Abstract
Seven transmembrane G protein-coupled receptors (GPCRs) have gained much interest in recent years as it is the largest class among cell surface receptors. G proteins lie in the heart of GPCRs signalling and therefore can be therapeutically targeted to overcome complexities in GPCR responses and signalling. G proteins are classified into four families (Gi, Gs, G12/13 and Gq); Gq is further subdivided into four classes. Among them Gαq and Gαq/11 isoforms are most crucial and ubiquitously expressed; these isoforms are almost 88% similar at their amino acid sequence but may exhibit functional divergences. However, uncertainties often arise about Gαq and Gαq/11 inhibitors, these G proteins might also have suitability to the invention of novel-specific inhibitors for each isoforms. YM-254890 and UBO-QIC are discovered as potent inhibitors of Gαq functions and also investigated in thrombin protease-activated receptor (PAR)-1 inhibitors and platelet aggregation inhibition. The most likely G protein involved in PAR-1 stimulates responses is one of the Gαq family isoforms. In this review, we highlight the molecular structures and pharmacological responses of Gαq family which may reflect the biochemical and molecular role of Gαq and Gαq/11. The advanced understanding of Gαq and Gαq/11 role in GPCR signalling may shed light on our understanding on cell biology, cellular physiology and pathophysiology and also lead to the development of novel therapeutic agents for a number of diseases.
Keywords: G proteins, Isoforms, G Protein-coupled receptors, GPCRs, PAR-1, Hyperelongation, Atherosclerosis, G alpha q
Introduction
Seven transmembrane G protein-coupled receptors (GPCRs) are the largest and most important class of cell surface receptors numbering in the hundreds [1–4]. There has been an enormous amount of work done on GPCRs and numerous processes and pathways have been discovered. These processes include aspects of biased signalling, action of receptor-directed antibodies, allosteric modulators, GPCR-heterodimer targeted compounds and transactivation of protein tyrosine kinase and more recently protein serine/threonine kinase receptors [4–10]. However, progress in exploiting this knowledge about GPCRs has not been translated into the pipeline of new therapeutic agents that might have been envisaged [11]. Thus, new areas of investigation in the area of GPCRs and GPCR signalling are required.
The major primary and proximal effectors of GPCR signalling are the guanosine triphosphate (GTP)-binding, GTPase active, G proteins. G proteins are a point of convergence in GPCR signalling and as such provide an opportunity to overcome some of the complexities that have been identified in therapeutically exploiting upstream GPCR responses and signalling [12–15]. G proteins are classified into four families according to their α subunits: Gi, Gs, G12/13 and Gq. The Gs and Gi families regulate adenylyl cyclase activity, while Gq activates phospholipase C (PLC) β and G12/13 can activate small GTPase families [16]. The Gq family consists of four members—Gq, G11, G14 and G15/16 [17, 18] and their respective α subunits are thus Gαq, Gα11, Gα14 and Gα15/16. Two of these isoforms, Gαq and Gαq/11, share 88% similarity at the amino acid sequence, and are the most prominent and ubiquitously expressed proteins in this family and are the topic of this review [19].
The Gαq family of G proteins which includes the Gαq and Gαq/11 isoforms are especially significant due to the vast range of cellular responses that are mediated by these protein effectors. In their customary role, Gαq proteins, activated by the upstream GPCRs, trigger PLC activity, which in turn hydrolyses membrane-bound phosphatidylinositol-4,5-bisphosphate (PIP2) to the generic diacylglycerol (DAG) which activates protein kinase C and inositol 1,4,5 trisphosphate (IP3) which in turn initiates the release of calcium ions from the sarcoplasmic reticulum. Though Gαq proteins play a critical role in GPCR-mediated signal transduction pathway, the role of heterotrimeric G proteins in GPCR signalling is discernibly the most under researched molecules in modern cell biology [20, 21].
There are some areas of science in which for various valid, but sometimes pragmatic or even investigator-determined reasons, terminology confuses non-specialist and even sometimes specialist readers. For example, in the intensely studied area of transforming growth factor-β (TGF-β) signalling, the immediate downstream target of the TGF-β type 1 receptor (TGFβR1) is the Smad transcription factor and its carboxy terminal phosphorylation (phosphoSmadC); however, the phenomena of indirect kinase-mediated phosphorylation of Smads in the linker region (phosphoSmadL) acquired the name, obviously confusingly, of “non-Smad signalling” or “Smad-independent signalling” [22–25]. For the Gαq family of G proteins the members are Gαq and Gαq/11—precisely the former member would be Gαq/q but this is truncated to simply Gαq. In some cases, the protein Gαq/11 is referred to as Gα11, a term which does not identify the protein as a Gαq family member [26]. The terminology, thus, creates serious opacity and compromises understanding in this area. This issue is further exemplified by the use of the phrase or the definition of a compound having activity as a selective Gαq inhibitor when that does not allow for the existence of the isoforms, Gαq and Gαq/11, where we speculate in this review that these two proteins might have different functions and might also be amenable to the discovery of inhibitors which are selective for each isoform [27].
A major area of confusion, which flows on from the lack of clarity in defining the two isoforms, is the role of inhibitors. We have previously reflected on the short-comings and confusion surrounding the pharmacological tools in this area [25]. Two natural products, YM-254890 from the bacteria Chromobacterium sp. QS3666 and UBO-QIC also known as FR300359 from the leaves of the plant, Ardisia crenata sims are very potent inhibitors of Gαq functions and have been investigated in the therapeutic context of inhibitors of the thrombin protease-activated receptor (PAR)-1 and the inhibition of platelet aggregation [21, 25, 28–30]. The availability of these compounds has been severely restricted; however, various groups [27], including our own [31], are proceeding along the pathway to the synthesis of these compounds which will almost certainly provide a base for experiments. These compounds will define more broadly the roles of the individual Gαq isoforms in GPCR signalling and will help us to understand the cell biology of these two isoforms.
In our recent studies of the role of PAR-1 in the stimulation of the expression of the genes which encode the enzymes that mediate the elongation of chondroitin/dermatan sulphate glycosaminoglycan (GAG) chains on proteoglycans, we have found that all of the signalling goes via the transactivation of protein tyrosine kinases (which are responsible for the generation of phosphoErk) and protein serine/threonine kinase receptors (which generate phosphoSmad2C) [32–34]. The most likely G protein involved in these responses is one of the Gαq family isoforms; however, there is little evidence for the involvement of calcium ions or protein kinase C in these responses which would reflect the traditional PLC mediated pathway [35]. We have not yet defined the role of G proteins in these responses but it is interesting to note that the response does not appear to arise from typical Gαq signalling raising the possibility that there may be novel pathways involving and downstream of Gαq.
