Abstract
Most research on nutritional effects on aging has focussed on the impact of manipulating single dietary factors such as total calorie intake or each of the macronutrients individually. More recent studies using a nutritional geometric approach called the Geometric Framework have facilitated an understanding of how aging is influenced across a landscape of diets that vary orthogonally in macronutrient and total energy content. Such studies have been performed using ad libitum feeding regimes, thus taking into account compensatory feeding responses that are inevitable in a non-constrained environment. Geometric Framework studies on insects and mice have revealed that diets low in protein and high in carbohydrates generate longest lifespans in ad libitum-fed animals while low total energy intake (caloric restriction by dietary dilution) has minimal effect. These conclusions are supported indirectly by observational studies in humans and a heterogeneous group of other types of interventional studies in insects and rodents. Due to compensatory feeding for protein dilution, low-protein, high-carbohydrate diets are often associated with increased food intake and body fat, a phenomenon called protein leverage. This could potentially be mitigated by supplementing these diets with interventions that influence body weight through physical activity and ambient temperature.
Keywords: Aging, Ageing, Caloric restriction, Geometric Framework, CPC diet, Dietary protein, Dietary carbohydrate
Introduction
The effect of diet on age-related health and lifespan is unequivocal; even Hippocrates recognized the link between overconsumption and early death [1]. In the 1500s, a Venetian nobleman, Luigi Cornaro famously proposed in his book, Discorsi della vita sobria that reducing the quantity of food eaten will increase health and lifespan. He self-experimented by reducing his own intake to 12 oz of food (and 14 oz wine) per day, and lived beyond 100 years [1, 2]. The beginning of the modern scientific study of nutrition and aging is often attributed to Clive McCay and colleagues who in 1935 reported that reducing the amount of food provided to rats in order to delay their growth led to an increased lifespan: “…individuals of both sexes attained extreme ages beyond those of either sex that grew normally” [3]. Of note, the diets McCay used in these early experiments were relatively high in protein and low in carbohydrates (casein 40 %, yeast 5 % vs starch 22 %, sucrose 10 %) [3].
Since that time, a reduction of food intake, known as caloric restriction, has become an established model for the study of aging, and is considered to be a robust and reproducible intervention for delaying aging and increasing lifespan. In most caloric restriction studies, access to food is reduced by 10–50 % of ad libitum intake and supplemented with micronutrients to prevent dietary deficiencies. The increase in maximum and/or median lifespan with caloric restriction has been reported across taxa ranging from yeast, worms, flies, mice and rats; with health and/or lifespan benefits reported in primates including humans [4–10]. Research into caloric restriction in animals has led to major advances in the understanding of the nutrient-sensing pathways that link diet and aging, including the sirtuin (SIRT), mechanistic target of rapamycin (mTOR), 5′ adenosine monophosphate-activated protein kinase (AMPK), insulin/insulin-like growth factor-1 (IGF-1)/Growth Hormone pathways and possibly fibroblast growth factor 21 (FGF21) [11, 12]. Remarkably, genetic and pharmacological manipulations of nutrient-sensing pathways are associated with delayed or accelerated aging in animal models [6, 13]. However, diet is a complex issue, and it has been debated whether the lifespan benefit of the caloric restriction intervention is secondary to a reduction in calories; or a reduction of one the macronutrients (protein, carbohydrates or fat); or the associated periodic deprivation and hunger that occurs once an animal has eaten its aliquot of food [14]. In an attempt to resolve these issues there have been investigations of dietary interventions such as reduced amounts of each of the macronutrients, and every-other-day feeding [15].
One of the major confounding factors in dietary studies of aging is compensatory feeding, where animals titrate food intake in order to meet endogenous targets for energy, macronutrients and micronutrients. Overall it has been found that dietary protein has the strongest impact on food intake, such that low-protein diets will lead to an increase in food intake and vice versa—this has been termed ‘protein leverage’ [16, 17]. Therefore in ad libitum-fed animals, a low-protein diet will also be a high-calorie diet, while a high-protein diet will also be a low-calorie diet. Meticulous evaluation of food intake is an essential first step in order to tease out the differential effects of calories versus each of the macronutrients [18]. The alternative approach, widely used in caloric restriction studies, is to provide animals with a reduced amount of food (on the assumption that it is all consumed) but this cannot differentiate between the effects of reduced calories and reduced macronutrients nor the effects of periodic food deprivation. Moreover, caloric restriction with reduced access to food has limited applicability to humans in developed countries where access to food is essentially unlimited. This makes research into optimal ad libitum-fed diets for aging and age-related health a priority.
One methodological approach that has been used recently to try to disentangle the effects of calories and macronutrients on health and aging is the Geometric Framework [14, 17, 19, 20]. In these studies, animals are ad libitum-fed one of many diets varying in the ratio of macronutrients and total energy content. Total energy content can be varied by dilution with a non-calorific or non-digestible filler such as cellulose. Each animal has ad libitum access to food quantity but is restricted to a single diet. Compensatory feeding occurs via increasing or decreasing intake of that particular diet, but the animal cannot regulate its nutrient intake by choosing between differing diets. Thus these experiments do not represent ‘dietary restriction’ in the sense of having reduced access to energy or macronutrients. The advantage of these experiments is that they permit evaluation of an outcome such as lifespan across a dietary landscape of different macronutrient concentrations, macronutrient ratios and calorie content. It is important to note that diets with different macronutrient ratios can lead to similar intakes of a macronutrient because of compensatory feeding, yet be associated with different phenotypic outcomes. These observations indicate that the ratios and/or interactions between macronutrients, not just total amounts consumed, can influence phenotype.
When applied to lifespan, the Geometric Framework method has shown that ad libitum-fed diets that are lower in protein and higher in carbohydrate (LPHC) are associated with longer lifespan, while moderately reduced total calorie intake either has no effect or is detrimental (Fig. 1) [21–27]. Many of these studies have conversely found that diets higher in protein and lower in carbohydrates (LCHP) are associated with improved reproductive outcomes, and when given the choice animals tend to prefer diets that optimise reproduction over lifespan. This provides some evolutionary ‘face validity’ to the results and is consistent with many evolutionary theories of aging [28]. To date, most of Geometric Framework studies of lifespan have been undertaken in insects especially Drosophila, with a recent study in mice, while there are some observational studies in humans that parallel the interventional experiments in animals.
Fig. 1.
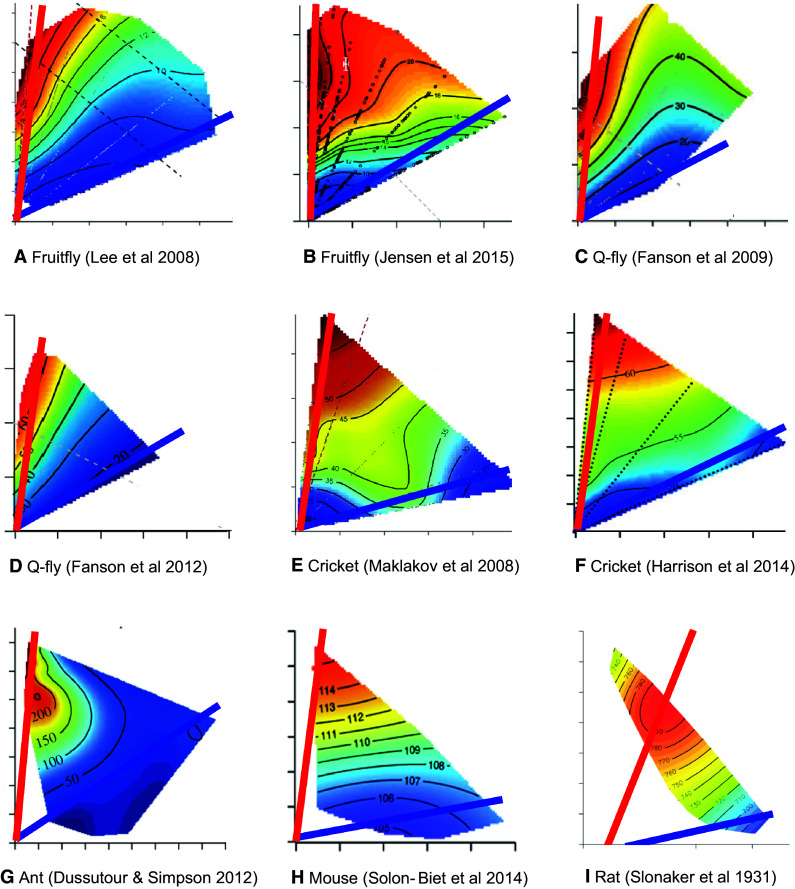
Published response surfaces for lifespan versus dietary macronutrients. In each figure, the x axis represents a measure of protein (dietary or intake; protein, casein or yeast) and the y axis represents a measure of carbohydrates (dietary or intake; carbohydrate or sucrose). The response surfaces vary from red which is the longest lifespan to blue which is the shortest lifespan. The red line represents the nutritional rail or PC ratio associated with the longest lifespan while the blue line represents that with the shortest lifespan. a–h from [21, 23–27, 30, 31] and i is a surface of simulated data parameterised from the results presented by Slonaker et al. in 1931 [55, 129]
The effects of diets with different ratios of protein and carbohydrates on lifespan
Insects
Although Clive McCay’s 1935 publication on caloric restriction in rats is well recognized, less known is the fact that in 1928 he published a paper showing that life expectancy was greatest in ad libitum-fed trout on low-protein diets [29]. This is possibly the first study on the effects of LPHC diets on aging. Most recent studies examining varying ratios of dietary protein and carbohydrate on lifespan have been undertaken in insect models including flies (drosophilid and tephritid fruit flies), crickets, ants and bees (Table 1). Ten of these studies have utilized a Geometric Framework methodology where lifespan was measured across a landscape of different dietary contents of protein, carbohydrate and energy [21–26, 30–33] (Fig. 1a–g). This is the most rigorous design for determining the effect of LPHC diets on lifespan and overall the studies have shown that the lowest protein to carbohydrate ratios (e.g. PC ratios ~1:10–1:16) are associated with longest lifespans. Many of the other studies, albeit with less dietary groups, also showed that the diets with lowest proportions of protein were linked with longest lifespans. Taken together such studies support the view that the dietary PC ratio influences lifespan with LPHC generating the longest lifespans.
