Abstract
Once viewed as a passive physiological state, sleep is a heterogeneous and complex sequence of brain states with essential effects on synaptic plasticity and neuronal functioning. Rapid-eye-movement (REM) sleep has been shown to promote calcium-dependent plasticity in principal neurons of the cerebral cortex, both during memory consolidation in adults and during post-natal development. This article reviews the plasticity mechanisms triggered by REM sleep, with a focus on the emerging role of kinases and immediate-early genes for the progressive corticalization of hippocampus-dependent memories. The body of evidence suggests that memory corticalization triggered by REM sleep is a systemic phenomenon with cellular and molecular causes.
Keywords: Plasticity, Long-term memory, Offline consolidation, Trace propagation, Cortical engagement
Introduction
Remembering is indispensable to all living animals. Memory formation is the biological transformation through which sensory, motor and cognitive experiences are encoded in the brain for future use. The long-term retrieval of stored information is only possible after memory consolidation, which is how memory traces stabilize over time. Memory corticalization is a hallmark of consolidation and can be operationally defined as the physiological process governing the progressive engagement of neocortical structures in the retrieval of memories acquired through experience. This phenomenon is reflected in electrophysiological, biochemical and gene regulatory changes that, altogether, allow the maturation of an observable memory trace into long-lasting neural representations, recognized in behavior as long-term memory (LTM) [1–5]. As recently acquired memories undergo long-term storage [3, 4, 6, 7], memories encoded by the hippocampus (HPC) are stabilized into neocortical regions, where cortico-cortical connections are slowly established and strengthened [8–10]. As a consequence, memory corticalization can support successful memory retrieval, can promote reconsolidation, or can prompt forgetting [11–14].
The standard systems consolidation model posits that declarative memories in humans, as well as spatial memories and certain kinds of conditioning in rodents, are dependent on the HPC for their acquisition, but become gradually independent from it over time [3, 8, 10, 15], as demonstrated by several studies of bilateral hippocampal lesions (Fig. 1a) [16]. Some recent optogenetic evidence detailing the circuitry responsible for fear memory retrieval is also consistent with the divergent model, the multiple trace theory [17, 18], which argues against the progressive HPC independence [11, 19]. Despite this contention (i.e., the hippocampal role in long-term memory), both theories agree about the increasing role of the neocortex in memory retrieval (i.e., corticalization). The quality of retrieval may be impaired in the conditions produced by several HPC interventions, as argued by multiple trace theory [11, 19]. However, most of the experimental approaches demonstrate successful retrieval related to increased neocortical involvement and decreased HPC engagement over time.
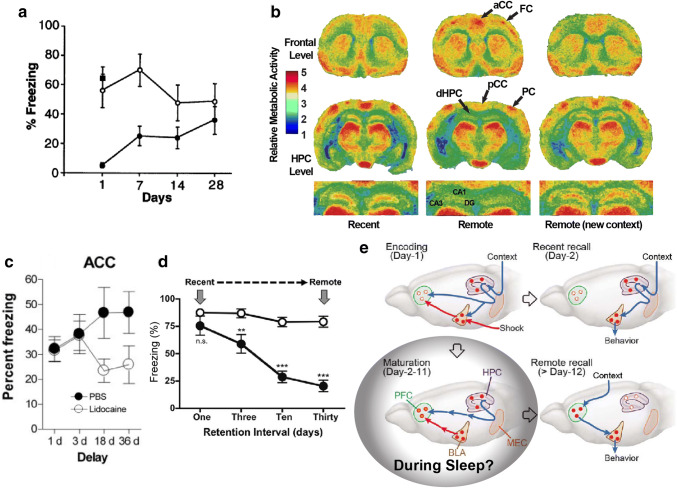
Fig. 1.
Evidence of memory corticalization. a Rats trained in a classical CFC protocol and submitted to electrolytic lesions bilaterally in the dorsal HPC (filled circle) or the cortex above it (filled square) 1, 7, 14 or 28 days after training show different patterns of behavior; indicating a progressive disengagement of the HPC in contextual fear memory retrieval. (open circle), control. [282]. b (14C)2-deoxyglucose color-coded autoradiographs from mice trained in a spatial discrimination task and tested after either a 5-days (recent—left column) or a 25-days (remote—middle and right columns) delay. The right column shows the results for a representative animal of the group that was trained in a given context and tested in a different one. Note the different activation pattern between recent and remote conditions within the highlighted regions. aCC anterior cingulate cortex, FC frontal cortex, pCC posterior cingulate cortex, PC parietal cortex, dHPC dorsal HPC, CA1 and CA3 Cornu Ammonis areas 1 and 3, respectively, DG dentate gyrus (modified from [23]). c Rats also trained in a CFC protocol but with a pharmacological inactivation of the ACC show a progressive engagement of the ACC in the retrieval of fear memory (modified from [56]). d Rats with excitotoxic lesions bilaterally in the dorsal HPC were trained ~ 12 days after surgery in a strong CFC protocol (prolonged exposure to the context and strong multiple shocks), which was previously shown to produce fear conditioning in the absence of the HPC. Animals were tested 1, 3, 10, or 30 days after training and, compared to animals with intact HPC, they showed a reduced memory persistence, indicating a requirement of the HPC to memory consolidation (modified from [78]). e Schematic of the regions related to encoding, maturation, recent and remote retrieval of CFC assessed at the level of neuronal engrams and cross-regional projections. These results corroborate previous findings related to the HPC-dependence for recent memory retrieval and consolidation, here described as PFC engram maturation, which becomes progressively sufficient for remote memory retrieval. It also raises the question about the mechanisms and behavioral states related to this maturation process. Is sleep involved?
(modified from [ 18 ])
For instance, rabbits subjected to trace eyeblink conditioning followed by bilateral hippocampal lesions 24 h after training showed significant memory impairment when retested. However, no memory deficit was observed when hippocampal lesions were made 30 days after training [20]. In rats subjected to the inhibitory avoidance task, memory formation in the HPC corresponds to a sequence of biochemical events that involve the successive activation of glutamate receptors and protein kinases, followed by changes in glutamate receptor subunits and immediate early gene (IEG) expression [21]. Retrieval a few days after training requires intact hippocampi and amygdalae, as well as entorhinal and parietal cortices. After 30 days, just the two latter are needed, and after 60 days only the parietal cortex is essential for retrieval. Altogether the data support the notion that long-term storage requires activity-dependent modifications of extra-hippocampal networks [21]. Socially acquired food preference in rats is also sensitive to post-training hippocampal lesions, with memory impairment for lesions performed 1–2 days after training, but not for lesions performed at longer intervals [22].
In agreement with these results, mice trained in a spatial discrimination task and subjected to functional brain imaging with (14C)2-deoxyglucose during retesting showed hippocampal disengagement and neocortical engagement 25 days after training, in comparison with an opposite trend 5 days after training (Fig. 1b) [23]. In humans’ functional magnetic resonance imaging (fMRI), the HPC and the posterior parietal cortex show increased activity and interconnectivity during early encoding of a spatial memory, followed by gradual dissociation across repeated training sessions, i.e., decreased HPC and increased posterior parietal cortical activity [24]. Additionally, humans trained in a faces remote memory test, and subjected to fMRI when retested, showed temporally graded changes in the medial temporal lobe (MTL), with hippocampal disengagement after a few years, but involvement of the entorhinal cortex for up to 20 years post-training [25]. In conclusion, human and rodent studies suggest a progressive relocation of information representation within brain structures. But, what are the mechanisms underlying the corticalization of memory traces? In the past 20 years, several lines of evidence indicate that sleep is directly involved in the HPC-guided maturation of memory traces in extra-hippocampal sites. Sleep comprises two main stages, rapid-eye-movement (REM) sleep (a.k.a. paradoxical sleep) and non-REM (NREM) sleep. In the present article we will review the main tenets of sleep-dependent memory corticalization, with emphasis on: (1) memory corticalization after contextual fear conditioning, (2) the role of post-learning REM sleep windows for LTM corticalization, (3) sleep-dependent cortical plasticity during development, (4) selective pruning and maintenance of cortical dendritic spines by post-training sleep, (5) the role of the HPC in the coordination of cortical reactivation, and (6) how sleep-dependent gene expression can contribute to the gradual hippocampofugal stabilization of mnemonic traces.
Memory corticalization after contextual fear conditioning
The experimental paradigm of contextual fear conditioning (CFC) offers one of the best-documented examples of memory corticalization over time. CFC is a behavioral paradigm defined as the association between a context (neutral stimulus) and an aversive stimulus (usually foot-shock) [26]. The standard protocol involves the placement of experimental animals in a specific context (usually an inescapable box) with multiple sensory cues (odor, visual, tactile and auditory) in which they receive one or multiple foot-shocks through a metal floor. During testing, animals are placed back in the context after different time delays to evaluate if the exposure to the previously neutral stimulus is sufficient to elicit the behavior related to the aversive stimulus (usually freezing). The percentage of time spent in freezing (complete immobility except for breathing movements) is understood as a measure of memory retrieval, i.e., animals are expressing a behavior indicating that they “remember” in which context the aversive stimulus was given [27].
In the temporal domain, this proxy of memory can be classified as recent or remote LTM. Since the HPC was shown to be necessary for the retrieval of memories during the first ~ 1–2 weeks after training (Fig. 1a) [16], we will refer to recent LTM in this review as those tested from 1 to 5 days after training (in which the HPC is still a protagonist), whereas remote LTM will comprise those assessed 7 days or more after training (in which memory retrieval do not need an intact HPC) [7, 10].
In this respect, the first week after training seems uniquely suited to investigate corticalization, and some findings corroborate toward this idea. For instance, the activation of the N-methyl-d-aspartate receptor (NMDAr) in the CA1 region during the first week after training is required for long-term potentiation (LTP) expression and LTM consolidation [28].
LTP and long-term depression (LTD) are supposed to be markers of structural plasticity and memory trace changing [29]. LTP was extensively studied in the HPC through its induction by electrical stimulation [30]. Direct evidence that LTP and LTD represent memory trace changing related to retrieval is scarce. However, it was shown that LTP is also induced in the HPC by spatial learning in vivo [31]. Additionally, infusion in the HPC of an inhibitor of protein kinase mzeta (PKMζ), which is selectively related to LTP maintenance, but not formation [32], hinders the persistence of LTP and spatial memory [33]. Moreover, LTP and LTD seem to induce the same processes in other regions [34]. For instance, learning also induces LTP in the cortex [35], and LTP- or LTD-like mechanisms modulate learning when triggered in the cortex [36] or amygdala [37].
Although some exceptions may exist [38], LTP and LTD are usually induced by NMDAr activation [39, 40]. This activation may elicit different types of LTP [39], but the maintenance of potentiation through stable synaptic changes requires gene expression and protein synthesis [39, 41–43]. Despite some controversy regarding the consolidation/reconsolidation model and its dependence on gene expression and protein synthesis due to technological and experimental limitations [44–49], there is compelling evidence that gene expression and protein synthesis represent the best alternative to explain how experience induces structural changes related to remembering and memory updating in the long-term [44, 45, 49].
The Ca2+/calmodulin-dependent protein kinase II (CaMKII), a kinase that has been related to LTP, synaptic plasticity and memory consolidation, is a main actor in the downstream pathway of the NMDAr activation [50, 51]. Although other molecular actors may be also involved [52], the LTP and LTM retrieval dependency on NMDAr activation within the first week after training is probably driven by CaMKII, since its inhibition within the same time window [53] produces similar effect as NMDAr impairment [28]. In fact, mice homozygous for a null mutation of α-CaMKII−/− show impaired HPC-dependent spatial learning [54], while the heterozygous strain, show normal acquisition and LTP in the HPC, but impaired persistence of spatial memory, neocortical engagement (especially the mPFC) in remote retrieval, as well as LTP in the neocortex [55, 56]. This is in line with the notion that the corticalization process is intensified during the first week after training and is dependent on the reactivation of the NMDAr, leading to gene expression and protein synthesis able to induce LTP initially in the HPC and later in the cortex.
In short, the NMDAr-dependent molecular cascade triggered by experience and conveyed by calcium entry into the cell leads to synaptic remodeling, dendritic spine turnover/stabilization and LTP terminating in synaptic consolidation [52, 57–61]. In this sense, during the first week after training, selected cortical regions would undergo gradual synaptic changes able to harbor some aspects of the memory trace necessary for remote LTM retrieval while HPC promptly established changes would fade out quickly. In agreement with this, new dendritic spines in the HPC were shown to survive for ~ 1–2 weeks while cortical spines were shown to be more stable [62]. Also, multiple evidence shows dendritic spine changes in several cortical regions following a multitude of experimental paradigms [18, 63, 64]. An important feature that may sustain the memory trace within cortical circuits is the laminar rearrangement of neuronal activity and synaptic changes, which would establish cortico-cortical connections that are supposed to build and stabilize neuronal assemblies related to LTM retrieval [65, 66]. Consistent with this hypothesis, recent LTM retrieval engages the cortical layer VI, whereas remote LTM engages the supragranular layers II/III and the granular layer IV [67, 68]. Due to its dense bidirectional cortico-cortical connections [65], layers II and III comprise an important site to look for structural changes related to memory corticalization. However, a question remains about which cortical regions to analyze.
Regions of the medial prefrontal cortex (mPFC), more specifically the prelimbic (PL), infralimbic (IL), and anterior cingulate cortex (ACC) regions, have been proposed to play a role in the encoding of remote memories, analogous to the role played by the HPC for recent memories [10]. For instance, the ACC has been shown to be important for the recovery of remote LTM (18–36 days), but not recent LTM (1–3 days after training) (Fig. 1c) [56]. In a follow-up study, Restivo and collaborators demonstrated an increased dendritic spine density in the HPC 48 h after CFC, while in the ACC a similar effect within layers II/III only occurred 37 days later [69]. Following this reasoning, they also showed that a bilateral dorsal hippocampal lesion immediately after CFC suppresses both the behavioral correlate of remote memory and the dendritic modifications previously observed in the ACC. In contrast, impairing hippocampal function 24 days after conditioning failed to produce these effects, which corroborates the notion that the memory trace relies on the HPC initially, including for its cortical stabilization, and evolves to depend less on this structure as it relies over time on neocortical areas, including the ACC.
In another study, Vetere and collaborators [70] used a viral vector-based strategy to activate the transcription of the MEF-2 gene (myocyte enhancer factor 2)—a negative regulator of synaptogenesis in vitro [71] and in vivo [72]—to inhibit dendritic modifications in ACC neurons within layers II/III resulting from CFC. The highest levels of dendritic modification in ACC neurons occurred in the window between 1 and 8 days after CFC, which was compatible with the time window of robust MEF-2 upregulation following viral vector micro-infusions into the ACC (up to ~ 7 days). Animals injected with the viral vector at 24-h post-conditioning showed a decreased formation of new dendritic spines in ACC neurons, as well as deficits in remote memory retrieval, 7 or 48 days after infusion. These morphological and behavioral changes were absent in animals injected with the viral vector 42 days after conditioning.
Together, these data suggest the existence of a window of opportunity for HPC-dependent memory corticalization, in which the activity of hippocampal neurons can transiently affect the anatomical structure of cortical neurons.
