Abstract
The human genome is under constant invasion by retrotransposable elements. The most successful of these are the Alu elements; with a copy number of over a million, they occupy about 10 % of the entire genome. Interestingly, the vast majority of these Alu insertions are located in gene-rich regions, and one-third of all human genes contains an Alu insertion. Alu sequences are often embedded in gene sequence encoding pre-mRNAs and mature mRNAs, usually as part of their intron or UTRs. Once transcribed, they can regulate gene expression as well as increase the number of RNA isoforms expressed in a tissue or a species. They also regulate the function of other RNAs, like microRNAs, circular RNAs, and potentially long non-coding RNAs. Mechanistically, Alu elements exert their effects by influencing diverse processes, such as RNA editing, exonization, and RNA processing. In so doing, they have undoubtedly had a profound effect on human evolution.
Keywords: Alu element, Retrotransposon, SINE, Exonization, RNA editing, circRNA, miRNA
Introduction to the Alu element
For several decades, it has been known that the human genome contains a large portion of repetitive sequences derived from transposable elements (TEs) [1]. Recent estimates suggest that as much as 70 % of the human genome is derived from elements that probably originated from TEs [2]. One of the most common types of TE in human and other primates is the Alu element, belonging to a class known as the conserved small interspersed elements (SINEs). Alu elements likely originated from the 7SL RNA gene, which encodes the non-coding RNA of the signal recognition particle (SRP) [3, 4]. A retrotransposon was plausibly formed from a partially deleted version of the 7SL RNA gene some time before the divergence of primates and rodents. This precursor evolved into B1 repeats in rodents and into the FLAM (free left Alu monomer) and FRAM (free right Alu monomer) elements in the primate lineage. Eventually, the FLAM and FRAM sequences were fused and able to amplify efficiently into what we today call an Alu element. The Alu repeats are divided into various subfamilies depending on when they were inserted into the genome: AluJ is the oldest element, AluS is of intermediate age, and AluY is the youngest [5–8]. The modern Alu element is about 280 nucleotides (nt) long, formed by the divergent FLAM and FRAM sequences separated by a short A-rich spacer (Fig. 1). The 3′ end of the element has an A-rich region, which plays an important role during amplification. The right arm has a unique 31 nt insertion, while the left arm contain two RNA polymerase III (Pol III) promoters [9, 10].
Fig. 1.
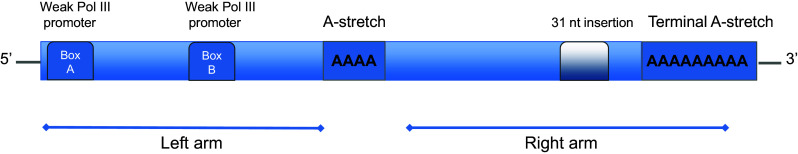
The genomic structure of an Alu element. The genomic Alu element is a dimeric structure composed of two related but not identical monomers: the left and right arm. The right arm has an additional 31 nucleotide insertion absent in the left arm. The left arm has two weak but functional RNA polymerase III internal promoters (A and B box). The two monomers are separated by an A-rich region and the element ends with a terminal A-stretch
With a copy number of over 1 million, Alus account for more than 10 % of the human genome. Still, their invasion is ongoing with an estimated insertion rate of one for every 20 births [11]. As with other repetitive elements, Alu elements were originally considered to be “selfish” or junk DNA [12, 13]. Today several lines of evidence show that the presence of repetitive elements, in particular Alu elements, has had a significant impact on the evolution of the human genome. Alu integration can potentially have both positive and negative consequences. On the one hand, integration into gene-rich regions can be deleterious if it disrupts gene expression. On the other hand, and perhaps more interestingly, Alu integration can contribute to diversity in gene expression. Because of their extended sequence homology, Alu elements can induce non-allelic recombination events that lead to both duplications and deletions of DNA segments, thereby accelerating evolution by several orders of magnitude.
The extensive integration of Alus into our genome has been known for years, but only recently we have started to appreciate the effects of transcribed Alus on our transcriptome. With the emergence of next-generation sequencing (NGS) technology, increasing evidence shows the important contributions of Alus as functional transcribed elements [14, 15]; they have been shown to influence gene expression by regulating splicing, polyadenylation, RNA editing, and the expression of non-coding RNAs. In this review, we will focus on how Alu elements influence variability within the transcriptome.
Genomic insertion of Alu elements
Alu elements require trans-acting factors for replication
On their own, Alu SINEs would be inactive. To replicate, they require help from the LINE-1 (L1) element, a fully-active autonomous family of retrotransposons in humans. When an Alu integrates into the genome, its new genomic location is crucial in determining whether it becomes an active Alu or an inactive retropseudosequence, like most of the other millions of repetitive elements [16]. Active Alus are rare. Their reintegration process is complex, not fully understood, and requires at least one factor derived from L1 elements. Overall, the internal RNA Pol III promoter in the left arm of the Alu sequence initiates transcription and produces an RNA that is reverse transcribed and integrated into a new location in the genome. Transcribed Alu RNAs are thought to assemble into ribonucleoprotein particles including the SRP/14 heterodimer and the polyA-binding-protein (PABP). These proteins, and at least one other unidentified protein, bind the Alu RNA and facilitate ribosomal association [17, 18]. At the ribosome, the Alu RNA associates with the ORF2 protein, translated from the L1 element. The ORF2 protein is used to make a replica of the Alu RNA that is integrated into a new genomic site using target-primed reverse transcription [19]. It is not known why only a handful of the more than 1 million genomic Alu elements can amplify and integrate into novel sites. One important factor is probably the sequence of the upstream flanking region that may recruit transcription factors that activate RNA Pol III [20].
Genomic distribution of Alu elements
The genomic distribution of Alu elements is uneven with a strong preference towards intervening sequences within gene-rich regions [21, 22]. Alu elements are more frequent in GC-rich domains, and about one-third of all genes contains Alu sequences, mostly in their flanking regions [22]. They are also enriched in highly expressed, pan-tissue housekeeping genes, and this is particularly true for the AluJ subclass [23–25]. It is also interesting to note that Alus are enriched in genes coding for zinc finger containing transcription factors [26].
Alus regulating transcription
DNA methylation is important for the regulation of gene expression, and Alu elements are rich in CpG residues, the dinucleotide targets for methylation. It has been estimated that about 25 % of all DNA methylation sites in the human genome are present in Alu sequences [27]. Methylated CpGs can easily mutate to TpG, which may explain why a higher density of methylation occurs in younger Alu elements than in older ones [27]. Identical Alu sequences may be methylated differently depending on their genomic location. A methylation pattern can be tissue dependent or influenced by its location within a gene. Alu sequences in intronic or 3′ untranslated regions (UTRs) are more commonly methylated than those in promoters, 5′UTRs and coding regions [27]. Although demethylation of an Alu may increase gene expression, thus far there are few studies that point towards Alu methylation as being a driving force for gene regulation.
As mentioned before, Alu elements are more prevalent near and within genes than elsewhere in the genome. This non-random distribution may result from the use of these elements for selective advantage. As an example, Alu elements contain a number of potential transcription factor (TF) binding sites. Some of these are common to certain Alu subfamilies, while others have emerged through mutations after Alu insertion [28]. Interestingly, this second class of sequences is frequently found in promoter regions of genes involved in development, while the first often is present in genes involved in ribosomal biogenesis and translation. Although most of the predicted TF binding sites have not been validated, it suggests that Alu sequences can influence gene expression directly at the promoter. Nevertheless, in a few cases, Alus have been shown to be involved in gene regulation by providing binding sites for transcription factors [29–34].
Alu induced RNA processing
Alu sequences also have functional consequences on the transcriptome. Several sequences within Alus have the potential to influence RNA processing events like splicing and polyadenylation. Since Alus tend to be inserted in introns, their likelihood of influencing mRNA maturation is substantial. Most Alu sequences contain motifs that are similar to splice sites [35, 36]. This is particularly true when they are in the antisense orientation because they encode a 5′ poly-T stretch that can serve as the required poly-pyrimidine tract upstream of a 3′ splice site. Alu insertions within introns can therefore influence alternative splicing. Two reports analyzing the genome-wide introduction of polyadenylation sites by retroelements in humans conclude that Alus also can provide polyadenylation sites [37, 38]. A near mimic of the canonical polyadenylation signal (AAUAAA) is present at three places in a sense-orientated Alu, although point mutations are required to form the complete signal. Nevertheless, few Alus are believed to influence polyadenylation; according to a genome-wide analysis of Alu elements in a position to influence polyadenylation, only ~1 % is active [37]. As discussed below, Alus use other mechanisms to affect the transcriptome far more frequently.
