Abstract
Alcoholic beverages are widely consumed, resulting in a staggering economic cost in different social and cultural settings. Types of alcohol consumption vary from light occasional to heavy, binge drinking, and chronic alcohol abuse at all ages. In general, heavy alcohol consumption is widely recognized as a major epidemiological risk factor for chronic diseases and is detrimental to many organs and tissues, including bones. Indeed, recent findings demonstrate that alcohol has a dose-dependent toxic effect in promoting imbalanced bone remodeling. This imbalance eventually results in osteopenia, an established risk factor for osteoporosis. Decreased bone mass and strength are major hallmarks of osteopenia, which is predominantly attributed not only to inhibition of bone synthesis but also to increased bone resorption through direct and indirect pathways. In this review, we present knowledge to elucidate the epidemiology, potential pathogenesis, and major molecular mechanisms and cellular effects that underlie alcoholism-induced bone loss in osteopenia. Novel therapeutic targets for correcting alcohol-induced osteopenia are also reviewed, such as modulation of proinflammatory cytokines and Wnt and mTOR signaling and the application of new drugs.
Keywords: Alcohol, Osteopenia, Mechanisms, Pathophysiology, Bone formation, Bone resorption
Introduction
Alcohol consumption has grown into one of the most severe substance abuse disorders worldwide. Heavy chronic alcohol consumption and alcohol-related diseases have various harmful effects on multiple organs, including the brain, heart, liver, muscles, and the skeleton [1, 2]. Although recent studies have indicated that moderate levels of alcohol intake may have benefits for bone parameters and mineral density [3], binge drinking and chronic, heavy alcohol abuse can be positively associated with impaired bone remodeling and an increased risk for bone fracture [4, 5]. The onset of alcoholic injury in gradual bone loss may eventually result in osteopenia, a disease with asymptomatic but substantial mortality and morbidity over the course of one to two decades in both males and females [6]. Therefore, effective treatments for this insidious bone loss should be applied before the clinical manifestations of alcohol abuse appear.
Different molecular and cellular mechanisms have been suggested to be responsible for the osteotoxicity of alcoholism, including a direct effect on osteoblasts and osteoclasts [7, 8], cells that differentiate from bone marrow mesenchymal stem cells (BMMSCs). Such mechanisms underlying ethanol action on bone cells may be regulated by the Wnt and mTOR pathways. In addition, systemic alterations, such as regulation of hormone homeostasis [9], changes in the parathyroid hormone (PTH)–vitamin D axis [10, 11], and growth hormone (GH)–insulin-like growth hormone (IGF) [12] signaling, and oxidative stress [13], have indirect effects on bone cells.
In recent decades, basic studies using human and mouse genetic approaches have considerably improved the understanding of the mechanisms that control alcohol-induced osteopenia through various signaling pathways [14, 15]. Here, we provide an overview of the current findings on the molecular mechanisms that underlie bone loss in alcohol-induced osteopenia and focus on potential therapeutic strategies that could ameliorate the substantial morbidity and mortality associated with disorders of bone imbalance.
The epidemiology of alcohol-induced osteopenia
To provide clinical context and disease prevention treatment, prevalence estimates for alcohol-induced bone diseases such as osteopenia and their comorbidity factors are necessary [16]. Bone loss associated with alcohol injury in males and females eventually results in osteopenia, a severe disease characterized by decreased bone formation as the principal cellular mechanism [6]. Furthermore, the onset of this bone loss process and structural deterioration will lead to increased risks for osteoporosis and delayed bone fracture healing. However, histological findings on the influence of alcohol consumption on bone metabolism are controversial [17], with evidence for dose-dependent effects on imbalances in both bone formation and resorption.
The definition of dose for chronic alcohol consumption varies in the literature. Recent studies have indicated that light alcohol consumption, mostly wine and defined as 1–10 g of ethanol per day [18], has positive effects on the skeletal health of postmenopausal women, as measured using lumbar spine bone mineral density (BMD). In addition, Dietary Guidelines for Americans defines 11–30 g of ethanol per day as moderate alcohol consumption. The effects of this level of consumption on BMD depend on factors such as age and sex hormone status and are associated with up to one drink per day for postmenopausal women and up to two drinks per day for men [19]. Other studies have found that long-term heavy alcohol consumption (up to 30 g of ethanol per day) [20] is associated with decreased BMD of forearm, spine, and trochanter [6], and a cross-sectional study involving 1198 male patients with osteoporosis in France demonstrated heavy alcohol consumption to be an important risk factor for up to 28.1% [21]. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) recently identified binge drinking as a new pattern of alcohol consumption defined as a blood alcohol concentration (BAC) level reaching 0.08% or more in adults [22]. Some other researchers have suggested that binge drinking in adults be defined as a BAC of 2–4.6 g/L [23]. However, binge drinking data on BACs in children and teenagers are insufficient, and precise epidemiological data are also lacking.
Alcohol-induced osteopenia is defined as a gradual systemic skeletal disorder characterized by imbalanced bone formation and bone resorption, resulting in increased bone fracture and delayed bone healing compared with nonalcoholic patients. In addition, the prevalence of alcohol consumption may have many multifactorial confounding aspects, and the multiple molecular mechanisms may thus further contribute to alcohol-induced bone loss.
Pathophysiology
All aspects of the pathogenesis of alcohol consumption in osteopenia have not yet been clearly elucidated [24]. Overall, the etiology of alcohol-induced modulation of bone remodeling is multifactorial and may involve both complex direct and indirect actions on bone [25].
Mechanisms for the direct effects of alcohol-induced bone turnover
Osteoblasts and osteoclasts
Most studies have shown that ethanol has various direct effects on bone cells [26]. Some animal-based and cell culture investigations have revealed that alcohol can alter osteoblast activity and proliferation in a dose-dependent manner [27]. Several experiments have reported significantly reduced osteoblast activity and induced BMD under the influence of chronic and binge alcohol consumption [28]. In contrast, light alcohol consumption (<27 drinks per week for men and <13 drinks per week for women) had almost no effect on BMD and bone metabolism. Increased BMD levels and reductions in bone remodeling markers have been reported in postmenopausal women with moderate alcohol consumption [29]. However, 7-week intermittent ethanol vapor intake led to decreased osteoblastogenesis and increased osteoclastogenesis in animal studies [30]. Osteocalcin, also known as BGLAP, originates from the late stages of bone formation, and levels were found to be decreased in alcoholics.
Many years ago, relevant reports of chronic or binge alcohol consumption focused mainly on inhibition of bone formation, whereas only a few studies investigated the toxic effect of alcohol on bone resorption [31]. In an in vitro study, cultured osteoclasts were activated by the alcohol exposure associated with social drinking (approximately two glasses per day), causing significantly increased bone resorption [32]. However, the number of osteoclasts was not associated with the effect of light or chronic alcohol consumption by humans. Therefore, the influence of covariates and indirect action on the osteoclast number and osteogenesis must be taken into account in future studies.
Proinflammatory cytokines
Recent investigations have provided evidence that under binge drinking and chronic alcohol exposure, proinflammatory cytokines can significantly regulate osteoclastogenesis and adipocyte differentiation [13, 33]. A study using IL-6 gene-knockout mice chronically fed alcohol for 4 months indicated that alcohol can induce serum IL-6 levels in wild-type mice but that osteoblast function and the histomorphometric index of bone formation were decreased in IL-6 gene-knockout mice. Further investigation demonstrated that RANKL (Receptor Activator of Nuclear Factor κB ligand) mRNA was only expressed in bone marrow cultures of the wild-type mice but not in the other groups [13]. Therefore, production of IL-6 and expression of RANKL regulate osteoblast function and osteoclastogenesis, which may result in decreased bone turnover.
