Abstract
Introduction
The skin is constantly exposed and responds to a wide range of biomechanical cues. The mechanobiology of skin has already been known and applied by clinicians long before the fundamental molecular mechanisms of mechanotransduction are elucidated.
Materials and methods
Despite increasing knowledge on the mediators of biomechanical signaling such as mitogen-associated protein kinases, Rho GTPases or FAK-ERK pathways, the key elements of mechano-responses transcription factors, and mechano-sensors remain unclear. Recently, canonical biochemical components of Hippo and Wnt signaling pathway YAP and β-catenin were found to exhibit undefined mechanical sensitivity. Mechanical forces were identified to be the dominant regulators of YAP/TAZ activity in a multicellular context. Furthermore, different voltage or ligand sensitive ion channels in the cell membrane exhibited their mechanical sensitivity as mechano-sensors. Additionally, a large number of microRNAs have been confirmed to regulate cellular behavior and contribute to various skin disorders under mechanical stimuli. Mechanosensitive (MS) microRNAs could not only be activated by distinct mechanical force pattern, but also responsively target MS sensors such as e-cadherin and cytoskeleton constituent RhoA.
Conclusion
Thus, a comprehensive understanding of this regulatory network of cutaneous mechanotransduction will facilitate the development of novel approaches to wound healing, hypertrophic scar formation, skin regeneration, and the progression or initiation of skin diseases.
Keywords: TRP, Piezo, Wound healing, Hypertrophic scar, Fibrosis, Scleroderma
Introduction
The skin is exposed to mechanical cues throughout life. As the outermost layer of human, it is an easily accessible tissue layer to investigate how mechanical force regulates adult stem cell renewal, lineage selection, and tissue assembly. Although fundamental mechanisms of mechanotransduction (the process of sensing mechanical cues and the subsequent biochemical response) [1] are still mostly unknown, the concept of Langer’s lines, topographical skin lines defined by skin mechanobiology, high mechanical stress regions for hypertrophic scar formation, has been widely used in clinical therapies. Decreasing mechanical force on the skin is the core principle for plastic surgeons to reduce pathologic scarring. Another example, skin expansion is a procedure that stimulates and promotes skin regeneration through continuous mechanical stretching provided by an underlying silicone expander. It has been used for a variety of plastic and reconstructive procedures due to its ability to regenerate additional normal skin [2].
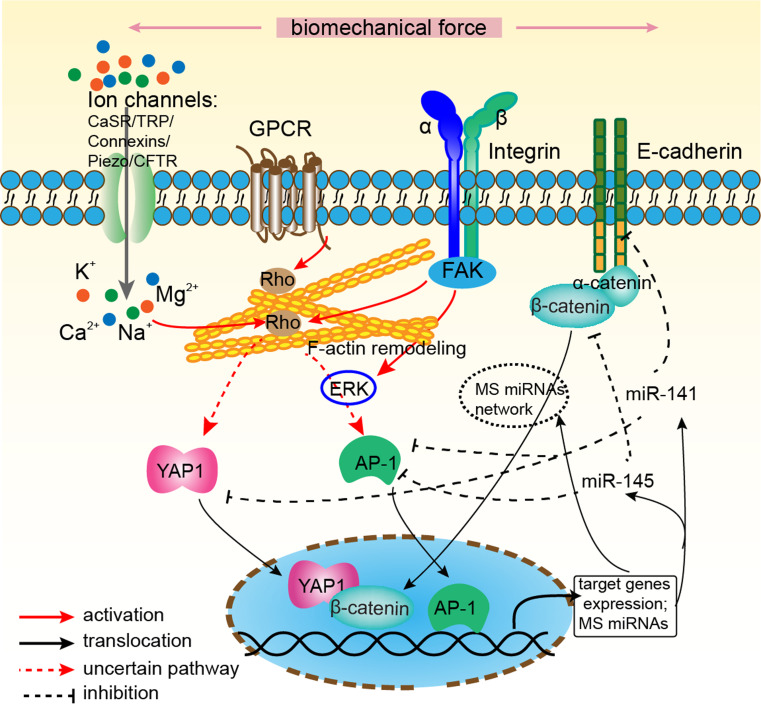
It is becoming well known that the mechanical environment has significant effects on cutaneous biology. As such, researchers and clinicians are pursuing the underlying molecular mechanisms of mechanotransduction pathway. Researches have demonstrated that mechanical force is transmitted across the cell membrane to activate downstream biochemical pathways including, but not limited to calcium-dependent pathways, nitric oxide (NO) signaling, MAPKs, Rho GTPases, and phosphoinositol-3-kinase (PI3K) [3]. A convincing study confirmed the association between mechanical force and pathologic scar through inflammatory FAK-ERK-MCP-1 pathways and that molecular strategies targeting FAK can effectively uncouple mechanical force from pathologic scar formation [4]. Besides, tissue architecture of cell–extracellular matrix (ECM), such as matrix stiffness and rigidity, cell–cell adhesions and the organization of cytoskeleton, controls the proliferation, migration, self-renewal, differentiation and cell death of stem cells (for more details seen in the excellent review of Wong et al. [5]). Notably, the process of epidermal stratification can be interpreted as a differentiation process induced by loss of cell-ECM contacts. However, except from the above signaling pathways, the role of most recent research revealed that mechano-related transcription factors and co-regulators, mechano-sensor ion channels, and a number of mechanosensitive microRNAs are not reviewed yet. Therefore, this review will focus on these molecular components of mechanotransduction in skin: mechanosensitive transcription regulators, ion channels, and microRNAs, which may provide basis for clinical therapies, rather than universal signaling passengers (Fig. 1).
Fig. 1.
Novel intracellular components of mechanotransduction in skin. Mechanical force activated multiple mechano-sensors have been described and include stretch-activated ion channels (Calcium-sensing receptor (CaSR), Transient receptor potential (TRP), Connexins and Piezos), integrins-focal adhesion kinase (FAK) complex, G-Protein-coupled receptors (GPCR) and cell-cell adhesion structure e-cadherin/α-catenin. Mechanical signal is transmitted across the cell membrane to activate multiple downstream biochemical pathways, Rho GTPases and F-actin remodeling of cytoskeleton. The convergence of these signals are mediated by three critical mechanosensitive (MS) transcription factors β-catenin, YAP and AP-1. While YAP could be activated by conformational change of GPCR directly or intermediate Rho GTPases, β-catenin could directly be regulated by the destruction of cell-cell adhesion structure. These transcription factors translocate into the nucleus and activate mechanoresponsive genes. Furthermore, plenty of MS microRNAs such as miR-141, miR-145 are expressed and target mechanosensitive elements, which may form a positive or negative feedback
Mechanosensitive (MS) transcription factors and co-regulators in skin
β-Catenin
The Wnt pathway is of central importance in regulating the development of skin and its appendages, hair follicles (HFs), interfollicular epidermal (IFE) stem cells renewal, and melanocytes [6]. Different members of the Wnt family are expressed in specific subsets of cells in developing and adult epidermis. Although loss of epidermal β-catenin resulted in loss of hair follicle specification, its role in IFE remains elusive [7]. Genetic evidence suggests that β-catenin is dispensable for interfollicular epidermal function [8], but a recent study of Lim et al. [9] showed that self-renewal of interfollicular epidermal stem cells is regulated via autocrine Wnt signaling. While conflicting evidences exist as to whether Wnt/β-catenin signaling is involved in IFE development and adult IFE maintenance, many Wnt signaling mutations do result in IFE phenotypes. In addition to its role as the key cytoplasmic effector of the Wnt pathway, β-catenin is also a component of intercellular adhesive junctions, binding to the cytoplasmic domain of E-cadherin. β-catenin then in turn binds to α-catenin, resulting in the formation of the cadherin–catenin complex [10]. Besides, α-catenin can bind to a variety of actin-binding proteins, indicating that the cadherin–catenin contact is essential to be subjected to mechanical stress, and β-catenin may function as mechano-mediator.
Direct regulation of Wnt signaling by biophysical signals has been demonstrated [11]. In osteoblasts, mechanical strain caused a rapid, transient accumulation of active β-catenin in the cytoplasm and its translocation into the nucleus in osteoblasts. Mechanical stimulation (3,600 cycles/day, 2 % strain) also can prevent adipogenic and improve osteogenic lineage differentiation of mesenchymal stem cells (MSCs) [12] by inactivating GSK3β and therefore inducing activation of both β-catenin and NFATc1 signaling. It has been found that mechanotransduction often caused conformational changes in the protein domains of β-catenin when β-catenin was subjected to tension. Using single-molecule force spectroscopy and molecular dynamics, armadillo repeat region (ARM) of β-catenin was found to be very sensitive to small changes in its structure and followed multiple unfolding pathways upon stretching. Simulations of the ARM/E-cadherin cytosolic tail complex emulated the most probable stress geometry occurring in vivo [13]. In skin, β-catenin was demonstrated to protect the epidermis of mice from mechanical stresses; while loss of β-catenin did not result in defects of epidermal differentiation and cell–cell contacts, tight junction protein localization was diminished in β-catenin knockout epidermis [14]. Another direct evidence of β-catenin in mechanotransduction in skin was that ROCK activation in mouse skin elevated tissue stiffness via increasing collagen production. β-catenin was stabilized by ROCK activation, leading to its nuclear accumulation, transcriptional activation, and consequent keratinocytes’ hyperproliferation and skin thickening [15]. So, β-catenin is indispensable to maintain tight junctions and mechanotransduction of skin under mechanical stretch.
Yes-associated protein (YAP)
The Hippo pathway has been demonstrated its function in precise skin size control [16, 17], with two key downstream effectors: YAP and its homolog transcriptional co-activator with PDZ-binding motif (TAZ). Transient overexpression of YAP resulted in enlarged liver and epidermis. However, sustained YAP overexpression eventually led to the development of liver and skin tumors. Clinical samples showed elevated expression and nuclear localization of YAP in various human cancers. Furthermore, YAP was inhibited by junctional proteins, such as angiomotin (AMOT) family proteins [18, 19], ZO-2, and alpha-catenin [20]. Besides, as the downstream components of canonical Hippo pathway, YAP/TAZ was also regulated by several extracellular signals including the G-protein-coupled receptors (GPCRs) signaling, the Wnt signaling, and mechanical stimulation. Rho GTPase and remodeling of F-actin, known effectors of GPCR signaling, were found to be required for YAP/TAZ regulation [21, 22]. Interestingly, GPCRs were also found to be mechano-sensor (discussed in section of Calcium channels).
