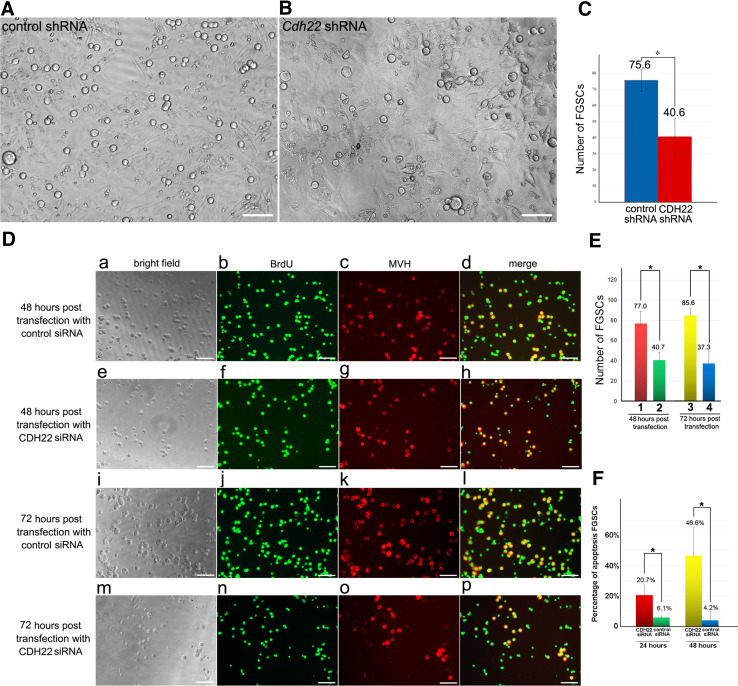
To determine the role of CDH22 in FGSCs, we interfered with the expression of CDH22 in FGSCs using a lentiviral shRNA knockdown system, which has been used in NIH-3T3 cells (Fig. S1 B–H). Due to the current lack of definitive FGSC markers, examination of BrdU+/MVH+ dual immunostaining and the expression of PCNA (a marker of cell replication) are convincing methods to discriminate between FGSCs and non-proliferative oocytes. Six days after the lentivirus infection, we observed a reduced number of FGSCs in the CDH22 knockdown group compared with the control group (Fig. 2A–B). Then, we counted the number of BrdU+/MVH+ cells (representing FGSCs) at 48 and 72 h post CDH22 siRNA transfection and observed that many BrdU+/MVH+ cells were significantly impacted by CDH22 siRNA (Fig. 2D). After 48-h treatment with CDH22 siRNA (Fig. 2D e–h, E), the number of BrdU+/MVH+ cells was reduced to 52.8% of those in the control group (Fig. 2D a–d, E). In the 72-h CDH22 siRNA treatment groups, the number of BrdU+/MVH+ cells (Fig. 2D m–p, E) was further decreased by a small amount compared with those in the 48-h treatment group (Fig. 2D e–h, E), indicating a continuous loss of FGSCs. On the other hand, the number of BrdU+/MVH+ cells in the 72-h treatment control group increased compared with that in the 48-h control group (Fig. 2D i–l, E), suggesting unimpaired proliferation in these control groups. Moreover, TUNEL staining was analyzed at 24 and 48 h post siRNA transfection to determine whether apoptosis is the reason for the reduced number of FGSCs at later time points. Approximately 20% apoptosis was observed in FGSCs at 24 h post CDH22 siRNA transfection, and the percentage increased to more than 40% at 48 h (Fig. 2F and Fig. S2), which indicates that the reduced number of FGSCs after CDH22 knockdown was due at least in part to increased apoptosis. These results suggest that CDH22 on the surface of FGSCs has a vital role in FGSC maintenance and self-renewal, and suppression of apoptosis.
Fig. 2.
