Abstract
Inflammation serves as the first line of defense in response to tissue injury, guiding the immune system to ensure preservation of the host. The inflammatory response can be divided into a quick initial phase mediated mainly by innate immune cells including neutrophils and macrophages, followed by a late phase that is dominated by lymphocytes. Early in the new millennium, a key component of the inflammatory reaction was discovered with the identification of a number of cytosolic sensor proteins (Nod-like receptors) that assembled into a common structure, the ‘inflammasome’. This structure includes an enzyme, caspase-1, which upon activation cleaves pro-forms of cytokines leading to subsequent release of active IL-1 and IL-18. This review focuses on the role of IL-18 in inflammatory responses with emphasis on autoimmune diseases.
Keywords: IL-18, Canonical & non-canonical IL-18 production, Autoimmune and inflammatory diseases
Introduction
Cytokines comprise a vital system of signaling proteins necessary for communication between cells. From the perspective of the immune system, they are indispensable for the development and maturation of immune cells, regulation of responses, both activating and tolerogenic, and response against antigens and clearance of dying cells. For many years, cytokines were mainly grouped under T helper (Th) subclasses based on the type of T cell responses they participated in. However, with the discovery of cytokines, such as IL-18 and IL-33, that are not loyal to activation of Th-subsets, a broader view is needed to understand the diversity cytokines bring to health and disease.
Discovery
The cytokine IL-18 was initially identified as a protein in sera from mice treated with bacterium Propionibacterium acnes and challenged with LPS, which induces interferon γ (IFNγ) in naïve spleen cells [1]. Subsequently, IFNγ-inducing factor (IGIF) was cloned from livers of these mice. The IGIF protein, mainly produced by Kuppfer cells as a 192 amino acids (aa) precursor, is cleaved to a mature protein of 157 aa [2]. Recombinant IGIF was also found to induce T cell proliferation and augment natural killer (NK) cell cytotoxic activity in primary spleen cells. Close to the discovery of murine IGIF, human IGIF was cloned (using murine IGIF cDNA as a probe) and expressed in E. coli [3]. Human IGIF similarly induced IFNγ in peripheral blood mononuclear cells (PBMCs). Though structurally similar to IL-1 family cytokines, IGIF is functionally and structurally distinct, and it was subsequently renamed interleukin 18 (IL-18) [3]. The IL-18 gene is located on chromosome 9 in mouse [4] and on chromosome 11 in humans [5].
The IL-1 family currently contains 11 members: IL-1α, IL-1β, IL-1 receptor agonist (IL-Ra), IL-18, IL-1F5, IL-1F6, IL-1F7, IL-1F8, IL-1F9, IL-1F10, and IL-33 [6]. IL-18 shares similarities with certain IL-1 family members, especially IL-1β and the recently discovered IL-33. Firstly, like IL-1β, IL-18 precursor lacks a signal peptide and requires IL-1β-converting enzyme (ICE) or caspase-1 to cleave the pro-form of the protein for IL-18 to become biologically active. Secondly, both IL-18 and IL-1β (and other IL-1 family members) have a unique ‘β-trefoil’ structure (β-pleated sheet), even though they share just around 17 % sequence identity [7]. Thirdly, they utilize downstream signaling molecules such as IRAK, MyD88, and TRAF-6, and activate NFκB translocation linking these cytokines at the level of gene regulation.
Production and release of IL-18
Canonical pathway
IL-18 production has been detected from a variety of cells including Kuppfer cells [2], monocytes [3], dendritic cells (DC), macrophages [8], keratinocytes [9], chondrocytes, intestinal epithelial cells [10], and synovial fibroblasts and osteoblasts [11]. Some cells important for innate immunity such as macrophages and DC constitutively express IL-18. Most cytokines such as IL-2 and IL-7 have an N-terminal signal peptide that is involved in their transport, through endoplasmic reticulum and Golgi complex, in small secretory vesicles where they can be released or stored for future use [12]. Cytokines in the IL-1 family, however, lack a signal peptide and therefore are not secreted in the so-called ‘classical’ pathway. Instead, they are expressed as a precursor protein that accumulates within the cytosol following translation on free ribosomes. Upon activation, the pro-domain is cleaved to release the active cytokine. Gu and colleagues reported a key finding that ICE (caspase-1) which is part of the NLR complex, cleaves a 24-kDa pro-IL-18 at Asp35 resulting in a 6-kDa and an 18-kDa protein, where the later co-migrated with rIL-18 and stimulated IFNγ production [13].
Nod-like receptors (NLRs) are cytosolic proteins that can identify microbial and non-microbial signals. NLRs form large cytosolic complexes containing different combinations of proteins, called ‘inflammasomes’, which were first identified as a caspase-activating complexes essential for generation of functional IL-1β by cleaving its pro-form [14]. NLRs have three distinct domains: (1) leucine-rich repeats (LRRs) required for ligand sensing, (2) a nucleotide binding domain (NBD) required for oligomerization, and (3) an effector domain (pyrin domain (PYD), caspase-associated recruitment domain (CARD), or BIR domain). Among the known inflammasomes, NLRP3 (Nod-like receptor 3) has been studied extensively. It is comprised of NLRP3, ASC, and pro-caspase-1. ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) is an adaptor protein that binds NLRP3 with its PYD domain and pro-caspase-1 with its CARD domain [15, 16]. Upon sensing intracellular danger signals, NLRP3 oligomerizes and recruits the ASC domain [17]. Subsequently, ASC recruits pro-caspase-1 via its CARD domain to the NLRP3–ASC complex [18]. Danger signals from viruses [19], bacteria [20], fungi [21], toxins [22], and dying cells have been shown to result in release of active caspase-1 via NLRP3 inflammasome activation [23]. For additional information on variations in inflammasome activation, we refer readers to the literature which has a number of reviews on this subject [24].
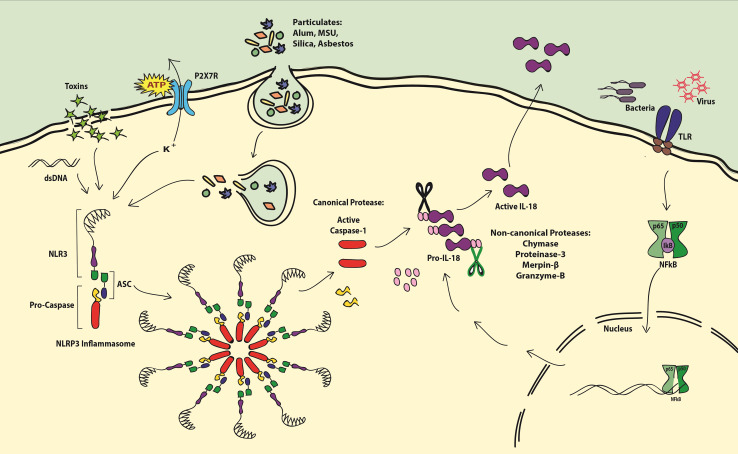
A common feature of NLRP3 activation is efflux of K+, which regulates lysosomal stability, where reduced K+ results in lysosomal damage and release of its contents [25]. Another characteristic feature of the NLRP3 inflammasome activation is ATP, which under homeostatic conditions is kept in balance by ATPases. However, ATP released from dying cells [26], cytotoxic T lymphocytes (CTLs) [27], microglia [28], and PAMP-primed monocytes [29] indicates ongoing stress. In effect, the extracellular ATP is bound by P2X7 receptor (P2X7R), which acts as a non-selective cation channel and in some cases forms large pores in the cell membrane [30]. It is well established that ATP bound to P2X7R results in production of active IL-1β [31] and IL-18 [29, 32]. Data proving this show that IL-18 release stimulated by ATP binding to P2X7R can be blocked by anti-P2X7R antibodies or oxidized ATP [33]. P2X7R appears to be crucial in the initiation of the IL-18-producing signal cascade as PBMCs from human subjects carrying a homozygous SNP in the gene (1513A/C) produced significantly lower IL-18 and were defective in ATP-induced uptake of ethidium+ when stimulated with LPS [34]. In relation to the other IL-1 family members, IL-18 is also evolutionarily close to IL-33 and shares many features of this cytokine. However, it has distinct features, where IL-33 is mostly released during necrosis, IL-18 needs cleavage by caspase-1 in a living cell to exert its function. After activation of caspase-1, IL-18 utilizes non-classical mechanisms for secretion. The proposed mechanisms include exocytosis of lysosomes via undefined protein transporters and release via microvesicles derived from plasma membrane blebs formed by evaginations (Fig. 1).
Fig. 1.
Production of IL-18. Inflammasome (NLRP3) is activated by several stimuli including extracellular ATP, toxins, PAMPs, particulates, and intracellular K+ efflux and dsDNA. Oligomerized NLRP3 inflammasome activates caspase-1 by cleaving pro-caspase. Pro-IL-18 is cleaved to produce active IL-18 by canonical protease caspase-1 or by non-canonical proteases such as chymase, proteinase-3, merpinβ, and granzyme-B. Pro-IL-18 production can be triggered by PAMPs via the activation of the NKκB pathway
Non-canonical production of active IL-18
Several studies suggest that caspase-1 is not the only IL-18-processing enzyme These additional non-canonical mechanisms include other caspases, granzyme B, proteinase 3, and merpins (Fig. 2).
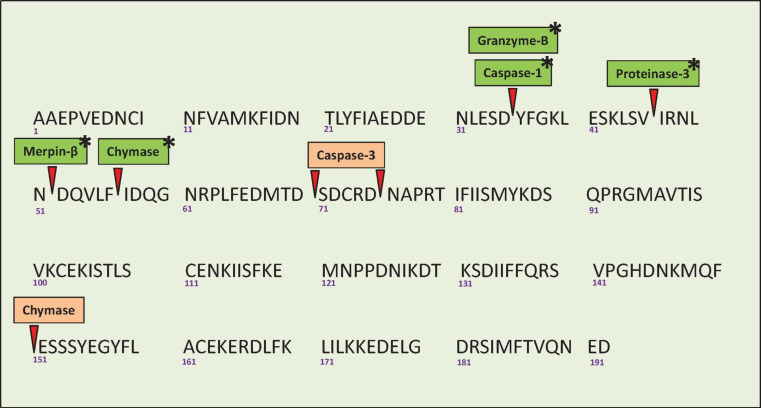
Fig. 2.
