Abstract
Articular cartilage is formed at the end of epiphyses in the synovial joint cavity and permanently contributes to the smooth movement of synovial joints. Most skeletal elements develop from transient cartilage by a biological process known as endochondral ossification. Accumulating evidence indicates that articular and growth plate cartilage are derived from different cell sources and that different molecules and signaling pathways regulate these two kinds of cartilage. As the first sign of joint development, the interzone emerges at the presumptive joint site within a pre-cartilage tissue. After that, joint cavitation occurs in the center of the interzone, and the cells in the interzone and its surroundings gradually form articular cartilage and the synovial joint. During joint development, the interzone cells continuously migrate out to the epiphyseal cartilage and the surrounding cells influx into the joint region. These complicated phenomena are regulated by various molecules and signaling pathways, including GDF5, Wnt, IHH, PTHrP, BMP, TGF-β, and FGF. Here, we summarize current literature and discuss the molecular mechanisms underlying joint formation and articular development.
Keywords: Articular cartilage, Joint, Interzone, Chondrocyte
Introduction
Osteoarthritis, a representative degenerative joint disease, is threatening the quality of life and daily activities of many elderly people. Many studies have been performed to understand its pathophysiology and develop disease-modifying drugs over decades. However, neither of these aims have yet been successfully achieved. However, an increasing number of developmental biology studies have revealed various kinds of molecules and signaling pathways involved in skeleton formation. In particular, achievements of cell biology and regenerative medicine research have enabled the induction of chondrocytes from pluripotent and somatic stem cells in vitro [1–7]. Moreover, recent studies indicate that chondrocytes are generated through several different biological steps according to their localization during skeleton formation [8, 9].
Articular cartilage is a highly specialized tissue that anatomically caps the end of epiphyses in the synovial joint cavity. Matured articular cartilage is also referred to as hyaline cartilage because of its translucent appearance that reflects its unique constituents, such as type II collagen, glycosaminoglycans (GAGs), and low cellularity [10]. In addition, articular cartilage does not have blood vessels, lymphatic vessels, or nerves [10]. Articular chondrocytes produce extracellular matrices and maintain their environment with very little or no cell turnover [11, 12]. GAGs, including chondroitin sulfate and hyaluronic acid, bind to each other via core proteins in cooperation with longitudinally orientated type II collagen [10, 13]. These protein complexes further form the intricate network of extracellular matrices, which are responsible for the distribution and absorption of mechanical forces loaded on the articular cartilage [14, 15]. Another structural feature of articular cartilage is the lubricated smooth surface composed of lubricin and horizontally oriented collagens, which attenuates friction generated through skeletal motion [15, 16]. Thus, articular cartilage consists of multiple layers that have differently oriented matrices and cell populations.
In vertebrate animals, most skeletal elements develop from transient cartilage by a biological process well known as endochondral ossification [17–19]. In the initial step, cartilage anlagen is formed through mesenchymal condensation at the presumptive site for bone. Cartilage anlagen grow to form a cartilage template, accompanied by chondrocyte proliferation and differentiation, which has a similar shape to future bones. At the center of the cartilage, chondrocytes undergo hypertrophic differentiation and then apoptosis, resulting in vascular invasion and ossification by osteoblasts. This sequential event longitudinally spreads to metaphysis. Later, another ossification site, termed as the secondary ossification center, is newly formed at the epiphysis and radially spreads within it. Part of the cartilage between the two ossification centers remains as a growth plate physis during skeletal growth, and other parts between the joint cavity and the secondary ossification center permanently remain as articular cartilage. Accumulating evidence indicates that articular and growth plate cartilage is derived from different cell sources and that different molecules and signaling pathways regulate these two kinds of cartilage [8, 9]. In this review, we introduce several crucial factors defining the inception of joint generation and development of articular cartilage.
Early stages of articular cartilage differentiation
Interzone emergence
The development of the synovial joint precedes articular cartilage formation. The timing of joint development depends on its site: forelimb and proximal joint formation generally precede hindlimb and distal joint formation, respectively [20–22]. Mice nascent limb joints are observed at around E12.5–E15.5, whereas articular cartilage is identified after birth [19]. The substantial morphological appearance of articular cartilage is observed in 2–4-week-old mice [12, 23, 24] and approximately 1-month-old rabbits [25].
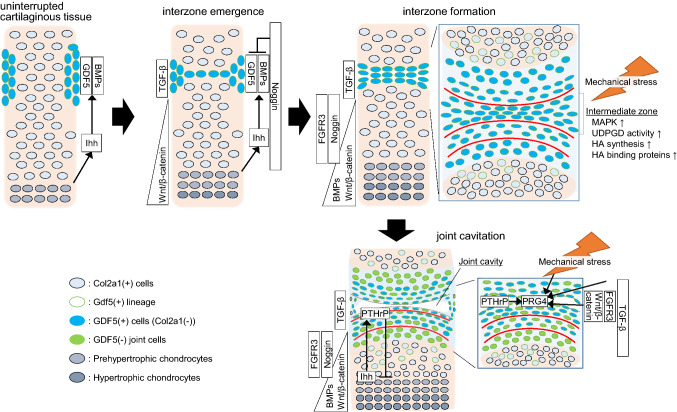
Notably, most parts of articular cartilage derive from different lineages from the growth plate cartilage. The first signs of joint development are presented by the appearance of condensed flattened cells at the presumptive joint site within a pre-cartilage tissue [26, 27] known as the interzone, the origin of the joint (Fig. 1). Removal of the interzone from a chick embryo leads to an uninterrupted long bone lacking joints [28], indicating that the interzone provides segmentation of skeletal elements in limbs. The interzone arises from mesenchymal/pre-cartilaginous tissue in which the cells initially express chondrocyte marker genes such as type II collagen, aggrecan, and matrillin-1 [11, 12, 24, 29–32]. Instead of the decreased expression of these chondrogenic markers, the interzone cells acquire the expression of growth differentiation factor 5 (Gdf5), formerly known as bone morphogenetic protein 14 (BMP14), or cartilage-derived morphogenetic protein 1 (CDMP1). Gdf5 is a representative marker for the interzone during early joint development [24, 29, 30, 33]. In addition to Gdf5, Wnt4, Wnt9a (formerly known as Wnt14), Wnt16, Erg, doublecortin, and Gli are also expressed in the interzone [34].
Fig. 1.
Joint formation and articular cartilage development in the early stage. The interzone emerges at the presumptive joint site within a pre-cartilage tissue. After that, joint cavitation occurs in the center of the interzone, and the cells in the interzone and its surroundings gradually form articular cartilage and synovial joints. During joint development, the interzone cells continuously migrate to the epiphyseal cartilage and the surrounding cells influx into the joint region. The width of each box indicates the area, where a particular molecule is expressed. GDF5 growth differentiation factor 5, BMP bone morphogenetic protein, Ihh Indian hedgehog, FGFR fibroblast growth factor receptor, TGF-β transforming growth factor-β, MAPK mitogen-activated protein kinase, UDPGD uridine diphosphoglucose dehydrogenase, HA hyaluronic acid, PTHrP parathyroid hormone-related protein, PRG4 proteoglycan 4, Col2a1 type II collagen, (+) positive, (−) negative
Joint cavitation
Joint cavitation is one of the most remarkable events specific to synovial joints and is a necessary step toward articular cartilage development (Fig. 1). Joint cavities are identified at around the same time as hypertrophic chondrocytes are observed in the center of adjacent cartilage templates. Previous studies have proposed that the cavity is generated through the apoptosis of the cells in the center of the interzone termed as the intermediate zone [35–38]. However, cell death is sparsely observed within a thin intermediate zone [36, 37]. Instead, recent literature suggests that the joint cavity develops by the filling of the fluidic extracellular matrix, particularly hyaluronan [11, 24, 31, 39–41]. Hyaluronan synthases, hyaluronan binding proteins, and the activity of uridine diphosphoglucose dehydrogenase (UDPGD) were specifically up-regulated at the intermediate zone before and during the detachment of cell–cell adhesion [11, 24, 31, 39, 40]. Indeed, mutant mice for hyaluronan synthetase 2 exhibit severe deformity of the joints [41]. These events are possibly regulated by mitogen-activated protein kinase (MAPK) signaling including p38 and Erk1/2, which are activated at the intermediate zone before the expression of hyaluronan related factors, and directly stimulate hyaluronan synthesis in the interzone cells in vitro [42, 43]. An extrinsic mechanical stimulus may be a potent candidate for an upstream of these pathways. Skeletal muscle paralysis in chick embryos causes joint cavitation failure [44–46]. While interzone generation and Gdf5 expression were not altered in this model [47], the activation of MAPK signaling and hyaluronan synthesis were decreased at the intermediate zone [42, 48]. Similar or severer phenotypes are observed in muscle-less mice (known as splotched-delay mutants) [49, 50]. Rolfe et al. carried out microarray analyses of muscle-less mice and showed associations of molecule signaling pathways including the transforming growth factor (TGF)-β superfamily, fibroblast growth factors (FGFs), hedgehogs, and Wnt [50]. Although little is known about how mechanical stimuli are transduced to intracellular signals, these findings indicate that movement, myogenesis, and muscle contraction play essential roles in cavitation and the healthy development of joints through these representative signaling pathways.
