Abstract
Pain is a significant health issue, and pain assessment is essential for proper diagnosis, follow-up, and effective management of pain. The conventional methods of pain assessment often suffer from subjectivity and variability. The main issue is to understand better how people experience pain. In recent years, artificial intelligence (AI) has been playing a growing role in improving clinical diagnosis and decision-making. The application of AI offers promising opportunities to improve the accuracy and efficiency of pain assessment. This review article provides an overview of the current state of AI in pain assessment and explores its potential for improving accuracy, efficiency, and personalized care. By examining the existing literature, research gaps, and future directions, this article aims to guide further advancements in the field of pain management. An online database search was conducted via multiple websites to identify the relevant articles. The inclusion criteria were English articles published between January 2014 and January 2024). Articles that were available as full text clinical trials, observational studies, review articles, systemic reviews, and meta-analyses were included in this review. The exclusion criteria were articles that were not in the English language, not available as free full text, those involving pediatric patients, case reports, and editorials. A total of (47) articles were included in this review. In conclusion, the application of AI in pain management could present promising solutions for pain assessment. AI can potentially increase the accuracy, precision, and efficiency of objective pain assessment.
Keywords: Pain assessment, Artificial intelligence (AI), AI in pain assessments, Machine learning (ML), Natural language processing (NLP), Computer vision (CV), Wearable devices and sensors, Virtual reality (VR)
Key Summary Points
| Why carry out this study? |
| To highlight the current status of the pain assessments. |
| To identify the practical tips for improving the pain assessment. |
| To identify the different applications of artificial intelligence (AI) technology for augmenting pain assessment. |
| To summarize the challenges of using the AI/machine learning (ML) technology in automated pain assessments. |
| To identify the future potential for fully automated pain management by integrating AI technology. |
| What was learned from the study? |
| Pain is a complex and multidimensional symptom that requires multimodal approaches for accurate assessments. |
| Various AI techniques such as ML, natural language processing (NLP), computer vision (CV), and wearable devices can be utilized to assess pain. |
| Evidence is promising regarding incorporating AI tools for objective, personalized, and accurate assessment of pain. |
| There are some limitations to consider in integrating AI in pain assessment, such as data quality and ethical considerations. |
| More collaborations between AI experts and healthcare professionals are required to enhance the integration of AI in pain management and improve the patient’s outcome. |
Introduction
Pain is an important health issue. It is defined by the International Association for the Study of Pain as “an unpleasant sensory and/or emotional experience associated with, or resembling that associated with, actual or potential tissue damage” [1]. It is a complex and multifactorial symptom. Patients may differ greatly, even with the same diagnosis [2]. Pain is a subjective experience that can be challenging to quantify, especially in people who are not able to report their pain experience or whose expression of pain is hard to interpret [3]. Assessment of pain is an important step for early diagnosis, monitoring disease progression, selecting a treatment plan, and validating the treatment effects [4]. Traditional methods for pain assessment rely mainly on patient self-report scores, which can be influenced by various factors such as cultural, psychological, and social biases. Moreover, the self-report scores are unfeasible for patients who cannot communicate or verbalize their pain experience properly [5]. AI-based approaches have emerged to improve pain assessment by providing automated objective, personalized, and standardized measures [6]. Artificial intelligence (AI) is the term used to describe the use of computers and technology to simulate intelligent behavior and critical thinking comparable to a human being [7]. AI can use data to perform intelligent tasks that humans usually undertake to mimic human cognitive functions, whether self-reports, behavioral scales, physiological markers, or medical records, to develop algorithms to better understand and assess pain and potentially predict treatment outcomes [8]. AI has several applications in the management. Various AI techniques such as machine learning, natural language processing, computer vision, and wearable devices and sensors can be utilized for the assessment, monitoring, and treatment of pain and for predicting the outcomes. They can be used to estimate the intensity of pain, classify different types of pain, continuously monitor pain, and predict treatment responses. The use of AI methods for pain assessment has gained significant attention due to its accuracy and efficiency in pain evaluation [9, 10]. This review highlights the current status of the use of AI in pain assessment, including different techniques, advantages, limitations, and challenges, and provides data for future directions.
Methodology
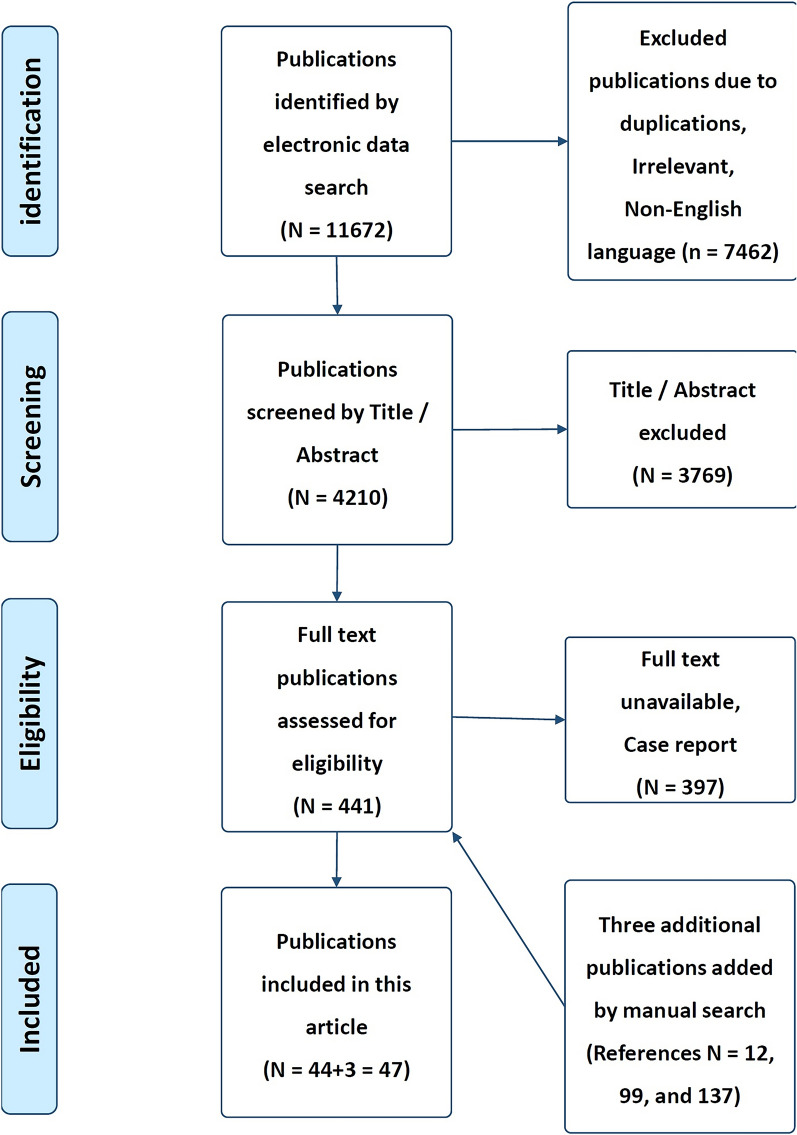
A computer search was conducted including literature from PubMed, EMBASE, MEDLINE, Web of Science, Cochrane, and Google Scholar. Manual screening of references from relevant sites was also conducted, and additional references were added. The search strategy included articles that were published during the last 10 years (from January 2014 to January 2024). The search strategy included the following keywords: pain assessment, artificial intelligence, AI in pain assessments, machine learning, natural language processing, computer vision, virtual reality, and wearable devices and sensors. Articles that met the inclusion criteria, such as articles published in the English language, relevant to the condition, presented information on the use of AI applications in pain assessments, and involving adult patients, were included. The search strategy included observational, cross-sectional, cohort, case–control, longitudinal studies, systematic reviews, and meta-analyses. The exclusion criteria included non-English language articles, inability to get the full articles, case reports, editorials, conference abstracts or expert opinions, and studies that did not test an AI-based intervention. Search strategy results in (11,672) publications. A total number of (11,672) articles were excluded due to different reasons (e.g., duplicated articles, non-English language, irrelevant, case report or full text unavailable). Final screening results in (44) publications were identified. An additional three publications were added by manual search through international societies (e.g., references no [12, 99, 137]). The final reviewing strategy of the literature search results in a total of (47) articles were included in this review (Fig. 1) [11]. The selected articles were screened by two independent reviewers using the same method of evaluation. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Fig. 1.
PRISMA flowchart of included studies [10]
Incorporation of Artificial Intelligence for Objective Pain assessment
Introducing Artificial Intelligence in Healthcare
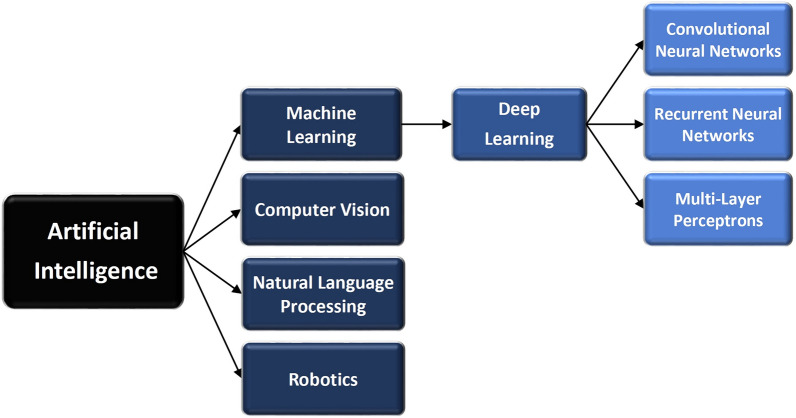
Artificial intelligence is defined as “the simulation of human intelligence processes by machines, especially computer systems. These processes include learning (the acquisition of information and rules for using the information), reasoning (using rules to reach approximate or definite conclusions), and self-correction.” [12]. AI is a growing branch of computer engineering that implements novel concepts and solutions to resolve complex challenges [13]. The opportunities for AI applications in healthcare from the emergency department, outpatient clinics or primary care, to home care, are huge [14]. The spectrum of AI includes but is not limited to, machine learning, deep learning, data mining, and natural language processing [15]. The medical field may benefit from two main categories of AI applications: physical and virtual. The physical subfield of AI in medicine involves medical equipment and care bots (intelligent robots), which assist in delivering medical care, while the virtual subfield includes machine learning (ML), natural language processing (NLP), and deep learning (DL) [16–18]. In general, machine learning is a subset of AI, while deep learning is a subset of machine learning (Fig. 2) [19, 120].
Fig. 2.
