Abstract
Blood vessel regression is an essential process for ensuring blood vessel networks function at optimal efficiency and for matching blood supply to the metabolic needs of tissues as they change over time. Angiogenesis is the major mechanism by which new blood vessels are produced, but the vessel growth associated with angiogenesis must be complemented by remodeling and maturation events including the removal of redundant vessel segments and cells to fashion the newly forming vasculature into an efficient, hierarchical network. This review will summarize recent findings on the role that endothelial cell apoptosis plays in vascular remodeling during angiogenesis and in vessel regression more generally.
Keywords: Vessel pruning, BCL2, Death receptors, Diabetes, Diabetic retinopathy
Introduction
Blood vessels are tubular structures composed of an inner lining of endothelial cells (ECs) surrounded by perivascular support cells (pericytes and smooth muscle) and layers of basement membrane [1]. The blood vasculature supports the metabolic needs of tissues by supplying oxygen and nutrients and removing waste products to facilitate tissue growth and maintain homeostasis. As tissues grow, so too does their demand for oxygen and nutrients, requiring a corresponding expansion of the vascular network to supply this need. Angiogenesis, defined as the sprouting growth of new vessels from pre-existing vessels [2–5], is the major mechanism responsible for the growth and expansion of blood vessel networks. Angiogenesis begins in utero [6] and continues into post-natal life during normal tissue growth. However, it can also be activated pathologically, contributing to diseases such as cancer, wet age-related macular degeneration, and diabetic retinopathy. Vessel network growth by angiogenesis is an imperfect process, as it generates excessive numbers of vessels that do not resemble a mature, hierarchical network. Achieving such hierarchy requires the selective regression of superfluous vessels (termed ‘pruning’), as well as the removal of excessive cells.
While vessel regression in the context of angiogenesis is thought to improve the functionality of the network, it can also serve to contract vessel networks as the metabolic demand of the tissue drops. For example, extensive vessel regression occurs in the corpus luteum during luteolysis [7] and the mammary gland during post-lactation involution [8]. Vessel regression can also remove vessel networks in their entirety. A particularly well-studied example of this is the hyaloid vasculature: a transient vessel network in the eye vitreous that is necessary for eye development, but is made redundant and undergoes regression when the retina becomes vascularized [9, 10]. In addition to these physiological examples, vessel regression can also be induced by stress stimuli. For example, wide-spread vessel regression, known as vaso-obliteration, occurs in the central retina of neonates exposed to hyperoxia in an animal model for retinopathy of prematurity [11], and neutralization of the survival-promoting, pro-angiogenic cytokine vascular endothelial growth factor (VEGF)-A, induces vessel regression in tumors and some normal tissues [12–14]. Vessel regression is also observed in several organs of diabetic patients, such as in the eye as a precursor to proliferative retinopathy and the kidney preceding nephropathy.
Apoptosis is a programmed form of cell death that contributes to tissue remodeling during development through the removal of redundant cells [15]. EC apoptosis has been reported in numerous examples of vessel ablation including hyaloid vessel regression [9], hyperoxia-induced retinal vaso-obliteration [16], mammary gland involution [8], and vessel regression following VEGF-A inhibition [13, 17, 18]. The importance of apoptosis to vessel regression in these cases is supported by studies showing that hyaloid regression [19–22], retinal vaso-obliteration [19], and the tumor growth-suppressing effects of VEGF-A neutralization [23] are all inhibited in mice lacking genes essential for apoptosis. EC apoptosis is also present during angiogenic vessel growth, both in vessel segments being pruned as part of the normal remodeling process, as well as in vessels not displaying overt signs of regression [20]. While the role of apoptosis in the examples of vessel ablation outlined above is well established, its contribution to the angiogenic process has until recently remained less so. Here, we review the role of EC apoptosis in angiogenesis as well as vessel regression more generally, with a focus on recent in vivo studies.
Angiogenesis
The growth of new vessels by sprouting angiogenesis is a multi-step process that can be broadly divided into two major processes: growth and maturation. The molecular regulation of sprouting angiogenesis and the accompanying cellular behavior have been the subject of numerous recent reviews and will not be covered in detail here [2–5]. During the growth phase, new vessel sprouts emerge from the existing vessels in response to VEGF-A [2]. These sprouts are led by a specialized EC type termed the ‘tip cell’, a highly migratory cell type that extends multiple filopodia and is thought to direct the migration of the sprout by sensing guidance cues [24–26]. Trailing the tip cells are the so-called ‘stalk cells’, which proliferate and contribute new cells to the growing sprout. Tip and stalk cell identity is dynamic, allowing for constant competition between ECs for the tip cell position. This competitive behavior has been hypothesized to ensure that migration is continually directed toward increasing concentrations of VEGF-A [27]. Vessel sprouts are blind-ended, but establish perfused vessel segments by anastomosing with other sprouts or vessels and subsequently forming a continuous lumen with them [5].
While sprouting angiogenesis is very effective at generating new vessels, the network that it initially produces is immature and requires maturation and remodeling events to transform it into a network with optimal, hierarchical structure. This process involves the selective removal of some vessels by pruning and the stabilization and maturation of others [5, 28, 29]. Vessel stabilization and maturation occurs through the deposition of extracellular matrix around the vessel and the recruitment of perivascular support cells such as pericytes. Pericytes stabilize vessels by secreting angiopoietin-1 (ANG1) which activates the TIE2 receptor on ECs, tightening junctions and reducing leakiness [30]; however, in some organs, pericytes can regulate ANG/TIE signaling in ECs independent of ANG1 secretion [31]. While the mechanisms that determine which vessel segments will regress and which will be stabilized remains poorly understood, it appears to be driven largely by hemodynamic cues, such that vessel segments experiencing low blood flow shear relative to their neighbors will be preferentially selected for regression [32, 33]. In addition to the removal of excessive vessel segments, extensive cellular rearrangements and a concurrent reduction in overall EC density also occur during angiogenic vessel remodeling [20, 34].
Much of our knowledge of the growth and maturation phases of angiogenesis in mammals has come from the study of the neonatal mouse retina. Vascularization of the mouse retina occurs entirely post-natally through sprouting angiogenesis in response to tissue hypoxia. Vessel sprouts emerge from the optic nerve head at the center of the retina at birth, and then expand radially, reaching the periphery of the retina around post-natal day (P)8 [35]. During retina angiogenesis, EC sprouting and proliferation occur at the leading edge of the growing network, while vessel remodeling and maturation occur more centrally. This division is not absolute, however, as EC proliferation also occurs in and around veins in the central portion of the retina [34], while vessel pruning occurs throughout the network [32]. The presence of EC apoptosis during retina angiogenesis is well documented [20, 32, 36, 37]. The distribution of apoptotic ECs within the newly forming vascular network changes over the course of the angiogenic response. While it is initially clustered around remodeling arteries, away from the proliferative regions of the vein and sprouting front, it becomes more uniformly distributed over time as the vessels shift away from proliferation and growth, but when remodeling and maturation processes are still continuing [20].
The retina is an attractive model for studying angiogenesis because of its significance to human health. Under-development or inappropriate re-activation of angiogenesis in the retinal vasculature underlies a wide range of vision-impairing diseases in humans [38, 39]. Furthermore, pathological re-activation of angiogenesis (neovascularization) in retinal vessels occurs in diseases such as proliferative diabetic retinopathy (PDR). This can result in the abnormal growth of vessels into the vitreous, greatly increasing the likelihood of vision impairment due to hemorrhage, scarring, and retinal detachment. Intravitreal neovascularization also occurs in retinopathy of prematurity, which can afflict pre-term infants of low birth-weight. This can be recapitulated in mice using the oxygen-induced retinopathy (OIR) model [11]. The OIR model is particularly relevant to the study of EC apoptosis and vessel regression. In this model, mice are reared briefly in a hyperoxic environment, which results in vaso-obliteration in the central retina. Upon return to normal air, the avascular central retina becomes hypoxic [40], triggering the over-production of pro-angiogenic cytokines such as VEGF-A [41] and causing the misdirected growth of vessels into the vitreous [42]. This model is relevant to the study of apoptosis and vessel regression, because extensive EC apoptosis accounts for the initial hyperoxia-induced vaso-obliteration [16, 19, 43], and the neovascular lesions undergo spontaneous regression over time [35]. Similar to the OIR model of ischemia-induced retinopathy, PDR is preceded by retinal capillary non-perfusion that is associated with increased EC and pericyte apoptosis [44]. This capillary loss presumably creates areas of focal ischemia that result in the abnormal production of pro-angiogenic cytokines including VEGF-A, driving neovascularization [45].
