Abstract
Bacteria use chemotaxis signaling pathways to sense environmental changes. Escherichia coli chemotaxis system represents an ideal model that illustrates fundamental principles of biological signaling processes. Chemoreceptors are crucial signaling proteins that mediate taxis toward a wide range of chemoeffectors. Recently, in deep study of the biochemical and structural features of chemoreceptors, the organization of higher-order clusters in native cells, and the signal transduction mechanisms related to the on–off signal output provides us with general insights to understand how chemotaxis performs high sensitivity, precise adaptation, signal amplification, and wide dynamic range. Along with the increasing knowledge, bacterial chemoreceptors can be engineered to sense novel chemoeffectors, which has extensive applications in therapeutics and industry. Here we mainly review recent advances in the E. coli chemotaxis system involving structure and organization of chemoreceptors, discovery, design, and characterization of chemoeffectors, and signal recognition and transduction mechanisms. Possible strategies for changing the specificity of bacterial chemoreceptors to sense novel chemoeffectors are also discussed.
Keywords: Chemotactic response, Receptor, Ligand, Molecular mechanism, Receptor design
Introduction
In the constantly changing environment, motile bacteria have the ability to perform chemotaxis toward a wide spectrum of environmental stimuli, which is mediated by a central two-component system composed of a histidine kinase and a response regulator and enhanced by additional signaling components (see reviews [1–6]). Chemoreceptors are functional signaling proteins located at the input end of the signaling pathway. They are responsible for detecting specific chemoeffectors with high specificity and transducing chemotactic signals to the downstream proteins [4, 5]. The adaptation process based on reversible receptor methylations is of crucial importance for survival. It allows bacteria to detect chemoeffector gradients with high sensitivity over a wide range of concentrations [1].
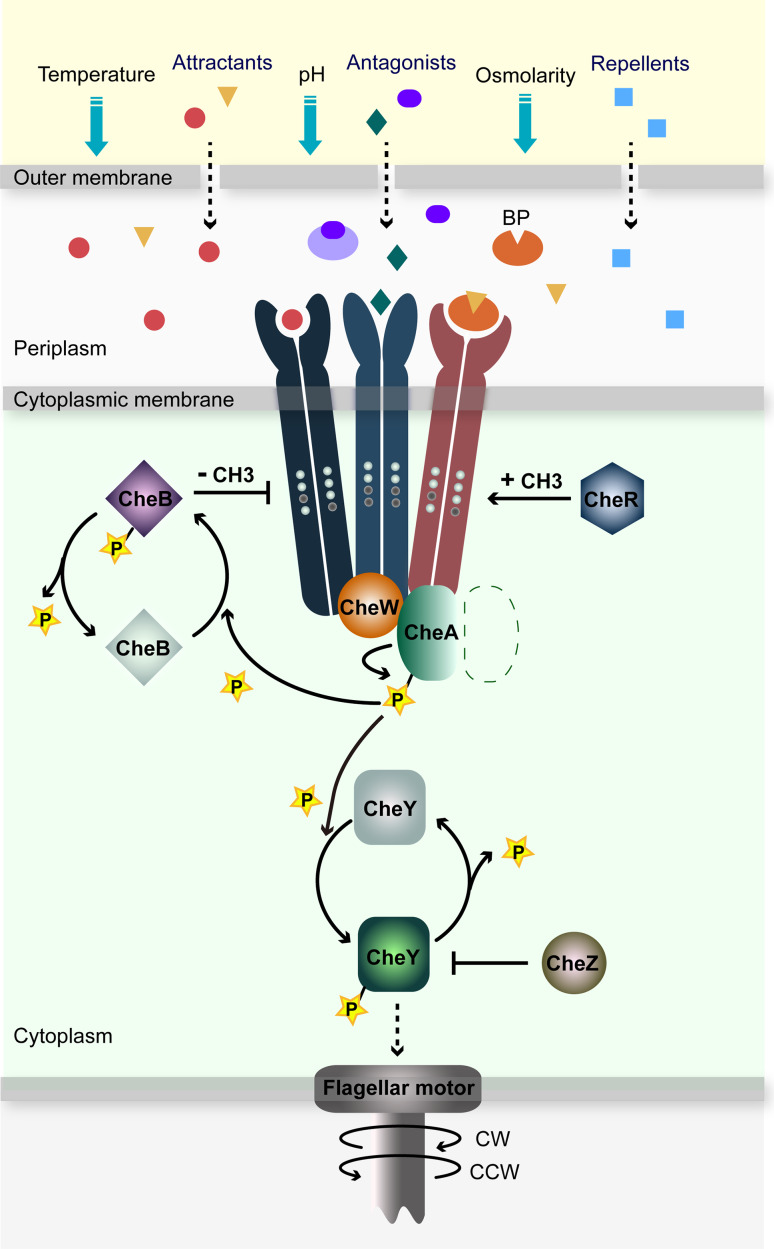
The Escherichia coli (E. coli) chemotaxis system is simple yet highly organized and evolutionarily optimized (Fig. 1). There are five kinds of chemoreceptors with different ligand specificities [4, 5]. Receptor Tsr (taxis to serine and repellents), Tar (taxis to aspartate and repellents), Tap (taxis to dipeptides) and Trg (taxis to ribose and galactose) are conventional transmembrane receptors with extracellular periplasmic domains that sense chemoeffectors directly or indirectly with the help of periplasmic substrate-binding proteins (BPs) [5]. The fifth chemoreceptor Aer (aerotaxis) does not have a periplasmic domain but an N-terminal Per-Arnt-Sim (PAS) domain, which binds with a flavin adenine dinucleotide to sense redox changes [7]. Three receptor homodimers form a heterotrimer and interact with the adaptor protein CheW and the histidine kinase CheA to assemble clusters of ternary signaling complexes [4, 5]. Autophosphorylation activity of CheA is modulated by sensing chemoeffectors. Repellent binding or attractant removing activates the autophosphorylation activity of CheA by inducing conformational changes of chemoreceptors and ternary signaling complexes. Its phosphate group is subsequently transferred to the response regulator CheY. The phosphorylated CheY (CheY-P) diffuses to the flagella motor. The phosphatase CheZ can rapidly dephosphorylate CheY-P. The interaction of CheY-P with flagellar motor induces clockwise (CW) rotation of one or several flagella and destabilizes the flagella bundle, causing a tumble movement of cells. Cell bodies are reoriented randomly. In contrast, attractant binding inhibits autophosphorylation activity of CheA. The inhibition of CheA reduces the level of CheY-P and induces counter-clockwise (CCW) rotation of the flagella, which forms a propelling flagella bundle and promotes run of cells [1].
Fig. 1.
The chemotaxis transduction network in E. coli [1, 5]. Several types of chemoreceptors (in different colors and shapes) specifically sense different kinds of chemoeffectors in the environment, including attractants (red spots or yellow triangles), repellents (blue squares), antagonists (green diamonds or purple ovals) and other environmental effectors (blue arrows). One trimer of receptor dimers, one CheW protein, and one CheA monomer (the other CheA monomer is shown as a dashed line) are shown in the figure. Receptor methylation sites are shown as white (unmethylated) or black (methylated) circles. The phosphoryl groups are shown as yellow stars
Adaptation process occurs following the initial responses to the ligand concentration changes. In E. coli, the methylation and demethylation of chemoreceptors on several specific glutamate residues are mediated by the methyltransferase CheR and the methylesterase CheB, respectively. The kinetic rate of CheR and CheB is slower than the time-scale of binding with a chemoeffector [8]. This lag provides a short-term memory for cells to compare the current ligand concentration (reflected by ligand occupancy) with that previously encountered (reflected by receptor methylation level) [5]. The adaptation regulates kinase activity by resetting it to a steady-state level. The activated CheA can phosphorylate CheB to CheB-P. CheB-P interacts with receptors and generates increased demethylation at the methylation sites. This demethylation-on conformation in turn modulates CheA activity to the baseline level. In contrast, the inactivated CheA inhibits CheB activity and induces an increase in methylation by CheR. This demethylation-off conformation in turn increases CheA activity to the baseline level [1, 4, 5]. The precise and robust adaptation is mediated by the feedback mechanism that couples kinase activity and receptor methylation [1, 5]. The first is provided by the substrate specificity of CheR and CheB. The conformations of the inactive or active receptors modulate the recognition of CheR or CheB, respectively. Thereby receptor adaptation can be controlled by its own activity/conformation [9]. The second feedback is provided by the CheA mediated CheB phosphorylation. The CheA activity in turn modulates receptor adaptation [1].
In this review we focus on the extensively characterized E. coli chemotaxis system and summarize the recent progress in understanding E. coli chemoreceptors and chemoeffectors. We divide the review into four sections: (1) In the first section we will summarize recent studies involving receptor structures and higher-order interactions of signaling complexes; (2) Then we will introduce recent advances in studying chemoeffectors, including attractants, repellents, antagonists, and environmental pH; (3) In the third section, the developments in understanding the mechanisms of chemotactic signaling in receptor homodimers and in higher-order of clusters will be summarized; (4) Finally, based on the increasing knowledge about the receptor structures, functions, and signal transduction mechanisms, the strategies for changing the specificity of bacterial chemoreceptors to sense novel chemoeffectors will be discussed.
Structure and organization of bacterial chemoreceptors
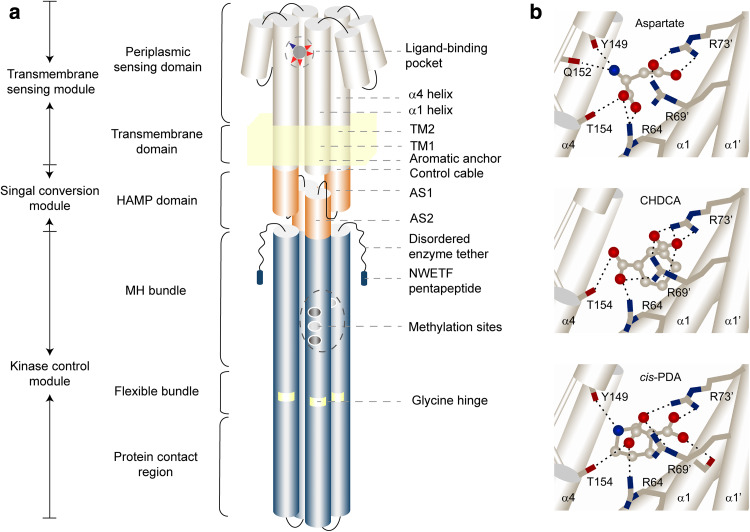
A conventional receptor homodimer contains a periplasmic sensing domain, a transmembrane helical region, a HAMP domain, a methylation helix (MH) bundle, a flexible bundle, and a protein contact region [4, 5] (Fig. 2a). According to the signaling mechanisms and functions, these elements can be divided into a transmembrane sensing module, a signal conversion module, and a kinase control module [5]. The transmembrane sensing module is composed of the periplasmic sensing domain and the transmembrane region; the signal conversion module is composed of the HAMP domain; and the kinase control module is composed of the MH bundle, the flexible bundle, and the protein contact region [5] (Fig. 2a). Structural information of receptor homodimers and higher-order clusters is necessary to discover the molecular mechanisms for chemoeffector sensing and signaling.
Fig. 2.
Illustration of a conventional chemoreceptor homodimer and the mechanism of chemoeffector binding and signaling. a Structural features of a conventional chemoreceptor homodimer in E. coli. The specific features and motifs are noted to the sides of the dimer. b The predicted interactions of Tar periplasmic sensing domain with attractant l-aspartate, cis-PDA, and antagonist CHDCA. The helices of receptor periplasmic sensing domains are shown as white cylinders. The binding molecules are shown as balls and sticks and the key interaction residues are shown as sticks. Oxygen atoms are shown in red and nitrogen atoms are shown in blue. Hydrogen bonds are indicated by black dashed lines. The interactions between the N–H group of attractants with the main-chain carbonyls of Tyr149 (Y149) and/or Gln152 (Q152) are crucial for eliciting chemotactic signals, which contribute to binding and signaling. The interactions of carboxyl groups of molecules with Arg64 (R64), Arg69′ (R69′) and Arg73′ (R73′) mainly contribute to binding [40]. a Drawn based on Refs. [5, 30], b drawn based on Ref. [40]
Structure of the chemoreceptor homodimer
Escherichia coli conventional chemoreceptors are extended helical bundles and coiled coils (Fig. 2a). The folds of chemoreceptor periplasmic sensing domains from different species are diverse. They can be divided into different types according to the structure features, including TarH, CHASE, Cache, GAF, or NIT [10, 11]. X-ray crystallography studies showed that the periplasmic domains of E. coli chemoreceptors are organized as a dimer of symmetric TarH four-helix bundles (α1–α4 and α1′–α4′) [12–15] (Fig. 2b). The α1 (α1′) and α4 (α4′) helices span the membrane to form transmembrane helices. The second transmembrane helix (TM2) typically has one or more aromatic residues near the cytoplasmic end [16–20]. They might interact with the lipid–water interface and regulate transmembrane signaling [16–20]. A control cable composed of several residues is a junction of the TM2 and the AS1 helix of the HAMP domain [21, 22]. It is an extension of TM2 and has a helix character [21–24]. There are one or more basic residues located in the control cable [22]. They probably interact with negatively charged lipid head groups of membrane and function as stop signals during the nascent receptor inserting into the membrane [22]. The X-ray structure of modified transmembrane helices and the HAMP domain of the receptor Af1503 from Archaeoglobus fulgidus (A. fulgidus) provides the first view of the connection between the transmembrane region and the HAMP domain [25].
