Abstract
E-Cadherin-based Adherens Junctions (AJs) are a defining feature of all epithelial sheets. Through the homophilic association of E-Cadherin molecules expressed on neighboring cells, they ensure intercellular adhesion amongst epithelial cells, and regulate many key aspects of epithelial biology. While their adhesive role requires these structures to remain stable, AJs are also extremely plastic. This plasticity allows for the adaptation of the cell to its changing environment: changes in neighbors after cell division, cell death, or cell movement, and changes in cell shape during differentiation. In this review we focus on the recent advances highlighting the critical role of the apico-basal polarity machinery, and in particular of the Par3/Bazooka scaffold, in the regulation and remodeling of AJs. We propose that by regulating key phosphorylation events on the core E-Cadherin complex components, Par3 and epithelial polarity promote meta-stable protein complexes governing the correct formation, localization, and functioning of AJ.
Keywords: E-Cadherin, Adherens Junctions, Remodeling, Epithelial polarity, Par3, Magi scaffolds
Introduction
In multicellular organisms, cells contact their neighbors to generate tissues and organs of very stereotypical shapes and forms. One of the most ubiquitous cell types is the epithelial cell. Epithelial cells organize as mono-layered or pseudo-stratified epithelial sheets that serve to create boundaries between different environments, for instance between the outside and inside of an organism, where the exchanges and fluxes of macromolecules, nutrients, metabolites, have to pass through and are therefore tightly controlled [1]. As such, maintaining the integrity of the epithelial sheets is crucial to their function.
Epithelial cells are polarized along their apico-basal (A/B) axis where the apical side faces the exterior of the organism or the lumen of the epithelial tube. This A/B polarity is established and maintained by the asymmetric segregation of evolutionarily conserved protein complexes which defines several lateral membrane domains (reviewed in [2–4]). Along these different lateral domains, different sets of intercellular junctions mediate cell–cell adhesion, integrity of the epithelial sheets, and the tightness of the barrier [5–7].
Adherens Junctions (AJs) are a defining feature of all epithelial sheets and constitute apical adhesive structures where the close membrane apposition between neighboring epithelial cells is mediated and strengthened by the homophilic interactions of single-pass transmembrane E-Cadherin (E-Cad) molecules. These structures are stabilized by the accumulation of a dense actin filaments-based cortical network, and in particular by the molecular links anchoring E-Cad clusters to the inner cytoskeleton (reviewed in [8–11]).
While stable adhesion is critical for epithelial sheet integrity and function, numerous studies also highlight the incredible plasticity of AJs, allowing for the destruction of ‘old’ cellular contacts and the creation of new ones [11–15]. This remodeling of the E-Cad complexes and AJs is intimately associated with changes in cell size, cell shape, and relative cell movements that are the basis of the global morphogenetic processes producing stereotypical tissues and organs fulfilling their functions during embryonic development and adaptive processes of adult tissues [8, 10, 16]. The study of AJ remodeling and dynamics is a very active field of research combining different model organisms and a very broad range of approaches from biochemical studies to monitor protein complexes, to bio-physics and mathematical modeling, to refined microscopic techniques in living tissues or organisms, allowing a description of this process from the molecular and cellular scale, up to the tissular scale and its implications during morphogenesis. While different organisms might use slightly different mechanisms to regulate AJs, the molecular conservation of the key players involved, as well as the ubiquity of AJ remodeling throughout the animal kingdom (see Table 1 for homology between human and Drosophila genes involved in AJ establishment and regulation), strongly suggest that common evolutionary rooted themes exist and that combining information gathered using different organisms and levels of analysis should stimulate the emergence of new hypotheses.
Table 1.
Human genes and their Drosophila orthologues involved in Adherens Junctions establishment and remodeling
| Human gene | Drosophila gene |
|---|---|
| E-Cadherin (CDH1) | E-Cadherin, a.k.a. shotgun (shg) |
| β-Catenin (CTNNB) | Armadillo (arm) |
| α-Catenin (CTNNA) | α-Catenin (α-Cat) |
| p120-Catenin (CTNND) | p120-Catenin (p120ctn) |
| Nectin | Echinoid (Ed)? |
| TIAM1 | still life (sif) |
| SRC | Src42A and Src64B |
| SYK | Shark |
| CSK | Csk |
| CK1α | Ck1α |
| CK1ε | discs overgrown (dco) |
| CK1γ | gilgamesh (gish) |
| ROCK | Rho-kinase (Rok) |
| PARD3 (Par3) | bazooka (baz) |
| PAR6 | par-6 |
| PKCζ | aPKC |
| CRB1, 2, 3 | Crumbs (crb) |
| PALS1 | Stardust (sdt) |
| PAR1 | par-1 |
| PTEN | Pten |
| MAGI1, 2, 3 | Magi |
| RASSF7, 8 | RASSF8 |
| ASPP1, 2 | ASPP |
The current review will first introduce the basic organization of E-Cad-based complexes found at AJs, and some key morphogenetic events that require AJ remodeling. Integrating information from different organisms, mainly the genetically tractable model organism Drosophila melanogaster and its powerful cellular and in vivo microscopic approaches, and mammalian tissue culture and its refined biochemical and macro-molecular complex analysis (see Table 1), this review will then describe the events that direct E-Cad complex formation, before focusing on the role of apical cortical scaffolds, in particular of Par3, and how they control the reversible assembly/disassembly of the E-Cad-based complexes to modulate the localization, size, and strength of AJs.
Core E-Cadherin complex composition and regulation
E-Cadherin
Epithelial cadherin (E-Cad, CDH1 in humans, shotgun in Drosophila; Table 1) is a member of the classical cadherins, together with the neural cadherin (N-Cad), placental cadherin (P-Cad), and the vascular endothelial cadherin (VE-Cad). E-Cad is an evolutionarily conserved large single-pass transmembrane glycoprotein involved in Ca2+-dependent cell–cell adhesion (reviewed in [17]). E-Cad, like all cadherins, harbors extracellular cadherin repeats with Ca2+ binding sites, which mediate homophilic interactions between cadherin molecules expressed on neighboring cells (trans-engagement). The highly conserved intracellular tail of E-Cad associates with many different cytoplasmic proteins, which are mainly composed of the α-, β- and p120-catenins (for reviews see [8, 9]; Fig. 1). These different E-Cad binding partners mediate and regulate the activity of E-Cad, and in particular its association with the actin-myosin cytoskeleton, its transport and recycling, and its interactions with the different A/B and planar polarity machineries at play in epithelial cells (reviewed in [5, 11, 15]). They represent therefore key players in the remodeling of E-Cad-based adhesion.
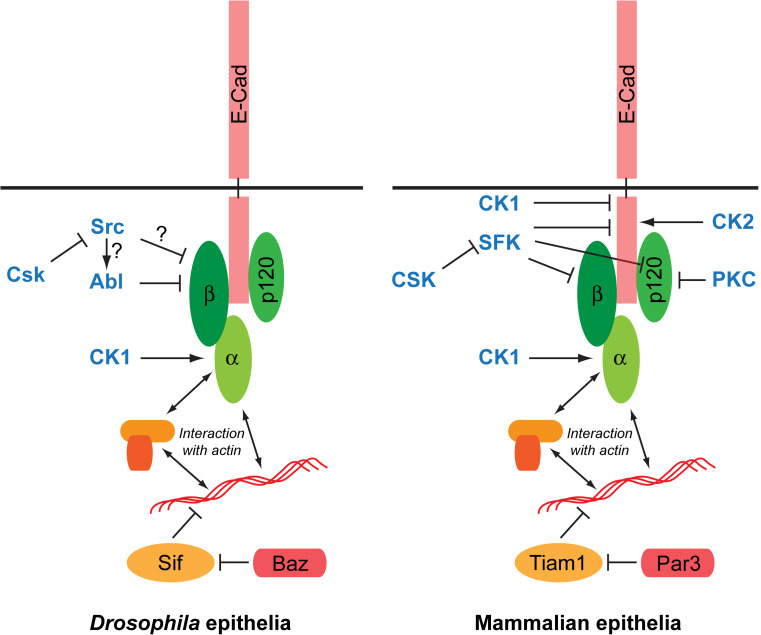
Fig. 1.
Core E-Cadherin/Catenin complexes at Adherens Junctions and their regulation by phosphorylation. Shown is a simplified comparison of the Drosophila (left) and mammalian (right) Adherens Junction (AJ) complexes and key kinases (in bold and in blue) regulating the association and stability between E-Cadherin and the different catenins. Arrows represent phosphorylations with an activating function (e.g., stabilization of complexes and AJs); bars represent inhibitory phosphorylations
The present review focuses on E-Cad, but a lot of E-Cad binding partners and regulators also control other classical cadherins, and the change of cadherin flavor (E-Cad, N-Cad, P-Cad), a.k.a. cadherin switch, occurs during normal development or during pathogenesis such as in certain cancers.
Catenins
The two main interactors of the E-Cad cytoplasmic domain are the β-catenin (β-Cat) and the p120-catenin (p120-Ctn). These two proteins are highly conserved throughout evolution and consist of repeats of the armadillo domain (β-Cat is also known as armadillo in Drosophila; Table 1).
The link to the actin cytoskeleton is mainly mediated by β-Cat via its association with α-Catenin (α-Cat; Fig. 1). It has been proposed that a complex composed of E-Cad, β-Cat, α-Cat, and actin is responsible for the association between E-Cad molecules and the actin/myosin network. Indeed, both in mammalian cell line studies and in Drosophila, direct linkage between α-Cat and E-Cad by the generation of E-Cad/α-Cat fusions can rescue most of E-Cad loss-of-function phenotypes, including remodeling [18, 19]. This model fits well with the observation that α-Cat plays a critical role in the transduction of mechanical tension to the AJ and E-Cad clusters [20, 21]. This model is further supported by the recent observation that under force, E-Cad/Catenin complexes bind directly to F-actin [22]. Besides its interaction with β-Cat, α-Cat can interact with many actin-binding proteins such as formin, vinculin, α-actinin, ZO-1, AF6/afadin, or EPLIN [23] (reviewed in [24, 25]), that could act as elements of an extra bridge between β-Cat and actin. These different proteins represent different possible levels of regulation and plasticity of the AJ in its relationship with the actin/myosin network (Fig. 1).
Many studies in mammalian systems have highlighted the key role of p120-Ctn in the regulation of AJs [26]. p120-Ctn has been shown to interact with various microtubule regulating proteins such as CLASP2 [27] or kinesin [28], but also with actin regulators such as the small GTPase Rho regulator p190RhoGAP [29] to mediate local Rho/Rac activity [29, 30], E-Cad endocytosis and turn-over [31, 32], and microtubule control of AJs. More specifically, p120-Ctn dynamically regulates Rho-GTPase activity at the Cadherin complex through transient interaction with several of its up- and downstream effectors, including ROCK1 [33]. These studies also demonstrate that p120-Ctn contributes to the maintenance of cell–cell adhesion by regulating E-Cadherin stability in epithelial cells. Surprisingly, p120-Ctn function is dispensable in Drosophila, suggesting a rather supportive role in the fly [34, 35]. However, a recent study in Drosophila demonstrated that p120-Ctn facilitates the endocytosis and recycling of the dynamic E-Cadherin-Par3 subcomplex (Par3 is known as Bazooka in Drosophila; Table 1), whereas its absence stabilizes this subcomplex at the membrane [36].
E-Cad and the Catenins are the substrates of key AJ-regulating kinases and phosphatases that modulate their protein/protein interactions, thereby regulating the strength of interaction between E-Cad and the Catenin complex and their levels at the membrane. These are key steps in modulating adhesive strength and AJ remodeling. The vast majority of these biochemical studies have been performed in human (and other mammalian) cell lines, thus the residues and mechanisms described below (1.3, 1.4, and 1.5) primarily apply to the human E-Cad/Catenin complexes (more details in Table 2).
Table 2.
