Abstract
Telemedicine, the provision of remote healthcare, has gained prominence, accelerated by the COVID-19 pandemic. It has the potential to replace routine in-person follow-up visits for patients with chronic inflammatory skin conditions. However, it remains unclear whether telemedicine can effectively substitute in-person consultations for this patient group. This systematic review assessed the effectiveness and safety of telemedicine compared with traditional in-person care for chronic inflammatory skin diseases. A comprehensive search in various databases identified 11 articles, including 5 randomized controlled trials (RCTs) and 1 clinical controlled trial (CCT). These studies evaluated telemedicine’s impact on patients with psoriasis and atopic dermatitis, with varying methods like video consultations and digital platforms. The findings tentatively suggest that telemedicine does not seem to be inferior compared with in-person care, particularly in terms of condition severity and quality of life for patients with chronic inflammatory skin diseases. However, these results should be interpreted with caution due to the inherent uncertainties in the evidence. There are indications that telemedicine can offer benefits such as cost-effectiveness, time savings, and reduced travel distances, but it is important to recognize these findings as preliminary, necessitating further validation through more extensive research.
SIGNIFICANCE
This study is essential because it explores whether using telemedicine, such as video calls or digital platforms, can be a good option for patients with chronic skin conditions. It is even more crucial in today’s world, where we have experienced the benefits of remote healthcare during the pandemic. If telemedicine proves effective, it could save patients time and money, making it easier for them to get the care they need without travelling. This research could help change the way we provide care for skin conditions, making it more convenient and accessible for everyone.
Key words: telemedicine, chronic inflammatory skin conditions, dermatology, remote healthcare, patient-friendly care
The field of telemedicine, which leverages technology to provide clinical care at a distance, has gained significant traction in dermatology. It encompasses various applications, ranging from diagnostic purposes to monitoring the management of diverse skin diseases (1, 2). Although telemedicine has been in existence for several years, its widespread adoption was catalysed by the COVID-19 pandemic, wherein in-person visits were often substituted by remote care out of necessity (3). Despite the easing of COVID-19 restrictions, telemedicine continues to play a prominent role in daily patient care.
Chronic inflammatory skin conditions, such as atopic dermatitis and psoriasis, impose a high demand for systemic medication, with patients typically undergoing strict periodic monitoring through in-person visits every 3 to 6 months (4–6). A systematic review conducted by Marasca et al. (7), analysing 69 case studies involving patients with chronic inflammatory skin conditions, suggested that telemedicine could serve as a patient-friendly solution. Additionally, telemedicine has the potential to reduce costs compared with in-person consultations, particularly by minimizing the number of physical visits, as demonstrated by studies on cost-effectiveness (2, 8, 9). However, previous reviews have not addressed the crucial aspect of preserving effectiveness when transitioning (partially) to remote care (7).
Despite the widespread utilization of telemedicine in current practice, it has not yet received formal recognition in the care of individuals with chronic skin conditions. Notably, telemedicine remains absent from existing (European) guidelines for psoriasis or atopic dermatitis (10, 11). However, it gains more attention, and the International Psoriasis Council formulated a call to action, based on several statements concerning telemedicine (12). The objective of this systematic review is to quantitatively evaluate the efficacy and safety outcomes of telemedicine compared with in-person care for chronic inflammatory skin diseases. This systematic review aims to contribute to the determination of whether telemedicine can be confidently recommended in clinical guidelines.
MATERIALS AND METHODS
This systematic review was prospectively registered in PROSPERO (CRD42022303032) and adheres to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline for transparent reporting (13).
Search strategy
On 1 May 2023, a comprehensive search was conducted in the following databases: MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and CINAHL. The strategy focused on identifying studies related to telemedicine and its application in chronic inflammatory skin conditions, including psoriasis, atopic dermatitis, hidradenitis suppurativa, and chronic urticaria, using a wide range of telehealth-related terms and specific disease keywords, without any restriction on the publication date. Additionally, grey literature was explored through trial registries such as ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP), along with reference checking. No restrictions were imposed on publication date or language. The complete search strategy is available in Appendix S1.
Inclusion criteria
To be eligible for inclusion in this systematic review, studies were required to meet the following criteria:
Study participants diagnosed with a chronic inflammatory skin condition.