Extra-hippocampal tagging at the time of encoding
This set of results obtained with CFC was replicated and advanced using the social transmission of food preference (STFP) test in rats [73]. The paradigm involves a natural, non-spatial learning task, in which the performance has been shown to depend on the HPC [74]. The expression of the memory trace decreases in the HPC as it increases the recruiting of the orbitofrontal cortex (OFC) [73], similarly to what has been described in the ACC after CFC. In addition to corroborating the previous findings that remote LTM requires hippocampal activity early after training (1–2 weeks), and that this is related to early synaptogenesis in prefrontal cortical areas, this study revealed that information-specific networks in the prefrontal cortex were tagged at the time of initial encoding and maturated thereafter through an AMPA- and NMDAr-dependent process which involves histone-tail acetylation through the MAPK/ERK pathway; a cascade of events necessary for the expression of synaptogenesis and the remote memory 30 days after training [73]. This supports the notion that neural signaling at specific synapses creates targets for subsequent plasticity [58, 75], a possible mechanism by which hippocampo-cortical dialogue can promote cortical plasticity.
Such early cortical tagging seems to also occur in the mPFC during CFC. Contrary to the classical finding of complete anterograde and limited retrograde amnesia after extensive medial temporal lobe resection in humans [15], rodents trained after dorsal or complete bilateral HPC lesions can express recent (24 h after training) contextual fear memory [76]. Freezing response was positively correlated with the duration of context exploration before the shock and with the number of applied shocks (one or three 1.5 mA shocks with 2-s duration) during training [76], suggesting the existence of two learning mechanisms, one dependent on the HPC and the other not. Later, it was proposed that this phenomenon depends on a network comprising the PL and IL sub-regions of mPFC, with special involvement of PL neurons projecting to the amygdala [77]. However, the evidence that this HPC-independent fear learning monotonically fades out within 30 days, compared to a stable retrieval in HPC intact animals tested 1, 3, 10, and 30 days after training (Fig. 1d) [78], suggests that, although not necessary for memory formation in specific conditions, hippocampal inputs to neocortical networks are required for memory consolidation and maintenance.
Recently, Kitamura and collaborators corroborated these results by showing that inputs from the basolateral amygdala (BLA) and the medial entorhinal cortex (MEC), the main gateway between the HPC and the neocortex [79], are necessary during conditioning to tag memory traces in the mPFC, whose maturation over days to weeks requires hippocampal activity [18]. The study used retrograde and anterograde tracing, optogenetics, confocal imaging of dendritic spines, calcium imaging and activity-dependent cell-labeling to provide robust evidence for the specificity of the projections and cells involved in the cellular and network consolidation of CFC over time (Fig. 1e).
These results are in line with the hypothesis that hippocampal guidance is required for the activity-dependent maturation of cortico-cortical connections involved in the consolidation of memory traces. Additionally, they suggest that prefrontal regions may act as network hubs that organize the expression of remote LTM [10]. Importantly, the consolidation of CFC involves other brain regions in addition to the HPC and the neocortex, such as the amygdala [80]. For instance, the selective ablation of neurons overexpressing CREB in the amygdala disrupts fear memory [81]. Furthermore, synaptic potentiation of the cortico-amygdala pathway is restricted to those neurons recruited during the training and expression of CFC [82]. The reactivation during memory retrieval of amygdala neurons activated at the time of encoding in a classical CFC task suggests that tagging also occurs in the amygdala [83]. Moreover, the same cell ensemble is recruited for recent or remote recall, but with a switch from the HPC-amygdala to the PFC-amygdala recall circuit [18].
A role for REM sleep in memory corticalization
It has long been known that declarative memory consolidation in humans is benefited by sleep [84–90]. In addition to the decrease in sensory inputs during sleep, which produce less information interference for previously encoded memory traces, sleep-dependent memory facilitation relies on brain activity that occurs specifically during the various quiescent state comprised by sleep. Since sleep can be divided into two main stages, REM and NREM sleep (which includes slow wave sleep, SWS), one may ask which sleep state better contributes to memory consolidation. The psychological evidence indicates that both NREM and REM sleep contribute to learning and memory [91], with a prevalence of NREM for declarative memories [92–94] and REM for procedural and emotional memories [95–98]—but see disagreements [99].
Based on very different experimental designs, several theories of how sleep-dependent mechanisms affect memory processing have been proposed to date. For instance, the reactivation of hippocampal place cells during SWS that follows spatial experience [100–103] could in principle trigger synaptic plasticity in cortical target structures, as hypothesized early on [104–106]. According to this basic notion of the role of sleep in memory processing, the rehearsal of specific patterns of neuronal activation during SWS would directly strengthen the synapses activated during waking, at the time of memory encoding [107, 108]. Also concerned exclusively with SWS, the synaptic homeostasis hypothesis (SHY) proposes that this sleep state promotes the elimination of weak synapses, producing a rescaling of memory traces that strengthens memories not in absolute terms, but only relatively [109]. In contrast, the sequential hypothesis proposes that while SWS weakens the ecologically irrelevant or competing memory traces, eliminating the non-adaptive ones, subsequent REM sleep actually strengthens the relevant, adaptive memory traces [110]. In partial agreement, the synaptic embossing theory proposes that the reactivation of cell assemblies during SWS is followed by synaptic upscaling and downscaling in complementary networks during REM sleep, affecting, respectively the synapses tagged or untagged by waking experience [13, 111–114]. Finally, the active systems consolidation theory proposes that system consolidation takes place during NREM rather than REM sleep, which would work passively providing the molecular milieu necessary for synaptic consolidation [89, 115]. This theory is mostly based on human studies from the same group [87, 92]. There is also some empirical support for this notion in animal models: An investigation of somatosensory evoked potentials showed a boosting effect associated with SWS and dependent on the joint activation of AMPA and NMDA receptors [116].
Although these theories are not all mutually compatible, they may just reflect different aspects of the same phenomenon. Are sleep-specific neural oscillations, neuronal activity and molecular mechanisms related to the molecular and cellular changes underlying memory processing and, more specifically, memory corticalization (Fig. 1e). Behavioral states in which subjects are not engaged in relevant experience (or “off-line” states), like waking immobility/rest (a.k.a., quiet waking) or NREM sleep, are characterized by large irregular activity in both HPC and neocortex. Unlike active waking or REM sleep, these states share an increased prevalence of low-frequency oscillations, and hippocampo-cortical sharp-wave ripple (SWR) events [117, 118]. On the other hand, thalamo-cortical spindles have been exclusively described during NREM sleep, a sleep state in which cortical neurons displaying classical inhibitory firing patterns (fast-rhythmic-bursting and fast-spiking) show decreased activity compared to waking [119]. During REM sleep, fast-spiking neurons are even more active than during waking, whereas fast-rhythmic-bursting neurons are less active than during NREM [119]. Neurons exhibiting a regular-spiking firing pattern are usually excitatory and show less variation across sleep states, with a trend of gradual increase across the waking → NREM → REM sequence [119].
Sorting neuronal types (excitatory pyramidal × inhibitory interneurons) using only firing patterns by intracellular recordings may lead to misclassification [120–123]. Likewise, differentiating these modes of activity using extracellularly recorded action potentials or imaging techniques (voltage sensitive dyes or calcium indicators) is challenging [124, 125]. Notwithstanding, single wire, tetrode and silicon probe recordings all suggest that SWS has the lowest mean firing rate among vigilant states, both in the HPC [126, 127] and neocortex [128, 129], while the discharge rate in both regions is increased during REM sleep episodes [126, 127, 129, 130]. REM sleep also shows minimal firing synchronicity among hippocampal neurons [126, 127].
However, recent studies using optical imaging suggests the discharge rate increment during REM sleep is restricted to inhibitory (parvalbumin-positive—PV +) neurons [131], which most probably correspond to the fast-spiking neurons previously detected through intracellular recordings [119]. Interestingly, only the subset of putative pyramidal cells or PV + cells most active during waking displayed sustained or increased activity from SWS to REM, respectively [131]. These results suggest that the long held notion that REM sleep is an active state was due to the biased extracellular detection of a subset of extremely active neurons, especially inhibitory PV + neurons. Nevertheless, the function of this inhibitory drive during REM sleep remains to be determined. One possibility is that it is required for the massive synaptic pruning elicited by REM sleep [114].
Yet, the assessment of neuronal activity across different sleep states reveals a glimpse of the different effects of NREM and REM sleep on neuronal excitability. The classical view argues that NREM sleep triggers a homeostatic process leading to net synaptic downscaling and decreases neuronal activity [128, 132]. However, in the cerebral cortex, neuronal activity is decreased after a REM sleep episode, with a stronger effect on those with low firing rates, while an episode of NREM sleep only significantly reduces the activity of neurons displaying high firing rates [130]. In the HPC, a detailed analysis of the effects of sleep states on neuronal excitability showed that putative pyramidal and inhibitory neurons gradually increase their firing rates across NREM sleep episodes. During REM sleep episodes, both neuronal types exhibit a rapid and pronounced activity reduction. The decreased firing rate and the increased synchrony measured during NREM episodes with an interleaved REM episode are correlated with theta power during the interleaved REM episode [126]. Altogether, these results corroborate the notion that REM sleep plays an important and previously ignored role in the modulation of neuronal activity in the HPC and cortex.
With regard to the mnemonic function during “off-line” states, a large body of evidence corroborates to the notion that they promote the reactivation of previous waking experiences [101–103, 133–136] during hippocampal SWR [137] and cortical up-states [138], which coincide with thalamo-cortical spindles and cortical unit activity in the mPFC during NREM sleep [118]. Also, it has recently been shown that the HPC-amygdala system is reactivated during SWR after an aversive task [139]. This coordinated activity represents a privileged window for hippocampal communication with dispersed regions relevant for memory consolidation [118, 139–145], or even for a top–down neuronal coordination between cortical regions, with relevant implications to perceptual memory consolidation [146]. This notion is supported by the fact that spatial learning can be improved by artificially increasing the coordination of NREM sleep electrophysiological markers between the HPC and the mPFC [147]. Moreover, cued and electrical stimulation during NREM sleep can enhance memory [87, 92, 148, 149]. Additionally, rewarding stimulation triggered by place cell activity firing mostly during SWR events can promote specific place field-directed behavior [150]. Importantly, the selective disruption of SWR, both during sleep [142] and rest [151], and abnormal hippocampal sharp wave-cortical spindle coupling [152] impairs spatial memory.
Memory reactivation during SWS has been proposed to serve as the first step to activity-dependent memory consolidation [153, 154], but at present it is unclear how it relates to protein synthesis able to stabilize long-lasting memory traces [58, 155, 156]. Indeed, studies of gene expression during sleep have demonstrated that REM sleep, not SWS, triggers the calcium-dependent phosphorylation events and transcriptional upregulation of gene expression related to LTP and LTD and, ultimately, to synaptic remodeling [157–165]. Importantly, these effects are experience-dependent, i.e., only occur in animals/brain regions affected by novel experience during waking.
The search for the mechanisms underlying the corticalization of memory traces was significantly advanced by research on experience-dependent IEG expression across the sleep–wake cycle. This thread began with the discovery that the mRNA levels of Zif-268 (a.k.a. Egr1) can be either down- or up-regulated in the brain during REM sleep, depending on the absence or presence, respectively, of novel experience before sleep [157]. The Zif-268 gene encodes a transcription factor with a major role in long-term learning [41], and its transcription is induced by post-synaptic calcium influx via NMDAr and activation of the MAPK(ERK)/CREB pathway [42]. The transcription factor Zif-268 binds promoter regions of hundreds of genes, and leads to the transcriptional upregulation of synapsins I and II, for instance [43]. The gene Arc, which encodes an effector protein directly related to the remodeling of dendritic spines [166], and other IEG involved in learning, such as Fos, CREB and phosphatases Ppp2ca and Ppp2r2d [41, 167–172], are also transcriptionally upregulated by REM sleep [159, 161, 165]. Importantly, similar experience-dependent activation was observed for calcium-dependent kinases upstream of IEG regulation [127, 159].
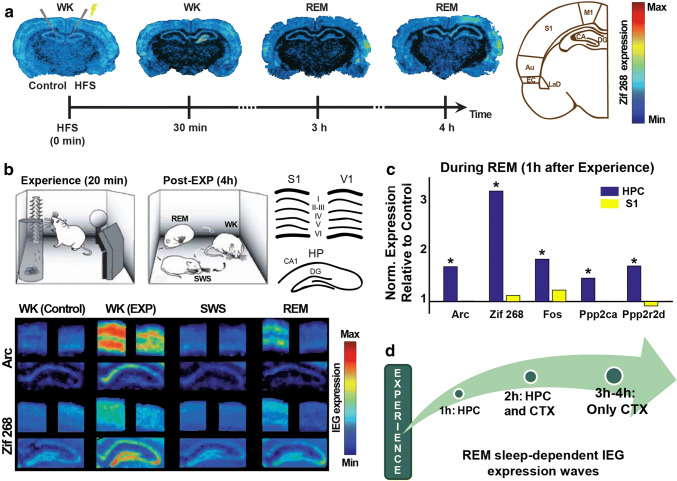
The discovery of experience-dependent IEG transcriptional upregulation in the neocortex and HPC during REM sleep prompted a follow-up study in which novel object exploration was replaced by the induction of LTP in the hippocampal dentate gyrus of freely behaving animals, using high-frequency electrical stimulation of the perforant path (Fig. 2a) [158]. This experimental design substantially narrowed the anatomical entry of novel synaptic changes, producing an initial upregulation of Zif-268 mRNA levels exclusively in the dentate gyrus. Overall the results revealed a series of 3 spatiotemporally separate waves of Zif-268 upregulation after LTP induction. The first wave began locally at the dentate gyrus 30 min after LTP induction, and after 3 h of waking, it reached proximal sites such as the hippocampal CA1 and CA3 fields, the laterodorsal nucleus of the amygdala and the auditory cortex (Fig. 2a). This wave was terminated by the first SWS episode after LTP induction. A second wave began during the first REM sleep episode after LTP in the entorhinal and auditory cortices, and in the laterodorsal nucleus of the amygdala. This wave propagated in the next 3 h of waking to distal brain regions, such as the primary somatosensory and motor cortices. The second wave was terminated by the following (second) SWS episode, and then a third wave began in all these extra-hippocampal regions during the ensuing REM sleep episode (Fig. 2a). Presumably, this third wave would end during a subsequent SWS episode, but this was not assessed in the study and remains to be determined. Importantly, the pharmacological inactivation of the HPC during REM sleep using a sodium channel blocker considerably decreased the transcriptional upregulation of Zif-268 in the neocortex and amygdala, which suggests that extra-hippocampal Zif-268 expression during REM sleep is subjected to hippocampal control [158].
Fig. 2.
