Abstract
Introduction
The COVID-19 pandemic has initiated an unparalleled global vaccination campaign, raising concerns about the vaccine's effects on various health conditions, including the risk of corneal transplant rejection. This systematic review aimed to identify the relationship between COVID-19 vaccination and rejection of corneal transplant, filling a significant gap in the existing medical literature.
Methods
A literature search was performed across multiple databases up to February 12, 2024, to identify studies evaluating the risk of corneal transplant rejection post-COVID-19 vaccination. Eligible studies were original research that reported outcomes of corneal graft rejection following vaccination. Nested Knowledge web software facilitated screening and data extraction. The Newcastle–Ottawa Scale was employed for quality assessment. A meta-analysis was conducted to calculate the aggregated relative risk (RR) utilizing R software version 4.3.
Results
Six studies were included in the qualitative synthesis, with four meeting the criteria for meta-analysis. These studies varied in geographic location, surgical techniques, and types of vaccines used. The pooled RR for corneal transplant rejection following COVID-19 vaccination was 0.816 (95% CI 0.178–1.453), indicating no significant risk of rejection. No statistical heterogeneity was observed among the studies (I2 = 0%).
Conclusions
This review and meta-analysis found no significant evidence that COVID-19 vaccination increases the risk of corneal graft rejection. However, the current evidence is insufficient to conclusively determine the vaccine's safety for corneal transplant recipients. These findings underscore the need for additional research to confirm these preliminary results and investigate the long-term effects of COVID-19 vaccination on corneal transplants, aiming to provide evidence-based guidance to healthcare providers and patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-024-00941-y.
Keywords: COVID-19 vaccine, Corneal transplantation, Meta-analysis, Systematic review, Ophthalmology
Key Summary Points
| The COVID-19 pandemic has led to global vaccination efforts, raising concerns about the vaccine's impact on health conditions, including the risk of corneal transplant rejection, a significant gap in medical literature. |
| This study aims to assess the relationship between COVID-19 vaccination and the risk of corneal transplant rejection, addressing a critical area of concern for patients and healthcare providers. |
| The meta-analysis of four studies, including a pooled relative risk (RR) of 0.816, showed no significant risk of corneal transplant rejection following COVID-19 vaccination. |
| This study contributes to the understanding that COVID-19 vaccination does not significantly increase the risk of corneal graft rejection, emphasizing the need for further research to explore the long-term vaccine safety for transplant recipients and potentially guiding vaccination policies for this patient group. |
Introduction
During the unparalleled worldwide health emergency induced by the COVID-19 pandemic, vaccination has risen as a cornerstone in the collective effort to control the spread of the SARS-CoV-2 virus and alleviate its profound impact on worldwide public health infrastructure [1–3]. The swift development, regulatory approval, and extensive administration of COVID-19 vaccines have played an instrumental role in decreasing transmission rates, reducing the severity of cases, and lowering mortality rates attributed to the virus [4]. As the vaccination initiative has expanded, there has been an escalating interest in discerning the potential ramifications of COVID-19 vaccines on various health conditions and medical interventions, particularly concerning organ transplantation procedures [5–8].
Corneal transplantation, or keratoplasty, represents a critical surgical intervention for restoring vision in individuals affected by corneal opacities, dystrophies, or scarring [9–11]. Despite the procedure's notable success rates, the postoperative period is fraught with the challenge of immune-mediated graft rejection, a significant cause of graft failure and subsequent vision loss [11]. The introduction of the COVID-19 vaccine has sparked a debate over its potential impact on the incidence or intensity of corneal transplant rejection [12]. This speculation is rooted in the notion that the vaccine's systemic immune activation could amplify the immune response to the transplanted corneal tissue, thereby elevating the risk of graft rejection [13].
The importance of addressing this concern is twofold; not only does it bear significance for patient care but it also has implications for the formulation of vaccination guidelines for individuals awaiting or having undergone corneal transplantation. Consequently, there is an urgent need to thoroughly examine the existing evidence to determine the relationship between COVID-19 vaccination and the risk of corneal transplant rejection [14–17]. While previous systematic reviews have delved into the characteristics and outcomes of corneal transplant rejection in the context of COVID-19 vaccination [18, 19], there still needs to be a gap in the literature regarding a comprehensive assessment of the risk associated with corneal graft rejection post-vaccination.