Exonization of Alu elements
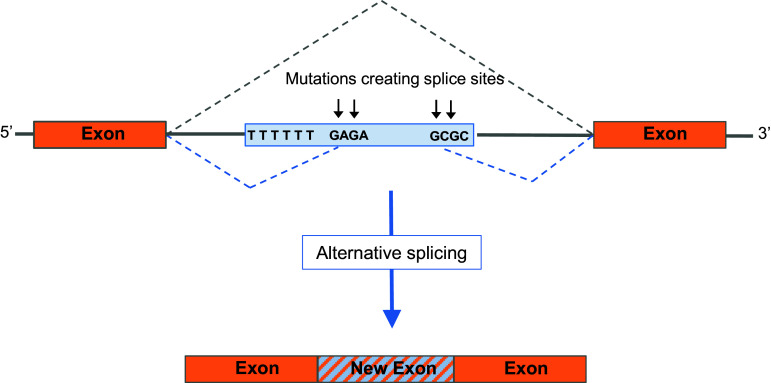
Fragments of Alu sequences appear in mature mRNAs, even within the open reading frame (ORF) [39–41]. In fact, computational analyses of the human genome predict that Alu elements are frequently part of ORFs [41, 42]. This can be explained by specific sequences within the Alu fragments that promote exonization [43–45]. Approximately 5 % of all alternatively spliced internal exons (exons flanked by introns at both sides) are derived from Alu elements [36, 46]. Alus have a great potential to become exonized because they can harbor up to ten potential 5′ splice sites and 13 potential 3′ splice sites. The vast majority of these sites (19 out of 23) are encoded in the antisense orientation. In addition, the A-rich region in the reverse orientation of an Alu element is well positioned to act as a pyrimidine-rich tract for 3′ splice site formation (Fig. 2). Older subfamilies of Alu elements are more commonly involved in exonization, probably because they have had a longer time to collect the mutations required for efficient splicing. In cases where an Alu element is inserted within a protein-coding region, it frequently leads to shortening of the protein via the introduction of a stop codon [36]. This is potentially deleterious to the organism. However, Alu exonization can also contribute new exons as well as additional coding sequence within an existing exon [26]. The majority of all Alu-derived exons are alternatively spliced and by consequence lead to proteome diversity [21, 47]. In a recent study of human brain transcripts, hundreds of genes were found to contain Alu exonized sequences [26]. The brain is known to require a large transcript variability for its function. Therefore, it is not surprising that Alu exonization is particularly prevalent in this area.
Fig. 2.
Exonization of an Alu element. An intronic Alu insertion (pale blue box) can, during evolution, acquire mutations within the Alu, creating functional alternative splice sites. As a consequence of these mutations, part of the Alu sequence is recognized as an exon and spliced into the mature transcript. Most Alu exonizations occur in the antisense orientation, probably because of the long poly(T) functioning as a poly-pyrimidine tract
One example of a functional Alu exonization occurs in the human ADAR2 gene [48]. Comparative analyses revealed that several splice variants of the ADAR2 transcript are conserved between vertebrates [49]. In humans, an additional splice variant has been detected where an AluJ exonization is integrated into the core of the catalytic domain [48, 50]. The additional amino acids generated by the Alu inclusion increase the distance between the critical Zn2+ chelating amino acids of the active site. The splice variants have the same RNA substrate specificity, but the catalytic activity of the ADAR2 enzyme with the AluJ inclusion is compromised. ADAR2 can also edit adenosines within inverted Alu repeats, as will be discussed below. It is intriguing, therefore, that the same enzyme known to modify Alu repeats also has a splice variant that arose from produces functional Alu exonization.
Regulation of Alu exonization
Alu elements that turn into exons generally introduce premature stop codons. This leads to mRNA degradation or aberrant proteins [51]. Therefore, newly acquired Alu elements within constitutively expressed mRNA isoforms are usually deleterious. Accordingly, organisms require a mechanism that protects the transcriptome against aberrant Alu exonization. One way to prevent Alu exonization is by interfering with splicing through protein-RNA interactions [52]. At least two RNA-binding proteins are known to impede the emergence of new Alu exons by interacting with antisense Alu RNA sequence. One of them is the abundantly expressed heterogeneous nuclear ribonucleoprotein C (hnRNP C). This factor interacts with the uridine tract in the antisense Alu sequence and prevents splicing by blocking the interaction of U2AF65 at the potential 3′ Alu splice site. Analysis of the genome-wide U2AF65 binding profile revealed increased binding to antisense Alu elements in hnRNP C depleted cells, suggesting that hnRNP C is acting globally to prevent transcribed Alus from mRNA inclusion [52]. Thus, even though Alu exonization might be of an evolutionary advantage, it is important to restrict the process.
A-to-I RNA editing of transcribed Alus
Inverted Alu repeats create long RNA duplexes
Alu sequences are present in the genome in both the sense and antisense orientation. When proximal and transcribed, inversely oriented Alus frequently base pair with each other. This was first discovered in cell lines when Robertson and Dickson showed that many Alu RNAs are cleaved by the double-strand specific ribonuclease RNase III [53]. More recently, transcriptome analyses have shown that inverted Alu repeats closer than 3500 nucleotides from each other frequently hybridize to form stem–loop structures. Over 700,000 Alu repeats meet this criteria and have been proposed to form duplex structures in RNA [54]. Since the mutation frequency in Alu repeats is relatively low, the stem of these structures is an almost perfect duplex of approximately 300 base pairs. This is also the reason why the primate transcriptome is prone to interact with double-stranded RNA-binding proteins such as the Adenosine DeAminase that acts on RNA (ADAR) editing enzymes.
Inverted Alu repeats are subjected to A-to-I editing
In all metazoans, double-stranded RNA (dsRNA) is subjected to A-to-I RNA modification by hydrolytic deamination. This was first discovered when long dsRNA molecules, injected into frog oocytes, became “unwound” into single stranded molecules [55]. It is the conserved family of ADAR enzymes that catalyze this reaction [56]. Mammalian genomes have two active members of the ADAR family, ADAR1 and ADAR2 [57–59]. These enzymes recognize largely double-stranded RNA as substrates. Since inosine, like guanosine, base pairs with cytosine, both the splicing and translation machineries will recognize inosine as guanosine [60]. Hitherto, only RNA polymerase II (Pol II) transcripts are known to be substrates for the ADAR enzymes, even though in principle any dsRNA could be edited. The two ADARs do not recognize a specific consensus sequence, although they do show preferences for the neighbors surrounding the edited A [61, 62]. Although the substrate specificity of the two ADAR enzymes is overlapping, some sites are only edited by one of them, indicating that there are other requirements for editing site selection than the immediately surrounding RNA sequence. The number of edited sites in a substrate is highly dependent on the length of the duplex. Short stems interrupted by bulges and internal loops are often edited at only a single site, while longer stems such as inverted Alu repeats can be edited at the majority of their adenosines (reviewed in [63]).
Initially, discoveries of novel A-to-I RNA editing sites were either found fortuitously or from bioinformatics and comparative genomics analyses restricted to coding regions. This resulted in the discovery of only a few editing sites within exons. Nevertheless, editing was found to have very important functional consequences on several brain-specific proteins involved in neurotransmission (reviewed in [64]). In 2004, several groups simultaneously discovered that the majority of all human editing events reside within non-coding regions, particularly in inverted Alu elements [65–68]. Recently, NGS technology and newly developed computational methods have revealed that more than 100 million editing sites within the human transcriptome; as before, the majority reside within Alu repeats [54, 69]. In spite of this remarkably large number, most sites have a low editing efficiency and their functions are largely unknown. The inescapable conclusion from these reports is that the vast majority of human editing sites are in repetitive sequences such as inverted Alu repeats, which are located in introns and UTRs. Although they would not be predicted to directly alter codons, they do have the potential to create or disrupt consensus splice sites and other functional sequences within RNAs. For example, it has been suggested that editing of Alus and other SINE elements within the 3′UTR of mRNAs can cause nuclear retention [70, 71]. Other studies show that several mRNAs with edited inverted repeats are localized to the cytoplasm and present on polysomes [72, 73]. The influence of editing on mRNA nuclear retention is therefore unclear and needs further investigation.
Editing of dsRNA may protect the cell from apoptosis
Having long dsRNA structures in the cell is a potential danger for an organism: Long duplexed viral RNA is known to trigger an immune response, a potent stressor on the cell. One pathway for this response is through autophosphorylation of the dsRNA-dependent protein kinase R (PKR). Phosphorylated PKR induces translational arrest and eventually leads to apoptosis (reviewed in [74]). This response is believed to prevent the spread of a viral infection. It has recently been reported that PKR also interacts with inverted Alu repeats during the early phases of mitosis [75]. This M-phase-specific activation may therefore contribute to the suppression of translation for the majority of mRNAs. During interphase, however, translation is required, so the cell needs a mechanism to prevent the dsRNA formed from inverted Alu elements from activating PKR. It has been suggested that this is accomplished by sequestering dsRNAs within the nucleus, away from the cytosolic PKR. The disintegration of the nuclear envelope during mitosis temporarily disrupts this separation and allows the interaction to occur. However, this does not explain why inverted Alu sequences located in the UTRs of cytosolic mRNAs do not activate PKR during interphase. It is reasonable to hypothesize that A-to-I editing can disrupt the duplexes enough to prevent PKR activation. However, this would imply that the editing activity is down-regulated during mitosis, although this is yet to be determined. A recent report has identified a role for ADAR1 in balancing the extent of endogenous dsRNA with the requirements of a cell; by introducing inosine within cellular dsRNA, ADAR1 differentiates them from invading exogenous RNAs and therefore controls the switch for the innate immunity response [76].