Molecular regulation of apoptosis
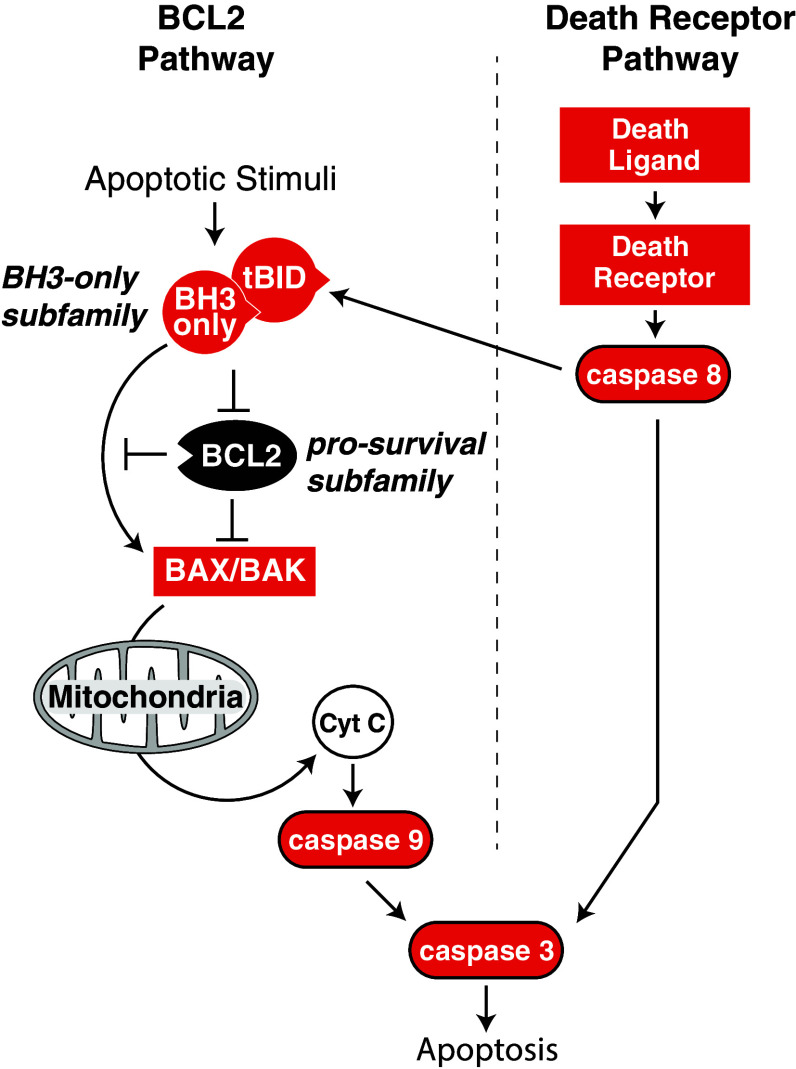
Two separate pathways control apoptosis: one is regulated by BCL2 family proteins and the other by the so-called ‘death receptors’. Both converge on the activation of caspases, intracellular proteases normally present in the cell as inactive zymogens. A cascade of caspases are activated during apoptosis, starting with initiator caspases (e.g., caspases 8 and 9) that in turn cleave and activate downstream effector caspases (e.g., caspases 3 and 7) which go on to cleave hundreds of target proteins and activate DNases, thereby demolishing the cell [46].
The BCL2-regulated apoptosis pathway
The BCL2-regulated pathway (also known as the ‘intrinsic’ or mitochondrial pathway) removes excess cells during development, but is also activated in response to cellular damage or stress. Mice in which the BCL2 pathway of apoptosis has been blocked show developmental defects including persistent interdigital webbing and accumulation of excess hematopoietic and neuronal cells [47]. Cells lacking the BCL2 apoptosis pathway are also resistant to a range of cellular stresses such as exposure to DNA damaging agents, ER stress, and withdrawal of trophic factor support [47, 48]. The BCL2 family proteins are related by the presence of short segments of sequence homology referred to as the BCL2-homology (BH) regions. These proteins are divided into three sub-families (Fig. 1). BCL2 is the prototypic member of the pro-survival sub-family, which also includes BCLxL, MCL1, BCLW, and A1. The other two sub-families are pro-apoptotic. The first of these, the BH3-only sub-family, is so-named because its members share only the BH3-region in common. Its members (BIM, BID, BAD, BIK, BMF, HRK, PUMA, and noxa) are regulated by apoptotic stimuli and initiate apoptosis via the BCL2 pathway. BAX and BAK (and possibly BOK) make up the other pro-apoptotic sub-family, and are responsible for the mitochondrial damage that commits a cell to apoptotic death. Interactions between the three BCL2 sub-families determine whether BAX and BAK become activated (Fig. 1). Pro-survival BCL2 family proteins inhibit cell death by preventing BH3-only proteins from binding to and activating BAX and BAK [49], and by directly binding to and inhibiting the activated forms of BAX and BAK [50]. There is selective affinity of BH3-only proteins for other BCL2 family members. While BIM, tBID, and PUMA appear to have affinity for all pro-survival BCL2 family proteins, others, such as BAD and noxa, bind only a limited subset [51–53]. Furthermore, only some BH3-only proteins (e.g., BIM and tBID) can directly bind to and activate BAX and BAK, thus are referred to as ‘activator’ BH3-only proteins [49]. Other BH3-only proteins displace the activator BH3-only proteins from the pro-survival proteins, thus freeing them to bind and activate BAX and BAK [49]. Once activated, BAX and BAK form oligomers at the outer mitochondrial membrane, which, through an ill-defined mechanism, permeabilize the mitochondria. This causes the release of apoptogenic factors such as cytochrome c and others that trigger the activation of the initiator caspase, caspase 9, and the subsequent caspase cascade [54].
Fig. 1.
Schematic overview of the BCL2-regulated and death receptor-regulated apoptosis pathways. In the BCL2 pathway, interactions between opposing factions of BCL2 family proteins (pro-apoptotic BH3-only sub-family proteins and pro-survival BCL2 sub-family proteins) determine whether the effector sub-family proteins BAK and BAX become activated. Activation of BAK and BAX results in mitochondrial permeability and release of apoptogenic factors such as cytochrome C, which, in turn, leads to activation of the caspase cascade via caspase 9. In the extrinsic pathway, death receptor-ligand engagement by their cognate receptor leads to activation of the caspase cascade via caspase 8. In some cell types, caspase 8 cleaves the BH3-only protein BID, yielding its active, truncated form (tBID) that can activate BAX and BAK
Death receptor-regulated apoptosis
The death receptor-mediated apoptosis pathway (otherwise known as the ‘extrinsic’ pathway) is particularly important in the adaptive immune system for killing infected cells and to prevent auto-immunity and tumorigenesis [55]. Apoptosis via this pathway is triggered when a death receptor on the surface of a cell is engaged by its cognate death ligand. Death receptors are a subset of the tumor necrosis factor (TNF) superfamily that are defined by the presence of a cytoplasmic death domain. These include Fas (CD95), TNFR1, death receptor (DR)3, and the TRAIL receptors DR4 and DR5 [56]. Their respective ligands are FasL, TNF, Apo3L, and TRAIL/Apo2L [56]. Ligand engagement of death receptors results in the recruitment of a “death-inducing signaling complex” (DISC) via their death domains [57]. The DISC activates the initiator caspase, caspase 8, which, in turn, activates the caspase cascade. In certain cell types, known as “type II” cells, the apoptotic response also requires caspase 8-mediated cleavage of the BH3 only protein BID, to produce its activated form, tBID, a direct activator of BAX and BAK, and thus the mitochondrial apoptosis pathway [58–60]. Therefore, while the BCL2 and death receptor apoptosis pathways generally act separately, type II cells require cross-talk between the two.
Death receptors do not solely transduce apoptotic signals. TNFR1, TRAIL, and FAS receptors can activate NF-κB and MAPK signaling pathways, leading to the expression of genes involved in inflammation, proliferation, and survival [55, 61, 62]. Under the right conditions (which can include inhibition of caspase 8), death receptors can also trigger necroptosis, a caspase-independent form of programmed necrosis [63]. Genetic inactivation of caspase 8 leads to embryonic lethality from cardiovascular defects [64]. These are likely caused by activation of necroptosis as co-deletion of the necroptosis pathway proteins RIPK3 or MLKL rescues the caspase 8 lethal phenotype [65–67]. This phenotype appears to be EC-specific as it is recapitulated when caspase 8 is deleted in endothelium using Tie1-cre [68].
Pathways regulating endothelial cell survival and death
Factors modulating endothelial cell survival
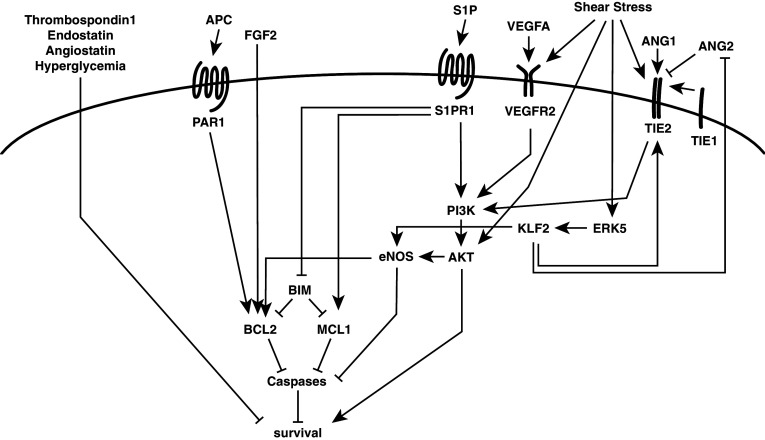
Pro-angiogenic growth factors promote EC survival (Fig. 2). VEGF-A promotes EC survival via activation of PI3K/AKT signaling [69–72]. In vivo, exogenous VEGF-A can prevent EC apoptosis [16, 73, 74], whereas blocking its activity causes EC apoptosis [13, 17, 18]. VEGF-A can be produced by ECs themselves and this autocrine VEGF-A production is believed to promote EC survival through intracellular signaling [75]. However, whereas signaling by extracellular VEGF-A prevents apoptotic cell death, intracellular VEGF-A signaling regulates autophagic cell death [76].
Fig. 2.
Overview of selected endothelial cell survival signaling pathways. EC survival is regulated via multiple signaling pathways, including growth factor receptor tyrosine kinases, G-protein coupled receptors, and mechanical forces, such as laminar blood flow shear stress. See main text for details
The ANG/TIE signaling axis is another important regulator of angiogenesis that also impinges on EC survival. It consists of two ligands (ANG1 and 2) and two receptors (TIE2 and the orphan receptor TIE1). ANG1 activates the TIE2 receptor and regulates both angiogenesis and vessel maturation, whereas ANG2 is a context-specific agonist or antagonist of TIE2 [30]. ANG1 promotes EC survival via activation of the PI3K/AKT pathway [77, 78]. Both the TIE1 and TIE2 receptors appear necessary to promote EC survival in cells that lose survival stimuli such as serum support or fluid shear [79]. While both TIE1 and TIE2 are required for the survival signal, deletion of TIE2 in quiescent adult vessels does not affect vascular integrity or stability, at least in the retina, suggesting that it is not required for EC survival (or barrier integrity) in all vessels [31]. ANG2, by contrast, is frequently expressed at sites of vessel remodeling (e.g.: hyaloid vessel regression or luteolysis) [29] and is required to induce hyaloid vessel regression by activating EC apoptosis [80, 81]. ANG2 is not required, however, for hyperoxic vaso-obliteration of neonatal retinal vessels [82], and thus, its role appears context-specific.
