Abstract
Anti-apoptotic Bcl-2-family members not only neutralize pro-apoptotic proteins but also directly regulate intracellular Ca2+ signaling from the endoplasmic reticulum (ER), critically controlling cellular health, survival, and death initiation. Furthermore, distinct Bcl-2-family members may selectively regulate inositol 1,4,5-trisphosphate receptor (IP3R): Bcl-2 likely acts as an endogenous inhibitor of the IP3R, preventing pro-apoptotic Ca2+ transients, while Bcl-XL likely acts as an endogenous IP3R-sensitizing protein promoting pro-survival Ca2+ oscillations. Furthermore, distinct functional domains in Bcl-2 and Bcl-XL may underlie the divergence in IP3R regulation. The Bcl-2 homology (BH) 4 domain, which targets the central modulatory domain of the IP3R, is likely to be Bcl-2’s determining factor. In contrast, the hydrophobic cleft targets the C-terminal Ca2+-channel tail and might be more crucial for Bcl-XL’s function. Furthermore, one amino acid critically different in the sequence of Bcl-2’s and Bcl-XL’s BH4 domains underpins their selective effect on Ca2+ signaling and distinct biological properties of Bcl-2 versus Bcl-XL. This difference is evolutionary conserved across five classes of vertebrates and may represent a fundamental divergence in their biological function. Moreover, these insights open novel avenues to selectively suppress malignant Bcl-2 function in cancer cells by targeting its BH4 domain, while maintaining essential Bcl-XL functions in normal cells. Thus, IP3R-derived molecules that mimic the BH4 domain’s binding site on the IP3R may function synergistically with BH3-mimetic molecules selectivity suppressing Bcl-2’s proto-oncogenic activity. Finally, a more general role for the BH4 domain on IP3Rs, rather than solely anti-apoptotic, may not be excluded as part of a complex network of molecular interactions.
Keywords: Bcl-2; Bcl-XL; BH4-domain targets; Inositol 1,4,5-trisphosphate receptors; Ca2+ signaling; Apoptosis
Anti-apoptotic Bcl-2-family members counteract pro-apoptotic Bcl-2-family members
Bcl-2-family members play a pivotal role in a cell’s decision to initiate apoptosis or to promote cell survival by controlling mitochondrial outer membrane permeabilization (MOMP) [1, 2]. Anti-apoptotic Bcl-2-family members (Bcl-2, Bcl-XL, Mcl-1, Bcl-W and Bfl-1) have a well-studied and characterized role in scaffolding the Bcl-2 homology (BH) 3 domain of pro-apoptotic Bcl-2-family members, thereby neutralizing their pro-apoptotic activity [3]. A network of interactions has been described in which anti-apoptotic Bcl-2-family members can scaffold the multi-domain pro-apoptotic proteins, Bax and Bak, the pro-apoptotic Bax/Bak-activator BH3-only proteins, Bid and Bim, or the sensitizer BH3-only proteins, Bad, Bik, Noxa, Hrk, Bmf, and Puma [2, 3]. The latter do not directly activate Bax/Bak, but target anti-apoptotic Bcl-2 proteins, thereby alleviating their repressive function on Bax, Bak, Bid, and Bim. Furthermore, these interactions seem to be dynamic and may be important to prevent the mitochondrial accumulation of pro-apoptotic proteins, like Bax [4, 5]. For instance, Bcl-XL binds Bax at the outer mitochondrial membrane, shuttling Bax back in the cytosol, where the Bcl-XL/Bax complex disassembles resulting in Bax accumulation in the cytosol. On the other hand, Bax activation by BH3-only proteins, like Bim/truncated Bid and Puma, causes a stepwise activation, involving its accumulation at mitochondrial membranes and its oligomerization to a death pore [6, 7]. Besides Bax/Bak, the mitochondrial permeability transition pore can mediate MOMP and cell death in response to apoptotic stimuli that elevate intracellular Ca2+ and induce mitochondrial calcium overload [8, 9]. The latter mechanism can be directly targeted and sensitized by Bad in Ca2+-dependent apoptosis through dephosphorylation of Bad by PP2A [10].
As summarized by Letai and coworkers [11], it is clear that while both activator-BH3-only proteins are targeted by all anti-apoptotic Bcl-2-family members, the interaction between anti-apoptotic proteins and the sensitizer BH3-only proteins display a high degree of selectivity [12–16]. For instance, while the BH3 domain of Bad mainly targets Bcl-2, Bcl-XL, Bcl-W, but not Mcl-1, the BH3 domain of Noxa mainly targets Mcl-1, but not Bcl-2, Bcl-XL, Bcl-W. The selectivity of BH3-only proteins towards anti-apoptotic Bcl-2-family members has been exploited to derive BH3-domain peptides and to set up a “BH3 profile” of cancer cells, identifying cancer cells as “primed for death” and helping to elucidate to which Bcl-2-family members these cancer cells are addicted [11, 12, 17]. This network also spurred the development of a novel class of anti-cancer drugs, the BH3-mimetic molecules, including the Bad BH3-mimetic ABT-737 (or its orally available variant ABT-263) [18–20].
Bcl-2 family members control Ca2+ signaling
The endoplasmic reticulum (ER) and mitochondria are closely connected
The first reports of Bcl-2 affecting ER Ca2+ arose in the beginning of the 90s [21, 22]. Since then, it has become increasingly clear that ER, the main intracellular Ca2+ store, is tightly controlled by Bcl-2-family members critically regulating Ca2+ fluxes from ER to mitochondria [23–27]. In particular, the close connection of the mitochondria and the ER, illustrates the critical role of ER Ca2+ homeostasis and ER Ca2+ release via inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs) during cell survival and cell death [28–31]. The latter channels are important components of the mitochondria-associated ER membranes (MAMs), which establish physical links between mitochondria and ER through interorganellar multi-protein complexes involving IP3Rs, glucose regulated protein (GRP) 75, voltage-dependent anion channels (VDACs), mitofusins, chaperones like phosphofurin acidic cluster sorting protein 2, and peptidic tethers [32, 33]. Recently, the ER-stress sensor PKR-like ER-regulated kinase (PERK) has been identified as a novel member of the MAMs [34]. As a consequence, both the steady-state Ca2+-filling level of the ER [35] as well as the IP3R activity [31] will affect the mitochondria. This is underpinned by recent studies of Foskett’s [36] and the Mikoshiba’s groups [37]. It was shown that constitutive Ca2+ transfer from the ER to the mitochondria through IP3Rs is essential for mitochondrial bioenergetics and for the production of ATP through oxidative phosphorylation. Suppressing this basal Ca2+ firing of IP3Rs causes the activation of AMP-activated kinase (AMPK) and subsequent induction of macroautophagy, a pro-survival lysosomal delivery pathway [36]. This concept is supported by previous studies showing that inhibition of IP3R signaling triggered autophagy [38, 39]. In this perspective, lowering the steady-state [Ca2+]ER levels may reduce the amount of Ca2+ that is released by spontaneous IP3R activity and consequently attenuate Ca2+-mediated cross-talk between ER and mitochondria. In addition, lowering the [Ca2+]ER causes the intraluminal ER chaperone, GRP78/BiP, to dissociate from IP3R1, leading to a decline in the amount of functional IP3R1 channels, further reducing IP3R1-mediated Ca2+ mobilization and inducing apoptotic cell death, as recently described [37].
Besides the spontaneous IP3R activity, agonist-triggered IP3R-mediated Ca2+ signals also affect cell survival and cell death. While repetitive and small Ca2+ oscillations seem to enhance mitochondrial bioenergetics, thereby promoting survival, large Ca2+ transients will inevitably lead to MOMP, thereby promoting cell death [23]. In the latter paradigm, decreasing steady-state [Ca2+]ER will help to avoid mitochondrial Ca2+ overloading and will promote survival, while increasing steady-state [Ca2+]ER will enhance apoptosis. Beyond apoptosis, IP3R activity also seems to be critical for proper autophagy induction during starvation [40, 41] and thus probably for survival responses during adverse conditions. Although the Ca2+-release pathways of the ER have been well established, the mitochondrial Ca2+-uptake pathways remained elusive for a long time. Now, recent work from the Rizzuto group found that VDAC1, but not VDAC2 or VDAC3, is specifically involved in transferring apoptotic Ca2+ signals across the outer membrane of the mitochondria [42]. This likely underlies the selective presence of VDAC1 in the MAMs. Furthermore, Ca2+ transfer across the inner membrane of the mitochondria is mediated by the recently identified mitochondrial Ca2+ uniporter (MCU) [43–45].