Table 1.
List of studies performed in insects investigating the relationship between various ratios of dietary protein and carbohydrates with the outcome of lifespan (PC protein:carbohydrate, YS yeast:sucrose, EAA essential amino acids, GF Geometric Framework)
| References | Year | Diets | GF | Miscellaneous outcomes | Diet with maximum lifespan |
|---|---|---|---|---|---|
| Fruitfly | |||||
| Van Herrewege et al. [117] | 1974 | 0, 1, 2, 3, 4, 5 % casein with 5, 4, 3, 2, 1, 0 % sucrose | Lowest casein (1 %) | ||
| Mair et al. [118] | 2005 | YS 1:2.5, 1:1, 2.5:1 |
Tetracycline did not influence outcomes Low-protein diets led to similar increase in lifespan as caloric restriction (43–65 vs 60.0–82.6 %) |
Lowest yeast (1:2.5) | |
| Min et al. [43] | 2006 | Casein 0, 0.5, 1, 2, 4 % | Fecundity increased with casein | Lowest casein (0.5 %) | |
| Lee et al. [21] | 2008 | Varying sucrose, yeast PC 0:1, 1:16, 1:8, 1:4, 1:2, 1:1, 9:1 | Yes |
Egg laying maximal PC 1:2 Body fat greatest in longest lived flies |
Lowest PC 1:16 |
| Skorupa et al. [22] | 2008 | YS 1:16, 1:8, 1:4, 1:2, 1:1, 2:1, 4:1, 8:1, 16:1 | Yes |
Fecundity maximal on high yeast Overeating and increased body fat on low-yeast high-sugar diets |
Intermediate YS 1:1 |
| Grandison et al. [34] | 2009 | Dilutional caloric restriction ± amino acid supplements |
Methionine improved fecundity without shortening lifespan Dependent on IGF-1 pathway |
Amino acids (except methionine) reversed lifespan benefit of caloric restriction | |
| Ja et al. [45] | 2009 | Varying yeast, sugar and cornflour ratios ± dilutional caloric restriction ± supplemental water | Concentrated diets reduce lifespan by limiting water intake | Lowest PC 1:15 | |
| Wang et al. [40] | 2011 | YS 1:9, 9:1 Also high fat, resveratrol | Beneficial effects of resveratrol on lifespan only seen on high PC diets | Lowest YS 1:9 | |
| Dick et al. [35] | 2011 | Dilutional caloric restriction ± amino acid supplements | Amino acids increase reproductive parameters | Amino acids reversed lifespan benefit of caloric restriction | |
| Lushchak et al. [119] | 2012 |
Varying yeast, sugar PC 1:95, 1:57, 1:38, 1:20 |
Low PC 1:57 | ||
| Bruce et al. [120] | 2013 | 12 different YS ratios with sucrose 1, 5, 20 % and yeast 0.25, 1, 4, 5 % | Low PC 1:10–1:20 | ||
| Zajitschek et al. [37] | 2013 | 4 yeast concentrations (40, 60, 100, 300 g/L) ± methionine |
Methionine reduced survival and no effect on reproductive fitness Social factors (single vs mixed sexes) influenced outcomes |
Lowest yeast (40 g/L) | |
| Emran et al. [36] | 2014 | Dilutional caloric restriction and essential amino acid restriction | Dietary effect of amino acids depends on mTOR but not insulin-like peptide | Essential amino acids reversed lifespan benefits of caloric restriction | |
| Zhu et al. [121] | 2014 | YS 1:3, 1:1, 3:1 |
Response of lifespan to YS depends on mitochondrial genes Response to caloric restriction depends on SIR2 |
Lowest YS (1:3) | |
| Piper et al. [122] | 2014 | Holidic diets nitrogen:sucrose 0:1, 1:2, 1:1, 2:1, 4:1, 8:1, 1:0 | Increased drug bioavailability with holidic diet | Intermediate nitrogen:sucrose 1:1, 2:1 | |
| Lee et al. [44] | 2015 | Synthetic diets PC 0:1, 1:16, 1:8, 1:4, 1:2, 1:1, 2:1, 4:1 |
Egg laying increased at highest PC Body fat greatest in longest lived flies |
Intermediate PC 1:4–1:2 | |
| Jensen et al. [23] | 2015 | Synthetic diets PC 0:1, 1:16, 1:8, 1:4, 1:2, 1:1, 2:1 | Yes | Reproductive outcomes optimal for PC 1:16 (M), 1:2 (F) | Lowest PC 1:16 |
| Q-fly | |||||
| Fanson et al. [25] | 2009 | YS 0:1, 1:21, 1:3.4, 1:1.6, 1.5:1, 5:1, 1:0 | Yes | Egg production optimal YS 1:3 | Lowest YS 1:21 |
| Fanson et al. [24] | 2012 | Synthetic amino acids and sucrose PC 0:1, 1:32, 1:16, 1:8, 1:4, 1:2 | Yes | Highest PC maximised reproductive factors | Lowest PC 1:32 |
| Mexican fruit fly | |||||
| Carey et al. [38] | 2008 |
YS 0:1, 1:24, 1:9, 1:3 Fixed diet allotment |
Lifespan effects of caloric restriction depend on protein content | Intermediate YS 1:9 | |
| Zou et al. [39] | 2010 | YS 1:24, 1:9, 1:3 | Lifespan effects of oregano and cranberry depend on YS ratio | Intermediate and higher YS 1:9–1:3 | |
| Liedo et al. [123] | 2012 | YS 0:1, 1:24, 1:9, 1:3 | Lifespan effects of acai and palmitic acid depend on YS ratio | Intermediate and higher YS 1:9–1:3 | |
| Cricket | |||||
| Maklakov et al. [30] | 2008 | PC 1:8, 1:5, 1:3, 1:1, 3:1, 5:1 | Yes | Egg production but not male calling increase with PC | Lowest PC 1:8 |
| Harrison et al. [26] | 2014 | PC 1:8, 1:5, 1:3, 1:1, 3:1 | Yes | Egg production and male calling increase with PC |
Intermediate PC 1:3 (males) Lowest PC 1:8 (females) |
| Ant | |||||
| Dussutour and Simpson [31] | 2012 | PC 1:5, 1:3, 3:1, 5:1 | Yes | Lowest PC 1:5 | |
| Bee | |||||
| Pirk et al. [124] | 2009 | Varying pollen, royal jelly, casein PC 0:1, 1:3, 3:1, 1:0 | Depended on source of protein | Lowest PC 0:1 | |
| Paoli et al. [125] | 2014 |
Varying sucrose and amino acids aa:sucrose 1:750, 1:500, 1:250, 1:100, 1:75, 1:50, 1:10, 1:5 |
Yes | Worker bees eat more carbohydrates with age | High aa increased mortality of young and forager bees |
| Paoli et al. [125] | 2014 |
Varying sucrose and essential amino acids EAA:sucrose 0:1, 1:500, 1:250, 1:100, 1:10, 1:5 |
Mediated by Sir2 | Lowest aa:sucrose (1:500) | |
| Stabler et al. [33] | 2015 |
Varying casein or essential amino acids EAA:carbohydrate 1:500–1:10 |
Yes | Highest mortality in high casein and high EAA diets | |
Several studies have shown that the lifespan extension seen with caloric restriction can be reversed by supplementation with essential amino acids [34–36]. This suggests that the benefits of caloric restriction might be mediated by reduced intake of protein and amino acids; an alternative explanation is that the benefits of caloric restriction can be reversed by a higher dietary PC ratio. In one study [34] but not another [37], methionine improved fecundity without shortening lifespan. The effects of interventions that delay aging such as caloric restriction [38] and various phytochemicals and pharmaceutical agents including resveratrol and rapamycin [36, 39, 40] also depend upon the underlying ratio of macronutrients. For example, resveratrol increases lifespan in mice on high fat diet [41] yet not in mice on standard chow diet [42].