This systematic review and meta-analysis seeks to bridge this gap by aggregating and scrutinizing data from various studies investigating the occurrence of corneal graft rejection after COVID-19 vaccination. The objective is to determine whether a statistically significant association exists between vaccination and an increased risk of rejection. This endeavor is vital for informing clinical decision-making processes, guiding vaccination policy for transplant recipients, and optimizing patient outcomes during the pandemic. By providing a detailed analysis of the evidence, this review aimed to contribute valuable insights to the body of knowledge on the efficacy and safety profile of COVID-19 vaccines in the context of corneal transplantation, thereby aiding healthcare professionals, policymakers, and patients in navigating the complexities of transplantation and vaccination during these challenging times.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to conduct the systematic review (Table S1). For the processes of screening and extracting data, we employed the Nested-Knowledge (Nested-Knowledge, Saint Paul, MN, USA) software. The protocol has been registered in PROSPERO.
Ethical Approval
Ethical approval is not required since this is a systematic review. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Eligibility Criteria
Studies were eligible for inclusion if they involved individuals who had undergone corneal transplantation and subsequently received a COVID-19 vaccine, reported outcomes specifically related to corneal graft rejection, and fell into the categories of either observational studies or clinical trials. On the other hand, exclusions were made for studies that did not explicitly address the incidence or outcomes of corneal transplant rejection after receiving the COVID-19 vaccine. Narrative reviews, case reports, editorial comments, and studies lacking primary data were also excluded from the analysis. The criteria for inclusion are detailed in Table S2.
Search Strategy
A literature search was performed across multiple electronic databases such as EMBASE, PubMed, and Web of Science, from their inception until February 12, 2024, to identify studies assessing the risk of corneal transplant rejection after receiving the COVID-19 vaccine. The search strategy was crafted to encompass a combination of keywords and MeSH terms related to "corneal transplantation," "keratoplasty," "COVID-19," "SARS-CoV-2," "vaccination," and "graft rejection." No language restrictions were imposed to ensure the inclusion of a wide range of relevant studies. Furthermore, we conducted a manual examination of the reference lists from the identified articles to discover additional studies that met our eligibility criteria. Google Scholar was also manually explored for additional relevant publications to enhance the search comprehensively. The details of the full search strategy are provided in Table S3.
Screening
For the screening process, we utilized the advanced capabilities of Nested Knowledge software, a specialized tool for managing and streamlining literature. The screening involved two steps: title and abstract, followed by full-text screening. Two independent reviewers screened the articles. A third reviewer resolved any discrepancies.
Data Extraction and Quality Assessment
Data from the included studies was collected by two separate reviewers using a uniform data collection template. The tagging function of Nested Knowledge software was used for data extraction. Data extracted included study design, participant characteristics, type of COVID-19 vaccine administered, incidence of graft rejection, and outcomes of interest, such as Relative Risk (RR)/Odds Ratio (OR)/Hazard Ratio (HR) for graft rejection. Disagreements among reviewers were settled via discussion, or when required, by seeking the opinion of a third reviewer. The quality assessment of the studies incorporated in this review was performed utilizing the Newcastle–Ottawa Scale (NOS) [20, 21].
Statistical Analysis
Meta-analytic techniques were employed to estimate the pooled RR of corneal transplant rejection after receiving the COVID-19 vaccine. Heterogeneity among studies was quantified using the I2 statistic, with values greater than 50% accounting for substantial heterogeneity [22, 23]. A random-effects model (REM) was utilized to accommodate the expected variability among studies. The tau-squared (τ2) statistic, calculated using the maximum likelihood estimation method, served as an additional measure of heterogeneity [24]. A p value of less than 0.05 was established as the criterion for statistical significance. The analyses were conducted using the "Meta" and "Metaphor" packages within R statistical software, Version 4.3 [20].
