Abstract
Macrophages play a crucial role in the innate immune system and contribute to a broad spectrum of pathologies, like in the defence against infectious agents, in inflammation resolution, and wound repair. In the past several years, microRNAs (miRNAs) have been demonstrated to play important roles in immune diseases by regulating macrophage functions. In this review, we will summarize the role of miRNAs in the differentiation of monocytes into macrophages, in the classical and alternative activation of macrophages, and in the regulation of phagocytosis and apoptosis. Notably, miRNAs preferentially target genes related to the cellular cholesterol metabolism, which is of key importance for the inflammatory activation and phagocytic activity of macrophages. miRNAs functionally link various mechanisms involved in macrophage activation and contribute to initiation and resolution of inflammation. miRNAs represent promising diagnostic and therapeutic targets in different conditions, such as infectious diseases, atherosclerosis, and cancer.
Keywords: Immune disorders, MicroRNA, Macrophage, Lipid metabolism, Inflammation
Introduction
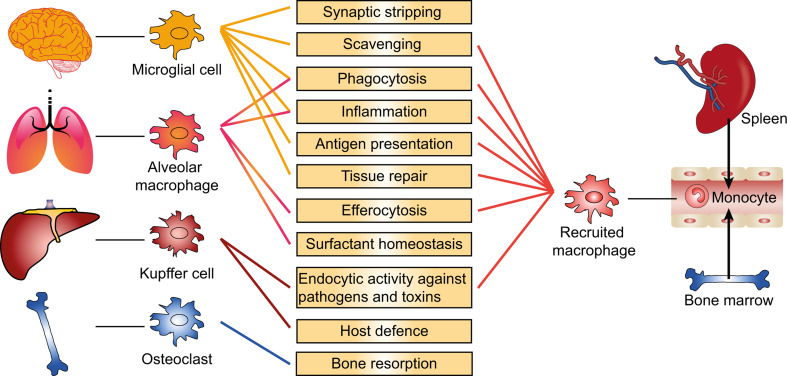
The eponymous function of macrophages is phagocytosis, for instance, of microorganisms, foreign material, or apoptotic cells, which plays a major role in the defence against infectious agents, in development, tissue homeostasis, inflammation resolution, and wound repair [1–3]. Tissue-resident macrophages exist in various tissues throughout the body where they are involved in immune surveillance (for instance, in the liver, lung, brain, and epidermis) and in tissue homeostasis through bone remodelling and regulation of haematopoiesis [4] (Fig. 1). In mice, most tissue-resident macrophages, including liver Kupffer cells and alveolar, splenic, and peritoneal macrophages, originate from embryonic precursors and self-renew by proliferation, whereas intestinal macrophages, which are physiologically exposed to microbial products, are constantly replaced by circulating monocytes [5–8]. In addition, inflammation triggers the accumulation of recruited macrophages derived from circulating monocytes, which are released from reservoirs in blood and bone marrow [2, 9, 10] (Fig. 1).
Fig. 1.
Macrophages perform important homeostatic and inflammatory functions. Tissue-resident macrophages exist in various tissues throughout the body where they maintain tissue homoeostasis, surveil the local microenvironment, and promote the accumulation of recruited macrophages in response to injuries or infections. Circulating monocytes from reservoirs in blood, spleen and bone marrow differentiate into macrophages at the inflammatory sites where they remove invading organisms or tissue debris, and promote inflammation resolution upon polarization into different subtypes of activated macrophages
The differentiation of monocytes into macrophages is morphologically characterized by an increase in cell size and vacuole content, plasma membrane extensions, and an increased number of mitochondria, and functionally by an enhanced phagocytosis capacity and inflammatory activation [11, 12]. Growth factors, like macrophage colony stimulating factor (M-CSF) and granulocyte–macrophage colony-stimulating factor (GM-CSF), induce the production of monocytes in the bone marrow, the differentiation of monocytes into macrophages and the proliferation of resident and recruited macrophages during inflammation [13–15]. M-CSF alone is sufficient for the differentiation of monocytes into macrophages and the surface expression of the M-CSF receptor (CSF1R) driven by the myeloid transcription factor purine-rich PU-box-binding protein 1 (PU.1) increased during the differentiation process [16–18]. M-CSF-mediated monocyte-to-macrophage differentiation is associated with the differential regulation of 2 % of the transcripts, including cell cycle genes and genes related to lipid and arachidonate metabolism [19]. Moreover, increased sterol regulatory element binding protein (SREBP)-1c-dependent fatty acid synthesis promotes phagocytosis and the development of cellular organelles, such as lysosomes, the endoplasmic reticulum, and the Golgi network during macrophage differentiation [20].
To identify pathogens, macrophages express pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), that recognize conserved motifs (so called pathogen-associated molecular patterns) on bacteria, fungi, and viruses [21]. PRRs like C-type lectin receptors and scavenger receptors trigger the phagocytosis of microbes, whereas TLRs, such as TLR2 and TLR4, may identify specific pathogens in phagosomes, which activate signalling pathways that promote phagosome maturation and inflammatory gene expression, and result in enhanced killing of infectious agents through oxidative and non-oxidative mechanisms [22–24]. Phagocytosis and lysosomal digestion of pathogens can lead to the presentation of processed antigens by major histocompatibility complex class II molecules on the surface of macrophages, which regulates the adaptive immune response by activating CD4+ T cells [25]. Conversely, T cells derived cytokines, such as interferon gamma (IFN-γ) and interleukin (IL)-17, greatly increase the antimicrobial activity of macrophages, which thus become a main effector cell type of the adaptive immune system [25]. In addition to phagocytosis, activated macrophages promote inflammation by secreting numerous factors, such as cytokines, proteinases, bioactive lipids, reactive oxygen and nitrogen intermediates, and complement components [26, 27] (Fig. 1). However, the effects of recruited macrophages are tightly controlled to prevent tissue destruction. Moreover, a phenotypic switch of inflammatory macrophages into a phenotype that promotes inflammation resolution is essential for the termination of the immune response [28].
The heterogeneity of recruited macrophages
The functional phenotypes of recruited macrophages range between two extremes, the pro-inflammatory and anti-inflammatory subtype, which contribute to different stages of the inflammatory response [29–33]. The initiation phase of an inflammatory response is characterized by pro-inflammatory macrophages, which secrete inflammatory cytokines, such as IL-12, IL-1β and tumor necrosis factor (TNF)-α, and exhibit increased microbicidal activity through production of reactive oxygen species (ROS) and reactive nitrogen species [33–35]. Anti-inflammatory macrophages play important roles in inflammation resolution and tissue repair by secreting anti-inflammatory cytokines, such as IL-10, which suppresses the activation of the nuclear factor-κB (NF-κB) signalling pathway [30, 34, 36]. In vitro, IL-4-mediated macrophage polarization into an anti-inflammatory phenotype (also known as alternatively activated or M2 macrophages) has only a limited effect on gene expression, whereas pro-inflammatory macrophage polarization (also known as classically activated or M1 macrophages) by lipopolysaccharide (LPS) and IFN-γ stimulation alters ~5 % of the macrophage transcriptome [19]. These changes of the gene expression during macrophage polarization are largely driven by the activation of transcription factors, such as NF-κB and peroxisome proliferator-activated receptor gamma (PPARγ) [37, 38]. In M1 macrophages, LPS-induced TLR4 signalling activates the NF-κB pathway and IFN-γ triggers Janus kinases (JAK)-mediated dimerization of signal transducers and activators of transcription 1 (STAT1), which results in increased expression of inflammatory genes and metabolic reprogramming [38, 39]. In addition to M-CSF-driven signalling via the phosphatidylinositol 3-kinase (PI3K)-thymoma viral proto-oncogene (Akt) pathway, IL-4 promotes anti-inflammatory gene expression and M2 polarization by activating STAT6 and PPARγ [37]. Notably, Akt1 activation promotes a M2 phenotype, whereas Akt2 induces a M1 macrophage subtype, indicating that the effect of Akt on macrophage polarization is isoform-specific [40, 41]. Moreover, activation of the transcription factors nuclear receptor subfamily 4 group A member 1 and Krüppel-like factor 4 promotes a M2 phenotype [42–46]. In contrast to anti-inflammatory macrophages, pro-inflammatory cells promote atherosclerosis and a switch from an anti-inflammatory into a pro-inflammatory macrophage phenotype in adipose tissue increases adipocyte dysfunction and drives obesity-induced insulin resistance, demonstrating that these macrophage subtypes have opposing roles in chronic inflammatory diseases [47–51]. In contrast to the effect of GM-CSF, treatment with M-CSF promotes the differentiation into macrophages with characteristics of the M2 phenotype, which may be the default pathway in macrophage differentiation [19].
In addition to the production and secretion of pro-inflammatory cytokines, energy metabolism differs fundamentally between M1 and M2 macrophages [35]. Activation of the transcription factor hypoxia-inducible factor-1 (HIF-1) by inflammatory activation shifts ATP synthesis from mitochondrial oxidative phosphorylation (OXPHOS) to aerobic glycolysis in macrophages and results in increased production of ROS through the mitochondrial electron transport chain by utilizing fatty acids, which results in increased NF-κB activation and enhanced bactericidal activity [52–57]. Moreover, nitric oxide production in inflammatory macrophages may contribute to mitochondrial ROS production by inhibiting cytochrome c oxidase [58–60]. M2 macrophages produce ATP by utilizing long-chain fatty acids in OXPHOS, which promotes an anti-inflammatory phenotype and improves macrophage survival [53, 55, 61–63], indicating that differences in nutrient utilization control macrophage activation and the phenotypic switch of macrophages during the progression of inflammation [35].
Resolution of inflammation requires the removal of tissue debris and apoptotic leukocytes (mainly neutrophils) by macrophages [64]. In contrast to infectious pathogens, apoptotic cells are engulfed through a distinct set of macrophage receptors, such as MERTK and CD36, which detect phosphatidylserine exposed on the surface of apoptotic cells [64, 65]. Induction of an M2 phenotype increases MERTK expression and enhances the clearance of apoptotic cells by macrophages (also known as efferocytosis) [66]. Moreover, the removal of apoptotic cells promotes anti-inflammatory gene expression in the efferocyte and thus contributes to inflammation resolution [67, 68]. Defective efferocytosis may lead to secondary necrosis of apoptotic leukocytes and the release of intracellular antigens, such as high-mobility group protein 1 and DNA, from the dying cells, which trigger an inflammatory response in macrophages and promote self-perpetuating chronic inflammation [69]. Thus, impaired efferocytosis may contribute to chronic inflammatory diseases, such as atherosclerosis and lung inflammation, systemic lupus erythematosus, and rheumatoid arthritis [70, 71]. Therefore, tight control of the different aspects of macrophage function is critical for the proper initiation and resolution of inflammation.
Although the M1 and M2 macrophage polarization concept helps to describe the immune response in a variety of diseases, a much broader transcriptional repertoire in macrophages exists and results in numerous functional subtypes, including macrophages (termed Mox macrophages) with impaired phagocytic and reduced inflammatory activity that develop in response to oxidized phospholipids and CXCL4-induced macrophages (termed M4 macrophages) characterized by reduced phagocytosis and a pro-inflammatory cytokine profile, [32, 72–76]. In vivo, a huge diversity of conditions activate macrophages and may result in an infinite spectrum of phenotypes driven by a highly dynamic network of transcriptional programs [33, 76–78]. Gene expression processes are intrinsically noisy and the regulation of transcriptional programs by transcription factors can result in large phenotypic variation [79, 80]. The inherently random nature of gene expression may greatly contribute to the enormous phenotypic heterogeneity of macrophages [81–83]. Short, non-coding microRNAs (miRNAs) negatively regulate gene expression by interacting with the 3′-UTR of mRNAs and thereby participate in negative feedback loops or incoherent feed-forward motifs that adapt gene expression networks. Thus, miRNAs confer phenotypic robustness by regulating transcriptional noise and may thereby play a crucial role in macrophage activation [84–86].
MicroRNA biogenesis and function
miRNAs are processed in a two-step process from RNA polymerase II-generated primary transcripts (pri-miRNAs) characterized by a hairpin structure with a double stranded stem [87]. In the nucleus, the endoribonucleolytic enzyme Drosha cleaves the pri-miRNAs at the 5′ and 3′ end into miRNA precursors (pre-miRNAs) that possess a 2-nt overhang at the 3′ end. After translocation of the pre-miRNAs into the cytoplasma by GTP-dependent binding to Exportin 5, the RNase III endonuclease Dicer recognizes the 2-nt overhang and cleaves the pre-miRNAs near the terminal loop of the hairpin into 21–25 nt long miRNA duplexes. Dicer assembles with RNA binding proteins, like TAR RNA binding protein or protein activator of interferon induced protein kinase EIF2AK2 (also known as PACT), and Argonaute (AGO) proteins to form a RNA-induced silencing complexes (RISCs)-loading complex, which mediates the transfer of miRNA duplexes from Dicer to AGO proteins in an ATP-dependent manner [88, 89]. In contrast to siRNA duplexes, which are separated by AGO2-mediated cleavage of one strand of the duplex (called slicing), the central mismatches in the miRNA duplexes prevent slicing [90]. Alternatively, the separation of the miRNA duplexes occurs through a process called unwinding in which the N-terminal end of the AGO proteins wedges into the miRNA double strand, presumably due to ATP-independent conformational change of the AGO protein, and thereby releases one of the strands (the passenger strand) from the RISC [91]. The mature RISC, a complex of an AGO protein and a single-stranded miRNA, is capable of target RNA binding. The first monophosphate and nucleotide of the 5′ end of the miRNA is anchored to the AGO proteins, which results in the presentation of the nucleotides 2–5 to recognize complementary RNA sequences. The canonical target sites interact through Watson–Crick complementary bases with the nucleotides 2–8 at the 5′ end of miRNAs (termed the “seed” sequence) and are usually located at the beginning or the end of long 3′ untranslated region (UTR) in the target mRNA. To silence the expression of the target mRNA, GW182 family proteins are recruited to the RISC, and inhibit mRNA translation or promote mRNA deadenylation and degradation by the exonuclease XRN1 [92]. Due to the short miRNA recognition sequence, an individual miRNA can target usually more than one mRNA and, conversely, one mRNA transcript contains binding sites for multiple miRNAs [93]. Therefore, mRNAs or other RNA molecules, such as long non-coding RNAs, which contain the same miRNA target site compete with each other for the binding to the same miRNA and thus one miRNA target can regulate the expression level of the competing endogenous RNAs in trans [94–96]. Of note, in quiescent cells, not all expressed miRNAs bind to targets and target-free miRNAs are stored in small AGO2 complexes until cells are activated [97, 98].
Considering the regulatory function of miRNAs in the cellular response to environmental fluctuation, miRNAs are essential regulatory elements for macrophage phenotype reprogramming in response to various inflammatory stimuli [99–102]. In this Review, we will discuss how miRNAs regulate the macrophage phenotype and function during an inflammatory response.