The structures of HAMP domains from different bacterial systems have been determined by nuclear magnetic resonance (NMR) and X-ray crystallography. The first HAMP domain structure is from the chemoreceptor Af1503 determined by NMR [26]. The HAMP monomer contains two amphipathic α-helix AS1 and AS2 joined by a non-helical connector. The homodimer shows a four-helical, parallel coiled coil. The AS1 and AS2 helices display a seven-residue pattern, with repeat residues labeled a–g (heptad repeat). The Af1503 HAMP domain shows a complementary x-da packing rather than a canonical knobs-into-holes packing [26]. The cross-linking studies and other characterized structures of native or mutant HAMP domains suggested similar overall structures but with some different helical arrangement [27–31]. For example, in the X-ray structures of three concatenated HAMP domains from the Pseudomonas aeruginosa (P. aeruginosa) receptor Aer2, the HAMP1 and HAMP3 are similar to the Af1503 structure, whereas HAMP2 has a different conformation in which the AS2 helices form an approximate two-helix coiled coil rather than a four-helix bundle [27, 29].
X-ray structures of part or full of the kinase control modules from E. coli Tsr, Thermotoga maritima (T. maritima) receptor TM1143 and TM14 have been determined [32–35]. These investigations showed that the kinase control modules are anti-parallel four-helix bundles [32–35]. A recent X-ray structure of a chimeric protein containing the Af1503 HAMP domain and the Tsr kinase control module showed that its core packing geometry alternates between canonical knobs-into-holes and complementary x-da [35]. The MH bundle has a complementary x-da packing geometry. A packing transition from x-da to knobs-into-holes occurs at the upstream of the glycine hinge in the flexible bundle, which is crucial in modulating kinase activity [36]. The following part of the flexible bundle and the protein contact region alternate to the complementary x-da packing geometry [35]. This packing transition is also consistent with the structures of receptor TM1143 and TM14 [33, 34]. A C-terminal NWETF pentapeptide associates CheR and CheB in E. coli Tar and Tsr [37, 38]. The carboxyl-terminal linker between the receptor body and this pentapeptide is an unstructured segment that serves as a disordered enzyme tether, which was indicated by the site-directed spin labeling and electron paramagnetic resonance spectroscopy studies [39].
Organization of the signaling complexes and higher-order clusters
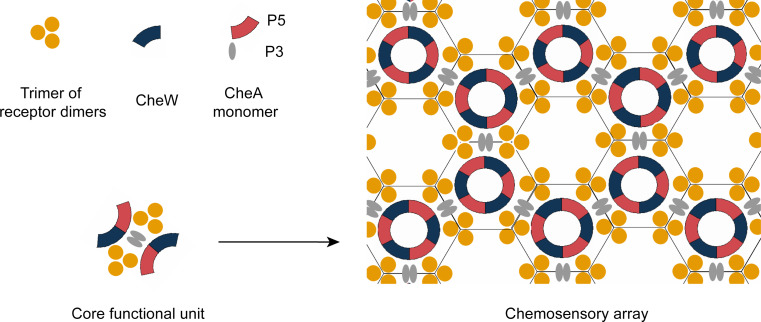
The ternary signaling complexes represent the basic sensory elements required for kinase regulation [41–44]. These ternary signaling complexes form clusters, which enhance CheA activity by about one hundred fold, making the chemotaxis sensory systems exhibit high sensitivity and wide dynamic range [4, 41, 42]. Chemoreceptors activate CheA and control its activity as a function of adaptational modification and ligand occupancy [41, 42, 45]. Hazelbauer and coworkers [42] used nanodiscs and showed that the interaction of CheA with one trimer of receptor dimers can fully activate kinase activity. The organization of the core functional unit of signaling complexes involves two trimers of receptor dimers interacting with two CheW proteins and one CheA dimer [41] (Fig. 3).
Fig. 3.
Illustration of the assembly of a chemosensory array. Only P3 and P5 domains of CheA are shown in the figure. The core functional unit of signaling complexes involves two trimers of receptor dimers interacting with two CheW proteins and one CheA dimer [41]. The CheA P5 domains and CheW form rings to link trimers of receptor dimers into hexagons. The CheA P3 domains link hexagons to form extended sensory arrays [44, 46]. This figure is drawn based on Refs. [44, 46, 47]
Recent electron-cryotomography and X-ray crystallography studies provided a global view of the native cluster architecture [44, 46–49]. The individual receptor dimers in a trimer unit could be visualized by electron-cryotomography. Combining with the structure information, models of array assembly have been suggested: The trimers of receptor dimers are linked in extended hexagonal lattices. The distance between the centers of adjacent hexagons is about 12 nm. CheA regulatory domains (P5) and CheW form rings linking trimers of receptor dimers into hexagons via multiple hydrophobic interactions. The CheA dimerization domains (P3) link neighboring hexagons to form extended and stable arrays [44, 46, 47] (Fig. 3). The interaction details of chemoreceptors, CheA and CheW have been studied to reveal the arrangement of the ternary complexes [50–58]. Recently, Underbakke et al. [51] identified the surfaces for the CheW–CheA and CheW–Tsr interactions in native E. coli membranes by using isotope-coded affinity tag (ICAT) footprinting, and proposed the CheA and Tsr binding sites on the CheW protein. Wang et al. [57] characterized the CheA–receptor interaction in T. maritima by NMR spectroscopy and validated the identified CheA-receptor binding sites in E. coli system. Li et al. [58] determined the X-ray structure of a receptor–CheA–CheW signaling complex in T. maritima. Their supposed interaction model was supported by Piasta et al., who used disulfide mapping and TAM-IDS (tryptophan and alanine mutation to identify docking sites) to test the receptor–CheA interaction interface in E. coli [56, 58]. The coordination of gene expression may be important for fully ordered complex arrays. Co-overexpression of chemoreceptors, CheA and CheW leads to a lower and less ordered CheA occupancy [44]. In addition, excess of CheW disrupts the receptor trimer assembly, possibly because CheW-receptor binding sites overlap with the receptor dimer–dimer interaction sites in a trimer unit [50]. So far, there is still much to learn about how chemoreceptors, CheW and CheA interact with each other, and how complex arrays are well organized. We hope that along with the fast development of the electron cryotomography, X-ray crystallography and other in vivo and in vitro approaches, the structural nature and interaction details of signaling complexes will be well characterized. This information will help us to fully understand the biophysical and biochemical properties of cluster arrays and the relationship between structures and signaling states.
Ligand spectra of bacterial chemoreceptors
Chemoreceptors from different bacteria can sense different chemoeffectors with high specificity. Generally, the chemoeffectors can be classified into two types. Type I chemoeffectors are chemical compounds or salts, such as amino acids, dipeptides, sugars, tricarboxylic acid cycle intermediates, aromatic molecules, metal ions or inorganic phosphate [2, 59–67], which can be classified into attractants, repellents and antagonist molecules. Type II chemoeffectors are other chemical effectors, for example pH [68]. Besides chemoeffectors, environmental stimuli such as temperature, osmotic pressure, redox, and oxygen change can also be sensed by chemoreceptors [7, 68–70]. In this review, we mainly focus on the chemoeffector spectra of E. coli chemotaxis system.
Type I chemoeffectors: attractants, repellents, and antagonists
Attractants
Escherichia coli exhibits different chemotactic behavior towards a wide range of chemoeffectors. The natural attractants are primarily nutrients, such as amino acids, sugars and dipeptides, which have influence on cell growth and survival. Tar and Tsr sense amino acids directly [12, 14, 71, 72]. Aspartate and serine are the most effective attractants, which induce E. coli chemotaxis at a concentration of nanomolar range [60, 73, 74]. Tar and Tsr also sense some other amino acids as weak attractants, but with different specificity and chemotactic strength [60, 74]. Sugars and dipeptides are indirectly binding attractants that are sensed through BPs. The ligand-bound BPs interact with specific chemoreceptors and induce chemotactic signals. Trg senses sugars such as d-ribose, d-glucose, and d-galactose through the periplasmic glucose/galactose binding protein [61, 75]. Tar senses maltose with the help of the periplasmic maltose binding protein (MBP) [76, 77]. Tap senses dipeptides such as Pro-Leu via the dipeptide binding protein [62]. The response sensitivity for the indirectly binding chemoeffectors is affected by the expression level of chemoreceptors and the corresponding BPs, which provide an additional flexibility in regulation [72]. The greater flexibility of the indirectly binding mode allows cells to well control the sensitivity to the specific ligands. However, the dynamic range for sensing indirectly binding chemoeffectors is narrower compared to the directly binding ones due to the limited occupancy of BPs [72].
Besides amino acids, sugars, and dipeptides, chemoreceptors also sense other physiological signals. Tsr has an attractant response to the interspecies quorum signal AI-2, which may be mediated by the periplasmic LsrB AI-2-binding protein [78]. Tsr also senses 3,4-dihydroxymandelic acid generated from norepinephrine as an attractant [79]. Tap senses pyrimidine thymine and uracil as attractants, but the mechanism is not clear. It has been shown that the response to pyrimidines is not through the periplasmic dipeptide binding protein [80]. These attractants may be mediated by directly binding with some receptor elements or other kinds of BPs.
In addition to the natural attractants, some non-natural attractants were discovered, designed, and synthesized for E. coli. These non-natural chemoeffectors might have potential applications in biotechnology and bioengineering. The well-known non-natural attractants are the analogues of natural attractants, such as α-methyl-aspartate and α-aminoisobutyrate, which are the analogues of aspartate and serine, respectively [73, 74]. We have used a novel strategy to search for new chemoeffectors for E. coli chemoreceptors by screening a large compound library using integrated virtual screening, isothermal titration calorimetry (ITC), microfluidics, and fluorescence resonance energy transfer (FRET) approaches. We discovered several novel attractants for E. coli chemoreceptor Tar, such as (±)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, guanidinosuccinic acid, and formimino-l-aspartate. These novel attractants bind directly to the ligand-binding pocket of Tar [40]. Besides screening potent ligands for the receptor ligand-binding pocket, attractants can also be designed targeting chemoreceptor clusters. Kiessling and coworkers [81–83] designed multivalent galactose derivatives and showed that these derivatives are potent ligands for the periplasmic galactose binding protein and trigger increased attractant responses via Trg. These multivalent ligands with extending valency are stronger attractants than galactose and corresponding monovalent ligands [81–83]. Borrok et al. [84] designed multivalent ligands containing multiple copies of leucine and found that these ligands function as potent attractants.
In addition to the attractants that target the ligand-binding pocket of receptor periplasmic domain or periplasmic binding proteins, attractants that target other receptor elements were also discovered. Pham and Parkinson suggested Tar sensing phenol as an attractant while Tsr sensing phenol as a repellent [85, 86], which is possibly because phenol diffuses into the membrane and perturbs the stability or position of the transmembrane helices and the segment joining TM2 to HAMP, thereby producing a conformational change in the HAMP domain that mimics the binding of an attractant in the periplasm [86]. For some aromatic chemoeffectors both function on Tar and Tsr, such as phenol and indole which are repellents for Tsr while attractants for Tar, they might target the common receptor elements and have similar mechanisms [86].
Repellents
A number of repellents for bacteria were also discovered. Tso and Adler [65] found and classified seven classes of repellent molecules for E. coli, including fatty acids and analogues, aliphatic alcohols, amino acids and analogues, indole and analogues, aromatic compounds, inorganic ions and mercaptans. Most of the repellents are harmful compounds for cells, but not all of them are. Some repellents are specific for particular chemoreceptors. For instance, metal ions such as cobalt (Co2+) and nickel (Ni2+) ions are specific for Tar. Chemoreceptor Tsr specifically senses some repellents such as leucine, tryptophan, valine, phenylalanine, indole, and glycerol [60, 65, 87].