Recently, mechanical signals were found to be transduced by two related transcriptional coactivators, YAP and TAZ [23]. To classify the complex crosstalk between these different inputs feeding on YAP/TAZ (mechanical stimulation, Hippo, Wnt, or GPCR signaling), Aragona et al. [24] demonstrated that mechanical forces were overarching regulators of YAP/TAZ in multicellular contexts, setting responsiveness to Hippo, WNT, and GPCR signaling. Another fascinating research found that YAP/TAZ could act as an intracellular mechanical rheostat that stored information from past physical environments and influenced the cells’ fate [25]. In fibroblasts, YAP function was required for cancer-associated fibroblasts to promote matrix stiffening, cancer cell invasion, and angiogenesis. Responsively, matrix stiffening further enhanced YAP activation, thus establishing a feed-forward self-reinforcing loop [26] during oncogenesis. So, YAP/TAZ was now widely recognized as mechano-sensors and mechanotransducers in regulating organ size and tumor growth (for more details seen in the excellent review of Low et al. [27]).
As the mechanical control checkpoint of multicellular growth, YAP controls epidermal growth and is involved in various skin diseases. YAP, through its interaction with α-catenin, acts as a critical mediator of crowd control in the epidermis of mice [20]. So, whether α-catenin could form a general mechano-sensor complex with β-catenin and YAP1, which orchestrates cellular responses to divergent mechanical stimuli, deserves more well-designed research. Increased cellular density limits stem cells expansion by inactivating YAP. Low basal cell density, as in a growing embryo or after wounding, would translate into nuclear YAP and proliferation. YAP/TAZ localization to the nucleus is required in skin wound healing [28], activation of stem/progenitor cell in the epidermis [29], promoting epidermal proliferation and inhibiting terminal differentiation [30]. During these pro-proliferation capacity, YAP also functioned in basal cell carcinoma [31] and contributed to the invasive and metastatic capacity of melanoma cells, with increased expression of TAZ and its downstream targets genes connective-tissue growth factor (CTGF) [32]. Collectively, YAP is the governor of epidermal proliferation and may act as a mechanical sensor in skin.
Activator protein 1 (AP-1)
The transcription factor AP-1 mediates gene expression in response to a variety of extracellular stimuli including growth factors, cytokines, and mechanical stress [33]. AP-1, a heterogeneous set of dimeric proteins, consists of members of the Jun, Fos, and ATF families. AP-1 proteins are now being recognized as critical regulators of oncogenesis, bone development, inflammation, skin physiology, and diseases [34]. In skin, AP-1 subunits expressed in keratinocytes are involved in the regulation of the proliferation and differentiation of epidermis. AP-1 target genes include transglutaminase and different members of the cytokeratin gene family such as Keratin1 (K1), K5, K14, K18, and K19 [35]. While Jun is regarded as a positive regulator of keratinocyte proliferation, JunB and JunD are often considered to be the negative regulators of cell proliferation. Fibroblasts overexpressing JunB showed reduced proliferation [36]. AP-1 has been demonstrated to function in wound healing [37], photoaging, melanoma, squamous cell carcinoma (for references, see [33, 34, 38]), psoriasis, as well as skin regeneration [39].
In addition to mediate inflammatory effect, AP-1 is also involved in mechanotransduction. AP-1 and NF-κB were found to be activated by cyclic strain and shear stress in endothelial cells [40, 41], inducing the expression of genes encoding for adhesion molecules, monocyte chemo-attractants, and growth factors. Then, AP-1- and NF-κB-mediated transcriptions were identified to function in bladder muscle cells [42], osteoblasts [43], lung parenchyma [44], and amnion cells [45], in response to mechanical forces. At the initiation of arteriogenesis, stretch-induced AP-1-mediated MCP-1 expression in vascular smooth muscle cells played as a key determinant [46]. With regard to the role of AP-1 in mechanotransduction of skin, previous research showed that by microarray analysis performed on mechanical stretched and normal human skin, significant difference was observed in 77 genes. Among these skin regeneration-related genes, AP-1 was up-regulated in mechanically stretched skin [39], implying that AP-1 may be involved in mechanotransduction of skin.
In brief, the above-mentioned three transcription factors and co-regulators in skin mechanobiology were the most studied, with their roles in skin summarized in Table 1. While β-catenin and YAP have been demonstrated to directly transduce mechanical stimulation in skin, AP-1 has only been found to be significantly up-regulated in mechanical stretched skin. More elaborate research is demanded to reveal the direct mechanosensitive facet of AP-1 in skin, especially epidermis.
Table 1.
Mechanosensitive transcription factors in skin
| Transcription regulators (homo sapiens) | Mechano-pattern | Effect | Skin diseases involved |
|---|---|---|---|
| β-Catenin | Stretch or strain; stiffness of ECM | Promote osteogenic differentiation of MSCs; protect epidermis from mechanical stresses; self-renewal of interfollicular epidermal stem cell | Maintain tight junction proteins of skin barrier [14]; epidermal hyperproliferation [15] |
| YAP | Stretch or strain; stiffness of ECM | Cell proliferation; cell crowd control; activate epidermal stem cell; inhibit epidermal terminal differentiation; skin cancers | Skin tumors [20]; stem/progenitor cell activation; wound healing [28] |
| AP-1 | Stretch or strain; fluid shear stress | Inflammation; initiation of arteriogenesis; adhesion; monocyte chemotaxis; induce expression of epidermal growth factor-like growth factor; activate osteoblast differentiation | Wound healing [37]; skin regeneration [39] |
Mechanosensitive ion channels of the skin
Mechanical stimuli are known to induce ionic currents in various types of cells. These currents are conducted through different ion channels in the cell membrane [47]. Mechanical sensitivity, much like voltage or ligand sensitivity, is a general characteristic of ion channels. Channels previously considered as “voltage-gated” such as K+ and Na+ channels were also found to be mechanically sensitive [48]. In this mechanosensory transduction system, the opening of stretch-activated channels is the first step, leading to various intracellular events. Although many ion channels are implicated in mechanosensation, few have been shown to be directly stretch activated. Ion channels which are identified to be MS in other cells have also been found to express and function in skin physiology. In the present review, without analysis of structure and function of MS ion channels, we focus on the physiological functions and related cutaneous diseases of four different MS ion channels: CaSR, TRP, Connexins, and Piezo.
Calcium channels (calcium-sensing receptor)
The major downstream effect of mechanosensitive ion channel activation in the epidermis is a change in cytoplasmic Ca2+ concentration [49]. Ca2+ oscillations have been observed in MSCs and are considered as both an indicator and a regulator of the MSC differentiation. Extracellular calcium is essential for initiating keratinocyte differentiation and maintaining epidermal functions [50]. Ca2+ concentration oscillation may be caused by mechanical signal [51] as found in human cardiac progenitor cells, keratinocytes, and myofibroblasts [52], indicating that mechanical forces might have the potential to directly regulate the fate of these cell types through modulating calcium signals [53]. It has been reported that changes in the plasma membrane tension can be transmitted to the mechanosensitive L- and N-type Ca2+ channel [54, 55]. Ca2+ influx can also be mediated by the nonspecific cation channels such as MS transient receptor potential (TRP) channels, connexins, and Piezos (described below), as well as voltage-gated calcium channels [56] and specific calcium-sensing receptors (CaSR) [57, 58].
The CaSR belongs to the family C of GPCRs which exhibit distinct functions in many physiological processes such as inflammation, cellular growth, and differentiation. Although the direct evidence of mechanical sensitivity has not been reported for CaSR yet, a multitude of researches suggested that GPCRs were involved in mechanosensation. Gq/11-protein-coupled receptors have recently been indicated in the sensing of fluid shear stress in endothelial cells (bradykinin B2 receptor) [59, 60] and mechanical stretch in cardiac myocytes (beta-adrenergic receptor) [61, 62] (For more details about G-protein-mediated stretch reception seen in [63]). In the skin, MS GPCRs are involved in collagen synthesis in cardiac and lung fibroblasts [64], and thought to activate fibroblast contraction during wound healing. Inhibition of CaSR expression in keratinocytes markedly impairs cell differentiation by reducing intracellular Ca2+ pools [58] and blocking E-cadherin mediated signaling [65, 66]. Abrogation of CaSR in vivo was shown to perturb the epidermal Ca2+ gradient and compromise differentiation and barrier functions [67]. However, CaSR transgenic mice exhibited advanced differentiation and epidermal permeability barrier formation during embryologic development and accelerated hair growth at birth.
Transient receptor potential (TRP) channels
Members of the TRP family are implicated in a wide variety of mechanical transduction processes in diverse organs and species. These channels can be activated by mechanical, as well as chemical and thermal stimulation. TRP channels play a role in cellular homeostasis and growth control, regulation of cell fate and survival, immune, and inflammatory mechanisms [68]. The TRP channel family is usually divided into seven subfamilies (TRPC, TRPV, TRPM, TRPN, TRPA, TRPP, and TRPML). These ion channels are non-selectively permeable to cations, including sodium, calcium, and magnesium. Evidences suggest that multiple TRP channels are critically involved in the regulation of cutaneous functions [69]. Among the TRP channels, TRPA1, TRPV1, and TRPV4 are the most studied TRP channels of the skin and their mutations lead to a variety of phenotypic disorders.
TRPA1 can induce marked changes in the expressions of certain adhesion and extracellular matrix proteins in keratinocytes as well as of molecules regulating cell fate and differentiation [70]. The role of TRPA1 in cutaneous inflammation has been further verified in rodent dermatitis models. TRPA1 activation enhanced the migration of dendritic cells to draining lymph nodes, and this effect was antagonized by the TRPA1 antagonist [71]. Activation of TRPV1 on epidermal keratinocytes resulted in the influx of Ca2+ into the cells, suppressing cell growth, and inducing apoptosis [68]. Furthermore, TRPV1 stimulation delayed barrier recovery, whereas administration of the TRPV1 antagonist accelerated barrier repair [72]. Stimulation of TRPV1 expressed by outer root sheath keratinocytes of human hair follicles also inhibited hair shaft elongation [73]. Besides, mechanical activation of TRPV1 caused intracutaneous release of a plethora of neuropeptides. The released peptides activated multiple types of skin cells, inducing the release of certain pro-inflammatory cytokines [73, 74]. Similarly, TRPV4, mediating the metabolic response of chondrocytes to mechanical stimuli [75], was also involved in epidermal barrier homeostasis [76, 77]. Most recent research showed that TRPV4 was abrogated both in premalignant lesions and non-melanoma skin cancer in cytokine-dependent manner [78].
