Abstract
Despite the recent promising results of clinical trials using human pluripotent stem cell (hPSC)-based cell therapies for age-related macular degeneration (AMD), the risk of teratoma formation resulting from residual undifferentiated hPSCs remains a serious and critical hurdle for broader clinical implementation. To mitigate the tumorigenic risk of hPSC-based cell therapy, a variety of approaches have been examined to ablate the undifferentiated hPSCs based on the unique molecular properties of hPSCs. In the present review, we offer a brief overview of recent attempts at selective elimination of undifferentiated hPSCs to decrease the risk of teratoma formation in hPSC-based cell therapy.
Keywords: Teratoma, Human pluripotent stem cells, Selective cell death, Apoptosis, Safe stem cell therapy
Introduction
The first clinical trial of human embryonic stem cell (hESC)-based cell therapy was approved by the FDA on January 23, 2009 and was launched by the Geron Corporation, a biotechnology company in the United States. The clinical trial was performed with oligodendrocyte progenitors (GRNOPC1) derived from hESCs to treat acute spinal cord injury patients through remyelination of damaged axons [1].
After the sudden discontinuation of the clinical trial for undisclosed reasons, the safety and efficacy of hESC-based cell therapy have been actively discussed in a number of articles [2–5]. Since the recent outstanding clinical results of retinal pigment epithelial (RPE) cells derived from hESCs used to regenerate vision following age-related macular degeneration (AMD) or Stargardt’s disease [6, 7], enormous efforts have been made to examine the efficacy and safety of this approach in phase I/II trials. At this time, ten hESC-based clinical trials are being undertaken worldwide for treatment of AMD or Stargardt’s disease, type I diabetes mellitus, severe heart failure, and spinal cord injury [8, 9].
The pluripotency and active cell proliferation capacity (or high clonogenic capacity with telomerase activity) are incomparable technical advantages of human pluripotent stem cells (hPSCs, which include hESCs and human induced pluripotent stem cells, iPSCs) over adult stem cells in terms of not only pluripotent differentiation potential but also a theoretically unlimited supply of desirable cell types. Therefore, hPSCs have been considered as a promising cell source for regenerative medicine. However, two characteristics of hPSCs (i.e., pluripotency and high clonogenic capacity with telomerase activity) are responsible for the formation of teratomas, benign tumors composed of three germ layers, which has been used to determine the ‘pluripotency’ of PSCs in vivo [10–12]. Ironically, the teratoma-forming capacity of the hPSCs that remain undifferentiated after the differentiation process (therefore, tumorigenic hPSCs) due to these unique characteristics contributes to one of the major hurdles to broader clinical implementation of hPSC-based cell therapy [5, 13].
It is notable that none of the preclinical studies with differentiated cells derived from hPSCs in mouse models indicate teratoma formation, although it is generally accepted that teratomas frequently develop after transplantation of cells derived from mouse ESCs into mouse models, regardless of the cell sorting and long-term culture techniques used after differentiation to minimize the possible engraftment of residual undifferentiated mESCs [14–16]. This difference in tumorigenicity between human and mouse PSCs that occurs after the engraftment of the cells derived from human or mouse ESCs in a mouse model results from host-dependent tumorigenesis. For example, as few as 500 mESCs can cause a 100% teratoma development rate in a mouse model (3 out of 3); however, 80,000 mESCs have only a 9% teratoma development rate in a rat model (2 out of 22) [17]. Importantly, it was also shown that a minimum of 10,000 hESCs are required to form a teratoma in a mouse model [18].
For hPSC-based cell therapy in human subjects, of which the tumorigenic effect cannot be determined using different species (such as mouse or rat), the risk of teratoma formation remains an important technical issue that must be fully resolved before the expansion of its clinical application [5]. Therefore, a variety of approaches using antibodies, suicide genes, stem cell-killing agents (defined as ‘stemotoxic agents’), and hypothetical ‘stem cells without tumorigenicity’ (stem cells that do not form a tumor in vivo) have been previously proposed to overcome this hurdle [19]. Since then, diverse approaches to this end have been actively studied, and one ‘stem cell-killing agent’ is even well defined for practical use [20]. Although there are already a few excellent review articles that summarize the strategies used to inhibit teratoma formation [21, 22], we aim to accentuate the important safety concerns of hPSC-based cell therapy and summarize the wide range of recent advances in preventing teratoma formation, such as the use of (1) small molecule-based selective elimination (2) genetic approach to introduce a suicide gene or miRNA switch, (3) antibodies targeting a surface-specific antigen (or antibody-guided toxins), (4) phototoxic approach, and (5) live detection and quantification of the residual hPSCs.
High mitochondrial priming in hPSCs
One of the distinct characteristics of hPSCs is their high susceptibility to DNA damage [23]. When differentiated somatic cells are challenged with genotoxic stimuli, such as ultraviolet light, ionizing radiation, or chemotherapeutic reagents, the cell cycle is arrested at G1/S to allow DNA damage repair before entering the S phase, unless the DNA damage is too severe to be repaired and triggers cell death [24]. The cell cycle checkpoint and DNA damage repair system are important for ensuring the genomic integrity of somatic cells. However, unlike in somatic cells, cell cycle checkpoints are absent or attenuated in mouse and human ESCs [25, 26]. Instead of activating cell cycle arrest, hESCs commit cell death even under low genotoxic stress [27]. High sensitivity to DNA damage and rapid apoptosis after even low-damage insults is some of the unique features of hESCs that allow them to avoid deleterious genomic mutations in their differentiated progeny [23].
Recent studies demonstrate that the high sensitivity to DNA damage in hESCs results from high induction of the mitochondrial cell death mechanism (referred to as ‘high mitochondrial priming’), which is mediated by cytoplasmic p53 [28] or prompt mitochondrial translocation of constitutively active BAX localized at the Golgi complex [29]. The details of mitochondria-dependent cell death in hESCs were extensively reviewed recently [30]. The high susceptibility of hPSCs to mitochondrial cell death compared with differentiated somatic cells is closely associated with the induction of selective cell death of hPSCs by a variety of small molecule-based approaches.
Small molecule-based selective elimination
Small molecules that induce selective cell death of hESCs to inhibit teratoma formation were first reported in 2004. The ceramide analog N-oleoyl serinol (S18) was shown to eliminate mouse and human ESCs through apoptosis and promote neural differentiation [31]. Since then, a variety of small molecules have been demonstrated to induce ESC-specific cell death and inhibit (or reduce) teratoma formation.
Activating ‘high mitochondrial priming’ for selective cell death of hESCs
As mentioned above, the high susceptibility of hESCs to cell death is due to the active mitochondria-dependent cell death mechanism that results from cytoplasmic p53 [28] (or mitochondrial translocation of p53 [32]) or rapid mitochondrial translocation of constitutively active BAX [29]. Additionally, pro-apoptotic proteins are highly upregulated, while a few anti-apoptotic proteins such as baculoviral IAP repeat-containing 5 (BIRC5) [32, 33] and B-cell CLL/lymphoma 10 (BCL10) [32] are expressed to maintain balance (or promote survival) [34]. Therefore, suppression or inhibition of hESC-specific anti-apoptotic proteins, such as BIRC5, with YM155 or quercetin (QC) was able to induce selective cell death of hPSCs while sparing differentiated smooth muscle cells and dopaminergic neurons [32]. Later, YM155 treatment was found to be more highly selective in eradicating teratoma formation by human iPSCs while sparing CD34+ hematopoietic stem cells (HSCs) in mouse models than other genetic approaches using a chemically inducible suicide gene system [35].
