Abstract
Recent high-throughput genome-wide sequencing studies have identified recurrent somatic mutations in myeloid neoplasms. An epigenetic regulator, Additional sex combs-like 1 (ASXL1), is one of the most frequently mutated genes in all subtypes of myeloid malignancies. ASXL1 mutations are also frequently detected in clonal hematopoiesis, which is associated with an increased risk of mortality. Therefore, it is important to understand how ASXL1 mutations contribute to clonal expansion and myeloid transformation in hematopoietic cells. Studies using ASXL1-depleted human hematopoietic cells and Asxl1 knockout mice have shown that deletion of wild-type ASXL1 protein leads to impaired hematopoiesis and accelerates myeloid malignancies via loss of interaction with polycomb repressive complex 2 proteins. On the other hand, ASXL1 mutations in myeloid neoplasms typically occur near the last exon and result in the expression of C-terminally truncated mutant ASXL1 protein. Biological studies and biochemical analyses of this variant have shed light on its dominant-negative and gain-of-function features in myeloid transformation via a variety of epigenetic changes. Based on these results, it would be possible to establish novel promising therapeutic strategies for myeloid malignancies harboring ASXL1 mutations by blocking interactions between ASXL1 and associating epigenetic regulators. Here, we summarize the clinical implications of ASXL1 mutations, the role of wild-type ASXL1 in normal hematopoiesis, and oncogenic functions of mutant ASXL1 in myeloid neoplasms.
Keywords: ASXL1, BAP1, HOX, Acute myeloid leukemia, AML, Myelodysplastic syndrome, MDS, MPN, CMML
Introduction
Myeloid malignancies are characterized by aberrant clonal expansion and differentiation defects of hematopoietic stem cells (HSCs), hematopoietic stem progenitor cells (HSPCs) or myeloid progenitor cells. Most myeloid malignancies are associated with high mortality due to limitations of the available therapeutic agents and high relapse rate. To investigate the causative mutations of myeloid malignancies, genome-wide sequencing studies have been performed and have revealed the mutational landscape [1–4].
An epigenetic modulator, Additional sex combs-like 1 (ASXL1), is one of the most frequently mutated genes in a variety of myeloid neoplasms such as myelodysplastic syndromes (MDS) [5–7], acute myeloid leukemia (AML) [7–9], myeloproliferative neoplasms (MPN) [10–16] and chronic myelomonogenous leukemia (CMML) [14, 17–20], and its mutations are always associated with poor prognosis. Additionally, ASXL1 mutations are frequently found in clonal hematopoiesis (CH) [also called clonal hematopoiesis of indeterminate potential (CHIP)], precursor states for hematologic neoplasms with somatic mutations in the absence of diagnostic criteria for hematologic malignancies [21–23]. Therefore, understanding the mechanism by which ASXL1 mutations contribute to myeloid transformation is clinically important. To understand the functions of ASXL1, ASXL1 knockdown or Asxl1 knockout mice studies have been performed [24–26]. These studies demonstrated that ASXL1 knockdown promoted the development of MDS/MPN disease and ASXL1 depletion resulted in impaired hematopoiesis due to loss of interaction with polycomb repressive complex 2 (PRC2). On the other hand, most ASXL1 mutations exist in the last exon and would produce C-terminally truncated mutant proteins of ASXL1 (hereinafter referred as to mutant ASXL1) by escaping from nonsense-mediated-decay [8, 27]. Overexpression of mutant ASXL1 impaired myeloid differentiation and induced MDS in mouse transplantation models [28]. There is also growing evidence indicating that the physiological expression of mutant ASXL1 protein perturbs hematopoiesis and promotes myeloid transformation by altering histone modifications in both a dominant-negative and gain-of-function manner [29]. In addition, novel promising therapeutic strategies targeting ASXL1 mutated malignancies have been investigated [30–33].
In this review, we will summarize the clinical significance of ASXL1 mutations in myeloid malignancies. We will also describe recent findings of ASXL1 functions from biochemical and biological perspectives, and will then introduce potential targeted therapies for myeloid malignancies harboring ASXL1 mutations.
Members of mammalian ASXL family
Mammalian ASXL family genes (ASXL1, ASXL2 and ASXL3) are paralogs of Drosophila Additional sex combs (Asx) [34, 35]. Asx was originally identified as an enhancer of the trithorax and polycomb group (ETP) genes to regulate Hox gene expression [36, 37]. Polycomb group (PcG) genes repress [38, 39], while trithorax group (TrxG) genes activate Hox gene expression [40, 41]. Thus, Drosophila Asx is involved in both gene activation and repression. In addition, Schermann et al. revealed that Asx and Calypso, the human ortholog of BRCA1-associated protein 1 (BAP1), formed a Polycomb-repressive deubiquitinase (PR-DUB), which removes monoubiquitination of histone H2A at lysine 119 (H2AK119ub) [42]. Collectively, Drosophila Asx is now thought to integrally control gene expression through exerting a variety of epigenetic modifications.
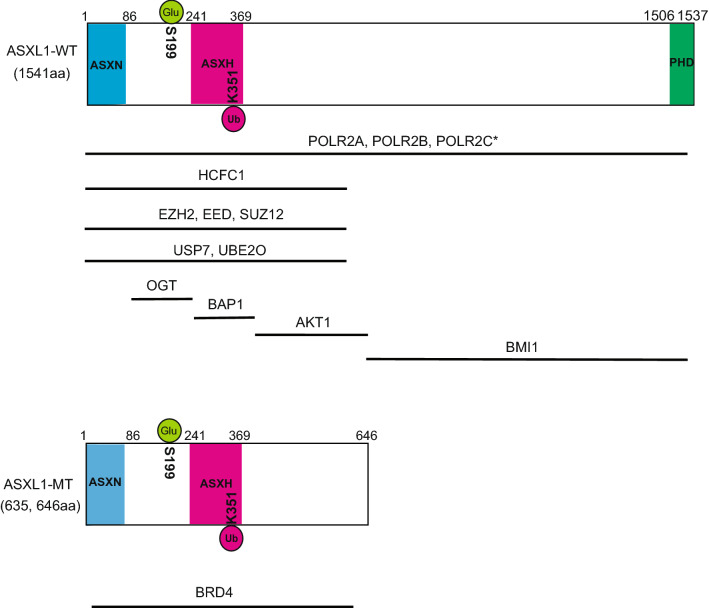
Mammalian ASXL1 is ubiquitously expressed [43]. Human ASXL1 gene is located on chromosome 20q11 and encodes a 1541 amino acids–protein [44]. ASXL1 has an N-terminus ASXN domain, an ASX homology (ASXH) domain at the N-terminus region, and a plant homeodomain (PHD) finger at the C-terminal region (Fig. 1). ASXN, ASXH, and PHD domains are shared among all three mammalian ASXL family proteins. The ASXN domain is structurally similar to a forkhead-box domain and predicted to be essential for the DNA-binding ability of ASXL family proteins [45]. The ASXH domain is highly conserved from Drosophila to mammalian and is also called as DEUBAD (deubiquitinase adaptor) because this domain binds a deubiquitinase BAP1 [42], suggesting the importance of the interaction between BAP1 and ASXL1. The PHD domain is a histone- or DNA-binding module, and recognizes different histone modification subtypes such as unmethylated H3K4 (H3K4me0) and trimethylated H3K4 (H3K4me3) [46, 47].
Fig. 1.
Schematic representation of the structure of wild-type ASXL1 (ASXL1-WT) and C-terminally truncated mutant ASXL1 (ASXL1-MT). Their known interacting partners and post translational modifications are also shown. *Binding sites are not identified
Germline mutations of ASXL1 and ASXL3 are identified in patients with Bohring–Opitz syndrome, which is characterized by severe developmental disorders [48, 49]. ASXL2 germline mutations are associated with the Shashi-Pena syndrome, which is a neurodevelopmental syndrome [50]. ASXL1 and ASXL2 are ubiquitously expressed in a variety of tissue, whereas ASXL3 expression is restricted to lymph node, eyes, lungs, skin, brain, and pituitary gland [43].
