Abstract
Objective
The efficacy of ultrasound-guided stellate ganglion block (SGB) in alleviating postoperative pain remains unclear. This meta-analysis was performed to determine the efficacy of ultrasound-guided SGB in relieving acute postoperative pain in patients undergoing surgery with general anesthesia.
Methods
This systematic review and meta-analysis focused on randomized controlled trials comparing SGB with control or placebo. The primary outcome was the pain score at 24 hours after surgery. A random-effects model was used to calculate the mean difference (MD) or risk ratio with a confidence interval (CI) of 95%.
Results
Eight studies involving 470 patients were included in the meta-analysis. The results revealed that ultrasound-guided SGB was significantly associated with a lower pain score at 24 hours after surgery (MD = −0.74; 95% CI = −1.39, −0.08; I2 = 86%; low evidence) and at 8 hours after surgery (MD = −0.65; 95% CI = −1.03, −0.28; I2 = 29%; moderate evidence).
Conclusion
Ultrasound-guided SGB is effective in alleviating acute postoperative pain. However, considering the limited number of trials performed to date, more large-scale and high-quality randomized controlled trials are required to confirm these findings.
Keywords: Acute postoperative pain, meta-analysis, stellate ganglion block, surgery, randomized controlled trial, ultrasound guidance, postoperative pain
Introduction
Acute postoperative pain is one of the most common complaints after surgery, with nearly 50% of patients experiencing moderate to severe pain within the first 24 hours postoperatively.1,2 Inadequate management of postoperative pain may increase the risk of autonomic instability, poor mobilization, chronic pain, and a longer hospital stay.3,4
Traditionally, opioids have been used as the primary pharmacological treatment for postoperative pain, although high doses of opioids can lead to postoperative nausea and vomiting, pruritus, and respiratory depression. 5 Multimodal analgesia techniques, such as regional nerve blocks and intravenous patient-controlled analgesia (PCA), have been proven effective in minimizing the stress response, pain intensity, and opioid consumption.
The stellate ganglion, also referred to as the cervicothoracic ganglion, is a sympathetic ganglion formed upon fusion of the inferior cervical ganglion with the first thoracic ganglion. 6 The stellate ganglion delivers sympathetic innervation to the upper extremities, head, neck, and heart. 7 Ultrasound-guided stellate ganglion block (SGB) is used to treat chronic neuropathic pain of the upper extremity, complex regional pain syndrome, and postherpetic neuralgia.8–10 However, the efficacy of ultrasound-guided SGB in relieving acute postoperative pain remains uncertain because of conflicting study results.11–17 In this context, the present meta-analysis was conducted to evaluate the efficacy of ultrasound-guided SGB in relieving acute postoperative pain after surgery under general anesthesia. The study hypothesis was that ultrasound-guided SGB can effectively alleviate acute postoperative pain.
Methods
This meta-analysis was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions 18 and the updated PRISMA 2020 statement guideline. 19 The study was prospective in nature and registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY202350022).
Literature search and participant selection criteria
PubMed, the Cochrane Library, and EMBASE were searched from their respective dates of inception until 6 April 2023, with the language restricted to English. The following queries were used in the PubMed search: (stellate ganglion OR cervicothoracic ganglion OR cervicothoracic ganglia OR stellate ganglia) AND (pain OR analgesia). The reference lists of the retrieved articles were also examined to identify other potentially eligible trials for inclusion.
Trials fulfilling the following criteria were included in the study: (1) population: adult patients undergoing surgery under general anesthesia, (2) intervention: ultrasound-guided SGB prior to surgery, (3) comparison: control or placebo, (4) design: randomized controlled trial (RCT), and (5) outcomes: acute pain score after surgery (visual analogue scale or numerical rating scale). The primary outcome used in the meta-analysis was the pain score at 24 hours after surgery. The secondary outcomes were acute postoperative pain at other time points (0, 2, 4, 6, 8, and 12 hours after surgery) and postoperative nausea and vomiting.