Results
Search Result
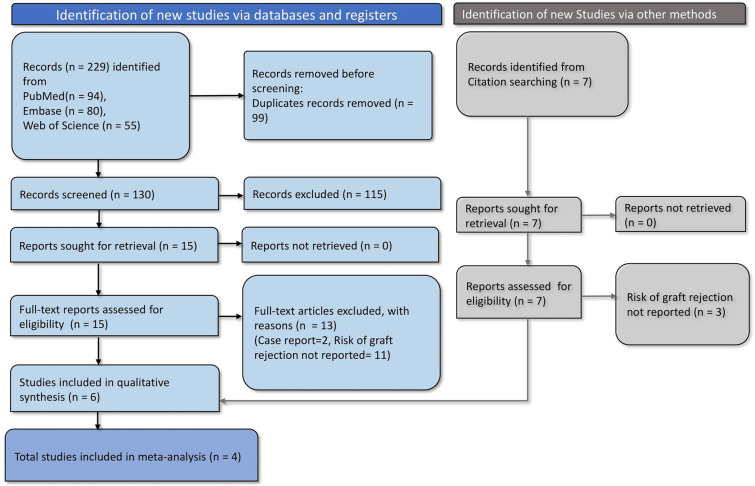
The search across various databases resulted in 229 records initially. Upon the removal of 99 duplicates prior to screening, we proceeded to screen 130 records. Out of these, 115 records were excluded for various reasons [25, 26], leaving 15 full-text articles to be assessed for eligibility. Additionally, records identified through citation searching and Google Scholar amounted to seven more articles retrieved for potential inclusion, of which four studies were included. Upon full-text review, 13 articles were excluded from the analysis: two were case reports, and 11 did not report the risk of graft rejection. Consequently, six studies were included in the systematic review. Of these, four studies provided data suitable for meta-analysis (Fig. 1).
Fig. 1.
PRISMA flowchart depicting study selection and screening process
Characteristics of Included Studies
Table 1 presents a summary of the characteristics of the studies included in the analysis. Three studies were from Japan [27–29], two from Italy [8, 30], and one from the USA [31]. All of the studies employed a retrospective design. The surgical techniques utilized across the studies included conventional penetrating keratoplasty (PK), deep anterior lamellar keratoplasty (DALK), Descemet stripping automated endothelial keratoplasty (DSAEK), Descemet membrane endothelial keratoplasty (DMEK), and mushroom PK. The type of COVID-19 vaccine was not reported in two of the studies. In contrast, one study exclusively involved an mRNA vaccine, and another study included a variety of vaccines such as BNT162b2, Moderna, Pfizer COVID-19 Vaccine, Johnson & Johnson's Janssen Vaccine, AZD1222, and Ad26.COV2.S. The quality assessment of the studies is presented in Table S4.
Table 1.
Characteristics of included studies
| Study | Country | Study design | Mean age (years) | Number of vaccinated patients | Surgical technique/graft | Type of vaccine | RR/OR/ HR (95% CI) for graft rejection | Follow-up days after vaccination | Quality of study |
|---|---|---|---|---|---|---|---|---|---|
| Busin (2022) [30] | Italy | Retrospective study | 58.6 | 785 | DALK, DMEK, DSAEK, PK conventional PK, mushroom PK, | BNT16262 (79.2%), mRNA (15.7%), AZD1222 (3.2%), Ad26.COV2. S (1.9%) | HR = 0.75 (0.10–5.52), IRR = 0.56 (0.13–2.28) | 60 days from the last dose | High |
| Culp (2022) [31] | USA | Retrospective study | NA | 784 | NA | Moderna or Pfizer COVID-19 Vaccine or J & J's Janssen Vaccine | RR = 0.92 (0.42–2.01) | NA | Unclear |
| Fujimoto (2021) [28] | Japan | Retrospective study | 72 | 29 | PK, DSAEK, ALK or DALK, LT | BNT162b2 | OR = 5.57 (1.18–25.57, RR = 4.47 (1.15–18.34) | 74 days | High |
| Fujimoto (2024) [29] | Japan | Retrospective cohort study | 67.95 | NA | PK, DSAEK, DALK, LT | SARS-CoV-2 mRNA vaccine | RR = 5.74 | 804 days | High |