Similarly, a combination of purvalanol A (Cdk1 inhibitor) and Taxol was used to suppress the expression of survivin (the protein encoded by BIRC5) to induce cell death of hESCs [33]. However, inhibition of survivin, whose expression is normally high in a variety of cancers [32, 33], for selective cell death of hPSCs could not be used to isolate HSCs because survivin is required for HSC survival [36].
A recent study also demonstrated that QC treatment produces reactive oxygen species (ROS) in hESCs but not in human dermal fibroblasts (hDFs), leading to activation of the mitochondrial cell death pathway through cyclophilin D, which is highly expressed in the mitochondria of hESCs [37].
Alternatively, etoposide treatment, which leads to DNA damage and activates ‘high mitochondrial priming’ [29], was sufficient to ‘purge the teratoma risk’ of mouse ESCs [38]. Recent studies from Hurskey N. E. et al. revealed that inhibition of CDK1 with small molecules, such as purvalanol A, Ro-3306, or dinaciclib, induces DNA damage and achieves selective cell death of mouse and human ESCs by inhibiting the anti-apoptotic molecule MCL1 in ESCs [39]. Likewise, PluriSIn#2, one of 15 pluripotent cell-specific inhibitors (PluriSIns), which were identified by Dr. Nissim Benvenisty’s group through high-throughput screening [40], induced selective cell death by suppressing the expression of topoisomerase II, which is important for maintaining DNA integrity [41]. It is also noteworthy that YM155, a known BIRC5 suppressant [42], which was used to induce hPSC cell death [32], was shown to induce DNA damage in cancer cells expressing high levels of solute carrier family 35 member F2 (SLC35F2) [43]. In line with this, SLC35F2 was previously shown to be a specific surface marker of hESCs [44], suggesting that the high sensitivity of hPSCs to YM155-dependent cell death [32] may result from the DNA damage-mediated mitochondrial priming occurring in hPSCs. If so, the use of YM155 to selectively eliminate hPSCs would be only applicable when the differentiated cells had low levels of SLC35F2.
Inhibiting the specific metabolism of hPSCs
PluriSIn#1, identified as the most selective compound for achieving hPSC-specific cell death among the other 15 PluriSIns identified via high-throughput screening, induces ER stress and apoptosis in hPSCs by inhibiting stearoyl-coA desaturase (SCD1) [40]. Later, PluriSIn#1 was found to be effective for isolating cardiomyocytes derived from iPSCs by selectively eliminating Nanog-positive cells [45]. Importantly, oleic acid biosynthesis via SCD1 is important for mouse embryonic development, suggesting that the unique metabolic processes of hPSCs would be a plausible target for purging residual hPSCs [40]. Similarly, an inhibitor of Erv1 oxidase (MitoBloCK-6, a mitochondrial protein import blocker from the laboratory of Carla Koehler), which is important in the mitochondrial disulfide relay system, was identified through chemical screening and selectively induces apoptotic cell death via cytochrome c release (a key event in mitochondrial cell death) in hESCs [46]. Similarly, differences in the glucose metabolism of hPSCs (e.g., high dependence on glutamine) were also used to selectively ablate residual hPSCs after cardiomyocyte differentiation using glucose- and glutamine-depleted culture medium supplemented with lactate [47].
However, considering the diverse molecular characteristics of desirable cells differentiated from hPSCs, this approach, targeting key metabolic enzymes or depleting a nutrient essential for hPSCs survival, would be limited to differentiated cells with low dependency on these enzymes or nutrients for their survival.
Other classes of small molecule-based selective elimination
In addition, through in-house compound library screening under hESCs and mouse embryonic fibroblast (MEF) co-culture conditions, JC011 was identified as a selective cell death inducer of hESCs to inhibit teratoma formation [48]. In a similar approach, screening of an in-house chemical library of cytotoxic small molecules, a derivative of okadaic acid, identified as 27-deoxy-27-oxookadaate, was found to have selective cytotoxicity to hESCs due to the low expression of ATP-binding cassette (ABC) transporters ABCB1 and ABCG2 in hESCs, leading to the accumulation of 27-deoxy-27-oxookadaate until the cytotoxic concentration was reached [49]. In this case, ABCB1 and ABCG2 expression in a certain type of differentiated cell would be an important indicator for the cytotoxicity of this compound to the differentiated cells, such as astrocytes, which are moderately sensitive to this compound [49]. Additionally, metformin treatment in vivo was shown to decrease teratoma size in an apoptosis-independent manner, although the mechanism of this finding was not clearly addressed [50].
Although the approach with small molecules would be highly effective and relatively simple, it would be difficult to guarantee the functional safety of all types of cells that differentiate from hPSCs considering their individual biological properties.
Genetic approach to introduce suicide genes and miRNA switches
The typical suicide gene approach, which uses selective expression of the thymidine kinase gene of the herpes simplex virus (HSVtk) in undifferentiated PSCs in combination with the guanosine analog prodrug ganciclovir (GCV) [51], was extensively applied to achieve selective cell death by GCV treatment [52–57]. To prevent the undesirable cytotoxicity of GCV treatment from activating the suicide gene system in the differentiated cells, which may cause cytotoxicity to a normal cell type, such as lymphoblastoid [58] or corneal endothelial cells [59], visual light (540–560 nm of green light) was used instead to activate a novel photosensitizer suicide gene system, inducing selective cell death of mouse and human ESCs but not endothelial cells derived from PSCs. This selective cell death in mouse ESCs is sufficient to completely inhibit teratoma formation in the mouse model [60]. Such high phototoxic selectivity was achieved by introducing the KillerRed (KR) gene (a genetically encoded photosensitizer) [61] to the EOS [early transposon promoter and Oct-4 (Pou5f1) and Sox2 enhancers] promoter, an artificial promoter initially designed to select fully reprogrammed iPSCs [62] and allow KR to be specifically expressed only in undifferentiated PSCs [60]. Importantly, endothelial cells derived from KR-expressing mESCs remained fully functional even in vivo and were able to repair the ischemic damage from the visual light exposure used to purge the undifferentiated mESCs [60].
Alternatively, inducible caspase-9 (iCasp9: iC9), which becomes an active dimer upon treatment with a synthetic chemical inducer of dimerization (CID) and which was initially developed as a safeguard system for T-cell therapy [63], was applied to PSCs for teratoma inhibition not only in vitro but also in vivo [64, 65]. This suicide system was also used to reduce the size of the teratoma and ablate iPSC-derived rejuvenated cytotoxic T lymphocytes, serving as a possible safeguard system in vivo [66]. A recent study reported that chemical inducers (e.g., CID) for activating the iCasp9 suicide systems were cytotoxic to CD34+ HSCs, while GCV itself showed a bystander effect on normal iPSCs [35]. Therefore, a chemical inducer to activate the suicide system should be carefully selected based on the differentiated cells to ensure the safety of the differentiated cells.
Furthermore, genetic approaches using a suicide gene have been largely criticized due to the additional risk of random insertion of the foreign gene into the hPSCs, which may cause unexpected genetic aberration, unless the insertion of the foreign gene is tightly controlled by new genetic editing technologies to the genomic safe harbor sites, such as AAVS1, ROSA26, or CCR5, as previously proposed [54, 60, 66]. Until then, this genetic approach with an inducible suicide system may remain at the ‘proof of concept’ level.
