Abstract
Two-dimensional (2D) AMX2 compounds are a family of mixed ionic and electronic conductors (where A is a monovalent metal ion, M is a trivalent metal, and X is a chalcogen) that offer a fascinating platform to explore intrinsic coupled ionic-electronic properties. However, the synthesis of 2D AMX2 compounds remains challenging due to their multielement characteristics and various by-products. Here, we report a separated-precursor-supply chemical vapor deposition strategy to manipulate the chemical reactions and evaporation of precursors, facilitating the successful fabrication of 20 types of 2D AMX2 flakes. Notably, a 10.4 nm-thick AgCrS2 flake shows superionic behavior at room temperature, with an ionic conductivity of 192.8 mS/cm. Room temperature ferroelectricity and reconfigurable positive/negative photovoltaic currents have been observed in CuScS2 flakes. This study not only provides an effective approach for the synthesis of multielement 2D materials with unique properties, but also lays the foundation for the exploration of 2D AMX2 compounds in electronic, optoelectronic, and neuromorphic devices.
Subject terms: Two-dimensional materials, Nanoscale materials
2D AMX2 compounds (where A is a monovalent metal ion, M is a trivalent metal, and X is a chalcogen) are a family of materials with coupled ionic-electronic properties. Here, the authors report a chemical vapor deposition strategy to fabricate 20 types of 2D AMX2 flakes, exhibiting superionic conductivity or room temperature ferroelectricity.
Introduction
The coupled ionic-electronic effects in two-dimensional (2D) materials have attracted tremendous interest in recent years as they endow the materials with diverse responses to external stimuli, further facilitating the development of next-generation electronic, optoelectronic, and neuromorphic devices1–5. For example, the hybrid of the ionic gate with 2D materials enables the modulation of the phase transition6–8 and band structures9–11 in 2D materials due to the strong gate control ability of the ionic gate. Furthermore, the extrinsic ionic states can be introduced into 2D materials through the pre-treatment, such as the intercalation of external ions4,12 and plasma treatment5,13. Subsequently, employing an electric field to control the migration of ions allows for emulating the function of biological neurons and synapses, showing the vast potential in the field of neuromorphic computing4,5,13. It should be noted that these additional modification techniques require complex processes and result in interface states4,11, thereby impeding the exploration of novel physical and chemical properties, as well as hindering the development of high-density integrated devices. The intrinsic ionic-electronic coupled 2D materials are expected to solve the above problems.
AMX2 is a family of mixed ionic-electronic conductors (where A is a monovalent metal ion, M is a trivalent transition or main group metal, and X is a chalcogen). The monovalent metal ions Cu+ and Ag+ have 3d-orbital electrons that exhibit second-order Jahn-Teller effect14, and normally possess a low ion migration barrier15. Thus, introducing the superionic conductivity16–18, multiferroics19,20, and magnetism21 properties within the AMX2. Meanwhile, the multielement characteristic and various atomic structures give the AMX2 rich band structures19,22,23, making the AMX2 compounds excellent systems for studying intrinsic coupled ionic-electronic properties. While a few demonstrations of the synthesis of 2D AMX2 have been reported16,24, the fabrication of most of these compounds remains elusive, hindering their exploration and application. Chemical vapor deposition (CVD) has been widely used in the synthesis of 2D materials25–34. For the synthesis of multielement compounds like AMX2, three kinds of precursors are required. In the common CVD process, the metal and chalcogen precursors transport along the same path, leading to uncontrollable pre-reactions and an unstable supply of precursors, thereby hindering the controllable synthesis of 2D AMX2.
In this work, we demonstrate a separated-precursor-supply strategy in which the suppressed by-reactions and controllable supply of precursors ensure the general synthesis of 20 distinct 2D AMX2, 18 types of which have never been reported. Interestingly, the as-grown AMX2 flakes exhibit unique electronic and ionic properties. A 10.4 nm AgCrS2 flake shows superionic conductor characteristics at room temperature with an ionic conductivity of up to 192.8 mS/cm. The as-grown CuScS2 flakes exhibit semiconductor ferroelectric properties, and show a Curie temperature reaching ~370 K. Notably, the reconfigurable positive/negative photovoltaic current can be observed in CuScS2 devices due to the adjustable ion migration drived by the external electric field. This work not only provides an effective strategy for synthesizing multielement 2D materials but also opens up opportunities for studying the properties and potential applications of a wide variety of 2D AMX2.
Results and discussion
General growth and characterization of AMX2
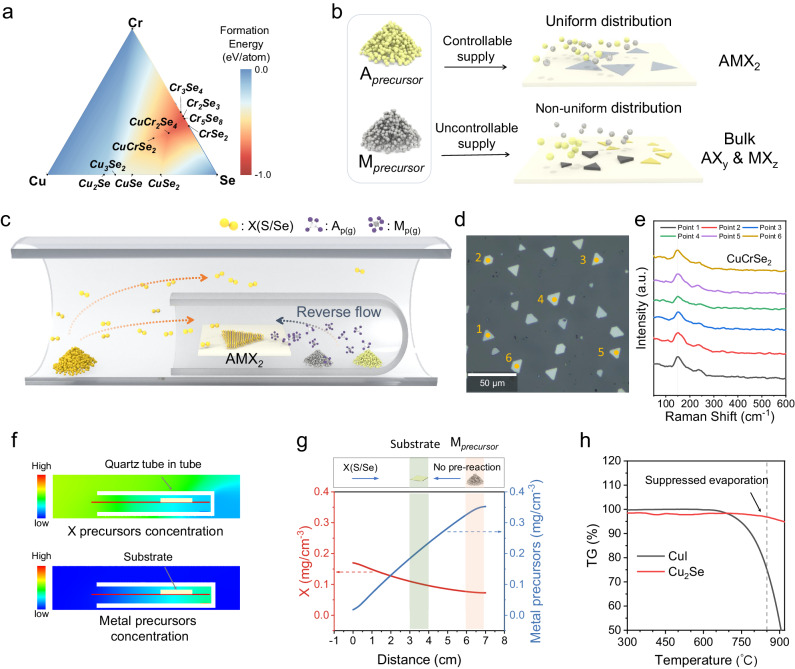
We first discuss the difficulties of controllable synthesis 2D AMX2. For multielement compounds like AMX2, during the CVD process, there are many possible reactions between metal and chalcogen precursors (Supplementary Fig. 1). And the formation energy of most binary products is less than that of AMX2 (Fig. 1a)35. Even if the AMX2 is more favorable thermodynamically, it is hard to control the reactions of precursors during transportation due to the premixing of the vaporized precursors in common CVD, which promotes the non-uniform distribution of precursors and results in undesired products (Fig. 1b). Taking the synthesis of CuCrSe2 as an example since other AMX2 compounds have similar troubles. In the common CVD process, the Se vapor will pass by the Cu and Cr precursors before reaching the substrate, resulting in uncontrollable pre-reactions (Supplementary Fig. 2). Due to the consistent exposure of the metal source to the Se vapor, the metal precursor powders undergo excessive selenization (Supplementary Fig. 3a), which will suppress the vaporization and destabilize the precursor supply, then giving rise to a large number of by-products such as CuxSe on the substrate (Supplementary Fig. 3b, c), hindering the controlled synthesis of AMX2.