REM sleep-dependent gene expression. a Activity-dependent expression of Zif-268 as induced by unilateral high-frequency stimulation (HFS) into the main gateway to the HPC, the perforant path. As time passes, Zif-268 expression spreads from the DG in the HPC to cortical and other limbic regions in a hippocampofugal manner, especially during REM sleep and selective to the stimulated hemisphere. DG dentate gyrus, CA cornu ammonis, EC entorhinal cortex, LaD dorsolateral nucleus of the amygdala and the auditory (Au), primary somatosensory (S1), and primary motor (M1) cortices (modified from [158]). b Top left: rats exposed to novel objects in the dark were left undisturbed for 4 h and then killed after reaching criteria for specific behavioral states (WK, SWS and REM). Top right: model to interpret IEG expression results. Bottom: IEG (Arc and Zif-268) expression showed a marked difference between animals exposed (WK—EXP) and not exposed (WK—control) to novel objects, including in V1, despite lights off during experience. During SWS, IEG expression was generally downregulated, followed by a significant enhanced expression during REM sleep restricted to S1 (modified from [161]). c Same protocol as in b, but with 1-h sleep deprivation after experience, followed by animals killed after reaching criteria for specific behavioral states. Plot shows the housekeeping gene-normalized expression of different plasticity-related genes measured by real-time PCR during REM sleep relative to the expression of the same genes from rats not exposed to novel objects (control). Note a significant expression of IEGs and phosphatases during REM sleep restricted to the HPC 1 h after the experience (modified from [165]). d Summarized model for the REM sleep-induced experience-dependent IEG expression waves
To investigate whether late post-experience REM sleep also correlates with IEG expression corticalization after more naturalistic training, rats were subjected to novel object exploration and then allowed to sleep freely for 4 h (Fig. 2b, top left), which corresponded in average to 28 full sleep–wake cycles. The results showed that REM sleep promotes the transcriptional upregulation of Zif-268 and Arc mRNA in the supragranular and infragranular layers of the neocortex, but not in the HPC (Fig. 2b), which suggests that REM sleep naturally produces hippocampofugal plasticity waves [161].
Importantly, Arc expression was proportional to the power of neural oscillations in the frequency range of cortical spindles (10–14 Hz). Neuronal firing rates during REM sleep showed robust temporal dependence across brain regions: while changes in cortical firing rates during object exploration predicted hippocampal firing rates during REM sleep in the first hour post-experience, changes in hippocampal firing rates during object exploration predicted cortical firing rates during REM sleep in the third hour post-experience. Along with other lines of evidence [157, 158, 165, 173], this corroborates the hypothesis that REM sleep is important for the stabilization of memory traces within cortico-cortical connections, specially in supragranular and infragranular layers [65].
More recently, these results were extended to the IEG Fos and two major phosphatases related to LTD [174, 175], Ppp2ca and Ppp2r2d (Fig. 2c) [165]. This result offers direct support to the notion that sleep-dependent memory processing is a complex, multifaceted process comprising both synaptic potentiation and synaptic depression, as proposed by the Synaptic Embossing Theory. Altogether, the results show that when the interval between novel stimulation and sleep was of 1 h, REM-dependent IEG reinduction occurred only in the HPC [165]. When this interval was around 2 h, the phenomenon occurred in both the HPC and the neocortex [157]. At 3–4 h intervals, the sites of IEG reinduction were restricted to the experience-related neocortical regions (Fig. 2d) [158, 161].
A sleep-dependent window for remote LTM consolidation?
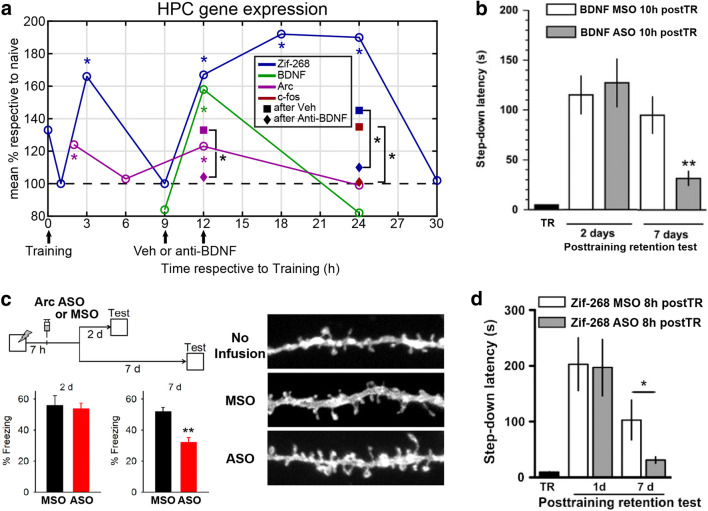
A different set of learning and memory studies, albeit unconcerned with the effects of sleep on IEG expression, corroborate the observation of multiple waves of IEG expression after learning (Fig. 3a). After inhibitory avoidance training, Katche and collaborators reported two waves of Zif-268 expression in the HPC at 3 h and 12–24 h (Fig. 3a) [170]. The inhibition of Zif-268 expression with antisense RNA during the 12–24 h window led to the selective impairment of remote LTM tested after 7 days, in comparison to recent LTM tested at a 24-h interval (Fig. 3d) [170]. Arc showed similar results, with two waves of expression in the HPC peaking at 2 and 12 h after CFC (Fig. 3a). The first wave occurred in the three main nodes of the hippocampal tri-synaptic pathway (DG, CA3, and CA1), while the second wave only appeared in CA1 [171]. Importantly, this late Arc expression was restricted to the cells activated during learning, and it was dependent on the Brain-Derived Neurotrophic Factor (BDNF), whose levels 12 h after fear conditioning have been implicated in LTM persistence and late Zif-268 expression (Fig. 3a, b) [176]. The selective inhibition of the late Arc wave with antisense spared recent memories, but impaired the retrieval of remote memories, as well as the remodeling of dendritic spines and the reactivation of the neuronal ensembles activated during training (Fig. 3c) [171]. Importantly, inhibition of neuronal activity and protein synthesis in the mPFC 12 h after fear learning has been shown to impair remote LTM assessed 7 days, but not recent LTM tested 2 days after training [177]. Similar to results from dorsal CA1 in the HPC [178], this study reported that remote, but not recent LTM is modulated by D1/D5 dopaminergic receptors in the mPFC 12 h after training. This set of results demonstrates that the experience-dependent upregulation of plasticity genes in the HPC 12 h after training is necessary for long-term memory persistence (Fig. 3). Neuronal activity, protein synthesis, and dopaminergic modulation in the mPFC within the same time window, lead to similar outcomes in the HPC. Since sleep was not controlled in these studies, it is quite possible that sleep-dependent plasticity in the HPC-cortical loop underlies the results.
Fig. 3.
A critical window for fear memory consolidation 12 h after training. a Compilation of results of IEG expression in the HPC after fear learning (inhibitory avoidance—IA or contextual fear conditioning—CFC). Note the higher IEG expression within the time window ~ 12 h after fear learning, and that BDNF expression inhibition by anti-BDNF antibody precludes c-fos, Arc, and Zif-268 expressions (modified from [170, 171, 176]). b BDNF antisense applied bilaterally in the HPC of rats 10 h after IA training hinders the expression of remote (7 days), but not recent memory tested 2 days after training (modified from [176]). c Top left: experimental protocol. Bottom left: the same pattern as in b is seen if Arc antisense is applied in the HPC of mice 7 h after CFC. Right: dendritic spines from the HPC of mice sacrificed 7 days after CFC. Note that the inhibition of Arc elevated expression ~ 12 h after CFC by antisense impairs the experience-dependent dendritic spine pruning (modified from [171]). d In the downstream pathway of the BDNF elevated expression, Zif-268 expression in the rat HPC 12 h after training is also selectively required for the remote memory retrieval 7 days after CFC, as shown by antisense application 8 h after training.
(modified from [170])
A first indication is that the inhibition of the elevated c-fos expression 12 h after training within the retrosplenial cortex (RSC) also selectively impairs the remote (7 days), but not the recent (2 days) LTM [179]. The RSC has been linked to memory formation [180], recent or remote retrieval [180, 181] and consolidation [182–184]. Moreover, the remote LTM impairment caused by protein synthesis inhibition in dorsal CA1 12 h after training in the inhibitory avoidance task can be rescued by dopaminergic stimulation in the RSC, and vice versa [179]. Additionally, the inhibition of the training-induced c-fos expression in the RSC 12 h after training hinders the c-fos expression within both dorsal CA1 and RSC for animals killed 24 h after training, and the inhibition of c-fos expression within dorsal CA1 produces the same effect, indicating an interplay between both regions 12 h after training [179]. Fos cell-labeling showed that, besides being strongly active during waking, the RSC is also activated during REM sleep hypersomnia [185]. We recently showed that ~ 70% of RSC neurons are more active during REM sleep than any other behavioral state; and that these neurons, along with RSC high-frequency oscillations (40–160 Hz) amplitude are coupled with the HPC theta rhythm during REM sleep, but not during active wake [129]. Although additional experiments are still ongoing, this evidence corroborates the idea that the HPC-RSC interplay 12 h after training required for LTM persistence probably take place during REM sleep.
Likewise, an earlier group of studies of the active (shuttle) avoidance task showed post-training REM sleep increases that lasted up to 7 days after training [186, 187]. This coincides with the temporal window of accelerated spine formation and maturation in the mPFC circuit, which is required for the expression of remote LTM for CFC [18, 70]. Most interesting, there are specific windows of REM sleep increase after learning [186, 187], and selective REM sleep deprivation between 9 and 12 h impairs learning [188], although total sleep deprivation within the first 3–5 h has also been reported to hinder memory formation [189, 190]. Of note, Ravassard and collaborators reported that REM sleep amounts within the 4 h time-window after training regulates LTP, the abundance of hippocampal neurons rich in Zif-268 protein, and contextual memory expression [172]. Specifically, less REM sleep decreases these features, and more REM sleep increases them.
It has also been shown that injection of a dopaminergic antagonist immediately after training in the hippocampal-dependent Novelty Preference Task selectively reduced REM sleep amount, impaired performance and down-regulated the protein levels of phosphorylated CaMKII, Zif-268, and BDNF in the HPC at specific time windows after training [191]. The anti-dopaminergic treatment down-regulated BDNF expression specifically 12 h after training [191]; in agreement with the 12 h peak of BDNF expression in the HPC related to the long-term persistence of aversive memories (Fig. 3a, b) [176].
It is possible that the interval 12 h after experience is simply one of multiple post-learning offline windows characterized by waves of enhanced plasticity, as suggested by studies from our group (Fig. 2) [157, 158, 161, 165].
In addition, the 12-h interval between training and plasticity windows may be particularly related to the physiology of learning. Since relevant experiences can only occur during waking periods, animals expressing a 12:12 h light–dark circadian rhythmicity are probably sleeping 12 h after a major learning event [192]. In other words, the 12-h time window seems especially suited for sleep-dependent memory consolidation.
Furthermore, circadian rhythmicity is a well-conserved biological feature spanning all phylogeny and has been implicated in memory formation, persistence and synaptic plasticity [193–195]. Cortical spine remodeling is, in fact, different between the dark sleep-poor and the light sleep-rich circadian periods [196]. Direct evidence has linked dendritic spine formation after motor learning with glucocorticoid variation across the circadian cycle in mice [197]. This study shows that low levels of endogenous corticosterone during the inactive phase (lights on), when mice are probably sleeping, are required for the preservation of dendritic spines formed after training, thus enabling performance improvement. This corticosterone decrease occurs 12 h after a corticosterone peak when training-dependent spine formation also peaks [197]. However, sleep was not controlled in this study and thus the additive effect of sleep along with glucocorticoids cannot be ruled out.
Selective pruning and maintenance of synapses by post-training REM sleep
Since synaptic plasticity is one of the key features of memory formation and consolidation [64], a key debate in the field concerns whether sleep inevitably promotes synaptic downscaling, as proposed by SHY [109, 198] or rather an experience-dependent mix of synaptic downscaling and upscaling, as proposed by the sequential hypothesis and the synaptic embossing theory [110, 111, 113, 199].
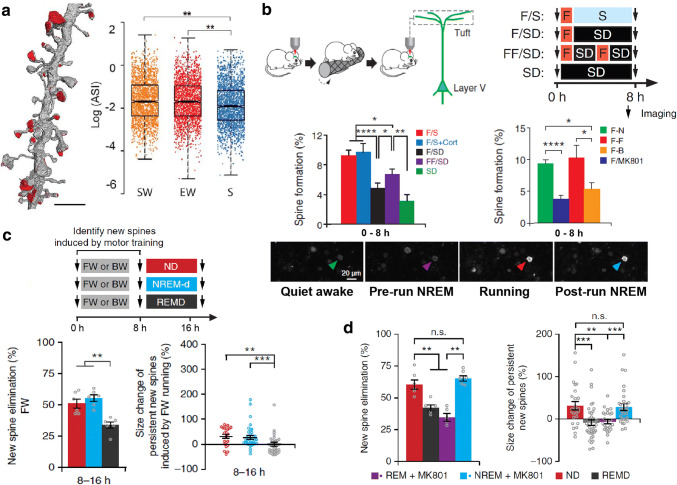
This debate began to be resolved empirically in recent years with the application of new technologies involving electron and two-photon microscopy and genetic manipulation to assess synaptic features in different conditions [200, 201]. While the so-called serial block-face scanning electron microscopy (SBEM) is more prone to evaluate the detailed synaptic morphology of brain slices in 3D [200], transcranial two-photon imaging can survey the dynamic of dendritic spines in vivo across long periods of time [201]. A static SBEM study comparing different groups of animals reported a net effect of total sleep (NREM + REM) in decreasing synaptic strength in primary motor and somatosensory cortices of mice that had explored novel objects (Fig. 4a) [202]. Reduction in axon–spine interface area was selective for small synapses, those that lacked endosomes, and those in spiny dendritic branches [202]. Overall, this study supported SHY by showing a sleep-dependent rescaling with a net decrease in synaptic strength, selective to recently formed small spines [109].
Fig. 4.
Sleep and cortical plasticity. a Distribution of axon–spine interface (ASI) sizes in layer II of primary motor (M1) and primary somatosensory (S1) cortices of mice in 3 different conditions: killed after SW, spontaneous wake at night; EW, enforced wake during the day through the exposure to novel objects or S, ad libitum sleep during the day. Results display a significant net reduction in ASI sizes after sleep (S) compared to the other groups [202]. b Top left: transcranial two-photon imaging to track motor cortex layer V pyramidal neuron apical dendritic spines from head-restrained wake transgenic mice before and after running in a rotarod paradigm. Top right and middle left: sleep deprivation during ~ 7 h immediately after motor training significantly attenuates the training-induced motor cortex spine formation. F forward running, SD sleep deprivation, S sleep, Cort. corticosterone injection. Bottom: two-photon calcium imaging of animals expressing GCaMP6 reveals a NREM sleep-related reactivation of cells activated during motor training. Middle right: injection of the NMDAr blocker MK801, as well as a different learning (B backward running) reduces the rate of training-induced spine formation in the motor cortex (modified from [208]). c Using the same two-photon imaging protocol of head-restrained mice as in b, the authors reported that the majority of the new spines formed in the motor cortex during the first 8 h after motor training are eliminated within the next 8 h, as opposed to the majority (~ 70%) of the persistent new spines which increase in size. Both processes were impaired by selective REM sleep deprivation within this late 8 h-time window. d Selective MK801 pulse injection into the motor cortex at the beginning of REM sleep episodes impaired the elimination of training-induced new spines and the size increment of persistent new spines.
(c and d modified from [ 114 ])
The contribution of NREM and REM sleep was not dissociated in this study, although SHY argues that NREM is a candidate protagonist. SHY hypothesizes the molecular milieu and the electrophysiological features of NREM sleep (slow wave activity, neuronal reactivation, SWR, spindles, etc.) would lead to activity-dependent down-selection [109, 203]. However, opposing theories argue that the same features support experience-dependent upscaling during sleep [108]. In fact both processes seem to be possible, since it was shown that HPC neuronal activity within SWR is related to LTD in circuits not related to a newly acquired memory [204], while specific synapses related to relevant new information undergo LTP [205].