Proto-oncogenes and tumor suppressors regulate intracellular Ca2+ signals and -release channels
As illustrated above, it is now clear that cell survival and cell death are tightly controlled by Ca2+ signaling. Hence, it is not surprising that proto-oncogenes, like anti-apoptotic Bcl-2-family members, protein kinase B (PKB)/Akt, Bax-Inhibitor-1 (BI-1) and tumor suppressors like promyelocytic leukemia (PML) and fragile histidine triad (FHIT) regulate ER Ca2+-release and mitochondrial Ca2+-uptake mechanisms [46–49]. IP3Rs are phosphorylated by the pro-survival kinase PKB/Akt, which is activated by phosphatidylinositol-3,4,5-trisphosphate (PIP3), thereby suppressing IP3R-channel activity and promoting survival [50, 51]. This is important, since phosphatase and tensin homolog (PTEN), a negative regulator of PKB/Akt signaling through dephosphorylation of PIP3 to phosphatidylinositol-4,5-bisphosphate, is one of the most frequent loss functions in human cancers [52]. Another negative regulator of PKB/Akt activity is the tumor suppressor PML, which recruits protein phosphatase 2A to the IP3R-PKB/Akt-protein complex in the MAMs and suppresses PKB/Akt activity [53–55]. At the mitochondrial level, FHIT seems to target MCU-driven mitochondrial Ca2+ uptake, thereby enhancing the transfer of Ca2+ into mitochondria during physiological signaling [56]. In addition, anti-apoptotic Bcl-2 proteins can regulate VDAC1-channel activity by directly binding, among other domains, its N-terminal tail, a region important for VDAC-1’s pro-apoptotic activity [57]. Importantly, some anti-apoptotic proteins do not target ER Ca2+-release or -uptake mechanisms, but function themselves as Ca2+-leak channels. For instance, ER-stress suppressor BI-1 has been shown to display endogenous Ca2+-leak activity as a Ca2+/H+ antiporter or Ca2+-release channel, thereby directly controlling the filling state of the ER Ca2+ stores [58–60]. In accordance with this, BI-1 overexpression has been proven to lower the Ca2+-filling state of the ER, a mechanism previously shown to act protective against apoptosis [35]. In this regard, we have also recently identified a putative Ca2+-channel pore in the C-terminal part of BI-1 [61]. In addition to this, BI-1 directly binds IP3Rs through their channel domains, thereby sensitizing these intracellular Ca2+-release channels to IP3 [62]. This mechanism seems to underlie the autophagy-promoting effect of BI-1, which required the presence of functional IP3R channels [63].
Bcl-2-family members control Ca2+ signaling from the ER
Besides these mechanisms, the best-studied protein family, regulating intracellular Ca2+ is the anti- and pro-apoptotic Bcl-2-family. Pinton et al. [64] elucidated a protective role of Bcl-2 at the ER. They found that Bcl-2 overexpression at the ER enhanced the ER Ca2+-leak rate and thus reduced the level of steady-state [Ca2+]ER, thereby dampening agonist-induced IP3R-mediated Ca2+ signals originating from the ER and thus reducing the transfer of Ca2+ to the mitochondria. This mechanism was underpinned by Scorrano and coworkers [65] who used mouse embryonic fibroblasts lacking Bax/Bak to increase the ratio of anti-apoptotic over pro-apoptotic Bcl-2 family members. Bax/Bak-deficient cells displayed decreased steady-state [Ca2+]ER levels, which protected the cells against apoptotic stimuli. The underlying mechanism involved the hypersensitization of the IP3R1 towards basal IP3 through a PKA-dependent phosphorylation of the IP3R, enhancing the basal IP3R-mediated Ca2+ leak from the ER [66]. In contrast, Distelhorst and coworkers initially proposed another mechanism for the protective role of Bcl-2 at the ER, pointing out that Bcl-2 maintained ER Ca2+ homeostasis [67]. Successively, Bcl-2 was also reported to recruit calcineurin/PP2B on IP3Rs [68, 69] or indirectly bind to the IP3R and suppress IP3R activity through the phosphatase PP1 [70]. More recent works elucidated direct binding of anti-apoptotic Bcl-2-family members to IP3Rs, finely regulating their Ca2+-flux properties and consequently cell death outcomes [71–73]. Additionally, Bcl-2-family members are able to indirectly regulate IP3R signaling by controlling the expression levels of IP3Rs. For instance, Bcl-XL overexpression has been shown to decrease the level of IP3Rs in cells by a decreased binding of the transcription factor nuclear factor of activated T cells (NFAT) cytoplasmic 2 to the IP3R promoter [74]. Next, anti-apoptotic Bcl-2 was proposed to up-regulate sarco/ER Ca2+-ATPase (SERCA) levels, thereby supporting sustained ER Ca2+ filling [75, 76]. This may be due to the direct molecular interactions found between some anti-apoptotic Bcl-2 family members and SERCA [75–77]. However, other studies indicated that the targeting of SERCA1, the skeletal muscle type isoform, by Bcl-2 seemed to destabilize and inactivate the SERCA protein by exposing thiol groups [78], thereby lowering the content of ER Ca2+ stores. The mechanisms may involve the translocation of SERCA1 from sarcoplasmic reticulum (SR) lipid-caveolae domains [79]. A recent paper from the same group showed that Bcl-2 also destabilized SERCA2b, the house-keeping isoform of the SERCA-protein family, while heat-shock proteins, chaperones, and other stress-regulated proteins attenuated the negative regulation of SERCA2b by Bcl-2 [80]. These findings are underpinned by recent observations in cystic fibrosis airway epithelium, which displayed decreased SERCA levels, increased Bcl-2 levels and the presence of SERCA/Bcl-2-protein complexes on ER membranes [81]. Finally, Bcl-2 was shown to counteract both the pro-apoptotic and paraptotic effects of p20, a cleaved form of Bap31, via regulation of ER Ca2+. Paraptosis is a form of caspase-independent non-apoptotic programmed cell death that is characterized by cytoplasmic vacuolation initiated by mitochondrial and ER swelling [82–84]. Bap31 is an ER-located protein that plays roles in protein trafficking [85] as well as ER-associated degradation [86]. In addition, Bap31 was shown to have anti-apoptotic qualities [87]. When Bap31 is cleaved by caspase 8, the resultant ER-located protein, p20, is known to have pro-apoptotic functions. This protein mobilizes ER Ca2+, resulting in MOMP. Via the above-described mechanisms, Bcl-2 is able to counteract these pro-apoptotic signals, allowing cell survival [88]. In addition, a recent paper describes a p20-initiated Bax/Bak-independent paraptotic death pathway [89]. Instead of mobilizing the ER Ca2+, p20 was shown to increase [Ca2+]ER leading to ER remodeling, vacuolization, and both caspase and Bax/Bak-independent paraptotic cell death. Here, the ability of Bcl-2 to lower [Ca2+]ER was shown to protect the cells from these events typically associated with paraptotic cell death.
Finally, in many cells, including pancreatic acinar cells, the ER can come in very close contact with the plasma membrane [90]. Thus, ER-localized Bcl-2 may have plasmalemmal targets and more general cell biological functions in regulating cellular Ca2+ homeostasis. A recent report showed that Bcl-2 suppresses cellular Ca2+ extrusion through the plasma membrane Ca2+ ATPase (PMCA), thereby determining the cell-death pathway that is engaged [91]. In this study, it was shown that Bcl-2-deficient pancreatic acinar cells extrude Ca2+ more efficiently, protecting them against excessive necrosis. At the same time, apoptosis was increased in cells exposed to reactive oxygen species (ROS) generated by menadione treatment. However, inhibition of PMCA using a peptide inhibitor promoted necrosis in menadione-treated cells, which may indicate that excessive Bcl-2 accumulation at the ER-plasma membrane junction inhibiting PMCA may be deleterious.
Irrespective of the underlying mechanism, it is clear that Bcl-2 proteins critically regulate ER Ca2+ homeostasis and dynamics. This is supported by a recent study, showing that chemical inhibitors of pro-survival Bcl-2-family members like the BH3-mimetic molecules BH3I-2′ and HA14-1 cause a pro-apoptotic depletion of the ER Ca2+ stores in part through activation of IP3R Ca2+-release channels [92].
Bcl-2-family members directly target IP3Rs
Bcl-2 and Bcl-XL directly target IP3Rs, but at different sites
More recent work indicated that Bcl-2 does not primarily act by altering the ER Ca2+-store content. Instead, Bcl-2 directly targets IP3Rs and functions as an endogenous regulator of IP3Rs [71, 93–97]. In this paradigm, Bcl-2 suppresses pro-apoptotic IP3R-mediated Ca2+ transients (provoked by strong T-cell-receptor stimulation), while maintaining or even promoting pro-survival Ca2+ oscillations (provoked by weak T-cell receptor stimulation). Moreover, Bcl-XL also directly binds IP3Rs and sensitizes IP3R-channel to sub-threshold [agonist] stimulation [72, 98]. IP3R/Bcl-XL-complex formation increases the frequency of Ca2+ oscillations, mitochondrial bioenergetics, and NFAT-mediated signaling in Bcl-XL-overexpressing DT40 cells, while not affecting global agonist-induced Ca2+ transients. Elegantly, it was shown that Bcl-XL protection against high [anti-IgM]-induced apoptosis was reduced in the absence of IP3Rs [98]. Furthermore, the effect of Bcl-XL on Ca2+ signaling depended on the type of IP3R isoform. Bcl-XL stimulated IP3R-mediated Ca2+ oscillations for all three isoforms while it lowered [Ca2+]ER in IP3R3-, but not in IP3R1- or IP3R2-, expressing DT40 cells.