As a tumor necrosis factor (TNF) family member, RANKL can bind to the cognate receptor RANK and osteoprotegerin (OPG), which plays an important role in alcoholic bone metabolism. Indeed, an increased level of OPG has a positive relationship with daily alcohol consumption that has been interpreted as being protective against alcohol-induced bone turnover [34]. Furthermore, crosstalk between OPG and the RANK/RANKL system in alcohol-induced bone loss was mediated by TNF-α and IL-6 [35]. The signaling pathway associated with increased IL-6 expression in osteoblasts may be regulated by induction of protein tyrosine kinase activity through the PKC (protein kinase C-dependent) pathway (Fig. 1) [2]. In addition, other osteoclastogenic cytokines, such as IL-1β and TNF-α, may have important roles in pathogenic alcohol-induced bone turnover disease [36].
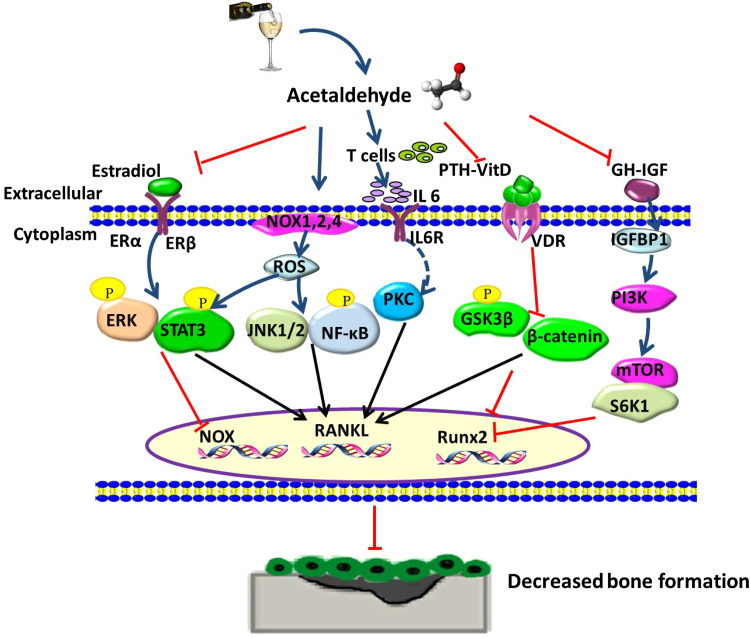
Fig. 1.
Schematic diagram of direct and indirect mechanisms effect on alcohol and its metabolism acetaldehyde (Acet) induced signaling cascades in osteopenia. In osteoblasts, decreased estradiol expression followed by phosphorylation of ERK/STAT3 cascades leads to increased RANKL level, and then binds to RANK to further decrease bone formation. Expression level of ROS is mainly induced by NOX enzyme family, NOX 1, 2, 4. Moreover, increased NOX activity depends on NF-κB, JNK, ERK signaling pathways to stimulate RANKL expression. Increased level of proinflammatory cytokine IL-6 may mediate the signal transportation through a protein kinase C-dependent pathway to increase RANKL expression. Inhibited PTH–vitamin D axis can be partly mediated by Wnt pathway with heavy alcohol consumption, which in turn promotes RANKL expression in osteoblasts. Decreased GH production and IGF-1 expression were found in osteoblasts, accompanied by increased IGF binding protein IGFBP1, with followed downstream signaling pathway mTOR/S6K1 to reversely inhibit Runx2 mRNA expression. NOX NADPH oxidase, ROS reactive oxygen species, PKC protein kinase C, PTH parathyroid hormone, GH growth hormone, IGF insulin-like growth factor, RANKL receptor activator of nuclear factor kappa-B ligand
Other proinflammatory cytokines such as TNF-α were also reported to have an important function in bone cell regulation. A recent study using liquid diets by distraction osteogenesis (DO) demonstrated that increased TNF-α and IL-1β in chronic alcohol abuse is associated with decreased tibial strength and inhibition of osteoblastogenesis [37]. Furthermore, treatment with a TNF receptor antagonist reversed alcohol-inhibited osteoblastogenesis during destruction osteogenesis. Regardless, the underlying mechanisms associated with TNF-α and inhibited osteogenesis are poorly understood. It has been reported that with alcohol exposure, increased levels of reactive oxygen species (ROS) may directly precede production of TNF-α in the bone marrow [38]. In addition, TNF-α induction in the bone marrow is blocked by treatment with N-acetylcysteine (NAC). More strikingly, NAC can reverse lineage commitment and differentiation of mesenchymal stromal cells toward adipocytes [39], indicating that oxidative stress may contribute to TNF-α-induced osteoblastogenesis inhibition.
The Wnt pathway
Another underlying mechanism appears to mediate alcohol-induced bone cell effects through activation of the Wnt/β-catenin pathway. Wnt signaling was described in the 1980s, and its essential roles in bone formation have been investigated in recent years [40]. This pathway is activated by association of the appropriate Wnt with a coreceptor complex, such as LRP5 (lipoprotein receptor-related protein 5) or LRP6 (lipoprotein receptor-related protein 6) [41]. β-Catenin exerts an effect on targeted gene transcription via accumulation in the cytoplasm and translocation to the nucleus. Moreover, the proliferation, differentiation and apoptosis of osteoblasts and osteoclasts are regulated by the Wnt/β-catenin pathway in alcoholics. This modulation by Wnt signaling may occur through increased mesenchymal bone marrow stem cell differentiation toward adipocytes [39]. The observed relationship suggests that ethanol can inhibit the Wnt/β-catenin pathway and activate adipogenetic genes and peroxisome proliferator-activated receptor γ (PPAR-γ) in bone marrow stem cells. The level of sclerostin, which is secreted by osteocytes, is significantly higher in alcohol abusers than in control subjects [42]; this elevation in sclerostin was related to decreased bone synthesis and increased bone resorption, and this effect may be modulated by antagonization of LRP5 and LRP6 binding and thus Wnt signaling [43].
The PI3K/AKT/mTOR pathway
Chronic alcohol consumption not only exerts a toxic effect on osteoblasts and osteoclasts but also has adverse effects on BMMSCs, multipotent progenitor stem cells with a strong capacity for differentiation into several specialized cell types, including osteoblasts and adipocytes [44, 45]. Furthermore, the effects of chronic alcohol consumption on BMMSCs differentiation toward adipocytes lead to increased fat accumulation in the bone marrow and contribute to bone loss with osteopenia [38, 46]. Several molecular pathways may be involved in regulating alcohol-induced BMMSC lineage differentiation in vitro, including the Wnt and mTOR pathways.
Signaling through mTOR, which belongs to the PI3K family of protein kinases, is a primary nutrient-sensing pathway [47], and interactions with other proteins regulate the proliferation of different cell types as well as differentiation and transcription. Recent studies have indicated that mTOR plays an important role in controlling mesenchymal stem cell lineage differentiation toward the adipogenic program: expression of the adipogenetic gene PPAR-γ increases and that of the osteogenetic gene Runx2 (Runt-related transcription factor 2) is inhibited [48]. In addition, the mTOR pathway may play an important role in osteoclastogenesis, and recent investigations have shown that inhibition of mTOR can promote osteoclast apoptosis and suppress osteoclast differentiation [49]. However, the role of the mTOR pathway in osteoclastogenesis remains unclear and requires further study.