Knockdown of CDH22 in FGSCs suppressed FGSC proliferation and induced apoptosis. The number of FGSCs manifestly decreased 6 days post Cdh22 shRNA lentivirus infection (B), compared with the control group (A). Statistical analysis of FGSC number in the Cdh22 shRNA or control shRNA lentivirus-infected group 6 days post infection (C). BrdU/MVH dual IF assays revealed that knockdown of Cdh22 led to a significant decrease in BrdU+/MVH+ cell number (D). After 48-h treatment with Cdh22 siRNA (D, e–h) or control siRNA (D, a–d), the morphology, BrdU staining, MVH staining and merged of BrdU and MVH staining of FGSCs were examined. In the 72-h treatment groups, morphology and BrdU/MVH staining after Cdh22 siRNA treatment (D, m–p) or their controls (D, i–l) were examined. Statistical analysis compared the number of BrdU+/MVH+ cells in each 200-fold insight view after Cdh22 siRNA transfection for 48 and 72 h; 1 and 3, control siRNA; 2 and 4 Cdh22 siRNA (E). The statistical analysis of TUNEL staining demonstrated alterations in apoptosis percentage at 24 and 48 h post Cdh22 siRNA transfection (F). The data represent the mean ± SD (*p < 0.05, **p < 0.01), Bar = 50 μm
Blockage of the JAK–STAT signaling pathway inhibits the self-renewal of mouse FGSCs
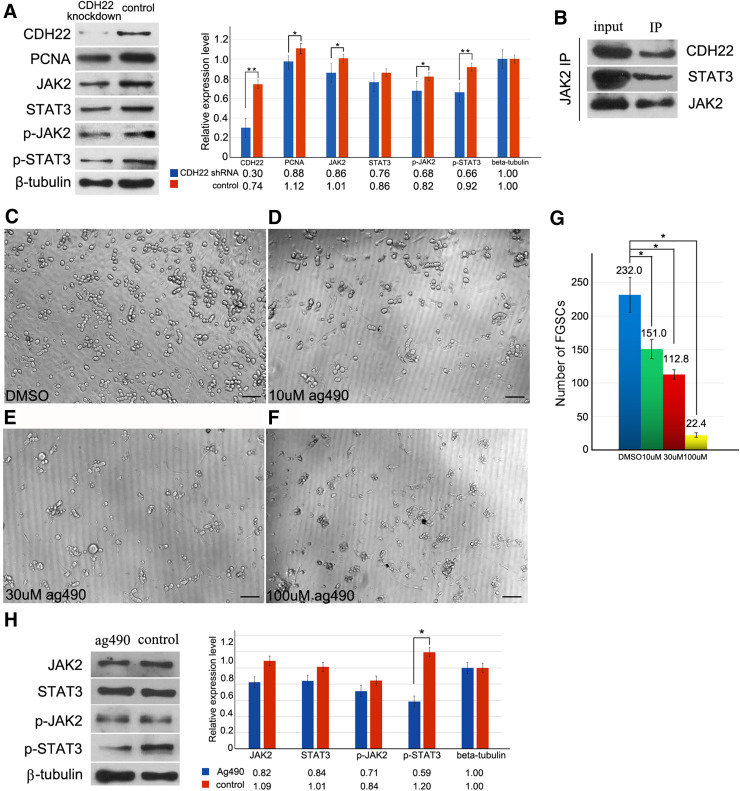
To reveal whether CDH22 links to the JAK–STAT signaling pathway as it does in rat SSCs, the expression levels and phosphorylation levels of JAK2 and STAT3 at the Tyr1007/1008 and Tyr705 sites [41, 44] were, respectively determined after CDH22 knockdown. We observed that knockdown of CDH22 caused a limited decrease in the endogenous expression levels of JAK2 and STAT3 in FGSCs but manifestly reduced the phosphorylation levels of p-JAK2 and p-STAT3 (Fig. 3a), suggesting that CDH22 was closely related to the phosphorylation of JAK2 and STAT3. Consistently, the expression level of PCNA, a downstream gene of STAT3, was down-regulated after disturbance of CDH22 expression (Fig. 3a). Then, we demonstrated that CDH22 was able to directly interact with JAK2 using an immunoprecipitation assay (Fig. 3b). These observations indicate that CDH22 interacts with the JAK–STAT signaling pathway in a manner similar to that observed in rat SSCs. To further confirm the role of JAK–STAT in FGSCs, we blocked the JAK–STAT signaling pathway to analyze FGSC self-renewal by suppressing JAK2 with the JAK inhibitor AG490. In contrast to the control group (Fig. 3c), FGSCs treated with 10 µM AG490 partially lost proliferation activity, and only a few of the chain-like FGSCs were maintained (Fig. 3d), indicating that proliferation was repressed in the majority of FGSCs. The statistical results revealed that after 10 µM AG490 treatment for 72 h, approximately 35% of FGSCs were lost compared to the control group (Fig. 3g), suggesting that disruption of JAK–STAT signaling impaired FGSC maintenance. In the 30 µM AG490 group, the FGSC number decreased to approximately 48% of that in the control group (Fig. 3g), and mitosis phase FGSCs and chain-like FGSCs were rarely observed (Fig. 3e). Moreover, 100 µM AG490 severely impaired FGSC maintenance (Fig. 3f), and the number of FGSCs in the 100 µM AG490 group after 72 h of treatment was sharply decreased in comparison with that in the DMSO group. Consistently, DMSO-treated FGSCs were maintained normally on feeder cells (Fig. 3c). Treatment with 10 µM AG490 blocked FGSC proliferation, and thus, the impact on the expression levels and phosphorylation levels of JAK2 and STAT3 in FGSCs treated with 10 µM AG490 was examined to analyze the molecular mechanism. We observed moderately reduced expression of JAK2 and STAT3 and a slightly decreased phosphorylation level at Tyr1007/1008 of JAK2 but a significantly decreased phosphorylation level at Tyr705 of STAT3 compared with the control cells (Fig. 3h). These results indicate that JAK–STAT signaling is required for FGSC self-renewal.
Fig. 3.