Regulators in the early stages
Growth differentiation factor 5
GDF5, a member of the TGF-β superfamily, was first identified as the gene responsible for brachypodism in mice that show altered skeletal morphology, in particular in distal joints [51]. Mutations of the human GDF5 gene causes skeletal malformations including brachydactyly [52] and chondrodysplasia [53–55], and mutations of its receptor BMPR1B also cause brachydactyly [56, 57]. These outcomes suggest that GDF5 is essential for healthy joint development. In the early stages, Gdf5 mRNA is faintly detected surrounding the pre-cartilage area, then restricted within the interzone with reinforced expression [35, 58–60]. In mice with brachypodism, Gdf5 is strongly expressed throughout the cartilage anlagen, out of the interzone, which results in the fusion of digit joints [59]. These data suggest the role of Gdf5 in the generation and maintenance of the interzone. While Gdf5 is detected in most synovial joints of limbs, some proximal joints like elbow and knee joints are not fused in brachypodism mice [61]. This eventuality is likely due to compensation by Gdf6, another member of the GDF family which is dominantly expressed in proximal joints [62]. Indeed, double mutations of Gdf5 and Gdf6 cause severe joint deformity which is not observed in each mutant [63, 64].
Several studies using Gdf5-Cre transgenic mice have shown that Gdf5-expressing cell lineage gives rise to all mature joint structures including articular cartilage, meniscus, ligaments, and synovium [65]. Therefore, interzone cells have been considered as progenitors for joints over the decades. Interestingly, the Gdf5-lineage progeny cells are also detected in subchondral bone as osteoblasts and osteocytes [9, 66], while Gdf5 expression is gradually down-regulated during joint development and diminished until birth [35, 36, 58]. Tsumaki et al. reported that Col11a2-Gdf5 transgenic mice, which continuously express Gdf5 in chondrocytes, display joint fusion with cartilage hyperplasia [67], as shown in brachypodism mice. Taken together with the transient expression of Gdf5, cell tracking based on the Gdf5-Cre system may not reflect the substantial fates of the interzone progeny. Recently, Gdf5-CreERT2 and Cre-dependent reporter mice, in which Gdf5-positive ((+)) cells can be labeled by tamoxifen administration, have provided novel findings [9, 12, 33]. Shwartz et al. demonstrated the embryonic stage-specific localization of Gdf5 (+) cells and their fate from E10.5 to E18.5 [33]. Gdf5 (+) cells continuously influx into the joint region from the surrounding tissues, and contrarily, the interzone/early joint cells migrate out to the epiphyseal cartilage losing Gdf5 expression [33]. Surprisingly, most cells that initially form the interzone do not give rise to articular cartilage but to transient cartilage, ligaments, and meniscus [33]. Decker et al. demonstrated the spatiotemporal distribution of Gdf5 (+) cells up to adulthood, and also observed the reciprocal cell migration between the interzone and the surrounding tissues [9, 12], as well as the previous report by Shwartz et al. [33]. It is now accepted that joint components are formed by the integration of peripheral cells in joint development. Epiphyseal chondrocytes migrate into the interzone at early stages [68], and the external regions of joints such as the synovium/joint capsule [69] and outer parts of the meniscus [30] are mainly composed of lately integrated cells. Thus, the fate of embryonic interzone cells, the surrounding cells, and their progeny cells may be determined by their spatiotemporally environment.
Although Gdf5 signaling has chondrogenic effects in vivo [67] and in vitro [58, 70–72], Gdf5 is unlikely to contribute directly to articular cartilage development, because the diminishment of Gdf5 expression is significant before articular cartilage appearance and some joints in brachypodism mice have normal articular cartilage [33, 61]. In adulthood, Gdf5 may be associated with homeostasis of the articular cartilage, because genome-wide association studies have revealed that GDF5 is one of the susceptible genes for osteoarthritis [73–75]; however, its role in adult articular cartilage remains unclear.
Wnt signaling
Wnt4, Wnt9a, and Wnt16 are expressed in the interzone and flanking areas at the early stages, before or simultaneously with Gdf5 expression in the interzone [31, 37, 49, 60, 76, 77]. The canonical Wnt signaling potently suppresses chondrogenesis of the limb bud mesenchymal cells in vitro [60, 70, 77, 78]. Several gain-of-function studies suggest that the canonical Wnt signaling provides cells with the interzone phenotype upstream of Gdf5 by suppressing chondrogenesis [60, 76]. Meanwhile, loss-of-function studies indicate that joint development is achieved in Wnt ligands knockout mice [77–79]. Spater et al. report that the deletion of Wnt9a does not affect the expression of joint markers, but causes ectopic chondrogenesis-like synovial chondromatosis in some joints, which is enhanced by the additional deletion of Wnt4 [77, 78]. The conditional knockout (cKO) of β-catenin using Col2a1-Cre or Gdf5-Cre slightly affects joint development [70, 80–82], while Guo et al. report the fusion of the wrist or knee in cKO using Col2a1-Cre or Dermo1-Cre, respectively [76]. These data should be carefully discussed, because alteration of the canonical Wnt signaling itself impairs chondrocyte differentiation and endochondral ossification [23, 77–79, 83].
Actual activity of the canonical Wnt signaling can be monitored using TOPGAL, a reporter containing a β-galactosidase gene under the control of LEF/TCF and β-catenin inducible promoter [84]. Yamagami et al. demonstrate that signaling activity is not detected in the interzone of the elbow and shoulder at E12.5–E14.5, whereas Wnt4 transcripts are detected there [85]. Kahn et al. show that Wnt4 and Wnt9a are predominant in the interzone of the elbow at E13.5, but TOPGAL activity is obscure [49]. The strong TOPGAL signal is observed in the cartilage anlagen at this stage, rather than in the interzone [49, 70, 85]. Although it is still controversial, WNT4 possibly inhibits canonical Wnt signaling [86, 87]. The role of Wnt in joint development is conflicting amongst reports, because Wnt ligand expression, their target cells, and their signaling pathways are complicated.
Besides these effects in the early stages of joint development, canonical Wnt signaling plays another role in specifying the superficial zone (SFZ) of the articular cartilage during the late phase. The SFZ is responsible for joint lubrication by producing lubricin (encoded by the Proteoglycan 4 (Prg4) gene) [88]. Koyama et al. report that flattened cells in the SFZ disappear and Prg4 expression is decreased in β-catenin cKO mice in Col2a1 (+) or Gdf5 (+) lineages [70]. Yasuhara et al. also showed a reduced number of SFZ cells and Prg4 expression in the articular cartilage of 7-week-old Col2a1-CreERT;β-cateninfl/fl mice which received tamoxifen administration at P7 [81]. The articular cartilage of the cKO mice has no stratified structure and is homogenously composed of chondrocyte-like round cells [81]. Yamagami et al. monitored TOPGAL activity in joints during development up to adulthood [85]. After joint cavitation, surface cells facing the cavity exhibit strong signal activity, which is observed until P7. The LacZ-positive cells are reduced in the articular and epiphyseal cartilage at P10 and eventually, are rarely found there at P50. Taken together, canonical Wnt signaling is less essential for the interzone/early joint development but orchestrates the integrity of joint formation through anti-chondrogenic effects in the SFZ of the articular cartilage.
Indian hedgehog—parathyroid hormone-related protein
Cartilage templates and early joints influence each other via paracrine signaling. Indian hedgehog (IHH) and parathyroid hormone-related protein (PTHrP) signaling have been intensively studied. IHH is widely involved in both the proliferation and differentiation of chondrocytes during endochondral development [89–91]. IHH is produced from pre-hypertrophic chondrocytes and up-regulates PTHrP expression in peri-articular chondrocytes [92, 93]. PTHrP inhibits the differentiation of proliferating chondrocytes into pre-hypertrophic chondrocytes [94–96]. This feedback loop determines the length of long bones [97]. Before the feedback loop, pre-hypertrophic chondrocytes around the center of anlagen are associated with interzone generation through the secretion of IHH. The loss of IHH causes not only dwarfism but also joint fusion in distal limb joints [89, 98]. In Ihh deficient mice, the interzone is absent or markedly hypoplastic [98]. Gdf5 (+) cells are observed at prospective joint sites in mutants, but they flank and surround uninterrupted joint sites [98]. The authors conclude that Ihh is indispensable for the recruitment and immigration of flanking cells into the interzone [99].
Ihh expression is initially detected in the ossification center, where cells first have pre-hypertrophic phenotypes and then undergo the endochondral ossification process. Although Ihh itself is not expressed in the presumptive joint sites [78, 89], Patched-1, a receptor of the IHH ligand, has been detected around the interzone and cartilage anlagen [78, 89, 100]. Gli1 and Gli3, major downstream transcription factors of IHH signaling, are expressed around the interzones and joints in the early stages [57, 59, 80, 98, 100, 101]. Gli3 knockout mice exhibit a malformation of phalanges and irregular joint shapes [101, 102]. Thus, the IHH signaling pathway regulates joint morphogenesis [103], and the cartilage anlagen contribute to development of the interzones and joints through secretion of IHH.
In addition to these spatial features of IHH-related molecules, the regulation of joint formation by IHH signaling is probably transient, since Patched-1 expression around the interzone is observed only from E12.5 to E13.5 in the elbow joints [89]. When IHH signaling is continuously activated in chondrocytes, Gdf5 (+) cells do not migrate into the interzone and joints are fused [80]. Furthermore, excessive IHH signaling activity in the interzone progeny induces ectopic cartilage formation in the knee [104]. Notably, joint morphology is not changed even when IHH signaling is disrupted in the interzone progeny (Gdf5-Cre;Smofl/fl) [104], contrary to the severe deformity of forelimb joints in Col2a1-Cre;Smofl/fl [91]. Zhou et al. demonstrate that Ihh deletion in adult articular cartilage does not alter joint phenotype. Instead, it attenuates osteoarthritis progression [105]. Other studies have also shown the association of IHH with osteoarthritis [106–108]. Taken together, the data suggest that IHH signaling is possibly less essential after interzone specification during joint development.