Fig. 1. Synthesis mechanism of chemical vapor deposition (CVD) growth AMX2.
a The formation energy of CuCrSe2 phase diagram35. b The kinetic growth process is influenced by the supply of metal precursors. A and M present the two kinds of metal elements of AMX2 compounds, and X represents the chalcogen element. The x and y in the AXy and MXz demonstrate the possible stoichiometric ratio of the binary by-products in the (a) (such as CuSe, Cu2Se, etc.). c Schematic image of the CVD setup. The orange and blue dash arrows represent the transportation paths of the vapored chalcogen precursor and metal precursors (Ap(g) and Mp(g)), respectively. d The large area optical image of the as-synthesized CuCrSe2 nanosheets. e The Raman spectra of the flakes in the (d). The vertical dash line located at 150 cm−1 demonstrates the consistent Raman peaks of the as-synthesized CuCrSe2 nanosheets. f The computational fluid dynamics (CFD) simulated distribution of X (S/Se) and metal precursors concentration. g The CFD simulated variation curve of precursor concentration along the red line in the (f). The green and pink shaded areas schematically represent the position of the substrate and metal precursors, respectively. h Thermogravimetric analysis (TGA) of CuI and Cu2Se powders. The black and red curves correspond to the weight-loss curves of CuI and Cu2Se, respectively. The vertical dash line located at 850°C demonstrates that the evaporation of excessively selenated metal precursors will be significantly suppressed.
To achieve the controllable synthesis of 2D AMX2 compounds, it is imperative to suppress undesired by-reactions. We have approached this challenge from a kinetic perspective. Specifically, we report a separated-precursor-supply strategy to suppress the by-reactions during mass transportation. The schematic representation of the CVD setup can be found in Fig. 1c and Supplementary Fig. 4a. First, stable source feeding of chalcogen is important. Here, we placed the resolidified chalcogen source, which is believed to realize stable source feeding and further reduce chalcogen vacancy forming in the CVD process36, at the upstream. More importantly, we should ensure the temporal and spatial uniform supply of two metal precursors to support the synthesis of 2D AMX2, which is much more difficult than the synthesis of binary compounds. We placed two kinds of metal precursors at the bottom of a one-side-sealed quartz tube, then the small quartz tube was placed downstream of the furnace tube (see Methods for more details). In this system, the transport process of metal precursors is protected by the small quartz tube and is separated from the transport process of chalcogen vapor (Supplementary Fig. 4b). Based on this method, we realized the uniform synthesis of the 2D AMX2 materials (Fig. 1d, e).
Computational fluid dynamics (CFD) simulations predict that the gas flow inside the small quartz tube is primarily directed towards the open side, opposing the flow direction of carrier gas, and exhibits significantly lower velocity compared to the external gas flow outside the small tube (Supplementary Fig. 6). This results in a reverse mass flow opposite to the Se precursor’s transport direction (Supplementary Figs. 6 and 7). As a consequence, the concentration of metal precursor vapors is lower at the tube’s open side and higher at the tube’s sealed side, and the Se vapors’ concentration distribution is opposite to that of the metal precursor vapor (Fig. 1f, g). The relatively high concentration of the metal precursor vapor in the small tube prevents excessive selenization of the metal precursor powder, ensuring the stable vaporization of the metal precursor during the whole CVD process. In contrast, without the confinement of the small quartz tube, the vapor of the metal precursor and Se precursor meet before reaching the substrate (Supplementary Fig. 8) which will facilitate the occurrence of by-reactions. And the concentration of chalcogen precursor is much higher than the metal precursors. This will result in excessive selenization or sulfurization, making the supply of metal precursor unstable (Fig. 1h). However, when the metal precursor is confined within the small quartz tube, its concentration is one order of magnitude higher compared to the situation without spatial confinement (Supplementary Fig. 9). The ample metal precursor supply, which matches the supply of chalcogen precursor, greatly suppress phase separation.
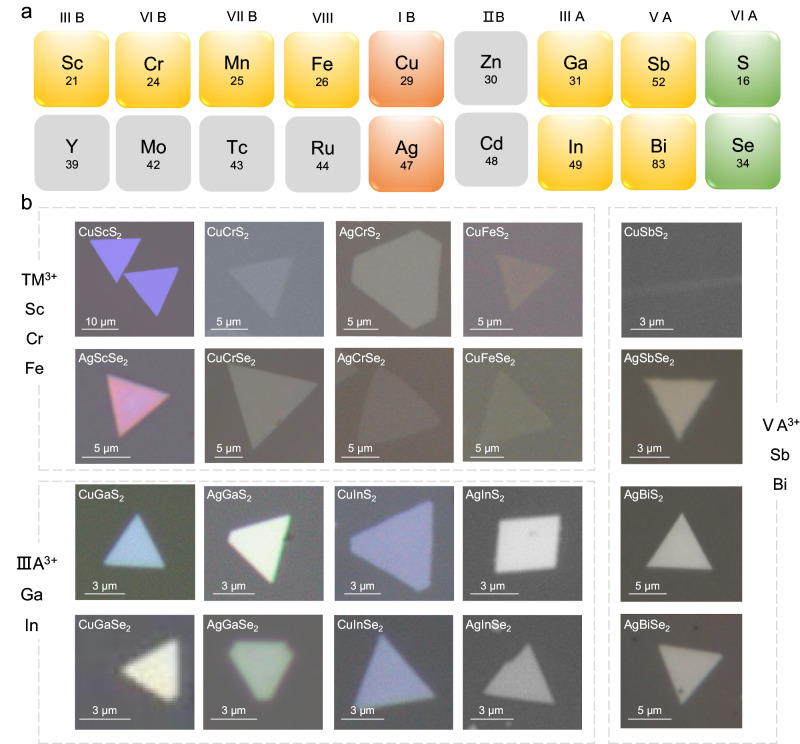
Taking the synthesis of 2D CuCrSe2 as an example again. Different from the common CVD, the separated-precursor-supply strategy protected the metal precursors from excessive selenization and suppressed the pre-reactions between the Cu/Cr precursors and Se precursors (Supplementary Fig. 10), thereby ensuring a stable supply of metal precursors and suppressing undesired by-products. Through this approach, 2D CuCrSe2 with consistent phase and uniform morphology can be obtained (Fig. 1d, e). Figure 2 shows a summary of optical images of the 20 kinds of 2D AMX2 materials prepared using this method. The corresponding synthesis conditions are described in the Methods, more details are summarized in Supplementary Table 1 and Supplementary Methods. To our best knowledge, 18 of them have not been previously synthesized using CVD or mechanical exfoliation methods (Supplementary Table 2). The synthesized AMX2 compounds are mainly selenides and sulfides and contain 9 metal elements including two monovalent metal ions (Cu+, Ag+); three transition metals (Sc, Cr, Fe); and four main group metals (Ga, In, Sb, Bi). To show our uniform growth, the larger area optical images with more flakes are shown in Supplementary Fig. 11. Most of the synthesized 2D AMX2 compounds exhibit triangular or hexagonal shapes, and a small fraction shows rhombic or nanoribbon morphologies. The thickness of most samples can be reduced to below 10 nm, and some can even reach few unit-cell thickness, such as CuCrS2 (2.56 nm), AgCrSe2 (1.86 nm), CuFeSe2 (1.9 nm), and CuSbS2 (0.79 nm) (Supplementary Fig. 12), which demonstrates the effectivity and generality of our growth methods.