Kinases phosphorylating AJ components in mammalian cells and consequences
| AJ component phosphorylated residue | Protein kinase | Biological consequences | Consequences on cell junctions | References |
|---|---|---|---|---|
| E-Cadherin | ||||
| Y753/754 | Src | Hakai -mediated ubiquitinylation and degradation | − | [40] |
| ND |
Src (PI3K dep.) |
Stabilize E-Cad-based junctions and collective cell movement | + | [57] |
| ND | Fyn | Hakai -mediated ubiquitinylation and degradation | − | [41] |
| ND | Syk | Stimulates localization of p120-Ctn at AJ | + | [59] |
| S846 | CK1 |
Inhibition AJ localization Decreased intercellular adhesion Weaker interaction with β-Cat More efficient internalization |
− | [75] |
| S838 | Ubiquitinylation via SCF-Skp2 & degradation | [76] | ||
| S684 | CK2 |
Increased E-Cad binding to β-Cat Protects E-Cad from degradation Strengthens intercell. adhesion |
+ | [63] |
| S686, S692 | GSK-3β |
Increased E-Cad binding to β-Cat Strengthened intercell. adhesion and epithelial barrier function |
+ | [158] |
| (S850, S853) | PKD1 | Increased cellular aggregation | + | [159] |
| α-Catenin | ||||
| Y177 | SFK |
Decreased cell adhesion Disrupted association of APC with cell membrane Enhanced β-Cat transactivation |
− | [160] |
| S641 | CK2α |
Disruption of the α/β-Cat complex Enhanced β-Cat transactivation |
− | [65] |
| CK1/CK2 | Phosphorylation is required for normal cadherin-catenin complex function | [66] | ||
| β-Catenin | ||||
| S45 | CK1 |
β-Cat Nuclear translocation β-TrCP-mediated ubiquitinylation and degradation |
− | [161] |
| S33, S37, T41 | GSK3 | β-TrCP-mediated ubiquitinylation and degradation | − |
[162] [163] |
| S37, T41 | JNK | Disruption of intercellular contacts | − | [78] |
| Y86, Y654 | Src |
Decreased affinity of β-Cat for E-Cad Disruption of intercellular contacts Impaired transactivating ability |
− | [44] |
| Y142 | Fyn | Disrupted β-Cat/α-Cat interaction | − | [48] |
| Y142 | Fer | Disrupted β-Cat/α-Cat interaction | − | [48] |
| Y142 |
FGFR2 FGFR3 |
Release of β-Cat from membranous Cadherin complexes Activation of the WNT/β-Cat signaling |
− | [49] |
| Y142 | EGFR |
β-Cat release from membranous cadherin complexes Activation of the WNT/β-Cat signaling |
− | [49] |
| Y142 | TRKA |
β-Cat release from membranous cadherin complexes Activation of the WNT/β-Cat signaling |
− | [49] |
| Y654 | RET | Impaired β-Cat/E-Cad interaction in AJ | − | [45] |
| p120-Catenin | ||||
| Y217, Y228 |
Src Src and Fer |
Increased p120-Ctn/E-Cad binding Increased affinity of p120-Ctn for RhoA and inhibition of RhoA activity |
+ |
[44] [46] |
| Y112 | Fyn |
Increased p120-Ctn/E-Cad binding Inhibited p120-Ctn/RhoA interaction |
+ | [46] |
| S879 |
PKCα (PDGFR- or Fyn dep.) |
AJ disassembly | − |
[71] [164] |
| S268 |
PKCɛ (Ras-dep.) |
p120-Ctn mislocalization from AJ EMT |
− | [72] |
| S268, S269 | CK1ε | Disrupting its interaction with E-Cad | − | [68] |
| Par3 | ||||
| S827 | aPKCζ/ι | Defects in the cell–cell contact-induced cell polarization | − | [127] |
| S144, S873 | Par1 (EMK1, MARK2) |
Creates binding sites for 14-3-3 proteins that antagonize the association of Par3 with aPKC PAR1 has an antagonistic role stabilizing the asymmetric localization of the aPKC complex |
− | [128] |
| T833 | ROCK | Disrupted interaction with aPKC and PAR-6 | − | [165] |
| Y1127 | EGFR/SFK | Reduced association of Par-3 with LIM kinase 2 | + | [157] |
Unless specified amino acid numbers correspond to the human orthologues
AJ adherens junction, + stabilizing effect on intercellular junctions, − destabilizing effect on intercellular junctions, ND not determined, SFK Src-family kinases, S serine, T threonine, Y tyrosine
Regulation of E-Cad/Catenin association by phosphorylation
In mammalian cells, the fine-tuned regulation of the E-Cad/Catenin complex and AJ stability by phosphorylation is being well studied and its complexity is only gradually being uncovered. Conversely, in Drosophila phosphorylation events are much less well documented and AJ dynamics and remodeling rely more on cellular and genetic approaches. Consequently, the below-mentioned phosphorylation-based E-Cad complex regulation studies mainly focus on mammalian cells, but when appropriate studies in Drosophila are also mentioned. Early observations evidenced that a short core region of the intracellular E-Cad part is highly phosphorylated on serine [37] and tyrosine residues [38, 39] and essential for Catenin binding (Table 2).
The Src kinase and other Src-family kinases (SFK) are critical protein-tyrosine kinases for the regulation of E-Cad and Catenin interactions (Fig. 1; Table 2). In mammalian cells, the Src kinase phosphorylates E-Cad on two consecutive tyrosine residues (Y753/754), thereby creating an interaction domain with the E3-ubiquitin ligase Hakai to promote ubiquitinylation and degradation of the E-Cad complex and disruption of the cell–cell contacts [40]. Other SFK members, such as Fyn, are also involved in Hakai-mediated E-Cad degradation [41], and chemical inhibition of SFKs can restore E-Cad mediated cell adhesion and reduce cancer metastasis in human cancer cell lines [42].
SFKs also phosphorylate β-Cat (Fig. 1; Table 2) resulting in a reduced association to E-Cad and α-Cat, and subsequent decreased cell–cell adhesion [43]. C-src-mediated β-Cat phosphorylation on Y654 reduces its affinity for E-Cad leading to AJ disruption [44]. The RET receptor kinase in epithelial cells also promotes the phosphorylation of the Y654 residue of β-Cat and likewise impairs its interaction with E-Cad at AJs [45].
The SFK kinase Fyn phosphorylates p120-Ctn on the Y112 residue of its N terminal regulatory domain, and inhibits its interaction with RhoA [46], thus potentially destabilizing AJs (see later; Fig. 1). In Drosophila, a similar negative role for p120-Ctn in cells with sensitized Src levels is suggested by the fact that in cells with increased Src activity, the associated AJ destabilization is suppressed when p120-Ctn is mutated [47] even though there is no direct proof of Src-mediated phosphorylation of p120-Ctn in Drosophila. Once phosphorylated by Fyn, p120-Ctn increases its affinity for E-Cad and, consequently, promotes the association of Fyn with the AJ complex [48]. This appears, however, as a destabilizing event since the p120-Ctn-mediated recruitment of Fyn leads to the phosphorylation of β-Cat on the Y142 residue to prevent the association with α-Cat. Other tyrosine kinases (e.g. FGFR2, FGFR3, EGFR and TRKA; Table 2) also directly phosphorylate β-Cat at Y142, releasing β-Cat from membranous Cadherin complexes, increasing the cytoplasmic β-Cat concentration, and ultimately activating the canonical WNT pathway signaling [49].
In Drosophila, there are only two Src kinases, Src42A and Src64B. When overexpressed, they induce a destabilization of E-Cad based AJs [50]. Src42A is found in a complex with E-Cad and with Arm, the Drosophila β-Cat orthologue (Table 1), and is able to promote the tyrosine phosphorylation of Arm [51]. It is therefore tempting to propose that this Y-phosphorylation of β-Cat/Arm is responsible for E-Cad/Catenin complex destabilization as observed in mammals [43, 44]. However, it remains unclear whether Drosophila Src directly phosphorylates Arm, or whether this is mediated by another kinase [51], such as the Abelson kinase [52] (Fig. 1). Indeed, the E-Cad and AJ destabilization observed after increased Src activity in Drosophila, are suppressed when Abl levels are impaired suggesting that Abl mediates, at least in part the effect of Src [53]. Abl-mediated phosphorylation of β-Cat/Arm is further supported by studies in Drosophila embryos, where asymmetrically localized Abl kinase directs planar polarized junctional remodeling during axis elongation through the tyrosine phosphorylation of β-Cat/Arm on the conserved Y667 residue, resulting in β-Cat/Arm accumulation in stable junctions parallel to the embryo antero-posterior axis, while Abl is found in shrinking junctions [52]. However, this effect has yet to be linked to Src kinases. Like its mammalian counterparts, the overall AJ destabilizing effect of Src in Drosophila is further supported by the observation that mutations for Csk, the C terminal Src kinase, a kinase that inhibits Src by phosphorylating its C terminal tail [54], promotes AJ destabilization and cell delamination [47, 55].
While overall SFK activity appears to promote E-Cad/Catenin complex destabilization and AJ destruction, the actual picture is much more dynamic, and SFK can also promote E-Cad/Catenin stability (see Table 2). For instance, Src signaling supports E-Cad signaling to PI3-kinase [56] and elevated SFK activity may also stabilize E-Cad-based junctions and collective cell movement [57], even though the unequivocal involvement of Src in a direct E-Cad phosphorylation has not been evidenced in these studies. Tyrosine phosphorylation of α-Cat enhances its translocation to the plasma membrane and its interaction with β-Cat, leading to enhanced actin polymerization and stabilization of AJs [58]. E-Cad and α-Cat are also phosphorylated by the Syk tyrosine kinase, which behaves as a tumor suppressor in epithelial cells. Their phosphorylation by Syk supports the proper localization of p120-Ctn at AJs [59], and the formation of cell–cell contacts [60].
SFKs also phosphorylate p120-Ctn directly, which is an essential regulator of Cadherin complexes in mammals which binds and stabilizes E-Cad to promote its adhesive and tumor suppressing function [26]. The Src-mediated phosphorylation pattern of p120-Ctn is complex and encompasses 8 major tyrosine sites [61] and conversely to its effect on β-Cat, it increases the affinities of p120-Ctn for E-Cad [44]. Tyrosine phosphorylation of p120-Ctn was not observed in v-Src-transformed cells expressing E-Cad mutants deleted of their cytoplasmic part, indicating that tyrosine phosphorylation of p120-Ctn depends on its complex formation with E-Cad and membrane localization [62]. Unlike the SFK Fyn, Src-mediated p120-Ctn phosphorylation (on Y217 and Y228) promotes increased affinity towards RhoA [46] and thus potentially AJ stabilization.
Another key kinase in the modulation of E-Cad/Catenin complexes is Casein kinase 2 (CK2; Fig. 1; Table 2). CK2 phosphorylates E-Cad on the S684, which probably in combination with the effect of glycogen synthase kinase-3β (GSK-3β), results in increased β-Cat binding and stronger cell–cell adhesion [63]. Accordingly, decreased E-Cad phosphorylation by CK2 is associated with the disruption of AJs [64]. While CK2 appears to stabilize junctions, EGFR-ERK mediated activation of CK2α promoting the phosphorylation of α-Cat on the S641 residue, results in the disruption of the α/β-Cat complex and weaker adhesion and promotion of the β-Cat transactivation [65]. Recently, several phosphorylation sites have been identified within mammalian and Drosophila α-Cat that is sequentially modified at CK2 and CK1 consensus sites. In mammalian cells, non-phosphorylatable forms of α-Cat showed defects in intercellular adhesion suggesting that these CK1 and CK2 sites are required for normal Cadherin-Catenin complex function [66].
CK1ε, not only phosphorylates α-Cat, it can also phosphorylate p120-Ctn [67]. In response to Wnt signaling, p120-catenin is phosphorylated at S268 and S269, disrupting its interaction with E-Cadherin [68] and therefore facilitating the activation of Rac1 signaling. Strikingly, this effect is inhibited by p120-Ctn tyrosine phosphorylation by Src or Fyn [69].