Repellent sensing is generally less understood compared to attractant sensing in bacterial chemotaxis. Possible receptor elements for sensing repellents are not clear. For the Ni2+ recognition, it was proposed that the periplasmic binding protein NikA binds Ni2+ and triggers repellent response via interacting with Tar [88]. However, recent study showed that repellent taxis to Ni2+ does not require the periplasmic Ni2+-binding protein NikA and suggested that Ni2+ might bind directly to Tar [89]. Another possibility for repellent sensing is through their effect on membrane fluidity. However, studies showed that for many repellents, the sensing is not mediated by their effect on the membrane fluidity [90].
Discovering how chemoeffectors interact with receptors is of great importance. Different chemoeffectors might bind with different receptor elements. The interaction details will help us to understand how chemotactic signals initiate in different elements and the relationship with the signal transduction. Different chemoeffectors might be mediated by different kinds of BPs. Discovery of these BPs will provide us with extensive insights into the chemotaxis networks. To further investigate the mechanisms of chemoeffector sensing, the receptor element that is responsible for effector sensing should be defined first. One might use a hybrid receptor of the target receptor and another receptor to discover the possible functional elements. Then random mutagenesis or site-specific mutagenesis relied on structure-based prediction can be performed to identify the key residues that are directly involved in the chemoeffector interaction. For the chemoeffectors that might be sensed through BPs, knocking out the related BPs might be helpful to determine whether these ligands are sensed indirectly.
Antagonists
Besides attractant and repellent, antagonist is another class of chemoeffector. They can compete with attractant binding, thereby inhibit chemotaxis response. Recently, antagonist molecules targeting the ligand-binding pockets of the receptors or BPs were reported [40, 91]. We discovered that cis-1,2-cyclohexane-dicarboxylic acid (CHDCA) is an antagonist for E. coli chemoreceptor Tar that directly binds to Tar ligand-binding pocket and competes with attractant binding without generating chemotaxis response [40]. Borrok et al. [91] designed an antagonist for the periplasmic glucose/galactose binding protein, which binds with the BP to lock it on the open state, so that inhibits cell glucose chemotaxis through the receptor Trg. As inhibiting chemotaxis of pathogenic bacteria is a potent therapeutic strategy to prevent disease [92, 93], designing antagonists of chemoreceptors that inhibit chemotaxis provides a novel therapeutic solution to prevent pathogenic bacteria [40].
Type II chemoeffectors: other chemical effectors
The unidirectional movement of bacteria chemotaxis, from low to high attractant concentrations or from high to low repellent concentrations, is of great physiologically significance in the nutrient or toxin gradients. However, in gradients of some other effectors, the physiological preferences of bacteria may correspond to an intermediate strength rather than to the highest or lowest level in the gradients [68, 94]. This bidirectional taxis benefits cells to modulate their environmental preferences and find optimum conditions.
The pH is a chemical effector in the environment. E. coli has an ability to use pH taxis to migrate toward a neutral pH condition from either extremely acidic or alkaline environments [65, 95]. Tsr and Tar are involved in pH taxis [96, 97]. Tar has an attractant response to a decreased pH and a repellent response to an increased pH, while Tsr has opposite responses [96–98]. Yang and Sourjik [68] suggested that in a pH gradient, cells use a ‘push–pull’ mechanism to regulate bidirectional pH taxis, which is achieved by tuning of the relative response strength of Tar and Tsr at the ambient external pH that is inversely correlated with the receptor methylation level. At basic pH, Tar is less methylated while Tsr has an increased methylation. The Tsr-mediated response weakens while the Tar-mediated response becomes stronger and dominated, making cells swim towards lower pH [68, 98]. At acidic pH, Tsr is less methylated, more sensitive to pH changes and dominates the response, making cells swim towards higher pH and accumulate at the preferred pH [68]. Tu and coworkers [95] developed an Ising-type model for a mixed cluster of receptors with opposing functions based on the ‘push–pull’ mechanism. Their studies provide insights for further understanding the bacterial pH sensing mechanisms.
Tar and Tsr can not only sense external (periplasmic) pH but also internal (cytoplasmic) pH. The external pH changes are primarily sensed by receptor periplasmic domains [68, 96]. However, the exact residues that influence pH sensing are still not known. The internal pH changes, such as a decreased cytoplasmic pH caused by addition of membrane permeable weak acids [97], are sensed by the cytoplasmic domain of the receptors. Several residues at the end of the HAMP domain are involved in this process [96, 99].
Although chemoeffectors for E. coli chemoreceptors are well known, many chemoreceptors from other bacterial species are of unknown function. Identifying the chemoeffectors for these chemoreceptors is of great physiological significance. Recently, Lacal et al. [64] discovered that some tricarboxylic acid (TCA) cycle intermediates are chemoeffectors for the chemoreceptor McpS from Pseudomonas putida (P. putida) using in vitro ITC approach and in vivo agarose-plug assays and capillary assays. Similar studies were performed for the chemoreceptor McpU from Sinorhizobium meliloti. Webb et al. [100] showed that McpU mediates chemotaxis toward host plants by direct proline sensing. Analogous approaches should be useful in determining the chemoeffectors of those chemoreceptors with unknown function.
Mechanisms of chemotactic signaling in chemoreceptors and signaling complexes
Chemoeffectors that bind directly or indirectly to the chemoreceptors trigger conformational changes in the three functional modules of chemoreceptors and control the kinase activity. So far, a series of structural, biochemical, and genetic studies have been carried out to investigate the nature of chemotactic signaling.
Signal transduction in the transmembrane sensing module
Much effort has been made to elucidate the molecular features of chemoeffector–chemoreceptor recognition and transmembrane signaling in the transmembrane sensing modules. The binding modes of chemoeffectors have been studied in detail for E. coli chemoreceptors, which provide molecular basis for understanding the signal relay process. Key residues involved in the interactions between Tsr with serine and Tar with aspartate have been well characterized [12–15, 71]. In the ligand-binding pocket of Tar, residue Arg64 from one monomer and Arg69′, Arg73′ from the other interact directly with the carboxyl groups of aspartate [12, 14, 101]. The side chain of Thr154 and the main-chain carbonyls of Tyr149 and Gln152 interact with the aspartate amino group directly [12, 14, 102–104]. Tyr149, Ser68, and Phe150 interact with aspartate through water molecules [12, 14, 102]. Mutation of Tyr149 influences ligand sensing [105], while mutation of Ser68 is not important for aspartate recognition [71] (Fig. 2a). Studies proposed that the different specificity of Tar and Tsr is probably resulted from different arrangement of the side chain of residue 68 (Asn in Tsr and Ser in Tar) [71]. Recently, structure information of the periplasmic sensing domains binding with or without ligands from other chemoreceptors is increasing [106, 107]. These structures and other in vivo or in vitro investigations will shed light on the mechanisms of chemoeffector recognitions and signaling in other kinds of chemoreceptors.
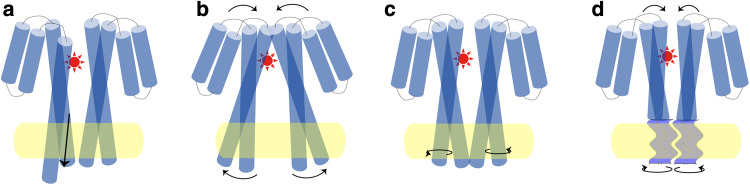
Molecular mechanisms have been proposed to explain the signal relay process in transmembrane sensing modules [26, 108–110]. Mechanisms proposed for E. coli chemoreceptors encompass, but are not limited to (1) Piston displacements, in which attractant binding generates a subtle piston-like, downward displacement of the α4-TM2 helix relative to the α1-TM1 helix within a monomer. This modest conformational change is relayed over long distances to the cytoplasm [111–113]. (2) Scissoring motions, in which attractant binding brings a rotation of one monomer with respect to the other along an axis parallel to the membrane [12, 13, 15]. (3) Rotation of helices, in which attractant binding elicits counter-clockwise rotation of α4-TM2/α4′-TM2′ helices along an axis perpendicular to the membrane [114]. (4) Supercoil unwinding of helices, in which attractant binding generates a partial unwinding of the negatively supercoiled monomers, resulting in orientation changes of cytoplasmic domains [115] (Fig. 4). Although various models are proposed, there are increasing evidences that support the piston displacement mechanism (see review [116]). Recently, functional studies of aromatic anchors of TM2 also support this mechanism [18]. Mutant receptors in which aromatic anchors were moved toward or away from the membrane had a decreased CW or increased CW signal output, respectively. This may be caused by the interaction between aromatic residues with the polar–hydrophobic interface that displaces the signaling helix downward or upward from the cytoplasm, in a manner similar to an attractant or a repellent interacting with the receptor [18]. Additionally, the piston mechanism is also conserved in interaction with indirectly binding chemoeffectors. Studies showed that the ligand-bound MBP might interact with the periplasmic domains of a Tar dimer asymmetrically and elicit a downward movement of the α4-TM2 helix [117].
Fig. 4.
Mechanisms of transmembrane signaling for E. coli chemoreceptors. a Mechanism of piston displacements [111–113], b mechanism of scissoring motions [12, 13, 15], c mechanism of rotation of helices [114], and d mechanism of supercoil unwinding of helices [115]. The helices of receptor periplasmic sensing domains are shown as blue cylinders. The attractants are shown in red
In order to decipher the relationship between the chemoeffector binding and chemotactic signaling, we compared the molecular binding patterns of novel attractant and antagonist molecules with Tar and provided new molecular insights in chemoeffector binding and signaling [40] (Fig. 2b). Our study showed that the interactions between the N–H groups or other hydrogen bond donor groups of attractants with the main-chain carbonyls of Tyr149 and/or Gln152 are crucial for triggering the piston-like downward shift of α4-TM2 helix and eliciting chemotactic signals. The molecular interactions of an attractant with Tar were then classified into two types: The first type of interactions mainly stabilizes ligand binding, primarily including the interactions of carboxyl groups of attractant with Arg64, Arg69′, and Arg73′. The second group of interactions contributes to binding and signaling, including the hydrogen bonds formed between the hydrogen bond donor groups of attractant and the main-chain carbonyls of Tyr149 and/or Gln152 on the α4 helix. Chemoeffector binding does not always lead to signaling. Ligands that bind to chemoreceptors with only the first type of interactions may function as antagonists [40]. This idea was confirmed by converting an antagonist CHDCA to an attractant cis-(2R, 3S)-2,3-piperidine dicarboxylic acid (cis-PDA) by simply replacing a CH2 group in CHDCA with a NH group (Fig. 2b).
The piston displacement mechanism is suggested as a conserved mechanism in some other chemoreceptors and two-component signaling systems [107, 118]. It would be interesting to study whether our chemoeffector binding and signaling mechanism holds true for these chemoreceptors or histidine kinases. Of course, we cannot exclude the possibility that different chemoreceptors might use different mechanisms for transmembrane signaling. For example, studies showed that the transducer protein NpHtrII from Natronomonas pharaonis (N. pharaonis) responds to the activation of the phototaxis receptor NpSRII by a 15° clockwise rotation of the TM2 and a lateral displacement by 0.9 Å of this helix [110]. Cross-linking studies showed that attractant binding to the Bacillus subtilis (B. subtilis) chemoreceptor McpB elicits a counter-clockwise rotation of the TM1 and TM1′ and trigger chemotactic signals [119]. More investigations would be useful in determining how signals are relayed in different chemoreceptors.