Role of microRNAs in recruited macrophages
MicroRNAs regulate monocyte-to-macrophage differentiation
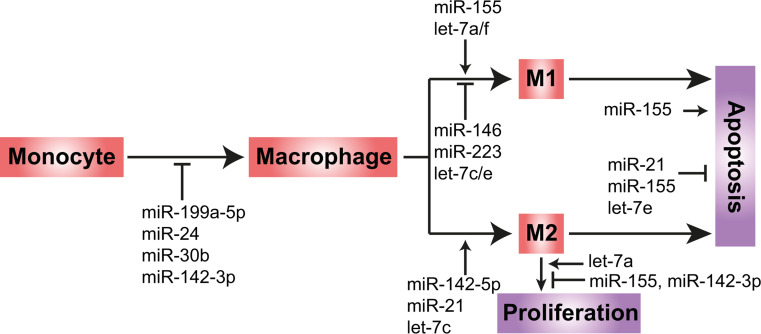
A large number of miRNAs are differentially regulated during the differentiation of monocytes into macrophages, for example, miR-24, -30b, -142-3p, and 199a-5p are down-regulated in this process [103, 104] (Fig. 2). Although miR-24, -30b and -142-3p have different seed sequences and are transcribed from different genes, all three miRNAs impair macrophage differentiation and activation. Overexpression of miR-24, miR-30b, or miR-142-3p in macrophages inhibits phagocytosis, reduces the expression of the mannose receptor (CD206) and inflammatory cytokines, such as IL-12 and TNF-α, and limits activation of PKC and NF-κB [103, 105, 106]. Similarly, PU.1-mediated down-regulation of miR-199a-5p promotes macrophage differentiation and proliferation by derepressing the type I serine/threonine kinase receptor of the transforming growth factor beta family activin A receptor type IB (ACVR1B) (also known as ALK4) and the CCAAT/enhancer binding protein alpha (C/EBP-α) expression [104, 107]. ACVR1B mediates signalling of the activin/inhibin/nodal pathway by heterodimerization with the type II receptors ACTRIIA/B and phosphorylation of SMAD2/3 proteins [107], and promotes activin A-induced pro-inflammatory macrophage polarization [108]. Although these miRNAs regulate monocyte differentiation in vitro, there is a lack of studies to demonstrate their roles in vivo.
Fig. 2.
Role of miRNAs in monocyte-to-macrophage differentiation and macrophage polarization. Differentiation of monocytes to macrophages is inhibited by miR-199a-5p, miR-24, miR-30b and miR-142-3p. M1 macrophage polarization requires miR-155 and let-7a/f, but is inhibited by miR-146, miR-223 and let-7c/e. Although miR-21 has been reported to play both pro- and anti-inflammatory roles, the latter role is more prominent. In addition, miR-142-5p and let-7c promote M2 macrophage polarization. In contrast to let-7a, miR-155 and miR-142-3p inhibit macrophage proliferation. miR-155 has both pro- and anti-apoptotic roles, whereas miR-21 and let-7e negatively regulate macrophage apoptosis
Expression and secretion of microRNAs in macrophage polarization
miRNAs are essential regulatory elements of macrophage polarization. Depletion of miRNAs by deletion of the Dicer gene leads to a marked reduction in the expression of pro-inflammatory genes, such as IL-6, TNF-α and IL-12, in macrophages stimulated with various TLR agonists [109]. Moreover, miRNA transcription is differentially regulated in M1 and M2 macrophages. MiR-155 and miR-147 are selectively up-regulated in M1 macrophages stimulated by LPS and IFN-γ [110–112], whereas the expression of miRNAs, like miR-125b-5p and miR-99a-5p, is increased in IL-4-treated macrophages [76]. Interestingly, IL-4 treatment also induces exosomal secretion of those miRNAs for which the number of mRNA targets decreased owing to transcription changes during alternative macrophage activation. For example, down-regulation of the miR-218-5p target Lyz2 in IL-4-stimulated macrophages causes enrichment of miR-218-5p in exosomes, whereas miR-16-5p, which does not target Lyz2, is not enriched in exosomes [113]. Thus, the exosomal secretion of miRNAs may represent a mechanism by which macrophages rapidly adapt their miRNA pool to changes of the transcriptome. Moreover, exosomal miRNAs secreted from macrophages may be transferred to other types of cells, like endothelial cells, and suppress mRNA targets in the recipient cell [113]. Conversely, anti-inflammatory miRNAs, such as miR-10a, are transferred from endothelial cells to monocytes through endothelial cell-derived extracellular vesicles, thereby repressing monocyte inflammatory activation [114]. Although secretion and uptake of miRNAs via microvesicles may contribute to macrophage polarization, their roles in the development of macrophage subtypes in vivo are currently incompletely understood.
The pro-inflammatory M1 microRNA: miR-155
A high miR-155 expression level is characteristic for M1 macrophages [110–112] (Fig. 3). LPS and mildly oxidized low-density lipoprotein (moxLDL) bind to TLR4 and increase miR-155 expression by activating the transcription factors E26 avian leukemia oncogene 2 (Ets2) and NF-κB, which bind to the promoter of miR-155 host gene BIC [115–118]. Moreover, IFN-γ up-regulates miR-155 by activating the JAK/STAT1 pathway in epithelial cells and tumour cells [119–121]. The different members of the Akt family have opposing effects on miR-155 expression. The M2-kinase Akt1 and the M1-kinase Akt2 down-regulates and up-regulates the expression of miR-155 in macrophages, respectively [40, 122]. Notably, miR-342-5p is a positive regulator of miR-155 expression level by suppressing the expression of Akt1 in M1 macrophages [102]. By contrast, the endogenous competitive RNA bone morphogenetic protein receptor type-2 (Bmpr2) prevents targeting of Akt1 by miR-342-5p in unstimulated macrophages. The transcriptional suppression of Bmpr2 during M1 polarization increases the availability of miR-342-5p and thereby leads to increased targeting of Akt1. Therefore, the competition between Bmpr2 and Akt1 for the binding to miR-342-5p regulates the expression of miR-155 (Fig. 3). Additionally, the YY1/histone deacetylase2/4 complex and the anti-inflammatory transcription factor Kruppel-like factor 2 transcriptionally repress miR-155 in oxLDL-treated macrophages [117, 123].
Fig. 3.
miR-155 and miR-342-5p form a functional pair to regulate macrophage activation. Bone morphogenetic protein receptor type-2 (Bmpr2) and thymoma viral proto-oncogene 1 (Akt1) compete for binding to miR-342-5p in resting macrophages. Transcriptional down-regulation of Bmpr2 in activated macrophages promotes the targeting of Akt1 by miR-342-5p and thereby up-regulates miR-155. Whereas basal miR-155 expression reduces macrophage proliferation through targeting of Csf1r, increased miR-155 levels in activated macrophages promote inflammatory gene expression and inhibit efferocytosis by targeting Bcl6
In vitro, Mir155 knockout and inhibition of miR-155 reduce the expression level of pro-inflammatory mediators, such as TNF-α, chemokine (C–C motif) ligand 2 (Ccl2) and IL-6 in macrophages treated with LPS or moxLDL and IFN-γ [110, 118, 122, 124]. Overexpression of miR-155 increases lipid uptake and ROS production in macrophages treated with oxLDL [117]. In addition, pro- and anti-apoptotic effects of miR-155 have been described. miR-155 inhibits oxLDL- and DNA damage-induced apoptosis of macrophage [125, 126]. By contrast, miR-155 is required for ESAT-6-induced apoptosis of macrophages [127]. However, miR-155 has no effect on the apoptosis of lesional macrophages in atherosclerosis and basal macrophage apoptosis [110]. These controversial effects of miR-155 on macrophage apoptosis might be related to distinct roles of miR-155 targets in the different apoptosis signalling pathways studied.
Several targets of miR-155 have been identified in macrophages, including suppressor of cytokine signaling 1 (SOCS1) and B cell leukemia/lymphoma 6 (Bcl6), which mediate the pro-inflammatory effects of miR-155 [110, 124]. Notably, disrupting the interaction between miR-155 and SOCS1 by mutating the miR-155 target site in the 3′-UTR of SOCS1 in mice impairs the immunological functions of T cells in vivo, suggesting a critical role of this single miRNA-mRNA interaction [128]. Additionally, miR-155 represses efferocytosis via targeting Bcl6, an inhibitor of the efferocytosis suppressor ras homolog gene family, member A [99] (Fig. 3). Several targets, including Fas-associated death domain-containing protein (FADD) and Trp53inp1 may mediate the anti-apoptotic role of miR-155, whereas SOCS1 mediates the pro-apoptotic role of miR-155 [125–127]. Targeting HMG-box transcription factor 1 contributes to the effect of miR-155 on foam cell formation after oxLDL treatment [117]. Furthermore, miR-155 inhibits macrophage proliferation by targeting Csf1r [99, 129].
miR-155 expression is increased in both murine and human lesions during the development of atherosclerosis, probably due to the existence of IFN-γ and modified LDL in the lesions which could activate the lesional macrophages [99, 110, 118]. miR-155 promotes CCL2 level by down-regulating Bcl6 in lesional macrophages, thereby enhancing the progression of atherosclerosis [110, 118]. By contrast, hematopoietic miR-155 inhibits the development of early atherosclerosis [130]. These controversial findings may be due to a stage-dependent effect of miR-155 during the progression of atherosclerosis, because increased miR-155 levels in macrophages from advanced lesions is associated with enhanced inflammation [99], suggesting a phenotypic switch from M2- to M1-like macrophages. In addition, miR-155 represses Csf1r expression, reduces lesional macrophage content and proliferation and decreases lesion size at the early stage of atherosclerosis [99]. However, in advanced lesions, the role of miR-155-regulated Csf1r expression in lesional macrophage accumulation is limited owing to the down-regulation of M-CSF. On the other hand, targeting of Bcl6 by miR-155 fosters atherosclerosis due to impaired macrophage efferocytosis and necrotic core formation at the late stage [99]. The stage-dependent role of miR-155 suggests that blocking the interaction between miR-155 and Bcl6 might be a potential therapeutic strategy for atherosclerosis.
The anti-inflammatory M2 microRNA: miR-223-3p
miR-223-3p is evolutionary conserved among various species and primarily expressed in hematopoietic cells [131]. Although its expression is down-regulated during monocyte-to-macrophage differentiation [132], miR-223-3p is still one of the most abundant miRNAs in macrophages [112, 133]. Moreover, miR-223-3p is highly enriched in microvesicles released from GM-CSF-treated human monocytes and vesicle-mediated transfer of miR-223-3p to recipient myeloid cells increases macrophage differentiation and survival [134]. In contrast to macrophage differentiation, miR-223-3p expression increases continuously during granulopoiesis and plays an essential role in the regulation of granulocyte differentiation and function by modestly suppressing hundreds of targets [135, 136]. Knockout of the Mir223 gene in mice increases the number of immature and hyperactive neutrophils in the circulation, while the proportion of monocytes in the peripheral blood and spleen is not altered [137]. Myeloid-specific transcription factors, such as PU.1 and C/EBP-α, induce the expression of miR-223-3p [133]. Accordingly, IL-4 stimulation induces miR-223-3p expression in murine macrophages in a PPARγ-dependent manner [137, 138], whereas LPS treatment down-regulates the expression level of miR-223-3p [137, 139].
The functional effects of miR-223-3p in macrophages are characterized by reduced expression of inflammatory cytokine, e.g. IL-1β, and of PPARγ in IL-4-treated macrophages [137]. In addition to the effect on Il1β mRNA expression, miR-223-3p limits IL-1β protein expression by targeting the inflammasome component Nlrp3 in macrophages [140, 141]. Overexpression of miR-223-3p can also reduce the expression of IL-6 and IL-1β in LPS-treated murine macrophages [137, 139]. Several targets of miR-223-3p have been identified in macrophages, including the Pbx/knotted 1 homeobox (Pknox1, also known as Prep-1), RAS p21 protein activator (GTPase activating protein) 1 (RASA1), nuclear factor of activated T cells 5 (NFAT5), STAT3, and IKKα, which might mediate the anti-inflammatory effects of miR-223-3p [132, 137, 139]. In murine macrophages, miR-223-3p-mediated targeting of NFAT5 and RASA1 results in reduced LPS-induced M1 polarization and enhanced IL-4-mediated M2 polarization [138], and targeting of STAT3 has been implicated in the regulation of IL-6 and IL-1β by miR-223-3p [139]. Pknox1 mediates the up-regulation of the anti-inflammatory cytokine IL-10 in macrophages that engulf apoptotic cells [68]; however, Pknox1 also increases the expression of IL-1β in LPS-treated (but not IL-4-treated) macrophages [137]. Thus, targeting of Pknox1 may explain the effects of miR-223-3p on IL-1β production in inflammatory macrophages. In contrast to the Pknox1 and RASA1 binding sites, the validated murine Nfat5 and Stat3 binding sites for miR-223-3p are not conserved in human [139].
The anti-inflammatory effects of miR-223-3p on macrophages may play a role in the pathogenesis of tuberculosis, because monocytes and monocyte-derived macrophages from patients with tuberculosis express higher levels of miR-223-3p and are less inflammatory than those from healthy subjects [142]. In mice, knockout of the Mir223 gene in bone marrow cells increases adipose tissue inflammation and insulin resistance, presumably owing to the shift of the adipose tissue macrophage phenotype towards an inflammatory M1 subtype [137]. Moreover, activation of the Csf1r/mitogen-activated protein kinase 1 (Erk)/Ets signaling pathway increases the expression of miR-223-3p in oncogenic M2-type tumor-infiltrating myeloid cells (TIMs) [143]. Conditional knockout of Dicer in myeloid cells, which leads to down-regulation of miR-223-3p in TIMs, reduces metastatic tumor burden, suggesting that the anti-inflammatory effects of miR-223-3p on macrophages may promote cancer [143].
The anti-inflammatory M1 microRNA: miR-146
The miR-146 family includes two members, miR-146a and miR-146b, which are encoded by genes located on different chromosomes. Although the sequences differ by two nucleotides, the seed sequences of miR-146a and miR-146b are identical, indicating that these two miRNAs target the same genes. LPS-induced TLR activation and stimulation with pro-inflammatory cytokines, like IL-1β and TNF-α, up-regulate the expression of miR-146a via pro-inflammatory NF-κB signaling, whereas Akt2 represses miR-146a expression in macrophages [111, 144, 145]. Moreover, apolipoprotein E (APOE) promotes miR-146a expression by up-regulating the transcription factor PU.1 [146]; however, the mechanism by which APOE induces PU.1 expression is unclear. In a negative feedback loop, the up-regulation of miR-146a results in endotoxin tolerance and limits IL-1β-induced inflammatory activation by repressing TNF receptor-associated factor 6 (TRAF6) and IL-1 receptor-associated kinase 1 (IRAK1) [144, 147]. Moreover, the pro-inflammatory effect of AKT2 is mediated by the downregulation of miR-146a and consequently the derepression of TRAF6 and interferon regulatory factor 5 (IRF5) [145]. In addition, miR-146a-mediated suppression of NOTCH1 decreases IL-6 production in macrophages by inhibiting LPS-induced NOTCH signalling [148–150]. These studies indicate that miR-146a plays an anti-inflammatory role by regulating a negative feedback loop comprising the TLR4-NF-κB pathway in vitro (Fig. 2).