Intervention via any form of telemedicine: synchronous (telephone consultations, video calls), asynchronous (e.g. mail-based communication, patient portals), and hybrid models. The telemedicine process must have involved the active participation of a qualified dermatologist.
Control via in-person care.
Study design was limited to randomized controlled trial (RCT), clinical controlled trial (CCT), or controlled before-and-after (CBA). Studies with case-control reports or non-comparative designs were excluded.
Outcomes of interest encompassed a range of factors, including patient and caretaker satisfaction, safety, disease activity scores (e.g. Psoriasis Area and Severity Index), and quality of life scores (e.g. Dermatology Life Quality Index). Additionally, secondary outcomes of interest included costs, cost-effectiveness, mobility (e.g. travel distance, carbon dioxide emission), and the duration and frequency of consultations. It is noteworthy that the measurement scales for these outcomes were not pre-specified.
Study selection and data extraction
After removing duplicates, 2 review authors (YW, SW) independently screened titles, abstracts, and subsequently full-text articles, applying the selection criteria. In the case of discrepancies, a third reviewer (WE) was consulted for resolution. If the full-text article was unavailable, 2 attempts were made to contact the authors and request the article.
Relevant information, including study characteristics (e.g. study design, sample size, follow-up duration, specific skin condition, type of remote healthcare, and in-person care), study population (e.g. age, sex), and study results (reported outcomes) were independently extracted from eligible studies by 2 authors (YW, SW) using a predefined template in Microsoft Excel (Microsoft Corp, Redmond, WA, USA). In instances where data were missing or unclear, we initiated contact with the original authors of the studies to request additional details. To maximize response rates, we followed up with up to 3 reminders, spaced at weekly intervals. Risk of bias was assessed independently by 2 authors (YW, SW) using the revised Cochrane risk of bias tool (14). If the 2 review authors disagreed, a third review author (WE) resolved the dispute.
Statistical analysis
We meticulously planned a meta-analysis to synthesize data from eligible studies, based on their clinical homogeneity in terms of participant and intervention characteristics. Also, we had planned to perform subgroup analyses to investigate variations in outcomes across different types of skin diseases, with a particular focus on examining the effects of various treatment types. However, due to heterogeneous data, we decided that it was not sound to actualize these plans.
For assessing the certainty of evidence, we used the Grading of Recommendations, Assessments, Development and Evaluations (GRADE) approach (15).
RESULTS
In our comprehensive review of the predefined databases, we extracted 1,351 records. After applying the inclusion criteria, we identified 11 reports that met the criteria, detailing findings from 6 unique studies (16–26). These 6 studies comprised 5 RCTs, detailed across 10 reports (16–25), and 1 CCT (26). The selection process is outlined in the PRISMA flow diagram (Fig. S1). For clarity, we present the results at the study level (n=6) rather than the report level (n = 11) and will refer to the study with the first published report.
Of the 6 included studies, 3 assessed telemedicine in a total of 394 patients with psoriasis, comprising both adults and children (18, 21, 26). Two studies were conducted in the United States and 1 in the Netherlands. The 3 other studies involved 428 patients with atopic dermatitis, also comprising both adults and children (16, 19, 22). These 3 studies were conducted in the United States, Norway, and The Netherlands. Detailed characteristics of the studies are summarized in Table I.
Table I.