The PC ratio has been observed to influence various measures of reproductive fitness, such as egg laying [21–26, 34, 35, 43, 44]. Overall, within studies the PC ratios that optimized lifespan were lower than those that optimized reproductive fitness. It has been suggested that protein is the key macronutrient required for reproduction, while carbohydrates are more important for somatic maintenance and consequently lifespan [24, 25]. Animals will preferentially choose diets with sufficient calories and protein to optimize reproduction and hence evolutionary fitness [21]. When faced with high protein diets that are harmful animals can simply reduce food intake. When faced with low-protein diets which are insufficient for reproduction, animals can increase food intake to achieve their protein target [16]. If the PC ratio and/or protein are too low to support reproduction or survival of offspring, then presumably there is evolutionary advantage in allocating resources towards somatic maintenance and survival, until the available diet improves [24, 25]. A very low PC diet maintained over a lifetime will be associated with increased lifespan at the cost of reduced reproductive output.
There are methodological issues which need to be considered when interpreting dietary interventions in flies [19]. Altering dietary protein and carbohydrates will lead to compensatory feeding therefore it is critical to accurately evaluate food intake before making conclusions about the effects of macronutrient intake on outcomes. Many of the caloric restriction experiments in flies rely on diluting the diet with water. It has been reported that the lifespan of flies with high concentration diets can be increased by providing supplementary water, perhaps suggesting that concentrated diets can reduce lifespan through dehydration; the benefits of caloric restriction observed in flies might simply reflect adequate water supply [45]. In the studies using the Geometric Framework to evaluate multiple diets with dietary dilution, this dehydration effect is unlikely since the diluted diets were associated with a shorter lifespan and in many studies, free water was made available separately to the diet [21–26]. It is also important that multiple diets are tested so that the entire dietary landscape can be assessed. This is particularly relevant when considering the effects of caloric restriction and pharmaceutical agents that have different effects depending upon the background PC ratio. Another issue is that many of the studies involve altering the concentration of yeast as a surrogate for protein, however, yeast is more than just protein and incorporates carbohydrates and a range of other substrates that could influence lifespan. This is unlikely to explain the effects PC on longevity and fecundity, because in a study in which yeast was replaced by a mixture of amino acids, the same patterns of response as seen with yeast-based diets was observed [23]. It should be acknowledged that laboratory studies of lifespan and nutrition do not take into account the additional stresses of a natural environment that might influence nutritional requirements including the need to respond to infections, injuries, foraging and cold [46].
Rodents
There has only been one study using the Geometric Framework to study aging and health outcomes in rodents [27]. In this study, mice were ad libitum-fed one of 25 diets varying in protein, carbohydrate and fat, with energy content varied through the addition of non-digestible fibre. In mammals, the third macronutrient fat also needs to be taken into account unlike the experimental insect systems that have been used where fat is not a major source of energy. One cohort of mice was sacrificed at 15 months of age to evaluate health and aging mechanisms, while the rest were maintained to study lifespan. Mice on the LPHC diets had the longest lifespans. Calorie intake had a negligible effect on lifespan and in fact the lowest energy intakes tended to be associated with shorter lifespans (Fig. 1h). LPHC diets were also associated with improved lipids, glucose tolerance and insulin. Further analysis showed that low protein intakes associated with LPHC diets were associated with a younger profile of splenic lymphocytes (CD4, CD8, CD4 memory and naïve cells), similar to benefits seen with standard caloric restriction [47]. Maximal longevity was achieved on diets containing a P:C ratio of 1:13 in males and 1:11 for females which were lower than those which optimized reproductive fitness (1:1 for testes mass, epididymal sperm counts, uterine mass and 3:1 for ovarian follicle number) [48]. The relationships between PC ratio and outcomes seen in mice are similar to those seen in insects. Dietary fat appeared to have minimal effect on outcomes in mice. This suggests that the effect of dietary PC on lifespan and reproduction is strongly conserved even in those species where fat has become a significant source of energy.
There are also studies that have investigated the effects of a smaller number of diets varying in protein content on aging and age-related health but not using the Geometric Framework methodology (Table 2). In a prescient study undertaken in 1931, Slonaker performed lifespan experiments in rats restricted to one of five diets varying in protein, fat and carbohydrate. Rats on the second lowest PC ratio (1:5) lived the longest and also demonstrated protein leverage, having increased food intake and body weights. Simulation modelling of the data and generation of a response surface revealed a similar pattern to that seen in more recent Geometric Framework and lifespan studies (Fig. 1i). The remainder of studies on rodents are heterogeneous and generally only compared two or three protein concentrations and often in parallel with standard caloric restriction interventions. The results of these are mixed with some early studies in rats finding increased lifespan on highest protein diets [49–51]. Notably these were usually strains of rats that become obese as they age in captivity (i.e. Wistar and Sprague–Dawley rats). Most of the other studies reported that lower protein diets are associated with an increase in lifespan compared with higher protein diets in both mice [27, 52–54] and rats [55–58] (see Fig. 2).
Table 2.
List of mouse and rat studies investigating two or more diets varying in protein or carbohydrates on the outcome of lifespan (PC protein:carbohydrate)
| References | Year | Species and strain | Diets | Miscellaneous outcomes | Diet with maximum lifespan |
|---|---|---|---|---|---|
| Slonaker et al. [55] | 1931 | rats | Five protein, carbohydrate and fat ratios (PC 1:7.5, 1:5, 1:3.6, 1:2.7, 1:2.1) | Second lowest protein (PC 1:5) | |
| Nakagawa et al. [49] | 1971 | Donryu rats | 10, 18, 27 % casein | Estrus delayed on low casein | Highest casein (27 %) |
| Ross et al. [50] | 1973 | SD rats | Casein 10, 22, 51 %, sucrose 71, 59, 30 %, (PC 1:7, 1:2.7, 1.7:1 in fixed quantities or ad libitum fed) |
Highest casein (51 %) Caloric restricted |
|
| Leto et al. [52, 53] | 1976 | C57Bl/6 mice | 4, 26 % casein (PC 1:2.5, 1:16) | Increased oxygen consumption and lower temperature on low-casein diet | Lowest casein (4 % casein) |
| Goodrick [54] | 1978 | A/J, C57Bl/6, hybrid mice | 4, 26 % casein | Size of effect depended on strain | Lowest casein (4 %) |
| Davis et al. [51] | 1983 | Wistar rats | 18, 30, 42 % casein with 2/3 caloric restriction (PC 1:3.3, 1:1.6, 10.9), or ad libitum 12, 20, 28 % (PC 1:5.5, 1:2.9, 1:1.8) |
Highest casein (42 %) Medium casein when caloric restricted |
|
| Horakova et al. [56] | 1988 | F344 rats | 12.6, 21 % casein (PC 1:5.3, 1:2.8) caloric restriction | Lowest casein (12.6 %) | |
| Yu et al. [57] | 1988 | F344 rats | 40 % CR (PC 1:3, 1:5) |
Lowest protein Caloric restricted |
|
| Maroro et al. [58] | 1989 | F344 rats | 21, 35 % casein (PC 1:2.8, 1:1.2 under 40 % caloric restriction) | Low casein delayed nephropathy |
Lowest casein (21 %) Caloric restricted |
| Preuss et al. [126] | 1997 | Wistar rats WKY,WAM | Five diets varying in protein, sucrose, fat (PC 1:1.2, 1:1.3, 1:2.9, 3.2:1, 5.1:1). High sucrose diets | No effects on lifespan (high sucrose diets) | |
| Solon-Biet et al. [27, 47, 48] | 2014 | C57Bl/6 mice | 25 diets varying in protein, fat, carbohydrates and energy (PC 3:1–1:15) |
High PC associated with increased measures of reproductive success Low protein intake improved lymphocyte patterns Low PC improved BP, insulin and lipoproteins |
Lowest PC |
Fig. 2.
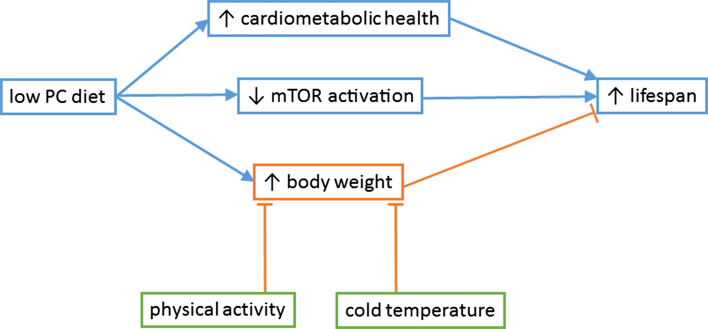
Physical activity and cold environmental temperature might reverse the weight gain associated with LPHC diets and act synergistically to increase lifespan. These effects might be mediated via countering effects of LPHC diets on mitochondrial uncoupling
Nakagawa et al. used a meta-analytic method to evaluate and compare the role of calories versus proteins on lifespan in 145 dietary restriction studies across 36 species [59]. They found that the lifespan effects of caloric restriction were greatest in females and in model laboratory species (yeast, nematodes, fruit flies and rodents). The proportion of protein intake, which ranged from 0 to 90 %, had a greater impact than caloric intake on life extension. The relationship between the hazard ratio for survival versus percentage protein in the diet was J-shaped with maximum survival occurring at about 30 % protein intake.
