Abstract
Carbohydrates establish the third alphabet of life. As part of cellular glycoconjugates, the glycans generate a multitude of signals in a minimum of space. The presence of distinct glycotopes and the glycome diversity are mapped by sugar receptors (antibodies and lectins). Endogenous (tissue) lectins can read the sugar-encoded information and translate it into functional aspects of cell sociology. Illustrated by instructive examples, each glycan has its own ligand properties. Lectins with different folds can converge to target the same epitope, while intrafamily diversification enables functional cooperation and antagonism. The emerging evidence for the concept of a network calls for a detailed fingerprinting. Due to the high degree of plasticity and dynamics of the display of genes for lectins the validity of extrapolations between different organisms of the phylogenetic tree yet is inevitably limited.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-016-2163-8) contains supplementary material, which is available to authorized users.
Keywords: Agglutinin, Antibodies, Glycobiology, Glycome, Lectins, Sialylation, Sugar code
Introduction
An ubiquitous constituent of all types of cells are the complex carbohydrates. When looking at the surfaces of pro- or eukaryotes, they are present in different forms. These range from capsular polysaccharides and lipopolysaccharides to the glycan part of glycoconjugates (glycoproteins, glycolipids and proteoglycans) [1, 2]. Historically, capsular polysaccharides enabled landmark discoveries of general importance, far beyond the field of glycosciences: they were the first non-protein compounds described to be antigens [1, 3]. This proof-of-principle evidence that a glycan becomes the target of recognition in adaptive immunity laid the fundament for research into vaccination, currently with synthetic carbohydrate antigens. Equally intriguing, enabling their synthesis in the case of Pneumococcus (Diplococcus pneumoniae) underlies the transition from the harmless to the deadly phenotype, and this conversion by transformation with deoxyribonuleic acid (DNA) was the first demonstration that this type of biomolecule is the chemical equivalent of a gene [4]. In structural terms, synthesis of the polymer of cellobiuronic acid, the disaccharide building block of the type III pneumococcal “specific soluble substance,” is reconstituted by the uptake of DNA from virulent bacteria [1]. For mammals, manipulations of glycosylation on the genetic level can have similarly far-reaching consequences, what underscores the significance of the sugary coating of proteins and the cell surface. For example, switching off the enzymatic route to the class of complex-type N-glycans is lethal [5–8]. Of medical relevance, the emerging insights into congenital diseases of glycosylation attest the intimate connection of deviations from normal glycan assembly to clinical manifestations [9, 10]. Conversely, clinical disorders (here the lysosomal storage diseases mucolipidosis II and III) were incentive for studies revealing a site-specific phosphorylation on high-mannose-type N-glycans to be a postal code for glycoprotein delivery to lysosomes [11, 12].
As the association with the term “complex” signifies, to characterize structural aspects of the oligo- and polysaccharides posed enormous challenges to analytical chemistry, a key reason “why glycobiology has apparently lagged so far behind the other fields (genomics, proteomics)” [13]. Explicitly, “glycoconjugates are much more complex, variegated, and difficult to study than proteins or nucleic acids” [13]. The reason lies in the unsurpassed potential for variability offered by carbohydrates. In contrast to the chemically constant connections of the peptide bond and phosphodiester linkages, the glycosidic bond allows three different parameters to be altered. In detail, (a) anomeric status (α,β), (b) positions of the glycosidic linkage (1,1; 1,2; 1,3; 1,4; 1,6 for a monosaccharide with C1-anomeric center that forms a disaccharide with a hexopyranoses), and (c) ring size (pyranose or furanose), along with the frequent introduction of branching and site-specific substitutions, account for the high-density coding capacity of glycans, the first (linear chains) and second (branched glycans) dimensions of the sugar code [14–16]. The fact that the enzymatic machinery for glycan synthesis is equipped to realize this potential [17–19] strengthens the concept of the sugar code.
By increasingly discerning the principles of glycan structure and assembly (for a review on analytical developments, please see [20]), “the polysaccharides of mammalian connective tissue, and glycoproteins, begin to make biochemical sense for the first time ever …. They have become interesting molecules to contemplate in relation to the life of the cells. The ugly ducklings have begun to look a little more like swans” [21]. That the glycans are “ideal for generating compact units with explicit informational properties” [22] and are hereby suited to serve as “multipurpose tool” [2] makes their occurrence in all three urkingdoms of life readily understandable. Actually, commonalities in the synthetic routes exist, studied and characterized in detail in eu- and archaebacteria as well as eukaryotes [2, 23–27], so that the mapping of the glycome complexity becomes an aim. Detection of distinct determinants, without any purification, requires tools as used in the practice of ELISAs or immunohistochemistry. As done for proteins, glycan presence can also conveniently be monitored by antibodies. Fittingly, nearly 20 glycotopes belong to the cluster of differentiation (CD) panel of antigens (for an introduction into the CD system and complete listing of these CD markers, please see [28]). In addition to antibodies tracing their antigens, the same purpose is fulfilled by another class of proteins separate from these effectors of adaptive immunity. Its discovery shares its roots with disclosing occurrence that of blood-group-specific antibodies in the fertile ground prepared by work on the agglutination of erythrocytes (for historical reviews, please see [29, 30]). Actually, cross-testing of sera and red blood cells had led to the detection of blood-group antigens and antibodies of respective specificity by K. Landsteiner [31–33]. In experimental terms, monitoring the bridging of erythrocytes by biological material other than serum was instrumental to find antibody-like activities (in further research shown to be proteins) with equally specific binding properties.
Lectins: general aspects
In pioneering studies, with snake (Crotalus durissus) venom and bean (Ricinus communis) extracts [34, 35], the capacity of constituents of these mixtures to cause coagulation or agglutination of red blood cells had been disclosed (for a review on haemagglutinins as blood-typing reagents, please see [36]). Accordingly, the protein responsible for bridging erythrocytes was referred to as a haemagglutinin [37] or “Phytagglutinin” [38]. In analogy to serum antibodies, preparations containing such proteins could even selectively mediate erythrocyte aggregation that depends on the blood-group status. In a survey of 2663 varieties of plant seeds tested for this activity, 90 (3 %) contained agglutinins with specificity to blood groups [39]. Pursuing the work on seed extracts started in 1888 by H. Stillmark’s thesis, K. Landsteiner had experimented with these plant haemagglutinins “and wrote, probably about 1914, a paper entitled “Pflanzliche Hämagglutinine” on the subject. This paper reached the stage of page proof, but was never published” [40]. Later, in 1945, data of this report were summarized in a book [41], and this information prompted W. C. Boyd, who coined the term ‘lectin’ (please see paragraph after next), to continue the search for antibody-like activities with blood-group specificity.
Drawing on the known susceptibility of antibodies toward inhibitors that block their antigen-binding sites, it was tempting to similarly reveal the nature of haptens for these reagents and hereby the immunologically active structures of the histo-blood groups. In the seminal case of the eel (Anguilla anguilla) serum agglutinin (at that time called an antibody), which bridges erythrocytes of the blood group H(0), a carbohydrate turned out to be active: the monosaccharide l-fucose (Fuc; as α-methyl derivative and as free sugar, weakly as β-methyl form, not at all as d-isomer) was found to be the most potent inhibitor [42]. This study with a sugar receptor contributed to disclose that the ABH(0) antigen status, the molecular basis of fatal complications in transfusion medicine, is laid down in letters of the sugar alphabet [43].
The underlying capacity of an agglutinin for selecting distinct determinants from the surface of erythrocytes as cognate binding partners led W. C. Boyd, who had been motivated by Landsteiner’s work to continue testing seed extracts, to propose “the word lectin from Latin lectus, the past principle of legere meaning to pick, choose or select” [44], “to call attention to their specificity without begging the question as to their nature” [40]. In other words, proteins with different folds will meet this definition, if they are specific, as antibodies are. The mentioned hapten studies indicated that the molecular target is a sugar. Hereby, they connect to previous work on concanavalin A, which “unites with starch, glycogen, mucins, etc.” [45]. “This significant observation was not followed up and became forgotten” [46], until the studies on agglutination with the antibody-like reagents put sugars into the spotlight, firmly associating the descriptive term ‘lectin’ with carbohydrate binding. Because different classes of proteins have this capacity, for example transporters for mono- or disaccharides and enzymes of carbohydrate metabolism and processing, the definition needed to be sharpened by negative selection criteria. The current version stipulates that immunoglobulins (Ig), enzymes processing the bound glycan or sensor/transport proteins for mono- to oligosaccharides are excluded [47, 48]. Of terminological interest, the question on how to classify proteins with more than one specific property needs to be answered: if a site for glycan recognition is embedded into bi- to multimodular proteins, combining different activities which might cooperate as is the case for the mediator of ordered cell migration of slime molds [49], then its presence justifies to call the protein, here discoidin I, a lectin. As detailed below in the context of mucin-type O-glycosylation, enzyme and lectin sites can both be present in the same protein.
Viewed from the perspective of understanding glycan functionality and of searching for probes to detect glycans other than antibodies, sugar receptors, which fulfil these criteria, afford a natural toolbox to ‘read’ sugar-encoded information. They establish the link between the “explicit informational properties” [22] and one aspect of the actual bioactivity of glycans. Obviously, the expectation for the size of the toolbox is rather high, on condition that the sugar code plays a prominent role in cell sociology.
Systematic research over the years has provided a satisfying answer. Indeed, a wide variety of folds and specificities are encountered among lectins [50–55], and their diversity is especially relevant for biomedical considerations. In detail, a recent compilation as Gallery of Lectins [55] lists 15 folds for animal/human lectins, with the range of sugar specificities covering the natural diversity of glycans (for a detailed survey of methods to characterize this property, please see Table 1 in [55]). Besides the endo-repertoire, also ′foreign′ glycans and glycoconjugates are targets of tissue lectins. To enumerate a few examples, bacterial cell wall peptidoglycan with the β1,4-linked N-acetylglucosamine (GlcNAc)/N-acetylmuramic acid disaccharide as building block, the substrate of lysozyme, is bound by the bactericidal C-type lectin RegIIIγ (murine)/hepatointestinal pancreatic/pancreatitis-associated protein (HIP/PAP; human) [56], microbial cell surface d-galactofuranose (β-Galf) and 3-deoxy-d-manno-oct-2-ulosonic acid (KDO) by human intelectin-1 (harboring a fold only weakly related to that of another class of defence proteins, i.e. ficolins) [57] and the mycobacterial cord factor trehalose-6,6′-dimycolate by the macrophage inducible C-type lectin Mincle (also called CLEC4E or Clecsf9) [58–61].
In addition to development of different folds a second route enables lectin diversification: in special cases such as the β-sandwich, the same fold has apparently acquired capacity for glycan binding independently at different occasions. Within a lectin family, the sequence of the carbohydrate recognition domain (CRD), quaternary structure and modular design are subject to changes, all factors contributing to the growth of a family. Thus, in addition to antibodies as effectors in adaptive immunity, which tailors the receptor to fit its target, lectins are the products of structural adaptations between diverse platforms of protein folds and glycan epitopes. Used as tools, these sugar receptors can map the presence of their cognate glycan(s) in any test system.
In this review, which is complementary to our previous survey on glycan biochemistry [2], the pairing of glycans with lectins is the central theme. Moving step by step through the paper, we will first illustrate the application of glycan mapping with plant/fungal lectins on tissue sections. We then proceed to explaining the concept to view common carbohydrate epitopes of natural glycan chains (first of N-glycans, then of O-glycans) as code words. In this part, we appraise the glycans’ potential for coding by presenting detailed information on binding partners in situ. As consequence, the systematic consideration of the effect of stepwise elongations of a glycan chain on its ligand properties uncovers an enormous versatility of teaming up glycans and lectins, called functional pairing. The intimate relationship between a glycan structure as part of a glycoprotein and its receptor(s) will then be taken to glycolipids (as counterreceptors). To complete the first part, we will look at an instructive example of dynamic remodeling of glycan structures, which has a switch-like impact on lectin binding. Its connection to cell activation or differentiation and the orchestrated co-regulation of glycan remodeling with expression of receptor(s) set forth to make the evidence truly compelling that glycans are endowed with “explicit informational properties.”
Having substantiated the route of glycan-lectin recognition as a hot topic in research on cell-activity regulation and herein necessarily mentioned various types of lectins, a closer look then needs to be given to a certain family, to trace principles of phylogenesis and to introduce the network concept for endogenous lectins. In this area, data base mining and structural study combined with engineering and expression analysis are answering the pertinent questions on evolutionary diversity and functional redundancy/neofunctionalization. To meet the challenge to pin down structure–activity relationships, that is to rationalize why lectins have the design as analyzed, shaping (bio)chemically defined models of glycoconjugates and cell (vesicle) surfaces for functional study with natural and engineered lectins is an emerging promising approach. With focus on lectins, deviations from a wild-type status are not only products of drawing-board design but arise spontaneously in populations. That genetic polymorphisms, on the level of various species and individuals of a species, offer natural test cases for such functional study will be documented in the final part. This section thus opens the door to let readers enter to get acquainted with the enormous inter- and intraspecies dynamics of genomic representation of lectins. As outlined above, we start illustrating the specific interplay of glycans with lectins in the natural context, that is in tissue sections, by presenting examples of lectin histochemistry (for reviews, please see [62–66]) in the electronic supplement. This section, shifted from the printed text upon request of reviewers, and thus as important as the printed part, is accompanied by its figures and references in the supplement.
Lectins and glycans: functional pairing
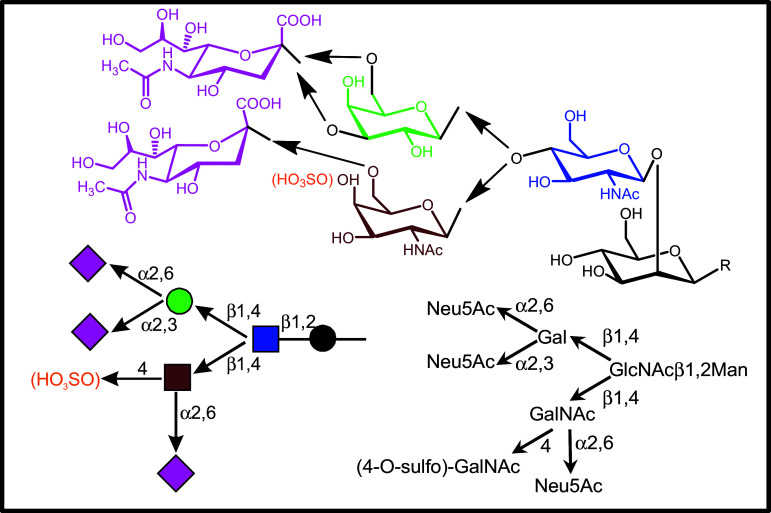
Looking first at branch maturation of complex-type N-glycans (please see Fig. 1 for structures of complex-type N-glycans), the synthetic pathway of branch elongation can physiologically already be arrested at the stage of the terminal GlcNAc, resulting in a product with an incomplete branch length (Fig. 1). The spatial accessibility of the GlcNAc moiety presented by the N-glycan core enables binding to the following human lectins:
The αM-chain of the αMβ2-integrin (CR3, CD11b/CD18, Mac-1), reactive with the N-terminal domain of a component of the von Willebrand factor receptor (GPIbα) of platelets and initiating their clearance by phagocytic cells [67, 68].
The C-type lectin langerin (CD207) of Langerhans cells, a protective pathogen receptor (this reactivity of the wild-type protein is even enhanced by a single nucleotide polymorphism (SNP; i.e. Lys313Ile) which reduces the affinity to 6′-sulfated Gal, the contact site for langerin in keratan sulfate) [69–71] (for further examples of the impact of natural sequence variability on lectin activity, please see section on lectin network).
The liver and lymph node sinusoidal endothelial cell C-type lectin LSECtin (CLEC4G), reactive with CD44 on T cells and viral glycoproteins, most efficiently blocked by the GlcNAcβ1,2Man disaccharide, the terminal part of this N-glycan [72–75]. The gene of this lectin is part of a cluster together with two closely related proteins (please see next paragraph and part on gene diversity in the last section).
Fig. 1.
Schematic illustration of the stepwise elongation of a branch of a complex-type N-glycan, starting from the β-GlcNAc-terminated structure. Gal/GalNAc moieties can then be added in alternative routes, and sialylation/sulfation completes the processing
In addition, lectins in host defense, especially C-type serum lectins (collectins) and the ficolins [76], may bind to glycoproteins carrying such determinants in clustered arrangement, multiantennary N-glycans presenting highly fucosylated termini affording an example for auto-reactivity of the collectin mannan-binding protein/lectin (MBP/L) [77–79]. Based on interaction studies with flaviviruses, the dendritic cell-specific ICAM-3-grabbing nonintegrin-related protein (DC-SIGNR (CD299, L-SIGN), CLEC4M; 77 % amino-acid identity to DC-SIGN) can also target GlcNAc-terminated N-glycans [80]. Interestingly, a member of the murine family of DC-SIGN-related proteins, i.e. SIGN-R2 (for information on gene display of this family, please see below), also has this ability [81].
As a consequence of the glycome’s complexity, presence of a terminal GlcNAc is not only found in N-glycans but also in O-glycans, here in β1,6-linkage in core 2/4/6 structures and β1,3/6-linkages in core 3 structures. Built from the same sugar unit but with changes in the anomeric position to α-linked GlcNAc and also to the 1,4-linkage position, O-glycans of stomach mucins harbor a further structural variation [82]. This type of presentation renders GlcNAc reactive with a plant lectin as tool preferring the α-anomeric presentation, i.e. Griffonia simplicifolia agglutinin-II (GSA-II) [50, 83], and a different human lectin, i.e. trefoil factor 2 (originally called pancreatic spasmolytic peptide) [84]. As part of the glycoprotein mucin 6, this epitope has antimicrobial activity against Helicobacter pylori and is discussed to exhibit suppressor properties against gastric cancer [85].
Overall, GlcNAc in β1,2-linkage in N-glycans is reactive with several tissue lectins. The mentioned natural structural variations in adding GlcNAc to cellular glycans (in terms of anomery and linkage positions) substantiate the complexity of the glycome and the predictions for broad-range functionality. Expectedly, other letters of the sugar alphabet have rather equal versatility, realized by a respective panel of products of glycogenes (glycosyltransferases), such as those facilitating the incorporation of l-fucose in α1,2/3/4/6-linkages [86–89].
Elongation of the N-glycan branch with the terminal β-GlcNAc by a β1,4-galactosyltransferase (β1,4GalT) abolishes the reactivities to the listed endogenous lectins. Accessibility at the branch end is thus mandatory. In general terms, each ‘code word’ thus really appears to have its own meaning. Of the seven enzymes with this specificity, proteins I-VI cooperate to introduce Gal into the N-glycan chain, with preferential activities on distinct acceptors described for each enzyme [90–92]. Interestingly, a distortion within this system can have far-reaching consequences on the presence of glycoproteins, for example reducing presence of the EGFR upon diminishing expression of β1,4GalT-I-VI [93]. The freely accessible β1,4-linked Gal of the N-glycan of a glycoprotein (ceruloplasmin in the pioneering study) is the reason why intravenously injected material with such epitopes (in this setting, after removal of sialic acid from the glycans) “disappears from the circulation within minutes and accumulates simultaneously in the parenchymal cells of the liver” [94]. This finding was explained by the presence of an endocytic sugar receptor in hepatocytes aptly called the hepatic asialoglycoprotein receptor. Its purification from detergent extracts of acetone powder of rabbit liver established the first case of an isolation of a mammalian lectin [95]. Clinically, this C-type lectin reduces the severity of disseminated intravascular coagulation during sepsis by eliminating platelets with terminal Gal moieties [96]. This process can also be a trigger for the production of thrombopoietin, connecting the lectin via Janus kinase 2/activator of transcription 3 signaling to platelet production [97]. Another C-type lectin, i.e. the blood-dendritic cell antigen-2 (BDCA-2/CLEC4C/CD303), shares binding to N-glycans of this type [98]. In the recognition process of the glycan, the hepatic C-type lectin will sense the 3′,4′-hydroxyl constellation with the 4′-axial position of Gal by coordination bonds with the Ca2+ of the lectin [48]. This active role of Ca2+, thus the strict dependence of lectin activity on presence of this cation at its place and on the formation of coordination bonds, is honored in terminology by the ′C′ as classification symbol, what explains the origin of the term ′C-type′.