Exonization through RNA editing
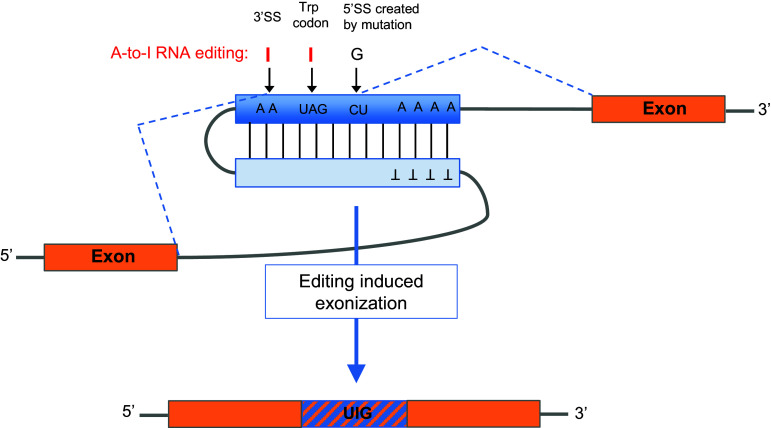
Exonization of Alus can occur via mechanisms besides the creation of splice sites through mutations. One way is for A-to-I editing to directly change the sequence of a pre-mRNA, as is the case for the nuclear prelamin A recognition factor (NARF). NARF interacts with and processes the carboxyl-terminal tail of prelamin A [77]. In higher primates, two proximate, inverted AluSx insertions are located within intron 7 of the NARF gene [78] (Fig. 3). In the downstream Alu element, a mutation from CU to GU was acquired that can function as a 5′ splice site. Sometime in the common ancestry of Great Apes, several RNA modifications were also introduced by A-to-I editing in the Alu stem–loop structure. The intron downstream of the new exon ends by AA, a site that usually cannot be recognized by the spliceosome (Fig. 3). However, in this case the second A is edited to I, converting it to a functional 3′ splice site (AG) and enabling exonization. In addition, the new Alu-derived exon contains a stop codon (UAG) that is efficiently edited to tryptophan (UGG), allowing read-through. Although examples of Alu exonization through RNA editing are rare thus far, this example demonstrates how evolution can act on both the DNA and RNA level to provide variants for natural selection. Interestingly, the Alu-derived exon in the NARF pre-mRNA is alternatively spliced in a tissue-specific manner, being eightfold more abundant in human brain tissue than in skeletal muscle [79]. This also highlights the complex evolution of novel exons via Alu editing.
Fig. 3.
Alu exonization through RNA editing in the human NARF transcript. Two inverted Alu elements fold into a dsRNA structure (blue and pale blue boxes), subjected to RNA editing. An AA is edited to AI and thereby read as an AG 3′ splice site. In addition, a UAG stop codon is edited to a UIG, read as a UGG Trp codon. Consequently, RNA editing within the Alu element leads to a functional exonization
Alus as cis-acting editing inducer elements
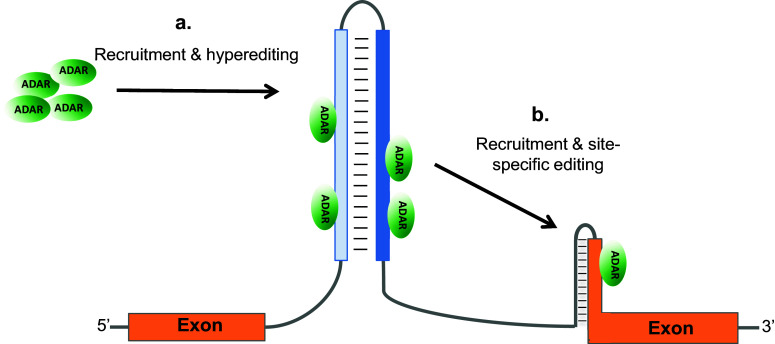
Recently, we have shown that inverted Alu repeats can recruit ADAR enzymes and induce editing at nearby sites, outside of the Alu sequences [80]. Our bioinformatics analysis revealed that there is an enrichment of site-selective editing events close to edited inverted Alu repeats. This observation suggests that site-selective editing within coding sequences is facilitated by inverted Alu repeats in primates. One example is within transcripts encoding the DNA repair enzyme NEIL1. These messages can undergo editing at the second and third positions of a lysine codon within exon 6 (K242; AAA). Editing at this position creates an arginine, a change which affects lesion-specific repair by the enzyme [81]. The edited site is located in a short stem–loop structure in which the complementary strand is located in an upstream intron (Fig. 4). About 220 nucleotides upstream this K/R site, two inverted Alu repeats form a large stem–loop structure, which is edited at several sites (Figs. 4, 5). Interestingly, editing of the NEIL1 transcript could not be detected in non-primates, organisms that lack Alu sequences but contain the short stem–loop harboring the K/R site. However, in primates like the rhesus macaque monkey, the NEIL1 transcript carries the upstream inverted Alu repeats and is edited at the K/R site to the same extent as it is in humans. Another example of Alu induced site-specific editing was found in the transcription factor GLI1, which is involved in the Hedgehog signaling pathway. Human GLI1 is edited at one site, changing an arginine to glycine (R/G) at position 701 [82]. As with NEIL1, the GLI1 R/G site is not edited in mice, which can be explained by the absence of inverted Alu repeats [80]. In contrast to NEIL1, the R/G site of GLI1 is not edited in rhesus monkey, which may be expected since one of the two Alu repeats present in humans is missing. Thus, a model is proposed where two adjacent, inverted Alu repeats function as a cis-acting recruitment element for ADAR, inducing editing at nearby sites and promoting primate-specific, or even human-specific, editing (Fig. 4). This model also explains why non-Alu editing sites are commonly found close to edited Alu repeats [69, 80].
Fig. 4.
Inverted Alus (blue and pale blue boxes) as cis-acting editing inducer elements (a). By attracting the ADAR enzyme and thereby increase the local ADAR concentration, site-specific editing of a nearby short hairpin is facilitated that can lead to recoding events (b)
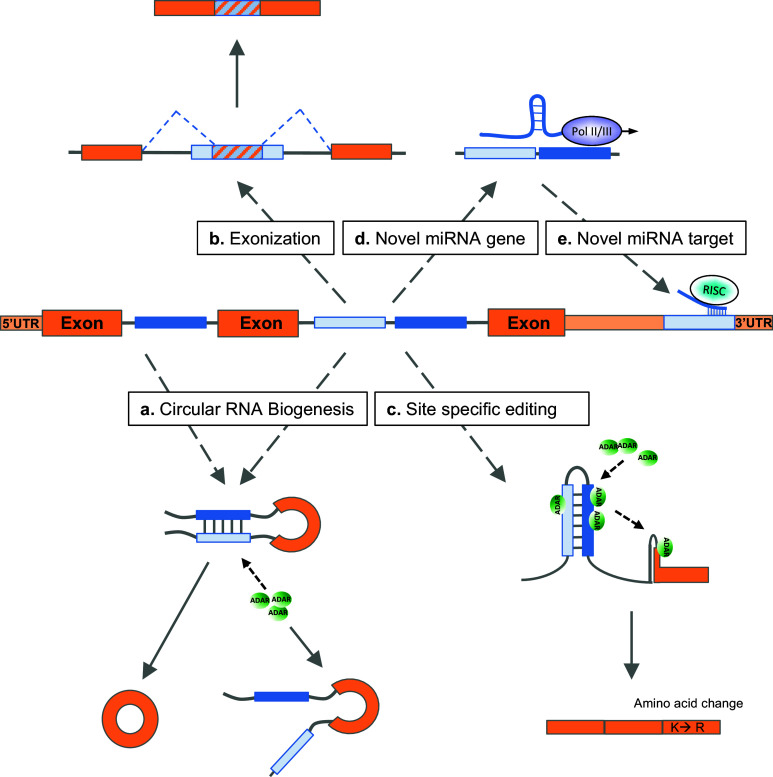
Fig. 5.