There have been some attempts to tease out which components of a low-protein diet increase lifespan in rodents. Low-methionine and low-tryptophan diets are associated with increased lifespan [60–63], while vegetable (soy) protein led to a greater lifespan than animal based (casein) protein [64]. In the Geometric Framework study of 25 diets, it was concluded that branched chain amino acids in protein might be important because mice on the LPHC diets had low circulating branched chain amino acids which correlated with decreased activation of hepatic mTOR [27]. Inhibition of mTOR, for example by rapamycin, is associated with increased lifespan [65]. Branched chain amino acids might also influence aging by increasing histone acetylation, because leucine catabolism increases acetic acid, and hence acetyl-CoA [66]. Histone de-acetylation, for example by resveratrol and other sirt1 agonists such as SIRT2104, is associated with increased lifespan in various animal models [67, 68]. Branched chain amino acids could explain some of the lifespan benefits [69] of vegetarian versus animal-based diets because animal-based proteins tend to be higher in branched amino acids.
Some short term studies (over 2–3 months in young rodents) have been undertaken to examine the effects of different PC ratios on outcomes, mostly cardio-metabolic, that might influence aging. In Wistar rats, 53 % dietary casein was associated with better insulin sensitivity and lower insulin levels than 14 % casein [70] while conversely in C57Bl/6 mice, 18 % dietary protein had better insulin sensitivity and cardiovascular function than 31 % dietary protein. ApoE−/− mice maintained on a diet containing 45 % protein and 12 % carbohydrates for 12 weeks developed more severe atherosclerosis than those on a diet containing 15 % protein and 43 % carbohydrates [71]. In a recent study ad libitum-fed and caloric restricted C57Bl mice were maintained on 5, 33 and 60 % protein diets (PC 1:15, 1:1.4, 3:1) for 8 weeks. The 5 % ad libitum diet was equivalent to the caloric restricted diets in terms of improvements in insulin, glucose, lipids and insulin sensitivity, despite increased food intake [72]. In a similar study in C57Bl mice, 3 months of caloric restriction and protein restriction (20, 16, 14, 12 %, with restricted food intake, PC 1:3.5, 1:4.6, 1:5.4, 1:6.5) were compared. The protein restricted mice had more body fat without any effect of protein on insulin, glucose tolerance or markers of oxidative stress [73–75]. The reasons for the differences between these two mouse studies are not clear. Mitchell et al. [73–75] restricted total energy intake in the LPHC diets while in our study [27], only the mice on the LPHC diets with the highest energy intakes achieved health and lifespan benefits. Mitchell et al. show found that mice under protein restriction consumed ~0.38 g/day of protein, whereas the mice showing health benefits in our study consumed less than half of this amount of protein at ~0.13 g/day. In another study of mice maintained on 5 different PC ratios for 12 weeks, LPHC diets were found to be associated with increased body temperature, increased white and brown adipose tissue and a reduction of uncoupling protein-1 (UCP1) and peroxisome proliferative activated receptor gamma coactivator 1 alpha (PGC-1α) expression in brown adipose tissue [76]. Deiodinase iodothyronine type II (DIO2) expression was increased which might explain the increase in body temperature given that UCP1 was down-regulated.
Humans
There are numerous trials, observational studies and reviews examining high and low carbohydrate or protein diets and their effects on obesity and metabolic outcomes, with conflicting conclusions. These give limited insights into the impact on human health of the ratio of dietary protein to carbohydrates because the effect of only one of the three macronutrients is usually reported. In a meta-analysis of 17 cohort studies of dietary carbohydrates, there was an association between low dietary carbohydrates and increased mortality [77]. Likewise, a meta-analysis of 19 clinical trials of low carbohydrate diets found no cardiometabolic or weight loss benefits compared with balanced diets [78]. A recent study suggested that low-protein diets might delay aging and cancer via decreased IGF-1 levels [9, 74]. These studies and reviews of the single macronutrient literature are consistent with the conclusion that LPHC diets are healthful in humans. On the other hand, low-carbohydrate high-protein (LCHP) diets have been advocated for weight loss and may be effective in the short term [79]. A systematic review of 13 clinical trials concluded that LCHP diets are as effective or more effective as low fat diets in the management of obesity [80] while another concluded that LCHP diets are effective in improving glycaemic control in people with diabetes mellitus [81].
There are a number observational studies explicitly evaluating the PC ratio and its effect on human lifespan and health outcomes relevant to aging (Table 3). These can be difficult to interpret because of the standard problem of residual confounding and the limited capacity of observational studies to establish efficacy of interventions. With regards to PC ratio, there are particular issues related to the health effects of animal-versus plant-based proteins, and high versus low glycaemic index carbohydrates which are likely to influence health outcomes independent of the PC ratio.
Table 3.
List of observational studies in humans (LCHP low-carbohydrate high-protein, PC protein:carbohydrate)
| References | Year | Cohort | Assessment of PC ratios | Outcomes related to PC |
|---|---|---|---|---|
| Lagiou et al. [83] | 2007 |
Swedish Women’s Lifestyle and Health Cohort N = 42,237 women 30–49 years followed for 12 years |
Deciles of carbohydrate and protein intake studied and an ‘LCHP additive score’ was created by adding the position in the deciles | Decreasing carbohydrate, increasing protein intake and ‘LCHP additive score’ associated with increased total and cardiovascular mortality |
| Trirchopoulou et al. [84] | 2007 |
EPIC study (Greek component) N = 22,944 adults 20–86 years, mean follow up 4.9 years |
Deciles of carbohydrate and protein intake studied and the ‘LCHP score’ was created by adding the position in the deciles | Decreasing carbohydrate and increasing LCHP score associated with increased mortality. LCHP score associated with cardiovascular and cancer mortality |
| Schulze et al. [87] | 2008 |
EPIC-Potsdam (German) study N = 25,067 aged 35–65 years followed up to 11 years |
Substituting carbohydrates with either fat or protein | Substituting carbohydrates for protein increased type 2 diabetes mellitus in men |
| Fung et al. [69] | 2010 |
Nurses’ Health Study N = 85,168 women 34–59 years followed up to 26 years Health Professional Follow-up Study N = 44,548 men 40–75 years followed up to 20 years |
Deciles of carbohydrate, protein and fat intake studied and a ‘low-carbohydrate diet score’ created by adding the deciles |
Low-carbohydrate score associated with increased mortality Animal-based diet and vegetable-based diets opposite effects on all cause and cardiovascular mortality |
| Sluijs et al. [88] | 2010 |
EPIC-NL (Dutch) study N = 38,094 aged 21–70 years followed 10 years |
Quartiles of carbohydrate, protein and fat intake | Substituting carbohydrates for animal protein increased risk of type 2 diabetes mellitus. No effect for vegetable proteins |
| Sjogren et al. [85] | 2010 | Swedish, N = 924 men aged 70 years followed average 10.1 years | Deciles of carbohydrate and protein intake with a ‘carbohydrate restriction’ score created by adding deciles | Carbohydrate restriction score associated with increased all cause and cardiovascular mortality |
| Lagiou et al. [89] | 2012 | Swedish, N = 43,396 women 30–49 years, followed up average 15.7 years | Deciles of carbohydrate and protein intake and a LCHP score created by adding the deciles | LCHP associated with increase cardiovascular disease including ischemic heart disease, peripheral arteriaL disease and stroke |
| Nilsson et al. [86] | 2012 | Swedish, N = 77,319 adults | Deciles of carbohydrate and protein intake and a LCHP score created by adding the deciles | No association between LCHP score and all-cause mortality |
In a meta-analysis primarily focussing particularly on protein intake and health, Pedersen et al. [82] reviewed the effects of LCHP diets. They concluded that the data are suggestive of a relationship between increased all-cause mortality and longterm LCHP diets. There was also a relationship between the risk of type 2 diabetes mellitus and long-term low-carbohydrate high-protein, high fat diets. A list of some of the cohort studies specifically investigating PC ratios is shown in Table 3. The majority of the eight studies grouped carbohydrate and protein intakes into deciles then generated a score based on adding the decile ranks so that participants with high ‘low-carbohydrate’ scores (or similar) had low-carbohydrate high-protein intakes, and vice versa. Four studies showed an increase in mortality with LCHP diets [69, 83–85], while one had no effect [86]. Two studies showed an increase in type 2 diabetes mellitus [87, 88] and one an increase in cardiovascular disease [89]. Where reported, animal based protein and diets had the worst effects, while vegetable-based proteins and diets nullified or reversed the trends. Such data would be amenable for analysis using the Geometric Framework which would tease out the effects of all the macronutrients, their interactions, and total calories.
Comparison of outcomes between LPHC diets and caloric restriction diets
Caloric restriction has well established health and aging benefits [4–7] but is not easily sustainable in humans or in animals with free access to food, thus diets such as the LPHC diet which involve ad libitum access to food are more feasible as a health intervention. Therefore it is relevant and important to compare the outcomes of LPHC diets with those of caloric restriction. Of importance, the comparison suggests similarities (mTOR inactivation) and differences (body fat, mitochondrial biogenesis) between the cellular mechanisms that influence aging and lifespan. A summary of some outcomes is shown in Table 4.