Terminal Galβ1,4GlcNAc (LacNAc), in addition, is the canonical ligand of an entire family of lectins. It is defined by the β-sandwich fold and the pivotal role of a Trp residue, whose aromatic electron system makes a C-H/π-contact with the B-face of the Gal moiety. These galactose-binding lectins (galectins; for details on modular design and gene diversity, please see below in the section on the network; [99–103]) additionally dock onto the 4′,6′-hydroxyl positioning via hydrogen bonding [48, 55]. LacNAc presentation in local vicinity, that is the formation of clusters, will determine the reactivity of glycoproteins to this class of adhesion/growth-regulatory lectins. The affinity of binding critically depends on the degree of loading of such clusters with lectin, a difference of about 6000-fold separating the KD-values of interaction of the first from that of the last epitope in a nonavalent glycoprotein (i.e. asialofetuin, used as probe for lectin localization in Fig. S3b) [104]. Owing to spatial factors of glycan presentation, tissue lectins are highly selective with respect to targeting glycoconjugates as counterreceptors so that only distinct glycoproteins or glycolipids are functional counterreceptors (for a six-level model of this selection process, please see [28]).
Along the biosynthetic route of processing, elongation of the GlcNAc-terminated branch can alternatively occur by β1,3-galactosyltransferases (building type I LacNAc [105], not shown) and by one of the two human β1,4-N-acetylgalactosaminyltransferases (β1,4GalNAcT-III/IV) that elongate glycans of distinct glycoproteins such as pituitary glycoprotein hormones based on sequence recognition (the Pro-Leu-Arg motif) [106]. The resulting GalNAcβ1,4GlcNAc (LacdiNAc) structure maintains reactivity to the hepatic receptor (and the homologous macrophage receptor; please see below) but restricts galectin reactivity, with galectin-3 but not galectin-1 remaining to be a strong binder [107, 108]. This glycan’s further site-specific processing by 4′-sulfation, shown in Fig. 1, gives LacdiNAc a new meaning. The fact that this substituted disaccharide is a common trait in vertebrate evolution intimates its physiological significance. Placed into N-glycans of the glycoprotein hormones such as lutropin, this determinant becomes a recognition signal for the uptake by the hepatic reticuloendothelial cell receptor, here for the β-trefoil domain of the bifunctional lectin, which also contains C-type lectin domains specific for Man and is thus known as (tandem-repeat-type) macrophage Man receptor [109–112]. Binding and ensuing endocytic uptake of the glycoprotein determine the circulatory half-life of glycoprotein hormones, one of the functionalities of site-specific glycan sulfation. In fact, this substitution has relevance beyond this functional aspect, reaching a rather broad significance to implement recognitive affinity and specificity, when present on GalNAc, Gal or GlcNAc residues for example in selectin-dependent lymphocyte homing [111, 113, 114] or the recognition of keratan sulfate by langerin mentioned above. As alternative to sulfation, LacdiNAc is a substrate for α2,6-sialylation by β-galactoside α2,6-sialyltransferase-II (ST6Gal-II), with about 3.1- to 3.5-fold higher activity on this disaccharide than LacNAc [115, 116].
The same applies to the extension of the LacNAc unit by sialylation in α2,3- or α2,6- linkage, which also adds a negative charge into the sugar chain (Fig. 1). The type of conjugating sialic acid to Gal, i.e. by 2,3- or 2,6-connections, is not random but depends on the nature of the individual glycan antenna relative to the core. The α2,6-sialyltransferase “appears to highly prefer” to act on the Galβ1,4GlcNAcβ1,2Manα1,3 branch so that it is the acceptor of the first sialic acid in respective enzyme assays [117]. The natural occurrence of α2,3/6-sialylated N-glycans, with overlapping and distinct patterns, has been illustrated by lectin histochemistry in Fig. S2e,f. Sialylation (as sulfation does) changes the character of the ‘sugar code word’. First, the negative charge adds a new docking point for lectins, modulating the reactivity of LacNAc by secondary interactions or becoming the key determinant sui generis for lectins with specificity to N-acetylneuraminic acid and its many derivatives, which belong to the family of sialic acids [118] (please see below). Next, a hydroxyl group of the Gal moiety is now occupied, and this blocks accommodation by a C-type lectin requiring the 3′-hydroxyl or a galectin requiring the 6′-hydroxyl group for contact building. Besides this difference in the position of the hydroxyl group, the inclusion of the 6′-exocyclic hydroxymethyl group into the linkage to the sialic acid has a further consequence: it adds a third bond to this system, giving the α2,6-linked sialylgalactose a third degree of spatial (rotational) freedom. Because the differences in energy levels between a group of low-energy conformers is below 2 kcal/mol, its overall internal degree of flexibility is rather high when compared to that of the α2,3-linked isomer [119]. In a binding process, arresting this flexible system into a rigid conformer would cause a substantial entropic penalty. Because glycan-lectin binding is often favored by the opportunity of selecting an energetically privileged conformer of the ligand, restricting the flexibility at this position is a major source of entropic penalty, in the case of PSL which accommodates α2,6-sialylated LacNAc (please see Fig. S1, reaching 73 % of this contribution in the thermodynamic balance sheet [120]). This type of sialylation shuts out reactivity to galectins but is tolerated by the hepatic asialoglycoprotein receptor, and N-glycans with α2,6-sialylgalactose termini are reactive with another C-type lectin, i.e. murine SIGN-R1 (also known as one of the murine proteins related to human DC-SIGN/DC-SIGNR; comparison of the respective gene displays in man and mouse given below) [121–124]. In contrast, α2,3-sialylation has opposite effects on lectin reactivity: it impairs asialoglycoprotein receptor binding, a means to prolong serum presence of therapeutic (neo)glycoproteins [125–127], or markedly enhances galectin reactivity, seen in the case of human tandem-repeat-type galectin-8 with nM affinity (for further structural information on galectins and their modular design, please see below) [128]. According to the given classification, sialylation differentially affects the affinity of terminal LacNAc to two lectin classes, and it is essential for a third group.
As already noted above, the terminal sialic acid becomes the contact site for the sialic acid-binding immunoglobulin-like lectins (siglecs), a class of the lectins with the immunoglobulin-like fold (I-type) [129–131]. Sialoadhesin (siglec-1) and myelin-associated glycoprotein (siglec-4) are specific for α2,3-sialylation, CD22 (siglec-2; with two forms differing in the number of Ig C2-set domains with six in CD22β and four in CD22α due to alternative splicing) is specific for α2,6-sialylated glycans, and this for glycan chains of different types of glycoconjugates (glycoproteins and gangliosides; please see below and in the electronic supplement).
In overview, each addition of a monosaccharide to the growing N-glycan branch (and its ensuing substitution) matters for binding properties of endogenous lectins. A glycan can thus be likened to a code word, and a structural change conveys a new message. Altering the structural context at anomeric and at linkage positions, elongating the chain or introducing substitutions are means to the same end. If this principle has fundamental value, then it must hold true also for O-glycans. We put this hypothesis to the test, here for the series of the mucin-type core 1 mono- to tetrasaccharides as shown in the electronic supplement.
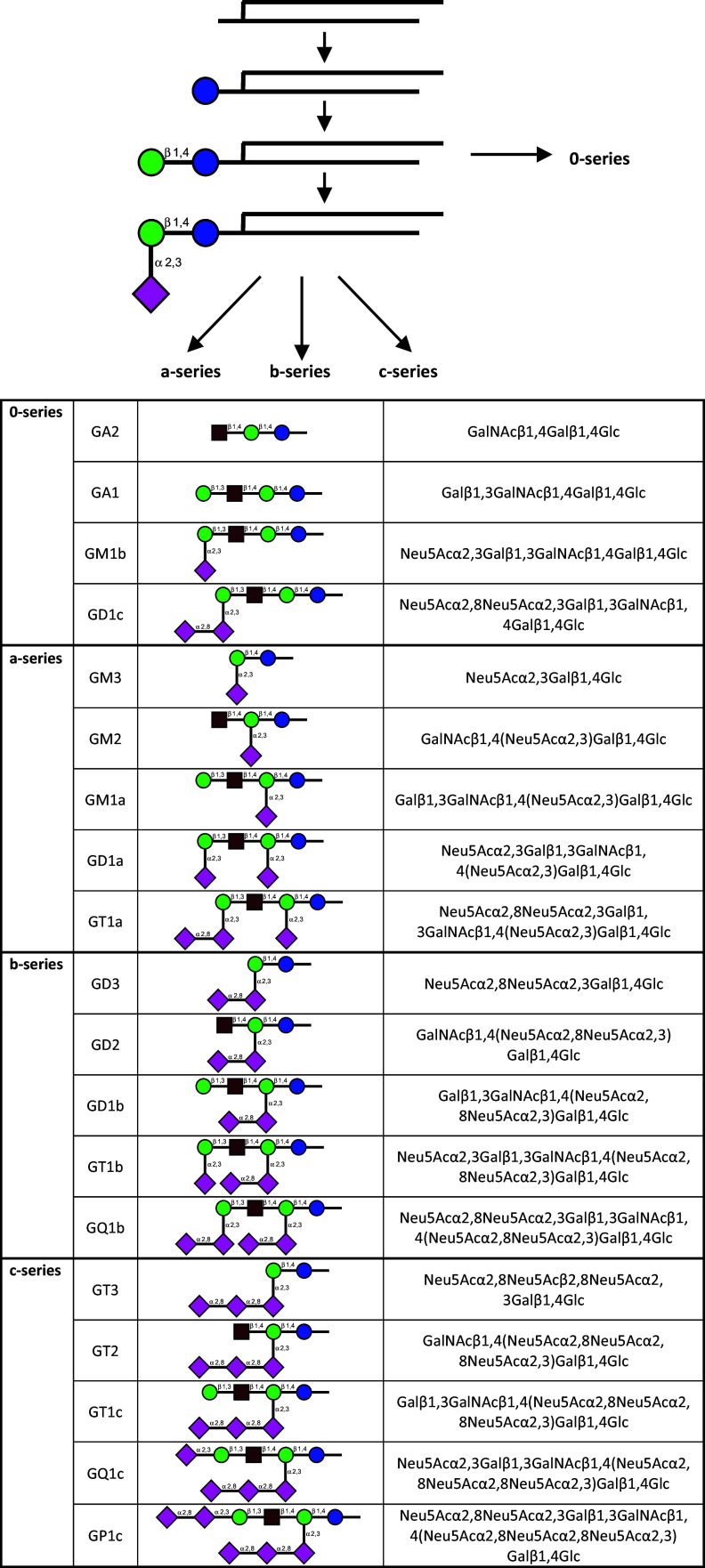
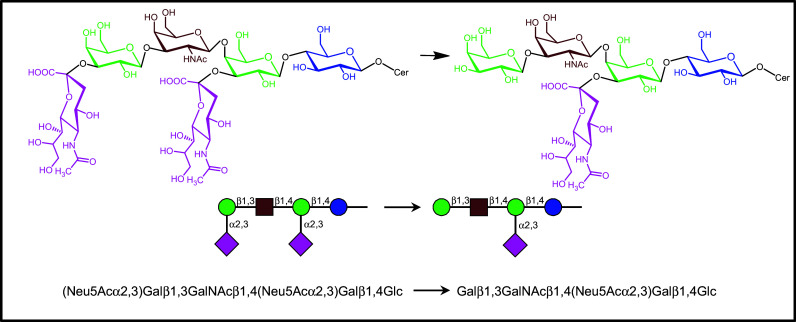
Given the account on the T(F) antigen and its α2,3-sialylation in the second part of the electronic supplement, this information immediately guides from the core 1 O-glycans to another class of glycoconjugates, i.e. glyco(sphingo)lipids. The sphingosine (elaborated to a ceramide by adding a second acyl group) is the second molecular backbone to present this epitope (here always as β-anomer) and many other glycans. Introduction of a sialic acid converts the neutral glycosphingolipid such as the gangliotetraosylceramide (with the terminal β-isomer of the T(F) antigen) into gangliosides, the major sialoglycoconjugate in the nervous system (the biosynthetic pathways for ganglio-series gangliosides with the different modes and positions of sialylation are summarized in Fig. 2; please note that intricate developmental regulation of presence of distinct gangliosides is a strong indicator of a broad functional significance of this type of glycan tailoring). As with the core 1 disaccharide, the terminal α2,3-sialylation of the pentasaccharide of ganglioside GM1 present in the a-series will produce the siglec-1 ligand GD1a, via the terminal sialic acid as contact site [S77]. GD1a is also a potent ligand for the tandem-repeat-type galectin-8 [132]. The two sialic acids presented by this ganglioside both are the docking sites for the adenovirus type 37, which causes the highly contagious epidemic keratoconjunctivitis [133]. Its fiber knops make direct contact with this part of the sugar chain [134]. By their prominent presentation of sialic acids shown in Fig. 2, gangliosides are often binding partners for siglecs. This reactivity warrants to briefly introduce ganglioside terminology. Using the abbreviations G (=ganglioside), then M (=monosialo), D (=disialo), T (=trisialo) and Q (=quatrasialo) and a number obtained from the length of the sugar chain of the neutral monosaccharides according to (5 − n), the Svennerholm nomenclature is established [135–138]. As is the case in biosynthesis of glycan chains for glycoproteins [S78], a step along the route, for example adding GalNAc to GM3/GD3 (Fig. 2), commits the product to its destiny, here branch extension without any further internal α2,8-sialylation. As contact site, the appearance of an α2,8-linked sialic acid, as in GD3, GT1b or GQ1b, makes the sugar chain reactive with distinct siglecs, especially siglecs-7 and -9 [139, 140]. With siglec-7, this moiety makes seven direct hydrogen bonds and-common for siglecs-the salt bridge between its carboxylate group and the conservative (signature) arginine (Arg124), added are three solvent-mediated hydrogen bonds and stacking with Trp132 [140]. Forming the contact pattern elicits a pronounced conformational shift of the C–C′ loop from its basic, bowl-like character to a hydrophobic, slightly convex shelf, an area of specificity determination covered by three moieties of the GT1b glycan. In other siglecs, conformational reordering can be confined to a minor extent, as seen in siglec-5 (CD170) [141] and siglec-1 [142]. Siglec-7 also binds the disialosyl globopentaosylceramide headgroup with α2,3/6-linked sialic acids in respective positions, and this interaction generates a negative signal for anti-tumoral cytotoxicity of natural killer cells [143]. Siglec-4, which offers two partners for salt bridges (Lys67, Arg118), shares the strong binding to GT1b and GQ1b [144].
Fig. 2.
Enzymatic routes of Golgi-based synthesis of gangliosides, starting from lactosylceramide in the 0-series, from GM3 in the a-series, from GD3 in the b-series and GT3 in the c-series (for details on terminology, please see [135, 136]). As in branch extensions of glycan chains on glycoproteins, the α2,8-sialylation leads to di- and trisialosides
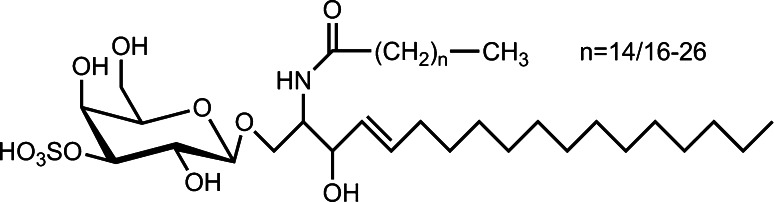
On the other side of the spectrum of chain length, a particular reactivity has been delineated: an interaction of GM3′s glycan with β-GlcNAc termini of N-glycans of the EGFR, a carbohydrate-carbohydrate interplay of physiological relevance [145–148]. This mode of recognition of a binding partner by β-GlcNAc adds to its ligand capacity to the tissue lectins listed above. When further reducing the length of the carbohydrate chain on the sphingolipid, already a single unit is sufficient for engagement in a recognition process. This principle is documented for the sulfatide, whose general structure is shown in Fig. 3. This glycoconjugate is another example of the association of a monosaccharide to lectins. While a potential role in eliciting inflammatory responses via a binding to the V-set-containing Ig-like receptor LMIR5/CD300b is emerging [149], its essential character as routing signal in apical and axonal transport of glycoproteins via galectin-4 is established, here with the headgroup presented by a scaffold with long-chain fatty acids (please see supplementary material, Fig. S5 in [150]) [151–154]. The heat shock protein (Hsp) 70s also has a specific site to bind this sulfogalactolipid, whose headgroup has been suggested to be a mimic of tyrosine phosphate [155, 156]. Loading to CD1a-c for T helper (type 1/2) cell priming, NK T cell recognition with formation of CD1d/sulfatide complexes as well as interaction with P- and L-selectins, with chemokines (e.g. IL-8/CXCL8, MCP-1/CCL2 or SDF-1α/CXCL12), with scavenger receptors and with bacterial receptors as adhesins further documents the wide activity profile of the sulfatide [28, 157–161]. Similar to the 4′-sulfated GalNAc headgroup of N-glycans, shown in Fig. 1, the combination of the monosaccharide (here with its free 4′, 6′-hydroxyl groups) and the 3′-substitution accounts for implementing specific ligand capacity.
Fig. 3.
Schematic illustration of the general sulfatide structure. The chain length of the fatty acid can vary so that different forms of this glycosphingolipid are physiologically present
In overview, a sugar headgroup presented by different backbones and its substitution or extensions are the source for a wide panel of binding partners for tissue lectins. All these relationships of functional pairing give incentive for designing and testing corresponding neoglycoconjugates in reverse lectin histochemistry, for GM1′s pentasaccharide for instance (please see Fig. 2, a-series) as lysoganglioside attached to a carrier protein [162, 163], this neoglycoconjugate, together with carrier-immobilized GlcNAc, helpful in differential diagnosis of mesothelioma and metastatic carcinoma of the pleura [164]. So far, in this context, the origin of glycan diversity in situ has mostly been considered to arise from the biosynthetic route. Taking into account the gradual appearance of the T(F) antigen on the erythrocyte surface by desialylation of the trisaccharide (please see Fig. S4) by bacterial sialidases, the reason why it takes time to make them responsive to serum antibodies against this antigen, as instructive example [28, S70], a well-controlled, specific and limited cleavage of a glycosidic bond can turn a cryptic signal into a physiological counterreceptor, with biomedical consequences as the polyagglutinability by removing sialic acid from the sialylated T(F) antigen is [S70, 165–168].
