Abstract
Considering the high mortality rate encountered in lung cancer, there is a strong need to explore new biomarkers for early diagnosis and also improved therapeutic targets to overcome this issue. The implementation of microRNAs as important regulators in cancer and other pathologies expanded the possibilities of lung cancer management and not only. MiR-21 represents an intensively studied microRNA in many types of cancer, including non-small cell lung cancer (NSCLC). Its role as an oncogene is underlined in multiple studies reporting the upregulated expression of this sequence in patients diagnosed with this malignancy; moreover, several studies associated this increased expression of miR-21 with a worse outcome within NSCLC patients. The same pattern is supported by the data existent in the Cancer Genome Atlas (TCGA). The carcinogenic advantage generated by miR-21 in NSCLC resides in the target genes involved in multiple pathways such as cell growth and proliferation, angiogenesis, invasion and metastasis, but also chemo- and radioresistance. Therapeutic modulation of miR-21 by use of antisense sequences entrapped in different delivery systems has shown promising results in impairment of NSCLC. Hereby, we review the mechanisms of action of miR-21 in cancer and the associated changes upon tumor cells together a focused perspective on NSCLC signaling, prognosis and therapy.
Electronic supplementary material
The online version of this article (10.1007/s00018-018-2877-x) contains supplementary material, which is available to authorized users.
Keywords: NSCLC, Resistance, Exosomes, miR-21, Oncogene
Introduction
Data collected into GLOBOCAN 2012 revealed that lung cancer classifies as the third most diagnosed cancer having also a high rate of mortality; the fact that makes this malignancy a major concern worldwide [1]. Non-small cell lung cancer (NSCLC) represents the most common subtype of lung cancer, with a higher incidence in developed countries [2]. The decreased survival rate is partially determined by late diagnosis and challenges in prevention, such as the poor impact of anti-smoking commercials [3]. The molecular alterations that characterize NSCLC are multiple and they work in networks leading to a molecular imbalance that generates a malignant phenotype. These modifications include changes in gene expression, copy number alterations, different methylation patterns, but also changes in the expression of non-coding RNAs (ncRNAs) [4]. It has been proved that the expression pattern of particular microRNAs (miRNAs) can be used as biomarkers for the histotype classification of NSCLC [5]. Therefore, the discovery of new biomarkers for early diagnosis and putative therapeutic targets for personalized medicine could significantly improve the clinical care and survival rate of lung cancer patients.
Once considered ‘junk DNA’, the non-coding part of the genome has proven to be of crucial importance, as the resulted transcripts of these sites exert modulatory functions upon target genes/molecules that further affect important signaling pathways [6]. These DNA regions are represented by different ncRNAs, and one major class is delineated by miRNAs. These small sequences constitute a class of ncRNAs encountered all over the genome [7], with nearly 22 nucleotides in final length after the process of maturation, form in which they can complementary bind to a messenger RNA (mRNA) of different genes [8–11]. The essential role of miRNAs results from the fact that one single molecule is able to target multiple mRNAs, thus influencing the expression of hundred of genes simultaneously [12–14]. The report is that about 30% of the genes are targeted by different miRNAs in humans [15].
The association of miRNAs with different types of cancer was first reported upon the illustration that downregulation of miR-15 and miR-16 was positively correlated with the presence of chronic lymphocytic leukemia phenotype in patients [6]. The involvement of miRNAs in several cancers was emphasized in a comprehensive review that underlines the implications of miRNAs in all hallmarks of cancer [16]. Besides, several other comprehensive studies are taking in discussion the importance of miRNAs in various types of cancer [17–22].
The present paper is focused on the role of miR-21 in lung cancer, more specifically in NSCLC. As this sequence was repeatedly reported as upregulated in this malignancy with further implications upon cancer progression and also patients’ prognosis, we are taking in discussion the possible utilization of this miRNA as early diagnosis tool and also therapeutic target. Moreover, specific mechanisms of action of miR-21 within the malignant environment are presented.
MiR-21 functions as an oncogene in several cancers
One of the first characterizations of pri-mir-21 appeared in 2004, being also one of the first miRNAs to be described with its processing steps [23]. Since then, deregulated expression of this miRNA together with its role has been reported in a wide range of malignancies (Table 1) [24, 25]. The aberrant expression appears because of altered molecular networks that may involve proteins such as signal transducer and activator of transcription 3 (STAT3), epidermal growth factor receptor (EGFR), transforming growth factor-β (TGF-β) [24, 26]. Moreover, it seems that miR-21 is also able to influence the p53 pathway [27], a well-known gene with decisive roles in cancer [28–30]. One of the first indications refers to the role of miR-21 in the apoptosis of glioblastoma cells. Reporting upregulated expression of miR-21 in tissue samples from glioblastoma patients, the researchers further demonstrated the association of high levels of miR-21 with the inhibition of apoptosis in several glioblastoma cell lines [31]. Abnormal expression of miR-21 in glioblastoma patients was confirmed within a further study, as it appears to be upregulated in tumor tissue when compared with non-tumor brain samples [32]. The possible use of miR-21 as a circulating biomarker for minimally invasive diagnosis approaches, can apply to glioblastoma patients, as its levels in plasma samples are significantly increased than in those with other neurological disorders [33]. In breast cancer patients, the expression of miR-21 appears to be upregulated in tumor compared with normal breast tissue [34]. Moreover, the expression of miR-21/miR-155/miR-365 in serum samples could help discriminate between breast cancer patients and healthy controls [35]. Another study found that in vitro and in vivo breast cancer models, as well as sera of actual patients treated with metformin exhibit a reduction in miR-21; this inhibition was further examined in vitro where SESN1 and CAB39L (direct target genes of miR-21) were found as increased, with further effects upon AMPK activation, mTOR reduction and also impairment of cell invasion and migration [36]. In the case of young gastric cancer patients, miR-21-5p was found as upregulated in tissue samples and the expression was correlated with recurrence in these individuals [37]. Hematological malignancies are also characterized by deregulated miR-21 profiles, as this miRNA was reported as overexpressed in patients with nucleophosmin 1 gene (NPM1)—mutant acute myeloid leukemia [38]. Another study refers to the elevated expression of miR-21 in colon cancer patients, expression that was also associated with high mortality rates in these patients [39]. Using in situ hybridization (ISH) and microarray analyses on tissue samples from patients with prostate cancer, Li et al. [40] showed that higher grades were associated with upregulated expression of miR-21 and its overexpression predicts poor biochemical recurrence-free survival. Likewise, using peripheral blood mononuclear cell samples for evaluation of mi-21 expression in prostate cancer patients, it was assessed that high expression level could be a useful predictor of the presence, recurrence and metastasis [41]. The high expression of miR-21 in hepatocellular carcinoma tissues obtained from patient samples is a measure for shorter disease-free survival (DFS) and overall survival (OS) in an Asian population [42]. In addition, the levels of miR-21 in serum samples from patients with the same malignancy can separate healthy volunteers from hepatocellular carcinoma patients, being also coupled with the clinical stage and the presence of metastases [43].
Table 1.
MiR-21 target genes and altered pathways in different types of cancer
| Cell line/animal model | Target genes | Pathway | References |
|---|---|---|---|
| Trp53 (–/–) miR-21 (–/–) mice | PTEN | TP53-associated apoptosis and senescence | [27] |
| MCF10A (ER-Src cells) | PTEN | PTEN/Akt/NF-κB | [26] |
| SUM159PT | SESN1 and CAB39L | Invasion and migration | [36] |
| CCR6+ Treg (from murine breast cancer) | PTEN | Cell proliferation | [47] |
| HeLa, HT-3 | RASA1 | EMT and cell migration | [48] |
| DU145 | PTEN | Angiogenesis | [49] |
PTEN phosphatase and tensin homolog, SESN1 sestrin 1, CAB39L calcium binding protein 39 like, RASA1 RAS P21 protein activator 1
The importance of miR-21 in regulating metastasis in different types of cancer is already known, as it was stated in several publications [44, 45]. In triple negative breast cancer (TNBC) cells, miR-21 modulates the invasive and metastatic potential of the cells, as part of the LPA1/Pi3 K/ZEB1/miR-21pathway that further regulates the lysophosphatidic acid (LPA) activity [46]. The aberrantly expressed miR-21 also mediates the activity of CCR6+Treg cells, through PTEN/Akt pathway, controlling their capacity to proliferate in breast cancer tumors [47]. MiR-21 contributes to epithelial-to-mesenchymal transition (EMT) in cervical cancer by modulating the expression of Rasa1 gene (RAS p21 protein activator 1). Therefore, by indirectly influencing the activity of Ras, miR-21 contributes to the migration potential of these cells [48]. Besides, miR-21 is known to modulate angiogenesis in prostate cancer cells; effect enabled by the indirect modulatory action upon HIF1α and VEGF [49].
All these investigations suggest that miR-21 functions as an oncogene in several types of cancer, making this molecule an important element that should be considered for early diagnosis, but also as therapeutic target for new precision medicine approaches (Fig. 1).
Fig. 1.
