Abstract
The thymus provides a specialized microenvironment in which a variety of stromal cells of both hematopoietic and non-hematopoietic origin regulate development and repertoire selection of T cells. Recent studies have been unraveling the inter- and intracellular signals and transcriptional networks for spatiotemporal regulation of development of thymic stromal cells, mainly thymic epithelial cells (TECs), and the molecular mechanisms of how different TEC subsets control T cell development and selection. TECs are classified into two functionally different subsets: cortical TECs (cTECs) and medullary TECs (mTECs). cTECs induce positive selection of diverse and functionally distinct T cells by virtue of unique antigen-processing systems, while mTECs are essential for establishing T cell tolerance via ectopic expression of peripheral tissue-restricted antigens and cooperation with dendritic cells. In addition to reviewing the role of the thymic stroma in conventional T cell development, we will discuss recently discovered novel functions of TECs in the development of unconventional T cells, such as natural killer T cells and γδT cells.
Keywords: Thymus, T cell, Repertoire selection, Thymic epithelial cell, cTEC, mTEC
Introduction
T lymphocytes (T cells) are central players in the adaptive immune system. Specific antigen recognition by T cells is dependent on their T cell antigen receptors (TCRs), αβTCR and γδTCR. T cells that express an αβTCR (αβT cells), as well as a coreceptor CD4 or CD8, are considered ‘conventional’ T cells in human and mouse. They are termed as such because αβTCR recognition of peptide antigens displayed by major histocompatibility complex (MHC) proteins plays a major role in immune responses against foreign antigens. Therefore, in this article, the terms ‘T cell’ and ‘TCR’ refer to αβT cell and αβTCR, respectively, unless otherwise specified. The specificity of antigen recognition by TCR is stringently established, such that T cells are reactive to foreign antigens but are tolerant to self-antigens [1, 2].
T cell development and TCR repertoire formation occur primarily in the thymus [3]. The thymus is an organ that provides a unique microenvironment composed of a variety of stromal cells, including thymic epithelial cells (TECs), endothelial cells, fibroblasts, and hematopoietic stromal cells such as dendritic cells (DCs) [4, 5]. These thymic stromal cells coordinate a three-dimensional meshwork architecture that hosts hematopoietic stem cell-derived T-lineage cells, called thymocytes, and critically supports their development. The thymus is subdivided into two histologically discrete regions, the cortex and medulla. The cortex is the outer region of the thymus, where a stromal meshwork houses densely packed immature thymocytes, while the medulla is the inner region with less densely localized mature thymocytes and enriched stromal cells. The most characteristic stromal components that distinguish cortical and medullary microenvironments are two different subsets of TECs: cortical TECs (cTECs) and medullary TECs (mTECs). cTECs and mTECs are both derived from endodermal epithelium, yet they display distinct phenotypes and functions in the regulation of T cell development. These TECs, along with other stromal cells, provide multiple signals to guide the differentiation, migration, proliferation, survival, and death of developing thymocytes, thus playing pivotal roles in forming the adaptive immune system [6, 7].
In this review, we first provide an overview of the stepwise process of T cell development in the thymus, and then review historical and recent studies on the development and function of TECs, particularly focusing on the contrasting roles of cortical and medullary microenvironments. We will also highlight the bidirectional interplay between TECs and developing thymocytes that is required for optimal development and repertoire formation of T cells. In addition, recently discovered functions of TEC subsets in controlling unconventional T cell development are also discussed.
Overview of thymic T cell development
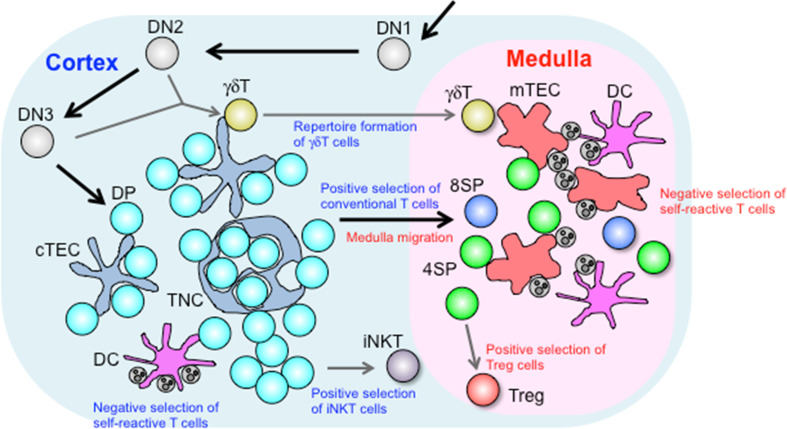
T cell development begins with seeding of the thymus by early T cell progenitors (ETPs) derived from hematopoietic stem cells in fetal liver or adult bone marrow [8]. These ETPs belong to CD4/CD8 double negative (DN) thymocytes and undergo developmental programs through DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−) stages. During DN2 and DN3 stages, V(D)J rearrangement at TCRγ, δ and β loci occurs. Production of a successfully rearranged TCR β-chain leads to further differentiation into the DN4 stage. This process, called ‘β selection’, ensures commitment to the αβT cell lineage, and DN4 thymocytes proliferate and express CD4 and CD8 coreceptors, giving rise to CD4/CD8 double positive (DP) thymocytes. These differentiation processes are associated with relocation of thymocytes [9]: in adult thymus, ETPs first arrive at the cortico-medullary junction, developing DN2 and DN3 thymocytes migrate through the cortex toward the subcapsular region, and the generation of DP thymocytes occurs in the outer cortex.
In the cortex, DP thymocytes undergo TCRα-VJ rearrangement, thereby expressing αβTCR on the cell surface. Interaction of αβTCR with peptide-MHC (pMHC) complexes presented in the cortical microenvironment leads to the fate decision of DP thymocytes. DP thymocytes that receive low avidity TCR interactions with self pMHC survive and differentiate into CD4 single positive (SP) or CD8SP thymocytes, in a process referred to as positive selection. In contrast, DP thymocytes expressing TCR strongly reactive to self pMHC (self-reactive cells) die by apoptosis, a process referred to as negative selection. Positively selected CD4SP or CD8SP thymocytes relocate to the medulla by chemotactic migration. In the medulla, mTECs express a variety of peripheral tissue-restricted antigens (TRAs) that are presented autonomously by mTECs or indirectly by DCs, such that SP thymocytes reactive to TRAs are deleted by negative selection or induced to differentiate into Foxp3+ regulatory T cells (Tregs). These medullary controls of T cell development are crucial for establishment of self-tolerance and preventing autoimmunity, and largely depend on autoimmune regulator (Aire), a nuclear factor expressed in mTECs. Consequently, mature SP thymocytes that have completed cortical and medullary selection processes—and which thereby express diverse yet self-tolerant TCRs—are released to the circulation as naïve T cells (Fig. 1).
Fig. 1.
Roles of thymic stromal cells in T cell development. Developing DN thymocytes migrate from the cortico-medullary junction toward the subcapsular region, and then back toward the medulla upon development to DP thymocytes. In the cortex, DP thymocytes begin to express TCR on their surface and are selected upon interaction with pMHC complexes displayed in the microenvironment. cTECs produce a unique set of MHC-bound peptides that are essential for inducing positive selection, while DCs critically contribute to induction of negative selection. Intimate interaction between DP thymocytes and cTECs results in formation of TNCs, which facilitate prolonged survival of inner DP thymocytes and secondary TCRα recombination. Positively selected cells migrate into the medulla in response to chemokines produced by mTECs. γδT cells diverge from the αβT cell lineage at the DN stage, and their repertoire formation is regulated by cTECs through unknown mechanisms. The cortical microenvironment is also important for positive selection of iNKT cells that depends on cell–cell interaction among DP thymocytes. In the medulla, SP thymocytes are screened for self-reactivity. SP thymocytes reactive to TRAs presented by mTECs or DCs are deleted by negative selection or induced to differentiate into Foxp3+ Treg cells
In addition to the mainstream conventional αβT cell development, the thymus also supports the development of unconventional (non-classical) T cells. γδT cells form a distinct T cell lineage expressing γδTCR that recognizes native non-peptide and peptide antigens such as stress-induced proteins. αβT and γδT cell development diverge at the DN2 and DN3 stages. Unlike αβT cell development, γδT cell development does not require antigen-specific interactions in the thymus [10]. γδT cell subsets expressing different TCR-Vγ chains are generated at defined periods during ontogeny and distribute to different epithelial and mucosal tissues [11]. Invariant natural killer T (iNKT) cells represent an unconventional αβT cell subset expressing invariant Vα14-Jα18 TCR that recognize glycolipid antigens presented by MHC-like CD1d molecules, and play roles in controlling innate and adaptive immune responses [12]. These iNKT cells are positively selected by CD1d/glycolipid complexes expressed on the surface of DP thymocytes [13].
T cell development as described above is controlled in the thymic microenvironment, mainly by TECs, and in turn, developing T cells critically regulate the development of TECs, such that T cell immunity can be finely tuned for optimal immune responses.
Generation of thymic epithelium
Thymic epithelial cells are derived from endodermal epithelium from the third pharyngeal pouch [14]. Early TEC development is controlled by specific transcription factors including FoxN1 (Whn), Tbx1, and Pax1 [15, 16]. FoxN1 is a major mediator of TEC development and function, as FoxN1 deficiency completely disrupts thymic T cell development in animals and human [17–20]. FoxN1 regulates the transcription of various target genes essential for hematopoietic function of the thymus, including cytokines, chemokines, and Notch ligands. FoxN1 expression is detected in almost all TECs during embryogenesis [21], in a manner dependent on Wnt signaling [22], while a fraction of TECs from adult mice lose the expression of FoxN1 but maintain the expression of its target genes [23], suggesting a FoxN1-independent mechanism for maintenance and function of postnatal TECs.
Other factors that regulate TEC development include the transcription factor p63 and its interacting partner Polycomb protein Cbx4. Both are strongly expressed in TECs and required for proliferation and maintenance of cTECs and mTECs [24–26].
Thymic cortex
cTEC development
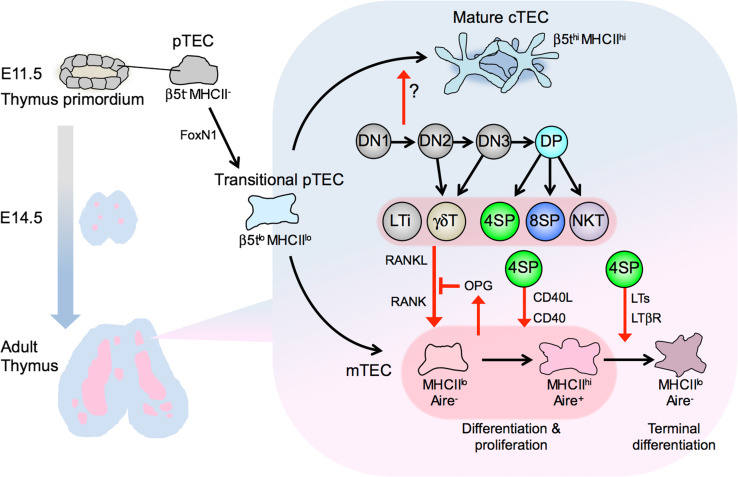
The stromal architecture in the thymic cortex is mainly composed of cTECs. cTECs can be identified by expression of marker proteins such as Keratin-8, Keratin-18, Cerebellar degeneration-related antigen 1 (CDR1), CD205, CD249 (Ly51), Interleukin (IL)-7, the thymoproteasome subunit β5t, and the atypical non-signaling chemokine receptor CCRL1 (CCX-CKR1). In contrast, mTECs are characterized by a different set of markers such as Keratin-5, Keratin-14, CD80, and Aire. Both cTECs and mTECs are derived from common endodermal progenitor cells identified in the third pharyngeal pouch [27, 28]. These common TEC progenitors progress to a transitional progenitor stage, a process dependent on the transcription factor FoxN1 [29, 30]. Such transitional TEC progenitors express cTEC-associated genes such as CD205, β5t, CCRL1, and IL-7, and give rise to both cTECs and mTECs, including the Aire+ subset [30–33]. It is still unclear how cTEC and mTEC lineage determination progresses: whether asymmetrically, in that the transitional progenitors undergo maturation into cTECs by default or lose cTEC traits upon differentiation to mTECs, or symmetrically, in that the transitional progenitors coexpress cTEC- and mTEC-associating genes and acquire enhanced expression of lineage-associating genes or lose ones for another lineage during lineage determination [34].
