Abstract
The different types of cells in the lung, from the conducting airway epithelium to the alveolar epithelium and the pulmonary vasculature, are interconnected by gap junctions. The specific profile of gap junction proteins, the connexins, expressed in these different cell types forms compartments of intercellular communication that can be further shaped by the release of extracellular nucleotides via pannexin1 channels. In this review, we focus on the physiology of connexins and pannexins and describe how this lung communication network modulates lung function and host defenses in conductive and respiratory airways.
Keywords: Lung, Intercellular communication, Connexin, Pannexin, Physiology, Disease
Introduction
The lung is the essential organ for breathing and, according to this vital function, is divided in 2 main portions, namely the respiratory portion, the site for gas exchange between air and blood, and the air-conducting portion, the duct for inhaled and exhaled air [1]. The air-conducting portion begins above the lungs, extending across most of the respiratory system from the nasal cavities to the intrapulmonary bronchi and the bronchioles, while the respiratory portion is composed of respiratory bronchioles, alveolar ducts, and alveoli entirely located within the lungs. Inhaled air needs to be cleaned from dust, pollen, chemicals, and pathogens present in the environment before it reaches the alveoli for gas exchange. Moreover, recent studies have revealed the presence of a respiratory microbiota, composed by commensal and opportunistic pathogen microbes residing in nose and lungs [2]. Thus, inherent to this organ, an appropriate lung function depends on host defense mechanisms, mainly represented by mucociliary clearance, secretion of antimicrobial molecules and surfactant, innate and adaptive immunity, and also inflammation in case of severe harmful stimuli [3]. The continuous exposure to inhaled hostile factors makes lungs in constant risk of injury and thereby tissue repair processes are also crucial for maintaining lung homeostasis. The latter requires efficient coordination between the different cell types constituting the organ. Here, we describe the cell-to-cell communication network provided by connexins (Cxs) and pannexins (Panxs) in conductive and respiratory airways, and we focus on the physiology of these channels in promoting lung function and host defense.
The lung communication network components: expression and function in lung development and aging
Cxs form gap junction channels enabling for direct cell-to-cell communication, a mechanism referred to as gap junctional intercellular communication (GJIC) [4]. Cxs oligomerize into hexameric structures to form a connexon at the plasma membrane. Connexons between cells in contact dock to form gap junctions, thus directly connecting their cytoplasm and allowing the passage of inorganic ions and small water-soluble molecules. In addition, connexons, also known as hemichannels, can provide direct access for the exchange of small molecules with the extracellular environment [5]. Oligomers of Panxs are similar to connexons at the level of structure and function, but they do not form gap junctions [6]. Panxs are glycoproteins that form single high-conductance plasma membrane channels allowing paracrine cell-to-cell communication [7]. In particular, Panx1, which is the most ubiquitously expressed of the 3 Panxs, has been described as a mechano-sensitive adenosine triphosphate (ATP) releasing channel involved in several biological processes. However, in some physiological context, it remains difficult to clearly discriminate between Cx hemichannels and Panx channels due to their close functional and pharmacological similarities [8, 9].
Specific patterns of Cx and Panx expression have been observed in the lungs depending on species, stages of differentiation, and cell types of the airway tree [10, 11]. In this paper, we review the expression of Cxs and Panxs in conductive and respiratory airways, and we discuss the involvement of the 2 protein families in the developing and adult lung.
Connexin and pannexin expression in conductive airways
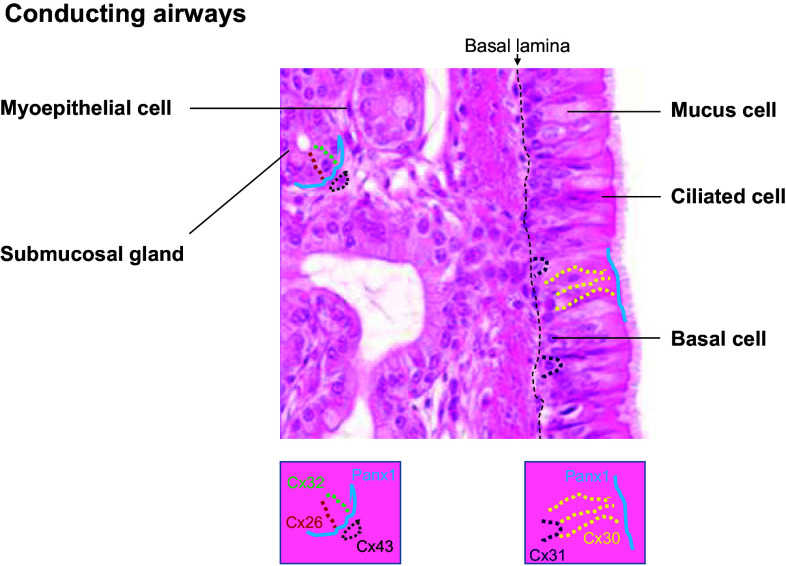
The air-conducting portion is lined by a pseudostratified epithelium, which is referred to as the airway epithelium, and is formed by basal, ciliated, and mucus-secreting goblet cells [1]. The conductive airways also contain submucosal glands, which are connected with the epithelium through ducts and are mainly composed by mucus-producing cells and serous cells (Fig. 1).
Fig. 1.
Expression profile of Panx1 and principal connexins in the adult conducting airways. Hematoxylin and eosin-stained section of human tracheal mucosa illustrating the surface airway epithelium and the tracheal glands. The localization of connexins and pannexin1 is depicted by a color code and schematically represented below the photograph (Panx1, blue line; Cx26, red dots; Cx30, yellow dots; Cx31; purple dots; Cx32, green dots; Cx43, brown dots)
Cx26 and Cx32 are prominent in airway epithelial cells of ferret fetus, but, while gap junctions formed by these Cxs persist in the glandular epithelium up to the weanling stage, their expression declines with ciliogenesis of the superficial airway epithelium and rapidly disappears after birth [12, 13]. Similarly, Cx26 and Cx43 are expressed in the undifferentiated human airway epithelium, but upon differentiation they rapidly disappear [13, 14]. Conversely, Cx30 and Cx31 remain detectable in human differentiated epithelium. Cx30 appears to have mostly apical or lateral localization and to mediate GJIC between ciliated cells and between basal and ciliated cells, whereas Cx31 is present between few cells localized basally and seems to preferentially connect basal cells [14]. In addition, primary cultures of differentiated human airway epithelial cells have also been found to express mRNAs for Cx30.3 and Cx31.1 [14]. Interestingly, the adult mouse airway epithelium exhibits a similar expression profile for Cx30 and Cx31, but, unlike what has been described in humans, Cx26 is present in adult epithelium, although its detection is discontinuous [14], and also Cx37 may be weakly expressed in murine airway epithelium [15–17]. In mouse submucosal glands, Cx26 and Cx32 are expressed between the epithelial cells, while Cx43 is observed between myoepithelial cells surrounding the glands [14].
Panx1 and Panx3 protein have been detected in whole murine lungs [18]. Primary cultures of differentiated human airway epithelial cells express mRNAs for Panx1 and Panx2, but not for Panx3 [19]. Panx1 is diffusely expressed in undifferentiated human airway epithelial cells, but its expression increases during differentiation and it is detected apically in ciliated and goblet cells. In addition, submucosal glands express Panx1 preferentially at the basolateral side of the glandular cells [19]. Figure 1 summarizes the profile of Cx and Panx expression in the conductive airways of the adult lung.
Connexin and pannexin expression in respiratory airways
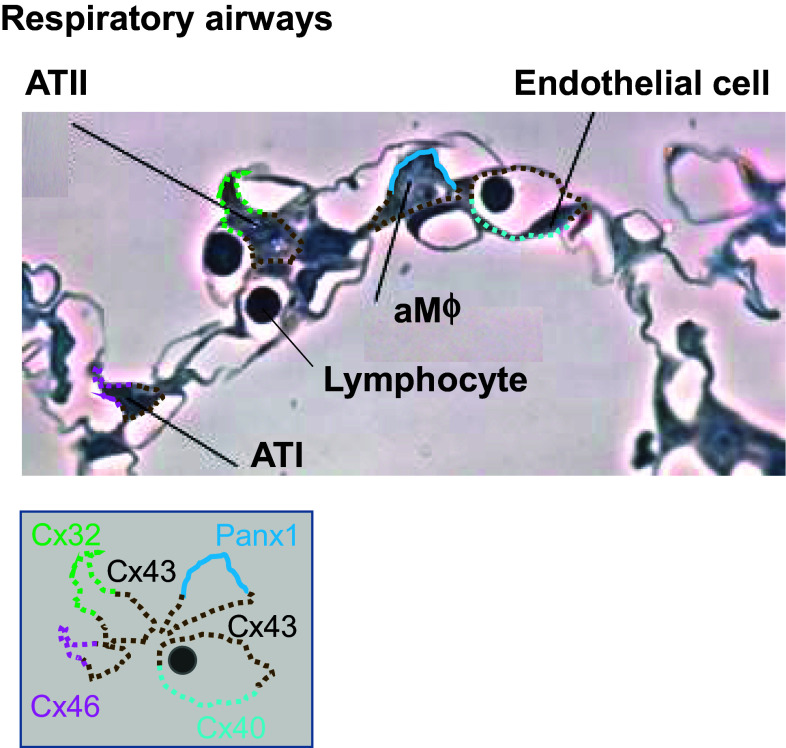
In the respiratory portion (Fig. 2), alveoli are lined by a thin epithelial monolayer composed of alveolar type I (ATI) and type II (ATII) cells that are in close contact with the endothelial monolayer of respiratory capillary network [1]. While ATI cells mediate gas exchange and constitute the majority of the alveolar surface, ATII cells absorb actively excessive alveolar liquid and produce surfactant, a major determinant of alveolar patency [20]. The lumen of some alveoli also hosts alveolar macrophages, lung-resident phagocytes that are located in close proximity to the epithelial surface and capillary endothelial cells [21].
Fig. 2.
