Abstract
Arthropod venoms feature the presence of cytolytic peptides believed to act synergetically with neurotoxins to paralyze prey or deter aggressors. Many of them are linear, i.e., lack disulfide bonds. When isolated from the venom, or obtained by other means, these peptides exhibit common properties. They are cationic; being mostly disordered in aqueous solution, assume amphiphilic α-helical structure in contact with lipid membranes; and exhibit general cytotoxicity, including antifungal, antimicrobial, hemolytic, and anticancer activities. To suit the pharmacological needs, the activity spectrum of these peptides should be modified by rational engineering. As an example, we provide a detailed review on latarcins (Ltc), linear cytolytic peptides from Lachesana tarabaevi spider venom. Diverse experimental and computational techniques were used to investigate the spatial structure of Ltc in membrane-mimicking environments and their effects on model lipid bilayers. The antibacterial activity of Ltc was studied against a panel of Gram-negative and Gram-positive bacteria. In addition, the action of Ltc on erythrocytes and cancer cells was investigated in detail with confocal laser scanning microscopy. In the present review, we give a critical account of the progress in the research of Ltc. We explore the relationship between Ltc structure and their biological activity and derive molecular characteristics, which can be used for optimization of other linear peptides. Current applications of Ltc and prospective use of similar membrane-active peptides are outlined.
Keywords: Biologically active compounds, Mechanism of action, Cytolytic toxin, Correlation analysis, Antimicrobial peptide, Structure–function relationship
Introduction
Organisms that utilize venom for predation, defense, and competitor deterrence are believed to possess evolutionary advantages [1–3]. Among the venomous taxa (including cnidarians, echinoderms, mollusks, vertebrates, and arthropods, encompassing ants, bees, centipedes, scorpions, spiders, and wasps), spiders are arguably the most successful representing one of the most abundant terrestrial predators. Spider genomes are similar in size to mammalian genomes and also feature a large amount of short exons and long introns [4]. Among the sources of the evolutionary advantage of spiders are the properties of their venom [5–7]: (1) they are fast acting; (2) minute dosages are enough to paralyze the victim or deter aggressors; (3) gene-encoded peptide neurotoxins are produced in the form of combinatorial libraries featuring hypermutations of essentially all residues, except for a few strictly conserved cysteines that stabilize the three-dimensional fold of the toxin; (4) there is functional synergy between the venom components. A combination of toxins, or toxin cabal (term coined by the Olivera laboratory to describe Conus venom [8]), acts synergistically, enhancing the venom potency.
Spider venoms contain [6, 7, 9–11] low molecular weight compounds (molecular mass below 1 kDa: salts, carbohydrates, amino acids, biogenic amines, nucleotides, etc.) and versatile proteins and peptides including enzymes (such as proteases, hyaluronidases, and phospholipases), neurotoxins (often disulfide-rich peptides affecting ion channels), and cytolytic peptides [12]. Pharmacological potential of spider venom is primarily associated with the disulfide-rich peptide neurotoxins. They represent a rich source of highly specific ligands for a great variety of calcium, potassium, and sodium channel subtypes of both insects and mammals [13–16]. Disulfide bridges provide extraordinary stability to the toxin molecules, granting a variety of delivery options for their therapeutic applications. These spider toxins are used as hits and leads for the development of therapeutics against a number of pathological conditions, such as cardiovascular disorders, chronic pain, and erectile dysfunction [17]. Also, they are considered as potential bioinsecticides and may be used for the development of transgenic plants encoding these peptides or baculoviruses [7, 18, 19]. Spider venom peptides lacking disulfide bonds (called linear peptides, LP in this review) exhibit, among others, antimicrobial and antifungal activity [6, 10, 12]. They are called many names, among them pore formers, linear cytolytic peptides, membrane-active antimicrobial peptides, or simply antimicrobial peptides (AMP). We consider them further in more detail.
Cytolytic LP from spider venom (Table 1) usually contain 20–35 amino acid residues with a high abundance of cationic residues (Lys and Arg) and are capable of interacting with lipid membranes, affecting their permeability [20]. Such peptides constitute a major fraction of the venoms of bees, bumblebees, wasps, and ants [12, 21–26]. Only several dozen LP have been described from spider venom compared to several hundred disulfide-containing neurotoxins [27]. Venoms of few well-studied spider representatives contain arrays of LP. A good example is short LP isolated from Lachesana tarabaevi spider venom that were called latarcins (Ltc) [28], which we discuss in detail here. They display membrane activity and cytotoxic effects against bacteria [28] and mammalian cells [29, 30]. Studies of their interaction with model lipid membranes [31–34] or with whole cells [29, 30] are a well-established methodology for characterization of membrane-active properties in LP. Similarly the venom of Cupiennius salei features the presence of numerous cupiennins [35], and Oxyopes takobius produces a number of oxyopinins [36, 37] (Table 1).
Table 1.
Cytolytic cysteine-free spider venom peptides
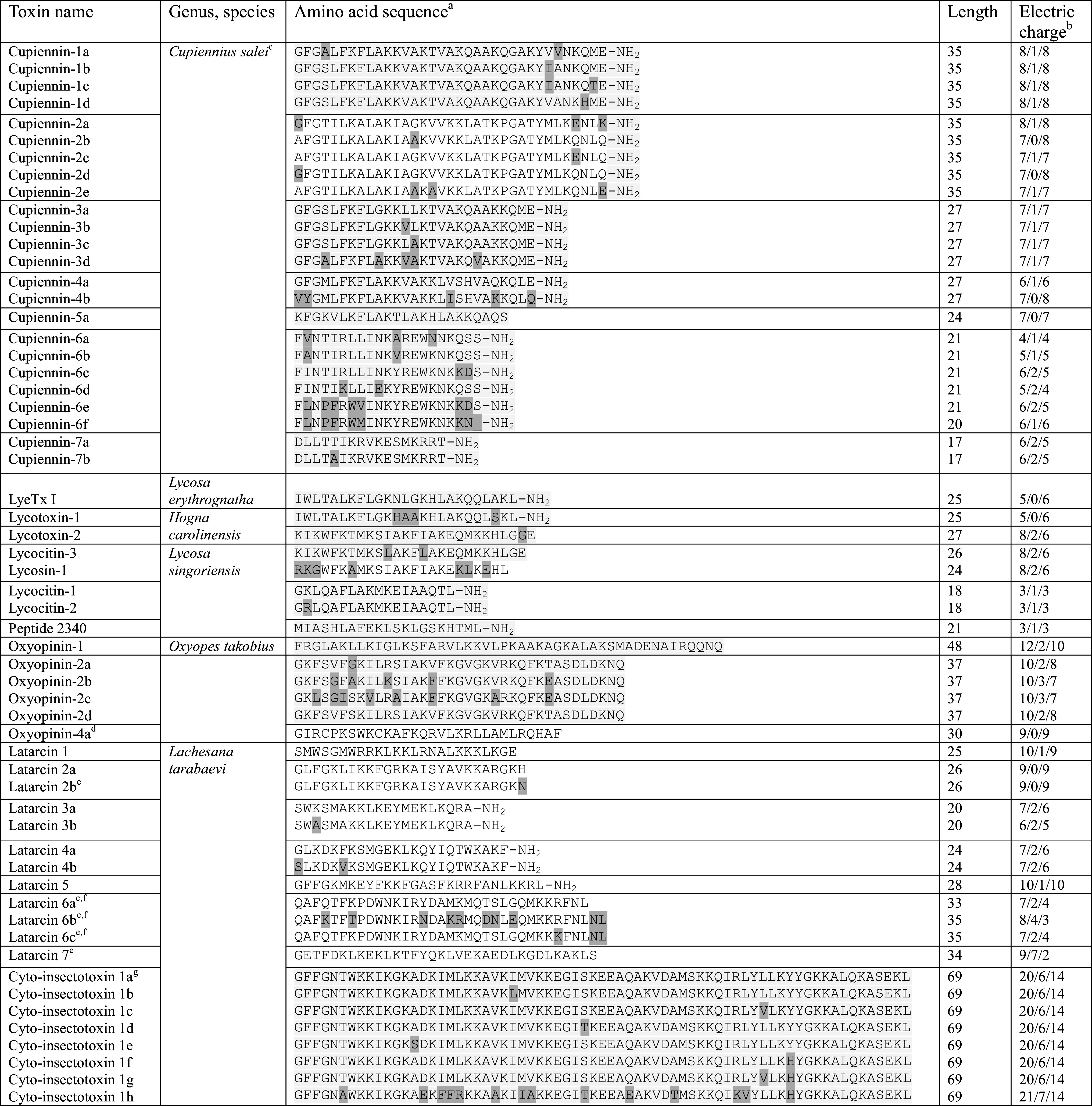
aThe amino acid sequences were retrieved from ArachnoServer (www.arachnoserver.org) and UniProt (www.uniprot.org) databases (both assessed on 17.06.2015). Amidated C-terminus is marked by adding "–NH2" to the sequence; differences in groups of highly similar peptides are highlighted by shading
bNumber of positively charged residues/negatively charged residues/total charge (at pH 7.0)
cThe names of cupiennins are given according to the work [189]
dThis peptide contains a pair of Cys residues and is included in the Table for reference only
eThese peptides were not found in the venom. They were identified by the analysis of the venom gland EST (expressed sequence tags)
fThe N-terminal residue is modified post-translationally to pyroglutamate
gThere are additional cyto-insectotoxins identified by the analysis of the venom gland EST; see details in [44]
What is the function of cytolytic LP in spider venom? Interestingly, their cationicity and capability to form amphiphilic α-helices upon interaction with phospholipid membranes make them similar to a number of host defense peptides, such as those produced by insect and arachnid hemocytes in response to microbial invasion [12]. It may be that the function of cytolytic LP from venom is protection of the venom glands from infection or prey sterilization. However, an offensive toxic function seems more likely. Synergy between major constituents of spider venom was proposed [38]. When injected into prey, the enzyme hyaluronidase may act as a spreading factor by destroying the extracellular matrix and thereby facilitating a better access of venom neurotoxins to their targets. By destroying cells, cytolytic LP may play a similar role. Moreover, they disturb cell membranes and affect cell excitability, which may augment the effect of neurotoxins. Positive cooperativity with neurotoxins has been suggested for cupiennins [39] and oxyopinins [36].
Synergism in action between cytolytic LP and neurotoxins seems to be secured also at the structural level. Some spider toxins contain a linear fragment attached either to the N-terminus (as in spiderines from O. takobius and Oxyopes lineatus [40, 41]), or the C-terminus of a disulfide-rich neurotoxin-like domain (as in CsTx-1 from Cupiennius salei [42] and latartoxins from L. tarabaevi [43]). Such molecules display the properties of both toxin groups. Cyto-insectotoxins are another type of linear peptides exhibiting two-domain organization [44]. They contain a pair of linear fragments connected by a short linker, which is reminiscent of the PQM motif (see below). Oxyopinin-4a (Table 1) isolated from the venom of O. takobius is a modular peptide comprising a linear part and a moiety constrained with a single disulfide bond [37].
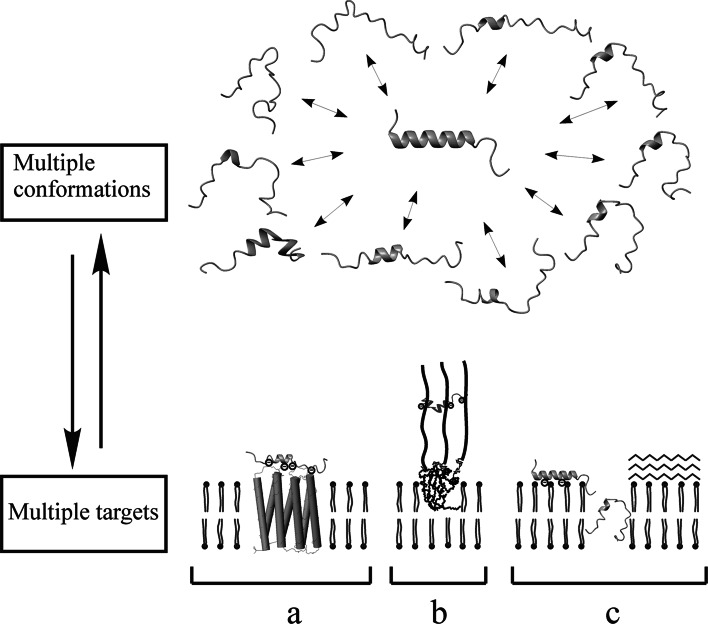
Cytolytic LP isolated from spider and scorpion venom were shown to exhibit multiple activities. Some LP are bradykinin-potentiating, some possess potent insecticidal activity, and many others are cytotoxic to mammalian cells, fungi, bacteria, trypanosomes, plasmodia, or human cancer cells [6, 20, 28, 39, 44–49]. This is probably because LP are rather flexible and can interact with a plethora of biological molecules, ranging from lipids and carbohydrates to proteins and nucleic acids (Fig. 1). Of note, membranolytic activity was reported for cytolytic LP. In addition, cupiennin 1a was shown to inhibit nitric oxide formation by neuronal nitric oxide synthase through complexation with the regulatory protein calmodulin [50].
Fig. 1.
Multiconformational and multitarget features of cytolytic LP. (Upper panel) multiconformational equilibrium of Ltc 1 exploited via Monte Carlo (MC) search in aqueous solution, starting from the structure of this peptide determined in detergent micelles (PDB code 2PCO, in the center). (Lower panel) possible targets of polycationic LP in a living cell membrane. From left to right: a membrane proteins possessing clusters of negative charge on their surface; b peripheral or transmembrane sialylated glycoproteins or glycolipids; c lipid bilayer itself. In the latter state, an equilibrium between surface-attached monomer (left) and membrane-penetrated form of the peptide molecule (center), giving rise to nonspecific pore formation, and olygomeric β-sheet aggregates on the surface of the membrane (right) are shown. The conformations of the peptides in the membrane-bound states are hypothetical. Negative charges on the membrane constituents are indicated with the encircled “−” sign. All peptide molecules are shown in a ribbon representation. The program MOLMOL [187] was used to prepare the drawing
Polycationic AMP are widely found not only in venoms, but also as part of the immune system in plants [51–54] and animals [55–57], where they often represent the first line of defense against invading microorganisms. Moreover, AMP are produced by bacteria as a tool for competition [58, 59]. AMP are becoming increasingly popular as lead compounds for antibacterial and anticancer therapy [60–66]. The antimicrobial properties of AMP were studied for a long time, and a number of them are in the process of clinical testing [67, 68]. To suit the pharmacological needs, it is necessary to understand the mechanism of AMP activity and establish the structure–activity relationships of peptides from natural sources, such as venoms. The goal of the present review is to summarize data on the structural properties of Ltc and their analogs in membrane-mimetic environments on the one hand, and mechanisms of their antibacterial, hemolytic, and anticancer activities on the other hand. Molecular characteristics related to these activities are described and approaches to rational engineering of Ltc are outlined. Pro and contra of the experiments with model lipid membranes and whole cells are discussed.
