Abstract
The pandemic rise in obesity has resulted in an increased incidence of metabolic complications. Non-alcoholic fatty liver disease is the hepatic manifestation of the metabolic syndrome and has become the most common chronic liver disease in large parts of the world. The adipose tissue expansion and hepatic fat accumulation characteristics of these disorders compromise local oxygen homeostasis. The resultant tissue hypoxia induces adaptive responses to restore oxygenation and tissue metabolism and cell survival. Hypoxia-inducible factors (HIFs) function as master regulators of this hypoxia adaptive response, and are in turn hydroxylated by prolyl hydroxylases (PHDs). PHDs are the main cellular oxygen sensors and regulate HIF proteasomal degradation in an oxygen-dependent manner. HIFs and PHDs are implicated in numerous physiological and pathological conditions. Extensive research using genetic models has revealed that hypoxia signaling is also a key mechanism in adipose tissue dysfunction, leading to adipose tissue fibrosis, inflammation and insulin resistance. Moreover, hypoxia affects liver lipid metabolism and deranges hepatic lipid accumulation. This review summarizes the molecular mechanisms through which the hypoxia adaptive response affects adipocyte and hepatic metabolism, and the therapeutic possibilities of modulating HIFs and PHDs in obesity and fatty liver disease.
Keywords: Obesity, Hypoxia, Hypoxia inducible factor, Lipid metabolism, Oxygen homeostasis, Angiogenesis
Introduction
Obesity and its associated comorbidities have become one of the defining diseases of the last decades. A recent analysis estimated that the number of overweight or obese individuals has risen to 2.1 billion people worldwide [1]. Consequently, non-alcoholic fatty liver disease (NAFLD) has become a global pandemic and is now the most common cause of chronic liver injury in large parts of the world [2, 3]. NAFLD comprises a spectrum of disease that ranges from hepatocellular steatosis to non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma. Simple steatosis should no longer be considered a benign condition, as it predisposes the liver to further injury, and is a precursor of and an independent risk factor for type 2 diabetes and cardiovascular disease [2, 4, 5].
Despite intensive research, satisfactory pharmacological treatment for either obesity or NAFLD is currently lacking, urging further research into the pathophysiological mechanisms underlying these disorders.
Complex multicellular organisms were able to evolve as a result of energy production through mitochondrial oxidative phosphorylation, which generates energy much more efficiently than alternative fermentation processes such as anaerobic glycolysis. Oxygen functions as the final electron acceptor in this mitochondrial respiratory chain, and is therefore vital for maintaining normal tissue homeostasis and metabolism [6, 7]. Accordingly, an evolutionary conserved pathway, termed the hypoxia-induced adaptive response, regulates adaptation to low oxygen tensions at the cellular level [8]. Both hypoxia and this adaptive response have been associated with many diseases, such as anemia, lung and cardiovascular disease, and cancer [6, 9].
Regarding obesity-related diseases, studies in animal models [10, 11] have demonstrated that white adipose tissue (WAT) becomes hypoxic as it expands in obesity. As WAT hypoxia is one of the first pathophysiological changes in obesity, it may underlie the observed link between obesity and low-grade chronic WAT inflammation [12, 13]. In human patients, the evidence for WAT hypoxia is more controversial. Some studies have shown a decrease in WAT oxygen levels as body fat increases [14, 15]. Nevertheless, others have not been able to find a metabolic signature characteristic of hypoxia in adipose tissues of obese subjects [16], whereas one study even found an increase in tissue oxygen levels, despite reduced WAT blood flow [17]. In recent years, there has been considerable interest in brown adipose tissue (BAT) as a potential anti-obesity target due to its capacity to burn excess energy in thermogenesis [18]. Research has indicated that obesity also causes BAT hypoxia, contributing to a loss of thermogenic capacity [19].
Adipose tissue expansion and inflammation cause ectopic lipid accumulation in muscle and liver tissue, leading to insulin resistance and liver steatosis [20, 21]. Because the liver is uniquely vascularized, receiving both oxygenated blood via the hepatic artery and deoxygenated blood via the portal vein, it is a hypoxia-sensitive organ. The blood that flows in the hepatic lobule is directed from the periportal region towards the central vein, creating an oxygen gradient with lower oxygen tensions in the perivenous regions of the liver lobule [22]. While it is known that acute and chronic alcohol exposure causes perivenous hypoxia [23, 24], more recently, a similar pattern was established in mice who were fed a high-fat diet (HFD) leading to NAFLD [25].
This review discusses the pathways involved in hypoxia signaling and the effects of modulating the hypoxia response on the development of adipose tissue dysfunction and NAFLD.
The hypoxia-induced adaptive response pathway
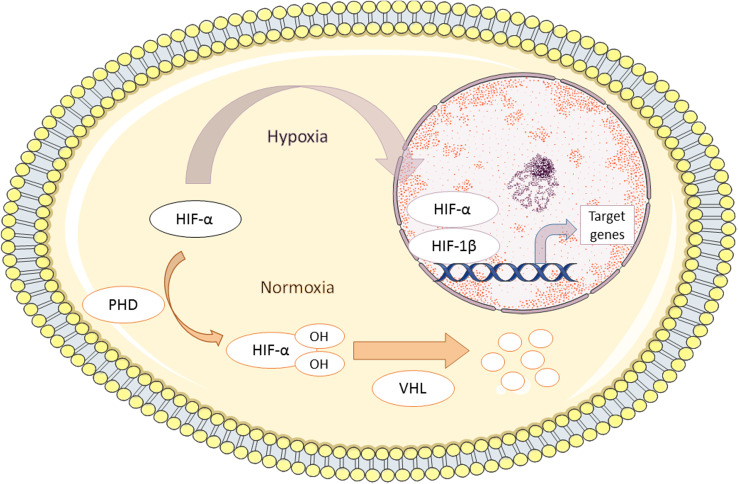
Hypoxia-inducible transcription factors (HIF) are the master regulators of the cellular and tissue response to hypoxic stress [26–28]. They are heterodimers consisting of an oxygen-sensitive HIF-α subunit and a stable, constitutively expressed β-subunit (HIF-1β), also known as ARNT (aryl hydrocarbon receptor nuclear translocator). Three forms of HIF-α, named HIF-1α, HIF-2α and HIF-3α, have been characterized. HIF-α subunit stability is strictly controlled by cellular oxygen concentrations (Fig. 1). Under normoxic conditions, HIF-α is subject to hydroxylation at specific proline residues by prolyl hydroxylase domain proteins (designated PHD1, PHD2 and PHD3). PHD activity requires oxygen and 2-oxoglutarate as co-substrates and ascorbic acid and iron as co-factors [29]. Hydroxylation allows recognition of HIF-α by the von Hippel Lindau (VHL) tumor suppressor gene, which acts as a substrate for the E3 ubiquitin ligase complex, resulting in polyubiquitination and proteasomal degradation [30]. Under hypoxic conditions, PHD activity is inhibited and HIF-α can translocate to the nucleus where it dimerizes with HIF-1β, thereby forming the active transcription factor HIF.
Fig. 1.