Expression profile of Panx1 and principal connexins in the adult respiratory airways. Semithin section of human lungs illustrating the respiratory airway epithelium and its relation with the capillary bed. The localization of connexins and pannexin1 is depicted by a color code and schematically represented below the photograph (Panx1, blue line; Cx26, red dots; Cx32, green dots; Cx43, brown dots; Cx40, light blue dots; Cx46, pink dots)
Cx26 and Cx43 have been detected in human epithelial cells of the respiratory airways [13, 22, 23]. However, most of our knowledge of Cx expression in the alveoli comes from murine models. Airway epithelial cells of mouse origin have been found to express Cx26, Cx32, Cx37, Cx43, and Cx46 [15, 17, 24]. In adult rat alveoli, Cx26, Cx32, and Cx40 show moderate staining by immunofluorescence, whereas Cx43 and Cx46 are strongly expressed [25–27]. While Cx46 is preferentially detected in ATI cells, Cx32 seems to be restricted in ATII cells for its location in the corners of alveoli. Interestingly, ATI and ATII cells are unable to form functional hetero-cellular gap junctions via Cx32 and, even though Cx32 has the potential to connect ATII cells, ATII cells are rarely adjacent to each other, suggesting an alternative role as hemichannels for Cx32 in these cells [26, 28]. In contrast, Cx43 is fairly distributed in both ATI and ATII cells and is the major Cx-mediating GJIC between the 2 cellular types [26]. In mouse, a subset of alveolar macrophages attached to the alveolar wall express Cx43 and form gap junctions with the epithelium [29]. Cx43 is also detected in alveolar endothelial cells together with Cx40 [30–32], whereas Cx37 is present in endothelium of large lung blood vessels, but not in alveolar capillaries [15].
In alveolar sections of mouse lungs, all the 3 Panxs have been observed. Panx1 and Panx3 localize more at the plasma membrane, while Panx2 is expressed in a perinuclear fashion [33]. However, the pattern of Panx expression within the different alveolar cells is not known. The only exception includes isolated human alveolar macrophages, where the mRNA profile of Panxs has been characterized [19, 34]. Human alveolar macrophages have high levels of Panx1 mRNA. Conversely, Panx2 expression is about 10-fold lower than Panx1 and Panx3 mRNA is not detected in these immune cells. Figure 2 summarizes the profile of Cx and Panx expression in the respiratory airways of the adult lung.
Connexins and pannexins in lung development and aging
Being the most abundantly Cx expressed in lung cells, Cx43 has been found to play a crucial role in lung development. Cx43 gene expression was shown to be downregulated during alveologenesis in a rat model of nitrofen-induced pulmonary hypoplasia, and upregulated in ill rats treated with retinoic acid, which is known to stimulate alveolar formation [35]. Cx43 knockout mice die at birth as a result of a failure in pulmonary gas exchange caused by a swelling and blockage of the right ventricular outflow tract from the heart [36]. A recent analysis of these knockout mice has also shown that their lungs are hypoplastic compared to wild-type mice, with smaller vessels and muscular layer and a strong delay in the development of the alveoli [37]. Airspaces are narrow and alveolar septa are thick, with a decrease in ATI cells and an enrichment of ATII cells in the mesenchyme. However, the mechanism by which Cx43 regulates lung differentiation is not clear. Failure in alveolar development in Cx43 knockout mice can be caused by a defect in vessel or epithelial formation, because Cx43 is expressed in both alveolar endothelial and epithelial cells. Since mice with endothelial-specific deletion of Cx43 have apparently normal lung development [30, 38], Cx43 is more likely to play roles in the proliferation/differentiation of epithelial or mesenchymal cells. Conversely, mice double knockout for Cx37 and Cx40, the other 2 main Cxs expressed in endothelial cells, die perinatally, and display severe vascular abnormalities affecting several organs compared to mice lacking either Cx40 or Cx37 alone. These include localized hemorrhages in lungs, with extravascular blood present in some of the airspace, which is probably the cause of death for inadequate pulmonary function [39].
The generation and characterization of Cx knockout mice have been also useful to understand the role of Cxs in the rising of aging-associated lung pathologies. As mentioned, knockout mice deficient for expression of either Cx40 [40] or endothelial Cx43 alone [38] do not exhibit obvious pulmonary alterations. However, adult mice lacking both Cx40 and endothelial Cx43 spontaneously develop lung dysfunction and fibrosis, leading to a shortened life span as they age [41]. As early as 16 weeks after birth, these mice begin to exhibit disruption of the alveolar airspaces. Upon aging, lung fibrosis gets worse with alveolar thickening, increased lung fibroblast content, and increased deposition of elastin and collagen in the extracellular matrix. Although the mechanistic basis linking Cx43 and Cx40 to lung fibrosis is not known, these results suggest that alveolar endothelial Cxs regulate intercellular communication between the vasculature and airspaces, and are important to maintain lung morphology and function. Moreover, lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have reduced Cx43 expression and decreased intercellular communication [42]. It remains to be elucidated whether impaired GJIC is involved in activation and proliferation of altered fibroblasts, which are central events in the pathogenesis of this fibrotic pathology [43].
Altered Cx expression and function have been also described in several lung cancer cells and exogenous tumorigenic agents have been associated with aberrant location or the absence of Cxs expressed in lung [10, 44]. These features have been observed in urethane-induced mouse lung adenomas, where both Cx32 and Cx43 were absent [24]. Interestingly, Cx32 knockout mice do not exhibit obvious pulmonary alterations, but they are more susceptible to benzene-induced lung toxicity [45] and have a higher incidence of chemical-induced bronchioloalveolar adenoma [46, 47]. Cx32-deficient non-tumor cells display increased proliferation compared to their wild-type counterparts and lung tumors originate from the proliferation-competent ATII cells, suggesting that Cx32 may regulate lung epithelial cell growth under stress conditions [46]. Similarly, a higher incidence of spontaneous and chemical-induced lung cancer has been described in Cx43+/− mice with reduced Cx43 expression in the lung [24, 48, 49]. Deletion of a single allele of Cx43 leads to lower GJIC capacity and increased cell proliferation of mouse ATII cells [50]. Furthermore, a large number of human lung cancer cell lines shows downregulation of Cx26 expression, which is linked to gene promoter methylation [23]. Nevertheless, the initial assumption suggesting that Cxs would act as tumor suppressor genes has been questioned repeatedly. Indeed, Ito and colleagues showed that the maintenance of Cx26 expression in human lung squamous cell carcinomas might be related to a higher ability to establish metastasis [51]. Thus, the roles of Cxs and gap junctions in lung carcinogenesis may depend on the stage of the cancer progression and the tumor cell phenotype [52].
Panx expression and function during lung development and aging are unknown. Panxs have been implicated in differentiation of keratinocytes [53], neurons [54, 55], chondrocytes [56], and osteoblasts [57] as well as in tumor progression of glioma cells [58, 59], keratinocytes [60], and mouse melanomas [61]. Surprisingly, given the ubiquitous expression of Panx1 in many tissues, Panx1 knockout mice do not present any major anatomical abnormalities [7]. Recent analysis of these mice has found that Panx1 regulates cellular properties of keratinocytes and dermal fibroblasts in skin development and wound healing [62]. Further studies are needed to address the involvement of Panxs in lung development and aging.
Connexin-dependent and pannexin-dependent communication in conductive airways
Mucociliary clearance
The first line of defense against pathogen invasion and external particles is the mucus lining the surface of the airway epithelium [63]. The secreted mucus layer acts as a physical and chemical trap for inhaled hostile factors, which are then removed from the lungs via cough reflex and mucociliary clearance [64]. Ciliary beating was shown to be regulated in a gap junction-dependent mechanism. Indeed, early studies demonstrated that Cx channels take part in the synchronization of beat frequency in airway cell culture of rabbit origin [65]. Later, it was suggested that the transmission of inositol triphosphate (IP3) from one cell to another via gap junctions mediates calcium wave propagation between airway epithelial cells and coordinate ciliary beating [66]. Another mechanism of cell-to-cell communication has been extensively described to coordinate ciliary beating and mucin secretion in response to mechanical stress [67]. This mechanism involves ATP release to the extracellular space, which in turn stimulates type-2 purinergic receptors (P2R) and induces calcium signaling in surrounding ciliated epithelial cells [68]. Recently, the release of ATP induced by hypotonic stress was found to correlate with functional expression of Panx1 channels in differentiated human airway epithelial cells [19, 69] as well as in murine airway tissues, while Panx1 knockout mice showed impaired ATP release [19, 70]. However, in other studies, both Cxs and Panxs were found to be involved in ATP release at airway mucosa [71, 72]. In addition, mucus-secreting airway epithelial cells are able to secrete ATP and other nucleotides within mucin granules by exocytosis [73].
Mucus clearance is facilitated by a thin periciliary liquid layer, which exhibits an optimal height for effective cilia beating [74]. This liquid layer together with the mucus layer forms the airway surface liquid (ASL). The ASL volume is determined by active transepithelial salt transport, involving chloride secretion via cystic fibrosis transmembrane conductance regulator (CFTR) channels and the calcium-activated chloride channel (CaCC) anoctamin1 (ANO1 or TMEM16A), while sodium absorption occurs through the amiloride-sensitive epithelial channel ENaC. Moreover, active chloride release mediates fluid secretion by serous cells to allow the transport of mucus from submucosal glands to the epithelial surface [75]. Interestingly, Cx43-mediated GJIC is required to stimulate mucus hydration in the human glandular epithelial cell line Calu-3 [76]. In fact, inhibition of gap junctions with mimetic-blocking peptides specific for Cx43 or with pharmacological inhibitors prevents the activation of CFTR-dependent chloride and fluid secretion in response to adenosine, protease-activated, and prostaglandin E2 (PGE2) receptor stimulation. These results have been confirmed in human primary airway epithelial cells, where pharmacological inhibition of GJIC results in a failure of PGE2 to increase ASL volume [76]. Pharmacological inhibition of Panx1 channels was also found to abolish the increase of ASL volume induced by lipoxin A4 (LXA4) in human airway epithelial cells [77]. LXA4-stimulated ASL volume increase by activating chloride secretion through CaCCs and by inhibiting ENaC-dependent sodium absorption [78, 79]. LXA4-induced fluid secretion was dependent on apical ATP release via Panx1 channels and on activation of the purinergic receptor P2RY11 [77].
Collectively, these findings draw a picture in which gap junctions and Panx channels integrate multiple signaling pathways to finely coordinate ciliary beating and mucus hydration. Therefore, the lung communication network provides a mean to maintain efficient mucociliary clearance and, thereby, a proper epithelial host defense [10].