To assess whether the effects of chronic alcohol consumption on the lineage commitment of BMMSCs occur through mTOR pathways, we used in vivo and in vitro BMMSC lines to study ethanol treatment-modulated cell differentiation involving the PI3K/AKT/mTOR pathway [46]. We found that high doses of ethanol (50/100 mM) suppressed the osteogenic differentiation of BMMSCs and that adipogenic differentiation of the cell lines was markedly increased with elevated levels of adipogenic genes PPAR-γ and LPL (lipoproteinlipase) (Fig. 3). Furthermore, bone microarchitecture analysis demonstrated that BMD was significantly decreased in the high-dose alcohol (30% ethanol in water) group compared to the controls. More importantly, the number of adipocytes in bone marrow cells was increased in the high-dose alcohol consumption group compared to the controls. These effects of ethanol on BMMSCs occurred mainly through mTOR signaling and included elevated levels of PI3K/AKT/P70S6K. Similar results were also found by western blot analysis, which indicated decreased osteogenic differentiation and increased adipogenic differentiation. In addition, treatment of P70S6K siRNA increased the expression of osteogenic genes Runx2 and ALP, but the adipocyte number and level of associated gene PPARγ decreased. However, similar effects were found with siRNA-mediated knockdown of raptor but not rictor in BMMSCs, suggesting that another mechanism may be involved in alcohol-impaired lineage BMMSC differentiation.
Fig. 3.
Schematic diagram of direct and indirect mechanisms effect on ethanol induced signaling cascades in osteopenia by bone marrow mesenchymal stem cells (BMMSCs). Ethanol treatment has direct effect on BMMSCs by ROS accumulation and Wnt inhibition, which in turn support a shift in BMMSCs differentiation towards adipocyte lineage over the osteoblast lineage. In addition, ethanol also increases TNF-α production in BMMSCs with ROS activation. Heavy alcohol consumption activates PI3K/AKT/mTOR signaling cascades in BMMSCs by inhibiting osteogenic differentiation and promoting adipogenic differentiation, with increased PPAR-γ production and inhibited Runx2 mRNA expression
Mechanisms of indirect effects of alcohol abuse on bone synthesis
The indirect action of alcohol-induced bone loss occurred secondary to target changes in the estradiol (E2) level, the regulation of the PTH–vitamin D axis, the level of growth-stimulated hepatic insulin (GH)-like growth factor type 1 (IGF-1), and the effects of oxidative stress.
Estrogen
Estrogen has been shown to prevent bone loss in both males and females [9], and experimental studies have reported decreased serum E2 levels in young female alcoholics [50]. A recent study demonstrated that alcohol dehydrogenase (ADH), an alcohol-metabolizing enzyme, is highly expressed in stromal osteoblasts and can suppress the cellular effects of alcohol-induced bone remodeling. Osteocalcin, a bone formation marker, is also suppressed [51]. In contrast, increased estrogen production in postmenopausal women is associated with the gain of bone remodeling that is associated with light alcohol consumption [6]. Mechanistically, the inhibitory effect of estrogen on heavy alcohol consumption mainly occurs through the estrogen receptor isoforms ERα and ERβ [52]. In addition, E2 and its receptor are reported to significantly inhibit alcohol-induced bone loss directly through osteoclastogenesis via induction of the RANK/RANKL/OPG system [53].
The cellular and molecular mediator of E2 action in alcohol-induced bone loss involves phosphorylation of mitogen-activated protein (MAP) kinase-related molecules, including stimulation of MAP kinase extracellular signal-regulated kinase (ERK) and its downstream target STAT3, which is phosphorylated following constitutive expression of RANKL mRNA in osteoblasts [51]. In addition, E2 can activate ERK in osteocytes for a short period, over 12–48 h [54], and acetaldehyde, a major byproduct of alcohol metabolism, triggers oxygen radical generation and induces RANKL mRNA through ERK in mouse osteoblasts (Fig. 1) [55]. As estrogen is considered to have an antioxidant effect on bone cells, its involvement in crosstalk and signaling needs further research [56].
Status of the PTH–vitamin D axis in alcohol abuse
Indirect effects on bone abnormalities at the hormone level have been associated with heavy alcohol consumption [57]. These changes may occur due to secondary interference with calcium regulation and bone mineral homeostasis through the PTH–vitamin D axis [10]. In an early study, 77 alcoholic patients (68 men and 9 women) who were heavy drinkers defined as those who consumed >150 g ethanol/day for prolonged periods (28.4 ± 11.4 years) exhibited significantly lower serum 25-hydroxyvitamin D3, IGF-1 and vitamin D levels compared with 28 controls [20]. Evidence has shown that PTH and its related protein (PTHrP) are involved in activating vitamin D, which plays a positive role in calcium homeostasis as well as microarchitectural remodeling of osteoblast function and differentiation [58].
The molecular mechanisms underlying PTH and vitamin D actions on alcohol-induced bone effects remain unclear. The effect of PTH on osteoblast cell number may require transcription factors such as Runx2 and CREB (CAMP response element-binding protein) [59]. Other observations suggest that intermittent PTH can ameliorate decreased bone mineralization, which is regulated by the Wnt pathway in heavy alcohol consumption, but that PTH fails to prevent alcohol-induced bone marrow adiposity [11, 60]. Overall, associations between adipocytic and osteogenic lineages remain unclear and require further research. Stimulation of vitamin D metabolism may be regulated by the vitamin D receptor (VDR) in osteoblasts, positively affecting bone matrix mineralization and cell differentiation [22]. In recent years, vitamin D deficiency in excessive alcohol consumption has been suggested to promote RANKL-regulated osteoclastogenesis and OPG induction [61]. However, administration of vitamin D supplements was found to cause decreased osteoclastogenesis through a downregulated RANKL/osteoprotegerin ratio, and impaired bone metabolism could subsequently be reversed in individuals with alcoholism [62].
GH–IGF1 signaling
Several groups have reported that the GH–IGF axis plays an important physiological role in lean body mass (LBM) accretion [63]. The GH–IGF axis is complex, involving numerous hormones in the blood and biological fluids [64] that may have important functions in the regulation of bone remodeling in adults [65]. Researchers have shown that damage to spontaneous GH excretion arises not only in heavy alcohol consumption but also in binge alcohol intake [66]. Further investigations showed that IGF-1 mRNA is significantly decreased in chronic alcoholics [67]. Moreover, an in vitro study demonstrated that treatment of osteoblasts and chondrocytes with GH may lead to cell differentiation and proliferation [68] and that the stimulatory mechanism may be regulated by the synthesis and secretion of IGF-1 [69]. Therefore, heavy alcohol consumption likely impairs the GH–IGF axis and results in an imbalance between osteogenesis and adipogenesis.
