Abstract
Bacteriocin production is a widespread phenomenon among bacteria. Bacteriocins hold great promise for the treatment of diseases caused by pathogenic bacteria and could be used in the future as alternatives to existing antibiotics. The anti-infective potential of bacteriocins for inhibiting pathogens has been shown in various food matrices including cheese, meat, and vegetables. However, their inhibition of pathogens in vivo remains unclear and needs more investigation, due mainly to difficulties associated with demonstrating their health benefits. Many bacteriocins produced by established or potential probiotic organisms have been evaluated as potential therapeutic agents and interesting findings have been documented in vitro as well as in a few in vivo studies. Some recent in vivo studies point to the efficacy of bacteriocin-based treatments of human and animal infections. While further investigation remains necessary before the possibilities for bacteriocins in clinical practice can be described more fully, this review provides an overview of their potential applications to human and veterinary health.
Keywords: Bacteriocins, Anti-infective properties, Pathogens, Clinical therapy
Introduction
Bacteriocins are peptides of bacterial origin with relatively narrow spectra of bacteriostatic or bactericidal activity directed primarily against species closely related to the producing strain [55]. They make up a heterogeneous family in terms of heat stability, molecular mass, mode of release and action, microbial target, and mechanism conferring protection to the producing strain [48, 57]. Bacteriocins were first identified almost 100 years ago and have since been found among most families of eubacteria as well as many actinomycetes. Although they have been described as universally produced, including by some members of the Archaea [107, 117], the vast majority of known bacteriocins are products of Gram-positive bacteria. Known producers among Gram-negative bacteria are few, and their bacteriocins are of less diverse nature [53].
Bacteriocin classification has been based on biochemical properties, genetics, and bioactivity profiles. The initial scheme proposed by Klaenhammer [72] grew as new bacteriocins were identified and characterized, but eventually became the subject of debate [22, 26, 35, 43, 56, 66, 155]. Four main classes, reviewed over 10 years ago [22], are currently recognized. Class I bacteriocins, also called the lantibiotics, are heat-stable, low molecular mass peptides (<5 kDa) characterized by the presence of unusual amino acids such as lanthionine or β-methyllanthionine produced by post-translational modification [149]. Class II bacteriocins are small heat-stable, unmodified peptides (<10 kDa) comprising three subclasses, namely class IIa (pediocin-like), class IIb (two-chain), and IIc (non-pediocin-like single-chain) [39]. Class III bacteriocins are large (>30 kDa) heat-labile proteins, while class IV bacteriocins include cyclic peptides with covalently linked N and C termini. A special group contains the so-called high molecular weight (HMW) bacteriocins, which have been shown by electron microscopy to be phage-tail-like molecules. Similarities between bacteriophages and HMW bacteriocins in terms of structure have been proven on the basis of morphological characters, immunological cross-reactivity, complementation, and DNA–DNA hybridization. The most thoroughly studied phage-tail-like bacteriocins, namely the F-type and R-type pyocins produced by Pseudomonas aeruginosa, have been reviewed [86]. A third type of pyocins called S, are colicin-like, soluble, and protease-sensitive proteins [86]. Archaea produce their own distinct family of bacteriocin-like antimicrobial substances known as archaeocins [116]. Known bacteriocins that have been isolated are listed in an online database called BACTIBASE [52, 53] at http://bactibase.pfba-lab-tun.org, which provides physicochemical, structural, microbiological, and taxonomic information on bacteriocins of both Gram-positive and Gram-negative bacteria.
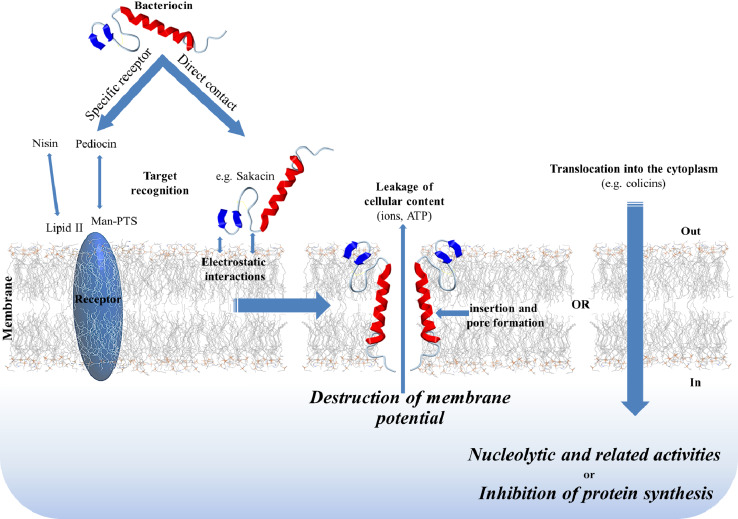
The lethal action of bacteriocins begins with entry into the target cell by interaction with specific cell surface molecules that act as receptors (Fig. 1). Several such receptors have been identified, including mannose-phosphotransferase systems (man-PTS) and lipid II. Class IIa bacteriocins target a phylogenetically defined subgroup of man-PTS on sensitive cells of genera such as Listeria, Enterococcus, and Lactobacillus [71]. The N-terminal domain of class II bacteriocins plays a crucial role in electrostatic binding to the membrane surface [41], while the C-terminal region is important for determining target specificity. Lipid II, which has an essential role in cell wall synthesis, serves as an anchoring receptor for the vancomycin/teicoplanin family of glycopeptides, mannopeptimycins, ramoplanins, katanosins/plusbacins, and lantibiotics, a subject previously reviewed [23]. While vancomycin binds the D-Ala-D-Ala peptide side chain of lipid II, the lantibiotic nisin binds the highly conserved sugar-pyrophosphate moiety. Vancomycin-resistant strains thus remain sensitive to nisin [12, 60]. Binding to the membrane surface (through specific or non-specific receptors) leads to subsequent insertion of the bacteriocin into the cytoplasmic membrane to form ion-permeable channels and ultimately large pores. Three models of pore formation have been proposed, namely the barrel-stave model, the carpet model, and the wedge model. Lantibiotics may form pores according to the barrel-stave or wedge models, while class II bacteriocins may function according to the barrel-stave or carpet models. These mechanisms have been previously reviewed [13, 21, 87]. Microbial cell death may result from efflux of metabolites, dissipation of vital ion gradients, leakage of cell contents, non-specific degradation of cellular DNA, inhibition of protein synthesis through specific cleavage of 16S rRNA, or by cell lysis resulting from inhibition of peptidoglycan synthesis [148]. For example, after translocation into the periplasmic space, colicin E2 kills the target cell by non-specific DNA cleavage, colicin E3 kills through inactivation of the ribosome, and colicin M inhibits murein biosynthesis by hydrolyzing the pyrophosphate linkage between bactoprenol and the murein precursor. These mechanisms have been reviewed previously [46, 73]. The modes of intracellular action of antibiotics and bacteriocins thus differ considerably. For example, macrolides are an important class of antibiotics that block protein synthesis by binding to the nascent peptide exit tunnel of the ribosome [69], while RNase-type colicins inhibit protein synthesis by cleaving a specific site near the 3′ end of 16S rRNA. The action of these colicins is the subject of a review [93].
Fig. 1.
Overview of the mode of action of bacteriocins
First-generation antibiotics have long been misused in human and veterinary medicine and in agriculture and are losing their effectiveness. The emergence of pathogens resistant to them is relentless and has long been a serious public health threat [118]. Of particular notoriety are ubiquitous pathogens such as Salmonella typhimurium, Staphylococcus aureus, and Listeria monocytogenes, all of which are having a major impact on human health and food safety. Many scientists have therefore tested bacteriocins as alternatives to antibiotics. While the effectiveness of bacteriocins and their producer strains as inhibitors of pathogens in food matrices has been shown repeatedly, their inhibitory activity in vivo remains uncertain and needs more investigation. Recent advances in bacteriocin identification and characterization have renewed interest in the study of their use as therapeutic agents, and support is accumulating for their efficacy in treating infections in humans and animals [30, 31, 69]. New bacteriocins with promising in vitro antimicrobial profiles and in vivo effectiveness are being identified and the use of this category of antimicrobial agent as alternatives or adjuncts to help alleviate the current problem of antibiotic overuse and resistance may now be seriously envisaged [37]. This review focuses on progress in bacteriocin applications since 2005.
Human applications
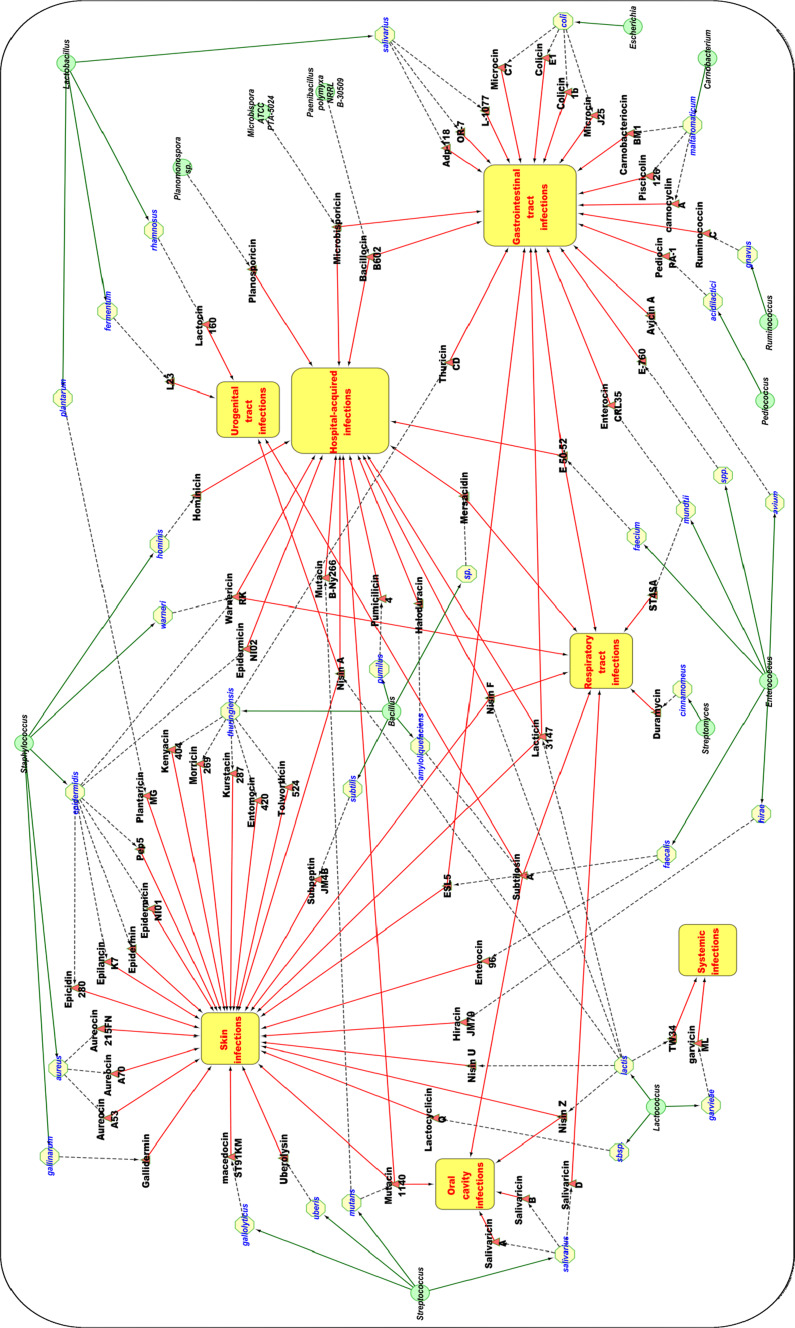
In recent years, bacteriocins have attracted increasing attention in medicine, since they are active at nanomolar concentrations and non-toxic to humans. Furthermore, their unique mechanism of action, highly specific activity, and their low propensity to generate resistance are attractive properties. Many bacteriocins have been assessed for potential application as therapeutic agents [139]. Bacteriocins have been used successfully to fight skin infections as well as oral, respiratory, gastrointestinal, and urogenital tract infections (Fig. 2; Table 1).
Fig. 2.
Review of therapeutic potential of bacteriocins (in vitro and in vivo studies). Green circles producer genus; yellow circles producer strain; red triangles bacteriocins; yellow rectangles anti-infective properties of bacteriocins
Table 1.