| Igarashi (2023) [27] | Japan | Retrospective cohort study | 73.3 | 74 | DMEK | NA | HR = 25.5 (2.10–309) | 474 days | High |
| Roberts (2023) [8] | Italy | Retrospective study | 56 | 82 | NA | NA | IRR = 0.53, p = 0.71 | NA | High |
DMEK Descemet membrane endothelial keratoplasty, NA not available/not applicable, HR hazard ratio, DALK deep anterior lamellar keratoplasty, DSAEK Descemet stripping automated endothelial keratoplasty, PK penetrating keratoplasty, BNT162b2 Pfizer-BioNTech COVID-19 vaccine, mRNA-based, mRNA Messenger RNA, AZD1222 AstraZeneca COVID-19 vaccine, Vaxzevria, Ad26.COV2.S Johnson & Johnson's Janssen COVID-19 vaccine, viral vector-based, IRR incidence rate ratio, OR odds ratio, CI confidence interval, LT lamellar transplantation, RR relative risk, SARS-CoV-2 Severe Acute Respiratory Syndrome Coronavirus 2
Risk of Corneal Transplant Rejection After Receiving COVID-19 Vaccine
The transplant rejection risk varied between studies. In Japan, Igarashi et al. (2023) [27] conducted a retrospective cohort study involving 74 vaccinated patients with an average age of 73.3 years who underwent DMEK. Although the specific type of vaccine was not reported, the study observed an HR of 25.5 (95% CI 2.10–309) for graft rejection over a 474-day follow-up period. From Italy, Busin et al. (2022) [30] analyzed 785 vaccinated patients with a mean age of 58.6 years. Various surgical techniques were utilized, such as DALK, DMEK, DSAEK, and different forms of PK. Vaccinations included BNT162b2 (79.2%), mRNA-based vaccines (15.7%), AZD1222 (3.2%), and Ad26.COV2.S (1.9%). The study reported an HR of 0.75 (95% CI 0.10–5.52) and an incidence rate ratio (IRR) of 0.56 (95% CI 0.13–2.28) following 60 days after the last vaccine dose. In another retrospective study, Roberts et al. (2023) reported that An IRR of 0.53 with a p value of 0.71 was reported, suggesting no significant association with graft rejection. Fujimoto et al. (2021) presented findings from a Japanese study with 29 patients, averaging 72 years old, who received various corneal transplants, including PK, DSAEK, ALK or DALK, and LT. The BNT162b2 vaccine yielded an OR of 5.57 (95% CI 1.18–25.57) and an RR of 4.47 (95% CI 1.15–18.34) within a 74-day follow-up. In the USA, Culp et al. (2022) included 784 patients in a retrospective study that did not disclose mean age or surgical techniques. The vaccines administered were Moderna, Pfizer COVID-19, or Johnson & Johnson's Janssen Vaccine, with a reported RR of 0.92 (95% CI 0.42–2.01), indicating no significant risk of rejection post-vaccination. Fujimoto et al. (2024) conducted a retrospective cohort study in Japan with an average patient age of 67.95 years. Though the number of vaccinated patients was not detailed, the study utilized PK, DSAEK, DALK, and LT with SARS-CoV-2 mRNA vaccines and reported an RR of 5.74 over an extensive 804-day follow-up.