Unlike in endochondral ossification, IHH and PTHrP seem to be independent in interzone generation and joint development. The genetic alteration of PTHrP causes the impairment of endochondral ossification, but no severe changes in joints [94–96, 109, 110]. Even after IHH signaling becomes silent, PTHrP-expressing cells exist in articular cartilage over a lifetime [110–112]. Recombinant human PTH (1–34) suppresses osteoarthritis development [113], and PTH/PTHrP signaling induces lubricin [114]. PTHrP possibly contributes to the postnatal development and homeostasis of articular cartilage.
Bone morphogenic protein signaling—Noggin
BMP signaling plays a central role in both chondrogenesis and osteogenesis [115]. Mice lacking Smad1, 5, and 8 canonical mediators of BMPs display severe chondrodysplasia both in appendicular and vertebral skeletons [116]. In addition to Gdf5, Bmp2 and Bmp4 are expressed in the interzone [34, 38, 65, 80, 117]. Although it has not been fully revealed, BMP and IHH signaling possibly regulate each other in the early stages [80, 89, 91, 116, 118]. Once the interzone is specialized, BMP signaling is negatively regulated. The phosphorylation of Smads are not observed in the presumptive joint site before cavitation, unlike in the adjacent epiphyseal cartilage [68, 119, 120]. Noggin and chordin, the BMP antagonists, contribute to this process. They directly bind to BMP2 [121], BMP4 [122], and GDF5 [35]. The expression pattern of these antagonists depends on the location of joints and species [31, 118, 123]. The mRNA of noggin is detected in the cartilage anlagen and temporally in the interzone [31, 37, 38, 69, 117, 124]. Chondrocyte-specific noggin transgenic mice display a marked impairment of skeleton formation [67], and noggin knockout causes the remarkable hyperplasia of cartilage templates lacking in articular cartilage [125, 126]. Similar phenotypes are observed in Col2a1-Cre; Nogginfl/fl mice or noggin antibody-injected chick embryos [68]. In humans, missense mutations of GDF5 cause synostosis, because the GDF5 mutants become insensitive to noggin [127]. Thus, noggin in the interzone contributes to the joint formation by antagonizing the chondrogenic effect of GDF5 [35, 36, 58].
As described above, the loss of embryo movement causes joint fusion. Notably, phosphorylated Smad1/5/8-positive cells are detected in the fused regions of paralyzed chick embryos and muscle-less mice [68, 119]. Considering that BMP signaling is regulated by the balance between ligands and antagonists, it is expected that the up-regulation of BMP ligands or down-regulation of BMP signaling by noggin or chordin may occur here. However, in the fused joints of muscle-less mice, noggin expression is up-regulated [49]. Singh et al. show that noggin expression is not altered and Bmp4 expression is down-regulated both in immobilized chicks and mice [119]. The interaction between mechanical loading and BMP signaling is not currently revealed.
After joint cavitation, noggin expression is converged within the epiphyseal cartilage and prevents diffusion of the BMP ligands from the hypertrophic chondrocytes and ossification center to the joint region [38, 68, 70, 117, 119]. Additionally, another BMP antagonist, gremlin 1, is associated with the regulation of BMP signaling after birth to adulthood [128–130]. BMP signaling and its various modulators are involved in the development, homeostasis, and pathophysiology of joints.
Transforming growth factor-β
TGF-β signaling plays a substantial role in the homeostasis of articular cartilage [131–133]. TGF-β ligands bind to TGF-β type II receptor (Tgfbr2), which leads to TGFβ type I receptor recruitment. This heterodimer complex activates intercellular signaling cascades, such as Smad2/3 or Smad-independent pathways. Age-related decreases of TGF-β signaling in chondrocytes, partially caused by the decreased expression of their receptors, is associated with cartilage degeneration [132, 134]. Currently, the intraarticular administration of human chondrocytes transduced with a viral vector containing the gene for Tgf-β1 transcription is undergoing a clinical trial for the treatment for osteoarthritis [135].
The role of TGF-β signaling in joint development is also prominent [136, 137]. The deletion of Tgfbr2 in the early mesenchyme (Prx1-Cre) results in the inhibition of interzone appearance in phalanges [36, 37]. Accordingly, the Gdf5 (+) lineage cannot enter into putative sites for the interzone in Prx1-Cre;Tgfbr2 fl/fl mice [36, 37], similar to Ihh null mice [98]. Furthermore, TGF-β signaling exerts suppressive effects against chondrogenesis in limb bud culture [36] as well as Wnt/β-catenin signaling [76, 80]. As mentioned previously, the generation of the interzone requires the inhibition of chondrogenesis within the pre-cartilage anlagen, where BMP signaling is activated. BMP and TGF-β signaling share a co-mediator, Smad4, which triggers the nuclear translocation of the Smad complex. Therefore, this competition may be significant in interzone formation. Smad4 deletion in the early mesenchyme causes the severe impairment of limb development, including joint creation [138, 139], whereas in chondrocytes, it predominantly affects endochondral ossification accompanied with less alteration of the joints [140].
Expression analyses have also provided significant evidence. Spagnoli et al. show that Tgfbr2 positive cells were detected explicitly in the interzone from E12.5 to E16.5 using Tgfbr2 reporter transgenic mice [37]. Apart from the transient expression in the interzone, Tgfbr2 expression is sustained in the joints and surrounding tissues until adulthood [69]. Joint intima including the perichondrium, synovium, enthesis, and articular cartilage surfaces in knees are expressed in neonates, while the epiphyseal cartilage is not [69]. These findings may support the hypothesis that articular and growth plate cartilage are derived from different cell sources [70].
Fibroblast growth factor 18/fibroblast growth factor receptor 3 signaling
FGF18 is among the FGF family members involved in articular cartilage. Fgf18 null mice die in the early neonatal period and display impaired skeletal development [141–143]. Mutations of its receptor FGFR3 also cause severe dwarfism and achondroplasia in mice and humans [144–146]. Thus, it is evident that FGF18/FGFR3 signaling is indispensable for skeletogenesis. FGFR3 expression is found in differentiating chondrocytes [142, 147–149], whereas FGF18 is secreted from the perichondrium [142, 150]. This interaction is one of the characteristic findings indicating that the morphology of the cartilage template is determined by the perichondrium [151, 152]. FGF18/FGFR3 signaling induces chondrogenesis from the limb mesenchyme in vitro [153], suppresses the proliferation and hypertrophy of growth plate chondrocytes in vivo [142, 143, 154, 155], and partially regulates subsequent osteogenesis [142–144, 154, 156].
FGF18 transcripts can be detected in the interzone cells [142, 143]; however, its role is unknown, because most Fgf18 null limb joints are unaffected [141–145]. Generally, FGF18/FGFR3 signaling seems to be deeply associated with IHH-PTHrP and the canonical Wnt signaling. Gain-of-function of FGFR3 signaling leads to the decreased expression of Ihh ligands and Pthrp receptors [93, 157, 158]. Postnatal chondrocyte-specific Fgfr3 deletion induces multiple chondroma-like lesions adjacent to the disordered growth plates by the up-regulation of Ihh signaling [159]. On the other hand, the constitutive activation of IHH signaling decreases Fgf18 expression and subsequent ectopic cartilage hyperplasia in joints [104]. Furthermore, the hedgehog-induced phenotypes are rescued by the stabilization of β-catenin or treatment with FGF18 [104]. Interestingly, Fgf18 is a direct transcriptional target of canonical Wnt signaling [160, 161]. Considering these findings, FGF18 may be involved in joint generation by mutually affecting the Ihh/PTHrP and the canonical Wnt signaling.
Adult articular chondrocytes also express FGF18 and FGFR3 [153, 162]. Mori et al. report that Fgf18 is dominantly expressed in articular cartilage compared with growth plate cartilage, both in infant and adult rats [163]. Fgfr3 knockout results in early osteoarthritis [164] with enhanced Ihh signaling [165, 166]. It is currently accepted that FGFR3 signaling exerts anabolic effects in healthy articular cartilage, while FGFR1 signaling exerts catabolic effects in articular chondrocytes [167]. Indeed, FGF2, a representative FGFR1 ligand, is up-regulated [168], and FGFR3/FGF18 is down-regulated in osteoarthritis cartilage [162, 169]. The therapeutic effects of FGF18 have been validated in various studies [163, 166, 170–172], and a clinical trial using recombinant human FGF18 for osteoarthritis is ongoing [135].
Late stages of articular cartilage differentiation
Interzone and joint development are regulated by various factors and signaling pathways as described above. Some of them may be dispensable during the specification of articular cartilage. Indeed, Gdf5, Wnt9a, Ihh, Bmp, and noggin disappear in the late stages of joint development [24, 34, 70]. Instead, the expression of structural proteins such as lubricin, tenascin-C, CD44, and type II collagen, which contribute to smooth movement and loading, becomes marked during differentiation toward adult articular cartilage [34, 70]. TGF-βs, FGF18, and PTHrP are continuously expressed from the interzone cells to matured articular chondrocytes, implying their extensive roles in articular cartilage. Currently, they all are considered as potential therapeutic agents for osteoarthritis [135].