Protein sequence of human pro-IL-18 showing the sites of cleavage for various proteolytic enzymes (source of IL-18 protein sequence: ensemble.org). Caspase-1-, granzyme-B-, proteinase-3-, merpin-β-, and chymase-mediated cleavage of pro-IL-18 results in biologically active IL-18 (Indicated in green with an asterix). Caspase-3 cleaves pro-IL-18 into functionally inactive proteins that are degraded (orange). Chymase-mediated cleavage results in two isoforms: a functionally active p16 with Ile57 N-terminal and Asp191 C-terminal (green with an asterix) and an inactive p20 with Ala1 N-terminal and Glu151 C-terminal that is degraded rapidly (orange)
Production of active IL-18 by other caspases involves an important mechanism of cell-to-cell-induced death were the engagement Fas-Ligand (FasL) by Fas (CD95) results in apoptosis. This process is essential for immune homeostasis and for deletion of tumorous, infected, and damaged cells and maintenance of tolerance. Macrophages from livers (Kupffer cells) of P. acnes-treated mice were shown to express Fas and were able to secrete biologically active (IFNγ-inducing) IL-18 when co-cultured with constitutively FasL-expressing cells or recombinant FasL. As further proof of existence of a non-canonical pathway, IL-18 secretion was also observed in caspase-1-deficient mice [35]. However, when general caspase inhibitors were used IL-18 production was inhibited, indicating the presence of caspase enzymes other than caspase-1 that can process pro-IL-18 [35]. A recent study by Bossaller et al. [36] demonstrated that non-canonical, Fas-mediated activation of IL-1β and IL-18 required the adapter molecule FADD (Fas-associated protein with Death Domain) and caspase-8. Caspase-3 cleaves both precursor and mature hIL-18 and generates two biologically inactive forms [37].
The detection of pro-IL-18 in non-hematopoietic cells (which lack caspase) such as keratinocytes [21] indicates the presence of a caspase-independent mechanism of active IL-18 production. Granzyme B, a protease expressed in NK cells and CTLs, is an important inducer of cell death. Omoto and colleagues have demonstrated that granzyme B can cleave pro-IL-18 at the same position as caspase-1 to produce biologically active IL-18, and that this process can occur in caspase-1-deficient cells [38]. Proteinase 3 (PR3), a serine proteinase mainly expressed in neutrophils, but also in monocytes, basophils, and macrophages, is important in many inflammatory processes including induction of apoptosis and release of IL-1β. PR3, together with LPS, has been reported to induce the secretion of active IL-18 in human oral epithelial cells in the presence of caspase-1-specific and general caspase family inhibitors [39]. Another serine proteinase expressed by mast cells, chymase, can cleave human recombinant pro-IL-18 to produce two different fragments, p20 and p16. The p16 fragment was stable and induced IFNγ production, but the p20 fragment was produced and degraded rapidly. [40]. Finally, merpins are tissue-specific metalloproteinases expressed in intestinal and kidney epithelial cells capable of cleaving a variety of substrates. Merpin-β, but not merpin-α, can cleave pro-IL-18 to produce active IL-18 in vitro, and merpin-β KO mice have reduced levels of serum IL-18 [41]. Together, this diversity of enzymes (Fig. 2) that induce secretion of active IL-18 from cells (hematopoietic lineage or otherwise) shows the importance of IL-18 in inducing an inflammatory responses.
The IL-18 receptor and downstream signaling events
The IL-18 receptor (IL-18R) is a heterodimer consisting of IL-18Rα and β chains. IL-18Rα was first identified using a Hodgkin’s disease cell line (L428), which bound rIL-18 and was different from the IL-1β receptor. An amino acid sequence of purified rhIL-18Rα matched that of a previously identified orphan receptor, IL-1 receptor-related protein 1 (IL-1Rrp1). Mechanistically, biologically active IL-18 binds free IL-18Rα on the cell surface resulting in the recruitment of the non-binding IL18Rβ chain (accessory protein-like, AcPL) forming a complex that initiates intracellular signaling events [26, 28, 30, 42–47].
Similar to the IL-1 signaling pathway, the IL-18 receptor complex enlists myeloid differentiation factor 88 (MyD88). MyD88−/− mice lack IL-18-mediated responses and NFκB and c-Jun N-terminal kinase (JNK)-mediated AP-1 activation [48]. MyD88 is most known for its function as an adaptor molecule (in TLR and IL-1R signaling pathways [49]). Here, it anchors IL-1R-associated kinases (IRAK) to an IL-18R-MyD88 complex. Experimental evidence for this is that NFκB and AP-1 activation was attenuated in IRAK4−/− mice [50] and antisense IRAK1-treated HepG2 cells [51]. This indicates specific roles for these two molecules in the IL-18 signaling pathway. The N-proximal region of IRAK is required for the NFκB signaling but not the JNK pathway, indicating that the IL-18-induced signaling can potentially branch into two separate pathways from this step [52].
In the NFκB pathway, phosphorylated IRAK dissociates from the complex to bind tumor necrosis factor receptor-associated factor 6 (TRAF6) [53, 54]. TRAF6 is then thought to phosphorylate NFκB-inducing kinase (NIK) as in the IL-1 signaling pathway [55]. However, the role of NIK in the IL-18 signaling cascade is not known. NIK phosphorylates IκB kinases (IKK). The IκB kinase complex is made of IKKα, IKKβ (catalytic in action)- and IKKγ (regulatory subunit). Phosphorylated IKK are targeted for ubiquitination and degraded rapidly, leaving NFκB free to translocate into the nucleus and initiate transcription of target genes such as IFNγ [56].
Apart from the NFκB pathway, several other factors have been implicated in IL-18 signaling. Activation of a human NK cell line (NK92) with IL-18 resulted in phosphorylation of STAT3 and mitogen-activated protein kinases (MAPK) p44erk−1 and p42erk−2 [57]. In neutrophils stimulated with IL-18, the p38 MAPK pathway was activated along with MEK/ERK, phosphatidylinositol-3 kinase (Pi 3-Kinase), and NFκB signaling cascades [58, 59]. Another pathway includes Jak kinases that phosphorylate STATs and induce gene expression, and Tyk2 is one of the four mammalian Jaks. Mice that lack tyk2 have reduced NK cell cytotoxicity and IFNγ production. This has been attributed to reduced levels of IL-18 receptor in tyk2−/− mice [60]. IL-18 also induces ERK-dependent phosphorylation of transcription factor nuclear factor of activated T cells (NFATc4) (Fig. 3) [61].
Fig. 3.
IL-18 signaling pathway. Active IL-18 is bound by IL18Rα which results in the recruitment of IL-18Rβ to the signaling complex. IL-18Rβ recruits MyD88 to trigger NFκB signaling cascade resulting in the production of IFNγ. IL-18-induced signaling can also activate other signaling pathways such as ERK and MAPK kinase-dependent pathways. IL-18BP can bind IL-18 and neutralize it
IL-18 binding protein
Improper regulation of a potent cytokine like IL-18 can result in potentially harmful inflammatory responses. A way to regulate this is to produce an endogenous antagonist that counteracts the response. Two groups independently reported an antagonizing protein that can bind IL-18 in humans and mouse: the IL-18 binding protein (IL-18BP) [62, 63]. mIL-18BP showed a 60–66 % identity to the human protein and both are potent in blocking the IFNγ-inducing capability of IL-18. mRNA of IL-18BP was found in abundance in naive mouse and human spleen cells, and its expression was also detected in other human tissues such as thymus, small intestine, and PBMCs [31], and in mouse tissues such as heart, lung, and placenta [29].
Alternate splicing of the IL-18BP gene has been proposed to explain the existence of four human (hIL-18BPa to d) and two mouse (mIL-18BPc-d) isoforms. The ability of these isoforms to bind and neutralize IL-18 was attributed primarily to the presence of an Ig domain and secondarily to a 29 amino acid C-terminal. Indeed, hIL-18BPa isoform with an Ig domain and 29 aa C-terminal showed highest affinity to human rIL-18 (and mIL-18BP), followed by hIL-18BPc. hIL-18BPb, and hIL-18BPd, completely lacked the Ig domain, and therefore were ineffective in binding and neutralizing human rIL-18. mIL18-BPd and mIL18-BPc have an identical Ig domain enabling their murine rIL-18-neutralizing activity [64]. Detection of cDNA for hIL-18BPb and hIL-18BPd in the human blood monocyte library has led to the speculation that the preferable production of these isoforms could result in an ineffective regulation of IFNγ production during inflammatory responses [32]. Also, as proof of a feedback mechanism, IFNγ has been shown to induce the expression and release of IL-18BP in human carcinoma/epithelial cell lines, indicating a self-limiting regulation of effector functions by IL-18 [65].
Microbes often evolve to suppress the immune system. Several poxviruses express IL-18BP homologous proteins that bind and effectively neutralize IL-18 and impair IFNγ production and NK cell cytotoxicity, aiding viral propagation in the host [66–68]. On the other hand, in instances where IL-18 plays a pathological role, IL-18BP has also been implicated in protection against diseases such as collagen-induced arthritis [69, 70], contact hypersensitivity [71], and protection against LPS-induced liver damage [72]. IL-18BP has therefore been suggested as a potential candidate for therapy in regulating Th1 responses. Also, when measuring serum levels of IL-18, the level of IL-18BP can give an exact value of free functional cytokine. Measuring IL-18BP levels may be an important indicator of the ongoing immune response, as sepsis patients were found to have significantly higher levels of IL-18BP and free IL-18 compared to healthy controls [73].