Origin of latarcins
The venom of L. tarabaevi spider (family Zodariidae or “ant” spiders) comprising at least 100 peptides was established to be a unique natural source of LP with uncommon structure (see Fig. 2 for venom separation). These peptides are subdivided into two groups, the molecular masses of which fall into the range of 2–5 and 7–9 kDa [28]. Separation of the former group resulted in identification of as many as 12 LP named latarcins (Table 1). Their length varies from 20 (Ltc 3a/3b) to 35 (Ltc 6b/6c) residues.
Fig. 2.
Separation of L. tarabaevi venom by HPLC. Crude venom (1 mg) was loaded on a Jupiter C5 column (2 × 150 mm, 300 Å, 5 μm, Phenomenex, USA) at a flow rate of 0.3 mL/min. A 30 min linear gradient of acetonitrile (0–60 %) in 0.1 % aqueous trifluoroacetic acid was applied. Eluent absorbance was monitored at 210 nm. Fractions containing Ltc are labeled. Adapted from [28]
The amount of Ltc in the crude venom was estimated to be several percent of total protein content. Nevertheless, these peptides are assumed to play a significant role in the overall effect of the venom. Analysis of the expressed sequence tags from the venom glands led to the identification of not only the mature peptide sequences, but also the complete precursor protein sequences (82–207 residues, see below). The total charge of the peptides varies from +2 to +10. Of note, besides positively charged amino acid residues (Lys, Arg), several Ltc contain also a number of negatively charged residues (Glu, Asp). The amino acid sequences of Ltc 6b and Ltc 7 feature the highest content of acidic residues (Table 1).
Several Ltc are homologous and highly similar peptides. Ltc 3a and 3b differ from each other by only one residue at position 3, and another pair, Ltc 4a and 4b, differs in two positions, 1 and 6. The similarity between other Ltc is low (see the next section).
What is known about biological activity of Ltc? It has been proposed that LP in spider venom facilitate the action of disulfide-rich neurotoxins by providing access to neurons [12, 28]. This likely arises due to the membrane activity of Ltc. Being mostly disordered in aqueous solution, these peptides become α-helical in membrane-mimetic environments [28]. They affect the permeability of planar bilayer membranes composed of either purely zwitterionic, or with admixture of anionic phospholipids. Most Ltc induce membrane breakdown at micromolar concentrations and negative transmembrane potentials [28, 33]. At the same time, several Ltc (1 and 7) do not disrupt planar membranes, but cause some conductance changes. This was attributed to specific peculiarities in the hydrophobic/hydrophilic properties of the helices formed by Ltc in lipid membranes [31, 32, 69].
Due to the absence of disulfide bonds, Ltc are flexible peptides probably capable of interacting with a wide range of biomolecules. Their cytotoxic activity against animal cells is of practical value and was broadly investigated (see below). While Ltc display poor insecticidal properties, antimicrobial activity of several Ltc against Gram-positive and Gram-negative bacteria falls into the low micromolar concentration range [28]. Erythrocytes and cancer cells, such as erythroleukemia K562, were found to be affected by Ltc [29, 30]. In all these cases, membrane-active properties of Ltc were supposed to underlie cytotoxicity.
Biosynthesis
Importantly, latarcins were the first group of LP from spider venom, for which the structures of corresponding precursor proteins were established [28] (Fig. 3). To date, the only other spider venom LP with a known precursor structure is Oxyopinin-4a from O. takobius [37]. Hence, latarcins are the primary spider venom LP, for which a putative biosynthetic pathway may be considered. All latarcins are excreted by the venom gland, and we find typical signal (pre-) peptides in the precursors. These signal peptides drive co-translational sorting of latarcin precursors into the ER, an entry to the secretory pathway, and are removed by the signal peptidase. In addition, all precursors contain one or several prosequence elements, which are cleaved off post-translationally by specific peptidases in the process known as limited proteolysis. In spider toxins, precursor processing is thought to occur at two types of specific sites, PQM (processing quadruplet motif: AAAR, where at least one A is Glu, and R = Arg) and iPQM (inverted PQM: RAAA) [70, 71]. However, the proteases involved in this process are still unknown. It is interesting to note in this regard the unequivocal reports of proteolytic activity in spider venom, which may actually relate to the toxin maturation process (for review, see [6]).
Fig. 3.
Schematic representation of latarcin precursors. Leader peptides, acidic prosequences, RPE, and latarcins are shown in different shades of gray. Propeptide processing motifs (PQM and iPQM, see text for details) are represented as triangles
Based on the number of released mature peptides, latarcin precursors may be split into three categories.
Simple precursors give rise to single mature peptides. They are conventional prepropeptides, with prosequences preceding the mature peptides and demarcated by PQM. A majority of latarcin precursors (together with the Oxyopinin-4a precursor) and most known spider neurotoxin precursors belong to this category.
Binary precursors are processed into two mature latarcins (Ltc 6a and Ltc 6b, or Ltc 6c). Each of these proteins contains two PQM.
Processing of complex precursors results in release of two types of peptides: latarcins (Ltc 4a or Ltc 4b) and so-called repetitive polypeptide elements (RPE). In each case, latarcins are the C-terminal parts of precursors. Four or five RPE are released due to cleavage at PQM and iPQM that define the N- and C-termini of the mature peptides.
Additional posttranslational modifications of latarcins include:
formation of pyroglutamic residues from N-terminal glutamines in Ltc 6a/6b/6c;
cleavage of the C-terminal lysine residue in Ltc 1;
C-terminal amidation in Ltc 3a, 3b, 4a, 4b, and 5, which is due to the conversion of the C-terminal glycine residues.
All these modifications are widespread among secreted molecules in animals, including AMP. Their functional relevance is debated, but most often assumed to ensure protection of the peptide termini from proteolysis. Synthetic peptides corresponding to immature latarcins 1 and 3b were produced and their activities compared to the mature LP [33]. Ltc 1-K containing a C-terminal lysine residue did not differ from Ltc 1. However, Ltc 3b-G containing a C-terminal glycine residue but no amidation showed significantly lower antimicrobial activity compared to Ltc 3b pointing to the functional importance of the modification.
This biosynthetic pathway may be common among spiders. Our data on Oxyopinin-4a and unpublished data on other cytolytic peptides support this assumption.
Similarity to other membrane-active peptides
Ltc present a number of structural features that are most common among membrane-active peptides, such as high net positive charge, amphiphilicity, and helix-forming propensity [28]. Nonetheless, the similarity of their primary structures to other known peptides is low (Table 2). We note two major types of sequence similarity between Ltc and other AMP. (1) Regular disposition of hydrophobic vs. charged residues along the sequences, such as in Ltc 1 and tammar wallaby cathelicidin [72], Ltc 2a/2b and artificial peptide KALA [73], Ltc 3a/3b and dahlein from frog skin [74], Ltc 5 and snake cathelicidins [75], Ltc 7 and frog skin brevinins [76]. (2) Common motifs are found in the N-terminal parts of peptides, whereas the C-termini are quite dissimilar. For example, this is the case for Ltc 4a/4b and toad alyteserins [77]. Of note, unusual peptides Ltc 6a/6b/6c featuring the presence of multiple Lys/Arg residues, interspersed with counter-charged Asp/Glu, exhibit similarity at both the N- and C-termini to a human neuropeptide [78].
Table 2.
Latarcins and similar peptides
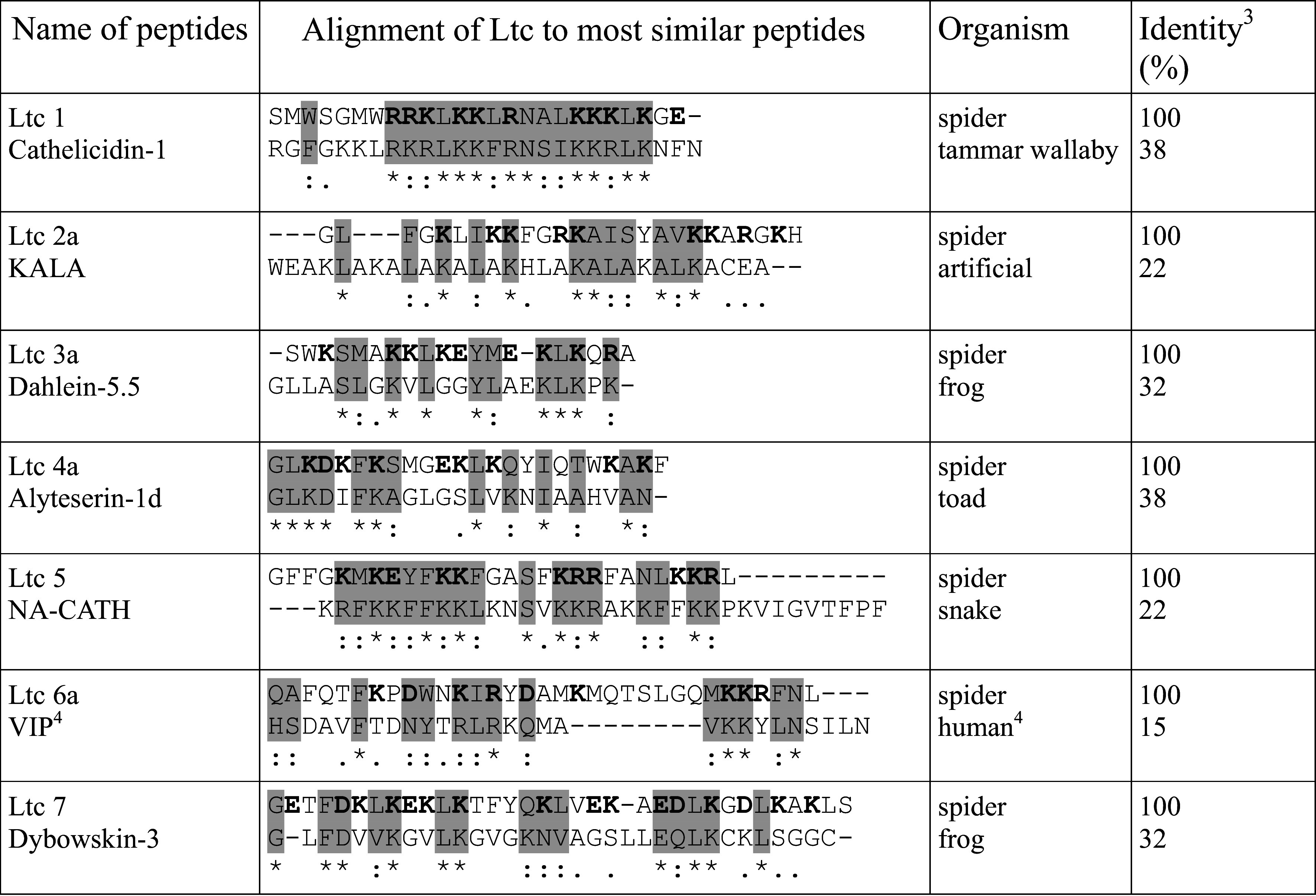
Charged residues in Ltc are marked bold
aAlignment was performed with the program ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/); identical (fully conserved) amino acids are marked with the “*” sign, “:” (colon sign) indicates conservation between groups of strongly similar properties, “.” (period) indicates conservation between groups of weakly similar properties, and regions with the highest similarity are shaded
bCalculated with the web server at http://www.uniprot.org/align/
cVasoactive intestinal polypeptide (neuropeptide)
There are two primary reasons for the similarity. One reason is a common molecular target. Similar sequence motifs (for instance, alterations of doublets of charged or hydrophobic residues) yield similar spatial organization and hence chemical properties and biological activity. The other reason is biosynthesis. We assume that the similarity in the frontmost N-terminal regions between otherwise unrelated peptides is related to the biosynthetic pathway. The latter implies production in the form of precursor, cleaved by specific proteases. The effectiveness of proteolysis at a specific site is usually affected by the neighboring residues [79], which are therefore selected to ensure the processing.
Structure in membrane-mimetic environments
Peptides featuring a high proportion of either negatively [80] or positively charged amino acid residues in their sequences [31, 32] are usually highly water-soluble and disordered in aqueous solution. According to CD spectroscopy [28] and 1H-NMR data [31, 32], this is the case for Ltc 1, 2a, 4a/4b, 5, 6a, and 7. Unlike these, Ltc 3a/3b exhibit an elevated α-helical content (Table 3), which could be due to helix-stabilizing interactions, such as N- or C-caps, and salt bridges favored by the amino acid sequence of the peptides [81].
Table 3.