A recent study showed that ASXL2 was essential for cardiac development and skeletal or metabolic homeostasis [51]. In myeloid malignancies, ASXL2 mutations are frequently found in AML harboring RUNX1-ETO fusion gene, whereas the frequency of ASXL2 mutations in other myeloid malignancies is much lower than that of ASXL1 mutations [52]. Interestingly, however, ASXL2 mutations are more frequently associated with RUNX1-ETO than ASXL1 mutations, making this particular fusion gene unique among many fusion genes. Asxl2-deficient mice showed more severe impaired hematopoiesis than Asxl1-deficient mice and development of MDS-like disease [53–55]. These results indicate that wild-type ASXL2 plays crucial roles as well as a tumor suppressor role in normal hematopoiesis. ASXL3 mutations are mainly detected in prostate cancers and pancreatic cancers, whereas the mutations are rarely found in hematological malignancies [56]. Although, ASXL2 and ASXL3 share conserved critical domains with ASXL1, the frequency of ASXL1 mutations are much higher than those of ASXL2 and ASXL3 mutations. The diversity of mutation frequencies within the ASXL family could be due to the differences in their unique binding partners, their binding sites on chromatin or histone modifications recognized by the PHD domain.
Clinical implications of ASXL1 mutations in myeloid malignancies
Somatic ASXL1 mutations are recurrently found in various myeloid malignancies including myelodysplastic syndromes (MDS) [5–7], acute myeloid leukemia (AML) [7–9] and myeloproliferative neoplasms (MPN) such as chronic myelogenous leukemia (CML), chronic neutrophic leukemia (CNL) and primary myelofibrosis (pMF) [10–16]. ASXL1 mutations are most frequently identified in patients with MPN/MDS overlap syndrome including chronic myelomonocytic leukemia (CMML) (50%) [14, 17–20] and juvenile myelomonocytic leukemia (JMML) [57, 58]. ASXL1 mutations are also detected in other myeloid malignancies such as blastic plasmacytoid dendritic cell neoplasm (BPDCN) [59] and systemic mastocytosis [60–62]. Additionally, ASXL1 mutations are found in aplastic anemia, a common cause of acquired bone marrow failure [63, 64]. Conversely, ASXL1 mutations are rarely found in lymphoid neoplasms [65].
The majority of ASXL1 mutations are frameshift or nonsense mutations localized at the last exon, exon 12. ASXL1 mutations frequently coexist with the following mutations; DNA methylation-related genes (TET2 [1], IDH1 [66], IDH2 [8, 66, 67]), spliceosomes (U2AF1 [68], SRSF2 [69]), transcriptional factors (CEBPA [9], RUNX1 [8, 67, 70, 71], GATA2 [72]), signal transducers (NRAS [14], JAK2 [70]), STAG2 [70] and SETBP1 [73–76]. However, ASXL1 mutations are mutually exclusive to DNMT3A [8, 67], FLT3-ITD [8, 67, 71, 77], NPM1 [8, 71, 77, 78] and SF3B1 [79] mutations. These positive and negative associations of mutations should be considered in functional analyses of these mutations.
ASXL1 mutations in acute myeloid leukemia
ASXL1 mutations are found in 5–11% of AML patients [71, 80] and independently confer poor prognosis [8, 9, 67, 71, 77]. ASXL1 mutations in AML are more common in older patients [9, 67, 71], in secondary leukemia [67] and in male patients [9, 67, 71]. In AML, ASXL1 mutations frequently coexist with RUNX1 mutations [8, 67, 71] and IDH2 mutations [67, 81], and are positively associated with FAB M0 karyotype [71, 77], t(8; 21) [52, 71, 82], trisomy 8 [67, 71] and del(7q)/− 7 chromosomal aberrations [67].
Notably, RUNX1 is the most frequently mutated gene in ASXL1-mutated AML. Coexistence of ASXL1 and RUNX1 mutations is related to poor prognosis in AML patients [67]. We previously reported that a RUNX1 frameshift mutation (RUNX1 S291fsX) indeed cooperates with an ASXL1 mutation to develop MDS/AML in a mouse model [29]. Further studies are required to reveal the precise mechanism by which ASXL1 mutation and RUNX1 mutation cooperatively induce myeloid malignancies.
ASXL1 mutations in myelodysplastic syndromes
ASXL1 mutations are found in 11–21% of patients with MDS and are also associated with adverse outcomes in MDS patients [1, 5, 83]. ASXL1 mutations are more frequently detected in patients with high-risk cases of MDS [6, 7]. DNA hypomethylating agents (HMA) such as azacitidine or decitabine are used for high-risk MDS patients. A recent study showed that TET2 mutations confer improved response to HMA; however, there was no association between ASXL1 mutations and response to HMA as there was with TET2 mutations [84]. Another study demonstrated that ASXL1 mutations are associated with shorter overall survival in MDS patients treated with HMA [85].
In MDS patients ASXL1 mutations frequently coexist with SETBP1 mutations [73–76]. SETBP1 mutations are localized in the SKI homologous region, resulting in increased stability of the SETBP1 protein [73, 76]. The presence of SETBP1 mutations is reported to be associated with quicker leukemic transformation of MDS and shorter survivals. In fact, Inoue et al. demonstrated that SETBP1 mutations rapidly drive leukemic transformation of MDS with ASXL1 mutations both in patients and in a mouse model [73].
ASXL1 mutations in chronic myelomonocytic leukemia
The ASXL1 mutation is the most frequently (40–50%) detected mutations in CMML patients. CMML patients harboring ASXL1 mutations have poorer prognosis [17, 18, 86, 87] and are categorized as a high-risk leukemic transformation group [17, 18]. Prognostic scores, including ASXL1 mutational status, divides CMML patients into three groups with distinct outcomes [17]. In CMML patients, ASXL1 mutations frequently coexist with TET2 mutations. Additional TET2 mutations are associated with shorter survival in the presence of ASXL1 mutations [88], while patients harboring TET2 mutations in the absence of ASXL1 mutations are categorized as favorable risk groups [89]. In CMML patients, hypomethylating agents are effective, but patients harboring ASXL1 mutations present a lower overall response rate (ORR) [90].
ASXL1 mutations in clonal hematopoiesis
Along with TET2 and DNMT3A mutations, ASXL1 mutations are frequently detected in clonal hematopoiesis (CH) as well [22]. Especially, CH is characterized by the presence of a somatic mutation common with hematological neoplasia without cytopenia nor dysplasia. CH is an independent risk factor in progression of myeloid malignancies [21, 23]. CH is also prevalent in aplastic anemia, and clones carrying ASXL1 mutations tend to increase in size over time [64].
A recent study revealed that CH carriers with DNMT3A, TET2, ASXL1 and JAK2 mutations are associated with atherosclerosis and coronary heart disease. Consistent with these clinical observations, Tet2-deficient mice showed enhanced progression of atherosclerosis than control mice [91, 92]. A recent study revealed that lack of Dnmt3a also accelerated atherosclerosis in mice [93]. Further studies are required to clarify whether CH with ASXL1 mutations also accelerate the development of atherosclerosis.
CH is frequently detected in solid tumor patients, particularly after chemotherapy [94]. The presence of CH in solid tumors is associated with higher recurrence ratio and adversely affects survival. It seems that chemotherapy promotes CH; PPM1D and TP53 mutations are particularly related to prior chemotherapy in CH with solid tumors [94]. Recently, there is a series of evidence that PPM1D mutations drive CH and confer resistance to chemotherapy [95, 96], but ASXL1 mutations that are unassociated with prior chemotherapy are frequently found in CH with solid tumors. On the other hand, it is also possible that CH enhances the growth of solid tumors. It will be interesting to investigate whether CH with ASXL1 mutations influence the growth of solid tumors.
The role of ASXL1 in normal hematopoiesis
To understand the roles of ASXL1 in normal hematopoiesis, several groups engineered and analyzed Asxl1 knockout mice (Table 1). Fisher et al. engineered and analyzed a constitutive Asxl1 knockout mouse. Constitutive disruption of Asxl1 led to partial perinatal lethality. Constitutive loss of Asxl1 also showed impaired B and T lymphopoiesis and impaired myeloid differentiation [97]. Wang et al. showed that heterozygous genetic Asxl1 knockout mice (Asxl1+/−) developed MDS/MPN [26]. Asxl1 loss led to an increase in apoptotic and mitotic cells in the bone marrow. Asxl1 loss also exhibited reduced hematopoietic stem cell (HSC)/hematopoietic stem progenitor cell (HSPC) populations and impaired hematopoietic repopulation ability. In addition, Zhang et al. demonstrated that deletion of Asxl1 cooperated with Nf1 haplo-insufficiency to activate multiple oncogenic pathways such as MYC, NRAS and BRD4, promoting myeloid transformation [98].
Table 1.