Signal transduction in the signal conversion module
The HAMP domain plays an important role in converting signals from the transmembrane sensing module. The molecular mechanisms for signal transduction by HAMP domains have been extensively studied (see recent commentaries and reviews [22, 120, 121]). So far, several mechanisms have been proposed to reveal the nature of HAMP kinase-on and kinase-off signaling states, although it is still in debate. These hypotheses mainly include (1) A cogwheel model is proposed based on the NMR structure of the Af1503 HAMP domain [26]. It suggests that the HAMP molecules interconvert between discrete complementary x-da and canonical knobs-into-holes packing conformations by a concerted 26° rotation of all four of HAMP helices. These two packing states might be associated with kinase-on and kinase-off output states [26]. Small side chains at position A291 would favor x-da packing conformation, whereas larger residues would favor knobs-into-holes packing [26, 31]. This idea is further supported by the structures of a series of Af1503 HAMP variants and functional analyses of the hybrid proteins with wild-type or mutant Af1503 HAMP domains [26, 31, 35]. (2) A helix rearrangement model is proposed based on the X-ray structures of three concatenated HAMP modules of Aer2 from P. aeruginosa [29]. It suggests that HAMP molecule switches between distinct two conformations, in which the structures of HAMP1 and HAMP3 are associated with kinase-on state, while that of HAMP2 is associated with kinase-off state. Furthermore, the structure and functional analyses of the Aer2 HAMP variants and hybrid chemoreceptors support this model [27, 29]. Exchange between these two conformers involves translation, rotation and tilt of the helices to interconvert between a four-helix bundle in HAMP1/HAMP3 and a two-helix coiled coil resembled by AS2 in HAMP2 [27]. (3) A scissor-like model is proposed based on disulfide mapping studies for Tar [30]. It proposed that chemoeffector signaling causes a scissors-type displacement of the C-terminal AS2 and AS2′ [30, 122]. The ‘scissor-open’ conformation is associated with the kinase-off state, while the ‘scissor-closed’ conformation is associated with the kinase-on state [122]. (4) The electron paramagnetic resonance (EPR) spectroscopy studies of the phototransducer NpHtrII from N. pharaonis suggest its HAMP1 domain switches between a compact and a dynamic HAMP conformation, which characterize the kinase-on state and the kinase-off state, respectively [123, 124]. However, the EPR and spin labeling studies of its HAMP1 and HAMP2 domains did not show the dynamic conformation. They showed that the HAMP1 and HAMP2 domains have alternating helical motions when relay signals, which lead to opposite output states [125]. (5) A biphasic dynamic bundle model is proposed based on a series of genetic studies for the E. coli Tsr HAMP domain [22, 120, 121, 126–128]. It proposes that the Tsr HAMP domain may traverse a dynamic range of packing stabilities. There are two different kinase-off states at the end of continuous dynamic states. Attractant signals might drive a high stable packing conformation HAMP(A), which inactivates CheA and triggers a kinase-off CCW(A) response. Mutant receptor that has a relatively severe destabilized HAMP(B) conformation elicits a kinase-off CCW(B) output. The HAMP domains in the intermediate stability states induce a kinase-on and CW signal output. The physiological modulating range of the HAMP domain spans the high stability HAMP(A) state through the intermediate stability states. Further destabilization of the HAMP domain leads to the kinase-off CCW(B) state, which is refractory to adaptation and cannot form signaling complex [126, 127]. This model is also supported by the functional analyses of the control cable [21, 24, 127]. A 1–2 Å piston movement of the TM2 may modulate the helicity of the control cable. Attractant signals might relax the control cable to relieve structural strain on the HAMP domain, making it pack stably; repellent signals might stiffen the control cable and exacerbate the structural clash with the HAMP domain, destabilizing the HAMP packing [21].
These models introduced above and other hypotheses [23, 28] share certain common features but also have different point of views. (1) The first four models support the static two-state model, suggesting that HAMP domains interconvert between two rigid conformations that trigger opposite signaling states. However, the dynamic bundle model proposes that HAMP domains have a dynamic range of packing stabilities [121, 128]. (2) The cogwheel model is supported by increasing experimental data. The helix rearrangement model, scissor-like model and studies on NpHtrII proteins reconcile with the cogwheel model that the helical rotation of HAMP domains is an important feature for signal relay. However, in other models, the helical translation or tilt, crossing angle changes or pivot movement also play important roles to relay signals. Therefore, the packing modes of different signaling states might not adhere to the uniform x-da or knobs-into-holes packing conformations, as the structures of HAMP domains in Aer2 [29]. Moreover, functional analyses of some Tsr HAMP variants could explain how HAMP mutations influence their structural stabilities to shift between the x-da and knobs-into-holes signaling conformations [127, 129]. However, some genetic studies of Tsr are not consistent with it. For instance, studies that deleted part or full of Tsr HAMP domain showed that AS2–AS2′ interaction could retain HAMP domain activity to induce kinase-off CCW(B) state. Therefore, the conformational changes of both entire AS1 and AS2 indicated by the cogwheel model are not necessary to relay signals [121, 128]. This idea is also supported by the studies of NpHtrII protein, which showed that stimulus induces local helical interaction changes rather than changes along the entire helices [125]. Furthermore, analyses of the mutations at the positions I229 (corresponding to A291 of Af1503 HAMP domain), S255 and L256 on the layer three of the Tsr HAMP bundle showed that HAMP alternations have multiple ‘on’ conformations [129], unlike the cogwheel model predicting that only the x-da conformer is associated with the kinase-on state. (3) The helix rearrangement model deduced from studies of poly-HAMP domains of Aer2 [27] are consistent with the EPR data from HAMP1 and HAMP2 of NpHtrII [125], suggesting that HAMP domains change between two conformers to modulate output states. The pulsed dipolar electron spin resonance spectroscopy (PDS) distributions of Aer2 HAMP1 are broader than those of Aer2 HAMP2, which might support the dynamic ‘on’ states proposed by the dynamic bundle model [27]. The helix rearrangement model predicts that the Tsr HAMP(A) and HAMP(B) states in the dynamic bundle model have the same structural mechanisms [27]. However, the dynamic bundle model proposed that the Tsr HAMP(A) and HAMP(B) states have different conformations and properties [128].
To test whether HAMP domains undergo two discrete conformations with opposing signaling states or traverse a range of dynamic conformations, additional high resolution structural information of mutant HAMP domains may prove useful, such as structures for mutant Tsr HAMP domains. Compared with these structures and their output signaling states, the relationship between their conformational structures and signaling activity might be revealed. Additionally, the in vitro and in vivo studies that have been successfully employed in some HAMP domains should also be performed in other HAMP-containing chemoreceptors. For example, the series of genetic analyses of Tsr HAMP domain might also be carried out in other chemoreceptors to observe whether other HAMP domains are also consistent with the dynamic bundle model [121]. In vitro methods, such as EPR or PDS measurements, might also be helpful to detect conformations of other HAMP domains. Because the mechanisms of signal input to HAMP domains and the output functions of chemoreceptors might be different, it is possible that different HAMP domains utilize different conformational signaling mechanisms [121]. However, in all the above cases, further studies are still needed to reveal the exact nature of the signal conversion in the HAMP domains.
Signal transduction in the kinase control module
The output of HAMP domains could modulate the dynamic and conformational properties of the kinase control modules. The dynamic bundle model suggests that the HAMP domain, MH bundle and protein contact region have opposing structural stabilities [126]. The HAMP domain and the MH bundle are oppositely coupled and modulated by both the piston motion from the TM2 to HAMP and the methylation process to the MH bundle [126, 127]. Acting through a phase stutter between the AS2 and MH1, tightly packed HAMP(A) conformation induced by attractants could in turn reduce the packing stability of the MH bundle, triggering a kinase-off CCW(A) output. The loosely packed HAMP(B) conformation may attenuate the helical phase clash between the AS2 and MH1 and enhance the stability of the MH bundle. The biphasic manner is that when the MH bundle packs either loosely or tightly, CheA is inactivated and the output is CCW. Only when the MH bundle has intermediate packing conformations, CheA can be activated and has a kinase-on output [126, 127]. The MH bundle and the protein contact region also have an opposite stability relationship, which might be mediated through the flexible bundle and the glycine hinge [122, 126]. Therefore, the attractant induced kinase-off state represents a tightly packed HAMP domain, a loosely packed MH bundle, and a tightly packed protein contact region. The kinase-on state has opposite packing conformations. This is an extension of the Yin–Yang model introduced by Swain et al. [122].
The inter-helix packing interactions of the MH bundle could provide a structural basis for the feedback control of adaptational modifications [121, 128]. The loose packing of the MH bundle in the kinase-off CCW(A) state may favor CheR recognition [128]. The methylation sites lie at the monomer–monomer interface in a receptor dimer. The methylation of Glu residues mediated by CheR might increase the stability of the MH bundle, because the negative charges of Glu residues are neutralized by the methylation process [130, 131]. In contrast, the tighter packing of the MH bundle in the kinase-on CW state may favor CheB recognition [128]. The demethylation mediated by CheB may reduce the stability of the MH bundle [128]. The conformational changes result from methylation could directly reverse the conformational changes that elicited by ligand binding and decrease the ligand affinity [130]. In the kinase-off CCW(B) state, the conformation of the MH bundle might prevent substrate recognition by both CheR and CheB [121, 128].
Signal transduction in trimer of chemoreceptor dimers and signaling complexes
The signaling mechanisms in higher-order arrays are less clear than in receptor homodimers. Increasing studies are carried out to reveal the nature of how signals pass through the trimer of receptor dimers and cluster arrays. Recently, several studies on discovering the function of trimer of dimers had been reported. Gosink et al. [132] characterized a number of amino acid replacements at a dimer contact residue Asn381 of E. coli Tsr and found that these mutants destroyed Tsr function and were unable to form ternary signaling complexes with CheA and CheW. Ortega et al. [133] conducted long molecular dynamics simulations and found that the cytoplasmic tips of Tsr in a trimer unit might fluctuate between two stable conformations in a signal-dependent manner. These two conformations are stabilized by alternative stacking arrangements of a conserved residue Phe396 that localizes on the protein contact region, which serves as a conformational switch [133]. Massazza et al. [134] studied seven-residue deletions in the flexible bundle of E. coli Tsr and indicated that the distortions introduced by length difference between receptors influence chemotactic function. Seven-residue deletion in only one helix alters the symmetry of the two helices of the cytoplasmic hairpin in a monomer, which results in a seriously impaired function. Symmetrical deletions of Tsr in both helices can still form trimer of dimers with full-length Tar, but with impaired signaling [134]. For the conformational changes in the trimer of receptor dimers, the in vivo anisotropy measurements of fluorescently labeled chemoreceptors showed that attractant binding might favor an expanded conformation among dimers in a trimer unit, whereas repellent might favor a compact conformation [135, 136]. However, there are no significant vertical movements between receptor dimers in signaling. The tight contact at the tips could preclude significant displacements among receptor dimers as indicated by recent cross-linking studies [137].
How chemotactic signals influence cluster arrays of signaling complexes is an important issue. The electron-cryotomography studies showed that the arrangement of signaling complexes is highly conserved between different signaling states [138]. Chemoreceptor arrays in Caulobacter crescentus kept the same hexagonal packing arrangement after stimulated by galactose [49, 138]. In E. coli, while chemoreceptors were mutationally locked in the well-defined kinase activity states, the packing of arrays were independent of the kinase activity [49]. The serine responses of purified E. coli Tsr receptors that reconstituted into liposomes indicated that the ligand-induced activity changes did not influence the receptor oligomerization state, which was consistent with the findings that ligand binding induced expanded conformation of trimer of receptor dimers without changing the number of associated receptors in the signaling complexes [56, 139]. The measurement of stimulus-induced dynamics in the fluorescence polarization of receptor clusters showed that the packing of E. coli receptors slowly decreased when a stimulus was applied. The process reversed when the stimulus was removed. However, despite these changes, the receptor clusters did not generally dissociate upon ligand binding [140]. Although chemosensory arrays are conserved in different signaling states, small conformational changes also occur in the signal transduction process. In the electron-cryotomography studies, the differences in the densities of CheA P1 domain and P2 domain showed that these two domains may be partially mobile in the kinase-on state, implying the possible role of CheA domain mobility in the kinase control [49]. Studies showed that attractant signals could perturb localized regions of the receptor–CheA interaction interface, implying an interface rearrangement triggered by attractant signaling [56]. More efforts are still required to reveal the conformational mechanisms of how signals pass through the cluster arrays of signaling complexes.
Changing the specificity of bacterial chemoreceptors
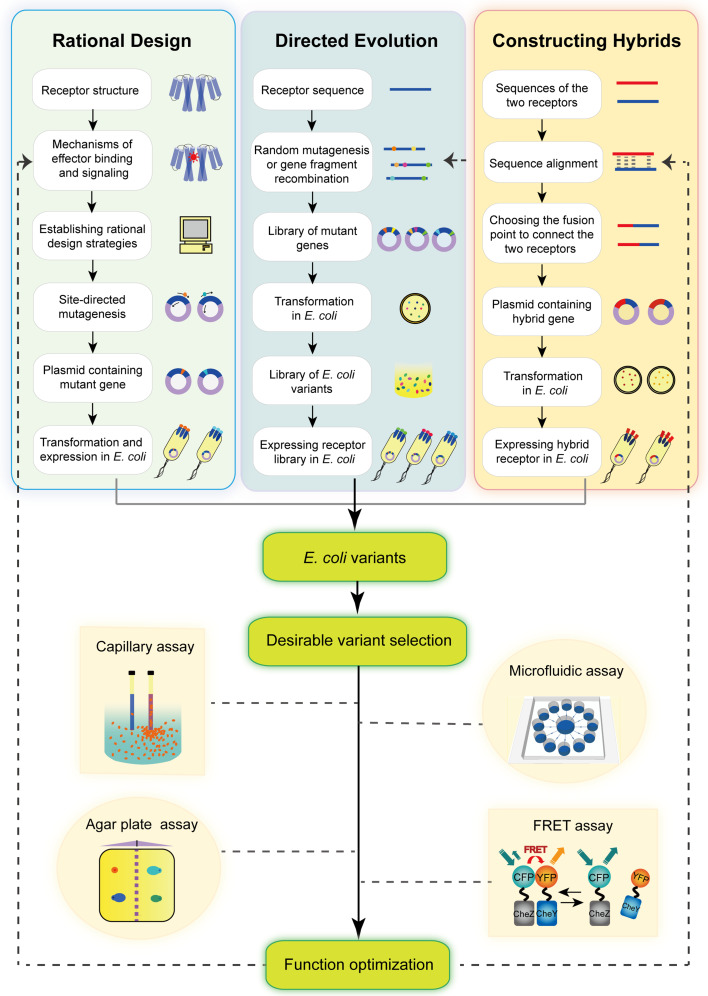
The high specificity of bacterial chemoreceptors allows cells to respond to particular environmental chemoeffectors. However, engineering bacteria for industrial and medical applications often requires novel sensors with enhanced or new specificity to desirable molecules [141]. Based on the increasing information about receptor sequences, structures, functions and mechanisms, engineering the specificity of bacterial chemoreceptors becomes one of the powerful approaches to achieve such goals. Rational design, directed evolution, and making hybrid receptors are commonly used methods to modify the receptor specificity to sense novel chemoeffectors (Fig. 5).