Fig. 2. Optical microscopy images of the 20 kinds of as-synthesized 2D AMX2 compounds.
a Summary of 2D AMX2 compounds that can be synthesized using this method. Orange, element A; yellow, element M; green, element X. b Optical images of the as-synthesized 2D AMX2 nanosheets.
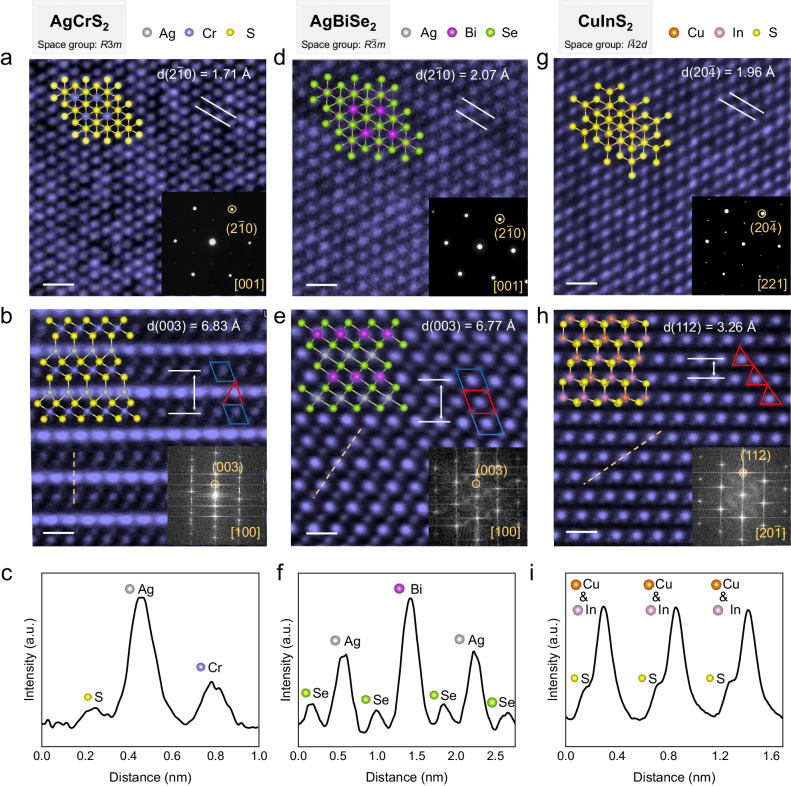
To elucidate the structural features of AMX2 compounds, we conducted high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) characterization on three representative materials, namely AgCrS2 with R3m space group, AgBiSe2 with Rm space group, and CuInS2 with I2d space group. For the as-grown AgCrS2. The High-resolution transmission electron microscopy (HRTEM) image along the [001] crystal direction reveals its hexagonal atomic arrangement, with a measured lattice spacing of d(20) = 1.71 Å (Fig. 3a). The cross-sectional HAADF-STEM image along the [100] crystal direction is shown in Fig. 3b, with a measured lattice spacing of d(003) = 6.83 Å. Based on the layered characteristics, the structure can be understood as alternating stacking of CrS2 layers and Ag+ ion layers along the c-axis. The brightness variation of the dashed line in Fig. 3b is depicted in Fig. 3c, where Ag exhibits the highest brightness, followed by Cr with intermediate brightness, and S appears the darkest. The 3d orbital electrons of Cr3+ in the material hybridize with the p orbital electrons of S, forming [CrS6] octahedral coordination structure (shown at the blue quadrilateral position in Fig. 3b). The [CrS6] octahedra are edge-connected to form the CrS2 layer (Supplementary Fig. 13). Meanwhile, Ag+ ions orderly occupy the tetrahedral sites between the CrS2 layers (shown at the red triangular position in Fig. 3b). This ordered tetrahedral occupancy results in the breaking of inversion symmetry, leading the material to exhibit a pronounced optical second harmonic generation (SHG) response (Supplementary Fig. 14g). Similarly, AgBiSe2 also exhibits typical layered structure characteristics, with Ag+ confined between the BiSe2 layers. The HRTEM image along the [001] crystal direction also demonstrates the characteristic hexagonal atomic arrangement, with a measured lattice spacing of d(20) = 2.07 Å (Fig. 3d). However, different with the AgCrS2, the Ag+ ions in the AgBiSe2 occupy the octahedral sites between the BiSe2 layers (shown at the red quadrilateral in Fig. 3e), with a measured lattice spacing of d(003) = 6.77 Å. The structure of CuInS2 is composed of [CuS4] and [InS4] tetrahedra. The exposed surface of the sample has a hexagonal atomic arrangement, namely the (112) plane (Fig. 3g). From HRTEM images and selected area electron diffraction (SAED) patterns, the annotated lattice spacings are d(20) = 1.96 Å and d(112) = 3.26 Å, respectively. The measured crystal plane spacings for these three materials are consistent with previous reports. Additionally, we provided Raman spectra, photoluminescence spectra, and optical SHG response for each sample (Supplementary Figs. 14–18), and we conducted HRTEM and energy-dispersive spectroscopy (EDS) to characterize the 20 kinds of as-grown AMX2 compounds (Supplementary Figs. 19–38). The synthesized AMX2 compounds exhibit good agreement with the expected phases and show high crystalline quality.