Physico-chemical properties of latarcins
| Toxin namea | Abbreviation | Database identifierb | Lengthc | Helical content in various environments (%)d | ΔGif, (kcal/mol)h | Hydrophobicity [μM−1] (in the presence of electrical potential)k | PDB code | ||
|---|---|---|---|---|---|---|---|---|---|
| Watere | Water/TFEf | SDSg | |||||||
| Latarcin 1 | Ltc 1 | Q1ELT9 | 88/25 | 7 | 49 | 45/62i | 0.68 (−1.35j) | 2 | 2PCOl |
| Latarcin 2a | Ltc 2a | Q1ELU1 | 84/26 | 0 | 64 | 40/62i | −1.61 (−2.4j) | 0.5 | 2G9Pm |
| Latarcin 2b | Ltc 2b | Q1ELU0 | 84/26 | n.d.n | n.d.n | n.d.n | −2.15o | – | |
| Latarcin 3a | Ltc 3a | Q1ELU3 | 82/20 | 18 | 56 | n.d.n | 2.85 (−1.21j) | 0.063 | – |
| Latarcin 3b | Ltc 3b | Q1ELU2 | 82/20 | 28 | 56 | n.d.n | 2.03 (−2.03j) | 0.026p | – |
| Latarcin 4a | Ltc 4a | Q1ELU5 | 207/24 | 4 | 74 | n.d.n | −2.28 (−4.31j) | 0.143 | – |
| Latarcin 4b | Ltc 4b | Q1ELU4 | 179/24 | 4 | 74 | n.d.n | −0.96 (−2.99j) | 0.2 | – |
| Latarcin 5 | Ltc 5 | Q1ELU9 | 93/28 | 0 | 44 | n.d.n | −1.97 (−4.0j) | 2.5 | – |
| Latarcin 6a | Ltc 6a | Q1ELU7 | 116/33 | 11 | 54 | n.d.n | −2.3 (−4.9j) | 0.059 | – |
| Latarcin 6b | Ltc 6b | Q1ELU8 | 116/35 | n.d.n | n.d.n | n.d.n | – | – | – |
| Latarcin 6c | Ltc 6c | Q1ELU7 | 116/35 | n.d.n | n.d.n | n.d.n | – | – | – |
| Latarcin 7 | Ltc 7 | Q1ELV0 | 97/34 | 10 | 66 | n.d.n | 6.29 (−5.73j) | 0.1q | – |
aThe amino acid sequences of the peptides are given in Table 1
bUniProtKB/Swiss-Prot
cNumber of residues in precursor/mature part
dHelical content in peptides estimated by CD spectroscopy using ellipticity at 220 nm and theoretical value for ellipticity of 100 % helical peptide of a given length (as in [80])
eExperimental value obtained from the ellipticity value at 220 nm in the CD spectra reported in the work [28], peptide concentration 0.16 mM, pH 7.5
fExperimental value in water/TFE (50 %, v/v), obtained in the work [28]
gObtained by dividing the number of residues in the α-helix to the total number of residues/or by averaging upfield shifts of α-protons (for details see Ref. [190])
hObtained with the MPEx program [133] utilizing interfacial scale for binding of the peptides with free N- and C-termini and helical content as in water/TFE mixture
iHelical content obtained as the number of “helical” residues in the NMR-derived structure of the peptide, divided by the total number of residues
jValues are obtained under the condition of protonation of Asp, Glu, and His residues
kHydrophobicity determined as a value reciprocal to the concentration that is necessary to disrupt planar membranes of DPhPC under negative voltages (−80/−100 mV), as reported in [28]
lPeptide with an extra lysine residue at the C-terminus (Ltc 1-K) was solubilized in micelles of deuterated SDS and investigated with 1H-NMR spectroscopy; see details in [32]
mIn SDS micelles; see details in [31]
nn.d., not determined
oThe same helical content was assumed as for Ltc 2a
pThe experimental condition for concentration >19 μM was converted to a precise value of C = 38 μM
qConductance changes without membrane rupture were reported to occur at C = 7 μM, and rupture was assumed to occur at C ~10 μM
Addition of trifluoroethanol (TFE) to an aqueous solution of any Ltc increases their α-helical content considerably (Table 3). α-Helicity is also elevated in detergent micelles, which are known to be a better mimic of lipid environment than isotropic solvents [80, 82]. In SDS micelles, the molecule of Ltc 2a is composed of a pair of helices (residues 2–10, 13–22) separated by Gly11 (Fig. 4a, left). The spatial structure of Ltc 1 peptide represents a disordered N-terminal fragment, a prominent amphiphilic α-helix (residues 8–23) and a short hydrophilic C-terminal region (Fig. 4a, right). The maps of hydrophobic–hydrophilic properties of α-helical regions of Ltc 2a and Ltc 1, calculated using the molecular hydrophobicity potential (MHP) approach [83, 84], are shown in Fig. 4b. Their inspection reveals that the N-terminal helix of Ltc 2a is clearly amphiphilic. In the C-terminal helix, no separation between polar and apolar residues is seen. Of note, the total hydrophobic surface in the N-terminal helix exceeds that in the C-terminus. A similar hydrophobicity gradient, also known as “oblique-oriented pattern”, was found in the so-called “tilted” peptides, which dip into the membrane interior [85, 86]. In contrast, Ltc 1 helix features a narrow hydrophobic pattern, non-interrupted by any helix-breaking residues. Helices of this type tend to orient parallel to the membrane plane (Fig. 4a, right). This distinguishes them from the “tilted-like” orientation of the N-terminal helix in Ltc 2a (Fig. 4a, left).
Fig. 4.
Structural properties of Ltc 1 and 2a and their membrane-perturbing effects. a Structure of Ltc 2a (left) and Ltc 1 (right) based on NMR data in SDS micelles and restrained MC simulations in implicit membrane and their membrane-binding mode. The “best” structure from the ensemble of 20 calculated (PDB codes of 2G9P, 2PCO) structures is shown. The peptides are depicted in a ribbon representation. The side chains of charged, neutral hydrophilic and interfacially localized aromatic, and hydrophobic residues are shown by blue, cyan, and red sticks, respectively. Implicit membrane is hatched gray. The N- and C-termini of the peptides are marked. (b) 2D isopotential map of MHP on the surface of the Ltc 2a (left) and Ltc 1 (right) helices. The value on the X axis is the rotation angle about the helix axis; the parameter on the Y axis is the distance along the helix axis. MHP is given in octanol–water logP units. Only the hydrophobic areas with MHP >0.09 are shown. Contour intervals are 0.015. The positions of residues are indicated by letters and numbers. The maps were prepared using the PLATINUM web server [156]. c Release of a fluorescent marker (CBF) from DOPE/DOPG (7:3) liposomes (diameter of ~100 nm) as a function of Ltc 2a (dotted line) and Ltc 1 (solid line) concentration. d 31P NMR spectra of DOPE/DOPG (7:3) liposomes in the absence (upper spectrum) or presence of Ltc 2a at an L/P ratio of 18:1 (dotted line) and Ltc 1 at an L/P ratio of 20:1 (solid line). The computer simulated spectrum, drawn in smooth solid line, is obtained using the P-FIT program [188] and superimposed on the spectrum of the liposomes in the absence of the peptides
In the presence of phospholipid vesicles, the structure of Ltc was shown to depend strongly on the lipid composition [34], in agreement with studies of other AMP [87]. Ltc 2a, Ltc 1-K, and Ltc 3b-G (see explanations above) were investigated in a suspension of liposomes composed of either zwitterionic dioleoylphosphatidylcholine (DOPC), anionic dioleoylphosphatidylglycerol (DOPG), or 7:3 (mol/mol) mixture of dioleoylphosphatidylethanolamine (DOPE) and DOPG. These Ltc displayed an α-helical structure in DOPC and DOPG vesicles. They induced leakage of carboxyfluorescein (CBF) from DOPG liposomes at lower peptide/lipid (P/L) ratios than from DOPC liposomes [33]. Apparently, this is caused by an electrostatic attraction between positively charged peptides and anionic liposomes [88]. Ltc 2a and Ltc 3b-G peptides are mainly α-helical in DOPE/DOPG liposomes (1:1, or 7:3 mol/mol). In contrast, Ltc 1-K features a combination of β-sheet and unordered structures [34]. This peptide does not induce CBF leakage from liposomes (Fig. 4c). It seems therefore that some peptides interact specifically with PE, remaining at the bilayer surface. This is in agreement with the effect of Ltc 2a and Ltc 1-K peptides on DOPE/DOPG liposomes as studied by 31P-NMR spectroscopy [32]. The 31P NMR spectrum of these liposomes exhibits a typical bilayer line shape either in the absence or presence of Ltc 1-K (Fig. 4d). Thus, the peptide binds electrostatically to the surface of the lipid bilayer. The 31P NMR spectrum of the liposomes in the presence of Ltc 2a, however, exhibits a broad isotropic signal (Fig. 4d), suggesting that the phospholipid bilayer is deteriorated by the bound peptide due to its hydrophobic partitioning.
Analysis of the amino acid sequences of other Ltc was performed with respect to their conformational preferences [32]. Ltc 3a/3b were suggested to contain putative rigid helical fragments of ~13 residues in length. Ltc 4a/4b and 5 show structural similarity to Ltc 2a, and thus a potential to form a helix-hinge-helix motif in membranes. The peptides of these two families were supposed to perturb membranes via either pore formation (Ltc 1 and 3a/3b) or the detergent-like “carpet” mechanism (Ltc 2a, 4a/4b and 5) [32]. The latter mechanism implies that peptide molecules get absorbed and form a “carpet” on the surface of target membranes, which spontaneously break down after the peptide concentration reaches a threshold [89].
The behavior of Ltc 1 and Ltc 2a peptides in phosphatidylethanolamine/phosphatidylglycerol (PE/PG) phospholipid bilayer was also assessed via an atomistic MD simulation [69]. In contrast to Ltc 1, insertion of Ltc 2a in the membrane induces significant changes in the dynamic behavior of lipids in the contact region. However, the “membrane response” has a local character and is caused by formation of specific peptide–lipid contacts. No penetration of Ltc 2a in the membrane core was observed, contrary to the 31P-NMR data (Fig. 4d, lower part, dotted spectrum). This discrepancy is likely due to the fact that the membrane destabilization is induced not by a single peptide molecule, but several of them, covering the membrane surface like a “carpet” [31].
In conclusion, we note that Ltc display conformational plasticity, which is dependent on the environment and governed by several factors. For lipid membranes, the crucial factor is their composition [90]. For instance, the presence of PE is known to strongly influence the peptide behavior [91, 92].
Biological effects
Antibacterial activity
That the bacterial plasma membrane is a primary target of AMP became a dogma [93–100]. However, a necessity to switch from the studies of AMP in model membrane systems to their investigations on cells is becoming clear [101, 102]. In cells, AMP interact first with an external barrier, the outer membrane of Gram-negative microorganisms, and peptidoglycan (PTG) layer of Gram-positive bacteria [103]. The size of the envelope exceeds considerably the width of the plasma membrane [104–107].
To determine the relationship between the molecular parameters of AMP and antimicrobial activity, regression and correlation analysis can be employed, which we had used previously to study the molecular determinants of antibacterial activity of cardiotoxins (or cytotoxins, CT) [108]. The latter are polycationic disulfide-rich membrane-active proteins from snake venom, carrying a positive charge, similar to that of Ltc [109, 110]. Due to structural rigidity of CT and availability of extensive data on their interaction with model phospholipid membranes, it became possible to specify molecular factors important either for destabilization of the plasma membrane or penetration through the outer envelope of bacteria [108]. We analyze in this way the respective data for Ltc.
The minimal inhibitory concentrations (MIC) of Ltc against a range of bacteria and the respective activities [where A = (MIC)−1] are listed in Table 4. The correlation (Pearson) coefficients between the activity of the peptides and their molecular properties are summarized in Table 5. The coefficients of correlation in the rows of activity/net charge for Ltc are nearly close to unity for Gram-negative bacteria and Gram-positive Bacillus subtilis. Most probably, positive charge is required both for passing through the outer membrane of Gram-negative bacteria and for interaction with their plasma membranes. The latter are known to contain a fraction of anionic phospholipids [87, 111]. We obtain slightly lower correlation coefficient values when only the number of positively charged residues in the peptides is taken into account. It seems that negatively charged residues do not favor the antibacterial effect. Indeed, the respective correlation coefficients are negative (Table 5). It is interesting to note that similar conclusions were drawn for CT [108, 112] and linear AMP of different origin [113, 114]. Of note, for LP an optimal charge, required to reach the maximal antimicrobial effect, depends on the overall length of the peptide [115, 116] and distribution of charged residues along the amino acid sequence [37].
Table 4.
Antibacterial/hemolytic properties of latarcins
| Latarcins | Gram-negative bacteria | Gram-positive bacteria | Hemolysis (rabbit erythrocytes, 20 % lysis) | |||
|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | A. globiformis | B. subtilis | |||
| DH5α | MH1 | |||||
| Ltc 1 | 1.0/1.0a | 0.7/1.43 | 4.1/0.244 | 0.5/2 | 1.0/1.0 | 80/0.013 |
| Ltc 2a | 0.5/2.0 | 0.7/1.43 | 6.7/0.149 | 0.7/1.43 | 0.4/2.5 | 6.0/0.167 |
| Ltc 3a | 2.5/0.4 | 6.0/0.17 | >40/0.013b | 0.3/3.33 | 1.2/0.83 | >120/0.004c |
| Ltc 3b | 23/0.04 | 28/0.04 | >45/0.011d | 0.7/1.43 | 2.9/0.35 | >120/0.004c |
| Ltc 4a | 4.5/0.22 | 3.2/0.32 | >35/0.014e | 0.3/3.33 | 1.1/0.91 | >120/0.004c |
| Ltc 4b | 4.4/0.23 | 4.4/0.23 | >35/0.014e | 0.3/1.33 | 1.1/0.91 | >120/0.004c |
| Ltc 5 | 0.6/1.67 | 0.6/1.67 | 18/0.056 | 1.1/0.91 | 0.6/1.67 | 40/0.025 |
| Ltc 6a | >70/0.01f | >70/0.01f | >70/0.01f | >70/0.01f | >70/0.01f | >120/0.004c |
| Ltc 7 | >70/0.01f | >70/0.01f | >70/0.01f | >70/0.01f | >70/0.01f | >120/0.004c |
The MIC values (presented in μM) are from [28]
aThe values in the Table are MIC/A, where A = (MIC)−1 [μM−1]
bTo obtain a definite value for activity, the MIC was assumed to be 80 μM, which satisfies the condition of >40 μM
cA value of C = 240 μM, satisfying the condition of >120 μM, was used
dA value of C = 90 μM, satisfying the condition of >45 μM, was used
eA value of C = 70 μM, satisfying the condition of >35 μM, was used
fA value of C = 140 μM, satisfying the ncondition of >70 μM, was used
Table 5.
Correlation coefficients between antibacterial or hemolytic activity and charge or hydrophobicity for latarcins
| Property | Gram-negative bacteria | Gram-positive bacteria | Hemolysis (rabbit erythrocytes, 20 % lysis) | |||
|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | A. globiformis | B. subtilis | |||
| DH5α | MH1 | |||||
| Full charge at pH7a | 0.92 | 0.94 | 0.77 | 0.05 | 0.85 | 0.64 |
| Positive chargeb | 0.80 | 0.85 | 0.72 | −0.34 | 0.59 | 0.53 |
| Negative chargec | −0.61 | −0.59 | −0.47 | −0.35 | −0.68 | −0.45 |
| Hydrophobicity (1)d | −0.39 | −0.40 | −0.15 | −0.19 | −0.48 | −0.28 |
| Hydrophobicity (2)e | 0.28 | 0.28 | 0.49 | 0.55 | 0.38 | 0.19 |
| Hydrophobicity (3)f | 0.67 | 0.86 | 0.62 | −0.14 | 0.45 | 0.08 |
Pearson coefficient (±0.01), or correlation in the row activity/charge (calculated, as in [108])
The following LP were taken into account: Ltc 1, 2a, 3a, 3b, 4a, 4b, 5, 6a, and 7, nine molecules in total
Antibacterial activity is assumed to be a value reciprocal to MIC, and the values of MIC were taken from [28]
aFull charge at pH 7 (as reported in Table 1)
bThe number of positively charged residues (Arg and Lys, reported in Table 1)
cThe result is obtained taking into account only the number of negatively charged residues
dIn the ΔG scale (see details in Table 3)
eIn the ΔG scale (the values in the brackets correspond to the conditions of protonation of Asp, Glu, and His residues)
fHydrophobicity was assumed to be the value proportional to the reciprocal of the concentration of AMP necessary to disrupt planar membranes composed from DPhPC; see Table 3 for the values
Due to basicity of peptidoglycan stem [117], the diffusion of polypeptides, bearing negatively charged residues, can be arrested [108]. Negative charge in AMP prevents their diffusion through the PTG layer of Gram-positive bacteria. When a polypeptide is disulfide rich and structurally rigid, like CT, a negative charge in certain positions within the molecule prevents completely their penetration through the PTG layer of Micrococcus luteus [108]. Most probably, the high number of negatively charged residues in Ltc 7 and Ltc 6a/6b/6c prevents these peptides from passing through PTG of B. subtilis.