Asxl1 knockout and mutant Asxl1 expressing mice studies
| Mice | Peripheral blood phenotypes | HSC/HSPC phenotypes | Myeloid malignancies | Histone modifications | Gene expressions | References |
|---|---|---|---|---|---|---|
| Asxl1tm1Bc mutant mice | Decreased matured B-cell | Decreased formation of myeloid/erythroid colonies | Asxl1tm1Bc mutant mice did not develop AML up to 58 weeks | Not described | Not described | [97] |
| Mx1-Cre/Vav-Cre conditional Asxl1 knockout mice | Leukocytopenia and anemia in old (> 6 months) mouse | Increase in LT-HSCs and LSK fractions | Development of Progressive MDS-like disease | Reduced global level of H3K27me3 | Increased expression of Hoxa7/9 | [25] |
| Constitutive Asxl1 knockout mice | Leukocytopenia, anemia, thrombocytopenia | Decrease in LSK fractions, increase in GMP fractions | Asxl1+/− mice developed mild MDS-like disease | Reduced global level of H3K27me3/H3K4me3 | Increased expression of Hoxa5/7/9/10 | [26] |
| Retroviral mutant ASXL1 expression in mouse BMT | Leukocytopenia, anemia, thrombocytopenia | Not described | MDS-like disease after a long latency (> 1 year) | Reduced global level of H3K27me3 | Increased expression of miR125a and Hoxa9 | [28] |
| Asxl1 Y588X mutant expressing transgenic mice | Anemia | Increase in ST-HSCs and LSK fractions | A part of mice developed myeloid malignancies | Increased level of H3K122ac at Prdm16 promoter locus | Increased expression of Prdm16 | [33] |
| Vav-Cre Rosa26 mutant Asxl1 knockin mice | Decreased in RBC count | Decrease in LT-HSCs and LSK fractions | Knockin mice alone did not develop MDS/AML but promoted MDS/AML development with RUNX1 mutant | Reduced global level of H2AK119ub/H3K4me3, reduced level of H3K27me3 at Hoxa loci | Decreased expression of Id3, Runx1, Sox6 and Tjp1 | [29] |
| Constitutive locus Asxl1 G643Wfs mutant knockin mice | Leukocytosis and increase in RBC count in aged (18 months) male mice | Competitive serial transplantation assay showed disadvantage | Knockin mice alone did not develop myeloid malignancies within 18 months | Change in distribution of H3K27me3 peak | Not described | [115] |
| Constitutive locus Asxl1 G643Wfs mutant knockin mice | Leukocytopenia/thrombocytosis in aged (12 months) mouse | Decrease in LT-HSCs and LSK fractions | A part of mice developed MDS/MPN disease in long latency (18–24 months) | Reduced level of H2AK119ub at p16Ink4a locus | Increased expression of Hoxa7/9/10 and p16Ink4a | [116] |
Abdel-Wahab et al. reported that hematopoietic cell-specific deletion of Asxl1 induced an MDS-like disease. They generated conditional Asxl1 knockout mice by crossing mice bearing floxed Asxl1 alleles with Vav-Cre or IFN-α-inducible Mx1-Cre transgenic mice [25]. Deletion of Asxl1 in hematopoietic cells resulted in age-dependent leukopenia and anemia with dysplasia. In the bone marrow of Asxl1−/− mice, the number of HSC/HSPC was increased, but the repopulating ability of these cells were impaired. They also showed that Asxl1 and Tet2 double knockout mice developed MDS more rapidly than Asxl1−/− or Tet2−/− mice. Zhang et al. found that systemic deletion of Asxl1 produced more severe hematological phenotypes than conditional deletion of Asxl1, implicating an important role for Asxl1 in the microenvironment to support hematopoiesis. They further showed that bone marrow stromal cells derived from CMML patients had decreased expression of ASXL1, and that loss of Asxl1 in the bone marrow niche led to a decrease in long-term (LT)-HSCs and myeloid lineage skewing in mice [99]. In human CD34-positive cord blood cells, it was shown that ASXL1 knockdown resulted in reduced erythropoiesis and impaired erythrocyte enucleation [100].
Taken together, these studies demonstrated an essential role of wild-type ASXL1 in maintaining normal hematopoiesis. Asxl1 deletion leads to impaired progenitor differentiation and often promotes the development of myeloid malignancies.
ASXL1 interaction partners
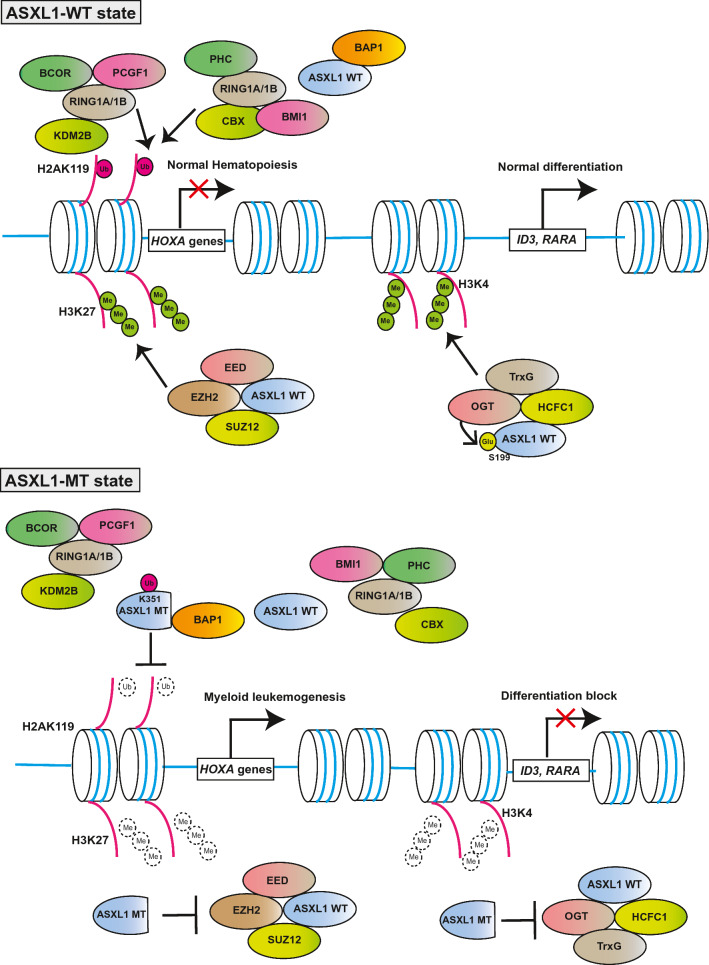
Schermann et al. revealed that, the mammalian ASXL1, like drosophila Asx and a deubiquitinase Calypso, bound the mammalian BAP. They also showed that ASXL1 and BAP1 formed a Polycomb-repressive deubiquitinase (PR-DUB), which removes monoubiquitination of histone H2A at lysine 119, catalyzed by PRC1 complexes [42]. Wild-type ASXL1 interacts with EZH2, EED and SUZ12 as well, main components of the polycomb repressive complex (PRC) 2 to help PRC2 functions [24]. Wild-type ASXL1 protein contributes to repress their target genes such as posterior HOXA genes via collaboration with PRC2 to induce a representative histone repressive mark H3K27me3. Therefore, ASXL1 depletion results in global reduction of the trimethylation of histone H3 at lysine 27 (H3K27me3), a representative repressive mark, leading to derepression of posterior HOXA genes. It was also reported that knockdown of wild-type Asxl1 caused myeloid transformation in concert with a NRAS mutant [24]. In addition, Wang et al. revealed that lineage− c-Kit+ cells of Asxl1-knockout bone marrow cells exhibited global reduction of both H3K27me3 and H3K4me3 [26]. Inoue et al. showed that ASXL1 interacted with OGT and HCFC1 by mass spectrometry, and found that the knockdown of ASXL1, OGT or HCFC1 decreased global levels of H3K4me3 and attenuated myeloid differentiation of HL-60 cells [31]. Previous reports showed that the OGT/HCFC1 complex bound and recruited trithorax homologues, such as MLL1, SET1/COMPASS and MLL5 [101–103]. These results indicate that wild-type ASXL1 could play pivotal roles as a scaffold to control the levels of H2AK119ub, H3K27me3 and H3K4me3, leading to epigenetic control of gene expression.
In addition, wild-type ASXL1 was shown to interact with non-histone proteins; ASXL1 directly bound AKT1 and ASXL1 deficiency led to p27-dependent cell cycle arrest, resulting in cellular senescence [104]. ASXL1 also interacts with the cohesion complex, including SMC1A, SMC3, and RAD21, and ASXL1 depletion leads to impaired telophase cohesion separation [105]. Moreover, ASXL1 interacts with RNA polymerase II (RNAPII) complex to regulate RNAPII transcriptional activity [99].