Kozhemyakina et al. identify Prg4 (+) cells in the SFZ as articular cartilage progenitors [173]. When Prg4 (+) cells are labeled at E17.5, their progeny cells compose all layers of articular cartilage in adulthood [173]. Even in 1-month-old mouse cartilage, Prg4 (+) cells slowly expand to the entire cartilage layers above the tidemark in 1 year [173]. Meanwhile, Decker et al. recently showed that articular cartilage is thickened mainly by zone-specific increases in cell volume in the late stage, and that cell proliferation or death plays a minor role [12]. Neonatal peri-articular chondrocytes actively proliferate, but underneath chondrocytes do not [12]. Although it is widely known that cell turnover is markedly suppressed and much less essential for the homeostasis of articular cartilage, it is surprising that the proliferation of articular chondrocytes is almost undetected in 2-week-old mice [12]. The peri-articular cartilage at this age contains fewer glycosaminoglycans, which are abundant in the underneath cartilage templates [12, 23, 110, 174]. These data may indicate that articular cartilage is not a residual of a cartilage template, rather it is newly formed by articular chondrocytes. Although whether the articular chondrocytes are derived from the interzone or the cartilage template is still controversial, joint components are probably constructed by the influx and efflux of cells during development [12, 33].
Regulators in the late stage
Lubricin
Lubricin, encoded by Prg4, is one of the major components of the synovial fluid, which is produced from synoviocytes and articular chondrocytes [175, 176]. Lubricin is responsible for joint lubricity [177, 178], and its expression is decreased in osteoarthritis [179, 180]. Exogenous lubricin injection is a promising treatment for osteoarthritis [181–183]. Prg4 expression is observed at the inception of joint cavitation and becomes intense during cavitation [88, 184]. Even after development, lubricin is dominantly expressed in the surface cells of the synovium and articular cartilage [88, 185]. Several factors, including mechanical loading, PTHrP, and TGF-β have been identified as upstream regulators of Prg4 [114, 186, 187]. The transient activation of the canonical Wnt signaling up-regulates SFZ cell growth and Prg4 expression, and its deletion impairs SFZ development along with Prg4 down-regulation [70, 81]. Meanwhile, the deletion of Prg4 in mice does not alter skeletal development in the neonatal period, and gradually causes abnormality at the surface of the articular cartilage with the deposition of an acellular layer with aging [88, 177, 188]. Although Prg4 is detected at the joint cavitation, it is likely less critical during joint formation and contributes to articular cartilage homeostasis.
Notch
Notch signaling regulates many asymmetric cellular developments via binding cell surface ligands (Jagged1, 2, Delta-like 1, 3, 4) and receptors (Notch1–4), whereby Notch intracellular domain (NICD) translocates into the nucleus and activates their downstream genes including Hes/Hey family members in concert with co-transcriptional regulator RBPjκ [189, 190]. In development, cells interfere with neighbor cells via Notch signaling, which yields cell diversity from a homogenous population. Notch signaling inhibits chondrogenesis in the early stage mesenchyme. Rbpjκ deletion in the limb mesenchyme enhances chondrogenesis, and NICD overexpression in chondrocytes severely impairs skeletal development [191, 192]. Moreover, notch signaling also regulates the survival, proliferation, and differentiation of chondrocytes during the endochondral ossification process via the RBPjκ-independent pathway [152].
In contrast to its robust role in endochondral ossification, Notch signaling is dispensable for joint formation and articular cartilage development. Joint structure and articular cartilage are almost normal in Rbpjκ cKO mice using Prx1-Cre and Col2a1-Cre [192, 193]. In the maturation and homeostasis of articular cartilage, the role of Notch signaling is controversial. Mirando et al. show that the chondrocyte-specific deletion of Rbpjκ at 1 month of age leads to a progressive osteoarthritis-like pathology in the subsequent course with aging [193], while Hosaka et al. demonstrate that the chondrocyte-specific deletion of Rbpjκ at 7 weeks suppresses osteoarthritis development in a surgically induced mouse model [194]. Furthermore, the up-regulation of Notch signaling in adult articular cartilage induces osteoarthritis [195]. Notably, Notch expression is detected in the SFZ cells of articular cartilage, which are considered a cartilage progenitor [184, 196, 197]. Notch expression and positive cells respond to osteoarthritic change with activation and altered distribution [184, 194, 198]. Considering that Notch is expressed in the SFZ and that articular cartilage homeostasis is disturbed when Rbpjκ is deleted at 2–4 weeks [193, 199], Notch signaling may be involved in the final differentiation or maturation of articular cartilage.
Conclusion
In this review, we introduced crucial factors involved in joint and articular cartilage development. Molecular mechanisms underlying endochondral ossification and joint specification have been well studied over decades. Additionally, articular cartilage homeostasis and the pathophysiology of osteoarthritis have been a research focus in recent years. On the other hand, molecules, signaling pathways, and cells that regulate the late stage differentiation and maturation of articular cartilage remain obscure. Furthermore, articular cartilage development in humans may be entirely different from mice, where articular cartilage is thinner and multi-layered. These issues may be obstacles to clinical application of the findings mentioned in this review.
Abbreviations
- BMP
Bone morphogenic protein
- CDMP1
Cartilage-derived morphogenetic protein 1
- cKO
Conditional knockout
- FGF
Fibroblast growth factor
- FGFR3
Fibroblast growth factor receptor 3
- GAG
Glycosaminoglycan
- GDF5
Growth differentiation factor 5
- IHH
Indian hedgehog
- MAPK
Mitogen-activated protein kinase
- NICD
Notch intracellular domain
- PTHrP
Parathyroid hormone-related protein
- Prg4
Progeoglycan 4
- SFZ
Superficial zone
- TGF-β
Transforming growth factor-β
- Tgfbr2
TGF-β type II receptor
- UDPGD
Uridine diphosphoglucose dehydrogenase
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oldershaw RA, Baxter MA, Lowe ET, et al. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28:1187–1194. doi: 10.1038/nbt.1683. [DOI] [PubMed] [Google Scholar]
- 2.Chijimatsu R, Ikeya M, Yasui Y, et al. Characterization of mesenchymal stem cell-like cells derived from human iPSCs via neural crest development and their application for osteochondral repair. Stem Cells Int. 2017 doi: 10.1155/2017/1960965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saito T, Yano F, Mori D, et al. Hyaline cartilage formation and tumorigenesis of implanted tissues derived from human induced pluripotent stem cells. Biomed Res. 2015;36:179–186. doi: 10.2220/biomedres.36.179. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita A, Morioka M, Yahara Y, et al. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Rep. 2015 doi: 10.1016/j.stemcr.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inui A, Iwakura T, Reddi A. Human stem cells and articular cartilage regeneration. Cells. 2012;1:994–1009. doi: 10.3390/cells1040994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S, Fu P, Cong R, et al. Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis. 2015;2:76–95. doi: 10.1016/j.gendis.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleary MA, van Osch GJ, Brama PA, et al. FGF, TGFbeta and Wnt crosstalk: embryonic to in vitro cartilage development from mesenchymal stem cells. J Tissue Eng Regen Med. 2015;9:332–342. doi: 10.1002/term.1744. [DOI] [PubMed] [Google Scholar]
- 8.Salva JE, Merrill AE. Signaling networks in joint development. Dev Dyn. 2017;246:262–274. doi: 10.1002/dvdy.24472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decker RS. Articular cartilage and joint development from embryogenesis to adulthood. Semin Cell Dev Biol. 2017;62:50–56. doi: 10.1016/j.semcdb.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archer CW, Morrison H, Pitsillides AA. Cellular aspects of the development of diarthrodial joints and articular cartilage. J Anat. 1994;184(Pt 3):447–456. [PMC free article] [PubMed] [Google Scholar]
- 12.Decker RS, Um HB, Dyment NA, et al. Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs. Dev Biol. 2017;426:56–68. doi: 10.1016/j.ydbio.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carballo CB, Nakagawa Y, Sekiya I, et al. Basic science of articular cartilage. Clin Sports Med. 2017;36:413–425. doi: 10.1016/j.csm.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Carter DR, Beaupre GS, Wong M, et al. The mechanobiology of articular cartilage development and degeneration. Clin Orthop Relat Res. 2004;427:S69–S77. doi: 10.1097/01.blo.0000144970.05107.7e. [DOI] [PubMed] [Google Scholar]
- 15.Camarero-Espinosa S, Rothen-Rutishauser B, Foster EJ, et al. Articular cartilage: from formation to tissue engineering. Biomater Sci. 2016;4:734–767. doi: 10.1039/c6bm00068a. [DOI] [PubMed] [Google Scholar]
- 16.Hughes LC, Archer CW, Ap Gwynn I. The ultrastructure of mouse articular cartilage: collagen orientation and implications for tissue functionality. A polarised light and scanning electron microscope study and review. Eur Cell Mater. 2005;9:68–84. doi: 10.22203/eCM.v009a09. [DOI] [PubMed] [Google Scholar]