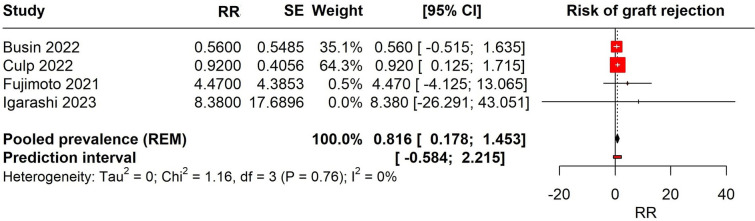
Meta-analysis
We performed a meta-analysis of four studies that reported the RR for corneal transplant rejection following COVID-19 vaccination (Fig. 2). The studies included in the analysis were Busin 2022, Culp 2022, Fujimoto 2021, and Igarashi 2023. The pooled RR was estimated using a REM, accounting for variation within and between studies. The pooled RR for the meta-analysis was 0.816 (95% CI 0.178–1.453), indicating no significant risk of corneal transplant rejection after receiving COVID-19 vaccine across the included studies. The prediction interval, which provides a range where the true effect is expected to lie in similar studies, was – 0.584 to 2.215. There was no observed heterogeneity among the studies (Tau2 = 0; Chi2 = 1.16, (p = 0.76); I2 = 0%).
Fig. 2.
Forest plot depicting the risk of corneal transplant rejection with COVID-19 vaccination
Publication Bias
Our ability to evaluate the presence of publication bias was constrained by the limited number of studies included in our meta-analysis. Typically, assessment methods such as funnel plots or Egger's test require more studies to provide reliable results. Due to this limitation, we cannot draw definitive conclusions about the potential for publication bias in this body of research.
Discussion
Our study represents the first attempt to perform a meta-analysis assessing the risk of corneal transplant rejection after COVID-19 vaccine administration. Our analysis revealed a pooled RR of 0.816 (95% CI 0.178–1.453) for corneal transplant rejection after receiving the COVID-19 vaccine. This finding suggests no significant increase in the risk of graft rejection among vaccinated individuals. The studies analyzed varied in geographic location, surgical techniques, and types of COVID-19 vaccines administered, reflecting a broad spectrum of clinical practices and patient experiences. Despite these variations, the overall lack of a significant association between COVID-19 vaccination and increased risk of corneal graft rejection may suggest a favorable safety profile for the vaccines in the context of corneal transplantation. Nonetheless, the available evidence still needs to provide a definitive conclusion.
While previous systematic reviews have broached the subject by collating case reports, our study provides a risk analysis of corneal transplant rejection with COVID-19 vaccination. For instance, a previous review [19] documented various types of corneal transplants, including 12 cases of penetrating keratoplasty, six of DMEK, four of DSAEK, and one instance of living-related conjunctival-limbal allograft. The onset of graft rejection post-vaccination ranged from as short as 1 day to up to 6 weeks. The leading clinical presentation was corneal edema, occurring in 20 eyes. Other notable symptoms included keratic precipitates and conjunctival or ciliary injection, each observed in 14 eyes. The primary treatment involved the frequent application of topical corticosteroids in 12 eyes and a regimen combining both topical and oral corticosteroids in four eyes. Corneal edema was identified as the most prevalent clinical ocular manifestation post-vaccination, affecting 87% of the cases, with keratic precipitates and conjunctival injection also being significant findings. Another systematic review has also reported similar findings [32].
Implications of this study are significant for clinical practice, particularly for the management of corneal transplant recipients in the era of COVID-19 vaccination. Our findings suggest that COVID-19 vaccines do not increase the risk of corneal graft rejection, which may alleviate concerns among healthcare providers and patients about the potential impact of vaccination on transplant outcomes. This could potentially support vaccination campaigns within this patient population, emphasizing the importance of protection against COVID-19 without compromising graft survival. Studies with larger cohorts are essential to validate our results and enable a more robust analysis of publication bias. Studies that are longitudinal in nature and include extended periods of follow-up could offer valuable insights into the enduring impacts of COVID-19 vaccination on the survival of corneal grafts. Further research should also aim to include diverse populations to ensure the applicability of the results across different patient demographics. Investigations into the relationship between different types of COVID-19 vaccines and corneal transplant rejection could uncover vaccine-specific risks or benefits. Additionally, mechanistic studies exploring the immunological responses to vaccination in the context of corneal transplantation could further our understanding of graft rejection. Comparative effectiveness research could situate the safety profile of COVID-19 vaccines within the broader context of vaccines administered to transplant recipients. Utilizing data from transplant registries might offer a comprehensive view of the post-vaccination outcomes on a global scale, providing valuable data for healthcare policy and practice. Addressing these research directions will be crucial in advancing our knowledge and ensuring the well-being of corneal transplant recipients during and beyond the pandemic.