The Hypoxia adaptive response system. Under normoxic conditions, hypoxia-inducible factor α (HIF-α) subunits are hydroxylated by prolyl hydroxylase (PHD) at specific proline residues (prolines 402 and 564 for HIF-1α and prolines 405 and 531 for HIF-2α). Hydroxylated HIFs are recognized by the von Hippel–Lindau (VHL) protein, leading to ubiquitinilation and proteasomal degradation. PHD activity is inhibited by hypoxia, allowing the nuclear translocation of HIF-α, where it dimerizes with HIF-1β to activate transcription of hypoxia target genes
HIF belongs to the PAS family of helix–loop–helix transcription factors, and binds DNA at so-called hypoxia responsive elements [31]. HIF regulates the expression of numerous downstream target genes, such as vascular endothelial growth factor (VEGF), erythropoietin and various glucose transporters (GLUTs). In this way, HIF increases oxygen delivery to the tissue, helps adapt the tissue to lower oxygen levels and lowers oxygen consumption. HIF-1α and HIF-2α share structural similarities and coordinate processes such as erythropoiesis, angiogenesis, glucose and lipid metabolism, proliferation, and inflammation [32]. Yet each isoform has non-redundant functions that vary in a tissue-specific way [33, 34]. In addition, the affinity of different PHD isoforms for HIF subunits and their relative abundance in different tissues and cells varies, which allows fine-tuning of a flexible hypoxia adaptive response to varying physiological oxygen tensions [35]. For instance, PHD2 shows greater affinity for HIF-1α, whereas PHD3 and, to a lesser extent, PHD1 predominantly hydroxylate HIF-2α [36].
Hypoxia signaling in adipose tissue
Adipose tissue has the unique property to expand drastically in adulthood, whereas most adult tissues retain a relatively stable size [37]. Although adipogenesis and angiogenesis are tightly interconnected, compensatory WAT angiogenesis is often inadequate to parallel WAT expansion and blood delivery to WAT is diminished [17, 38]. As adipocyte cell expansion also limits oxygen diffusion, oxygen supply can become insufficient, although some inconsistencies exist in the literature regarding WAT oxygen levels in obese humans [14, 16, 17, 39]. Moreover, the increase in free fatty acids in obesity directly increases WAT oxygen consumption through activation of adenine nucleotide translocase 2 (ANT2), a mitochondrial inner-membrane protein normally involved in BAT thermogenesis [40]. ANT2 specifically stimulates the uncoupling of mitochondrial oxidative metabolism, causing energy to be dissipated as heat instead of being coupled to ATP production [41, 42]. The activation of ANT2 suggests that, in obesity, oxygen consumption in WAT is increased in a compensatory attempt to increase energy expenditure.
Importantly, research in animal models has shown that WAT hypoxia and HIF-1α upregulation occur as early as 1–3 days after starting a high-fat diet, before inflammation and insulin resistance develop [40, 43]. This WAT hypoxia exerts a profound influence on tissue homeostasis and dysregulates the expression of many adipokines and pro-inflammatory cytokines [11, 44]. As a consequence, adipose tissue is polarized towards a pro-inflammatory phenotype, further contributing to the attraction of circulating macrophages towards the hypoxic regions [11]. These data support the hypothesis that hypoxia is one of the key features in the development of low grade inflammation and adipose tissue dysfunction [13, 39]. Therefore, the ability to modulate the hypoxia response might hold important therapeutic potential (Table 1).
Table 1.
Summary of key studies on adipocyte-specific HIF modulation
| Gene modulation | Study protocol | Body weight | Glucose tolerance | Adipocyte phenotype | Adipose tissue inflammation | Vasculature | Hepatic metabolism | References |
|---|---|---|---|---|---|---|---|---|
| HIF-1α overexpression | aP2-HIF-1α-ODD transgene construct | Increased | Impaired | Increased cell size | Increased | Not affected | Impaired insulin sensitivity, TG increase | Halberg et al. [43] |
| HIF-1α or HIF-1β deletion | aP2-Cre × HIF-1αf/f/HIF-1βf/f | Decreased | Improved | Smaller adipocytes | Decreased | ND | Increased insulin sensitivity | Jiang et al. [47] |
| HIF-1β deletion | aP2-Cre × HIF-1βf/f | Decreased | Improved | Smaller adipocytes | Unchanged | Reduced permeability | Unchanged lipid accumulation or insulin sensitivity | Lee et al. [53] |
|
HIF-1α deletion HIF-2α deletion |
aP2-Cre × HIF-1αf/f aP2-Cre × HIF-2αf/f |
Unchanged Increased |
Improved Impaired |
Decreased cell mass and size ND |
Decreased Increased |
Not affected ND |
Decreased steatosis and inflammation ND |
Lee et al. [40] |
| HIF-1α deletion | aP2-Dominant-negative (dn)-HIF-1α construct | Increased | Impaired |
‘Whitening’ of BAT Increased WAT mass |
Increased inflammation and fibrosis | Impaired (BAT) | ND | Zhang et al. [69] |
| HIF-2α deletion | Fabp4-Cre × HIF-2αf/f | Increased | Impaired |
‘Whitening’ of BAT Increased WAT mass |
Increased inflammation and fibrosis | Impaired |
Increased steatosis and TG accumulation Progression to NASH |
Garcia-Martin et al. [51] |
| HIF-1α inhibition |
Compound PX-478 Apn-rtTA × TRE-dn-HIF-1α |
Reduced | Improved | Decreased cell mass and size | Decreased | ND | Decreased steatosis | Sun et al. [48] |
| PHD2 inhibition | aP2-Cre × PHD2f/f | Reduced | Improved | Smaller adipocytes | Reduced | Mildly increased | ND | Matsuura et al. [60] |
| PHD2 inhibition | aP2-Cre × PHD2f/f | Increased | Unaffected | Larger adipocytes | Unaffected | Increased | Decreased liver TG | Michailidou et al. [62] |
| PHD2 inhibition |
PHD2gt/gt mice FG-4497 administration |
Reduced Reduced |
Improved Improved |
Smaller adipocytes Smaller adipocytes |
Decreased Decreased |
Not affected ND |
Decreased steatosis ND |
Rahtu-Korpela et al. [114] |
Outcome values in comparison with WT mice after HFD feeding
ND not determined, TG triglycerides
HIF isoform specificity in adipose tissue
HIF-1α can be artificially stabilized by deleting the HIF-α oxygen-dependent degradation domain, thus conferring resistance to hydroxylation by PHDs [45]. Using this approach, Halberg et al. [43] found a HIF-1α dose-dependent increase in adipocyte cell mass. HIF-1α transgene hemizygotic mice gained more weight and experienced decreased glucose tolerance compared to their wild-type (WT) littermates. This led to WAT fibrosis through upregulation of various collagens and the collagen cross-linking protein lysyl oxidase. Fibrosis preceded infiltration by immune cells, and inhibition of lysyl oxidase resulted in a reversal of glucose intolerance, fibrosis, and inflammation, indicating that HIF-associated fibrosis is a central mechanism of WAT dysfunction [43] (Fig. 2a).
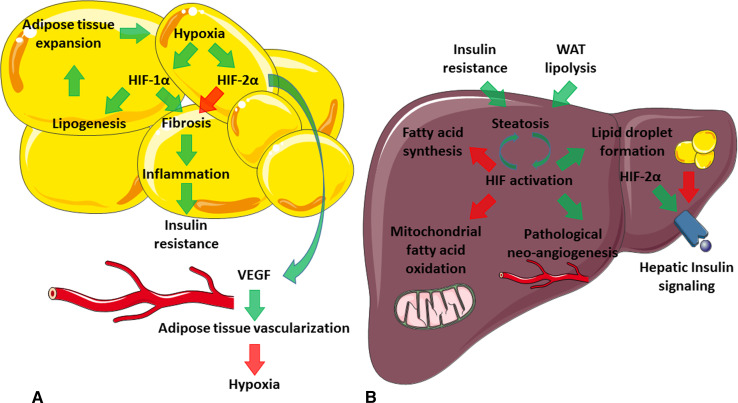
Fig. 2.