These findings demonstrated that ASXL1 interacts with a variety of molecules, important for transcription and translation, and that its loss or mutations cause aberrant histone modifications and dysregulated transcription as well as other cellular functions such as cell division and cell signaling, leading to various diseases (Fig. 2).
Fig. 2.
Overview of effects on histone modifications by wild-type ASXL1 (ASXL1-WT) and C-terminally truncated mutant ASXL1 (ASXL1-MT)
Posttranslational modifications of ASXL1
Notably, posttranslational modifications of ASXL1 influence its stability and function. Inoue et al. demonstrated that ASXL1 was ubiquitinated at lysine 351. The deubiquitinase USP7 stabilizes ASXL1 by removing polyubiquitin chain [106]. ASXL1 lysine 351 is subject to not only polyubiquitination but also monoubiquitination, in the presence of BAP1 [30]. Interestingly, monoubiquitination of mutant ASXL1 at lysine 351, in turn, activates the catalytic function of associating BAP1. Recent mechanistic analysis of mutant ASXL1 protein revealed the ‘gain of function’ features of ASXL1 mutations. BAP1, a strong interacting partner of ASXL1, is frequently mutated in renal cell carcinoma, mesothelioma and uveal melanoma, implicating BAP1 as a tumor suppressor [107–109]. However, BAP1 is rarely mutated in acute myeloid leukemia [110]. There are a series of experimental evidence that BAP1 plays tumor-promoting roles in myeloid neoplasms. Balasubramani et al. showed that the cancer-associated ASXL1 mutant protein aberrantly enhanced the catalytic function of BAP1, leading to a profound decrease in H2AK119ub [111]. Sahtoe et al. also biochemically demonstrated that the ASXH domain of ASXL1 was essential in increasing BAP1′s affinity to ubiquitin on H2A [112]. We showed the mutually reinforcing effects between the monoubiquitinated form of mutant ASXL1 and BAP1 in myeloid leukemogenesis by dysregulating HOXA and IRF8 genes [30], which are responsible for leukemogenesis and monopoiesis, respectively. We also demonstrated that depletion of endogenous BAP1 abrogated the leukemogenesis induced by mutant ASXL1, demonstrating pivotal roles of BAP1 in mutant ASXL1-induced cell transformation. Recently, Daou et al. showed that monoubiquitination of wild-type ASXL2 at lysine 370, which corresponds to lysine 351 of ASXL1, was indispensable for activation of the catalytic function of BAP1, and was catalyzed by UBE2E family proteins [113]. Whether monoubiquitination of mutant ASXL1 at lysine 351 is also catalyzed by UBE2E family proteins remains to be elucidated. In addition to ubiquitination, Inoue et al. demonstrated that glycosylation of ASXL1 at serine 199 by OGT (O-linked N-acetylglucosamine transferase) was important for its stability [31]. Functional significance of other modifications of ASXL1 such as phosphorylation, sumoylation, and methylation remains to be elucidated.
Mutant ASXL1 protein gains functions leading to myeloid transformation
As described above, Asxl1 deficiency leads to the development of myeloid diseases in mouse models, suggesting that ASXL1 mutations are loss-of-function mutations. However, accumulating evidence suggests that mutant ASXL1 proteins gain functions that promote myeloid leukemogenesis. Most ASXL1 mutations in myeloid malignancies are heterozygous frameshift or nonsense mutations localized near the 5′ end of the last exon [20]. Mutant ASXL1 transcripts are, therefore, predicted to escape from nonsense-mediated decay, resulting in production of the C-terminally truncated ASXL1 protein [114]. In cell lines derived from patients with hematological malignancies, mutant ASXL1 proteins were indeed detected by western blot and mTRAQ-based mass spectrometric analyses [27].
Hence, several groups have investigated whether the presence of the C-terminally truncated forms of ASXL1 protein induce myeloid transformation. Inoue et al. showed that mutant ASXL1 proteins (ASXL1-MT) interacted with PRC2 components and interfere with its catalytic activity. Forced expression of ASXL1-MT inhibited wild-type ASXL1 functions and caused MDS/AML development in mouse bone marrow transplantation models via derepression of miR125a and Hoxa genes caused by decreased H3K27me3 [28]. Yang et al. established C-terminally truncated mutant of Asxl1(Asxl1Y588X)-expressing transgenic mice mimicking human ASXL1 Y591X mutation and demonstrated that transgenic Asxl1Y588X expression led to myeloid malignancies [33]. Nagase et al. engineered a conditional Rosa26 locus ASXL1-MT knock-in mice (Asxl1-MT KI mice) mimicking human ASXL1 E635RfsX15 mutation, derived from patients with MDS/AML, and characterized the phenotype [29]. Asxl1-MT KI mice showed mild anemia and a modest block in erythroid differentiation associated with increased number of platelets, and repopulation ability of HSCs was attenuated. However, Asxl1-MT KI mice did not develop any hematological malignancies. Co-expression of a RUNX1 frameshift mutation cooperatively induced MDS/AML in Asxl1-MT KI mice. In addition, a retrovirus-mediated insertional mutagenesis study exhibited the susceptibility of Asxl1-MT KI bone marrow cells to myeloid leukemia. Thus, mutant Asxl1 promotes leukemia susceptibility.
Several groups generated and analyzed Asxl1 mutant knock-in mice at the endogenous Asxl1 locus. Hsu et al. established endogenous locus Asxl1G643fs mutant knock-in mice mimicking human ASXL1 G646WfsX12 mutation (Asxl1tm/+) [115]. Asxl1tm/+ mice showed enhanced colony-forming activity of HSPCs and modestly impaired repopulation ability of HSCs. They showed that MN1 overexpression was observed in patients harboring ASXL1 mutations, and that MN1 overexpression increased the frequency of long-term culture initiation cells. However, Asxl1G643fs mutant knock-in mice alone did not develop hematological malignancies within 18 months of follow-up. On the other hand, Uni et al. generated endogenous locus knock-in mice of Asxl1G643fs mutant and identified different phenotypes [116], although it is not clear why theoretically the exact same KI mice gave different phenotypes. The locus KI mice developed by Uni et al. presented decreased number of HSC and increased apoptotic cells, and leukopenia and thrombocytosis were observed at 12 months old, with some mice developing MDS/MPN-like disease after a long latency period (about 18–24 months). Consistent with the previous mouse studies of mutant ASXL1, expression of Hoxa genes in Asxl1G643fs/+ mice was dysregulated. In addition, they focused on upregulation of senescence-related genes including p16Ink4a in Asxl1G643fs/+ mice because young Asxl1G643fs/+ mice (3 months old) showed myeloid-skewing differentiation like aged mice. In relation to this observation, it was previously reported that the ASXL1/BAP1 axis was implicated in upregulation of p15Ink4b, supported by the fact that the promoter activity of INK4B-ARF-INK4A locus was suppressed by H2AK119ub modification [117]. Uni et al. demonstrated that wild-type, but not mutant ASXL1 proteins, interacted with BMI1, a key component of PRC1. The level of H2AK119ub was decreased at the p16Ink4a promoter locus, and Ink4a expression was derepressed in Asxl1G643fs mutant knock-in mice. They also found that p16Ink4a knockout rescued decreased HSC numbers and aberrant apoptosis in Asxl1G643fs mutant knock-in mice.
Collectively, these findings indicate that mutant ASXL1 at physiological expression levels alone is insufficient to induce myeloid transformation but impairs hematopoiesis and promotes susceptibility to myeloid malignancies by altering histone modifications. The distinct phenotypes of Asxl1 mutant knock-in mice among several groups could be caused by the differences in the cites of Asxl1 mutations or the levels and the hematopoietic lineages of Asxl1 expression.
Potential therapies for myeloid malignancies harboring ASXL1 mutations
Recent studies pave the way to novel therapeutic strategies for ASXL1-mutated myeloid malignancies. First, ASXL proteins/BAP1 complex promotes gene activation via opposing PRC1-mediated monoubiquitination of H2AK119 [118]. As described above, ASXL1-MT, but not wild-type ASXL1, strongly enhanced the catalytic activity of BAP1, resulting in profound reduction of H2AK119ub [30, 111]. In hematopoietic cells, hyperactive ASXL1-MT/BAP1 complex upregulates HOXA genes resulting in myeloid transformation [30]. Therefore, enzymatic activity of BAP1 or BAP1–ASXL1 binding is a potential therapeutic target for ASXL1-mutated myeloid malignancies. Guo et al. also revealed that the endogenous Bap1 activity is essential for pathogenesis of myeloid malignancies of Asxl1Y588X transgenic mice [119].