Fig. 3. Structural analysis of selected three as-synthesized 2D AMX2 compounds.
a, d, g High-resolution transmission electron microscopy (HRTEM) images of AgCrS2, AgBiSe2, and CuInS2 along the direction out of the plane of the as-grown nanosheets, scale bar: 0.5 nm. The insets display the top view of atomic structure models and selected area electron diffraction (SAED) patterns. b, e, h Cross-sectional high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images of AgCrS2, AgBiSe2, and CuInS2, scale bar: 0.5 nm. The insets display the side view of the atomic structure models and the fast Fourier transform (FFT) patterns. The blue and red polygons represent the octahedral and tetrahedron sites. c, f, i Intensity profiles of the orange dash lines in (b), (e), and (h).
In summary, the as-grown AMX2 compounds typically possess a quasi-2D layered structure, where monovalent metal ions Cu+ and Ag+, known for their strong migration characteristics17,18, are confined within the interlayer space of MX2 (Supplementary Fig. 13). This means that most AMX2 materials possess inherent migratable ions, and have natural 2D ion migration pathways. As a result, AMX2 materials exhibit intrinsic ion characteristics18,23,37. Additionally, the 3d orbital electrons of the transition metal in the MX2 layers may introduce ferromagnetic or antiferromagnetic characteristics to the materials19,20,38 (Supplementary Table 3). In addition to their layered structure characteristics, 14 kinds of the as-grown 2D AMX2 possess features with broken inversion symmetry (Supplementary Table 4), endowing them with optical SHG properties, as well as piezoelectric and ferroelectric properties24,39.
The ionic and electronic properties of AMX2 compounds
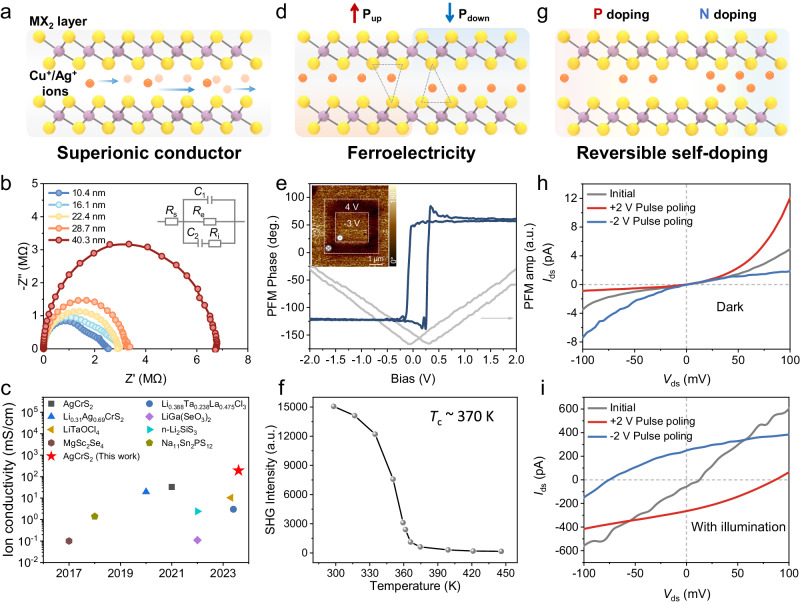
Building upon the structural attributes of AMX2, Cu+/Ag+ ions can undergo hopping between the tetrahedral or octahedral sites within the MX2 interlayer space when the temperature is higher than a certain point18,37. Simultaneously, under the influence of an external electric field, ions can exhibit directed migration, thereby manifesting superionic conductor features16,40 (Fig. 4a). We commence our investigation by delving into the ionic migration properties of AMX2, using AgCrS2 as an illustrative example, we fabricated two-terminal electrode devices and employed Au as a blocking electrode for testing ion conductivity. The impedance spectra of samples with different thicknesses (Fig. 4b) can all be fitted with two semicircles. These curves exhibit characteristic mixed ion-electron conductivity features16,23. According to the equivalent circuit of the mixed ionic-electronic conductor model (illustrated in the inset of Fig. 4b), ionic conductivity can be obtained by fitting the electrochemical impedance curves, and the detail of the fitting process is described in Methods and Supplementary Fig. 39. In a 10.4 nm AgCrS2 nanosheet, we measured an ionic conductivity of 192.8 mS/cm. Interestingly, we observed that the ionic conductivity increases as the sample thickness decreases (Supplementary Fig. 40). This trend is consistent with the previous report on AgCrS2 samples obtained via electrochemical exfoliation16. However, our samples exhibit a higher ionic conductivity, which could be attributed to the superior crystalline quality resulting from our synthesis method. Compared to other ion conductors, the ionic conductivity of the AgCrS2 nanosheets synthesized by us remains at a relatively high level (Fig. 4c)16,40–46.
Fig. 4. The ionic and electronic properties of two representative as-synthesized 2D AMX2 compounds.
a, d, g Schematic diagrams of the superionic conductor (a), ferroelectricity (d), and reversible self-doping (g) properties that arise from the ionic-electronic coupling effects. The blue arrows in (a) present the directed long-distance migration of the Cu+/Ag+ ions within the interlayer. The dashed triangles in (d) show the two enantiomeric tetrahedral sites of Cu+/Ag+ ions, corresponding to the up and down ferroelectric polarization states of the materials. b The impedance spectroscopic measurement for AgCrS2 nanosheets with different thicknesses. The inset shows the equivalent circuit, where Rs, Re, and Ri correspond to the contact resistance, electronic resistance, and ionic resistance, respectively, and C1 and C2 are the constant phase elements. The detailed calculation process of ionic conductivity is shown in Supplementary Fig. 39. c The comparison of ionic conductivity with other reported superionic conductors16,40–46. e Piezoresponse force microscopy (PFM) phase and amplitude hysteresis loop of CuScS2 nanosheet, the inset is the ferroelectric domains (white dashed square area) after forward and reverse DC bias polarization. The out-of-plane arrow symbols represent the Pup and Pdown states of the nanosheet after polarization. f Temperature-dependent optical second harmonic generation (SHG) measurement of a CuScS2 nanosheet, demonstrating a ferroelectric Curie temperature (Tc) of ~ 370 K. h, i Memristor behavior (h) and switchable photovoltaic behavior under illumination (λ = 532 nm, 256.6 mW/cm2) (i) after positive/negative pulse polarization of the CuScS2-based device.