This review sets out the current knowledge of the Gαq family of G proteins including the molecular structures and pharmacological responses on the basis that new experimental data will shortly become available. We assume that these data will be helpful to define the biochemical and/or molecular role of Gαq and Gαq/11 and perhaps will help us to answer some of the very interesting questions such as the role of G protein flipping in GPCR biased signalling and GPCR transactivation-dependent cell signalling [9].
Role of G proteins and Gαq proteins specifically in GPCR signalling
The proximal effectors of GPCR signalling are the G proteins, so-called because of their ability to bind GTP and guanosine diphosphate (GDP). There are monomeric G proteins such as Ras (downstream of GPCRs) but the G proteins which are involved in GPCR proximal signalling are part of heterotrimeric complexes involving a poly functional α subunit and a supporting, sometimes functional, βγ dimer [36–39]. The α and γ subunits have membrane binding domains which serve as lipid anchors in the cell membrane (Fig. 1). When the GPCR is not activated, in the absence of a cognate ligand, the G protein α subunit is bound to GDP and the whole complex of GPCR and GDP bound G α subunit, is quiescent. Upon ligand engagement with the GPCR, the receptor changes conformation leading to changes in the G protein α subunit and exchange of the bound GDP for GTP. The heterotrimeric G protein complex simultaneously dissociates into the GTP-bound α subunit and the independent βγ subunit. The GTP-bound Gα subunit remains anchored to the cell membrane but it is now capable of two-dimensional lateral movement in the membrane, away for the GPCR, where it can play multiple functional roles and continue the signal transduction. Thus, it is this change in conformation, modified and activated the GTP-bound and membrane-anchored G protein which is the effector of integrating and promulgating the original GPCR signal (Fig. 1). In the present context activated GTP-bound Gαq family proteins bind and activate PLC with the well-known distal cascade arising from the hydrolysis of PIP2 in the membrane, leading to activation of two signalling pathways and ultimately changes in gene expression and, to the extent that the gene expression profile defines phenotype, modifications in cellular phenotype. Whereas, in contrast to kinase receptors [40], GPCRs do not possess enzymatic activity, Gα subunits possess GTPase enzymatic activity (see domain analysis in Fig. 2). The GTPase activity of G proteins catalyses the hydrolysis of the GTP to GDP and reverses the activation state of the Gα subunit which can then restore its relationship with an inactive GPCR (Fig. 1). Although a considerable amount of work has led to the description of this elegant biochemical process, there is much which is unknown such as the range of partners that can bind to activated Gαq subunit, the pathways that can be initiated and whether or not there are distinct pathways associated with Gαq and Gαq/11 isoforms. All of this new information will be revealed from studies with YM-254890 and UBO-QIC and their derivatives or surrogates and it is expected that these studies will shed some major light in the area of GPCR signalling. For noting, compound YM-254890 was intermittently available a decade or so ago from Yamaguichi Pharmaceuticals which, following a corporate takeover, subsequently became the property of Astellas Pharmaceuticals. YM-254890 has only recently become available commercially from a generic biotech company. UBO-QIC was made commercially available for a short time a couple of years ago; however, at the present time, it is not available commercially and its only supply is from the publishing source [21].
Fig. 1.
G protein coupled receptor-mediated activation of signal transduction pathway: Membrane-bound GPCR is associated with Gα, Gβ and Gγ proteins where Gα protein is bound with GDP which is functionally inactive form. In the presence of extracellular ligand, GDP is replaced by GTP and both Gα and Gβγ protein heterodimer relocate along the membrane to associate with the specific ion channels and activate intracellular signalling pathways. In the presence of modulator, the Gα activates the GTPase activity and hydrolyses the GTP and the pocket is occupied by the GDP which is coupled with the release of ligand from GPCR
Fig. 2.
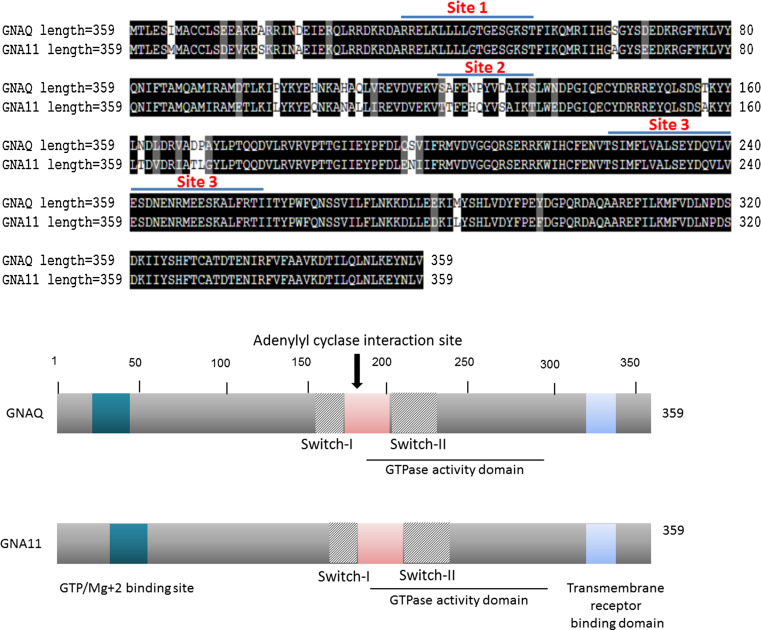
Gαq (GNAQ) and Gαq/11(GNA11) share very high sequence homology (upper panel) and functionally active domain structures (lower panel). Human Gαq and Gαq/11 proteins were compared to find sequence homology. The dark shed region represents the highest homology. Sequences with absolutely no similarity are not shaded and AA belongs to the same group are marked with light shade. The GTP-binding domain (Site 1), adenylyl cyclase interaction site (Site 2) and transmembrane receptor binding site (Site 3) are very conserved. AA sequence of both proteins were analysed by NCBI CDD (conserved domain database) to find out functionally homologous domain. Diagram of both proteins were generated by arbitrarily marking the possible functionally active sites (lower panel). Both proteins have GTP/Mg+2 ion binding site, GTPase activity domain and transmembrane domain at the identical location
Functional analysis of Gαq and Gαq/11
Insofar as the functions of Gαq and Gαq/11 are concerned, having identical numbers of amino acids and a very high amino acid (AA) sequence homology would suggest that the proteins have similar if not identical functions. However, consideration of AA sequence and analysis of the functional domain homology indicated that, besides their known role as activators of PLC, they might actually have additional and distinct biochemical functions. The primary structures of Gαq and Gαq/11 are comprised of several α-helices and β-strands. Conserved domain database analysis located a GTP/Mg2 + binding site, around the first 50 AA, close to the N-terminal end. Besides this GTP/Mg2+ binding site, both proteins have a conserved adenylyl cyclase interaction site, which is located almost at the central part of the protein and a C-terminal end transmembrane receptor binding site (Fig. 2, upper panel). The adenylyl cyclase site on Gαq is located close to 180AA from the N-terminal end of both proteins and has around 60% sequence homology with the cognate site in the much larger Gαs protein (1024AA) which is traditionally associated with adenylate cyclase signalling [41]. Structural analysis also indicated a switch region, which is located almost at the central part of the proteins and this region is responsible for changing the conformation of these proteins depending upon their binding to GTP or GDP (Fig. 2, lower panel). The inherent GTPase activity of Gαq and Gαq/11 has been established almost a decade ago; however, the domain, which is responsible for this activity, is not very well defined. Our conserved functional domain search indicated that the GTPase activity domain of Gαq is similar to both the RAS as well as ARF-like superfamily of small GTPases, which includes Ras, Rho, Rab, and Sar1/Arf families of proteins. The domain is located between 178-282AA where several β-strands are surrounded by an α-helix which is the predicted structure of the enzymatic activity. On the other hand, in the case of Gαq/11 the predicted site, associated with the GTPase activity, is located between 184-278AA and it is more similar to the ARF-like but not the RAS-like superfamily family of small GTPases. Therefore, it can be speculated that these two proteins are perhaps linked to different signal transduction pathways to activate specific intracellular signalling cascades. Analysis of a human gene database also showed that both proteins are linked to the distinct signal transduction pathways. For example, Gαq is associated with thyroid stimulating hormone and serotonin receptor 2 and ELK-SRF/GATA4 signalling pathway where as Gαq/11 is not.