Innate immune response and inflammation
A successful host response is essential for an orchestrated pathogen clearing in the lungs. The airway innate immune response begins with the identification of pathogen-associated molecular patterns (PAMPs) through pathogen recognition receptors (PRRs) expressed on the host epithelium [80]. PRRs include, among others, toll-like receptors (TLRs), which are transmembrane proteins that can be expressed either on the cell surface or in endosomal compartments [81]. Once TLRs sense a pathogen, they activate signaling pathways, such as mitogen-activated protein kinases (MAPKs), and transcription factors, including nuclear factor-κB (NF-κB), to initiate an inflammatory response characterized by the production of cytokines and chemokines, and by apoptosis [81, 82]. In addition, activation of TLRs stimulates epithelial secretion of defense molecules, such as antimicrobial peptides [83, 84] and mucus [85], as well as epithelial fluid efflux for enhancing mucociliary clearance [86–88].
In the human airway epithelial cell line 1HAEo−, binding of bacterial lipoprotein to TLR2 induces immediate calcium-dependent signaling that leads to activation of NF-κB and release of interleukin-8 (IL-8), a powerful chemo-attractant for neutrophil recruitment to the infected area [89]. Interestingly, in lungs of mice infected with the respiratory opportunistic pathogen Pseudomonas aeruginosa, recruitment of neutrophils is significantly inhibited by intraperitoneal administration of a pharmacological blocker of gap junctions. The results have been confirmed in vitro, where the same blocker reduces IL-8 production in response to the pathogen. Cx43-based gap junctions were shown to transiently amplify this TRL2-induced pro-inflammatory signaling by communicating calcium fluxes from stimulated to adjacent non-stimulated cells [89]. However, this signaling amplification can be attenuated in the presence of lipopolysaccharide (LPS), tumor necrosis factor-α (TNF-α), and lysophosphatidic acid, which were found to inhibit GJIC by a mechanism dependent on phosphorylation of the Cx43 C-terminus by the tyrosine kinase c-Src [90–92]. Indeed, Pseudomonas aeruginosa induced tyrosine phosphorylation of Cx43 and decreased GJIC in airway epithelial cells 4 h post-stimulation prevented excessive NF-kB activation [89]. Thus, this gating regulation of Cx channels enables the epithelium to immediately respond to bacterial infections, but later it limits the extent of inflammation by a delayed negative feedback elicited by pro-inflammatory mediators. It is worthwhile to note that in cystic fibrosis (CF) airway epithelial cells, this negative feedback does not occur, which may contribute to the severity of the disease [90–92]. CF, a genetic disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, is characterized by excessive and destructive airway inflammation [93].
Gap junctions were also described to be finely regulated during P. aeruginosa infection of polarized submucosal glandular Calu-3 cells [94]. P. aeruginosa flagellin activates cell surface receptors, probably TLR5, to elicit an intracellular MAPK-dependent signaling cascade leading to increased expression of Cx43 and enhancement of GJIC. Based on the crucial role of GJIC in ASL regulation, up-regulation of Cx43 expression and function may boost CFTR activation and fluid transport in order to counteract bacterial infection [76]. Cx43 was oppositely modulated by MAPKs. Thus, p38 stimulated Cx43 up-regulation, while c-Jun N-terminal kinases (JNK) exerted a negative regulation. Interestingly, Cx43-mediated cell-to-cell communication did not affect IL-8 secretion in these glandular epithelial cells, but modulated apoptosis. Apoptosis is important for bacterial clearance from the lungs and for the stimulation of tissue repair [82, 95]. In addition to strongly enhanced GJIC, inhibition of JNK increased the number of apoptotic cells during bacterial infection. The latter effect was prevented by lentiviral expression of a Cx43-specific short hairpin RNA [94]. Therefore, gap junctions exert a pro-apoptotic role in glandular epithelial cells infected by Pseudomonas aeruginosa, but the tightly regulated expression of Cx43, mediated by JNK signaling, confers a mechanism to modulate the survival/apoptosis balance. Again, it is worthwhile to mention that this regulation of Cx43 was altered in CF-like conditions. Indeed, CFTR inhibition in Calu-3 cells was associated with decreased Cx43 expression and reduced apoptosis [94]. Thus, defective regulation of Cx43 in CF airway epithelial cells may contribute to the reduced apoptosis and bacterial killing that has been observed in this disease [96, 97].
Airway epithelial repair
The recovery of an intact epithelium following infection, inflammation, or injury is critical for restoration of lung homeostasis. The repair process requires the migration and proliferation of local progenitor cells of undamaged areas, which gives rise after re-differentiation to all cell types constituting the airway epithelium [98]. In mice, basal cells have been identified as epithelial progenitor cells, which are able once activated to self-renew and to regenerate ciliated and mucus-secreting cells [98].
Cx and Panx channels have both been involved in the process of airway epithelial repair. As described above, airway epithelial cells release ATP through Panx1 channels in response to various stimuli, including LXA4. In addition to induce fluid secretion, LXA4 stimulates epithelial repair [99]. Pharmacological inhibition of Panx1 channels has been found to abolish proliferation of the human airway epithelial cell line NuLi-1 stimulated by LXA4, resulting in decrease wound closure [77]. ATP, upon its release via Panx1 channels, triggers airway epithelial repair by stimulation of P2RY11 receptors, activation of KATP potassium channels, and phosphorylation of extracellular signal-regulated kinases [99]. In contrast, in a wound/healing model of primary cultures of differentiated human airway epithelial cells, Cx26 has been shown to act as a negative regulator of epithelial cell proliferation [100]. Cx26 is not detectable in differentiated airway epithelial cells [14], but upon wounding, its expression is transiently induced in activated basal cells. Intracellular and some junctional staining of Cx26 have been detected 12 h post-wounding in basal cells at the edge of the wound. Its expression markedly increases with time, reaching the peak at 48 h after injury, when it localizes only at cell–cell contacts and it strongly decreases after wound closure [100]. Activation of cell proliferation upon injury is required to trigger Cx26 expression in repairing cells, but the signaling factors that regulate Cx26 induction during repair remained to be identified. Interestingly, Cx26 silencing in immortalized cell lines using small interfering RNA and in non-differentiated primary human airway epithelial cells using lentiviral-transduced short hairpin RNA enhances cell proliferation [100]. The signaling molecules/pathways mediated by GJIC that controls proliferation within repairing epithelial basal cells are unknown. Recently, it has been reported that Cx26-based gap junctions redistribute cyclic adenosine monophosphate (cAMP) between cancer cells that, in turn, block cell division [101]. Thus, induction of Cx26-mediated intercellular communication by proliferative signals in repairing basal cells may represent a feedback mechanism to repress their proliferation and progressively promote differentiation. Interestingly, as described for Cx43, Cx26 regulation is altered in CF cells. At the early phase of wound repair, primary culture of CF-differentiated human airway epithelial cells exhibits prompt induction of proliferation and Cx26 expression even in regions far from the edge of the wound, as well as an enhanced initial rate of wound closure [100]. Furthermore, in CF and other lung diseases characterized by extensive tissue injury and chronic P. aeruginosa infection, proper Cx function in repairing cells might be also disturbed by quorum-sensing molecule C12 (N-3-oxo-dodecanoyl-l-homoserine lactone), which is highly produced by bacterial biofilm [102]. While an intact polarized epithelium is able to inactivate this molecule, recent data show that C12 disrupts gap junctions and epithelial integrity by inducing cell shrinkage and blebbing in non-polarized airway epithelial cells [103]. These effects result in impaired airway epithelial cell repair in wound/healing in vitro model.
Connexin-dependent and pannexin-dependent communication in respiratory airways
Hypoxic pulmonary vasoconstriction
Hypoxic pulmonary vasoconstriction (HPV) is a physiological mechanism that improves lung ventilation/perfusion ratio and arterial oxygenation [104]. Pulmonary arteries constrict in lung areas with low oxygen levels in order to redirect blood flow to alveoli with higher oxygen supply, thereby increasing the total area involved in gaseous exchange. Although pulmonary arterial smooth muscle cells are thought to constitute both the sensor and the transducer of the hypoxic signal as well as its contractile effector, significant controversy remains concerning the underlying mechanisms of local alveolar oxygen sensing and of signal transduction activating vasoconstriction [104].
Interestingly, a recent study has shown that the site for oxygen sensing is located at the alveolo-capillary level with subsequent propagation of the hypoxic signal to upstream arterioles [105]. Inhibition of gap junctions with mimetic-blocking peptides specific for Cx40 or with pharmacological inhibitors largely attenuates HPV in the lungs of wild-type mice. HPV is also impaired in Cx40 knockout mice and in mice with endothelial-specific deletion of Cx40, clearly demonstrating the relevance of Cx40-based GJIC at the lung vascular endothelium in this mechanism [105]. Hypoxic conditions in wild-type mice induce endothelial membrane depolarization which starts in alveolar capillaries and propagates to neighboring arterioles. The depolarization in upstream vessels is prevented in Cx40 knockout mice. Therefore, HPV originates at the alveolar site, where hypoxia triggers endothelial membrane depolarization in the capillaries, which is transmitted by electric coupling via Cx40-based gap junctions to the upstream arterioles. At this site, the hypoxic-transmitted signal elicits vasoconstriction through a signaling cascade induced by endothelial membrane depolarization and involving activation of endothelial voltage-dependent α1G subtype calcium channels, cytosolic phospholipase A2, and formation of epoxyeicosatrienoic acids, which may stimulate arterial smooth muscle cell contraction [105].
Strikingly, although HPV is a physiological process, it can lead to pulmonary hypertension under conditions of chronic lung hypoxia [104]. Interestingly, chronic hypoxic pulmonary hypertension is attenuated in Cx40 knockout mice, suggesting that Cx40 is not only required for physiological HPV, but is also involved in lung vascular remodeling and pulmonary hypertension in response to chronic hypoxia [105]. Furthermore, induction of pulmonary hypertension in rats by chronic hypoxia or pharmacological treatment increases the expression of Cx43 at the lung arterial wall [106, 107]. Since inhibition of Cx43 with blocking peptides tends to decrease HPV [105], the role of this Cx in mediating HPV may become deleterious during pulmonary hypertension, representing a potential target for the treatment of this disease [10].