SHY argues that synaptic upscaling would take place preferentially during waking periods, while activity-dependent down-selection during SWS would lead to the renormalization of synaptic weights [132]. In this context, sleep would favor global downscaling, with the pruning of weak synapses and comparatively less depression of stronger synapses [109]. Computation simulations showed that such a model would increase signal-to-noise ratio and could in principle promote memory consolidation, gist extraction, and the integration of new information among consolidated ones, while upscaling during sleep would be detrimental to these functions [206, 207]. More recently, the proponents of SHY argued that some downscaling may happen during waking, and some upscaling may happen during sleep, but these phenomena would be exceptional and irrelevant for memory consolidation [109].
However, quite different conclusions were reached with the use of two-photon microscopy to address the same problem dynamically, by imaging individual synapses over time, as mice learned tasks and were selectively deprived of specific sleep states [114]. The researchers used mice genetically engineered to express a fluorescent protein that labels cell membranes, which were imaged trans-cranially through a delicate thinning of the skull [201]. This method allows individual dendritic spines to be tracked with exquisite detail over time, and its application to the sleep and memory field provided unprecedented direct insights regarding the theoretical controversy in the field.
For instance, in mice subjected to 7 h of total sleep deprivation immediately after motor training, the number of new spines drops dramatically in the dendrites of layer V pyramidal cells in the motor cortex (Fig. 4b, top and middle left) [208]. The sleep-dependent formation of new synapses was shown to rely on neuronal reactivation and NMDAr activation during NREM sleep (Fig. 4b, bottom and middle right), but was insensitive to selective REM sleep deprivation [208].
It is worth considering that, in this study; the deprivation of REM sleep was incomplete, with ~ 21% of the REM sleep preserved. Interestingly, neuronal activity in the cortex during NREM sleep is stronger in the first hours after learning [161], and REM sleep rebounds after sleep deprivation lead to enhanced LTP and increased Zif-268 expression [162, 172]. Moreover, in the neocortex, a single minute of REM sleep is enough to renormalize the global firing rate increase produced by waking activity lasting several minutes [130]. Most methods for REM sleep deprivation are directly or indirectly based on the early detection and interruption of REM sleep, as was the case in this study. These procedures succeed in reducing the duration of REM episodes and the total time in REM sleep, but most often fail to reduce the number of REM sleep episodes [114]. It is thus possible that the marked NMDAr activation during NREM sleep, summed with brief entrances into REM sleep during short rebound episodes, may have circumvented the attempt to prevent the effects of REM sleep on the formation of dendritic spines during the initial consolidation period of motor learning.
In a follow-up study with a similar approach, Wen-biao Gan and his team carefully investigated the influence of different sleep states in a later phase of consolidation (> 8 h after motor learning) by assessing the persistence of dendritic spines [114]. It was found that REM sleep, but not NREM sleep, plays a key role in the pruning of newly formed spines after motor learning (Fig. 4c), facilitating subsequent spine formation after a different motor learning, which is related to improved performance. Moreover, REM sleep strengthens the majority (> 70%) of the persistent new spines formed after learning (Fig. 4c), facilitating their long-term survival [114].
Although pruning after motor learning task in the neocortex was restricted to REM sleep, structural plasticity in the hippocampus may depend on NREM sleep [204]. In this study, the authors showed that SWR complex decrease Schaffer-evoked fEPSP in CA1. The excitability reduction was restricted to those neurons that were not activated during the exploration of a new environment and it was accompanied by decreased spine volume as a function of time (or accumulated SWR events), mainly the ones with tiny and stubby morphologies [204]. The downscale of unspecific spines has been linked to Hebbian potentiation of specific excitatory synapses in the visual cortex [209]. Although sleep was not assessed in this last study, synapse remodeling was dependent on Arc-mediated plasticity. Interestingly, the REM-dependent pruning and strengthening of newly formed spines after learning was dependent on the activation of the NMDAr (Fig. 4d), increased calcium signaling specifically in the dendrites related to the mnemonic trace, and CaMKII activation, which in turn, can interact with Arc [210] (for a review see [211]).
These phenomena were observed both during development and adult learning [114]. Additionally, the time of training (8:00 or 20:00) did not modify the formation of dendritic spines [208], and was insensitive to corticosterone levels as well as the REM sleep-dependent late selective pruning/strengthening of dendritic spines [114, 208]. These results corroborate to the idea that synaptic remodeling was sleep-specific and not just a result of hormonal modulation or circadian rhythmicity.
A recent study detailed early activity-dependent dendritic spine remodeling in the visual cortex by pairing optogenetic stimulation to visual stimulation in vivo [209]. It was found that a small subset of spines undergo rapid and strong structural LTP (sLTP), followed by additional increases in amplitude in the following hours. Concurrently, nearby spines undergo rapid weak depotentiation (structural LTD—sLTD), followed by prominent depotentiation in the next few hours [209]. Interestingly, AMPA receptors redistribution mediated these effects via targeted expression of the IEG Arc after NMDAr-activation [209]. Since Arc expression is upregulated within previously excited cortical circuits during REM sleep in the first hours after experience (Fig. 2b) [161], this further suggests that REM sleep may be necessary for dendritic spine remodeling early after experience, and not only at longer time delays [114].
Although theta rhythm was not quantified in these studies, selective structural remodeling may rely on hippocampal theta oscillations, since numerous studies implicate theta oscillations with synaptic plasticity [212–216]. Additionally, neocortical neurons and oscillations phase-lock to hippocampal theta rhythm during REM sleep [129, 217], and selective REM sleep theta disruption impairs spatial memory [218]. Altogether, the active monitoring of individual spines supports the notion that REM sleep is required for the early bidirectional spine remodeling and later selection of specific new cortical spines to undergo elimination, strengthening, and persistence related to previous experience.
We conclude that both upscaling and downscaling happen during waking and sleep [111, 113]. During waking, sensory inputs are conveyed to hippocampo-cortical circuits so as to induce massive synaptic upscaling as well as downscaling. Sleep seems to be tuned to the refinement of these circuits through reactivation-dependent neuronal upscaling, followed by generalized synaptic downscaling plus selective upscaling. This goes against the notion that upscaling during sleep would be exceptional. Instead, the evidence argues in favor of a crucial role of REM sleep in the early remodeling of existing spines and selection of experience-related recently formed spines. Prior difficulties in finding evidence of experience-dependent upscaling of specific synapses during sleep are likely related to the fact that only ~ 0.04% of the new synapses formed in the neocortical region in relation to a specific training (for instance, motor cortex after motor learning) persist until the end of a mouse’s life [64].
Sleep-dependent cortical plasticity during development
The effort to elucidate sleep-dependent cortical plasticity involved not only adult animals undergoing learning and therefore synaptic plasticity in selected circuits, but also developing animals undergoing high levels of plasticity across multiple circuits [219]. Notwithstanding the major differences between these levels of analysis, there is a substantial amount of parallelism [114]. REM sleep amounts are specifically and dramatically elevated in humans and other mammals in the postnatal period [220–222], in parallel with elevated synaptic plasticity (Fig. 5a, left) [196, 219, 223]. Experimental monocular deprivation (MD) is a very common paradigm used to study plasticity in the visual cortex during critical periods of development [224, 225]. MD causes ocular dominance plasticity (ODP), which stands for the response shift of visual cortex cells in favor of the intact eye [225]. ODP is understood as a physiological process of cortical network response adaptation to naturalistic stimuli through a competitive Hebbian interaction, followed by non-Hebbian homeostatic plasticity to remodel the network [225–227].
Fig. 5.
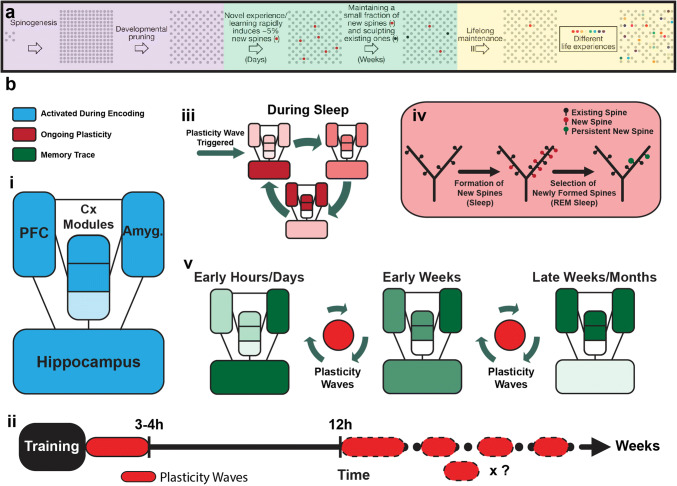
Plasticity across life and the framework for contextual fear conditioning corticalization during REM sleep. a Framework of dendritic spine dynamics across the mouse life. Left (purple): spine dynamics during the postnatal period showing accelerated spinogenesis followed by experience-dependent pruning during a critical developmental period. Middle (green): adult new learning induces a similar process by which spines are rapidly formed and posteriorly selected (pruning and strengthening). Right (yellow): a small fraction of spines from different life experiences is maintained along with a majority of persistent spines from development (modified from [64]). b Framework for REM sleep-dependent CFC corticalization. i Key regions related to CFC memory. ii After training, an initial sleep-dependent plasticity wave (0–3/4 h) is related to memory formation. 12 h after training, a second plasticity wave is critical for memory persistence, although not yet related to sleep (dashed line around ellipse). During the following days, other plasticity waves would proceed with the corticalization process. iii Each plasticity wave initiates in the HPC, probably by previous NREM reactivation of memory traces, and continues in a hippocampofugal manner during REM sleep, episode after episode, gradually reaching the neocortex and other related regions (e.g., amygdala). iv Within regions undergoing plasticity during sleep, experience-related cells and dendrites are reactivated and undergo branch-specific clustered plasticity [58, 208], leading to sleep-dependent formation of new spines, followed by REM sleep-dependent pruning of the majority of new and a minimum of pre-existing spines, along with the reinforcement of the majority of the persistent ones. v In summary, during the first hours/days the CFC memory trace relies mainly on the HPC, amygdala and other related cortical regions to produce retrieval as described in [18]. As time passes, sleep-dependent plasticity waves would lead to a gradual stabilization of the CFC memory trace, making the retrieval less dependent on the HPC and progressively dependent on the interplay between cortical regions and the amygdala, until the HPC is no longer required for remote LTM expression
Using this paradigm, Frank and collaborators showed that NREM sleep enhances ODP in the developing cat visual cortex after 6 h of MD [228]. Despite an increase in REM sleep amount, particularly in the first 2 h after MD, this study was not conclusive about the role of REM sleep in cortical plasticity during development.
Further studies showed that cortical activity is required for sleep-dependent plasticity after MD, since reversible inactivation of the primary visual cortex (V1) during sleep (REM and NREM) precluded ODP in a critical time window that could not be recovered by additional post-inactivation sleep [229, 230]. Also, multiunit activity [231] and firing rates of putative principal neurons in layers 2/3 [232] recorded from V1 during both NREM and REM sleep are elevated after MD and positively correlated with ocular dominance change. The mechanism governing this cortical activity-dependent synaptic plasticity during sleep is dependent on NMDAr and cAMP-dependent protein kinase (PKA), since their specific blockade during sleep impairs ODP [231]. Moreover, during post-MD sleep, there is an increase in the phosphorylation of CaMKII and extracellular signal-regulated kinase (ERK) [231], two LTP-related kinases in the downstream pathway of the NMDAr activation [233, 234].
ODP is positively correlated with NREM sleep amount [228] and with the spike-field coherence in V1, which was calculated using the discharge of principal neurons and delta (0.5–4 Hz) and spindle (7–14 Hz) oscillations [232]. More recently, REM sleep was shown to be required for proper ERK phosphorylation and ODP expression [235]. NMDAr-mediated activation of CaMKII comprise a necessary step for cortical ERK phosphorylation [236], and NMDAr and CaMKII activity in the cortex of adult mice are prominent also during REM sleep [114]. REM sleep deprivation is related to reduced NMDAr surface expression [237] and NMDAr-LTP in the HPC [238]. Signaling through the mammalian target of rapamycin (mTOR) is another mechanism involved in the late phase of NMDAr-dependent hippocampal LTP, synaptic plasticity and memory consolidation [239, 240]. mTOR signaling inhibition by rapamycin specifically during sleep, but not waking, impairs ODP and decreases the levels of various synaptic plasticity-related proteins (Arc, PSD-95, BDNF and eF1A). Rapamycin also decreases the phosphorylation of a target downstream to mTOR, the eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) [240, 241]. Experiments in adult mice using the orientation-specific response potentiation, a learning paradigm to assess plasticity in the visual cortex, shows the same pattern of sleep deprivation-dependent impairment in V1 response potentiation as the developing visual cortex [242].
On the other hand, extended recordings from V1 of young rats suggest that a resetting of firing rates after neuronal activity decay due to prolonged MD (days) may take place only during waking [243]. This finding is at odds with the body of evidence reviewed above [229–232], for reasons yet unclear. One possible explanation is that this work did not assess the response shift of the neurons in the deprived hemisphere after short-term MD (6 h), but the homeostatic effect of different behavioral states to bring neurons up to its baseline firing rate set-point across many days. The everyday homeostatic drive is more prone to downscale circuits excited during experience [109], than upscale local circuits to its baseline set-point after prolonged stimuli deprivation. It is possible that these homeostatic processes comprise different mechanisms. Since external input to deprived circuits is limited during waking, sleep may be involved in only stabilizing the circuit to sustain the wake-dependent increased neuronal activity instead of reducing excessive activation as in normally stimulated cortical circuits [130]. Although it is still unclear how complex structural changes affect neuronal firing rate and vice versa [244], Zuo and collaborators showed that prolonged sensory deprivation shifts the balance of dendritic spine remodeling towards formation [245]. While dendritic spine formation is sustained, spine elimination is reduced in the deprived cortex, with a stronger effect in young compared to adult mice [245]. Since REM sleep is related to spine elimination during development [114], the increased firing rate during waking towards the set-point showed by [243] may be sustained by sleep-dependent structural changes, since they do not return to the previous firing pattern after complete sleep epochs. Although other homeostatic processes may participate as neuronal regulation of intrinsic excitability [246], they may be also sleep-dependent [247].
This evidence is in line with REM sleep-dependent dendritic spine alterations and molecular mechanisms of adult learning and development in the motor cortex [114]. In conclusion, despite the many differences that exist between developing and adult animals, they share key activity-dependent mechanisms of memory consolidation during NREM and REM sleep [114, 248, 249].
Corticalization as a systemic phenomenon with REM sleep-dependent molecular causes
A comprehensive view of the literature supports a framework by which the HPC-dependent memory trace of relevant experiences is consolidated during REM sleep, with increasing dependence on neocortical structures and HPC-independence over time (Fig. 1e, 5b). During the experience, a subset of cells within cortical and subcortical (i.e., HPC and amygdala) structures are tagged (Fig. 5bi). During subsequent sleep, tagged cells go through a maturation process that is transiently HPC-dependent, and increasingly more cortical, as cortico-cortical connections get to be strengthened (Fig. 5bv), in parallel with the integration of the newly acquired memories within the pre-existing network of stable memories (Fig. 5a, middle and right).