Proposed model of HIF actions in obesity and fatty liver. Green arrows activation; red arrows inhibition. a White adipose tissue (WAT) expansion in obesity causes regional hypoxia, activating the hypoxia adaptive response pathway. HIF-1α increases collagen deposition and crosslinking, causing WAT fibrosis. This in turn underlies the inflammation and insulin resistance characteristic of the metabolic syndrome. HIF-2α has positive effects on WAT, most likely through counteracting the HIF-1α pathway. In addition, HIF-2α also stimulates WAT angiogenesis, improving oxygen delivery. b Insulin resistance and WAT lipolysis cause ectopic lipid deposition in the liver. Hepatic steatosis is associated with perivenous hypoxia, again activating HIF signaling. Hypoxia has complicated effects, yet in general lowers oxygen and energy consumption through inhibition of both oxidative metabolism and fatty acid synthesis. Fatty acids are stored as lipid droplets to protect against the toxic effects of free fatty acids. Liver hypoxia is associated with pathological neo-angiogenesis, which further deranges metabolic function. The role of HIF-2α in NAFLD is still under debate, as it also improves hepatic metabolism, in particular hepatic insulin signaling
In addition, a Cre recombinase under the control of the human adipocyte fatty acid-binding protein (aP2) promotor has been used to obtain adipose-tissue specific knock-out (KO) of HIF-1α [46]. This protected against diet-induced glucose intolerance and WAT inflammation [40, 47]. HIF-1α KO increased energy expenditure, in part by increasing physical activity and in part by increasing fatty acid β-oxidation [47], in keeping with the role of HIFs in reducing oxidative metabolism [6]. These results were reproduced by the administration of PX-478, a selective HIF-1α inhibitor. PX-478 treatment reduced HFD-induced weight gain, adipocyte size, and serum leptin levels, suppressed WAT fibrosis and inflammation, and increased glucose tolerance [48]. In agreement with these data, temporally induced overexpression of a defective HIF-1α protein by means of a doxycycline sensitive transgene resulted in the same metabolic phenotype [48].
Conversely, HIF-2α is a promotor of adipocyte differentiation [49] and plays a protective role in adipose tissue. HFD feeding downregulates WAT HIF-2α levels [47], and HIF-2α haplodeficient mice displayed more glucose and insulin intolerance and showed increased expression of fibrotic collagen genes and pro-inflammatory macrophages in WAT compared to WT littermates [50]. Adipocyte-specific HIF-2α ablation had similar effects [40, 51]. Nevertheless, some ambiguity remains, as another group reported that VHL deletion, resulting in HIF-α accumulation, caused WAT inflammation. This effect was HIF-2α-dependent and resulted in critical hypertrophic cardiomyopathy, independent of weight gain or insulin intolerance [52].
A combined adipocyte-specific deletion of HIF-1α and HIF-2α phenotypically resembled HIF-1α KO mice, indicating that HIF-1α is the dominant isoform in WAT [40]. Correspondingly, deletion of HIF-1β, which impairs both HIF-1α and HIF-2α signaling, had comparable effects to HIF-1α deletion [47, 53].
At the molecular level, hypoxia induces a switch from aerobic to anaerobic metabolism. An increase in anaerobic glycolysis depends on increased glucose transport, which is highly upregulated in WAT by hypoxia [39]. Indeed, glucose uptake and lactate production progressively increase as oxygen levels drop in adipose tissue, with major shifts in glucose utilization between oxygen tensions of 10 and 3 % O2 [54]. As one animal study showed that oxygen levels were about 45 mm Hg (6.5 % O2) in lean mice and 15 mm Hg (2 % O2) in obese mice [10], these results show that adipocytes are highly sensitive to oxygen. A relatively small expansion of WAT can change oxygen tensions, which in turn can drastically impact WAT gene expression.
Interestingly, WAT HIF-1β ablation decreased glucose uptake through downregulation of the HIF-targets GLUT1 and 4, with a secondary decrease in glucose uptake and lipogenesis [53]. It has been suggested that this mechanism is involved in the reduced fat accumulation in HIF-1α and HIF-1β adipose KO mice. This hypothesis needs to be corroborated, as high-altitude hypoxia has also been known to induce marked weight loss through HIF signaling [55]. Moreover, insulin induces translocation and activation of GLUT4 in adipose tissue, whereas HIF-1β ablation greatly improved insulin sensitivity, so that an increase rather than a decrease in GLUT4 levels was to be expected [53, 56].
Role of PHDs to modulate HIFs in adipose tissue
A relatively novel approach in modifying the hypoxia adaptive response in obesity and metabolic disease has focused on inhibition of PHDs, inspired by promising applications of PHD inhibitors (PHI) in animal models of anemia and ischemic disease [57]. Two recently published clinical trials using the PHI roxadustat for anemia in chronic kidney disease show potential benefit, via upregulation of HIF-dependent genes in iron metabolism and hemoglobin synthesis [58, 59]. Trials investigating PHIs in coronary and peripheral vascular ischemia are underway [57].
Concerning adipose tissue, Matsuura et al. [60] found that adipocyte-specific deletion of PHD2 diminished HFD-induced weight gain. Adipocytes were smaller and glucose and insulin tolerance were likewise improved, secondary to a HIF-induced switch to anaerobic glycolysis. This resulted in inefficient metabolism, comparable to the weight-reducing effects of hypoxia in high altitude and chronic lung disease [55, 61]. However, Michailidou et al. [62] stated different conclusions using an almost identical study protocol. Their mice gained considerable weight but maintained normal glucose tolerance and experienced a remarkable reduction in liver triglyceride content. Reasons for this discrepancy could include an upregulation of PHD3 found only in one of these studies [60]. Indeed, PHD3 upregulation compensates for decreased PHD activity in continued hypoxia to adapt cells to a novel set point [35, 63]. A similar mechanism could account for the observed effect in the study performed by Matsuura et al., as PHD2 deletion artificially pre-adapts tissues to a lower oxygen set point [60].
Modulation of HIFs and PHDs in adipose tissue have thus resulted in discrepant outcomes, which are not easily reconciled. In this context, it is important to note that both HIF-1α and PHDs stand at cross-roads of diverse signaling pathways. PHDs not only sense oxygen but also various metabolites, such as citric acid cycle intermediates and reactive oxygen species [64, 65], whereas HIF-1α expression is, for example, also affected by adipocyte differentiation and insulin levels [66]. Furthermore, PHDs regulate targets other than HIF-1α, such as the key glycolytic enzyme pyruvate kinase M2 and the nuclear factor kappa-light chain-enhancer of activated B cells (NFκB) pathway [65, 67].
Concerning the role of PHDs in hypoxia, the hypoxia autoregulatory feedback mechanism could possibly be exploited by PHD inhibition to create a therapeutic window of HIF-1α and HIF-2α upregulation. A study by the group of Giaccia [68] provided the first proof-of-concept by demonstrating that liver-specific PHD3 deletion improves hepatic insulin signaling, mainly through HIF-2α stabilization. A further rise in HIF-2α (and HIF-1α) levels, obtained by simultaneous PHD1 and/or PHD2 deletions, did not further improve insulin signaling and even caused liver steatosis.