Blockage of the JAK–STAT signaling pathway suppressed FGSC proliferation. Western blotting results revealed that knockdown of Cdh22 induced a slight down-regulation of JAK2 and STAT3 in FGSCs but an obvious decrease in phosphorylation levels of p-JAK2 and p-STAT3 (a). Immunoprecipitation was used to examine the binding of CDH22 and JAK2 in FGSCs (b). The morphology of FGSCs treated with vehicle (c) or the JAK2 inhibitor AG490 at concentrations of 10 µM (d), 30 µM (e), and 100 µM (f) for 72 h and the statistical analysis of FGSC number in each of the groups (g). Treatment with 10 µM AG490 impacted the expression levels and phosphorylation levels of JAK2 and STAT3 in FGSCs, in which a significantly decreased phosphorylation level at Tyr705 of STAT3 was observed compared with the control (Fig. 4H). The data represent the mean ± SD (*p < 0.05). Bar = 50 μm
Interaction of CDH22 and β-catenin in FGSCs
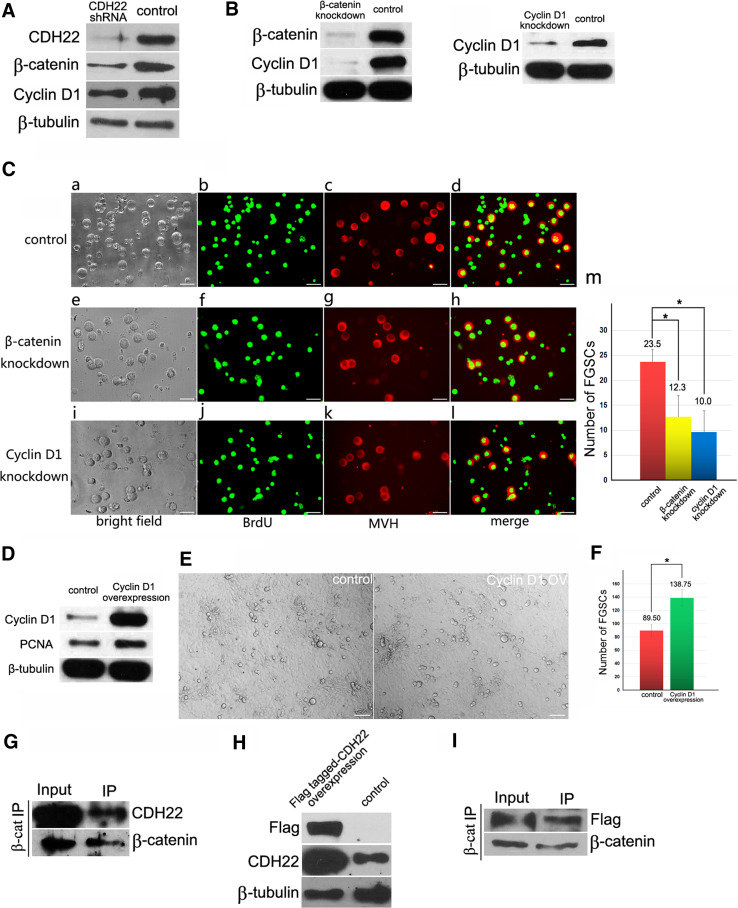
Given that mouse Cdh22 has only one coding protein, we investigated whether CHD22 interacts with β-catenin. The expression of Cdh22 in FGSCs was silenced using Cdh22 shRNA lentivirus, and a progressive reduction in β-catenin was observed in the knockdown group compared with the controls (Fig. 4A). Consistently, the expression of Cyclin D1, which is downstream of β-catenin, was also significantly down-regulated by Cdh22 shRNA, which partially explains why FGSC growth was arrested after blockage of CDH22 expression. To further confirm the regulatory role of β-catenin in FGSCs, the expression of β-catenin and Cyclin D1 was interrupted with siRNA (Fig. 4B). Then, BrdU/MVH dual IF was used to estimate the FGSC number, and we observed that blockage of either β-catenin (Fig. 4C e–h, m) or Cyclin D1 (Fig. 4C i–l, m) caused a significant decrease in FGSCs compared with the control (Fig. 4C a–d, m), and disturbance of Cyclin D1 led to a more severe loss in FGSC number compared with that in the β-catenin knockdown group (Fig. 4C e–m). Subsequently, FGSCs were transfected with a Cyclin D1 overexpression plasmid to analyze its role in FGSC proliferation. An increased number of FGSCs and an enhanced expression level of PCNA was observed 72 h post transfection (Fig. 4D–F), suggesting that Cyclin D1 promotes FGSC proliferation. This indicates that CDH22-mediated regulation of FGSC proliferation likely involves not only the JAK–STAT signaling pathway but also cadherin–catenin signaling. Immunoprecipitation confirmed the interaction between endogenous CDH22 and β-catenin (Fig. 4G). Moreover, the Cdh22 ORF was cloned into pcDNA3-Flag, and FGSCs were then transfected with this construct for immunoprecipitation using Flag as a tag to further confirm the binding of CDH22 and β-catenin in FGSCs (Fig. 4H). Flag-tagged CDH22 was also pulled down by antibody against β-catenin (Fig. 4I). These results demonstrate that, unlike Cdh22 in rats, the only protein form encoded by mouse Cdh22 contains a β-catenin-binding domain and regulates FGSC self-renewal via interaction with β-catenin to activate its downstream genes.