Most probably, when the concentration of Ltc exceeds the MIC value, the lipopolysaccharide (LPS) layer of both tested Escherichia coli strains (Table 4) is perturbed and a fraction of the peptides reaches the plasma membrane [118]. This conclusion agrees with a recent observation that AMP capable of fluidizing LPS encompass moieties with a high local density of positive charge [119–121]. The peptide motifs featuring extra positive charge density include: (1) a boomerang motif, containing several Lys (Arg) residues, bordered by aromatic residues on both sides [119]; (2) instead of aromatic residues, a disulfide bond is introduced [37, 122–124]; (3) cyclization of the peptide backbone, like in polymixins B and M [125, 126]. In each case, the charge density is increased due to the close positioning of the positively charged residues, which otherwise tend to move away because of electric repulsion. Aromatic residues of the boomerang motif, disulfide bonds, or backbone cyclization serve as a “clutch” holding the charged residues in close proximity. It seems that Ltc possess an additional motif of this kind, i.e., regular diads and triads of Lys (Arg) residues.
An interesting observation is that in the case of E. coli, the MIC values of Ltc 3a and 3b differ by an order of magnitude, although the peptides differ by the presence of a single lysine residue (Table 1). Probably, this residue is necessary to create a threshold value of charge density for fluidizing the outer membrane. The integrity of this membrane depends upon coordination of LPS molecules in the outer monolayer by divalent cations (Mg2+, Ca2+) [127]. Their displacement by polycationic peptides and direct interaction of the peptides with LPS molecules [128] may facilitate penetration of AMP through the outer membrane.
After passing through the outer membrane or the PTG, AMP interact with the plasma membrane. We suppose that partitioning of an AMP monomer in the plasma membrane predetermines peptide activity. This agrees with the fact that interfacial partitioning of peptide monomers is a limiting step in the membrane deterioration process as shown by Ladokhin and White on melittin, a cationic LP from the honeybee venom, and supported by other groups [129–132]. The energetics of Ltc partitioning into membrane can be accounted for with the MPEX program [133] (Table 5, row for Hydrophobicity 1), where the interfacial Wimley–White scale for peptide transfer from water to membrane interface is implemented. The corresponding ΔGif values are given in Table 3. If charge neutralization at the membrane interface takes place [88], the correlation coefficient becomes positive (Table 5, row for Hydrophobicity 2). Finally, the largest correlation is achieved if the electric potential across the bacterial membrane is taken into account. Indeed, Ltc 1 is incapable of disrupting model PE/PG bilayer in the absence of the membrane potential (Fig. 4c, d). At the same time, both Ltc 1 and 2a exhibit similar MIC values against E. coli (Table 4), whose plasma membrane consists of a PE/PG mixture [87]. When hydrophobicity is defined as a value, reciprocal to the peptide concentration, which is necessary to disrupt diphytanoylphosphatidylcholine (DPhPC) planar membrane (Table 3), the correlation coefficient reaches its maximum (Table 5, Hydrophobicity 3). Probably, both peptides permeabilize equally well the LPS layer of these bacteria. Then Ltc 1 interacts with the plasma membrane and forms pores [32]. Microsecond-long coarse-grained MD simulations of Ltc 1 at the surface of PE/PG bilayer demonstrate the capacity of the peptide to cluster lipid molecules [134]. The peptide adsorption site is enriched with negatively charged PG molecules, surrounded at the periphery with neutral PE. It is suggested that the boundaries between these lipid regions are the loci of membrane destabilization [87, 111, 135]. Having a distinctly different MHP pattern of the helix (Fig. 4b), Ltc 2a disintegrates the plasma membrane via the carpet mechanism.
A special comment on the correlations obtained for the Gram-positive bacterium Arthrobacter globiformis is warranted. No positive correlation between charge, hydrophobicity, and activities of Ltc was obtained (Table 5). One of the reasons could be that A. globiformis peptidoglycan is electrically neutral and thinner than in other Gram-positive bacteria [136]. The plasma membrane of this bacterium contains a fraction of glycolipids (up to ~25 % of total lipid [137]), unlike in other surveyed bacteria where glycolipids are absent [138]. This might be the reason for the distinct hydrophobicity/activity correlation of A. globiformis, compared to other bacteria.
In conclusion, we note that the antibacterial effect of Ltc, and of course other AMP, depends critically on their capability to pass through the outer bacterial envelope. A minimum hydrophobicity is required for Ltc to deteriorate the bacterial plasma membrane, across which electric potential is present.
Hemolysis
High antibacterial activity of AMP is usually coupled with high unwanted lytic activity against erythrocytes [139]. The balance between antimicrobial and hemolytic effects has been discussed in numerous works [116, 140–151]. The hemolytic effect is especially pronounced for Ltc 2a (Table 4). It was investigated in detail using a fluorescently labeled analog of this peptide [29]. The interaction of Ltc 2a with erythrocytes was found to be a multi-stage process (Fig. 5). Clearly, hemolysis occurs when the concentration of the membrane-bound Ltc 2a exceeds a threshold level. Externally exposed glycoproteins do not block the peptide access to the plasma membrane, although Ltc 2a is capable of interacting with sialic acid homopolymers [34]. Probably, the interaction with erythrocytes is a two-step process. First, Ltc 2a peptides are trapped by sialic acid residues exposed to the surface of erythrocytes. Then they diffuse to the erythrocyte membrane and partition into its outer monolayer. This causes an expansion of the external membrane leaflet relative to the inner leaflet, resulting in spicule formation without membrane permeabilization (the stage of echinocyte formation). If the concentration of the bound Ltc 2a further increases, small transient pores with a diameter of ~2 nm are formed. Such pores likely produce osmotic shock, leading to an increase of membrane tension. As a result, the shape of the cell becomes spherical (the stage of echinocyte-to-spherocyte transformation). The lifetime of transient pores in tensed membranes can increase substantially, thus facilitating the peptide access to the inner leaflet. Here, complexes can form between Ltc 2a and anionic phosphatidylserine (PS), which is present in this leaflet. Accumulation of these complexes results in formation of stable lipid–peptide pores of the size ~13 nm. Through these pores, hemoglobin leakage occurs and erythrocyte ghosts are formed. Interestingly, Ltc 1, exhibiting lower hemolytic activity compared to Ltc 2a, was shown to induce similar transformations of the erythrocyte shape and hemoglobin leakage, although it required a longer time [30]. Lower content of PE (~30 % of total lipid [152]) in erythrocyte plasma membrane compared to E. coli (up to ~80 % [138]) seems to facilitate hydrophobic partitioning of Ltc 1 in the erythrocyte membrane, even at moderate membrane potential [153].
Fig. 5.
Hemolytic effect of latarcin Ltc 2a. a A real-time analysis of alterations of erythrocyte shape induced by Ltc 2a. Shape of many erythrocytes is not disturbed during the observation period. Some erythrocytes are transformed to echinocytes that have a long lifetime (an example is marked with an arrowhead). Other erythrocytes are subjected to hemolysis and undergo the following sequence of transformations: discocyte→ echinocyte → spherocyte → ghost (an example is marked with an arrow). Bar length is 5 μm. b Binding of rhodamine-labeled Ltc 2a (Rh-Ltc 2a) to erythrocytes and their lysis. A real-time analysis with confocal laser scanning microscopy. Column I shows bright-field images of cells. Column II shows confocal fluorescence images of the cells showing the distribution of Rh-Ltc 2a at the erythrocyte membrane. Bar length is 5 μm. An example when echinocyte formation is accompanied with an intense binding of Ltc 2a at some region of the membrane, is marked with an arrow. An example when a discocyte transforms to an echinocyte without detectable membrane binding of Ltc 2a is marked with an arrowhead. Typical statistically valid examples are shown in a and b. Time after peptide addition is indicated in each panel. Adapted from [29]
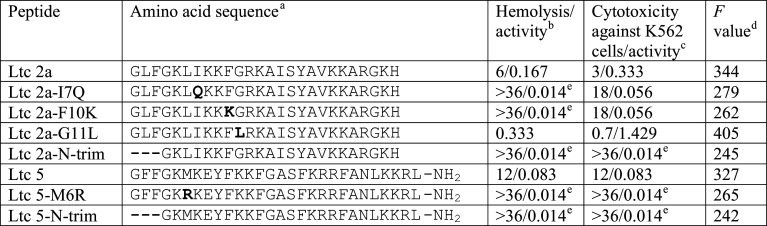
Other Ltc exhibit lower hemolytic activity compared to Ltc 2a (Table 4). Correlation coefficients in the row charge(s)/hemolytic activity imply that positive charge of the peptides augments lytic activity and negative charge is not favorable for it (Table 5). This conclusion agrees with an observation of cupiennin-1a, revealing that reduction of sialic acids from erythrocytes also reduces the hemolytic activity of the peptide [45]. To relate the hemolytic properties of AMP with their hydrophobicity, the following approach was suggested [154]. Efremov and coworkers analyzed the hydrophobic properties of peptides available in the APD database [155] and suggested using the so-called F values to characterize the hydrophobicity of AMP: F = (<mhp> × Aphob)1/2. Here, <mhp> is the average surface hydrophobicity calculated for a peptide in an α-helical conformation, and Aphob is the total hydrophobic area [156]. Analysis of AMP sequences in APD shows that the peptides feature a hydrophobicity gradient. More hydrophobic residues are located at the N-terminus as compared to the C-terminus. A hydrophobicity gradient is also found in other biologically active peptides [80, 85]. Using F-scores, a number of point mutations in Ltc 2a were suggested to decrease its hemolytic activity (summarized in Table 6). Through variation of the hydrophobic/hydrophilic balance in a 13-residue N-terminal fragment of the peptide, hemolytic activity can be modulated. Replacements of hydrophobic isoleucine and phenylalanine residues to hydrophilic glutamine or lysine (I7Q and F10K mutants) increase the effective peptide concentration inducing 50 % hemolysis (EC50) above ~36 μM [154]. The effect of these mutations agrees with the findings obtained for a number of helical AMP. Namely, disruption of the hydrophobic face by the introduction of a polar or charged residue results in significant reduction of hemolytic activity with no attenuation of the antimicrobial properties [139]. Indeed, the MIC of the mutant peptides against E. coli increases by only a factor of 2, compared to the parent Ltc 2a. A similar effect was noted for an Ltc 2a analog truncated by the three residues from the N-terminus. An inverse effect was noted for the G11L mutant, which lowered the value of EC50 by a factor of 2, without affecting the MIC against E. coli [154]. Recently this peptide, Ltc 2a-G11A, was investigated in more detail [157]. The modified peptide was found to be more disruptive against supported phospholipid bilayers, the lipid composition of which was similar to that of mammalian cell membranes. Cholesterol was shown to attenuate the activity of both the mutant and native Ltc 2a peptides [158].
Table 6.
Modified latarcins and their hemolytic and cytotoxic properties
aMutated residues are marked in bold
bHuman erythrocytes, EC50 [μM]/Activity = EC−150. EC50 is reported in [154]
cErythroleukemia K562 cells, EC50 concentration [μM], activity = (EC50)−1
dThe values are reported in [154]. They are dimensionless values; for details of the calculations, see text
eA value of EC50 = 72 μM, satisfying the condition of >36 μM, was used
A number of analogs of Ltc 5 were synthesized [154]. Reduction of hemolytic activity of Ltc 5 can be achieved by M6R replacement (EC50 >36 μM, increase by more than a factor of 3). MIC against E. coli increases by only a factor ~2 compared to native Ltc 5. Similar effects were observed for analogs of Ltc 5 [154] and Ltc 1 [30], which were truncated by three residues from the N-terminus.
The correlation coefficient between the hemolytic activity of Ltc analogs and their F values (Table 6) amounts to 0.95. This high value supports the view that Ltc 2a and 5 belong to the same structural group of highly hemolytic peptides and that the F-score is a reliable predictor of hemolytic activity of α-helical AMP. In a similar manner, HTL-scores were used to characterize the cytotoxic activity of β-sheet polypeptides, CT [110]. We assume that F- and HTL-scores can be used to predict hemolytic and cytotoxic activity of linear and disulfide-rich peptides, respectively.
Anticancer potential
Cationic LP are known for their anticancer properties [159], and interest in this feature has a long history [62, 160–163]. Several LP, e.g., brevinin from frog skin, were shown to kill different tumor cells (Jurkat, BJAB, MCF-7, L929, and A549) at lower concentrations compared to commercial doxorubicin and cisplatin drugs [164]. Peptides are considered to be a novel class of anticancer agents due to their capability to specifically target cancer cells with lower toxicity to normal tissues [66]. Strategies are described aiming at increasing peptide selectivity toward specific cells, while reducing toxicity [162]. Improvements in drug delivery associated with cancer-targeting peptides have also been reported [165–171].
Ltc 2a was shown to possess cytotoxicity against human erythroleukemia K562 cells [29]. The plasma membrane of these cells is affected by the peptide (Fig. 6). Time-dependent membrane blebbing was observed: at EC50 ~3 μM, multiple small blebs grew and changed in shape, and coalesced into several huge blebs. The fact that the cell membrane is severely compromised was indicated by its permeability to a fluorescent marker, propidium iodide. Ltc 2a binding to the outer membrane leaflet of K562 cells seems to trigger PS externalization. 15 min after the addition of the peptide, redistribution of PS toward the outer leaflet of the membrane was detected in ~32 % of the cells. Interestingly, apoptosis was not activated by the peptide.
Fig. 6.
Interactions of fluorescein-labeled Ltc 2a with K562 cells. A real-time analysis was performed with confocal laser scanning microscopy. Column I shows bright-field images of cells. Bar length is 10 µm. Arrowheads mark sack-like cells. Plasma membrane of such cells adopts a spherical shape without blebs, and it is separated from the light-contrast cytoplasmic cellular structures by a transparent cytosol gap. Columns II and III are confocal fluorescent images showing distribution of Fl-Ltc 2a in the cells (column II) and propidium iodide accumulation in cells with compromised membrane (column III). Time after peptide addition is indicated in each row of images. Adapted from [29]
The analogs of Ltc 2a and five peptides, which were synthesized to probe hemolytic activity, were also tested against human erythroleukemia K562 cells (Table 6). The correlation coefficient between the cytotoxic activity and F-scores of these peptides (Table 6) amounts to 0.86. Apparently, the peptides partition similarly to the plasma membrane of erythrocytes and erythroleukemia cells, because they feature similar phospholipid composition (PC/PE/PS/cholesterol = 50:30:7:7) [152, 172].
