Abstract
Current evidence indicates that a subpopulation of cancer cells, named cancer stem cells (CSCs) or tumor-initiating cells, are responsible for the initiation, growth, metastasis, therapy resistance and recurrence of cancers. CSCs share core regulatory pathways with normal stem cells; however, CSCs rely on distinct reprogrammed pathways to maintain stemness and to contribute to the progression of cancers. The specific targeting of CSCs, together with conventional chemotherapy or radiotherapy, may achieve stable remission or cure cancer. Therefore, the identification of CSCs and a better understanding of the complex characteristics of CSCs will provide invaluable diagnostic, therapeutic and prognostic targets for clinical application. In this review, we will introduce the dysregulated properties of CSCs in cancers and discuss the possible challenges in targeting CSCs for cancer treatment.
Keywords: Cancer-initiating cell, Self-renewal, Tumorigenesis, Tumor metastasis
Introduction
Cancer stem cells (CSCs), or tumor-initiating cells, are a subset of cancer cells with the abilities to self-renew and differentiate and to drive the growth and metastasis of tumors, whereas the majority of cancer cells have only limited proliferative potential [1, 2]. CSCs were first identified in acute myeloid leukemia (AML) [3, 4] and were subsequently discovered in breast cancer [5] and other types of solid tumors. A distinctive repertoire of cell surface markers are used to identify and enrich CSCs from human tumor tissues and cancer cell lines (Table 1). Compared to low-tumorigenic bulk cancer cells and normal stem cells, CSCs exhibit dysregulated signaling pathways and abnormal phenotypes. Recent studies have demonstrated that CSCs are involved in the initiation, growth, metastasis, therapy resistance and recurrence of human cancers [1, 2]. Here, we summarize the characteristics of CSCs in cancers and discuss the possibility of targeting CSCs as a therapeutic strategy for the treatment of human cancers.
Table 1.
Cancer stem cell markers in tumors
| Types of tumor | CSC markers | References |
|---|---|---|
| Breast cancer | CD44+CD24−, ALDH1+ | [5, 6] |
| Glioblastoma | CD133+, CD15+ | [7, 8] |
| Colon cancer | LGR5+, CD133+, CD44+v6 | [9–11] |
| Liver cancer | CD24+, CD133+, CD90+ | [12, 13] |
| Lung cancer | CD133+, ALDH1+ | [14, 15] |
| Leukemia | CD34+CD38−, CD117 | [16–18] |
| Melanoma | CD20+, CD271+ | [19, 20] |
| Gastric cancer | CD44+, Lgr5+ | [21, 22] |
| Ovarian cancer | CD44+CD117+, CD133+ | [23, 24] |
| Pancreatic cancer | CD44+CD24+EpCAM+ | [25] |
| Prostate cancer | CD44, TRA-1-60+CD151+CD166+ | [26, 27] |
| Head and neck cancer | CD44+, c-MET+ | [28, 29] |
| Osteosarcoma | CD133+, CD117+Stro-1+ | [30, 31] |
| Chondrosarcoma | CD133+ | [32] |
| Synovial sarcoma | CD133+ | [33] |
| Ewing’s sarcoma | CD133+, ALDH+ | [34, 35] |
| Rhabdomyosarcoma | CD133+ | [36] |
Dysregulated self-renewal and differentiation capacities of CSCs
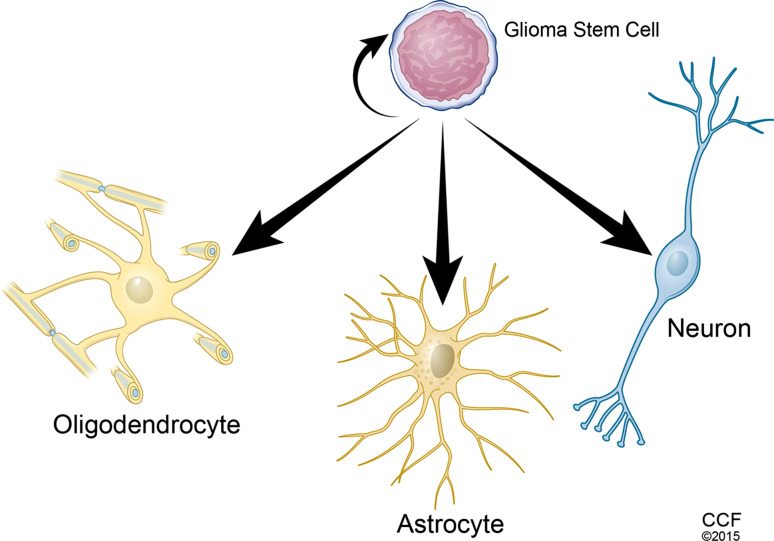
Normal stem cells have evolved various defense mechanisms to prevent tumor development. Normal stem cells also exhibit regulated life cycles that include tightly controlled self-renewal and committed lineage differentiation. Current evidence demonstrates that dysfunction of self-renewal- and differentiation-related genes endows normal stem cells and/or their differentiated progeny with the capacity for continuous self-renewal and dysregulated differentiation, which may promote the bypass of certain protective mechanisms in cells, ultimately resulting in cancers. Combined p53 and PTEN mutations can promote c-Myc activation to enhance self-renewal capacity and impair differentiation of glioblastoma-initiating cells [37]. CSCs can be established by overexpressing H-Ras (L61) in p53-deficient neural stem cells, while Sox11 prevents tumorigenesis of CSCs by inducing neural differentiation [38]; however, only a fraction of cancer cells display the capacity to give rise to cancer cells in the long term [39]. Similar to normal stem cells, CSCs also display a committed differentiation ability. Glioblastoma stem cells (GSCs) can differentiate into astrocytes, oligodendrocytes or neurons [40] (Fig. 1). Interestingly, GSCs can transdifferentiate into vascular endothelial cells and pericytes to support vessel function and tumor growth [41–44].
Fig. 1.
Schema of glioblastoma stem cells (GSCs) displaying self-renewal and multiple-lineage differentiation. GSCs can maintain their stemness through self-renewal and differentiation into oligodendrocytes, astrocytes and neurons
In addition to glioblastoma, the self-renewal and differentiation abilities of CSCs are also documented in other types of cancers. CSCs from prostate cancer specimens can differentiate into three prostate epithelial cell lineages and reconstitute the original human tumor in vivo [45]. Head and neck squamous cell carcinoma encompasses a subpopulation of CD44+ cancer cells that can be serially passaged and that reproduce the original tumor heterogeneity. CD44+ cells express high levels of BMI1, whereas CD44− cancer cells express the differentiation marker involucrin and resemble differentiated squamous epithelium cells [28]. Colon CSCs are able to form large lumen-containing colonies, which consist of three types of differentiated colon epithelial cells in three-dimensional matrigel culture. Some single cells from these colonies can reconstitute themselves and form tumors in immunodeficient mice [46]. Notably, CSC-derived differentiated cancer cells are usually major components of tumors and also play important roles in sustaining tumor growth.
Dysregulation of cell death in CSCs
In addition to sustained proliferation, cancer cells can disrupt the balance between proliferation and death by evading signals from apoptotic factors. Compared with other cells, CSCs are more resistant to apoptosis. CD133+ colon CSCs secrete IL-4 to protect themselves from apoptosis [47]. When nutrition transport is prevented by blood vessel growth blockage, most colon cancer cells will die, while CD133+ colon CSCs are apoptosis resistant [48]. Moreover, colon CSCs can escape the apoptosis stimulant by entering into a reversible quiescent state [49]; however, some treatment strategies can induce apoptosis sensitivity in CSCs. For example, the removal of phosphatase Wip1 inhibits APC-driven polyposis through lowering the threshold for p53-dependent apoptosis of colon CSCs [50]. BMI1 deficiency promotes cell death and delays cell cycle progression in lung cancers [51]. In conjunction with irradiation, TRAIL-expressing mesenchymal stem cells (MSCs) enhance glioma stem cells to undergo apoptosis [52]. Transient exposure of leukemia stem cells (LSCs) to a DNA methylation inhibitor causes an antitumor “memory” without immediate toxicity [53]. Delta12-prostaglandin-J3, an omega-3 fatty acid-derived metabolite, selectively targets LSCs for apoptosis in murine bone marrow and spleen [54]. Niclosamide inhibits the formation of spheroids and induces the apoptosis of breast CSCs [55].