Fig. 5.
Three strategies for changing the specificity of chemoreceptors. The rational design strategy starts with the full understanding of receptor structures, mechanisms, and functions. Residues that may bind to a novel chemoeffector are predicted rationally. The variant genes are obtained by site-directed mutagenesis and then expressed in E. coli [40, 142]. The directed evolution strategy needs mutant gene libraries that can be prepared by random mutagenesis or gene fragment recombination [141–143]. Libraries of E. coli variants are obtained after gene transformation and expression. Making hybrid chemoreceptors is another strategy to change the ligand specificity. Two receptor genes can be fused together according to the sequence conservation [141]. The non-native periplasmic sensing domains in hybrid receptors enable E. coli to sense novel ligands. The methods for selecting desirable E. coli variants that can sense novel chemoeffectors are various, such as capillary assay [60], agar plate assay [143], microfluidic assay [40], and FRET assay [68]. The three strategies can be successively used in combination to optimize the desired function
Rational design approach
The rational design approach by site-directed mutagenesis is an effective strategy for protein engineering. It usually requires both the availability of the protein structure and the knowledge about the relationship between sequence, structure, mechanism, and function [142]. High-throughput selection is not needed for this strategy, so it is expected to be low cost and efficient.
For the specificity design of chemoreceptors in bacterial chemotaxis, although the understandings of receptor–effector recognition and signal transduction mechanisms in chemoreceptors are increasing, it is still thought to be rather difficult because one might design a novel chemoeffector that can bind to a receptor, however, binding may not drive right conformational changes to elicit chemotactic signals [143]. The knowledge on the ligand binding and signaling mechanism is one of the key points for successful rational design.
We have studied the key interaction features for attractant binding and signaling in the E. coli chemoreceptor Tar and showed that the chemoreceptor specificity can be rationally designed on the basis of the binding and signaling mechanism [40]. The ligand-binding pocket of Tar is divided into two regions: region I mainly including residues Arg64, Arg69′ and Arg73′, which forms interactions with carboxyl groups of attractant that mainly contribute to binding; region II mainly including the main-chain carbonyls of Tyr149 and/or Gln152 on the α4 helix, which forms interactions with the hydrogen bond donor group of attractant that contribute to both binding and signaling. We hypothesized that Tar can be rationally designed to sense novel chemoeffectors according to the following strategies: the target chemoeffector should keep interactions with the region II of the binding pocket, which trigger the conformational changes for the chemotactic signaling; while the region I of the binding pocket should be rationally changed to form new interactions with the novel chemoeffector to stabilize binding. On the basis of these analyses, we rationally designed the ligand-binding pocket of Tar to respond to l-arginine, a basic amino acid that cannot be sensed by the wild-type Tar. By using site-directed mutagenesis and microfluidic technology, an E. coli mutant strain R69′ER73′E that had an attractant response to arginine was successfully obtained [40]. In addition to arginine, other types of amino acids can also be sensed via rational design based on these strategies. A series of Tar variants that had novel or enhanced specificity to different amino acids were identified, and these Tar variants showed different recognition spectra for the 20 natural amino acids from the wild-type Tar (to be published). The sensitivities of these variant receptors can be further optimized by using computational methods. Our rational design strategies might probably be applied in other chemoreceptors.
Directed evolution approach
Directed evolution is another powerful approach to engineer proteins with improved activity or novel function [141, 144]. This strategy does not rely on the structural knowledge. A library of protein variants with large diversity was used to screen for the desirable mutations [142]. Various methods such as random mutagenesis by error-prone PCR [145] or recombination of gene fragments [146] can be used to obtain such libraries. The generated libraries are then assayed using high-throughput technologies to identify variants with desirable function. Successive rounds of selections are usually needed to improve the function [143].
Goulian and coworkers have successfully used this strategy to engineer E. coli to sense new ligands. They used error-prone PCR to generate mutant libraries and selected the functional variants on the semisolid agar plates. They successfully evolved E. coli chemoreceptor Tar to recognize new compounds, such as cysteic acid and phenylalanine. The variant that had an enhanced sensitivity to glutamate was also found [143]. On the basis of this work, Goldberg et al. [147] used directed evolution to select Tar variant that performed chemotaxis toward phenylacetic acid. Cells expressing penicillin acylase, an enzyme that hydrolyzes phenylacetyl glycine to produce phenylacetic acid, could migrate up the gradient of phenylacetyl glycine.
Making hybrid chemoreceptor approach
Making hybrid chemoreceptors is an approach that fuses periplasmic sensing domain from a non-native protein, such as from a foreign chemoreceptor or a histidine kinase, to the cytoplasmic signaling domain of the target chemoreceptor [141]. It is a potent strategy for changing the receptor specificity because the protein scaffold of the periplasmic sensing domain can be totally replaced, so changes in ligand specificity can be achieved.
Based on the evolutionary conservation, some hybrid receptors were successfully constructed. These hybrids could be constructed between two chemoreceptors from the same bacterial species, such as the Tar–Tsr [96], Tsr–Tar [68], Trg–Tsr [148], Tap–Tar [149], Tar–Tap [149], Aer–Tsr [150] hybrids in E. coli, and the McpB–McpC hybrid in B. subtilis [151]. Functional hybrids with each component from different bacterial species could also be constructed. The Pp0584–Tar and Pp5020–Tar are hybrids with the sensing domains of chemoreceptors Pp0584 and Pp5020 from P. putida and the cytoplasmic domain of Tar from E. coli [86]. These two hybrids sense phenol as an attractant [86]. In addition, the hybrids could be constructed between a two-component sensor and a chemoreceptor. Ward et al. [152] constructed a hybrid receptor of NarX-Tar, which contains the N-terminal domains from the E. coli sensor kinase NarX and C-terminal domains from Tar. As NarX is a sensor kinase that detects nitrate and nitrite, the new hybrid receptor gains novel function to mediate repellent responses to nitrate and nitrite [152]. Sensor kinase NarX was also fused to C-terminal signaling domain of chemoreceptor FrzCD from Myxococcus xanthus (M. xanthus) to get a hybrid receptor NarX-FrzCD, which can be used to study the chemotaxis behavior of M. xanthus [153]. Because hybrid receptors change the original specificity and gain abilities to sense new molecules, it can be a fast and efficient way to engineer proteins with novel functions, and become more and more important in the field of bacterial engineering.
Combined and successive rounds of rational design, directed evolution, or making hybrids are usually needed to obtain desirable variants with the optimum function. For example, one can use rational design or making hybrid approach to change the specificity of a chemoreceptor, and then use directed evolution to improve its sensitivity to the novel chemoeffectors. Chemoreceptor engineering could be applied in various fields. One application is that it can be used in bioremediation. Chemoreceptors can be engineered to sense some environmental pollutions, such as some organic compounds. Combined with synthetic biology approaches, one might design bacteria to perform chemotaxis to those organic compounds and degradation them. Another application is that it can be used in biodetection. Chemoreceptors can be engineered to sense and detect some harmful compounds containing in food or water. For example, one can use engineered bacteria to detect the level of antibiotics in surface water.
Conclusions
Chemoreceptors are central signaling components that enable bacteria to sense chemoeffector gradients with high sensitivity and wide dynamic range. Recent studies of E. coli chemotaxis system on the structure of chemoreceptor homodimers, the organization of signaling complex clusters, the novel chemoeffector discovery and design, and the signal recognition and transduction mechanisms, provide deep insights in understanding the molecular details of chemoeffector sensing and signaling process. On the other hand, changing the specificity of chemoreceptors to recognize and respond to novel chemoeffectors is an interesting issue in recent years. It based on broaden knowledge of structures, functions and mechanisms of chemotactic sensory arrays. The studies on E. coli chemotaxis system can shed light on understanding other intracellular networks in both prokaryotes and eukaryotes, and provide potent applications in industry and therapeutics.
Acknowledgments
We thank Dr. Yiling Yang, Dr. Abiola Pollard, and Dr. Fan Jin at the Max Planck Institute for Terrestrial Microbiology for discussions. This work was supported in part by the National Natural Science Foundation of China (No. 21173013).
