Abstract
Necrosis has long been considered as a passive event resulting from a cell extrinsic stimulus, such as pathogen infection. Recent advances have refined this view and it is now well established that necrosis is tightly regulated at the cell level. Regulated necrosis can occur in the context of host–pathogen interactions, and can either participate in the control of infection or favor it. Here, we review the two main pathways implicated so far in bacteria-associated regulated necrosis: caspase 1-dependent pyroptosis and RIPK1/RIPK3-dependent necroptosis. We present how these pathways are modulated in the context of infection by a series of model bacterial pathogens.
Keywords: Bacteria, Cell death, Regulated necrosis
Introduction
Eukaryotes and bacteria have coevolved for millions of years by interacting in multiple ways, from symbiosis to pathogenesis. This selected a plethora of molecular pathways that link microbial fate to host cell biology. Our vision of microbes has drastically changed since their first observation by Antonie van Leeuwenhoek. Bacteria have since mainly been considered in the context of infection, but are also recognized as symbionts and commensals, as evidenced by the increasingly documented role of the intestinal bacterial flora on many aspects of human health [1–5]. The effects of bacteria on host health reflect their impact on host cell metabolism. Yet, the manipulation of cell homeostasis by microbes can also threaten cell viability. Cell death induced by bacteria was considered for a long time as necrosis, and this premature death was seen as an acute event, deleterious for host integrity. Indeed, cells killed by bacteria may exhibit hallmarks of necrotic death such as organelle swelling, rupture of the plasma membrane and release of cytosolic content [6]. These features contrast with apoptosis, the regulated cell death pathway described in the early 1970s, characterized by nuclear condensation and retention of organelle and membrane integrity [7]. Apoptosis was long considered as the sole pathway of regulated cell death implicated in physiological processes, and bacteria-induced necrosis as a hallmark of pathology, even though bacteria have also been shown to trigger apoptosis [8, 9]. It should be noted that the distinction between apoptosis and necrosis is no longer based on morphological criteria only. Many studies have indeed defined the biochemical pathways leading to cell death [10], and this led to revisit reports dealing with bacteria-associated cell death. A better understanding of the cellular pathways leading to cell death have also shown that necrosis, as apoptosis, can be regulated. Recent reports on cell death pathways have also generated some degree of confusion in the nomenclature with the appearance of neologisms such as parthanatos [11], paraptosis [12], ferroptosis [13], pyronecrosis [14], which significance will likely be refined by mechanistic studies. The term necroptosis is the most frequently used for regulated necrosis, even if it was coined to describe a specific subtype of cell death [15]. For simplicity, we have chosen to use the general term “regulated necrosis” throughout this review to refer to all the forms of regulated cell death distinct from apoptosis. Even if regulated necrosis is far more documented in the context of viral infections, many examples of bacteria-associated necroptosis have been reported, and constitute the focus of this review.
To kill or to be killed: the fate of pathogens facing their hosts
By definition, pathogenic bacteria do not primarily share common interests with their hosts. However, the host constitutes the environment in which they feed and grow, at least transiently. If, as François Jacob coined it, «the dream of a bacterium is to become two bacteria», their unregulated proliferation can compromise host viability and be self-deleterious. The success of bacterial pathogens in the context of infections depends on the balance between their growth within the host and their control by the immune system. Since host cell regulated necrosis is characterized by cellular swelling and cell membrane rupture, it leads to the extracellular release of intracellular content triggering innate and adaptive immune responses, which will compromise pathogen survival. Successful invaders have selected ways of regulating host cell necrosis to dampen the activation of immune responses and promote their intracellular growth. Bacterial pathogens can also directly impair immune functions by activating selectively the death of phagocytic and bactericidal immune cells such as macrophages. Therefore, infection is a tradeoff that integrates the spatiotemporal regulation of host cell death. Bacterial pathogenesis is dynamic and strongly depends on the cell, tissue and host contexts. In-depth in vivo studies are, therefore, required to precisely apprehend the relevance of regulated cell necrosis along the infection process.
Many recent studies have addressed the mechanisms of bacteria-associated regulated necrosis. Most reports have used cultured macrophages, professional phagocytes being a major line of defense against invading bacteria. Two main pathways have been described, depending either on caspase 1 or RIPK1/RIPK3 kinases. These pathways constitute the core of the regulated necrosis machinery, which can be modulated by bacteria to induce or inhibit necrotic cell death. The molecular mechanisms of regulated necrosis have been reviewed in detail [16–18]. This review provides a brief description of these mechanisms, and illustrates how bacteria can modulate caspase 1 or RIPK1/RIPK3 cell death pathways.