In addition to its other roles in the vasculature such as vasodilation and suppression of inflammation, nitric oxide (NO) produced in ECs by endothelial nitric oxide synthase (eNOS) can prevent EC apoptosis caused by a range of stimuli [83]. NO directly inhibits caspase activation through S-nitrosylation of the active site of caspases, activation of ERK1/2, suppression of JNK, and upregulation of BCL2 [83, 84]. eNOS is activated by AKT in response to a range of stimuli including shear stress [85], VEGF-A [86], and sphingosine 1-phosphate (S1P). S1P, via its G-protein-coupled receptors SIPR1 (and to a lesser extent S1PR3) activates eNOS via AKT in a calcium-dependent manner to promote EC survival [87, 88]. An S1P receptor agonist delays apoptosis in ECs following serum starvation by upregulating the expression of pro-survival MCL1 and delaying the induction of BIM [89]. S1P and other lysophospholipids are present in high-density lipoprotein complexes and they have been proposed to impart the anti-apoptotic activity of HDL on ECs via G-protein-coupled receptor activation of eNOS via AKT [90]. Loss of S1PR1 in embryos results in EC apoptosis; however, this is subsequent to other vascular abnormalities and loss in post-natal retinal vasculature resulted in a hyper-sprouting phenotype with no obvious evidence for impaired EC survival [91], and thus, the regulation of EC survival is likely secondary to other roles for S1P in vivo. Other G-protein-coupled receptors contribute to EC survival. Activation of protease-activated receptor 1 (PAR1) by activated protein C prevents EC apoptosis in response to high glucose by preventing the downregulation of BCL2 and the translocation of BAX to mitochondria [92]. Kaposi sarcoma is a malignancy of the endothelium caused by Kaposi sarcoma-associated herpesvirus (KSHV) [93]. A key molecule in the pathogenesis of Kaposi sarcoma is a G-protein-coupled receptor encoded by the virus (referred to as KSHV-GPCR), which has been shown to promote the survival of serum deprived ECs through activation of PI3K/AKT signaling [94].
Other factors reported to promote EC survival include erythropoietin which acts via PI3K/AKT (and MEK/ERK) activation [95], FGF2 [72, 96, 97], and both extracellular matrix and cell–cell contacts by ECs [98]. In contrast, endogenous angiogenesis inhibitors (thrombospondin-1, endostatin, and angiostatin) have been reported to induce EC apoptosis [98].
Blood flow-mediated endothelial cell survival
Laminar shear stress imparted by unidirectional blood flow is an important stimulus for shaping vascular networks for maximal flow efficiency, which suppresses inflammation (such as in atherosclerosis) and promotes EC survival [99]. A range of endothelial cell-surface receptors can sense shear stress, including integrins, receptor tyrosine kinases, G-protein-coupled receptors, and intercellular junction proteins [100]. Blood flow shear regulates EC survival through AKT signaling, which, in turn, upregulates eNOS [85, 101] (Fig. 2). Ligand-independent activation of VEGFR2 occurs in response to shear stress, resulting in activation of PI3K/AKT and eNOS by forming a complex with VE-cadherin and beta-catenin [102, 103]. The MAP kinase ERK5 is activated in ECs by laminar blood flow shear and promotes EC survival and anti-inflammatory responses [104, 105]. ERK5 upregulates eNOS via the flow-responsive transcription factor Kruppel-like factor 2 (KLF2) [104]. KLF2 also modulates the ANG/TIE axis by upregulating TIE2 but downregulating ANG2 in response to flow [79, 104]. This may enhance ANG1/TIE2 survival signaling under laminar flow conditions, but shear stress can also promote EC survival by directly activating TIE2, thereby triggering activation of the PI3K/AKT pathway [106]. TIE1 is upregulated under conditions of low flow, and during angiogenesis, this has been proposed to promote EC survival in vessels undergoing angiogenic vessel pruning (which is associated with reduced blood flow) through increased expression of TIE2 [79]. Despite its positive regulation by blood flow shear, deletion of TIE2 from adult quiescent vasculature demonstrates that it is not continuously required for EC survival by all vessels, at least in the quiescent state [31]. Similarly, mice lacking ERK5 specifically in the quiescent endothelium survive normally and do not show elevated levels of EC apoptosis, suggesting that ERK5 may not be required for vascular maintenance under steady-state conditions [107]. On challenge, however, ERK5 mice have increased atherosclerotic lesion formation demonstrating an important role for ERK5 in protecting against atherosclerosis [107]; however, the extent to which this depends on its anti-apoptotic function as opposed to its anti-inflammatory role is not clear.
Pericyte regulation of endothelial cell survival
In addition to their role in promoting vascular stability and integrity, pericytes can promote EC viability through the production of the pro-survival cytokines ANG1 [30] and VEGF-A [108]. A protective role for pericytes was suggested by early studies, which showed that the level of pericyte investment determined retinal vessel sensitivity to hyperoxia-induced regression [109]. However, extensive studies using more specific markers for pericytes have since shown that all retinal vessels are covered by pericytes, including those that are sensitive to hyperoxia-induced apoptosis [110]. Nonetheless, EC apoptosis during retina angiogenesis increases dramatically in the absence of pericytes [31], and pericyte-specific deletion of the BH3-only gene Bim leads to an increase in EC number in retinal vessels, likely due to increased pericyte numbers [111]. In the retina, pericytes promote TIE2 signaling, which, in turn, suppresses FOXO1 activation [31]. While FOXOs are transcriptional inducers of apoptosis, FOXO1 upregulation is unlikely to be the cause of increased EC apoptosis observed in the absence of pericytes, because the expression of constitutively active FOXO1 in ECs in vivo does not cause increased EC apoptosis [112]. Loss of pericytes causes significant vascular abnormalities in the angiogenic retina and the temporal sequence of these events and EC apoptosis needs to be determined to better understand the relationship between pericytes and EC survival in this context [31]. While pericytes are typically regarded as being a major source of ANG1, this appears not to be the case in the retina, where pericytes appear instead to promote TIE2 signaling by regulating the expression of angiopoietin receptors on the surface of ECs [31]. The sensitivity of tumor-associated ECs to apoptosis following VEGF-A inhibition has also been suggested to depend on pericyte coverage [113]. However, tumors grown in mice deficient in pericytes did not show enhanced sensitivity to VEGF-A inhibition [114] and pericyte coverage does not determine the sensitivity of normal blood vessels to VEGF-A inhibitors [14].
Pericytes have also been found to promote EC apoptosis in some contexts. In hyaloid vessels, pericytes produce ANG2, which reduces pro-survival ANG1/TIE2 signaling on hyaloid ECs [81]. During retina angiogenesis, pericytes have been proposed to promote apoptotic vessel regression through the production of endosialin [115]; however, the increased vessel density reported in endosialin −/− mice needs to be reconciled with the reduced vessel density seen in the absence of pericytes themselves [31] and the lack of a vessel regression defect in EC-apoptosis resistant mice [20].
BCL2-pathway regulation of endothelial cell apoptosis
There is strong evidence that the BCL2 pathway regulates EC survival during normal angiogenesis and vessel regression. The BH3-only protein BIM is an essential initiator of EC apoptosis, consistent with its ability to bind to all pro-survival BCL2 family proteins. BIM is required for hyaloid vessel regression [19, 21], tumor-associated EC apoptosis in response to VEGF-A inhibition [23], and EC apoptosis during retina angiogenesis [19, 21, 111]. Hyperoxia-induced vaso-obliteration of central retina vessels is also prevented in Bim −/− mice [19], but interestingly not in EC- or pericyte-specific Bim mutants, suggesting that death of several cell types in the neurovascular unit may contribute to overall vessel regression in this model [111]. While in vitro studies have suggested that FOXO3 (a negatively regulated target of PI3K/AKT signaling) promotes the expression of Bim in ECs [116], retina EC apoptosis was not altered in Foxo3 −/− mice [21]. Redundancy with other FOXO family members is unlikely as retina EC apoptosis was also not affected in mice in which the FOXO binding sites were mutated in the BIM promoter [21], or in mice where ECs expressed a constitutively nuclear FOXO1 [112]. These results argue against an essential requirement for FOXO regulation of BIM during normal angiogenesis in vivo. Loss of BIM does not protect against all EC apoptosis, however [19, 21], likely due to redundancy with other BH3-only proteins. Other BH3-only proteins expressed in blood vessels in vivo include BIK in hyaloid endothelium [117] and PUMA in retinal endothelium [118]; however, loss of BIK did not impair hyaloid vessel regression [117], and PUMA reportedly has an unusual pro-survival, pro-proliferative role in ECs [118]. Therefore, which BH3-only proteins other than BIM may be important for EC apoptosis remains unclear. The importance of the BCL2 pathway to EC apoptosis was further demonstrated in mice in which ECs were engineered to be apoptosis resistant through genetic inactivation of the apoptosis effectors BAK and BAX. These mice were generated through the EC-specific deletion of Bax on an otherwise Bak −/− background (referred to as Bak −/− Bax EC/EC mice) [20]. EC apoptosis that normally occurs during retina angiogenesis was completely blocked in Bak −/− Bax EC/EC mice, while hyaloid vessel apoptosis and regression was significantly impaired [20].
Endothelial cell survival during angiogenesis depends on pro-survival BCL2 family proteins
Knockout mouse studies have revealed a diverse range of cell types and contexts in which individual pro-survival BCL2 family proteins promote cell survival [119]. Because ECs are diverse in terms of their identity, function and environmental context, it is unlikely that a single pro-survival protein will be responsible for the survival of all ECs in all circumstances. Recent studies investigating the role of BCL2 family proteins during angiogenesis have suggested that distinct pro-survival BCL2 family proteins do indeed have context-specific roles for promoting EC survival.
MCL1 has been shown to act as a dose-dependent survival factor for ECs during angiogenesis. EC-specific deletion of Mcl1 resulted in an increase in EC apoptosis and a reduction in vessel density during retina angiogenesis [120]. However, the reduction in vessel density was not a consequence of increased vessel regression. Instead, the location of the apoptotic ECs in the mutants likely holds the key to explaining the reduction in vasculature observed. In MCL1 mutants, the distribution of apoptotic ECs was substantially altered, with large numbers of apoptotic ECs in the proliferative regions of the sprouting front and the remodeling veins, areas in which EC apoptosis does not normally occur [120]. There are several ways in which the ectopic apoptosis in these growth regions may impact on vessel density. First, MCL1 may be essential for the survival of proliferating ECs themselves. Second, loss of MCL1 may reduce the number of ECs available to proliferate. Finally, ectopic apoptosis in the sprouting front could deplete tip cells, thereby reducing new vessel growth. Tip cell numbers determine vascular density: over-production of tip cells results in the growth of excessive vessels [121], while a deficiency in tip cells results in sparse vasculature [122].