CSCs and angiogenesis
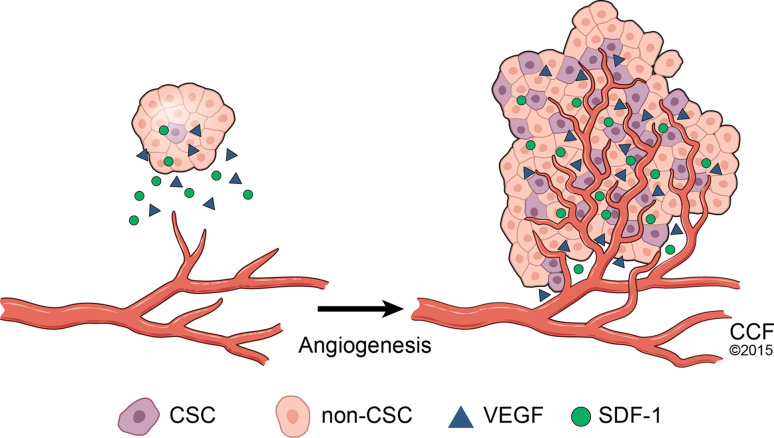
Angiogenesis is considered as an important property of tumors and is required for tumor growth and metastasis. CSCs have the capacity to give rise to angiogenesis, whereas differentiated tumor cells are non-angiogenic [56]. CD105-positive renal CSCs release microvesicles to stimulate angiogenesis and the formation of premetastatic niches, which results in cancer cell metastasis to the lungs [57]. In glioblastoma, GSCs promote angiogenesis by secreting vascular endothelial growth factor (VEGF) and stromal-derived factor 1 (SDF-1) [58, 59] (Fig. 2). In contrast, VEGF promotes cancer stemness by stimulating angiogenesis in a paracrine manner and providing a perivascular niche for CSCs [60]. SDF-1 and its receptor CXCR4 can stimulate glioma stem cells to secrete VEGF to promote glioma growth and angiogenesis [61]. CSCs can promote vasculogenesis by serving as tumor vasculogenic progenitors [62]. CD133+ liver CSCs promote tumor angiogenesis by upregulating IL-8 and CXCL1 signaling [63]. When endothelial cells are selectively eliminated in glioblastomas, the self-renewal ability of CSCs is downregulated, suggesting that endothelial cells are also critical for the maintenance of GSCs [64]; however, anti-angiogenic therapies have the potential to trigger a more invasive and metastatic phenotype in some tumors [65]. The anti-angiogenic agent increases the population of breast CSCs by generating a hypoxic niche [66]. Hypoxia inducible factors (HIFs) contribute to angiogenesis by binding to the HIF element and activating downstream pathways. Unlike HIF-1α, which is expressed in abundant hypoxic niche cells, HIF-2α is only expressed in GSCs and regulates the self-renewal and survival of GSCs but not that of non-stem tumor cells or normal neural progenitors [67]. HIF-2α might, therefore, represent a promising target to inhibit tumor angiogenesis. Interestingly, in glioblastoma, subsets of GSCs are able to transdifferentiate into vascular endothelium cells [41, 42]; however, a new study demonstrates that GSCs preferentially transdifferentiate into vascular pericytes but not endothelial cells. The selective deletion of GSC-derived pericytes in vivo impairs glioblastoma tumor growth and progression [43, 44].
Fig. 2.
Schema of tumor angiogenesis. During tumor angiogenesis, cancer stem cells release molecules that send signals to surrounding endothelial cells and encourage the growth of new blood vessels. Blood vessels are depicted as red tissues
Increased invasive and metastatic capabilities in CSCs
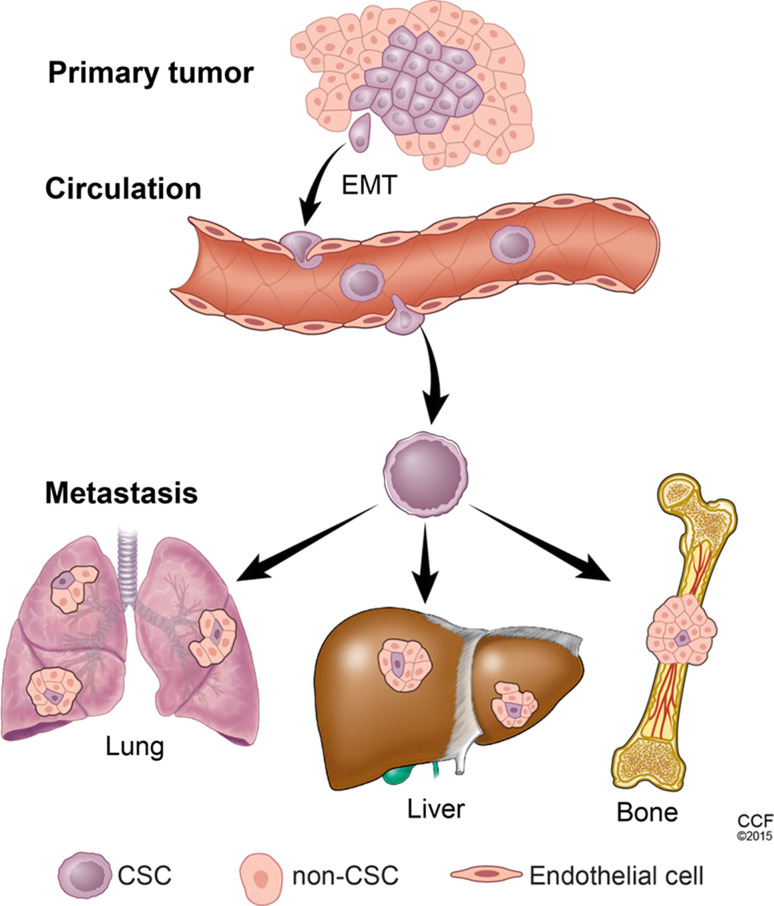
Metastasis can be divided into several steps. First, tumor cells disseminate into the surrounding tissues and enter capillaries. Second, the disseminated tumor cells are circulated in the blood and reach their target organs. Finally, tumor cells infiltrate from the blood and colonize to form metastatic tumors (Fig. 3). Recent evidence reveals that the epithelial to mesenchymal transition (EMT) is involved in cancer cell invasion and metastasis to distant organ sites [68]. Moreover, EMT contributes to the generation of CSCs with mesenchymal cell-like properties [69]. Our work reveals that Twist2 endows breast and liver cancer cells with mesenchymal phenotypes and stemness [70, 71]. Interestingly, CSCs display a combination of epithelial and mesenchymal phenotypes, indicating that cancer cells may adapt EMT programming to gain stem cell status [72]. Multiple genes contribute to regulate both self-renewal and EMT. The EMT activator Twist1 can interact with BRD4 to control the properties of basal breast CSCs by regulating Wnt5A [73]. YAP is important for stem cell pluripotency [74] and has also been implicated in regulating EMT [75].
Fig. 3.
Schema of metastasis. Cancer stem cells from a primary tumor disseminate to distant organs through the circulation and subsequently initiate macrometastatic deposition
Although EMT is very important for tumor dissemination, mesenchymal to epithelial transition is required for the colonization of tumor cells. Yang et al. [76] reported that the reversion of EMT is required for the disseminated tumor cells to proliferate and metastasize to distant sites. miR-200s promote the expression of E-cadherin by inhibiting ZEB but also regulate metastatic colonization by targeting metastasis-suppressing proteins [77]. Breast CSCs secrete TGF-β to stimulate stromal cells in the lungs expressing periostin, which interacts with Wnt1 and Wnt3A to augment Wnt signaling in CSCs [78]. CD133+CXCR4+ CSCs are found in the invasive front of pancreatic tumors, and deletion of this subpopulation abrogates tumor metastasis [79]. Autocrine CCL5 signaling activates the NF-κB pathway, leading to enhanced matrix metallopeptidase 9 (MMP9) secretion that promotes ovarian CSC invasion of stromal tissue [80]. CD117+Stro-1+ osteosarcoma CSCs show high invasive capacities, and CSC-derived tumors metastasize at a higher frequency [31]. CD44v6-expressing colorectal CSCs can initiate the process of tumor metastasis [11]. Therefore, CSCs are crucial for metastatic colonization [2].
CSCs and genomic instability
Genomic instability provides the engine power for the genetic mutations and epigenetic alterations that promote tumorigenesis and tumor progression. Cancer can be caused by the sequential accumulation of genetic mutations and epigenetic modifications that initiate the transformation of neoplasms. Shiras et al. [81] found that genomic instability in glioblastomas promotes the development of an immortalized clone into a CSC-like clone with the capacity of self-renewal, indicating that genomic instability contributes to the formation of CSCs.
Chromosome translocation is involved in the initiation of CSCs and tumorigenesis. The translocation of chromosomes 9 and 22 generates the new fusion protein Bcr-Ab1, and the continuously activated Bcr-Ab1 results in unregulated cell division in chronic myeloid leukemia (CML) [82]. Recently, a fused FGFR-TACC gene was found in glioblastoma. The tyrosine kinase coding domains of FGFR are translocated to the coding domains of TACC. The FGFR-TACC fusion protein exhibits tumor-initiating activity [83]. Similarly, chromosomal inversion between 3q21 and 3q26 brings the GATA2 distal hematopoietic enhancer into close proximity with the EV1 gene, leading to the hyper-activation of EV1 and the formation of AML [84, 85]. The active site mutation (R132H) of isocitrate dehydrogenase (IDH) was found in a high percentage of glioblastoma and AML patients [86, 87]. This type of mutant IDH acquires the ability to catalyze the NADPH-dependent reduction of α-ketoglutarate to R(–)-2-hydroxyglutarate but loses the ability to catalyze the conversion of isocitrate to α-ketoglutarate [88]. IDH1 (R132H) transgenic mice exhibit an increased number of early hematopoietic progenitors [89]. The IDH1 (R132H) mutation can also prevent some of the histone demethylation that is required for specific stem cell differentiation [90]. This evidence indicates that point mutations can affect stem cell maintenance and the incidence of CSC formation. Although random mutations are abundant, only one or two mutations are usually enough to drive the malignant progression of AML. Subsequent, continuous gene mutations may then contribute to cancer progression or relapse [91]. Interestingly, ultraviolet (UV) and mitomycin C, which induce DNA damage, can increase the quantity of CSCs in nasopharyngeal carcinoma [92]. DNA methylation and histone deacetylase inhibitors can induce redifferentiation in CSCs by promoting the re-expression of silenced genes [93].