In mice, knockout of the Mir146a gene results in hyper-responsivity to LPS owing to increased production of TNF-α, IL-6 and IL-1β in bone marrow-derived macrophages [151]. Moreover, the effect of miR-146 treatment has been studied in several mouse models of inflammatory diseases. In hyperlipidemic mice, treatment with miR-146a mimics reduces Ly-6Chigh monocytosis, the lesional macrophage number, the macrophage inflammatory response, and atherosclerosis [146]. Systemic delivery of miR-146a encapsulated in polyethylenimine nanoparticles inhibits renal fibrosis and macrophage infiltration in the kidney by inhibiting pro-fibrotic and inflammatory signaling pathways following unilateral ureteral obstruction [152]. The administration of miR-146a and miR-146b mimics prevents liver graft injury probably by reducing NF-κB-mediated inflammation through targeting TRAF6 and IRAK1 in Kupffer cells [153]. Taken together, delivery of miR-146 mimics may be a therapeutic approach to limit inflammation by negatively regulating TLR4-NF-κB signaling in macrophages.
The anti-apoptotic and anti-inflammatory M1- and M2 microRNA: miR-21
The human pri-miR-21 is located immediately downstream of the vacuole membrane protein-1 gene [also known as transmembrane protein 19 (TMEM49)] and transcriptionally regulated by its own promoter in the 10th intron of the TMEM49 gene [154]. Processing of the pre-miR-21 results in two mature miRNA, miR-21-5p (miR-21) and miR-21-3p (miR-21*). While the human miR-21 sequence is evolutionary highly conserved between species, miR-21* is mainly expressed in humans and rodents. In contrast to most miRNAs, miR-21-5p is overexpressed in several cancer cell types [155, 156] and promotes cancer development mainly by inhibiting apoptosis but also by increasing proliferation [157–160]. LPS treatment induces miR-21 expression in murine macrophages mediated by the TLR adaptor protein MyD88 and downstream activation of NF-κB signalling [161]. In M2-macrophages, stimulation with IL-10 and CSF1R-induced PI3K and ERK1/2 signalling up-regulate miR-21 expression [112, 162]. Moreover, engulfment of apoptotic cells increases miR-21 expression in macrophages [163].
Overexpression of miR-21 promotes IL-10 and represses IL-1β expression in macrophages [161–163], indicating an anti-inflammatory role of miR-21. In line with its anti-apoptotic effects, miR-21 inhibits glucose-induced macrophage apoptosis by blocking caspase3 activation [164]. The anti-apoptotic and anti-inflammatory effects of miR-21 (Fig. 2) are mediated by suppression of programmed cell death 4 (PDCD4) or phosphatase and tensin homolog (PTEN) [161, 162, 164–168]. Whereas PDCD4 promotes apoptosis via inhibiting p53 transcription or the translation of anti-apoptotic proteins, PTEN induces apoptosis by blocking the pro-survival PI3K/Akt pathway [169, 170]. In addition, PDCD4 is required for LPS-induced NF-κB activation and IL-6 production, whereas PTEN can promote both pro- and anti-inflammatory macrophage activation maybe due to the diverse contribution of the Akt isoforms to macrophage polarization [40, 161, 162, 171, 172]. Although both miR-21 and miR-21* are up-regulated in human atherosclerotic plaques [173, 174] and the circulating miR-21 levels are reduced in patients with unstable plaques [173], the role of the differential regulation of miR-21 in macrophages is currently incompletely understood in vivo.
The chameleon-like let-7 microRNA family: c and e versus a and f
In humans, the let-7 miRNA family contains 13 members (let-7a-1/-2/-3, let-7b, let-7c, let-7d, let-7e, let-7f-1/-2, let-7g, let-7i, and miR-98) that share the same seed sequence. The let-7 miRNAs are expressed from nine different chromosomes and individual let-7 family members, such as let-7a and let-7f, are encoded in multiple genomic loci [175]. Human let-7a is expressed from three paralogs into the precursor miRNAs let-7a-1–let-7d (which includes let-7a-1, let-7f-1, and let-7d), let-7a-2, and let-7a-3/let-7b [176, 177]. Let-7 miRNAs are abundantly expressed in various cell types, including macrophages. LPS stimulation regulates the expression of the various let-7 miRNAs differently. In murine macrophages, LPS treatment down-regulates the expression of let-7a and let-7c (through polycomb repressor complex 2-mediated histone trimethylation), and up-regulates let-7e expression by activating Akt1 [122, 178–180]. By contrast, in human macrophages, LPS increases the expression of let-7a and decreases the expression of let-7f [181, 182]. Moreover, infection of macrophages with Salmonella typhimurium and Mycobacterium tuberculosis down-regulates the expression of several let-7 family members, such as let-7a and let-7f, presumably mediated by LPS and mycobacterium-derived ESAT-6, respectively [179, 183]. These in vitro results demonstrate that the regulation of the individual let-7 family members varies in macrophages and also differs between humans and mice.
The effect of let-7 family members on the macrophage activation is not uniform. Interestingly, the 3′-UTRs of the pro-inflammatory IL-6 and the anti-inflammatory IL-10, which both contain only few conserved miRNA binding sites, are both targeted by let-7a and let-7d [179]. Accordingly, Salmonella infection post-transcriptionally increases the expression of these cytokines in macrophages presumably by down-regulating let-7 family members. In LPS-treated murine macrophages, the up-regulation of let-7e suppresses not only the expression of IL-6, but also the expression of several other pro-inflammatory cytokines, such as TNF-α, CCL2, and CCL3, clearly demonstrating an anti-inflammatory role of let-7e [122]. This more general anti-inflammatory effect of let-7e may be mediated by the suppression of TLR4 through interaction of let-7e with a non-canonical binding site in the murine TLR4 3′-UTR containing three G:U wobbles in the seed match [122]. Although let-7i can also target human TLR4 through a canonical binding site that is not conserved in mice, it is unclear whether the interaction of let-7i with TLR4 plays a role in the inflammatory activation of macrophages [184]. Notably, let-7e can also target caspase 3 and may thus increase the survival of inflammatory macrophages [185]. The anti-inflammatory effect of let-7 family members like let-7c may be mediated by the targeting of the transcription factor C/EBP-δ, which contains a highly conserved binding site in its 3′-UTR sequence [178]. NF-κB activation in LPS-treated macrophages up-regulates C/EBP-δ transcription, which in turn amplifies NF-κB-induced IL-6 and TLR4 expression [186, 187]. Moreover, compared to other let-7 family members, let-7c greatly suppresses the expression of the p21-activated kinase 1, a serine/threonine kinase that is essential for LPS-induced NF-κB activation [180]. Accordingly, down-regulation of let-7c in LPS-treated macrophages mediates the increased expression of several inflammatory cytokines, such as TNF-α, IL-6, IL-1β, IL-12, and of iNOs [178, 180]. Conversely, high expression levels of let-7c in M2-type macrophages promote the IL-4-induced up-regulation of M2 markers and contribute to the high efferocytosis capability of M2 macrophages by increasing the expression of CD36, indicating that let-7c plays an important role in alternative macrophage polarization [178].
In contrast to the anti-inflammatory role of some let-7 family members, let-7f induces the expression of inflammatory cytokines, such as IL-1β, TNF-α, chemokine (C-X-C motif) ligand 1, and CCL2, and of iNOs in macrophages infected with M. tuberculosis [183]. Thus, suppression of let-7f expression in infected macrophages enhances the survival of M. tuberculosis and may promote mycobacterial disease [183]. Let-7f increases the inflammatory activation of macrophages by increased NF-κB activation owing to the targeting of tumor necrosis factor alpha-induced protein 3 (also known as A20), which negatively regulates TLR signaling by deubiquitinylation of TRAF6 [183, 188]. In addition, another negative regulator of NF-κB activation, NFKB inhibitor interacting Ras-like protein 2 (NKIRAS2, also known as κB-Ras2), contains a highly conserved binding site for let-7 miRNAs in its 3′-UTR. Let-7a can target NKIRAS2 in human macrophages and may thus generate a positive feed-forward loop in which NF-κB-mediated up-regulation of let-7a promotes NF-κB activation by suppressing NKIRAS2 [182, 189]. Additionally, let-7a enhances the proliferation of macrophages stimulated with LPS/IFN-γ by inducing S phase entry, which is mediated by increased expression of cell cycle activator E2F transcription factor 2 (E2F2) and reduced expression of cell cycle inhibitor E2F5 [189]. Taken together, although let-7 family members harbor the same seed sequence, they have specific individual function in macrophages (Fig. 2), indicating that non-seed sequences may be crucial for specific target recognition of miRNA family members.
miR-142-3p and miR-142-5p: a miRNA pair in macrophages
Like miR-155-5p and miR-223-3p, miR-142-3p expression driven by the transcription factor PU.1 is highly specific for hematopoietic cells [190, 191]. However, miR-155-5p can suppress miR-142-3p by targeting PU.1 [190]. In mice, the Mir142 gene is embedded in the first intron of a long non-coding RNA with unknown function [192]. Mir142 knockout mice are viable and do not show any gross abnormalities except an enlarged spleen due to an increase in B cells and myeloid cells, and a combined immunodeficiency [193]. In macrophages, miR-142-3p targets the transcription factor early growth response 2 (Egr2), which shifts the effect of PU.1 during myelopoiesis towards the macrophage cell fate [194, 195]. Conversely, M-CSF-induced Egr2 promotes macrophage proliferation by negatively regulating miR-142-3p expression [194]. Moreover, miR-142-3p mediates the anti-proliferative effect of miR-223-3p on hematopoietic cells, because miR-223-3p up-regulates miR-142-3p expression through targeting of C/EBP-β and LIM domain only 2 [196]. In addition, miR-142-3p limits IL-6 signalling by suppressing Il6 mRNA and the IL-6 receptor protein IL6st in dendritic cells and macrophages, respectively [197, 198]. In mice, the mutual inhibition of miR-142-3p and C/EBP-β isoform LAP* expression may further amplify IL-6 signalling due to derepression of IL6st [197]. Whereas miR-142-3p targets Il6st through a canonical binding site in its 3′-UTR, isoform-specific suppression of C/EBP-β is mediated via a noncanonical site in the coding sequence of LAP* [197, 199]. Notably, miR-142-3p is up-regulated in tumor infiltrating macrophages through the CSF1-ETS2 pathway and overexpression of miR-142-3p in myeloid cells delays tumor growth following immunotherapy with tumor-specific T lymphocytes [143, 197]. This antitumor activity of miR-142-3p has been attributed to reduced IL-6-signaling in tumor macrophages, because increased IL-6 signalling induces the expression of M-CSFR and the IL-4 receptor and may, thus, skew the macrophage phenotype towards an M2 phenotype [197, 200, 201]. However, miR-142-3p also suppresses the pro-inflammatory cytokines TNF-α and IL-12 in inflammatory and alternatively activated macrophages, indicating a more complex role of miR-142-3p in macrophage polarization [105]. Moreover, miR-142-3p limits the phagocytic capability of macrophages by suppressing the Wiskott-Aldrich syndrome-like (Wasl) mRNA encoding for a protein that is a crucial regulator of Rho GTPases and thereby regulates cytoskeletal reorganization [106, 193, 202]. Accordingly, up-regulation of miR-142-3p in macrophages infected with mycobacteria reduces the phagocytic clearance of M. tuberculosis, indicating that regulation of this miRNA is a microbial strategy to prevent phagocytic clearance by macrophages [106].
In contrast to most miRNA genes, which give rise only to one mature miRNA strand, the processing of the primary miR-142 transcript results in the expression of miR-142-5p in addition to miR-142-3p in macrophages. Similar to the cooperative function of the two mature miR-126 strands in endothelial cells, miR-142-5p also plays an essential role in macrophages [203, 204]. IL-4 and IL-13 treatment induce miR-142-5p expression in macrophages, which in turn promotes the expression of M2 markers, such as CCL18, CCL17, and TGF-β1, and enhances the profibrogenic activity of macrophages by targeting SOCS1, a negative regulator of STAT6 phosphorylation [203].
miRNAs control macrophage cholesterol homeostasis
The cellular cholesterol content plays an essential role in macrophages by regulating PRRs and phagocytosis, and is regulated by the opposing actions of the transcription factors liver X receptor (LXR) and SREBP-2 [205]. LXR is activated by oxysterols, which are synthesized when cells accumulate excess cholesterol, in a negative feedback loop to reduce cellular cholesterol levels (e.g., by increasing cholesterol efflux), whereas low cholesterol levels activate SREBP-2 and thereby up-regulate genes that increase cholesterol uptake (e.g., LDL receptor) and synthesis (e.g., HMG-CoA reductase) [206]. Cholesterol efflux through ATP binding cassette (ABC) subfamily A member 1 (ABCA1) and subfamily G member 1 (ABCG1) plays a crucial role in the regulation of the cellular cholesterol content, because macrophages like many other cells cannot degrade cholesterol. During the phagocytosis of apoptotic cells, macrophages permanently face the challenge to tackle an enormous cholesterol load and to have enough cholesterol available to manage the massive membrane turnover [207]. Notably, the uptake of apoptotic cells greatly increases cholesterol efflux to the lipoprotein ApoA1 by LXR-mediated up-regulation of ABCA1 and thereby maintains their phagocytosis capacity and prevents apoptosis [208–211].