This controlled bioactivity of IGF-1 may be partly attributable to its high affinity for binding to its target protein IGFBP1 [64]. In vitro and in vivo studies have reported that chronic alcohol consumption not only inhibits the biological activity of IGF-1 but also increases IGFBP1 production [70]. The mechanism of this reciprocal relationship between IGF-1 and IGFBP1 may be partly due to IGF-1 resistance (Fig. 1) [71, 72]. In addition, acute alcohol intake impairs IGF activity in stimulating threonine phosphorylation of ribosomal protein S6 kinase-1 (S6K-1/Thr389; S6K-1/Thr421) [72], and this defect might be mediated through the mTOR pathway [73, 74]. However, this mechanism has not been fully defined, as phosphorylation of another mTOR substrate, 4E-binding protein-1 (4E-BP1), is not inhibited [71]. Other nutritional states and the route of alcohol administration may also play contributory roles in IGF-1 resistance [75].
Oxidative stress
Recent studies using gene array approaches suggest that ROS act as regulators of the effects of chronic alcohol exposure in the inhibition of bone formation [7, 76]. Production of ROS may contribute to plasma membrane lipid and nucleic acid damage [77], and the toxic effect of alcohol-induced bone pathology may, therefore, derive from intracellular ROS production [78]. Indeed, chronic alcohol consumption is reported to accelerate ROS generation through increased expression of NADPH oxidase (NOX) in osteoblasts in a rat model [79]. Several sources of oxidative stress have been reported in alcohol-induced bone cells. The plasma membrane-associated NOX enzyme family (NOX1, 2, 4) has been identified in osteoblasts in vitro, with NOX4 being the major constitutive subtype to generate superoxide in these cells [80]. Moreover, mRNA expression of three members of the NOX family is significantly upregulated in osteoblasts after alcohol exposure [81], and NOX4 is considered the most responsive agent for regulating cell proliferation and apoptosis (Fig. 1) [82]. As a result, alcohol-induced NOX expression has been associated with bone resorption. Consistently, oxidative stress ultimately leads to an imbalanced relationship between ROS production and the biological capacity to detoxify ROS, which may contribute to decreased bone formation [78].
Different animal studies have helped to identify these pathogenic mechanisms [83]. One study using rat osteoblast cell lines from neonatal calvariae demonstrated that induced oxidative stress promotes activation of the senescence pathway and upregulates RANKL mRNA production in osteoblasts [81]. In contrast, NOX production was blocked by E2 and dietary NAC, which could reverse the effect of alcohol on RANKL mRNA expression. This is consistent with a previous report demonstrating that NAC is able to block alcohol-induced oxidative stress in vivo [84]. In addition, treatment with diphenyleneiodonium, a specific NOX inhibitor, effectively abolishes RANKL mRNA induction in osteoblasts [81], suggesting the involvement of converging crosstalk between E2 and alcohol-induced RANKL gene expression.
Several signal transduction pathways have been reported to regulate ROS production. Increased NAPDH oxidase activity by ROS, which increases RANKL expression, depends on prolonged nuclear accumulation of MAP kinase signaling [85], and the signal-regulated kinase pathway of ERK [39]. This association was further observed in co-treatment of osteoblasts with alcohol and the ERK inhibitor PD98095, which prevented RANKL expression [79]. The downstream transducer and activator of STAT3 transcription are reported to be the ERK target, modulating RANKL expression in osteoblasts [86]. In addition, AG490, an inhibitor of STAT3, appears to prevent alcohol-induced RANKL mRNA induction in these cells. Furthermore, several other intracellular signaling cascades, including the NF-κB, JNK and the Wnt pathways, have also been implicated in increased NOX activity in alcohol-induced bone loss in osteoblasts [87].
However, information is lacking with regard to the relative signaling role of NOX enzymes in osteoclastic bone resorption and osteoclastogenesis [88]. Nonetheless, an in vitro experiment demonstrated that increased NOX1 and -2 may stimulate osteoclast activity and prevent osteoclast apoptosis due to alcohol exposure [79] and that NOX4 participates in the shift lineage commitment and differentiation of preadipocytes [82]. Another study has suggested that RANKL can increase osteoclast differentiation in osteoclast precursors downstream of RANKL and RANK binding. In addition, with increased ROS production, ethanol can affect osteoclasts or other precursors regulated by RANKL signaling and may also have an influence on ROS primarily through MAPK and STAT3 signaling [89].
Some studies have demonstrated that oxidative stress has a lineage association with Wnt/β-catenin signaling and osteoblastogenesis [39]. Indeed, Wnt signaling is a potential target in alcohol-induced bone loss [41]. In addition to accumulating ROS and interfering with osteoblast differentiation in vivo, chronic alcohol abuse has direct effects on mesenchymal stem cells, ultimately leading to dampened β-catenin nuclear translocation and decreased Wnt-dependent T cell factor/lymphoid enhancer factor (TCF/LEF) transcription, partly through GSK3β gene production [76, 90].
Potential therapeutic strategies to prevent alcohol-induced bone disease
As described in this review, chronic alcohol consumption has toxic effects on bone regulation by decreasing bone formation and increasing osteogenesis in bone degradation. This impaired bone remodeling is influenced by proinflammatory factors, the Wnt/β-catenin pathway, the mTOR pathway and RANKL/RANK signaling toward osteoblasts, osteoclasts and BMMSCs. In addition, indirect actions should be taken into account, including nutritional and hormonal factors. Therefore, the treatment of alcohol-induced osteopenia should focus on increasing osteogenesis and decreasing osteoclastic activity.
It has been reported that the Wnt pathway can be targeted by antagonizing sclerostin, which is produced by osteocytes [91, 92]. Consistent with this, blocking sclerostin increases bone formation and has a beneficial effect on bone mass in osteopenic animals [93]. Moreover, an anti-sclerostin antibody was recently used in postmenopausal woman to increase bone formation and strength [94]. Another class of drugs for treating postmenopausal osteoporosis that could be theoretically effective in alcohol-induced bone loss includes the RNAK/RANKL pathway inhibitor denosumab [95]. This human monoclonal antibody can decrease osteoclast activity and bone resorption by inhibiting RANKL and its binding to RANK. In fact, one study demonstrated that denosumab reduced vertebral and hip fractures in postmenopausal women compared to controls [96].
As a highly specific medicine, rapamycin targets the mTOR pathway [97], and previous studies have demonstrated that rapamycin can be used as a novel nonsteroidal anticancer drug [98] and may play a critical immunosuppressive role in tumor immunity [99]. Mechanistically, this effect appears to be regulated by natural crosstalk between mTOR and rapamycin [47]. In addition, rapamycin binding to FKBP12 (FK506-binding protein 12, targeted intracellular receptor) causes functional alterations in certain amino acid residues in the mTOR complex assembly [100]. Consistent with this finding, in one of our studies, we found that rapamycin treatment of BMMSCs was able to apparently rescue alcohol-induced osteopenia [46]. However, concerning the multifunctional roles of rapamycin in immune-regulatory and osteoporosis therapies, the effect and safety of this drug in preclinical tests and more detailed clinical trials need to be assessed in future studies.
It has been shown that vitamin D supplementation with eldecalcitol can protect against cortical bone loss through inhibition of NF-κB ligand mRNA in mice fed a liquid ethanol diet (30% ethanol in water) [14]. Clinical studies are necessary, however, to investigate the effects of vitamin D supplements and therapy with bisphosphonates in people with chronic alcoholism [101]. Bisphosphonates, which inhibit bone resorption, have been widely used to treat bone diseases in postmenopausal women [102]. Alendronate also has positive effects on bone mineralization in the femur during chronic alcohol consumption [103] and, it could compensate for the decreased biomechanical properties of femurs affected by ethanol [103]. Similar results were found in another study, suggesting that bisphosphonates can decrease production of urinary amino telopeptides, including expression of the osteoblastogenesis genes collagen-I and serum osteocalcin [104].