CSCs, immune surveillance and inflammatory responses
Immune evasion or dysfunction may contribute to the development of tumors. Tumors transplanted in immunodeficient mice grow faster than those in normal animals. There is also a higher incidence of malignant neoplasms in organ transplant recipients and HIV-infected individuals [94]. These findings indicate that the immune system plays an important role in preventing tumorigenesis. CSCs might successfully escape immune surveillance and reconstitute a new tumor mass in new organs or recipients. GSCs contribute to the immune evasion of tumors by inhibiting T-cell proliferation and activation and by inducing regulatory T cell apoptosis [95]. CSCs in renal carcinoma escape immune surveillance by expressing low levels of Fas, natural killer receptors and complement regulatory proteins [96]. A recent study demonstrates that EMT contributes to the inhibition of cytotoxic T lymphocyte-mediated breast tumor cell lysis [97], indicating that CSCs potentially escape from immune surveillance via the EMT program. In addition, AML stem cells attenuate macrophage-mediated phagocytosis through SIRPα/CD47 signaling [98].
To some extent, tumors can be regarded as unhealed chronic inflammatory diseases. Chronic inflammation also contributes to the development of cancer. Hepatitis B infection is the main risk factor for hepatocellular carcinoma (HCC) in Asia. Helicobacter pylori is the most common bacteria found in the human stomach and is a high risk factor for gastric cancer [99]. Inflammation can lead to aberrant DNA methylation in PcG target genes, which promotes the malignant transformation of intestinal tissues [100]. IL-6 is the most common inflammatory cytokine secreted by T cells and macrophages to stimulate immune responses [101]. The cytokine IL-6, which is generated by inflammatory cells, transforms prostate stem cells to prostate CSCs [102]. IL-6 cooperates with tumor-associated macrophage-derived MFG-E8 to promote CSC self-renewal and anticancer drug resistance by activating STAT3 and Sonic Hedgehog pathways [103]. Let-7 inhibition promotes IL-6-mediated activation of the STAT3 signal, which is necessary for the transformation of breast cells to CSCs [104]. Interferon regulatory factor 7 promotes the maintenance of glioma stem cells by activating IL-6 and Notch signaling [105]. Both GSCs and tumor-associated macrophages are enriched in the perivascular niche. Recently, GSCs have been found to secrete periostin to recruit monocyte-derived macrophages from the peripheral blood to support tumor growth [106]. Other immune and inflammatory cells and their secreted factors also play important roles in regulating CSCs. Therefore, CSCs may not only escape immune destruction but they may also hijack and adapt the reprogrammed immune responses and inflammatory cells and cytokines to promote self-maintenance and proliferation.
CSCs and dysregulated metabolism
In contrast to normal cells, which rely on mitochondrial oxidative phosphorylation to produce energy, cancer cells employ glycolysis, even in the presence of oxygen. This phenomenon is termed the “Warburg effect” [107]. This aerobic glycolysis metabolism provides cancer cells with an acidic environment that promotes cancer cell invasion into normal stromal tissue. Moreover, the metabolites generated by glycolysis can be used as intermediate materials to support the rapidly proliferating cells. The embryonic M2 isoform of pyruvate kinase (PKM2) is exclusively expressed in cancer cells and contributes to the Warburg effect. Aerobic glycolysis can be switched off by expressing PKM1 instead of PKM2, leading to reduced lactate production, increased oxygen consumption and a reduced ability to form tumors [108]. When cancer cells are stimulated by certain growth factors, phosphotyrosine signaling can regulate PKM2 to divert glucose metabolites toward anabolic processes instead of energy production [109]. Thus, it has been proposed that only cells experiencing the Warburg effect undergo the genetic aberrations that transform cells into CSCs [110].
CSCs are also maintained by the metabolic switch that is caused by gene dysfunction-mediated decreased oxygen consumption and low levels of reactive oxygen species [111]. Compared with non-CSCs, CSCs preferentially perform glycolysis over oxidative phosphorylation. Forced activation of pyruvate dehydrogenase, a key regulator of oxidative phosphorylation, inhibits CSC self-renewal in vitro and in vivo [112]. AMPK activation establishes a metabolic barrier to reprogramming and imposes a normalized metabolic flow away from glycolysis, the process required to promote stemness and pluripotency [113]. Glycolytic and aldehyde dehydrogenase (ALDH) activities are elevated in mesenchymal GSCs. Inhibition of ALDH1A3 sensitizes mesenchymal GSCs to irradiation [114]. Dichloroacetate inhibits pyruvate dehydrogenase kinase and shifts the metabolism from glycolysis to glucose oxidation, but it cannot promote the production of reactive oxygen species. In conjunction with irradiation, dichloroacetate induces Bax-dependent apoptosis [115]. VHL loss of function in renal carcinoma cells significantly increases HIF-1 activity and the switch from oxidative phosphorylation to glycolysis. Inhibition of HIF-1 can make cancer cells more sensitive to chemotherapy [116]; however, one study showed that GSCs are less glycolytic than differentiated glioma cells. CSCs rely on oxidative phosphorylation to produce more energy that correlates with radioresistance [117]. Most studies support the role of glycolysis in maintaining the stemness of CSCs. Therefore, metabolic therapy is a potential avenue for human cancer treatment.
CSCs and therapeutic resistance
Currently, therapeutic resistance is a fundamental obstacle to human cancer radiotherapy and chemotherapy. CSCs have been shown to contribute to therapeutic resistance and cancer treatment failure [118]. CSCs have channel proteins to efflux chemical compounds. Moreover, compared with non-CSCs, CSCs activate higher levels of survival signal pathways, which make them difficult to be eradicated.
The ABC super-family of transporters is a class of transmembrane proteins that induce multidrug resistance by effluxing drugs out of cells, thus reducing their toxicity [119]. Stem cells are able to pump out Hoechst 33342, so stem cells capable of exporting this dye are known as side population cells [120]. Side population cells express high levels of the transporter genes, ABCA3 and ABCG2, and exhibit a greater ability to expel cytotoxic drugs, leading to an enhanced survival of CSCs [121]. LSCs display a higher drug efflux than non-CSCs and contribute to leukemia relapse [122]. Therefore, ABC transporters in CSCs are potential targets for cancer treatment.
CD133+ GSCs contribute to radioresistance by preferentially activating DNA damage repair pathways in these cells compared with non-CSCs [123]; however, the detailed mechanism of radioresistance in CSCs is not well defined. c-Myc is involved in radioresistance by activating CHK1 and CHK2 in nasopharyngeal CSCs [124]. CD133+ HCC cells promote chemoresistance through the preferential activation of the Akt and Bcl-2 survival signaling pathways [125]. TGF-β is induced as a negative feedback mechanism in breast cancer after chemotherapy treatment. The increased TGF-β signal contributes to breast cancer recurrence through the IL-8-mediated expansion of CSCs, and inhibition of the TGF-β pathway prevents the development of drug-resistant CSCs [126]. Temozolomide is one of the common chemical drugs used in treating GBM patients; however, subsets of quiescent GSCs escape the drug’s effects and are responsible for tumor relapse after treatment [127]. The quiescent bladder CSCs can be activated into proliferative cycles when tumors are exposed to chemotherapy. Blocking this PGE-induced CSC repopulation by a PGE-neutralizing antibody significantly attenuates chemoresistance in bladder tumors [128]. Receptor kinase inhibitor is a common cancer treatment with fewer side effects. A recent report reveals that treatment with high concentrations of an EGFR inhibitor in lung cancer patients results in not only EMT features but also stem cell-like properties [129]. In addition to involvement in the metastatic cascade and acquirement of stemness of cancer cells, the EMT program contributes to radioresistance and chemoresistance. The EMT inducer ZEB1 is found to be stabilized by ATM and to interact with USP7 to stabilize CHK1, which promotes the DNA damage repair response in breast CSCs [130]. miR-30c sensitizes breast cancer cells to paclitaxel and doxorubicin by regulating TWF1 that promotes EMT [131]. Moreover, some cancers evade drugs through a loss of the expression of key proteins. For example, prostate CSCs that fail to express the androgen receptor do not respond to hormonal treatment, leading to the failure of hormone-based therapies [132]. Overall, CSCs can employ diverse mechanisms to acquire therapeutic resistance, and thus, targeting CSCs directly may be more effective than current treatment regimes and may improve the overall survival of cancer patients.
CSCs as therapeutic targets in cancer
Currently, drug candidates that target CSCs are being screened (Table 2). Salinomycin can specifically induce the loss of expression of breast CSC genes and reduce the proportion of CSCs in breast cancers [150]. Another drug, thioridazine, antagonizes dopamine receptors to selectively impair leukemic CSCs without affecting normal blood stem cells [151]. Finally, DECA-14 was identified to specifically target neuroblastoma CSCs without affecting normal pediatric stem cells [152].
Table 2.