Free cholesterol complexed with sphingolipids or phospholipids is mainly localized in the plasma membrane and forms highly dynamic membrane microdomains, called lipid rafts, which provide a platform for the aggregation of surface receptors and intracellular signalling molecules, like CD14, TLR4, and Myd88 following LPS stimulation [212–216]. Moreover, plasma membrane cholesterol promotes pro-inflammatory CD40 signalling and plays an essential role in the phagocytosis of pathogens [217–219]. Conversely, TLR4-mediated inflammatory activation of macrophages increases intracellular cholesterol levels by reducing cholesterol efflux through ABCA1 and ABCG1, which may in turn enhance TLR signalling by increasing the cholesterol content in lipid rafts [220–224]. However, increased cellular free cholesterol levels in the ER membrane can induce apoptosis through activation of the unfolded protein response [225]. To tightly adjust the potentially cytotoxic increased free cholesterol level, inflammatory macrophages esterify free cholesterol in the ER membrane via acetyl-CoA acetyltransferase 1 (ACAT1) to cholesteryl esters (CE), which are stored together with triglycerides in lipid droplets [221, 226, 227]. Excessive storage of CEs in lipid droplet may completely fill the cytoplasm of macrophages, which leads to the histologic picture of a foam cell. Foam cell formation suppresses inflammatory genes, up-regulates LXR target genes, and down-regulates SREBP2 target genes [228]. However, lipid droplet formation in macrophages can have pro-inflammatory effects, such as increased IL-1β secretion by activating the NLRP3 inflammasome [229]. The essential role of cellular cholesterol homeostasis in macrophage function dictates the handling of oxidatively modified LDL, which shares binding motifs with some pathogen-associated molecular patterns and is therefore recognized by TLR4 and macrophage scavenger receptors, such as CD36, but no longer by LDL receptors [230]. Oxidation of LDL occurs in the subendothelial space at arterial bifurcations, where the unrestricted uptake of oxLDL by macrophages leads to foam cell formation and atherosclerosis [204]. Taken together, intracellular cholesterol homeostasis is closely linked to macrophage functions, such as inflammatory activation and phagocytosis, through ABC transporters [231].
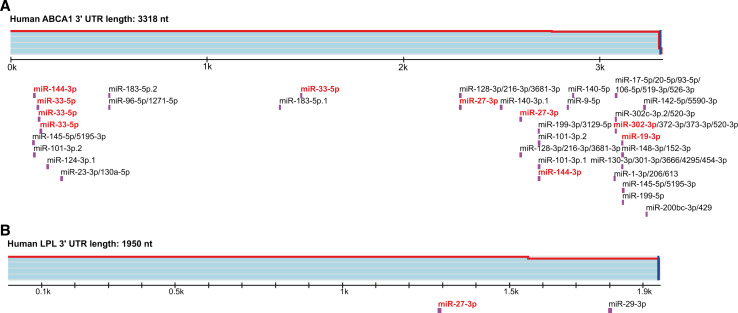
Intriguingly, numerous conserved miRNA binding sites (e.g., for miR-33-5p, miR-27-3p, miR-145, miR-19-3p, and miR-144-3p) were confirmed in the 3′-UTR of the ABCA1 mRNA (Fig. 4), suggesting that regulation of the cholesterol efflux by miRNAs has been an evolutionary advantage [232–236]. The role of miRNAs in cholesterol metabolism has been recently reviewed [237]. Here, we will focus on how miRNAs link cellular cholesterol metabolism with the innate immune response (Fig. 5).
Fig. 4.
The ABCA1 mRNA is a preferred miRNA target. Distribution of highly conserved miRNA binding sites in the 3′-UTR of human ABCA1 (a) and LPL (b) predicted by Targetscan (http://www.targetscan.org/). The miRNA target sites labeled in red have been confirmed experimentally in macrophages (see text for details)
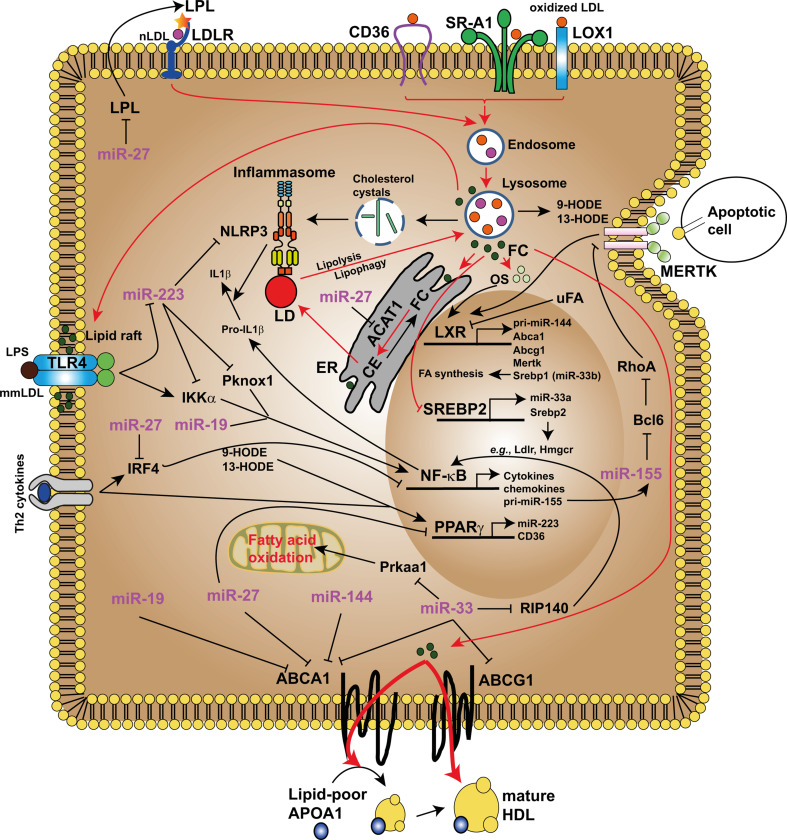
Fig. 5.
miRNAs connect lipid metabolism and macrophage functions. LDL is taken up by macrophages via LDL receptor (LDLR) and lipoprotein lipase (LPL) enhances the uptake of LDL. However, modified LDL (e.g., oxidized LDL) cannot be recognized by LDLR, but by scavenger receptors (CD36, SR-A1 and LOX1). Lipoproteins engulfed by macrophages are transported to endosomes and then to lysosomes, where they are hydrolysed to generate free cholesterol (FC). In addition, 9- and 13-hydroxyoctadecadienoic acid (HODE) are formed upon peroxidation of the most abundant fatty acid in LDL, linoleic acid. 9- and 13-HODE activate PPARγ, which upregulates the expression of CD36. Free cholesterol is trafficked to the endoplasmic reticulum (ER) where excess cholesterol is re-esterificated to cholesteryl fatty acid esters (CE) by acyl-CoA acyltransferase-1 (ACAT1) and stored in cytoplasmic lipid droplet (LD). CEs in the lipid droplet can be transported back to lysosomes either via lipolysis or an autophagic process termed ‘lipophagy’. Incorporation of FC into lipid rafts in the plasma membrane increases the activation of toll-like receptor 4 (TLR4) following stimulation with LPS or mildly modified LDL (mmLDL). In addition to the uptake of apoptotic cells, excess cholesterol metabolized into oxysterols (OS) activate LXR and thus induce the transcription of ABCA1 and ABCG1, which enhances the efflux of cholesterol efflux via apolipoprotein A1 (APOA1) and HDL particles. Moreover, cholesterol loading inhibits the uptake and synthesis of cholesterol by inhibiting SREBP2 activation. Excessive cholesterol uptake by scavenger receptors may lead to the formation of cholesterol crystals that activate the NLRP3 inflammasome and promote IL-1β production. The roles of miRNAs in cellular lipid homeostasis and macrophage functions are described in the text
The role of the highly conserved miRNA miR-33 in macrophage cholesterol metabolism has been extensively studied. The MIR33A gene (MIR33-P1) encoding miR-33a-5p has been acquired in the bilaterian ancestor of protostomes and deuterostomes, and a paralog of this gene MIR33B (MIR33-P2) occurred in vertebrates encoding miR-33b-5p, which differs in two nucleotides outside the seed sequence from miR-33a-5p [238]. However, several vertebrate species, including mice, have lost the MIR33-P2 gene and retained only MIR33-P1, whereas the MIR33 gene family is completely absent in the zebrafish genome. The MIR33-P1 gene is located in intron 16 of the human, mouse, cow and chicken SREBP2 gene, which is a key transcription factor in cholesterol metabolism by inducing expression of the LDL receptor and cholesterol biosynthesis genes [239]. The expression of the MIR33-P1 gene is co-regulated with its host gene by the cellular cholesterol content in macrophages [239, 240]. miR-33a-5p targets the 3′-UTR of several genes involved in cholesterol homeostasis including ABCA1 and thereby limits cholesterol efflux from macrophages to ApoA1 and increases macrophage apoptosis induced by free cholesterol loading [235, 240, 241]. Moreover, miR-33 targets PPARγ coactivator-1α in macrophages, which enhances mitochondrial ATP production required for the ATP-dependent cholesterol efflux via ABCA1 [242]. Thus, up-regulation of miR-33a-5p and SREBP-2 by cholesterol depletion act synergistically to increase the macrophage cholesterol content [235, 240].
The MIR33-P2 gene is located in the intron of the SREBP1 gene and both genes are up-regulated through activation of LXR [243]. The SREBP1 gene expression can give rise to two transcription factors SREBP1a and SREBP1c, which primarily promote fatty acid synthesis. Unsaturated fatty acids inhibit LXR activation and thereby reduce SREBP1c expression and fatty acid synthesis [244, 245]. miR-33b is co-expressed with its host gene, but suppresses SREBP1 mRNA by targeting its 3′-UTR and thereby counter-regulates the effect of SREBP1c on fatty acid metabolism [246]. Knockin of the MIR33-P2 gene into the murine SRBF1 locus decreased the expression of ABCA1 and ABCG1 (only a target of miR-33 in mice) in macrophages and the cholesterol efflux to ApoA1 and HDL, indicating that expression of the MIR33 paralog enhances the effect of miR-33a on cholesterol efflux in humans [243].
miR-33 is up-regulated by LPS stimulation in M1 macrophages and promotes a pro-inflammatory phenotype [247, 248]; however, this effect of miR-33 is independent of ABCA1, but mediated through targeting of the protein kinase, AMP-activated, alpha 1 catalytic subunit (AMPK), which codes for a kinase that promotes ATP production via fatty acid oxidation [248]. In addition, AMPK mediates the effect of miR-33 on the expression of aldehyde dehydrogenase family 1, subfamily A2 (Aldh1a2) in macrophages by regulating the energy metabolism. Aldh1a2 is up-regulated in M2 macrophages and produces athero-protective retinoic acid. The expression of miR-33 in M1 macrophages has been linked to decreased secretion of retinoic acid, which promotes the generation of regulatory T cells [248]. Although these data would indicate that miR-33 expression in macrophages reduces atherosclerosis, the current evidence for this conclusion is contradictory. Several studies in animal models of atherosclerosis have demonstrated that systemic inhibition of miR-33 reduces lesion formation. However, it is difficult to attribute these effects clearly to the role of miR-33 in macrophages, because miR-33 affects many other tissues, such as liver and pancreas. For instance, Mir33 knockout mice develop steatohepatitis, insulin resistance, and an increased body weight on a high fat diet [246]. Deletion of the miR-33 gene only in hematopoietic cells, however, does not substantially decrease atherosclerosis [241].
By contrast, feeding mice with a high fat diet down-regulates miR-33 in peritoneal macrophages and de-represses receptor-interacting protein-140 (RIP140), a coactivator of NF-κB, which increases inflammatory cytokine expression and may thereby contribute to the increased survival rate of mice fed a high fat diet in a sepsis model [249]. An anti-inflammatory role of miR-33 has also been reported in aged macrophages. Aging increases the expression of miR-33 in murine macrophages, which results in senescence-associated suppression of ABCA1 expression and cholesterol efflux [250]. Thus, macrophages from aged mice contain more cholesterol and accumulate more lipid droplets than those from younger mice [250]. Surprisingly, the aging-induced disturbance in cellular cholesterol homeostasis is associated with an anti-inflammatory M2 phenotype, characterized by increased IL-10 production. This M2 phenotype of aged macrophages also promotes endothelial cell proliferation and contributes to pathological angiogenesis related to age-related macular degeneration [250]. Moreover, inhibition of miR-33 up-regulated the expression of inflammatory genes like TNF-α and IL-1β in peritoneal macrophages from young and aged mice, indicating an anti-inflammatory role of this miRNA [250].
In addition to miR-33, miRNAs from the miR-19-3p member are predicted by Targetscan to bind the 3′-UTR of the ABCA1 mRNA via a highly conserved binding site (Fig. 4). The miR-19-3p family consists of three members transcribed from two different genetic loci. miR-19a-3p and miR-19b-3p, which differ in one nucleotide at position 11 of the miRNA, are embedded in the polycistronic primary pri-miR-17~92 transcript that contains six mature miRNAs [251]. The miR-19b hairpin is also transcribed within the pri-miR-106~363 cluster. Lv et al. confirmed the binding site for miR-19-3p in the ABCA1 3′-UTR using miR-19b-3p mimics and demonstrated that miR-19b-3p decreases cholesterol efflux in human macrophages and reverse cholesterol transport pathway in mice [236]. Moreover, miR-19-3p family members target several genes, such as A20 and its cofactor ring finger protein 11, and lysine-specific demethylase 2A (also known as FBXL11), which negatively regulate NF-κB activation and thereby inhibit inflammatory macrophage activation [109]. Although both miR-19a-3p and miR-19b-3p promote NF-κB activation, miR-19a-3p is more effective than its sister strand miR-19b-3p [109]. As expected from the effect of miR-19-3p on cholesterol efflux and NF-κB activation, systemic inhibition of miR-19b-3p reduced, whereas treatment with miR-19b-3p mimics enhanced atherosclerosis in mice, indicating that the effect of miR-19-3p on macrophages is pro-atherogenic [236]. However, HIF-1α up-regulates miR-19a-3p in atherosclerotic endothelial cells, which increases endothelial inflammation and atherosclerosis [252]. Therefore, further studies are needed to determine the role of the macrophage miR-19-3p family members in disease.
Another miRNA family that plays a central role in macrophage cholesterol metabolism is miR-27-3p. miR-27a-3p is transcribed together with miR-23 and miR-24 from the miR-23~27~24 gene cluster and miR-27b-3p (which differs from miR-27a-3p in one nucleotide at position 18) is included in a gene cluster with miR-23b. The ABCA1 3′-UTR contains two highly conserved binding sites for miR-27 family members (Fig. 4); however, only one binding site is targeted by miR-27a-3p and miR-27b-3p [232]. Akin to miR-33, miR-27a/b-3p decreases ABCA1 expression and cholesterol efflux in human macrophage cell line [232]. However, miR-27a/b-3p also limits the uptake of cholesterol via oxLDL partly by targeting lipoprotein lipase (LPL), which enhances the lipid uptake through a nonenzymatic bridging mechanism [232]. In contrast to the ABCA1 3′-UTR, only two conserved binding sites are predicted in the 1950-nt long LPL 3′-UTR by Targetscan including miR-27-3p and miR-29-3p (Fig. 4), indicating a less redundant control of the LPL expression by miRNAs. In addition, miR-27a/b-3p targets PPARγ in macrophages, which may contribute to the reduced oxLDL uptake by down-regulating CD36 expression [232, 253]. Moreover, miR-27a/b-3p targets human ACAT1 mRNA through a non-canonical seed match including a G:U wobble at position 1 of the miRNA, which results in reduced formation of cholesteryl ester [232]. In summary, different from miR-33, miR-27a/b-3p promotes a general inhibition of cellular cholesterol metabolism and a shift from cholesteryl esters to free cholesterol (by suppressing multiple targets) rather than a total increase of the cellular cholesterol content [232] (Fig. 5). Up- and down-regulation of miR-27a/b-3p expression by LPS treatment has been reported in human macrophages [111, 253], whereas IL-4 increases and LPS reduces miR-27-3p expression in mouse macrophages [171, 254, 255]. In addition to the targeting of PPARγ, miR-27a-3p may enhance inflammatory cytokine expression in LPS-treated macrophages by targeting the 3′-UTR of IL-10, which inhibits the inflammatory activation in an autocrine manner, and of interferon regulatory factor 4, a negative regulator of TLR signalling [254, 255]. In addition, miR-27b-3p may increase inflammatory macrophage activation by targeting the RNA-binding protein ZFP36 (also known as tristetraprolin), which limits the expression of various inflammatory genes by mediating their rapid decay through binding to AU-rich element motifs in their 3′-UTR [171, 256]. Thus, miR-27-3p may limit M2 polarization and decrease tumor growth promoted by the M2 phenotype of tumor-associated macrophages [255].
