Abstract
Pulmonary arterial hypertension (PAH) is characterized by persistent pulmonary vasoconstriction and pulmonary vascular remodeling. The pathogenic mechanisms of PAH remain to be fully clarified and measures of effective prevention are lacking. Recent studies; however, have indicated that epigenetic processes may exert pivotal influences on PAH pathogenesis. In this review, we summarize the latest research findings regarding epigenetic regulation in PAH, focusing on the roles of non-coding RNAs, histone modifications, ATP-dependent chromatin remodeling and DNA methylation, and discuss the potential of epigenetic-based therapies for PAH.
Keywords: Epigenetic, Pulmonary arterial hypertension, miRNAs, lncRNAs, HDACs, PASMCs, PAECs
Introduction
Pulmonary arterial hypertension (PAH) is a fatal disease, characterized by a progressive increase in pulmonary artery pressure that is accompanied by pulmonary vascular remodeling, increased vasomotor tone and compensatory right ventricular hypertrophy, leading to heart failure and death. Five categories of PAH have been defined according to clinical, etiological, and hemodynamic features, but share common pathogenesis [1]. The vascular remodeling can be extensive, involving medial hypertrophy and formation of neointima and plexogenic lesions; moreover, these changes, individually and collectively, are associated with excessive cell proliferation, apoptosis resistance, phenotypic transition, metabolic shifts, and the recruitment of circulating inflammatory cells [2, 3].
Researchers are just beginning to elucidate the myriad of molecular factors underlying the causes and consequences of vascular remodeling. Endothelial cells (ECs) appear to be involved in the earliest stages, exerting direct modulating effects on other cells, such as pulmonary artery smooth muscle cells (PASMCs), fibroblasts and even other ECs, through paracrine signaling [4]. The initiation of different signaling pathways under these conditions can support sustained vasoconstriction and proliferation, as well as the anti-apoptotic phenotype. EC signaling is also capable of recruiting pro-inflammatory cells, which in the pulmonary arteries then sustain the inflammatory microenvironment and result in increased vascular wall thickness and muscularization of the small pulmonary arterioles [5–7]. The physiological imbalances related to PAH pathogenesis have known associations with genetic susceptibilities and non-homeostatic responses in gene regulation that occur via epigenetic modification [8].
The term epigenetics in its contemporary usage emerged in the 1990s, but for some years after it was applied with somewhat variable intent [9]. A consensus definition of the concept of epigenetic trait as a “stably heritable phenotype resulting from changes in a chromosome without alterations in the DNA sequence” was formulated at a Cold Spring Harbor meeting in 2008. During this time, intensive investigations sought to uncover the multitude of epigenetic mechanisms that contribute to normal physiology as well as pathogenesis of a wide array of human diseases, from metabolic dysfunctions to infectious diseases; as a result the beneficial and detrimental roles of chromatin activation, DNA methylation, histone modification and non-coding RNA (ncRNA) regulation have been extensively studied.
Accumulating evidence supports the hypothesis that epigenetic changes are involved in PAH [10, 11]. Furthermore, various epigenetic mechanisms have been confirmed to exert a profound influence on PAH, including miR deregulation, DNA methylation and histone modification [5]. Gaining a detailed understanding of the general and specific epigenetic principles in PAH may be a key for developing new and more effective treatments for PAH.
Herein, we review the latest advances in the understanding of epigenetic regulation in PAH, focusing on the role of ncRNAs, histone modifications, ATP-dependent chromatin remodeling, and DNA methylation and we discuss the potential for epigenetic-based therapy for PAH.
ncRNAs involvement in PAH
Around 98% of all RNA transcripts do not possess protein coding capability [12]. It is now clear that many, if not all, of them regulate individual steps of gene expression, including transcription, RNA processing and translation [13]. The ncRNAs can be broadly classified according to their size, representing the long ncRNAs (lncRNAs), which are longer than 200 nucleotides (nts) and can range up to hundreds of thousands of nts in length, and the small ncRNAs, which are less than 200 nts in length; the latter classification includes the much more recently discovered micro (mi) RNAs, which are ~22 nts in length [14].
In this section, we will discuss the effects and underlying mechanisms of miRNAs and lncRNAs on PAH pathogenesis. The information for the particular miRNAs and lncRNAs discussed herein are summarized in Table 1.
Table 1.
Non-coding RNAs involved in PAH development
| ncRNA | Expression in PAH | Function | Target mRNA and signaling pathway | Models | References |
|---|---|---|---|---|---|
| miRNAs | |||||
| miR-let-7g | ↓ | Promote PASMC proliferation and pulmonary vascular remodeling | C-myc-Bmi-1-p16 signaling | Rat-hypoxia | [87] |
| miR-9 | ↑ | Promote PASMC phenotypic switch (inhibit SMC differentiation) | HIF1α-miR-9 | Rat-hypoxia | [61] |
| miR-17/92 | ↑ | Promote EC injury; Induce PASMC proliferation and reduce PASMC apoptosis | p21, STAT3-miR-17/92-BMPR2 pathway, HIF-1α and MFN2 |
Mouse-hypoxia Rat-MCT, hypoxia Human-PAH |
[29, 67, 89, 90, 105] |
| miR-20a | ↑ | Promote PASMC proliferation and migration; inhibit PASMC differentiation | PRKG1, BMPR2 | Mouse-hypoxia HPASMC-hypoxia | [49, 50] |
| miR-21 | ↑ | Promote PAEC and PASMC proliferation, migration; vasoconstriction and pulmonary vascular remodeling | PDCD4, SPRY2, PPARα, WWP1, SATB1, PTEN, BMPR2 and RhoB |
Mouse-hypoxia Rat-MCT Human-PAH HPASMC/HPAEC-hypoxia |
[30, 52, 53, 72] |
| miR-23a | ↑ | Promote PASMC phenotypic transformation | HIF1α-miR-23a |
Rat-hypoxia HPASMC-hypoxia |
[62] |
| miR-26b | ↓ | Promote PASMC proliferation and pulmonary vascular remodeling | CTGF and CCND1 | Rat and PASMC-MCT | [88] |
| miR-27a | ↑ | Promote PAEC proliferation | PPARγ-ET-1 |
Mouse-hypoxia HPAEC-hypoxia |
[40] |
| miR-27b | ↑ | Promote vasoconstriction and remodeling | PPARγ-Hsp90-eNOS |
Rat-MCT HPAEC |
[41] |
| miR-29 | ↑ | Regulate energy metabolism and promote pulmonary vascular remodeling | PPARγ |
Human-PAH Bmpr2 mutant Mice-16αOHE |
[54] |
| miR-34a | ↓ | Promote PASMC proliferation and migration; Reduce SMC apoptosis | PDGFA |
Rat-hypoxia HPASMC-hypoxia |
[73] |
| miR-103/107 | ↓ | Promote PASMC proliferation and pulmonary vascular remodeling | HIF-1β | Rat-hypoxia | [70] |
| miR-124 | ↓ | Promote PASMC and fibroblast proliferation and migration | SMCs: NFATc1 CAMTA1 and PTBP1; Fibroblasts: MCP-1, PTBP1, Notch1/PTEN/FOXO3/p21 and p27 signaling |
Mouse-hypoxia Human and calve PAH |
[82, 93] |
| miR-126 | ↓ | Promote PAEC proliferation, migration and angiogenesis | Spred-1 | Human-PAH, plexiform lesions | [43, 44] |
| miR-130/301 | ↑ | Promote PASMC and PAEC proliferation | PPARγ-STAT3-miR-204-Src signaling, CDKN1A(p21) (SMC); PPARγ-apelin-miR-424/503-FGF2 signaling (EC) | Human-PAH Mouse-hypoxia | [42, 46, 71] |
| miR-135a | ↑ | Pulmonary vascular remodeling | BMPR2 | Mouse-OVA and PM | [55] |
| miR-138 | ↑ | Reduce PASMC apoptosis; Promote HMVEC dysfunction | S100A1; HIF-1α-miR-138-Mst1-akt signaling |
Rat-hypoxia PASMC-hypoxia HMVEC-hypoxia |
[36, 37, 63] |
| miR-140-5p | ↓ | Promote PASMC proliferation, migration and pulmonary vascular remodeling | Smurf1-BMP signaling | Human-PAH Rat-MCT, sugen5416/hypoxia | [56] |
| miR-143-3p | ↑ | Promote PASMC and PAEC migration (crosstalk) and pulmonary vascular remodeling |
Mouse-hypoxia Human and calve PAH |
[58] | |
| miR-145 | ↑ | Promote development of PAH | KLF4, KLF5, Smad4, Smad5 |
Mouse-hypoxia, BMPR2 deficient Human heritable and idiopathic PAH, BMPR2 mutation |
[44] |
| miR-143/145 | ↓ | Promote PASMC phenotypic switch and pulmonary vascular remodeling | BMP4, TGF-β, myocardin | Human-PAH, plexiform lesions | [44, 59] |
| miR-150 | ↓ | Poor survival | Human-PAH plasma | [23] | |
| miR-190 | ↑ | Enhance vasoconstriction and Ca2+ influx in PASMCs | KCNQ5 | Rat-hypoxia | [86] |
| miR-193 | ↓ | Promote PASMC proliferation | LOXs and IGF1R |
Mouse-hypoxia Rat-MCT |
[74] |
| miR-199a-5p | ↑ | Promote vasoconstriction | Smad3 | Rat-MCT | [31] |
| miR-204 | ↓ | Promote cell survival, proliferation and apoptosis resistance, calcified lesion | SHP2-Src/STAT3 axis and PARP1-NFAT/HIF1α signaling pathway, RUNX2 |
Human-PAH, plexiform lesions Mouse-hypoxia Rat-MCT, sugen5416/hypoxia |
[44, 83–85] |
| miR-206 | ↓ | Promote PASMC proliferation; reduce apoptosis | Notch3 and HIF-1α/Fhl-1 pathway | Mouse and rat-hypoxia | [68, 69] |
| miR-210 | ↑ |
Reduce PASMC apoptosis Regulate cell metabolism |
HIF-1α-miR-210-E2F3 signaling; ISCU1/2-Iron-sulfur (Fe-S) clusters |
Mouse-sugen5416/hypoxia PASMC-hypoxia |
[64, 65] |
| miR-214 | ↑ | Regulate right ventricular hypertrophy | PTEN | Mouse and rat-sugen5416/hypoxia | [106] |
| miR-223 | ↓ | Promote PASMC proliferation and migration; reduce apoptosis | PARP1, RhoB, MLC2 and IGF-1R |
Human-PAH Rat-MCT, hypoxia Mouse-hypoxia |
[75–77] |
| miR-322 | ↑ | Promote PASMC proliferation and migration |
BMPR1a and Smad5 (HIF-1α-miR-322-BMP-Smad signaling pathway) |
Mouse and rat-hypoxia PASMC-hypoxia |
[66] |
| miR–328 | ↓ | Promote vasoconstriction and remodeling; reduce SMC apoptosis | CaV1.2 and IGF1R | Rat-hypoxia | [78] |
| miR-424/503 | ↓ | Promote PAEC and PASMC proliferation (crosstalk) and pulmonary vascular remodeling | Apelin/MEF2-miR-424/503-FGF2, FGFR1 pathway | Rat-MCT, sugen5416/hypoxia | [45, 107] |
| lncRNAs | |||||
| MALAT-1 | ↑ | Promote EC proliferation, migration and vessel growth | Cell cycle regulators (CCNE1,CCNA2, p21) and XBP1, |
Mouse Retinal Angiogenesis Model EC-hypoxia |
[100, 101] |
| MANTIS | ↓ | Regulate angiogenesis | Interact with BRG1 |
Human-PAH Rat-MCT |
[102] |
| lncRNA-p21 | Promote SMC and fibroblast proliferation and neointimal hyperplasia; inhibit SMC apoptosis | P53, Thy-1 | Human-coronary artery disease Mouse-ARDS and lung fibroblasts—LPS | [103, 104] | |
Cell types: PAEC, pulmonary artery endothelial cell; PASMC, pulmonary artery smooth muscle cell
Target genes/proteins: ACE, angiotensin-converting enzyme; BMPR, bone morphogenetic protein receptor; BRG1, Brahma-related gene 1, CAMTA1, calmodulin-binding transcription activator 1; CaV1.2, L-type calcium channel subunit alpha 1C; CCNA2, cyclin A2;CCND1, cyclin D1; CCNE1, cyclin E1; CTGF, connective tissue growth factor; E2/F3, transcription factor E2F3; Elk-1, ETS domain-containing protein 1; FGF2, fibroblast growth factor 2; FGFR1, fibroblast growth factor receptor 1; Fhl-1, four and a half LIM domains protein 1; FOXO3, forkhead box O3; HIF, hypoxia-inducible factor; IGF1R, insulin growth factor 1 receptor; ISCU, iron-sulfur cluster assembly enzyme; KCNQ5, potassium voltage-gated channel subfamily KQT member 5 protein; KLF4, Kruppel-like factor 4; KLF5, Kruppel-like factor 5; LOXs, lipoxygenases; LPS, lipopolysaccharide; MCP-1, monocyte chemotactic protein-1; MCT, monocrotaline; MLC2, myosin light chain of myosin II; MFN2, Mitofusin 2; Mst 1, macrophage stimulating protein 1; NFAT, nuclear factor of activated T cells; Nkx2.5, NK2 transcription factor related, locus 5; Notch1, neurogenic locus notch homolog 3 protein 1; Notch3, neurogenic locus notch homolog protein 3; p21, cyclin-dependent kinase inhibitor 1; p27, cyclin-dependent kinase inhibitor 1B; PARP1, poly(ADP-ribose) polymerase-1; PDCD4, programmed cell death protein 4; PDGFA, platelet-derived growth factor receptor alpha; PPAR, peroxisome proliferator–activated receptor alpha; PPARγ, peroxisome proliferator-activated receptor-gamma; PRKG1, protein kinase, cGMP-dependent, type I; PTBP1, polypyrimidine tract-binding protein 1; PTEN, phosphatase and tensin homolog; RhoB, Ras homolog gene family, member B; RUNX2, Runt-related transcription factor 2; S100A1, S100 calcium-binding protein A1; SATB1, special AT-rich sequence-binding protein-1; SHP, src homology region 2 domain-containing phosphatase; Smad, Sma and Mad-related protein; SMURF1, SMAD-specific E3 ubiquitin protein ligase 1; Sp-1, specificity protein 1; Spred-1, Sprouty-related protein-1; SPRY2, Sprouty homolog 2; Src, src kinase; STAT3, signal transducer and activator of transcription 3; TGF-β, transforming growth factor β; Thy-1, thymocyte differentiation antigen-1; WWP1, WW domain-containing protein 1; XBP1, X box-binding protein 1
miRNAs
miRNAs silence gene expression by binding to the 3′-untranslated regions of messenger (m)RNAs and inhibiting translation or promoting degradation of the mRNAs [15, 16]. It is estimated that each miRNA expressed in a given cell may target about 100–200 mRNAs for down-regulation [17], and that about 60% of human protein coding genes are regulated by miRNAs. Interestingly, studies of miRNAs in PAH have suggested key roles in the pathogenic mechanism [18–20], making them novel candidates for therapeutic intervention [21]. Furthermore, a panel of circulatory miRNAs, including miR-126, miR-150, miR-193, and miR-204 appear to be promising biomarkers for PAH diagnosis and prognosis [22, 23]. These collective studies have also suggested that miRNAs may contribute to the development of PAH by regulating the cellular components known to be critically involved in the disease process, namely the PASMCs, and the pulmonary artery endothelial cells (PAECs) and fibroblasts.