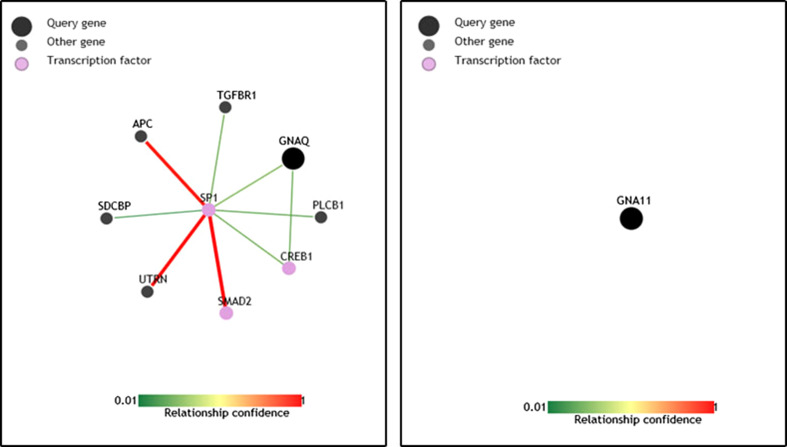
We used pathway analysis (an online network analysis programme) to identify the biological network they are involved. In this analysis, Gαq is well connected to the transcription factor SP1, SP1-SMAD2 and SP1-Activate Protein C driven signalling pathways. Surprisingly, our analysis did not identify any network where Gαq/11 is involved (Fig. 3). As mentioned above, the limited availability to specific tools (pharmacological inhibitors) to address the possibility that Gαq and Gαq/11 having specific functions it is only speculation that Gαq and Gαq/11 might have different functions but there is sufficient information to make this a proposal worthy of further investigation.
Fig. 3.
Pathway analysis for Gαq and Gαq/11. Both Gαq and Gαq/11 proteins were placed separately in PathwayNet analysis programme (an online network analysis programme developed by Princeton University) to identify the molecular network they belong to based on the existing information. No network association was identified so far where Gαq/11 is involved. These figures are reproduced from our analysis in PathwayNet
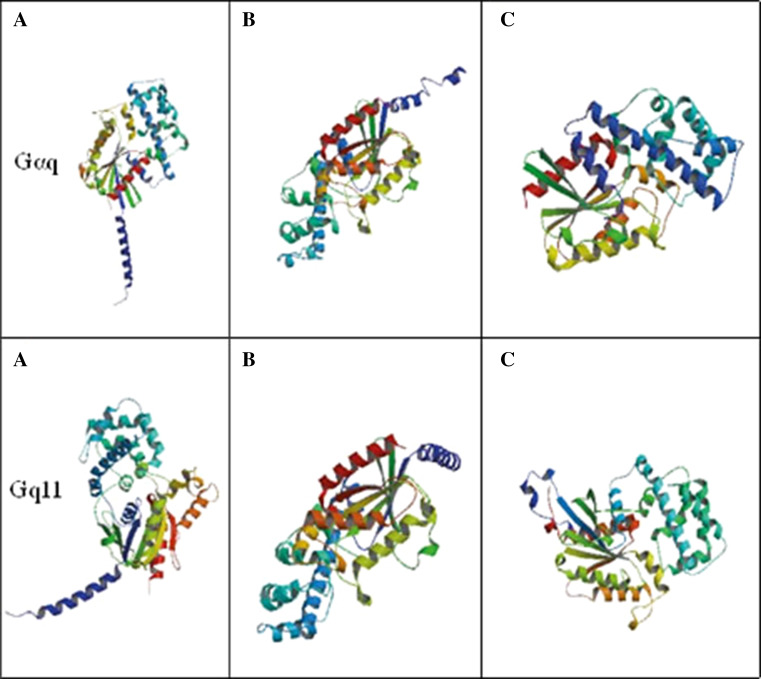
The speculation, that Gαq and Gαq/11 might have distinct functional roles, is also supported by an examination of the ribbon structures of the two proteins (Fig. 4). Ribbon diagrams are three-dimensional representations of protein structure as described above in terms of α-helices shown as coiled ribbons or β-strands shown as thick tubes. Ribbon diagrams show a visualisation of the twists, folds and unfolded areas of proteins and in the current context, they are used to show that the two isoforms of Gαq proteins, Gαq and Gαq/11 actually have discernibly different three-dimensional structures. For example, when we compared Fig. 4a of both proteins, we found a group of β-sheet is surrounded by two α-helixes in Gαq. On the other hand, those β-sheets are closer to one of the α-helixes in Gαq/11. Thus, the derived ribbon structures show a visual depiction of the structural features described above and clearly show differences empirically capable of yielding functional differences between Gαq and Gαq/11.
Fig. 4.