Categories of artificial intelligence [12]
Artificial intelligence (AI) is a broad field that involves machine learning, deep learning, computer vision, natural language processing, and robotics [12]. Its main goal is to develop smart tools that can carry out cognitive functions such as problem-solving, sentiment analysis, and decision-making. Machine learning (ML) algorithms enable systems to learn and improve from experience without being explicitly programmed. It is a subset of AI and delivers predictions and decisions by identifying patterns in its training data. Deep learning (DL) is a subset of machine learning that uses artificial neural networks to model and understand complex patterns and relationships in data. With this model, an algorithm can determine whether a prediction is accurate through a neural network without human intervention. Deep learning models can build extensive knowledge over time, acting as a brain of sorts [12, 19, 20]. Computer vision (CV) is AI systems that can analyze and understand visual data, such as images and videos. Natural language processing (NLP) refers to AI systems that can understand and generate human language, enabling tasks like speech recognition and language translation. Robotics refers to AI systems that can control and interact with physical robots to perform tasks in the physical world [12].
The last few decades have shown a rapidly growing role of AI in healthcare. The implementation of AI in healthcare can automate patient assessment and eliminate human bias. AI will benefit and improve clinical diagnoses, deliver rapid results, offer continuous monitoring of the patients, facilitate decision-making and help select the treatment plan [21].
Augmented Intelligence as opposed to Artificial Intelligence is being applied to augment human cognition but does not replace it. Augmented intelligence is designed to enhance human capabilities by the effective use of information from the huge data sets to augment and support human cognition [22]. Augmented intelligence can be used in different aspects of medicine and guides the physician in early disease recognition, accurate diagnosis, and decision-making for complex diseases [23]. Both AI and augmented intelligence are likely to play an important role in the future. AI is capable of performing tasks that require human intelligence to achieve and replace humans. While augmented intelligence reflects the enhanced capabilities of human clinical decision-making by combining human intelligence and machine-derived outputs to improve health [24].
AI Techniques in Pain Assessment
Various AI interventions have been utilized in recognizing, assessing, understanding, and treating pain, each with its own effectiveness and applicability [15]. It is important to note that the choice of a model will depend on the specific task and dataset and that different models may perform better for different tasks [10, 25]. Several studies have found promising findings of AI-based pain detection through facial expressions [25]. ML and AI can use data, whether self-report measures, physiological markers, or medical records, to create algorithms to better understand pain, assess changes to pain, and potentially predict treatment outcomes [27, 28]. These technologies can help improve the reliability of pain assessment tools. For example, ML algorithms can be used to assess pain by establishing a baseline and then looking at the patient’s changing facial expressions and analyzing and assessing their vocal sounds [5, 10]. Following are some examples of different AI techniques used for pain assessment and how they are beneficial in improving patient outcomes (see Fig. 2).
Machine Learning (ML) Algorithms
ML is defined as the discovery and testing of algorithms that assist pattern recognition, classification, and prediction based on models built from existing data [29]. ML technology has the capability of teaching computers to recognize patterns and images by supplying them with data and an algorithm [30]. ML algorithms, such as support vector machines, random forests, and deep learning models, have been applied to pain assessment tasks. ML techniques are highly beneficial in accurate pain classification, prediction, estimating pain intensity, and identifying personalized treatment in various conditions, like chronic low back pain or neuropathic pain [31]. They have shown promising results in different pain conditions and can handle complex and nonlinear relationships within the data. They can analyze various data sources, such as physiological signals, self-reported scores, imaging data and estimate pain intensity using EEG signals. ML applications can be used in smart units, digital tools, smart watches, smart documentation, and electronic medical records [32].
Several studies developed novel models for pain recognition with ML by analyzing facial expressions. They were able to automatically detect pain successfully with relatively high accuracy in more than 95% of the subjects [33–36]. Other studies used the AI-based approach to analyze clinical notes and patients’ records with pain assessment information to identify components related to pain classifications and severity [37]. Results of a recent review provide evidence that machine learning, data mining, and natural language processing can improve efficient pain recognition and pain assessment, analyze self-reported pain data, predict pain, and help clinicians and patients to manage chronic pain more effectively [33–36]. These promising results may help in the creation of new pain assessment instruments with human language technology.
Another application of AI/ML is for patients with severe dementia and those who cannot verbalize or communicate; a novel method of pain assessment that combines a number of technologies, including automated facial recognition and analysis, smart computing, affective computing, and cloud computing could be developed. This new system could help pain assessment in these challenging cases [38, 39].
Deep Learning and Neural Networks
Deep learning is a branch within the area of ML, AI, as well as data science and analytics, due to its learning capabilities from the given data. Deep learning models have been used to recognize and classify pain-related facial expressions [39]. Deep learning techniques, including convolutional neural networks (CNNs), recurrent neural networks (RNNs), and multilayer perceptron (MLP), are the three primary types of deep networks that are extensively used in many different applications, including complex pain-related data [19]. Medical images such as X-rays or MRI scans can be processed to extract meaningful features and patterns, shaping the diagnosis and treatment plan. Electroencephalogram (EEG) readings can also be analyzed to assess pain levels objectively [41]. DL can also be used to analyze itself and, in that way, overcome unexpected challenges and improve overall outcomes [40].
Natural Language Processing (NLP)
NLP denotes the field of study that emphasizes the interactions between human language and computers [42, 43]. NLP represents an evolution in the language analysis for automated pain assessment of patient-reported outcomes, enhancing the efficiency of pain assessment. NLP has proven effective in analyzing text-based pain assessments, such as pain descriptions from patient reports, pain diaries, or electronic health records [10]. NLP techniques include language feature extraction, classification, and prediction. NLP depends on the interaction between computer science and human language, combining AI and linguistics [10]. NLP has several practical applications in medicine. For example, it can enable computerized clinical decision-support systems, improve healthcare management, and can also be used for building tele-triage services and other aims [44]. NLP can be used to analyze data from social media and identify pain-related experiences. NLP can analyze the language used to describe pain, assist in pain assessment and patient triage, and identify pain-related trends or patterns, with the goal of understanding, extracting, and retrieving data from unstructured written and spoken texts, such as patient descriptions of their pain experiences. NLP has been able to extract information on pain intensity, pain location, and duration from social media data (e.g., pain intensity, location, and duration) [45, 46].
Computer Vision
Computer vision is the field of AI that deals with how computers can gain a high-level of understanding of the patient from digital images or videos. The main applications of computer vision are feature extraction and image segmentation. The main goal of feature extraction is to retrieve a restricted number of relevant features from an image in order to facilitate subsequent tasks, such as classification or regression. On the other hand, image segmentation is labeling each pixel of an image with a corresponding class to detect the relevant elements of an image [47].
Computer vision techniques have demonstrated effectiveness in pain assessment by analyzing visual signs or signals like facial expressions, body postures, or movement patterns. Facial expression analysis, for example, can use deep learning models and computer vision to detect and classify facial signs and behavior related to pain, such as grimacing or frowning [48, 49]. CV/ML pain assessment models derived from automated facial expression measurements performed well in detecting clinically significant pain and estimating pain severity at rest and during movement in the postoperative setting in youth. The automated facial expression measurements positively correlated with the patient’s self-reported pain scores [50].
A study conducted at the University of California San Diego examined computer vision-based deep learning models to predict pain measurements using facial images in 77 patients. The computer focused on facial expressions, particularly the eyebrows, lips, and nose. The study found that the Visual Analogue Scale (VAS) is less accurate than the Critical Care Pain Observation Tool (CPOT). This is attributed to two important factors: first, VAS is a subjective measurement that can be more influenced by emotional and other factors than CPOT; second, there is a gap in the continuous observation methods for pain during the perioperative period. The AI model could help improve patient care through real-time continuous and unbiased pain detection [50, 51].
Moreover, computer vision techniques can be applied to automatically identify pain-related facial expressions in specific populations, such as patients with dementia, non-verbal expressions, or impaired communication. Computer vision algorithms have also been utilized in pain assessment during movement analysis based on movement patterns of the patients [5, 48].
Robotics
AI systems can control and interact with physical robots to perform tasks in the physical world. A robot is a type of programmable machine that is designed to perform specific tasks, interact with the surroundings, and perform duties autonomously. Robots perform the tasks with little or no human intervention and with speed and precision. In healthcare, robotics can enhance patient care, quality assurance, and assist medical professionals. Robotic surgery can assist surgeons during complex procedures, with less invasive techniques, therefore reducing the risk of complications and improving outcomes with enhanced precision and accuracy [52].
Robotic technology can also be used in the field of pain management. Most devices and different techniques were used to differentiate perceived pain or anxiety for the treatment of procedural pain. Overall results showed beneficial effects for using robotic technologies to manage pain for both acute and chronic pain conditions [53]. A recent study used social assistive robots for the assessment and management of pain in residents with dementia, their families and caregivers as well. All received five sessions/week for 3 weeks. Results are promising for using robotic technology with some limitations related mainly to staff training [54].
AI-Based Pain Assessment Tools
AI-based pain assessment tools utilize various high-tech tools to improve the efficiency, accuracy, and objectivity of pain assessment. These tools can assist healthcare professionals in properly assessing, diagnosing, monitoring, and managing pain more effectively. The following represent a few examples of the AI-based tools for pain assessments.
Wearable Devices and Mobile Applications
The World Health Organization defines mobile health technologies as medical and public health practices supported by mobile devices [55]. Smart wearable devices or sensors are AI tools that can enable continuous monitoring of human physiological activities, vital signs, and body movements without any disturbance to the activities of daily living [57]. Wearable-based devices equipped with AI technology offer the potential for real-time continuous pain monitoring and personalized interventions [57]. Wearable devices have the potential to continuously collect a huge amount of data from patients to help in the follow-up and management of many chronic conditions, such as diabetes, heart conditions, and chronic pain [58]. These tools collect data on pain levels, physical activity, sleep patterns, and medication adherence. Wearable devices include, for example: wristbands, smart watches, wearable mobile sensors, and smart wearable shirts [5]. Recent literature reviews on the clinical impact of wearable devices and behavior change have shown promising effectiveness for digital technology [59].
Using AI, wearable smart devices can capture and analyze digital biomarkers of pain. “Digital bio-markers” is a term recently defined as the “objective, quantifiable, physiological, and behavioral measures collected using digital devices that are portable, wearable, implantable, or digestible” [60]. Digital devices can capture vital signs such as heart rate, respiration, blood volume pulse, and skin temperature; physiological data such as skin conductance, brain activity, muscle activity, and pattern of body movements; and behavioral signals such as speech, facial expressions, and body movements [4, 5]. Numerous biosignal methods have been used to assess pain: electrocardiography (ECG), electromyography (EMG), electrodermal activity (EDA), photoplethysmography (PPG), blood oxygen saturation (SpO2), and near-infrared spectroscopy (NIRS) [61]. Wearable-based AI systems have also been explored for pain assessment in non-verbal populations, such as infants undergoing vaccinations or individuals with communication impairments and dementia [5, 61].