Finally, different PKC isoforms regulate p120-Ctn association with the E-Cad/Cat complexes. There are eight PKC-dependent Ser/Thr phosphorylation sites in mammalian p120-Ctn [70], and signaling events that activate PKC induce rapid phosphorylation of p120-Ctn, suggesting that p120-Ctn activity is regulated, in part, by one or more PKC isoforms. For instance, physiologic activation of several receptor tyrosine kinases such as PDGFR, induce rapid and robust p120-Ctn phosphorylation at S879, an effect mediated by PKCα, a conventional PKC isoform shown to be implicated in AJ disruption [71]. p120-Ctn is also phosphorylated at S268 in a strictly PKCɛ-dependent manner and is a key effector of the Ras-PKCɛ oncogenic signaling axis [72].
In conclusion, regulation of the E-Cad/Catenin complexes by phosphorylation is coordinated by an increasingly diverse series of phosphorylation events. Even though reviewed here, they are strongly dependent on cell type and stimulus, and might not all occur at the same time in the same cell. This largely accounts for the current apparent contradictory effects (strengthening versus disrupting AJ) (see Table 2). It is also possible that contradictory events occur simultaneously, to maintain the plasticity of the AJs, allowing rapid remodeling. The relative dynamics of many of these destruction/creation of proteins/protein contacts will ultimately tilt the balance towards strengthening or disrupting of AJs. It is therefore impossible in this review to present a complete and clear-cut/unambiguous unifying model on these dynamic regulations. We did, however, opt not to simplify but realistically represent the current provisional view with the many questions remaining.
Regulation of E-Cad/Catenin levels by phosphorylations
Cadherins associate with β-Cat in the endoplasmic reticulum shortly after their synthesis, and uncomplexed cadherins are degraded [73, 74], highlighting one of the mechanisms by which phosphorylations can regulate AJ strength. Alternatively, rather than this indirect effect on protein stability, key phosphorylation events may directly control E-Cad and Catenins degradation.
CK1 co-localizes with E-Cad and phosphorylates its cytoplasmic domain on the highly conserved S846 residue (Fig. 1). Constitutively phosphorylated E-Cad on S846 is unable to localize at cell–cell contacts, has a decreased intercellular adhesive activity, and binds weakly with β-Cat [75]. Indeed CK1-mediated E-Cad phosphorylation triggers its ubiquitinylation by the SCF-Skp2 E3 ubiquitin ligase complex, and its subsequent degradation [76].
Both in mammals and Drosophila, CK1 also mediates the phosphorylation and degradation of β-Cat, even though this process has been mainly documented in the regulation of the Wnt signaling-dedicated cytoplasmic pool of β-Cat (reviewed in [77]). Alternatively the membrane-associated pool of β-Cat could also be controlled by phosphorylation-primed degradation. Lee and collaborators reported that the c-Jun amino-terminal kinase (JNK) binds to the E-Cad/β-Cat complex and phosphorylates β-Cat at S37 and T41, sites that are shared with GSK-3β, disrupting intercellular contacts [78].
Role of Phosphatases in E-Cad/Catenin complex
AJs are dynamic structures that need to adapt and are subject to constant stabilization, destruction, and renewal. The phosphorylations described above are therefore counterbalanced by de-phosphorylation events. For instance, the protein Ser/Thr phosphatase-6 catalytic subunit (PP6c) accumulates at AJs, associates directly with E-Cad, and opposes CK1 to maintain cell surface localization of E-Cad [79]. Similarly, PP2A activity is required for cell–cell adhesion [80].
Several phosphatases mediate β-Cat de-phosphorylation, such as PP1cγ (on the T41 and S45 residues, previously phosphorylated by GSK3 and CK1, respectively [81]), the protein-tyrosine phosphatase LAR (leukocyte common antigen related) [82], or the receptor-like protein-tyrosine phosphatase PCP-2 [83] to stabilize AJs. Similarly, de-phosphorylation of p120-Ctn by phosphatases (for instance by the CD148 transmembrane tyrosine phosphatase [84]) increases the homophilic binding affinity of E-Cad, thus directly demonstrating that cell surface E-Cad is allosterically regulated by p120-Ctn [85].
When are Adherens Junctions remodeled?
The primary function of E-Cad based AJs is to mediate intercellular adhesion between neighboring cells, ensuring the integrity of the epithelium. It is therefore essential that AJs are maintained as stable structures to prevent weakening and ultimately rupture of the epithelial sheet, with its potential damaging effects on the homeostasis of the tissue and survival of the organism. AJs also represent anchoring points with respect to the A/B axis of epithelial cells, and as such prevent the mixing between the apical and basal determinants, the Crumbs/Patj/Pals1/Par-6/aPKC and Scrib/Dlg/Lgl/Par-1 complexes, respectively [2–4, 86]. The antagonistic activities of these apical and basal determinants, together with their interactions with AJs components help position and stabilize the exact position of the AJs, defining therefore the relative sizes of the apical and baso-lateral membranes, and as a consequence contribute to the accurate positioning of the other asymmetrically distributed compartments, organelles and traffic in epithelial cells.
Despite the need for stability, AJs also exhibit a tremendous plasticity and are highly remodeled during development and morphogenesis. AJs shrink and expand, change in size and strength, are dissociated and reformed, to accommodate for the changes in cell shape and to mediate relative cell movements, as epithelial cells adapt to their environment and follow stereotypical developmental morphogenesis. While not exhaustive, AJ remodeling occurs for instance in the following morphogenetic events:
When epithelial cells move with respect to each other, such as during the convergent and extension movements of the Drosophila embryo ectodermal cells. During this morphogenetic process, global extrinsic and intrinsic forces command epithelial cell intercalation along a dorso-ventral axis generating a global antero-posterior extension. To achieve this, planar polarized AJs are destroyed and created whilst cells “slide” with respect to each other (reviewed in [8, 11, 13, 87]).
When epithelial cells divide and undergo mitosis, new AJ material accumulates at the newly created interface between the neighboring cells that partly involve a redistribution of the cortical material [88–90].
When epithelial cells change shape, such as during apical constriction of columnar epithelial cells of vertebrate sensory placodes or during neural tube closure. Apical constriction reduces the total apical circumference of epithelial cells and hence is accompanied by a shrinking of AJs around the apex (reviewed in [16, 87]).
When epithelia change their overall epithelial type to become either squamous or columnar. While all the previous examples implicated a remodeling of AJs along the plane of the epithelium, AJs can also be remodeled along the apico-basal axis of cells by changing their position and size, affecting the respective sizes of the apical and baso-lateral membranes. This is occurring when epithelial cells transition from a cuboidal “classical” epithelial structure, to a flatter squamous, or thicker columnar epithelium [16]. Such transitions are documented for instance when some of the cuboidal follicle cells surrounding the developing female Drosophila egg chamber become flatter and adopt a squamous type around the anterior nurse cells, while others located around the posterior oocyte become taller and columnar [91, 92].
Establishment of E-Cadherin-based Adherens Junctions
The mechanisms and the sequence of events leading to the formation of AJs is a very active field of research and recent advances have been the subject of many excellent reviews [8, 9, 11, 14, 15]. Even though this topic is slightly outside of the primary focus of this review, notions about the mechanisms at play, and the current model describing how AJs form, are necessary to understand AJ remodeling. Indeed, in many instances, AJ remodeling appears as a partial re-use or modulation of AJ establishment.
While many details remain to be elucidated, E-Cad based AJs establishment and stabilization in metazoans, is controlled by a sequence of events involving local adhesion triggered by transmembrane adhesion molecules, the progressive recruitment of E-Cad/Catenin complexes to the membrane apposition sites, the cross-talks and dynamic interactions between the actin cytoskeleton and E-Cad clusters, and the shift from a dynamic Rac-controlled filamentous actin cytoskeleton to a more stable Rho-controlled bundled actin cytoskeleton [10, 11].
Based on work in cell culture, a model emerges where AJ formation starts with the contact of actin based protrusions between two neighboring cells. This first contact is triggered by the homophilic interaction ‘in trans’ between E-Cad molecules initiating the formation of little clusters of E-Cad connected to the actin radial cytoskeleton [93]. These little clusters of E-Cad engagement activate Rac signaling, in particular through the activation of the RacGEF TIAM1, to promote a branched actin network and more protrusions, ultimately expanding the initial small clusters of E-Cad both by coalescence and by the diffusion of free E-Cad monomers that finally become engaged and retained on-site. This leads to the formation of discontinuous and very dynamic spot AJs. In parallel to activating Arp2/3 filamentous actin nucleation, Rac signaling also recruits p190RhoGAP to the maturing AJs, inhibiting therefore Rho signaling at these early junctions. Alternatively, these initial contacts could be mediated or reinforced by the trans-engagement of immunoglobulin-like adhesion molecules of the Nectin family, to help recruit free E-Cad complexes to the apposition sites [11, 15].
Once junctions grow, the scaffolds Par3 (PARD3 in humans, Bazooka in Drosophila; Table 1) and α-Cat are recruited to the expanding junctions and inhibit Rac signaling. The joint inhibitions of the RacGEF TIAM1 by Par3 (or its orthologue Sif by Baz in Drosophila, see later for the role of Par3 in AJ regulation; Fig. 1) [94, 95] and of Arp2/3 and p190RhoGAP by α-Cat [96, 97] result in the activation of Rho signaling and the formation of stable actin bundles that run parallel to the cell–cell contact surface and stabilize mature AJs. This ultimately results in the formation of a subcortical actin ring at the apex of epithelial cells supporting a continuous ring of E-Cad based AJs.
While this model accounts primarily for AJ formation at the interface between connecting mammalian cells, most of it can be extrapolated to other systems. There are, however, many little variations depending on the epithelial cell type, or the nature of the initial contact. For instance two main differences can be highlighted in the extensively studied Drosophila paradigms of AJ remodeling.
First, Drosophila does not appear to have a clear Nectin orthologue, even though the IgCAM molecule Echinoid (Ed) is taking over some of its Ca2+-independent adhesive role in parallel to the action of E-Cad complexes (Table 1). For instance the interaction between Nectins and Afadin is paralleled in Drosophila in the interaction between Ed and Canoe. The link between Ed and Baz (orthologue of Par3; Table 1) will be discussed later.
Second, in the developing Drosophila embryo, the first AJs are formed during lateral membrane creation, isolating little patches of cytoplasm around individual nuclei out of the initial syncytial embryo. In this intensely studied model, there is no initial apposition of membrane from two isolated cells, and E-Cad accumulation appears primarily mediated by targeted traffic of E-Cad/Catenin complexes (reviewed in [8]). Most epithelia of the Drosophila embryo, larva, and adult, are inherited from this initial ‘epithelization’ and AJ creation. It is noteworthy that in the developing mammalian organism, AJs are also inherited and extended between dividing cells. The situation is therefore different from the meeting of isolated cells seen in culture conditions, suggesting that the mechanisms describing AJ initiation after apposition of membrane might be more specific than general.
Despite little differences particular for each system, a few key features of AJs formation with respect to their necessary remodeling can be highlighted:
The formation of AJs is a multi-step process that is reversible allowing for the remodeling of E-Cad based AJs.
The many components of E-Cad complexes and accessory proteins and the complex interactions they mutually engage in, offer many points of regulation, in particular through post-translational modifications (Fig. 1; Table 2).
AJ formation and evolution is driven by the cross-talks and feed-backs between E-Cad clusters and the underlying actin cytoskeleton. In particular, the switch between Rac (flexible), and Rho (stable) signaling is critical for the transition from more mobile spot-like AJs to more stable belt-like AJs.
A few protein scaffolds, and in particular afadin/AF-6 [93] and Par3/Baz control locally this switch between Rac and Rho signaling, and offer key entry points to regulate AJ remodeling.