Alternatively, micro-RNA (miRNA) switch technology, a transgene-free genetic approach targeting cell-specific miRNA, which was originally developed for the purification of desirable cells [67], was applied to selectively eliminate undifferentiated hPSCs with puromycin resistance using an miR-302a switch [68].
Targeting the specific surface markers of hPSCs with antibodies or proteins
Cell sorting using antibodies for surface proteins specific to the differentiated cell types has been primarily used for isolating desirable cell types after differentiation. However, certain types of cells, such as ventricular cardiomyocytes, lack a specific surface protein, making pure isolation technically challenging after differentiation [69]. Alternatively, molecular beacons (MBs), oligonucleotide hybridization probes that specifically bind to intracellular mRNAs, have been developed to isolate or enrich a desired cell type that lacks surface markers [69–72].
On the other hand, considering the unique surface marker expression profile of hPSCs [44], a set of antibodies recognizing the unique surface proteins of hPSCs has been produced [73, 74]. Antibodies against stage-specific embryonic antigens (SSEAs), such as SSEA-3 and SSEA-4, and tumor-related antigen (TRA)-1-60 and TRA-1-81 were used not only to identify but also to sort out the undifferentiated PSCs [75].
Therefore, separation based on an antibody against a specific surface protein (e.g., SSEA-5) through fluorescence-activated cell sorting (FACS) [76] or selective cell death with a cytotoxic antibody (e.g., against claudin-6 [77] or podocalyxin-like protein-1 (PODXL) [74]) would be a valid approach to reduce the potential for teratoma formation in heterogeneously differentiated cultures.
Similar to the antibody approach, rBC2LCN (recombinant N-terminal domain of the BC2L-C lectin derived from Burkholderia cenocepacia) was identified as a lectin probe that specifically binds to hyperglycosylated podocalyxin as a cell surface ligand in hPSCs [78]. Therefore, strategies involving the addition of a recombinant toxin protein (catalytic domain of Pseudomonas aeruginosa exotoxin A) conjugated to the lectin probe (rBC2LCN-PE23) were found to be effective at selectively eliminating undifferentiated hPSCs [79]. Importantly, rBC2LCN-PE23 has been recently commercialized as an ‘undifferentiated hPSCs elimination reagent’ (http://www.wako-chem.co.jp).
Phototoxic approach
An antibody conjugated with a gold nanoparticle, which absorbs the energy from laser pulses to produce heat, was previously developed to induce lethal membrane damage exclusively to the labeled cells [80]. Using an antibody against TRA-1-60 and TRA-1-81, a surface antigen specific to hPSCs, laser exposure and subsequent photothermolysis were sufficient to induce selective cell death and inhibit teratoma formation [81]. Alternatively, a fluorescence rhodamine compound (rosamine, a compound designated yellow1: CDy1), which was found to specifically interact with a protein in the mitochondria of PSCs [82], was used to induce selective cell death of both mouse and human PSCs [83]. Of note, exposure to green light at 532 nm produces ROS and selectively kills CDy1-stained PSCs but not the endothelial cells derived from PSCs. Importantly, teratoma formation after transplantation of mouse ESCs in the mouse model was completely blocked by CDy1 staining and light exposure [83].
It is also noteworthy that the amount of green light used to induce hPSC death with a photosensitizing gene (e.g., KR) [60] or fluorescence probe [83] while ensuring the functional safety of endothelial cells would not be suitable for RPE cells considering the high photosensitivity of RPE cells [84].
Detecting and evaluating residual hPSCs
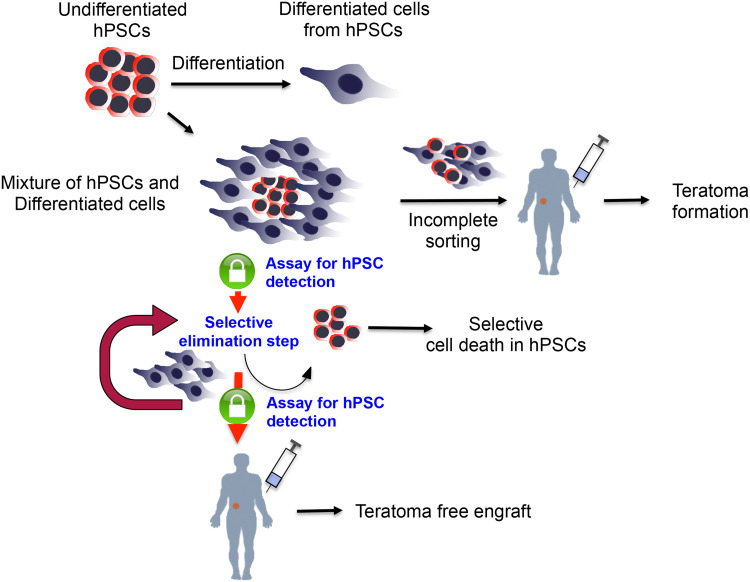
After differentiation, the residual undifferentiated hPSCs should be carefully quantified to determine whether an additional step is required to eliminate the undifferentiated hPSCs with the aforementioned techniques. Furthermore, even after the treatment, further validation to specifically quantify the residual hPSCs as a safeguard to ensure complete ablation can also be performed, as shown in Fig. 1.
Fig. 1.
An example of the procedure of human pluripotent stem cell-based cell therapy for assuring teratoma-free cell therapy. After differentiation to the desirable cell type, an assay should be performed to check for possible contamination of tumorigenic hPSCs, even after enriching the desirable cell types. Likewise, even after additional steps have been taken for ablating residual tumorigenic hPSCs, cell therapy should only be conducted after a highly sensitive assay has been performed that validates that no tumorigenic hPSCs remain in order to guarantee teratoma-free cell therapy
Invasive approaches
Conventionally, semi quantitative real-time PCR or flow cytometry has been widely used to monitor the residual undifferentiated PSCs [85]. Dr. Yoji Sato’s group recently developed highly sensitive real-time PCR [86, 87] or droplet digital PCR [88] with a PSC-specific gene (e.g., Lin28) to examine the possible contamination of undifferentiated hPSCs at the single-cell level following their differentiation into RPE cells or cardiomyocytes. Although flow cytometry using mostly PSC-specific surface antibodies has been widely employed to monitor undifferentiated PSCs after differentiation, the proportion of the marker-positive population is markedly affected by the gating technique [86]. Therefore, several strict controls should be prepared to define the desirable population by flow cytometry. However, despite their high sensitivity, both real-time PCR and flow cytometry techniques are invasive approaches that irreversibly consume a large quantity of the differentiated cells (more than 10,000 cells). Because considerable time and resources are required to differentiate desirable cell types from hPSCs (for example, 30–50 days for differentiation of RPE cells from hESCs [89]), unrecoverable consumption of differentiated cells to ensure safety should be reconsidered.
Non-invasive approaches
Recently, a hPSC-specific glycoprotein was identified [90], and rBC2LCN, a recombinant lectin probe, was identified as a specific probe [91] for podocalyxin, a heavily glycosylated type 1 transmembrane protein prominent in hPSCs [92]. Specific interaction with rBC2LCN was sufficient for live-cell imaging of hPSCs in a cost-effective manner [93]. Furthermore, it was also demonstrated that hyperglycosylated podocalyxin is secreted into the hPSC culture medium. Therefore, simple determination of the concentration of hyperglycosylated podocalyxin in the culture medium using the rBC2LCN-based sandwich assay system (named the Glycostem test) can selectively quantify the teratoma-forming (or tumorigenic) undifferentiated hPSCs present after differentiation [78].