Activation of LXR in macrophages up-regulates the expression of ABCA1 and ABCG1 in response to cholesterol load and increases cholesterol efflux. Moreover, LXR also regulates the expression of miRNAs in macrophages, which are predicted to target lipid-related genes [234]. Notably, one of the miRNAs up-regulated by LXR activation is miR-144-3p, which targets ABCA1 at least via two highly conserved binding sites and reduces cholesterol efflux synergistically with miR-33 (Fig. 5), indicating that LXR activation induces a negative feedback regulation of ABCA1 through miR-144-3p [234, 257]. In addition, miR-144-3p increases inflammatory cytokine secretion in human macrophage foam cells. Systemic treatment with miR-144-3p increased the formation of atherosclerotic lesions in mice; however, it is unclear whether this effect is mediated by the role of miR-144-3p in macrophages, because HDL cholesterol levels and the reverse cholesterol transport were reduced most likely owing to the downregulation of ABCA1 in the liver [257].
Foam cell formation results in the differential regulation of several miRNAs in murine macrophages, which are predicted to target ABCA1, such as miR-302a-3p, miR-106b, and miR-216a [258]. Like miR-106b, cholesterol loading by oxLDL down-regulates the expression of miR-302a-3p in macrophages. The miR-302a hairpin is embedded in a polycistronic primary transcript containing also the miR-367, the miR-302d, the miR-302c, and the miR-302b hairpin, which is transcribed from an intron of its host gene La ribonucleoprotein domain family member 7 as an independent transcriptional unit [259]. All miR-302-3p family members have the same seed sequence and differ only in one nucleotide at position 18 or 19 of the miRNAs. miR-302a targets ABCA1 mRNA and limits cholesterol efflux to ApoA1 in macrophages. Moreover, inhibition of miR-302a reduces atherosclerosis and increases HDL cholesterol levels in mice, probably owing to increased ABCA1 expression in the liver [258].
Conclusions
miRNAs play crucial roles in many aspects of macrophages and thereby affect many pathological conditions, like infection, tumor growth, atherosclerosis, and macular degeneration by connecting inflammatory activation, cholesterol homeostasis, cell survival and proliferation, and phagocytosis. Targeting miRNAs through the application of modified oligonucleotides may become an effective therapeutic strategy for immune diseases [260, 261]. However, the function of miRNAs is highly context and cell type dependent, and can change during disease progression, like macrophage miR-155 in atherosclerosis, owing to changes in miRNA and target mRNA expression levels. Unfortunately, the miRNA-regulated context-specific network is still poorly understood, which hampers the drug development. Moreover, developing carriers to deliver miRNAs in a cell-type specific manner would greatly increase potential applications of miRNA-based therapies.
Acknowledgments
A.S. acknowledges support from the Deutsche Forschungsgemeinschaft (SFB 1123-B4), the German Center for Cardiovascular Research, and the German Federal Ministry of Education and Research (Grant number 01KU1213A). A.S. and Y.W. acknowledge support from Else Kröner-Fresenius-Stiftung (Grant number 2014_A219).
Abbreviations
- M-CSF
Macrophage colony stimulating factor
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- CSF1R
Macrophage colony stimulating factor receptor
- PU.1
Purine-rich PU-box-binding protein 1
- SREBP
Sterol regulatory element binding protein
- PRRs
Pattern-recognition receptors
- TLRs
Toll-like receptors
- IFN-γ
Interferon gamma
- IL
Interleukin
- TNF
Tumor necrosis factor
- ROS
Reactive oxygen species
- NF-κB
Nuclear factor-κB
- LPS
Lipopolysaccharide
- PPARγ
Peroxisome proliferator-activated receptor gamma
- JAK
Janus kinases
- STAT1
Signal transducers and activators of transcription 1
- PI3K
Phosphatidylinositol 3-kinase
- Akt
Thymoma viral proto-oncogene
- HIF-1
Hypoxia-inducible factor-1
- OXPHOS
Oxidative phosphorylation
- miRNAs
MicroRNAs
- UTR
Untranslated region
- C/EBP-α
CCAAT/enhancer binding protein alpha
- ACVR1B
Activin A receptor type IB
- Ets E26
Avian leukemia oncogene
- Bmpr2
Bone morphogenetic protein receptor type-2
- OxLDL
Oxidized low-density lipoprotein
- SOCS1
Suppressor of cytokine signaling 1
- Bcl6
B cell leukemia/lymphoma 6
- FADD
Fas-associated death domain-containing protein
- Ccl2
Chemokine (C–C motif) ligand 2
- Erk
Mitogen-activated protein kinase 1
- APOE
Apolipoprotein E
- TRAF6
TNF receptor-associated factor 6
- IRAK1
IL-1 receptor-associated kinase 1
- IRF5
Interferon regulatory factor 5
- TMEM49
Transmembrane protein 19
- PDCD4
Programmed cell death 4
- PTEN
Phosphatase and tensin homolog
- Egr2
Early growth response 2
- LXR
Liver X receptor
- ABCA1
ATP binding cassette subfamily A member 1
- ABCG1
ATP binding cassette subfamily G member 1
- ACAT1
Acetyl-CoA acetyltransferase 1
- CE
Cholesteryl ester
References
- 1.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefater JA, Ren S, Lang RA, Duffield JS. Metchnikoff’s policemen: macrophages in development, homeostasis and regeneration. Trends Mol Med. 2011;17:743–752. doi: 10.1016/j.molmed.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 8.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 9.Liddiard K, Rosas M, Davies LC, Jones SA, Taylor PR. Macrophage heterogeneity and acute inflammation. Eur J Immunol. 2011;41:2503–2508. doi: 10.1002/eji.201141743. [DOI] [PubMed] [Google Scholar]
- 10.Guilliams M, van de Laar L. A Hitchhiker’s guide to myeloid cell subsets: practical implementation of a novel mononuclear phagocyte classification system. Front Immunol. 2015;6:406. doi: 10.3389/fimmu.2015.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann C, Sorg C. Sequential expression of functions during macrophage differentiation in murine bone marrow liquid cultures. Eur J Immunol. 1980;10:834–840. doi: 10.1002/eji.1830101107. [DOI] [PubMed] [Google Scholar]
- 12.Ross JA, Auger MJ. The biology of the macrophage. In: Burke B, Lewis CE, editors. The macrophage. New York: Oxford University Press; 2002. pp. 3–72. [Google Scholar]
- 13.Mossadegh-Keller N, Sarrazin S, Kandalla PK, Espinosa L, Stanley ER, Nutt SL, Moore J, Sieweke MH. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature. 2013;497:239–243. doi: 10.1038/nature12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 2013;34:81–89. doi: 10.1016/j.it.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Davies LC, Rosas M, Jenkins SJ, Liao CT, Scurr MJ, Brombacher F, Fraser DJ, Allen JE, Jones SA, Taylor PR. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun. 2013;4:1886. doi: 10.1038/ncomms2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang DE, Hetherington CJ, Chen HM, Tenen DG. The macrophage transcription factor PU.1 directs tissue-specific expression of the macrophage colony-stimulating factor receptor. Mol Cell Biol. 1994;14:373–381. doi: 10.1128/MCB.14.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mouchemore KA, Pixley FJ. CSF-1 signaling in macrophages: pleiotrophy through phosphotyrosine-based signaling pathways. Crit Rev Clin Lab Sci. 2012;49:49–61. doi: 10.3109/10408363.2012.666845. [DOI] [PubMed] [Google Scholar]
- 18.Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. 2014;6:a021857. doi: 10.1101/cshperspect.a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 20.Ecker J, Liebisch G, Englmaier M, Grandl M, Robenek H, Schmitz G. Induction of fatty acid synthesis is a key requirement for phagocytic differentiation of human monocytes. Proc Natl Acad Sci USA. 2010;107:7817–7822. doi: 10.1073/pnas.0912059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 22.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 23.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 24.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takemura R, Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984;246:C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- 28.Natarelli L, Schober A. MicroRNAs and the response to injury in atherosclerosis. Hamostaseologie. 2015;35:142–150. doi: 10.5482/HAMO-14-10-0051. [DOI] [PubMed] [Google Scholar]
- 29.Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, Roy S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185:2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72:4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lichtnekert J, Kawakami T, Parks WC, Duffield JS. Changes in macrophage phenotype as the immune response evolves. Curr Opin Pharmacol. 2013;13:555–564. doi: 10.1016/j.coph.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall CJ, Sanderson LE, Crosier KE, Crosier PS. Mitochondrial metabolism, reactive oxygen species, and macrophage function-fishing for insights. J Mol Med (Berl) 2014;92:1119–1128. doi: 10.1007/s00109-014-1186-6. [DOI] [PubMed] [Google Scholar]
- 36.Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013;183:1352–1363. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 38.Tugal D, Liao X, Jain MK. Transcriptional control of macrophage polarization. Arterioscler Thromb Vasc Biol. 2013;33:1135–1144. doi: 10.1161/ATVBAHA.113.301453. [DOI] [PubMed] [Google Scholar]
- 39.Su X, Yu Y, Zhong Y, Giannopoulou EG, Hu X, Liu H, Cross JR, Ratsch G, Rice CM, Ivashkiv LB. Interferon-gamma regulates cellular metabolism and mRNA translation to potentiate macrophage activation. Nat Immunol. 2015;16:838–849. doi: 10.1038/ni.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED, Ieronymaki E, Androulidaki A, Venihaki M, Margioris AN, Stathopoulos EN, Tsichlis PN, Tsatsanis C. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci USA. 2012;109:9517–9522. doi: 10.1073/pnas.1119038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weichhart T, Hengstschlager M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15:599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamers AA, Vos M, Rassam F, Marinkovic G, Kurakula K, van Gorp PJ, de Winther MP, Gijbels MJ, de Waard V, de Vries CJ. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res. 2012;110:428–438. doi: 10.1161/CIRCRESAHA.111.260760. [DOI] [PubMed] [Google Scholar]
- 44.Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, Flask CA, Kim J, Doreian BW, Lu KQ, Kaestner KH, Hamik A, Clement K, Jain MK. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma N, Lu Y, Zhou G, Liao X, Kapil P, Anand P, Mahabeleshwar GH, Stamler JS, Jain MK. Myeloid Kruppel-like factor 4 deficiency augments atherogenesis in ApoE−/− mice–brief report. Arterioscler Thromb Vasc Biol. 2012;32:2836–2838. doi: 10.1161/ATVBAHA.112.300471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardilo-Reis L, Gruber S, Schreier SM, Drechsler M, Papac-Milicevic N, Weber C, Wagner O, Stangl H, Soehnlein O, Binder CJ. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med. 2012;4:1072–1086. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babaev VR, Hebron KE, Wiese CB, Toth CL, Ding L, Zhang Y, May JM, Fazio S, Vickers KC, Linton MF. Macrophage deficiency of Akt2 reduces atherosclerosis in Ldlr null mice. J Lipid Res. 2014;55:2296–2308. doi: 10.1194/jlr.M050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab . 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;112:61–67. doi: 10.1016/j.imlet.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 51.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newsholme P, Gordon S, Newsholme EA. Rates of utilization and fates of glucose, glutamine, pyruvate, fatty acids and ketone bodies by mouse macrophages. Biochem J . 1987;242:631–636. doi: 10.1042/bj2420631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 56.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hall CJ, Boyle RH, Astin JW, Flores MV, Oehlers SH, Sanderson LE, Ellett F, Lieschke GJ, Crosier KE, Crosier PS. Immunoresponsive gene 1 augments bactericidal activity of macrophage-lineage cells by regulating beta-oxidation-dependent mitochondrial ROS production. Cell Metab . 2013;18:265–278. doi: 10.1016/j.cmet.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 58.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J . 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.James AM, Collins Y, Logan A, Murphy MP. Mitochondrial oxidative stress and the metabolic syndrome. Trends Endocrinol Metab . 2012;23:429–434. doi: 10.1016/j.tem.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 60.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 61.Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511:167–176. doi: 10.1038/nature13312. [DOI] [PubMed] [Google Scholar]
- 62.Huang SCC, Everts B, Ivanova Y, O’Sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O’Neil CM, Yan C, Du H, Abumrad NA, Urban JF, Artyomov MN, Pearce EL, Pearce EJ. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab . 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 65.Penberthy KK, Ravichandran KS. Apoptotic cell recognition receptors and scavenger receptors. Immunol Rev. 2016;269:44–59. doi: 10.1111/imr.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189:3508–3520. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, Ma X. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity. 2007;27:952–964. doi: 10.1016/j.immuni.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 72.Erbel C, Tyka M, Helmes CM, Akhavanpoor M, Rupp G, Domschke G, Linden F, Wolf A, Doesch A, Lasitschka F, Katus HA, Gleissner CA. CXCL4-induced plaque macrophages can be specifically identified by co-expression of MMP7+S100A8+ in vitro and in vivo. Innate Immun. 2015;21:255–265. doi: 10.1177/1753425914526461. [DOI] [PubMed] [Google Scholar]
- 73.Gleissner CA, Shaked I, Little KM, Ley K. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J Immunol. 2010;184:4810–4818. doi: 10.4049/jimmunol.0901368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, Krause M, Schworer AM, Wagner U, Muller-Brusselbach S, Muller R. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer. 2014;134:32–42. doi: 10.1002/ijc.28335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E, Freeman TC, Ulas T, Schultze JL. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hume DA. The many alternative faces of macrophage activation. Front Immunol. 2015;6:370. doi: 10.3389/fimmu.2015.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nilsson R, Bajic VB, Suzuki H, di Bernardo D, Bjorkegren J, Katayama S, Reid JF, Sweet MJ, Gariboldi M, Carninci P, Hayashizaki Y, Hume DA, Tegner J, Ravasi T. Transcriptional network dynamics in macrophage activation. Genomics. 2006;88:133–142. doi: 10.1016/j.ygeno.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 79.Raj A, van Oudenaarden A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell. 2008;135:216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet. 2011;12:846–860. doi: 10.1038/nrg3079. [DOI] [PubMed] [Google Scholar]