Regulation of Adherens Junctions by the Par3/Baz scaffolds
The regulation of E-Cad-based AJs is highly dependent on the interactions of AJs with the cytoskeleton and the many factors that can regulate the formation of dynamic protein complexes centered on the E-Cad C terminal-tail (see ‘‘Regulation of E-Cad/Catenin association by phosphorylation’’ and ‘‘Regulation of E-Cad/Catenin levels by phosphorylations’’). From studies with mammalian cell culture and in vivo models such as the developmental morphogenesis in Drosophila, the scaffold Par3 has emerged as a critical node of AJ regulation. Par3 was first identified in the worm Caenorhabditis elegans as one of the critical factors controlling the initial polarization of the worm embryo. Worms mutant for PAR-3 show a partition defective phenotype during the first cell division, hence the name PAR-3 [98]. PAR-3 orthologues are found in Drosophila (Bazooka, Baz, [99]) and vertebrates (known as PARD3 in human) and form a family of evolutionarily conserved protein scaffolds, possessing multiple protein/protein interaction domains (for reviews see [3, 4, 100]; Table 1). These different domains and the many protein partners they interact with regulate Par3 localization and activity, and through their effect on the actin cytoskeleton and on protein kinases and/or phosphatases, they mediate the action of Par3 on AJ dynamics.
Par3 as a localization clue for Adherens Junctions
Studies performed in the developing Drosophila embryo have suggested a pivotal role of Par3/Baz in the formation and regulation of AJs [8]. Indeed, in the cellularizing early embryo, small clusters of E-Cad/Catenin complexes co-localize apically with clusters of Par3/Baz protein, but their initial localizations appear independent [101]. However, in the absence of Par3/Baz these small initial E-Cad clusters fail to grow and expand as spot AJs [102], suggesting that early AJs require Par3/Baz function, at least to expand, even though the exact mechanisms remain unclear but likely involve redundant mechanisms affecting the cytoskeleton and trafficking [102]. As development proceeds (gastrulating embryo and later stages) these early spot AJs mature to more robust and stable belt AJs (zonulae adherens) encircling the whole circumference of the epithelial cell apex [8]. Strikingly, at this stage, E-Cad-based AJs accumulate where the Par3/Baz protein is enriched [103, 104]. This correlation is also seen in the gastrulating Drosophila embryo during germ-band extension when Par3/Baz, excluded from the shrinking myosin II-rich membranes by a direct phosphorylation by Rho-kinase ROCK (Rok in Drosophila; Fig. 2), is planar polarized and accumulates along the expanding cell–cell junctions running parallel to the antero-posterior axis of the embryo, and co-localizes with an enrichment of the AJ component Arm [105–107]. The current evidence supports therefore an important role for Par3/Baz as a landmark for AJs localization, regulation and remodeling in these different Drosophila epithelia [103, 108] (Fig. 2).
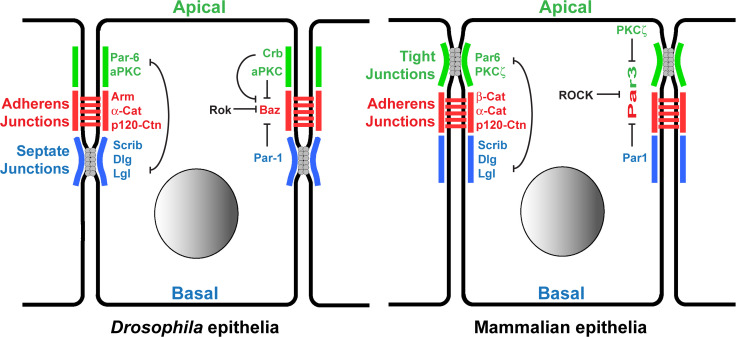
Fig. 2.
Apical restriction of Par3/Baz localization by inhibitory kinases. Structures of epithelial cells in Drosophila (left) and in mammals (right), with the different protein orthologues implicated in Par3/Baz restriction, are presented. AJs, (shown in red) are localized apically at the interface between the apical and basal membranous domains characterized by antagonizing protein complexes: the apical Par6/PKCζ (green), and the baso-lateral Dlg/Lgl/Scrib/Par1 (blue). Par3 accumulates at the level of AJs due to a double restriction by the apical kinase PKCζ and by the basal kinase Par1. The domains of these two kinases stay segregated due to the mutual exclusion of the apical (green) and baso-lateral (blue) protein complex networks. Par3 levels are further modulated along the circumference of the AJ domain by the antagonizing Rho-Kinase (ROCK)
In mammalian cells, the role of Par3 has been primarily associated with the formation and stabilization of ZO-1-containing tight junctions (TJs Fig. 2; [94]). However, TJs and E-Cad-based AJs are in very close proximity in mammalian cells [7] and photon microscopy is insufficient to unmistakably separate them. Moreover, TJs and AJs share common mechanisms of formation and most likely influence and stabilize each other. For instance, in MDCK cells, Par3 depletion results in both AJ (marked with E-Cad) and TJ (marked with ZO-1) defects [109] suggesting that beyond its role on TJ, a role for Par3 in AJs might have been overlooked. Indeed, mouse primary mammary epithelial cells depleted of Par3 in the context of Notch or Ras oncogenic activation, exhibit increased tumor formation and cell invasiveness, and a dramatic disorganization of AJs and E-Cad expression at the plasma membrane [110]. More direct evidence of the conserved role of Par3 in mammalian AJs stabilization was recently obtained in MCF-10A immortalized human mammary cells stably transfected with the ErbB2 tyrosine kinase receptor. In these cells, Par3 knock-down by shRNA triggered decreased cell–cell adhesion, increased E-Cad membrane mobility and less stable AJs [111].
Mechanisms of Adherens Junction regulation by Par3
Even though Par3 regulates AJ formation and stability at multiple steps, the exact mechanisms by which it exerts its actions require further studies. Through its different binding partners Par3 has been shown to regulate E-Cad/Catenin complex turn-over and traffic, the dynamics of the actin cytoskeleton, and the linkage between E-Cad and the underlying actin network. It is noteworthy that these different processes are all very sensitive to protein complex formation and to the phosphorylation status of the proteins involved, and in particular E-Cad and the Catenins (Fig. 1; Table 2). The link between Par3 and the local activity of kinases and phosphatases represents therefore a promising avenue for future studies.
In multiple organisms, from invertebrates to humans, Par3 has been shown to interact with Par6 and atypical PKC (PKCζ/aPKC; Table 1), to form the so-called Par complex [3, 4, 100, 112, 113]. This complex is required to establish and maintain apico-basal polarity by defining the identity of the apical side. In Drosophila, despite a physical interaction, Par3/Baz is actually segregated from Par-6/aPKC in mature epithelial cells, and accumulates at the level of AJs (Fig. 2). Par-6 and aPKC, together with the small GTPase cdc42 but independently of Par3/Baz, control E-Cad endocytosis preventing its apical accumulation [114–116]. Because reports have suggested that Par3 could have an inhibitory effect on PKCζ/aPKC [112, 113], it is tantalizing to propose that the accumulation of Par3/Baz at the level of AJs prevents cdc42/Par-6/aPKC mediated E-Cad endocytosis to stabilize E-Cad complexes at the AJ level, even though this has not yet been formally demonstrated. In mammalian cells, Par3 has been observed both at AJs and the more apical TJs suggesting that Par3 might not actually segregate from the Par6/PKCζ territory (Fig. 2). However, being confined in the same broad domain does not exclude mutual antagonisms in more discrete sub-domains.
In mammalian cells, Par3 has been shown to interact with the RacGEF Tiam1 [94, 117, 118]. Functional assays in MDCK cultured epithelial cells show that Par3 inhibits Tiam1 activity leading to an inhibition of Rac1 signaling to stabilize TJ formation [94]. Similar observations have been made using the Drosophila pupal notum columnar epithelia where Par3/Baz inhibits the activity of Sif, the Drosophila orthologue of Tiam1 [115], supporting a model where Par3/Baz through the inhibition of Tiam1, inhibits Rac1 signaling to the actin cytoskeleton and its protrusive activity to promote the switch towards Rho signaling and its effect on actin cable formation and consequent stabilization of AJs (Reviewed in [11]; Fig. 1).
Par3 also binds to the FERM domain containing protein Nf2 (a.k.a. Merlin), which serves as a linker with α-Cat. Nf2 is required for Par3 and E-Cad stabilization at AJs in mouse keratinocytes [119] suggesting a model where Nf2 and Par3 through the recruitment of α-Cat stabilize E-Cad complexes at AJs. However, in Drosophila, the two Nf2 orthologues, Expanded and Merlin, act redundantly on membrane protein trafficking, and opposite to what could be expected from the before-mentioned study, E-Cad (as well as other membrane proteins) accumulate at the plasma membrane in expanded and merlin mutant clones [120], indicating that more studies are required to evaluate whether this role of Nf2 is limited to mouse keratinocytes or more general.
Par3 apico-basal restriction
Par3 localization is one of the critical factors that regulate AJs. Stabilizing/recruiting Par3 to certain sub-domains of the plasma membrane will govern local stabilization of the E-Cad complex clustering, controlling where AJs are stable. Remodeling patterns of AJs correlate with Par3 membrane association. Par3/Baz accumulates at the interface of the apical and baso-lateral membrane domains where AJs form [4, 121]. In Drosophila and in mammals, it appears to be achieved by a conjunction of exclusion mechanisms from both the most apical and baso-lateral domains (Fig. 2).
In Drosophila, apical Par3/Baz is excluded by the conjunction of two mechanisms (Fig. 2). First, following its physical binding to the apical Par-6/aPKC complex, Par3/Baz is phosphorylated by aPKC on S980 which further prevents its association with aPKC, resulting in the release of Par3/Baz from the Par-6/aPKC apical complex [108, 122]. However, exclusion from the most apical part of the cell is also achieved by a second mechanism involving the large transmembrane protein and apical marker Crumbs (Crb). Par-6 can bind, via its PDZ domain, to both Baz and the Crb intracellular cytoplasmic C tail. These interactions have been shown to be mutually exclusive, and the presence of Crb at the most apical part of epithelial cells excludes Baz by engaging Par-6, [108, 122]. The system is further refined as other apical factors such as Stardust (Sdt), the Drosophila orthologue of the mammalian Pals1, can also bind to Par3/Baz, and Crb or Par-6, to modulate complex formation [123]. This intricate network of interactions between Par3/Baz, Par-6, aPKC, Crb, and Pals1/Sdt that matures in epithelial cells to ultimately resolve with the apical exclusion of Par3/Baz, appears to correspond to the re-wiring of the initial network responsible for setting up the apico-basal polarity [3, 4, 100]. Evolving from this initial network to the mature epithelial network likely involves subtle modifications in some of its parameters such as relative abundance of the different factors, strength of the different protein/protein interactions, and presence or absence of additional factors regulating protein complex formation such as accessory scaffolds, and phosphatases that could control the balance between phosphorylated and un-phosphorylated S980.
In Drosophila epithelial cells, Par3/Baz is also excluded by the activity of the baso-lateral resident kinase Par-1 (also known as EMK1/MARK2 in humans; Fig. 2). Par-1 phosphorylates Par3/Baz on S151 and S1085 generating 14-3-3 protein binding sites preventing Par3/Baz oligomerization and its binding to aPKC [124]. This results in the exclusion of Par3/Baz from the baso-lateral membranes, even though the mechanisms are not entirely clear, but might involve a recently described Par3/Baz-centrosome pathway where the authors speculate that 14-3-3-bound Par3/Baz could be targeted to the microtubules [125].
This double exclusion mechanism of Par3/Baz from the most apical and basal part is generally conserved in mammalian epithelial cells. Par3 is phosphorylated by the Ser/Thr kinases PKCζ/ι (atypical protein kinase C zeta/iota; Table 1) on S827 preventing its binding to PKCζ, and promoting the spreading of Par3 to the junctional part of the cell. Par3 is also a substrate of Par1 (EMK1/MARK2) creating 14-3-3 binding sites excluding Par3 from the most basal part of epithelial cells [126–128]. The Ser/Thr protein phosphatase 1 (PP1) is required for the de-phosphorylation of Par3 at several key serine residues, thereby regulating its association with 14-3-3 proteins and PKCζ [129].