Alternatively, a few fluorescent chemical probes, such as the Kyoto probe 1 (KP1) [94] and the aforementioned CDy1, were demonstrated to be highly specific to hPSCs [82]. In particular, CDy1 was later applied to quantify or isolate the undifferentiated hPSCs using FACS [95] and to selectively kill the stained, undifferentiated PSCs using visible light [83].
On the other hand, dual fluorescence resonance energy transfer (FRET) MBs that specifically bind to Oct-4, originally developed for identification and isolation of hESCs [96], could also be used to quantify and even sort out the residual hPSCs. The efficiency of this approach has not yet been experimentally determined.
Due to recent advances in bioengineering techniques, a cell-chip system has been widely applied to monitor the cellular response, including differentiation, of the stem cells [97]. Similarly, a cell-chip system detecting the unique electrochemical potential of hPSCs has been designed. The intensity of the electrochemical potential generated from the live hPSCs without any labeling demonstrated a clear linear relationship with cell number, even in mixed cell conditions with differentiated progeny, allowing the extrapolation of the exact number of residual hPSCs in the mixed condition [98]. Very recently, a surface-enhanced Raman scattering (SERS) assay based on nanoparticles conjugated with the hPSC surface markers SSEA-5 and TRA-1-60 was demonstrated to trace as few as a single hPSC in 106 cells [99]. Therefore, when such an assay system allowing live detection of hPSCs is optimized and further improved, it would be highly useful for monitoring the presence of residual hPSCs after differentiation and for deciding whether an additional step to ablate the residual hPSCs in the mixture is necessary for safety assurance. Finally, immediately prior to cell transplantation, the differentiated cell population would be again used for final validation of safety for teratoma-free cell therapy (Fig. 1).
Concluding remarks
As mentioned above, a variety of strategies, summarized in Table 1, have been examined to selectively ablate undifferentiated PSCs from differentiated cells for teratoma-free hPSC-based cell therapy with no or low cytotoxicity of the differentiated cells for quality assurance as well. However, considering the diversity of desirable cell types for future hPSC-based cell therapy, it would be hardly possible to presume that one methodology may selectively eliminate residual hPSCs without damaging the diverse types of differentiated cell type, of which properties would be varied. In this line, each methodology should be carefully selected depending on the molecular characteristics of desirable cells. Besides, not only low cytotoxicity of the differentiated cells, as listed in Table 1, but also the functional safety of each methodology to the differentiated cells in vivo, as shown previously [60, 100], should be intensively examined to apply each method to practical use. Accordingly, continuous effort should be applied further to develop novel strategies to ensure the safety of the differentiated cells as well as the efficacy of eliminating the undifferentiated hPSCs for future teratoma-free hPSC-based cell therapy.
Table 1.
Strategies to selectively eliminate the tumorigenic hPSCs
| Strategies | Name | Mode of action or target | Cell model | Refs. |
|---|---|---|---|---|
| Small molecules | Quercetin | BIRC5 repression, CypD interaction |
hPSCs vs hDFs, hASMCs, hiPSC-derived SMCs, hESC-derived dopaminergic neurons |
[32, 37] |
| YM155 | BIRC5 repression or DNA damage (?) |
hPSCs vs hDFs, hASMCs, hiPSC-derived SMCs, hESC-derived dopaminergic neurons |
[32] | |
| Taxol and purvalanol A | BIRC5 repression | hESC-derived teratoma | [33] | |
| Purvalanol A, Ro-3306, Dinaciclib | CDK inhibition | Human and mouse ESCs vs mES-diff, hESC-derived pancreatic progenitor cells | [39] | |
| Etoposide | DNA damage | mPSCs vs MEFs | [38] | |
| PluriSIn#2 | Suppression of Topoisomerase II alpha | hPSCs vs hPSC-derived various cell types, fibroblasts | [41] | |
| Metformin | Unknown | miPSCs vs MEFs | [50] | |
| JC011 | Unknown | hPSCs vs MRC-5, human neonatal cardiomyocytes | [48] | |
| S18 (N-oleoyl serinol) | Ceramide analog, PKC delta inhibition | Human and mouse ESCs | [31] | |
| 27-deoxy-27-oxookadaate | ABCB1 and ABCG2 | hiPSCs vs adrenal gland, liver, bronchia and prostate cells | [49] | |
| Targeting hPSCs’ specific metabolism | PluriSIn#1 | Oleate synthesis inhibitor to inhibit stearoyl-coA desaturase (SCD1) | hPSCs vs fibroblasts, hepatocytes, cardiomyoctes, NSCs, MSCs | [40] |
| MitoBloCK-6 | Erv1 oxidase | hESCs vs hDFs | [46] | |
| Glucose and glutamine free medium with lactate supplement | High dependency of glucose and glutamine metabolism | hPSCs vs hPSC-derived cardiomyocytes | [47] | |
| HSVtk and GCV | Inhibition of DNA elongation | hPSCs vs MRC-5 | [52–57] | |
| Introducing suicide gene and miRNA switch | KillerRed with visual light | Oxidative damage | hESCs, mPSCs vs mESC-derived endothelial cells | [60] |
| Inducible caspase 9 | Apoptosis induction | hiPSCs | [64, 65] | |
| miR-302a switch | micro-RNA-302a | hiPSCs vs NHDF, hiPSC-derived dopaminergic-like neuronal cells | [68] | |
| SSEA-5 mAb | SSEA-5 | hPSCs vs RA-induced differentiated cell mixtures | [76] | |
| Targeting hPSCs’ specific surface markers with antibody or protein | Claudin-6 mAb | Claudin-6 | hPSCs vs ectodermal and mesodermal cell types | [77] |
| mAb 84 | Podocalyxin-like Protein 1 | hESCs, hECs vs hEBs, mESC, mouse fibroblasts | [74] | |
| rBC2LCN-PE23 | Hyperglycosylated podocalyxin | hPSCs vs Human fibroblasts, hADSCs | [79] | |
| Antibody conjugated gold nanoparticle with laser exposure | Photothermolysis | hESCs vs hESC-derived neural precursors | [81] | |
| Phototoxic approach | CDy1 with visual light | Oxidative damage | Human and mouse PSCs vs hESC-derived endothelial cells | [82] |
PSCs pluripotent stem cells, ESCs embryonic stem cells, EB embryonic body, EC embryonic carcinoma, DFs dermal fibroblasts, ASMCs aortic smooth muscle cells, NSCs neural stem cells, MSCs mesenchymal stem cells, MRC-5 human lung fibroblasts, NHDF normal human dermal fibroblasts, MEFs mouse embryonic fibroblasts, ADSCs adipose-derived mesenchymal stem cells, RA retinoic acid
In addition, it is also important to develop an approach to selectively eliminate the hPSCs in vivo after accidental transplantation into patients. To this end, the iCasp9 suicide system was shown to reduce the teratoma size in vivo [64], and subsequent treatment with metformin lowered teratoma formation after the transplantation of mouse iPSCs [50]. Such a methodology to limit the teratoma formation in vivo should be more intensively verified to minimize unexpected side effects in human patients.
As with drugs, safety should be considered to be of a similarly high priority as efficacy in hPSC-based stem cell therapy for future clinical outcomes. Therefore, after serious assessment of the risk–benefit ratio, hPSC-based therapy should be performed when the benefit to the human patient is considered to be greater than the potential risks. From this point of view, continuous attempts to lower the risk of teratoma formation may improve the clinical application of hPSC-based therapy by increasing the therapeutic index in the future.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (Nos. 2011–0030043, 2009–0093822 and 2017R1A2A2A05000766).