IL-18 in autoimmune diseases
Pleiotropic effects of IL-18, specifically the induction of IFNγ, is essential for immunity against invading pathogens. However, improper regulation of IL-18 can potentially lead to inflammation and destruction of self.
Type 1 diabetes (T1D)
Type 1 diabetes results from autoimmune destruction of insulin-producing pancreatic β cells. Of interest, genes for both mouse and human IL-18 are located in regions associated with susceptibility to T1D (Idd2) [6, 74]. Studies on the association of single nucleotide polymorphisms (SNP) in the promoter region of the human IL-18 gene (-607 (C/A) and -137 (G/C) reported varying strengths of association among different patient groups. Earlier studies have reported positive association of these SNPs with T1D [75, 76]. However, a recent study involving a large cohort, with thousands of patients and control samples, failed to show any association [77]. Polymorphisms in the human IL-18BP promoter, exon, and 3′-UTR regions were also not associated with T1D [78]. In one of the early reports on IL-18 in human T1D, elevated levels of IL-18 were detected in serum of 14 % of newly diagnosed T1D patients. Interestingly, 68 % of the first-degree relatives of T1D patients who had tested positive for two or more T1D associated autoantibodies, but had not yet developed the disease, also tested positive for serum IL-18. This indicates an early and potential predictive role of IL-18 measures in human disease [79]. Two separate studies have also reported increased serum IL-18 levels in patients, and this increase was correlated with glycated hemoglobin (HbA1C) levels suggesting a link between hyperglycemia and IL-18 [80, 81]. Increased serum IL-18 has been associated with diabetic kidney damage (nephropathy) [82], diabetic ketoacidosis [83], and microangiopathy [84].
In non-obese diabetic (NOD) mice, a well-studied model for autoimmune diabetes, systemic IL-18 mRNA was linked to progression from benign to destructive insulitis [85]. Administration of exogenous IL-18 to 10-week old female NOD mice has been reported to protect from insulitis/development of diabetes [86]. However, administration of IL-18 in the form of IL-18 expressing plasmid delivery promoted the disease in 4-week old NOD mice [87]. This stark contrast can be attributed to the double role of IL-18 as a regulator of Th1 and Th2 responses based on the existing cytokine environment [88]. IL-18 has minor effects on normal function of rat islet β cells [89], while in response to inflammatory stimuli, they express IL-18 mRNA, but not IL-18 receptor mRNA. This is thought to exacerbate their ongoing autoimmune destruction [90, 91]. Surprisingly, the IL-18 processing enzyme caspase-1 was not required for development of spontaneous or streptozotocin-induced disease in NOD mice [92]. A recent study demonstrated that IL-18−/− NOD mice had reduced islet reactive T cells compared to WT NOD mice. The authors observed that IL-18 is required for islet reactive T cell expansion as transferred CD8+ islet reactive T cells (from 8.3 NODScid mouse) into IL18−/− NOD mice proliferated significantly less compared to those transferred to WT NOD mice. This defective proliferation could be corrected partially by injection of rIL-18. The number of CD44hi, CD4+, and CD8+ T cells expressing IL-18Rα were significantly lower in IL18−/− NOD mice, indicating that IL-18 responsiveness is a hallmark of activated T cells. This study provides evidence indicating that IL-18 shifts the cytokine profile of effector T cells from IL-17F-producing to IFNγ-producing cells, highlighting a novel role for IL-18 in the pathogenesis of T1D [93]. The role of IL-18 in T1D is further strengthened by evidence that incidence of the disease is significantly reduced by blocking IL-18 with an IL-18BP-Fc fusion molecule in cyclophosphamide-induced and diabetogenic cell transferred-induced disease models [94]. In summary, results from animal and human studies indicate a role for IL-18 in T1D disease pathogenesis which could primarily be its IFNγ-inducing capability.
Multiple sclerosis (MS)
Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system (CNS) where myelinated axons are progressively destroyed. Initially, the disease is characterized by reversible neurological deficits followed by extensive deterioration of the CNS. The study of MS has been facilitated by experimental autoimmune encephalomyelitis (EAE) in mice, where demyelinating inflammation, similar to human MS, is induced by administration of antigens such as myelin basic protein (MBP) and myelin oligodendrocyte glycoprotein (MOG). In EAE mice, caspase-1 mRNA levels in blood correlated with disease severity. Also, caspase-1−/− mice were significantly protected from the disease and administration of caspase inhibitors gave a similar effect [95]. In addition, IL-18−/− mice were resistant to MOG peptide-induced EAE and displayed defective autoreactive Th1 responses and had lower levels of circulating anti-MOG35–55 IgG and IgG2b [96]. However, another study reported that IL-18−/− mice and WT mice were equally susceptible to EAE, but mice lacking IL-18Rα were resistant to EAE [97]. Differences in animal health status and degree of backcrossing onto the C57BL/6 background have been speculated to be the reason for this discrepancy. More importantly, antibodies blocking (but not depleting) IL-18Rα in IL-18−/− mice rendered them disease-resistant, indicating the existence of IL-18Rα ligands other than IL-18. IL-18Rα-deficient mice lacked the presence of persistent immune cells such as CD4+ T cells, B cells, macrophages, and granulocytes from CNS. IL-18Rα signaling on APCs was critical for the secretion of IL-23p40, a factor essential for maintaining pathogenic IL-17 secreting effector T cells [61].
Support for the existence of alternate ligands for IL-18Rα comes from studies where spleen cells from IL-18Rα−/− mice produced significantly more pro-inflammatory cytokines compared to those from WT or IL-18−/− mice in response to concanavalin A stimulation [98]. Further, another study from the same group showed that mouse embryonic fibroblast (MEF) cells lacking IL-18Rα produced more proinflammatory cytokines in response to IL-1β and TNFα compared to WT or those that lacked IL-18. An effect they could reproduce by blocking IL-18Rα using antibodies or siRNA. MEF cells lacking IL-18Rα displayed reduced suppressor of cytokine signaling (SOCS) -1 and -3 at mRNA and protein levels. These data indicate the existence of one or more alternate IL-18Rα ligands that deliver an anti-inflammatory signal through SOCS-1 and SOCS-3 signaling [99].
In cerebrospinal fluid cells and PBMCs of MS patients IL-18R1 mRNA was significantly increased compared to controls, but SNPs in the IL-18R1 gene were not associated with the disease in a large case–control study. The authors speculate that the IL-18R1 gene could be regulated by components from other parts of the genome or that it is a part of the MS inflammatory cascade [100]. Furthermore, IL-18-neutralizing IL-18BP was found up-regulated in CNS-resident microglial cells in an IFNγ-dependent manner [101]. IFNγ−/−, IL-18−/−, and WT mice treated with adenoviral vector-expressing IL-18BP displayed significantly reduced disease incidence and severity, and this correlated with inhibition of TH17 responses. Another possible mechanism is that IL-18Rα is free to bind its alternate, anti-inflammatory ligand(s) as IL-18 is neutralized by IL-18BP.
In relation to disease progression, human MS patients with chronic progressive MS had the highest levels of serum IL-18, followed by those with acute relapsing–remitting MS and stable relapsing–remitting MS [102, 103]. Increased caspase-1 levels were also reported in cerebrospinal fluid from patients with acute MS [104]. In MS patients who never received any immunomodulatory drugs, mRNA and protein levels of caspase-1 and IL-18 were detected in PBMCs [105]. In vitro stimulation of PBMCs from MS patients with anti-CD3/CD28 resulted in significantly higher IL-18 production, especially in those suffering from secondary progressive MS. CD4+, but not CD8+, T cells via CD40–CD40L interaction were responsible for this IL-18 production subsequently resulting in higher IFNγ levels in MS patients [106]. These studies indicate an early role for IL-18 in mediating an autoimmune response, via Th1 and Th17 effector pathways. Data from acute relapsing–remitting MS patients indicate that IL-18 is also essential for progression of the disease. However, results from EAE model indicate a more prominent role of the IL-18Rα in disease pathogenesis, suggesting the existence of another ligand with potential role for regulating inflammation in the CNS.
Myasthenia gravis (MG)
Myasthenia gravis (MG), an autoimmune disease affecting the neuromuscular junctions, is mediated by autoantibodies against the nicotinic acetylcholine receptor (AChR). Experimental autoimmune MG (EAMG) can be induced in mice by treating with AChR in complete Freund’s adjuvant. Disease development in humans and rodents is dependent on T cell help to B cells in autoantibody production [107]. IL-18 adds strongly to the pathogenesis as IL-18−/− mice are resistant to EAMG [108]. Blocking IL-18 using anti-IL-18 antibodies or the CD40–CD40L interaction effectively reduced disease severity, even in acute and chronic stages. This effect was attributed to up-regulated TGF-β and CTLA-4 and down-regulation of Th1 cytokines and CD40L [88, 109]. Treatment of EAMG rats with ex vivo-generated regulatory T cells resulted in reduced disease incidence and severity and a reduction in mRNA levels of IL-10 and IL-18 [110]. MG patients had higher levels of serum IL-18, and in subjects treated with immunosuppressive drugs, serum IL-18 was significantly reduced compared to that at disease diagnosis [111].
Rheumatoid arthritis (RA)
The role of IL-18 in RA has been extensively reviewed by Volin and Koch [112]; therefore, in this section, we will briefly discuss the key findings. Rheumatoid arthritis is a Th1-driven chronic, systemic inflammatory disease primarily targeting the synovial joints. In relation to pathology, IL-18 mRNA and protein expression was detected in synovial tissue from RA patients but not in those suffering from osteoarthritis (joint disorder due to aging). The severity of inflammation and cartilage destruction in collagen-induced arthritis (CIA) in mice was enhanced by administration of rIL-18 [113] and the same was dramatically reduced in IL-18−/− mice [114]. Furthermore, CIA was restored in IL-18−/− mice treated with rIL-18 [71]. Neutralizing IL-18 in mouse CIA, either by anti-IL-18 IgG antibodies or by rhIL-18BP, resulted in significant reduction of the disease phenotype [72].