Connexins
Connexins are structurally conserved gap junction (GJ) protein abundantly expressed in bone and skin cells [79], playing a central role in cell-to-cell communication and mechanotransduction. Channel opening and closure are tightly regulated by changes in cytosolic pH, voltage, as well as mechanical stimulation [80]. This process is critical for the maintenance of cellular homeostasis and regulation of proliferation, differentiation, and apoptosis [79, 81]. In vitro studies suggested that gap junctional intercellular communication (GJIC) sensitized bone cells to mechanical signals. Additionally, mechanical signals detected by osteocytes were communicated to osteoblasts via GJIC [80, 82–84]. Connexin43 (Cx43) is the predominant GJ protein in bone. Cx43 expression was enhanced by loading in bones in vivo as well as in cultured osteoblasts and osteocytes, thus preventing osteocyte apoptosis [82, 83]. Under mechanical loading, the c-terminus domain of Cx43 with integrin α5 and β1 led to activation of PI3 K, the kinases FAK/Src, and the ERK pathway, eventually promoting osteocyte survival.
Connexin has also been demonstrated to function in keratinocytes differentiation, wound healing, and skin diseases. Up to 10 different connexins are differentially expressed throughout the terminal differentiation. Cx43 is the main connexin found in basal proliferating cells [85]. Cx43 knockdown resulted in impaired epidermal differentiation and barrier function [86]. During wound healing, Cx43 knockout mice showed shorter wound closure times, making itself a potential therapeutic target to improve wound healing [87]. Cx43 expression at wound margins declined rapidly following injury, returning to normal levels following re-epithelization. The reduction of Cx43 at wound edges is related with the mechanical force which was produced by intrinsic myofibroblats and many extrinsic forces. Another skin disorder associated with connexin is melanoma. The loss of the ability to form heterocellular contacts and exhibit GJIC with keratinocytes was found to be a contributor or suppressor to melanoma growth within the epidermis [88]. While Cx32 knockout mice are more prone to developing chemical- and radiation-induced tumors [89, 90], the role of connexins during the onset and progression of melanoma tumorigenesis is still controversial. Most recently, it was found that Cx43 could act as a tumor suppressor during melanoma tumorigenesis by significantly reducing cellular proliferation and anchorage-independent growth [90].
Piezos
Piezo1 and Piezo2 were first identified as MS channels in the cell line Neuro2A. Piezo proteins have 2,100–4,700 amino acids which contain approximately 24–39 transmembrane segments showing no homology to other already known voltage sensitive channels [91]. Piezos do not require any additional proteins for their opening, but can directly sense lipid membrane extension [92]. Although currents mediated by Piezo proteins are non-selective cationic currents, Piezo1 protein was classified as a real ion channel that conducts both K+ and Na+. The currents mediated by both Piezo1 and Piezo2 can be pharmacologically inhibited by mechanosensitive channel blockers [93]. Piezo1 expression has been observed in bladder, colon, kidney, lung, and skin which all constantly undergo mechanotransduction. Recent studies found that Piezo1 is present in the mouse and human bladder urothelium and has a functional role in stretch-evoked Ca2+ influx and ATP release in mouse urothelial cell [94] and human neural stem cells [95], indicating the potential MS property of Piezos.
The role of Piezo proteins in skin has not been investigated until most recently. Piezo2 was previously shown to be present at low levels in the skin [91]. However, Woo et al. [96] found that Piezo2 was specifically expressed in Merkel cells (~0.05–0.1 % of total epithelial cells from dorsal skin) in whisker pad, dorsal skin, and foot pad. Using Piezo2 knockout mice, they found that Merkel cells were indeed mechanosensitive, and Piezo2 was required in Merkel cells to produce their mechanical currents. Piezo2 ablation impaired proper mechanosensory encoding in Merkel cell–neurite complexes in the intact skin. The results indicated that Piezo2 was the Merkel-cell mechanotransduction channel and provided the first line of evidence that Piezo channels have a physiological role in mechanosensation in mammals. The function of Piezo proteins in non-Merkel cells of the skin needs further investigation.
In summary, direct evidence of ion channels to be MS was only found in Connexin43 and Piezo2 in the skin. Although Ca2+ and TRP channels that are also expressed in the skin and play a vital role in skin barrier formation and inflammation have been shown to be activated by various mechanical stimuli in non-cutaneous cells, they have not been demonstrated to mediate a mechanical signal yet (seen in Table 2). Besides, many mechanically activated currents are non-selective cationic currents, such as Ca2+ and Connexins, could be conducted through different MS ion channels in the cell membrane. So, the mechanism how these MS ion channels are orchestrated by cutaneous cells under mechanical condition and the identification of the downstream signaling pathways for these currents deserve more further research.
Table 2.
Mechanosensitive ion channels and skin disorders
| Channel family | MS channel isoforms | Skin disorders involved | Effect |
|---|---|---|---|
| Ca2+ channels | GPCRs [60–62] | Epidermal differentiation and barrier functions | Advance epidermal differentiation and permeability barrier formation [65–67] |
| TRP channels | TRPA1 [97, 98] | Skin barrier | Accelerate skin permeability barrier recovery [70] |
| Skin inflammation | Contact dermatitis [71] | ||
| TRPV1 [99] | Skin barrier | Decrease proliferation and induce apoptosis of keratinocytes; inhibit skin barrier recovery [72] | |
| Skin inflammation | Induce release of pro-inflammatory cytokines [73, 74] | ||
| TRPV4 [100] | Skin barrier | Strengthen the tight junction barrier in human epidermal keratinocytes [76, 77] | |
| Non-melanoma skin cancer | Decreased TRPV4 expression as an early biomarker of skin carcinogenesis [78] | ||
| Connexins | Connexin43 [82–84, 101, 102] | Wound healing | Enhance wound closure [85, 87, 103, 104] |
| Melanoma | Serve as tumor suppressor [88, 90, 105] | ||
| Epidermal homeostasis | Epidermal differentiation and barrier function [81, 86, 106] | ||
| Piezo | Piezo1 and piezo2 [91, 93] | Merkel cells sensory afferents | Touch-sensitive currents of Merkel cells [96] |
| Cl− channels | CFTR [107] | Hyperpigmentation | Mediate the procedure of treatment of melasma with estrogen [108] |
The association between mechanosensitive microRNAs and skin disorders
MiRNAs are shown to be involved in the mechanical regulation of epidermal biology and functions. The role of MS miRNAs has been widely studied in endothelial cells (ECs) [109, 110]. In vascular endothelial cells, shear stress regulated ECs redox and inflammatory state, cell cycle, cytoskeleton and gap junctions. Furthermore, different shear stress patterns determine distinct endothelial function. While pulsatile shear stress (PS) act as atheroprotective flow, oscillatory shear stress (OS) was identified to be an atheroprone factor, suggesting the specific role of shear stress in endothelial response. Recent studies underline the importance of miRNAs in skin development and epidermal differentiation, wound healing, fibrosis, and skin cancers [111]. For example, miR-203 has been shown to be indispensable in skin mechanobiology. AP-1 subunits c-Jun and JunB were found to drive miR-203 expression in keratinocytes, acting as a switch between keratinocyte proliferation and differentiation. MiR-200 and miR-205 positively regulate E-cadherin and seem to be essential in maintaining epithelial stability (For more details about microRNAs in skin seen in [111–113]). So, in this review, we will focus on the role of reported MS miRNAs in skin disorders, such as wound healing, hypertrophic scar, inflammation or related scleroderma fibrosis, and skin cancers (Table 3).
Table 3.
Mechanosensitive microRNAs and associated skin disorders
| MS miRNAs | Targeted genes (under mechanical stimuli) | Effect | MS pattern | Direct effect | Skin disorders involved |
|---|---|---|---|---|---|
| miR-21 [116, 118] | Phosphatase and tensin homolog (PTEN) | Down | Shear stress | Decrease apoptosis and activate the NO pathway |
Hypertrophic scar [140] Scleroderma fibrosis [124] |
| miR-23b [146] | Retinoblastoma (Rb) | Up | Shear stress | Growth arrest of endothelial cells |
Radiation skin injury [147] Epidermal differentiation [148] |
| miR-24 [149] | Subtilisin-like proprotein convertase (FURIN) | Up | Mechanical stretch | Induction of TGFβ1 |
Epidermal differentiation [150] radiation skin injury [151, 152] melanoma [153] |
| miR-26a [137] | Glycogen synthase kinase-3β (GSK-3β) | Up | Mechanical stretch | Hypertrophy of human airway smooth muscle cells |
Squamous cell carcinoma [155] Epidermal differentiation [148] |
| miR-34a [156] | Forkhead box j2 (Foxj2) | Up | Shear stress | Promotes endothelial differentiation of endothelial progenitor cells |
Non-melanoma skin cancer [157, 158] Merkel cell carcinoma [159] Epidermal proliferation [161] |
| miR-17-92a [125] | Cyclin D1 | Down | Shear stress | Cell cycle arrest of endothelial cells |
Epidermal Langerhans cells [162] Scleroderma fibrosis [126] Basal cell carcinoma [163] Mycosis fungoides [164] |
| miR-144/451 [165] | AMPKα1 | Down | Mechanical stretch | Promote contractile differentiation of smooth muscle cells | None |
| miR-145 [166–168] | Angiotensin-converting enzyme (ACE) | Down | Mechanical stretch | Angiotensin-converting enzyme expression |
Squamous cell carcinoma [155] Melanoma [169] Basal cell carcinoma [163] |
| Up | Shear stress | ||||
| miR-146a [127] | IRAK1 and TRAF6 | Up | Oscillatory pressure | Induce inflammation in lung epithelia |
Chronic inflammation [128, 171] Non-melanoma skin cancers [172] |
| miR-155 [117] | RhoA and myosin light chain kinase (MYLK) | Up | Shear stress | Cytoskeleton organization of endothelial cells |
Wound healing [173] |
MS microRNAs in wound healing, hypertrophic scar, and keloid
Acute wounds undergo a healing process of complex biochemical and cellular events. All the phases are influenced by mechanical forces which are generated by wound contraction and traction forces of ECM and myofibroblasts. It has been well described that the mechanical stretching of fibroblasts can regulate the expression of matrix and inflammatory genes involved in scar formation, suggesting that wound fibrosis can be modulated by fibroblast mechanosensitivity [4]. Keratinocytes are also mechanically responsive: mechanical stretch promotes keratinocyte proliferation and migration in vitro [114]. Thus, clinical application of mechanical forces provides an alternative method for the acceleration of cutaneous wound healing and prevention of hypertrophic scars. Compression treatment is a frequently used therapy for postburn hypertrophic scars. Silicone gel sheeting has been shown effective in reducing tension between the scar and normal skin. Paper or plastic adhesive tape is also used to prevent excessive scarring by decreasing wound tension [115].