Wearable sensors used in pain detection in perioperative settings are based on the reaction of the autonomic nervous system to formulate a valuable index as a pain reference. The two most common medical devices that are used to assess perioperative pain are the “analgesia nociception index” [62, 63], which is based on heart rate variability, and the “surgical pleth index” based on plethysmography [64, 65].
A novel approach by health systems integrates information from electronic health records (EHRs) with wearable sensors. Integrating patient data into wearable devices launches a new era of health technology [66, 67]. Accordingly, several healthcare systems have incorporated devices that can connect wearable devices to EHRs. Integrating these new applications could increase patient data transparency and improve patient care quality [68, 69]. However, they lack system interoperability, cannot be easily integrated into EHR systems, and raise privacy concerns [66, 67].
Virtual Reality (VR) and Augmented Reality (AR)
VR is defined as “simulations that use various combinations of interaction between devices and sensory display systems” [70]. VR and AR technologies have the potential to make interactive, customizable, 3D virtual patients “digital twins” effective for educating healthcare professionals with vast applications and to improve the clinicians’ skills. Also, they are valuable for undergraduate basic and clinical sciences learning [71, 72]. VR and AR technologies are used to create environments that simulate pain-inducing scenarios to analyze user responses, such as physiological changes or behavioral reactions. These technologies can also be used as distraction techniques during painful procedures because they command an individual’s focus, reducing their awareness of pain [73]. The immersive nature of VR and AR can transport patients to different environments, engage them in interactive activities, and, thus, alter their perceptions of pain [73, 74]. VR can be used as an alternative therapy for pain management in children and adults [72].
Internet of Things (IoT) Integration
The IoT includes wireless sensor networks, radio frequency identification, universal computation, and machine learning [75, 76]. The IoT allows multiple devices with unique identities to sense, transmit, process, and exchange information to provide automated, remote, and real-time pain monitoring by using of electronic diaries. Clinical studies showed they were more accurate in capturing and recording pain signals with the IoT compared to conventional methods [75, 77]. IoT devices, such as mobile, Internet-connected smart sensors embedded in clothing or furniture, can capture pain-related data like physiological indicators, pain behaviors, body posture, movement, and pressure distribution [78]. AI/ML algorithms can analyze these data and provide real-time and personalized pain assessment tools. They also showed a direct relationship between pain and mental illness, these results may help to improve the understanding of mental illness as a possible enhancer of pain episodes and functionality [79]. For example, they can suggest modifications or adjustments to seating positions. Such devices demonstrated efficacy in some patients with fibromyalgia and other musculoskeletal pain, such as low back pain [3, 79].
Smart Home
Smart home technology is a wireless device system that senses, observes, and recognizes pain-related behaviors in the home setting. They can collect, transfer, store, and analyze data over a network. These tools may be more accurate and minimize the reporting biases found in other clinical methods [59, 80]. Smart home technology continuously monitors routine daily activities and behaviors [81, 82]. Recognizing human behaviors may be helpful in quantifying functional pain interference, thereby creating new ways of assessing pain and supporting people living with pain [80, 83].
Smart home ML models can differentiate behavior markers between groups [84, 85], and predict behavior-based sleep and wake patterns [82]. Automated pain assessment using smart home devices can develop consistent, reliable, more objective pain assessments, detecting patterns in behaviors and activities that exacerbate or relieve pain, and responses to pain medications [80, 86]. Therefore, smart homes are creating new ways of assessing pain and supporting people living with pain [80, 83]. A recent study showed that smart home technology could be used overnight in patients with opioid-use disorders to detect pain, sleep disturbances, opioid withdrawal symptoms, and apnea events [87]. Smart home devices can be applied to detect pain and behavioral changes in special populations, such as patients with dementia and intellectual disability [5, 39, 86].
Applications of AI in Pain Assessment
Assessments of Pain Intensity
AI-driven pain assessment has enabled the development of automated pain assessment tools, such as smartphone applications and wearable devices, to provide real-time monitoring and analysis of pain-related data. Various models based on machine learning have been developed to detect pain and pain intensity objectively and accurately [8]. These smart tools can offer efficient and accurate assessment and management of pain [10]. Implementing AI technology can analyze multiple data, including self-report pain scales, facial expressions, and behavioral and physiological signals, to estimate pain intensity objectively. This multimodal model can provide a more accurate and more comprehensive pain assessment compared to the subjective self-report alone [5, 61].
Several studies showed varying levels of accuracy in the assessments of pain intensity, with overall promising results for the use of AI for automated pain assessment [61]. However, more clinical studies on automated pain recognition are required before approval and can be practically implemented [88]. Fontaine et al. [36] evaluated postoperative pain in adults and showed the following results: sensitivity in pain detection (89.7%), for severe pain (77.5%), with accuracy of estimating the pain intensity (53%), while Bargshady et al. [89] showed a more accurate estimation of shoulder pain as the following: accuracy (89–94%) in two groups of patients. For the estimation of self-induced shoulder pain, Barua et al. [90] showed more accuracy of pain intensity (95.57%).
Classification of the Type of Pain
AI and ML incorporate large datasets with complex compositions, combining multiple modalities from different sources and allow data sharing to extract information from the data that can be transformed into knowledge about the patient’s experience of pain [91]. These data can assist clinicians in identifying the underlying mechanisms of pain and offer appropriate treatment strategies [27]. They can classify pain into nociceptive, neuropathic, or inflammatory pain.
ML algorithms use medical images (e.g., CT and MRI) and reports acquired in the context of a painful condition, i.e., low back pain [92]. An example of this process is its use in disk fissures, where nerve ingrowth may occur along granulation tissue. This has been used to classify fissures and the pain caused by a discography. The dataset included pain-positive discograms and a similar number of control disks [91, 92]. ML programming algorithms can be used to differentiate between individual disks with positive and negative pain provocation on discography. The image diagnostic method was completed with a very high success of 99% and an accuracy of 97%. However, pain was only captured at 71% precision and 69% accuracy [92].
Continuous Monitoring and Management of Pain
AI/ML can offer real-time pain continuous monitoring and assessments of pain levels and detect early signs of pain exacerbation. Several studies report promising correlations between pain scores and objectively measured signals derived from wearable devices. These measurements can provide real-time monitoring and assessment of pain [55].
Furthermore, these smart tools can suggest treatment plans and personalized interventions, such as relaxation techniques or medical treatments [10]. For example, wearable devices or mobile applications could track pain levels throughout the day, alert healthcare providers, and prevent pain-related complications [55]. A recent study compared postoperative analgesia by traditional management versus the application of the newly introduced “analgesia quality index” (AQI) through the application of AI-patient controlled analgesia (AI-PCA) and showed that the AQI decreased the incidence of moderate-to-severe pain, improved the quality of analgesia, and may provide guidance for optimum postoperative pain management [93]. However, the monitoring of physical activity may be affected by many factors, such as personal mood, weather conditions, and other psychosocial factors [94, 95].
Predictive Models
Based on patient characteristics and electronic medical records, applications of AI/ML algorithms can predict pain outcomes, possible treatment responses, and how a treatment will be received. Implementations of these smart algorithms can help choose the best interventions; personalized pain management options are made possible by this predictive capability, which maximizes therapeutic efficacy and minimizes side effects [4, 96].
In the perioperative setting, AL/ML can be applied to predict persistent postoperative pain after surgery. Accordingly, a preventive strategy can be implemented to minimize the risk of the developing more persistent type of pain. ML is used for predicting persistent postoperative pain in women after breast surgery using psychological, demographic, and clinical factors. Prediction of persistent pain with a cross-validated accuracy of 86% and a negative predictive value of approximately 95% [97]. Prediction capability and predictive analytics based on ML may save time and money in clinical trials without compromising accuracy [32].
Decision-Making
AI enables the analysis of a large volume of data, including medical records, imaging results, clinical notes, and guidelines. ML techniques could comprehensively analyze the pain-related data, and therefore a personalized decision-making process could be conducted. AI/ML-based technology can improve the decision-making process by providing a solution for clinicians and researchers to extract hidden knowledge from the huge amount of available data that cannot be discovered if relying only on human effort. Also, ML can support healthcare providers in decision-making regarding pain assessment [98].
AI algorithms can aid healthcare providers and clinicians in pain assessments. Accordingly, they can make decisions for accurate pain diagnosis, minimizing errors, and increasing treatment efficacy [75]. Automated decision-making can be applied to patients who cannot communicate or are otherwise unable to verbalize their pain experience. Alternative methods, such as AI, can be used for assessment and management plans [5].
Research and Data Analysis
To assess the efficacy and safety of AI-based pain assessment tools, long-term studies will provide valuable insights into their real-world impact. Longitudinal data can provide insights into the reliability and effectiveness of AI models over time, enabling evidence-based decision-making [10, 15]. AI is ideally suitable for analyzing large and complex medical research datasets [99].
AI/ML-based technologies provide novel methods and efficient programming interfaces to analyze vast amounts of data to assist medical professionals in making more informed decisions while reducing human errors [32]. AI technology has the capacity to capture data, process it, perform dynamic analyses, and produce results that can be effectively used for medical intervention [98].
AI can perform a comparative analysis using “big data” so that information from a patient is compared with massive datasets and digital images collected from other patients in related settings [98, 100]. Using ML applications for clinical research ensures that data are accessed in real time and it manages the trial associates, and electronic records are used to eliminate database errors [101, 102].
They could improve clinical research by applying advanced predictive analytics to clinical trial applicants, evaluating a broader range of data and reducing the costs and time required for medical tests. AI/ML can be used to identify the optimal trial sample sizes for greater efficacy and eliminate errors [32, 103, 104]. Furthermore, ChatGPT is an AI-based conversational agent that utilizes NLP and machine learning algorithms to simulate human-like conversations [105].
Advantages of AI in Pain Assessment
The advantages of AI in pain assessment include the following:
Standard and Accurate Assessment
The utilization of AI algorithms in pain assessment enables the analysis of large-scale datasets, pattern identification, and the emergence of trends and correlations within these datasets, leading to valuable insights into pain mechanisms, risk factors, and treatment efficacy. Clinical studies showed that the application of AI-based algorithms is associated with better pain relief and improved quality of analgesia [8, 15, 93, 106]. Clinical application can provide guidance for optimum pain management in a postoperative setting [93, 106]. Evidence suggests that facial expressions of pain are sensitive and specific to pain and that these expressions can be distinguished from facial expressions associated with basic emotions [107, 108].
Objective Assessment of Pain
Accurate pain assessment facilitates the early detection, diagnosis, and continuous monitoring of pain. The main elements for pain assessments include pain severity, distribution, and duration [109]. AI-based tools provide more objective measures of pain, reducing reliance on subjective self-reports. This objectivity can eliminate individual differences or cultural variations [5, 8].
Digital therapeutics have been successfully used in the monitoring and treatment of opioid-use disorder [110] and to assists patients decrease and manage their opioid use [111]. A recent review found a significant decrease in pain, indicating a clear significant impact of digital health interventions in pain management or opioid-use reduction [110].