Possible Alu-induced RNA processing events. a Inverted Alus on each side of an exon that form a dsRNA structure may induce exonic RNA circularization. b An intronic Alu with a mutated or edited sequence can induce alternative splicing and/or Alu exonization. c Inverted Alu elements forming a dsRNA structure frequently induce A-to-I editing at nearby sites. d Within introns Alus can contribute to maturation of miRNAs. e Alu elements in 3′UTRs may act as miRNA targets
Alus and the biogenesis of circular RNA
A recent interesting finding is that inverted Alu repeats appear to promote the formation of circular RNAs (circRNAs) by facilitating back-splicing of pre-mRNAs [83]. Recently, increased attention has been focused on circRNAs as they are abundant and found in a number of different eukaryotes [84–86]. Derived from pre-mRNAs, they have been described in both human and mouse [87–89]. Unlike linear mRNAs, circRNAs are not polyadenylated or capped; instead the 5′ and 3′ ends are covalently linked, making them circular. In the past, circRNAs tended to be excluded because of commonly used poly(A)-based purifications. However, recently, ribosome-depletion purifications have largely replaced polyA selection, and this has led to the surprising discovery of thousands of circRNAs in the mammalian transcriptome [83, 85, 86, 90]. circRNAs appear to be tissue specific and developmentally regulated [85, 91, 92]. They are mainly produced through back-splicing of a pre-mRNA when a downstream 3′ splice site is joined to a 5′ splice site at an upstream exon. Moreover, a variety of studies demonstrates that the spliceosome is involved in exon circularization [86–89, 93–95]. Although the underlying mechanisms are still under investigation, it is clear that complementary sequences in the flanking intronic regions are necessary but not sufficient for circRNA formation. Several studies demonstrate that in humans, the complementary sequences often consist of inverted Alu repeats [83, 96, 97]. A genome-wide screen reveled that introns flanking circularized exons usually are much longer than average and are sixfold more likely to contain complementary Alu repeats [97]. It was demonstrated that there is a competition between inverted Alu repeats within a single intron and inverted repeats from flanking introns. Only the pairing of inverted repeats in separate introns, flanked by one or more exons, can bring the exon–intron junctions in close enough proximity to each other to promote circularization. Hence, it is clear not only that Alu repeats contribute to the formation of circRNAs but also that they can be involved in alternative circularization events between different exons. The requirement of inverted Alu repeats for circRNA formation, and the fact that inverted Alus are heavily edited, raises the question of whether ADARs are involved in the production of circRNAs. ADAR binding to inverted repeats might provide stability and thereby promote circularization. On the other hand, ADAR may inhibit circularization by converting adenosines to inosines, thereby reducing complementarity of the ends. Indeed, two recent reports suggest the latter hypothesis by showing that ADAR1 antagonizes the formation of circRNAs (Fig. 5) [91, 98].
Interestingly, many of the validated circRNAs are highly expressed and for some genes the expression of circRNAs even exceeds that of their linear counterparts. Nevertheless, the function of circRNAs have been determined in only a few cases. An interesting example is found in a circRNA derived from the antisense of the cerebellar degeneration-related protein 1 transcript (CDR1as). This circRNA functions as a microRNA (miRNA) sponge, harboring about 70 conserved binding sites for miR-7 and thereby buffering its activity [85, 90]. Even though it is tempting to suggest that circRNAs in general function as sponges to sequester and regulate miRNAs, only very few have been proven to have this function. Other potential functions that have been suggested are in the sequestration of RNA-binding proteins (RBPs), base pairing with other RNAs, or even in the production of novel peptides [99]. Another straight-forward function may be to regulate mRNA expression, as the circRNA’s synthesis would act as a competitor.
The role of Alu elements in the functionality of miRNAs
Alu-mediated expansion and evolution of miRNA genes
The small, non-coding miRNAs are predicted to regulate the majority of all mammalian messages by interacting with the 3′UTR [100–102]. Several characteristics of Alu elements make them putative sources of novel miRNAs. Most known miRNA genes reside within introns and are probably co-expressed together with their host gene [103]. Alus are also often located within intronic sequences of protein-coding genes, increasing their likelihood of contributing to the birth of novel miRNAs. Furthermore, nearly 50 % of all miRNA genes are located in clusters that often share sequence homology [104–106]. This organization coincides with the frequent Alu insertions into the same genomic region. To be processed into a bona fide miRNA, a primary (pri-)miRNA transcript containing one or more short hairpin structures is subjected to two endonucleolytic cleavage events. The first, by the nuclear Drosha ribonuclease, cuts it into an approximately 70–90 nucleotides long precursor (pre-)miRNA. The second, by the cytoplasmic ribonuclease Dicer, creates a short miRNA duplex of about 22 nucleotides. Inverted Alu repeats that form RNA hairpins are candidates for precursors of miRNAs. The first report showing that TE-insertions correlated with the production of functional miRNAs came in 2005 from the Smalheiser and Torvik laboratory [107]. By comparing pre-miRNA sequences with known repeat elements, they showed that transcription across repeat elements present in opposite orientations could explain the origin of several miRNAs. Since then, several independent investigations have increased the identification of TE-derived miRNA loci in various mammal- and primate-specific genomes [108–113]. In the most recent report, an extensive analysis showed that about 15 % of all known miRNA genomic loci have a TE-origin [114]. miRNAs derived from TEs have been shown to produce functional miRNAs processed by the same cellular machinery as other miRNAs that lack a TE-origin [115–117]. Another feature that links Alu element insertion and miRNA gene origin is the fact that a substantial fraction of human miRNA genes are not evolutionary conserved [118]. This may reflect the unique occurrence of Alu sequences in the primate lineage and the diverse integration of Alus between different primates.
One interesting example of how Alus have contributed to the rapid expansion of primate-specific miRNA genes is the miRNA cluster on human chromosome 19 (C19MC) [118]. This is the largest miRNA cluster in humans and spans over 100 kilobases. It consists of 46 Alu-embedded pre-miRNA genes that are both primate- and tissue specific [118–120]. In total, 50 % of the entire genomic region consists of repeat elements, and 90 % of these correspond to Alus. Based on the high enrichment of Alus in this region, the miRNA expansion in this cluster was proposed to be generated by Alu-mediated recombination events [121], leading to segmental duplications of 400–700 nt long cassettes which include one miRNA surrounded by several Alu elements in the reverse orientation [110, 120].
Alu-directed transcriptional regulation of miRNA
Transcription of miRNA genes is mainly mediated by RNA Pol II [122–124]. Most of the miRNAs encoded in the C19MC cluster lie within introns of a pre-mRNA with non-coding exons [125]. However, transcription of some of the miRNAs from C19MC are thought to be initiated from Alu-derived Pol III promoters [119]. Possibly Pol II generates the steady-state “bulk” transcription of these miRNAs, whereas Pol III may mediate elevated transcriptional output under specialized conditions, such as cellular stress. In addition, several of the Alu associated miRNAs from the C19MC cluster were shown to be elevated in gastric cancer cells upon demethylation of Alu-embedded Pol II promoters [126]. The role of cis-acting elements involved in miRNA transcriptional regulation, and the possible cross-talk between Pol II and Pol III transcription, needs to be explored further.
Alus as donors of novel miRNA target sites
For most well-defined miRNAs, the specificity largely resides within the complementarity between the 5′ “seed region” of the mature miRNA and the 3′UTR of the mRNA. Upon, miRNA-RISC binding to the mRNA target, protein synthesis is inhibited through translational silencing mechanisms, which include degradation, deadenylation, and/or translational repression [127–129]. Due to the fact that Alu insertions have contributed to a substantial fraction of primate 3′UTR sequences [130], the question arises whether this has affected miRNA-dependent gene regulation. In order to investigate if Alu element insertion has provided novel miRNA target sites, several laboratories, using computational approaches, predicted miRNA seed-matches within Alu elements residing in 3′UTRs. In this way, 30 human miRNAs candidates were identified [131]. More recently, Hoffman et al. reported that 16 % of all human genes contain at least one Alu element in their 3′UTR [116]. In total, this means almost 5000 Alus that create nearly 30 000 putative target sites with perfect match to seed sequences of 400 miRNAs, conserved among mammals. Even though, the majority of 3′UTR miRNA seed-matches reside outside of Alu elements, in a few cases, they are enriched in defined hotspots within Alus [110, 131]. Interestingly, these sites were only present in Alus in the sense orientation and overlapped with the most conserved region of the Alu consensus sequence. These sites were also preserved in the mouse Alu-like B1 SINE consensus sequence, indicating that they originated from a common Alu ancestor. Furthermore, this hotspot perfectly overlaps with an experimentally verified 6-base unpaired loop within the Alu element [132]. This position possibly facilitates the miRNA’s access to its target and indicates that the target site is real. A few Alu-derived miRNA targets have been experimentally verified [116, 117]. However, the majority of Alu-embedded miRNA targets sites seem to be non-functional or less potent than targets residing outside of Alus [116, 117]. In general, miRNA targets reside in proximal or distal portions of 3′UTRs, whereas Alus tend to reside in the center. Furthermore, a high AU-content surrounding miRNA targets correlates with their effectiveness [133], and miRNA targets within Alus have a significantly lower surrounding AU content. Moreover, analyses of interactions between miRNAs and their targets show that the miRNA machinery avoids targets within Alus [116].
Inverted Alu repeats have the potential to generate both miRNAs and their targets. This opens a role for Alu-derived miRNAs as regulators of active transposition. It has also been proposed that miRNAs might target Alus to repress active Alu transposition, e.g., during cellular stress when Alu transcription is elevated [110, 121].