Molecular mechanism of the caspase 1-mediated regulated necrosis (also referred as pyroptosis)
Jurg Tschopp and his group have highlighted and elucidated the function of the inflammasome, the macromolecular complex that senses external dangers, such as infection. The inflammasome is composed of an effector (caspase 1), an adaptor (ASC protein) and a sensor (a member of the NOD-like receptor family (NLR)). The role of caspase 1 is to cleave and activate the pro-inflammatory interleukins pro-IL-1β and pro-IL-18 [19, 20]. The release of IL-1β and IL-18 initiates the immune response. Facultative intracellular bacteria have been shown to trigger a form of pro-inflammatory cell death called pyroptosis, in opposition to apoptosis. Even if pyroptosis involves caspase 1 activity, IL-1β and IL-18 are not directly involved in the cell death process [21]; caspase 1 is rather involved in pore formation within the cellular membrane leading to swelling and osmotic lysis of cells [22].
Molecular mechanism of RIPK1-RIPK3-mediated regulated necrosis (also referred as necroptosis)
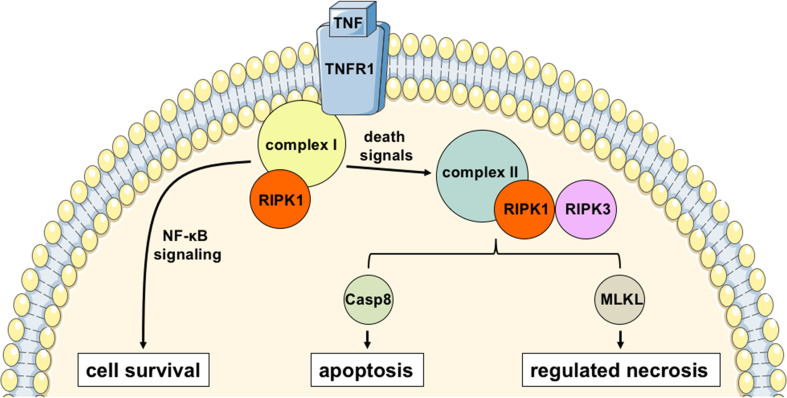
Most of the knowledge regarding necroptosis is based on the study of tumor necrosis factor (TNF)-induced necrosis. As its name indicates, TNF is a cytokine that was shown 40 years ago to trigger tumor necrosis [23]. Yet, several studies have shown that TNF signaling can mediate either apoptosis or necrosis depending on the biological context [24, 25]. Briefly, after binding of TNF to TNF-receptor 1 (TNFR1), an oligomeric structure called complex 1 is formed with the kinase RIPK1 and other cofactors which activates the NF-κB pathway promoting cell survival [26]. Under a variety of death signals which are not fully deciphered, RIPK1 kinase activity is stimulated and this leads to cell death: complex I is destabilized and RIPK1 engages in a cytosolic structure called complex II together with RIPK3 and caspase 8 [26, 27]. Activation of caspase 8 mediates apoptosis, but in conditions in which caspase 8 is not activated, RIPK1 and RIPK3 mutually phosphorylate each other and get activated [28–30]. In the absence of active caspase 8, RIPK3 can phosphorylate MLKL, and promote necroptosis [31], by modulating the osmotic pressure via its action on ion channels, resulting in cell swelling, membrane rupture and ultimately cell death [32–34] (Fig. 1).
Fig. 1.
TNF-mediated regulated cell death. Upon stimulation with TNF, a RIPK1-bound oligomeric structure called complex I induces NF-kB activation leading to cell survival. The complex I is, then destabilized, releasing free RIPK1 kinase that binds to a cytoplasmic structure called complex II. RIPK1 and RIPK3 can be cleaved within this complex in a caspase 8-dependent manner, inducing apoptosis. Alternatively, when caspase 8 action is prevented (artifically or by the action of pathogen effectors), RIPK1 and RIPK3 associate in a complex called necrosome that recruits MLKL triggering the regulated necrosis
Pathogenic mycobacteria
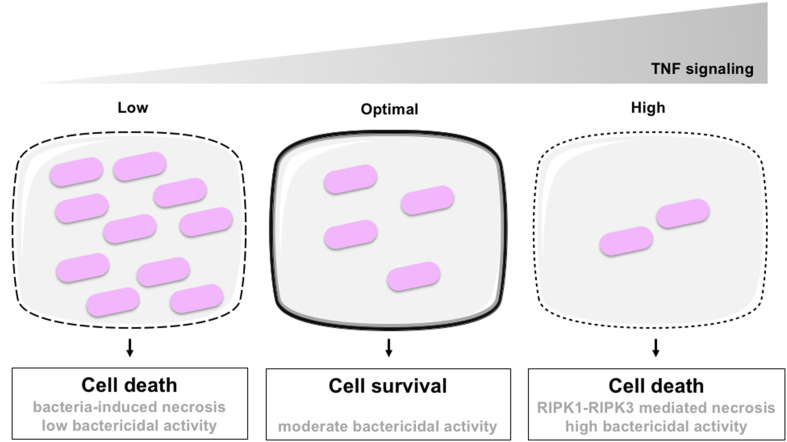
The infection by Mycobacterium spp. constitutes one of the best illustrations of the life-or-death situation that constitute host–pathogen interactions. Mycobacterium tuberculosis (Mt) is a facultative intracellular bacterium, the causative agent cause of tuberculosis, one of the most prevalent and persistent human infections. It is, according to the World Health Organization (WHO), the greatest bacterial killer worldwide with nearly 1.5 million deaths annually. By using zebrafish larvae infected with Mycobacterium marinum (Mm), a closely related pathogenic mycobacterium species as a model for Mt, it has been shown that TNF signaling triggers reactive oxygen species production and mediates resistance to Mm by stimulating the mycobactericidal activity of infected macrophages [35]. This leads to the decrease of the intracellular bacterial burden and protects infected macrophages to bacteria-induced necrosis. But in case of massive infection, TNF signaling also triggers the RIPK1-RIPK3 necrotic pathway leading to the death of heavily infected macrophages [36] (Fig. 2). In other words, macrophages undergo cell death if TNF signaling is too weak and bacteria proliferate, but they also undergo RIPK1-RIPK3-mediated necrosis if the TNF signaling is too strong.