Efficiency, Time-Saving, and Low Costs
AI provides innovative solutions in the healthcare sector by enhancing patient care and improving its effectiveness and efficiency without increasing costs [112–114]. In clinical settings, AI/ML algorithms can quickly manage huge amounts of data, enabling efficient pain assessment and decision-making. This saves time for healthcare providers, allowing them to focus on treatment planning and patient care. It also frees up healthcare providers’ time to focus on patient care rather than searching or entering information [15, 32].
Technologically advanced hospitals are now exploring the use of AI technologies to help improve the accuracy of practice [115] and lower the cost of operations by presenting detailed information on various treatment options [115, 116].
Personalized Approach
AI models can consider individual patient characteristics, genetic factors, medical records, and imaging to tailor personalized pain assessment and management strategies. This strategy can improve treatment outcomes and patient satisfaction. Another benefit of ML in the healthcare business is giving individualized therapies that are more dynamic and efficient by combining personal health with predictive analytics [103, 104]. ML-based tools are used to provide various treatment alternatives and individualized treatments and improve the overall efficiency of hospitals and healthcare systems while lowering the cost of care [32].
Early Detection and Preventive Intervention
AI-based technology can detect changes in the patient’s behavior or even slight changes in the pain intensity, duration, type or patterns. Additionally, AL/ML algorithms can enable early intervention to prevent the progression of acute pain to persistent and chronic conditions. This can be achieved by analyzing the patient's data, medical records, imaging, risk factors, or pain predictors and suggesting interventions. ML has the ability of predicting and recommending preventive strategies. Accordingly, physicians can implement preventive and preemptive strategies for pain management [117, 118]. One of AI's most important benefits is promoting the preventive strategy in the healthcare system that will render all humans healthy [119, 120]. AI can also assist in the self-management of musculoskeletal pain [121]. This form of pain intervention outlines rehabilitation and exercise for treatment and recovery and has been shown to be as effective as in-person treatment [122].
Pain Detection in Complex Situations
AI can be helpful in the detection of pain in difficult and complex situations, such as in patients who are unable to communicate verbally, critically ill patients, pediatric patients, and patients with dementia. Moreover, it can be applied during the perioperative period while the patients are unconscious and still under anesthesia [5, 88, 123, 124].
Differentiate True versus False Pain
AI technologies are applied to diagnosis data to reduce false positives and false negatives [125]. Some studies suggest that AI/ML can differentiate true pain versus faked pain and identify malingering [126]. This is of great importance in different situations, such as detecting patients seeking compensation [127], requesting unjustifiable sick leaves, and preventing unnecessary narcotics prescriptions for drug abusers and people with a substance-use disorder; this has the potential to reduce health care costs [26, 88, 107].
Remote Health Care
Remote patient monitoring is an emerging field of health care, which concerns the assessment and management of the patient with the goal of treating or diagnosing illness using information technology and telecommunication tools [6, 119]. Jiang [128] developed a wearable telehealth system to monitor the patients and elderly [6]. Telehealth systems are also gaining importance, especially with the COVID-19 pandemic [129]. Remote health care offers many advantages. It helps to save time, effort, and resources for both patients and health care system [129, 130].
Challenges and Limitations
Ethical Considerations
As AI becomes more integrated into health care services, addressing the ethical considerations such as obtaining informed consent, maintaining confidentiality and patient privacy, data security, and potential biases take on extreme importance. In a clinical setting, it is important to know the potential harms that should be balanced against the expected benefits [131]. The value of transparency cuts across both with trust in the medical profession and health care systems at stake [131].
-
Data privacy and security:
The AI/ML algorithms depend on vast amounts of sensitive patient data. One of the concerns regarding digital health is data privacy. Ensuring data privacy and security is of utmost importance. Strong privacy measures and adherence to regulatory frameworks are crucial to maintaining patient confidentiality and trust in AI-driven pain assessment systems [132, 133]. Furthermore, raw data acquired from patients and hospitals are used by AI/ML systems, and ethical considerations should be considered while collecting these data [133]. It is crucial to develop guidelines and regulations to ensure transparency, fairness, and accountability in AI algorithms and their deployment [133, 134].
-
Considerations related to patient privacy:
Concerns are being raised regarding patient privacy and autonomy. For example, patients should provide informed consent beforehand, as they may refuse facial analysis. Furthermore, algorithms might be trained for particular demographics, further marginalizing already vulnerable groups [26, 135, 136]. Additionally, using AI/ML algorithms to detect pain through facial expressions has several limitations [26, 135, 136]. Their application may be limited by many factors, e.g., the patient’s age, gender, behavior, as well as the head position and movements. Moreover, some medical conditions can affect facial shape and mobility, such as Parkinson’s, stroke, facial injury, or deformity. All these factors can reduce the accuracy and sensitivity of the AI/ML to automatically detect pain experiences [26, 135, 136].
The “WHO” reiterates the importance of applying ethical principles and appropriate governance, as enumerated in the WHO guidance on the ethics and governance of AI for health, when designing, developing, and deploying AI for health. The six core principles identified by WHO are: (1) protect autonomy; (2) promote human well-being, human safety, and the public interest; (3) ensure transparency, explainability, and intelligibility; (4) foster responsibility and accountability; (5) ensure inclusiveness and equity; (6) promote AI that is responsive and sustainable [137].
-
Interpretability, explainability, and transparency:
The lack of transparency in making a decision is referred to as the “black box” of AI. Since AI models often operate as black boxes, it can be challenging to understand the underlying decision-making process. This lack of interpretability can raise ethical concerns and limit clinical adoption [131]. Efforts have been made in the recent years to enhance the interpretability, explainability, and transparency of AI/ML algorithms with deep learning models. It is essential to mimic human judgment regarding the interpretation and explainable skills to provide clear explanations for the assessments, build trust, and help clinicians understand the decision-making process [138, 139].
Data Quality, Potentials of Error, and Bias
The risk of biases within the data built into AI algorithms could lead to inaccurate pain assessments and wrong decisions [26, 131]. It is essential to consider the potential for errors and inaccuracies in pain detection models, which could lead to inappropriate decisions, such as misdiagnosis, inappropriate treatment, unnecessary surgery, patient injury, or even significant legal and financial implications [140]. Sometimes the data collected from hospitals is inaccurate or of insufficient quality. Data errors are among the top challenges in medical data processing via AI. False decisions in automated diagnosis may have very harmful results [141]. Moreover, some studies have found that ML algorithms are prone to misinterpreting unpleasant disgust as pain in facial expressions [141].
The Complex Nature of Pain
Automatic pain detection is challenging because it is complex, subjective, and subject to a variety of factors, such as personal and social-cultural perspectives, as well as past experiences of pain [143]. Several studies worldwide are engaged in the field of automatic pain assessments, but the lack of adequate knowledge can represent an obstacle to the potential translation into clinical practice. Such limitations contribute to the gap between the development of an AI algorithm and its application [10, 144]. The most important limitation of these studies is attributed to the tools that determine only the presence or absence of pain but not pain quality or characteristics [145].
Human–Machine Interaction
AI technology may improve the accuracy of assessments, diagnosis, and care planning. It will be an integral part of health care services and will be incorporated into several aspects of clinical care [146]. However, the role of healthcare providers in the setting of AI-based pain assessment should be carefully considered. Establishing effective human–machine interactions and prioritizing clinical expertise and judgment are essential to ensure the responsible use of AI tools. The increasing focus of AI has raised the question of whether AI-based systems will ultimately replace physicians in some specializations or augment physicians’ role without actually replacing them. Research studies conclude that AI-based systems will augment physicians and are unlikely to replace the traditional physician–patient relationship [147]. Collaborative efforts between AI experts and healthcare professionals are necessary to ensure the smooth integration of AI tools into clinical workflows [8].
Future Perspectives for AI in Pain Assessment
AI is a rapidly growing new field that shows effectiveness and benefits for application in many aspects of health care. The AI models should be designed to continuously learn and adapt from new data, improving their accuracy and performance over time. This continuous learning and adaptation process will enhance the effectiveness and reliability of AI-based pain assessment systems. The future of AI in pain assessment involves different aspects.
Integration with Telemedicine
The integration of AI in telemedicine platforms has emerged as an innovative approach. It has the potential to change the delivery of healthcare services and enhance remote patient care, and improve overall healthcare outcomes. This virtual system can provide patient information and assessments, offer basic medical advice, and schedule appointments [129]. AI in telemedicine can facilitate the way for the patients to interact with healthcare providers remotely and can assist in the diagnostic process [129, 147]. AI algorithms can analyze patient-reported data, video consultations, and other relevant information to provide clinicians with valuable insights for accurate pain evaluation and treatment recommendations. More research is required to facilitate the ethical and practical implications of AI integration in telemedicine to ensure patient safety and improve health outcomes [129, 147, 149].
Integration of Behavioral and Biometric Data
AI-based technology can integrate behavioral and biometric data, such as facial expressions, body movements, or physiological signals. This will offer a more comprehensive, multimodal pain assessment and enhance the accuracy of pain evaluation [10, 149].
Digital Twins
Digital Twins are described by Fuller et al. [150] as an effortless data integration between a physical and virtual machine in either direction. In pain assessment and management, digital twins can be conceptualized as virtual customized representations of an individual’s pain experience. These digital twins can be created by collecting and analyzing various data sources, such as physiological measurements, medical records, and patient-reported outcomes. AI algorithms can then analyze this data to identify patterns and correlations that may further deepen our understanding of pain’s underlying causes and mechanisms.
One of the key advantages of digital twins is their ability to capture the multidimensional nature of pain. Pain is a complex and subjective experience influenced by various factors such as genetics, environment, and psychological state. By integrating data from different sources, digital twins can provide a holistic view of an individual’s pain experience, enabling healthcare providers to tailor treatment plans accordingly [150–152].
AI algorithms can also analyze the data from digital twins to predict pain outcomes and optimize treatment strategies. For example, machine learning algorithms can identify patterns in the data that may indicate the effectiveness of different interventions or predict the likelihood of chronic pain development. These patterns assist healthcare providers in their clinical decision-making process, personalizing care, and improving patient outcomes [151, 152].
Furthermore, digital twins could also facilitate remote monitoring, telemedicine, and telehealth for pain management [153]. Patients could use wearable devices or smartphone apps to collect data on their pain levels, activity levels, and medication usage, which can be integrated into their digital twin [154]. Healthcare providers could remotely access this data and make real-time adjustments to the treatment plan, reducing the need for in-person visits and improving patient convenience.