Fig. 4.
Interaction of CDH22 and β-catenin in FGSCs. Knockdown of CDH22 in FGSCs decreased the expression of β-catenin and the downstream β-catenin gene Cyclin D1 (A). Validation of knockdown efficiency of β-catenin and Cyclin D1 in FGSCs using western blotting (B). Bright field and BrdU/MVH dual IF staining in FGSCs 48 h post transfection of control siRNA (C, a–d), β-catenin siRNA (C, e–h) or Cyclin D1 siRNA (C, i–l). The statistical comparison of FGSC number in control siRNA, β-catenin siRNA and Cyclin D1 siRNA groups 48 h post transfection (C, m). Overexpression of Cyclin D1 in FGSCs was detected using western blotting (D). Morphology of FGSCs 72 h post transfection with control (E) or Cyclin D1 overexpression plasmid (F). An immunoprecipitation assay demonstrated that CDH22 directly binds with β-catenin in mouse ovary (G). Overexpression of CDH22 Flag fusion protein in FGSCs (H) and pull-down of Flag-tagged CDH22 with an antibody against β-catenin (I)
Extrinsic CDH22 facilitates FGSC maintenance
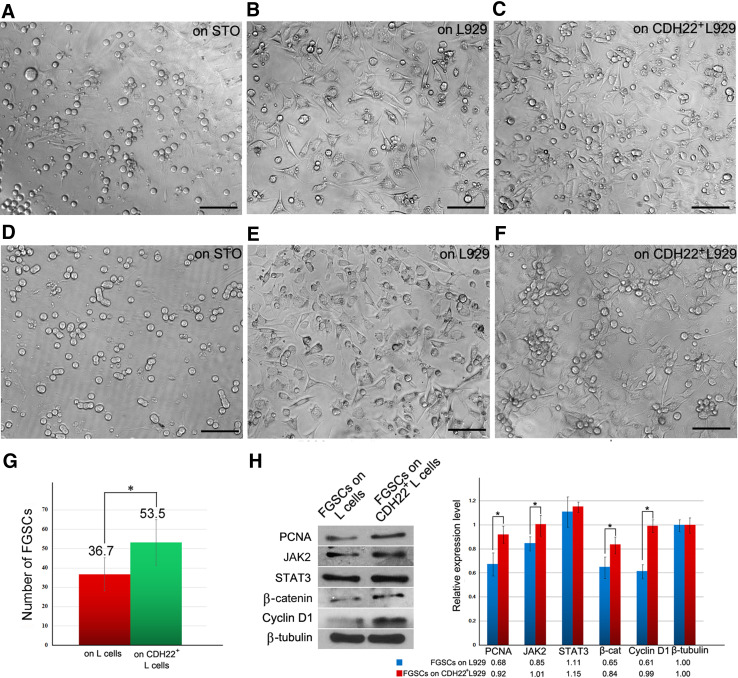
The IHC results revealed that CDH22 expression in the ovary is not restricted to germ cells but also is present in the surrounding somatic cells, suggesting that CDH22 may function in the ovary microenvironment. To preliminarily investigate the effect of extrinsic CDH22 on FGSCs, L929 fibroblasts were infected with lentivirus for Cdh22 expression (Fig. S1 I–J); L929 fibroblasts are intrinsically deficient in cadherin. Wild-type or CDH22+ L929 fibroblasts were mitosis-inactivated with mitomycin C for co-culture with FGSCs as feeders. As expected, FGSCs on L929 feeders exhibited a reduced growth ratio (Fig. 5b) compared with those on STO feeders (Fig. 5a), suggesting that L929 fibroblasts are not suitable to maintain the growth of FGSCs, likely due to the lack of cadherin. However, FGSCs on CDH22+ L929 fibroblasts (Fig. 5c) partially recovered growth compared with those on wild-type L929 fibroblasts (Fig. 5b). After 5 days, the number of FGSCs cultured on CDH22+ L929 fibroblasts was nearly 1.5-fold that of FGSCs cultured on L929 fibroblasts (Fig. 5g). Notably, FGSCs were maintained and formed colonies on CDH22+ L929 cells after two passages (Fig. 5f), while FGSCs on wild-type L929 feeders were almost extinguished (Fig. 5e). This suggests that CDH22 may facilitate FGSC adhesion and proliferation. Moreover, FGSCs cultured on wild-type or CDH22+ L929 feeders for 5 days were harvested for western blot analysis. The results revealed that replenishment of CDH22 in feeders enhanced the expression levels of PCNA, β-catenin and Cyclin D1 in co-cultured FGSCs (Fig. 5h). The expression levels of JAK2 and STAT3 were comparable to those of FGSCs cultured on L929 cells, indicating that extrinsic CDH22 promotion of FGSC self-renewal might be related to β-catenin. Collectively, these data suggest that extrinsic CDH22 also promotes the maintenance of FGSCs in vitro.