Drugs that target cancer stem cells
| Drugs | Targets | CSC types | Mechanisms | References |
|---|---|---|---|---|
| WZB117 | GLUT1 | Pancreatic, ovarian, glioblastoma | Regulating metabolism | [133] |
| PTC-209 | BMI-1 | Colorectal | Inhibiting self-renewal | [134] |
| PF-2341066 | c-Met | Head and neck squamous carcinomas | Eliminating CSC, inhibiting metastasis | [135] |
| ABT-737 | BAD | Breast | Inducing CSC apoptosis | [136] |
| SP600125 | JNK | Pancreatic | Inhibiting self-renewal | [137] |
| AD-01 | CD44 | Breast | Inhibiting self-renewal, inducing differentiation | [138] |
| All-trans retinoic acid | Nuclear receptor | Breast | Inhibiting self-renewal | [139] |
| IIIA4 | EphA3 | Glioblastoma | Inducing apoptosis | [140] |
| 1B50-1 | Calcium channel | Liver | Inhibiting self-renewal | [141] |
| Rituximab | CD20 | Melanoma | Inhibiting metastasis | [142] |
| GSI | γ-Secretase | Ovarian | Inhibiting self-renewal | [143] |
| C3B3 | HLA class I | Myeloma | Inhibiting self-renewal | [144] |
| Echinomycin | HIF1α | Leukemia | Inhibiting self-renewal | [145] |
| GLPG0187 | α(v)-Integrins | Prostate | Inhibiting metastasis | [146] |
| Transtuzumab | HER2 | Breast | Inhibiting self-renewal | [147] |
| 7G3 | CD123 | Acute myeloid leukemia | Impairing homing to bone marrow | [148] |
| 21M18 | DLL4 | Colon | Inhibiting self-renewal | [149] |
The Bcl-2 family proteins are important anti-apoptotic proteins. The inactivation of Bcl-2, Bcl-xl, and Mcl-1 induced by an EGFR inhibitor, lapatinib, significantly promotes breast CSC apoptosis, suggesting that the Bcl-2 family proteins can be used as therapeutic targets in breast cancer [153]. ABT-737, a Bcl-2 inhibitor, can specifically kill AML stem cells without affecting normal hematopoietic cells by inducing the disruption of the BCL-2/BAX complex and the BAK-dependent activation of the apoptotic pathway [154]. Docetaxel exposure can target the Notch and Hedgehog pathways to deplete CSCs by inhibiting Akt and Bcl-2 activities in prostate cancer [155]. The inhibition of TGF-β signaling by a compound was also identified to dramatically decrease the tumorigenicity of glioma stem cells by inducing cell differentiation [156].
A prostate stem cell antigen-based vaccine could affect long-term protection against prostate cancer progression in transgenic mice [157]. In addition, vaccination using dendritic cells that present CSC-associated antigen to stimulate cytotoxic T lymphocytes against CSCs prolongs the survival of animals bearing CSC-derived glioblastoma [158]. The monoclonal antibody against the epitope of CSCs exhibits an anti-cancer effect by suppressing the invasion of cancers or the expansion of CSCs [159, 160].
siRNA also exhibits therapeutic potential in targeting CSCs. A recent study has shown that the targeting of thymosin β4 by siRNA leads to the loss of chemoresistance in breast CSCs [161]. Inhibiting c-Myc expression by siRNA significantly suppresses CSC maintenance [162]. Recently, we reported that the transcription factor, ZFX, could regulate c-Myc to maintain the tumorigenic potential of GSCs [163]. Therefore, using siRNA to target CSC transcription factors represents an additional cancer treatment strategy.
CSCs show high glycolytic activity and low mitochondrial respiration, indicating that CSCs are more resistant to metabolic drugs [164]. In view of the phenomenon that CSCs display deregulated characteristics and drug resistance, combination therapy may be a better option for targeting CSCs. Sorafenib has been used to treat prostate cancer by inhibiting tyrosine protein kinases (VEGFR and PDGFR) and Raf kinases; however, sorafenib treatment can result in NF-κB activation, which promotes the survival of cancer cells. Treatment in combination with sulforaphane can completely suppress sorafenib-induced NF-κB activity and decrease ALDH1 activity in pancreatic CSCs [165]. Synergistic treatment with TRAIL and PI3K inhibitor-perifosine leads to the decrease of CD34+ cells in AML patients [166]. Blockade of Akt/HIF-1α signaling with inhibitors can suppress tumor growth and prolong the survival of animals by eliminating liver CSCs [167]. In addition, inducing differentiation by arsenic trioxide has been identified as an effective strategy to treat acute promyelocytic leukemia [168, 169], but treatment that induces differentiation has a limited effect on solid tumors.
Recently, the CSC conference held in Cleveland led to more knowledge regarding CSC-targeting therapy [170]. Because CSCs exhibit preferentially active signaling pathways than non-stem cancer cells or normal cells, targeting signaling pathways is paid particular attention in clinical trials. For example, OMP-18R5, a monoclonal antibody targeting the Wnt receptor FZD7, inhibits the growth of breast, pancreatic, and colon cancer and is currently in clinical trial phase 1 [171]. Tarextumab (anti-Notch2/3) inhibits CSC self-renewal, induces cell differentiation and displays broad-spectrum anti-tumor ability. Clinical results demonstrate that the diabetic drug metformin has an antineoplastic effect by inhibiting CSC self-renewal and tumor metastasis [172]. More clinical evaluations of metformin on other solid tumors are ongoing.
Concluding remarks
Targeting CSCs provides a promising prospect for cancer treatment with few side effects and a better prognosis; however, the ratios of CSCs vary greatly in different patients. Moreover, CSCs themselves are heterogeneous and evolve continuously within a patient [173], and the different properties and phenotypes of CSCs do not necessarily coexist in the same subpopulation of CSCs. CSCs are heterogeneous among the different types of tumors. The strategy targeting CSCs in one specific tumor may not be effective in other types of tumors. Furthermore, non-tumorigenic cells might be transformed to tumorigenic cells in the presence of the appropriate microenvironmental cues [174–176]. Currently, whether the reversion of non-CSCs to CSCs is a universal phenomenon among all types of tumors remains unknown. Most importantly, both CSCs and non-stem cancer cells have to adapt multiple strategies to overcome various specific microenvironmental growth barriers [177]. Therefore, synergistically targeting CSCs and non-CSCs, together with targeting the tumor microenvironment, should be considered to achieve better therapeutic effects and less adverse reactions.
As shown in Table 1, most types of CSCs share the same cell surface protein as the CSC marker. Moreover, normal stem cells have the same antigen markers and properties with CSCs. For example, CD133 is the marker for neural stem cells and GSCs. Therefore, the drug used for targeting CSCs may inevitably attack normal stem cells. Due to the short lifespan and the severe symptoms of patients with malignant tumors, the negative effects of CSC-targeting therapy are difficult to be detected or are often neglected. Therefore, much research remains to identify the specific markers that only exist in the CSCs and not in normal stem cells.
The drug or antibody delivery efficiency is also a limitation of targeting CSCs. CSCs are only a small population of tumor cells. Some CSCs are located in the hypoxic niche where there are fewer blood vessels. Therefore, whether the compound drug and antibody are able to target CSCs in hypoxic regions is still unknown. Furthermore, the blood–prostate barrier and blood–brain barrier are other concerns that need to be resolved. These types of barriers lead to drug delivery failure, which results in primary or metastatic tumors that cannot be effectively regressed. Therefore, developing notable drugs that effectively target CSCs will also be a major project in the next generation. Taken together, we still need to explore the characteristics of CSCs, which will lead to a better understanding of tumorigenesis and metastasis and will lay a solid foundation for cancer treatment in the future.
Acknowledgments
We would like to thank David Schumick from the Medical Art and Photography Centers at the Cleveland Clinic for creating and modifying the figures in this study. Reprinted with the permission of the Cleveland Clinic Center for Medical Art and Photography © 2015. All rights reserved. This work was supported by grants from the National Nature Science Foundation of China (Nos. 81372841, 31171339, 31071302), the Fundamental Research Funds for the Central Universities, the Program for New Century Excellent Talents in University (NCET-11-0296) and the Scientific Fund for Distinguished Young Investigator of Fujian Province (2010J06013).
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Qi Luo, Email: luoqixmzsh@126.com.