Conclusion
In recent years, some important mechanisms underlying alcohol-induced bone pathology in humans and animal models have been identified. The effects of alcohol consumption on BMD and bone mass are dose and duration dependent [105]. Although the effect of light chronic alcohol consumption is not deleterious to bones, the exact effects depend on other factors including age, sex hormone status, and nutrition levels. Chronic and binge alcohol consumption have harmful effects on bone tissues and represent a risk for osteopenia. However, the optimal amount of daily alcohol consumption for a positive effect on bone tissues can hardly be estimated based on current studies because general population studies are heterogeneous globally. Indeed, different mechanisms may contribute to the effects of chronic heavy alcohol consumption on the activity of osteoblasts and osteoclasts and also its inhibition of bone formation. Some emerging evidence suggests that the effects of typical cell changes might be modulated in part by Wnt and mTOR signaling [39, 106], leading to decreased bone formation associated with increased adipogenesis in the bone marrow (Fig. 3). The harmful effects of chronic alcohol consumption on bone tissues may also be regulated in indirect ways, including by estrogen, the PTH–vitamin D axis, GH–IGF levels, and effects of oxidative stress (Figs. 1, 2). All of these prompted us to provide novel therapeutic approaches, such as modulation of the corresponding signaling (Wnt and mTOR pathway) and inhibition of the RANKL/RANK pathway in bone cells. Studying these direct and indirect effects of alcohol-induced bone pathology may translate into efficient therapeutics, not only for preventing the development of alcohol-induced bone disorders but also for correcting imbalanced bone remodeling in human metabolic skeletal disorders. Regardless, the cellular mechanisms for alcohol-induced osteopenia are not so simple. More investigations into osteoclast apoptosis and activity will lead us to a better understanding of bone pathology in alcoholics.
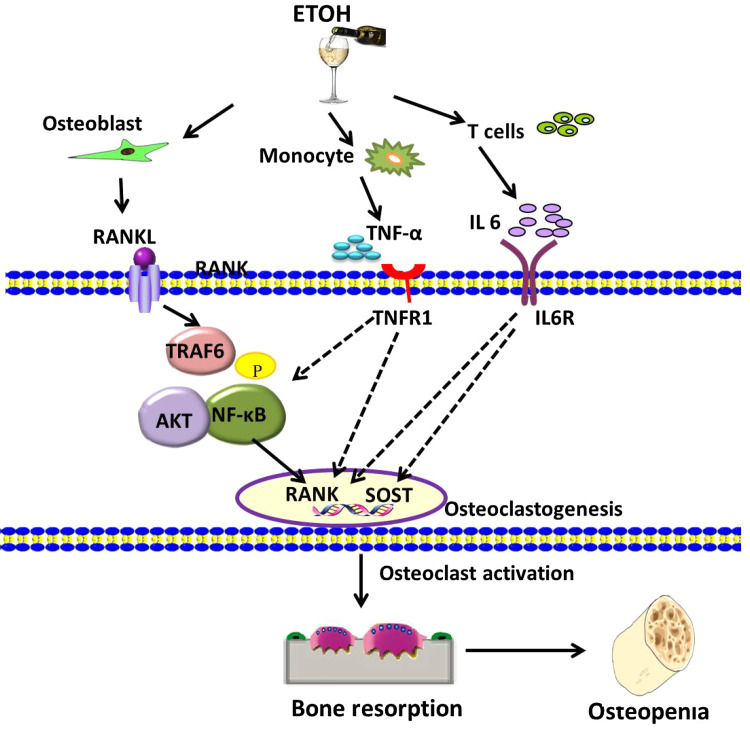
Fig. 2.
Direct and indirect mechanisms effect on ethanol (ETOH)-induced osteoclast activation and osteoclastogenesis via RANKL/RANK signaling cascades in osteopenia. Other inflammatory cytokines, including TNF-α and IL-6, also have directly positive effect on osteoclasts. However, their downstream signaling cascades remain unsolved and need further research. RANK receptor activator of nuclear factor kappa-B, RANKL RANK ligand, SOST sclerostin
References
- 1.Miranda RC, Pietrzykowski AZ, Tang Y, et al. MicroRNAs: master regulators of ethanol abuse and toxicity. Alcohol Clin Exp Res. 2010;34:575–587. doi: 10.1111/j.1530-0277.2009.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakkalakal DA. Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res. 2005;29:2077–2090. doi: 10.1097/01.alc.0000192039.21305.55. [DOI] [PubMed] [Google Scholar]
- 3.Jonsson IM, Verdrengh M, Brisslert M, et al. Ethanol prevents development of destructive arthritis. Proc Natl Acad Sci USA. 2007;104:258–263. doi: 10.1073/pnas.0608620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callaci JJ, Juknelis D, Patwardhan A, Wezeman FH. Binge alcohol treatment increases vertebral bone loss following ovariectomy: compensation by intermittent parathyroid hormone. Alcohol Clin Exp Res. 2006;30:665–672. doi: 10.1111/j.1530-0277.2006.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampson HW. Alcohol and other factors affecting osteoporosis risk in women. Alcohol Res Health. 2002;26:292–298. [PMC free article] [PubMed] [Google Scholar]
- 6.Turner RT. Skeletal response to alcohol. Alcohol Clin Exp Res. 2000;24:1693–1701. doi: 10.1111/j.1530-0277.2000.tb01971.x. [DOI] [PubMed] [Google Scholar]
- 7.Callaci JJ, Himes R, Lauing K, Roper P. Long-term modulations in the vertebral transcriptome of adolescent-stage rats exposed to binge alcohol. Alcohol Alcohol. 2010;45:332–346. doi: 10.1093/alcalc/agq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyer SA, Buckendahl P, Sampson HW. Alcohol consumption inhibits osteoblastic cell proliferation and activity in vivo. Alcohol. 1998;16:337–341. doi: 10.1016/S0741-8329(98)00025-1. [DOI] [PubMed] [Google Scholar]
- 9.Turner RT, Sibonga JD. Effects of alcohol use and estrogen on bone. Alcohol Res Health. 2001;25:276–281. [PMC free article] [PubMed] [Google Scholar]
- 10.Duggal S, Simpson ME, Keiver K. Effect of chronic ethanol consumption on the response of parathyroid hormone to hypocalcemia in the pregnant rat. Alcohol Clin Exp Res. 2007;31:104–112. doi: 10.1111/j.1530-0277.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- 11.Howe KS, Iwaniec UT, Turner RT. The effects of low dose parathyroid hormone on lumbar vertebrae in a rat model for chronic alcohol abuse. Osteoporos Int. 2011;22:1175–1181. doi: 10.1007/s00198-010-1304-4. [DOI] [PubMed] [Google Scholar]
- 12.Wezeman FH, Gong Z. Bone marrow triglyceride accumulation and hormonal changes during long-term alcohol intake in male and female rats. Alcohol Clin Exp Res. 2001;25:1515–1522. doi: 10.1111/j.1530-0277.2001.tb02155.x. [DOI] [PubMed] [Google Scholar]
- 13.Dai J, Lin D, Zhang J, et al. Chronic alcohol ingestion induces osteoclastogenesis and bone loss through IL-6 in mice. J Clin Investig. 2000;106:887–895. doi: 10.1172/JCI10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercer KE, Wynne RA, Lazarenko OP, et al. Vitamin D supplementation protects against bone loss associated with chronic alcohol administration in female mice. J Pharmacol Exp Ther. 2012;343:401–412. doi: 10.1124/jpet.112.197038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frost M, Wraae K, Gudex C, et al. Chronic diseases in elderly men: underreporting and underdiagnosis. Age Ageing. 2012;41:177–183. doi: 10.1093/ageing/afr153. [DOI] [PubMed] [Google Scholar]
- 17.Williams FM, Cherkas LF, Spector TD, MacGregor AJ. The effect of moderate alcohol consumption on bone mineral density: a study of female twins. Ann Rheum Dis. 2005;64:309–310. doi: 10.1136/ard.2004.022269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganry O, Baudoin C, Fardellone P. Effect of alcohol intake on bone mineral density in elderly women: the EPIDOS study. Am J Epidemiol. 2000;151:773–780. doi: 10.1093/oxfordjournals.aje.a010277. [DOI] [PubMed] [Google Scholar]