Cu+/Ag+ ions can not only undergo long-distance migration but also experience local displacements, giving rise to ferroelectricity, specifically ion displacement-induced ferroelectricity47. By applying a vertical electric field, we can drive ion displacements between the two enantiomeric tetrahedral sites within the MX2 interlayer space, resulting in spontaneous polarization reversal and exhibiting ferroelectric properties (Fig. 4d). Taking CuScS2 as an example. The SHG mapping shows the uniform non-centrosymmetric crystal structure and the single domain characteristics of the as-synthesized CuScS2 flake (Supplementary Fig. 41). Then, we employed piezoresponse force microscopy (PFM) testing methods to investigate the material’s room-temperature ferroelectric properties. The as-grown CuScS2 flakes exhibited distinct ferroelectric phase hysteresis loops and typical amplitude butterfly curves (Fig. 4e). Simultaneously, we performed domain read-write operations on the flakes. The PFM phase image shows two stable opposite polarization domain regions (the inset of Fig. 4e), validating the out-of-plane ferroelectric properties of CuScS2 at room temperature. We also tried to reveal the in-plane (IP) polarization of the CuScS2 flakes. However, there is no typical IP PFM phase and amplitude hysteresis loop (as Supplementary Fig. 42 shows), suggesting no IP polarization in the as-synthesized CuScS2 flakes. Furthermore, to reveal the temperature stability of the ionic displacement-type ferroelectricity in CuScS2, which is critical to the applications, we employed high-temperature SHG measurements to characterize its ferroelectric Curie temperature (TC). As the temperature increases to above 370 K, the SHG signal of CuScS2 nearly quenches (Fig. 4f and Supplementary Fig. 43a), indicating the disruption of the ordered occupancy of Cu+ ions in the interlayer tetrahedral positions (Supplementary Fig. 43b). This implies that the TC of CuScS2 is approximately 370 K, demonstrating significant potential for extensive applications in the field of ferroelectricity.
The movement of ions within the material often results in concentration gradients, leading to different doping effects48. By employing electrical pulses to control the migration of ions within the material, reversible self-doping characteristics can be achieved (Fig. 4g), consequently leading to interesting electrical and optoelectronic properties, which is exemplified by 2D CuScS2. First-principles calculations predict that the migration barrier for a Cu+ ion to move through the tetrahedral-octahedral-tetrahedral path within the CuScS2 is 0.24 eV (Supplementary Fig. 44). Such a low migration energy barrier is comparable to that for intercalated Li+ ions in transition metal dichalcogenides (TMDs)49, indicating that a relatively small external electric field is sufficient to drive the migration of Cu+ ions. Applying electric pulses for polarization is an effective approach for achieving controlled migration of interlayer ions as we can modulate the pulse width and amplitude precisely50. Firstly, we investigated the ionic-electronic coupled properties of CuScS2-based devices under dark conditions using triangular electric pulses (Supplementary Fig. 45a). As shown in Fig. 4h, the initial state I-V curve of the device is symmetric in the positive and negative voltage ranges. After poling the device with a forward bias pulse, the I-V curve exhibits characteristic diode-like behavior, indicating the generation of a potential barrier within the device under the influence of the electric pulse. Furthermore, upon applying a reverse bias pulse, the rectification direction of the device is reversed, indicating that the direction of internal potential barriers within the material can be modulated by the electric pulse. This reconfigurable potential barrier allows for continuous modulation of the device resistance through electric pulses, enabling memristive functionality (Supplementary Fig. 45b–d). Under illumination, compared to the initial state, the device exhibits a noticeable photovoltaic response after poling by 2 V/0.5 s electric pulse. The device demonstrates a photovoltaic short-circuit current (Isc) of ~270 pA when the source-drain bias voltage (Vds) is zero and shows a distinct open-circuit voltage (Voc) of ~85 mV. Similar to the dark situation, the photovoltaic response direction also reverses after the device undergoes reverse poling electric pulses (Fig. 4i). This reconfigurable photovoltaic response exhibits great stability during I-t testing (Supplementary Fig. 46). To reveal the mechanism of this reconfigurable photovoltaic response, we firstly analyzed the possible bulk photovoltaic effect (BPVE) in the ferroelectric CuScS2. Based on the single-domain characteristic of as-synthesized CuScS2 (Supplementary Fig. 41), the BPVE would induce obvious Isc at the initial-state device. However, the Isc of the initial-state device is ignorable (Fig. 4i), demonstrating that the BPVE is negligible here. Moreover, the undetectable IP ferroelectricity of the as-synthesized CuScS2 also proves this point. Then, we consider that the long-distance migration of the Cu+ ions could induce reversible self-doping, which also can give rise to the reconfigurable photovoltaic response. Thus, we conducted cross-sectional EDS scanning tests on the polarized devices and observed that the distribution of Sc and S elements in the material remains uniformly distributed. However, the content of Cu elements beneath the electrode is significantly higher than in the channel region (Supplementary Fig. 47). This confirms the migration of Cu+ ions after poling by the electric pulse. Moreover, the first-principles calculations indicate that the local absence or accumulation of Cu+ ions resulting from ion migration will introduce significant p-type or n-type doping in the material (Supplementary Fig. 48), which is similar to the doping effect of point defects5. This coupling effect between ion migration and charge doping induces the emergence of the modulated potential barrier within 2D CuScS2 and finally gives rise to the memristive behavior and reconfigurable photovoltaic response, which is crucial to the logic and neuromorphic devices5,51.
In summary, we have demonstrated a separated-precursor-supply CVD method to control the reactions and vaporization of precursors. 20 kinds of 2D AMX2 compounds have been synthesized successfully showing the practicality of our approach. Detailed structural analysis and comprehensive characterization have revealed the high crystalline quality of the prepared AMX2 materials. Notably, the as-grown 2D AMX2 flakes show intriguing ionic and electronic properties. A high ionic conductivity of 192.8 mS/cm can be observed in a 10.4 nm AgCrS2 flake at room temperature. The synthesized 2D CuScS2 flakes show ion displacement-induced ferroelectricity at room temperature. Meanwhile, the reconfigurable photovoltaic response based on the coupling of ions migration and charge doping also can be observed in CuScS2. The achievement of generally synthesizing 2D AMX2 compounds offers new insights into the vapor-phase synthesis of multielement 2D materials and provides an excellent material choice for exploration in electronics, optoelectronics, and neuromorphics.