At the molecular level, striking differences between Bcl-2 and Bcl-XL for IP3R binding were observed. While Bcl-2 binds to the central, modulatory domain of the IP3R [95, 96], Bcl-XL binds the C-terminal region close to the Ca2+-channel pore [72, 98] (Fig. 1). This C-terminal tail is also involved in the control of IP3R-channel gating through the N-terminal suppressor domain of the IP3-binding domain [99]. Thus, Bcl-XL may enhance the coupling between the N-terminal IP3-binding domain and C-terminal channel-pore opening, underlying the observed IP3R sensitization. The latter region has been proposed to display structural features that mimic the BH3 domain of BH3-only proteins [100]. In this respect, one expects that the hydrophobic cleft formed by BH3, BH1, and BH2 of all anti-apoptotic Bcl-2-family members may participate in the binding the IP3R. Finally, it has been recently described that not only Bcl-XL but also Bcl-2 and Mcl-1 target this site on IP3Rs and cause IP3R sensitization [73]. In addition to this site, Bcl-2 possesses an additional binding site on the IP3R with distinct molecular and functional properties. Indeed, Bcl-2 directly binds to a site between amino acids 1389–1408 of IP3R1. Bcl-2 binding to this central, modulatory domain of the IP3R causes an inhibition of the Ca2+-flux properties of IP3R in response to agonist stimulation (Fig. 1). Furthermore, a peptide corresponding to the Bcl-2-binding site on IP3Rs (a.a. 1389–1408), IP3R-derived peptide (IDP), completely abolishes the binding of Bcl-2 to the IP3Rs [95]. A cell-permeable version of IDP enhances IP3R-mediated Ca2+ signaling, thereby potentiating apoptotic signals, similarly to strong TCR stimulation. In this respect, IDP derepresses Bcl-2’s inhibitory function on IP3R1 by specifically targeting its BH4 domain and not the BH3-binding hydrophobic cleft. The only domain of Bcl-2 sufficient for binding, inhibiting, and protecting against IP3R1-mediated apoptosis [96, 101] is indeed the BH4 domain. This indicates that IDP targets Bcl-2 independently of the compounds that target the hydrophobic cleft, like the BH3-mimetic tools, ABT-737 and HA14-1. Combining IDP with ABT-737 enhanced the potency of ABT-737 to induce cell death in lymphocytes obtained from chronic lymphocytic leukemia (CLL) patients [102]. Furthermore, applying a stabilized cell-permeable form of IDP (TAT-IDPDD/AA) potently induced cell death through excessive IP3R-mediated Ca2+-release events in CLL cells, while TAT-IDPDD/AA did not significantly reduce the survival of normal lymphocytes [103].
Fig. 1.
Differential regulation of IP3R channels by Bcl-2 versus Bcl-XL. The Ca2+-flux properties of IP3R are thought to be critically controlled by Bcl-2-family members to promote cell survival or protect against cell death. We hypothesize that distinct Bcl-2-family members target distinct IP3R domains. In this paradigm, Bcl-2 through its BH4 domain may primarily target the central, modulatory domain of the IP3R, thereby reducing large global pro-apoptotic Ca2+ transients (left), while Bcl-XL through its hydrophobic cleft (HC) or another domain may primarily target the C-terminal tail of the IP3R close to the channel pore, thereby increasing IP3R sensitivity to basal IP3 levels and promoting pro-survival Ca2+ oscillations (right). It should be noted that the C-terminal domain of IP3Rs has been proposed to harbors BH3-like domains and may also recruit Bcl-2. In addition, there is increasing evidence that other Bcl-2-family members may target IP3Rs, like NrZ, the zebrafish homologue of Bcl-2L10, through its BH4 domain and Mcl-1 through its hydrophobic cleft (HC) or another domain may primarily target the C-terminal tail of the IP3R close to the channel pore
Bcl-2 and Bcl-XL regulate various IP3R-dependent physiological and pathophysiological processes
Bcl-2 and Bcl-XL-mediated regulation of IP3Rs is not only relevant for cell death and cancer but also for other physiological processes like embryonic development and pathophysiological conditions, like muscle dystrophy, type-2 diabetes, and bipolar disorders.
A recent paper by Gillet et al. revealed that Nrz (the zebrafish orthologue of human Nrh/Bcl-2L10) through its BH4 domain binds and regulates IP3R-mediated Ca2+ signaling in the developing zebrafish embryo, acting as an inhibitor of IP3R function [104]. In more detail, Nrz is proposed to suppress Ca2+ signaling in yolk syncytial layer (YSL) to facilitate proper blastomere migration from the animal to vegetative pool (known as epiboly, a process that happens before the onset of gastrulation [105]) [104]. Completion of epiboly is characterized by the formation of an acto-myosin contractile ring close to the vegetative pool of the enveloping layer and the deep cell layer. Therefore, it is critically important that during epiboly Ca2+ signaling in the YSL is suppressed to prevent premature acto-myosin contractions. This is supported by recent findings showing that nrz morphants displayed elevated Ca2+ signaling in the YSL causing Ca2+-dependent myosin light chain (MLC) phosphorylation by MLC kinase, thereby affecting cytoskeletal dynamics and cell movements [104]. As a consequence, nrz morphants undergo developmental arrest before the onset of gastrulation, resulting in embryonic death without the activation of caspases [106]. This indicates that Bcl-2 family members as critical Ca2+ regulators not only control apoptosis but also developmental processes through Ca2+-dependent processes like acto-myosin contraction and/or cell movements [107]. The molecular determinants underpinning this role have not been fully characterized yet but it is intriguing that both Bcl-2’s and Nrz’s BH4 domains bind and modulate IP3Rs despite their very divergent primary sequence. Eventually, this may suggest that the concept of Bcl-2-dependent regulation of IP3Rs is dynamically conserved during evolution.
In Duchenne muscle dystrophy, a lethal disease caused by deficiency in dystrophin, a cytoskeletal protein, the degeneration of muscle is associated with disrupted intracellular Ca2+ homeostasis [108]. Overexpression of Bcl-2 in myotubes obtained from dystrophic (mdx) mice decreases subsarcolemmal and mitochondrial Ca2+ elevations in response to stimulation of the nicotinic acetylcholine receptor [109]. The central role of IP3Rs in this process was underpinned by experiments performed on saponin-permeabilized myotubes. Myotubes obtained from mdx mice displayed more IP3-induced Ca2+ responses than their wild-type counterparts, while Bcl-2 overexpression suppressed these IP3R-dependent Ca2+ signals. These observations correlate with the increased susceptibility of mdx myotubes to apoptotic stimuli, which could be counteracted by overexpressing Bcl-2 or an IP3 sponge.
In the vascular smooth muscle of type 2 diabetes mouse models, the level of Bcl-XL, but not of Bcl-2, seemed elevated, while IP3R levels remained constant [110]. Importantly, the rate of IP3R-mediated Ca2+ release from the SR of vascular smooth muscle of type 2 diabetes mouse models was similar to their wild-type counterparts. This enhanced IP3R activity by Bcl-XL was counteracted by ABT-737, suggesting IP3R regulation by Bcl-XL through its hydrophobic cleft.
Very recently, a single-nucleotide polymorphism (SNP) in the Bcl-2 gene (rs956572) associated with bipolar disorder seemed to affect Ca2+ signaling in the lymphoblasts of bipolar disorder patients [111, 112]. This Bcl-2-deficient SNP variant AA is known to be associated with reduced Bcl-2-mRNA and -protein levels and directly affects the brain by significantly decreasing grey matter volume in the ventral striatum of healthy subjects [113]. The striatum’s ventral region is important for the neurobiology and pathophysiology of mood disorders [114]. Particularly, Bcl-2-deficient SNP variant AA caused elevated cytosolic [Ca2+] and increased IP3R-mediated Ca2+ release without affecting basal ER and mitochondrial Ca2+ levels [111]. These properties were associated with a decline in the Bcl-2-mRNA and -protein levels. In addition, increased IP3R-mediated Ca2+ release could be mimicked by treating lymphoblasts from subjects presenting the normal Bcl-2 SNP variant GG with the Bcl-2 inhibitor BH3-I. Therefore, it is likely that IP3Rs from lymphoblasts containing the Bcl-2-deficient SNP variant AA are largely depleted from Bcl-2. Nevertheless, this study suggests a critical role for IP3R/Bcl-2 complexes in regulating intracellular Ca2+ dynamics in the brain to control emotional regulation and reward processing.
Collectively, these examples show that Bcl-2 and Bcl-XL display different functional properties towards IP3R regulation, underpinning that distinct IP3R and Bcl-2/Bcl-XL protein domains are responsible for this phenomenon.