Gaoliang Ouyang, Email: oygldz@xmu.edu.cn.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Ouyang G. Periostin: a bridge between cancer stem cells and their metastatic niche. Cell Stem Cell. 2012;10:111–112. doi: 10.1016/j.stem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 8.Read TA, Fogarty MP, Markant SL, McLendon RE, Wei Z, Ellison DW, Febbo PG, Wechsler-Reya RJ. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 2009;15:135–147. doi: 10.1016/j.ccr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G, Medema JP. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 11.Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S, Cocorullo G, Gulotta G, Dieli F, De Maria R, Stassi G. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14:342–356. doi: 10.1016/j.stem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo Vullo S, Camerini T, Mariani L, Delia D, Calabrò E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, Katz RL. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feuring-Buske M, Hogge DE. Hoechst 33342 efflux identifies a subpopulation of cytogenetically normal CD34(+)CD38(−) progenitor cells from patients with acute myeloid leukemia. Blood. 2001;97:3882–3889. doi: 10.1182/blood.v97.12.3882. [DOI] [PubMed] [Google Scholar]
- 17.Escribano L, Ocqueteau M, Almeida J, Orfao A, San Miguel JF. Expression of the c-kit (CD117) molecule in normal and malignant hematopoiesis. Leuk Lymphoma. 1998;30:459–466. doi: 10.3109/10428199809057558. [DOI] [PubMed] [Google Scholar]
- 18.Hosen N, Park CY, Tatsumi N, Oji Y, Sugiyama H, Gramatzki M, Krensky AM, Weissman IL. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci USA. 2007;104:11008–11013. doi: 10.1073/pnas.0704271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinc A, Somasundaram R, Wagner C, Hörmann M, Karanikas G, Jalili A, Bauer W, Brunner P, Grabmeier-Pfistershammer K, Gschaider M, Lai CY, Hsu MY, Herlyn M, Stingl G, Wagner SN. Targeting CD20 in melanoma patients at high risk of disease recurrence. Mol Ther. 2012;20:1056–1062. doi: 10.1038/mt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, Longaker MT, Weissman IL. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curley MD, Therrien VA, Cummings CL, Sergent PA, Koulouris CR, Friel AM, Roberts DJ, Seiden MV, Scadden DT, Rueda BR, Foster R. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27:2875–2883. doi: 10.1002/stem.236. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 26.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 27.Rajasekhar VK, Studer L, Gerald W, Socci ND, Scher HI. Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-kappaB signalling. Nat Commun. 2011;2:162. doi: 10.1038/ncomms1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seiwert TY, Jagadeeswaran R, Faoro L, Janamanchi V, Nallasura V, El Dinali M, Yala S, Kanteti R, Cohen EE, Lingen MW, Martin L, Krishnaswamy S, Klein-Szanto A, Christensen JG, Vokes EE, Salgia R. The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma. Cancer Res. 2009;69:3021–3031. doi: 10.1158/0008-5472.CAN-08-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tirino V, Desiderio V, d’Aquino R, De Francesco F, Pirozzi G, Graziano A, Galderisi U, Cavaliere C, De Rosa A, Papaccio G, Giordano A. Detection and characterization of CD133+ cancer stem cells in human solid tumours. PLoS ONE. 2008;3:e3469. doi: 10.1371/journal.pone.0003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adhikari AS, Agarwal N, Wood BM, Porretta C, Ruiz B, Pochampally RR, Iwakuma T. CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Res. 2010;70:4602–4612. doi: 10.1158/0008-5472.CAN-09-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, Fazioli F, Pirozzi G, Papaccio G. Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumorigenicity in vivo. FASEB J. 2011;25:2022–2030. doi: 10.1096/fj.10-179036. [DOI] [PubMed] [Google Scholar]
- 33.Liu A, Feng B, Gu W, Cheng X, Tong T, Zhang H, Hu Y. The CD133+ subpopulation of the SW982 human synovial sarcoma cell line exhibits cancer stem-like characteristics. Int J Oncol. 2013;42:1399–1407. doi: 10.3892/ijo.2013.1826. [DOI] [PubMed] [Google Scholar]
- 34.Suva ML, Riggi N, Stehle JC, Baumer K, Tercier S, Joseph JM, Suvà D, Clément V, Provero P, Cironi L, Osterheld MC, Guillou L, Stamenkovic I. Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res. 2009;69:1776–1781. doi: 10.1158/0008-5472.CAN-08-2242. [DOI] [PubMed] [Google Scholar]
- 35.Awad O, Yustein JT, Shah P, Gul N, Katuri V, O’Neill A, Kong Y, Brown ML, Toretsky JA, Loeb DM. High ALDH activity identifies chemotherapy-resistant Ewing’s sarcoma stem cells that retain sensitivity to EWS-FLI1 inhibition. PLoS ONE. 2010;5:e13943. doi: 10.1371/journal.pone.0013943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter D, Satheesha S, Albrecht P, Bornhauser BC, D’Alessandro V, Oesch SM, Rehrauer H, Leuschner I, Koscielniak E, Gengler C, Moch H, Bernasconi M, Niggli FK, Schäfer BW. CD133 positive embryonal rhabdomyosarcoma stem-like cell population is enriched in rhabdospheres. PLoS ONE. 2011;6:e19506. doi: 10.1371/journal.pone.0019506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, Stommel JM, Dunn KL, Wiedemeyer R, You MJ, Brennan C, Wang YA, Ligon KL, Wong WH, Chin L, DePinho RA. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hide T, Takezaki T, Nakatani Y, Nakamura H, Kuratsu J, Kondo T. Sox11 prevents tumorigenesis of glioma-initiating cells by inducing neuronal differentiation. Cancer Res. 2009;69:7953–7959. doi: 10.1158/0008-5472.CAN-09-2006. [DOI] [PubMed] [Google Scholar]
- 39.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 41.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, De Maria R. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 42.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 43.Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, Min W, McLendon RE, Rich JN, Bao S. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu AY, Ouyang G. Tumor angiogenesis: a new source of pericytes. Curr Biol. 2013;23:R565–R568. doi: 10.1016/j.cub.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 45.Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007;67:4807–4815. doi: 10.1158/0008-5472.CAN-06-4608. [DOI] [PubMed] [Google Scholar]
- 46.Ashley N, Yeung TM, Bodmer WF. Stem cell differentiation and lumen formation in colorectal cancer cell lines and primary tumors. Cancer Res. 2013;73:5798–5809. doi: 10.1158/0008-5472.CAN-13-0454. [DOI] [PubMed] [Google Scholar]
- 47.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, Stassi G. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Lin SP, Lee YT, Yang SH, Miller SA, Chiou SH, Hung MC, Hung SC. Colon cancer stem cells resist antiangiogenesis therapy-induced apoptosis. Cancer Lett. 2013;328:226–234. doi: 10.1016/j.canlet.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 49.Touil Y, Igoudjil W, Corvaisier M, Dessein AF, Vandomme J, Monté D, Stechly L, Skrypek N, Langlois C, Grard G, Millet G, Leteurtre E, Dumont P, Truant S, Pruvot FR, Hebbar M, Fan F, Ellis LM, Formstecher P, Van Seuningen I, Gespach C, Polakowska R, Huet G. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin Cancer Res. 2014;20:837–846. doi: 10.1158/1078-0432.CCR-13-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demidov ON, Timofeev O, Lwin HN, Kek C, Appella E, Bulavin DV. Wip1 phosphatase regulates p53-dependent apoptosis of stem cells and tumorigenesis in the mouse intestine. Cell Stem Cell. 2007;1:180–190. doi: 10.1016/j.stem.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 51.Liu L, Andrews LG, Tollefsbol TO. Loss of the human polycomb group protein BMI1 promotes cancer-specific cell death. Oncogene. 2006;25:4370–4375. doi: 10.1038/sj.onc.1209454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SM, Oh JH, Park SA, Ryu CH, Lim JY, Kim DS, Chang JW, Oh W, Jeun SS. Irradiation enhances the tumor tropism and therapeutic potential of tumor necrosis factor-related apoptosis-inducing ligand-secreting human umbilical cord blood-derived mesenchymal stem cells in glioma therapy. Stem Cells. 2010;28:2217–2228. doi: 10.1002/stem.543. [DOI] [PubMed] [Google Scholar]
- 53.Tsai HC, Li H, Van Neste L, Cai Y, Robert C, Rassool FV, Shin JJ, Harbom KM, Beaty R, Pappou E, Harris J, Yen RW, Ahuja N, Brock MV, Stearns V, Feller-Kopman D, Yarmus LB, Lin YC, Welm AL, Issa JP, Minn I, Matsui W, Jang YY, Sharkis SJ, Baylin SB, Zahnow CA. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell. 2012;21:430–446. doi: 10.1016/j.ccr.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hegde S, Kaushal N, Ravindra KC, Chiaro C, Hafer KT, Gandhi UH, Thompson JT, van den Heuvel JP, Kennett MJ, Hankey P, Paulson RF, Prabhu KS. Delta12-prostaglandin J3, an omega-3 fatty acid-derived metabolite, selectively ablates leukemia stem cells in mice. Blood. 2011;118:6909–6919. doi: 10.1182/blood-2010-11-317750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang YC, Chao TK, Chang CC, Yo YT, Yu MH, Lai HC. Drug screening identifies niclosamide as an inhibitor of breast cancer stem-like cells. PLoS ONE. 2013;8:e74538. doi: 10.1371/journal.pone.0074538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Achilles EG, Fernandez A, Allred EN, Kisker O, Udagawa T, Beecken WD, Flynn E, Folkman J. Heterogeneity of angiogenic activity in a human liposarcoma: a proposed mechanism for “no take” of human tumors in mice. J Natl Cancer Inst. 2001;93:1075–1081. doi: 10.1093/jnci/93.14.1075. [DOI] [PubMed] [Google Scholar]