Fig. 5.
Extrinsic CDH22 promotes self-renewal of FGSCs. FGSCs on L929 feeders for 5 days exhibited a reduced growth ratio (b) compared with cells on STO feeders (a), while FGSCs on CDH22+ L929 fibroblasts partially recovered growth (c). Statistical results showing that the number of FGSCs cultured on L929 cells decreased to approximately 68% of those grown on CDH22+ L929 (g). After two passages, FGSCs on STO cells grew normally (d), and FGSCs formed colonies on CDH22+ L929 cells (f), while FGSCs on L929 feeders were nearly extinguished (e). FGSCs on feeders were harvested for western blotting (h). The expression levels of PCNA, JAK2, β-catenin and Cyclin D1 were up-regulated in FGSCs cultured on CDH22+ L929. The data represent the mean ± SD (*p < 0.05). Bar = 50 μm
Discussion
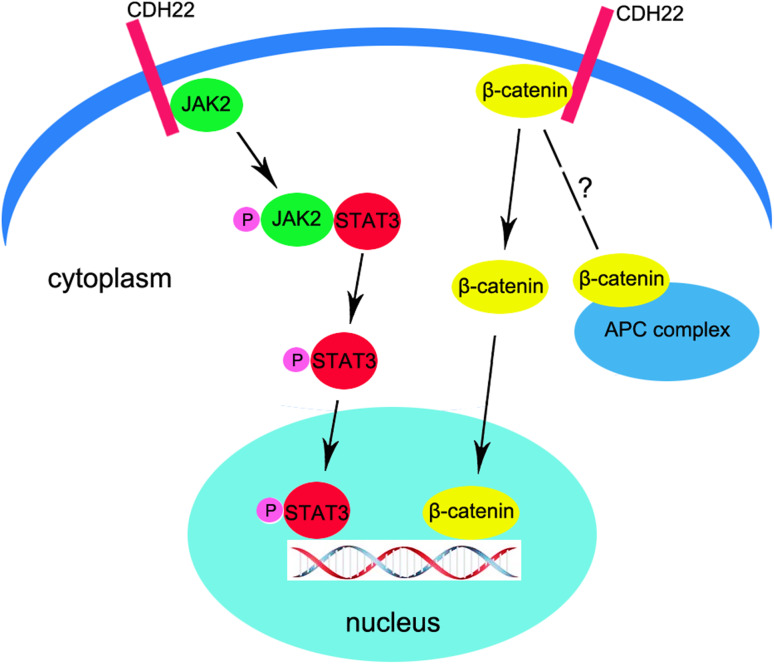
Based on the above data, we summarized a brief regulatory pattern of CDH22 on FGSC self-renewal (Fig. 6). CDH22 interacts with the JAK–STAT signaling pathway via binding and phosphorylation of JAK2, which subsequently activates STAT3 to regulate transcription. Moreover, mouse CDH22 was demonstrated to bind with β-catenin, and loss of CDH22 leads to down-regulation of Cyclin D1, indicating that CDH22 is also able to regulate FGSC fates via β-catenin. Knockdown of CDH22 resulted in a high percentage of apoptosis in FGSCs, suggesting that CDH22 also likely participates in the maintenance of FGSCs. These results preliminarily suggested the mechanism of FGSC self-renewal. However, the mechanism of activation and translocation of β-catenin in FGSCs is unclear, and the relationship between CDH22 and the Wnt signaling pathway is unknown.
Fig. 6.
A preliminary illustration of the self-renewal mechanism of FGSCs mediated by CDH22. CDH22 interacts with the JAK-STAT signaling pathway via binding and phosphorylation of JAK2, which subsequently activates STAT3 to regulate transcription. In addition, CDH22 binds with β-catenin, and loss of CDH22 leads to down-regulation of CyclinD1, indicating that CDH22 regulates FGSC fate via β-catenin. However, the mechanism of activation and translocation of β-catenin in FGSCs is unclear, and the relationship between CDH22 and the Wnt signaling pathway is unknown
In this study, we observed the expression pattern of several types of cadherins and integrins in FGSCs, which is very similar to that in SSCs. This indicates that cadherins and integrins are routinely functional molecules for germ line stem cells and can be used as their markers. However, a previous study reported that CDH1 deficiency did not have any impact on SSC colony proliferation or homing [45], suggesting that not all of these surface markers are indispensable for germ line stem cells. One possibility is the existence of alternative molecules to replenish their function, especially because SSCs are enriched in cadherins or integrins on their surface. Combined with previous studies, which demonstrated the role of CDH22 in rat SSC self-renewal, we wanted to ask whether CDH22 is an alternative surface molecule to maintain self-renewal of SSCs after CDH1 deficiency. This study reveals that CDH22 is required by mouse FGSCs for their maintenance and self-renewal, mainly by promotion of FGSCs proliferation and suppression of apoptosis. JAK–STAT and β-catenin have been identified as two key factors for FGSCs proliferation, but the underlying molecular mechanism of suppression apoptosis by CDH22 has not been studied yet. Since disturbance of CDH22 also caused increased apoptosis in rat SSCs [41, 42], we will focus on the relationship between CDH22 and FGSCs apoptosis in further investigation, to answer whether CDH22 has a more indispensable role than CDH1 in maintenance of germ line stem cells, especially in suppression of apoptosis.