3D protein structure and functional domain analysis of Gαq (GNAQ) and Gαq/11(GNA11). The 3D ribbon structures of both proteins were derived based on ModBase, a database of comparative protein structure model analysis. A few of the all possible models with similar alignment were picked up to compare the 3D structure. The protein structures are reproduced from ModBase [69]
Tissue distribution of Gαq and Gαq/11
Tissue-specific gene (and protein) expression studies are very important to understand the biology of any target peptide or protein, and they are also useful for drug development and biomarker discovery. Several new databases were developed recently generated to correlate gene expression and a variety of human diseases [42, 43]. We accessed one of such databases (Gene Cards® Human Gene Database) to investigate the tissue-specific distributions of Gαq and Gαq/11. Both Gαq and Gαq/11 proteins are expressed in the majority of the human tissues and organs such as heart, smooth muscle, neurons, brain, pancreas, thyroid, colon and uterus (Fig. 5). Interestingly, expression was not observed in a few organs such as breast, stomach and spleen indicating that these two proteins may have common as well as other functions specific to particular tissues. The observed similar organ and tissue-specific expression patterns also suggest that expression of both genes are regulated by a common upstream regulatory mechanism, which is, absent or inactivated in tissues or organs where their expression is restricted. However, in the absence of functional data it is not possible to speculate further on the role of these two isoforms in all the tissues.
Fig. 5.
Gαq family proteins—tissue distribution. Tissue distribution of Gαq and Gαq/11 is very similar. The tissue distribution profiles of both proteins across the major human tissues were adopted from the public data base Gene Cards [70]
Possible Gαq pathways other than through PLC
We have recently discovered that the GPCR, the thrombin receptor, PAR-1, which was known to transactivate the protein tyrosine kinase receptor, epidermal growth factor receptor (EGFR) can also transactivate the protein serine/threonine kinase TGFβR1 [32, 33, 44]. Detailed investigations of the distal components of these signalling pathways and comparison with those reported for the GPCR transactivation of EGFR show that the distal mechanisms are completely distinct. For example, the PAR-1 transactivation of EGFR involves matrix metalloproteinases (MMPs) and the cell surface cleavage of a cognate ligand whereas the transactivation of TGFβR1 does not involve MMPs [44].
In the cardiovascular studies in our laboratory, the functional read-out is the process of thrombin-stimulated elongation of the chondroitin/dermatan sulphate GAG chains on the proteoglycan biglycan secreted by vascular smooth muscle cells (VSMC), as it relates to enhanced lipid binding which is associated with the early step in atherosclerosis [45–47]. GAG hyperelongation has been investigated as changes in the apparent size of the GAG chains and more recently we studied the regulation of gene expression for the enzymes which are rate limiting for the hyperelongation of GAG chains on biglycan [33, 44]. Our data indicate that PLC and its downstream pathways are not responsible for GAG hyperelongation. Thus, mimicking the increase in intracellular calcium with the calcium ionophore, ionomycin, does not stimulate GAG elongation [35]. Calcium channel blockers partially block growth factor-stimulated GAG hyperelongation but the mechanism of inhibition does not depend solely upon the blocking of calcium channels [35]. TGF-β, which is a very efficacious stimulator of GAG hyperelongation has no effect on cellular IP3 levels [48]. When VSMCs were transfected with angiotensin (AII) Type 1 receptors there was a huge increase in AII stimulated IP3 production but no effect on proteoglycan synthesis (Little, Thomas and Ivey, unpublished observations). In accordance with these unpublished findings, Yang et al. [49] studying Gαq coupled glutamate receptor (mGluR) responses in cultured neurones and found that the stimulation of the phosphorylation of the mitogen-activated protein (MAP) kinase JNK, occurred via transactivation of the EGFR and did not involve the traditional PLC, PKC and IP3 pathways. These results suggested that the PLC pathway and the pathway to the transactivation of the EGFR are occurring in parallel and therefore implying that there is an unknown mechanism which links the role of the Gαq to the transactivation of EGFR [49].
As previously mentioned, our conserved functional domain search indicated that the GTPase activity domain of Gαq is similar to both the RAS as well as ARF like superfamily of small GTPases, which includes Ras, Rho, Rab, and Sar1/Arf families of proteins whereas for Gαq/11 the GTP/GDP binding site is more similar to the ARF-like but not the RAS-like superfamily family of small GTPases. This information provides support for the existence of Gαq/Gαq/11 signalling pathways distinct from the PLC pathways although these pathways have been very little explored. New roles are merging for the β-arrestin molecule other than its action of heterotrimeric G protein desensitisation and the internalisation of GPCRs. Data reveal that β-arrestin molecules can act as signal transducers of Erk activation independent of G proteins [50, 51]. The increase in expression of β-arrestin1 allows for β-arrestin2 to regulate Erk activity in HEK cells expressing angiotensin type II receptor in [52, 53]. However, overexpression of β-arrestin1 leads to activation of small GTPase, RhoA [52]. Thus β-arrestin signalling can activate Gαq mediated pathways independent of PLC.
Taken together, these data suggest that multiple pathways including the process of growth factor-stimulated GAG hyperelongation do not involve PLC and its downstream pathways. As responses through GPCRs must, by definition, involve G proteins, if PAR-1-mediated GAG hyperelongation includes a G protein, presumably a Gαq family G protein, but that does not involve activation of PLC, then this would possibly be an alternative pathway(s) of PAR-1 signalling. Thus, as most signal transduction pathways involve protein:protein interactions, it would be interesting to investigate the potential signal transduction binding partners for the conformationally modified, activated, GTP bound and membrane anchored G protein which arises after the stimulation of PAR-1 in VSMCs.
Pharmacology of Gαq inhibitors
UBO-QIC/FR300359
The plant derived compound, FR300359, also known as UBO-QIC, is like the better known as YM-254890, a cyclic depsipeptide [21, 25]. UBO-QIC is isolated from the Ardisia crenata sims plant [54], a plant of the primrose family with multiple common names including Christmas berry and Australian holly and it is native to East Asia but an invasive species in the south-eastern United States and elsewhere.
UBO-QIC inhibits platelet aggregation in rabbits and causes dose-related hypotension in anaesthetised normotensive rodents which is consistent with the observed effect on blood pressure in Gαq knock down mice [55, 56]. The blood pressure lowering effect was attributed to the ability of UBO-QIC to partially mediate nitric oxide release from endothelial cells and inhibit calcium migration caused by voltage-dependent and receptor operated channels [57]. In HEK cells transfected with Transient receptor potential cation channel subfamily V member 4 (TRPV4), PAR-2 or G protein-coupled receptor 11, mediated intracellular calcium release was abolished by UBO-QIC when compared to control; however, extracellular calcium influx through the TRPV4 ion channel was unaffected thus showing that PAR-2 coupling to TRPV4 is not mediated by Gαq signalling [30]. Neither of the above studies assessed the level of expression of Gαq and Gαq/11 nor were there other studies that addressed the specificity of action of UBO-QIC towards these isoforms.