Study population characteristics
| Study | Study design and setting | Total n of patients (age, years, mean; SD) | Treatment | Telemedicine | |
|---|---|---|---|---|---|
| Remote | In-person | ||||
| Skin condition: Psoriasis | |||||
| Armstrong, 2018 (USA) Armstrong, 2019 Ford, 2019 Young, 2019 |
RCT, outpatient | 148 (49; 14) | 148 (49; 14) | Topical (67.7%), light therapy (35.5%), non-biologic systemic therapy (38.5%), biologics (19.9%) | Asynchronously – store and forward: collaborative connected-health delivery model |
|
| |||||
| Chambers, 2012 (USA) Parsi, 2012 |
RCT, outpatient | 32 (51) | 32 (43) | Not reported | Asynchronously – store and forward: E-medicine platform (RelayHealth®) |
|
| |||||
| Oostveen, 2014 (Netherlands) | CCT, outpatient | 17 (10.2; 4.0) | 17 (11.4; 3.4) | Topical (dithranol combined with ascorbic acid, and Cremor Lanette I) | Synchronously – video calls |
| Skin condition: Atopic dermatitis | |||||
| Armstrong, 2015 (USA) Kornmehl, 2017 |
RCT, outpatient | 78 (27; 10) | 78 (28; 10) | Not reported (systemically treated patients were excluded) | Asynchronously – store and forward: direct-access online website for their dermatologic care |
|
| |||||
| Bergmo, 2009 (Norway) | RCT, outpatient | 50 (4.6; 95% CI 3.7–5.5) | 48 (5.3; 95% CI 4.3–6.3) | Not reported | Asynchronously – store and forward: software that enables a secure messaging system for users |
|
| |||||
| Van Os-Medendorp, 2012 (Netherlands) |
RCT, outpatient | 53 adults (31; 13) 38 parents of children (age children 2.9; 1.7) |
56 adults (32; 11) 45 parents of children (age children 2.7; 1.6) |
Topical | Asynchronously – store and forward: eczema portal including e-consultations with the dermatology nurse |
CCT: controlled clinical trial; CI: confidence interval; RCT: randomized controlled trial; SD: standard deviation.
Risk of bias
The risk of bias assessment revealed uncertainty in most domains (Fig. 1). Specifically, 4 studies (16, 18, 19, 21) raised some concerns regarding potential bias, and 1 study (22) was determined to have a high risk of bias.
Fig. 1.
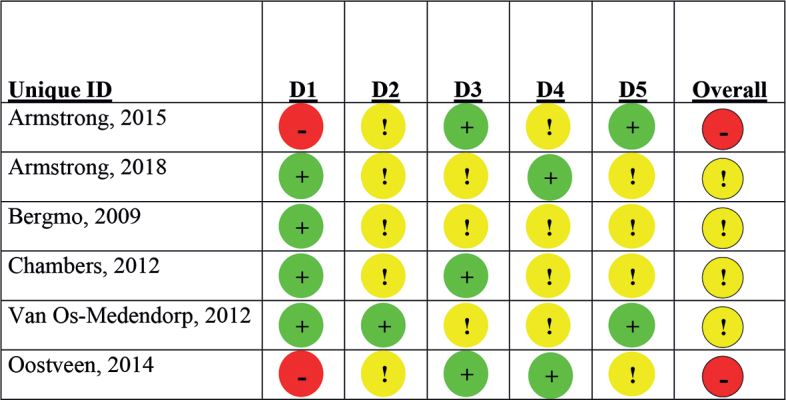
Risk of bias assessment with the revised Cochrane Risk of bias tool (13). Symbols: +: low risk; !: some concerns; -: high risk. D1: randomization process; D2: deviations from the intended interventions; D3: missing outcome data; D4: measurement of the outcome; D5: selection of the reported result.
Notably, 4 studies reported an adequate randomization process, while 1 study lacked information on the random allocation sequence (22) and 1 study had a non-randomized design (26). Blinding of patients and healthcare providers was not feasible in all studies due to the nature of the interventions. Three studies lacked blinding of outcome assessors (16, 22, 26), while others provided no information on this aspect. Moreover, 3 studies experienced significant loss-to-follow-up rates (>20%) (16, 19, 21).
Overall, these findings indicate that, while the included studies contribute valuable insights, there are some concerns regarding the potential for bias, randomization procedures, and blinding in certain cases.
Remote care
All included RCTs (16–25) employed online platforms for remote patient support, facilitating secure information exchange on disease severity, and enabling asynchronous communication with healthcare providers. Baseline in-person visits were conducted to assess disease severity and introduce patients to the online platforms. In contrast, the CCT utilized synchronous communication by replacing in-person visits with scheduled video calls (26).
Remote versus in-person healthcare for patients with psoriasis
Chambers et al. (18) followed patients for 24 weeks and organized a physical visit for both groups at baseline and after 24 weeks. At 8 and 16 weeks, patients in the intervention group shared information concerning their health status and were able to ask questions, while the control group had in-person consultations. Armstrong et al. (21) followed patients for a 12-month period. The intervention group were able to reach out to their dermatologist for questions through the platform, whereafter the dermatologist could make treatment recommendations, prescribe medications, and provide educational materials. The control group received in-person care. The frequency of consultations was determined by the dermatologist and the patient. Results were measured after 12 months. Oostveen et al. (26) assessed children and gave them or their parents the option to choose between regular in-person day care or day care with telemedicine, using Skype video calls. Initially, all patients attended the day care centre for 4 days per week, and then visits were reduced to twice per week. Starting from the second week, the telemedicine group replaced 1 visit per week with a scheduled video call, while patients treated themselves daily at home, with the option to call for extra instructions. Treatment ended upon achieving clearance or near-clearance results.