miRNAs and endothelial dysfunction in PAH
The PAEC houses in innermost intimal layer of pulmonary vascular and immediate contact with the blood supply and able to detect changes in pressure, circulating factors and oxygenation. Proliferating and dysfunctional PAECs are considered as characteristic features of intimal thickening in PAH, and the targeting activities of miRNAs have been implicated in proliferation and apoptosis of these cell types through different signaling pathway.
BMPR2 signaling
Bone morphogenetic protein receptor (BMPR) 2 is a receptor for the transforming growth factor (TGF)-β/BMP superfamily, the loss of function has been linked to cellular pathophenotypes, including proliferation, cell survival [24, 25], endothelial dysfunction [26], repression of mitochondrial metabolism, and endothelial-to-mesenchymal transition and then leading to the development of PAH [27, 28]. miR-17/92 has been shown to mediate the endothelial injury that triggers PAH, itself being transcriptionally regulated by signal transducer and activator of transcription (STAT3) and targeting either BMPR2 directly to result in cell proliferation and apoptosis resistance [29]. The increased miR-21 is also known to target BMPR2 and to suppress Rho/Rho kinase activity, as shown in cultured human PAECs, in hypoxia, accompanying increased angiogenesis and vasoconstriction [30]. miR-199a-5p has also been reported as involved in PAH by targeting to the BMPR-Sma and Mad-related protein (Smad) signaling pathway; specifically, it was shown to be significantly increased in PAH rat models, while anti-miR-199a-5p was shown to increase nitric oxide (NO) level and decrease cytoplasmic Ca2+ level in PAECs, thereby decreasing the pulmonary artery pressure and right ventricular hypertrophy by attenuating the expression of Smad3, which was confirmed to be the target gene of miR-199a-5p [31].
HIFs signaling
Hypoxia-inducible factors (HIFs) are transcription factors that respond to decreased available oxygen in the cellular environment, or hypoxia. Active HIF transcription factors are comprised of an oxygen-regulated α-subunit and a constitutive β-subunit, and there are three HIF-α isoforms in humans, including HIF-1α, HIF-2α and HIF-3α [32]. Chronic hypoxia, or prolonged low oxygen exposure, is a key trigger for pulmonary vascular remodeling and PAH. The alteration of HIFs signaling in various PAH-relevant pathways contributing to vasocontraction, vascular cell proliferation, metabolic shifts and inflammation [33–35]. The transcription factor HIF-1α, a major mediator of these effects, modulates various miRNAs and reciprocally is regulated by miRNAs. The up-regulation of miR-138 has been shown to be HIF-1α-dependent and involved in hypoxia-induced EC dysfunction by regulating nitric oxide (NO) expression through S100A1 [36, 37]. miR-138 have also been shown to inhibit hypoxia-induced proliferation of endothelial progenitor cells [38].
PPARγ signaling
Peroxisome proliferator-activated receptor-γ (PPARγ) is a nuclear hormone receptor that interacts with retinoid receptor (RXR) form heterodimer, upon binding with its ligand, triggering transcriptional activation of its target genes. The reduced expression of PPARγ has been shown to contribute to PAH progression by promoting proliferative and inhibiting apoptotic program in the pulmonary vasculature [39]. miR-27a was found to be increased under hypoxia, and to promote PAEC proliferation by regulating endothelin-1 (ET-1) expression through PPARγ [40]. miR-27b have also been shown to be up-regulated in monocrotaline (MCT)-induced PAH and inversely correlated with the levels of PPARγ, which was shown to mediate the disruption of endothelial nitric oxide synthase (eNOS) coupling to heat shock protein (Hsp)90 and the suppression of NO production associated with the PAH phenotype [41]. miR-130/301 was also up-regulated and this contributes to the proliferation of PAECs through the activation of PPARγ [42].
Related growth factors signaling
Growth factors play an important role in regulating a variety of cellular processes, such as cellular growth, proliferation and differentiation, through binding to specific receptors on the surface of their target cells. Lots of growth factors signaling pathway play important role in the formation of PAH. Some miRNAs have been shown to be down-regulated in PAH models and their participation in the pathogenesis is a promising topic of study. For instance, miR-126 was found to be down-regulated in plexiform lesions of patients with severe PAH, and contribute to the proliferation, migration of endothelial cells and angiogenesis by regulating several growth factors expression, such as vascular endothelial growth factor-A (VEGF-A) and fibroblast growth factor (FGF), through sprouty-related, EVH1 domain-containing protein-1 (Spred-1) [43, 44]. The down-regulation of miR-424 and miR-503 in hypoxia has been associated with, and proposed to result to, the FGF signaling (FGF2 and FGFR1) which is known to play a key role in regulating proliferation of PAECs and in maintaining pulmonary vascular homeostasis [45]. Bertero et al. [42] reported that miR-424/503-FGF2 signaling have also been regulated by up-regulated miR-130/301 in PAECs through the activation of PPARγ.
Whereas EC dysfunction that can directly lead to intimal thickening and remodeling, probably equally important are the indirect signals capable of inducing PASMCs contraction, hypertrophy, and even hyperplasia. For instance, the down-regulation of miR-424 and miR-503 is known to play a key role in regulating proliferation of PAECs. And interestingly, the alteration of miR-424 and miR-503 in PAECs also contribute to the proliferation of PASMCs through PAEC-PASMC crosstalk [45]. The up-regulation of miR-130/301 in PAECs also link to the contractile function of PASMCs through the activation of endothelin-1 and its receptors [46].
miRNAs and PASMCs in PAH
Pulmonary vascular medial thickening, attributable mostly to PASMC proliferation and hypertrophy, is the main feature of pulmonary artery remodeling of PAH. PASMCs exhibit extraordinary plasticity in adult animals in response to a number of environmental cues. Under hypoxia, PASMCs undergo phenotypic switch from a contractile-differentiated to a proliferative/migratory-dedifferentiated phenotype [47]. The dedifferentiated smooth muscle cells are characterized by high rates of proliferation and migration, increased expression of the extracellular matrix (ECM) proteins and low expression of contractile proteins, which are comprised of smooth muscle actin-α (α-SMA), smooth muscle-myosin heavy chain (SM-MHC), calponin, caldesmon and sm22-α [48]. This process plays a major role in the development of vascular remodeling and PAH. Studies have shown that numbers of miRNAs dysregulated in PAH substantially contribute to its pathogenesis through targeting several signaling pathways, such as BMPR2, HIFs, PPARγ and NFAT signaling.
BMPR2 signaling
The expression of miR-20a was increased in lung, pulmonary artery and serum of hypoxia PAH modeled mice, and in human PASMCs exposed to hypoxia, thereby promoting the proliferation and migration of human PASMCs; the authors also showed that it inhibits the differentiation of PASMCs, which is otherwise accompanied by decreased BMPR2 [49] and cGMP-dependent protein kinase, type I(PRKG1) [50] expression. And the decreased PRKG expression has been demonstrated strongly related to the decrease expression levels of smooth muscle cell contractile markers in hypoxia [51]. miR-21 was also up-regulated in hypoxia-induced PASMCs, affecting proliferation and migration as well as pulmonary vascular remodeling and PAH by regulating multiple related gene targets, including BMPR2 [52, 53]. miR-29 has also been shown to be up-regulated in lung tissue of PAH patients and BMPR2 mutant mice, and anti-miR-29 had improvements in hemodynamic profile, histology, and markers of dysregulated energy metabolism [54]. Similarly, the marked increase in miR-135a induced by combined Th2 antigen (Ovalbumin, OVA) and urban particulate matter (PM) in mice has been shown to contribute the pulmonary artery remodeling by regulating BMPR2 [55]. The increased miR-199a-5p, involved in PAECs dysfunction, also contributed to PASMCs proliferation by targeting the BMPR-Sma3 [31]. The expression of miR-140-5p is reduced in patients with PAH and experimental models of PAH. This dysregulation of miRNA induces PASMC proliferation, migration and promote the development of PAH by allowing for activation of SMAD-specific E3 ubiquitin protein ligase 1 (SMURF1)—a regulator of BMP signaling [56].
In other studies, miR-145 was shown to be up-regulated in lung tissue of patients with idiopathic and heritable PAH and also in the lungs of hypoxia or BMPR2-deficient mice, with miR-145 deficiency and anti-miR-mediated reduction shown to provide significant protection from the development of PAH [57]. Similarly, the marked increase of miR-143-3p in PAH models of calf and mice, and in samples from PAH patients, and in PASMCs exposed to TGF-β, causing a significant increase in PASMCs migration. The up-regulation of 143-3p in PASMCs also contribute to the migration of PAECs through PASMC-PAEC crosstalk [58]. Conversely, miR-143/145 were also found to be down-regulated in plexiform lesions of patients with severe PAH [44]. And interestingly, the down-regulation of miR-143/145 were also found in aortic artery SMCs, and cooperatively targeted a network of transcription factors, including Kruppel-like factor (Kfl)1, Klf5 and myocardin that regulates smooth muscle cell-specific genes’ expression, further regulate SMC fate and plasticity [59, 60].