Although PHD inhibition is a promising approach, further research in this area is needed to explore these discordant results.
Hypoxia-induced angiogenesis in adipose tissue
One of the central hypoxia-adaptive mechanisms induced by HIFs, in virtually every tissue, is the stimulation of angiogenesis through VEGF upregulation.
Zhang et al. [69] found that HIF-1α inhibition in BAT results in a marked disruption of BAT angiogenesis, and secondarily in impaired thermogenic activity, glucose intolerance and WAT inflammation [69]. It is therefore surprising that neither HIF-1α overexpression nor deletion significantly altered angiogenesis in WAT [40, 43]. Conversely, adipocyte HIF-2α deletion resulted in deficient vascularization in both WAT and BAT [51]. Michailidou et al. further observed a twofold increase in WAT vessel density as a result of adipocyte-specific PHD2 deletion, mediated by HIF2α stabilization [62]. This was accompanied by a 50 % increase in WAT mass, but not with metabolic dysregulation. Mechanistically, a prophylactic vascular remodeling of the adipose tissue was induced by HIF upregulation, allowing a further increase in WAT mass whilst preventing WAT inflammation and ectopic fat deposition, a major cause of insulin resistance development [62, 70, 71]. Several studies have indeed shown that transgenic overexpression of VEGF prevents and even reverts HFD-induced metabolic dysfunction through increased vascularization [72–74]. Accordingly, WAT contained less hypoxic areas and HIF-1α protein levels were significantly decreased compared to WT controls [73, 74]. VEGF also induced transdifferentiation of white to thermogenic beige adipocytes [18, 72, 75].
In summary, VEGF is a potent inducer of adipose tissue angiogenesis and can pre-adapt WAT to hypoxia [37]. This mechanism is HIF-2α dependent, while HIF-1α intriguingly seems to regulate vascularization in brown but not white adipose tissue (Fig. 2a).
Hypoxia signaling in fatty liver disease
Cross-talk between adipose tissue hypoxia and fatty liver disease
Adipose tissue hypoxia also stimulates basal lipolysis, another key feature of adipose tissue dysfunction. Although a study by Lolmede et al. could not demonstrate an influence of hypoxia on basal lipolysis in vitro [76], other groups subsequently reported an increase in lipolysis in vitro and in vivo in hypoxic conditions [77–79]. Corresponding to the latter studies, lipolysis is inversely correlated with WAT oxygen tensions in human patients [80]. The resultant release of fatty acids into the bloodstream leads to re-esterification and deposition of fatty acids in insulin-sensitive organs such as the liver, causing both insulin resistance and NAFLD [20, 21].
Accordingly, adipocyte HIF modulation affected liver lipid accumulation. Use of the hyperinsulinemic-euglycemic clamp technique to quantify insulin resistance revealed that adipocyte HIF-1α KO leads to decreased gluconeogenesis and thus improved insulin sensitivity [40, 47]. Moreover, both genetic and pharmacological HIF-1α inhibition were able to prevent NAFLD [40, 47, 48] as well as hepatocyte inflammation [40], which is an essential step in the progression to NASH [81], while adipocyte-specific HIF-1α overexpression had the opposite effects [43].
Hypoxia affects hepatic lipid metabolism
Because of the unique vascularization of the liver, there exists an oxygen gradient across the liver lobule, with lower oxygen tensions in the perivenous regions [22]. Research in animal models has shown that liver lipid deposition induces perivenous hypoxia and HIF-1α activation [25].
Important evidence for a pathophysiological role of hypoxia on hepatic lipid metabolism is provided by the now well established independent link between the obstructive sleep apnea syndrome, causing intermittent hypoxia, and NAFLD development and stage [82–84]. In experimental models of high-fat diet-induced obesity, exposure to intermittent hypoxia exacerbated insulin intolerance and hepatic steatosis [85]. Additionally, HIF-1α haplodeficiency limited steatosis development when mice were submitted to intermittent hypoxia, suggesting a role for HIF-1α in sleep apnea-related NAFLD [86].
Hypoxia signaling exerts its effects through a direct regulation of hepatic lipid metabolism (Fig. 2b). Breakdown of fatty acids through mitochondrial β-oxidation consumes large amounts of oxygen and is a major HIF target [87]. Hepatic fatty acid β-oxidation is regulated by the transcriptional control of peroxisome proliferator-activated receptor α (PPARα) over key mitochondrial oxidative enzymes [88]. Gene expression analysis revealed that PPARα and its target genes are indeed downregulated in hepatocyte HIF-2α overexpressing mice [89, 90], whereas HIF-1α KO in hepatocytes leads to an induction of oxidative enzymes [91]. PPARα agonism has beneficial effects in preventing NAFLD, NASH and progression to fibrosis [92]. Unsurprisingly, it has since long been an attractive pharmacological target [93], and promising new PPAR agonists are currently being developed for the treatment of metabolic disorders [94].
The effect of hypoxia signaling on lipogenesis, another oxygen-consuming metabolic process, is more ambiguous. Qu et al. [90] have shown a downregulation of key lipogenic enzymes 2 weeks after genetic induction of HIF-α in hepatocytes, in an attempt to limit energy consumption in hypoxic cells [87]. Nevertheless, 3 days after HIF-α induction a marked upregulation of lipogenic enzymes was observed. Possibly, this upregulation was secondary to a HIF-mediated increased uptake of glucose by hepatocytes [6, 95].
Moreover, the lipid binding protein adipocyte differentiation-related protein (ADRP) was upregulated in hepatocyte-specific HIF overexpressing mice [89, 96]. ADRP strongly promotes lipid droplet formation in various tissues [97, 98]. Formation of lipid droplets may represent a protective mechanism against the direct toxic effects of free fatty acids, which cause oxidative stress and inflammation [81], although this initiates a vicious circle of lipid deposition and hypoxia.
Extrapolation from experimental data to the role of hypoxia in human liver metabolism is not straightforward. The intricate regulation of hepatic lipid metabolism by HIFs is made even more complex by zonation of metabolism and oxygen supply in the liver. Perivenous hepatocytes, prone to hypoxia, are more engaged in lipogenesis, whereas the well-oxygenated periportal hepatocytes are more involved in β-oxidation [99]. Hence, whereas overexpression HIF-α isoforms in mouse models inhibits fatty acid oxidation, perivenous hypoxia would not affect periportal β-oxidation in human NAFLD [87]. In patients with NAFLD, β-oxidation is indeed typically increased instead of reduced [100]. And although hypoxia inhibits lipogenesis, lipogenic genes are upregulated in human NAFLD and play a central role in hepatic fat accumulation [101, 102]. Therefore, zonated modulation of the hypoxia response in fatty liver disease could provide interesting insights.
Hepatocyte-specific HIF modulation in fatty liver disease
To modulate the hypoxia response system in vivo, hepatocyte-selective ablation and overexpression models of HIFs have been used. These were obtained either by injection of adenoviral particles or by hepatocyte-specific Cre-mediated recombination, in most cases by use of the albumin-Cre mouse strain [103] (Table 2).
Table 2.