Compliance with ethical standards
Competing interests
None.
References
- 1.Alper J. Geron gets green light for human trial of ES cell-derived product. Nat Biotechnol. 2009;27(3):213–214. doi: 10.1038/nbt0309-213a. [DOI] [PubMed] [Google Scholar]
- 2.Lebkowski J. GRNOPC1: the world’s first embryonic stem cell-derived therapy. Interview with Jane Lebkowski. Regen Med. 2011;6(6 Suppl):11–13. doi: 10.2217/rme.11.77. [DOI] [PubMed] [Google Scholar]
- 3.Strauss S. Geron trial resumes, but standards for stem cell trials remain elusive. Nat Biotechnol. 2010;28(10):989–990. doi: 10.1038/nbt1010-989. [DOI] [PubMed] [Google Scholar]
- 4.Baker M. Stem-cell pioneer bows out. Nature. 2011;479(7374):459. doi: 10.1038/479459a. [DOI] [PubMed] [Google Scholar]
- 5.Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19(8):998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. The Lancet. 2015;385(9967):509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 8.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Kimbrel EA, Lanza R. Current status of pluripotent stem cells: moving the first therapies to the clinic. Nat Rev Drug Discov. 2015;14(10):681–692. doi: 10.1038/nrd4738. [DOI] [PubMed] [Google Scholar]
- 10.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang EJ, Fulati A, Lee HS, Sritanaudomchai H, Masterson K, Larson J, Eaton D, Sadler-Fredd K, Battaglia D, Lee D, Wu DA, Jensen J, Patton P, Gokhale S, Stouffer RL, Wolf D, Mitalipov S. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153(6):1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heslop JA, Hammond TG, Santeramo I, Tort Piella A, Hopp I, Zhou J, Baty R, Graziano EI, Proto Marco B, Caron A, Skold P, Andrews PW, Baxter MA, Hay DC, Hamdam J, Sharpe ME, Patel S, Jones DR, Reinhardt J, Danen EH, Ben-David U, Stacey G, Bjorquist P, Piner J, Mills J, Rowe C, Pellegrini G, Sethu S, Antoine DJ, Cross MJ, Murray P, Williams DP, Kitteringham NR, Goldring CE, Park BK. Concise review: workshop review: understanding and assessing the risks of stem cell-based therapies. Stem Cells Transl Med. 2015;4(4):389–400. doi: 10.5966/sctm.2014-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujikawa T, Oh SH, Pi L, Hatch HM, Shupe T, Petersen BE. Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells. Am J Pathol. 2005;166(6):1781–1791. doi: 10.1016/S0002-9440(10)62488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon J, Lee HS, Kang JM, Park J, Leung A, Hong SH, Chung SM, Kim KS. Stem cell grafting improves both motor and cognitive impairments in a genetic model of Parkinson’s disease, the aphakia (ak) mouse. Cell Transpl. 2013;22(7):1263–1279. doi: 10.3727/096368912X657242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Investig Ophthalmol Vis Sci. 2004;45(12):4251–4255. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- 17.Erdo F, Buhrle C, Blunk J, Hoehn M, Xia T, Fleischmann B, Focking M, Kustermann E, Kolossov E, Hescheler T, Hossmann KA, Trapp T. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood F Metab. 2003;23(7):780–785. doi: 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- 18.Lee AS, Tang C, Cao F, Xie X, van der Bogt K, Hwang A, Connolly AJ, Robbins RC, Wu JC. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle. 2009;8(16):2608–2612. doi: 10.4161/cc.8.16.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27(5):1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-David U, Benvenisty N. Chemical ablation of tumor-initiating human pluripotent stem cells. Nat Protoc. 2014;9(3):729–740. doi: 10.1038/nprot.2014.050. [DOI] [PubMed] [Google Scholar]
- 21.Malecki M (2014) ‘Above all, do no harm’: safeguarding pluripotent stem cell therapy against iatrogenic tumorigenesis. Stem Cell Res Ther 5 (3):73. doi:10.1186/scrt462 [DOI] [PMC free article] [PubMed]
- 22.Rodrigues GM, Rodrigues CA, Fernandes TG, Diogo MM, Cabral JM. Clinical-scale purification of pluripotent stem cell derivatives for cell-based therapies. Biotechnol J. 2015;10(8):1103–1114. doi: 10.1002/biot.201400535. [DOI] [PubMed] [Google Scholar]
- 23.Weissbein U, Benvenisty N, Ben-David U. Quality control: genome maintenance in pluripotent stem cells. J Cell Biol. 2014;204(2):153–163. doi: 10.1083/jcb.201310135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison JC, Haber JE. Surviving the breakup: the DNA damage checkpoint. Annu Rev Genet. 2006;40:209–235. doi: 10.1146/annurev.genet.40.051206.105231. [DOI] [PubMed] [Google Scholar]
- 25.Hong Y, Stambrook PJ. Restoration of an absent G1 arrest and protection from apoptosis in embryonic stem cells after ionizing radiation. Proc Natl Acad Sci USA. 2004;101(40):14443–14448. doi: 10.1073/pnas.0401346101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filion TM, Qiao M, Ghule PN, Mandeville M, van Wijnen AJ, Stein JL, Lian JB, Altieri DC, Stein GS. Survival responses of human embryonic stem cells to DNA damage. J Cell Physiol. 2009;220(3):586–592. doi: 10.1002/jcp.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desmarais JA, Hoffmann MJ, Bingham G, Gagou ME, Meuth M, Andrews PW. Human embryonic stem cells fail to activate CHK1 and commit to apoptosis in response to DNA replication stress. Stem Cells. 2012;30(7):1385–1393. doi: 10.1002/stem.1117. [DOI] [PubMed] [Google Scholar]
- 28.Liu JC, Guan X, Ryan JA, Rivera AG, Mock C, Agrawal V, Letai A, Lerou PH, Lahav G. High mitochondrial priming sensitizes hESCs to DNA-damage-induced apoptosis. Cell Stem Cell. 2013;13(4):483–491. doi: 10.1016/j.stem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumitru R, Gama V, Fagan BM, Bower JJ, Swahari V, Pevny LH, Deshmukh M. Human embryonic stem cells have constitutively active Bax at the Golgi and are primed to undergo rapid apoptosis. Mol Cell. 2012 doi: 10.1016/j.molcel.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.TeSlaa T, Setoguchi K, Teitell MA. Mitochondria in human pluripotent stem cell apoptosis. Semin Cell Dev Biol. 2016;52:76–83. doi: 10.1016/j.semcdb.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bieberich E, Silva J, Wang G, Krishnamurthy K, Condie BG. Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants. J Cell Biol. 2004;167(4):723–734. doi: 10.1083/jcb.200405144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee MO, Moon SH, Jeong HC, Yi JY, Lee TH, Shim SH, Rhee YH, Lee SH, Oh SJ, Lee MY, Han MJ, Cho YS, Chung HM, Kim KS, Cha HJ. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1303669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blum B, Bar-Nur O, Golan-Lev T, Benvenisty N. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat Biotechnol. 2009;27(3):281–287. doi: 10.1038/nbt.1527. [DOI] [PubMed] [Google Scholar]