HIFs signaling
Various miRNAs have been reported to be regulated by HIF-1α under hypoxic conditions. It has been demonstrated that miR-9 [61] and miR-23a [62] are both induced by hypoxia and subsequently involved in a hypoxia-induced phenotypic switch of the rat PASMCs. Specifically, the up-regulation of miR-9 and miR-23a were shown to be mediated by HIF-1α—a key transcription factor for hypoxia-induced gene transcription. Up-regulation of miR-138 under hypoxic conditions has been shown to be HIF-1α-dependent and plays a role in SMCs apoptosis and hypoxic pulmonary vascular remodeling by targeting macrophage stimulating protein (Mst) 1 [63]. Similarly, the marked increase of miR-210 under hypoxic conditions has also been shown to be HIF-1α-dependent and transcription factor E2F3, and iron-sulfur cluster assembly enzyme (ISCU)1/2 were identified as its targets responsible for PAH in PASMCs and in PAH mouse model [64, 65]. miR-322 has also been shown to be up-regulated in lungs of chronically hypoxic mice and rats, and in primary cultured rat PASMCs under hypoxic conditions has been shown to be HIF-1α-dependent, and that this change in miR expression promoted hypoxia-induced cell proliferation and migration by directly targeting BMPR1a and Smad5 [66].
The transcription factor HIF-1 has also been regulated by lots of miRNAs. Chen et al. [67] suggest that the increased expression of miR-17/92 in PASMCs under hypoxic conditions contributes to PASMC proliferation by directly targeting of PHD2 and then induce HIF-1α expression. Additionally, a plethora of other miRNAs down-regulated under hypoxic conditions has been considered as contributors to PAH. Among them, miR-206 has been found to be decreased both in animal models of PAH and in cultured PASMCs, with the dysregulation being shown to cause increased proliferation and reduced apoptosis of PASMCs likely due to the up-regulation of Notch3 [68] or by targeting HIF-1α [69]. miR-103/107 down-regulation was observed in remodeled intrapulmonary vascular in HPH rats and hypoxia-exposured PASMCs, and inversely correlated with the expression of HIF-1β, but not HIF-1α [70].
PPARγ signaling
Bertero et al. [42] first demonstrated that miR-130/301 was up-regulated in different PAH models and in patients with PAH, leading to sustainment of the PAH-PASMC pro-proliferative phenotype through the activation of PPARγ [42, 46] and cyclin-dependent kinase inhibitor(CDKN) 1 (alias p21) [71]. The authors subsequent findings suggested that down-regulation of miR-204 could result from the activation of miR-130/301 in PASMCs [42, 46]. PPARγ agonists (rosiglitazone) has reported to attenuate hypoxia-induced HPASMC proliferation, vascular remodeling and PAH through inhibition of hypoxia-induced miR-21 expression, which emerged as an important miRNA that contributes to PAH pathogenesis [72].
Related growth factors signaling
Wang et al. [73] has demonstrated that the down-regulation of miR-34a in rat distal PAs and HPASMCs is associated with the proliferation of PASMCs by enhancing platelet-derived growth factor receptor alpha (PDGFRA) expression. Decreased miR-193 expression has been observed in both the lung tissue and serum from patients with PAH as well as from rodent models of PAH [74]; this dysregulation of miRNA induces PASMC proliferation by allowing for activation of insulin growth factor 1 receptor (IGF1R) and lipoxygenases (LOXs). miR-223 has also been found to be down-regulated in patients and animal models of PAH, thereby significantly allowing PAH-PASMCs proliferation and resistance to apoptosis by targeting IGF-1and its receptor IGF-1R [75], poly(ADP-ribose) polymerase-1(PARP-1) [76], RhoB and myosin light chain of myosin II (MLC2) [77]. Similarly, the marked decrease in miR-328 induced by hypoxia has been shown to contribute to the pulmonary artery constriction and remodeling by regulating IGF-1R and L-type calcium channel-alpha 1C (CaV1.2) in hypoxic pulmonary hypertension [78].
NFAT signaling
The nuclear factor of activated T cells (NFAT), originally identified in T lymphocytes represents a family of Ca2+-dependent transcription factors comprising five isoforms: NFATc1 to -c4 and NFAT5. NFAT was activated and dephosphorylated by calcineurin, a Ca2+/calmodulin-dependent phosphatase, and then regulate diverse cellular process including growth and survival and are involved in the development of cancer and cardiovascular diseases [79]. It has been observed that NFAT family members are involved in the pathogenesis of PAH. NFAT has been shown to be up-regulated and activated in PAH patients, mouse model of PAH and hypoxia-treated PASMC, which is associated with increased cell proliferation and resistance to apoptosis by regulating membrane potential, Kv1.5 and increasing [Ca2+] influx [80, 81]. miR-124 has been shown to be down-regulated by hypoxia in human PASMCs and lung cells of hypoxia-induced PAH mouse model, consistent with the activation of nuclear factor of activated T cells (NFAT) during this process; in contrast, overexpression of miR-124 was shown to not only inhibit human PASMC proliferation, but to maintain its differentiated phenotype by repressing the NFAT pathway [82]. In line with those findings, down-regulated expression of miR-204 has also been found in human patient samples and in rodent models of PAH, the latter of which showed that rescue of miR-204 reverses the disease state [44, 83]. Various studies have begun to elucidate the mechanisms by which miR-204 might be involved in PAH, namely through activation of the PARP-1signaling pathway via regulating NFATc2 and HIF-1α [84], or through regulation of the Src/STAT3 axis [44, 83] and Runt-related transcription factor 2 (RUNX2) expression [85]. Another miRNA, miR-190, was reported to be significantly increased in the pulmonary artery and PASMCs under hypoxic conditions, and this differential expression was associated with the accompanying vasoconstriction responses and Ca2+ influx through targeting to the voltage-gated K(+) channel subfamily member, Kcnq5 [86].
Cyclin related signaling
miR-let-7g was down-regulated in remodeled pulmonary arteries of hypoxia PAH modeled rats, and in PASMCs exposed to hypoxia, with the dysregulation being shown to cause increased proliferation of PASMCs by regulating C-myc-Bmi-1-cyclin-dependent kinase inhibitor 2A(p16) signaling pathway [87]. miR-26b was also down-regulated in MCT induced PAH model rat, and in PASMCs exposed to MCT, and inversely correlated with the expression of connective tissue growth factor (CTGF) and cyclin D1 (CCND1),which were involved in pulmonary vascular remodeling by promoting PASMCs proliferation [88]. miR-17 was significantly increased in hypoxia or MCT induced PAH models, and the treatment of miR-17 inhibitor significantly decrease the proliferation of PASMCs, and right ventricular systolic pressure, and pulmonary vascular remodeling. The beneficial effects may be related to the up-regulation of cyclin-dependent kinase inhibitor 1 (p21) [89] and Mitofusin 2 (MFN2) [90]. Conversely, Bockmeyer et al. [44] showed that miR-17 expression was down-regulated in plexiform lesions of patients with severe PAH.
miRNAs and fibroblasts in PAH
The thickening of pulmonary vascular adventitia in PAH attributable to a significant increase in collagen and ECM protein deposition, proliferation of resident fibroblasts and possibly macrophages, as well as recruitment of circulating immune and progenitor cells [91]. Hypoxia can directly induce the differentiation of pulmonary artery adventitial fibroblasts into myofibroblasts, which likely play an important pathophysiological role in contributing to abnormalities of tone and structure of the pulmonary artery in PAH [92]. Wang et al. [93] demonstrated that miR-124 was decreased in hypertensive pulmonary adventitial fibroblasts, contributing to an epigenetically reprogrammed, highly proliferative, migratory and inflammatory phenotype via direct binding to polypyrimidine tract-binding protein 1 (PTBP1) and subsequent regulation of Notch1/phosphatase and tensin homolog/FOXO3/p21Cip1 and p27Kip1 signaling; moreover, it was shown that miR-124 expression was suppressed by histone deacetylases and that treatment of hypertensive fibroblasts with histone deacetylase inhibitors increased miR-124 expression and decreased proliferation and production of the monocyte chemotactic protein-1 (MCP-1).
lncRNAs
lncRNAs, non-protein coding transcripts longer than 200 nucleotides, represent an important component of animal genome regulation [94], exerting multiple developmental and cell-type-specific regulatory functions, and with greatly expanded numbers in multicellular animals and plants [95]. The lncRNAs regulate gene transcription under normal physiologic conditions through a variety of epigenetic, transcriptional and posttranscriptional mechanisms, such as splicing and epithelial-mesenchymal transition [96–98]. Recent research interest in the lncRNAs has segued into their potential roles in various pathogenic pathways, such as those underlying cardiovascular diseases, cancer and, more recently, PAH.
Comparative microarray analysis of lncRNAs and mRNAs in lung tissues from a hypoxia-induced pulmonary hypertension (HPH) rat model and a control group identified a total of 362 lncRNAs as significantly differentially expressed [99]. One of these lncRNAs, the metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) which is known to control phenotypic switch of ECs and regulates vessel growth, was significantly increased under hypoxia. Moreover, genetic ablation of MALAT1 has been shown to inhibit proliferation of ECs and to reduce vessel growth [100]. Zhuo et al. [101] further indicated that the single nucleotide polymorphism (SNP) alteration from rs619586A to G in MALAT1 could directly upregulate X box-binding protein 1 (XBP1) expression, and consequentially inhibiting the vascular endothelial cells proliferation and migration in vitro, and decreasing the risk of PAH in Chinese people. LncRNA-MANTIS was down-regulated in patients with idiopathic pulmonary arterial hypertension (IPAH) and in rats treated with monocrotaline and the deletion or silencing of MANTIS inhibited angiogenic sprouting and alignment of endothelial cells in response to shear stress [102]. In a study of the neointimal hyperplasia observed in a model of carotid artery injury (which shows vascular remodeling similar to that in PAH), lncRNA-p21 expression was found to be decreased and linked to imbalance between proliferation and apoptosis of vascular smooth muscle cells [103]. Conversely, lncRNA-p21 expression showed a time-dependent increase in a mouse model of acute respiratory distress syndrome (ARDS) and in lipopolysaccharide-treated lung fibroblasts, subsequently contributing to elevated cell proliferation; moreover, the mechanism by which lncRNA-p21 promotes pulmonary fibrosis in ARDS was shown to involve inhibition of the expression of thymocyte differentiation antigen-1(Thy-1) through inhibition of acetylation of H3 and H4 at the Thy-1 promoter [104].
Collectively, these lncRNA findings support the ongoing research interest in lncRNAs contributions to PAH, likely through regulation of cellular proliferation.
Chromatin remodeling in PAH
Chromatin remodeling is the enzyme-assisted process to facilitate access of nucleosomal DNA by remodeling the structure, composition and positioning of nucleosomes. Such remodeling is principally carried out by; (1) histone modifications by specific enzymes and (2) ATP-dependent chromatin remodeling [108]. Although remodeled chromatin is not always inherited, and not all epigenetic inheritance involves chromatin remodeling. Chromatin remodeling is essential to several important biological processes, including DNA replication and repair, chromosome segregation, embryonic development, apoptosis and cell-cycle progression.
Histone modification in PAH
The nucleosome, the fundamental unit of eukaryotic chromatin, is composed of four core histones (H2A, H2B, H3 and H4) surrounded by 146 bp of DNA. The linker histones (H1 and H5) bind the nucleosome at the entry and exit sites of the DNA, thus locking the DNA into place [109]. The H3 and H4 histones have long tails protruding from the nucleosome, which can be covalently modified on specific residues, catalyzed by histone-modifying enzymes. These tail modifications include methylation, acetylation, phosphorylation, ubiquitination, SUMOylation, citrullination and ADP-ribosylation [110]. The posttranslational modifications of histone tails are important components of the regulatory genomic landscape in eukaryotes, and it has been confirmed that most of the well-studied histone modifications are involved in control of transcription.
Among the histones, modifications to H3 and H4 have been found to be largely conserved across the eukaryotes analyzed. Acetylation modifications involve the lysine residues and are coupled by histone acetyltransferases and removed by histone deacetylases (HDACs), which was classified into five subgroups: class I (HDAC 1, 2, 3, 8), class IIa (HDAC 4, 5, 7, 9), class IIb (HDAC 6, 10), class III (sirt1-7) and class IV (HDAC 11) [111]. Acetylation of lysine residues within nucleosomal histone tails also plays central roles for epigenetic control of gene expression, and is usually associated with active transcription. On the other hand, particular trimethylations of H3, trimethylation of H3 lysine 4 (H3K4Me3) [112] and trimethylation of H3 lysine 36 (H3K36Me3) [113], appear to be exclusively associated with active transcription. Some methylations of H3 and H4 are associated with repressed genes; these include trimethylation of H3 lysine 27 (H3K27Me3) [114], di- and trimethylation of H3 lysine 9 (H3K9Me2/3) [115] and trimethylation of H4 lysine 20 (H4K20Me3) [116].