Synovial fibroblasts are thought to be play an important role in the pathogenesis of RA, and they, in many ways, distinguish RA from other joint diseases [115]. In vitro stimulation of RA synovial fibroblasts with IL-18-induced the expression of surface vascular cell adhesion molecule-1 (VCAM-1, essential for leukocyte–connective tissue interaction) [116] and neutrophil chemoattractants [117]. IL-18 response is regulated in a positive feedback loop in RA where synovial fibroblasts and macrophages produce IL-18, which stimulates synovial macrophages to produce TNFα, which in turn acts on synovial fibroblasts to produce more IL-18. Apart from mediating inflammation and recruitment of leukocytes to the joint, IL-18 also induces angiogenesis [118, 119]. Taken together, these data thus indicate an important role for IL-18 in RA and especially in the affected tissue of the joint.
Psoriasis
Psoriasis is a chronic inflammatory disease of the skin. Human keratinocytes have been shown to constitutively express IL-18 protein that was able to induce IFNγ production in PBMCs. Immunohistochemistry data from skin sections in healthy and psoriasis patients showed increased levels of IL-18 protein and caspase-1 in the latter [120–122]. IL-18 protein levels were higher in active/progressive psoriatic lesions compared with those that are stable and established [123]. Increased levels of IL-18 were also observed in serum from patients [124]. Human Langerhans cells (epidermal DC) express IL-18R and, when stimulated by IL-18, display filamentous actin polymerization as a sign of increased migratory tendency [125]. Also in connection to the skin, stimulation of human keratinocyte cell line HaCaT with UVB radiation induced IL-18 production and this was dependent on reactive oxygen intermediates [126]. In a recent study on primary human keratinocytes, IL-18BP was up-regulated upon stimulation with IL-27 [127]. These data suggest that IL-18 is involved in early stages of psoriatic inflammatory response and also suggest the potential use of IL-18BP in therapeutic intervention.
Systemic lupus erythematosus (SLE)
SLE is connected to the inability to clear and respond correctly to dying apoptotic cells. As IL-18 secretion is part of the response to cell death, it has several connections to this systemic disease. Several studies reported increased serum IL-18 in SLE patients [128–130]. Patients with Lupus nephritis (LN) had higher levels of serum IL-18 compared to those without LN, and kidney biopsies from the former showed IL-18-positive glomeruli [130]. In a mouse model for SLE (lpr/lpr), lymph node cells were found hyper-responsive to IL-18 (increased IFNγ production). Interestingly, the IL-18Rα was similarly expressed in lpr/lpr and control mice, but the IL-18Rβ chain was significantly increased in the lpr/lpr mice. IL-18Rβ chain is the signal-inducing component of the IL-18 receptor complex, and therefore Th1-mediated disease in these mice could be due to increased IFNγ induction [131]. In SLE prone lpr/lpr mice vaccinated with a cDNA vector expressing IL-18, auto-antibodies to IL-18 were generated in vivo, and these mice had reduced IFNγ and renal damage. Also, mortality was significantly delayed compared to empty vector vaccinated mice [132]. Data from lpr/lpr mice from a young age showed increased levels of IL-18, especially evident in the lymph nodes. Interestingly, IL-18BP mRNA was also higher, but detectable protein levels of the same were very low, indicating unstable protein expression or rapid degradation. These findings suggest that imbalance in the regulation of IL-18 (via IL-18BP) can be another cause for disease in mice [133].
IL-18 in inflammatory disorders
Atopic eczema (AE)
Atopic eczema (AE) is a chronic inflammatory skin disease with complex etiology including genetic predisposition and environmental triggers such as infection. The disease etiology is thought to be driven initially by a Th2-like response involving IL-4 and IL-13, followed by a Th1-like response [134]. Elevated levels of IL-18 have been reported in serum from AE patients and in a mouse model for the disease [135]. The yeast Malassezia furfur is a known trigger for AE, and stimulation of monocyte-derived DC from human PBMCs with M. furfur resulted in increased IL-18 production along with TNFα and IL-1β [136]. We have previously reported increased free IL-18 levels in plasma of AE patients. However, the levels IL-18BP were similar to that in healthy controls, indicating a role for free IL-18 in disease pathogenesis.
In relation to allergy, IL-18 can also act as a Th2 response inducer. Injection of IL-12 and IL-18 inhibits IgE production in helminth-infected WT mice. In contrast, IL-12+IL-18 induced IgE in IFNγ−/− mice. Also, IL-18 alone was found to induce IL-4 from basophils in both WT and IFNγ−/− mice [18]. Furthermore, IL-18-induced IgE response was found to be dependent on CD4+ T cells, IL-4, and STAT6 [17, 137].
Administration of IL-18 to mice lacking CD4+ T cells or class II−/− failed to induce an IgE response, indicating a role for CD1d restricted, natural killer T (NKT) and CD4+ T cells. As NKT cells express high levels of IL-18Rα and CD4+ T cells are poor expressers of the same, it has been speculated that IL-18-stimulated NKT cells are the major source of IL-4 required for CD4+ T cell help to B cells in IgE production [137]. In a recent report, we have demonstrated that IL-18 induced self-reactive IgM, IgG, and IgE responses in mice. Innate-like B cells that produce these antibodies are located in the spleen [marginal zone B cells (MZB)] and peritoneum (B1a cells). We observed that ex vivo stimulation of IL-18-treated mouse spleen and peritoneal cells results in IgM, IgG, and IgE production from spleen but not the peritoneal cells, indicating a role for MZBs in self-reactive antibody response. In vivo, IL-18 induced MZB expansion and increased their CD1d expression, and resulted in isotype switch and production of innate antibodies in extracellular foci and immature germinal centers (GC). Interestingly, mice lacking NKT cells had significantly higher IgE responses, an increased rate of plasma cell differentiation, and a shift towards mature GC phenotype, indicating that NKT cells regulate innate B cell responses [138]. IL-21 produced by NKT cells has been shown to activate NKT cells cytotoxicity and prevent IgE production [139, 140]. Indeed, we observed an increase of IL-21 expression in spleen after IL-18 injections. Cytotoxicity of NKT cells is brought about by release of perforin and up-regulation of FasL (CD178). NKT cell-dependent killing of B cells has been linked to the expression of lipid ligand-presenting CD1d molecules on their surface [141]. IL-18-injected perforin−/− or FasL−/− mice had increased IgE production. These data suggest that NKT cells regulate IL-18-induced B cell activation by preventing self-reactive B cells from entering GCs [138].
In humans, rIL-18 or IL-18 from AE patient serum was able to activate NFκB signaling in iNKT cell lines (derived from PBMCs) even in the absence of exogenous lipid ligands for iNKT cells [142]. Prolonged stimulation of iNKT cell lines with IL-18 (levels similar to those observed in AE patients) resulted in a selective decrease in the CD4+ iNKT cell population, a phenomenon we detected in AE patients. These data indicate that IL-18 contributes to AE pathogenesis by activating iNKT cells and skewing their population in a CD1d-dependent manner [142].
Atherosclerosis
Atherosclerosis is chronic inflammatory disease of the vascular system. A typical atherosclerotic plaque contains a lipid core and immune cells such as macrophages, T cells, and mast cells. Plaque rupture, followed by platelet aggregation and coagulation, results in thrombus (blood clot) formation that can block the lumen of the blood vessel, resulting in myocardial infarction and ischemic stroke [143]. Higher concentrations of IL-18 were first observed in patients with myocardial infarction [144]. Shortly afterwards, Mallat et al. [145] reported localization of IL-18 to plaque macrophages and increased IL-18R expression in plaque macrophages and endothelial cells in human atherosclerotic plaques. Interestingly, they noticed significantly higher levels of IL-18 mRNA in unstable, symptomatic plaques compared to stable, asymptomatic ones [145]. Administration of plasmid encoding IL-18BP to apolipoprotein-E−/− (ApoE−/−) mice resulted in a stable plaque phenotype and reduced atherosclerosis [146]. Administration of rIL-18 to ApoE−/− mice resulted in IFNγ-dependent exacerbation of the disease, as IFNγ−/− ApoE−/− mice treated with IL-18 had significantly reduced lesions [147]. ApoE−/− and IL-18−/− double-knockout mice displayed reduced atherosclerosis. Although they had higher serum cholesterol, IFNγ signaling was reduced and plaque stability was increased [148]. IL-18 treatment of SCID/ApoE−/− mice induced atherosclerotic lesions and IFNγ production, indicating that IL-18 can induce atherosclerosis even in the absence of T cells, particularly by macrophages and NK cells in this case [149]. These data suggest a critical role for IL-18, especially in its ability to induce IFNγ production. Angiotensin-II, an atherogenic protein, has been shown to enhance IL-18-induced inflammatory responses by increasing IL-18Rα expression [150]. IL-18 has been suggested as a biomarker for predicting cardiovascular disease [151]; however, contrasting data in this regard warrant further studies [152].
Future perspectives
As a general theme, IL-18 plays a pathogenic role in all so far studied inflammatory diseases either by promoting Th1- or Th2-related responses. For the future, it will be essential to define the definite steps involved, firstly in IL-18-mediated signaling cascades involved in various diseases, and secondly to identify the triggers for activation of such pathways. Of particular interest would be the study of innate cells such as macrophages and NKT cells that produce and respond to IL-18, respectively, in different types of immune responses. Further, the role of IL-18BP and its regulation in inflammatory conditions would open new avenues for therapeutic approaches. Also, the evidence for the existence of anti-inflammatory, alternate ligand(s) for IL-18Rα warrants further investigation. To summarize, restoring the immune homeostasis through specific regulation of the action of IL-18 in inflammatory conditions could pave way for effective therapies in the future.