MS microRNAs, found in non-cutaneous cells, play essential roles in wound healing or hypertrophic scar. In ECs, shear stress forces increased the expression of miR-21 and miR-155 which influence endothelial biology by decreasing apoptosis [116] and inducing changes in morphology and F-actin organization [117]. In human aortic smooth muscle cells, mechanical stretch modulated miR-21 expression, thus participating in cellular proliferation and apoptosis [118]. Both miR-21and miR-155 are involved in wound healing and the formation of hypertrophic scars, mainly in activated and migrating keratinocytes of the epidermis and mesenchymal cells of the dermis [119, 120]. Antagonizing miR-21 caused significant delay of wound closure with impaired collagen deposition. Interestingly, wounds treated with miR-21 antagomir exhibited significantly impaired wound contraction at an early stage of wound healing, which provided direct evidence of miR-21 to be an MS microRNA during wound healing [119]. MiR-155 was up-regulated in wound tissue when compared with healthy skin. However, lacking expression of miR-155 resulted in an accelerated wound closure. Besides, miR-155 targets two key regulators of the cytoskeleton organization: RhoA and myosin light chain kinase (MYLK) [117], which are key components in mechanotransduction. More research is required to understand the exact function of miR-155 in wound healing. But it is undoubted that biomechanical force and MS miRNAs play a vital role in wound closure and formation of hypertrophic scar.
MS microRNAs in scleroderma fibrosis and chronic inflammatory diseases
Scleroderma is a complex systemic autoimmune disease characterized by extensive chronic fibrosis, primarily of the skin. Overproduction of pathological ECM components by fibroblasts plays a major role in the pathogenesis of scleroderma. Importantly, functional alterations of scleroderma are accompanied by changes in the mechanical properties and morphology of fibroblasts. The architecture of the fibrosis-associated ECM is fundamentally different from that of the normal tissue stroma. Previous data showed that ongoing myofibroblast activation and TGF-β signaling can be driven by altered mechanical tension in a feed-forward loop [121]. Compared to normal dermal fibroblasts, significant differences in cellular stiffness have been detected in dermal fibroblasts derived from sclerodermal lesions using atomic force microscopy [122]. Another explanation for the overproduction of ECM in scleroderma is the activation of fibroblasts by cytokines and growth factors, endothelin1 and thrombin [123], as well as chronic inflammation. Matrix-generated biomechanical tension via integrin signaling seems to be involved in this process. These findings suggest an association between scleroderma, chronic inflammation, and mechanotransduction.
Besides TGF-β signaling and inflammatory cytokines, miRs have recently been identified to play a role in the pathogenesis of inflammatory cutaneous diseases such as scleroderma, psoriasis, and diabetic wound healing. While TGF-β leads to an increased expression of miR-21 and decreased expression of fibrosis-inhibitor gene Smad7, overexpression of miR-21 in fibroblasts decreases the expression of Smad7, suggesting that miR-21 may act as a potential therapeutic target of scleroderma [124]. MiR-19a is another MS microRNA that is involved in the pathogenesis of scleroderma. Using microRNA chip array, miR-19a expression in ECs was found to be regulated by laminar shear stress [125]. MiR-19a level was also shown to be significantly higher in the serum and dermal fibroblasts of scleroderma patients [126]. Similarly, miR-146a is rapidly up-regulated by oscillatory pressure in airway epithelial cells, playing an important role in mechanically induced inflammation in lung epithelia [127]. IRAK1 and TRAF6, two important mediators of inflammatory responses, were then identified as targets of miR-146a. In cutaneous diseases, miR-146a is known to be up-regulated both in lesions and peripheral blood mononuclear cells (PBMCs) of psoriatic patients, correlated with IRAK1 expression [128]. Thus, mechanical cues may contribute to the persistent inflammation of psoriasis mediated by miR-146a. Chronic inflammation is also thought to play a central role in the pathogenesis of diabetic wound. Notably, miR-146a expression is markedly down-regulated in diabetic mouse wounds [128].
MS microRNAs in melanoma and non-melanoma skin cancers
Cutaneous melanoma is a highly malignant tumor type due to its tendency to metastases. Recent work has identified BRAF and NRAS mutations in melanoma, which lead to constitutive activation of MAPK and phosphoinositide 3-kinase (PI3K) pathway [129]. Both MAPK and PI3 K pathways were found to mediate biomechanical signal, implying the potential role of BRAF and NRAS in mechanotransduction of melanoma. It has been shown that many stromal components are able to activate varieties of cellular processes in melanoma [130]. Cutaneous melanoma cells have varied morphological characteristics, and the stroma can show myxoid or desmoplastic changes. Members of the matrix metalloproteinase (MMP) family are expressed by melanoma cells [131], which indicates that frequent ECM remodeling and collagen deposition happened during melanoma progression. The ECM stiffness can regulate melanoma cells and tissue behavior by initiating biomechanical signaling cascades in cells through interactions with a number of specialized transmembrane ECM receptors. Furthermore, tumor growth cannot be sustained unless the tumor cells attract and stimulate fibroblasts, which are the main source of extracellular matrix. Recruited fibroblasts are converted into myofibroblasts, differentiated to fibrocytes that secrete fibrillated extracellular matrix components, which further stiffen the ECM [132]. Importantly, biophysical force generated by contraction of myofibroblasts and stiff ECM in the microenvironment has a significant role in melanoma growth and metastases.
A large number of MS miRNAs were demonstrated to act in melanoma. MiR-21 was shown to be MS in ECs and aortic smooth muscle cells, and significantly increased in primary melanoma tissues as compared to benign nevi. Upregulation of miR-21 in melanocytes resulted in increased proliferation and decreased apoptosis [133]. MiR-21 has also been found in plasma and its expression was associated with tumor burden in cutaneous melanoma and squamous cell carcinoma patients [134]. MS miR-23b on the other hand mediated flow-regulation of Rb phosphorylation in EC growth. MiR-23b was found to be decreased in melanomas, as assessed by an in-depth analysis of the miRs transcriptome [135, 136]. MiR-26a is an MS gene to mechanical stretch and plays an important role in the regulation of human airway smooth muscle hypertrophy [137]. MiR-26a was identified to be specifically down-regulated in human melanoma cells [138] and squamous cell carcinoma. Another example, miR-34a can modulate differentiation of endothelial progenitor cells in response to shear stress. In melanoma, miR-34a was found to be tumor suppressive by targeting ULBP2, a natural killer cell immunoreceptor NKG2D ligand and it is widely expressed in tumor cells [139]. Furthermore, miR-34a was also found in non-melanoma skin cancers (Table 3). Taken together, these findings imply a strong association between mechanotransduction and skin cancers. These MS miRs modulators (antagonist or agonist) may serve as promising novel therapies for subsets of melanoma.
What is known and unknown about the regulatory network induced by mechanical force?
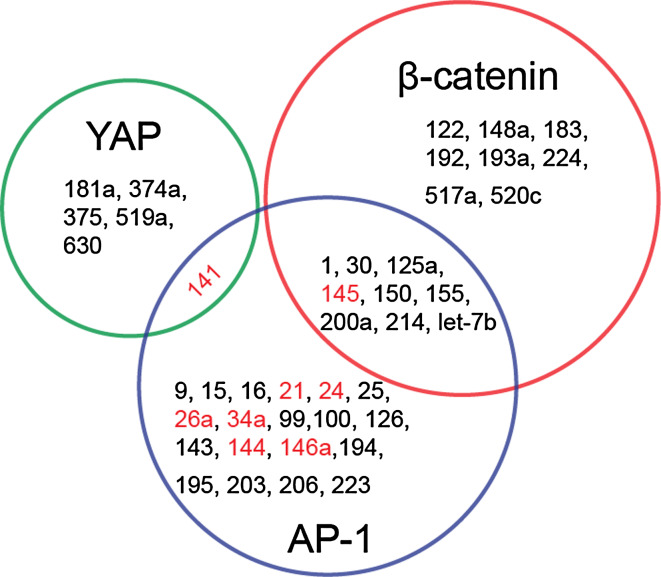
Interestingly, some MS miRNAs are found to target MS transcription factors (Table 4). miRNAs regulate gene expression by binding to the 3′ untranslated region of the target mRNAs. Specific binding of MS miRNAs to MS transcription factors (YAP1, β-catenin and AP-1) is validated and gathered, according to an open-access miRNAs database miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/micrornapredictedtarget.html). For example, YAP1 can be regulated by mir-141, 181a, 374a, 375, 519a, and 630, during which mir-141 (belong to mir-200 family) is abundantly expressed in skin. Mir-141 targets the E-cadherin transcriptional repressors ZEB1 and ZEB2, inducing invasion and metastasis of skin tumor cells [180, 181]. Furthermore, both YAP1 and AP-1 are regulated by mir-141. β-catenin and AP-1 can be targeted by many miRNAs, during which mir-21, 24, 26a, 34a, 141, 144, 145, and 146a are identified to be MS (Fig. 2). Mir-145 targets junctional adhesion molecule A and the actin-bundling protein fascin, modulating cancer cell motility [182]. Mir-145 is also expressed through the phosphoinositide-3 kinase (PI-3K)/Akt and p53 pathways, and then targets the expression of c-Myc [183]. Most listed MS miRNAs were found to regulate AP-1, but direct evidence that these miRNAs are activated and target AP-1 expression under mechanical stimulation is still missing.
Table 4.
Mechanosensitive transcription regulators related to microRNAs
| Transcription factors (homo sapiens) | MiRNAs validateda | MS miRNAs | Effect |
|---|---|---|---|
| YAP1 | mir-141, 181a, 374a, 375, 519a, 630 | mir-141 | Target the E-cadherin transcriptional repressors ZEB1 and ZEB2, inducing invasion and metastasis of skin tumor cells [180, 181] |
| β-Catenin | mir-1, 30, 122, 125a, 145, 148a, 150, 155, 183, 192, 193a, 200a, 214, 224, 517a, 520c; let-7b | mir-145 | Target the junctional adhesion molecule A, the actin-bundling protein fascin and c-Myc [182, 183], involved in melanoma and non-melanoma skin tumors |
| AP-1 | mir-1, 9, 15, 16, 21, 24, 25, 26a, 30, 34a, 99, 100, 125a, 126, 141, 143, 144, 145,146a, 150, 155, 194, 195, 200a, 214, 203, 206, 223; let-7b | mir-21, 24, 26a, 34a, 141, 144, 145, 146a | mir-21 targets a number of tumor suppressors; mir-24 can directly repress cytoskeletal modulators in keratinocytes [150]; mir-26a can repress gene expression of SODD (silencer of death domains) in melanocytes [138]; mir-34a: repressed by p63 to maintain keratinocyte cycle progression [161] |
aResults from miRWalk. (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/micrornapredictedtarget.html)
Fig. 2.
