Abstract
Cell motility is defined as cell movement in the three-dimensional space leading to repositioning of the cell. Atypical protein kinase C (aPKC, including ζ and λ/ι) are a subfamily of PKC. Different from classic PKC and novel PKC, the activation of atypical PKC is not dependent on diacylglycerol or calcium. PKCζ can be activated by lipid components, such as phosphatidylinositols, phosphatidic acid, arachidonic acid, and ceramide. Both phosphatidylinositol (3,4,5)-trisphosphate and PDK1 are necessary for the complete and stable activation of PKCζ. Atypical PKC is involved in the regulation of cell polarization, directional sensing, formation of filopodia, and cell motility. It is essential for migration and invasion of multiple cancer cell types. Particularly, atypical PKC has been found in the regulation of the motility of hematopoietic cells. It also participates in the regulation of proteolytic activity of podosomes and invadopodia. It has been found that atypical PKC can work coordinately with other PKC subfamily members and other signaling pathways. Research on the roles of atypical PKC in cell motility may lead to new therapeutic strategies for cancer and other diseases.
Keywords: Cell migration, Cell invasion, Podosome, Invadopodia, Lamellipodia, MMP
Introduction
Protein kinase C (PKC) is a family of protein serine/threonine kinases. PKCs serve as the transducers and modulators in many signaling networks in intracellular signal transduction. PKCs are important regulators of a variety of essential cellular functions, including cell proliferation [1], gene expression, cell differentiation, cytoskeleton organization, apoptosis [2], and cell migration [3]. PKC family members are involved in the pathogenesis of many disorders, such as Alzheimer disease [4], lung diseases [5], platelet activation and thrombus formation [6], cancer progression and metastasis [7, 8], diabetic kidney diseases [9] and diabetic retinopathy and macular edema [10]. PKCs play a vital role in cell migration and invasion in various types of cells. PKCs are highly involved in the formation and function of the newly discovered cellular structures—podosomes and invadopodia. Accumulating evidences show that atypical PKC is important for cell migration and invasion. In this review, we will briefly introduce the PKC family and the mechanisms of atypical PKC activation, and focus on several special features of atypical PKC in cell migration and invasion.
PKC family: structural–functional relationships
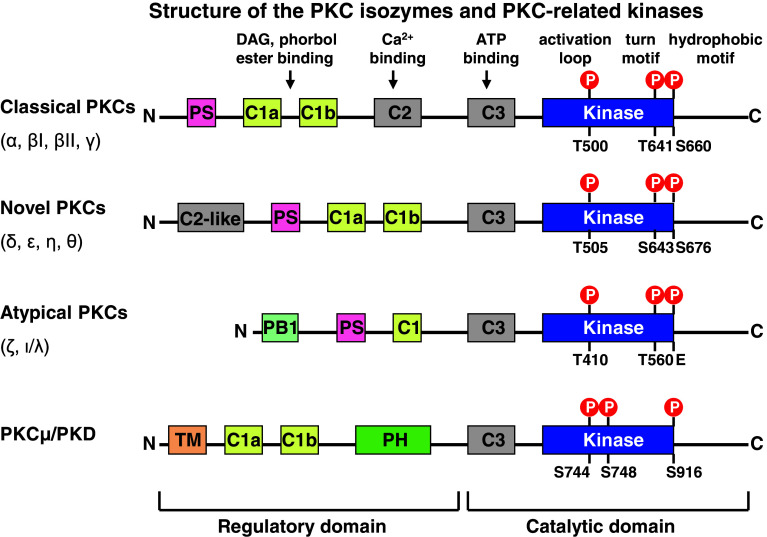
PKC is a family of serine/threonine kinases implicated in the transduction of signals coupled to receptor-mediated hydrolysis of membrane phospholipids [11]. According to sequence homology and sensitivity to activators, at least 10 isoforms encoded by 9 different genes have been described (Fig. 1) [12]. The various PKCs are grouped into three subfamilies: (1) classic or conventional PKCs (α, βI, βII and γ); (2) novel PKCs (δ, ε, η and θ); and (3) atypical PKCs (ζ and λ/ι) [13]. In addition, PKCμ or PKD1 is another serine/threonine protein kinase, which has similar structure and function as other PKCs. It contains two cysteine-rich domains that bind diacylglycerol or phorbol esters, but it lacks the Ca2+ binding domain found in cPKCs. PKCμ also has a pleckstrin homology (PH) domain that regulates its kinase activity, but does not harbor the typical PKC autoinhibitory pseudosubstrate motif (Fig. 1) [14].
Fig. 1.
Molecular structure of PKCs. PKC is a family of serine/threonine kinases with at least 10 isoforms encoded by nine different genes. PKCs are grouped into three subfamilies: (1) classic or conventional PKCs (α, βI, βII and γ); (2) novel PKCs (δ, ε, η and θ); and (3) atypical PKCs (ζ and λ/ι). PKCμ or PKD1 is another serine/threonine protein kinase with similar structure and functions as PKCs
The general structure of the classic PKCs includes conserved domains (C1–C4) separated by variable sequences (V1–V5) (Fig. 1) [15]. C1–C2 represents the regulatory portion of each enzyme to interact with specific activators, while C3–C4 form the catalytic region responsible for both the substrate binding and the kinase activity [15]. The catalytic domain is characterized by a high degree of homology among the various kinases [16]. Classic and novel PKC isoforms contain two cysteine-rich C1 domains (C1a and C1b). The region encompassing these two C1 domains could be denoted as the C1 region [15, 17]. These C1 domains represent the docking-sites for phosphatidylserine [18], and the physiological activator DAG as well as its analogous phorbol esters [i.e. PMA (phorbol 12-myristate 13-acetate), PDBu (phorbol-12,13-dibutyrate)] [19]. The C2 region in the conventional PKCs (cPKCs) contains the Ca2+ binding site. The novel PKCs (nPKCs) are not responsible to Ca2+ because of the absence of the Ca2+ binding site in the C2-like domain; their recruitment to membrane is dependent on a C1 region with higher affinity for phospholipids [16, 20, 21]. C3 bears the ATP binding lobe, while the C4 kinase domain contains the substrate docking sequence (Fig. 1) [15]. All known PKCs are characterized by a pseudosubstrate or autoinhibitory region, adjacent to C1 at the N-terminus, which keeps them in an inactive conformation (Fig. 1) [16, 22].
Individual PKC isoforms can translocate to subcellular locations other than the plasma membrane, including membrane vesicles, nuclear structures, and cytoskeletal components. The subcellular location of a specific PKC isoform may directly control its access to potential substrates thus performing distinct functions [23]. Specific PKC isoforms can associate with a range of cytoskeletal proteins including intermediate filaments proteins, membrane-cytoskeletal cross-linking proteins, and components of the actin filaments and microtubules [23].