Data from RCTs demonstrated equivalence in safety, disease activity score (PASI), and quality of life scores (DLQI) for patients receiving telemedicine compared with in-person care (Table II). The exception was the Patient Global Assessment measured by Armstrong et al. (21) with a mean difference of –0.11 (95% CI, –0.32 to 0.10), which exceeded the equivalence margin, with the telemedicine group displaying greater improvement. The CCT found no significant differences in safety, disease activity, or quality of life (26).
Table II.
Outcomes for the comparison of remote care versus inpatient care in patients with psoriasis
| Study | Safety | Disease severity score (mean difference [95% CI]) | Quality of life scores (mean difference [95% CI]) | Patient and caretaker satisfaction | Costs and cost-efficacy | Mobility | Length and frequency of consultation |
|---|---|---|---|---|---|---|---|
| Armstrong, 2018 (20,21) Follow-up 12 months |
Rates of adverse events were similar between the online (42 [28.4%]) and in-person (51 [34.5%]) | PASI: –0.27 [95% CI −0.85 to 0.31] BSA: −0.05% [95% CI −1.58% to 1.48%] PtGA: −0.11 [95% CI −0.32 to 0.10]* |
DLQI: −0.45 [95% CI −1.29 to 0.38] Skindex-16: −0.83 [95% CI − 5.18 to 3.51] |
Not quantitatively investigated Patients found CCH to be safe, accessible, equitable, efficient, effective, and patient-centred Providers found CCH to be useful for providing psoriasis care |
Not investigated | Mean ± SD travel distance: In person care: 174.8 ± 577.4 km/person Online: 327 km (2.2 ± 14.2 km/person Mean ± SD transportation and waiting time: In person care 4.0 ± 4.5 h/person) Online: 0.1h ± 0.4) h/person) p = 0.0001 |
Not investigated |
| Chambers, 2012 (17,18) Follow-up 24 weeks |
Not investigated | PASI: 0.1 [95% CI −2.2 to 2.30] IGA: no significant differences (p = 0.74; p = 0.8; p = 0.16). |
DLQI: 1.1 [95% CI −4.1 to 2.0] | Not investigated | CEA: incremental cost-effectiveness ratio US$16,318.75 per QALY saved by the in-office group over the online group | Not investigated | Frequency of online visits is 1.7 times lower than in-person visits |
| Oostveen, 2014 (26) | Number of irritation events during treatment: –0.2 [95% CI –2.0 to 1.6] |
PASI between the groups did not significantly differ (–67.2% for regular day care vs –71.3% for telemedicine, p = 0.62) | CDLQI: –2.0 [95% CI −5.15 to 1.5] | Not investigated | Not investigated | Not investigated | Not investigated |
BSA: Body Surface Area; CCH: Chronic Cutaneous Hyperkeratosis; CEA: Cost-Effectiveness Analysis; CI: confidence interval; (C)DLQI: (Children’s) Dermatology Life Quality Index; IGA: Investigator Global Assessment; PASI: Psoriasis Area and Severity Index; PtGA: Patient Global Assessment; QALY: Quality-Adjusted Life Year; SD: standard deviation.
Quantitative data regarding the satisfaction of patient and caretaker were not available. However, Armstrong et al. reported that patients expressed positive views regarding telemedicine, emphasizing its safety, accessibility, equity, efficiency, effectiveness, and patient-centred nature. Caretakers also found telemedicine to be valuable in facilitating psoriasis care (21).
Chambers et al. (17, 18) found an economic advantage for telemedicine, with follow-up costs 1.7 times lower than in-person care (US$315 vs US $576) within 24 weeks. The cost-effectiveness ratio was US $704.7/QALY for telemedicine versus US $1244.28/QALY for in-person care. Over a 12-month period, Armstrong et al. (21) reported statistically significant reductions in travel distance (total reduction of 25,544 km travelled for the online group) and transportation and waiting time (–4 hours per patient) in the online group.