Summary of key studies on hepatocyte-specific HIF modulation
| Gene modulation | Study protocol | Phenotype | Lipogenic genes | β-Oxidation | References |
|---|---|---|---|---|---|
| HIF-1α and/or HIF-2α overexpression | Alb-Cre × VHLf/f, HIF-1dPA and/or HIF-2dPA |
Severe steatosis in Alb-Cre; VHLf/f and Alb-Cre; HIF1dPA/HIF2dPA mice Moderate steatosis in Alb-Cre; HIF1dPA mice |
ND | ND | Kim et al. [105] |
| HIF-1α and/or HIF-2α overexpression | Alb-Cre × VHLf/f ± HIF-1αf/f and/or HIF-2αf/f |
Severe steatosis in mice overexpressing HIF-2α (±HIF-1α) HIF-1α overexpression alone does not cause steatosis |
Downregulation | Suppression | Rankin et al. [89] |
| Temporally induced HIF-1α and/or HIF-2α overexpression | SA-Cre-ERT2 × VHLf/f ± HIF-1αf/f and/or HIF-2αf/f |
Severe steatosis in mice overexpressing HIF-2α (±HIF-1α) HIF-1α overexpression does not cause steatosis |
Upregulated after 3 days, downregulated after 14 days | Suppression | Qu et al. [90] |
|
HIF-1α deletion PHD inhibition |
Alb-Cre × HIF-1αf/f + Lieber-DeCarli diet Dimethyloxalylglycine |
Exacerbated steatosis Increased serum TG Decreased steatosis and liver TG |
Upregulated Downregulated |
Induction ND |
Nishiyama et al. [91] |
|
HIF-1α overexpression HIF-1α deletion |
Alb-Cre × HIF-1dPA + alcoholic diet Alb-Cre × HIF-1αf/f + alcoholic diet |
Increased steatosis and liver TG, increased serum ALT No increase in ALT, protected against steatosis |
ND ND |
Suppression ND |
Nath et al. [96] |
| HIF-1β deletion | Adenoviral Cre injection in HIF-1βf/f mice ± high-fat diet |
Increased gluconeogenesis Mild insulin resistance Decreased liver TG content |
Upregulated | Not affected | Wang et al. [112] |
ND not determined, ALT alanine aminotransferase, TG triglycerides
The first studies showing the significance of HIF modulation in hepatic steatosis used conditional VHL deletions, which prevented proteasomal degradation of hydroxylated HIFs. This resulted in increased vascularization, cavernous liver hemangiomas and severe hepatic steatosis [104]. This phenotype was copied by simultaneous HIF-1α and HIF-2α overexpression using degradation-resistant HIF variants (HIF-1dPA and HIF-2dPA) [105]. Furthermore, HIF-1α activation resulted in moderate steatosis, whereas HIF-2α overexpression greatly increased liver microvascular density with only minimal lipid deposition [105].
By inducing simultaneous deletions in VHL and HIF-1α and/or HIF-2α, to reverse the overexpression of specific HIFs after VHL deletion, a dominant role for HIF-2α in fatty liver disease was asserted, as deletion of HIF-2α, but not of HIF-1α, prevented the severe steatosis associated with VHL deletion [89]. Qu et al. [90] have confirmed these data and showed that temporal disruption of VHL causes severe steatohepatitis with progression to fibrosis. Again, deletion of HIF-2α but not HIF-1α rescued this phenotype.
Conversely, several papers have convincingly shown pathophysiological effects of HIF-1α in fatty liver disease. Using degradation-resistant HIF-1dPA mice, Nath et al. [96] demonstrated that hepatocyte HIF-1α overexpression causes hepatomegaly and liver triglyceride accumulation, which were further exacerbated by liquid ethanol administration, whereas hepatocyte-specific HIF-1α KO provided protection against steatosis. Comparable results were obtained in models of diet-induced obesity, as antisense nucleotide treatment, resulting in both hepatocyte and adipocyte HIF downregulation, protected against adverse metabolic effects of HFD feeding [106]. However, Nishiyama et al. [91] postulated the opposite hypothesis of HIF-1α as an adaptive mechanism, as they found that hepatocyte HIF-1α deletion aggravated lipid deposition in an alcoholic model of fatty liver disease. The same group also reported the development of insulin resistance when these mice were fed a HFD [107]. These discrepant results are difficult to reconcile, as both groups [91, 96] have used identical genetic approaches. However, different control animals were used by the two groups, which may have biased the outcome. It has also been suggested that differences in the intestinal flora between the two research group animal facilities and between the different mice strains could have influenced steatosis progression, as it is well known that the gut microbiome contributes to the development of NAFLD [96, 108–110]. Research in related fields, such as liver fibrosis, nonetheless suggests that HIF-1α is a mediator of liver disease progression rather than a protective regulator [111].
In addition to HIF1α modulation, hepatocytic deletion of HIF-1β, targeting all HIF signaling pathways, has resulted in increased gluconeogenesis and mild whole-body insulin resistance, yet slightly lowers hepatic triglyceride content [112]. Consistently, another group reported a decrease in steatosis in hepatocyte HIF-1β null mice when subjected to binge ethanol models [113].
Taken together, these studies indicate that both HIF-1α and HIF-2α promote hepatic lipid accumulation. Which HIF-α isoform plays the dominant role, however, remains to be elucidated. The difference in the genetic mouse models used may explain some of the discrepancies. For instance, indications for a pathological role of HIF-2α in WAT [52] and fatty liver disease [89, 90] have solely been obtained in mice with tissue-specific VHL deletions by showing that additional HIF-2α KO reversed these effects. Further research with other genetic approaches to HIF-2α could help to clarify this discussion.
Role of PHD inhibition as a treatment strategy in fatty liver disease
Parallel to PHD inhibition in obesity, it also represents an attractive target in fatty liver disease. Rahtu-Korpela et al. [114] have used hypomorphic PHD2gt/gt mice, which express decreased amounts of PHD2 and show differing levels of HIF-1α and HIF-2α stabilization across various tissues [115]. Whereas 1-year-old control littermates spontaneously developed insulin resistance and hepatic steatosis, PHD2gt/gt mice were protected against metabolic dysregulation through an upregulation of insulin receptor substrate 2 (Irs2) [114]. These mice retained improved metabolic parameters after a HFD. As only hepatic HIF-2α was stabilized, these results are concordant with a study by Wei et al. [116]. They found that VEGF inhibition and the concomitant impaired angiogenesis caused local hypoxia and specifically stabilized HIF-2α, which in turn improved insulin signaling through Irs2 induction. Therefore, these studies postulate a beneficial effect of moderate HIF-2α upregulation in fatty liver disease through PHD inhibition, contrary to the studies that used VHL deletion to stabilize HIFs discussed in the previous paragraph.
Remarkably, pharmacological PHD inhibition with the compound FG-4497 led to highly similar results in the models of metabolic dysregulation, suggesting that treatment could not only prevent but also reverse metabolic dysfunction [114]. In accord with this study, Nishiyama et al. [91] demonstrated a marked improvement in alcoholic fatty liver disease upon treatment with the PHI dimethyloxalylglycine. Although isoform-specific PHD inhibitors are not yet available, these could represent a new approach in the treatment of obesity and its associated metabolic disorders.
Hypoxia-induced angiogenesis in fatty liver disease
In chronic liver disease, especially hepatocellular carcinoma [28, 117], hypoxia-induced angiogenesis is an attractive therapeutic target, yet its role in NAFLD is not extensively researched. We have shown that increased angiogenesis and VEGF upregulation are hallmarks of NAFLD progression, whereas treatment with anti-VEGF receptor 2 antibodies prevents disease progression [118]. Wei et al. [116] have additionally demonstrated that VEGF inhibition improves hepatic insulin signaling through induction of hypoxia and stabilization of HIF-2α.