In vitro studies on the pro-survival effects of VEGF-A and FGF2 found that they induced the expression of BCL2 in ECs [72, 96, 123, 124]. Mice lacking VE-cadherin are embryonic lethal, with extensive EC apoptosis attributed to the downregulation of BCL2 due to reduced PI3K/AKT signaling by VEGF-A [72]. Furthermore, over-expression of BCL2 enhances EC survival [96, 123, 124]. While these data suggest that BCL2 may promote EC survival in response to angiogenic cues, in vivo data have suggested that this role may be context-specific. EC-specific deletion of Bcl2 had no effect on sprouting angiogenesis in the neonatal retina or on neovascularization in the OIR model, but it was required for the angiogenic response during laser-induced choroidal neovascularization [125]. While BCL2 was not required for vessel growth or EC proliferation during sprouting angiogenesis, EC-specific BCL2 mutants did show increased EC death and fewer ECs at later time points when a reduction in overall EC density is normally occurring [125]. This would suggest that while BCL2 is dispensable for normal angiogenic vessel growth, it is required during late-stage vessel maturation and for certain cases of pathological angiogenesis.
The other members of the pro-survival sub-family (BCLW, A1, and BCLxL) have each been implicated in EC survival; however, detailed investigation into their functional requirement during angiogenesis in vivo has to date been lacking. BCLW is reportedly increased in ECs by ‘intracrine’ VEGF-A signaling [126], although the significance of this is not immediately clear given that Bclw −/− mice are viable and healthy well into adulthood [127], whereas EC-specific VEGF-A mutants are not [75]. Furthermore, ‘intracrine’ VEGF-A promotes EC survival by preventing autophagic cell death, not apoptosis [76], whereas the inhibition of pro-survival BCL2 proteins only triggers autophagy as a downstream consequence of apoptosis induction [128]. A1 is upregulated in ECs by VEGF-A and the inflammatory cytokines TNFα and IL-1, and can inhibit apoptosis caused by TNFα stimulation when over-expressed [123, 129, 130]. Investigation of A1’s roles in vivo has been hampered by gene duplication events and only recently has a mouse lacking all isoforms of A1 been generated and shown to be viable [131]. BCLxL is induced by EPO via PI3K/AKT [95], but not by VEGF-A, which also promotes EC survival via activation of PI3K/AKT [123, 124]. BCLxL is also downregulated concurrent with increased EC apoptosis in Ets1/2 double knockouts in vivo [132], and by Angiotensin-II in vitro [133]. Bclx −/− mice are embryonic lethal with neural and hematopoiesis defects [134]. Thus, while there is evidence for these pro-survival proteins in EC survival, their role during angiogenesis has yet to be determined.
The data for pro-survival BCL2 family proteins to date suggest a model in which MCL1 is required for the survival of ECs during the growth phase of angiogenesis, particularly in those regions of the vasculature where proliferation and sprouting are occurring, whereas BCL2 appears to be required for EC survival during the later stages of vessel maturation. Other cell types, particularly lymphocytes, are known to switch their dependence on pro-survival proteins depending on their stage of development or maturation [135–137] and the above studies suggest that ECs will do the same.
Death receptor regulation of endothelial cell apoptosis
Death receptors have also been investigated for their involvement in angiogenesis and blood vessel regression. Studies in the rat using FasL-neutralizing antibodies have suggested that Fas-dependent EC apoptosis was required for vessel pruning during normal retina angiogenesis and hyperoxia-induced retinal vaso-obliteration [138]. In mice, however, both retina vessel development and vaso-obliteration were reportedly normal in Fasl loss of function mutants (Fasl gld/gld) [139, 140]. Despite having apparently normal vessel regression, Fasl gld/gld mice did show an increase in neovascular tuft formation and fewer TUNEL+ cells within the tufts in the OIR model, suggesting that Fas signaling may limit neovascularization by inducing EC death [139, 140]. Laser-induced choroidal neovascularization was also increased in both Fasl gld/gld and Fas mutant (Fas lpr/lpr) mice, again suggesting that Fas-induced EC death may prevent pathological angiogenesis in the eye [141].
TRAIL has been reported to either cause EC apoptosis or promote angiogenic behavior in in vitro assays. These divergent outcomes have been suggested to depend on the concentration of TRAIL used, with higher doses favoring apoptosis [142]. Mice have a single death-inducing TRAIL receptor, DR5, which is expressed on normal retinal ECs, OIR-induced neovascular tufts and tumor-associated ECs in vivo [143, 144]. In vivo administration of cross-linked TRAIL to tumor-bearing mice caused extensive tumor hemorrhage with accompanying EC apoptosis [144]. While Trail −/− mice exhibited a small, transient reduction in the capillary free area around retinal arteries, they did not show any impairment in hyperoxia-induced vaso-obliteration [143]. Trail −/− mice showed increased neovascularization in the OIR model, along with delayed tuft regression, suggesting that like Fas, EC apoptosis by DR5 may limit neovascularization and promote its spontaneous regression [143].
Mice lacking caspase 8 (on an Mlkl −/− background) have also been investigated to assess the sum of death receptor-induced apoptosis on the angiogenic vasculature. While these mutants showed normal levels of EC apoptosis in the retina at P5, there was possibly a small reduction at P6; however, this may have been an indirect consequence of reduced weight gain in the mutants at this age [20]. Nonetheless, the potential reduction seen at P6 was minimal compared to that seen in Bak −/− Bax EC/EC mice. Overall, the above studies would suggest that any contribution by death receptors to EC apoptosis during normal angiogenesis and vessel regression is likely minor by comparison to mice in which BCL2-pathway apoptosis is inhibited. Instead, the role appears more directed to restricting pathological angiogenesis, and given that death receptors can activate pro-inflammatory signaling pathways with the potential to impact pathological angiogenesis responses, the contribution of EC survival regulation in this context is not always clear.
Can endothelial cell apoptosis initiate vessel regression?
Studies of the pupillary membrane that overlies the developing lens suggested that apoptosis could initiate vessel regression. In those vessels, dying ECs were observed to protrude into the vessel lumen obstructing blood flow, followed by synchronous death of the remaining ECs in the affected vessel due to the disruption to blood flow shear [145]. However, while EC apoptosis occurs in the pupillary membrane vessels, more recent studies have suggested that it may not be essential for initiating their regression. Mice with germline deletion of the apoptosis effectors BAK and BAX do not show persistent pupillary membrane vessels, despite a block in EC apoptosis [22]. Instead, vessel stretching during growth of the lens may provide the stimulus for pupillary membrane vessel retraction [146].
Apoptosis likely initiates vessel regression in the context of the hyaloid network. Hyaloid vessel regression is dependent both on macrophage-derived Wnt7b [147] and ANG2 [81]. Mice lacking Wnt7b or Ang2 have persistent hyaloids and less EC apoptosis [80, 81, 147]. Consistent with a role in preventing EC apoptosis, high levels of retinal VEGF-A can also lead to persistent hyaloids [148, 149]. Mice in which EC apoptosis is blocked through genetic inactivation of BAK and BAX contain persistent, perfused hyaloid vessels [20, 22]. The presence of perfused hyaloids in these mice raises the possibility that the model originally proposed in the pupillary membrane vessel regression of apoptotic EC causing the initial vessel blockage may apply in the hyaloid network. However, definitive proof for this will require live-imaging of hyaloid regression. While these results strongly suggest that apoptosis can initiate hyaloid vessel regression, this requirement may not be absolute as some hyaloid vessel regression still occurs in Bak −/− Bax EC/EC mice [20].
The role of endothelial cell apoptosis during angiogenesis
The presence of dying ECs during angiogenesis has been recognized since the 1960s [150]. While not referred to specifically as apoptotic death (a term not coined until 1972 [151]), subsequent molecular analysis using methods such as the detection of activated caspase 3 has confirmed the presence of apoptosis. However, the role that this plays during angiogenesis, particularly in regard to vessel pruning, has until recently remained unclear.
Apoptosis during angiogenic vessel pruning
During angiogenic maturation of the retinal vasculature, characteristic avascular zones form around maturing arteries. This has been attributed to a reduction in Vegfa expression around arteries, likely due to the diffusion of oxygen from arterial blood, causing vessels in this region to regress [152]. Consistent with this, VEGF-A inhibition or exposure of pups to increasing levels of oxygen widens the avascular zone [43, 152]. As hyperoxia causes apoptotic vaso-obliteration concurrent with reduced VEGF-A expression [16], the reduction in vessel density around arteries has similarly been attributed to EC apoptosis [152]. In line with this hypothesis, EC apoptosis in the neonatal retina is frequently observed in regressing vessels around maturing arteries [20, 32, 115, 120]. Several mouse mutants are also consistent with a role for EC apoptosis in vessel pruning. Reduced EC apoptosis in mice lacking Endosialin [115] or the BH3-only protein BIM [19] was associated with increased retinal vessel density. Conversely, EC-specific deletion of the angiopoietin receptor TIE1 [79] or Wnt pathway genes [37, 153] or over-expression of FGD5 [154] each showed reduced vessel density correlated with increased EC apoptosis.