- 17.Kozhemyakina E, Lassar AB, Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 2015;142:817–831. doi: 10.1242/dev.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol. 2013 doi: 10.1101/cshperspect.a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefebvre V, Bhattaram P. Vertebrate skeletogenesis. Curr Top Dev Biol. 2010;90:291–317. doi: 10.1016/s0070-2153(10)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lhuaire M, Martinez A, Kaplan H, et al. Human developmental anatomy: microscopic magnetic resonance imaging (muMRI) of four human embryos (from carnegie stage 10 to 20) Ann Anat. 2014;196:402–409. doi: 10.1016/j.aanat.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Lhuaire M, Tonnelet R, Renard Y, et al. Developmental anatomy of the liver from computerized three-dimensional reconstructions of four human embryos (from carnegie stage 14 to 23) Ann Anat. 2015;200:105–113. doi: 10.1016/j.aanat.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 22.O’Rahilly R, Muller F. Developmental stages in human embryos: revised and new measurements. Cells Tissues Organs. 2010;192:73–84. doi: 10.1159/000289817. [DOI] [PubMed] [Google Scholar]
- 23.Chen M, Zhu M, Awad H, et al. Inhibition of β-catenin signaling causes defects in postnatal cartilage development. J Cell Sci. 2008;121:1455–1465. doi: 10.1242/jcs.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dy P, Smits P, Silvester A, et al. Synovial joint morphogenesis requires the chondrogenic action of Sox5 and Sox6 in growth plate and articular cartilage. Dev Biol. 2010;341:346–359. doi: 10.1016/j.ydbio.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kavanagh E, Abiri M, Bland YS, et al. Division and death of cells in developing synovial joints and long bones. Cell Biol Int. 2002;26:679–688. doi: 10.1006/cbir.2002.0918. [DOI] [PubMed] [Google Scholar]
- 26.Mitrovic DR. Development of the metatarsophalangeal joint of the chick embryo: morphological, ultrastructural and histochemical studies. Am J Anat. 1977;150:333–347. doi: 10.1002/aja.1001500207. [DOI] [PubMed] [Google Scholar]
- 27.Ito MM, Kida MY. Morphological and biochemical re-evaluation of the process of cavitation in the rat knee joint: cellular and cell strata alterations in the interzone. J Anat. 2000;197(Pt 4):659–679. doi: 10.1046/j.1469-7580.2000.19740659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holder N. An experimental investigation into the early development of the chick elbow joint. J Embryol Exp Morphol. 1977;39:115–127. [PubMed] [Google Scholar]
- 29.Hyde G, Dover S, Aszodi A, et al. Lineage tracing using matrillin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev Biol. 2007;304:825–833. doi: 10.1016/j.ydbio.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyde G, Boot-Handford RP, Wallis GA. Col2a1 lineage tracing reveals that the meniscus of the knee joint has a complex cellular origin. J Anat. 2008;213:531–538. doi: 10.1111/j.1469-7580.2008.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winslow BB, Burke AC. Atypical molecular profile for joint development in the avian costal joint. Dev Dyn. 2010;239:2547–2557. doi: 10.1002/dvdy.22388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy JM, Heinegård D, McIntosh A, et al. Distribution of cartilage molecules in the developing mouse joint. Matrix Biol. 1999;18:487–497. doi: 10.1016/s0945-053x(99)00042-6. [DOI] [PubMed] [Google Scholar]
- 33.Shwartz Y, Viukov S, Krief S, et al. Joint development involves a continuous influx of Gdf5-positive cells. Cell Rep. 2016;15:2577–2587. doi: 10.1016/j.celrep.2016.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh PN, Ray A, Azad K, et al. A comprehensive mRNA expression analysis of developing chicken articular cartilage. Gene Expr Patterns. 2016;20:22–31. doi: 10.1016/j.gep.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Merino R, Macias D, Ganan Y, et al. Expression and function of Gdf-5 during digit skeletogenesis in the embryonic chick leg bud. Dev Biol. 1999;206:33–45. doi: 10.1006/dbio.1998.9129. [DOI] [PubMed] [Google Scholar]
- 36.Seo H-S, Serra R. Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev Biol. 2007;310:304–316. doi: 10.1016/j.ydbio.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spagnoli A, O’Rear L, Chandler RL, et al. TGF-beta signaling is essential for joint morphogenesis. J Cell Biol. 2007;177:1105–1117. doi: 10.1083/jcb.200611031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seemann P, Schwappacher R, Kjaer KW, et al. Activating and deactivating mutations in the receptor interaction site of GDF5 cause symphalangism or brachydactyly type A2. J Clin Investig. 2005;115:2373–2381. doi: 10.1172/jci25118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dowthwaite GP, Edwards JCW, Pitsillides AA. An essential role for the interaction between hyaluronan and hyaluronan binding proteins during joint development. J Histochem Cytochem. 1998;46:641–651. doi: 10.1177/002215549804600509. [DOI] [PubMed] [Google Scholar]
- 40.Pitsillides AA, Archer CW, Prehm P, et al. Alterations in hyaluronan synthesis during developing joint cavitation. J Histochem Cytochem. 1995;43:263–273. doi: 10.1177/43.3.7868856. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto K, Li Y, Jakuba C, et al. Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb. Development. 2009;136:2825–2835. doi: 10.1242/dev.038505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bastow ER, Lamb KJ, Lewthwaite JC, et al. Selective activation of the MEK-ERK pathway is regulated by mechanical stimuli in forming joints and promotes pericellular matrix formation. J Biol Chem. 2005;280:11749–11758. doi: 10.1074/jbc.m414495200. [DOI] [PubMed] [Google Scholar]
- 43.Lewthwaite JC, Bastow ER, Lamb KJ, et al. A specific mechanomodulatory role for p38 MAPK in embryonic joint articular surface cell MEK-ERK pathway regulation. J Biol Chem. 2006;281:11011–11018. doi: 10.1074/jbc.m510680200. [DOI] [PubMed] [Google Scholar]
- 44.Osborne AC, Lamb KJ, Lewthwaite JC, et al. Short-term rigid and flaccid paralyses diminish growth of embryonic chick limbs and abrogate joint cavity formation but differentially preserve pre-cavitated joints. J Musculoskelet Neuronal Interact. 2002;2:448–456. [PubMed] [Google Scholar]
- 45.Drachman DB, Sokoloff L. The role of movement in embryonic joint development. Dev Biol. 1966;14:401–420. doi: 10.1016/0012-1606(66)90022-4. [DOI] [Google Scholar]
- 46.Mitrovic D. Development of the articular cavity in paralyzed chick embryos and in chick embryo limb buds cultured on chorioallantoic membranes. Acta Anat (Basel) 1982;113:313–324. doi: 10.1159/000145566. [DOI] [PubMed] [Google Scholar]
- 47.Kavanagh E, Church VL, Osborne AC, et al. Differential regulation of GDF-5 and FGF-2/4 by immobilisation in ovo exposes distinct roles in joint formation. Dev Dyn. 2006;235:826–834. doi: 10.1002/dvdy.20679. [DOI] [PubMed] [Google Scholar]
- 48.Roddy KA, Prendergast PJ, Murphy P. Mechanical influences on morphogenesis of the knee joint revealed through morphological, molecular and computational analysis of immobilised embryos. PLoS One. 2011;6:e17526. doi: 10.1371/journal.pone.0017526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahn J, Shwartz Y, Blitz E, et al. Muscle contraction is necessary to maintain joint progenitor cell fate. Dev Cell. 2009;16:734–743. doi: 10.1016/j.devcel.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Rolfe RA, Nowlan NC, Kenny EM, et al. Identification of mechanosensitive genes during skeletal development: alteration of genes associated with cytoskeletal rearrangement and cell signalling pathways. BMC Genom. 2014;15:48. doi: 10.1186/1471-2164-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Storm EE, Huynh TV, Copeland NG, et al. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- 52.Polinkovsky A, Robin NH, Thomas JT, et al. Mutations in CDMP1 cause autosomal dominant brachydactyly type C. Nat Genet. 1997;17:18–19. doi: 10.1038/ng0997-18. [DOI] [PubMed] [Google Scholar]
- 53.Thomas JT, Kilpatrick MW, Lin K, et al. Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nat Genet. 1997;17:58–64. doi: 10.1038/ng0997-58. [DOI] [PubMed] [Google Scholar]
- 54.Thomas JT, Lin K, Nandedkar M, et al. A human chondrodysplasia due to a mutation in a TGF-beta superfamily member. Nat Genet. 1996;12:315–317. doi: 10.1038/ng0396-315. [DOI] [PubMed] [Google Scholar]
- 55.Faiyaz-Ul-Haque M, Ahmad W, Zaidi SH, et al. Mutation in the cartilage-derived morphogenetic protein-1 (CDMP1) gene in a kindred affected with fibular hypoplasia and complex brachydactyly (DuPan syndrome) Clin Genet. 2002;61:454–458. doi: 10.1034/j.1399-0004.2002.610610.x. [DOI] [PubMed] [Google Scholar]
- 56.Yi SE, Daluiski A, Pederson R, et al. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development. 2000;127:621–630. doi: 10.1242/dev.127.3.621. [DOI] [PubMed] [Google Scholar]
- 57.Baur ST, Mai JJ, Dymecki SM. Combinatorial signaling through BMP receptor IB and GDF5: shaping of the distal mouse limb and the genetics of distal limb diversity. Development. 2000;127:605–619. doi: 10.1242/dev.127.3.605. [DOI] [PubMed] [Google Scholar]
- 58.Francis-West PH, Abdelfattah A, Chen P, et al. Mechanisms of GDF-5 action during skeletal development. Development. 1999;126:1305–1315. doi: 10.1242/dev.126.6.1305. [DOI] [PubMed] [Google Scholar]
- 59.Storm EE, Kingsley DM. GDF5 coordinates bone and joint formation during digit development. Dev Biol. 1999;209:11–27. doi: 10.1006/dbio.1999.9241. [DOI] [PubMed] [Google Scholar]