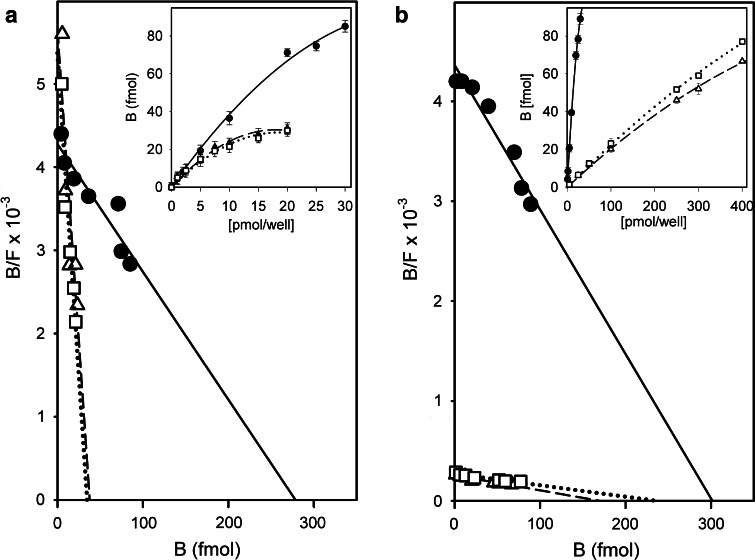
A prominent example for the impact of glycan remodeling is the neuraminidase (Neu3)-dependent desialylation of ganglioside GD1a (Fig. 4) (for an overview on mammalian sialidases, please see [169]). Cell surface presence of this enzyme makes it possible to reprogram this aspect of glycan display. Overall, a new epitope (GM1) replaces its precursor (GD1a), both of them with their own ligand properties. The conversion to ganglioside GM1 has been identified as a switch for neuronal differentiation and neurite outgrowth [170–177]. Underlying a contribution to the regulation of these processes, GD1a is a contact partner for siglec-4, this recognition sending a negative signal [178–180]. After losing GD1a, the resulting gain in ganglioside GM1 will lead to a positive signal [181, 182], one effector route operating via the binding to a cross-linking galectin [182–186] (a further route involves direct GM1 association to receptors such as Trk, the high-affinity tyrosine kinase-type receptor for nerve growth factor, or GM1 activity as coreceptor for fibroblast growth factor 2 [187–189]). On human neuroblastoma (SK-N-MC) cells, growth regulation goes hand in hand with increased cell surface presence of galectin-1, a truly effective functional pairing between a distinct receptor (lectin) and its glycan-based counterreceptor. Homodimeric galectin-1, which does not bind GD1a’s glycan (the terminal sialic acid precluding the interplay), and also galectins-2 and -7 are active in this respect (for structural illustrations, please see below in the section on the lectin network). Underlining the natural versatility of this pathway, orchestrated upregulation of GM1/galectin-1 is also active in effector (presenting GM1)/regulatory T cell (delivering galectin-1) communication. Engaging α5β1-integrin, which forms complexes with GM1, and their cross-linking by homodimeric galectin-1, a downstream protein, which is upregulated by stimulation of effector T cells, becomes activated and elicits the cellular response, i.e. the transient receptor potential [canonical] channel 5 (TRPC5). Channel opening allows the intracellular Ca2+ level in effector T cells to rise in order to attenuate auto-aggression [190–192]. Autophosphorylation of focal adhesion kinase as well as activation of phospholipase Cγ and phosphoinositide-3 kinase are part of the signaling pathway, toward effector T cell anergy/apoptosis and, similarly, axonogenesis in cultures of murine cerebellar neurons. The dependence of cell reactivity on ganglioside GM1 had been ascertained in neuroblastoma cells by blocking ganglioside synthesis at the level of biosynthesis of glucosylceramide, what leads to drastically diminished galectin-1 binding (Fig. 5a) [193]. The pentameric cholera toxin and presence of a neuraminidase inhibitor similarly precluded lectin binding [183]. The importance of a clustered presentation of the ganglioside for high-affinity binding was documented by depleting the membrane of cholesterol (Fig. 5b) [193]. Work on (glyco)lipid monolayers as models for cell surfaces reducing the complexity of surface composition and enabling to establish density variations for ganglioside GM1 corroborated these conclusions drawn from experiments with cells [194].
Fig. 4.
Dynamic glycan remodeling by the (cell surface) ganglioside neuraminidase (Neu3). The hydrolytic removal of the branch-end sialic acid converts GD1a to GM1 (for details on ensuing consequences of this processing for ligand properties, please see text)
Fig. 5.
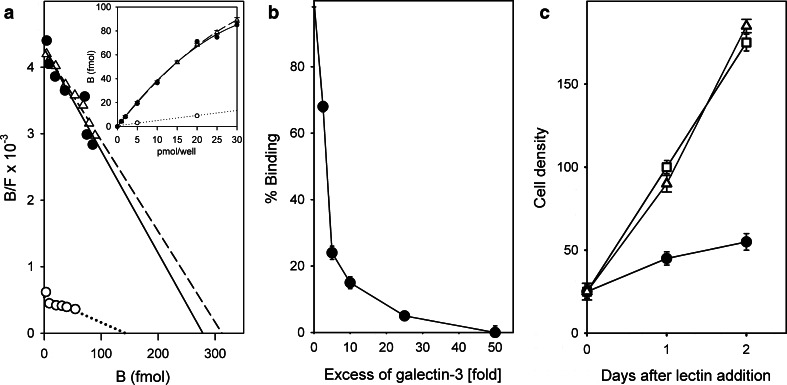
Effect of exposure of human neuroblastoma (SK-N-MC) cells to inhibitors of glucosylceramide biosynthesis to block generation of gangliosides [a, for 10 days; 50 µM N-butyldeoxynojirimycin (open triangle) or 10 µM threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (open square)] and to reagents for cholesterol depletion [b; 5 µM hydroxypropyl-β-cyclodextrin (triangle) or 3 µg/ml filipin III (open square)] on binding of radioiodinated galectin-1 (untreated control cells: filled circle). Data of the binding curves (inset) were algebraically processed by Scatchard analysis (from [193])
On the level of the pentasaccharide, galectin-1 selects a distinct conformer allowing it to come into contact with both branches, different from the glycan’s shape when entering the binding site of cholera toxin [184]. Thus, the same ligand can be selected in different shapes (conformers) by two lectins. This is a starting point for rational drug design with the aim to minimize reactivity of a drug hitting the toxic lectin for the endogenous receptor and an example for the third dimension of the sugar code. That glycans often adopt only few low-energy conformations, the reason to liken them to ‘a bunch of keys’ [195], helps keep the entropic penalty upon binding low. When the lectin responds to ligand binding with restructuring and a reduction of the gyration radius, as first detected for human galectin-1 by small angle neutron scattering and fluorescence correlation spectroscopy [196, 197], with impact on its structural entropy, as detectable by NMR spectroscopy or monitoring hydrogen–deuterium exchange [198–200], then the binding process is further fueled. The particular structural features of the pairing can modulate the extent of this favorable contribution.
On the side of the lectins targeting the same glycan, functional antagonism between cholera toxin and galectin-1 and also between two binders from the galectin family (galectins-1 and -3) had been detected in this system: these two proteins, i.e. the bacterial lectin and galectin-3, compete with galectin-1 for GM1 binding but are not active as growth regulators in the neuroblastoma cells [201, 202]. As shown in Fig. 6, both homodimeric galectin-1 and the chimera-type galectin-3 with its non-triple-helical collagen-like tail associated to the lectin domain, as shown schematically in Fig. 7, bind with high affinity to neuroblastoma (SK-N-MC) cells (Fig. 6a). Proteolytic removal of this tail abolishes the high-affinity binding (Fig. 6a), and this at the distinct threshold between collagen-like repeats IV/V, as delineated when testing stepwisely truncated deletion variants [203]. Evidently, this section is crucial for an aggregation of galectin-3 in the presence of ligand. Adding increasing amounts of galectin-3 to galectin-1 reduced its cell-surface binding (Fig. 6b) and negative effect on cell growth (Fig. 6c), establishing clear evidence for functional antagonism.
Fig. 6.
Galectin binding to human neuroblastoma cells and functional competition between galectins in cell growth regulation. a Radioiodinated homodimeric galectin-1 (filled circle solid line) and chimera-type galectin-3 (open triangle dashed line) bind with similar affinity to human neuroblastoma cells, whereas proteolytic removal of the N-terminal stalk section from galectin-3 impairs binding (open circle dotted line); inset binding curves. b Reduction of cell surface binding of labeled galectin-1 by increasing concentrations of label-free galectin-3. c Impact of galectin-1 on cell growth (filled circle) relative to the mock-treated control (open square) and the neutralizing effect of galectin-3 applied at a 10-fold excess relative to the concentration of galectin-1 (open triangle) (from Figs. 6–8 in [201], extended by Fig. 2E, F in [192])
Fig. 7.
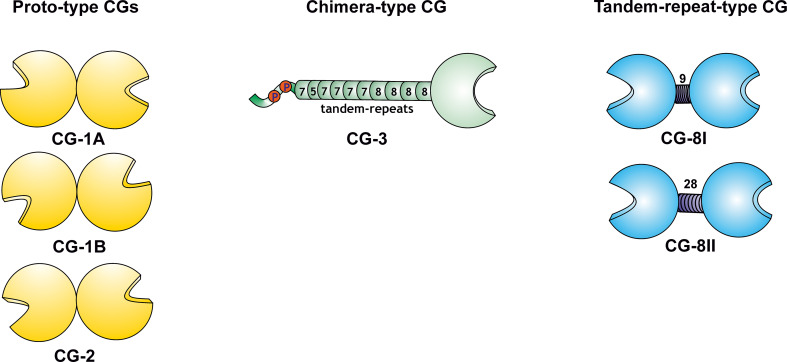
Schematic illustration of the three types of modular arrangement of galectins, with the five canonical CGs as example. The proto-type (homodimeric) proteins with similarity to mammalian galectins-1 and -2 (the paralogue pair CG-1A/-B and CG-2; relative positioning of CRDs given as in respective crystal structures), the chimera-type CG-3 (constituted by the C-terminal carbohydrate recognition domain (CRD), 10 Gly/Pro-rich sequence repeats with a length of five to eight amino acids and an N-terminal peptide with two sites for Ser phosphorylation) and the tandem-repeat-type CG (with two different CRDs separated by a linker, its two lengths arising from alternative splicing) with similarity to mammalian galectin-8, thus referred to as CG-8
Evidently, as shown above for PILRs, lectins of the same family can form a network with opposing activities, in this case via structural differences within the complexes of lectin with its cell surface counterreceptors. In addition to differences in counterreceptor selection on the cell surface, as documented for T cell growth regulation [204], the modular architecture of lectins will determine the spatial characteristics of these complexes and hereby their capacity for triggering signaling. Thus, the individual structural aspects of each lectin (for the CRD and for the modular assembly) and expression profiles will be key determinants of a lectin network. Both properties will be analyzed for galectins in the next section. A search for the best suited organism, with full representation of galectin display at a minimum number of proteins, will be helpful to simplify the task of monitoring expression profiles.
The lectin network: case study of galectins
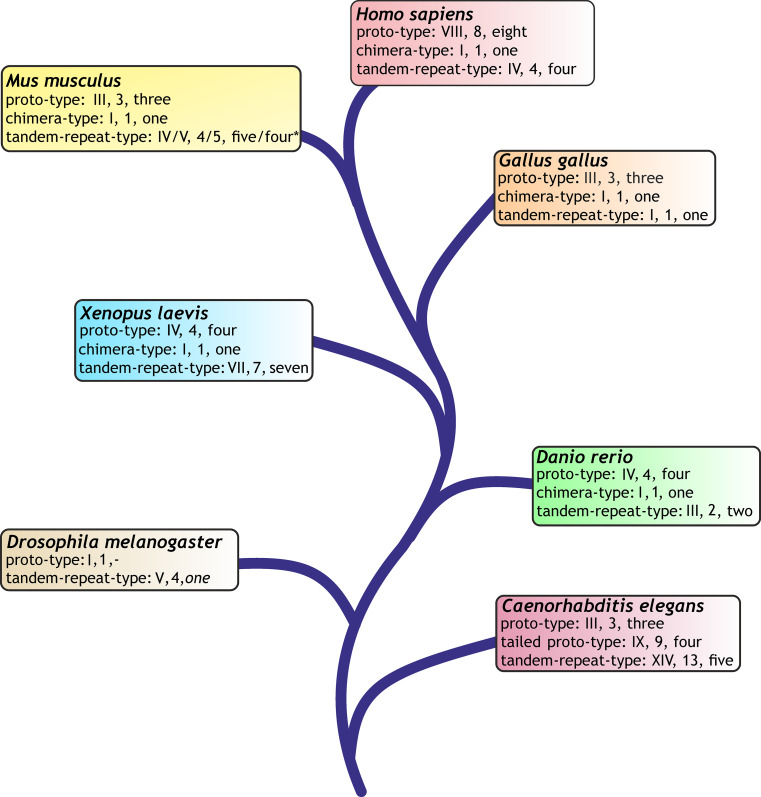
The CRD of galectins is presented in three types of structural organization (Fig. 7) [102, 103, 205]. This level of variability in protein architecture is relatively small compared to that of C-type lectins but offers more modes of design than the siglecs with their V-set lectin domain on top of a chain of C2-set Ig-like modules or the two tandem-repeat-type P-type lectins binding mannose-6-phosphate (“P” stands for the phosphomannosyl specificity), a routing signal for lysosomal enzymes already mentioned in the introduction, and four other proteins with homology to that CRD [130, 206–208]. A fundamental question concerns the functional meaning of the three types of molecular architecture including the range of natural variations, e.g. arising from an alternative splicing. As documented above in Fig. 6c by a functional assay, even functional antagonism is possible. The accurate dissection of the mode of cooperation and of structure–activity relationships is aided by availability of defined tools/assays. Using chemically programmable test systems, i.e. vesicle-like glycodendrimersomes self-assembled from amphiphilic glycodendrimers, for assessing capacity of lectins to bridge two particles as in haemagglutination (trans-interactions) as ligands as well as engineered and natural variants as receptors, new structure–activity correlations have already been discerned, also including to measure the response to precisely defined changes in the density of (cell) surface glycans [209–212]. So covalent connection of the two CRDs of galectin-1 by a linker enhanced its capacity for trans-interactions [211, 213], presence of a natural sequence variation in galectin-8 (at position 19, far from the contact sites with the sugar [128]) reduced it [210, 212]. These results encourage systematic testing. In phylogenetic overview, these three types of molecular design are recurring themes among galectins in diverse organisms. Of note, the respective phylogenetic survey of the galectin family among a wide panel of organisms (please see [103] for details) disclosed different degrees of diversification for each type and the total number of genes (Fig. 8).
Fig. 8.
Phylogenetic tree of model organisms and their display of canonical galectins (for modes of modular organization, please see Fig. 7) on the level of the gene (Roman number), transcript (Arabic number) and produced protein (numerical information). For details on search algorithms and data bases, please see [103, 253]
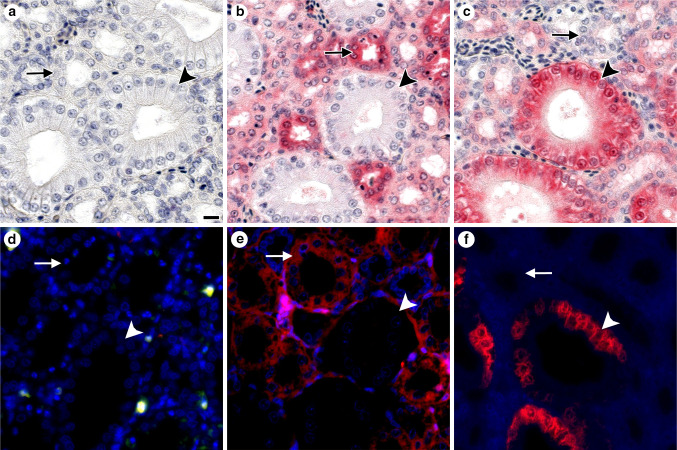
To discern principles of the galectin network most readily, an organism with representation of all three structural types and a relatively small number of canonical proteins will be a suitable study object as model. According to Fig. 8, the chicken is thus the choice meeting these criteria, and Fig. S3a,b had already presented evidence for presence of Lac/LacNAc-specific binding, a hallmark of galectin reactivity, in chicken kidney. As the comparison between glyco- and immunohistochemical staining profiles in kidney cortex attests, reactivity to the lactoside part of (neo)glycoconjugates can be attributed to galectin presence, here chicken galectins (CG)-1A, -2, -3, and -8 (Fig. S3c, d). Absence of reactivity for CG-1B served as negative control. Each lectin has its distinct expression profile (Fig. S3c, Fig. 9) (for further information, please see Table 2 in [103] and [214, 215]). What is furthermore noteworthy is that the CGs are not only present extracellularly but also at different sites within the cell, as illustrated in Fig. 10. Obviously, these proteins appear to be multifunctional, and their spectrum of binding partners includes intracellular proteins [216, 217]. A compilation of identified ligands, separated into the categories of glycoconjugates and protein, is presented for mammalian galectins-1 and -3 in Table 1. As seen for lectins, a glycoconjugate such as the ganglioside GM1 can also be present at more than one site, that is it is also detectable in the nuclear membrane and sarco/endoplasmic reticulum [218–220]. CGs, as mammalian galectins do, thus have their distinct expression profiles at various sites of the cell. Considering the potential of functional cooperation/antagonism, monitoring of the complete set of lectins (fingerprinting) is advisable in such immunohistochemical studies also in organisms with more complex galectin display.
Fig. 9.
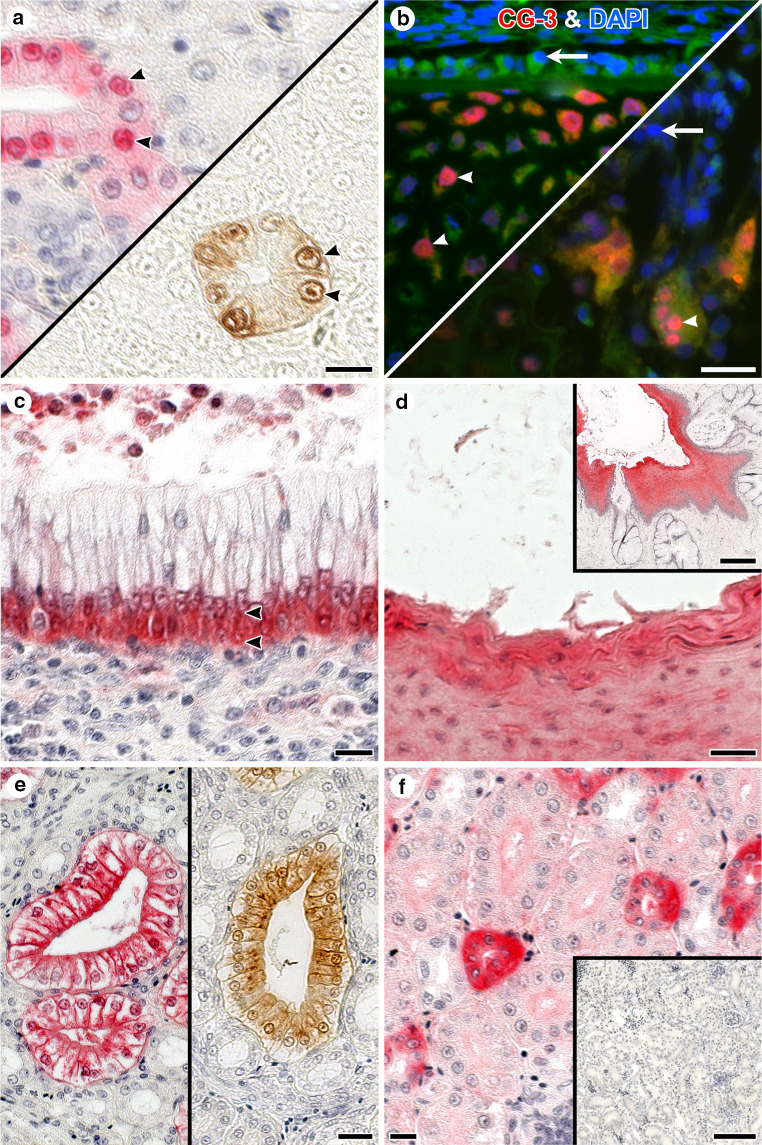
Immunohistochemical detection of CGs by light (a–c) and fluorescence (d–f) microscropy in sections of adult chicken kidney illustrating non-overlapping staining profiles (please see also Fig. S3c, d). For fluorescence microscopy, an Alexa Fluor 568-labeled second-step antibody (goat anti-rabbit IgG) was used to visualize sites of binding by the primary (CG-specific) antibody preparation (please see also Fig. 10). Application of anti-CG-1B served as control to exclude antigen-independent staining (a, d). Epithelial cells of collecting tubules (arrowheads) were negative for CG-1A (b, e), but positive for CG-3 (c, f). Intensity of staining of the epithelial cells of thick loops (arrows) was mostly strong for CG-1A (b, e), but negative to weak for CG-3 (c, f). The following concentrations of antibodies were used after systematic testing in each case to reach an optimal signal-to-background ratio: anti-CG-1B: 0.5 µg/ml (a, d), anti-CG-1A: 1 µg/ml (b, e) and anti-CG-3: 0.016 µg/ml (c) or 0.06 µg/ml (f). Scale bar in a is 10 µm and applies to microphotographs a–f (for details on tissue preparation and the immunohistochemical protocol, please see [214, 215, 274, 275]; Table S1 in [214] summarizes the immunohistochemical data reported in [214, 274, 275], Figs. 10i and 11 in [214] as well as Figs. 8 and 9 in [186] present respective microphotographs)
Fig. 10.