In addition, it has been shown that ASXL1-MT, but not wildtype ASXL1, bound Bromodomain-containing 4 (BRD4) [33], a well-known oncoprotein in myeloid malignancies [120]. BRD4 activates pTEFb complex and induces acetylation of H3 at lysine 122 (H3K122Ac), resulting in phosphorylation of RNA polymerase II and gene activation. In the Asxl1Y588X transgenic mice, the level of H3K122Ac at the promoter locus of Prdm16 was increased, resulting in dysregulated expression of Prdm16 [33]. Bone marrow cells from Asxl1Y588X transgenic mice showed higher sensitivity to the BRD4 inhibitor than those from normal mice.
A previous study showed that combined expression of ASXL1-MT and SETBP1-MT rapidly developed MDS/AML in mice and the leukemia cells showed repression of TGFβ pathway genes [73]. Nano-liquid chromatography–mass spectrometry analysis revealed physical interaction between mutant ASXL1 and HDAC1 [30]. Saika et al. demonstrated that decrease in acetylation levels of histone H3K14 and H4K5 at TGFβ pathway genes in leukemia cells transformed by ASXL1-MT and SETBP1-MT [32]. They also showed that mutant ASXL1-induced leukemia conferred high sensitivity to an HDAC inhibitor, vorinostat. Vorinostat restored acetylation of histone H3K14 and H4K5 and the expression of TGFβ pathway genes.
On the other hand, it is effective to reactivate the functions of wild-type ASXL1 which are weakened by hemizygous ASXL1 mutations. Wild-type ASXL1/OGT complex is required for maintaining the level of H3K4me3 [31]. Depletion of ASXL1 or OGT led to impaired myeloid differentiation and global loss of the level of H3K4me3. In addition, OGT directly bound and stabilized wild-type ASXL1. Therefore, enhancing OGT activity is a reasonable strategy for restoring tumor suppressive functions of wild-type ASXL1. Intriguingly, an OGA inhibitor, which elicits the OGT activity, was effective in suppressing growth of leukemia cells expressing the mutant ASXL1 by restoring the tumor suppressor roles of wild-type ASXL1–OGT axis [31].
Taken together, inhibition of either BAP1, BRD4, HDACs or OGA has been shown to suppress leukemia with ASXL1 mutations in mouse models. These findings need to be validated using patient derived xenograft (PDX) models in future studies.
Conclusions and future perspectives
ASXL1 mutations are often associated with poor prognosis. Therefore, it is important to understand the precise mechanisms by which ASXL1 mutations contribute to myeloid transformation. Recent biological analyses demonstrated that mutant ASXL1 plays pivotal roles in leukemogenesis and leads to increased susceptibility to myeloid transformation by altering histone modifications. Meanwhile, unlike other epigenetic factors such as EZH2 and TET2, ASXL1 itself has no catalytic function. Hence, ASXL1 binding partners have been intensively investigated and biochemical analyses of these binding partners have shed light on the potential therapeutic strategies for myeloid malignancies harboring ASXL1 mutations.
While mutant ASXL1 causes dysregulations of histone modifications, resulting in myeloid malignancies, wild-type ASXL1 should also play crucial roles in epigenetic regulations under the physiological conditions via interacting a variety of epigenetic factors. In addition, ASXL1 have various post-transcriptional modifications probably induced by outside stimuli. Therefore, investigation of epigenetic control by wild-type ASXL1 may clarify how the outside stimuli are converted to the transcriptional profiles via altering epigenetics.
Acknowledgements
This work was supported by a Grant-in-Aid Scientific Research B from the Ministry of Education, Culture, Sports, Science and Technology of Japan (15H04855, TK), a Grant from the Tokyo Biochemical Research Foundation (TK), and a Grant from the Uehara Memorial Foundation (TK).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364(26):2496. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research N. Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makishima H, Yoshizato T, Yoshida K, Sekeres MA, Radivoyevitch T, Suzuki H, et al. Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet. 2017;49(2):204. doi: 10.1038/ng.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thol F, Friesen I, Damm F, Yun H, Weissinger EM, Krauter J, et al. Prognostic significance of ASXL1 mutations in patients with myelodysplastic syndromes. J Clin Oncol. 2011;29(18):2499. doi: 10.1200/JCO.2010.33.4938. [DOI] [PubMed] [Google Scholar]
- 6.Boultwood J, Perry J, Pellagatti A, Fernandez-Mercado M, Fernandez-Santamaria C, Calasanz MJ, et al. Frequent mutation of the polycomb-associated gene ASXL1 in the myelodysplastic syndromes and in acute myeloid leukemia. Leukemia. 2010;24(5):1062. doi: 10.1038/leu.2010.20. [DOI] [PubMed] [Google Scholar]
- 7.Rocquain J, Carbuccia N, Trouplin V, Raynaud S, Murati A, Nezri M, et al. Combined mutations of ASXL1, CBL, FLT3, IDH1, IDH2, JAK2, KRAS, NPM1, NRAS, RUNX1, TET2 and WT1 genes in myelodysplastic syndromes and acute myeloid leukemias. BMC Cancer. 2010;10:401. doi: 10.1186/1471-2407-10-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnittger S, Eder C, Jeromin S, Alpermann T, Fasan A, Grossmann V, et al. ASXL1 exon 12 mutations are frequent in AML with intermediate risk karyotype and are independently associated with an adverse outcome. Leukemia. 2013;27(1):82. doi: 10.1038/leu.2012.262. [DOI] [PubMed] [Google Scholar]
- 9.Metzeler KH, Becker H, Maharry K, Radmacher MD, Kohlschmidt J, Mrozek K, et al. ASXL1 mutations identify a high-risk subgroup of older patients with primary cytogenetically normal AML within the ELN favorable genetic category. Blood. 2011;118(26):6920. doi: 10.1182/blood-2011-08-368225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbuccia N, Murati A, Trouplin V, Brecqueville M, Adelaide J, Rey J, et al. Mutations of ASXL1 gene in myeloproliferative neoplasms. Leukemia. 2009;23(11):2183. doi: 10.1038/leu.2009.141. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Wahab O, Manshouri T, Patel J, Harris K, Yao J, Hedvat C, et al. Genetic analysis of transforming events that convert chronic myeloproliferative neoplasms to leukemias. Cancer Res. 2010;70(2):447. doi: 10.1158/0008-5472.CAN-09-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24(6):1128. doi: 10.1038/leu.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein BL, Williams DM, O’Keefe C, Rogers O, Ingersoll RG, Spivak JL, et al. Disruption of the ASXL1 gene is frequent in primary, post-essential thrombocytosis and post-polycythemia vera myelofibrosis, but not essential thrombocytosis or polycythemia vera: analysis of molecular genetics and clinical phenotypes. Haematologica. 2011;96(10):1462. doi: 10.3324/haematol.2011.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel-Wahab O, Pardanani A, Patel J, Wadleigh M, Lasho T, Heguy A, et al. Concomitant analysis of EZH2 and ASXL1 mutations in myelofibrosis, chronic myelomonocytic leukemia and blast-phase myeloproliferative neoplasms. Leukemia. 2011;25(7):1200. doi: 10.1038/leu.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott MA, Pardanani A, Hanson CA, Lasho TL, Finke CM, Belachew AA, et al. ASXL1 mutations are frequent and prognostically detrimental in CSF3R-mutated chronic neutrophilic leukemia. Am J Hematol. 2015;90(7):653. doi: 10.1002/ajh.24031. [DOI] [PubMed] [Google Scholar]
- 16.Makishima H, Jankowska AM, McDevitt MA, O’Keefe C, Dujardin S, Cazzolli H, et al. CBL, CBLB, TET2, ASXL1, and IDH1/2 mutations and additional chromosomal aberrations constitute molecular events in chronic myelogenous leukemia. Blood. 2011;117(21):e198. doi: 10.1182/blood-2010-06-292433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itzykson R, Kosmider O, Renneville A, Gelsi-Boyer V, Meggendorfer M, Morabito M, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol. 2013;31(19):2428. doi: 10.1200/JCO.2012.47.3314. [DOI] [PubMed] [Google Scholar]