Polycationic peptides, and among them cobra CT, were shown to be internalized into mammalian cancer cells [173] and damage mitochondria inducing apoptosis [174]. Similar effects were induced by melittin [175], mastoparan [176] and its analogs [177], and spider venom peptide lycosin-1 [178]. Being internalized, these peptides interfere with cell signaling pathways by attenuating the activity of the key proteins. Cobra CT, mastoparan, melittin, and polymyxin B were shown to interact with the phospholipid-binding domain of protein kinases and inhibit them, eventually killing the cell [179]. Melittin was shown to inhibit calmodulin [176], and CT affect metalloproteinases [180]. Lycosin-1 up-regulates p27, inhibiting cell proliferation [178]. Although such effects have not yet been reported for Ltc, they cannot be excluded.
Noteworthy, plasma membrane permeabilization by cationic LP can be potentiated by external electric pulses applied to the cell suspension [167]. These results bring new perspectives for local destruction of solid tumors using the combined “peptide–electric pulses” synergistic treatment. Poly-ionic peptides, like Ltc, seem to be promising candidates for this treatment.
Conclusions and future prospects
Concerning the activities of Ltc considered in the present review, we arrive at the following conclusions: (1) positive charge density in the peptides exceeding a threshold value, and represented by alternation of diads (triads) of Lys or Arg residues in the sequence, is required to fluidize the LPS layer of Gram-negative bacteria; (2) absence of anionic residues (Asp or Glu) in the peptides correlates with their ability to permeate the outer membrane and PTG layer; (3) Ltc-induced destabilization of bacterial plasma membrane is promoted by the transmembrane potential; (4) the hemolytic activity of Ltc can be manipulated by modification of the sequence within ~13 amino acid residues at the N-terminus and related hydrophobicity, or the F-score; (5) high positive correlation between cytotoxic activity against mammalian cells and the F-scores of Ltc agrees with the experimental evidence that the plasma membrane destabilization is the main mechanism of cell death.
Potential pharmacological applications of Ltc are manifold. Corzo and coworkers evaluated Ltc 3a activity in the presence of the following antibiotics: ampicillin, chloramphenicol, kanamycin, novobiocin, and streptomycin. The best antimicrobial combinations were obtained with mixtures of Ltc 3a and kanamycin. Overall, these data show a motivating outlook for potential clinical treatments of bacterial infections using AMP and commercial antibiotics. Lazarev and coworkers used Ltc 1, 2a, 3a, 4b, and 5 as well as cyto-insectotoxin 1a sequences to produce therapeutic plasmids to combat Chlamydia [181, 182]. HEK293 cells were transfected with recombinant constructs encoding these peptides, and gene expression was induced by doxycycline. As a result, the cells became resistant to the infection. This methodology is claimed to be effective against diverse epithelial infections caused by bacteria or fungi; and protection or rescue of infected cell lines in laboratory practice seems more plausible.
Increasing antibiotic resistance in Gram-negative bacteria, particularly in Pseudomonas aeruginosa, presents a global medical challenge due to their ability to rapidly develop resistance to multiple classes of antibiotics [183]. Ltc were shown to be active against this bacterium at a concentration of ~10 μM (Table 4) and may therefore be of practical value.
In the study of Liu and coworkers [184], as many as 39 AMP with broad-spectrum antimicrobial activity were selected to combat Gram-positive Bacillus anthracis (anthrax). The MIC of peptides against B. anthracis and their hemolytic activities against human erythrocytes were determined and therapeutic indices (TI) were calculated. Papillosin and Ltc 2a displayed TI higher than that of SMAP-29, the most potent AMP against this bacterium.
Antiviral activity is common for many AMP. Recently, peptides from the APD database [155, 185] were tested for activity against human immunodeficiency virus [186]. The following criteria were applied: (1) length <25 residues and absence of cysteine residues; (2) charge >0 (anionic peptides are known to be inactive); (3) no toxicity to mammalian cells. Of the 30 peptides selected, Ltc 3a was shown to possess the required activity, exhibiting TI above unity. However, the remaining Ltc have not yet been tested for antiviral properties.
The scope of applications of Ltc and other linear cytolytic peptides is rather wide. This fits in the multitarget concept, reflected in Fig. 1. We suppose that future applications will be biased toward those in which the capability of the peptides to differentiate between membranes with low and high transmembrane potential. It seems to be directly related to the biological function of these peptides to affect electroexcitable tissues. Ltc and similar peptides may be used as hits for targeting infections caused by Gram-negative bacteria. Local destruction of solid tumors by the combined “peptide–electric pulses” treatment is also of interest.
Acknowledgments
This work was supported by the Russian Foundation for Basic Research (grants 13-04-02128 awarded to P.V.D. and 13-04-00825 awarded to R.G.E.), the Program “Molecular and Cellular Biology” of the Russian Academy of Sciences, the grant NSh-1924.2014.4 from the President of the Russian Federation, and grant 14-24-00118 of the Russian Science Foundation awarded to E.V.G. Access to computational facilities of the Joint Supercomputer Center of the Russian Academy of Sciences (Moscow) is appreciated. P.V.D. expresses sincere gratitude to Maria Astapova, Lidiya Baryshnikova, and Stella Yevstigneyeva for providing valuable microbiological information. We thank Lucia Kuhn-Nentwig for advice on the cupiennin classification.
Abbreviations
- AMP
Antimicrobial peptides
- CBF
Carboxyfluorescein
- CD
Circular dichroism
- CL
Cardiolipin
- CSA
Chemical shift anisotropy
- CT
Cobra venom cytotoxins (cardiotoxins)
- DOPC
Dioleoylphosphatidylcholine
- DOPE
Dioleoylphosphatidylethanolamine
- DOPG
Dioleoylphosphatidylglycerol
- DPhPC
Diphytanoylphosphatidylcholine
- EC50
Effective concentration producing a half-maximal effect
- EST
Expressed sequence tags
- GAG
Glycosaminoglycan
- iPQM
Inverted PQM
- LP
Linear peptides
- LPS
Lipopolysaccharide
- Ltc
Latarcins
- MC
Monte Carlo method
- MD
Molecular dynamics
- MHP
Molecular hydrophobicity potential
- MIC
Minimal inhibitory concentration
- PE
Phosphatidylethanolamine
- PG
Phosphatidylglycerol
- P/L
Peptide to lipid molar ratio
- PQM
Processing quadruplet motif
- PS
Phosphatidylserine
- PTG
Peptidoglycan
- RPE
Repetitive polypeptide elements
- SDS
Sodium dodecyl sulfate
- TFE
Trifluoroethanol
- TI
Therapeutic index
- n.d.
Not determined
References
- 1.Fry BG, Roelants K, Champagne DE, Scheib H, Tyndall JD, King GF, Nevalainen TJ, Norman JA, Lewis RJ, Norton RS, Renjifo C, de la Vega RC. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annu Rev Genomics Hum Genet. 2009;10:483–511. doi: 10.1146/annurev.genom.9.081307.164356. [DOI] [PubMed] [Google Scholar]
- 2.Casewell NR, Wuster W, Vonk FJ, Harrison RA, Fry BG. Complex cocktails: the evolutionary novelty of venoms. Trends Ecol Evol. 2013;28(4):219–229. doi: 10.1016/j.tree.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 3.von Reumont BM, Campbell LI, Jenner RA. Quo vadis venomics? A roadmap to neglected venomous invertebrates. Toxins (Basel) 2014;6(12):3488–3551. doi: 10.3390/toxins6123488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanggaard KW, Bechsgaard JS, Fang X, Duan J, Dyrlund TF, Gupta V, Jiang X, Cheng L, Fan D, Feng Y, Han L, Huang Z, Wu Z, Liao L, Settepani V, Thogersen IB, Vanthournout B, Wang T, Zhu Y, Funch P, Enghild JJ, Schauser L, Andersen SU, Villesen P, Schierup MH, Bilde T, Wang J. Spider genomes provide insight into composition and evolution of venom and silk. Nat Commun. 2014;5:3765. doi: 10.1038/ncomms4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escoubas P, Diochot S, Corzo G. Structure and pharmacology of spider venom neurotoxins. Biochimie. 2000;82(9–10):893–907. doi: 10.1016/S0300-9084(00)01166-4. [DOI] [PubMed] [Google Scholar]
- 6.Vassilevski AA, Kozlov SA, Grishin EV. Molecular diversity of spider venom. Biochemistry (Moscow) 2009;74(13):1505–1534. doi: 10.1134/S0006297909130069. [DOI] [PubMed] [Google Scholar]
- 7.King GF, Hardy MC. Spider-venom peptides: structure, pharmacology, and potential for control of insect pests. Annu Rev Entomol. 2013;58:475–496. doi: 10.1146/annurev-ento-120811-153650. [DOI] [PubMed] [Google Scholar]
- 8.Terlau H, Shon KJ, Grilley M, Stocker M, Stuhmer W, Olivera BM. Strategy for rapid immobilization of prey by a fish-hunting marine snail. Nature. 1996;381(6578):148–151. doi: 10.1038/381148a0. [DOI] [PubMed] [Google Scholar]
- 9.Rash LD, Hodgson WC. Pharmacology and biochemistry of spider venoms. Toxicon. 2002;40(3):225–254. doi: 10.1016/S0041-0101(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 10.Estrada G, Villegas E, Corzo G. Spider venoms: a rich source of acylpolyamines and peptides as new leads for CNS drugs. Nat Prod Rep. 2007;24(1):145–161. doi: 10.1039/B603083C. [DOI] [PubMed] [Google Scholar]
- 11.Gremski LH, Trevisan-Silva D, Ferrer VP, Matsubara FH, Meissner GO, Wille AC, Vuitika L, Dias-Lopes C, Ullah A, de Moraes FR, Chavez-Olortegui C, Barbaro KC, Murakami MT, Arni RK, Senff-Ribeiro A, Chaim OM, Veiga SS. Recent advances in the understanding of brown spider venoms: from the biology of spiders to the molecular mechanisms of toxins. Toxicon. 2014;83:91–120. doi: 10.1016/j.toxicon.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn-Nentwig L. Antimicrobial and cytolytic peptides of venomous arthropods. Cell Mol Life Sci. 2003;60(12):2651–2668. doi: 10.1007/s00018-003-3106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bode F, Sachs F, Franz MR. Tarantula peptide inhibits atrial fibrillation. Nature. 2001;409(6816):35–36. doi: 10.1038/35051165. [DOI] [PubMed] [Google Scholar]
- 14.Sp Mouhat, Jouirou B, Mosbah A, De Waard M, Sabatier J-M. Diversity of folds in animal toxins acting on ion channels. Biochem J. 2004;378(Pt 3):717–726. doi: 10.1042/BJ20031860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutertre S, Lewis RJ. Use of venom peptides to probe ion channel structure and function. J Biol Chem. 2010;285(18):13315–13320. doi: 10.1074/jbc.R109.076596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King GF. Venoms as a platform for human drugs: translating toxins into therapeutics. Expert Opin Biol Ther. 2011;11(11):1469–1484. doi: 10.1517/14712598.2011.621940. [DOI] [PubMed] [Google Scholar]
- 17.Saez NJ, Senff S, Jensen JE, Er SY, Herzig V, Rash LD, King GF. Spider-venom peptides as therapeutics. Toxins (Basel) 2010;2(12):2851–2871. doi: 10.3390/toxins2122851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitches E, Edwards MG, Mee C, Grishin E, Gatehouse AM, Edwards JP, Gatehouse JA. Fusion proteins containing insect-specific toxins as pest control agents: snowdrop lectin delivers fused insecticidal spider venom toxin to insect haemolymph following oral ingestion. J Insect Physiol. 2004;50(1):61–71. doi: 10.1016/j.jinsphys.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Bonning BC, Pal N, Liu S, Wang Z, Sivakumar S, Dixon PM, King GF, Miller WA. Toxin delivery by the coat protein of an aphid-vectored plant virus provides plant resistance to aphids. Nat Biotechnol. 2014;32(1):102–105. doi: 10.1038/nbt.2753. [DOI] [PubMed] [Google Scholar]
- 20.Kourie JI, Shorthouse AA. Properties of cytotoxic peptide-formed ion channels. Am J Physiol Cell Physiol. 2000;278(6):C1063–C1087. doi: 10.1152/ajpcell.2000.278.6.C1063. [DOI] [PubMed] [Google Scholar]
- 21.Habermann E. Bee and wasp venoms. Science (New York, NY) 1972;177(46):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 22.Bernheimer AW, Rudy B. Interactions between membranes and cytolytic peptides. Biochim Biophys Acta. 1986;864(1):123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- 23.Pluzhnikov KA, Kozlov SA, Vassilevski AA, Vorontsova OV, Feofanov AV, Grishin EV. Linear antimicrobial peptides from Ectatomma quadridens ant venom. Biochimie. 2014;107 Pt B:211–215. doi: 10.1016/j.biochi.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Wanandy T, Gueven N, Davies NW, Brown SG, Wiese MD. Pilosulins: a review of the structure and mode of action of venom peptides from an Australian ant Myrmecia pilosula. Toxicon. 2015;98(54):61. doi: 10.1016/j.toxicon.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Argiolas A, Pisano JJ. Bombolitins, a new class of mast cell degranulating peptides from the venom of the bumblebee Megabombus pennsylvanicus. J Biol Chem. 1985;260(3):1437–1444. [PubMed] [Google Scholar]
- 26.Moreau SJ. “It stings a bit but it cleans well”: venoms of Hymenoptera and their antimicrobial potential. J Insect Physiol. 2013;59(2):186–204. doi: 10.1016/j.jinsphys.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Herzig V, Wood DLA, Newell F, Chaumeil PA, Kaas Q, Binford GJ, Nicholson GM, Gorse D, King GF. ArachnoServer 2.0, an updated online resource for spider toxin sequences and structures. Nucleic Acids Res. 2011;39(Database):D653–D657. doi: 10.1093/nar/gkq1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozlov SA, Vassilevski AA, Feofanov AV, Surovoy AY, Karpunin DV, Grishin EV. Latarcins, antimicrobial and cytolytic peptides from the venom of the spider Lachesana tarabaevi (Zodariidae) that exemplify biomolecular diversity. J Biol Chem. 2006;281(30):20983–20992. doi: 10.1074/jbc.M602168200. [DOI] [PubMed] [Google Scholar]