Abbreviations
- BPs
Periplasmic substrate-binding proteins
- CW
Clockwise
- CCW
Counter-clockwise
- TM2
The second transmembrane helix
- HAMP
Histidine kinase, adenylyl cyclase, methyl-accepting chemotaxis proteins and phosphatase
- MH
Methylation helix
- NMR
Nuclear magnetic resonance
- MBP
Maltose binding protein
- CHDCA
cis-1,2-Cyclohexane-dicarboxylic acid
- cis-PDA
cis-(2R, 3S)-2,3-Piperidine dicarboxylic acid
- ITC
Isothermal titration calorimetry
- FRET
Fluorescence resonance energy transfer
- EPR
Electron paramagnetic resonance
- PDS
Pulsed dipolar electron spin resonance spectroscopy
- E. coli
Escherichia coli
- A. fulgidus
Archaeoglobus fulgidus
- P. aeruginosa
Pseudomonas aeruginosa
- T. maritima
Thermotoga maritima
- P. putida
Pseudomonas putida
- N. pharaonis
Natronomonas pharaonis
- B. subtilis
Bacillus subtilis
- M. xanthus
Myxococcus xanthus
References
- 1.Sourjik V, Wingreen NS. Responding to chemical gradients: bacterial chemotaxis. Curr Opin Cell Biol. 2012;24:262–268. doi: 10.1016/j.ceb.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krell T, Lacal J, Munoz-Martinez F, Reyes-Darias JA, Cadirci BH, Garcia-Fontana C, Ramos JL. Diversity at its best: bacterial taxis. Environ Microbiol. 2011;13:1115–1124. doi: 10.1111/j.1462-2920.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 3.Sourjik V, Armitage JP. Spatial organization in bacterial chemotaxis. EMBO J. 2010;29:2724–2733. doi: 10.1038/emboj.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hazelbauer GL, Lai WC. Bacterial chemoreceptors: providing enhanced features to two-component signaling. Curr Opin Microbiol. 2010;13:124–132. doi: 10.1016/j.mib.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazelbauer GL, Falke JJ, Parkinson JS. Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends Biochem Sci. 2008;33:9–19. doi: 10.1016/j.tibs.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter SL, Wadhams GH, Armitage JP. Signal processing in complex chemotaxis pathways. Nat Rev Microbiol. 2011;9:153–165. doi: 10.1038/nrmicro2505. [DOI] [PubMed] [Google Scholar]
- 7.Bibikov SI, Biran R, Rudd KE, Parkinson JS. A signal transducer for aerotaxis in Escherichia coli . J Bacteriol. 1997;179:4075–4079. doi: 10.1128/jb.179.12.4075-4079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goy MF, Springer MS, Adler J. Sensory transduction in Escherichia coli: role of a protein methylation reaction in sensory adaptation. Proc Natl Acad Sci USA. 1977;74:4964–4968. doi: 10.1073/pnas.74.11.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan G, Schulmeister S, Sourjik V, Tu Y. Adapt locally and act globally: strategy to maintain high chemoreceptor sensitivity in complex environments. Mol Syst Biol. 2011;7:475. doi: 10.1038/msb.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacal J, Garcia-Fontana C, Munoz-Martinez F, Ramos JL, Krell T. Sensing of environmental signals: classification of chemoreceptors according to the size of their ligand binding regions. Environ Microbiol. 2010;12:2873–2884. doi: 10.1111/j.1462-2920.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- 11.Shu CJ, Ulrich LE, Zhulin IB. The NIT domain: a predicted nitrate-responsive module in bacterial sensory receptors. Trends Biochem Sci. 2003;28:121–124. doi: 10.1016/S0968-0004(03)00032-X. [DOI] [PubMed] [Google Scholar]
- 12.Milburn MV, Prive GG, Milligan DL, Scott WG, Yeh J, Jancarik J, Koshland DE, Jr, Kim SH. Three-dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand. Science. 1991;254:1342–1347. doi: 10.1126/science.1660187. [DOI] [PubMed] [Google Scholar]
- 13.Yeh JI, Biemann HP, Pandit J, Koshland DE, Kim SH. The three-dimensional structure of the ligand-binding domain of a wild-type bacterial chemotaxis receptor. Structural comparison to the cross-linked mutant forms and conformational changes upon ligand binding. J Biol Chem. 1993;268:9787–9792. [PubMed] [Google Scholar]
- 14.Yeh JI, Biemann HP, Prive GG, Pandit J, Koshland DE, Jr, Kim SH. High-resolution structures of the ligand binding domain of the wild-type bacterial aspartate receptor. J Mol Biol. 1996;262:186–201. doi: 10.1006/jmbi.1996.0507. [DOI] [PubMed] [Google Scholar]
- 15.Chi YI, Yokota H, Kim SH. Apo structure of the ligand-binding domain of aspartate receptor from Escherichia coli and its comparison with ligand-bound or pseudoligand-bound structures. FEBS Lett. 1997;414:327–332. doi: 10.1016/s0014-5793(97)01027-2. [DOI] [PubMed] [Google Scholar]
- 16.Miller AS, Falke JJ. Side chains at the membrane–water interface modulate the signaling state of a transmembrane receptor. Biochemistry. 2004;43:1763–1770. doi: 10.1021/bi0360206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Draheim RR, Bormans AF, Lai RZ, Manson MD. Tryptophan residues flanking the second transmembrane helix (TM2) set the signaling state of the Tar chemoreceptor. Biochemistry. 2005;44:1268–1277. doi: 10.1021/bi048969d. [DOI] [PubMed] [Google Scholar]
- 18.Draheim RR, Bormans AF, Lai RZ, Manson MD. Tuning a bacterial chemoreceptor with protein–membrane interactions. Biochemistry. 2006;45:14655–14664. doi: 10.1021/bi061259i. [DOI] [PubMed] [Google Scholar]
- 19.Adase CA, Draheim RR, Manson MD. The residue composition of the aromatic anchor of the second transmembrane helix determines the signaling properties of the aspartate/maltose chemoreceptor Tar of Escherichia coli . Biochemistry. 2012;51:1925–1932. doi: 10.1021/bi201555x. [DOI] [PubMed] [Google Scholar]
- 20.Adase CA, Draheim RR, Rueda G, Desai R, Manson MD. Residues at the cytoplasmic end of transmembrane helix 2 determine the signal output of the TarEc chemoreceptor. Biochemistry. 2013;52:2729–2738. doi: 10.1021/bi4002002. [DOI] [PubMed] [Google Scholar]
- 21.Kitanovic S, Ames P, Parkinson JS. Mutational analysis of the control cable that mediates transmembrane signaling in the Escherichia coli serine chemoreceptor. J Bacteriol. 2011;193:5062–5072. doi: 10.1128/JB.05683-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkinson JS. Signaling mechanisms of HAMP domains in chemoreceptors and sensor kinases. Annu Rev Microbiol. 2010;64:101–122. doi: 10.1146/annurev.micro.112408.134215. [DOI] [PubMed] [Google Scholar]
- 23.Park H, Im W, Seok C. Transmembrane signaling of chemotaxis receptor tar: insights from molecular dynamics simulation studies. Biophys J. 2011;100:2955–2963. doi: 10.1016/j.bpj.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright GA, Crowder RL, Draheim RR, Manson MD. Mutational analysis of the transmembrane helix 2-HAMP domain connection in the Escherichia coli aspartate chemoreceptor tar. J Bacteriol. 2011;193:82–90. doi: 10.1128/JB.00953-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmann MD, Dunin-Horkawicz S, Hulko M, Martin J, Coles M, Lupas AN. A soluble mutant of the transmembrane receptor Af1503 features strong changes in coiled-coil periodicity. J Struct Biol. 2014;186:357–366. doi: 10.1016/j.jsb.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Hulko M, Berndt F, Gruber M, Linder JU, Truffault V, Schultz A, Martin J, Schultz JE, Lupas AN, Coles M. The HAMP domain structure implies helix rotation in transmembrane signaling. Cell. 2006;126:929–940. doi: 10.1016/j.cell.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 27.Airola MV, Sukomon N, Samanta D, Borbat PP, Freed JH, Watts KJ, Crane BR. HAMP domain conformers that propagate opposite signals in bacterial chemoreceptors. PLoS Biol. 2013;11:e1001479. doi: 10.1371/journal.pbio.1001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watts KJ, Johnson MS, Taylor BL. Different conformations of the kinase-on and kinase-off signaling states in the Aer HAMP domain. J Bacteriol. 2011;193:4095–4103. doi: 10.1128/JB.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Airola MV, Watts KJ, Bilwes AM, Crane BR. Structure of concatenated HAMP domains provides a mechanism for signal transduction. Structure. 2010;18:436–448. doi: 10.1016/j.str.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swain KE, Falke JJ. Structure of the conserved HAMP domain in an intact, membrane-bound chemoreceptor: a disulfide mapping study. Biochemistry. 2007;46:13684–13695. doi: 10.1021/bi701832b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferris HU, Dunin-Horkawicz S, Mondejar LG, Hulko M, Hantke K, Martin J, Schultz JE, Zeth K, Lupas AN, Coles M. The mechanisms of HAMP-mediated signaling in transmembrane receptors. Structure. 2011;19:378–385. doi: 10.1016/j.str.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Kim KK, Yokota H, Kim SH. Four-helical-bundle structure of the cytoplasmic domain of a serine chemotaxis receptor. Nature. 1999;400:787–792. doi: 10.1038/23512. [DOI] [PubMed] [Google Scholar]
- 33.Park SY, Borbat PP, Gonzalez-Bonet G, Bhatnagar J, Pollard AM, Freed JH, Bilwes AM, Crane BR. Reconstruction of the chemotaxis receptor-kinase assembly. Nat Struct Mol Biol. 2006;13:400–407. doi: 10.1038/nsmb1085. [DOI] [PubMed] [Google Scholar]
- 34.Pollard AM, Bilwes AM, Crane BR. The structure of a soluble chemoreceptor suggests a mechanism for propagating conformational signals. Biochemistry. 2009;48:1936–1944. doi: 10.1021/bi801727m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferris HU, Zeth K, Hulko M, Dunin-Horkawicz S, Lupas AN. Axial helix rotation as a mechanism for signal regulation inferred from the crystallographic analysis of the E. coli serine chemoreceptor. J Struct Biol. 2014;186:349–356. doi: 10.1016/j.jsb.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Coleman MD, Bass RB, Mehan RS, Falke JJ. Conserved glycine residues in the cytoplasmic domain of the aspartate receptor play essential roles in kinase coupling and on-off switching. Biochemistry. 2005;44:7687–7695. doi: 10.1021/bi0501479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiomi D, Zhulin IB, Homma M, Kawagishi I. Dual recognition of the bacterial chemoreceptor by chemotaxis-specific domains of the CheR methyltransferase. J Biol Chem. 2002;277:42325–42333. doi: 10.1074/jbc.M202001200. [DOI] [PubMed] [Google Scholar]
- 38.Barnakov AN, Barnakova LA, Hazelbauer GL. Location of the receptor–interaction site on CheB, the methylesterase response regulator of bacterial chemotaxis. J Biol Chem. 2001;276:32984–32989. doi: 10.1074/jbc.M105925200. [DOI] [PubMed] [Google Scholar]
- 39.Bartelli NL, Hazelbauer GL. Direct evidence that the carboxyl-terminal sequence of a bacterial chemoreceptor is an unstructured linker and enzyme tether. Protein Sci. 2011;20:1856–1866. doi: 10.1002/pro.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bi S, Yu D, Si G, Luo C, Li T, Ouyang Q, Jakovljevic V, Sourjik V, Tu Y, Lai L. Discovery of novel chemoeffectors and rational design of Escherichia coli chemoreceptor specificity. Proc Natl Acad Sci USA. 2013;110:16814–16819. doi: 10.1073/pnas.1306811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li M, Hazelbauer GL. Core unit of chemotaxis signaling complexes. Proc Natl Acad Sci USA. 2011;108:9390–9395. doi: 10.1073/pnas.1104824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, Khursigara CM, Subramaniam S, Hazelbauer GL. Chemotaxis kinase CheA is activated by three neighbouring chemoreceptor dimers as effectively as by receptor clusters. Mol Microbiol. 2011;79:677–685. doi: 10.1111/j.1365-2958.2010.07478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Studdert CA, Parkinson JS. Insights into the organization and dynamics of bacterial chemoreceptor clusters through in vivo crosslinking studies. Proc Natl Acad Sci USA. 2005;102:15623–15628. doi: 10.1073/pnas.0506040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briegel A, Wong ML, Hodges HL, Oikonomou CM, Piasta KN, Harris MJ, Fowler DJ, Thompson LK, Falke JJ, Kiessling LL, Jensen GJ. New insights into bacterial chemoreceptor array structure and assembly from electron cryotomography. Biochemistry. 2014;53:1575–1585. doi: 10.1021/bi5000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li G, Weis RM. Covalent modification regulates ligand binding to receptor complexes in the chemosensory system of Escherichia coli . Cell. 2000;100:357–365. doi: 10.1016/s0092-8674(00)80671-6. [DOI] [PubMed] [Google Scholar]