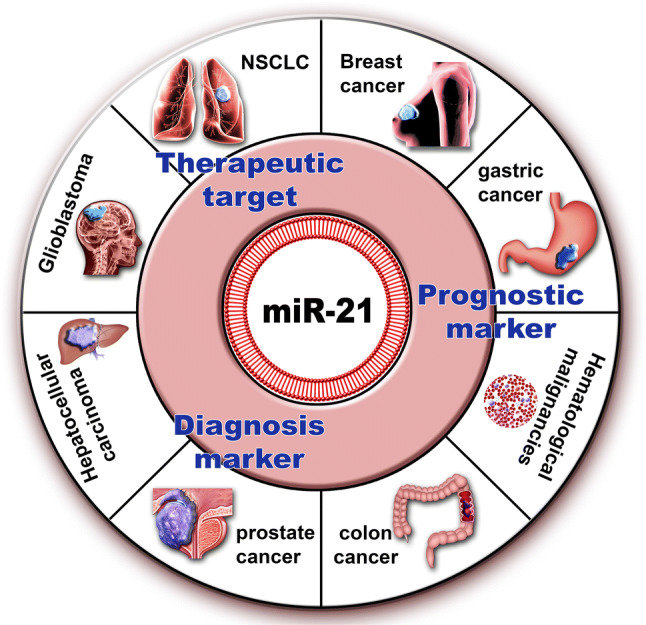
Mir-21 is upregulated in several types of cancer, contributing to the malignant transformation. The ubiquitous presence of upregulated miR-21 in many cancers reveals the importance of this miRNA as diagnosis/prognostic marker and therapeutic target for precision medicine
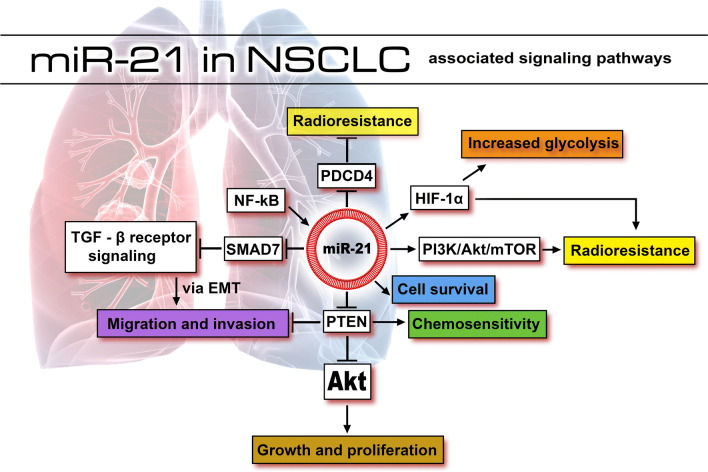
In NSCLC, it was stated that NF-kB influences the expression of miR-21 through a mechanism that involves the binding of NF-kB to the promoter region of miR-21, leading to its upregulation [50]. As reminded, the mechanism of action of miR-21 as an oncogene in different types of cancer involves the modulation of several genes (Supplemental Fig. 1—miR-21 target genes based on strong experimental evidences) that are part of important signaling pathways, such as PDCD4/NF-κB/TNF-α [51], PTEN/PI3K/AKT [52], or PTEN/Akt/HIF-1α pathway [53]. In NSCLC, the publications in which the target genes of miR-21 were evaluated in cell lines, identified PTEN [50, 54, 55], PDCD4 [56], HIF1α and hMSH2 [56] as direct targets (Table 2). PTEN is a well-known tumor suppressor gene, frequently mutated or inactivated in NSCLC cell lines [57–59]. By targeting PTEN transcript in NSCLC, miR-21 influences the growth and invasion of these cells [54].
Table 2.
Validated target genes for miR-21 in NSCLC cell lines
| Cell line | Target genes | Pathway | References |
|---|---|---|---|
| A549 | PTEN | Cell growth, invasion | [54] |
| NCI-H446, A549, NCI-H460 | hMSH2 | Cell proliferation | [60] |
| A549 | SMAD7 | Cell invasion/chemosensitivity | [61] |
| A549 | PTEN | Cell growth, metastasis, chemo-/radioresistance | [55] |
| A549 | PTEN | Cell viability/cisplatin sensitivity | [50] |
| A549 | HIF1α | Radioresistance | [62] |
| A549 | PDCD4 | Radioresistance | [56] |
hMSH2 human MutS protein homolog 2, PTEN phosphatase and tensin homolog, HIF1α hypoxia-inducible factors 1 alpha, PDCD4 programmed cell death protein 4, SMAD7 mothers against decapentaplegic homolog 7
Reports have shown that miR-21 intervenes in EMT by modulating the expression of SMAD7 in NSCLC cells. Besides, as SMAD7 represents the target for carboplatin therapy, miR-21 expression could serve as an important biomarker for carboplatin chemosensitivity screening of patients with NSCLC [61]. The signaling pathways disturbed according to the validated target genes of miR-21 in NSCLC cell lines, and their mechanism of action are illustrated in Fig. 2.
Fig. 2.
Processes influenced by the action of miR-21 in NSCLC, according to the published literature. By inhibiting the expression of Akt via PTEN, miR-21 increases the rate of growth and proliferation. The inhibition of PTEN also results in impairment of migration and invasion and in sensitivity to chemotherapy. Sensitivity to radiotherapy is controlled via inhibition of PDCD4 or activation of HIF-1α gene and PI3 K/Akt/mTOR pathway by miR-21. Increased glycolysis appears as a result of stimulated expression of HIF-1α by miR-21. The action of stimulating the migration and invasion is reflected in the inhibition of SMAD7 that modulates the expression of TGF-β
Mechanisms and regulation of miR-21 biogenesis and dysregulation in cancer
MiR-21 is localized in the 17q23.2 chromosome region within an intron of vacuole membrane protein 1 (VMP1) coding region—its human homologue being TMEM49. The same publication revealed the fact that AP-1 promotes the transcription of miR-21, process dependent on chromatin remodeling complexes [63]. Post-transcriptional regulation of miR-21 is also backed by the fact that when pre-miR-21 of exogenous origins is expressed in A549 and MCF-7 cells, mature miR-21 is not produced [64].
The expression of miR-21 appears to be regulated by different factors. In prostate cancer, it has been reported that reactive oxygen species (ROS) play an important role in regulating its expression. Within this context, it was previously described that in prostate cancer, ROS resulting from NADPH (nicotinamide adenine dinucleotide phosphate) oxidase activity, is necessary for miR-21 expression. The enhanced expression of NADPH and miR-21 contributes to the invasive potential of prostate cancer cells [65]. Mir-21 expression in also indirectly regulated by CD24, which activates Src, leading to the upregulation of miR-21 in RKO and Geo colorectal cell lines [66].
In glioma cells, the biogenesis of miR-21 is modulated by DDX23 (DEAD-box RNA helicase), a gene with implications in mRNA splicing [67]. DDX23 influences the expression of miR-21 during the processing steps of pri-miR-21 to the precursor one, by intervening at the level of Drosha protein [68]. In glioblastoma cell lines, miR-21 appears to be regulated by MPS1 (Monopolar spindle 1), a key protein whose action is necessary to ensure spindle stability [69], which in turn, modulates the expression of PDCD4 and MSH2 (MutS protein homolog 2), with further effects on cell proliferation [70]. Multiple myeloma represents another pathology characterized by miR-21 deregulation. In this malignancy, the STAT3 pathway through IL-6 (Interleukin-6) is responsible for the upregulation of miR-21 [71].
Another mechanism involved in the regulation of miR-21 refers to the epigenetic changes that disturb the expression of this miRNA. Therefore, in colorectal cancer, it was demonstrated that miR-21 is under epigenetic regulation, the promoter of miR-21 being activated or repressed upon the action of histone modifications [72]. In addition, the mechanism of miR-21 modulation by retinoblastoma binding protein 2 (RBP2) in chronic myeloid leukemia cells implies also epigenetic changes, such as decreased H3K4 trimethylation in its promoter [73].
Deregulated expression of miR-21 in lung cancer
Regarding the expression of miR-21 in lung cancer, specifically NSCLC, the studies published up to date have reported upregulated levels of this sequence in diagnosed patients (Supplemental Table 1). One of the first publication that mentions the possible role of miR-21 expression profile in NSCLC patients as prognostic factor was in 2008, when Markou et al. [74] measured the levels of the miRNA in tissue samples from NSCLC patients and reported the upregulated levels in tumor tissue compared with its paired normal controls. The investigation went further, as it was observed that overexpression of miR-21 resulted in poor overall survival within these patients. The results were confirmed by the work of Cho et al. [75], which validated the upregulation of miR-21 in tumor tissue vs. non-tumor ones through microarray analysis and quantitative reverse transcription polymerase chain reaction (qRT-PCR). The feasible value of miR-21 as a non-invasive biomarker for NSCLC diagnosis, assessed from sputum samples, was confirmed with high specificity and sensitivity, as its expression was significantly higher than in sputum samples from cancer-free individuals [76]. The same observation was made on plasma samples. In this sense, upregulated expression of miR-21 could stand as a biomarker for distinguishing between NSCLC patients and healthy plasma donors [77]. Another important aspect concerning the deregulated expression of miR-21 in NSCLC refers to its relevance in gefitinib resistance/sensitivity. This feature of miR-21 was assessed in pc-9 and pc-9/GR (Gefitinib-resistant) cells, where upregulation of miR-21 correlated with downregulation of PTEN, further inducing low-gefitinib sensitivity and decreasing the overall survival of these patients [78].