Our review is subject to several limitations. The number of available studies that reported an association between the COVID-19 vaccine and corneal transplant rejection was very limited. The inherent biases of retrospective study designs, which constituted all the included studies, may affect the reliability of the results. Additionally, the variability in reporting the type of COVID-19 vaccines and the lack of detailed immunological profiles of the recipients represent gaps in the data. More studies are needed in the future for a better understanding of the condition.
Conclusions
Our analysis has revealed no significant evidence to suggest that COVID-19 vaccination increases the risk of corneal graft rejection. However, the evidence currently available is insufficient to conclusively determine the safety of COVID-19 vaccines on corneal transplant recipients. Further research is necessary to verify these findings and investigate the long-term effects of COVID-19 vaccination on corneal transplant outcomes. This will enable healthcare providers to offer evidence-based advice to their patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the Nested-Knowledge, Saint Paul, MN, USA for providing access to the software. Qatar National Library funded the publication of this article.
Author Contributions
Conceptualization: Bijaya K Padhi, Mahalaqua Nazli Khatib, Parul Chawla Gupta; Data curation: Mahalaqua Nazli Khatib, Quazi Syed Zahiruddin, Arkadiusz Dziedzic; Formal analysis: Shilpa Gaidhane, Arkadiusz Dziedzic; Investigation: Shilpa Gaidhane, Prakasini Satapathy, Parul Chawla Gupta; Methodology: Bijaya K Padhi, Arkadiusz Dziedzic, Neelima Kukreti; Project administration: Abhay M Gaidhane; Resources: Quazi Syed Zahiruddin, Prakasini Satapathy; Software: Sarvesh Rustagi; Supervision: Abhay M Gaidhane; Validation: Neelima Kukreti, Sarvesh Rustagi; Visualization: Hashem Abu Serhan; Writing – original draft: Mahalaqua Nazli Khatib; Writing – review & editing: Hashem Abu Serhan, Prakasini Satapathy.
Funding
This author received no funding for conducting the study. The journal’s Rapid Service Fee was funded by Qatar National Library through the author Hashem Abu Serhan.
Data Availability
The data are with the authors and available on request.
Declarations
Conflict of Interest
All named authors confirm that they have no conflicts of interest to report.
Ethical Approval
Ethical approval is not required since it is a systematic review. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Footnotes
Parul Chawla Gupta and Mahalaqua Nazli Khatib contributed equally to this work.
References
- 1.Agyarko R, Al Slail F, Garrett DO, Gentry B, Gresham L, Underwood MLK, et al. Modernizing global health security to prevent, detect, and respond. NY: Elsevier; 2024. The imperative for global cooperation to prevent and control pandemics; pp. 53–69. [Google Scholar]
- 2.Dzinamarira T, Tungwarara N, Chitungo I, Chimene M, Iradukunda PG, Mashora M, et al. Unpacking the implications of SARS-CoV-2 breakthrough infections on COVID-19 vaccination programs. Vaccines. 2022;10(2):252. doi: 10.3390/vaccines10020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adu P, Popoola T, Medvedev ON, Collings S, Mbinta J, Aspin C, Simpson CR. Implications for COVID-19 vaccine uptake: a systematic review. J Infect Public Health. 2023;16(3):441–466. doi: 10.1016/j.jiph.2023.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Han X, Lin X, Zhu X, Wei Y. Impact of vaccine measures on the transmission dynamics of COVID-19. PLoS ONE. 2023;18(8):e0290640. doi: 10.1371/journal.pone.0290640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Luo D, Mei B, Du J, Liu X, Xie H, et al. Immunogenicity of COVID-19 vaccines in solid organ transplant recipients: a systematic review and meta-analysis. Clin Microbiol Infect. 