Before investigating the association between CDH22 on FGSCs and the effect on supporting FGSC proliferation, we observed that some regions of preantral follicles contained weak CDH22 signals. This suggests that CDH22 is involved in multiple functions during ovary development, and some of these functions are the same as those in pre-meiotic FGSCs and in post-meiotic oocytes. In addition to the roles of CDH22 in FGSC maintenance and self-renewal verified in this study, it is of interest to further reveal the potential functions in post-meiotic germ cells.
Another issue for FGSC research is that no specific markers for FGSCs have been identified. We reveal that FGSCs share many marker genes with primordial germ cells and oocytes, such as E-cadherin and Fragilis. Thus, BrdU/MVH dual staining is probably the best standard to identify FGSCs. This study concludes that CDH22 is a potential marker for mouse FGSCs, but it requires a combination of other markers to distinguish FGSCs from oocytes and somatic cells due to low specificity. Moreover, the identification of CDH22 as a surface adhesion molecule on FGSCs demonstrates that gonocytes in the testis and FGSCs in the ovary share some molecular signatures and similar biological functions. Interestingly, CDH22 does not involve completely different biological mechanisms in rats and mice. In both species, JAK–STAT is identified as a direct signaling pathway. It has been reported that activation of STAT3 by JAK2 initiates a maintenance response, allowing stem cells to survive in their microenvironments [46]. We observed that CDH22 is associated with JAK–STAT and caused slight down-regulation of JAK2 and STAT3 expression but a dramatic loss of p-JAK2 and p-STAT3 phosphorylation after exposure to Cdh22 shRNA-lentivirus in FGSCs. A similar regulatory mechanism that involves CDH22 was reported in rats, indicating that self-renewal of FGSCs requires complicated crosstalk between extrinsic signals from the niche and intrinsic regulators of survival signals via CDH22. Next, we demonstrated that JAK–STAT signaling contributes to cell proliferation. The inhibition of JAK–STAT caused by AG490 led to rapid loss of FGSCs, accompanied by a decrease in the AG490 target p-STAT3. This result suggests that the phosphorylation status of STAT3 is important for FGSC maintenance. Therefore, we speculate that cooperation between JAK2 and STAT3 guarantee proliferation and growth of FGSCs. This leads us to assume that a similar mechanism of CDH22-dependent proliferation is important for FGSCs.
Although the interaction between CDH22 and the JAK–STAT signaling pathway in FGSCs and SSCs was demonstrated, the function and mechanism of CDH22 in the regulation of stem cell fates have not been fully revealed, especially the β-catenin-related mechanism. A previous study indicated that, unlike other cadherin family members, the short splicing pattern of Cdh22 lacks a β-catenin binding domain in rats. We provide evidence of the existence of a β-catenin binding domain in mouse CDH22 according to immunoprecipitation assays in vivo and vitro. This assumption was corroborated by data demonstrating that knockdown of CDH22 expression in FGSCs also impacted expression of β-catenin and the subsequent downstream target Cyclin D1. In this scenario, CDH22 on FGSCs not only effects adhesion with feeder cells in vitro but also leads to activation of JAK–STAT and β-catenin signaling and subsequent expression of β-catenin and downstream genes. It is noteworthy that β-catenin is involved in multiple pathways in regulating cell fate. Thus, the loss of CDH22 may cause growth arrest not only by suppressing the expression of β-catenin and the downstream target Cyclin D1, but the action pattern of β-catenin during this process is not clear, i.e., whether β-catenin interacts with the Wnt signaling pathway after release from CDH22. These results suggest that different biological mechanisms exist between rats and mice, and further study is still required to fully reveal how CDH22 functions in regulating FGSC fate.
Moreover, understanding how CDH22 drives FGSC establishment via extrinsic signals from the microenvironment is of great interest, and the critical step is to identify signals transduced by CDH22 from somatic cells. Routinely used feeders for germ line stem cell culture, such as STO, MEF and 3T3 cells, express CDH22 (Fig. S1 A). Thus, we verified the role of CDH22 using the endogenously cadherin-deficient cell line L929 fibroblasts as feeders. Notably, extremely weak CDH22 signal was detected in L929 fibroblasts (Fig. S1 A), indicating that this cell actually expresses a very low level of cadherin. The low growth ratio of FGSCs suggests the pivotal role of extrinsic CDH22 for cell maintenance. We also observed that extrinsic CDH22 impacts expression of β-catenin and Cyclin D1 in FGSCs. Thus, we assume that extrinsic CDH22 likely functions in a structural role to interact with certain surface proteins on FGSCs to maintain adhesion, which probably promotes FGSC proliferation. In future investigations, the binding partners that interact with external CDH22 and transmit the signal to the FGSC nucleus must be determined. This also will provide clues to the function and mechanism of CDH22 in the surrounding somatic cells in the ovary microenvironment.