- 60.Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–351. doi: 10.1016/S0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 61.Harada M, Takahara M, Zhe P, et al. Developmental failure of the intra-articular ligaments in mice with absence of growth differentiation factor 5. Osteoarthr Cartil. 2007;15:468–474. doi: 10.1016/j.joca.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Investig. 1997;100:321–330. doi: 10.1172/jci119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Storm EE, Kingsley DM. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development. 1996;122:3969–3979. doi: 10.1242/dev.122.12.3969. [DOI] [PubMed] [Google Scholar]
- 64.Settle SH, Jr, Rountree RB, Sinha A, et al. Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev Biol. 2003;254:116–130. doi: 10.1016/S0012-1606(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 65.Rountree RB, Schoor M, Chen H, et al. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004;2:e355. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roelofs AJ, Zupan J, Riemen AHK, et al. Joint morphogenetic cells in the adult mammalian synovium. Nat Commun. 2017 doi: 10.1038/ncomms15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsumaki N, Nakase T, Miyaji T, et al. Bone morphogenetic protein signals are required for cartilage formation and differently regulate joint development during skeletogenesis. J Bone Miner Res. 2002;17:898–906. doi: 10.1359/jbmr.2002.17.5.898. [DOI] [PubMed] [Google Scholar]
- 68.Ray A, Singh PN, Sohaskey ML, et al. Precise spatial restriction of BMP signaling is essential for articular cartilage differentiation. Development. 2015;142:1169–1179. doi: 10.1242/dev.110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li T, Longobardi L, Myers TJ, et al. Joint TGF-beta type II receptor-expressing cells: ontogeny and characterization as joint progenitors. Stem Cells Dev. 2013;22:1342–1359. doi: 10.1089/scd.2012.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koyama E, Shibukawa Y, Nagayama M, et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316:62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coleman CM, Vaughan EE, Browe DC, et al. Growth differentiation factor-5 enhances in vitro mesenchymal stromal cell chondrogenesis and hypertrophy. Stem Cells Dev. 2013;22:1968–1976. doi: 10.1089/scd.2012.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu FL, Lin LH, Sytwu HK, et al. GDF-5 is suppressed by IL-1beta and enhances TGF-beta3-mediated chondrogenic differentiation in human rheumatoid fibroblast-like synoviocytes. Exp Mol Pathol. 2010;88:163–170. doi: 10.1016/j.yexmp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 73.Southam L, Rodriguez-Lopez J, Wilkins JM, et al. An SNP in the 5′-UTR of GDF5 is associated with osteoarthritis susceptibility in Europeans and with in vivo differences in allelic expression in articular cartilage. Hum Mol Genet. 2007;16:2226–2232. doi: 10.1093/hmg/ddm174. [DOI] [PubMed] [Google Scholar]
- 74.Miyamoto Y, Mabuchi A, Shi D, et al. A functional polymorphism in the 5′ UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat Genet. 2007;39:529–533. doi: 10.1038/2005. [DOI] [PubMed] [Google Scholar]
- 75.Wu DD, Li GM, Jin W, et al. Positive selection on the osteoarthritis-risk and decreased-height associated variants at the GDF5 gene in East Asians. PLoS One. 2012;7:e42553. doi: 10.1371/journal.pone.0042553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo X, Day TF, Jiang X, et al. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18:2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spater D, Hill TP, Gruber M, et al. Role of canonical Wnt-signalling in joint formation. Eur Cell Mater. 2006;12:71–80. doi: 10.22203/eCM.v012a09. [DOI] [PubMed] [Google Scholar]
- 78.Spater D, Hill TP, O’Sullivan RJ, et al. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. 2006;133:3039–3049. doi: 10.1242/dev.02471. [DOI] [PubMed] [Google Scholar]
- 79.Lee H-H, Behringer RR. Conditional expression of Wnt4 during chondrogenesis leads to dwarfism in mice. PLoS One. 2007;2:e450. doi: 10.1371/journal.pone.0000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mak KK, Chen MH, Day TF, et al. Wnt/beta-catenin signaling interacts differentially with Ihh signaling in controlling endochondral bone and synovial joint formation. Development. 2006;133:3695–3707. doi: 10.1242/dev.02546. [DOI] [PubMed] [Google Scholar]
- 81.Yasuhara R, Ohta Y, Yuasa T, et al. Roles of β-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab Investig. 2011;91:1739. doi: 10.1038/labinvest.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cantley L, Saunders C, Guttenberg M, et al. Loss of β-catenin induces multifocal periosteal chondroma-like masses in mice. Am J Pathol. 2013;182:917–927. doi: 10.1016/j.ajpath.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuasa T, Kondo N, Yasuhara R, et al. Transient activation of Wnt/{beta}-catenin signaling induces abnormal growth plate closure and articular cartilage thickening in postnatal mice. Am J Pathol. 2009;175:1993–2003. doi: 10.2353/ajpath.2009.081173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 85.Yamagami T, Molotkov A, Zhou CJ. Canonical Wnt signaling activity during synovial joint development. J Mol Histol. 2009;40:311. doi: 10.1007/s10735-009-9242-1. [DOI] [PubMed] [Google Scholar]
- 86.Bernard P, Fleming A, Lacombe A, et al. Wnt4 inhibits beta-catenin/TCF signalling by redirecting beta-catenin to the cell membrane. Biol Cell. 2008;100:167–177. doi: 10.1042/bc20070072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bernardi H, Gay S, Fedon Y, et al. Wnt4 activates the canonical beta-catenin pathway and regulates negatively myostatin: functional implication in myogenesis. Am J Physiol Cell Physiol. 2011;300:C1122–C1138. doi: 10.1152/ajpcell.00214.2010. [DOI] [PubMed] [Google Scholar]
- 88.Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Investig. 2005;115:622–631. doi: 10.1172/jci22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kobayashi T, Soegiarto DW, Yang Y, et al. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Investig. 2005;115:1734–1742. doi: 10.1172/jci24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mak KK, Kronenberg HM, Chuang P-T, et al. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development. 2008;135:1947–1956. doi: 10.1242/dev.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Minina E, Wenzel HM, Kreschel C, et al. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development. 2001;128:4523–4534. doi: 10.1242/dev.128.22.4523. [DOI] [PubMed] [Google Scholar]
- 93.Minina E, Kreschel C, Naski MC, et al. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell. 2002;3:439–449. doi: 10.1016/S1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 94.Weir EC, Philbrick WM, Amling M, et al. Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc Natl Acad Sci USA. 1996;93:10240–10245. doi: 10.1073/pnas.93.19.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lanske B, Karaplis AC, Lee K, et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 96.Karaplis AC, Luz A, Glowacki J, et al. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–289. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- 97.Chung UI, Schipani E, McMahon AP, et al. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Investig. 2001;107:295–304. doi: 10.1172/jci11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koyama E, Ochiai T, Rountree RB, et al. Synovial joint formation during mouse limb skeletogenesis. Ann N Y Acad Sci. 2007;1116:100–112. doi: 10.1196/annals.1402.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Decker RS, Koyama E, Pacifici M. Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix Biol. 2014;39:5–10. doi: 10.1016/j.matbio.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Niedermaier M, Schwabe GC, Fees S, et al. An inversion involving the mouse Shh locus results in brachydactyly through dysregulation of Shh expression. J Clin Investig. 2005;115:900–909. doi: 10.1172/jci23675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koziel L, Wuelling M, Schneider S, et al. Gli3 acts as a repressor downstream of Ihh in regulating two distinct steps of chondrocyte differentiation. Development. 2005;132:5249–5260. doi: 10.1242/dev.02097. [DOI] [PubMed] [Google Scholar]
- 102.Huang B-L, Trofka A, Furusawa A, et al. An interdigit signalling centre instructs coordinate phalanx-joint formation governed by 5′Hoxd–Gli3 antagonism. Nat Commun. 2016 doi: 10.1038/ncomms12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gritli-Linde A, Lewis P, McMahon AP, et al. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev Biol. 2001;236:364–386. doi: 10.1006/dbio.2001.0336. [DOI] [PubMed] [Google Scholar]