Bcl-2 and Bcl-XL display different BH4-domain properties at the level of the IP3R
Despite the fact that Bcl-2 and Bcl-XL are highly similar in sequence and structure, the BH4-domain biology of Bcl-2 and Bcl-XL seems totally different [101]. Both their BH4 domains protect against IP3R-mediated apoptosis, but only the BH4-Bcl-2 domain binds and inhibits IP3Rs. Indeed, IDP seems to inhibit only Bcl-2 by targeting its BH4 domain without affecting Bcl-XL’s anti-apoptotic function. This is an important therapeutic advantage over the existing BH3-mimetic molecules. For instance, ABT-737 acts as a Bad BH3-mimetic molecule, indicating that it does not discriminate between Bcl-2 and Bcl-XL, thus inhibiting both proteins. This may not be desirable in cancer patients and cause adverse effects, since Bcl-2 and Bcl-XL have distinct biological functions. While some types of cancer cells may need the elevated Bcl-2 levels to compensate for the on-going upstream pro-apoptotic signaling and the elevated levels of BH3-only proteins, normal cells may still need Bcl-XL for their survival. Potent Bcl-2 inhibitors, like the BH3 mimetics ABT-737 and ABT-263, which target the hydrophobic cleft of both Bcl-2 and Bcl-XL, are already in clinical development and enhance the therapeutic potency of different chemotherapeutical drugs in solid and hematologic malignancies [20, 115–119]. However, in single-use regiments, these compounds lead to in vivo dose-dependent transient thrombocytopenia [120] and thrombocytopathy [121] in a similar range as they kill cancer cells (like CLL). The former is due to the inhibition of Bcl-XL, which is essential to sustain platelet survival by limiting Bax activity [122]. Since BH3-mimetic molecules do not discriminate between the hydrophobic cleft of Bcl-2 and Bcl-XL, the treatment of Bcl-2-dependent malignancies by BH3 mimetics like ABT-263 will provoke side effects in patients by limiting the life span of platelets. Hence, specifically targeting the BH4 domain of Bcl-2 with IDP may be a very promising approach to promote cell death via the induction of pro-apoptotic Ca2+ signaling in Bcl-2-dependent malignancies [102].
Collectively, these data indicate that Bcl-2 and Bcl-XL likely have distinct properties at the level of the IP3R (Fig. 1). We propose that the predominant effect of Bcl-2 is executed via its BH4 domain targeting the central, modulatory domain of the IP3R, imposing IP3R inhibition and ultimately preventing large pro-apoptotic Ca2+ transients. For Bcl-XL, we anticipate a dominant role for its hydrophobic cleft targeting the BH3 structure near the C-terminal Ca2+-channel pore of the IP3R, optimizing IP3R-channel gating and sensitivity towards IP3. We do not exclude that Bcl-2 too targets this C-terminal site, but its BH4-domain biology seems to overcome this sensitizing effect.
The conserved Lys17 in the BH4 domain of Bcl-2 determines its selective action on IP3Rs
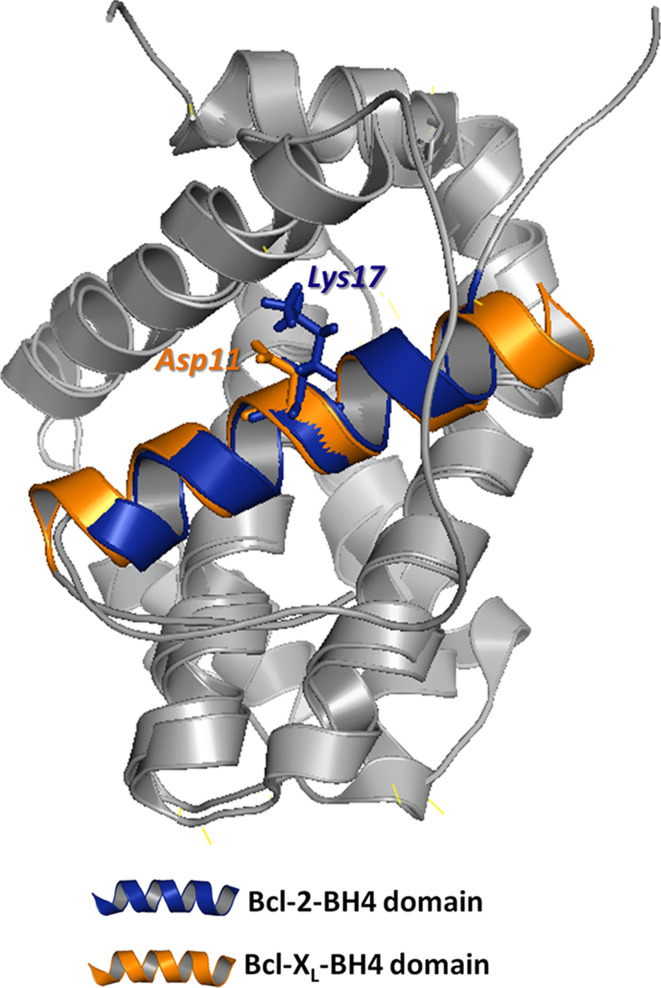
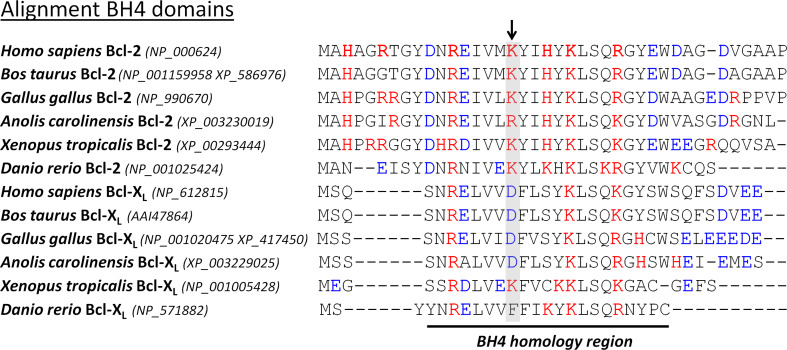
Recently, we elucidated one factor in the selective action of Bcl-2 and Bcl-XL on IP3Rs [101]. While most residues are conserved among the BH4 domains of Bcl-2 and Bcl-XL, we identified a critical difference in one single surface-accessible residue in the center of this domain (Fig. 2). We found that Lys17 in BH4-Bcl-2 is not conserved in BH4-Bcl-XL, in which it corresponds to an Asp residue. We performed a plethora of molecular and functional studies to pinpoint this residue as the underlying factor responsible for the difference in BH4-domain biology between Bcl-2 and Bcl-XL. Indeed, replacing Asp11 by Lys in BH4-Bcl-XL led to a variant that is able to bind and inhibit IP3Rs, while replacing Lys17 by Asp in BH4-Bcl-2 led to a variant that completely lost its IP3R-binding and inhibitory properties. The importance of this critical difference for the biological properties of these proteins is highlighted by the fact that altering this residue in full-length Bcl-2 impairs its ability to regulate IP3Rs and to protect against Ca2+-mediated apoptosis. This is further highlighted by the fact that this critical difference in residues is conserved among the five classes of vertebrates in both Bcl-2 and Bcl-XL (Fig. 3). Indeed, all vertebrate Bcl-2 orthologues contain a positively charged amino acid in the center of their BH4 domain, while all vertebrate Bcl-XL orthologues contain a negatively charged amino acid. This means that already in the first appearances of Bcl-2 and Bcl-XL during evolution, this selective function may have been important.
Fig. 2.
A representation of the overlapping Bcl-2 and Bcl-XL structures. Their respective BH4 domains (blue for Bcl-2, orange for Bcl-XL) have been indicated together with the critical difference between Bcl-2 (Lys17) and Bcl-XL (Asp11), which determines the ability of Bcl-2, but not of Bcl-XL, to interact with the central, modulatory domain of the IP3R
Fig. 3.
Sequence alignment of the BH4 domain of different Bcl-2 and Bcl-XL homologues in the different classes of vertebrates. Bcl-2 and Bcl-XL homologues were obtained from the DeathBase [152]. The number between brackets indicates the accession number of the protein database of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/protein). This analysis reveals that the amino acid Lys17 in human Bcl-2 is conserved as a positively charged residue during evolution. The amino acid Asp11 in human Bcl-XL also seems conserved as a negatively charged residue during evolution, although Xenopus Bcl-XL contains a Lys and zebrafish Bcl-XL contains a Phe in the corresponding position. The positively charged (red) and negatively charged (blue) amino acids are depicted in color. The conserved critical Lys residue in the Bcl-2 homologues and its corresponding residue in the Bcl-XL homologues are displayed on a gray background and are indicated by an arrow
Therefore, while Bcl-2 and Bcl-XL were considered alike in their respect to regulating Ca2+ signaling, we propose selective functions for Bcl-2 and Bcl-XL at the level of the IP3R. This idea impinges on the selective environment in which Bcl-2 and Bcl-XL seems to operate. Bcl-2 seems to operate at different intracellular membranes, including the ER, while Bcl-XL seems to mainly operate at mitochondrial membranes and in the cytosol. On the one hand, excessive Bcl-2 expression at the mitochondria seems to be toxic for the cells and leads to apoptotic cell death, while Bcl-2 expression at the ER promotes bona fide anti-apoptotic responses [22]. On the other hand, a very recent and elegant study using the recomplementation of Bcl-X−/−L cells with either ER or mitochondrial-targeted Bcl-XL showed that the presence of Bcl-XL is a conditio sine qua non for proper protection against apoptotic stimuli [123]. Strikingly, ER-targeted Bcl-XL expression in Bcl-X−/−L cells was able to regulate ER Ca2+ homeostasis, but this was not sufficient to protect against apoptotic stimuli. The latter required mitochondrial Bcl-XL, since Bcl-XL expression in wild-type cells containing endogenous Bcl-XL provided apoptosis protection. Thus, the ER seems part of the natural environment, in which Bcl-2 would operate in protecting against apoptosis, while the mitochondria may be the natural environment for Bcl-XL-mediated protection against apoptosis. Finally, alignment of the BH4 domain of the other Bcl-2-family members indicates that the BH4 domain of Bcl-XL resembles Bcl-2 one’s the most. Thus, since BH4-Bcl-XL is not able to target IP3Rs, this may suggest a unique role for the BH4 domain of Bcl-2 among the other Bcl-2-family members in repressing pro-apoptotic IP3R function.