Another aspect worth mentioning is the fact that miR-21 is often part of panels of genes/miRNAs proposed as biomarkers for NSCLC early detection. In this concern, along with miR-221, miR-145, miR-20a, and miR-223, miR-21 is present as a member of the 5 miRNAs signature from plasma samples with high potential as minimally invasive method for early diagnosis of NSCLC patients [79]. Similarly, another panel of miRNAs (miR-20a, miR-21, miR-223, and miR-145) detected in plasma samples that includes also miR-21, could stand as a potential tool for early diagnosis, as their elevated levels could discriminate between NSCLC patients and healthy controls [80]. Upregulated expression of miR-21 is also encountered in serum samples of NSCLC patients together with downregulated expression of tumor suppressor miRNAs: miR-148a/b and miR-152, profile that could also serve as a biomarker tool for NSCLC screening [81].
Exosomal miR-21 expression in lung cancer
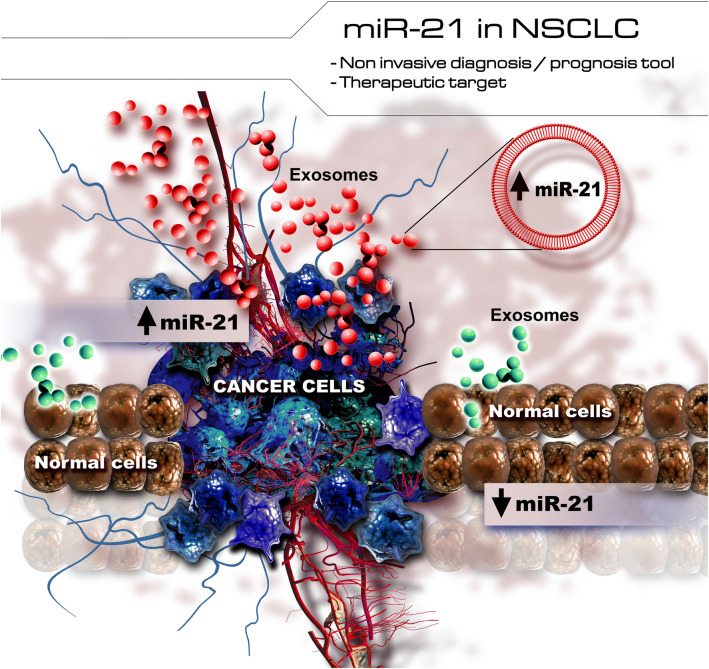
MiRNAs can exert their functions not only within the cell in which they are expressed but also in other parts of the body, their small size enabling them to travel together with the biological fluids—including through small nanoparticles released by cells [82, 83]. Exosomes are vesicles derived from donor cells with a size ranging between 20 and 120 nm [84, 85]. Their composition includes the presence of proteins usually localized in the cell at the cytosol level, in the membranes of endocytic compartments and also plasma membrane [86, 87], suggesting that exosomes are derived from lipid structures called intraluminal vesicles and are extravasated from the cell in a process that involves the fusion with the plasma membrane [88]. Their role mainly consist in the ensuring of intracellular communication through transportation of different regulatory small molecules [89]. The exosomal cargo contains also miRNAs and mRNAs, thus underlining the importance of miRNAs in processes of cell communication [90]. Exosomes have been reported to play key roles in cancer via internalization and transport of regulatory molecular cargos with further direct consequences upon the target cell and also tumor development [91]. MiRNAs that are exported via exosomes, with their oncogenic/tumor suppressor potential, contribute also to the involvement of exosomes in cancer progression [92, 93], but can be also exploited as potential biomarkers for diagnosis/prognosis due to the presence in liquid samples (Fig. 3).
Fig. 3.
Upregulation of miR-21 in NSCLC cells and exosomes released from malignant transformed cells. The expression of miR-21 in exosomes harvested from NSCLC patients could be used as minimal invasive biomarkers for early diagnosis/prognosis
In NSCLC, several studies investigated the association between the levels of exosomal miR-21 and the prognostic of patients affected by this pathology. Therefore, the upregulation of miR-21-5p in exosomes isolated from plasma was confirmed, indicating that exosomal miR-21 could stand as a possible biomarker for minimally invasive diagnosis of NSCLC [94]. As it can be observed in Table 3, miR-21 present in exosomes can offer valuable information concerning the prognosis of patients with NSCLC. Accordingly, it was stated that using Cox proportional hazard model, high miR-21-5p levels in plasma exosomes of these patients determined a poor overall survival (OS) [95].
Table 3.
List of publications evaluating the correlation between the expression levels of miR-21 and the outcome of patients with NSCLC
| Expression of miR-21 | Outcome of NSCLC patients | Sample size/type | Statistical method | References |
|---|---|---|---|---|
| ↑ | Shorter RFS (p = 0.042) and OS (p = 0.043) | 210 patients/tissue | Log-rank test | [96] |
| ↑ | Poor prognosis for OS (p = 0.011) | 423 patients (TCGA data) | Cox regression analysis | [101] |
| ↑ | Poor OS (p = 0.003) | 10 patients/plasma exosomes | Cox proportional hazard analysis | [95] |
| ↑ | Poor DFS (p = 0.016) | 195 patients/plasma exosomes | Log-rank test/multivariate Cox analysis | [106] |
| ↑ | Worse cancer-specific survival (p = 0.018/0.014) and RFS (p = 0.0005) | 317 patients/tissue | Univariate Cox analysis | [97] |
| ↑ | Reduced OS (p = 0.027) | 48 patients/tissue | Log-rank test | [74] |
| ↓ | Worse DSS (p = 0.027) | 95 lymph-node positive patients/tumor cells | Multivariate analysis | [105] |
| ↑ | Lower DFI (p = 0.022—tissue/p = 0.045—plasma) and OS (p = 0.037—tissue/p = 0.065—plasma) |
40 patients/tissue 37 patients/plasma |
Log-rank test | [107] |
| ↑ | Worse OS (p = 0.0045) | 261 patients (gefitinib treatment)/plasma | Univariate and multivariate analysis | [102] |
| ↑ | Shorter DFS (p = 0.008) | 58 patients/tissue | Cox proportional hazard regression model | [98] |
| ↑ | Poor OS (p = 0.015) | 88 patients/serum | Univariate and multivariate analysis | [103] |
| ↑ | Shorter OS and PFS (p < 0.001) | 204 patients/tissue | Multivariate logistic regression | [99] |
| ↑ |
Shorter OS (p = 0.0065—tissue) Poor prognosis (p = 0.019—serum) |
70 patients/serum and tissue |
Log-rank test Univariate cox analysis |
[108] |
| ↑ | Lower survival time (p = 0.001) | 80 patients/serum | Pearson correlation | [104] |
| ↑ | Lower PFS (p < 0.001) | 108 patients/plasma | Kaplan–Meier analysis | [109] |
| ↑ | Shorter DFS | 46 patients/tissue | Kaplan–Meier analysis | [78] |
| ↑ | Lower PFS (p = 0.0003) and OS (p = 0.0045) | 80 patients/tissue | Kaplan–Meier analysis | [100] |
NSCLC non-small cell lung cancer, RFS relapse/recurrence-free survival, OS overall survival, PFS progression-free survival, DFS disease-free survival, DSS disease-specific survival, DFI disease-free interval
MiR-21 prognostic value in lung cancer
The large number of studies that assessed the expression of miR-21 in patients with NSCLC and investigated the association with survival, indicate that this molecule is actively involved in the modulation of malignant transformation and progression. Table 3 illustrates the studies that reported the connection of high miR-21 expression and its prognostic significance. The majority of the results indicate that independent of the sample type, upregulation of miR-21 is correlated with negative outcome in tissue [96–100], plasma [95, 101, 102] or serum samples [103, 104]. One particular case is represented by the work of Stenvold et al., where they used ISH for the evaluation of miR-21 independent expression on tumor cells and stromal cells. Therefore, it was reported that the upregulation of miR-21 in tumor cells positively correlates with better prognosis, these results being limited only to lymph-node positive NSCLC patients. When the expression was evaluated in the stroma, overexpression of miR-21 appeared to negatively correlate with the prognosis of patients with lymph-node negative status [105].
MiR-21 turned out to be an important biomarker in predicting brain metastases in patients with NSCLC. In this regard, a recent publication reported that when dividing the patients with NSCLC according to their brain metastasis status (present/absent), the expression of miR-21 is higher in those with positive for brain metastases [110].