Illustration of the diversity of subcellular localization of CGs. In light microscopy, red coloring is based on alkaline phosphatase/Vector Red substrate reaction, brown color is generated by horseradish peroxidase/diaminobenzidine (H2O2) reaction. In both cases, an enzyme-second antibody conjugate was applied. Counterstaining with hemalaun was generally done except for panel a (bottom right). a Presence of CG-3 was detected in cell nuclei of collecting tubules (arrowheads) in sections of kidney using two different histochemical detection systems. b Fluorescent CG-3-dependent staining of nuclei (magenta, arrowheads) of hypertrophic chondrocytes (left) and multinucleated osteoclasts (right) in developing bone after applying the first-step anti-CG-3 antibody, then Alexa Fluor 568-labeled second-step antibody (red) and finally 4′,6-diamidino-2-phenylindole (DAPI) for counterstaining of nuclei (blue, arrows). c Subnuclear (arrowheads) presence of CG-3 in epithelial cells of the ureter. d and inset to d Extracellular presence of CG-3 in the stratified squamous epithelium of the esophagus shown at two levels of magnification. e Presence of CG-2 in collecting ducts of kidney was confined to the apical cytoplasm of the epithelial cells, visualized by two detection systems as in a. f Intense signal for cytoplasmic CG-8 presence in epithelial cells of distal tubules of the adult kidney (inset: control without incubation step with first-step antibody). Antibodies were used in the following concentrations after systematic testing in each case to reach an optimal signal-to-background ratio: anti-CG-3 in a, c 0.016 µg/ml, in b 0.5 µg/ml, and in d 1 µg/ml; e anti-CG-2: 0.0625 µg/ml; f anti-CG-8: 2 µg/ml. Scale bars are 10 µm (a, c, f), 20 µm (b, d, e), 100 µm (inset to f), and 200 µm (inset to d) (for details on the immunohistochemical protocol, please see [214, 215]; Table 2 in [103], along with microphotographs in Fig. 8 [103], summarizes respective observations, also as originally given for CG-2/CG-8 in [274, 275])
Table 1.
Overview of documented binding partners for mammalian galectins-1 and -3 reactive with the glycan part of cellular glycoconjugates or with proteins
| Type of binding partner | Galectin-1 | Galectin-3 |
|---|---|---|
| Glycoconjugate | Ovarian carcinoma antigen CA125, CD2, CD3, CD4, CD6, CD7, CD43, CD45, CD69, CD95 (Fas), CD146, CD166 (ALCAM), carcinoembryonic antigen (CEA), fibronectin (tissue), gastrointestinal mucin, hsp90-like glycoprotein, β1-integrin (CD29), α1/α4/α5/α7β1-, αMβ3- and α4β7-integrins, cell adhesion molecule L1, keratan sulfate, laminin, lamp-1, Mac-2-binding protein, nephrin, neuropilin-1, receptor protein-tyrosine phosphatase (RPTPβ), thrombospondin, Thy-1, tissue plasminogen activator, von Willebrand factor, chondroitin sulfate proteoglycan, distinct neutral glycolipids, ganglioside GM1 | CD6, CD7, CD11b of CD11b/CD18 (Mac-1 antigen, CR3), CD13 (aminopeptidase N), CD32, CD43, CD44, CD45, CD66a,b, CD71, CD95, CD98, CD147, CD166 (ALCAM), CEA, colon cancer mucin, corneal mucin (MUC16), pancreas cancer mucin-4 and MUC1-D (N-glycan at Asn36), cubilin, C4.4A (member of Ly6 family), desmoglein-2, epidermal growth factor receptor, glycoform of IgE, haptoglobin β-subunit (after desialylation), hensin (DMBT-1), insulin-like growth factor-1 receptor, β1-integrin (CD29), α4/α5/β1- and ανβ3-integrins, keratan sulfate, LI-cadherin, laminin, lamp-1/-2, Mac-2-binding protein, Mac-3, MAG, MP20 (tetraspanin), Na+/K+-ATPase, NG2 proteoglycan, NKp30, TCR complex, tenascin, tissue plasminogen activator, transforming growth factor-β receptor, vascular cell adhesion molecule-1, vascular endothelial growth factor receptor 2, von Willebrand factor, ganglioside GM1 |
| Protein | B lymphocyte adaptor molecule of 32 kDa (Bam32), CaV1.2 L-type calcium channel (α1-subunit), Gemin4, oncogenic H-Ras, OCA-B, pre-B cell receptor (human, not murine system) | AGE products, Alix/AIP-1, ATP synthase b-subunit, axin, bax, bcl-2, β-catenin, Cys/His-rich protein, Gemin4, glycogen synthase kinase-3β, hnRNP Q, mSufu, Mer receptor tyrosine kinase, non-receptor tyrosine kinases c-Abl and Arg, nucleoporin Nup98, nucling, oncogenic K-Ras, OCA-B, pCIP, PIAS1, synexin (annexin VII), TTF-1 |
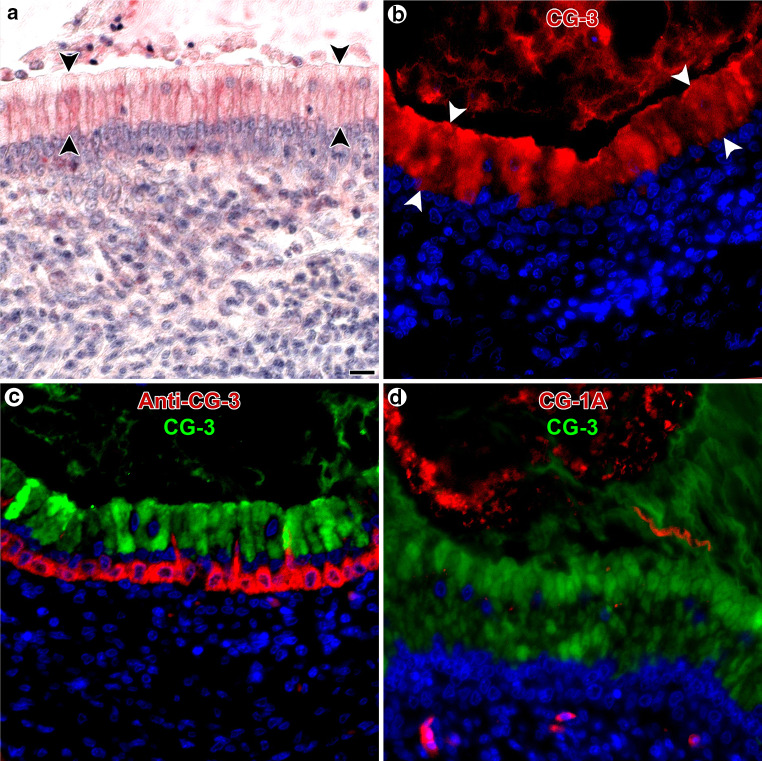
The availability of the panel of galectins not only facilitates the immunohistochemical monitoring but also the application of the tissue lectins as histochemical tools. The feasibility to apply lectins as probes has been shown for plant/fungal lectins in Fig. S2. Light and fluorescence microscopy can be performed to visualize accessible sites with galectin reactivity, and the resulting staining is specific and distinct (Fig. 11a, b). For human galectins, the same conclusion upon systematic mapping had been reached in a study on immuno- and galectin histochemical staining of the human eye [221]. Intracellular reactivity can reflect aspects of functionality: in line with an involvement of galectins-1 and -3 in pre-mRNA splicing, nuclear positivity was expected and could be verified by galectin cyto- and histochemistry [222–224]. The double staining with antibody and labeled lectin revealed a spatial separation in chicken ureter for CG-3 (Fig. 11c). In human peripheral nerves, accessible sites for a galectin could also be detected along with galectin presence [225]. Underlining that galectins of the same organism can differ in their binding properties, the staining profiles of CG-1A and CG-3 show no overlap (Fig. 11d). This series of microphotographs, together with those of Fig. S3c, teaches the lesson that each family member has its individual profile of localization and reactivity, the latter reflecting differences in target selection, as delineated by ligand-binding assays [226–228].
Fig. 11.
Histochemical detection of accessible binding sites for labeled CGs by light (a) and fluorescence (b–d) microscopy in sections of adult chicken ureter. a, b Staining by labeled CG-3 was most prominent in the apical part of cells in the pseudo-stratified epithelial lining of the ureter’s mucosa (arrowheads). c Consecutive application of anti-CG-3-specific first-step antibody detecting endogenous CG-3 with Alexa Fluor 488-labeled second-step (goat anti-rabbit) antibody and then of Alexa Fluor 555-labeled CG-3 to detect binding sites for the lectin led to visualization of CG-3-binding sites in the aforementioned apical parts of the respective epithelial cells, clearly separated from CG–3’s presence in the basal part of these cells. d Differences in reactivity profiles for two CGs were visualized with Alexa Fluor 488-labeled CG-3 and Alexa Fluor 555-labeled CG-1A. Binding sites for CG-3 were found in the epithelial cells of the ureter’s mucosa, whereas CG-1A strongly bound in the ureter’s lumen. Color coding for assignment of red/green to the respective probe is given in b–d, nuclear staining with DAPI adds the blue color. Reagents were used in the following concentrations after systematic testing in each case to optimal signal-to-background ratio: CG-3: 1 µg/ml (a) or 8 µg/ml (b, c, d), anti-CG-3: 0.125 µg/ml (c), CG-1A: 8 µg/ml (d). Scale bar in a is 10 µm and applies to microphotographs a–d (for details on the galectin histochemical processing, please see [215, 234, 239]; from experiments for Fig. 10 in [186], further microphotographs on CG histochemistry in Fig. 8 in [215])
Equally important, any other tissue lectin qualifies for this application such as a siglec [229] or a selectin [230]. These authors, by carefully examining fixation protocols, advise “that special attention should be paid when choosing a tissue section fixation protocol for in situ detection of L-selectin ligands in particular, and for lectin ligands in general, especially when using this method to detect biologically relevant ligands for the protein of interest. This may avoid relevant ligands being missed or wrong conclusions being drawn based on the binding pattern observed”. As to glycolipids such as ganglioside GM1, acetone and methanol will significantly extract or completely deplete them from the specimen [231], flanking the given conclusion.
Taken to the clinical level, the immunohistochemical monitoring of different family members in a human tissue provides information on the positive cell types and their status of differentiation. These data are of value for example in differential tumor diagnosis and the quest to identify new prognostic markers [232–235]. Here, thorough functional studies in vitro are then needed to pinpoint mechanistic pathways that link galectin appearance to pathophysiological manifestations that may or may not be associated with the expression status in vivo [236–238]. As noted above and illustrated in Fig. 11, the comprehensive testing of tissue lectins is technically feasible, and its application can also result in prognostic information, what is the case for intra- and extracellular binding sites of galectin-3 [234, 239]. Positivity of head and neck tumors (stages III/IV) correlated with relapse-free and overall survival (n = 53; p = 0.0039, p = 0.0259), of colon tumors with overall survival (n = 99; p = 0.0183) and pN status (p = 0.0069), albeit not as independent marker. As noted above for nuclear staining, the compilation of binding partners for galectins-1 and -3, the two most often analyzed lectins in tumor pathology, gives an idea of typical targets when positive reactions occur (Table 1). Strikingly, the NWGR motif of galectin-3 is not only a contact site for Gal by C-H/π-interactions but also for the anti-apoptotic Bcl-2 known to form homo- and heterodimers (with Bax) and for the pro-apoptotic Bax [240–242]. Of particular importance, inhibition of the galectin-3–Bcl-2 interaction by lactose should thus not be interpreted as evidence for lactose-dependent binding but as lactose-inhibitable reaction, either by directly blocking the active site or an allosteric rearrangement.
Turning back to the network concept, the presence of a combination of galectins detected by the immunohistochemical fingerprinting in situ, as is the case in areas of severe degeneration in osteoarthritis [243], and the possibility for functional antagonism will inspire to proceed from assaying individual lectins to putting galectins together in such physiological mixtures. This approach will characterize their inherent and not at all fully defined potential for additive and antagonistic effects. The following information on the glycobiological effects of reconstituting the tumor suppressor p16INK4a in human pancreatic carcinoma cells (Capan-1) illustrates its perspectives: in addition to a co-regulation of lectin and its counterreceptor (the scaffold such as the α5-integrin and its glycosylation such as reduced α2,6-sialylation by throttling sialic acid biosynthesis for galectin-1-dependent anoikis induction [244, 245]), even regulation within the network can occur (in this case by downregulating the anti-apoptotic galectin-3, which is a competitive inhibitor of galectin-1 on the level of the cell surface and an anti- apoptotic intracellular effector [246]). These lines of evidence give direction to the detailed analysis of the network in each organism, and a further look at phylogenetic information will support this conclusion, adding a strong caveat to extrapolations among organisms. In fact, lectin display has species-specific traits.
Lectin genes in phylogenesis: diversity and dynamics
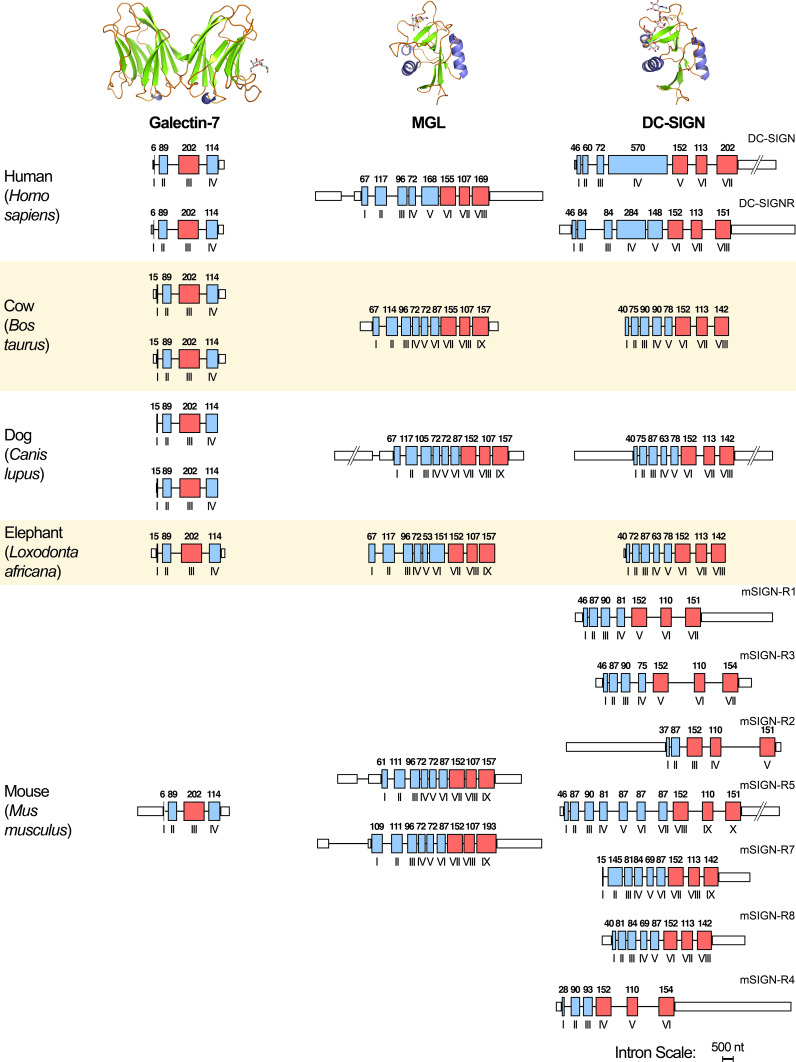
Among the galectins, evolutionary origin of certain proteins can be unique, that is restricted to distinct organisms. Two instructive cases are the occurrence of tandem-repeat-type galectin-6, a close relative of galectin-4, with a presence/absence polymorphism in mice [247–249] and of the proto-type galectin-5, closely related to the C-terminal CRD of galectin-9, in rats [250–252]. On the level of common genes, differences, too, have arisen: copy-number variation of genes is documented among galectins (that in the case of the proto-type galectin-7 shown in Fig. 12) [253]. Beyond galectins, for other lectins already mentioned, the opposite constellation with two operative genes in mice and one in humans is seen for MGL, mentioned above as Tn/sTn and asialoglycoprotein receptor (Fig. 12) [254, 255]. The genes for the C-type lectin DC-SIGN and related proteins present an even greater diversity (Fig. 12), with two genes (DC-SIGN (CD209)/DC-SIGNR (CD299, L-SIGN)) in humans and seven active SIGN-related (SIGN-R) genes in mice as product of serial duplications [81, 256, 257]. L-SIGN homologues only exist in man and in non-human primates (two genes in chimpanzee), and here as result of recent duplications and deletions [258, 259]. Adding LSECtin (CLEC4G), the mentioned GlcNAcβ1,2Man receptor, the copy number of its gene reaches up to three in horse, revealing distinct evolutionary processes for each of the C-type lectins in this cluster (for man located at chromosome 19p13,3) in each species [260]. Among the C-type lectins, to list further cases, hominids have lost one of the two functional genes of the serum collectin MBP/L by a stop-codon mutation [261]. Conversely, no murine orthologue of BDCA-2, a specific marker of human plasmacytoid dendritic cells (its reactivity with Gal-terminated N-glycans had been described above), is detectable by data base mining (H. Kaltner, unpublished). Cattle acquired its characteristic host defence proteins, here especially conglutinin (the first collectin described in 1906 [262]), by gene duplications in the locus of the bovine gene for surfactant protein D [263]. The interplay of duplications, conversion and pairing with deletions has shaped the species-dependent representation of the CD33-related siglecs, currently resulting in nine genes in humans and only four in mice [264]. Looking beyond the coding sequence, an interesting phenomenon, i.e. the occurrence of hammerhead ribozyme motifs in the 3′-untranslated region of rodent mRNA for osteoclast inhibitory lectin (OCIL; also referred to as C-type lectin domain family 2, member D, CLEC2D), points to further functionally hot spots for interspecies comparisons, here for posttranscriptional gene regulation with impact on protein production [265, 266].
Fig. 12.
Schematic illustration of the copy number and architecture of genes for proto-type galectin-7, MGL and DC-SIGN/DC-SIGNR (domain structure placed in the top section in each case) of selected mammals. Exons are drawn as boxes (colored) in red when coding for the CRD, otherwise presented in blue, introns as lines (length drawn in proportions). Lengths of exons in base pairs are given in Arabic numbers, order of exons in Roman numerals (for technical details, please see [214, 253])
As a means of fine-tuning of lectin activity, common occurrence of splice variants and SNPs likely broaden the functional spectrum, a challenge for research with biomedical perspective. These two natural routes to sequence diversity alter structural features of lectins, for instance by removing the membrane-spanning or neck regions or by altering the length of linkers between CRDs, the susceptibility of sites for proteolytic processing or the architecture of the lectin site. Galectin-8, with its linker length variation by alternative splicing and with one of its SNPs associated with onset of autoimmune diseases [267] studied functionally in aggregation assays with glycodendrimersomes (please see above) and in vitro, is an instructive example for two different levels of altering lectin structure and the challenge of delineating structure–activity relationships. Already the substitution of a single amino acid can make its presence felt remarkably, as the study of SNP variants of this galectin (Phe19Tyr) [128] or the already mentioned case of the C-type lectin langerin (Lys313Ile) [71] attests. For OCIL, the Asn19Lys substitution has been found to be associated with a decrease of bone mineral density in women older than 53 years [268]. Not surprisingly, early-stop-codon mutations can be harmful, e.g. by increasing susceptibility to fungal infections in the case of dectin-1 (Tyr238X) [269], and deviations from the normal status of lectin genes can even be identified to underlie clinical aberrations, for the 3MC syndrome (a rare autosomal recessive disorder causing, among other manifestations, facial dysmorphism and hearing loss) in the collectin-11 gene (also called collectin kidney 1), which arose by duplication prior to the separation of the collectins MBP/L and surfactant proteins A and D [270, 271]. These precedents encourage rational engineering of biomedically relevant lectins by introducing seemingly minute alterations. Applying this strategy in a systematic manner led to get rid of the undesired mitogenic activity of a potent antiviral lectin, a step toward its optimization for clinical testing [272].