As a HPC-dependent memory trace is relocated to the neocortex during sleep, how does the representation change? How does the new memory maintain subjective identity with the original memory, despite the engagement of very different, only partially, overlapping neuronal sets? The mechanisms underlying trace change and in particular the displacement of the main routes required for retrieval remain to a large extent uncharted. The computational simulation of LTP during sleep in a homeostatic artificial fully connected neural network fed with real action potentials from a rat’s HPC showed that the presence or absence of LTP can explain trace strengthening and weakening [127], respectively. However, the most interesting and unexpected consequence of sleep-dependent LTP is trace restructuring, characterized by a rank reorganization of synaptic weights that could in principle account for sleep-enhanced creativity [85, 88, 250–254], understood as the generation of new memory traces from pre-existing ones. The research suggests that even little amounts of LTP during sleep can greatly change memories [127]. It is important, although, to understand the mechanisms that regulate the where, when, and how much of LTP is given that avoids detrimental effects like over-restructuring which would include new traces with loss or an excessive cost for old ones, as proposed theoretically by the stability plasticity-dilemma [255].
Another important question that remains open has to do with the determination of the conditions both necessary and sufficient to trigger synaptic plasticity during sleep. While some minimum amount of REM sleep is required [127, 157, 158, 161, 172], neocortical spindles and hippocampal SWR during the immediately precedent NREM sleep, involving both SWS and intermediate sleep, appear to be important as well [127, 161], possibly contributing to the NREM sleep-dependent increase in intracellular calcium theoretically [107], and recently verified empirically [204, 256]. Interestingly, Seibt and collaborators reported that calcium activity was higher in rodents’ somatosensory cortex during intermediate sleep. They also found a positive correlation between calcium activity during REM sleep and spindle power during the previous SWS-intermediate sleep joint episode [256]. These results are in line with ours, which correlated the amount of spindles immediately before REM sleep initiation with the amount of phosphorylated CaMKII protein immediately after REM sleep episodes [127].
It is important to point out that dendritic calcium spikes were dramatically more prominent during late REM sleep in the motor cortex after motor learning than during NREM sleep, and were abolished by pulsed infusion of the NMDAr inhibitor MK801, along with the impairment in the pruning and strengthening of the experience-dependent newly formed dendritic spines [114]. This indicates that NMDAr activation and calcium influx during REM sleep is also important for dendritic spine remodeling and memory persistence.
In this framework, NREM reactivation may be reinforcing experience-related tagged circuits by inducing transient synaptic facilitation, while REM sleep may induce the necessary protein synthesis able to promote structural changes and memory persistence; which is in line with the synaptic tagging and capture (STC) theory of cellular consolidation [75, 257]. However, no direct evidence has been given that corroborates this proposition, and the mechanisms linking neural processing and calcium activity during SWS to neural processing and calcium activity during subsequent REM sleep, and the role of cortical spindles at SWS/REM transitions in that coupling, remain to be determined.
An explanation may rely on the change in neuronal excitability across different behavioral states, which indicate that plasticity initiates during hippocampal SWR and cortical spindles, but is implemented during REM sleep [258]. PV + cells, which induce somatic inhibition onto pyramidal neurons in the cortex, are thought to be related to the generation of cortical spindles and theta rhythm, and show increased activity during spindles and REM sleep [131, 259–263]. In contrast, somatostatin positive (SOM +) are related to dendritic inhibition and show decreased activity during spindles and REM sleep [131, 262]. It is hypothesized that this shift in the excitatory/inhibitory balance during NREM cortical spindles would open a window for reactivated memories to reach disinhibited distal dendrites, while PV + perisomatic inhibitory drive would provide temporal windows for coincident detection through spike-time dependent plasticity and that the same would happen during REM sleep theta oscillations [262]. This is in line with recent findings, since there is a decouple between L5 pyramidal neurons’ output firing and dendritic activity during spindles [256].
However, this hypothesis is not clear about how this would happen during REM sleep, and some points should be considered in this interpretation. (1) Intra and juxtacellular recordings show pyramidal cell firing rate invariance across different behavioral states [119, 256], concurrent with increased perisomatic PV + inhibition during spindles and REM sleep [131, 262]; (2) Cortical spindles are short-lasted, while theta rhythm is a continuous oscillation [264, 265]; (3) Cortical spindles correlate with dendritic calcium activity and CaMKII phosphorylation during the ensuing REM sleep [127, 256]; (4) Although calcium activity is usually much higher during NREM than during REM [256], the pattern is inverse in recently stimulated cortical regions [114].
This indicates that spindle-related plasticity may not be entirely mirrored to REM sleep. Instead of been continuously suppressed during PV + increased inhibitory activity as during spindles, the sustained pyramidal activity during REM sleep suggests that inhibition is transient, probably coupled to the ongoing theta rhythm [260]. Additionally, the spindle-related activity in the neocortex may reactivate memory traces [262] to undergo intense synaptic remodeling during REM sleep [114]. The mechanisms underlying the memory function of sleep are not entirely elucidated, but the current evidence leads to the notion that NREM sleep induces synaptic downscaling through the addition of many new weak synapses, while REM sleep triggers massive synaptic pruning with the strengthening of selected synapses [114, 208, 262]. A non-exclusive hypothesis is the possibility that NREM sleep reactivates relevant distinct features of the experience, which need to be rehearsed within the pre-existing memory traces in the cortical network during REM sleep to be integrated in an optimized manner [266]. The tagging and optogenetic manipulation of experience-related region-specific networks [18] during sleep, along with the assessment of structural change dynamics [201] may shed some light on this issue.
One more important question that remains unanswered is the characterization of the neurotransmitter milieu during REM sleep that permits synaptic plasticity. Dopaminergic neurons from the ventral tegmental area (VTA) fire in burst during REM sleep [267], but experience-dependent VTA reactivation during REM sleep was not observed in recent studies, which found increased reactivation during quiet waking and SWS [268, 269]. Noradrenergic neurons in the locus coeruleus (LC) show similar dynamics [270], and there is evidence of coupling between noradrenergic and dopaminergic release [271]. It has been hypothesized that dopaminergic and noradrenergic neurons may release neurotransmitters from VTA and LC axonal terminals, respectively, even in the absence of action potentials, due to subthreshold variations in membrane potentials [157, 158].
It is also possible that REM sleep-dependent plasticity is governed by cholinergic mechanisms [272, 273]. For instance, it was shown that the NMDAr-dependent glutamatergic inputs from PFC into the VTA regulate the dopaminergic modulation of the HPC-dependent memory consolidation 12 h after training [177, 178, 274], which is consistent with the mPFC-HPC loop that sustain memory persistence by HPC dopaminergic stimulation 12 h after mPFC dopaminergic inhibition during training. Interestingly, this regulation of VTA activity by mPFC was modulated by cholinergic inputs from the pedunculopontine tegmental nucleus (PPTg) [274]. Increased PPTg cholinergic activity induces burst firing of VTA dopaminergic neurons, leading to massive dopamine release [275]. Although a large body of evidence implicates PPTg activity in the initiation and maintenance of REM sleep [276–281], and VTA dopaminergic neurons fire in burst during REM sleep [267], to the best of our knowledge, a direct evidence that the VTA-dopaminergic modulation of learning and memory is related to PPTg cholinergic inputs during REM sleep was not yet demonstrated.
Finally, the search for the mechanisms underlying memory corticalization must consider that this system-level phenomenon may derive from the molecular properties of the IEG response, and in particular Zif-268, whose expression effectively couples early dendritic changes (e.g., calcium entry through NMDA receptors, CaMKII phosphorylation) to late axonal changes (e.g., changes in synapsin levels), as revised in [111]. Our investigations spanned a window until 4 h after the HPC-dependent relevant experiences (Fig. 2d). Sleep and IEG expression in the first hours after training are usually related to memory formation [170, 172, 189, 190], while the elevated IEG expression in the HPC 12 h after training is related to memory persistence (Fig. 3) [170, 171, 176–178]. Since HPC-dependent memories still depend on the HPC for retrieval (Fig. 1a) [16, 282] and corticalization (Fig. 1d, e) [18, 69, 78] for little more than 1 week, it is possible that other rounds of hippocampofugal plasticity waves take place after this initial 4 h (Fig. 5bii,v), until the trace may be considered mostly cortical (Fig. 5bv).
Additionally, the REM sleep-dependent analysis of dendritic spine remodeling was restricted to the newly training-induced early formed spines [114], reinforcing REM sleep’s role in newly formed spine selection (elimination or strengthening), an effect that was present even for the late time windows examined (8–36 h). Due to the difficulty of completely depriving animals of REM sleep (discussed in “Selective pruning and maintenance of synapses by post-training REM sleep”), the role of this stage in training-induced spine formation during the initial hours after training remains a possibility [208]. The training itself generates patterns of stimulation that rapidly induce potentiation and a marginal depression during waking [209]. But the corticalization process that lasts for ~ 1–2 weeks after training might need sleep for the formation and selection of dendritic spines during this period to integrate the new memory into pre-existing cortical networks (Fig. 5d) [114, 208]. Indeed, the role of complete sleep cycles (NREM and REM) during longer delays remains to be assessed.
However, the training-induced formation of new spines in the cortex and their selection for survival also lasts days to weeks [18, 64, 70, 114, 197, 208]. REM sleep amount is elevated for ~ 1 week after training [186, 187], overlapping with the regional-dependence and spine remodeling induced by training. Altogether, these evidences point to the notion that hippocampofugal plasticity waves recur in time during this period (~ 1–2 weeks), with a critical importance for the 12 h window, in which HPC-cortical networks would be engaged in Zif-268-dependent memory trace maturation in the neocortex during sleep (Fig. 5bii), which is associated with repeated rounds of cortical dendritic spine formation and selection (Fig. 5a, middle, 5biii,iv). This would ensure an optimized inclusion of new experiences into pre-existing networks (Fig. 5A, right), maybe to efficiently accommodate ever-growing complex information into increasingly stable networks, from early periods of development to late adulthood (Fig. 5a) [64]. This is indicated by the fact that sleep contributes to the consolidation of new information related to an already consolidated schema (related or similar experience) [283–291]. Some mechanisms have been proposed to support this idea [203, 292]. Importantly, the inclusion of new hippocampal-dependent information within an already consolidated schema in rodents undergoes accelerated corticalization and involves the same regions (HPC, mPFC and RSC) and molecular factors (NMDAr activation, dopaminergic modulation, Zif-268 and Arc upregulation) as the classical ones reviewed in this work for CFC of “naïve” animals [288, 293–296].
Critical experiments to test this hypothesis require, for instance, blocking Zif-268 expression during specific REM sleep episodes, like the experiments recently performed to block NMDAr activation using MK801 micro-injections [114]. Although the slow dynamics of the current methods to block gene expression make this experiment impossible at present, ongoing experiments with antisense are targeting periods enriched in REM sleep. The hypothesis is that under translational inhibition of Zif-268 expression during REM-enriched periods during critical windows after training (12 h), memory corticalization will be impaired, and the memory will be much more sensitive to interference after that.
Finally, it is important to investigate the role in memory trace stabilization of successive waves of Zif-268 expression during multiple post-training REM sleep episodes (Fig. 5bii), for instance using the CFC paradigm in which the memory trace is dependent initially on the HPC and later on the PFC [18, 56, 69, 70]. Altogether, the data are compatible with the notion that REM sleep is a privileged window for systems consolidation and structural plasticity, with a likely instructive role of the HPC in the stabilization of memory traces in the cerebral cortex.
Funding
Support obtained from the Federal University of Rio Grande do Norte, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Grants 308775/2015-5 and 408145/2016-1 to SR; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (STIC AmSud 062/2015) to SR; Fundação de Amparo à Pesquisa do Rio Grande do Norte Grant Pronem 003/2011 to SR; Fundação de Amparo à Pesquisa do Estado de São Paulo Grant #2013/07699-0 Center for Neuromathematics to SR, Pew Latin American Fellows Program to SR; CAPES/COFECUB Grant 783/13 to CMQ; CAPES PhD scholarship to DGAF.