- 29.Vorontsova OV, Egorova NS, Arseniev AS, Feofanov AV. Haemolytic and cytotoxic action of latarcin Ltc2a. Biochimie. 2011;93(2):227–241. doi: 10.1016/j.biochi.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Samsonova OV, Kudryashova KS, Feofanov AV. N-terminal moiety of antimicrobial peptide Ltc1-K increases its toxicity for eukaryotic cells. Acta Naturae. 2011;3(2):68–78. [PMC free article] [PubMed] [Google Scholar]
- 31.Dubovskii PV, Volynsky PE, Polyansky AA, Chupin VV, Efremov RG, Arseniev AS. Spatial structure and activity mechanism of a novel spider antimicrobial peptide. Biochemistry. 2006;45(35):10759–10767. doi: 10.1021/bi060635w. [DOI] [PubMed] [Google Scholar]
- 32.Dubovskii PV, Volynsky PE, Polyansky AA, Karpunin DV, Chupin VV, Efremov RG, Arseniev AS. Three-dimensional structure/hydrophobicity of latarcins specifies their mode of membrane activity. Biochemistry. 2008;47(11):3525–3533. doi: 10.1021/bi702203w. [DOI] [PubMed] [Google Scholar]
- 33.Vassilevski AA, Kozlov SA, Zhmak MN, Kudelina IA, Dubovskii PV, Shatursky OY, Arseniev AS, Grishin EV. Synthetic analogues of antimicrobial peptides from the venom of the Central Asian spider Lachesana tarabaevi. Russ J Bioorg Chem. 2007;33(4):376–382. doi: 10.1134/S1068162007040024. [DOI] [PubMed] [Google Scholar]
- 34.Kuznetsov AS, Dubovskii PV, Vorontsova OV, Feofanov AV, Efremov RG. Interaction of linear cationic peptides with phospholipid membranes and polymers of sialic acid. Biochemistry (Moscow) 2014;79(5):459–468. doi: 10.1134/S0006297914050101. [DOI] [PubMed] [Google Scholar]
- 35.Trachsel C, Siegemund D, Kampfer U, Kopp LS, Buhr C, Grossmann J, Luthi C, Cunningham M, Nentwig W, Kuhn-Nentwig L, Schurch S, Schaller J. Multicomponent venom of the spider Cupiennius salei: a bioanalytical investigation applying different strategies. FEBS J. 2012;279(15):2683–2694. doi: 10.1111/j.1742-4658.2012.08650.x. [DOI] [PubMed] [Google Scholar]
- 36.Corzo G, Villegas E, Gomez-Lagunas F, Possani LD, Belokoneva OS, Nakajima T. Oxyopinins, large amphipathic peptides isolated from the venom of the wolf spider Oxyopes kitabensis with cytolytic properties and positive insecticidal cooperativity with spider neurotoxins. J Biol Chem. 2002;277(26):23627–23637. doi: 10.1074/jbc.M200511200. [DOI] [PubMed] [Google Scholar]
- 37.Dubovskii PV, Vassilevski AA, Samsonova OV, Egorova NS, Kozlov SA, Feofanov AV, Arseniev AS, Grishin EV. Novel lynx spider toxin shares common molecular architecture with defense peptides from frog skin. FEBS J. 2011;278(22):4382–4393. doi: 10.1111/j.1742-4658.2011.08361.x. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn-Nentwig L, Schaller J, Nentwig W. Biochemistry, toxicology and ecology of the venom of the spider Cupiennius salei (Ctenidae) Toxicon. 2004;43(5):543–553. doi: 10.1016/j.toxicon.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Kuhn-Nentwig L, Muller J, Schaller J, Walz A, Dathe M, Nentwig W. Cupiennin 1, a new family of highly basic antimicrobial peptides in the venom of the spider Cupiennius salei (Ctenidae) J Biol Chem. 2002;277(13):11208–11216. doi: 10.1074/jbc.M111099200. [DOI] [PubMed] [Google Scholar]
- 40.Vassilevski AA, Sachkova MY, Ignatova AA, Kozlov SA, Feofanov AV, Grishin EV. Spider toxins comprising disulfide-rich and linear amphipathic domains: a new class of molecules identified in the lynx spider Oxyopes takobius. FEBS J. 2013;280(23):6247–6261. doi: 10.1111/febs.12547. [DOI] [PubMed] [Google Scholar]
- 41.Sachkova MY, Slavokhotova AA, Grishin EV, Vassilevski AA. Genes and evolution of two-domain toxins from lynx spider venom. FEBS Lett. 2014;588(5):740–745. doi: 10.1016/j.febslet.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 42.Kuhn-Nentwig L, Fedorova IM, Luscher BP, Kopp LS, Trachsel C, Schaller J, Vu XL, Seebeck T, Streitberger K, Nentwig W, Sigel E, Magazanik LG. A venom-derived neurotoxin, CsTx-1, from the spider Cupiennius salei exhibits cytolytic activities. J Biol Chem. 2012;287(30):25640–25649. doi: 10.1074/jbc.M112.339051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuzmenkov AI, Fedorova IM, Vassilevski AA, Grishin EV. Cysteine-rich toxins from Lachesana tarabaevi spider venom with amphiphilic C-terminal segments. Biochim Biophys Acta. 2013;1828(2):724–731. doi: 10.1016/j.bbamem.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Vassilevski AA, Kozlov SA, Samsonova OV, Egorova NS, Karpunin DV, Pluzhnikov KA, Feofanov AV, Grishin EV. Cyto-insectotoxins, a novel class of cytolytic and insecticidal peptides from spider venom. Biochem J. 2008;411(3):687–696. doi: 10.1042/BJ20071123. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn-Nentwig L, Willems J, Seebeck T, Shalaby T, Kaiser M, Nentwig W. Cupiennin 1a exhibits a remarkably broad, non-stereospecific cytolytic activity on bacteria, protozoan parasites, insects, and human cancer cells. Amino Acids. 2011;40(1):69–76. doi: 10.1007/s00726-009-0471-0. [DOI] [PubMed] [Google Scholar]
- 46.Santos DM, Verly RM, Pilo-Veloso D, de Maria M, de Carvalho MA, Cisalpino PS, Soares BM, Diniz CG, Farias LM, Moreira DF, Frezard F, Bemquerer MP, Pimenta AM, de Lima ME. LyeTx I, a potent antimicrobial peptide from the venom of the spider Lycosa erythrognatha. Amino Acids. 2010;39(1):135–144. doi: 10.1007/s00726-009-0385-x. [DOI] [PubMed] [Google Scholar]
- 47.Zeng X-C, Corzo G, Hahin R. Scorpion venom peptides without disulfide bridges. IUBMB Life. 2005;57(1):13–21. doi: 10.1080/15216540500058899. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, Cao L, Zhong M, Zhang Y, Han C, Li Q, Yang J, Zhou D, Shi W, He B, Liu F, Yu J, Sun Y, Cao Y, Li Y, Li W, Guo D, Cao Z, Yan H. Anti-HIV-1 activity of a new scorpion venom peptide derivative Kn2-7. PLoS One. 2012;7(4):e34947. doi: 10.1371/journal.pone.0034947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong W, Li T, Song Y, Zhang R, Zeng Z, Han S, Zhang X, Wu Y, Li W, Cao Z. Inhibitory activity and mechanism of two scorpion venom peptides against herpes simplex virus type 1. Antiviral Res. 2014;102:1–10. doi: 10.1016/j.antiviral.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pukala TL, Doyle JR, Llewellyn LE, Kuhn-Nentwig L, Apponyi MA, Separovic F, Bowie JH. Cupiennin 1a, an antimicrobial peptide from the venom of the neotropical wandering spider Cupiennius salei, also inhibits the formation of nitric oxide by neuronal nitric oxide synthase. FEBS J. 2007;274(7):1778–1784. doi: 10.1111/j.1742-4658.2007.05726.x. [DOI] [PubMed] [Google Scholar]
- 51.Egorov TA, Odintsova TI, Pukhalsky VA, Grishin EV. Diversity of wheat anti-microbial peptides. Peptides. 2005;26(11):2064–2073. doi: 10.1016/j.peptides.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Dubovskii PV, Vassilevski AA, Slavokhotova AA, Odintsova TI, Grishin EV, Egorov TA, Arseniev AS. Solution structure of a defense peptide from wheat with a 10-cysteine motif. Biochem Biophys Res Commun. 2011;411(1):14–18. doi: 10.1016/j.bbrc.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 53.Craik DJ. Host-defense activities of cyclotides. Toxins (Basel) 2012;4(2):139–156. doi: 10.3390/toxins4020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goyal RK, Mattoo AK. Multitasking antimicrobial peptides in plant development and host defense against biotic/abiotic stress. Plant Sci. 2014;228C:135–149. doi: 10.1016/j.plantsci.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Yount NY, Yeaman MR. Structural congruence among membrane-active host defense polypeptides of diverse phylogeny. Biochim Biophys Acta. 2006;1758(9):1373–1386. doi: 10.1016/j.bbamem.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 56.Fjell CD, Jenssen H, Fries P, Aich P, Griebel P, Hilpert K, Hancock RE, Cherkasov A. Identification of novel host defense peptides and the absence of alpha-defensins in the bovine genome. Proteins. 2008;73(2):420–430. doi: 10.1002/prot.22059. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, Falla TJ. Potential therapeutic application of host defense peptides. Methods Mol Biol. 2010;618:303–327. doi: 10.1007/978-1-60761-594-1_19. [DOI] [PubMed] [Google Scholar]
- 58.Diep DB, Nes IF. Ribosomally synthesized antibacterial peptides in Gram positive bacteria. Curr Drug Targets. 2002;3(2):107–122. doi: 10.2174/1389450024605409. [DOI] [PubMed] [Google Scholar]
- 59.Duquesne S, Destoumieux-Garzon D, Peduzzi J, Rebuffat S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat Prod Rep. 2007;24(4):708–734. doi: 10.1039/b516237h. [DOI] [PubMed] [Google Scholar]
- 60.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19(3):491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bechinger B, Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim Biophys Acta. 2006;1758(9):1529–1539. doi: 10.1016/j.bbamem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Hoskin DW, Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta. 2008;1778(2):357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 64.Yount NY, Yeaman MR. Emerging themes and therapeutic prospects for anti-infective peptides. Annu Rev Pharmacol Toxicol. 2012;52:337–360. doi: 10.1146/annurev-pharmtox-010611-134535. [DOI] [PubMed] [Google Scholar]
- 65.Riedl S, Zweytick D, Lohner K. Membrane-active host defense peptides–challenges and perspectives for the development of novel anticancer drugs. Chem Phys Lipids. 2011;164(8):766–781. doi: 10.1016/j.chemphyslip.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu D, Gao Y, Qi Y, Chen L, Ma Y, Li Y. Peptide-based cancer therapy: opportunity and challenge. Cancer Lett. 2014;351(1):13–22. doi: 10.1016/j.canlet.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Haney E, Hancock R. Peptide design for antimicrobial and immunomodulatory applications. Biopolymers. 2013;100(6):572–583. doi: 10.1002/bip.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teixeira V, Feio MJ, Bastos M. Role of lipids in the interaction of antimicrobial peptides with membranes. Prog Lipid Res. 2012;51(2):149–177. doi: 10.1016/j.plipres.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Polyansky AA, Volynsky PE, Efremov RG. Computer simulations of membrane-lytic peptides: perspectives in drug design. J Bioinform Comput Biol. 2007;5(2B):611–626. doi: 10.1142/S0219720007002783. [DOI] [PubMed] [Google Scholar]
- 70.Kozlov S, Malyavka A, McCutchen B, Lu A, Schepers E, Herrmann R, Grishin E. A novel strategy for the identification of toxinlike structures in spider venom. Proteins. 2005;59(1):131–140. doi: 10.1002/prot.20390. [DOI] [PubMed] [Google Scholar]
- 71.Kozlov SA, Grishin EV. The universal algorithm of maturation for secretory and excretory protein precursors. Toxicon. 2007;49(5):721–726. doi: 10.1016/j.toxicon.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 72.Daly KA, Digby MR, Lefevre C, Nicholas KR, Deane EM, Williamson P. Identification, characterization and expression of cathelicidin in the pouch young of tammar wallaby (Macropus eugenii) Comp Biochem Physiol B Biochem Mol Biol. 2008;149(3):524–533. doi: 10.1016/j.cbpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Wyman TB, Nicol F, Zelphati O, Scaria PV, Plank C, Szoka FC., Jr Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry. 1997;36(10):3008–3017. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]
- 74.Wegener KL, Brinkworth CS, Bowie JH, Wallace JC, Tyler MJ. Bioactive dahlein peptides from the skin secretions of the Australian aquatic frog Litoria dahlii: sequence determination by electrospray mass spectrometry. Rapid Commun Mass Spectrom. 2001;15(18):1726–1734. doi: 10.1002/rcm.429. [DOI] [PubMed] [Google Scholar]
- 75.Zhao H, Gan TX, Liu XD, Jin Y, Lee WH, Shen JH, Zhang Y. Identification and characterization of novel reptile cathelicidins from elapid snakes. Peptides. 2008;29(10):1685–1691. doi: 10.1016/j.peptides.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Kim SS, Shim MS, Chung J, Lim DY, Lee BJ. Purification and characterization of antimicrobial peptides from the skin secretion of Rana dybowskii. Peptides. 2007;28(8):1532–1539. doi: 10.1016/j.peptides.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 77.Conlon JM, Demandt A, Nielsen PF, Leprince J, Vaudry H, Woodhams DC. The alyteserins: two families of antimicrobial peptides from the skin secretions of the midwife toad Alytes obstetricans (Alytidae) Peptides. 2009;30(6):1069–1073. doi: 10.1016/j.peptides.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 78.El Karim IA, Linden GJ, Orr DF, Lundy FT. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J Neuroimmunol. 2008;200(1–2):11–16. doi: 10.1016/j.jneuroim.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 79.Keil B. Specificity of proteolysis. Berlin: Springer-Verlag; 1992. [Google Scholar]
- 80.Dubovskii PV, Li H, Takahashi S, Arseniev AS, Akasaka K. Structure of an analog of fusion peptide from hemagglutinin. Protein Sci. 2000;9(4):786–798. doi: 10.1110/ps.9.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yakimov A, Rychkov G, Petukhov M. De novo design of stable alpha-helices. Methods Mol Biol. 2014;1216:1–14. doi: 10.1007/978-1-4939-1486-9_1. [DOI] [PubMed] [Google Scholar]
- 82.Rizo J, Blanco FJ, Kobe B, Bruch MD, Gierasch LM. Conformational behavior of Escherichia coli OmpA signal peptides in membrane mimetic environments. Biochemistry. 1993;32(18):4881–4894. doi: 10.1021/bi00069a025. [DOI] [PubMed] [Google Scholar]