Conclusions
Carbohydrates, the third alphabet of life, are the chemical toolbox for generating biochemical oligomers (‚words’) with unsurpassed coding capacity. Indeed, this potential is realized by an elaborate synthetic machinery so that the cellular glycome covers a broad range of sugar-encoded messages, as epitomized by calling glycans “multipurpose tools” in our previous review [2]. Using sugar receptors (antibodies and lectins) for their detection, the fine-tuned spatiotemporal regulation of their expression supports the assumed physiological significance. Even more importantly, the search for endogenous receptors has allowed to propose a functional pairing between glycan epitopes and endogenous (tissue) lectins, which we have herein exemplified for two series of common N- and O-glycans, for gangliosides and a case of dynamic remodeling. Matching the glycans’ diversity, emergence of folds with capacity to bind carbohydrates is not a rare event, and diversification within lectin families mapped by fingerprinting even resulted in functionally antagonistic group members, posing highly tempting challenges to define the structure–activity relationships. In addition, the occurrence of variability on the level of the individual lectin by genetic polymorphisms and alternative splicing let intriguing questions on functionality arise. That we encounter a large degree of interspecies differences sets limits to extrapolations of conclusions between species, although a focus on a model will teach fundamental lessons on principles of lectins cooperating in networks and their interplay with distinct aspects of the glycome. That being said, we can reason that the network concept and the pairing are likely to give a functional meaning to what still appears mysterious in aspects, i.e. the diversity of complex carbohydrates.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 4 (TIFF 10834 kb)
Acknowledgments
We are indebted to Drs. B. Friday, R. Gabius, M. Ilsüm, A. Leddoz, A.W.L. Nose and B. Onil for inspiring discussions and the reviewers for their valuable input.
Abbreviations
- BDCA-2
Blood-dendritic cell antigen-2
- CG
Chicken galectin
- CD
Cluster of differentiation
- CRD
Carbohydrate recognition domain
- DAPI
4′,6-Diamidino-2-phenylindole
- DC-SIGN(R)
Dendritic cell-specific ICAM-3-grabbing nonintegrin(-related protein)
- DNA
Deoxyribonucleic acid
- EGF(R)
Epidermal growth factor (receptor)
- Gal
Galactose
- GalNAc
N-acetylgalactosamine
- GlcNAc
N-acetylglucosamine
- IgG
Immunoglobulin G
- KDO
3-deoxy-d-manno-oct-2-ulosonic acid
- Lac
Lactose
- LacNAc
N-acetyllactosamine
- LSECtin
Liver and lymph node sinusoidal endothelial cell C-type lectin
- Man
Mannose
- MBP/L
Mannose (or mannan)-binding protein/lectin
- MGL
Macrophage galactose(-binding C)-type lectin
- MUC1
Mucin1
- OCIL
Osteoclast inhibitory lectin
- PILR
Paired Ig-like receptors
- Siglec
Sialic acid-binding immunoglobulin-like lectin
- SNP
Single nucleotide polymorphism
- T(F) antigen
Thomsen(-Friedenreich) antigen
- TGF(R)
Transforming growth factor (receptor)
- Tn antigen
T antigen nouvelle
- TRPC5
Transient receptor potential [canonical] channel 5
References
- 1.Sharon N (1975) Complex Carbohydrates. Their Chemistry, Biosynthesis, and Functions. Addison-Wesley Publ. Co., Reading, MA, USA
- 2.Reuter G, Gabius H-J. Eukaryotic glycosylation: whim of nature or multipurpose tool? Cell Mol Life Sci. 1999;55(3):368–422. doi: 10.1007/s000180050298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haji-Ghassemi O, Blackler RJ, Young NM, Evans SV. Antibody recognition of carbohydrate epitopes. Glycobiology. 2015;25(9):920–952. doi: 10.1093/glycob/cwv037. [DOI] [PubMed] [Google Scholar]
- 4.Avery OT, Macleod CM, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type I. J Exp Med. 1944;79(2):137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioffe E, Stanley P. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc Natl Acad Sci USA. 1994;91(2):728–732. doi: 10.1073/pnas.91.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metzler M, Gertz A, Sarkar M, Schachter H, Schrader JW, Marth JD. Complex asparagine-linked oligosaccharides are required for morphogenic events during post-implantation development. EMBO J. 1994;13(9):2056–2065. doi: 10.1002/j.1460-2075.1994.tb06480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honke K, Taniguchi N. Animal models to delineate glycan functionality. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 385–401. [Google Scholar]
- 8.Schachter H. Complex N-glycans: the story of the “yellow brick road”. Glycoconj J. 2014;31(1):1–5. doi: 10.1007/s10719-013-9507-5. [DOI] [PubMed] [Google Scholar]
- 9.Hennet T. Diseases of glycosylation. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 365–383. [Google Scholar]
- 10.Hennet T, Cabalzar J. Congenital disorders of glycosylation: a concise chart of glycocalyx dysfunction. Trends Biochem Sci. 2015;40(7):377–384. doi: 10.1016/j.tibs.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Sly WS. The missing link in lysosomal enzyme targeting. J Clin Invest. 2000;105:563–564. doi: 10.1172/JCI9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kollmann K, Pohl S, Marschner K, Encarnacao M, Sakwa I, Tiede S, Poorthuis BJ, Lubke T, Muller-Loennies S, Storch S, Braulke T. Mannose phosphorylation in health and disease. Eur J Cell Biol. 2010;89(1):117–123. doi: 10.1016/j.ejcb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Roseman S. Reflections on glycobiology. J Biol Chem. 2001;276(45):41527–41542. doi: 10.1074/jbc.R100053200. [DOI] [PubMed] [Google Scholar]
- 14.Laine RA. The information-storing potential of the sugar code. In: Gabius H-J, Gabius S, editors. Glycosciences: Status and Perspectives. London - Weinheim: Chapman & Hall; 1997. pp. 1–14. [Google Scholar]
- 15.Gabius H-J, André S, Kaltner H, Siebert H-C. The sugar code: functional lectinomics. Biochim Biophys Acta. 2002;1572(2–3):165–177. doi: 10.1016/S0304-4165(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 16.Rüdiger H, Gabius H-J. The biochemical basis and coding capacity of the sugar code. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 3–13. [Google Scholar]
- 17.Buddecke E. Proteoglycans. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 199–216. [Google Scholar]
- 18.Patsos G, Corfield A. O-Glycosylation: structural diversity and function. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 111–137. [Google Scholar]
- 19.Zuber C, Roth J. N-Glycosylation. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 87–110. [Google Scholar]
- 20.Lee YC. Tracing the development of structural elucidation of N-glycans. Trends Glycosci Glycotechnol. 2009;21:53–69. doi: 10.4052/tigg.21.53. [DOI] [Google Scholar]
- 21.Rees DA. Shapely polysaccharides. Biochem J. 1972;126(2):257–273. doi: 10.1042/bj1260257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winterburn PJ, Phelps CF. The significance of glycosylated proteins. Nature. 1972;236:147–151. doi: 10.1038/236147a0. [DOI] [PubMed] [Google Scholar]
- 23.Wilson IBH, Paschinger H, Rendic D. Glycosylation of model and ‘lower’ organisms. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 139–154. [Google Scholar]
- 24.Jarrell KF, Ding Y, Meyer BH, Albers SV, Kaminski L, Eichler J. N-Linked glycosylation in Archaea: a structural, functional, and genetic analysis. Microbiol Mol Biol Rev. 2014;78(2):304–341. doi: 10.1128/MMBR.00052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tytgat HL, Lebeer S. The sweet tooth of bacteria: common themes in bacterial glycoconjugates. Microbiol Mol Biol Rev. 2014;78(3):372–417. doi: 10.1128/MMBR.00007-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corfield AP, Berry M. Glycan variation and evolution in the eukaryotes. Trends Biochem Sci. 2015;40(7):351–359. doi: 10.1016/j.tibs.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Tan FY, Tang CM, Exley RM. Sugar coating: bacterial protein glycosylation and host-microbe interactions. Trends Biochem Sci. 2015;40(7):342–350. doi: 10.1016/j.tibs.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Gabius H-J, Kaltner H, Kopitz J, André S. The glycobiology of the CD system: a dictionary for translating marker designations into glycan/lectin structure and function. Trends Biochem Sci. 2015;40(7):360–376. doi: 10.1016/j.tibs.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Kocourek J (1986) Historical background. In: Liener IE, Sharon N, Goldstein IJ (eds) The Lectins. Properties, Functions and Applications in Biology and Medicine. Academic Press, New York, pp 1–32
- 30.Rüdiger H, Gabius H-J. The history of lectinology. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 261–268. [Google Scholar]
- 31.Landsteiner K. Ueber Agglutinationserscheinungen normalen menschlichen Blutes. Wien Klin Wochenschr. 1901;46:1132–1134. [PubMed] [Google Scholar]
- 32.Hughes-Jones NC, Gardner B. Red cell agglutination: the first description by Creite (1869) and further observations made by Landois (1875) and Landsteiner (1901) Br J Haematol. 2002;119(4):889–893. doi: 10.1046/j.1365-2141.2002.03675.x. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz HP, Dorner F. Karl Landsteiner and his major contributions to haematology. Br J Haematol. 2003;121(4):556–565. doi: 10.1046/j.1365-2141.2003.04295.x. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell SW (1860) Researches upon the venom of the rattlesnake. Smithsonian Contributions to Knowledge XII (Article VI):89-90
- 35.Stillmark H (1888) Ueber Ricin, ein giftiges Ferment aus den Samen von Ricinus comm. L. und einigen anderen Euphorbiaceen. Inaugural-Dissertation, Dorpat: Schnakenburg’s Buchdruckerei
- 36.Kilpatrick DC, Green C. Lectins as blood typing reagents. Adv Lectin Res. 1992;5:51–94. [Google Scholar]
- 37.Elfstrand M (1898) Ueber blutkörperchenagglutinierende Eiweisse. In: Kobert R (ed) Görbersdorfer Veröffentlichungen. Enke, Stuttgart, pp 1-159
- 38.Krüpe M. Blutgruppenspezifische pflanzliche Eiweißkörper. Enke, Stuttgart: Phytagglutinine; 1956. [Google Scholar]
- 39.Allen NK, Brilliantine L. A survey of hemagglutinins in various seeds. J Immunol. 1969;102(5):1295–1299. [PubMed] [Google Scholar]
- 40.Boyd WC. The lectins: their present status. Vox Sang. 1963;8:1–32. doi: 10.1111/j.1423-0410.1963.tb04146.x. [DOI] [PubMed] [Google Scholar]
- 41.Landsteiner K. The Specificity of Serological Reactions. Cambridge: Harvard University Press; 1945. [Google Scholar]
- 42.Watkins WM, Morgan WTJ. Neutralisation of the anti-H agglutinin in eel serum by simple sugars. Nature. 1952;169:825–826. doi: 10.1038/169825a0. [DOI] [PubMed] [Google Scholar]
- 43.Watkins WM. A half century of blood-group antigen research: some personal recollections. Trends Glycosci Glycotechnol. 1999;11:391–411. doi: 10.4052/tigg.11.391. [DOI] [Google Scholar]
- 44.Boyd WC. The proteins of immune reactions. In: Neurath H, Bailey K, editors. The Proteins. New York: Academic Press; 1954. pp. 756–844. [Google Scholar]
- 45.Sumner JB, Howell SF. The identification of a hemagglutinin of the jack bean with concanavalin A. J Bacteriol. 1936;32:227–237. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bird GWG. Lectins in immunohematology. Transfusion Med Rev. 1989;3:55–62. doi: 10.1016/S0887-7963(89)70068-7. [DOI] [PubMed] [Google Scholar]
- 47.Barondes SH. Bifunctional properties of lectins: lectins redefined. Trends Biochem Sci. 1988;13:480–482. doi: 10.1016/0968-0004(88)90235-6. [DOI] [PubMed] [Google Scholar]
- 48.Gabius H-J, André S, Jiménez-Barbero J, Romero A, Solís D. From lectin structure to functional glycomics: principles of the sugar code. Trends Biochem Sci. 2011;36(6):298–313. doi: 10.1016/j.tibs.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Gabius HJ, Springer WR, Barondes SH. Receptor for the cell binding site of discoidin I. Cell. 1985;42(2):449–456. doi: 10.1016/0092-8674(85)90102-3. [DOI] [PubMed] [Google Scholar]
- 50.Goldstein IJ, Poretz RD (1986) Isolation, physicochemical characterization, and carbohydrate-binding specificity of lectins. In: Liener IE, Sharon N, Goldstein IJ (eds) The Lectins. Properties, Functions, and Applications in Biology and Medicine. Academic Press, Orlando, pp 33-247
- 51.Kilpatrick DC. Handbook of Animal Lectins. Chichester: J. Wiley & Sons; 2000. [Google Scholar]
- 52.Loris R. Principles of structures of animal and plant lectins. Biochim Biophys Acta. 2002;1572:198–208. doi: 10.1016/S0304-4165(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 53.Holgersson S, Gustafsson A, Gaunitz S. Bacterial and viral lectins. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 279–300. [Google Scholar]
- 54.Rüdiger H, Gabius H-J. Plant lectins. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 301–315. [Google Scholar]
- 55.Solís D, Bovin NV, Davis AP, Jiménez-Barbero J, Romero A, Roy R, Smetana K, Jr, Gabius H-J . A guide into glycosciences: how chemistry, biochemistry and biology cooperate to crack the sugar code. Biochim Biophys Acta. 2015;1850:186–235. doi: 10.1016/j.bbagen.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Lehotzky RE, Partch CL, Mukherjee S, Cash HL, Goldman WE, Gardner KH, Hooper LV. Molecular basis for peptidoglycan recognition by a bactericidal lectin. Proc Natl Acad Sci USA. 2010;107(17):7722–7727. doi: 10.1073/pnas.0909449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wesener DA, Wangkanont K, McBride R, Song X, Kraft MB, Hodges HL, Zarling LC, Splain RA, Smith DF, Cummings RD, Paulson JC, Forest KT, Kiessling LL. Recognition of microbial glycans by human intelectin-1. Nat Struct Mol Biol. 2015;22(8):603–610. doi: 10.1038/nsmb.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsunaga I, Moody DB. Mincle is a long sought receptor for mycobacterial cord factor. J Exp Med. 2009;206(13):2865–2868. doi: 10.1084/jem.20092533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206(13):2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Furukawa A, Kamishikiryo J, Mori D, Toyonaga K, Okabe Y, Toji A, Kanda R, Miyake Y, Ose T, Yamasaki S, Maenaka K. Structural analysis for glycolipid recognition by the C-type lectins Mincle and MCL. Proc Natl Acad Sci USA. 2013;110(43):17438–17443. doi: 10.1073/pnas.1312649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jegouzo SA, Harding EC, Acton O, Rex MJ, Fadden AJ, Taylor ME, Drickamer K. Defining the conformation of human mincle that interacts with mycobacterial trehalose dimycolate. Glycobiology. 2014;24(12):1291–1300. doi: 10.1093/glycob/cwu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicolson GL. The interactions of lectins with animal cell surfaces. Int Rev Cytol. 1974;39:89–190. doi: 10.1016/S0074-7696(08)60939-0. [DOI] [PubMed] [Google Scholar]
- 63.Roth J. The lectins: molecular probes in cell biology and membrane research. Exp Pathol. 1978;3(Suppl 1):1–186. [Google Scholar]
- 64.Spicer SS, Schulte BA. Diversity of cell glycoconjugates shown histochemically: a perspective. J Histochem Cytochem. 1992;40:1–38. doi: 10.1177/40.1.1370305. [DOI] [PubMed] [Google Scholar]
- 65.Danguy A, Akif F, Pajak B, Gabius H-J. Contribution of carbohydrate histochemistry to glycobiology. Histol Histopathol. 1994;9:155–171. [PubMed] [Google Scholar]
- 66.Roth J. Lectins for histochemical demonstration of glycans. Histochem Cell Biol. 2011;136(2):117–130. doi: 10.1007/s00418-011-0848-5. [DOI] [PubMed] [Google Scholar]
- 67.Josefsson EC, Gebhard HH, Stossel TP, Hartwig JH, Hoffmeister KM. The macrophage αMβ2 integrin αM lectin domain mediates the phagocytosis of chilled platelets. J Biol Chem. 2005;280(18):18025–18032. doi: 10.1074/jbc.M501178200. [DOI] [PubMed] [Google Scholar]
- 68.Hoffmeister K, Falet H. Platelet glycoproteins as lectin in hematology. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 485–493. [Google Scholar]
- 69.de Jong MAWP, Vriend LE, Theelen B, Taylor ME, Fluitsma D, Boekhout T, Geijtenbeek TB. C-Type lectin Langerin is a β-glucan receptor on human Langerhans cells that recognizes opportunistic and pathogenic fungi. Mol Immunol. 2010;47(6):1216–1225. doi: 10.1016/j.molimm.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holla A, Skerra A. Comparative analysis reveals selective recognition of glycans by the dendritic cell receptors DC-SIGN and Langerin. Protein Eng Des Sel. 2011;24(9):659–669. doi: 10.1093/protein/gzr016. [DOI] [PubMed] [Google Scholar]
- 71.Feinberg H, Rowntree TJ, Tan SL, Drickamer K, Weis WI, Taylor ME. Common polymorphisms in human langerin change specificity for glycan ligands. J Biol Chem. 2013;288(52):36762–36771. doi: 10.1074/jbc.M113.528000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dominguez-Soto A, Aragoneses-Fenoll L, Martin-Gayo E, Martinez-Prats L, Colmenares M, Naranjo-Gomez M, Borras FE, Munoz P, Zubiaur M, Toribio ML, Delgado R, Corbi AL. The DC-SIGN-related lectin LSECtin mediates antigen capture and pathogen binding by human myeloid cells. Blood. 2007;109(12):5337–5345. doi: 10.1182/blood-2006-09-048058. [DOI] [PubMed] [Google Scholar]
- 73.Tang L, Yang J, Tang X, Ying W, Qian X, He F. The DC-SIGN family member LSECtin is a novel ligand of CD44 on activated T cells. Eur J Immunol. 2010;40(4):1185–1191. doi: 10.1002/eji.200939936. [DOI] [PubMed] [Google Scholar]
- 74.Pipirou Z, Powlesland AS, Steffen I, Pohlmann S, Taylor ME, Drickamer K. Mouse LSECtin as a model for a human Ebola virus receptor. Glycobiology. 2011;21(6):806–812. doi: 10.1093/glycob/cwr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang F, Ren S, Zuo Y. DC-SIGN, DC-SIGNR and LSECtin: C-type lectins for infection. Int Rev Immunol. 2014;33(1):54–66. doi: 10.3109/08830185.2013.834897. [DOI] [PubMed] [Google Scholar]
- 76.Thomsen T, Schlosser A, Holmskov U, Sorensen GL. Ficolins and FIBCD1: soluble and membrane bound pattern recognition molecules with acetyl group selectivity. Mol Immunol. 2011;48(4):369–381. doi: 10.1016/j.molimm.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 77.Terada M, Khoo KH, Inoue R, Chen CI, Yamada K, Sakaguchi H, Kadowaki N, Ma BY, Oka S, Kawasaki T, Kawasaki N. Characterization of oligosaccharide ligands expressed on SW1116 cells recognized by mannan-binding protein. A highly fucosylated polylactosamine type N-glycan. J Biol Chem. 2005;280(12):10897–10913. doi: 10.1074/jbc.M413092200. [DOI] [PubMed] [Google Scholar]