Mechanosensitive transcription regulators and microRNAs. Validated gene-microRNA interactions are gathered from the miRWalk database. MicroRNAs which target MS transcription factors β-catenin, YAP and AP-1 are shown by Venn Diagram respectively. These microRNAs, which have been confirmed to be activated directly or responsively by mechanical stimuli, are shown in red
However, it is still largely unknown about the regulatory network of miRNAs under mechanical stimuli conditions. For example, pulsatile flow down-regulates miR-17 which targets SOD2, GPx2, and TrxR2. In contrast, oscillatory flow down-regulates SOD1 by miR-872 [109], indicating that different mechanical patterns were sensed and mediated by different membrane proteins and downstream signaling pathways. Although diverse mechanotransduction pathways under specific patterns of mechanical stimuli have been identified, the underlying complex mechanisms require more further investigation to understand how slight difference in mechanical patterns may cause tremendous divergence of cell responses. Mechanical stimuli-induced miRNAs could responsively regulate MS cell sensors such as e-cadherin, cytoskeleton constituent RhoA, and MS transcription factors such as YAP1, β-catenin, and AP-1 (Table 4). Moreover, it remains largely unknown how mechanical force regulates the network of miRNAs. Therefore, it is still unknown if these mechanical-induced feedback regulations are positive or negative.
Conclusion and future directions
Taken together, the exploitation of cutaneous mechanobiology will help researchers and clinicians to gain a more comprehensive understanding of wound healing, hypertrophic scar formation, skin regeneration, and skin tissue engineering. However, different magnitude of mechanical force also seems to exert distinct biological effects by targeting different genes. MS transcription factors and microRNAs interweave in a complex regulatory network of cellular-responses. On the other hand, the reciprocal cell-ECM remodeling mechanisms of this circuit and the model of skin stem cells (interfollicle or hair follicle) activation under mechanical stimuli remain unknown. Identification of the mechanical property divergence of ECM under pathological or physiological conditions or stem cells niche, the association of ECM mechanical modulus with different Rho GTPases, and the convergence of mechano-sensors with canonical biochemical receptors may be the possible steps to overcome these challenges. Advances in nanotechnology, molecular imaging, and gene manipulation will further accelerate the investigation of the mechanically activated regulatory network within the cells. Finally, in-depth research of these questions is urgently needed not only in cutaneous biology, skin, and hair follicle morphogenesis, but also in the progression and initiation of skin diseases like cancers.
Conflict of interest
The authors state no conflict of interest.
Abbreviations
- YAP
Yes-associated protein
- AP-1
Activator protein 1
- ECM
Extracellular matrix
- MS
Mechanosensitive
- MAPKs
Mitogen-associated protein kinases
- FAK
Focal adhesion kinase
- ERK
Extracellular regulated protein kinases
- PI3K
Phosphoinositol-3-kinase
- HF
Hair follicles
- IFE
Interfollicular epidermal
- MSCs
Mesenchymal stem cells
- GPCRs
G-Protein-coupled receptors
- CaSR
Calcium-sensing receptor
- TRP
Transient receptor potential
- ECs
Endothelial cells
- Cx
Connexin
Footnotes
J. Wang and Y. Zhang authors contributed equally to this work.
References
- 1.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou SB, Wang J, Chiang CA, Sheng LL, Li QF. Mechanical stretch upregulates SDF-1alpha in skin tissue and induces migration of circulating bone marrow-derived stem cells into the expanded skin. Stem Cells. 2013;31:2703–2713. doi: 10.1002/stem.1479. [DOI] [PubMed] [Google Scholar]
- 3.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Bio. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2012;18:148–152. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong VW, Akaishi S, Longaker MT, Gurtner GC. Pushing back: wound mechanotransduction in repair and regeneration. J Invest Dermatol. 2011;131:2186–2196. doi: 10.1038/jid.2011.212. [DOI] [PubMed] [Google Scholar]
- 6.Lim X, Nusse R (2013) Wnt signaling in skin development, homeostasis, and disease. Cold Spring Harb Perspect Biol 5. doi:10.1101/cshperspect.a008029 [DOI] [PMC free article] [PubMed]
- 7.Watt FM, Collins CA. Role of beta-catenin in epidermal stem cell expansion, lineage selection, and cancer. Cold Spring Harb Symp Quant Biol. 2008;73:503–512. doi: 10.1101/sqb.2008.73.011. [DOI] [PubMed] [Google Scholar]
- 8.Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, et al. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science. 2013;342:1226–1230. doi: 10.1126/science.1239730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yonemura S. A mechanism of mechanotransduction at the cell-cell interface: emergence of alpha-catenin as the center of a force-balancing mechanism for morphogenesis in multicellular organisms. Bioessays News Rev Mol Cell Dev Biol. 2011;33:732–736. doi: 10.1002/bies.201100064. [DOI] [PubMed] [Google Scholar]
- 11.Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chem. 2009;284:34607–34617. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valbuena A, Vera AM, Oroz J, Menendez M, Carrion-Vazquez M. Mechanical properties of beta-catenin revealed by single-molecule experiments. Biophys J. 2012;103:1744–1752. doi: 10.1016/j.bpj.2012.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray S, Foote HP, Lechler T. beta-Catenin protects the epidermis from mechanical stresses. J Cell Biol. 2013;202:45–52. doi: 10.1083/jcb.201212140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei QY, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Gene Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller E, Yang J, DeRan M, Wu C, Su AI, Bonamy GM, et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 24.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13:645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588:2663–2670. doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Lee MJ. Ran Byun M, Furutani-Seiki M, Hong JH, Jung HS. YAP and TAZ regulate skin wound healing. J Invest Dermatol. 2014;134:518–525. doi: 10.1038/jid.2013.339. [DOI] [PubMed] [Google Scholar]
- 29.Beverdam A, Claxton C, Zhang X, James G, Harvey KF, Key B. Yap Controls Stem/Progenitor Cell Proliferation in the Mouse Postnatal Epidermis. J Invest Dermatol. 2012;133:1497–1505. doi: 10.1038/jid.2012.430. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci USA. 2011;108:2270–2275. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan T, Xu Y, Qin Z, Robichaud P, Betcher S, Calderone K, et al. Elevated YAP and its downstream targets CCN1 and CCN2 in basal cell carcinoma: impact on keratinocyte proliferation and stromal cell activation. Am J Pathol. 2014;184:937–943. doi: 10.1016/j.ajpath.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nallet-Staub F, Marsaud V, Li L, Gilbert C, Dodier S, Bataille V, et al. Pro-invasive activity of the Hippo pathway effectors YAP and TAZ in cutaneous melanoma. J Invest Dermatol. 2014;134:123–132. doi: 10.1038/jid.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zenz R, Eferl R, Scheinecker C, Redlich K, Smolen J, Schonthaler HB, et al. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res Ther. 2008;10:201. doi: 10.1186/ar2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angel P, Szabowski A, Schorpp-Kistner M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene. 2001;20:2413–2423. doi: 10.1038/sj.onc.1204380. [DOI] [PubMed] [Google Scholar]
- 35.Eckert RL, Welter JF. Transcription factor regulation of epidermal keratinocyte gene expression. Mol Biol Rep. 1996;23:59–70. doi: 10.1007/BF00357073. [DOI] [PubMed] [Google Scholar]
- 36.Passegue E, Wagner EF. JunB suppresses cell proliferation by transcriptional activation of p16(INK4a) expression. EMBO J. 2000;19:2969–2979. doi: 10.1093/emboj/19.12.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yates S, Rayner TE. Transcription factor activation in response to cutaneous injury: role of AP-1 in reepithelialization. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2002;10:5–15. doi: 10.1046/j.1524-475x.2002.10902.x. [DOI] [PubMed] [Google Scholar]
- 38.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 39.Yang M, Liang Y, Sheng L, Shen G, Liu K, Gu B, et al. A preliminary study of differentially expressed genes in expanded skin and normal skin: implications for adult skin regeneration. Arch Dermatol Res. 2011;303:125–133. doi: 10.1007/s00403-011-1123-2. [DOI] [PubMed] [Google Scholar]
- 40.Du W, Mills I, Sumpio BE. Cyclic strain causes heterogeneous induction of transcription factors, AP-1, CRE binding protein and NF-kB, in endothelial cells: species and vascular bed diversity. J Biomech. 1995;28:1485–1491. doi: 10.1016/0021-9290(95)00096-8. [DOI] [PubMed] [Google Scholar]
- 41.Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 42.Park JM, Adam RM, Peters CA, Guthrie PD, Sun Z, Klagsbrun M, et al. AP-1 mediates stretch-induced expression of HB-EGF in bladder smooth muscle cells. Am J Physiol. 1999;277:C294–C301. doi: 10.1152/ajpcell.1999.277.2.C294. [DOI] [PubMed] [Google Scholar]
- 43.Peverali FA, Basdra EK, Papavassiliou AG. Stretch-mediated activation of selective MAPK subtypes and potentiation of AP-1 binding in human osteoblastic cells. Mol Med. 2001;7:68–78. [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar A, Lnu S, Malya R, Barron D, Moore J, Corry DB, et al. Mechanical stretch activates nuclear factor-kappaB, activator protein-1, and mitogen-activated protein kinases in lung parenchyma: implications in asthma. FASEB J Off Publ Fed Am Soc Exp Biol. 2003;17:1800–1811. doi: 10.1096/fj.02-1148com. [DOI] [PubMed] [Google Scholar]
- 45.Mohan AR, Sooranna SR, Lindstrom TM, Johnson MR, Bennett PR. The effect of mechanical stretch on cyclooxygenase type 2 expression and activator protein-1 and nuclear factor-kappaB activity in human amnion cells. Endocrinology. 2007;148:1850–1857. doi: 10.1210/en.2006-1289. [DOI] [PubMed] [Google Scholar]
- 46.Demicheva E, Hecker M, Korff T. Stretch-induced activation of the transcription factor activator protein-1 controls monocyte chemoattractant protein-1 expression during arteriogenesis. Circ Res. 2008;103:477–484. doi: 10.1161/CIRCRESAHA.108.177782. [DOI] [PubMed] [Google Scholar]