Atypical PKC activation and interaction with other proteins
The atypical subclass of PKC family is comprised of two isoforms: PKCζ and PKCλ in the mouse, and PKCζ and PKCι in humans. The murine PKCλ and human PKCι share 98 % amino acid identify. PKCζ and PKCλ/PKCι share over 70 % amino acid identity [24]. They have four functional domains: a unique PB1 domain at the N-terminus, a pseudosubstrate sequence, a C1 domain consisting of a single cysteine rich zinc-finger motif, and a kinase domain in the C-terminus [25]. The C1 domain of aPKC isotypes is considered as atypical, due to the lack of specific amino acid residues that constitute the phorbol ester-binding pocket. Thus, aPKCs do not respond to DAG and phorbol esters [26]. PKCζ can be activated by lipid components, such as phosphatidylinositols [27], phosphatidic acid [28], arachidonic acid, and ceramide [29]. Thr410 in the activation loop of aPKCs is phosphorylated by pyruvate dehydrogenase kinase 1 (PDK1) that binds to the hydrophobic motif [25]. The PKCζ directly interacts with PIP3, which releases PS-dependent auto-inhibition [30, 31]. Both PIP3 and PDK1 are necessary for the complete and stable activation of PKCζ. In rat alveolar epithelial cells, hypoxia-induced activation of AMP-activated protein kinase α1 (AMPK α1) binds and directly phosphorylates PKCζ at Thr410 [32].
aPKC in cell polarization and directional migration
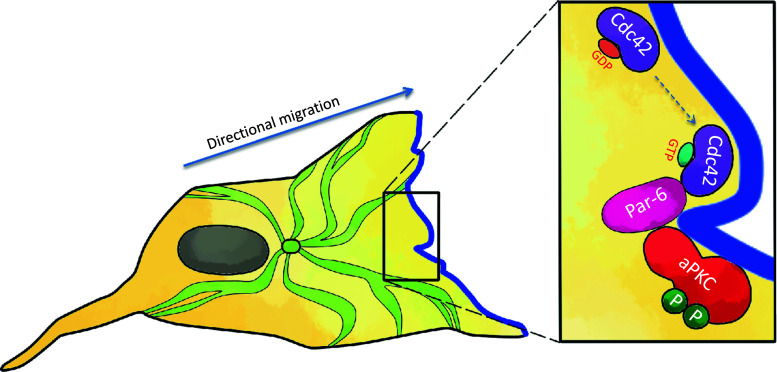
Directional sensing and polarization are crucial steps for cell migration [33]. It is well known that the three Rho GTPases, Rho, Rac, and Cdc42, play essential roles in controlling the organization of the actin cytoskeleton in different cell types [34]. There is growing evidence that they can also influence the microtubule cytoskeleton, which is essential for polarization and directional cell migration. Scratching a confluent monolayer of primary rat astrocytes leads to polarization of cells. Cells form microtubule-dependent protrusions and a microtubule organization center (MTOC) toward the wound (Fig. 2). Cdc42 is required for initiating the formation of protrusions and the MTOC, and Rac activity is essential for the development of the protrusions [35]. In the nematode C. elegans, Par (partitioning-defective) proteins and atypical PKC-3 are essential for asymmetric cell division and polarized growth [36, 37]. In mammalian cells, active Cdc42 can associate with the PDZ domain of mammalian Par6, and, through its N-terminal PB1 domain, interacts with the PB1 domain of aPKC (Fig. 2) [38–40]. Wounding induces strong activation of PKCζ in rat astrocytes. Par6, Cdc42, and PKCζ become associated with the plasma membrane at the leading edge of migrating cells. Par6 and PKCζ are required for the correct orientation of the protrusion and the microtubule organization center [35].
Fig. 2.
Role of atypical PKC in directional sensing in cell migration. Activation of Cdc42, by an external polarity cue, leads to binding of GTP-bound Cdc42 with the PDZ domains of Par-6. Par-6 and atypical PKC are linked through their PB1 domains (phox-bem domains). This allows phosphorylation and activation of the atypical PKC. The active complex can phosphorylate substrates to determine and maintain cell polarity in directional migration
Wnt signaling pathway is also known for its involvement in cytoskeletal remodeling and cell motility. Inhibition of Wnt5a, but not Wnt1 or Wnt3a, by siRNA-blocked reorientation of fibroblasts after scratching monolayers. Components of Wnt pathways interact with aPKC family members to regulate cell migration by controlling cell polarity [41]. Dishevelled (Dvl) is a scaffold protein that transduces signals both in the canonical (β-catenin-dependent) and the non-canonical (β-catenin-independent) Wnt pathways. After scratching of cell monolayers, Dvl2 and aPKC formed a complex, which was dependent on both Wnt and Cdc42. Therefore, Wnt and Dvl can cooperate with Cdc42/Par6/aPKC to promote cell polarity for migration [42]. A recent study by Ishida-Takagishi and colleagues further demonstrated that Daple (Dishevelled-associating protein with a high frequency of leucine residues) is an upstream protein of Dvl2. Daple regulates Wnt5a-mediated activation of Rac and formation of lamellipodia through its interaction with Dvl, and increases the association of Dvl with aPKCλ. Daple deficiency impairs migration of fibroblasts and epithelial cells in vivo in a skin wound-healing assay [43]. PKCζ has been found as a positive modulator of canonical Wnt signaling pathway in tumoral colon cell lines [44]. Obviously, further studies on the cross-talk between Wnt and aPKC pathways will be necessary, which may provide new mechanisms for cell motility.
The interaction and co-localization of Rac, PKCζ, and Par-6 at the leading edge of migrating cells could be a target for anti-cancer therapy. A synthetic anti-tumor compound, triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO)-imidazolide (CDDO-Im), can localize to the polarity complex at the leading edge of migrating cells. It disrupts the localization of PKCζ, Par-6, and TGFβ receptors from the leading edge of migrating cells, and reduces TGFβ-dependent cell migration [45]. In non-small cell lung cancer (NSCLC) cells, PKCι activates Rac1 by formation of a complex with Par-6α and Rac1, which drives anchorage-independent growth and invasion through activation of MMP-10. Knockdown of PKCι, Par-6α, or Rac1 with shRNA inhibits NSCLC transformation and MMP-10 expression. The complex of PKCι/Par6α/Rac1 is crucial for mediating these effects. Dominant-negative PKCι inhibits tumorigenicity and MMP-10 expression in subcutaneous tumors formed by NSCLC cells [46]. The discovery of the complex of aPKC/Par-6/Rho family GTPases shows an essential regulatory mechanism in cell migration and invasion.