Fig. 2.
TNF and Mycobacterium-induced regulated the cell death. Intracellular Mycobacterium proliferates within phagocytes when the TNF signaling is absent or too weak, leading to the death of infected cells by unregulated necrosis (left). When the TNF signaling is moderate, bactericidal activity of macrophages is stimulated, limiting bacterial growth and favoring cell survival (middle). However, when TNF signaling is too high, RIPK1-RIPK3-mediated necrosis is stimulated even if bacterial growth is controlled. Adapted from [36]
The in vivo depletion of macrophages leads to bacterial extracellular growth resulting in a higher bacterial burden and a cording morphology of bacteria, a Mycobacterium-specific feature correlated with local dissemination [35, 37]. This illustrates that macrophages are key for the early control of infection. However, infected macrophages have been shown to be motile and implicated in the dissemination of Mm [37]. The necrosis of infected macrophages is therefore a host defense mechanism to prevent pathogen dissemination and contain locally the infection at the cost of local tissue damages.
An additional layer of complexity in Mt infection is the formation of granuloma. A tuberculous granuloma is classically composed of a central core of necrotic cells surrounded by concentric layers of macrophages and lymphocytes [38]. Granuloma physically wall off bacteria from neighboring tissues, and have therefore, long been considered as a host defense mechanism. Nevertheless, granuloma formation induces the recruitment of monocyte-derived macrophages that serve as a replicative niche for bacteria [39]. In this context, granulomas favor the dissemination of bacteria during the early steps of the disease. At later stages, granuloma can reflect a host–pathogen equilibrium that promotes latent infection, a cardinal feature of tuberculosis.
Salmonella
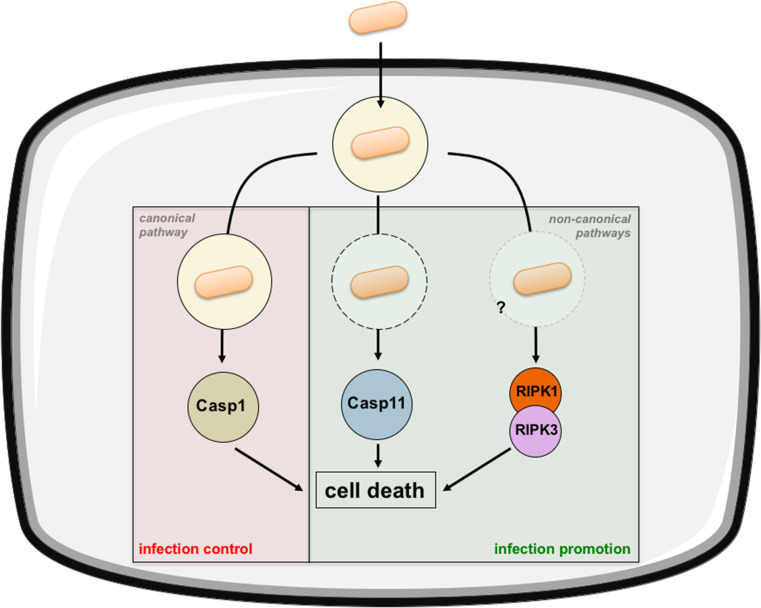
Salmonellosis is one of the major foodborne bacterial infections. In the US, non-typhoidal Salmonella enterica (Se) (mainly serovars Enteritidis and Typhimurium) affects 1 million individuals and causes around 400 deaths each year [40]. Se is a facultative intracellular pathogen that infects and kills a variety of cell types including macrophages and epithelial cells in vitro and in vivo [41–43]. The death pathway triggered by Se in infected macrophages was first described as apoptosis 20 years ago [8]. But this view has been revisited by more recent studies. First, it has been shown that macrophage death requires caspase 1 activation and not caspases 3 and 8 [42, 44]. Pyroptosis was, therefore, proposed as an explaining mechanism of Salmonella-induced macrophage death. It was later shown that Casp1 −/− mice were more susceptible to Se infection [45, 46] supporting a role for macrophage pyroptosis as a host defense mechanism. A model has been proposed where dying macrophages release free extracellular bacteria that are phagocytized by neutrophils and killed by reactive oxygen species [47]. However, the central role of caspase 1 has recently been profoundly challenged. First, it has been shown that commonly used Casp1 −/− mice also lack caspase 11 and are actually Casp1 −/− Casp11 −/− double knockout [48]. This has a strong significance since caspase 11 alone can induce macrophage death in response to intracytosolic Se infection [49, 50]. Indeed, although macrophage death is alleviated in Casp11 −/− as compared to Casp1 −/− Casp11 −/− double knockout mice, it occurs by a noncanonical pathway involving type-I interferon signaling. Interestingly, bacterial burdens are higher in Casp1 −/− mice than in Casp1 −/− Casp11 −/− mice, indicating that noncanonical caspase 11-mediated macrophage death promotes Se infection [49] (Fig. 3).