Data from the Cancer Genome Atlas (TCGA) strengthen the importance of miR-21 in the context of the present article. Thus, to achieve a more comprehensive view upon the effects of hsa-miR-21 expression in lung cancer, TCGA miRNA-seq data were downloaded from the UCSC (University of California Santa Cruz) Cancer Browser in the form of a data matrix containing the normalized, log2 transformed miRNA expression levels for 515 tumor tissues and 46 peritumoral normal tissues, together with the corresponding clinical parameters. After removing the normal tissues and selecting the samples with complete survival information, Kaplan–Meier survival analysis was performed for 486 samples for which miR-21 expression levels were separated into high-expression and low-expression according to the median. The test presented statistical significance, with a p value of 0.047, as seen in Fig. 3, meaning that the expression of miR-21 in lung adenocarcinoma patients can be correlated with the overall survival of these patients. Precisely, upregulated expression of miR-21 is associated with poor overall survival of patients with LUAD (Fig. 4).
Fig. 4.
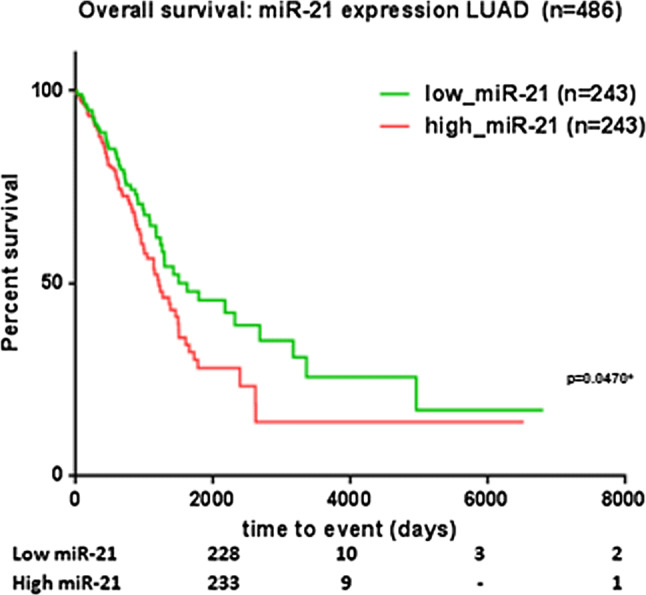
MiR-21 expression associated with cancer-specific mortality in lung adenocarcinoma patients. Kaplan–Meier curve on TCGA data from patients with LUAD (n = 486). Patients were separated in low- and high-expression based on the median value; TCGA The Cancer Genome Atlas, LUAD lung adenocarcinoma; *p < 0.05
Association with long non-coding RNAs in NSCLC
Long non-coding RNAs (lncRNAs) represent a class of ncRNAs, along with miRNAs, with gene sequences that are not transcribed into proteins, but unlike miRNAs, this class contain transcripts that have more than 200 nucleotides in length and their post-transcription modifications include alternative splicing and 5′ capping [111, 112]. LncRNAs role in cancer is now an established fact, as their involvement in modulation of mRNAs stability, splicing and translation [113] has indirect repercussions on the disruption of signaling pathways that contribute to neoplastic transformations [114].
As it was formerly stated, upregulated miR-21 expression correlates with the presence of NSCLC. Contrarily, GAS5 (Growth Arrest Specific 5) lncRNA is found with as poorly expressed in NSCLC, possibly indicating an association between this lncRNA and miR-21. Therefore, the link was explored, and the obtained data suggested that miR-21 affects the sensitivity of H157 and H460 cells to cisplatin by regulating the expression of GAS5 through interfering with PTEN pathway [115]. H19, an lncRNA whose role was assessed in extensive studies, was studied together with miR-21 in the context of NSCLC. It was revealed that upregulated expression of both mir-21 and H19 appear in patients with NSCLC and that their expression, taken together, has prognostic value for these individuals. These results indicate the fact that a panel consisting of miR-21 and H19 could stand as a biomarker of diagnosis/prognosis [116]. Supplemental Table 2 presents the putative mir21—lncRNAs interactions retrieved from miRWalk database and sorted according to the seed length, demonstrating other possible regulatory mechanisms involving miR-21 and lncRNAs that are not yet backed up by research data.
MiR-21 delivery systems for targeted therapy
Concerning the connection between miR-21 expression and other features that characterize malignant cells, such as resistance to therapy, there are several publications that assessed the implication of miR-21 in blocking the response to therapy. It was underlined fact that upregulation of miR-21 in various types of cancer is correlated with resistance to different categories of chemotherapeutics, such as cisplatin, gemcitabine, and teniposide [117]. The association between plasma levels of miR-21 and the acquired resistance to the treatment consisting of epidermal growth factor receptor (EGFR) and tyrosine kinase inhibitors (TKIs) was assessed in patients that followed the above-mentioned therapies and the disease continued to progress after the treatment. The conclusion was that in these patients, miR-21 levels were more increased than at baseline [118]. The radiosensitivity mediated by PI3K/Akt pathway represents also an aspect controlled by miR-21. Accordingly, it was reported that in A549 cells, the inhibition of miR-21 improved the sensitivity to radiotherapy, followed by increased apoptosis and decreased cell proliferation [119]. Furthermore, the action of miR-21 in mediating radiation-induced bystander effects through TGF-β1-miR-21-ROS pathway was previously described. Therefore, upregulated expression of miR-21 in bystander H1299 cells determined increased ROS levels and also DNA damage. As expected, inhibited expression of miR-21 significantly reduced cell proliferation [120]. In plasma samples, the high levels of miR-21 in NSCLC patients divided based on their reaction to cisplatin-based therapy, respectively, responders and non-responders, could be a useful predictor of the therapeutic response, and constructive, worse outcome [109].
The use of different delivery systems for miRNAs with tumor suppressor properties could represent a major improvement for personalized treatment in cancer patients. The limitations of this treatment option are imputed to the deficient stability of these molecules, but also to the faulty delivery systems used [121–123].
Given the ubiquitous character of miR-21 as an oncogene in a large number of cancers, several publications evaluated the effect of miR-21 or antisense miR-21 (anti-miR-21) in different cancer cell lines. In this interest, using poly[lactic-co-glycolic]acid (PLGA) nanoparticles as carriers for antimiR-21 in combination with temozolomide in glioma cell lines (miR-21 is overexpressed in glioblastoma), it was demonstrated that PLGA is an efficient delivery system for miRNAs, leading to the downregulation of the endogenous sequence and further reducing the number of viable cells [124]. An efficient delivery system for antisense-oligodeoxynucleotide (antisense-ODN) against miR-21 in glioblastoma cells was also developed by Song et al., using a R3V6 complex stable enough to deliver anti-miR-21 and inhibit its effects on cells. Therefore, anti-miR-21 administrated in R3V6 reduced the survival of glioma cells showing also less cytotoxicity than PEI25k (polyethylenimine) system [125]. It was previously shown that combination therapies could have a significant impact on glioblastoma cells [126], strategy that could work also with miR-21 inhibitors combined with other targeted agents (e.g., metformin, sorafenib). Lentiviral vectors are also used as delivery systems for miR-21 inhibitors, precisely for multiple myeloma cells using in vitro and in vivo models with anti-proliferative effects [127].
Anti-miR-21 was also used in mice models, and orthotropic injection of 3WJ-EGFRapt nanoparticles carrying anti-miR-21 appeared to specifically target the TNBC tumor and release the miRNA within these cells. The inactivation of miR-21 was observed upon the effect on its target genes, PDCD4 and PTEN, their expression being upregulated in the targeted TNBC cells [128]. In solid cancer tumors, graphene represents a valuable biomaterial for targeted therapy due to its characteristics, such as biocompatibility and the possibility to be functionalized [129]. A complex that incorporated graphene was utilized for in vitro studies of breast cancer tumors as carrier for miR-21 to overcome multi-drug resistance (MDR). Precisely, the researchers used polyethylenimine (PEI)/poly (sodium 4-) (PSS)/graphene oxide (GO), a complex easy to be internalized by target cells, as a delivery system for adriamycin and anti-miR-21; their results showed that this complex managed to keep its properties and also efficiently released the anti-miR-21 sequence [130]. In pancreatic cancer, nanoparticles consisting of PEG-PEI-IONPs (polyethylene glycol–polyethylenimine–magnetic iron oxide nanoparticles) were used to co-deliver gemcitabine and anti-miR-21 in pancreatic cancer cells, where the inhibition upon migration and proliferation of these cells confirmed the appropriate delivery and release of the therapeutic agents. The overexpressed PDCD4 and PTEN and underexpressed E-cadherin and Vimentin validated the inhibition of miR-21 [131]. Nanocapsules containing anti-miR-21 trapped inside the shell through in situ polymerization were used upon glioblastoma and breast cancer cell lines, and the results evidenced the fact that nanocapsules carrying anti-miR-21 are more efficient than lipofectamine. The same therapeutic strategy was used on murine models, where the tumor growth was suppressed as a result of angiogenesis inhibition in mice injected with U87 tumor cells [132].
Natural compounds delivered within cancer cells were also associated with modulatory capacity upon miR-21 levels—another perspective upon modifications of miRNAs level [133, 134]. Besides the suppressive action upon miRNAs, these compounds have additional inhibitory effects on cancer progression and can be also delivered inside targeted nanostructures [9, 135, 136].