Histone modifications act in diverse biological processes, such as gene regulation, DNA repair, chromosome condensation (mitosis) and spermatogenesis (meiosis) [117]. Consequently, they play important roles in various pathogeneses, and studies have begun to uncover their contribution to PAH as well. Several studies have demonstrated that early epigenetic changes, such as the maternal nutrient restriction consequence of increased histone acetylation, can result in a highly sensitive phenotype to hypoxia later in life, thereby causing more significant PAH or pulmonary vascular remodeling [118]. These events are similar to the effects of epigenetics in mitochondrial stress-induced longevity [119, 120].
Class I HDACs are dramatically elevated in the pulmonary arteries of humans with PAH and in the lungs and vessels from PAH animal models, and have been reported to contribute to pulmonary vascular remodeling by promoting smooth muscle cell proliferation [121]. Inhibition of class I HDACs alleviates the PDGF-induced smooth muscle cell proliferation and migration by inhibiting Akt phosphorylation and cyclin D1 expression [122]. Zhao et al. [123] showed that expression of HDAC1 and HDAC5 was increased in rats exposed to hypoxia and in human idiopathic PAH and that these differential expressions were associated with fibroblast proliferation and inflammatory cytokines’ expression by regulating p21, FOXO3 and survivin levels. The class I HDACs was also reported to regulate the activation of distinct adventitial fibroblasts and monocytes/macrophages, both of which are associated with changes in cytokine/chemokine expression and pro-inflammatory response activation [124]. In addition, IGF-1 expression is also regulated by class I HDACs and their inhibition (via apicidin treatment,) reduces chronic hypoxia-induced activation of IGF-1/pAKT signaling in lungs and attenuates neonatal hypoxia-induced PAH, as well as global DNA methylation [125].
HDACs have also been reported to regulate oxidative stress injury in PAH. Moreover, the HDAC inhibitors scriptaid have been reported to attenuate expression of the NADPH oxidases (Nox) in human lung fibroblasts and human lung microvascular endothelial cells (HLMVECs), which have been proposed to contribute to PAH and other cardiovascular diseases via blockade of the binding of both the RNA polymerase II and the histone acetyltransferase p300 to the Nox promoter regions and to decrease histone activation markers (H3K4me3 and H3K9ac) at these promoter sites as well [126]. Finally, histone deacetylation, specifically via class I HDAC3, has been shown to decrease superoxide dismutase 3 (SOD3) expression in PASMCs, and consequently it was proposed that HDAC inhibitors may protect PAH in part by increasing PASMC SOD3 expression [127].
The impaired myocyte enhancer factor 2 (MEF2) activities in PAH-PAECs has been shown to be regulated by 2 class IIa HDACs, namely HDAC4 and HDAC5. And selectively inhibition of class IIa HDACs led to restoration of MEF2 activity and its target expression in PAECs, decreased cell migration and proliferation, and rescue MCT and hypoxia-induced PAH [107].
Moreover, histone lysine methylation mediated by G9a—a key enzyme for histone H3 dimethylation at position lysine-9—is involved in cell proliferation, migration, contractility and global DNA methylation, companied by increased p21 expression, as shown in fetal PASMCs [128]. Our unpublished data have also revealed that H3K9me3 can also play an important role in smooth muscle cell proliferation and phenotypic switch, contributing to the development of HPH.
Our laboratory very recently identified an H3K4me3-dependent pathway that contributes to hypoxia pulmonary hypertension (HPH). EC-specific silencing of ASH2 and WDR5, two key components of the histone H3K4 methyltransferase complex, ameliorated HPH in mice, and this effect was enhanced by overexpression of ASH2 and WDR5, but dampened by their depletion. These events were associated with hypoxia-induced transactivation of cell adhesion molecules (CAMs), supporting establishment of a pro-inflammatory milieu that contributes to the pathogenesis of chronic hypoxia-induced pulmonary hypertension [129]. The ASH2 and WDR5 components were also found to activate endothelin (ET-1)-induced pro-inflammatory transcription in vascular smooth muscle cells, which contribute to vascular inflammation [130], and to induce the transactivation of ET-1 in vascular ECs [131]. Interestingly, the increased synthesis of ET-1 by human vascular ECs in response to hypoxia underlies the persistent vasoconstriction observed in patients with pulmonary hypertension [131].
ATP-dependent chromatin remodeling in PAH
Unlike the prokaryotic organisms, eukaryotic genes are wrapped by histones into individual nucleosomes to form chromatin. The process of unwrapping the high-order DNA structure requires a group of highly conserved proteins called chromatin remodeling complex. And there are at least five families of chromatin remodeling complexes in eukaryotes: SWI/SNF, ISWI, NuRD/Mi-2/CHD, INO80, and SWR1. Especially, ATP-dependent chromatin remodeling complexes could reposition (slide, twist or loop) nucleosomes along the DNA depend on the common ATPase domain and energy from the hydrolysis of ATP [133].
We have discovered that the Brahma-related gene 1 (Brg1) and brahma (Brm)—two key catalytic components of the mammalian chromatin remodeling complex—are induced in cultured ECs upon hypoxia challenge as well as in pulmonary arteries in an animal model of HPH. Brg1 and Brm can activate transcription of the CAMs by altering the chromatin structure surrounding the CAMs’ promoters, and EC-specific deletion of Brg1/Brm was found to ameliorate vascular inflammation and HPH in mice [134]. Brg1 and Brm can also be recruited onto the ET-1 promoter, causing subsequent modulation of ET-1 transactivation by impacting histone modifications [135]. Furthermore, Brg1 and Brm have been shown to be required for ET-1-dependent induction of pro-inflammatory mediators, performing this function by communicating with ASH2, and to be responsible for vascular inflammation inflicted by ET-1 [130]. Collectively, these data suggest that Brg1 and Brm provide the crucial epigenetic link to hypoxia-induced CAM induction and leukocyte adhesion that engenders endothelial malfunction and pathogenesis of HPH.
DNA methylation in PAH
DNA methylation occurs on cytosine residues in cytosine-phospho-guanine (CpG) regions of the genome and is essential for normal development [136]. The process is regulated by DNA methyltransferases (DNMTs), which add methyl groups to the DNA. The addition of the methyl group typically serves to repress gene transcription, and about 60–90% of all CpGs are methylated in mammals [137]. Studies have shown that DNA methylation plays an important role in the pathogenesis of PAH. For instance, Archer et al. [138] determined that the observed decreased expression of SOD2 in pulmonary arteries and plexiform lesions, which is responsible for the hyperproliferative PAH phenotype, is due to hypermethylation of CpG islands in the SOD2 gene; moreover, reversal of the methylation status via DNMT1 inhibition rescued the SOD2 expression and restored the ratio of proliferation to apoptosis. The epigenetic silencing of SOD2 was also reported to contribute to the normoxic activation of HIF-1α that is implicated in the Warburg effect of cancer cells and in PAH-PASMC proliferation [139, 140]. Interestingly, Nozik et al. [127] suggested that the decreased expression of SOD3 in patients with IPAH and animal models was not regulated by DNA methylation.
Hypoxia also significantly enhances the expression of DNMT3A in visceral smooth muscle cells, which is associated with the phenotypic switch of these cells [141]. IGF-1, which is regulated by HDACs, has also been shown to be controlled by DNA methylation, and the apicidin-mediated inhibition of HDACs decreases global DNA methylation levels in lungs [129], suggesting a role in PAH. A study applying the methylated DNA immunoprecipitation microarray to investigate the differential profile of the extrauterine growth restriction (EUGR)-induced PAH rat model showed that the hypermethylated genes are vascular development-associated and that the hypomethylated genes are late-differentiation-associated and related to signal transduction [142]. Thus, epigenetic dysregulation appears to be a strong mechanism for propagating the cellular memory of early postnatal events, causing changes in the expression of genes and long-term susceptibility to pulmonary hypertension. Nevertheless, Pousada et al. [143] reported that BMPR2, regulated by miRNAs and associated with the development of PAH, was not methylated in the promoter region in PAH patients and controls.
PAH Treatment basing on epigenetics
PAH is characterized by sustained vasoconstriction and progressive remodeling of pulmonary arteries [144]. Despite considerable advances in PAH treatment, this devastating disease still carries a prognosis worse than many cancers [2]. Current pharmacological treatments of PAH are primarily vasodilators, and offer a significant increase in survival, but there remains no cure other than transplantation, therefore, the new strategies and effective biomarkers used for assessing disease severity and response to treatment are urgently required [145]. We have summarized that epigenetic modification processes exert pivotal influences on PAH pathogenesis by regulating vasoconstriction and pulmonary vascular remodeling (Fig. 1). And some studies have already demonstrated that epigenetic-based therapy, such as inhibition of HDACs and restoration of miRNA expression can reverse PAH in different animal models. Additionally, multiple studies in cancers indicated that targeting of epigenetic regulation pathways is currently evolving as a major therapeutic strategy in the treatment of several cancers [146–148]. Thus, we summarize the latest research findings regarding epigenetic-based therapies for PAH, mainly focusing on histone modifications and non-coding RNAs.
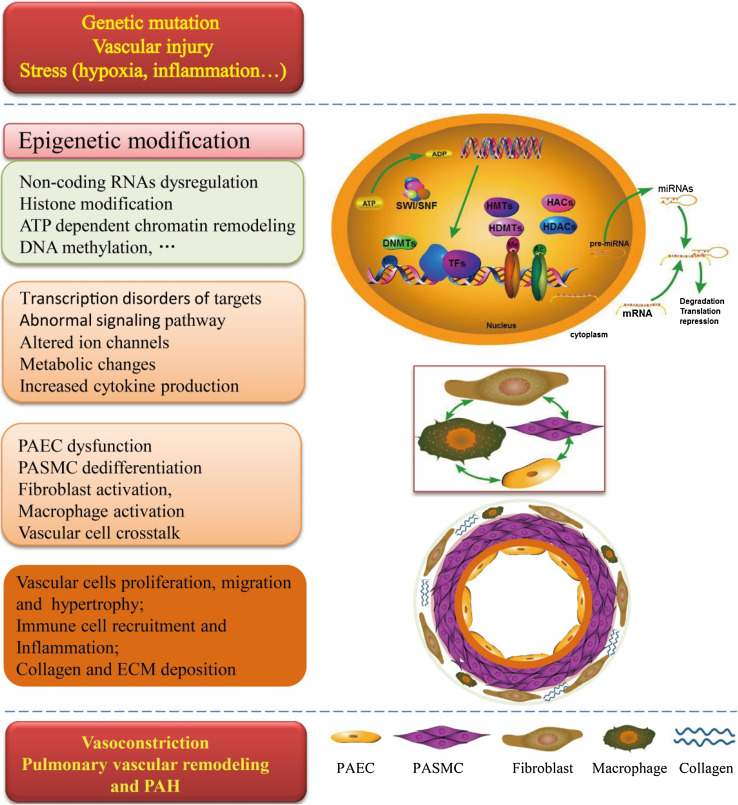
Fig. 1.
Epigenetic regulation in the pathogenesis of pulmonary arterial hypertension. Several triggers, including general mutation, vascular injury and some stress, has been known risk factors and drivers of PAH pathogenesis. With alterations in epigenetic modification, such as non-coding RNAs dysregulation, histone acetylation and methylation, ATP-dependent chromatin remodeling and DNA methylation, the most common downstream molecular, signaling pathway, ion channels, metabolic, and cytokine production consequences in PAH are changed. As a consequence of this perturbed signaling, there is an increase in PAEC dysfunction, PASMC dedifferentiation, fibroblast and macrophage activation, which is associated with vascular cells proliferation, migration and hypertrophy, Immune cell recruitment and Inflammation, and collagen and ECM deposition. These changes drive vasoconstriction and both inward and outward vascular remodeling. This culminates in vasoconstriction and remodeling of the pulmonary vessels, which can result in increased pulmonary vascular resistance and right ventricular afterload, eventually leading to right heart failure and death. ECM extracellular matrix, PAEC pulmonary artery endothelial cells, PASMCs pulmonary artery smooth muscle cells
High levels of HDAC expression and activity are found in PAH, and research has shown the potential of HDAC inhibitors in repressing the development of PAH via induction of anti-inflammatory and anti-proliferative effects. Some recent studies have also shown that effectiveness of the HDAC inhibitors valproic acid (VPA) and suberoylanilide hydroxamic acid(SAHA)in hypoxia-induced models of PAH, is sufficient to support their development as a therapeutic strategy in PAH patients [122, 123]. This efficacy is based on their abilities to inhibit the highly proliferative phenotype of fibroblasts and PASMCs and down-regulate the expression of inflammatory cytokines, such as MCP-1 and interleukin-6 (IL-6). Coincidentally, both Lan group [132] and Chen group [126] showed that daily administration of VPA therapy prevented and partially reversed the development of combined MCT- and chronic hypoxia-induced severe PAH in rats, and decreased inflammation and proliferation in the remodeled pulmonary arteries. Class I HDAC inhibitors (MGCD0103) have also been shown to be capable of reducing pulmonary arterial wall thickening and maintained right ventricular function [121, 127]. Furthermore, selective inhibition of class IIa HDACs has been shown to rescue PAH by decreasing PAECs migration and proliferation of PAECs [107]. The information for the particular inhibitors of HDACs discussed herein has been summarized in Table 2.