Table 4.
Comparison of outcomes of studies in animals of ad libitum LPHC diets and standard caloric restriction regimens
| Outcome | Low PC diet with ad libitum access to food | Caloric restriction with reduced amounts of food |
|---|---|---|
| Food intake | ↑ | ↓ |
| Body weight | ↑ | ↓ |
| Body fat | ↑ | ↓ |
| Temperature | ↑ | ↓ |
| Activity | ↔ | ↑↓ |
| Insulin | ↓ | ↓ |
| LDLc | ↓ | ↓ |
| HDLc | ↑ | ↑ |
| Mitochondrial number | ↓ | ↑ |
| Mitochondrial free radicals | ↑ | ↓ |
| PGC-1α | ↓ | ↑ |
| Uncoupling protein | ↓ | ↔ |
| mTOR phosphorylation | ↓ | ↓ |
| AMPK phosphorylation | ?↓ | ↑ |
| Reproductive fitness | ↓ | ↓ |
| Lifespan | ↑ | ↑↑ |
One of the key differences is that LPHC diets are associated with increased food intake and subsequent increase in body weight and body fat compared to caloric restriction where these are all decreased. The increase in body fat with LPHC diets has been seen in both Drosophila [21, 44] and mice [27, 76] and is an expected consequence of protein leverage, present in most species including humans [16, 90]. In a short-term study of mice it was found that LPHC diets increase both white and brown adipose tissue [76], which may have opposing effects on metabolic health. Given the association between obesity and poor health outcomes, this raises the question as to whether the increase in body weight and body fat is an undesirable side effect of LPHC diets, or whether it represents ‘healthy obesity’ [91]. In a study of the effects of caloric restriction on 41 recombinant inbred stains of mice it was found that those mice with the least reduction in body fat were more likely to have an increased lifespan [92]. In an older study of Ob/Ob mice, it was found that caloric restriction increased lifespan despite maintenance of high levels of body fat [93]. Such studies suggest that body fat is not an impediment to the life extending properties of nutritional interventions.
It is also of interest that LPHC diets are associated with reduced mitochondrial numbers (assessed by citrate synthase activity [27]), increased hydrogen peroxide formation (assessed by Seahorse method [27]) and reduced expression of the key regulator of mitochondrial biogenesis, PGC-1α [76]. Again this is different to what is seen in caloric restriction where there is an increase in mitochondrial number associated with increased PGC-1α expression and reduced free radical production [94, 95]. Mitochondrial dysfunction is a major feature of old age and probably has a mechanistic role in the aging process itself [94–97]. Therefore the paradox that LPHC and caloric restriction diets both increase lifespan but with opposing effects on mitochondria requires explanation. The concept of ‘mitohormesis’ might provide a mechanism [95, 98] whereby low levels of oxidative stress are postulated to induce systemic defence mechanisms that are beneficial for aging, such as endogenous antioxidant enzymes. Thus LPHC diets might increase hydrogen peroxide production sufficient to generate hormetic benefits, but not an excess that will lead to mitochondrial damage. The effects of LPHC diets on antioxidant defences are unreported. On the other hand, caloric restriction reduces harm from excess oxidative stress by directly improving mitochondrial function and reducing the production of mitochondrial free radicals. The difference between the effects of LPHC and caloric restriction diets on mitochondria are consistent with a recent finding in fruitflies that lifespan correlates with mTOR activation but not mitochondrial function or free radical production [99].
The reduction in mitochondrial number with LPHC diets is consistent with inactivation of mTOR [100]. Caloric restriction diets are also associated with inactivation of mTOR but with increased expression of PGC-1α and mitochondrial number. Presumably with caloric restriction the inactivation of mTOR is overridden by activation of SIRT1 and subsequent increase in PGC-1α [101]. Another explanation could be via activation of AMPK. AMPK is phosphorylated in caloric restriction which activates mitochondrial biogenesis [94]. The effect of LPHC diets on AMPK has not been reported but it would be expected that phosphorylation should be reduced secondary to the increase in food intake. The differences in the numbers of mitochondria between caloric restriction and LPHC diets are unlikely to be related to the effect of energy intake on mitochondrial fission and fusion, because it has been found that dietary energy excess stimulates fission (an increase in mitochondrial numbers) while deficiency stimulates fusion (a reduction in mitochondrial numbers)—this is the opposite to the effects seen with LPHC and caloric restriction [102].
Cellular mechanisms linking LPHC diets and ageing
Evidence for the mechanisms linking LPHC diets and age-related health is still limited and has been reviewed elsewhere [12]. By comparison, the nutrient sensing pathways for caloric restriction have been extensively studied and include four canonical pathways: mTOR, sirtuin, AMPK and insulin/IGF-1/Growth hormone [6]. In our study of mice, we found that LPHC diets were associated with a small reduction in phosphorylation of hepatic mTOR which was correlated with lower circulating branched chain amino acids and higher glucose levels [103]. Insulin levels were lowest in the mice on the LPHC diets. The effects of LPHC on other nutrient sensing pathways AMPK and sirtuins have not yet been reported. Another plausible candidate mechanism is FGF21 which has been found to be influenced by dietary protein and has many downstream effects on metabolism and mitochondrial function that would be expected to influence aging [12].
Will exercise- and cold-induced weight loss enhance or detract from the beneficial effect of LPHC diets?
There is accruing evidence that LPHC diets in ad libitum-fed animals are associated with increased lifespan. However there is some evidence for potentially adverse effects of these diets related to weight gain and mitochondrial function. On the other hand, these ‘adverse effects’ may not be adverse at all, but in fact represent ‘healthy obesity’ and ‘mitohormesis’ and thus be beneficial. Or of course, they may simply be neutral epiphenomena. One way to evaluate these issues would be to combine LPHC diets with exercise and/or cold, which are interventions that might counter the effects of LPHC diets on body fat and mitochondria.
Physical activity and exercise are both associated with improved health and reduced body weight. However, exercise has not been shown to increase lifespan, and in association with caloric restriction may even detract from the lifespan gains induced by caloric restriction [104]. This is perhaps not surprising given that physical activity requires energy input which is reduced in caloric restriction. Caloric restriction has usually been reported to be associated with increased physical activity dependent on Sirt1 [105] however recent studies reported that severe caloric restriction in mice leads to marked physical inactivity [73, 92]. On the other hand, there is the potential for synergy between exercise and LPHC diets because exercise might reduce the increased weight associated with LPHC diets, while the increased food intake that occurs with LPHC diets will provide the energy input required to sustain physical activity.
Environmental temperature is another variable that could be synergistic with LPHC diets. Experimentally lowering body temperature extends lifespan in some but not all species [106, 107], has been proposed as a method for weight loss in obesity [108] and does reduce body weight and body fat in experimental animal models [107]. Caloric restriction reduces body temperature [73] while LPHC diets are associated with an increase in body temperature [73, 76]. Intriguingly, rats housed under a low temperature increase intake of carbohydrates presumably to provide energy for maintenance of temperature [109]. This led to a reduction in their dietary PC ratio which might contribute to the longevity effects of low temperature reported in some studies.
Mechanistically, the effects of LPHC diets, exercise and cold ambient temperature could interact via effects on mitochondrial biogenesis, mitochondrial protein leak and uncoupling protein-1, UCP1. UCP1 is found in brown and beige fat where it dissipates energy as heat. UCP1 expression is increased by cold, overfeeding, exercise and sympathomimetics [110] and has been proposed as a potential therapeutic target for obesity [111]. Increased lifespan has been generated by genetic overexpression of UCP1 and by chemical uncoupling with 2,4-dinitrophenol [112–114]. In a short study in mice, UCP1 expression in brown adipose tissue was reduced despite overfeeding with LPHC diets, therefore interventions that increase UCP1 could be useful in combatting weight gain with compensatory feeding in these diets. Physical activity also upregulates PGC-1α and mitochondrial numbers [115] which might overcome the reduction of mitochondrial numbers [27] and down regulation of PGC-1α [76] that we reported with LPHC diets. Physical activity increases mitochondrial content and function even in older people [116].
In summary, it is plausible that physical activity and cold will enhance the lifespan benefits of LPHC diets by reducing body weight and increasing mitochondrial numbers and uncoupling. If this was not confirmed, then it would necessitate a rethinking of the current views on obesity and lifespan, and on the role of mitochondria in aging.
Conclusions
There is accruing evidence that LPHC diets are associated with increased lifespan in ad libitum-fed insects and mice, albeit at the cost of reduced reproductive fitness. This conclusion is supported by observational data in human populations. In undertaking this review it became apparent that there were many diverse terminologies used to describe diets with varying protein and carbohydrate ratios and the effects on health and aging. Future research might be unified by a single unique terminology—we propose the ‘CPC diet’ (correct ratio of proteins to carbohydrates; and the Charles Perkins Centre where much of the research in this field has been instigated). The main adverse effect of CPC diets is increased body weight, which potentially could be addressed by supplementing CPC diets with interventions such as physical activity and reduced ambient temperature.