Data extraction and quality assessment
The data were extracted by Yan Zhao and confirmed by Xiangli Xiao. The following extracted data were systematically recorded and arranged in a pre-existing Excel spreadsheet: first author, year of publication, population, number of patients, American Society of Anesthesiologists physical status, surgical procedure, intervention (specific type and concentration of local anesthetic, vertebral puncture), comparison, and outcomes. For reports with only graphical data, the GetData Graph Digitizer was employed to interpolate the data. 20 The median and interquartile range were approximated to the mean and respective standard deviation using the following formula: median = mean and standard deviation = quartile distance/1.35. 21
The methodological quality of the trials included in the present meta-analysis was assessed using a risk-of-bias table. 22 Each item in the table was categorized as having a low, unclear, or high risk of bias based on sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other potential sources of bias.
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool was used to evaluate the certainty of the main results of all included studies. 23 The evidence quality was classified as very low, low, moderate, or high based on the following factors: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Any uncertainty that emerged was resolved by consensus.
Statistical analysis
The mean difference (MD) with 95% confidence interval (CI) was estimated for continuous outcomes, and the relative risk with 95% CI was estimated for dichotomous outcomes. A random-effects model was adopted to obtain the most conservative effects estimate. The Cochrane Q test and I2 statistic were adopted to calculate the heterogeneity across the included trials, and p < 0.1 or I2 > 50% indicated significant heterogeneity.24,25 Further, subgroup analyses involving stratification based on the surgical procedure were performed to evaluate the robustness of the results. Publication bias was assessed using funnel plots and Begg’s and Egger’s tests.26,27 Review Manager Version 5.4 (Nordic Cochrane Centre, Copenhagen, Denmark) and Stata Version 12.0 (StataCorp LLC, College Station, TX, USA) were employed to perform the meta-analyses, considering p < 0.05 to be statistically significant.
Results
Study identification and characteristics
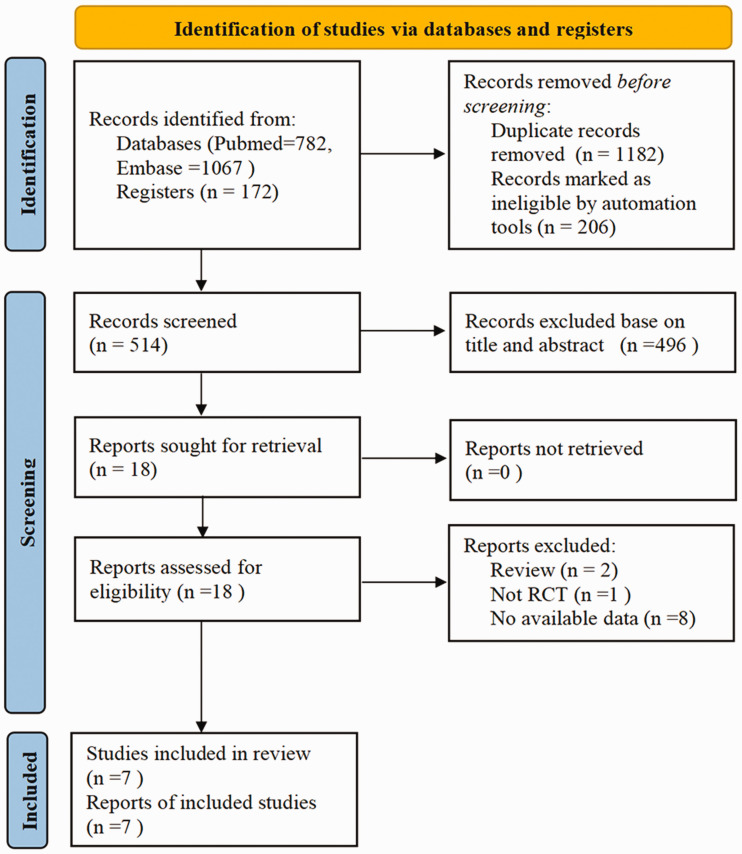
In total, 2021 articles were retrieved in the initial comprehensive search. Among these articles, 1182 were excluded because of duplication and 496 were excluded after screening the titles and abstracts. Finally, seven RCTs fulfilling all the inclusion criteria were retained for the meta-analysis.11–17 The entire selection process is illustrated in Figure 1.
Figure 1.
PRISMA flow diagram of study selection. RCT, randomized controlled trial.