Atypical PKCs in filopodia formation and cell motility
Cell motility is defined as cell movement in the three-dimensional space leading to repositioning of the cell. Components of motile behavior include cell spreading, generation of filopodia, lamellipodia, and ruffles. Expression of water channel aquaporin-9 (AQP9) induces formation of numerous filopodia extensions in different cell lines, associated with an increase in active Cdc42. Mutation of the putative PKC-binding or PKC-phosphorylation sites in AQP9, significantly reduced the number of filopodia and active Cdc42. Furthermore, active PKCζ phosphorylates AQP9 and myristoylated PKCζ pseudosubstrate inhibits the formation of filopodia in AQP9-expressing cells [47]. Increased water influx through AQP9 and activation of Cdc42 and PKCζ are critically involved in the formation of membrane protrusions of filopodia.
In certain cell types and conditions, aPKC isozymes could be the key PKC isoform to determine the cell motility. Laudanna and co-workers developed a motility score to quantify cell motility based on time-lapse video microscopy and digital image analysis. To determine the role of PKC isozymes in mediating cell motility in human pancreatic adenocarcinoma cells, they treated cells with myristoylated peptides specific for classic (α), novel (δ and ε), and atypical (ζ) PKCs. Only myristoylated pseudosubstrated peptide for PKCζ inhibited cell motility in a dose-dependent manner. Scrambling the peptide sequence abolished this inhibitory effect. In motile cells, PKCζ is constitutively associated with the plasma membrane, whereas, in non-motile cells, PKCζ is excluded from the plasma membrane, suggesting the membrane-associated intracellular distribution of PKCζ plays a critical role in maintaining pancreatic cancer cell motility [48].
Thrombin induces migration of retinal pigment epithelial cells through stimulation of release of chemokines, MCP1 and GRO, and activation of related chemokine receptors, CXCR-2 and CCR-2. Thrombin-induced MCP1 and GRO expression/secretion, and cell migration of retinal pigment epithelial cells, can be completely prevented by the inhibitory PKCζ pseudosubstrate [49].
Atypical PKC in cancer cell migration and invasion
Atypical PKCs are involved in the regulation of cancer cell migration and invasion. In human squamous carcinomas SASH1 cells, PKCζ-dependent phosphorylation of RhoGDI-1, and subsequent activation of RhoGTPases, is the mechanism that mediates superoxide-induced cell migration. Although the protein levels of PKCα, β, γ, δ, ε, ζ, and μ are found in these cells, only PKCζ formed a complex with RhoGD1, which is further enhanced by superoxide stimulation [50]. In human breast cancer MDA-MB-231, MCF-7, and T47D cells, EGF induced PKCζ translocation from the cytosol to the plasma membrane and activation of PKCζ probably via PI3 K. PKCζ is an essential component of the EGF-stimulated chemotactic signaling pathway in these human breast cancer cells. The myristoylated PKCζ peusosubstrate peptide blocked the chemotaxis. By contrast, inhibitors of classic and novel PKC, such as Gö6976, Gö6850, or calphostin C, only slightly impaired EGF-induced chemotaxis [51]. In astrocytoma cells, PTEN deficiency results in a marked increase in cell invasiveness that can be suppressed by a PKCζ-specific pseudosubstrate peptide inhibitor [52].
On the other hand, PKCζ has shown inhibitory effects on invasive and metastatic abilities in rat prostate cancer cells. PKCζ mRNA levels were reduced markedly in metastatic Dunning R-3327 rat prostate tumors relative to the non-metastatic Dunning H tumor and normal rat prostate [53]. In Dunning R-3327 MAT-LyLu rat prostate tumor cells stably transfected with PKCζ, 9 independent clones of PKCζ-expressing cells exhibited a lower tendency to metastasize to lungs relative to vector-transfected cell clones, and the ability of four PKCζ overexpressing MAT-LyLu cell clones to invade through Matrigel in a Boyden chamber assay was greatly reduced [54]. These results contradict the observations of the role of PKCζ in promoting cell motility in other cell types. Whether this is specific to this type of prostate tumors in rats or PKCζ may have different functions in cell migration/invasion needs to be further investigated.
PKCζ and hematopoietic cell migration
Mobilization and recruitment of hematopoietic cells are important mechanisms in inflammatory responses. Recruitment of hematopoietic stem cells and progenitor cells are also important for proper tissue repair and regeneration. In comparison with cPKCs and nPKCs, aPKCs, especially PKCζ, appear to play a major role in motility in hematopoietic cell linage, stem cells, and progenitor cells (Table 1). Stromal cell-derived factor-1 (SDF-1, also named CXCL12) is a powerful chemoattractant for human CD34 + CD38(lo−/−) hematopoietic stem cells. PKCζ is directly involved in the SDF-1 signaling in immature human CD34+-enriched cells and in leukemic pre-B acute lymphocytic leukemia G2 cells. SDF-1 triggered PKCζ phosphorylation, translocation to the plasma membrane, and kinase activation. Chemotaxis, cell polarization, and adhesion of CD34+ cells to bone marrow stromal cells are PKCζ-dependent. PI3 K is an activator of PKCζ, and Pyk-2 and ERK1/2 are downstream targets of SDF-1-induced PKCζ activation [55]. Moreover, in vivo engraftment of human CD34+-enriched cells to the bone marrow of NOD/SCID mice is PKCζ-dependent [55]. Although SDF-1 and its receptor CXCR4 play the central role in hematopoietic stem cell/hematopoietic progenitor cell homing, additional co-regulators are required to provide the specificity of such cells to lodge and be retained in particular niches. CD164 is an adhesion receptor that regulates the adhesion of CD34+ cells to bone marrow stromal and the recruitment of CD34+ CD38(lo/−) cells into cycles, and associates with CXCR4. Blocking CD164 with monoclonal antibody, or knocking-down CD164 with siRNA, significantly inhibits migration of CD133+ hematopoietic progenitor cells toward SDF-1- and reduced SDF-1-induced phosphorylation of PKCζ and Akt [56].
Table 1.