In general, HIF-2α seems to play a major role in blood vessel normalization and homeostasis and can impact tissue function and metabolism in various organs [34, 119]. Thus, the HIF/PHD pathways could represent an attractive therapeutic strategy to remodel the hypoxia-induced angiogenic response in obesity and NAFLD.
Conclusion and future perspectives
The prevention and treatment of obesity and the associated non-alcoholic fatty liver disease has become one of the major priorities in public health care. Unfortunately, sustained weight loss is difficult to achieve for many patients, and no pharmacological treatment can satisfactorily remedy these conditions. Therefore, a greater understanding of the underlying pathological mechanisms is required. Hypoxia signaling pathways represent an attractive target in obesity and NAFLD. Although HIF modulation in adipose tissue and fatty liver has yielded conflicting results, prolyl hydroxylase inhibition is a promising approach as studies have consistently yielded beneficial effects.
It is of significance that pharmacological HIF-1α and PHD inhibition were able to prevent or reverse the metabolic dysregulation associated with obesity, thus proving the therapeutic potential of tweaking the hypoxia response. As there are still many gaps in our understanding of hypoxia in metabolic disorders, further research is warranted to determine the physiological functions of each HIF isoform, and to explore their cell-specific role in adipose and hepatic tissue.
Acknowledgments
The authors would like to thank Margot Guillemin for her critical reading of the manuscript.
Compliance with ethical standards
Financial support
S.L. and X.V. received a research Grant from the Fund for Scientific Research (FWO Flanders, FWO15/ASP/146 and 1700214N, respectively). H.V.V. is a senior clinical researcher of the FWO Flanders.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
L. Devisscher and A. Geerts contributed equally to this manuscript.
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of non-alcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence and outcomes. Hepatology. 2015 doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47(3):181–190. doi: 10.1016/j.dld.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T, Shinjo S, Arai T, Kanai M, Goda N. Hypoxia and fatty liver. World J Gastroenterol. 2014;20(41):15087–15097. doi: 10.3748/wjg.v20.i41.15087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nath B, Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology. 2012;55(2):622–633. doi: 10.1002/hep.25497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer BF, Clegg DJ. Oxygen sensing and metabolic homeostasis. Mol Cell Endocrinol. 2014;397(1–2):51–58. doi: 10.1016/j.mce.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293(4):E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 11.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32(3):451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 12.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 13.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347–355. doi: 10.1079/BJN20041213. [DOI] [PubMed] [Google Scholar]
- 14.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58(3):718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI, Kurz A. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100(2):274–280. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodson L, Humphreys SM, Karpe F, Frayn KN. Metabolic signatures of human adipose tissue hypoxia in obesity. Diabetes. 2013;62(5):1417–1425. doi: 10.2337/db12-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goossens GH, Bizzarri A, Venteclef N, et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124(1):67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 18.Chechi K, Nedergaard J, Richard D. Brown adipose tissue as an anti-obesity tissue in humans. Obes Rev. 2014;15(2):92–106. doi: 10.1111/obr.12116. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu I, Aprahamian T, Kikuchi R, Shimizu A, Papanicolaou KN, MacLauchlan S, Maruyama S, Walsh K. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest. 2014;124(5):2099–2112. doi: 10.1172/JCI71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126(1):12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sattar N, Gill JM. Type 2 diabetes as a disease of ectopic fat? BMC Med. 2014;12:123. doi: 10.1186/s12916-014-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31(2):255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- 23.Arteel GE, Iimuro Y, Yin M, Raleigh JA, Thurman RG. Chronic enteral ethanol treatment causes hypoxia in rat liver tissue in vivo. Hepatology. 1997;25(4):920–926. doi: 10.1002/hep.510250422. [DOI] [PubMed] [Google Scholar]
- 24.Arteel GE, Raleigh JA, Bradford BU, Thurman RG. Acute alcohol produces hypoxia directly in rat liver tissue in vivo: role of Kupffer cells. Am J Physiol. 1996;271(3 Pt 1):G494–G500. doi: 10.1152/ajpgi.1996.271.3.G494. [DOI] [PubMed] [Google Scholar]
- 25.Mantena SK, Vaughn DP, Andringa KK, Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW, Darley-Usmar VM, Bailey SM. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417(1):183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993;268(29):21513–21518. [PubMed] [Google Scholar]
- 27.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92(12):5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, Van Vlierberghe H. Angiogenesis in chronic liver disease and its complications. Liver Int. 2011;31(2):146–162. doi: 10.1111/j.1478-3231.2010.02369.x. [DOI] [PubMed] [Google Scholar]
- 29.Ichiki T, Sunagawa K. Novel roles of hypoxia response system in glucose metabolism and obesity. Trends Cardiovasc Med. 2014;24(5):197–201. doi: 10.1016/j.tcm.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Kaelin WG., Jr Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2(9):673–682. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- 31.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12(1):9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 33.Loboda A, Jozkowicz A, Dulak J. HIF-1 versus HIF-2–is one more important than the other? Vascul Pharmacol. 2012;56(5–6):245–251. doi: 10.1016/j.vph.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev Cell. 2009;16(2):167–179. doi: 10.1016/j.devcel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Stiehl DP, Wirthner R, Koditz J, Spielmann P, Camenisch G, Wenger RH. Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem. 2006;281(33):23482–23491. doi: 10.1074/jbc.M601719200. [DOI] [PubMed] [Google Scholar]
- 36.Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279(37):38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 37.Corvera S, Gealekman O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta. 2014;1842(3):463–472. doi: 10.1016/j.bbadis.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christiaens V, Lijnen HR. Angiogenesis and development of adipose tissue. Mol Cell Endocrinol. 2010;318(1–2):2–9. doi: 10.1016/j.mce.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93(1):1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 40.Lee YS, Kim JW, Osborne O, et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell. 2014;157(6):1339–1352. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Busiello RA, Savarese S, Lombardi A. Mitochondrial uncoupling proteins and energy metabolism. Front Physiol. 2015;6:36. doi: 10.3389/fphys.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trayhurn P, Alomar SY. Oxygen deprivation and the cellular response to hypoxia in adipocytes–perspectives on white and brown adipose tissues in obesity. Front Endocrinol (Lausanne) 2015;6:19. doi: 10.3389/fendo.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halberg N, Khan T, Trujillo ME, et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29(16):4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56(4):901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 45.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95(14):7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409(6821):729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 47.Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes. 2011;60(10):2484–2495. doi: 10.2337/db11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol Cell Biol. 2013;33(5):904–917. doi: 10.1128/MCB.00951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimba S, Wada T, Hara S, Tezuka M. EPAS1 promotes adipose differentiation in 3T3-L1 cells. J Biol Chem. 2004;279(39):40946–40953. doi: 10.1074/jbc.M400840200. [DOI] [PubMed] [Google Scholar]