However, other lines of evidence do not support a role for EC apoptosis in angiogenic vessel pruning. No correlation between vessel regression and EC apoptosis could be found during retina angiogenesis in the rat [36], and while the majority of apoptotic ECs are found in regressing vessels during mouse retina angiogenesis [20], only 5% of all regressing vessels actually contain apoptotic ECs [32]. Although some reports suggested that EC apoptosis was responsible for the reduced vasculature in Wnt pathway mutants [37, 153], others have not supported this [155]. The direct correlation between EC apoptosis and vessel regression reported in many mutants affecting retina angiogenesis is not always observed. Mice lacking the pro-survival protein MCL1 in ECs did not show an increase in vessel regression, despite increases in EC apoptosis to levels that exceeded that of other mutants [120]. Given that the genes in those mutants that showed a direct correlation between apoptosis and regression regulate processes other than just apoptosis, it seems likely that the disruption of those functions, not cell survival control, will be responsible for the regression phenotypes observed. In contrast, MCL1 appears to regulate only apoptosis in ECs [120]. Despite Bim −/− mice having increased retinal vasculature [19], this may be independent of a reduction in apoptosis as ECs isolated from these mice show increased migratory activity [156, 157], and Bim also regulates pericyte numbers which, in turn, could influence vascular patterning [111]. Finally, time-lapse imaging studies in zebrafish have shown that EC apoptosis is not common during vessel pruning [33, 158, 159].
Endothelial cell migration, not apoptosis drives angiogenic vessel pruning
Analysis of retina angiogenesis in the EC apoptosis-resistant Bak −/− Bax EC/EC mice has directly tested whether apoptosis is required for vessel pruning. Despite the lack of EC apoptosis in these mice, vessel pruning around arteries still occurred, albeit with delayed kinetics [20]. In the capillary plexus region, where apoptosis is infrequent, blocking apoptosis was not found to have any effect on vessel pruning [20]. Apoptosis was also found to be largely dispensable for vessel regression in zebrafish when it was associated with vessel pruning [158]. These results strongly argue that apoptosis does not cause vessel regression during angiogenic vessel pruning. This is further supported by the finding that increased EC apoptosis in the MCL1 mutants did not result in an increase in vessel regression, as would be expected if apoptosis caused vessel regression [120].
Detailed, time-lapse imaging in a range of vessel beds in zebrafish has shown that during vessel pruning, ECs migrate out of regressing vessel segments and into neighboring, perfused vessels, eliminating the need for apoptotic disposal of ECs [33, 158, 159]. The steps involved in the dissolution and regression of the vessel segment (i.e., loss of lumen and disassembly of cell–cell junctions) appear to be the reverse of those involved in anastomotic vessel assembly [32, 159]. These studies showed that differences in blood flow shear between neighboring vessels promoted the movement of ECs out of the vessel with the lower shear, leading to its regression [33]. EC migration also occurs in response to hemodynamic cues during vessel remodeling and pruning in the mouse retina [32], with the sensitivity of ECs to flow being dependent on non-canonical Wnt signaling [155]. In the absence of non-canonical Wnt signaling, ECs showed a heightened sensitivity to flow that resulted in premature vessel pruning and a subsequent reduction in retinal vasculature [155]. Therefore, EC migration as the mechanism of vessel regression during angiogenic vessel pruning appears to be highly conserved both between species and across vessel networks.
Apoptosis removes endothelial cells deprived of blood flow during vessel regression
Blood flow shear has important survival-promoting effects on ECs. For example, vessel non-perfusion precedes the onset of EC apoptosis in mice and rats subjected to hyperoxia-induced vaso-obliteration [36, 43], and abundant EC apoptosis occurs in non-perfused hyaloid vessels. During angiogenic vessel pruning, the failure of ECs to integrate into adjacent, perfused vessels would be expected to deprive them of the survival-promoting effects of blood flow shear, causing them to undergo apoptosis. Indeed, EC apoptosis in the mouse retina frequently occurs during the regression of longer vessel segments [32]. These cells likely die because they fail to relocate into neighboring vessels where they would receive the protective benefit of blood flow [32]. EC apoptosis is also observed in vascular segments that become disconnected from the circulation due to excessive pruning [36]. Consistent with this, persistent ECs were observed isolated from the circulation in the retinas of apoptosis-resistant Bak −/− Bax EC/EC mice [20]. Such cells were also found in the remnant hyaloid network of Bak −/− Bax EC/EC mice, suggesting a flow-disrupting event that is independent of apoptosis can initiate the regression of some hyaloid vessel segments, followed by the apoptotic clearance of the ECs from the flow-disrupted vessel [20]. It appears then that during angiogenic vessel pruning, EC apoptosis does not initiate the vessel regression event, and only removes ECs from a minority of vessels undergoing regression. In those few instances where EC apoptosis does occur during vessel pruning (most notably in vessels around arteries), it serves first to improve the overall efficiency with which vessel pruning occurs, and second to clear away ECs that become isolated from the circulation. This would imply that during angiogenic vessel pruning, EC apoptosis is a consequence of vessel regression, not a cause.
Causes of endothelial cell apoptosis during angiogenic vessel pruning
The findings that EC apoptosis does not drive vessel pruning raises the question of why it is so prominent in the regressing capillaries and side branches around maturing arteries. As discussed in the previous section, EC apoptosis is often found in longer regressing segments in cells that probably fail to integrate into neighboring, perfused vessels [32]. As these longer regressing segments are frequently located around arteries [32], this may be one explanation for the concentration of EC apoptosis in that area. This explanation may not explain all EC apoptosis around arteries, as longer segments are not the only ones that regress in that region. Another possible cause is the low level of pro-survival Vegfa expressed around the oxygen-rich arteries [152]. However, while inhibiting VEGF-A widens the avascular zone around arteries, it does not cause wide-spread capillary regression, and, therefore, cannot be solely responsible for the localized distribution of EC apoptosis [43]. Based on computer modeling, arterial side branches in the retina experience higher blood flow velocity and wall shear than plexus capillaries [32, 160], which may make the ECs in these vessels particularly sensitive to the loss of blood flow that occurs during pruning. In culture, ECs alter their expression of apoptosis regulators depending on the strength of flow [161]. However, this also may not fully account for why apoptosis is focused around arteries as the same computer modeling suggested that some venous side branches near the central retina also experience higher blood flow and wall shear than plexus capillaries [160]. The decision of some arterial side-branch ECs to undergo apoptosis may prove multi-factorial. For example, the effect of pruning a high-flow vessel in a low VEGF-A environment could trigger EC apoptosis, as the sum of survival-promoting signals drops below a minimum threshold. Such a threshold would be less likely to be crossed by ECs located in plexus and venous regions, either because of higher levels of VEGF-A or because the ECs are pre-conditioned to a low-flow environment. The decision of arterial side-branch ECs to undergo apoptosis during vessel pruning is, therefore, likely to be complex and may depend on a number of factors such as extracellular milieu, flow status prior to regression, and their capacity to successfully migrate into a protective environment during regression.
Apoptosis reduces endothelial cell density during vessel maturation
During the later stages of vessel maturation in the retinal vasculature, the distribution of EC apoptosis becomes more wide-spread [20]. This occurs concurrently with a reduction in overall EC density [20, 34]. This reduction in EC density fails to occur in the apoptosis-resistant Bak −/− Bax EC/EC mice, leading adult retinal vessels to contain a higher than normal number of ECs [20]. The increase in EC density had the consequence of increasing the diameter of capillaries, but interestingly, not larger caliber vessels (radial artery and vein) [20]. Whether the increased vessel cellularity and capillary diameter has any consequences for vessel or neural retina function has yet to be determined, but these findings show that the reduction in EC density that accompanies vessel maturation requires their active removal by apoptosis. Survival of ECs during this stage of maturation may be dependent on the pro-survival activity of BCL2, as EC apoptosis was increased at this time in mice lacking BCL2 in ECs [125].
Endothelial cell apoptosis and vessel regression in diabetes
Diabetes causes endothelial dysfunction and an increased state of vessel regression and EC apoptosis in complications such as retinopathy and nephropathy. Diabetes is also a risk factor for atherosclerosis in which EC apoptosis is observed due to disturbed flow. During diabetic retinopathy, capillary drop-out is associated with increased EC and pericyte apoptosis [44]. Pericytes are lost before ECs in diabetic vessel regression [45], which is preceded by increased levels of ANG2 and can be reduced in ANG2 mutants [162]. Furthermore, hyperglycemia induces Ang2 expression in ECs [163]. Elegant studies recently showed that the pericyte loss alone in the mature adult retina is not sufficient to cause capillary loss or vessel destabilization [31]. Instead, loss of pericytes is likely permissive for the progression of diabetic retinopathy when accompanied by other vascular stresses or insults such as hyperglycemia or increased VEGF-A found in patients with proliferative diabetic retinopathy [31, 164]. Endothelial dysfunction and subsequent loss of vessels is an important contributor to diabetes pathogenesis in nephropathy [165]. Microvascular rarefaction has been shown to precede the reduction in renal function in a rat model of diabetes [166]. Hyperglycemia, which is characteristic of diabetes patients, can trigger EC apoptosis [167–169] and has been shown to increase the ratio of BAX to BCL2 within HUVECs and increase cleavage of caspase 3 and apoptosis. VEGF-A and activated protein C are each able to attenuate hyperglycemia-induced EC apoptosis [92, 169]. In accordance with this, VEGF-A levels were decreased in kidney biopsies taken from patients with diabetic nephropathy [170] and loss of this EC survival factor may be one of the factors influencing apoptosis of ECs and rarefaction in this disease. Consistent with this, deletion of VEGF-A in a mouse model of diabetic nephropathy leads to acceleration of nephropathy [171]. Similarly, thrombospondin-mediated production of activated protein C prevents glomerular EC apoptosis in diabetic mice and activated protein C prevents EC apoptosis in response to hyperglycemia in vitro [92].
Conclusions and future directions
The recent findings summarized here indicate context-specific roles for EC apoptosis during vessel remodeling and regression. While apoptosis is associated with vessel regression in a range of cases, it has only been shown to be necessary in cases of vessel ablation. In the context of angiogenesis, apoptosis removes supernumerary cells from the network, or occurs as a consequence of vessel regression, serving to improve the efficiency of the regression process or to clear away vessel segments and cells that become isolated from the circulation. It is important to recognize that this interpretation comes mainly from analysis of normal angiogenesis in the retina and it is yet to be established whether it will hold true for vascular development in other tissues. Furthermore, the precise contribution of EC apoptosis to stress-induced vessel regression such as occurs in vascular complications associated with diabetes, or disease progression in atherosclerosis is yet to be fully explored. The limited existing evidence from apoptosis-resistant animal models suggests that apoptosis will be required for vessel regression at least in certain stress-induced contexts, potentially distinguishing it from the situation of normal angiogenic remodeling. However, the context-specific role for apoptosis in initiating vessel regression along with the diverse regulatory inputs that govern an EC’s decision to live or die depending on circumstance means that extrapolations from one pathological context to another may not be any more informative than extending observations in developmental vessel regression to the pathological context in question. As such, further research is required to fully understand how apoptosis contributes to pathological vessel regression on a case by case basis. The recent work on MCL1 and BCL2 in vivo has demonstrated that pro-survival BCL2 family proteins are required for EC survival during angiogenesis, in a context-specific manner. These findings raise numerous questions. Are the other pro-survival BCL2 family proteins important in EC survival? How is the requirement for particular BCL2 family proteins in distinct EC subsets regulated? In what pathological contexts are each required? Are they required for the survival of quiescent vasculature, and finally, can this knowledge be exploited for therapeutic benefit in the treatment of neovascular disease? These questions and others highlight how much remains to be understood about apoptosis control in ECs.