PKCζ and hematopoietic cell migration
| Cell type | Chemo-attractants | Cellular activity | Role of PKC | Reference |
|---|---|---|---|---|
| Human CD34 + enriched cells,Leukemic pre-B acute lymphocytic leukemia G2 cells | SDF-1 (CXCL12) | Chemotaxis, cell polarization, adhesion to bone marrow stromal cells | PI3 K → PKCζ → Pyk2/ERK1/2 | [55] |
| Human CD34 + enriched cells | SDF-1 (CXCL12) | Engraftment to bone marrow of NOS/SCID mice in vivo | [55] | |
| CD133 + hematopoietic progenitor cells | SDF-1 (CXCL12) | Migration | [56] | |
| Human acute monocytic leukemia THP-1 cells,Murine peritoneal macrophages | CSF-1 | Chemotaxis | PKCζ/LIMK → actin polymerization, cofilin phosphorylation | [57] |
| Human peripheral monocytes, Murine macrophage-like cell line J774.1 | Superoxide, fMLP | Motility | PKCζ → RhoDGI-1 | [50] |
PKCζ is required for migration of macrophages. PKCζ peudosubstrate peptide inhibitor or knockdown of PKCζ by siRNA impaired CSF-1-induced chemotaxis of human acute monocytic leukemia THP-1 cells and impaired migration of mouse peritoneal macrophages. Chemoattractant-induced actin polymerization, mediated by LIMK, is an important mechanism for chemotaxis. LIMK regulates actin cytoskeleton by phosphorylating the actin depolymerizing factor/cofilin. CSF-1 stimulation increases interaction between PKCζ and LIMK. CSF-1-induced transient actin polymerization and phosphorylation of LIMK and cofilin are reduced by PKCζ siRNA pretreatment [57]. In human peripheral monocytes and murine macrophage-like cell line J774.1, PKCζ is essential for transducing the motility signal induced by superoxide and a chemotactic peptide, fMLP. PKCζ is activated to phosphorylate RhoGDI-1, which liberates RhoGTPases, leading to their activation. These events can be inhibited by myristoylated PKCζ pseudosubstrate peptide [50] (Table 1).
Interaction between PKCζ other PKC family member in cell migration
It has been noticed that, in many cases, aPKCs needs to interact with other PKC family members in a coordinated manner to control the cell migration and/or invasion. In human pulmonary artery endothelial cells, sphingosine 1-phosphate (S1P) stimulation activates G(i). Overexpression of dominant negative (dn) PKCε or PKCζ (but not PKCα or PKCδ) blocked S1P-induced cell migration. Further studies have demonstrated that PKCε is an upstream regulator of PKCζ. It can activate phospholipase D2 and then control the activity of PKCζ. The major effect of PKCζ on cell migration is to control Rac1 activation. Either dnPKCζ or myristoylated PKCζ peptide inhibitor blocked S1P-induced Rac activation [58].
In primary human mesangial cells, connective tissue growth factor (CTGF)-stimulated cell migration is mediated through a PKCζ–GSK3β signaling axis. CTGF-induced cell migration is associated with cytoskeletal rearrangement, a loss of focal adhesions, tyrosine dephosphorylation of FAK and paxillin, increased activity of protein tyrosine phosphatase SHP-2, decreased RhoA and Rac1 activity, and increased Cdc42 activity. These changes are associated with the phosphorylation and translocation of PKCζ to the leading edge of migrating cells. Inhibition of CTGF-induced PKCζ activity with a myristolated PKCζ pseudosubstrate peptide inhibitor or transient transfection of human mesangial cells with a dnPKCζ leads to a decrease in CTGF-induced cell migration [59]. This effect can be impaired under diseased conditions. In high ambient glucose culture to model the diabetic milieu, basal PKCζ and GSK3β phosphorylation levels are increased and CTGF-stimulated PKCζ and GSK3β phosphorylation is impaired [60]. Interestingly, inhibition of PKCβ with LY379196 and PKCβ siRNA reduced basal PKCζ and GSK3β phosphorylation in human mesangial cells exposed to high glucose. Under these conditions, CTGF-induced PKCζ phosphorylation and cell migration was recovered [60]. The interaction between different PKC family members determines the cellular responsiveness to high glucose and CTGF, and subsequently controls cell migration.
PKCζ and podosome and invadopodia
Podosomes are unique actin-rich structures, which protrude into the extracellular matrix, resulting in localized remodeling activities associated with enhanced invasiveness [61, 62]. Podosomes, can not only establish close contact to the substratum but also degrade components of extracellular matrix to assist motile cells to cross tissue boundaries; thus, it has been called the foot and mouth of the cell [61]. Although podosomes in different cell types share similar functions—promoting matrix degradation and cell invasion—their morphologies appear to be diverse. Linder summarized that podosomes are dot-like structures attached to substrate, and containing actin regulators and plaque proteins with the number of around 20–100 per cell with the maximum size of 1 μm in diameter and 0.4 μm in depth [63]. Podosomes function to degrade local extracellular matrix and therefore promote invasion through underlying tissue boundaries. Podosomes may also function to anchor a cell to the extracellular matrix, and to sense substrate rigidity and transmit forces [64].
Invadopodia are actin-based outward protrusions at the ventral surface of tumor cells and transformed cells which mediate proteolysis of the extracellular matrix [65]. The size of invadopodia varies from 1 to 8 μm in diameter and may reach 2–3 μm or more than 10 μm in length [66]. Invadopida usually assemble into clusters around membrane invaginations proximal to the Golgi complex in the cytoplasm [67]. Invadopodia show stability once formed, and prolonged protease secretion to degrade the extracellular matrix [68]. Usually a cell can form a few invadopodia. Generally, invadopodia share similar molecular protein components with podosomes. However, the same protein in different structures may play a distinct role.