- 78.Kawasaki N, Lin CW, Inoue R, Khoo KH, Kawasaki N, Ma BY, Oka S, Ishiguro M, Sawada T, Ishida H, Hashimoto T, Kawasaki T. Highly fucosylated N-glycan ligands for mannan-binding protein expressed specifically on CD26 (DPPVI) isolated from a human colorectal carcinoma cell line, SW1116. Glycobiology. 2009;19(4):437–450. doi: 10.1093/glycob/cwn158. [DOI] [PubMed] [Google Scholar]
- 79.Kawasaki N, Kawasaki T. Recognition of endogenous ligands by C-type lectins: interaction of serum mannan-binding protein with tumor-associated oligosaccharide epitopes. Trends Glycosci Glycotechnol. 2010;22(125):141–151. doi: 10.4052/tigg.22.141. [DOI] [Google Scholar]
- 80.Davis CW, Mattei LM, Nguyen HY, Ansarah-Sobrinho C, Doms RW, Pierson TC. The location of asparagine-linked glycans on West Nile virions controls their interactions with CD209 (dendritic cell-specific ICAM-3 grabbing nonintegrin) J Biol Chem. 2006;281(48):37183–37194. doi: 10.1074/jbc.M605429200. [DOI] [PubMed] [Google Scholar]
- 81.Powlesland AS, Ward EM, Sadhu SK, Guo Y, Taylor ME, Drickamer K. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins. J Biol Chem. 2006;281(29):20440–20449. doi: 10.1074/jbc.M601925200. [DOI] [PubMed] [Google Scholar]
- 82.Nakayama J, Yeh JC, Misra AK, Ito S, Katsuyama T, Fukuda M. Expression cloning of a human α1,4-N-acetylglucosaminyltransferase that forms GlcNAcα1,4Galβ-R, a glycan specifically expressed in the gastric gland mucous cell-type mucin. Proc Natl Acad Sci USA. 1999;96(16):8991–8996. doi: 10.1073/pnas.96.16.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.André S, Kaltner H, Kayser K, Murphy PV, Gabius H-J (2016) Merging carbohydrate chemistry with lectin histochemistry to study inhibition of lectin binding by glycoclusters in the natural tissue context. Histochem Cell Biol 145(2):185–199. doi:10.1007/s00418-015-1383-6 [DOI] [PubMed]
- 84.Hoffmann W. TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more. Int J Oncol. 2015;47(3):806–816. doi: 10.3892/ijo.2015.3090. [DOI] [PubMed] [Google Scholar]
- 85.Nakayama J. Dual roles of gastric gland mucin-specific O-glycans in prevention of gastric cancer. Acta Histochem Cytochem. 2014;47(1):1–9. doi: 10.1267/ahc.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Becker DJ, Lowe JB. Fucose: biosynthesis and biological functions in mammals. Glycobiology. 2003;13:41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 87.Martinez-Duncker I, Mollicone R, Candelier J-J, Breton C, Oriol R. A new superfamily of protein-O-fucosyltransferases, α2-fucosyltransferases, and α6-fucosyltransferases: phylogeny and identification of conserved peptide motifs. Glycobiology. 2003;13(12):1C–5C. doi: 10.1093/glycob/cwg113. [DOI] [PubMed] [Google Scholar]
- 88.Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16(12):158R–184R. doi: 10.1093/glycob/cwl040. [DOI] [PubMed] [Google Scholar]
- 89.Aplin JD, Jones CJ. Fucose, placental evolution and the glycocode. Glycobiology. 2012;22(4):470–478. doi: 10.1093/glycob/cwr156. [DOI] [PubMed] [Google Scholar]
- 90.Furukawa K, Sato T. β1,4-Galactosylation of N-glycans is a complex process. Biochim Biophys Acta. 1999;1473(1):54–66. doi: 10.1016/S0304-4165(99)00169-5. [DOI] [PubMed] [Google Scholar]
- 91.Guo S, Sato T, Shirane K, Furukawa K. Galactosylation of N-linked oligosaccharides by human β1,4-galactosyltransferases I, II, III, IV, V, and VI expressed in Sf-9 cells. Glycobiology. 2001;11(10):813–820. doi: 10.1093/glycob/11.10.813. [DOI] [PubMed] [Google Scholar]
- 92.Hennet T. The galactosyltransferase family. Cell Mol Life Sci. 2002;59(7):1081–1095. doi: 10.1007/s00018-002-8489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gabius H-J, van de Wouwer M, André S, Villalobo A. Down-regulation of the epidermal growth factor receptor by altering N-glycosylation: emerging role of β1,4-galactosyltransferases. Anticancer Res. 2012;32(5):1565–1572. [PubMed] [Google Scholar]
- 94.Morell AG, van den Hamer CJA, Scheinberg IH, Ashwell G. Physical and chemical studies on ceruloplasmin. IV. Preparation of radioactive, sialic acid-free ceruloplasmin labeled with tritium on terminal d-galactose residues. J Biol Chem. 1966;241(16):3745–3749. [PubMed] [Google Scholar]
- 95.Hudgin RL, Pricer WEJ, Ashwell G, Stockert RJ, Morell AG. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974;249(17):5536–5543. [PubMed] [Google Scholar]
- 96.Grewal PK, Uchiyama S, Ditto D, Varki N, Le DT, Nizet V, Marth JD. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14(6):648–655. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grozovsky R, Begonja AJ, Liu K, Visner G, Hartwig JH, Falet H, Hoffmeister KM. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat Med. 2015;21(1):47–54. doi: 10.1038/nm.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Riboldi E, Daniele R, Parola C, Inforzato A, Arnold PL, Bosisio D, Fremont DH, Bastone A, Colonna M, Sozzani S. Human C-type lectin domain family 4, member C (CLEC4C/BDCA-2/CD303) is a receptor for asialo-galactosyl-oligosaccharides. J Biol Chem. 2011;286(41):35329–35333. doi: 10.1074/jbc.C111.290494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barondes SH. Soluble lectins: a new class of extracellular proteins. Science. 1984;223:1259–1264. doi: 10.1126/science.6367039. [DOI] [PubMed] [Google Scholar]
- 100.Barondes SH. Galectins: a personal review. Trends Glycosci Glycotechnol. 1997;9:1–7. doi: 10.4052/tigg.9.1. [DOI] [Google Scholar]
- 101.Hirabayashi J. Recent topics on galectins. Trends Glycosci Glycotechnol. 1997;9:1–180. doi: 10.4052/tigg.9.1. [DOI] [Google Scholar]
- 102.Cooper DNW. Galectinomics: finding themes in complexity. Biochim Biophys Acta. 2002;1572:209–231. doi: 10.1016/S0304-4165(02)00310-0. [DOI] [PubMed] [Google Scholar]
- 103.Kaltner H, Gabius H-J. A toolbox of lectins for translating the sugar code: the galectin network in phylogenesis and tumors. Histol Histopathol. 2012;27(4):397–416. doi: 10.14670/HH-27.397. [DOI] [PubMed] [Google Scholar]
- 104.Dam TK, Gabius H-J, André S, Kaltner H, Lensch M, Brewer CF. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry. 2005;44:12564–12571. doi: 10.1021/bi051144z. [DOI] [PubMed] [Google Scholar]
- 105.Togayachi A, Narimatsu H. Functional analysis of β1,3-N-acetylglucosaminyltransferases and regulation of immunological function by polylactosamine. Trends Glycosci Glycotechnol. 2012;24:95–111. doi: 10.4052/tigg.24.95. [DOI] [Google Scholar]
- 106.Fiete D, Beranek M, Baenziger JU. Molecular basis for protein-specific transfer of N-acetylgalactosamine to N-linked glycans by the glycosyltransferases β1,4-N-acetylgalactosaminyl transferase 3 (β4GalNAc-T3) and β4GalNAc-T4. J Biol Chem. 2012;287(34):29194–29203. doi: 10.1074/jbc.M112.371567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park EI, Baenziger JU. Closely related mammals have distinct asialoglycoprotein receptor carbohydrate specificities. J Biol Chem. 2004;279(39):40954–40959. doi: 10.1074/jbc.M406647200. [DOI] [PubMed] [Google Scholar]
- 108.van den Berg TK, Honing H, Franke N, van Remoortere A, Schiphorst WECM, Liu F-T, Deelder AM, Cummings RD, Hokke CH, van Die I. LacdiNAc-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J Immunol. 2004;173(3):1902–1907. doi: 10.4049/jimmunol.173.3.1902. [DOI] [PubMed] [Google Scholar]
- 109.Fiete D, Srivastava V, Hindsgaul O, Baenziger JU. A hepatic reticuloendothelial cell receptor specific for SO4-4GalNAcβ1,4GlcNAcβ1,2Manα that mediates rapid clearance of lutropin. Cell. 1991;67(6):1103–1110. doi: 10.1016/0092-8674(91)90287-9. [DOI] [PubMed] [Google Scholar]
- 110.Fiete D, Beranek MC, Baenziger JU. The macrophage/endothelial cell mannose receptor cDNA encodes a protein that binds oligosaccharides terminating with SO4-4-GalNAcβ1,4GlcNAcβ or Man at independent sites. Proc Natl Acad Sci USA. 1997;94:11256–11261. doi: 10.1073/pnas.94.21.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hooper LV, Manzella SM, Baenziger JU. The biology of sulfated oligosaccharides. In: Gabius H-J, Gabius S, editors. Glycosciences: Status and Perspectives. London - Weinheim: Chapman & Hall; 1997. pp. 261–276. [Google Scholar]
- 112.East L, Isacke CM. The mannose receptor family. Biochim Biophys Acta. 2002;1572(2–3):364–386. doi: 10.1016/S0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 113.Chapman E, Best MD, Hanson SR, Wong CH. Sulfotransferases: structure, mechanism, biological activity, inhibition, and synthetic utility. Angew Chem Int Ed. 2004;43(27):3526–3548. doi: 10.1002/anie.200300631. [DOI] [PubMed] [Google Scholar]
- 114.Kawashima H. Two roles of mucin sulfation. Trends Glycosci Glycotechnol. 2010;22:211–225. doi: 10.4052/tigg.22.211. [DOI] [Google Scholar]
- 115.Laporte B, Gonzalez-Hilarion S, Maftah A, Petit JM. The second bovine β-galactoside-α2,6-sialyltransferase (ST6Gal II): genomic organization and stimulation of its in vitro expression by IL-6 in bovine mammary epithelial cells. Glycobiology. 2009;19(10):1082–1093. doi: 10.1093/glycob/cwp094. [DOI] [PubMed] [Google Scholar]
- 116.Takashima S, Tsuji S. Functional diversity of mammalian sialyltransferases. Trends Glycosci Glycotechnol. 2011;23:178–193. doi: 10.4052/tigg.23.178. [DOI] [Google Scholar]
- 117.Joziasse DH, Schiphorst WECM, van den Eijnden DH, van Kuik JA, van Halbeek H, Vliegenthart JFG. Branch specificity of bovine colostrum CMP-sialic acid: Galβ1-4GlcNAc-R α2-6-sialyltransferase. Sialylation of bi-, tri-, and tetraantennary oligosaccharides and glycopeptides of the N-acetyllactosamine type. J Biol Chem. 1987;262(5):2025–2033. [PubMed] [Google Scholar]
- 118.Reuter G, Gabius H-J. Sialic acids. Structure, analysis, metabolism, and recognition. Biol Chem Hoppe-Seyler. 1996;377:325–342. doi: 10.1515/bchm3.1996.377.6.325. [DOI] [PubMed] [Google Scholar]
- 119.Siebert H-C, Rosen J, Seyrek K, Kaltner H, André S, Bovin NV, Nyholm P-G, Sinowatz F, Gabius H-J. α2,3/α2,6-Sialylation of N-glycans: non-synonymous signals with marked developmental regulation in bovine reproductive tracts. Biochimie. 2006;88:399–410. doi: 10.1016/j.biochi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 120.Kadirvelraj R, Grant OC, Goldstein IJ, Winter HC, Tateno H, Fadda E, Woods RJ. Structure and binding analysis of Polyporus squamosus lectin in complex with the Neu5Acα2-6Galβ1-4GlcNAc human-type influenza receptor. Glycobiology. 2011;21(7):973–984. doi: 10.1093/glycob/cwr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ahmad N, Gabius H-J, Kaltner H, André S, Kuwabara I, Liu F-T, Oscarson S, Norberg T, Brewer CF. Thermodynamic binding studies of cell surface carbohydrate epitopes to galectins-1, -3 and -7. Evidence for differential binding specificities. Can J Chem. 2002;80:1096–1104. doi: 10.1139/v02-162. [DOI] [Google Scholar]
- 122.Park EI, Manzella SM, Baenziger JU. Rapid clearance of sialylated glycoproteins by the asialoglycoprotein receptor. J Biol Chem. 2003;278(7):4597–4602. doi: 10.1074/jbc.M210612200. [DOI] [PubMed] [Google Scholar]
- 123.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105(50):19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Silva-Martin N, Bartual SG, Ramirez-Aportela E, Chacon P, Park CG, Hermoso JA. Structural basis for selective recognition of endogenous and microbial polysaccharides by macrophage receptor SIGN-R1. Structure. 2014;22(11):1595–1606. doi: 10.1016/j.str.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 125.André S, Unverzagt C, Kojima S, Dong X, Fink C, Kayser K, Gabius H-J. Neoglycoproteins with the synthetic complex biantennary nonasaccharide or its α2,3/α2,6-sialylated derivatives: their preparation, the assessment of their ligand properties for purified lectins, for tumor cells in vitro and in tissue sections and their biodistribution in tumor-bearing mice. Bioconjugate Chem. 1997;8:845–855. doi: 10.1021/bc970164d. [DOI] [PubMed] [Google Scholar]
- 126.André S, Kozár T, Schuberth R, Unverzagt C, Kojima S, Gabius H-J. Substitutions in the N-glycan core as regulators of biorecognition: the case of core-fucose and bisecting GlcNAc moieties. Biochemistry. 2007;46:6984–6995. doi: 10.1021/bi7000467. [DOI] [PubMed] [Google Scholar]
- 127.André S, Kozár T, Kojima S, Unverzagt C, Gabius H-J. From structural to functional glycomics: core substitutions as molecular switches for shape and lectin affinity of N-glycans. Biol Chem. 2009;390(7):557–565. doi: 10.1515/BC.2009.072. [DOI] [PubMed] [Google Scholar]
- 128.Ruiz FM, Scholz BA, Buzamet E, Kopitz J, André S, Menendez M, Romero A, Solís D, Gabius H-J. Natural single amino acid polymorphism (F19Y) in human galectin-8: detection of structural alterations and increased growth-regulatory activity on tumor cells. FEBS J. 2014;281(5):1446–1464. doi: 10.1111/febs.12716. [DOI] [PubMed] [Google Scholar]
- 129.Gabius H-J. Animal lectins. Eur J Biochem. 1997;243(3):543–576. doi: 10.1111/j.1432-1033.1997.t01-1-00543.x. [DOI] [PubMed] [Google Scholar]
- 130.Angata T, Brinkman-van der Linden ECM. I-type lectins. Biochim Biophys Acta. 2002;1572(2–3):294–316. doi: 10.1016/S0304-4165(02)00316-1. [DOI] [PubMed] [Google Scholar]
- 131.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14(10):653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ideo H, Seko A, Ishizuka I, Yamashita K. The N-terminal carbohydrate recognition domain of galectin-8 recognizes specific glycosphingolipids with high affinity. Glycobiology. 2003;13(10):713–723. doi: 10.1093/glycob/cwg094. [DOI] [PubMed] [Google Scholar]
- 133.Nilsson EC, Storm RJ, Bauer J, Johansson SM, Lookene A, Angstrom J, Hedenstrom M, Eriksson TL, Frangsmyr L, Rinaldi S, Willison HJ, Pedrosa Domellof F, Stehle T, Arnberg N. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med. 2011;17(1):105–109. doi: 10.1038/nm.2267. [DOI] [PubMed] [Google Scholar]
- 134.Burmeister WP, Guilligay D, Cusack S, Wadell G, Arnberg N. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J Virol. 2004;78(14):7727–7736. doi: 10.1128/JVI.78.14.7727-7736.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Svennerholm L. Chromatographic separation of human brain gangliosides. J Neurochem. 1963;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- 136.Chester MA. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of glycolipids–recommendations 1997. Eur J Biochem. 1998;257(2):293–298. doi: 10.1046/j.1432-1327.1998.2570293.x. [DOI] [PubMed] [Google Scholar]
- 137.Kopitz J. Glycolipids. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 177–198. [Google Scholar]
- 138.Schengrund CL. Gangliosides: glycosphingolipids essential for normal neural development and function. Trends Biochem Sci. 2015;40(7):397–406. doi: 10.1016/j.tibs.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 139.Rapoport E, Mikhalyov I, Zhang J, Crocker P, Bovin N. Ganglioside binding pattern of CD33-related siglecs. Bioorg Med Chem Lett. 2003;13(4):675–678. doi: 10.1016/S0960-894X(02)00998-8. [DOI] [PubMed] [Google Scholar]
- 140.Attrill H, Imamura A, Sharma RS, Kiso M, Crocker PR, van Aalten DM. Siglec-7 undergoes a major conformational change when complexed with the α(2,8)-disialylganglioside GT1b. J Biol Chem. 2006;281(43):32774–32783. doi: 10.1074/jbc.M601714200. [DOI] [PubMed] [Google Scholar]
- 141.Zhuravleva MA, Trandem K, Sun PD. Structural implications of Siglec-5-mediated sialoglycan recognition. J Mol Biol. 2008;375(2):437–447. doi: 10.1016/j.jmb.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zaccai NR, May AP, Robinson RC, Burtnick LD, Crocker PR, Brossmer R, Kelm S, Jones EY. Crystallographic and in silico analysis of the sialoside-binding characteristics of the Siglec sialoadhesin. J Mol Biol. 2007;365(5):1469–1479. doi: 10.1016/j.jmb.2006.10.084. [DOI] [PubMed] [Google Scholar]
- 143.Kawasaki Y, Ito A, Withers DA, Taima T, Kakoi N, Saito S, Arai Y. Ganglioside DSGb5, preferred ligand for Siglec-7, inhibits NK cell cytotoxicity against renal cell carcinoma cells. Glycobiology. 2010;20(11):1373–1379. doi: 10.1093/glycob/cwq116. [DOI] [PubMed] [Google Scholar]
- 144.Collins BE, Ito H, Sawada N, Ishida H, Kiso M, Schnaar RL. Enhanced binding of the neural siglecs, myelin-associated glycoprotein and Schwann cell myelin protein, to Chol-1 (α-series) gangliosides and novel sulfated Chol-1 analogs. J Biol Chem. 1999;274(53):37637–37643. doi: 10.1074/jbc.274.53.37637. [DOI] [PubMed] [Google Scholar]
- 145.Yoon SJ, Nakayama K, Hikita T, Handa K, Hakomori SI. Epidermal growth factor receptor tyrosine kinase is modulated by GM3 interaction with N-linked GlcNAc termini of the receptor. Proc Natl Acad Sci USA. 2006;103(50):18987–18991. doi: 10.1073/pnas.0609281103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kawashima N, Yoon SJ, Itoh K, Nakayama K. Tyrosine kinase activity of epidermal growth factor receptor is regulated by GM3 binding through carbohydrate to carbohydrate interactions. J Biol Chem. 2009;284(10):6147–6155. doi: 10.1074/jbc.M808171200. [DOI] [PubMed] [Google Scholar]
- 147.Bucior I, Burger MM, Fernàndez-Busquets X. Carbohydrate-carbohydrate interactions. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 347–362. [Google Scholar]