The GRADE criteria were employed for the assessment of all outcomes. Disease severity, quality of life, and mobility attained a rating of “low.” Meanwhile, other outcomes were categorized as “very low” (Table SI).
Remote versus in-person healthcare for patients with atopic dermatitis
Bergmo et al. (19) assessed children with atopic dermatitis, while the 2 other studies focused on adults (16, 22). At baseline, all patients or parents received training on how to use the platform and how to take and generate high-quality pictures. After that, patients were randomized to telemedicine or care as usual. Van Os-Medendorp et al. (16) scheduled a second in-person consultation after 6 weeks of follow-up to discuss any concerns for both groups. After that, no more in-person consultations took place in the intervention groups, while the control groups received the usual in-person consultations. Two studies (16, 22) proactively scheduled appointments for the usual care group with their dermatologist, while 1 study (19) allowed patients to arrange healthcare themselves from any healthcare provider. Outcomes were measured after 12 months.
All 3 studies (16, 19, 22) demonstrated equivalence or no significant difference in disease activity score or quality of life between telemedicine compared with in-person care (Table III). Differences in reported outcome measures between studies made a meta-analysis impossible.
Table III.
Outcomes for the comparison of remote care versus inpatient care in patients with atopic dermatitis
| Study | Safety | Disease severity score (mean difference [95% CI]) | Quality of life scores | Patient and caretaker satisfaction | Costs and cost-efficiency | Mobility | Length and frequency of consultation |
|---|---|---|---|---|---|---|---|
| Armstrong, 2015 (22, 23, 25) Follow-up 12 months |
Not reported | POEM: 0.24 (SD 6.59) [90% CI −1.70 to 1.23] IGA: 5.1% [90% CI 1.7%–8.6%] |
CDLQI: 0.23 [90% CI, –2.21 to 2.67], DLQI: 0.72 [90% CI, –0.97 to 2.41] SF-12 PCS: 0.34 [90% CI, –1.16 to 1.84] SF-12 MCS: 0.51 [90% CI, –1.11 to 2.13] |
Not investigated | Not investigated | Not investigated | Not investigated |
|
| |||||||
| Bergmo, 2009 (19) Follow-up 12 months |
Not reported | SCORAD: no interaction between the groups (p = 0.55) | Not investigated | Not investigated | Not investigated | Not investigated | Not investigated |
|
| |||||||
| Van Os-Medendorp, 2012 Follow-up 12 months (16) |
Not reported | P for interaction of disease severity (p = 0.04; however, at each time point no significant differences), Extent and severity of AD (p = 1.00) |
DLQI (p = 0.45) | Not investigated | Direct costs: €24 [95% CI –360 to 383], Indirect costs: –€618 [95% CI –2502 to 1,143] Total costs –€594 [95% CI –2545 to 1,227] per patient |
Not investigated | Not investigated |
AD: atopic dermatitis; (C)DLQI: (Children’s) Dermatology Life Quality Index; CI: confidence interval; IGA: Investigator Global Assessment; POEM: Patient-Oriented Eczema Measure; SCORAD: Severity Scoring of Atopic Dermatitis; SD: standard deviation; SF-12 PCS/MCS: Short Form Physical Component Score/Mental Component Score; VAS: visual analogue scale.
One study measured costs (16). Direct costs per year were found to be €24 higher (95% CI –360 to 383) in the telemedicine group while the indirect costs, reduced productivity during paid work and unpaid labour, were found to be €618 lower (95% CI –2,502 to 1,143) per patient, resulting in a non-significant saving of €594 (95% CI –2,545 to 1,227) per patient per year in the telemedicine compared with the usual care group. No results were found on the outcomes of safety, patient and caretaker satisfaction, mobility, or length or frequency of consultation.
In the context of GRADE assessment, the evaluation of disease severity and quality of life outcomes yielded a classification of “low certainty”, while the appraisal of cost-effectiveness revealed a classification of “very low certainty” (Table SII). Notably, the included studies did not encompass measurement of other outcomes.
DISCUSSION
This systematic review offers valuable insights into the efficacy of remote telemedicine for managing chronic inflammatory skin diseases, particularly focusing on psoriasis and atopic dermatitis. Our findings suggest that telemedicine is equivalent or not significantly different from traditional in-person care in terms of safety, disease activity, and quality of life outcomes. Moreover, remote healthcare offers several advantages, including potential cost savings, plus reduced travel distance and time for patients, making it an attractive option for both patients and healthcare providers. However, it is important to note that these results are based on evidence of low to very low certainty.