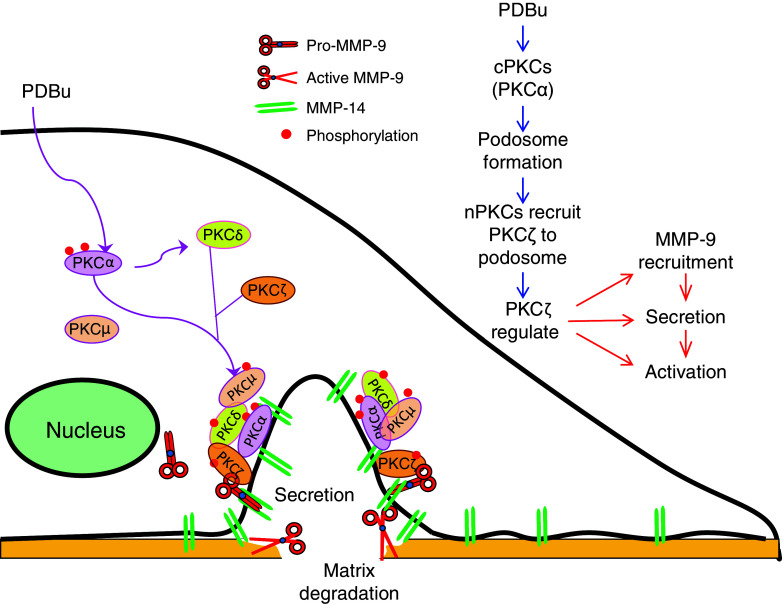
PKCs, as important cytoskeleton regulators and effecters, can drive the formation of podosome and invadopodia, and regulate the appropriate functionality of these cellular structures during the process of cell motility. Migration and invasion of epithelial cells are important processes for airway branching, lung growth and development, and repair after damage of epithelium in the lung [69]. A phorbol ester PKC activator, PDBu induced podosome assembly in human bronchial epithelial cells with increased cell invasion activity [70]. The formation of these podosomes is mainly mediated through the redistribution of conventional PKCs, especially PKCα, from the cytosol to the podosomes, while atypical PKCζ plays a dominant role locally at the podosomes to regulate the proteolytic activity through the recruitment, release, and activation of MMP-9. The novel PKCs, especially PKCδ, is responsible for the recruitment of PKCζ to the podosomes [71] (Fig. 3). This coordinated regulation of multiple PKC isozymes in the assembly and activity of podosomes is very interesting. Similar mechanisms may exist in other PKC-related cell migration and invasion.
Fig. 3.
Role of PKCζ and its interaction with other PKC family members in PDBu-induced podosome formation and enzymatic activity. In human bronchial epithelial cells, PDBu-induced translocation/activation of classical PKC, especially PKCα, controls podosome formation. Novel PKC, especially PKCδ, recruits atypical PKC (PKCζ), which further regulates MMP-9 recruitment, secretion, and activation
PI3 K/Akt- and Src-related signaling play roles in podosome formation and function [72–75]. In human bronchial epithelial cells, they are involved in regulating PDBu-induced podosome formation, whereas MEK/ERK and JNK are involved in the podosome enzymatic activities. They are downstream signals of PKCζ as their activation is determined by the activity of PKCζ. However, interestingly, the recruitment of PKCζ and MMP-9 to podosomes requires MEK/ERK and JNK activity [76]. The interdependent relationship between PKC family members and other signal transduction molecules in the regulation of podosome formation and function suggest that cell migration and invasion are regulated by a large array of signaling proteins as a network. Systems biology-based methods should be considered to explore these cellular processes.
In v-Src-transformed NIH 3T3 cells, both aPKC isoforms (PKCζ and PKCλ) are required for migration, invasion, and polarization during cell migration, with PKCλ playing a more important role. This may be due to its relatively higher expression in these cells, or may be due to intrinsic differences between these two isoforms. In these cells, the PKCλ isoform is also more important for podosome assembly and extracellular matrix degradation [77].
Podosomes and invadopodia are recently discovered transient cell surface structures essential for degradation of extracellular matrix during cell migration and invasion. Although PKC is highly involved in the regulation of podosome formation and functionality, the roles of individual PKC isoforms in podosome formation and function, and their crosstalk and the underlining mechanisms, are largely unknown. These need further study in the near future.
Role of PKCζ in the regulation of MMPs
In terms of cell invasion, PKCζ mainly enhances cell invasion into the underlying matrix through up-regulation of MMPs. The stable overexpression of PKCζ in immortalized mammary epithelial NMuMG cells induced phenotypic alterations associated with malignant transformation and tumor progression. PKCζ overexpression markedly altered the adhesive, spreading, and migratory abilities. Overexpression of PKCζ significantly increased expression of urokinase-type plasminogen activator and MMP-9 (but not MMP-2 and MMP-3) [78]. During glioma cell invasion through the brain extracellular matrix, secretion and activation of MMP-9 is one of the key processes. In rat C6 glioma cells, PKCζ regulated transcription of the MMP-9 gene induced by IL-1 and TNF-α via the NF-κB signaling pathway. IL-1 and TNF-α activated PKCζ, and overexpressing PKCζ, but not PKCε, up-regulated MMP-9 activity and increased MMP-9 promoter activity [79]. Ursolic acid (UA) is a triterpenoid compound that has been shown to have antioxidant and anti-carcinogenic properties. UA reduced IL-1β- or TNF-α-induced rat C6 glioma cell invasion through suppressing the association of PKCζ with the aPKC-interacting protein, ZIP/p62, interrupting NF-κB signaling and subsequently down-regulating the MMP-9 expression and activity [80].
Conclusion and perspective
Although other PKC family members are also involved in the regulation of cell motility, there are several features that highlight the contributions of atypical PKCs in this important cellular function. The involvement of atypical PKCs in cell polarity, especially in directional sensing and migration, is essential. The formation of a leading edge complex, cellular protrusions, and a microtubule organization center are initiation steps that determined the direction of cell migration. Through a literature review, we also noticed that most reports on the regulation of hematopoietic cells and stem cells are on atypical PKCs. This does not exclude the role of other PKC family members; nonetheless, the current knowledge suggests that this could be a unique or crucial role for this subfamily of PKCs. In comparison with cell migration, cell invasion is less studied, due to the three-dimensional nature of this type of cell motility. The discovery of podosomes and invadopodia, and techniques developed for these studies, opens a new area of research. The role of PKCζ on the regulation of enzymatic activity of podosmes is also interesting.
This review also suggests that the interplays among different PKC family members and interaction with other signaling pathways are important. Individual PKC isoforms may sometimes have opposing functions. Partly, this may be due to so-called antagonistic actions of related PKC isoforms. One challenge to the understanding of the specific role of individual PKC isoforms is the lack of specific PKC inhibitors. The new generation of PKC inhibitors, such as more selective catalytic inhibitors, peptide (-mimetic) inhibitors, protein substrate site inhibitors, effectors site-directed inhibitors, C2-directed and PB1-directed inhibitors, and antisense oligonucleotide inhibitors, have shown more selectivity [81]. In addition, specific tools, such as siRNA and genetic disruption of individual PKC isoforms in mice, have allowed more detailed analysis of the function of specific isoforms of this PKC large family. With these new tools, it is necessary to study the interaction among PKC isoforms.
In summary, it is obvious that atypical PKC is essential for regulation of cell motility, and it is also crucial to investigate the mechanisms of how PKC regulate cell motility in vivo. These studies may lead to new therapeutic strategies for cancer and other diseases.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR) operating Grants MOP-13270, MOP-42546 and MOP-119514 to M.L. H.X. was a recipient of the Peterborough K.M. Hunter Graduate Studentship for Cancer Research.