Other selective BH4-domain targets for Bcl-2 and Bcl-XL?
Although a number BH4 domain targets of Bcl-2 and/or Bcl-XL have been identified, a selective role for both proteins has not been investigated. In this respect, most studies either focused on the BH4 domain of Bcl-2 or of Bcl-XL, providing a growing list of novel targets beyond IP3Rs, including calcineurin/PP2b, VDACs, Raf-1, Ras, CED-4, paxillin, NF-kB, BI-1 and apoptosis-stimulating of p53 protein 2 (ASPP2) [96]. However, some findings in the literature seem to hint towards a selective regulation of these targets by Bcl-2 versus Bcl-XL and vice versa [124–126]. Nevertheless, in many cases, firm evidence is lacking, because a side-by-side comparison of the regulation of these targets by Bcl-2 versus Bcl-XL, or by protein domains derived from them, has never been performed. Since VDAC1 and BI-1 directly control Ca2+-signaling events and apoptosis, we first discuss their BH4-mediated regulation. Finally, we focus on the pro-apoptotic ASPP2 protein, which has been proposed to display selective Bcl-2/Bcl-XL-binding properties.
VDACs
VDACs are transport proteins located on the outer mitochondrial membranes responsible for exchanging metabolites and ATP between cytosol and mitochondria and for the flux of Ca2+ ions from ER into the mitochondria [127, 128]. VDAC proteins seem to be essential for both cell growth and apoptosis [129, 130]. The role in cell growth seems to involve their ability to transport metabolites and energy, but may also be attributed to its Ca2+-flux properties and its localization in MAM’s [127]. This flux of Ca2+ from the ER into the mitochondria is essential for proper mitochondrial bioenergetics [36]. This correlates with recent observations from White and coworkers showing that Bcl-XL, at the mitochondrial membranes, enhanced VDAC1-mediated Ca2+ flux into the mitochondria, thereby promoting ATP production and increasing mitochondrial bioenergetics (Carl White, pers. comm.). However, Bcl-XL’s regulation of mitochondrial Ca2+ uptake might be different during apoptosis, since this anti-apoptotic protein was previously shown to delay Ca2+-mediated MOMP in neuronal cell models [131]. Although the role of VDAC proteins in mitochondria-dependent cell death has always been controversial, recent evidence showed that VDAC1, but neither VDAC2 nor VDAC3, relays IP3R-mediated pro-apoptotic Ca2+ signals into the mitochondria [42]. Additionally, the expression of VDAC1 appears to critically control apoptosis likely by the formation IP3R/VDAC1 complexes, which are enhanced during apoptotic stress, and by the formation of VDAC1 oligomers [42]. The oligomerization of VDAC1 has been shown to be coupled to its ability to induce apoptosis [132]. Further reports, from Shoshan-Barmatz’s laboratory, indicate that anti-apoptotic proteins, like Bcl-2 and hexokinase I and II bind mainly the N-terminal part of VDAC1 and suppress VDAC’s apoptotic function [57, 133–135]. The Bcl-2/Bcl-XL protein domain regulating VDAC1 activity was proposed to be its BH4 domain [126]. Indeed, the isolated BH4 domains of both Bcl-2 and Bcl-XL were sufficient to inhibit VDAC1 activity in isolated mitochondria and to prevent apoptosis in intact cells. Likewise, solely a Bcl-XL not lacking the BH4 domain could display these anti-apoptotic properties. However, in this study [126], a complete, quantitative comparison between the properties of the BH4 domain of Bcl-2 and Bcl-XL for preventing apoptosis through targeting VDAC1 was not performed. Nevertheless, a sucrose-driven liposomal swelling assay mediated by reconstitution of recombinant VDAC1 into the liposomes showed that both BH4-Bcl-2 and BH4-Bcl-XL inhibited VDAC1 activity, but BH4-Bcl-XL seemed more potent than BH4-Bcl-2. In any case, a full side-by-side and quantitative comparison between BH4-Bcl-2 and BH4-Bcl-XL is needed to unravel their differences in regulating VDAC1 activity and to characterize the importance of Asp11 in BH4-Bcl-XL and Lys17 in BH4-Bcl-2 for these properties. Furthermore, it will be necessary to determine how the properties of isolated BH4 domains are reflected in the regulation of VDAC1 by full-length Bcl-2 and Bcl-XL. Indeed, it seems likely that other protein domains of Bcl-2 and Bcl-XL besides the BH4 domain are involved in the direct interaction with VDAC1, since Bcl-XL lacking its BH4 domain still interacts with VDAC1 [126, 136]. In addition, the mechanism by which these BH4 domains target VDAC1 is poorly characterized and may involve a complex network of protein interactions.
Bax Inhibitor-1
Seminal work from Reed’s laboratory elucidated BI-1 as a highly conserved ER-localized six/seven-transmembrane domain protein that protects cells against apoptosis and counteracts ER stress [137, 138]. Part of BI-1’s anti-apoptotic properties have been attributed to its role in controlling ER Ca2+ homeostasis through its H+/Ca2+-antiporter activity [59, 60, 139]. BI-1 overexpression leads to enhanced ER Ca2+ leak and decreases the steady-state ER Ca2+ levels, while cells deficient for BI-1 display an increase in [Ca2+]ER. These BI-1 properties seemed to be highly dependent on its C-terminal domain [59, 140, 141]. These findings are compatible with the recently identified Ca2+-channel pore in the membrane-embedded part of the C-terminal domain of BI-1 [61]. Furthermore, there is now mounting evidence that other BI-1-related proteins like human Golgi anti-apoptotic protein (hGAAP) and TMBIM6/GRINA also control ER Ca2+ homeostasis potentially by regulating IP3Rs [142, 143]. While BI-1’s name refers to its discovery as a high-copy suppressor of Bax-induced cell death in yeast, BI-1 is targeted and regulated by anti-apoptotic Bcl-2-family members. Bcl-2 seems to bind BI-1 through its BH4 domain [138]. Furthermore, the BH4 domain of Bcl-2 stimulates BI-1’s H+/Ca2+ anti-porter activity by promoting BI-1 oligomerization [139]. In fact, the regulation of the Ca2+-flux properties of BI-1 by anti-apoptotic Bcl-2-family members may underlie the conflicting evidence on whether Bcl-2-family members can lower the ER Ca2+-store content or not. Reed and coworkers showed that Bcl-XL required the presence of BI-1 to lower [Ca2+]ER, since overexpression of Bcl-XL in BI-1-deficient cells failed to decrease the ER Ca2+-store content, indicating a critical role for BI-1 as downstream targets of Bcl-2 proteins in lowering [Ca2+]ER [60]. In these studies, both Bcl-2 and Bcl-XL seemed to similarly affect the Ca2+-leak properties of BI-1. While it seems likely that these effects are mediated through their BH4 domains, it is not known whether BH4-Bcl-2 and BH4-Bcl-XL are equally potent in controlling BI-1 properties.
ASPP2
ASPP2 provokes mitochondrial-dependent cell death by activating tumor suppressors like p53 and by counteracting pro-survival mechanisms like NF-κB and Bcl-2 [144, 145]. Two variants of the pro-apoptotic protein ASPP2 have been discovered: one variant binds to the tumor suppressor p53 and stimulates its pro-apoptotic activity by enhancing the expression of pro-apoptotic proteins at the transcriptional level; the other variant binds to and counteracts the anti-apoptotic Bcl-2 proteins, leading to apoptosis by promoting the release of pro-apoptotic proteins, like BH3-only proteins, from Bcl-2 [146, 147]. Structural studies elucidated four ankyrin repeats and an SH3 domain in the C-terminal part of ASPP2, responsible for interaction with other proteins, including p53, NF-κB, and Bcl-2 [146, 148–151]. An elegant study combining molecular modeling with biophysical analysis revealed the molecular properties of the interaction of C-terminal domain of ASPP2 with anti-apoptotic Bcl-2-family members [125]. Using a peptide array screening, both the BH4 domains as well as the hydrophobic cleft, involved in scaffolding pro-apoptotic BH3 domains, were identified as ASPP2-binding sites. Using quantitative biophysical methods, it was shown that the binding affinity of ASPP2 to BH4-Bcl-2 was about tenfold higher than to BH4-Bcl-XL or to the Bcl-2-hydrophobic cleft. This indicates a dual selectivity in ASPP2-binding properties of anti-apoptotic Bcl-2-family members. Strikingly, a critical role in the high-affinity binding of BH4-Bcl-2 to ASPP2 was attributed to the surface-exposed Lys17. Lysine’s additional positive charge seemed critical, since replacing Lys17 by an alanine or an aspartate (like in BH4-Bcl-XL) caused a significant reduction in the binding affinity to ASPP2 or completely abolished ASPP2 binding, respectively. Docking studies revealed that SH3 domain targeted the BH4 domain of Bcl-2/Bcl-XL, while the ankyrin repeats targeted the hydrophobic cleft of Bcl-2/Bcl-XL. Hence, ASPP2 may counteract the anti-apoptotic function of both Bcl-2 and Bcl-XL but with different efficiency. In this way, ASPP2 may discriminate between Bcl-2 and Bcl-XL targets. Therefore, pro-apoptotic targets of Bcl-2 and Bcl-XL may be released in a selective manner or time frame upon ASPP2 binding to Bcl-2 and/or Bcl-XL. In this respect, ASPP2 levels may control the properties of proteins that are targeted by both the BH4 domain and the hydrophobic cleft of Bcl-2 anti-apoptotic proteins.