Generation of transitional TEC progenitors does not require lymphocyte-derived signals, since Keratin-8+ cells are detected in Rag2/γc double-deficient mice, in which the development of T, B, and NK cells is completely impaired [35], and β5t expression in TECs is readily detectable in CD3εTg26 mice, in which thymocyte development arrests at DN1 due to the unidentified mechanism caused by genomic insertion of multiple copies of a human CD3ε transgene [36]. The expression of cTEC-specific markers or functional proteins, such as CD249, CCRL1, β5t and MHC class II, gradually increases along cTEC ontogeny [29, 33, 36, 37]. Mature MHC class IIhi cTECs are detectable in Rag1-deficient mice (arrested at DN3) but not in CD3εTg26 mice (arrested at DN1) [29], indicating that the functional maturation of cTECs requires thymocyte development beyond the DN1 stage. This is consistent with an early report that the meshwork architecture of thymic cortical epithelium is disturbed in CD3εTg26 mice [38]. Maturation of cTECs was restored in CD3εTg26 mice by transfer of wild-type T-progenitor cells [39]. Together, these results indicate that maturation of cTECs requires signals delivered by developing thymocytes, likely through as of yet unidentified intercellular signals (Fig. 2).
Fig. 2.
Development of thymic epithelial cells is induced by various subsets of thymocytes. cTECs and mTECs arise from common progenitor TECs (pTECs) in endodermal epithelium from the third pharyngeal pouch. pTECs differentiate into ‘transitional’ pTECs that express cTEC-associated genes such as β5t and IL-7. This process is critically regulated by the transcription factor FoxN1 but independent of lymphocytes. Thymocyte development beyond DN1 induces maturation of cTECs expressing high levels of β5t. The development of mTECs is triggered in embryonic thymus by LTi cells and γδT cells that express RANKL. In postnatal thymus, SP thymocytes and NKT cells express RANKL to promote the differentiation and proliferation of Aire-expressing mTECs. RANKL-stimulated mTECs produce OPG to self-tune their development. CD40L expressed in CD4SP thymocytes cooperates with RANKL to promote mTEC development. CD4SP thymocytes also express LTs, which induce terminal differentiation of mTECs
Early T cell development and migration in the cortex
Cortical TECs are the predominant source of Notch ligands, cytokines, and chemokines required for early T cell development. Delta-like 4 (Dll4), a Notch ligand expressed by cTECs, is essential and sufficient for T-lineage determination of early lymphoid progenitors in the thymus [40–42]. IL-7 is also predominantly produced by cTECs [43] and promotes survival, proliferation, and differentiation of thymocytes [44–46]. Outward migration of DN thymocytes from the cortico-medullary junction to the subcapsular region is mediated by chemokines CCL25 and CXCL12, produced by cTECs, and their receptors CCR9 and CXCR4, respectively, expressed on DN thymocytes [47–50]. CXCL12-CXCR4 signaling also promotes β selection [51]. CCRL1, an atypical non-signaling chemokine receptor highly expressed in cTECs [52], promotes outward migration of DN thymocytes via still-unknown mechanisms [53, 54]. Vascular-cell adhesion molecule-1 (VCAM-1), expressed by cTECs, and its receptor integrins α4β1 and α4β7, expressed by DN thymocytes, are also important for intimate stromal interaction and outward migration of DN thymocytes [55]. DN thymocytes turn back inward and differentiate into DP thymocytes at the subcapsular region, where transforming growth factor (TGF) β is expressed and exerts negative feedback on DN to DP differentiation [56].
The majority of DP thymocytes move randomly in the cortex, likely scanning pMHC ligands for with newly generated TCR in the cortical microenvironment [57]. Successful TCR interaction with pMHC ligands leads to a stop of the ‘random walk’ migration and a prolonged duration of thymocyte–stromal interaction [58], which is required for efficient positive selection. Indeed, DP thymocytes with enhanced migratory activity to cortical chemokines CCL25 and CXCL12 in vitro and dysregulated migration in in vivo thymic cortex undergo less positive selection but unaltered negative selection [59, 60], indicating that properly regulated migration and stromal interaction of DP thymocytes is distinctly required for positive selection.
Positive selection in the cortex
The most recognized function of cTECs is the induction of T cell-positive selection. As described above, low affinity TCR engagement by pMHC complexes induces positive selection of functional T cells, whereas high affinity TCR-pMHC interaction leads to negative selection of self-reactive (potentially harmful) T cells. Recent studies support the idea that cTECs have unique proteolytic and antigen-processing capabilities to produce MHC-associating peptides that are essential for positive selection.
For the MHC class I system, cTECs are equipped with a unique type of proteasome. Proteasomes are multi-subunit protease complexes responsible for producing MHC class I-associating peptides as well as for turnover of intracellular proteins [61]. Peptides with C-terminal hydrophobic anchor residues are produced by chymotrypsin-like activity of the proteasomes, which is mediated by β5 catalytic subunits. Unlike most somatic cells that express ‘standard proteasomes’ containing β5 subunits or immune cells and interferon (IFN) γ-stimulated cells that express β5i subunit-containing ‘immunoproteasomes’ [62, 63], cTECs express a specialized type of proteasome, called a ‘thymoproteasome’, that contains the β5t subunit [64, 65]. β5t is exclusively expressed by cTECs throughout the lifespan of mice [36], thus representing a specific marker of cTECs. In mice deficient for β5t, cTECs express β5- and β5i-containing proteasomes and display a spectrum of MHC class I-associating peptides that are different from those in β5t-sufficient cTECs [66, 67]. In these mice, positive selection of MHC class I-restricted thymocytes is substantially reduced, leading to a marked reduction (20 % of wild-type) and altered repertoire of CD8 T cells, indicating that optimal positive selection of CD8 T cells requires the β5t-dependent peptide repertoire in cTECs. The β5t-dependent peptides are also essential for functionally conditioning antigen responsiveness of positively selected CD8 T cells [68]. A recent study identified unique cleavage motifs in β5t-dependent MHC class I-associating peptides that confer low affinity TCR interaction and capabilities to efficiently induce positive selection [69]. This uniqueness of the peptide motifs might be attributed to the peptide cleavage preference between β5t and the other subunits β5 and β5i [64, 69]. Collectively, these aspects indicate that cTECs regulate positive selection of CD8 T cells by producing a unique set of MHC class I-associating peptides that exhibit low affinity for TCR [70].
In the MHC class II system, various lysosomal proteases produce peptide antigens [71]. cTECs highly express lysosomal proteases cathepsin L and thymus-specific serine protease (TSSP) [71, 72]. Mice deficient for cathepsin L show a reduced positive selection of polyclonal CD4 T cells [73, 74]. TSSP-deficient mice show a defective positive selection of CD4 T cells with certain TCR specificities [75, 76], including diabetogenic self-reactive CD4 T cells [77]. It was also shown that cTECs exhibit high levels of constitutive macroautophagy [78], a cellular process that facilitates loading of endogenously generated peptides onto MHC class II molecules. Mice with defective macroautophagy induction, specifically in TECs, show altered repertoire selection of certain CD4 T cells [79]. These data strongly support the idea that cTECs have unique protein degradation and antigen-processing machineries for inducing positive selection of CD4 T cells, although the nature of MHC class II-associating peptides produced by cTECs remains to be elucidated.
Negative selection in the cortex
Thymic cortex is also the place where self-reactive thymocytes are deleted by negative selection. A recent study estimated that nearly 6 times as many thymocytes undergo negative selection compared with positive selection, and 75 % of negative selection occurs in the cortex [79], most frequently in the inner cortical region [80]. However, negative selection, by any experimental model tested, was observed to be normal in β5t-deficient mice [66] and TSSP-deficient mice [75], indicating that cTEC-specific peptides are not required for cortical negative selection. Involvement of cTECs in negative selection in the cortex was also challenged by our recent finding that negative selection was not affected in the TN mutant mice that intrinsically lack mature cTECs (see below) [37]. Rather, it is the cortex-resident DCs that appear to be responsible for negative selection in the cortex [80].
Thymic nurse cell
Recently, a long-argued topic in cTEC function was revisited. In 1980, a group reported the discovery of unique multicellular complexes in cell suspensions prepared by enzymatically dissociating thymus tissues [81, 82]. These complexes were termed ‘thymic nurse cells’ (TNC) for the large thymic epithelial cells that had engulfed multiple (up to 50) living lymphocytes within their intracellular vesicles. These studies, as well as many later studies (reviewed in [83]), hypothesized that TNCs provide a unique microenvironment for T cell selection, although the precise cell lineage and function of TNC-forming thymic epithelium had long remained elusive. A recent report found that approximately 10–15 % of β5t-expressing cTECs, but not mTECs, form thymocyte-wrapping complexes in adult mouse thymus that are identical to previously described TNCs [84]. The formation of TNC requires normal development of cTECs, as cTEC-deficient mice have no TNCs in the thymus ([37] and our unpublished data). TNCs are poorly formed in embryonic thymus from normal mice or in adult thymus from ‘positive-selector’ TCR transgenic mice, but readily detectable in the ‘null-selector’ mouse thymus. The majority of TNC-enveloped lymphocytes are long-lived, unselected DP thymocytes undergoing secondary TCRα-VJ rearrangements. Thus, TNCs are formed upon persistent cTEC-DP thymocyte interactions and facilitate secondary TCRα rearrangements. Given that the efficiency of secondary TCRα rearrangements is controlled by DP thymocyte survival [85], the microenvironments within intra-TNC vesicles may ensure survival of enclosed DP thymocytes. Secondary TCRα rearrangement is required for multiplying the opportunities for positive selection and thereby maximizing the developmental efficiency of functional T cells [86]. The mechanisms by which unselected thymocytes are enclosed into and positively selected thymocytes are released from the TNC complexes, and how intra-TNC microenvironments promote survival and/or continued TCR rearrangement in DP thymocytes, remain to be studied.
Taken together, SP thymocytes that passed positive and negative selection in the cortex set out on a new journey toward the medulla, to be further screened for TCR reactivity to self.
New aspects of cTEC function: unconventional T cell development
To date, a few studies have reported mutant mice lacking normal cTEC development. Preferential loss of cTECs and disorganized thymic cortical architecture were observed in Keratin-5-driven Stat3-deficient mice [87], Eph4-deficient mice [88], and transgenic insertional mutant mice called Tg66 [89], although the molecular basis of the cTEC deficiency in these mice remains unclear. A study using mice transgenic for the human diphtheria toxin receptor under the control of the CCRL1 promoter demonstrated that diphtheria toxin-inducible depletion of cTECs resulted in nearly complete loss of DN and DP thymocytes, confirming that cTECs are essential for thymic cortical architecture and thereby maintenance of cortical thymocytes [52].
Recently, we established a spontaneous mutant mouse line, called TN that exhibits an almost complete loss of mature cTECs yet only a modest effect on mTECs [37]. A missense mutation in the gene encoding β5t was responsible for this phenotype. The mutant β5t inhibits normal proteasome assembly and cell survival, resulting in substantial loss of β5thigh mature cTECs and accumulation of β5tlow transitional TEC progenitors. Therefore, the TN mouse is a novel animal model that intrinsically and specifically lacks mature cTECs. The thymus from TN mice shows a disorganized cortical architecture, massive loss of thymic cellularity, impaired positive selection, and altered αβTCR repertoire: all in agreement with the above-described functions of cTECs in forming the cortical microenvironment and inducing positive selection. cTEC deficiency also caused a reduction in iNKT cell development, possibly due to inefficient cell–cell interaction among DP thymocytes in the disorganized cortical microenvironment.