- 18.Gelsi-Boyer V, Trouplin V, Roquain J, Adelaide J, Carbuccia N, Esterni B, et al. ASXL1 mutation is associated with poor prognosis and acute transformation in chronic myelomonocytic leukaemia. Br J Haematol. 2010;151(4):365. doi: 10.1111/j.1365-2141.2010.08381.x. [DOI] [PubMed] [Google Scholar]
- 19.Patnaik MM, Padron E, LaBorde RR, Lasho TL, Finke CM, Hanson CA, et al. Mayo prognostic model for WHO-defined chronic myelomonocytic leukemia: ASXL1 and spliceosome component mutations and outcomes. Leukemia. 2013;27(7):1504. doi: 10.1038/leu.2013.88. [DOI] [PubMed] [Google Scholar]
- 20.Gelsi-Boyer V, Brecqueville M, Devillier R, Murati A, Mozziconacci MJ, Birnbaum D. Mutations in ASXL1 are associated with poor prognosis across the spectrum of malignant myeloid diseases. J Hematol Oncol. 2012;5:12. doi: 10.1186/1756-8722-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel-Wahab O, Adli M, LaFave LM, Gao J, Hricik T, Shih AH, et al. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell. 2012;22(2):180. doi: 10.1016/j.ccr.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdel-Wahab O, Gao J, Adli M, Dey A, Trimarchi T, Chung YR, et al. Deletion of Asxl1 results in myelodysplasia and severe developmental defects in vivo. J Exp Med. 2013;210(12):2641. doi: 10.1084/jem.20131141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Li Z, He Y, Pan F, Chen S, Rhodes S, et al. Loss of Asxl1 leads to myelodysplastic syndrome-like disease in mice. Blood. 2014;123(4):541. doi: 10.1182/blood-2013-05-500272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue D, Matsumoto M, Nagase R, Saika M, Fujino T, Nakayama KI, et al. Truncation mutants of ASXL1 observed in myeloid malignancies are expressed at detectable protein levels. Exp Hematol. 2016;44(3):172. doi: 10.1016/j.exphem.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Inoue D, Kitaura J, Togami K, Nishimura K, Enomoto Y, Uchida T, et al. Myelodysplastic syndromes are induced by histone methylation-altering ASXL1 mutations. J Clin Investig. 2013;123(11):4627. doi: 10.1172/JCI70739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagase R, Inoue D, Pastore A, Fujino T, Hou HA, Yamasaki N, et al. Expression of mutant Asxl1 perturbs hematopoiesis and promotes susceptibility to leukemic transformation. J Exp Med. 2018;215(6):1729. doi: 10.1084/jem.20171151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asada S, Goyama S, Inoue D, Shikata S, Takeda R, Fukushima T, et al. Mutant ASXL1 cooperates with BAP1 to promote myeloid leukaemogenesis. Nat Commun. 2018;9(1):2733. doi: 10.1038/s41467-018-05085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue D, Fujino T, Sheridan P, Zhang YZ, Nagase R, Horikawa S, et al. A novel ASXL1–OGT axis plays roles in H3K4 methylation and tumor suppression in myeloid malignancies. Leukemia. 2018;32(6):1327. doi: 10.1038/s41375-018-0083-3. [DOI] [PubMed] [Google Scholar]
- 32.Saika M, Inoue D, Nagase R, Sato N, Tsuchiya A, Yabushita T, et al. ASXL1 and SETBP1 mutations promote leukaemogenesis by repressing TGFbeta pathway genes through histone deacetylation. Sci Rep. 2018;8(1):15873. doi: 10.1038/s41598-018-33881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, Kurtenbach S, Guo Y, Lohse I, Durante MA, Li J, et al. Gain of function of ASXL1 truncating protein in the pathogenesis of myeloid malignancies. Blood. 2018;131(3):328. doi: 10.1182/blood-2017-06-789669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katoh M, Katoh M. Identification and characterization of ASXL2 gene in silico. Int J Oncol. 2003;23(3):845. [PubMed] [Google Scholar]
- 35.Katoh M, Katoh M. Identification and characterization of ASXL3 gene in silico. Int J Oncol. 2004;24(6):1617. [PubMed] [Google Scholar]
- 36.Sinclair DA, Milne TA, Hodgson JW, Shellard J, Salinas CA, Kyba M, et al. The Additional sex combs gene of Drosophila encodes a chromatin protein that binds to shared and unique polycomb group sites on polytene chromosomes. Development. 1998;125(7):1207. doi: 10.1242/dev.125.7.1207. [DOI] [PubMed] [Google Scholar]
- 37.Milne TA, Sinclair DA, Brock HW. The Additional sex combs gene of Drosophila is required for activation and repression of homeotic loci, and interacts specifically with polycomb and super sex combs. Mol Gen Genet. 1999;261(4–5):753. doi: 10.1007/s004380050018. [DOI] [PubMed] [Google Scholar]
- 38.Beuchle D, Struhl G, Muller J. Polycomb group proteins and heritable silencing of Drosophila Hox genes. Development. 2001;128(6):993. doi: 10.1242/dev.128.6.993. [DOI] [PubMed] [Google Scholar]
- 39.Cao R, Tsukada Y, Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell. 2005;20(6):845. doi: 10.1016/j.molcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Klymenko T, Muller J. The histone methyltransferases trithorax and Ash1 prevent transcriptional silencing by polycomb group proteins. EMBO Rep. 2004;5(4):373. doi: 10.1038/sj.embor.7400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuettengruber B, Bourbon HM, Di Croce L, Cavalli G. Genome regulation by polycomb and trithorax: 70 years and counting. Cell. 2017;171(1):34. doi: 10.1016/j.cell.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, et al. Histone H2A deubiquitinase activity of the polycomb repressive complex PR–DUB. Nature. 2010;465(7295):243. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher CL, Randazzo F, Humphries RK, Brock HW. Characterization of Asxl1, a murine homolog of additional sex combs, and analysis of the Asx-like gene family. Gene. 2006;369:109. doi: 10.1016/j.gene.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 44.Fisher CL, Berger J, Randazzo F, Brock HW. A human homolog of additional sex combs, ADDITIONAL SEX COMBS-LIKE 1, maps to chromosome 20q11. Gene. 2003;306:115. doi: 10.1016/S0378-1119(03)00430-X. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez-Pulido L, Kong L, Ponting CP. A common ancestry for BAP1 and Uch37 regulators. Bioinformatics. 2012;28(15):1953. doi: 10.1093/bioinformatics/bts319. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez R, Zhou MM. The PHD finger: a versatile epigenome reader. Trends Biochem Sci. 2011;36(7):364. doi: 10.1016/j.tibs.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell. 2007;25(1):15. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 48.Hoischen A, van Bon BW, Rodriguez-Santiago B, Gilissen C, Vissers LE, de Vries P, et al. De novo nonsense mutations in ASXL1 cause Bohring-Opitz syndrome. Nat Genet. 2011;43(8):729. doi: 10.1038/ng.868. [DOI] [PubMed] [Google Scholar]
- 49.Bainbridge MN, Hu H, Muzny DM, Musante L, Lupski JR, Graham BH, et al. De novo truncating mutations in ASXL3 are associated with a novel clinical phenotype with similarities to Bohring-Opitz syndrome. Genome Med. 2013;5(2):11. doi: 10.1186/gm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shashi V, Pena LD, Kim K, Burton B, Hempel M, Schoch K, et al. De novo truncating variants in ASXL2 are associated with a unique and recognizable clinical phenotype. Am J Hum Genet. 2016;99(4):991. doi: 10.1016/j.ajhg.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Izawa T, Rohatgi N, Fukunaga T, Wang QT, Silva MJ, Gardner MJ, et al. ASXL2 regulates glucose, lipid, and skeletal homeostasis. Cell Rep. 2015;11(10):1625. doi: 10.1016/j.celrep.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Micol JB, Duployez N, Boissel N, Petit A, Geffroy S, Nibourel O, et al. Frequent ASXL2 mutations in acute myeloid leukemia patients with t(8; 21)/RUNX1-RUNX1T1 chromosomal translocations. Blood. 2014;124(9):1445. doi: 10.1182/blood-2014-04-571018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, He F, Zhang P, Chen S, Shi H, Sun Y, et al. Loss of Asxl2 leads to myeloid malignancies in mice. Nat Commun. 2017;8:15456. doi: 10.1038/ncomms15456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Micol JB, Pastore A, Inoue D, Duployez N, Kim E, Lee SC, et al. ASXL2 is essential for haematopoiesis and acts as a haploinsufficient tumour suppressor in leukemia. Nat Commun. 2017;8:15429. doi: 10.1038/ncomms15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madan V, Han L, Hattori N, Teoh WW, Mayakonda A, Sun QY, et al. ASXL2 regulates hematopoiesis in mice and its deficiency promotes myeloid expansion. Haematologica. 2018;103(12):1980. doi: 10.3324/haematol.2018.189928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duployez N, Micol JB, Boissel N, Petit A, Geffroy S, Bucci M, et al. Unlike ASXL1 and ASXL2 mutations, ASXL3 mutations are rare events in acute myeloid leukemia with t(8; 21) Leuk Lymphoma. 2016;57(1):199. doi: 10.3109/10428194.2015.1037754. [DOI] [PubMed] [Google Scholar]