This dual exclusion mechanism stabilizes Par3/Baz at the interface of the apical and basal domains, where AJs will form (Fig. 2). Nevertheless, since apical and baso-lateral domains antagonize each other’s activity (Fig. 2), the dynamic equilibrium between them will ultimately define their relative sizes [4, 86], and thus the size and localization of the domain competent to accumulate Par3/Baz and form AJs. Movements and remodeling of AJs along the apico-basal axis, or apical fattening of AJs seen during epithelial type transitions (squamous–cuboidal–columnar, see chapter 2) or during morphogenesis (such as apical constriction, see chapter 2) are ultimately controlled by these antagonistic interactions and their consequences on Par3/Baz localization [16]. It would be interesting to re-examine the morphogenesis and AJ remodeling models to study whether patterns of AJ stability could be correlated with subtle changes in the relative sizes of these lateral Par3 exclusion domains.
Par3 retention
Baz/Par3 is not only excluded from both the apical and basal sides of epithelial cells, it is also specifically retained at the level of AJs (Fig. 3).
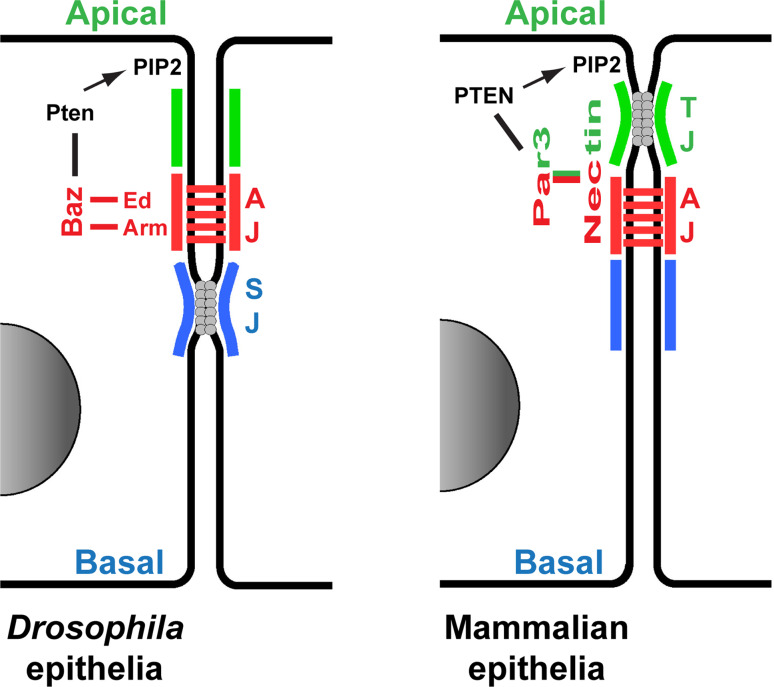
Fig. 3.
Adherens Junction retention of Par3/Baz. Comparison between the Drosophila (left) and mammalian (right) mechanisms to anchor Par3/Baz at AJs. Lines (black, red and green) indicate physical interaction. PTEN promotes the production of PIP2 phospholipids, a major component of the plasma membrane, anchoring Par3/Baz at the membrane
Par3 and Baz bind to phospho-inositides, and in particular PIP2 and PIP3 that are found along the apical and basal membranes, and the PIP2/PIP3-binding domain of Par3/Baz is critical for its membrane localization [104, 130]. Recently, studies in Drosophila have suggested that apically-enriched PIP2 is the crucial phospho-inositide for Baz membrane recruitment and AJ formation in the follicular epithelial cells [131]. Interestingly, in both mammals and Drosophila, Par3/Baz binds the PTEN phosphatase [132, 133], which converts PIP3 to PIP2. PTEN was recently shown to control morphogenesis in the Drosophila wing epithelia, at least in part through AJ remodeling and myosin II localization [134]. It is tempting to propose that the localized PIP2/PIP3 balance controlled by PTEN, impacts subtle changes in the ability of Baz to associate with the membrane, and affects AJ remodeling and dynamics, even though this remains yet to be proven.
Par3 binds several AJ resident proteins involved in its retention at the interface between apical and basal domains. In Drosophila, the PDZ domains of Par3/Baz bind both to the C termini of the adhesion molecule Echinoid (Ed) and the β-Cat orthologue Arm [135] (Fig. 3). It remains, however, controversial whether these two interactions are critical, or if they are required in different epithelial tissues since AJs form normally when these interactions are impaired [102, 136]. More subtle effects, and redundant action between Arm and Ed, or with an as yet un-identified other AJ resident factor cannot be excluded. It is noteworthy that Par3 binds to the C terminus of the Nectin AJ regulators in mammalian cells, weak orthologues of Ed [109, 137]. This evolutionarily conserved interaction suggests a key role for the Nectin-Par3 interaction in AJ regulation, even though this still remains unclear in Drosophila.
A new apical complex regulating Par3 apical localization
Several studies made in mammalian cells demonstrated that the p53-binding partner ASPP2 is localized at the level of the apical junction complexes in MDCK and mouse neuro-epithelial cells. It overlaps there with Par3 and the TJ marker ZO-1, as well as with the most apical part of the AJs [138, 139] (Fig. 4). More importantly, ASPP2 binds to Par3 and is required for the correct Par3 apical localization. TJs are strongly delayed in ASPP2 knock-down cells, as well as in cells overexpressing the ASPP2 destabilizing enzyme Siah2 (an E3 ubiquitin-protein ligase) [138–140]. ASPP2 was also shown to interact with β-Cat at AJs (Fig. 4) in human mammary cells MCF-10A, where it was proposed to inhibit β-Cat accumulation and transport to the nucleus, thus preventing ZEB1 expression and E-Cad gene repression [141]. It emerges that ASPP2 is an apical junction promoter, and in particular AJs, via the binding to both Par3 and β-Cat. In Drosophila, the unique ASPP orthologue is required for E-Cad based AJ integrity at least in part through its activation of Csk activity (an inhibitor of the Src kinase [55], see ‘‘Regulation of E-Cad/Catenin association by phosphorylation’’). Furthermore, in the developing Drosophila pupal eye ASPP is required both for Par3/Baz recruitment at the membrane at AJ level, and for the integrity of the E-Cad belt found around the cortex of inter-ommatidial epithelial cells [142]. No direct binding between ASPP and Par3/Baz has been documented in the fly, so the actual mechanism by which ASPP brings Par3/Baz to the membrane in Drosophila remains elusive.
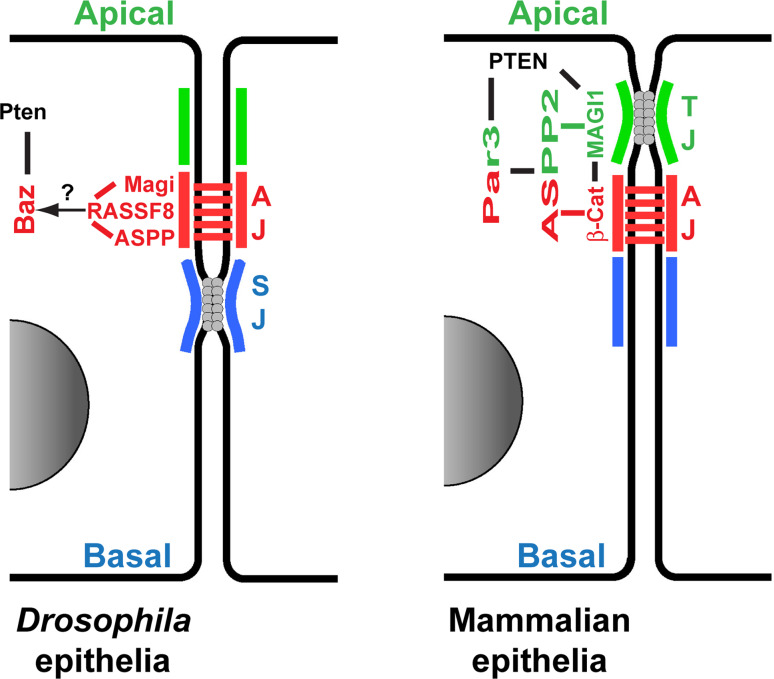
Fig. 4.
A MAGI-ASPP network to localize Par3/Baz at the Adherens Junction. Comparison between the Drosophila (left) and mammalian (right) emerging role of the MAGI-ASPP network in AJ regulation. Lines (black, red and green) indicate physical interaction. Further work on this network will allow to clarify the exact localizations and interactions between the different members of the complexes, and between the MAGI-ASPP network and the other factors regulating E-Cad complexes and Par3 localization presented in Figs. 1, 2, and 3
A remaining key question is what brings ASPP to the cortex and at the level of future AJs? The situation in mammalian cells is complex: while ASPP2 is required for correct localization of Par3 at junction sites, reciprocally Par3 is required for ASPP2 localization [138, 139, 143]. This could reflect the early role of Par3 in setting up the apico-basal axis, rather than its more dedicated role in junction specification. In the Drosophila pupal eye, ASPP localization is not dramatically affected in Par3/Baz mutant cells, ruling out Par3/Baz as the primary anchor of ASPP in the fly [142]. However, ASPP correct AJ localization is mediated by its physical interaction with the RASSF8 scaffolding protein, and RASSF8 or ASPP mutant cells show similar a pattern of interrupted AJs [144]. We have recently shown that in Drosophila the scaffold Magi is the upstream anchor, and that through its physical interaction with RASSF8, Magi controls ASPP and Par3/Baz localization at AJs and regulates the integrity of E-Cad junctions [142] (Fig. 4). The genetic requirement for Magi and RASSF8 are, however, limited to a small time window during pupal eye development suggesting that the Magi-RASSF8-ASPP axis of Par3/Baz localization at AJs is redundant with other mechanisms [142], such as interaction with β-Cat [135], or with the double exclusion machineries described before (Fig. 2 and Fig. 3). In the nematode C. elegans, MAGI-1 interacts genetically with the Cadherin/Catenins complex to define junction domains [145]. In mammalian cells, there are three Magi orthologues, MAGI1, 2, and 3. They have been shown to regulate TJ and AJ formation and invasiveness (reviewed in [146]). Of note, this AJ stabilizing effect of MAGI scaffolds has been proposed to be mediated by physical interactions with β-Cat [147, 148], with PTEN [149, 150], or with regulators of the junction remodeling small GTPase Rap1 [151, 152]. A role for MAGI scaffolds on the actin cytoskeleton is therefore suggested by this link with Rap1 signaling [153], but also by the physical interactions of MAGI1 with the actin binding proteins alpha-actinin-4 and synaptopodin in MDCK cells [154]. Interestingly, in biochemical studies, MAGI1 was shown to bind directly to ASPP2 [155], suggesting that a common ASPP-MAGI axis regulating AJs, likely through the localization of Par3, could be conserved from Drosophila to human (Fig. 4). Whether mammalian orthologues of RASSF8 are also involved in the complex remains to be explored, but a role of the RASSF7-10 scaffold family (the RASSF with the Ras association domain in the N terminal) is supported by the observation that RASSF8 is found at the level of AJs co-localizing with E-Cad and β-Cat in several human lung cancer cell lines, and that RASSF8 depletion leads to AJ destabilization [156], reminiscent of the role proposed for its Drosophila counterpart.
Many open questions regarding this emerging MAGI-RASSF8-ASSP complex remain, including the evolutionary conservation of some of the protein interactions in the complex, and the potential cross regulations with the other Par3 localization and AJ regulation machineries.
Conclusions
E-Cad based AJs are a crucial structure of epithelial cells. Even though their primary role is to mediate intercellular adhesion, they participate in the overall polarization of epithelial cells to segregate apical and basal membrane compartments, and to polarize organelles and intracellular traffic. They engage in complex feedback interactions with the actin-myosin cytoskeleton network and are hotspots to integrate tissue force sensing, and to assemble cell signaling platforms. Despite these key cellular functions, AJs that seem stable on a short time-scale appear very plastic on a longer time-scale, a feature that is critical for tissue morphogenesis and cell shape changes. In this review, we have discussed the recent findings regarding the ever more elaborate mechanisms ensuring the correct localization and remodeling of AJs by the epithelial polarity machineries and in particular the Par3 apical scaffold. The correct localization and stability of Par3 scaffolds is under the tight control of many other scaffolds, and ultimately regulated by numerous phosphorylation/de-phosphorylation events such as by the apico-basal polarized aPKC/PKCζ and PAR1 kinases, or the planar polarized ROCK kinase, in Drosophila and mammals. The recent identification in Drosophila of a Magi/RASSF8/ASPP complex regulating both Par3/Baz recruitment and the activity of Csk, a negative regulator of SFK, opens the exciting prospect that SFK activity could be regulating Par3 localization and/or activity as was suggested by studies in human cell lines, where Par3 is phosphorylated on tyrosine by the SFK members c-Src and c-Yes, releasing the LIM kinase 2 (LIMK2) to promote junction formation [157]. The integration of Par3/Baz, and other key apical scaffolds, with the reversible phosphorylation events controlling the stability and strength of the E-Cad complexes will contribute to a better understanding of the dynamics controlling AJ stability and remodeling during normal epithelial development and pathology.