Surfactant secretion
In the lower airways, surfactant released by ATII cells is required for the reduction of alveolar surface tension to prevent collapse of the lungs (atelectasis) during the ventilatory cycle and for protection against reactive oxygen lung injuries and infections [20]. Lung surfactant consists of glycerophospholipids packaged into specialized organelles termed lamellar bodies, which are delivered by exocytosis to the apical cell surface. This exocytosis is triggered by an increase in cytosolic calcium concentration. ATI cells, which represent 90 % of alveolar cells and that are more subjected to mechanical stress, promote the release of surfactants by ATII cells through calcium wave propagation [26, 108]. In isolated intact rat alveoli, photo-excited release of caged calcium inside the alveolar epithelial cells was performed to study the transmission of calcium [108]. This technique led to an increase of calcium concentration in the cytosol of selected cells and induced ATII cell secretion, even when photo-excited cells were ATI cells. Furthermore, GJIC also enables calcium wave propagation from one alveolus to the neighboring ones. Interestingly, the interalveolar communication involving ATI and ATII cells was blocked by mimetic-blocking peptides specific for Cx43 [108]. Thus, calcium signaling is propagated within the alveolar epithelium through Cx43-based gap junctions, leading to ATII surfactant secretion. It is important to note that in response to mechanical stretch transmission of calcium waves from ATI to ATII cells and the resulting surfactant secretion can also occur via paracrine stimulation of P2R through ATP release [27, 109]. Pharmacological and peptide inhibitors of Cxs have no effect on this paracrine cell-to-cell communication [109], suggesting that in alveolar epithelial cells, ATP can be release through Panx channels and/or by exocytosis. Therefore, propagation of calcium signals, either via gap junctions or via paracrine ATP release, is crucial to modulate surfactant secretion and may also help to adjust surfactant production to stimuli such as changes in pulmonary blood pressure [110, 111].
Alveolar macrophage-driven immune response
In healthy humans, alveolar macrophages are most likely the only immune cells present in the alveoli, representing the sentinel phagocytic cells of the innate immune system in the lungs [21]. The innate immune response of macrophage-like cells involves the activation of inflammasomes, which are large multiprotein complexes for proper maturation and secretion of the pro-inflammatory cytokines IL-1β and IL-18, and for induction of apoptosis/pyroptosis [112]. Although an early report has found that Panx1 interacts with P2X7 receptor to activate inflammasomes [34], its requirement for this innate immune response is still controversial [113].
Induction of inflammasome signaling, ascribed primarily to the alveolar macrophage, has been demonstrated to impair Pseudomonas aeruginosa clearance and to increase apoptosis/pyroptosis and mortality in murine acute pneumonia [114]. Interestingly, pharmacological inhibition of Panx1 channels by probenecid enhanced bacterial clearance by reducing levels of IL-1β in lung of mice affected by acute P. aeruginosa pneumonia [115]. In addition, the authors showed that probenecid also inhibited in vitro IL-1β secretion by murine alveolar macrophages upon bacterial infection. Therefore, probenecid inhibition of Panx1-dependent inflammasome activation in alveolar macrophages has been proposed as therapeutic option to reduce the negative consequences of inflammation in acute P. aeruginosa pneumonia [115]. Conversely, in agreement with previous studies showing that inflammasome activation is required for protective immunity in the lung against Streptococcus pneumoniae infection [116, 117], probenecid treatment in mice infected with S. pneumoniae did not improve the clearance of this bacterium from the lung. On the contrary, the number of viable S. pneumoniae was even augmented in bronchoalveolar lavage (BAL) of treated mice [115].
A recent study has demonstrated intercellular communication occurring in the alveolar lumen between resident macrophages and epithelial cells [29]. Formation of gap junctions between sessile macrophages and the epithelium was demonstrated by cell-specific photo-excited release of caged calcium and fluorescence recovery after photobleaching (FRAP). In 40 % of alveolar macrophages, uncaging-induced calcium waves spread from the epithelium to macrophages and in the opposite direction. Treatment with mimetic peptides specific for Cx43 abolished this communication. FRAP techniques and Cx43 staining revealed the presence of “Cx43-high” coupled and “Cx43-low” uncoupled alveolar macrophage populations. Cx43 expression was more abundant in murine macrophages attached to the alveolar wall than non-adherent macrophages isolated by BAL, indicating that this resident population is able to form gap junctions with the alveolar epithelium. Furthermore, exposure of lung alveoli to Escherichia coli LPS-induced cyclic and synchronized calcium spikes in both macrophages and epithelial cells. The spikes propagated between different alveolar macrophages, often separated by several alveoli, across the intervening epithelium. These calcium waves were inhibited by Cx43 blockers, but not by P2R antagonists, ruling out a role for Cx hemichannels in this cell-to-cell communication mechanism. Mice with either alveolar macrophage-specific or alveolar epithelial-specific deletion of Cx43 lacked LPS-induced synchronized calcium spikes. Importantly, in response to LPS treatment, these mice also showed an increase in different inflammatory parameters, including nuclear translocation of NF-κB, production of pro-inflammatory cytokines and chemokines, recruitment of neutrophils to alveoli, and higher mortality as compared to wild-type mice. The anti-inflammatory signals induced by calcium wave transmission involved calcium/calmodulin-dependent kinase kinase (CAMKK) and phosphorylation in alveolar epithelial cells of its downstream target, namely the pro-survival kinase Akt. All together, these findings indicate that in the alveolar lumen, Cx43-based GJIC between resident macrophages and the epithelium modulates the extent of the inflammatory response against bacterial pathogens and protects from acute lung injury.
Leukocyte recruitment in the alveolar lumen
Chemokines and inflammatory mediators activate and guide leukocytes to inflamed tissues by stimulating chemotaxis, a complex process that involves recognition of chemotactic gradients, cell polarization, and directed migration [118]. Panx1 channels have been demonstrated to control several aspects of human neutrophil chemotaxis by mediating ATP release and autocrine purinergic signaling [119, 120]. Extracellular nucleotides also act as potent mediators of lung inflammation, providing a paracrine mechanism for intercellular communication between leukocytes and cells present in the alveoli [121]. In a murine model of lung fibrosis induced by airway administration of bleomycin, ATP released into the alveolar lumen was shown to constitute a major endogenous danger signal that engages the P2X7 receptor Panx1 axis, leading to IL-1β maturation, leukocyte recruitment, and lung fibrosis [122]. Indeed, acute inflammation was reduced by mimetic-blocking peptides specific for Panx1. Furthermore, based on in vitro experiments, it has been proposed that airway and alveolar epithelial cells underlie increased levels of luminal ATP during lung fibrosis [122]. However, ATP release by pulmonary epithelial cells in response to bleomycin treatment was only partially inhibited by Panx blockers, suggesting that other mechanisms, such as Cx hemichannels, exocytosis, and/or membrane leakage of damaged cells, might be responsible for increased levels of extracellular nucleotides. Similarly, stress inflammatory conditions also induce massive release of ATP from endothelial cells [121]. Activated leukocytes were also proposed as a source of enhanced levels of extracellular ATP through release via Cx37 hemichannels for monocytes [123] and Cx43 hemichannels for neutrophils [124], although Panx1 channels may fulfill similar functions.
The ability of gap junctions to propagate inflammatory signals has been clearly shown to be crucial in the endothelium to mediate the adhesion and the transmigration of neutrophils in the alveolar space of inflamed lungs. The lung microvascular Cx40 and Cx43 show opposite expression patterns and function during acute lung inflammation [10]. Cx40 expression was found to decrease in response to lung injury in rabbits and this phenotype has been associated with increased pulmonary vascular permeability [125, 126]. Intranasal instillation of E. coli LPS in mice also reduced pulmonary Cx40 expression, but the development of inflammation did not differ between wild-type and Cx40 knockout mice [127]. In contrast, increased recruitment of neutrophils to the alveoli was observed in mice with endothelial-specific deletion of Cx40 early during the inflammatory response to intratracheal instillation of LPS from P. aeruginosa [32]. Furthermore, murine lung capillaries lacking endothelial Cx40 showed decreased activity of ecto-5′-nucleotidase (CD73), the ectoenzyme that hydrolyzes extracellular nucleotides to generate adenosine. Several studies indicate that production of adenosine has a protective role in acute lung inflammation [128]. Adenosine produced by CD73 prevents leukocyte adhesion to the endothelium via an intracellular cAMP signaling triggered by stimulation of A2B receptors [129]. Interestingly, targeting endothelial Cx40 in vitro reduced GJIC and CD73 expression and activity, resulting in enhanced leukocyte adhesion to a mouse endothelial cell line. Moreover, it has been shown that adenosine enhances Cx40-mediated GJIC, enabling the propagation of anti-adhesion signaling between endothelial cells [32].
In contrast to Cx40, Cx43 expression increases during the acute phase of inflammation at alveolar septa of damaged lungs [130]. Inhibition of Cx43-based gap junctions was found to block the increase of endothelial barrier permeability, which is responsible for alveolar edema, induced by lung acid injury [131]. Intratracheal instillation of P. aeruginosa LPS also increased the expression of Cx43 in mouse alveolar compartment [31]. In this study, the pro-inflammatory role for this Cx was demonstrated in vivo using Cx43+/− mice with reduced Cx43 expression in the lung. These mice showed decreased neutrophil recruitment to the alveolar space 24 h after induction of lung inflammation. Conversely, mice expressing a truncated Cx43 protein at amino acid 257, causing the formation of Cx43 channels that remain mostly open due to lack of regulatory motifs present in the C-terminus [132], exhibit increased neutrophil recruitment during acute lung inflammation [31]. Furthermore, mimetic-blocking peptides specific for Cx43 reduced adhesion of leukocytes to the surface of murine alveolar and endothelial cell lines, while treatment of wild-type mice with peptides reduced recruitment of neutrophils and promoted the resolution of inflammation in response to LPS instillation. Consistent with this model, imaging of the intact perfused lung showed evidence of calcium waves that propagate along pulmonary vessels through Cx43-based gap junctions [30]. A consequence of this Cx43-dependent conduction is the expression at the surface of venular endothelial cells of the leukocyte adhesion molecule P-selectin in response to an increase in calcium level in the alveolar capillary bed, thereby facilitating the transmigration of neutrophils and leukocytes across the thin alveolar endothelial-epithelial barrier. Thus, Cx40-based and Cx43-based gap junctions with their specificity in propagating pro-inflammatory or anti-inflammatory signals between endothelial cells appear as key modulators of neutrophil recruitment during acute lung inflammation. While Cx40 delays the adhesion of neutrophils to the endothelial cells early during the inflammatory response, Cx43 promotes their transmigration across the alveolar wall during the acute phase of inflammation.