The growing interest in digital healthcare solutions worldwide (7) suggests that telemedicine is likely to be utilized increasingly (27). However, for wider implementation, it is crucial to address several knowledge gaps identified through our review.
First, the perspectives of patients and healthcare providers on telemedicine have not been specifically addressed in the included studies, likely due to our primary focus on quantitative studies such as RCTs and CCTs. Qualitative studies, like those by Armstrong et al. (21), suggest that patients generally perceive remote care as safe, accessible, and effective. However, the impact of remote healthcare on patient–nurse relationships and the professional dynamics between nurses and doctors, as highlighted by Mossfeldt Nickelsen (28), indicates that a comprehensive understanding of the factors influencing patient and care providers’ preferences and acceptance of remote care modalities is essential. The successful integration of remote care into clinical practice necessitates the active involvement and consideration of both patients’ and healthcare providers’ viewpoints.
Second, the direct application of traditional clinical assessment tools, such as the Psoriasis Area and Severity Index (PASI) in telemedicine, without validation, poses significant challenges. Our review reveals that in-office assessments were predominantly conducted, highlighting the limitations of exclusively using remote care for skin condition monitoring. Therefore, development and validation of specific assessment tools for remote healthcare are imperative to ensure their suitability and reliability in digital contexts.
Another significant knowledge gap identified in this review is the scarcity of data on remote care for patients with chronic inflammatory skin conditions other than psoriasis and atopic dermatitis. Investigating the feasibility and effectiveness of remote care for conditions like acne and hidradenitis suppurativa could provide valuable insights, given that these conditions also impose a substantial burden on patients.
Furthermore, evaluating the effect of telemedicine modalities on different patient demographics is crucial, taking into account individual health conditions, socioeconomic backgrounds, and access to technology. Significantly, research by Vittrup et al. (29) illuminates the challenges faced by patients with atopic dermatitis and hidradenitis suppurativa, who may struggle with telemedicine services that rely heavily on written communication due to comparatively lower academic achievements. This finding underscores the necessity for telehealth platforms to integrate a variety of communication methods, thereby ensuring equitable access to dermatological care and mitigating any existing disparities in healthcare provision.
Additionally, the included studies demonstrated equivalence or no significant differences in various outcomes, yet the certainty of the evidence was generally low or very low due to potential risk of bias, clinical heterogeneity, and limited sample sizes. The varying study designs, outcome measures, and participant characteristics prevented conducting a meta-analysis, underscoring the need for well-designed, large-scale, and homogeneous studies in the future. Furthermore, the limited data on adverse events in our reviewed studies points to a notable gap in our understanding of the safety of telemedicine for these conditions.
The economic evaluations included in our review indicate potential cost savings with remote care, though these savings are significantly influenced by factors such as healthcare providers’ reimbursement mechanisms and the initial setup costs of remote systems. Long-term, comprehensive studies are essential to fully understand the cost-effectiveness of remote care across different healthcare systems.
Recent research (30) highlights the difficulty in choosing appropriate measures for evaluating the cost-effectiveness of Digital Health Interventions (DHIs). Traditional metrics might not adequately reflect DHIs’ full impact, particularly in reducing appointments. It is also crucial to consider the equitable distribution of costs and benefits among DHI users. Extended analyses are suggested to assess the equity impacts and influence of health and social determinants, offering a more detailed evaluation of DHIs’ value, especially for those in remote areas.
Lastly, determining the best-suited context and phase of care for remote healthcare implementation remains a crucial challenge. This review mainly included studies examining care around the period of diagnosis; therefore, the applicability and effectiveness of remote care in the monitoring phase for patients with chronic skin diseases are yet to be fully explored. Research specifically targeting this phase of care could provide valuable insights into the potential benefits and limitations of remote care in long-term disease management.