Acknowledgments
The authors would like to thank the members of the PC and AD labs, and S. Zaessinger for critical reading. The PC lab is supported by grants from the “Plan Cancer/INCa” (ASC14021FSA) and the “Fondation ARC pour la recherche sur le cancer” (SL220110603480). The AD lab is supported by Grants from the “Fondation ARC pour la recherche sur le cancer”, “Marie Curie CIG”, and “ATIP/Avenir programme”.
References
- 1.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141:757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Tepass U. The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu Rev Cell Dev Biol. 2012;28:655–685. doi: 10.1146/annurev-cellbio-092910-154033. [DOI] [PubMed] [Google Scholar]
- 5.Nelson WJ, Dickinson DJ, Weis WI. Roles of cadherins and catenins in cell-cell adhesion and epithelial cell polarity. Prog Mol Biol Transl Sci. 2013;116:3–23. doi: 10.1016/B978-0-12-394311-8.00001-7. [DOI] [PubMed] [Google Scholar]
- 6.Wei Q, Huang H. Insights into the role of cell–cell junctions in physiology and disease. Int Rev Cell Mol Biol. 2013;306:187–221. doi: 10.1016/B978-0-12-407694-5.00005-5. [DOI] [PubMed] [Google Scholar]
- 7.Harder JL, Margolis B. SnapShot: tight and adherens junction signaling. Cell. 2008;133:1118.e1–1118.e2. doi: 10.1016/j.cell.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Harris TJC. Adherens junction assembly and function in the Drosophila embryo. Int Rev Cell Mol Biol. 2012;293:45–83. doi: 10.1016/B978-0-12-394304-0.00007-5. [DOI] [PubMed] [Google Scholar]
- 9.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192:907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Röper K. Integration of cell–cell adhesion and contractile actomyosin activity during morphogenesis. Curr Top Dev Biol. 2015;112:103–127. doi: 10.1016/bs.ctdb.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Collinet C, Lecuit T. Stability and dynamics of cell–cell junctions. Prog Mol Biol Transl Sci. 2013;116:25–47. doi: 10.1016/B978-0-12-394311-8.00002-9. [DOI] [PubMed] [Google Scholar]
- 12.de Beco S, Gueudry C, Amblard F, Coscoy S. Endocytosis is required for E-Cadherin redistribution at mature adherens junctions. Proc Natl Acad Sci U S A. 2009;106:7010–7015. doi: 10.1073/pnas.0811253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecuit T, Yap AS. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol. 2015;17:533–539. doi: 10.1038/ncb3136. [DOI] [PubMed] [Google Scholar]
- 14.Yap AS, Crampton MS, Hardin J. Making and breaking contacts: the cellular biology of cadherin regulation. Curr Opin Cell Biol. 2007;19:508–514. doi: 10.1016/j.ceb.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanov AI, Naydenov NG. Dynamics and regulation of epithelial adherens junctions: recent discoveries and controversies. Int Rev Cell Mol Biol. 2013;303:27–99. doi: 10.1016/B978-0-12-407697-6.00002-7. [DOI] [PubMed] [Google Scholar]
- 16.St Johnston D, Sanson B. Epithelial polarity and morphogenesis. Curr Opin Cell Biol. 2011;23:540–546. doi: 10.1016/j.ceb.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 18.Desai R, Sarpal R, Ishiyama N, Pellikka M, Ikura M, Tepass U. Monomeric α-catenin links cadherin to the actin cytoskeleton. Nat Cell Biol. 2013;15:261–273. doi: 10.1038/ncb2685. [DOI] [PubMed] [Google Scholar]
- 19.Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-Cadherin-alpha catenin fusion molecules. J Cell Biol. 1994;127:235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavey M, Rauzi M, Lenne P-F, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 2008;453:751–756. doi: 10.1038/nature06953. [DOI] [PubMed] [Google Scholar]
- 21.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 22.Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, et al. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014;346:1254211. doi: 10.1126/science.1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A. 2008;105:13–19. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bershadsky A. Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 2004;14:589–593. doi: 10.1016/j.tcb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Yonemura S. Cadherin-actin interactions at adherens junctions. Curr Opin Cell Biol. 2011;23:515–522. doi: 10.1016/j.ceb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Kourtidis A, Ngok SP, Anastasiadis PZ. p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog Mol Biol Transl Sci. 2013;116:409–432. doi: 10.1016/B978-0-12-394311-8.00018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahbazi MN, Megias D, Epifano C, Akhmanova A, Gundersen GG, Fuchs E, et al. CLASP2 interacts with p120-catenin and governs microtubule dynamics at adherens junctions. J Cell Biol. 2013;203:1043–1061. doi: 10.1083/jcb.201306019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Kojima S, Borisy GG, Green KJ. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol. 2003;163:547–557. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, et al. p120-catenin and p190RhoGAP regulate cell–cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 30.Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J Biol Chem. 2005;280:24095–24103. doi: 10.1074/jbc.M414447200. [DOI] [PubMed] [Google Scholar]
- 33.Smith AL, Dohn MR, Brown MV, Reynolds AB. Association of Rho-associated protein kinase 1 with E-cadherin complexes is mediated by p120-catenin. Mol Biol Cell. 2012;23:99–110. doi: 10.1091/mbc.E11-06-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myster SH, Cavallo R, Anderson CT, Fox DT, Peifer M. Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential Adherens Junction component. J Cell Biol. 2003;160:433–449. doi: 10.1083/jcb.200211083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacquelet A, Lin L, Rorth P. Binding site for p120/delta-catenin is not required for Drosophila E-cadherin function in vivo. J Cell Biol. 2003;160:313–319. doi: 10.1083/jcb.200207160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bulgakova NA, Brown NH. Drosophila p120-catenin is crucial for endocytosis of the dynamic E-cadherin-Bazooka complex. J Cell Sci. 2016;129:477–482. doi: 10.1242/jcs.177527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stappert J, Kemler R. A short core region of E-cadherin is essential for catenin binding and is highly phosphorylated. Cell Adhes Commun. 1994;2:319–327. doi: 10.3109/15419069409014207. [DOI] [PubMed] [Google Scholar]
- 38.Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, et al. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M. Cadherin-mediated cell–cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HEM, Behrens J, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 41.Smyth D, Leung G, Fernando M, McKay DM. Reduced surface expression of epithelial E-cadherin evoked by interferon-gamma is Fyn kinase-dependent. PLoS One. 2012;7:e38441. doi: 10.1371/journal.pone.0038441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nam J-S, Ino Y, Sakamoto M, Hirohashi S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin Cancer Res Off J Am Assoc Cancer Res. 2002;8:2430–2436. [PubMed] [Google Scholar]
- 43.Hu P, O’Keefe EJ, Rubenstein DS. Tyrosine phosphorylation of human keratinocyte beta-catenin and plakoglobin reversibly regulates their binding to E-cadherin and alpha-catenin. J Invest Dermatol. 2001;117:1059–1067. doi: 10.1046/j.0022-202x.2001.01523.x. [DOI] [PubMed] [Google Scholar]
- 44.Roura S, Miravet S, Piedra J, García de Herreros A, Duñach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 45.Fernández-Sánchez ME, Barbier S, Whitehead J, Béalle G, Michel A, Latorre-Ossa H, et al. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature. 2015;523:92–95. doi: 10.1038/nature14329. [DOI] [PubMed] [Google Scholar]
- 46.Castaño J, Solanas G, Casagolda D, Raurell I, Villagrasa P, Bustelo XR, et al. Specific phosphorylation of p120-catenin regulatory domain differently modulates its binding to RhoA. Mol Cell Biol. 2007;27:1745–1757. doi: 10.1128/MCB.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vidal M, Larson DE, Cagan RL. Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev Cell. 2006;10:33–44. doi: 10.1016/j.devcel.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Piedra J, Miravet S, Castaño J, Pálmer HG, Heisterkamp N, García de Herreros A, et al. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol Cell Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krejci P, Aklian A, Kaucka M, Sevcikova E, Prochazkova J, Masek JK, et al. Receptor tyrosine kinases activate canonical WNT/β-catenin signaling via MAP kinase/LRP6 pathway and direct β-catenin phosphorylation. PLoS ONE. 2012;7:e35826. doi: 10.1371/journal.pone.0035826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shindo M, Wada H, Kaido M, Tateno M, Aigaki T, Tsuda L, et al. Dual function of Src in the maintenance of adherens junctions during tracheal epithelial morphogenesis. Dev Camb Engl. 2008;135:1355–1364. doi: 10.1242/dev.015982. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi M, Takahashi F, Ui-Tei K, Kojima T, Saigo K. Requirements of genetic interactions between Src42A, armadillo and shotgun, a gene encoding E-cadherin, for normal development in Drosophila. Dev Camb Engl. 2005;132:2547–2559. doi: 10.1242/dev.01850. [DOI] [PubMed] [Google Scholar]
- 52.Tamada M, Farrell DL, Zallen JA. Abl regulates planar polarized junctional dynamics through β-catenin tyrosine phosphorylation. Dev Cell. 2012;22:309–319. doi: 10.1016/j.devcel.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh J, Aaronson SA, Mlodzik M. Drosophila Abelson kinase mediates cell invasion and proliferation through two distinct MAPK pathways. Oncogene. 2010;29:4033–4045. doi: 10.1038/onc.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pedraza LG, Stewart RA, Li D-M, Xu T. Drosophila Src-family kinases function with Csk to regulate cell proliferation and apoptosis. Oncogene. 2004;23:4754–4762. doi: 10.1038/sj.onc.1207635. [DOI] [PubMed] [Google Scholar]
- 55.Langton PF, Colombani J, Aerne BL, Tapon N. Drosophila ASPP regulates C-terminal Src kinase activity. Dev Cell. 2007;13:773–782. doi: 10.1016/j.devcel.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Pang J-H, Kraemer A, Stehbens SJ, Frame MC, Yap AS. Recruitment of phosphoinositide 3-kinase defines a positive contribution of tyrosine kinase signaling to E-cadherin function. J Biol Chem. 2005;280:3043–3050. doi: 10.1074/jbc.M412148200. [DOI] [PubMed] [Google Scholar]
- 57.Veracini L, Grall D, Schaub S, la Forest Beghelli-de, Divonne S, Etienne-Grimaldi M-C, Milano G, et al. Elevated Src family kinase activity stabilizes E-cadherin-based junctions and collective movement of head and neck squamous cell carcinomas. Oncotarget. 2015;6:7570–7583. doi: 10.18632/oncotarget.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burks J, Agazie YM. Modulation of alpha-catenin Tyr phosphorylation by SHP2 positively effects cell transformation induced by the constitutively active FGFR3. Oncogene. 2006;25:7166–7179. doi: 10.1038/sj.onc.1209728. [DOI] [PubMed] [Google Scholar]
- 59.Larive RM, Urbach S, Poncet J, Jouin P, Mascré G, Sahuquet A, et al. Phosphoproteomic analysis of Syk kinase signaling in human cancer cells reveals its role in cell–cell adhesion. Oncogene. 2009;28:2337–2347. doi: 10.1038/onc.2009.99. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X, Shrikhande U, Alicie BM, Zhou Q, Geahlen RL. Role of the protein tyrosine kinase Syk in regulating cell-cell adhesion and motility in breast cancer cells. Mol Cancer Res MCR. 2009;7:634–644. doi: 10.1158/1541-7786.MCR-08-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mariner DJ, Anastasiadis P, Keilhack H, Böhmer FD, Wang J, Reynolds AB. Identification of Src phosphorylation sites in the catenin p120ctn. J Biol Chem. 2001;276:28006–28013. doi: 10.1074/jbc.M102443200. [DOI] [PubMed] [Google Scholar]