- 19.McGuire S. U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv Nutr. 2011;2:293–294. doi: 10.3945/an.111.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvisa-Negrín J, González-Reimers E, Santolaria-Fernández F, et al. Osteopenia in alcoholics: effect of alcohol abstinence. Alcohol Alcohol. 2009;44:468–475. doi: 10.1093/alcalc/agp038. [DOI] [PubMed] [Google Scholar]
- 21.Audran M, Cortet B. Prevalence of osteoporosis in male patients with risk factors. Presse Med. 2011;40:e489–e498. doi: 10.1016/j.lpm.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Kizilgul M, Ozcelik O, Delibasi T. Bone health and vitamin D status in alcoholic liver disease. Indian J Gastroenterol. 2016;35:253–259. doi: 10.1007/s12664-016-0652-1. [DOI] [PubMed] [Google Scholar]
- 23.Menecier P, Girard A, Badila P, Rotheval L, Lefranc D, Menecier-Ossia L. Acute alcoholic intoxication at the hospital: a clinical stake. A prospective study of one year in a general hospital. Rev Med Interne. 2009;30:316–321. doi: 10.1016/j.revmed.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Mikosch P. Alcohol and bone. Wien Med Wochenschr. 2014;164:15–24. doi: 10.1007/s10354-013-0258-5. [DOI] [PubMed] [Google Scholar]
- 25.Shankar K, Hidestrand M, Haley R, et al. Different molecular mechanisms underlie ethanol-induced bone loss in cycling and pregnant rats. Endocrinology. 2006;147:166–178. doi: 10.1210/en.2005-0529. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Li Y, Mao K, Li J, Cui Q, Wang GJ. Alcohol-induced adipogenesis in bone and marrow: a possible mechanism for osteonecrosis. Clin Orthop Relat Res. 2003;410:213–224. doi: 10.1097/01.blo.0000063602.67412.83. [DOI] [PubMed] [Google Scholar]
- 27.Friday KE, Howard GA. Ethanol inhibits human bone cell proliferation and function in vitro. Metabolism. 1991;40:562–565. doi: 10.1016/0026-0495(91)90044-W. [DOI] [PubMed] [Google Scholar]
- 28.Jugdaohsingh R, O’Connell MA, Sripanyakorn S, Powell JJ. Moderate alcohol consumption and increased bone mineral density: potential ethanol and non-ethanol mechanisms. Proc Nutr Soc. 2006;65:291–310. doi: 10.1079/PNS2006508. [DOI] [PubMed] [Google Scholar]
- 29.Høidrup S, Grønbaek M, Gottschau A, Lauritzen JB, Schroll M. Alcohol intake, beverage preference, and risk of hip fracture in men and women. Copenhagen Centre for Prospective Population Studies. Am J Epidemiol. 1999;149:993–1001. doi: 10.1093/oxfordjournals.aje.a009760. [DOI] [PubMed] [Google Scholar]
- 30.Torricelli P, Fini M, Giavaresi G, et al. Intermittent exposure to ethanol vapor affects osteoblast behaviour more severely than estrogen deficiency does in vitro study on rat osteoblasts. Toxicology. 2007;237:168–176. doi: 10.1016/j.tox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Díez-Ruiz A, García-Saura PL, García-Ruiz P, González-Calvin JL, Gallego-Rojo F, Fuchs D. Bone mineral density, bone turnover markers and cytokines in alcohol-induced cirrhosis. Alcohol Alcohol. 2010;45:427–430. doi: 10.1093/alcalc/agq037. [DOI] [PubMed] [Google Scholar]
- 32.Cheung RC, Gray C, Boyde A, Jones SJ. Effects of ethanol on bone cells in vitro resulting in increased resorption. Bone. 1995;16:143–147. doi: 10.1016/8756-3282(95)80025-L. [DOI] [PubMed] [Google Scholar]
- 33.Yao Z, Zhang J, Dai J, Keller ET. Ethanol activates NFkappaB DNA binding and p56lck protein tyrosine kinase in human osteoblast-like cells. Bone. 2001;28:167–173. doi: 10.1016/S8756-3282(00)00425-7. [DOI] [PubMed] [Google Scholar]
- 34.García-Valdecasas-Campelo E, González-Reimers E, Santolaria-Fernández F, et al. Serum osteoprotegerin and RANKL levels in chronic alcoholic liver disease. Alcohol Alcohol. 2006;41:261–266. doi: 10.1093/alcalc/agl004. [DOI] [PubMed] [Google Scholar]
- 35.Fábrega E, Orive A, García-Suarez C, García-Unzueta M, Antonio AJ, Pons-Romero F. Osteoprotegerin and RANKL in alcoholic liver cirrhosis. Liver Int. 2005;25:305–310. doi: 10.1111/j.1478-3231.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- 36.Perrien DS, Brown EC, Fletcher TW, et al. Interleukin-1 and tumor necrosis factor antagonists attenuate ethanol-induced inhibition of bone formation in a rat model of distraction osteogenesis. J Pharmacol Exp Ther. 2002;303:904–908. doi: 10.1124/jpet.102.039636. [DOI] [PubMed] [Google Scholar]
- 37.Wahl EC, Aronson J, Liu L, et al. Chronic ethanol exposure inhibits distraction osteogenesis in a mouse model: role of the TNF signaling axis. Toxicol Appl Pharmacol. 2007;220:302–310. doi: 10.1016/j.taap.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankar K, Hidestrand M, Liu X, et al. Chronic ethanol consumption inhibits postlactational anabolic bone rebuilding in female rats. J Bone Miner Res. 2008;23:338–349. doi: 10.1359/jbmr.071023. [DOI] [PubMed] [Google Scholar]
- 39.Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Badger TM, Ronis MJ. A role for ethanol-induced oxidative stress in controlling lineage commitment of mesenchymal stromal cells through inhibition of Wnt/beta-catenin signaling. J Bone Miner Res. 2010;25:1117–1127. doi: 10.1002/jbmr.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galli C, Passeri G, Macaluso GM. Osteocytes and WNT: the mechanical control of bone formation. J Dent Res. 2010;89:331–343. doi: 10.1177/0022034510363963. [DOI] [PubMed] [Google Scholar]
- 41.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith C, Gasparetto M, Jordan C, Pollyea DA, Vasiliou V. The effects of alcohol and aldehyde dehydrogenases on disorders of hematopoiesis. Adv Exp Med Biol. 2015;815:349–359. doi: 10.1007/978-3-319-09614-8_20. [DOI] [PubMed] [Google Scholar]
- 43.Ronis MJ, Mercer K, Chen JR. Effects of nutrition and alcohol consumption on bone loss. Curr Osteoporos Rep. 2011;9:53–59. doi: 10.1007/s11914-011-0049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 45.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Kou X, Chen C, et al. Chronic high dose alcohol induces osteopenia via activation of mTOR signaling in bone marrow mesenchymal stem cells. Stem Cells. 2016;34:2157–2168. doi: 10.1002/stem.2392. [DOI] [PubMed] [Google Scholar]