Conclusions
An essential role of anti-apoptotic Bcl-2 family proteins is due to their regulation of intracellular Ca2+ dynamics. Here, we have discussed a selective function of Bcl-2 as endogenous IP3R inhibitors versus Bcl-XL as endogenous IP3R sensitizers. We propose that distinct functional domains of Bcl-2 and Bcl-XL underlie their divergence in IP3R-functional regulation. In more detail, Bcl-2 acts on the IP3Rs primarily via its BH4 domain on the receptor central, modulatory domain while Bcl-XL via its hydrophobic BH3-domain-binding cleft and on the C-terminal channel-pore domain. We identified a conserved molecular determinant (Lys17) that is critical for the inhibitory action of the BH4 domain of Bcl-2 on IP3Rs and that is evolutionary conserved among all Bcl-2 orthologues in the five classes of vertebrates. It is one of the most striking differences in surface-accessible residues between BH4-Bcl-2 and BH4-Bcl-XL underlying the selective action of BH4-Bcl-2 on IP3Rs. Furthermore, since the sequence of the BH4 domains of other Bcl-2 family members including Mcl-1 deviates a lot from Bcl-2, this suggests a unique role for the BH4 domain of Bcl-2 as an endogenous inhibitor of the IP3R channel. However, this concept may be too simplistic, considering the recent data showing that the zebrafish’s Bcl-2-related protein Nrz is still able to bind and control IP3Rs activity via its BH4 domain. These data may suggest a broader role for the BH4 domain biology in Ca2+ signaling beyond apoptosis modulation, either by a distinct regulation of the Ca2+-flux properties of IP3R channels or by selective binding and regulation of Ca2+-transport systems in both the ER and the mitochondria. In conclusion, future research is needed to fully characterize BH4-domain biology in the context of Bcl2’s proteins physiological and pathophysiological activities, especially considering the growing list of its potential molecular targets besides the IP3Rs.
Acknowledgments
Work in the author’s laboratory has been supported by research grants from the Research Council of the KU Leuven (OT/STRT1/10/044), from the Research Foundation—Flanders (FWO) grants G.0788.11 and G.0571.12, and from the Royal Flemish Academy of Belgium for Science and the Arts (Research Award from the Octaaf Dupont Foundation 2010).
Footnotes
G. Monaco, T. Vervliet and H. Akl contributed equally to this work.
References
- 1.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chipuk JE, et al. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edlich F, et al. Bcl-XL retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soriano ME, Scorrano L. Traveling Bax and forth from mitochondria to control apoptosis. Cell. 2011;145:15–17. doi: 10.1016/j.cell.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao Y, Marassi FM. BAX and BAK caught in the act. Mol Cell. 2009;36:353–354. doi: 10.1016/j.molcel.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forte M, Bernardi P. Genetic dissection of the permeability transition pore. J Bioenerg Biomembr. 2005;37:121–128. doi: 10.1007/s10863-005-6565-9. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner HK, et al. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J Biol Chem. 2009;284:20796–20803. doi: 10.1074/jbc.M109.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy SS, et al. Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. Mol Cell. 2009;33:377–388. doi: 10.1016/j.molcel.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng J, et al. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Certo M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Kim H, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 15.Kuwana T, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Opferman JT, et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 17.Del Gaizo Moore V, et al. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Ming L, Yu J. BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat. 2007;10:207–217. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park CM, et al. Discovery of an orally bioavailable small molecule inhibitor of prosurvival B-cell lymphoma 2 proteins. J Med Chem. 2008;51:6902–6915. doi: 10.1021/jm800669s. [DOI] [PubMed] [Google Scholar]
- 20.Tse C, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 21.Baffy G, et al. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993;268:6511–6519. [PubMed] [Google Scholar]
- 22.Lam M, et al. Evidence that Bcl-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Nat Acad Sci USA. 1994;91:6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomenius MJ, Distelhorst CW. Bcl-2 on the endoplasmic reticulum: protecting the mitochondria from a distance. J Cell Sci. 2003;116:4493–4499. doi: 10.1242/jcs.00829. [DOI] [PubMed] [Google Scholar]
- 24.Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- 25.Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13:1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- 26.De Smedt H, Verkhratsky A, Muallem S. Ca2+ signaling mechanisms of cell survival and cell death: an introduction. Cell Calcium. 2011;50:207–210. doi: 10.1016/j.ceca.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Zhivotovsky B, Orrenius S. Calcium and cell death mechanisms: a perspective from the cell death community. Cell Calcium. 2011;50:211–221. doi: 10.1016/j.ceca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Romagnoli A, et al. Endoplasmic reticulum/mitochondria calcium cross-talk. Novartis Found Symp. 2007;287:122–131. doi: 10.1002/9780470725207.ch9. [DOI] [PubMed] [Google Scholar]
- 29.Zecchini E, et al. Mitochondrial calcium signalling: message of life and death. Ital J Biochem. 2007;56:235–242. [PubMed] [Google Scholar]
- 30.Giorgi C, et al. Ca2+ signaling, mitochondria and cell death. Curr Mol Med. 2008;8:119–130. doi: 10.2174/156652408783769571. [DOI] [PubMed] [Google Scholar]
- 31.Pinton P, et al. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giorgi C, et al. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizzuto R, et al. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verfaillie T et al (2012) PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS based ER stress. Cell Death Differ. doi:10.1038/cdd.2012.74 [DOI] [PMC free article] [PubMed]
- 35.Pinton P, et al. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardenas C, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higo T, et al. Mechanism of ER stress-induced brain damage by IP3 receptor. Neuron. 2010;68:865–878. doi: 10.1016/j.neuron.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Criollo A, et al. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 2007;14:1029–1039. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- 39.Vicencio JM, et al. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ. 2009;16:1006–1017. doi: 10.1038/cdd.2009.34. [DOI] [PubMed] [Google Scholar]
- 40.Decuypere JP, et al. IP 3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy. 2011;7:1472–1489. doi: 10.4161/auto.7.12.17909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decuypere JP, Bultynck G, Parys JB. A dual role for Ca2+ in autophagy regulation. Cell Calcium. 2011;50:242–250. doi: 10.1016/j.ceca.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 42.De Stefani D, et al. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ. 2012;19:267–273. doi: 10.1038/cdd.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Stefani D, et al. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jean-Quartier C, et al. Studying mitochondrial Ca2+ uptake: a revisit. Mol Cell Endocrinol. 2012;353:114–127. doi: 10.1016/j.mce.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sammels E, et al. Intracellular Ca2+ storage in health and disease: a dynamic equilibrium. Cell Calcium. 2010;47:297–314. doi: 10.1016/j.ceca.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Decuypere JP, et al. The IP3 receptor-mitochondria connection in apoptosis and autophagy. Biochim Biophys Acta. 2011;1813:1003–1013. doi: 10.1016/j.bbamcr.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Decuypere JP, et al. IP3 receptors, mitochondria, and Ca signaling: implications for aging. J Aging Res. 2011;2011:920178. doi: 10.4061/2011/920178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mekahli D, et al. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol. 2011;3:1–30. doi: 10.1101/cshperspect.a004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marchi S, et al. Selective modulation of subtype III IP3R by Akt regulates ER Ca2+ release and apoptosis. Cell Death Dis. 2012;3:e304. doi: 10.1038/cddis.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szado T, et al. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Nat Acad Sci USA. 2008;105:2427–2432. doi: 10.1073/pnas.0711324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carnero A. The PKB/AKT pathway in cancer. Curr Pharm Des. 2010;16:34–44. doi: 10.2174/138161210789941865. [DOI] [PubMed] [Google Scholar]