Acknowledgments
We acknowledge funding from the Aging and Alzheimers Research Institute, NHMRC grants #571328 and #1084267 and our co-authors in studies cited in this review. RdC and SJM are funded by the Intramural Program of the National Institute on Aging, NIH.
References
- 1.Schafer D. Aging, longevity, and diet: historical remarks on calorie intake reduction. Gerontology. 2005;51:126–130. doi: 10.1159/000082198. [DOI] [PubMed] [Google Scholar]
- 2.Howell TH. The art of living long by Luigi Cornaro. Age Ageing. 1987;16:194–195. doi: 10.1093/ageing/16.3.194. [DOI] [PubMed] [Google Scholar]
- 3.McCay C, Crowell M, Maynard L. The effect of retarded growth upon the length of life and upon ultimate size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 4.Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everitt AV, Rattan SI, Le Couteur DG, de Cabo R. Calorie restriction, aging and longevity. New York: Springer Press; 2010. [Google Scholar]
- 6.de Cabo R, Le Couteur DG (2015) The biology of ageing. In: Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, Loscalzo J (eds) Harrisons principles of internal medicine, 19th edn. McGraw Hill Education, New York, p 94e
- 7.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizza W, Veronese N, Fontana L. What are the roles of calorie restriction and diet quality in promoting healthy longevity? Ageing Res Rev. 2014;13:38–45. doi: 10.1016/j.arr.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, Smith SR, Stein RI, Scott TM, Stewart TM, Saltzman E, Klein S, Bhapkar M, Martin CK, Gilhooly CH, Holloszy JO, Hadley EC, Roberts SB, Group CS (2015) A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci 70:1097–1104 [DOI] [PMC free article] [PubMed]
- 11.Guarente L, Picard F. Calorie restriction—the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Solon-Biet SM, Mitchell SJ, de Cabo R, Raubenheimer D, Le Couteur DG, Simpson SJ. Macronutrients and caloric intake in health and longevity. J Endocrinol. 2015;226:R17–R28. doi: 10.1530/JOE-15-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Couteur DG, McLachlan AJ, Quinn RJ, Simpson SJ, de Cabo R. Aging biology and novel targets for drug discovery. J Gerontol A Biol Sci Med Sci. 2012;67:169–174. doi: 10.1093/gerona/glr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson SJ, Le Couteur DG, Raubenheimer D. Putting the balance back in diet. Cell. 2015;161:18–23. doi: 10.1016/j.cell.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 15.Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R. Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci. 2010;65:695–703. doi: 10.1093/gerona/glq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes Rev. 2005;6:133–142. doi: 10.1111/j.1467-789X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 17.Simpson SJ, Raubenheimer D. The nature of nutrition. A unifying framework form animal adaption to human obesity. Princeton: Princeton University Press; 2012. [Google Scholar]
- 18.Le Couteur DG, Wilder SM, de Cabo R, Simpson SJ. The evolution of research on ageing and nutrition. J Gerontol A Biol Sci Med Sci. 2014;69:1–2. doi: 10.1093/gerona/glt130. [DOI] [PubMed] [Google Scholar]
- 19.Simpson SJ, Raubenheimer D. Caloric restriction and aging revisited: the need for a geometric analysis of the nutritional bases of aging. J Gerontol A Biol Sci Med Sci. 2007;62:707–713. doi: 10.1093/gerona/62.7.707. [DOI] [PubMed] [Google Scholar]
- 20.Simpson SJ, Raubenheimer D. Perspective: tricks of the trade. Nature. 2014;508:S66. doi: 10.1038/508S66a. [DOI] [PubMed] [Google Scholar]
- 21.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci USA. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster . Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen K, McClure C, Priest NK, Hunt J. Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster . Aging Cell. 2015;14:605–615. doi: 10.1111/acel.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fanson BG, Taylor PW. Protein:carbohydrate ratios explain life span patterns found in Queensland fruit fly on diets varying in yeast:sugar ratios. Age (Dordr) 2012;34:1361–1368. doi: 10.1007/s11357-011-9308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanson BG, Weldon CW, Perez-Staples D, Simpson SJ, Taylor PW. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni) Aging Cell. 2009;8:514–523. doi: 10.1111/j.1474-9726.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- 26.Harrison SJ, Raubenheimer D, Simpson SJ, Godin JG, Bertram SM (2014) Towards a synthesis of frameworks in nutritional ecology: interacting effects of protein, carbohydrate and phosphorus on field cricket fitness. Proc Biol Sci 281(1792). doi:10.1098/rspb.2014.0539 [DOI] [PMC free article] [PubMed]
- 27.Solon-Biet S, McMahon A, Ballard JWO, Ruohonen K, Wu L, Cogger V, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney G, Sinclair D, Raubenheimer D, Le Couteur D, Simpson S. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holliday R. Food, fertility and longevity. Biogerontology. 2006;7:139–141. doi: 10.1007/s10522-006-9012-3. [DOI] [PubMed] [Google Scholar]
- 29.McCay CM, Bing FC, Dilley WE. Factor H in the nutrition of trout. Science. 1928;67:249–250. doi: 10.1126/science.67.1731.249. [DOI] [PubMed] [Google Scholar]
- 30.Maklakov AA, Simpson SJ, Zajitschek F, Hall MD, Dessmann J, Clissold F, Raubenheimer D, Bonduriansky R, Brooks RC. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr Biol. 2008;18:1062–1066. doi: 10.1016/j.cub.2008.06.059. [DOI] [PubMed] [Google Scholar]
- 31.Dussutour A, Simpson SJ. Ant workers die young and colonies collapse when fed a high-protein diet. Proc Biol Sci. 2012;279:2402–2408. doi: 10.1098/rspb.2012.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paoli PP, Donley D, Stabler D, Saseendranath A, Nicolson SW, Simpson SJ, Wright GA. Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids. 2014;46:1449–1458. doi: 10.1007/s00726-014-1706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stabler D, Paoli PP, Nicolson SW, Wright GA. Nutrient balancing of the adult worker bumblebee (Bombus terrestris) depends on the dietary source of essential amino acids. J Exp Biol. 2015;218:793–802. doi: 10.1242/jeb.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dick KB, Ross CR, Yampolsky LY. Genetic variation of dietary restriction and the effects of nutrient-free water and amino acid supplements on lifespan and fecundity of Drosophila. Genet Res (Camb) 2011;93:265–273. doi: 10.1017/S001667231100019X. [DOI] [PubMed] [Google Scholar]
- 36.Emran S, Yang M, He X, Zandveld J, Piper MD. Target of rapamycin signalling mediates the lifespan-extending effects of dietary restriction by essential amino acid alteration. Aging (Albany NY) 2014;6:390–398. doi: 10.18632/aging.100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zajitschek F, Zajitschek SR, Friberg U, Maklakov AA. Interactive effects of sex, social environment, dietary restriction, and methionine on survival and reproduction in fruit flies. Age (Dordr) 2013;35:1193–1204. doi: 10.1007/s11357-012-9445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carey JR, Harshman LG, Liedo P, Muller HG, Wang JL, Zhang Z. Longevity-fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell. 2008;7:470–477. doi: 10.1111/j.1474-9726.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou S, Carey JR, Liedo P, Ingram DK, Yu B, Ghaedian R. Prolongevity effects of an oregano and cranberry extract are diet dependent in the Mexican fruit fly (Anastrepha ludens) J Gerontol A Biol Sci Med Sci. 2010;65:41–50. doi: 10.1093/gerona/glp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Wheeler CT, Alberico T, Sun X, Seeberger J, Laslo M, Spangler E, Kern B, de Cabo R, Zou S (2011) The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. Age (Dordr) 35:69–81 [DOI] [PMC free article] [PubMed]
- 41.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Min KJ, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster . Mech Ageing Dev. 2006;127:643–646. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Lee KP. Dietary protein:carbohydrate balance is a critical modulator of lifespan and reproduction in Drosophila melanogaster: a test using a chemically defined diet. J Insect Physiol. 2015;75:12–19. doi: 10.1016/j.jinsphys.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Ja WW, Carvalho GB, Zid BM, Mak EM, Brummel T, Benzer S. Water- and nutrient-dependent effects of dietary restriction on Drosophila lifespan. Proc Natl Acad Sci USA. 2009;106:18633–18637. doi: 10.1073/pnas.0908016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adler MI, Bonduriansky R. Why do the well-fed appear to die young? A new evolutionary hypothesis for the effect of dietary restriction on lifespan. BioEssays. 2014;36:439–450. doi: 10.1002/bies.201300165. [DOI] [PubMed] [Google Scholar]