Acknowledgements
The work of the authors is supported by the National Health and Medical Research Council, Australia (Project Grant: 1125536), Australian Government Research Training Program Scholarships (to ECW and ZLG), and the L.E.W Carty Charitable Fund. The work is made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
Abbreviations
- EC
Endothelial cell
- OIR
Oxygen-induced retinopathy
- DISC
Death-inducing signaling complex
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
References
- 1.Neufeld S, Planas-Paz L, Lammert E. Blood and lymphatic vascular tube formation in mouse. Semin Cell Dev Biol. 2014;31:115–123. doi: 10.1016/j.semcdb.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development. 2011;138:4569–4583. doi: 10.1242/dev.062323. [DOI] [PubMed] [Google Scholar]
- 3.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Siekmann AF, Affolter M, Belting HG. The tip cell concept 10 years after: new players tune in for a common theme. Exp Cell Res. 2013;319:1255–1263. doi: 10.1016/j.yexcr.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Betz C, Lenard A, Belting HG, Affolter M. Cell behaviors and dynamics during angiogenesis. Development. 2016;143:2249–2260. doi: 10.1242/dev.135616. [DOI] [PubMed] [Google Scholar]
- 6.Walls JR, Coultas L, Rossant J, Henkelman RM. Three-dimensional analysis of vascular development in the mouse embryo. PLoS One. 2008;3:e2853. doi: 10.1371/journal.pone.0002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modlich U, Kaup FJ, Augustin HG. Cyclic angiogenesis and blood vessel regression in the ovary: blood vessel regression during luteolysis involves endothelial cell detachment and vessel occlusion. Lab Invest. 1996;74:771–780. [PubMed] [Google Scholar]
- 8.Walker NI, Bennett RE, Kerr JF. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am J Anat. 1989;185:19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- 9.Ito M, Yoshioka M. Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat Embryol. 1999;200:403–411. doi: 10.1007/s004290050289. [DOI] [PubMed] [Google Scholar]
- 10.Saint-Geniez M, D’Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- 11.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 12.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baffert F, Thurston G, Rochon-Duck M, Le T, Brekken R, McDonald DM. Age-related changes in vascular endothelial growth factor dependency and angiopoietin-1-induced plasticity of adult blood vessels. Circ Res. 2004;94:984–992. doi: 10.1161/01.RES.0000125295.43813.1F. [DOI] [PubMed] [Google Scholar]
- 14.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Zhang Y, Cao Z, Ji H, Yang X, Iwamoto H, Wahlberg E, Lanne T, Sun B, Cao Y. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc Natl Acad Sci USA. 2013;110:12018–12023. doi: 10.1073/pnas.1301331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, McDonald DM. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290:H547–H559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Park S, Fei P, Sorenson CM. Bim is responsible for the inherent sensitivity of the developing retinal vasculature to hyperoxia. Dev Biol. 2011;349:296–309. doi: 10.1016/j.ydbio.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson EC, Koenig MN, Grant ZL, Whitehead L, Trounson E, Dewson G, Coultas L. Apoptosis regulates endothelial cell number and capillary vessel diameter but not vessel regression during retinal angiogenesis. Development. 2016;143:2973–2982. doi: 10.1242/dev.137513. [DOI] [PubMed] [Google Scholar]
- 21.Koenig MN, Naik E, Rohrbeck L, Herold MJ, Trounson E, Bouillet P, Thomas T, Voss AK, Strasser A, Coultas L. Pro-apoptotic BIM is an essential initiator of physiological endothelial cell death independent of regulation by FOXO3. Cell Death Differ. 2014;21:1687–1695. doi: 10.1038/cdd.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn P, Lindsten T, Tolentino M, Thompson CB, Bennett J, Dunaief JL. Persistent fetal ocular vasculature in mice deficient in bax and bak. Arch Ophthalmol. 2005;123:797–802. doi: 10.1001/archopht.123.6.797. [DOI] [PubMed] [Google Scholar]
- 23.Naik E, O’Reilly LA, Asselin-Labat ML, Merino D, Lin A, Cook M, Coultas L, Bouillet P, Adams JM, Strasser A. Destruction of tumor vasculature and abated tumor growth upon VEGF blockade is driven by proapoptotic protein Bim in endothelial cells. J Exp Med. 2011;208:1351–1358. doi: 10.1084/jem.20100951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 28.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 29.Korn C, Augustin HG. Mechanisms of vessel pruning and regression. Dev Cell. 2015;34:5–17. doi: 10.1016/j.devcel.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 31.Park DY, Lee J, Kim J, Kim K, Hong S, Han S, Kubota Y, Augustin HG, Ding L, Kim JW, Kim H, He Y, Adams RH, Koh GY. Plastic roles of pericytes in the blood-retinal barrier. Nat Commun. 2017;8:15296. doi: 10.1038/ncomms15296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franco CA, Jones ML, Bernabeu MO, Geudens I, Mathivet T, Rosa A, Lopes FM, Lima AP, Ragab A, Collins RT, Phng LK, Coveney PV, Gerhardt H. Dynamic endothelial cell rearrangements drive developmental vessel regression. PLoS Biol. 2015;13:e1002125. doi: 10.1371/journal.pbio.1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Q, Jiang L, Li C, Hu D, Bu JW, Cai D, Du JL. Haemodynamics-driven developmental pruning of brain vasculature in zebrafish. PLoS Biol. 2012;10:e1001374. doi: 10.1371/journal.pbio.1001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehling M, Adams S, Benedito R, Adams RH. Notch controls retinal blood vessel maturation and quiescence. Development. 2013;140:3051–3061. doi: 10.1242/dev.093351. [DOI] [PubMed] [Google Scholar]
- 35.Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Lofqvist C, Hellstrom A, Smith LE. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes S, Chang-Ling T. Roles of endothelial cell migration and apoptosis in vascular remodeling during development of the central nervous system. Microcirculation. 2000;7:317–333. doi: 10.1111/j.1549-8719.2000.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 37.Korn C, Scholz B, Hu J, Srivastava K, Wojtarowicz J, Arnsperger T, Adams RH, Boutros M, Augustin HG, Augustin I. Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development. 2014;141:1757–1766. doi: 10.1242/dev.104422. [DOI] [PubMed] [Google Scholar]
- 38.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 39.Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology. 2013;120:106–114. doi: 10.1016/j.ophtha.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 40.Scott A, Powner MB, Fruttiger M. Quantification of vascular tortuosity as an early outcome measure in oxygen induced retinopathy (OIR) Exp Eye Res. 2014;120:55–60. doi: 10.1016/j.exer.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 41.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S, Uemura A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest. 2011;121:1974–1985. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lobov IB, Cheung E, Wudali R, Cao J, Halasz G, Wei Y, Economides A, Lin HC, Papadopoulos N, Yancopoulos GD, Wiegand SJ. The Dll4/Notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow. Blood. 2011;117:6728–6737. doi: 10.1182/blood-2010-08-302067. [DOI] [PubMed] [Google Scholar]
- 44.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–2890. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011;60:9–16. doi: 10.2337/db10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green DR, Llambi F. Cell Death Signaling. Cold Spring Harb Perspect Biol. 2015;7:a006080. doi: 10.1101/cshperspect.a006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/S1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/S1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 50.Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 52.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 54.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 55.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10:348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- 57.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/S0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 59.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/S0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 60.Kaufmann T, Strasser A, Jost PJ. Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ. 2012;19:42–50. doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Miguel D, Lemke J, Anel A, Walczak H, Martinez-Lostao L. Onto better TRAILs for cancer treatment. Cell Death Differ. 2016;23:733–747. doi: 10.1038/cdd.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 64.Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, Lapidot T, Soffer D, Sobe T, Avraham KB, Goncharov T, Holtmann H, Lonai P, Wallach D. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/S1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 65.Alvarez-Diaz S, Dillon CP, Lalaoui N, Tanzer MC, Rodriguez DA, Lin A, Lebois M, Hakem R, Josefsson EC, O’Reilly LA, Silke J, Alexander WS, Green DR, Strasser A. The pseudokinase MLKL and the kinase ripk3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity. 2016;45:513–526. doi: 10.1016/j.immuni.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, Ramakrishnan P, Lapidot T, Wallach D. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- 69.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 71.Gratton JP, Morales-Ruiz M, Kureishi Y, Fulton D, Walsh K, Sessa WC. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J Biol Chem. 2001;276:30359–30365. doi: 10.1074/jbc.M009698200. [DOI] [PubMed] [Google Scholar]
- 72.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/S0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 73.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114:1219–1228. doi: 10.1001/archopht.1996.01100140419009. [DOI] [PubMed] [Google Scholar]