- 83.Efremov RG, Alix AJ. Environmental characteristics of residues in proteins: three-dimensional molecular hydrophobicity potential approach. J Biomol Struct Dyn. 1993;11(3):483–507. doi: 10.1080/07391102.1993.10508011. [DOI] [PubMed] [Google Scholar]
- 84.Efremov RG, Nolde DE, Konshina AG, Syrtcev NP, Arseniev AS. Peptides and proteins in membranes: what can we learn via computer simulations? Curr Med Chem. 2004;11(18):2421–2442. doi: 10.2174/0929867043364496. [DOI] [PubMed] [Google Scholar]
- 85.Efremov RG, Nolde DE, Volynsky PE, Chernyavsky AA, Dubovskii PV, Arseniev AS. Factors important for fusogenic activity of peptides: molecular modeling study of analogs of fusion peptide of influenza virus hemagglutinin. FEBS Lett. 1999;462(1–2):205–210. doi: 10.1016/S0014-5793(99)01505-7. [DOI] [PubMed] [Google Scholar]
- 86.Glaser RW, Sachse C, Durr UH, Wadhwani P, Afonin S, Strandberg E, Ulrich AS. Concentration-dependent realignment of the antimicrobial peptide PGLa in lipid membranes observed by solid-state 19F-NMR. Biophys J. 2005;88(5):3392–3397. doi: 10.1529/biophysj.104.056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Epand RM, Rotem S, Mor A, Berno B, Epand RF. Bacterial membranes as predictors of antimicrobial potency. J Am Chem Soc. 2008;130(43):14346–14352. doi: 10.1021/ja8062327. [DOI] [PubMed] [Google Scholar]
- 88.Beschiaschvili G, Seelig J. Melittin binding to mixed phosphatidylglycerol/phosphatidylcholine membranes. Biochemistry. 1990;29(1):52–58. doi: 10.1021/bi00453a007. [DOI] [PubMed] [Google Scholar]
- 89.Oren Z, Shai Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers. 1998;47(6):451–463. doi: 10.1002/(SICI)1097-0282(1998)47:6<451::AID-BIP4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 90.Matsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta. 1999;1462(1–2):1–10. doi: 10.1016/S0005-2736(99)00197-2. [DOI] [PubMed] [Google Scholar]
- 91.Dennison SR, Morton LH, Harris F, Phoenix DA. The impact of membrane lipid composition on antimicrobial function of an alpha-helical peptide. Chem Phys Lipids. 2008;151(2):92–102. doi: 10.1016/j.chemphyslip.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 92.Cheng JT, Hale JD, Elliott M, Hancock RE, Straus SK. The importance of bacterial membrane composition in the structure and function of aurein 2.2 and selected variants. Biochim Biophys Acta. 2011;1808(3):622–633. doi: 10.1016/j.bbamem.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 93.Toke O. Antimicrobial peptides: new candidates in the fight against bacterial infections. Biopolymers. 2005;80(6):717–735. doi: 10.1002/bip.20286. [DOI] [PubMed] [Google Scholar]
- 94.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 95.Wimley WC, Hristova K. Antimicrobial peptides: successes, challenges and unanswered questions. J Membr Biol. 2011;239(1–2):27–34. doi: 10.1007/s00232-011-9343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Henriques ST, Craik DJ. Importance of the cell membrane on the mechanism of action of cyclotides. ACS Chem Biol. 2012;7(4):626–636. doi: 10.1021/cb200395f. [DOI] [PubMed] [Google Scholar]
- 97.Jean-Francois F, Elezgaray J, Berson P, Vacher P, Dufourc EJ. Pore formation induced by an antimicrobial peptide: electrostatic effects. Biophys J. 2008;95(12):5748–5756. doi: 10.1529/biophysj.108.136655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uggerhoj LE, Poulsen TJ, Munk JK, Fredborg M, Sondergaard TE, Frimodt-Moller N, Hansen PR, Wimmer R. Rational design of alpha-helical antimicrobial peptides: do’s and don’ts. ChemBioChem. 2015;16(2):242–253. doi: 10.1002/cbic.201402581. [DOI] [PubMed] [Google Scholar]
- 99.Misiewicz J, Afonin S, Grage SL, van den Berg J, Strandberg E, Wadhwani P, Ulrich AS. Action of the multifunctional peptide BP100 on native biomembranes examined by solid-state NMR. J Biomol NMR. 2015;61(3–4):287–298. doi: 10.1007/s10858-015-9897-8. [DOI] [PubMed] [Google Scholar]
- 100.Lee DK, Bhunia A, Kotler SA, Ramamoorthy A. Detergent-type membrane fragmentation by MSI-78, MSI-367, MSI-594, and MSI-843 antimicrobial peptides and inhibition by cholesterol: a solid-state nuclear magnetic resonance study. Biochemistry. 2015;54(10):1897–1907. doi: 10.1021/bi501418m. [DOI] [PubMed] [Google Scholar]
- 101.He J, Krauson AJ, Wimley WC. Toward the de novo design of antimicrobial peptides: lack of correlation between peptide permeabilization of lipid vesicles and antimicrobial, cytolytic, or cytotoxic activity in living cells. Biopolymers. 2014;102(1):1–6. doi: 10.1002/bip.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freire JM, Gaspar D, Veiga AS, Castanho MA. Shifting gear in antimicrobial and anticancer peptides biophysical studies: from vesicles to cells. J Pept Sci. 2015;21(3):178–185. doi: 10.1002/psc.2741. [DOI] [PubMed] [Google Scholar]
- 103.Papo N, Shai Y. Can we predict biological activity of antimicrobial peptides from their interactions with model phospholipid membranes? Peptides. 2003;24(11):1693–1703. doi: 10.1016/j.peptides.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 104.Silva MT, Sousa JC. Ultrastructure of the cell wall and cytoplasmic membrane of Gram-negative bacteria with different fixation techniques. J Bacteriol. 1973;113(2):953–962. doi: 10.1128/jb.113.2.953-962.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goodsell DS. Inside a living cell. Trends Biochem Sci. 1991;16(6):203–206. doi: 10.1016/0968-0004(91)90083-8. [DOI] [PubMed] [Google Scholar]
- 106.Amro NA, Kotra LP, Wadu-Mesthrige K, Bulychev A, Mobashery S, Liu G. High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: structural basis for permeability. Langmuir. 2000;16(6):2789–2796. doi: 10.1021/la991013x. [DOI] [Google Scholar]
- 107.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67(4):593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dubovskii PV, Vorontsova OV, Utkin YN, Arseniev AS, Efremov RG, Feofanov AV. Cobra cytotoxins: determinants of antibacterial activity. Mendeleev Commun. 2015;21(1):70–71. doi: 10.1016/j.mencom.2015.01.026. [DOI] [Google Scholar]
- 109.Konshina AG, Dubovskii PV, Efremov RG. Structure and dynamics of cardiotoxins. Curr Protein Pept Sci. 2012;13(6):570–584. doi: 10.2174/138920312803582960. [DOI] [PubMed] [Google Scholar]
- 110.Dubovskii PV, Konshina AG, Efremov RG. Cobra cardiotoxins: membrane interactions and pharmacological potential. Curr Med Chem. 2014;21(3):270–287. doi: 10.2174/09298673113206660315. [DOI] [PubMed] [Google Scholar]
- 111.Epand RM, Epand RF. Domains in bacterial membranes and the action of antimicrobial agents. Mol BioSyst. 2009;5(6):580–587. doi: 10.1039/b900278m. [DOI] [PubMed] [Google Scholar]
- 112.Dubovskii PV, Utkin YN. Cobra cytotoxins: structural organization and antibacterial activity. Acta Naturae. 2014;6(3):11–18. [PMC free article] [PubMed] [Google Scholar]
- 113.Travis SM, Anderson NN, Forsyth WR, Espiritu C, Conway BD, Greenberg EP, McCray PB, Jr, Lehrer RI, Welsh MJ, Tack BF. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect Immun. 2000;68(5):2748–2755. doi: 10.1128/IAI.68.5.2748-2755.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Won A, Pripotnev S, Ruscito A, Ianoul A. Effect of point mutations on the secondary structure and membrane interaction of antimicrobial peptide anoplin. J Phys Chem B. 2011;115(10):2371–2379. doi: 10.1021/jp108343g. [DOI] [PubMed] [Google Scholar]
- 115.Beven L, Castano S, Dufourcq J, Wieslander A, Wroblewski H. The antibiotic activity of cationic linear amphipathic peptides: lessons from the action of leucine/lysine copolymers on bacteria of the class Mollicutes. Eur J Biochem. 2003;270(10):2207–2217. doi: 10.1046/j.1432-1033.2003.03587.x. [DOI] [PubMed] [Google Scholar]
- 116.Ma Q, Jiao W, Lv Y, Dong N, Zhu X, Shan A. Structure-function relationship of Val/Arg-rich peptides: effects of net charge and pro on activity. Chem Biol Drug Des. 2014;84(3):348–353. doi: 10.1111/cbdd.12325. [DOI] [PubMed] [Google Scholar]
- 117.Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32(2):149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 118.Domadia PN, Bhunia A, Ramamoorthy A, Bhattacharjya S. Structure, interactions, and antibacterial activities of MSI-594 derived mutant peptide MSI-594F5A in lipopolysaccharide micelles: role of the helical hairpin conformation in outer-membrane permeabilization. J Am Chem Soc. 2010;132(51):18417–18428. doi: 10.1021/ja1083255. [DOI] [PubMed] [Google Scholar]
- 119.Mohanram H, Bhattacharjya S. Resurrecting inactive antimicrobial peptides from the lipopolysaccharide trap. Antimicrob Agents Chemother. 2014;58(4):1987–1996. doi: 10.1128/AAC.02321-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bai Y, Liu S, Jiang P, Zhou L, Li J, Tang C, Verma C, Mu Y, Beuerman RW, Pervushin K. Structure-dependent charge density as a determinant of antimicrobial activity of peptide analogues of defensin. Biochemistry. 2009;48(30):7229–7239. doi: 10.1021/bi900670d. [DOI] [PubMed] [Google Scholar]
- 121.Chai H, Allen WE, Hicks RP. Spectroscopic investigations of the binding mechanisms between antimicrobial peptides and membrane models of Pseudomonas aeruginosa and Klebsiella pneumoniae. Bioorg Med Chem. 2014;22(15):4210–4222. doi: 10.1016/j.bmc.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 122.Cujova S, Slaninova J, Monincova L, Fucik V, Bednarova L, Stokrova J, Hovorka O, Voburka Z, Straka J, Cerovsky V. Panurgines, novel antimicrobial peptides from the venom of communal bee Panurgus calcaratus (Hymenoptera: andrenidae) Amino Acids. 2013;45(1):143–157. doi: 10.1007/s00726-013-1482-4. [DOI] [PubMed] [Google Scholar]
- 123.Hwang H, Hyun S, Kim Y, Yu J. Reduction of helical content by insertion of a disulfide bond leads to an antimicrobial peptide with decreased hemolytic activity. ChemMedChem. 2013;8(1):59–62. doi: 10.1002/cmdc.201200463. [DOI] [PubMed] [Google Scholar]
- 124.Mohanram H, Bhattacharjya S. Beta-boomerang antimicrobial and antiendotoxic peptides: lipidation and disulfide bond effects on activity and structure. Pharmaceuticals (Basel) 2014;7(4):482–501. doi: 10.3390/ph7040482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Velkov T, Thompson PE, Nation RL, Li J. Structure–activity relationships of polymyxin antibiotics. J Med Chem. 2010;53(5):1898–1916. doi: 10.1021/jm900999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Velkov T, Roberts KD, Nation RL, Thompson PE, Li J. Pharmacology of polymyxins: new insights into an ‘old’ class of antibiotics. Future Microbiol. 2013;8(6):711–724. doi: 10.2217/fmb.13.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Clifton LA, Skoda MW, Le Brun AP, Ciesielski F, Kuzmenko I, Holt SA, Lakey JH. Effect of divalent cation removal on the structure of Gram-negative bacterial outer membrane models. Langmuir. 2015;31(1):404–412. doi: 10.1021/la504407v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bhattacharjya S, Ramamoorthy A. Multifunctional host defense peptides: functional and mechanistic insights from NMR structures of potent antimicrobial peptides. FEBS J. 2009;276(22):6465–6473. doi: 10.1111/j.1742-4658.2009.07357.x. [DOI] [PubMed] [Google Scholar]
- 129.Ladokhin AS, White SH. Folding of amphipathic alpha-helices on membranes: energetics of helix formation by melittin. J Mol Biol. 1999;285(4):1363–1369. doi: 10.1006/jmbi.1998.2346. [DOI] [PubMed] [Google Scholar]
- 130.Ladokhin AS, White SH. ‘Detergent-like’ permeabilization of anionic lipid vesicles by melittin. Biochim Biophys Acta. 2001;1514(2):253–260. doi: 10.1016/S0005-2736(01)00382-0. [DOI] [PubMed] [Google Scholar]
- 131.Almeida PF, Pokorny A. Mechanisms of antimicrobial, cytolytic, and cell-penetrating peptides: from kinetics to thermodynamics. Biochemistry. 2009;48(34):8083–8093. doi: 10.1021/bi900914g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McKeown AN, Naro JL, Huskins LJ, Almeida PF. A thermodynamic approach to the mechanism of cell-penetrating peptides in model membranes. Biochemistry. 2011;50(5):654–662. doi: 10.1021/bi1013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Snider C, Jayasinghe S, Hristova K, White SH. MPEx: a tool for exploring membrane proteins. Protein Sci. 2009;18(12):2624–2628. doi: 10.1002/pro.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Polyansky AA, Ramaswamy R, Volynsky PE, Sbalzarini IF, Marrink SJ, Efremov RG. Antimicrobial peptides induce growth of phosphatidylglycerol domains in a model bacterial membrane. J Phys Chem Lett. 2010;1(20):3108–3111. doi: 10.1021/jz101163e. [DOI] [Google Scholar]
- 135.Epand RF, Maloy WL, Ramamoorthy A, Epand RM. Probing the “charge cluster mechanism” in amphipathic helical cationic antimicrobial peptides. Biochemistry. 2010;49(19):4076–4084. doi: 10.1021/bi100378m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Conn HJ, Dimmick I. Soil bacteria similar in morphology to Mycobacterium and Corynebacterium. J Bacteriol. 1947;54(3):291–303. doi: 10.1128/jb.54.3.291-303.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shaw N, Stead D. Lipid composition of some species of Arthrobacter. J Bacteriol. 1971;107(1):130–133. doi: 10.1128/jb.107.1.130-133.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang G, Mishra B, Epand RF, Epand RM. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim Biophys Acta. 2014;1838(9):2160–2172. doi: 10.1016/j.bbamem.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Matsuzaki K. Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta. 2009;1788(8):1687–1692. doi: 10.1016/j.bbamem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 140.Jin Y, Mozsolits H, Hammer J, Zmuda E, Zhu F, Zhang Y, Aguilar MI, Blazyk J. Influence of tryptophan on lipid binding of linear amphipathic cationic antimicrobial peptides. Biochemistry. 2003;42(31):9395–9405. doi: 10.1021/bi034338s. [DOI] [PubMed] [Google Scholar]