The use of nanoparticles to deliver anti-miR-21 in NSCLC studies appears to be a domain insufficiently explored, as there are limited publications of this type; one study used calcium phosphate nanoparticles as carriers for anti-miR-21 in NSCLC cell lines. The anti-miR-21 was used to overcome radioresistance, determined and maintained by the presence of ALDH1+ and CD133+ cancer stem cells. By targeting these cells and releasing miR-21 and miR-95 inhibitors within them, subsequently inhibition of miR-21 and miR-95 was achieved, and to the lung cancer tumors were sensitized to radiation therapy [137].
Conclusions
MiR-21 is one of the most intensively studied miRNA, and its role as an oncogene was proven in many types of cancer with an upregulated expression in patients affected by these malignancies. The deregulated expression of miR-21 affects pathways that control cell proliferation, migration, survival, but also angiogenesis. The already mentioned publications suggest a possible role of miR-21 as a valuable target for personalized treatment of cancer patients. The limited number of target genes for miR-21 in NSCLC validated up to date, represents a major advantage, as its inhibition would only disrupt a restrained amount of signaling pathways, and its action would be specifically targeted and easily controlled. The advancements made within biomaterials development and nanodelivery systems are encouraging for the future use of miR-21 as part of the standard treatment for different types of cancers, and particularly for NSCLC. The use of anti-miR-21 agents encapsulated in different functionalized nanoparticles—to prevent their degradation, minimize their interaction with cell structures and maximize their uptake—brings new insights into the management of NSCLC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was granted by Competitiveness Operational Program, 2014–2020, entitled “Clinical and economical impact of personalized targeted anti-microRNA therapies in reconverting lung cancer chemoresistance”—CANTEMIR, no. 35/01.09.2016, MySMIS 103375 and PhD fellowship (PCD 2017) no. 1300/51/13.01.2017 entitled, “Next Generation sequencing (NGS) in personalized medicine”.
Abbreviations
- NSCLC
Non-small cell lung cancer
- PTEN
Phosphatase and tensin homolog
- LUAD
Lung adenocarcinoma
- TCGA
The Cancer Genome Atlas
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, et al. The 2015 World Health Organization Classification of lung tumors: impact of genetic, clinical and radiologic advances Since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 3.Office of the Surgeon General (US) Office on Smoking and Health (US) The health consequences of smoking: a report of the surgeon general. Atlanta, GA: Centers for Disease Control and Prevention (US); 2004. [PubMed] [Google Scholar]
- 4.Pikor LA, Ramnarine VR, Lam S, Lam WL. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer (Amst, Neth) 2013;82(2):179–189. doi: 10.1016/j.lungcan.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Voortman J, Goto A, Mendiboure J, Sohn JJ, Schetter AJ, Saito M, et al. MicroRNA expression and clinical outcomes in patients treated with adjuvant chemotherapy after complete resection of non-small cell lung carcinoma. Can Res. 2010;70(21):8288–8298. doi: 10.1158/0008-5472.CAN-10-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99(24):15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14(10A):1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortolin-Cavaille ML, Dance M, Weber M, Cavaille J. C19MC microRNAs are processed from introns of large Pol-II, non-protein-coding transcripts. Nucleic Acids Res. 2009;37(10):3464–3473. doi: 10.1093/nar/gkp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulei D, Mehterov N, Nabavi SM, Atanasov AG, Berindan-Neagoe I. Targeting ncRNAs by plant secondary metabolites: the ncRNAs game in the balance towards malignancy inhibition. Biotechnol Adv. 2017 doi: 10.1016/j.biotechadv.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Redis RS, Berindan-Neagoe I, Pop VI, Calin GA. Non-coding RNAs as theranostics in human cancers. J Cell Biochem. 2012;113(5):1451–1459. doi: 10.1002/jcb.24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pop-Bica C, Gulei D, Cojocneanu-Petric R, Braicu C, Petrut B, Berindan-Neagoe I. Understanding the role of non-coding RNAs in bladder cancer: from dark matter to valuable therapeutic targets. Int J Mol Sci. 2017;18(7):1514. doi: 10.3390/ijms18071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu SD, Lin FM, Wu WY, Liang C, Huang WC, Chan WL, et al. miRTarBase: a database curates experimentally validated microRNA-target interactions. Nucl Acids Res. 2011;39(Database issue):D163–D169. doi: 10.1093/nar/gkq1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braicu C, Calin GA, Berindan-Neagoe I. MicroRNAs and cancer therapy—from bystanders to major players. Curr Med Chem. 2013;20(29):3561–3573. doi: 10.2174/0929867311320290002. [DOI] [PubMed] [Google Scholar]
- 14.Gulei D, Magdo L, Jurj A, Raduly L, Cojocneanu-Petric R, Moldovan A, et al. The silent healer: miR-205-5p up-regulation inhibits epithelial to mesenchymal transition in colon cancer cells by indirectly up-regulating E-cadherin expression. Cell Death Dis. 2018;9(2):66. doi: 10.1038/s41419-017-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouellet DL, Perron MP, Gobeil LA, Plante P, Provost P. MicroRNAs in gene regulation: when the smallest governs it all. J Biomed Biotechnol. 2006;2006(4):69616. doi: 10.1155/JBB/2006/69616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berindan-Neagoe I, Monroig Pdel C, Pasculli B, Calin GA. MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA Cancer J Clin. 2014;64(5):311–336. doi: 10.3322/caac.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xi X, Li T, Huang Y, Sun J, Zhu Y, Yang Y, et al. RNA biomarkers: frontier of precision medicine for cancer. Non-Coding RNA. 2017;3(1):9. doi: 10.3390/ncrna3010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang HD, et al. CircRNA: a novel type of biomarker for cancer. Breast cancer. 2018;25(1):1–7. doi: 10.1007/s12282-017-0793-9. [DOI] [PubMed] [Google Scholar]
- 19.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20(8):460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Bertoli G, Cava C, Castiglioni I. MicroRNAs: new biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics. 2015;5(10):1122–1143. doi: 10.7150/thno.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budisan L, Gulei D, Zanoaga OM, Irimie AI, Sergiu C, Braicu C, et al. Dietary intervention by phytochemicals and their role in modulating coding and non-coding genes in cancer. Int J Mol Sci. 2017;18(6):1178. doi: 10.3390/ijms18061178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berindan-Neagoe I, Calin GA. Molecular pathways: microRNAs, cancer cells, and microenvironment. Clin Cancer Res. 2014;20(24):6247–6253. doi: 10.1158/1078-0432.CCR-13-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10(12):1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13(1):39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X, Ren Y, Moore L, Mei M, You Y, Xu P, et al. Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab Investig. 2010;90(2):144–155. doi: 10.1038/labinvest.2009.126. [DOI] [PubMed] [Google Scholar]
- 26.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39(4):493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma X, Choudhury SN, Hua X, Dai Z, Li Y. Interaction of the oncogenic miR-21 microRNA and the p53 tumor suppressor pathway. Carcinogenesis. 2013;34(6):1216–1223. doi: 10.1093/carcin/bgt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berindan-Neagoe I, Balacescu O, Burz C, Braicu C, Balacescu L, Tudoran O, et al. p53 gene therapy using RNA interference. J BUON. 2009;14(Suppl 1):S51–S59. [PubMed] [Google Scholar]
- 29.Braicu C, Pileczki V, Irimie A, Berindan-Neagoe I. p53siRNA therapy reduces cell proliferation, migration and induces apoptosis in triple negative breast cancer cells. Mol Cell Biochem. 2013;381(1–2):61–68. doi: 10.1007/s11010-013-1688-5. [DOI] [PubMed] [Google Scholar]