- 75.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Domigan CK, Warren CM, Antanesian V, Happel K, Ziyad S, Lee S, Krall A, Duan L, Torres-Collado AX, Castellani LW, Elashoff D, Christofk HR, van der Bliek AM, Potente M, Iruela-Arispe ML. Autocrine VEGF maintains endothelial survival through regulation of metabolism and autophagy. J Cell Sci. 2015;128:2236–2248. doi: 10.1242/jcs.163774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones N, Master Z, Jones J, Bouchard D, Gunji Y, Sasaki H, Daly R, Alitalo K, Dumont DJ. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J Biol Chem. 1999;274:30896–30905. doi: 10.1074/jbc.274.43.30896. [DOI] [PubMed] [Google Scholar]
- 78.Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–29. doi: 10.1161/01.RES.86.1.24. [DOI] [PubMed] [Google Scholar]
- 79.Savant S, La Porta S, Budnik A, Busch K, Hu J, Tisch N, Korn C, Valls AF, Benest AV, Terhardt D, Qu X, Adams RH, Baldwin HS, Ruiz de Almodovar C, Rodewald HR, Augustin HG. The orphan receptor Tie1 controls angiogenesis and vascular remodeling by differentially regulating Tie2 in Tip and stalk cells. Cell Rep. 2015;12:1761–1773. doi: 10.1016/j.celrep.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/S1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 81.Rao S, Lobov IB, Vallance JE, Tsujikawa K, Shiojima I, Akunuru S, Walsh K, Benjamin LE, Lang RA. Obligatory participation of macrophages in an angiopoietin 2-mediated cell death switch. Development. 2007;134:4449–4458. doi: 10.1242/dev.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192:182–187. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- 83.Dimmeler S, Zeiher AM. Nitric oxide-an endothelial cell survival factor. Cell Death Differ. 1999;6:964–968. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- 84.Rossig L, Haendeler J, Hermann C, Malchow P, Urbich C, Zeiher AM, Dimmeler S. Nitric oxide down-regulates MKP-3 mRNA levels: involvement in endothelial cell protection from apoptosis. J Biol Chem. 2000;275:25502–25507. doi: 10.1074/jbc.M002283200. [DOI] [PubMed] [Google Scholar]
- 85.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 86.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kwon YG, Min JK, Kim KM, Lee DJ, Billiar TR, Kim YM. Sphingosine 1-phosphate protects human umbilical vein endothelial cells from serum-deprived apoptosis by nitric oxide production. J Biol Chem. 2001;276:10627–10633. doi: 10.1074/jbc.M011449200. [DOI] [PubMed] [Google Scholar]
- 88.Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J Biol Chem. 2001;276:12420–12426. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- 89.Rutherford C, Childs S, Ohotski J, McGlynn L, Riddick M, MacFarlane S, Tasker D, Pyne S, Pyne NJ, Edwards J, Palmer TM. Regulation of cell survival by sphingosine-1-phosphate receptor S1P1 via reciprocal ERK-dependent suppression of Bim and PI-3-kinase/protein kinase C-mediated upregulation of Mcl-1. Cell Death Dis. 2013;4:e927. doi: 10.1038/cddis.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nofer JR, van der Giet M, Tolle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Godecke A, Ishii I, Kleuser B, Schafers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI200418004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gaengel K, Niaudet C, Hagikura K, Lavina B, Muhl L, Hofmann JJ, Ebarasi L, Nystrom S, Rymo S, Chen LL, Pang MF, Jin Y, Raschperger E, Roswall P, Schulte D, Benedito R, Larsson J, Hellstrom M, Fuxe J, Uhlen P, Adams R, Jakobsson L, Majumdar A, Vestweber D, Uv A, Betsholtz C. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell. 2012;23:587–599. doi: 10.1016/j.devcel.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 92.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13:1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 93.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 94.Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 2001;61:2641–2648. [PubMed] [Google Scholar]
- 95.Zhande R, Karsan A. Erythropoietin promotes survival of primary human endothelial cells through PI3K-dependent, NF-kappaB-independent upregulation of Bcl-xL. Am J Physiol Heart Circ Physiol. 2007;292:H2467–H2474. doi: 10.1152/ajpheart.00649.2006. [DOI] [PubMed] [Google Scholar]
- 96.Karsan A, Yee E, Poirier GG, Zhou P, Craig R, Harlan JM. Fibroblast growth factor-2 inhibits endothelial cell apoptosis by Bcl-2-dependent and independent mechanisms. Am J Pathol. 1997;151:1775–1784. [PMC free article] [PubMed] [Google Scholar]
- 97.Araki S, Simada Y, Kaji K, Hayashi H. Role of protein kinase C in the inhibition by fibroblast growth factor of apoptosis in serum-depleted endothelial cells. Biochem Biophys Res Commun. 1990;172:1081–1085. doi: 10.1016/0006-291X(90)91557-9. [DOI] [PubMed] [Google Scholar]
- 98.Chavakis E, Dimmeler S. Regulation of endothelial cell survival and apoptosis during angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:887–893. doi: 10.1161/01.ATV.0000017728.55907.A9. [DOI] [PubMed] [Google Scholar]
- 99.Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest. 2016;126:821–828. doi: 10.1172/JCI83083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 2014;34:2191–2198. doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher AM. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res. 1998;83:334–341. doi: 10.1161/01.RES.83.3.334. [DOI] [PubMed] [Google Scholar]
- 102.Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci USA. 2002;99:9462–9467. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 104.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr, Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abe J, Berk BC. Novel mechanisms of endothelial mechanotransduction. Arterioscler Thromb Vasc Biol. 2014;34:2378–2386. doi: 10.1161/ATVBAHA.114.303428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee HJ, Koh GY. Shear stress activates Tie2 receptor tyrosine kinase in human endothelial cells. Biochem Biophys Res Commun. 2003;304:399–404. doi: 10.1016/S0006-291X(03)00649-1. [DOI] [PubMed] [Google Scholar]
- 107.Le NT, Heo KS, Takei Y, Lee H, Woo CH, Chang E, McClain C, Hurley C, Wang X, Li F, Xu H, Morrell C, Sullivan MA, Cohen MS, Serafimova IM, Taunton J, Fujiwara K, Abe J. A crucial role for p90RSK-mediated reduction of ERK5 transcriptional activity in endothelial dysfunction and atherosclerosis. Circulation. 2013;127:486–499. doi: 10.1161/CIRCULATIONAHA.112.116988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 109.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 110.Fruttiger M. Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci. 2002;43:522–527. [PubMed] [Google Scholar]
- 111.Wang S, Zaitoun IS, Johnson RP, Jamali N, Gurel Z, Wintheiser CM, Strasser A, Lindner V, Sheibani N, Sorenson CM. Bim expression in endothelial cells and pericytes is essential for regression of the fetal ocular vasculature. PLoS One. 2017;12:e0178198. doi: 10.1371/journal.pone.0178198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wilhelm K, Happel K, Eelen G, Schoors S, Oellerich MF, Lim R, Zimmermann B, Aspalter IM, Franco CA, Boettger T, Braun T, Fruttiger M, Rajewsky K, Keller C, Bruning JC, Gerhardt H, Carmeliet P, Potente M. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. 2016;529:216–220. doi: 10.1038/nature16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nisancioglu MH, Betsholtz C, Genove G. The absence of pericytes does not increase the sensitivity of tumor vasculature to vascular endothelial growth factor-A blockade. Cancer Res. 2010;70:5109–5115. doi: 10.1158/0008-5472.CAN-09-4245. [DOI] [PubMed] [Google Scholar]
- 115.Simonavicius N, Ashenden M, van Weverwijk A, Lax S, Huso DL, Buckley CD, Huijbers IJ, Yarwood H, Isacke CM. Pericytes promote selective vessel regression to regulate vascular patterning. Blood. 2012;120:1516–1527. doi: 10.1182/blood-2011-01-332338. [DOI] [PubMed] [Google Scholar]
- 116.Czymai T, Viemann D, Sticht C, Molema G, Goebeler M, Schmidt M. FOXO3 modulates endothelial gene expression and function by classical and alternative mechanisms. J Biol Chem. 2010;285:10163–10178. doi: 10.1074/jbc.M109.056663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Coultas L, Bouillet P, Stanley EG, Brodnicki TC, Adams JM, Strasser A. Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death. Mol Cell Biol. 2004;24:1570–1581. doi: 10.1128/MCB.24.4.1570-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang F, Li Y, Tang Z, Kumar A, Lee C, Zhang L, Zhu C, Klotzsche-von Ameln A, Wang B, Gao Z, Zhang S, Langer HF, Hou X, Jensen L, Ma W, Wong W, Chavakis T, Liu Y, Cao Y, Li X. Proliferative and survival effects of PUMA promote angiogenesis. Cell Rep. 2012;2:1272–1285. doi: 10.1016/j.celrep.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 119.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 120.Watson EC, Whitehead L, Adams RH, Dewson G, Coultas L. Endothelial cell survival during angiogenesis requires the pro-survival protein MCL1. Cell Death Differ. 2016;23:1371–1379. doi: 10.1038/cdd.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007;7:327–331. doi: 10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- 122.Benedito R, Roca C, Sörensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 123.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 124.Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zaitoun IS, Johnson RP, Jamali N, Almomani R, Wang S, Sheibani N, Sorenson CM. Endothelium expression of Bcl-2 is essential for normal and pathological ocular vascularization. PLoS One. 2015;10:e0139994. doi: 10.1371/journal.pone.0139994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–2917. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, de Kretser D, Metcalf D, Kontgen F, Adams JM, Cory S. Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci USA. 1998;95:12424–12431. doi: 10.1073/pnas.95.21.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lindqvist LM, Heinlein M, Huang DC, Vaux DL. Prosurvival Bcl-2 family members affect autophagy only indirectly, by inhibiting Bax and Bak. Proc Natl Acad Sci USA. 2014;111:8512–8517. doi: 10.1073/pnas.1406425111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Duriez PJ, Wong F, Dorovini-Zis K, Shahidi R, Karsan A. A1 functions at the mitochondria to delay endothelial apoptosis in response to tumor necrosis factor. J Biol Chem. 2000;275:18099–18107. doi: 10.1074/jbc.M908925199. [DOI] [PubMed] [Google Scholar]