Summary list of the bacteriocins discussed in this article
| Bacteriocin | Producer strain | Class | Anti-infective effects | In vitro/vivo | Reference |
|---|---|---|---|---|---|
| Nisin A | Lactococcus lactis subsp. | Ia | Multidrug-resistant strain | In vitro | [99] |
| Human mastitis | In vivo (human model) | [40] | |||
| Spermicidal activity | In vitro | [105] | |||
| Bovine mastitis | In vivo (bovine model) | – | |||
| Nisin F | Lactococcus lactis subsp. | Ia | Multidrug-resistant strain | In vitro | [100] |
| Multidrug-resistant strain | In vivo (murine model) | [11, 140, 141] | |||
| Cutaneous diseases | In vivo (murine model) | [34] | |||
| Pneumonia | In vitro/in vivo (rat model) | [32, 33] | |||
| Nisin Z | Lactococcus lactis subsp. | Ia | Bovine mastitis | In vivo (bovine model) | [17, 152] |
| Oral candidiasis | In vitro | [2] | |||
| Ruminococcin C | Ruminococcus gnavus E1 | Ia | Gastrointestinal disease | In vivo (rat model) | [29] |
| Microbisporicin | Microbispora ATCC PTA-5024 | I | Multidrug-resistant strain | In vitro/in vivo (animal model) | [20, 65] |
| Gastrointestinal disease | In vitro | [78] | |||
| Mutacin 1140 | Streptococcus mutans | I | Cutaneous infection | In vitro | [45] |
| Multidrug-resistant strain | In vitro | [45] | |||
| Oral caries | In vitro/in vivo (animal + human models) | [58, 59, 154] | |||
| Hominicin | Staphylococcus hominis MBBL2-9 | I | Multidrug-resistant strain | In vitro | [70] |
| Gallidermin | Staphylococcus gallinarum Tü3928 | I | Cutaneous infection | In vitro/in vivo (rat model) | [83] |
| Mutacin B-Ny266 | Streptococcus mutans ATCC 202022 | I | Multidrug-resistant strain | In vitro/in vivo (murine model) | [88] |
| Mersacidin | Bacillus sp. strain HIL Y-85,54728 | I | Rhinitis/multidrug-resistant strain | mouse model | [76] |
| Duramycin | Streptomyces cinnamomeus NRRL B-1699 | I | Cystic fibrosis | In vivo (human model) | [49, 123] |
| Salivaricin A | Streptococcus salivarius K12 | I | Halitosis | In vitro/in vivo (animal + human models) | [14–16] |
| Salivaricin B | |||||
| Salivaricin D | Streptococcus salivarius 5M6c | I | Pneumoniae | In vitro | [8] |
| Nisin U | Lactococcus lactis spp. | I | Bovine mastitis | In vitro | [150] |
| Macedocin ST91KM | Streptococcus gallolyticus subsp. macedonicus ST91KM | I | Bovine mastitis | In vitro | [96, 97] |
| Pep5 | Staphylococcus epidermidis 5 | I | Bovine mastitis | In vitro | [142] |
| Epidermin | Staphylococcus epidermidis | I | Bovine mastitis | In vitro | [142] |
| Epilancin K7 | Staphylococcus epidermidis | I | Bovine mastitis | In vitro | [142] |
| Epicidin 280 | Staphylococcus epidermidis | I | Bovine mastitis | In vitro | [142] |
| Lacticin 3147 | Lactococcus lactis DPC 3147 | Ib | Multidrug-resistant strain | In vitro | [99] |
| Multidrug-resistant strain | In vivo (murine model) | [98] | |||
| Gastrointestinal disease | In vitro (fermentation model) | [102] | |||
| Bovine mastitis | In vivo (bovine model) | [27, 28, 74] | |||
| Thuricin CD | Bacillus thuringiensis DPC 6431 | Ib | Gastrointestinal disease | In vitro/distal colon model | [103, 104] |
| Haloduracin | Bacillus halodurans C-125 | Ib | Multidrug-resistant strain | In vitro | [91] |
| Planosporicin | Planomonospora sp. DSM14920 | IB | Multidrug-resistant strain | In vitro/in vivo (mouse model) | [19] |
| Avicin A | Enterococcus avium | IIa | Gastrointestinal disease | In vitro | [7] |
| Piscicolin 126 | Carnobacterium maltaromaticum UAL307 | IIa | Gastrointestinal disease | In vitro | [85] |
| Carnobacteriocin BM1 | IIa | Gastrointestinal disease | |||
| Epidermicin NI01 | Staphylococcus epidermidis 224 | II | Cutaneous infection | In vitro | [112] |
| Multidrug-resistant strain | |||||
| Cutaneous infection | |||||
| Pediocin PA-1 | Pediococcus acidilactici UL5 | IIa | Gastrointestinal disease | In vivo (murine model) | [31] |
| Enterocin CRL35 | Enterococcus mundtii CRL35 | IIa | Gastrointestinal disease | In vivo (murine model) | [109] |
| ST4SA | Enterococcus mundtii | IIa | Middle ear infection | In vitro | [75] |
| Bacillocin B602 | Paenibacillus polymyxa NRRL B-30509 | IIa | Campylobacter infection | In vivo (Chickens + turkey models) | [24, 125] |
| Multidrug-resistant strain | In vitro | [130] | |||
| E 50-52 | Enterococcus faecium NRRL B-30746 | IIa | Gastrointestinal diseases | In situ (chicken) | [132] |
| Tuberculosis | mouse model | [122] | |||
| Multidrug-resistant strain | In vitro | [130] | |||
| L-1077 | Lactobacillus salivarius NRRL B-50053 | IIa | Gastrointestinal diseases | In vitro/in situ (chicken model) | [131] |
| Enterocin 96 | Enterococcus faecalis WHE96 | II | Cutaneous infections | In vitro | [64] |
| Hiracin JM79 | Enterococcus hirae DCH5 | II | Cutaneous infections | In vitro | [111] |
| Adp-118 | Lactobacillus salivarius UCC118 | IIb | Gastrointestinal disease | In vivo (murine + pig models) | [25, 106] |
| E-760 | Enterococcus spp. NRRL B-30745 | IIb | Campylobacter infection | In situ (chicken model) | [79] |
| Carnocyclin A | Carnobacterium maltaromaticum UAL307 | Cyclic | Gastrointestinal disease | In vitro | [85] |
| Subtilosin A | Bacillus amyloliquefaciens | Cyclic | Periodontal disease | In vitro | [119] |
| Pneumonia | In vitro | [119] | |||
| Bacterial vaginosis | In vitro | [129] | |||
| Spermicidal activity | In vivo (animal model) | [120, 127] | |||
| Garvicin ML | Lactococcus garvieae DCC43 | Cyclic | Lactococcosis | In vitro | [9, 110] |
| Microcin J25 | Escherichia coli | Gastrointestinal disease | In vivo (murine model) | [81] | |
| Microcin C7 | Escherichia coli H22 | Gastrointestinal disease | In vitro/in vivo (murine model) | [30] | |
| Colicin 1b | |||||
| Colicin E1 | |||||
| Plantaricin MG | Lactobacillus plantarum KLDS1.0391 | UN | Cutaneous infection | In vitro | [47] |
| Subpeptin JM4B | Bacillus subtilis JM4 | UN | Cutaneous infection | In vitro | [153] |
| ESL5 | Enterococcus faecalis SL-5 | UN | Cutaneous infection | In vitro/in vivo (human model) | [68] |
| Gastrointestinal disease | |||||
| Pumicilicin 4 | Bacillus pumilus WAPB4 | UN | Multidrug-resistant strain | In vitro | [5] |
| Warnericin RK | Staphylococcus warneri RK | UN | Multidrug-resistant strain | In vitro | [3] |
| Staphylococcus epidermidis A487 | Pneumonia | In vitro | [146] | ||
| Lactocin 160 | Lactobacillus rhamnosus 160 | UN | Bacterial vaginosis | In vitro/in-vivo (rabbit model) | [38, 138] |
| L23 | Lactobacillus fermentum L23 | UN | Bacterial vaginosis | In vitro | [94] |
| TW34 | Lactococcus lactis TW34 | UN | Lactococcosis | In vitro | [115] |
| Lactocyclicin Q | Lactococcus ssp. QU12 | UN | Cutaneous infections | In vitro | [113] |
| Uberolysin | Streptococcus uberis strain 42 | UN | Bovine mastitis | In vitro | [151] |
| Aureocin A70 | Staphylococcus aureus A70 | UN | Bovine mastitis | In vitro | [142] |
| Aureocin A53 | Staphylococcus aureus A53 | UN | Bovine mastitis | In vitro | [142] |
| Aureocin 215FN | Staphylococcus aureus 215FN | UN | Bovine mastitis | In vitro | [142] |
| Morricin 269 | Bacillus thuringiensis subsp. Morrisoni (LBIT 269) | BLIS | Bovine mastitis | In vitro | [6] |
| Kurstacin 287 | Bacillus thuringiensis subsp. Kurstaki (LBIT 287) | BLIS | Bovine mastitis | In vitro | [6] |
| Kenyacin 404 | Bacillus thuringiensis subsp. Kenyae (LBIT 404) | BLIS | Bovine mastitis | In vitro | [6] |
| Entomocin 420 | Bacillus thuringiensis subsp. Entomocidus (LBIT 420) | BLIS | Bovine mastitis | In vitro | [6] |
| Tolworthcin 524 | Bacillus thuringiensis subsp. Tolworthi (LBIT 524) | BLIS | Bovine mastitis | In vitro | [6] |
| OR-7 | Lactobacillus salivarius NRRL B-30514 | Campylobacter infection | In vitro (chicken model) | [126] |
UN unclassified, BLIS bacteriocin-like inhibitory substance
Hospital-acquired infections
The increasing emergence of antibiotic resistance among nosocomial and community-acquired pathogens is a serious public health threat and will have a major impact on antimicrobial treatments in the future [118]. Currently, the greatest cause for concern is infection by S. aureus, enterococci and pneumococci strains displaying acquired resistance to six or more antibiotics. Advances in bacteriocin identification and characterization have prompted renewed interest in the use of these molecules as anti-infective agents. An increasing number of bacteriocins are reportedly potent inhibitors of staphylococci of clinical relevance. Of particular interest are the modes of action of bacteriocins, which differ markedly from those of antibiotics. In a recent study, the antimicrobial activities of lacticin 3147 and nisin A against a common set of methicillin-resistant S. aureus (MRSA) and vancomycin-resistant enterococci (VRE) were assessed [99]. The minimum inhibitory concentration (MIC) is defined as the lowest concentration of antimicrobial agent that completely inhibits the growth of a test organism. Nisin A was thus found more effective against MRSA (MIC = 0.5–4.1 μg/ml), while lacticin 3147 was more potent against VRE (MIC = 2–8.3 μg/ml). More recently, the in vivo activity of lacticin 3147 against luminescent S. aureus was investigated using BALB/c mice infected via the intraperitoneal route with 106 colony-forming units (CFUs) of S. aureus Xen 29, and treated subcutaneously with the lantibiotic (50.85 mg/kg of Ltnα and 43.8 mg/kg of Ltnβ) after 1.5 h. Lacticin 3147 treatment brought a significant reduction of pathogen levels in the liver, spleen, and kidneys. The capacity to inhibit systemic infection by S. aureus makes lacticin 3147 a promising candidate for potential therapeutic applications [98].
In a prior study, Piper and coworkers evaluated the in vitro activity of nisin variants A, F, Q, and Z against MRSA [100], finding variant F the most active, inhibiting strains ST 525 (MIC = 1.2 μg/ml), EC 676 (MIC = 5.1 μg/ml), EC 725 (MIC = 2.5 μg/ml), VISA 22900 (MIC = 41.4 μg/ml), VISA 22781 (MIC = 82.8 μg/ml), hVISA 35197 (MIC = 2.5 μg/ml), S. aureus 8325-4 (MIC = 5.1 μg/ml) and Lactococcus lactis HP (MIC = 0.064 μg/ml). The ability of nisin F to control S. aureus infection in the peritoneal cavity was assessed in a murine model [11]. In this trial, C57BL/6 mice were infected intraperitoneally with 108 CFU of S. aureus Xen 36 and treated with a single dose of nisin F (640 AU) 4 h postinfection. Growth of the pathogen was inhibited for only 15 min, after which re-emergence of pathogen bioluminescence was observed. It was presumed that degradation by proteolytic enzymes and/or inactivation through non-specific binding at physiological pH values limited the inhibitory activity. Injected into the peritoneum, nisin F did not alter bacterial diversity in the mouse gastrointestinal tract [141].
More recently, the feasibility of nisin F-loaded brushite cement was evaluated [140]. Incorporation of nisin F into the formula did not cause substantial changes in cement structure or chemistry. The resulting bone cement clearly inhibited in vitro the growth of S. aureus Xen 36 for at least 72 h. In vivo studies were then carried out using BALB/c mice infected with bioluminescent S. aureus Xen 36 [140]. Nisin F-loaded brushite cement was implanted in a subcutaneous pocket and the mice were then infected with the pathogen. As expected, nisin-F-loaded cement protected the mice from S. aureus infection for 7 days and no viable bacterial cells were detected in the implants. Incorporation of nisin F into brushite cement resulted in a high burst release, followed by a slow steady release. However, the release trend was slightly more rapid than reported for other drugs. Release kinetics could be improved by altering porosity of the cement or by incorporating polymeric molecules associated with the desired bacteriocin. A two-peptide lantibiotic called haloduracin, produced by Bacillus halodurans C-125, was also compared to nisin [91]. Some of the tested strains (vancomycin-resistant Enterococcus) were more sensitive to haloduracin (MIC = 781 nM) than to nisin (MIC = 12,500 nM). Conversely, nisin was more effective than haloduracin against methicillin-resistant S. aureus C5 in the micromolar range (MIC = 4,690 and 1,560 μM, respectively). Haloduracin was more stable at physiological pH than was nisin, which is of interest for therapeutic applications. E 50-52 and B602, two pediocin-like bacteriocins produced respectively by Enterococcus faecium NRRL B-30746 and Paenibacillus polymyxa NRRL B-30509, were shown to be active against multidrug-resistant bacteria (MDR) [130]. All tested MRSA isolates were highly sensitive to both B602 (MIC < 0.025 μg/ml) and E 50-52 (MIC ≤ 0.05–0.2 μg/ml). E 50-52 was active against isolates of Gram-negative Acinetobacter baumannii and Proteus spp., while S. aureus isolates showed susceptibilities in narrower ranges, with MIC ranging from ≤0.05 to 0.2 μg/ml. Similarly, isolates of Gram-negative organisms such as A. baumannii, Citrobacter freundii, Escherichia coli, Klebsiella pneumoniae, and Proteus spp. were sensitive to B602, with MIC from ≤0.025 to 0.05 μg/ml. MRSA isolates were similarly susceptible to this bacteriocin, with MIC ≤0.025 μg/ml. Compared to published MIC of nisin for MRSA and methicillin-sensitive S. aureus (MSSA), varying from 0.5 to 4.1 μg/ml [99], these isolates were 100 times more sensitive.
Planosporicin is a type B lantibiotic produced by Planomonospora sp. DSM14920 and displaying a wide antimicrobial spectrum [19]. Patented as antibiotic 97518, planosporicin has MIC values of 2–16 μg/ml against S. aureus (including MRSA), 4 μg/ml against Streptococcus pneumoniae and ≤0.25 μg/ml against Clostridium perfringens. Although no known lipid II binding motif is present in planosporicin, its mechanism of action involves inhibition of cell wall biosynthesis. The authors developed a murine model of Streptococcus pyogenes infection to evaluate the in vivo antibiotic activity of planosporicin (MIC = 0.5 μg/ml). The peptide protected mice with an ED50 of 3.75 mg/kg by both intravenous and subcutaneous administration. No acute toxicity was observed during this trial. Castiglione and coworkers [19, 20] reported comparisons of the bioactivity of microbisporicin, planosporicin, actagardine, mersacidin, and nisin. Microbisporicin is produced by Microbispora ATCC PTA-5024 and secreted as isoforms A1 and A2 with distinguishing post-translational modifications 5-chloro-trypthopan and mono (in A2) or bis-hydroxylated (in A1) proline [20]. The activity of microbisporicin appeared comparable to or better than those of reference lantibiotics such as planosporicin, actagardine, mersacidin, and nisin. Microbisporicin, also called NAI-107 or antibiotic 107891, was active against Gram-positive bacteria including MRSA, glycopeptide-intermediate S. aureus (GISA) and VRE [65] and against Gram-negative bacteria including Moraxella catarrhalis, Neisseria spp. and Haemophilus influenzae, which were insensitive to most of the lantibiotics and only moderately susceptible to nisin. The strong bactericidal activity of microbisporicin observed in vitro was also confirmed in vivo in animal models of severe infection induced by MDR Gram-positive pathogens [20, 65].