- 47.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 48.Sachs F. Stretch-activated ion channels: what are they? Physiology. 2010;25:50–56. doi: 10.1152/physiol.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trepat X, Deng L, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, et al. Universal physical responses to stretch in the living cell. Nature. 2007;447:592–595. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bikle DD, Ratnam A, Mauro T, Harris J, Pillai S. Changes in calcium responsiveness and handling during keratinocyte differentiation. Potential role of the calcium receptor. J Clin Investig. 1996;97:1085–1093. doi: 10.1172/JCI118501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruder WC, Pratt ED, Brandy NZ, LaVan DA, LeDuc PR, Antaki JF. Calcium signaling is gated by a mechanical threshold in three-dimensional environments. Sci Rep. 2012;2:554. doi: 10.1038/srep00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godbout C, Follonier Castella L, Smith EA, Talele N, Chow ML, Garonna A, et al. The mechanical environment modulates intracellular calcium oscillation activities of myofibroblasts. PLoS One. 2013;8:e64560. doi: 10.1371/journal.pone.0064560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun Y, Chen CS, Fu J. Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annual Rev Biophys. 2012;41:519–542. doi: 10.1146/annurev-biophys-042910-155306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calabrese B, Tabarean IV, Juranka P, Morris CE. Mechanosensitivity of N-type calcium channel currents. Biophys J. 2002;83:2560–2574. doi: 10.1016/S0006-3495(02)75267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kraichely RE, Strege PR, Sarr MG, Kendrick ML, Farrugia G. Lysophosphatidyl choline modulates mechanosensitive L-type Ca2+ current in circular smooth muscle cells from human jejunum. Am J Physiol Gastrointest Liver Physiol. 2009;296:G833–G839. doi: 10.1152/ajpgi.90610.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oda Y, Tu CL, Chang W, Crumrine D, Komuves L, Mauro T, et al. The calcium sensing receptor and its alternatively spliced form in murine epidermal differentiation. J Biol Chem. 2000;275:1183–1190. doi: 10.1074/jbc.275.2.1183. [DOI] [PubMed] [Google Scholar]
- 58.Tu CL, Chang W, Bikle DD. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J Biol Chem. 2001;276:41079–41085. doi: 10.1074/jbc.M107122200. [DOI] [PubMed] [Google Scholar]
- 59.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 60.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA. 2006;103:15463–15468. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malhotra R, D’Souza KM, Staron ML, Birukov KG, Bodi I, Akhter SA. G alpha(q)-mediated activation of GRK2 by mechanical stretch in cardiac myocytes: the role of protein kinase C. J Biol Chem. 2010;285:13748–13760. doi: 10.1074/jbc.M110.109272. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Storch U, MederosySchnitzler M, Gudermann T. G protein-mediated stretch reception. Am J Physiol Heart Circ Physiol. 2012;H302:1241–1249. doi: 10.1152/ajpheart.00818.2011. [DOI] [PubMed] [Google Scholar]
- 64.D’Souza KM, Malhotra R, Philip JL, Staron ML, Theccanat T, Jeevanandam V, et al. G protein-coupled receptor kinase-2 is a novel regulator of collagen synthesis in adult human cardiac fibroblasts. J Biol Chem. 2011;286:15507–15516. doi: 10.1074/jbc.M111.218263. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Tu CL, Chang W, Bikle DD. The calcium-sensing receptor-dependent regulation of cell-cell adhesion and keratinocyte differentiation requires Rho and filamin A. J Invest Dermatol. 2011;131:1119–1128. doi: 10.1038/jid.2010.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tu CL, Chang W, Xie Z, Bikle DD. Inactivation of the calcium sensing receptor inhibits E-cadherin-mediated cell-cell adhesion and calcium-induced differentiation in human epidermal keratinocytes. J Biol Chem. 2008;283:3519–3528. doi: 10.1074/jbc.M708318200. [DOI] [PubMed] [Google Scholar]
- 67.Tu CL, Crumrine DA, Man MQ, Chang W, Elalieh H, You M, et al. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J Invest Dermatol. 2012;132:2350–2359. doi: 10.1038/jid.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsutsumi M, Denda S, Ikeyama K, Goto M, Denda M. Exposure to low temperature induces elevation of intracellular calcium in cultured human keratinocytes. J Invest Dermatol. 2010;130:1945–1948. doi: 10.1038/jid.2010.33. [DOI] [PubMed] [Google Scholar]
- 69.Toth BI, Olah A, Szollosi AG, Biro T. TRP channels in the skin. Br J Pharmacol. 2014;171:2568–2581. doi: 10.1111/bph.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Denda M, Tsutsumi M, Goto M, Ikeyama K, Denda S. Topical application of TRPA1 agonists and brief cold exposure accelerate skin permeability barrier recovery. J Invest Dermatol. 2010;130:1942–1945. doi: 10.1038/jid.2010.32. [DOI] [PubMed] [Google Scholar]
- 71.Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J Off Publ Fed Am Soc Exp Biol. 2013;27:3549–3563. doi: 10.1096/fj.13-229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toth BI, Dobrosi N, Dajnoki A, Czifra G, Olah A, Szollosi AG, et al. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J Invest Dermatol. 2011;131:1095–1104. doi: 10.1038/jid.2010.421. [DOI] [PubMed] [Google Scholar]
- 73.Bodo E, Biro T, Telek A, Czifra G, Griger Z, Toth BI, et al. A hot new twist to hair biology: involvement of vanilloid receptor-1 (VR1/TRPV1) signaling in human hair growth control. Am J Pathol. 2005;166:985–998. doi: 10.1016/S0002-9440(10)62320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Southall MD, Li T, Gharibova LS, Pei Y, Nicol GD, Travers JB. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J Pharmacol Exp Ther. 2003;304:217–222. doi: 10.1124/jpet.102.040675. [DOI] [PubMed] [Google Scholar]
- 75.O’Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci USA. 2014;111:1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akazawa Y, Yuki T, Yoshida H, Sugiyama Y, Inoue S. Activation of TRPV4 strengthens the tight-junction barrier in human epidermal keratinocytes. Skin Pharmacol Physiol. 2013;26:15–21. doi: 10.1159/000343173. [DOI] [PubMed] [Google Scholar]
- 77.Sokabe T, Fukumi-Tominaga T, Yonemura S, Mizuno A, Tominaga M. The TRPV4 channel contributes to intercellular junction formation in keratinocytes. J Biol Chem. 2010;285:18749–18758. doi: 10.1074/jbc.M110.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fusi C, Materazzi S, Minocci D, Maio V, Oranges T, Massi D, et al. Transient receptor potential vanilloid 4 (TRPV4) is downregulated in keratinocytes in human non-melanoma skin cancer. J Invest Dermatol. 2014;134:2408–2417. doi: 10.1038/jid.2014.145. [DOI] [PubMed] [Google Scholar]
- 79.Richard G. Connexins: a connection with the skin. Exp Dermatol. 2000;9:77–96. doi: 10.1034/j.1600-0625.2000.009002077.x. [DOI] [PubMed] [Google Scholar]
- 80.Plotkin LI, Bellido T. Beyond gap junctions: Connexin43 and bone cell signaling. Bone. 2013;52:157–166. doi: 10.1016/j.bone.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scott CA, Tattersall D, O’Toole EA, Kelsell DP. Connexins in epidermal homeostasis and skin disease. Biochim Biophys Acta. 2012;1818:1952–1961. doi: 10.1016/j.bbamem.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, et al. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci USA. 2012;109:3359–3364. doi: 10.1073/pnas.1115967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E, et al. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem. 2008;283:26374–26382. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ziambaras K, Lecanda F, Steinberg TH, Civitelli R. Cyclic stretch enhances gap junctional communication between osteoblastic cells. J Bone Miner Res Off J Am Soc Bone and Miner Res. 1998;13:218–228. doi: 10.1359/jbmr.1998.13.2.218. [DOI] [PubMed] [Google Scholar]
- 85.Martin PE, Easton JA, Hodgins MB, Wright CS. Connexins: sensors of epidermal integrity that are therapeutic targets. FEBS Lett. 2014;588:1304–1314. doi: 10.1016/j.febslet.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 86.Langlois S, Maher AC, Manias JL, Shao Q, Kidder GM, Laird DW. Connexin levels regulate keratinocyte differentiation in the epidermis. J Biol Chem. 2007;282:30171–30180. doi: 10.1074/jbc.M703623200. [DOI] [PubMed] [Google Scholar]
- 87.Brandner JM, Houdek P, Husing B, Kaiser C, Moll I. Connexins 26, 30, and 43: differences among spontaneous, chronic, and accelerated human wound healing. J Invest Dermatol. 2004;122:1310–1320. doi: 10.1111/j.0022-202X.2004.22529.x. [DOI] [PubMed] [Google Scholar]
- 88.Rezze GG, Fregnani JH, Duprat J, Landman G. Cell adhesion and communication proteins are differentially expressed in melanoma progression model. Hum Pathol. 2011;42:409–418. doi: 10.1016/j.humpath.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 89.King TJ, Lampe PD. The gap junction protein connexin32 is a mouse lung tumor suppressor. Cancer Res. 2004;64:7191–7196. doi: 10.1158/0008-5472.CAN-04-0624. [DOI] [PubMed] [Google Scholar]
- 90.Ableser MJ, Penuela S, Lee J, Shao Q, Laird DW. Connexin43 reduces melanoma growth within a keratinocyte microenvironment and during tumorigenesis in vivo. J Biol Chem. 2014;289:1592–1603. doi: 10.1074/jbc.M113.507228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gottlieb PA, Sachs F. Piezo1: properties of a cation selective mechanical channel. Channels. 2012;6:214–219. doi: 10.4161/chan.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyamoto T, Mochizuki T, Nakagomi H, Kira S, Watanabe M, Takayama Y, et al. Functional role for Piezo1 in stretch-evoked Ca2+ influx and ATP release in urothelial cell cultures. J Biol Chem. 2014;289:16565–16575. doi: 10.1074/jbc.M113.528638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DT, et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci USA. 2014;111:16148–16153. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509:622–626. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- 98.Senatore S. Rami Reddy V, Semeriva M, Perrin L, Lalevee N. Response to mechanical stress is mediated by the TRPA channel painless in the Drosophila heart. PLoS Genet. 2010;6:e1001088. doi: 10.1371/journal.pgen.1001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng NH, Lee HH, Shiang JC, Ma MC. Transient receptor potential vanilloid type 1 channels act as mechanoreceptors and cause substance P release and sensory activation in rat kidneys. Am J Physiol Renal Physiol. 2008;294:F316–F325. doi: 10.1152/ajprenal.00308.2007. [DOI] [PubMed] [Google Scholar]