Integrating Multimodal Approaches
Since pain is a multidimensional phenomenon, a promising direction is to combine different data from different sources and various channels to supplement each other and finally lead to significant improvement in the accuracy, specificity, and sensitivity of the assessments [8]. Integrating multiple modalities from different sources, such as self-reports, electronic medical reports, physiological and behavioral signals, and imaging data can allow a more comprehensive understanding of pain and facilitate fully automated and personalized treatment approaches [48]. A recent systematic review demonstrated the importance of multimodal approaches for automatic pain assessment, especially in clinical settings [8]. Recent approaches involve multimodal strategies by combining behaviors with neurophysiological findings can enhance the accuracy of AI-based technology in pain assessments [6, 10].
Brain–Computer Interaction (BCI)
Brain–computer interfaces (BCI) are defined as communication and control systems in which the thoughts of the human mind are translated into real-world interactions without the use of the common neural pathways and muscles [155]. Recent advances in BCI development are offering numerous application in healthcare [156], including novel pain assessment and management approaches. BCIs are devices that establish a direct communication pathway between the brain and an external device, such as a computer. When deployed safely and responsibly, they could be leveraged to measure and interpret brain activity associated with pain perception.
BCIs could provide objective measures of pain, overcoming the limitations of self-reporting, which can be subjective and influenced by various factors. By analyzing brain activity patterns, AI algorithms can identify neural signatures of pain and quantify its intensity. This objective assessment could help healthcare providers better understand and manage pain, especially in cases where patients are unable to communicate their pain levels, such as infants or individuals with severe cognitive impairments [157–159].
BCIs could also be used to develop closed-loop systems for pain management [160]. By continuously monitoring brain activity, AI algorithms could be deployed to detect pain-related changes and trigger interventions, such as drug delivery or neuromodulation techniques, to alleviate pain in real-time. This personalized and adaptive approach has the potential to provide more effective pain relief and minimize the risk of adverse effects [161].
Limitations
This review is mainly limited to adults and excluded pediatric populations. The applications of AI for automated pain assessment are very beneficial in the most vulnerable groups of patients, e.g., pediatrics, geriatric patients with dementia, intellectual disability and unconscious patients. However, those most vulnerable groups were not included in this review. This is a narrative review and not a prospective study or a systematic review. This is a very new topic and few clinical studies have been conducted; some of the studies we found were pilot studies. Finally, the integration of AI in health care generally and in pain management specifically is relatively a new technology and still in early development. Further research is required to identify the knowledge gaps, risk–benefit ratio, and proper applications of the AI-based technology in the healthcare systems.
Summary and Conclusions
This comprehensive review shows how incorporating AI/ML-based technologies offers a promising opportunity to deliver unbiased, objective, and personalized pain assessments. Over the past few years, studies have demonstrated tremendous progress in providing accurate, effective, continuous, and real-time pain assessments. These studies show how AI/ML-based therapies could enhance the ability to identify, predict, and provide effective self-management of pain. These new methods may also help prevent pain before it starts. There are obstacles and limitations regarding the application of AI/ML-based technology in healthcare in general and pain management specifically. The main goals are to enhance accuracy, objectivity, and personalization in pain management through the application of AI. Furthermore, the integrations between clinical practice guidelines, health care authorities, and human–computer interaction with AI/ML-based technology are the key steps for improving pain management and overall patient outcomes. The ethical considerations and successful use of AI in clinical practice will depend on overcoming obstacles pertaining to patient privacy, data security, quality, transparency, and interpretability. Sustained investigation, multidisciplinary research, and cooperation between AI professionals and health care providers by establishing effective human–machine interaction are essential to ensure the responsible use of AI tools. In the near future, it is essential to fully utilize AI technology in the evaluation and management of pain and enhance overall patient outcomes. A multimodal approach could also help pain assessment for nonverbal patients or those who have limited language skills or other communication barriers. Further research is required to develop an updated model, improve the accuracy of pain assessments, work in a more complex environment, and practically implement AI in real-world healthcare settings to improve their effectiveness, safety, and impact on patient outcomes.
Acknowledgments
Authorship.
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article.
Author Contributions
All authors reviewed the results and approved the final version of the manuscript. Salah N. El-Tallawy (Corresponding Author): concept and design, writing, searching, and supervision for all steps. Joseph V. Pergolizzi: helped supervise the project, study screening, writing, and editing. Ingrid Vasiliu-Feltes: writing, reviewing, and making intellectual contributions on revisions. Rania S. Ahmed: searching, study screening, and editing. JoAnn K LeQuang: draft manuscript preparation, writing, and editing. Hamdy N El-Tallawy: final revision, and preparation of the reviewer responses. Giustino Varrassi: searching, study screening, and editing. Mohamed S. Nagiub: searching, study screening, and editing.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Giustino Varrassi works as an Editorial Board Member for Pain and Therapy journal. All other authors have no conflict of interest.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161:1976–1982. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strand N, Maloney J, Francio VT, et al. Advances in pain medicine: a review of new technologies. Curr Pain Headache Rep. 2022;26:605–616. doi: 10.1007/s11916-022-01062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werner P, Lopez-Martinez D, Walter S, Al-Hamadi A, Gruss S, Picard R. Automatic recognition methods supporting pain assessment: a survey. IEEE Trans Affect Comput. 2019 doi: 10.1109/TAFFC.2019.2946774. [DOI] [Google Scholar]
- 4.Chen J, Abbod M, Shieh JS. Pain and stress detection using wearable sensors and devices—a review. Sensors. 2021;21:1030. doi: 10.3390/s21041030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Tallawy SN, Ahmed RS, Nagiub MS. Pain management in the most vulnerable intellectual disability: a review. Pain Ther. 2023;12:939–961. doi: 10.1007/s40122-023-00526-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali O, Abdelbaki W, Shrestha A, et al. A systematic literature review of artificial intelligence in the healthcare sector: benefits, challenges, methodologies, and functionalities. J Innov Knowl. 2023 doi: 10.1016/j.jik.2023.100333. [DOI] [Google Scholar]
- 7.Amisha, Malik P, Pathania M, Rathaur VK. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328–2331. doi: 10.4103/jfmpc.jfmpc_440_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gkikas S, Tsiknakis M. Automatic assessment of pain based on deep learning methods: a systematic review. Comput Methods Programs Biomed. 2023;231:107365. doi: 10.1016/j.cmpb.2023.107365. [DOI] [PubMed] [Google Scholar]
- 9.Dialani P. AI in Healthcare: AI in Pain Management, a New Application. Analytics Insight, March 2021. https://www.analyticsinsight.net/ai-in-healthcare-ai-in-pain-management-a-new-application/
- 10.Cascella M, Schiavo D, Cuomo A, et al. Artificial intelligence for automatic pain assessment: research methods and perspectives. Pain Res Manage. 2023 doi: 10.1155/2023/6018736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 12.NVIDIA. Artificial Intelligence. Available at: https://www.nvidia.com/en-us/glossary/data-science/artificial-intelligence/
- 13.Hamet P, Tremblay J. Artificial intelligence in medicine. Metabolism. 2017;69:S36–S40. doi: 10.1016/j.metabol.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Priya Dialani. AI in Healthcare: AI in Pain Management, a New Application. Analytics Insight. 2021. https://www.analyticsinsight.net/ai-in-healthcare-ai-in-pain-management-a-new-application/
- 15.Zhang M, Zhu L, Shih-Yin L, et al. Using artificial intelligence to improve pain assessment and pain management: a scoping review. J Am Med Inform Assoc. 2023;30(3):570–587. doi: 10.1093/jamia/ocac231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornet G. Robot companions and ethics a pragmatic approach of ethical design. Int J Bioethics. 2013;24(4):49–58. doi: 10.3917/jib.243.0049. [DOI] [PubMed] [Google Scholar]
- 17.Jung W, Lee KE, Suh BJ, Seok H, Lee DW. Deep learning for osteoarthritis classification in temporo-mandibular joint. Oral Dis. 2023;29:1050–1059. doi: 10.1111/odi.14056. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, He M, Jiang Z, Wu Z, et al. Survey on natural language processing in medical image analysis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47:981–993. doi: 10.11817/j.issn.1672-7347.2022.220376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taye MM. Understanding of machine learning with deep learning: architectures, workflow, applications and future directions. Computers. 2023;12(5):91. doi: 10.3390/computers12050091. [DOI] [Google Scholar]
- 20.Janiesch C, Zschech P, Heinrich K. Machine learning and deep learning. Electron Markets. 2021;31:685–695. doi: 10.1007/s12525-021-00475-2. [DOI] [Google Scholar]
- 21.Myszczynska MA, Ojamies PN, Lacoste AM, et al. Applications of machine learning to diagnosis and treatment of neurodegenerative diseases. Nat Rev Neurol. 2020;16(8):440–456. doi: 10.1038/s41582-020-0377-8. [DOI] [PubMed] [Google Scholar]
- 22.Hassani H, Silva ES, Unger S, TajMazinani M, Feely SM. Artificial Intelligence (AI) or Intelligence Augmentation (IA): What is the future? AI 2020;1:143–55. 10.3390/ai1020008
- 23.Bazoukis G, Hall J, Loscalzo J, Antman EM, Fuster V, Armoundas AA. The inclusion of augmented intelligence in medicine: a framework for successful implementation. Cell Reports Med. 2022;3:100485. doi: 10.1016/j.xcrm.2021.100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crigger E, Reinbold K, Hanson C, Kao A, Blake K, Irons M. Trustworthy augmented intelligence in health care. J Med Syst. 2022;46:12. doi: 10.1007/s10916-021-01790-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian Y. Artificial intelligence image recognition method based on convolutional neural network algorithm. IEEE Access. 2020;8:125731–125744. doi: 10.1109/ACCESS.2020.3006097. [DOI] [Google Scholar]
- 26.De Sario GD, Haider CR, Maita KC, et al. Using AI to detect pain through facial expressions: a review. Bioengineering. 2023;10:548. doi: 10.3390/bioengineering10050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadjiat Y, Arendt-Nielsen L. Digital health in pain assessment, diagnosis, and management: overview and perspectives. Front Pain Res. 2023;4:1097379. doi: 10.3389/fpain.2023.1097379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Societal Impact of Pain. SIP position on digital health: pain assessment and quality indicators. (2022). Available at: https://www.sipplatform.eu/files/editor/newsroom/News/2021/SIP_Position_on_Digital_Health_FINAL_2.pdf (Accessed July 5, 2022).
- 29.Tarca AL, Carey VJ, Chen XW, Romero R, Draghici S. Machine learning and its applications to biology. PLoS Comput Biol. 2007;3(6):e116. doi: 10.1371/journal.pcbi.0030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallol-Ragolta A, Liu S, Cummins N, Schuller B. A curriculum learning approach for pain intensity recognition from facial expressions. In: 2020 15th IEEE international conference on automatic face and gesture recognition (FG 2020); 16 November 2020. IEEE: 829–33.