Fig. 3.
Salmonella-induced regulated cell death. Extracellular Salmonella is phagocytized leading to its internalization within a vacuole. From there, it will manipulate host cell metabolism thanks to secretion of virulence factors. It can induce cell death via the canonical pyroptotic pathway through caspase 1 activation. Cytoplasmic (extravacuolar) bacteria are also able to induce cell death in a noncanonical pathway by activating caspase 11. Finally, Salmonella can induce cell death via the RIPK1/RIPK3 pathway. Caspase 1-associated pyroptosis has a deleterious effect on bacteria limiting the infection whereas caspase 11-associated pyroptosis and RIPK1/RIPK3 necroptosis favor bacterial colonization
Another recent study has confirmed that type-I interferon signaling (IFNβ but not IFNα) is involved in Se-induced macrophage death [51]. Yet, Ifnar1 −/− mice (mice that lack the type-I interferon receptor) were shown to be less susceptible to Se infection than wt mice, arguing for a role of bacterial-induced macrophage death in the promotion of the infection [51]. But in this study, a link between type-I interferon signaling and RIPK1-RIPK3-mediated necrosis was clearly demonstrated. Authors have also observed a direct molecular interaction between IFNAR1 and RIPK1 reminiscent of the well-described TNFR-RIPK1 interaction that leads to necroptosis (see Fig. 1). Even if they have shown that inhibition of RIPK1 is much more effective than inhibition of caspase 1 in preventing Se-induced macrophage death [51], the exact functional relationship between RIPK1-RIPK3-mediated necrosis, caspase 1 and caspase 11-induced pyroptosis and their relative impact on infection remains to be addressed to fully elucidate Se-associated macrophage cell death (Fig. 3).
Listeria monocytogenes
Listeriosis is caused by Listeria monocytogenes (Lm), one of the foodborne pathogens associated with the highest mortality rate [40]. Lm is a facultative intracellular pathogen that can survive and replicate in professional phagocytes as well as in nonphagocytic cells. Once intracellular, Lm escapes from the phagosome before its fusion with lysosomes via the action of the pore-forming toxin listeriolysin O (LLO) [52]. LLO-producing bacteria induce the production of TNF [53] and activate NF-κB [54]. Of note, purified LLO in itself is sufficient to induce NF-κB signaling and lymphocyte apoptosis [55]. Even if they did not use LLO, authors of a very recent paper have shown that pore-forming toxins of the LLO family induce necroptosis arguing for a probable role of LLO as a direct inducer of RIPK1-RIPK3-mediated regulated necrosis [56].
It has been shown that Lm-induced macrophage death is distinct from apoptosis [57] and that Lm induces pyroptosis of cultured bone marrow derived macrophages [58]. These data have been challenged by results from us and others showing a Lm-induced non-pyroptotic necrotic death of macrophages in vitro [56] and in vivo [59, 60]. Indeed, during the liver stage of mouse listeriosis, we have observed a death of the embryonically derived resident macrophages, known as Kupffer cells [59]. This death is caspase 1-independent, ruling out pyroptosis. Furthermore, it is not observed when mice are infected with a ∆hly isogenic mutant unable to produce LLO, a result reinforced by a recent study that links pore-forming toxins to RIPK1-RIPK3 mediated necrosis [56]. We have also observed a defect in macrophage death in Ifnar1 −/− mice as previously reported in vitro and in vivo [51, 61–65] pointing out to a role of type-I interferon signaling in Lm-induced macrophage death. Finally, we found that chemical inhibitors of RIPK1, necrostatin 1 and necrostatin 1 s, strongly reduce the Lm-induced macrophage mortality arguing for a RIPK1-mediated necrosis [59]. By contrast, we observed that RIPK3 was not directly involved in the death process, an original feature already observed upon adenoviral infection, arguing for a RIPK1-dependent, but RIPK3-independent regulated necroptosis, which mechanism remains to be elucidated [60]. It seems that Lm infection could be favored by macrophage necrosis at least during the very early steps [60], but the global impact of this liver resident macrophage death on the infection outcome has not yet been precisely assessed.