- 81.Kouno T, de Hoon M, Mar JC, Tomaru Y, Kawano M, Carninci P, Suzuki H, Hayashizaki Y, Shin JW. Temporal dynamics and transcriptional control using single-cell gene expression analysis. Genome Biol. 2013;14:R118. doi: 10.1186/gb-2013-14-10-r118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramsey S, Ozinsky A, Clark A, Smith KD, de Atauri P, Thorsson V, Orrell D, Bolouri H. Transcriptional noise and cellular heterogeneity in mammalian macrophages. Philos Trans R Soc Lond B Biol Sci. 2006;361:495–506. doi: 10.1098/rstb.2005.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raser JM, O’Shea EK. Noise in gene expression: origins, consequences, and control. Science. 2005;309:2010–2013. doi: 10.1126/science.1105891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siciliano V, Garzilli I, Fracassi C, Criscuolo S, Ventre S, di Bernardo D. miRNAs confer phenotypic robustness to gene networks by suppressing biological noise. Nat Commun. 2013;4:2364. doi: 10.1038/ncomms3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmiedel JM, Klemm SL, Zheng Y, Sahay A, Bluthgen N, Marks DS, van Oudenaarden A. Gene expression. MicroRNA control of protein expression noise. Science. 2015;348:128–132. doi: 10.1126/science.aaa1738. [DOI] [PubMed] [Google Scholar]
- 87.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 88.Yoda M, Kawamata T, Paroo Z, Ye X, Iwasaki S, Liu Q, Tomari Y. ATP-dependent human RISC assembly pathways. Nat Struct Mol Biol. 2010;17:17–23. doi: 10.1038/nsmb.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang HW, Noland C, Siridechadilok B, Taylor DW, Ma E, Felderer K, Doudna JA, Nogales E. Structural insights into RNA processing by the human RISC-loading complex. Nat Struct Mol Biol. 2009;16:1148–1153. doi: 10.1038/nsmb.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilson RC, Doudna JA. Molecular mechanisms of RNA interference. Annu Rev Biophys. 2013;42:217–239. doi: 10.1146/annurev-biophys-083012-130404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kwak PB, Tomari Y. The N domain of Argonaute drives duplex unwinding during RISC assembly. Nat Struct Mol Biol. 2012;19:145–151. doi: 10.1038/nsmb.2232. [DOI] [PubMed] [Google Scholar]
- 92.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Helwak A, Kudla G, Dudnakova T, Tollervey D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell. 2013;153:654–665. doi: 10.1016/j.cell.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bosson AD, Zamudio JR, Sharp PA. Endogenous miRNA and target concentrations determine susceptibility to potential ceRNA competition. Mol Cell. 2014;56:347–359. doi: 10.1016/j.molcel.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yuan Y, Liu B, Xie P, Zhang MQ, Li Y, Xie Z, Wang X. Model-guided quantitative analysis of microRNA-mediated regulation on competing endogenous RNAs using a synthetic gene circuit. Proc Natl Acad Sci USA. 2015;112:3158–3163. doi: 10.1073/pnas.1413896112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.La Rocca G, Olejniczak SH, Gonzalez AJ, Briskin D, Vidigal JA, Spraggon L, DeMatteo RG, Radler MR, Lindsten T, Ventura A, Tuschl T, Leslie CS, Thompson CB. In vivo, Argonaute-bound microRNAs exist predominantly in a reservoir of low molecular weight complexes not associated with mRNA. Proc Natl Acad Sci USA. 2015;112:767–772. doi: 10.1073/pnas.1424217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eichhorn SW, Guo H, McGeary SE, Rodriguez-Mias RA, Shin C, Baek D, Hsu SH, Ghoshal K, Villen J, Bartel DP. mRNA destabilization is the dominant effect of mammalian microRNAs by the time substantial repression ensues. Mol Cell. 2014;56:104–115. doi: 10.1016/j.molcel.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wei Y, Zhu M, Corbalan-Campos J, Heyll K, Weber C, Schober A. Regulation of Csf1r and Bcl6 in macrophages mediates the stage-specific effects of microRNA-155 on atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:796–803. doi: 10.1161/ATVBAHA.114.304723. [DOI] [PubMed] [Google Scholar]
- 100.Wei Y, Schober A, Weber C. Pathogenic arterial remodeling: the good and bad of microRNAs. Am J Physiol Heart Circ Physiol. 2013;304:H1050–H1059. doi: 10.1152/ajpheart.00267.2012. [DOI] [PubMed] [Google Scholar]
- 101.Wei Y, Nazari-Jahantigh M, Neth P, Weber C, Schober A. MicroRNA-126, -145, and -155: a therapeutic triad in atherosclerosis? Arterioscler Thromb Vasc Biol. 2013;33:449–454. doi: 10.1161/ATVBAHA.112.300279. [DOI] [PubMed] [Google Scholar]
- 102.Wei Y, Nazari-Jahantigh M, Chan L, Zhu M, Heyll K, Corbalan-Campos J, Hartmann P, Thiemann A, Weber C, Schober A. The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1- and microRNA-155-dependent pathway during atherosclerosis. Circulation. 2013;127:1609–1619. doi: 10.1161/CIRCULATIONAHA.112.000736. [DOI] [PubMed] [Google Scholar]
- 103.Fordham JB, Naqvi AR, Nares S. Regulation of miR-24, miR-30b, and miR-142-3p during macrophage and dendritic cell differentiation potentiates innate immunity. J Leukoc Biol. 2015;98:195–207. doi: 10.1189/jlb.1A1014-519RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin HS, Gong JN, Su R, Chen MT, Song L, Shen C, Wang F, Ma YN, Zhao HL, Yu J, Li WW, Huang LX, Xu XH, Zhang JW. miR-199a-5p inhibits monocyte/macrophage differentiation by targeting the activin A type 1B receptor gene and finally reducing C/EBPalpha expression. J Leukoc Biol. 2014;96:1023–1035. doi: 10.1189/jlb.1A0514-240R. [DOI] [PubMed] [Google Scholar]
- 105.Naqvi AR, Fordham JB, Nares S. miR-24, miR-30b, and miR-142-3p regulate phagocytosis in myeloid inflammatory cells. J Immunol. 2015;194:1916–1927. doi: 10.4049/jimmunol.1401893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bettencourt P, Marion S, Pires D, Santos LF, Lastrucci C, Carmo N, Blake J, Benes V, Griffiths G, Neyrolles O, Lugo-Villarino G, Anes E. Actin-binding protein regulation by microRNAs as a novel microbial strategy to modulate phagocytosis by host cells: the case of N-Wasp and miR-142-3p. Front Cell Infect Microbiol. 2013;3:19. doi: 10.3389/fcimb.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sierra-Filardi E, Puig-Kroger A, Blanco FJ, Nieto C, Bragado R, Palomero MI, Bernabeu C, Vega MA, Corbi AL. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood. 2011;117:5092–5101. doi: 10.1182/blood-2010-09-306993. [DOI] [PubMed] [Google Scholar]
- 109.Gantier MP, Stunden HJ, McCoy CE, Behlke MA, Wang D, Kaparakis-Liaskos M, Sarvestani ST, Yang YH, Xu D, Corr SC, Morand EF, Williams BR. A miR-19 regulon that controls NF-kappaB signaling. Nucleic Acids Res. 2012;40:8048–8058. doi: 10.1093/nar/gks521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, Weber C, Schober A. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122:4190–4202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Graff JW, Dickson AM, Clay G, McCaffrey AP, Wilson ME. Identifying functional microRNAs in macrophages with polarized phenotypes. J Biol Chem. 2012;287:21816–21825. doi: 10.1074/jbc.M111.327031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cobos Jimenez V, Bradley EJ, Willemsen AM, van Kampen AH, Baas F, Kootstra NA. Next-generation sequencing of microRNAs uncovers expression signatures in polarized macrophages. Physiol Genomics. 2014;46:91–103. doi: 10.1152/physiolgenomics.00140.2013. [DOI] [PubMed] [Google Scholar]
- 113.Squadrito ML, Baer C, Burdet F, Maderna C, Gilfillan GD, Lyle R, Ibberson M, De Palma M. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep. 2014;8:1432–1446. doi: 10.1016/j.celrep.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 114.Njock MS, Cheng HS, Dang LT, Nazari-Jahantigh M, Lau AC, Boudreau E, Roufaiel M, Cybulsky MI, Schober A, Fish JE. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood. 2015;125:3202–3212. doi: 10.1182/blood-2014-11-611046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Quinn SR, Mangan NE, Caffrey BE, Gantier MP, Williams BR, Hertzog PJ, McCoy CE, O’Neill LA. The role of Ets2 transcription factor in the induction of microRNA-155 (miR-155) by lipopolysaccharide and its targeting by interleukin-10. J Biol Chem. 2014;289:4316–4325. doi: 10.1074/jbc.M113.522730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bala S, Marcos M, Kodys K, Csak T, Catalano D, Mandrekar P, Szabo G. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor alpha (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;286:1436–1444. doi: 10.1074/jbc.M110.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tian FJ, An LN, Wang GK, Zhu JQ, Li Q, Zhang YY, Zeng A, Zou J, Zhu RF, Han XS, Shen N, Yang HT, Zhao XX, Huang S, Qin YW, Jing Q. Elevated microRNA-155 promotes foam cell formation by targeting HBP1 in atherogenesis. Cardiovasc Res. 2014;103:100–110. doi: 10.1093/cvr/cvu070. [DOI] [PubMed] [Google Scholar]
- 118.Du F, Yu F, Wang Y, Hui Y, Carnevale K, Fu M, Lu H, Fan D. MicroRNA-155 deficiency results in decreased macrophage inflammation and attenuated atherogenesis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2014;34:759–767. doi: 10.1161/ATVBAHA.113.302701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malyshev I, Malyshev Y. Current concept and update of the macrophage plasticity concept: intracellular mechanisms of reprogramming and M3 macrophage “switch” phenotype. Biomed Res Int. 2015;2015:341308. doi: 10.1155/2015/341308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kutty RK, Nagineni CN, Samuel W, Vijayasarathy C, Hooks JJ, Redmond TM. Inflammatory cytokines regulate microRNA-155 expression in human retinal pigment epithelial cells by activating JAK/STAT pathway. Biochem Biophys Res Commun. 2010;402:390–395. doi: 10.1016/j.bbrc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin CC, Jiang W, Mitra R, Cheng F, Yu H, Zhao Z. Regulation rewiring analysis reveals mutual regulation between STAT1 and miR-155-5p in tumor immunosurveillance in seven major cancers. Sci Rep. 2015;5:12063. doi: 10.1038/srep12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, Margioris AN, Tsichlis PN, Tsatsanis C. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31:220–231. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.He S, Yang L, Li D, Li M. Kruppel-like factor 2-mediated suppression of microRNA-155 reduces the proinflammatory activation of macrophages. PLoS One. 2015;10:e0139060. doi: 10.1371/journal.pone.0139060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pankratz F, Bemtgen X, Zeiser R, Leonhardt F, Kreuzaler S, Hilgendorf I, Smolka C, Helbing T, Hoefer I, Esser JS, Kustermann M, Moser M, Bode C, Grundmann S. MicroRNA-155 exerts cell-specific antiangiogenic but proarteriogenic effects during adaptive neovascularization. Circulation. 2015;131:1575–1589. doi: 10.1161/CIRCULATIONAHA.114.014579. [DOI] [PubMed] [Google Scholar]
- 125.Zhu GF, Yang LX, Guo RW, Liu H, Shi YK, Wang H, Ye JS, Yang ZH, Liang X. miR-155 inhibits oxidized low-density lipoprotein-induced apoptosis of RAW264.7 cells. Mol Cell Biochem. 2013;382:253–261. doi: 10.1007/s11010-013-1741-4. [DOI] [PubMed] [Google Scholar]
- 126.Koch M, Mollenkopf HJ, Klemm U, Meyer TF. Induction of microRNA-155 is TLR- and type IV secretion system-dependent in macrophages and inhibits DNA-damage induced apoptosis. Proc Natl Acad Sci USA. 2012;109:E1153–E1162. doi: 10.1073/pnas.1116125109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang S, Li F, Jia S, Zhang K, Jiang W, Shang Y, Chang K, Deng S, Chen M. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes apoptosis of macrophages via targeting the microRNA155-SOCS1 interaction. Cell Physiol Biochem. 2015;35:1276–1288. doi: 10.1159/000373950. [DOI] [PubMed] [Google Scholar]
- 128.Lu LF, Gasteiger G, Yu IS, Chaudhry A, Hsin JP, Lu Y, Bos PD, Lin LL, Zawislak CL, Cho S, Sun JC, Leslie CS, Lin SW, Rudensky AY. A single miRNA-mRNA interaction affects the immune response in a context- and cell-type-specific manner. Immunity. 2015;43:52–64. doi: 10.1016/j.immuni.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.O’Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, Baltimore D. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med. 2008;205:585–594. doi: 10.1084/jem.20072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Donners MM, Wolfs IM, Stoger LJ, van der Vorst EP, Pottgens CC, Heymans S, Schroen B, Gijbels MJ, de Winther MP. Hematopoietic miR155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. PLoS One. 2012;7:e35877. doi: 10.1371/journal.pone.0035877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 132.Li T, Morgan MJ, Choksi S, Zhang Y, Kim YS, Liu ZG. MicroRNAs modulate the noncanonical transcription factor NF-kappaB pathway by regulating expression of the kinase IKKalpha during macrophage differentiation. Nat Immunol. 2010;11:799–805. doi: 10.1038/ni.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Taïbi F, Metzinger-Le Meuth V, Massy ZA, Metzinger L. miR-223: an inflammatory oncomiR enters the cardiovascular field. Biochim Biophys Acta. 2014;1842:1001–1009. doi: 10.1016/j.bbadis.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 134.Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K, Shah P, Wisler J, Eubank TD, Tridandapani S, Paulaitis ME, Piper MG, Marsh CB. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121:984–995. doi: 10.1182/blood-2011-08-374793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fukao T, Fukuda Y, Kiga K, Sharif J, Hino K, Enomoto Y, Kawamura A, Nakamura K, Takeuchi T, Tanabe M. An evolutionarily conserved mechanism for microRNA-223 expression revealed by microRNA gene profiling. Cell. 2007;129:617–631. doi: 10.1016/j.cell.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 136.Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, Li H, Wang G, Evans AR, Safe S, Wu C, Zhou B. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125:2892–2903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- 138.Ying W, Tseng A, Chang RC, Morin A, Brehm T, Triff K, Nair V, Zhuang G, Song H, Kanameni S, Wang H, Golding MC, Bazer FW, Chapkin RS, Safe S, Zhou B. MicroRNA-223 is a crucial mediator of PPARgamma-regulated alternative macrophage activation. J Clin Invest. 2015;125:4149–4159. doi: 10.1172/JCI81656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen Q, Wang H, Liu Y, Song Y, Lai L, Han Q, Cao X, Wang Q. Inducible microRNA-223 down-regulation promotes TLR-triggered IL-6 and IL-1beta production in macrophages by targeting STAT3. PLoS One. 2012;7:e42971. doi: 10.1371/journal.pone.0042971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol. 2012;189:4175–4181. doi: 10.4049/jimmunol.1201516. [DOI] [PubMed] [Google Scholar]