References
- 1.Hebb DO. The organization of behavior: a neuropsychological theory. New York: Wiley; 1949. [Google Scholar]
- 2.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037//0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 3.McGaugh JL. Memory—a century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 4.Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Hu Y, Tsien JZ. Molecular and systems mechanisms of memory consolidation and storage. Prog Neurobiol. 2006;79(3):123–135. doi: 10.1016/j.pneurobio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Goelet P, Castellucci VF, Schacher S, Kandel ER. The long and the short of long–term memory—a molecular framework. Nature. 1986;322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- 7.Nader K. Memory traces unbound. Trends Neurosci. 2003;26(2):65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 8.McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 9.Lavenex P, Amaral DG (2000) Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus 10(4):420–430. https://doi.org/10.1002/1098-1063(2000)10:4<420::aid-hipo8>3.0.co;2-5 [DOI] [PubMed]
- 10.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6(2):119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 11.Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7(2):217–227. doi: 10.1016/S0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 12.Dudai Y, Karni A, Born J. The consolidation and transformation of memory. Neuron. 2015;88(1):20–32. doi: 10.1016/j.neuron.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Poe GR. Sleep is for forgetting. J Neurosci. 2017;37(3):464–473. doi: 10.1523/JNEUROSCI.0820-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards BA, Frankland PW. The persistence and transience of memory. Neuron. 2017;94(6):1071–1084. doi: 10.1016/j.neuron.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 15.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anagnostaras SG, Gale GD, Fanselow MS (2001) Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus 11(1):8–17. https://doi.org/10.1002/1098-1063(2001)11:1<8::aid-hipo1015>3.0.co;2-7 [DOI] [PubMed]
- 17.Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. Dynamics of retrieval strategies for remote memories. Cell. 2011;147(3):678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, Redondo RL, Tonegawa S. Engrams and circuits crucial for systems consolidation of a memory. Science. 2017;356(6333):73–78. doi: 10.1126/science.aam6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadel L, Hardt O. Update on memory systems and processes. Neuropsychopharmacology. 2011;36(1):251–273. doi: 10.1038/npp.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109(2):195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- 21.Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68(3):285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 22.Winocur G, McDonald RM, Moscovitch M (2001) Anterograde and retrograde amnesia in rats with large hippocampal lesions. Hippocampus 11(1):18–26. https://doi.org/10.1002/1098-1063(2001)11:1<18::aid-hipo1016>3.0.co;2-5 [DOI] [PubMed]
- 23.Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400(6745):671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- 24.Brodt S, Pöhlchen D, Flanagin VL, Glasauer S, Gais S, Schönauer M. Rapid and independent memory formation in the parietal cortex. Proc Natl Acad Sci USA. 2016;113(46):13251–13256. doi: 10.1073/pnas.1605719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haist F, Bowden Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci. 2001;4(11):1139–1145. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- 26.Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30(2):188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci Off J Pavlov. 1980;15(4):177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290(5494):1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- 29.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Larson J, Munkácsy E. Theta-burst LTP. Brain Res. 2015;1621:38–50. doi: 10.1016/j.brainres.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313(5790):1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 32.Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mzeta maintains late-phase long-term potentiation. J Neurosci. 2005;25(8):1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313(5790):1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 34.Feldman DE, Nicoll RA, Malenka RC. Synaptic plasticity at thalamocortical synapses in developing rat somatosensory cortex: LTP, LTD, and silent synapses. J Neurobiol. 1999;41(1):92–101. doi: 10.1002/(SICI)1097-4695(199910)41:1<92::AID-NEU12>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 35.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290(5491):533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez-Durán LF, Martínez-Moreno A, Escobar ML. Bidirectional modulation of taste aversion extinction by insular cortex LTP and LTD. Neurobiol Learn Mem. 2017;142(Pt A):85–90. doi: 10.1016/j.nlm.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511(7509):348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volianskis A, France G, Jensen MS, Bortolotto ZA, Jane DE, Collingridge GL. Long-term potentiation and the role of N-methyl-d-aspartate receptors. Brain Res. 2015;1621:5–16. doi: 10.1016/j.brainres.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park P, Volianskis A, Sanderson TM, Bortolotto ZA, Jane DE, Zhuo M, Kaang B-K, Collingridge GL. NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Philos Trans R Soc Lond B Biol Sci. 2014;369(1633):20130131. doi: 10.1098/rstb.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dore K, Aow J, Malinow R. The emergence of NMDA receptor metabotropic function: insights from imaging. Front Synaptic Neurosci. 2016;8:20. doi: 10.3389/fnsyn.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4(3):289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- 42.Bozon B, Kelly A, Josselyn SA, Silva AJ, Davis S, Laroche S. MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Philos Trans R Soc Lond B Biol Sci. 2003;358(1432):805–814. doi: 10.1098/rstb.2002.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav Brain Res. 2003;142(1–2):17–30. doi: 10.1016/S0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- 44.Alberini CM. The role of protein synthesis during the labile phases of memory: revisiting the skepticism. Neurobiol Learn Mem. 2008;89(3):234–246. doi: 10.1016/j.nlm.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10(3):224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg T, Gal-Ben-Ari S, Dieterich DC, Kreutz MR, Ziv NE, Gundelfinger ED, Rosenblum K. The roles of protein expression in synaptic plasticity and memory consolidation. Front Mol Neurosci. 2014;7:86. doi: 10.3389/fnmol.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gisquet-Verrier P, Lynch JF, Cutolo P, Toledano D, Ulmen A, Jasnow AM, Riccio DC. Integration of new information with active memory accounts for retrograde amnesia: a challenge to the consolidation/reconsolidation hypothesis? J Neurosci. 2015;35(33):11623–11633. doi: 10.1523/JNEUROSCI.1386-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Memory. Engram cells retain memory under retrograde amnesia. Science. 2015;348(6238):1007–1013. doi: 10.1126/science.aaa5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JLC, Nader K, Schiller D. An update on memory reconsolidation updating. Trends Cogn Sci. 2017;21(7):531–545. doi: 10.1016/j.tics.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3(3):175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 51.Achterberg KG, Buitendijk GH, Kool MJ, Goorden SM, Post L, Slump DE, Silva AJ, van Woerden GM, Kushner SA, Elgersma Y. Temporal and region-specific requirements of αCaMKII in spatial and contextual learning. J Neurosci. 2014;34(34):11180–11187. doi: 10.1523/JNEUROSCI.0640-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Peng RY. Basic roles of key molecules connected with NMDAR signaling pathway on regulating learning and memory and synaptic plasticity. Mil Med Res. 2016;3(1):26. doi: 10.1186/s40779-016-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Shimizu E, Tang YP, Cho M, Kyin M, Zuo W, Robinson DA, Alaimo PJ, Zhang C, Morimoto H, Zhuo M, Feng R, Shokat KM, Tsien JZ. Inducible protein knockout reveals temporal requirement of CaMKII reactivation for memory consolidation in the brain. Proc Natl Acad Sci USA. 2003;100(7):4287–4292. doi: 10.1073/pnas.0636870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva A, Paylor R, Wehner J, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257(5067):206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 55.Frankland PW, O’Brien C, Ohno M, Kirkwood A, Silva AJ. Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411(6835):309–313. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- 56.Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304(5672):881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 57.Bock J, Braun K. Blockade of N-methyl-d-aspartate receptor activation suppresses learning-induced synaptic elimination. Proc Natl Acad Sci USA. 1999;96(5):2485–2490. doi: 10.1073/pnas.96.5.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Govindarajan A, Kelleher RJ, Tonegawa S. A clustered plasticity model of long-term memory engrams. Nat Rev Neurosci. 2006;7(7):575–583. doi: 10.1038/nrn1937. [DOI] [PubMed] [Google Scholar]
- 59.De Roo M, Klauser P, Muller D. LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS Biol. 2008;6(9):e219. doi: 10.1371/journal.pbio.0060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clopath C. Synaptic consolidation: an approach to long-term learning. Cogn Neurodyn. 2012;6(3):251–257. doi: 10.1007/s11571-011-9177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caroni P, Chowdhury A, Lahr M. Synapse rearrangements upon learning: from divergent-sparse connectivity to dedicated sub-circuits. Trends Neurosci. 2014;37(10):604–614. doi: 10.1016/j.tins.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 62.Attardo A, Fitzgerald JE, Schnitzer MJ. Impermanence of dendritic spines in live adult CA1 hippocampus. Nature. 2015;523(7562):592–596. doi: 10.1038/nature14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- 64.Yang G, Pan F, Gan W-B. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462(7275):920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller R. Neural assemblies and laminar interactions in the cerebral cortex. Biol Cybern. 1996;75(3):253–261. doi: 10.1007/s004220050292. [DOI] [PubMed] [Google Scholar]
- 66.Takashima A, Nieuwenhuis IL, Jensen O, Talamini LM, Rijpkema M, Fernández G. Shift from hippocampal to neocortical centered retrieval network with consolidation. J Neurosci. 2009;29(32):10087–10093. doi: 10.1523/JNEUROSCI.0799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maviel T. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305(5680):96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- 68.Sacco T, Sacchetti B. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science. 2010;329(5992):649–656. doi: 10.1126/science.1183165. [DOI] [PubMed] [Google Scholar]
- 69.Restivo L, Vetere G, Bontempi B, Ammassari-Teule M. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J Neurosci. 2009;29(25):8206–8214. doi: 10.1523/JNEUROSCI.0966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vetere G, Restivo L, Cole CJ, Ross PJ, Ammassari-Teule M, Josselyn SA, Frankland PW. Spine growth in the anterior cingulate cortex is necessary for the consolidation of contextual fear memory. Proc Natl Acad Sci USA. 2011;108(20):8456–8460. doi: 10.1073/pnas.1016275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flavell SW, Cowan CW, Kim T-K, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311(5763):1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 72.Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59(4):621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lesburgueres E, Gobbo OL, Alaux-Cantin S, Hambucken A, Trifilieff P, Bontempi B. Early tagging of cortical networks is required for the formation of enduring associative memory. Science. 2011;331(6019):924–928. doi: 10.1126/science.1196164. [DOI] [PubMed] [Google Scholar]
- 74.Alvarez P, Lipton PA, Melrose R, Eichenbaum H. Differential effects of damage within the hippocampal region on memory for a natural, nonspatial odor–odor association. Learn Mem. 2001;8(2):79–86. doi: 10.1101/lm.38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frey U, Morris RG. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21(5):181–188. doi: 10.1016/S0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- 76.Wiltgen BJ. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26(20):5484–5491. doi: 10.1523/jneurosci.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zelikowsky M, Bissiere S, Hast TA, Bennett RZ, Abdipranoto A, Vissel B, Fanselow MS. Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proc Natl Acad Sci USA. 2013;110(24):9938–9943. doi: 10.1073/pnas.1301691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zelikowsky M, Bissiere S, Fanselow MS. Contextual fear memories formed in the absence of the dorsal hippocampus decay across time. J Neurosci. 2012;32(10):3393–3397. doi: 10.1523/JNEUROSCI.4339-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Witter M. Entorhinal cortex. Scholarpedia J. 2011;6(10):4380. doi: 10.4249/scholarpedia.4380. [DOI] [Google Scholar]
- 80.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16(6):317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 81.Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12(11):1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nonaka A, Toyoda T, Miura Y, Hitora-Imamura N, Naka M, Eguchi M, Yamaguchi S, Ikegaya Y, Matsuki N, Nomura H. Synaptic plasticity associated with a memory engram in the basolateral amygdala. J Neurosci. 2014;34(28):9305–9309. doi: 10.1523/JNEUROSCI.4233-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317(5842):1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 84.Jenkins JG, Dallenbach KM. Obliviscence during sleep and waking. Am J Psychol. 1924;35(4):605–612. doi: 10.2307/1414040. [DOI] [Google Scholar]
- 85.Stickgold R, Scott L, Rittenhouse C, Hobson JA. Sleep-induced changes in associative memory. J Cogn Neurosci. 1999;11(2):182–193. doi: 10.1162/089892999563319. [DOI] [PubMed] [Google Scholar]
- 86.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437(7063):1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 87.Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 88.Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci. 2009;106(25):10130–10134. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010 doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 90.Diekelmann S. Sleep for cognitive enhancement. Front Syst Neurosci. 2014;8:46. doi: 10.3389/fnsys.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93(2):681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315(5817):1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 93.Tucker MA, Fishbein W. Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep. 2008;31(2):197–203. doi: 10.1093/sleep/31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruch S, Markes O, Duss SB, Oppliger D, Reber TP, Koenig T, Mathis J, Roth C, Henke K. Sleep stage II contributes to the consolidation of declarative memories. Neuropsychologia. 2012;50(10):2389–2396. doi: 10.1016/j.neuropsychologia.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 95.Laureys S, Peigneux P, Phillips C, Fuchs S, Degueldre C, Aerts J, Del Fiore G, Petiau C, Luxen A, van der Linden M, Cleeremans A, Smith C, Maquet P. Experience-dependent changes in cerebral functional connectivity during human rapid eye movement sleep. Neuroscience. 2001;105(3):521–525. doi: 10.1016/S0306-4522(01)00269-X. [DOI] [PubMed] [Google Scholar]
- 96.Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19(5):1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8(2):112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith C. Sleep states and memory processes in humans: procedural versus declarative memory systems. Sleep Med Rev. 2001;5(6):491–506. doi: 10.1053/smrv.2001.0164. [DOI] [PubMed] [Google Scholar]
- 99.Ackermann S, Rasch B. Differential effects of non-REM and REM sleep on memory consolidation? Curr Neurol Neurosci Rep. 2014;14(2):430. doi: 10.1007/s11910-013-0430-8. [DOI] [PubMed] [Google Scholar]
- 100.Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci. 1989;9(8):2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265(5172):676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 102.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36(6):1183–1194. doi: 10.1016/S0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 103.Diba K, Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10(10):1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262(841):23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 105.Chrobak JJ, Buzsáki G. Selective activation of deep layer (V–VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. J Neurosci. 1994;14(10):6160–6170. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4(3):374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 107.Sejnowski TJ, Destexhe A. Why do we sleep? Brain Res. 2000;886(1–2):208–223. doi: 10.1016/S0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- 108.Timofeev I, Chauvette S. Sleep slow oscillation and plasticity. Curr Opin Neurobiol. 2017;44:116–126. doi: 10.1016/j.conb.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 109.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Giuditta A, Ambrosini MV, Montagnese P, Mandile P, Cotugno M, Zucconi GG, Vescia S. The sequential hypothesis of the function of sleep. Behav Brain Res. 1995;69(1–2):157–166. doi: 10.1016/0166-4328(95)00012-i. [DOI] [PubMed] [Google Scholar]