Acknowledgments
We thank the Swedish Research Council, Torsten Söderberg foundation, Swedish Cancer foundation, King V’s 80 year foundation, Swedish Rheumatism association, Theme Centre for Cardiovascular Research, Karolinska Instituet, Swedish Society for Medical Research (SSMF), O.E. and Edla Johanssons Foundation, Center for Allergy Research at Karolinska Institutet (CfA), Svenska Sällskapet för Medicinsk Forskning and Olle Engkvist Foundations for research grant support. T.H. is supported by a PhD fellowship from Karolinska Institutet.
Contributor Information
Saikiran K. Sedimbi, Email: Saikiran.Sedimbi@ki.se
Mikael C. I. Karlsson, Email: Mikael.Karlsson@ki.se
References
- 1.Nakamura K, Okamura H, Nagata K, Komatsu T, Tamura T. Purification of a factor which provides a costimulatory signal for gamma interferon production. Infect Immun. 1993;61:64–70. doi: 10.1128/iai.61.1.64-70.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 3.Ushio S, Namba M, Okura T, Hattori K, Nukada Y, Akita K, Tanabe F, Konishi K, Micallef M, Fujii M, et al. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–4279. [PubMed] [Google Scholar]
- 4.Rothe H, Jenkins NA, Copeland NG, Kolb H. Active stage of autoimmune diabetes is associated with the expression of a novel cytokine, IGIF, which is located near Idd2. J Clin Invest. 1997;99:469–474. doi: 10.1172/JCI119181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolan KF, Greaves DR, Waldmann H. The human interleukin 18 gene IL18 maps to 11q22.2-q22.3, closely linked to the DRD2 gene locus and distinct from mapped IDDM loci. Genomics. 1998;51:161–163. doi: 10.1006/geno.1998.5336. [DOI] [PubMed] [Google Scholar]
- 6.Barksby HE, Lea SR, Preshaw PM, Taylor JJ. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. 2007;149:217–225. doi: 10.1111/j.1365-2249.2007.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato Z, Jee J, Shikano H, Mishima M, Ohki I, Ohnishi H, Li A, Hashimoto K, Matsukuma E, Omoya K, et al. The structure and binding mode of interleukin-18. Nat Struct Biol. 2003;10:966–971. doi: 10.1038/nsb993. [DOI] [PubMed] [Google Scholar]
- 8.Stoll S, Jonuleit H, Schmitt E, Muller G, Yamauchi H, Kurimoto M, Knop J, Enk AH. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur J Immunol. 1998;28:3231–3239. doi: 10.1002/(SICI)1521-4141(199810)28:10<3231::AID-IMMU3231>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 9.Stoll S, Muller G, Kurimoto M, Saloga J, Tanimoto T, Yamauchi H, Okamura H, Knop J, Enk AH. Production of IL-18 (IFN-gamma-inducing factor) messenger RNA and functional protein by murine keratinocytes. J Immunol. 1997;159:298–302. [PubMed] [Google Scholar]
- 10.Takeuchi M, Nishizaki Y, Sano O, Ohta T, Ikeda M, Kurimoto M. Immunohistochemical and immuno-electron-microscopic detection of interferon-gamma-inducing factor (“interleukin-18”) in mouse intestinal epithelial cells. Cell Tissue Res. 1997;289:499–503. doi: 10.1007/s004410050895. [DOI] [PubMed] [Google Scholar]
- 11.Udagawa N, Horwood NJ, Elliott J, Mackay A, Owens J, Okamura H, Kurimoto M, Chambers TJ, Martin TJ, Gillespie MT. Interleukin-18 (interferon-gamma-inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-gamma to inhibit osteoclast formation. J Exp Med. 1997;185:1005–1012. doi: 10.1084/jem.185.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood. 2011;118:9–18. doi: 10.1182/blood-2010-08-265892. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 14.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 15.Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J, Reed JC. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J Immunol. 2003;171:6154–6163. doi: 10.4049/jimmunol.171.11.6154. [DOI] [PubMed] [Google Scholar]
- 16.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan JA, Gao X, Huang MT, O’Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182:6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 22.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 23.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 24.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 25.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 26.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 27.Filippini A, Taffs RE, Sitkovsky MV. Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc Natl Acad Sci USA. 1990;87:8267–8271. doi: 10.1073/pnas.87.21.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 31.Dubyak GR. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol. 2012;14:1697–1706. doi: 10.1111/cmi.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perregaux DG, McNiff P, Laliberte R, Conklyn M, Gabel CA. ATP acts as an agonist to promote stimulus-induced secretion of IL-1 beta and IL-18 in human blood. J Immunol. 2000;165:4615–4623. doi: 10.4049/jimmunol.165.8.4615. [DOI] [PubMed] [Google Scholar]
- 33.Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- 34.Sluyter R, Dalitz JG, Wiley JS. P2X7 receptor polymorphism impairs extracellular adenosine 5′-triphosphate-induced interleukin-18 release from human monocytes. Genes Immun. 2004;5:588–591. doi: 10.1038/sj.gene.6364127. [DOI] [PubMed] [Google Scholar]
- 35.Tsutsui H, Kayagaki N, Kuida K, Nakano H, Hayashi N, Takeda K, Matsui K, Kashiwamura S, Hada T, Akira S, et al. Caspase-1-independent, Fas/Fas ligand-mediated IL-18 secretion from macrophages causes acute liver injury in mice. Immunity. 1999;11:359–367. doi: 10.1016/s1074-7613(00)80111-9. [DOI] [PubMed] [Google Scholar]
- 36.Bossaller L, Chiang PI, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VA, Mocarski ES, Subramanian D, Green DR, Silverman N, et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akita K, Ohtsuki T, Nukada Y, Tanimoto T, Namba M, Okura T, Takakura-Yamamoto R, Torigoe K, Gu Y, Su MS, et al. Involvement of caspase-1 and caspase-3 in the production and processing of mature human interleukin 18 in monocytic THP.1 cells. J Biol Chem. 1997;272:26595–26603. doi: 10.1074/jbc.272.42.26595. [DOI] [PubMed] [Google Scholar]
- 38.Omoto Y, Yamanaka K, Tokime K, Kitano S, Kakeda M, Akeda T, Kurokawa I, Gabazza EC, Tsutsui H, Katayama N, et al. Granzyme B is a novel interleukin-18 converting enzyme. J Dermatol Sci. 2010;59:129–135. doi: 10.1016/j.jdermsci.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Sugawara S, Uehara A, Nochi T, Yamaguchi T, Ueda H, Sugiyama A, Hanzawa K, Kumagai K, Okamura H, Takada H. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J Immunol. 2001;167:6568–6575. doi: 10.4049/jimmunol.167.11.6568. [DOI] [PubMed] [Google Scholar]
- 40.Omoto Y, Tokime K, Yamanaka K, Habe K, Morioka T, Kurokawa I, Tsutsui H, Yamanishi K, Nakanishi K, Mizutani H. Human mast cell chymase cleaves pro-IL-18 and generates a novel and biologically active IL-18 fragment. J Immunol. 2006;177:8315–8319. doi: 10.4049/jimmunol.177.12.8315. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee S, Bond JS. Prointerleukin-18 is activated by meprin beta in vitro and in vivo in intestinal inflammation. J Biol Chem. 2008;283:31371–31377. doi: 10.1074/jbc.M802814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torigoe K, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikata T, Murakami T, Sanou O, Kojima H, Fujii M, et al. Purification and characterization of the human interleukin-18 receptor. J Biol Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- 43.Hoshino K, Tsutsui H, Kawai T, Takeda K, Nakanishi K, Takeda Y, Akira S. Cutting edge: generation of IL-18 receptor-deficient mice: evidence for IL-1 receptor-related protein as an essential IL-18 binding receptor. J Immunol. 1999;162:5041–5044. [PubMed] [Google Scholar]
- 44.Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 45.Thomassen E, Bird TA, Renshaw BR, Kennedy MK, Sims JE. Binding of interleukin-18 to the interleukin-1 receptor homologous receptor IL-1Rrp1 leads to activation of signaling pathways similar to those used by interleukin-1. J Interf Cytokine Res. 1998;18:1077–1088. doi: 10.1089/jir.1998.18.1077. [DOI] [PubMed] [Google Scholar]
- 46.Born TL, Thomassen E, Bird TA, Sims JE. Cloning of a novel receptor subunit, AcPL, required for interleukin-18 signaling. J Biol Chem. 1998;273:29445–29450. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- 47.Cheung H, Chen NJ, Cao Z, Ono N, Ohashi PS, Yeh WC. Accessory protein-like is essential for IL-18-mediated signaling. J Immunol. 2005;174:5351–5357. doi: 10.4049/jimmunol.174.9.5351. [DOI] [PubMed] [Google Scholar]