Kostenis and her colleagues [21] have recently reported a major study on the characterisation of UBO-QIC. To understand G protein specificity of UBO-QIC, they used an experimental approach based on bioluminescence resonance energy transfer (BRET) that relies on co-transfection of GPCRs with a panel of Gα subunit sensors. These sensor based studies were proven to be a valuable tool to monitor the activation associated conformational changes within G proteins upon stimulation of GPCR. Upon pre-treatment with UBO-QIC (FR3 00359), it was observed that agonist BRET responses of Gαq- and Gα11-containing heterotrimers were completely prevented but those of all other Gα isoforms were not affected [21, 58–61]. Inhibition of Gαq, Gα11 and Gα14 signalling by UBO-QIC was observed at micromolar concentrations whereas, signalling via other Gαq family member, Gα16 was completely unaffected in the presence of excess UBO-QIC. Thus, their results clearly showed that UBO-QIC is selective for the inhibition of Gαq, Gα11 and Gα14. By using CRISPR-Cas9-mediated genome editing and rescue experiments, these authors [21] showed unambiguously that UBO-QIC selectively blocks the activity of Gαq and Gαq/11. This work was taken further in exploring the therapeutic potential of targeting G proteins in a model of oncogenesis involving the malignancy of melanoma cells. UBO-QIC blocked melanoma cell proliferation and migration and triggered differentiation [21] underpinning the need for further proof of concept studies on the targeting of G proteins, particularly, Gαq family of proteins in disease models.
Very recently, Jacobson et al. [62] reported the effect of UBO-QIC on other subunits of G proteins, such as βγ subunits. Their studies showed that at lower concentration, UBO-QIC inhibited multiple examples of known Gαq signalling pathways as well as other non-Gαq-events such as A1 adenosine and M2 muscarinic acetylcholine production, indicating that its effect is not limited to Gαq. UBO-QIC completely inhibited signalling responses activated by Gq-coupled P2Y1 receptor and Gi-coupled A1A or P2Y12 receptor. UBO-QIC completely blocked Gq mediated Akt activity and only partially inhibited Gi mediated activity. This shows that the full selectively spectrum of UBO-QIC is still unknown and thus the need for a specific Gαq antagonist.
YM-254890 and related cyclic depsipeptides
The compound known as YM-254890, a cyclic depsipeptide isolated from the bacteria Chromobacterium sp. QS3666, has been used as a Gαq family inhibitor. YM-254890 inhibits the signal transduction of Gαq by inhibiting the exchange of GDP for GTP preventing the activation of the G protein, rather than GPCR-Gαq interactions [63]. When bound to GDP, the non-polar side chains of YM-254890 form hydrogen bonds with the Switch I region of Gαq, however, this is a conformation that cannot be maintained when bound with GTP [64]. At a molecular level YM derivatives appear to inhibit Gαq by binding to a pocket in the Gαq that stabilises the protein in an inactive conformation which prevents the activation of the Gαq by a cognate GPCR [65].
YM-254890 inhibits platelet aggregation induced by ADP, a response that is mediated via GPCRs, P2Y1 and P2Y12 receptors [29]. YM-254890 has no effect on a specific P2Y12 signal transduction pathway which is conventionally associated with the Gi signalling pathways, indicating that the compound has some specificity for Gαq. It was also shown that YM-254890 inhibited Gαq-coupled GPCR signalling by inhibiting calcium mobilisation in P2Y1-expressing C6-15 cells but not cAMP accumulation which is coupled to the P2Y12 receptor-mediated signal transduction pathway [63]. Aside from antiplatelet activity, by electrically inducing carotid artery thrombosis in rodents, YM-254890 was also shown to have antithrombotic and thrombolytic effects [28].
YM-254890 was discovered and developed by Yamaguichi Pharmaceuticals Japan; Yamaguichi was subsequently acquired by Astellas Pharmaceuticals, Japan. This compound was made available to researchers 10 years ago for a short period of time and a small number of interesting studies were published. The initial results indicated that YM-254890 is a useful tool for investigating Gαq/11-coupled receptor signalling and the physiological roles of Gαq/11. Gαq knockout mice have blood pressure lower than the control [56]. Therefore, it is possible that high blood pressure in patients can be controlled by modulating the activity of Gαq, This indicates the potential of YM-254890 as a Gαq inhibitor to be an anti-hypertensive agent. There are very limited data available on the selectivity of YM-254890 on other G protein subunits; thus, more studies are needed to profile this inhibitor.
The peptide antagonist GP-2A
In 2004, Tanski et al. [66] discovered a competitive Gαq inhibitor, G Protein antagonist- 2A, also known as GP-2A. GP-2A is a peptide that selectively inhibits the action of Gαq by M1 muscarinic cholinergic receptors. GP-2A, as presented here, should be distinguished from the small chemical entity known as GP-2A which is a pyrazole derivative and a selective cannabinoid receptor CB2 agonist [67]. The effect of GP-2A on the signalling pathway of Gαq and its role in cell proliferation with rat pulmonary artery smooth muscle cells have been investigated. Angiotensin II-mediated proliferation, PLCβ activation and Erk1/2 phosphorylation were inhibited by more than 50% in the presence of GP-2A [66]. The EGFR can be activated by EGF to generate an intracellular signalling pathway leading to the phosphorylation of several downstream effector proteins such as Erk1/2 [68]. Tanski et al. have evaluated angiotensin II, as a specific Gαq agonist, to effectively reduce Erk1/2 activation mediated by PLCβ via Gαq in the presence of GP-2A indicating its association with the phosphorylation of Erk1/2 in rat pulmonary artery smooth muscle cells [66]. GP-2A is commercially available; so further studies on its peptide and potentially other derivatives might provide more information on the role of Gαq family members in diverse biological processes and diseases.
Future medicinal chemistry and pharmacological developments
Gαq family of proteins has not been sufficiently studied to understand fully their role in cell signalling and cell biology. Numerous groups have been involved in the synthesis of the depsipeptides YM-254890 and UBO-QIC/FR300359, however limited data is available on the actions of these peptides. Our effort to synthesise YM-254890 produced structurally most of the molecule but this compound did not inhibit thrombin-stimulated increase of intracellular calcium, which is a response most likely to be mediated by Gαq family proteins (Little and Brimble, unpublished data). The remaining residues between the molecules we reported and YM-254890 must provide the inhibitory interaction with the Gαq protein. Other groups have taken alternative pathways to synthesise simplified form of YM-254890 derivatives [27] but the resultant molecules are at least tenfold less potent than YM-254890.