The contribution of transcribed Alus and editing to primate evolution
As discussed above, it is evident that Alus, as non-coding components of primate genomes, can modulate the transcriptome in a variety of ways. Transcribed repetitive Alu elements can no longer be considered as “junk” non-coding RNA. Their contribution to primate-specific genome evolution has now been explored and accepted. Presently, a more pertinent question is to what extent they have contributed to the primate specification. The genomes of humans and chimpanzees are nearly 99 % identical [134, 135]. The similarity is even greater when only coding regions are compared [136]. Still the morphological, cognitive, and behavioral differences between the two are striking. Although there are multiple explanations for this phenotypic variation, mobile elements, and especially retrotransposons, are major contributors [137–139]. The number of mobile elements are similar between humans, chimpanzees, and rhesus macaques [1, 140, 141]. However, a large fraction of the retrotransposons has been inserted independently, at different locations, and this has served to shape each genome differently [138]. Furthermore, Alu repeats are by far the most abundant class of retrotransposon insertions in both humans and chimpanzees, but the number of insertions in humans is 3.4-fold higher. The majority of these insertions (>95 %) in both species are AluY repeats, members of the youngest and most active Alu family [140]. Most of the chimpanzee AluY repeats belong to the subfamily AluYc1 which is very similar to the original Alu sequence in the common ancestor. Most of the human-specific AluY repeats belongs to two new subfamilies (AluYa5 and AluYb8) that have evolved since the chimpanzee-human split and vary substantially from the ancestor Alu sequence [140]. Compared to chimpanzees, almost three times more of the newly inserted Alu sequences in humans are located within genes (1477 versus 497) [138]. It is therefore conceivable that Alus have a greater potential to affect gene expression in humans. Interestingly, newly inserted Alus are enriched in genes involved in nervous system development in both human and chimpanzee [142].
In primates, the central nervous system is the most complex organ, requiring extensive transcript variability. With over 100 million A-to-I editing sites within inversely orientated transcribed Alu sequences in the majority of all human genes [54], the potential to influence evolution at the transcriptome level is immense. Furthermore, the fact that the editing enzymes are particularly abundant in the brain leads to the speculation that editing is also involved in primate speciation [142–145]. Interestingly, editing within position-specific Alu sequences is more conserved between different human individuals than editing between identical Alus within the same individual [146]. This low variance of editing levels between individuals could be an indication of tight regulation and functionality of editing events. Moreover, there is a higher level of editing in human Alu repeats than in Alus from other primates [142]. The contribution of Alus to alternative splicing leads to further transcriptome variability. As previously discussed, Alus are frequently exonized in humans and almost all of these exons are alternatively spliced. Most introduce stop codons, either directly or through frame-shifts, events which lead to degradation of the transcript through nonsense-mediated decay [36]. However, editing within Alu repeats can remove premature termination codons and thereby create new exons that lead to new isoforms, as is the case for the NARF messages. In this way, Alu elements and RNA editing work in concert to modulate the transcriptome rather than the genome. A great advantage to modulating transcripts rather than genes is the possibility in keeping several isoforms in parallel. In this way, new gene functions can evolve without jeopardizing essential pathways.
Summary
Alu elements are an important engine for functional diversity within the primate transcriptome. As building blocks of extra genetic material, retroelements are used to invent new ways to vary mRNA. The almost 300 nt long Alu element is an ideal player for several reasons: (1) Alus are frequently inserted into non-coding regions of pre-mRNAs, (2) when transcribed, they easily form stable secondary structures that seed a number of different RNA processing events, and (3) small changes to their sequence make them targets for a number of RNA-binding proteins that regulate gene expression. Depending on its location and specific sequence, the Alu element can induce different RNA processing events (Fig. 5). If two inverted Alus reside on each side of an exon, they can form a double-stranded RNA structure that may induce back-splicing and the formation of circular RNA. An intronic Alu with a mutated or edited sequence can induce alternative splicing or Alu exonization. Inverted repeat elements can also contribute to transcript variations in a more fine-tuned manner by inducing A-to-I editing within coding sequence. Also, Alus in introns and 3′UTRs can provide both miRNAs and their target sequences. In this review, we have only highlighted a few examples of how Alu elements may contribute to transcriptome variation in primates. These effects certainly combine with the better explored genomic variations that Alus create. Future studies will most likely reveal additional mechanisms on how these elements modulate our genetics.
Acknowledgments
We would like to thank Joshua Rosenthal, Lars Wieslander, and Petra Björk for their input and for critically reading the paper. This work was supported by the Swedish Research Council, grant K2013-66X-20702-06-4.
References
- 1.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 2.de Koning AP, Gu W, Castoe TA, Batzer MA, Pollock DD. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet. 2011;7:e1002384. doi: 10.1371/journal.pgen.1002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ullu E, Tschudi C. Alu sequences are processed 7SL RNA genes. Nature. 1984;312:171–172. doi: 10.1038/312171a0. [DOI] [PubMed] [Google Scholar]
- 4.Kriegs JO, Churakov G, Jurka J, Brosius J, Schmitz J. Evolutionary history of 7SL RNA-derived SINEs in Supraprimates. Trends Genet TIG. 2007;23:158–161. doi: 10.1016/j.tig.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Willard C, Nguyen HT, Schmid CW. Existence of at least three distinct Alu subfamilies. J Mol Evol. 1987;26:180–186. doi: 10.1007/BF02099850. [DOI] [PubMed] [Google Scholar]
- 6.Britten RJ, Baron WF, Stout DB, Davidson EH. Sources and evolution of human Alu repeated sequences. Proc Natl Acad Sci USA. 1988;85:4770–4774. doi: 10.1073/pnas.85.13.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurka J, Smith T. A fundamental division in the Alu family of repeated sequences. Proc Natl Acad Sci USA. 1988;85:4775–4778. doi: 10.1073/pnas.85.13.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labuda D, Striker G. Sequence conservation in Alu evolution. Nucleic Acids Res. 1989;17:2477–2491. doi: 10.1093/nar/17.7.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuhrman SA, Deininger PL, LaPorte P, Friedmann T, Geiduschek EP. Analysis of transcription of the human Alu family ubiquitous repeating element by eukaryotic RNA polymerase III. Nucleic Acids Res. 1981;9:6439–6456. doi: 10.1093/nar/9.23.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis IM. RNA polymerase III. Genes, factors and transcriptional specificity. Eur J Biochem. 1993;212:1–11. doi: 10.1111/j.1432-1033.1993.tb17626.x. [DOI] [PubMed] [Google Scholar]
- 11.Xing J, Zhang Y, Han K, Salem AH, Sen SK, Huff CD, Zhou Q, Kirkness EF, Levy S, Batzer MA, et al. Mobile elements create structural variation: analysis of a complete human genome. Genome Res. 2009;19:1516–1526. doi: 10.1101/gr.091827.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orgel LE, Crick FH. Selfish DNA: the ultimate parasite. Nature. 1980;284:604–607. doi: 10.1038/284604a0. [DOI] [PubMed] [Google Scholar]
- 13.Orgel LE, Crick FH, Sapienza C. Selfish DNA. Nature. 1980;288:645–646. doi: 10.1038/288645a0. [DOI] [PubMed] [Google Scholar]
- 14.Jacques PE, Jeyakani J, Bourque G. The majority of primate-specific regulatory sequences are derived from transposable elements. PLoS Genet. 2013;9:e1003504. doi: 10.1371/journal.pgen.1003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowley M, Oakey RJ. Transposable elements re-wire and fine-tune the transcriptome. PLoS Genet. 2013;9:e1003234. doi: 10.1371/journal.pgen.1003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer MF. SINEs and LINEs: highly repeated short and long interspersed sequences in mammalian genomes. Cell. 1982;28:433–434. doi: 10.1016/0092-8674(82)90194-5. [DOI] [PubMed] [Google Scholar]