Kim and coworkers [70] purified a lantibiotic from Staphylococcus hominis MBBL 2–9. Hominicin showed potent activity against MRSA ATCC 11435 (MIC = 0.96 μg/ml) and VISA CCARM 3501 (MIC = 3.82 μg/ml), but none against VRE CCARM 5024. The authors also compared the potency of hominicin and other antimicrobial peptides against MRSA, VISA, and S. aureus, obtaining an MIC of 0.06 μg/ml for hominicin against S. aureus, just slightly higher than that of nisin (0.05 μg/ml) and lower than those of gramicidin (1.76 μg/ml), polymyxin B (0.16 μg/ml), colistin (0.13 μg/ml) and bacitracin (0.16 μg/ml) [70]. Hominicin was more active than nisin against MRSA and VISA. Similarly, pumilicin 4, a 2-kDa bacteriocin produced by Bacillus pumilus WAPB4 isolated from water [5] showed broad spectrum antibacterial activity including MRSA and VRE. Pumilicin 4 (at 80 AU/ml) induced 3 and 2.5 log reduction of MRSA and VRE counts, respectively. Mutacin B-Ny266, a lantibiotic produced by Streptococcus mutans Ny266 (ATCC 202022) was shown to be active in vitro against VREF 0032 (MIC = 2.7 μg/ml), MRSA 0034 (MIC = 2 μg/ml) and E. coli 0025 (MIC = 1.7 μg/ml) but not active at the highest tested concentration (64 μg/ml) against Candida albicans or Pseudomonas aeruginosa [88]. The efficacy of mutacin B-Ny266 against staphylococci infection was also shown, protecting mice infected intraperitoneally with 3.1 × 107 CFU of S. aureus Smith (MSSA) and injected with a single intraperitoneal dose of bacteriocin (1 mg/kg) immediately after infection [88].
Respiratory infections
Community-acquired pneumonia is a frequent infectious respiratory disease that remains a major cause of morbidity in both the developing and developed world [82, 101]. While S. pneumoniae is the most frequently isolated pathogen, pneumonia may be caused also by a wide variety of pathogens including H. influenzae and M. catarrhalis, S. aureus, Enterobacteriaceae and P. aeruginosa. Less common pneumonia etiologies include S. pyogenes, Neisseria meningitidis, Pasteurella multocida, and H. influenzae type b. [82]. Legionella pneumophila, another pathogen responsible for a severe pneumonia, infects humans through inhalation of contaminated aerosols [89]. Produced by Staphylococcus warneri RK, warnericin RK was the first bacteriocin found to have anti-Legionella activity [146]. This peptide exhibits a very narrow spectrum of activity almost limited to the Legionella genus, and a hemolytic activity. More recently, Staphylococcus epidermidis strain A487 was shown to produce the same bacteriocin (called peptide 487 by the authors) with strong activity against MRSA in vitro [3]. Bacteriocins inhibitory to S. pneumoniae, S. aureus, and P. aeruginosa in vitro have been purified recently and characterized microbiologically, including subtilosin A [119], L23 [94], mutacin 1140 [45], and salivaricin D [8]. Acinetobacter baumannii, P. aeruginosa, S. pneumonia, S. aureus, and E. faecium, all known to cause otitis media, one of the most common infections in young children [114], are inhibited by a class IIa bacteriocin called peptide ST4SA, produced by Enterococcus mundtii [75]. Peptide ST4SA was more active than other antibiotics in vitro and remained active when incubated in blood and middle ear fluid and could thus be useful for the treatment of middle ear infections.
Nisin F has been reported to inhibit clinical strains of S. aureus in vitro [33]. In a recent in vivo trial, Nisin F administered intranasally for four consecutive days inhibited the growth of S. aureus in the respiratory tract of immunocompromised Wistar rats [32] and prevented the onset of symptoms of pneumonia, mucosal hyperplasia, blood contamination, or necrotic lesions. The authors concluded that nisin F is nontoxic and could be used to treat respiratory tract infections caused by S. aureus. However, these findings remain to be confirmed in humans. The in vivo activity of the bacteriocin mersacidin was assessed against MRSA colonizing nasal epithelia in a mouse rhinitis and MRSA carrier model recently developed by Kruszewska and collaborators [76]. Administered intranasally twice daily over three days, mersacidin completely killed MRSA. Furthermore, no bacteremia was detected in animals post-treated with the bacteriocin.
The antimycobacterial activity of bacteriocin E50-52 was evaluated in vivo using a murine model of tuberculosis infection developed by Sosunov and collaborators [122]. C57BL/6JCit (B6) mice were challenged with Mycobacterium tuberculosis strain H37Rv to induce acute tuberculosis. During this trial, bacteriocin E50-52 was compared to rifampicin, a clinically used antibiotic. A 5-day treatment with bacteriocin/liposome complex was sufficient to increase animal life span. The effect was slightly weaker than that of rifampicin injected at tenfold higher concentrations. Carroll and coworkers [18] have reported the in vitro susceptibility of clinically relevant strains of mycobacteria to the lantibiotics lacticin 3147 and nisin A. Lacticin 3147 was particularly active against M. tuberculosis H37Ra (MIC90 = 7.5 μg/ml; MIC90 values are defined as the lowest concentration of bacteriocin that prevent the growth of >90 % of the bacterial population), M. avium subsp. paratuberculosis ATCC 19698 (MIC90 = 15 μg/ml) and M. kansasii CIT11/06 (MIC90 = 60 μg/ml). Although inhibitory, nisin A was less potent against the tested mycobacteria, with MIC90 values of 60 μg/ml for M. kansasii and >60 μg/ml for M. avium subsp. paratuberculosis and M. tuberculosis H37Ra.
In patients with cystic fibrosis (CF), chronic lung infection with P. aeruginosa occurs in infancy and early childhood, damaging the epithelial surfaces. The company AOP Orphan Pharmaceuticals AG have developed a therapeutic product for CF treatment based on the lantibiotic duramycin (Moli1901). A phase II placebo-controlled, double-blind, single-center trial was successfully completed using an inhaled aerosol Moli1901 in 24 patients with CF and stable lung disease [49]. Pulmonary pharmacokinetics and safety of nebulized duramycin were studied in healthy human volunteers [123]. Pyocin S2, a HMW bacteriocin isolated recently from P. aeruginosa PA01, was found more effective than aztreonam or tobramycin (two approved antibiotics for treatment of chronic CF lung infection) against biofilms of P. aeruginosa clinical isolate YHP14, showing 4-log reductions in cell survival at a concentration equivalent to the highest concentration of antibiotic. The ability of pyocin S2 to provide protection against an infectious dose (104 CFU) of P. aeruginosa YHP14 in the caterpillar Galleria mellonella was then tested. Viable counts of pathogen 3 h post-infection in larvae treated with pyocin S2 (27 μg/g) were as low as 10–40 CFU, compared to 5–10 × 108 CFU in untreated larvae at the time of death. The authors also found pyocin S2 to be nontoxic to the host in the absence of infection [121].
Skin infections
Community-acquired MRSA infection has become epidemic, with skin and soft-tissue infections being the most frequent forms of disease. S. aureus may colonize intact skin only transiently and remain present in nasal mucosa without the occurrence of any pathogenic event [63]. However, the bacteria can colonize broken skin to utilize the nutrient-rich bloodstream, express a wide range of virulence factors that disrupt the epithelial barrier, and diminish neutrophil functions and cell-mediated immune responses [63]. Staphylococci cause an array of cutaneous and systemic infections including impetigo, furuncles, subcutaneous abscesses, staphylococcal scalded skin syndrome (SSSS), toxic shock syndrome, and neonatal toxic shock syndrome-like exanthematous disease or NTED [63]. Acne vulgaris is another common human skin disease, mostly caused by pathogenic Propionibacterium acnes and S. epidermidis [90]. C57BL/6 mice challenged with a bioluminescent strain of S. aureus (Xen 36) and then treated with subcutaneous administration of nisin F (256 AU at 24 and 48 h) did not resist subcutaneous skin infections any better than did control mice [34], although the lantibiotic did appear to stimulate the immune system. The authors concluded that nisin F is ineffective as a treatment for deep dermal staphylococcal infections. Previously, strains of Streptococcus salivarius that produce a bacteriocin-like substance (BLIS) have been shown to inhibit growth of P. acnes [10]. In this study, the prevalence of S. salivarius in the oropharynx of patients with acne vulgaris was evaluated. A total of 33 human clinical isolates of S. salivarius were tested for BLIS-producing capacity based on antagonism of three streptococci and P. acnes. One-third of the isolated S. salivarius strains inhibited growth of P. acnes [10]. Enterococcus faecalis SL-5 isolated from fecal specimen of a healthy Korean adult was found to produce bacteriocin ESL5, which inhibited P. acnes, Bacillus cereus, Bacillus subtilis, L. monocytogenes, and S. aureus [68]. A double-blind, randomized, placebo-controlled phase III trial was then conducted to evaluate the therapeutic effect of E. faecalis SL-5 in patients suffering from mild to moderate acne using a lotion containing E. faecalis SL-5. With a twice-per-day treatment, the lotion significantly reduced inflammatory pustule-like lesions in patients. However, the authors noted the presence of a mild unpleasant odor and skin dryness, reported by both treated and untreated patients.
Gallidermin is a well-characterized lantibiotic that has shown great therapeutic potential against several pathogens including P. acnes and S. aureus. Entrapped in anionic niosomes incorporated into a gel, gallidermin was used to treat P. acnes and S. aureus infections in rats by transdermal absorption [83]. The gel formulation provided superior cumulative flux into the skin with no risk of systemic effects. The entrapment efficiency was about 45 %. However, the gallidermin–niosome system decreased the in vitro antibacterial activity by half compared to nonencapsulated gallidermin. Several antistaphylococcal class II bacteriocins, including enterocin 96 from E. faecalis WHE96 [64], hiracin JM79 from Enterococcus hirae DCH5 [111] and lactocyclicin Q from Lactococcus subsp QU12 [113] have been purified and characterized in vitro in recent years. These have been shown to possess a wide spectrum of activity inhibitory also to enterococci, lactobacilli, and L. monocytogenes.
Mutacin 1140 (MU1140), a 22-amino-acid lantibiotic produced by S. mutans, has a promising role in promoting colonization of the oral cavity by the producer strain [45]. All tested Gram-positive bacterial strains (n = 30) were sensitive to MU1140, while none of the 28 Gram-negative species or the yeasts tested showed sensitivity. MU1140 was particularly active against E. faecalis (MIC = 16–32 μg/ml), S. aureus (MIC = 16 μg/ml), S. epidermidis (MIC = 16 μg/ml), Streptococcus agalactiae (MIC = 4 μg/ml), S. pneumoniae ATCC 49619 (MIC = 4 μg/ml), and S. pyogenes (MIC = 0.5 μg/ml). This study also demonstrated the susceptibility of vancomycin-resistant and oxacillin-resistant S. aureus, E. faecalis, and E. faecium strains to this lantibiotic [45]. Initially isolated from fermented cream, Lactobacillus plantarum KLDS1.0391 produces plantaricin MG, another bacteriocin with a broad spectrum of activity [47]. Partially purified plantaricin MG inhibited S. aureus, Pseudomonas fluorescens ATCC 17485, Pseudomonas putida ATCC 13525, E. coli, and S. typhimurium during in vitro studies. Likewise, Wu and coworkers [153] reported the susceptibility of Shigella flexeneri to subpeptin JM4B, a dodecapeptide bacteriocin obtained from B. subtilis JM4. Subpeptin JM4B was also active in vitro against S. aureus, P. aeruginosa, Salmonella sp., and E. faecalis. More recently, a new class II bacteriocin produced by S. epidermidis 224 isolated from human skin has been identified [112]. Epidermicin NI01 has been shown to be active against a range of clinically relevant pathogens including S. epidermidis (MIC = 0.063–4 μg/ml), Staphylococcus saprophyticus (MIC = 0.5–1 μg/ml), S. hominis (MIC = 1 μg/ml), S. warneri (MIC = 1 μg/ml), E. faecalis (MIC = 1 μg/ml), VRE (MIC = 0.5–2 μg/ml), MRSA (MIC = 1–2 μg/ml) and S. aureus 1195 (MIC = 2 μg/ml). Toxicity data indicate that peptide NI01 at 100 × MIC has no adverse effects on human blood cells or on human fibroblast cell lines, which is encouraging with respect to clinical applications.
Mastitis is an inflammation of the breast, usually caused by an infection during lactation. Staphylococcus albus and S. aureus are the pathogens most commonly associated with human mastitis, while E. coli and streptococci are involved less frequently. Fernández and collaborators [40] have found nisin effective for the treatment of staphylococcal mastitis during lactation. During this trial, eight women who did not respond to cloxacillin, clindamycin, amoxicillin/clavulanic acid and/or erythromycin were treated with nisin solution (6 μg/ml) applied to the nipple and mammary areola after each suckling episode for 2 weeks. The treatment reduced staphylococcal counts considerably and led to the complete disappearance of clinical signs of mastitis.