We have recently been able to successfully perform siRNA-(GE Dharmacon M-010960-02-0005 and M-008562-00-0005) based selective knock down of Gαq and Gαq/11 VSMCs (Little, Osman and Kamato, unpublished observations). This result will allow us to look for Gαq- and Gαq/11-specific responses. We are currently speculating, based on our preliminary data, that in some systems or some cells, UBO-QIC/FR300359 and YM-254890 might behave as Gαq and Gαq/11 selective inhibitors, respectively, or that YM-254890 is a selective Gαq/11 inhibitor, but these speculations obviously require further experimental data. These speculations also relate to the medicinal chemistry in that the substituents absent from our recently reported molecule might not only provide the potent inhibitory activity towards Gq family proteins but also be a selective inhibitor of Gαq or Gαq/11.
Finally, due to the very high homology in AA sequences, the molecular approaches are undoubtedly required to define the role of Gαq and Gαq/11 isoforms in particular to the cellular responses. However, molecular approaches in this area, for example G protein knock down, can lead to rebound increases in other G proteins with unexpected results [13]. Ultimately the combined approaches of molecular interventions with pharmacological tools will be required to define the role of Gαq family of proteins in cell biology and this approach would potentially identify a pathophysiological role and their specific inhibitors.
Conclusions
The two Gαq family proteins, Gαq and Gαq/11, warrant intense investigation for their ability to provide extremely useful information on the understanding of the functioning of GPCRs and also as potential therapeutic targets in their own right. Although such as all-encompassing target may intuitively be too broad to provide the desired specificity in a therapeutic target and be limited by off-target effects, the outcome is simply not clear until the target is evaluated in several diseases. Furthermore, in a conceptual sense, targets may often be activated or hyperactivated in a particular disease state and it is this state that elevates the target to a therapeutic target over and above the role in normal physiology.
G proteins are most likely at the centre of the phenomena of biased signalling which has enormous potential in explaining and exploiting the therapeutic potential of many GPCRs. We consider it is likely that biased signalling arises, at least in one form, from the ability of different GPCR biased ligands to “flip” the proximal G protein partner from one G protein to another, for example from Gαq to Gαs and thus elicit a qualitatively different response. However, how far does such a paradigm extend? What of Gαq and Gαq/11—can a biased agonist flip the proximal signalling partner from one Gαq isoform to another with different effects on phenotype or is this the limit of the paradigm of biased signalling? These questions will best be answered with the broad availability of the pharmacological tools specially YM-254890 and UBO-QIC and their derivatives. When more confirmatory information is available on the potency and the (Gαq versus Gαq/11) specificity of these important agents, then it can be established with the two depsipeptides coupled with siRNA studies whether or not the two isoforms are involved in biased signalling and indeed if there are independent signalling pathways arising from each of these two G proteins.
In summary, an advanced understanding of the role of Gαq and Gαq/11 in GPCR signalling will provide an enormous addition to our understanding of cell biology and potentially of cellular physiology and pathophysiology, and may lead to the discovery and development of new therapeutic agents capable of modulating diseases process in years that have not yet been envisaged.
References
- 1.Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf). 2007;190(1):9–19. doi: 10.1111/j.1365-201X.2007.01693.x. [DOI] [PubMed] [Google Scholar]
- 2.Lodowski DT, Angel TE, Palczewski K. Comparative analysis of GPCR crystal structures. Photochem Photobiol. 2009;85(2):425–430. doi: 10.1111/j.1751-1097.2008.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crouch MF, Osmond RI. New strategies in drug discovery for GPCRs: high throughput detection of cellular ERK phosphorylation. Comb Chem High Throughput Screen. 2008;11(5):344–356. doi: 10.2174/138620708784534806. [DOI] [PubMed] [Google Scholar]
- 4.Kamato D, Rostam MA, Bernard R, Piva TJ, Mantri N, Guidone D, et al. The expansion of GPCR transactivation-dependent signalling to include serine/threonine kinase receptors represents a new cell signalling frontier. Cell Mol Life Sci. 2015;72(4):799–808. doi: 10.1007/s00018-014-1775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao P, Metcalf M, Bunnett NW. Biased signaling of protease-activated receptors. Front Endocrinol. 2014;5:67. doi: 10.3389/fendo.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulon S, Cande C, Bunnett NW, Hollenberg MD, Chignard M, Pidard D. Proteinase-activated receptor-2 and human lung epithelial cells: disarming by neutrophil serine proteinases. Am J Respir Cell Mol Biol. 2003;28(3):339–346. doi: 10.1165/rcmb.4908. [DOI] [PubMed] [Google Scholar]
- 7.Burch ML, Osman N, Getachew R, Al-Aryahi S, Poronnik P, Zheng W, et al. G protein coupled receptor transactivation: extending the paradigm to include serine/threonine kinase receptors. Int J Biochem Cell Biol. 2012;44(5):722–727. doi: 10.1016/j.biocel.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Little PJ. GPCR responses in vascular smooth muscle can occur predominantly through dual transactivation of kinase receptors and not classical Galphaq protein signalling pathways. Life Sci. 2013;92(20–21):951–956. doi: 10.1016/j.lfs.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Little PJ, Hollenberg MD, Kamato D, Thomas W, Chen J, Wang T, et al. Integrating the GPCR transactivation-dependent and biased signalling paradigms in the context of PAR-1 signalling. Br J Pharmacol. 2016;173(20):2992–3000. doi: 10.1111/bph.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christopoulos A. Advances in G protein-coupled receptor allostery: from function to structure. Mol Pharmacol. 2014;86(5):463–478. doi: 10.1124/mol.114.094342. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson KA. New paradigms in GPCR drug discovery. Biochem Pharmacol. 2015;98(4):541–555. doi: 10.1016/j.bcp.2015.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390(6655):88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 13.Krumins AM, Gilman AG. Targeted knockdown of G protein subunits selectively prevents receptor-mediated modulation of effectors and reveals complex changes in non-targeted signaling proteins. J Biol Chem. 2006;281(15):10250–10262. doi: 10.1074/jbc.M511551200. [DOI] [PubMed] [Google Scholar]