The most unexpected and significant finding from the study of TN mice is the influence of mature cTEC deficiency on development and repertoire formation of γδT cells. It has been known that thymic development of γδT cell subsets is ontogenically regulated and that γδT subsets show different tissue distribution and effector functions [11]. Recent studies have highlighted an IL-17-producing subset of γδT (γδT17) cells, which includes Vγ4+ and Vγ6+ cells in mice, as being essential for various infections, inflammations, and malignancies [90], although regulation of thymic development of γδT17 cells remains unclear. In the thymus from cTEC-deficient TN mice, while the frequency of total γδT cells is unaltered, the proportion of γδT17 cells is greatly increased [37]. Among these γδT17 cells, the Vγ6+ subset robustly increased, whereas the Vγ4+ subset decreased, resulting in the marked skewing from Vγ4 to Vγ6 in the TCR repertoire of γδT17 cells and the perturbation of γδT17-dependent inflammatory responses in peripheral tissues. The γδT17 repertoire is unaffected by β5t deficiency and mTEC development. Thus, normal cTEC development contributes to optimal repertoire formation not only of conventional αβT cells but also of unconventional ‘innate type’ γδT cells. The thymus from TN mice may provide a ‘fetal type’ microenvironment that specifically supports the predominant development of Vγ6+ γδT17 cells [91, 92]. It is also possible that the thymus lacking mature cTECs has altered expression of as of yet unidentified selecting ligand molecule(s) or cell-surface proteins that mediate differentiation or deletion of γδT17 cell subsets: for example, as mTECs regulate development of Vγ5+Vδ1+ γδT cells via expression of a B7-family protein called ‘selection and upkeep of intraepithelial T cells (Skint1)’ [93].
Thymic medulla
mTEC development
Medullary TECs emerge from TEC progenitors expressing cTEC-associated genes, and are distinguished by the expression of proteins such as Keratin-5, Keratin-14, CD80, Aire, Claudin-3, and Claudin-4 and their reactivity with the fucose-binding lectin Ulex europaeus agglutinin 1 (UEA1) [94, 95]. mTECs are further classified into two subsets, mTEChi (MHC class IIhi CD80hi) cells and mTEClo (MHC class IIlo CD80lo) cells [94], and the mTEChi cells represent functionally mature mTECs expressing Aire (Fig. 2). Several reports show that mTEClo cells can give rise to mTEChi cells [96–98], but recent lineage tracing studies show that Aire+ mTEChi cells progress to an Aire− mTEClo stage [99, 100], indicating that mTEClo is a heterogeneous cell population including developing immature mTECs and developed mature ‘post-Aire’ mTECs. The ‘post-Aire’ mTEClo cells represent a distinct mTEC subpopulation expressing chemokines such as CCL21 [101]. This cell subset also includes terminally differentiated mTECs, characterized by the expression of Involucrin and the stratified squamous epithelia resembling Hassall’s corpuscles, as observed in the human thymus [102, 103].
Early studies, mostly conducted in the 1990s, indicated that thymic medulla formation is defective in mice with T cell development arrested at early stages [104]. Particularly, mice deficient for positive selection showed a marked reduction of thymic medullary regions and mTEC cellularity without affecting overall thymus size and cortical architecture [94, 105–107], indicating that the positively selected SP thymocytes induce the development of mTECs, which, in turn, provide a microenvironment for selection and maturation of SP thymocytes. This mTEC-thymocyte interdependency is referred to as ‘thymic crosstalk’.
Over a span of two decades, a series of studies has revealed that the signaling pathways for the activation of nuclear factor-κB (NF-κB) are required for mTEC development. Mice deficient for TNF receptor-associated factor 6 (TRAF6), NF-κB-inducing kinase (NIK), IκB-kinase α (IKK α), Bcl-3, NF-κB2 (p52), or RelB, exhibit defective development of Aire+ mTECs and thymic medulla formation in an mTEC-autonomous manner [108–116]. These NF-κB pathways for thymic medulla formation are activated by TNFR superfamily receptors, receptor for activating NF-κB (RANK), CD40, and lymphotoxin β receptor (LTβR), expressed on mTECs, and their TNF superfamily ligands RANKL, CD40L, and lymphotoxins (LTs), respectively, are expressed by lymphoid cells, mostly SP thymocytes [117–120]. This configuration of receptor-ligand expression provides an explanation for early observations of ‘thymic crosstalk’ and mechanism for later findings of NF-κB involvement. Indeed, TNF superfamily ligand-mediated mTEC development is ensured by TCR–ligand interactions between self-reactive SP thymocytes and mTECs [121–125].
RANKL, a major mediator of mTEC development, is produced by lymphoid tissue inducer (LTi) cells and γδT cells in the embryonic thymus and by SP thymocytes and iNKT cells in postnatal thymus [118, 120, 126, 127] (Fig. 2). CD40L and LTs are expressed predominantly by SP thymocytes [117, 120, 126]. These TNFSF ligands have cooperative as well as distinct non-redundant functions in mTEC development. RANKL and CD40L synergistically promote development and proliferation of Aire+ mTECs [119, 120], while LTs regulate the development of a distinct subset of mTECs expressing CCL21 [101, 128, 129]. LT signals also regulate the expression of RANK in mTECs [130] and the terminal differentiation of mTECs [103].
RANKL signaling in mTECs up-regulates the transcription factor Spi-B, which in turn induces the expression of some TRAs, co-stimulatory molecules, and osteoprotegerin (OPG) [131]. OPG is an inhibitory decoy receptor for RANKL and represses RANKL-mediated mTEC development and expansion [98, 120, 131]. The fact that mTEC development is primarily dependent on interaction with SP thymocytes and controlled by the RANKL-OPG negative feedback system indicates that the cellularity and function of mTECs must be properly adjusted, such that self-reactive SP thymocytes can be moderately, not excessively, deleted in the thymic medulla.
mTEC development is promoted by coordination between RANKL and type I interferon signals [132] and negatively regulated by TGFβ signaling [133]. It was also reported that the microRNA production by the endoribonuclease Dicer, and specifically microRNA miR-29a, was essential for postnatal maintenance of mTECs [134, 135].
Medulla migration and emigration of thymocytes
Double positive thymocytes that received positive selection signals differentiate into CD4SP or CD8SP thymocytes and express the chemokine receptor CCR7 on the cell surface [136]. CCR7 ligand chemokines, CCL19 and CCL21, are produced by mTECs [136] and medullary fibroblasts [137], and attract CCR7-expressing SP thymocytes from the cortex to the medulla [136, 138, 139]. During medullary residency, which is estimated to be 4–5 days [140], SP thymocytes are exposed to antigens presented by mTECs and DCs. CCR7-mediated medullary migration is required to ensure negative selection of self-reactive SP thymocytes [128, 141]. Indeed, mice deficient for CCR7 or CCR7 ligand chemokines exhibit organ-specific autoimmunity [139, 142, 143]. CCR7 signals also direct the migration of γδT cells to the medulla [144]. mTECs produce another chemokine XCL1 that mediates medullary accumulation of thymus-resident DCs [145].
SP thymocytes that have completed developmental programs and repertoire selection are exported from the thymus into circulation. Export of thymocytes is controlled by chemotactic signaling via sphingosine-1 phosphate (S1P) and its receptor S1PR1. Mature SP thymocytes express high levels of S1PR1 and then migrate toward a gradient of S1P [139, 146, 147], which is provided by neural crest-derived perivascular cells (pericytes) in the cortico-medullary junctions [148] and circulating blood [149]. However, the mechanism that determines the timing of thymocyte emigration such that only mature yet self-tolerant SP thymocytes are permitted to exit the thymus remains largely unclear. It is speculated that, in mature SP thymocytes that have completed self-reactivity screening, cessation of TCR signaling leads to down-regulation of CD69—an inhibitor of S1PR1 surface expression—resulting in up-regulation of S1PR1 expression, thereby rendering SP thymocytes responsive to S1P and primed for thymic exit [150].
TRA expression by mTECs
In the medulla, a diverse array of TRAs—whose expression is primarily restricted to peripheral tissues—are transcribed in mTECs, particularly mTEChi cells [151–154], in a phenomenon termed ‘promiscuous gene expression’. SP thymocytes reactive to these TRAs are ejected from the conventional T cell pool through deletion by negative selection or differentiation to Foxp3+ Tregs (see below). As shown by many studies, T cells produced in mice lacking normal mTEC development caused autoimmune disorders, indicating that mTECs are essential for establishing central tolerance [99, 109, 111–119, 155]. TRA expression represents a mosaic pattern, as each TRA protein is expressed in only 1–3 % of mTECs, such that a maximal number and sufficient epitope density of TRAs can be displayed to SP thymocytes [7]. A single mTEC expresses a set of TRA genes, which are clustered in chromosomes and colocalized to nuclear subdomains [156].
A substantial fraction of TRAs is controlled by Aire [157], a nuclear protein predominantly expressed in mTECs [158–160]. Aire-driven TRA expression is crucial for negative selection of TRA-reactive SP thymocytes [161–163] and generation of Foxp3+ Tregs [164–166] in the medulla. Genetic deficiency of Aire results in autoimmune polyendocrinopathy syndrome type 1 (APS1) or autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APECED) in human [167, 168], and similar organ-specific autoimmune disorders in mice [157, 169, 170], indicating that Aire is essential for establishment of self-tolerance. Accruing evidence indicates that Aire has no obvious DNA binding domain [171, 172] but instead epigenetically regulates transcriptional elongation and pre-mRNA processing of target TRA genes [154, 173–175]. However, some groups propose another mechanistic view whereby Aire controls the differentiation program of mTECs to enable TRA expression. This is because Aire deficiency in mice causes abnormal medulla organization and mTEC development [97, 100, 102, 176–178] and defective T cell tolerance against transcriptionally unrepressed TRAs [163, 170]. Indeed, Aire also regulates expression of a large number of non-TRA proteins such as cytokines, chemokines, MHC class II peptide-loading factors, posttranslational modifiers, and proteases [145, 163, 179–181]. Several studies showed that Aire regulated the expression of some microRNAs, including the miR-376 family members, which were located in the genome within an Aire-dependent TRA gene [182], and miR-29a, which affected Aire-dependent TRA expression and maintenance of TEC cellularity in aged mice [134, 183].
Also, it should be noted that more than half (60 %, estimated by [181]; 64 %, estimated by [184]) of total TRAs expressed in mTECs are Aire-independent [154, 157], indicating that additional transcriptional or epigenetic mechanisms must be responsible for the induction of Aire-independent gene expression in mTECs. Some Aire-independent TRAs are regulated by LTβR signaling [129, 185], although the downstream regulator(s) remain to be identified.
Although mTEC-dependent tolerance induction is essential for protection against autoimmunity, this system has a possible demerit in anti-tumor immunity. Because self-antigens expressed in mTECs include tumor-associated antigens, tumor-specific T cells can be deleted in the thymic medulla and fail to reach peripheral targets [165, 186–188]. Given the double-edged potential of mTECs and the medullary microenvironment, it seems reasonable that the capacity and function of the thymic medulla is finely modulated by the RANKL-OPG feedback mechanism [98, 120, 131]. It has been experimentally shown that suppression of RANKL-mediated mTEC development and maintenance can rescue tumor-specific T cells from medullary deletion and attenuate tumor progression in mice [98, 131]. Further study may lead to the development of new therapeutic approaches to control T cell tolerance and anti-tumor immunity.
mTEC-DC interactions for central tolerance
In addition to mTECs as the lead player, thymic DCs also play a pivotal role in inducing T cell tolerance in the thymic medulla. Thymic DCs are predominantly localized in the medulla, with a small fraction sparsely localized in the cortex [138, 144]. As well as peripheral DCs, thymic DCs are derived from hematopoietic precursor cells [189], some through intrathymic differentiation and others from peripheral circulation [190].
It has been shown that thymic DCs contribute to T cell tolerance, through direct presentation of endogenously expressed antigens and indirect presentation of antigens expressed by other cells. Mtv-encoded superantigens as well as TRAs expressed by mTECs and blood-borne antigens can be presented by thymic DCs to developing thymocytes, to induce negative selection [191–194]. Furthermore, a subpopulation of peripheral DCs can be recruited to the thymic medulla and present peripheral antigens to induce negative selection [190, 195, 196].