- 104.Rockel JS, Yu C, Whetstone H, et al. Hedgehog inhibits β-catenin activity in synovial joint development and osteoarthritis. J Clin Investig. 2016;126:1649–1663. doi: 10.1172/jci80205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou J, Chen Q, Lanske B, et al. Disrupting the Indian hedgehog signaling pathway in vivo attenuates surgically induced osteoarthritis progression in Col2a1-CreERT2; Ihhfl/fl mice. Arthritis Res Ther. 2014;16:R11. doi: 10.1186/ar4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang C, Wei X, Chen C, et al. Indian hedgehog in synovial fluid is a novel marker for early cartilage lesions in human knee joint. Int J Mol Sci. 2014;15:7250–7265. doi: 10.3390/ijms15057250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shuang F, Zhou Y, Hou S-X, et al. Indian Hedgehog signaling pathway members are associated with magnetic resonance imaging manifestations and pathological scores in lumbar facet joint osteoarthritis. Sci Rep. 2015 doi: 10.1038/srep10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Woods S, Barter MJ, Elliott HR, et al. miR-324-5p is up regulated in end-stage osteoarthritis and regulates Indian Hedgehog signalling by differing mechanisms in human and mouse. Matrix Biol. 2018 doi: 10.1016/j.matbio.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo X, Mak KK, Taketo MM, et al. The Wnt/β-catenin pathway interacts differentially with PTHrP signaling to control chondrocyte hypertrophy and final maturation. PLoS One. 2009;4:e6067. doi: 10.1371/journal.pone.0006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Macica C, Liang G, Nasiri A, et al. Genetic evidence of the regulatory role of parathyroid hormone-related protein in articular chondrocyte maintenance in an experimental mouse model. Arthritis Rheum. 2011;63:3333–3343. doi: 10.1002/art.30515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen X, Macica CM, Nasiri A, et al. Regulation of articular chondrocyte proliferation and differentiation by Indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum. 2008;58:3788–3797. doi: 10.1002/art.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen X, Macica CM, Dreyer BE, et al. Initial characterization of PTH-related protein gene-driven lacZ expression in the mouse. J Bone Miner Res. 2006;21:113–123. doi: 10.1359/jbmr.051005. [DOI] [PubMed] [Google Scholar]
- 113.Sampson ER, Hilton MJ, Tian Y, et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med. 2011 doi: 10.1126/scitranslmed.3002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ogawa H, Kozhemyakina E, Hung HH, et al. Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes Dev. 2014;28:127–139. doi: 10.1101/gad.231969.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 2016;12:203–221. doi: 10.1038/nrendo.2016.12. [DOI] [PubMed] [Google Scholar]
- 116.Retting KN, Song B, Yoon BS, et al. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Francis-West PH, Parish J, Lee K, et al. BMP/GDF-signalling interactions during synovial joint development. Cell Tissue Res. 1999;296:111–119. doi: 10.1007/s004410051272. [DOI] [PubMed] [Google Scholar]
- 118.Pathi S, Rutenberg JB, Johnson RL, et al. Interaction of Ihh and BMP/Noggin signaling during cartilage differentiation. Dev Biol. 1999;209:239–253. doi: 10.1006/dbio.1998.9181. [DOI] [PubMed] [Google Scholar]
- 119.Singh PNP, Shea CA, Sonker SK, et al. Precise spatial restriction of BMP signaling in developing joints is perturbed upon loss of embryo movement. Development. 2018 doi: 10.1242/dev.153460. [DOI] [PubMed] [Google Scholar]
- 120.Singh PNP, Yadav US, Azad K, et al. NFIA and GATA3 are crucial regulators of embryonic articular cartilage differentiation. Development. 2018 doi: 10.1242/dev.156554. [DOI] [PubMed] [Google Scholar]
- 121.Zhu W, Kim J, Cheng C, et al. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone. 2006;39:61–71. doi: 10.1016/j.bone.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/S0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 123.Egawa S, Saito D, Abe G, et al. Morphogenetic mechanism of the acquisition of the dinosaur-type acetabulum. R Soc Open Sci. 2018 doi: 10.1098/rsos.180604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Amarilio R, Viukov SV, Sharir A, et al. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 125.Brunet LJ, McMahon JA, McMahon AP, et al. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 126.Wijgerde M, Karp S, McMahon J, et al. Noggin antagonism of BMP4 signaling controls development of the axial skeleton in the mouse. Dev Biol. 2005;286:149–157. doi: 10.1016/j.ydbio.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 127.Seemann P, Brehm A, König J, et al. Mutations in GDF5 reveal a key residue mediating BMP inhibition by NOGGIN. PLoS Genet. 2009;5:e1000747. doi: 10.1371/journal.pgen.1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garrison P, Yue S, Hanson J, et al. Spatial regulation of bone morphogenetic proteins (BMPs) in postnatal articular and growth plate cartilage. PLoS One. 2017;12:e0176752. doi: 10.1371/journal.pone.0176752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gelse K, Ekici AB, Cipa F, et al. Molecular differentiation between osteophytic and articular cartilage–clues for a transient and permanent chondrocyte phenotype. Osteoarthr Cartil. 2012;20:162–171. doi: 10.1016/j.joca.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 130.Chang SH, Mori D, Kobayashi H, et al. Excessive mechanical loading promotes osteoarthritis through the gremlin-1–NF-κB pathway. Nat Commun. 2019;10:1442. doi: 10.1038/s41467-019-09491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shen J, Li J, Wang B, et al. Deletion of the transforming growth factor beta receptor type II gene in articular chondrocytes leads to a progressive osteoarthritis-like phenotype in mice. Arthritis Rheum. 2013;65:3107–3119. doi: 10.1002/art.38122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Finnson KW, Chi Y, Bou-Gharios G, et al. TGF-b signaling in cartilage homeostasis and osteoarthritis. Front Biosci (Schol Ed) 2012;4:251–268. doi: 10.2741/s266. [DOI] [PubMed] [Google Scholar]
- 133.Hayes AJ, MacPherson S, Morrison H, et al. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl.) 2001;203:469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- 134.van Caam A, Madej W, Thijssen E, et al. Expression of TGFbeta-family signalling components in ageing cartilage: age-related loss of TGFbeta and BMP receptors. Osteoarthr Cartil. 2016;24:1235–1245. doi: 10.1016/j.joca.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 135.Oo WM, Yu SP, Daniel MS, et al. Disease-modifying drugs in osteoarthritis: current understanding and future therapeutics. Expert Opin Emerg Drugs. 2018;23:331–347. doi: 10.1080/14728214.2018.1547706. [DOI] [PubMed] [Google Scholar]
- 136.Pelton RW, Saxena B, Jones M, et al. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991;115:1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu M, Chen G, Li Y-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lim J, Tu X, Choi K, et al. BMP-Smad4 signaling is required for precartilaginous mesenchymal condensation independent of Sox9 in the mouse. Dev Biol. 2015;400:132–138. doi: 10.1016/j.ydbio.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bénazet JD, Pignatti E, Nugent A, et al. Smad4 is required to induce digit ray primordia and to initiate the aggregation and differentiation of chondrogenic progenitors in mouse limb buds. Development. 2012;139:4250–4260. doi: 10.1242/dev.084822. [DOI] [PubMed] [Google Scholar]
- 140.Zhang J, Tan X, Li W, et al. Smad4 is required for the normal organization of the cartilage growth plate. Dev Biol. 2005;284:311–322. doi: 10.1016/j.ydbio.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 141.Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn. 2008;237:18–27. doi: 10.1002/dvdy.21388. [DOI] [PubMed] [Google Scholar]
- 142.Ohbayashi N, Shibayama M, Kurotaki Y, et al. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 2002;16:870–879. doi: 10.1101/gad.965702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Z, Xu J, Colvin JS, et al. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859–869. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen L, Adar R, Yang X, et al. Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis. J Clin Investig. 1999;104:1517–1525. doi: 10.1172/jci6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shiang R, Thompson LM, Zhu YZ, et al. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 146.Deng C, Li C, Chen L, et al. A Ser365 → Cys mutation of fibroblast growth factor receptor 3 in mouse downregulates Ihh/PTHrP signals and causes severe achondroplasia. Hum Mol Genet. 2001;10:457–466. doi: 10.1093/hmg/10.5.457. [DOI] [PubMed] [Google Scholar]
- 147.Peters K, Ornitz D, Werner S, et al. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev Biol. 1993;155:423–430. doi: 10.1006/dbio.1993.1040. [DOI] [PubMed] [Google Scholar]
- 148.Molténi A, Modrowski D, Hott M, et al. Differential expression of fibroblast growth factor receptor-1, -2, and -3 and syndecan-1, -2, and -4 in neonatal rat mandibular condyle and calvaria during osteogenic differentiation in vitro. Bone. 1999;24:337–347. doi: 10.1016/s8756-3282(98)00191-4. [DOI] [PubMed] [Google Scholar]
- 149.Hellingman CA, Koevoet W, Kops N, et al. Fibroblast growth factor receptors in in vitro and in vivo chondrogenesis: relating tissue engineering using adult mesenchymal stem cells to embryonic development. Tissue Eng Part A. 2010;16:545–556. doi: 10.1089/ten.tea.2008.0551. [DOI] [PubMed] [Google Scholar]
- 150.Lazarus JE, Hegde A, Andrade AC, et al. Fibroblast growth factor expression in the postnatal growth plate. Bone. 2007;40:577–586. doi: 10.1016/j.bone.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 151.Hojo H, Ohba S, Taniguchi K, et al. Hedgehog-Gli activators direct osteo-chondrogenic function of bone morphogenetic protein toward osteogenesis in the perichondrium. J Biol Chem. 2013;288:9924–9932. doi: 10.1074/jbc.m112.409342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kohn A, Dong Y, Mirando AJ, et al. Cartilage-specific RBPjkappa-dependent and -independent Notch signals regulate cartilage and bone development. Development. 2012;139:1198–1212. doi: 10.1242/dev.070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ellsworth JL, Berry J, Bukowski T, et al. Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthr Cartil. 2002;10:308–320. doi: 10.1053/joca.2002.0514. [DOI] [PubMed] [Google Scholar]