- 141.Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey AA, Pich D, McInnes IB, Hammerschmidt W, O’Neill LA, Masters SL. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1beta production. J Immunol. 2012;189:3795–3799. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- 142.Liu Y, Wang R, Jiang J, Yang B, Cao Z, Cheng X. miR-223 is upregulated in monocytes from patients with tuberculosis and regulates function of monocyte-derived macrophages. Mol Immunol. 2015;67:475–481. doi: 10.1016/j.molimm.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 143.Mathsyaraja H, Thies K, Taffany DA, Deighan C, Liu T, Yu L, Fernandez SA, Shapiro C, Otero J, Timmers C, Lustberg MB, Chalmers J, Leone G, Ostrowski MC. CSF1-ETS2-induced microRNA in myeloid cells promote metastatic tumor growth. Oncogene. 2015;34:3651–3661. doi: 10.1038/onc.2014.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vergadi E, Vaporidi K, Theodorakis EE, Doxaki C, Lagoudaki E, Ieronymaki E, Alexaki VI, Helms M, Kondili E, Soennichsen B, Stathopoulos EN, Margioris AN, Georgopoulos D, Tsatsanis C. Akt2 deficiency protects from acute lung injury via alternative macrophage activation and miR-146a induction in mice. J Immunol. 2014;192:394–406. doi: 10.4049/jimmunol.1300959. [DOI] [PubMed] [Google Scholar]
- 146.Li K, Ching D, Luk FS, Raffai RL. Apolipoprotein E enhances microRNA-146a in monocytes and macrophages to suppress nuclear factor-kappaB-driven inflammation and atherosclerosis. Circ Res. 2015;117:e1–e11. doi: 10.1161/CIRCRESAHA.117.305844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nahid MA, Satoh M, Chan EK. Interleukin 1beta-responsive microRNA-146a is critical for the cytokine-induced tolerance and cross-tolerance to toll-like receptor ligands. J Innate Immun. 2015;7:428–440. doi: 10.1159/000371517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.He Y, Sun X, Huang C, Long XR, Lin X, Zhang L, Lv XW, Li J. MiR-146a regulates IL-6 production in lipopolysaccharide-induced RAW264.7 macrophage cells by inhibiting Notch1. Inflammation. 2014;37:71–82. doi: 10.1007/s10753-013-9713-0. [DOI] [PubMed] [Google Scholar]
- 149.Monsalve E, Ruiz-Garcia A, Baladron V, Ruiz-Hidalgo MJ, Sanchez-Solana B, Rivero S, Garcia-Ramirez JJ, Rubio A, Laborda J, Diaz-Guerra MJ. Notch1 upregulates LPS-induced macrophage activation by increasing NF-kappaB activity. Eur J Immunol. 2009;39:2556–2570. doi: 10.1002/eji.200838722. [DOI] [PubMed] [Google Scholar]
- 150.Palaga T, Buranaruk C, Rengpipat S, Fauq AH, Golde TE, Kaufmann SH, Osborne BA. Notch signaling is activated by TLR stimulation and regulates macrophage functions. Eur J Immunol. 2008;38:174–183. doi: 10.1002/eji.200636999. [DOI] [PubMed] [Google Scholar]
- 151.Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J, Sun G, Tay J, Linsley PS, Baltimore D. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morishita Y, Imai T, Yoshizawa H, Watanabe M, Ishibashi K, Muto S, Nagata D. Delivery of microRNA-146a with polyethylenimine nanoparticles inhibits renal fibrosis in vivo. Int J Nanomedicine. 2015;10:3475–3488. doi: 10.2147/IJN.S82587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jiang W, Ni Q, Tan L, Kong L, Lu Y, Xu X, Kong L. The microRNA-146a/b attenuates acute small-for-size liver graft injury in rats. Liver Int. 2015;35:914–924. doi: 10.1111/liv.12674. [DOI] [PubMed] [Google Scholar]
- 154.Ribas J, Lupold SE. The transcriptional regulation of miR-21, its multiple transcripts, and their implication in prostate cancer. Cell Cycle. 2010;9:923–929. doi: 10.4161/cc.9.5.10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 156.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 158.Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- 159.Hatley ME, Patrick DM, Garcia MR, Richardson JA, Bassel-Duby R, van Rooij E, Olson EN. Modulation of K-Ras-dependent lung tumorigenesis by microRNA-21. Cancer Cell. 2010;18:282–293. doi: 10.1016/j.ccr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 161.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, Johnson DS, Chen Y, O’Neill LA. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 162.Caescu CI, Guo X, Tesfa L, Bhagat TD, Verma A, Zheng D, Stanley ER. Colony stimulating factor-1 receptor signaling networks inhibit mouse macrophage inflammatory responses by induction of microRNA-21. Blood. 2015;125:e1–e13. doi: 10.1182/blood-2014-10-608000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol. 2014;192:1120–1129. doi: 10.4049/jimmunol.1300613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shang YY, Fang NN, Wang F, Wang H, Wang ZH, Tang MX, Peng J, Zhang Y, Zhang W, Zhong M. MicroRNA-21, induced by high glucose, modulates macrophage apoptosis via programmed cell death 4. Mol Med Rep. 2015;12:463–469. doi: 10.3892/mmr.2015.3398. [DOI] [PubMed] [Google Scholar]
- 165.Ma X, Kumar M, Choudhury SN, Becker Buscaglia LE, Barker JR, Kanakamedala K, Liu MF, Li Y. Loss of the miR-21 allele elevates the expression of its target genes and reduces tumorigenesis. Proc Natl Acad Sci USA. 2011;108:10144–10149. doi: 10.1073/pnas.1103735108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H(2)O(2)-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol. 2009;47:5–14. doi: 10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu ZL, Wang H, Liu J, Wang ZX. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol Cell Biochem. 2013;372:35–45. doi: 10.1007/s11010-012-1443-3. [DOI] [PubMed] [Google Scholar]
- 168.Cheng Y, Zhu P, Yang J, Liu X, Dong S, Wang X, Chun B, Zhuang J, Zhang C. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res. 2010;87:431–439. doi: 10.1093/cvr/cvq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wedeken L, Singh P, Klempnauer KH. Tumor suppressor protein Pdcd4 inhibits translation of p53 mRNA. J Biol Chem. 2011;286:42855–42862. doi: 10.1074/jbc.M111.269456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liwak U, Thakor N, Jordan LE, Roy R, Lewis SM, Pardo OE, Seckl M, Holcik M. Tumor suppressor PDCD4 represses internal ribosome entry site-mediated translation of antiapoptotic proteins and is regulated by S6 kinase 2. Mol Cell Biol. 2012;32:1818–1829. doi: 10.1128/MCB.06317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sahin E, Haubenwallner S, Kuttke M, Kollmann I, Halfmann A, Dohnal AM, Chen L, Cheng P, Hoesel B, Einwallner E, Brunner J, Kral JB, Schrottmaier WC, Thell K, Saferding V, Bluml S, Schabbauer G. Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses. J Immunol. 2014;193:1717–1727. doi: 10.4049/jimmunol.1302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wu C, Xue Y, Wang P, Lin L, Liu Q, Li N, Xu J, Cao X. IFN-gamma primes macrophage activation by increasing phosphatase and tensin homolog via downregulation of miR-3473b. J Immunol. 2014;193:3036–3044. doi: 10.4049/jimmunol.1302379. [DOI] [PubMed] [Google Scholar]
- 173.Fan X, Wang E, Wang X, Cong X, Chen X. MicroRNA-21 is a unique signature associated with coronary plaque instability in humans by regulating matrix metalloproteinase-9 via reversion-inducing cysteine-rich protein with Kazal motifs. Exp Mol Pathol. 2014;96:242–249. doi: 10.1016/j.yexmp.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 174.Raitoharju E, Lyytikainen LP, Levula M, Oksala N, Mennander A, Tarkka M, Klopp N, Illig T, Kahonen M, Karhunen PJ, Laaksonen R, Lehtimaki T. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011;219:211–217. doi: 10.1016/j.atherosclerosis.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 175.Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 176.Lee H, Han S, Kwon CS, Lee D. Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell. 2016;7:100–113. doi: 10.1007/s13238-015-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Altuvia Y, Landgraf P, Lithwick G, Elefant N, Pfeffer S, Aravin A, Brownstein MJ, Tuschl T, Margalit H. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005;33:2697–2706. doi: 10.1093/nar/gki567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M, Abraham E, Liu G. MicroRNA let-7c regulates macrophage polarization. J Immunol. 2013;190:6542–6549. doi: 10.4049/jimmunol.1202496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schulte LN, Eulalio A, Mollenkopf HJ, Reinhardt R, Vogel J. Analysis of the host microRNA response to Salmonella uncovers the control of major cytokines by the let-7 family. EMBO J. 2011;30:1977–1989. doi: 10.1038/emboj.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang W, Liu H, Liu W, Liu Y, Xu J. Polycomb-mediated loss of microRNA let-7c determines inflammatory macrophage polarization via PAK1-dependent NF-kappaB pathway. Cell Death Differ. 2015;22:287–297. doi: 10.1038/cdd.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Naqvi AR, Fordham JB, Khan A, Nares S. MicroRNAs responsive to Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis LPS modulate expression of genes regulating innate immunity in human macrophages. Innate Immun. 2014;20:540–551. doi: 10.1177/1753425913501914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Murphy AJ, Guyre PM, Pioli PA. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J Immunol. 2010;184:5029–5037. doi: 10.4049/jimmunol.0903463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kumar M, Sahu SK, Kumar R, Subuddhi A, Maji RK, Jana K, Gupta P, Raffetseder J, Lerm M, Ghosh Z, van Loo G, Beyaert R, Gupta UD, Kundu M, Basu J. MicroRNA let-7 modulates the immune response to Mycobacterium tuberculosis infection via control of A20, an inhibitor of the NF-kappaB pathway. Cell Host Microbe. 2015;17:345–356. doi: 10.1016/j.chom.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 184.Chen XM, Splinter PL, O’Hara SP, LaRusso NF. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J Biol Chem. 2007;282:28929–28938. doi: 10.1074/jbc.M702633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sharbati J, Lewin A, Kutz-Lohroff B, Kamal E, Einspanier R, Sharbati S. Integrated microRNA-mRNA-analysis of human monocyte derived macrophages upon Mycobacterium avium subsp. hominissuis infection. PLoS One. 2011;6:e20258. doi: 10.1371/journal.pone.0020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Litvak V, Ramsey SA, Rust AG, Zak DE, Kennedy KA, Lampano AE, Nykter M, Shmulevich I, Aderem A. Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat Immunol. 2009;10:437–443. doi: 10.1038/ni.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Balamurugan K, Sharan S, Klarmann KD, Zhang Y, Coppola V, Summers GH, Roger T, Morrison DK, Keller JR, Sterneck E. FBXW7alpha attenuates inflammatory signalling by downregulating C/EBPdelta and its target gene Tlr4. Nat Commun. 2013;4:1662. doi: 10.1038/ncomms2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hamerman JA, Pottle J, Ni M, He Y, Zhang ZY, Buckner JH. Negative regulation of TLR signaling in myeloid cells-implications for autoimmune diseases. Immunol Rev. 2016;269:212–227. doi: 10.1111/imr.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chafin CB, Regna NL, Caudell DL, Reilly CM. MicroRNA-let-7a promotes E2F-mediated cell proliferation and NFkappaB activation in vitro. Cell Mol Immunol. 2014;11:79–83. doi: 10.1038/cmi.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sun Y, Sun J, Tomomi T, Nieves E, Mathewson N, Tamaki H, Evers R, Reddy P. PU.1-dependent transcriptional regulation of miR-142 contributes to its hematopoietic cell-specific expression and modulation of IL-6. J Immunol. 2013;190:4005–4013. doi: 10.4049/jimmunol.1202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foa R, Schliwka J, Fuchs U, Novosel A, Muller RU, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, De Vita G, Frezzetti D, Trompeter HI, Hornung V, Teng G, Hartmann G, Palkovits M, Di Lauro R, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M, Tuschl T. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shrestha A, Carraro G, El Agha E, Mukhametshina R, Chao CM, Rizvanov A, Barreto G, Bellusci S. Generation and Validation of miR-142 knock out mice. PLoS One. 2015;10:e0136913. doi: 10.1371/journal.pone.0136913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kramer NJ, Wang WL, Reyes EY, Kumar B, Chen CC, Ramakrishna C, Cantin EM, Vonderfecht SL, Taganov KD, Chau N, Boldin MP. Altered lymphopoiesis and immunodeficiency in miR-142 null mice. Blood. 2015;125:3720–3730. doi: 10.1182/blood-2014-10-603951. [DOI] [PubMed] [Google Scholar]
- 194.Lagrange B, Martin RZ, Droin N, Aucagne R, Paggetti J, Largeot A, Itzykson R, Solary E, Delva L, Bastie JN. A role for miR-142-3p in colony-stimulating factor 1-induced monocyte differentiation into macrophages. Biochim Biophys Acta. 2013;1833:1936–1946. doi: 10.1016/j.bbamcr.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 195.Laslo P, Spooner CJ, Warmflash A, Lancki DW, Lee HJ, Sciammas R, Gantner BN, Dinner AR, Singh H. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126:755–766. doi: 10.1016/j.cell.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 196.Sun W, Shen W, Yang S, Hu F, Li H, Zhu TH. miR-223 and miR-142 attenuate hematopoietic cell proliferation, and miR-223 positively regulates miR-142 through LMO2 isoforms and CEBP-beta. Cell Res. 2010;20:1158–1169. doi: 10.1038/cr.2010.134. [DOI] [PubMed] [Google Scholar]