The trials included in the present study were published between the years 2004 and 2022, and their sample sizes ranged from 30 to 90. The trials investigated the effects of SGB in patients who underwent various kinds of surgeries: breast surgeries in three studies,15–17 abdominal surgeries in two studies,13,14 and upper limb and thoracoscopic surgeries in the remaining studies.11,12 Five studies compared SGB with placebo,11,13,14,17,18 and two studies compared SGB with multimodal systemic analgesia or PCA.12,15 Two studies used lidocaine for SGB,11,14 two studies used bupivacaine,12,15 and three studies used ropivacaine.13,16,17 The concentration of the local anesthetic drugs differed across the included studies. The characteristics of the RCTs included in the present study are summarized in Table 1.
Table 1.
Characteristics of included studies.
| Study | Population | ASA physical status | Surgical procedure | Patients (n) | Stellate ganglion block | Comparison | Outcomes |
|---|---|---|---|---|---|---|---|
| Kumar et al. 11 (2014) | Adults aged 18–60 years | I–II | Upper limb fracture surgery | 30 | Injection of lidocaine (3 mL) at the level of the C7 vertebra before surgery | 0.9% Saline (3 mL) | Postoperative pain score at 0, 2, 4, 6, 8, 12, 24, and 48 hours |
| Choi et al. 12 (2015) | Adults | NA | Arthroscopic rotator cuff repair | 40 | Injection of 0.375% levobupivacaine (4 mL) at the level of the C7 vertebra before surgery | Postoperative intravenous PCA | Postoperative pain score at 0, 2, 4, 6, 8, 12, 24, and 48 hours |
| Wu et al. 13 (2020) | Adults | I–III | Video-assisted thoracic surgery | 87 | Injection of 0.5% ropivacaine (5 mL) at the level of the C7 vertebra before surgery | No injection | Postoperative pain score at 0, 2, 4, 6, 24, and 48 hours; PONV |
| Rahimzadeh et al. 14 (2020) | Adults aged 18–50 years | I–II | Laparoscopic gynecologic surgery | 40 | Injection of 1% lidocaine (10 mL) | Distilled water (10 mL) | Postoperative pain score at 24 hours |
| Salman et al. 15 (2021) | Adults aged 18–75 years | II | Mastectomy with axillary dissection | 80 | Injection of 0.5% bupivacaine (5 mL) at the level of the C6 vertebra before surgery + multimodal systemic analgesia | Multimodal systemic analgesia | Postoperative pain score at 24 hours |
| Yang et al. 16 (2023) | Women aged 18–70 years | I–II | Breast cancer surgery | 60 | Injection of 0.25% ropivacaine (6 mL) at the level of the C6 vertebra before surgery | No injection | Postoperative pain score at 0, 2, 4, 8, and 24 hours; recovery time of anal exhaust |
| Yang et al. 17 (2022) | Women aged 18–65 years | I–II | Modified radical mastectomy | 48 | Injection of 0.5% ropivacaine (3 mL) at the level of the C6 vertebra before surgery | No injection | Postoperative pain score at 24 and 48 hours |
ASA, American society of Anesthesiologists; PCA, patient-controlled analgesia; PONV, postoperative nausea and vomiting; NA, not reported.