Interestingly, it was also shown that cytotoxic CD8+ T cells, which are well known to kill Lm-infected cells mainly by secretion of perforins, can also mediate anti-listerial immunity via a perforin-independent but TNF-dependent mechanism [66]. Even if direct evidence is lacking, one could speculate that TNF produced by cytotoxic CD8+ T cells triggers TNF-mediated necrosis in infected cells (Fig. 1).
Shigella
Shigellosis, also known as bacillary dysentery, is a human foodborne bacterial infection highly prevalent in children in developing countries. Bacteria from the Shigella genus invade the lamina propria of the gut after crossing the intestinal barrier via Peyer patches M cells [67] and kill resident macrophages [68]. Shigella was long known to induce the death of infected macrophages in vitro [9]. Based on the classical criteria used at that time, the death pathway was assigned to as apoptosis. It was later shown that Shigella infection and killing of macrophages triggers caspase 1 activation, IL-1β and IL-18 release, and pyroptosis [69]. The killing of macrophages is required for an efficient invasion of the intestinal epithelium via the basal pole after translocation via M cells. Surprisingly, macrophages are not the only professional phagocytes killed by Shigella. It has been shown that neutrophils can also die and produce neutrophil extracellular traps (NETs) in response to Shigella infection [70]. These NETs are composed of neutrophil DNA that is externalized and physically trap and kill bacteria. The release of DNA is a form of cellular suicide and can be now considered as a specialized form of regulated cell death (NETosis) triggered by bacteria such as Shigella and involved in bacterial clearance.
Interestingly, Shigella has also been shown to induce nonmyeloid necrosis by triggering mitochondrial dysfunction in cultured epithelial cells [71]. However, Shigella can modulate epithelial cell necrosis by activating a pathway involving Nod1, RIPK2, and NF-κB which delay cell death. This pro-survival pathway could explain the observed in vivo resistance of epithelial cells to Shigella-induced cell death. In this model, Shigella could favor epithelial cell survival to maintain alive their ecological niche [71]. A more recent study has added a supplemental level of complexity: upon infection, Shigella induces a genotoxic stress which activates p53 in the host cell and favors the apoptosis of the infected cell [72]. A Shigella virulence factor, VirA, activates calpain which in turn induces the degradation of p53. This allows infected cells to survive and serve as a reservoir for Shigella multiplication prior to the future invasion of neighboring cells [72]. Finally, another recent study has revealed that the Shigella protein OspC3 modulates caspase 11-dependent pyroptotic death of several human epithelial cell lines, providing the first evidence of a modulation by a bacterial effector of the noncanonical pyroptotic pathway [73]. To summarize, Shigella exhibits a unique capability to hijack cell survival or cell death pathways and promote infection.
Yersinia
The Yersinia genus is composed of several species, three of which are pathogenic for humans, Yersinia pestis, the causative agent of the plague, and Yersinia pseudotuberculosis and Yersinia enterocolitica, associated with enteritis and mesenteric adenitis. Yersinia crosses the intestinal barrier via Peyer patches M cells specifically, thanks to a surface protein called invasin [74–76]. But interestingly, as compared to the aforementioned foodborne bacteria, Yersinia prevents its phagocytosis by macrophages. It injects virulence factors within targeted cells via a type-III secretion system. The main toxin injected is YopJ in Y. pestis and Y. pseudotuberculosis and its ortholog YopP in Y. enterolitica. As for Salmonella, Yersinia-associated macrophage death was first thought to be apoptotic [77, 78], before the requirement of caspase 1 was demonstrated, reclassifying Yersinia-induced macrophage death as pyroptosis [79]. Triggering phagocyte death was originally viewed as a bacterial weapon destroying one of the host first lines of defense [80]. Nevertheless, this has been revisited by a study where authors have used a system in which the cytotoxicity of Yersinia is modulatable [81]. A YopP-producing strain of Y. pseudotuberculosis was shown to induce more macrophage, dendritic cell and B cell death in mesenteric lymph nodes, as compared to the isogenic strain expressing YopJ. Strikingly, the virulence of the YopP-expressing so called “super killer” strain was attenuated as compared with the YopJ-expressing control strain with a colonization of spleen and lymph node diminished after an infection with the YopP-expressing strain [81]. Interestingly, ∆yopJ and YopP-expressing strains exhibit comparable host colonization potentials, both invading less efficiently the host than the wt YopJ-expressing strain. These data indicate that bacteria unable to induce cell death or that overkill host cells have a reduced colonizing ability whereas the more invasive bacteria are the ones that induce an intermediate level of cell death [81]. These observations have been corroborated by another study with Y. pestis, where the authors have shown that YopP-expressing Y. pestis exhibits high cytotoxic activity against macrophages in vitro as compared to the wild-type YopJ-expressing Y. pestis [82]. Once again, this high cytotoxicity correlates with reduced colonization of organs.