In this study, we report an adhesion molecule, CDH22, that drives the proliferation of mouse FGSCs and preliminarily explore the related mechanisms. One of the paramount tasks in the future will be to reveal the role of CDH22 in vivo because we are far from a complete understanding of the precise mechanisms of FGSC self-renewal mediated by CDH22. Determining this role will provide important hints for reproductive biology and clinical applications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 3 (TIFF 18789 kb)
Acknowledgements
This work was supported by National Natural Science Foundation of China (81200472) and Fundamental Research Funds for the Central Universities in China (KYTZ201602).
Abbreviations
- BrdU
5-Bromo-2′-deoxyuridine
- CDH22
Cadherin-22
- FGSCs
Female germ line stem cells
- DMSO
Dimethyl sulphoxide
- JAK–STAT
Janus kinase–signal transducer and activator of transcription
- PCNA
Proliferating cell nuclear antigen
Author contributions
XZ, collection and/or assembly of data, data analysis and interpretation. YY, collection and/or assembly of data, data analysis and interpretation. QX, collection and/or assembly of data, data analysis and interpretation. HS, collection and/or assembly of data, and data analysis. RW, collection and/or assembly of data, and data analysis. JW, collection and/or assembly of data, and data analysis. KZ, conception and design, financial support, manuscript writing, and final approval of manuscript.
Compliance with ethical standards
Conflict of interest
The authors have no potential conflicts of interest.
References
- 1.Zuckerman S. The number of oocytes in the mature ovary. Horm Res. 1951;6:63–108. [Google Scholar]
- 2.Borum K. Oogenesis in the mouse : a study of the meiotic prophase. Exp Cell Res. 1961;24(3):495–507. doi: 10.1016/0014-4827(61)90449-9. [DOI] [PubMed] [Google Scholar]
- 3.Peters H. Migration of gonocytes into the mammalian gonad and their differentiation. Philos Trans R Soc Lond. 1970;259(828):91–101. doi: 10.1098/rstb.1970.0048. [DOI] [PubMed] [Google Scholar]
- 4.Mclaren A. Meiosis and differentiation of mouse germ cells. Symp Soc Exp Biol. 1984;38(38):7. [PubMed] [Google Scholar]
- 5.Faddy MJ, Jones EC, Edwards RG. An analytical model for ovarian follicle dynamics. J Exp Zool. 1976;197(2):173. doi: 10.1002/jez.1401970203. [DOI] [PubMed] [Google Scholar]
- 6.Perez GI, et al. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21(2):200–203. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- 7.Faddy MJ. Follicle dynamics during ovarian ageing. Mol Cell Endocrinol. 2000;163(1–2):43–48. doi: 10.1016/S0303-7207(99)00238-5. [DOI] [PubMed] [Google Scholar]
- 8.Tilly JL. Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2(11):838–848. doi: 10.1038/35099086. [DOI] [PubMed] [Google Scholar]
- 9.Johnson J, et al. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 10.Johnson J, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122(2):303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y, et al. GSK3 inhibitor-BIO regulates proliferation of female germline stem cells from the postnatal mouse ovary. Cell Prolif. 2012;45(4):287–298. doi: 10.1111/j.1365-2184.2012.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai Y, et al. Location and characterization of female germline stem cells (FGSCs) in juvenile porcine ovary. Cell Prolif. 2013;46(5):516–528. doi: 10.1111/cpr.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura S, et al. Identification of germline stem cells in the ovary of the teleost medaka. Science. 2010;328(5985):1561–1563. doi: 10.1126/science.1185473. [DOI] [PubMed] [Google Scholar]
- 14.Zou K, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11(5):631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 15.White YAR, et al. Oocyte formation by mitotically-active germ cells purified from ovaries of reproductive age women. Nat Med. 2012;18(3):413–421. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo K, et al. Germ stem cells are active in postnatal mouse ovary under physiological conditions. Mol Hum Reprod. 2016;22(5):316–328. doi: 10.1093/molehr/gaw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, et al. Experimental evidence showing that no mitotically active female germ line progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci USA. 2012;109(31):12580. doi: 10.1073/pnas.1206600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei L, Spradling AC. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc Natl Acad Sci USA. 2013;110(21):8585. doi: 10.1073/pnas.1306189110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods DC, Tilly JL. Reply to Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat Med. 2015;21(10):1116–1118. doi: 10.1038/nm.3964. [DOI] [PubMed] [Google Scholar]