- 57.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 58.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 59.Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, Hoffman RM, Kerbel RS. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69:7243–7251. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A, Mascre G, Drogat B, Dekoninck S, Haigh JJ, Carmeliet P, Blanpain C. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- 61.Ping YF, Yao XH, Jiang JY, Zhao LT, Yu SC, Jiang T, Lin MC, Chen JH, Wang B, Zhang R, Cui YH, Qian C, Jm Wang, Bian XW. The chemokine CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT signalling. J Pathol. 2011;224:344–354. doi: 10.1002/path.2908. [DOI] [PubMed] [Google Scholar]
- 62.Shen R, Ye Y, Chen L, Yan Q, Barsky SH, Gao JX. Precancerous stem cells can serve as tumor vasculogenic progenitors. PLoS ONE. 2008;3:e1652. doi: 10.1371/journal.pone.0001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang KH, Ma S, Lee TK, Chan YP, Kwan PS, Tong CM, Ng IO, Man K, To KF, Lai PB, Lo CM, Guan XY, Chan KW. CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology. 2012;55:807–820. doi: 10.1002/hep.24739. [DOI] [PubMed] [Google Scholar]
- 64.Hovinga KE, Shimizu F, Wang R, Panagiotakos G, Van Der Heijden M, Moayedpardazi H, Correia AS, Soulet D, Major T, Menon J, Tabar V. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 2010;28:1019–1029. doi: 10.1002/stem.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 66.Conley SJ, Gheordunescu E, Kakarala P, Newman B, Korkaya H, Heath AN, Clouthier SG, Wicha MS. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci USA. 2012;109:2784–2789. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, Hjelmeland AB, Rich JN. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 69.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fang X, Cai Y, Liu J, Wang Z, Wu Q, Zhang Z, Yang CJ, Yuan L, Ouyang G. Twist2 contributes to breast cancer progression by promoting an epithelial–mesenchymal transition and cancer stem-like cell self-renewal. Oncogene. 2011;30:4707–4720. doi: 10.1038/onc.2011.181. [DOI] [PubMed] [Google Scholar]
- 71.Liu AY, Cai Y, Mao Y, Lin Y, Zheng H, Wu T, Huang Y, Fang X, Lin S, Feng Q, Huang Z, Yang T, Luo Q, Ouyang G. Twist2 promotes self-renewal of liver cancer stem-like cells by regulating CD24. Carcinogenesis. 2014;35:537–545. doi: 10.1093/carcin/bgt364. [DOI] [PubMed] [Google Scholar]
- 72.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells—what challenges do they pose? Nat Rev Drug Discov. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang Q, Lin Y, Li J, Kang T, Tao M, Rusinova E, Zhang G, Wang C, Zhu H, Yao J, Zeng YX, Evers BM, Zhou MM, Zhou BP. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell. 2014;25:210–225. doi: 10.1016/j.ccr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, Ding S, Guan KL. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, Zwang Y, Roberts TM, Root DE, Jacks T, Hahn WC. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial–mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celià-Terrassa T, Mercatali L, Khan Z, Goodarzi H, Hua Y, Wei Y, Hu G, Garcia BA, Ragoussis J, Amadori D, Harris AL, Kang Y. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med. 2011;17:1101–1108. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 79.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Long H. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-kappaB-mediated MMP-9 upregulation. Stem Cells. 2012;30:2309–2319. doi: 10.1002/stem.1194. [DOI] [PubMed] [Google Scholar]
- 81.Shiras A, Chettiar ST, Shepal V, Rajendran G, Prasad GR, Shastry P. Spontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells. 2007;25:1478–1489. doi: 10.1634/stemcells.2006-0585. [DOI] [PubMed] [Google Scholar]
- 82.Advani AS, Pendergast AM. Bcr-Abl variants: biological and clinical aspects. Leuk Res. 2002;26:713–720. doi: 10.1016/s0145-2126(01)00197-7. [DOI] [PubMed] [Google Scholar]
- 83.Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, Liu EM, Reichel J, Porrati P, Pellegatta S, Qiu K, Gao Z, Ceccarelli M, Riccardi R, Brat DJ, Guha A, Aldape K, Golfinos JG, Zagzag D, Mikkelsen T, Finocchiaro G, Lasorella A, Rabadan R, Iavarone A. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337:1231–1235. doi: 10.1126/science.1220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamazaki H, Suzuki M, Otsuki A, Shimizu R, Bresnick EH, Engel JD, Yamamoto M. A remote GATA2 hematopoietic enhancer drives leukemogenesis in inv(3)(q21;q26) by activating EVI1 expression. Cancer Cell. 2014;25:415–427. doi: 10.1016/j.ccr.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Groschel S, Sanders MA, Hoogenboezem R, de Wit E, Bouwman BA, Erpelinck C, van der Velden VH, Havermans M, Avellino R, van Lom K, Rombouts EJ, van Duin M, Döhner K, Beverloo HB, Bradner JE, Döhner H, Löwenberg B, Valk PJ, Bindels EM, de Laat W, Delwel R. A single oncogenic enhancer rearrangement causes concomitant EVI1 and GATA2 deregulation in leukemia. Cell. 2014;157:369–381. doi: 10.1016/j.cell.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 86.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD, Fulton LA, Locke DP, Magrini VJ, Abbott RM, Vickery TL, Reed JS, Robinson JS, Wylie T, Smith SM, Carmichael L, Eldred JM, Harris CC, Walker J, Peck JB, Du F, Dukes AF, Sanderson GE, Brummett AM, Clark E, McMichael JF, Meyer RJ, Schindler JK, Pohl CS, Wallis JW, Shi X, Lin L, Schmidt H, Tang Y, Haipek C, Wiechert ME, Ivy JV, Kalicki J, Elliott G, Ries RE, Payton JE, Westervelt P, Tomasson MH, Watson MA, Baty J, Heath S, Shannon WD, Nagarajan R, Link DC, Walter MJ, Graubert TA, DiPersio JF, Wilson RK, Ley TJ. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Vander Heiden MG, Su SM. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sasaki M, Knobbe CB, Munger JC, Lind EF, Brenner D, Brüstle A, Harris IS, Holmes R, Wakeham A, Haight J, You-Ten A, Li WY, Schalm S, Su SM, Virtanen C, Reifenberger G, Ohashi PS, Barber DL, Figueroa ME, Melnick A, Zúñiga-Pflücker JC, Mak TW. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488:656–659. doi: 10.1038/nature11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A, Wellen KE, O’Rourke DM, Berger SL, Chan TA, Levine RL, Mellinghoff IK, Thompson CB. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, Wartman LD, Lamprecht TL, Liu F, Xia J, Kandoth C, Fulton RS, McLellan MD, Dooling DJ, Wallis JW, Chen K, Harris CC, Schmidt HK, Kalicki-Veizer JM, Lu C, Zhang Q, Lin L, O’Laughlin MD, McMichael JF, Delehaunty KD, Fulton LA, Magrini VJ, McGrath SD, Demeter RT, Vickery TL, Hundal J, Cook LL, Swift GW, Reed JP, Alldredge PA, Wylie TN, Walker JR, Watson MA, Heath SE, Shannon WD, Varghese N, Nagarajan R, Payton JE, Baty JD, Kulkarni S, Klco JM, Tomasson MH, Westervelt P, Walter MJ, Graubert TA, DiPersio JF, Ding L, Mardis ER, Wilson RK. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150:264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liang Y, Zhong Z, Huang Y, Deng W, Cao J, Tsao G, Liu Q, Pei D, Kang T, Zeng YX. Stem-like cancer cells are inducible by increasing genomic instability in cancer cells. J Biol Chem. 2010;285:4931–4940. doi: 10.1074/jbc.M109.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tysnes BB. Tumor-initiating and -propagating cells: cells that we would like to identify and control. Neoplasia. 2010;12:506–515. doi: 10.1593/neo.10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 95.Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, Gumin J, Henry V, Colman H, Sawaya R, Lang FF, Heimberger AB. Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res. 2010;16:461–473. doi: 10.1158/1078-0432.CCR-09-1983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Zhong Y, Guan K, Guo S, Zhou C, Wang D, Ma W, Zhang Y, Li C, Zhang S. Spheres derived from the human SK-RC-42 renal cell carcinoma cell line are enriched in cancer stem cells. Cancer Lett. 2010;299:150–160. doi: 10.1016/j.canlet.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 97.Akalay I, Janji B, Hasmim M, Noman MZ, André F, De Cremoux P, Bertheau P, Badoual C, Vielh P, Larsen AK, Sabbah M, Tan TZ, Keira JH, Hung NT, Thiery JP, Mami-Chouaib F, Chouaib S. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res. 2013;73:2418–2427. doi: 10.1158/0008-5472.CAN-12-2432. [DOI] [PubMed] [Google Scholar]