Alveolar barrier repair
Recovery of proper respiratory function after lung injury requires the restoration of an intact and a functional alveolar barrier able to drain alveolar edema fluid and secrete surfactant [133]. Although ATII cells have been classically considered the only cells that can repopulate denuded alveolar epithelium, recent studies in murine model have identified progenitor cells in strategically located niches in the distal lung that contributes to alveolar repair mechanisms [133]. Cell-based therapies that have been shown to reduce lung injury and to enhance lung repair in murine models could be beneficial for treating patients with severe acute lung inflammation and injury. Novel treatment strategies include the administration of allogeneic mesenchymal stem or stromal cells (MSCs), which are connective tissue progenitor cells with multilineage differentiation potential [134]. These stem cells can be isolated not only from adult bone marrow, but also from several non-hematopoietic organs, including the lungs [135]. Interestingly, once engrafted in the lungs, MSCs are able to communicate with locally resident alveolar epithelial cells via gap junctions [136]. In a recent elegant study, Islam and colleagues have proposed an intriguing function for gap junctions in the context of MSC-based therapy for severe lung injury [137]. Using live-lung FRAP microscopy and Cx43-blocking peptides, Cx43-dependent GJIC between MSCs and alveolar epithelial cells was confirmed in murine lung after intratracheal administration of mice bone marrow-derived MSCs (BMSCs). However, formation of gap junctions occurred only under acute lung inflammation induced by E. coli LPS. In control mice treated with phosphate buffer, BMSCs were detected outside of the alveoli in pleural interstitia and in thoracic lymph nodes. As previously described, LPS increased the expression of Cx43 in alveoli, creating a Cx43-rich alveolar environment that is required for the attachment of BMSCs. Indeed, GJIC was essential for BMSCs placement at the alveoli, because mouse BMSCs expressing a dominant-negative mutant of Cx43 (Cx43T154A), resulting in the formation of closed gap junctions [138], did not communicate with epithelial cells and migrated more out of the lung [137]. LPS-induced acute lung inflammation and injury were markedly reduced by instillation of mouse BMSCs, as indicated by decreased leukocyte recruitment and alveolar barrier permeability, restoration of surfactant secretion and increase survival. In contrast, administration of BMSCs expressing Cx43T154A or small interfering RNA against Cx43 did not ameliorate the severity of the disease. The beneficial effects exerted by BMSCs required GJIC and were dependent on the ability of these cells to transfer mitochondria and supply bioenergy to the damage alveolar epithelial cells. In fact, the establishment of GJIC during alveolar attachment was shown to trigger an intracellular calcium rise in mouse BMSCs, which in turn formed nanotubes and released microvesicles containing mitochondria. BMSC-derived microvesicles were then engulfed via endocytosis by ATII cells, thereby, transferring their mitochondrial contents to the alveolar epithelium. This transfer resulted in enhanced intracellular ATP production in the inflamed lung, providing supplemental energy that may help the recovery of the alveolar cells [137].
Although other paracrine and cell contact-independent mechanisms are likely to also contribute to improved lung healing and function by MSCs in acute inflammatory diseases [133], these findings indicate that GJIC is crucial to mediate some therapeutic effects of MSCs in the repair of the alveolar barrier function. These findings should open the way for future studies addressing the role of Cxs and Panxs in lung-resident stem cells and their involvement in lung repair.
Concluding remarks
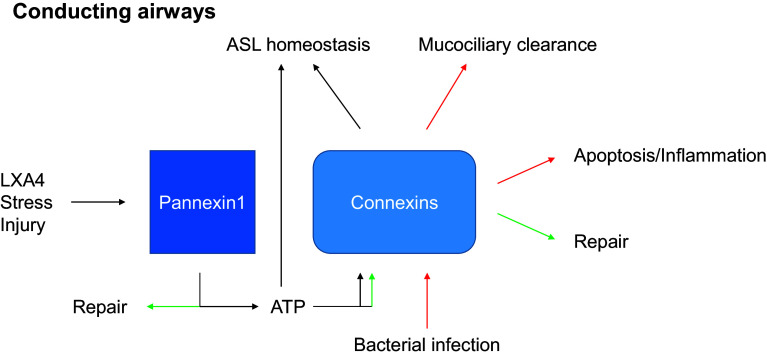
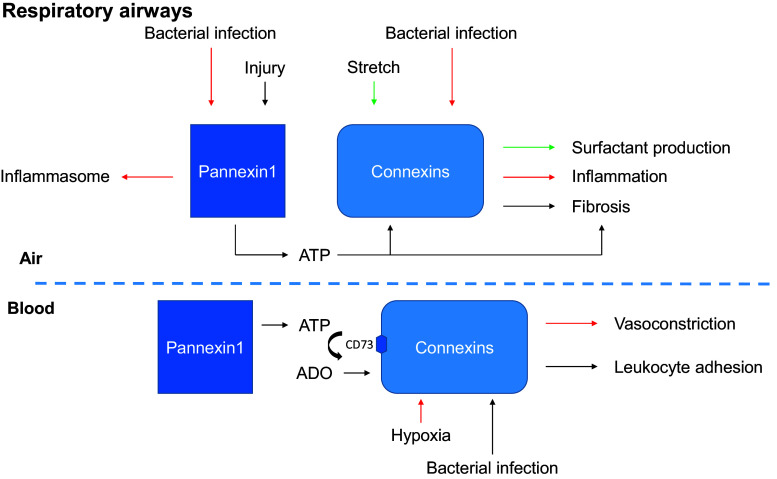
The principal function of the lungs is to bring oxygen from the atmosphere into the bloodstream while maintaining the sterility of the peripheral tissue. Respiration induces stretches of the respiratory epithelium, which provides a mechanism for surfactant secretion. The oxygen/carbon dioxide ratio contributes to contraction of the respiratory vessel walls in order to direct blood flow to areas with optimal oxygen supply. The inhaled air is filtered in the conductive airways by mucociliary clearance and, when needed, bacterial clearance is promoted via several inflammatory mechanisms involving macrophages, endothelial, and epithelial cells. In response to injury a repair process is engaged, although fibrosis can also occur [3]. To fulfill these critical functions, the different cells of the lung act as integrated systems enabled by Cxs and Panxs, which regulate extracellular and intercellular signaling, control the flow of metabolites, and restrict the flow of toxic agents. The roles of Panx1 and Cxs in the airway epithelium and the alveolar barrier of the adult lung are summarized in Figs. 3 and 4. While a diversity of gap junction proteins is involved in coupling epithelial cells and non-epithelial cells in the lung, Cx43, Cx40, and Panx1 appear so far to have critical roles in intercellular communications among epithelial cells, endothelial cells, and macrophages. Clearly, more has to be understood regarding the physiological roles of Cxs and Panxs in lung functions, which may provide the foundation to determine whether targeting these intercellular communication pathways represent an efficient approach to the treatment of lung pathologies [10].
Fig. 3.
Principal functions of Panx1 and connexins in the adult conducting airways. The functions mediated by Cxs or Panx1 for given stimulus are indicated by a color code (ASL airway surface liquid, LXA4 lipoxin A4, ATP adenosine triphosphate)
Fig. 4.
Principal functions of Panx1 and connexins in the adult respiratory airways. The functions mediated by Cxs or Panx1 for given stimulus are indicated by a color code. The basal lamina separating the air-blood compartments is indicated by a blue dashed line (ADO adenosine, ATP adenosine triphosphate, CD73 ecto-5′-nucleotidase)
Acknowledgments
This work was supported by the Swiss National Science Foundation and the “Fondation pour des Bourses d’Etudes Italo-Suisses.”
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Abbreviations
- A2B
Adenosine 2B receptor
- ANO1
Anoctamin1
- ASL
Airway surface liquid
- ATI
Alveolar type I cells
- ATII
Alveolar type II cells
- ATP
Adenosine triphosphate
- BAL
Bronchoalveolar lavage
- BMSC
Bone marrow-derived mesenchymal stem or stromal cells
- C12
N-3-oxo-dodecanoyl-l-homoserine lactone
- CaCC
Calcium-activated chloride channels
- CAMKK
Ca2+/calmodulin-dependent kinase kinase
- cAMP
Cyclic adenosine monophosphate
- CD73
Ecto-5′-nucleotidase
- CF
Cystic fibrosis
- CFTR
Cystic fibrosis transmembrane conductance regulator
- Cx(s)
Connexin(s)
- ENaC
Amiloride-sensitive epithelial channel
- FRAP
Fluorescence recovery after photobleaching
- GJIC
Gap junctional intercellular communication
- HPV
Hypoxic pulmonary vasoconstriction
- IL-8
Interleukin-8
- IP3
Inositol triphosphate
- JNK
c-Jun N-terminal kinases
- LPS
Lipopolysaccharide
- LXA4
Lipoxin A4
- MAPK
Mitogen-activated protein kinase
- MSC
Mesenchymal stem or stromal cell
- NF-κB
Nuclear factor-κB
- PAMP
Pathogen-associated molecular pattern
- Panx(s)
Pannexin(s)
- PGE2
Prostaglandin E2
- P2R
Purinergic receptor
- PRR
Pathogen recognition receptor
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor-α
References
- 1.Kierszenbaum AL. Histology and cell biology : an introduction to pathology. St. Louis: Mosby; 2002. [Google Scholar]
- 2.Gollwitzer ES, Marsland BJ. Microbiota abnormalities in inflammatory airway diseases: potential for therapy. Pharmacol Ther. 2014;141(1):32–39. doi: 10.1016/j.pharmthera.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16(1):27–35. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodenough DA, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol. 2009;1(1):a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saez JC, Leybaert L. Hunting for connexin hemichannels. FEBS Lett. 2014;588(8):1205–1211. doi: 10.1016/j.febslet.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, Naus CC, Penuela S, Scemes E, Spray DC, Thompson RJ, Zhao HB, Dahl G. Pannexin channels are not gap junction hemichannels. Channels (Austin) 2011;5(3):193–197. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta. 2013;1828(1):15–22. doi: 10.1016/j.bbamem.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Vinken M, Rogiers V, Bukauskas FF, Bultynck G, Leybaert L. Paracrine signaling through plasma membrane hemichannels. Biochim Biophys Acta. 2013;1828(1):35–50. doi: 10.1016/j.bbamem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl G, Qiu F, Wang J. The bizarre pharmacology of the ATP release channel pannexin1. Neuropharmacology. 2013;75:583–593. doi: 10.1016/j.neuropharm.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Losa D, Chanson M, Crespin S. Connexins as therapeutic targets in lung disease. Expert Opin Ther Targets. 2011;15(8):989–1002. doi: 10.1517/14728222.2011.584875. [DOI] [PubMed] [Google Scholar]
- 11.Johnson LN, Koval M. Cross-talk between pulmonary injury, oxidant stress, and gap junctional communication. Antioxid Redox Signal. 2009;11(2):355–367. doi: 10.1089/ars.2008.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carson JL, Collier AM, Hu SC, McLachlan JB. Variability in distribution and populations of gap junctions in ferret trachea during postnatal development. Am J Physiol. 1995;268(4 Pt 1):L576–L583. doi: 10.1152/ajplung.1995.268.4.L576. [DOI] [PubMed] [Google Scholar]