- 57.Sugimoto Y, Muramatsu H, Makishima H, Prince C, Jankowska AM, Yoshida N, et al. Spectrum of molecular defects in juvenile myelomonocytic leukaemia includes ASXL1 mutations. Br J Haematol. 2010;150(1):83. doi: 10.1111/j.1365-2141.2010.08196.x. [DOI] [PubMed] [Google Scholar]
- 58.Perez B, Kosmider O, Cassinat B, Renneville A, Lachenaud J, Kaltenbach S, et al. Genetic typing of CBL, ASXL1, RUNX1, TET2 and JAK2 in juvenile myelomonocytic leukaemia reveals a genetic profile distinct from chronic myelomonocytic leukaemia. Br J Haematol. 2010;151(5):460. doi: 10.1111/j.1365-2141.2010.08393.x. [DOI] [PubMed] [Google Scholar]
- 59.Menezes J, Acquadro F, Wiseman M, Gomez-Lopez G, Salgado RN, Talavera-Casanas JG, et al. Exome sequencing reveals novel and recurrent mutations with clinical impact in blastic plasmacytoid dendritic cell neoplasm. Leukemia. 2014;28(4):823. doi: 10.1038/leu.2013.283. [DOI] [PubMed] [Google Scholar]
- 60.Jawhar M, Schwaab J, Schnittger S, Meggendorfer M, Pfirrmann M, Sotlar K, et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia. 2016;30(1):136. doi: 10.1038/leu.2015.284. [DOI] [PubMed] [Google Scholar]
- 61.Pardanani AD, Lasho TL, Finke C, Zblewski DL, Abdelrahman RA, Wassie EA, et al. ASXL1 and CBL mutations are independently predictive of inferior survival in advanced systemic mastocytosis. Br J Haematol. 2016;175(3):534. doi: 10.1111/bjh.13865. [DOI] [PubMed] [Google Scholar]
- 62.Damaj G, Joris M, Chandesris O, Hanssens K, Soucie E, Canioni D, et al. ASXL1 but not TET2 mutations adversely impact overall survival of patients suffering systemic mastocytosis with associated clonal hematologic non-mast-cell diseases. PLoS One. 2014;9(1):e85362. doi: 10.1371/journal.pone.0085362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang J, Ge M, Lu S, Shi J, Li X, Zhang J, et al. Mutations of ASXL1 and TET2 in aplastic anemia. Haematologica. 2015;100(5):e172. doi: 10.3324/haematol.2014.120931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshizato T, Dumitriu B, Hosokawa K, Makishima H, Yoshida K, Townsley D, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373(1):35. doi: 10.1056/NEJMoa1414799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin CC, Hou HA, Chou WC, Kuo YY, Liu CY, Chen CY, et al. IDH mutations are closely associated with mutations of DNMT3A, ASXL1 and SRSF2 in patients with myelodysplastic syndromes and are stable during disease evolution. Am J Hematol. 2014;89(2):137. doi: 10.1002/ajh.23596. [DOI] [PubMed] [Google Scholar]
- 67.Paschka P, Schlenk RF, Gaidzik VI, Herzig JK, Aulitzky T, Bullinger L, et al. ASXL1 mutations in younger adult patients with acute myeloid leukemia: a study by the German-Austrian Acute Myeloid Leukemia Study Group. Haematologica. 2015;100(3):324. doi: 10.3324/haematol.2014.114157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thol F, Kade S, Schlarmann C, Loffeld P, Morgan M, Krauter J, et al. Frequency and prognostic impact of mutations in SRSF2, U2AF1, and ZRSR2 in patients with myelodysplastic syndromes. Blood. 2012;119(15):3578. doi: 10.1182/blood-2011-12-399337. [DOI] [PubMed] [Google Scholar]
- 69.Wu SJ, Kuo YY, Hou HA, Li LY, Tseng MH, Huang CF, et al. The clinical implication of SRSF2 mutation in patients with myelodysplastic syndrome and its stability during disease evolution. Blood. 2012;120(15):3106. doi: 10.1182/blood-2012-02-412296. [DOI] [PubMed] [Google Scholar]
- 70.Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616. doi: 10.1182/blood-2013-08-518886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chou WC, Huang HH, Hou HA, Chen CY, Tang JL, Yao M, et al. Distinct clinical and biological features of de novo acute myeloid leukemia with additional sex comb-like 1 (ASXL1) mutations. Blood. 2010;116(20):4086. doi: 10.1182/blood-2010-05-283291. [DOI] [PubMed] [Google Scholar]
- 72.Micol JB, Abdel-Wahab O. Collaborating constitutive and somatic genetic events in myeloid malignancies: ASXL1 mutations in patients with germline GATA2 mutations. Haematologica. 2014;99(2):201. doi: 10.3324/haematol.2013.101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Inoue D, Kitaura J, Matsui H, Hou HA, Chou WC, Nagamachi A, et al. SETBP1 mutations drive leukemic transformation in ASXL1-mutated MDS. Leukemia. 2015;29(4):847. doi: 10.1038/leu.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meggendorfer M, Bacher U, Alpermann T, Haferlach C, Kern W, Gambacorti-Passerini C, et al. SETBP1 mutations occur in 9% of MDS/MPN and in 4% of MPN cases and are strongly associated with atypical CML, monosomy 7, isochromosome i(17)(q10), ASXL1 and CBL mutations. Leukemia. 2013;27(9):1852. doi: 10.1038/leu.2013.133. [DOI] [PubMed] [Google Scholar]
- 75.Makishima H. Somatic SETBP1 mutations in myeloid neoplasms. Int J Hematol. 2017;105(6):732. doi: 10.1007/s12185-017-2241-1. [DOI] [PubMed] [Google Scholar]
- 76.Makishima H, Yoshida K, Nguyen N, Przychodzen B, Sanada M, Okuno Y, et al. Somatic SETBP1 mutations in myeloid malignancies. Nat Genet. 2013;45(8):942. doi: 10.1038/ng.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pratcorona M, Abbas S, Sanders MA, Koenders JE, Kavelaars FG, Erpelinck-Verschueren CA, et al. Acquired mutations in ASXL1 in acute myeloid leukemia: prevalence and prognostic value. Haematologica. 2012;97(3):388. doi: 10.3324/haematol.2011.051532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carbuccia N, Trouplin V, Gelsi-Boyer V, Murati A, Rocquain J, Adelaide J, et al. Mutual exclusion of ASXL1 and NPM1 mutations in a series of acute myeloid leukemias. Leukemia. 2010;24(2):469. doi: 10.1038/leu.2009.218. [DOI] [PubMed] [Google Scholar]
- 79.Lin CC, Hou HA, Chou WC, Kuo YY, Wu SJ, Liu CY, et al. SF3B1 mutations in patients with myelodysplastic syndromes: the mutation is stable during disease evolution. Am J Hematol. 2014;89(8):E109. doi: 10.1002/ajh.23734. [DOI] [PubMed] [Google Scholar]
- 80.Metzeler KH, Herold T, Rothenberg-Thurley M, Amler S, Sauerland MC, Gorlich D, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. 2016;128(5):686. doi: 10.1182/blood-2016-01-693879. [DOI] [PubMed] [Google Scholar]
- 81.Molenaar RJ, Thota S, Nagata Y, Patel B, Clemente M, Przychodzen B, et al. Clinical and biological implications of ancestral and non-ancestral IDH1 and IDH2 mutations in myeloid neoplasms. Leukemia. 2015;29(11):2134. doi: 10.1038/leu.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krauth MT, Eder C, Alpermann T, Bacher U, Nadarajah N, Kern W, et al. High number of additional genetic lesions in acute myeloid leukemia with t(8; 21)/RUNX1-RUNX1T1: frequency and impact on clinical outcome. Leukemia. 2014;28(7):1449. doi: 10.1038/leu.2014.4. [DOI] [PubMed] [Google Scholar]
- 83.Lin Y, Zheng Y, Wang ZC, Wang SY. Prognostic significance of ASXL1 mutations in myelodysplastic syndromes and chronic myelomonocytic leukemia: a meta-analysis. Hematology. 2016;21(8):454. doi: 10.1080/10245332.2015.1106815. [DOI] [PubMed] [Google Scholar]
- 84.Bejar R, Lord A, Stevenson K, Bar-Natan M, Perez-Ladaga A, Zaneveld J, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014;124(17):2705. doi: 10.1182/blood-2014-06-582809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Traina F, Visconte V, Elson P, Tabarroki A, Jankowska AM, Hasrouni E, et al. Impact of molecular mutations on treatment response to DNMT inhibitors in myelodysplasia and related neoplasms. Leukemia. 2014;28(1):78. doi: 10.1038/leu.2013.269. [DOI] [PubMed] [Google Scholar]