Methods
CVD growth
2D AMX2 compounds were synthesized by separated-precursor-supply CVD. Ultrahigh purity Ar (purity 99.999%) and H2 (purity 99.999%) were used as the carrier gases. All the raw precursors were bought from Alfa Aesar with a purity higher than 99%. Freshly exfoliated mica was chosen as the growth substrate. A single temperature zone tube furnace (diameter, one inch) was used as the reaction instrument with a heating zone length of ~30 cm (Supplementary Fig. 5). A porcelain boat containing chalcogen precursor was placed upstream and heated to 150°C for sulfur (300°C for selenium). Two metal precursors were placed at the bottom of a one-side sealed small quartz tube with a length of 7 cm and a diameter of 10 mm. The growth substrate was placed in the small quartz tube too, 0.5-4 cm away from the metal precursors. Then, the small quartz tube was put downstream of the tube furnace and followed by the heating process. The heating rate is 50°C/min or 30°C/min for different AMX2, and the reactions were carried out at 1 atm pressure. The schematic image of the CVD setup can be seen in Fig. 1c and Supplementary Fig 4a. The detailed growth parameters and descriptions are shown in Supplementary Table 1 and Supplementary Methods. The detailed characterizations of the morphology, phase, and atomic structures of as-synthesized ultrathin 2D AMX2 are shown in Supplementary Figs. 11, 12, 14–38.
CFD simulations
To reveal the mass flow during the experiment, we did the numerical finite element simulation. During the modeling, we followed the real experiment setup of the tube furnace, the details can be seen in Supplementary Fig. 5. The transient model is used to simulate the real CVD process. The gravity and convection heat transfer were considered. Argon and air are considered ideal gases. The shear stress transfer (SST) model is used here. It is a low Reynolds number and comprehensive turbulence model, which can give more accurate near-wall results52. Firstly, we conduct a steady analysis of the system. Similar to the real condition of the heating process of the furnace, we assume the system is stable when the temperature of the monitor point reaches the goal point and does not change again. Based on this result, the transient analysis was conducted to reveal the mass transportation process of two precursors.
TG-DSC
TG-DSC testing was conducted using a DIAMOND TG/DTA thermal analyzer. Approximately 5 mg of the sample was added to an alumina crucible and heated from 20 °C to 900 °C at a rate of 50 K per minute in a pure argon atmosphere.
Characterizations
The morphologies of the as-grown AMX2 nanosheets were characterized by an optical microscope (BX51, OLYMPUS) and atomic force microscope (Dimension icon, Bruker). Raman, photoluminescence, and SHG spectra were obtained by a confocal Raman system (Alpha 300 R, WITec) equipped with a 532 nm CW laser and a high-temperature test chamber (TS1000EV, Linkam). Femtosecond laser (Verdi, Coherent) was applied as the excitation source of SHG measurement. For the cross-sectional HAADF-STEM and EDS measurements, the samples were prepared by focus ion beam (Helios NanoLab G3 UC, FEI). The atomic resolution HAADF-STEM images were obtained by a double CS-corrected transmission electron microscopy (Titan Themis G2 60-300, FEI). For the TEM measurements, the samples were prepared with a poly (methyl methacrylate) (PMMA) assisted transfer method. TEM, SAED, and EDS were performed on an FEI Tecnai G2 F30 instrument.
Impedance spectra measurements
AgCrS2 nanosheets were transferred to the silica substrate. Then, the Au/AgCrS2/Au devices were fabricated by electron-beam lithography (EBL, FEI Quanta 650 SEM & Raith Elphy Plus) and thermal evaporation coating (Angstrom Engineering, Nexdep). The ionic conductivity of AgCrS2 nanosheets is obtained by fitting the electrochemical impedance spectra (Autolab PGSTAT 302 N) of the Au/AgCrS2/Ag devices at room temperature. The testing frequency range is 1 Hz to 1 MHz. To avoid electromagnetic interference the whole process was operated in a Faraday cage.
PFM measurements
The PFM measurements were conducted on the AFM platform (Dimension icon, Bruker). CuScS2 nanosheets were transferred to the silica substrate that was covered with gold. A DC voltage was applied to the conductive tip coated with Pt/Ir to reverse the ferroelectric domain of the sample.
Device fabricating and electrical measurements
All the devices were transferred on the silicon substrate with a 300-nm-thick oxide film using a PMMA-assisted method. The Bi/Au was chosen to be the contact electrode. The electrical measurements were carried out with a semiconductor characterization system (4200SCS, Keithley) and a cryogenic probe station (CRX-6.5, Lake Shore).
Theory calculations
The present first principle DFT calculations are performed by Vienna Ab initio Simulation Package (VASP)53 with the projector augmented wave (PAW) method54. The exchange-functional is treated using the generalized gradient approximation (GGA) of Perdew-Burke-Ernzerhof (PBE)55 functional. The energy cutoff for the plane wave basis expansion was set to 450 eV and the force on each atom less than 0.02 eV/Å was set for the convergence criterion of geometry relaxation. The Brillouin zone integration is performed using 5 × 5 × 5 and 9 × 9 × 9 k-point sampling for structure optimization and electronic structure calculation, respectively. The self-consistent calculations apply a convergence energy threshold of 10−5 eV. Transition state searching was performed using the climbing-image nudged elastic band (CI-NEB) method56.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52322210, 22375069, 52172144, and U21A2069), the National Key Research and Development Program of China (2021YFA1200500), and the Innovation Project of Optics Valley Laboratory (OVL2023PY007). Here, the authors also thank the technical support from the Analytical and Testing Center at Huazhong University of Science and Technology.
Author contributions
X.Z., T.Z., and X.X. conceived and designed the experiments. X.X. and Y.C. synthesized the materials. X.X., P.L., Y.C., and D.L. performed the AFM, Raman, and SHG characterizations of the samples. X.X. and H.L. performed the HRTEM and HAADF-STEM characterizations and worked on the analysis of the results. X.X. performed the electrochemical impedance measurements. X.X., P.L., Y.C., H.W., and X.S. performed device fabrication and measurement. X.X. and Z.L. performed the PFM measurements. X.X. and Y.C. wrote the paper with inputs from T.Z., X.Z., and J.W. All authors participated in discussions and approved the manuscript.
Peer review
Peer review information
Nature Communications thanks Xidong Duan, Wei-jin Hu and Jing Peng for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiang Xu, Yunxin Chen.