- 111.Ribeiro S, Nicolelis MAL. Reverberation, storage, and postsynaptic propagation of memories during sleep. Learn Mem. 2004;11(6):686–696. doi: 10.1101/lm.75604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Poe GR, Walsh CM, Bjorness TE. Cognitive neuroscience of sleep. Prog Brain Res. 2010;185:1–19. doi: 10.1016/B978-0-444-53702-7.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ribeiro S. Sleep and plasticity. Pflugers Arch. 2012;463(1):111–120. doi: 10.1007/s00424-011-1031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li W, Ma L, Yang G, Gan W-B. REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci. 2017;20(3):427–437. doi: 10.1038/nn.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res. 2011;76(2):192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chauvette S, Seigneur J, Timofeev I. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron. 2012;75(6):1105–1113. doi: 10.1016/j.neuron.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92(3):1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khodagholy D, Gelinas JN, Buzsáki G. Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science. 2017;358(6361):369–372. doi: 10.1126/science.aan6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85(5):1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 120.Gray CM, McCormick DA. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science. 1996;274(5284):109–113. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- 121.Thomson AM, West DC, Hahn J, Deuchars J. Single axon IPSPs elicited in pyramidal cells by three classes of interneurons in slices of rat neocortex. J Physiol. 1996;496(Pt 1):81–102. doi: 10.1113/jphysiol.1996.sp021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steriade M, Timofeev I, Dürmüller N, Grenier F. Dynamic properties of corticothalamic neurons and local cortical interneurons generating fast rhythmic (30–40 Hz) spike bursts. J Neurophysiol. 1998;79(1):483–490. doi: 10.1152/jn.1998.79.1.483. [DOI] [PubMed] [Google Scholar]
- 123.Roxin A, Brunel N, Hansel D, Mongillo G, van Vreeswijk C. On the distribution of firing rates in networks of cortical neurons. J Neurosci. 2011 doi: 10.1523/jneurosci.1677-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rahmati V, Kirmse K, Marković D, Holthoff K, Kiebel SJ. Inferring neuronal dynamics from calcium imaging data using biophysical models and Bayesian inference. PLoS Comput Biol. 2016;12(2):e1004736. doi: 10.1371/journal.pcbi.1004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rahmati V, Kirmse K, Holthoff K, Kiebel SJ. Ultra-fast accurate reconstruction of spiking activity from calcium imaging data. J Neurophysiol. 2018 doi: 10.1152/jn.00934.2017. [DOI] [PubMed] [Google Scholar]
- 126.Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsáki G. REM sleep reorganizes hippocampal excitability. Neuron. 2012;75(6):1001–1007. doi: 10.1016/j.neuron.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Blanco W, Pereira CM, Cota VR, Souza AC, Rennó-Costa C, Santos S, Dias G, Guerreiro AMG, Tort ABL, Neto AD, Ribeiro S. Synaptic homeostasis and restructuring across the sleep-wake cycle. PLoS Comput Biol. 2015;11(5):e1004241. doi: 10.1371/journal.pcbi.1004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63(6):865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koike BDV, Farias KS, Billwiller F, Almeida-Filho D, Libourel PA, Tiran-Cappello A, Parmentier R, Blanco W, Ribeiro S, Luppi PH, Queiroz CM. Electrophysiological evidence that the retrosplenial cortex displays a strong and specific activation phased with hippocampal theta during paradoxical (REM) sleep. J Neurosci. 2017;37(33):8003–8013. doi: 10.1523/JNEUROSCI.0026-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Watson BO, Levenstein D, Greene JP, Gelinas JN, Buzsáki G. Network homeostasis and state dynamics of neocortical sleep. Neuron. 2016;90(4):839–852. doi: 10.1016/j.neuron.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Niethard N, Hasegawa M, Itokazu T, Oyanedel CN, Born J, Sato TR. Sleep-stage-specific regulation of cortical excitation and inhibition. Curr Biol. 2016;26(20):2739–2749. doi: 10.1016/j.cub.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 132.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 133.Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271(5257):1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 134.Paller KA, Voss JL. Memory reactivation and consolidation during sleep. Learn Mem. 2004;11(6):664–670. doi: 10.1101/lm.75704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ribeiro S, Gervasoni D, Soares ES, Zhou Y, Lin S-C, Pantoja J, Lavine M, Nicolelis MAL. Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas. PLoS Biol. 2004;2(1):E24. doi: 10.1371/journal.pbio.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O’Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. Play it again: reactivation of waking experience and memory. Trends Neurosci. 2010;33(5):220–229. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 137.Buzsáki G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25(10):1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Destexhe A, Hughes SW, Rudolph M, Crunelli V. Are corticothalamic ‘up’ states fragments of wakefulness? Trends Neurosci. 2007;30(7):334–342. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Girardeau G, Inema I, Buzsáki G. Reactivations of emotional memory in the hippocampus-amygdala system during sleep. Nat Neurosci. 2017;20(11):1634–1642. doi: 10.1038/nn.4637. [DOI] [PubMed] [Google Scholar]
- 140.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21(5):1123–1128. doi: 10.1016/S0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 141.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10(1):100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 142.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12(10):1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 143.Peyrache A, Khamassi M, Benchenane K, Wiener SI, Battaglia FP. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci. 2009;12(7):919–926. doi: 10.1038/nn.2337. [DOI] [PubMed] [Google Scholar]
- 144.Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61(4):587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang DV, Ikemoto S. Coordinated interaction between hippocampal sharp-wave ripples and anterior cingulate unit activity. J Neurosci. 2016;36(41):10663–10672. doi: 10.1523/JNEUROSCI.1042-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miyamoto D, Hirai D, Fung CC, Inutsuka A, Odagawa M, Suzuki T, Boehringer R, Adaikkan C, Matsubara C, Matsuki N, Fukai T, McHugh TJ, Yamanaka A, Murayama M. Top-down cortical input during NREM sleep consolidates perceptual memory. Science. 2016;352(6291):1315–1318. doi: 10.1126/science.aaf0902. [DOI] [PubMed] [Google Scholar]
- 147.Maingret N, Girardeau G, Todorova R, Goutierre M, Zugaro M. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat Neurosci. 2016;19(7):959–964. doi: 10.1038/nn.4304. [DOI] [PubMed] [Google Scholar]
- 148.Latchoumane CV, Ngo HV, Born J, Shin HS. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron. 2017;95(2):424–435. doi: 10.1016/j.neuron.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 149.Schouten DI, Pereira SIR, Tops M, Louzada FM. State of the art on targeted memory reactivation: sleep your way to enhanced cognition. Sleep Med Rev. 2017;32:123–131. doi: 10.1016/j.smrv.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 150.de Lavilléon G, Lacroix MM, Rondi-Reig L, Benchenane K. Explicit memory creation during sleep demonstrates a causal role of place cells in navigation. Nat Neurosci. 2015;18(4):493–495. doi: 10.1038/nn.3970. [DOI] [PubMed] [Google Scholar]
- 151.Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336(6087):1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gelinas JN, Khodagholy D, Thesen T, Devinsky O, Buzsaki G. Interictal epileptiform discharges induce hippocampal–cortical coupling in temporal lobe epilepsy. Nat Med. 2016;22(6):641–648. doi: 10.1038/nm.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rasch B, Born J. Maintaining memories by reactivation. Curr Opin Neurobiol. 2007;17(6):698–703. doi: 10.1016/j.conb.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 154.Ramadan W, Eschenko O, Sara SJ. Hippocampal sharp wave/ripples during sleep for consolidation of associative memory. PLoS One. 2009;4(8):e6697. doi: 10.1371/journal.pone.0006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Furini CRG, Myskiw JC, Benetti F, Izquierdo I. New frontiers in the study of memory mechanisms. Rev Bras Psiquiatr. 2013;35(2):173–177. doi: 10.1590/1516-4446-2012-1046. [DOI] [PubMed] [Google Scholar]
- 156.Alberini CM, Kandel ER. The regulation of transcription in memory consolidation. Cold Spring Harb Perspect Biol. 2015;7(1):a021741. doi: 10.1101/cshperspect.a021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ribeiro S, Goyal V, Mello CV, Pavlides C. Brain gene expression during REM sleep depends on prior waking experience. Learn Mem. 1999;6(5):500–508. doi: 10.1101/lm.6.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ribeiro S, Mello CV, Velho T, Gardner TJ, Jarvis ED, Pavlides C. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J Neurosci. 2002;22(24):10914–10923. doi: 10.1523/JNEUROSCI.22-24-10914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ulloor J, Datta S. Spatio-temporal activation of cyclic AMP response element-binding protein, activity-regulated cytoskeletal-associated protein and brain-derived nerve growth factor: a mechanism for pontine-wave generator activation-dependent two-way active-avoidance memory processing in the rat. J Neurochem. 2005;95(2):418–428. doi: 10.1111/j.1471-4159.2005.03378.x. [DOI] [PubMed] [Google Scholar]
- 160.Frank MG, Benington JH. The role of sleep in memory consolidation and brain plasticity: dream or reality? Neuroscientist. 2006;12(6):477–488. doi: 10.1177/1073858406293552. [DOI] [PubMed] [Google Scholar]
- 161.Ribeiro S, Shi X, Engelhard M, Zhou Y, Zhang H, Gervasoni D, Lin S-C, Wada K, Lemos NA, Nicolelis MA. Novel experience induces persistent sleep-dependent plasticity in the cortex but not in the hippocampus. Front Neurosci. 2007;1(1):43. doi: 10.3389/neuro.01.1.1.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ravassard P, Pachoud B, Comte JC, Mejia-Perez C, Scote-Blachon C, Gay N, Claustrat B, Touret M, Luppi PH, Salin PA. Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep. 2009;32(2):227–240. doi: 10.1093/sleep/32.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol. 2013;23(17):R774–R788. doi: 10.1016/j.cub.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Luo J, Phan TX, Yang Y, Garelick MG, Storm DR. Increases in cAMP, MAPK activity, and CREB phosphorylation during REM sleep: implications for REM sleep and memory consolidation. J Neurosci. 2013;33(15):6460–6468. doi: 10.1523/JNEUROSCI.5018-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Calais JB, Ojopi EB, Morya E, Sameshima K, Ribeiro S. Experience-dependent upregulation of multiple plasticity factors in the hippocampus during early REM sleep. Neurobiol Learn Mem. 2015;122:19–27. doi: 10.1016/j.nlm.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 166.Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, Wibrand K. The Arc of synaptic memory. Exp Brain Res. 2010;200(2):125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Malkani S, Wallace KJ, Donley MP, Rosen JB. An egr-1 (zif268) antisense oligodeoxynucleotide infused into the amygdala disrupts fear conditioning. Learn Mem. 2004;11(5):617–624. doi: 10.1101/lm.73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lonergan ME, Gafford GM, Jarome TJ, Helmstetter FJ. Time-dependent expression of Arc and zif268 after acquisition of fear conditioning. Neural Plast. 2010;2010:139891. doi: 10.1155/2010/139891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14(3):279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Katche C, Goldin A, Gonzalez C, Bekinschtein P, Medina JH. Maintenance of long-term memory storage is dependent on late posttraining Egr-1 expression. Neurobiol Learn Mem. 2012;98(3):220–227. doi: 10.1016/j.nlm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 171.Nakayama D, Iwata H, Teshirogi C, Ikegaya Y, Matsuki N, Nomura H. Long-delayed expression of the immediate early gene Arc/Arg3.1 refines neuronal circuits to perpetuate fear memory. J Neurosci. 2015;35(2):819–830. doi: 10.1523/JNEUROSCI.2525-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ravassard P, Hamieh AM, Joseph MA, Fraize N, Libourel P-A, Lebarillier L, Arthaud S, Meissirel C, Touret M, Malleret G, Salin P-A. REM sleep-dependent bidirectional regulation of hippocampal-based emotional memory and LTP. Cereb Cortex. 2016;26(4):1488–1500. doi: 10.1093/cercor/bhu310. [DOI] [PubMed] [Google Scholar]
- 173.Ravassard P, Hamieh AM, Malleret G, Salin PA. Paradoxical sleep: a vigilance state to gate long-term brain plasticity? Neurobiol Learn Mem. 2015;122:4–10. doi: 10.1016/j.nlm.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 174.Norman ED, Thiels E, Barrionuevo G, Klann E. Long-term depression in the hippocampus in vivo is associated with protein phosphatase-dependent alterations in extracellular signal-regulated kinase. J Neurochem. 2000;74(1):192–198. doi: 10.1046/j.1471-4159.2000.0740192.x. [DOI] [PubMed] [Google Scholar]
- 175.Thiels E, Kanterewicz BI, Knapp LT, Barrionuevo G, Klann E. Protein phosphatase-mediated regulation of protein kinase C during long-term depression in the adult hippocampus in vivo. J Neurosci. 2000;20(19):7199–7207. doi: 10.1523/JNEUROSCI.20-19-07199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LRM, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007;53(2):261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 177.Gonzalez MC, Kramar CP, Tomaiuolo M, Katche C, Weisstaub N, Cammarota M, Medina JH. Medial prefrontal cortex dopamine controls the persistent storage of aversive memories. Front Behav Neurosci. 2014;8:408. doi: 10.3389/fnbeh.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rossato JI, Bevilaqua LRM, Izquierdo I, Medina JH, Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325(5943):1017–1020. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- 179.Katche C, Dorman G, Gonzalez C, Kramar CP, Slipczuk L, Rossato JI, Cammarota M, Medina JH. On the role of retrosplenial cortex in long-lasting memory storage. Hippocampus. 2013;23(4):295–302. doi: 10.1002/hipo.22092. [DOI] [PubMed] [Google Scholar]
- 180.Katche C, Dorman G, Slipczuk L, Cammarota M, Medina JH. Functional integrity of the retrosplenial cortex is essential for rapid consolidation and recall of fear memory. Learn Mem. 2013;20(4):170–173. doi: 10.1101/lm.030080.112. [DOI] [PubMed] [Google Scholar]
- 181.Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. Direct reactivation of a coherent neocortical memory of context. Neuron. 2014;84(2):432–441. doi: 10.1016/j.neuron.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Haijima A, Ichitani Y. Anterograde and retrograde amnesia of place discrimination in retrosplenial cortex and hippocampal lesioned rats. Learn Mem. 2008;15(7):477–482. doi: 10.1101/lm.862308. [DOI] [PubMed] [Google Scholar]
- 183.Haijima A, Ichitani Y. Dissociable anterograde amnesic effects of retrosplenial cortex and hippocampal lesions on spontaneous object recognition memory in rats. Hippocampus. 2012;22(9):1868–1875. doi: 10.1002/hipo.22021. [DOI] [PubMed] [Google Scholar]
- 184.Katche C, Medina JH. Requirement of an early activation of BDNF/c-Fos cascade in the retrosplenial cortex for the persistence of a long-lasting aversive memory. Cereb Cortex. 2017;27(2):1060–1067. doi: 10.1093/cercor/bhv284. [DOI] [PubMed] [Google Scholar]
- 185.Renouard L, Billwiller F, Ogawa K, Clément O, Camargo N, Abdelkarim M, Gay N, Scoté-Blachon C, Touré R, Libourel P-A, Ravassard P, Salvert D, Peyron C, Claustrat B, Léger L, Salin P, Malleret G, Fort P, Luppi P-H. The supramammillary nucleus and the claustrum activate the cortex during REM sleep. Sci Adv. 2015;1(3):e1400177. doi: 10.1126/sciadv.1400177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Smith C, Young J, Young W. Prolonged increases in paradoxical sleep during and after avoidance-task acquisition. Sleep. 1980;3(1):67–81. [PubMed] [Google Scholar]
- 187.Smith C, Lapp L. Prolonged increases in both PS and number of REMs following a shuttle avoidance task. Physiol Behav. 1986;36(6):1053–1057. doi: 10.1016/0031-9384(86)90479-8. [DOI] [PubMed] [Google Scholar]
- 188.Smith C, Butler S. Paradoxical sleep at selective times following training is necessary for learning. Physiol Behav. 1982;29(3):469–473. doi: 10.1016/0031-9384(82)90268-2. [DOI] [PubMed] [Google Scholar]
- 189.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10(3):168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Prince TM, Wimmer M, Choi J, Havekes R, Aton S, Abel T. Sleep deprivation during a specific 3-hour time window post-training impairs hippocampal synaptic plasticity and memory. Neurobiol Learn Mem. 2014;109:122–130. doi: 10.1016/j.nlm.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.França AS, Lobão-Soares B, Muratori L, Nascimento G, Winne J, Pereira CM, Jeronimo SM, Ribeiro S. D2 dopamine receptor regulation of learning, sleep and plasticity. Eur Neuropsychopharmacol. 2015;25(4):493–504. doi: 10.1016/j.euroneuro.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 192.Yasenkov R, Deboer T (2012) Circadian modulation of sleep in rodents. In: Progress in brain research, pp 203–218. 10.1016/b978-0-444-59427-3.00012-5 [DOI] [PubMed]
- 193.Gerstner JR, Yin JCP. Circadian rhythms and memory formation. Nat Rev Neurosci. 2010;11(8):577–588. doi: 10.1038/nrn2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Frank MG. Circadian regulation of synaptic plasticity. Biology. 2016;5(3):31. doi: 10.3390/biology5030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GCK, Scheiner ZS, Storm DR. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nat Neurosci. 2008;11(9):1074–1082. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yang G, Gan WB. Sleep contributes to dendritic spine formation and elimination in the developing mouse somatosensory cortex. Dev Neurobiol. 2012;72(11):1391–1398. doi: 10.1002/dneu.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan W-B. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16(6):698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62(2):143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 199.Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13(5):309–321. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 200.Denk W, Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2004;2(11):e329. doi: 10.1371/journal.pbio.0020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yang G, Pan F, Chang PC, Gooden F, Gan W-B (2013) Transcranial two-photon imaging of synaptic structures in the cortex of awake head-restrained mice. In: Methods in molecular biology, pp 35–43. 10.1007/978-1-62703-411-1_3 [DOI] [PMC free article] [PubMed]