- 31.Matsangidou M, Liampas A, Pittara M, Pattichi CS, Zis P. Machine learning in pain medicine: an up-to-date systematic review. Pain Ther. 2021;10:1067–1084. doi: 10.1007/s40122-021-00324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Javaid M, Haleem A, Singh RP, Suman R, Rab S. Significance of machine learning in healthcare: features, pillars and applications. Int J Intell Netw. 2022;3:58–73. doi: 10.1016/j.ijin.2022.05.002. [DOI] [Google Scholar]
- 33.Dutta P, Nachamai M. Facial pain expression recognition in real-time videos. J Healthcare Eng. 2018 doi: 10.1155/2018/7961427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kharghanian R, Peiravi A, Moradi F. Pain detection from facial images using unsupervised feature learning approach. Annu Int Conf IEEE Eng Med Biol Soc. 2016;2016:419–422. doi: 10.1109/EMBC.2016.7590729. [DOI] [PubMed] [Google Scholar]
- 35.Lucey P, Cohn JF, Matthews I, et al. Automatically detecting pain in video through facial action units. IEEE Trans Syst Man Cybern B Cybern. 2011;41(3):664–674. doi: 10.1109/TSMCB.2010.2082525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontaine D, Vielzeuf V, Genestier P, Limeux P, Santucci-Sivilotto S, Mory E, Darmon N, Lanteri-Minet M, Mokhtar M, Laine M, Vistoli D, DEFI study group Artificial intelligence to evaluate postoperative pain based on facial expression recognition. Eur J Pain. 2022;26(6):1282–1291. doi: 10.1002/ejp.1948. [DOI] [PubMed] [Google Scholar]
- 37.Fodeh SJ, Finch D, Bouayad L, Luther S, Kerns RD, Brandt C. Classifying clinical notes with pain assessment. Stud Health Technol Inform. 2017;245:1261. [PubMed] [Google Scholar]
- 38.Atee M, Hoti K, Hughes JD. A technical note on the PainChekTM system: a web portal and mobile medical device for assessing pain in people with dementia. Front Aging Neurosci. 2018;10:117. doi: 10.3389/fnagi.2018.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Tallawy SN, Ahmed RS, Shabi SM, Al-Zabidi FZ, Zaidi AZ, Varrassi G, Perglozzi GV, LeQuang JA, Paladini A. The challenges of pain assessment in geriatric patients with dementia: a review. Cureus. 2023;15(11):e49639. doi: 10.7759/cureus.49639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarker IH. Deep learning: a comprehensive overview on techniques, taxonomy, applications and research directions. SN Comput Sci. 2021;2:420. doi: 10.1007/s42979-021-00815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yen C, Lin C-L, Chiang M-C. Exploring the frontiers of neuroimaging: a review of recent advances in understanding brain functioning and disorders. Life. 2023;13(7):1472. doi: 10.3390/life13071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xafi V, Owen Schaefer GO, Labude MK, et al. An ethics framework for big data in health and research. Asian Bioethics Rev. 2019;11:227–254. doi: 10.1007/s41649-019-00099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadiku MNO, Zhou Y, Musa SM. Natural language processing. Int J Adv Sci Res Eng. 2018;4:68–70. doi: 10.31695/IJASRE.2018.32708. [DOI] [Google Scholar]
- 44.Voytovich L, Greenberg C. Natural Language processing: practical applications in medicine and investigation of contextual autocomplete. Acta Neurochir Suppl. 2022;134:207–214. doi: 10.1007/978-3-030-85292-4_24. [DOI] [PubMed] [Google Scholar]
- 45.Liu K, Hogan WR, Crowley RS. Natural Language Processing methods and systems for biomedical ontology learning. J Biomed Inform. 2011;44(1):163–179. doi: 10.1016/j.jbi.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlson LA, Hooten PM. Pain—linguistics and natural language processing. Mayo Clin Proc. 2020;4(3):346–347. doi: 10.1016/j.mayocpiqo.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Antoni F, Russo F, Ambrosio L, et al. Artificial intelligence and computer vision in low back pain: a systematic review. Int J Environ Res Public Health. 2021;18:10909. doi: 10.3390/ijerph182010909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prkachin KM, Hammal Z. Computer mediated automatic detection of pain-related behavior: prospect, progress. Perils Front Pain Res. 2021;2:788606. doi: 10.3389/fpain.2021.788606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egede JO, Song S, Olugbade TA et al. EMOPAIN challenge 2020: multimodal pain evaluation from facial and bodily expressions. arXiv. 2020; arXiv.2001.07739v07733. 10.1109/FG47880.2020.00078.
- 50.Sikka K, Ahmed AA, Diaz D et al. Automated assessment of children’s postoperative pain using computer vision. PEDIATRICS 2015. 10.1542/peds.2015-0029. [DOI] [PMC free article] [PubMed]
- 51.Heintz T. AI pain recognition system could help detect patients’ pain before, during and after surgery. A study presented at the annual meeting of the ASA 2023, Oct. 13 to 17 in San Francisco. Available at: https://www.asahq.org/about-asa/newsroom/news-releases/2023/10/ai-pain-recognition-system
- 52.Soori M, Arezoo B, Dastres R. Artificial intelligence, machine learning and deep learning in advanced robotics, a review. Cogn Robot. 2023;3:54–70. doi: 10.1016/j.cogr.2023.04.001. [DOI] [Google Scholar]
- 53.Higgins A, Llewellyn A, Dures E, Caleb-Solly P. Robotics technology for pain treatment and management: a review. In: Social Robotics: 14th International Conference, ICSR 2022, Florence, Italy, December 13–16, 2022, Proceedings, Part I. Springer-Verlag, Berlin, Heidelberg, p. 534–45. 10.1007/978-3-031-24667-8_47.
- 54.Lihui Pu, Coppieters MW, Smalbrugge M, Jones C, Byrnes J, Todorovic M, Moyle W. Implementing PainChek and PARO to support pain assessment and management in residents with dementia: a qualitative study. Pain Manag Nurs. 2023;24(6):587–594. doi: 10.1016/j.pmn.2023.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Leroux A, Rzasa-Lynn R, Crainiceanu C, Sharma T. Wearable devices: current status and opportunities in pain assessment and management. Digit Biomark. 2021;5(1):89–102. doi: 10.1159/000515576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ploner M, May ES. Electroencephalography and magnetoencephalography in pain research-current state and future perspectives. Pain. 2018;159(2):206–211. doi: 10.1097/j.pain.0000000000001087. [DOI] [PubMed] [Google Scholar]
- 57.Dinh-Le C, Chuang R, Chokshi S, Mann D. Wearable health technology and electronic health record integration: scoping review and future directions. JMIR Mhealth Uhealth. 2019;7(9):e12861. doi: 10.2196/12861:10.2196/12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharon T. Self-tracking for health and the quantified self: re-articulating autonomy, solidarity, and authenticity in an age of personalized healthcare. Philos Technol. 2016;30(1):93–121. doi: 10.1007/s13347-016-0215-5. [DOI] [Google Scholar]
- 59.Benromano T, Pick CG, Merick R, Defrin R. Physiological and behavioral responses to calibrated noxious stimuli among individuals with cerebral palsy and intellectual disability. Pain Med. 2017;18:441–453. doi: 10.1093/pm/pnw155. [DOI] [PubMed] [Google Scholar]
- 60.Babrak LM, Menetski J, Rebhan M, et al. Traditional and digital biomarkers: two worlds apart? Digit Biomark. 2019;3(2):92–102. doi: 10.1159/000502000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vallejo-De la Cueva A, Aretxabala-Cortajarena N, Quintano-Rodero A, Rodriguez-Nuñez C, Pelegrin-Gaspar PM, Gil-Garcia ZI, Margüello-Fernandez AA, Aparicio-Cilla L, Parraza-Diez N. Pupillary dilation reflex and behavioural pain scale: Study of diagnostic test. Intensive Crit Care Nurs. 2023;74:103332. doi: 10.1016/j.iccn.2022.103332. [DOI] [PubMed] [Google Scholar]
- 62.Jeanne M, Clément C, De Jonckheere J, Logier R, Tavernier B. Variations of the analgesia nociception index during general anaesthesia for laparoscopic abdominal surgery. J Clin Monit. 2012;26:289–294. doi: 10.1007/s10877-012-9354-0. [DOI] [PubMed] [Google Scholar]
- 63.Abdullayev R, Uludag O, Celik B. Analgesia Nociception Index: Assessment of acute postoperative pain. Braz J Anesthesiol (English Ed) 2019;69:396–402. doi: 10.1016/j.bjane.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thee C, Ilies C, Gruenewald M, Kleinschmidt A, Steinfath M, Bein B. Reliability of the surgical Pleth index for assessment of postoperative pain. Eur J Anaesthesiol. 2015;32:44–48. doi: 10.1097/EJA.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 65.Ledowski T, Schneider M, Gruenewald M, Goyal R, Teo S, Hruby J. Surgical pleth index: prospective validation of the score to predict moderate-to-severe postoperative pain. Br J Anaesth. 2019;123:e328–e332. doi: 10.1016/j.bja.2018.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wicklund E. mHealth Intelligence. Telehealth, mHealth Put The Spotlight on Interoperability. 2018. [2018–10–12], at HIMSS18 https://mhealthintelligence.com/news/telehealth-mhealth-put-the-spotlight-on-interoperability-at-himss18
- 67.Gay V, Leijdekkers P. Bringing health and fitness data together for connected health care: mobile apps as enablers of interoperability. J Med Internet Res. 2015;17(11):e260. doi: 10.2196/jmir.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nangalia V, Prytherch DR, Smith GB. Health technology assessment review: remote monitoring of vital signs–current status and future challenges. Crit Care. 2010;14(5):233. doi: 10.1186/cc9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cannon B. How Cedars Sinai & Dartmouth-Hitchcock are using wearable tech to improve patient experience. MedCity News. 2016. [2018–10–10]. Available at: https://medcitynews.com/2016/08/cedars-sinai-dartmouth-hitchcock-using-wearable-tech-improve-patient-experience/
- 70.Trost Z, Zielke M, Guck A, Nowlin L, Zakhidov D, France CR, et al. The promise and challenge of virtual gaming technologies for chronic pain: the case of graded exposure for low back pain. Pain Manag. 2015;5:197–206. doi: 10.2217/pmt.15.6. [DOI] [PubMed] [Google Scholar]
- 71.El Miedany, Y. Virtual reality and augmented reality. In: Rheumatology teaching. Cham: Springer International. Publishing; 2019; p. 403–27. 10.1007/978-3-319-98213-7_20
- 72.Viderman D, Tapinova K, Dossov M, Seitenov S, Abdildin YG. Virtual reality for pain management: an umbrella review. Front Med. 2023;10:1203670. doi: 10.3389/fmed.2023.1203670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ventola CL. Virtual reality in pharmacy: opportunities for clinical, research, and educational applications. PT. 2019;44(5):267–276. [PMC free article] [PubMed] [Google Scholar]