Acute postoperative pain scores
Pain score at 24 hours after surgery
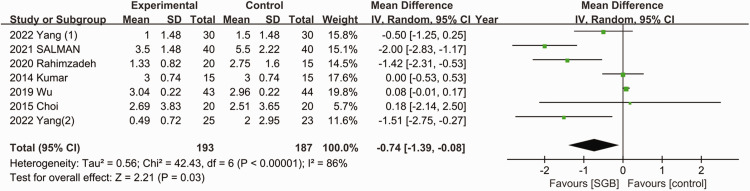
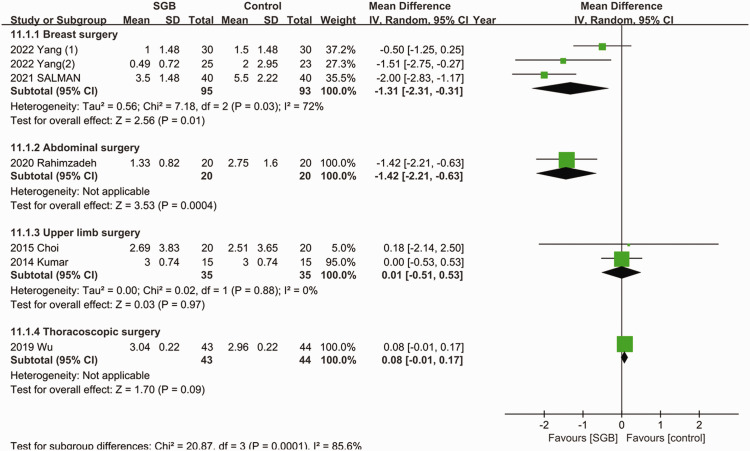
As presented in Figure 2, all seven studies reported the postoperative pain scores.11–17 Ultrasound-guided SGB was associated with a lower pain score at 24 hours after surgery (MD = −0.74; 95% CI = −1.39, −0.08; p = 0.03; I2 = 86%). The subgroup analysis (Figure 3) indicated that the use of ultrasound-guided SGB alone reduced postoperative pain in abdominal surgeries (MD = −1.42; 95% CI = −2.21, −0.63; p = 0.009; I2 = 63%) and breast surgeries (MD = −1.31; 95% CI = −2.31, −0.31; p = 0.01; I2 = 72%). Among patients who underwent upper limb and thoracoscopic surgeries, the postoperative pain scores were not lower in the SGB group.
Figure 2.
Forest plot of pain score at 24 hours after surgery. SD, standard deviation; CI, confidence interval; SGB, stellate ganglion block.
Figure 3.
Forest plot for subgroup analysis of pain score at 24 hours after surgery. SD, standard deviation; CI, confidence interval; SGB, stellate ganglion block.
Pain score at other time points after surgery
No difference in pain scores was noted between the SGB group and the control group at 0, 2, 4, 6, and 12 hours after surgery, although a trend toward lower pain scores was observed at 2 hours after surgery (MD = −0.22; 95% CI = −0.54, 0.11) and 6 hours after surgery (MD = −0.54; 95% CI = −0.19, 0.12) (Supplementary Figures 1 and 2). Ultrasound-guided SGB was associated with a lower pain score at 8 hours after surgery (MD = −0.65; 95% CI = −1.03, −0.28; p = 0.01) without significant heterogeneity (I2 = 29%).
Quality assessment and publication bias
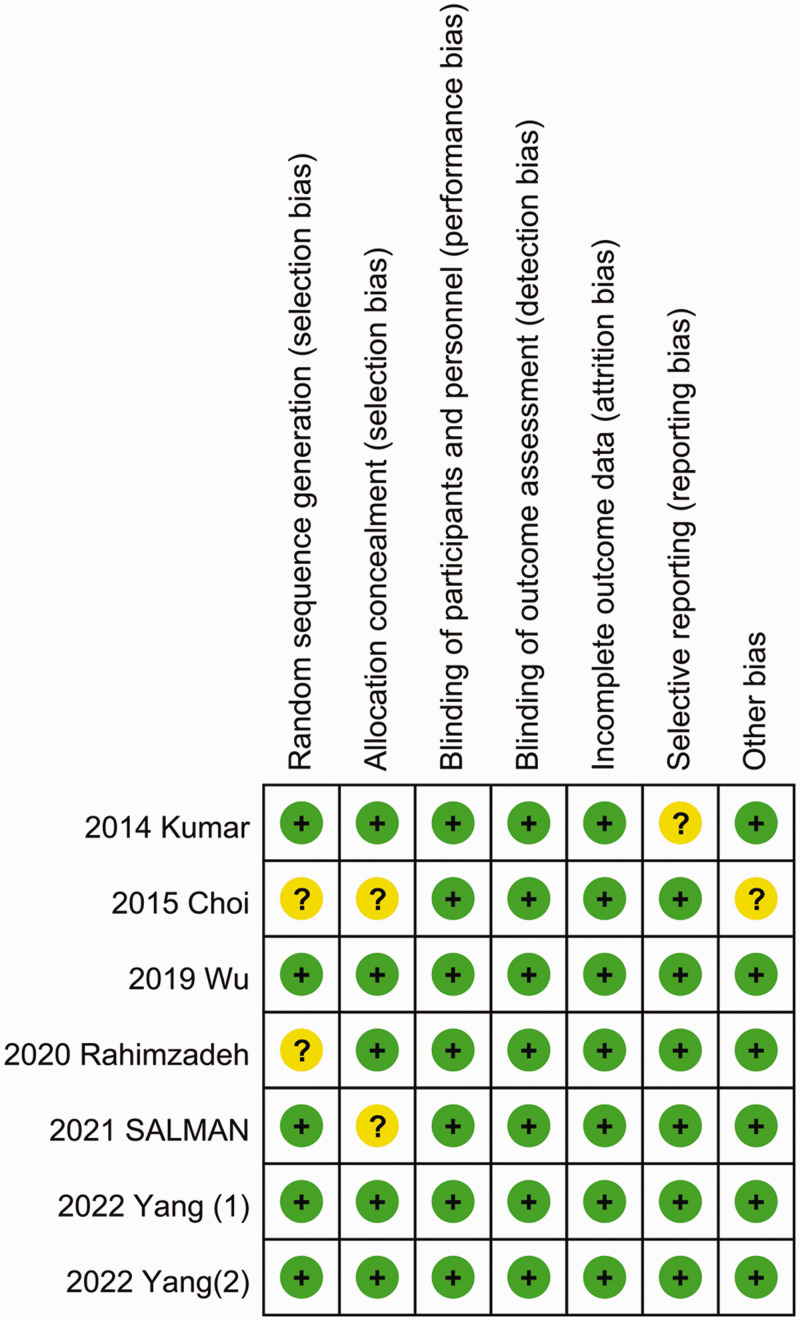