Studying the long-term effects of telemedicine is essential to comprehensively assess its impact on patients and healthcare systems. While current evidence indicates positive outcomes and potential benefits in the short term, understanding the sustainability and durability of these effects is crucial for informed decision-making and policy development. Long-term studies can shed light on whether the benefits observed in the early stages of telemedicine persist over time and whether any unforeseen challenges or limitations arise with prolonged use. Investigating the long-term patient satisfaction, disease management, and cost-effectiveness of telemedicine will provide valuable insights into its role as a sustainable and viable solution for delivering dermatological care. Additionally, exploring the potential impact of telemedicine on patient–provider relationships and adherence to treatment plans in the long run will be vital in ensuring the successful integration of this technology into routine clinical practice. Overall, a comprehensive evaluation of the long-term effects of telemedicine will provide a more complete picture of its potential benefits and limitations, supporting evidence-based decisions for its widespread adoption and implementation. In conclusion, telemedicine shows promise as an effective alternative for patients with chronic inflammatory skin diseases like psoriasis and atopic dermatitis, offering outcomes comparable to traditional care regarding safety, disease activity, and quality of life. It also presents potential cost savings and increased accessibility, making it attractive for both patients and healthcare providers. However, further investigation is essential to address existing knowledge gaps, including qualitative analyses of patient and provider perspectives, identifying and validating outcome tools for remote outcome assessment, conducting large-scale and well-designed studies and economic evaluations, and identifying the best-suited context for remote care implementation. By bridging these gaps, we can fully unlock the benefits of telemedicine and successfully integrate it into clinical practice for chronic skin conditions.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to express their sincere gratitude to the individuals whose invaluable contributions were essential in bringing this article to fruition. Tenzin Nlgisang, MD, played a pivotal role by meticulously reviewing the study selection and providing invaluable assistance in extracting data from an additional study.
The authors’ appreciation is extended to the following individuals for their significant contributions to the manuscript: Prof. Marjolein de Bruin-Weller, MD; and Dr Shiarra Stewart, MD and Ilse van Ee (Dutch Psoriasis Patient Federation). Their insights and expertise greatly enriched the content.
Special thanks are due to Prof. dr. Leonard Wittkamp for his contributions to the design of this study.
Funding Statement
Funding sources This research was funded by ZonMw (project number 80-83900-98-20013) and started on 3-1-2022. ZonMw did not influence this research in any way.
Conflict of interest statement
The research was conducted with the utmost objectivity and impartiality, without any external influences or affiliations that could potentially bias the results or interpretation of the findings.
All authors declare no conflicts of interest.
REFERENCES
- 1.Trettel A, Eissing L, Augustin M. Telemedicine in dermatology: findings and experiences worldwide – a systematic literature review. J Eur Acad Dermatol Venereol 2018; 32: 215–224. [DOI] [PubMed] [Google Scholar]
- 2.Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol 2018; 19: 253–260. [DOI] [PubMed] [Google Scholar]
- 3.Elsner P. Teledermatology in the times of COVID-19: a systematic review. J Dtsch Dermatol Ges 2020; 18: 841–845. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA 2020; 323: 1945–1960. [DOI] [PubMed] [Google Scholar]
- 5.Ständer S. Atopic dermatitis. N Engl J Med 2021; 384: 1136–1143. [DOI] [PubMed] [Google Scholar]
- 6.Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, et al. Joint AAD–NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol 2019; 80: 1029–1072. [DOI] [PubMed] [Google Scholar]
- 7.Marasca C, Annunziata MC, Camela E, Di Guida A, Fornaro L, Megna M, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med 2022; 11: 1511.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snoswell C, Finnane A, Janda M, Soyer HP, Whitty JA. Cost-effectiveness of store-and-forward teledermatology: a systematic review. JAMA Dermatol 2016; 152: 702–708. [DOI] [PubMed] [Google Scholar]