- 47.Le Couteur DG, Tay SS, Solon-Biet S, Bertolino P, McMahon AC, Cogger VC, Colakoglu F, Warren A, Holmes AJ, Pichaud N, Horan M, Correa C, Melvin RG, Turner N, Ballard JW, Ruohonen K, Raubenheimer D, Simpson SJ. The influence of macronutrients on splanchnic and hepatic lymphocytes in aging mice. J Gerontol A Biol Sci Med Sci. 2014;70:1499–1507. doi: 10.1093/gerona/glu196. [DOI] [PubMed] [Google Scholar]
- 48.Solon-Biet SM, Walters KA, Simanainen UK, McMahon AC, Ruohonen K, Ballard JW, Raubenheimer D, Handelsman DJ, Le Couteur DG, Simpson SJ. Macronutrient balance, reproductive function, and lifespan in aging mice. Proc Natl Acad Sci USA. 2015;112:3481–3486. doi: 10.1073/pnas.1422041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakagawa I, Masana Y. Effect of protein nutrition on growth and life span in the rat. J Nutr. 1971;101:613–620. doi: 10.1093/jn/101.5.613. [DOI] [PubMed] [Google Scholar]
- 50.Ross MH, Bras G. Influence of protein under- and overnutrition on spontaneous tumor prevalence in the rat. J Nutr. 1973;103:944–963. doi: 10.1093/jn/103.7.944. [DOI] [PubMed] [Google Scholar]
- 51.Davis TA, Bales CW, Beauchene RE. Differential effects of dietary caloric and protein restriction in the aging rat. Exp Gerontol. 1983;18:427–435. doi: 10.1016/0531-5565(83)90021-9. [DOI] [PubMed] [Google Scholar]
- 52.Leto S, Kokkonen GC, Barrows CH., Jr Dietary protein, life-span, and biochemical variables in female mice. J Gerontol. 1976;31:144–148. doi: 10.1093/geronj/31.2.144. [DOI] [PubMed] [Google Scholar]
- 53.Leto S, Kokkonen GC, Barrows CH. Dietary protein life-span, and physiological variables in female mice. J Gerontol. 1976;31:149–154. doi: 10.1093/geronj/31.2.149. [DOI] [PubMed] [Google Scholar]
- 54.Goodrick CL. Body weight increment and length of life: the effect of genetic constitution and dietary protein. J Gerontol. 1978;33:184–190. doi: 10.1093/geronj/33.2.184. [DOI] [PubMed] [Google Scholar]
- 55.Slonaker JR. The effect of different per cents of protein in the diet I Growth. Am J Physiol. 1931;96:547–556. [Google Scholar]
- 56.Horakova M, Deyl Z, Hausmann J, Macek K. The effect of low protein-high dextrin diet and subsequent food restriction upon life prolongation in Fischer 344 male rats. Mech Ageing Dev. 1988;45:1–7. doi: 10.1016/0047-6374(88)90014-0. [DOI] [PubMed] [Google Scholar]
- 57.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 58.Masoro EJ, Iwasaki K, Gleiser CA, McMahan CA, Seo EJ, Yu BP. Dietary modulation of the progression of nephropathy in aging rats: an evaluation of the importance of protein. Am J Clin Nutr. 1989;49:1217–1227. doi: 10.1093/ajcn/49.6.1217. [DOI] [PubMed] [Google Scholar]
- 59.Nakagawa S, Lagisz M, Hector KL, Spencer HG. Comparative and meta-analytic insights into life-extension via dietary restriction. Aging Cell. 2012;11:401–409. doi: 10.1111/j.1474-9726.2012.00798.x. [DOI] [PubMed] [Google Scholar]
- 60.De Marte ML, Enesco HE. Influence of low tryptophan diet on survival and organ growth in mice. Mech Ageing Dev. 1986;36:161–171. doi: 10.1016/0047-6374(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 61.Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Gerontol. 2003;38:47–52. doi: 10.1016/S0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]
- 62.Lopez-Torres M, Barja G. Lowered methionine ingestion as responsible for the decrease in rodent mitochondrial oxidative stress in protein and dietary restriction possible implications for humans. Biochim Biophys Acta. 2008;1780:1337–1347. doi: 10.1016/j.bbagen.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Pamplona R, Barja G. Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochim Biophys Acta. 2006;1757:496–508. doi: 10.1016/j.bbabio.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 64.Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. Influence of the restriction of individual dietary components on longevity and age-related disease of Fischer rats: the fat component and the mineral component. J Gerontol. 1988;43:B13–B21. doi: 10.1093/geronj/43.1.B13. [DOI] [PubMed] [Google Scholar]
- 65.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol Metab. 2014;25:558–566. doi: 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins proper targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mercken EM, Mitchell SJ, Martin-Montalvo A, Minor RK, Almeida M, Gomes AP, Scheibye-Knudsen M, Palacios HH, Licata JJ, Zhang YQ, Becker KG, Khraiwesh H, Gonzalez-Reyes JA, Villalba JM, Baur JA, Elliott P, Westphal C, Vlasuk GP, Ellis JL, Sinclair DA, Bernier M, de Cabo R. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell. 2014;13:787–796. doi: 10.1111/acel.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010;153:289–298. doi: 10.7326/0003-4819-153-5-201009070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blouet C, Mariotti F, Azzout-Marniche D, Bos C, Mathe V, Tome D, Huneau JF. The reduced energy intake of rats fed a high-protein low-carbohydrate diet explains the lower fat deposition, but macronutrient substitution accounts for the improved glycemic control. J Nutr. 2006;136:1849–1854. doi: 10.1093/jn/136.7.1849. [DOI] [PubMed] [Google Scholar]
- 71.Foo SY, Heller ER, Wykrzykowska J, Sullivan CJ, Manning-Tobin JJ, Moore KJ, Gerszten RE, Rosenzweig A. Vascular effects of a low-carbohydrate high-protein diet. Proc Natl Acad Sci USA. 2009;106:15418–15423. doi: 10.1073/pnas.0907995106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Solon-Biet SM, Mitchell SJ, Coogan SC, Cogger VC, Gokarn R, McMahon AC, Raubenheimer D, de Cabo R, Simpson SJ, Le Couteur DG. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Rep. 2015;11:1529–1534. doi: 10.1016/j.celrep.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchell SE, Delville C, Konstantopedos P, Derous D, Green CL, Chen L, Han JD, Wang Y, Promislow DE, Douglas A, Lusseau D, Speakman JR. The effects of graded levels of calorie restriction: III. Impact of short term calorie and protein restriction on mean daily body temperature and torpor use in the C57BL/6 mouse. Oncotarget. 2015;6:18314–18337. doi: 10.18632/oncotarget.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitchell SE, Delville C, Konstantopedos P, Hurst J, Derous D, Green C, Chen L, Han JJ, Wang Y, Promislow DE, Lusseau D, Douglas A, Speakman JR. The effects of graded levels of calorie restriction: II. Impact of short term calorie and protein restriction on circulating hormone levels, glucose homeostasis and oxidative stress in male C57BL/6 mice. Oncotarget. 2015;6:23213–23237. doi: 10.18632/oncotarget.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitchell SE, Tang Z, Kerbois C, Delville C, Konstantopedos P, Bruel A, Derous D, Green C, Aspden RM, Goodyear SR, Chen L, Han JJ, Wang Y, Promislow DE, Lusseau D, Douglas A, Speakman JR. The effects of graded levels of calorie restriction: I. impact of short term calorie and protein restriction on body composition in the C57BL/6 mouse. Oncotarget. 2015;6:15902–15930. doi: 10.18632/oncotarget.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang X, Hancock DP, Gosby AK, McMahon AC, Solon SM, Le Couteur DG, Conigrave AD, Raubenheimer D, Simpson SJ. Effects of dietary protein to carbohydrate balance on energy intake, fat storage, and heat production in mice. Obesity (Silver Spring) 2013;21:85–92. doi: 10.1002/oby.20007. [DOI] [PubMed] [Google Scholar]
- 77.Noto H, Goto A, Tsujimoto T, Noda M. Low-carbohydrate diets and all-cause mortality: a systematic review and meta-analysis of observational studies. PLoS One. 2013;8:e55030. doi: 10.1371/journal.pone.0055030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naude CE, Schoonees A, Senekal M, Young T, Garner P, Volmink J. Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: a systematic review and meta-analysis. PLoS One. 2014;9:e100652. doi: 10.1371/journal.pone.0100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 80.Hession M, Rolland C, Kulkarni U, Wise A, Broom J. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes Rev. 2009;10:36–50. doi: 10.1111/j.1467-789X.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- 81.Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97:505–516. doi: 10.3945/ajcn.112.042457. [DOI] [PubMed] [Google Scholar]
- 82.Pedersen AN, Kondrup J, Borsheim E. Health effects of protein intake in healthy adults: a systematic literature review. Food Nutr Res. 2013 doi: 10.3402/fnr.v57i0.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lagiou P, Sandin S, Weiderpass E, Lagiou A, Mucci L, Trichopoulos D, Adami HO. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J Intern Med. 2007;261:366–374. doi: 10.1111/j.1365-2796.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 84.Trichopoulou A, Psaltopoulou T, Orfanos P, Hsieh CC, Trichopoulos D. Low-carbohydrate-high-protein diet and long-term survival in a general population cohort. Eur J Clin Nutr. 2007;61:575–581. doi: 10.1038/sj.ejcn.1602557. [DOI] [PubMed] [Google Scholar]