Antigen presentation by mTECs and thymic DCs also induces development of Foxp3+ Treg cells, which are essential for protection from autoimmunity [197, 198]. Foxp3+ Treg cells differentiate from Foxp3− CD25+ CD4SP or Foxp3+ CD25− CD4SP precursor cells, a process that requires cytokine signals and TCR-CD28 co-stimulatory signals [199–201]. In the thymus, the majority of Foxp3+ Treg cells are detected in the medulla [164, 202], where mTECs and DCs present antigens with co-stimulatory molecules. In mice deficient for mTEC development or expression of MHC class II on mTECs, thymic development of Foxp3+ Treg cells is impaired [111, 112, 116, 203]. Studies using neo-self antigen transgenic mice showed the generation of Foxp3+ Treg cells specific for self-antigen expressed by Aire+ mTECs [164, 204]. It was also reported that Foxp3+ Treg cells reactive to endogenous self-antigens are generated in an Aire-dependent manner [165, 166]. These data provide a link between Aire-dependent TRA expression and development of TRA-specific Treg cells. A recent report estimated that a substantial portion (about half) of Aire-dependent negative selection and Treg development are mediated by indirect presentation of TRAs by thymic DCs [205]. This mTEC-DC cooperation might be dependent on unidirectional, intercellular transfer of mTEC-derived proteins to DCs [206, 207]. For optimal Treg cell induction, these tripartite interactions among mTECs, DCs, and CD4SP thymocytes, require medullary accumulation of thymic DCs, which depends on the chemokine XCL1 [145], as well as CCR7-mediated medullary migration of CD4SP thymocytes [208]. Thymic DCs can also induce development of Foxp3+ Tregs reactive to blood-borne antigens [194, 195].
Unique swirled epithelial structures composed of terminally differentiated mTECs, called ‘Hassall’s corpuscles’, may provide the microenvironment for the generation of Treg cells. Hassall’s corpuscles produce thymic stromal lymphopoietin (TSLP) [209], which was shown to activate immature thymic DCs to promote the expression of co-stimulatory molecules [210, 211]. TSLP-activated thymic DCs induce differentiation of CD4SP thymocytes into Foxp3+ Tregs [209].
The number of thymic Foxp3+ Tregs is likely controlled by the mTEC cellularity and size of the medulla, as the thymus from OPG-deficient mice contains the increased number of Foxp3+ Tregs [131]. A recent report showed that the increased Foxp3+ Tregs in OPG-deficient thymus included a substantial number of recirculating Tregs that re-entered the thymus from the periphery [212], suggesting that mTECs provide intrathymic niches for peripheral Tregs.
Unconventional T cell development in the medulla
Medullary TECs play a role in γδT cell development, in a manner different from that of cTECs. mTECs from fetal thymus express Skint1, a B7-family protein required for intrathymic maturation of Vγ5Vδ1+ epidermal γδT cells [93, 213, 214]. Skint1 is considered to induce strong, agonist-like signals to Vγ5Vδ1 TCR and a differentiation program toward an IFNγ-producing lineage [215]. Given that cTECs and mTECs regulate distinct subpopulations of γδT cells, it is possible that cTECs and mTECs provide a distinct set of putative ligands or selecting molecules for modulating γδT cell immunity. In fetal mouse thymus, Vγ5Vδ1+ γδT cells closely associate with mTECs, and foster Aire+ mTEC development by expression of RANKL [93], implying a bidirectional crosstalk as well between RANKL-expressing γδT cells and Skint1-expressing mTECs.
It was also shown that mTECs were required for optimal maturation of iNKT cells and that developing iNKT cells express RANKL and CD40L to promote development of Aire+ mTECs [127], suggesting that thymic crosstalk interactions also occur between iNKT cells and mTECs, although the intrathymic distribution of developing iNKT cells remains to be determined. Mature iNKT cells express the chemokine receptor CXCR3, which is required for thymic retention in response to its ligand CXCL10 produced in the medulla [216].
A series of studies demonstrated that the thymic medulla supports the development of natural IL-17-producing T helper (nTh17) cells, a recently described unconventional CD4+ αβT cell subset that potentially contributes to protective and pathological inflammatory responses. Intrathymic development of nTh17 cells requires MHC class II expression on mTECs but not on cTECs [217], and is induced by self-antigen recognition and the cytokines IL-6 and TGFβ [218]. It was also reported that RelB-dependent Aire+ mTECs are required for nTh17 cell development [219], suggesting a novel mTEC-mediated regulatory mechanism of inflammatory and autoimmune responses.
Concluding remarks
Here, we focused on the developmental mechanisms and functions of thymic stromal cells—namely, TECs. cTECs and mTECs are derived from common progenitors and upon differentiation and maturation acquire distinct functional characteristics essential for supporting T cell development. cTECs shape the functional T cell repertoire through positive selection, whereas mTECs trim the self-reactive repertoire through negative selection and cell fate conversion into Tregs. The development of TEC subsets is largely dependent on the signals from developing T cells, and these crosstalk interactions are indispensable for organization and fine-tuning of the thymic microenvironment. It should also be noted that cTECs and mTECs are important for the development not only of conventional T cells but also of unconventional T cells that bridge innate and adaptive immunity. Given this cellular and molecular basis for orchestrating the development and function of thymic stromal cells, current and forthcoming studies will provide invaluable information toward in vivo regeneration and reconstitution of thymic tissue for future therapeutic application.
Acknowledgments
This work was supported by Grants to T.N. from the Ministry of Education, Culture, Sports, and Technology in Japan (25111516, 25460606), National Center for Global Health and Medicine (24-112), Astellas Foundation, Inamori Foundation, Kanae Foundation, Naito Foundation, and Ichiro Kanehara Foundation.
Contributor Information
Takeshi Nitta, Email: nit-im@m.u-tokyo.ac.jp.
Harumi Suzuki, Email: hsuzuki@ri.ncgm.go.jp.
References
- 1.Von Boehmer H. Selection of the T-cell repertoire: receptor-controlled checkpoints in T-cell development. Adv Immunol. 2004;84(84):201–238. doi: 10.1016/S0065-2776(04)84006-9. [DOI] [PubMed] [Google Scholar]
- 2.Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate ‘decisions’ and effector function. Nat Immunol. 2014;15(9):815–823. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller JF. Immunological function of thymus. Lancet. 1961;2(720):748–749. doi: 10.1016/S0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 4.Boyd RL, Tucek CL, Godfrey DI, Izon DJ, Wilson TJ, Davidson NJ, Bean AGD, Ladyman HM, Ritter MA, Hugo P. The thymic microenvironment. Immunol Today. 1993;14(9):445–459. doi: 10.1016/0167-5699(93)90248-J. [DOI] [PubMed] [Google Scholar]
- 5.Gray DH, Ueno T, Chidgey AP, Malin M, Goldberg GL, Takahama Y, Boyd RL. Controlling the thymic microenvironment. Curr Opin Immunol. 2005;17(2):137–143. doi: 10.1016/j.coi.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Anderson G, Takahama Y. Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol. 2012;33(6):256–263. doi: 10.1016/j.it.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014;14(6):377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhandoola A, Sambandam A, Allman D, Meraz A, Schwarz B. Early T lineage progenitors: new insights, but old questions remain. J Immunol. 2003;171(11):5653–5658. doi: 10.4049/jimmunol.171.11.5653. [DOI] [PubMed] [Google Scholar]
- 9.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6(2):127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 10.Jensen KDC, Su X, Shin S, Li L, Youssef S, Yarnasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien Y-H. Thymic selection determines gamma delta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29(1):90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carding SR, Egan PJ. gamma delta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2(5):336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 12.Kronenberg M, Engel I. On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Curr Opin Immunol. 2007;19(2):186–193. doi: 10.1016/j.coi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CDId. Nat Immunol. 2001;2(10):971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 14.Gordon J, Wilson VA, Blair NF, Sheridan J, Farley A, Wilson L, Manley NR, Blackburn CC. Functional evidence for a single endodermal origin for the thymic epithelium. Nat Immunol. 2004;5(5):546–553. doi: 10.1038/ni1064. [DOI] [PubMed] [Google Scholar]
- 15.Anderson G, Lane PJL, Jenkinson EJ. Generating intrathymic microenvironments to establish T-cell tolerance. Nat Rev Immunol. 2007;7(12):954–963. doi: 10.1038/nri2187. [DOI] [PubMed] [Google Scholar]
- 16.Boehm T. Thymus development and function. Curr Opin Immunol. 2008;20(2):178–184. doi: 10.1016/j.coi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the Winged-Helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372(6501):103–107. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- 18.Blackburn CC, Augustine CL, Li R, Harvey RP, Malin MA, Boyd RL, Miller JFAP, Morahan G. The nu gene acts cell-autonomously and is required for differentiation of thymic epithelial progenitors. Proc Natl Acad Sci USA. 1996;93(12):5742–5746. doi: 10.1073/pnas.93.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nehls M, Kyewski B, Messerle M, Waldschutz R, Schuddekopf K, Smith AJH, Boehm T. Two genetically separable steps in the differentiation of thymic epithelium. Science. 1996;272(5263):886–889. doi: 10.1126/science.272.5263.886. [DOI] [PubMed] [Google Scholar]
- 20.Vigliano I, Gorrese M, Fusco A, Vitiello L, Amorosi S, Panico L, Ursini MV, Calcagno G, Racioppi L, Del Vecchio L, Pignata C. FOXN1 mutation abrogates prenatal T-cell development in humans. J Med Genet. 2011;48(6):413–416. doi: 10.1136/jmg.2011.089532. [DOI] [PubMed] [Google Scholar]
- 21.Zuklys S, Gill J, Keller MP, Hauri-Hohl M, Zhanybekova S, Balciunaite G, Na KJ, Jeker LT, Hafen K, Tsukamoto N, Amagai T, Taketo MM, Krenger W, Hollander GA. Stabilized beta-catenin in thymic epithelial cells blocks thymus development and function. J Immunol. 2009;182(5):2997–3007. doi: 10.4049/jimmunol.0713723. [DOI] [PubMed] [Google Scholar]
- 22.Balciunaite G, Keller MP, Balciunaite E, Piali L, Zuklys S, Mathieu YD, Gill J, Boyd R, Sussman DJ, Hollander GA. Wnt glycoproteins regulate the expression of FoxNI, the gene defective in nude mice. Nat Immunol. 2002;3(11):1102–1108. doi: 10.1038/ni850. [DOI] [PubMed] [Google Scholar]
- 23.Itoi M, Tsukamoto N, Amagai T. Expression of DII4 and CCL25 in Foxn1-negative epithelial cells in the post-natal thymus. Int Immunol. 2007;19(2):127–132. doi: 10.1093/intimm/dxl129. [DOI] [PubMed] [Google Scholar]
- 24.Senoo M, Pinto F, Crum CP, McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129(3):523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 25.Candi E, Rufini A, Terrinoni A, Giamboi-Miraglia A, Lena AM, Mantovani R, Knight R, Melino G. Delta Np63 regulates thymic development through enhanced expression of FgfR2 and Jag2. Proc Natl Acad Sci USA. 2007;104(29):11999–12004. doi: 10.1073/pnas.0703458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu B, Liu YF, Du YR, Mardaryev AN, Yang W, Chen H, Xu ZM, Xu CQ, Zhang XR, Botchkarev VA, Zhang Y, Xu GL. Cbx4 regulates the proliferation of thymic epithelial cells and thymus function. Development. 2013;140(4):780–788. doi: 10.1242/dev.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi SW, Jenkinson WE, Anderson G, Jenkinson EJ. Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature. 2006;441(7096):988–991. doi: 10.1038/nature04813. [DOI] [PubMed] [Google Scholar]