Gastrointestinal tract infections
Oral cavity
The oral cavity is not simply the entrance to the gastrointestinal tract, but harbors some of the most varied and extensive indigenous microbiota in the human body. The oral cavity provides a continuous source of infectious agents. Oral disorders can lead to dental caries (cavities), periodontal diseases, gingivitis, halitosis, and periodontitis. Probiotics have been used successfully to control gastrointestinal diseases, as reviewed in [42]. Much attention has been given recently to the health-promoting properties of probiotics in the treatment of periodontal disease, reviewed in [136]. Porphyromonas gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans are regarded as the principal potential periodontal pathogens [4]. Therapy consists of replacing resident pathogens with a nonpathogenic bacteriocin-producing variant. Described by Shelburne and collaborators [119], subtilosin A inhibited in vitro growth of P. gingivalis (MIC = 3.125–6.25 μg/ml). The Lactobacillus paracasei strain HL32 produces a 114-kDa bacteriocin that has been shown to inhibit P. gingivalis selectively, making it a candidate for periodontal treatment [92]. However, this bacteriocin appears toxic to human erythrocytes and would therefore not be suitable for systemic use. Since the creation of S. mutans BCS3-L1 (in the year 2000, using recombinant DNA technology) for use in the prevention of dental caries, Hillman and collaborators have improved this producer of mutacin 1140 but not lactic acid [59]. Additional mutations have been introduced to allow rapid elimination of the strain in case of adverse side effects and to increase genetic stability. The newly transformed strain A2JM has been tested in vitro and in vivo in animal models. The authors believe that based on the apparent safety and efficacy, A2JM is ready for testing in human clinical trials [59]. They have also developed and patented a probiotic mouthwash called ProBiora3. The product consists of a blend of Streptococcus uberis KJ2, Streptococcus oralis KJ3, and Streptococcus rattus JH145 (MU1140 producing and lactic acid-deficient). Studies of ProBiora3 in rats [58] and human volunteers [154] have been carried out to assess its safety and effectiveness as a mouthwash.
Halitosis or oral malodor results from metabolic activity of oral bacteria that produce volatile sulphur compounds, valeric acid, butyric acid, and putrescine [80]. S. salivarius K12 produces two lantibiotics, salivaricin A, and salivaricin B and has been shown active against bacterial species involved in halitosis, inhibiting Micrococcus luteus I1, Streptococcus anginosis T29, Eubacterium saburreum ATCC 33271, and Micromonas micros ATCC 33270 [15]. Further toxicology studies in animals have not revealed any negative effect of multiple dosing [14]. Meanwhile, S. salivarius K12 has been evaluated for safety and human tolerance in a randomized, placebo-controlled, double-blind trial [16]. Clinical trials of Lactobacillus salivarius strain WB21 [62] for probiotic control of halitosis and Lactobacillus reuteri (Prodentis) for preventing periodontal disease [147] have also been carried out. In a recent screening, 357 oral strains of Lactobacillus isolated from children were evaluated as inhibitors of oral pathogens [135]. Six of these were strong inhibitors of P. gingivalis and A. actinomycetemcomitans in vitro. Further characterization and safety analyses are necessary before testing any of these isolates in clinical trials.
Oral candidiasis and denture-associated stomatitis are oral infections caused by C. albicans. Nisin Z has been evaluated in vitro for efficacy against C. albicans adhesion and transition following contact with normal human gingival cells [2]. At concentrations of 25 μg/ml, nisin Z reduced C. albicans adhesion to gingival monolayer cultures and inhibited germ tube formation in vitro.
Gastric and intestinal infections
The human gastrointestinal (GI) tract harbors trillions of microorganisms (approximately 1011 per gram of feces) that carry out vital processes for normal digestive functions of the host and play an important albeit not yet fully understood role in the maturation of human immunity and defense against pathogens [145]. Intestinal floral equilibrium may be altered by several factors, such as infection or antibiotic therapy, resulting in diarrhea and hence exposure of the subject to additional and often more dangerous infections, for example C. difficile [84]. Several bacteriocins have been tested as inhibitors of GI pathogenic bacteria such as Helicobacter pylori, C. difficile, L. monocytogenes, and Salmonella (Fig. 1).
Clostridium difficile
C. difficile is the most common identifiable cause of bacteria-associated diarrhea in developed nations and the major cause of gastroenteritis in nursing homes and other long-term-care facilities [67]. Microbisporicin shows promising activity in vitro against several clostridia, with MIC values <0.125 μg/ml against C. difficile, C. perfringens, Clostridium butyricum, Clostridium beijerinckii, and Clostridium septicum [78]. Microbisporicin also inhibits L. monocytogenes (MIC = 0.125 μg/ml). The impact of this bacteriocin on the intestinal microbiota needs to be studied in vivo before it can be considered for use as an orally administered therapeutic agent.
Lacticin 3147, a two-component lantibiotic produced by L. lactis, has significant antimicrobial activity against pathogenic isolates of C. difficile [102], bactericidal in the same concentration range as vancomycin and metronidazole, the most commonly used antibiotics. At 18 μg/ml, it totally eliminated C. difficile (106 CFU/ml) from a model fecal environment within 30 min with only minor effects on commensal flora such as Lactobacillus and Bifidobacterium. However, porcine studies indicate that gastric acidity is a hurdle for lacticin 3147, to be overcome by suitable technology (e.g., encapsulation) before it can be considered for use as an orally administered therapeutic agent [44]. Despite the effectiveness of lacticin 3147 against L. monocytogenes in vitro, its producer strain failed to prevent infection by the pathogen in a mouse model [37]. Bacillus thuringiensis DPC 6431, isolated from human feces, has been shown to produce thuricin CD [104]. Identified as a two-peptide lantibiotic, thuricin CD has impressive in vitro activity against clostridia including 21 clinical isolates of C. difficile, Clostridium histolyticum NCIMB 503, Clostridium indolis NCIMB 9731, Clostridium lituseburense NCIMB 10637, C. perfringens LMG 10468, C. perfringens LMG 11264, and Clostridium tyrobutyricum NCIMB 8243. It has little impact on most other genera, including gastrointestinal commensals such as Bifidobacterium, Enterococcus, and Lactobacillus. Comparing the impact of vancomycin, metronidazole, lacticin 3147, and thuricin CD on microbiota composition in a model of the human distal colon, Rea and coworkers [103] found that although effective against C. difficile, the antibiotics and lacticin 3147 had a broad spectrum of activity making them less suited to therapeutic use in the gastrointestinal tract. In contrast, the narrow spectrum of activity of thuricin CD represents a therapeutic advantage, killing C. difficile but having little impact on microbiota composition.
Recently isolated directly from the dominant fecal microbiota of a healthy man, Ruminococcus gnavus E1 was used to colonize the digestive tract of axenic rats. Under these conditions, it was found to express the rumA genes weakly and produce a bacteriocin called ruminococcin C or RumC in the cecum [29]. RumC is active against C. perfringens and resistant to high temperature and acidic pH.
Listeria monocytogenes
Listeria monocytogenes is an opportunistic and invasive pathogen transmitted to animals and humans by ingestion of contaminated food. It causes listeriosis, an infection with serious consequences (septicemia, meningitis, encephalitis, spontaneous abortion) among high-risk individuals (the elderly, immunocompromised individuals, pregnant women) and potentially fatal outcome [143]. One strain of particular note is Carnobacterium maltaromaticum UAL307, isolated from fresh pork. Strain UAL307 produces several bacteriocins, including piscicolin 126, carnobacteriocin BM1, and carnocyclin A, and exhibits potent activity against a number of Gram-positive organisms, including numerous Listeria species [85]. While piscicolin 126 and carnobacteriocin BM1 belong to subclass IIa, carnocyclin A has a cyclic structure consisting of 60 amino acids. This cyclic peptide inhibited all of the Gram-positive strains tested, including pathogens such as L. monocytogenes and S. aureus. Likewise, Enterococcus avium isolated from feces of healthy infants was found to produce avicin A, a class IIa bacteriocin active (MIC < 1.33 μg/ml) against L. monocytogenes [7]. The bacteriocin Abp-118 is a broad-spectrum class IIb bacteriocin produced by L. salivarius strain UCC118, which was originally isolated from the intestine of a human patient. This bacteriocin has been shown to protect in vivo against L. monocytogenes invasion [25]. Mice fed L. salivarius UCC118 for 3 days and then challenged with L. monocytogenes were significantly protected against infection of the liver and spleen [25]. It was later shown that feeding L. salivarius UCC118 for 7 days affected intestinal microbiota diversity in mice and pigs less than antibiotic treatment did [106].
Previously, the efficacy of pediocin PA-1 and its producer strain Pediococcus acidilactici UL5 was assessed in vivo in a murine model of L. monocytogenes infection (2 × 109 CFU) [31]. In this trial, the intragastric administration of 1010 CFU of P. acidilactici UL5 failed to protect against pathogen infection in mice. Researchers were surprised to note increased invasiveness of L. monocytogenes in the intestine, liver, and spleen tissues of P. acidilactici-treated mice compared to control mice. However, they did note a 2-log reduction of fecal listerial counts following post-infection administration of pediocin PA-1 in three daily doses of 250 μg. Enterocin CRL35 is a class IIa bacteriocin produced by E. mundtii CRL35 and active against Listeria. In this instance, the effectiveness of enterocin CRL35 and its producing strain were evaluated in a murine model of pregnancy-associated L. monocytogenes infection [109]. Oral administration of 65 μg of enterocin CRL35 (every 12 h for 3 days) provided 1.6-log and 1-log decreases in L. monocytogenes FBUNT (MIC = 1.6 ng/ml) in spleen and liver respectively on day 3 postinfection. By day 7, L. monocytogenes was undetectable in these organs in CRL35-treated animals. Furthermore, no bacteria were recovered from the bloodstream of mothers or fetuses. Administering 2 × 109 CFU of E. mundtii CRL35 5 h prior to infection resulted in reduced counts of L. monocytogenes FBUNT in liver and spleen. Although E. mundtii CRL35 was shown to inhibit or delay Listeria translocation, it did not prevent it completely. Both pediocin PA-1 and enterocin CRL35 were shown more effective than their producer strains as inhibitors of L. monocytogenes translocation.
Salmonella
Salmonella is a major foodborne pathogen that causes gastroenteritis, enteric fever, bacteremia, and subsequent focal infections [1]. In recent years, the emergence of antibiotic-resistant Salmonella due to the overuse of antimicrobial agents has become a public health concern. Bacteriocins could replace antibiotics currently used to treat infections. The efficacy of microcin J25 (MccJ25) has been evaluated in a murine model of Salmonella infection [81]. In mice receiving an intraperitoneal dose of 106 CFU of Salmonella Newport and treated with MccJ25 2 h later and then daily for the next 6 days, reductions in pathogen viable count were 2–3 log in both the spleen and the liver compared to control mice. The authors also reported low hemolytic activity for MccJ25.
E. coli H22 has been shown to produce several bacteriocins, including microcin C7 and colicins 1b and E1, and to inhibit several pathogenic or potentially pathogenic enterobacteria [30]. Pathogens Enterobacter agglomerans, E. coli, K. pneumoniae, Morganella morganii, Salmonella enterica, S. flexeneri, and Yersinia enterocolitica were inhibited in vitro by strain H22. The in vitro inhibition of S. flexeneri was shown mediated by microcin C7. A further trial involving a germ-free mouse model confirmed the efficacy of H22 in reducing fecal population of S. flexneri to undetectable levels.
Urogenital tract infections
Bacterial vaginosis (BV) is a common vaginal infection associated with a variety of serious health risks in women of reproductive age. BV is characterized by a decrease in the lactobacilli population and by an increase in the number of other organisms. Several bacteria have been implicated in BV, in particular Gardnerella vaginalis, Mycoplasma hominis, and Mobiluncus curtisii [77]. One of the greatest concerns in conventional treatment with antibiotics such as clindamycin and metronidazole is inhibition of healthy vaginal microflora along with the pathogens. Clinicians and microbiologists are therefore looking for alternative approaches that treat BV without killing Lactobacillus. Lactocin 160, a bacteriocin produced by Lactobacillus rhamnosus 160 (a healthy vaginal isolate), specifically inhibits BV-associated pathogens such as G. vaginalis and Prevotella bivia without affecting the healthy microflora [138]. Furthermore, both in vitro and in vivo toxicity studies have shown that lactocin 160 does not irritate vaginal epithelial tissue, is neither hemolytic for human erythrocytes nor toxic to vaginal lactobacilli and therefore could be safe for use in BV treatment [38]. More recently, Turovskiy and Chikindas [137] reported that zinc lactate and soap-nut extract act synergistically with lactocin 160 against G. vaginalis. Produced by Bacillus amyloliquefaciens isolated from a fermented dairy beverage, subtilosin A [128] has been shown in vitro to inhibit L. monocytogenes, G. vaginalis, and S. agalactiae without affecting healthy vaginal lactobacilli. Moreover, synergy with glycerol monolaurate, lauric arginate, and ε-poly-l-lysine make it effective at lower concentrations [129]. Its safety, nontoxicity, and specificity toward pathogenic G. vaginalis make subtilosin A a promising candidate for the treatment of BV. Obtained in partially purified form from Lactobacillus fermentum L23, an isolate from vaginal flora of healthy women, bacteriocin L23 (at 640 AU/ml) inhibits several Gram-positive and Gram-negative pathogenic strains and Candida species [94], producing large zones of inhibition of E. coli (∅ = 41 mm), P. aeruginosa (∅ = 44 mm), Proteus mirabilis (∅ = 40 mm), K. pneumoniae (∅ = 34 mm), Klebsiella oxytoca (∅ = 30 mm), Enterobacter aerogenes (∅ = 49 mm), S. epidermidis (∅ = 21 mm), S. saprophyticus (∅ = 30 mm), S. aureus (∅ = 36 mm), C. albicans (∅ = 15 mm), and Candida glabrata (∅ = 13 mm). This bacteriocin has good potential for inactivation of pathogens in the urogenital tract and its activity should be confirmed in vivo.
Spermicidal activity
The spermicidal activity of bacteriocins has received much attention in conjunction with their potent antibacterial activity and lack of harmful effects on human tissues. Although nisin has significant spermicidal activity [105], it appears harmful to healthy vaginal microbiota, making it unsuitable for this type of commercial application [127]. In contrast, subtilosin appears inoffensive to vaginal lactobacilli and is lethal to horse/pony, cattle, boar, rat [120], and human [127] spermatozoa. Subtilosin therefore appears to have great potential for use as a contraceptive.
Animal applications
As the incidence of disease caused by drug-resistant pathogens increases in the human population, debate grows around the systematic use of antibiotics to protect livestock and enhance growth performance. This use of antibiotics is being blamed increasingly for the emergence of antibiotic resistance and is regarded more and more as an unnecessary risk to human health [36]. The use of bacteriocins and their producer strains (probiotics) in animal feed is proposed as a safe alternative to running such risks (Fig. 2; Table 1).