- 17.Hsu K, Chang DY, Maraia RJ. Human signal recognition particle (SRP) Alu-associated protein also binds Alu interspersed repeat sequence RNAs. Characterization of human SRP9. J Biol Chem. 1995;270:10179–10186. doi: 10.1074/jbc.270.14.8319. [DOI] [PubMed] [Google Scholar]
- 18.West N, Roy-Engel AM, Imataka H, Sonenberg N, Deininger P. Shared protein components of SINE RNPs. J Mol Biol. 2002;321:423–432. doi: 10.1016/S0022-2836(02)00542-9. [DOI] [PubMed] [Google Scholar]
- 19.Dewannieux M, Esnault C, Heidmann T. LINE-mediated retrotransposition of marked Alu sequences. Nat Genet. 2003;35:41–48. doi: 10.1038/ng1223. [DOI] [PubMed] [Google Scholar]
- 20.Roy AM, West NC, Rao A, Adhikari P, Aleman C, Barnes AP, Deininger PL. Upstream flanking sequences and transcription of SINEs. J Mol Biol. 2000;302:17–25. doi: 10.1006/jmbi.2000.4027. [DOI] [PubMed] [Google Scholar]
- 21.Sela N, Mersch B, Gal-Mark N, Lev-Maor G, Hotz-Wagenblatt A, Ast G. Comparative analysis of transposed element insertion within human and mouse genomes reveals Alu’s unique role in shaping the human transcriptome. Genome Biol. 2007;8:R127. doi: 10.1186/gb-2007-8-6-r127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grover D, Mukerji M, Bhatnagar P, Kannan K, Brahmachari SK. Alu repeat analysis in the complete human genome: trends and variations with respect to genomic composition. Bioinformatics. 2004;20:813–817. doi: 10.1093/bioinformatics/bth005. [DOI] [PubMed] [Google Scholar]
- 23.Kim TM, Jung YC, Rhyu MG. Alu and L1 retroelements are correlated with the tissue extent and peak rate of gene expression, respectively. J Korean Med Sci. 2004;19:783–792. doi: 10.3346/jkms.2004.19.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganapathi M, Srivastava P, Das Sutar SK, Kumar K, Dasgupta D, Pal Singh G, Brahmachari V, Brahmachari SK. Comparative analysis of chromatin landscape in regulatory regions of human housekeeping and tissue specific genes. BMC Bioinform. 2005;6:126. doi: 10.1186/1471-2105-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eller CD, Regelson M, Merriman B, Nelson S, Horvath S, Marahrens Y. Repetitive sequence environment distinguishes housekeeping genes. Gene. 2007;390:153–165. doi: 10.1016/j.gene.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen S, Lin L, Cai JJ, Jiang P, Kenkel EJ, Stroik MR, Sato S, Davidson BL, Xing Y. Widespread establishment and regulatory impact of Alu exons in human genes. Proc Natl Acad Sci USA. 2011;108:2837–2842. doi: 10.1073/pnas.1012834108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie H, Wang M, Bonaldo Mde F, Smith C, Rajaram V, Goldman S, Tomita T, Soares MB. High-throughput sequence-based epigenomic analysis of Alu repeats in human cerebellum. Nucleic Acids Res. 2009;37:4331–4340. doi: 10.1093/nar/gkp393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polak P, Domany E. Alu elements contain many binding sites for transcription factors and may play a role in regulation of developmental processes. BMC Genom. 2006;7:133. doi: 10.1186/1471-2164-7-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babich V, Aksenov N, Alexeenko V, Oei SL, Buchlow G, Tomilin N. Association of some potential hormone response elements in human genes with the Alu family repeats. Gene. 1999;239:341–349. doi: 10.1016/S0378-1119(99)00391-1. [DOI] [PubMed] [Google Scholar]
- 30.Le Goff W, Guerin M, Chapman MJ, Thillet J. A CYP7A promoter binding factor site and Alu repeat in the distal promoter region are implicated in regulation of human CETP gene expression. J Lipid Res. 2003;44:902–910. doi: 10.1194/jlr.M200423-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Norris J, Fan D, Aleman C, Marks JR, Futreal PA, Wiseman RW, Iglehart JD, Deininger PL, McDonnell DP. Identification of a new subclass of Alu DNA repeats which can function as estrogen receptor-dependent transcriptional enhancers. J Biol Chem. 1995;270:22777–22782. doi: 10.1074/jbc.270.39.22777. [DOI] [PubMed] [Google Scholar]
- 32.Oei SL, Babich VS, Kazakov VI, Usmanova NM, Kropotov AV, Tomilin NV. Clusters of regulatory signals for RNA polymerase II transcription associated with Alu family repeats and CpG islands in human promoters. Genomics. 2004;83:873–882. doi: 10.1016/j.ygeno.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Piedrafita FJ, Molander RB, Vansant G, Orlova EA, Pfahl M, Reynolds WF. An Alu element in the myeloperoxidase promoter contains a composite SP1-thyroid hormone-retinoic acid response element. J Biol Chem. 1996;271:14412–14420. doi: 10.1074/jbc.271.24.14412. [DOI] [PubMed] [Google Scholar]
- 34.Vansant G, Reynolds WF. The consensus sequence of a major Alu subfamily contains a functional retinoic acid response element. Proc Natl Acad Sci USA. 1995;92:8229–8233. doi: 10.1073/pnas.92.18.8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makalowski W, Mitchell GA, Labuda D. Alu sequences in the coding regions of mRNA: a source of protein variability. Trends Genet TIG. 1994;10:188–193. doi: 10.1016/0168-9525(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 36.Sorek R, Ast G, Graur D. Alu-containing exons are alternatively spliced. Genome Res. 2002;12:1060–1067. doi: 10.1101/gr.229302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C, Ara T, Gautheret D. Using Alu elements as polyadenylation sites: a case of retroposon exaptation. Mol Biol Evol. 2009;26:327–334. doi: 10.1093/molbev/msn249. [DOI] [PubMed] [Google Scholar]
- 38.Roy-Engel AM, El-Sawy M, Farooq L, Odom GL, Perepelitsa-Belancio V, Bruch H, Oyeniran OO, Deininger PL. Human retroelements may introduce intragenic polyadenylation signals. Cytogenet Genome Res. 2005;110:365–371. doi: 10.1159/000084968. [DOI] [PubMed] [Google Scholar]
- 39.Makalowski W. Genomic scrap yard: how genomes utilize all that junk. Gene. 2000;259:61–67. doi: 10.1016/S0378-1119(00)00436-4. [DOI] [PubMed] [Google Scholar]
- 40.Yulug IG, Yulug A, Fisher EM. The frequency and position of Alu repeats in cDNAs, as determined by database searching. Genomics. 1995;27:544–548. doi: 10.1006/geno.1995.1090. [DOI] [PubMed] [Google Scholar]
- 41.Nekrutenko A, Li WH. Transposable elements are found in a large number of human protein-coding genes. Trends Genet TIG. 2001;17:619–621. doi: 10.1016/S0168-9525(01)02445-3. [DOI] [PubMed] [Google Scholar]
- 42.Wu M, Li L, Sun Z. Transposable element fragments in protein-coding regions and their contributions to human functional proteins. Gene. 2007;401:165–171. doi: 10.1016/j.gene.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Lev-Maor G, Sorek R, Shomron N, Ast G. The birth of an alternatively spliced exon: 3′ splice-site selection in Alu exons. Science. 2003;300:1288–1291. doi: 10.1126/science.1082588. [DOI] [PubMed] [Google Scholar]
- 44.Ram O, Schwartz S, Ast G. Multifactorial interplay controls the splicing profile of Alu-derived exons. Mol Cell Biol. 2008;28:3513–3525. doi: 10.1128/MCB.02279-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorek R, Lev-Maor G, Reznik M, Dagan T, Belinky F, Graur D, Ast G. Minimal conditions for exonization of intronic sequences: 5′ splice site formation in alu exons. Mol Cell. 2004;14:221–231. doi: 10.1016/S1097-2765(04)00181-9. [DOI] [PubMed] [Google Scholar]
- 46.Gotea V, Makalowski W. Do transposable elements really contribute to proteomes? Trends Genet TIG. 2006;22:260–267. doi: 10.1016/j.tig.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Sorek R. The birth of new exons: mechanisms and evolutionary consequences. RNA. 2007;13:1603–1608. doi: 10.1261/rna.682507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerber A, O’Connell MA, Keller W. Two forms of human double-stranded RNA-specific editase 1 (hRED1) generated by the insertion of an Alu cassette. RNA. 1997;3:453–463. [PMC free article] [PubMed] [Google Scholar]
- 49.Slavov D, Crnogorac-Jurcevic T, Clark M, Gardiner K. Comparative analysis of the DRADA A-to-I RNA editing gene from mammals, pufferfish and zebrafish. Gene. 2000;250:53–60. doi: 10.1016/S0378-1119(00)00175-X. [DOI] [PubMed] [Google Scholar]
- 50.Agranat L, Sperling J, Sperling R. A novel tissue-specific alternatively spliced form of the A-to-I RNA editing enzyme ADAR2. RNA Biol. 2010;7:253–262. doi: 10.4161/rna.7.2.11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vorechovsky I. Transposable elements in disease-associated cryptic exons. Hum Genet. 2010;127:135–154. doi: 10.1007/s00439-009-0752-4. [DOI] [PubMed] [Google Scholar]
- 52.Zarnack K, Konig J, Tajnik M, Martincorena I, Eustermann S, Stevant I, Reyes A, Anders S, Luscombe NM, Ule J. Direct competition between hnRNP C and U2AF65 protects the transcriptome from the exonization of Alu elements. Cell. 2013;152:453–466. doi: 10.1016/j.cell.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robertson HD, Dickson E. Structure and distribution of Alu family sequences or their analogs within heterogeneous nuclear RNA of HeLa, KB, and L cells. Mol Cell Biol. 1984;4:310–316. doi: 10.1128/MCB.4.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bazak L, Haviv A, Barak M, Jacob-Hirsch J, Deng P, Zhang R, Isaacs FJ, Rechavi G, Li JB, Eisenberg E, et al. A-to-I RNA editing occurs at over a hundred million genomic sites, located in a majority of human genes. Genome Res. 2014;24:365–376. doi: 10.1101/gr.164749.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bass BL, Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987;48:607–613. doi: 10.1016/0092-8674(87)90239-X. [DOI] [PubMed] [Google Scholar]