Table 2.
Inhibitors of HDACs involved in PAH development
| Inhibitors | Targets HDACs | Function | Targets genes/proteins and Signaling pathway | Models | References |
|---|---|---|---|---|---|
| Apicidin | Class I | Inhibit fibroblast and monocytes/macrophages activation; attenuate right ventricular hypertrophy and pulmonary vascular remodeling | Inhibit pro-inflammatory cytokines and pro-fibrogenic mediators (TIMP1) expression; Inhibit IGF-1/pAKT signaling; |
Human-PAH Rat-hypoxia Neonatal mouse-hypoxia Fibroblasts, PASMC and PAEC |
[124] [125] |
| MC1855 | Class I | Inhibit PASMC proliferation and migration | Inhibit Akt Phosphorylation and Cyclin D1 expression | Rat PASMC-PDGF | [122] |
| MC1568 | I Class IIa | Inhibit PAEC proliferation and migration | Increase MEF2-miRNA-424/503, connexins 37/40, and Klf 2 and 4 |
Rat-MCT, sugen5416/hypoxia PAEC |
[107] |
| MGCD0103 | Class I | Inhibit PASMC proliferation pulmonary vascular remodeling | Increase FOXO3a, P27;SOD3 |
Rat-hypoxia PASMC-hypoxia |
[121] [127] |
| SAHA | Class I, II and IV | Inhibit fibroblast and PASMC proliferation and inflammation | Increase p21 and FOXO3 levels, reduced the expression of survivin and cytokines |
Human-PAH Rat-hypoxia Bovine vascular fibroblasts, PASMC-PDGF |
[123] [124] |
| Scriptaid | Class I, II and IV | Reduce PAH | Reduce Nox transcription and ROS production |
Rat-MCT HLF and HLMVEC |
[126] |
| VPA | Class I | Inhibit fibroblast and PASMC proliferation and inflammation | Increase p21 and FOXO3 levels, reduced the expression of survivin and cytokines |
Human-PAH Rat-hypoxia, MCT Bovine vascular fibroblasts, PASMC-PDGF |
[123] [132] |
Drugs: MCT, monocrotaline; SAHA, suberoylanilide hydroxamic acid; TSA, trichostatin A; VPA, valproic acid
Cell types: PAEC, pulmonary artery endothelial cells; PASMCs, pulmonary artery smooth muscle cells; HLF, human lung fibroblasts; HLMVEC, Human lung microvascular endothelial cells
Target genes/proteins: FOXO, forkhead box O; GF-1, insulin growth factor 1; KLF4, Kruppel-like factor; MEF2, myocyte enhancer factor; Nox, NADPH oxidase; p21, cyclin-dependent kinase inhibitor 1; p27, cyclin-dependent kinase inhibitor 1B; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; SOD, superoxide dismutase; TIMP1, TIMP metallopeptidase inhibitor 1
Findings from recent studies are supportive for the clinical application of HDAC inhibitors as PAH treatment, and the underlying mechanism appears to involve regulation of Tregs in particular [149–151]. A study of this Treg-related protective mechanism against PAH conducted by Chu et al. [152] indicated that the mechanism is further associated with suppression of the inflammatory response, inhibition of (human) PASMC proliferation and regulation of the cell cycle. Yet, other studies have yielded some troubling findings, showing potential detrimental effects of therapeutically applied HDAC inhibitors, particularly on the right ventricle and in decreasing normal angiogenesis [153]. Thus, the therapeutic role of HDAC inhibitors in PAH remains controversial. Based on these studies; however, we can speculate that selective HDAC inhibitors might be beneficial for PAH if they can be delivered directly to the lungs, thereby avoiding the detrimental right ventricle effects.
Interestingly, miRNA mimics and antagonists (known as antogomiRs) have recently entered into clinical trials for patients with liver cancer [154] and hepatitis C [155]. Some mimics and antagomiRs have recently been applied to experimental PAH models [156, 157], and in one of those studies miR-204 expression rescue of the PAH-reduced miR-204 was shown to reverse the disease in rats [83]. Treatment with nebulized miR-140-5p mimic also prevents the development of experimental PAH in rats [56]. Similarly, inhibition of miR-17, which is up-regulated in PAH rats, was shown to improve PAH [89], antagomiR-135a injected into mice showed to reverse PAH [55] and anti-miR-199a-5p [31] and anti-miR-27b [41] were shown to overcome the PAH-related significant increase and promote the NO level and decrease the pulmonary artery pressure and the extent of right ventricular hypertrophy. Thus, while numerous studies have provided clear demonstrations of miRNAs’ association with the development of PAH, the clinical utility of these findings currently remains unclear. Nonetheless, the collective findings put forth the promise of RNAi therapy for PAH and support its ongoing investigation.
Conclusion and perspectives
The past two decades of PAH research have yielded a better understanding of the pathophysiological processes and new therapies that have emerged, but the effective prevention of PAH remains an unmet objective and diagnosis still occurs largely at the late stage and relies on invasive methods. The physiological imbalances, resulting from stress, are linked with non-homeostatic responses in gene regulation that occur via epigenetic modification. A significant amount of research studies have examined, and begun to elucidate, the myriad roles of miRNAs and HDACs in the development of PAH; however, other histone modifications, such as methylation and phosphorylation, as well as DNA methylation and mitochondrial epigenetics should be studied in the same manner, as they are likely to be important contributors to the pathogenesis of PAH and may represent manipulable targets of therapy. While some studies have already demonstrated that restoration of miRNA expression and inhibition of HDACs can reverse PAH in different animal models, no epigenetic-based therapy for PAH has yet reached clinical or preclinical development. There is a lacuna between the extent of findings from animal studies and those from humans, especially in relation to therapeutics, and further research is needed to fully understand the epigenetics of PAH. In all, epigenetic regulation presents a flicker of hope for new therapeutic strategies and targets of PAH in the future.
Acknowledgements
This work was supported by Natural Science Foundation of China (Nos. 81501626, 81471814, J1310001).
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Contributor Information
Bing Ni, Phone: +86 23 68752389, Email: nibing@tmmu.edu.cn.
Yuqi Gao, Phone: +86 23 68752399, Email: gaoy66@yahoo.com.
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Investig. 2012;122(12):4306–4313. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8(8):443–455. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aaronson PI, Robertson TP, Ward JP. Endothelium-derived mediators and hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol. 2002;132(1):107–120. doi: 10.1016/S1569-9048(02)00053-8. [DOI] [PubMed] [Google Scholar]
- 5.Vaillancourt M, Ruffenach G, Meloche J, Bonnet S. Adaptation and remodelling of the pulmonary circulation in pulmonary hypertension. Can J Cardiol. 2015;31(4):407–415. doi: 10.1016/j.cjca.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(1 Suppl):S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99(7):675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 8.Mishra A, Mohammad G, Norboo T, Newman JH, Pasha MA. Lungs at high-altitude: genomic insights into hypoxic responses. J Appl Physiol. 2015;119(1):1–15. doi: 10.1152/japplphysiol.00513.2014. [DOI] [PubMed] [Google Scholar]
- 9.Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 10.Gamen E, Seeger W, Pullamsetti SS. The emerging role of epigenetics in pulmonary hypertension. Eur Respir J. 2016;48(3):903–917. doi: 10.1183/13993003.01714-2015. [DOI] [PubMed] [Google Scholar]
- 11.Chelladurai P, Seeger W, Pullamsetti SS. Epigenetic mechanisms in pulmonary arterial hypertension: the need for global perspectives. Eur Respir Rev. 2016;25(140):135–140. doi: 10.1183/16000617.0036-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, Xue C, Marinov GK, Khatun J, Williams BA, Zaleski C, Rozowsky J, Roder M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Bar NS, Batut P, Bell K, Bell I, Chakrabortty S, Chen X, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Falconnet E, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Gunawardena H, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Luo OJ, Park E, Persaud K, Preall JB, Ribeca P, Risk B, Robyr D, Sammeth M, Schaffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Wrobel J, Yu Y, Ruan X, Hayashizaki Y, Harrow J, Gerstein M, Hubbard T, Reymond A, Antonarakis SE, Hannon G, Giddings MC, Ruan Y, Wold B, Carninci P, Guigo R, Gingeras TR. Landscape of transcription in human cells. Nature. 2012;489(7414):101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157(1):77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 14.St Laurent G, Wahlestedt C, Kapranov P. The Landscape of long noncoding RNA classification. Trends Genet. 2015;31(5):239–251. doi: 10.1016/j.tig.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Yao H, Lin S, Zhu X, Shen Z, Lu G, Poon WS, Xie D, Lin MC, Kung HF. Transcriptional and epigenetic regulation of human microRNAs. Cancer Lett. 2013;331(1):1–10. doi: 10.1016/j.canlet.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–826. doi: 10.1016/S0092-8674(01)00616-X. [DOI] [PubMed] [Google Scholar]
- 17.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 18.Meloche J, Pflieger A, Vaillancourt M, Graydon C, Provencher S, Bonnet S. miRNAs in PAH: biomarker, therapeutic target or both? Drug Discov Today. 2014;19(8):1264–1269. doi: 10.1016/j.drudis.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Rothman AM, Chico TJ, Lawrie A. MicroRNA in pulmonary vascular disease. Progr Mol Biol Transl Sci. 2014;124:43–63. doi: 10.1016/B978-0-12-386930-2.00003-3. [DOI] [PubMed] [Google Scholar]
- 20.Grant JS, White K, MacLean MR, Baker AH. MicroRNAs in pulmonary arterial remodeling. Cell Mol Life Sci. 2013;70(23):4479–4494. doi: 10.1007/s00018-013-1382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, Denby L, Dempsie Y, Long L, Morrell NW, Baker AH. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30(4):716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 22.Potus F, Graydon C, Provencher S, Bonnet S. Vascular remodeling process in pulmonary arterial hypertension, with focus on miR-204 and miR-126 (2013 Grover Conference series) Pulm Circ. 2014;4(2):175–184. doi: 10.1086/675980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes CJ, Wharton J, Boon RA, Roexe T, Tsang H, Wojciak-Stothard B, Chakrabarti A, Howard LS, Gibbs JS, Lawrie A, Condliffe R, Elliot CA, Kiely DG, Huson L, Ghofrani HA, Tiede H, Schermuly R, Zeiher AM, Dimmeler S, Wilkins MR. Reduced microRNA-150 is associated with poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;187(3):294–302. doi: 10.1164/rccm.201205-0839OC. [DOI] [PubMed] [Google Scholar]
- 24.Morrell NW. Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling? Proc Am Thorac Soc. 2006;3(8):680–686. doi: 10.1513/pats.200605-118SF. [DOI] [PubMed] [Google Scholar]
- 25.Diebold I, Hennigs JK, Miyagawa K, Li CG, Nickel NP, Kaschwich M, Cao A, Wang L, Reddy S, Chen PI, Nakahira K, Alcazar MA, Hopper RK, Ji L, Feldman BJ, Rabinovitch M. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 2015;21(4):596–608. doi: 10.1016/j.cmet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Vattulainen S, Aho J, Orcholski M, Rojas V, Yuan K, Helenius M, Taimen P, Myllykangas S, De Jesus Perez V, Koskenvuo JW, Alastalo TP. Loss of bone morphogenetic protein receptor 2 is associated with abnormal DNA repair in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2014;50(6):1118–1128. doi: 10.1165/rcmb.2013-0349OC. [DOI] [PubMed] [Google Scholar]