- 34.Liu JC, Lerou PH, Lahav G. Stem cells: balancing resistance and sensitivity to DNA damage. Trends Cell Biol. 2014;24(5):268–274. doi: 10.1016/j.tcb.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bedel A, Beliveau F, Lamrissi-Garcia I, Rousseau B, Moranvillier I, Rucheton B, Guyonnet-Duperat V, Cardinaud B, de Verneuil H, Moreau-Gaudry F, Dabernat S. Preventing pluripotent cell teratoma in regenerative medicine applied to hematology disorders. Stem Cells Transl Med. 2016 doi: 10.5966/sctm.2016-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung CG, Xu Y, Mularski B, Liu H, Gurbuxani S, Crispino JD. Requirements for survivin in terminal differentiation of erythroid cells and maintenance of hematopoietic stem and progenitor cells. J Exp Med. 2007;204(7):1603–1611. doi: 10.1084/jem.20062395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SY, Jeong HC, Hong SK, Lee MO, Cho SJ, Cha HJ. Quercetin induced ROS production triggers mitochondrial cell death of human embryonic stem cells. Oncotarget. 2016 doi: 10.18632/oncotarget.11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith AJ, Nelson NG, Oommen S, Hartjes KA, Folmes CD, Terzic A, Nelson TJ. Apoptotic susceptibility to DNA damage of pluripotent stem cells facilitates pharmacologic purging of teratoma risk. Stem Cells Transl Med. 2012;1(10):709–718. doi: 10.5966/sctm.2012-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huskey NE, Guo T, Evason KJ, Momcilovic O, Pardo D, Creasman KJ, Judson RL, Blelloch R, Oakes SA, Hebrok M, Goga A. CDK1 inhibition targets the p53-NOXA-MCL1 axis, selectively kills embryonic stem cells, and prevents teratoma formation. Stem Cell Rep. 2015;4(3):374–389. doi: 10.1016/j.stemcr.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-David U, Gan QF, Golan-Lev T, Arora P, Yanuka O, Oren YS, Leikin-Frenkel A, Graf M, Garippa R, Boehringer M, Gromo G, Benvenisty N. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12(2):167–179. doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Ben-David U, Cowell IG, Austin CA, Benvenisty N. Brief reports: controlling the survival of human pluripotent stem cells by small molecule-based targeting of topoisomerase II alpha. Stem Cells. 2015;33(3):1013–1019. doi: 10.1002/stem.1888. [DOI] [PubMed] [Google Scholar]
- 42.Yamanaka K, Nakahara T, Yamauchi T, Kita A, Takeuchi M, Kiyonaga F, Kaneko N, Sasamata M. Antitumor activity of YM155, a selective small-molecule survivin suppressant, alone and in combination with docetaxel in human malignant melanoma models. Clin Cancer Res. 2011;17(16):5423–5431. doi: 10.1158/1078-0432.CCR-10-3410. [DOI] [PubMed] [Google Scholar]
- 43.Winter GE, Radic B, Mayor-Ruiz C, Blomen VA, Trefzer C, Kandasamy RK, Huber KV, Gridling M, Chen D, Klampfl T, Kralovics R, Kubicek S, Fernandez-Capetillo O, Brummelkamp TR, Superti-Furga G. The solute carrier SLC35F2 enables YM155-mediated DNA damage toxicity. Nat Chem Biol. 2014;10(9):768–773. doi: 10.1038/nchembio.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolle G, Ho M, Zhou Q, Chy HS, Krishnan K, Cloonan N, Bertoncello I, Laslett AL, Grimmond SM. Identification of human embryonic stem cell surface markers by combined membrane-polysome translation state array analysis and immunotranscriptional profiling. Stem Cells. 2009;27(10):2446–2456. doi: 10.1002/stem.182. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Pan Y, Qin G, Chen L, Chatterjee TK, Weintraub NL, Tang Y. Inhibition of stearoyl-coA desaturase selectively eliminates tumorigenic Nanog-positive cells: improving the safety of iPS cell transplantation to myocardium. Cell Cycle. 2014;13(5):762–771. doi: 10.4161/cc.27677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dabir DV, Hasson SA, Setoguchi K, Johnson ME, Wongkongkathep P, Douglas CJ, Zimmerman J, Damoiseaux R, Teitell MA, Koehler CM. A small molecule inhibitor of redox-regulated protein translocation into mitochondria. Dev Cell. 2013;25(1):81–92. doi: 10.1016/j.devcel.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tohyama S, Fujita J, Hishiki T, Matsuura T, Hattori F, Ohno R, Kanazawa H, Seki T, Nakajima K, Kishino Y, Okada M, Hirano A, Kuroda T, Yasuda S, Sato Y, Yuasa S, Sano M, Suematsu M, Fukuda K. Glutamine oxidation is indispensable for survival of human pluripotent stem cells. Cell Metab. 2016;23(4):663–674. doi: 10.1016/j.cmet.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Richards M, Phoon CW, Goh GT, Seng EK, Guo XM, Tan CM, Chan WK, Lee JM. A new class of pluripotent stem cell cytotoxic small molecules. PloS ONE. 2014;9(3):e85039. doi: 10.1371/journal.pone.0085039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuo TF, Mao D, Hirata N, Khambu B, Kimura Y, Kawase E, Shimogawa H, Ojika M, Nakatsuji N, Ueda K, Uesugi M. Selective elimination of human pluripotent stem cells by a marine natural product derivative. J Am Chem Soc. 2014;136(28):9798–9801. doi: 10.1021/ja501795c. [DOI] [PubMed] [Google Scholar]
- 50.Vazquez-Martin A, Cufi S, Lopez-Bonet E, Corominas-Faja B, Oliveras-Ferraros C, Martin-Castillo B, Menendez JA. Metformin limits the tumourigenicity of iPS cells without affecting their pluripotency. Sci Rep. 2012;2:964. doi: 10.1038/srep00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fillat C, Carrio M, Cascante A, Sangro B. Suicide gene therapy mediated by the Herpes Simplex virus thymidine kinase gene/Ganciclovir system: fifteen years of application. Curr Gene Ther. 2003;3(1):13–26. doi: 10.2174/1566523033347426. [DOI] [PubMed] [Google Scholar]
- 52.Kotini AG, de Stanchina E, Themeli M, Sadelain M, Papapetrou EP. Escape mutations, ganciclovir resistance, and teratoma formation in human iPSCs expressing an HSVtk suicide gene. Mol Ther Nucleic Acids. 2016;5:e284. doi: 10.1038/mtna.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuldiner M, Itskovitz-Eldor J, Benvenisty N. Selective ablation of human embryonic stem cells expressing a “suicide” gene. Stem Cells. 2003;21(3):257–265. doi: 10.1634/stemcells.21-3-257. [DOI] [PubMed] [Google Scholar]
- 54.Ou W, Li P, Reiser J. Targeting of herpes simplex virus 1 thymidine kinase gene sequences into the OCT4 locus of human induced pluripotent stem cells. PloS ONE. 2013;8(11):e81131. doi: 10.1371/journal.pone.0081131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hara A, Aoki H, Taguchi A, Niwa M, Yamada Y, Kunisada T, Mori H. Neuron-like differentiation and selective ablation of undifferentiated embryonic stem cells containing suicide gene with Oct-4 promoter. Stem Cells Dev. 2008;17(4):619–627. doi: 10.1089/scd.2007.0235. [DOI] [PubMed] [Google Scholar]