- 100.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 101.Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol. 2005;289:C633–C643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 102.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 103.Dunn CA, Lampe PD. Injury-triggered Akt phosphorylation of Cx43: a ZO-1-driven molecular switch that regulates gap junction size. J Cell Sci. 2014;127:455–464. doi: 10.1242/jcs.142497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang CM, Lincoln J, Cook JE, Becker DL. Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes. 2007;56:2809–2817. doi: 10.2337/db07-0613. [DOI] [PubMed] [Google Scholar]
- 105.Villares GJ, Dobroff AS, Wang H, Zigler M, Melnikova VO, Huang L, et al. Overexpression of protease-activated receptor-1 contributes to melanoma metastasis via regulation of connexin 43. Cancer Res. 2009;69:6730–6737. doi: 10.1158/0008-5472.CAN-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gerner L, Youssef G, O’Shaughnessy RF. The protein phosphatase 2A regulatory subunit Ppp2r2a is required for Connexin-43 dephosphorylation during epidermal barrier acquisition. Exp Dermatol. 2013;22:754–756. doi: 10.1111/exd.12234. [DOI] [PubMed] [Google Scholar]
- 107.Zhang WK, Wang D, Duan Y, Loy MM, Chan HC, Huang P. Mechanosensitive gating of CFTR. Nat Cell Biol. 2010;12:507–512. doi: 10.1038/ncb2053. [DOI] [PubMed] [Google Scholar]
- 108.Kim NH, Cheong KA, Lee TR, Lee AY. PDZK1 upregulation in estrogen-related hyperpigmentation in melasma. J Invest Dermatol. 2012;132:2622–2631. doi: 10.1038/jid.2012.175. [DOI] [PubMed] [Google Scholar]
- 109.Marin T, Gongol B, Chen Z, Woo B, Subramaniam S, Chien S, et al. Mechanosensitive microRNAs-role in endothelial responses to shear stress and redox state. Free Radic Biol Med. 2013;64:61–68. doi: 10.1016/j.freeradbiomed.2013.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yehya N, Yerrapureddy A, Tobias J, Margulies SS. MicroRNA modulate alveolar epithelial response to cyclic stretch. BMC Genom. 2012;13:154. doi: 10.1186/1471-2164-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Banerjee J, Chan YC, Sen CK. MicroRNAs in skin and wound healing. Physiol Genomics. 2011;43:543–556. doi: 10.1152/physiolgenomics.00157.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schneider MR. MicroRNAs as novel players in skin development, homeostasis and disease. Br J Dermatol. 2012;166:22–28. doi: 10.1111/j.1365-2133.2011.10568.x. [DOI] [PubMed] [Google Scholar]
- 113.Sand M, Gambichler T, Sand D, Skrygan M, Altmeyer P, Bechara FG. MicroRNAs and the skin: tiny players in the body’s largest organ. J Dermatol Sci. 2009;53:169–175. doi: 10.1016/j.jdermsci.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 114.Wong VW, Longaker MT, Gurtner GC. Soft tissue mechanotransduction in wound healing and fibrosis. Semin Cell Dev Biol. 2012;23:981–986. doi: 10.1016/j.semcdb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 115.Yagmur C, Akaishi S, Ogawa R, Guneren E. Mechanical receptor-related mechanisms in scar management: a review and hypothesis. Plast Reconstr Surg. 2010;126:426–434. doi: 10.1097/PRS.0b013e3181df715d. [DOI] [PubMed] [Google Scholar]
- 116.Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun. 2010;393:643–648. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weber M, Kim S, Patterson N, Rooney K, Searles CD. MiRNA-155 targets myosin light chain kinase and modulates actin cytoskeleton organization in endothelial cells. Am J Physiol Heart Circ Physiol. 2014;306:H1192–H1203. doi: 10.1152/ajpheart.00521.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Song J, Hu B, Qu H, Bi C, Huang X, Zhang M. Mechanical stretch modulates microRNA 21 expression, participating in proliferation and apoptosis in cultured human aortic smooth muscle cells. PLoS One. 2012;7:e47657. doi: 10.1371/journal.pone.0047657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang T, Feng Y, Sun H, Zhang L, Hao L, Shi C, et al. miR-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am J Pathol. 2012;181:1911–1920. doi: 10.1016/j.ajpath.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 120.Pastar I, Khan AA, Stojadinovic O, Lebrun EA, Medina MC, Brem H, et al. Induction of specific microRNAs inhibits cutaneous wound healing. J Biol Chem. 2012;287:29324–29335. doi: 10.1074/jbc.M112.382135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Karsdal MA, Nielsen MJ, Sand JM, Henriksen K, Genovese F, Bay-Jensen AC, et al. Extracellular matrix remodeling: the common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev Technol. 2013;11:70–92. doi: 10.1089/adt.2012.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reich A, Meurer M, Eckes B, Friedrichs J, Muller DJ. Surface morphology and mechanical properties of fibroblasts from scleroderma patients. J Cell Mol Med. 2009;13:1644–1652. doi: 10.1111/j.1582-4934.2008.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ogawa R, Hsu CK. Mechanobiological dysregulation of the epidermis and dermis in skin disorders and in degeneration. J Cell Mol Med. 2013;17:817–822. doi: 10.1111/jcmm.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu H, Luo H, Li Y, Zhou Y, Jiang Y, Chai J, et al. MicroRNA-21 in scleroderma fibrosis and its function in TGF-beta-regulated fibrosis-related genes expression. J Clin Immunol. 2013;33:1100–1109. doi: 10.1007/s10875-013-9896-z. [DOI] [PubMed] [Google Scholar]
- 125.Qin X, Wang X, Wang Y, Tang Z, Cui Q, Xi J, et al. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci USA. 2010;107:3240–3244. doi: 10.1073/pnas.0914882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sing T, Jinnin M, Yamane K, Honda N, Makino K, Kajihara I, et al. microRNA-92a expression in the sera and dermal fibroblasts increases in patients with scleroderma. Rheumatology. 2012;51:1550–1556. doi: 10.1093/rheumatology/kes120. [DOI] [PubMed] [Google Scholar]
- 127.Huang Y, Crawford M, Higuita-Castro N, Nana-Sinkam P, Ghadiali SN. miR-146a regulates mechanotransduction and pressure-induced inflammation in small airway epithelium. FASEB J Off Publ Fed Am Soc Exp Biol. 2012;26:3351–3364. doi: 10.1096/fj.11-199240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu J, Wu W, Zhang L, Dorset-Martin W, Morris MW, Mitchell ME, et al. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: correction with mesenchymal stem cell treatment. Diabetes. 2012;61:2906–2912. doi: 10.2337/db12-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Smalley KS. Understanding melanoma signaling networks as the basis for molecular targeted therapy. J Invest Dermatol. 2010;130:28–37. doi: 10.1038/jid.2009.177. [DOI] [PubMed] [Google Scholar]
- 130.Clarijs R, Ruiter DJ, De Waal RM. Pathophysiological implications of stroma pattern formation in uveal melanoma. J Cell Physiol. 2003;194:267–271. doi: 10.1002/jcp.10214. [DOI] [PubMed] [Google Scholar]
- 131.Hofmann UB, Westphal JR, Van Muijen GN, Ruiter DJ. Matrix metalloproteinases in human melanoma. J Invest Dermatol. 2000;115:337–344. doi: 10.1046/j.1523-1747.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- 132.Ruiter D, Bogenrieder T, Elder D, Herlyn M. Melanoma-stroma interactions: structural and functional aspects. Lancet Oncol. 2002;3:35–43. doi: 10.1016/s1470-2045(01)00620-9. [DOI] [PubMed] [Google Scholar]
- 133.Satzger I, Mattern A, Kuettler U, Weinspach D, Niebuhr M, Kapp A, et al. microRNA-21 is upregulated in malignant melanoma and influences apoptosis of melanocytic cells. Exp Dermatol. 2012;21:509–514. doi: 10.1111/j.1600-0625.2012.01510.x. [DOI] [PubMed] [Google Scholar]
- 134.Saldanha G, Potter L, Shendge P, Osborne J, Nicholson S, Yii N, et al. Plasma microRNA-21 is associated with tumor burden in cutaneous melanoma. J Invest Dermatol. 2013;133:1381–1384. doi: 10.1038/jid.2012.477. [DOI] [PubMed] [Google Scholar]
- 135.Philippidou D, Schmitt M, Moser D, Margue C, Nazarov PV, Muller A, et al. Signatures of microRNAs and selected microRNA target genes in human melanoma. Cancer Res. 2010;70:4163–4173. doi: 10.1158/0008-5472.CAN-09-4512. [DOI] [PubMed] [Google Scholar]
- 136.Kozubek J, Ma Z, Fleming E, Duggan T, Wu R, Shin DG, et al. In-depth characterization of microRNA transcriptome in melanoma. PLoS One. 2013;8:e72699. doi: 10.1371/journal.pone.0072699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mohamed JS, Lopez MA, Boriek AM. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3beta. J Biol Chem. 2010;285:29336–29347. doi: 10.1074/jbc.M110.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reuland SN, Smith SM, Bemis LT, Goldstein NB, Almeida AR, Partyka KA, et al. MicroRNA-26a is strongly downregulated in melanoma and induces cell death through repression of silencer of death domains (SODD) J Invest Dermatol. 2013;133:1286–1293. doi: 10.1038/jid.2012.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Heinemann A, Zhao F, Pechlivanis S, Eberle J, Steinle A, Diederichs S, et al. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Cancer Res. 2012;72:460–471. doi: 10.1158/0008-5472.CAN-11-1977. [DOI] [PubMed] [Google Scholar]
- 140.Zhu HY, Li C, Bai WD, Su LL, Liu JQ, Li Y, et al. MicroRNA-21 Regulates hTERT via PTEN in Hypertrophic Scar Fibroblasts. PLoS One. 2014;9:e97114. doi: 10.1371/journal.pone.0097114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guinea-Viniegra J, Jimenez M, Schonthaler HB, Navarro R, Delgado Y, Concha-Garzon MJ, et al. Targeting miR-21 to treat psoriasis. Science translational medicine. 2014;6:225re1. doi: 10.1126/scitranslmed.3008089. [DOI] [PubMed] [Google Scholar]
- 142.Meisgen F, Xu N, Wei T, Janson PC, Obad S, Broom O, et al. MiR-21 is up-regulated in psoriasis and suppresses T cell apoptosis. Exp Dermatol. 2012;21:312–314. doi: 10.1111/j.1600-0625.2012.01462.x. [DOI] [PubMed] [Google Scholar]