- 27.Hopper RK, Moonen JR, Diebold I, Cao A, Rhodes CJ, Tojais NF, Hennigs JK, Gu M, Wang L, Rabinovitch M. In pulmonary arterial hypertension, reduced BMPR2 promotes endothelial-to-mesenchymal transition via HMGA1 and its target slug. Circulation. 2016;133(18):1783–1794. doi: 10.1161/CIRCULATIONAHA.115.020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orriols M, Gomez-Puerto MC, Ten Dijke P. BMP type II receptor as a therapeutic target in pulmonary arterial hypertension. Cell Mol Life Sci: CMLS; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res. 2009;104(10):1184–1191. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 30.Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, Cottrill KA, Shaik RS, Waxman AB, Zhang YY, Maron BA, Hartner JC, Fujiwara Y, Orkin SH, Haley KJ, Barabasi AL, Loscalzo J, Chan SY. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125(12):1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Liu G, Zhang H, Wang J. MiRNA-199a-5p influences pulmonary artery hypertension via downregulating Smad3. Biochem Biophys Res Commun. 2016;473(4):859–866. doi: 10.1016/j.bbrc.2016.03.140. [DOI] [PubMed] [Google Scholar]
- 32.Wilkins SE, Abboud MI, Hancock RL, Schofield CJ. Targeting protein–protein interactions in the HIF system. Chem Med Chem. 2016;11(8):773–786. doi: 10.1002/cmdc.201600012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei W, He Y, Shui X, Li G, Yan G, Zhang Y, Huang S, Chen C, Ding Y. Expression and analyses of the HIF-1 pathway in the lungs of humans with pulmonary arterial hypertension. Mol Med Rep. 2016;14(5):4383–4390. doi: 10.3892/mmr.2016.5752. [DOI] [PubMed] [Google Scholar]
- 34.Dunham-Snary KJ, Wu D, Sykes EA, Thakrar A, Parlow LR, Mewburn JD, Parlow JL, Archer SL. Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest. 2017;151(1):181–192. doi: 10.1016/j.chest.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, Tuder RM. Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol. 2010;176(3):1130–1138. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sen A, Ren S, Lerchenmuller C, Sun J, Weiss N, Most P, Peppel K. MicroRNA-138 regulates hypoxia-induced endothelial cell dysfunction by targeting S100A1. PLoS One. 2013;8(11):e78684. doi: 10.1371/journal.pone.0078684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sen A, Most P, Peppel K. Induction of microRNA-138 by pro-inflammatory cytokines causes endothelial cell dysfunction. FEBS Lett. 2014;588(6):906–914. doi: 10.1016/j.febslet.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou W, Zhou W, Zeng Q, Xiong J. MicroRNA-138 inhibits hypoxia-induced proliferation of endothelial progenitor cells via inhibition of HIF-1alpha-mediated MAPK and AKT signaling. Exp Ther Med. 2017;13(3):1017–1024. doi: 10.3892/etm.2017.4091. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Rabinovitch M. PPARgamma and the pathobiology of pulmonary arterial hypertension. Adv Exp Med Biol. 2010;661:447–458. doi: 10.1007/978-1-60761-500-2_29. [DOI] [PubMed] [Google Scholar]
- 40.Kang BY, Park KK, Green DE, Bijli KM, Searles CD, Sutliff RL, Hart CM. Hypoxia mediates mutual repression between microRNA-27a and PPARgamma in the pulmonary vasculature. PLoS One. 2013;8(11):e79503. doi: 10.1371/journal.pone.0079503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bi R, Bao C, Jiang L, Liu H, Yang Y, Mei J, Ding F. MicroRNA-27b plays a role in pulmonary arterial hypertension by modulating peroxisome proliferator-activated receptor gamma dependent Hsp90-eNOS signaling and nitric oxide production. Biochem Biophys Res Commun. 2015;460(2):469–475. doi: 10.1016/j.bbrc.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 42.Bertero T, Lu Y, Annis S, Hale A, Bhat B, Saggar R, Saggar R, Wallace WD, Ross DJ, Vargas SO, Graham BB, Kumar R, Black SM, Fratz S, Fineman JR, West JD, Haley KJ, Waxman AB, Chau BN, Cottrill KA, Chan SY. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Investig. 2014;124(8):3514–3528. doi: 10.1172/JCI74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bockmeyer CL, Maegel L, Janciauskiene S, Rische J, Lehmann U, Maus UA, Nickel N, Haverich A, Hoeper MM, Golpon HA, Kreipe H, Laenger F, Jonigk D. Plexiform vasculopathy of severe pulmonary arterial hypertension and microRNA expression. J Heart Lung Transpl. 2012;31(7):764–772. doi: 10.1016/j.healun.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, McLean DL, Park H, Comhair SA, Greif DM, Erzurum SC, Chun HJ. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. 2013;19(1):74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertero T, Cottrill K, Krauszman A, Lu Y, Annis S, Hale A, Bhat B, Waxman AB, Chau BN, Kuebler WM, Chan SY. The microRNA-130/301 family controls vasoconstriction in pulmonary hypertension. J Biol Chem. 2015;290(4):2069–2085. doi: 10.1074/jbc.M114.617845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75(3):487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 48.Owens GK. Molecular control of vascular smooth muscle cell differentiation and phenotypic plasticity. Novartis Found Symp. 2007;283:174–191. doi: 10.1002/9780470319413.ch14. [DOI] [PubMed] [Google Scholar]
- 49.Brock M, Samillan VJ, Trenkmann M, Schwarzwald C, Ulrich S, Gay RE, Gassmann M, Ostergaard L, Gay S, Speich R, Huber LC. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J. 2014;35(45):3203–3211. doi: 10.1093/eurheartj/ehs060. [DOI] [PubMed] [Google Scholar]
- 50.Zeng Y, Pan Y, Liu H, Kang K, Wu Y, Hui G, Peng W, Ramchandran R, Raj JU, Gou D. MiR-20a regulates the PRKG1 gene by targeting its coding region in pulmonary arterial smooth muscle cells. FEBS Lett. 2014;588(24):4677–4685. doi: 10.1016/j.febslet.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 51.Zhou W, Negash S, Liu J, Raj JU. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase and myocardin. Am J Physiol Lung Cell Mol Physiol. 2009;296(5):L780–L789. doi: 10.1152/ajplung.90295.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol. 2010;299(6):L861–L871. doi: 10.1152/ajplung.00201.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang S, Banerjee S, Freitas A, Cui H, Xie N, Abraham E, Liu G. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2012;302(6):L521–L529. doi: 10.1152/ajplung.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, Talati M, Fessel JP, Hemnes AR, Gladson S, French J, Shay S, Trammell A, Phillips JA, Hamid R, Cogan JD, Dawson EP, Womble KE, Hedges LK, Martinez EG, Wheeler LA, Loyd JE, Majka SJ, West J, Austin ED. Estrogen metabolite 16alpha-hydroxyestrone exacerbates bone morphogenetic protein receptor type II-associated pulmonary arterial hypertension through microRNA-29-mediated modulation of cellular metabolism. Circulation. 2016;133(1):82–97. doi: 10.1161/CIRCULATIONAHA.115.016133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee HW, Park SH. Elevated microRNA-135a is associated with pulmonary arterial hypertension in experimental mouse model. Oncotarget. 2017 doi: 10.18632/oncotarget.16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rothman AM, Arnold ND, Pickworth JA, Iremonger J, Ciuclan L, Allen RM, Guth-Gundel S, Southwood M, Morrell NW, Thomas M, Francis SE, Rowlands DJ, Lawrie A. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Investig. 2016;126(7):2495–2508. doi: 10.1172/JCI83361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caruso P, Dempsie Y, Stevens HC, McDonald RA, Long L, Lu R, White K, Mair KM, McClure JD, Southwood M, Upton P, Xin M, van Rooij E, Olson EN, Morrell NW, MacLean MR, Baker AH. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res. 2012;111(3):290–300. doi: 10.1161/CIRCRESAHA.112.267591. [DOI] [PubMed] [Google Scholar]
- 58.Deng L, Blanco FJ, Stevens H, Lu R, Caudrillier A, McBride M, McClure JD, Grant J, Thomas M, Frid M, Stenmark K, White K, Seto AG, Morrell NW, Bradshaw AC, MacLean MR, Baker AH. MicroRNA-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res. 2015;117(10):870–883. doi: 10.1161/CIRCRESAHA.115.306806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256):705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105(2):158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shan F, Li J, Huang QY. HIF-1 alpha-induced up-regulation of miR-9 contributes to phenotypic modulation in pulmonary artery smooth muscle cells during hypoxia. J Cell Physiol. 2014;229(10):1511–1520. doi: 10.1002/jcp.24593. [DOI] [PubMed] [Google Scholar]
- 62.Yan L, Gao H, Li C, Han X, Qi X. Effect of miR-23a on anoxia-induced phenotypic transformation of smooth muscle cells of rat pulmonary arteries and regulatory mechanism. Oncol Lett. 2017;13(1):89–98. doi: 10.3892/ol.2016.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li S, Ran Y, Zhang D, Chen J, Li S, Zhu D. MicroRNA-138 plays a role in hypoxic pulmonary vascular remodelling by targeting Mst1. Biochem J. 2013;452(2):281–291. doi: 10.1042/BJ20120680. [DOI] [PubMed] [Google Scholar]
- 64.Gou D, Ramchandran R, Peng X, Yao L, Kang K, Sarkar J, Wang Z, Zhou G, Raj JU. miR-210 has an antiapoptotic effect in pulmonary artery smooth muscle cells during hypoxia. Am J Physiol Lung Cell Mol Physiol. 2012;303(8):L682–L691. doi: 10.1152/ajplung.00344.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White K, Lu Y, Annis S, Hale AE, Chau BN, Dahlman JE, Hemann C, Opotowsky AR, Vargas SO, Rosas I, Perrella MA, Osorio JC, Haley KJ, Graham BB, Kumar R, Saggar R, Saggar R, Wallace WD, Ross DJ, Khan OF, Bader A, Gochuico BR, Matar M, Polach K, Johannessen NM, Prosser HM, Anderson DG, Langer R, Zweier JL, Bindoff LA, Systrom D, Waxman AB, Jin RC, Chan SY. Genetic and hypoxic alterations of the microRNA-210-ISCU1/2 axis promote iron-sulfur deficiency and pulmonary hypertension. EMBO Mol Med. 2015;7(6):695–713. doi: 10.15252/emmm.201404511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeng Y, Liu H, Kang K, Wang Z, Hui G, Zhang X, Zhong J, Peng W, Ramchandran R, Raj JU, Gou D. Hypoxia inducible factor-1 mediates expression of miR-322: potential role in proliferation and migration of pulmonary arterial smooth muscle cells. Sci Rep. 2015;5:12098. doi: 10.1038/srep12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen TJ, Zhou QY, Tang HY, Bozkanat M, Yuan JXJ, Raj JU, Zhou GF. miR-17/20 controls prolyl hydroxylase 2 (PHD2)/hypoxia-inducible factor 1 (HIF1) to regulate pulmonary artery smooth muscle cell proliferation. J Am Heart Assoc. 2016 doi: 10.1161/JAHA.116.004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jalali S, Ramanathan GK, Parthasarathy PT, Aljubran S, Galam L, Yunus A, Garcia S, Cox RR, Jr, Lockey RF, Kolliputi N. Mir-206 regulates pulmonary artery smooth muscle cell proliferation and differentiation. PLoS One. 2012;7(10):e46808. doi: 10.1371/journal.pone.0046808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yue J, Guan J, Wang X, Zhang L, Yang Z, Ao Q, Deng Y, Zhu P, Wang G. MicroRNA-206 is involved in hypoxia-induced pulmonary hypertension through targeting of the HIF-1alpha/Fhl-1 pathway. Lab Investig J Tech Methods Pathol. 2013;93(7):748–759. doi: 10.1038/labinvest.2013.63. [DOI] [PubMed] [Google Scholar]