- 30.Chira S, Gulei D, Hajitou A, Berindan-Neagoe I. Restoring the p53 ‘Guardian’ phenotype in p53-deficient tumor cells with CRISPR/Cas9. Trends Biotechnol. 2018;36(7):653–660. doi: 10.1016/j.tibtech.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Can Res. 2005;65(14):6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 32.Lakomy R, Sana J, Hankeova S, Fadrus P, Kren L, Lzicarova E, et al. MiR-195, miR-196b, miR-181c, miR-21 expression levels and O-6-methylguanine-DNA methyltransferase methylation status are associated with clinical outcome in glioblastoma patients. Cancer Sci. 2011;102(12):2186–2190. doi: 10.1111/j.1349-7006.2011.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivo D’Urso P, Fernando D’Urso O, Damiano Gianfreda C, Mezzolla V, Storelli C, Marsigliante S. miR-15b and miR-21 as circulating biomarkers for diagnosis of glioma. Curr Genom. 2015;16(5):304–311. doi: 10.2174/1389202916666150707155610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang GL, Zhang XH, Guo GL, Huang KT, Yang KY, Shen X, et al. Clinical significance of miR-21 expression in breast cancer: SYBR-Green I-based real-time RT-PCR study of invasive ductal carcinoma. Oncol Rep. 2009;21(3):673–679. [PubMed] [Google Scholar]
- 35.Han JG, Jiang YD, Zhang CH, Yang YM, Pang D, Song YN, et al. A novel panel of serum miR-21/miR-155/miR-365 as a potential diagnostic biomarker for breast cancer. Ann Surg Treat Res. 2017;92(2):55–66. doi: 10.4174/astr.2017.92.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pulito C, Mori F, Sacconi A, Goeman F, Ferraiuolo M, Pasanisi P, et al. Metformin-induced ablation of microRNA 21-5p releases Sestrin-1 and CAB39L antitumoral activities. Cell Discov. 2017;3:17022. doi: 10.1038/celldisc.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SK, Park YS, Ahn JY, Do EJ, Kim D, Kim JE, et al. MiR 21-5p as a predictor of recurrence in young gastric cancer patients. J Gastroenterol Hepatol. 2016;31(8):1429–1435. doi: 10.1111/jgh.13300. [DOI] [PubMed] [Google Scholar]
- 38.Riccioni R, Lulli V, Castelli G, Biffoni M, Tiberio R, Pelosi E, et al. miR-21 is overexpressed in NPM1-mutant acute myeloid leukemias. Leuk Res. 2015;39(2):221–228. doi: 10.1016/j.leukres.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Oue N, Anami K, Schetter AJ, Moehler M, Okayama H, Khan MA, et al. High miR-21 expression from FFPE tissues is associated with poor survival and response to adjuvant chemotherapy in colon cancer. Int J Cancer. 2014;134(8):1926–1934. doi: 10.1002/ijc.28522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li T, Li RS, Li YH, Zhong S, Chen YY, Zhang CM, et al. miR-21 as an independent biochemical recurrence predictor and potential therapeutic target for prostate cancer. J Urol. 2012;187(4):1466–1472. doi: 10.1016/j.juro.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 41.Huang W, Kang XL, Cen S, Wang Y, Chen X. High-level expression of microRNA-21 in peripheral blood mononuclear cells is a diagnostic and prognostic marker in prostate cancer. Genet Test Mol Biomark. 2015;19(9):469–475. doi: 10.1089/gtmb.2015.0088. [DOI] [PubMed] [Google Scholar]
- 42.Wang WY, Zhang HF, Wang L, Ma YP, Gao F, Zhang SJ, et al. miR-21 expression predicts prognosis in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2014;38(6):715–719. doi: 10.1016/j.clinre.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Guo X, Lv X, Lv X, Ma Y, Chen L, Chen Y. Circulating miR-21 serves as a serum biomarker for hepatocellular carcinoma and correlated with distant metastasis. Oncotarget. 2017;8(27):44050–44058. doi: 10.18632/oncotarget.17211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Wang J. MicroRNA-mediated breast cancer metastasis: from primary site to distant organs. Oncogene. 2012;31(20):2499–2511. doi: 10.1038/onc.2011.444. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H, Li Y, Lai M. The microRNA network and tumor metastasis. Oncogene. 2010;29(7):937–948. doi: 10.1038/onc.2009.406. [DOI] [PubMed] [Google Scholar]
- 46.Sahay D, Leblanc R, Grunewald TG, Ambatipudi S, Ribeiro J, Clezardin P, et al. The LPA1/ZEB1/miR-21-activation pathway regulates metastasis in basal breast cancer. Oncotarget. 2015;6(24):20604–20620. doi: 10.18632/oncotarget.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu Y, Wang C, Li Y, Zhao J, Chen C, Zhou Y, et al. MiR-21 controls in situ expansion of CCR6(+) regulatory T cells through PTEN/AKT pathway in breast cancer. Immunol Cell Biol. 2015;93(8):753–764. doi: 10.1038/icb.2015.37. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Zhan X, Yan D, Wang Z. Circulating MicroRNA-21 Is Involved in Lymph Node Metastasis in Cervical Cancer by Targeting RASA1. Int J Gynecol Cancer. 2016;26(5):810–816. doi: 10.1097/IGC.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 49.Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, et al. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS One. 2011;6(4):e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Z, Fang S, Di Y, Ying W, Tan Y, Gu W. Modulation of NF-kappaB/miR-21/PTEN pathway sensitizes non-small cell lung cancer to cisplatin. PLoS One. 2015;10(3):e0121547. doi: 10.1371/journal.pone.0121547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su Q, Li L, Liu Y, Zhou Y, Wang J, Wen W. Ultrasound-targeted microbubble destruction-mediated microRNA-21 transfection regulated PDCD4/NF-kappaB/TNF-alpha pathway to prevent coronary microembolization-induced cardiac dysfunction. Gene Ther. 2015;22(12):1000–1006. doi: 10.1038/gt.2015.59. [DOI] [PubMed] [Google Scholar]
- 52.Gui F, Hong Z, You Z, Wu H, Zhang Y. MiR-21 inhibitor suppressed the progression of retinoblastoma via the modulation of PTEN/PI3 K/AKT pathway. Cell Biol Int. 2016;40(12):1294–1302. doi: 10.1002/cbin.10678. [DOI] [PubMed] [Google Scholar]
- 53.Song L, Liu S, Zhang L, Yao H, Gao F, Xu D, et al. MiR-21 modulates radiosensitivity of cervical cancer through inhibiting autophagy via the PTEN/Akt/HIF-1alpha feedback loop and the Akt-mTOR signaling pathway. Tumour Biol. 2016;37(9):12161–12168. doi: 10.1007/s13277-016-5073-3. [DOI] [PubMed] [Google Scholar]
- 54.Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC) Clin Chim Acta. 2010;411(11–12):846–852. doi: 10.1016/j.cca.2010.02.074. [DOI] [PubMed] [Google Scholar]
- 55.Liu ZL, Wang H, Liu J, Wang ZX. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol Cell Biochem. 2013;372(1–2):35–45. doi: 10.1007/s11010-012-1443-3. [DOI] [PubMed] [Google Scholar]
- 56.Jiang LP, He CY, Zhu ZT. Role of microRNA-21 in radiosensitivity in non-small cell lung cancer cells by targeting PDCD4 gene. Oncotarget. 2017;8(14):23675–23689. doi: 10.18632/oncotarget.15644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forgacs E, Biesterveld EJ, Sekido Y, Fong K, Muneer S, Wistuba II, et al. Mutation analysis of the PTEN/MMAC1 gene in lung cancer. Oncogene. 1998;17(12):1557–1565. doi: 10.1038/sj.onc.1202070. [DOI] [PubMed] [Google Scholar]
- 58.Kohno T, Takahashi M, Manda R, Yokota J. Inactivation of the PTEN/MMAC1/TEP1 gene in human lung cancers. Genes Chromosom Cancer. 1998;22(2):152–156. doi: 10.1002/(SICI)1098-2264(199806)22:2<152::AID-GCC10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 59.Hosoya Y, Gemma A, Seike M, Kurimoto F, Uematsu K, Hibino S, et al. Alteration of the PTEN/MMAC1 gene locus in primary lung cancer with distant metastasis. Lung cancer (Amst, Neth) 1999;25(2):87–93. doi: 10.1016/S0169-5002(99)00052-5. [DOI] [PubMed] [Google Scholar]
- 60.Zhong Z, Dong Z, Yang L, Gong Z. miR-21 induces cell cycle at S phase and modulates cell proliferation by down-regulating hMSH2 in lung cancer. J Cancer Res Clin Oncol. 2012;138(10):1781–1788. doi: 10.1007/s00432-012-1287-y. [DOI] [PubMed] [Google Scholar]
- 61.Lin L, Tu HB, Wu L, Liu M, Jiang GN. MicroRNA-21 regulates non-small cell lung cancer cell invasion and chemo-sensitivity through SMAD7. Cell Physiol Biochem. 2016;38(6):2152–2162. doi: 10.1159/000445571. [DOI] [PubMed] [Google Scholar]
- 62.Jiang S, Wang R, Yan H, Jin L, Dou X, Chen D. MicroRNA-21 modulates radiation resistance through upregulation of hypoxia-inducible factor-1alpha-promoted glycolysis in non-small cell lung cancer cells. Mol Med Rep. 2016;13(5):4101–4107. doi: 10.3892/mmr.2016.5010. [DOI] [PubMed] [Google Scholar]
- 63.Fujita S, Ito T, Mizutani T, Minoguchi S, Yamamichi N, Sakurai K, et al. miR-21 Gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J Mol Biol. 2008;378(3):492–504. doi: 10.1016/j.jmb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Li C, Nguyen HT, Zhuang Y, Lin Y, Flemington EK, Guo W, et al. Post-transcriptional up-regulation of miR-21 by type I collagen. Mol Carcinog. 2011;50(7):563–570. doi: 10.1002/mc.20742. [DOI] [PubMed] [Google Scholar]