- 50.Choe SS, Shin KC, Ka S, Lee YK, Chun JS, Kim JB. Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes. 2014;63(10):3359–3371. doi: 10.2337/db13-1965. [DOI] [PubMed] [Google Scholar]
- 51.Garcia-Martin R, Alexaki VI, Qin N, et al. Adipocyte-specific HIF2α deficiency exacerbates obesity-induced brown adipose tissue dysfunction and metabolic dysregulation. Mol Cell Biol. 2015;36(3):376–393. doi: 10.1128/MCB.00430-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin Q, Huang Y, Booth CJ, Haase VH, Johnson RS, Celeste Simon M, Giordano FJ, Yun Z. Activation of hypoxia-inducible factor-2 in adipocytes results in pathological cardiac hypertrophy. J Am Heart Assoc. 2013;2(6):e000548. doi: 10.1161/JAHA.113.000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee KY, Gesta S, Boucher J, Wang XL, Kahn CR. The differential role of Hif1β/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation. Cell Metab. 2011;14(4):491–503. doi: 10.1016/j.cmet.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wood IS, Stezhka T, Trayhurn P. Modulation of adipokine production, glucose uptake and lactate release in human adipocytes by small changes in oxygen tension. Pflugers Arch. 2011;462(3):469–477. doi: 10.1007/s00424-011-0985-7. [DOI] [PubMed] [Google Scholar]
- 55.Palmer BF, Clegg DJ. Ascent to altitude as a weight loss method: the good and bad of hypoxia inducible factor activation. Obesity (Silver Spring) 2014;22(2):311–317. doi: 10.1002/oby.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cushman SW, Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980;255(10):4758–4762. [PubMed] [Google Scholar]
- 57.Selvaraju V, Parinandi NL, Adluri RS, Goldman JW, Hussain N, Sanchez JA, Maulik N. Molecular mechanisms of action and therapeutic uses of pharmacological inhibitors of HIF-prolyl 4-hydroxylases for treatment of ischemic diseases. Antioxid Redox Signal. 2014;20(16):2631–2665. doi: 10.1089/ars.2013.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Besarab A, Chernyavskaya E, Motylev I, et al. Roxadustat (FG-4592): correction of Anemia in Incident Dialysis Patients. J Am Soc Nephrol. 2015;27(4):1225–1233. doi: 10.1681/ASN.2015030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Besarab A, Provenzano R, Hertel J, Zabaneh R, Klaus SJ, Lee T, Leong R, Hemmerich S, Yu KH, Neff TB. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant. 2015;30(10):1665–1673. doi: 10.1093/ndt/gfv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuura H, Ichiki T, Inoue E, Nomura M, Miyazaki R, Hashimoto T, Ikeda J, Takayanagi R, Fong GH, Sunagawa K. Prolyl hydroxylase domain protein 2 plays a critical role in diet-induced obesity and glucose intolerance. Circulation. 2013;127(21):2078–2087. doi: 10.1161/CIRCULATIONAHA.113.001742. [DOI] [PubMed] [Google Scholar]
- 61.Raguso CA, Guinot SL, Janssens JP, Kayser B, Pichard C. Chronic hypoxia: common traits between chronic obstructive pulmonary disease and altitude. Curr Opin Clin Nutr Metab Care. 2004;7(4):411–417. doi: 10.1097/01.mco.0000134372.78438.09. [DOI] [PubMed] [Google Scholar]
- 62.Michailidou Z, Morton NM, Moreno Navarrete JM, West CC, Stewart KJ, Fernandez-Real JM, Schofield CJ, Seckl JR, Ratcliffe PJ. Adipocyte pseudohypoxia suppresses lipolysis and facilitates benign adipose tissue expansion. Diabetes. 2015;64(3):733–745. doi: 10.2337/db14-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minamishima YA, Moslehi J, Padera RF, Bronson RT, Liao R, Kaelin WG., Jr A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Mol Cell Biol. 2009;29(21):5729–5741. doi: 10.1128/MCB.00331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell. 2005;7(1):77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 65.Wong BW, Kuchnio A, Bruning U, Carmeliet P. Emerging novel functions of the oxygen-sensing prolyl hydroxylase domain enzymes. Trends Biochem Sci. 2013;38(1):3–11. doi: 10.1016/j.tibs.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 66.He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J. Regulation of HIF-1α activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab. 2011;300(5):E877–E885. doi: 10.1152/ajpendo.00626.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145(5):732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taniguchi CM, Finger EC, Krieg AJ, et al. Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nat Med. 2013;19(10):1325–1330. doi: 10.1038/nm.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X, Lam KS, Ye H, Chung SK, Zhou M, Wang Y, Xu A. Adipose tissue-specific inhibition of hypoxia-inducible factor 1α induces obesity and glucose intolerance by impeding energy expenditure in mice. J Biol Chem. 2010;285(43):32869–32877. doi: 10.1074/jbc.M110.135509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59(2):713–723. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome—an allostatic perspective. Biochim Biophys Acta. 2010;1801(3):338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 72.Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA. 2012;109(15):5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sung HK, Doh KO, Son JE, et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013;17(1):61–72. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 74.Elias I, Franckhauser S, Ferre T, et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61(7):1801–1813. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.During MJ, Liu X, Huang W, Magee D, Slater A, McMurphy T, Wang C, Cao L. Adipose VEGF links the white-to-brown fat switch with environmental, genetic, and pharmacological stimuli in male mice. Endocrinology. 2015;156(6):2059–2073. doi: 10.1210/en.2014-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lolmede K, de Saint Durand, Front V, Galitzky J, Lafontan M, Bouloumie A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int J Obes Relat Metab Disord. 2003;27(10):1187–1195. doi: 10.1038/sj.ijo.0802407. [DOI] [PubMed] [Google Scholar]
- 77.Yin J, Gao Z, He Q, Zhou D, Guo Z, Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab. 2009;296(2):E333–E342. doi: 10.1152/ajpendo.90760.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiong Y, Qu Z, Chen N, Gong H, Song M, Chen X, Du J, Xu C. The local corticotropin-releasing hormone receptor 2 signalling pathway partly mediates hypoxia-induced increases in lipolysis via the cAMP-protein kinase A signalling pathway in white adipose tissue. Mol Cell Endocrinol. 2014;392(1–2):106–114. doi: 10.1016/j.mce.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 79.Hashimoto T, Yokokawa T, Endo Y, Iwanaka N, Higashida K, Taguchi S. Modest hypoxia significantly reduces triglyceride content and lipid droplet size in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2013;440(1):43–49. doi: 10.1016/j.bbrc.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 80.Pasarica M, Rood J, Ravussin E, Schwarz JM, Smith SR, Redman LM. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab. 2010;95(8):4052–4055. doi: 10.1210/jc.2009-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103(2):71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jouet P, Sabate JM, Maillard D, Msika S, Mechler C, Ledoux S, Harnois F, Coffin B. Relationship between obstructive sleep apnea and liver abnormalities in morbidly obese patients: a prospective study. Obes Surg. 2007;17(4):478–485. doi: 10.1007/s11695-007-9085-3. [DOI] [PubMed] [Google Scholar]