- 28.Bleul CC, Corbeaux T, Reuter A, Fisch P, Monting JS, Boehm T. Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature. 2006;441(7096):992–996. doi: 10.1038/nature04850. [DOI] [PubMed] [Google Scholar]
- 29.Shakib S, Desanti GE, Jenkinson WE, Parnell SM, Jenkinson EJ, Anderson G. Checkpoints in the development of thymic cortical epithelial cells. J Immunol. 2009;182(1):130–137. doi: 10.4049/jimmunol.182.1.130. [DOI] [PubMed] [Google Scholar]
- 30.Baik S, Jenkinson EJ, Lane PJL, Anderson G, Jenkinson WE. Generation of both cortical and Aire(+) medullary thymic epithelial compartments from CD205(+) progenitors. Eur J Immunol. 2013;43(3):589–594. doi: 10.1002/eji.201243209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribeiro AR, Rodrigues PM, Meireles C, Di Santo JP, Alves NL. Thymocyte selection regulates the homeostasis of IL-7-expressing thymic cortical epithelial cells in vivo. J Immunol. 2013;191(3):1200–1209. doi: 10.4049/jimmunol.1203042. [DOI] [PubMed] [Google Scholar]
- 32.Ohigashi I, Zuklys S, Sakata M, Mayer CE, Zhanybekova S, Murata S, Tanaka K, Hollaender GA, Takahama Y. Aire-expressing thymic medullary epithelial cells originate from beta 5t-expressing progenitor cells. Proc Natl Acad Sci USA. 2013;110(24):9885–9890. doi: 10.1073/pnas.1301799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro AR, Meireles C, Rodrigues PM, Alves NL. Intermediate expression of CCRL1 reveals novel subpopulations of medullary thymic epithelial cells that emerge in the postnatal thymus. Eur J Immunol. 2014;44(10):2918–2924. doi: 10.1002/eji.201444585. [DOI] [PubMed] [Google Scholar]
- 34.Alves NL, Takahama Y, Ohigashi I, Ribeiro AR, Baik S, Anderson G, Jenkinson WE. Serial progression of cortical and medullary thymic epithelial microenvironments. Eur J Immunol. 2014;44(1):16–22. doi: 10.1002/eji.201344110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klug DB, Carter C, Gimenez-Conti IB, Richie ER. Cutting edge: thymocyte-independent and thymocyte-dependent phases of epithelial patterning in the fetal thymus. J Immunol. 2002;169(6):2842–2845. doi: 10.4049/jimmunol.169.6.2842. [DOI] [PubMed] [Google Scholar]
- 36.Ripen AM, Nitta T, Murata S, Tanaka K, Takahama Y. Ontogeny of thymic cortical epithelial cells expressing the thymoproteasome subunit beta 5t. Eur J Immunol. 2011;41(5):1278–1287. doi: 10.1002/eji.201041375. [DOI] [PubMed] [Google Scholar]
- 37.Nitta T, Muro R, Shimizu Y, Nitta S, Oda H, Ohte Y, Goto M, Yanobu-Takanashi R, Narita T, Takayanagi H, Yasuda H, Okamura T, Murata S, Suzuki H. The thymic cortical epithelium determines the TCR repertoire of IL-17-producing gamma delta T cells. EMBO Rep. 2015;16(5):638–653. doi: 10.15252/embr.201540096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollander GA, Wang B, Nichogiannopoulou A, Platenburg PP, van Ewijk W, Burakoff SJ, Gutierrez-Ramos JC, Terhorst C. Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature. 1995;373(6512):350–353. doi: 10.1038/373350a0. [DOI] [PubMed] [Google Scholar]
- 39.Roberts NA, Desanti GE, Withers DR, Scott HR, Jenkinson WE, Lane PJ, Jenkinson EJ, Anderson G. Absence of thymus crosstalk in the fetus does not preclude hematopoietic induction of a functional thymus in the adult. Eur J Immunol. 2009;39(9):2395–2402. doi: 10.1002/eji.200939501. [DOI] [PubMed] [Google Scholar]
- 40.Hozumi K, Mailhos C, Negishi N, Hirano K, Yahata T, Ando K, Zuklys S, Hollander GA, Shima DT, Habu S. Delta-like 4 is indispensable in thymic environment specific for T cell development. J Exp Med. 2008;205(11):2507–2513. doi: 10.1084/jem.20080134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch U, Fiorini E, Benedito R, Besseyrias V, Schuster-Gossler K, Pierres M, Manley NR, Duarte A, Macdonald HR, Radtke F. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J Exp Med. 2008;205(11):2515–2523. doi: 10.1084/jem.20080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calderon L, Boehm T. Synergistic, context-dependent, and hierarchical functions of epithelial components in thymic microenvironments. Cell. 2012;149(1):159–172. doi: 10.1016/j.cell.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 43.Hara T, Shitara S, Imai K, Miyachi H, Kitano S, Yao H, Tani-ichi S, Ikuta K. Identification of IL-7-producing cells in primary and secondary lymphoid organs using IL-7-GFP knock-in mice. J Immunol. 2012;189(4):1577–1584. doi: 10.4049/jimmunol.1200586. [DOI] [PubMed] [Google Scholar]
- 44.Moore TA, von Freeden-Jeffry U, Murray R, Zlotnik A. Inhibition of gamma delta T cell development and early thymocyte maturation in IL-7−/− mice. J Immunol. 1996;157(6):2366–2373. [PubMed] [Google Scholar]
- 45.Shitara S, Hara T, Liang B, Wagatsuma K, Zuklys S, Hollander GA, Nakase H, Chiba T, Tani-ichi S, Ikuta K. IL-7 produced by thymic epithelial cells plays a major role in the development of thymocytes and TCRgammadelta + intraepithelial lymphocytes. J Immunol. 2013;190(12):6173–6179. doi: 10.4049/jimmunol.1202573. [DOI] [PubMed] [Google Scholar]
- 46.Boudil A, Matei IR, Shih HY, Bogdanoski G, Yuan JS, Chang SG, Montpellier B, Kowalski PE, Voisin V, Bashir S, Bader GD, Krangel MS, Guidos CJ. IL-7 coordinates proliferation, differentiation and Tcra recombination during thymocyte beta-selection. Nat Immunol. 2015;16(4):397–405. doi: 10.1038/ni.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plotkin J, Prockop SE, Lepique A, Petrie HT. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J Immunol. 2003;171(9):4521–4527. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 48.Benz C, Heinzel K, Bleul CC. Homing of immature thymocytes to the subcapsular microenvironment within the thymus is not an absolute requirement for T cell development. Eur J Immunol. 2004;34(12):3652–3663. doi: 10.1002/eji.200425248. [DOI] [PubMed] [Google Scholar]
- 49.Ara T, Itoi M, Kawabata K, Egawa T, Tokoyoda K, Sugiyama T, Fujii N, Amagai T, Nagasawa T. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J Immunol. 2003;170(9):4649–4655. doi: 10.4049/jimmunol.170.9.4649. [DOI] [PubMed] [Google Scholar]
- 50.Misslitz A, Pabst O, Hintzen G, Ohl L, Kremmer E, Petrie HT, Forster R. Thymic T cell development and progenitor localization depend on CCR7. J Exp Med. 2004;200(4):481–491. doi: 10.1084/jem.20040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trampont PC, Tosello-Trampont AC, Shen Y, Duley AK, Sutherland AE, Bender TP, Littman DR, Ravichandran KS. CXCR4 acts as a costimulator during thymic beta-selection. Nat Immunol. 2010;11(2):162–170. doi: 10.1038/ni.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rode I, Boehm T. Regenerative capacity of adult cortical thymic epithelial cells. Proc Natl Acad Sci USA. 2012;109(9):3463–3468. doi: 10.1073/pnas.1118823109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bunting MD, Comerford I, Seach N, Hammett MV, Asquith DL, Korner H, Boyd RL, Nibbs RJ, McColl SR. CCX-CKR deficiency alters thymic stroma impairing thymocyte development and promoting autoimmunity. Blood. 2013;121(1):118–128. doi: 10.1182/blood-2012-06-434886. [DOI] [PubMed] [Google Scholar]
- 54.Lucas B, White AJ, Ulvmar MH, Nibbs RJ, Sitnik KM, Agace WW, Jenkinson WE, Anderson G, Rot A. CCRL1/ACKR4 is expressed in key thymic microenvironments but is dispensable for T lymphopoiesis at steady state in adult mice. Eur J Immunol. 2015;45(2):574–583. doi: 10.1002/eji.201445015. [DOI] [PubMed] [Google Scholar]
- 55.Prockop SE, Palencia S, Ryan CM, Gordon K, Gray D, Petrie HT. Stromal cells provide the matrix for migration of early lymphoid progenitors through the thymic cortex. J Immunol. 2002;169(8):4354–4361. doi: 10.4049/jimmunol.169.8.4354. [DOI] [PubMed] [Google Scholar]
- 56.Takahama Y, Letterio JJ, Suzuki H, Farr AG, Singer A. Early progression of thymocytes along the CD4/CD8 developmental pathway is regulated by a subset of thymic epithelial cells expressing transforming growth factor beta. J Exp Med. 1994;179(5):1495–1506. doi: 10.1084/jem.179.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bousso P, Bhakta NR, Lewis RS, Robey E. Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science. 2002;296(5574):1876–1880. doi: 10.1126/science.1070945. [DOI] [PubMed] [Google Scholar]
- 58.Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6(2):143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 59.Phee H, Dzhagalov I, Mollenauer M, Wang Y, Irvine DJ, Robey E, Weiss A. Regulation of thymocyte positive selection and motility by GIT2. Nat Immunol. 2010;11(6):503–511. doi: 10.1038/ni.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korthals M, Schilling K, Reichardt P, Mamula D, Schluter T, Steiner M, Langnase K, Thomas U, Gundelfinger E, Premont RT, Tedford K, Fischer KD. AlphaPIX RhoGEF supports positive selection by restraining migration and promoting arrest of thymocytes. J Immunol. 2014;192(7):3228–3238. doi: 10.4049/jimmunol.1302585. [DOI] [PubMed] [Google Scholar]
- 61.Sijts EJ, Kloetzel PM. The role of the proteasome in the generation of MHC class I ligands and immune responses. Cell Mol Life Sci. 2011;68(9):1491–1502. doi: 10.1007/s00018-011-0657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka K, Kasahara M. The MHC class I ligand-generating system: roles of immunoproteasomes and the interferon-gamma-inducible proteasome activator PA28. Immunol Rev. 1998;163:161–176. doi: 10.1111/j.1600-065X.1998.tb01195.x. [DOI] [PubMed] [Google Scholar]
- 63.Kloetzel PM. Antigen processing by the proteasome. Nat Rev Mol Cell Biol. 2001;2(3):179–187. doi: 10.1038/35056572. [DOI] [PubMed] [Google Scholar]
- 64.Murata S, Sasaki K, Kishimoto T, S-i Niwa, Hayashi H, Takahama Y, Tanaka K. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science. 2007;316(5829):1349–1353. doi: 10.1126/science.1141915. [DOI] [PubMed] [Google Scholar]
- 65.Tomaru U, Ishizu A, Murata S, Miyatake Y, Suzuki S, Takahashi S, Kazamaki T, Ohara J, Baba T, Iwasaki S, Fugo K, Otsuka N, Tanaka K, Kasahara M. Exclusive expression of proteasome subunit {beta}5t in the human thymic cortex. Blood. 2009;113(21):5186–5191. doi: 10.1182/blood-2008-11-187633. [DOI] [PubMed] [Google Scholar]
- 66.Nitta T, Murata S, Sasaki K, Fujii H, Ripen AM, Ishimaru N, Koyasu S, Tanaka K, Takahama Y. Thymoproteasome shapes immunocompetent repertoire of CD8+ T cells. Immunity. 2010;32(1):29–40. doi: 10.1016/j.immuni.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 67.Xing Y, Jameson SC, Hogquist KA. Thymoproteasome subunit-beta 5T generates peptide-MHC complexes specialized for positive selection. Proc Natl Acad Sci USA. 2013;110(17):6979–6984. doi: 10.1073/pnas.1222244110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takada K, Van Laethem F, Xing Y, Akane K, Suzuki H, Murata S, Tanaka K, Jameson SC, Singer A, Takahama Y. TCR affinity for thymoproteasome-dependent positively selecting peptides conditions antigen responsiveness in CD8(+) T cells. Nat Immunol. 2015;16(10):1069–1076. doi: 10.1038/ni.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sasaki K, Takada K, Ohte Y, Kondo H, Sorimachi H, Tanaka K, Takahama Y, Murata S. Thymoproteasomes produce unique peptide motifs for positive selection of CD8(+) T cells. Nat Commun. 2015;6:7484. doi: 10.1038/ncomms8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahama Y, Nitta T, Mat Ripen A, Nitta S, Murata S, Tanaka K. Role of thymic cortex-specific self-peptides in positive selection of T cells. Semin Immunol. 2010;22(5):287–293. doi: 10.1016/j.smim.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 71.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3(6):472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 72.Bowlus CL, Ahn J, Chu T, Gruen JR. Cloning of a novel MHC-encoded serine peptidase highly expressed by cortical epithelial cells of the thymus. Cell Immunol. 1999;196(2):80–86. doi: 10.1006/cimm.1999.1543. [DOI] [PubMed] [Google Scholar]