- 98.Theocharides AP, Jin L, Cheng PY, Prasolava TK, Malko AV, Ho JM, Poeppl AG, van Rooijen N, Minden MD, Danska JS, Dick JE, Wang JC. Disruption of SIRPalpha signaling in macrophages eliminates human acute myeloid leukemia stem cells in xenografts. J Exp Med. 2012;209:1883–1899. doi: 10.1084/jem.20120502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 100.Hahn MA, Hahn T, Lee DH, Esworthy RS, Kim BW, Riggs AD, Chu FF, Pfeifer GP. Methylation of polycomb target genes in intestinal cancer is mediated by inflammation. Cancer Res. 2008;68:10280–10289. doi: 10.1158/0008-5472.CAN-08-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Köhler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 102.Maitland NJ, Collins AT. Inflammation as the primary aetiological agent of human prostate cancer: a stem cell connection? J Cell Biochem. 2008;105:931–939. doi: 10.1002/jcb.21843. [DOI] [PubMed] [Google Scholar]
- 103.Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci USA. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jin X, Kim SH, Jeon HM, Beck S, Sohn YW, Yin J, Kim JK, Lim YC, Lee JH, Kim SH, Kang SH, Pian X, Song MS, Park JB, Chae YS, Chung YG, Lee SH, Choi YJ, Nam DH, Choi YK, Kim H. Interferon regulatory factor 7 regulates glioma stem cells via interleukin-6 and Notch signalling. Brain. 2012;135:1055–1069. doi: 10.1093/brain/aws028. [DOI] [PubMed] [Google Scholar]
- 106.Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, Rich JN, Bao S. Periostin secreted by glioblastoma stem cells recruits M2 tumor-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17:170–182. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 109.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 110.Menendez JA, Joven J, Cufí S, Corominas-Faja B, Oliveras-Ferraros C, Cuyàs E, Martin-Castillo B, López-Bonet E, Alarcón T, Vazquez-Martin A. The Warburg effect version 2.0: metabolic reprogramming of cancer stem cells. Cell Cycle. 2013;12:1166–1179. doi: 10.4161/cc.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, Lorkiewicz P, St Clair D, Hung MC, Evers BM, Zhou BP. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–331. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feng W, Gentles A, Nair RV, Huang M, Lin Y, Lee CY, Cai S, Scheeren FA, Kuo AH, Diehn M. Targeting unique metabolic properties of breast tumor initiating cells. Stem Cells. 2014;32:1734–1745. doi: 10.1002/stem.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vazquez-Martin A, Vellon L, Quirós PM, Cufí S, Ruiz de Galarreta E, Oliveras-Ferraros C, Martin AG, Martin-Castillo B, López-Otín C, Menendez JA. Activation of AMP-activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells. Cell Cycle. 2012;11:974–989. doi: 10.4161/cc.11.5.19450. [DOI] [PubMed] [Google Scholar]
- 114.Mao P, Joshi K, Li J, Kim SH, Li P, Santana-Santos L, Luthra S, Chandran UR, Benos PV, Smith L, Wang M, Hu B, Cheng SY, Sobol RW, Nakano I. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci USA. 2013;110:8644–8649. doi: 10.1073/pnas.1221478110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morfouace M, Lalier L, Bahut M, Bonnamain V, Naveilhan P, Guette C, Oliver L, Gueguen N, Reynier P, Vallette FM. Comparison of spheroids formed by rat glioma stem cells and neural stem cells reveals differences in glucose metabolism and promising therapeutic applications. J Biol Chem. 2012;287:33664–33674. doi: 10.1074/jbc.M111.320028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–3671. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vlashi E, Lagadec C, Vergnes L, Matsutani T, Masui K, Poulou M, Popescu R, Della Donna L, Evers P, Dekmezian C, Reue K, Christofk H, Mischel PS, Pajonk F. Metabolic state of glioma stem cells and nontumorigenic cells. Proc Natl Acad Sci USA. 2011;108:16062–16067. doi: 10.1073/pnas.1106704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jackson M, Hassiotou F, Nowak A. Glioblastoma stem-like cells: at the root of tumor recurrence and a therapeutic target. Carcinogenesis. 2015 doi: 10.1093/carcin/bgu243. [DOI] [PubMed] [Google Scholar]
- 119.Tan B, Piwnica-Worms D, Ratner L. Multidrug resistance transporters and modulation. Curr Opin Oncol. 2000;12:450–458. doi: 10.1097/00001622-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 120.Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 121.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wulf GG, Wang RY, Kuehnle I, Weidner D, Marini F, Brenner MK, Andreeff M, Goodell MA. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–1173. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- 123.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 124.Wang WJ, Wu SP, Liu JB, Shi YS, Huang X, Zhang QB, Yao KT. MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res. 2013;73:1219–1231. doi: 10.1158/0008-5472.CAN-12-1408. [DOI] [PubMed] [Google Scholar]
- 125.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 126.Bhola NE, Balko JM, Dugger TC, Kuba MG, Sánchez V, Sanders M, Stanford J, Cook RS, Arteaga CL. TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest. 2013;123:1348–1358. doi: 10.1172/JCI65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kurtova AV, Xiao J, Mo Q, Pazhanisamy S, Krasnow R, Lerner SP, Chen F, Roh TT, Lay E, Ho PL, Chan KS. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517:209–213. doi: 10.1038/nature14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shien K, Toyooka S, Yamamoto H, Soh J, Jida M, Thu KL, Hashida S, Maki Y, Ichihara E, Asano H, Tsukuda K, Takigawa N, Kiura K, Gazdar AF, Lam WL, Miyoshi S. Acquired resistance to EGFR inhibitors is associated with a manifestation of stem cell-like properties in cancer cells. Cancer Res. 2013;73:3051–3061. doi: 10.1158/0008-5472.CAN-12-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang P, Wei Y, Wang L, Debeb BG, Yuan Y, Zhang J, Yuan J, Wang M, Chen D, Sun Y, Woodward WA, Liu Y, Dean DC, Liang H, Hu Y, Ang KK, Hung MC, Chen J, Ma L. ATM-mediated stabilization of ZEB1 promotes DNA damage response and radioresistance through CHK1. Nat Cell Biol. 2014;16:864–875. doi: 10.1038/ncb3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bockhorn J, Dalton R, Nwachukwu C, Huang S, Prat A, Yee K, Chang YF, Huo D, Wen Y, Swanson KE, Qiu T, Lu J, Park SY, Dolan ME, Perou CM, Olopade OI, Clarke MF, Greene GL, Liu H. MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11. Nat Commun. 2013;4:1393. doi: 10.1038/ncomms2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maitland NJ, Collins AT. Prostate cancer stem cells: a new target for therapy. J Clin Oncol. 2008;26:2862–2870. doi: 10.1200/JCO.2007.15.1472. [DOI] [PubMed] [Google Scholar]
- 133.Shibuya K, Okada M, Suzuki S, Seino M, Seino S, Takeda H, Kitanaka C. Targeting the facilitative glucose transporter GLUT1 inhibits the self-renewal and tumor-initiating capacity of cancer stem cells. Oncotarget. 2015;6:651–661. doi: 10.18632/oncotarget.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kreso A, van Galen P, Pedley NM, Lima-Fernandes E, Frelin C, Davis T, Cao L, Baiazitov R, Du W, Sydorenko N, Moon YC, Gibson L, Wang Y, Leung C, Iscove NN, Arrowsmith CH, Szentgyorgyi E, Gallinger S, Dick JE, O’Brien CA. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med. 2014;20:29–36. doi: 10.1038/nm.3418. [DOI] [PubMed] [Google Scholar]
- 135.Sun S, Liu S, Duan SZ, Zhang L, Zhou H, Hu Y, Zhou X, Shi C, Zhou R, Zhang Z. Targeting the c-Met/FZD8 signaling axis eliminates patient-derived cancer stem-like cells in head and neck squamous carcinomas. Cancer Res. 2014;74:7546–7559. doi: 10.1158/0008-5472.CAN-14-0826. [DOI] [PubMed] [Google Scholar]
- 136.Sastry KS, Al-Muftah MA, Li P, Al-Kowari MK, Wang E, Ismail Chouchane A, Kizhakayil D, Kulik G, Marincola FM, Haoudi A, Chouchane L. Targeting proapoptotic protein BAD inhibits survival and self-renewal of cancer stem cells. Cell Death Differ. 2014;21:1936–1949. doi: 10.1038/cdd.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Okada M, Shibuya K, Sato A, Seino S, Suzuki S, Seino M, Kitanaka C. Targeting the K-Ras–JNK axis eliminates cancer stem-like cells and prevents pancreatic tumor formation. Oncotarget. 2014;5:5100–5112. doi: 10.18632/oncotarget.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McClements L, Yakkundi A, Papaspyropoulos A, Harrison H, Ablett MP, Jithesh PV, McKeen HD, Bennett R, Donley C, Kissenpfennig A, McIntosh S, McCarthy HO, O’Neill E, Clarke RB, Robson T. Targeting treatment-resistant breast cancer stem cells with FKBPL and its peptide derivative, AD-01, via the CD44 pathway. Clin Cancer Res. 2013;19:3881–3893. doi: 10.1158/1078-0432.CCR-13-0595. [DOI] [PubMed] [Google Scholar]