- 53.Giorgi C, et al. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science. 2010;330:1247–1251. doi: 10.1126/science.1189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones AW, Szabadkai G. Ca2+ transfer from the ER to mitochondria: channeling cell death by a tumor suppressor. Dev Cell. 2010;19:789–790. doi: 10.1016/j.devcel.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 55.Pinton P, Giorgi C, Pandolfi PP. The role of PML in the control of apoptotic cell fate: a new key player at ER-mitochondria sites. Cell Death Differ. 2011;18:1450–1456. doi: 10.1038/cdd.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rimessi A, et al. Intramitochondrial calcium regulation by the FHIT gene product sensitizes to apoptosis. Proc Nat Acad Sci USA. 2009;106:12753–12758. doi: 10.1073/pnas.0906484106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arbel N, Shoshan-Barmatz V. Voltage-dependent anion channel 1-based peptides interact with Bcl-2 to prevent antiapoptotic activity. J Biol Chem. 2010;285:6053–6062. doi: 10.1074/jbc.M109.082990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer AE, et al. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Nat Acad Sci USA. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim HR, et al. Bax Inhibitor-1 Is a pH-dependent regulator of Ca2+ channel activity in the endoplasmic reticulum. J Biol Chem. 2008;283:15946–15955. doi: 10.1074/jbc.M800075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu C, et al. BI-1 regulates endoplasmic reticulum Ca2+ homeostasis downstream of Bcl-2 family proteins. J Biol Chem. 2008;283:11477–11484. doi: 10.1074/jbc.M708385200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bultynck G, et al. The C terminus of Bax inhibitor-1 forms a Ca2+-permeable channel pore. J Biol Chem. 2012;287:2544–2557. doi: 10.1074/jbc.M111.275354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kiviluoto S, et al. Bax Inhibitor-1 is a novel IP3 receptor-interacting and-sensitizing protein. Cell Death Dis. 2012;3:e367. doi: 10.1038/cddis.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sano R, et al. Endoplasmic reticulum protein BI-1 regulates Ca2+-mediated bioenergetics to promote autophagy. Genes Dev. 2012;26:1041–1054. doi: 10.1101/gad.184325.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pinton P, et al. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scorrano L, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 66.Oakes SA, et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Nat Acad Sci USA. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He H, et al. Maintenance of calcium homeostasis in the endoplasmic reticulum by Bcl-2. J Cell Biol. 1997;138:1219–1228. doi: 10.1083/jcb.138.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erin N, Billingsley ML. Domoic acid enhances Bcl-2-calcineurin-inositol-1,4,5-trisphosphate receptor interactions and delayed neuronal death in rat brain slices. Brain Res. 2004;1014:45–52. doi: 10.1016/j.brainres.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 69.Erin N, Bronson SK, Billingsley ML. Calcium-dependent interaction of calcineurin with Bcl-2 in neuronal tissue. Neuroscience. 2003;117:541–555. doi: 10.1016/S0306-4522(02)00933-8. [DOI] [PubMed] [Google Scholar]
- 70.Xu L, et al. Suppression of IP3-mediated calcium release and apoptosis by Bcl-2 involves the participation of protein phosphatase 1. Mol Cell Biochem. 2007;295:153–165. doi: 10.1007/s11010-006-9285-5. [DOI] [PubMed] [Google Scholar]
- 71.Chen R, et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White C, et al. The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nat Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eckenrode EF, et al. Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J Biol Chem. 2010;285:13678–13684. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li C, et al. Bcl-XL affects Ca2+ homeostasis by altering expression of inositol 1,4,5-trisphosphate receptors. Proc Nat Acad Sci USA. 2002;99:9830–9835. doi: 10.1073/pnas.152571899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuo TH, et al. Modulation of endoplasmic reticulum calcium pump by Bcl-2. Oncogene. 1998;17:1903–1910. doi: 10.1038/sj.onc.1202110. [DOI] [PubMed] [Google Scholar]
- 76.Kobrinsky EM, Kirchberger MA. Evidence for a role of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase in thapsigargin and Bcl-2 induced changes in Xenopus laevis oocyte maturation. Oncogene. 2001;20:933–941. doi: 10.1038/sj.onc.1204153. [DOI] [PubMed] [Google Scholar]
- 77.Vento MT, et al. Praf2 is a novel Bcl-XL/Bcl-2 interacting protein with the ability to modulate survival of cancer cells. PLoS One. 2010;5:e15636. doi: 10.1371/journal.pone.0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dremina ES, et al. Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) Biochem J. 2004;383:361–370. doi: 10.1042/BJ20040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dremina ES, Sharov VS, Schoneich C. Displacement of SERCA from SR lipid caveolae-related domains by Bcl-2: a possible mechanism for SERCA inactivation. Biochemistry. 2006;45:175–184. doi: 10.1021/bi050800s. [DOI] [PubMed] [Google Scholar]
- 80.Dremina ES, Sharov VS, Schoneich C. Heat-shock proteins attenuate SERCA inactivation by the anti-apoptotic protein Bcl-2: possible implications for the ER Ca2+-mediated apoptosis. Biochem J. 2012;444:127–139. doi: 10.1042/BJ20111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahmad S, et al. Bcl-2 suppresses sarcoplasmic/endoplasmic reticulum Ca2+-ATPase expression in cystic fibrosis airways: role in oxidant-mediated cell death. Am J Respir Crit Care Med. 2009;179:816–826. doi: 10.1164/rccm.200807-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Broker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11:3155–3162. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- 83.Galluzzi L, et al. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sperandio S, de Belle I, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci USA. 2000;97:14376–14381. doi: 10.1073/pnas.97.26.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ladasky JJ, et al. Bap31 enhances the endoplasmic reticulum export and quality control of human class I MHC molecules. J Immunol. 2006;177:6172–6181. doi: 10.4049/jimmunol.177.9.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang B, et al. BAP31 interacts with Sec61 translocons and promotes retrotranslocation of CFTRDeltaF508 via the derlin-1 complex. Cell. 2008;133:1080–1092. doi: 10.1016/j.cell.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 87.Wang B, et al. Uncleaved BAP31 in association with A4 protein at the endoplasmic reticulum is an inhibitor of Fas-initiated release of cytochrome c from mitochondria. J Biol Chem. 2003;278:14461–14468. doi: 10.1074/jbc.M209684200. [DOI] [PubMed] [Google Scholar]
- 88.Breckenridge DG, et al. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heath-Engel HM, Wang B, Shore GC. Bcl2 at the endoplasmic reticulum protects against a Bax/Bak-independent paraptosis-like cell death pathway initiated via p20Bap31. Biochim Biophys Acta. 2012;1823:335–347. doi: 10.1016/j.bbamcr.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 90.Lur G, et al. Ribosome-free terminals of rough ER allow formation of STIM1 puncta and segregation of STIM1 from IP3 receptors. Curr Biol. 2009;19:1648–1653. doi: 10.1016/j.cub.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferdek PE, et al. A novel role for Bcl-2 in regulation of cellular calcium extrusion. Curr Biol. 2012;22:1241–1246. doi: 10.1016/j.cub.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gerasimenko J, et al. Inhibitors of Bcl-2 protein family deplete ER Ca2+ stores in pancreatic acinar cells. Pflugers Arch. 2010;460:891–900. doi: 10.1007/s00424-010-0859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhong F, et al. Bcl-2 differentially regulates Ca2+ signals according to the strength of T cell receptor activation. J Cell Biol. 2006;172:127–137. doi: 10.1083/jcb.200506189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hanson CJ, et al. Bcl-2 suppresses Ca2+ release through inositol 1,4,5-trisphosphate receptors and inhibits Ca2+ uptake by mitochondria without affecting ER calcium store content. Cell Calcium. 2008;44:324–338. doi: 10.1016/j.ceca.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 95.Rong YP, et al. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s inhibition of apoptotic calcium signals. Mol Cell. 2008;31:255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rong YP, et al. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc Nat Acad Sci USA. 2009;106:14397–14402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Distelhorst CW, Bootman MD. Bcl-2 interaction with the inositol 1,4,5-trisphosphate receptor: role in Ca(2+) signaling and disease. Cell Calcium. 2011;50:234–241. doi: 10.1016/j.ceca.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li C, et al. Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Nat Acad Sci USA. 2007;104:12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chan J, et al. Structural studies of inositol 1,4,5-trisphosphate receptor: coupling ligand binding to channel gating. J Biol Chem. 2010;285:36092–36099. doi: 10.1074/jbc.M110.140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Foskett JK, et al. Bcl-xL regulation of InsP3 receptor gating mediated by dual Ca2+ release channel BH3 domains. Biophys J. 2009;96:391a. doi: 10.1016/j.bpj.2008.12.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Monaco G, et al. Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ. 2012;19:295–309. doi: 10.1038/cdd.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rong YP, et al. Targeting Bcl-2 based on the interaction of its BH4 domain with the inositol 1,4,5-trisphosphate receptor. Biochim Biophys Acta. 2009;1793:971–978. doi: 10.1016/j.bbamcr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhong F, et al. Induction of Ca2+-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2-IP3 receptor interaction. Blood. 2011;117:2924–2934. doi: 10.1182/blood-2010-09-307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Popgeorgiev N, et al. The apoptotic regulator Nrz controls cytoskeletal dynamics via the regulation of Ca2+ trafficking in the zebrafish blastula. Dev Cell. 2011;20:663–676. doi: 10.1016/j.devcel.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 105.Solnica-Krezel L. Gastrulation in zebrafish: all just about adhesion? Curr Opin Genet Dev. 2006;16:433–441. doi: 10.1016/j.gde.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 106.Arnaud E, et al. The zebrafish bcl-2 homologue Nrz controls development during somitogenesis and gastrulation via apoptosis-dependent and -independent mechanisms. Cell Death Differ. 2006;13:1128–1137. doi: 10.1038/sj.cdd.4401797. [DOI] [PubMed] [Google Scholar]