- 202.de Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, Cirelli C. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science. 2017;355(6324):507–510. doi: 10.1126/science.aah5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lewis PA, Durrant SJ. Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn Sci. 2011;15(8):343–351. doi: 10.1016/j.tics.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 204.Norimoto H, Makino K, Gao M, Shikano Y, Okamoto K, Ishikawa T, Sasaki T, Hioki H, Fujisawa S, Ikegaya Y. Hippocampal ripples down-regulate synapses. Science. 2018;359(6383):1524–1527. doi: 10.1126/science.aao0702. [DOI] [PubMed] [Google Scholar]
- 205.Sadowski JH, Jones MW, Mellor JR. Sharp-wave ripples orchestrate the induction of synaptic plasticity during reactivation of place cell firing patterns in the hippocampus. Cell Rep. 2016;14(8):1916–1929. doi: 10.1016/j.celrep.2016.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hashmi A, Nere A, Tononi G. Sleep-dependent synaptic down-selection (II): single-neuron level benefits for matching, selectivity, and specificity. Front Neurol. 2013 doi: 10.3389/fneur.2013.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nere A, Hashmi A, Cirelli C, Tononi G. Sleep-dependent synaptic down-selection (I): modeling the benefits of sleep on memory consolidation and integration. Front Neurol. 2013;4:143. doi: 10.3389/fneur.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yang G, Lai CSW, Cichon J, Ma L, Li W, Gan W-B. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344(6188):1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.El-Boustani S, Ip JPK, Breton-Provencher V, Knott GW, Okuno H, Bito H, Sur M. Locally coordinated synaptic plasticity of visual cortex neurons in vivo. Science. 2018;360(6395):1349–1354. doi: 10.1126/science.aao0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76(2):99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 211.Nikolaienko O, Patil S, Eriksen MS, Bramham CR. Arc protein: a flexible hub for synaptic plasticity and cognition. Semin Cell Dev Biol. 2018;77:33–42. doi: 10.1016/j.semcdb.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 212.Pavlides C, Greenstein YJ, Grudman M, Winson J. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of θ-rhythm. Brain Res. 1988;439(1–2):383–387. doi: 10.1016/0006-8993(88)91499-0. [DOI] [PubMed] [Google Scholar]
- 213.Hölscher C, Anwyl R, Rowan MJ. Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can be depotentiated by stimulation on the negative phase in area CA1 in vivo. J Neurosci. 1997;17(16):6470–6477. doi: 10.1523/JNEUROSCI.17-16-06470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Poe GR, Nitz DA, McNaughton BL, Barnes CA. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 2000;855(1):176–180. doi: 10.1016/S0006-8993(99)02310-0. [DOI] [PubMed] [Google Scholar]
- 215.Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J Neurosci. 2003;23(37):11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Booth V, Poe GR. Input source and strength influences overall firing phase of model hippocampal CA1 pyramidal cells during theta: relevance to REM sleep reactivation and memory consolidation. Hippocampus. 2006;16(2):161–173. doi: 10.1002/hipo.20143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60(4):683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Boyce R, Glasgow SD, Williams S, Adamantidis A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science. 2016;352(6287):812–816. doi: 10.1126/science.aad5252. [DOI] [PubMed] [Google Scholar]
- 219.Frank MG (2014) Sleep and synaptic plasticity in the developing and adult brain. In: Current topics in behavioral neurosciences, pp 123–149. 10.1007/7854_2014_305 [DOI] [PMC free article] [PubMed]
- 220.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152(3722):604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 221.Thurber A, Jha SK, Coleman T, Frank MG. A preliminary study of sleep ontogenesis in the ferret (Mustela putorius furo) Behav Brain Res. 2008;189(1):41–51. doi: 10.1016/j.bbr.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Frank MG (2011) The ontogeny and function(s) of REM sleep. In: Rapid eye movement sleep, pp 49–57. 10.1017/cbo9780511921179.008
- 223.Lohmann C, Kessels HW. The developmental stages of synaptic plasticity. J Physiol. 2013;592(1):13–31. doi: 10.1113/jphysiol.2012.235119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hubel DH, Wiesel TN. Anatomical demonstration of columns in the monkey striate cortex. Nature. 1969;221(5182):747–750. doi: 10.1038/221747a0. [DOI] [PubMed] [Google Scholar]
- 225.Frank MG. Sleep and plasticity in the visual cortex: more than meets the eye. Curr Opin Neurobiol. 2017;44:8–12. doi: 10.1016/j.conb.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Crair MC, Shah RD (2009) Long-term potentiation and long-term depression in experience-dependent plasticity. In: Encyclopedia of neuroscience, pp 561–570. 10.1016/b978-008045046-9.01213-4
- 227.Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75(2):230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30(1):275–287. doi: 10.1016/S0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 229.Jha SK, Jones BE, Coleman T, Steinmetz N, Law C-T, Griffin G, Hawk J, Dabbish N, Kalatsky VA, Frank MG. Sleep-dependent plasticity requires cortical activity. J Neurosci. 2005;25(40):9266–9274. doi: 10.1523/JNEUROSCI.2722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Frank MG, Jha SK, Coleman T. Blockade of postsynaptic activity in sleep inhibits developmental plasticity in visual cortex. NeuroReport. 2006;17(13):1459–1463. doi: 10.1097/01.wnr.0000233100.05408.e4. [DOI] [PubMed] [Google Scholar]
- 231.Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61(3):454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Aton SJ, Broussard C, Dumoulin M, Seibt J, Watson A, Coleman T, Frank MG. Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. Proc Natl Acad Sci USA. 2013;110(8):3101–3106. doi: 10.1073/pnas.1208093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405(6789):955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 234.Poser S, Storm DR. Role of Ca2+-stimulated adenylyl cyclases in LTP and memory formation. Int J Dev Neurosci. 2001;19(4):387–394. doi: 10.1016/S0736-5748(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 235.Dumoulin Bridi MC, Aton SJ, Seibt J, Renouard L, Coleman T, Frank MG. Rapid eye movement sleep promotes cortical plasticity in the developing brain. Sci Adv. 2015;1(6):e1500105. doi: 10.1126/sciadv.1500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.El Gaamouch F, Buisson A, Moustie O, Lemieux M, Labrecque S, Bontempi B, De Koninck P, Nicole O. Interaction between CaMKII and GluN2B controls ERK-dependent plasticity. J Neurosci. 2012;32(31):10767–10779. doi: 10.1523/jneurosci.5622-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol. 2006;570(Pt 3):553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23(10):2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bekinschtein P, Katche C, Slipczuk LN, Igaz LM, Cammarota M, Izquierdo I, Medina JH. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiol Learn Mem. 2007;87(2):303–307. doi: 10.1016/j.nlm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 240.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33(2):67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Seibt J, Dumoulin MC, Aton SJ, Coleman T, Watson A, Naidoo N, Frank MG. Protein synthesis during sleep consolidates cortical plasticity in vivo. Curr Biol. 2012;22(8):676–682. doi: 10.1016/j.cub.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Aton SJ, Suresh A, Broussard C, Frank MG. Sleep promotes cortical response potentiation following visual experience. Sleep. 2014;37(7):1163–1170. doi: 10.5665/sleep.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hengen KB, Torrado Pacheco A, McGregor JN, Van Hooser SD, Turrigiano GG. Neuronal firing rate homeostasis is inhibited by sleep and promoted by wake. Cell. 2016;165(1):180–191. doi: 10.1016/j.cell.2016.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Puentes-Mestril C, Aton SJ. Linking network activity to synaptic plasticity during sleep: hypotheses and recent data. Front Neural Circuits. 2017;11:61. doi: 10.3389/fncir.2017.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zuo Y, Yang G, Kwon E, Gan W-B. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436(7048):261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
- 246.Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- 247.Levenstein D, Watson BO, Rinzel J, Buzsáki G. Sleep regulation of the distribution of cortical firing rates. Curr Opin Neurobiol. 2017;44:34–42. doi: 10.1016/j.conb.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26(7):369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 249.He H-Y, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006;26(11):2951–2955. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lewin I, Gombosh D (1973) Increase in REM time as a function of the need for divergent thinking. In: European Congress on sleep research, pp 399–403. 10.1159/000428097
- 251.Walker MP, Liston C, Hobson JA, Stickgold R. Cognitive flexibility across the sleep–wake cycle: REM-sleep enhancement of anagram problem solving. Cogn Brain Res. 2002;14(3):317–324. doi: 10.1016/s0926-6410(02)00134-9. [DOI] [PubMed] [Google Scholar]
- 252.Barsky MM, Tucker MA, Stickgold R. REM sleep enhancement of probabilistic classification learning is sensitive to subsequent interference. Neurobiol Learn Mem. 2015;122:63–68. doi: 10.1016/j.nlm.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Landmann N, Kuhn M, Maier J-G, Spiegelhalder K, Baglioni C, Frase L, Riemann D, Sterr A, Nissen C. REM sleep and memory reorganization: potential relevance for psychiatry and psychotherapy. Neurobiol Learn Mem. 2015;122:28–40. doi: 10.1016/j.nlm.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 254.Whitehurst LN, Cellini N, McDevitt EA, Duggan KA, Mednick SC. Autonomic activity during sleep predicts memory consolidation in humans. Proc Natl Acad Sci USA. 2016;113(26):7272–7277. doi: 10.1073/pnas.1518202113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Abraham WC, Robins A. Memory retention—the synaptic stability versus plasticity dilemma. Trends Neurosci. 2005;28(2):73–78. doi: 10.1016/j.tins.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 256.Seibt J, Richard CJ, Sigl-Glöckner J, Takahashi N, Kaplan DI, Doron G, de Limoges D, Bocklisch C, Larkum ME. Cortical dendritic activity correlates with spindle-rich oscillations during sleep in rodents. Nat Commun. 2017;8(1):684. doi: 10.1038/s41467-017-00735-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Morris RGM. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 2006;23(11):2829–2846. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- 258.Mizuseki K, Miyawaki H. Hippocampal information processing across sleep/wake cycles. Neurosci Res. 2017;118:30–47. doi: 10.1016/j.neures.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 259.Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, Bähner F, Both M, Tort AB, Kopell NJ, Wisden W, Monyer H. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci USA. 2009;106(9):3561–3566. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Amilhon B, Huh CY, Manseau F, Ducharme G, Nichol H, Adamantidis A, Williams S. Parvalbumin interneurons of hippocampus tune population activity at theta frequency. Neuron. 2015;86(5):1277–1289. doi: 10.1016/j.neuron.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 261.Averkin RG, Szemenyei V, Bordé S, Tamás G. Identified cellular correlates of neocortical ripple and high-gamma oscillations during spindles of natural sleep. Neuron. 2016;92(4):916–928. doi: 10.1016/j.neuron.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Niethard N, Burgalossi A, Born J. Plasticity during sleep is linked to specific regulation of cortical circuit activity. Front Neural Circuits. 2017;11:65. doi: 10.3389/fncir.2017.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ognjanovski N, Schaeffer S, Wu J, Mofakham S, Maruyama D, Zochowski M, Aton SJ. Parvalbumin-expressing interneurons coordinate hippocampal network dynamics required for memory consolidation. Nat Commun. 2017;8:15039. doi: 10.1038/ncomms15039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 265.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 266.Norman KA, Newman EL, Perotte AJ. Methods for reducing interference in the complementary learning systems model: oscillating inhibition and autonomous memory rehearsal. Neural Netw. 2005;18(9):1212–1228. doi: 10.1016/j.neunet.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 267.Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32(6):1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- 268.Valdes JL, McNaughton BL, Fellous JM. Offline reactivation of experience-dependent neuronal firing patterns in the rat ventral tegmental area. J Neurophysiol. 2015;114(2):1183–1195. doi: 10.1152/jn.00758.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Gomperts SN, Kloosterman F, Wilson MA. VTA neurons coordinate with the hippocampal reactivation of spatial experience. Elife. 2015 doi: 10.7554/eLife.05360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Eschenko O, Sara SJ. Learning-dependent, transient increase of activity in noradrenergic neurons of locus coeruleus during slow wave sleep in the rat: brain stem-cortex interplay for memory consolidation? Cereb Cortex. 2008;18(11):2596–2603. doi: 10.1093/cercor/bhn020. [DOI] [PubMed] [Google Scholar]
- 271.Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernández G, Deisseroth K, Greene RW, Morris RGM. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature. 2016;537(7620):357. doi: 10.1038/nature19325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25(17):4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lin SC, Gervasoni D, Nicolelis MA. Fast modulation of prefrontal cortex activity by basal forebrain noncholinergic neuronal ensembles. J Neurophysiol. 2006;96(6):3209–3219. doi: 10.1152/jn.00524.2006. [DOI] [PubMed] [Google Scholar]
- 274.Lima RH, Radiske A, Köhler CA, Gonzalez MC, Bevilaqua LR, Rossato JI, Medina JH, Cammarota M. Nicotine modulates the long-lasting storage of fear memory. Learn Mem. 2013;20(3):120–124. doi: 10.1101/lm.029900.112. [DOI] [PubMed] [Google Scholar]
- 275.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6(9):968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 276.Leonard CS, Llinás R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study. Neuroscience. 1994;59(2):309–330. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 277.Datta S, Siwek DF. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J Neurophysiol. 1997;77(6):2975–2988. doi: 10.1152/jn.1997.77.6.2975. [DOI] [PubMed] [Google Scholar]
- 278.Rye DB. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20(9):757–788. doi: 10.1093/sleep/20.9.757. [DOI] [PubMed] [Google Scholar]
- 279.Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;280(3):R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- 280.Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J Neurophysiol. 2002;87(4):1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- 281.Lu J, Sherman D, Devor M, Saper CB. A putative flip–flop switch for control of REM sleep. Nature. 2006;441(7093):589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 282.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 283.Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J Neurosci. 2010;30(43):14356–14360. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Dragoi G, Tonegawa S. Development of schemas revealed by prior experience and NMDA receptor knock-out. Elife. 2013;2:e01326. doi: 10.7554/eLife.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Tamminen J, Lambon Ralph MA, Lewis PA. The role of sleep spindles and slow-wave activity in integrating new information in semantic memory. J Neurosci. 2013;33(39):15376–15381. doi: 10.1523/JNEUROSCI.5093-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Durrant SJ, Cairney SA, McDermott C, Lewis PA. Schema-conformant memories are preferentially consolidated during REM sleep. Neurobiol Learn Mem. 2015;122:41–50. doi: 10.1016/j.nlm.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 287.Hennies N, Lambon Ralph MA, Kempkes M, Cousins JN, Lewis PA. Sleep spindle density predicts the effect of prior knowledge on memory consolidation. J Neurosci. 2016;36(13):3799–3810. doi: 10.1523/JNEUROSCI.3162-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Gilboa A, Marlatte H. Neurobiology of schemas and schema-mediated memory. Trends Cogn Sci. 2017 doi: 10.1016/j.tics.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 289.Groch S, Schreiner T, Rasch B, Huber R, Wilhelm I. Prior knowledge is essential for the beneficial effect of targeted memory reactivation during sleep. Sci Rep. 2017;7:39763. doi: 10.1038/srep39763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Noack H, Schick W, Mallot H, Born J. Sleep enhances knowledge of routes and regions in spatial environments. Learn Mem. 2017;24(3):140–144. doi: 10.1101/lm.043984.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Tamminen J, Lambon Ralph MA, Lewis PA. Targeted memory reactivation of newly learned words during sleep triggers REM-mediated integration of new memories and existing knowledge. Neurobiol Learn Mem. 2017;137:77–82. doi: 10.1016/j.nlm.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 292.Ohki T, Takei Y. Neural mechanisms of mental schema: a triplet of delta, low beta/spindle and ripple oscillations. Eur J Neurosci. 2018 doi: 10.1111/ejn.13844. [DOI] [PubMed] [Google Scholar]
- 293.Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM. Schemas and memory consolidation. Science. 2007;316(5821):76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- 294.Bethus I, Tse D, Morris RGM. Dopamine and memory: modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. J Neurosci. 2010;30(5):1610–1618. doi: 10.1523/JNEUROSCI.2721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RGM. Schema-dependent gene activation and memory encoding in neocortex. Science. 2011;333(6044):891–895. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- 296.Wang S-H, Tse D, Morris RGM. Anterior cingulate cortex in schema assimilation and expression. Learn Mem. 2012;19(8):315–318. doi: 10.1101/lm.026336.112. [DOI] [PMC free article] [PubMed] [Google Scholar]