- 62.Ozawa M, Ohkubo T. Tyrosine phosphorylation of p120(ctn) in v-Src transfected L cells depends on its association with E-cadherin and reduces adhesion activity. J Cell Sci. 2001;114:503–512. doi: 10.1242/jcs.114.3.503. [DOI] [PubMed] [Google Scholar]
- 63.Lickert H, Bauer A, Kemler R, Stappert J. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/beta-catenin interaction and strengthens cell-cell adhesion. J Biol Chem. 2000;275:5090–5095. doi: 10.1074/jbc.275.7.5090. [DOI] [PubMed] [Google Scholar]
- 64.Serres M, Filhol O, Lickert H, Grangeasse C, Chambaz EM, Stappert J, et al. The disruption of adherens junctions is associated with a decrease of E-cadherin phosphorylation by protein kinase CK2. Exp Cell Res. 2000;257:255–264. doi: 10.1006/excr.2000.4895. [DOI] [PubMed] [Google Scholar]
- 65.Ji H, Wang J, Nika H, Hawke D, Keezer S, Ge Q, et al. EGF-induced ERK activation promotes CK2-mediated disassociation of alpha-Catenin from beta-Catenin and transactivation of beta-Catenin. Mol Cell. 2009;36:547–559. doi: 10.1016/j.molcel.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Escobar DJ, Desai R, Ishiyama N, Folmsbee SS, Novak MN, Flozak AS, et al. α-Catenin phosphorylation promotes intercellular adhesion through a dual-kinase mechanism. J Cell Sci. 2015;128:1150–1165. doi: 10.1242/jcs.163824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casagolda D, Del Valle-Pérez B, Valls G, Lugilde E, Vinyoles M, Casado-Vela J, et al. A p120-catenin-CK1epsilon complex regulates Wnt signaling. J Cell Sci. 2010;123:2621–2631. doi: 10.1242/jcs.067512. [DOI] [PubMed] [Google Scholar]
- 68.Del Valle-Pérez B, Casagolda D, Lugilde E, Valls G, Codina M, Dave N, et al. Wnt controls the transcriptional activity of Kaiso through CK1ε-dependent phosphorylation of p120-catenin. J Cell Sci. 2011;124:2298–2309. doi: 10.1242/jcs.082693. [DOI] [PubMed] [Google Scholar]
- 69.Valls G, Codina M, Miller RK, Del Valle-Pérez B, Vinyoles M, Caelles C, et al. Upon Wnt stimulation, Rac1 activation requires Rac1 and Vav2 binding to p120-catenin. J Cell Sci. 2012;125:5288–5301. doi: 10.1242/jcs.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia X, Mariner DJ, Reynolds AB. Adhesion-associated and PKC-modulated changes in serine/threonine phosphorylation of p120-catenin. Biochemistry (Mosc) 2003;42:9195–9204. doi: 10.1021/bi034597h. [DOI] [PubMed] [Google Scholar]
- 71.Brown MV, Burnett PE, Denning MF, Reynolds AB. PDGF receptor activation induces p120-catenin phosphorylation at serine 879 via a PKCalpha-dependent pathway. Exp Cell Res. 2009;315:39–49. doi: 10.1016/j.yexcr.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dann SG, Golas J, Miranda M, Shi C, Wu J, Jin G, et al. p120 catenin is a key effector of a Ras-PKCɛ oncogenic signaling axis. Oncogene. 2014;33:1385–1394. doi: 10.1038/onc.2013.91. [DOI] [PubMed] [Google Scholar]
- 73.Hinck L, Näthke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. 2001;105:391–402. doi: 10.1016/S0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- 75.Dupre-Crochet S, Figueroa A, Hogan C, Ferber EC, Bialucha CU, Adams J, et al. Casein kinase 1 is a novel negative regulator of E-cadherin-based cell–cell contacts. Mol Cell Biol. 2007;27:3804–3816. doi: 10.1128/MCB.01590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inuzuka H, Gao D, Finley LWS, Yang W, Wan L, Fukushima H, et al. Acetylation-dependent regulation of Skp2 function. Cell. 2012;150:179–193. doi: 10.1016/j.cell.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stamos JL, Weis WI. The β-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee M-H, Koria P, Qu J, Andreadis ST. JNK phosphorylates beta-catenin and regulates adherens junctions. FASEB J Off Publ Fed Am Soc Exp Biol. 2009;23:3874–3883. doi: 10.1096/fj.08-117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohama T, Wang L, Griner EM, Brautigan DL. Protein Ser/Thr phosphatase-6 is required for maintenance of E-cadherin at adherens junctions. BMC Cell Biol. 2013;14:42. doi: 10.1186/1471-2121-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takahashi K, Nakajima E, Suzuki K. Involvement of protein phosphatase 2A in the maintenance of E-cadherin-mediated cell-cell adhesion through recruitment of IQGAP1. J Cell Physiol. 2006;206:814–820. doi: 10.1002/jcp.20524. [DOI] [PubMed] [Google Scholar]
- 81.Marchesini N, Jones JA, Hannun YA. Confluence induced threonine41/serine45 phospho-beta-catenin dephosphorylation via ceramide-mediated activation of PP1cgamma. Biochim Biophys Acta. 2007;1771:1418–1428. doi: 10.1016/j.bbalip.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Müller T, Choidas A, Reichmann E, Ullrich A. Phosphorylation and free pool of beta-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J Biol Chem. 1999;274:10173–10183. doi: 10.1074/jbc.274.15.10173. [DOI] [PubMed] [Google Scholar]
- 83.Yan H-X, Yang W, Zhang R, Chen L, Tang L, Zhai B, et al. Protein-tyrosine phosphatase PCP-2 inhibits beta-catenin signaling and increases E-cadherin-dependent cell adhesion. J Biol Chem. 2006;281:15423–15433. doi: 10.1074/jbc.M602607200. [DOI] [PubMed] [Google Scholar]
- 84.Takahashi K, Matafonov A, Sumarriva K, Ito H, Lauhan C, Zemel D, et al. CD148 tyrosine phosphatase promotes cadherin cell adhesion. PLoS One. 2014;9:e112753. doi: 10.1371/journal.pone.0112753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shashikanth N, Petrova YI, Park S, Chekan J, Maiden S, Spano M, et al. Allosteric regulation of E-Cadherin adhesion. J Biol Chem. 2015;290:21749–21761. doi: 10.1074/jbc.M115.657098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5:53–58. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- 87.Heisenberg C-P, Bellaïche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 88.Founounou N, Loyer N, Le Borgne R. Septins regulate the contractility of the actomyosin ring to enable adherens junction remodeling during cytokinesis of epithelial cells. Dev Cell. 2013;24:242–255. doi: 10.1016/j.devcel.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 89.Herszterg S, Leibfried A, Bosveld F, Martin C, Bellaiche Y. Interplay between the dividing cell and its neighbors regulates adherens junction formation during cytokinesis in epithelial tissue. Dev Cell. 2013;24:256–270. doi: 10.1016/j.devcel.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 90.Guillot C, Lecuit T. Adhesion disengagement uncouples intrinsic and extrinsic forces to drive cytokinesis in epithelial tissues. Dev Cell. 2013;24:227–241. doi: 10.1016/j.devcel.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 91.Grammont M. Adherens junction remodeling by the Notch pathway in Drosophila melanogaster oogenesis. J Cell Biol. 2007;177:139–150. doi: 10.1083/jcb.200609079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kolahi KS, White PF, Shreter DM, Classen A-K, Bilder D, Mofrad MRK. Quantitative analysis of epithelial morphogenesis in Drosophila oogenesis: new insights based on morphometric analysis and mechanical modeling. Dev Biol. 2009;331:129–139. doi: 10.1016/j.ydbio.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mandai K, Rikitake Y, Shimono Y, Takai Y. Afadin/AF-6 and canoe: roles in cell adhesion and beyond. Prog Mol Biol Transl Sci. 2013;116:433–454. doi: 10.1016/B978-0-12-394311-8.00019-4. [DOI] [PubMed] [Google Scholar]
- 94.Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005;7:262–269. doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- 95.Georgiou M, Baum B. Polarity proteins and Rho GTPases cooperate to spatially organise epithelial actin-based protrusions. J Cell Sci. 2010;123:1089–1098. doi: 10.1242/jcs.060772. [DOI] [PubMed] [Google Scholar]
- 96.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, et al. Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol. 2012;14:818–828. doi: 10.1038/ncb2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Etemad-Moghadam B, Guo S, Kemphues KJ. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell. 1995;83:743–752. doi: 10.1016/0092-8674(95)90187-6. [DOI] [PubMed] [Google Scholar]
- 99.Kuchinke U, Grawe F, Knust E. Control of spindle orientation in Drosophila by the Par-3-related PDZ-domain protein Bazooka. Curr Biol CB. 1998;8:1357–1365. doi: 10.1016/S0960-9822(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 100.Wodarz A. Establishing cell polarity in development. Nat Cell Biol. 2002;4:E39–E44. doi: 10.1038/ncb0202-e39. [DOI] [PubMed] [Google Scholar]
- 101.Harris TJC, Peifer M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila . J Cell Biol. 2004;167:135–147. doi: 10.1083/jcb.200406024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McGill MA, McKinley RFA, Harris TJC. Independent cadherin-catenin and Bazooka clusters interact to assemble adherens junctions. J Cell Biol. 2009;185:787–796. doi: 10.1083/jcb.200812146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McKinley RFA, Yu CG, Harris TJC. Assembly of Bazooka polarity landmarks through a multifaceted membrane-association mechanism. J Cell Sci. 2012;125:1177–1190. doi: 10.1242/jcs.091884. [DOI] [PubMed] [Google Scholar]
- 104.Krahn MP, Klopfenstein DR, Fischer N, Wodarz A. Membrane targeting of Bazooka/PAR-3 is mediated by direct binding to phosphoinositide lipids. Curr Biol CB. 2010;20:636–642. doi: 10.1016/j.cub.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 105.Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–355. doi: 10.1016/S1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 106.Sde Simões M, Blankenship JT, Weitz O, Farrell DL, Tamada M, Fernandez-Gonzalez R, et al. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev Cell. 2010;19:377–388. doi: 10.1016/j.devcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 108.Walther RF, Pichaud F. Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr Biol CB. 2010;20:1065–1074. doi: 10.1016/j.cub.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 109.Ooshio T, Fujita N, Yamada A, Sato T, Kitagawa Y, Okamoto R, et al. Cooperative roles of Par-3 and afadin in the formation of adherens and tight junctions. J Cell Sci. 2007;120:2352–2365. doi: 10.1242/jcs.03470. [DOI] [PubMed] [Google Scholar]
- 110.McCaffrey LM, Montalbano J, Mihai C, Macara IG. Loss of the Par3 polarity protein promotes breast tumorigenesis and metastasis. Cancer Cell. 2012;22:601–614. doi: 10.1016/j.ccr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xue B, Krishnamurthy K, Allred DC, Muthuswamy SK. Loss of Par3 promotes breast cancer metastasis by compromising cell–cell cohesion. Nat Cell Biol. 2013;15:189–200. doi: 10.1038/ncb2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3–PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2:540–547. doi: 10.1038/35019592. [DOI] [PubMed] [Google Scholar]
- 113.Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 114.Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol. 2008;183:1129–1143. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol CB. 2008;18:1631–1638. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 116.Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol CB. 2008;18:1639–1648. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 117.Mertens AEE, Rygiel TP, Olivo C, van der Kammen R, Collard JG. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol. 2005;170:1029–1037. doi: 10.1083/jcb.200502129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, et al. PAR-6–PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol. 2005;7:270–277. doi: 10.1038/ncb1227. [DOI] [PubMed] [Google Scholar]