- 107.Bonneau B, et al. Cytoskeleton dynamics in early zebrafish development: a matter of phosphorylation? BioArchitecture. 2011;1:1–5. doi: 10.4161/bioa.18116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Allen DG, et al. Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol. 2010;88:83–91. doi: 10.1139/Y09-058. [DOI] [PubMed] [Google Scholar]
- 109.Basset O, et al. Bcl-2 overexpression prevents calcium overload and subsequent apoptosis in dystrophic myotubes. Biochem J. 2006;395:267–276. doi: 10.1042/BJ20051265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Velmurugan GV, White C. Calcium homeostasis in vascular smooth muscle cells is altered in type 2 diabetes by Bcl-2 protein modulation of InsP3R calcium release channels. Am J Physiol Heart Circ Physiol. 2011;302(1):H124–H134. doi: 10.1152/ajpheart.00218.2011. [DOI] [PubMed] [Google Scholar]
- 111.Machado-Vieira R, et al. The Bcl-2 gene polymorphism rs956572AA increases inositol 1,4,5-trisphosphate receptor-mediated endoplasmic reticulum calcium release in subjects with bipolar disorder. Biol Psychiatry. 2011;69:344–352. doi: 10.1016/j.biopsych.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Uemura T, et al. Bcl-2 SNP rs956572 associates with disrupted intracellular calcium homeostasis in bipolar I disorder. Bipolar Disord. 2011;13:41–51. doi: 10.1111/j.1399-5618.2011.00897.x. [DOI] [PubMed] [Google Scholar]
- 113.Salvadore G, et al. Bcl-2 polymorphism influences gray matter volume in the ventral striatum in healthy humans. Biol Psychiatry. 2009;66:804–807. doi: 10.1016/j.biopsych.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 115.Lock R, et al. Initial testing (stage 1) of the BH3 mimetic ABT-263 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:1181–1189. doi: 10.1002/pbc.21433. [DOI] [PubMed] [Google Scholar]
- 116.Vogler M, et al. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2009;16:360–367. doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- 117.Azmi AS, Mohammad RM. Non-peptidic small molecule inhibitors against Bcl-2 for cancer therapy. J Cell Physiol. 2009;218:13–21. doi: 10.1002/jcp.21567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.High LM, et al. The Bcl-2 homology domain 3 mimetic ABT-737 targets the apoptotic machinery in acute lymphoblastic leukemia resulting in synergistic in vitro and in vivo interactions with established drugs. Mol Pharmacol. 2010;77:483–494. doi: 10.1124/mol.109.060780. [DOI] [PubMed] [Google Scholar]
- 119.Ackler S, et al. The Bcl-2 inhibitor ABT-263 enhances the response of multiple chemotherapeutic regimens in hematologic tumors in vivo. Cancer Chemother Pharmacol. 2010;66:869–880. doi: 10.1007/s00280-009-1232-1. [DOI] [PubMed] [Google Scholar]
- 120.Vogler M, et al. BCL2/BCL-XL inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood. 2011;117:7145–7154. doi: 10.1182/blood-2011-03-344812. [DOI] [PubMed] [Google Scholar]
- 121.Schoenwaelder SM, et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood. 2011;118:1663–1674. doi: 10.1182/blood-2011-04-347849. [DOI] [PubMed] [Google Scholar]
- 122.Mason KD, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 123.Eno CO, et al. Distinct roles of mitochondria- and ER-localized Bcl-xL in apoptosis resistance and Ca2+ homeostasis. Mol Biol Cell. 2012;23:2605–2618. doi: 10.1091/mbc.E12-02-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haughn L, et al. BCL-2 and BCL-XL restrict lineage choice during hematopoietic differentiation. J Biol Chem. 2003;278:25158–25165. doi: 10.1074/jbc.M212849200. [DOI] [PubMed] [Google Scholar]
- 125.Katz C, et al. Molecular basis of the interaction between the antiapoptotic Bcl-2 family proteins and the proapoptotic protein ASPP2. Proc Natl Acad Sci USA. 2008;105:12277–12282. doi: 10.1073/pnas.0711269105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shimizu S, et al. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc Natl Acad Sci USA. 2000;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shoshan-Barmatz V, et al. The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death. Curr Pharm Des. 2006;12:2249–2270. doi: 10.2174/138161206777585111. [DOI] [PubMed] [Google Scholar]
- 128.Shoshan-Barmatz V, et al. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Aspects Med. 2010;31:227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 129.Zaid H, et al. The voltage-dependent anion channel-1 modulates apoptotic cell death. Cell Death Differ. 2005;12:751–760. doi: 10.1038/sj.cdd.4401599. [DOI] [PubMed] [Google Scholar]
- 130.Abu-Hamad S, Sivan S, Shoshan-Barmatz V. The expression level of the voltage-dependent anion channel controls life and death of the cell. Proc Natl Acad Sci USA. 2006;103:5787–5792. doi: 10.1073/pnas.0600103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tornero D, Posadas I, Cena V. Bcl-x(L) blocks a mitochondrial inner membrane channel and prevents Ca2+ overload-mediated cell death. PLoS One. 2011;6:e20423. doi: 10.1371/journal.pone.0020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Keinan N, Tyomkin D, Shoshan-Barmatz V. Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis. Mol Cell Biol. 2010;30:5698–5709. doi: 10.1128/MCB.00165-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abu-Hamad S, et al. Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: mapping the site of binding. J Biol Chem. 2008;283:13482–13490. doi: 10.1074/jbc.M708216200. [DOI] [PubMed] [Google Scholar]
- 134.Abu-Hamad S, et al. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J Cell Sci. 2009;122:1906–1916. doi: 10.1242/jcs.040188. [DOI] [PubMed] [Google Scholar]
- 135.Geula S, Ben-Hail D, Shoshan-Barmatz V. Structure-based analysis of VDAC1: n-terminus location, translocation, channel gating and association with anti-apoptotic proteins. Biochem J. 2012;444:475–485. doi: 10.1042/BJ20112079. [DOI] [PubMed] [Google Scholar]
- 136.Malia TJ, Wagner G. NMR structural investigation of the mitochondrial outer membrane protein VDAC and its interaction with antiapoptotic Bcl-xL. Biochemistry. 2007;46:514–525. doi: 10.1021/bi061577h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu Q, Reed JC. Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol Cell. 1998;1:337–346. doi: 10.1016/S1097-2765(00)80034-9. [DOI] [PubMed] [Google Scholar]
- 138.Chae HJ, et al. BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol Cell. 2004;15:355–366. doi: 10.1016/j.molcel.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 139.Ahn T, et al. Cardiolipin, phosphatidylserine, and BH4 domain of Bcl-2 family regulate Ca2+/H+ antiporter activity of human Bax inhibitor-1. Cell Calcium. 2010;47:387–396. doi: 10.1016/j.ceca.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 140.Westphalen BC, et al. BI-1 protects cells from oxygen glucose deprivation by reducing the calcium content of the endoplasmic reticulum. Cell Death Differ. 2005;12:304–306. doi: 10.1038/sj.cdd.4401547. [DOI] [PubMed] [Google Scholar]
- 141.Henke N, et al. The ancient cell death suppressor BAX inhibitor-1. Cell Calcium. 2011;50:251–260. doi: 10.1016/j.ceca.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 142.de Mattia F, et al. Human Golgi antiapoptotic protein modulates intracellular calcium fluxes. Mol Biol Cell. 2009;20:3638–3645. doi: 10.1091/mbc.E09-05-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rojas-Rivera D, et al. TMBIM3/GRINA is a novel unfolded protein response (UPR) target gene that controls apoptosis through the modulation of ER calcium homeostasis. Cell Death Differ. 2012;19:1013–1016. doi: 10.1038/cdd.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sullivan A, Lu X. ASPP: a new family of oncogenes and tumour suppressor genes. Br J Cancer. 2007;96:196–200. doi: 10.1038/sj.bjc.6603525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vives V, Slee EA, Lu X. ASPP2: a gene that controls life and death in vivo. Cell Cycle. 2006;5:2187–2190. doi: 10.4161/cc.5.19.3266. [DOI] [PubMed] [Google Scholar]
- 146.Benyamini H, Friedler A. The ASPP interaction network: electrostatic differentiation between pro- and anti-apoptotic proteins. J Mol Recognit. 2011;24:266–274. doi: 10.1002/jmr.1048. [DOI] [PubMed] [Google Scholar]
- 147.Kampa KM, Bonin M, Lopez CD. New insights into the expanding complexity of the tumor suppressor ASPP2. Cell Cycle. 2009;8:2871–2876. doi: 10.4161/cc.8.18.9474. [DOI] [PubMed] [Google Scholar]
- 148.Ahn J, et al. Insight into the structural basis of pro- and antiapoptotic p53 modulation by ASPP proteins. J Biol Chem. 2009;284:13812–13822. doi: 10.1074/jbc.M808821200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Benyamini H, et al. A model for the interaction between NF-kappa-B and ASPP2 suggests an I-kappa-B-like binding mechanism. Proteins. 2009;77:602–611. doi: 10.1002/prot.22473. [DOI] [PubMed] [Google Scholar]
- 150.Rotem S, et al. The structure and interactions of the proline-rich domain of ASPP2. J Biol Chem. 2008;283:18990–18999. doi: 10.1074/jbc.M708717200. [DOI] [PubMed] [Google Scholar]
- 151.Rotem S, Katz C, Friedler A. Insights into the structure and protein-protein interactions of the pro-apoptotic protein ASPP2. Biochem Soc Trans. 2007;35:966–969. doi: 10.1042/BST0350966. [DOI] [PubMed] [Google Scholar]
- 152.Díez J, Walter D, Muñoz-Pinedo C, Gabaldón T. DeathBase: a database on structure, evolution and function of proteins involved in apoptosis and other forms of cell death. Cell Death Differ. 2010;17:735–736. doi: 10.1038/cdd.2009.215. [DOI] [PubMed] [Google Scholar]