- 46.Briegel A, Li X, Bilwes AM, Hughes KT, Jensen GJ, Crane BR. Bacterial chemoreceptor arrays are hexagonally packed trimers of receptor dimers networked by rings of kinase and coupling proteins. Proc Natl Acad Sci USA. 2012;109:3766–3771. doi: 10.1073/pnas.1115719109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, Hu B, Morado DR, Jani S, Manson MD, Margolin W. Molecular architecture of chemoreceptor arrays revealed by cryoelectron tomography of Escherichia coli minicells. Proc Natl Acad Sci USA. 2012;109:E1481–E1488. doi: 10.1073/pnas.1200781109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khursigara CM, Lan G, Neumann S, Wu X, Ravindran S, Borgnia MJ, Sourjik V, Milne J, Tu Y, Subramaniam S. Lateral density of receptor arrays in the membrane plane influences sensitivity of the E. coli chemotaxis response. EMBO J. 2011;30:1719–1729. doi: 10.1038/emboj.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briegel A, Ames P, Gumbart JC, Oikonomou CM, Parkinson JS, Jensen GJ. The mobility of two kinase domains in the Escherichia coli chemoreceptor array varies with signalling state. Mol Microbiol. 2013;89:831–841. doi: 10.1111/mmi.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cardozo MJ, Massazza DA, Parkinson JS, Studdert CA. Disruption of chemoreceptor signalling arrays by high levels of CheW, the receptor-kinase coupling protein. Mol Microbiol. 2010;75:1171–1181. doi: 10.1111/j.1365-2958.2009.07032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Underbakke ES, Zhu Y, Kiessling LL. Protein footprinting in a complex milieu: identifying the interaction surfaces of the chemotaxis adaptor protein CheW. J Mol Biol. 2011;409:483–495. doi: 10.1016/j.jmb.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boukhvalova MS, Dahlquist FW, Stewart RC. CheW binding interactions with CheA and Tar. Importance for chemotaxis signaling in Escherichia coli . J Biol Chem. 2002;277:22251–22259. doi: 10.1074/jbc.M110908200. [DOI] [PubMed] [Google Scholar]
- 53.Boukhvalova M, VanBruggen R, Stewart RC. CheA kinase and chemoreceptor interaction surfaces on CheW. J Biol Chem. 2002;277:23596–23603. doi: 10.1074/jbc.M202288200. [DOI] [PubMed] [Google Scholar]
- 54.Mehan RS, White NC, Falke JJ. Mapping out regions on the surface of the aspartate receptor that are essential for kinase activation. Biochemistry. 2003;42:2952–2959. doi: 10.1021/bi027127g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ames P, Studdert CA, Reiser RH, Parkinson JS. Collaborative signaling by mixed chemoreceptor teams in Escherichia coli . Proc Natl Acad Sci USA. 2002;99:7060–7065. doi: 10.1073/pnas.092071899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piasta KN, Ulliman CJ, Slivka PF, Crane BR, Falke JJ. Defining a key receptor–CheA kinase contact and elucidating its function in the membrane-bound bacterial chemosensory array: a disulfide mapping and TAM-IDS Study. Biochemistry. 2013;52:3866–3880. doi: 10.1021/bi400385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Vu A, Lee K, Dahlquist FW. CheA–receptor interaction sites in bacterial chemotaxis. J Mol Biol. 2012;422:282–290. doi: 10.1016/j.jmb.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X, Fleetwood AD, Bayas C, Bilwes AM, Ortega DR, Falke JJ, Zhulin IB, Crane BR. The 3.2 A resolution structure of a receptor: CheA:CheW signaling complex defines overlapping binding sites and key residue interactions within bacterial chemosensory arrays. Biochemistry. 2013;52:3852–3865. doi: 10.1021/bi400383e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lacal J, Munoz-Martinez F, Reyes-Darias JA, Duque E, Matilla M, Segura A, Calvo JJ, Jimenez-Sanchez C, Krell T, Ramos JL. Bacterial chemotaxis towards aromatic hydrocarbons in Pseudomonas . Environ Microbiol. 2011;13:1733–1744. doi: 10.1111/j.1462-2920.2011.02493.x. [DOI] [PubMed] [Google Scholar]
- 60.Hedblom ML, Adler J. Chemotactic response of Escherichia coli to chemically synthesized amino acids. J Bacteriol. 1983;155:1463–1466. doi: 10.1128/jb.155.3.1463-1466.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adler J, Hazelbauer GL, Dahl MM. Chemotaxis toward sugars in Escherichia coli . J Bacteriol. 1973;115:824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manson MD, Blank V, Brade G, Higgins CF. Peptide chemotaxis in E. coli involves the Tap signal transducer and the dipeptide permease. Nature. 1986;321:253–256. doi: 10.1038/321253a0. [DOI] [PubMed] [Google Scholar]
- 63.Hutz A, Schubert K, Overmann J. Thalassospira sp. isolated from the oligotrophic eastern Mediterranean Sea exhibits chemotaxis toward inorganic phosphate during starvation. Appl Environ Microbiol. 2011;77:4412–4421. doi: 10.1128/AEM.00490-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lacal J, Alfonso C, Liu X, Parales RE, Morel B, Conejero-Lara F, Rivas G, Duque E, Ramos JL, Krell T. Identification of a chemoreceptor for tricarboxylic acid cycle intermediates: differential chemotactic response towards receptor ligands. J Biol Chem. 2010;285:23126–23136. doi: 10.1074/jbc.M110.110403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tso WW, Adler J. Negative chemotaxis in Escherichia coli . J Bacteriol. 1974;118:560–576. doi: 10.1128/jb.118.2.560-576.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krell T, Lacal J, Guazzaroni ME, Busch A, Silva-Jimenez H, Fillet S, Reyes-Darias JA, Munoz-Martinez F, Rico-Jimenez M, Garcia-Fontana C, Duque E, Segura A, Ramos JL. Responses of Pseudomonas putida to toxic aromatic carbon sources. J Biotechnol. 2012;160:25–32. doi: 10.1016/j.jbiotec.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 67.Rico-Jimenez M, Munoz-Martinez F, Garcia-Fontana C, Fernandez M, Morel B, Ortega A, Ramos JL, Krell T. Paralogous chemoreceptors mediate chemotaxis towards protein amino acids and the non-protein amino acid gamma-aminobutyrate (GABA) Mol Microbiol. 2013;88:1230–1243. doi: 10.1111/mmi.12255. [DOI] [PubMed] [Google Scholar]
- 68.Yang Y, Sourjik V. Opposite responses by different chemoreceptors set a tunable preference point in Escherichia coli pH taxis. Mol Microbiol. 2012;86:1482–1489. doi: 10.1111/mmi.12070. [DOI] [PubMed] [Google Scholar]
- 69.Li C, Boileau AJ, Kung C, Adler J. Osmotaxis in Escherichia coli . Proc Natl Acad Sci USA. 1988;85:9451–9455. doi: 10.1073/pnas.85.24.9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oleksiuk O, Jakovljevic V, Vladimirov N, Carvalho R, Paster E, Ryu WS, Meir Y, Wingreen NS, Kollmann M, Sourjik V. Thermal robustness of signaling in bacterial chemotaxis. Cell. 2011;145:312–321. doi: 10.1016/j.cell.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tajima H, Imada K, Sakuma M, Hattori F, Nara T, Kamo N, Homma M, Kawagishi I. Ligand specificity determined by differentially arranged common ligand-binding residues in bacterial amino acid chemoreceptors Tsr and Tar. J Biol Chem. 2011;286:42200–42210. doi: 10.1074/jbc.M111.221887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neumann S, Hansen CH, Wingreen NS, Sourjik V. Differences in signalling by directly and indirectly binding ligands in bacterial chemotaxis. EMBO J. 2010;29:3484–3495. doi: 10.1038/emboj.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Springer MS, Goy MF, Adler J. Sensory transduction in Escherichia coli: a requirement for methionine in sensory adaptation. Proc Natl Acad Sci USA. 1977;74:183–187. doi: 10.1073/pnas.74.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mesibov R, Adler J. Chemotaxis toward amino acids in Escherichia coli . J Bacteriol. 1972;112:315–326. doi: 10.1128/jb.112.1.315-326.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harayama S, Palva ET, Hazelbauer GL. Transposon-insertion mutants of Escherichia coli K12 defective in a component common to galactose and ribose chemotaxis. Mol Gen Genet. 1979;171:193–203. doi: 10.1007/BF00270005. [DOI] [PubMed] [Google Scholar]
- 76.Hazelbauer GL. Maltose chemoreceptor of Escherichia coli . J Bacteriol. 1975;122:206–214. doi: 10.1128/jb.122.1.206-214.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brass JM, Manson MD. Reconstitution of maltose chemotaxis in Escherichia coli by addition of maltose-binding protein to calcium-treated cells of maltose regulon mutants. J Bacteriol. 1984;157:881–890. doi: 10.1128/jb.157.3.881-890.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hegde M, Englert DL, Schrock S, Cohn WB, Vogt C, Wood TK, Manson MD, Jayaraman A. Chemotaxis to the quorum-sensing signal AI-2 requires the Tsr chemoreceptor and the periplasmic LsrB AI-2-binding protein. J Bacteriol. 2011;193:768–773. doi: 10.1128/JB.01196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pasupuleti S, Sule N, Cohn WB, MacKenzie DS, Jayaraman A, Manson MD. Chemotaxis of Escherichia coli to norepinephrine (NE) requires conversion of NE to 3,4-dihydroxymandleic acid (DHMA) J Bacteriol. 2014 doi: 10.1128/JB.02065-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu X, Parales RE. Chemotaxis of Escherichia coli to pyrimidines: a new role for the signal transducer tap . J Bacteriol. 2008;190:972–979. doi: 10.1128/JB.01590-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gestwicki JE, Strong LE, Kiessling LL. Tuning chemotactic responses with synthetic multivalent ligands. Chem Biol. 2000;7:583–591. doi: 10.1016/s1074-5521(00)00002-8. [DOI] [PubMed] [Google Scholar]
- 82.Gestwicki JE, Strong LE, Borchardt SL, Cairo CW, Schnoes AM, Kiessling LL. Designed potent multivalent chemoattractants for Escherichia coli . Bioorg Med Chem. 2001;9:2387–2393. doi: 10.1016/s0968-0896(01)00162-6. [DOI] [PubMed] [Google Scholar]
- 83.Gestwicki JE, Kiessling LL. Inter-receptor communication through arrays of bacterial chemoreceptors. Nature. 2002;415:81–84. doi: 10.1038/415081a. [DOI] [PubMed] [Google Scholar]
- 84.Borrok MJ, Kolonko EM, Kiessling LL. Chemical probes of bacterial signal transduction reveal that repellents stabilize and attractants destabilize the chemoreceptor array. ACS Chem Biol. 2008;3:101–109. doi: 10.1021/cb700211s. [DOI] [PubMed] [Google Scholar]
- 85.Imae Y, Oosawa K, Mizuno T, Kihara M, Macnab RM. Phenol: a complex chemoeffector in bacterial chemotaxis. J Bacteriol. 1987;169:371–379. doi: 10.1128/jb.169.1.371-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pham HT, Parkinson JS. Phenol sensing by Escherichia coli chemoreceptors: a nonclassical mechanism. J Bacteriol. 2011;193:6597–6604. doi: 10.1128/JB.05987-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oosawa K, Imae Y. Glycerol and ethylene glycol: members of a new class of repellents of Escherichia coli chemotaxis. J Bacteriol. 1983;154:104–112. doi: 10.1128/jb.154.1.104-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Pina K, Navarro C, McWalter L, Boxer DH, Price NC, Kelly SM, Mandrand-Berthelot MA, Wu LF. Purification and characterization of the periplasmic nickel-binding protein NikA of Escherichia coli K12. Eur J Biochem. 1995;227:857–865. doi: 10.1111/j.1432-1033.1995.tb20211.x. [DOI] [PubMed] [Google Scholar]
- 89.Englert DL, Adase CA, Jayaraman A, Manson MD. Repellent taxis in response to nickel ion requires neither Ni2+ transport nor the periplasmic NikA binding protein. J Bacteriol. 2010;192:2633–2637. doi: 10.1128/JB.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eisenbach M, Constantinou C, Aloni H, Shinitzky M. Repellents for Escherichia coli operate neither by changing membrane fluidity nor by being sensed by periplasmic receptors during chemotaxis. J Bacteriol. 1990;172:5218–5224. doi: 10.1128/jb.172.9.5218-5224.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borrok MJ, Zhu Y, Forest KT, Kiessling LL. Structure-based design of a periplasmic binding protein antagonist that prevents domain closure. ACS Chem Biol. 2009;4:447–456. doi: 10.1021/cb900021q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Josenhans C, Suerbaum S. The role of motility as a virulence factor in bacteria. Int J Med Microbiol. 2002;291:605–614. doi: 10.1078/1438-4221-00173. [DOI] [PubMed] [Google Scholar]
- 93.Lux R, Moter A, Shi W. Chemotaxis in pathogenic spirochetes: directed movement toward targeting tissues? J Mol Microbiol Biotechnol. 2000;2:355–364. [PubMed] [Google Scholar]
- 94.Li C, Adler J. Escherichia coli shows two types of behavioral responses to osmotic upshift. J Bacteriol. 1993;175:2564–2567. doi: 10.1128/jb.175.9.2564-2567.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu B, Tu Y. Precision sensing by two opposing gradient sensors: how does Escherichia coli find its preferred pH level? Biophys J. 2013;105:276–285. doi: 10.1016/j.bpj.2013.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krikos A, Conley MP, Boyd A, Berg HC, Simon MI. Chimeric chemosensory transducers of Escherichia coli . Proc Natl Acad Sci USA. 1985;82:1326–1330. doi: 10.1073/pnas.82.5.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Slonczewski JL, Macnab RM, Alger JR, Castle AM. Effects of pH and repellent tactic stimuli on protein methylation levels in Escherichia coli . J Bacteriol. 1982;152:384–399. doi: 10.1128/jb.152.1.384-399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khan S, Spudich JL, McCray JA, Trentham DR. Chemotactic signal integration in bacteria. Proc Natl Acad Sci USA. 1995;92:9757–9761. doi: 10.1073/pnas.92.21.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Umemura T, Matsumoto Y, Ohnishi K, Homma M, Kawagishi I. Sensing of cytoplasmic pH by bacterial chemoreceptors involves the linker region that connects the membrane-spanning and the signal-modulating helices. J Biol Chem. 2002;277:1593–1598. doi: 10.1074/jbc.M109930200. [DOI] [PubMed] [Google Scholar]