- 13.Carson JL, Reed W, Moats-Staats BM, Brighton LE, Gambling TM, Hu SC, Collier AM. Connexin 26 expression in human and ferret airways and lung during development. Am J Respir Cell Mol Biol. 1998;18(1):111–119. doi: 10.1165/ajrcmb.18.1.2789. [DOI] [PubMed] [Google Scholar]
- 14.Wiszniewski L, Sanz J, Scerri I, Gasparotto E, Dudez T, Lacroix JS, Suter S, Gallati S, Chanson M. Functional expression of connexin30 and connexin31 in the polarized human airway epithelium. Differentiation. 2007;75(5):382–392. doi: 10.1111/j.1432-0436.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 15.Traub O, Hertlein B, Kasper M, Eckert R, Krisciukaitis A, Hulser D, Willecke K. Characterization of the gap junction protein connexin37 in murine endothelium, respiratory epithelium, and after transfection in human HeLa cells. Eur J Cell Biol. 1998;77(4):313–322. doi: 10.1016/S0171-9335(98)80090-3. [DOI] [PubMed] [Google Scholar]
- 16.Park SJ, Lee KS, Kim SR, Min KH, Lee KY, Choe YH, Park SY, Hong SH, Lee YC. Change of connexin 37 in allergen-induced airway inflammation. Exp Mol Med. 2007;39(5):629–640. doi: 10.1038/emm.2007.69. [DOI] [PubMed] [Google Scholar]
- 17.Udaka N, Miyagi Y, Ito T. Connexin expression in mouse lung tumor. Cancer Lett. 2007;246(1–2):224–229. doi: 10.1016/j.canlet.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120(Pt 21):3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- 19.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41(5):525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L259–L271. doi: 10.1152/ajplung.00112.2007. [DOI] [PubMed] [Google Scholar]
- 21.Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol. 2015;16(1):36–44. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 22.Cesen-Cummings K, Fernstrom MJ, Malkinson AM, Ruch RJ. Frequent reduction of gap junctional intercellular communication and connexin43 expression in human and mouse lung carcinoma cells. Carcinogenesis. 1998;19(1):61–67. doi: 10.1093/carcin/19.1.61. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Huhn D, Knosel T, Pacyna-Gengelbach M, Deutschmann N, Petersen I. Downregulation of connexin 26 in human lung cancer is related to promoter methylation. Int J Cancer. 2005;113(1):14–21. doi: 10.1002/ijc.20498. [DOI] [PubMed] [Google Scholar]
- 24.Avanzo JL, Mesnil M, Hernandez-Blazquez FJ, da Silva TC, Fukumasu H, Mori CM, Yamasaki H, Dagli ML. Altered expression of connexins in urethane-induced mouse lung adenomas. Life Sci. 2006;79(23):2202–2208. doi: 10.1016/j.lfs.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 25.Lee YC, Yellowley CE, Li Z, Donahue HJ, Rannels DE. Expression of functional gap junctions in cultured pulmonary alveolar epithelial cells. Am J Physiol. 1997;272(6 Pt 1):L1105–L1114. doi: 10.1152/ajplung.1997.272.6.L1105. [DOI] [PubMed] [Google Scholar]
- 26.Abraham V, Chou ML, George P, Pooler P, Zaman A, Savani RC, Koval M. Heterocellular gap junctional communication between alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1085–L1093. doi: 10.1152/ajplung.2001.280.6.L1085. [DOI] [PubMed] [Google Scholar]
- 27.Isakson BE, Evans WH, Boitano S. Intercellular Ca2+ signaling in alveolar epithelial cells through gap junctions and by extracellular ATP. Am J Physiol Lung Cell Mol Physiol. 2001;280(2):L221–L228. doi: 10.1152/ajplung.2001.280.2.L221. [DOI] [PubMed] [Google Scholar]
- 28.De Vuyst E, Decrock E, De Bock M, Yamasaki H, Naus CC, Evans WH, Leybaert L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell. 2007;18(1):34–46. doi: 10.1091/mbc.E06-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506(7489):503–506. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parthasarathi K, Ichimura H, Monma E, Lindert J, Quadri S, Issekutz A, Bhattacharya J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest. 2006;116(8):2193–2200. doi: 10.1172/JCI26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarieddine MZ, Scheckenbach KE, Foglia B, Maass K, Garcia I, Kwak BR, Chanson M. Connexin43 modulates neutrophil recruitment to the lung. J Cell Mol Med. 2009;13(11–12):4560–4570. doi: 10.1111/j.1582-4934.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chadjichristos CE, Scheckenbach KE, van Veen TA, Richani Sarieddine MZ, de Wit C, Yang Z, Roth I, Bacchetta M, Viswambharan H, Foglia B, Dudez T, van Kempen MJ, Coenjaerts FE, Miquerol L, Deutsch U, Jongsma HJ, Chanson M, Kwak BR. Endothelial-specific deletion of connexin40 promotes atherosclerosis by increasing CD73-dependent leukocyte adhesion. Circulation. 2010;121(1):123–131. doi: 10.1161/CIRCULATIONAHA.109.867176. [DOI] [PubMed] [Google Scholar]
- 33.Lohman AW, Billaud M, Straub AC, Johnstone SR, Best AK, Lee M, Barr K, Penuela S, Laird DW, Isakson BE. Expression of pannexin isoforms in the systemic murine arterial network. J Vasc Res. 2012;49(5):405–416. doi: 10.1159/000338758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25(21):5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruttenstock EM, Doni T, Dingemann J, Puri P. Prenatal retinoic acid upregulates connexin 43 (Cx43) gene expression in pulmonary hypoplasia in the nitrofen-induced congenital diaphragmatic hernia rat model. J Pediatr Surg. 2012;47(2):336–340. doi: 10.1016/j.jpedsurg.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267(5205):1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 37.Nagata K, Masumoto K, Esumi G, Teshiba R, Yoshizaki K, Fukumoto S, Nonaka K, Taguchi T. Connexin43 plays an important role in lung development. J Pediatr Surg. 2009;44(12):2296–2301. doi: 10.1016/j.jpedsurg.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 38.Liao Y, Day KH, Damon DN, Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci USA. 2001;98(17):9989–9994. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon AM, McWhorter AR. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol. 2002;251(2):206–220. doi: 10.1006/dbio.2002.0826. [DOI] [PubMed] [Google Scholar]
- 40.de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86(6):649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- 41.Koval M, Billaud M, Straub AC, Johnstone SR, Zarbock A, Duling BR, Isakson BE. Spontaneous lung dysfunction and fibrosis in mice lacking connexin 40 and endothelial cell connexin 43. Am J Pathol. 2011;178(6):2536–2546. doi: 10.1016/j.ajpath.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trovato-Salinaro A, Trovato-Salinaro E, Failla M, Mastruzzo C, Tomaselli V, Gili E, Crimi N, Condorelli DF, Vancheri C. Altered intercellular communication in lung fibroblast cultures from patients with idiopathic pulmonary fibrosis. Respir Res. 2006;7:122. doi: 10.1186/1465-9921-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vancheri C. Common pathways in idiopathic pulmonary fibrosis and cancer. Eur Respir Rev. 2013;22(129):265–272. doi: 10.1183/09059180.00003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trosko JE, Ruch RJ. Gap junctions as targets for cancer chemoprevention and chemotherapy. Curr Drug Targets. 2002;3(6):465–482. doi: 10.2174/1389450023347371. [DOI] [PubMed] [Google Scholar]
- 45.Yoon BI, Hirabayashi Y, Kawasaki Y, Tsuboi I, Ott T, Kodama Y, Kanno J, Kim DY, Willecke K, Inoue T. Exacerbation of benzene pneumotoxicity in connexin 32 knockout mice: enhanced proliferation of CYP2E1-immunoreactive alveolar epithelial cells. Toxicology. 2004;195(1):19–29. doi: 10.1016/j.tox.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 46.King TJ, Lampe PD. The gap junction protein connexin32 is a mouse lung tumor suppressor. Cancer Res. 2004;64(20):7191–7196. doi: 10.1158/0008-5472.CAN-04-0624. [DOI] [PubMed] [Google Scholar]
- 47.King TJ, Gurley KE, Prunty J, Shin JL, Kemp CJ, Lampe PD. Deficiency in the gap junction protein connexin32 alters p27Kip1 tumor suppression and MAPK activation in a tissue-specific manner. Oncogene. 2005;24(10):1718–1726. doi: 10.1038/sj.onc.1208355. [DOI] [PubMed] [Google Scholar]
- 48.Fukumasu H, Avanzo JL, Sanches DS, Mennecier G, Mori CM, Dagli ML. Higher susceptibility of spontaneous and NNK-induced lung neoplasms in connexin 43 deficient CD1× AJ F1 mice: paradoxical expression of connexin 43 during lung carcinogenesis. Mol Carcinog. 2013;52(7):497–506. doi: 10.1002/mc.21884. [DOI] [PubMed] [Google Scholar]
- 49.de Oliveira KD, Tedardi MV, Cogliati B, Dagli ML. Higher incidence of lung adenocarcinomas induced by DMBA in connexin 43 heterozygous knockout mice. Biomed Res Int. 2013;2013:618475. doi: 10.1155/2013/618475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avanzo JL, Mennecier G, Mesnil M, Hernandez-Blazquez FJ, Fukumasu H, da Silva TC, Rao KV, Dagli ML. Deletion of a single allele of Cx43 is associated with a reduction in the gap junctional intercellular communication and increased cell proliferation of mouse lung pneumocytes type II. Cell Prolif. 2007;40(3):411–421. doi: 10.1111/j.1365-2184.2007.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ito A, Koma Y, Uchino K, Okada T, Ohbayashi C, Tsubota N, Okada M. Increased expression of connexin 26 in the invasive component of lung squamous cell carcinoma: significant correlation with poor prognosis. Cancer Lett. 2006;234(2):239–248. doi: 10.1016/j.canlet.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 52.Cronier L, Crespin S, Strale PO, Defamie N, Mesnil M. Gap junctions and cancer: new functions for an old story. Antioxid Redox Signal. 2009;11(2):323–338. doi: 10.1089/ars.2008.2153. [DOI] [PubMed] [Google Scholar]
- 53.Celetti SJ, Cowan KN, Penuela S, Shao Q, Churko J, Laird DW. Implications of pannexin 1 and pannexin 3 for keratinocyte differentiation. J Cell Sci. 2010;123(Pt 8):1363–1372. doi: 10.1242/jcs.056093. [DOI] [PubMed] [Google Scholar]