- 14.Rodbell M. The role of GTP-binding proteins in signal transduction: from the sublimely simple to the conceptually complex. Curr Top Cell Regul. 1992;32:1–47. doi: 10.1016/B978-0-12-152832-4.50003-3. [DOI] [PubMed] [Google Scholar]
- 15.Hurowitz EH, Melnyk JM, Chen YJ, Kouros-Mehr H, Simon MI, Shizuya H. Genomic characterization of the human heterotrimeric G protein alpha, beta, and gamma subunit genes. DNA Res. 2000;7(2):111–120. doi: 10.1093/dnares/7.2.111. [DOI] [PubMed] [Google Scholar]
- 16.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296(5573):1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 17.Strathmann M, Simon MI. G protein diversity: a distinct class of alpha subunits is present in vertebrates and invertebrates. Proc Natl Acad Sci USA. 1990;87(23):9113–9117. doi: 10.1073/pnas.87.23.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkie TM, Scherle PA, Strathmann MP, Slepak VZ, Simon MI. Characterization of G-protein alpha subunits in the Gq class: expression in murine tissues and in stromal and hematopoietic cell lines. Proc Natl Acad Sci USA. 1991;88(22):10049–10053. doi: 10.1073/pnas.88.22.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizuno N, Itoh H. Functions and regulatory mechanisms of Gq-signaling pathways. Neurosignals. 2009;17(1):42–54. doi: 10.1159/000186689. [DOI] [PubMed] [Google Scholar]
- 20.Kostenis E, Waelbroeck M, Milligan G. Techniques: promiscuous Galpha proteins in basic research and drug discovery. Trends Pharmacol Sci. 2005;26(11):595–602. doi: 10.1016/j.tips.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Schrage R, Schmitz AL, Gaffal E, Annala S, Kehraus S, Wenzel D, et al. The experimental power of FR900359 to study Gq-regulated biological processes. Nat Commun. 2015;6:10156. doi: 10.1038/ncomms10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118(Pt 16):3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 23.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19(1):128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S, et al. Transforming growth factor-beta signalling: role and consequences of Smad linker region phosphorylation. Cell Signal. 2013;25(10):2017–2024. doi: 10.1016/j.cellsig.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Kamato D, Thach L, Bernard R, Chan V, Zheng W, Kaur H, et al. Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Galpha/q,11. Front Cardiovasc Med. 2015;2:14. doi: 10.3389/fcvm.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pahlavan S, Oberhofer M, Sauer B, Ruppenthal S, Tian Q, Scholz A, et al. Galphaq and Galpha11 contribute to the maintenance of cellular electrophysiology and Ca2+ handling in ventricular cardiomyocytes. Cardiovasc Res. 2012;95(1):48–58. doi: 10.1093/cvr/cvs162. [DOI] [PubMed] [Google Scholar]
- 27.Rensing DT, Uppal S, Blumer KJ, Moeller KD. Toward the selective inhibition of G proteins: total synthesis of a simplified YM-254890 analog. Org Lett. 2015;17(9):2270–2273. doi: 10.1021/acs.orglett.5b00944. [DOI] [PubMed] [Google Scholar]
- 28.Kawasaki T, Taniguchi M, Moritani Y, Hayashi K, Saito T, Takasaki J, et al. Antithrombotic and thrombolytic efficacy of YM-254890, a G q/11 inhibitor, in a rat model of arterial thrombosis. Thromb Haemost. 2003;90(3):406–413. doi: 10.1160/TH03-02-0115. [DOI] [PubMed] [Google Scholar]
- 29.Taniguchi M, Nagai K, Arao N, Kawasaki T, Saito T, Moritani Y, et al. YM-254890, a novel platelet aggregation inhibitor produced by Chromobacterium sp. QS3666. J Antibiot (Tokyo) 2003;56(4):358–363. doi: 10.7164/antibiotics.56.358. [DOI] [PubMed] [Google Scholar]
- 30.Grace MS, Lieu T, Darby B, Abogadie FC, Veldhuis N, Bunnett NW, et al. The tyrosine kinase inhibitor bafetinib inhibits PAR2-induced activation of TRPV4 channels in vitro and pain in vivo. Br J Pharmacol. 2014;171(16):3881–3894. doi: 10.1111/bph.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaur H, Harris PW, Little PJ, Brimble MA. Total synthesis of the cyclic depsipeptide YM-280193, a platelet aggregation inhibitor. Org Lett. 2015;17(3):492–495. doi: 10.1021/ol503507g. [DOI] [PubMed] [Google Scholar]
- 32.Burch ML, Ballinger ML, Yang SN, Getachew R, Itman C, Loveland K, et al. Thrombin stimulation of proteoglycan synthesis in vascular smooth muscle is mediated by protease-activated receptor-1 transactivation of the transforming growth factor beta type I receptor. J Biol Chem. 2010;285(35):26798–26805. doi: 10.1074/jbc.M109.092767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burch ML, Getachew R, Osman N, Febbraio MA, Little PJ. Thrombin-mediated proteoglycan synthesis utilizes both protein-tyrosine kinase and serine/threonine kinase receptor transactivation in vascular smooth muscle cells. J Biol Chem. 2013;288(10):7410–7419. doi: 10.1074/jbc.M112.400259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little PJ, Burch ML, Getachew R, Al-aryahi S, Osman N. Endothelin-1 stimulation of proteoglycan synthesis in vascular smooth muscle is mediated by endothelin receptor transactivation of the transforming growth factor-[beta] type I receptor. J Cardiovasc Pharmacol. 2010;56(4):360–368. doi: 10.1097/FJC.0b013e3181ee6811. [DOI] [PubMed] [Google Scholar]
- 35.Survase S, Ivey ME, Nigro J, Osman N, Little PJ. Actions of calcium channel blockers on vascular proteoglycan synthesis: relationship to atherosclerosis. Vasc Health Risk Manag. 2005;1(3):199–208. [PMC free article] [PubMed] [Google Scholar]
- 36.Hall A. The cellular functions of small GTP-binding proteins. Science. 1990;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- 37.Hein P, Bunemann M. Coupling mode of receptors and G proteins. Naunyn Schmiedebergs Arch Pharmacol. 2009;379(5):435–443. doi: 10.1007/s00210-008-0383-7. [DOI] [PubMed] [Google Scholar]
- 38.Hendriks-Balk MC, Peters SL, Michel MC, Alewijnse AE. Regulation of G protein-coupled receptor signalling: focus on the cardiovascular system and regulator of G protein signalling proteins. Eur J Pharmacol. 2008;585(2–3):278–291. doi: 10.1016/j.ejphar.2008.02.088. [DOI] [PubMed] [Google Scholar]
- 39.Hubbard KB, Hepler JR. Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell Signal. 2006;18(2):135–150. doi: 10.1016/j.cellsig.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 41.Lefkowitz RJ, Caron MG, Michel T, Stadel JM. Mechanisms of hormone receptor-effector coupling: the beta-adrenergic receptor and adenylate cyclase. Fed Proc. 1982;41(10):2664–2670. [PubMed] [Google Scholar]