- 56.Bass BL, Nishikura K, Keller W, Seeburg PH, Emeson RB, O’Connell MA, Samuel CE, Herbert A. A standardized nomenclature for adenosine deaminases that act on RNA. RNA. 1997;3:947–949. [PMC free article] [PubMed] [Google Scholar]
- 57.Kim U, Garner TL, Sanford T, Speicher D, Murray JM, Nishikura K. Purification and characterization of double-stranded RNA adenosine deaminase from bovine nuclear extracts. J Biol Chem. 1994;269:13480–13489. [PubMed] [Google Scholar]
- 58.O’Connell MA, Krause S, Higuchi M, Hsuan JJ, Totty NF, Jenny A, Keller W. Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Mol Cell Biol. 1995;15:1389–1397. doi: 10.1128/MCB.15.3.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melcher T, Maas S, Herb A, Sprengel R, Seeburg PH, Higuchi M. A mammalian RNA editing enzyme. Nature. 1996;379:460–464. doi: 10.1038/379460a0. [DOI] [PubMed] [Google Scholar]
- 60.Basilio C, Wahba AJ, Lengyel P, Speyer JF, Ochoa S. Synthetic polynucleotides and the amino acid code. V. Proc Natl Acad Sci USA. 1962;48:613–616. doi: 10.1073/pnas.48.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li JB, Levanon EY, Yoon JK, Aach J, Xie B, Leproust E, Zhang K, Gao Y, Church GM. Genome-wide identification of human RNA editing sites by parallel DNA capturing and sequencing. Science. 2009;324:1210–1213. doi: 10.1126/science.1170995. [DOI] [PubMed] [Google Scholar]
- 62.Polson AG, Bass BL. Preferential selection of adenosines for modification by double-stranded RNA adenosine deaminase. EMBO J. 1994;13:5701–5711. doi: 10.1002/j.1460-2075.1994.tb06908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wahlstedt H, Öhman M. Site-selective versus promiscuous A-to-I editing. Wiley Interdiscip Rev RNA. 2011;2:761–771. doi: 10.1002/wrna.89. [DOI] [PubMed] [Google Scholar]
- 64.Tariq A, Jantsch MF. Transcript diversification in the nervous system: a to I RNA editing in CNS function and disease development. Front Neurosci. 2012;6:99. doi: 10.3389/fnins.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Athanasiadis A, Rich A, Maas S. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol. 2004;2:e391. doi: 10.1371/journal.pbio.0020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blow M, Futreal PA, Wooster R, Stratton MR. A survey of RNA editing in human brain. Genome Res. 2004;14:2379–2387. doi: 10.1101/gr.2951204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim DD, Kim TT, Walsh T, Kobayashi Y, Matise TC, Buyske S, Gabriel A. Widespread RNA editing of embedded alu elements in the human transcriptome. Genome Res. 2004;14:1719–1725. doi: 10.1101/gr.2855504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levanon EY, Eisenberg E, Yelin R, Nemzer S, Hallegger M, Shemesh R, Fligelman ZY, Shoshan A, Pollock SR, Sztybel D, et al. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat Biotechnol. 2004;22:1001–1005. doi: 10.1038/nbt996. [DOI] [PubMed] [Google Scholar]
- 69.Ramaswami G, Zhang R, Piskol R, Keegan LP, Deng P, O’Connell MA, Li JB. Identifying RNA editing sites using RNA sequencing data alone. Nat Methods. 2013;10:128–132. doi: 10.1038/nmeth.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen LL, DeCerbo JN, Carmichael GG. Alu element-mediated gene silencing. EMBO J. 2008;27:1694–1705. doi: 10.1038/emboj.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prasanth KV, Prasanth SG, Xuan Z, Hearn S, Freier SM, Bennett CF, Zhang MQ, Spector DL. Regulating gene expression through RNA nuclear retention. Cell. 2005;123:249–263. doi: 10.1016/j.cell.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 72.Capshew CR, Dusenbury KL, Hundley HA. Inverted Alu dsRNA structures do not affect localization but can alter translation efficiency of human mRNAs independent of RNA editing. Nucleic Acids Res. 2012;40:8637–8645. doi: 10.1093/nar/gks590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hundley HA, Krauchuk AA, Bass BL. C. elegans and H. sapiens mRNAs with edited 3′ UTRs are present on polysomes. RNA. 2008;14:2050–2060. doi: 10.1261/rna.1165008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nallagatla SR, Toroney R, Bevilacqua PC. Regulation of innate immunity through RNA structure and the protein kinase PKR. Curr Opin Struct Biol. 2011;21:119–127. doi: 10.1016/j.sbi.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim Y, Lee JH, Park JE, Cho J, Yi H, Kim VN. PKR is activated by cellular dsRNAs during mitosis and acts as a mitotic regulator. Genes Dev. 2014;28:1310–1322. doi: 10.1101/gad.242644.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mannion NM, Greenwood SM, Young R, Cox S, Brindle J, Read D, Nellaker C, Vesely C, Ponting CP, McLaughlin PJ, et al. The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell reports. 2014;9:1482–1494. doi: 10.1016/j.celrep.2014.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barton RM, Worman HJ. Prenylated prelamin A interacts with Narf, a novel nuclear protein. J Biol Chem. 1999;274:30008–30018. doi: 10.1074/jbc.274.42.30008. [DOI] [PubMed] [Google Scholar]
- 78.Lev-Maor G, Sorek R, Levanon EY, Paz N, Eisenberg E, Ast G. RNA-editing-mediated exon evolution. Genome Biol. 2007;8:R29. doi: 10.1186/gb-2007-8-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lev-Maor G, Goren A, Sela N, Kim E, Keren H, Doron-Faigenboim A, Leibman-Barak S, Pupko T, Ast G. The “alternative” choice of constitutive exons throughout evolution. PLoS Genet. 2007;3:e203. doi: 10.1371/journal.pgen.0030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Daniel C, Silberberg G, Behm M, Öhman M. Alu elements shape the primate transcriptome by cis-regulation of RNA editing. Genome Biol. 2014;15:R28. doi: 10.1186/gb-2014-15-2-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yeo J, Goodman RA, Schirle NT, David SS, Beal PA. RNA editing changes the lesion specificity for the DNA repair enzyme NEIL1. Proc Natl Acad Sci USA. 2010;107:20715–20719. doi: 10.1073/pnas.1009231107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimokawa T, Rahman MF, Tostar U, Sonkoly E, Stahle M, Pivarcsi A, Palaniswamy R, Zaphiropoulos PG. RNA editing of the GLI1 transcription factor modulates the output of Hedgehog signaling. RNA Biol. 2013;10:321–333. doi: 10.4161/rna.23343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15:409. doi: 10.1186/s13059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 86.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, Kinzler KW, Vogelstein B. Scrambled exons. Cell. 1991;64:607–613. doi: 10.1016/0092-8674(91)90244-S. [DOI] [PubMed] [Google Scholar]
- 88.Cocquerelle C, Mascrez B, Hetuin D, Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J. 1993;7:155–160. doi: 10.1096/fasebj.7.1.7678559. [DOI] [PubMed] [Google Scholar]
- 89.Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, Goodfellow P, Lovell-Badge R. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell. 1993;73:1019–1030. doi: 10.1016/0092-8674(93)90279-Y. [DOI] [PubMed] [Google Scholar]
- 90.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 91.Rybak-Wolf A, Stottmeister C, Glazar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 92.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chao CW, Chan DC, Kuo A, Leder P. The mouse formin (Fmn) gene: abundant circular RNA transcripts and gene-targeted deletion analysis. Mol Med. 1998;4:614–628. [PMC free article] [PubMed] [Google Scholar]
- 94.Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;6:e1001233. doi: 10.1371/journal.pgen.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, Kjems J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011;30:4414–4422. doi: 10.1038/emboj.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liang D, Wilusz JE. Short intronic repeat sequences facilitate circular RNA production. Genes Dev. 2014;28:2233–2247. doi: 10.1101/gad.251926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L. Complementary sequence-mediated exon circularization. Cell. 2014;159:134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 98.Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, Piechotta M, Levanon EY, Landthaler M, Dieterich C, et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2014;10:170–177. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 99.Wilusz JE, Sharp PA. Molecular biology. A circuitous route to noncoding RNA. Science. 2013;340:440–441. doi: 10.1126/science.1238522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol. 2013;14:475–488. doi: 10.1038/nrm3611. [DOI] [PubMed] [Google Scholar]