Other examples of bacteria-induced host cell death
Mycobacteria, Salmonella, Listeria, Shigella, and Yersinia species are exquisite tools to characterize bacteria-induced regulated necrosis. Nevertheless, as this field is just emerging, a growing number of host–pathogen interactions leading to regulated necrosis are reported. Helicobacter pylori is a bacterium which colonizes about half of the world’s population and is associated with gastric adenocarcinoma. H. pylori induce a form of regulated necrosis via the virulence factor VacA, at least in vitro [83]. Even if the relevance of this phenotype in cancer development has not been assessed, experimental data suggest that VacA-mediated regulated necrosis may contribute to gastric inflammation, an identified risk factor for the development of ulcer disease and gastric adenocarcinoma.
Regulated necrosis was also observed upon infection by enteropathogenic species from the genus Clostridium, Clostridium septicum [84] and Clostridium perfringens [85], uropathogenic Escherichia coli [86]. In addition, studies using Staphylococcus aureus have also revealed a bacteria-induced macrophage necrotic death; although it remains unclear if the process is caspase 1-dependent pyroptosis [87] or RIPK1-RIPK3-mediated necroptosis [88].
Finally, very recently, a study has shed light on the impact of early necroptosis of macrophages on infection outcome [56]. Using various pore-forming toxin-producing bacterial pathogens, authors have shown that prevention of bacteria-induced regulated necrosis could be beneficial for the host, at least in a murine model of Serratia marcescens-induced hemorrhagic pneumonia [56]. These results fit with those obtained earlier with Salmonella [51] and argue for a model where early necroptosis favors bacterial infection and is detrimental for the host.
Conclusions
Bacterial infections modulate host cell responses. One of these responses is necrotic cell death. The description of apoptosis 40 years ago was instrumental in showing that cell death is not necessarily detrimental, as it is involved in embryonic development and tissue homeostasis. The recent years have revealed that necrosis can also be regulated, which can be beneficial for the host during pathology. Nevertheless, the outcome of bacteria-induced host necrotic cell death can vary according to infection parameters such as the intensity of infection, the time point, the infected organ and cell types and the pathogenic agent. In this context, studies with immortalized cell lines in which by definition cell death pathways are perturbed show some limitations. Integrative studies of bacteria-associated host cell necrosis in vivo will be critically needed to fully explore the spatiotemporal modulation of host cell death by bacteria in their physiological environment. The underlying host- and pathogen-driven mechanisms that will be uncovered could be critical to a basic understanding of regulated cell death, but also important to develop targeted anti-infective strategies.
Acknowledgments
We apologize to the many colleagues whose work has not been discussed. We thank the members of the Biology of Infection Unit for helpful discussions. Research in the Biology of Infection Unit is supported by Institut Pasteur, Institut National de la Santé et de la Recherche Médicale, Agence Nationale de la Recherche, LabEx IBEID, the Proantilis EU program and the European Research Council.
References
- 1.Van Praet JT, Donovan E, Vanassche I, Drennan MB, Windels F, Dendooven A, Allais L, Cuvelier CA, van de Loo F, Norris PS, Kruglov AA, Nedospasov SA, Rabot S, Tito R, Raes J, Gaboriau-Routhiau V, Cerf-Bensussan N, Van de Wiele T, Eberl G, Ware CF, Elewaut D. Commensal microbiota influence systemic autoimmune responses. EMBO J. 2015;34(4):466–474. doi: 10.15252/embj.201489966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 3.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338(6103):120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MR, Kamboj M, Pamer EG. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Investig. 2010;120(12):4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G, Nomenclature Committee on Cell D Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16(1):3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93(18):9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 10.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19(1):107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci USA. 2006;103(48):18314–18319. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sperandio S, de Belle I, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci. 2000;97(26):14376–14381. doi: 10.1073/pnas.97.26.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willingham SB, Bergstralh DT, O’Connor W, Morrison AC, Taxman DJ, Duncan JA, Barnoy S, Venkatesan MM, Flavell RA, Deshmukh M, Hoffman HM, Ting JP. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe. 2007;2(3):147–159. doi: 10.1016/j.chom.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 16.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 17.Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol. 2014;35:24–32. doi: 10.1016/j.semcdb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15(2):135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 19.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 20.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 21.Monack DM, Detweiler CS, Falkow S. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell Microbiol. 2001;3(12):825–837. doi: 10.1046/j.1462-5822.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- 22.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8(11):1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 23.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988;141(8):2629–2634. [PubMed] [Google Scholar]