Contributor Information
Xing Zhou, Email: zhoux0903@hust.edu.cn.
Tianyou Zhai, Email: zhaity@hust.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-48690-7.
References
- 1.Sangwan VK, Hersam MC. Neuromorphic nanoelectronic materials. Nat. Nanotechnol. 2020;15:517–528. doi: 10.1038/s41565-020-0647-z. [DOI] [PubMed] [Google Scholar]
- 2.Fuller EJ, et al. Parallel programming of an ionic floating-gate memory array for scalable neuromorphic computing. Science. 2019;364:6. doi: 10.1126/science.aaw5581. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, et al. Two-dimensional materials for next-generation computing technologies. Nat. Nanotechnol. 2020;15:545–557. doi: 10.1038/s41565-020-0724-3. [DOI] [PubMed] [Google Scholar]
- 4.Zhu X, Li D, Liang X, Lu WD. Ionic modulation and ionic coupling effects in MoS2 devices for neuromorphic computing. Nat. Mater. 2019;18:141–148. doi: 10.1038/s41563-018-0248-5. [DOI] [PubMed] [Google Scholar]
- 5.Li T, et al. Reconfigurable, non-volatile neuromorphic photovoltaics. Nat. Nanotechnol. 2023;18:1303–1310. doi: 10.1038/s41565-023-01446-8. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Li D, Wu C-L, Hwang HY, Cui Y. Electrostatic gating and intercalation in 2D materials. Nat. Rev. Mater. 2022;8:41–53. doi: 10.1038/s41578-022-00473-6. [DOI] [Google Scholar]
- 7.Li LJ, et al. Controlling many-body states by the electric-field effect in a two-dimensional material. Nature. 2016;529:185–189. doi: 10.1038/nature16175. [DOI] [PubMed] [Google Scholar]
- 8.Wu C-L, et al. Gate-induced metal–insulator transition in MoS2 by solid superionic conductor LaF3. Nano Lett. 2018;18:2387–2392. doi: 10.1021/acs.nanolett.7b05377. [DOI] [PubMed] [Google Scholar]
- 9.Alam MH, et al. Lithium-ion electrolytic substrates for sub-1V high-performance transition metal dichalcogenide transistors and amplifiers. Nat. Commun. 2020;11:3203. doi: 10.1038/s41467-020-17006-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domaretskiy D, et al. Quenching the bandgap of two-dimensional semiconductors with a perpendicular electric field. Nat. Nanotechnol. 2022;17:1078–1083. doi: 10.1038/s41565-022-01183-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee S-J, et al. Programmable devices based on reversible solid-state doping of two-dimensional semiconductors with superionic silver iodide. Nat. Electron. 2020;3:630–637. doi: 10.1038/s41928-020-00472-x. [DOI] [Google Scholar]
- 12.Edwards B, et al. Giant valley-Zeeman coupling in the surface layer of an intercalated transition metal dichalcogenide. Nat. Mater. 2023;22:459–465. doi: 10.1038/s41563-022-01459-z. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H-T, et al. Reconfigurable perovskite nickelate electronics for artificial intelligence. Science. 2022;375:533–539. doi: 10.1126/science.abj7943. [DOI] [PubMed] [Google Scholar]
- 14.Burdett JK, Eisenstein O. From three- to four-coordination in copper(I) and silver(I) Inorg. Chem. 1992;31:1758–1762. doi: 10.1021/ic00036a007. [DOI] [Google Scholar]
- 15.Ma J, Wei S-H. Origin of novel diffusions of Cu and Ag in semiconductors: the case of CdTe. Phys. Rev. Lett. 2013;110:235901. doi: 10.1103/PhysRevLett.110.235901. [DOI] [PubMed] [Google Scholar]
- 16.Peng J, et al. Stoichiometric two-dimensional non-van der Waals AgCrS2 with superionic behaviour at room temperature. Nat. Chem. 2021;13:1235–1240. doi: 10.1038/s41557-021-00800-4. [DOI] [PubMed] [Google Scholar]
- 17.Rettie AJE, et al. A two-dimensional type I superionic conductor. Nat. Mater. 2021;20:1683–1688. doi: 10.1038/s41563-021-01053-9. [DOI] [PubMed] [Google Scholar]
- 18.Niedziela JL, et al. Selective breakdown of phonon quasiparticles across superionic transition in CuCrSe2. Nat. Phys. 2019;15:73–78. doi: 10.1038/s41567-018-0298-2. [DOI] [Google Scholar]
- 19.Zhong T, Li X, Wu M, Liu J-M. Room-temperature multiferroicity and diversified magnetoelectric couplings in 2D materials. Natl Sci. Rev. 2020;7:373–380. doi: 10.1093/nsr/nwz169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damay F, et al. Magnetoelastic coupling and unconventional magnetic ordering in the multiferroic triangular lattice AgCrS2. Phys. Rev. B. 2011;83:184413. doi: 10.1103/PhysRevB.83.184413. [DOI] [Google Scholar]
- 21.Peng J, et al. Even–odd‐layer‐dependent ferromagnetism in 2D non‐van‐der‐Waals CrCuSe2. Adv. Mater. 2023;35:2209365. doi: 10.1002/adma.202209365. [DOI] [PubMed] [Google Scholar]
- 22.Pang C, et al. High-performance inorganically connected CuInSe2 nanocrystal thin-film transistors and integrated circuits based on the solution process of colloidal synthesis, ligand exchange, and surface treatment. Chem. Mater. 2021;33:8775–8785. doi: 10.1021/acs.chemmater.1c02877. [DOI] [Google Scholar]
- 23.Harikesh PC, et al. Cubic NaSbS2 as an ionic–electronic coupled semiconductor for switchable photovoltaic and neuromorphic device applications. Adv. Mater. 2020;32:1906976. doi: 10.1002/adma.201906976. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, et al. High-TC two-dimensional ferroelectric CuCrS2 grown via chemical vapor deposition. ACS Nano. 2022;16:8141–8149. doi: 10.1021/acsnano.2c01470. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, et al. Chemical vapour deposition. Nat. Rev. Methods Prim. 2021;1:5. doi: 10.1038/s43586-020-00005-y. [DOI] [Google Scholar]