2023;29(4):441–456. doi: 10.1016/j.cmi.2022.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo D, Chen X, Du J, Mei B, Wang A, Kuang F, et al. Immunogenicity of COVID-19 vaccines in chronic liver disease patients and liver transplant recipients: a systematic review and meta-analysis. Liver Int. 2023;43(1):34–48. doi: 10.1111/liv.15403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbulut S, Yagin FH, Sahin TT, Garzali IU, Tuncer A, Akyuz M, et al. Effect of COVID-19 pandemic on patients who have undergone liver transplantation: retrospective cohort study. J Clin Med. 2023;12(13):4466. doi: 10.3390/jcm12134466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts HW, Wilkins MR, Malik M, Talachi-Langroudi M, Myerscough J, Pellegrini M, et al. A lack of an association between COVID-19 vaccination and corneal graft rejection: results of a large multi-country population based study. Eye. 2023;37(11):2316–2319. doi: 10.1038/s41433-022-02341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Wang T, Tuli SS, Steigleman WA, Shah AA. Overview of corneal transplantation for the nonophthalmologist. Transplant Direct. 2023;9(2):e1434. doi: 10.1097/TXD.0000000000001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemaya M, Hemaya M, Habeeb A. Evaluating keratoplasty for Fuchs’ endothelial corneal dystrophy: a literature review. Cureus. 2023 doi: 10.7759/cureus.33639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wajima H, Hayashi T, Kobayashi A, Nishino T, Mori N, Yokogawa H, et al. Graft rejection episodes after keratoplasty in Japanese eyes. Sci Rep. 2023;13(1):2635. doi: 10.1038/s41598-023-29659-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen NE, Zhang J, McGhee CN. COVID-19 vaccination and corneal allograft rejection-a review. Front Cell Infect Microbiol. 2023 doi: 10.3389/fcimb.2023.1307655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh RB, Li J, Parmar UPS, Jeng BH, Jhanji V. Vaccine-associated corneal graft rejection following SARS-CoV-2 vaccination: a CDC-VAERS database analysis. Br J Ophthalmol. 2024;108(1):17–22. doi: 10.1136/bjo-2022-322512. [DOI] [PubMed] [Google Scholar]
- 14.Raiker R, Akosman S, Foos W, Pakhchanian H, Mishra S, Geist C, Belyea DA. Examining the influence of COVID-19 infection and pandemic restrictions on the risk of corneal transplant rejection or failure: a multicenter study. Semin Ophthalmol. 2023;38(8):777–783. doi: 10.1080/08820538.2023.2234495. [DOI] [PubMed] [Google Scholar]
- 15.Kuziez L, Eleiwa TK, Chauhan MZ, Sallam AB, Elhusseiny AM, Saeed HN. Corneal adverse events associated with SARS-CoV-2/COVID-19 vaccination: a systematic review. Vaccines. 2023;11(1):166. doi: 10.3390/vaccines11010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wasai Y, Yamada N, Ariyoshi N, Haraguchi A, Funatsu M, Mikuni M, et al. SARS-CoV-2 infection status in corneal preservation solution and COVID-19 prevalence after corneal transplantation. Sci Rep. 2024;14(1):3766. doi: 10.1038/s41598-024-53863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bushi G, Shabil M, Padhi BK, Ahmed M, Pandey P, Satapathy P, et al. Prevalence of acute kidney injury among dengue cases: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2024;118(1):1–11. doi: 10.1093/trstmh/trad067. [DOI] [PubMed] [Google Scholar]