References
- 1.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Guerrico AM, Meshki J, Xiao L, Benavides F, Conti CJ, Kazanietz MG. Molecular mechanisms of protein kinase C-induced apoptosis in prostate cancer cells. J Biochem Mol Biol. 2005;38:639–645. doi: 10.5483/BMBRep.2005.38.6.639. [DOI] [PubMed] [Google Scholar]
- 3.Carter CA, Kane CJ. Therapeutic potential of natural compounds that regulate the activity of protein kinase C. Curr Med Chem. 2004;11:2883–2902. doi: 10.2174/0929867043364090. [DOI] [PubMed] [Google Scholar]
- 4.de Barry J, Liegeois CM, Janoshazi A. Protein kinase C as a peripheral biomarker for Alzheimer’s disease. Exp Gerontol. 2010;45:64–69. doi: 10.1016/j.exger.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Dempsey EC, Cool CD, Littler CM. Lung disease and PKCs. Pharmacol Res. 2007;55:545–559. doi: 10.1016/j.phrs.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Harper MT, Poole AW. Diverse functions of protein kinase C isoforms in platelet activation and thrombus formation. J Thromb Haemost. 2010;8:454–462. doi: 10.1111/j.1538-7836.2009.03722.x. [DOI] [PubMed] [Google Scholar]
- 7.Gonelli A, Mischiati C, Guerrini R, Voltan R, Salvadori S, Zauli G. Perspectives of protein kinase C (PKC) inhibitors as anti-cancer agents. Mini Rev Med Chem. 2009;9:498–509. doi: 10.2174/138955709787847967. [DOI] [PubMed] [Google Scholar]
- 8.Ali AS, Ali S, El-Rayes BF, Philip PA, Sarkar FH. Exploitation of protein kinase C: a useful target for cancer therapy. Cancer Treat Rev. 2009;35:1–8. doi: 10.1016/j.ctrv.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Tuttle KR. Protein kinase C-beta inhibition for diabetic kidney disease. Diabetes Res Clin Pract. 2008;82(Suppl 1):S70–S74. doi: 10.1016/j.diabres.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 10.Galvez MI. Rubosixtaurin and other PKC inhibitors in diabetic retinopathy and macular edema. Review. Curr Diabetes Rev. 2009;5:14–17. doi: 10.2174/157339909787314167. [DOI] [PubMed] [Google Scholar]
- 11.Lang D, Beermann ML, Hauser G, Cressman CM, Shea TB. Phospholipids inhibit proteolysis of protein kinase C alpha by mM calcium-requiring calpain. Neurochem Res. 1995;20:1361–1364. doi: 10.1007/BF00992512. [DOI] [PubMed] [Google Scholar]
- 12.Parker PJ, Murray-Rust J. PKC at a glance. J Cell Sci. 2004;117:131–132. doi: 10.1242/jcs.00982. [DOI] [PubMed] [Google Scholar]
- 13.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 14.Vertommen D, Rider M, Ni Y, Waelkens E, Merlevede W, Vandenheede JR, Van Lint J. Regulation of protein kinase D by multisite phosphorylation. Identification of phosphorylation sites by mass spectrometry and characterization by site-directed mutagenesis. J Biol Chem. 2000;275:19567–19576. doi: 10.1074/jbc.M001357200. [DOI] [PubMed] [Google Scholar]
- 15.Oliva JL, Griner EM, Kazanietz MG. PKC isozymes and diacylglycerol-regulated proteins as effectors of growth factor receptors. Growth Factors. 2005;23:245–252. doi: 10.1080/08977190500366043. [DOI] [PubMed] [Google Scholar]
- 16.Corbalan-Garcia S, Gomez-Fernandez JC. Protein kinase C regulatory domains: the art of decoding many different signals in membranes. Biochim Biophys Acta Mol Cell Biol Lipids. 2006;1761:633–654. doi: 10.1016/j.bbalip.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Ono Y, Fujii T, Igarashi K, Kuno T, Tanaka C, Kikkawa U, Nishizuka Y. Phorbol ester binding to protein kinase-C requires a cysteine-rich zinc-finger-like sequence. Proc Natl Acad Sci USA. 1989;86:4868–4871. doi: 10.1073/pnas.86.13.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurley JH, Newton AC, Parker PJ, Blumberg PM, Nishizuka Y. Taxonomy and function of C1 protein kinase C homology domains. Protein Sci. 1997;6:477–480. doi: 10.1002/pro.5560060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Fernandez JC, Torrecillas A, Corbalan-Garcia S. Diacylglycerols as activators of protein kinase C (Review) Mol Membr Biol. 2004;21:339–349. doi: 10.1080/09687860400010508. [DOI] [PubMed] [Google Scholar]
- 20.Rizo J, Sudhof TC. C-2-domains, structure and function of a universal Ca2+-binding domain. J Biol Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 21.Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci. 1996;5:2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colon-Gonzalez F, Kazanietz MG. C1 domains exposed: from diacylglycerol binding to protein–protein interactions. Biochim Biophys Acta. 2006;1761:827–837. doi: 10.1016/j.bbalip.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Keenan C, Kelleher D. Protein kinase C and the cytoskeleton. Cell Signal. 1998;10:225–232. doi: 10.1016/S0898-6568(97)00121-6. [DOI] [PubMed] [Google Scholar]
- 24.Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/S0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 25.Hirai T, Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem (Tokyo) 2003;133:1–7. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- 26.Ways DK, Cook PP, Webster C, Parker PJ. Effect of phorbol esters on protein kinase-C-zeta. J Biol Chem. 1992;267:4799–4805. [PubMed] [Google Scholar]
- 27.Nakanishi H, Brewer KA, Exton JH. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- 28.Limatola C, Schaap D, Moolenaar WH, van Blitterswijk WJ. Phosphatidic acid activation of protein kinase C-zeta overexpressed in COS cells: comparison with other protein kinase C isotypes and other acidic lipids. Biochem J. 1994;304(Pt 3):1001–1008. doi: 10.1042/bj3041001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller G, Ayoub M, Storz P, Rennecke J, Fabbro D, Pfizenmaier K. PKC zeta is a molecular switch in signal transduction of TNF-alpha, bifunctionally regulated by ceramide and arachidonic acid. EMBO J. 1995;14:1961–1969. doi: 10.1002/j.1460-2075.1995.tb07188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takenawa T, Itoh T. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim Biophys Acta. 2001;1533:190–206. doi: 10.1016/S1388-1981(01)00165-2. [DOI] [PubMed] [Google Scholar]