- 130.Karsan A, Yee E, Kaushansky K, Harlan JM. Cloning of human Bcl-2 homologue: inflammatory cytokines induce human A1 in cultured endothelial cells. Blood. 1996;87:3089–3096. [PubMed] [Google Scholar]
- 131.Schenk RL, Tuzlak S, Carrington EM, Zhan Y, Heinzel S, Teh CE, Gray DH, Tai L, Lew AM, Villunger A, Strasser A, Herold MJ. Characterisation of mice lacking all functional isoforms of the pro-survival BCL-2 family member A1 reveals minor defects in the haematopoietic compartment. Cell Death Differ. 2017;24:534–545. doi: 10.1038/cdd.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wei G, Srinivasan R, Cantemir-Stone CZ, Sharma SM, Santhanam R, Weinstein M, Muthusamy N, Man AK, Oshima RG, Leone G, Ostrowski MC. Ets1 and Ets2 are required for endothelial cell survival during embryonic angiogenesis. Blood. 2009;114:1123–1130. doi: 10.1182/blood-2009-03-211391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee YH, Mungunsukh O, Tutino RL, Marquez AP, Day RM. Angiotensin-II-induced apoptosis requires regulation of nucleolin and Bcl-xL by SHP-2 in primary lung endothelial cells. J Cell Sci. 2010;123:1634–1643. doi: 10.1242/jcs.063545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 135.Vikstrom IB, Slomp A, Carrington EM, Moesbergen LM, Chang C, Kelly GL, Glaser SP, Jansen JH, Leusen JH, Strasser A, Huang DC, Lew AM, Peperzak V, Tarlinton DM. MCL-1 is required throughout B-cell development and its loss sensitizes specific B-cell subsets to inhibition of BCL-2 or BCL-XL. Cell Death Dis. 2016;7:e2345. doi: 10.1038/cddis.2016.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dzhagalov I, Dunkle A, He YW. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521–528. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khaw SL, Merino D, Anderson MA, Glaser SP, Bouillet P, Roberts AW, Huang DC. Both leukaemic and normal peripheral B lymphoid cells are highly sensitive to the selective pharmacological inhibition of prosurvival Bcl-2 with ABT-199. Leukemia. 2014;28:1207–1215. doi: 10.1038/leu.2014.1. [DOI] [PubMed] [Google Scholar]
- 138.Ishida S, Yamashiro K, Usui T, Kaji Y, Ogura Y, Hida T, Honda Y, Oguchi Y, Adamis AP. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med. 2003;9:781–788. doi: 10.1038/nm877. [DOI] [PubMed] [Google Scholar]
- 139.Barreiro R, Schadlu R, Herndon J, Kaplan HJ, Ferguson TA. The role of Fas-FasL in the development and treatment of ischemic retinopathy. Invest Ophthalmol Vis Sci. 2003;44:1282–1286. doi: 10.1167/iovs.02-0478. [DOI] [PubMed] [Google Scholar]
- 140.Davies MH, Eubanks JP, Powers MR. Increased retinal neovascularization in Fas ligand-deficient mice. Invest Ophthalmol Vis Sci. 2003;44:3202–3210. doi: 10.1167/iovs.03-0050. [DOI] [PubMed] [Google Scholar]
- 141.Kaplan HJ, Leibole MA, Tezel T, Ferguson TA. Fas ligand (CD95 ligand) controls angiogenesis beneath the retina. Nat Med. 1999;5:292–297. doi: 10.1038/6509. [DOI] [PubMed] [Google Scholar]
- 142.Cantarella G, Di Benedetto G, Ribatti D, Saccani-Jotti G, Bernardini R. Involvement of caspase 8 and c-FLIPL in the proangiogenic effects of the tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) FEBS J. 2014;281:1505–1513. doi: 10.1111/febs.12720. [DOI] [PubMed] [Google Scholar]
- 143.Hubert KE, Davies MH, Stempel AJ, Griffith TS, Powers MR. TRAIL-deficient mice exhibit delayed regression of retinal neovascularization. Am J Pathol. 2009;175:2697–2708. doi: 10.2353/ajpath.2009.090099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wilson NS, Yang A, Yang B, Couto S, Stern H, Gogineni A, Pitti R, Marsters S, Weimer RM, Singh M, Ashkenazi A. Proapoptotic activation of death receptor 5 on tumor endothelial cells disrupts the vasculature and reduces tumor growth. Cancer Cell. 2012;22:80–90. doi: 10.1016/j.ccr.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 145.Meeson A, Palmer M, Calfon M, Lang R. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development. 1996;122:3929–3938. doi: 10.1242/dev.122.12.3929. [DOI] [PubMed] [Google Scholar]
- 146.Poche RA, Hsu CW, McElwee ML, Burns AR, Dickinson ME. Macrophages engulf endothelial cell membrane particles preceding pupillary membrane capillary regression. Dev Biol. 2015;403:30–42. doi: 10.1016/j.ydbio.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rao S, Chun C, Fan J, Kofron JM, Yang MB, Hegde RS, Ferrara N, Copenhagen DR, Lang RA. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494:243–246. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Okabe K, Kobayashi S, Yamada T, Kurihara T, Tai-Nagara I, Miyamoto T, Mukouyama YS, Sato TN, Suda T, Ema M, Kubota Y. Neurons limit angiogenesis by titrating VEGF in retina. Cell. 2014;159:584–596. doi: 10.1016/j.cell.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 150.Ashton N. Oxygen and the growth and development of retinal vessels. In vivo and in vitro studies. The XX Francis I. Proctor Lecture. Am J Ophthalmol. 1966;62:412–435. doi: 10.1016/0002-9394(66)91322-5. [DOI] [PubMed] [Google Scholar]
- 151.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Claxton S, Fruttiger M. Role of arteries in oxygen induced vaso-obliteration. Exp Eye Res. 2003;77:305–311. doi: 10.1016/S0014-4835(03)00153-2. [DOI] [PubMed] [Google Scholar]
- 153.Scholz B, Korn C, Wojtarowicz J, Mogler C, Augustin I, Boutros M, Niehrs C, Augustin HG. Endothelial RSPO3 controls vascular stability and pruning through non-canonical WNT/Ca(2+)/NFAT signaling. Dev Cell. 2016;36:79–93. doi: 10.1016/j.devcel.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 154.Cheng C, Haasdijk R, Tempel D, van de Kamp EH, Herpers R, Bos F, Den Dekker WK, Blonden LA, de Jong R, Burgisser PE, Chrifi I, Biessen EA, Dimmeler S, Schulte-Merker S, Duckers HJ. Endothelial cell-specific FGD5 involvement in vascular pruning defines neovessel fate in mice. Circulation. 2012;125:3142–3158. doi: 10.1161/CIRCULATIONAHA.111.064030. [DOI] [PubMed] [Google Scholar]
- 155.Franco CA, Jones ML, Bernabeu MO, Vion AC, Barbacena P, Fan J, Mathivet T, Fonseca CG, Ragab A, Yamaguchi TP, Coveney PV, Lang RA, Gerhardt H. Non-canonical Wnt signalling modulates the endothelial shear stress flow sensor in vascular remodelling. Elife. 2016;5:e07727. doi: 10.7554/eLife.07727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grutzmacher C, Park S, Elmergreen TL, Tang Y, Scheef EA, Sheibani N, Sorenson CM. Opposing effects of bim and bcl-2 on lung endothelial cell migration. Am J Physiol Lung Cell Mol Physiol. 2010;299:L607–L620. doi: 10.1152/ajplung.00390.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sheibani N, Morrison ME, Gurel Z, Park S, Sorenson CM. BIM deficiency differentially impacts the function of kidney endothelial and epithelial cells through modulation of their local microenvironment. Am J Physiol Renal Physiol. 2012;302:F809–F819. doi: 10.1152/ajprenal.00498.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kochhan E, Lenard A, Ellertsdottir E, Herwig L, Affolter M, Belting HG, Siekmann AF. Blood flow changes coincide with cellular rearrangements during blood vessel pruning in zebrafish embryos. PLoS One. 2013;8:e75060. doi: 10.1371/journal.pone.0075060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lenard A, Daetwyler S, Betz C, Ellertsdottir E, Belting HG, Huisken J, Affolter M. Endothelial cell self-fusion during vascular pruning. PLoS Biol. 2015;13:e1002126. doi: 10.1371/journal.pbio.1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bernabeu MO, Jones ML, Nielsen JH, Kruger T, Nash RW, Groen D, Schmieschek S, Hetherington J, Gerhardt H, Franco CA, Coveney PV. Computer simulations reveal complex distribution of haemodynamic forces in a mouse retina model of angiogenesis. J R Soc Interface. 2014;11:20140543. doi: 10.1098/rsif.2014.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bartling B, Tostlebe H, Darmer D, Holtz J, Silber RE, Morawietz H. Shear stress-dependent expression of apoptosis-regulating genes in endothelial cells. Biochem Biophys Res Commun. 2000;278:740–746. doi: 10.1006/bbrc.2000.3873. [DOI] [PubMed] [Google Scholar]
- 162.Hammes HP, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T, Renner O, Breier G, Brownlee M, Deutsch U. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes. 2004;53:1104–1110. doi: 10.2337/diabetes.53.4.1104. [DOI] [PubMed] [Google Scholar]
- 163.Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I, Suske G, Ahmed N, Thornalley PJ, Sarthy VP, Hammes HP, Brownlee M. Methylglyoxal modification of mSin3A links glycolysis to angiopoietin-2 transcription. Cell. 2007;128:625. doi: 10.1016/j.cell.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 164.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 165.van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediat Inflamm. 2010;2010:792393. doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maric-Bilkan C, Flynn ER, Chade AR. Microvascular disease precedes the decline in renal function in the streptozotocin-induced diabetic rat. Am J Physiol Renal Physiol. 2012;302:F308–F315. doi: 10.1152/ajprenal.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 168.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 169.Yang Z, Mo X, Gong Q, Pan Q, Yang X, Cai W, Li C, Ma JX, He Y, Gao G. Critical effect of VEGF in the process of endothelial cell apoptosis induced by high glucose. Apoptosis. 2008;13:1331–1343. doi: 10.1007/s10495-008-0257-y. [DOI] [PubMed] [Google Scholar]
- 170.Lindenmeyer MT, Kretzler M, Boucherot A, Berra S, Yasuda Y, Henger A, Eichinger F, Gaiser S, Schmid H, Rastaldi MP, Schrier RW, Schlondorff D, Cohen CD. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol. 2007;18:1765–1776. doi: 10.1681/ASN.2006121304. [DOI] [PubMed] [Google Scholar]
- 171.Sivaskandarajah GA, Jeansson M, Maezawa Y, Eremina V, Baelde HJ, Quaggin SE. Vegfa protects the glomerular microvasculature in diabetes. Diabetes. 2012;61:2958–2966. doi: 10.2337/DB11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]