Skin infections
Mastitis is the most significant and costly disease of dairy cattle. It is caused by bacterial intramammary infection, S. aureus, S. uberis, and Streptococcus dysgalactiae being the pathogens most frequently involved [51]. The strong activity of many of bacteriocins against mastitis pathogens has spurred interest in their potential application to the control of udder infections, as reviewed in [95]. Although bacteriocin-based products have been developed successfully in the past, only nisin and lacticin 3147 have been applied to the control of mastitis on a commercial scale. Nisin is used in the product Wipe Out® Dairy Wipes (Immucell, Portland, ME, USA), marketed as a teat disinfectant prior to milking. Nisin Z has been tested in the treatment of clinical [17] and subclinical mastitis [152]. The nisin Z used in these studies was only about 50 % pure, and some udder irritation was observed. Another commercial product, Mast Out® (Immucell, Portland, ME, USA) has been submitted recently for FDA approval. An intramammary infusion product, Mast Out contains pharmaceutical-grade nisin A to lessen irritation. Lacticin 3147, a lantibiotic produced by L. lactis DPC3147 and highly inhibitory to many mastitis-causing pathogens, has received much attention in this regard. Lacticin 3147 has been evaluated for use as a dry cow therapy in teat seal formulations [27, 28]. Two patents were filed in 2010 for this use of the bacteriocin. In recent trials, Klostermann and collaborators [74] found that 10-min treatment with teat dip containing lacticin 3147 reduced counts of S. aureus, S. dysgalactiae, and S. uberis on the teats of lactating cows by 80, 97, and 90 %, respectively. In recent years, several potential antimastitis bacteriocins, such as nisin U [150], uberolysin [151], and bacteriocin ST91KM [96, 97], have been characterized. Varella Coelho and collaborators [142] report the activity of several staphylococcal bacteriocins (Pep5, epidermin, epilancin K7, epicidin 280, aureocins A70, A53, and 215FN) against S. aureus and S. agalactiae involved in bovine mastitis. Bacteriocins (morricin 269, kurstacin 287, kenyacin 404, entomocin 420, and tolworthcin 524) produced by different isolates of B. thuringiensis have been tested against a collection of S. aureus isolates involved in subclinical bovine mastitis [6]. While S. aureus isolates have shown a high level of resistance to commercial antibiotics, they appear sensitive to bacteriocins, in particular morricin 269 and kurstacin 287. Several bacteriocins are being evaluated for their efficacy in treatment or prevention of infections. Mastitis treatment could be enhanced by using a mixture of bacteriocins displaying synergistic antimastitis activity.
Systemic infection
Lactococcosis is an emerging disease worldwide that causes systemic septicemia in numerous fish species and major economic losses both in marine and freshwater aquaculture. Lactococcus garvieae is the etiological agent of the disease [144]. A novel circular bacteriocin called garvicin ML (GarML), produced by L. garvieae DCC43 isolated from Mallard ducks (Anas platyrhynchos) [110], has been shown to inhibit various Gram-positive bacterial strains in vitro including L. garvieae, C. perfringens, L. monocytogenes, and S. pneumoniae [9]. However, no activity against any Gram-negative bacteria was noted. The bacteriocin producer L. lactis TW34 isolated from the intestinal tract of Odontesthes platensis also inhibits L. garvieae [115]. Crude TW34 bacteriocin was also found to inhibit Lactococcus piscium, Carnobacterium piscicola, Streptococcus iniae, E. faecalis, and L. monocytogenes. L. lactis TW34 and L. garvieae DCC43 could provide an alternative for the control of lactococcosis and should therefore be considered for future challenge experiments with fish.
Gastrointestinal tract infections
Campylobacter infection is a leading cause of diarrheal disease and foodborne gastroenteritis worldwide. Campylobacter jejuni or Campylobacter coli are the species typically involved. Transmission of Campylobacter to humans occurs by ingestion of contaminated food or water, including undercooked poultry, fresh produce, and unpasteurized milk, or by direct contact with animals. The bacterium is zoonotic and poultry is considered a major reservoir for transmission to humans [61]. Considerable progress towards isolation of anti-Campylobacter poultry commensal bacteria and associated bacteriocins has been made in the past few years, as reviewed in [133]. Several potent anti-Campylobacter bacteriocins produced by commensal bacteria isolated from the chicken intestine have been purified and characterized, including bacillocin B602 from P. polymyxa [134], E50-52, and E-760 from Enterococcus spp. [24, 79], OR-7 and L-1077 from L. salivarius [126, 131]. Purified B602 from P. polymyxa NRRL B-30509 has been microencapsulated in polyvinylpyrrolidone and incorporated into chicken [125] and turkey [24] feed. In both trials, B602 feeds reduced Campylobacter colonization to undetectable levels in chickens and turkeys experimentally infected with C. jejuni and C. coli, respectively. Similar results were obtained with bacteriocin E50-52, which reduced Campylobacter counts by six log cycles in the ceca of broiler chickens infected with C. jejuni and Salmonella enteritidis [132]. Moreover, this class IIa bacteriocin inhibited Yersinia spp., Salmonella spp., E. coli O157:H7, Shigella dysenteriae, M. morganii, Staphylococcus spp. and Listeria spp., with MIC ranging from 0.025 to 32 μg/ml. Similar use of purified bacteriocin E-760 with broad-spectrum activity against both Gram-positive and Gram-negative bacteria has also been reported [79], with an impressive 8-log reduction of Campylobacter counts in broiler chickens. Svetoch and coworkers isolated a bacterial strain identified as L. salivarius 1077 (NRRL B-50053) from poultry intestinal material and found that it produces a class IIa bacteriocin (L-1077) found most active against C. jejuni L-4 (MIC = 0.09 μg/ml) and also active against Salmonella sp., E. coli O157:H7, Yersinia sp., Staphylococcus sp., L. monocytogenes, and C. perfringens, with MIC values ranging from 0.09 to 1.5 μg/ml [131]. Administration of L-1077-containing feed significantly reduced colonization of broiler chickens experimentally challenged with C. jejuni and S. enteritidis by more than four CFU log cycles. Previously, Stern and collaborators [124] found that the producer strain was ineffective for controlling C. jejuni by competitive exclusion in broiler chicken trials. The mechanism for C. jejuni colonization control is independent of the bacteria-producing bacteriocins but rather seems to be related on the secreted and purified bacteriocin [124].
Data mining of bacteriocin anti-infective properties
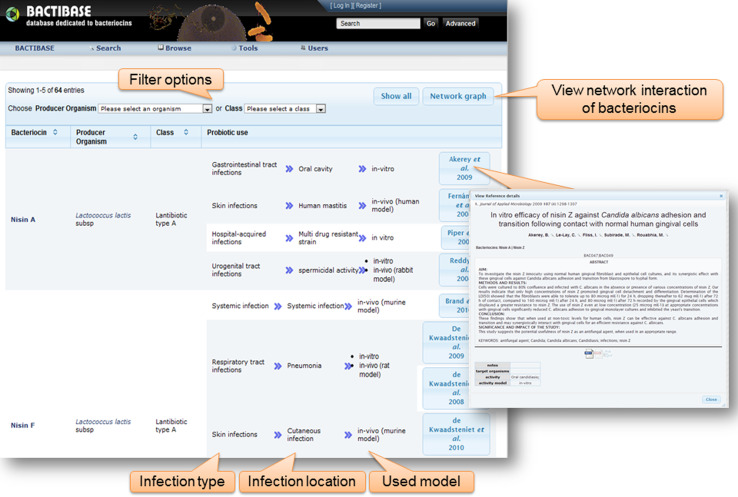
The increasing interest of researchers in bacteriocins is reflected in the number of papers that can be found in PubMed describing them [54]. The BACTIBASE database offers a more complete picture of bacteriocins. Peptide sequence data stored in the database have been accumulating rapidly [53, 54]. However, data relating to therapeutic applications of bacteriocin have been scattered in separate resources for many years. To address this deficiency, we have developed a literature area in BACTIBASE, which groups papers focused on bacteriocins and bacteriocin-like substances (BLIS) that have been evaluated as potential therapeutic agents. Compilation of documents relevant to the anti-infective properties of bacteriocins was achieved using various knowledge databases including PubMed, Google Scholar, and ISI Web of Science. A Web-based interface was developed and made available at http://bactibase.pfba-lab-tun.org/antiinfective. As illustrated in Fig. 2, data are displayed in tabular format. The Web interface also hosts an interactive copy of the network graph presented in Fig. 2. The table can be sorted or filtered according to producer organism or class. If it exists, detailed information for each entry in BACTIBASE can be viewed by clicking on the peptide name. A link is also provided to all bacteriocins produced by a specific organism in BACTIBASE. The right column includes infection type and location, model used (in vitro, or in vivo + animal) and publications by author name. The user can also click on the publications button to get detailed information on the study, including the abstract, studied bacteriocins, targeted strains, infection type and location, and experimental model (Fig. 3). These visualization techniques help users explore the data more conveniently.
Fig. 3.
Web-based user interface for therapeutic application of bacteriocins
Conclusions
Bacteriocin production is a widespread phenomenon among prokaryotes. The anti-infective potential of bacteriocins for inhibiting pathogens has been shown in various food matrices including cheese, meat, and vegetables. However, the nature of their in vivo inhibitory activities against pathogens remains unclear and needs more investigation, mainly because of difficulties associated with demonstrating their health benefits. Many bacteriocins produced by established or potential probiotic strains have been assessed for potential application as therapeutic agents in studies in vitro as well as a few in vivo. Some in vivo studies carried out over the past decade point to the efficacy of bacteriocin-based treatments of human and animal infections [30, 31, 68]. Furthermore, investigation of the pharmacokinetics and pharmacodynamics of lantibiotics demonstrated their high therapeutic efficacy (e.g., MU1140), as reviewed in [140]. Although extensive progress has been made with respect to our understanding of bacteriocin structure/function, regulation and immunity, additional thorough investigation of the factors influencing cell survival, bacteriocin production, and bacteriocin activity is required in order to obtain greater consistency between observed in vitro inhibition and in vivo results. One concern regarding the use of bacteriocins as anti-infective agents would be the possible occurrence of spontaneously acquired or natural resistance. Such phenomena could result from the failure of the bacteriocin to bind to the target strain (altered receptor or membrane composition) or from rapid inactivation of the bacteriocin by proteases produced by the target organism. However, experience shows that this occurs at very low frequencies, for example, 10−8 to 10−9 CFU observed for lacticin 3147 [50]. In addition, resistance acquired against one bacteriocin does not make a strain resistant to many other bacteriocins. Only bacteriocins sharing the same mode of action might lose their effectiveness. The use of mixtures of bacteriocins having different mechanisms of action would make the development of resistance highly unlikely.
Several challenges must still be met before more bacteriocins can be brought to the market. All drug candidates must be cleared of potential toxicity issues as well as questions regarding in vivo stability and appropriate delivery route. Loss of peptide activity in vivo due to proteolytic action is also of concern. For example, nisin shows promising activities in vitro and in animal models against numerous clinically relevant bacteria strains. However, its inherent chemical instability and poor solubility at pH 7.0 place a technological hurdle before its application as a therapeutic agent. Unlike nisin, lacticin 3147 is soluble and active at physiological pH and therefore might be more suitable for human clinical applications. Both nisin and lacticin 3147 are broad-spectrum antimicrobials with activity against most Gram-positive species, including those that would be considered beneficial to human gut health, such as Lactobacillus and Bifidobacterium, which constitutes a concern for their application in GI tract diseases. It is noted with interest that thuricin CD did not produce major alterations of GI populations, a contributing factor in recurrent C. difficile infection [108]. Similarly, class IIa bacteriocins are less disruptive to the intestinal microbiota equilibrium. For example, the safety of pediocin PA-1 and its producer strain for common intestinal bacterial equilibrium has been demonstrated in a murine model [31]. Several factors influence the in vivo effectiveness of bacteriocins produced in situ, including producer strain survival, specific activity, the animal model, and the targeted pathogen. The efficacy of bacteriocin production in vivo still remains a challenge, in spite of generally high bacteriocin specific activities, due mainly to low production levels and non-specific interactions with other hydrophobic molecules.
While further investigation remains necessary before the possibilities for bacteriocins in clinical practice can be described more fully, this review provided an overview of their potential applications to human and veterinary health. Bacteriocins hold great promise for the treatment of diseases caused by pathogenic bacteria and may eventually be employed as alternatives to existing antibiotics. Bacteriocins will have to go through the same rigorous and expensive research and validation process as all other compounds before they can be approved for use as therapeutic agents.