- 54.Vogt A, Hormuzdi SG, Monyer H. Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Brain Res Mol Brain Res. 2005;141(1):113–120. doi: 10.1016/j.molbrainres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Swayne LA, Sorbara CD, Bennett SA. Pannexin 2 is expressed by postnatal hippocampal neural progenitors and modulates neuronal commitment. J Biol Chem. 2010;285(32):24977–24986. doi: 10.1074/jbc.M110.130054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwamoto T, Nakamura T, Doyle A, Ishikawa M, de Vega S, Fukumoto S, Yamada Y. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem. 2010;285(24):18948–18958. doi: 10.1074/jbc.M110.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ishikawa M, Iwamoto T, Nakamura T, Doyle A, Fukumoto S, Yamada Y. Pannexin 3 functions as an ER Ca(2+) channel, hemichannel, and gap junction to promote osteoblast differentiation. J Cell Biol. 2011;193(7):1257–1274. doi: 10.1083/jcb.201101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lai CP, Bechberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC. Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer Res. 2007;67(4):1545–1554. doi: 10.1158/0008-5472.CAN-06-1396. [DOI] [PubMed] [Google Scholar]
- 59.Lai CP, Bechberger JF, Naus CC. Pannexin2 as a novel growth regulator in C6 glioma cells. Oncogene. 2009;28(49):4402–4408. doi: 10.1038/onc.2009.283. [DOI] [PubMed] [Google Scholar]
- 60.Cowan KN, Langlois S, Penuela S, Cowan BJ, Laird DW. Pannexin1 and Pannexin3 exhibit distinct localization patterns in human skin appendages and are regulated during keratinocyte differentiation and carcinogenesis. Cell Commun Adhes. 2012;19(3–4):45–53. doi: 10.3109/15419061.2012.712575. [DOI] [PubMed] [Google Scholar]
- 61.Penuela S, Gyenis L, Ablack A, Churko JM, Berger AC, Litchfield DW, Lewis JD, Laird DW. Loss of pannexin 1 attenuates melanoma progression by reversion to a melanocytic phenotype. J Biol Chem. 2012;287(34):29184–29193. doi: 10.1074/jbc.M112.377176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Penuela S, Kelly JJ, Churko JM, Barr KJ, Berger AC, Laird DW. Panx1 regulates cellular properties of keratinocytes and dermal fibroblasts in skin development and wound healing. J Invest Dermatol. 2014;134(7):2026–2035. doi: 10.1038/jid.2014.86. [DOI] [PubMed] [Google Scholar]
- 63.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109(5):571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanderson MJ, Chow I, Dirksen ER. Intercellular communication between ciliated cells in culture. Am J Physiol. 1988;254(1 Pt 1):C63–C74. doi: 10.1152/ajpcell.1988.254.1.C63. [DOI] [PubMed] [Google Scholar]
- 66.Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258(5080):292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 67.Davis CW, Lazarowski E. Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling. Respir Physiol Neurobiol. 2008;163(1–3):208–213. doi: 10.1016/j.resp.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol. 2000;150(6):1349–1360. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ohbuchi T, Takenaga F, Hohchi N, Wakasugi T, Ueta Y, Suzuki H. Possible contribution of pannexin-1 to ATP release in human upper airway epithelia. Physiol Rep. 2014;2(2):e00227. doi: 10.1002/phy2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O’Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011;286(30):26277–26286. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seminario-Vidal L, Kreda S, Jones L, O’Neal W, Trejo J, Boucher RC, Lazarowski ER. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of rho- and Ca2+-dependent signaling pathways. J Biol Chem. 2009;284(31):20638–20648. doi: 10.1074/jbc.M109.004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richter K, Kiefer KP, Grzesik BA, Clauss WG, Fronius M. Hydrostatic pressure activates ATP-sensitive K+ channels in lung epithelium by ATP release through pannexin and connexin hemichannels. FASEB J. 2014;28(1):45–55. doi: 10.1096/fj.13-229252. [DOI] [PubMed] [Google Scholar]
- 73.Kreda SM, Seminario-Vidal L, van Heusden CA, O’Neal W, Jones L, Boucher RC, Lazarowski ER. Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J Physiol. 2010;588(Pt 12):2255–2267. doi: 10.1113/jphysiol.2009.186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Randell SH, Boucher RC, University of North Carolina Virtual Lung G, Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol. 2006;35(1):20–28. doi: 10.1165/rcmb.2006-0082SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wine JJ, Joo NS. Submucosal glands and airway defense. Proc Am Thorac Soc. 2004;1(1):47–53. doi: 10.1513/pats.2306015. [DOI] [PubMed] [Google Scholar]
- 76.Scheckenbach KE, Losa D, Dudez T, Bacchetta M, O’Grady S, Crespin S, Chanson M. Prostaglandin E(2)regulation of cystic fibrosis transmembrane conductance regulator activity and airway surface liquid volume requires gap junctional communication. Am J Respir Cell Mol Biol. 2011;44(1):74–82. doi: 10.1165/rcmb.2009-0361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Higgins G, Buchanan P, Perriere M, Al-Alawi M, Costello RW, Verriere V, McNally P, Harvey BJ, Urbach V. Activation of P2RY11 and ATP release by lipoxin A4 restores the airway surface liquid layer and epithelial repair in cystic fibrosis. Am J Respir Cell Mol Biol. 2014;51(2):178–190. doi: 10.1165/rcmb.2012-0424OC. [DOI] [PubMed] [Google Scholar]
- 78.Verriere V, Higgins G, Al-Alawi M, Costello RW, McNally P, Chiron R, Harvey BJ, Urbach V. Lipoxin A4 stimulates calcium-activated chloride currents and increases airway surface liquid height in normal and cystic fibrosis airway epithelia. PLoS ONE. 2012;7(5):e37746. doi: 10.1371/journal.pone.0037746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Alawi M, Buchanan P, Verriere V, Higgins G, McCabe O, Costello RW, McNally P, Urbach V, Harvey BJ. Physiological levels of lipoxin A4 inhibit ENaC and restore airway surface liquid height in cystic fibrosis bronchial epithelium. Physiol Rep. 2014 doi: 10.14814/phy2.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kovach MA, Standiford TJ. Toll like receptors in diseases of the lung. Int Immunopharmacol. 2011;11(10):1399–1406. doi: 10.1016/j.intimp.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barton GM, Kagan JC. A cell biological view of toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9(8):535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henson PM, Tuder RM. Apoptosis in the lung: induction, clearance and detection. Am J Physiol Lung Cell Mol Physiol. 2008;294(4):L601–L611. doi: 10.1152/ajplung.00320.2007. [DOI] [PubMed] [Google Scholar]
- 83.Hertz CJ, Wu Q, Porter EM, Zhang YJ, Weismuller KH, Godowski PJ, Ganz T, Randell SH, Modlin RL. Activation of toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol. 2003;171(12):6820–6826. doi: 10.4049/jimmunol.171.12.6820. [DOI] [PubMed] [Google Scholar]
- 84.Yu FS, Cornicelli MD, Kovach MA, Newstead MW, Zeng X, Kumar A, Gao N, Yoon SG, Gallo RL, Standiford TJ. Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. J Immunol. 2010;185(2):1142–1149. doi: 10.4049/jimmunol.1000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ben Mohamed F, Garcia-Verdugo I, Medina M, Balloy V, Chignard M, Ramphal R, Touqui L. A crucial role of Flagellin in the induction of airway mucus production by Pseudomonas aeruginosa . PLoS ONE. 2012;7(7):e39888. doi: 10.1371/journal.pone.0039888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kunzelmann K, Scheidt K, Scharf B, Ousingsawat J, Schreiber R, Wainwright B, McMorran B. Flagellin of Pseudomonas aeruginosa inhibits Na+ transport in airway epithelia. FASEB J. 2006;20(3):545–546. doi: 10.1096/fj.05-4454fje. [DOI] [PubMed] [Google Scholar]
- 87.Illek B, Fu Z, Schwarzer C, Banzon T, Jalickee S, Miller SS, Machen TE. Flagellin-stimulated Cl- secretion and innate immune responses in airway epithelia: role for p38. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L531–L542. doi: 10.1152/ajplung.90292.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buyck JM, Verriere V, Benmahdi R, Higgins G, Guery B, Matran R, Harvey BJ, Faure K, Urbach V. P. aeruginosa LPS stimulates calcium signaling and chloride secretion via CFTR in human bronchial epithelial cells. J Cyst Fibros. 2013;12(1):60–67. doi: 10.1016/j.jcf.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 89.Martin FJ, Prince AS. TLR2 regulates gap junction intercellular communication in airway cells. J Immunol. 2008;180(7):4986–4993. doi: 10.4049/jimmunol.180.7.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chanson M, Berclaz PY, Scerri I, Dudez T, Wernke-Dollries K, Pizurki L, Pavirani A, Fiedler MA, Suter S. Regulation of gap junctional communication by a pro-inflammatory cytokine in cystic fibrosis transmembrane conductance regulator-expressing but not cystic fibrosis airway cells. Am J Pathol. 2001;158(5):1775–1784. doi: 10.1016/S0002-9440(10)64133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang S, Jornot L, Wiszniewski L, Rochat T, Suter S, Lacroix JS, Chanson M. Src signaling links mediators of inflammation to Cx43 gap junction channels in primary and transformed CFTR-expressing airway cells. Cell Commun Adhes. 2003;10(4–6):279–285. doi: 10.1080/cac.10.4-6.279.285. [DOI] [PubMed] [Google Scholar]
- 92.Huang S, Dudez T, Scerri I, Thomas MA, Giepmans BN, Suter S, Chanson M. Defective activation of c-Src in cystic fibrosis airway epithelial cells results in loss of tumor necrosis factor-alpha-induced gap junction regulation. J Biol Chem. 2003;278(10):8326–8332. doi: 10.1074/jbc.M208264200. [DOI] [PubMed] [Google Scholar]
- 93.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352(19):1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 94.Losa D, Kohler T, Bellec J, Dudez T, Crespin S, Bacchetta M, Boulanger P, Hong SS, Morel S, Nguyen TH, van Delden C, Chanson M. Pseudomonas aeruginosa-induced apoptosis in airway epithelial cells is mediated by gap junctional communication in a JNK-dependent manner. J Immunol. 2014;192(10):4804–4812. doi: 10.4049/jimmunol.1301294. [DOI] [PubMed] [Google Scholar]