- 83.Polotsky VY, Patil SP, Savransky V, et al. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med. 2009;179(3):228–234. doi: 10.1164/rccm.200804-608OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Musso G, Cassader M, Olivetti C, Rosina F, Carbone G, Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14(5):417–431. doi: 10.1111/obr.12020. [DOI] [PubMed] [Google Scholar]
- 85.Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring) 2011;19(11):2167–2174. doi: 10.1038/oby.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li J, Bosch-Marce M, Nanayakkara A, Savransky V, Fried SK, Semenza GL, Polotsky VY. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1α. Physiol Genomics. 2006;25(3):450–457. doi: 10.1152/physiolgenomics.00293.2005. [DOI] [PubMed] [Google Scholar]
- 87.Goda N, Kanai M. Hypoxia-inducible factors and their roles in energy metabolism. Int J Hematol. 2012;95(5):457–463. doi: 10.1007/s12185-012-1069-y. [DOI] [PubMed] [Google Scholar]
- 88.Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR α in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116(3):571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q, Haase VH. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol. 2009;29(16):4527–4538. doi: 10.1128/MCB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qu A, Taylor M, Xue X, Matsubara T, Metzger D, Chambon P, Gonzalez FJ, Shah YM. Hypoxia-inducible transcription factor 2α promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology. 2011;54(2):472–483. doi: 10.1002/hep.24400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nishiyama Y, Goda N, Kanai M, et al. HIF-1α induction suppresses excessive lipid accumulation in alcoholic fatty liver in mice. J Hepatol. 2012;56(2):441–447. doi: 10.1016/j.jhep.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 92.Tailleux A, Wouters K, Staels B. Roles of PPARs in NAFLD: potential therapeutic targets. Biochim Biophys Acta. 2012;1821(5):809–818. doi: 10.1016/j.bbalip.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 93.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 94.Sahebkar A, Chew GT, Watts GF. New peroxisome proliferator-activated receptor agonists: potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert Opin Pharmacother. 2014;15(4):493–503. doi: 10.1517/14656566.2014.876992. [DOI] [PubMed] [Google Scholar]
- 95.Hamaguchi T, Iizuka N, Tsunedomi R, et al. Glycolysis module activated by hypoxia-inducible factor 1α is related to the aggressive phenotype of hepatocellular carcinoma. Int J Oncol. 2008;33(4):725–731. [PubMed] [Google Scholar]
- 96.Nath B, Levin I, Csak T, Petrasek J, Mueller C, Kodys K, Catalano D, Mandrekar P, Szabo G. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53(5):1526–1537. doi: 10.1002/hep.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Magnusson B, Asp L, Bostrom P, Ruiz M, Stillemark-Billton P, Linden D, Boren J, Olofsson SO. Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler Thromb Vasc Biol. 2006;26(7):1566–1571. doi: 10.1161/01.ATV.0000223345.11820.da. [DOI] [PubMed] [Google Scholar]
- 98.Bostrom P, Magnusson B, Svensson PA, Wiklund O, Boren J, Carlsson LM, Stahlman M, Olofsson SO, Hulten LM. Hypoxia converts human macrophages into triglyceride-loaded foam cells. Arterioscler Thromb Vasc Biol. 2006;26(8):1871–1876. doi: 10.1161/01.ATV.0000229665.78997.0b. [DOI] [PubMed] [Google Scholar]
- 99.Hijmans BS, Grefhorst A, Oosterveer MH, Groen AK. Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. Biochimie. 2014;96:121–129. doi: 10.1016/j.biochi.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 100.Kohjima M, Enjoji M, Higuchi N, et al. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2007;20(3):351–358. [PubMed] [Google Scholar]
- 101.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, Kellum JM, Min H, Luketic VA, Sanyal AJ. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48(6):1810–1820. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis. 2000;26(2):149–150. doi: 10.1002/(SICI)1526-968X(200002)26:2<149::AID-GENE16>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 104.Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel–Lindau tumor suppressor. Proc Natl Acad Sci USA. 2001;98(4):1583–1588. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim WY, Safran M, Buckley MR, Ebert BL, Glickman J, Bosenberg M, Regan M, Kaelin WG., Jr Failure to prolyl hydroxylate hypoxia-inducible factor α phenocopies VHL inactivation in vivo. EMBO J. 2006;25(19):4650–4662. doi: 10.1038/sj.emboj.7601300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shin MK, Drager LF, Yao Q, Bevans-Fonti S, Yoo DY, Jun JC, Aja S, Bhanot S, Polotsky VY. Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1α. PLoS One. 2012;7(10):e46562. doi: 10.1371/journal.pone.0046562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ochiai D, Goda N, Hishiki T, Kanai M, Senoo-Matsuda N, Soga T, Johnson RS, Yoshimura Y, Suematsu M. Disruption of HIF-1α in hepatocytes impairs glucose metabolism in diet-induced obesity mice. Biochem Biophys Res Commun. 2011;415(3):445–449. doi: 10.1016/j.bbrc.2011.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Le Roy T, Llopis M, Lepage P, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62(12):1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 109.Diamant M, Blaak EE, de Vos WM. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes Rev. 2011;12(4):272–281. doi: 10.1111/j.1467-789X.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 110.Mehal WZ. HIF-1α is a major and complex player in alcohol induced liver diseases. J Hepatol. 2012;56(2):311–312. doi: 10.1016/j.jhep.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 111.Moon JO, Welch TP, Gonzalez FJ, Copple BL. Reduced liver fibrosis in hypoxia-inducible factor-1α-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2009;296(3):G582–G592. doi: 10.1152/ajpgi.90368.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang XL, Suzuki R, Lee K, et al. Ablation of ARNT/HIF1β in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab. 2009;9(5):428–439. doi: 10.1016/j.cmet.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ni HM, Bhakta A, Wang S, Li Z, Manley S, Huang H, Copple B, Ding WX. Role of hypoxia inducing factor-1β in alcohol-induced autophagy, steatosis and liver injury in mice. PLoS One. 2014;9(12):e115849. doi: 10.1371/journal.pone.0115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rahtu-Korpela L, Karsikas S, Horkko S, et al. HIF prolyl 4-hydroxylase-2 inhibition improves glucose and lipid metabolism and protects against obesity and metabolic dysfunction. Diabetes. 2014;63(10):3324–3333. doi: 10.2337/db14-0472. [DOI] [PubMed] [Google Scholar]
- 115.Hyvarinen J, Hassinen IE, Sormunen R, Maki JM, Kivirikko KI, Koivunen P, Myllyharju J. Hearts of hypoxia-inducible factor prolyl 4-hydroxylase-2 hypomorphic mice show protection against acute ischemia-reperfusion injury. J Biol Chem. 2010;285(18):13646–13657. doi: 10.1074/jbc.M109.084855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wei K, Piecewicz SM, McGinnis LM, et al. A liver Hif-2α-Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition. Nat Med. 2013;19(10):1331–1337. doi: 10.1038/nm.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heindryckx F, Kuchnio A, Casteleyn C, Coulon S, Olievier K, Colle I, Geerts A, Libbrecht L, Carmeliet P, Van Vlierberghe H. Effect of prolyl hydroxylase domain-2 haplodeficiency on the hepatocarcinogenesis in mice. J Hepatol. 2012;57(1):61–68. doi: 10.1016/j.jhep.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 118.Coulon S, Legry V, Heindryckx F, et al. Role of vascular endothelial growth factor in the pathophysiology of nonalcoholic steatohepatitis in two rodent models. Hepatology. 2013;57(5):1793–1805. doi: 10.1002/hep.26219. [DOI] [PubMed] [Google Scholar]
- 119.Coulon C, Georgiadou M, Roncal C, De Bock K, Langenberg T, Carmeliet P. From vessel sprouting to normalization: role of the prolyl hydroxylase domain protein/hypoxia-inducible factor oxygen-sensing machinery. Arterioscler Thromb Vasc Biol. 2010;30(12):2331–2336. doi: 10.1161/ATVBAHA.110.214106. [DOI] [PubMed] [Google Scholar]