- 73.Nakagawa T, Roth W, Wong P, Nelson A, Farr A, Deussing J, Villadangos JA, Ploegh H, Peters C, Rudensky AY. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280(5362):450–453. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 74.Honey K, Nakagawa T, Peters C, Rudensky A. Cathepsin L regulates CD4(+) T cell selection independently of its effect on invariant chain: a role in the generation of positively selecting peptide ligands. J Exp Med. 2002;195(10):1349–1358. doi: 10.1084/jem.20011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gommeaux J, Gregoire C, Nguessan P, Richelme M, Malissen M, Guerder S, Malissen B, Carrier A. Thymus-specific serine protease regulates positive selection of a subset of CD4(+) thymocytes. Eur J Immunol. 2009;39(4):956–964. doi: 10.1002/eji.200839175. [DOI] [PubMed] [Google Scholar]
- 76.Viret C, Lamare C, Guiraud M, Fazilleau N, Bour A, Malissen B, Carrier A, Guerder S. Thymus-specific serine protease contributes to the diversification of the functional endogenous CD4 T cell receptor repertoire. J Exp Med. 2011;208(1):3–11. doi: 10.1084/jem.20100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Viret C, Leung-Theung-Long S, Serre L, Lamare C, Vignali DA, Malissen B, Carrier A, Guerder S. Thymus-specific serine protease controls autoreactive CD4 T cell development and autoimmune diabetes in mice. J Clin Invest. 2011;121(5):1810–1821. doi: 10.1172/JCI43314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455(7211):396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 79.Stritesky GL, Xing Y, Erickson JR, Kalekar LA, Wang X, Mueller DL, Jameson SC, Hogquist KA. Murine thymic selection quantified using a unique method to capture deleted T cells. Proc Natl Acad Sci USA. 2013;110(12):4679–4684. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205(11):2575–2584. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wekerle H, Ketelsen UP. Thymic nurse cells–Ia-bearing epithelium involved in T-lymphocyte differentiation? Nature. 1980;283(5745):402–404. doi: 10.1038/283402a0. [DOI] [PubMed] [Google Scholar]
- 82.Wekerle H, Ketelsen UP, Ernst M. Thymic nurse cells. Lymphoepithelial cell complexes in murine thymuses: morphological and serological characterization. J Exp Med. 1980;151(4):925–944. doi: 10.1084/jem.151.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pezzano M, Samms M, Martinez M, Guyden J. Questionable thymic nurse cell. Microbiol Mol Biol Rev. 2001;65(3):390–403. doi: 10.1128/MMBR.65.3.390-403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakagawa Y, Ohigashi I, Nitta T, Sakata M, Tanaka K, Murata S, Kanagawa O, Takahama Y. Thymic nurse cells provide microenvironment for secondary T cell receptor alpha rearrangement in cortical thymocytes. Proc Natl Acad Sci USA. 2012;109(50):20572–20577. doi: 10.1073/pnas.1213069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo J, Hawwari A, Li H, Sun Z, Mahanta SK, Littman DR, Krangel MS, He YW. Regulation of the TCRalpha repertoire by the survival window of CD4(+)CD8(+) thymocytes. Nat Immunol. 2002;3(5):469–476. doi: 10.1038/ni791. [DOI] [PubMed] [Google Scholar]
- 86.Ni PP, Solomon B, Hsieh CS, Allen PM, Morris GP. The ability to rearrange dual TCRs enhances positive selection, leading to increased allo- and autoreactive T cell repertoires. J Immunol. 2014;193(4):1778–1786. doi: 10.4049/jimmunol.1400532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sano S, Takahama Y, Sugawara T, Kosaka H, Itami S, Yoshikawa K, Miyazaki J, van Ewijk W, Takeda J. Stat3 in thymic epithelial cells is essential for postnatal maintenance of thymic architecture and thymocyte survival. Immunity. 2001;15(2):261–273. doi: 10.1016/S1074-7613(01)00180-7. [DOI] [PubMed] [Google Scholar]
- 88.Munoz JJ, Alfaro D, Garcia-Ceca J, Alonso CL, Jimenez E, Zapata A. Thymic alterations in EphA4-deficient mice. J Immunol. 2006;177(2):804–813. doi: 10.4049/jimmunol.177.2.804. [DOI] [PubMed] [Google Scholar]
- 89.Assarsson E, Chambers BJ, Hogstrand K, Berntman E, Lundmark C, Fedorova L, Imreh S, Grandien A, Cardell S, Rozell B, Ljunggren H-G. Severe defect in thymic development in an insertional mutant mouse model. J Immunol. 2007;178(8):5018–5027. doi: 10.4049/jimmunol.178.8.5018. [DOI] [PubMed] [Google Scholar]
- 90.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gamma delta T cells to immunology. Nat Rev Immunol. 2013;13(2):88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haas JD, Ravens S, Dueber S, Sandrock I, Oberdoerfer L, Kashani E, Chennupati V, Foehse L, Naumann R, Weiss S, Krueger A, Foerster R, Prinz I. Development of interleukin-17-producing gamma delta T cells is restricted to a functional embryonic wave. Immunity. 2012;37(1):48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 92.Cai Y, Xue F, Fleming C, Yang J, Ding C, Ma Y, Liu M, Zhang HG, Zheng J, Xiong N, Yan J. Differential developmental requirement and peripheral regulation for dermal Vgamma4 and Vgamma6T17 cells in health and inflammation. Nat Commun. 2014;5:3986. doi: 10.1038/ncomms4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roberts NA, White AJ, Jenkinson WE, Turchinovich G, Nakamura K, Withers DR, McConnell FM, Desanti GE, Benezech C, Parnell SM, Cunningham AF, Paolino M, Penninger JM, Simon AK, Nitta T, Ohigashi I, Takahama Y, Caamano JH, Hayday AC, Lane PJL, Jenkinson EJ, Anderson G. Rank signaling links the development of invariant gamma delta T cell progenitors and Aire(+) medullary epithelium. Immunity. 2012;36(3):427–437. doi: 10.1016/j.immuni.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006;108(12):3777–3785. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- 95.Hamazaki Y, Fujita H, Kobayashi T, Choi Y, Scott HS, Matsumoto M, Minato N. Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat Immunol. 2007;8(3):304–311. doi: 10.1038/ni1438. [DOI] [PubMed] [Google Scholar]
- 96.Gabler J, Arnold J, Kyewski B. Promiscuous gene expression and the developmental dynamics of medullary thymic epithelial cells. Eur J Immunol. 2007;37(12):3363–3372. doi: 10.1002/eji.200737131. [DOI] [PubMed] [Google Scholar]
- 97.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204(11):2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khan IS, Mouchess ML, Zhu ML, Conley B, Fasano KJ, Hou Y, Fong L, Su MA, Anderson MS. Enhancement of an anti-tumor immune response by transient blockade of central T cell tolerance. J Exp Med. 2014;211(5):761–768. doi: 10.1084/jem.20131889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Metzger TC, Khan IS, Gardner JM, Mouchess ML, Johannes KP, Krawisz AK, Skrzypczynska KM, Anderson MS. Lineage tracing and cell ablation identify a post-Aire-expressing thymic epithelial cell population. Cell Rep. 2013;5(1):166–179. doi: 10.1016/j.celrep.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nishikawa Y, Nishijima H, Matsumoto M, Morimoto J, Hirota F, Takahashi S, Luche H, Fehling HJ, Mouri Y, Matsumoto M. Temporal lineage tracing of Aire-expressing cells reveals a requirement for Aire in their maturation program. J Immunol. 2014;192(6):2585–2592. doi: 10.4049/jimmunol.1302786. [DOI] [PubMed] [Google Scholar]
- 101.Lkhagvasuren E, Sakata M, Ohigashi I, Takahama Y. Lymphotoxin beta receptor regulates the development of CCL21-expressing subset of postnatal medullary thymic epithelial cells. J Immunol. 2013;190(10):5110–5117. doi: 10.4049/jimmunol.1203203. [DOI] [PubMed] [Google Scholar]
- 102.Yano M, Kuroda N, Han H, Meguro-Horike M, Nishikawa Y, Kiyonari H, Maemura K, Yanagawa Y, Obata K, Takahashi S, Ikawa T, Satoh R, Kawamoto H, Mouri Y, Matsumoto M. Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. J Exp Med. 2008;205(12):2827–2838. doi: 10.1084/jem.20080046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.White AJ, Nakamura K, Jenkinson WE, Saini M, Sinclair C, Seddon B, Narendran P, Pfeffer K, Nitta T, Takahama Y, Caamano JH, Lane PJL, Jenkinson EJ, Anderson G. Lymphotoxin signals from positively selected thymocytes regulate the terminal differentiation of medullary thymic epithelial cells. J Immunol. 2010;185(8):4769–4776. doi: 10.4049/jimmunol.1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shores EW, Van Ewijk W, Singer A. Disorganization and restoration of thymic medullary epithelial cells in T cell receptor-negative scid mice: evidence that receptor-bearing lymphocytes influence maturation of the thymic microenvironment. Eur J Immunol. 1991;21(7):1657–1661. doi: 10.1002/eji.1830210711. [DOI] [PubMed] [Google Scholar]
- 105.Philpott KL, Viney JL, Kay G, Rastan S, Gardiner EM, Chae S, Hayday AC, Owen MJ. Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science. 1992;256(5062):1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 106.Surh CD, Ernst B, Sprent J. Growth of epithelial-cells in the thymic medulla is under the control of mature t-cells. J Exp Med. 1992;176(2):611–616. doi: 10.1084/jem.176.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Negishi I, Motoyama N, Nakayama K, Nakayama K, Senju S, Hatakeyama S, Zhang Q, Chan AC, Loh DY. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376(6539):435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 108.Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373(6514):531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 109.Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80(2):331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 110.Naspetti M, Aurrand-Lions M, DeKoning J, Malissen M, Galland F, Lo D, Naquet P. Thymocytes and RelB-dependent medullary epithelial cells provide growth-promoting and organization signals, respectively, to thymic medullary stromal cells. Eur J Immunol. 1997;27(6):1392–1397. doi: 10.1002/eji.1830270615. [DOI] [PubMed] [Google Scholar]
- 111.Kajiura F, Sun S, Nomura T, Izumi K, Ueno T, Bando Y, Kuroda N, Han H, Li Y, Matsushima A, Takahama Y, Sakaguchi S, Mitani T, Matsumoto M. NF-kappa B-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J Immunol. 2004;172(4):2067–2075. doi: 10.4049/jimmunol.172.4.2067. [DOI] [PubMed] [Google Scholar]
- 112.Akiyama T, Maeda S, Yamane S, Ogino K, Kasai M, Kajiura F, Matsumoto M, Inoue J. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science. 2005;308(5719):248–251. doi: 10.1126/science.1105677. [DOI] [PubMed] [Google Scholar]
- 113.Kinoshita D, Hirota F, Kaisho T, Kasai M, Izumi K, Bando Y, Mouri Y, Matsushima A, Niki S, Han H, Oshikawa K, Kuroda N, Maegawa M, Irahara M, Takeda K, Akira S, Matsumoto M. Essential role of IkappaB kinase alpha in thymic organogenesis required for the establishment of self-tolerance. J Immunol. 2006;176(7):3995–4002. doi: 10.4049/jimmunol.176.7.3995. [DOI] [PubMed] [Google Scholar]
- 114.Zhu M, Chin RK, Christiansen PA, Lo JC, Liu X, Ware C, Siebenlist U, Fu YX. NF-kappaB2 is required for the establishment of central tolerance through an Aire-dependent pathway. J Clin Invest. 2006;116(11):2964–2971. doi: 10.1172/JCI28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang B, Wang Z, Ding J, Peterson P, Gunning WT, Ding HF. NF-kappaB2 is required for the control of autoimmunity by regulating the development of medullary thymic epithelial cells. J Biol Chem. 2006;281(50):38617–38624. doi: 10.1074/jbc.M606705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang X, Wang H, Claudio E, Brown K, Siebenlist U. A role for the IkappaB family member Bcl-3 in the control of central immunologic tolerance. Immunity. 2007;27(3):438–452. doi: 10.1016/j.immuni.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTbetaR. J Exp Med. 2003;198(5):757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rossi SW, Kim M-Y, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, Lane PJL, Anderson G. RANK signals from CD4(+)3(−) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204(6):1267–1272. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, Nitta T, Takahama Y, J-i Inoue. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29(3):423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 120.Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, Yokota Y, Yamada H, Yoshikai Y, J-I Inoue, Akiyama T, Takahama Y. The cytokine RANKL produced by positively selected thymocytes Fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29(3):438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 121.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert F-X, Scott HS, Takahama Y, Hollaender GA, Reith W. Autoantigen-specific interactions with CD4(+) thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29(3):451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 122.Irla M, Guerri L, Guenot J, Serge A, Lantz O, Liston A, Imhof BA, Palmer E, Reith W. Antigen recognition by autoreactive CD4(+) thymocytes drives homeostasis of the thymic medulla. PLoS ONE. 2012;7(12):e52591. doi: 10.1371/journal.pone.0052591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Irla M, Guenot J, Sealy G, Reith W, Imhof BA, Serge A. Three-dimensional visualization of the mouse thymus organization in health and immunodeficiency. J Immunol. 2013;190(2):586–596. doi: 10.4049/jimmunol.1200119. [DOI] [PubMed] [Google Scholar]