- 95.Grassme H, Kirschnek S, Riethmueller J, Riehle A, von Kurthy G, Lang F, Weller M, Gulbins E. CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa . Science. 2000;290(5491):527–530. doi: 10.1126/science.290.5491.527. [DOI] [PubMed] [Google Scholar]
- 96.Cannon CL, Kowalski MP, Stopak KS, Pier GB. Pseudomonas aeruginosa-induced apoptosis is defective in respiratory epithelial cells expressing mutant cystic fibrosis transmembrane conductance regulator. Am J Respir Cell Mol Biol. 2003;29(2):188–197. doi: 10.1165/rcmb.4898. [DOI] [PubMed] [Google Scholar]
- 97.Grassme H, Becker KA, Zhang Y, Gulbins E. CFTR-dependent susceptibility of the cystic fibrosis-host to Pseudomonas aeruginosa . Int J Med Microbiol. 2010;300(8):578–583. doi: 10.1016/j.ijmm.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 98.Wansleeben C, Barkauskas CE, Rock JR, Hogan BL. Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip Rev Dev Biol. 2013;2(1):131–148. doi: 10.1002/wdev.58. [DOI] [PubMed] [Google Scholar]
- 99.Buchanan PJ, McNally P, Harvey BJ, Urbach V. Lipoxin A(4)-mediated KATP potassium channel activation results in cystic fibrosis airway epithelial repair. Am J Physiol Lung Cell Mol Physiol. 2013;305(2):L193–L201. doi: 10.1152/ajplung.00058.2013. [DOI] [PubMed] [Google Scholar]
- 100.Crespin S, Bacchetta M, Bou Saab J, Tantilipikorn P, Bellec J, Dudez T, Nguyen TH, Kwak BR, Lacroix JS, Huang S, Wiszniewski L, Chanson M. Cx26 regulates proliferation of repairing basal airway epithelial cells. Int J Biochem Cell Biol. 2014;52:152–160. doi: 10.1016/j.biocel.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 101.Chandrasekhar A, Kalmykov EA, Polusani SR, Mathis SA, Zucker SN, Nicholson BJ. Intercellular redistribution of cAMP underlies selective suppression of cancer cell growth by connexin26. PLoS ONE. 2013;8(12):e82335. doi: 10.1371/journal.pone.0082335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shiner EK, Rumbaugh KP, Williams SC. Inter-kingdom signaling: deciphering the language of acyl homoserine lactones. FEMS Microbiol Rev. 2005;29(5):935–947. doi: 10.1016/j.femsre.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 103.Losa D, Kohler T, Bacchetta M, Bou Saab J, Frieden M, van Delden C, Chanson M. Airway epithelial cell integrity protects from cytotoxicity of P. aeruginosa quorum-sensing signals. Am J Respir Cell Mol Biol. 2015 doi: 10.1165/rcmb.2014-0405OC. [DOI] [PubMed] [Google Scholar]
- 104.Ward JP, McMurtry IF. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol. 2009;9(3):287–296. doi: 10.1016/j.coph.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L, Yin J, Nickles HT, Ranke H, Tabuchi A, Hoffmann J, Tabeling C, Barbosa-Sicard E, Chanson M, Kwak BR, Shin HS, Wu S, Isakson BE, Witzenrath M, de Wit C, Fleming I, Kuppe H, Kuebler WM. Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J Clin Invest. 2012;122(11):4218–4230. doi: 10.1172/JCI59176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yen CH, Leu S, Lin YC, Kao YH, Chang LT, Chua S, Fu M, Wu CJ, Sun CK, Yip HK. Sildenafil limits monocrotaline-induced pulmonary hypertension in rats through suppression of pulmonary vascular remodeling. J Cardiovasc Pharmacol. 2010;55(6):574–584. doi: 10.1097/FJC.0b013e3181d9f5f4. [DOI] [PubMed] [Google Scholar]
- 107.Billaud M, Dahan D, Marthan R, Savineau JP, Guibert C. Role of the gap junctions in the contractile response to agonists in pulmonary artery from two rat models of pulmonary hypertension. Respir Res. 2011;12:30. doi: 10.1186/1465-9921-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ichimura H, Parthasarathi K, Lindert J, Bhattacharya J. Lung surfactant secretion by interalveolar Ca2+ signaling. Am J Physiol Lung Cell Mol Physiol. 2006;291(4):L596–L601. doi: 10.1152/ajplung.00036.2006. [DOI] [PubMed] [Google Scholar]
- 109.Patel AS, Reigada D, Mitchell CH, Bates SR, Margulies SS, Koval M. Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am J Physiol Lung Cell Mol Physiol. 2005;289(3):L489–L496. doi: 10.1152/ajplung.00074.2005. [DOI] [PubMed] [Google Scholar]
- 110.Ashino Y, Ying X, Dobbs LG, Bhattacharya J. [Ca(2+)](i) oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am J Physiol Lung Cell Mol Physiol. 2000;279(1):L5–L13. doi: 10.1152/ajplung.2000.279.1.L5. [DOI] [PubMed] [Google Scholar]
- 111.Wang PM, Fujita E, Bhattacharya J. Vascular regulation of type II cell exocytosis. Am J Physiol Lung Cell Mol Physiol. 2002;282(5):L912–L916. doi: 10.1152/ajplung.00303.2001. [DOI] [PubMed] [Google Scholar]
- 112.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 113.Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol. 2011;186(11):6553–6561. doi: 10.4049/jimmunol.1100478. [DOI] [PubMed] [Google Scholar]
- 114.Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest. 2013;123(4):1630–1637. doi: 10.1172/JCI66142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wonnenberg B, Tschernig T, Voss M, Bischoff M, Meier C, Schirmer SH, Langer F, Bals R, Beisswenger C. Probenecid reduces infection and inflammation in acute Pseudomonas aeruginosa pneumonia. Int J Med Microbiol. 2014;304(5–6):725–729. doi: 10.1016/j.ijmm.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 116.Kafka D, Ling E, Feldman G, Benharroch D, Voronov E, Givon-Lavi N, Iwakura Y, Dagan R, Apte RN, Mizrachi-Nebenzahl Y. Contribution of IL-1 to resistance to Streptococcus pneumoniae infection. Int Immunol. 2008;20(9):1139–1146. doi: 10.1093/intimm/dxn071. [DOI] [PubMed] [Google Scholar]
- 117.McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, Moran B, Fitzgerald KA, Tschopp J, Petrilli V, Andrew PW, Kadioglu A, Lavelle EC. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 2010;6(11):e1001191. doi: 10.1371/journal.ppat.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11(3):201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3(125):ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bao Y, Chen Y, Ledderose C, Li L, Junger WG. Pannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophils. J Biol Chem. 2013;288(31):22650–22657. doi: 10.1074/jbc.M113.476283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Burnstock G, Brouns I, Adriaensen D, Timmermans JP. Purinergic signaling in the airways. Pharmacol Rev. 2012;64(4):834–868. doi: 10.1124/pr.111.005389. [DOI] [PubMed] [Google Scholar]
- 122.Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, Ryffel B, Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182(6):774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 123.Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, Chanson M, Goodenough DA, Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med. 2006;12(8):950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- 124.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99(10):1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 125.Zhang J, Wang W, Sun J, Li Q, Liu J, Zhu H, Chen T, Wang H, Yu S, Sun G, Chen W, Yi D. Gap junction channel modulates pulmonary vascular permeability through calcium in acute lung injury: an experimental study. Respiration. 2010;80(3):236–245. doi: 10.1159/000274384. [DOI] [PubMed] [Google Scholar]
- 126.Li Q, Zhang J, Wang W, Liu J, Zhu H, Chen W, Chen T, Yu S, Wang H, Sun G, Yi D. Connexin40 modulates pulmonary permeability through gap junction channel in acute lung injury after thoracic gunshot wounds. J Trauma. 2010;68(4):802–809. doi: 10.1097/TA.0b013e3181bb80ea. [DOI] [PubMed] [Google Scholar]
- 127.Rignault S, Haefliger JA, Waeber B, Liaudet L, Feihl F. Acute inflammation decreases the expression of connexin 40 in mouse lung. Shock. 2007;28(1):78–85. doi: 10.1097/shk.0b013e3180310bd1. [DOI] [PubMed] [Google Scholar]
- 128.Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 2009;24:298–306. doi: 10.1152/physiol.00022.2009. [DOI] [PubMed] [Google Scholar]
- 129.Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med. 1998;188(8):1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kasper M, Traub O, Reimann T, Bjermer L, Grossmann H, Muller M, Wenzel KW. Upregulation of gap junction protein connexin43 in alveolar epithelial cells of rats with radiation-induced pulmonary fibrosis. Histochem Cell Biol. 1996;106(4):419–424. doi: 10.1007/BF02473301. [DOI] [PubMed] [Google Scholar]
- 131.Parthasarathi K. Endothelial connexin43 mediates acid-induced increases in pulmonary microvascular permeability. Am J Physiol Lung Cell Mol Physiol. 2012;303(1):L33–L42. doi: 10.1152/ajplung.00219.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Moreno AP, Chanson M, Elenes S, Anumonwo J, Scerri I, Gu H, Taffet SM, Delmar M. Role of the carboxyl terminal of connexin43 in transjunctional fast voltage gating. Circ Res. 2002;90(4):450–457. doi: 10.1161/hh0402.105667. [DOI] [PubMed] [Google Scholar]
- 133.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 134.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S, Wang Z, Liao H, Toews GB, Krebsbach PH, Peters-Golden M, Pinsky DJ, Martinez FJ, Thannickal VJ. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest. 2007;117(4):989–996. doi: 10.1172/JCI29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Badri L, Walker NM, Ohtsuka T, Wang Z, Delmar M, Flint A, Peters-Golden M, Toews GB, Pinsky DJ, Krebsbach PH, Lama VN. Epithelial interactions and local engraftment of lung-resident mesenchymal stem cells. Am J Respir Cell Mol Biol. 2011;45(4):809–816. doi: 10.1165/rcmb.2010-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Beahm DL, Oshima A, Gaietta GM, Hand GM, Smock AE, Zucker SN, Toloue MM, Chandrasekhar A, Nicholson BJ, Sosinsky GE. Mutation of a conserved threonine in the third transmembrane helix of alpha- and beta-connexins creates a dominant-negative closed gap junction channel. J Biol Chem. 2006;281(12):7994–8009. doi: 10.1074/jbc.M506533200. [DOI] [PubMed] [Google Scholar]