- 74.Javaid M, Haleem A. Virtual reality applications toward medical field. Clin Epidemiol Global Health. 2020;8:600–605. doi: 10.1016/j.cegh.2019.12.010. [DOI] [Google Scholar]
- 75.Argüello Prada EJ. The Internet of Things (IoT) in pain assessment and management: an overview. Inform Med Unlocked. 2020;18:100298. doi: 10.1016/j.imu.2020.100298. [DOI] [Google Scholar]
- 76.Gubbi J, Buyyab G, Marusica S, Palaniswami M. Internet of things (IoT): a vision, architectural elements. Future Generat Comput Syst. 2013;29(7):1645–1660. doi: 10.1016/j.future.2013.01.010. [DOI] [Google Scholar]
- 77.Silva BN, Khan M, Han K. Internet of things: a comprehensive review of enabling technologies, architecture, and challenges. IETE Tech Rev. 2018;35(2):205–220. doi: 10.1080/02564602.2016.1276416. [DOI] [Google Scholar]
- 78.Gkikas S, Manolis TM. Automatic assessment of pain based on deep learning methods: a systematic review. Comput Methods Programs Biomed. 2023;231:107365. doi: 10.1016/j.cmpb.2023.107365. [DOI] [PubMed] [Google Scholar]
- 79.Parolini F, Goethel M, Becker K, et al. Breaking barriers: artificial intelligence interpreting the interplay between mental illness and pain as defined by the international association for the study of pain. Biomedicines. 2023;11(7):2042. doi: 10.3390/biomedicines11072042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fritz RL, Wilson M, Dermody G, Schmitter-Edgecombe M, Cook DJ. Automated smart home assessment to support pain management: multiple methods analysis. J Med Internet Res. 2020;22(11):e23943. doi: 10.2196/23943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.LeBaron V, Hayes J, Gordon K, Alam R, Homdee N, Martinez Y, et al. Leveraging smart health technology to empower patients and family caregivers in managing cancer pain: protocol for a feasibility study. JMIR Res Protoc. 2019;8(12):e16178. doi: 10.2196/16178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams JA, Cook DJ. Forecasting behavior in smart homes based on sleep and wake patterns. THC. 2017;25(1):89–110. doi: 10.3233/thc-161255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berger SE, Baria AT. Assessing pain research: a narrative review of emerging pain methods, their technosocial implications, and opportunities for multidisciplinary approaches. Front Pain Res. 2022;3:896276. doi: 10.3389/fpain.2022.896276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cook DJ, Schmitter-Edgecombe M, Dawadi P. Analyzing activity behavior and movement in a naturalistic environment using smart home techniques. IEEE J Biomed Health Inform. 2015;19(6):1882–1892. doi: 10.1109/jbhi.2015.2461659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sprint GL, Cook DJ, Fritz R. Behavioral differences between subject groups identified using smart homes and change point detection. IEEE J Biomed Health Inform. 2020 doi: 10.1109/jbhi.2020.2999607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Debes C, Merentitis A, Sukhanov S, Niessen M, Frangiadakis N, Bauer A. Monitoring activities of daily living in smart homes: understanding human behavior. IEEE Signal Process Mag. 2016;33(2):81–94. doi: 10.1109/MSP.2015.2503881. [DOI] [Google Scholar]
- 87.Wilson M, Fritz R, Finlay M, Cook DJ. Piloting smart home sensors to detect overnight respiratory and withdrawal symptoms in adults prescribed opioids. Pain Manag Nurs. 2023;24:4–11. doi: 10.1016/j.pmn.2022.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walter S, Gruss S, Frisch S, Liter J, Jerg-Bretzke L, Zujalovic B, Barth E. “What about automated pain recognition for routine clinical use?” A survey of physicians and nursing staff on expectations, requirements, and acceptance. Front Med. 2020;7:566278. doi: 10.3389/fmed.2020.566278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bargshady G, Zhou X, Deo RC, Soar J, Whittaker F, Wang H. The modeling of human facial pain intensity based on Temporal Convolutional Networks trained with video frames in HSV color space. Appl Soft Comput J. 2020;97:106805. doi: 10.1016/j.asoc.2020.106805. [DOI] [Google Scholar]
- 90.Barua PD, Baygin N, Dogan S, et al. Automated detection of pain levels using deep feature extraction from shutter blinds-based dynamic-sized horizontal patches with facial images. Sci Rep. 2022;12(1):17297. doi: 10.1038/s41598-022-21380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lotscha J, Ultsch A, Mayera B, Kringel D. Artificial intelligence and machine learning in pain research: a data scientometric analysis. Pain Report. 2022;9(7):e1044. doi: 10.1097/PR9.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hinton GE, Salakhutdinov RR. Reducing the dimensionality of data with neural networks. Science. 2006;313:504–507. doi: 10.1126/science.1127647. [DOI] [PubMed] [Google Scholar]
- 93.Wang D, Guo Y, Yin Q, Cao H, Chen X, Qian H, Ji M, Zhang J. Analgesia quality index improves the quality of postoperative pain management: a retrospective observational study of 14,747 patients between 2014 and 2021. BMC Anesthesiol. 2023;23:281. doi: 10.1186/s12871-023-02240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Naranjo-Hernández D, Reina-Tosina J, Roa LM. Sensor technologies to manage the physiological traits of chronic pain: a review. Sensors (Basel) 2020;20(2):365. doi: 10.3390/s20020365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yen T, Mohler J, Dohm M, Laksari K, Najafi B, Toosizadeh N. The effect of pain relief on daily physical activity: in-home objective physical activity assessment in chronic low back pain patients after paravertebral spinal block. Sensors. 2018;18(9):3048. doi: 10.3390/s18093048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fan B. Artificial intelligence in pain management. In: Xia M, Jiang H, editors. Artificial intelligence in anesthesiology. Singapore: Springer; 2023. [Google Scholar]
- 97.Lotsch J, Sipila R, Tasmuth T, Kringel D, Estlander AM, Meretoja T, Kalso E, Ultsch A. Machine-learning-derived classifier predicts absence of persistent pain after breast cancer surgery with high accuracy. Breast Cancer Res Treat. 2018;171:399–411. doi: 10.1007/s10549-018-4841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alia O, Abdelbaki W, Shrestha A, et al. A systematic literature review of artificial intelligence in the healthcare sector: benefits, challenges, methodologies, and functionalities. J Innov Knowl. 2023;8:100333. doi: 10.1016/j.jik.2023.100333. [DOI] [Google Scholar]
- 99.Academy of Royal Medical Colleges. Artificial Intelligence in Healthcare. 2019. Available at: https://www.aomrc.org.uk/wpcontent/uploads/2019/01/Artificial_intelligence_in_healthcare_0119.pdf (accessed on 11 Jan 2023).
- 100.Somasundaram M, Junaid KAM, Mangadu S. Artificial intelligence (AI) enabled intelligent quality management system (IQMS) for personalized learning path. Procedia Comput Sci. 2020;172:438–442. doi: 10.1016/j.procs.2020.05.096. [DOI] [Google Scholar]
- 101.Kushwaha S, Bahl S, Bagha AK, Parmar KS, Javaid M, Haleem A, Singh RP. Significant applications of machine learning for COVID-19 pandemic. J Ind Integrat Manage. 2020;5(4):453–479. doi: 10.1142/S2424862220500268. [DOI] [Google Scholar]
- 102.Rudin C, Ustun B. Optimized scoring systems: toward trust in machine learning for healthcare and criminal justice. Interfaces. 2018;48(5):449–466. doi: 10.1287/inte.2018.0957. [DOI] [Google Scholar]
- 103.Siddique S, Chow JC. Machine learning in healthcare communication. Encyclopedia. 2021;1(1):220–239. doi: 10.3390/encyclopedia1010021. [DOI] [Google Scholar]
- 104.Waring J, Lindvall C, Umeton R. Automated machine learning: review of the state-of-the-art and opportunities for healthcare. Artif Intell Med. 2020;104:101822. doi: 10.1016/j.artmed.2020.101822. [DOI] [PubMed] [Google Scholar]
- 105.Hassani H, Silva ES. The role of ChatGPT in data science: how AI-assisted conversational interfaces are revolutionizing the field. Big Data and Cogn Comput. 2023;7:62. doi: 10.3390/bdcc7020062. [DOI] [Google Scholar]
- 106.Wang R, Wang S, Duan N, Wang Q. From patient-controlled analgesia to artificial intelligence-assisted patient-controlled analgesia: practices and perspectives. Front Med (Lausanne) 2020;7:145. doi: 10.3389/fmed.2020.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simon D, Craig KD, Gosselin F, Belin P, Rainville P. Recognition and discrimination of prototypical dynamic expressions of pain and emotions. Pain. 2008;135:55–64. doi: 10.1016/j.pain.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 108.Williams A. Facial expression of pain: an evolutionary account. Behav Brain Sci. 2002;25:439–455. doi: 10.1017/S0140525X02000080. [DOI] [PubMed] [Google Scholar]
- 109.De Ruddere L, Tait R. Facing others in pain: why context matters. In: Vervoort T, Karos K, Trost Z, Prkachin K, editors. Social and interpersonal dynamics in pain. Cham: Springer; 2018. [Google Scholar]
- 110.Giravi HY, Biskupiak Z, Tyler L, Bulaj G. Adjunct digital interventions improve opioid-based pain management: impact of virtual reality and Mobile applications on patient-centered pharmacy care. Front Digit Health. 2022;4:884047. doi: 10.3389/fdgth.2022.884047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Magee M, McNeilage A, Avery N, Glare P, Ashton-James C. Mhealth interventions to support prescription opioid tapering in patients with chronic pain: qualitative study of patients’ perspectives. JMIR Form Res. 2021;5:e25969. doi: 10.2196/25969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wahl B, Cossy-Gantner A, Germann S, Schwalbe NR. Artificial intelligence (AI) and global health: how can AI contribute to health in resource-poor settings? BMJ Glob Health. 2018;3(4):e000798. doi: 10.1136/bmjgh-2018-000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pee LG, Pan S, Cui L. Artificial intelligence in healthcare robots: a social informatics study of knowledge embodiment. J Assoc Inf Sci Technol. 2019 doi: 10.1002/asi.24145. [DOI] [Google Scholar]
- 114.Dhieb N, Ghazzai H, Besbes H, Massoud YA. Secure AI-Driven architecture for automated insurance systems: fraud detection and risk measurement. IEEE Access. 2020;8:58546–58558. doi: 10.1109/ACCESS.2020.2983300. [DOI] [Google Scholar]