- 143.Joyce CE, Zhou X, Xia J, Ryan C, Thrash B, Menter A, et al. Deep sequencing of small RNAs from human skin reveals major alterations in the psoriasis miRNAome. Hum Mol Genet. 2011;20:4025–4040. doi: 10.1093/hmg/ddr331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Darido C, Georgy SR, Wilanowski T, Dworkin S, Auden A, Zhao Q, et al. Targeting of the tumor suppressor GRHL3 by a miR-21-dependent proto-oncogenic network results in PTEN loss and tumorigenesis. Cancer Cell. 2011;20:635–648. doi: 10.1016/j.ccr.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 145.Bhandari A, Gordon W, Dizon D, Hopkin AS, Gordon E, Yu Z, et al. The Grainyhead transcription factor Grhl3/Get1 suppresses miR-21 expression and tumorigenesis in skin: modulation of the miR-21 target MSH2 by RNA-binding protein DND1. Oncogene. 2013;32:1497–1507. doi: 10.1038/onc.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang KC, Garmire LX, Young A, Nguyen P, Trinh A, Subramaniam S, et al. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proc Natl Acad Sci USA. 2010;107:3234–3239. doi: 10.1073/pnas.0914825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kraemer A, Chen IP, Henning S, Faust A, Volkmer B, Atkinson MJ, et al. UVA and UVB irradiation differentially regulate microRNA expression in human primary keratinocytes. PLoS One. 2013;8:e83392. doi: 10.1371/journal.pone.0083392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hildebrand J, Rutze M, Walz N, Gallinat S, Wenck H, Deppert W, et al. A comprehensive analysis of microRNA expression during human keratinocyte differentiation in vitro and in vivo. J Invest Dermatol. 2011;131:20–29. doi: 10.1038/jid.2010.268. [DOI] [PubMed] [Google Scholar]
- 149.Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. MicroRNA-24 regulates the processing of latent TGFbeta1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J Cell Physiol. 2011;226:1407–1414. doi: 10.1002/jcp.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Amelio I, Lena AM, Viticchie G, Shalom-Feuerstein R, Terrinoni A, Dinsdale D, et al. miR-24 triggers epidermal differentiation by controlling actin adhesion and cell migration. J Cell Biol. 2012;199:347–363. doi: 10.1083/jcb.201203134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang S, Wang W, Gu Q, Xue J, Cao H, Tang Y, et al. Protein and miRNA profiling of radiation-induced skin injury in rats: the protective role of peroxiredoxin-6 against ionizing radiation. Free Radic Biol Med. 2014;69:96–107. doi: 10.1016/j.freeradbiomed.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 152.Cha HJ, Lee KS, Lee GT, Lee KK, Hong JT, Lee SN, et al. Altered miRNA expression profiles are involved in the protective effects of troxerutin against ultraviolet B radiation in normal human dermal fibroblasts. Int J Mol Med. 2014;33:957–963. doi: 10.3892/ijmm.2014.1647. [DOI] [PubMed] [Google Scholar]
- 153.Sand M, Skrygan M, Sand D, Georgas D, Gambichler T, Hahn SA, et al. Comparative microarray analysis of microRNA expression profiles in primary cutaneous malignant melanoma, cutaneous malignant melanoma metastases, and benign melanocytic nevi. Cell Tissue Res. 2013;351:85–98. doi: 10.1007/s00441-012-1514-5. [DOI] [PubMed] [Google Scholar]
- 154.Ryu B, Hwang S, Alani RM. MicroRNAs as an emerging target for melanoma therapy. J Invest Dermatol. 2013;133:1137–1139. doi: 10.1038/jid.2012.505. [DOI] [PubMed] [Google Scholar]
- 155.Sand M, Skrygan M, Georgas D, Sand D, Hahn SA, Gambichler T, et al. Microarray analysis of microRNA expression in cutaneous squamous cell carcinoma. J Dermatol Sci. 2012;68:119–126. doi: 10.1016/j.jdermsci.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 156.Cheng BB, Qu MJ, Wu LL, Shen Y, Yan ZQ, Zhang P, et al. MicroRNA-34a targets Forkhead box j2 to modulate differentiation of endothelial progenitor cells in response to shear stress. J Mol Cell Cardiol. 2014;74C:4–12. doi: 10.1016/j.yjmcc.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 157.Herbert KJ, Cook AL, Snow ET. SIRT1 inhibition restores apoptotic sensitivity in p53-mutated human keratinocytes. Toxicol Appl Pharmacol. 2014;277:288–297. doi: 10.1016/j.taap.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 158.Lefort K, Brooks Y, Ostano P, Cario-Andre M, Calpini V, Guinea-Viniegra J, et al. A miR-34a-SIRT6 axis in the squamous cell differentiation network. EMBO J. 2013;32:2248–2263. doi: 10.1038/emboj.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xie H, Lee L, Caramuta S, Hoog A, Browaldh N, Bjornhagen V, et al. MicroRNA expression patterns related to merkel cell polyomavirus infection in human merkel cell carcinoma. J Invest Dermatol. 2014;134:507–517. doi: 10.1038/jid.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cozzolino AM, Pedace L, Castori M, De Simone P, Preziosi N, Sperduti I, et al. Analysis of the miR-34a locus in 62 patients with familial cutaneous melanoma negative for CDKN2A/CDK4 screening. Fam Cancer. 2012;11:201–208. doi: 10.1007/s10689-011-9502-6. [DOI] [PubMed] [Google Scholar]
- 161.Antonini D, Russo MT, De Rosa L, Gorrese M, Del Vecchio L, Missero C. Transcriptional repression of miR-34 family contributes to p63-mediated cell cycle progression in epidermal cells. J Invest Dermatol. 2010;130:1249–1257. doi: 10.1038/jid.2009.438. [DOI] [PubMed] [Google Scholar]
- 162.Zhou L, Qi RQ, Liu M, Xu YP, Li G, Weiland M, et al. microRNA miR-17-92 cluster is highly expressed in epidermal Langerhans cells but not required for its development. Genes Immun. 2014;15:57–61. doi: 10.1038/gene.2013.61. [DOI] [PubMed] [Google Scholar]
- 163.Sand M, Skrygan M, Sand D, Georgas D, Hahn SA, Gambichler T, et al. Expression of microRNAs in basal cell carcinoma. Br J Dermatol. 2012;167:847–855. doi: 10.1111/j.1365-2133.2012.11022.x. [DOI] [PubMed] [Google Scholar]
- 164.Maj J, Jankowska-Konsur A, Sadakierska-Chudy A, Noga L, Reich A. Altered microRNA expression in mycosis fungoides. Br J Dermatol. 2012;166:331–336. doi: 10.1111/j.1365-2133.2011.10669.x. [DOI] [PubMed] [Google Scholar]
- 165.Turczynska KM, Bhattachariya A, Sall J, Goransson O, Sward K, Hellstrand P, et al. Stretch-sensitive down-regulation of the miR-144/451 cluster in vascular smooth muscle and its role in AMP-activated protein kinase signaling. PLoS One. 2013;8:e65135. doi: 10.1371/journal.pone.0065135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hu B, Song JT, Qu HY, Bi CL, Huang XZ, Liu XX, et al. Mechanical stretch suppresses microRNA-145 expression by activating extracellular signal-regulated kinase 1/2 and upregulating angiotensin-converting enzyme to alter vascular smooth muscle cell phenotype. PLoS One. 2014;9:e96338. doi: 10.1371/journal.pone.0096338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kohlstedt K, Trouvain C, Boettger T, Shi L, Fisslthaler B, Fleming I. AMP-activated protein kinase regulates endothelial cell angiotensin-converting enzyme expression via p53 and the post-transcriptional regulation of microRNA-143/145. Circ Res. 2013;112:1150–1158. doi: 10.1161/CIRCRESAHA.113.301282. [DOI] [PubMed] [Google Scholar]
- 168.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 169.Dynoodt P, Speeckaert R, De Wever O, Chevolet I, Brochez L, Lambert J, et al. miR-145 overexpression suppresses the migration and invasion of metastatic melanoma cells. Int J Oncol. 2013;42:1443–1451. doi: 10.3892/ijo.2013.1823. [DOI] [PubMed] [Google Scholar]
- 170.Yamashita J, Iwakiri T, Fukushima S, Jinnin M, Miyashita A, Hamasaki T, et al. The rs2910164 G>C polymorphism in microRNA-146a is associated with the incidence of malignant melanoma. Melanoma Res. 2013;23:13–20. doi: 10.1097/CMR.0b013e32835c5b30. [DOI] [PubMed] [Google Scholar]
- 171.Xia P, Fang X, Zhang ZH, Huang Q, Yan KX, Kang KF, et al. Dysregulation of miRNA146a versus IRAK1 induces IL-17 persistence in the psoriatic skin lesions. Immunol Lett. 2012;148:151–162. doi: 10.1016/j.imlet.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 172.Farzan SF, Karagas MR, Christensen BC, Li Z, Kuriger JK, Nelson HH, et al. RNASEL and MIR146A SNP-SNP interaction as a susceptibility factor for non-melanoma skin cancer. PLoS One. 2014;9:e93602. doi: 10.1371/journal.pone.0093602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.van Solingen C, Araldi E, Chamorro-Jorganes A, Fernandez-Hernando C, Suarez Y. Improved repair of dermal wounds in mice lacking microRNA-155. J Cell Mol Med. 2014;18:1104–1112. doi: 10.1111/jcmm.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moyal L, Barzilai A, Gorovitz B, Hirshberg A, Amariglio N, Jacob-Hirsch J, et al. miR-155 is involved in tumor progression of mycosis fungoides. Exp Dermatol. 2013;22:431–433. doi: 10.1111/exd.12161. [DOI] [PubMed] [Google Scholar]
- 175.Kopp KL, Ralfkiaer U, Gjerdrum LM, Helvad R, Pedersen IH, Litman T, et al. STAT5-mediated expression of oncogenic miR-155 in cutaneous T-cell lymphoma. Cell Cycle. 2013;12:1939–1947. doi: 10.4161/cc.24987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kopp KL, Ralfkiaer U, Nielsen BS, Gniadecki R, Woetmann A, Odum N, et al. Expression of miR-155 and miR-126 in situ in cutaneous T-cell lymphoma. APMIS Acta Pathol Microbiol Immunol Scand. 2013;121:1020–1024. doi: 10.1111/apm.12162. [DOI] [PubMed] [Google Scholar]
- 177.Monsalvez V, Montes-Moreno S, Artiga MJ, Rodriguez ME, Sanchez-Espiridion B, Lozano M, et al. MicroRNAs as prognostic markers in indolent primary cutaneous B-cell lymphoma. Mod Pathol Off J U S Can Acad Pathol Inc. 2013;26:171–181. doi: 10.1038/modpathol.2012.149. [DOI] [PubMed] [Google Scholar]
- 178.Benner MF, Ballabio E, van Kester MS, Saunders NJ, Vermeer MH, Willemze R, et al. Primary cutaneous anaplastic large cell lymphoma shows a distinct miRNA expression profile and reveals differences from tumor-stage mycosis fungoides. Exp Dermatol. 2012;21:632–634. doi: 10.1111/j.1600-0625.2012.01548.x. [DOI] [PubMed] [Google Scholar]
- 179.Ralfkiaer U, Hagedorn PH, Bangsgaard N, Lovendorf MB, Ahler CB, Svensson L, et al. Diagnostic microRNA profiling in cutaneous T-cell lymphoma (CTCL) Blood. 2011;118:5891–5900. doi: 10.1182/blood-2011-06-358382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 181.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gotte M, Mohr C, Koo CY, Stock C, Vaske AK, Viola M, et al. miR-145-dependent targeting of junctional adhesion molecule A and modulation of fascin expression are associated with reduced breast cancer cell motility and invasiveness. Oncogene. 2010;29:6569–6580. doi: 10.1038/onc.2010.386. [DOI] [PubMed] [Google Scholar]
- 183.Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci USA. 2009;106:3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]