- 48.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 49.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki N, Chen NJ, Millar DG, Suzuki S, Horacek T, Hara H, Bouchard D, Nakanishi K, Penninger JM, Ohashi PS, et al. IL-1 receptor-associated kinase 4 is essential for IL-18-mediated NK and Th1 cell responses. J Immunol. 2003;170:4031–4035. doi: 10.4049/jimmunol.170.8.4031. [DOI] [PubMed] [Google Scholar]
- 51.Guo F, Wu S. Antisense IRAK-1 oligonucleotide blocks activation of NF-kappa B and AP-1 induced by IL-18. Immunopharmacology. 2000;49:241–246. doi: 10.1016/s0162-3109(00)00200-9. [DOI] [PubMed] [Google Scholar]
- 52.Wald D, Commane M, Stark GR, Li X. IRAK and TAK1 are required for IL-18-mediated signaling. Eur J Immunol. 2001;31:3747–3754. doi: 10.1002/1521-4141(200112)31:12<3747::aid-immu3747>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 53.Kojima H, Takeuchi M, Ohta T, Nishida Y, Arai N, Ikeda M, Ikegami H, Kurimoto M. Interleukin-18 activates the IRAK-TRAF6 pathway in mouse EL-4 cells. Biochem Biophys Res Commun. 1998;244:183–186. doi: 10.1006/bbrc.1998.8236. [DOI] [PubMed] [Google Scholar]
- 54.Kanakaraj P, Ngo K, Wu Y, Angulo A, Ghazal P, Harris CA, Siekierka JJ, Peterson PA, Fung-Leung WP. Defective interleukin (IL)-18-mediated natural killer and T helper cell type 1 responses in IL-1 receptor-associated kinase (IRAK)-deficient mice. J Exp Med. 1999;189:1129–1138. doi: 10.1084/jem.189.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 56.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 57.Kalina U, Kauschat D, Koyama N, Nuernberger H, Ballas K, Koschmieder S, Bug G, Hofmann WK, Hoelzer D, Ottmann OG. IL-18 activates STAT3 in the natural killer cell line 92, augments cytotoxic activity, and mediates IFN-gamma production by the stress kinase p38 and by the extracellular regulated kinases p44erk-1 and p42erk-21. J Immunol. 2000;165:1307–1313. doi: 10.4049/jimmunol.165.3.1307. [DOI] [PubMed] [Google Scholar]
- 58.Wyman TH, Dinarello CA, Banerjee A, Gamboni-Robertson F, Hiester AA, England KM, Kelher M, Silliman CC. Physiological levels of interleukin-18 stimulate multiple neutrophil functions through p38 MAP kinase activation. J Leukoc Biol. 2002;72:401–409. [PubMed] [Google Scholar]
- 59.Fortin CF, Ear T, McDonald PP. Autocrine role of endogenous interleukin-18 on inflammatory cytokine generation by human neutrophils. FASEB J. 2009;23:194–203. doi: 10.1096/fj.08-110213. [DOI] [PubMed] [Google Scholar]
- 60.Shimoda K, Tsutsui H, Aoki K, Kato K, Matsuda T, Numata A, Takase K, Yamamoto T, Nukina H, Hoshino T, et al. Partial impairment of interleukin-12 (IL-12) and IL-18 signaling in Tyk2-deficient mice. Blood. 2002;99:2094–2099. doi: 10.1182/blood.v99.6.2094. [DOI] [PubMed] [Google Scholar]
- 61.Chandrasekar B, Patel DN, Mummidi S, Kim JW, Clark RA, Valente AJ. Interleukin-18 suppresses adiponectin expression in 3T3-L1 adipocytes via a novel signal transduction pathway involving ERK1/2-dependent NFATc4 phosphorylation. J Biol Chem. 2008;283:4200–4209. doi: 10.1074/jbc.M708142200. [DOI] [PubMed] [Google Scholar]
- 62.Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 63.Aizawa Y, Akita K, Taniai M, Torigoe K, Mori T, Nishida Y, Ushio S, Nukada Y, Tanimoto T, Ikegami H, et al. Cloning and expression of interleukin-18 binding protein. FEBS Lett. 1999;445:338–342. doi: 10.1016/s0014-5793(99)00148-9. [DOI] [PubMed] [Google Scholar]
- 64.Kim SH, Eisenstein M, Reznikov L, Fantuzzi G, Novick D, Rubinstein M, Dinarello CA. Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc Natl Acad Sci USA. 2000;97:1190–1195. doi: 10.1073/pnas.97.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paulukat J, Bosmann M, Nold M, Garkisch S, Kampfer H, Frank S, Raedle J, Zeuzem S, Pfeilschifter J, Muhl H. Expression and release of IL-18 binding protein in response to IFN-gamma. J Immunol. 2001;167:7038–7043. doi: 10.4049/jimmunol.167.12.7038. [DOI] [PubMed] [Google Scholar]
- 66.Xiang Y, Moss B. IL-18 binding and inhibition of interferon gamma induction by human poxvirus-encoded proteins. Proc Natl Acad Sci USA. 1999;96:11537–11542. doi: 10.1073/pnas.96.20.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith VP, Bryant NA, Alcami A. Ectromelia, vaccinia and cowpox viruses encode secreted interleukin-18-binding proteins. J Gen Virol. 2000;81:1223–1230. doi: 10.1099/0022-1317-81-5-1223. [DOI] [PubMed] [Google Scholar]
- 68.Krumm B, Meng X, Wang Z, Xiang Y, Deng J. A unique bivalent binding and inhibition mechanism by the yatapoxvirus interleukin 18 binding protein. PLoS Pathog. 2012;8:e1002876. doi: 10.1371/journal.ppat.1002876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banda NK, Vondracek A, Kraus D, Dinarello CA, Kim SH, Bendele A, Senaldi G, Arend WP. Mechanisms of inhibition of collagen-induced arthritis by murine IL-18 binding protein. J Immunol. 2003;170:2100–2105. doi: 10.4049/jimmunol.170.4.2100. [DOI] [PubMed] [Google Scholar]
- 70.Plater-Zyberk C, Joosten LA, Helsen MM, Sattonnet-Roche P, Siegfried C, Alouani S, van De Loo FA, Graber P, Aloni S, Cirillo R, et al. Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J Clin Invest. 2001;108:1825–1832. doi: 10.1172/JCI12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plitz T, Saint-Mezard P, Satho M, Herren S, Waltzinger C, Carvalho Bittencourt M, Kosco-Vilbois MH, Chvatchko Y. IL-18 binding protein protects against contact hypersensitivity. J Immunol. 2003;171:1164–1171. doi: 10.4049/jimmunol.171.3.1164. [DOI] [PubMed] [Google Scholar]
- 72.Faggioni R, Cattley RC, Guo J, Flores S, Brown H, Qi M, Yin S, Hill D, Scully S, Chen C, et al. IL-18-binding protein protects against lipopolysaccharide-induced lethality and prevents the development of Fas/Fas ligand-mediated models of liver disease in mice. J Immunol. 2001;167:5913–5920. doi: 10.4049/jimmunol.167.10.5913. [DOI] [PubMed] [Google Scholar]
- 73.Novick D, Schwartsburd B, Pinkus R, Suissa D, Belzer I, Sthoeger Z, Keane WF, Chvatchko Y, Kim SH, Fantuzzi G, et al. A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine. 2001;14:334–342. doi: 10.1006/cyto.2001.0914. [DOI] [PubMed] [Google Scholar]
- 74.Sarvetnick N. IFN-gamma, IGIF, and IDDM. J Clin Invest. 1997;99:371–372. doi: 10.1172/JCI119167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kretowski A, Mironczuk K, Karpinska A, Bojaryn U, Kinalski M, Puchalski Z, Kinalska I. Interleukin-18 promoter polymorphisms in type 1 diabetes. Diabetes. 2002;51:3347–3349. doi: 10.2337/diabetes.51.11.3347. [DOI] [PubMed] [Google Scholar]
- 76.Ide A, Kawasaki E, Abiru N, Sun F, Kobayashi M, Fukushima T, Takahashi R, Kuwahara H, Kita A, Oshima K, et al. Association between IL-18 gene promoter polymorphisms and CTLA-4 gene 49A/G polymorphism in Japanese patients with type 1 diabetes. J Autoimmun. 2004;22:73–78. doi: 10.1016/j.jaut.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Saleh NM, Raj SM, Smyth DJ, Wallace C, Howson JM, Bell L, Walker NM, Stevens HE, Todd JA. Genetic association analyses of atopic illness and proinflammatory cytokine genes with type 1 diabetes. Diabetes Metab Res Rev. 2011;27:838–843. doi: 10.1002/dmrr.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nolsoe RL, Pociot F, Novick D, Rubinstein M, Kim SH, Dinarello CA, Mandrup-Poulsen T. Mutation scan of a type 1 diabetes candidate gene: the human interleukin-18 binding protein gene. Ann NY Acad Sci. 2003;1005:332–339. doi: 10.1196/annals.1288.053. [DOI] [PubMed] [Google Scholar]
- 79.Nicoletti F, Conget I, Di Marco R, Speciale AM, Morinigo R, Bendtzen K, Gomis R. Serum levels of the interferon-gamma-inducing cytokine interleukin-18 are increased in individuals at high risk of developing type I diabetes. Diabetologia. 2001;44:309–311. doi: 10.1007/s001250051619. [DOI] [PubMed] [Google Scholar]
- 80.Katakami N, Kaneto H, Matsuhisa M, Yoshiuchi K, Kato K, Yamamoto K, Umayahara Y, Kosugi K, Hori M, Yamasaki Y. Serum interleukin-18 levels are increased and closely associated with various soluble adhesion molecule levels in type 1 diabetic patients. Diabetes Care. 2007;30:159–161. doi: 10.2337/dc06-1768. [DOI] [PubMed] [Google Scholar]
- 81.Altinova AE, Yetkin I, Akbay E, Bukan N, Arslan M. Serum IL-18 levels in patients with type 1 diabetes: relations to metabolic control and microvascular complications. Cytokine. 2008;42:217–221. doi: 10.1016/j.cyto.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 82.Mahmoud RA, el-Ezz SA, Hegazy AS. Increased serum levels of interleukin-18 in patients with diabetic nephropathy. Ital J Biochem. 2004;53:73–81. [PubMed] [Google Scholar]