The quality assessment results revealed an unclear risk of bias for four of the included studies 11,12,14,15 and a low risk of bias for three studies.13,16,17 The risk-of-bias assessment results are summarized in Figure 4. Table 2 provides an overview of the level of certainty for the primary findings. The level of evidence of all studies evaluated using the GRADE tool was classified as very low to moderate. Specifically, the pain scores at 0, 12, and 24 hours after surgery were categorized as low, while the pain scores at 8 hours after surgery were classified as moderate.
Figure 4.
Risk-of-bias summary.
Table 2.
GRADE profile.
| Outcomes | Numbers of participants (studies) | Certainty of the evidence (GRADE) | Effect |
|
|---|---|---|---|---|
| Relative effect(95% CI) | Absolute effect(95% CI) | |||
| Pain score at 24 hours after surgery | 380(6 RCTs) | ⨁⨁◯◯Low a ,b | – | MD 0.74 lower(1.39 lower to 0.08 lower) |
| Pain score at 0 hours after surgery | 130(3 RCTs) | ⨁⨁◯◯Low a ,c | – | MD 0.01 higher(0.39 lower to 0.40 higher) |
| Pain score at 2 hours after surgery | 217(4 RCTs) | ⨁◯◯◯Very low a ,b,c | – | MD 0.22 lower(0.54 lower to 0.11 higher) |
| Pain score at 4 hours after surgery | 217(4 RCTs) | ⨁◯◯◯Very low a ,b,c | – | MD 0.05 higher(0.85 lower to 0.95 higher) |
| Pain score at 6 hours after surgery | 205(4 RCTs) | ⨁◯◯◯Very low b ,c | – | MD 0.54 lower(1.19 lower to 0.12 higher) |
| Pain score at 8 hours after surgery | 130(3 RCTs) | ⨁⨁⨁◯Moderate a | – | MD 0.65 lower(1.03 lower to 0.28 lower) |
| Pain score at 12 hours after surgery | 70(2 RCTs) | ⨁⨁◯◯Low a ,c | – | MD 1.00 higher(0.04 higher to 2.04 higher) |
Some of the included trials had an unclear risk of bias.
Heterogeneity across trials was observed.
The included trials had wide 95% CIs.
CI, confidence interval; MD, mean difference; RCT, randomized controlled trial; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation.
No publication bias was detected by visual inspection of the funnel plots or by the formal statistical tests (Begg’s test and Egger’s test) (Supplementary Figures 3 and 4).