- 70.Deng B, Du J, Hu R, Wang AP, Wu WH, Hu CP, Li YJ, Li XH. MicroRNA-103/107 is involved in hypoxia-induced proliferation of pulmonary arterial smooth muscle cells by targeting HIF-1 beta. Life Sci. 2016;147:117–124. doi: 10.1016/j.lfs.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 71.Brock M, Haider TJ, Vogel J, Gassmann M, Speich R, Trenkmann M, Ulrich S, Kohler M, Huber LC. The hypoxia-induced microRNA-130a controls pulmonary smooth muscle cell proliferation by directly targeting CDKN1A. Int J Biochem Cell Biol. 2015;61:129–137. doi: 10.1016/j.biocel.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 72.Green DE, Murphy TC, Kang BY, Searles CD, Hart CM. PPARgamma ligands attenuate hypoxia-induced proliferation in human pulmonary artery smooth muscle cells through modulation of microRNA-21. PLoS One. 2015;10(7):e0133391. doi: 10.1371/journal.pone.0133391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang P, Xu J, Hou Z, Wang F, Song Y, Wang J, Zhu H, Jin H. miRNA-34a promotes proliferation of human pulmonary artery smooth muscle cells by targeting PDGFRA. Cell Prolif. 2016;49(4):484–493. doi: 10.1111/cpr.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma S, Umar S, Potus F, Iorga A, Wong G, Meriwether D, Breuils-Bonnet S, Mai D, Navab K, Ross D, Navab M, Provencher S, Fogelman AM, Bonnet S, Reddy ST, Eghbali M. Apolipoprotein A–I mimetic peptide 4F rescues pulmonary hypertension by inducing microRNA-193-3p. Circulation. 2014;130(9):776–785. doi: 10.1161/CIRCULATIONAHA.114.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi L, Kojonazarov B, Elgheznawy A, Popp R, Dahal BK, Bohm M, Pullamsetti SS, Ghofrani HA, Godecke A, Jungmann A, Katus HA, Muller OJ, Schermuly RT, Fisslthaler B, Seeger W, Fleming I. miR-223-IGF-IR signalling in hypoxia- and load-induced right-ventricular failure: a novel therapeutic approach. Cardiovasc Res. 2016;111(3):184–193. doi: 10.1093/cvr/cvw065. [DOI] [PubMed] [Google Scholar]
- 76.Meloche J, Le Guen M, Potus F, Vinck J, Ranchoux B, Johnson I, Antigny F, Tremblay E, Breuils-Bonnet S, Perros F, Provencher S, Bonnet S. miR-223 reverses experimental pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2015;309(6):C363–C372. doi: 10.1152/ajpcell.00149.2015. [DOI] [PubMed] [Google Scholar]
- 77.Zeng Y, Zhang X, Kang K, Chen J, Wu Z, Huang J, Lu W, Chen Y, Zhang J, Wang Z, Zhai Y, Qu J, Ramchandran R, Raj JU, Wang J, Gou D. MicroRNA-223 attenuates hypoxia-induced vascular remodeling by targeting RhoB/MLC2 in pulmonary arterial smooth muscle cells. Sci Rep. 2016;6:24900. doi: 10.1038/srep24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guo L, Qiu Z, Wei L, Yu X, Gao X, Jiang S, Tian H, Jiang C, Zhu D. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-alpha1C. Hypertension. 2012;59(5):1006–1013. doi: 10.1161/HYPERTENSIONAHA.111.185413. [DOI] [PubMed] [Google Scholar]
- 79.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17(18):2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 80.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA. 2007;104(27):11418–11423. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen R, Yan J, Liu P, Wang Z, Wang C, Zhong W, Xu L. The role of nuclear factor of activated T cells in pulmonary arterial hypertension. Cell Cycle. 2017;16(6):508–514. doi: 10.1080/15384101.2017.1281485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang K, Peng X, Zhang X, Wang Y, Zhang L, Gao L, Weng T, Zhang H, Ramchandran R, Raj JU, Gou D, Liu L. MicroRNA-124 suppresses the transactivation of nuclear factor of activated T cells by targeting multiple genes and inhibits the proliferation of pulmonary artery smooth muscle cells. J Biol Chem. 2013;288(35):25414–25427. doi: 10.1074/jbc.M113.460287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208(3):535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, Graydon C, Courboulin A, Breuils-Bonnet S, Tremblay E, Couture C, Michelakis ED, Provencher S, Bonnet S. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014;129(7):786–797. doi: 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- 85.Ruffenach G, Chabot S, Tanguay VF, Courboulin A, Boucherat O, Potus F, Meloche J, Pflieger A, Breuils-Bonnet S, Nadeau V, Paradis R, Tremblay E, Girerd B, Hautefort A, Montani D, Fadel E, Dorfmuller P, Humbert M, Perros F, Paulin R, Provencher S, Bonnet S. Role for runt-related transcription factor 2 in proliferative and calcified vascular lesions in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;194(10):1273–1285. doi: 10.1164/rccm.201512-2380OC. [DOI] [PubMed] [Google Scholar]
- 86.Li SS, Ran YJ, Zhang DD, Li SZ, Zhu D. MicroRNA-190 regulates hypoxic pulmonary vasoconstriction by targeting a voltage-gated K(+) channel in arterial smooth muscle cells. J Cell Biochem. 2014;115(6):1196–1205. doi: 10.1002/jcb.24771. [DOI] [PubMed] [Google Scholar]
- 87.Zhang WF, Xiong YW, Zhu TT, Xiong AZ, Bao HH, Cheng XS. MicroRNA let-7g inhibited hypoxia-induced proliferation of PASMCs via G0/G1 cell cycle arrest by targeting c-myc. Life Sci. 2017;170:9–15. doi: 10.1016/j.lfs.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 88.Wang R, Ding X, Zhou S, Li M, Sun L, Xu X, Fei G. Microrna-26b attenuates monocrotaline-induced pulmonary vascular remodeling via targeting connective tissue growth factor (CTGF) and cyclin D1 (CCND1) Oncotarget. 2016;7(45):72746–72757. doi: 10.18632/oncotarget.10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pullamsetti SS, Doebele C, Fischer A, Savai R, Kojonazarov B, Dahal BK, Ghofrani HA, Weissmann N, Grimminger F, Bonauer A, Seeger W, Zeiher AM, Dimmeler S, Schermuly RT. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2012;185(4):409–419. doi: 10.1164/rccm.201106-1093OC. [DOI] [PubMed] [Google Scholar]
- 90.Lu Z, Li S, Zhao S, Fa X. Upregulated miR-17 regulates hypoxia-mediated human pulmonary artery smooth muscle cell proliferation and apoptosis by targeting mitofusin 2. Med Sci Monit Int Med J Exp Clin Res. 2016;22:3301–3308. doi: 10.12659/MSM.900487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stenmark KR, Yeager ME, El Kasmi KC, Nozik-Grayck E, Gerasimovskaya EV, Li M, Riddle SR, Frid MG. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol. 2013;75:23–47. doi: 10.1146/annurev-physiol-030212-183802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Short M, Nemenoff RA, Zawada WM, Stenmark KR, Das M. Hypoxia induces differentiation of pulmonary artery adventitial fibroblasts into myofibroblasts. Am J Physiol Cell Physiol. 2004;286(2):C416–C425. doi: 10.1152/ajpcell.00169.2003. [DOI] [PubMed] [Google Scholar]
- 93.Wang D, Zhang H, Li M, Frid MG, Flockton AR, McKeon BA, Yeager ME, Fini MA, Morrell NW, Pullamsetti SS, Velegala S, Seeger W, McKinsey TA, Sucharov CC, Stenmark KR. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res. 2014;114(1):67–78. doi: 10.1161/CIRCRESAHA.114.301633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marques AC, Ponting CP. Intergenic lncRNAs and the evolution of gene expression. Curr Opin Genet Dev. 2014;27:48–53. doi: 10.1016/j.gde.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 95.Gaiti F, Fernandez-Valverde SL, Nakanishi N, Calcino AD, Yanai I, Tanurdzic M, Degnan BM. Dynamic and widespread lncRNA expression in a sponge and the origin of animal complexity. Mol Biol Evol. 2015;32(9):2367–2382. doi: 10.1093/molbev/msv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 97.Li L, Song X. In vivo functions of long non-coding RNAs. Hereditas. 2014;36(3):228–236. [PubMed] [Google Scholar]
- 98.Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genom Proteom Bioinf. 2016;14(1):42–54. doi: 10.1016/j.gpb.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang X, Yan C, Xu X, Dong L, Su H, Hu Y, Zhang R, Ying K. Long noncoding RNA expression profiles of hypoxic pulmonary hypertension rat model. Gene. 2016;579(1):23–28. doi: 10.1016/j.gene.2015.12.044. [DOI] [PubMed] [Google Scholar]
- 100.Michalik KM, You X, Manavski Y, Doddaballapur A, Zornig M, Braun T, John D, Ponomareva Y, Chen W, Uchida S, Boon RA, Dimmeler S. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. 2014;114(9):1389–1397. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 101.Zhuo Y, Zeng Q, Zhang P, Li G, Xie Q, Cheng Y. Functional polymorphism of lncRNA MALAT1 contributes to pulmonary arterial hypertension susceptibility in Chinese people. Clin Chem Lab Med. 2017;55(1):38–46. doi: 10.1515/cclm-2016-0056. [DOI] [PubMed] [Google Scholar]
- 102.Leisegang MS, Fork C, Josipovic I, Richter F, Preussner J, Hu J, Miller MJ, Epah JN, Hofmann P, Gunther S, Moll F, Valasarajan C, Heidler J, Ponomareva Y, Freiman TM, Maegdefessel L, Plate KH, Mittelbronn M, Uchida S, Kunne C, Stellos K, Schermuly RT, Weissmann N, Devraj K, Wittig I, Boon RA, Dimmeler S, Pullamsetti SS, Looso M, Miller FJ, Brandes RP. Long noncoding RNA MANTIS facilitates endothelial angiogenic function. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.116.026991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen C, Cai Y, Huang H, Yang Y, Liu Y, Xu Z, He D, Zhang X, Hu X, Pinello L, Zhong D, He F, Yuan GC, Wang DZ, Zeng C. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014;130(17):1452–1465. doi: 10.1161/CIRCULATIONAHA.114.011675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou WQ, Wang P, Shao QP, Wang J. Lipopolysaccharide promotes pulmonary fibrosis in acute respiratory distress syndrome (ARDS) via lincRNA-p21 induced inhibition of Thy-1 expression. Mol Cell Biochem. 2016;419(1–2):19–28. doi: 10.1007/s11010-016-2745-7. [DOI] [PubMed] [Google Scholar]
- 105.Taguchi A, Yanagisawa K, Tanaka M, Cao K, Matsuyama Y, Goto H, Takahashi T. Identification of hypoxia-inducible factor-1 alpha as a novel target for miR-17-92 microRNA cluster. Can Res. 2008;68(14):5540–5545. doi: 10.1158/0008-5472.CAN-07-6460. [DOI] [PubMed] [Google Scholar]
- 106.Stevens HC, Deng L, Grant JS, Pinel K, Thomas M, Morrell NW, MacLean MR, Baker AH, Denby L. Regulation and function of miR-214 in pulmonary arterial hypertension. Pulm Circ. 2016;6(1):109–117. doi: 10.1086/685079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim J, Hwangbo C, Hu X, Kang Y, Papangeli I, Mehrotra D, Park H, Ju H, McLean DL, Comhair SA, Erzurum SC, Chun HJ. Restoration of impaired endothelial myocyte enhancer factor 2 function rescues pulmonary arterial hypertension. Circulation. 2015;131(2):190–199. doi: 10.1161/CIRCULATIONAHA.114.013339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teif VB, Rippe K. Predicting nucleosome positions on the DNA: combining intrinsic sequence preferences and remodeler activities. Nucleic Acids Res. 2009;37(17):5641–5655. doi: 10.1093/nar/gkp610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marino-Ramirez L, Kann MG, Shoemaker BA, Landsman D. Histone structure and nucleosome stability. Expert Rev Proteom. 2005;2(5):719–729. doi: 10.1586/14789450.2.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 111.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338(1):17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 112.Krogan NJ, Dover J, Khorrami S, Greenblatt JF, Schneider J, Johnston M, Shilatifard A. COMPASS, a histone H3 (Lysine 4) methyltransferase required for telomeric silencing of gene expression. J Biol Chem. 2002;277(13):10753–10755. doi: 10.1074/jbc.C200023200. [DOI] [PubMed] [Google Scholar]