- 124.Jenkinson SR, Williams JA, Jeon H, Zhang J, Nitta T, Ohigashi I, Kruhlak M, Zuklys S, Sharrow S, Adams A, Granger L, Choi Y, Siebenlist U, Bishop GA, Hollander GA, Takahama Y, Hodes RJ. TRAF3 enforces the requirement for T cell cross-talk in thymic medullary epithelial development. Proc Natl Acad Sci USA. 2013;110(52):21107–21112. doi: 10.1073/pnas.1314859111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Williams JA, Zhang J, Jeon H, Nitta T, Ohigashi I, Klug D, Kruhlak MJ, Choudhury B, Sharrow SO, Granger L, Adams A, Eckhaus MA, Jenkinson SR, Richie ER, Gress RE, Takahama Y, Hodes RJ. Thymic medullary epithelium and thymocyte self-tolerance require cooperation between CD28-CD80/86 and CD40-CD40L costimulatory pathways. J Immunol. 2014;192(2):630–640. doi: 10.4049/jimmunol.1302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.White AJ, Withers DR, Parnell SM, Scott HS, Finke D, Lane PJL, Jenkinson EJ, Anderson G. Sequential phases in the development of Aire-expressing medullary thymic epithelial cells involve distinct cellular input. Eur J Immunol. 2008;38(4):942–947. doi: 10.1002/eji.200738052. [DOI] [PubMed] [Google Scholar]
- 127.White AJ, Jenkinson WE, Cowan JE, Parnell SM, Bacon A, Jones ND, Jenkinson EJ, Anderson G. An essential role for medullary thymic epithelial cells during the intrathymic development of invariant NKT cells. J Immunol. 2014;192(6):2659–2666. doi: 10.4049/jimmunol.1303057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu M, Chin RK, Tumanov AV, Liu X, Fu YX. Lymphotoxin beta receptor is required for the migration and selection of autoreactive T cells in thymic medulla. J Immunol. 2007;179(12):8069–8075. doi: 10.4049/jimmunol.179.12.8069. [DOI] [PubMed] [Google Scholar]
- 129.Seach N, Ueno T, Fletcher AL, Lowen T, Mattesich M, Engwerda CR, Scott HS, Ware CF, Chidgey AP, Gray DH, Boyd RL. The lymphotoxin pathway regulates Aire-independent expression of ectopic genes and chemokines in thymic stromal cells. J Immunol. 2008;180(8):5384–5392. doi: 10.4049/jimmunol.180.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mouri Y, Yano M, Shinzawa M, Shimo Y, Hirota F, Nishikawa Y, Nii T, Kiyonari H, Abe T, Uehara H, Izumi K, Tamada K, Chen L, Penninger JM, Inoue J, Akiyama T, Matsumoto M. Lymphotoxin signal promotes thymic organogenesis by eliciting RANK expression in the embryonic thymic stroma. J Immunol. 2011;186(9):5047–5057. doi: 10.4049/jimmunol.1003533. [DOI] [PubMed] [Google Scholar]
- 131.Akiyama N, Shinzawa M, Miyauchi M, Yanai H, Tateishi R, Shimo Y, Ohshima D, Matsuo K, Sasaki I, Hoshino K, Wu G, Yagi S, Inoue J, Kaisho T, Akiyama T. Limitation of immune tolerance-inducing thymic epithelial cell development by Spi-B-mediated negative feedback regulation. J Exp Med. 2014;211(12):2425–2438. doi: 10.1084/jem.20141207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Otero DC, Baker DP, David M. IRF7-dependent IFN-beta production in response to RANKL promotes medullary thymic epithelial cell development. J Immunol. 2013;190(7):3289–3298. doi: 10.4049/jimmunol.1203086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hauri-Hohl M, Zuklys S, Hollander GA, Ziegler SF. A regulatory role for TGF-beta signaling in the establishment and function of the thymic medulla. Nat Immunol. 2014;15(6):554–561. doi: 10.1038/ni.2869. [DOI] [PubMed] [Google Scholar]
- 134.Papadopoulou AS, Dooley J, Linterman MA, Pierson W, Ucar O, Kyewski B, Zuklys S, Hollander GA, Matthys P, Gray DH, De Strooper B, Liston A. The thymic epithelial microRNA network elevates the threshold for infection-associated thymic involution via miR-29a mediated suppression of the IFN-alpha receptor. Nat Immunol. 2012;13(2):181–187. doi: 10.1038/ni.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zuklys S, Mayer CE, Zhanybekova S, Stefanski HE, Nusspaumer G, Gill J, Barthlott T, Chappaz S, Nitta T, Dooley J, Nogales-Cadenas R, Takahama Y, Finke D, Liston A, Blazar BR, Pascual-Montano A, Hollander GA. MicroRNAs control the maintenance of thymic epithelia and their competence for T lineage commitment and thymocyte selection. J Immunol. 2012;189(8):3894–3904. doi: 10.4049/jimmunol.1200783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ueno T, Saito F, Gray DHD, Kuse S, Hieshima K, Nakano H, Kakiuchi T, Lipp M, Boyd RL, Takahama Y. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med. 2004;200(4):493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gray DH, Tull D, Ueno T, Seach N, Classon BJ, Chidgey A, McConville MJ, Boyd RL. A unique thymic fibroblast population revealed by the monoclonal antibody MTS-15. J Immunol. 2007;178(8):4956–4965. doi: 10.4049/jimmunol.178.8.4956. [DOI] [PubMed] [Google Scholar]
- 138.Kwan J, Killeen N. CCR7 directs the migration of thymocytes into the thymic medulla. J Immunol. 2004;172(7):3999–4007. doi: 10.4049/jimmunol.172.7.3999. [DOI] [PubMed] [Google Scholar]
- 139.Kurobe H, Liu C, Ueno T, Saito F, Ohigashi I, Seach N, Arakaki R, Hayashi Y, Kitagawa T, Lipp M, Boyd RL, Takahama Y. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24(2):165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 140.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204(11):2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nitta T, Nitta S, Lei Y, Lipp M, Takahama Y. CCR7-mediated migration of developing thymocytes to the medulla is essential for negative selection to tissue-restricted antigens. Proc Natl Acad Sci USA. 2009;106(40):17129–17133. doi: 10.1073/pnas.0906956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hopken UE, Wengner AM, Loddenkemper C, Stein H, Heimesaat MM, Rehm A, Lipp M. CCR7 deficiency causes ectopic lymphoid neogenesis and disturbed mucosal tissue integrity. Blood. 2007;109(3):886–895. doi: 10.1182/blood-2006-03-013532. [DOI] [PubMed] [Google Scholar]
- 143.Davalos-Misslitz AC, Rieckenberg J, Willenzon S, Worbs T, Kremmer E, Bernhardt G, Forster R. Generalized multi-organ autoimmunity in CCR7-deficient mice. Eur J Immunol. 2007;37(3):613–622. doi: 10.1002/eji.200636656. [DOI] [PubMed] [Google Scholar]
- 144.Reinhardt A, Ravens S, Fleige H, Haas JD, Oberdorfer L, Lyszkiewicz M, Forster R, Prinz I. CCR7-mediated migration in the thymus controls gammadelta T-cell development. Eur J Immunol. 2014;44(5):1320–1329. doi: 10.1002/eji.201344330. [DOI] [PubMed] [Google Scholar]
- 145.Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bosl MR, Hollander GA, Hayashi Y, Malefyt Rde W, Nitta T, Takahama Y. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208(2):383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 147.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279(15):15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 148.Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010;328(5982):1129–1135. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316(5822):295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 150.Love PE, Bhandoola A. Signal integration and crosstalk during thymocyte migration and emigration. Nat Rev Immunol. 2011;11(7):469–477. doi: 10.1038/nri2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Klein L, Kyewski B. “Promiscuous” expression of tissue antigens in the thymus: a key to T-cell tolerance and autoimmunity? J Mol Med (Berl) 2000;78(9):483–494. doi: 10.1007/s001090000146. [DOI] [PubMed] [Google Scholar]
- 152.Klein L, Klugmann M, Nave KA, Tuohy VK, Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat Med. 2000;6(1):56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- 153.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2(11):1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 154.Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202(1):33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.DeKoning J, DiMolfetto L, Reilly C, Wei Q, Havran WL, Lo D. Thymic cortical epithelium is sufficient for the development of mature T cells in relB-deficient mice. J Immunol. 1997;158(6):2558–2566. [PubMed] [Google Scholar]
- 156.Pinto S, Michel C, Schmidt-Glenewinkel H, Harder N, Rohr K, Wild S, Brors B, Kyewski B. Overlapping gene coexpression patterns in human medullary thymic epithelial cells generate self-antigen diversity. Proc Natl Acad Sci USA. 2013;110(37):E3497–E3505. doi: 10.1073/pnas.1308311110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Anderson MS, Venanzi ES, Klein L, Chen ZB, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298(5597):1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 158.Heino M, Peterson P, Kudoh J, Nagamine K, Lagerstedt A, Ovod V, Ranki A, Rantala I, Nieminen M, Tuukkanen J, Scott HS, Antonarakis SE, Shimizu N, Krohn K. Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochem Biophys Res Commun. 1999;257(3):821–825. doi: 10.1006/bbrc.1999.0308. [DOI] [PubMed] [Google Scholar]
- 159.Heino M, Peterson P, Sillanpaa N, Guerin S, Wu L, Anderson G, Scott HS, Antonarakis SE, Kudoh J, Shimizu N, Jenkinson EJ, Naquet P, Krohn KJ. RNA and protein expression of the murine autoimmune regulator gene (Aire) in normal, RelB-deficient and in NOD mouse. Eur J Immunol. 2000;30(7):1884–1893. doi: 10.1002/1521-4141(200007)30:7<1884::AID-IMMU1884>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 160.Zuklys S, Balciunaite G, Agarwal A, Fasler-Kan E, Palmer E, Hollander GA. Normal thymic architecture and negative selection are associated with Aire expression, the gene defective in the autoimmune-polyendocrinopathy–candidiasis–ectodermal dystrophy (APECED) J Immunol. 2000;165(4):1976–1983. doi: 10.4049/jimmunol.165.4.1976. [DOI] [PubMed] [Google Scholar]