- 47.Yang H, Rudge DG, Koos JD, Vaidialingam B, Yang HJ, Pavletich NP. mTOR kinase structure, mechanism and regulation. Nature. 2013;497:217–223. doi: 10.1038/nature12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin SK, Fitter S, Dutta AK, et al. Brief report: the differential roles of mTORC1 and mTORC2 in mesenchymal stem cell differentiation. Stem Cells. 2015;33:1359–1365. doi: 10.1002/stem.1931. [DOI] [PubMed] [Google Scholar]
- 49.Dai Q, Xie F, Han Y, et al. Inactivation of regulatory-associated protein of mTOR (raptor)/mammalian target of rapamycin complex 1 (mTORC1) signaling in osteoclasts increases bone mass by inhibiting osteoclast differentiation in mice. J Biol Chem. 2017;292:196–204. doi: 10.1074/jbc.M116.764761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dorgan JF, Reichman ME, Judd JT, et al. The relation of reported alcohol ingestion to plasma levels of estrogens and androgens in premenopausal women (Maryland, United States) Cancer Causes Control. 1994;5:53–60. doi: 10.1007/BF01830726. [DOI] [PubMed] [Google Scholar]
- 51.Chen JR, Haley RL, Hidestrand M, et al. Estradiol protects against ethanol-induced bone loss by inhibiting up-regulation of receptor activator of nuclear factor-kappaB ligand in osteoblasts. J Pharmacol Exp Ther. 2006;319:1182–1190. doi: 10.1124/jpet.106.109454. [DOI] [PubMed] [Google Scholar]
- 52.Monroe DG, Getz BJ, Johnsen SA, Riggs BL, Khosla S, Spelsberg TC. Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERalpha or ERbeta. J Cell Biochem. 2003;90:315–326. doi: 10.1002/jcb.10633. [DOI] [PubMed] [Google Scholar]
- 53.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–696. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 54.Chen JR, Plotkin LI, Aguirre JI, et al. Transient versus sustained phosphorylation and nuclear accumulation of ERKs underlie anti-versus pro-apoptotic effects of estrogens. J Biol Chem. 2005;280:4632–4638. doi: 10.1074/jbc.M411530200. [DOI] [PubMed] [Google Scholar]
- 55.Bai XC, Lu D, Liu AL, et al. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J Biol Chem. 2005;280:17497–17506. doi: 10.1074/jbc.M409332200. [DOI] [PubMed] [Google Scholar]
- 56.Lean JM, Davies JT, Fuller K, et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Investig. 2003;112:915–923. doi: 10.1172/JCI200318859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Díez A, Puig J, Serrano S, et al. Alcohol-induced bone disease in the absence of severe chronic liver damage. J Bone Miner Res. 1994;9:825–831. doi: 10.1002/jbmr.5650090608. [DOI] [PubMed] [Google Scholar]
- 58.Malik P, Gasser RW, Kemmler G, et al. Low bone mineral density and impaired bone metabolism in young alcoholic patients without liver cirrhosis: a cross-sectional study. Alcohol Clin Exp Res. 2009;33:375–381. doi: 10.1111/j.1530-0277.2008.00847.x. [DOI] [PubMed] [Google Scholar]
- 59.Swarthout JT, D’Alonzo RC, Selvamurugan N, Partridge NC. Parathyroid hormone-dependent signaling pathways regulating genes in bone cells. Gene. 2002;282:1–17. doi: 10.1016/S0378-1119(01)00798-3. [DOI] [PubMed] [Google Scholar]
- 60.Lombardi G, Di SC, Rubino M, et al. The roles of parathyroid hormone in bone remodeling: prospects for novel therapeutics. J Endocrinol Investig. 2011;34:18–22. [PubMed] [Google Scholar]
- 61.Anderson PH, Sawyer RK, Moore AJ, May BK, O’Loughlin PD, Morris HA. Vitamin D depletion induces RANKL-mediated osteoclastogenesis and bone loss in a rodent model. J Bone Miner Res. 2008;23:1789–1797. doi: 10.1359/jbmr.080616. [DOI] [PubMed] [Google Scholar]
- 62.Baldock PA, Thomas GP, Hodge JM, et al. Vitamin D action and regulation of bone remodeling: suppression of osteoclastogenesis by the mature osteoblast. J Bone Miner Res. 2006;21:1618–1626. doi: 10.1359/jbmr.060714. [DOI] [PubMed] [Google Scholar]
- 63.Lang CH, Frost RA, Summer AD, Vary TC. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. Int J Biochem Cell Biol. 2005;37:2180–2195. doi: 10.1016/j.biocel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 64.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 65.Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int. 2012;23:1–16. doi: 10.1007/s00198-011-1787-7. [DOI] [PubMed] [Google Scholar]
- 66.Maddalozzo GF, Turner RT, Edwards CH, et al. Alcohol alters whole body composition, inhibits bone formation, and increases bone marrow adiposity in rats. Osteoporos Int. 2009;20:1529–1538. doi: 10.1007/s00198-009-0836-y. [DOI] [PubMed] [Google Scholar]
- 67.Lang CH, Fan J, Lipton BP, Potter BJ, McDonough KH. Modulation of the insulin-like growth factor system by chronic alcohol feeding. Alcohol Clin Exp Res. 1998;22:823–829. doi: 10.1111/j.1530-0277.1998.tb03874.x. [DOI] [PubMed] [Google Scholar]
- 68.Lang CH, Liu X, Nystrom G, Wu D, Cooney RN, Frost RA. Acute effects of growth hormone in alcohol-fed rats. Alcohol Alcohol. 2000;35:148–158. doi: 10.1093/alcalc/35.2.148. [DOI] [PubMed] [Google Scholar]
- 69.Menagh PJ, Turner RT, Jump DB, et al. Growth hormone regulates the balance between bone formation and bone marrow adiposity. J Bone Miner Res. 2010;25:757–768. doi: 10.1359/jbmr.091015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lang CH, Vary TC, Frost RA. Acute in vivo elevation of insulin-like growth factor (IGF) binding protein-1 decreases plasma free IGF-I and muscle protein synthesis. Endocrinology. 2003;144:3922–3933. doi: 10.1210/en.2002-0192. [DOI] [PubMed] [Google Scholar]
- 71.Kumar V, Frost RA, Lang CH. Alcohol impairs insulin and IGF-I stimulation of S6K1 but not 4E-BP1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E917–E928. doi: 10.1152/ajpendo.00181.2002. [DOI] [PubMed] [Google Scholar]
- 72.Shah OJ, Anthony JC, Kimball SR, Jefferson LS. 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab. 2000;279:E715–E729. doi: 10.1152/ajpendo.2000.279.4.E715. [DOI] [PubMed] [Google Scholar]
- 73.Ronis MJ, Wands JR, Badger TM, de la Monte SM, Lang CH, Calissendorff J. Alcohol-induced disruption of endocrine signaling. Alcohol Clin Exp Res. 2007;31:1269–1285. doi: 10.1111/j.1530-0277.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 74.Bakker AD, Gakes T, Hogervorst JM, de Wit GM, Klein-Nulend J, Jaspers RT. Mechanical stimulation and IGF-1 enhance mRNA translation rate in osteoblasts via activation of the AKT-mTOR pathway. J Cell Physiol. 2016;231:1283–1290. doi: 10.1002/jcp.25228. [DOI] [PubMed] [Google Scholar]