- 26.Zhu J, et al. Low-thermal-budget synthesis of monolayer molybdenum disulfide for silicon back-end-of-line integration on a 200 mm platform. Nat. Nanotechnol. 2023;18:456–463. doi: 10.1038/s41565-023-01375-6. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, et al. Composition and phase engineering of metal chalcogenides and phosphorous chalcogenides. Nat. Mater. 2022;22:450–458. doi: 10.1038/s41563-022-01291-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhang P, et al. Flux-assisted growth of atomically thin materials. Nat. Synth. 2022;1:864–872. doi: 10.1038/s44160-022-00165-7. [DOI] [Google Scholar]
- 29.Zhou Z, et al. Stack growth of wafer-scale van der Waals superconductor heterostructures. Nature. 2023;621:499–505. doi: 10.1038/s41586-023-06404-x. [DOI] [PubMed] [Google Scholar]
- 30.Li J, et al. General synthesis of two-dimensional van der Waals heterostructure arrays. Nature. 2020;579:368–374. doi: 10.1038/s41586-020-2098-y. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, et al. Uniform nucleation and epitaxy of bilayer molybdenum disulfide on sapphire. Nature. 2022;605:69–75. doi: 10.1038/s41586-022-04523-5. [DOI] [PubMed] [Google Scholar]
- 32.Fu J-H, et al. Oriented lateral growth of two-dimensional materials on c-plane sapphire. Nat. Nanotechnol. 2023;18:1289–1294. doi: 10.1038/s41565-023-01445-9. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, et al. Dual-coupling-guided epitaxial growth of wafer-scale single-crystal WS2 monolayer on vicinal a-plane sapphire. Nat. Nanotechnol. 2022;17:33–38. doi: 10.1038/s41565-021-01004-0. [DOI] [PubMed] [Google Scholar]
- 34.Xia Y, et al. 12-inch growth of uniform MoS2 monolayer for integrated circuit manufacture. Nat. Mater. 2023;22:1324–1331. doi: 10.1038/s41563-023-01671-5. [DOI] [PubMed] [Google Scholar]
- 35.Jain A, et al. Commentary: The materials project: a materials genome approach to accelerating materials innovation. APL Mater. 2013;1:011002. doi: 10.1063/1.4812323. [DOI] [Google Scholar]
- 36.Wu Q, et al. Resolidified chalcogen precursors for high‐quality 2D semiconductor growth. Angew. Chem. Int. Ed. 2023;62:e202301501. doi: 10.1002/anie.202301501. [DOI] [PubMed] [Google Scholar]
- 37.Li B, et al. Liquid-like thermal conduction in intercalated layered crystalline solids. Nat. Mater. 2018;17:226–230. doi: 10.1038/s41563-017-0004-2. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi H, et al. Spin-orbit-derived giant magnetoresistance in a layered magnetic semiconductor AgCrSe2. Phys. Rev. Mater. 2022;6:054602. doi: 10.1103/PhysRevMaterials.6.054602. [DOI] [Google Scholar]
- 39.Shiomi Y, Akiba T, Takahashi H, Ishiwata S. Giant piezoelectric response in superionic polar semiconductor. Adv. Electron. Mater. 2018;4:1800174. doi: 10.1002/aelm.201800174. [DOI] [Google Scholar]
- 40.Peng J, et al. Fast lithium ion conductivity in layered (li-Ag)CrS2. J. Am. Chem. Soc. 2020;142:18645–18651. doi: 10.1021/jacs.0c08448. [DOI] [PubMed] [Google Scholar]
- 41.Yin Y-C, et al. A LaCl3-based lithium superionic conductor compatible with lithium metal. Nature. 2023;616:77–83. doi: 10.1038/s41586-023-05899-8. [DOI] [PubMed] [Google Scholar]
- 42.Jun K, et al. Lithium superionic conductors with corner-sharing frameworks. Nat. Mater. 2022;21:924–931. doi: 10.1038/s41563-022-01222-4. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka Y, et al. New oxyhalide solid electrolytes with high lithium ionic conductivity > 10 mS cm−1 for all‐solid‐state batteries. Angew. Chem. Int. Ed. 2023;62:e202217581. doi: 10.1002/anie.202217581. [DOI] [PubMed] [Google Scholar]
- 44.Huang W, et al. Anomalously high ionic conductivity of Li2SiS3-type conductors. J. Am. Chem. Soc. 2022;144:4989–4994. doi: 10.1021/jacs.1c13178. [DOI] [PubMed] [Google Scholar]
- 45.Canepa P, et al. High magnesium mobility in ternary spinel chalcogenides. Nat. Commun. 2017;8:1759. doi: 10.1038/s41467-017-01772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, et al. Na11Sn2PS12: a new solid state sodium superionic conductor. Energy Environ. Sci. 2018;11:87–93. doi: 10.1039/C7EE03083E. [DOI] [Google Scholar]
- 47.Brehm JA, et al. Tunable quadruple-well ferroelectric van der Waals crystals. Nat. Mater. 2020;19:43–48. doi: 10.1038/s41563-019-0532-z. [DOI] [PubMed] [Google Scholar]
- 48.Gong Y, et al. Spatially controlled doping of two-dimensional SnS2 through intercalation for electronics. Nat. Nanotechnol. 2018;13:294–299. doi: 10.1038/s41565-018-0069-3. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y, et al. 2D layered materials for fast‐charging lithium‐ion battery anodes. Small. 2023;19:2301574. doi: 10.1002/smll.202301574. [DOI] [PubMed] [Google Scholar]
- 50.Yu J, et al. Simultaneously ultrafast and robust two-dimensional flash memory devices based on phase-engineered edge contacts. Nat. Commun. 2023;14:5662. doi: 10.1038/s41467-023-41363-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pi L, et al. Broadband convolutional processing using band-alignment-tunable heterostructures. Nat. Electron. 2022;5:248–254. doi: 10.1038/s41928-022-00747-5. [DOI] [Google Scholar]
- 52.Menter FR, Kuntz M, Langtry R. Ten years of industrial experience with the SST turbulence model. Turbul. Heat. Mass Transf. 2003;4:625–632. [Google Scholar]
- 53.Kresse G, Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996;6:15–50. doi: 10.1016/0927-0256(96)00008-0. [DOI] [PubMed] [Google Scholar]
- 54.Blöchl PE. Projector augmented-wave method. Phys. Rev. B. 1994;50:17953–17979. doi: 10.1103/PhysRevB.50.17953. [DOI] [PubMed] [Google Scholar]
- 55.Perdew JP, et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B. 1992;46:6671–6687. doi: 10.1103/PhysRevB.46.6671. [DOI] [PubMed] [Google Scholar]
- 56.Henkelman G, Uberuaga BP, Jónsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000;113:9901–9904. doi: 10.1063/1.1329672. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon request.