- 25.Fady C, Gardner A, Jacoby F, Briskin K, Tu Y, Schmid I, Lichtenstein A. Atypical apoptotic cell death induced in L929 targets by exposure to tumor necrosis factor. J Interferon Cytokine Res. 1995;15(1):71–80. doi: 10.1089/jir.1995.15.71. [DOI] [PubMed] [Google Scholar]
- 26.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1(6):489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 27.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–190. doi: 10.1016/S0092-8674(03)00521-X. [DOI] [PubMed] [Google Scholar]
- 28.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 31.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1–2):213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 32.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16(1):55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, Han J. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24(1):105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54(1):133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Clay H, Volkman HE, Ramakrishnan L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity. 2008;29(2):283–294. doi: 10.1016/j.immuni.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153(3):521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clay H, Davis JM, Beery D, Huttenlocher A, Lyons SE, Ramakrishnan L. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe. 2007;2(1):29–39. doi: 10.1016/j.chom.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulrichs T, Kosmiadi GA, Trusov V, Jorg S, Pradl L, Titukhina M, Mishenko V, Gushina N, Kaufmann SH. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol. 2004;204(2):217–228. doi: 10.1002/path.1628. [DOI] [PubMed] [Google Scholar]
- 39.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136(1):37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis. 2011;17(1):7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richter-Dahlfors A, Buchan AM, Finlay BB. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186(4):569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96(5):2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JM, Eckmann L, Savidge TC, Lowe DC, Witthoft T, Kagnoff MF. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Investig. 1998;102(10):1815–1823. doi: 10.1172/JCI2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38(1):31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 45.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, Galan JE. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203(6):1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raupach B, Peuschel SK, Monack DM, Zychlinsky A. Caspase-1-mediated activation of interleukin-1beta (IL-1beta) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006;74(8):4922–4926. doi: 10.1128/IAI.00417-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 49.Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490(7419):288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao EA. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339(6122):975–978. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13(10):954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaillard JL, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vazquez MA, Sicher SC, Wright WJ, Proctor ML, Schmalzried SR, Stallworth KR, Crowley JC, Lu CY. Differential regulation of TNF-alpha production by listeriolysin-producing versus nonproducing strains of Listeria monocytogenes . J Leukoc Biol. 1995;58(5):556–562. doi: 10.1002/jlb.58.5.556. [DOI] [PubMed] [Google Scholar]
- 54.Kayal S, Lilienbaum A, Poyart C, Memet S, Israel A, Berche P. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-kappa B and upregulation of adhesion molecules and chemokines. Mol Microbiol. 1999;31(6):1709–1722. doi: 10.1046/j.1365-2958.1999.01305.x. [DOI] [PubMed] [Google Scholar]
- 55.Carrero JA, Calderon B, Unanue ER. Listeriolysin O from Listeria monocytogenes is a lymphocyte apoptogenic molecule. J Immunol. 2004;172(8):4866–4874. doi: 10.4049/jimmunol.172.8.4866. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez-Juarbe N, Gilley RP, Hinojosa CA, Bradley KM, Kamei A, Gao G, Dube PH, Bergman MA, Orihuela CJ. Pore-forming toxins induce macrophage necroptosis during acute bacterial Pneumonia. PLoS Pathog. 2015;11(12):e1005337. doi: 10.1371/journal.ppat.1005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barsig J, Kaufmann SH. The mechanism of cell death in Listeria monocytogenes-infected murine macrophages is distinct from apoptosis. Infect Immun. 1997;65(10):4075–4081. doi: 10.1128/iai.65.10.4075-4081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cervantes J, Nagata T, Uchijima M, Shibata K, Koide Y. Intracytosolic Listeria monocytogenes induces cell death through caspase-1 activation in murine macrophages. Cell Microbiol. 2008;10(1):41–52. doi: 10.1111/j.1462-5822.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 59.Bleriot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015;42(1):145–158. doi: 10.1016/j.immuni.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 60.Di Paolo NC, Doronin K, Baldwin LK, Papayannopoulou T, Shayakhmetov DM. The transcription factor IRF3 triggers “defensive suicide” necrosis in response to viral and bacterial pathogens. Cell reports. 2013;3(6):1840–1846. doi: 10.1016/j.celrep.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stockinger S, Materna T, Stoiber D, Bayr L, Steinborn R, Kolbe T, Unger H, Chakraborty T, Levy DE, Muller M, Decker T. Production of type I IFN sensitizes macrophages to cell death induced by Listeria monocytogenes . J Immunol. 2002;169(11):6522–6529. doi: 10.4049/jimmunol.169.11.6522. [DOI] [PubMed] [Google Scholar]