- 161.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4(4):350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 162.Liston A, Gray DH, Lesage S, Fletcher AL, Wilson J, Webster KE, Scott HS, Boyd RL, Peltonen L, Goodnow CC. Gene dosage–limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med. 2004;200(8):1015–1026. doi: 10.1084/jem.20040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Anderson MS, Venanzi ES, Chen ZB, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23(2):227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 164.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8(4):351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 165.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, Socci ND, Savage PA. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339(6124):1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348(6234):589–594. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N. Positional cloning of the APECED gene. Nat Genet. 1997;17(4):393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 168.Aaltonen J, Bjorses P, Perheentupa J, HorelliKuitunen N, Palotie A, Peltonen L, Lee YS, Francis F, Hennig S, Thiel C, Lehrach H, Yaspo ML. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17(4):399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 169.Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kampe O, Eskelin P, Pelto-Huikko M, Peltonen L. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11(4):397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 170.Kuroda N, Mitani T, Takeda N, Ishimaru N, Arakaki R, Hayashi Y, Bando Y, Izumi K, Takahashi T, Nomura T, Sakaguchi S, Ueno T, Takahama Y, Uchida D, Sun SJ, Kajiura F, Mouri Y, Han HW, Matsushima A, Yamada G, Matsumoto M. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of aire-deficient mice. J Immunol. 2005;174(4):1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 171.Giraud M, Yoshida H, Abramson J, Rahl PB, Young RA, Mathis D, Benoist C. Aire unleashes stalled RNA polymerase to induce ectopic gene expression in thymic epithelial cells. Proc Natl Acad Sci USA. 2012;109(2):535–540. doi: 10.1073/pnas.1119351109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Waterfield M, Khan IS, Cortez JT, Fan U, Metzger T, Greer A, Fasano K, Martinez-Llordella M, Pollack JL, Erle DJ, Su M, Anderson MS. The transcriptional regulator Aire coopts the repressive ATF7ip-MBD1 complex for the induction of immunotolerance. Nat Immunol. 2014;15(3):258–265. doi: 10.1038/ni.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Venanzi ES, Melamed R, Mathis D, Benoist C. The variable immunological self: genetic variation and nongenetic noise in Aire-regulated transcription. Proc Natl Acad Sci USA. 2008;105(41):15860–15865. doi: 10.1073/pnas.0808070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Abramson J, Giraud M, Benoist C, Mathis D. Aire’s partners in the molecular control of immunological tolerance. Cell. 2010;140(1):123–135. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 175.Giraud M, Jmari N, Du L, Carallis F, Nieland TJ, Perez-Campo FM, Bensaude O, Root DE, Hacohen N, Mathis D, Benoist C. An RNAi screen for Aire cofactors reveals a role for Hnrnpl in polymerase release and Aire-activated ectopic transcription. Proc Natl Acad Sci USA. 2014;111(4):1491–1496. doi: 10.1073/pnas.1323535111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gillard GO, Farr AG. Contrasting models of promiscuous gene expression by thymic epithelium. J Exp Med. 2005;202(1):15–19. doi: 10.1084/jem.20050976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gillard GO, Dooley J, Erickson M, Peltonen L, Farr AG. Aire-dependent alterations in medullary thymic epithelium indicate a role for Aire in thymic epithelial differentiation. J Immunol. 2007;178(5):3007–3015. doi: 10.4049/jimmunol.178.5.3007. [DOI] [PubMed] [Google Scholar]
- 178.Dooley J, Erickson M, Farr AG. Alterations of the medullary epithelial compartment in the Aire-deficient thymus: implications for programs of thymic epithelial differentiation. J Immunol. 2008;181(8):5225–5232. doi: 10.4049/jimmunol.181.8.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ruan QG, Tung K, Eisenman D, Setiady Y, Eckenrode S, Yi B, Purohit S, Zheng WP, Zhang Y, Peltonen L, She JX. The autoimmune regulator directly controls the expression of genes critical for thymic epithelial function. J Immunol. 2007;178(11):7173–7180. doi: 10.4049/jimmunol.178.11.7173. [DOI] [PubMed] [Google Scholar]
- 180.Laan M, Kisand K, Kont V, Moll K, Tserel L, Scott HS, Peterson P. Autoimmune regulator deficiency results in decreased expression of CCR4 and CCR7 ligands and in delayed migration of CD4+ thymocytes. J Immunol. 2009;183(12):7682–7691. doi: 10.4049/jimmunol.0804133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.St-Pierre C, Trofimov A, Brochu S, Lemieux S, Perreault C. Differential features of AIRE-induced and AIRE-independent promiscuous gene expression in thymic epithelial cells. J Immunol. 2015;195(2):498–506. doi: 10.4049/jimmunol.1500558. [DOI] [PubMed] [Google Scholar]
- 182.Macedo C, Evangelista AF, Marques MM, Octacilio-Silva S, Donadi EA, Sakamoto-Hojo ET, Passos GA. Autoimmune regulator (Aire) controls the expression of microRNAs in medullary thymic epithelial cells. Immunobiology. 2013;218(4):554–560. doi: 10.1016/j.imbio.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 183.Ucar O, Tykocinski LO, Dooley J, Liston A, Kyewski B. An evolutionarily conserved mutual interdependence between Aire and microRNAs in promiscuous gene expression. Eur J Immunol. 2013;43(7):1769–1778. doi: 10.1002/eji.201343343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sansom SN, Shikama-Dorn N, Zhanybekova S, Nusspaumer G, Macaulay IC, Deadman ME, Heger A, Ponting CP, Hollander GA. Population and single-cell genomics reveal the Aire dependency, relief from Polycomb silencing, and distribution of self-antigen expression in thymic epithelia. Genome Res. 2014;24(12):1918–1931. doi: 10.1101/gr.171645.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chin RK, Zhu M, Christiansen PA, Liu W, Ware C, Peltonen L, Zhang X, Guo L, Han S, Zheng B, Fu YX. Lymphotoxin pathway-directed, autoimmune regulator-independent central tolerance to arthritogenic collagen. J Immunol. 2006;177(1):290–297. doi: 10.4049/jimmunol.177.1.290. [DOI] [PubMed] [Google Scholar]
- 186.Bos R, van Duikeren S, van Hall T, Kaaijk P, Taubert R, Kyewski B, Klein L, Melief CJ, Offringa R. Expression of a natural tumor antigen by thymic epithelial cells impairs the tumor-protective CD4+ T-cell repertoire. Cancer Res. 2005;65(14):6443–6449. doi: 10.1158/0008-5472.CAN-05-0666. [DOI] [PubMed] [Google Scholar]
- 187.Cloosen S, Arnold J, Thio M, Bos GM, Kyewski B, Germeraad WT. Expression of tumor-associated differentiation antigens, MUC1 glycoforms and CEA, in human thymic epithelial cells: implications for self-tolerance and tumor therapy. Cancer Res. 2007;67(8):3919–3926. doi: 10.1158/0008-5472.CAN-06-2112. [DOI] [PubMed] [Google Scholar]
- 188.Zhu ML, Nagavalli A, Su MA. Aire deficiency promotes TRP-1-specific immune rejection of melanoma. Cancer Res. 2013;73(7):2104–2116. doi: 10.1158/0008-5472.CAN-12-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wu L, Shortman K. Heterogeneity of thymic dendritic cells. Semin Immunol. 2005;17(4):304–312. doi: 10.1016/j.smim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 190.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7(10):1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 191.Moore NC, Anderson G, McLoughlin DE, Owen JJ, Jenkinson EJ. Differential expression of Mtv loci in MHC class II-positive thymic stromal cells. J Immunol. 1994;152(10):4826–4831. [PubMed] [Google Scholar]
- 192.Ferrero I, Anjuere F, MacDonald HR, Ardavin C. In vitro negative selection of viral superantigen-reactive thymocytes by thymic dendritic cells. Blood. 1997;90(5):1943–1951. [PubMed] [Google Scholar]
- 193.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200(8):1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Atibalentja DF, Byersdorfer CA, Unanue ER. Thymus-blood protein interactions are highly effective in negative selection and regulatory T cell induction. J Immunol. 2009;183(12):7909–7918. doi: 10.4049/jimmunol.0902632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Baba T, Badr Mel S, Tomaru U, Ishizu A, Mukaida N. Novel process of intrathymic tumor-immune tolerance through CCR2-mediated recruitment of Sirpalpha + dendritic cells: a murine model. PLoS ONE. 2012;7(7):e41154. doi: 10.1371/journal.pone.0041154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, Nguyen L, Ghodsi A, Adler S, Butcher EC. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36(3):438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 198.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202(7):901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lio CWJQ, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28(1):100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tai X, Erman B, Alag A, Mu J, Kimura M, Katz G, Guinter T, McCaughtry T, Etzensperger R, Feigenbaum L, Singer DS, Singer A. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity. 2013;38(6):1116–1128. doi: 10.1016/j.immuni.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor FoxP3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 203.Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJL, Jenkinson EJ, Jenkinson WE, Anderson G. The thymic medulla is required for Foxp3(+) regulatory but not conventional CD4(+) thymocyte development. J Exp Med. 2013;210(4):675–681. doi: 10.1084/jem.20122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. 2010;11(6):U512–U580. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 205.Perry JSA, Lio CWJ, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, Hsieh CS. Distinct contributions of aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41(3):414–426. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Millet V, Naquet P, Guinamard RR. Intercellular MHC transfer between thymic epithelial and dendritic cells. Eur J Immunol. 2008;38(5):1257–1263. doi: 10.1002/eji.200737982. [DOI] [PubMed] [Google Scholar]
- 207.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206(7):1505–1513. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fuertbauer E, Zaujec J, Uhrin P, Raab I, Weber M, Schachner H, Bauer M, Schutz GJ, Binder BR, Sixt M, Kerjaschki D, Stockinger H. Thymic medullar conduits-associated podoplanin promotes natural regulatory T cells. Immunol Lett. 2013;154(1–2):31–41. doi: 10.1016/j.imlet.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 209.Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, Liu YJ. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436(7054):1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 210.Watanabe N, Hanabuchi S, Soumelis V, Yuan W, Ho S, de Waal Malefyt R, Liu YJ. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004;5(4):426–434. doi: 10.1038/ni1048. [DOI] [PubMed] [Google Scholar]
- 211.Hanabuchi S, Lto T, Park WR, Watanabe N, Shaw JL, Roman E, Arima K, Wang YH, Voo KS, Cao W, Liu YJ. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3(+) regulatory T cells in human thymus. J Immunol. 2010;184(6):2999–3007. doi: 10.4049/jimmunol.0804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.McCarthy NI, Cowan JE, Nakamura K, Bacon A, Baik S, White AJ, Parnell SM, Jenkinson EJ, Jenkinson WE, Anderson G. Osteoprotegerin-mediated homeostasis of rank + thymic epithelial cells does not limit Foxp3+ regulatory T cell development. J Immunol. 2015;195(6):2675–2682. doi: 10.4049/jimmunol.1501226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, Tigelaar RE, Lifton RP. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gamma delta T cells. Nat Genet. 2008;40(5):656–662. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Barbee SD, Woodward MJ, Turchinovich G, Mention J-J, Lewis JM, Boyden LM, Lifton RP, Tigelaar R, Hayday AC. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci USA. 2011;108(8):3330–3335. doi: 10.1073/pnas.1010890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Turchinovich G, Hayday AC. Skint-1 identifies a common molecular mechanism for the development of interferon-gamma-secreting versus interleukin-17-secreting gamma delta T cells. Immunity. 2011;35(1):59–68. doi: 10.1016/j.immuni.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 216.Drennan MB, Franki AS, Dewint P, Van Beneden K, Seeuws S, van de Pavert SA, Reilly EC, Verbruggen G, Lane TE, Mebius RE, Deforce D, Elewaut D. Cutting edge: the chemokine receptor CXCR3 retains invariant NKT cells in the thymus. J Immunol. 2009;183(4):2213–2216. doi: 10.4049/jimmunol.0901213. [DOI] [PubMed] [Google Scholar]
- 217.Kim JS, Smith-Garvin JE, Koretzky GA, Jordan MS. The requirements for natural Th17 cell development are distinct from those of conventional Th17 cells. J Exp Med. 2011;208(11):2201–2207. doi: 10.1084/jem.20110680. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 218.Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, Craft J. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10(10):U1125–U1128. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jenkinson WE, McCarthy NI, Dutton EE, Cowan JE, Parnell SM, White AJ, Anderson G. Natural Th17 cells are critically regulated by functional medullary thymic microenvironments. J Autoimmun. 2015;63:13–22. doi: 10.1016/j.jaut.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]