- 65.Jajoo S, Mukherjea D, Kaur T, Sheehan KE, Sheth S, Borse V, et al. Essential role of NADPH oxidase-dependent reactive oxygen species generation in regulating microRNA-21 expression and function in prostate cancer. Antioxid Redox Signal. 2013;19(16):1863–1876. doi: 10.1089/ars.2012.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muppala S, Mudduluru G, Leupold JH, Buergy D, Sleeman JP, Allgayer H. CD24 induces expression of the oncomir miR-21 via Src, and CD24 and Src are both post-transcriptionally downregulated by the tumor suppressor miR-34a. PLoS One. 2013;8(3):e59563. doi: 10.1371/journal.pone.0059563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mathew R, Hartmuth K, Mohlmann S, Urlaub H, Ficner R, Luhrmann R. Phosphorylation of human PRP28 by SRPK2 is required for integration of the U4/U6-U5 tri-snRNP into the spliceosome. Nat Struct Mol Biol. 2008;15(5):435–443. doi: 10.1038/nsmb.1415. [DOI] [PubMed] [Google Scholar]
- 68.Yin J, Park G, Lee JE, Choi EY, Park JY, Kim TH, et al. DEAD-box RNA helicase DDX23 modulates glioma malignancy via elevating miR-21 biogenesis. Brain. 2015;138(Pt 9):2553–2570. doi: 10.1093/brain/awv167. [DOI] [PubMed] [Google Scholar]
- 69.Liu X, Winey M. The MPS1 family of protein kinases. Annu Rev Biochem. 2012;81:561–585. doi: 10.1146/annurev-biochem-061611-090435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maachani UB, Tandle A, Shankavaram U, Kramp T, Camphausen K. Modulation of miR-21 signaling by MPS1 in human glioblastoma. Oncotarget. 2016;7(33):52912–52927. doi: 10.18632/oncotarget.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loffler D, Brocke-Heidrich K, Pfeifer G, Stocsits C, Hackermuller J, Kretzschmar AK, et al. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood. 2007;110(4):1330–1333. doi: 10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- 72.Ferraro A, Kontos CK, Boni T, Bantounas I, Siakouli D, Kosmidou V, et al. Epigenetic regulation of miR-21 in colorectal cancer: ITGB4 as a novel miR-21 target and a three-gene network (miR-21-ITGBeta4-PDCD4) as predictor of metastatic tumor potential. Epigenetics. 2014;9(1):129–141. doi: 10.4161/epi.26842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou M, Zeng J, Wang X, Wang X, Huang T, Fu Y, et al. Histone demethylase RBP2 decreases miR-21 in blast crisis of chronic myeloid leukemia. Oncotarget. 2015;6(2):1249–1261. doi: 10.18632/oncotarget.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Markou A, Tsaroucha EG, Kaklamanis L, Fotinou M, Georgoulias V, Lianidou ES. Prognostic value of mature microRNA-21 and microRNA-205 overexpression in non-small cell lung cancer by quantitative real-time RT-PCR. Clin Chem. 2008;54(10):1696–1704. doi: 10.1373/clinchem.2007.101741. [DOI] [PubMed] [Google Scholar]
- 75.Cho WC, Chow AS, Au JS. Restoration of tumour suppressor hsa-miR-145 inhibits cancer cell growth in lung adenocarcinoma patients with epidermal growth factor receptor mutation. Eur J Cancer. 2009;45(12):2197–2206. doi: 10.1016/j.ejca.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 76.Xie Y, Todd NW, Liu Z, Zhan M, Fang H, Peng H, et al. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer. 2010;67(2):170–176. doi: 10.1016/j.lungcan.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei J, Gao W, Zhu CJ, Liu YQ, Mei Z, Cheng T, et al. Identification of plasma microRNA-21 as a biomarker for early detection and chemosensitivity of non-small cell lung cancer. Chin J Cancer. 2011;30(6):407–414. doi: 10.5732/cjc.010.10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen H, Zhu F, Liu J, Xu T, Pei D, Wang R, et al. Alteration in Mir-21/PTEN expression modulates gefitinib resistance in non-small cell lung cancer. PLoS One. 2014;9(7):e103305. doi: 10.1371/journal.pone.0103305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geng Q, Fan T, Zhang B, Wang W, Xu Y, Hu H. Five microRNAs in plasma as novel biomarkers for screening of early-stage non-small cell lung cancer. Respir Res. 2014;15:149. doi: 10.1186/s12931-014-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H, Mao F, Shen T, Luo Q, Ding Z, Qian L, et al. Plasma miR-145, miR-20a, miR-21 and miR-223 as novel biomarkers for screening early-stage non-small cell lung cancer. Oncol Lett. 2017;13(2):669–676. doi: 10.3892/ol.2016.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang JS, Li BJ, Lu HW, Chen Y, Lu C, Zhu RX, et al. Serum miR-152, miR-148a, miR-148b, and miR-21 as novel biomarkers in non-small cell lung cancer screening. Tumour Biol. 2015;36(4):3035–3042. doi: 10.1007/s13277-014-2938-1. [DOI] [PubMed] [Google Scholar]
- 82.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids–the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8(8):467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zaharie F, Muresan MS, Petrushev B, Berce C, Gafencu GA, Selicean S, et al. Exosome-carried microRNA-375 inhibits cell progression and dissemination via Bcl-2 blocking in colon cancer. J Gastrointest Liver Dis JGLD. 2015;24(4):435–443. doi: 10.15403/jgld.2014.1121.244.375. [DOI] [PubMed] [Google Scholar]
- 84.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–3799. [PubMed] [Google Scholar]
- 85.Braicu C, Tomuleasa C, Monroig P, Cucuianu A, Berindan-Neagoe I, Calin GA. Exosomes as divine messengers: are they the Hermes of modern molecular oncology? Cell Death Differ. 2015;22(1):34–45. doi: 10.1038/cdd.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 87.Gulei D, Irimie AI, Cojocneanu-Petric R, Schultze JL, Berindan-Neagoe I. Exosomes-small players, big sound. Bioconjugate Chem. 2018;29(3):635–648. doi: 10.1021/acs.bioconjchem.8b00003. [DOI] [PubMed] [Google Scholar]
- 88.Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81. doi: 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 89.Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16(4):415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 90.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 91.Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JE, et al. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT) J Proteome Res. 2009;8(3):1304–1314. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7(3):e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bullock M, Silva A, Kanlikilicer-Unaldi P, Filant J, Rashed M, Sood A, et al. Exosomal non-coding RNAs: diagnostic, prognostic and therapeutic applications in cancer. Non-Coding RNA. 2015;1(1):53. doi: 10.3390/ncrna1010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou X, Wen W, Shan X, Zhu W, Xu J, Guo R, et al. A six-microRNA panel in plasma was identified as a potential biomarker for lung adenocarcinoma diagnosis. Oncotarget. 2017;8(4):6513–6525. doi: 10.18632/oncotarget.14311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Q, Yu Z, Yuan S, Xie W, Li C, Hu Z, et al. Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget. 2017;8(8):13048–13058. doi: 10.18632/oncotarget.14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gallach S, Jantus-Lewintre E, Calabuig-Farinas S, Montaner D, Alonso S, Sirera R, et al. MicroRNA profiling associated with non-small cell lung cancer: next generation sequencing detection, experimental validation, and prognostic value. Oncotarget. 2017;8:56143. doi: 10.18632/oncotarget.18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saito M, Schetter AJ, Mollerup S, Kohno T, Skaug V, Bowman ED, et al. The association of microRNA expression with prognosis and progression in early-stage, non-small cell lung adenocarcinoma: a retrospective analysis of three cohorts. Clin Cancer Res. 2011;17(7):1875–1882. doi: 10.1158/1078-0432.CCR-10-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao W, Lu X, Liu L, Xu J, Feng D, Shu Y. MiRNA-21: a biomarker predictive for platinum-based adjuvant chemotherapy response in patients with non-small cell lung cancer. Cancer Biol Ther. 2012;13(5):330–340. doi: 10.4161/cbt.19073. [DOI] [PubMed] [Google Scholar]
- 99.Tian L, Shan W, Zhang Y, Lv X, Li X, Wei C. Up-regulation of miR-21 expression predicate advanced clinicopathological features and poor prognosis in patients with non-small cell lung cancer. Pathol Oncol Res POR. 2016;22(1):161–167. doi: 10.1007/s12253-015-9979-7. [DOI] [PubMed] [Google Scholar]
- 100.Capodanno A, Boldrini L, Proietti A, Ali G, Pelliccioni S, Niccoli C, et al. Let-7g and miR-21 expression in non-small cell lung cancer: correlation with clinicopathological and molecular features. Int J Oncol. 2013;43(3):765–774. doi: 10.3892/ijo.2013.2003. [DOI] [PubMed] [Google Scholar]
- 101.Li C, Yin Y, Liu X, Xi X, Xue W, Qu Y. Non-small cell lung cancer associated microRNA expression signature: integrated bioinformatics analysis, validation and clinical significance. Oncotarget. 2017;8(15):24564–24578. doi: 10.18632/oncotarget.15596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shen Y, Tang D, Yao R, Wang M, Wang Y, Yao Y, et al. microRNA expression profiles associated with survival, disease progression, and response to gefitinib in completely resected non-small-cell lung cancer with EGFR mutation. Med Oncol. 2013;30(4):750. doi: 10.1007/s12032-013-0750-1. [DOI] [PubMed] [Google Scholar]