- 56.Rong Z, Fu X, Wang M, Xu Y. A scalable approach to prevent teratoma formation of human embryonic stem cells. J Biol Chem. 2012;287(39):32338–32345. doi: 10.1074/jbc.M112.383810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng F, Ke Q, Chen F, Cai B, Gao Y, Ye C, Wang D, Zhang L, Lahn BT, Li W, Xiang AP. Protecting against wayward human induced pluripotent stem cells with a suicide gene. Biomaterials. 2012;33(11):3195–3204. doi: 10.1016/j.biomaterials.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 58.Janoly-Dumenil A, Rouvet I, Bleyzac N, Bertrand Y, Aulagner G, Zabot MT. Effect of duration and intensity of ganciclovir exposure on lymphoblastoid cell toxicity. Antivir Chem Chemother. 2009;19(6):257–262. doi: 10.1177/095632020901900605. [DOI] [PubMed] [Google Scholar]
- 59.Choi WS, Koh JW, Chung TY, Hyon JY, Wee WR, Shin YJ. Cytotoxicity of ganciclovir on cultured human corneal endothelial cells. Antivir Ther. 2013;18(6):813–820. doi: 10.3851/IMP2556. [DOI] [PubMed] [Google Scholar]
- 60.Cho SJ, Kim SY, Jeong HC, Cheong H, Kim D, Park SJ, Choi JJ, Kim H, Chung HM, Moon SH, Cha HJ. Repair of Ischemic injury by pluripotent stem cell based cell therapy without teratoma through selective photosensitivity. Stem Cell Rep. 2015;5(6):1067–1080. doi: 10.1016/j.stemcr.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. A genetically encoded photosensitizer. Nat Biotechnol. 2006;24(1):95–99. doi: 10.1038/nbt1175. [DOI] [PubMed] [Google Scholar]
- 62.Hotta A, Cheung AY, Farra N, Vijayaragavan K, Seguin CA, Draper JS, Pasceri P, Maksakova IA, Mager DL, Rossant J, Bhatia M, Ellis J. Isolation of human iPS cells using EOS lentiviral vectors to select for pluripotency. Nat Methods. 2009;6(5):370–376. doi: 10.1038/nmeth.1325. [DOI] [PubMed] [Google Scholar]
- 63.Straathof KC, Pule MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, Heslop HE, Spencer DM, Rooney CM. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105(11):4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yagyu S, Hoyos V, Del Bufalo F, Brenner MK. An inducible caspase-9 suicide gene to improve the safety of therapy using human induced pluripotent stem cells. Mol Ther. 2015;23(9):1475–1485. doi: 10.1038/mt.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu C, Hong SG, Winkler T, Spencer DM, Jares A, Ichwan B, Nicolae A, Guo V, Larochelle A, Dunbar CE. Development of an inducible caspase-9 safety switch for pluripotent stem cell-based therapies. Mol Ther Methods Clin Dev. 2014;1:14053. doi: 10.1038/mtm.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ando M, Nishimura T, Yamazaki S, Yamaguchi T, Kawana-Tachikawa A, Hayama T, Nakauchi Y, Ando J, Ota Y, Takahashi S, Nishimura K, Ohtaka M, Nakanishi M, Miles JJ, Burrows SR, Brenner MK, Nakauchi H. A safeguard system for induced pluripotent stem cell-derived rejuvenated T cell therapy. Stem Cell Rep. 2015;5(4):597–608. doi: 10.1016/j.stemcr.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miki K, Endo K, Takahashi S, Funakoshi S, Takei I, Katayama S, Toyoda T, Kotaka M, Takaki T, Umeda M, Okubo C, Nishikawa M, Oishi A, Narita M, Miyashita I, Asano K, Hayashi K, Osafune K, Yamanaka S, Saito H, Yoshida Y. Efficient detection and purification of cell populations using synthetic MicroRNA switches. Cell Stem Cell. 2015;16(6):699–711. doi: 10.1016/j.stem.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Parr CJC, Katayama S, Miki K, Kuang Y, Yoshida Y, Morizane A, Takahashi J, Yamanaka S, Saito H (2016) MicroRNA-302 switch to identify and eliminate undifferentiated human pluripotent stem cells. Sci Rep doi:10.1038/Srep32532 [DOI] [PMC free article] [PubMed]
- 69.Ban K, Wile B, Cho KW, Kim S, Song MK, Kim SY, Singer J, Syed A, Yu SP, Wagner M, Bao G, Yoon YS. Non-genetic purification of ventricular cardiomyocytes from differentiating embryonic stem cells through molecular beacons targeting IRX-4. Stem Cell Rep. 2015;5(6):1239–1249. doi: 10.1016/j.stemcr.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bizy A, Guerrero-Serna G, Hu B, Ponce-Balbuena D, Willis BC, Zarzoso M, Ramirez RJ, Sener MF, Mundada LV, Klos M, Devaney EJ, Vikstrom KL, Herron TJ, Jalife J. Myosin light chain 2-based selection of human iPSC-derived early ventricular cardiac myocytes. Stem Cell Res. 2013;11(3):1335–1347. doi: 10.1016/j.scr.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jha R, Wile B, Wu Q, Morris AH, Maher KO, Wagner MB, Bao G, Xu C. Molecular beacon-based detection and isolation of working-type cardiomyocytes derived from human pluripotent stem cells. Biomaterials. 2015;50:176–185. doi: 10.1016/j.biomaterials.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wile BM, Ban K, Yoon YS, Bao G. Molecular beacon-enabled purification of living cells by targeting cell type-specific mRNAs. Nat Protoc. 2014;9(10):2411–2424. doi: 10.1038/nprot.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi HS, Kim H, Won A, Kim JJ, Son CY, Kim KS, Ko JH, Lee MY, Kim CH, Ryu CJ. Development of a decoy immunization strategy to identify cell-surface molecules expressed on undifferentiated human embryonic stem cells. Cell Tissue Res. 2008;333(2):197–206. doi: 10.1007/s00441-008-0632-6. [DOI] [PubMed] [Google Scholar]
- 74.Choo AB, Tan HL, Ang SN, Fong WJ, Chin A, Lo J, Zheng L, Hentze H, Philp RJ, Oh SK, Yap M. Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells. 2008;26(6):1454–1463. doi: 10.1634/stemcells.2007-0576. [DOI] [PubMed] [Google Scholar]
- 75.Fong CY, Peh GS, Gauthaman K, Bongso A. Separation of SSEA-4 and TRA-1-60 labelled undifferentiated human embryonic stem cells from a heterogeneous cell population using magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS) Stem Cell Rev. 2009;5(1):72–80. doi: 10.1007/s12015-009-9054-4. [DOI] [PubMed] [Google Scholar]
- 76.Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, Mosley AR, Inlay MA, Ardehali R, Chavez SL, Pera RR, Behr B, Wu JC, Weissman IL, Drukker M. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29(9):829–834. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tateno H, Onuma Y, Ito Y, Hiemori K, Aiki Y, Shimizu M, Higuchi K, Fukuda M, Warashina M, Honda S, Asashima M, Hirabayashi J. A medium hyperglycosylated podocalyxin enables noninvasive and quantitative detection of tumorigenic human pluripotent stem cells. Sci Rep. 2014;4:4069. doi: 10.1038/srep04069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tateno H, Onuma Y, Ito Y, Minoshima F, Saito S, Shimizu M, Aiki Y, Asashima M, Hirabayashi J. Elimination of tumorigenic human pluripotent stem cells by a recombinant lectin-toxin fusion protein. Stem Cell Rep. 2015;4(5):811–820. doi: 10.1016/j.stemcr.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin CP, Kelly MW, Sibayan SA, Latina MA, Anderson RR. Selective cell killing by microparticle absorption of pulsed laser radiation. IEEE J Sel Top Quantum Electron. 1999;5(4):963–968. doi: 10.1109/2944.796318. [DOI] [Google Scholar]