- 148.Lopez PH, Schnaar RL. Gangliosides in cell recognition and membrane protein regulation. Curr Opin Struct Biol. 2009;19(5):549–557. doi: 10.1016/j.sbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Phongsisay V, Iizasa E, Hara H, Yamasaki S. 3-O-sulfo-β-D-galactose moiety of endogenous sulfoglycolipids is a potential ligand for immunoglobulin-like receptor LMIR5. Mol Immunol. 2015;63(2):595–599. doi: 10.1016/j.molimm.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 150.Gabius H-J. The magic of the sugar code. Trends Biochem Sci. 2015;40(7):341. doi: 10.1016/j.tibs.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 151.Delacour D, Gouyer V, Zanetta J-P, Drobecq H, Leteurtre E, Grard G, Moreau-Hannedouche O, Maes E, Pons A, André S, Le Bivic A, Gabius H-J, Manninen A, Simons K, Huet G. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol. 2005;169(3):491–501. doi: 10.1083/jcb.200407073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stechly L, Morelle W, Dessein AF, André S, Grard G, Trinel D, Dejonghe MJ, Leteurtre E, Drobecq H, Trugnan G, Gabius H-J, Huet G. Galectin-4-regulated delivery of glycoproteins to the brush border membrane of enterocyte-like cells. Traffic. 2009;10(4):438–450. doi: 10.1111/j.1600-0854.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 153.Velasco S, Díez-Revuelta N, Hernández-Iglesias T, Kaltner H, André S, Gabius H-J, Abad-Rodríguez J. Neuronal galectin-4 is required for axon growth and for the organization of axonal membrane L1 delivery and clustering. J Neurochem. 2013;125(1):49–62. doi: 10.1111/jnc.12148. [DOI] [PubMed] [Google Scholar]
- 154.Abad-Rodríguez J, Díez-Revuelta N. Axon glycoprotein routing in nerve polarity, function, and repair. Trends Biochem Sci. 2015;40(7):385–396. doi: 10.1016/j.tibs.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 155.Mamelak D, Mylvaganam M, Whetstone H, Hartmann E, Lennarz W, Wyrick PB, Raulston J, Han H, Hoffman P, Lingwood CA. Hsp70 s contain a specific sulfogalactolipid binding site. Differential aglycone influence on sulfogalactosyl ceramide binding by recombinant prokaryotic and eukaryotic hsp70 family members. Biochemistry. 2001;40(12):3572–3582. doi: 10.1021/bi001643u. [DOI] [PubMed] [Google Scholar]
- 156.Lingwood C, Mylvaganam M, Minhas F, Binnington B, Branch DR, Pomes R. The sulfogalactose moiety of sulfoglycosphingolipids serves as a mimic of tyrosine phosphate in many recognition processes. Prediction and demonstration of Src homology 2 domain/sulfogalactose binding. J Biol Chem. 2005;280(13):12542–12547. doi: 10.1074/jbc.M413724200. [DOI] [PubMed] [Google Scholar]
- 157.Hartmann E, Lingwood CA, Reidl J. Heat-inducible surface stress protein (Hsp70) mediates sulfatide recognition of the respiratory pathogen Haemophilus influenzae . Infect Immun. 2001;69(5):3438–3441. doi: 10.1128/IAI.69.5.3438-3441.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shamshiev A, Gober HJ, Donda A, Mazorra Z, Mori L, De Libero G. Presentation of the same glycolipid by different CD1 molecules. J Exp Med. 2002;195(8):1013–1021. doi: 10.1084/jem.20011963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Duchesneau P, Gallagher E, Walcheck B, Waddell TK. Up-regulation of leukocyte CXCR4 expression by sulfatide: an L-selectin-dependent pathway on CD4 + T cells. Eur J Immunol. 2007;37(10):2949–2960. doi: 10.1002/eji.200737118. [DOI] [PubMed] [Google Scholar]
- 160.Simonis D, Schlesinger M, Seelandt C, Borsig L, Bendas G. Analysis of SM4 sulfatide as a P-selectin ligand using model membranes. Biophys Chem. 2010;150(1–3):98–104. doi: 10.1016/j.bpc.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 161.Takahashi T, Suzuki T. Role of sulfatide in normal and pathological cells and tissues. J Lipid Res. 2012;53(8):1437–1450. doi: 10.1194/jlr.R026682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tiemeyer M, Yasuda Y, Schnaar RL. Ganglioside-specific binding protein on rat brain membranes. J Biol Chem. 1989;264:1671–1681. [PubMed] [Google Scholar]
- 163.Gabius S, Kayser K, Hellmann KP, Ciesiolka T, Trittin A, Gabius H-J. Carrier-immobilized derivatized lysoganglioside GM1 is a ligand for specific binding sites in various human tumor cell types and peripheral blood lymphocytes and monocytes. Biochem Biophys Res Commun. 1990;169:239–244. doi: 10.1016/0006-291X(90)91459-6. [DOI] [PubMed] [Google Scholar]
- 164.Kayser K, Böhm G, Blum S, Beyer M, Zink S, André S, Gabius H-J. Glyco- and immunohistochemical refinement of the differential diagnosis between mesothelioma and metastatic carcinoma and survival analysis of patients. J Pathol. 2001;193:175–180. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH772>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 165.Sonnino S, Aureli M, Loberto N, Chigorno V, Prinetti A. Fine tuning of cell functions through remodeling of glycosphingolipids by plasma membrane-associated glycohydrolases. FEBS Lett. 2010;584(9):1914–1922. doi: 10.1016/j.febslet.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 166.Lewis AL, Lewis WG. Host sialoglycans and bacterial sialidases: a mucosal perspective. Cell Microbiol. 2012;14(8):1174–1182. doi: 10.1111/j.1462-5822.2012.01807.x. [DOI] [PubMed] [Google Scholar]
- 167.Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology. 2012;22(7):880–896. doi: 10.1093/glycob/cws057. [DOI] [PubMed] [Google Scholar]
- 168.Pshezhetsky AV, Ashmarina LI. Desialylation of surface receptors as a new dimension in cell signaling. Biochemistry (Moscow) 2013;78(7):736–745. doi: 10.1134/S0006297913070067. [DOI] [PubMed] [Google Scholar]
- 169.Miyagi T. Mammalian sialidases and their functions. Trends Glycosci Glycotechnol. 2010;22:162–172. doi: 10.4052/tigg.22.162. [DOI] [Google Scholar]
- 170.Pitto M, Chigorno V, Giglioni A, Valsecchi M, Tettamanti G. Sialidase in cerebellar granule cells differentiating in culture. J Neurochem. 1989;53(5):1464–1470. doi: 10.1111/j.1471-4159.1989.tb08539.x. [DOI] [PubMed] [Google Scholar]
- 171.Wu G, Ledeen RW. Stimulation of neurite outgrowth in neuroblastoma cells by neuraminidase: putative role of GM1 ganglioside in differentiation. J Neurochem. 1991;56(1):95–104. doi: 10.1111/j.1471-4159.1991.tb02567.x. [DOI] [PubMed] [Google Scholar]
- 172.Kopitz J, von Reitzenstein C, Mühl C, Cantz M. Role of plasma membrane ganglioside sialidase of human neuroblastoma cells in growth control and differentiation. Biochem Biophys Res Comm. 1994;199(3):1188–1193. doi: 10.1006/bbrc.1994.1356. [DOI] [PubMed] [Google Scholar]
- 173.Kopitz J, Mühl C, Ehemann V, Lehmann C, Cantz M. Effects of cell surface ganglioside sialidase inhibition on growth control and differentiation of human neuroblastoma cells. Eur J Cell Biol. 1997;73:1–7. [PubMed] [Google Scholar]
- 174.Abad-Rodríguez J, Piddini E, Hasegawa T, Miyagi T, Dotti CG. Plasma membrane ganglioside sialidase regulates axonal growth and regeneration in hippocampal neurons in culture. J Neurosci. 2001;21(21):8387–8395. doi: 10.1523/JNEUROSCI.21-21-08387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Da Silva JS, Hasegawa T, Miyagi T, Dotti CG, Abad-Rodríguez J. Asymmetric membrane ganglioside sialidase activity specifies axonal fate. Nat Neurosci. 2005;8(5):606–615. doi: 10.1038/nn1442. [DOI] [PubMed] [Google Scholar]
- 176.Abad-Rodríguez J, Robotti A. Regulation of axonal development by plasma membrane gangliosides. J Neurochem. 2007;103(Suppl 1):47–55. doi: 10.1111/j.1471-4159.2007.04713.x. [DOI] [PubMed] [Google Scholar]
- 177.Kappagantula S, Andrews MR, Cheah M, Abad-Rodríguez J, Dotti CG, Fawcett JW. Neu3 sialidase-mediated ganglioside conversion is necessary for axon regeneration and is blocked in CNS axons. J Neurosci. 2014;34(7):2477–2492. doi: 10.1523/JNEUROSCI.4432-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vyas AA, Blixt O, Paulson JC, Schnaar RL. Potent glycan inhibitors of myelin-associated glycoprotein enhance axon outgrowth in vitro. J Biol Chem. 2005;280(16):16305–16310. doi: 10.1074/jbc.M500250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem. 2007;100(6):1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- 180.Minami A, Suzuki T. Distribution of sialidase activity and the role of sialidase in the brain. Trends Glycosci Glycotechnol. 2012;24:112–121. doi: 10.4052/tigg.24.112. [DOI] [Google Scholar]
- 181.Ledeen RW, Wu G. Neurobiology meets glycosciences. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 495–516. [Google Scholar]
- 182.Wu G, Lu Z-H, André S, Gabius H-J, Ledeen RW (2016) Functional interplay between ganglioside GM1 and cross-linking galectin-1 induces axon-like neuritogenesis via integrin-based signaling and TRPC5-dependent Ca2+ influx. J Neurochem 136(3):550–563. doi: 10.1111/jnc.13418 [DOI] [PMC free article] [PubMed]
- 183.Kopitz J, von Reitzenstein C, Burchert M, Cantz M, Gabius H-J. Galectin-1 is a major receptor for ganglioside GM1, a product of the growth-controlling activity of a cell surface ganglioside sialidase, on human neuroblastoma cells in culture. J Biol Chem. 1998;273(18):11205–11211. doi: 10.1074/jbc.273.18.11205. [DOI] [PubMed] [Google Scholar]
- 184.Siebert H-C, André S, Lu S-Y, Frank M, Kaltner H, van Kuik JA, Korchagina EY, Bovin NV, Tajkhorshid E, Kaptein R, Vliegenthart JFG, von der Lieth C-W, Jiménez-Barbero J, Kopitz J, Gabius H-J. Unique conformer selection of human growth-regulatory lectin galectin-1 for ganglioside GM1 versus bacterial toxins. Biochemistry. 2003;42(50):14762–14773. doi: 10.1021/bi035477c. [DOI] [PubMed] [Google Scholar]
- 185.André S, Kaltner H, Lensch M, Russwurm R, Siebert H-C, Fallsehr C, Tajkhorshid E, Heck AJR, von Knebel-Döberitz M, Gabius H-J, Kopitz J. Determination of structural and functional overlap/divergence of five proto-type galectins by analysis of the growth-regulatory interaction with ganglioside GM1 in silico and in vitro on human neuroblastoma cells. Int J Cancer. 2005;114:46–57. doi: 10.1002/ijc.20699. [DOI] [PubMed] [Google Scholar]
- 186.André S, Kaltner H, Manning JC, Murphy PV, Gabius H-J. Lectins: getting familiar with translators of the sugar code. Molecules. 2015;20(2):1788–1823. doi: 10.3390/molecules20021788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mutoh T, Tokuda A, Miyadai T, Hamaguchi M, Fujiki N. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc Natl Acad Sci USA. 1995;92(11):5087–5091. doi: 10.1073/pnas.92.11.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rusnati M, Urbinati C, Tanghetti E, Dell’Era P, Lortat-Jacob H, Presta M. Cell membrane GM1 ganglioside is a functional coreceptor for fibroblast growth factor 2. Proc Natl Acad Sci USA. 2002;99(7):4367–4372. doi: 10.1073/pnas.072651899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Prinetti A, Loberto N, Chigorno V, Sonnino S. Glycosphingolipid behaviour in complex membranes. Biochim Biophys Acta. 2009;1788(1):184–193. doi: 10.1016/j.bbamem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 190.Wang J, Lu ZH, Gabius H-J, Rohowsky-Kochan C, Ledeen RW, Wu G. Cross-linking of GM1 ganglioside by galectin-1 mediates regulatory T cell activity involving TRPC5 channel activation: possible role in suppressing experimental autoimmune encephalomyelitis. J Immunol. 2009;182(7):4036–4045. doi: 10.4049/jimmunol.0802981. [DOI] [PubMed] [Google Scholar]
- 191.Wu G, Lu ZH, Gabius H-J, Ledeen RW, Bleich D. Ganglioside GM1 deficiency in effector T cells from NOD mice induces resistance to regulatory T cell suppression. Diabetes. 2011;60(9):2341–2349. doi: 10.2337/db10-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ledeen RW, Wu G, André S, Bleich D, Huet G, Kaltner H, Kopitz J, Gabius H-J. Beyond glycoproteins as galectin counterreceptors: tumor/effector T cell growth control via ganglioside GM1. Ann N Y Acad Sci. 2012;1253:206–221. doi: 10.1111/j.1749-6632.2012.06479.x. [DOI] [PubMed] [Google Scholar]
- 193.Kopitz J, Bergmann M, Gabius H-J. How adhesion/growth-regulatory galectins-1 and -3 attain cell specificity: case study defining their target on neuroblastoma cells (SK-N-MC) and marked affinity regulation by affecting microdomain organization of the membrane. IUBMB Life. 2010;62(8):624–628. doi: 10.1002/iub.358. [DOI] [PubMed] [Google Scholar]
- 194.Majewski J, André S, Jones E, Chi E, Gabius H-J. X-ray reflectivity and grazing incidence diffraction studies of interaction between huma adhesion/growth-regulatory galectin-1 and DPPE:GM1 lipid monolayer at the air/water interface. Biochemistry (Moscow) 2015;80:943–956. doi: 10.1134/S0006297915070135. [DOI] [PubMed] [Google Scholar]
- 195.Hardy BJ. The glycosidic linkage flexibility and time-scale similarity hypotheses. J Mol Struct. 1997;395–396:187–200. doi: 10.1016/S0166-1280(96)04866-X. [DOI] [Google Scholar]
- 196.He L, André S, Siebert H-C, Helmholz H, Niemeyer B, Gabius H-J. Detection of ligand- and solvent-induced shape alterations of cell-growth-regulatory human lectin galectin-1 in solution by small angle neutron and X-ray scattering. Biophys J. 2003;85(1):511–524. doi: 10.1016/S0006-3495(03)74496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Göhler A, André S, Kaltner H, Sauer M, Gabius H-J, Doose S. Hydrodynamic properties of human adhesion/growth-regulatory galectins studied by fluorescence correlation spectroscopy. Biophys J. 2010;98(12):3044–3053. doi: 10.1016/j.bpj.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Diehl C, Genheden S, Modig K, Ryde U, Akke M. Conformational entropy changes upon lactose binding to the carbohydrate recognition domain of galectin-3. J Biomol NMR. 2009;45(1–2):157–169. doi: 10.1007/s10858-009-9356-5. [DOI] [PubMed] [Google Scholar]
- 199.Diehl C, Engstrom O, Delaine T, Hakansson M, Genheden S, Modig K, Leffler H, Ryde U, Nilsson UJ, Akke M. Protein flexibility and conformational entropy in ligand design targeting the carbohydrate recognition domain of galectin-3. J Am Chem Soc. 2010;132(41):14577–14589. doi: 10.1021/ja105852y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ruiz FM, Gilles U, Lindner I, André S, Romero A, Reusch D, Gabius H-J. Combining crystallography and hydrogen-deuterium exchange to study galectin-ligand complexes. Chem Eur J. 2015;21(39):13558–13568. doi: 10.1002/chem.201501961. [DOI] [PubMed] [Google Scholar]
- 201.Kopitz J, von Reitzenstein C, André S, Kaltner H, Uhl J, Ehemann V, Cantz M, Gabius H-J. Negative regulation of neuroblastoma cell growth by carbohydrate-dependent surface binding of galectin-1 and functional divergence from galectin-3. J Biol Chem. 2001;276(38):35917–35923. doi: 10.1074/jbc.M105135200. [DOI] [PubMed] [Google Scholar]
- 202.Kopitz J, Ballikaya S, André S, Gabius H-J. Ganglioside GM1/galectin-dependent growth regulation in human neuroblastoma cells: special properties of bivalent galectin-4 and significance of linker length for ligand selection. Neurochem Res. 2012;37(6):1267–1276. doi: 10.1007/s11064-011-0693-x. [DOI] [PubMed] [Google Scholar]
- 203.Kopitz J, Vértesy S, André S, Fiedler S, Schnölzer M, Gabius H-J. Human chimera-type galectin-3: defining the critical tail length for high-affinity glycoprotein/cell surface binding and functional competition with galectin-1 in neuroblastoma cell growth regulation. Biochimie. 2014;104:90–99. doi: 10.1016/j.biochi.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 204.Villalobo A, Nogales-Gonzáles A, Gabius H-J. A guide to signaling pathways connecting protein-glycan interaction with the emerging versatile effector functionality of mammalian lectins. Trends Glycosci Glycotechnol. 2006;18(99):1–37. doi: 10.4052/tigg.18.1. [DOI] [Google Scholar]
- 205.Kasai K-i, Hirabayashi J. Galectins: a family of animal lectins that decipher glycocodes. J Biochem. 1996;119:1–8. doi: 10.1093/oxfordjournals.jbchem.a021192. [DOI] [PubMed] [Google Scholar]
- 206.Dahms NM, Hancock MK. P-Type lectins. Biochim Biophys Acta. 2002;1572(2–3):317–340. doi: 10.1016/S0304-4165(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 207.Gready JN, Zelensky AN. Routes in lectin evolution: case study on the C-type lectin-like domains. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 329–346. [Google Scholar]
- 208.Satoh T. Molecular and structural basis for sugar recognition by mannose-6-phosphate receptor homology domain-containing lectins and proteins. Trends Glycosci Glycotechnol. 2012;24:193–202. doi: 10.4052/tigg.24.193. [DOI] [Google Scholar]
- 209.Percec V, Leowanawat P, Sun HJ, Kulikov O, Nusbaum CD, Tran TM, Bertin A, Wilson DA, Peterca M, Zhang S, Kamat NP, Vargo K, Moock D, Johnston ED, Hammer DA, Pochan DJ, Chen Y, Chabre YM, Shiao TC, Bergeron-Brlek M, André S, Roy R, Gabius H-J, Heiney PA. Modular synthesis of amphiphilic Janus glycodendrimers and their self-assembly into glycodendrimersomes and other complex architectures with bioactivity to biomedically relevant lectins. J Am Chem Soc. 2013;135(24):9055–9077. doi: 10.1021/ja403323y. [DOI] [PubMed] [Google Scholar]
- 210.Zhang S, Moussodia R-O, Vértesy S, André S, Klein ML, Gabius H-J, Percec V. Unraveling functional significance of natural variations of a human galectin by glycodendrimersomes with programmable glycan surface. Proc Natl Acad Sci USA. 2015;112(18):5585–5590. doi: 10.1073/pnas.1506220112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang S, Moussodia R-O, Murzeau C, Sun HJ, Klein ML, Vértesy S, André S, Roy R, Gabius H-J, Percec V. Dissecting molecular aspects of cell interactions using glycodendrimersomes with programmable glycan presentation and engineered human lectins. Angew Chem Int Ed. 2015;54(13):4036–4040. doi: 10.1002/anie.201410882. [DOI] [PubMed] [Google Scholar]
- 212.Zhang S, Xiao Q, Sherman SE, Muncan A, Ramos Vicente AD, Wang Z, Hammer DA, Williams D, Chen Y, Pochan DJ, Vértesy S, André S, Klein ML, Gabius H-J, Percec V. Glycodendrimersomes from sequence-defined Janus glycodendrimers reveal high activity and sensor capacity for the agglutination by natural variants of human lectins. J Am Chem Soc. 2015;137(41):13334–13344. doi: 10.1021/jacs.5b08844. [DOI] [PubMed] [Google Scholar]
- 213.Vértesy S, Michalak M, Miller MC, Schnölzer M, André S, Kopitz J, Mayo KH, Gabius H-J. Structural significance of galectin design: impairment of homodimer stability by linker insertion and partial reversion by ligand presence. Protein Eng Des Sel. 2015;28(7):199–210. doi: 10.1093/protein/gzv014. [DOI] [PubMed] [Google Scholar]
- 214.Kaltner H, Kübler D, López-Merino L, Lohr M, Manning JC, Lensch M, Seidler J, Lehmann WD, André S, Solís D, Gabius H-J. Toward comprehensive analysis of the galectin network in chicken: unique diversity of galectin-3 and comparison of its localization profile in organs of adult animals to the other four members of this lectin family. Anat Rec. 2011;294(3):427–444. doi: 10.1002/ar.21341. [DOI] [PubMed] [Google Scholar]