Discussion
The present meta-analysis indicated, with a moderate to low level of certainty, that ultrasound-guided SGB is associated with alleviation of acute postoperative pain at 8 and 24 hours after surgery.
Previous studies have indicated that ultrasound-guided SGB reduces the intensity of pain by inhibiting the sympathetic nervous system.8,28 Sympathetic excitation may lead to catecholamine release and exacerbate the inflammatory response during the perioperative period. 29 Inflammation and sympathetic nervous system activation are critical contributors to postoperative pain. 30 According to recent theories, the pain relief experienced upon ultrasound-guided SGB is linked to the regulation of nerve growth factor (NGF), which plays a crucial role in several signaling pathways associated with acute stress. 31 In addition, NGF activates sympathetic nerves and increases the release of norepinephrine, which promotes sensitization of the peripheral nociceptors and increases the perception of pain. 32 Ultrasound-guided SGB blocks the injurious afferent sympathetic pathways, thereby effectively reducing the NGF level and decreasing sympathetic activity, resulting in pain reduction. 33
Several previous studies showed that ultrasound-guided SGB was associated with reduced postoperative pain scores in patients undergoing upper limb surgery.11,30,34 However, Choi et al. 12 indicated that ultrasound-guided SGB was not associated with a reduction of the postoperative pain score at any time point after arthroscopic shoulder surgery. One possible explanation for this is that the intensity of pain experienced during arthroscopic shoulder surgery is not as high as that experienced after open upper limb surgery. In addition, tramadol was administered intravenously when the patients’ visual analogue scale score was >4, 12 which might have interfered with the postoperative pain score to a certain extent, and minor differences might not have been detected in the small patient population. Similarly, in another study included in the present meta-analysis (the study by Wu et al. 13 ), ultrasound-guided SGB did not reduce the acute postoperative pain score in patients undergoing thoracoscopic surgery. This might have occurred because the small incisions used in thoracoscopic surgery and the routine use of PCA after the surgery decreased the difference in the pain scores between the SGB group and the control group.
Postoperative pain alleviation is of great concern to clinicians. In the present study, therefore, we sought to provide further useful information to clinicians in their attempts to improve the surgical and anesthetic techniques used for postoperative pain relief. Ultrasound-guided SGB is superior to traditional nerve blocks because it does not produce motor or sensory blockade, thereby enabling the surgeon to assess the motor function immediately after surgery. 11 A small amount of injectate is sufficient for a successful block. According to a previous study, 4 mL of 0.2% ropivacaine used in ultrasound-guided SGB was effective in maintaining good analgesia. 35 All studies included in the present meta-analysis used low-dose local anesthetics, and no local anesthetic toxicity was recorded. However, anatomic variation and incorrect identification of relevant structures can lead to failure of the block. 36 Moreover, the stellate ganglion is situated close to the inferior thyroid artery, carotid artery, and vertebral artery, necessitating vigilance on the part of clinicians to avoid intravascular injection and post-pharyngeal hematoma. 37 However, these complications were not reported in the studies included in the present meta-analysis. In addition, enhancing clinicians’ proficiency is crucial to minimizing errors and complications during the ultrasound-guided SGB procedure. Whether SGB can replace or serve as an adjunct to certain traditional nerve blocks, such as the brachial plexus block, is a valuable focus of further research. Although a few studies have addressed this question, it seems unlikely that SGB can entirely supplant the brachial plexus block. This is because the efficacy of SGB in alleviating postoperative pain through sympathetic nerve blockade may be limited. Our meta-analysis suggests that SGB may only reduce postoperative pain in some surgeries. Because of the limited number of studies and their small sample sizes, future high-quality research is needed to better understand the role of SGB in various surgical settings.