References
- 1.Acheson D, Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis. 2001;32:263–269. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 2.Akerey B, Le-Lay C, Fliss I, Subirade M, Rouabhia M. In vitro efficacy of nisin Z against Candida albicans adhesion and transition following contact with normal human gingival cells. J Appl Microbiol. 2009;107:1298–1307. doi: 10.1111/j.1365-2672.2009.04312.x. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mahrous MM, Jack RW, Sandiford SK, Tagg JR, Beatson SA, Upton M. Identification of a haemolysin-like peptide with antibacterial activity using the draft genome sequence of Staphylococcus epidermidis strain A487. FEMS Immunol Med Microbiol. 2011;62:273–282. doi: 10.1111/j.1574-695X.2011.00811.x. [DOI] [PubMed] [Google Scholar]
- 4.Armitage GC, Robertson PB. The biology, prevention, diagnosis and treatment of periodontal diseases: scientific advances in the United States. J Am Dental Assoc. 2009;140:36S–43S. doi: 10.14219/jada.archive.2009.0356. [DOI] [PubMed] [Google Scholar]
- 5.Aunpad R, Na-Bangchang K. Pumilicin 4, a novel bacteriocin with anti-MRSA and anti-VRE activity Produced by newly isolated bacteria Bacillus pumilus strain WAPB4. Curr Microbiol. 2007;55:308–313. doi: 10.1007/s00284-006-0632-2. [DOI] [PubMed] [Google Scholar]
- 6.Barboza-Corona JE, de la Fuente-Salcido N, Alva-Murillo N, Ochoa-Zarzosa A, López-Meza JE. Activity of bacteriocins synthesized by Bacillus thuringiensis against Staphylococcus aureus isolates associated to bovine mastitis. Veterinary Microbiol. 2009;138:179–183. doi: 10.1016/j.vetmic.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Birri DJ, Brede DA, Forberg T, Holo H, Nes IF. Molecular and genetic characterization of a novel bacteriocin locus in Enterococcus avium isolates from infants. Appl Environ Microbiol. 2010;76:483–492. doi: 10.1128/AEM.01597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birri DJ, Brede DA, Nes IF. Salivaricin D, a novel intrinsically trypsin-resistant lantibiotic from Streptococcus salivarius 5M6c isolated from a healthy Infant. Appl Environ Microbiol. 2012;78:402–410. doi: 10.1128/AEM.06588-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrero J, Brede DA, Skaugen M, Diep DB, Herranz C, Nes IF, Cintas LM, Hernandez PE. Characterization of Garvicin ML, a novel circular bacteriocin produced by Lactococcus garvieae DCC43, isolated from mallard ducks (Anas platyrhynchos) Appl Environ Microbiol. 2011;77:369–373. doi: 10.1128/AEM.01173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowe WP, Filip JC, DiRienzo JM, Volgina A, Margolis DJ. Inhibition of Propionibacterium acnes by bacteriocin-like inhibitory substances (BLIS) produced by Streptococcus salivarius . J Drugs Dermatol. 2006;5:868–870. [PMC free article] [PubMed] [Google Scholar]
- 11.Brand AM, De Kwaadsteniet M, Dicks LMT. The ability of nisin F to control Staphylococcus aureus infection in the peritoneal cavity, as studied in mice. Lett Appl Microbiol. 2010;51:645–649. doi: 10.1111/j.1472-765X.2010.02948.x. [DOI] [PubMed] [Google Scholar]
- 12.Breukink E, Wiedemann I, Kraaij Cv, Kuipers OP, Sahl H-G, de Kruijff B. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science. 1999;286:2361–2364. doi: 10.1126/science.286.5448.2361. [DOI] [PubMed] [Google Scholar]
- 13.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 14.Burton J, Chilcott C, Wescombe P, Tagg J. Extended safety data for the oral cavity probiotic Streptococcus salivarius K12. Probiotics Antimicrob Proteins. 2010;2:135–144. doi: 10.1007/s12602-010-9045-4. [DOI] [PubMed] [Google Scholar]
- 15.Burton JP, Chilcott CN, Moore CJ, Speiser G, Tagg JR. A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. J Appl Microbiol. 2006;100:754–764. doi: 10.1111/j.1365-2672.2006.02837.x. [DOI] [PubMed] [Google Scholar]
- 16.Burton JP, Cowley S, Simon RR, McKinney J, Wescombe PA, Tagg JR. Evaluation of safety and human tolerance of the oral probiotic Streptococcus salivarius K12: a randomized, placebo-controlled, double-blind study. Food Chem Toxicol. 2011;49:2356–2364. doi: 10.1016/j.fct.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 17.Cao LT, Wu JQ, Xie F, Hu SH, Mo Y. Efficacy of nisin in treatment of clinical mastitis in lactating dairy cows. J Dairy Sci. 2007;90:3980–3985. doi: 10.3168/jds.2007-0153. [DOI] [PubMed] [Google Scholar]
- 18.Carroll J, Draper LA, O’Connor PM, Coffey A, Hill C, Ross RP, Cotter PD, O’Mahony J. Comparison of the activities of the lantibiotics nisin and lacticin 3147 against clinically significant mycobacteria. Int J Antimicrob Agents. 2010;36:132–136. doi: 10.1016/j.ijantimicag.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Castiglione F, Cavaletti L, Losi D, Lazzarini A, Carrano L, Feroggio M, Ciciliato I, Corti E, Candiani G, Marinelli F, Selva E. A novel lantibiotic acting on bacterial cell wall synthesis produced by the uncommon Actinomycete Planomonospora sp. Biochemistry. 2007;46:5884–5895. doi: 10.1021/bi700131x. [DOI] [PubMed] [Google Scholar]
- 20.Castiglione F, Lazzarini A, Carrano L, Corti E, Ciciliato I, Gastaldo L, Candiani P, Losi D, Marinelli F, Selva E, Parenti F. Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multiresistant pathogens. Chem Biol. 2008;15:22–31. doi: 10.1016/j.chembiol.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee C, Paul M, Xie L, van der Donk WA. Biosynthesis and mode of action of lantibiotics. Chem Rev. 2005;105:633–684. doi: 10.1021/cr030105v. [DOI] [PubMed] [Google Scholar]
- 22.Cintas LM, Casaus MP, Herranz C, Nes IF, Hernandez PE. Review: bacteriocins of lactic acid bacteria. Food Sci Technol Int. 2001;7:281–305. [Google Scholar]
- 23.Clardy J, Fischbach MA, Walsh CT. New antibiotics from bacterial natural products. Nat Biotech. 2006;24:1541–1550. doi: 10.1038/nbt1266. [DOI] [PubMed] [Google Scholar]
- 24.Cole K, Farnell MB, Donoghue AM, Stern NJ, Svetoch EA, Eruslanov BN, Volodina LI, Kovalev YN, Perelygin VV, Mitsevich EV, Mitsevich IP, Levchuk VP, Pokhilenko VD, Borzenkov VN, Svetoch OE, Kudryavtseva TY, Reyes-Herrera I, Blore PJ, de los Santos FS, Donoghue DJ. Bacteriocins reduce Campylobacter colonization and alter gut morphology in Turkey poults. Poult Sci. 2006;85:1570–1575. doi: 10.1093/ps/85.9.1570. [DOI] [PubMed] [Google Scholar]
- 25.Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CGM. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci. 2007;104:7617–7621. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotter PD, Hill C, Ross RP. Bacteriocins: developing innate immunity for food. Nat Rev Micro. 2005;3:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 27.Crispie F, Flynn J, Ross RP, Hill C, Meaney W. Update on the development of a novel dry cow therapy using a bismuth-based intramammary teat seal in combination with the bacteriocin lacticin 3147. Irish Veterinary J. 2004;57:652–656. doi: 10.1186/2046-0481-57-11-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crispie F, Twomey D, Flynn J, Hill C, Ross P, Meaney W. The lantibiotic lacticin 3147 produced in a milk-based medium improves the efficacy of a bismuth-based teat seal in cattle deliberately infected with Staphylococcus aureus . J Dairy Res. 2005;72:159–167. doi: 10.1017/S0022029905000816. [DOI] [PubMed] [Google Scholar]
- 29.Crost EH, Ajandouz EH, Villard C, Geraert PA, Puigserver A, Fons M. Ruminococcin C, a new anti-Clostridium perfringens bacteriocin produced in the gut by the commensal bacterium Ruminococcus gnavus E1. Biochimie. 2011;93:1487–1494. doi: 10.1016/j.biochi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Cursino L, Šmajs D, Šmarda J, Nardi RMD, Nicoli JR, Chartone-Souza E, Nascimento AMA. Exoproducts of the Escherichia coli strain H22 inhibiting some enteric pathogens both in vitro and in vivo. J Appl Microbiol. 2006;100:821–829. doi: 10.1111/j.1365-2672.2006.02834.x. [DOI] [PubMed] [Google Scholar]
- 31.Dabour N, Zihler A, Kheadr E, Lacroix C, Fliss I. In vivo study on the effectiveness of pediocin PA-1 and Pediococcus acidilactici UL5 at inhibiting Listeria monocytogenes. Int J Food Microbiol. 2009;133:225–233. doi: 10.1016/j.ijfoodmicro.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 32.De Kwaadsteniet M, Doeschate KT, Dicks LMT. Nisin F in the treatment of respiratory tract infections caused by Staphylococcus aureus . Lett Appl Microbiol. 2009;48:65–70. doi: 10.1111/j.1472-765X.2008.02488.x. [DOI] [PubMed] [Google Scholar]
- 33.de Kwaadsteniet M, ten Doeschate K, Dicks LMT. Characterization of the structural gene encoding Nisin F, a new lantibiotic produced by a Lactococcus lactis subsp. lactis isolate from freshwater catfish (Clarias gariepinus) Appl Environ Microbiol. 2008;74:547–549. doi: 10.1128/AEM.01862-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Kwaadsteniet M, van Reenen C, Dicks L. Evaluation of nisin F in the treatment of subcutaneous skin infections, as monitored by using a bioluminescent strain of Staphylococcus aureus . Probiotics Antimicrob Proteins. 2010;2:61–65. doi: 10.1007/s12602-009-9017-8. [DOI] [PubMed] [Google Scholar]
- 35.Diep DB, Nes IF. Ribosomally synthesized antibacterial peptides in Gram-positive bacteria. Curr Drug Targets. 2002;3:107–122. doi: 10.2174/1389450024605409. [DOI] [PubMed] [Google Scholar]
- 36.Diez-Gonzalez F. Applications of bacteriocins in livestock. Curr Issues Intest Microbiol. 2007;8:15–23. [PubMed] [Google Scholar]
- 37.Dobson A, Crispie F, Rea MC, O’Sullivan O, Casey PG, Lawlor PG, Cotter PD, Ross P, Gardiner GE, Hill C. Fate and efficacy of lacticin 3147-producing Lactococcus lactis in the mammalian gastrointestinal tract. FEMS Microbiol Ecol. 2011;76:602–614. doi: 10.1111/j.1574-6941.2011.01069.x. [DOI] [PubMed] [Google Scholar]
- 38.Dover SE, Aroutcheva AA, Faro S, Chikindas ML (2007) Safety study of an antimicrobial peptide lactocin 160, produced by the vaginal Lactobacillus rhamnosus. Infect Dis Obstet Gynecol. doi:10.1155/2007/78248 [DOI] [PMC free article] [PubMed]
- 39.Drider D, Fimland G, Hechard Y, McMullen L, Prevost H. The continuing story of class IIa bacteriocins. Microbiol Mol Biol Rev. 2006;70:564–582. doi: 10.1128/MMBR.00016-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernández L, Delgado S, Herrero H, Maldonado A, Rodríguez JM. The bacteriocin nisin, an effective agent for the treatment of Staphylococcal Mastitis during lactation. J Human Lactation. 2008;24:311–316. doi: 10.1177/0890334408317435. [DOI] [PubMed] [Google Scholar]
- 41.Fimland G, Blingsmo OR, Sletten K, Jung G, Nes IF, Nissen-Meyer J. New biologically active hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins: the C-terminal region is important for determining specificity. Appl Environ Microbiol. 1996;62:3313–3318. doi: 10.1128/aem.62.9.3313-3318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fliss I, Hammami R, Le Lay C (2011) Biological control of human digestive microbiota using antimicrobial cultures and bacteriocins. In: Lacroix C (ed) Protective cultures, antimicrobial metabolites and bacteriophages for food and beverage biopreservation. Woodhead Publ Ltd, Abington Hall Abington, Cambridge Cb1 6ah, Cambs, UK, pp 240–263
- 43.Franz CMAP, Van Belkum MJ, Holzapfel WH, Abriouel H, Gálvez A. Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol Rev. 2007;31:293–310. doi: 10.1111/j.1574-6976.2007.00064.x. [DOI] [PubMed] [Google Scholar]
- 44.Gardiner GE, Rea MC, O’Riordan B, O’Connor P, Morgan SM, Lawlor PG, Lynch PB, Cronin M, Ross RP, Hill C. Fate of the two-component lantibiotic lacticin 3147 in the gastrointestinal tract. Appl Environ Microbiol. 2007;73:7103–7109. doi: 10.1128/AEM.01117-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghobrial OG, Derendorf H, Hillman JD. Pharmacodynamic activity of the lantibiotic MU1140. Int J Antimicrob Agents. 2009;33:70–74. doi: 10.1016/j.ijantimicag.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gillor O, Kirkup BC, Riley MA (2004) Colicins and microcins: the next-generation antimicrobials. In: Advances in applied microbiology, vol 54. Academic Press, London, pp 129–146 [DOI] [PubMed]
- 47.Gong HS, Meng XC, Wang H. Plantaricin MG active against Gram-negative bacteria produced by Lactobacillus plantarum KLDS1.0391 isolated from “Jiaoke”, a traditional fermented cream from China. Food Control. 2009;21:89–96. doi: 10.1016/j.foodcont.2009.04.005. [DOI] [Google Scholar]
- 48.Gordon DM, Oliver E, Littlefield-Wyer J. The diversity of bacteriocins in Gram-negative bacteria. In: Riley MA, Chavan M, editors. Bacteriocins: ecology and evolution. Berlin Heidelberg New York: Springer; 2007. pp. 5–18. [Google Scholar]
- 49.Grasemann H, Stehling F, Brunar H, Widmann R, Laliberte TW, Molina L, Döring G, Ratjen F. Inhalation of Moli 1901 in patients with cystic fibrosis. Chest. 2007;131:1461–1466. doi: 10.1378/chest.06-2085. [DOI] [PubMed] [Google Scholar]
- 50.Guinane CM, Cotter PD, Hill C, Ross RP. Spontaneous resistance in Lactococcus lactis IL1403 to the lantibiotic lacticin 3147. FEMS Microbiol Lett. 2006;260:77–83. doi: 10.1111/j.1574-6968.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- 51.Halasa T, Huijps K, Osterás O, Hogeveen H. Economic effects of bovine mastitis and mastitis management: a review. Veterinary Q. 2007;29:18–31. doi: 10.1080/01652176.2007.9695224. [DOI] [PubMed] [Google Scholar]