- 100.Webb BA, Hildreth S, Helm RF, Scharf BE. Sinorhizobium meliloti chemoreceptor McpU mediates chemotaxis toward host plant exudates through direct proline sensing. Appl Environ Microbiol. 2014;80:3404–3415. doi: 10.1128/AEM.00115-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wolff C, Parkinson JS. Aspartate taxis mutants of the Escherichia coli tar chemoreceptor. J Bacteriol. 1988;170:4509–4515. doi: 10.1128/jb.170.10.4509-4515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bjorkman AM, Dunten P, Sandgren MO, Dwarakanath VN, Mowbray SL. Mutations that affect ligand binding to the Escherichia coli aspartate receptor: implications for transmembrane signaling. J Biol Chem. 2001;276:2808–2815. doi: 10.1074/jbc.M009593200. [DOI] [PubMed] [Google Scholar]
- 103.Lee L, Imae Y. Role of threonine residue 154 in ligand recognition of the tar chemoreceptor in Escherichia coli . J Bacteriol. 1990;172:377–382. doi: 10.1128/jb.172.1.377-382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang Y, Park H, Inouye M. Ligand binding induces an asymmetrical transmembrane signal through a receptor dimer. J Mol Biol. 1993;232:493–498. doi: 10.1006/jmbi.1993.1405. [DOI] [PubMed] [Google Scholar]
- 105.Gardina P, Conway C, Kossman M, Manson M. Aspartate and maltose-binding protein interact with adjacent sites in the Tar chemotactic signal transducer of Escherichia coli . J Bacteriol. 1992;174:1528–1536. doi: 10.1128/jb.174.5.1528-1536.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goers Sweeney E, Henderson JN, Goers J, Wreden C, Hicks KG, Foster JK, Parthasarathy R, Remington SJ, Guillemin K. Structure and proposed mechanism for the pH-sensing Helicobacter pylori chemoreceptor TlpB. Structure. 2012;20:1177–1188. doi: 10.1016/j.str.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pineda-Molina E, Reyes-Darias JA, Lacal J, Ramos JL, Garcia-Ruiz JM, Gavira JA, Krell T. Evidence for chemoreceptors with bimodular ligand-binding regions harboring two signal-binding sites. Proc Natl Acad Sci USA. 2012;109:18926–18931. doi: 10.1073/pnas.1201400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scott WG, Stoddard BL. Transmembrane signalling and the aspartate receptor. Structure. 1994;2:877–887. doi: 10.1016/s0969-2126(94)00088-3. [DOI] [PubMed] [Google Scholar]
- 109.Chervitz SA, Falke JJ. Molecular mechanism of transmembrane signaling by the aspartate receptor: a model. Proc Natl Acad Sci USA. 1996;93:2545–2550. doi: 10.1073/pnas.93.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moukhametzianov R, Klare JP, Efremov R, Baeken C, Goppner A, Labahn J, Engelhard M, Buldt G, Gordeliy VI. Development of the signal in sensory rhodopsin and its transfer to the cognate transducer. Nature. 2006;440:115–119. doi: 10.1038/nature04520. [DOI] [PubMed] [Google Scholar]
- 111.Milligan DL, Koshland DE., Jr Intrasubunit signal transduction by the aspartate chemoreceptor. Science. 1991;254:1651–1654. doi: 10.1126/science.1661030. [DOI] [PubMed] [Google Scholar]
- 112.Lynch BA, Koshland DE., Jr The fifth Datta Lecture. Structural similarities between the aspartate receptor of bacterial chemotaxis and the trp repressor of E. coli. Implications for transmembrane signaling. FEBS Lett. 1992;307:3–9. doi: 10.1016/0014-5793(92)80891-j. [DOI] [PubMed] [Google Scholar]
- 113.Ottemann KM, Xiao W, Shin YK, Koshland DE., Jr A piston model for transmembrane signaling of the aspartate receptor. Science. 1999;285:1751–1754. doi: 10.1126/science.285.5434.1751. [DOI] [PubMed] [Google Scholar]
- 114.Maruyama IN, Mikawa YG, Maruyama HI. A model for transmembrane signalling by the aspartate receptor based on random-cassette mutagenesis and site-directed disulfide cross-linking. J Mol Biol. 1995;253:530–546. doi: 10.1006/jmbi.1995.0571. [DOI] [PubMed] [Google Scholar]
- 115.Kim SH. “Frozen” dynamic dimer model for transmembrane signaling in bacterial chemotaxis receptors. Protein Sci. 1994;3:159–165. doi: 10.1002/pro.5560030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Falke JJ, Hazelbauer GL. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem Sci. 2001;26:257–265. doi: 10.1016/s0968-0004(00)01770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Y, Gardina PJ, Kuebler AS, Kang HS, Christopher JA, Manson MD. Model of maltose-binding protein/chemoreceptor complex supports intrasubunit signaling mechanism. Proc Natl Acad Sci USA. 1999;96:939–944. doi: 10.1073/pnas.96.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Falke JJ, Erbse AH. The piston rises again. Structure. 2009;17:1149–1151. doi: 10.1016/j.str.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Szurmant H, Bunn MW, Cho SH, Ordal GW. Ligand-induced conformational changes in the Bacillus subtilis chemoreceptor McpB determined by disulfide crosslinking in vivo. J Mol Biol. 2004;344:919–928. doi: 10.1016/j.jmb.2004.09.093. [DOI] [PubMed] [Google Scholar]
- 120.Manson MD. Not too loose, not too tight–just right. Biphasic control of the Tsr HAMP domain. Mol Microbiol. 2011;80:573–576. doi: 10.1111/j.1365-2958.2011.07578.x. [DOI] [PubMed] [Google Scholar]
- 121.Stewart V. The HAMP signal-conversion domain: static two-state or dynamic three-state? Mol Microbiol. 2014;91:853–857. doi: 10.1111/mmi.12516. [DOI] [PubMed] [Google Scholar]
- 122.Swain KE, Gonzalez MA, Falke JJ. Engineered socket study of signaling through a four-helix bundle: evidence for a Yin–Yang mechanism in the kinase control module of the aspartate receptor. Biochemistry. 2009;48:9266–9277. doi: 10.1021/bi901020d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Doebber M, Bordignon E, Klare JP, Holterhues J, Martell S, Mennes N, Li L, Engelhard M, Steinhoff HJ. Salt-driven equilibrium between two conformations in the HAMP domain from Natronomonas pharaonis: the language of signal transfer? J Biol Chem. 2008;283:28691–28701. doi: 10.1074/jbc.M801931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klare JP, Bordignon E, Engelhard M, Steinhoff HJ. Transmembrane signal transduction in archaeal phototaxis: the sensory rhodopsin II–transducer complex studied by electron paramagnetic resonance spectroscopy. Eur J Cell Biol. 2011;90:731–739. doi: 10.1016/j.ejcb.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 125.Wang J, Sasaki J, Tsai AL, Spudich JL. HAMP domain signal relay mechanism in a sensory rhodopsin-transducer complex. J Biol Chem. 2012;287:21316–21325. doi: 10.1074/jbc.M112.344622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou Q, Ames P, Parkinson JS. Biphasic control logic of HAMP domain signalling in the Escherichia coli serine chemoreceptor. Mol Microbiol. 2011;80:596–611. doi: 10.1111/j.1365-2958.2011.07577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou Q, Ames P, Parkinson JS. Mutational analyses of HAMP helices suggest a dynamic bundle model of input–output signalling in chemoreceptors. Mol Microbiol. 2009;73:801–814. doi: 10.1111/j.1365-2958.2009.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ames P, Zhou Q, Parkinson JS. HAMP domain structural determinants for signalling and sensory adaptation in Tsr, the Escherichia coli serine chemoreceptor. Mol Microbiol. 2014;91:875–886. doi: 10.1111/mmi.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lai RZ, Parkinson JS (2014) Functional suppression of HAMP domain signaling defects in the E. coli serine chemoreceptor. J Mol Biol. doi:10.1016/j.jmb.2014.08.003 [DOI] [PMC free article] [PubMed]
- 130.Lai WC, Beel BD, Hazelbauer GL. Adaptational modification and ligand occupancy have opposite effects on positioning of the transmembrane signalling helix of a chemoreceptor. Mol Microbiol. 2006;61:1081–1090. doi: 10.1111/j.1365-2958.2006.05296.x. [DOI] [PubMed] [Google Scholar]
- 131.Starrett DJ, Falke JJ. Adaptation mechanism of the aspartate receptor: electrostatics of the adaptation subdomain play a key role in modulating kinase activity. Biochemistry. 2005;44:1550–1560. doi: 10.1021/bi048089z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gosink KK, Zhao Y, Parkinson JS. Mutational analysis of N381, a key trimer contact residue in Tsr, the Escherichia coli serine chemoreceptor. J Bacteriol. 2011;193:6452–6460. doi: 10.1128/JB.05887-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ortega DR, Yang C, Ames P, Baudry J, Parkinson JS, Zhulin IB. A phenylalanine rotameric switch for signal-state control in bacterial chemoreceptors. Nat Commun. 2013;4:2881. doi: 10.1038/ncomms3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Massazza DA, Izzo SA, Gasperotti AF, Herrera Seitz MK, Studdert CA. Functional and structural effects of seven-residue deletions on the coiled-coil cytoplasmic domain of a chemoreceptor. Mol Microbiol. 2012;83:224–239. doi: 10.1111/j.1365-2958.2011.07928.x. [DOI] [PubMed] [Google Scholar]
- 135.Vaknin A, Berg HC. Direct evidence for coupling between bacterial chemoreceptors. J Mol Biol. 2008;382:573–577. doi: 10.1016/j.jmb.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vaknin A, Berg HC. Physical responses of bacterial chemoreceptors. J Mol Biol. 2007;366:1416–1423. doi: 10.1016/j.jmb.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Massazza DA, Parkinson JS, Studdert CA. Cross-linking evidence for motional constraints within chemoreceptor trimers of dimers. Biochemistry. 2011;50:820–827. doi: 10.1021/bi101483r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Briegel A, Beeby M, Thanbichler M, Jensen GJ. Activated chemoreceptor arrays remain intact and hexagonally packed. Mol Microbiol. 2011;82:748–757. doi: 10.1111/j.1365-2958.2011.07854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sferdean FC, Weis RM, Thompson LK. Ligand affinity and kinase activity are independent of bacterial chemotaxis receptor concentration: insight into signaling mechanisms. Biochemistry. 2012;51:6920–6931. doi: 10.1021/bi3007466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Frank V, Vaknin A. Prolonged stimuli alter the bacterial chemosensory clusters. Mol Microbiol. 2013;88:634–644. doi: 10.1111/mmi.12215. [DOI] [PubMed] [Google Scholar]
- 141.Salis H, Tamsir A, Voigt C. Engineering bacterial signals and sensors. Contrib Microbiol. 2009;16:194–225. doi: 10.1159/000219381. [DOI] [PubMed] [Google Scholar]
- 142.Bornscheuer UT, Pohl M. Improved biocatalysts by directed evolution and rational protein design. Curr Opin Chem Biol. 2001;5:137–143. doi: 10.1016/s1367-5931(00)00182-4. [DOI] [PubMed] [Google Scholar]
- 143.Derr P, Boder E, Goulian M. Changing the specificity of a bacterial chemoreceptor. J Mol Biol. 2006;355:923–932. doi: 10.1016/j.jmb.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 144.Zhao H. Directed evolution of novel protein functions. Biotechnol Bioeng. 2007;98:313–317. doi: 10.1002/bit.21455. [DOI] [PubMed] [Google Scholar]
- 145.Cadwell RC, Joyce GF (2006) Mutagenic PCR. CSH Protoc. doi:10.1101/pdb.prot4143 [DOI] [PubMed]
- 146.Coco WM, Levinson WE, Crist MJ, Hektor HJ, Darzins A, Pienkos PT, Squires CH, Monticello DJ. DNA shuffling method for generating highly recombined genes and evolved enzymes. Nat Biotechnol. 2001;19:354–359. doi: 10.1038/86744. [DOI] [PubMed] [Google Scholar]
- 147.Goldberg SD, Derr P, DeGrado WF, Goulian M. Engineered single- and multi-cell chemotaxis pathways in E. coli . Mol Syst Biol. 2009;5:283. doi: 10.1038/msb.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Feng X, Baumgartner JW, Hazelbauer GL. High- and low-abundance chemoreceptors in Escherichia coli: differential activities associated with closely related cytoplasmic domains. J Bacteriol. 1997;179:6714–6720. doi: 10.1128/jb.179.21.6714-6720.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Weerasuriya S, Schneider BM, Manson MD. Chimeric chemoreceptors in Escherichia coli: signaling properties of Tar–Tap and Tap–Tar hybrids. J Bacteriol. 1998;180:914–920. doi: 10.1128/jb.180.4.914-920.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Repik A, Rebbapragada A, Johnson MS, Haznedar JO, Zhulin IB, Taylor BL. PAS domain residues involved in signal transduction by the Aer redox sensor of Escherichia coli . Mol Microbiol. 2000;36:806–816. doi: 10.1046/j.1365-2958.2000.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kristich CJ, Glekas GD, Ordal GW. The conserved cytoplasmic module of the transmembrane chemoreceptor McpC mediates carbohydrate chemotaxis in Bacillus subtilis . Mol Microbiol. 2003;47:1353–1366. doi: 10.1046/j.1365-2958.2003.03375.x. [DOI] [PubMed] [Google Scholar]
- 152.Ward SM, Delgado A, Gunsalus RP, Manson MD. A NarX–Tar chimera mediates repellent chemotaxis to nitrate and nitrite. Mol Microbiol. 2002;44:709–719. doi: 10.1046/j.1365-2958.2002.02902.x. [DOI] [PubMed] [Google Scholar]
- 153.Xu Q, Black WP, Mauriello EM, Zusman DR, Yang Z. Chemotaxis mediated by NarX–FrzCD chimeras and nonadapting repellent responses in Myxococcus xanthus . Mol Microbiol. 2007;66:1370–1381. doi: 10.1111/j.1365-2958.2007.05996.x. [DOI] [PubMed] [Google Scholar]