To the best of our knowledge, the present meta-analysis is the first to evaluate the efficacy of ultrasound-guided SGB in relieving acute postoperative pain. The meta-analysis was conducted strictly in accordance with the Cochrane Handbook and the PRISMA statement. However, a few limitations must be noted. First, significant heterogeneity was detected across the included studies. This heterogeneity can be attributed to the different surgeries performed and the diverse types, concentrations, and dosages of local anesthetics used in these studies. The differences in the surgical duration and the proficiency of the SGB procedure could also have contributed to the heterogeneity. Second, although our analysis showed statistically significant reductions in pain scores at 24 hours after surgery, the mean difference was small. Considering the small sample size in most of the trials included in the present meta-analysis, the findings of the present study must be interpreted with caution. Further large-scale and high-quality research is needed to confirm the analgesic effect of SGB. Third, the GRADE analysis revealed that most outcomes had a very low to moderate level of evidence, and the risk-of-bias analysis showed that more than half of the included studies had unclear risk. Finally, the effect of ultrasound-guided SGB in terms of reducing the consumption of opioids and the safety of using SGB were not evaluated in the present meta-analysis because of the unavailability of sufficient relevant data across the included studies.
Conclusion
Ultrasound-guided SGB is effective in alleviating acute postoperative pain. However, considering the low evidence of outcomes and limited number of trials, more studies are warranted to definitively determine the analgesic effect of ultrasound-guided SGB on patients undergoing surgery under general anesthesia.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605241252237 for Efficacy of ultrasound-guided stellate ganglion block in relieving acute postoperative pain: a systematic review and meta-analysis by Yan Zhao and Xiangli Xiao in Journal of International Medical Research
Authors’ contributions: YZ: Study conception and design, data acquisition and analysis, drafting of the article, and critical revision of the article for important intellectual content;
XX: Study conception and design, data analysis and confirmation, and revision of the article.
The authors declare that there is no conflict of interest.
Funding: The study was supported by the Beihai City Science and Technology Planning Project (201995060).
ORCID iD: Xiangli Xiao https://orcid.org/0009-0008-6180-3321
Availability of data and materials
All data relevant to the study are included in the article or have been uploaded as supplementary information.
Consent for publication
Consent for publication was not applicable because of the nature of this study (systematic review and meta-analysis).
Ethics approval and consent to participate
Ethics approval and consent to participate were not applicable because of the nature of this study (systematic review and meta-analysis).
References
- 1.Gerbershagen HJ, Aduckathil S, Van Wijck AJ, et al. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013; 118: 934–944. [DOI] [PubMed] [Google Scholar]
- 2.Walker EMK, Bell M, Cook TM, et al. Patient reported outcome of adult perioperative anaesthesia in the United Kingdom: a cross-sectional observational study. Br J Anaesth 2016; 117: 758–766. [DOI] [PubMed] [Google Scholar]
- 3.McDonnell A, Nicholl J, Read SM. Acute pain teams and the management of postoperative pain: a systematic review and meta-analysis. J Adv Nurs 2003; 41: 261–273. [DOI] [PubMed] [Google Scholar]
- 4.Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet 2019; 393: 1537–1546. [DOI] [PubMed] [Google Scholar]
- 5.De Boer HD, Detriche O, Forget P. Opioid-related side effects: postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol 2017; 31: 499–504. [DOI] [PubMed] [Google Scholar]
- 6.Fudim M, Boortz-Marx R, Ganesh A, et al. Stellate ganglion blockade for the treatment of refractory ventricular arrhythmias: a systematic review and meta-analysis. Cardiovasc Electrophysiol 2017; 28: 1460–1467. [DOI] [PubMed] [Google Scholar]
- 7.Wen S, Chen L, Wang TH, et al. The efficacy of ultrasound-guided stellate ganglion block in alleviating postoperative pain and ventricular arrhythmias and its application prospects. Neurol Sci 2021; 42: 3121–3133. [DOI] [PubMed] [Google Scholar]
- 8.Yoo Y, Lee CS, Kim YC, et al. A randomized comparison between 4, 6 and 8 mL of local anesthetic for ultrasound-guided stellate ganglion block. J Clin Med 2019; 8: 1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makharita MY, Amr YM, El-Bayoumy Y. Effect of early stellate ganglion blockade for facial pain from acute herpes zoster and incidence of postherpetic neuralgia. Pain Physician 2012; 15: 467–474. [PubMed] [Google Scholar]