- 197.Sonda N, Simonato F, Peranzoni E, Cali B, Bortoluzzi S, Bisognin A, Wang E, Marincola FM, Naldini L, Gentner B, Trautwein C, Sackett SD, Zanovello P, Molon B, Bronte V. miR-142-3p prevents macrophage differentiation during cancer-induced myelopoiesis. Immunity. 2013;38:1236–1249. doi: 10.1016/j.immuni.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 198.Sun Y, Varambally S, Maher CA, Cao Q, Chockley P, Toubai T, Malter C, Nieves E, Tawara I, Wang Y, Ward PA, Chinnaiyan A, Reddy P. Targeting of microRNA-142-3p in dendritic cells regulates endotoxin-induced mortality. Blood. 2011;117:6172–6183. doi: 10.1182/blood-2010-12-325647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huber R, Pietsch D, Panterodt T, Brand K. Regulation of C/EBPbeta and resulting functions in cells of the monocytic lineage. Cell Signal. 2012;24:1287–1296. doi: 10.1016/j.cellsig.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 200.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Bronneke HS, Estevez E, Allen TL, Mesaros A, Partridge L, Febbraio MA, Chawla A, Wunderlich FT, Bruning JC. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15:423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chomarat P, Banchereau J, Davoust J, Karolina Palucka A. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 202.Thrasher AJ. Wasp in immune-system organization and function. Nat Rev Immunol. 2002;2:635–646. doi: 10.1038/nri884. [DOI] [PubMed] [Google Scholar]
- 203.Su S, Zhao Q, He C, Huang D, Liu J, Chen F, Chen J, Liao JY, Cui X, Zeng Y, Yao H, Su F, Liu Q, Jiang S, Song E. miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program. Nat Commun. 2015;6:8523. doi: 10.1038/ncomms9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schober A, Nazari-Jahantigh M, Weber C. MicroRNA-mediated mechanisms of the cellular stress response in atherosclerosis. Nat Rev Cardiol. 2015;12:361–374. doi: 10.1038/nrcardio.2015.38. [DOI] [PubMed] [Google Scholar]
- 205.Maxfield FR, van Meer G. Cholesterol, the central lipid of mammalian cells. Curr Opin Cell Biol. 2010;22:422–429. doi: 10.1016/j.ceb.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol. 2015;16:907–917. doi: 10.1038/ni.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kiss RS, Elliott MR, Ma Z, Marcel YL, Ravichandran KS. Apoptotic cells induce a phosphatidylserine-dependent homeostatic response from phagocytes. Curr Biol. 2006;16:2252–2258. doi: 10.1016/j.cub.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 209.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 210.Fond AM, Lee CS, Schulman IG, Kiss RS, Ravichandran KS. Apoptotic cells trigger a membrane-initiated pathway to increase ABCA1. J Clin Invest. 2015;125:2748–2758. doi: 10.1172/JCI80300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yvan-Charvet L, Pagler TA, Seimon TA, Thorp E, Welch CL, Witztum JL, Tabas I, Tall AR. ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ Res. 2010;106:1861–1869. doi: 10.1161/CIRCRESAHA.110.217281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ruysschaert JM, Lonez C. Role of lipid microdomains in TLR-mediated signalling. Biochim Biophys Acta. 2015;1848:1860–1867. doi: 10.1016/j.bbamem.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 213.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 214.Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J Immunol. 2011;187:1529–1535. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Steck TL, Lange Y. Cell cholesterol homeostasis: mediation by active cholesterol. Trends Cell Biol. 2010;20:680–687. doi: 10.1016/j.tcb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Plociennikowska A, Hromada-Judycka A, Borzecka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2015;72:557–581. doi: 10.1007/s00018-014-1762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rub A, Dey R, Jadhav M, Kamat R, Chakkaramakkil S, Majumdar S, Mukhopadhyaya R, Saha B. Cholesterol depletion associated with Leishmania major infection alters macrophage CD40 signalosome composition and effector function. Nat Immunol. 2009;10:273–280. doi: 10.1038/ni.1705. [DOI] [PubMed] [Google Scholar]
- 218.Gatfield J, Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science. 2000;288:1647–1650. doi: 10.1126/science.288.5471.1647. [DOI] [PubMed] [Google Scholar]
- 219.Kannan S, Audet A, Huang H, Chen LJ, Wu M. Cholesterol-rich membrane rafts and Lyn are involved in phagocytosis during Pseudomonas aeruginosa infection. J Immunol. 2008;180:2396–2408. doi: 10.4049/jimmunol.180.4.2396. [DOI] [PubMed] [Google Scholar]
- 220.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Funk JL, Feingold KR, Moser AH, Grunfeld C. Lipopolysaccharide stimulation of RAW 264.7 macrophages induces lipid accumulation and foam cell formation. Atherosclerosis. 1993;98:67–82. doi: 10.1016/0021-9150(93)90224-I. [DOI] [PubMed] [Google Scholar]
- 222.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51:3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Koseki M, Hirano K, Masuda D, Ikegami C, Tanaka M, Ota A, Sandoval JC, Nakagawa-Toyama Y, Sato SB, Kobayashi T, Shimada Y, Ohno-Iwashita Y, Matsuura F, Shimomura I, Yamashita S. Increased lipid rafts and accelerated lipopolysaccharide-induced tumor necrosis factor-alpha secretion in Abca1-deficient macrophages. J Lipid Res. 2007;48:299–306. doi: 10.1194/jlr.M600428-JLR200. [DOI] [PubMed] [Google Scholar]
- 225.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 226.Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Melo RC, D’Avila H, Wan HC, Bozza PT, Dvorak AM, Weller PF. Lipid bodies in inflammatory cells: structure, function, and current imaging techniques. J Histochem Cytochem. 2011;59:540–556. doi: 10.1369/0022155411404073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, Raetz CR, Wang EW, Kelly SL, Sullards MC, Murphy RC, Merrill AH, Jr, Brown HA, Dennis EA, Li AC, Ley K, Tsimikas S, Fahy E, Subramaniam S, Quehenberger O, Russell DW, Glass CK. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhang Y, Li Q, Rao E, Sun Y, Grossmann ME, Morris RJ, Cleary MP, Li B. Epidermal fatty acid binding protein promotes skin inflammation induced by high-fat diet. Immunity. 2015;42:953–964. doi: 10.1016/j.immuni.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Azzam KM, Fessler MB. Crosstalk between reverse cholesterol transport and innate immunity. Trends Endocrinol Metab . 2012;23:169–178. doi: 10.1016/j.tem.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhang M, Wu JF, Chen WJ, Tang SL, Mo ZC, Tang YY, Li Y, Wang JL, Liu XY, Peng J, Chen K, He PP, Lv YC, Ouyang XP, Yao F, Tang DP, Cayabyab FS, Zhang DW, Zheng XL, Tian GP, Tang CK. MicroRNA-27a/b regulates cellular cholesterol efflux, influx and esterification/hydrolysis in THP-1 macrophages. Atherosclerosis. 2014;234:54–64. doi: 10.1016/j.atherosclerosis.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 233.Sala F, Aranda JF, Rotllan N, Ramirez CM, Aryal B, Elia L, Condorelli G, Catapano AL, Fernandez-Hernando C, Norata GD. MiR-143/145 deficiency attenuates the progression of atherosclerosis in Ldlr−/− mice. Thromb Haemost. 2014;112:796–802. doi: 10.1160/TH13-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ramirez CM, Rotllan N, Vlassov AV, Davalos A, Li M, Goedeke L, Aranda JF, Cirera-Salinas D, Araldi E, Salerno A, Wanschel A, Zavadil J, Castrillo A, Kim J, Suarez Y, Fernandez-Hernando C. Control of cholesterol metabolism and plasma high-density lipoprotein levels by microRNA-144. Circ Res. 2013;112:1592–1601. doi: 10.1161/CIRCRESAHA.112.300626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lv YC, Tang YY, Peng J, Zhao GJ, Yang J, Yao F, Ouyang XP, He PP, Xie W, Tan YL, Zhang M, Liu D, Tang DP, Cayabyab FS, Zheng XL, Zhang DW, Tian GP, Tang CK. MicroRNA-19b promotes macrophage cholesterol accumulation and aortic atherosclerosis by targeting ATP-binding cassette transporter A1. Atherosclerosis. 2014;236:215–226. doi: 10.1016/j.atherosclerosis.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 237.DiMarco DM, Fernandez ML. The regulation of reverse cholesterol transport and cellular cholesterol homeostasis by microRNAs. Biology. 2015;4:494–511. doi: 10.3390/biology4030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fromm B, Billipp T, Peck LE, Johansen M, Tarver JE, King BL, Newcomb JM, Sempere LF, Flatmark K, Hovig E, Peterson KJ. A uniform system for the annotation of vertebrate microRNA genes and the evolution of the human microRNAome. Annu Rev Genet. 2015;49:213–242. doi: 10.1146/annurev-genet-120213-092023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Horie T, Ono K, Horiguchi M, Nishi H, Nakamura T, Nagao K, Kinoshita M, Kuwabara Y, Marusawa H, Iwanaga Y, Hasegawa K, Yokode M, Kimura T, Kita T. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci USA. 2010;107:17321–17326. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Horie T, Baba O, Kuwabara Y, Chujo Y, Watanabe S, Kinoshita M, Horiguchi M, Nakamura T, Chonabayashi K, Hishizawa M, Hasegawa K, Kume N, Yokode M, Kita T, Kimura T, Ono K. MicroRNA-33 deficiency reduces the progression of atherosclerotic plaque in ApoE−/− mice. J Am Heart Assoc. 2012;1:e003376. doi: 10.1161/JAHA.112.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Karunakaran D, Thrush AB, Nguyen MA, Richards L, Geoffrion M, Singaravelu R, Ramphos E, Shangari P, Ouimet M, Pezacki JP, Moore KJ, Perisic L, Maegdefessel L, Hedin U, Harper ME, Rayner KJ. Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by anti-miR33 in atherosclerosis. Circ Res. 2015;117:266–278. doi: 10.1161/CIRCRESAHA.117.305624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Horie T, Nishino T, Baba O, Kuwabara Y, Nakao T, Nishiga M, Usami S, Izuhara M, Nakazeki F, Ide Y, Koyama S, Sowa N, Yahagi N, Shimano H, Nakamura T, Hasegawa K, Kume N, Yokode M, Kita T, Kimura T, Ono K. MicroRNA-33b knock-in mice for an intron of sterol regulatory element-binding factor 1 (Srebf1) exhibit reduced HDL-C in vivo. Sci Rep. 2014;4:5312. doi: 10.1038/srep05312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI0215593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jeon TI, Osborne TF. SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol Metab . 2012;23:65–72. doi: 10.1016/j.tem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Horie T, Nishino T, Baba O, Kuwabara Y, Nakao T, Nishiga M, Usami S, Izuhara M, Sowa N, Yahagi N, Shimano H, Matsumura S, Inoue K, Marusawa H, Nakamura T, Hasegawa K, Kume N, Yokode M, Kita T, Kimura T, Ono K. MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice. Nat Commun. 2013;4:2883. doi: 10.1038/ncomms3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhao GJ, Tang SL, Lv YC, Ouyang XP, He PP, Yao F, Chen WJ, Lu Q, Tang YY, Zhang M, Fu Y, Zhang DW, Yin K, Tang CK. Antagonism of betulinic acid on LPS-mediated inhibition of ABCA1 and cholesterol efflux through inhibiting nuclear factor-kappaB signaling pathway and miR-33 expression. PLoS One. 2013;8:e74782. doi: 10.1371/journal.pone.0074782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ouimet M, Ediriweera HN, Gundra UM, Sheedy FJ, Ramkhelawon B, Hutchison SB, Rinehold K, van Solingen C, Fullerton MD, Cecchini K, Rayner KJ, Steinberg GR, Zamore PD, Fisher EA, Loke P, Moore KJ. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Invest. 2015;125:4334–4348. doi: 10.1172/JCI81676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ho PC, Chang KC, Chuang YS, Wei LN. Cholesterol regulation of receptor-interacting protein 140 via microRNA-33 in inflammatory cytokine production. Faseb J. 2011;25:1758–1766. doi: 10.1096/fj.10-179267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sene A, Khan AA, Cox D, Nakamura RE, Santeford A, Kim BM, Sidhu R, Onken MD, Harbour JW, Hagbi-Levi S, Chowers I, Edwards PA, Baldan A, Parks JS, Ory DS, Apte RS. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab . 2013;17:549–561. doi: 10.1016/j.cmet.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;20:1603–1614. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Akhtar S, Hartmann P, Karshovska E, Rinderknecht FA, Subramanian P, Gremse F, Grommes J, Jacobs M, Kiessling F, Weber C, Steffens S, Schober A. Endothelial hypoxia-inducible factor-1alpha promotes atherosclerosis and monocyte recruitment by upregulating microRNA-19a. Hypertension. 2015;66:1220–1226. doi: 10.1161/HYPERTENSIONAHA.115.05886. [DOI] [PubMed] [Google Scholar]
- 253.Jennewein C, von Knethen A, Schmid T, Brune B. MicroRNA-27b contributes to lipopolysaccharide-mediated peroxisome proliferator-activated receptor gamma (PPARgamma) mRNA destabilization. J Biol Chem. 2010;285:11846–11853. doi: 10.1074/jbc.M109.066399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Xie N, Cui H, Banerjee S, Tan Z, Salomao R, Fu M, Abraham E, Thannickal VJ, Liu G. miR-27a regulates inflammatory response of macrophages by targeting IL-10. J Immunol. 2014;193:327–334. doi: 10.4049/jimmunol.1400203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ma S, Liu M, Xu Z, Li Y, Guo H, Ge Y, Liu Y, Zheng D, Shi J. A double feedback loop mediated by microRNA-23a/27a/24-2 regulates M1 versus M2 macrophage polarization and thus regulates cancer progression. Oncotarget. 2015 doi: 10.18632/oncotarget.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sanduja S, Blanco FF, Young LE, Kaza V, Dixon DA. The role of tristetraprolin in cancer and inflammation. Front Biosci (Landmark Ed) 2012;17:174–188. doi: 10.2741/3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hu YW, Hu YR, Zhao JY, Li SF, Ma X, Wu SG, Lu JB, Qiu YR, Sha YH, Wang YC, Gao JJ, Zheng L, Wang Q. An agomir of miR-144-3p accelerates plaque formation through impairing reverse cholesterol transport and promoting pro-inflammatory cytokine production. PLoS One. 2014;9:e94997. doi: 10.1371/journal.pone.0094997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Meiler S, Baumer Y, Toulmin E, Seng K, Boisvert WA. MicroRNA 302a is a novel modulator of cholesterol homeostasis and atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:323–331. doi: 10.1161/ATVBAHA.114.304878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gao Z, Zhu X, Dou Y. The miR-302/367 cluster: a comprehensive update on its evolution and functions. Open Biol. 2015;5:150138. doi: 10.1098/rsob.150138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X, Khatsenko OG, Kaimal V, Lees CJ, Fernandez-Hernando C, Fisher EA, Temel RE, Moore KJ. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478:404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]