- 52.Hammami R, Zouhir A, Ben Hamida J, Fliss I. BACTIBASE: a new web-accessible database for bacteriocin characterization. BMC Microbiol. 2007;7:89. doi: 10.1186/1471-2180-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hammami R, Zouhir A, Le Lay C, Ben Hamida J, Fliss I. BACTIBASE second release: a database and tool platform for bacteriocin characterization. BMC Microbiol. 2010;10:22. doi: 10.1186/1471-2180-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammami R, Zouhir A, Le Lay C, Ben Hamida J, Fliss I. Database mining for bacteriocin discovery. Wallingford: Cabi Publishing; 2011. [Google Scholar]
- 55.Hatakka K, Saxelin M. Probiotics in intestinal and non-intestinal infectious diseases—clinical evidence. Curr Pharm Des. 2008;14:1351–1367. doi: 10.2174/138161208784480162. [DOI] [PubMed] [Google Scholar]
- 56.Heng NC, Tagg JR. What’s in a name? Nat Rev Micro: Class distinction for bacteriocins; 2006. [Google Scholar]
- 57.Heng NCK, Wescombe PA, Burton JP, Jack RW, Tagg JR. The diversity of bacteriocins in Gram-positive bacteria. In: Riley MA, Chavan M, editors. Bacteriocins: ecology and evolution. Berlin Heidelberg New York: Springer; 2007. pp. 45–92. [Google Scholar]
- 58.Hillman JD, McDonell E, Hillman CH, Zahradnik RT, Soni MG. Safety assessment of ProBiora3, a probiotic mouthwash: subchronic toxicity study in rats. Int J Toxicol. 2009;28:357–367. doi: 10.1177/1091581809340705. [DOI] [PubMed] [Google Scholar]
- 59.Hillman JD, Mo J, McDonell E, Cvitkovitch D, Hillman CH. Modification of an effector strain for replacement therapy of dental caries to enable clinical safety trials. J Appl Microbiol. 2007;102:1209–1219. doi: 10.1111/j.1365-2672.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- 60.Hsu S-TD, Breukink E, Tischenko E, Lutters MAG, de Kruijff B, Kaptein R, Bonvin AMJJ, van Nuland NAJ. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat Struct Mol Biol. 2004;11:963–967. doi: 10.1038/nsmb830. [DOI] [PubMed] [Google Scholar]
- 61.Humphrey T, O’Brien S, Madsen M. Campylobacters as zoonotic pathogens: a food production perspective. Int J Food Microbiol. 2007;117:237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Iwamoto T, Suzuki N, Tanabe K, Takeshita T, Hirofuji T. Effects of probiotic Lactobacillus salivarius WB21 on halitosis and oral health: an open-label pilot trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2010;110:201–208. doi: 10.1016/j.tripleo.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 63.Iwatsuki K, Yamasaki O, Morizane S, Oono T. Staphylococcal cutaneous infections: invasion, evasion and aggression. J Dermatol Sci. 2006;42:203–214. doi: 10.1016/j.jdermsci.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 64.Izquierdo E, Wagner C, Marchioni E, Aoude-Werner D, Ennahar S. Enterocin 96, a novel class II bacteriocin produced by Enterococcus faecalis WHE 96, isolated from Munster cheese. Appl Environ Microbiol. 2009;75:4273–4276. doi: 10.1128/AEM.02772-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jabes D, Brunati C, Candiani G, Riva S, Romano’ G, Donadio S (2011) Efficacy of the new lantibiotic NAI-107 in experimental infections induced by MDR Gram-positive pathogens. Antimicrob Agents Chemother. AAC.01288-01210. doi:10.1128/aac.01288-10 [DOI] [PMC free article] [PubMed]
- 66.Jack RW, Tagg JR, Ray B. Bacteriocins of Gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37:178–187. doi: 10.3109/1040841X.2011.556598. [DOI] [PubMed] [Google Scholar]
- 68.Kang B, Seo J-G, Lee G-S, Kim J-H, Kim S, Han Y, Kang H, Kim H, Rhee J, Chung M-J, Park Y. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J Microbiol. 2009;47:101–109. doi: 10.1007/s12275-008-0179-y. [DOI] [PubMed] [Google Scholar]
- 69.Kannan K, Mankin AS. Macrolide antibiotics in the ribosome exit tunnel: species-specific binding and action. Annals N Y Acad Sci. 2011;1241:33–47. doi: 10.1111/j.1749-6632.2011.06315.x. [DOI] [PubMed] [Google Scholar]
- 70.Kim PI, Sohng JK, Sung C, Joo H-S, Kim E-M, Yamaguchi T, Park D, Kim B-G. Characterization and structure identification of an antimicrobial peptide, hominicin, produced by Staphylococcus hominis MBBL 2–9. Biochem Biophys Res Commun. 2010;399:133–138. doi: 10.1016/j.bbrc.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 71.Kjos M, Nes IF, Diep DB. Class II one-peptide bacteriocins target a phylogenetically defined subgroup of mannose phosphotransferase systems on sensitive cells. Microbiology. 2009;155:2949–2961. doi: 10.1099/mic.0.030015-0. [DOI] [PubMed] [Google Scholar]
- 72.Klaenhammer TR. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–85. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 73.Kleanthous C. Swimming against the tide: progress and challenges in our understanding of colicin translocation. Nat Rev Micro. 2010;8:843–848. doi: 10.1038/nrmicro2454. [DOI] [PubMed] [Google Scholar]
- 74.Klostermann K, Crispie F, Flynn J, Meaney WJ, Paul Ross R, Hill C. Efficacy of a teat dip containing the bacteriocin lacticin 3147 to eliminate Gram-positive pathogens associated with bovine mastitis. J Dairy Res. 2010;77:231–238. doi: 10.1017/S0022029909990239. [DOI] [PubMed] [Google Scholar]
- 75.Knoetze H, Todorov SD, Dicks LMT. A class IIa peptide from Enterococcus mundtii inhibits bacteria associated with otitis media. Int J Antimicrob Agents. 2008;31:228–234. doi: 10.1016/j.ijantimicag.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 76.Kruszewska D, Sahl H-G, Bierbaum G, Pag U, Hynes SO, Ljungh Å. Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitis model. J Antimicrob Chemother. 2004;54:648–653. doi: 10.1093/jac/dkh387. [DOI] [PubMed] [Google Scholar]
- 77.Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, Romero R (2011) The vaginal microbiome: new information about genital tract flora using molecular-based techniques. BJOG Int J Obstet Gynaecol 118:533–549. doi:10.1111/j.1471-0528.2010.02840.x [DOI] [PMC free article] [PubMed]
- 78.Lazzarini A, Gastaldo L, Candiani G, Ciciliato I, Losi D, Marinelli F, Selva E, Parenti F (2008) Antibiotic 107891, its factors A1 and A2, pharmaceutically acceptable salts and compositions, and use thereof. US Patent #07351687
- 79.Line JE, Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, Levchuk VP, Svetoch OE, Seal BS, Siragusa GR, Stern NJ. Isolation and purification of Enterocin E-760 with broad antimicrobial activity against Gram-positive and Gram-negative bacteria. Antimicrob Agents Chemother. 2008;52:1094–1100. doi: 10.1128/AAC.01569-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loesche WJ, Kazor C. Microbiology and treatment of halitosis. Periodontology. 2002;2000(28):256–279. doi: 10.1034/j.1600-0757.2002.280111.x. [DOI] [PubMed] [Google Scholar]
- 81.Lopez FE, Vincent PA, Zenoff AM, Salomón RA, Farías RN. Efficacy of microcin J25 in biomatrices and in a mouse model of Salmonella infection. J Antimicrob Chemother. 2007;59:676–680. doi: 10.1093/jac/dkm009. [DOI] [PubMed] [Google Scholar]
- 82.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Musher DM, Niederman MS, Torres A, Whitney CG. Infectious diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manosroi A, Khanrin P, Lohcharoenkal W, Werner RG, Götz F, Manosroi W, Manosroi J. Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes. Int J Pharm. 2010;392:304–310. doi: 10.1016/j.ijpharm.2010.03.064. [DOI] [PubMed] [Google Scholar]
- 84.Mao Y, Zhu C, Boedeker EC. Foodborne enteric infections. Curr Opin Gastroenterol. 2003;19:11–22. doi: 10.1097/00001574-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 85.Martin-Visscher LA, van Belkum MJ, Garneau-Tsodikova S, Whittal RM, Zheng J, McMullen LM, Vederas JC. Isolation and characterization of Carnocyclin A, a novel circular bacteriocin produced by Carnobacterium maltaromaticum UAL307. Appl Environ Microbiol. 2008;74:4756–4763. doi: 10.1128/AEM.00817-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Michel-Briand Y, Baysse C. The pyocins of Pseudomonas aeruginosa . Biochimie. 2002;84:499–510. doi: 10.1016/S0300-9084(02)01422-0. [DOI] [PubMed] [Google Scholar]
- 87.Moll GN, Konings WN, Driessen AJM. Bacteriocins: mechanism of membrane insertion and pore formation. Antonie van Leeuwenhoek. 1999;76:185–198. doi: 10.1023/A:1002002718501. [DOI] [PubMed] [Google Scholar]
- 88.Mota-Meira M, Morency H, Lavoie MC. In vivo activity of mutacin B-Ny266. J Antimicrob Chemother. 2005;56:869–871. doi: 10.1093/jac/dki295. [DOI] [PubMed] [Google Scholar]
- 89.Muder RR, Yu VL, Woo AH. Mode of transmission of Legionella pneumophila: a critical review. Arch Intern Med. 1986;146:1607–1612. doi: 10.1001/archinte.1986.00360200183030. [DOI] [PubMed] [Google Scholar]
- 90.Nishijima S, Kurokawa I, Katoh N, Watanabe K. The bacteriology of acne vulgaris and antimicrobial susceptibility of Propionibacterium acnes and Staphylococcus epidermidis isolated from acne lesions. J Dermatol. 2000;27:318–323. doi: 10.1111/j.1346-8138.2000.tb02174.x. [DOI] [PubMed] [Google Scholar]
- 91.Oman TJ, van der Donk WA. Insights into the mode of action of the two-peptide lantibiotic haloduracin. ACS Chem Biol. 2009;4:865–874. doi: 10.1021/cb900194x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pangsomboon K, Bansal S, Martin GP, Suntinanalert P, Kaewnopparat S, Srichana T. Further characterization of a bacteriocin produced by Lactobacillus paracasei HL32. J Appl Microbiol. 2009;106:1928–1940. doi: 10.1111/j.1365-2672.2009.04146.x. [DOI] [PubMed] [Google Scholar]
- 93.Papadakos G, Wojdyla JA, Kleanthous C. Nuclease colicins and their immunity proteins. Q Rev Biophys. 2012;45:57–103. doi: 10.1017/S0033583511000114. [DOI] [PubMed] [Google Scholar]
- 94.Pascual L, Daniele M, Giordano W, Pájaro M, Barberis I. Purification and partial characterization of novel bacteriocin L23 produced by Lactobacillus fermentum L23. Curr Microbiol. 2008;56:397–402. doi: 10.1007/s00284-007-9094-4. [DOI] [PubMed] [Google Scholar]
- 95.Pieterse R, Todorov SD. Bacteriocins: exploring alternatives to antibiotics in mastitis treatment. Brazilian J Microbiol. 2010;41:542–562. doi: 10.1590/S1517-83822010000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pieterse R, Todorov SD, Dicks LMT. Bacteriocin ST91KM, produced by Streptococcus gallolyticus subsp. macedonicus ST91KM, is a narrow-spectrum peptide active against bacteria associated with mastitis in dairy cattle (Clinical report) Can J Microbiol. 2008;54:525. doi: 10.1139/w08-040. [DOI] [PubMed] [Google Scholar]
- 97.Pieterse R, Todorov SD, Dicks LMT. Mode of action and in vitro susceptibility of mastitis pathogens to macedocin ST91KM and preparation of a teat seal containing the bacteriocin. Brazilian J Microbiol. 2010;41:133–145. doi: 10.1590/S1517-83822010000100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Piper C, Casey PG, Hill C, Cotter PD (2012) The lantibiotic Lacticin 3147 prevents systemic spread of Staphylococcus aureus in a murine infection model. Int J Microbiol. doi:10.1155/2012/806230 [DOI] [PMC free article] [PubMed]
- 99.Piper C, Draper LA, Cotter PD, Ross RP, Hill C. A comparison of the activities of lacticin 3147 and nisin against drug-resistant Staphylococcus aureus and Enterococcus species. J Antimicrob Chemother. 2009;64:546–551. doi: 10.1093/jac/dkp221. [DOI] [PubMed] [Google Scholar]
- 100.Piper C, Hill C, Cotter PD, Ross RP. Bioengineering of a Nisin A-producing Lactococcus lactis to create isogenic strains producing the natural variants Nisin F, Q and Z. Microb Biotechnol. 2011;4:375–382. doi: 10.1111/j.1751-7915.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ranganathan SC, Sonnappa S. Pneumonia and other respiratory infections. Pediatr Clin North Am. 2009;56:135–156. doi: 10.1016/j.pcl.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rea MC, Clayton E, O’Connor PM, Shanahan F, Kiely B, Ross RP, Hill C. Antimicrobial activity of lacticin 3147 against clinical Clostridium difficile strains. J Med Microbiol. 2007;56:940–946. doi: 10.1099/jmm.0.47085-0. [DOI] [PubMed] [Google Scholar]
- 103.Rea MC, Dobson A, O’Sullivan O, Crispie F, Fouhy F, Cotter PD, Shanahan F, Kiely B, Hill C, Ross RP. Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc Natl Acad Sci. 2010 doi: 10.1073/pnas.1001224107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rea MC, Sit CS, Clayton E, O’Connor PM, Whittal RM, Zheng J, Vederas JC, Ross RP, Hill C. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile . Proc Natl Acad Sci. 2010;107:9352–9357. doi: 10.1073/pnas.0913554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reddy KVR, Aranha C, Gupta SM, Yedery RD. Evaluation of antimicrobial peptide nisin as a safe vaginal contraceptive agent in rabbits: in vitro and in vivo studies. Reproduction. 2004;128:117–126. doi: 10.1530/rep.1.00028. [DOI] [PubMed] [Google Scholar]
- 106.Riboulet-Bisson E, Sturme MHJ, Jeffery IB, O’Donnell MM, Neville BA, Forde BM, Claesson MJ, Harris H, Gardiner GE, Casey PG, Lawlor PG, O’Toole PW, Ross RP. Effect of Lactobacillus salivarius bacteriocin Abp118 on the mouse and pig intestinal microbiota. PLoS One. 2012;7:e31113. doi: 10.1371/journal.pone.0031113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Riley MA, Wertz JE. BACTERIOCINS: evolution, ecology, and application. Annu Rev Microbiol. 2002;56:117–137. doi: 10.1146/annurev.micro.56.012302.161024. [DOI] [PubMed] [Google Scholar]