- 83.Dong G, Liang L, Fu J, Zou C. Serum interleukin-18 levels are raised in diabetic ketoacidosis in Chinese children with type 1 diabetes mellitus. Indian Pediatr. 2007;44:732–736. [PubMed] [Google Scholar]
- 84.Kuryliszyn-Moskal A, Dubicki A, Zarzycki W, Zonnenberg A, Gorska M. Microvascular abnormalities in capillaroscopy correlate with higher serum IL-18 and sE-selectin levels in patients with type 1 diabetes complicated by microangiopathy. Folia Histochem Cytobiol. 2011;49:104–110. doi: 10.5603/fhc.2011.0015. [DOI] [PubMed] [Google Scholar]
- 85.Rothe H, Hibino T, Itoh Y, Kolb H, Martin S. Systemic production of interferon-gamma inducing factor (IGIF) versus local IFN-gamma expression involved in the development of Th1 insulitis in NOD mice. J Autoimmun. 1997;10:251–256. doi: 10.1006/jaut.1997.0135. [DOI] [PubMed] [Google Scholar]
- 86.Rothe H, Hausmann A, Casteels K, Okamura H, Kurimoto M, Burkart V, Mathieu C, Kolb H. IL-18 inhibits diabetes development in nonobese diabetic mice by counterregulation of Th1-dependent destructive insulitis. J Immunol. 1999;163:1230–1236. [PubMed] [Google Scholar]
- 87.Oikawa Y, Shimada A, Kasuga A, Morimoto J, Osaki T, Tahara H, Miyazaki T, Tashiro F, Yamato E, Miyazaki J, et al. Systemic administration of IL-18 promotes diabetes development in young nonobese diabetic mice. J Immunol. 2003;171:5865–5875. doi: 10.4049/jimmunol.171.11.5865. [DOI] [PubMed] [Google Scholar]
- 88.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/s1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 89.Krook H, Wallstrom J, Sandler S. Function of rat pancreatic islets exposed to interleukin-18 in vitro. Autoimmunity. 1999;29:263–267. doi: 10.3109/08916939908994745. [DOI] [PubMed] [Google Scholar]
- 90.Hong TP, Andersen NA, Nielsen K, Karlsen AE, Fantuzzi G, Eizirik DL, Dinarello CA, Mandrup-Poulsen T. Interleukin-18 mRNA, but not interleukin-18 receptor mRNA, is constitutively expressed in islet beta-cells and up-regulated by interferon-gamma. Eur Cytokine Netw. 2000;11:193–205. [PubMed] [Google Scholar]
- 91.Frigerio S, Hollander GA, Zumsteg U. Functional IL-18 Is produced by primary pancreatic mouse islets and NIT-1 beta cells and participates in the progression towards destructive insulitis. Horm Res. 2002;57:94–104. doi: 10.1159/000057959. [DOI] [PubMed] [Google Scholar]
- 92.Schott WH, Haskell BD, Tse HM, Milton MJ, Piganelli JD, Choisy-Rossi CM, Reifsnyder PC, Chervonsky AV, Leiter EH. Caspase-1 is not required for type 1 diabetes in the NOD mouse. Diabetes. 2004;53:99–104. doi: 10.2337/diabetes.53.1.99. [DOI] [PubMed] [Google Scholar]
- 93.Marleau AM, Sarvetnick NE. IL-18 is required for self-reactive T cell expansion in NOD mice. J Autoimmun. 2011;36:263–277. doi: 10.1016/j.jaut.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zaccone P, Phillips J, Conget I, Cooke A, Nicoletti F. IL-18 binding protein fusion construct delays the development of diabetes in adoptive transfer and cyclophosphamide-induced diabetes in NOD mouse. Clin Immunol. 2005;115:74–79. doi: 10.1016/j.clim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 95.Furlan R, Martino G, Galbiati F, Poliani PL, Smiroldo S, Bergami A, Desina G, Comi G, Flavell R, Su MS, et al. Caspase-1 regulates the inflammatory process leading to autoimmune demyelination. J Immunol. 1999;163:2403–2409. [PubMed] [Google Scholar]
- 96.Shi FD, Takeda K, Akira S, Sarvetnick N, Ljunggren HG. IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-gamma by NK cells. J Immunol. 2000;165:3099–3104. doi: 10.4049/jimmunol.165.6.3099. [DOI] [PubMed] [Google Scholar]
- 97.Gutcher I, Urich E, Wolter K, Prinz M, Becher B. Interleukin 18-independent engagement of interleukin 18 receptor-alpha is required for autoimmune inflammation. Nat Immunol. 2006;7:946–953. doi: 10.1038/ni1377. [DOI] [PubMed] [Google Scholar]
- 98.Lewis EC, Dinarello CA. Responses of IL-18- and IL-18 receptor-deficient pancreatic islets with convergence of positive and negative signals for the IL-18 receptor. Proc Natl Acad Sci USA. 2006;103:16852–16857. doi: 10.1073/pnas.0607917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nold-Petry CA, Nold MF, Nielsen JW, Bustamante A, Zepp JA, Storm KA, Hong JW, Kim SH, Dinarello CA. Increased cytokine production in interleukin-18 receptor alpha-deficient cells is associated with dysregulation of suppressors of cytokine signaling. J Biol Chem. 2009;284:25900–25911. doi: 10.1074/jbc.M109.004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gillett A, Thessen Hedreul M, Khademi M, Espinosa A, Beyeen AD, Jagodic M, Kockum I, Harris RA, Olsson T. Interleukin 18 receptor 1 expression distinguishes patients with multiple sclerosis. Mult Scler. 2010;16:1056–1065. doi: 10.1177/1352458510364634. [DOI] [PubMed] [Google Scholar]
- 101.Millward JM, Lobner M, Wheeler RD, Owens T. Inflammation in the central nervous system and Th17 responses are inhibited by IFN-gamma-Induced IL-18 binding protein. J Immunol. 2010;185:2458–2466. doi: 10.4049/jimmunol.0902153. [DOI] [PubMed] [Google Scholar]
- 102.Nicoletti F, Di Marco R, Mangano K, Patti F, Reggio E, Nicoletti A, Bendtzen K, Reggio A. Increased serum levels of interleukin-18 in patients with multiple sclerosis. Neurology. 2001;57:342–344. doi: 10.1212/wnl.57.2.342. [DOI] [PubMed] [Google Scholar]
- 103.Losy J, Niezgoda A. IL-18 in patients with multiple sclerosis. Acta Neurol Scand. 2001;104:171–173. doi: 10.1034/j.1600-0404.2001.00356.x. [DOI] [PubMed] [Google Scholar]
- 104.Franciotta D, Martino G, Zardini E, Furlan R, Bergamaschi R, Gironi M, Bergami A, Angelini G, De Benedetti F, Pignatti P, et al. Caspase-1 levels in biological fluids from patients with multiple sclerosis and from patients with other neurological and non-neurological diseases. Eur Cytokine Netw. 2002;13:99–103. [PubMed] [Google Scholar]
- 105.Huang WX, Huang P, Hillert J. Increased expression of caspase-1 and interleukin-18 in peripheral blood mononuclear cells in patients with multiple sclerosis. Mult Scler. 2004;10:482–487. doi: 10.1191/1352458504ms1071oa. [DOI] [PubMed] [Google Scholar]
- 106.Karni A, Koldzic DN, Bharanidharan P, Khoury SJ, Weiner HL. IL-18 is linked to raised IFN-gamma in multiple sclerosis and is induced by activated CD4(+) T cells via CD40-CD40 ligand interactions. J Neuroimmunol. 2002;125:134–140. doi: 10.1016/s0165-5728(02)00018-8. [DOI] [PubMed] [Google Scholar]
- 107.Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330:1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 108.Shi FD, Wang HB, Li H, Hong S, Taniguchi M, Link H, Van Kaer L, Ljunggren HG. Natural killer cells determine the outcome of B cell-mediated autoimmunity. Nat Immunol. 2000;1:245–251. doi: 10.1038/79792. [DOI] [PubMed] [Google Scholar]
- 109.Souroujon MC, Maiti PK, Feferman T, Im SH, Raveh L, Fuchs S. Suppression of myasthenia gravis by antigen-specific mucosal tolerance and modulation of cytokines and costimulatory factors. Ann NY Acad Sci. 2003;998:533–536. doi: 10.1196/annals.1254.069. [DOI] [PubMed] [Google Scholar]
- 110.Aricha R, Feferman T, Fuchs S, Souroujon MC. Ex vivo generated regulatory T cells modulate experimental autoimmune myasthenia gravis. J Immunol. 2008;180:2132–2139. doi: 10.4049/jimmunol.180.4.2132. [DOI] [PubMed] [Google Scholar]
- 111.Jander S, Stoll G. Increased serum levels of the interferon-gamma-inducing cytokine interleukin-18 in myasthenia gravis. Neurology. 2002;59:287–289. doi: 10.1212/wnl.59.2.287. [DOI] [PubMed] [Google Scholar]
- 112.Volin MV, Koch AE. Interleukin-18: a mediator of inflammation and angiogenesis in rheumatoid arthritis. J Interferon Cytokine Res. 2011;31:745–751. doi: 10.1089/jir.2011.0050. [DOI] [PubMed] [Google Scholar]
- 113.Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, Kennedy K, Carter R, Wei XQ, Xu D, et al. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104:1393–1401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wei XQ, Leung BP, Arthur HM, McInnes IB, Liew FY. Reduced incidence and severity of collagen-induced arthritis in mice lacking IL-18. J Immunol. 2001;166:517–521. doi: 10.4049/jimmunol.166.1.517. [DOI] [PubMed] [Google Scholar]
- 115.Huber LC, Distler O, Tarner I, Gay RE, Gay S, Pap T. Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology (Oxford) 2006;45:669–675. doi: 10.1093/rheumatology/kel065. [DOI] [PubMed] [Google Scholar]
- 116.Morel JC, Park CC, Zhu K, Kumar P, Ruth JH, Koch AE. Signal transduction pathways involved in rheumatoid arthritis synovial fibroblast interleukin-18-induced vascular cell adhesion molecule-1 expression. J Biol Chem. 2002;277:34679–34691. doi: 10.1074/jbc.M206337200. [DOI] [PubMed] [Google Scholar]