- 31.Takenawa T, Itoh T. Membrane targeting and remodeling through phosphoinositide-binding domains. IUBMB Life. 2006;58:296–303. doi: 10.1080/15216540600732039. [DOI] [PubMed] [Google Scholar]
- 32.Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LA, Chandel NS, Sznajder JI. Alpha 1-AMP-activated protein kinase regulates hypoxia-induced Na, K-ATPase endocytosis via direct phosphorylation of PKC zeta. Mol Cell Biol. 2009;29:3455–3464. doi: 10.1128/MCB.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 35.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/S0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 36.Hung TJ, Kemphues KJ. PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development. 1999;126:127–135. doi: 10.1242/dev.126.1.127. [DOI] [PubMed] [Google Scholar]
- 37.Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, Ohno S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 39.Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2:540–547. doi: 10.1038/35019592. [DOI] [PubMed] [Google Scholar]
- 40.Qiu RG, Abo A, Steven Martin G. A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Curr Biol. 2000;10:697–707. doi: 10.1016/S0960-9822(00)00535-2. [DOI] [PubMed] [Google Scholar]
- 41.Luna-Ulloa LB, Hernandez-Maqueda JG, Castaneda-Patlan MC, Robles-Flores M. Protein kinase C in Wnt signaling: implications in cancer initiation and progression. IUBMB Life. 2011;63:873–879. doi: 10.1002/iub.559. [DOI] [PubMed] [Google Scholar]
- 42.Schlessinger K, McManus EJ, Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol. 2007;178:355–361. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishida-Takagishi M, Enomoto A, Asai N, Ushida K, Watanabe T, Hashimoto T, Kato T, Weng L, Matsumoto S, Asai M, Murakumo Y, Kaibuchi K, Kikuchi A, Takahashi M. The Dishevelled-associating protein Daple controls the non-canonical Wnt/Rac pathway and cell motility. Nat Commun. 2012;3:859. doi: 10.1038/ncomms1861. [DOI] [PubMed] [Google Scholar]
- 44.Luna-Ulloa LB, Hernandez-Maqueda JG, Santoyo-Ramos P, Castaneda-Patlan MC, Robles-Flores M. Protein kinase C zeta is a positive modulator of canonical Wnt signaling pathway in tumoral colon cell lines. Carcinogenesis. 2011;32:1615–1624. doi: 10.1093/carcin/bgr190. [DOI] [PubMed] [Google Scholar]
- 45.To C, Kulkarni S, Pawson T, Honda T, Gribble GW, Sporn MB, Wrana JL, Di Guglielmo GM. The synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid-imidazolide alters transforming growth factor beta-dependent signaling and cell migration by affecting the cytoskeleton and the polarity complex. J Biol Chem. 2008;283:11700–11713. doi: 10.1074/jbc.M704064200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frederick LA, Matthews JA, Jamieson L, Justilien V, Thompson EA, Radisky DC, Fields AP. Matrix metalloproteinase-10 is a critical effector of protein kinase Ciota-Par6alpha-mediated lung cancer. Oncogene. 2008;27:4841–4853. doi: 10.1038/onc.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loitto VM, Huang C, Sigal YJ, Jacobson K. Filopodia are induced by aquaporin-9 expression. Exp Cell Res. 2007;313:1295–1306. doi: 10.1016/j.yexcr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 48.Laudanna C, Sorio C, Tecchio C, Butcher EC, Bonora A, Bassi C, Scarpa A. Motility analysis of pancreatic adenocarcinoma cells reveals a role for the atypical zeta isoform of protein kinase C in cancer cell movement. Lab Invest. 2003;83:1155–1163. doi: 10.1097/01.LAB.0000081390.92179.F3. [DOI] [PubMed] [Google Scholar]
- 49.Palma-Nicolas JP, Lopez E, Lopez-Colome AM. Thrombin stimulates RPE cell motility by PKC-zeta- and NF-kappaB-dependent gene expression of MCP-1 and CINC-1/GRO chemokines. J Cell Biochem. 2010;110:948–967. doi: 10.1002/jcb.22608. [DOI] [PubMed] [Google Scholar]
- 50.Kuribayashi K, Nakamura K, Tanaka M, Sato T, Kato J, Sasaki K, Takimoto R, Kogawa K, Terui T, Takayama T, Onuma T, Matsunaga T, Niitsu Y. Essential role of protein kinase C zeta in transducing a motility signal induced by superoxide and a chemotactic peptide, fMLP. J Cell Biol. 2007;176:1049–1060. doi: 10.1083/jcb.200607019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun R, Gao P, Chen L, Ma D, Wang J, Oppenheim JJ, Zhang N. Protein kinase C zeta is required for epidermal growth factor-induced chemotaxis of human breast cancer cells. Cancer Res. 2005;65:1433–1441. doi: 10.1158/0008-5472.CAN-04-1163. [DOI] [PubMed] [Google Scholar]
- 52.Xiao A, Yin C, Yang C, Di Cristofano A, Pandolfi PP, Van Dyke T. Somatic induction of Pten loss in a preclinical astrocytoma model reveals major roles in disease progression and avenues for target discovery and validation. Cancer Res. 2005;65:5172–5180. doi: 10.1158/0008-5472.CAN-04-3902. [DOI] [PubMed] [Google Scholar]
- 53.Powell CT, Fair WR, Heston WD. Differential expression of protein kinase C isozyme messenger RNAs in dunning R-3327 rat prostatic tumors. Cell Growth Differ. 1994;5:143–149. [PubMed] [Google Scholar]
- 54.Powell CT, Gschwend JE, Fair WR, Brittis NJ, Stec D, Huryk R. Overexpression of protein kinase C-zeta (PKC-zeta) inhibits invasive and metastatic abilities of Dunning R-3327 MAT-LyLu rat prostate cancer cells. Cancer Res. 1996;56:4137–4141. [PubMed] [Google Scholar]
- 55.Petit I, Goichberg P, Spiegel A, Peled A, Brodie C, Seger R, Nagler A, Alon R, Lapidot T. Atypical PKC-zeta regulates SDF-1-mediated migration and development of human CD34+ progenitor cells. J Clin Invest. 2005;115:168–176. doi: 10.1172/JCI21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forde S, Tye BJ, Newey SE, Roubelakis M, Smythe J, McGuckin CP, Pettengell R, Watt SM. Endolyn (CD164) modulates the CXCL12-mediated migration of umbilical cord blood CD133+ cells. Blood. 2007;109:1825–1833. doi: 10.1182/blood-2006-05-023028. [DOI] [PubMed] [Google Scholar]