- 215.Kaltner H, Singh T, Manning JC, Raschta AS, André S, Sinowatz F, Gabius H-J. Network monitoring of adhesion/growth-regulatory galectins: localization of the ive canonical chicken proteins in embryonic and maturing bone and cartilage and their introduction as histochemical tools. Anat Rec. 2015;298(12):2051–2070. doi: 10.1002/ar.23265. [DOI] [PubMed] [Google Scholar]
- 216.Wang JL, Gray RM, Haudek KC, Patterson RJ. Nucleocytoplasmic lectins. Biochim Biophys Acta. 2004;1673(1–2):75–93. doi: 10.1016/j.bbagen.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 217.Smetana K, Jr, André S, Kaltner H, Kopitz J, Gabius H-J. Context-dependent multifunctionality of galectin-1: a challenge for defining the lectin as therapeutic target. Expert Opin Ther Targets. 2013;17(4):379–392. doi: 10.1517/14728222.2013.750651. [DOI] [PubMed] [Google Scholar]
- 218.Kozireski-Chuback D, Wu G, Ledeen RW. Developmental appearance of nuclear GM1 in neurons of the central and peripheral nervous systems. Brain Res Dev Brain Res. 1999;115(2):201–208. doi: 10.1016/S0165-3806(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 219.Ledeen RW, Wu G. GM1 in the nuclear envelope regulates nuclear calcium through association with a nuclear sodium-calcium exchanger. J Neurochem. 2007;103(Suppl 1):126–134. doi: 10.1111/j.1471-4159.2007.04722.x. [DOI] [PubMed] [Google Scholar]
- 220.Nowycky MC, Wu G, Ledeen RW. Glycobiology of ion transport in the nervous system. Adv Neurobiol. 2014;9:321–342. doi: 10.1007/978-1-4939-1154-7_15. [DOI] [PubMed] [Google Scholar]
- 221.Schlötzer-Schrehardt U, André S, Janko C, Kaltner H, Kopitz J, Gabius H-J, Herrmann M. Adhesion/growth-regulatory galectins in the human eye: localization profiles and tissue reactivities as a standard to detect disease-associated alterations. Graefes Arch Clin Exp Ophthalmol. 2012;250(8):1169–1180. doi: 10.1007/s00417-012-2021-9. [DOI] [PubMed] [Google Scholar]
- 222.Park JW, Voss PG, Grabski S, Wang JL, Patterson RJ. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001;29(17):3595–3602. doi: 10.1093/nar/29.17.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Purkrábková T, Smetana K, Jr, Dvoránková B, Holíková Z, Böck C, Lensch M, André S, Pytlík R, Liu F-T, Klíma J, Smetana K, Motlik J, Gabius H-J. New aspects of galectin functionality in nuclei of cultured bone marrow stromal and epidermal cells: biotinylated galectins as tool to detect specific binding sites. Biol Cell. 2003;95:535–545. doi: 10.1016/j.biolcel.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 224.Haudek KC, Patterson RJ, Wang JL. SR proteins and galectins: what’s in a name? Glycobiology. 2010;20(10):1199–1207. doi: 10.1093/glycob/cwq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gabius H-J, Wosgien B, Hendrys M, Bardosi A. Lectin localization in human nerve by biochemically defined lectin-binding glycoproteins, neoglycoprotein and lectin-specific antibody. Histochemistry. 1991;95:269–277. doi: 10.1007/BF00266777. [DOI] [PubMed] [Google Scholar]
- 226.Solís D, Romero A, Kaltner H, Gabius H-J, Díaz-Mauriño T. Different architecture of the combining sites of two chicken galectins revealed by chemical-mapping studies with synthetic ligand derivatives. J Biol Chem. 1996;271:12744–12748. doi: 10.1074/jbc.271.22.12744. [DOI] [PubMed] [Google Scholar]
- 227.Wu AM, Singh T, Liu J-H, Krzeminski M, Russwurm R, Siebert H-C, Bonvin AMJJ, André S, Gabius H-J. Activity-structure correlations in divergent lectin evolution: fine specificity of chicken galectin CG-14 and computational analysis of flexible ligand docking for CG-14 and the closely related CG-16. Glycobiology. 2007;17:165–184. doi: 10.1093/glycob/cwl062. [DOI] [PubMed] [Google Scholar]
- 228.Rapoport EM, Matveeva VK, Kaltner H, André S, Vokhmyanina OA, Pazynina GV, Severov VV, Ryzhov IM, Korchagina EY, Belyanchikov IM, Gabius H-J, Bovin NV. Comparative lectinology: delineating glycan-specificity profiles of the chicken galectins using neoglycoconjugates in a cell assay. Glycobiology. 2015;25(7):726–734. doi: 10.1093/glycob/cwv012. [DOI] [PubMed] [Google Scholar]
- 229.Lohr M, Kaltner H, Schwartz-Albiez R, Sinowatz F, Gabius H-J. Towards functional glycomics by lectin histochemistry: strategic probe selection to monitor core and branch-end substitutions and detection of cell-type and regional selectivity in adult mouse testis and epididymis. Anat Histol Embryol. 2010;39(6):481–493. doi: 10.1111/j.1439-0264.2010.01019.x. [DOI] [PubMed] [Google Scholar]
- 230.Celie JW, Beelen RHJ, van den Born J. Effect of fixation protocols on in situ detection of L-selectin ligands. J Immunol Methods. 2005;298(1–2):155–159. doi: 10.1016/j.jim.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 231.Schwarz A, Futerman AH. Determination of the localization of gangliosides using anti-ganglioside antibodies: comparison of fixation methods. J Histochem Cytochem. 1997;45(4):611–618. doi: 10.1177/002215549704500413. [DOI] [PubMed] [Google Scholar]
- 232.Nagy N, Legendre H, Engels O, André S, Kaltner H, Wasano K, Zick Y, Pector J-C, Decaestecker C, Gabius H-J, Salmon I, Kiss R. Refined prognostic evaluation in colon cancer using immunohistochemical galectin fingerprinting. Cancer. 2003;97:1849–1858. doi: 10.1002/cncr.11268. [DOI] [PubMed] [Google Scholar]
- 233.Remmelink M, de Leval L, Decaestecker C, Duray A, Crompot E, Sirtaine N, André S, Kaltner H, Leroy X, Gabius H-J, Saussez S. Quantitative immunohistochemical fingerprinting of adhesion/growth-regulatory galectins in salivary gland tumours: divergent profiles with diagnostic potential. Histopathology. 2011;58(4):543–556. doi: 10.1111/j.1365-2559.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 234.Dawson H, André S, Karamitopoulou E, Zlobec I, Gabius H-J. The growing galectin network in colon cancer and clinical relevance of cytoplasmic galectin-3 reactivity. Anticancer Res. 2013;33(8):3053–3059. [PubMed] [Google Scholar]
- 235.Katzenmaier E-M, André S, Kopitz J, Gabius H-J. Impact of sodium butyrate on the network of adhesion/growth-regulatory galectins in human colon cancer in vitro. Anticancer Res. 2014;34(10):5429–5438. [PubMed] [Google Scholar]
- 236.Rorive S, Belot N, Decaestecker C, Lefranc F, Gordower L, Micik S, Maurage C-A, Kaltner H, Ruchoux M-M, Danguy A, Gabius H-J, Salmon I, Kiss R, Camby I. Galectin-1 is highly expressed in human gliomas with relevance for modulation of invasion of tumor astrocytes into the brain parenchyma. Glia. 2001;33(3):241–255. doi: 10.1002/1098-1136(200103)33:3<241::AID-GLIA1023>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 237.Camby I, Belot N, Lefranc F, Sadeghi N, de Launoit Y, Kaltner H, Musette S, Darro F, Danguy A, Salmon I, Gabius H-J, Kiss R. Galectin-1 modulates human glioblastoma cell migration into the brain through modifications to the actin cytoskeleton and levels of expression of small GTPases. J Neuropathol Exp Neurol. 2002;61(7):585–596. doi: 10.1093/jnen/61.7.585. [DOI] [PubMed] [Google Scholar]
- 238.Hann A, Gruner A, Chen Y, Gress TM, Buchholz M. Comprehensive analysis of cellular galectin-3 reveals no consistent oncogenic function in pancreatic cancer cells. PLoS ONE. 2011;6(6):e20859. doi: 10.1371/journal.pone.0020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Plzák J, Betka J, Smetana K, Jr, Chovanec M, Kaltner H, André S, Kodet R, Gabius H-J. Galectin-3: an emerging prognostic indicator in advanced head and neck carcinoma. Eur J Cancer. 2004;40(15):2324–2330. doi: 10.1016/j.ejca.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 240.Yang RY, Hsu DK, Liu F-T. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996;93:6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Akahani S, Nangia-Makker P, Inohara H, Kim H-RC, Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997;57:5272–5276. [PubMed] [Google Scholar]
- 242.Harazono Y, Kho DH, Balan V, Nakajima K, Zhang T, Hogan V, Raz A. Galectin-3 leads to attenuation of apoptosis through Bax heterodimerization in human thyroid carcinoma cells. Oncotarget. 2014;5(20):9992–10001. doi: 10.18632/oncotarget.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Toegel S, Bieder D, André S, Kayser K, Walzer SM, Hobusch G, Windhager R, Gabius H-J. Human osteoarthritic knee cartilage: fingerprinting of adhesion/growth-regulatory galectins in vitro and in situ indicates differential upregulation in severe degeneration. Histochem Cell Biol. 2014;142(4):373–388. doi: 10.1007/s00418-014-1234-x. [DOI] [PubMed] [Google Scholar]
- 244.André S, Sanchez-Ruderisch H, Nakagawa H, Buchholz M, Kopitz J, Forberich P, Kemmner W, Böck C, Deguchi K, Detjen KM, Wiedenmann B, von Knebel-Döberitz M, Gress TM, Nishimura S-I, Rosewicz S, Gabius H-J. Tumor suppressor p16INK4a: modulator of glycomic profile and galectin-1 expression to increase susceptibility to carbohydrate-dependent induction of anoikis in pancreatic carcinoma cells. FEBS J. 2007;274:3233–3256. doi: 10.1111/j.1742-4658.2007.05851.x. [DOI] [PubMed] [Google Scholar]
- 245.Amano M, Eriksson H, Manning JC, Detjen KM, André S, Nishimura S-I, Lehtiö J, Gabius H-J. Tumour suppressor p16INK4a: anoikis-favouring decrease in N/O-glycan/cell surface sialylation by down-regulation of enzymes in sialic acid biosynthesis in tandem in a pancreatic carcinoma model. FEBS J. 2012;279(21):4062–4080. doi: 10.1111/febs.12001. [DOI] [PubMed] [Google Scholar]
- 246.Sanchez-Ruderisch H, Fischer C, Detjen KM, Welzel M, Wimmel A, Manning JC, André S, Gabius H-J. Tumor suppressor p16INK4a: downregulation of galectin-3, an endogenous competitor of the pro-anoikis effector galectin-1, in a pancreatic carcinoma model. FEBS J. 2010;277(17):3552–3563. doi: 10.1111/j.1742-4658.2010.07764.x. [DOI] [PubMed] [Google Scholar]
- 247.Gitt MA, Colnot C, Poirier F, Nani KJ, Barondes SH, Leffler H. Galectin-4 and galectin-6 are two closely related lectins expressed in mouse gastrointestinal tract. J Biol Chem. 1998;273:2954–2960. doi: 10.1074/jbc.273.5.2954. [DOI] [PubMed] [Google Scholar]
- 248.Gitt MA, Xia YR, Atchison RE, Lusis AJ, Barondes SH, Leffler H. Sequence, structure, and chromosomal mapping of the mouse Lgals6 gene, encoding galectin-6. J Biol Chem. 1998;273(5):2961–2970. doi: 10.1074/jbc.273.5.2961. [DOI] [PubMed] [Google Scholar]
- 249.Houzelstein D, Goncalves IR, Orth A, Bonhomme F, Netter P. Lgals6, a 2-million-year-old gene in mice: a case of positive Darwinian selection and presence/absence polymorphism. Genetics. 2008;178(3):1533–1545. doi: 10.1534/genetics.107.082792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gitt MA, Wiser MF, Leffler H, Herrmann J, Xia Y, Massa SM, Cooper DNW, Lusis AJ, Barondes SH. Sequence and mapping of galectin-5, a β-galactoside-binding lectin, found in rat erythrocytes. J Biol Chem. 1995;270:5032–5038. doi: 10.1074/jbc.270.10.5032. [DOI] [PubMed] [Google Scholar]
- 251.Wada J, Kanwar YS. Identification and characterization of galectin-9, a novel β-galactoside-binding mammalian lectin. J Biol Chem. 1997;272(9):6078–6086. doi: 10.1074/jbc.272.9.6078. [DOI] [PubMed] [Google Scholar]
- 252.Lensch M, Lohr M, Russwurm R, Vidal M, Kaltner H, André S, Gabius H-J. Unique sequence and expression profiles of rat galectins-5 and -9 as a result of species-specific gene divergence. Int J Biochem Cell Biol. 2006;38(10):1741–1758. doi: 10.1016/j.biocel.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 253.Kaltner H, Raschta A-S, Manning JC, Gabius H-J. Copy-number variation of functional galectin genes: studying animal galectin-7 (p53-induced gene 1 in man) and tandem-repeat-type galectins-4 and -9. Glycobiology. 2013;23(10):1152–1163. doi: 10.1093/glycob/cwt052. [DOI] [PubMed] [Google Scholar]
- 254.Suzuki N, Yamamoto K, Toyoshima S, Osawa T, Irimura T. Molecular cloning and expression of cDNA encoding human macrophage C-type lectin. J Immunol. 1996;156:128–135. [PubMed] [Google Scholar]
- 255.Tsuiji M, Fujimori M, Ohashi Y, Higashi N, Onami TM, Hedrick SM, Irimura T. Molecular cloning and characterization of a novel mouse macrophage C-type lectin, mMGL2, which has a distinct carbohydrate specificity from mMGL1. J Biol Chem. 2002;277(32):28892–28901. doi: 10.1074/jbc.M203774200. [DOI] [PubMed] [Google Scholar]
- 256.Soilleux EJ, Barten R, Trowsdale J. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J Immunol. 2000;165(6):2937–2942. doi: 10.4049/jimmunol.165.6.2937. [DOI] [PubMed] [Google Scholar]
- 257.Park CG, Takahara K, Umemoto E, Yashima Y, Matsubara K, Matsuda Y, Clausen BE, Inaba K, Steinman RM. Five mouse homologues of the human dendritic cell C-type lectin. DC-SIGN. Int Immunol. 2001;13(10):1283–1290. doi: 10.1093/intimm/13.10.1283. [DOI] [PubMed] [Google Scholar]
- 258.Bashirova AA, Wu L, Cheng J, Martin TD, Martin MP, Benveniste RE, Lifson JD, KewalRamani VN, Hughes A, Carrington M. Novel member of the CD209 (DC-SIGN) gene family in primates. J Virol. 2003;77(1):217–227. doi: 10.1128/JVI.77.1.217-227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Huang YW, Dryman BA, Li W, Meng XJ. Porcine DC-SIGN: molecular cloning, gene structure, tissue distribution and binding characteristics. Dev Comp Immunol. 2009;33(4):464–480. doi: 10.1016/j.dci.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Huang YW, Meng XJ. Identification of a porcine DC-SIGN-related C-type lectin, porcine CLEC4G (LSECtin), and its order of intron removal during splicing: comparative genomic analyses of the cluster of genes CD23/CLEC4G/DC-SIGN among mammalian species. Dev Comp Immunol. 2009;33(6):747–760. doi: 10.1016/j.dci.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Guo N, Mogues T, Weremowicz S, Morton CC, Sastry KN. The human ortholog of rhesus mannose-binding protein-A gene is an expressed pseudogene that localizes to chromosome 10. Mamm Genome. 1998;9(3):246–249. doi: 10.1007/s003359900735. [DOI] [PubMed] [Google Scholar]
- 262.Bordet J, Gay FP. Sur les relations des sensibilisatrices avec l’alexine. Ann Inst Pasteur. 1906;20:467–498. [Google Scholar]
- 263.Gjerstorff M, Hansen S, Jensen B, Dueholm B, Horn P, Bendixen C, Holmskov U. The genes encoding bovine SP-A, SP-D, MBL-A, conglutinin, CL-43 and CL-46 form a distinct collectin locus on Bos taurus chromosome 28 (BTA28) at position q.1.8-1.9. Anim Genet. 2004;35(4):333–337. doi: 10.1111/j.1365-2052.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- 264.Cao H, Crocker PR. Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation? Immunology. 2011;132(1):18–26. doi: 10.1111/j.1365-2567.2010.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Martick M, Horan LH, Noller HF, Scott WG. A discontinuous hammerhead ribozyme embedded in a mammalian messenger RNA. Nature. 2008;454(7206):899–902. doi: 10.1038/nature07117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Talini G, Branciamore S, Gallori E. Ribozymes: flexible molecular devices at work. Biochimie. 2011;93(11):1998–2005. doi: 10.1016/j.biochi.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 267.Pál Z, Antal P, Srivastava SK, Hullám G, Semsei AF, Gál J, Svébis M, Soós G, Szalai C, André S, Gordeeva E, Nagy G, Kaltner H, Bovin NV, Molnár MJ, Falus A, Gabius H-J, Buzás EI. Non-synonymous single nucleotide polymorphisms in genes for immunoregulatory galectins: association of galectin-8 (F19Y) occurrence with autoimmune diseases in a Caucasian population. Biochim Biophys Acta. 2012;1820:1512–1518. doi: 10.1016/j.bbagen.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 268.Pineda B, Laporta P, Cano A, Garcia-Perez MA. The Asn19Lys substitution in the osteoclast inhibitory lectin (OCIL) gene is associated with a reduction of bone mineral density in postmenopausal women. Calcif Tissue Int. 2008;82(5):348–353. doi: 10.1007/s00223-008-9135-4. [DOI] [PubMed] [Google Scholar]
- 269.Marakalala MJ, Kerrigan AM, Brown GD. Dectin-1: a role in antifungal defense and consequences of genetic polymorphisms in humans. Mamm Genome. 2011;22(1–2):55–65. doi: 10.1007/s00335-010-9277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rooryck C, Diaz-Font A, Osborn DP, Chabchoub E, Hernandez-Hernandez V, Shamseldin H, Kenny J, Waters A, Jenkins D, Kaissi AA, Leal GF, Dallapiccola B, Carnevale F, Bitner-Glindzicz M, Lees M, Hennekam R, Stanier P, Burns AJ, Peeters H, Alkuraya FS, Beales PL. Mutations in lectin complement pathway genes COLEC11 and MASP1 cause 3MC syndrome. Nat Genet. 2011;43(3):197–203. doi: 10.1038/ng.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Selman L, Hansen S. Structure and function of collectin liver 1 (CL-L1) and collectin 11 (CL-11, CL-K1) Immunobiology. 2012;217(9):851–863. doi: 10.1016/j.imbio.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 272.Swanson MD, Boudreaux DM, Salmon L, Chugh J, Winter HC, Meagher JL, André S, Murphy PV, Oscarson S, Roy R, King S, Kaplan MH, Goldstein IJ, Tarbet EB, Hurst BL, Smee DF, de la Fuente C, Hoffmann HH, Xue Y, Rice CM, Schols D, Garcia JV, Stuckey JA, Gabius H-J, Al-Hashimi HM, Markovitz DM. Engineering a therapeutic lectin by uncoupling mitogenicity from antiviral activity. Cell. 2015;163(3):746–758. doi: 10.1016/j.cell.2015.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Gabius H-J. Animal and human lectins. In: Gabius H-J, editor. The Sugar Code. Wiley-VCH, Weinheim, Germany: Fundamentals of glycosciences; 2009. pp. 317–328. [Google Scholar]
- 274.Kaltner H, Solís D, Kopitz J, Lensch M, Lohr M, Manning JC, Mürnseer M, Schnölzer M, André S, Sáiz JL, Gabius H-J. Prototype chicken galectins revisited: characterization of a third protein with distinctive hydrodynamic behaviour and expression pattern in organs of adult animals. Biochem J. 2008;409(2):591–599. doi: 10.1042/BJ20070419. [DOI] [PubMed] [Google Scholar]
- 275.Kaltner H, Solís D, André S, Lensch M, Manning JC, Mürnseer M, Saíz JL, Gabius H-J. Unique chicken tandem-repeat-type galectin: implications of alternative splicing and a distinct expression profile compared to those of the three proto-type proteins. Biochemistry. 2009;48(20):4403–4416. doi: 10.1021/bi900083q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 4 (TIFF 10834 kb)