- 62.O’Connell RM, Saha SK, Vaidya SA, Bruhn KW, Miranda GA, Zarnegar B, Perry AK, Nguyen BO, Lane TF, Taniguchi T, Miller JF, Cheng G. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med. 2004;200(4):437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Auerbuch V, Brockstedt DG, Meyer-Morse N, O’Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes . J Exp Med. 2004;200(4):527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med. 2004;200(4):535–540. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solodova E, Jablonska J, Weiss S, Lienenklaus S. Production of IFN-beta during Listeria monocytogenes infection is restricted to monocyte/macrophage lineage. PLoS One. 2011;6(4):e18543. doi: 10.1371/journal.pone.0018543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White DW, Harty JT. Perforin-deficient CD8+ T cells provide immunity to Listeria monocytogenes by a mechanism that is independent of CD95 and IFN-gamma but requires TNF-alpha. J Immunol. 1998;160(2):898–905. [PubMed] [Google Scholar]
- 67.Wassef JS, Keren DF, Mailloux JL. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect Immun. 1989;57(3):858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zychlinsky A, Thirumalai K, Arondel J, Cantey JR, Aliprantis AO, Sansonetti PJ. In vivo apoptosis in Shigella flexneri infections. Infect Immun. 1996;64(12):5357–5365. doi: 10.1128/iai.64.12.5357-5365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, Takeda K, Zychlinsky A. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12(5):581–590. doi: 10.1016/S1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- 70.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 71.Carneiro LA, Travassos LH, Soares F, Tattoli I, Magalhaes JG, Bozza MT, Plotkowski MC, Sansonetti PJ, Molkentin JD, Philpott DJ, Girardin SE. Shigella induces mitochondrial dysfunction and cell death in nonmyleoid cells. Cell Host Microbe. 2009;5(2):123–136. doi: 10.1016/j.chom.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 72.Bergounioux J, Elisee R, Prunier AL, Donnadieu F, Sperandio B, Sansonetti P, Arbibe L. Calpain activation by the Shigella flexneri effector VirA regulates key steps in the formation and life of the bacterium’s epithelial niche. Cell Host Microbe. 2012;11(3):240–252. doi: 10.1016/j.chom.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 73.Kobayashi T, Ogawa M, Sanada T, Mimuro H, Kim M, Ashida H, Akakura R, Yoshida M, Kawalec M, Reichhart JM, Mizushima T, Sasakawa C. The Shigella OspC3 effector inhibits caspase-4, antagonizes inflammatory cell death, and promotes epithelial infection. Cell Host Microbe. 2013;13(5):570–583. doi: 10.1016/j.chom.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 74.Hanski C, Kutschka U, Schmoranzer HP, Naumann M, Stallmach A, Hahn H, Menge H, Riecken EO. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect Immun. 1989;57(3):673–678. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Autenrieth IB, Firsching R. Penetration of M cells and destruction of Peyer’s patches by Yersinia enterocolitica: an ultrastructural and histological study. J Med Microbiol. 1996;44(4):285–294. doi: 10.1099/00222615-44-4-285. [DOI] [PubMed] [Google Scholar]
- 76.Marra A, Isberg RR. Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer’s patch intestinal epithelium. Infect Immun. 1997;65(8):3412–3421. doi: 10.1128/iai.65.8.3412-3421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Monack DM, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94(19):10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mills SD, Boland A, Sory MP, van der Smissen P, Kerbourch C, Finlay BB, Cornelis GR. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94(23):12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bergsbaken T, Cookson BT. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3(11):e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Monack DM, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188(11):2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brodsky IE, Medzhitov R. Reduced secretion of YopJ by Yersinia limits in vivo cell death but enhances bacterial virulence. PLoS Pathog. 2008;4(5):e1000067. doi: 10.1371/journal.ppat.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zauberman A, Tidhar A, Levy Y, Bar-Haim E, Halperin G, Flashner Y, Cohen S, Shafferman A, Mamroud E. Yersinia pestis endowed with increased cytotoxicity is avirulent in a bubonic plague model and induces rapid protection against pneumonic plague. PLoS One. 2009;4(6):e5938. doi: 10.1371/journal.pone.0005938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Radin JN, Gonzalez-Rivera C, Ivie SE, McClain MS, Cover TL. Helicobacter pylori VacA induces programmed necrosis in gastric epithelial cells. Infect Immun. 2011;79(7):2535–2543. doi: 10.1128/IAI.01370-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kennedy CL, Smith DJ, Lyras D, Chakravorty A, Rood JI. Programmed cellular necrosis mediated by the pore-forming alpha-toxin from Clostridium septicum . PLoS Pathog. 2009;5(7):e1000516. doi: 10.1371/journal.ppat.1000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Autheman D, Wyder M, Popoff M, D’Herde K, Christen S, Posthaus H. Clostridium perfringens beta-toxin induces necrostatin-inhibitable, calpain-dependent necrosis in primary porcine endothelial cells. PLoS One. 2013;8(5):e64644. doi: 10.1371/journal.pone.0064644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schaale K, Peters KM, Murthy AM, Fritzsche AK, Phan MD, Totsika M, Robertson AA, Nichols KB, Cooper MA, Stacey KJ, Ulett GC, Schroder K, Schembri MA, Sweet MJ. Strain- and host species-specific inflammasome activation, IL-1beta release, and cell death in macrophages infected with uropathogenic Escherichia coli . Mucosal Immunol. 2015 doi: 10.1038/mi.2015.44. [DOI] [PubMed] [Google Scholar]
- 87.Accarias S, Lugo-Villarino G, Foucras G, Neyrolles O, Boullier S, Tabouret G. Pyroptosis of resident macrophages differentially orchestrates inflammatory responses to Staphylococcus aureus in resistant and susceptible mice. Eur J Immunol. 2015;45(3):794–806. doi: 10.1002/eji.201445098. [DOI] [PubMed] [Google Scholar]
- 88.Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, Wachtel S, Bueno S, Prince A. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 2015;11(4):e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]