- 18.Moura-Coelho N, Cunha JP, Papa-Vettorazzi R, Gris Ó, Güell JL. Acute corneal allograft rejection following SARS-CoV-2 vaccination: a systematic review. Acta Ophthalmol. 2023;101(1):e1–e13. doi: 10.1111/aos.15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujio K, Sung J, Nakatani S, Yamamoto K, Iwagami M, Fujimoto K, et al. Characteristics and clinical ocular manifestations in patients with acute corneal graft rejection after receiving the COVID-19 vaccine: a systematic review. J Clin Med. 2022;11(15):4500. doi: 10.3390/jcm11154500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bushi G, Padhi BK, Shabil M, Satapathy P, Rustagi S, Pradhan KB, et al. Cardiovascular disease outcomes associated with obstructive sleep apnea in diabetics: a systematic review and meta-analysis. Diseases. 2023;11(3):103. doi: 10.3390/diseases11030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shabil M, Bushi G, Beig MA, Rais MA, Ahmed M, Padhi BK. Cardiovascular manifestation in tuberculosis cases: a systematic review and meta-analysis. Curr Probl Cardiol. 2023;48(7):101666. doi: 10.1016/j.cpcardiol.2023.101666. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi AP, Satapathy P, Rustagi S, Hermis AH, Sah R, Padhi BK. Comments on" Shigellosis in Southeast Asia: a systematic review and meta-analysis. Travel Med Infect Dis. 2023;54:102593. doi: 10.1016/j.tmaid.2023.102593. [DOI] [PubMed] [Google Scholar]
- 23.Swarup SS, Padhi BK, Satapathy P, Shabil M, Bushi G, Gandhi AP, et al. Cardiovascular consequences of financial stress: a systematic review and meta-analysis. Curr Probl Cardiol. 2023;49:102153. doi: 10.1016/j.cpcardiol.2023.102153. [DOI] [PubMed] [Google Scholar]
- 24.Shabil M, Bushi G, Yadav A, Ahmed M, Kishore J, Lekamwasam S, Joshi A. Effect of metformin on cardiovascular outcomes: a systematic review and meta-analysis of observational studies and RCTs. The Evidence. 2023;1(1):23–34. [Google Scholar]
- 25.Shabil M, Kumar VU, Dhingra S, Ravichandiran V, Parihar VK, Kumar N, et al. Current scenario and strategies to tackle cardiovascular disease risk in HIV geriatrics. Curr Pharmacol Rep. 2023;9(6):523–539. doi: 10.1007/s40495-023-00332-0. [DOI] [Google Scholar]
- 26.Shabil M, Murti K, Kumar VU, Kumar R, Kumar N, Dhingra S, et al. Older PLHIV are at higher cardiovascular risk with poor quality of life. Curr HIV Res. 2023;21(6):354–360. doi: 10.2174/011570162X277586231218104922. [DOI] [PubMed] [Google Scholar]
- 27.Igarashi A, Shimizu T, Takeda M, Ida Y, Ishida A, Yuda K, et al. Incidence of graft rejection in Descemet membrane endothelial keratoplasty after COVID-19 mRNA vaccination. Cornea. 2022;13:10–97. doi: 10.1097/ICO.0000000000003335. [DOI] [PubMed] [Google Scholar]
- 28.Fujimoto H, Kiryu J. Incidence of corneal transplant rejection following BNT162b2 SARS-CoV-2 messenger RNA vaccination. J Ophthalmol Res. 2021;4(3):279–288. doi: 10.26502/fjor.2644-00240046. [DOI] [Google Scholar]
- 29.Fujimoto H. Rejection of corneal transplant after administration of SARS-CoV-2 messenger RNA vaccine. J Ophthalmol Res. 2024;7:13–15. doi: 10.26502/fjor.2644-00240089. [DOI] [Google Scholar]
- 30.Busin M, Zauli G, Pellegrini M, Virgili G, Yu AC. COVID-19 vaccination may not increase rates of corneal graft rejection. Cornea. 2022;41(12):1536–1538. doi: 10.1097/ICO.0000000000003101. [DOI] [PubMed] [Google Scholar]
- 31.Culp CJ, Pakhchanian H, Raiker R, Chan C, Foos WF, Belyea D. Risk of corneal graft rejection in COVID-19 vaccinated patients in the 120-day postoperative period: a multi-healthcare system analysis. Investig Ophthalmol Vis Sci. 2022;63(7):4351-A0288. [Google Scholar]
- 32.Huang L-Y, Chiang C-C, Li Y-L, Lai H-Y, Hsieh Y-C, Wu Y-H, Tsai Y-Y. Corneal complications after COVID-19 vaccination: a systemic review. J Clin Med. 2022;11(22):6828. doi: 10.3390/jcm11226828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are with the authors and available on request.