- 20.Zarate-Garcia L, et al. FACS-sorted putative oogonial stem cells from the ovary are neither DDX4-positive nor germ cells. Sci Rep. 2016;6:27991. doi: 10.1038/srep27991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Ao J, Wu J. Systematic identification and comparison of expressed profiles of lncRNAs and circRNAs with associated co-expression and ceRNA networks in mouse germline stem cells. Oncotarget. 2017;8(16):26573–26590. doi: 10.18632/oncotarget.15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C, et al. Tracing and characterizing the development of transplanted female germline stem cells in vivo. Mol Ther. 2017;25(6):1408–1419. doi: 10.1016/j.ymthe.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding X, et al. Human GV oocytes generated by mitotically active germ cells obtained from follicular aspirates. Sci Rep. 2016;6:28218. doi: 10.1038/srep28218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, et al. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3(2):132–141. doi: 10.1093/jmcb/mjq043. [DOI] [PubMed] [Google Scholar]
- 25.Zou K, et al. Improved efficiency of female germline stem cell purification using fragilis-based magnetic bead sorting. Stem Cells Dev. 2011;20(12):2197–2204. doi: 10.1089/scd.2011.0091. [DOI] [PubMed] [Google Scholar]
- 26.Woods DC, Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc. 2013;8(5):966–988. doi: 10.1038/nprot.2013.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, et al. Conversion of female germline stem cells from neonatal and prepubertal mice into pluripotent stem cells. J Mol Cell Biol. 2014;6(2):164–171. doi: 10.1093/jmcb/mju004. [DOI] [PubMed] [Google Scholar]
- 28.Xie W, Wang H, Wu J. Similar morphological and molecular signatures shared by female and male germline stem cells. Sci Rep. 2014;4:5580. doi: 10.1038/srep05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou L, et al. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol Hum Reprod. 2014;20(3):271–281. doi: 10.1093/molehr/gat081. [DOI] [PubMed] [Google Scholar]
- 30.Khosravifarsani S, et al. Isolation and enrichment of mouse female germ line stem cells. Cell J. 2015;16(4):406–415. doi: 10.22074/cellj.2015.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park ES, Tilly JL. Use of DEAD-box polypeptide-4 (Ddx4) gene promoter-driven fluorescent reporter mice to identify mitotically active germ cells in post-natal mouse ovaries. Mol Hum Reprod. 2015;21(1):58. doi: 10.1093/molehr/gau071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Z, et al. Improvement in isolation and identification of mouse oogonial stem cells. Stem Cell Int. 2016;2016(19):1–10. doi: 10.1155/2016/2749461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C, Wu J. Production of offspring from a germline stem cell line derived from prepubertal ovaries of germline reporter mice. Mol Hum Reprod. 2016;22(7):457. doi: 10.1093/molehr/gaw030. [DOI] [PubMed] [Google Scholar]
- 34.Pacchiarotti J, et al. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79(3):159–170. doi: 10.1016/j.diff.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Park ES, Woods DC, Tilly JL. Bone morphogenetic protein 4 (BMP4) promotes mammalian oogonial stem cell differentiation via Smad1/5/8 signaling. Fertil Steril. 2013;100(5):1468–1475. doi: 10.1016/j.fertnstert.2013.07.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song X, Xie T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc Natl Acad Sci USA. 2002;99(23):14813–14818. doi: 10.1073/pnas.232389399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tokuda M, et al. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol Reprod. 2007;76(1):130–141. doi: 10.1095/biolreprod.106.053181. [DOI] [PubMed] [Google Scholar]
- 38.Baronsky T, et al. Reduction in E-cadherin expression fosters migration of Xenopus laevis primordial germ cells. Integr Biol. 2016;8(3):349–358. doi: 10.1039/C5IB00291E. [DOI] [PubMed] [Google Scholar]
- 39.Sugimoto K, et al. Molecular cloning and characterization of a newly identified member of the cadherin family, PB-cadherin. J Biol Chem. 1996;271(19):11548–11556. doi: 10.1074/jbc.271.19.11548. [DOI] [PubMed] [Google Scholar]
- 40.Wu J, et al. Expression of a novel factor, short-type PB-cadherin, in Sertoli cells and spermatogenic stem cells of the neonatal rat testis. J Endocrinol. 2003;176(3):381. doi: 10.1677/joe.0.1760381. [DOI] [PubMed] [Google Scholar]
- 41.Wu J, Jester W, Orth J. Short-type PB-cadherin promotes survival of gonocytes and activates JAK-STAT signalling. Dev Biol. 2005;284(2):437–450. doi: 10.1016/j.ydbio.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 42.Wu J, et al. Short-type PB-cadherin promotes self-renewal of spermatogonial stem cells via multiple signaling pathways. Cell Signal. 2008;20(6):1052. doi: 10.1016/j.cellsig.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Kitajima K, Koshimizu U, Nakamura T. Expression of a novel type of classic cadherin, PB-cadherin in developing brain and limb buds. Dev Dyn. 1999;215(3):206–214. doi: 10.1002/(SICI)1097-0177(199907)215:3<206::AID-AJA3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 44.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8(4):241. doi: 10.1016/S1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 45.Kanatsushinohara M, et al. Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell. 2008;3(5):533. doi: 10.1016/j.stem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Kiger AA, et al. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294(5551):2542. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 3 (TIFF 18789 kb)