- 154.Karuppaiah K, Yu K, Lim J, et al. FGF signaling in the osteoprogenitor lineage non-autonomously regulates postnatal chondrocyte proliferation and skeletal growth. Development. 2016;143:1811–1822. doi: 10.1242/dev.131722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Colvin JS, Bohne BA, Harding GW, et al. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- 156.Wen X, Li X, Tang Y, et al. Chondrocyte FGFR3 regulates bone mass by inhibiting osteogenesis. J Biol Chem. 2016;291:24912–24921. doi: 10.1074/jbc.m116.730093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li M, Seki Y, Freitas PHL, et al. FGFR3 down-regulates PTH/PTHrP receptor gene expression by mediating JAK/STAT signaling in chondrocytic cell line. J Electron Microsc (Tokyo) 2010;59:227–236. doi: 10.1093/jmicro/dfq002. [DOI] [PubMed] [Google Scholar]
- 158.Naski MC, Colvin JS, Coffin JD, et al. Repression of hedgehog signaling and BMP4 expression in growth plate cartilage by fibroblast growth factor receptor 3. Development. 1998;125:4977–4988. doi: 10.1242/dev.125.24.4977. [DOI] [PubMed] [Google Scholar]
- 159.Zhou S, Xie Y, Tang J, et al. FGFR3 deficiency causes multiple chondroma-like lesions by upregulating hedgehog signaling. PLoS Genet. 2015;11:e1005214. doi: 10.1371/journal.pgen.1005214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Reinhold MI, Naski MC. Direct interactions of Runx2 and canonical Wnt signaling induce FGF18. J Biol Chem. 2007;282:3653–3663. doi: 10.1074/jbc.m608995200. [DOI] [PubMed] [Google Scholar]
- 161.Shimokawa T, Furukawa Y, Sakai M, et al. Involvement of the FGF18 gene in colorectal carcinogenesis, as a novel downstream target of the beta-catenin/T-cell factor complex. Cancer Res. 2003;63:6116–6120. [PubMed] [Google Scholar]
- 162.Li X, Ellman MB, Kroin JS, et al. Species-specific biological effects of FGF-2 in articular cartilage: implication for distinct roles within the FGF receptor family. J Cell Biochem. 2012;113:2532–2542. doi: 10.1002/jcb.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mori Y, Saito T, Chang SH, et al. Identification of fibroblast growth factor-18 as a molecule to protect adult articular cartilage by gene expression profiling. J Biol Chem. 2014;289:10192–10200. doi: 10.1074/jbc.m113.524090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Valverde-Franco G, Binette JS, Li W, et al. Defects in articular cartilage metabolism and early arthritis in fibroblast growth factor receptor 3 deficient mice. Hum Mol Genet. 2006;15:1783–1792. doi: 10.1093/hmg/ddl100. [DOI] [PubMed] [Google Scholar]
- 165.Zhou S, Xie Y, Li W, et al. Conditional deletion of Fgfr3 in chondrocytes leads to osteoarthritis-like defects in temporomandibular joint of adult mice. Sci Rep. 2016;6:24039. doi: 10.1038/srep24039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tang J, Su N, Zhou S, et al. Fibroblast growth factor receptor 3 inhibits osteoarthritis progression in the knee joints of adult mice. Arthritis Rheumatol. 2016;68:2432–2443. doi: 10.1002/art.39739. [DOI] [PubMed] [Google Scholar]
- 167.Ellman MB, Yan D, Ahmadinia K, et al. Fibroblast growth factor control of cartilage homeostasis. J Cell Biochem. 2013;114:735–742. doi: 10.1002/jcb.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yan D, Chen D, Cool SM, et al. Fibroblast growth factor receptor 1 is principally responsible for fibroblast growth factor 2-induced catabolic activities in human articular chondrocytes. Arthritis Res Ther. 2011;13:R130. doi: 10.1186/ar3441. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 169.Shu CC, Jackson MT, Smith MM, et al. Ablation of perlecan domain 1 heparan sulfate reduces progressive cartilage degradation, synovitis, and osteophyte size in a preclinical model of posttraumatic osteoarthritis. Arthritis Rheumatol. 2016;68:868–879. doi: 10.1002/art.39529. [DOI] [PubMed] [Google Scholar]
- 170.Moore EE, Bendele AM, Thompson DL, et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthr Cartil. 2005;13:623–631. doi: 10.1016/j.joca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 171.Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol. 2014;10:11–22. doi: 10.1038/nrrheum.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang Z, Wang Y, Li M, et al. Fibroblast growth factor 18 increases the trophic effects of bone marrow mesenchymal stem cells on chondrocytes isolated from late stage osteoarthritic patients. Stem Cells Int. 2014 doi: 10.1155/2014/125683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kozhemyakina E, Zhang M, Ionescu A, et al. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 2015;67:1261–1273. doi: 10.1002/art.39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tavella S, Biticchi R, Schito A, et al. Targeted expression of SHH affects chondrocyte differentiation, growth plate organization, and Sox9 expression. J Bone Miner Res. 2004;19:1678–1688. doi: 10.1359/jbmr.040706. [DOI] [PubMed] [Google Scholar]
- 175.Schumacher BL, Block JA, Schmid TM, et al. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144–152. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- 176.Schumacher BL, Hughes CE, Kuettner KE, et al. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17:110–120. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 177.Coles JM, Zhang L, Blum JJ, et al. Loss of cartilage structure, stiffness, and frictional properties in mice lacking PRG4. Arthritis Rheum. 2010;62:1666–1674. doi: 10.1002/art.27436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ruan MZC, Erez A, Guse K, et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med. 2013;5:176ra34. doi: 10.1126/scitranslmed.3005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kosinska MK, Ludwig TE, Liebisch G, et al. Articular joint lubricants during osteoarthritis and rheumatoid arthritis display altered levels and molecular species. PLoS One. 2015;10:e0125192. doi: 10.1371/journal.pone.0125192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ogawa H, Matsumoto K, Terabayashi N, et al. Association of lubricin concentration in synovial fluid and clinical status of osteoarthritic knee. Mod Rheumatol. 2017;27:489–492. doi: 10.1080/14397595.2016.1209829. [DOI] [PubMed] [Google Scholar]
- 181.Flannery CR, Zollner R, Corcoran C, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60:840–847. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 182.Cui Z, Xu C, Li X, et al. Treatment with recombinant lubricin attenuates osteoarthritis by positive feedback loop between articular cartilage and subchondral bone in ovariectomized rats. Bone. 2015;74:37–47. doi: 10.1016/j.bone.2014.12.065. [DOI] [PubMed] [Google Scholar]
- 183.Bao JP, Chen WP, Wu LD. Lubricin: a novel potential biotherapeutic approaches for the treatment of osteoarthritis. Mol Biol Rep. 2011;38:2879–2885. doi: 10.1007/s11033-010-9949-9. [DOI] [PubMed] [Google Scholar]
- 184.Grogan SP, Miyaki S, Asahara H, et al. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee Y, Choi J, Hwang NS. Regulation of lubricin for functional cartilage tissue regeneration: a review. Biomater Res. 2018;22:9. doi: 10.1186/s40824-018-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Matsuzaki T, Alvarez-Garcia O, Mokuda S, et al. FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci Transl Med. 2018 doi: 10.1126/scitranslmed.aan0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Niikura T, Reddi AH. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007;56:2312–2321. doi: 10.1002/art.22659. [DOI] [PubMed] [Google Scholar]
- 188.Hill A, Waller KA, Cui Y, et al. Lubricin restoration in a mouse model of congenital deficiency. Arthritis Rheumatol. 2015;67:3070–3081. doi: 10.1002/art.39276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 190.Kovall RA, Gebelein B, Sprinzak D, et al. The canonical Notch signaling pathway: structural and biochemical insights into shape, sugar, and force. Dev Cell. 2017;41:228–241. doi: 10.1016/j.devcel.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dong Y, Jesse AM, Kohn A, et al. RBPjκ-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. Development (Cambridge, England) 2010;137:1461–1471. doi: 10.1242/dev.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mead TJ, Yutzey KE. Notch pathway regulation of chondrocyte differentiation and proliferation during appendicular and axial skeleton development. Proc Natl Acad Sci USA. 2009;106:14420–14425. doi: 10.1073/pnas.0902306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mirando AJ, Liu Z, Moore T, et al. RBP-Jkappa-dependent Notch signaling is required for murine articular cartilage and joint maintenance. Arthritis Rheum. 2013;65:2623–2633. doi: 10.1002/art.38076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hosaka Y, Saito T, Sugita S, et al. Notch signaling in chondrocytes modulates endochondral ossification and osteoarthritis development. Proc Natl Acad Sci USA. 2013;110:1875–1880. doi: 10.1073/pnas.1207458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu Z, Chen J, Mirando AJ, et al. A dual role for NOTCH signaling in joint cartilage maintenance and osteoarthritis. Sci Signal. 2015;8:ra71. doi: 10.1126/scisignal.aaa3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dowthwaite GP, Bishop JC, Redman SN, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 197.Li L, Newton PT, Bouderlique T, et al. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice. FASEB J. 2017;31:1067–1084. doi: 10.1096/fj.201600918r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Karlsson C, Brantsing C, Egell S, et al. Notch1, Jagged1, and HES5 are abundantly expressed in osteoarthritis. Cells Tissues Organs. 2008;188:287–298. doi: 10.1159/000121610. [DOI] [PubMed] [Google Scholar]
- 199.Liu Z, Ren Y, Mirando AJ, et al. Notch signaling in postnatal joint chondrocytes, but not subchondral osteoblasts, is required for articular cartilage and joint maintenance. Osteoarthr Cartil. 2016;24:740–751. doi: 10.1016/j.joca.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]