- 141.Lee KH, Shin SY, Hong JE, Yang ST, Kim JI, Hahm KS, Kim Y. Solution structure of termite-derived antimicrobial peptide, spinigerin, as determined in SDS micelle by NMR spectroscopy. Biochem Biophys Res Commun. 2003;309(3):591–597. doi: 10.1016/j.bbrc.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 142.Lee K, Shin SY, Kim K, Lim SS, Hahm KS, Kim Y. Antibiotic activity and structural analysis of the scorpion-derived antimicrobial peptide IsCT and its analogs. Biochem Biophys Res Commun. 2004;323(2):712–719. doi: 10.1016/j.bbrc.2004.08.144. [DOI] [PubMed] [Google Scholar]
- 143.Nguyen LT, Schibli DJ, Vogel HJ. Structural studies and model membrane interactions of two peptides derived from bovine lactoferricin. J Pept Sci. 2005;11(7):379–389. doi: 10.1002/psc.629. [DOI] [PubMed] [Google Scholar]
- 144.Konno K, Hisada M, Naoki H, Itagaki Y, Fontana R, Rangel M, Oliveira JS, Cabrera MP, Neto JR, Hide I, Nakata Y, Yasuhara T, Nakajima T. Eumenitin, a novel antimicrobial peptide from the venom of the solitary eumenine wasp Eumenes rubronotatus. Peptides. 2006;27(11):2624–2631. doi: 10.1016/j.peptides.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 145.Zhu WL, Nan YH, Hahm KS, Shin SY. Cell selectivity of an antimicrobial peptide melittin diastereomer with D-amino acid in the leucine zipper sequence. J Biochem Mol Biol. 2007;40(6):1090–1094. doi: 10.5483/BMBRep.2007.40.6.1090. [DOI] [PubMed] [Google Scholar]
- 146.Won A, Khan M, Gustin S, Akpawu A, Seebun D, Avis TJ, Leung BO, Hitchcock AP, Ianoul A. Investigating the effects of L- to D-amino acid substitution and deamidation on the activity and membrane interactions of antimicrobial peptide anoplin. Biochim Biophys Acta. 2011;1808(6):1592–1600. doi: 10.1016/j.bbamem.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 147.Rangel M, Cabrera MP, Kazuma K, Ando K, Wang X, Kato M, Nihei K, Hirata IY, Cross TJ, Garcia AN, Faquim-Mauro EL, Franzolin MR, Fuchino H, Mori-Yasumoto K, Sekita S, Kadowaki M, Satake M, Konno K. Chemical and biological characterization of four new linear cationic alpha-helical peptides from the venoms of two solitary eumenine wasps. Toxicon. 2011;57(7–8):1081–1092. doi: 10.1016/j.toxicon.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 148.Lin CH, Tzen JT, Shyu CL, Yang MJ, Tu WC. Structural and biological characterization of mastoparans in the venom of Vespa species in Taiwan. Peptides. 2011;32(10):2027–2036. doi: 10.1016/j.peptides.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 149.Jin F, Sun Q, Xu X, Li L, Gao G, Xu Y, Yu X, Ren S. cDNA cloning and characterization of the antibacterial peptide cecropin 1 from the diamondback moth, Plutella xylostella L. Protein Expr Purif. 2012;85(2):230–238. doi: 10.1016/j.pep.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 150.Rifflet A, Gavalda S, Tene N, Orivel J, Leprince J, Guilhaudis L, Genin E, Vetillard A, Treilhou M. Identification and characterization of a novel antimicrobial peptide from the venom of the ant Tetramorium bicarinatum. Peptides. 2012;38(2):363–370. doi: 10.1016/j.peptides.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 151.Kim SJ, Kim JS, Lee YS, Sim DW, Lee SH, Bahk YY, Lee KH, Kim EH, Park SJ, Lee BJ, Won HS. Structural characterization of de novo designed L5K5 W model peptide isomers with potent antimicrobial and varied hemolytic activities. Molecules. 2013;18(1):859–876. doi: 10.3390/molecules18010859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kleinig H, Zentgraf H, Comes P, Stadler J. Nuclear membranes and plasma membranes from hen erythrocytes. II. Lipid composition. J Biol Chem. 1971;246(9):2996–3000. [PubMed] [Google Scholar]
- 153.Jay AW, Burton AC. Direct measurement of potential difference across the human red blood cell membrane. Biophys J. 1969;9(2):115–121. doi: 10.1016/S0006-3495(69)86372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Polyansky AA, Vassilevski AA, Volynsky PE, Vorontsova OV, Samsonova OV, Egorova NS, Krylov NA, Feofanov AV, Arseniev AS, Grishin EV, Efremov RG. N-terminal amphipathic helix as a trigger of hemolytic activity in antimicrobial peptides: a case study in latarcins. FEBS Lett. 2009;583(14):2425–2428. doi: 10.1016/j.febslet.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 155.Wang G, Li X, Wang Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009;37(Database issue):D933–D937. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pyrkov TV, Chugunov AO, Krylov NA, Nolde DE, Efremov RG. PLATINUM: a web tool for analysis of hydrophobic/hydrophilic organization of biomolecular complexes. Bioinformatics (Oxford, England) 2009;25(9):1201–1202. doi: 10.1093/bioinformatics/btp111. [DOI] [PubMed] [Google Scholar]
- 157.Idiong G, Won A, Ruscito A, Leung BO, Hitchcock AP, Ianoul A. Investigating the effect of a single glycine to alanine substitution on interactions of antimicrobial peptide latarcin 2a with a lipid membrane. Eur Biophys J. 2011;40(9):1087–1100. doi: 10.1007/s00249-011-0726-z. [DOI] [PubMed] [Google Scholar]
- 158.Won A, Ruscito A, Ianoul A. Imaging the membrane lytic activity of bioactive peptide latarcin 2a. Biochim Biophys Acta. 2012;1818(12):3072–3080. doi: 10.1016/j.bbamem.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 159.Baker MA, Maloy WL, Zasloff M, Jacob LS. Anticancer efficacy of Magainin2 and analogue peptides. Cancer Res. 1993;53(13):3052–3057. [PubMed] [Google Scholar]
- 160.Mader JS, Hoskin DW. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin Investig Drugs. 2006;15(8):933–946. doi: 10.1517/13543784.15.8.933. [DOI] [PubMed] [Google Scholar]
- 161.Schweizer F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol. 2009;625(1–3):190–194. doi: 10.1016/j.ejphar.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 162.Gaspar D, Veiga AS, Castanho MA. From antimicrobial to anticancer peptides. A review. Front Microbiol. 2013;4:294. doi: 10.3389/fmicb.2013.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Guo X, Ma C, Du Q, Wei R, Wang L, Zhou M, Chen T, Shaw C. Two peptides, TsAP-1 and TsAP-2, from the venom of the Brazilian yellow scorpion, Tityus serrulatus: evaluation of their antimicrobial and anticancer activities. Biochimie. 2013;95(9):1784–1794. doi: 10.1016/j.biochi.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 164.Savelyeva A, Ghavami S, Davoodpour P, Asoodeh A, Los MJ. An overview of Brevinin superfamily: structure, function and clinical perspectives. Adv Exp Med Biol. 2014;818:197–212. doi: 10.1007/978-1-4471-6458-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yin CM, Wong JH, Xia J, Ng TB. Studies on anticancer activities of lactoferrin and lactoferricin. Curr Protein Pept Sci. 2013;14(6):492–503. doi: 10.2174/13892037113149990066. [DOI] [PubMed] [Google Scholar]
- 166.Lemeshko VV. Potential-dependent membrane permeabilization and mitochondrial aggregation caused by anticancer polyarginine-KLA peptides. Arch Biochem Biophys. 2010;493(2):213–220. doi: 10.1016/j.abb.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 167.Lemeshko VV. Electrical potentiation of the membrane permeabilization by new peptides with anticancer properties. Biochim Biophys Acta. 2013;1828(3):1047–1056. doi: 10.1016/j.bbamem.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 168.Yao L, Daniels J, Moshnikova A, Kuznetsov S, Ahmed A, Engelman DM, Reshetnyak YK, Andreev OA. pHLIP peptide targets nanogold particles to tumors. Proc Natl Acad Sci USA. 2013;110(2):465–470. doi: 10.1073/pnas.1219665110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Weerakkody D, Moshnikova A, Thakur MS, Moshnikova V, Daniels J, Engelman DM, Andreev OA, Reshetnyak YK. Family of pH (low) insertion peptides for tumor targeting. Proc Natl Acad Sci USA. 2013;110(15):5834–5839. doi: 10.1073/pnas.1303708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Viola-Villegas NT, Carlin SD, Ackerstaff E, Sevak KK, Divilov V, Serganova I, Kruchevsky N, Anderson M, Blasberg RG, Andreev OA, Engelman DM, Koutcher JA, Reshetnyak YK, Lewis JS. Understanding the pharmacological properties of a metabolic PET tracer in prostate cancer. Proc Natl Acad Sci USA. 2014;111(20):7254–7259. doi: 10.1073/pnas.1405240111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Karabadzhak AG, An M, Yao L, Langenbacher R, Moshnikova A, Adochite RC, Andreev OA, Reshetnyak YK, Engelman DM. pHLIP-FIRE, a cell insertion-triggered fluorescent probe for imaging tumors demonstrates targeted cargo delivery in vivo. ACS Chem Biol. 2014;9(11):2545–2553. doi: 10.1021/cb500388m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Maccarrone M, Nieuwenhuizen WE, Dullens HF, Catani MV, Melino G, Veldink GA, Vliegenthart JF, Finazzo Agro A. Membrane modifications in human erythroleukemia K562 cells during induction of programmed cell death by transforming growth factor beta 1 or cisplatin. Eur J Biochem. 1996;241(1):297–302. doi: 10.1111/j.1432-1033.1996.0297t.x. [DOI] [PubMed] [Google Scholar]
- 173.Feofanov AV, Sharonov GV, Dubinnyi MA, Astapova MV, Kudelina IA, Dubovskii PV, Rodionov DI, Utkin YN, Arseniev AS. Comparative study of structure and activity of cytotoxins from venom of the cobras Naja oxiana, Naja kaouthia, and Naja haje. Biochemistry (Moscow) 2004;69(10):1148–1157. doi: 10.1023/B:BIRY.0000046890.46901.7e. [DOI] [PubMed] [Google Scholar]
- 174.Wang C-H, W-g Wu. Amphiphilic beta-sheet cobra cardiotoxin targets mitochondria and disrupts its network. FEBS Lett. 2005;579(14):3169–3174. doi: 10.1016/j.febslet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 175.Orsolic N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012;31(1–2):173–194. doi: 10.1007/s10555-011-9339-3. [DOI] [PubMed] [Google Scholar]
- 176.Heinen TE, da Veiga AB. Arthropod venoms and cancer. Toxicon. 2011;57(4):497–511. doi: 10.1016/j.toxicon.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 177.Jones S, Martel C, Belzacq-Casagrande AS, Brenner C, Howl J. Mitoparan and target-selective chimeric analogues: membrane translocation and intracellular redistribution induces mitochondrial apoptosis. Biochim Biophys Acta. 2008;1783(5):849–863. doi: 10.1016/j.bbamcr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 178.Liu Z, Deng M, Xiang J, Ma H, Hu W, Zhao Y, Li DW, Liang S. A novel spider peptide toxin suppresses tumor growth through dual signaling pathways. Curr Mol Med. 2012;12(10):1350–1360. doi: 10.2174/156652412803833643. [DOI] [PubMed] [Google Scholar]
- 179.Raynor RL, Bin Z, Kuo JF. Membrane interactions of amphiphilic polypeptides mastoparan, melittin, polymyxin B, and cardiotoxin. Differential inhibition of protein kinase C, Ca2+/calmodulin-dependent protein kinase II and synaptosomal membrane Na, K-ATPase, and Na+ pump and differentiation of HL60 cells. J Biol Chem. 1991;266(5):2753–2758. [PubMed] [Google Scholar]
- 180.Tsai P-C, Hsieh C-Y, Chiu C-C, Wang C-K, Chang L-S, Lin S-R. Cardiotoxin III suppresses MDA-MB-231 cell metastasis through the inhibition of EGF/EGFR-mediated signaling pathway. Toxicon. 2012;60(5):734–743. doi: 10.1016/j.toxicon.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 181.Lazarev VN, Polina NF, Shkarupeta MM, Kostrjukova ES, Vassilevski AA, Kozlov SA, Grishin EV, Govorun VM. Spider venom peptides for gene therapy of Chlamydia infection. Antimicrob Agents Chemother. 2011;55(11):5367–5369. doi: 10.1128/AAC.00449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lazarev VN, Shkarupeta MM, Polina NF, Kostrjukova ES, Vassilevski AA, Kozlov SA, Grishin EV, Govorun VM. Antimicrobial peptide from spider venom inhibits Chlamydia trachomatis infection at an early stage. Arch Microbiol. 2013;195(3):173–179. doi: 10.1007/s00203-012-0863-5. [DOI] [PubMed] [Google Scholar]
- 183.Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 2009;22(4):582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dawson RM, Fox MA, Atkins HS, Liu C-Q. Potent antimicrobial peptides with selectivity for Bacillus anthracis over human erythrocytes. Int J Antimicrob Agents. 2011;38(3):237–242. doi: 10.1016/j.ijantimicag.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 185.Wang Z, Wang G. APD: the antimicrobial peptide database. Nucleic Acids Res. 2004;32(Database issue):D590–D592. doi: 10.1093/nar/gkh025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang G, Watson KM, Peterkofsky A, Buckheit RW., Jr Identification of novel human immunodeficiency virus type 1-inhibitory peptides based on the antimicrobial peptide database. Antimicrob Agents Chemother. 2010;54(3):1343–1346. doi: 10.1128/AAC.01448-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Koradi R, Billeter M, Wuthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J Mol Graph. 1996;14(1):51–55. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 188.Dubinnyi MA, Lesovoy DM, Dubovskii PV, Chupin VV, Arseniev AS. Modeling of 31P-NMR spectra of magnetically oriented phospholipid liposomes: a new analytical solution. Solid State Nucl Magn Reson. 2006;29(4):305–311. doi: 10.1016/j.ssnmr.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 189.Kuhn-Nentwig L, Schaller J, Schürch S, Nentwig W (2016) The Venom of Cupiennius salei (Ctenidae). In: Gopalakrishnakone P, Corzo GA, de Lima ME, Diego-Garcia E (eds) Spider Venoms. Toxinology. Springer, in press
- 190.Dubovskii PV. Unusual titration of the membrane-bound artificial hemagglutinin fusion peptide. Eur Biophys J. 2012;41(12):1077–1084. doi: 10.1007/s00249-012-0867-8. [DOI] [PubMed] [Google Scholar]