- 139.Bhat-Nakshatri P, Goswami CP, Badve S, Sledge GW, Jr, Nakshatri H. Identification of FDA-approved drugs targeting breast cancer stem cells along with biomarkers of sensitivity. Sci Rep. 2013;3:2530. doi: 10.1038/srep02530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Day BW, Stringer BW, Al-Ejeh F, Ting MJ, Wilson J, Ensbey KS, Jamieson PR, Bruce ZC, Lim YC, Offenhäuser C, Charmsaz S, Cooper LT, Ellacott JK, Harding A, Leveque L, Inglis P, Allan S, Walker DG, Lackmann M, Osborne G, Khanna KK, Reynolds BA, Lickliter JD, Boyd AW. EphA3 maintains tumorigenicity and is a therapeutic target in glioblastoma multiforme. Cancer Cell. 2013;23:238–248. doi: 10.1016/j.ccr.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 141.Zhao W, Wang L, Han H, Jin K, Lin N, Guo T, Chen Y, Cheng H, Lu F, Fang W, Wang Y, Xing B, Zhang Z. 1B50-1, a mAb raised against recurrent tumor cells, targets liver tumor-initiating cells by binding to the calcium channel α2δ1 subunit. Cancer Cell. 2013;23:541–556. doi: 10.1016/j.ccr.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 142.Schlaak M, Schmidt P, Bangard C, Kurschat P, Mauch C, Abken H. Regression of metastatic melanoma in a patient by antibody targeting of cancer stem cells. Oncotarget. 2012;3:22–30. doi: 10.18632/oncotarget.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McAuliffe SM, Morgan SL, Wyant GA, Tran LT, Muto KW, Chen YS, Chin KT, Partridge JC, Poole BB, Cheng KH, Daggett J, Jr, Cullen K, Kantoff E, Hasselbatt K, Berkowitz J, Muto MG, Berkowitz RS, Aster JC, Matulonis UA, Dinulescu DM. Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc Natl Acad Sci USA. 2012;109:E2939–E2948. doi: 10.1073/pnas.1206400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ikegame A, Ozaki S, Tsuji D, Harada T, Fujii S, Nakamura S, Miki H, Nakano A, Kagawa K, Takeuchi K, Abe M, Watanabe K, Hiasa M, Kimura N, Kikuchi Y, Sakamoto A, Habu K, Endo M, Itoh K, Yamada-Okabe H, Matsumoto T. Small molecule antibody targeting HLA class I inhibits myeloma cancer stem cells by repressing pluripotency-associated transcription factors. Leukemia. 2012;26:2124–2134. doi: 10.1038/leu.2012.78. [DOI] [PubMed] [Google Scholar]
- 145.Wang Y, Liu Y, Malek SN, Zheng P, Liu Y. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8:399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van der Horst G, van den Hoogen C, Buijs JT, Cheung H, Bloys H, Pelger RC, Lorenzon G, Heckmann B, Feyen J, Pujuguet P, Blanque R, Clément-Lacroix P, van der Pluijm G. Targeting of α(v)-integrins in stem/progenitor cells and supportive microenvironment impairs bone metastasis in human prostate cancer. Neoplasia. 2011;13:516–525. doi: 10.1593/neo.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, Sozzi G, Fontanella E, Menard S, Tagliabue E. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res. 2009;15:2010–2021. doi: 10.1158/1078-0432.CCR-08-1327. [DOI] [PubMed] [Google Scholar]
- 148.Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, Guthridge MA, Thomas D, Barry EF, Boyd A, Gearing DP, Vairo G, Lopez AF, Dick JE, Lock RB. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 149.Hoey T, Yen WC, Axelrod F, Basi J, Donigian L, Dylla S, Fitch-Bruhns M, Lazetic S, Park IK, Sato A, Satyal S, Wang X, Clarke MF, Lewicki J, Gurney A. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 150.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sachlos E, Risueño RM, Laronde S, Shapovalova Z, Lee JH, Russell J, Malig M, McNicol JD, Fiebig-Comyn A, Graham M, Levadoux-Martin M, Lee JB, Giacomelli AO, Hassell JA, Fischer-Russell D, Trus MR, Foley R, Leber B, Xenocostas A, Brown ED, Collins TJ, Bhatia M. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149:1284–1297. doi: 10.1016/j.cell.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 152.Smith KM, Datti A, Fujitani M, Grinshtein N, Zhang L, Morozova O, Blakely KM, Rotenberg SA, Hansford LM, Miller FD, Yeger H, Irwin MS, Moffat J, Marra MA, Baruchel S, Wrana JL, Kaplan DR. Selective targeting of neuroblastoma tumour-initiating cells by compounds identified in stem cell-based small molecule screens. EMBO Mol Med. 2010;2:371–384. doi: 10.1002/emmm.201000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lang JY, Hsu JL, Meric-Bernstam F, Chang CJ, Wang Q, Bao Y, Yamaguchi H, Xie X, Woodward WA, Yu D, Hortobagyi GN, Hung MC. BikDD eliminates breast cancer initiating cells and synergizes with lapatinib for breast cancer treatment. Cancer Cell. 2011;20:341–356. doi: 10.1016/j.ccr.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling X, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 155.Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, Bonal DM, Charytonowicz E, Gladoun N, de la Iglesia-Vicente J, Petrylak DP, Benson MC, Silva JM, Cordon-Cardo C. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012;22:373–388. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ikushima H, Todo T, Ino Y, Takahashi M, Miyazawa K, Miyazono K. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5:504–514. doi: 10.1016/j.stem.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 157.Garcia-Hernandez Mde L, Gray A, Hubby B, Klinger OJ, Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008;68:861–869. doi: 10.1158/0008-5472.CAN-07-0445. [DOI] [PubMed] [Google Scholar]
- 158.Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J, Konda B, Black KL, Yu JS. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells. 2009;27:1734–1740. doi: 10.1002/stem.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu M, Yuan Y, Xia Y, Achilefu S. Monoclonal antibody CC188 binds a carbohydrate epitope expressed on the surface of both colorectal cancer stem cells and their differentiated progeny. Clin Cancer Res. 2008;14:7461–7469. doi: 10.1158/1078-0432.CCR-07-4430. [DOI] [PubMed] [Google Scholar]
- 160.Majeti R. Monoclonal antibody therapy directed against human acute myeloid leukemia stem cells. Oncogene. 2011;30:1009–1019. doi: 10.1038/onc.2010.511. [DOI] [PubMed] [Google Scholar]
- 161.Steiniger SC, Coppinger JA, Krüger JA, Yates J, 3rd, Janda KD. Quantitative mass spectrometry identifies drug targets in cancer stem cell-containing side population. Stem Cells. 2008;26:3037–3046. doi: 10.1634/stemcells.2008-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Civenni G, Malek A, Albino D, Garcia-Escudero R, Napoli S, Di Marco S, Pinton S, Sarti M, Carbone GM, Catapano CV. RNAi-mediated silencing of Myc transcription inhibits stem-like cell maintenance and tumorigenicity in prostate cancer. Cancer Res. 2013;73:6816–6827. doi: 10.1158/0008-5472.CAN-13-0615. [DOI] [PubMed] [Google Scholar]
- 163.Fang X, Huang Z, Zhou W, Wu Q, Sloan AE, Ouyang G, McLendon RE, Yu JS, Rich JN, Bao S. The zinc finger transcription factor ZFX Is required for maintaining the tumorigenic potential of glioblastoma stem cells. Stem Cells. 2014;32:2033–2047. doi: 10.1002/stem.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yuan S, Wang F, Chen G, Zhang H, Feng L, Wang L, Colman H, Keating MJ, Li X, Xu RH, Wang J, Huang P. Effective elimination of cancer stem cells by a novel drug combination strategy. Stem Cells. 2013;31:23–34. doi: 10.1002/stem.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rausch V, Liu L, Kallifatidis G, Baumann B, Mattern J, Gladkich J, Wirth T, Schemmer P, Büchler MW, Zöller M, Salnikov AV, Herr I. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res. 2010;70:5004–5013. doi: 10.1158/0008-5472.CAN-10-0066. [DOI] [PubMed] [Google Scholar]
- 166.Tazzari PL, Tabellini G, Ricci F, Papa V, Bortul R, Chiarini F, Evangelisti C, Martinelli G, Bontadini A, Cocco L, McCubrey JA, Martelli AM. Synergistic proapoptotic activity of recombinant TRAIL plus the Akt inhibitor Perifosine in acute myelogenous leukemia cells. Cancer Res. 2008;68:9394–9403. doi: 10.1158/0008-5472.CAN-08-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lau CK, Yang ZF, Ho DW, Ng MN, Yeoh GC, Poon RT, Fan ST. An Akt/hypoxia-inducible factor-1alpha/platelet-derived growth factor-BB autocrine loop mediates hypoxia-induced chemoresistance in liver cancer cells and tumorigenic hepatic progenitor cells. Clin Cancer Res. 2009;15:3462–3471. doi: 10.1158/1078-0432.CCR-08-2127. [DOI] [PubMed] [Google Scholar]
- 168.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, Liu MM, He KL, Niu C, Ma J, Zhang P, Zhang TD, Paul P, Naoe T, Kitamura K, Miller W, Waxman S, Wang ZY, de The H, Chen SJ, Chen Z. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 169.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 170.Adorno-Cruz V, Kibria G, Liu X, Doherty M, Junk DJ, Guan D, Hubert C, Venere M, Mulkearns-Hubert E, Sinyuk M, Alvarado A, Caplan AI, Rich J, Gerson SL, Lathia J, Liu H. Cancer stem cells: targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. Cancer Res. 2015;75:924–929. doi: 10.1158/0008-5472.CAN-14-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, Lam A, Lazetic S, Ma S, Mitra S, Park IK, Pickell K, Sato A, Satyal S, Stroud M, Tran H, Yen WC, Lewicki J, Hoey T. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rattan R, Ali Fehmi R, Munkarah A. Metformin: an emerging new therapeutic option for targeting cancer stem cells and metastasis. J Oncol. 2012;2012:928127. doi: 10.1155/2012/928127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 174.Suva ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD, Riggi N, Chi AS, Cahill DP, Nahed BV, Curry WT, Martuza RL, Rivera MN, Rossetti N, Kasif S, Beik S, Kadri S, Tirosh I, Wortman I, Shalek AK, Rozenblatt-Rosen O, Regev A, Louis DN, Bernstein BE. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell. 2014;157:580–594. doi: 10.1016/j.cell.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, Arendt LM, Kuperwasser C, Bierie B, Weinberg RA. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 177.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8:56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]