- 113.Strahl BD, Grant PA, Briggs SD, Sun ZW, Bone JR, Caldwell JA, Mollah S, Cook RG, Shabanowitz J, Hunt DF, Allis CD. Set2 is a nucleosomal histone H3-selective methyltransferase that mediates transcriptional repression. Mol Cell Biol. 2002;22(5):1298–1306. doi: 10.1128/MCB.22.5.1298-1306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuzmichev A, Nishioka K, Erdjument-Bromage H, Tempst P, Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 2002;16(22):2893–2905. doi: 10.1101/gad.1035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rea S, Eisenhaber F, O’Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, Jenuwein T. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406(6796):593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 116.Schotta G, Lachner M, Sarma K, Ebert A, Sengupta R, Reuter G, Reinberg D, Jenuwein T. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev. 2004;18(11):1251–1262. doi: 10.1101/gad.300704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song N, Liu J, An S, Nishino T, Hishikawa Y, Koji T. Immunohistochemical analysis of histone H3 modifications in germ cells during mouse spermatogenesis. Acta Histochem Cytochem. 2011;44(4):183–190. doi: 10.1267/ahc.11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu XF, Lv Y, Gu WZ, Tang LL, Wei JK, Zhang LY, Du LZ. Epigenetics of hypoxic pulmonary arterial hypertension following intrauterine growth retardation rat: epigenetics in PAH following IUGR. Respir Res. 2013;14:20. doi: 10.1186/1465-9921-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Merkwirth C, Jovaisaite V, Durieux J, Matilainen O, Jordan SD, Quiros PM, Steffen KK, Williams EG, Mouchiroud L, Tronnes SU, Murillo V, Wolff SC, Shaw RJ, Auwerx J, Dillin A. Two conserved histone demethylases regulate mitochondrial stress-induced longevity. Cell. 2016;165(5):1209–1223. doi: 10.1016/j.cell.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tian Y, Garcia G, Bian Q, Steffen KK, Joe L, Wolff S, Meyer BJ, Dillin A. Mitochondrial stress induces chromatin reorganization to promote longevity and UPR(mt) Cell. 2016;165(5):1197–1208. doi: 10.1016/j.cell.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cavasin MA, Demos-Davies K, Horn TR, Walker LA, Lemon DD, Birdsey N, Weiser-Evans MC, Harral J, Irwin DC, Anwar A, Yeager ME, Li M, Watson PA, Nemenoff RA, Buttrick PM, Stenmark KR, McKinsey TA. Selective class I histone deacetylase inhibition suppresses hypoxia-induced cardiopulmonary remodeling through an antiproliferative mechanism. Circ Res. 2012;110(5):739–748. doi: 10.1161/CIRCRESAHA.111.258426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Galletti M, Cantoni S, Zambelli F, Valente S, Palazzini M, Manes A, Pasquinelli G, Mai A, Galie N, Ventura C. Dissecting histone deacetylase role in pulmonary arterial smooth muscle cell proliferation and migration. Biochem Pharmacol. 2014;91(2):181–190. doi: 10.1016/j.bcp.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 123.Zhao L, Chen CN, Hajji N, Oliver E, Cotroneo E, Wharton J, Wang D, Li M, McKinsey TA, Stenmark KR, Wilkins MR. Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation. 2012;126(4):455–467. doi: 10.1161/CIRCULATIONAHA.112.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, Strassheim D, Meyrick B, Yeager ME, Flockton AR, McKeon BA, Lemon DD, Horn TR, Anwar A, Barajas C, Stenmark KR. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. Journal of immunology. 2011;187(5):2711–2722. doi: 10.4049/jimmunol.1100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang Q, Sun M, Ramchandran R, Raj JU. IGF-1 signaling in neonatal hypoxia-induced pulmonary hypertension: role of epigenetic regulation. Vascul Pharmacol. 2015;73:20–31. doi: 10.1016/j.vph.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen F, Li X, Aquadro E, Haigh S, Zhou J, Stepp DW, Weintraub NL, Barman SA, Fulton DJ. Inhibition of histone deacetylase reduces transcription of NADPH oxidases and ROS production and ameliorates pulmonary arterial hypertension. Free Radical Biol Med. 2016;99:167–178. doi: 10.1016/j.freeradbiomed.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nozik-Grayck E, Woods C, Stearman RS, Venkataraman S, Ferguson BS, Swain K, Bowler RP, Geraci MW, Ihida-Stansbury K, Stenmark KR, McKinsey TA, Domann FE. Histone deacetylation contributes to low extracellular superoxide dismutase expression in human idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2016;311(1):L124–L134. doi: 10.1152/ajplung.00263.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang Q, Lu Z, Singh D, Raj JU. BIX-01294 treatment blocks cell proliferation, migration and contractility in ovine foetal pulmonary arterial smooth muscle cells. Cell Prolif. 2012;45(4):335–344. doi: 10.1111/j.1365-2184.2012.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen D, Yang Y, Cheng X, Fang F, Xu G, Yuan Z, Xia J, Kong H, Xie W, Wang H, Fang M, Gao Y, Xu Y. Megakaryocytic leukemia 1 directs a histone H3 lysine 4 methyltransferase complex to regulate hypoxic pulmonary hypertension. Hypertension. 2015;65(4):821–833. doi: 10.1161/HYPERTENSIONAHA.114.04585. [DOI] [PubMed] [Google Scholar]
- 130.Yang Y, Cheng X, Tian W, Zhou B, Wu X, Xu H, Fang F, Fang M, Xu Y. MRTF-A steers an epigenetic complex to activate endothelin-induced pro-inflammatory transcription in vascular smooth muscle cells. Nucleic Acids Res. 2014;42(16):10460–10472. doi: 10.1093/nar/gku776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weng X, Yu L, Liang P, Li L, Dai X, Zhou B, Wu X, Xu H, Fang M, Chen Q, Xu Y. A crosstalk between chromatin remodeling and histone H3K4 methyltransferase complexes in endothelial cells regulates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2015;82:48–58. doi: 10.1016/j.yjmcc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 132.Lan B, Hayama E, Kawaguchi N, Furutani Y, Nakanishi T. Therapeutic efficacy of valproic acid in a combined monocrotaline and chronic hypoxia rat model of severe pulmonary hypertension. PLoS One. 2015;10(1):e0117211. doi: 10.1371/journal.pone.0117211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saha A, Wittmeyer J, Cairns BR. Chromatin remodelling: the industrial revolution of DNA around histones. Nat Rev Mol Cell Biol. 2006;7(6):437–447. doi: 10.1038/nrm1945. [DOI] [PubMed] [Google Scholar]
- 134.Chen D, Fang F, Yang Y, Chen J, Xu G, Xu Y, Gao Y. Brahma-related gene 1 (Brg1) epigenetically regulates CAM activation during hypoxic pulmonary hypertension. Cardiovasc Res. 2013;100(3):363–373. doi: 10.1093/cvr/cvt214. [DOI] [PubMed] [Google Scholar]
- 135.Yang Y, Chen D, Yuan Z, Fang F, Cheng X, Xia J, Fang M, Xu Y, Gao Y. Megakaryocytic leukemia 1 (MKL1) ties the epigenetic machinery to hypoxia-induced transactivation of endothelin-1. Nucleic Acids Res. 2013;41(12):6005–6017. doi: 10.1093/nar/gkt311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Strobl JS. A role for DNA methylation in vertebrate gene expression? Mol Endocrinol. 1990;4(2):181–183. doi: 10.1210/mend-4-2-181. [DOI] [PubMed] [Google Scholar]
- 137.Ehrlich M, Gama-Sosa MA, Huang LH, Midgett RM, Kuo KC, McCune RA, Gehrke C. Amount and distribution of 5-methylcytosine in human DNA from different types of tissues of cells. Nucleic Acids Res. 1982;10(8):2709–2721. doi: 10.1093/nar/10.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121(24):2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ryan J, Dasgupta A, Huston J, Chen KH, Archer SL. Mitochondrial dynamics in pulmonary arterial hypertension. J Mol Med. 2015;93(3):229–242. doi: 10.1007/s00109-015-1263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Archer SL. Acquired mitochondrial abnormalities, including epigenetic inhibition of superoxide dismutase 2, in pulmonary hypertension and cancer: therapeutic implications. Adv Exp Med Biol. 2016;903:29–53. doi: 10.1007/978-1-4899-7678-9_3. [DOI] [PubMed] [Google Scholar]
- 141.Jiang JX, Aitken KJ, Sotiropoulos C, Kirwan T, Panchal T, Zhang N, Pu S, Wodak S, Tolg C, Bagli DJ. Phenotypic switching induced by damaged matrix is associated with DNA methyltransferase 3A (DNMT3A) activity and nuclear localization in smooth muscle cells (SMC) PLoS One. 2013;8(8):e69089. doi: 10.1371/journal.pone.0069089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang L, Tang L, Wei J, Lao L, Gu W, Hu Q, Lv Y, Fu L, Du L. Extrauterine growth restriction on pulmonary vascular endothelial dysfunction in adult male rats: the role of epigenetic mechanisms. J Hypertens. 2014;32(11):2188–2198. doi: 10.1097/HJH.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pousada G, Baloira A, Valverde D. Methylation analysis of the BMPR2 gene promoter region in patients with pulmonary arterial hypertension. Arch Bronconeumol. 2016;52(6):293–298. doi: 10.1016/j.arbres.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 144.Thompson AA, Lawrie A. Targeting vascular remodeling to treat pulmonary arterial hypertension. Trends Mol Med. 2017;23(1):31–45. doi: 10.1016/j.molmed.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 145.Barrier M, Meloche J, Jacob MH, Courboulin A, Provencher S, Bonnet S. Today’s and tomorrow’s imaging and circulating biomarkers for pulmonary arterial hypertension. Cell Mol Life Sci. 2012;69(17):2805–2831. doi: 10.1007/s00018-012-0950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Perri F, Longo F, Giuliano M, Sabbatino F, Favia G, Ionna F, Addeo R, Scarpati GDV, Di Lorenzo G, Pisconti S. Epigenetic control of gene expression: potential implications for cancer treatment. Crit Rev Oncol Hemat. 2017;111:166–172. doi: 10.1016/j.critrevonc.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 147.Zhou WQ, Feng XY, Han H, Guo SC, Wang GD. Synergistic effects of combined treatment with histone deacetylase inhibitor suberoylanilide hydroxamic acid and TRAIL on human breast cancer cells. Sci Rep. 2016 doi: 10.1038/srep28004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim SM, Park KC, Jeon JY, Kim BW, Kim HK, Chang HJ, Choi SH, Park CS, Chang HS. Potential anti-cancer effect of N-hydroxy-7-(2-naphthylthio) heptanomide (HNHA), a novel histone deacetylase inhibitor, for the treatment of thyroid cancer. BMC Cancer. 2015 doi: 10.1186/s12885-015-1982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang J, Saren G, Jiang H. HDAC inhibition: a novel therapeutic target for attenuating pulmonary hypertension by regulating Tregs. Int J Cardiol. 2015;198:176–177. doi: 10.1016/j.ijcard.2015.06.172. [DOI] [PubMed] [Google Scholar]
- 150.Gaowa S, Zhou W, Yu L, Zhou X, Liao K, Yang K, Lu Z, Jiang H, Chen X. Effect of Th17 and Treg axis disorder on outcomes of pulmonary arterial hypertension in connective tissue diseases. Mediators Inflamm. 2014;2014:247372. doi: 10.1155/2014/247372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tamosiuniene R, Tian W, Dhillon G, Wang L, Sung YK, Gera L, Patterson AJ, Agrawal R, Rabinovitch M, Ambler K, Long CS, Voelkel NF, Nicolls MR. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res. 2011;109(8):867–879. doi: 10.1161/CIRCRESAHA.110.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chu Y, Xiangli X, Xiao W. Regulatory T cells protect against hypoxia-induced pulmonary arterial hypertension in mice. Mol Med Rep. 2015;11(4):3181–3187. doi: 10.3892/mmr.2014.3106. [DOI] [PubMed] [Google Scholar]
- 153.Bogaard HJ, Mizuno S, Hussaini AA, Toldo S, Abbate A, Kraskauskas D, Kasper M, Natarajan R, Voelkel NF. Suppression of histone deacetylases worsens right ventricular dysfunction after pulmonary artery banding in rats. Am J Respir Crit Care Med. 2011;183(10):1402–1410. doi: 10.1164/rccm.201007-1106OC. [DOI] [PubMed] [Google Scholar]
- 154.Bouchie A. First microRNA mimic enters clinic. Nat Biotechnol. 2013;31(7):577. doi: 10.1038/nbt0713-577. [DOI] [PubMed] [Google Scholar]
- 155.Kamo Y, Ichikawa T, Miyaaki H, Uchida S, Yamaguchi T, Shibata H, Honda T, Taura N, Isomoto H, Takeshima F, Nakao K. Significance of miRNA-122 in chronic hepatitis C patients with serotype 1 on interferon therapy. Hepatol Res. 2015;45(1):88–96. doi: 10.1111/hepr.12317. [DOI] [PubMed] [Google Scholar]
- 156.Bienertova-Vasku J, Novak J, Vasku A. MicroRNAs in pulmonary arterial hypertension: pathogenesis, diagnosis and treatment. J Am Soc Hypertens. 2015;9(3):221–234. doi: 10.1016/j.jash.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 157.Meloche J, Paulin R, Provencher S, Bonnet S. Therapeutic potential of microRNA modulation in pulmonary arterial hypertension. Curr Vasc Pharmacol. 2015;13(3):331–340. doi: 10.2174/15701611113119990010. [DOI] [PubMed] [Google Scholar]