- 117.Morel JC, Park CC, Kumar P, Koch AE. Interleukin-18 induces rheumatoid arthritis synovial fibroblast CXC chemokine production through NFkappaB activation. Lab Invest. 2001;81:1371–1383. doi: 10.1038/labinvest.3780351. [DOI] [PubMed] [Google Scholar]
- 118.Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001;167:1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- 119.Amin MA, Mansfield PJ, Pakozdi A, Campbell PL, Ahmed S, Martinez RJ, Koch AE. Interleukin-18 induces angiogenic factors in rheumatoid arthritis synovial tissue fibroblasts via distinct signaling pathways. Arthr Rheum. 2007;56:1787–1797. doi: 10.1002/art.22705. [DOI] [PubMed] [Google Scholar]
- 120.Naik SM, Cannon G, Burbach GJ, Singh SR, Swerlick RA, Wilcox JN, Ansel JC, Caughman SW. Human keratinocytes constitutively express interleukin-18 and secrete biologically active interleukin-18 after treatment with pro-inflammatory mediators and dinitrochlorobenzene. J Invest Dermatol. 1999;113:766–772. doi: 10.1046/j.1523-1747.1999.00750.x. [DOI] [PubMed] [Google Scholar]
- 121.Ohta Y, Hamada Y, Katsuoka K. Expression of IL-18 in psoriasis. Arch Dermatol Res. 2001;293:334–342. doi: 10.1007/s004030100240. [DOI] [PubMed] [Google Scholar]
- 122.Johansen C, Moeller K, Kragballe K, Iversen L. The activity of caspase-1 is increased in lesional psoriatic epidermis. J Invest Dermatol. 2007;127:2857–2864. doi: 10.1038/sj.jid.5700922. [DOI] [PubMed] [Google Scholar]
- 123.Companjen A, van der Wel L, van der Fits L, Laman J, Prens E. Elevated interleukin-18 protein expression in early active and progressive plaque-type psoriatic lesions. Eur Cytokine Netw. 2004;15:210–216. [PubMed] [Google Scholar]
- 124.Gangemi S, Merendino RA, Guarneri F, Minciullo PL, DiLorenzo G, Pacor M, Cannavo SP. Serum levels of interleukin-18 and s-ICAM-1 in patients affected by psoriasis: preliminary considerations. J Eur Acad Dermatol Venereol. 2003;17:42–46. doi: 10.1046/j.1468-3083.2003.00647.x. [DOI] [PubMed] [Google Scholar]
- 125.Gutzmer R, Langer K, Mommert S, Wittmann M, Kapp A, Werfel T. Human dendritic cells express the IL-18R and are chemoattracted to IL-18. J Immunol. 2003;171:6363–6371. doi: 10.4049/jimmunol.171.12.6363. [DOI] [PubMed] [Google Scholar]
- 126.Cho D, Seung Kang J, Hoon Park J, Kim YI, Hahm E, Lee J, Yang Y, Jeon J, Song H, Park H, et al. The enhanced IL-18 production by UVB irradiation requires ROI and AP-1 signaling in human keratinocyte cell line (HaCaT) Biochem Biophys Res Commun. 2002;298:289–295. doi: 10.1016/s0006-291x(02)02433-6. [DOI] [PubMed] [Google Scholar]
- 127.Wittmann M, Doble R, Bachmann M, Pfeilschifter J, Werfel T, Muhl H. IL-27 regulates IL-18 binding protein in skin resident cells. PLoS ONE. 2012;7:e38751. doi: 10.1371/journal.pone.0038751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wong CK, Li EK, Ho CY, Lam CW. Elevation of plasma interleukin-18 concentration is correlated with disease activity in systemic lupus erythematosus. Rheumatology (Oxford) 2000;39:1078–1081. doi: 10.1093/rheumatology/39.10.1078. [DOI] [PubMed] [Google Scholar]
- 129.Wong CK, Ho CY, Li EK, Tam LS, Lam CW. Elevated production of interleukin-18 is associated with renal disease in patients with systemic lupus erythematosus. Clin Exp Immunol. 2002;130:345–351. doi: 10.1046/j.1365-2249.2002.01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Park MC, Park YB, Lee SK. Elevated interleukin-18 levels correlated with disease activity in systemic lupus erythematosus. Clin Rheumatol. 2004;23:225–229. doi: 10.1007/s10067-004-0867-x. [DOI] [PubMed] [Google Scholar]
- 131.Neumann D, Del Giudice E, Ciaramella A, Boraschi D, Bossu P. Lymphocytes from autoimmune MRL lpr/lpr mice are hyperresponsive to IL-18 and overexpress the IL-18 receptor accessory chain. J Immunol. 2001;166:3757–3762. doi: 10.4049/jimmunol.166.6.3757. [DOI] [PubMed] [Google Scholar]
- 132.Bossu P, Neumann D, Del Giudice E, Ciaramella A, Gloaguen I, Fantuzzi G, Dinarello CA, Di Carlo E, Musiani P, Meroni PL, et al. IL-18 cDNA vaccination protects mice from spontaneous lupus-like autoimmune disease. Proc Natl Acad Sci USA. 2003;100:14181–14186. doi: 10.1073/pnas.2336094100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Favilli F, Anzilotti C, Martinelli L, Quattroni P, De Martino S, Pratesi F, Neumann D, Beermann S, Novick D, Dinarello CA, et al. IL-18 activity in systemic lupus erythematosus. Ann NY Acad Sci. 2009;1173:301–309. doi: 10.1111/j.1749-6632.2009.04742.x. [DOI] [PubMed] [Google Scholar]
- 134.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 135.Tanaka T, Tsutsui H, Yoshimoto T, Kotani M, Matsumoto M, Fujita A, Wang W, Higa S, Koshimoto T, Nakanishi K, et al. Interleukin-18 is elevated in the sera from patients with atopic dermatitis and from atopic dermatitis model mice, NC/Nga. Int Arch Allergy Immunol. 2001;125:236–240. doi: 10.1159/000053821. [DOI] [PubMed] [Google Scholar]
- 136.Buentke E, Heffler LC, Wallin RP, Lofman C, Ljunggren HG, Scheynius A. The allergenic yeast Malassezia furfur induces maturation of human dendritic cells. Clin Exp Allergy. 2001;31:1583–1593. doi: 10.1046/j.1365-2222.2001.01199.x. [DOI] [PubMed] [Google Scholar]
- 137.Hoshino T, Yagita H, Ortaldo JR, Wiltrout RH, Young HA. In vivo administration of IL-18 can induce IgE production through Th2 cytokine induction and up-regulation of CD40 ligand (CD154) expression on CD4+ T cells. Eur J Immunol. 2000;30:1998–2006. doi: 10.1002/1521-4141(200007)30:7<1998::AID-IMMU1998>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 138.Enoksson SL, Grasset EK, Hagglof T, Mattsson N, Kaiser Y, Gabrielsson S, McGaha TL, Scheynius A, Karlsson MC. The inflammatory cytokine IL-18 induces self-reactive innate antibody responses regulated by natural killer T cells. Proc Natl Acad Sci USA. 2011;108:E1399–E1407. doi: 10.1073/pnas.1107830108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 140.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 141.Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr Mol Med. 2009;9:4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- 142.Lind SM, Kuylenstierna C, Moll M, D Jordö E, Winqvist O, Lundeberg L, Karlsson MA, T Linder M, Johansson C, Scheynius A et al (2009) IL-18 skews the invariant NKT-cell population via autoreactive activation in atopic eczema. Eur J Immunol 39:2293–2301 [DOI] [PubMed]
- 143.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 144.Seta Y, Kanda T, Tanaka T, Arai M, Sekiguchi K, Yokoyama T, Kurimoto M, Tamura J, Kurabayashi M. Interleukin 18 in acute myocardial infarction. Heart. 2000;84:668. doi: 10.1136/heart.84.6.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mallat Z, Corbaz A, Scoazec A, Besnard S, Leseche G, Chvatchko Y, Tedgui A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104:1598–1603. doi: 10.1161/hc3901.096721. [DOI] [PubMed] [Google Scholar]
- 146.Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, Tedgui A. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res. 2001;89:E41–E45. doi: 10.1161/hh1901.098735. [DOI] [PubMed] [Google Scholar]
- 147.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ Res. 2002;90:E34–E38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 148.Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F, Hansson GK. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 149.Tenger C, Sundborger A, Jawien J, Zhou X. IL-18 accelerates atherosclerosis accompanied by elevation of IFN-gamma and CXCL16 expression independently of T cells. Arterioscler Thromb Vasc Biol. 2005;25:791–796. doi: 10.1161/01.ATV.0000153516.02782.65. [DOI] [PubMed] [Google Scholar]
- 150.Sahar S, Dwarakanath RS, Reddy MA, Lanting L, Todorov I, Natarajan R. Angiotensin II enhances interleukin-18 mediated inflammatory gene expression in vascular smooth muscle cells: a novel cross-talk in the pathogenesis of atherosclerosis. Circ Res. 2005;96:1064–1071. doi: 10.1161/01.RES.0000168210.10358.f4. [DOI] [PubMed] [Google Scholar]
- 151.Blankenberg S, Tiret L, Bickel C, Peetz D, Cambien F, Meyer J, Rupprecht HJ, AtheroGene I. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002;106:24–30. doi: 10.1161/01.cir.0000020546.30940.92. [DOI] [PubMed] [Google Scholar]
- 152.Chapman CM, McQuillan BM, Beilby JP, Thompson PL, Hung J. Interleukin-18 levels are not associated with subclinical carotid atherosclerosis in a community population. The Perth Carotid Ultrasound Disease Assessment Study (CUDAS) Atherosclerosis. 2006;189:414–419. doi: 10.1016/j.atherosclerosis.2005.12.026. [DOI] [PubMed] [Google Scholar]