- 9.López-Liria R, Valverde-Martínez MÁ, López-Villegas A, Bautista-Mesa RJ, Vega-Ramírez FA, Peiró S, et al. Teledermatology versus face-to-face dermatology: an analysis of cost-effectiveness from eight studies from Europe and the United States. Int J Environ Res Public Health 2022; 19: 2534.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018; 32: 657–682. [DOI] [PubMed] [Google Scholar]
- 11.Nast A, Spuls PI, van der Kraaij G, Gisondi P, Paul C, Ormerod AD, et al. European S3-Guideline on the systemic treatment of psoriasis vulgaris – Update Apremilast and Secukinumab – EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol 2017; 31: 1951–1963. [DOI] [PubMed] [Google Scholar]
- 12.El Komy MHM, Chiricozzi A, van de Kerkhof P, Armstrong A, Diamei V, Hsu C, et al. Telemedicine and psoriasis: a review based on statements of the telemedicine working group of the International Psoriasis Council. JEADV Clin Practice 2023; 2: 19–31. [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: 4898. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Os-Medendorp H, Koffijberg H, Eland-De Kok PCM, Van Der Zalm A, De Bruin-Weller MS, Pasmans SGMA, et al. E-health in caring for patients with atopic dermatitis: a randomized controlled cost-effectiveness study of internet-guided monitoring and online self-management training. Br J Dermatol 2012; 166: 1060–1068. [DOI] [PubMed] [Google Scholar]
- 17.Parsi K, Chambers CJ, Armstrong AW. Cost-effectiveness analysis of a patient-centered care model for management of psoriasis. J Am Acad Dermatol 2012; 66: 563–570. [DOI] [PubMed] [Google Scholar]
- 18.Chambers CJ, Parsi KK, Schupp C, Armstrong AW. Patient-centered online management of psoriasis: a randomized controlled equivalency trial. J Am Acad Dermatol 2012; 66: 948–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergmo TS, Wangberg SC, Schopf TR, Solvoll T. Web-based consultations for parents of children with atopic dermatitis: results of a randomized controlled trial. Acta Paediatr 2009; 98: 316–320. [DOI] [PubMed] [Google Scholar]
- 20.Ford AR, Gibbons CM, Torres J, Kornmehl HA, Singh S, Young PM, et al. Access to dermatological care with an innovative online model for psoriasis management: results from a randomized controlled trial. Telemed J E Health 2019; 25: 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong AW, Chambers CJ, Maverakis E, Cheng MY, Dunnick CA, Chren MM, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open 2018; 1: e183062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong AW, Johnson MA, Lin S, Maverakis E, Fazel N, Liu FT. Patient-centered, direct-access online care for management of atopic dermatitis: a randomized clinical trial. JAMA Dermatol 2015; 151: 154–160. [DOI] [PubMed] [Google Scholar]
- 23.Kornmehl H, Singh S, Johnson MA, Armstrong AW. Direct-access online care for the management of atopic dermatitis: a randomized clinical trial examining patient quality of life. Telemed J E Health 2017; 23: 726–732. [DOI] [PubMed] [Google Scholar]
- 24.Young PM, Chen AY, Ford AR, Cheng MY, Lane CJ, Armstrong AW. Effects of online care on functional and psychological outcomes in patients with psoriasis: a randomized controlled trial. J Am Acad Dermatol 2023; 88: 364–370. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong AW, Ford AR, Chambers CJ, Maverakis E, Dunnick CA, Chren MM, et al. Online Care Versus In-Person Care for Improving Quality of Life in Psoriasis: A Randomized Controlled Equivalency Trial. J Invest Dermatol 2019; 139: 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oostveen AM, Beulens CA, Van De Kerkhof PCM, De Jong EMGJ, Seyger MMB. The effectiveness and safety of short-contact dithranol therapy in paediatric psoriasis: a prospective comparison of regular day care and day care with telemedicine. Br J Dermatol 2014; 170: 454–457. [DOI] [PubMed] [Google Scholar]
- 27.Kohn LL, Pickett K, Day JA, Torres-Zegarra C, Plost G, Gurnee E, et al. When is synchronous telehealth acceptable for pediatric dermatology? Pediatr Dermatol 2022; 39: 236–242. [DOI] [PubMed] [Google Scholar]
- 28.Mossfeldt Nickelsen NC. The infrastructure of telecare: implications for nursing tasks and the nurse–doctor relationship. Sociol Health Illn 2019; 41: 67–80. [DOI] [PubMed] [Google Scholar]
- 29.Vittrup I, Andersen YMF, Skov L, Wu JJ, Agner T, Thomsen SF, et al. The association between atopic dermatitis, cognitive function and school performance in children and young adults. Br J Dermatol 2023; 188: 341-349 [DOI] [PubMed] [Google Scholar]
- 30.Benedetto V, Filipe L, Harris C, Spencer J, Hickson C, Clegg A. Analytical frameworks and outcome measures in economic evaluations of digital health interventions: a methodological systematic review. Med Decis Making 2023; 43: 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