- 80.Terstegge S, Winter F, Rath BH, Laufenberg I, Schwarz C, Leinhaas A, Levold F, Dolf A, Haupt S, Koch P, Endl E, Brustle O. Laser-assisted photoablation of human pluripotent stem cells from differentiating cultures. Stem Cell Rev. 2010;6(2):260–269. doi: 10.1007/s12015-010-9114-9. [DOI] [PubMed] [Google Scholar]
- 81.Im CN, Kang NY, Ha HH, Bi X, Lee JJ, Park SJ, Lee SY, Vendrell M, Kim YK, Lee JS, Li J, Ahn YH, Feng B, Ng HH, Yun SW, Chang YT. A fluorescent rosamine compound selectively stains pluripotent stem cells. Angew Chem. 2010;49(41):7497–7500. doi: 10.1002/anie.201002463. [DOI] [PubMed] [Google Scholar]
- 82.Cho S-J, Kim S-Y, Park S-J, Song N, Kwon H-Y, Kang N-Y, Moon S-H, Chang Y-T, Cha H-J (2016) Photodynamic approach for teratoma-free pluripotent stem cell therapy using CDy1 and Visible Light. ACS Cent Sci [DOI] [PMC free article] [PubMed]
- 83.Cachafeiro M, Bemelmans AP, Samardzija M, Afanasieva T, Pournaras JA, Grimm C, Kostic C, Philippe S, Wenzel A, Arsenijevic Y. Hyperactivation of retina by light in mice leads to photoreceptor cell death mediated by VEGF and retinal pigment epithelium permeability. Cell Death Dis. 2013;4:e781. doi: 10.1038/cddis.2013.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cunningham JJ, Ulbright TM, Pera MF, Looijenga LH. Lessons from human teratomas to guide development of safe stem cell therapies. Nat Biotechnol. 2012;30(9):849–857. doi: 10.1038/nbt.2329. [DOI] [PubMed] [Google Scholar]
- 85.Kuroda T, Yasuda S, Kusakawa S, Hirata N, Kanda Y, Suzuki K, Takahashi M, Nishikawa S, Kawamata S, Sato Y. Highly sensitive in vitro methods for detection of residual undifferentiated cells in retinal pigment epithelial cells derived from human iPS cells. PloS ONE. 2012;7(5):e37342. doi: 10.1371/journal.pone.0037342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuroda T, Yasuda S, Sato Y. In vitro detection of residual undifferentiated cells in retinal pigment epithelial cells derived from human induced pluripotent stem cells. Methods Mol Biol. 2014;1210:183–192. doi: 10.1007/978-1-4939-1435-7_14. [DOI] [PubMed] [Google Scholar]
- 87.Kuroda T, Yasuda S, Matsuyama S, Tano K, Kusakawa S, Sawa Y, Kawamata S, Sato Y. Highly sensitive droplet digital PCR method for detection of residual undifferentiated cells in cardiomyocytes derived from human pluripotent stem cells. Regen Ther. 2015;2:17–23. doi: 10.1016/j.reth.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maruotti J, Wahlin K, Gorrell D, Bhutto I, Lutty G, Zack DJ. A simple and scalable process for the differentiation of retinal pigment epithelium from human pluripotent stem cells. Stem Cells Transl Med. 2013;2(5):341–354. doi: 10.5966/sctm.2012-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tateno H, Toyota M, Saito S, Onuma Y, Ito Y, Hiemori K, Fukumura M, Matsushima A, Nakanishi M, Ohnuma K, Akutsu H, Umezawa A, Horimoto K, Hirabayashi J, Asashima M. Glycome diagnosis of human induced pluripotent stem cells using lectin microarray. J Biol Chem. 2011;286(23):20345–20353. doi: 10.1074/jbc.M111.231274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Onuma Y, Tateno H, Hirabayashi J, Ito Y, Asashima M. rBC2LCN, a new probe for live cell imaging of human pluripotent stem cells. Biochem Biophys Res Commun. 2013;431(3):524–529. doi: 10.1016/j.bbrc.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 91.Tateno H, Matsushima A, Hiemori K, Onuma Y, Ito Y, Hasehira K, Nishimura K, Ohtaka M, Takayasu S, Nakanishi M, Ikehara Y, Nakanishi M, Ohnuma K, Chan T, Toyoda M, Akutsu H, Umezawa A, Asashima M, Hirabayashi J. Podocalyxin is a glycoprotein ligand of the human pluripotent stem cell-specific probe rBC2LCN. Stem Cells Transl Med. 2013;2(4):265–273. doi: 10.5966/sctm.2012-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tateno H, Onuma Y, Ito Y. Live-cell imaging of human pluripotent stem cells by a novel lectin probe rBC2LCN. Methods Mol Biol. 2014;1200:313–318. doi: 10.1007/978-1-4939-1292-6_26. [DOI] [PubMed] [Google Scholar]
- 93.Hirata N, Nakagawa M, Fujibayashi Y, Yamauchi K, Murata A, Minami I, Tomioka M, Kondo T, Kuo TF, Endo H, Inoue H, Sato S, Ando S, Kawazoe Y, Aiba K, Nagata K, Kawase E, Chang YT, Suemori H, Eto K, Nakauchi H, Yamanaka S, Nakatsuji N, Ueda K, Uesugi M. A chemical probe that labels human pluripotent stem cells. Cell Rep. 2014;6(6):1165–1174. doi: 10.1016/j.celrep.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 94.Kang NY, Yun SW, Ha HH, Park SJ, Chang YT. Embryonic and induced pluripotent stem cell staining and sorting with the live-cell fluorescence imaging probe CDy1. Nat Protoc. 2011;6(7):1044–1052. doi: 10.1038/nprot.2011.350. [DOI] [PubMed] [Google Scholar]
- 95.King FW, Liszewski W, Ritner C, Bernstein HS. High-throughput tracking of pluripotent human embryonic stem cells with dual fluorescence resonance energy transfer molecular beacons. Stem Cells Dev. 2011;20(3):475–484. doi: 10.1089/scd.2010.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bagnaninchi PO, Drummond N. Real-time label-free monitoring of adipose-derived stem cell differentiation with electric cell-substrate impedance sensing. Proc Natl Acad Sci USA. 2011;108(16):6462–6467. doi: 10.1073/pnas.1018260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yea CH, Jeong HC, Moon SH, Lee MO, Kim KJ, Choi JW, Cha HJ. In situ label-free quantification of human pluripotent stem cells with electrochemical potential. Biomaterials. 2016;75:250–259. doi: 10.1016/j.biomaterials.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 98.Han J, Qian X, Wu Q, Jha R, Duan J, Yang Z, Maher KO, Nie S, Xu C. Novel surface-enhanced Raman scattering-based assays for ultra-sensitive detection of human pluripotent stem cells. Biomaterials. 2016;105:66–76. doi: 10.1016/j.biomaterials.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ben-David U, Nudel N, Benvenisty N. Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat Commun. 2013;4:1992. doi: 10.1038/ncomms2992. [DOI] [PubMed] [Google Scholar]
- 100.Kim KT, Jeong HC, Kim CY, Kim EY, Heo SH, Cho SJ, Hong KS, Cha HJ (2017) Intact wound repair activity of human mesenchymal stem cells after YM155 mediated selective ablation of undifferentiated human embryonic stem cells. J Dermatol Sci. doi:10.1016/j.jdermsci.2017.01.011 [DOI] [PubMed]