- 108.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 109.Salvucci E, Saavedra L, Hebert EM, Haro C, Sesma F. Enterocin CRL35 inhibits Listeria monocytogenes in a murine model. Foodborne Pathog Dis. 2012;9:68–74. doi: 10.1089/fpd.2011.0972. [DOI] [PubMed] [Google Scholar]
- 110.Sánchez J, Basanta A, Gómez-Sala B, Herranz C, Cintas LM, Hernández PE. Antimicrobial and safety aspects, and biotechnological potential of bacteriocinogenic enterococci isolated from mallard ducks (Anas platyrhynchos) Int J Food Microbiol. 2007;117:295–305. doi: 10.1016/j.ijfoodmicro.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 111.Sánchez J, Diep DB, Herranz C, Nes IF, Cintas LM, Hernández PE. Amino acid and nucleotide sequence, adjacent genes, and heterologous expression of hiracin JM79, a sec-dependent bacteriocin produced by Enterococcus hirae DCH5, isolated from Mallard ducks (Anas platyrhynchos) FEMS Microbiol Lett. 2007;270:227–236. doi: 10.1111/j.1574-6968.2007.00673.x. [DOI] [PubMed] [Google Scholar]
- 112.Sandiford S, Upton M. Identification, characterization, and recombinant expression of epidermicin NI01, a novel unmodified bacteriocin produced by Staphylococcus epidermidis that displays potent activity against staphylococci. Antimicrob Agents Chemother. 2012;56:1539–1547. doi: 10.1128/AAC.05397-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sawa N, Zendo T, Kiyofuji J, Fujita K, Himeno K, Nakayama J, Sonomoto K. Identification and characterization of Lactocyclicin Q, a novel cyclic bacteriocin produced by Lactococcus sp. strain QU 12. Appl Environ Microbiol. 2009;75:1552–1558. doi: 10.1128/AEM.02299-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Segal N, Leibovitz E, Dagan R, Leiberman A. Acute otitis media-diagnosis and treatment in the era of antibiotic resistant organisms: updated clinical practice guidelines. Int J Pediatr Otorhinolaryngol. 2005;69:1311–1319. doi: 10.1016/j.ijporl.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 115.Sequeiros C, Vallejo M, Marguet E, Olivera N. Inhibitory activity against the fish pathogen Lactococcus garvieae produced by Lactococcus lactis TW34, a lactic acid bacterium isolated from the intestinal tract of a Patagonian fish. Arch Microbiol. 2010;192:237–245. doi: 10.1007/s00203-010-0552-1. [DOI] [PubMed] [Google Scholar]
- 116.Shand R, Leyva K (2007) Peptide and protein antibiotics from the domain archaea: halocins and sulfolobicins, pp 93–109
- 117.Shand RF, Leyva KJ (2008) Archaeal antimicrobials: an undiscovered country. In: Norfolk BP (ed) Archaea: new models for prokaryotic biology. Caister Academic, Wymondham, pp 233–242
- 118.Shears P. Resistance as a worldwide problem. In: Lewis K, Salyers AA, Taber HW, Wax RG, editors. Bacterial resistance to antimicrobials: mechanisms, genetics, medical practice, and public health. 2. New York: Marcel Dekker; 2007. pp. 363–376. [Google Scholar]
- 119.Shelburne CE, An FY, Dholpe V, Ramamoorthy A, Lopatin DE, Lantz MS. The spectrum of antimicrobial activity of the bacteriocin subtilosin A. J Antimicrob Chemother. 2007;59:297–300. doi: 10.1093/jac/dkl495. [DOI] [PubMed] [Google Scholar]
- 120.Silkin L, Hamza S, Kaufman S, Cobb SL, Vederas JC. Spermicidal bacteriocins: lacticin 3147 and subtilosin A. Bioorg Med Chem Lett. 2008;18:3103–3106. doi: 10.1016/j.bmcl.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 121.Smith K, Martin L, Rinaldi A, Rajendran R, Ramage G, Walker D. Activity of Pyocin S2 against Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2012;56:1599–1601. doi: 10.1128/AAC.05714-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sosunov V, Mischenko V, Eruslanov B, Svetoch E, Shakina Y, Stern N, Majorov K, Sorokoumova G, Selishcheva A, Apt A. Antimycobacterial activity of bacteriocins and their complexes with liposomes. J Antimicrob Chemother. 2007;59:919–925. doi: 10.1093/jac/dkm053. [DOI] [PubMed] [Google Scholar]
- 123.Steiner I, Errhalt P, Kubesch K, Hubner M, Holy M, Bauer M, Müller M, Hinterberger S, Widmann R, Mascher D, Freissmuth M, Kneussl M. Pulmonary pharmacokinetics and safety of nebulized duramycin in healthy male volunteers. Naunyn-Schmiedeberg Arch Pharmacol. 2008;378:323–333. doi: 10.1007/s00210-008-0293-8. [DOI] [PubMed] [Google Scholar]
- 124.Stern NJ, Eruslanov BV, Pokhilenko VD, Kovalev YN, Volodina LL, Perelygin VV, Mitsevich EV, Mitsevich IP, Borzenkov VN, Levchuk VP, Svetoch OE, Stepanshin YG, Svetoch EA. Bacteriocins reduce Campylobacter jejuni colonization while bacteria-producing bacteriocins are ineffective. Microb Ecol Health Dis. 2008;20:74–79. doi: 10.1080/08910600802030196. [DOI] [Google Scholar]
- 125.Stern NJ, Svetoch EA, Eruslanov BV, Kovalev YN, Volodina LI, Perelygin VV, Mitsevich EV, Mitsevich IP, Levchuk VP. Paenibacillus polymyxa purified bacteriocin to control Campylobacter jejuni in chickens. J Food Protect. 2005;68:1450–1453. doi: 10.4315/0362-028x-68.7.1450. [DOI] [PubMed] [Google Scholar]
- 126.Stern NJ, Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, Pokhilenko VD, Levchuk VP, Svetoch OE, Seal BS. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob Agents Chemother. 2006;50:3111–3116. doi: 10.1128/AAC.00259-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sutyak KE, Anderson RA, Dover SE, Feathergill KA, Aroutcheva AA, Faro S, Chikindas ML (2008) Spermicidal activity of the safe natural antimicrobial peptide subtilosin. Infect Dis Obstet Gynecol. doi:10.1155/2008/540758 [DOI] [PMC free article] [PubMed]
- 128.Sutyak KE, Wirawan RE, Aroutcheva AA, Chikindas ML. Isolation of the Bacillus subtilis antimicrobial peptide subtilosin from the dairy product-derived Bacillus amyloliquefaciens . J Appl Microbiol. 2008;104:1067–1074. doi: 10.1111/j.1365-2672.2007.03626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sutyak Noll K, Prichard MN, Khaykin A, Sinko PJ, Chikindas ML. The natural antimicrobial peptide subtilosin acts synergistically with glycerol monolaurate, lauric arginate and ε-poly-l-lysine against bacterial vaginosis-associated pathogens but not human lactobacilli. Antimicrob Agents Chemother. 2012 doi: 10.1128/AAC.05861-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Svetoch E, Eruslanov B, Kovalev Y, Mitsevich E, Mitsevich I, Levchuk V, Fursova N, Perelygin V, Stepanshin Y, Teymurasov M, Seal B, Stern N. Antimicrobial activities of bacteriocins E 50–52 and B 602 against antibiotic-resistant strains involved in nosocomial infections. Probiotics Antimicrob Proteins. 2009;1:136–142. doi: 10.1007/s12602-009-9027-6. [DOI] [PubMed] [Google Scholar]
- 131.Svetoch EA, Eruslanov BV, Levchuk VP, Perelygin VV, Mitsevich EV, Mitsevich IP, Stepanshin J, Dyatlov I, Seal BS, Stern NJ. Isolation of Lactobacillus salivarius 1077 (NRRL B-50053) and characterization of its bacteriocin, including the antimicrobial activity spectrum. Appl Environ Microbiol. 2011;77:2749–2754. doi: 10.1128/AEM.02481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, Borzenkov VN, Levchuk VP, Svetoch OE, Kovalev YN, Stepanshin YG, Siragusa GR, Seal BS, Stern NJ. Diverse antimicrobial killing by Enterococcus faecium E 50–52 bacteriocin. J Agric Food Chem. 2008;56:1942–1948. doi: 10.1021/jf073284g. [DOI] [PubMed] [Google Scholar]
- 133.Svetoch EA, Stern NJ. Bacteriocins to control Campylobacter spp. in poultry—a review. Poultry Sci. 2010;89:1763–1768. doi: 10.3382/ps.2010-00659. [DOI] [PubMed] [Google Scholar]
- 134.Svetoch EA, Stern NJ, Eruslanov BV, Kovalev YN, Volodina LI, Perelygin VV, Mitsevich EV, Mitsevich IP, Pokhilenko VD, Borzenkov VN, Levchuk VP, Svetoch OE, Kudriavtseva TY. Isolation of Bacillus circulans and Paenibacillus polymyxa strains inhibitory to Campylobacter jejuni and characterization of associated Bacteriocins. J Food Protect. 2005;68:11–17. doi: 10.4315/0362-028x-68.1.11. [DOI] [PubMed] [Google Scholar]
- 135.Teanpaisan R, Piwat S, Dahlén G. Inhibitory effect of oral Lactobacillus against oral pathogens. Lett Appl Microbiol. 2011;53:452–459. doi: 10.1111/j.1472-765X.2011.03132.x. [DOI] [PubMed] [Google Scholar]
- 136.Teughels W, Loozen G, Quirynen M. Do probiotics offer opportunities to manipulate the periodontal oral microbiota? J Clin Periodontol. 2011;38:159–177. doi: 10.1111/j.1600-051X.2010.01665.x. [DOI] [PubMed] [Google Scholar]
- 137.Turovskiy Y, Chikindas M. Zinc lactate and sapindin act synergistically with lactocin 160 against Gardnerella vaginalis . Probiotics Antimicrob Proteins. 2011;3:144–149. doi: 10.1007/s12602-011-9068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Turovskiy Y, Ludescher R, Aroutcheva A, Faro S, Chikindas M. Lactocin 160, a bacteriocin produced by vaginal Lactobacillus rhamnosus, targets cytoplasmic membranes of the vaginal pathogen, Gardnerella vaginalis . Probiotics Antimicrob Proteins. 2009;1:67–74. doi: 10.1007/s12602-008-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van Heel AJ, Montalban-Lopez M, Kuipers OP. Evaluating the feasibility of lantibiotics as an alternative therapy against bacterial infections in humans. Exp Opin Drug Metabol Toxicol. 2011;7:675–680. doi: 10.1517/17425255.2011.573478. [DOI] [PubMed] [Google Scholar]
- 140.van Staden AD, Brand AM, Dicks LMT. Nisin F-loaded brushite bone cement prevented the growth of Staphylococcus aureus in vivo. J Appl Microbiol. 2012 doi: 10.1111/j.1365-2672.2012.05241.x. [DOI] [PubMed] [Google Scholar]
- 141.van Staden DA, Brand AM, Endo A, Dicks LMT. Nisin F, intraperitoneally injected, may have a stabilizing effect on the bacterial population in the gastro-intestinal tract, as determined in a preliminary study with mice as model. Lett Appl Microbiol. 2011;53:198–201. doi: 10.1111/j.1472-765X.2011.03091.x. [DOI] [PubMed] [Google Scholar]
- 142.Varella Coelho ML, JD Santos Nascimento, Fagundes PC, Madureira DJ, Oliveira SSD, Vasconcelos de Paiva Brito MA, Freire Bastos MDCD. Activity of staphylococcal bacteriocins against Staphylococcus aureus and Streptococcus agalactiae involved in bovine mastitis. Res Microbiol. 2007;158:625–630. doi: 10.1016/j.resmic.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 143.Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vendrell D, Balcázar JL, Ruiz-Zarzuela I, de Blas I, Gironés O, Múzquiz JL. Lactococcus garvieae in fish: a review. Comp Immunol Microbiol Infect Dis. 2006;29:177–198. doi: 10.1016/j.cimid.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 145.Verberkmoes NC, Russell AL, Shah M, Godzik A, Rosenquist M, Halfvarson J, Lefsrud MG, Apajalahti J, Tysk C, Hettich RL, Jansson JK. Shotgun metaproteomics of the human distal gut microbiota. ISME J. 2008;3:179–189. doi: 10.1038/ismej.2008.108. [DOI] [PubMed] [Google Scholar]
- 146.Verdon J, Berjeaud J-M, Lacombe C, Héchard Y. Characterization of anti-Legionella activity of warnericin RK and delta-lysin I from Staphylococcus warneri . Peptides. 2008;29:978–984. doi: 10.1016/j.peptides.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 147.Vivekananda MR, Vandana KL, Bhat KG (2010) Effect of the probiotic Lactobacilli reuteri (Prodentis) in the management of periodontal disease: a preliminary randomized clinical trial [DOI] [PMC free article] [PubMed]
- 148.Vriezen JAC, Valliere M, Riley MA. The evolution of reduced microbial killing. Genome Biol Evol. 2009;2009:400–408. doi: 10.1093/gbe/evp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Willey JM, van der Donk WA. Lantibiotics: peptides of diverse structure and function. Annu Rev Microbiol. 2007;61:477–501. doi: 10.1146/annurev.micro.61.080706.093501. [DOI] [PubMed] [Google Scholar]
- 150.Wirawan RE, Klesse NA, Jack RW, Tagg JR. Molecular and genetic characterization of a novel nisin variant produced by Streptococcus uberis . Appl Environ Microbiol. 2006;72:1148–1156. doi: 10.1128/AEM.72.2.1148-1156.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wirawan RE, Swanson KM, Kleffmann T, Jack RW, Tagg JR. Uberolysin: a novel cyclic bacteriocin produced by Streptococcus uberis . Microbiology. 2007;153:1619–1630. doi: 10.1099/mic.0.2006/005967-0. [DOI] [PubMed] [Google Scholar]
- 152.Wu J, Hu S, Cao L. Therapeutic effect of Nisin Z on subclinical mastitis in lactating cows. Antimicrob Agents Chemother. 2007;51:3131–3135. doi: 10.1128/AAC.00629-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu S, Jia S, Sun D, Chen M, Chen X, Zhong J, Huan L. Purification and characterization of two novel antimicrobial peptides subpeptin JM4-A and subpeptin JM4-B produced by Bacillus subtilis JM4. Curr Microbiol. 2005;51:292–296. doi: 10.1007/s00284-005-0004-3. [DOI] [PubMed] [Google Scholar]
- 154.Zahradnik RT, Magnusson I, Walker C, McDonell E, Hillman CH, Hillman JD. Preliminary assessment of safety and effectiveness in humans of ProBiora3 ™, a probiotic mouthwash. J Appl Microbiol. 2009;107:682–690. doi: 10.1111/j.1365-2672.2009.04243.x. [DOI] [PubMed] [Google Scholar]
- 155.Zouhir A, Hammami R, Fliss I, Hamida J. A new structure-based classification of Gram-positive bacteriocins. Protein J. 2010;29:432–439. doi: 10.1007/s10930-010-9270-4. [DOI] [PubMed] [Google Scholar]