- 85.Sjogren P, Becker W, Warensjo E, Olsson E, Byberg L, Gustafsson IB, Karlstrom B, Cederholm T. Mediterranean and carbohydrate-restricted diets and mortality among elderly men: a cohort study in Sweden. Am J Clin Nutr. 2010;92:967–974. doi: 10.3945/ajcn.2010.29345. [DOI] [PubMed] [Google Scholar]
- 86.Nilsson LM, Winkvist A, Eliasson M, Jansson JH, Hallmans G, Johansson I, Lindahl B, Lenner P, Van Guelpen B. Low-carbohydrate, high-protein score and mortality in a northern Swedish population-based cohort. Eur J Clin Nutr. 2012;66:694–700. doi: 10.1038/ejcn.2012.9. [DOI] [PubMed] [Google Scholar]
- 87.Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Carbohydrate intake and incidence of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Br J Nutr. 2008;99:1107–1116. doi: 10.1017/S0007114507853360. [DOI] [PubMed] [Google Scholar]
- 88.Sluijs I, Beulens JW, van der AD, Spijkerman AM, Grobbee DE, van der Schouw YT (2010) Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 33:43–48 [DOI] [PMC free article] [PubMed]
- 89.Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344:e4026. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Simpson SJ, Batley R, Raubenheimer D. Geometric analysis of macronutrient intake in humans: the power of protein? Appetite. 2003;41:123–140. doi: 10.1016/S0195-6663(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 91.Wildman RP. Healthy obesity. Curr Opin Clin Nutr Metab Care. 2009;12:438–443. doi: 10.1097/MCO.0b013e32832c6db7. [DOI] [PubMed] [Google Scholar]
- 92.Liao CY, Rikke BA, Johnson TE, Gelfond JA, Diaz V, Nelson JF. Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell. 2011;10:629–639. doi: 10.1111/j.1474-9726.2011.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harrison DE, Archer JR, Astle CM. Effects of food restriction on aging: separation of food intake and adiposity. Proc Natl Acad Sci USA. 1984;81:1835–1838. doi: 10.1073/pnas.81.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lopez-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008;43:813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gonzalez-Freire M, de Cabo R, Bernier M, Sollott SJ, Fabbri E, Navas P, Ferrucci L. Reconsidering the role of mitochondria in aging. J Gerontol A Biol Sci Med Sci. 2015;70:1334–1342. doi: 10.1093/gerona/glv070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miquel J, Economos AC, Fleming J, Johnson JE., Jr Mitochondrial role in cell aging. Exp Gerontol. 1980;15:575–591. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- 98.Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Exp Gerontol. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 99.Scialo F, Sriram A, Naudi A, Ayala V, Jove M, Pamplona R, Sanz A. Target of rapamycin activation predicts lifespan in fruit flies. Cell Cycle. 2015;14:2949–2958. doi: 10.1080/15384101.2015.1071745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wei Y, Zhang YJ, Cai Y, Xu MH. The role of mitochondria in mTOR-regulated longevity. Biol Rev Camb Philos Soc. 2015;90:167–181. doi: 10.1111/brv.12103. [DOI] [PubMed] [Google Scholar]
- 101.Guarente L. Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol. 2007;72:483–488. doi: 10.1101/sqb.2007.72.024. [DOI] [PubMed] [Google Scholar]
- 102.Rambold AS, Kostelecky B, Lippincott-Schwartz J. Fuse or die: shaping mitochondrial fate during starvation. Commun Integr Biol. 2011;4:752–754. doi: 10.4161/cib.17667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holloszy JO (1997) Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol (1985) 82:399–403 [DOI] [PubMed]
- 105.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 106.Conti B. Considerations on temperature, longevity and aging. Cell Mol Life Sci. 2008;65:1626–1630. doi: 10.1007/s00018-008-7536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vaanholt LM, Daan S, Schubert KA, Visser GH. Metabolism and aging: effects of cold exposure on metabolic rate, body composition, and longevity in mice. Physiol Biochem Zool. 2009;82:314–324. doi: 10.1086/589727. [DOI] [PubMed] [Google Scholar]
- 108.Landsberg L. Core temperature: a forgotten variable in energy expenditure and obesity? Obes Rev. 2012;13(Suppl 2):97–104. doi: 10.1111/j.1467-789X.2012.01040.x. [DOI] [PubMed] [Google Scholar]
- 109.Simpson SJ, Raubenheimer D. Geometric analysis of macronutrient selection in the rat. Appetite. 1997;28:201–213. doi: 10.1006/appe.1996.0077. [DOI] [PubMed] [Google Scholar]
- 110.Slocum N, Durrant JR, Bailey D, Yoon L, Jordan H, Barton J, Brown RH, Clifton L, Milliken T, Harrington W, Kimbrough C, Faber CA, Cariello N, Elangbam CS. Responses of brown adipose tissue to diet-induced obesity, exercise, dietary restriction and ephedrine treatment. Exp Toxicol Pathol. 2013;65:549–557. doi: 10.1016/j.etp.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 111.Busiello RA, Savarese S, Lombardi A. Mitochondrial uncoupling proteins and energy metabolism. Front Physiol. 2015;6:36. doi: 10.3389/fphys.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008;7:552–560. doi: 10.1111/j.1474-9726.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 113.Mookerjee SA, Divakaruni AS, Jastroch M, Brand MD. Mitochondrial uncoupling and lifespan. Mech Ageing Dev. 2010;131:463–472. doi: 10.1016/j.mad.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gates AC, Bernal-Mizrachi C, Chinault SL, Feng C, Schneider JG, Coleman T, Malone JP, Townsend RR, Chakravarthy MV, Semenkovich CF. Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metab. 2007;6:497–505. doi: 10.1016/j.cmet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 115.Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, Hepple RT, Kraus WE, Reid MB, Bodine SC, Jakicic JM, Fleg JL, Williams JP, Joseph L, Evans M, Maruvada P, Rodgers M, Roary M, Boyce AT, Drugan JK, Koenig JI, Ingraham RH, Krotoski D, Garcia-Cazarin M, McGowan JA, Laughlin MR. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab. 2015;22:4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 116.Broskey NT, Greggio C, Boss A, Boutant M, Dwyer A, Schlueter L, Hans D, Gremion G, Kreis R, Boesch C, Canto C, Amati F. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J Clin Endocrinol Metab. 2014;99:1852–1861. doi: 10.1210/jc.2013-3983. [DOI] [PubMed] [Google Scholar]
- 117.Van Herrewege J. Nutritional requirements of adult Drosophila melanogaster: the influence of the casein concentration on the duration of life. Exp Gerontol. 1974;9:191–198. doi: 10.1016/0531-5565(74)90036-9. [DOI] [PubMed] [Google Scholar]
- 118.Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lushchak OV, Gospodaryov DV, Rovenko BM, Glovyak AD, Yurkevych IS, Klyuba VP, Shcherbij MV, Lushchak VI. Balance between macronutrients affects life span and functional senescence in fruit fly Drosophila melanogaster . J Gerontol A Biol Sci Med Sci. 2012;67:118–125. doi: 10.1093/gerona/glr184. [DOI] [PubMed] [Google Scholar]
- 120.Bruce KD, Hoxha S, Carvalho GB, Yamada R, Wang HD, Karayan P, He S, Brummel T, Kapahi P, Ja WW. High carbohydrate-low protein consumption maximizes Drosophila lifespan. Exp Gerontol. 2013;48:1129–1135. doi: 10.1016/j.exger.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu CT, Ingelmo P, Rand DM. GxGxE for lifespan in Drosophila: mitochondrial, nuclear, and dietary interactions that modify longevity. PLoS Genet. 2014;10:e1004354. doi: 10.1371/journal.pgen.1004354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Piper MD, Blanc E, Leitao-Goncalves R, Yang M, He X, Linford NJ, Hoddinott MP, Hopfen C, Soultoukis GA, Niemeyer C, Kerr F, Pletcher SD, Ribeiro C, Partridge L. A holidic medium for Drosophila melanogaster . Nat Methods. 2014;11:100–105. doi: 10.1038/nmeth.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liedo P, Carey JR, Ingram DK, Zou S. The interplay among dietary fat, sugar, protein and acai (Euterpe oleracea Mart.) pulp in modulating lifespan and reproduction in a Tephritid fruit fly. Exp Gerontol. 2012;47:536–539. doi: 10.1016/j.exger.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pirk CW, Boodhoo C, Human H, Nicolson SW. The importance of portein type and protein to carbohydrate ratio for survival and ovarian activation of caged honeybees (Apis mellifera scutella) Apidologie. 2009;41:62–72. doi: 10.1051/apido/2009055. [DOI] [Google Scholar]
- 125.Paoli PP, Wakeling LA, Wright GA, Ford D. The dietary proportion of essential amino acids and Sir2 influence lifespan in the honeybee. Age (Dordr) 2014;36:9649. doi: 10.1007/s11357-014-9649-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Preuss HG. Effects of diets containing different proportions of macronutrients on longevity of normotensive Wistar rats. Geriatr Nephrol Urol. 1997;7:81–86. doi: 10.1023/A:1008249001782. [DOI] [PubMed] [Google Scholar]
- 127.Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am J Physiol Endocrinol Metab. 2005;289:E429–E438. doi: 10.1152/ajpendo.00435.2004. [DOI] [PubMed] [Google Scholar]
- 129.Slonaker JR. The effect of different per cents of protein in the diet III Intake and expenditure of energy. Am J Physiol. 1931;97:15–21. [Google Scholar]