- 86.Sallman DA, Komrokji R, Cluzeau T, Vaupel C, Al Ali NH, Lancet J, et al. ASXL1 frameshift mutations drive inferior outcomes in CMML without negative impact in MDS. Blood Cancer J. 2017;7(12):633. doi: 10.1038/s41408-017-0004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cui Y, Tong H, Du X, Li B, Gale RP, Qin T, et al. Impact of TET2, SRSF2, ASXL1 and SETBP1 mutations on survival of patients with chronic myelomonocytic leukemia. Exp Hematol Oncol. 2015;4:14. doi: 10.1186/s40164-015-0009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cui Y, Tong H, Du X, Li B, Gale RP, Qin T, et al. TET2 mutations were predictive of inferior prognosis in the presence of ASXL1 mutations in patients with chronic myelomonocytic leukemia. Stem Cell Investig. 2016;3:50. doi: 10.21037/sci.2016.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patnaik MM, Lasho TL, Vijayvargiya P, Finke CM, Hanson CA, Ketterling RP, et al. Prognostic interaction between ASXL1 and TET2 mutations in chronic myelomonocytic leukemia. Blood Cancer J. 2016;6:e385. doi: 10.1038/bcj.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duchmann M, Yalniz FF, Sanna A, Sallman D, Coombs CC, Renneville A, et al. Prognostic role of gene mutations in chronic myelomonocytic leukemia patients treated with hypomethylating agents. EBioMedicine. 2018;31:174. doi: 10.1016/j.ebiom.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355(6327):842. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rauch PJ, Silver AJ, Gopakumar J, McConkey M, Sinha E, Fefer M, et al. Loss-of-function mutations in Dnmt3a and Tet2 lead to accelerated atherosclerosis and convergent macrophage phenotypes in mice. Blood. 2018;132(Suppl 1):745. [Google Scholar]
- 94.Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21(3):374. doi: 10.1016/j.stem.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsu JI, Dayaram T, Tovy A, De Braekeleer E, Jeong M, Wang F, et al. PPM1D mutations drive clonal hematopoiesis in response to cytotoxic chemotherapy. Cell Stem Cell. 2018;23(5):700. doi: 10.1016/j.stem.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kahn JD, Miller PG, Silver AJ, Sellar RS, Bhatt S, Gibson C, et al. PPM1D-truncating mutations confer resistance to chemotherapy and sensitivity to PPM1D inhibition in hematopoietic cells. Blood. 2018;132(11):1095. doi: 10.1182/blood-2018-05-850339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fisher CL, Pineault N, Brookes C, Helgason CD, Ohta H, Bodner C, et al. Loss-of-function additional sex combs like 1 mutations disrupt hematopoiesis but do not cause severe myelodysplasia or leukemia. Blood. 2010;115(1):38. doi: 10.1182/blood-2009-07-230698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang P, He F, Bai J, Yamamoto S, Chen S, Zhang L, et al. Chromatin regulator Asxl1 loss and Nf1 halpoinsufficiency cooperate to accelerate myeloid malignancy. J Clin Investig. 2018;128(12):5383. doi: 10.1172/JCI121366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang P, Chen Z, Li R, Guo Y, Shi H, Bai J, et al. Loss of ASXL1 in the bone marrow niche dysregulates hematopoietic stem and progenitor cell fates. Cell Discov. 2018;4:4. doi: 10.1038/s41421-017-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shi H, Yamamoto S, Sheng M, Bai J, Zhang P, Chen R, et al. ASXL1 plays an important role in erythropoiesis. Sci Rep. 2016;6:28789. doi: 10.1038/srep28789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tyagi S, Chabes AL, Wysocka J, Herr W. E2F activation of S phase promoters via association with HCF-1 and the MLL family of histone H3K4 methyltransferases. Mol Cell. 2007;27(1):107. doi: 10.1016/j.molcel.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 102.Zhou P, Wang Z, Yuan X, Zhou C, Liu L, Wan X, et al. Mixed lineage leukemia 5 (MLL5) protein regulates cell cycle progression and E2F1-responsive gene expression via association with host cell factor-1 (HCF-1) J Biol Chem. 2013;288(24):17532. doi: 10.1074/jbc.M112.439729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deplus R, Delatte B, Schwinn MK, Defrance M, Mendez J, Murphy N, et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J. 2013;32(5):645. doi: 10.1038/emboj.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Youn HS, Kim TY, Park UH, Moon ST, An SJ, Lee YK, et al. Asxl1 deficiency in embryonic fibroblasts leads to cellular senescence via impairment of the AKT-E2F pathway and Ezh2 inactivation. Sci Rep. 2017;7(1):5198. doi: 10.1038/s41598-017-05564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Z, Zhang P, Yan A, Guo Z, Ban Y, Li J, et al. ASXL1 interacts with the cohesin complex to maintain chromatid separation and gene expression for normal hematopoiesis. Sci Adv. 2017;3(1):e1601602. doi: 10.1126/sciadv.1601602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inoue D, Nishimura K, Kozuka-Hata H, Oyama M, Kitamura T. The stability of epigenetic factor ASXL1 is regulated through ubiquitination and USP7-mediated deubiquitination. Leukemia. 2015;29(11):2257. doi: 10.1038/leu.2015.90. [DOI] [PubMed] [Google Scholar]
- 107.Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43(7):668. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43(10):1018. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pena-Llopis S, Vega-Rubin-de-Celis S, Liao A, Leng N, Pavia-Jimenez A, Wang S, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44(7):751. doi: 10.1038/ng.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Balasubramani A, Larjo A, Bassein JA, Chang X, Hastie RB, Togher SM, et al. Cancer-associated ASXL1 mutations may act as gain-of-function mutations of the ASXL1–BAP1 complex. Nat Commun. 2015;6:7307. doi: 10.1038/ncomms8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sahtoe DD, van Dijk WJ, Ekkebus R, Ovaa H, Sixma TK. BAP1/ASXL1 recruitment and activation for H2A deubiquitination. Nat Commun. 2016;7:10292. doi: 10.1038/ncomms10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Daou S, Barbour H, Ahmed O, Masclef L, Baril C, Sen Nkwe N, et al. Monoubiquitination of ASXLs controls the deubiquitinase activity of the tumor suppressor BAP1. Nat Commun. 2018;9(1):4385. doi: 10.1038/s41467-018-06854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kitamura T. ASXL1 mutations gain a function. Blood. 2018;131(3):274. doi: 10.1182/blood-2017-12-816595. [DOI] [PubMed] [Google Scholar]
- 115.Hsu YC, Chiu YC, Lin CC, Kuo YY, Hou HA, Tzeng YS, et al. The distinct biological implications of Asxl1 mutation and its roles in leukemogenesis revealed by a knock-in mouse model. J Hematol Oncol. 2017;10(1):139. doi: 10.1186/s13045-017-0508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Uni M, Masamoto Y, Sato T, Kamikubo Y, Arai S, Hara E, et al. Modeling ASXL1 mutation revealed impaired hematopoiesis caused by derepression of p16Ink4a through aberrant PRC1-mediated histone modification. Leukemia. 2018;33(1):191. doi: 10.1038/s41375-018-0198-6. [DOI] [PubMed] [Google Scholar]
- 117.Wu X, Bekker-Jensen IH, Christensen J, Rasmussen KD, Sidoli S, Qi Y, et al. Tumor suppressor ASXL1 is essential for the activation of INK4B expression in response to oncogene activity and anti-proliferative signals. Cell Res. 2015;25(11):1205. doi: 10.1038/cr.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Campagne A, Lee MK, Zielinski D, Michaud A, Le Corre S, Dingli F, et al. BAP1 complex promotes transcription by opposing PRC1-mediated H2A ubiquitylation. Nat Commun. 2019;10(1):348. doi: 10.1038/s41467-018-08255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guo Y, Yang H, Chen S, Zhang P, Li R, Nimer SD, et al. Reduced BAP1 activity prevents ASXL1 truncation-driven myeloid malignancy in vivo. Leukemia. 2018;32(8):1834. doi: 10.1038/s41375-018-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]