- 115.Zhou L. A rapid, accurate and machine-agnostic segmentation and quantification method for CT-Based COVID-19 diagnosis. IEEE Trans Med Imaging. 2020;39(8):2638–2652. doi: 10.1109/TMI.2020.3001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sqalli MT, Al-Thani D. AI-supported health coaching model for patients with chronic diseases. The 16th International Symposium on Wireless Communication Systems, 2019; (p. 452−6). 10.1109/ISWCS.2019.8877113
- 117.Esteva A, Robicquet A, Ramsundar B, Kuleshov V, DePristo M, Chou K, Dean J. A guide to deep learning in healthcare. Nat Med. 2019;25(1):24–29. doi: 10.1038/s41591-018-0316-z. [DOI] [PubMed] [Google Scholar]
- 118.Alotaibi S, Mehmood R, Katib I, Rana O, Albeshri A. Sehaa: a big data analytics tool for healthcare symptoms and diseases detection using Twitter, Apache Spark, and machine learning. Appl Sci. 2020;10(4):1398. doi: 10.3390/app10041398. [DOI] [Google Scholar]
- 119.Jaiman V, Urovi V. A consent model for block chain-based health data sharing platforms. IEEE Access. 2020;8:143734–143745. doi: 10.1109/ACCESS.2020.3014565. [DOI] [Google Scholar]
- 120.Samuel J, Kashyap R, Samuel Y, Pelaez A. Adaptive cognitive fit: Artificial intelligence augmented management of information facets and representations. Int J Inf Manage. 2022;65:102505. doi: 10.1016/j.ijinfomgt.102505. [DOI] [Google Scholar]
- 121.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290(18):2443–2454. doi: 10.1001/JAMA.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 122.Prkachin KM, Solomon PE. The structure, reliability and validity of pain expression: evidence from patients with shoulder pain. Pain. 2008;139(2):267–274. doi: 10.1016/j.pain.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 123.Al Darwish ZQ, Hamdi R, Fallatah S. Evaluation of pain assessment tools in patients receiving mechanical ventilation. AACN Adv Crit Care. 2016;27:162–172. doi: 10.4037/aacnacc2016287. [DOI] [PubMed] [Google Scholar]
- 124.Jiménez-Moreno C, Aristizábal-Nieto JK, Giraldo-Salazar OL. Classification of facial expression of post-surgical pain in children: evaluation of convolutional neural networks. Vis Electron. 2020;15:7–16. doi: 10.14483/issn.2248-4728. [DOI] [Google Scholar]
- 125.De la Torre J, Marin J, Ilarri S, Marin JJ. Applying machine learning for healthcare: a case study on cervical pain assessment with motion capture. Appl Sci. 2020;10(17):5942. doi: 10.3390/app10175942. [DOI] [Google Scholar]
- 126.Bartlett MS, Littlewort GC, Frank MG, Lee K. Automatic decoding of facial movements reveals deceptive pain expressions. Curr Biol. 2014;24(7):738–743. doi: 10.1016/j.cub.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Greve KW, Ord JS, Bianchini KJ, Curtis KL. Prevalence of malingering in patients with chronic pain referred for psychologic evaluation in a medico-legal context. Arch Phys Med Rehabil. 2009;90(7):1117–1126. doi: 10.1016/j.apmr.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 128.Jiang W, Majumder S, Kumar S, et al. A wearable tele-health system towards monitoring COVID-19 and chronic diseases. IEEE Rev Biomed Eng. 2022;15:61–84. doi: 10.1109/RBME.2021.3069815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.El-Tallawy SN, Perglozzi JV, Ahmed RS, et al. Pain management in the post-COVID era-an update: a narrative review. Pain Ther. 2023;12(2):423–448. doi: 10.1007/s40122-023-00486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ahmed I, Jeon G, Piccialli F. A Deep-Learning-based smart healthcare system for patient’s discomfort detection at the edge of Internet of Things. IEEE Internet Things J. 2021;8(13):10318–10326. doi: 10.1109/JIOT.2021.3052067. [DOI] [Google Scholar]
- 131.Lysaght T, Lim HY, Xafis V, Ngiam KY. AI-assisted decision-making in healthcare the application of an ethics framework for big data in health and research. Asian Bioethics Rev. 2019;11:299–314. doi: 10.1007/s41649-019-00096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990– 2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018; 392 (10159):1789–1858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed]
- 133.Xafis V, Schaefer GO, Labude MK, et al. An ethics framework for big data in health and research. Asian Bioethics Review. 2019;11:227–254. doi: 10.1007/s41649-019-00099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shaban-Nejad A, Michalowski M, Brownstein J, Buckeridge D. Guest editorial explainable AI: towards fairness, accountability, transparency and trust in healthcare. IEEE J Biomed Health Inform. 2021;25(7):2374–2375. doi: 10.1109/JBHI.2021.3088832. [DOI] [Google Scholar]
- 135.Balthazar P, Harri P, Prater A, Safdar NM. Protecting your patients’ interests in the era of big data, artificial intelligence, and predictive analytics. J Am Coll Radiol. 2018;15:580–586. doi: 10.1016/j.jacr.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 136.Martinez-Martin N. What are important ethical implications of using facial recognition technology in health care? AMA J Ethics. 2019;21(2):E180–E187. doi: 10.1001/amajethics.2019.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.World Health Organization. The WHO guidance on the ethics and governance of AI for health. WHO Guideline 28 June 2021; Available at: https://www.who.int/news/item/16-05-2023-who-calls-for-safe-and-ethical-ai-for-health
- 138.Chaddad A, Peng J, Xu J, Bouridane A. Survey of explainable AI techniques in healthcare. Sensors. 2023;23:634. doi: 10.3390/s23020634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nazar M, Alam MM, Yafi E, Su’ud MM. A systematic review of human-computer interaction and explainable artificial intelligence in healthcare with artificial intelligence techniques. IEEE Access. 2021;9:153316–153348. doi: 10.1109/ACCESS.2021.3127881. [DOI] [Google Scholar]
- 140.Prkachin KM, Hammal Z. Computer mediated automatic detection of pain-related behavior: prospect, progress, perils. Front Pain Res. 2021;2:788606. doi: 10.3389/fpain.2021.788606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nikolaev AV. Quantitative evaluation of an automated cone-based breast ultrasound scanner for MRI−3D US image fusion. IEEE Trans Med Imaging. 2021;40(4):1229–1239. doi: 10.1109/TMI.2021.3050525. [DOI] [PubMed] [Google Scholar]
- 142.Dirupo G, Garlasco P, Chappuis C, Sharvit G, Corradi-Dell’Acqua C. State-specific and supraordinal components of facial response to pain. IEEE Trans Affect Comput. 2022;13:793–804. doi: 10.1109/TAFFC.2020.2965105. [DOI] [Google Scholar]
- 143.Grouper H. Eisenberg E, Pud D. More insight on the role of personality traits and sensitivity to experimental pain. J Pain Res. 2021;14:1837–1844. doi: 10.2147/JPR.S309729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ferryman K. Rethinking the AI Chasm. Am J Bioeth. 2022;22(5):29–30. doi: 10.1080/15265161.2022.2055218. [DOI] [PubMed] [Google Scholar]
- 145.Cascella M, Monaco F, Nocerino D, et al. Bibliometric network analysis on rapid-onset opioids for breakthrough cancer pain treatment. J Pain Symptom Manage. 2022;63(6):1041–1050. doi: 10.1016/j.jpainsymman.2022.01.023. [DOI] [PubMed] [Google Scholar]
- 146.Esmaeilzadeh P. Use of AI-based tools for healthcare purposes: a survey study from consumers’ perspectives. BMC Med Inform Decis Mak. 2020;20:170. doi: 10.1186/s12911-020-01191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ahuja AS. The impact of artificial intelligence in medicine on the future role of the physician. Peer J. 2019;7:e7702. doi: 10.7717/peerj.7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tahereh R, Parisa P, Soheila K et al. Integrating artificial intelligence into telemedicine: revolutionizing healthcare delivery. (2023); Publisher: Kindle, ISBN: 979-8862996500. 10.5281/zenodo.8395812
- 149.Al-Kuwaiti A, Nazer K, Al-Reedy A, Al-Shehri S, Al-Muhanna A, Subbarayalu AV, Al Muhanna D, Al-Muhanna FA. A review of the role of artificial intelligence in healthcare. J Pers Med. 2023;13(6):951. doi: 10.3390/jpm13060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fuller A, Fan Z, Day C, Barlow C. Digital twin: enabling technologies, challenges and open research. IEEE Access. 2020;8:108952–108971. doi: 10.1109/ACCESS.2020.2998358. [DOI] [Google Scholar]
- 151.Voigt I, Inojosa H, Dillenseger A, Haase R, Akgün K, Ziemssen T. Digital twins for multiple sclerosis. Front Immunol. 2021;12:669811. doi: 10.3389/fimmu.2021.669811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gazerani P. Intelligent digital twins for personalized migraine care. J Pers Med. 2023;13(8):1255. doi: 10.3390/jpm13081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Poonsuph R. The design blueprint for a large-scale telehealth platform. Int J Telemed Appl. 2022;5:2022. doi: 10.1155/2022/8486508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Volkov I, Radchenko G, Tchernykh A. Digital twins, internet of things and mobile medicine: a review of current platforms to support smart healthcare. Program Comput Softw. 2021;47:578–590. doi: 10.1134/S0361768821080284. [DOI] [Google Scholar]
- 155.Ai Q, Liu Q, Meng W, Xie SQ. Introduction. In: Ai Q, Liu Q, Meng W, Xie SQ, editors. Advanced rehabilitative technology. Academic Press; 2018. pp. 1–10. [Google Scholar]
- 156.Karikari E, Koshechkin KA. Review on brain–computer interface technologies in healthcare. Biophys Rev. 2023;15(5):1351–1358. doi: 10.1007/s12551-023-01138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee MB, Kramer DR, Peng T, Barbaro MF, Liu CY, Kellis S, Lee B. Brain–computer interfaces in quadriplegic patients. Neurosurg Clin. 2019;30(2):275–281. doi: 10.1016/j.nec.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 158.Vassantachart AY, Yeo E, Chau B. Virtual and augmented reality-based treatments for phantom limb pain: a systematic review. Innov Clin Neurosci. 2022;19(10–12):48. [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang J, Li J, Huang Z, Huang D, Yu H, Li Z. Recent progress in wearable brain–computer interface (BCI) devices based on electroencephalogram (EEG) for medical applications: a review. Health Data Sci. 2023. [DOI] [PMC free article] [PubMed]
- 160.Mang J, Xu Z, Qi Y, Zhang T. Favoring the cognitive-motor process in the closed-loop of BCI mediated post-stroke motor function recovery: challenges and approaches. Front Neurorobotics. 2023;17. [DOI] [PMC free article] [PubMed]
- 161.Ma Y, Gong A, Nan W, Ding P, Wang F, Fu Y. Personalized brain–computer interface and its applications. J Personaliz Med. 2022;13(1):46. doi: 10.3390/jpm13010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.