- 42.Liu X, Yu X, Zack DJ, Zhu H, Qian J. TiGER: a database for tissue-specific gene expression and regulation. BMC Bioinform. 2008;9:271. doi: 10.1186/1471-2105-9-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X, Ye Y, Wang G, Huang H, Yu D, Liang S. VeryGene: linking tissue-specific genes to diseases, drugs, and beyond for knowledge discovery. Physiol Genom. 2011;43(8):457–460. doi: 10.1152/physiolgenomics.00178.2010. [DOI] [PubMed] [Google Scholar]
- 44.Kamato D, Thach L, Getachew R, Burch M, Hollenberg MD, Zheng W, et al. Protease activated receptor-1 mediated dual kinase receptor transactivation stimulates the expression of glycosaminoglycan synthesizing genes. Cell Signal. 2016;28(1):110–119. doi: 10.1016/j.cellsig.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Little PJ, Chait A, Bobik A. Cellular and cytokine-based inflammatory processes as novel therapeutic targets for the prevention and treatment of atherosclerosis. Pharmacol Ther. 2011;131(3):255–268. doi: 10.1016/j.pharmthera.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Little PJ, Osman N, O’Brien KD. Hyperelongated biglycan: the surreptitious initiator of atherosclerosis. Curr Opin Lipidol. 2008;19:448–454. doi: 10.1097/MOL.0b013e32830dd7c4. [DOI] [PubMed] [Google Scholar]
- 47.Nigro J, Osman N, Dart AM, Little PJ. Insulin resistance and atherosclerosis. Endocr Rev. 2006;27(3):242–259. doi: 10.1210/er.2005-0007. [DOI] [PubMed] [Google Scholar]
- 48.Burch ML, Yang SN, Ballinger ML, Getachew R, Osman N, Little PJ. TGF-b stimulates biglycan synthesis via p38 and ERK phosphorylation of the linker region of Smad 2. Cell Mol Life Sci. 2010;67:2077–2090. doi: 10.1007/s00018-010-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang L, Mao L, Chen H, Catavsan M, Kozinn J, Arora A, et al. A signaling mechanism from G alpha q-protein-coupled metabotropic glutamate receptors to gene expression: role of the c-Jun N-terminal kinase pathway. J Neurosci. 2006;26(3):971–980. doi: 10.1523/JNEUROSCI.4423-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, et al. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100(19):10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, et al. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281(2):1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 52.Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem. 2005;280(9):8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- 53.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279(34):35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 54.Miyamae S. Influence of magnesium and extracellular calcium reduction on ouabain-treated sinoatrial node cells in rabbit heart. Pharmacol Toxicol. 1989;65(3):192–197. doi: 10.1111/j.1600-0773.1989.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 55.Fujioka M, Koda S, Morimoto Y, Biemann K. Structure of Fr900359, a cyclic depsipeptide from Ardisia-Crenata sims. J Org Chem. 1988;53(12):2820–2825. doi: 10.1021/jo00247a030. [DOI] [Google Scholar]
- 56.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14(1):64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 57.Zaima K, Deguchi J, Matsuno Y, Kaneda T, Hirasawa Y, Morita H. Vasorelaxant effect of FR900359 from Ardisia crenata on rat aortic artery. J Nat Med. 2013;67(1):196–201. doi: 10.1007/s11418-012-0644-0. [DOI] [PubMed] [Google Scholar]
- 58.Kukkonen JP. G-protein inhibition profile of the reported Gq/11 inhibitor UBO-QIC. Biochem Biophys Res Commun. 2016;469(1):101–107. doi: 10.1016/j.bbrc.2015.11.078. [DOI] [PubMed] [Google Scholar]
- 59.Carr R, 3rd, Koziol-White C, Zhang J, Lam H, An SS, Tall GG, et al. Interdicting Gq activation in airway disease by receptor-dependent and receptor-independent mechanisms. Mol Pharmacol. 2016;89(1):94–104. doi: 10.1124/mol.115.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inamdar V, Patel A, Manne BK, Dangelmaier C, Kunapuli SP. Characterization of UBO-QIC as a Galphaq inhibitor in platelets. Platelets. 2015;26(8):771–778. doi: 10.3109/09537104.2014.998993. [DOI] [PubMed] [Google Scholar]
- 61.Karpinsky-Semper D, Volmar CH, Brothers SP, Slepak VZ. Differential effects of the Gbeta5-RGS7 complex on muscarinic M3 receptor-induced Ca2+ influx and release. Mol Pharmacol. 2014;85(5):758–768. doi: 10.1124/mol.114.091843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao ZG, Jacobson KA. On the selectivity of the Galphaq inhibitor UBO-QIC: a comparison with the Galphai inhibitor pertussis toxin. Biochem Pharmacol. 2016;107:59–66. doi: 10.1016/j.bcp.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takasaki J, Saito T, Taniguchi M, Kawasaki T, Moritani Y, Hayashi K, et al. A novel Galphaq/11-selective inhibitor. J Biol Chem. 2004;279(46):47438–47445. doi: 10.1074/jbc.M408846200. [DOI] [PubMed] [Google Scholar]
- 64.Nishimura A, Kitano K, Takasaki J, Taniguchi M, Mizuno N, Tago K, et al. Structural basis for the specific inhibition of heterotrimeric Gq protein by a small molecule. Proc Natl Acad Sci USA. 2010;107(31):13666–13671. doi: 10.1073/pnas.1003553107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitatsuji C, Kurogochi M, Nishimura S, Ishimori K, Wakasugi K. Molecular basis of guanine nucleotide dissociation inhibitor activity of human neuroglobin by chemical cross-linking and mass spectrometry. J Mol Biol. 2007;368(1):150–160. doi: 10.1016/j.jmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Tanski WJ, Roztocil E, Hemady EA, Williams JA, Davies MG. Role of Galphaq in smooth muscle cell proliferation. J Vasc Surg. 2004;39(3):639–644. doi: 10.1016/j.jvs.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 67.Murineddu G, Lazzari P, Ruiu S, Sanna A, Loriga G, Manca I, et al. Tricyclic pyrazoles. 4. Synthesis and biological evaluation of analogues of the robust and selective CB2 cannabinoid ligand 1-(2′,4′-dichlorophenyl)-6-methyl-N-piperidin-1-yl-1,4-dihydroindeno[1,2-c]pyrazo le-3-carboxamide. J Med Chem. 2006;49(25):7502–7512. doi: 10.1021/jm060920d. [DOI] [PubMed] [Google Scholar]
- 68.Akhtar S, Yousif MH, Chandrasekhar B, Benter IF. Activation of EGFR/ERBB2 via pathways involving ERK1/2, P38 MAPK, AKT and FOXO enhances recovery of diabetic hearts from ischemia-reperfusion injury. PLoS ONE. 2012;7(6):e39066. doi: 10.1371/journal.pone.0039066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pieper U, Webb BM, Dong GQ, Schneidman-Duhovny D, Fan H, Kim SJ, et al. ModBase, a database of annotated comparative protein structure models and associated resources. Nucleic Acids Res. 2014;42:D336–D346. doi: 10.1093/nar/gkt1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R, et al. Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics. 2005;21(5):650–659. doi: 10.1093/bioinformatics/bti042. [DOI] [PubMed] [Google Scholar]