- 101.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rodriguez J, Vives L, Jorda M, Morales C, Munoz M, Vendrell E, Peinado MA. Genome-wide tracking of unmethylated DNA Alu repeats in normal and cancer cells. Nucleic Acids Res. 2008;36:770–784. doi: 10.1093/nar/gkm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 105.Mourelatos Z, Dostie J, Paushkin S, Sharma A, Charroux B, Abel L, Rappsilber J, Mann M, Dreyfuss G. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev. 2002;16:720–728. doi: 10.1101/gad.974702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Altuvia Y, Landgraf P, Lithwick G, Elefant N, Pfeffer S, Aravin A, Brownstein MJ, Tuschl T, Margalit H. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005;33:2697–2706. doi: 10.1093/nar/gki567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smalheiser NR, Torvik VI. Mammalian microRNAs derived from genomic repeats. Trends Genet TIG. 2005;21:322–326. doi: 10.1016/j.tig.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 108.Borchert GM, Holton NW, Williams JD, Hernan WL, Bishop IP, Dembosky JA, Elste JE, Gregoire NS, Kim JA, Koehler WW, et al. Comprehensive analysis of microRNA genomic loci identifies pervasive repetitive-element origins. Mob Genet Elem. 2011;1:8–17. doi: 10.4161/mge.1.1.15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dahary D, Shalgi R, Pilpel Y. CpG Islands as a putative source for animal miRNAs: evolutionary and functional implications. Mol Biol Evol. 2011;28:1545–1551. doi: 10.1093/molbev/msq315. [DOI] [PubMed] [Google Scholar]
- 110.Lehnert S, Van Loo P, Thilakarathne PJ, Marynen P, Verbeke G, Schuit FC. Evidence for co-evolution between human microRNAs and Alu-repeats. PLoS One. 2009;4:e4456. doi: 10.1371/journal.pone.0004456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Piriyapongsa J, Jordan IK. A family of human microRNA genes from miniature inverted-repeat transposable elements. PLoS One. 2007;2:e203. doi: 10.1371/journal.pone.0000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Piriyapongsa J, Marino-Ramirez L, Jordan IK. Origin and evolution of human microRNAs from transposable elements. Genetics. 2007;176:1323–1337. doi: 10.1534/genetics.107.072553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yuan Z, Sun X, Jiang D, Ding Y, Lu Z, Gong L, Liu H, Xie J. Origin and evolution of a placental-specific microRNA family in the human genome. BMC Evol Biol. 2010;10:346. doi: 10.1186/1471-2148-10-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roberts JT, Cooper EA, Favreau CJ, Howell JS, Lane LG, Mills JE, Newman DC, Perry TJ, Russell ME, Wallace BM, et al. Continuing analysis of microRNA origins: formation from transposable element insertions and noncoding RNA mutations. Mob Genet Elem. 2013;3:e27755. doi: 10.4161/mge.27755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ahn K, Gim JA, Ha HS, Han K, Kim HS. The novel MER transposon-derived miRNAs in human genome. Gene. 2013;512:422–428. doi: 10.1016/j.gene.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 116.Hoffman Y, Dahary D, Bublik DR, Oren M, Pilpel Y. The majority of endogenous microRNA targets within Alu elements avoid the microRNA machinery. Bioinformatics. 2013;29:894–902. doi: 10.1093/bioinformatics/btt044. [DOI] [PubMed] [Google Scholar]
- 117.Spengler RM, Oakley CK, Davidson BL. Functional microRNAs and target sites are created by lineage-specific transposition. Hum Mol Genet. 2014;23:1783–1793. doi: 10.1093/hmg/ddt569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 119.Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol. 2006;13:1097–1101. doi: 10.1038/nsmb1167. [DOI] [PubMed] [Google Scholar]
- 120.Zhang R, Wang YQ, Su B. Molecular evolution of a primate-specific microRNA family. Mol Biol Evol. 2008;25:1493–1502. doi: 10.1093/molbev/msn094. [DOI] [PubMed] [Google Scholar]
- 121.Bailey JA, Liu G, Eichler EE. An Alu transposition model for the origin and expansion of human segmental duplications. Am J Hum Genet. 2003;73:823–834. doi: 10.1086/378594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smalheiser NR. EST analyses predict the existence of a population of chimeric microRNA precursor-mRNA transcripts expressed in normal human and mouse tissues. Genome Biol. 2003;4:403. doi: 10.1186/gb-2003-4-7-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bortolin-Cavaille ML, Dance M, Weber M, Cavaille J. C19MC microRNAs are processed from introns of large Pol-II, non-protein-coding transcripts. Nucleic Acids Res. 2009;37:3464–3473. doi: 10.1093/nar/gkp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saito Y, Suzuki H, Tsugawa H, Nakagawa I, Matsuzaki J, Kanai Y, Hibi T. Chromatin remodeling at Alu repeats by epigenetic treatment activates silenced microRNA-512-5p with downregulation of Mcl-1 in human gastric cancer cells. Oncogene. 2009;28:2738–2744. doi: 10.1038/onc.2009.140. [DOI] [PubMed] [Google Scholar]
- 127.Olsen PH, Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev Biol. 1999;216:671–680. doi: 10.1006/dbio.1999.9523. [DOI] [PubMed] [Google Scholar]
- 128.Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci USA. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- 130.Daskalova E, Baev V, Rusinov V, Minkov I. 3′UTR-located ALU elements: donors of potential miRNA target sites and mediators of network miRNA-based regulatory interactions. Evolut Bioinform Online. 2006;2:103–120. [PMC free article] [PubMed] [Google Scholar]
- 131.Smalheiser NR, Torvik VI. Alu elements within human mRNAs are probable microRNA targets. Trends Genet TIG. 2006;22:532–536. doi: 10.1016/j.tig.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 132.Sinnett D, Richer C, Deragon JM, Labuda D. Alu RNA secondary structure consists of two independent 7 SL RNA-like folding units. J Biol Chem. 1991;266:8675–8678. [PubMed] [Google Scholar]
- 133.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ebersberger I, Metzler D, Schwarz C, Paabo S. Genomewide comparison of DNA sequences between humans and chimpanzees. Am J Hum Genet. 2002;70:1490–1497. doi: 10.1086/340787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen FC, Li WH. Genomic divergences between humans and other hominoids and the effective population size of the common ancestor of humans and chimpanzees. Am J Hum Genet. 2001;68:444–456. doi: 10.1086/318206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wildman DE, Uddin M, Liu G, Grossman LI, Goodman M. Implications of natural selection in shaping 99.4% nonsynonymous DNA identity between humans and chimpanzees: enlarging genus Homo. Proc Natl Acad Sci USA. 2003;100:7181–7188. doi: 10.1073/pnas.1232172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hedges DJ, Callinan PA, Cordaux R, Xing J, Barnes E, Batzer MA. Differential alu mobilization and polymorphism among the human and chimpanzee lineages. Genome Res. 2004;14:1068–1075. doi: 10.1101/gr.2530404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mills RE, Bennett EA, Iskow RC, Luttig CT, Tsui C, Pittard WS, Devine SE. Recently mobilized transposons in the human and chimpanzee genomes. Am J Hum Genet. 2006;78:671–679. doi: 10.1086/501028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Han K, Konkel MK, Xing J, Wang H, Lee J, Meyer TJ, Huang CT, Sandifer E, Hebert K, Barnes EW, et al. Mobile DNA in Old World monkeys: a glimpse through the rhesus macaque genome. Science. 2007;316:238–240. doi: 10.1126/science.1139462. [DOI] [PubMed] [Google Scholar]
- 140.Chimpanzee S, Analysis C. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
- 141.Rhesus Macaque Genome S. Analysis C. Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 142.Paz-Yaacov N, Levanon EY, Nevo E, Kinar Y, Harmelin A, Jacob-Hirsch J, Amariglio N, Eisenberg E, Rechavi G. Adenosine-to-inosine RNA editing shapes transcriptome diversity in primates. Proc Natl Acad Sci USA. 2010;107:12174–12179. doi: 10.1073/pnas.1006183107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gommans WM, Mullen SP, Maas S. RNA editing: a driving force for adaptive evolution? BioEssays News Rev Mol Cell Dev Biol. 2009;31:1137–1145. doi: 10.1002/bies.200900045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mattick JS. Deconstructing the dogma: a new view of the evolution and genetic programming of complex organisms. Ann N Y Acad Sci. 2009;1178:29–46. doi: 10.1111/j.1749-6632.2009.04991.x. [DOI] [PubMed] [Google Scholar]
- 145.Levanon EY, Eisenberg E. Does RNA editing compensate for Alu invasion of the primate genome? BioEssays. 2014;37:175–181. doi: 10.1002/bies.201400163. [DOI] [PubMed] [Google Scholar]
- 146.Greenberger S, Levanon EY, Paz-Yaacov N, Barzilai A, Safran M, Osenberg S, Amariglio N, Rechavi G, Eisenberg E. Consistent levels of A-to-I RNA editing across individuals in coding sequences and non-conserved Alu repeats. BMC Genom. 2010;11:608. doi: 10.1186/1471-2164-11-608. [DOI] [PMC free article] [PubMed] [Google Scholar]