- 75.Lang CH, Pruznak AM, Deshpande N, Palopoli MM, Frost RA, Vary TC. Alcohol intoxication impairs phosphorylation of S6K1 and S6 in skeletal muscle independently of ethanol metabolism. Alcohol Clin Exp Res. 2004;28:1758–1767. doi: 10.1097/01.ALC.0000145787.66405.59. [DOI] [PubMed] [Google Scholar]
- 76.Himes R, Wezeman FH, Callaci JJ. Identification of novel bone-specific molecular targets of binge alcohol and ibandronate by transcriptome analysis. Alcohol Clin Exp Res. 2008;32:1167–1180. doi: 10.1111/j.1530-0277.2008.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Novitskiy G, Traore K, Wang L, Trush MA, Mezey E. Effects of ethanol and acetaldehyde on reactive oxygen species production in rat hepatic stellate cells. Alcohol Clin Exp Res. 2006;30:1429–1435. doi: 10.1111/j.1530-0277.2006.00171.x. [DOI] [PubMed] [Google Scholar]
- 78.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 79.Chen JR, Shankar K, Nagarajan S, Badger TM, Ronis MJ. Protective effects of estradiol on ethanol-induced bone loss involve inhibition of reactive oxygen species generation in osteoblasts and downstream activation of the extracellular signal-regulated kinase/signal transducer and activator of transcription 3/receptor activator of nuclear factor-kappaB ligand signaling cascade. J Pharmacol Exp Ther. 2008;324:50–59. doi: 10.1124/jpet.107.130351. [DOI] [PubMed] [Google Scholar]
- 80.Serrander L, Cartier L, Bedard K, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen JR, Lazarenko OP, Shankar K, et al. Inhibition of NADPH oxidases prevents chronic ethanol-induced bone loss in female rats. J Pharmacol Exp Ther. 2011;336:734–742. doi: 10.1124/jpet.110.175091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schröder K, Wandzioch K, Helmcke I, Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol. 2009;29:239–245. doi: 10.1161/ATVBAHA.108.174219. [DOI] [PubMed] [Google Scholar]
- 83.Colston JT, de la Rosa SD, Strader JR, Anderson MA, Freeman GL. H2O2 activates Nox4 through PLA2-dependent arachidonic acid production in adult cardiac fibroblasts. FEBS Lett. 2005;579:2533–2540. doi: 10.1016/j.febslet.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 84.Shankar K, Liu X, Singhal R, et al. Chronic ethanol consumption leads to disruption of vitamin D3 homeostasis associated with induction of renal 1,25 dihydroxyvitamin D3-24-hydroxylase (CYP24A1) Endocrinology. 2008;149:1748–1756. doi: 10.1210/en.2007-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Torres M. Mitogen-activated protein kinase pathways in redox signaling. Front Biosci. 2003;8:d369–d391. doi: 10.2741/999. [DOI] [PubMed] [Google Scholar]
- 86.O’Brien CA, Gubrij I, Lin SC, Saylors RL, Manolagas SC. STAT3 activation in stromal/osteoblastic cells is required for induction of the receptor activator of NF-kappaB ligand and stimulation of osteoclastogenesis by gp130-utilizing cytokines or interleukin-1 but not 1,25-dihydroxyvitamin D3 or parathyroid hormone. J Biol Chem. 1999;274:19301–19308. doi: 10.1074/jbc.274.27.19301. [DOI] [PubMed] [Google Scholar]
- 87.Wu RF, Ma Z, Myers DP, Terada LS. HIV-1 Tat activates dual Nox pathways leading to independent activation of ERK and JNK MAP kinases. J Biol Chem. 2007;282:37412–37419. doi: 10.1074/jbc.M704481200. [DOI] [PubMed] [Google Scholar]
- 88.Lee NK, Choi YG, Baik JY, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 89.Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79:1348–1356. doi: 10.1189/jlb.1005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yeh CH, Chang JK, Wang YH, Ho ML, Wang GJ. Ethanol may suppress Wnt/beta-catenin signaling on human bone marrow stroma cells: a preliminary study. Clin Orthop Relat Res. 2008;466:1047–1053. doi: 10.1007/s11999-008-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marie PJ. Osteoblast dysfunctions in bone diseases: from cellular and molecular mechanisms to therapeutic strategies. Cell Mol Life Sci. 2015;72:1347–1361. doi: 10.1007/s00018-014-1801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 93.Paszty C, Turner CH, Robinson MK. Sclerostin: a gem from the genome leads to bone-building antibodies. J Bone Miner Res. 2010;25:1897–1904. doi: 10.1002/jbmr.161. [DOI] [PubMed] [Google Scholar]
- 94.van Bezooijen RL, ten Dijke P, Papapoulos SE, Löwik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005;16:319–327. doi: 10.1016/j.cytogfr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 95.González-Reimers E, Quintero-Platt G, Rodríguez-Rodríguez E, Martínez-Riera A, Alvisa-Negrín J, Santolaria-Fernández F. Bone changes in alcoholic liver disease. World J Hepatol. 2015;7:1258–1264. doi: 10.4254/wjh.v7.i9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cummings SR, San MJ, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 97.Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19:373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 99.Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- 100.Mukhopadhyay S, Frias MA, Chatterjee A, Yellen P, Foster DA. The enigma of rapamycin dosage. Mol Cancer Ther. 2016;15:347–353. doi: 10.1158/1535-7163.MCT-15-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wezeman FH, Juknelis D, Himes R, Callaci JJ. Vitamin D and ibandronate prevent cancellous bone loss associated with binge alcohol treatment in male rats. Bone. 2007;41:639–645. doi: 10.1016/j.bone.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.López-Larramona G, Lucendo AJ, González-Delgado L. Alcoholic liver disease and changes in bone mineral density. Rev Esp Enferm Dig. 2013;105:609–621. doi: 10.4321/S1130-01082013001000006. [DOI] [PubMed] [Google Scholar]
- 103.Wezeman FH, Emanuele MA, Moskal SF, Steiner J, Lapaglia N. Alendronate administration and skeletal response during chronic alcohol intake in the adolescent male rat. J Bone Miner Res. 2000;15:2033–2041. doi: 10.1359/jbmr.2000.15.10.2033. [DOI] [PubMed] [Google Scholar]
- 104.Rudic JS, Giljaca V, Krstic MN, Bjelakovic G, Gluud C. Bisphosphonates for osteoporosis in primary biliary cirrhosis. Cochrane Database Syst Rev. 2011;12:CD009144. doi: 10.1002/14651858.CD009144.pub2. [DOI] [PubMed] [Google Scholar]
- 105.Kouda K, Iki M, Fujita Y, et al. Alcohol intake and bone status in elderly Japanese men: baseline data from the Fujiwara-kyo osteoporosis risk in men (FORMEN) study. Bone. 2011;49:275–280. doi: 10.1016/j.bone.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 106.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]