- 10.Park JH, Min YS, Chun SM, et al. Effects of stellate ganglion block on breast cancer-related lymphedema: comparison of various injectates. Pain Physician 2015; 18: 93–99. [PubMed] [Google Scholar]
- 11.Kumar N, Thapa D, Gombar S, et al. Analgesic efficacy of pre-operative stellate ganglion block on postoperative pain relief: a randomised controlled trial. Anaesthesia 2014; 69: 954–960. [DOI] [PubMed] [Google Scholar]
- 12.Choi EM, Kim EM, Chung MH, et al. Effects of ultrasound-guided stellate ganglion block on acute pain after arthroscopic shoulder surgery. Pain Physician 2015; 18: E379–388. [PubMed] [Google Scholar]
- 13.Wu CN, Wu XH, Yu DN, et al. A single-dose of stellate ganglion block for the prevention of postoperative dysrhythmias in patients undergoing thoracoscopic surgery for cancer: a randomised controlled double-blind trial. Eur J Anaesthesiol 2020; 37: 323–331. [DOI] [PubMed] [Google Scholar]
- 14.Rahimzadeh P, Mahmoudi K, Khodaverdi M, et al. Effects of ultrasound guided ganglion stellate blockade on intraoperative and postoperative hemodynamic responses in laparoscopic gynecologic surgery. Wideochir Inne Tech Maloinwazyjne 2020; 15: 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salman AS, Abbas DN, Elrawas MM, et al. Postmastectomy pain syndrome after preoperative stellate ganglion block: a randomized controlled trial. Minerva Anestesiol 2021; 87: 786–793. [DOI] [PubMed] [Google Scholar]
- 16.Yang RZ, Li YZ, Liang M, et al. Stellate ganglion block improves postoperative sleep quality and analgesia in patients with breast cancer: a randomized controlled trial. Pain Ther 2023; 12: 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Wu Q, Wang H, et al. Effects of ultrasound-guided stellate ganglion block on postoperative quality of recovery in patients undergoing breast cancer surgery: a randomized controlled clinical trial. J Healthc Eng 2022; 2022: 7628183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
- 19.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giang HTN, Ahmed AM, Fala RY, et al. Methodological steps used by authors of systematic reviews and meta-analyses of clinical trials: a cross-sectional study. BMC Med Res Methodol 2019; 19: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gøtzsche PC, Cochrane Bias Methods Group et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imani F, Hemati K, Rahimzadeh P, et al. Effectiveness of stellate ganglion block under fluoroscopy or ultrasound guidance in upper extremity CRPS. J Clin Diagn Res 2016; 10: UC09–UC12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie A, Zhang X, Ju F, et al. Effects of the ultrasound-guided stellate ganglion block on hemodynamics. Comput Intell Neurosci 2022; 2022: 2056969. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.McDonnell JG, Finnerty O, Laffey JG. Stellate ganglion blockade for analgesia following upper limb surgery. Anaesthesia 2011; 66: 611–614. [DOI] [PubMed] [Google Scholar]
- 31.Uchida K, Tateda T, Hino H. Novel mechanism of action hypothesized for stellate ganglion block related to melatonin. Med Hypotheses 2002; 59: 446–449. [DOI] [PubMed] [Google Scholar]
- 32.Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep 2015; 17: 599. [DOI] [PubMed] [Google Scholar]
- 33.Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med 2016; 16: 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajagopalan V, Chouhan RS, Pandia MP, et al. Effect of stellate ganglion block on intraoperative propofol and fentanyl consumption in patients with complex regional pain syndrome undergoing surgical repair of brachial plexus injury: a randomized, double-blind, placebo-controlled trial. Neurol India 2020; 68: 617–623. [DOI] [PubMed] [Google Scholar]
- 35.Jung G, Kim BS, Shin KB, et al. The optimal volume of 0.2% ropivacaine required for an ultrasound-guided stellate ganglion block. Korean J Anesthesiol 2011; 60: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soneji N, Peng PW. Ultrasound-guided pain interventions – a review of techniques for peripheral nerves. Korean J Pain 2013; 26: 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narouze S. Ultrasound-guided stellate ganglion block: safety and efficacy. Curr Pain Headache Rep 2014; 18: 424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605241252237 for Efficacy of ultrasound-guided stellate ganglion block in relieving acute postoperative pain: a systematic review and meta-analysis by Yan Zhao and Xiangli Xiao in Journal of International Medical Research
Data Availability Statement
All data relevant to the study are included in the article or have been uploaded as supplementary information.