- 119.Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell. 2010;19:727–739. doi: 10.1016/j.devcel.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. The tumor suppressors Merlin and expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol CB. 2006;16:702–709. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 121.Harris TJC, Peifer M. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J Cell Biol. 2005;170:813–823. doi: 10.1083/jcb.200505127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morais-de-Sá E, Mirouse V, St Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell. 2010;141:509–523. doi: 10.1016/j.cell.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Krahn MP, Bückers J, Kastrup L, Wodarz A. Formation of a Bazooka-Stardust complex is essential for plasma membrane polarity in epithelia. J Cell Biol. 2010;190:751–760. doi: 10.1083/jcb.201006029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/S0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- 125.Jiang T, McKinley RFA, McGill MA, Angers S, Harris TJC. A Par-1–Par-3-centrosome cell polarity pathway and its tuning for isotropic cell adhesion. Curr Biol CB. 2015;25:2701–2708. doi: 10.1016/j.cub.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 126.Hirose T, Izumi Y, Nagashima Y, Tamai-Nagai Y, Kurihara H, Sakai T, et al. Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J Cell Sci. 2002;115:2485–2495. doi: 10.1242/jcs.115.12.2485. [DOI] [PubMed] [Google Scholar]
- 127.Nagai-Tamai Y, Mizuno K, Hirose T, Suzuki A, Ohno S. Regulated protein-protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells Devoted Mol Cell Mech. 2002;7:1161–1171. doi: 10.1046/j.1365-2443.2002.00590.x. [DOI] [PubMed] [Google Scholar]
- 128.Hurd TW, Fan S, Liu CJ, Kweon HK, Hakansson K, Margolis B. Phosphorylation-dependent binding of 14-3-3 to the polarity protein Par3 regulates cell polarity in mammalian epithelia. Curr Biol CB. 2003;13:2082–2090. doi: 10.1016/j.cub.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 129.Traweger A, Wiggin G, Taylor L, Tate SA, Metalnikov P, Pawson T. Protein phosphatase 1 regulates the phosphorylation state of the polarity scaffold Par-3. Proc Natl Acad Sci U S A. 2008;105:10402–10407. doi: 10.1073/pnas.0804102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Horikoshi Y, Hamada S, Ohno S, Suetsugu S. Phosphoinositide binding by par-3 involved in par-3 localization. Cell Struct Funct. 2011;36:97–102. doi: 10.1247/csf.11005. [DOI] [PubMed] [Google Scholar]
- 131.Claret S, Jouette J, Benoit B, Legent K, Guichet A. PI(4,5)P2 produced by the PI4P5K SKTL controls apical size by tethering PAR-3 in Drosophila epithelial cells. Curr Biol CB. 2014;24:1071–1079. doi: 10.1016/j.cub.2014.03.056. [DOI] [PubMed] [Google Scholar]
- 132.von Stein W, Ramrath A, Grimm A, Müller-Borg M, Wodarz A. Direct association of Bazooka/PAR-3 with the lipid phosphatase PTEN reveals a link between the PAR/aPKC complex and phosphoinositide signaling. Dev Camb Engl. 2005;132:1675–1686. doi: 10.1242/dev.01720. [DOI] [PubMed] [Google Scholar]
- 133.Feng W, Wu H, Chan L-N, Zhang M. Par-3-mediated junctional localization of the lipid phosphatase PTEN is required for cell polarity establishment. J Biol Chem. 2008;283:23440–23449. doi: 10.1074/jbc.M802482200. [DOI] [PubMed] [Google Scholar]
- 134.Bardet P-L, Guirao B, Paoletti C, Serman F, Léopold V, Bosveld F, et al. PTEN controls junction lengthening and stability during cell rearrangement in epithelial tissue. Dev Cell. 2013;25:534–546. doi: 10.1016/j.devcel.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 135.Wei S-Y, Escudero LM, Yu F, Chang L-H, Chen L-Y, Ho Y-H, et al. Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Dev Cell. 2005;8:493–504. doi: 10.1016/j.devcel.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 136.Laplante C, Nilson LA. Differential expression of the adhesion molecule Echinoid drives epithelial morphogenesis in Drosophila. Dev Camb Engl. 2006;133:3255–3264. doi: 10.1242/dev.02492. [DOI] [PubMed] [Google Scholar]
- 137.Takekuni K, Ikeda W, Fujito T, Morimoto K, Takeuchi M, Monden M, et al. Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J Biol Chem. 2003;278:5497–5500. doi: 10.1074/jbc.C200707200. [DOI] [PubMed] [Google Scholar]
- 138.Sottocornola R, Royer C, Vives V, Tordella L, Zhong S, Wang Y, et al. ASPP2 binds Par-3 and controls the polarity and proliferation of neural progenitors during CNS development. Dev Cell. 2010;19:126–137. doi: 10.1016/j.devcel.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 139.Cong W, Hirose T, Harita Y, Yamashita A, Mizuno K, Hirano H, et al. ASPP2 regulates epithelial cell polarity through the PAR complex. Curr Biol CB. 2010;20:1408–1414. doi: 10.1016/j.cub.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 140.Kim H, Claps G, Möller A, Bowtell D, Lu X, Ronai ZA. Siah2 regulates tight junction integrity and cell polarity through control of ASPP2 stability. Oncogene. 2014;33:2004–2010. doi: 10.1038/onc.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Y, Bu F, Royer C, Serres S, Larkin JR, Soto MS, et al. ASPP2 controls epithelial plasticity and inhibits metastasis through β-catenin-dependent regulation of ZEB1. Nat Cell Biol. 2014;16:1092–1104. doi: 10.1038/ncb3050. [DOI] [PubMed] [Google Scholar]
- 142.Zaessinger S, Zhou Y, Bray SJ, Tapon N, Djiane A. Drosophila MAGI interacts with RASSF8 to regulate E-Cadherin-based adherens junctions in the developing eye. Dev Camb Engl. 2015;142:1102–1112. doi: 10.1242/dev.116277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hauri S, Wepf A, van Drogen A, Varjosalo M, Tapon N, Aebersold R, et al. Interaction proteome of human Hippo signaling: modular control of the co-activator YAP1. Mol Syst Biol. 2013;9:713. doi: 10.1002/msb.201304750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Langton PF, Colombani J, Chan EHY, Wepf A, Gstaiger M, Tapon N. The dASPP-dRASSF8 complex regulates cell–cell adhesion during Drosophila retinal morphogenesis. Curr Biol CB. 2009;19:1969–1978. doi: 10.1016/j.cub.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 145.Lynch AM, Grana T, Cox-Paulson E, Couthier A, Cameron M, Chin-Sang I, et al. A genome-wide functional screen shows MAGI-1 is an L1CAM-dependent stabilizer of apical junctions in C. elegans . Curr Biol CB. 2012;22:1891–1899. doi: 10.1016/j.cub.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Feng X, Jia S, Martin TA, Jiang WG. Regulation and involvement in cancer and pathological conditions of MAGI1, a tight junction protein. Anticancer Res. 2014;34:3251–3256. [PubMed] [Google Scholar]
- 147.Kotelevets L, van Hengel J, Bruyneel E, Mareel M, van Roy F, Chastre E. Implication of the MAGI-1b/PTEN signalosome in stabilization of adherens junctions and suppression of invasiveness. FASEB J Off Publ Fed Am Soc Exp Biol. 2005;19:115–117. doi: 10.1096/fj.04-1942fje. [DOI] [PubMed] [Google Scholar]
- 148.Subauste MC, Nalbant P, Adamson ED, Hahn KM. Vinculin controls PTEN protein level by maintaining the interaction of the adherens junction protein beta-catenin with the scaffolding protein MAGI-2. J Biol Chem. 2005;280:5676–5681. doi: 10.1074/jbc.M405561200. [DOI] [PubMed] [Google Scholar]
- 149.Wu X, Hepner K, Castelino-Prabhu S, Do D, Kaye MB, Yuan XJ, et al. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci U S A. 2000;97:4233–4238. doi: 10.1073/pnas.97.8.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu Y, Dowbenko D, Spencer S, Laura R, Lee J, Gu Q, et al. Interaction of the tumor suppressor PTEN/MMAC with a PDZ domain of MAGI3, a novel membrane-associated guanylate kinase. J Biol Chem. 2000;275:21477–21485. doi: 10.1074/jbc.M909741199. [DOI] [PubMed] [Google Scholar]
- 151.Mino A, Ohtsuka T, Inoue E, Takai Y. Membrane-associated guanylate kinase with inverted orientation (MAGI)-1/brain angiogenesis inhibitor 1-associated protein (BAP1) as a scaffolding molecule for Rap small G protein GDP/GTP exchange protein at tight junctions. Genes Cells Devoted Mol Cell Mech. 2000;5:1009–1016. doi: 10.1046/j.1365-2443.2000.00385.x. [DOI] [PubMed] [Google Scholar]
- 152.Sakurai A, Fukuhara S, Yamagishi A, Sako K, Kamioka Y, Masuda M, et al. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell. 2006;17:966–976. doi: 10.1091/mbc.E05-07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pannekoek W-J, Kooistra MRH, Zwartkruis FJT, Bos JL. Cell–cell junction formation: the role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim Biophys Acta. 2009;1788:790–796. doi: 10.1016/j.bbamem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 154.Patrie KM, Drescher AJ, Welihinda A, Mundel P, Margolis B. Interaction of two actin-binding proteins, synaptopodin and alpha-actinin-4, with the tight junction protein MAGI-1. J Biol Chem. 2002;277:30183–30190. doi: 10.1074/jbc.M203072200. [DOI] [PubMed] [Google Scholar]
- 155.Pirozzi G, McConnell SJ, Uveges AJ, Carter JM, Sparks AB, Kay BK, et al. Identification of novel human WW domain-containing proteins by cloning of ligand targets. J Biol Chem. 1997;272:14611–14616. doi: 10.1074/jbc.272.23.14611. [DOI] [PubMed] [Google Scholar]
- 156.Lock FE, Underhill-Day N, Dunwell T, Matallanas D, Cooper W, Hesson L, et al. The RASSF8 candidate tumor suppressor inhibits cell growth and regulates the Wnt and NF-kappaB signaling pathways. Oncogene. 2010;29:4307–4316. doi: 10.1038/onc.2010.192. [DOI] [PubMed] [Google Scholar]
- 157.Wang Y, Du D, Fang L, Yang G, Zhang C, Zeng R, et al. Tyrosine phosphorylated Par3 regulates epithelial tight junction assembly promoted by EGFR signaling. EMBO J. 2006;25:5058–5070. doi: 10.1038/sj.emboj.7601384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Severson EA, Kwon M, Hilgarth RS, Parkos CA, Nusrat A. Glycogen Synthase Kinase 3 (GSK-3) influences epithelial barrier function by regulating occludin, claudin-1 and E-cadherin expression. Biochem Biophys Res Commun. 2010;397:592–597. doi: 10.1016/j.bbrc.2010.05.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jaggi M, Rao PS, Smith DJ, Wheelock MJ, Johnson KR, Hemstreet GP, et al. E-cadherin phosphorylation by protein kinase D1/protein kinase C{mu} is associated with altered cellular aggregation and motility in prostate cancer. Cancer Res. 2005;65:483–492. [PubMed] [Google Scholar]
- 160.Choi SH, Estarás C, Moresco JJ, Yates JR, Jones KA. α-Catenin interacts with APC to regulate β-catenin proteolysis and transcriptional repression of Wnt target genes. Genes Dev. 2013;27:2473–2488. doi: 10.1101/gad.229062.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, et al. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 163.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/S0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 164.Hsu K-L, Fan H-J, Chen Y-C, Huang Y-S, Chen C-H, Wu J-C, et al. Protein kinase C-Fyn kinase cascade mediates the oleic acid-induced disassembly of neonatal rat cardiomyocyte adherens junctions. Int J Biochem Cell Biol. 2009;41:1536–1546. doi: 10.1016/j.biocel.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 165.Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, et al. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell. 2008;14:205–215. doi: 10.1016/j.devcel.2007.11.021. [DOI] [PubMed] [Google Scholar]