- 103.Wang ZX, Bian HB, Wang JR, Cheng ZX, Wang KM, De W. Prognostic significance of serum miRNA-21 expression in human non-small cell lung cancer. J Surg Oncol. 2011;104(7):847–851. doi: 10.1002/jso.22008. [DOI] [PubMed] [Google Scholar]
- 104.Zhao W, Zhao JJ, Zhang L, Xu QF, Zhao YM, Shi XY, et al. Serum miR-21 level: a potential diagnostic and prognostic biomarker for non-small cell lung cancer. Int J Clin Exp Med. 2015;8(9):14759–14763. [PMC free article] [PubMed] [Google Scholar]
- 105.Stenvold H, Donnem T, Andersen S, Al-Saad S, Valkov A, Pedersen MI, et al. High tumor cell expression of microRNA-21 in node positive non-small cell lung cancer predicts a favorable clinical outcome. BMC Clin Pathol. 2014;14(1):9. doi: 10.1186/1472-6890-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dejima H, Iinuma H, Kanaoka R, Matsutani N, Kawamura M. Exosomal microRNA in plasma as a non-invasive biomarker for the recurrence of non-small cell lung cancer. Oncol Lett. 2017;13(3):1256–1263. doi: 10.3892/ol.2017.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Markou A, Sourvinou I, Vorkas PA, Yousef GM, Lianidou E. Clinical evaluation of microRNA expression profiling in non small cell lung cancer. Lung Cancer. 2013;81(3):388–396. doi: 10.1016/j.lungcan.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 108.Liu XG, Zhu WY, Huang YY, Ma LN, Zhou SQ, Wang YK, et al. High expression of serum miR-21 and tumor miR-200c associated with poor prognosis in patients with lung cancer. Med Oncol. 2012;29(2):618–626. doi: 10.1007/s12032-011-9923-y. [DOI] [PubMed] [Google Scholar]
- 109.Zhu J, Qi Y, Wu J, Shi M, Feng J, Chen L. Evaluation of plasma microRNA levels to predict insensitivity of patients with advanced lung adenocarcinomas to pemetrexed and platinum. Oncol Lett. 2016;12(6):4829–4837. doi: 10.3892/ol.2016.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dong J, Zhang Z, Gu T, Xu SF, Dong LX, Li X, et al. The role of microRNA-21 in predicting brain metastases from non-small cell lung cancer. OncoTargets Ther. 2017;10:185–194. doi: 10.2147/OTT.S116619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 112.Tuck AC, Tollervey D. A transcriptome-wide atlas of RNP composition reveals diverse classes of mRNAs and lncRNAs. Cell. 2013;154(5):996–1009. doi: 10.1016/j.cell.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martens-Uzunova ES, Bottcher R, Croce CM, Jenster G, Visakorpi T, Calin GA. Long noncoding RNA in prostate, bladder, and kidney cancer. Eur Urol. 2014;65(6):1140–1151. doi: 10.1016/j.eururo.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 115.Cao L, Chen J, Ou B, Liu C, Zou Y, Chen Q. GAS5 knockdown reduces the chemo-sensitivity of non-small cell lung cancer (NSCLC) cell to cisplatin (DDP) through regulating miR-21/PTEN axis. Biomed Pharmacother. 2017;93:570–579. doi: 10.1016/j.biopha.2017.06.089. [DOI] [PubMed] [Google Scholar]
- 116.Zhou Y, Sheng B, Xia Q, Guan X, Zhang Y. Association of long non-coding RNA H19 and microRNA-21 expression with the biological features and prognosis of non-small cell lung cancer. Cancer Gene Ther. 2017;24:317. doi: 10.1038/cgt.2017.20. [DOI] [PubMed] [Google Scholar]
- 117.Geretto M, Pulliero A, Rosano C, Zhabayeva D, Bersimbaev R, Izzotti A. Resistance to cancer chemotherapeutic drugs is determined by pivotal microRNA regulators. Am J Cancer Res. 2017;7(6):1350–1371. [PMC free article] [PubMed] [Google Scholar]
- 118.Li B, Ren S, Li X, Wang Y, Garfield D, Zhou S, et al. MiR-21 overexpression is associated with acquired resistance of EGFR-TKI in non-small cell lung cancer. Lung Cancer (Amst, Neth) 2014;83(2):146–153. doi: 10.1016/j.lungcan.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 119.Ma Y, Xia H, Liu Y, Li M. Silencing miR-21 sensitizes non-small cell lung cancer A549 cells to ionizing radiation through inhibition of PI3 K/Akt. Biomed Res Int. 2014;2014:617868. doi: 10.1155/2014/617868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jiang Y, Chen X, Tian W, Yin X, Wang J, Yang H. The role of TGF-beta1-miR-21-ROS pathway in bystander responses induced by irradiated non-small-cell lung cancer cells. Br J Cancer. 2014;111(4):772–780. doi: 10.1038/bjc.2014.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pereira DM, Rodrigues PM, Borralho PM, Rodrigues CM. Delivering the promise of miRNA cancer therapeutics. Drug Discovery Today. 2013;18(5–6):282–289. doi: 10.1016/j.drudis.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 122.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tomuleasa C, Braicu C, Irimie A, Craciun L, Berindan-Neagoe I. Nanopharmacology in translational hematology and oncology. Int J Nanomed. 2014;9:3465–3479. doi: 10.2147/IJN.S60488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ananta JS, Paulmurugan R, Massoud TF. Tailored nanoparticle codelivery of antimiR-21 and antimiR-10b augments glioblastoma cell kill by temozolomide: toward a “Personalized” anti-microRNA therapy. Mol Pharm. 2016;13(9):3164–3175. doi: 10.1021/acs.molpharmaceut.6b00388. [DOI] [PubMed] [Google Scholar]
- 125.Song H, Oh B, Choi M, Oh J, Lee M. Delivery of anti-microRNA-21 antisense-oligodeoxynucleotide using amphiphilic peptides for glioblastoma gene therapy. J Drug Target. 2015;23(4):360–370. doi: 10.3109/1061186X.2014.1000336. [DOI] [PubMed] [Google Scholar]
- 126.Aldea MD, Petrushev B, Soritau O, Tomuleasa CI, Berindan-Neagoe I, Filip AG, et al. Metformin plus sorafenib highly impacts temozolomide resistant glioblastoma stem-like cells. J BUON. 2014;19(2):502–511. [PubMed] [Google Scholar]
- 127.Leone E, Morelli E, Di Martino MT, Amodio N, Foresta U, Gulla A, et al. Targeting miR-21 inhibits in vitro and in vivo multiple myeloma cell growth. Clin Cancer Res. 2013;19(8):2096–2106. doi: 10.1158/1078-0432.CCR-12-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shu D, Li H, Shu Y, Xiong G, Carson WE, 3rd, Haque F, et al. Systemic delivery of anti-miRNA for suppression of triple negative breast cancer utilizing RNA nanotechnology. ACS Nano. 2015;9(10):9731–9740. doi: 10.1021/acsnano.5b02471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dong H, Ding L, Yan F, Ji H, Ju H. The use of polyethylenimine-grafted graphene nanoribbon for cellular delivery of locked nucleic acid modified molecular beacon for recognition of microRNA. Biomaterials. 2011;32(15):3875–3882. doi: 10.1016/j.biomaterials.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 130.Zhi F, Dong H, Jia X, Guo W, Lu H, Yang Y, et al. Functionalized graphene oxide mediated adriamycin delivery and miR-21 gene silencing to overcome tumor multidrug resistance in vitro. PLoS One. 2013;8(3):e60034. doi: 10.1371/journal.pone.0060034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Y, Chen Y, Li J, Zhang Z, Huang C, Lian G, et al. Co-delivery of microRNA-21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci. 2017;108(7):1493–1503. doi: 10.1111/cas.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu C, Wen J, Meng Y, Zhang K, Zhu J, Ren Y, et al. Efficient delivery of therapeutic miRNA nanocapsules for tumor suppression. Adv Mater. 2015;27(2):292–297. doi: 10.1002/adma.201403387. [DOI] [PubMed] [Google Scholar]
- 133.Lin Q, Ma L, Liu Z, Yang Z, Wang J, Liu J, et al. Targeting microRNAs: a new action mechanism of natural compounds. Oncotarget. 2017;8(9):15961–15970. doi: 10.18632/oncotarget.14392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sethi S, Li Y, Sarkar FH. Regulating miRNA by natural agents as a new strategy for cancer treatment. Curr Drug Targets. 2013;14(10):1167–1174. doi: 10.2174/13894501113149990189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Braicu C, Pilecki V, Balacescu O, Irimie A, Neagoe IB. The relationships between biological activities and structure of flavan-3-ols. Int J Mol Sci. 2011;12(12):9342–9353. doi: 10.3390/ijms12129342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cojocneanu Petric R, Braicu C, Raduly L, Zanoaga O, Dragos N, Monroig P, et al. Phytochemicals modulate carcinogenic signaling pathways in breast and hormone-related cancers. OncoTargets Ther. 2015;8:2053–2066. doi: 10.2147/OTT.S83597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang J, Zhang C, Hu L, He Y, Shi Z, Tang S, et al. Abnormal expression of miR-21 and miR-95 in cancer stem-like cells is associated with radioresistance of lung cancer. Cancer Invest. 2015;33(5):165–171. doi: 10.3109/07357907.2015.1019676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.