- 57.Guo H, Ma Y, Zhang B, Sun B, Niu R, Ying G, Zhang N. Pivotal advance: PKCzeta is required for migration of macrophages. J Leukoc Biol. 2009;85:911–918. doi: 10.1189/jlb.0708429. [DOI] [PubMed] [Google Scholar]
- 58.Gorshkova I, He D, Berdyshev E, Usatuyk P, Burns M, Kalari S, Zhao Y, Pendyala S, Garcia JG, Pyne NJ, Brindley DN, Natarajan V. Protein kinase C-epsilon regulates sphingosine 1-phosphate-mediated migration of human lung endothelial cells through activation of phospholipase D2, protein kinase C-zeta, and Rac1. J Biol Chem. 2008;283:11794–11806. doi: 10.1074/jbc.M800250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crean JK, Furlong F, Finlay D, Mitchell D, Murphy M, Conway B, Brady HR, Godson C, Martin F. Connective tissue growth factor [CTGF]/CCN2 stimulates mesangial cell migration through integrated dissolution of focal adhesion complexes and activation of cell polarization. FASEB J. 2004;18:1541–1543. doi: 10.1096/fj.04-1546fje. [DOI] [PubMed] [Google Scholar]
- 60.Furlong F, Crean J, Thornton L, O’Leary R, Murphy M, Martin F. Dysregulated intracellular signaling impairs CTGF-stimulated responses in human mesangial cells exposed to high extracellular glucose. Am J Physiol Renal Physiol. 2007;292:F1691–F1700. doi: 10.1152/ajprenal.00342.2006. [DOI] [PubMed] [Google Scholar]
- 61.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 62.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/S0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 63.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Collin O, Na S, Chowdhury F, Hong M, Shin ME, Wang F, Wang N. Self-organized podosomes are dynamic mechanosensors. Curr Biol. 2008;18:1288–1294. doi: 10.1016/j.cub.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stylli SS, Kaye AH, Lock P. Invadopodia: at the cutting edge of tumour invasion. J Clin Neurosci. 2008;15:725–737. doi: 10.1016/j.jocn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Mueller SC, Chen WT. Cellular invasion into matrix beads: localization of beta 1 integrins and fibronectin to the invadopodia. J Cell Sci. 1991;99(Pt 2):213–225. doi: 10.1242/jcs.99.2.213. [DOI] [PubMed] [Google Scholar]
- 67.Baldassarre M, Pompeo A, Beznoussenko G, Castaldi C, Cortellino S, McNiven MA, Luini A, Buccione R. Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol Biol Cell. 2003;14:1074–1084. doi: 10.1091/mbc.E02-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 69.Xiao H, Li DX, Liu M. Knowledge translation: airway epithelial cell migration and respiratory diseases. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-1044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao H, Eves R, Yeh C, Kan W, Xu F, Mak AS, Liu M. Phorbol ester-induced podosomes in normal human bronchial epithelial cells. J Cell Physiol. 2009;218:366–375. doi: 10.1002/jcp.21609. [DOI] [PubMed] [Google Scholar]
- 71.Xiao H, Bai XH, Kapus A, Lu WY, Mak AS, Liu M. The protein kinase C cascade regulates recruitment of matrix metalloprotease 9 to podosomes and its release and activation. Mol Cell Biol. 2010;30:5545–5561. doi: 10.1128/MCB.00382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gatesman A, Walker VG, Baisden JM, Weed SA, Flynn DC. Protein kinase Calpha activates c-Src and induces podosome formation via AFAP-110. Mol Cell Biol. 2004;24:7578–7597. doi: 10.1128/MCB.24.17.7578-7597.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker VG, Ammer A, Cao Z, Clump AC, Jiang BH, Kelley LC, Weed SA, Zot H, Flynn DC. PI3 K activation is required for PMA-directed activation of cSrc by AFAP-110. Am J Physiol Cell Physiol. 2007;293:C119–C132. doi: 10.1152/ajpcell.00525.2006. [DOI] [PubMed] [Google Scholar]
- 74.Dorfleutner A, Cho Y, Vincent D, Cunnick J, Lin H, Weed SA, Stehlik C, Flynn DC. Phosphorylation of AFAP-110 affects podosome lifespan in A7r5 cells. J Cell Sci. 2008;121:2394–2405. doi: 10.1242/jcs.026187. [DOI] [PubMed] [Google Scholar]
- 75.Xiao H, Han B, Lodyga M, Bai XH, Wang Y, Liu M. The actin-binding domain of actin filament-associated protein (AFAP) is involved in the regulation of cytoskeletal structure. Cell Mol Life Sci. 2012;69:1137–1151. doi: 10.1007/s00018-011-0812-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao H, Bai X-H, Wang Y, Kim H, Mak AS, Liu M. MEK/ERK pathway mediates PKC activation-induced recruitment of PKC zeta and MMP-9 to podosomes. J Cell Physiol. 2012 doi: 10.1002/jcp.24146. [DOI] [PubMed] [Google Scholar]
- 77.Rodriguez EM, Dunham EE, Martin GS. Atypical protein kinase C activity is required for extracellular matrix degradation and invasion by Src-transformed cells. J Cell Physiol. 2009;221:171–182. doi: 10.1002/jcp.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Urtreger AJ, Grossoni VC, Falbo KB, Kazanietz MG, Bal de Kier Joffe EM. Atypical protein kinase C-zeta modulates clonogenicity, motility, and secretion of proteolytic enzymes in murine mammary cells. Mol Carcinog. 2005;42:29–39. doi: 10.1002/mc.20066. [DOI] [PubMed] [Google Scholar]
- 79.Esteve PO, Chicoine E, Robledo O, Aoudjit F, Descoteaux A, Potworowski EF, St-Pierre Y. Protein kinase C-zeta regulates transcription of the matrix metalloproteinase-9 gene induced by IL-1 and TNF-alpha in glioma cells via NF-kappa B. J Biol Chem. 2002;277:35150–35155. doi: 10.1074/jbc.M108600200. [DOI] [PubMed] [Google Scholar]
- 80.Huang HC, Huang CY, Lin-Shiau SY, Lin JK. Ursolic acid inhibits IL-1beta or TNF-alpha-induced C6 glioma invasion through suppressing the association ZIP/p62 with PKC-zeta and downregulating the MMP-9 expression. Mol Carcinog. 2009;48:517–531. doi: 10.1002/mc.20490. [DOI] [PubMed] [Google Scholar]
- 81.Roffey J, Rosse C, Linch M, Hibbert A, McDonald NQ, Parker PJ. Protein kinase C intervention: the state of play. Curr Opin Cell Biol. 2009;21:268–279. doi: 10.1016/j.ceb.2009.01.019. [DOI] [PubMed] [Google Scholar]